User login
The Next Generation of Anticoagulants?
The popularity of the next generation of anticoagulation therapies could be dependent on whether reversing agents for the newest drugs can be developed, says a hospitalist who heads an antithrombotic clinic.
In October, the FDA approved dabigatran etexilate (Pradaxa) for atrial fibrillation (AF) patients. In a noninferiority study published last month, investigators found that treatment with oral rivaroxaban alone (15mg twice daily for three weeks, followed by 20mg once daily) showed effectiveness versus subcutaneous enoxaparin followed by a vitamin K antagonist. In relation to the primary outcome of recurrent DVT, rivaroxaban had noninferior efficacy (36 events [2.1%], vs. 51 events, 0.44 to 1.04; P<0.001) (N Engl J Med. 2010;363:2499-2510).
Another study, dubbed ROCKET-AF (PDF) and unveiled at an American Heart Association meeting in November, reported that rivaroxaban was noninferior to warfarin in the treatment of stroke and non-CNS embolism. Study patients treated with rivaroxaban exhibited significantly less events (1.71) per 100 patient-years (188 patients) compared with those on warfarin (2.16; 241 patients; P<0.001 for noninferiority, P=0.018 for superiority).
A third medication, apixaban, which also acts as a direct
fact Xa inhibitor, is currently being tested in clinical trials.
Geno Merli, MD, senior vice president and chief medical officer at Thomas Jefferson University Hospital and head of the Jefferson Antithrombotic Therapy Service, both in Philadelphia, says one of the most pressing issues with the Xa inhibitors is that there is not yet a reversing agent for the drugs should complications arise. “I can reverse Coumadin,” Dr. Merli says. “I can give vitamin K or fresh frozen plasma. You’re giving back the factors that were affected.”
Dr. Merli adds that pharmaceutical companies already are working on development of reversing agents and antibodies, but until those are approved, some physicians might shy away from new anticoagulant therapies. Still, he encourages physicians to get the medications added to their respective hospitals’ medicine cabinets as quickly as feasible.
“You’ve got to have it on your formulary because you have to know the drug,” Dr. Merli says. “You have to have it for the doctor who will choose to use it or the patient who comes in already on it.”
The popularity of the next generation of anticoagulation therapies could be dependent on whether reversing agents for the newest drugs can be developed, says a hospitalist who heads an antithrombotic clinic.
In October, the FDA approved dabigatran etexilate (Pradaxa) for atrial fibrillation (AF) patients. In a noninferiority study published last month, investigators found that treatment with oral rivaroxaban alone (15mg twice daily for three weeks, followed by 20mg once daily) showed effectiveness versus subcutaneous enoxaparin followed by a vitamin K antagonist. In relation to the primary outcome of recurrent DVT, rivaroxaban had noninferior efficacy (36 events [2.1%], vs. 51 events, 0.44 to 1.04; P<0.001) (N Engl J Med. 2010;363:2499-2510).
Another study, dubbed ROCKET-AF (PDF) and unveiled at an American Heart Association meeting in November, reported that rivaroxaban was noninferior to warfarin in the treatment of stroke and non-CNS embolism. Study patients treated with rivaroxaban exhibited significantly less events (1.71) per 100 patient-years (188 patients) compared with those on warfarin (2.16; 241 patients; P<0.001 for noninferiority, P=0.018 for superiority).
A third medication, apixaban, which also acts as a direct
fact Xa inhibitor, is currently being tested in clinical trials.
Geno Merli, MD, senior vice president and chief medical officer at Thomas Jefferson University Hospital and head of the Jefferson Antithrombotic Therapy Service, both in Philadelphia, says one of the most pressing issues with the Xa inhibitors is that there is not yet a reversing agent for the drugs should complications arise. “I can reverse Coumadin,” Dr. Merli says. “I can give vitamin K or fresh frozen plasma. You’re giving back the factors that were affected.”
Dr. Merli adds that pharmaceutical companies already are working on development of reversing agents and antibodies, but until those are approved, some physicians might shy away from new anticoagulant therapies. Still, he encourages physicians to get the medications added to their respective hospitals’ medicine cabinets as quickly as feasible.
“You’ve got to have it on your formulary because you have to know the drug,” Dr. Merli says. “You have to have it for the doctor who will choose to use it or the patient who comes in already on it.”
The popularity of the next generation of anticoagulation therapies could be dependent on whether reversing agents for the newest drugs can be developed, says a hospitalist who heads an antithrombotic clinic.
In October, the FDA approved dabigatran etexilate (Pradaxa) for atrial fibrillation (AF) patients. In a noninferiority study published last month, investigators found that treatment with oral rivaroxaban alone (15mg twice daily for three weeks, followed by 20mg once daily) showed effectiveness versus subcutaneous enoxaparin followed by a vitamin K antagonist. In relation to the primary outcome of recurrent DVT, rivaroxaban had noninferior efficacy (36 events [2.1%], vs. 51 events, 0.44 to 1.04; P<0.001) (N Engl J Med. 2010;363:2499-2510).
Another study, dubbed ROCKET-AF (PDF) and unveiled at an American Heart Association meeting in November, reported that rivaroxaban was noninferior to warfarin in the treatment of stroke and non-CNS embolism. Study patients treated with rivaroxaban exhibited significantly less events (1.71) per 100 patient-years (188 patients) compared with those on warfarin (2.16; 241 patients; P<0.001 for noninferiority, P=0.018 for superiority).
A third medication, apixaban, which also acts as a direct
fact Xa inhibitor, is currently being tested in clinical trials.
Geno Merli, MD, senior vice president and chief medical officer at Thomas Jefferson University Hospital and head of the Jefferson Antithrombotic Therapy Service, both in Philadelphia, says one of the most pressing issues with the Xa inhibitors is that there is not yet a reversing agent for the drugs should complications arise. “I can reverse Coumadin,” Dr. Merli says. “I can give vitamin K or fresh frozen plasma. You’re giving back the factors that were affected.”
Dr. Merli adds that pharmaceutical companies already are working on development of reversing agents and antibodies, but until those are approved, some physicians might shy away from new anticoagulant therapies. Still, he encourages physicians to get the medications added to their respective hospitals’ medicine cabinets as quickly as feasible.
“You’ve got to have it on your formulary because you have to know the drug,” Dr. Merli says. “You have to have it for the doctor who will choose to use it or the patient who comes in already on it.”
In the Literature: Research You Need to Know
Clinical question: What is the relative efficacy of trimethoprim/sulfamethoxazole (TMP/sulfa) versus ciprofloxacin for the treatment of severe exacerbations of COPD?
Background: The use of antimicrobials in the treatment of COPD exacerbations is well accepted, with the original studies using amoxicillin, TMP/sulfa, and tetracyclines. Whether newer antimicrobial agents offer greater efficacy versus these standard agents remains uncertain.
Study design: Randomized, double-blind, placebo-controlled (double-dummy), noninferiority trial.
Setting: Two academic medical ICUs in Tunisia.
Synopsis: Consecutive patients (n=170) with severe exacerbations of COPD requiring mechanical ventilation were randomized to standard medical therapy plus either TMP/sulfa or ciprofloxacin. Patients had a prior diagnosis of COPD and the clinical presence of purulent sputum and respiratory failure. The study excluded those who were immunosuppressed, had significant hepatic or renal disease, pneumonia, recent antibiotic use, active cancer, or inability to take oral medications.
The primary endpoint of hospital death and the need for an additional course of antibiotics was no different between the groups (16.4% with TMP/sulfa versus 15.3% with ciprofloxacin). The mean exacerbation-free interval, days of mechanical ventilation, and length of stay were no different. Noninferiority was defined as <10% relative difference.
Bottom line: TMP/sulfa was noninferior to ciprofloxacin in the treatment of severe exacerbations of COPD requiring mechanical ventilation.
Citation: Nouira S, Marghli S, Besbes L, et al. Standard versus newer antibacterial agents in the treatment of severe acute exacerbations of chronic obstructive pulmonary disease: a randomized trial of trimethoprim-sulfamethoxazole versus ciprofloxacin. Clin Inf Dis. 2010;51:143-149.
For more physician reviews of HM-related research, visit our website.
Clinical question: What is the relative efficacy of trimethoprim/sulfamethoxazole (TMP/sulfa) versus ciprofloxacin for the treatment of severe exacerbations of COPD?
Background: The use of antimicrobials in the treatment of COPD exacerbations is well accepted, with the original studies using amoxicillin, TMP/sulfa, and tetracyclines. Whether newer antimicrobial agents offer greater efficacy versus these standard agents remains uncertain.
Study design: Randomized, double-blind, placebo-controlled (double-dummy), noninferiority trial.
Setting: Two academic medical ICUs in Tunisia.
Synopsis: Consecutive patients (n=170) with severe exacerbations of COPD requiring mechanical ventilation were randomized to standard medical therapy plus either TMP/sulfa or ciprofloxacin. Patients had a prior diagnosis of COPD and the clinical presence of purulent sputum and respiratory failure. The study excluded those who were immunosuppressed, had significant hepatic or renal disease, pneumonia, recent antibiotic use, active cancer, or inability to take oral medications.
The primary endpoint of hospital death and the need for an additional course of antibiotics was no different between the groups (16.4% with TMP/sulfa versus 15.3% with ciprofloxacin). The mean exacerbation-free interval, days of mechanical ventilation, and length of stay were no different. Noninferiority was defined as <10% relative difference.
Bottom line: TMP/sulfa was noninferior to ciprofloxacin in the treatment of severe exacerbations of COPD requiring mechanical ventilation.
Citation: Nouira S, Marghli S, Besbes L, et al. Standard versus newer antibacterial agents in the treatment of severe acute exacerbations of chronic obstructive pulmonary disease: a randomized trial of trimethoprim-sulfamethoxazole versus ciprofloxacin. Clin Inf Dis. 2010;51:143-149.
For more physician reviews of HM-related research, visit our website.
Clinical question: What is the relative efficacy of trimethoprim/sulfamethoxazole (TMP/sulfa) versus ciprofloxacin for the treatment of severe exacerbations of COPD?
Background: The use of antimicrobials in the treatment of COPD exacerbations is well accepted, with the original studies using amoxicillin, TMP/sulfa, and tetracyclines. Whether newer antimicrobial agents offer greater efficacy versus these standard agents remains uncertain.
Study design: Randomized, double-blind, placebo-controlled (double-dummy), noninferiority trial.
Setting: Two academic medical ICUs in Tunisia.
Synopsis: Consecutive patients (n=170) with severe exacerbations of COPD requiring mechanical ventilation were randomized to standard medical therapy plus either TMP/sulfa or ciprofloxacin. Patients had a prior diagnosis of COPD and the clinical presence of purulent sputum and respiratory failure. The study excluded those who were immunosuppressed, had significant hepatic or renal disease, pneumonia, recent antibiotic use, active cancer, or inability to take oral medications.
The primary endpoint of hospital death and the need for an additional course of antibiotics was no different between the groups (16.4% with TMP/sulfa versus 15.3% with ciprofloxacin). The mean exacerbation-free interval, days of mechanical ventilation, and length of stay were no different. Noninferiority was defined as <10% relative difference.
Bottom line: TMP/sulfa was noninferior to ciprofloxacin in the treatment of severe exacerbations of COPD requiring mechanical ventilation.
Citation: Nouira S, Marghli S, Besbes L, et al. Standard versus newer antibacterial agents in the treatment of severe acute exacerbations of chronic obstructive pulmonary disease: a randomized trial of trimethoprim-sulfamethoxazole versus ciprofloxacin. Clin Inf Dis. 2010;51:143-149.
For more physician reviews of HM-related research, visit our website.
Pneumonia Readmission Validation
Hospital readmissions are emblematic of the numerous challenges facing the US health care system. Despite high levels of spending, nearly 20% of Medicare beneficiaries are readmitted within 30 days of hospital discharge, many readmissions are considered preventable, and rates vary widely by hospital and region.1 Further, while readmissions have been estimated to cost taxpayers as much as $17 billion annually, the current fee‐for‐service method of paying for the acute care needs of seniors rewards hospitals financially for readmission, not their prevention.2
Pneumonia is the second most common reason for hospitalization among Medicare beneficiaries, accounting for approximately 650,000 admissions annually,3 and has been a focus of national quality‐improvement efforts for more than a decade.4, 5 Despite improvements in key processes of care, rates of readmission within 30 days of discharge following a hospitalization for pneumonia have been reported to vary from 10% to 24%.68 Among several factors, readmissions are believed to be influenced by the quality of both inpatient and outpatient care, and by care‐coordination activities occurring in the transition from inpatient to outpatient status.912
Public reporting of hospital performance is considered a key strategy for improving quality, reducing costs, and increasing the value of hospital care, both in the US and worldwide.13 In 2009, the Centers for Medicare & Medicaid Services (CMS) expanded its reporting initiatives by adding risk‐adjusted hospital readmission rates for acute myocardial infarction, heart failure, and pneumonia to the Hospital Compare website.14, 15 Readmission rates are an attractive focus for public reporting for several reasons. First, in contrast to most process‐based measures of quality (eg, whether a patient with pneumonia received a particular antibiotic), a readmission is an adverse outcome that matters to patients and families.16 Second, unlike process measures whose assessment requires detailed review of medical records, readmissions can be easily determined from standard hospital claims. Finally, readmissions are costly, and their prevention could yield substantial savings to society.
A necessary prerequisite for public reporting of readmission is a validated, risk‐adjusted measure that can be used to track performance over time and can facilitate comparisons across institutions. Toward this end, we describe the development, validation, and results of a National Quality Forum‐approved and CMS‐adopted model to estimate hospital‐specific, risk‐standardized, 30‐day readmission rates for Medicare patients hospitalized with pneumonia.17
METHODS
Data Sources
We used 20052006 claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files to develop and validate the administrative model. The Medicare Enrollment Database was used to determine Medicare fee‐for‐service enrollment and mortality statuses. A medical record model, used for additional validation of the administrative model, was developed using information abstracted from the charts of 75,616 pneumonia cases from 19982001 as part of the National Pneumonia Project, a CMS quality improvement initiative.18
Study Cohort
We identified hospitalizations of patients 65 years of age and older with a principal diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification codes 480.XX, 481, 482.XX, 483.X, 485, 486, 487.0) as potential index pneumonia admissions. Because our focus was readmission for patients discharged from acute care settings, we excluded admissions in which patients died or were transferred to another acute care facility. Additionally, we restricted analysis to patients who had been enrolled in fee‐for‐service Medicare Parts A and B, for at least 12 months prior to their pneumonia hospitalization, so that we could use diagnostic codes from all inpatient and outpatient encounters during that period to enhance identification of comorbidities.
Outcome
The outcome was 30‐day readmission, defined as occurrence of at least one hospitalization for any cause within 30 days of discharge after an index admission. Readmissions were identified from hospital claims data, and were attributed to the hospital that had discharged the patient. A 30‐day time frame was selected because it is a clinically meaningful period during which hospitals can be expected to collaborate with other organizations and providers to implement measures to reduce the risk of rehospitalization.
Candidate and Final Model Variables
Candidate variables for the administrative claims model were selected by a clinician team from 189 diagnostic groups included in the Hierarchical Condition Category (HCC) clinical classification system.19 The HCC clinical classification system was developed for CMS in preparation for all‐encounter risk adjustment for Medicare Advantage (managed care). Under the HCC algorithm, the 15,000+ ICD‐9‐CM diagnosis codes are assigned to one of 189 clinically‐coherent condition categories (CCs). We used the April 2008 version of the ICD‐9‐CM to CC assignment map, which is maintained by CMS and posted at
The final risk‐adjustment model included 39 variables selected by the team of clinicians and analysts, primarily based on their clinical relevance but also with knowledge of the strength of their statistical association with readmission outcome (Table 1). For each patient, the presence or absence of these conditions was assessed from multiple sources, including secondary diagnoses during the index admission, principal and secondary diagnoses from hospital admissions in the 12 months prior to the index admission, and diagnoses from hospital outpatient and physician encounters 12 months before the index admission. A small number of CCs were considered to represent potential complications of care (eg, bleeding). Because we did not want to adjust for complications of care occurring during the index admission, a patient was not considered to have one of these conditions unless it was also present in at least one encounter prior to the index admission.
| Variable | Frequencies | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Intercept | 2.395 | 0.021 | ||||
| Age 65 (years above 65, continuous) | 0.0001 | 0.001 | 1.000 | 0.998 | 1.001 | |
| Male | 45 | 0.071 | 0.012 | 1.073 | 1.048 | 1.099 |
| History of CABG | 5.2 | 0.179 | 0.027 | 0.836 | 0.793 | 0.881 |
| Metastatic cancer and acute leukemia (CC 7) | 4.3 | 0.177 | 0.029 | 1.194 | 1.128 | 1.263 |
| Lung, upper digestive tract, and other severe cancers (CC 8) | 6.0 | 0.256 | 0.024 | 1.292 | 1.232 | 1.354 |
| Diabetes and DM complications (CC 15‐20, 119, 120) | 36 | 0.059 | 0.012 | 1.061 | 1.036 | 1.087 |
| Disorders of fluid/electrolyte/acid‐base (CC 22, 23) | 34 | 0.149 | 0.013 | 1.160 | 1.131 | 1.191 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 46 | 0.118 | 0.012 | 1.126 | 1.099 | 1.153 |
| Other psychiatric disorders (CC 60) | 12 | 0.108 | 0.017 | 1.114 | 1.077 | 1.151 |
| Cardio‐respiratory failure and shock (CC 79) | 16 | 0.114 | 0.016 | 1.121 | 1.087 | 1.156 |
| Congestive heart failure (CC 80) | 39 | 0.151 | 0.014 | 1.163 | 1.133 | 1.194 |
| Chronic atherosclerosis (CC 83, 84) | 47 | 0.051 | 0.013 | 1.053 | 1.027 | 1.079 |
| Valvular and rheumatic heart disease (CC 86) | 23 | 0.062 | 0.014 | 1.064 | 1.036 | 1.093 |
| Arrhythmias (CC 92, 93) | 38 | 0.126 | 0.013 | 1.134 | 1.107 | 1.163 |
| Vascular or circulatory disease (CC 104‐106) | 38 | 0.088 | 0.012 | 1.092 | 1.066 | 1.119 |
| COPD (CC 108) | 58 | 0.186 | 0.013 | 1.205 | 1.175 | 1.235 |
| Fibrosis of lung and other chronic lung disorders (CC 109) | 17 | 0.086 | 0.015 | 1.090 | 1.059 | 1.122 |
| Renal failure (CC 131) | 17 | 0.147 | 0.016 | 1.158 | 1.122 | 1.196 |
| Protein‐calorie malnutrition (CC 21) | 7.9 | 0.121 | 0.020 | 1.129 | 1.086 | 1.173 |
| History of infection (CC 1, 3‐6) | 35 | 0.068 | 0.012 | 1.071 | 1.045 | 1.097 |
| Severe hematological disorders (CC 44) | 3.6 | 0.117 | 0.028 | 1.125 | 1.064 | 1.188 |
| Decubitus ulcer or chronic skin ulcer (CC 148, 149) | 10 | 0.101 | 0.018 | 1.106 | 1.067 | 1.146 |
| History of pneumonia (CC 111‐113) | 44 | 0.065 | 0.013 | 1.067 | 1.041 | 1.094 |
| Vertebral fractures (CC 157) | 5.1 | 0.113 | 0.024 | 1.120 | 1.068 | 1.174 |
| Other injuries (CC 162) | 32 | 0.061 | 0.012 | 1.063 | 1.038 | 1.089 |
| Urinary tract infection (CC 135) | 26 | 0.064 | 0.014 | 1.066 | 1.038 | 1.095 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal, and other cancers and tumors (CC 9‐10) | 16 | 0.050 | 0.016 | 1.051 | 1.018 | 1.084 |
| End‐stage renal disease or dialysis (CC 129, 130) | 1.9 | 0.131 | 0.037 | 1.140 | 1.060 | 1.226 |
| Drug/alcohol abuse/dependence/psychosis (CC 51‐53) | 12 | 0.081 | 0.017 | 1.084 | 1.048 | 1.121 |
| Septicemia/shock (CC 2) | 6.3 | 0.094 | 0.022 | 1.098 | 1.052 | 1.146 |
| Other gastrointestinal disorders (CC 36) | 56 | 0.073 | 0.012 | 1.076 | 1.051 | 1.102 |
| Acute coronary syndrome (CC 81, 82) | 8.3 | 0.126 | 0.019 | 1.134 | 1.092 | 1.178 |
| Pleural effusion/pneumothorax (CC 114) | 12 | 0.083 | 0.017 | 1.086 | 1.051 | 1.123 |
| Other urinary tract disorders (CC 136) | 24 | 0.059 | 0.014 | 1.061 | 1.033 | 1.090 |
| Stroke (CC 95, 96) | 10 | 0.047 | 0.019 | 1.049 | 1.011 | 1.088 |
| Dementia and senility (CC 49, 50) | 27 | 0.031 | 0.014 | 1.031 | 1.004 | 1.059 |
| Hemiplegia, paraplegia, paralysis, functional disability (CC 67‐69, 100‐102, 177, 178) | 7.4 | 0.068 | 0.021 | 1.070 | 1.026 | 1.116 |
| Other lung disorders (CC 115) | 45 | 0.005 | 0.012 | 1.005 | 0.982 | 1.030 |
| Major psychiatric disorders (CC 54‐56) | 11 | 0.038 | 0.018 | 1.038 | 1.003 | 1.075 |
| Asthma (CC 110) | 12 | 0.006 | 0.018 | 1.006 | 0.972 | 1.041 |
Model Derivation
For the development of the administrative claims model, we randomly sampled half of 2006 hospitalizations that met inclusion criteria. To assess model performance at the patient level, we calculated the area under the receiver operating curve (AUC), and calculated observed readmission rates in the lowest and highest deciles on the basis of predicted readmission probabilities. We also compared performance with a null model, a model that adjusted for age and sex, and a model that included all candidate variables.20
Risk‐Standardized Readmission Rates
Using hierarchical logistic regression, we modeled the log‐odds of readmission within 30 days of discharge from an index pneumonia admission as a function of patient demographic and clinical characteristics, and a random hospital‐specific intercept. This strategy accounts for within‐hospital correlation, or clustering, of observed outcomes, and models the assumption that underlying differences in quality among hospitals being evaluated lead to systematic differences in outcomes. We then calculated hospital‐specific readmission rates as the ratio of predicted‐to‐expected readmissions (similar to observed/expected ratio), multiplied by the national unadjusted ratea form of indirect standardization. Predicted number of readmissions in each hospital is estimated given the same patient mix and its estimated hospital‐specific intercept. Expected number of readmissions in each hospital is estimated using its patient mix and the average hospital‐specific intercept. To assess hospital performance in any given year, we re‐estimate model coefficients using that year's data.
Model Validation: Administrative Claims
We compared the model performance in the development sample with its performance in the sample from the 2006 data that was not selected for the development set, and separately among pneumonia admissions in 2005. The model was recalibrated in each validation set.
Model Validation: Medical Record Abstraction
We developed a separate medical record‐based model of readmission risk using information from charts that had previously been abstracted as part of CMS's National Pneumonia Project. To select variables for this model, the clinician team: 1) reviewed the list of variables that were included in a medical record model that was previously developed for validating the National Quality Forum‐approved pneumonia mortality measure; 2) reviewed a list of other potential candidate variables available in the National Pneumonia Project dataset; and 3) reviewed variables that emerged as potentially important predictors of readmission, based on a systematic review of the literature that was conducted as part of measure development. This selection process resulted in a final medical record model that included 35 variables.
We linked patients in the National Pneumonia Project cohort to their Medicare claims data, including claims from one year before the index hospitalization, so that we could calculate risk‐standardized readmission rates in this cohort separately using medical record and claims‐based models. This analysis was conducted at the state level, for the 50 states plus the District of Columbia and Puerto Rico, because medical record data were unavailable in sufficient numbers to permit hospital‐level comparisons. To examine the relationship between risk‐standardized rates obtained from medical record and administrative data models, we estimated a linear regression model describing the association between the two rates, weighting each state by number of index hospitalizations, and calculated the correlation coefficient and the intercept and slope of this equation. A slope close to 1 and an intercept close to 0 would provide evidence that risk‐standardized state readmission rates from the medical record and claims models were similar. We also calculated the difference between state risk‐standardized readmission rates from the two models.
Analyses were conducted with the use of SAS version 9.1.3 (SAS Institute Inc, Cary, NC). Models were fitted separately for the National Pneumonia Project and 2006 cohort. We estimated the hierarchical models using the GLIMMIX procedure in SAS. The Human Investigation Committee at the Yale School of Medicine approved an exemption for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation and Performance
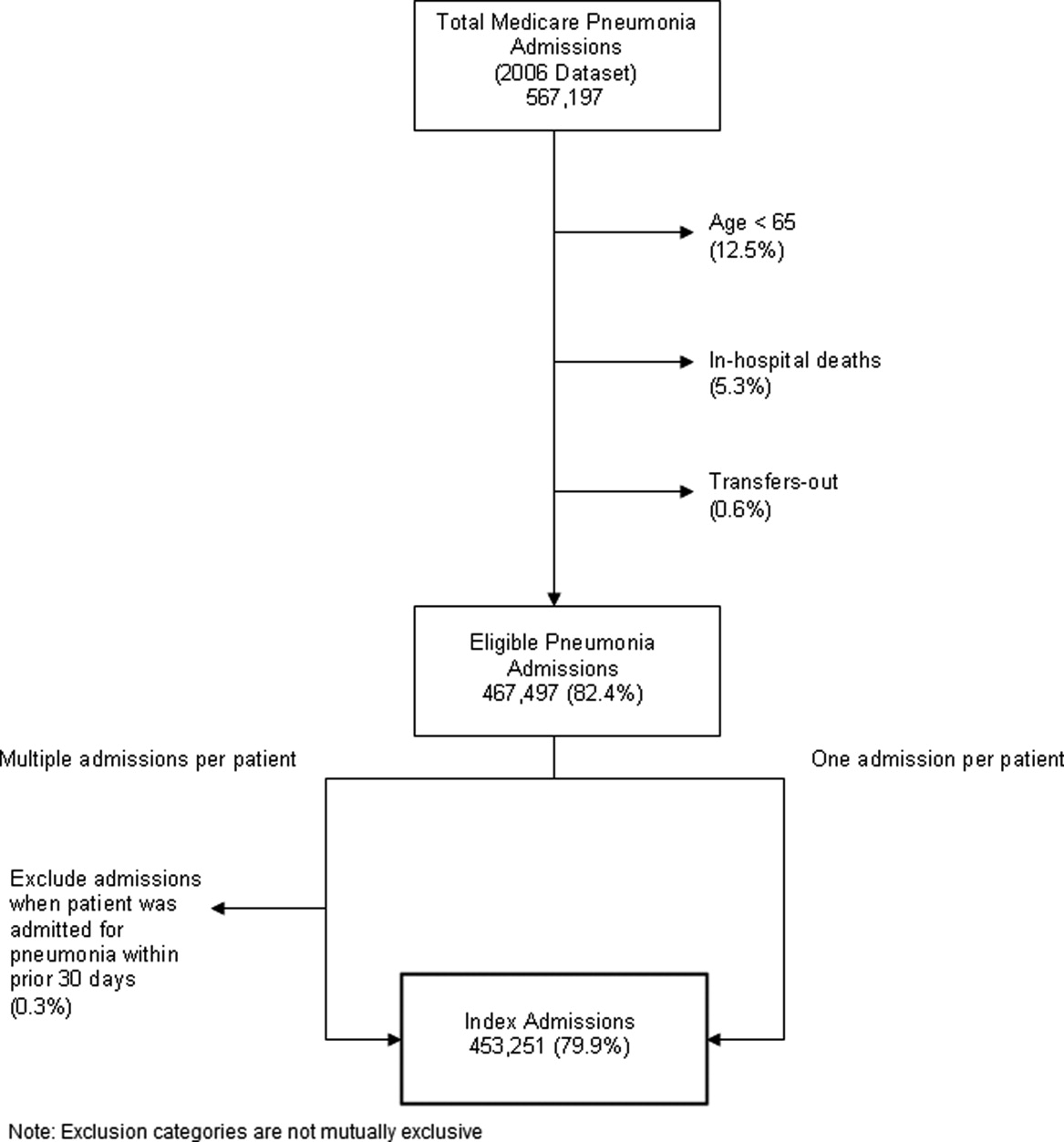
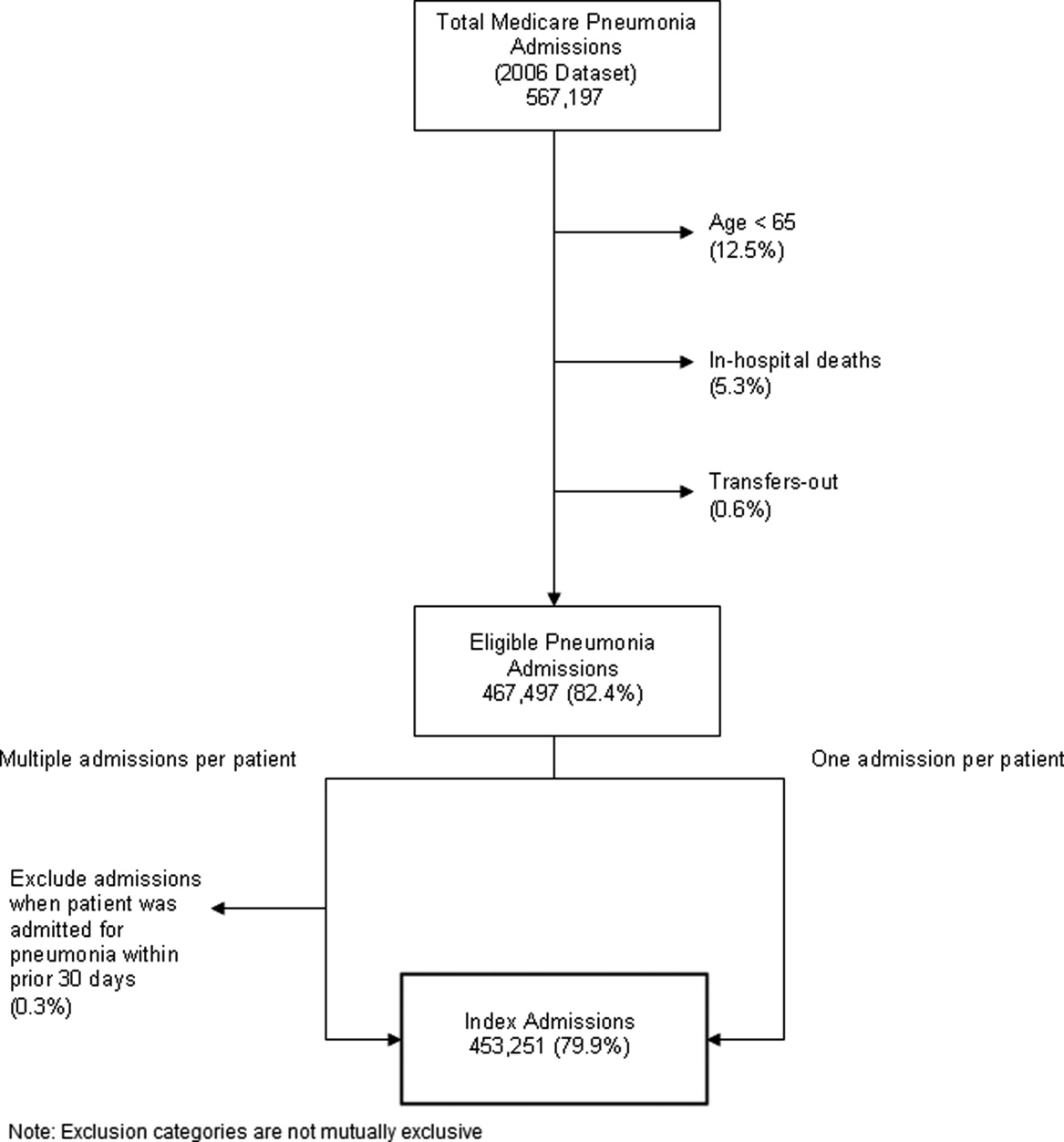
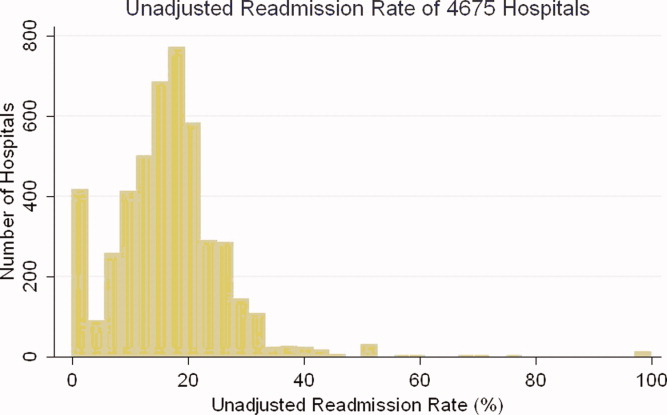
After exclusions were applied, the 2006 sample included 453,251 pneumonia hospitalizations (Figure 1). The development sample consisted of 226,545 hospitalizations at 4675 hospitals, with an overall unadjusted 30‐day readmission rate of 17.4%. In 11,694 index cases (5.2%), the patient died within 30 days without being readmitted. Median readmission rate was 16.3%, 25th and 75th percentile rates were 11.1% and 21.3%, and at the 10th and 90th percentile, hospital readmission rates ranged from 4.6% to 26.7% (Figure 2).
The claims model included 39 variables (age, sex, and 37 clinical variables) (Table 1). The mean age of the cohort was 80.0 years, with 55.5% women and 11.1% nonwhite patients. Mean observed readmission rate in the development sample ranged from 9% in the lowest decile of predicted pneumonia readmission rates to 32% in the highest predicted decile, a range of 23%. The AUC was 0.63. For comparison, a model with only age and sex had an AUC of 0.51, and a model with all candidate variables had an AUC equal to 0.63 (Table 2).
| Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | ||||
| |||||||||
| Development sample | |||||||||
| 2006 | (1st half) N = 226,545 | (0, 1) | (0.09, 0.32) | 0.63 | 0 | 82.62 | 7.39 | 9.99 | 6,843 (40) |
| Validation sample | |||||||||
| 2006 | (2nd half) N = 226,706 | (0.002, 0.997) | (0.09, 0.31) | 0.63 | 0 | 82.55 | 7.45 | 9.99 | 6,870 (40) |
| 2005 | N = 536,015 | (0.035, 1.008) | (0.08, 0.31) | 0.63 | 0 | 82.67 | 7.31 | 10.03 | 16,241 (40) |
Hospital Risk‐Standardized Readmission Rates
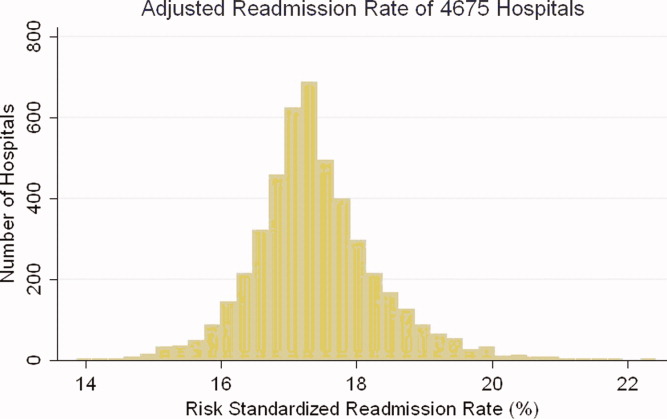
Risk‐standardized readmission rates varied across hospitals (Figure 3). Median risk‐standardized readmission rate was 17.3%, and the 25th and 75th percentiles were 16.9% and 17.9%, respectively. The 5th percentile was 16.0% and the 95th percentile was 19.1%. Odds of readmission for a hospital one standard deviation above average was 1.4 times that of a hospital one standard deviation below average.
Administrative Model Validation
In the remaining 50% of pneumonia index hospitalizations from 2006, and the entire 2005 cohort, regression coefficients and standard errors of model variables were similar to those in the development data set. Model performance using 2005 data was consistent with model performance using the 2006 development and validation half‐samples (Table 2).
Medical Record Validation
After exclusions, the medical record sample taken from the National Pneumonia Project included 47,429 cases, with an unadjusted 30‐day readmission rate of 17.0%. The final medical record risk‐adjustment model included a total of 35 variables, whose prevalence and association with readmission risk varied modestly (Table 3). Performance of the medical record and administrative models was similar (areas under the ROC curve 0.59 and 0.63, respectively) (Table 4). Additionally, in the administrative model, predicted readmission rates ranged from 8% in the lowest predicted decile to 30% in the highest predicted decile, while in the medical record model, the corresponding rates varied from 10% to 26%.
| Variable | Percent | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Age 65, mean (SD) | 15.24 (7.87) | 0.003 | 0.002 | 0.997 | 0.993 | 1.000 |
| Male | 46.18 | 0.122 | 0.025 | 1.130 | 1.075 | 1.188 |
| Nursing home resident | 17.71 | 0.035 | 0.037 | 1.036 | 0.963 | 1.114 |
| Neoplastic disease | 6.80 | 0.130 | 0.049 | 1.139 | 1.034 | 1.254 |
| Liver disease | 1.04 | 0.089 | 0.123 | 0.915 | 0.719 | 1.164 |
| History of heart failure | 28.98 | 0.234 | 0.029 | 1.264 | 1.194 | 1.339 |
| History of renal disease | 8.51 | 0.188 | 0.047 | 1.206 | 1.100 | 1.323 |
| Altered mental status | 17.95 | 0.009 | 0.034 | 1.009 | 0.944 | 1.080 |
| Pleural effusion | 21.20 | 0.165 | 0.030 | 1.179 | 1.111 | 1.251 |
| BUN 30 mg/dl | 23.28 | 0.160 | 0.033 | 1.174 | 1.100 | 1.252 |
| BUN missing | 14.56 | 0.101 | 0.185 | 0.904 | 0.630 | 1.298 |
| Systolic BP <90 mmHg | 2.95 | 0.068 | 0.070 | 1.070 | 0.932 | 1.228 |
| Systolic BP missing | 11.21 | 0.149 | 0.425 | 1.160 | 0.504 | 2.669 |
| Pulse 125/min | 7.73 | 0.036 | 0.047 | 1.036 | 0.945 | 1.137 |
| Pulse missing | 11.22 | 0.210 | 0.405 | 1.234 | 0.558 | 2.729 |
| Respiratory rate 30/min | 16.38 | 0.079 | 0.034 | 1.082 | 1.012 | 1.157 |
| Respiratory rate missing | 11.39 | 0.204 | 0.240 | 1.226 | 0.765 | 1.964 |
| Sodium <130 mmol/L | 4.82 | 0.136 | 0.057 | 1.145 | 1.025 | 1.280 |
| Sodium missing | 14.39 | 0.049 | 0.143 | 1.050 | 0.793 | 1.391 |
| Glucose 250 mg/dl | 5.19 | 0.005 | 0.057 | 0.995 | 0.889 | 1.114 |
| Glucose missing | 15.44 | 0.156 | 0.105 | 0.855 | 0.696 | 1.051 |
| Hematocrit <30% | 7.77 | 0.270 | 0.044 | 1.310 | 1.202 | 1.428 |
| Hematocrit missing | 13.62 | 0.071 | 0.135 | 0.932 | 0.715 | 1.215 |
| Creatinine 2.5 mg/dL | 4.68 | 0.109 | 0.062 | 1.115 | 0.989 | 1.258 |
| Creatinine missing | 14.63 | 0.200 | 0.167 | 1.221 | 0.880 | 1.695 |
| WBC 6‐12 b/L | 38.04 | 0.021 | 0.049 | 0.979 | 0.889 | 1.079 |
| WBC >12 b/L | 41.45 | 0.068 | 0.049 | 0.934 | 0.848 | 1.029 |
| WBC missing | 12.85 | 0.167 | 0.162 | 1.181 | 0.860 | 1.623 |
| Immunosuppressive therapy | 15.01 | 0.347 | 0.035 | 1.415 | 1.321 | 1.516 |
| Chronic lung disease | 42.16 | 0.137 | 0.028 | 1.147 | 1.086 | 1.211 |
| Coronary artery disease | 39.57 | 0.150 | 0.028 | 1.162 | 1.100 | 1.227 |
| Diabetes mellitus | 20.90 | 0.137 | 0.033 | 1.147 | 1.076 | 1.223 |
| Alcohol/drug abuse | 3.40 | 0.099 | 0.071 | 0.906 | 0.788 | 1.041 |
| Dementia/Alzheimer's disease | 16.38 | 0.125 | 0.038 | 1.133 | 1.052 | 1.222 |
| Splenectomy | 0.44 | 0.016 | 0.186 | 1.016 | 0.706 | 1.463 |
| Model | Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||
|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | |||
| ||||||||
| Medical Record Model Development Sample (NP) | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.10, 0.26) | 0.59 | 0 | 83.04 | 5.28 | 11.68 | 710 (35) |
| Linked Administrative Model Validation Sample | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.08, 0.30) | 0.63 | 0 | 83.04 | 6.94 | 10.01 | 1,414 (40) |
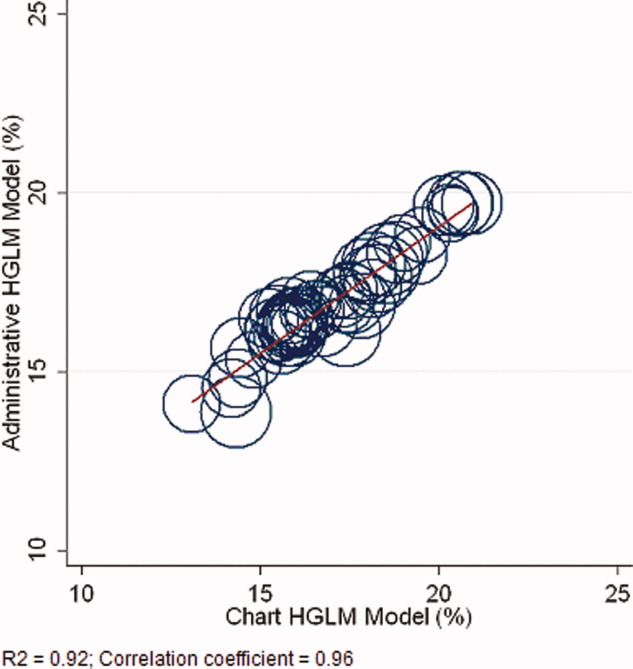
The correlation coefficient of the estimated state‐specific standardized readmission rates from the administrative and medical record models was 0.96, and the proportion of the variance explained by the model was 0.92 (Figure 4).
DISCUSSION
We have described the development, validation, and results of a hospital, 30‐day, risk‐standardized readmission model for pneumonia that was created to support current federal transparency initiatives. The model uses administrative claims data from Medicare fee‐for‐service patients and produces results that are comparable to a model based on information obtained through manual abstraction of medical records. We observed an overall 30‐day readmission rate of 17%, and our analyses revealed substantial variation across US hospitals, suggesting that improvement by lower performing institutions is an achievable goal.
Because more than one in six pneumonia patients are rehospitalized shortly after discharge, and because pneumonia hospitalizations represent an enormous expense to the Medicare program, prevention of readmissions is now widely recognized to offer a substantial opportunity to improve patient outcomes while simultaneously lowering health care costs. Accordingly, promotion of strategies to reduce readmission rates has become a key priority for payers and quality‐improvement organizations. These range from policy‐level attempts to stimulate change, such as publicly reporting hospital readmission rates on government websites, to establishing accreditation standardssuch as the Joint Commission's requirement to accurately reconcile medications, to the creation of quality improvement collaboratives focused on sharing best practices across institutions. Regardless of the approach taken, a valid, risk‐adjusted measure of performance is required to evaluate and track performance over time. The measure we have described meets the National Quality Forum's measure evaluation criteria in that it addresses an important clinical topic for which there appears to be significant opportunities for improvement, the measure is precisely defined and has been subjected to validity and reliability testing, it is risk‐adjusted based on patient clinical factors present at the start of care, is feasible to produce, and is understandable by a broad range of potential users.21 Because hospitalists are the physicians primarily responsible for the care of patients with pneumonia at US hospitals, and because they frequently serve as the physician champions for quality improvement activities related to pneumonia, it is especially important that they maintain a thorough understanding of the measures and methodologies underlying current efforts to measure hospital performance.
Several features of our approach warrant additional comment. First, we deliberately chose to measure all readmission events rather than attempt to discriminate between potentially preventable and nonpreventable readmissions. From the patient perspective, readmission for any reason is a concern, and limiting the measure to pneumonia‐related readmissions could make it susceptible to gaming by hospitals. Moreover, determining whether a readmission is related to a potential quality problem is not straightforward. For example, a patient with pneumonia whose discharge medications were prescribed incorrectly may be readmitted with a hip fracture following an episode of syncope. It would be inappropriate to treat this readmission as unrelated to the care the patient received for pneumonia. Additionally, while our approach does not presume that every readmission is preventable, the goal is to reduce the risk of readmissions generally (not just in narrowly defined subpopulations), and successful interventions to reduce rehospitalization have typically demonstrated reductions in all‐cause readmission.9, 22 Second, deaths that occurred within 30 days of discharge, yet that were not accompanied by a hospital readmission, were not counted as a readmission outcome. While it may seem inappropriate to treat a postdischarge death as a nonevent (rather than censoring or excluding such cases), alternative analytic approaches, such as using a hierarchical survival model, are not currently computationally feasible with large national data sets. Fortunately, only a relatively small proportion of discharges fell into this category (5.2% of index cases in the 2006 development sample died within 30 days of discharge without being readmitted). An alternative approach to handling the competing outcome of death would have been to use a composite outcome of readmission or death. However, we believe that it is important to report the outcomes separately because factors that predict readmission and mortality may differ, and when making comparisons across hospitals it would not be possible to determine whether differences in rate were due to readmission or mortality. Third, while the patient‐level readmission model showed only modest discrimination, we intentionally excluded covariates such as race and socioeconomic status, as well as in‐hospital events and potential complications of care, and whether patients were discharged home or to a skilled nursing facility. While these variables could have improved predictive ability, they may be directly or indirectly related to quality or supply factors that should not be included in a model that seeks to control for patient clinical characteristics. For example, if hospitals with a large share of poor patients have higher readmission rates, then including income in the model will obscure differences that are important to identify. While we believe that the decision to exclude such factors in the model is in the best interest of patients, and supports efforts to reduce health inequality in society more generally, we also recognize that hospitals that care for a disproportionate share of poor patients are likely to require additional resources to overcome these social factors. Fourth, we limited the analysis to patients with a principal diagnosis of pneumonia, and chose not to also include those with a principal diagnosis of sepsis or respiratory failure coupled with a secondary diagnosis of pneumonia. While the broader definition is used by CMS in the National Pneumonia Project, that initiative relied on chart abstraction to differentiate pneumonia present at the time of admission from cases developing as a complication of hospitalization. Additionally, we did not attempt to differentiate between community‐acquired and healthcare‐associated pneumonia, however our approach is consistent with the National Pneumonia Project and Pneumonia Patient Outcomes Research Team.18 Fifth, while our model estimates readmission rates at the hospital level, we recognize that readmissions are influenced by a complex and extensive range of factors. In this context, greater cooperation between hospitals and other care providers will almost certainly be required in order to achieve dramatic improvement in readmission rates, which in turn will depend upon changes to the way serious illness is paid for. Some options that have recently been described include imposing financial penalties for early readmission, extending the boundaries of case‐based payment beyond hospital discharge, and bundling payments between hospitals and physicians.2325
Our measure has several limitations. First, our models were developed and validated using Medicare data, and the results may not apply to pneumonia patients less than 65 years of age. However, most patients hospitalized with pneumonia in the US are 65 or older. In addition, we were unable to test the model with a Medicare managed care population, because data are not currently available on such patients. Finally, the medical record‐based validation was conducted by state‐level analysis because the sample size was insufficient to carry this out at the hospital level.
In conclusion, more than 17% of Medicare beneficiaries are readmitted within 30 days following discharge after a hospitalization for pneumonia, and rates vary substantially across institutions. The development of a valid measure of hospital performance and public reporting are important first steps towards focusing attention on this problem. Actual improvement will now depend on whether hospitals and partner organizations are successful at identifying and implementing effective methods to prevent readmission.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
- Medicare Payment Advisory Commission.Report to the Congress: Promoting Greater Efficiency in Medicare.2007.
- ,,,,. HCUP Facts and Figures: Statistics on Hospital‐based Care in the United States, 2007.2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed November 7, 2009.
- Centers for Medicare 353(3):255–264.
- ,,,.Trends in postdischarge mortality and readmissions: has length of stay declined too far?Arch Intern Med.2004;164(5):538–544.
- ,,,.Short‐term outcomes and their predictors for patients hospitalized with community‐acquired pneumonia.Heart Lung.2004;33(5):301–307.
- ,,, et al.Improved clinical outcomes with utilization of a community‐acquired pneumonia guideline.Chest.2006;130(3):794–799.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159(21):2562–2572.
- ,.Hospital readmissions as a measure of quality of health care: advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- Corrigan JM, Eden J, Smith BM, eds.Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Committee on Enhancing Federal Healthcare Quality Programs.Washington, DC:National Academies Press,2003.
- Medicare.gov—Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Search/Welcome.asp?version=default1(1):29–37.
- ,,,,.Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement.Health Aff.2007;26(1):75–85.
- NQF‐Endorsed® Standards. Available at: http://www.qualityforum.org/Measures_List.aspx. Accessed November 6,2009.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164(6):637–644.
- ,,. Diagnostic Cost Group Hierarchical Condition Category Models for Medicare Risk Adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc;2000. Available at: http://www.cms.hhs.gov/Reports/Reports/ItemDetail.asp?ItemID=CMS023176. Accessed November 7, 2009.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.1st ed.New York:Springer;2006.
- National Quality Forum—Measure Evaluation Criteria.2008. Available at: http://www.qualityforum.org/uploadedFiles/Quality_Forum/Measuring_Performance/Consensus_Development_Process%E2%80%99s_Principle/EvalCriteria2008–08‐28Final.pdf?n=4701.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- .Paying for care episodes and care coordination.N Engl J Med.2007;356(11):1166–1168.
- .Health care reform—toward more freedom, and responsibility, for physicians.N Engl J Med.2009;361(6):623–628.
- .Beyond pay for performance—emerging models of provider‐payment reform.N Engl J Med.2008;359(12):1197–1200.
Hospital readmissions are emblematic of the numerous challenges facing the US health care system. Despite high levels of spending, nearly 20% of Medicare beneficiaries are readmitted within 30 days of hospital discharge, many readmissions are considered preventable, and rates vary widely by hospital and region.1 Further, while readmissions have been estimated to cost taxpayers as much as $17 billion annually, the current fee‐for‐service method of paying for the acute care needs of seniors rewards hospitals financially for readmission, not their prevention.2
Pneumonia is the second most common reason for hospitalization among Medicare beneficiaries, accounting for approximately 650,000 admissions annually,3 and has been a focus of national quality‐improvement efforts for more than a decade.4, 5 Despite improvements in key processes of care, rates of readmission within 30 days of discharge following a hospitalization for pneumonia have been reported to vary from 10% to 24%.68 Among several factors, readmissions are believed to be influenced by the quality of both inpatient and outpatient care, and by care‐coordination activities occurring in the transition from inpatient to outpatient status.912
Public reporting of hospital performance is considered a key strategy for improving quality, reducing costs, and increasing the value of hospital care, both in the US and worldwide.13 In 2009, the Centers for Medicare & Medicaid Services (CMS) expanded its reporting initiatives by adding risk‐adjusted hospital readmission rates for acute myocardial infarction, heart failure, and pneumonia to the Hospital Compare website.14, 15 Readmission rates are an attractive focus for public reporting for several reasons. First, in contrast to most process‐based measures of quality (eg, whether a patient with pneumonia received a particular antibiotic), a readmission is an adverse outcome that matters to patients and families.16 Second, unlike process measures whose assessment requires detailed review of medical records, readmissions can be easily determined from standard hospital claims. Finally, readmissions are costly, and their prevention could yield substantial savings to society.
A necessary prerequisite for public reporting of readmission is a validated, risk‐adjusted measure that can be used to track performance over time and can facilitate comparisons across institutions. Toward this end, we describe the development, validation, and results of a National Quality Forum‐approved and CMS‐adopted model to estimate hospital‐specific, risk‐standardized, 30‐day readmission rates for Medicare patients hospitalized with pneumonia.17
METHODS
Data Sources
We used 20052006 claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files to develop and validate the administrative model. The Medicare Enrollment Database was used to determine Medicare fee‐for‐service enrollment and mortality statuses. A medical record model, used for additional validation of the administrative model, was developed using information abstracted from the charts of 75,616 pneumonia cases from 19982001 as part of the National Pneumonia Project, a CMS quality improvement initiative.18
Study Cohort
We identified hospitalizations of patients 65 years of age and older with a principal diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification codes 480.XX, 481, 482.XX, 483.X, 485, 486, 487.0) as potential index pneumonia admissions. Because our focus was readmission for patients discharged from acute care settings, we excluded admissions in which patients died or were transferred to another acute care facility. Additionally, we restricted analysis to patients who had been enrolled in fee‐for‐service Medicare Parts A and B, for at least 12 months prior to their pneumonia hospitalization, so that we could use diagnostic codes from all inpatient and outpatient encounters during that period to enhance identification of comorbidities.
Outcome
The outcome was 30‐day readmission, defined as occurrence of at least one hospitalization for any cause within 30 days of discharge after an index admission. Readmissions were identified from hospital claims data, and were attributed to the hospital that had discharged the patient. A 30‐day time frame was selected because it is a clinically meaningful period during which hospitals can be expected to collaborate with other organizations and providers to implement measures to reduce the risk of rehospitalization.
Candidate and Final Model Variables
Candidate variables for the administrative claims model were selected by a clinician team from 189 diagnostic groups included in the Hierarchical Condition Category (HCC) clinical classification system.19 The HCC clinical classification system was developed for CMS in preparation for all‐encounter risk adjustment for Medicare Advantage (managed care). Under the HCC algorithm, the 15,000+ ICD‐9‐CM diagnosis codes are assigned to one of 189 clinically‐coherent condition categories (CCs). We used the April 2008 version of the ICD‐9‐CM to CC assignment map, which is maintained by CMS and posted at
The final risk‐adjustment model included 39 variables selected by the team of clinicians and analysts, primarily based on their clinical relevance but also with knowledge of the strength of their statistical association with readmission outcome (Table 1). For each patient, the presence or absence of these conditions was assessed from multiple sources, including secondary diagnoses during the index admission, principal and secondary diagnoses from hospital admissions in the 12 months prior to the index admission, and diagnoses from hospital outpatient and physician encounters 12 months before the index admission. A small number of CCs were considered to represent potential complications of care (eg, bleeding). Because we did not want to adjust for complications of care occurring during the index admission, a patient was not considered to have one of these conditions unless it was also present in at least one encounter prior to the index admission.
| Variable | Frequencies | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Intercept | 2.395 | 0.021 | ||||
| Age 65 (years above 65, continuous) | 0.0001 | 0.001 | 1.000 | 0.998 | 1.001 | |
| Male | 45 | 0.071 | 0.012 | 1.073 | 1.048 | 1.099 |
| History of CABG | 5.2 | 0.179 | 0.027 | 0.836 | 0.793 | 0.881 |
| Metastatic cancer and acute leukemia (CC 7) | 4.3 | 0.177 | 0.029 | 1.194 | 1.128 | 1.263 |
| Lung, upper digestive tract, and other severe cancers (CC 8) | 6.0 | 0.256 | 0.024 | 1.292 | 1.232 | 1.354 |
| Diabetes and DM complications (CC 15‐20, 119, 120) | 36 | 0.059 | 0.012 | 1.061 | 1.036 | 1.087 |
| Disorders of fluid/electrolyte/acid‐base (CC 22, 23) | 34 | 0.149 | 0.013 | 1.160 | 1.131 | 1.191 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 46 | 0.118 | 0.012 | 1.126 | 1.099 | 1.153 |
| Other psychiatric disorders (CC 60) | 12 | 0.108 | 0.017 | 1.114 | 1.077 | 1.151 |
| Cardio‐respiratory failure and shock (CC 79) | 16 | 0.114 | 0.016 | 1.121 | 1.087 | 1.156 |
| Congestive heart failure (CC 80) | 39 | 0.151 | 0.014 | 1.163 | 1.133 | 1.194 |
| Chronic atherosclerosis (CC 83, 84) | 47 | 0.051 | 0.013 | 1.053 | 1.027 | 1.079 |
| Valvular and rheumatic heart disease (CC 86) | 23 | 0.062 | 0.014 | 1.064 | 1.036 | 1.093 |
| Arrhythmias (CC 92, 93) | 38 | 0.126 | 0.013 | 1.134 | 1.107 | 1.163 |
| Vascular or circulatory disease (CC 104‐106) | 38 | 0.088 | 0.012 | 1.092 | 1.066 | 1.119 |
| COPD (CC 108) | 58 | 0.186 | 0.013 | 1.205 | 1.175 | 1.235 |
| Fibrosis of lung and other chronic lung disorders (CC 109) | 17 | 0.086 | 0.015 | 1.090 | 1.059 | 1.122 |
| Renal failure (CC 131) | 17 | 0.147 | 0.016 | 1.158 | 1.122 | 1.196 |
| Protein‐calorie malnutrition (CC 21) | 7.9 | 0.121 | 0.020 | 1.129 | 1.086 | 1.173 |
| History of infection (CC 1, 3‐6) | 35 | 0.068 | 0.012 | 1.071 | 1.045 | 1.097 |
| Severe hematological disorders (CC 44) | 3.6 | 0.117 | 0.028 | 1.125 | 1.064 | 1.188 |
| Decubitus ulcer or chronic skin ulcer (CC 148, 149) | 10 | 0.101 | 0.018 | 1.106 | 1.067 | 1.146 |
| History of pneumonia (CC 111‐113) | 44 | 0.065 | 0.013 | 1.067 | 1.041 | 1.094 |
| Vertebral fractures (CC 157) | 5.1 | 0.113 | 0.024 | 1.120 | 1.068 | 1.174 |
| Other injuries (CC 162) | 32 | 0.061 | 0.012 | 1.063 | 1.038 | 1.089 |
| Urinary tract infection (CC 135) | 26 | 0.064 | 0.014 | 1.066 | 1.038 | 1.095 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal, and other cancers and tumors (CC 9‐10) | 16 | 0.050 | 0.016 | 1.051 | 1.018 | 1.084 |
| End‐stage renal disease or dialysis (CC 129, 130) | 1.9 | 0.131 | 0.037 | 1.140 | 1.060 | 1.226 |
| Drug/alcohol abuse/dependence/psychosis (CC 51‐53) | 12 | 0.081 | 0.017 | 1.084 | 1.048 | 1.121 |
| Septicemia/shock (CC 2) | 6.3 | 0.094 | 0.022 | 1.098 | 1.052 | 1.146 |
| Other gastrointestinal disorders (CC 36) | 56 | 0.073 | 0.012 | 1.076 | 1.051 | 1.102 |
| Acute coronary syndrome (CC 81, 82) | 8.3 | 0.126 | 0.019 | 1.134 | 1.092 | 1.178 |
| Pleural effusion/pneumothorax (CC 114) | 12 | 0.083 | 0.017 | 1.086 | 1.051 | 1.123 |
| Other urinary tract disorders (CC 136) | 24 | 0.059 | 0.014 | 1.061 | 1.033 | 1.090 |
| Stroke (CC 95, 96) | 10 | 0.047 | 0.019 | 1.049 | 1.011 | 1.088 |
| Dementia and senility (CC 49, 50) | 27 | 0.031 | 0.014 | 1.031 | 1.004 | 1.059 |
| Hemiplegia, paraplegia, paralysis, functional disability (CC 67‐69, 100‐102, 177, 178) | 7.4 | 0.068 | 0.021 | 1.070 | 1.026 | 1.116 |
| Other lung disorders (CC 115) | 45 | 0.005 | 0.012 | 1.005 | 0.982 | 1.030 |
| Major psychiatric disorders (CC 54‐56) | 11 | 0.038 | 0.018 | 1.038 | 1.003 | 1.075 |
| Asthma (CC 110) | 12 | 0.006 | 0.018 | 1.006 | 0.972 | 1.041 |
Model Derivation
For the development of the administrative claims model, we randomly sampled half of 2006 hospitalizations that met inclusion criteria. To assess model performance at the patient level, we calculated the area under the receiver operating curve (AUC), and calculated observed readmission rates in the lowest and highest deciles on the basis of predicted readmission probabilities. We also compared performance with a null model, a model that adjusted for age and sex, and a model that included all candidate variables.20
Risk‐Standardized Readmission Rates
Using hierarchical logistic regression, we modeled the log‐odds of readmission within 30 days of discharge from an index pneumonia admission as a function of patient demographic and clinical characteristics, and a random hospital‐specific intercept. This strategy accounts for within‐hospital correlation, or clustering, of observed outcomes, and models the assumption that underlying differences in quality among hospitals being evaluated lead to systematic differences in outcomes. We then calculated hospital‐specific readmission rates as the ratio of predicted‐to‐expected readmissions (similar to observed/expected ratio), multiplied by the national unadjusted ratea form of indirect standardization. Predicted number of readmissions in each hospital is estimated given the same patient mix and its estimated hospital‐specific intercept. Expected number of readmissions in each hospital is estimated using its patient mix and the average hospital‐specific intercept. To assess hospital performance in any given year, we re‐estimate model coefficients using that year's data.
Model Validation: Administrative Claims
We compared the model performance in the development sample with its performance in the sample from the 2006 data that was not selected for the development set, and separately among pneumonia admissions in 2005. The model was recalibrated in each validation set.
Model Validation: Medical Record Abstraction
We developed a separate medical record‐based model of readmission risk using information from charts that had previously been abstracted as part of CMS's National Pneumonia Project. To select variables for this model, the clinician team: 1) reviewed the list of variables that were included in a medical record model that was previously developed for validating the National Quality Forum‐approved pneumonia mortality measure; 2) reviewed a list of other potential candidate variables available in the National Pneumonia Project dataset; and 3) reviewed variables that emerged as potentially important predictors of readmission, based on a systematic review of the literature that was conducted as part of measure development. This selection process resulted in a final medical record model that included 35 variables.
We linked patients in the National Pneumonia Project cohort to their Medicare claims data, including claims from one year before the index hospitalization, so that we could calculate risk‐standardized readmission rates in this cohort separately using medical record and claims‐based models. This analysis was conducted at the state level, for the 50 states plus the District of Columbia and Puerto Rico, because medical record data were unavailable in sufficient numbers to permit hospital‐level comparisons. To examine the relationship between risk‐standardized rates obtained from medical record and administrative data models, we estimated a linear regression model describing the association between the two rates, weighting each state by number of index hospitalizations, and calculated the correlation coefficient and the intercept and slope of this equation. A slope close to 1 and an intercept close to 0 would provide evidence that risk‐standardized state readmission rates from the medical record and claims models were similar. We also calculated the difference between state risk‐standardized readmission rates from the two models.
Analyses were conducted with the use of SAS version 9.1.3 (SAS Institute Inc, Cary, NC). Models were fitted separately for the National Pneumonia Project and 2006 cohort. We estimated the hierarchical models using the GLIMMIX procedure in SAS. The Human Investigation Committee at the Yale School of Medicine approved an exemption for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation and Performance
After exclusions were applied, the 2006 sample included 453,251 pneumonia hospitalizations (Figure 1). The development sample consisted of 226,545 hospitalizations at 4675 hospitals, with an overall unadjusted 30‐day readmission rate of 17.4%. In 11,694 index cases (5.2%), the patient died within 30 days without being readmitted. Median readmission rate was 16.3%, 25th and 75th percentile rates were 11.1% and 21.3%, and at the 10th and 90th percentile, hospital readmission rates ranged from 4.6% to 26.7% (Figure 2).


The claims model included 39 variables (age, sex, and 37 clinical variables) (Table 1). The mean age of the cohort was 80.0 years, with 55.5% women and 11.1% nonwhite patients. Mean observed readmission rate in the development sample ranged from 9% in the lowest decile of predicted pneumonia readmission rates to 32% in the highest predicted decile, a range of 23%. The AUC was 0.63. For comparison, a model with only age and sex had an AUC of 0.51, and a model with all candidate variables had an AUC equal to 0.63 (Table 2).
| Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | ||||
| |||||||||
| Development sample | |||||||||
| 2006 | (1st half) N = 226,545 | (0, 1) | (0.09, 0.32) | 0.63 | 0 | 82.62 | 7.39 | 9.99 | 6,843 (40) |
| Validation sample | |||||||||
| 2006 | (2nd half) N = 226,706 | (0.002, 0.997) | (0.09, 0.31) | 0.63 | 0 | 82.55 | 7.45 | 9.99 | 6,870 (40) |
| 2005 | N = 536,015 | (0.035, 1.008) | (0.08, 0.31) | 0.63 | 0 | 82.67 | 7.31 | 10.03 | 16,241 (40) |
Hospital Risk‐Standardized Readmission Rates
Risk‐standardized readmission rates varied across hospitals (Figure 3). Median risk‐standardized readmission rate was 17.3%, and the 25th and 75th percentiles were 16.9% and 17.9%, respectively. The 5th percentile was 16.0% and the 95th percentile was 19.1%. Odds of readmission for a hospital one standard deviation above average was 1.4 times that of a hospital one standard deviation below average.

Administrative Model Validation
In the remaining 50% of pneumonia index hospitalizations from 2006, and the entire 2005 cohort, regression coefficients and standard errors of model variables were similar to those in the development data set. Model performance using 2005 data was consistent with model performance using the 2006 development and validation half‐samples (Table 2).
Medical Record Validation
After exclusions, the medical record sample taken from the National Pneumonia Project included 47,429 cases, with an unadjusted 30‐day readmission rate of 17.0%. The final medical record risk‐adjustment model included a total of 35 variables, whose prevalence and association with readmission risk varied modestly (Table 3). Performance of the medical record and administrative models was similar (areas under the ROC curve 0.59 and 0.63, respectively) (Table 4). Additionally, in the administrative model, predicted readmission rates ranged from 8% in the lowest predicted decile to 30% in the highest predicted decile, while in the medical record model, the corresponding rates varied from 10% to 26%.
| Variable | Percent | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Age 65, mean (SD) | 15.24 (7.87) | 0.003 | 0.002 | 0.997 | 0.993 | 1.000 |
| Male | 46.18 | 0.122 | 0.025 | 1.130 | 1.075 | 1.188 |
| Nursing home resident | 17.71 | 0.035 | 0.037 | 1.036 | 0.963 | 1.114 |
| Neoplastic disease | 6.80 | 0.130 | 0.049 | 1.139 | 1.034 | 1.254 |
| Liver disease | 1.04 | 0.089 | 0.123 | 0.915 | 0.719 | 1.164 |
| History of heart failure | 28.98 | 0.234 | 0.029 | 1.264 | 1.194 | 1.339 |
| History of renal disease | 8.51 | 0.188 | 0.047 | 1.206 | 1.100 | 1.323 |
| Altered mental status | 17.95 | 0.009 | 0.034 | 1.009 | 0.944 | 1.080 |
| Pleural effusion | 21.20 | 0.165 | 0.030 | 1.179 | 1.111 | 1.251 |
| BUN 30 mg/dl | 23.28 | 0.160 | 0.033 | 1.174 | 1.100 | 1.252 |
| BUN missing | 14.56 | 0.101 | 0.185 | 0.904 | 0.630 | 1.298 |
| Systolic BP <90 mmHg | 2.95 | 0.068 | 0.070 | 1.070 | 0.932 | 1.228 |
| Systolic BP missing | 11.21 | 0.149 | 0.425 | 1.160 | 0.504 | 2.669 |
| Pulse 125/min | 7.73 | 0.036 | 0.047 | 1.036 | 0.945 | 1.137 |
| Pulse missing | 11.22 | 0.210 | 0.405 | 1.234 | 0.558 | 2.729 |
| Respiratory rate 30/min | 16.38 | 0.079 | 0.034 | 1.082 | 1.012 | 1.157 |
| Respiratory rate missing | 11.39 | 0.204 | 0.240 | 1.226 | 0.765 | 1.964 |
| Sodium <130 mmol/L | 4.82 | 0.136 | 0.057 | 1.145 | 1.025 | 1.280 |
| Sodium missing | 14.39 | 0.049 | 0.143 | 1.050 | 0.793 | 1.391 |
| Glucose 250 mg/dl | 5.19 | 0.005 | 0.057 | 0.995 | 0.889 | 1.114 |
| Glucose missing | 15.44 | 0.156 | 0.105 | 0.855 | 0.696 | 1.051 |
| Hematocrit <30% | 7.77 | 0.270 | 0.044 | 1.310 | 1.202 | 1.428 |
| Hematocrit missing | 13.62 | 0.071 | 0.135 | 0.932 | 0.715 | 1.215 |
| Creatinine 2.5 mg/dL | 4.68 | 0.109 | 0.062 | 1.115 | 0.989 | 1.258 |
| Creatinine missing | 14.63 | 0.200 | 0.167 | 1.221 | 0.880 | 1.695 |
| WBC 6‐12 b/L | 38.04 | 0.021 | 0.049 | 0.979 | 0.889 | 1.079 |
| WBC >12 b/L | 41.45 | 0.068 | 0.049 | 0.934 | 0.848 | 1.029 |
| WBC missing | 12.85 | 0.167 | 0.162 | 1.181 | 0.860 | 1.623 |
| Immunosuppressive therapy | 15.01 | 0.347 | 0.035 | 1.415 | 1.321 | 1.516 |
| Chronic lung disease | 42.16 | 0.137 | 0.028 | 1.147 | 1.086 | 1.211 |
| Coronary artery disease | 39.57 | 0.150 | 0.028 | 1.162 | 1.100 | 1.227 |
| Diabetes mellitus | 20.90 | 0.137 | 0.033 | 1.147 | 1.076 | 1.223 |
| Alcohol/drug abuse | 3.40 | 0.099 | 0.071 | 0.906 | 0.788 | 1.041 |
| Dementia/Alzheimer's disease | 16.38 | 0.125 | 0.038 | 1.133 | 1.052 | 1.222 |
| Splenectomy | 0.44 | 0.016 | 0.186 | 1.016 | 0.706 | 1.463 |
| Model | Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||
|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | |||
| ||||||||
| Medical Record Model Development Sample (NP) | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.10, 0.26) | 0.59 | 0 | 83.04 | 5.28 | 11.68 | 710 (35) |
| Linked Administrative Model Validation Sample | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.08, 0.30) | 0.63 | 0 | 83.04 | 6.94 | 10.01 | 1,414 (40) |
The correlation coefficient of the estimated state‐specific standardized readmission rates from the administrative and medical record models was 0.96, and the proportion of the variance explained by the model was 0.92 (Figure 4).

DISCUSSION
We have described the development, validation, and results of a hospital, 30‐day, risk‐standardized readmission model for pneumonia that was created to support current federal transparency initiatives. The model uses administrative claims data from Medicare fee‐for‐service patients and produces results that are comparable to a model based on information obtained through manual abstraction of medical records. We observed an overall 30‐day readmission rate of 17%, and our analyses revealed substantial variation across US hospitals, suggesting that improvement by lower performing institutions is an achievable goal.
Because more than one in six pneumonia patients are rehospitalized shortly after discharge, and because pneumonia hospitalizations represent an enormous expense to the Medicare program, prevention of readmissions is now widely recognized to offer a substantial opportunity to improve patient outcomes while simultaneously lowering health care costs. Accordingly, promotion of strategies to reduce readmission rates has become a key priority for payers and quality‐improvement organizations. These range from policy‐level attempts to stimulate change, such as publicly reporting hospital readmission rates on government websites, to establishing accreditation standardssuch as the Joint Commission's requirement to accurately reconcile medications, to the creation of quality improvement collaboratives focused on sharing best practices across institutions. Regardless of the approach taken, a valid, risk‐adjusted measure of performance is required to evaluate and track performance over time. The measure we have described meets the National Quality Forum's measure evaluation criteria in that it addresses an important clinical topic for which there appears to be significant opportunities for improvement, the measure is precisely defined and has been subjected to validity and reliability testing, it is risk‐adjusted based on patient clinical factors present at the start of care, is feasible to produce, and is understandable by a broad range of potential users.21 Because hospitalists are the physicians primarily responsible for the care of patients with pneumonia at US hospitals, and because they frequently serve as the physician champions for quality improvement activities related to pneumonia, it is especially important that they maintain a thorough understanding of the measures and methodologies underlying current efforts to measure hospital performance.
Several features of our approach warrant additional comment. First, we deliberately chose to measure all readmission events rather than attempt to discriminate between potentially preventable and nonpreventable readmissions. From the patient perspective, readmission for any reason is a concern, and limiting the measure to pneumonia‐related readmissions could make it susceptible to gaming by hospitals. Moreover, determining whether a readmission is related to a potential quality problem is not straightforward. For example, a patient with pneumonia whose discharge medications were prescribed incorrectly may be readmitted with a hip fracture following an episode of syncope. It would be inappropriate to treat this readmission as unrelated to the care the patient received for pneumonia. Additionally, while our approach does not presume that every readmission is preventable, the goal is to reduce the risk of readmissions generally (not just in narrowly defined subpopulations), and successful interventions to reduce rehospitalization have typically demonstrated reductions in all‐cause readmission.9, 22 Second, deaths that occurred within 30 days of discharge, yet that were not accompanied by a hospital readmission, were not counted as a readmission outcome. While it may seem inappropriate to treat a postdischarge death as a nonevent (rather than censoring or excluding such cases), alternative analytic approaches, such as using a hierarchical survival model, are not currently computationally feasible with large national data sets. Fortunately, only a relatively small proportion of discharges fell into this category (5.2% of index cases in the 2006 development sample died within 30 days of discharge without being readmitted). An alternative approach to handling the competing outcome of death would have been to use a composite outcome of readmission or death. However, we believe that it is important to report the outcomes separately because factors that predict readmission and mortality may differ, and when making comparisons across hospitals it would not be possible to determine whether differences in rate were due to readmission or mortality. Third, while the patient‐level readmission model showed only modest discrimination, we intentionally excluded covariates such as race and socioeconomic status, as well as in‐hospital events and potential complications of care, and whether patients were discharged home or to a skilled nursing facility. While these variables could have improved predictive ability, they may be directly or indirectly related to quality or supply factors that should not be included in a model that seeks to control for patient clinical characteristics. For example, if hospitals with a large share of poor patients have higher readmission rates, then including income in the model will obscure differences that are important to identify. While we believe that the decision to exclude such factors in the model is in the best interest of patients, and supports efforts to reduce health inequality in society more generally, we also recognize that hospitals that care for a disproportionate share of poor patients are likely to require additional resources to overcome these social factors. Fourth, we limited the analysis to patients with a principal diagnosis of pneumonia, and chose not to also include those with a principal diagnosis of sepsis or respiratory failure coupled with a secondary diagnosis of pneumonia. While the broader definition is used by CMS in the National Pneumonia Project, that initiative relied on chart abstraction to differentiate pneumonia present at the time of admission from cases developing as a complication of hospitalization. Additionally, we did not attempt to differentiate between community‐acquired and healthcare‐associated pneumonia, however our approach is consistent with the National Pneumonia Project and Pneumonia Patient Outcomes Research Team.18 Fifth, while our model estimates readmission rates at the hospital level, we recognize that readmissions are influenced by a complex and extensive range of factors. In this context, greater cooperation between hospitals and other care providers will almost certainly be required in order to achieve dramatic improvement in readmission rates, which in turn will depend upon changes to the way serious illness is paid for. Some options that have recently been described include imposing financial penalties for early readmission, extending the boundaries of case‐based payment beyond hospital discharge, and bundling payments between hospitals and physicians.2325
Our measure has several limitations. First, our models were developed and validated using Medicare data, and the results may not apply to pneumonia patients less than 65 years of age. However, most patients hospitalized with pneumonia in the US are 65 or older. In addition, we were unable to test the model with a Medicare managed care population, because data are not currently available on such patients. Finally, the medical record‐based validation was conducted by state‐level analysis because the sample size was insufficient to carry this out at the hospital level.
In conclusion, more than 17% of Medicare beneficiaries are readmitted within 30 days following discharge after a hospitalization for pneumonia, and rates vary substantially across institutions. The development of a valid measure of hospital performance and public reporting are important first steps towards focusing attention on this problem. Actual improvement will now depend on whether hospitals and partner organizations are successful at identifying and implementing effective methods to prevent readmission.
Hospital readmissions are emblematic of the numerous challenges facing the US health care system. Despite high levels of spending, nearly 20% of Medicare beneficiaries are readmitted within 30 days of hospital discharge, many readmissions are considered preventable, and rates vary widely by hospital and region.1 Further, while readmissions have been estimated to cost taxpayers as much as $17 billion annually, the current fee‐for‐service method of paying for the acute care needs of seniors rewards hospitals financially for readmission, not their prevention.2
Pneumonia is the second most common reason for hospitalization among Medicare beneficiaries, accounting for approximately 650,000 admissions annually,3 and has been a focus of national quality‐improvement efforts for more than a decade.4, 5 Despite improvements in key processes of care, rates of readmission within 30 days of discharge following a hospitalization for pneumonia have been reported to vary from 10% to 24%.68 Among several factors, readmissions are believed to be influenced by the quality of both inpatient and outpatient care, and by care‐coordination activities occurring in the transition from inpatient to outpatient status.912
Public reporting of hospital performance is considered a key strategy for improving quality, reducing costs, and increasing the value of hospital care, both in the US and worldwide.13 In 2009, the Centers for Medicare & Medicaid Services (CMS) expanded its reporting initiatives by adding risk‐adjusted hospital readmission rates for acute myocardial infarction, heart failure, and pneumonia to the Hospital Compare website.14, 15 Readmission rates are an attractive focus for public reporting for several reasons. First, in contrast to most process‐based measures of quality (eg, whether a patient with pneumonia received a particular antibiotic), a readmission is an adverse outcome that matters to patients and families.16 Second, unlike process measures whose assessment requires detailed review of medical records, readmissions can be easily determined from standard hospital claims. Finally, readmissions are costly, and their prevention could yield substantial savings to society.
A necessary prerequisite for public reporting of readmission is a validated, risk‐adjusted measure that can be used to track performance over time and can facilitate comparisons across institutions. Toward this end, we describe the development, validation, and results of a National Quality Forum‐approved and CMS‐adopted model to estimate hospital‐specific, risk‐standardized, 30‐day readmission rates for Medicare patients hospitalized with pneumonia.17
METHODS
Data Sources
We used 20052006 claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files to develop and validate the administrative model. The Medicare Enrollment Database was used to determine Medicare fee‐for‐service enrollment and mortality statuses. A medical record model, used for additional validation of the administrative model, was developed using information abstracted from the charts of 75,616 pneumonia cases from 19982001 as part of the National Pneumonia Project, a CMS quality improvement initiative.18
Study Cohort
We identified hospitalizations of patients 65 years of age and older with a principal diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification codes 480.XX, 481, 482.XX, 483.X, 485, 486, 487.0) as potential index pneumonia admissions. Because our focus was readmission for patients discharged from acute care settings, we excluded admissions in which patients died or were transferred to another acute care facility. Additionally, we restricted analysis to patients who had been enrolled in fee‐for‐service Medicare Parts A and B, for at least 12 months prior to their pneumonia hospitalization, so that we could use diagnostic codes from all inpatient and outpatient encounters during that period to enhance identification of comorbidities.
Outcome
The outcome was 30‐day readmission, defined as occurrence of at least one hospitalization for any cause within 30 days of discharge after an index admission. Readmissions were identified from hospital claims data, and were attributed to the hospital that had discharged the patient. A 30‐day time frame was selected because it is a clinically meaningful period during which hospitals can be expected to collaborate with other organizations and providers to implement measures to reduce the risk of rehospitalization.
Candidate and Final Model Variables
Candidate variables for the administrative claims model were selected by a clinician team from 189 diagnostic groups included in the Hierarchical Condition Category (HCC) clinical classification system.19 The HCC clinical classification system was developed for CMS in preparation for all‐encounter risk adjustment for Medicare Advantage (managed care). Under the HCC algorithm, the 15,000+ ICD‐9‐CM diagnosis codes are assigned to one of 189 clinically‐coherent condition categories (CCs). We used the April 2008 version of the ICD‐9‐CM to CC assignment map, which is maintained by CMS and posted at
The final risk‐adjustment model included 39 variables selected by the team of clinicians and analysts, primarily based on their clinical relevance but also with knowledge of the strength of their statistical association with readmission outcome (Table 1). For each patient, the presence or absence of these conditions was assessed from multiple sources, including secondary diagnoses during the index admission, principal and secondary diagnoses from hospital admissions in the 12 months prior to the index admission, and diagnoses from hospital outpatient and physician encounters 12 months before the index admission. A small number of CCs were considered to represent potential complications of care (eg, bleeding). Because we did not want to adjust for complications of care occurring during the index admission, a patient was not considered to have one of these conditions unless it was also present in at least one encounter prior to the index admission.
| Variable | Frequencies | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Intercept | 2.395 | 0.021 | ||||
| Age 65 (years above 65, continuous) | 0.0001 | 0.001 | 1.000 | 0.998 | 1.001 | |
| Male | 45 | 0.071 | 0.012 | 1.073 | 1.048 | 1.099 |
| History of CABG | 5.2 | 0.179 | 0.027 | 0.836 | 0.793 | 0.881 |
| Metastatic cancer and acute leukemia (CC 7) | 4.3 | 0.177 | 0.029 | 1.194 | 1.128 | 1.263 |
| Lung, upper digestive tract, and other severe cancers (CC 8) | 6.0 | 0.256 | 0.024 | 1.292 | 1.232 | 1.354 |
| Diabetes and DM complications (CC 15‐20, 119, 120) | 36 | 0.059 | 0.012 | 1.061 | 1.036 | 1.087 |
| Disorders of fluid/electrolyte/acid‐base (CC 22, 23) | 34 | 0.149 | 0.013 | 1.160 | 1.131 | 1.191 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 46 | 0.118 | 0.012 | 1.126 | 1.099 | 1.153 |
| Other psychiatric disorders (CC 60) | 12 | 0.108 | 0.017 | 1.114 | 1.077 | 1.151 |
| Cardio‐respiratory failure and shock (CC 79) | 16 | 0.114 | 0.016 | 1.121 | 1.087 | 1.156 |
| Congestive heart failure (CC 80) | 39 | 0.151 | 0.014 | 1.163 | 1.133 | 1.194 |
| Chronic atherosclerosis (CC 83, 84) | 47 | 0.051 | 0.013 | 1.053 | 1.027 | 1.079 |
| Valvular and rheumatic heart disease (CC 86) | 23 | 0.062 | 0.014 | 1.064 | 1.036 | 1.093 |
| Arrhythmias (CC 92, 93) | 38 | 0.126 | 0.013 | 1.134 | 1.107 | 1.163 |
| Vascular or circulatory disease (CC 104‐106) | 38 | 0.088 | 0.012 | 1.092 | 1.066 | 1.119 |
| COPD (CC 108) | 58 | 0.186 | 0.013 | 1.205 | 1.175 | 1.235 |
| Fibrosis of lung and other chronic lung disorders (CC 109) | 17 | 0.086 | 0.015 | 1.090 | 1.059 | 1.122 |
| Renal failure (CC 131) | 17 | 0.147 | 0.016 | 1.158 | 1.122 | 1.196 |
| Protein‐calorie malnutrition (CC 21) | 7.9 | 0.121 | 0.020 | 1.129 | 1.086 | 1.173 |
| History of infection (CC 1, 3‐6) | 35 | 0.068 | 0.012 | 1.071 | 1.045 | 1.097 |
| Severe hematological disorders (CC 44) | 3.6 | 0.117 | 0.028 | 1.125 | 1.064 | 1.188 |
| Decubitus ulcer or chronic skin ulcer (CC 148, 149) | 10 | 0.101 | 0.018 | 1.106 | 1.067 | 1.146 |
| History of pneumonia (CC 111‐113) | 44 | 0.065 | 0.013 | 1.067 | 1.041 | 1.094 |
| Vertebral fractures (CC 157) | 5.1 | 0.113 | 0.024 | 1.120 | 1.068 | 1.174 |
| Other injuries (CC 162) | 32 | 0.061 | 0.012 | 1.063 | 1.038 | 1.089 |
| Urinary tract infection (CC 135) | 26 | 0.064 | 0.014 | 1.066 | 1.038 | 1.095 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal, and other cancers and tumors (CC 9‐10) | 16 | 0.050 | 0.016 | 1.051 | 1.018 | 1.084 |
| End‐stage renal disease or dialysis (CC 129, 130) | 1.9 | 0.131 | 0.037 | 1.140 | 1.060 | 1.226 |
| Drug/alcohol abuse/dependence/psychosis (CC 51‐53) | 12 | 0.081 | 0.017 | 1.084 | 1.048 | 1.121 |
| Septicemia/shock (CC 2) | 6.3 | 0.094 | 0.022 | 1.098 | 1.052 | 1.146 |
| Other gastrointestinal disorders (CC 36) | 56 | 0.073 | 0.012 | 1.076 | 1.051 | 1.102 |
| Acute coronary syndrome (CC 81, 82) | 8.3 | 0.126 | 0.019 | 1.134 | 1.092 | 1.178 |
| Pleural effusion/pneumothorax (CC 114) | 12 | 0.083 | 0.017 | 1.086 | 1.051 | 1.123 |
| Other urinary tract disorders (CC 136) | 24 | 0.059 | 0.014 | 1.061 | 1.033 | 1.090 |
| Stroke (CC 95, 96) | 10 | 0.047 | 0.019 | 1.049 | 1.011 | 1.088 |
| Dementia and senility (CC 49, 50) | 27 | 0.031 | 0.014 | 1.031 | 1.004 | 1.059 |
| Hemiplegia, paraplegia, paralysis, functional disability (CC 67‐69, 100‐102, 177, 178) | 7.4 | 0.068 | 0.021 | 1.070 | 1.026 | 1.116 |
| Other lung disorders (CC 115) | 45 | 0.005 | 0.012 | 1.005 | 0.982 | 1.030 |
| Major psychiatric disorders (CC 54‐56) | 11 | 0.038 | 0.018 | 1.038 | 1.003 | 1.075 |
| Asthma (CC 110) | 12 | 0.006 | 0.018 | 1.006 | 0.972 | 1.041 |
Model Derivation
For the development of the administrative claims model, we randomly sampled half of 2006 hospitalizations that met inclusion criteria. To assess model performance at the patient level, we calculated the area under the receiver operating curve (AUC), and calculated observed readmission rates in the lowest and highest deciles on the basis of predicted readmission probabilities. We also compared performance with a null model, a model that adjusted for age and sex, and a model that included all candidate variables.20
Risk‐Standardized Readmission Rates
Using hierarchical logistic regression, we modeled the log‐odds of readmission within 30 days of discharge from an index pneumonia admission as a function of patient demographic and clinical characteristics, and a random hospital‐specific intercept. This strategy accounts for within‐hospital correlation, or clustering, of observed outcomes, and models the assumption that underlying differences in quality among hospitals being evaluated lead to systematic differences in outcomes. We then calculated hospital‐specific readmission rates as the ratio of predicted‐to‐expected readmissions (similar to observed/expected ratio), multiplied by the national unadjusted ratea form of indirect standardization. Predicted number of readmissions in each hospital is estimated given the same patient mix and its estimated hospital‐specific intercept. Expected number of readmissions in each hospital is estimated using its patient mix and the average hospital‐specific intercept. To assess hospital performance in any given year, we re‐estimate model coefficients using that year's data.
Model Validation: Administrative Claims
We compared the model performance in the development sample with its performance in the sample from the 2006 data that was not selected for the development set, and separately among pneumonia admissions in 2005. The model was recalibrated in each validation set.
Model Validation: Medical Record Abstraction
We developed a separate medical record‐based model of readmission risk using information from charts that had previously been abstracted as part of CMS's National Pneumonia Project. To select variables for this model, the clinician team: 1) reviewed the list of variables that were included in a medical record model that was previously developed for validating the National Quality Forum‐approved pneumonia mortality measure; 2) reviewed a list of other potential candidate variables available in the National Pneumonia Project dataset; and 3) reviewed variables that emerged as potentially important predictors of readmission, based on a systematic review of the literature that was conducted as part of measure development. This selection process resulted in a final medical record model that included 35 variables.
We linked patients in the National Pneumonia Project cohort to their Medicare claims data, including claims from one year before the index hospitalization, so that we could calculate risk‐standardized readmission rates in this cohort separately using medical record and claims‐based models. This analysis was conducted at the state level, for the 50 states plus the District of Columbia and Puerto Rico, because medical record data were unavailable in sufficient numbers to permit hospital‐level comparisons. To examine the relationship between risk‐standardized rates obtained from medical record and administrative data models, we estimated a linear regression model describing the association between the two rates, weighting each state by number of index hospitalizations, and calculated the correlation coefficient and the intercept and slope of this equation. A slope close to 1 and an intercept close to 0 would provide evidence that risk‐standardized state readmission rates from the medical record and claims models were similar. We also calculated the difference between state risk‐standardized readmission rates from the two models.
Analyses were conducted with the use of SAS version 9.1.3 (SAS Institute Inc, Cary, NC). Models were fitted separately for the National Pneumonia Project and 2006 cohort. We estimated the hierarchical models using the GLIMMIX procedure in SAS. The Human Investigation Committee at the Yale School of Medicine approved an exemption for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation and Performance
After exclusions were applied, the 2006 sample included 453,251 pneumonia hospitalizations (Figure 1). The development sample consisted of 226,545 hospitalizations at 4675 hospitals, with an overall unadjusted 30‐day readmission rate of 17.4%. In 11,694 index cases (5.2%), the patient died within 30 days without being readmitted. Median readmission rate was 16.3%, 25th and 75th percentile rates were 11.1% and 21.3%, and at the 10th and 90th percentile, hospital readmission rates ranged from 4.6% to 26.7% (Figure 2).


The claims model included 39 variables (age, sex, and 37 clinical variables) (Table 1). The mean age of the cohort was 80.0 years, with 55.5% women and 11.1% nonwhite patients. Mean observed readmission rate in the development sample ranged from 9% in the lowest decile of predicted pneumonia readmission rates to 32% in the highest predicted decile, a range of 23%. The AUC was 0.63. For comparison, a model with only age and sex had an AUC of 0.51, and a model with all candidate variables had an AUC equal to 0.63 (Table 2).
| Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | ||||
| |||||||||
| Development sample | |||||||||
| 2006 | (1st half) N = 226,545 | (0, 1) | (0.09, 0.32) | 0.63 | 0 | 82.62 | 7.39 | 9.99 | 6,843 (40) |
| Validation sample | |||||||||
| 2006 | (2nd half) N = 226,706 | (0.002, 0.997) | (0.09, 0.31) | 0.63 | 0 | 82.55 | 7.45 | 9.99 | 6,870 (40) |
| 2005 | N = 536,015 | (0.035, 1.008) | (0.08, 0.31) | 0.63 | 0 | 82.67 | 7.31 | 10.03 | 16,241 (40) |
Hospital Risk‐Standardized Readmission Rates
Risk‐standardized readmission rates varied across hospitals (Figure 3). Median risk‐standardized readmission rate was 17.3%, and the 25th and 75th percentiles were 16.9% and 17.9%, respectively. The 5th percentile was 16.0% and the 95th percentile was 19.1%. Odds of readmission for a hospital one standard deviation above average was 1.4 times that of a hospital one standard deviation below average.

Administrative Model Validation
In the remaining 50% of pneumonia index hospitalizations from 2006, and the entire 2005 cohort, regression coefficients and standard errors of model variables were similar to those in the development data set. Model performance using 2005 data was consistent with model performance using the 2006 development and validation half‐samples (Table 2).
Medical Record Validation
After exclusions, the medical record sample taken from the National Pneumonia Project included 47,429 cases, with an unadjusted 30‐day readmission rate of 17.0%. The final medical record risk‐adjustment model included a total of 35 variables, whose prevalence and association with readmission risk varied modestly (Table 3). Performance of the medical record and administrative models was similar (areas under the ROC curve 0.59 and 0.63, respectively) (Table 4). Additionally, in the administrative model, predicted readmission rates ranged from 8% in the lowest predicted decile to 30% in the highest predicted decile, while in the medical record model, the corresponding rates varied from 10% to 26%.
| Variable | Percent | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Age 65, mean (SD) | 15.24 (7.87) | 0.003 | 0.002 | 0.997 | 0.993 | 1.000 |
| Male | 46.18 | 0.122 | 0.025 | 1.130 | 1.075 | 1.188 |
| Nursing home resident | 17.71 | 0.035 | 0.037 | 1.036 | 0.963 | 1.114 |
| Neoplastic disease | 6.80 | 0.130 | 0.049 | 1.139 | 1.034 | 1.254 |
| Liver disease | 1.04 | 0.089 | 0.123 | 0.915 | 0.719 | 1.164 |
| History of heart failure | 28.98 | 0.234 | 0.029 | 1.264 | 1.194 | 1.339 |
| History of renal disease | 8.51 | 0.188 | 0.047 | 1.206 | 1.100 | 1.323 |
| Altered mental status | 17.95 | 0.009 | 0.034 | 1.009 | 0.944 | 1.080 |
| Pleural effusion | 21.20 | 0.165 | 0.030 | 1.179 | 1.111 | 1.251 |
| BUN 30 mg/dl | 23.28 | 0.160 | 0.033 | 1.174 | 1.100 | 1.252 |
| BUN missing | 14.56 | 0.101 | 0.185 | 0.904 | 0.630 | 1.298 |
| Systolic BP <90 mmHg | 2.95 | 0.068 | 0.070 | 1.070 | 0.932 | 1.228 |
| Systolic BP missing | 11.21 | 0.149 | 0.425 | 1.160 | 0.504 | 2.669 |
| Pulse 125/min | 7.73 | 0.036 | 0.047 | 1.036 | 0.945 | 1.137 |
| Pulse missing | 11.22 | 0.210 | 0.405 | 1.234 | 0.558 | 2.729 |
| Respiratory rate 30/min | 16.38 | 0.079 | 0.034 | 1.082 | 1.012 | 1.157 |
| Respiratory rate missing | 11.39 | 0.204 | 0.240 | 1.226 | 0.765 | 1.964 |
| Sodium <130 mmol/L | 4.82 | 0.136 | 0.057 | 1.145 | 1.025 | 1.280 |
| Sodium missing | 14.39 | 0.049 | 0.143 | 1.050 | 0.793 | 1.391 |
| Glucose 250 mg/dl | 5.19 | 0.005 | 0.057 | 0.995 | 0.889 | 1.114 |
| Glucose missing | 15.44 | 0.156 | 0.105 | 0.855 | 0.696 | 1.051 |
| Hematocrit <30% | 7.77 | 0.270 | 0.044 | 1.310 | 1.202 | 1.428 |
| Hematocrit missing | 13.62 | 0.071 | 0.135 | 0.932 | 0.715 | 1.215 |
| Creatinine 2.5 mg/dL | 4.68 | 0.109 | 0.062 | 1.115 | 0.989 | 1.258 |
| Creatinine missing | 14.63 | 0.200 | 0.167 | 1.221 | 0.880 | 1.695 |
| WBC 6‐12 b/L | 38.04 | 0.021 | 0.049 | 0.979 | 0.889 | 1.079 |
| WBC >12 b/L | 41.45 | 0.068 | 0.049 | 0.934 | 0.848 | 1.029 |
| WBC missing | 12.85 | 0.167 | 0.162 | 1.181 | 0.860 | 1.623 |
| Immunosuppressive therapy | 15.01 | 0.347 | 0.035 | 1.415 | 1.321 | 1.516 |
| Chronic lung disease | 42.16 | 0.137 | 0.028 | 1.147 | 1.086 | 1.211 |
| Coronary artery disease | 39.57 | 0.150 | 0.028 | 1.162 | 1.100 | 1.227 |
| Diabetes mellitus | 20.90 | 0.137 | 0.033 | 1.147 | 1.076 | 1.223 |
| Alcohol/drug abuse | 3.40 | 0.099 | 0.071 | 0.906 | 0.788 | 1.041 |
| Dementia/Alzheimer's disease | 16.38 | 0.125 | 0.038 | 1.133 | 1.052 | 1.222 |
| Splenectomy | 0.44 | 0.016 | 0.186 | 1.016 | 0.706 | 1.463 |
| Model | Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||
|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | |||
| ||||||||
| Medical Record Model Development Sample (NP) | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.10, 0.26) | 0.59 | 0 | 83.04 | 5.28 | 11.68 | 710 (35) |
| Linked Administrative Model Validation Sample | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.08, 0.30) | 0.63 | 0 | 83.04 | 6.94 | 10.01 | 1,414 (40) |
The correlation coefficient of the estimated state‐specific standardized readmission rates from the administrative and medical record models was 0.96, and the proportion of the variance explained by the model was 0.92 (Figure 4).

DISCUSSION
We have described the development, validation, and results of a hospital, 30‐day, risk‐standardized readmission model for pneumonia that was created to support current federal transparency initiatives. The model uses administrative claims data from Medicare fee‐for‐service patients and produces results that are comparable to a model based on information obtained through manual abstraction of medical records. We observed an overall 30‐day readmission rate of 17%, and our analyses revealed substantial variation across US hospitals, suggesting that improvement by lower performing institutions is an achievable goal.
Because more than one in six pneumonia patients are rehospitalized shortly after discharge, and because pneumonia hospitalizations represent an enormous expense to the Medicare program, prevention of readmissions is now widely recognized to offer a substantial opportunity to improve patient outcomes while simultaneously lowering health care costs. Accordingly, promotion of strategies to reduce readmission rates has become a key priority for payers and quality‐improvement organizations. These range from policy‐level attempts to stimulate change, such as publicly reporting hospital readmission rates on government websites, to establishing accreditation standardssuch as the Joint Commission's requirement to accurately reconcile medications, to the creation of quality improvement collaboratives focused on sharing best practices across institutions. Regardless of the approach taken, a valid, risk‐adjusted measure of performance is required to evaluate and track performance over time. The measure we have described meets the National Quality Forum's measure evaluation criteria in that it addresses an important clinical topic for which there appears to be significant opportunities for improvement, the measure is precisely defined and has been subjected to validity and reliability testing, it is risk‐adjusted based on patient clinical factors present at the start of care, is feasible to produce, and is understandable by a broad range of potential users.21 Because hospitalists are the physicians primarily responsible for the care of patients with pneumonia at US hospitals, and because they frequently serve as the physician champions for quality improvement activities related to pneumonia, it is especially important that they maintain a thorough understanding of the measures and methodologies underlying current efforts to measure hospital performance.
Several features of our approach warrant additional comment. First, we deliberately chose to measure all readmission events rather than attempt to discriminate between potentially preventable and nonpreventable readmissions. From the patient perspective, readmission for any reason is a concern, and limiting the measure to pneumonia‐related readmissions could make it susceptible to gaming by hospitals. Moreover, determining whether a readmission is related to a potential quality problem is not straightforward. For example, a patient with pneumonia whose discharge medications were prescribed incorrectly may be readmitted with a hip fracture following an episode of syncope. It would be inappropriate to treat this readmission as unrelated to the care the patient received for pneumonia. Additionally, while our approach does not presume that every readmission is preventable, the goal is to reduce the risk of readmissions generally (not just in narrowly defined subpopulations), and successful interventions to reduce rehospitalization have typically demonstrated reductions in all‐cause readmission.9, 22 Second, deaths that occurred within 30 days of discharge, yet that were not accompanied by a hospital readmission, were not counted as a readmission outcome. While it may seem inappropriate to treat a postdischarge death as a nonevent (rather than censoring or excluding such cases), alternative analytic approaches, such as using a hierarchical survival model, are not currently computationally feasible with large national data sets. Fortunately, only a relatively small proportion of discharges fell into this category (5.2% of index cases in the 2006 development sample died within 30 days of discharge without being readmitted). An alternative approach to handling the competing outcome of death would have been to use a composite outcome of readmission or death. However, we believe that it is important to report the outcomes separately because factors that predict readmission and mortality may differ, and when making comparisons across hospitals it would not be possible to determine whether differences in rate were due to readmission or mortality. Third, while the patient‐level readmission model showed only modest discrimination, we intentionally excluded covariates such as race and socioeconomic status, as well as in‐hospital events and potential complications of care, and whether patients were discharged home or to a skilled nursing facility. While these variables could have improved predictive ability, they may be directly or indirectly related to quality or supply factors that should not be included in a model that seeks to control for patient clinical characteristics. For example, if hospitals with a large share of poor patients have higher readmission rates, then including income in the model will obscure differences that are important to identify. While we believe that the decision to exclude such factors in the model is in the best interest of patients, and supports efforts to reduce health inequality in society more generally, we also recognize that hospitals that care for a disproportionate share of poor patients are likely to require additional resources to overcome these social factors. Fourth, we limited the analysis to patients with a principal diagnosis of pneumonia, and chose not to also include those with a principal diagnosis of sepsis or respiratory failure coupled with a secondary diagnosis of pneumonia. While the broader definition is used by CMS in the National Pneumonia Project, that initiative relied on chart abstraction to differentiate pneumonia present at the time of admission from cases developing as a complication of hospitalization. Additionally, we did not attempt to differentiate between community‐acquired and healthcare‐associated pneumonia, however our approach is consistent with the National Pneumonia Project and Pneumonia Patient Outcomes Research Team.18 Fifth, while our model estimates readmission rates at the hospital level, we recognize that readmissions are influenced by a complex and extensive range of factors. In this context, greater cooperation between hospitals and other care providers will almost certainly be required in order to achieve dramatic improvement in readmission rates, which in turn will depend upon changes to the way serious illness is paid for. Some options that have recently been described include imposing financial penalties for early readmission, extending the boundaries of case‐based payment beyond hospital discharge, and bundling payments between hospitals and physicians.2325
Our measure has several limitations. First, our models were developed and validated using Medicare data, and the results may not apply to pneumonia patients less than 65 years of age. However, most patients hospitalized with pneumonia in the US are 65 or older. In addition, we were unable to test the model with a Medicare managed care population, because data are not currently available on such patients. Finally, the medical record‐based validation was conducted by state‐level analysis because the sample size was insufficient to carry this out at the hospital level.
In conclusion, more than 17% of Medicare beneficiaries are readmitted within 30 days following discharge after a hospitalization for pneumonia, and rates vary substantially across institutions. The development of a valid measure of hospital performance and public reporting are important first steps towards focusing attention on this problem. Actual improvement will now depend on whether hospitals and partner organizations are successful at identifying and implementing effective methods to prevent readmission.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
- Medicare Payment Advisory Commission.Report to the Congress: Promoting Greater Efficiency in Medicare.2007.
- ,,,,. HCUP Facts and Figures: Statistics on Hospital‐based Care in the United States, 2007.2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed November 7, 2009.
- Centers for Medicare 353(3):255–264.
- ,,,.Trends in postdischarge mortality and readmissions: has length of stay declined too far?Arch Intern Med.2004;164(5):538–544.
- ,,,.Short‐term outcomes and their predictors for patients hospitalized with community‐acquired pneumonia.Heart Lung.2004;33(5):301–307.
- ,,, et al.Improved clinical outcomes with utilization of a community‐acquired pneumonia guideline.Chest.2006;130(3):794–799.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159(21):2562–2572.
- ,.Hospital readmissions as a measure of quality of health care: advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- Corrigan JM, Eden J, Smith BM, eds.Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Committee on Enhancing Federal Healthcare Quality Programs.Washington, DC:National Academies Press,2003.
- Medicare.gov—Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Search/Welcome.asp?version=default1(1):29–37.
- ,,,,.Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement.Health Aff.2007;26(1):75–85.
- NQF‐Endorsed® Standards. Available at: http://www.qualityforum.org/Measures_List.aspx. Accessed November 6,2009.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164(6):637–644.
- ,,. Diagnostic Cost Group Hierarchical Condition Category Models for Medicare Risk Adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc;2000. Available at: http://www.cms.hhs.gov/Reports/Reports/ItemDetail.asp?ItemID=CMS023176. Accessed November 7, 2009.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.1st ed.New York:Springer;2006.
- National Quality Forum—Measure Evaluation Criteria.2008. Available at: http://www.qualityforum.org/uploadedFiles/Quality_Forum/Measuring_Performance/Consensus_Development_Process%E2%80%99s_Principle/EvalCriteria2008–08‐28Final.pdf?n=4701.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- .Paying for care episodes and care coordination.N Engl J Med.2007;356(11):1166–1168.
- .Health care reform—toward more freedom, and responsibility, for physicians.N Engl J Med.2009;361(6):623–628.
- .Beyond pay for performance—emerging models of provider‐payment reform.N Engl J Med.2008;359(12):1197–1200.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
- Medicare Payment Advisory Commission.Report to the Congress: Promoting Greater Efficiency in Medicare.2007.
- ,,,,. HCUP Facts and Figures: Statistics on Hospital‐based Care in the United States, 2007.2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed November 7, 2009.
- Centers for Medicare 353(3):255–264.
- ,,,.Trends in postdischarge mortality and readmissions: has length of stay declined too far?Arch Intern Med.2004;164(5):538–544.
- ,,,.Short‐term outcomes and their predictors for patients hospitalized with community‐acquired pneumonia.Heart Lung.2004;33(5):301–307.
- ,,, et al.Improved clinical outcomes with utilization of a community‐acquired pneumonia guideline.Chest.2006;130(3):794–799.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159(21):2562–2572.
- ,.Hospital readmissions as a measure of quality of health care: advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- Corrigan JM, Eden J, Smith BM, eds.Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Committee on Enhancing Federal Healthcare Quality Programs.Washington, DC:National Academies Press,2003.
- Medicare.gov—Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Search/Welcome.asp?version=default1(1):29–37.
- ,,,,.Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement.Health Aff.2007;26(1):75–85.
- NQF‐Endorsed® Standards. Available at: http://www.qualityforum.org/Measures_List.aspx. Accessed November 6,2009.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164(6):637–644.
- ,,. Diagnostic Cost Group Hierarchical Condition Category Models for Medicare Risk Adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc;2000. Available at: http://www.cms.hhs.gov/Reports/Reports/ItemDetail.asp?ItemID=CMS023176. Accessed November 7, 2009.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.1st ed.New York:Springer;2006.
- National Quality Forum—Measure Evaluation Criteria.2008. Available at: http://www.qualityforum.org/uploadedFiles/Quality_Forum/Measuring_Performance/Consensus_Development_Process%E2%80%99s_Principle/EvalCriteria2008–08‐28Final.pdf?n=4701.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- .Paying for care episodes and care coordination.N Engl J Med.2007;356(11):1166–1168.
- .Health care reform—toward more freedom, and responsibility, for physicians.N Engl J Med.2009;361(6):623–628.
- .Beyond pay for performance—emerging models of provider‐payment reform.N Engl J Med.2008;359(12):1197–1200.
Copyright © 2010 Society of Hospital Medicine
Turn to ACGME for Transfer, Resident Supervision Rules
I have doubts: Are there any guidelines about “bouncing” patients between teaching and nonteaching services in a teaching hospital?
Srikanth Seethala, MD
Pittsburgh
Dr. Hospitalist responds: Several thoughts came to my mind when I read your question. What did you mean by the term “bouncing”? When you refer to “nonteaching service,” are you referring to the cohort of inpatients in your teaching hospital cared for by attending physicians without the involvement of trainees? Of course, the most obvious question is what is causing your “doubt”?
As you may know, all U.S. postgraduate physician training programs are governed by the rules and standards set forth by the Accreditation Council for Graduate Medical Education (ACGME). You can find all of ACGME’s rules online at www.acgme.org. Regardless of whether you are a trainee or an attending physician, the ACGME expects the same interpretation and enforcement of their standards.
Our general medical service is divided into the resident-covered service and a separate, nonresident-covered service. Resident-covered service means IM residents are involved in the care of the patient under the supervision of an attending physician. No residents are involved in patient care on the nonresident-covered service. The development of our nonresident-covered service was clearly a product of ACGME duty-hour standards, which were originally enacted in 2003 and recently revised.
Our IM program has the same number of residents that we did before the new rules were put in place. Before 2003, we did not have a nonresident-covered medical service because we had a sufficient number of residents to care for all patients on our medical service. We found that the 2003 standards restricted the number of hours our residents could work in our hospital, so despite no change in the size of our medical service or the number of residents, we found ourselves without sufficient numbers of residents to meet the clinical demand. To meet this demand, we developed a hospitalist-run, nonresident-covered medical service.
We discussed a number of issues during the planning stages of our new service:
- How many hospitalist full-time equivalents (FTEs) would we need to staff this service?
- Would we have hospitalists physically in the hospital 24/7 or take call from outside the hospital?
- How much would it cost?
- Do we have two groups of hospitalist staff, one for the resident-covered service and a separate one for the nonresident-covered service? Or do we maintain one cohort of hospitalists and ask the staff to work on both the resident- and nonresident-covered services?
- Do we ask our hospitalists to rotate month by month or week by week, separately on the resident- and then the nonresident-covered service? Or do we ask hospitalists to see both patients on any given day?
- Do we geographically cohort our resident-covered patients on floors separate from our nonresident-covered patients?
The new rules fueled a lot of discussion between educators and trainees. Your question about the transfer of patients between resident- and nonresident-covered services does not surprise me. Some training programs tried to minimize the necessary number of attending level staff in the hospital by allowing trainees to “cross-cover,” or essentially care for patients on the nonresident-covered service, when the attending staff was not present in the hospital. It is my understanding that trainees are never allowed to cross-cover patients on the nonresident-covered service.
To my knowledge, however, there are no rules against transferring patients from the nonresident-covered service to the resident-covered service, or vice versa. TH
I have doubts: Are there any guidelines about “bouncing” patients between teaching and nonteaching services in a teaching hospital?
Srikanth Seethala, MD
Pittsburgh
Dr. Hospitalist responds: Several thoughts came to my mind when I read your question. What did you mean by the term “bouncing”? When you refer to “nonteaching service,” are you referring to the cohort of inpatients in your teaching hospital cared for by attending physicians without the involvement of trainees? Of course, the most obvious question is what is causing your “doubt”?
As you may know, all U.S. postgraduate physician training programs are governed by the rules and standards set forth by the Accreditation Council for Graduate Medical Education (ACGME). You can find all of ACGME’s rules online at www.acgme.org. Regardless of whether you are a trainee or an attending physician, the ACGME expects the same interpretation and enforcement of their standards.
Our general medical service is divided into the resident-covered service and a separate, nonresident-covered service. Resident-covered service means IM residents are involved in the care of the patient under the supervision of an attending physician. No residents are involved in patient care on the nonresident-covered service. The development of our nonresident-covered service was clearly a product of ACGME duty-hour standards, which were originally enacted in 2003 and recently revised.
Our IM program has the same number of residents that we did before the new rules were put in place. Before 2003, we did not have a nonresident-covered medical service because we had a sufficient number of residents to care for all patients on our medical service. We found that the 2003 standards restricted the number of hours our residents could work in our hospital, so despite no change in the size of our medical service or the number of residents, we found ourselves without sufficient numbers of residents to meet the clinical demand. To meet this demand, we developed a hospitalist-run, nonresident-covered medical service.
We discussed a number of issues during the planning stages of our new service:
- How many hospitalist full-time equivalents (FTEs) would we need to staff this service?
- Would we have hospitalists physically in the hospital 24/7 or take call from outside the hospital?
- How much would it cost?
- Do we have two groups of hospitalist staff, one for the resident-covered service and a separate one for the nonresident-covered service? Or do we maintain one cohort of hospitalists and ask the staff to work on both the resident- and nonresident-covered services?
- Do we ask our hospitalists to rotate month by month or week by week, separately on the resident- and then the nonresident-covered service? Or do we ask hospitalists to see both patients on any given day?
- Do we geographically cohort our resident-covered patients on floors separate from our nonresident-covered patients?
The new rules fueled a lot of discussion between educators and trainees. Your question about the transfer of patients between resident- and nonresident-covered services does not surprise me. Some training programs tried to minimize the necessary number of attending level staff in the hospital by allowing trainees to “cross-cover,” or essentially care for patients on the nonresident-covered service, when the attending staff was not present in the hospital. It is my understanding that trainees are never allowed to cross-cover patients on the nonresident-covered service.
To my knowledge, however, there are no rules against transferring patients from the nonresident-covered service to the resident-covered service, or vice versa. TH
I have doubts: Are there any guidelines about “bouncing” patients between teaching and nonteaching services in a teaching hospital?
Srikanth Seethala, MD
Pittsburgh
Dr. Hospitalist responds: Several thoughts came to my mind when I read your question. What did you mean by the term “bouncing”? When you refer to “nonteaching service,” are you referring to the cohort of inpatients in your teaching hospital cared for by attending physicians without the involvement of trainees? Of course, the most obvious question is what is causing your “doubt”?
As you may know, all U.S. postgraduate physician training programs are governed by the rules and standards set forth by the Accreditation Council for Graduate Medical Education (ACGME). You can find all of ACGME’s rules online at www.acgme.org. Regardless of whether you are a trainee or an attending physician, the ACGME expects the same interpretation and enforcement of their standards.
Our general medical service is divided into the resident-covered service and a separate, nonresident-covered service. Resident-covered service means IM residents are involved in the care of the patient under the supervision of an attending physician. No residents are involved in patient care on the nonresident-covered service. The development of our nonresident-covered service was clearly a product of ACGME duty-hour standards, which were originally enacted in 2003 and recently revised.
Our IM program has the same number of residents that we did before the new rules were put in place. Before 2003, we did not have a nonresident-covered medical service because we had a sufficient number of residents to care for all patients on our medical service. We found that the 2003 standards restricted the number of hours our residents could work in our hospital, so despite no change in the size of our medical service or the number of residents, we found ourselves without sufficient numbers of residents to meet the clinical demand. To meet this demand, we developed a hospitalist-run, nonresident-covered medical service.
We discussed a number of issues during the planning stages of our new service:
- How many hospitalist full-time equivalents (FTEs) would we need to staff this service?
- Would we have hospitalists physically in the hospital 24/7 or take call from outside the hospital?
- How much would it cost?
- Do we have two groups of hospitalist staff, one for the resident-covered service and a separate one for the nonresident-covered service? Or do we maintain one cohort of hospitalists and ask the staff to work on both the resident- and nonresident-covered services?
- Do we ask our hospitalists to rotate month by month or week by week, separately on the resident- and then the nonresident-covered service? Or do we ask hospitalists to see both patients on any given day?
- Do we geographically cohort our resident-covered patients on floors separate from our nonresident-covered patients?
The new rules fueled a lot of discussion between educators and trainees. Your question about the transfer of patients between resident- and nonresident-covered services does not surprise me. Some training programs tried to minimize the necessary number of attending level staff in the hospital by allowing trainees to “cross-cover,” or essentially care for patients on the nonresident-covered service, when the attending staff was not present in the hospital. It is my understanding that trainees are never allowed to cross-cover patients on the nonresident-covered service.
To my knowledge, however, there are no rules against transferring patients from the nonresident-covered service to the resident-covered service, or vice versa. TH
FPHM: A License to Drive Change
I was musing one morning about my day ahead. I was doing one of those subcortical activities of daily living in which the mind can wander freely. Have you ever jumped in the car with the intention of stopping at the store on the way home, only to find yourself pulling into your driveway after spending the drive contemplating those issues on your plate that day? You drive home on autopilot. It occurred to me that it can happen in much the same way in our daily practice of medicine—how easy it is to slip into autopilot when admitting patients and doing our daily rounds.
During this particular morning, I mulled over many things: the translocation between chromosomes 9 and 22 in CML, the obstructive PFTs one generally sees in cadmium exposure, debating whether to give corticosteroids or to induce delivery in a 33-week pregnant woman with HELLP syndrome. Maybe you’re wondering: Am I a physician practicing in a remote rural area that has no access to oncologists? Do I practice in an underserved industrial town next to an old battery factory? Or am I an old-fashioned GP who still delivers babies?
No, no, and no. I am a board-certified internist who was preparing for my Maintenance of Certification (MOC) examination.
A New Way of Thinking
I am 42 years old, I have a busy medical practice, I am the medical director of the 14-person HM group at Wentworth-Douglass Hospital in Dover, N.H., and I am the mother of two children, ages 9 and 11. And I found myself, on top of all these things, a student, too.
I’ve been practicing medicine for 11 years. I’ve gone from practicing primary care in a small community in Maine to working at a larger community medical center in New Hampshire, becoming a hospitalist in 2005, then taking on the job of director of my hospitalist group in 2006. With more than a decade of experience under my belt, I felt I had the depth of knowledge experience brings.
However, as I traveled through the process of preparing for the American Board of Internal Medicine’s (ABIM’s) new Focused Practice in Hospital Medicine (FPHM) secure exam, it began to dawn on me: Medicine is a complicated profession that not only requires careful attention to the details of every case, it also demands it. In order to avoid the pitfall of practicing distracted medicine, we must carefully foster our own continuing education.
Going through the studying process has enabled me to think about the medicine I practice in a much more academic way. True, I don’t necessarily need to know some of the things I’ve encountered in my study sessions for my everyday practice, but I find myself spouting off random facts to anyone who will listen—colleagues, nurses, even patients. “Did you know that only about one-fourth of crystalloid remains in the intravascular space, where the rest goes into the tissues?” “If the triglycerides in this fluid are greater than 115, this is a chylothorax!” I’m paying attention again to the theory and pathophysiology behind medical illnesses, not just to the drudgery of writing routine orders or checking off boxes on a protocol.
It has not been easy. Although I’ve known I needed to recertify in internal medicine since I took the exam the first time, I did not actively start looking into the exam and preparing until about a year and a half before my exam, when I talked to a colleague who had already started preparing. That’s when I learned that this was not only an exam, but also a process. This process is intended by the board to be an active part of maintaining certification during the 10 years before it is due again, not just to be crammed into the last year or two before certification expires. I recommend to anyone going through this process to familiarize yourself with the ABIM website (www.abim.org). Initially, it was a little unwieldy to maneuver around the site, and it wasn’t entirely clear to me what exactly was needed to recertify until I spent some time maneuvering through the site.
HM-Focused Pathway
To add to this, at around the time I was getting ready to register for the exam, it was announced that this would be the first year ABIM would be offering the FPHM pathway, which is designed to recognize those of us who concentrate our practices on hospital medicine. This to me was an excellent opportunity to recertify in a field in which I actively practice, hopefully making the exam more applicable to what I do, but the flip side was that no one would have taken this particular exam before. Admittedly, when I first signed up, I felt like I was either a guinea pig or a pioneer.
To obtain the FPHM, one must do not one but two projects requiring turning in data on process-improvement projects. Hospitalists who intend to certify with the FPHM will be well served by participating in safety, quality, and process-improvement projects, as we often already do. These projects can be used to complete the required Practice Improvement Modules.
Furthermore, I found that doing such projects is the best way to prepare for the new content, which deals specifically with HM on the actual exam. The internal-medicine topics were covered, just as they are in the nonfocused exam, and anyone who reviews for the exam with available study aids (e.g. review books, courses, or practice questions) will have adequate exposure to these topics.
However, a colleague in my HM group chose not to take the focused practice exam, largely because there was no previously established review material to use as study aids. I anticipate that future study aids will contain references to these questions, but for now I felt that material was adequately covered just by completing the Practice Improvement Modules and by being involved in process improvement projects at my hospital. In fact, attendance at one Institute for Healthcare Improvement (www.ihi.org/IHI/Programs) conference would probably cover the topics nicely.
To all of my colleages considering the MOC in FPHM exam, I wish you luck. I feel that any practicing hospitalist is likely to be able to satisfy the requirements of the FPHM pathway without doing too much more than they would in their daily practice or their usual exam preparation. I also found the ABIM staff useful and helpful, and recommend you use the “contact ABIM” link on their website with any questions.
Focused practice is exactly what we should be driving for. TH
Dr. Ammann is medical director of the hospital medicine division at Wentworth-Douglass Hospital in Dover, N.H.
I was musing one morning about my day ahead. I was doing one of those subcortical activities of daily living in which the mind can wander freely. Have you ever jumped in the car with the intention of stopping at the store on the way home, only to find yourself pulling into your driveway after spending the drive contemplating those issues on your plate that day? You drive home on autopilot. It occurred to me that it can happen in much the same way in our daily practice of medicine—how easy it is to slip into autopilot when admitting patients and doing our daily rounds.
During this particular morning, I mulled over many things: the translocation between chromosomes 9 and 22 in CML, the obstructive PFTs one generally sees in cadmium exposure, debating whether to give corticosteroids or to induce delivery in a 33-week pregnant woman with HELLP syndrome. Maybe you’re wondering: Am I a physician practicing in a remote rural area that has no access to oncologists? Do I practice in an underserved industrial town next to an old battery factory? Or am I an old-fashioned GP who still delivers babies?
No, no, and no. I am a board-certified internist who was preparing for my Maintenance of Certification (MOC) examination.
A New Way of Thinking
I am 42 years old, I have a busy medical practice, I am the medical director of the 14-person HM group at Wentworth-Douglass Hospital in Dover, N.H., and I am the mother of two children, ages 9 and 11. And I found myself, on top of all these things, a student, too.
I’ve been practicing medicine for 11 years. I’ve gone from practicing primary care in a small community in Maine to working at a larger community medical center in New Hampshire, becoming a hospitalist in 2005, then taking on the job of director of my hospitalist group in 2006. With more than a decade of experience under my belt, I felt I had the depth of knowledge experience brings.
However, as I traveled through the process of preparing for the American Board of Internal Medicine’s (ABIM’s) new Focused Practice in Hospital Medicine (FPHM) secure exam, it began to dawn on me: Medicine is a complicated profession that not only requires careful attention to the details of every case, it also demands it. In order to avoid the pitfall of practicing distracted medicine, we must carefully foster our own continuing education.
Going through the studying process has enabled me to think about the medicine I practice in a much more academic way. True, I don’t necessarily need to know some of the things I’ve encountered in my study sessions for my everyday practice, but I find myself spouting off random facts to anyone who will listen—colleagues, nurses, even patients. “Did you know that only about one-fourth of crystalloid remains in the intravascular space, where the rest goes into the tissues?” “If the triglycerides in this fluid are greater than 115, this is a chylothorax!” I’m paying attention again to the theory and pathophysiology behind medical illnesses, not just to the drudgery of writing routine orders or checking off boxes on a protocol.
It has not been easy. Although I’ve known I needed to recertify in internal medicine since I took the exam the first time, I did not actively start looking into the exam and preparing until about a year and a half before my exam, when I talked to a colleague who had already started preparing. That’s when I learned that this was not only an exam, but also a process. This process is intended by the board to be an active part of maintaining certification during the 10 years before it is due again, not just to be crammed into the last year or two before certification expires. I recommend to anyone going through this process to familiarize yourself with the ABIM website (www.abim.org). Initially, it was a little unwieldy to maneuver around the site, and it wasn’t entirely clear to me what exactly was needed to recertify until I spent some time maneuvering through the site.
HM-Focused Pathway
To add to this, at around the time I was getting ready to register for the exam, it was announced that this would be the first year ABIM would be offering the FPHM pathway, which is designed to recognize those of us who concentrate our practices on hospital medicine. This to me was an excellent opportunity to recertify in a field in which I actively practice, hopefully making the exam more applicable to what I do, but the flip side was that no one would have taken this particular exam before. Admittedly, when I first signed up, I felt like I was either a guinea pig or a pioneer.
To obtain the FPHM, one must do not one but two projects requiring turning in data on process-improvement projects. Hospitalists who intend to certify with the FPHM will be well served by participating in safety, quality, and process-improvement projects, as we often already do. These projects can be used to complete the required Practice Improvement Modules.
Furthermore, I found that doing such projects is the best way to prepare for the new content, which deals specifically with HM on the actual exam. The internal-medicine topics were covered, just as they are in the nonfocused exam, and anyone who reviews for the exam with available study aids (e.g. review books, courses, or practice questions) will have adequate exposure to these topics.
However, a colleague in my HM group chose not to take the focused practice exam, largely because there was no previously established review material to use as study aids. I anticipate that future study aids will contain references to these questions, but for now I felt that material was adequately covered just by completing the Practice Improvement Modules and by being involved in process improvement projects at my hospital. In fact, attendance at one Institute for Healthcare Improvement (www.ihi.org/IHI/Programs) conference would probably cover the topics nicely.
To all of my colleages considering the MOC in FPHM exam, I wish you luck. I feel that any practicing hospitalist is likely to be able to satisfy the requirements of the FPHM pathway without doing too much more than they would in their daily practice or their usual exam preparation. I also found the ABIM staff useful and helpful, and recommend you use the “contact ABIM” link on their website with any questions.
Focused practice is exactly what we should be driving for. TH
Dr. Ammann is medical director of the hospital medicine division at Wentworth-Douglass Hospital in Dover, N.H.
I was musing one morning about my day ahead. I was doing one of those subcortical activities of daily living in which the mind can wander freely. Have you ever jumped in the car with the intention of stopping at the store on the way home, only to find yourself pulling into your driveway after spending the drive contemplating those issues on your plate that day? You drive home on autopilot. It occurred to me that it can happen in much the same way in our daily practice of medicine—how easy it is to slip into autopilot when admitting patients and doing our daily rounds.
During this particular morning, I mulled over many things: the translocation between chromosomes 9 and 22 in CML, the obstructive PFTs one generally sees in cadmium exposure, debating whether to give corticosteroids or to induce delivery in a 33-week pregnant woman with HELLP syndrome. Maybe you’re wondering: Am I a physician practicing in a remote rural area that has no access to oncologists? Do I practice in an underserved industrial town next to an old battery factory? Or am I an old-fashioned GP who still delivers babies?
No, no, and no. I am a board-certified internist who was preparing for my Maintenance of Certification (MOC) examination.
A New Way of Thinking
I am 42 years old, I have a busy medical practice, I am the medical director of the 14-person HM group at Wentworth-Douglass Hospital in Dover, N.H., and I am the mother of two children, ages 9 and 11. And I found myself, on top of all these things, a student, too.
I’ve been practicing medicine for 11 years. I’ve gone from practicing primary care in a small community in Maine to working at a larger community medical center in New Hampshire, becoming a hospitalist in 2005, then taking on the job of director of my hospitalist group in 2006. With more than a decade of experience under my belt, I felt I had the depth of knowledge experience brings.
However, as I traveled through the process of preparing for the American Board of Internal Medicine’s (ABIM’s) new Focused Practice in Hospital Medicine (FPHM) secure exam, it began to dawn on me: Medicine is a complicated profession that not only requires careful attention to the details of every case, it also demands it. In order to avoid the pitfall of practicing distracted medicine, we must carefully foster our own continuing education.
Going through the studying process has enabled me to think about the medicine I practice in a much more academic way. True, I don’t necessarily need to know some of the things I’ve encountered in my study sessions for my everyday practice, but I find myself spouting off random facts to anyone who will listen—colleagues, nurses, even patients. “Did you know that only about one-fourth of crystalloid remains in the intravascular space, where the rest goes into the tissues?” “If the triglycerides in this fluid are greater than 115, this is a chylothorax!” I’m paying attention again to the theory and pathophysiology behind medical illnesses, not just to the drudgery of writing routine orders or checking off boxes on a protocol.
It has not been easy. Although I’ve known I needed to recertify in internal medicine since I took the exam the first time, I did not actively start looking into the exam and preparing until about a year and a half before my exam, when I talked to a colleague who had already started preparing. That’s when I learned that this was not only an exam, but also a process. This process is intended by the board to be an active part of maintaining certification during the 10 years before it is due again, not just to be crammed into the last year or two before certification expires. I recommend to anyone going through this process to familiarize yourself with the ABIM website (www.abim.org). Initially, it was a little unwieldy to maneuver around the site, and it wasn’t entirely clear to me what exactly was needed to recertify until I spent some time maneuvering through the site.
HM-Focused Pathway
To add to this, at around the time I was getting ready to register for the exam, it was announced that this would be the first year ABIM would be offering the FPHM pathway, which is designed to recognize those of us who concentrate our practices on hospital medicine. This to me was an excellent opportunity to recertify in a field in which I actively practice, hopefully making the exam more applicable to what I do, but the flip side was that no one would have taken this particular exam before. Admittedly, when I first signed up, I felt like I was either a guinea pig or a pioneer.
To obtain the FPHM, one must do not one but two projects requiring turning in data on process-improvement projects. Hospitalists who intend to certify with the FPHM will be well served by participating in safety, quality, and process-improvement projects, as we often already do. These projects can be used to complete the required Practice Improvement Modules.
Furthermore, I found that doing such projects is the best way to prepare for the new content, which deals specifically with HM on the actual exam. The internal-medicine topics were covered, just as they are in the nonfocused exam, and anyone who reviews for the exam with available study aids (e.g. review books, courses, or practice questions) will have adequate exposure to these topics.
However, a colleague in my HM group chose not to take the focused practice exam, largely because there was no previously established review material to use as study aids. I anticipate that future study aids will contain references to these questions, but for now I felt that material was adequately covered just by completing the Practice Improvement Modules and by being involved in process improvement projects at my hospital. In fact, attendance at one Institute for Healthcare Improvement (www.ihi.org/IHI/Programs) conference would probably cover the topics nicely.
To all of my colleages considering the MOC in FPHM exam, I wish you luck. I feel that any practicing hospitalist is likely to be able to satisfy the requirements of the FPHM pathway without doing too much more than they would in their daily practice or their usual exam preparation. I also found the ABIM staff useful and helpful, and recommend you use the “contact ABIM” link on their website with any questions.
Focused practice is exactly what we should be driving for. TH
Dr. Ammann is medical director of the hospital medicine division at Wentworth-Douglass Hospital in Dover, N.H.
What Is the Best Treatment of an Adult Patient with Hypercalcemia of Malignancy?
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.
Shared/Split Service
In response to internal and external pressures to minimize length of stay, adhere to limitations on the maximum number of admitted patients, focus on evidence-based care, and improve outcomes of care, hospitalists have incorporated nonphysician providers (NPPs), such as acute-care nurse practitioners (ACNPs), into their group practices.1 HM groups employing these practitioners must be aware of state and federal regulations, as well as billing and documentation standards surrounding NPP services.
Consider the following common hospitalist scenario: A nurse practitioner evaluates a 67-year-old patient admitted for chronic obstructive bronchitis and progressing shortness of breath. The nurse practitioner documents the service and provides the attending physician with an update on the patient’s status. Later in the day, the physician makes rounds and concurs with the patient’s current plan of care.
The above scenario represents a shared/split service in which two providers from the same group perform a service for the same patient on the same calendar day. The Centers for Medicare & Medicaid Services (CMS) allows these visits to be combined and reported under a single provider’s name if the shared/split billing criteria are met and appropriately documented.
Eligible Providers
The shared/split billing option only applies to services rendered by the attending physician and specified NPPs: nurse practitioners, physician assistants, clinical nurse specialists, and certified nurse-midwives. Both the attending physician and the NPP must be part of the same group practice, either through direct employment or a leased arrangement that contractually links the two individuals. The “leased” relationship often occurs when the facility directly employs the NPP but arranges for the NPP to provide services exclusively for the physician group. It is imperative that the bills for the NPP services are captured and reported by one entity—the hospitalist group.
Several other NPPs (e.g. clinical psychologists or certified registered nurse anesthetists) are recognized by CMS but are ineligible for shared/split billing and must report their services under a different Medicare billing option. Additionally, shared/split services do not apply to physicians in training (interns, residents, fellows) or students.
Qualifying Services
Medicare reimburses services that are considered reasonable and necessary and not otherwise excluded from coverage. From a clinical perspective, NPPs might provide any service permitted by the state scope of practice and performed under the appropriate level of supervision or collaboration as depicted in licensure requirements. These typically comprise visits or procedures rendered by ancillary staff or considered a “physician” service.
Alternatively, shared/split billing regulations limit the types of services that can be reported under this methodology, recognizing only evaluation and management (E/M) services provided in explicit facility-based settings: EDs, outpatient hospital clinics, or inpatient hospitals. Critical-care services and procedures are excluded.
Physician Involvement
The NPP and the physician must have a face-to-face encounter with the same patient on the same calendar day, and there are no constraints on which provider should perform the initial encounter of the day.2
The extent of each provider’s involvement is left to provider discretion and/or local Medicare contractor requirements. Some contractors refer to the physician performing a “substantive” service but do not elaborate with specific service parameters, leaving the physician to determine the critical or key portion of his/her service. A corresponding, detailed notation alleviates any misconceptions of physician involvement.
Documentation by the attending physician should include an attestation that unequivocally demonstrates their personal encounter with the patient—for example, “Patient seen and examined by me.” Additionally, both the NPP and the physician should document the name of the individual with whom the service is shared/split—for example, “Agree with note by ____.” This allows for better charge capture; alerts coders, auditors, and payor representatives to consider both notes in support of the billed service; and ensures that the correct notes are sent to the payor in the event of claim denial and subsequent appeal.
Each provider must document their portion of the rendered service, date and legibly sign their corresponding note, and select the visit level supported by the cumulative encounter—for example, “Pulse oximetry 94% on room air. Audible rhonchi at bilateral lung bases. Start O2 2L nasal cannula. Obtain CXR.”
Only one claim can be submitted for a shared/split service. The services might either be reported with the physician’s NPI or the NPP’s NPI. Reimbursement is dependent upon this designation. The physician NPI generates 100% of the Medicare allowable rate; the NPP NPI limits reimbursement to 85% of the allowable physician rate.
Non-Medicare Claims
The shared/split billing policy only applies to Medicare beneficiaries. Due to excessive costs of NPP credentialing and enrollment, most non-Medicare insurers do not issue NPP provider numbers.
Effective June 1, 2010, Aetna began to enroll and reimburse NPP services, but it has not yet outlined a policy that parallels Medicare’s shared/split billing policy. However, lack of payor policy does not preclude payment for shared NPP services; it necessitates additional—and initial—efforts to obtain recognition and corresponding reimbursement.
After determining which insurers have applicable shared/split billing policies, develop a reasonable guideline to offer those payors who do not recognize the billing option. Alert the payor, in writing, that policy implementation will take place in a predetermined timeframe unless the payor can provide an alternate billing option. Some experts suggest physician groups outline the following key issues when structuring a billing option:
- Types of NPP involved in patient care;
- Category of services provided (e.g. E/M, procedures);
- Service location(s) (ED, inpatient, or outpatient hospital);
- Physician involvement;
- Mechanism for reporting services; and
- Documentation requirements.
This can be performed for any of the NPP billing options and is not limited to shared/split billing. Be sure to obtain payor response before initiating the shared/split billing process.
Summary
NPPs are involved in numerous services within the hospital, and often share/split services with hospitalists. Successful reporting requires understanding of and adherence to federal, state, and billing guidelines.
It is important to identify NPP employment relationships, the NPP’s role in the provision of services, the state supervisory or collaborative rules, and local payor interpretations to prevent misrepresentations, misunderstandings, or erroneous reporting. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Howie JN, Erickson M. Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J Crit Care. 2002; 11(5):448-458.
- Medicare Claims Processing Manual: Chapter 12, Section 30.6.1B. Centers for Medicare and Medicaid Services website. Available at: www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed Nov. 14, 2010.
- Pohlig, C. Nonphysician providers in your practice. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians; 2010.
- Medicare Benefit Policy Manual: Chapter 15, Section 190-200. CMS website. Available at: www.cms.hhs.gov/manuals/Downloads/bp102c15.pdf. Accessed Nov. 14, 2010.
In response to internal and external pressures to minimize length of stay, adhere to limitations on the maximum number of admitted patients, focus on evidence-based care, and improve outcomes of care, hospitalists have incorporated nonphysician providers (NPPs), such as acute-care nurse practitioners (ACNPs), into their group practices.1 HM groups employing these practitioners must be aware of state and federal regulations, as well as billing and documentation standards surrounding NPP services.
Consider the following common hospitalist scenario: A nurse practitioner evaluates a 67-year-old patient admitted for chronic obstructive bronchitis and progressing shortness of breath. The nurse practitioner documents the service and provides the attending physician with an update on the patient’s status. Later in the day, the physician makes rounds and concurs with the patient’s current plan of care.
The above scenario represents a shared/split service in which two providers from the same group perform a service for the same patient on the same calendar day. The Centers for Medicare & Medicaid Services (CMS) allows these visits to be combined and reported under a single provider’s name if the shared/split billing criteria are met and appropriately documented.
Eligible Providers
The shared/split billing option only applies to services rendered by the attending physician and specified NPPs: nurse practitioners, physician assistants, clinical nurse specialists, and certified nurse-midwives. Both the attending physician and the NPP must be part of the same group practice, either through direct employment or a leased arrangement that contractually links the two individuals. The “leased” relationship often occurs when the facility directly employs the NPP but arranges for the NPP to provide services exclusively for the physician group. It is imperative that the bills for the NPP services are captured and reported by one entity—the hospitalist group.
Several other NPPs (e.g. clinical psychologists or certified registered nurse anesthetists) are recognized by CMS but are ineligible for shared/split billing and must report their services under a different Medicare billing option. Additionally, shared/split services do not apply to physicians in training (interns, residents, fellows) or students.
Qualifying Services
Medicare reimburses services that are considered reasonable and necessary and not otherwise excluded from coverage. From a clinical perspective, NPPs might provide any service permitted by the state scope of practice and performed under the appropriate level of supervision or collaboration as depicted in licensure requirements. These typically comprise visits or procedures rendered by ancillary staff or considered a “physician” service.
Alternatively, shared/split billing regulations limit the types of services that can be reported under this methodology, recognizing only evaluation and management (E/M) services provided in explicit facility-based settings: EDs, outpatient hospital clinics, or inpatient hospitals. Critical-care services and procedures are excluded.
Physician Involvement
The NPP and the physician must have a face-to-face encounter with the same patient on the same calendar day, and there are no constraints on which provider should perform the initial encounter of the day.2
The extent of each provider’s involvement is left to provider discretion and/or local Medicare contractor requirements. Some contractors refer to the physician performing a “substantive” service but do not elaborate with specific service parameters, leaving the physician to determine the critical or key portion of his/her service. A corresponding, detailed notation alleviates any misconceptions of physician involvement.
Documentation by the attending physician should include an attestation that unequivocally demonstrates their personal encounter with the patient—for example, “Patient seen and examined by me.” Additionally, both the NPP and the physician should document the name of the individual with whom the service is shared/split—for example, “Agree with note by ____.” This allows for better charge capture; alerts coders, auditors, and payor representatives to consider both notes in support of the billed service; and ensures that the correct notes are sent to the payor in the event of claim denial and subsequent appeal.
Each provider must document their portion of the rendered service, date and legibly sign their corresponding note, and select the visit level supported by the cumulative encounter—for example, “Pulse oximetry 94% on room air. Audible rhonchi at bilateral lung bases. Start O2 2L nasal cannula. Obtain CXR.”
Only one claim can be submitted for a shared/split service. The services might either be reported with the physician’s NPI or the NPP’s NPI. Reimbursement is dependent upon this designation. The physician NPI generates 100% of the Medicare allowable rate; the NPP NPI limits reimbursement to 85% of the allowable physician rate.
Non-Medicare Claims
The shared/split billing policy only applies to Medicare beneficiaries. Due to excessive costs of NPP credentialing and enrollment, most non-Medicare insurers do not issue NPP provider numbers.
Effective June 1, 2010, Aetna began to enroll and reimburse NPP services, but it has not yet outlined a policy that parallels Medicare’s shared/split billing policy. However, lack of payor policy does not preclude payment for shared NPP services; it necessitates additional—and initial—efforts to obtain recognition and corresponding reimbursement.
After determining which insurers have applicable shared/split billing policies, develop a reasonable guideline to offer those payors who do not recognize the billing option. Alert the payor, in writing, that policy implementation will take place in a predetermined timeframe unless the payor can provide an alternate billing option. Some experts suggest physician groups outline the following key issues when structuring a billing option:
- Types of NPP involved in patient care;
- Category of services provided (e.g. E/M, procedures);
- Service location(s) (ED, inpatient, or outpatient hospital);
- Physician involvement;
- Mechanism for reporting services; and
- Documentation requirements.
This can be performed for any of the NPP billing options and is not limited to shared/split billing. Be sure to obtain payor response before initiating the shared/split billing process.
Summary
NPPs are involved in numerous services within the hospital, and often share/split services with hospitalists. Successful reporting requires understanding of and adherence to federal, state, and billing guidelines.
It is important to identify NPP employment relationships, the NPP’s role in the provision of services, the state supervisory or collaborative rules, and local payor interpretations to prevent misrepresentations, misunderstandings, or erroneous reporting. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Howie JN, Erickson M. Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J Crit Care. 2002; 11(5):448-458.
- Medicare Claims Processing Manual: Chapter 12, Section 30.6.1B. Centers for Medicare and Medicaid Services website. Available at: www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed Nov. 14, 2010.
- Pohlig, C. Nonphysician providers in your practice. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians; 2010.
- Medicare Benefit Policy Manual: Chapter 15, Section 190-200. CMS website. Available at: www.cms.hhs.gov/manuals/Downloads/bp102c15.pdf. Accessed Nov. 14, 2010.
In response to internal and external pressures to minimize length of stay, adhere to limitations on the maximum number of admitted patients, focus on evidence-based care, and improve outcomes of care, hospitalists have incorporated nonphysician providers (NPPs), such as acute-care nurse practitioners (ACNPs), into their group practices.1 HM groups employing these practitioners must be aware of state and federal regulations, as well as billing and documentation standards surrounding NPP services.
Consider the following common hospitalist scenario: A nurse practitioner evaluates a 67-year-old patient admitted for chronic obstructive bronchitis and progressing shortness of breath. The nurse practitioner documents the service and provides the attending physician with an update on the patient’s status. Later in the day, the physician makes rounds and concurs with the patient’s current plan of care.
The above scenario represents a shared/split service in which two providers from the same group perform a service for the same patient on the same calendar day. The Centers for Medicare & Medicaid Services (CMS) allows these visits to be combined and reported under a single provider’s name if the shared/split billing criteria are met and appropriately documented.
Eligible Providers
The shared/split billing option only applies to services rendered by the attending physician and specified NPPs: nurse practitioners, physician assistants, clinical nurse specialists, and certified nurse-midwives. Both the attending physician and the NPP must be part of the same group practice, either through direct employment or a leased arrangement that contractually links the two individuals. The “leased” relationship often occurs when the facility directly employs the NPP but arranges for the NPP to provide services exclusively for the physician group. It is imperative that the bills for the NPP services are captured and reported by one entity—the hospitalist group.
Several other NPPs (e.g. clinical psychologists or certified registered nurse anesthetists) are recognized by CMS but are ineligible for shared/split billing and must report their services under a different Medicare billing option. Additionally, shared/split services do not apply to physicians in training (interns, residents, fellows) or students.
Qualifying Services
Medicare reimburses services that are considered reasonable and necessary and not otherwise excluded from coverage. From a clinical perspective, NPPs might provide any service permitted by the state scope of practice and performed under the appropriate level of supervision or collaboration as depicted in licensure requirements. These typically comprise visits or procedures rendered by ancillary staff or considered a “physician” service.
Alternatively, shared/split billing regulations limit the types of services that can be reported under this methodology, recognizing only evaluation and management (E/M) services provided in explicit facility-based settings: EDs, outpatient hospital clinics, or inpatient hospitals. Critical-care services and procedures are excluded.
Physician Involvement
The NPP and the physician must have a face-to-face encounter with the same patient on the same calendar day, and there are no constraints on which provider should perform the initial encounter of the day.2
The extent of each provider’s involvement is left to provider discretion and/or local Medicare contractor requirements. Some contractors refer to the physician performing a “substantive” service but do not elaborate with specific service parameters, leaving the physician to determine the critical or key portion of his/her service. A corresponding, detailed notation alleviates any misconceptions of physician involvement.
Documentation by the attending physician should include an attestation that unequivocally demonstrates their personal encounter with the patient—for example, “Patient seen and examined by me.” Additionally, both the NPP and the physician should document the name of the individual with whom the service is shared/split—for example, “Agree with note by ____.” This allows for better charge capture; alerts coders, auditors, and payor representatives to consider both notes in support of the billed service; and ensures that the correct notes are sent to the payor in the event of claim denial and subsequent appeal.
Each provider must document their portion of the rendered service, date and legibly sign their corresponding note, and select the visit level supported by the cumulative encounter—for example, “Pulse oximetry 94% on room air. Audible rhonchi at bilateral lung bases. Start O2 2L nasal cannula. Obtain CXR.”
Only one claim can be submitted for a shared/split service. The services might either be reported with the physician’s NPI or the NPP’s NPI. Reimbursement is dependent upon this designation. The physician NPI generates 100% of the Medicare allowable rate; the NPP NPI limits reimbursement to 85% of the allowable physician rate.
Non-Medicare Claims
The shared/split billing policy only applies to Medicare beneficiaries. Due to excessive costs of NPP credentialing and enrollment, most non-Medicare insurers do not issue NPP provider numbers.
Effective June 1, 2010, Aetna began to enroll and reimburse NPP services, but it has not yet outlined a policy that parallels Medicare’s shared/split billing policy. However, lack of payor policy does not preclude payment for shared NPP services; it necessitates additional—and initial—efforts to obtain recognition and corresponding reimbursement.
After determining which insurers have applicable shared/split billing policies, develop a reasonable guideline to offer those payors who do not recognize the billing option. Alert the payor, in writing, that policy implementation will take place in a predetermined timeframe unless the payor can provide an alternate billing option. Some experts suggest physician groups outline the following key issues when structuring a billing option:
- Types of NPP involved in patient care;
- Category of services provided (e.g. E/M, procedures);
- Service location(s) (ED, inpatient, or outpatient hospital);
- Physician involvement;
- Mechanism for reporting services; and
- Documentation requirements.
This can be performed for any of the NPP billing options and is not limited to shared/split billing. Be sure to obtain payor response before initiating the shared/split billing process.
Summary
NPPs are involved in numerous services within the hospital, and often share/split services with hospitalists. Successful reporting requires understanding of and adherence to federal, state, and billing guidelines.
It is important to identify NPP employment relationships, the NPP’s role in the provision of services, the state supervisory or collaborative rules, and local payor interpretations to prevent misrepresentations, misunderstandings, or erroneous reporting. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Howie JN, Erickson M. Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J Crit Care. 2002; 11(5):448-458.
- Medicare Claims Processing Manual: Chapter 12, Section 30.6.1B. Centers for Medicare and Medicaid Services website. Available at: www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed Nov. 14, 2010.
- Pohlig, C. Nonphysician providers in your practice. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians; 2010.
- Medicare Benefit Policy Manual: Chapter 15, Section 190-200. CMS website. Available at: www.cms.hhs.gov/manuals/Downloads/bp102c15.pdf. Accessed Nov. 14, 2010.
Turbulence Ahead
After the wild ride of 2010, public-policy watchers could be forgiven for fervently hoping that 2011 offers a calmer year on the healthcare front.
Fat chance.
The turbulence could begin immediately, with the seating of the 112th Congress on Jan. 3. “I think the first question that’s on everybody’s mind is, ‘What will the Republican majority in the House do to Obama’s healthcare reform initiative?’ ” says Eric Siegal, MD, SFHM, a member of SHM’s Public Policy Committee (PPC) and a clinical assistant professor of medicine at the University of Wisconsin School of Medicine and Public Health.
For most issues of direct concern to hospitalists, he says, especially those centered on healthcare delivery, “the wheels were in motion” long before the reform bill became law. Dr. Siegal also says most healthcare experts support the substance of accountable care organizations (ACOs), pay for performance, and reforming Medicare in ways that reward quality instead of quantity. “I think that ship is out of the harbor,” he says.
Throughout the year, the PPC will focus on three priorities identified in the Affordable Care Act: hospital value-based purchasing, bundled payments including ACOs, and hospital readmissions and transitions of care.
—Bill Vaughan, senior policy analyst, Consumers Union, Washington, D.C.
Debate, Delay, Defund?
There are several ways that Congress can delay or thwart the launch of specific reform initiatives. The first is to hold hearings about reform measures, Dr. Siegal says, “with the hope that they can somehow undermine it by raising questions about either the finances of it or about the implications for average Americans in terms of what kind of healthcare they’re going to get.” Such tactics carry significant risk, however, because highlighting specific aspects of the reform law could actually increase overall public support. “It has the potential to backfire on them,” he says.
“Repeal won’t happen anytime soon,” predicts Pat Conway, MD, chair of the PPC and director of hospital medicine at Cincinnati Children’s Hospital. “However, Congress could gut or significantly reduce funding to multiple programs within the bill, and then if you significantly reduce the funding, this may make it nearly impossible for those programs to be successful.”
Rough estimates suggest that some $150 billion worth of programs over the 10-year life of the healthcare reform act remain unfunded and are at risk. As an example, Dr. Conway cites wording in the bill that authorizes a program to help ease patient transitions in and out of the hospital. “If you reduce that funding to near zero, hospitals and hospitalists may still be successful, but you’ve essentially removed the program to learn how to be successful,” he says.
Bill Vaughan, a senior policy analyst in healthcare with the Washington, D.C.-based organization Consumers Union, says targeted riders could be added to appropriations bills. For instance, one rider could prohibit the Centers for Medicare & Medicaid Services (CMS) from spending any money to help develop government-supported insurance exchanges. Another could prevent the IRS from collecting money to be channeled into the trust fund for the Patient-Centered Outcomes Research Institute and its focus on comparative-effectiveness research. “There’s no end to mischief,” Vaughan says. “There are as many opportunities as the day is long.”
A major confrontation could arrive in March or April, when the U.S. runs into its debt ceiling. A continuing resolution would then be required to continue the appropriations process (and increase the U.S. debt ceiling past its current limit of $14.3 trillion). At that point or soon thereafter, Vaughan says, an opportunity could arise for legislators to say they won’t vote for a critical appropriations bill unless it includes certain spending reductions cited by one of several commissions tasked with recommending ways to reduce the deficit. “That could include hospital cuts, more doctor cuts, significant cost shifting to beneficiaries, higher copays,” he says.
Amid a “firestorm of ideas” on how to further cut Medicare and Medicaid spending, ideas once deemed radical could gain more traction. Some legislators have tossed around the idea of shutting down the government, if need be. “There’s nothing on the radar scope but static and fuzz,” Vaughan says. “It is totally unclear what is going to happen.”
Dearth of Drugs
Another trend generating both uncertainty and headaches in the nation’s hospitals is an unprecedented prescription drug shortage that could last well into the New Year, based on the number of medicines now in scarce supply across the country. In mid-November, for example, the American Society of Clinical Oncology announced “severe and worsening shortages of many critical therapies,” including doxorubicin, leucovorin, etoposide, nitrogen mustard, vincristine, propofol, and morphine.
Valerie Jensen, associate director of the FDA’s drug shortage program, told the Associated Press that her agency was seeing a record number of drug shortfalls in 2010. In mid-November, the FDA’s Current Drug Shortages list (www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050792.htm) included multiple formulations of 50 different medicines. Why so many? Jensen blamed the scarcity, in part, on the fact that many older drugs are not as profitable as newer ones. Manufacturing issues or delays and increased demand were the two biggest official reasons, though the FDA reported that at least eight formulations had been pulled or held from the market.
Vaughan says he’s heard plenty of buzz about the problem showing up quickly and unexpectedly in hospitals. Drug companies are supposed to give the FDA six months’ notice if they stop producing a drug, he says, but there’s no penalty if they don’t. “It’s amazing the number of people who are starting to worry about it,” he says. TH
Bryn Nelson is a freelance medical writer based in Seattle.
After the wild ride of 2010, public-policy watchers could be forgiven for fervently hoping that 2011 offers a calmer year on the healthcare front.
Fat chance.
The turbulence could begin immediately, with the seating of the 112th Congress on Jan. 3. “I think the first question that’s on everybody’s mind is, ‘What will the Republican majority in the House do to Obama’s healthcare reform initiative?’ ” says Eric Siegal, MD, SFHM, a member of SHM’s Public Policy Committee (PPC) and a clinical assistant professor of medicine at the University of Wisconsin School of Medicine and Public Health.
For most issues of direct concern to hospitalists, he says, especially those centered on healthcare delivery, “the wheels were in motion” long before the reform bill became law. Dr. Siegal also says most healthcare experts support the substance of accountable care organizations (ACOs), pay for performance, and reforming Medicare in ways that reward quality instead of quantity. “I think that ship is out of the harbor,” he says.
Throughout the year, the PPC will focus on three priorities identified in the Affordable Care Act: hospital value-based purchasing, bundled payments including ACOs, and hospital readmissions and transitions of care.
—Bill Vaughan, senior policy analyst, Consumers Union, Washington, D.C.
Debate, Delay, Defund?
There are several ways that Congress can delay or thwart the launch of specific reform initiatives. The first is to hold hearings about reform measures, Dr. Siegal says, “with the hope that they can somehow undermine it by raising questions about either the finances of it or about the implications for average Americans in terms of what kind of healthcare they’re going to get.” Such tactics carry significant risk, however, because highlighting specific aspects of the reform law could actually increase overall public support. “It has the potential to backfire on them,” he says.
“Repeal won’t happen anytime soon,” predicts Pat Conway, MD, chair of the PPC and director of hospital medicine at Cincinnati Children’s Hospital. “However, Congress could gut or significantly reduce funding to multiple programs within the bill, and then if you significantly reduce the funding, this may make it nearly impossible for those programs to be successful.”
Rough estimates suggest that some $150 billion worth of programs over the 10-year life of the healthcare reform act remain unfunded and are at risk. As an example, Dr. Conway cites wording in the bill that authorizes a program to help ease patient transitions in and out of the hospital. “If you reduce that funding to near zero, hospitals and hospitalists may still be successful, but you’ve essentially removed the program to learn how to be successful,” he says.
Bill Vaughan, a senior policy analyst in healthcare with the Washington, D.C.-based organization Consumers Union, says targeted riders could be added to appropriations bills. For instance, one rider could prohibit the Centers for Medicare & Medicaid Services (CMS) from spending any money to help develop government-supported insurance exchanges. Another could prevent the IRS from collecting money to be channeled into the trust fund for the Patient-Centered Outcomes Research Institute and its focus on comparative-effectiveness research. “There’s no end to mischief,” Vaughan says. “There are as many opportunities as the day is long.”
A major confrontation could arrive in March or April, when the U.S. runs into its debt ceiling. A continuing resolution would then be required to continue the appropriations process (and increase the U.S. debt ceiling past its current limit of $14.3 trillion). At that point or soon thereafter, Vaughan says, an opportunity could arise for legislators to say they won’t vote for a critical appropriations bill unless it includes certain spending reductions cited by one of several commissions tasked with recommending ways to reduce the deficit. “That could include hospital cuts, more doctor cuts, significant cost shifting to beneficiaries, higher copays,” he says.
Amid a “firestorm of ideas” on how to further cut Medicare and Medicaid spending, ideas once deemed radical could gain more traction. Some legislators have tossed around the idea of shutting down the government, if need be. “There’s nothing on the radar scope but static and fuzz,” Vaughan says. “It is totally unclear what is going to happen.”
Dearth of Drugs
Another trend generating both uncertainty and headaches in the nation’s hospitals is an unprecedented prescription drug shortage that could last well into the New Year, based on the number of medicines now in scarce supply across the country. In mid-November, for example, the American Society of Clinical Oncology announced “severe and worsening shortages of many critical therapies,” including doxorubicin, leucovorin, etoposide, nitrogen mustard, vincristine, propofol, and morphine.
Valerie Jensen, associate director of the FDA’s drug shortage program, told the Associated Press that her agency was seeing a record number of drug shortfalls in 2010. In mid-November, the FDA’s Current Drug Shortages list (www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050792.htm) included multiple formulations of 50 different medicines. Why so many? Jensen blamed the scarcity, in part, on the fact that many older drugs are not as profitable as newer ones. Manufacturing issues or delays and increased demand were the two biggest official reasons, though the FDA reported that at least eight formulations had been pulled or held from the market.
Vaughan says he’s heard plenty of buzz about the problem showing up quickly and unexpectedly in hospitals. Drug companies are supposed to give the FDA six months’ notice if they stop producing a drug, he says, but there’s no penalty if they don’t. “It’s amazing the number of people who are starting to worry about it,” he says. TH
Bryn Nelson is a freelance medical writer based in Seattle.
After the wild ride of 2010, public-policy watchers could be forgiven for fervently hoping that 2011 offers a calmer year on the healthcare front.
Fat chance.
The turbulence could begin immediately, with the seating of the 112th Congress on Jan. 3. “I think the first question that’s on everybody’s mind is, ‘What will the Republican majority in the House do to Obama’s healthcare reform initiative?’ ” says Eric Siegal, MD, SFHM, a member of SHM’s Public Policy Committee (PPC) and a clinical assistant professor of medicine at the University of Wisconsin School of Medicine and Public Health.
For most issues of direct concern to hospitalists, he says, especially those centered on healthcare delivery, “the wheels were in motion” long before the reform bill became law. Dr. Siegal also says most healthcare experts support the substance of accountable care organizations (ACOs), pay for performance, and reforming Medicare in ways that reward quality instead of quantity. “I think that ship is out of the harbor,” he says.
Throughout the year, the PPC will focus on three priorities identified in the Affordable Care Act: hospital value-based purchasing, bundled payments including ACOs, and hospital readmissions and transitions of care.
—Bill Vaughan, senior policy analyst, Consumers Union, Washington, D.C.
Debate, Delay, Defund?
There are several ways that Congress can delay or thwart the launch of specific reform initiatives. The first is to hold hearings about reform measures, Dr. Siegal says, “with the hope that they can somehow undermine it by raising questions about either the finances of it or about the implications for average Americans in terms of what kind of healthcare they’re going to get.” Such tactics carry significant risk, however, because highlighting specific aspects of the reform law could actually increase overall public support. “It has the potential to backfire on them,” he says.
“Repeal won’t happen anytime soon,” predicts Pat Conway, MD, chair of the PPC and director of hospital medicine at Cincinnati Children’s Hospital. “However, Congress could gut or significantly reduce funding to multiple programs within the bill, and then if you significantly reduce the funding, this may make it nearly impossible for those programs to be successful.”
Rough estimates suggest that some $150 billion worth of programs over the 10-year life of the healthcare reform act remain unfunded and are at risk. As an example, Dr. Conway cites wording in the bill that authorizes a program to help ease patient transitions in and out of the hospital. “If you reduce that funding to near zero, hospitals and hospitalists may still be successful, but you’ve essentially removed the program to learn how to be successful,” he says.
Bill Vaughan, a senior policy analyst in healthcare with the Washington, D.C.-based organization Consumers Union, says targeted riders could be added to appropriations bills. For instance, one rider could prohibit the Centers for Medicare & Medicaid Services (CMS) from spending any money to help develop government-supported insurance exchanges. Another could prevent the IRS from collecting money to be channeled into the trust fund for the Patient-Centered Outcomes Research Institute and its focus on comparative-effectiveness research. “There’s no end to mischief,” Vaughan says. “There are as many opportunities as the day is long.”
A major confrontation could arrive in March or April, when the U.S. runs into its debt ceiling. A continuing resolution would then be required to continue the appropriations process (and increase the U.S. debt ceiling past its current limit of $14.3 trillion). At that point or soon thereafter, Vaughan says, an opportunity could arise for legislators to say they won’t vote for a critical appropriations bill unless it includes certain spending reductions cited by one of several commissions tasked with recommending ways to reduce the deficit. “That could include hospital cuts, more doctor cuts, significant cost shifting to beneficiaries, higher copays,” he says.
Amid a “firestorm of ideas” on how to further cut Medicare and Medicaid spending, ideas once deemed radical could gain more traction. Some legislators have tossed around the idea of shutting down the government, if need be. “There’s nothing on the radar scope but static and fuzz,” Vaughan says. “It is totally unclear what is going to happen.”
Dearth of Drugs
Another trend generating both uncertainty and headaches in the nation’s hospitals is an unprecedented prescription drug shortage that could last well into the New Year, based on the number of medicines now in scarce supply across the country. In mid-November, for example, the American Society of Clinical Oncology announced “severe and worsening shortages of many critical therapies,” including doxorubicin, leucovorin, etoposide, nitrogen mustard, vincristine, propofol, and morphine.
Valerie Jensen, associate director of the FDA’s drug shortage program, told the Associated Press that her agency was seeing a record number of drug shortfalls in 2010. In mid-November, the FDA’s Current Drug Shortages list (www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050792.htm) included multiple formulations of 50 different medicines. Why so many? Jensen blamed the scarcity, in part, on the fact that many older drugs are not as profitable as newer ones. Manufacturing issues or delays and increased demand were the two biggest official reasons, though the FDA reported that at least eight formulations had been pulled or held from the market.
Vaughan says he’s heard plenty of buzz about the problem showing up quickly and unexpectedly in hospitals. Drug companies are supposed to give the FDA six months’ notice if they stop producing a drug, he says, but there’s no penalty if they don’t. “It’s amazing the number of people who are starting to worry about it,” he says. TH
Bryn Nelson is a freelance medical writer based in Seattle.
In the Literature: HM-Related Research You Need to Know
In This Edition
Literature at a Glance
A guide to this month’s studies
- Characteristics of CA-MRSA
- Association of gurgling with morbidity and mortality
- Antibiotics for active ulcerative colitis
- TIPS for cirrhosis-induced variceal bleeding
- Steroid dose, route in COPD exacerbations
- Effect of reminders and stop orders on urinary catheter use
- Outcomes of chest-compression-only CPR
- Albumin levels and risk of surgical-site infections
Characteristics of Community-Acquired methicillin-resistant Staphylococcus aureus Pneumonia in an Academic Medical Center
Clinical question: What are the clinical features and epidemiology of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) pneumonia?
Background: CA-MRSA is an emerging cause of pneumonia. The genetic makeup of most CA-MRSA strains is different than that of nosocomial MRSA. Typically, CA-MRSA is resistant to methicillin, beta-lactams, and erythromycin, but it retains susceptibility to trimethoprim-sulfamethoxazole (TMP/sulfa) and clindamycin.
In addition, the most common strain of CA-MRSA carries the Panton-Valentine leukocidin (PVL) toxin, which is associated with necrotizing pneumonia and high mortality rates.
Study design: Retrospective case series.
Setting: A 1,100-bed teaching hospital in Chicago.
Synopsis: Of the 5,955 discharges with a diagnosis-related group (DRG) code of pneumonia, 15 met criteria for CA-MRSA, or <1% of all inpatient community-acquired pneumonia cases. All 15 CA-MRSA strains were positive for PVL.
Seven of the 15 patients never were admitted to the ICU, while seven patients required mechanical ventilation. Seven patients were immunocompromised; one patient presented with preceding influenza; seven patients presented with hemoptysis; and eight patients demonstrated findings of lung necrosis on CT scan. Two patients died; both were immunocompromised.
Although the initial antibiotic regimen varied considerably, 14 patients ultimately received either clindamycin or linezolid.
Bottom line: CA-MRSA pneumonia is an uncommon subset of community-acquired pneumonia admissions. Approximately half the patients admitted with CA-MRSA presented with features of severe pneumonia. Nearly all were treated with antibiotics that inhibit exotoxin production, and the associated mortality rate of 13% was lower than previously reported.
Citation: Lobo JL, Reed KD, Wunderink RG. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest. 2010;138(1):130-136.
Gurgling Breath Sounds in Hospitalized Patients Might Predict Subsequent Pneumonia Development
Clinical question: Can gurgling sounds over the glottis during speech or quiet breathing predict hospital-acquired pneumonia (HAP)?
Background: HAP is a relatively frequent complication of hospitalization. HAP usually portends an increase in morbidity and mortality. Patients in the hospital might have disease states that inhibit the reflexes that normally eliminate secretions from above or below their glottis, increasing the risk of pneumonia.
Study design: Prospective cohort.
Setting: A 350-bed community teaching hospital in Bridgeport, Conn.
Synopsis: All patients admitted to a respiratory-care unit and general medical ward from December 2008 to April 2009 underwent auscultation over their glottis by study personnel. Patients with gurgles heard during speech or quiet breathing on auscultation and patients without gurgles were entered into the study in a 1:3 fashion, until 20 patients with gurgles and 60 patients without gurgles had been enrolled. Patients were followed for the development of clinical and radiographic evidence of HAP, ICU transfer, and in-hospital death.
Both dementia and treatment with opiates were independent predictors of gurgle in multivariate analysis. HAP occurred in 55% of the patients with gurgle versus 1.7% of patients without gurgle. In addition, 50% of the patients with gurgle required transfer to the ICU, compared with only 3.3% of patients without gurgle. In-hospital mortality was 30% among patients with gurgle versus 11.7% among patients without gurgle.
Bottom line: In patients admitted to the medical service of a community teaching hospital, gurgling sounds heard over the glottis during speech or quiet inspiration are independently associated with the development of HAP, ICU transfer, and in-hospital mortality.
Citation: Vazquez R, Gheorghe C, Ramos F, Dadu R, Amoateng-Adjepong Y, Manthous CA. Gurgling breath sounds may predict hospital-acquired pneumonia. Chest. 2010;138(2):284-288.
Treatment of Active Ulcerative Colitis with Triple Antibiotic Therapy Provides Better Response than Placebo
Clinical question: Does combination antibiotic therapy induce and/or maintain remission of active ulcerative colitis (UC)?
Background: Mouse models and other experimental evidence have suggested a pathogenic role for microbes in the development and/or exacerbation of ulcerative colitis, although antibiotic human trials have produced conflicting results. Recently, Fusobacterium varium was shown to be present in the gastrointestinal (GI) tract of most UC patients, and a pilot study using targeted antibacterials demonstrated efficacy in treating active UC.
Study design: Randomized, double-blind, placebo-controlled, multicenter trial.
Setting: Eleven hospitals in Japan.
Synopsis: Patients with mild to severe chronic relapsing UC were randomly assigned to either combination antibiotic therapy or placebo. All previous UC treatment regimens were continued in study patients, with the exception of steroids, which were tapered slowly if possible. Patients in the antibiotic group received a two-week combination therapy of amoxicillin, tetracycline, and metronidazole. Patients were followed weekly or monthly and underwent periodic exams and colonoscopies to assess clinical and endoscopic improvement for 12 months.
One hundred five patients were enrolled in each group. The clinical response rate at one year in patients treated with antibiotics was 44.8% versus 22.8% in the placebo group. Remission at one year was achieved in 26.7% of patients treated with antibiotics versus 14.9% of placebo patients. Endoscopic response rates and steroid discontinuation rates were higher in the antibiotic-treated groups. Effects were most pronounced in the group of patients with active disease.
Bottom line: Triple antibiotic therapy with amoxicillin, tetracycline, and metronidazole, when compared with placebo, was associated with improvement in clinical symptoms, endoscopic findings, remission rates, and steroid withdrawal in patients with active ulcerative colitis.
Citation: Ohkusa T, Kato K, Terao S, et al. Newly developed antibiotic combination therapy for ulcerative colitis: a double-blind placebo-controlled multicenter trial. Am J Gastroenterol. 2010;105(8):1820-1829.
Early TIPS Outperformed Optimal Medical Therapy in Patients with Advanced Cirrhosis and Variceal Bleeding
Clinical question: Does early treatment with a transjugular intrahepatic portosystemic shunt (TIPS) improve outcomes in patients with advanced cirrhosis and variceal bleeding?
Background: Current management guidelines for variceal bleeding include treatment with vasoactive drugs and serial endoscopy, yet treatment failure occurs in 10% to 15% of patients. TIPS is highly effective in controlling bleeding in such patients, but it historically has been reserved for patients who repeatedly fail preventive strategies.
Study design: Randomized controlled trial.
Setting: Nine European centers.
Synopsis: Sixty-three patients with advanced cirrhosis and acute esophageal variceal bleeding treated with optimal medical therapy were randomized within 24 hours of admission to either 1) early TIPS (polytetrafluoroethylene-covered stents) within 72 hours of randomization, or 2) ongoing optimal medical therapy with vasoactive drugs, treatment with a nonselective beta-blocker, and endoscopic band ligation.
During the median 16-month follow-up, rebleeding or failure to control bleeding occurred in 45% of patients in the optimal medical therapy group versus 3% of patients in the early TIPS group. One-year actuarial survival was 61% in the optimal medical therapy group versus 86% in the early-TIPS group. Remarkably, encephalopathy was less common in the early-TIPS group, and adverse events as a whole were similar in both groups.
Bottom line: Early use of TIPS was superior to optimal medical therapy for patients with advanced cirrhosis hospitalized for acute variceal bleeding at high risk for treatment failure.
Citation: García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370-2379.
Low-Dose Oral Corticosteroids As Effective As High-Dose Intravenous Therapy in COPD Exacerbations
Clinical question: In patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease (COPD), what are the outcomes of those initially treated with low doses of steroids administered orally compared with those initially treated with higher doses intravenously?
Background: COPD affects 6% of adults in the U.S., and acute exacerbation of COPD is one of the leading causes of hospitalization nationwide. Systemic corticosteroids are beneficial for patients hospitalized with acute exacerbation of COPD; however, optimal dose and route of administration are uncertain.
Study design: Retrospective cohort.
Setting: Four hundred fourteen U.S. acute-care hospitals; most were small to midsize nonteaching facilities serving urban patient populations.
Synopsis: Almost 80,000 patients admitted to a non-ICU setting with a diagnosis of acute exacerbation of COPD from 2006 to 2007, and who received systemic corticosteroids during the first two hospital days, were included in the study. In contrast to clinical guidelines recommending the use of low-dose oral corticosteroids, 92% of study participants were treated initially with intravenous steroids, whereas 8% received oral treatment. The primary composite outcome measure—need for mechanical ventilation after the second hospital day, inpatient mortality, or readmission for COPD within 30 days—was no worse in patients treated with oral steroids. Risk of treatment failure, length of stay, and cost were significantly lower among orally treated patients.
Bottom line: High-dose intravenous steroids appear to be no more effective than low-dose oral steroids for acute exacerbation of COPD. The authors recommend a randomized controlled trial be conducted to compare these two management strategies.
Citation: Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367.
Reminders and Stop Orders Reduce Catheter-Associated Urinary Tract Infections
Clinical question: Do interventions that remind clinicians of the presence of urinary catheters and prompt timely removal decrease the rate of catheter-associated urinary tract infections (CA-UTI)?
Background: CA-UTI is a common yet preventable hospital-acquired infection. Many catheters are placed unnecessarily, remain in use without physician awareness, and are not removed promptly when no longer needed.
Study design: Systematic review and meta-analysis of 13 preintervention and postintervention quasi-experimental trials and one randomized controlled trial.
Setting: Studies conducted in the U.S., Canada, Europe, and Asia.
Synopsis: This literature search revealed 14 articles that used a reminder or stop-order intervention to prompt removal of urinary catheters and reported pre- and postintervention outcomes for CA-UTI rates, duration of urinary catheter use, and recatheterization need. Five studies used stop orders and nine studies used reminder interventions.
Use of a stop order or reminder reduced the rate of CA-UTI (episodes per 1,000 catheter days) by 52%. Mean duration of catheterization decreased by 37%, which resulted in 2.61 fewer days of catheterization per patient in the intervention versus control groups. Recatheterization rates were similar in the control and intervention groups.
Bottom line: Urinary catheter reminders and stop orders are low-cost strategies that appear to reduce the rate of CA-UTI.
Citation: Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560.
Chest-Compression-Only Bystander CPR Increases Survival
Clinical question: Is bystander cardiopulmonary resuscitation (CPR) with chest compressions alone or chest compressions with rescue breathing superior in out-of-hospital adult cardiac arrest?
Background: Out-of-hospital cardiac arrest claims hundreds of thousands of lives each year. Early initiation of CPR by a layperson can increase a patient’s chances of surviving and having a favorable long-term neurologic recovery. Although traditional CPR consists of chest compression with rescue breathing, chest compression alone might be more acceptable to many laypersons and has the potential advantage of fewer compression interruptions.
Study design: Multicenter randomized trial.
Setting: Two EMSs in Washington state and one in London.
Synopsis: Patients were initially eligible for this study if a dispatcher determined that the patient was unconscious and not breathing, and that bystander CPR was not yet under way. If the caller was willing to undertake CPR with the dispatcher’s assistance, a randomization envelope containing CPR instructions was opened. Patients with arrest due to trauma, drowning, or asphyxiation were excluded, as were those under 18 years of age.
No significant difference was observed between the two groups in the percentage of patients who survived to hospital discharge or who survived with a favorable neurologic outcome. However, subgroup analyses showed a trend toward a higher percentage of patients surviving to hospital discharge with chest compressions alone, as compared with chest compressions with rescue breathing for patients with a cardiac cause of arrest and for those with shockable rhythms.
Bottom line: Dispatcher CPR instruction consisting of chest compression alone was noninferior to conventional CPR with rescue breathing, and it showed a trend toward better outcomes in cardiac cause of arrest.
Citation: Rea TD, Fahrenbruch C, Culley L, et al. CPR with chest compression alone or with rescue breathing. N Engl J Med. 2010;363(5):423-433.
Low Albumin Is Associated with Postoperative Wound Infections
Clinical question: What is the relationship between preoperative serum albumin levels and postoperative surgical-site infections (SSI)?
Background: Poor nutritional status is associated with adverse surgical outcomes. Serum albumin can both reflect nutritional status and function as a negative acute phase reactant, i.e., decreases in the setting of inflammation. It is uncertain whether low preoperative albumin levels are associated with postoperative SSI risk.
Study design: Retrospective cohort with multivariate analysis.
Setting: Four centers in Ireland.
Synopsis: Patients undergoing GI surgery (n=524) were prospectively followed as part of an SSI database. Demographic data, American Society of Anesthesia class, serum albumin levels, and presence and severity of SSI data were collected on all patients. Follow-up extended to 30 days.
SSI developed in 20% of patients. Patients who developed a SSI had lower serum albumin levels (mean 3.0 g/dL versus 3.6 g/dL). A serum albumin level less than 3.0 g/dL was associated with greater risk of SSI (relative risk 5.68), deeper SSI, and prolonged length of stay.
Bottom line: After controlling for other variables, serum albumin lower than 3.0 g/dL is independently associated with SSI frequency and severity.
Citation: Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252 (2):325-329. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Characteristics of CA-MRSA
- Association of gurgling with morbidity and mortality
- Antibiotics for active ulcerative colitis
- TIPS for cirrhosis-induced variceal bleeding
- Steroid dose, route in COPD exacerbations
- Effect of reminders and stop orders on urinary catheter use
- Outcomes of chest-compression-only CPR
- Albumin levels and risk of surgical-site infections
Characteristics of Community-Acquired methicillin-resistant Staphylococcus aureus Pneumonia in an Academic Medical Center
Clinical question: What are the clinical features and epidemiology of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) pneumonia?
Background: CA-MRSA is an emerging cause of pneumonia. The genetic makeup of most CA-MRSA strains is different than that of nosocomial MRSA. Typically, CA-MRSA is resistant to methicillin, beta-lactams, and erythromycin, but it retains susceptibility to trimethoprim-sulfamethoxazole (TMP/sulfa) and clindamycin.
In addition, the most common strain of CA-MRSA carries the Panton-Valentine leukocidin (PVL) toxin, which is associated with necrotizing pneumonia and high mortality rates.
Study design: Retrospective case series.
Setting: A 1,100-bed teaching hospital in Chicago.
Synopsis: Of the 5,955 discharges with a diagnosis-related group (DRG) code of pneumonia, 15 met criteria for CA-MRSA, or <1% of all inpatient community-acquired pneumonia cases. All 15 CA-MRSA strains were positive for PVL.
Seven of the 15 patients never were admitted to the ICU, while seven patients required mechanical ventilation. Seven patients were immunocompromised; one patient presented with preceding influenza; seven patients presented with hemoptysis; and eight patients demonstrated findings of lung necrosis on CT scan. Two patients died; both were immunocompromised.
Although the initial antibiotic regimen varied considerably, 14 patients ultimately received either clindamycin or linezolid.
Bottom line: CA-MRSA pneumonia is an uncommon subset of community-acquired pneumonia admissions. Approximately half the patients admitted with CA-MRSA presented with features of severe pneumonia. Nearly all were treated with antibiotics that inhibit exotoxin production, and the associated mortality rate of 13% was lower than previously reported.
Citation: Lobo JL, Reed KD, Wunderink RG. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest. 2010;138(1):130-136.
Gurgling Breath Sounds in Hospitalized Patients Might Predict Subsequent Pneumonia Development
Clinical question: Can gurgling sounds over the glottis during speech or quiet breathing predict hospital-acquired pneumonia (HAP)?
Background: HAP is a relatively frequent complication of hospitalization. HAP usually portends an increase in morbidity and mortality. Patients in the hospital might have disease states that inhibit the reflexes that normally eliminate secretions from above or below their glottis, increasing the risk of pneumonia.
Study design: Prospective cohort.
Setting: A 350-bed community teaching hospital in Bridgeport, Conn.
Synopsis: All patients admitted to a respiratory-care unit and general medical ward from December 2008 to April 2009 underwent auscultation over their glottis by study personnel. Patients with gurgles heard during speech or quiet breathing on auscultation and patients without gurgles were entered into the study in a 1:3 fashion, until 20 patients with gurgles and 60 patients without gurgles had been enrolled. Patients were followed for the development of clinical and radiographic evidence of HAP, ICU transfer, and in-hospital death.
Both dementia and treatment with opiates were independent predictors of gurgle in multivariate analysis. HAP occurred in 55% of the patients with gurgle versus 1.7% of patients without gurgle. In addition, 50% of the patients with gurgle required transfer to the ICU, compared with only 3.3% of patients without gurgle. In-hospital mortality was 30% among patients with gurgle versus 11.7% among patients without gurgle.
Bottom line: In patients admitted to the medical service of a community teaching hospital, gurgling sounds heard over the glottis during speech or quiet inspiration are independently associated with the development of HAP, ICU transfer, and in-hospital mortality.
Citation: Vazquez R, Gheorghe C, Ramos F, Dadu R, Amoateng-Adjepong Y, Manthous CA. Gurgling breath sounds may predict hospital-acquired pneumonia. Chest. 2010;138(2):284-288.
Treatment of Active Ulcerative Colitis with Triple Antibiotic Therapy Provides Better Response than Placebo
Clinical question: Does combination antibiotic therapy induce and/or maintain remission of active ulcerative colitis (UC)?
Background: Mouse models and other experimental evidence have suggested a pathogenic role for microbes in the development and/or exacerbation of ulcerative colitis, although antibiotic human trials have produced conflicting results. Recently, Fusobacterium varium was shown to be present in the gastrointestinal (GI) tract of most UC patients, and a pilot study using targeted antibacterials demonstrated efficacy in treating active UC.
Study design: Randomized, double-blind, placebo-controlled, multicenter trial.
Setting: Eleven hospitals in Japan.
Synopsis: Patients with mild to severe chronic relapsing UC were randomly assigned to either combination antibiotic therapy or placebo. All previous UC treatment regimens were continued in study patients, with the exception of steroids, which were tapered slowly if possible. Patients in the antibiotic group received a two-week combination therapy of amoxicillin, tetracycline, and metronidazole. Patients were followed weekly or monthly and underwent periodic exams and colonoscopies to assess clinical and endoscopic improvement for 12 months.
One hundred five patients were enrolled in each group. The clinical response rate at one year in patients treated with antibiotics was 44.8% versus 22.8% in the placebo group. Remission at one year was achieved in 26.7% of patients treated with antibiotics versus 14.9% of placebo patients. Endoscopic response rates and steroid discontinuation rates were higher in the antibiotic-treated groups. Effects were most pronounced in the group of patients with active disease.
Bottom line: Triple antibiotic therapy with amoxicillin, tetracycline, and metronidazole, when compared with placebo, was associated with improvement in clinical symptoms, endoscopic findings, remission rates, and steroid withdrawal in patients with active ulcerative colitis.
Citation: Ohkusa T, Kato K, Terao S, et al. Newly developed antibiotic combination therapy for ulcerative colitis: a double-blind placebo-controlled multicenter trial. Am J Gastroenterol. 2010;105(8):1820-1829.
Early TIPS Outperformed Optimal Medical Therapy in Patients with Advanced Cirrhosis and Variceal Bleeding
Clinical question: Does early treatment with a transjugular intrahepatic portosystemic shunt (TIPS) improve outcomes in patients with advanced cirrhosis and variceal bleeding?
Background: Current management guidelines for variceal bleeding include treatment with vasoactive drugs and serial endoscopy, yet treatment failure occurs in 10% to 15% of patients. TIPS is highly effective in controlling bleeding in such patients, but it historically has been reserved for patients who repeatedly fail preventive strategies.
Study design: Randomized controlled trial.
Setting: Nine European centers.
Synopsis: Sixty-three patients with advanced cirrhosis and acute esophageal variceal bleeding treated with optimal medical therapy were randomized within 24 hours of admission to either 1) early TIPS (polytetrafluoroethylene-covered stents) within 72 hours of randomization, or 2) ongoing optimal medical therapy with vasoactive drugs, treatment with a nonselective beta-blocker, and endoscopic band ligation.
During the median 16-month follow-up, rebleeding or failure to control bleeding occurred in 45% of patients in the optimal medical therapy group versus 3% of patients in the early TIPS group. One-year actuarial survival was 61% in the optimal medical therapy group versus 86% in the early-TIPS group. Remarkably, encephalopathy was less common in the early-TIPS group, and adverse events as a whole were similar in both groups.
Bottom line: Early use of TIPS was superior to optimal medical therapy for patients with advanced cirrhosis hospitalized for acute variceal bleeding at high risk for treatment failure.
Citation: García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370-2379.
Low-Dose Oral Corticosteroids As Effective As High-Dose Intravenous Therapy in COPD Exacerbations
Clinical question: In patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease (COPD), what are the outcomes of those initially treated with low doses of steroids administered orally compared with those initially treated with higher doses intravenously?
Background: COPD affects 6% of adults in the U.S., and acute exacerbation of COPD is one of the leading causes of hospitalization nationwide. Systemic corticosteroids are beneficial for patients hospitalized with acute exacerbation of COPD; however, optimal dose and route of administration are uncertain.
Study design: Retrospective cohort.
Setting: Four hundred fourteen U.S. acute-care hospitals; most were small to midsize nonteaching facilities serving urban patient populations.
Synopsis: Almost 80,000 patients admitted to a non-ICU setting with a diagnosis of acute exacerbation of COPD from 2006 to 2007, and who received systemic corticosteroids during the first two hospital days, were included in the study. In contrast to clinical guidelines recommending the use of low-dose oral corticosteroids, 92% of study participants were treated initially with intravenous steroids, whereas 8% received oral treatment. The primary composite outcome measure—need for mechanical ventilation after the second hospital day, inpatient mortality, or readmission for COPD within 30 days—was no worse in patients treated with oral steroids. Risk of treatment failure, length of stay, and cost were significantly lower among orally treated patients.
Bottom line: High-dose intravenous steroids appear to be no more effective than low-dose oral steroids for acute exacerbation of COPD. The authors recommend a randomized controlled trial be conducted to compare these two management strategies.
Citation: Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367.
Reminders and Stop Orders Reduce Catheter-Associated Urinary Tract Infections
Clinical question: Do interventions that remind clinicians of the presence of urinary catheters and prompt timely removal decrease the rate of catheter-associated urinary tract infections (CA-UTI)?
Background: CA-UTI is a common yet preventable hospital-acquired infection. Many catheters are placed unnecessarily, remain in use without physician awareness, and are not removed promptly when no longer needed.
Study design: Systematic review and meta-analysis of 13 preintervention and postintervention quasi-experimental trials and one randomized controlled trial.
Setting: Studies conducted in the U.S., Canada, Europe, and Asia.
Synopsis: This literature search revealed 14 articles that used a reminder or stop-order intervention to prompt removal of urinary catheters and reported pre- and postintervention outcomes for CA-UTI rates, duration of urinary catheter use, and recatheterization need. Five studies used stop orders and nine studies used reminder interventions.
Use of a stop order or reminder reduced the rate of CA-UTI (episodes per 1,000 catheter days) by 52%. Mean duration of catheterization decreased by 37%, which resulted in 2.61 fewer days of catheterization per patient in the intervention versus control groups. Recatheterization rates were similar in the control and intervention groups.
Bottom line: Urinary catheter reminders and stop orders are low-cost strategies that appear to reduce the rate of CA-UTI.
Citation: Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560.
Chest-Compression-Only Bystander CPR Increases Survival
Clinical question: Is bystander cardiopulmonary resuscitation (CPR) with chest compressions alone or chest compressions with rescue breathing superior in out-of-hospital adult cardiac arrest?
Background: Out-of-hospital cardiac arrest claims hundreds of thousands of lives each year. Early initiation of CPR by a layperson can increase a patient’s chances of surviving and having a favorable long-term neurologic recovery. Although traditional CPR consists of chest compression with rescue breathing, chest compression alone might be more acceptable to many laypersons and has the potential advantage of fewer compression interruptions.
Study design: Multicenter randomized trial.
Setting: Two EMSs in Washington state and one in London.
Synopsis: Patients were initially eligible for this study if a dispatcher determined that the patient was unconscious and not breathing, and that bystander CPR was not yet under way. If the caller was willing to undertake CPR with the dispatcher’s assistance, a randomization envelope containing CPR instructions was opened. Patients with arrest due to trauma, drowning, or asphyxiation were excluded, as were those under 18 years of age.
No significant difference was observed between the two groups in the percentage of patients who survived to hospital discharge or who survived with a favorable neurologic outcome. However, subgroup analyses showed a trend toward a higher percentage of patients surviving to hospital discharge with chest compressions alone, as compared with chest compressions with rescue breathing for patients with a cardiac cause of arrest and for those with shockable rhythms.
Bottom line: Dispatcher CPR instruction consisting of chest compression alone was noninferior to conventional CPR with rescue breathing, and it showed a trend toward better outcomes in cardiac cause of arrest.
Citation: Rea TD, Fahrenbruch C, Culley L, et al. CPR with chest compression alone or with rescue breathing. N Engl J Med. 2010;363(5):423-433.
Low Albumin Is Associated with Postoperative Wound Infections
Clinical question: What is the relationship between preoperative serum albumin levels and postoperative surgical-site infections (SSI)?
Background: Poor nutritional status is associated with adverse surgical outcomes. Serum albumin can both reflect nutritional status and function as a negative acute phase reactant, i.e., decreases in the setting of inflammation. It is uncertain whether low preoperative albumin levels are associated with postoperative SSI risk.
Study design: Retrospective cohort with multivariate analysis.
Setting: Four centers in Ireland.
Synopsis: Patients undergoing GI surgery (n=524) were prospectively followed as part of an SSI database. Demographic data, American Society of Anesthesia class, serum albumin levels, and presence and severity of SSI data were collected on all patients. Follow-up extended to 30 days.
SSI developed in 20% of patients. Patients who developed a SSI had lower serum albumin levels (mean 3.0 g/dL versus 3.6 g/dL). A serum albumin level less than 3.0 g/dL was associated with greater risk of SSI (relative risk 5.68), deeper SSI, and prolonged length of stay.
Bottom line: After controlling for other variables, serum albumin lower than 3.0 g/dL is independently associated with SSI frequency and severity.
Citation: Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252 (2):325-329. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Characteristics of CA-MRSA
- Association of gurgling with morbidity and mortality
- Antibiotics for active ulcerative colitis
- TIPS for cirrhosis-induced variceal bleeding
- Steroid dose, route in COPD exacerbations
- Effect of reminders and stop orders on urinary catheter use
- Outcomes of chest-compression-only CPR
- Albumin levels and risk of surgical-site infections
Characteristics of Community-Acquired methicillin-resistant Staphylococcus aureus Pneumonia in an Academic Medical Center
Clinical question: What are the clinical features and epidemiology of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) pneumonia?
Background: CA-MRSA is an emerging cause of pneumonia. The genetic makeup of most CA-MRSA strains is different than that of nosocomial MRSA. Typically, CA-MRSA is resistant to methicillin, beta-lactams, and erythromycin, but it retains susceptibility to trimethoprim-sulfamethoxazole (TMP/sulfa) and clindamycin.
In addition, the most common strain of CA-MRSA carries the Panton-Valentine leukocidin (PVL) toxin, which is associated with necrotizing pneumonia and high mortality rates.
Study design: Retrospective case series.
Setting: A 1,100-bed teaching hospital in Chicago.
Synopsis: Of the 5,955 discharges with a diagnosis-related group (DRG) code of pneumonia, 15 met criteria for CA-MRSA, or <1% of all inpatient community-acquired pneumonia cases. All 15 CA-MRSA strains were positive for PVL.
Seven of the 15 patients never were admitted to the ICU, while seven patients required mechanical ventilation. Seven patients were immunocompromised; one patient presented with preceding influenza; seven patients presented with hemoptysis; and eight patients demonstrated findings of lung necrosis on CT scan. Two patients died; both were immunocompromised.
Although the initial antibiotic regimen varied considerably, 14 patients ultimately received either clindamycin or linezolid.
Bottom line: CA-MRSA pneumonia is an uncommon subset of community-acquired pneumonia admissions. Approximately half the patients admitted with CA-MRSA presented with features of severe pneumonia. Nearly all were treated with antibiotics that inhibit exotoxin production, and the associated mortality rate of 13% was lower than previously reported.
Citation: Lobo JL, Reed KD, Wunderink RG. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest. 2010;138(1):130-136.
Gurgling Breath Sounds in Hospitalized Patients Might Predict Subsequent Pneumonia Development
Clinical question: Can gurgling sounds over the glottis during speech or quiet breathing predict hospital-acquired pneumonia (HAP)?
Background: HAP is a relatively frequent complication of hospitalization. HAP usually portends an increase in morbidity and mortality. Patients in the hospital might have disease states that inhibit the reflexes that normally eliminate secretions from above or below their glottis, increasing the risk of pneumonia.
Study design: Prospective cohort.
Setting: A 350-bed community teaching hospital in Bridgeport, Conn.
Synopsis: All patients admitted to a respiratory-care unit and general medical ward from December 2008 to April 2009 underwent auscultation over their glottis by study personnel. Patients with gurgles heard during speech or quiet breathing on auscultation and patients without gurgles were entered into the study in a 1:3 fashion, until 20 patients with gurgles and 60 patients without gurgles had been enrolled. Patients were followed for the development of clinical and radiographic evidence of HAP, ICU transfer, and in-hospital death.
Both dementia and treatment with opiates were independent predictors of gurgle in multivariate analysis. HAP occurred in 55% of the patients with gurgle versus 1.7% of patients without gurgle. In addition, 50% of the patients with gurgle required transfer to the ICU, compared with only 3.3% of patients without gurgle. In-hospital mortality was 30% among patients with gurgle versus 11.7% among patients without gurgle.
Bottom line: In patients admitted to the medical service of a community teaching hospital, gurgling sounds heard over the glottis during speech or quiet inspiration are independently associated with the development of HAP, ICU transfer, and in-hospital mortality.
Citation: Vazquez R, Gheorghe C, Ramos F, Dadu R, Amoateng-Adjepong Y, Manthous CA. Gurgling breath sounds may predict hospital-acquired pneumonia. Chest. 2010;138(2):284-288.
Treatment of Active Ulcerative Colitis with Triple Antibiotic Therapy Provides Better Response than Placebo
Clinical question: Does combination antibiotic therapy induce and/or maintain remission of active ulcerative colitis (UC)?
Background: Mouse models and other experimental evidence have suggested a pathogenic role for microbes in the development and/or exacerbation of ulcerative colitis, although antibiotic human trials have produced conflicting results. Recently, Fusobacterium varium was shown to be present in the gastrointestinal (GI) tract of most UC patients, and a pilot study using targeted antibacterials demonstrated efficacy in treating active UC.
Study design: Randomized, double-blind, placebo-controlled, multicenter trial.
Setting: Eleven hospitals in Japan.
Synopsis: Patients with mild to severe chronic relapsing UC were randomly assigned to either combination antibiotic therapy or placebo. All previous UC treatment regimens were continued in study patients, with the exception of steroids, which were tapered slowly if possible. Patients in the antibiotic group received a two-week combination therapy of amoxicillin, tetracycline, and metronidazole. Patients were followed weekly or monthly and underwent periodic exams and colonoscopies to assess clinical and endoscopic improvement for 12 months.
One hundred five patients were enrolled in each group. The clinical response rate at one year in patients treated with antibiotics was 44.8% versus 22.8% in the placebo group. Remission at one year was achieved in 26.7% of patients treated with antibiotics versus 14.9% of placebo patients. Endoscopic response rates and steroid discontinuation rates were higher in the antibiotic-treated groups. Effects were most pronounced in the group of patients with active disease.
Bottom line: Triple antibiotic therapy with amoxicillin, tetracycline, and metronidazole, when compared with placebo, was associated with improvement in clinical symptoms, endoscopic findings, remission rates, and steroid withdrawal in patients with active ulcerative colitis.
Citation: Ohkusa T, Kato K, Terao S, et al. Newly developed antibiotic combination therapy for ulcerative colitis: a double-blind placebo-controlled multicenter trial. Am J Gastroenterol. 2010;105(8):1820-1829.
Early TIPS Outperformed Optimal Medical Therapy in Patients with Advanced Cirrhosis and Variceal Bleeding
Clinical question: Does early treatment with a transjugular intrahepatic portosystemic shunt (TIPS) improve outcomes in patients with advanced cirrhosis and variceal bleeding?
Background: Current management guidelines for variceal bleeding include treatment with vasoactive drugs and serial endoscopy, yet treatment failure occurs in 10% to 15% of patients. TIPS is highly effective in controlling bleeding in such patients, but it historically has been reserved for patients who repeatedly fail preventive strategies.
Study design: Randomized controlled trial.
Setting: Nine European centers.
Synopsis: Sixty-three patients with advanced cirrhosis and acute esophageal variceal bleeding treated with optimal medical therapy were randomized within 24 hours of admission to either 1) early TIPS (polytetrafluoroethylene-covered stents) within 72 hours of randomization, or 2) ongoing optimal medical therapy with vasoactive drugs, treatment with a nonselective beta-blocker, and endoscopic band ligation.
During the median 16-month follow-up, rebleeding or failure to control bleeding occurred in 45% of patients in the optimal medical therapy group versus 3% of patients in the early TIPS group. One-year actuarial survival was 61% in the optimal medical therapy group versus 86% in the early-TIPS group. Remarkably, encephalopathy was less common in the early-TIPS group, and adverse events as a whole were similar in both groups.
Bottom line: Early use of TIPS was superior to optimal medical therapy for patients with advanced cirrhosis hospitalized for acute variceal bleeding at high risk for treatment failure.
Citation: García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370-2379.
Low-Dose Oral Corticosteroids As Effective As High-Dose Intravenous Therapy in COPD Exacerbations
Clinical question: In patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease (COPD), what are the outcomes of those initially treated with low doses of steroids administered orally compared with those initially treated with higher doses intravenously?
Background: COPD affects 6% of adults in the U.S., and acute exacerbation of COPD is one of the leading causes of hospitalization nationwide. Systemic corticosteroids are beneficial for patients hospitalized with acute exacerbation of COPD; however, optimal dose and route of administration are uncertain.
Study design: Retrospective cohort.
Setting: Four hundred fourteen U.S. acute-care hospitals; most were small to midsize nonteaching facilities serving urban patient populations.
Synopsis: Almost 80,000 patients admitted to a non-ICU setting with a diagnosis of acute exacerbation of COPD from 2006 to 2007, and who received systemic corticosteroids during the first two hospital days, were included in the study. In contrast to clinical guidelines recommending the use of low-dose oral corticosteroids, 92% of study participants were treated initially with intravenous steroids, whereas 8% received oral treatment. The primary composite outcome measure—need for mechanical ventilation after the second hospital day, inpatient mortality, or readmission for COPD within 30 days—was no worse in patients treated with oral steroids. Risk of treatment failure, length of stay, and cost were significantly lower among orally treated patients.
Bottom line: High-dose intravenous steroids appear to be no more effective than low-dose oral steroids for acute exacerbation of COPD. The authors recommend a randomized controlled trial be conducted to compare these two management strategies.
Citation: Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367.
Reminders and Stop Orders Reduce Catheter-Associated Urinary Tract Infections
Clinical question: Do interventions that remind clinicians of the presence of urinary catheters and prompt timely removal decrease the rate of catheter-associated urinary tract infections (CA-UTI)?
Background: CA-UTI is a common yet preventable hospital-acquired infection. Many catheters are placed unnecessarily, remain in use without physician awareness, and are not removed promptly when no longer needed.
Study design: Systematic review and meta-analysis of 13 preintervention and postintervention quasi-experimental trials and one randomized controlled trial.
Setting: Studies conducted in the U.S., Canada, Europe, and Asia.
Synopsis: This literature search revealed 14 articles that used a reminder or stop-order intervention to prompt removal of urinary catheters and reported pre- and postintervention outcomes for CA-UTI rates, duration of urinary catheter use, and recatheterization need. Five studies used stop orders and nine studies used reminder interventions.
Use of a stop order or reminder reduced the rate of CA-UTI (episodes per 1,000 catheter days) by 52%. Mean duration of catheterization decreased by 37%, which resulted in 2.61 fewer days of catheterization per patient in the intervention versus control groups. Recatheterization rates were similar in the control and intervention groups.
Bottom line: Urinary catheter reminders and stop orders are low-cost strategies that appear to reduce the rate of CA-UTI.
Citation: Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560.
Chest-Compression-Only Bystander CPR Increases Survival
Clinical question: Is bystander cardiopulmonary resuscitation (CPR) with chest compressions alone or chest compressions with rescue breathing superior in out-of-hospital adult cardiac arrest?
Background: Out-of-hospital cardiac arrest claims hundreds of thousands of lives each year. Early initiation of CPR by a layperson can increase a patient’s chances of surviving and having a favorable long-term neurologic recovery. Although traditional CPR consists of chest compression with rescue breathing, chest compression alone might be more acceptable to many laypersons and has the potential advantage of fewer compression interruptions.
Study design: Multicenter randomized trial.
Setting: Two EMSs in Washington state and one in London.
Synopsis: Patients were initially eligible for this study if a dispatcher determined that the patient was unconscious and not breathing, and that bystander CPR was not yet under way. If the caller was willing to undertake CPR with the dispatcher’s assistance, a randomization envelope containing CPR instructions was opened. Patients with arrest due to trauma, drowning, or asphyxiation were excluded, as were those under 18 years of age.
No significant difference was observed between the two groups in the percentage of patients who survived to hospital discharge or who survived with a favorable neurologic outcome. However, subgroup analyses showed a trend toward a higher percentage of patients surviving to hospital discharge with chest compressions alone, as compared with chest compressions with rescue breathing for patients with a cardiac cause of arrest and for those with shockable rhythms.
Bottom line: Dispatcher CPR instruction consisting of chest compression alone was noninferior to conventional CPR with rescue breathing, and it showed a trend toward better outcomes in cardiac cause of arrest.
Citation: Rea TD, Fahrenbruch C, Culley L, et al. CPR with chest compression alone or with rescue breathing. N Engl J Med. 2010;363(5):423-433.
Low Albumin Is Associated with Postoperative Wound Infections
Clinical question: What is the relationship between preoperative serum albumin levels and postoperative surgical-site infections (SSI)?
Background: Poor nutritional status is associated with adverse surgical outcomes. Serum albumin can both reflect nutritional status and function as a negative acute phase reactant, i.e., decreases in the setting of inflammation. It is uncertain whether low preoperative albumin levels are associated with postoperative SSI risk.
Study design: Retrospective cohort with multivariate analysis.
Setting: Four centers in Ireland.
Synopsis: Patients undergoing GI surgery (n=524) were prospectively followed as part of an SSI database. Demographic data, American Society of Anesthesia class, serum albumin levels, and presence and severity of SSI data were collected on all patients. Follow-up extended to 30 days.
SSI developed in 20% of patients. Patients who developed a SSI had lower serum albumin levels (mean 3.0 g/dL versus 3.6 g/dL). A serum albumin level less than 3.0 g/dL was associated with greater risk of SSI (relative risk 5.68), deeper SSI, and prolonged length of stay.
Bottom line: After controlling for other variables, serum albumin lower than 3.0 g/dL is independently associated with SSI frequency and severity.
Citation: Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252 (2):325-329. TH
NEW FEATURE: POLICY CORNER: An inside look at the most pressing policy issues (updated 01.04.2011)
The Centers for Medicare & Medicaid Services (CMS) in November announced the official launch of the Center for Medicare & Medicaid Innovation (CMMI). The CMI was authorized under the Affordable Care Act (ACA) to test innovative ways to reduce costs, while preserving or enhancing the quality. This sounds very similar to many other reform initiatives, so why have a separate center when ACOs, value-based purchasing, and payment bundling already are in the ACA?
A quick glance at the CMMI website didn’t provide much detail beyond uplifting language about the promise that the center represents. Don Berwick, MD, the new CMS administrator, has even gone so far as to call the center the “jewel in the crown” of the ACA.
Inspirational language aside, the center can be summed up using a simple analogy: The “other” ACA initiatives (bundling, VBP, etc.) are like a factory floor. The tools are in place, the processes are more or less defined, and they will be carried out regardless of the degree of positive impact. CMMI is more like a research and development lab, with the freedom to tinker with new ideas before wide-scale implementation.
The keys to CMMI success are twofold. First, it will implement pilot projects rather than demonstrations. A pilot gives the Secretary of Health and Human Services the power to implement and expand promising projects without Congressional approval. A demonstration requires Congressional approval for its continuation.. Second, CMMI does not require proposals to be budget neutral. Initial training and staffing costs alone can disqualify a program on budget neutrality grounds. Since CMMI does not require budget neutrality, promising programs with significant start-up costs are less likely to be cast aside.
Dr. Berwick has asked for provider partnership and input, and says he “would like to help forge an unprecedented level of shared aim, shared vision, and synergy in action among the public and private stewards and leaders of healthcare.” This vision and a $10 billion appropriation over the next decade present a tremendous opportunity for SHM’s quality initiatives, and the promising hospitalist-created protocol.
However, this large appropriation presents both the greatest strength and the greatest threat to the center. With the Republican takeover of the House of Representatives, the CMMI budget likely is to be a target for the “repeal, replace, or revise” agenda. Therefore, increasing awareness of CMMI’s role will be imperative over the coming months. Hospitalists can help by educating themselves, then passing their knowledge along to those who might not understand the importance of the center. TH
The Centers for Medicare & Medicaid Services (CMS) in November announced the official launch of the Center for Medicare & Medicaid Innovation (CMMI). The CMI was authorized under the Affordable Care Act (ACA) to test innovative ways to reduce costs, while preserving or enhancing the quality. This sounds very similar to many other reform initiatives, so why have a separate center when ACOs, value-based purchasing, and payment bundling already are in the ACA?
A quick glance at the CMMI website didn’t provide much detail beyond uplifting language about the promise that the center represents. Don Berwick, MD, the new CMS administrator, has even gone so far as to call the center the “jewel in the crown” of the ACA.
Inspirational language aside, the center can be summed up using a simple analogy: The “other” ACA initiatives (bundling, VBP, etc.) are like a factory floor. The tools are in place, the processes are more or less defined, and they will be carried out regardless of the degree of positive impact. CMMI is more like a research and development lab, with the freedom to tinker with new ideas before wide-scale implementation.
The keys to CMMI success are twofold. First, it will implement pilot projects rather than demonstrations. A pilot gives the Secretary of Health and Human Services the power to implement and expand promising projects without Congressional approval. A demonstration requires Congressional approval for its continuation.. Second, CMMI does not require proposals to be budget neutral. Initial training and staffing costs alone can disqualify a program on budget neutrality grounds. Since CMMI does not require budget neutrality, promising programs with significant start-up costs are less likely to be cast aside.
Dr. Berwick has asked for provider partnership and input, and says he “would like to help forge an unprecedented level of shared aim, shared vision, and synergy in action among the public and private stewards and leaders of healthcare.” This vision and a $10 billion appropriation over the next decade present a tremendous opportunity for SHM’s quality initiatives, and the promising hospitalist-created protocol.
However, this large appropriation presents both the greatest strength and the greatest threat to the center. With the Republican takeover of the House of Representatives, the CMMI budget likely is to be a target for the “repeal, replace, or revise” agenda. Therefore, increasing awareness of CMMI’s role will be imperative over the coming months. Hospitalists can help by educating themselves, then passing their knowledge along to those who might not understand the importance of the center. TH
The Centers for Medicare & Medicaid Services (CMS) in November announced the official launch of the Center for Medicare & Medicaid Innovation (CMMI). The CMI was authorized under the Affordable Care Act (ACA) to test innovative ways to reduce costs, while preserving or enhancing the quality. This sounds very similar to many other reform initiatives, so why have a separate center when ACOs, value-based purchasing, and payment bundling already are in the ACA?
A quick glance at the CMMI website didn’t provide much detail beyond uplifting language about the promise that the center represents. Don Berwick, MD, the new CMS administrator, has even gone so far as to call the center the “jewel in the crown” of the ACA.
Inspirational language aside, the center can be summed up using a simple analogy: The “other” ACA initiatives (bundling, VBP, etc.) are like a factory floor. The tools are in place, the processes are more or less defined, and they will be carried out regardless of the degree of positive impact. CMMI is more like a research and development lab, with the freedom to tinker with new ideas before wide-scale implementation.
The keys to CMMI success are twofold. First, it will implement pilot projects rather than demonstrations. A pilot gives the Secretary of Health and Human Services the power to implement and expand promising projects without Congressional approval. A demonstration requires Congressional approval for its continuation.. Second, CMMI does not require proposals to be budget neutral. Initial training and staffing costs alone can disqualify a program on budget neutrality grounds. Since CMMI does not require budget neutrality, promising programs with significant start-up costs are less likely to be cast aside.
Dr. Berwick has asked for provider partnership and input, and says he “would like to help forge an unprecedented level of shared aim, shared vision, and synergy in action among the public and private stewards and leaders of healthcare.” This vision and a $10 billion appropriation over the next decade present a tremendous opportunity for SHM’s quality initiatives, and the promising hospitalist-created protocol.
However, this large appropriation presents both the greatest strength and the greatest threat to the center. With the Republican takeover of the House of Representatives, the CMMI budget likely is to be a target for the “repeal, replace, or revise” agenda. Therefore, increasing awareness of CMMI’s role will be imperative over the coming months. Hospitalists can help by educating themselves, then passing their knowledge along to those who might not understand the importance of the center. TH