User login
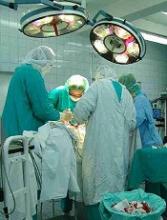
Is same-day discharge feasible and safe for women undergoing vaginal hysterectomy?
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
Tight inflammation control could reduce CV risk in men with gout
MADRID – Optimizing anti-inflammatory treatment may help to reduce the risk of heart-related problems in men with crystal-proven gout, judging from 7-year follow up data from a prospective study.
Five factors were found to increase substantially the risk for cardiovascular (CV) events in the 251-patient study: including: high levels of C-reactive protein (CRP); renal insufficiency; daily intake of more than 20 g of alcohol; current coronary heart disease (CHD); and a family history of premature CV events.
The odds ratio (OR) for any CV event was 5.71 for CRP levels greater than 5 mg/L on multivariate analysis. This increased to 10.31 and 14.26 when nonfatal and fatal CV events were considered separately.
Odds ratios for renal insufficiency, defined as a creatinine clearance of less than 60 mL/min per 1.73 m2, were 4.76 for any CV event and 8.42 for fatal CV events. Respective values for a family history of CV events before the age of 55 years was 3.09 considering any CV event, but 7.53 if consideration was limited to fatal events only. Current CHD also increased the risk for any CV event (OR, 3.67) and for nonfatal events (OR, 10.41).
Alcohol intake of more than 20g/day carried an OR of 4.23 for any CV event.
"We have a cardiologist in our team to ensure the reliability of the cardiovascular outcomes," said Dr. Victoria Barskova of the Research Institute of Rheumatology in Moscow, who presented the findings at the annual European Congress of Rheumatology (Ann. Rheum. Dis. 2013;72[suppl. 3]:95). She explained that the study was designed to prospectively determine baseline factors that might influence the development of CV events in male patients with crystal-proven gout.
Between 2003 and 2006, a total of 407 men were screened and 301 deemed eligible for the study. Of these, 251 had follow-up data to 2010-2012. Data collected at baseline and at follow-up included patient demographics, including family history of gout and early CV disease, smoking history, and alcohol consumption. Comorbidities and gout characteristics were also assessed and all patients had an ECG and echocardiogram.
Comparing baseline clinical features with follow-up visit data, Dr. Barskova noted that the number of allopurinol users increased from 16% to 57% (P = .00001), although regular allopurinol use was not found to decrease the risk for CV events.
There was an increase in the percentage of patients with diabetes from 18% to 43% (P = .00001), chronic heart disease from 35% to 53% (P = .00001), and heart failure from 10% to 28% (P = .0001). Alcohol use significantly decreased (92% vs. 63%; P less than .0001).
The frequency of subcutaneous tophi and chronic arthritis comparing the first and last visits was the same.
Just under a quarter (23.1%, n = 58) of patients experienced a CV event. There were 32 (13%) deaths reported of which 22 were from cardiovascular causes. There were 36 nonfatal CV events.
"In our cohort of patients with crystal proven gout, the following independent risk factors for all CV events have been highlighted: CRP, renal insufficiency, alcohol intake, coronary heart disease, and a family history of CV events," Dr. Barskova said in conclusion.
The key message for rheumatologists, she added, is that "tight control of inflammation, which is measured not only by the obvious arthritis, but also by the serum CRP level, may have a positive effect on cardiovascular events in patients with gout."
MADRID – Optimizing anti-inflammatory treatment may help to reduce the risk of heart-related problems in men with crystal-proven gout, judging from 7-year follow up data from a prospective study.
Five factors were found to increase substantially the risk for cardiovascular (CV) events in the 251-patient study: including: high levels of C-reactive protein (CRP); renal insufficiency; daily intake of more than 20 g of alcohol; current coronary heart disease (CHD); and a family history of premature CV events.
The odds ratio (OR) for any CV event was 5.71 for CRP levels greater than 5 mg/L on multivariate analysis. This increased to 10.31 and 14.26 when nonfatal and fatal CV events were considered separately.
Odds ratios for renal insufficiency, defined as a creatinine clearance of less than 60 mL/min per 1.73 m2, were 4.76 for any CV event and 8.42 for fatal CV events. Respective values for a family history of CV events before the age of 55 years was 3.09 considering any CV event, but 7.53 if consideration was limited to fatal events only. Current CHD also increased the risk for any CV event (OR, 3.67) and for nonfatal events (OR, 10.41).
Alcohol intake of more than 20g/day carried an OR of 4.23 for any CV event.
"We have a cardiologist in our team to ensure the reliability of the cardiovascular outcomes," said Dr. Victoria Barskova of the Research Institute of Rheumatology in Moscow, who presented the findings at the annual European Congress of Rheumatology (Ann. Rheum. Dis. 2013;72[suppl. 3]:95). She explained that the study was designed to prospectively determine baseline factors that might influence the development of CV events in male patients with crystal-proven gout.
Between 2003 and 2006, a total of 407 men were screened and 301 deemed eligible for the study. Of these, 251 had follow-up data to 2010-2012. Data collected at baseline and at follow-up included patient demographics, including family history of gout and early CV disease, smoking history, and alcohol consumption. Comorbidities and gout characteristics were also assessed and all patients had an ECG and echocardiogram.
Comparing baseline clinical features with follow-up visit data, Dr. Barskova noted that the number of allopurinol users increased from 16% to 57% (P = .00001), although regular allopurinol use was not found to decrease the risk for CV events.
There was an increase in the percentage of patients with diabetes from 18% to 43% (P = .00001), chronic heart disease from 35% to 53% (P = .00001), and heart failure from 10% to 28% (P = .0001). Alcohol use significantly decreased (92% vs. 63%; P less than .0001).
The frequency of subcutaneous tophi and chronic arthritis comparing the first and last visits was the same.
Just under a quarter (23.1%, n = 58) of patients experienced a CV event. There were 32 (13%) deaths reported of which 22 were from cardiovascular causes. There were 36 nonfatal CV events.
"In our cohort of patients with crystal proven gout, the following independent risk factors for all CV events have been highlighted: CRP, renal insufficiency, alcohol intake, coronary heart disease, and a family history of CV events," Dr. Barskova said in conclusion.
The key message for rheumatologists, she added, is that "tight control of inflammation, which is measured not only by the obvious arthritis, but also by the serum CRP level, may have a positive effect on cardiovascular events in patients with gout."
MADRID – Optimizing anti-inflammatory treatment may help to reduce the risk of heart-related problems in men with crystal-proven gout, judging from 7-year follow up data from a prospective study.
Five factors were found to increase substantially the risk for cardiovascular (CV) events in the 251-patient study: including: high levels of C-reactive protein (CRP); renal insufficiency; daily intake of more than 20 g of alcohol; current coronary heart disease (CHD); and a family history of premature CV events.
The odds ratio (OR) for any CV event was 5.71 for CRP levels greater than 5 mg/L on multivariate analysis. This increased to 10.31 and 14.26 when nonfatal and fatal CV events were considered separately.
Odds ratios for renal insufficiency, defined as a creatinine clearance of less than 60 mL/min per 1.73 m2, were 4.76 for any CV event and 8.42 for fatal CV events. Respective values for a family history of CV events before the age of 55 years was 3.09 considering any CV event, but 7.53 if consideration was limited to fatal events only. Current CHD also increased the risk for any CV event (OR, 3.67) and for nonfatal events (OR, 10.41).
Alcohol intake of more than 20g/day carried an OR of 4.23 for any CV event.
"We have a cardiologist in our team to ensure the reliability of the cardiovascular outcomes," said Dr. Victoria Barskova of the Research Institute of Rheumatology in Moscow, who presented the findings at the annual European Congress of Rheumatology (Ann. Rheum. Dis. 2013;72[suppl. 3]:95). She explained that the study was designed to prospectively determine baseline factors that might influence the development of CV events in male patients with crystal-proven gout.
Between 2003 and 2006, a total of 407 men were screened and 301 deemed eligible for the study. Of these, 251 had follow-up data to 2010-2012. Data collected at baseline and at follow-up included patient demographics, including family history of gout and early CV disease, smoking history, and alcohol consumption. Comorbidities and gout characteristics were also assessed and all patients had an ECG and echocardiogram.
Comparing baseline clinical features with follow-up visit data, Dr. Barskova noted that the number of allopurinol users increased from 16% to 57% (P = .00001), although regular allopurinol use was not found to decrease the risk for CV events.
There was an increase in the percentage of patients with diabetes from 18% to 43% (P = .00001), chronic heart disease from 35% to 53% (P = .00001), and heart failure from 10% to 28% (P = .0001). Alcohol use significantly decreased (92% vs. 63%; P less than .0001).
The frequency of subcutaneous tophi and chronic arthritis comparing the first and last visits was the same.
Just under a quarter (23.1%, n = 58) of patients experienced a CV event. There were 32 (13%) deaths reported of which 22 were from cardiovascular causes. There were 36 nonfatal CV events.
"In our cohort of patients with crystal proven gout, the following independent risk factors for all CV events have been highlighted: CRP, renal insufficiency, alcohol intake, coronary heart disease, and a family history of CV events," Dr. Barskova said in conclusion.
The key message for rheumatologists, she added, is that "tight control of inflammation, which is measured not only by the obvious arthritis, but also by the serum CRP level, may have a positive effect on cardiovascular events in patients with gout."
AT THE EULAR CONGRESS 2013
Major finding: The odds ratio for any CV event was 5.71 for CRP levels greater than 5 mg/L on multivariate analysis. This increased to 10.31 and 14.26 when nonfatal and fatal CV events were considered separately.
Data source: Single-center, prospective cohort study of 251 male patients with crystal-proven gout with 6.9 years’ mean follow-up.
Disclosures: The research was supported by the Russian Academy of Medical Sciences. Dr. Barskova has not relevant conflicts of interest.
Mammas, don't let your babies grow up to be doctors
When country singer Ed Bruce released "Mammas, Don’t Let Your Babies Grow Up to be Cowboys" in 1975, he suggested that they should consider becoming doctors instead, but if a new national survey of doctors is to be believed, that’s not such a good career move either.
The survey, conducted by the Georgia-based staffing company Jackson Healthcare, found that 59% of physicians would be unlikely to encourage a young person to become a doctor. The findings are based on the responses of 3,456 physicians who completed e-mailed surveys between March 7 and April 1, 2013.
Their dissatisfaction with medical practice is reflected in the career satisfaction numbers in the survey. Only 20% of physicians said that they were very satisfied in their work, while 39% were somewhat satisfied and 42% were somewhat or very dissatisfied.
The satisfied ones tended to be those employed by a hospital or working at a physician-owned practice where they had no ownership stake, according to the survey.
Satisfied doctors reported that they worked 11 hours a day or less and were supported by nurse practitioners or physician assistants.
In contrast, dissatisfied doctors tended to own their practices, work as locum tenens physicians, or work for a hospital-owned practice. They also worked longer hours and had few physician extenders.
Maybe the next generation will take Ed Bruce’s other suggested career path and become lawyers.
When country singer Ed Bruce released "Mammas, Don’t Let Your Babies Grow Up to be Cowboys" in 1975, he suggested that they should consider becoming doctors instead, but if a new national survey of doctors is to be believed, that’s not such a good career move either.
The survey, conducted by the Georgia-based staffing company Jackson Healthcare, found that 59% of physicians would be unlikely to encourage a young person to become a doctor. The findings are based on the responses of 3,456 physicians who completed e-mailed surveys between March 7 and April 1, 2013.
Their dissatisfaction with medical practice is reflected in the career satisfaction numbers in the survey. Only 20% of physicians said that they were very satisfied in their work, while 39% were somewhat satisfied and 42% were somewhat or very dissatisfied.
The satisfied ones tended to be those employed by a hospital or working at a physician-owned practice where they had no ownership stake, according to the survey.
Satisfied doctors reported that they worked 11 hours a day or less and were supported by nurse practitioners or physician assistants.
In contrast, dissatisfied doctors tended to own their practices, work as locum tenens physicians, or work for a hospital-owned practice. They also worked longer hours and had few physician extenders.
Maybe the next generation will take Ed Bruce’s other suggested career path and become lawyers.
When country singer Ed Bruce released "Mammas, Don’t Let Your Babies Grow Up to be Cowboys" in 1975, he suggested that they should consider becoming doctors instead, but if a new national survey of doctors is to be believed, that’s not such a good career move either.
The survey, conducted by the Georgia-based staffing company Jackson Healthcare, found that 59% of physicians would be unlikely to encourage a young person to become a doctor. The findings are based on the responses of 3,456 physicians who completed e-mailed surveys between March 7 and April 1, 2013.
Their dissatisfaction with medical practice is reflected in the career satisfaction numbers in the survey. Only 20% of physicians said that they were very satisfied in their work, while 39% were somewhat satisfied and 42% were somewhat or very dissatisfied.
The satisfied ones tended to be those employed by a hospital or working at a physician-owned practice where they had no ownership stake, according to the survey.
Satisfied doctors reported that they worked 11 hours a day or less and were supported by nurse practitioners or physician assistants.
In contrast, dissatisfied doctors tended to own their practices, work as locum tenens physicians, or work for a hospital-owned practice. They also worked longer hours and had few physician extenders.
Maybe the next generation will take Ed Bruce’s other suggested career path and become lawyers.
Leukocytosis After ICD Implantation; A Misleading Case of Herpes; Calculating the Risks of C difficile; Keeping Weight Off: A Losing Battle?
Fraud, errors were behind delay of apixaban approval, report shows

Credit: Esther Dyson
Medication mistakes, reporting errors, and record changes are what led the US Food and Drug Administration (FDA) to delay approval of the anticoagulant apixaban (Eliquis), according to a report in Pharmaceutical Approvals Monthly.
The report reveals that a number of patients enrolled on the ARISTOTLE trial received the wrong medication or the wrong dose, some serious adverse events went unreported, and employees who worked at trial sites in China altered records to cover up noncompliance with “good clinical practice.”
Apixaban won FDA approval last December as prophylaxis for stroke and systemic embolism in patients with nonvalvular atrial fibrillation. And that approval was based on results of the ARISTOTLE trial.
Prior to the approval, the FDA twice rejected a new drug application filed by apixaban’s developers, Pfizer and Bristol-Myers Squibb. But the agency’s reasons were not immediately made public.
Now, the Pharmaceutical Approvals Monthly report and FDA documents show that “data irregularities” were behind the decision.
In January 2012, Bristol-Myers Squibb notified the FDA of the cover-up attempt that occurred at (at least) 1 trial site in China. The company had learned that its senior manager at a site in Shanghai and an employee from a contract research organization had altered source records to conceal good clinical practice violations. (The employees were subsequently fired.)
According to FDA documents, the cover-up included failure to report 4 potential adverse events, late reports on 3 other events, and the omission of 3 patient outcomes. In addition, there were errors in patient names and dates, some Chinese and English records didn’t match up, and some patient records disappeared before a site visit by FDA inspectors.
Only 35 patients enrolled in the ARISTOTLE trial were treated at the Shanghai site, but the employees who perpetrated the fraud had also worked at 24 of the 36 trial sites in China.
So the FDA performed analyses excluding data from the Shanghai site alone, from the 24 sites where the employees worked, and from all 36 sites in China. And they found that apixaban’s efficacy still held up.
However, there was still the issue of ARISTOTLE participants receiving the wrong medication or the wrong dose. According to Bristol-Myers Squibb, the trial’s double-blind design allowed for dispensing errors.
Initially, the company said this meant that 7.3% of patients who were set to receive apixaban and 1.2% of patients who were set to receive warfarin may have received the wrong drug or dose at some point during the study. However, after additional review, the company said those percentages were likely much lower.
Regardless of the actual percentages, the FDA said this information suggests a pattern of inadequate oversight. And an independent review by FDA medical team leader Thomas Marciniak appears to support that statement. In addition to the aforementioned errors, his review revealed records of doctor visits taking place after patients’ deaths.
In spite of the errors and the fraud, the FDA said it remained convinced of apixaban’s efficacy. So the agency decided to approve the drug and leave out any mention of the data irregularities on the drug’s label. ![]()

Credit: Esther Dyson
Medication mistakes, reporting errors, and record changes are what led the US Food and Drug Administration (FDA) to delay approval of the anticoagulant apixaban (Eliquis), according to a report in Pharmaceutical Approvals Monthly.
The report reveals that a number of patients enrolled on the ARISTOTLE trial received the wrong medication or the wrong dose, some serious adverse events went unreported, and employees who worked at trial sites in China altered records to cover up noncompliance with “good clinical practice.”
Apixaban won FDA approval last December as prophylaxis for stroke and systemic embolism in patients with nonvalvular atrial fibrillation. And that approval was based on results of the ARISTOTLE trial.
Prior to the approval, the FDA twice rejected a new drug application filed by apixaban’s developers, Pfizer and Bristol-Myers Squibb. But the agency’s reasons were not immediately made public.
Now, the Pharmaceutical Approvals Monthly report and FDA documents show that “data irregularities” were behind the decision.
In January 2012, Bristol-Myers Squibb notified the FDA of the cover-up attempt that occurred at (at least) 1 trial site in China. The company had learned that its senior manager at a site in Shanghai and an employee from a contract research organization had altered source records to conceal good clinical practice violations. (The employees were subsequently fired.)
According to FDA documents, the cover-up included failure to report 4 potential adverse events, late reports on 3 other events, and the omission of 3 patient outcomes. In addition, there were errors in patient names and dates, some Chinese and English records didn’t match up, and some patient records disappeared before a site visit by FDA inspectors.
Only 35 patients enrolled in the ARISTOTLE trial were treated at the Shanghai site, but the employees who perpetrated the fraud had also worked at 24 of the 36 trial sites in China.
So the FDA performed analyses excluding data from the Shanghai site alone, from the 24 sites where the employees worked, and from all 36 sites in China. And they found that apixaban’s efficacy still held up.
However, there was still the issue of ARISTOTLE participants receiving the wrong medication or the wrong dose. According to Bristol-Myers Squibb, the trial’s double-blind design allowed for dispensing errors.
Initially, the company said this meant that 7.3% of patients who were set to receive apixaban and 1.2% of patients who were set to receive warfarin may have received the wrong drug or dose at some point during the study. However, after additional review, the company said those percentages were likely much lower.
Regardless of the actual percentages, the FDA said this information suggests a pattern of inadequate oversight. And an independent review by FDA medical team leader Thomas Marciniak appears to support that statement. In addition to the aforementioned errors, his review revealed records of doctor visits taking place after patients’ deaths.
In spite of the errors and the fraud, the FDA said it remained convinced of apixaban’s efficacy. So the agency decided to approve the drug and leave out any mention of the data irregularities on the drug’s label. ![]()

Credit: Esther Dyson
Medication mistakes, reporting errors, and record changes are what led the US Food and Drug Administration (FDA) to delay approval of the anticoagulant apixaban (Eliquis), according to a report in Pharmaceutical Approvals Monthly.
The report reveals that a number of patients enrolled on the ARISTOTLE trial received the wrong medication or the wrong dose, some serious adverse events went unreported, and employees who worked at trial sites in China altered records to cover up noncompliance with “good clinical practice.”
Apixaban won FDA approval last December as prophylaxis for stroke and systemic embolism in patients with nonvalvular atrial fibrillation. And that approval was based on results of the ARISTOTLE trial.
Prior to the approval, the FDA twice rejected a new drug application filed by apixaban’s developers, Pfizer and Bristol-Myers Squibb. But the agency’s reasons were not immediately made public.
Now, the Pharmaceutical Approvals Monthly report and FDA documents show that “data irregularities” were behind the decision.
In January 2012, Bristol-Myers Squibb notified the FDA of the cover-up attempt that occurred at (at least) 1 trial site in China. The company had learned that its senior manager at a site in Shanghai and an employee from a contract research organization had altered source records to conceal good clinical practice violations. (The employees were subsequently fired.)
According to FDA documents, the cover-up included failure to report 4 potential adverse events, late reports on 3 other events, and the omission of 3 patient outcomes. In addition, there were errors in patient names and dates, some Chinese and English records didn’t match up, and some patient records disappeared before a site visit by FDA inspectors.
Only 35 patients enrolled in the ARISTOTLE trial were treated at the Shanghai site, but the employees who perpetrated the fraud had also worked at 24 of the 36 trial sites in China.
So the FDA performed analyses excluding data from the Shanghai site alone, from the 24 sites where the employees worked, and from all 36 sites in China. And they found that apixaban’s efficacy still held up.
However, there was still the issue of ARISTOTLE participants receiving the wrong medication or the wrong dose. According to Bristol-Myers Squibb, the trial’s double-blind design allowed for dispensing errors.
Initially, the company said this meant that 7.3% of patients who were set to receive apixaban and 1.2% of patients who were set to receive warfarin may have received the wrong drug or dose at some point during the study. However, after additional review, the company said those percentages were likely much lower.
Regardless of the actual percentages, the FDA said this information suggests a pattern of inadequate oversight. And an independent review by FDA medical team leader Thomas Marciniak appears to support that statement. In addition to the aforementioned errors, his review revealed records of doctor visits taking place after patients’ deaths.
In spite of the errors and the fraud, the FDA said it remained convinced of apixaban’s efficacy. So the agency decided to approve the drug and leave out any mention of the data irregularities on the drug’s label. ![]()
Hospital Compare's data makes it a site to see
On one of my recent Internet excursions I spent some time on a highly useful website, Hospital Compare. This site is sponsored by Medicare.gov and provides a plethora of useful information for health care consumers who want to know quality metrics on health care facilities in their area. There is quality data on over 4,000 Medicare-certified hospitals across the country.
Hospital Compare is a very user-friendly site. Even those among us who are not so Web savvy can navigate this site in a snap. Just type in your zip code and up pops a list of hospitals in your specified area. You can even narrow your search by requesting what type of hospital you are interested in – acute care Veterans Affairs, acute care, children’s, or critical care access hospitals.
Next, putting a check in the boxes next to hospitals you want to compare produces a grid describing the different facilities. Users can find out which hospitals provide emergency services and whether lab results can be tracked between visits.
Of more interest to hospitalists is the next tab: Patient Survey Results. Here you can find out how well physicians and nurses on your staff communicated with patients during their hospitalization versus how well other health care providers in your region performed.
Scrolling through the tabs, you will then find tabs for Timely & Effective Care, which compares inpatient core measures, such as the percentage of patients with MI given aspirin at discharge and the percentage of heart failure patients receiving an ACE inhibitor or an angiotensin receptor blocker. Users can even find out the average wait time in the ED prior to being admitted to a particular hospital.
There is information on how to save money on prescription drugs, advanced directives and long term care, Medicare rights and forms, readmissions, hospital complications, 30-day death rates, health care–associated infections, and much more. While perusing that site, I also came upon yet another great site, Nursing Home Compare. Medicare.gov’s Nursing Home Compareis also chock-full of information for patients and family members who want to find the best care possible. Not only does it rate nursing homes, it gives ratings for rehabilitation facilities as well.
I was shocked to find out that one nursing home near my home had a terrible health inspection rating, while another one close by received flying colors for this metric. This type of information is invaluable for patients who are already sick and vulnerable. This site even provides information about fire safety ratings and details of health inspection reports and complaints.
Patients often ask me what I think about various facilities in the area, and I have had very little valuable information to offer them. It seemed like I always deferred that question to the social worker. Now, I can refer them to this website to help them make very important choices for their health care as well as care for their loved ones.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
On one of my recent Internet excursions I spent some time on a highly useful website, Hospital Compare. This site is sponsored by Medicare.gov and provides a plethora of useful information for health care consumers who want to know quality metrics on health care facilities in their area. There is quality data on over 4,000 Medicare-certified hospitals across the country.
Hospital Compare is a very user-friendly site. Even those among us who are not so Web savvy can navigate this site in a snap. Just type in your zip code and up pops a list of hospitals in your specified area. You can even narrow your search by requesting what type of hospital you are interested in – acute care Veterans Affairs, acute care, children’s, or critical care access hospitals.
Next, putting a check in the boxes next to hospitals you want to compare produces a grid describing the different facilities. Users can find out which hospitals provide emergency services and whether lab results can be tracked between visits.
Of more interest to hospitalists is the next tab: Patient Survey Results. Here you can find out how well physicians and nurses on your staff communicated with patients during their hospitalization versus how well other health care providers in your region performed.
Scrolling through the tabs, you will then find tabs for Timely & Effective Care, which compares inpatient core measures, such as the percentage of patients with MI given aspirin at discharge and the percentage of heart failure patients receiving an ACE inhibitor or an angiotensin receptor blocker. Users can even find out the average wait time in the ED prior to being admitted to a particular hospital.
There is information on how to save money on prescription drugs, advanced directives and long term care, Medicare rights and forms, readmissions, hospital complications, 30-day death rates, health care–associated infections, and much more. While perusing that site, I also came upon yet another great site, Nursing Home Compare. Medicare.gov’s Nursing Home Compareis also chock-full of information for patients and family members who want to find the best care possible. Not only does it rate nursing homes, it gives ratings for rehabilitation facilities as well.
I was shocked to find out that one nursing home near my home had a terrible health inspection rating, while another one close by received flying colors for this metric. This type of information is invaluable for patients who are already sick and vulnerable. This site even provides information about fire safety ratings and details of health inspection reports and complaints.
Patients often ask me what I think about various facilities in the area, and I have had very little valuable information to offer them. It seemed like I always deferred that question to the social worker. Now, I can refer them to this website to help them make very important choices for their health care as well as care for their loved ones.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
On one of my recent Internet excursions I spent some time on a highly useful website, Hospital Compare. This site is sponsored by Medicare.gov and provides a plethora of useful information for health care consumers who want to know quality metrics on health care facilities in their area. There is quality data on over 4,000 Medicare-certified hospitals across the country.
Hospital Compare is a very user-friendly site. Even those among us who are not so Web savvy can navigate this site in a snap. Just type in your zip code and up pops a list of hospitals in your specified area. You can even narrow your search by requesting what type of hospital you are interested in – acute care Veterans Affairs, acute care, children’s, or critical care access hospitals.
Next, putting a check in the boxes next to hospitals you want to compare produces a grid describing the different facilities. Users can find out which hospitals provide emergency services and whether lab results can be tracked between visits.
Of more interest to hospitalists is the next tab: Patient Survey Results. Here you can find out how well physicians and nurses on your staff communicated with patients during their hospitalization versus how well other health care providers in your region performed.
Scrolling through the tabs, you will then find tabs for Timely & Effective Care, which compares inpatient core measures, such as the percentage of patients with MI given aspirin at discharge and the percentage of heart failure patients receiving an ACE inhibitor or an angiotensin receptor blocker. Users can even find out the average wait time in the ED prior to being admitted to a particular hospital.
There is information on how to save money on prescription drugs, advanced directives and long term care, Medicare rights and forms, readmissions, hospital complications, 30-day death rates, health care–associated infections, and much more. While perusing that site, I also came upon yet another great site, Nursing Home Compare. Medicare.gov’s Nursing Home Compareis also chock-full of information for patients and family members who want to find the best care possible. Not only does it rate nursing homes, it gives ratings for rehabilitation facilities as well.
I was shocked to find out that one nursing home near my home had a terrible health inspection rating, while another one close by received flying colors for this metric. This type of information is invaluable for patients who are already sick and vulnerable. This site even provides information about fire safety ratings and details of health inspection reports and complaints.
Patients often ask me what I think about various facilities in the area, and I have had very little valuable information to offer them. It seemed like I always deferred that question to the social worker. Now, I can refer them to this website to help them make very important choices for their health care as well as care for their loved ones.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
New and Noteworthy Information—July 2013
In patients with arterial disease, low baseline diastolic blood pressure may correspond with increased progression of subcortical atrophy, researchers reported online ahead of print June 10 in JAMA Neurology. The association may not depend on subsequent change in blood pressure. Researchers measured blood pressure for 663 participants at baseline and at a mean of 3.9 years later. Change in brain parenchymal fraction, cortical gray matter fraction, and ventricular fraction were quantified as indicators of progression of global, cortical, and subcortical brain atrophy. Patients with lower baseline diastolic blood pressure or mean arterial pressure had more progression of subcortical atrophy. Of patients with higher baseline blood pressure, those with declining blood pressure over time had less progression of subcortical atrophy, compared with those with increasing blood pressure.
Diffusion tensor imaging (DTI) of the basal ganglia and cerebellum may accurately diagnose Parkinson’s disease and other movement disorders, according to a study published online ahead of print May 14 in Movement Disorders. Investigators studied 72 subjects with DTI at 3 T. The technique distinguished between controls and patients with movement disorder with 92% sensitivity and 88% specificity. DTI also distinguished between controls and patients with parkinsonism (93% sensitivity, 91% specificity), Parkinson’s disease and atypical parkinsonism (90% sensitivity, 100% specificity), Parkinson’s disease and multiple system atrophy (94% sensitivity, 100% specificity), Parkinson’s disease and progressive supranuclear palsy (87% sensitivity, 100% specificity), multiple system atrophy and progressive supranuclear palsy (90% sensitivity, 100% specificity), and Parkinson’s disease and essential tremor (92% sensitivity, 87% specificity).
Heading a soccer ball may be associated with abnormal white matter microstructure and poor neurocognitive performance, according to research published online ahead of print June 11 in Radiology. Thirty-seven amateur soccer players answered a questionnaire about heading in the previous 12 months and lifetime history of concussions. Investigators performed diffusion-tensor MRI on the players and evaluated their cognitive function. The players had headed a median of 432 times during the previous year. Heading was associated with lower fractional anisotropy (FA) at three locations in temporo-occipital white matter with a threshold that varied according to location. Heading more than 1,800 times per year was associated with lower levels of FA and poorer memory scores. Concussion history and demographic features were not significantly associated with FA or cognitive performance.
Restless legs syndrome (RLS) may entail an increased risk of mortality, independent of known risk factors, according to a study published online ahead of print June 12 in Neurology. Researchers conducted a prospective cohort study of 18,425 American men without diabetes, arthritis, or renal failure. RLS was assessed using standardized questions. During eight years of follow-up, 2,765 deaths occurred. In an age-adjusted model, RLS was associated with a 39% increased risk of mortality. The association between RLS and mortality was slightly attenuated after further adjustment for BMI, lifestyle factors, chronic conditions, sleep duration, and other sleep-related disorders. When patients with major chronic conditions (eg, cancer, high blood pressure, cardiovascular disease, and other comorbidities) were excluded, the adjusted hazard ratio for RLS was 1.92.
Patients with stroke who are about to undergo surgery can safely continue to take aspirin or warfarin in many instances, according to a guideline published in the May 28 Neurology. Researchers systematically reviewed the literature to assess evidence about the periprocedural management of antithrombotic drugs in patients with ischemic cerebrovascular disease. Stroke patients undergoing dental procedures should routinely continue aspirin, according to the guideline. Stroke patients undergoing invasive ocular anesthesia, cataract surgery, dermatologic procedures, transrectal ultrasound–guided prostate biopsy, spinal or epidural procedures, and carpal tunnel surgery should probably continue aspirin. Stroke patients requiring warfarin should routinely continue it when undergoing dental procedures and probably continue it for dermatologic procedures. Some patients undergoing EMG, prostate procedures, inguinal herniorrhaphy, and endothermal ablation of the great saphenous vein should possibly continue warfarin.
Exposure to pesticides or solvents could be a risk factor for Parkinson’s disease, investigators concluded in the May 28 Neurology. Researchers performed a meta-analysis of prospective cohort and case-control studies that estimated the risk of Parkinson’s disease associated with exposure to pesticides or solvents. Parkinson’s disease was associated with farming, and the association between the disease and pesticides was highly significant in the studies in which Parkinson’s disease diagnosis was self-reported. In high-quality case-control studies, exposure to any pesticides, herbicides, and solvents increased the risk of Parkinson’s disease. Exposure to paraquat or maneb/mancozeb was associated with about a twofold increase in risk. Study quality was not a source of heterogeneity among prospective studies. In case-control studies, study quality was a source of heterogeneity in risk estimates for some exposures.
Atrial fibrillation may be associated with faster cognitive decline, according to a study published online ahead of print June 5 in Neurology. Researchers studied 5,150 men and women 65 or older who did not have atrial fibrillation or a history of stroke at baseline. Atrial fibrillation was identified by examining hospital discharge records and annual ECGs. A total of 552 patients (10.7%) developed incident atrial fibrillation during a mean seven years of follow-up. Mean Modified Mini-Mental State Examination (MMMSE) scores declined faster in patients with incident atrial fibrillation than in patients without atrial fibrillation. The predicted five-year decline in mean MMMSE score from age 80 to age 85 was −6.4 points for participants without atrial fibrillation and −10.3 points for participants with incident atrial fibrillation at age 80.
The level of interleukin 17F (IL-17F) in a patient with relapsing-remitting multiple sclerosis (MS) may not predict his or her response to treatment with interferon beta, according to research published online ahead of print June 3 in JAMA Neurology. Researchers analyzed serum samples from 239 randomly selected patients treated with interferon beta-1b (250 μg) for at least two years. Using clinical and MRI criteria, the investigators found that levels of IL-17F measured at baseline and month six did not correlate with lack of response to treatment after two years. Relapses and new lesions on MRI were not associated with pretreatment serum IL-17F levels. The results did not change when patients with neutralizing antibodies were excluded. Patients with levels of IL-17F higher than 200 pg/mL had poor response and clinical or radiologic activity.
Earlier treatment with thrombolytics may be associated with reduced mortality for patients with stroke, according to data published in the June 19 JAMA. Researchers analyzed data from 58,353 patients with acute ischemic stroke who were treated with t-PA within 4.5 hours of symptom onset in 1,395 hospitals. Patients’ median age was 72, 50.3% of patients were women, and median time to treatment was 144 minutes. A total of 5,142 in-hospital deaths occurred. Faster time to treatment, in 15-minute increments, was associated with reduced in-hospital mortality, reduced symptomatic intracranial hemorrhage, increased achievement of independent ambulation at discharge, and increased discharge to home. Patient factors most strongly associated with shorter time to treatment included greater stroke severity, arrival by ambulance, and arrival during regular hours.
High serum iron levels may be associated with a decreased risk of developing Parkinson’s disease, according to a study published June 4 in PLOS Medicine. Researchers investigated three polymorphisms in the genes HFE and TMPRSS6. For each polymorphism, they performed a meta-analysis of studies investigating the genetic effect on iron levels and a meta-analysis of studies investigating the genetic effect on the risk of Parkinson’s disease. Using three Mendelian randomization analyses, the investigators estimated the effect of iron on Parkinson’s disease for the three polymorphisms. Every 10 µg/dl increase in blood iron corresponded to a 3% reduction in the risk of Parkinson’s disease. Thus, increased blood iron levels may have a protective effect against Parkinson’s disease, but the underlying mechanism remains unclear, said the researchers.
Diabetes and dementia may have a bidirectional association, according to research published online ahead of print June 10 in JAMA Internal Medicine. Investigators examined 783 older adults with diabetes who were participating in a prospective population-based study. Subjects’ mean age was 74, and their baseline Modified Mini-Mental State Examination scores were 80 or higher. Approximately 47% of subjects were African American, and 47% were female. During the 12-year follow-up, 61 participants had a reported hypoglycemic event, and 148 developed dementia. Subjects who had a hypoglycemic event had a twofold increased risk for developing dementia, compared with those who did not have a hypoglycemic event. Subjects with diabetes who developed dementia had a greater risk for having a subsequent hypoglycemic event, compared with participants who did not develop dementia.
Cardiovascular biomarkers may help identify patients with subclinical cerebral injury, according to research published online ahead of print May 9 in Stroke. A total of 1,920 subjects received a brain MRI and had their levels of N-terminal brain natriuretic peptide (NT-proBNP) and cardiac troponin T (hs-cTnT) measured. Of the total group, 1,112 had a follow-up MRI between nine and 13 years later. Individuals with the highest NT-proBNP levels had significantly more silent brain infarcts and white matter lesions on the baseline MRI and more incident silent brain infarcts and white matter lesion progression on the follow-up MRI. Individuals with the highest hs-cTnT levels had more silent brain infarcts and white matter lesions on the initial MRI and more white matter lesion progression on the follow-up MRI.
Stroke symptoms or transient ischemic attack (TIA) may be strongly associated with incident cognitive impairment, researchers concluded in a study published online ahead of print June 19 in Neurology. The investigators studied 23,830 participants without cognitive impairment or history of stroke enrolled in the Reasons for Geographic and Racial Differences in Stroke Study. Subjects reported stroke symptoms and TIA every six months and were screened annually for cognitive impairment. Among Caucasians, the odds ratio for incident cognitive impairment was 2.08 for subjects reporting at least one stroke symptom or TIA, compared with those reporting no stroke symptom or TIA. Among African Americans, the odds ratio was 1.66 using the same modeling. The magnitude of impact was largest among participants with fewer traditional stroke risk factors.
—Erik Greb
Senior Associate Editor
In patients with arterial disease, low baseline diastolic blood pressure may correspond with increased progression of subcortical atrophy, researchers reported online ahead of print June 10 in JAMA Neurology. The association may not depend on subsequent change in blood pressure. Researchers measured blood pressure for 663 participants at baseline and at a mean of 3.9 years later. Change in brain parenchymal fraction, cortical gray matter fraction, and ventricular fraction were quantified as indicators of progression of global, cortical, and subcortical brain atrophy. Patients with lower baseline diastolic blood pressure or mean arterial pressure had more progression of subcortical atrophy. Of patients with higher baseline blood pressure, those with declining blood pressure over time had less progression of subcortical atrophy, compared with those with increasing blood pressure.
Diffusion tensor imaging (DTI) of the basal ganglia and cerebellum may accurately diagnose Parkinson’s disease and other movement disorders, according to a study published online ahead of print May 14 in Movement Disorders. Investigators studied 72 subjects with DTI at 3 T. The technique distinguished between controls and patients with movement disorder with 92% sensitivity and 88% specificity. DTI also distinguished between controls and patients with parkinsonism (93% sensitivity, 91% specificity), Parkinson’s disease and atypical parkinsonism (90% sensitivity, 100% specificity), Parkinson’s disease and multiple system atrophy (94% sensitivity, 100% specificity), Parkinson’s disease and progressive supranuclear palsy (87% sensitivity, 100% specificity), multiple system atrophy and progressive supranuclear palsy (90% sensitivity, 100% specificity), and Parkinson’s disease and essential tremor (92% sensitivity, 87% specificity).
Heading a soccer ball may be associated with abnormal white matter microstructure and poor neurocognitive performance, according to research published online ahead of print June 11 in Radiology. Thirty-seven amateur soccer players answered a questionnaire about heading in the previous 12 months and lifetime history of concussions. Investigators performed diffusion-tensor MRI on the players and evaluated their cognitive function. The players had headed a median of 432 times during the previous year. Heading was associated with lower fractional anisotropy (FA) at three locations in temporo-occipital white matter with a threshold that varied according to location. Heading more than 1,800 times per year was associated with lower levels of FA and poorer memory scores. Concussion history and demographic features were not significantly associated with FA or cognitive performance.
Restless legs syndrome (RLS) may entail an increased risk of mortality, independent of known risk factors, according to a study published online ahead of print June 12 in Neurology. Researchers conducted a prospective cohort study of 18,425 American men without diabetes, arthritis, or renal failure. RLS was assessed using standardized questions. During eight years of follow-up, 2,765 deaths occurred. In an age-adjusted model, RLS was associated with a 39% increased risk of mortality. The association between RLS and mortality was slightly attenuated after further adjustment for BMI, lifestyle factors, chronic conditions, sleep duration, and other sleep-related disorders. When patients with major chronic conditions (eg, cancer, high blood pressure, cardiovascular disease, and other comorbidities) were excluded, the adjusted hazard ratio for RLS was 1.92.
Patients with stroke who are about to undergo surgery can safely continue to take aspirin or warfarin in many instances, according to a guideline published in the May 28 Neurology. Researchers systematically reviewed the literature to assess evidence about the periprocedural management of antithrombotic drugs in patients with ischemic cerebrovascular disease. Stroke patients undergoing dental procedures should routinely continue aspirin, according to the guideline. Stroke patients undergoing invasive ocular anesthesia, cataract surgery, dermatologic procedures, transrectal ultrasound–guided prostate biopsy, spinal or epidural procedures, and carpal tunnel surgery should probably continue aspirin. Stroke patients requiring warfarin should routinely continue it when undergoing dental procedures and probably continue it for dermatologic procedures. Some patients undergoing EMG, prostate procedures, inguinal herniorrhaphy, and endothermal ablation of the great saphenous vein should possibly continue warfarin.
Exposure to pesticides or solvents could be a risk factor for Parkinson’s disease, investigators concluded in the May 28 Neurology. Researchers performed a meta-analysis of prospective cohort and case-control studies that estimated the risk of Parkinson’s disease associated with exposure to pesticides or solvents. Parkinson’s disease was associated with farming, and the association between the disease and pesticides was highly significant in the studies in which Parkinson’s disease diagnosis was self-reported. In high-quality case-control studies, exposure to any pesticides, herbicides, and solvents increased the risk of Parkinson’s disease. Exposure to paraquat or maneb/mancozeb was associated with about a twofold increase in risk. Study quality was not a source of heterogeneity among prospective studies. In case-control studies, study quality was a source of heterogeneity in risk estimates for some exposures.
Atrial fibrillation may be associated with faster cognitive decline, according to a study published online ahead of print June 5 in Neurology. Researchers studied 5,150 men and women 65 or older who did not have atrial fibrillation or a history of stroke at baseline. Atrial fibrillation was identified by examining hospital discharge records and annual ECGs. A total of 552 patients (10.7%) developed incident atrial fibrillation during a mean seven years of follow-up. Mean Modified Mini-Mental State Examination (MMMSE) scores declined faster in patients with incident atrial fibrillation than in patients without atrial fibrillation. The predicted five-year decline in mean MMMSE score from age 80 to age 85 was −6.4 points for participants without atrial fibrillation and −10.3 points for participants with incident atrial fibrillation at age 80.
The level of interleukin 17F (IL-17F) in a patient with relapsing-remitting multiple sclerosis (MS) may not predict his or her response to treatment with interferon beta, according to research published online ahead of print June 3 in JAMA Neurology. Researchers analyzed serum samples from 239 randomly selected patients treated with interferon beta-1b (250 μg) for at least two years. Using clinical and MRI criteria, the investigators found that levels of IL-17F measured at baseline and month six did not correlate with lack of response to treatment after two years. Relapses and new lesions on MRI were not associated with pretreatment serum IL-17F levels. The results did not change when patients with neutralizing antibodies were excluded. Patients with levels of IL-17F higher than 200 pg/mL had poor response and clinical or radiologic activity.
Earlier treatment with thrombolytics may be associated with reduced mortality for patients with stroke, according to data published in the June 19 JAMA. Researchers analyzed data from 58,353 patients with acute ischemic stroke who were treated with t-PA within 4.5 hours of symptom onset in 1,395 hospitals. Patients’ median age was 72, 50.3% of patients were women, and median time to treatment was 144 minutes. A total of 5,142 in-hospital deaths occurred. Faster time to treatment, in 15-minute increments, was associated with reduced in-hospital mortality, reduced symptomatic intracranial hemorrhage, increased achievement of independent ambulation at discharge, and increased discharge to home. Patient factors most strongly associated with shorter time to treatment included greater stroke severity, arrival by ambulance, and arrival during regular hours.
High serum iron levels may be associated with a decreased risk of developing Parkinson’s disease, according to a study published June 4 in PLOS Medicine. Researchers investigated three polymorphisms in the genes HFE and TMPRSS6. For each polymorphism, they performed a meta-analysis of studies investigating the genetic effect on iron levels and a meta-analysis of studies investigating the genetic effect on the risk of Parkinson’s disease. Using three Mendelian randomization analyses, the investigators estimated the effect of iron on Parkinson’s disease for the three polymorphisms. Every 10 µg/dl increase in blood iron corresponded to a 3% reduction in the risk of Parkinson’s disease. Thus, increased blood iron levels may have a protective effect against Parkinson’s disease, but the underlying mechanism remains unclear, said the researchers.
Diabetes and dementia may have a bidirectional association, according to research published online ahead of print June 10 in JAMA Internal Medicine. Investigators examined 783 older adults with diabetes who were participating in a prospective population-based study. Subjects’ mean age was 74, and their baseline Modified Mini-Mental State Examination scores were 80 or higher. Approximately 47% of subjects were African American, and 47% were female. During the 12-year follow-up, 61 participants had a reported hypoglycemic event, and 148 developed dementia. Subjects who had a hypoglycemic event had a twofold increased risk for developing dementia, compared with those who did not have a hypoglycemic event. Subjects with diabetes who developed dementia had a greater risk for having a subsequent hypoglycemic event, compared with participants who did not develop dementia.
Cardiovascular biomarkers may help identify patients with subclinical cerebral injury, according to research published online ahead of print May 9 in Stroke. A total of 1,920 subjects received a brain MRI and had their levels of N-terminal brain natriuretic peptide (NT-proBNP) and cardiac troponin T (hs-cTnT) measured. Of the total group, 1,112 had a follow-up MRI between nine and 13 years later. Individuals with the highest NT-proBNP levels had significantly more silent brain infarcts and white matter lesions on the baseline MRI and more incident silent brain infarcts and white matter lesion progression on the follow-up MRI. Individuals with the highest hs-cTnT levels had more silent brain infarcts and white matter lesions on the initial MRI and more white matter lesion progression on the follow-up MRI.
Stroke symptoms or transient ischemic attack (TIA) may be strongly associated with incident cognitive impairment, researchers concluded in a study published online ahead of print June 19 in Neurology. The investigators studied 23,830 participants without cognitive impairment or history of stroke enrolled in the Reasons for Geographic and Racial Differences in Stroke Study. Subjects reported stroke symptoms and TIA every six months and were screened annually for cognitive impairment. Among Caucasians, the odds ratio for incident cognitive impairment was 2.08 for subjects reporting at least one stroke symptom or TIA, compared with those reporting no stroke symptom or TIA. Among African Americans, the odds ratio was 1.66 using the same modeling. The magnitude of impact was largest among participants with fewer traditional stroke risk factors.
—Erik Greb
Senior Associate Editor
In patients with arterial disease, low baseline diastolic blood pressure may correspond with increased progression of subcortical atrophy, researchers reported online ahead of print June 10 in JAMA Neurology. The association may not depend on subsequent change in blood pressure. Researchers measured blood pressure for 663 participants at baseline and at a mean of 3.9 years later. Change in brain parenchymal fraction, cortical gray matter fraction, and ventricular fraction were quantified as indicators of progression of global, cortical, and subcortical brain atrophy. Patients with lower baseline diastolic blood pressure or mean arterial pressure had more progression of subcortical atrophy. Of patients with higher baseline blood pressure, those with declining blood pressure over time had less progression of subcortical atrophy, compared with those with increasing blood pressure.
Diffusion tensor imaging (DTI) of the basal ganglia and cerebellum may accurately diagnose Parkinson’s disease and other movement disorders, according to a study published online ahead of print May 14 in Movement Disorders. Investigators studied 72 subjects with DTI at 3 T. The technique distinguished between controls and patients with movement disorder with 92% sensitivity and 88% specificity. DTI also distinguished between controls and patients with parkinsonism (93% sensitivity, 91% specificity), Parkinson’s disease and atypical parkinsonism (90% sensitivity, 100% specificity), Parkinson’s disease and multiple system atrophy (94% sensitivity, 100% specificity), Parkinson’s disease and progressive supranuclear palsy (87% sensitivity, 100% specificity), multiple system atrophy and progressive supranuclear palsy (90% sensitivity, 100% specificity), and Parkinson’s disease and essential tremor (92% sensitivity, 87% specificity).
Heading a soccer ball may be associated with abnormal white matter microstructure and poor neurocognitive performance, according to research published online ahead of print June 11 in Radiology. Thirty-seven amateur soccer players answered a questionnaire about heading in the previous 12 months and lifetime history of concussions. Investigators performed diffusion-tensor MRI on the players and evaluated their cognitive function. The players had headed a median of 432 times during the previous year. Heading was associated with lower fractional anisotropy (FA) at three locations in temporo-occipital white matter with a threshold that varied according to location. Heading more than 1,800 times per year was associated with lower levels of FA and poorer memory scores. Concussion history and demographic features were not significantly associated with FA or cognitive performance.
Restless legs syndrome (RLS) may entail an increased risk of mortality, independent of known risk factors, according to a study published online ahead of print June 12 in Neurology. Researchers conducted a prospective cohort study of 18,425 American men without diabetes, arthritis, or renal failure. RLS was assessed using standardized questions. During eight years of follow-up, 2,765 deaths occurred. In an age-adjusted model, RLS was associated with a 39% increased risk of mortality. The association between RLS and mortality was slightly attenuated after further adjustment for BMI, lifestyle factors, chronic conditions, sleep duration, and other sleep-related disorders. When patients with major chronic conditions (eg, cancer, high blood pressure, cardiovascular disease, and other comorbidities) were excluded, the adjusted hazard ratio for RLS was 1.92.
Patients with stroke who are about to undergo surgery can safely continue to take aspirin or warfarin in many instances, according to a guideline published in the May 28 Neurology. Researchers systematically reviewed the literature to assess evidence about the periprocedural management of antithrombotic drugs in patients with ischemic cerebrovascular disease. Stroke patients undergoing dental procedures should routinely continue aspirin, according to the guideline. Stroke patients undergoing invasive ocular anesthesia, cataract surgery, dermatologic procedures, transrectal ultrasound–guided prostate biopsy, spinal or epidural procedures, and carpal tunnel surgery should probably continue aspirin. Stroke patients requiring warfarin should routinely continue it when undergoing dental procedures and probably continue it for dermatologic procedures. Some patients undergoing EMG, prostate procedures, inguinal herniorrhaphy, and endothermal ablation of the great saphenous vein should possibly continue warfarin.
Exposure to pesticides or solvents could be a risk factor for Parkinson’s disease, investigators concluded in the May 28 Neurology. Researchers performed a meta-analysis of prospective cohort and case-control studies that estimated the risk of Parkinson’s disease associated with exposure to pesticides or solvents. Parkinson’s disease was associated with farming, and the association between the disease and pesticides was highly significant in the studies in which Parkinson’s disease diagnosis was self-reported. In high-quality case-control studies, exposure to any pesticides, herbicides, and solvents increased the risk of Parkinson’s disease. Exposure to paraquat or maneb/mancozeb was associated with about a twofold increase in risk. Study quality was not a source of heterogeneity among prospective studies. In case-control studies, study quality was a source of heterogeneity in risk estimates for some exposures.
Atrial fibrillation may be associated with faster cognitive decline, according to a study published online ahead of print June 5 in Neurology. Researchers studied 5,150 men and women 65 or older who did not have atrial fibrillation or a history of stroke at baseline. Atrial fibrillation was identified by examining hospital discharge records and annual ECGs. A total of 552 patients (10.7%) developed incident atrial fibrillation during a mean seven years of follow-up. Mean Modified Mini-Mental State Examination (MMMSE) scores declined faster in patients with incident atrial fibrillation than in patients without atrial fibrillation. The predicted five-year decline in mean MMMSE score from age 80 to age 85 was −6.4 points for participants without atrial fibrillation and −10.3 points for participants with incident atrial fibrillation at age 80.
The level of interleukin 17F (IL-17F) in a patient with relapsing-remitting multiple sclerosis (MS) may not predict his or her response to treatment with interferon beta, according to research published online ahead of print June 3 in JAMA Neurology. Researchers analyzed serum samples from 239 randomly selected patients treated with interferon beta-1b (250 μg) for at least two years. Using clinical and MRI criteria, the investigators found that levels of IL-17F measured at baseline and month six did not correlate with lack of response to treatment after two years. Relapses and new lesions on MRI were not associated with pretreatment serum IL-17F levels. The results did not change when patients with neutralizing antibodies were excluded. Patients with levels of IL-17F higher than 200 pg/mL had poor response and clinical or radiologic activity.
Earlier treatment with thrombolytics may be associated with reduced mortality for patients with stroke, according to data published in the June 19 JAMA. Researchers analyzed data from 58,353 patients with acute ischemic stroke who were treated with t-PA within 4.5 hours of symptom onset in 1,395 hospitals. Patients’ median age was 72, 50.3% of patients were women, and median time to treatment was 144 minutes. A total of 5,142 in-hospital deaths occurred. Faster time to treatment, in 15-minute increments, was associated with reduced in-hospital mortality, reduced symptomatic intracranial hemorrhage, increased achievement of independent ambulation at discharge, and increased discharge to home. Patient factors most strongly associated with shorter time to treatment included greater stroke severity, arrival by ambulance, and arrival during regular hours.
High serum iron levels may be associated with a decreased risk of developing Parkinson’s disease, according to a study published June 4 in PLOS Medicine. Researchers investigated three polymorphisms in the genes HFE and TMPRSS6. For each polymorphism, they performed a meta-analysis of studies investigating the genetic effect on iron levels and a meta-analysis of studies investigating the genetic effect on the risk of Parkinson’s disease. Using three Mendelian randomization analyses, the investigators estimated the effect of iron on Parkinson’s disease for the three polymorphisms. Every 10 µg/dl increase in blood iron corresponded to a 3% reduction in the risk of Parkinson’s disease. Thus, increased blood iron levels may have a protective effect against Parkinson’s disease, but the underlying mechanism remains unclear, said the researchers.
Diabetes and dementia may have a bidirectional association, according to research published online ahead of print June 10 in JAMA Internal Medicine. Investigators examined 783 older adults with diabetes who were participating in a prospective population-based study. Subjects’ mean age was 74, and their baseline Modified Mini-Mental State Examination scores were 80 or higher. Approximately 47% of subjects were African American, and 47% were female. During the 12-year follow-up, 61 participants had a reported hypoglycemic event, and 148 developed dementia. Subjects who had a hypoglycemic event had a twofold increased risk for developing dementia, compared with those who did not have a hypoglycemic event. Subjects with diabetes who developed dementia had a greater risk for having a subsequent hypoglycemic event, compared with participants who did not develop dementia.
Cardiovascular biomarkers may help identify patients with subclinical cerebral injury, according to research published online ahead of print May 9 in Stroke. A total of 1,920 subjects received a brain MRI and had their levels of N-terminal brain natriuretic peptide (NT-proBNP) and cardiac troponin T (hs-cTnT) measured. Of the total group, 1,112 had a follow-up MRI between nine and 13 years later. Individuals with the highest NT-proBNP levels had significantly more silent brain infarcts and white matter lesions on the baseline MRI and more incident silent brain infarcts and white matter lesion progression on the follow-up MRI. Individuals with the highest hs-cTnT levels had more silent brain infarcts and white matter lesions on the initial MRI and more white matter lesion progression on the follow-up MRI.
Stroke symptoms or transient ischemic attack (TIA) may be strongly associated with incident cognitive impairment, researchers concluded in a study published online ahead of print June 19 in Neurology. The investigators studied 23,830 participants without cognitive impairment or history of stroke enrolled in the Reasons for Geographic and Racial Differences in Stroke Study. Subjects reported stroke symptoms and TIA every six months and were screened annually for cognitive impairment. Among Caucasians, the odds ratio for incident cognitive impairment was 2.08 for subjects reporting at least one stroke symptom or TIA, compared with those reporting no stroke symptom or TIA. Among African Americans, the odds ratio was 1.66 using the same modeling. The magnitude of impact was largest among participants with fewer traditional stroke risk factors.
—Erik Greb
Senior Associate Editor
Two Distinct Pathways to Hospital Leadership
Thadeo Catacutan, MD, sat in a ballroom at the Gaylord Resort & Convention Center in National Harbor, Md., on a sunny spring day at HM13, just thinking about his future after spending seven years as a hospitalist at Cleveland Clinic. He wondered if additional training in business administration would provide him with the advancement opportunities and professional fulfillment he sought.
“It’s been hard to determine what I really want to do,” he said. “After seven years, is this the peak? Are there other opportunities?
“I realize that if you are not going to specialize, you have to go into a leadership career track,” he said. “Some would say you can just be a clinician, but in the long term, I don’t think that is a sustainable path. You will just burn out.”
Dr. Catacutan isn’t the only one concerned. Nearly 200 physicians joined him for an HM13 breakout session titled “Career Tracks for Hospitalists.” The session provided attendees two viewpoints on career advancement: the executive pathway and the hospital leadership pathway. And although the session’s speakers demonstrated distinct routes to senior positions, there’s a shared path for each, including:
- Weighing choices carefully. The reasons for seeking a new career path are personal and not the same for everyone. Keep that in mind.
- Thinking about return on investment. Physicians should consider what they’re getting for their effort, whether that’s the cost of an advanced degree or the value of time spent volunteering on a hospital committee.
- Contemplating what you like about your job. When considering a career move, remember the adage that the grass is always greener.
“The way you get to take on leadership opportunities is by acceptance,” said session moderator Michael Guthrie, MD, MBA, executive in residence at the University of Colorado. “Say ‘yes.’ Say ‘I’m curious’ or ‘How can I make a contribution?’ You will stumble. You will make mistakes. You will be clumsy. … But it is that process that teaches you what you need to know.”
Path: Advanced Training
Michael Ruhlen, MD, MHCM, FACHE, SFHM, said he needed to know how to communicate better with the administrators he regularly met with to discuss his pediatric hospitalist program.
“I found that I wasn’t always speaking the same language when I talked to administrators about the value that hospitalists brought to their individual institutions,” he said. “No matter how hard I tried, I could always walk into a room with a master’s-prepared administrator who could absolutely prove to me that my hospitalist program would cost them far more money than it would ever be worth—and they simply could not afford it.”
To address what he calls a “knowledge deficit,” Dr. Ruhlen earned his master’s degree in health-care management from the Harvard School of Public Health in Boston. Armed with new business acumen, he rose to vice president of medical affairs at Toledo Children’s Hospital in Ohio, and even served as interim president for a short time before he became vice president and chief medical officer of Carolina HealthCare Systems in Charlotte, N.C.
“Communications, public speaking, and change management was a very big component of what we were taught,” he added, “as well as performance improvement, which is where my career has drifted.”
Dr. Ruhlen said hospitalists are perfectly positioned for leadership roles in hospitals. He also said that “institutions led by physicians appear to have better performance metrics” and that physicians “owe it to our patients to try to take back medicine.”
One thing Dr. Ruhlen wished medical training would address better is population health, a topic he became “enamored” with in business school. “It’s a very interesting body of work, interesting body of language,” he said. “I think it will be critically important as we move into a realm of significant health reform.”
Path: Quality Expertise
Greg Maynard, MD, MSC, SFHM, took a different route to hospital leadership. He didn’t seek an advanced degree in business and hasn’t bounced from one job to another climbing the corporate ladder. Instead, he’s devoted his time and energy to quality improvement (QI) as clinical professor of medicine in the division of hospital medicine at the University of California at San Diego.
Dr. Maynard encouraged hospitalists to consider becoming experts in hospital quality and patient safety, a path that has led him to national recognition. “You won’t get bored,” he said. “Your career will find you.”
Dr. Maynard, who serves as senior vice president of SHM’s Center for Healthcare Improvement and Innovation, said every QI project “seems to have its time,” and he warned hospitalists that dealing with frustration is part of the job description.
“Some projects I initiated didn’t get off the ground, no matter why I did it,” he explained. “For example, transitions of care—when we started looking at it, nobody in the hospital cared. The administrators didn’t care about discharge summaries or teachback communication strategies. They only cared when readmissions came in focus.”
He and his team needed to learn to “satisfy ourselves” and change what they could change as hospitalists. The hard part, he said, was being patient.
“We knew it was coming; we just had to wait and not get rankled,” he added. “I had to learn to not take things personally. Be patient and wait for the opportunity.”
Dr. Maynard advised hospitalists to learn to “say no, or ‘I would love to but I just can’t.’” He also said hospitalists should not be afraid to ask for help.
Return on Investment
Dr. Guthrie, who received his MBA nearly 40 years ago, said times have changed and business schools have adapted to a new economic landscape. Opportunities for physicians to receive advanced training are much greater now, with physicians earning advanced degrees in public health and health administration, as well as MBAs.
Many of the top schools offer work-friendly course schedules, including night and weekend courses and plenty of online options. Some, such as the University of Massachusetts, offer a 100% online MBA program.
Still, he warned hospitalists to consider their goals along with the time, energy, and financial commitment that post-graduate work requires.
“The investment is huge,” he said.
It’s exactly what Dr. Catacutan is contemplating. Should he go back to school, pursue leadership within the walls of his hospital and executive courses like SHM’s Leadership Academy, or should he be satisfied as a full-time clinician?
“Is it really worth my time, especially with family and kids?” he asked rhetorically. “It’s a personal decision.” TH
Richard Quinn is a freelance writer in New Jersey.
Thadeo Catacutan, MD, sat in a ballroom at the Gaylord Resort & Convention Center in National Harbor, Md., on a sunny spring day at HM13, just thinking about his future after spending seven years as a hospitalist at Cleveland Clinic. He wondered if additional training in business administration would provide him with the advancement opportunities and professional fulfillment he sought.
“It’s been hard to determine what I really want to do,” he said. “After seven years, is this the peak? Are there other opportunities?
“I realize that if you are not going to specialize, you have to go into a leadership career track,” he said. “Some would say you can just be a clinician, but in the long term, I don’t think that is a sustainable path. You will just burn out.”
Dr. Catacutan isn’t the only one concerned. Nearly 200 physicians joined him for an HM13 breakout session titled “Career Tracks for Hospitalists.” The session provided attendees two viewpoints on career advancement: the executive pathway and the hospital leadership pathway. And although the session’s speakers demonstrated distinct routes to senior positions, there’s a shared path for each, including:
- Weighing choices carefully. The reasons for seeking a new career path are personal and not the same for everyone. Keep that in mind.
- Thinking about return on investment. Physicians should consider what they’re getting for their effort, whether that’s the cost of an advanced degree or the value of time spent volunteering on a hospital committee.
- Contemplating what you like about your job. When considering a career move, remember the adage that the grass is always greener.
“The way you get to take on leadership opportunities is by acceptance,” said session moderator Michael Guthrie, MD, MBA, executive in residence at the University of Colorado. “Say ‘yes.’ Say ‘I’m curious’ or ‘How can I make a contribution?’ You will stumble. You will make mistakes. You will be clumsy. … But it is that process that teaches you what you need to know.”
Path: Advanced Training
Michael Ruhlen, MD, MHCM, FACHE, SFHM, said he needed to know how to communicate better with the administrators he regularly met with to discuss his pediatric hospitalist program.
“I found that I wasn’t always speaking the same language when I talked to administrators about the value that hospitalists brought to their individual institutions,” he said. “No matter how hard I tried, I could always walk into a room with a master’s-prepared administrator who could absolutely prove to me that my hospitalist program would cost them far more money than it would ever be worth—and they simply could not afford it.”
To address what he calls a “knowledge deficit,” Dr. Ruhlen earned his master’s degree in health-care management from the Harvard School of Public Health in Boston. Armed with new business acumen, he rose to vice president of medical affairs at Toledo Children’s Hospital in Ohio, and even served as interim president for a short time before he became vice president and chief medical officer of Carolina HealthCare Systems in Charlotte, N.C.
“Communications, public speaking, and change management was a very big component of what we were taught,” he added, “as well as performance improvement, which is where my career has drifted.”
Dr. Ruhlen said hospitalists are perfectly positioned for leadership roles in hospitals. He also said that “institutions led by physicians appear to have better performance metrics” and that physicians “owe it to our patients to try to take back medicine.”
One thing Dr. Ruhlen wished medical training would address better is population health, a topic he became “enamored” with in business school. “It’s a very interesting body of work, interesting body of language,” he said. “I think it will be critically important as we move into a realm of significant health reform.”
Path: Quality Expertise
Greg Maynard, MD, MSC, SFHM, took a different route to hospital leadership. He didn’t seek an advanced degree in business and hasn’t bounced from one job to another climbing the corporate ladder. Instead, he’s devoted his time and energy to quality improvement (QI) as clinical professor of medicine in the division of hospital medicine at the University of California at San Diego.
Dr. Maynard encouraged hospitalists to consider becoming experts in hospital quality and patient safety, a path that has led him to national recognition. “You won’t get bored,” he said. “Your career will find you.”
Dr. Maynard, who serves as senior vice president of SHM’s Center for Healthcare Improvement and Innovation, said every QI project “seems to have its time,” and he warned hospitalists that dealing with frustration is part of the job description.
“Some projects I initiated didn’t get off the ground, no matter why I did it,” he explained. “For example, transitions of care—when we started looking at it, nobody in the hospital cared. The administrators didn’t care about discharge summaries or teachback communication strategies. They only cared when readmissions came in focus.”
He and his team needed to learn to “satisfy ourselves” and change what they could change as hospitalists. The hard part, he said, was being patient.
“We knew it was coming; we just had to wait and not get rankled,” he added. “I had to learn to not take things personally. Be patient and wait for the opportunity.”
Dr. Maynard advised hospitalists to learn to “say no, or ‘I would love to but I just can’t.’” He also said hospitalists should not be afraid to ask for help.
Return on Investment
Dr. Guthrie, who received his MBA nearly 40 years ago, said times have changed and business schools have adapted to a new economic landscape. Opportunities for physicians to receive advanced training are much greater now, with physicians earning advanced degrees in public health and health administration, as well as MBAs.
Many of the top schools offer work-friendly course schedules, including night and weekend courses and plenty of online options. Some, such as the University of Massachusetts, offer a 100% online MBA program.
Still, he warned hospitalists to consider their goals along with the time, energy, and financial commitment that post-graduate work requires.
“The investment is huge,” he said.
It’s exactly what Dr. Catacutan is contemplating. Should he go back to school, pursue leadership within the walls of his hospital and executive courses like SHM’s Leadership Academy, or should he be satisfied as a full-time clinician?
“Is it really worth my time, especially with family and kids?” he asked rhetorically. “It’s a personal decision.” TH
Richard Quinn is a freelance writer in New Jersey.
Thadeo Catacutan, MD, sat in a ballroom at the Gaylord Resort & Convention Center in National Harbor, Md., on a sunny spring day at HM13, just thinking about his future after spending seven years as a hospitalist at Cleveland Clinic. He wondered if additional training in business administration would provide him with the advancement opportunities and professional fulfillment he sought.
“It’s been hard to determine what I really want to do,” he said. “After seven years, is this the peak? Are there other opportunities?
“I realize that if you are not going to specialize, you have to go into a leadership career track,” he said. “Some would say you can just be a clinician, but in the long term, I don’t think that is a sustainable path. You will just burn out.”
Dr. Catacutan isn’t the only one concerned. Nearly 200 physicians joined him for an HM13 breakout session titled “Career Tracks for Hospitalists.” The session provided attendees two viewpoints on career advancement: the executive pathway and the hospital leadership pathway. And although the session’s speakers demonstrated distinct routes to senior positions, there’s a shared path for each, including:
- Weighing choices carefully. The reasons for seeking a new career path are personal and not the same for everyone. Keep that in mind.
- Thinking about return on investment. Physicians should consider what they’re getting for their effort, whether that’s the cost of an advanced degree or the value of time spent volunteering on a hospital committee.
- Contemplating what you like about your job. When considering a career move, remember the adage that the grass is always greener.
“The way you get to take on leadership opportunities is by acceptance,” said session moderator Michael Guthrie, MD, MBA, executive in residence at the University of Colorado. “Say ‘yes.’ Say ‘I’m curious’ or ‘How can I make a contribution?’ You will stumble. You will make mistakes. You will be clumsy. … But it is that process that teaches you what you need to know.”
Path: Advanced Training
Michael Ruhlen, MD, MHCM, FACHE, SFHM, said he needed to know how to communicate better with the administrators he regularly met with to discuss his pediatric hospitalist program.
“I found that I wasn’t always speaking the same language when I talked to administrators about the value that hospitalists brought to their individual institutions,” he said. “No matter how hard I tried, I could always walk into a room with a master’s-prepared administrator who could absolutely prove to me that my hospitalist program would cost them far more money than it would ever be worth—and they simply could not afford it.”
To address what he calls a “knowledge deficit,” Dr. Ruhlen earned his master’s degree in health-care management from the Harvard School of Public Health in Boston. Armed with new business acumen, he rose to vice president of medical affairs at Toledo Children’s Hospital in Ohio, and even served as interim president for a short time before he became vice president and chief medical officer of Carolina HealthCare Systems in Charlotte, N.C.
“Communications, public speaking, and change management was a very big component of what we were taught,” he added, “as well as performance improvement, which is where my career has drifted.”
Dr. Ruhlen said hospitalists are perfectly positioned for leadership roles in hospitals. He also said that “institutions led by physicians appear to have better performance metrics” and that physicians “owe it to our patients to try to take back medicine.”
One thing Dr. Ruhlen wished medical training would address better is population health, a topic he became “enamored” with in business school. “It’s a very interesting body of work, interesting body of language,” he said. “I think it will be critically important as we move into a realm of significant health reform.”
Path: Quality Expertise
Greg Maynard, MD, MSC, SFHM, took a different route to hospital leadership. He didn’t seek an advanced degree in business and hasn’t bounced from one job to another climbing the corporate ladder. Instead, he’s devoted his time and energy to quality improvement (QI) as clinical professor of medicine in the division of hospital medicine at the University of California at San Diego.
Dr. Maynard encouraged hospitalists to consider becoming experts in hospital quality and patient safety, a path that has led him to national recognition. “You won’t get bored,” he said. “Your career will find you.”
Dr. Maynard, who serves as senior vice president of SHM’s Center for Healthcare Improvement and Innovation, said every QI project “seems to have its time,” and he warned hospitalists that dealing with frustration is part of the job description.
“Some projects I initiated didn’t get off the ground, no matter why I did it,” he explained. “For example, transitions of care—when we started looking at it, nobody in the hospital cared. The administrators didn’t care about discharge summaries or teachback communication strategies. They only cared when readmissions came in focus.”
He and his team needed to learn to “satisfy ourselves” and change what they could change as hospitalists. The hard part, he said, was being patient.
“We knew it was coming; we just had to wait and not get rankled,” he added. “I had to learn to not take things personally. Be patient and wait for the opportunity.”
Dr. Maynard advised hospitalists to learn to “say no, or ‘I would love to but I just can’t.’” He also said hospitalists should not be afraid to ask for help.
Return on Investment
Dr. Guthrie, who received his MBA nearly 40 years ago, said times have changed and business schools have adapted to a new economic landscape. Opportunities for physicians to receive advanced training are much greater now, with physicians earning advanced degrees in public health and health administration, as well as MBAs.
Many of the top schools offer work-friendly course schedules, including night and weekend courses and plenty of online options. Some, such as the University of Massachusetts, offer a 100% online MBA program.
Still, he warned hospitalists to consider their goals along with the time, energy, and financial commitment that post-graduate work requires.
“The investment is huge,” he said.
It’s exactly what Dr. Catacutan is contemplating. Should he go back to school, pursue leadership within the walls of his hospital and executive courses like SHM’s Leadership Academy, or should he be satisfied as a full-time clinician?
“Is it really worth my time, especially with family and kids?” he asked rhetorically. “It’s a personal decision.” TH
Richard Quinn is a freelance writer in New Jersey.
No survival benefit for routine surveillance scans in classical Hodgkin disease
CHICAGO – Routine surveillance imaging does not improve clinical outcomes in patients with classic Hodgkin disease who are in first complete remission and it sharply increases costs, researchers reported at the annual meeting of the American Society of Clinical Oncology.
The team, led by Dr. Sai Ravi Pingali, retrospectively reviewed the charts of 241 adult patients who achieved a complete remission after first-line therapy.
In 68%, the treating physicians’ planned approach was routine surveillance imaging, which consisted of radiologic imaging with scans every few months, plus clinical exams and laboratory testing. In the other 32%, the planned approach was clinical surveillance, meaning that radiologic imaging was performed only if concerning signs or symptoms occurred.
The two groups had statistically indistinguishable overall survival, and in both groups, all patients experiencing relapse successfully achieved a second complete remission with salvage therapy.
In the routinely imaged group, scanning increased costs by nearly $20,000 per patient and by almost $600,000 per each relapse detected. In addition, patients were exposed to the associated radiation.
"We were unable to detect an overall survival benefit associated with routine surveillance imaging, although I have to acknowledge that our study was limited in power given the small number of deaths and relapses," commented Dr. Pingali, an oncologist with the Medical College of Wisconsin Affiliated Hospitals in Milwaukee.
"Relapses in both ... groups were effectively salvaged with autologous stem cell transplantation, arguing against a critical advantage of detection of asymptomatic relapse. Also, we need to keep in mind that the costs associated with routine surveillance imaging are significant, and it is also associated with potential risks, both in terms of radiation exposure and unnecessary work-up," he added.
"We do not feel that potential risks and costs without overall survival benefit or any other clinical benefit justify the practice of routine surveillance imaging in classical Hodgkin lymphoma patients who have achieved a complete remission after first-line therapy. We recommend that such patients be followed clinically," Dr. Pingali concluded.
Invited discussant Dr. Leo Gordon of Northwestern University in Chicago, agreed that accumulating data argue against routine imaging for surveillance in this context and noted that insurers will likely not continue to cover scans having no proven benefit. The data should prompt a revision of guidelines and reeducation of clinicians and patients, he said.
"For translational researchers and investigators and academics, I think we need to convince journal reviewers that a manuscript is acceptable if scans are not so frequent. And for industry trials, I think we need to discuss with the Food and Drug Administration the endpoint of progression-free survival and that those endpoints may not only be driven by scans but by more mundane parameters," he said—namely, the history and physical examination.
But session comoderator Dr. Gilles A. Salles of Hospices Civils de Lyon, Université Claude Bernard, France, expressed reservations, noting that the study did not provide information on how patients were allocated to groups and the time frame of relapse.
"It may be different whether relapses occur early, in the first year, or they occur later, and that may have some implications for the surveillance," he said. "I understand that you and many others jumped over the idea that we should immediately stop. A few people may think that we need more solid data, despite the provocative and quality data that were presented, to really make this jump in clinical practice. That’s my personal opinion."
Dr. Pingali and his team retrospectively reviewed the charts of adult patients who received a new diagnosis of classical Hodgkin lymphoma between 2000 and 2010 at three institutions, achieved complete remission after first-line therapy and had at least 2 years of follow-up.
The routine surveillance imaging and clinical surveillance groups had similar demographic and disease characteristics, Dr. Pingali reported. The former were significantly more likely to have received ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, and dacarbazine) and less likely to have received the Stanford V regimen as first-line therapy, and they were significantly less likely to have received radiation therapy.
With a median duration of follow-up of about 4 years, the groups did not differ significantly with respect to overall survival. "When we look at the 5-year time point, when typically the surveillance CT scans are discontinued, the curves are pretty much superimposable," he pointed out.
There were five deaths in the routine surveillance imaging group, one of which was from relapsed disease; the other deaths were from cancer, heart failure, hip fracture, and myelodysplastic syndrome. There were four deaths in the clinical surveillance group: two were from non-Hodgkin lymphoma and two from unknown causes while the patient was in confirmed remission.
All of the six patients in the routinely imaged group and all of the five patients in the clinically followed group experiencing a relapse achieved another complete remission with second-line therapy.
The mean number of scans received was 1.14 in the clinical surveillance group – usually the scan performed after first-line treatment to confirm remission, according to Dr. Pingali – and 4.25 in the routine surveillance imaging group. The ratio of scans to detected relapses was 18 vs. 124.
The extra charges incurred from scans using the routine surveillance imaging approach were $18,896/patient and $593,698/relapse.
"It is important to note that this does not include additional costs from the work-up of the false-positive scans and also the wages lost," he noted.
Dr. Pingali disclosed no relevant conflicts of interest. Dr. Gordon disclosed that he receives honoraria from Genentech and research funding from Millennium and Pharmacyclics. Dr. Salles disclosed serving as an advisor or consultant for Calistoga Pharmaceuticals, Celgene; Genentech, Janssen Pharmaceutica, and Roche. He has served as a speaker or a member of a speakers bureau and has received grants for clinical research from Celgene and Roche.
CHICAGO – Routine surveillance imaging does not improve clinical outcomes in patients with classic Hodgkin disease who are in first complete remission and it sharply increases costs, researchers reported at the annual meeting of the American Society of Clinical Oncology.
The team, led by Dr. Sai Ravi Pingali, retrospectively reviewed the charts of 241 adult patients who achieved a complete remission after first-line therapy.
In 68%, the treating physicians’ planned approach was routine surveillance imaging, which consisted of radiologic imaging with scans every few months, plus clinical exams and laboratory testing. In the other 32%, the planned approach was clinical surveillance, meaning that radiologic imaging was performed only if concerning signs or symptoms occurred.
The two groups had statistically indistinguishable overall survival, and in both groups, all patients experiencing relapse successfully achieved a second complete remission with salvage therapy.
In the routinely imaged group, scanning increased costs by nearly $20,000 per patient and by almost $600,000 per each relapse detected. In addition, patients were exposed to the associated radiation.
"We were unable to detect an overall survival benefit associated with routine surveillance imaging, although I have to acknowledge that our study was limited in power given the small number of deaths and relapses," commented Dr. Pingali, an oncologist with the Medical College of Wisconsin Affiliated Hospitals in Milwaukee.
"Relapses in both ... groups were effectively salvaged with autologous stem cell transplantation, arguing against a critical advantage of detection of asymptomatic relapse. Also, we need to keep in mind that the costs associated with routine surveillance imaging are significant, and it is also associated with potential risks, both in terms of radiation exposure and unnecessary work-up," he added.
"We do not feel that potential risks and costs without overall survival benefit or any other clinical benefit justify the practice of routine surveillance imaging in classical Hodgkin lymphoma patients who have achieved a complete remission after first-line therapy. We recommend that such patients be followed clinically," Dr. Pingali concluded.
Invited discussant Dr. Leo Gordon of Northwestern University in Chicago, agreed that accumulating data argue against routine imaging for surveillance in this context and noted that insurers will likely not continue to cover scans having no proven benefit. The data should prompt a revision of guidelines and reeducation of clinicians and patients, he said.
"For translational researchers and investigators and academics, I think we need to convince journal reviewers that a manuscript is acceptable if scans are not so frequent. And for industry trials, I think we need to discuss with the Food and Drug Administration the endpoint of progression-free survival and that those endpoints may not only be driven by scans but by more mundane parameters," he said—namely, the history and physical examination.
But session comoderator Dr. Gilles A. Salles of Hospices Civils de Lyon, Université Claude Bernard, France, expressed reservations, noting that the study did not provide information on how patients were allocated to groups and the time frame of relapse.
"It may be different whether relapses occur early, in the first year, or they occur later, and that may have some implications for the surveillance," he said. "I understand that you and many others jumped over the idea that we should immediately stop. A few people may think that we need more solid data, despite the provocative and quality data that were presented, to really make this jump in clinical practice. That’s my personal opinion."
Dr. Pingali and his team retrospectively reviewed the charts of adult patients who received a new diagnosis of classical Hodgkin lymphoma between 2000 and 2010 at three institutions, achieved complete remission after first-line therapy and had at least 2 years of follow-up.
The routine surveillance imaging and clinical surveillance groups had similar demographic and disease characteristics, Dr. Pingali reported. The former were significantly more likely to have received ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, and dacarbazine) and less likely to have received the Stanford V regimen as first-line therapy, and they were significantly less likely to have received radiation therapy.
With a median duration of follow-up of about 4 years, the groups did not differ significantly with respect to overall survival. "When we look at the 5-year time point, when typically the surveillance CT scans are discontinued, the curves are pretty much superimposable," he pointed out.
There were five deaths in the routine surveillance imaging group, one of which was from relapsed disease; the other deaths were from cancer, heart failure, hip fracture, and myelodysplastic syndrome. There were four deaths in the clinical surveillance group: two were from non-Hodgkin lymphoma and two from unknown causes while the patient was in confirmed remission.
All of the six patients in the routinely imaged group and all of the five patients in the clinically followed group experiencing a relapse achieved another complete remission with second-line therapy.
The mean number of scans received was 1.14 in the clinical surveillance group – usually the scan performed after first-line treatment to confirm remission, according to Dr. Pingali – and 4.25 in the routine surveillance imaging group. The ratio of scans to detected relapses was 18 vs. 124.
The extra charges incurred from scans using the routine surveillance imaging approach were $18,896/patient and $593,698/relapse.
"It is important to note that this does not include additional costs from the work-up of the false-positive scans and also the wages lost," he noted.
Dr. Pingali disclosed no relevant conflicts of interest. Dr. Gordon disclosed that he receives honoraria from Genentech and research funding from Millennium and Pharmacyclics. Dr. Salles disclosed serving as an advisor or consultant for Calistoga Pharmaceuticals, Celgene; Genentech, Janssen Pharmaceutica, and Roche. He has served as a speaker or a member of a speakers bureau and has received grants for clinical research from Celgene and Roche.
CHICAGO – Routine surveillance imaging does not improve clinical outcomes in patients with classic Hodgkin disease who are in first complete remission and it sharply increases costs, researchers reported at the annual meeting of the American Society of Clinical Oncology.
The team, led by Dr. Sai Ravi Pingali, retrospectively reviewed the charts of 241 adult patients who achieved a complete remission after first-line therapy.
In 68%, the treating physicians’ planned approach was routine surveillance imaging, which consisted of radiologic imaging with scans every few months, plus clinical exams and laboratory testing. In the other 32%, the planned approach was clinical surveillance, meaning that radiologic imaging was performed only if concerning signs or symptoms occurred.
The two groups had statistically indistinguishable overall survival, and in both groups, all patients experiencing relapse successfully achieved a second complete remission with salvage therapy.
In the routinely imaged group, scanning increased costs by nearly $20,000 per patient and by almost $600,000 per each relapse detected. In addition, patients were exposed to the associated radiation.
"We were unable to detect an overall survival benefit associated with routine surveillance imaging, although I have to acknowledge that our study was limited in power given the small number of deaths and relapses," commented Dr. Pingali, an oncologist with the Medical College of Wisconsin Affiliated Hospitals in Milwaukee.
"Relapses in both ... groups were effectively salvaged with autologous stem cell transplantation, arguing against a critical advantage of detection of asymptomatic relapse. Also, we need to keep in mind that the costs associated with routine surveillance imaging are significant, and it is also associated with potential risks, both in terms of radiation exposure and unnecessary work-up," he added.
"We do not feel that potential risks and costs without overall survival benefit or any other clinical benefit justify the practice of routine surveillance imaging in classical Hodgkin lymphoma patients who have achieved a complete remission after first-line therapy. We recommend that such patients be followed clinically," Dr. Pingali concluded.
Invited discussant Dr. Leo Gordon of Northwestern University in Chicago, agreed that accumulating data argue against routine imaging for surveillance in this context and noted that insurers will likely not continue to cover scans having no proven benefit. The data should prompt a revision of guidelines and reeducation of clinicians and patients, he said.
"For translational researchers and investigators and academics, I think we need to convince journal reviewers that a manuscript is acceptable if scans are not so frequent. And for industry trials, I think we need to discuss with the Food and Drug Administration the endpoint of progression-free survival and that those endpoints may not only be driven by scans but by more mundane parameters," he said—namely, the history and physical examination.
But session comoderator Dr. Gilles A. Salles of Hospices Civils de Lyon, Université Claude Bernard, France, expressed reservations, noting that the study did not provide information on how patients were allocated to groups and the time frame of relapse.
"It may be different whether relapses occur early, in the first year, or they occur later, and that may have some implications for the surveillance," he said. "I understand that you and many others jumped over the idea that we should immediately stop. A few people may think that we need more solid data, despite the provocative and quality data that were presented, to really make this jump in clinical practice. That’s my personal opinion."
Dr. Pingali and his team retrospectively reviewed the charts of adult patients who received a new diagnosis of classical Hodgkin lymphoma between 2000 and 2010 at three institutions, achieved complete remission after first-line therapy and had at least 2 years of follow-up.
The routine surveillance imaging and clinical surveillance groups had similar demographic and disease characteristics, Dr. Pingali reported. The former were significantly more likely to have received ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, and dacarbazine) and less likely to have received the Stanford V regimen as first-line therapy, and they were significantly less likely to have received radiation therapy.
With a median duration of follow-up of about 4 years, the groups did not differ significantly with respect to overall survival. "When we look at the 5-year time point, when typically the surveillance CT scans are discontinued, the curves are pretty much superimposable," he pointed out.
There were five deaths in the routine surveillance imaging group, one of which was from relapsed disease; the other deaths were from cancer, heart failure, hip fracture, and myelodysplastic syndrome. There were four deaths in the clinical surveillance group: two were from non-Hodgkin lymphoma and two from unknown causes while the patient was in confirmed remission.
All of the six patients in the routinely imaged group and all of the five patients in the clinically followed group experiencing a relapse achieved another complete remission with second-line therapy.
The mean number of scans received was 1.14 in the clinical surveillance group – usually the scan performed after first-line treatment to confirm remission, according to Dr. Pingali – and 4.25 in the routine surveillance imaging group. The ratio of scans to detected relapses was 18 vs. 124.
The extra charges incurred from scans using the routine surveillance imaging approach were $18,896/patient and $593,698/relapse.
"It is important to note that this does not include additional costs from the work-up of the false-positive scans and also the wages lost," he noted.
Dr. Pingali disclosed no relevant conflicts of interest. Dr. Gordon disclosed that he receives honoraria from Genentech and research funding from Millennium and Pharmacyclics. Dr. Salles disclosed serving as an advisor or consultant for Calistoga Pharmaceuticals, Celgene; Genentech, Janssen Pharmaceutica, and Roche. He has served as a speaker or a member of a speakers bureau and has received grants for clinical research from Celgene and Roche.
AT THE ASCO ANNUAL MEETING 2013
Major finding: The ratio of scans to detected relapses was 18 for the clinical surveillance group and 124 for the routine scan group. The extra charges incurred from scans using the routine surveillance imaging approach were $18,896/patient and $593,698/relapse.
Data source: A retrospective analysis of 241 patients with classical Hodgkin lymphoma in first complete remission
Disclosures: Dr. Pingali disclosed no relevant conflicts of interest. Dr. Gordon disclosed that he receives honoraria from Genentech and research funding from Millennium and Pharmacyclics. Dr. Salles disclosed serving as an advisor or consultant for Calistoga Pharmaceuticals, Celgene; Genentech, Janssen Pharmaceutica, and Roche. He has served as a speaker or a member of a speakers bureau and has received grants for clinical research from Celgene and Roche.
Fusion protein controls surgery-related bleeding in hemophilia B
AMSTERDAM—A recombinant factor IX Fc fusion protein (rFIXFc) can control bleeding among hemophilia B patients undergoing major surgery, according to data from the B-LONG study presented at ISTH 2013.
The goal of the phase 3 B-LONG study was to evaluate the safety, efficacy, and pharmacokinetics (PK) of rFIXFc among male patients with hemophilia B.
Previously released data from the study suggested rFIXFc can safely prevent bleeding in these patients, and the product stays in the body more than twice as long as the recombinant factor IX therapy BeneFIX.
At ISTH 2013, Jerry Powell, MD, of the University of California at Davis, presented an analysis of B-LONG data that demonstrated rFIXFc’s effects among hemophilia B patients who underwent major surgery (e-Poster PA 2.07-4).
The B-LONG study was sponsored by Biogen Idec and Sobi, the companies developing rFIXFc (also known as eftrenonacog alfa) as Alprolix.
The study included 123 male subjects with severe hemophilia B (≤2 IU/dL [2%] endogenous FIX). Patients were 12 years of age or older They had no current or previous FIX inhibitors and a history of 100 or more documented prior exposure days to FIX products.
Patients received rFIXFc in 1 of 4 treatment arms:
- Weekly prophylaxis starting at 50 IU/kg, with PK-driven dose adjustments (n=63)
- Individualized interval prophylaxis starting at 100 IU/kg every 10 days, with PK-driven interval adjustments (n=29)
- On-demand treatment at 20 IU/kg to 100 IU/kg (n=27)
- Perioperative management (n=12, including 8 from arms 1-3).
The patients who required major surgery were placed in arm 4. Investigators and surgeons decided upon treatment for these patients based on considerations of their rFIXFc PK profile, the type of planned surgery, and the patients’ clinical status.
The 12 patients underwent a total of 14 major surgeries, including arthroscopic meniscectomy of knee (n=1), arthroscopic ankle fusion (n=1), knee replacements (n=5), and other (n=7).
The investigators/surgeons rated hemostasis as “excellent” in 13 of the surgeries and “good” for 1 procedure.
The median estimated blood loss was 65.5 mL (range, 0.0 to 300.0 mL) during surgery and 0.0 mL (range, 0.0 to 500 mL) after surgery. None of the patients required blood transfusions during surgery, but 2 patients received transfusions postoperatively.
In most of the surgeries—85.7%—patients required a single injection of rFIXFc to maintain hemostasis during the operation. The median dose was 90.9 IU/kg per injection.
Most patients required 1 to 2 injections of rFIXFc the day before and the day of surgery. And most patients required 2 to 3 injections from days 1 to 3 after surgery. So the median rFIXFc consumption was 146.1 IU/kg on the day of surgery, 164.6 IU/kg from days 1 to 3 after surgery, and 277.1 IU/kg for days 4 to 14 after surgery.
A majority of patients—83.3% (10/12)—experienced 1 or more adverse events related to treatment. Three patients experienced 6 adverse events, but these were resolved, and investigators said they were unrelated to rFIXFc treatment.
Additional analyses of B-LONG data were presented at ISTH 2013, including an analysis of rFIXFc in the treatment of bleeding episodes and a PK analysis of rFIXFc. ![]()
AMSTERDAM—A recombinant factor IX Fc fusion protein (rFIXFc) can control bleeding among hemophilia B patients undergoing major surgery, according to data from the B-LONG study presented at ISTH 2013.
The goal of the phase 3 B-LONG study was to evaluate the safety, efficacy, and pharmacokinetics (PK) of rFIXFc among male patients with hemophilia B.
Previously released data from the study suggested rFIXFc can safely prevent bleeding in these patients, and the product stays in the body more than twice as long as the recombinant factor IX therapy BeneFIX.
At ISTH 2013, Jerry Powell, MD, of the University of California at Davis, presented an analysis of B-LONG data that demonstrated rFIXFc’s effects among hemophilia B patients who underwent major surgery (e-Poster PA 2.07-4).
The B-LONG study was sponsored by Biogen Idec and Sobi, the companies developing rFIXFc (also known as eftrenonacog alfa) as Alprolix.
The study included 123 male subjects with severe hemophilia B (≤2 IU/dL [2%] endogenous FIX). Patients were 12 years of age or older They had no current or previous FIX inhibitors and a history of 100 or more documented prior exposure days to FIX products.
Patients received rFIXFc in 1 of 4 treatment arms:
- Weekly prophylaxis starting at 50 IU/kg, with PK-driven dose adjustments (n=63)
- Individualized interval prophylaxis starting at 100 IU/kg every 10 days, with PK-driven interval adjustments (n=29)
- On-demand treatment at 20 IU/kg to 100 IU/kg (n=27)
- Perioperative management (n=12, including 8 from arms 1-3).
The patients who required major surgery were placed in arm 4. Investigators and surgeons decided upon treatment for these patients based on considerations of their rFIXFc PK profile, the type of planned surgery, and the patients’ clinical status.
The 12 patients underwent a total of 14 major surgeries, including arthroscopic meniscectomy of knee (n=1), arthroscopic ankle fusion (n=1), knee replacements (n=5), and other (n=7).
The investigators/surgeons rated hemostasis as “excellent” in 13 of the surgeries and “good” for 1 procedure.
The median estimated blood loss was 65.5 mL (range, 0.0 to 300.0 mL) during surgery and 0.0 mL (range, 0.0 to 500 mL) after surgery. None of the patients required blood transfusions during surgery, but 2 patients received transfusions postoperatively.
In most of the surgeries—85.7%—patients required a single injection of rFIXFc to maintain hemostasis during the operation. The median dose was 90.9 IU/kg per injection.
Most patients required 1 to 2 injections of rFIXFc the day before and the day of surgery. And most patients required 2 to 3 injections from days 1 to 3 after surgery. So the median rFIXFc consumption was 146.1 IU/kg on the day of surgery, 164.6 IU/kg from days 1 to 3 after surgery, and 277.1 IU/kg for days 4 to 14 after surgery.
A majority of patients—83.3% (10/12)—experienced 1 or more adverse events related to treatment. Three patients experienced 6 adverse events, but these were resolved, and investigators said they were unrelated to rFIXFc treatment.
Additional analyses of B-LONG data were presented at ISTH 2013, including an analysis of rFIXFc in the treatment of bleeding episodes and a PK analysis of rFIXFc. ![]()
AMSTERDAM—A recombinant factor IX Fc fusion protein (rFIXFc) can control bleeding among hemophilia B patients undergoing major surgery, according to data from the B-LONG study presented at ISTH 2013.
The goal of the phase 3 B-LONG study was to evaluate the safety, efficacy, and pharmacokinetics (PK) of rFIXFc among male patients with hemophilia B.
Previously released data from the study suggested rFIXFc can safely prevent bleeding in these patients, and the product stays in the body more than twice as long as the recombinant factor IX therapy BeneFIX.
At ISTH 2013, Jerry Powell, MD, of the University of California at Davis, presented an analysis of B-LONG data that demonstrated rFIXFc’s effects among hemophilia B patients who underwent major surgery (e-Poster PA 2.07-4).
The B-LONG study was sponsored by Biogen Idec and Sobi, the companies developing rFIXFc (also known as eftrenonacog alfa) as Alprolix.
The study included 123 male subjects with severe hemophilia B (≤2 IU/dL [2%] endogenous FIX). Patients were 12 years of age or older They had no current or previous FIX inhibitors and a history of 100 or more documented prior exposure days to FIX products.
Patients received rFIXFc in 1 of 4 treatment arms:
- Weekly prophylaxis starting at 50 IU/kg, with PK-driven dose adjustments (n=63)
- Individualized interval prophylaxis starting at 100 IU/kg every 10 days, with PK-driven interval adjustments (n=29)
- On-demand treatment at 20 IU/kg to 100 IU/kg (n=27)
- Perioperative management (n=12, including 8 from arms 1-3).
The patients who required major surgery were placed in arm 4. Investigators and surgeons decided upon treatment for these patients based on considerations of their rFIXFc PK profile, the type of planned surgery, and the patients’ clinical status.
The 12 patients underwent a total of 14 major surgeries, including arthroscopic meniscectomy of knee (n=1), arthroscopic ankle fusion (n=1), knee replacements (n=5), and other (n=7).
The investigators/surgeons rated hemostasis as “excellent” in 13 of the surgeries and “good” for 1 procedure.
The median estimated blood loss was 65.5 mL (range, 0.0 to 300.0 mL) during surgery and 0.0 mL (range, 0.0 to 500 mL) after surgery. None of the patients required blood transfusions during surgery, but 2 patients received transfusions postoperatively.
In most of the surgeries—85.7%—patients required a single injection of rFIXFc to maintain hemostasis during the operation. The median dose was 90.9 IU/kg per injection.
Most patients required 1 to 2 injections of rFIXFc the day before and the day of surgery. And most patients required 2 to 3 injections from days 1 to 3 after surgery. So the median rFIXFc consumption was 146.1 IU/kg on the day of surgery, 164.6 IU/kg from days 1 to 3 after surgery, and 277.1 IU/kg for days 4 to 14 after surgery.
A majority of patients—83.3% (10/12)—experienced 1 or more adverse events related to treatment. Three patients experienced 6 adverse events, but these were resolved, and investigators said they were unrelated to rFIXFc treatment.
Additional analyses of B-LONG data were presented at ISTH 2013, including an analysis of rFIXFc in the treatment of bleeding episodes and a PK analysis of rFIXFc. ![]()