User login
Choosing Wisely in Hospital Medicine
The overuse of medical tests and treatments is a growing concern. A recent survey revealed that 2 in 5 primary care physicians perceive that patients in their own practice are receiving too much care.[1] Twenty‐eight percent of the physicians indicated they provide more care than they should. When queried about reasons for the aggressiveness of care, responses included fear of malpractice litigation, adherence to clinical performance measures that require following protocols, and inadequacy of time spent with patients. Overutilization of healthcare resources is a complex issue promulgated not only by the factors cited by the physicians but also a culture in the United States habituated to believe more care is better care.[2, 3, 4] In 2010, $2.6 trillion was spent on healthcare, an increase of $1.3 trillion between 2000 and 2010.5 As much as 30% of healthcare spending may be wasted.[6] Because physicians influence approximately 80% of healthcare expenditures, including ordering tests and treatments, it is imperative that physicians take a leadership role in reversing this trend.[7]
In response to this need, several physician‐led projects have emerged.[8, 9, 10] One such initiative is the American Board of Internal Medicine Foundation's (ABIM‐F's) Choosing Wisely campaign.[11] The ABIM‐F contacted a variety of specialty societies and asked each to identify the 5 top tests or treatments relevant to their specialty that may frequently be overused. Phase 1 of the Choosing Wisely campaign was launched in April 2012 with 9 specialty societies participating. The second phase was unveiled in February 2013 and comprised of 16 additional groups including the Society of Hospital Medicine (SHM). The SHM represents 35,000 hospitalists in the United States whose primary focus is the general medical care of hospitalized patients. This is especially important because almost one‐third of total US healthcare expenditures are on hospital care,[12] and hospitalists care for an increasing number of hospitalized patients.[13] In this article, we describe the used to derive the adult hospital medicine Choosing processes Wisely list, review the tests and treatments that the SHM's Choosing Wisely Subcommittee chose, and discuss potential next steps in implementation of the adult hospital medicine recommendations.
METHODOLOGY
Upon invitation to participate in the Choosing Wisely campaign, SHM's Hospital Quality and Patient Safety (HQPS) Committee formally convened the Choosing Wisely Subcommittee. The subcommittee identified and executed a methodology (see Supporting Figure 1 and Supporting Table 1 in the online version of this article) to create the list of 5 tests and treatments that the SHM submitted to the ABIM‐F. All subcommittee members participated fully in the voting and refinement process. The Choosing Wisely Subcommittee worked closely with the SHM's Pediatrics Choosing Wisely Subcommittee to develop both adult and pediatric lists.
Convening the Choosing Wisely Subcommittee
The HQPS Committee convened a subcommittee consisting of 9 members. The subcommittee represented a diverse group of hospitalists reflecting different institution types, geographic regions, and experience. All Choosing Wisely Subcommittee members signed conflict of interest statements and reported no conflict related to the conclusions, implications, or opinions stated. The subcommittee did not consult other external stakeholders in the development of recommendations.
Identification and Refinement of Potential Wasteful Practices
To generate an initial list of potential recommendations, members of all of the SHM committees were surveyed and asked to submit 5 tests and treatments that are inappropriately used or overused. SHM staff removed duplicates and categorized submissions by topic, highlighting overlapping recommendations. Tests and treatments that are used infrequently and items included in phase 1 society lists were also excluded. Subcommittee members then ranked the resultant list using a 5‐point Likert scale. All SHM members were then given the opportunity to rank their agreement with the tests and treatments on the list, as refined at the time based upon their own experience and consideration of the following criteria: tests and procedures within the control and purview of hospital medicine, the frequency with which the tests or procedures occur, and the significance of associated costs. This was accomplished via electronic survey.
Establishing an Evidence Base
SHM staff conducted a literature review of the list of tests and treatments that was further refined by the SHM membership's ranking using a standard template. Two reviewers (W.N. and J.G.) conducted an independent literature review of the remaining tests and treatments using PubMed, MEDLINE, and Cochrane Library. The reviewers also conducted generic Internet searches. The literature review included all literature published through 2012 as well as nonEnglish language publications. The reviewers included clinical research guidelines and primary and secondary research studies. Studies included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, reviewed the harm associated with the administration of a test or treatment, and explored the cost associated with the test or treatment as well as the overall strength of evidence. Additionally, the reference lists included in articles were reviewed to identify supplementary literature sources. The reviewers read and analyzed the articles identified in the initial search for relevant subject matter and summarized the findings in a table.
Delphi Panels
A Delphi scoring process was utilized to complete list refinement.[14] Subcommittee members anonymously voted via email for the strength of the test and treatment recommendation based upon specific criteria. To assist with this process, they received a copy of the completed literature review and an evidence summary of the literature. The following categories were used to guide the scoring: validity/evidence base to support, feasibility of implementation, frequency of occurrence, cost of occurrence, yield/emmpact, harm, and potential to improve. Results were aggregated and shared with the Choosing Wisely Subcommittee. The subcommittee conferred a final time, editing the recommendations for clarification and improved wording. A second anonymous vote was then conducted for the remaining tests and treatments through a revised scoring spreadsheet. The penultimate list was presented to the SHM's Board. Upon the Board's approval, the final list was submitted to the ABIM‐F.
RESULTS
The results of each stage of the list development process are shown in the online supporting information (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). The initial survey of SHM committee members garnered in excess of 150 tests and treatments from approximately 40 SHM committee members. The subsequent list refinement by SHM staff narrowed this list to 65 items, which were then further reduced to 15 items after ranking by members of the subcommittee (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). Voting by members of the general SHM membership further reduced the list to 11 tests and treatments.
The final list of 5 tests and treatments submitted to the ABIM‐F were:
- Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
- Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal (GI) complications.
- Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
- Do not order continuous telemetry monitoring outside of the intensive care unit (ICU) without using a protocol that governs continuation.
- Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability (Table 1).
|
| Test/Treatment Recommendations |
| Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis).[21, 50] |
| Do not prescribe GI prophylaxis to medical inpatients without clear‐cut indication or high risk for GI complication.[24] |
| Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms.[29, 51] |
| Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes.[35, 43, 52, 53] |
| Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.[44, 54, 55] |
RECOMMENDATIONS
Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
Despite guidelines identifying appropriate indications for the placement of urinary catheters, urinary tract infections due to catheter use remain the most frequent type of infection in acute care settings. Nearly 1 in every 5 patients in the hospital receives an indwelling catheter, and up to half are placed inappropriately.[15] Twenty‐six percent of patients who have indwelling catheters for 2 to 10 days will develop bacteriuria; subsequently, 24% of those patients will develop a catheter‐associated urinary tract infection (CAUTI).[15] More than 13,000 deaths due to CAUTI occur annually.[16] In addition to urinary tract infections and their complications, additional adverse outcomes related to indwelling catheters include formation of encrustations and restrictions to flow, prolonged hospital stay, and exposure to multidrug resistant organisms due to increased use of antibiotics. Evidence suggests that infections due to catheters are frequently preventable.[17, 18]
The economic burden associated with indwelling catheter complications is also substantial. Each episode of symptomatic urinary tract infection adds $676 in incremental costs, and catheter‐related bacteremia costs at least $2836.15 According to Scott, nearly 450,000 CAUTIs were estimated to have occurred in 2007, resulting in direct medical costs of between $340 to $370 million.[19]
Several organizations simultaneously released guidelines to provide a roadmap for appropriate catheter use and prevention of CAUTIs.[20, 21] Despite explicit guidelines, the Centers for Disease Control and Prevention recently reported that there was no improvement in CAUTIs between 2010 and 2011.[22] Implementing these strategies for CAUTI reduction include establishing a multidisciplinary team that applies a clear protocol, with daily reminders about catheters and stop orders for catheter discontinuation.
Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
Stress ulcer prophylaxis in the hospital with proton pump inhibitors (PPIs) or histamine‐2 antagonists are common. As many as 71% of patients admitted to the hospital receive some form of prophylaxis without appropriate indication.[23] Guidelines exist for appropriate use; however, therapy is commonly used in the inpatient setting for indications not investigated or supported by the literature.[24]
Inappropriate prescribing practices have been associated with multiple adverse events, including drug interactions, hospital‐acquired infections, and increased costs of care. Although consensus among physicians regarding whether GI prophylaxis causes harm is lacking, studies demonstrate a strong correlation between use of PPIs and common adverse events such as pneumonia and Clostridium difficile infection.[25, 26] For instance, inpatients receiving PPIs were 3.6 times more likely to develop C. difficile‐associated diarrhea than inpatients not exposed to PPIs.[27]
The American Society of Health‐System Pharmacists Therapeutic Guidelines on Stress Ulcer Prophylaxis provide guidance regarding the optimal indication for administration of acid‐suppression medication for patients in the hospital setting. The clinical guidelines specify that stress ulcer prophylaxis is not recommended for adult patients in non‐ICU settings. The recommendations are applicable to general medical and surgical patients with fewer than 2 risk factors for clinically important bleeding. Indications for use of stress ulcer prophylaxis in the ICU include coagulopathy and mechanical ventilation.[24]
Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
Anemia is a frequent comorbid condition in hospitalized patients. Correcting anemia by means of allogeneic blood transfusions with the goal of maximizing oxygen delivery is common practice in many hospitals. Varied threshold levels of hemoglobin and hematocrit are used, which is unsupported by evidence.[28, 29]
Acute anemia with normovolemic hemodilution has been proven safe in patients with coronary artery disease, heart valve disease, and the elderly. A restrictive transfusion approach with hemoglobin cutoff of 7 g/dL, as opposed to higher thresholds, has shown improved outcomes (lower mortality and lower rate of rebleeding) in adult and pediatric critical care as well as surgical patients.[30] Large studies in patients with acute myocardial infarction demonstrated that restrictive transfusional strategies are associated with decreased in‐hospital mortality, rate of reinfarction, and worsening heart failure, as well as 30‐day mortality.[31] A randomized trial in patients with active GI bleeding showed that a restrictive strategy of hemoglobin threshold of 7 g/dL was associated with improved outcomes (less mortality, less rate of rebleeding), compared with a strategy to transfuse patients with hemoglobin less than 9 g/dL.[32] In addition, increased awareness of the high cost of blood ($700$900 per unit) associated with the blood banking process as well as risk of potential infectious and noninfectious adverse reactions (eg, human immunodeficiency virus, hepatitis C virus, transfusion‐related lung injury, transfusion‐related circulatory overload) must be considered in the risk/benefit equation.[28]
Based on current available evidence, the American Association of Blood Banks recommends adhering to a restrictive transfusion strategy (7 g/dL) in hospitalized stable patients, and this threshold is raised to 8 g/dL in patients with preexisting cardiovascular disease or with active symptoms.[28] This should be combined with techniques such as preoperative anemia optimization by hematinics replacement (eg, iron, vitamin B12, folate, erythropoietin), intraoperative strategies (eg, antifibrinolytics, hypotension, normovolemic hemodilution, etc.), and postoperative strategies (eg, intraoperative cell salvage). These strategies have been shown to result in parsimonious red blood cell utilization as well as in substantial healthcare cost savings.[33]
Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
Telemetry use in the hospital is common and clearly has a role for patients with certain cardiac conditions and those at risk for cardiac events. Telemetry is resource intensive, requiring dedicated multidisciplinary staff with specialized training. Many hospitals lack the ability to maintain and staff telemetry beds.[34] Physicians may overestimate the role of telemetry in guiding patient management.[35] One study concluded that only 12.6% of patients on a non‐ICU cardiac telemetry unit required telemetric monitoring, and only 7% received modified management as a result of telemetry findings.[36]
Inappropriate utilization of telemetry can be linked to increased length of stay or boarding in the emergency department, reduced hospital throughput, increased ambulance diversion, and increased operational costs.[37] In addition, the use of telemetry can lead to a false sense of security and alarm fatigue.[38] Telemetry artifacts may result in unnecessary testing and procedures for patients.[39] Furthermore, to accommodate the need for telemetry, frequent room changes may occur that may lead to decreased patient satisfaction. Low‐risk chest pain patients (hemodynamically stable with negative biomarkers, no electrocardiogram changes, and no indication for invasive procedure) do not require telemetry monitoring, because it rarely affects direct care of these patients.[36, 40] A 2009 study concluded that telemetry monitoring does not affect the care or the outcome of low‐risk patients.[41] Patients with other diagnoses, such as chronic obstructive pulmonary disease exacerbation or hemodynamically stable pulmonary embolism, and those requiring blood transfusions, are often placed in monitored beds without evidence that this will impact their care.[37]
The American Heart Association has published guidelines on the use of cardiac telemetry.[35] Patients are risk stratified into 3 categories, with class III patients being those who are low risk and do not require telemetry. Seventy percent of patients with the top 10 diagnoses that were admitted from the emergency department may clinically warrant telemetry.[37] Implementing a systematic evidence‐based approach to telemetry use can decrease unnecessary telemetry days,[42] reduce costs, and avoid unnecessary testing for rhythm artifacts.[39, 43]
Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability.
Although unnecessary laboratory testing is widely perceived as ineffective and wasteful, no national guideline or consensus statement exists regarding the utility or timing of repetitive laboratory testing. Multiple studies showed no difference in readmission rates, transfers to ICUs, lengths of stay, rates of adverse events, or mortality when the frequency of laboratory testing was reduced. Charges for daily laboratory testing were estimated to be $150/patient/day.[44] In a study at a university‐associated teaching hospital, an intervention to reduce the frequency of laboratory testing was associated with a total decrease of nearly 98,000 tests over a 3‐year period.[45] The cost savings in this study was estimated to be almost $2 million over the same time period. A second study at a teaching hospital, involving a computerized physician order entry (CPOE)‐based intervention, showed a reduction of almost 72,000 tests over a 1‐year period, which reduced the total number of inpatient phlebotomies by approximately 21%.[46]
The cost of routine, daily laboratory testing for a given patient or health system is not insignificant. When healthcare providers are made aware of the cost of daily laboratory testing, this might reduce the number of laboratory tests ordered and result in significant savings for a health system, as well as improve the patient experience.[44]
Developing guidelines or strategies to reduce repetitive laboratory testing in the face of clinical or laboratory stability would likely produce significant cost savings for both the individual patient as well as the health system, and could possibly would likely improve the hospital experience for many patients. Widespread adoption of CPOE by the US healthcare system has the potential to facilitate decision support that can change laboratory ordering practices.
DISCUSSION
Eliminating waste in healthcare is a priority for physicians,[6, 7, 8, 9, 10] and the ABIM‐F's Choosing Wisely campaign is a key component of this effort.[11] The SHM chose 5 tests and treatments relevant to the specialty of hospital medicine that occur at a high frequency, have significant cost and affect to patients, and that can feasibly be impacted. Given that a high percentage of healthcare costs occur in the hospital[5] and hospitalists care for an increasing number of these patients,[13] successful implementation of the SHM's adult hospital medicine Choosing Wisely list has great potential to decrease waste in the hospital, reduce harm, and improve patient outcomes.
The methodology chosen to develop the adult Choosing Wisely recommendations was intended to be both pragmatic and evidence based. A broad range of opinions was solicited, including from the SHM's general membership. The final refinement included a literature review and a Delphi process.
Review of cost and utilization data to determine the scope of the problem was used for decision‐making by subcommittee members to formulate the SHM's recommendations. For some recommendations, there were significant data, whereas for others, this information was sparse. As has been noted, we were unable to identify the total number of patients in the United States who receive telemetry on an annual basis, and thus were unable to make an estimate about the total population that would be impacted by improved utilization. However, several studies do indicate inappropriate use in significant patient populations and widespread use of the resource. Similarly, we were able to identify the costs associated with a CBC, but were unable to calculate the total number of CBCs administered annually. In the absence of these data, subcommittee members utilized other criteria, including frequency of test or treatment, patient harm or benefit, and utility for making treatment/management decisions.
In general, the tests and treatments contained in the adult hospital medicine Choosing Wisely list are not requested by patients. As such, physicians' choices play a greater role, potentially magnifying the impact hospitalists could make. Overuse of medical tests is multifactorial, and culture plays a significant role in the United States.[2, 3, 4] Although each of the tests and treatments identified by the SHM is within the purview of hospitalists, ensuring that guidelines are reliably followed will require interdisciplinary process changes. Ample opportunity exists to partner with nurses (urinary catheters and telemetry), pharmacists (stress ulcer prophylaxis), blood banks, and laboratories (transfusions and lab testing), as well as other healthcare providers and physicians in multiple specialties.
Successful implementation of each guideline will require improvement of systems within hospitals to drive reliability.[47] Provider education, training programs, protocols and reminders may prove to be significant catalysts in overcoming misinformation or no information about specific guidelines. More importantly, interdisciplinary teams will need to assess the current practice patterns within their hospitals prior to implementing solutions that standardize and automate the ordering processes for these tests and treatments.[48] Additionally, the culture within individual patient care units will need to be modified.[49] The challenge of changing the behavior of multiple stakeholders and hardwiring systems changes represent significant potential barriers to success.
There are several potential concerns with the recommendations. Concepts such as high risk and clinical stability exist in several of the recommendations. In most cases, specific guidelines exist that explicitly define the appropriate use of the test or treatment. Where they do not, implementers will need to define the operational definitions, such as the number of normal CBCs that define stability. Although the recommendations are based on the best evidence available, consensus still plays a role. As has been noted, the risk of malpractice litigation influences physicians' decisions.[1] Although evidence‐based recommendations such as these help shape the standard of care and mitigate risk, they may not completely eliminate this concern. Providers should always weigh the risks and benefits of any test or treatment. Finally, the approach taken to establish the list was both pragmatic and evidence based. Published evidence was not reviewed until the list was honed to 11. When the evidence was reviewed, the strength of the evidence was judged in a subjective manner by members of the committee as part of the Delphi panel voting.
CONCLUSION
As healthcare providers enter an era of more cost conscious decision‐making about provision of care based upon necessity, hospitalists have an excellent opportunity to impact overutilization. The 5 recommendations comprising the adult hospital medicine Choosing Wisely list offer an explicit starting point. The SHM hopes to lead this process during the coming months and years and to offer additional recommendations, providing a foundation for hospitalists to decrease unnecessary tests and treatments and improve healthcare value.
Acknowledgments
The authors thank the additional members of the Choosing Wisely subcommittee of the SHM's Healthcare Quality and Patient Safety Committee: Krishna Das, MD; Shelley Taylor, MD; Kevin O'Leary, MD; and Nasim Afsarmanesh, MD. The authors also thank SHM staff who were involved in all facets of the recommendation development process, particularly Brendon Shank, who provided significant input into the survey and dissemination process.
Disclosure
Nothing to report.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- , , , et al. Evidence that consumers are skeptical about evidence‐based health care. Health Aff (Millwood). 2010;29:1400–1406.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Too much treatment? Aggressive medical care can lead to more pain, with no gain. Consum Rep. 2008;73:40–44.
- Centers for Medicare and Medicaid Services. Historical national health expenditure data. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed February 12, 2013.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- . Change the microenvironment: delivery system reform essential to controlling costs. Available at: http://www.commonwealthfund.org/Publications/Commentaries/2009/Apr/Change‐the‐Microenvironment.aspx. Accessed February 12, 2013.
- Costs of care. Available at: http://www.costsofcare.org. Accessed February 12, 2013.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170:749–750.
- , , , ; Clinical Guidelines Committee of the American College of Physicians. High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- ABIM Foundation. U.S. physician groups identify commonly used tests or procedures they say are often not necessary. Available at: http://www.abimfoundation.org/News/ABIM‐Foundation‐News/2012/Choosing‐Wisely.aspx. Accessed February 12, 2013.
- , . Addressing requests by patients for nonbeneficial interventions. JAMA. 2012;307:149–150.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102–1112.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28:68–75.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- , , , et al. A compendium of strategies to prevent healthcare‐associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1): S12–S21.
- , , , et al. Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S41–S50.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Available at: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Accessed February 12, 2013.
- , , , , . Healthcare Infection Control Practices Advisory Committee, guideline for prevention of catheter‐associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319–326.
- , , , et al. Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663.
- , , , et al. 2011 National and State Healthcare‐Associated Infection Standardized Infection Ratio Report. Available at: http://www.cdc.gov/hai/pdfs/SIR/SIR‐Report_02_07_2013.pdf. Accessed February 13, 2013.
- , . Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64:1396–1400.
- . ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56:347–379.
- , , , , , . Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA. 2004;292:1955–1960.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301:2120–2128.
- , , , . Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile‐associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58.
- , , , et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31.
- , , , . Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818.
- , , , , . Association of blood transfusion with increased mortality in myocardial infarction: a meta‐analysis and diversity‐adjusted study sequential analysis. JAMA Intern Med 2013;173:132–139.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , . Economic impact of inappropriate blood transfusions in coronary artery bypass graft surgery. Am J Med. 1993;94:509–514.
- , , , , , . The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97:1483–1487.
- , , , et al. ACC/AHA guidelines for ambulatory electrocardiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Circulation. 1999;100:886–893.
- , , , et al. Role of telemetry monitoring in the non‐intensive care unit. Am J Cardiol. 1995;76:960–965.
- , . When do patients need admission to a telemetry bed? J Emerg Med. 2007;33:53–60.
- , . Electrocardiographic monitoring in the hospitalized patient: a diagnostic intervention of uncertain clinical impact. Am J Emerg Med. 2008;26:1047–1055.
- , , , , . Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med. 1999;341:1270–1274.
- , , , . Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in‐hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci. 2005;60:605–606.
- , , , , . Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8:125–126.
- Agency for Healthcare Research and Quality. Winawer N. Redesign of telemetry unit admission and transfer criteria leads to improved patient flow and reduced emergency department waiting times. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2239. Accessed February 12, 2013.
- , , , et al. Is telemetry monitoring necessary in low‐risk suspected acute chest pain syndromes? Chest. 2002;122:517–523.
- , . Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146:524–527.
- , , , et al. A cost‐effective method for reducing the volume of laboratory tests in a university‐associated teaching hospital. Mt Sinai J Med. 2006;73:787–794.
- , , , et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200–206.
- . Making noncatastrophic health care processes reliable: learning to walk before running in creating high‐reliability organizations. Health Serv Res. 2006;41:1677–1689.
- , , , et al. What have we learned about interventions to reduce medical errors? Annu Rev Public Health. 2010;31:479–497.
- , . Safe Patients, Smart Hospitals: How One Doctor's Checklist Can Help Us Change Health Care From The Inside Out. New York, NY: Hudson Street Press; 2010.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877–884.
- Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703.
- , , , , , . Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76:368–372.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–1653.
- , , , , . Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520–524.
The overuse of medical tests and treatments is a growing concern. A recent survey revealed that 2 in 5 primary care physicians perceive that patients in their own practice are receiving too much care.[1] Twenty‐eight percent of the physicians indicated they provide more care than they should. When queried about reasons for the aggressiveness of care, responses included fear of malpractice litigation, adherence to clinical performance measures that require following protocols, and inadequacy of time spent with patients. Overutilization of healthcare resources is a complex issue promulgated not only by the factors cited by the physicians but also a culture in the United States habituated to believe more care is better care.[2, 3, 4] In 2010, $2.6 trillion was spent on healthcare, an increase of $1.3 trillion between 2000 and 2010.5 As much as 30% of healthcare spending may be wasted.[6] Because physicians influence approximately 80% of healthcare expenditures, including ordering tests and treatments, it is imperative that physicians take a leadership role in reversing this trend.[7]
In response to this need, several physician‐led projects have emerged.[8, 9, 10] One such initiative is the American Board of Internal Medicine Foundation's (ABIM‐F's) Choosing Wisely campaign.[11] The ABIM‐F contacted a variety of specialty societies and asked each to identify the 5 top tests or treatments relevant to their specialty that may frequently be overused. Phase 1 of the Choosing Wisely campaign was launched in April 2012 with 9 specialty societies participating. The second phase was unveiled in February 2013 and comprised of 16 additional groups including the Society of Hospital Medicine (SHM). The SHM represents 35,000 hospitalists in the United States whose primary focus is the general medical care of hospitalized patients. This is especially important because almost one‐third of total US healthcare expenditures are on hospital care,[12] and hospitalists care for an increasing number of hospitalized patients.[13] In this article, we describe the used to derive the adult hospital medicine Choosing processes Wisely list, review the tests and treatments that the SHM's Choosing Wisely Subcommittee chose, and discuss potential next steps in implementation of the adult hospital medicine recommendations.
METHODOLOGY
Upon invitation to participate in the Choosing Wisely campaign, SHM's Hospital Quality and Patient Safety (HQPS) Committee formally convened the Choosing Wisely Subcommittee. The subcommittee identified and executed a methodology (see Supporting Figure 1 and Supporting Table 1 in the online version of this article) to create the list of 5 tests and treatments that the SHM submitted to the ABIM‐F. All subcommittee members participated fully in the voting and refinement process. The Choosing Wisely Subcommittee worked closely with the SHM's Pediatrics Choosing Wisely Subcommittee to develop both adult and pediatric lists.
Convening the Choosing Wisely Subcommittee
The HQPS Committee convened a subcommittee consisting of 9 members. The subcommittee represented a diverse group of hospitalists reflecting different institution types, geographic regions, and experience. All Choosing Wisely Subcommittee members signed conflict of interest statements and reported no conflict related to the conclusions, implications, or opinions stated. The subcommittee did not consult other external stakeholders in the development of recommendations.
Identification and Refinement of Potential Wasteful Practices
To generate an initial list of potential recommendations, members of all of the SHM committees were surveyed and asked to submit 5 tests and treatments that are inappropriately used or overused. SHM staff removed duplicates and categorized submissions by topic, highlighting overlapping recommendations. Tests and treatments that are used infrequently and items included in phase 1 society lists were also excluded. Subcommittee members then ranked the resultant list using a 5‐point Likert scale. All SHM members were then given the opportunity to rank their agreement with the tests and treatments on the list, as refined at the time based upon their own experience and consideration of the following criteria: tests and procedures within the control and purview of hospital medicine, the frequency with which the tests or procedures occur, and the significance of associated costs. This was accomplished via electronic survey.
Establishing an Evidence Base
SHM staff conducted a literature review of the list of tests and treatments that was further refined by the SHM membership's ranking using a standard template. Two reviewers (W.N. and J.G.) conducted an independent literature review of the remaining tests and treatments using PubMed, MEDLINE, and Cochrane Library. The reviewers also conducted generic Internet searches. The literature review included all literature published through 2012 as well as nonEnglish language publications. The reviewers included clinical research guidelines and primary and secondary research studies. Studies included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, reviewed the harm associated with the administration of a test or treatment, and explored the cost associated with the test or treatment as well as the overall strength of evidence. Additionally, the reference lists included in articles were reviewed to identify supplementary literature sources. The reviewers read and analyzed the articles identified in the initial search for relevant subject matter and summarized the findings in a table.
Delphi Panels
A Delphi scoring process was utilized to complete list refinement.[14] Subcommittee members anonymously voted via email for the strength of the test and treatment recommendation based upon specific criteria. To assist with this process, they received a copy of the completed literature review and an evidence summary of the literature. The following categories were used to guide the scoring: validity/evidence base to support, feasibility of implementation, frequency of occurrence, cost of occurrence, yield/emmpact, harm, and potential to improve. Results were aggregated and shared with the Choosing Wisely Subcommittee. The subcommittee conferred a final time, editing the recommendations for clarification and improved wording. A second anonymous vote was then conducted for the remaining tests and treatments through a revised scoring spreadsheet. The penultimate list was presented to the SHM's Board. Upon the Board's approval, the final list was submitted to the ABIM‐F.
RESULTS
The results of each stage of the list development process are shown in the online supporting information (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). The initial survey of SHM committee members garnered in excess of 150 tests and treatments from approximately 40 SHM committee members. The subsequent list refinement by SHM staff narrowed this list to 65 items, which were then further reduced to 15 items after ranking by members of the subcommittee (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). Voting by members of the general SHM membership further reduced the list to 11 tests and treatments.
The final list of 5 tests and treatments submitted to the ABIM‐F were:
- Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
- Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal (GI) complications.
- Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
- Do not order continuous telemetry monitoring outside of the intensive care unit (ICU) without using a protocol that governs continuation.
- Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability (Table 1).
|
| Test/Treatment Recommendations |
| Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis).[21, 50] |
| Do not prescribe GI prophylaxis to medical inpatients without clear‐cut indication or high risk for GI complication.[24] |
| Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms.[29, 51] |
| Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes.[35, 43, 52, 53] |
| Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.[44, 54, 55] |
RECOMMENDATIONS
Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
Despite guidelines identifying appropriate indications for the placement of urinary catheters, urinary tract infections due to catheter use remain the most frequent type of infection in acute care settings. Nearly 1 in every 5 patients in the hospital receives an indwelling catheter, and up to half are placed inappropriately.[15] Twenty‐six percent of patients who have indwelling catheters for 2 to 10 days will develop bacteriuria; subsequently, 24% of those patients will develop a catheter‐associated urinary tract infection (CAUTI).[15] More than 13,000 deaths due to CAUTI occur annually.[16] In addition to urinary tract infections and their complications, additional adverse outcomes related to indwelling catheters include formation of encrustations and restrictions to flow, prolonged hospital stay, and exposure to multidrug resistant organisms due to increased use of antibiotics. Evidence suggests that infections due to catheters are frequently preventable.[17, 18]
The economic burden associated with indwelling catheter complications is also substantial. Each episode of symptomatic urinary tract infection adds $676 in incremental costs, and catheter‐related bacteremia costs at least $2836.15 According to Scott, nearly 450,000 CAUTIs were estimated to have occurred in 2007, resulting in direct medical costs of between $340 to $370 million.[19]
Several organizations simultaneously released guidelines to provide a roadmap for appropriate catheter use and prevention of CAUTIs.[20, 21] Despite explicit guidelines, the Centers for Disease Control and Prevention recently reported that there was no improvement in CAUTIs between 2010 and 2011.[22] Implementing these strategies for CAUTI reduction include establishing a multidisciplinary team that applies a clear protocol, with daily reminders about catheters and stop orders for catheter discontinuation.
Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
Stress ulcer prophylaxis in the hospital with proton pump inhibitors (PPIs) or histamine‐2 antagonists are common. As many as 71% of patients admitted to the hospital receive some form of prophylaxis without appropriate indication.[23] Guidelines exist for appropriate use; however, therapy is commonly used in the inpatient setting for indications not investigated or supported by the literature.[24]
Inappropriate prescribing practices have been associated with multiple adverse events, including drug interactions, hospital‐acquired infections, and increased costs of care. Although consensus among physicians regarding whether GI prophylaxis causes harm is lacking, studies demonstrate a strong correlation between use of PPIs and common adverse events such as pneumonia and Clostridium difficile infection.[25, 26] For instance, inpatients receiving PPIs were 3.6 times more likely to develop C. difficile‐associated diarrhea than inpatients not exposed to PPIs.[27]
The American Society of Health‐System Pharmacists Therapeutic Guidelines on Stress Ulcer Prophylaxis provide guidance regarding the optimal indication for administration of acid‐suppression medication for patients in the hospital setting. The clinical guidelines specify that stress ulcer prophylaxis is not recommended for adult patients in non‐ICU settings. The recommendations are applicable to general medical and surgical patients with fewer than 2 risk factors for clinically important bleeding. Indications for use of stress ulcer prophylaxis in the ICU include coagulopathy and mechanical ventilation.[24]
Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
Anemia is a frequent comorbid condition in hospitalized patients. Correcting anemia by means of allogeneic blood transfusions with the goal of maximizing oxygen delivery is common practice in many hospitals. Varied threshold levels of hemoglobin and hematocrit are used, which is unsupported by evidence.[28, 29]
Acute anemia with normovolemic hemodilution has been proven safe in patients with coronary artery disease, heart valve disease, and the elderly. A restrictive transfusion approach with hemoglobin cutoff of 7 g/dL, as opposed to higher thresholds, has shown improved outcomes (lower mortality and lower rate of rebleeding) in adult and pediatric critical care as well as surgical patients.[30] Large studies in patients with acute myocardial infarction demonstrated that restrictive transfusional strategies are associated with decreased in‐hospital mortality, rate of reinfarction, and worsening heart failure, as well as 30‐day mortality.[31] A randomized trial in patients with active GI bleeding showed that a restrictive strategy of hemoglobin threshold of 7 g/dL was associated with improved outcomes (less mortality, less rate of rebleeding), compared with a strategy to transfuse patients with hemoglobin less than 9 g/dL.[32] In addition, increased awareness of the high cost of blood ($700$900 per unit) associated with the blood banking process as well as risk of potential infectious and noninfectious adverse reactions (eg, human immunodeficiency virus, hepatitis C virus, transfusion‐related lung injury, transfusion‐related circulatory overload) must be considered in the risk/benefit equation.[28]
Based on current available evidence, the American Association of Blood Banks recommends adhering to a restrictive transfusion strategy (7 g/dL) in hospitalized stable patients, and this threshold is raised to 8 g/dL in patients with preexisting cardiovascular disease or with active symptoms.[28] This should be combined with techniques such as preoperative anemia optimization by hematinics replacement (eg, iron, vitamin B12, folate, erythropoietin), intraoperative strategies (eg, antifibrinolytics, hypotension, normovolemic hemodilution, etc.), and postoperative strategies (eg, intraoperative cell salvage). These strategies have been shown to result in parsimonious red blood cell utilization as well as in substantial healthcare cost savings.[33]
Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
Telemetry use in the hospital is common and clearly has a role for patients with certain cardiac conditions and those at risk for cardiac events. Telemetry is resource intensive, requiring dedicated multidisciplinary staff with specialized training. Many hospitals lack the ability to maintain and staff telemetry beds.[34] Physicians may overestimate the role of telemetry in guiding patient management.[35] One study concluded that only 12.6% of patients on a non‐ICU cardiac telemetry unit required telemetric monitoring, and only 7% received modified management as a result of telemetry findings.[36]
Inappropriate utilization of telemetry can be linked to increased length of stay or boarding in the emergency department, reduced hospital throughput, increased ambulance diversion, and increased operational costs.[37] In addition, the use of telemetry can lead to a false sense of security and alarm fatigue.[38] Telemetry artifacts may result in unnecessary testing and procedures for patients.[39] Furthermore, to accommodate the need for telemetry, frequent room changes may occur that may lead to decreased patient satisfaction. Low‐risk chest pain patients (hemodynamically stable with negative biomarkers, no electrocardiogram changes, and no indication for invasive procedure) do not require telemetry monitoring, because it rarely affects direct care of these patients.[36, 40] A 2009 study concluded that telemetry monitoring does not affect the care or the outcome of low‐risk patients.[41] Patients with other diagnoses, such as chronic obstructive pulmonary disease exacerbation or hemodynamically stable pulmonary embolism, and those requiring blood transfusions, are often placed in monitored beds without evidence that this will impact their care.[37]
The American Heart Association has published guidelines on the use of cardiac telemetry.[35] Patients are risk stratified into 3 categories, with class III patients being those who are low risk and do not require telemetry. Seventy percent of patients with the top 10 diagnoses that were admitted from the emergency department may clinically warrant telemetry.[37] Implementing a systematic evidence‐based approach to telemetry use can decrease unnecessary telemetry days,[42] reduce costs, and avoid unnecessary testing for rhythm artifacts.[39, 43]
Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability.
Although unnecessary laboratory testing is widely perceived as ineffective and wasteful, no national guideline or consensus statement exists regarding the utility or timing of repetitive laboratory testing. Multiple studies showed no difference in readmission rates, transfers to ICUs, lengths of stay, rates of adverse events, or mortality when the frequency of laboratory testing was reduced. Charges for daily laboratory testing were estimated to be $150/patient/day.[44] In a study at a university‐associated teaching hospital, an intervention to reduce the frequency of laboratory testing was associated with a total decrease of nearly 98,000 tests over a 3‐year period.[45] The cost savings in this study was estimated to be almost $2 million over the same time period. A second study at a teaching hospital, involving a computerized physician order entry (CPOE)‐based intervention, showed a reduction of almost 72,000 tests over a 1‐year period, which reduced the total number of inpatient phlebotomies by approximately 21%.[46]
The cost of routine, daily laboratory testing for a given patient or health system is not insignificant. When healthcare providers are made aware of the cost of daily laboratory testing, this might reduce the number of laboratory tests ordered and result in significant savings for a health system, as well as improve the patient experience.[44]
Developing guidelines or strategies to reduce repetitive laboratory testing in the face of clinical or laboratory stability would likely produce significant cost savings for both the individual patient as well as the health system, and could possibly would likely improve the hospital experience for many patients. Widespread adoption of CPOE by the US healthcare system has the potential to facilitate decision support that can change laboratory ordering practices.
DISCUSSION
Eliminating waste in healthcare is a priority for physicians,[6, 7, 8, 9, 10] and the ABIM‐F's Choosing Wisely campaign is a key component of this effort.[11] The SHM chose 5 tests and treatments relevant to the specialty of hospital medicine that occur at a high frequency, have significant cost and affect to patients, and that can feasibly be impacted. Given that a high percentage of healthcare costs occur in the hospital[5] and hospitalists care for an increasing number of these patients,[13] successful implementation of the SHM's adult hospital medicine Choosing Wisely list has great potential to decrease waste in the hospital, reduce harm, and improve patient outcomes.
The methodology chosen to develop the adult Choosing Wisely recommendations was intended to be both pragmatic and evidence based. A broad range of opinions was solicited, including from the SHM's general membership. The final refinement included a literature review and a Delphi process.
Review of cost and utilization data to determine the scope of the problem was used for decision‐making by subcommittee members to formulate the SHM's recommendations. For some recommendations, there were significant data, whereas for others, this information was sparse. As has been noted, we were unable to identify the total number of patients in the United States who receive telemetry on an annual basis, and thus were unable to make an estimate about the total population that would be impacted by improved utilization. However, several studies do indicate inappropriate use in significant patient populations and widespread use of the resource. Similarly, we were able to identify the costs associated with a CBC, but were unable to calculate the total number of CBCs administered annually. In the absence of these data, subcommittee members utilized other criteria, including frequency of test or treatment, patient harm or benefit, and utility for making treatment/management decisions.
In general, the tests and treatments contained in the adult hospital medicine Choosing Wisely list are not requested by patients. As such, physicians' choices play a greater role, potentially magnifying the impact hospitalists could make. Overuse of medical tests is multifactorial, and culture plays a significant role in the United States.[2, 3, 4] Although each of the tests and treatments identified by the SHM is within the purview of hospitalists, ensuring that guidelines are reliably followed will require interdisciplinary process changes. Ample opportunity exists to partner with nurses (urinary catheters and telemetry), pharmacists (stress ulcer prophylaxis), blood banks, and laboratories (transfusions and lab testing), as well as other healthcare providers and physicians in multiple specialties.
Successful implementation of each guideline will require improvement of systems within hospitals to drive reliability.[47] Provider education, training programs, protocols and reminders may prove to be significant catalysts in overcoming misinformation or no information about specific guidelines. More importantly, interdisciplinary teams will need to assess the current practice patterns within their hospitals prior to implementing solutions that standardize and automate the ordering processes for these tests and treatments.[48] Additionally, the culture within individual patient care units will need to be modified.[49] The challenge of changing the behavior of multiple stakeholders and hardwiring systems changes represent significant potential barriers to success.
There are several potential concerns with the recommendations. Concepts such as high risk and clinical stability exist in several of the recommendations. In most cases, specific guidelines exist that explicitly define the appropriate use of the test or treatment. Where they do not, implementers will need to define the operational definitions, such as the number of normal CBCs that define stability. Although the recommendations are based on the best evidence available, consensus still plays a role. As has been noted, the risk of malpractice litigation influences physicians' decisions.[1] Although evidence‐based recommendations such as these help shape the standard of care and mitigate risk, they may not completely eliminate this concern. Providers should always weigh the risks and benefits of any test or treatment. Finally, the approach taken to establish the list was both pragmatic and evidence based. Published evidence was not reviewed until the list was honed to 11. When the evidence was reviewed, the strength of the evidence was judged in a subjective manner by members of the committee as part of the Delphi panel voting.
CONCLUSION
As healthcare providers enter an era of more cost conscious decision‐making about provision of care based upon necessity, hospitalists have an excellent opportunity to impact overutilization. The 5 recommendations comprising the adult hospital medicine Choosing Wisely list offer an explicit starting point. The SHM hopes to lead this process during the coming months and years and to offer additional recommendations, providing a foundation for hospitalists to decrease unnecessary tests and treatments and improve healthcare value.
Acknowledgments
The authors thank the additional members of the Choosing Wisely subcommittee of the SHM's Healthcare Quality and Patient Safety Committee: Krishna Das, MD; Shelley Taylor, MD; Kevin O'Leary, MD; and Nasim Afsarmanesh, MD. The authors also thank SHM staff who were involved in all facets of the recommendation development process, particularly Brendon Shank, who provided significant input into the survey and dissemination process.
Disclosure
Nothing to report.
The overuse of medical tests and treatments is a growing concern. A recent survey revealed that 2 in 5 primary care physicians perceive that patients in their own practice are receiving too much care.[1] Twenty‐eight percent of the physicians indicated they provide more care than they should. When queried about reasons for the aggressiveness of care, responses included fear of malpractice litigation, adherence to clinical performance measures that require following protocols, and inadequacy of time spent with patients. Overutilization of healthcare resources is a complex issue promulgated not only by the factors cited by the physicians but also a culture in the United States habituated to believe more care is better care.[2, 3, 4] In 2010, $2.6 trillion was spent on healthcare, an increase of $1.3 trillion between 2000 and 2010.5 As much as 30% of healthcare spending may be wasted.[6] Because physicians influence approximately 80% of healthcare expenditures, including ordering tests and treatments, it is imperative that physicians take a leadership role in reversing this trend.[7]
In response to this need, several physician‐led projects have emerged.[8, 9, 10] One such initiative is the American Board of Internal Medicine Foundation's (ABIM‐F's) Choosing Wisely campaign.[11] The ABIM‐F contacted a variety of specialty societies and asked each to identify the 5 top tests or treatments relevant to their specialty that may frequently be overused. Phase 1 of the Choosing Wisely campaign was launched in April 2012 with 9 specialty societies participating. The second phase was unveiled in February 2013 and comprised of 16 additional groups including the Society of Hospital Medicine (SHM). The SHM represents 35,000 hospitalists in the United States whose primary focus is the general medical care of hospitalized patients. This is especially important because almost one‐third of total US healthcare expenditures are on hospital care,[12] and hospitalists care for an increasing number of hospitalized patients.[13] In this article, we describe the used to derive the adult hospital medicine Choosing processes Wisely list, review the tests and treatments that the SHM's Choosing Wisely Subcommittee chose, and discuss potential next steps in implementation of the adult hospital medicine recommendations.
METHODOLOGY
Upon invitation to participate in the Choosing Wisely campaign, SHM's Hospital Quality and Patient Safety (HQPS) Committee formally convened the Choosing Wisely Subcommittee. The subcommittee identified and executed a methodology (see Supporting Figure 1 and Supporting Table 1 in the online version of this article) to create the list of 5 tests and treatments that the SHM submitted to the ABIM‐F. All subcommittee members participated fully in the voting and refinement process. The Choosing Wisely Subcommittee worked closely with the SHM's Pediatrics Choosing Wisely Subcommittee to develop both adult and pediatric lists.
Convening the Choosing Wisely Subcommittee
The HQPS Committee convened a subcommittee consisting of 9 members. The subcommittee represented a diverse group of hospitalists reflecting different institution types, geographic regions, and experience. All Choosing Wisely Subcommittee members signed conflict of interest statements and reported no conflict related to the conclusions, implications, or opinions stated. The subcommittee did not consult other external stakeholders in the development of recommendations.
Identification and Refinement of Potential Wasteful Practices
To generate an initial list of potential recommendations, members of all of the SHM committees were surveyed and asked to submit 5 tests and treatments that are inappropriately used or overused. SHM staff removed duplicates and categorized submissions by topic, highlighting overlapping recommendations. Tests and treatments that are used infrequently and items included in phase 1 society lists were also excluded. Subcommittee members then ranked the resultant list using a 5‐point Likert scale. All SHM members were then given the opportunity to rank their agreement with the tests and treatments on the list, as refined at the time based upon their own experience and consideration of the following criteria: tests and procedures within the control and purview of hospital medicine, the frequency with which the tests or procedures occur, and the significance of associated costs. This was accomplished via electronic survey.
Establishing an Evidence Base
SHM staff conducted a literature review of the list of tests and treatments that was further refined by the SHM membership's ranking using a standard template. Two reviewers (W.N. and J.G.) conducted an independent literature review of the remaining tests and treatments using PubMed, MEDLINE, and Cochrane Library. The reviewers also conducted generic Internet searches. The literature review included all literature published through 2012 as well as nonEnglish language publications. The reviewers included clinical research guidelines and primary and secondary research studies. Studies included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, reviewed the harm associated with the administration of a test or treatment, and explored the cost associated with the test or treatment as well as the overall strength of evidence. Additionally, the reference lists included in articles were reviewed to identify supplementary literature sources. The reviewers read and analyzed the articles identified in the initial search for relevant subject matter and summarized the findings in a table.
Delphi Panels
A Delphi scoring process was utilized to complete list refinement.[14] Subcommittee members anonymously voted via email for the strength of the test and treatment recommendation based upon specific criteria. To assist with this process, they received a copy of the completed literature review and an evidence summary of the literature. The following categories were used to guide the scoring: validity/evidence base to support, feasibility of implementation, frequency of occurrence, cost of occurrence, yield/emmpact, harm, and potential to improve. Results were aggregated and shared with the Choosing Wisely Subcommittee. The subcommittee conferred a final time, editing the recommendations for clarification and improved wording. A second anonymous vote was then conducted for the remaining tests and treatments through a revised scoring spreadsheet. The penultimate list was presented to the SHM's Board. Upon the Board's approval, the final list was submitted to the ABIM‐F.
RESULTS
The results of each stage of the list development process are shown in the online supporting information (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). The initial survey of SHM committee members garnered in excess of 150 tests and treatments from approximately 40 SHM committee members. The subsequent list refinement by SHM staff narrowed this list to 65 items, which were then further reduced to 15 items after ranking by members of the subcommittee (see Supporting Figure 1 and Supporting Table 1 in the online version of this article). Voting by members of the general SHM membership further reduced the list to 11 tests and treatments.
The final list of 5 tests and treatments submitted to the ABIM‐F were:
- Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
- Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal (GI) complications.
- Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
- Do not order continuous telemetry monitoring outside of the intensive care unit (ICU) without using a protocol that governs continuation.
- Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability (Table 1).
|
| Test/Treatment Recommendations |
| Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis).[21, 50] |
| Do not prescribe GI prophylaxis to medical inpatients without clear‐cut indication or high risk for GI complication.[24] |
| Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms.[29, 51] |
| Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes.[35, 43, 52, 53] |
| Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.[44, 54, 55] |
RECOMMENDATIONS
Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
Despite guidelines identifying appropriate indications for the placement of urinary catheters, urinary tract infections due to catheter use remain the most frequent type of infection in acute care settings. Nearly 1 in every 5 patients in the hospital receives an indwelling catheter, and up to half are placed inappropriately.[15] Twenty‐six percent of patients who have indwelling catheters for 2 to 10 days will develop bacteriuria; subsequently, 24% of those patients will develop a catheter‐associated urinary tract infection (CAUTI).[15] More than 13,000 deaths due to CAUTI occur annually.[16] In addition to urinary tract infections and their complications, additional adverse outcomes related to indwelling catheters include formation of encrustations and restrictions to flow, prolonged hospital stay, and exposure to multidrug resistant organisms due to increased use of antibiotics. Evidence suggests that infections due to catheters are frequently preventable.[17, 18]
The economic burden associated with indwelling catheter complications is also substantial. Each episode of symptomatic urinary tract infection adds $676 in incremental costs, and catheter‐related bacteremia costs at least $2836.15 According to Scott, nearly 450,000 CAUTIs were estimated to have occurred in 2007, resulting in direct medical costs of between $340 to $370 million.[19]
Several organizations simultaneously released guidelines to provide a roadmap for appropriate catheter use and prevention of CAUTIs.[20, 21] Despite explicit guidelines, the Centers for Disease Control and Prevention recently reported that there was no improvement in CAUTIs between 2010 and 2011.[22] Implementing these strategies for CAUTI reduction include establishing a multidisciplinary team that applies a clear protocol, with daily reminders about catheters and stop orders for catheter discontinuation.
Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
Stress ulcer prophylaxis in the hospital with proton pump inhibitors (PPIs) or histamine‐2 antagonists are common. As many as 71% of patients admitted to the hospital receive some form of prophylaxis without appropriate indication.[23] Guidelines exist for appropriate use; however, therapy is commonly used in the inpatient setting for indications not investigated or supported by the literature.[24]
Inappropriate prescribing practices have been associated with multiple adverse events, including drug interactions, hospital‐acquired infections, and increased costs of care. Although consensus among physicians regarding whether GI prophylaxis causes harm is lacking, studies demonstrate a strong correlation between use of PPIs and common adverse events such as pneumonia and Clostridium difficile infection.[25, 26] For instance, inpatients receiving PPIs were 3.6 times more likely to develop C. difficile‐associated diarrhea than inpatients not exposed to PPIs.[27]
The American Society of Health‐System Pharmacists Therapeutic Guidelines on Stress Ulcer Prophylaxis provide guidance regarding the optimal indication for administration of acid‐suppression medication for patients in the hospital setting. The clinical guidelines specify that stress ulcer prophylaxis is not recommended for adult patients in non‐ICU settings. The recommendations are applicable to general medical and surgical patients with fewer than 2 risk factors for clinically important bleeding. Indications for use of stress ulcer prophylaxis in the ICU include coagulopathy and mechanical ventilation.[24]
Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke.
Anemia is a frequent comorbid condition in hospitalized patients. Correcting anemia by means of allogeneic blood transfusions with the goal of maximizing oxygen delivery is common practice in many hospitals. Varied threshold levels of hemoglobin and hematocrit are used, which is unsupported by evidence.[28, 29]
Acute anemia with normovolemic hemodilution has been proven safe in patients with coronary artery disease, heart valve disease, and the elderly. A restrictive transfusion approach with hemoglobin cutoff of 7 g/dL, as opposed to higher thresholds, has shown improved outcomes (lower mortality and lower rate of rebleeding) in adult and pediatric critical care as well as surgical patients.[30] Large studies in patients with acute myocardial infarction demonstrated that restrictive transfusional strategies are associated with decreased in‐hospital mortality, rate of reinfarction, and worsening heart failure, as well as 30‐day mortality.[31] A randomized trial in patients with active GI bleeding showed that a restrictive strategy of hemoglobin threshold of 7 g/dL was associated with improved outcomes (less mortality, less rate of rebleeding), compared with a strategy to transfuse patients with hemoglobin less than 9 g/dL.[32] In addition, increased awareness of the high cost of blood ($700$900 per unit) associated with the blood banking process as well as risk of potential infectious and noninfectious adverse reactions (eg, human immunodeficiency virus, hepatitis C virus, transfusion‐related lung injury, transfusion‐related circulatory overload) must be considered in the risk/benefit equation.[28]
Based on current available evidence, the American Association of Blood Banks recommends adhering to a restrictive transfusion strategy (7 g/dL) in hospitalized stable patients, and this threshold is raised to 8 g/dL in patients with preexisting cardiovascular disease or with active symptoms.[28] This should be combined with techniques such as preoperative anemia optimization by hematinics replacement (eg, iron, vitamin B12, folate, erythropoietin), intraoperative strategies (eg, antifibrinolytics, hypotension, normovolemic hemodilution, etc.), and postoperative strategies (eg, intraoperative cell salvage). These strategies have been shown to result in parsimonious red blood cell utilization as well as in substantial healthcare cost savings.[33]
Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
Telemetry use in the hospital is common and clearly has a role for patients with certain cardiac conditions and those at risk for cardiac events. Telemetry is resource intensive, requiring dedicated multidisciplinary staff with specialized training. Many hospitals lack the ability to maintain and staff telemetry beds.[34] Physicians may overestimate the role of telemetry in guiding patient management.[35] One study concluded that only 12.6% of patients on a non‐ICU cardiac telemetry unit required telemetric monitoring, and only 7% received modified management as a result of telemetry findings.[36]
Inappropriate utilization of telemetry can be linked to increased length of stay or boarding in the emergency department, reduced hospital throughput, increased ambulance diversion, and increased operational costs.[37] In addition, the use of telemetry can lead to a false sense of security and alarm fatigue.[38] Telemetry artifacts may result in unnecessary testing and procedures for patients.[39] Furthermore, to accommodate the need for telemetry, frequent room changes may occur that may lead to decreased patient satisfaction. Low‐risk chest pain patients (hemodynamically stable with negative biomarkers, no electrocardiogram changes, and no indication for invasive procedure) do not require telemetry monitoring, because it rarely affects direct care of these patients.[36, 40] A 2009 study concluded that telemetry monitoring does not affect the care or the outcome of low‐risk patients.[41] Patients with other diagnoses, such as chronic obstructive pulmonary disease exacerbation or hemodynamically stable pulmonary embolism, and those requiring blood transfusions, are often placed in monitored beds without evidence that this will impact their care.[37]
The American Heart Association has published guidelines on the use of cardiac telemetry.[35] Patients are risk stratified into 3 categories, with class III patients being those who are low risk and do not require telemetry. Seventy percent of patients with the top 10 diagnoses that were admitted from the emergency department may clinically warrant telemetry.[37] Implementing a systematic evidence‐based approach to telemetry use can decrease unnecessary telemetry days,[42] reduce costs, and avoid unnecessary testing for rhythm artifacts.[39, 43]
Do not perform repetitive complete blood count (CBC) and chemistry testing in the face of clinical and lab stability.
Although unnecessary laboratory testing is widely perceived as ineffective and wasteful, no national guideline or consensus statement exists regarding the utility or timing of repetitive laboratory testing. Multiple studies showed no difference in readmission rates, transfers to ICUs, lengths of stay, rates of adverse events, or mortality when the frequency of laboratory testing was reduced. Charges for daily laboratory testing were estimated to be $150/patient/day.[44] In a study at a university‐associated teaching hospital, an intervention to reduce the frequency of laboratory testing was associated with a total decrease of nearly 98,000 tests over a 3‐year period.[45] The cost savings in this study was estimated to be almost $2 million over the same time period. A second study at a teaching hospital, involving a computerized physician order entry (CPOE)‐based intervention, showed a reduction of almost 72,000 tests over a 1‐year period, which reduced the total number of inpatient phlebotomies by approximately 21%.[46]
The cost of routine, daily laboratory testing for a given patient or health system is not insignificant. When healthcare providers are made aware of the cost of daily laboratory testing, this might reduce the number of laboratory tests ordered and result in significant savings for a health system, as well as improve the patient experience.[44]
Developing guidelines or strategies to reduce repetitive laboratory testing in the face of clinical or laboratory stability would likely produce significant cost savings for both the individual patient as well as the health system, and could possibly would likely improve the hospital experience for many patients. Widespread adoption of CPOE by the US healthcare system has the potential to facilitate decision support that can change laboratory ordering practices.
DISCUSSION
Eliminating waste in healthcare is a priority for physicians,[6, 7, 8, 9, 10] and the ABIM‐F's Choosing Wisely campaign is a key component of this effort.[11] The SHM chose 5 tests and treatments relevant to the specialty of hospital medicine that occur at a high frequency, have significant cost and affect to patients, and that can feasibly be impacted. Given that a high percentage of healthcare costs occur in the hospital[5] and hospitalists care for an increasing number of these patients,[13] successful implementation of the SHM's adult hospital medicine Choosing Wisely list has great potential to decrease waste in the hospital, reduce harm, and improve patient outcomes.
The methodology chosen to develop the adult Choosing Wisely recommendations was intended to be both pragmatic and evidence based. A broad range of opinions was solicited, including from the SHM's general membership. The final refinement included a literature review and a Delphi process.
Review of cost and utilization data to determine the scope of the problem was used for decision‐making by subcommittee members to formulate the SHM's recommendations. For some recommendations, there were significant data, whereas for others, this information was sparse. As has been noted, we were unable to identify the total number of patients in the United States who receive telemetry on an annual basis, and thus were unable to make an estimate about the total population that would be impacted by improved utilization. However, several studies do indicate inappropriate use in significant patient populations and widespread use of the resource. Similarly, we were able to identify the costs associated with a CBC, but were unable to calculate the total number of CBCs administered annually. In the absence of these data, subcommittee members utilized other criteria, including frequency of test or treatment, patient harm or benefit, and utility for making treatment/management decisions.
In general, the tests and treatments contained in the adult hospital medicine Choosing Wisely list are not requested by patients. As such, physicians' choices play a greater role, potentially magnifying the impact hospitalists could make. Overuse of medical tests is multifactorial, and culture plays a significant role in the United States.[2, 3, 4] Although each of the tests and treatments identified by the SHM is within the purview of hospitalists, ensuring that guidelines are reliably followed will require interdisciplinary process changes. Ample opportunity exists to partner with nurses (urinary catheters and telemetry), pharmacists (stress ulcer prophylaxis), blood banks, and laboratories (transfusions and lab testing), as well as other healthcare providers and physicians in multiple specialties.
Successful implementation of each guideline will require improvement of systems within hospitals to drive reliability.[47] Provider education, training programs, protocols and reminders may prove to be significant catalysts in overcoming misinformation or no information about specific guidelines. More importantly, interdisciplinary teams will need to assess the current practice patterns within their hospitals prior to implementing solutions that standardize and automate the ordering processes for these tests and treatments.[48] Additionally, the culture within individual patient care units will need to be modified.[49] The challenge of changing the behavior of multiple stakeholders and hardwiring systems changes represent significant potential barriers to success.
There are several potential concerns with the recommendations. Concepts such as high risk and clinical stability exist in several of the recommendations. In most cases, specific guidelines exist that explicitly define the appropriate use of the test or treatment. Where they do not, implementers will need to define the operational definitions, such as the number of normal CBCs that define stability. Although the recommendations are based on the best evidence available, consensus still plays a role. As has been noted, the risk of malpractice litigation influences physicians' decisions.[1] Although evidence‐based recommendations such as these help shape the standard of care and mitigate risk, they may not completely eliminate this concern. Providers should always weigh the risks and benefits of any test or treatment. Finally, the approach taken to establish the list was both pragmatic and evidence based. Published evidence was not reviewed until the list was honed to 11. When the evidence was reviewed, the strength of the evidence was judged in a subjective manner by members of the committee as part of the Delphi panel voting.
CONCLUSION
As healthcare providers enter an era of more cost conscious decision‐making about provision of care based upon necessity, hospitalists have an excellent opportunity to impact overutilization. The 5 recommendations comprising the adult hospital medicine Choosing Wisely list offer an explicit starting point. The SHM hopes to lead this process during the coming months and years and to offer additional recommendations, providing a foundation for hospitalists to decrease unnecessary tests and treatments and improve healthcare value.
Acknowledgments
The authors thank the additional members of the Choosing Wisely subcommittee of the SHM's Healthcare Quality and Patient Safety Committee: Krishna Das, MD; Shelley Taylor, MD; Kevin O'Leary, MD; and Nasim Afsarmanesh, MD. The authors also thank SHM staff who were involved in all facets of the recommendation development process, particularly Brendon Shank, who provided significant input into the survey and dissemination process.
Disclosure
Nothing to report.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- , , , et al. Evidence that consumers are skeptical about evidence‐based health care. Health Aff (Millwood). 2010;29:1400–1406.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Too much treatment? Aggressive medical care can lead to more pain, with no gain. Consum Rep. 2008;73:40–44.
- Centers for Medicare and Medicaid Services. Historical national health expenditure data. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed February 12, 2013.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- . Change the microenvironment: delivery system reform essential to controlling costs. Available at: http://www.commonwealthfund.org/Publications/Commentaries/2009/Apr/Change‐the‐Microenvironment.aspx. Accessed February 12, 2013.
- Costs of care. Available at: http://www.costsofcare.org. Accessed February 12, 2013.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170:749–750.
- , , , ; Clinical Guidelines Committee of the American College of Physicians. High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- ABIM Foundation. U.S. physician groups identify commonly used tests or procedures they say are often not necessary. Available at: http://www.abimfoundation.org/News/ABIM‐Foundation‐News/2012/Choosing‐Wisely.aspx. Accessed February 12, 2013.
- , . Addressing requests by patients for nonbeneficial interventions. JAMA. 2012;307:149–150.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102–1112.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28:68–75.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- , , , et al. A compendium of strategies to prevent healthcare‐associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1): S12–S21.
- , , , et al. Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S41–S50.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Available at: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Accessed February 12, 2013.
- , , , , . Healthcare Infection Control Practices Advisory Committee, guideline for prevention of catheter‐associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319–326.
- , , , et al. Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663.
- , , , et al. 2011 National and State Healthcare‐Associated Infection Standardized Infection Ratio Report. Available at: http://www.cdc.gov/hai/pdfs/SIR/SIR‐Report_02_07_2013.pdf. Accessed February 13, 2013.
- , . Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64:1396–1400.
- . ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56:347–379.
- , , , , , . Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA. 2004;292:1955–1960.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301:2120–2128.
- , , , . Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile‐associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58.
- , , , et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31.
- , , , . Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818.
- , , , , . Association of blood transfusion with increased mortality in myocardial infarction: a meta‐analysis and diversity‐adjusted study sequential analysis. JAMA Intern Med 2013;173:132–139.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , . Economic impact of inappropriate blood transfusions in coronary artery bypass graft surgery. Am J Med. 1993;94:509–514.
- , , , , , . The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97:1483–1487.
- , , , et al. ACC/AHA guidelines for ambulatory electrocardiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Circulation. 1999;100:886–893.
- , , , et al. Role of telemetry monitoring in the non‐intensive care unit. Am J Cardiol. 1995;76:960–965.
- , . When do patients need admission to a telemetry bed? J Emerg Med. 2007;33:53–60.
- , . Electrocardiographic monitoring in the hospitalized patient: a diagnostic intervention of uncertain clinical impact. Am J Emerg Med. 2008;26:1047–1055.
- , , , , . Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med. 1999;341:1270–1274.
- , , , . Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in‐hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci. 2005;60:605–606.
- , , , , . Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8:125–126.
- Agency for Healthcare Research and Quality. Winawer N. Redesign of telemetry unit admission and transfer criteria leads to improved patient flow and reduced emergency department waiting times. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2239. Accessed February 12, 2013.
- , , , et al. Is telemetry monitoring necessary in low‐risk suspected acute chest pain syndromes? Chest. 2002;122:517–523.
- , . Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146:524–527.
- , , , et al. A cost‐effective method for reducing the volume of laboratory tests in a university‐associated teaching hospital. Mt Sinai J Med. 2006;73:787–794.
- , , , et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200–206.
- . Making noncatastrophic health care processes reliable: learning to walk before running in creating high‐reliability organizations. Health Serv Res. 2006;41:1677–1689.
- , , , et al. What have we learned about interventions to reduce medical errors? Annu Rev Public Health. 2010;31:479–497.
- , . Safe Patients, Smart Hospitals: How One Doctor's Checklist Can Help Us Change Health Care From The Inside Out. New York, NY: Hudson Street Press; 2010.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877–884.
- Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703.
- , , , , , . Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76:368–372.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–1653.
- , , , , . Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520–524.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- , , , et al. Evidence that consumers are skeptical about evidence‐based health care. Health Aff (Millwood). 2010;29:1400–1406.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Too much treatment? Aggressive medical care can lead to more pain, with no gain. Consum Rep. 2008;73:40–44.
- Centers for Medicare and Medicaid Services. Historical national health expenditure data. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed February 12, 2013.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- . Change the microenvironment: delivery system reform essential to controlling costs. Available at: http://www.commonwealthfund.org/Publications/Commentaries/2009/Apr/Change‐the‐Microenvironment.aspx. Accessed February 12, 2013.
- Costs of care. Available at: http://www.costsofcare.org. Accessed February 12, 2013.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170:749–750.
- , , , ; Clinical Guidelines Committee of the American College of Physicians. High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- ABIM Foundation. U.S. physician groups identify commonly used tests or procedures they say are often not necessary. Available at: http://www.abimfoundation.org/News/ABIM‐Foundation‐News/2012/Choosing‐Wisely.aspx. Accessed February 12, 2013.
- , . Addressing requests by patients for nonbeneficial interventions. JAMA. 2012;307:149–150.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102–1112.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28:68–75.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- , , , et al. A compendium of strategies to prevent healthcare‐associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1): S12–S21.
- , , , et al. Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S41–S50.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Available at: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Accessed February 12, 2013.
- , , , , . Healthcare Infection Control Practices Advisory Committee, guideline for prevention of catheter‐associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319–326.
- , , , et al. Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663.
- , , , et al. 2011 National and State Healthcare‐Associated Infection Standardized Infection Ratio Report. Available at: http://www.cdc.gov/hai/pdfs/SIR/SIR‐Report_02_07_2013.pdf. Accessed February 13, 2013.
- , . Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64:1396–1400.
- . ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56:347–379.
- , , , , , . Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA. 2004;292:1955–1960.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301:2120–2128.
- , , , . Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile‐associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58.
- , , , et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31.
- , , , . Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818.
- , , , , . Association of blood transfusion with increased mortality in myocardial infarction: a meta‐analysis and diversity‐adjusted study sequential analysis. JAMA Intern Med 2013;173:132–139.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , . Economic impact of inappropriate blood transfusions in coronary artery bypass graft surgery. Am J Med. 1993;94:509–514.
- , , , , , . The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97:1483–1487.
- , , , et al. ACC/AHA guidelines for ambulatory electrocardiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Circulation. 1999;100:886–893.
- , , , et al. Role of telemetry monitoring in the non‐intensive care unit. Am J Cardiol. 1995;76:960–965.
- , . When do patients need admission to a telemetry bed? J Emerg Med. 2007;33:53–60.
- , . Electrocardiographic monitoring in the hospitalized patient: a diagnostic intervention of uncertain clinical impact. Am J Emerg Med. 2008;26:1047–1055.
- , , , , . Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med. 1999;341:1270–1274.
- , , , . Inpatient telemetry does not need to be used in the management of older patients hospitalized with chest pain at low risk for in‐hospital coronary events and mortality. J Gerontol A Biol Sci Med Sci. 2005;60:605–606.
- , , , , . Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8:125–126.
- Agency for Healthcare Research and Quality. Winawer N. Redesign of telemetry unit admission and transfer criteria leads to improved patient flow and reduced emergency department waiting times. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2239. Accessed February 12, 2013.
- , , , et al. Is telemetry monitoring necessary in low‐risk suspected acute chest pain syndromes? Chest. 2002;122:517–523.
- , . Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146:524–527.
- , , , et al. A cost‐effective method for reducing the volume of laboratory tests in a university‐associated teaching hospital. Mt Sinai J Med. 2006;73:787–794.
- , , , et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200–206.
- . Making noncatastrophic health care processes reliable: learning to walk before running in creating high‐reliability organizations. Health Serv Res. 2006;41:1677–1689.
- , , , et al. What have we learned about interventions to reduce medical errors? Annu Rev Public Health. 2010;31:479–497.
- , . Safe Patients, Smart Hospitals: How One Doctor's Checklist Can Help Us Change Health Care From The Inside Out. New York, NY: Hudson Street Press; 2010.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877–884.
- Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703.
- , , , , , . Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76:368–372.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–1653.
- , , , , . Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520–524.
Copyright © 2013 Society of Hospital Medicine
Focusing on Value
Over the last 30 years, rounds of therapeutic treatments with cost consciousness and cost containment have been administered to the healthcare industry, with generally disappointing clinical response. The last treatment cycle came in the 1990s, with the combination therapy of prospective payment and managed care, treatments that produced a transient remission in cost inflation but that left the healthcare system spent and decidedly unenthusiastic about another round of intensive therapy. For the next 15 years or so, the underlying conditions remained untreated, and unsurprisingly, runaway healthcare inflation returned. To continue this metaphor only a bit further, in 2013 the healthcare system is again facing intensive treatments, but in this case the treatments seem more likely to produce a strong and durable clinical response.
Although some argue that current efforts shall also pass, we believe that the present day is clearly different. A major difference is the implementation of the Affordable Care Act, which creates new structures to facilitate and incentives to promote cost reductions. More importantly, there has been a sea change in how the publicnot just payors or employersview healthcare costs. The ideas that care is too expensive and that much of it adds no value to patients have gained wide acceptance across the political spectrum, among patients, and increasingly among physicians.
It was in this context that the American Board of Internal Medicine Foundation (ABIMF) launched its Choosing Wisely campaign in 2011.[1] The stated goal of the campaign was to promote important conversations [between doctors and patients] necessary to ensure the right care is delivered at the right time. Importantly, this careful framing successfully avoided the caricatures of rationing or death panels, reactions that doomed prior efforts to engage all stakeholders in a reasoned national dialogue about costs and value.
The ABIMF chose an approach of having physicians identify tests and procedures that may be unnecessary in certain situations. Working with Consumer Reports, the Foundation asked a wide range of medical specialty societies to develop their own list of tests and procedures that could potentially be avoided with no harm to patients. The vast majority, 25 as of July 2013, chose to participate.
In February 2013, the Society of Hospital Medicine (SHM) joined the initiative when it posted adult and pediatric versions of Five Things Physicians and Patients Should Question.[2] We are pleased to publish summaries of the recommendations and the processes by which the 2 working groups produced their lists in the Journal of Hospital Medicine.[3, 4]
In reading these articles, we are struck by the importance of the SHM's work to reduce costs and improve value. However, it really is a first step: both articles must now catalyze a body of work to create and sustain meaningful change.
Although many of the 10 targets have strong face validity, it is not clear whether they are in fact the most common, costly, or low‐value practices under the purview of hospitalists. Given the fact that the selection process involved both evidence‐based reviews and consensus, it is possible that other, potentially more contentious, practices may provide even more bang for the buck, or in this case, nonbuck.
Nevertheless, these are quibbles. These lists are good starting points, and in fact many hospitalist groups, including our own, are using the SHM practices as a foundation for our waste‐reduction efforts. The next challenge will be translating these recommendations into actionable measures and then clinical practice. For example, 1 of the adult recommendations is to avoid repeat blood counts and chemistries in patients who are clinically stable. Concepts of clinical stability are notoriously difficult to define within specific patient subgroups, much less across the diverse patient populations seen by hospitalists. One approach here would be to narrow the focus (eg, do not order repeated blood counts in patients with gastrointestinal bleeding whose labs have been stable for 48 hours), but this step would limit the cost savings. Other measures, such as those related to urinary catheters, are more clearly defined and seem closer to being widely adoptable.
For all these measures, the ultimate question remains: How much can actually be saved and how do we measure the savings? The marginal cost of a complete blood count is extraordinarily small in comparison to an entire hospital stay, but it is possible that eliminating redundant testing also reduces the costs related to follow‐up of false positive findings. Reducing the use of urinary catheters can cut the costs of urinary tract infections and the complications of treatment, but these costs could be offset by the higher‐level nursing care needed to mobilize patients earlier or assist patients in toileting, squeezing the proverbial balloon. For all these measures, it is unclear whether what might be relatively small variable cost reductions related to specific tests/procedures can lead to subsequent reduction in fixed costs related to facilities and equipment, where more than 70% of healthcare costs lie.[5] In other words, reducing the number of lab technicians and the amount of laboratory equipment needed will lead to far greater cost reductions than reducing individual test utilization.
None of this is to say that the Choosing Wisely campaign is without merit. To the contrary, the campaign and the efforts of the SHM are early and critical steps in changing the behavior of a profession. Since the early days of hospital medicine, hospitalists have embraced cost reduction and value improvement as a central focus. By successfully engaging consumers and the community of medical specialties, Choosing Wisely has created a language and a framework that will allow our field and others to tackle the crucial work of resource stewardship with new purpose, and we hope, unprecedented success.
Disclosures
Dr. Wachter is immediate past‐chair of the American Board of Internal Medicine (ABIM) and serves on the ABIM Foundation's Board of Trustees. Dr. Auerbach receives honoraria from the American Board of Internal Medicine as a contributor to the Maintenance of Certification question pool.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Are you choosing wisely? 2013. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement8:486–492.
- , , , et al. Choosing Wisely in inpatient pediatrics: 5 opportunities for improved healthcare value. J Hosp Med. 2013;8:479–485.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281:644–649.
Over the last 30 years, rounds of therapeutic treatments with cost consciousness and cost containment have been administered to the healthcare industry, with generally disappointing clinical response. The last treatment cycle came in the 1990s, with the combination therapy of prospective payment and managed care, treatments that produced a transient remission in cost inflation but that left the healthcare system spent and decidedly unenthusiastic about another round of intensive therapy. For the next 15 years or so, the underlying conditions remained untreated, and unsurprisingly, runaway healthcare inflation returned. To continue this metaphor only a bit further, in 2013 the healthcare system is again facing intensive treatments, but in this case the treatments seem more likely to produce a strong and durable clinical response.
Although some argue that current efforts shall also pass, we believe that the present day is clearly different. A major difference is the implementation of the Affordable Care Act, which creates new structures to facilitate and incentives to promote cost reductions. More importantly, there has been a sea change in how the publicnot just payors or employersview healthcare costs. The ideas that care is too expensive and that much of it adds no value to patients have gained wide acceptance across the political spectrum, among patients, and increasingly among physicians.
It was in this context that the American Board of Internal Medicine Foundation (ABIMF) launched its Choosing Wisely campaign in 2011.[1] The stated goal of the campaign was to promote important conversations [between doctors and patients] necessary to ensure the right care is delivered at the right time. Importantly, this careful framing successfully avoided the caricatures of rationing or death panels, reactions that doomed prior efforts to engage all stakeholders in a reasoned national dialogue about costs and value.
The ABIMF chose an approach of having physicians identify tests and procedures that may be unnecessary in certain situations. Working with Consumer Reports, the Foundation asked a wide range of medical specialty societies to develop their own list of tests and procedures that could potentially be avoided with no harm to patients. The vast majority, 25 as of July 2013, chose to participate.
In February 2013, the Society of Hospital Medicine (SHM) joined the initiative when it posted adult and pediatric versions of Five Things Physicians and Patients Should Question.[2] We are pleased to publish summaries of the recommendations and the processes by which the 2 working groups produced their lists in the Journal of Hospital Medicine.[3, 4]
In reading these articles, we are struck by the importance of the SHM's work to reduce costs and improve value. However, it really is a first step: both articles must now catalyze a body of work to create and sustain meaningful change.
Although many of the 10 targets have strong face validity, it is not clear whether they are in fact the most common, costly, or low‐value practices under the purview of hospitalists. Given the fact that the selection process involved both evidence‐based reviews and consensus, it is possible that other, potentially more contentious, practices may provide even more bang for the buck, or in this case, nonbuck.
Nevertheless, these are quibbles. These lists are good starting points, and in fact many hospitalist groups, including our own, are using the SHM practices as a foundation for our waste‐reduction efforts. The next challenge will be translating these recommendations into actionable measures and then clinical practice. For example, 1 of the adult recommendations is to avoid repeat blood counts and chemistries in patients who are clinically stable. Concepts of clinical stability are notoriously difficult to define within specific patient subgroups, much less across the diverse patient populations seen by hospitalists. One approach here would be to narrow the focus (eg, do not order repeated blood counts in patients with gastrointestinal bleeding whose labs have been stable for 48 hours), but this step would limit the cost savings. Other measures, such as those related to urinary catheters, are more clearly defined and seem closer to being widely adoptable.
For all these measures, the ultimate question remains: How much can actually be saved and how do we measure the savings? The marginal cost of a complete blood count is extraordinarily small in comparison to an entire hospital stay, but it is possible that eliminating redundant testing also reduces the costs related to follow‐up of false positive findings. Reducing the use of urinary catheters can cut the costs of urinary tract infections and the complications of treatment, but these costs could be offset by the higher‐level nursing care needed to mobilize patients earlier or assist patients in toileting, squeezing the proverbial balloon. For all these measures, it is unclear whether what might be relatively small variable cost reductions related to specific tests/procedures can lead to subsequent reduction in fixed costs related to facilities and equipment, where more than 70% of healthcare costs lie.[5] In other words, reducing the number of lab technicians and the amount of laboratory equipment needed will lead to far greater cost reductions than reducing individual test utilization.
None of this is to say that the Choosing Wisely campaign is without merit. To the contrary, the campaign and the efforts of the SHM are early and critical steps in changing the behavior of a profession. Since the early days of hospital medicine, hospitalists have embraced cost reduction and value improvement as a central focus. By successfully engaging consumers and the community of medical specialties, Choosing Wisely has created a language and a framework that will allow our field and others to tackle the crucial work of resource stewardship with new purpose, and we hope, unprecedented success.
Disclosures
Dr. Wachter is immediate past‐chair of the American Board of Internal Medicine (ABIM) and serves on the ABIM Foundation's Board of Trustees. Dr. Auerbach receives honoraria from the American Board of Internal Medicine as a contributor to the Maintenance of Certification question pool.
Over the last 30 years, rounds of therapeutic treatments with cost consciousness and cost containment have been administered to the healthcare industry, with generally disappointing clinical response. The last treatment cycle came in the 1990s, with the combination therapy of prospective payment and managed care, treatments that produced a transient remission in cost inflation but that left the healthcare system spent and decidedly unenthusiastic about another round of intensive therapy. For the next 15 years or so, the underlying conditions remained untreated, and unsurprisingly, runaway healthcare inflation returned. To continue this metaphor only a bit further, in 2013 the healthcare system is again facing intensive treatments, but in this case the treatments seem more likely to produce a strong and durable clinical response.
Although some argue that current efforts shall also pass, we believe that the present day is clearly different. A major difference is the implementation of the Affordable Care Act, which creates new structures to facilitate and incentives to promote cost reductions. More importantly, there has been a sea change in how the publicnot just payors or employersview healthcare costs. The ideas that care is too expensive and that much of it adds no value to patients have gained wide acceptance across the political spectrum, among patients, and increasingly among physicians.
It was in this context that the American Board of Internal Medicine Foundation (ABIMF) launched its Choosing Wisely campaign in 2011.[1] The stated goal of the campaign was to promote important conversations [between doctors and patients] necessary to ensure the right care is delivered at the right time. Importantly, this careful framing successfully avoided the caricatures of rationing or death panels, reactions that doomed prior efforts to engage all stakeholders in a reasoned national dialogue about costs and value.
The ABIMF chose an approach of having physicians identify tests and procedures that may be unnecessary in certain situations. Working with Consumer Reports, the Foundation asked a wide range of medical specialty societies to develop their own list of tests and procedures that could potentially be avoided with no harm to patients. The vast majority, 25 as of July 2013, chose to participate.
In February 2013, the Society of Hospital Medicine (SHM) joined the initiative when it posted adult and pediatric versions of Five Things Physicians and Patients Should Question.[2] We are pleased to publish summaries of the recommendations and the processes by which the 2 working groups produced their lists in the Journal of Hospital Medicine.[3, 4]
In reading these articles, we are struck by the importance of the SHM's work to reduce costs and improve value. However, it really is a first step: both articles must now catalyze a body of work to create and sustain meaningful change.
Although many of the 10 targets have strong face validity, it is not clear whether they are in fact the most common, costly, or low‐value practices under the purview of hospitalists. Given the fact that the selection process involved both evidence‐based reviews and consensus, it is possible that other, potentially more contentious, practices may provide even more bang for the buck, or in this case, nonbuck.
Nevertheless, these are quibbles. These lists are good starting points, and in fact many hospitalist groups, including our own, are using the SHM practices as a foundation for our waste‐reduction efforts. The next challenge will be translating these recommendations into actionable measures and then clinical practice. For example, 1 of the adult recommendations is to avoid repeat blood counts and chemistries in patients who are clinically stable. Concepts of clinical stability are notoriously difficult to define within specific patient subgroups, much less across the diverse patient populations seen by hospitalists. One approach here would be to narrow the focus (eg, do not order repeated blood counts in patients with gastrointestinal bleeding whose labs have been stable for 48 hours), but this step would limit the cost savings. Other measures, such as those related to urinary catheters, are more clearly defined and seem closer to being widely adoptable.
For all these measures, the ultimate question remains: How much can actually be saved and how do we measure the savings? The marginal cost of a complete blood count is extraordinarily small in comparison to an entire hospital stay, but it is possible that eliminating redundant testing also reduces the costs related to follow‐up of false positive findings. Reducing the use of urinary catheters can cut the costs of urinary tract infections and the complications of treatment, but these costs could be offset by the higher‐level nursing care needed to mobilize patients earlier or assist patients in toileting, squeezing the proverbial balloon. For all these measures, it is unclear whether what might be relatively small variable cost reductions related to specific tests/procedures can lead to subsequent reduction in fixed costs related to facilities and equipment, where more than 70% of healthcare costs lie.[5] In other words, reducing the number of lab technicians and the amount of laboratory equipment needed will lead to far greater cost reductions than reducing individual test utilization.
None of this is to say that the Choosing Wisely campaign is without merit. To the contrary, the campaign and the efforts of the SHM are early and critical steps in changing the behavior of a profession. Since the early days of hospital medicine, hospitalists have embraced cost reduction and value improvement as a central focus. By successfully engaging consumers and the community of medical specialties, Choosing Wisely has created a language and a framework that will allow our field and others to tackle the crucial work of resource stewardship with new purpose, and we hope, unprecedented success.
Disclosures
Dr. Wachter is immediate past‐chair of the American Board of Internal Medicine (ABIM) and serves on the ABIM Foundation's Board of Trustees. Dr. Auerbach receives honoraria from the American Board of Internal Medicine as a contributor to the Maintenance of Certification question pool.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Are you choosing wisely? 2013. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement8:486–492.
- , , , et al. Choosing Wisely in inpatient pediatrics: 5 opportunities for improved healthcare value. J Hosp Med. 2013;8:479–485.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281:644–649.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- Are you choosing wisely? 2013. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement8:486–492.
- , , , et al. Choosing Wisely in inpatient pediatrics: 5 opportunities for improved healthcare value. J Hosp Med. 2013;8:479–485.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281:644–649.
Procalcitonin‐Guided Antibiotic Therapy
Many serum biomarkers have been identified in recent years with a wide range of potential applications, including diagnosis of local and systemic infections, differentiation of bacterial and fungal infections from viral syndromes or noninfectious conditions, prognostic stratification of patients, and enhanced management of antibiotic therapy. Currently, there are at least 178 serum biomarkers that have potential roles to guide antibiotic therapy, and among these, procalcitonin has been the most extensively studied biomarker.[1, 2]
Procalcitonin is the prohormone precursor of calcitonin that is expressed primarily in C cells of the thyroid gland. Conversion of procalcitonin to calcitonin is inhibited by various cytokines and bacterial endotoxins. Procalcitonin's primary diagnostic utility is thought to be in establishing the presence of bacterial infections, because serum procalcitonin levels rise and fall rapidly in bacterial infections.[3, 4, 5] In healthy individuals, procalcitonin levels are very low. In systemic infections, including sepsis, procalcitonin levels are generally greater than 0.5 to 2 ng/mL, but often reach levels 10 ng/mL, which correlates with severity of illness and a poor prognosis. In patients with respiratory tract infections, procalcitonin levels are less elevated, and a cutoff of 0.25 ng/mL seems to be most predictive of a bacterial respiratory tract infection requiring antibiotic therapy.[6, 7, 8] Procalcitonin levels decrease to 0.25 ng/mL as infection resolves, and a decline in procalcitonin level may guide decisions about discontinuation of antibiotic therapy.[5]
The purpose of this systematic review was to synthesize comparative studies examining the use of procalcitonin to guide antibiotic therapy in patients with suspected local or systemic infections in different patient populations. We are aware of 6 previously published systematic reviews evaluating the utility of procalcitonin guidance in the management of infections.[9, 10, 11, 12, 13, 14] Our systematic review included more studies and pooled patients into the most clinically similar groups compared to other systematic reviews.
METHODS
This review is based on a comparative effectiveness review prepared for the Agency for Healthcare Research and Quality's Effective Health Care Program.[15] A standard protocol consistent with the Methods Guide for Effectiveness and Comparative Effectiveness Reviews[16] was followed. A detailed description of the methods is available online (
Study Question
In selected populations of patients with suspected local or systemic infection, what are the effects of using procalcitonin measurement plus clinical criteria for infection to guide initiation, intensification, and/or discontinuation of antibiotic therapy when compared to clinical criteria for infection alone?
Search Strategy
MEDLINE and EMBASE were searched from January 1, 1990 through December 16, 2011, and the Cochrane Controlled Trials register was searched with no date restriction for randomized and nonrandomized comparative studies using the following search terms: procalcitonin AND chronic obstructive pulmonary disease; COPD; critical illness; critically ill; febrile neutropenia; ICU; intensive care; intensive care unit; postoperative complication(s); postoperative infection(s); postsurgical infection(s); sepsis; septic; surgical wound infection; systemic inflammatory response syndrome OR postoperative infection. In addition, a search for systematic reviews was conducted in MEDLINE, the Cochrane Database of Systematic Reviews, and Web sites of the National Institute for Clinical Excellence, the National Guideline Clearinghouse, and the Health Technology Assessment Programme. Gray literature, including databases with regulatory information, clinical trial registries, abstracts and conference papers, grants and federally funded research, and manufacturing information was searched from January 1, 2006 to June 28, 2011.
Study Selection
A single reviewer screened abstracts and selected studies looking at procalcitonin‐guided antibiotic therapy. Second and third reviewers were consulted to screen articles when needed. Studies were included if they fulfilled all of the following criteria: (1) randomized, controlled trial or nonrandomized comparative study; (2) adult and/or pediatric patients with known or suspected local or systemic infection, including critically ill patients with sepsis syndrome or ventilator‐associated pneumonia, adults with respiratory tract infections, neonates with sepsis, children with fever of unknown source, and postoperative patients at risk of infection; (3) interventions included initiation, intensification, and/or discontinuation of antibiotic therapy guided by procalcitonin plus clinical criteria; (4) primary outcomes included antibiotic usage (antibiotic prescription rate, total antibiotic exposure, duration of antibiotic therapy, and days without antibiotic therapy); and (5) secondary outcomes included morbidity (antibiotic adverse events, hospital and/or intensive care unit length of stay), mortality, and quality of life.
Studies with any of the following criteria were excluded: published in non‐English language, not reporting primary data from original research, not a randomized, controlled trial or nonrandomized comparative study, not reporting relevant outcomes.
Data Extraction and Quality Assessment
A single reviewer abstracted data and a second reviewer confirmed accuracy. Disagreements between reviewers were resolved by group discussion among the research team and final quality rating was assigned by consensus adjudication. Data elements were abstracted into the following categories: quality assessment, applicability and clinical diversity assessment, and outcome assessment. Quality of included studies was assessed using the US Preventive Services Task Force framework[17] by at least 2 independent reviewers. Three quality categories were used: good, fair, and poor.
Data Synthesis and Analysis
The decision to incorporate formal data synthesis in this review was made after completing the formal literature search, and the decision to pool studies was based on the specific number of studies with similar questions and outcomes. If a meta‐analysis could be performed, subgroup and sensitivity analyses were based on clinical similarity of available studies and reporting of mean and standard deviation. The pooling method involved inverse variance weighting and a random effects model.
The strength of evidence was graded using the Methods Guide,[16] a system based on the Grading of Recommendations Assessment, Development and Evaluation Working Group.[18] The following domains were addressed: risk of bias, consistency, directness, and precision. The overall strength of evidence was graded as high, moderate, low, or insufficient. The final strength of evidence grading was made by consensus adjudication among the authors.
RESULTS
Of the 2000 studies identified through the literature search, 1986 were excluded and 14 studies[19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32] were included. Search of gray literature yielded 4 published studies.[33, 34, 35, 36] A total of 18 randomized, controlled trials comparing procalcitonin guidance to use of clinical criteria alone to manage antibiotic therapy in patients with infections were included. The PRISMA diagram (Figure 1) depicts the flow of search screening and study selection. We sought, but did not find, nonrandomized comparative studies of populations, comparisons, interventions, and outcomes that were not adequately studied in randomized, controlled trials.
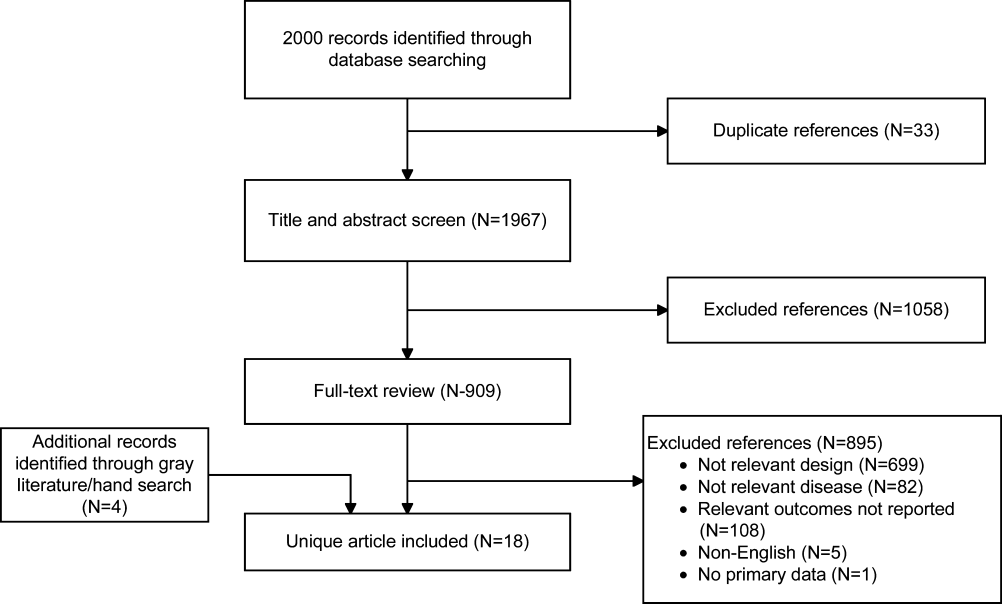
Data were pooled into clinically similar groups that were reviewed separately: (1) adult intensive care unit (ICU) patients, including patients with ventilator‐associated pneumonia; (2) adult patients with respiratory tract infections; (3) neonates with suspected sepsis; (4) children between 1 to 36 months of age with fever of unknown source; and (5) postoperative patients at risk of infection. Tables summarizing study quality and outcome measures with strength of evidence are available online (
| Outcome | Author, Year | N | PCT‐Guided Therapya | Controla | Difference PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ABT Duration, d | Hochreiter, 2009[22] | 110 | 5.9 | 7.9 | 2.0 (2.5 to 1.5) | 0.001 |
| Nobre, 2008[19] | 79 | 66 | 9.5 (ITT), 10 (PP) | 2.6 (5.5 to 0.3), 3.2 (1.1 to5.4) | 0.15, 0.003 | |
| Schroeder, 2009[20] | 27 | 6.6 | 8.3 | 1.7 (2.4 to 1.0) | 0.001 | |
| Stolz, 2009[21] | 101 | 10 (616)b | 15 (1023)b | 5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 10.3 | 13.3 | 3.0 (4.20 to 1.80) | 0.0001 | |
| Days without ABTs, day 28 | Nobre, 2008[19] | 79 | 15.3, 17.4 | 13, 13.6 | 2.3 (5.9 to 1.8), 3.8 (0.1 to 7.5)c | 0.28, 0.04 |
| Stolz, 2009[21] | 101 | 13 (221)b | 9.5 (1.517)b | 3.5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 14.3 | 11.6 | 2.7 (1.4 to 4.1) | 0.001 | |
| Total ABT exposured | Nobre, 2008[19] | 79 | 541 | 644 | 1.1e (0.9 to 1.3), 1.3e (1.1 to 1,5)c | 0.07, 0.0002 |
| 504 | 655 | |||||
| Stolz, 2009[21] | 101 | 1077 | 1341 | |||
| Bouadma, 2010[23]d | 621 | 653 | 812 | 159 (185 to 131) | 0.001 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ABT duration, days | Jensen, 2011[33] | 1200 | 6 (311)b | 4 (310)b | NR | NR |
| Days spent in ICU on 3 ABTs | Jensen, 2011[33] | 1200 | 3570/5447 (65.5%) | 2721/4717 (57.7%) | 7.9% (6.0 to 9.7) | 0.002 |
| Adult patients with respiratory tract infections | ||||||
| ABT duration, da | Schuetz, 2009[2][5] | 1359 | 5.7 | 8.7 | 3.0 | |
| Christ‐Crain, 2004[30] | 243 | 10.9 | 12.8 | 1.9 (3.1 to 0.7) | 0.002 | |
| Kristoffersen, 2009[26] | 210 | 5.1 | 6.8 | 1.7 | ||
| Briel, 2008[27] | 458 | 6.2 | 7.1 | 1.0 (1.7 to 0.4) | 0.05 | |
| Long, 20113[5] | 162 | 5 (36)f | 7 (59)f | 2.0 | 0.001 | |
| Burkhardt, 2010[34] | 550 | 7.8 | 7.7 | 0.1 (0.7 to 0.9) | 0.8 | |
| Christ‐Crain, 2006[29] | 302 | 5.8 | 12.9 | 7.1(8.4 to 5.8) | 0.0001 | |
| Antibiotic prescription rate, % | Schuetz, 2009[2][5] | 1359 | 506/671 (75.4%) | 603/688 (87.6%) | 12.2% (16.3 to 8.1) | 0.05 |
| Christ‐Crain, 2004[30] | 243 | 55/124 (44.4%) | 99/119 (83.2%) | 38.8% (49.9 to 27.8) | 0.0001 | |
| Kristoffersen, 2009[26] | 210 | 88/103 (85.4%) | 85/107 (79.4%) | 6.0% (4.3 to 16.2) | 0.25 | |
| Briel, 2008[27] | 458 | 58/232 (25.0%) | 219/226 (96.9%) | 72% (78 to 66) | 0.05 | |
| Long, 20113[5] | 162 | NR (84.4%) | NR (97.5%) | 13.1% | 0.004 | |
| Stolz, 2007[28] | 208 | 41/102 (40.2%) | 76/106 (71.7%) | 31.5% (44.3 to 18.7) | 0.0001 | |
| Christ‐Crain, 2006[29] | 302 | 128/151 (84.8%) | 149/151 (98.79%) | 13.9% (19.9 to 7.9) | 0.0001 | |
| Burkhardt, 2010[34] | 550 | 84/275 (30.5%) | 89/275 (32.4%) | 1.8% (9.6 to 5.9) | 0.701 | |
| Total ABT exposure | Stolz, 2007[28] | 208 | NR | NR | 31.5% (18.7 to 44.3) | 0.0001 |
| Long, 20113[5] | 162 | NR | NR | NR | ||
| Christ‐Crain, 2006[29] | 302 | 136g | 323g | |||
| Christ‐Crain, 2004[30] | 243 | 332g | 661g | |||
| Neonates with sepsis | ||||||
| ABTs 72 hours, % | Stocker, 2010[31] | All neonates (N=121) | 33/60 (55%) | 50/61 (82%) | 27.0 (42.8 to 11.1) | 0.002 |
| Infection proven/probably (N=21) | 9/9 (100%) | 12/12 (100%) | 0% (0 to 0) | NA | ||
| Infection possible (N=40) | 13/21 (61.9%) | 19/19 (100%) | 38.1 (58.9 to 17.3) | 0.003 | ||
| Infection unlikely (N=60) | 11/30 (36.7%) | 19/30 (63.3%) | 26.6 (51.1 to 2.3) | 0.038 | ||
| ABT duration, h | Stocker, 2010[31] | All neonates (N=121) | 79.1 | 101.5 | 22.4 | 0.012 |
| Infection proven/probably (N=21) | 177.8 | 170.8 | 7 | NSS | ||
| Infection possible (N=40) | 83.4 | 111.5 | 28.1 | 0.001 | ||
| Infection unlikely (N=60) | 46.5 | 67.4 | 20.9 | 0.001 | ||
| Children ages 136 months with fever of unknown source | ||||||
| Antibiotic prescription rate, % | Manzano, 2010[36] | All children (N=384) | 48/192 (25%) | 54/192 (28.0%) | 3.1 (12.0 to 5.7) | 0.49 |
| No SBI or neutropenia (N=312) | 14/158 (9%) | 16/154 (10%) | 1.5 (8.1 to 5.0) | 0.65 | ||
| Adult postoperative patients at risk of infection | ||||||
| ABT duration, d | Chromik, 2006[32] | All patients (N=20) | 5.5 | 9 | 3.5 | 0.27 |
| Outcome | Author, Year | N | PCTa | Controla | Difference, PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ICU LOS, days | Hochreiter, 2009[22] | 110 | 15.5 | 17.7 | 2.2 | 0.046 |
| Nobre, 2008[19] | 79 | 4 | 7 | 4.6 (8.2 to 1.0) | 0.02 | |
| Schroeder, 2009[20] | 27 | 16.4 | 16.7 | 0.3 (5.6 to 5.0) | NSS | |
| Bouadma, 2010[23] | 621 | 15.9 | 14.4 | 1.5 (0.9 to 3.1) | 0.23 | |
| Hospital LOS, days | Nobre, 2008[19] | 79 | 17 | 23.5 | 2.5 (6.5 to 1.5) | 0.85 |
| Stolz, 2009[21] | 101 | 26 (721)b | 26 (16.822.3)b | 0 | 0.15 | |
| Bouadma, 2010[23] | 621 | 26.1 | 26.4 | 0.3 (3.2 to 2.7) | 0.87 | |
| ICU‐free days alive, 128 | Stolz, 2009[21] | 101 | 10 (018)b | 8.5 (018)c | 1.5 | 0.53 |
| SOFA day 28 | Bouadma, 2010[23] | 621 | 1.5 | 0.9 | 0.6 (0.0, 1.1) | 0.037 |
| SOFA score max | Schroeder, 2009[20] | 27 | 7.3 | 8.3 | 8.1 (4.1 to 1.7) | NSS |
| SAPS II score | Hochreiter, 2009[22] | 110 | 40.1 | 40.5 | 0.4 (6.4 to 5.6) | >0.05 |
| Days without MV | Stolz, 2009[21] | 101 | 21 (224)b | 19 (8.522.5)b | 2.0 | 0.46 |
| Bouadma, 2010[23] | 621 | 16.2 | 16.9 | 0.7 (2.4 to 1.1) | 0.47 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ICU LOS, da | Svoboda, 2007[24] | 72 | 16.1 | 19.4 | 3.3 (7.0 to 0.4) | 0.09 |
| Jensen, 2011[33] | 1200 | 6 (312)b | 5 (311)b | 1 | 0.004 | |
| SOFA scorea | Svoboda, 2007[24] | 72 | 7.9 | 9.3 | 1.4 (2.8 to 0.0) | 0.06 |
| Days on MVa | Svoboda, 2007[24] | 72 | 10.3 | 13.9 | 3.6 (7.6 to 0.4) | 0.08 |
| Jensen, 2011[33] | 1200 | 3569 (65.5%) | 2861 (60.7%) | 4.9% (3 to 6.7) | 0.0001 | |
| Percent days in ICU with GFR 60 | Jensen, 2011[33] | 1200 | 2796 (51.3%) | 2187 (46.4%) | 5.0 % (3.0 to 6.9) | 0.0001 |
| Adult patients with respiratory tract infections | ||||||
| Hospital LOS, da | Schuetz, 2009[2][5] | 1359 | 9.4 | 9.2 | 0.2 | |
| Christ‐Crain, 2004[30] | 224 | 10.78.9 | 11.210.6 | 0.5 (3.0 to 2.0) | 0.69 | |
| Kristoffersen, 2009[26] | 210 | 5.9 | 6.7 | 0.8 | 0.22 | |
| Stolz, 2007[28] | 208 | 9 (115)b | 10 (115)b | 1 | 0.96 | |
| Christ‐Crain, 2006[29] | 302 | 12.09.1 | 13.09.0 | 1 (3.0 to 1.0) | 0.34 | |
| ICU admission, % | Schuetz, 2009[2][5] | 1359 | 43/671 (6.4%) | 60/688 (8.7%) | 2.3% (5.2 to 0.4) | 0.12 |
| Christ‐Crain, 2004[30] | 224 | 5/124 (4.0%) | 6/119 (5.0%) | 1.0% (6.2 to 4.2) | 0.71 | |
| Kristoffersen, 2009[26] | 210 | 7/103 (6.8%) | 5/107 (4.7%) | 2.1% (4.2 to 8.4) | 0.51 | |
| Stolz, 2007[28] | 208 | 8/102 (7.8%) | 11/106 (10.4%) | 2.5% (10.3 to 5.3) | 0.53 | |
| Christ‐Crain, 2006[29] | 302 | 20/151 (13.2%) | 21/151 (13.94%) | 0.7% (8.4 to 7.1) | 0.87 | |
| Antibiotic adverse events | Schuetz, 2009[2][5]c | 1359 | 133/671 (19.8%) | 193/688 (28.1%) | 8.2% (12.7 to 3.7) | |
| Briel, 2008[27]d | 458 | 2.34.6 days | 3.66.1 days | 1.1 days (2.1 to 0.1) | 0.05 | |
| Burkhardt, 2010[34]e | 550 | 11 /59 (18.6%) | 16/101 (15.8%) | 2.8% (9.4 to 15.0) | 0.65 | |
| Restricted activity, df | Briel, 2008[27] | 458 | 8.73.9 | 8.63.9 | 0.2 (0.4 to 0.9) | >0.05 |
| Burkhardt, 2010[34] | 550 | 9.1 | 8.8 | 0.25 (0.52 to 1.03) | >0.05 | |
| Neonates with sepsis | ||||||
| Recurrence of infection | Stocker, 2010[31] | 121 | 32% | 39% | 7 | 0.45 |
| Children ages 136 months with fever of unknown source | ||||||
| Hospitalization rate | Manzano, 2010[36] | All children (N=384) | 50/192 (26%) | 48/192 (25%) | 1 (8 to 10) | 0.81 |
| No SBI or neutropenia (N=312) | 16/158 (10%) | 11/154 (7%) | 3 (3 to 10) | 0.34 | ||
| Adult postoperative patients at risk of infection | ||||||
| Hospital LOS, days | Chromik, 2006[32] | 20 | 18 | 30 | 12 | 0.057 |
| Local wound infection, % | Chromik, 2006[32] | 20 | 1/10 | 2/10 | 10 (41.0 to 21.0) | 0.53 |
| Systemic infection, % | Chromik, 2006[32] | 20 | 3/10 | 7/10 | 40.0 (80.2 to 0.2) | 0.07 |
| Sepsis/SIRS, % | Chromik, 2006[32] | 20 | 2/10 | 8/10 | 60.0 (95.1 to 24.9) | 0.007 |
| Mortality | Mortality | Difference | ||||
|---|---|---|---|---|---|---|
| Outcome | Author, Year | N | PCT‐Guided Therapy | Control | PCT‐CTRL (95% CI) | P Value |
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| 28‐day mortality | Nobre, 2008[19] | 79 | 8/39 (20.5%) | 8/40 (20.0%) | 0.5 (17.2 to 18.2), | 0.95 |
| 5/31 (16.1%) | 6/37 (16.2%) | 0.1 (17.7 to 17.5)a | 0.99 | |||
| Stolz, 2009[21] | 101 | 8/51 (15.7%) | 12/50 (24.0%) | 8.3 (23.8 to 7.2) | 0.29 | |
| Bouadma, 2010[23] | 621 | 65/307 (21.2%) | 64/314 (20.4%) | 0.8 (5.6 to 7.2) | 0.81 | |
| 60‐day mortality | Bouadma, 2010[23] | 621 | 92/307 (30.0%) | 82/314 (26.1%) | 3.9 (3.2 to 10.9) | 0.29 |
| In‐hospital mortality | Nobre, 2008[19] | 79 | 9/39 (23.1%) | 9/40 (22.5%) | 0.6 (17.9 to 19.1) | 0.95 |
| 6/31 (19.4%) | 7/37 (18.9%) | 0.4+ (18.3 to 19.2) | 0.96 | |||
| Stolz, 2009[21] | 101 | 10/51 (19.6%) | 14/50 (28.0%) | 8.4, (24.9 to 8.1) | 0.32 | |
| Hochreiter, 2009[22] | 110 | 15/57 (26.3%) | 14/53 (26.4%) | 0.1, (16.6 to 16.4) | 0.99 | |
| Schroeder, 2009[20] | 27 | 3/14 (21.4%) | 3/13 (23.1%) | 1.7, (33.1 to 29.8) | 0.92 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| 28‐day mortality | Svoboda, 2007[24] | 72 | 10/38 (26.3%) | 13/34 (38.2%) | 11.9 (33.4 to 9.6) | 0.28 |
| 28‐day mortality | Jensen, 2011[33] | 1200 | 190/604 (31.5%) | 191/596 (32.0%) | 0.6 (4.7 to 5.9) | 0.83 |
| Adult patients with respiratory tract infections | ||||||
| 6‐month mortality | Stolz, 2007[28] | 208 | 5/102 (4.9%) | 9/106 (8.5%) | 3.6% (10.3 to 3.2%) | 0.30 |
| 6‐week mortality | Christ‐Crain, 2006[29] | 302 | 18/151 (11.9%) | 20/151 (13.2%) | 1.3% (8.8 to 6.2) | 0.73 |
| 28‐day mortality | Christ‐Crain, 2004[30] | 243 | 4/124(3.2%) | 4/119 (3.4%) | 0.1% (4.6 to 4.4) | 0.95 |
| Schuetz, 2009 (30‐day)[25] | 1359 | 34/671(5.1%) | 33/688(4.8%) | 0.3% (2.1 to 2.5) | 0.82 | |
| Briel, 2008[27] | 458 | 0/231(0%) | 1/224 (0.4%) | 0.4% (1.3 to 0.4) | 0.31 | |
| Burkhardt, 2010[34] | 550 | 0/275(0%) | 0/275 (0%) | 0 | ||
| Kristoffersen, 2009[26] | 210 | 2/103(1.9%) | 1/107 (0.9%) | 1.0% (2.2 to 4.2) | 0.54 | |
| Long, 20113[5] | 162 | 0/81 (0%) | 0/81 (0%) | 0 | ||
| Neonates with sepsis | ||||||
| Mortality (in‐hospital) | Stocker, 2010[31] | 121 | 0% | 0% | 0 (0 to 0) | NA |
| Children ages 136 months with fever of unknown source | ||||||
| Mortality | Manzano, 2010[36] | 384 | All children | 0% | 0% | 0 (0 to 0) |
| Adult postoperative patients at risk of infection | ||||||
| Mortality | Chromik, 2006[32] | 20 | 1/10 (10%) | 3/10 (30%) | 20 (54.0 to 14.0) | 0.07 |
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Discontinuation
Five studies[19, 20, 21, 22, 23] (N=938) addressed procalcitonin‐guided discontinuation of antibiotic therapy in adult ICU patients. Four studies conducted superiority analyses for mortality with procalcitonin‐guided therapy, whereas 1 study conducted a noninferiority analysis. Absolute procalcitonin values for discontinuation of antibiotics ranged from 0.25 to 1 ng/mL. Physicians in control groups administered antibiotics according to their standard practice.
Antibiotic Usage
The absolute reduction in duration of antibiotic usage with procalcitonin guidance in these studies ranged from 1.7 to 5 days, and the relative reduction ranged from 21% to 38%. Meta‐analysis of antibiotic duration in adult ICU patients was performed (Figure 2A).
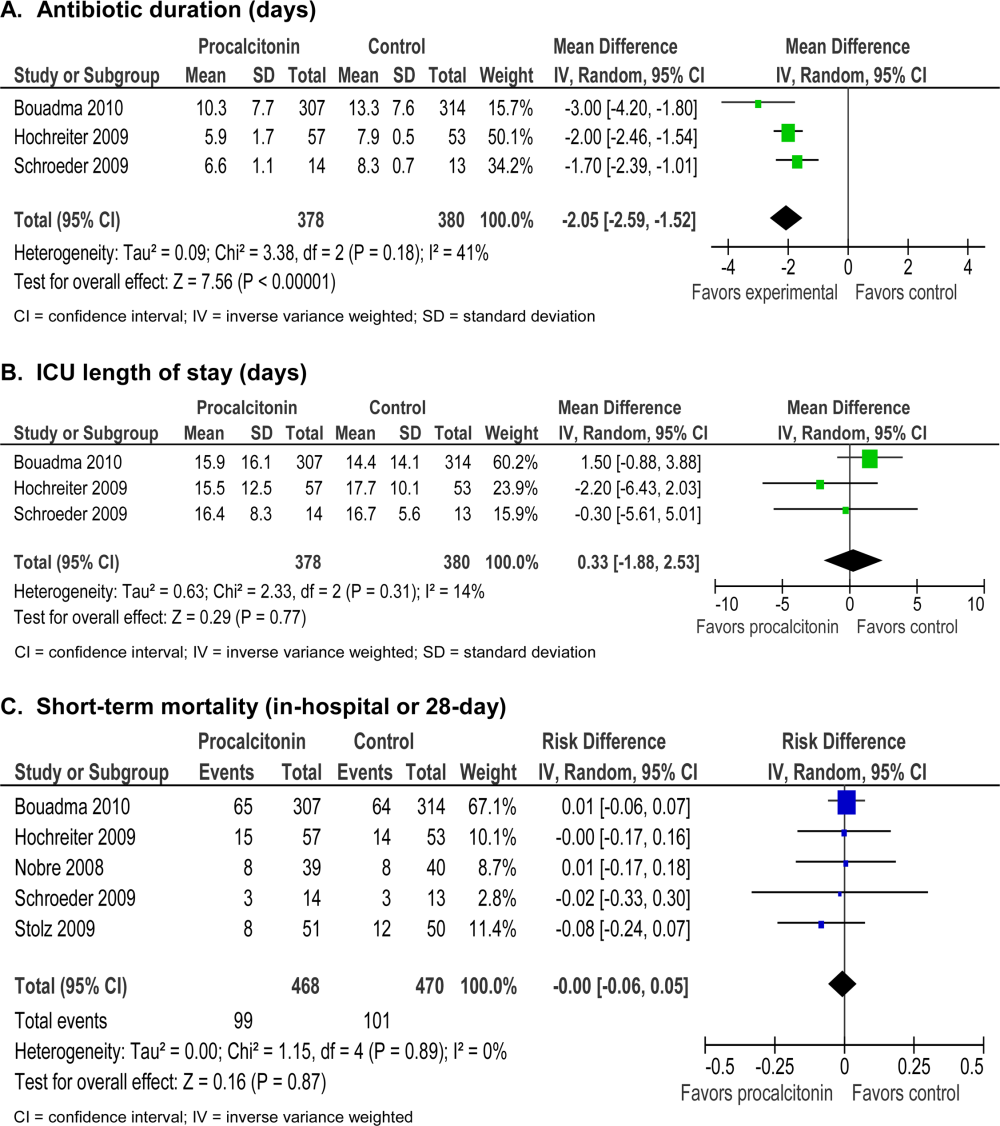
Morbidity
Procalcitonin‐guided antibiotic discontinuation did not increase morbidity, including ICU length of stay (LOS). Meta‐analysis of ICU LOS is displayed in Figure 2B. Limited data on adverse antibiotic events were reported (Table 2).
Mortality
There was no increase in mortality as a result of shorter duration of antibiotic therapy. Meta‐analysis of short‐term mortality (28‐day or in‐hospital mortality) showed a mortality difference of 0.43% favoring procalcitonin‐guided therapy, and a 95% confidence interval (CI) of 6% to 5% (Figure 2C).
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Intensification
Two studies[24, 33] (N=1272) addressed procalcitonin‐guided intensification of antibiotic therapy in adult ICU patients. The Jensen et al. study[33] was a large (N=1200), high‐quality study that used a detailed algorithm for broadening antibiotic therapy in patients with elevated procalcitonin. The Jensen et al. study also educated physicians about empiric therapy and intensification of antibiotic therapy. A second study[24] was too small (N=72) and lacked sufficient details to be informative.
Antibiotic Usage
The Jensen et al. study found a 2‐day increase, or 50% relative increase, in the duration of antibiotic therapy and a 7.9% absolute increase (P=0.002) in the number of days on 3 antibiotics with procalcitonin‐guided intensification.
Morbidity
The Jensen et al. study showed a significant 1‐day increase in ICU LOS (P=0.004) and a significant increase in organ dysfunction. Specifically, patients had a highly statistically significant 5% increase in days on mechanical ventilation (P0.0001) and 5% increase in days with abnormal renal function (P0.0001).
Mortality
The Jensen et al. study was a superiority trial powered to test a 7.5% decrease in 28‐day mortality, but no significant difference in mortality was observed with procalcitonin‐guided intensification (31.5% vs 32.0, P=0.83).
Adult Patients With Respiratory Tract Infections
Eight studies[25, 26, 27, 28, 29, 30, 34, 35] (N=3492) addressed initiation and/or discontinuation of antibiotics in adult patients with acute upper and lower respiratory tract infections, including community‐acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease, and acute bronchitis. Settings included primary care clinics, emergency departments, and hospital wards. Physicians in control groups administered antibiotics according to their own standard practices and/or evidence‐based guidelines. All studies encouraged initiation of antibiotics with procalcitonin levels >0.25 ng/mL, and 4 studies strongly encouraged antibiotics with procalcitonin levels >0.5 ng/mL.
Antibiotic Usage
Procalcitonin guidance reduced antibiotic duration, antibiotic prescription rate, and total antibiotic exposure. Absolute reduction in antibiotic duration ranged from 1 to 7 days, and relative reductions ranged from 13% to 55%. Four of the 8 studies reported sufficient details to be pooled into a meta‐analysis (Figure 3A) with a statistically significant pooled mean difference of 2.35 days favoring procalcitonin (95% CI: 4.38 to 0.33). Procalcitonin guidance also reduced antibiotic prescription rate with absolute reductions ranging from 2% to 7% and relative reductions ranging from 1.8% to 72%. Meta‐analysis of prescription rates from 8 studies (Figure 3B) yielded a statistically significant pooled risk difference of 22% (95% CI: 41% to 4%). Total antibiotic exposure was consistently reduced in the 4 studies reporting this outcome.
Morbidity
Procalcitonin guidance did not increase hospital LOS or ICU admission rates. Meta‐analysis of ICU admission rates from 5 studies (Figure 3C) produced a risk difference of 1%, with a narrow 95% CI (4% to 1%). There was insufficient evidence to judge the effect on days of restricted activity or antibiotic adverse events.
Mortality
Procalcitonin guidance did not increase mortality, and meta‐analysis of 4 studies (Figure 3D) produced a risk difference of 0.3% with a narrow 95% CI (1% to 2%), with no statistical heterogeneity (I2=0%).
Neonates With Sepsis
One study[31] (N=121) evaluated procalcitonin‐guided antibiotic therapy for suspected neonatal sepsis. Neonatal sepsis was suspected on the basis of risk factors and clinical signs and symptoms. Antibiotic initiation or discontinuation was based on a procalcitonin nomogram. Antibiotic therapy in the control group was based on the physician's assessment. The quality of this study was rated good, and strength of evidence was rated moderate for antibiotic usage and insufficient for morbidity and mortality outcomes.
Antibiotic Usage
Duration of antibiotic therapy was decreased by 22.4 hours (P=0.012), a 24% relative reduction, and the proportion of neonates on antibiotics 72 hours was reduced by 27% (P=0.002). The largest reduction in antibiotic duration was seen in the 80% to 85% of neonates who were categorized as having possible or infection or unlikely to have infection.
Morbidity
A statistically insignificant 7% reduction in rate of recurrence of infection was seen with procalcitonin‐guided antibiotic therapy (P=0.45).
Mortality
No in‐hospital deaths occurred in either the procalcitonin or control group.
Children Ages 1 to 36 Months With Fever of Unknown Source
One study[36] (N=384) evaluated procalcitonin‐guided antibiotic therapy for fever of unknown source in children 1 to 36 months of age, but the overall strength of evidence was judged insufficient to draw conclusions.
Antibiotic Usage
A statistically insignificant reduction of 3.1% in antibiotic prescription rate was seen with procalcitonin‐guided antibiotic therapy (P=0.49).
Morbidity
Rate of hospitalization was relatively low, and no significant difference was seen between procalcitonin and control groups.
Mortality
In‐hospital mortality was reported as 0% in both arms.
Adult Postoperative Patients at Risk of Infection
One study[32] (N =250) monitored procalcitonin in consecutive patients after colorectal surgery to identify patients at risk of infection who might benefit from prophylactic antibiotic therapy. Two hundred thirty patients had normal procalcitonin levels. Twenty patients with elevated procalcitonin levels (>1.5 ng/mL) were randomized to receive prophylactic antibiotic therapy with ceftriaxone or no antibiotics. The strength of evidence was judged insufficient to draw conclusions from this study.
Antibiotic Usage
Duration of antibiotic therapy was reduced by 3.5% but was not statistically insignificant (P=0.27).
Morbidity
Procalcitonin guidance reduced the incidence of sepsis/systemic inflammatory response syndrome by 60% (p=0.007). The incidences of local and systemic infection were reduced with procalcitonin guidance but were not statistically significant (10%, P=0.53; and 40%, P=0.07, respectively).
Mortality
Mortality was 20% higher in the control arm but was not statistically significant (P=0.07).
DISCUSSION
Summary of the Main Findings
Diagnosis of sepsis or other serious infections in critically ill patients is challenging because clinical criteria for diagnosis overlap with noninfectious causes of the systemic inflammatory response syndrome. Initiation of antibiotic therapy for presumed sepsis is necessary while diagnostic evaluation is ongoing, because delaying antibiotic therapy is associated with increased mortality.[37, 38, 39] Our review found that procalcitonin guidance significantly reduced antibiotic usage in adult ICU patients by reducing the duration of antibiotic therapy, rather than decreasing the initiation of antibiotics, without increasing morbidity or mortality.
In contrast, the use of procalcitonin as an indicator of need for intensification of antibiotic therapy in adult ICU patients should be discouraged because this approach was associated with increased morbidity. The large, well‐designed study by Jensen[33] showed that antibiotic intensification in response to elevated procalcitonin measurement was associated with increased morbidity: a longer ICU LOS, an increase in days on mechanical ventilation, and an increase in days with abnormal renal function. The authors concluded that the increased morbidity could only be explained by clinical harms of increased exposure to broad‐spectrum antibiotics.
Clinical and microbiological evaluations are neither sensitive nor specific for differentiating bacterial from viral respiratory tract infections. Procalcitonin can guide initiation of antibiotic therapy in adults with suspected bacterial respiratory tract infection. Our review showed that procalcitonin guidance significantly reduced antibiotic usage with respect to antibiotic prescription rate, duration of antibiotic therapy, and total exposure to antibiotic therapy in adult patients with respiratory tract infections.
The role of procalcitonin‐guided therapy in other populations is less clear. One study in postoperative colorectal surgery patients reported that elevated procalcitonin levels may identify patients at risk for infection who benefit from prophylactic antibiotic therapy.[32] Patients with elevated procalcitonin levels who received prophylactic antibiotic therapy had a significant decrease in the incidence and severity of systemic infections, whereas patients with normal procalcitonin levels did not require any additional surgical or medical therapy. Although these findings are promising, more data in postoperative patients are needed.
The utility of procalcitonin in pediatric settings is a significant gap in the present literature. One study[31] in neonates with suspected sepsis showed a significant decrease in the proportion of neonates started on empiric antibiotic therapy and a decrease in the duration of antibiotic therapy with procalcitonin guidance. However, there was insufficient evidence that procalcitonin guidance does not increase morbidity or mortality.
Comparison to Other Systematic Reviews
Six systematic reviews of procalcitonin guidance in the management of patients with infections were published prior to our review.[9, 10, 11, 12, 13, 14] Our systematic review differs from past reviews in the number of studies included and the pooling of studies according to patient population, type and severity of infection, and different uses of procalcitonin measurements, either for initiation, discontinuation, or intensification of antibiotic therapy. Previous systematic reviews included 7 to 14 studies, whereas ours included 18 randomized, controlled trials. One previous review[13] included and pooled the Jensen et al. study[33] with other studies of adult ICU patients. We evaluated the Jensen et al. study separately because it uniquely looked at procalcitonin‐guided antibiotic intensification in adult ICU patients, in contrast to other studies that looked at procalcitonin‐guided antibiotic discontinuation. We addressed pediatric populations separately from adult patients, and recognizing that there are distinct groups within the pediatric population, we separately grouped neonates and children ages 1 to 36 months. Despite these differences, our review and other systematic reviews, we came to similar conclusions: procalcitonin‐guided antibiotic decision making compared to clinical criteria‐guided antibiotic decision making reduces antibiotic usage without increasing morbidity or mortality.
Limitations
An important limitation of this review was the uncertainty about the noninferiority margin for morbidity and mortality in adult ICU patients. Only the Bouadma et al. study[23] did a power analysis and predefined a margin for noninferiority for 28‐ and 60‐day mortality. Meta‐analysis of all 5 ICU studies showed a pooled point estimate of 0.43% in mortality and a 95% CI of 6% to 5% for difference in mortality between procalcitonin‐guided therapy versus standard care. A 10% noninferiority margin for mortality has been recommended by the Infectious Diseases Society of America and American College of Chest Physicians, but there is concern that a 10% margin for mortality may be too high. Presently, 2 large trials are in progress that may yield more precise estimates of mortality in the future.
Differences in reporting of total antibiotic exposure and morbidity outcomes limited our ability to pool data. Total antibiotic exposure is conventionally reported as mean days per 1000 days of follow‐up, but some studies only reported relative or absolute differences. Likewise, morbidity was reported with different severity of illness scales, including Sepsis‐Related Organ Failure Assessment, Simplified Acute Physiology (SAP) II, SAP III, and Acute Physiology and Chronic Health Evaluation II, which limited comparisons across studies.
Research Gaps
We identified gaps in the available literature and opportunities for future research. First, the safety and efficacy of procalcitonin‐guided antibiotic therapy needs to be studied in patient populations excluded from current randomized controlled studies, such as immunocompromised patients and pregnant women. Patients who are immunocompromised or have chronic conditions, such as cystic fibrosis, account for a significant percentage of community‐acquired respiratory tract infections and are often treated empirically.[29, 30] Second, standardized reporting of antibiotic adverse events and emergence of antibiotic resistance is needed. Strategies to reduce antibiotic usage have been associated with reductions in antibiotic adverse events, such as Clostridium difficile colitis and superinfection with multi‐drug resistant Gram‐negative bacteria.[37, 40, 41] Few studies in our review reported allergic reactions or adverse events of antibiotic therapy, [25, 27, 34] and only 1 reported antibiotic resistance.[19] Third, procalcitonin guidance should be compared to other strategies to reduce antibiotic usage, such as structured implementation of practice guidelines and antibiotic stewardship programs.[42] One single‐arm study describes how procalcitonin can be used in antibiotic stewardship programs to decrease the duration of antibiotic therapy,[43] but additional studies are needed. Finally, generalizing results from those studies that were conducted primarily in Europe would depend on similar use of and adherence to study‐based algorithms. Newer observational studies have demonstrated reduced antibiotic usage with implementation of procalcitonin algorithms in real‐life settings in Europe, but algorithm adherence was significantly less in the United States.[44, 45]
In summary, our systematic review found that procalcitonin‐guided antibiotic therapy can significantly reduce antibiotic usage in adult ICU patients without affecting morbidity or mortality. Procalcitonin should not be used to guide intensification of antibiotic therapy in adult ICU patients because this approach may increase morbidity. In adults with respiratory infections, procalcitonin guidance can significantly reduce antibiotic usage without adversely affecting morbidity or mortality. There is insufficient evidence to recommend procalcitonin‐guided antibiotic therapy in neonates with sepsis, children with fever of unknown source, or postoperative patients at risk for infection.
Acknowledgments
Disclosures: This project was funded under contract HHSA 2902007‐10058 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services. The authors of this article are responsible for its content, including any clinical treatment recommendations. No statement in this article should be construed as an official position of AHRQ or of the US Department of Health and Human Services. There are no conflicts of interest reported by any of the authors.
- , . Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15.
- , . Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298.
- , , . Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888–889.
- , , , et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–1608.
- , , , et al. Procalcitonin kinetics as a prognostic marker of ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48–53.
- , , , , . Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clin Infect Dis. 2004;39(2):206–217.
- , . Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30(3):556–573.
- , , , et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26(3):664–672.
- , , , , . Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection. 2009;37(6):497–507.
- , . Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53(4):379–387.
- , , , , . Procalcitonin‐guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta‐analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229–2241.
- , , , . Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331.
- , , , , , . An ESCIM systematic review and meta‐analysis of procalcitonin‐guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med. 2012;38:940–949.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
- , , , , , . Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under contract no. 290–2007‐10058‐I. Procalcitonin‐guided antibiotic therapy. Comparative effectiveness review No. 78. AHRQ publication no. 12(13)‐EHC124‐EF. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Published Accessed October 2012.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ publication no. 10(11)‐EHC063‐EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , , . Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505.
- , , , et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226.
- , , , et al. Procalcitonin for reduced antibiotic exposure in ventilator‐associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375.
- , , , et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83.
- , , , et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474.
- , , , , . Can procalcitonin help us in timing of re‐intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology. 2007;54(74):359–363.
- , , , et al. Effect of procalcitonin‐based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin Microbiol Infect. 2009;15(5):481–487.
- , , , et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–2007; discussion 2007–2008.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131(1):9–19.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363(9409):600–607.
- , , , , . Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology. 2010;97(2):165–174.
- , , , , , . Pre‐emptive antibiotic treatment vs “standard” treatment in patients with elevated serum procalcitonin levels after elective colorectal surgery: a prospective randomised pilot study. Langenbecks Arch Surg. 2006;391(3):187–194.
- , , , et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–2058.
- , , , et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–607.
- , , , , , . Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community‐acquired pneumonia. Respirology. 2011;16(5):819–824.
- , , , , , . Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. Am J Emerg Med. 2010;28(6):647–653.
- , , , , , . Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia. Crit Care Med. 2001;29(6):1109–1115.
- , , , et al. Diagnostic value of procalcitonin, interleukin‐6, and interleukin‐8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402.
- , , , . Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462–474.
- , , , , . Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24(9):699–706.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- , , , , . Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). Eur J Clin Microbiol Infect Dis. 2011;30(7):853–855.
- , , , et al. Effectiveness and safety of procalcitonin‐guided antibiotic therapy in lower respiratory tract infections in “real life.” Arch Intern Med. 2012;172(9):715–722.
- , , , et al. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post‐study survey. Eur J Clin Microbiol Infect Dis. 2012;29(3):269–277.
Many serum biomarkers have been identified in recent years with a wide range of potential applications, including diagnosis of local and systemic infections, differentiation of bacterial and fungal infections from viral syndromes or noninfectious conditions, prognostic stratification of patients, and enhanced management of antibiotic therapy. Currently, there are at least 178 serum biomarkers that have potential roles to guide antibiotic therapy, and among these, procalcitonin has been the most extensively studied biomarker.[1, 2]
Procalcitonin is the prohormone precursor of calcitonin that is expressed primarily in C cells of the thyroid gland. Conversion of procalcitonin to calcitonin is inhibited by various cytokines and bacterial endotoxins. Procalcitonin's primary diagnostic utility is thought to be in establishing the presence of bacterial infections, because serum procalcitonin levels rise and fall rapidly in bacterial infections.[3, 4, 5] In healthy individuals, procalcitonin levels are very low. In systemic infections, including sepsis, procalcitonin levels are generally greater than 0.5 to 2 ng/mL, but often reach levels 10 ng/mL, which correlates with severity of illness and a poor prognosis. In patients with respiratory tract infections, procalcitonin levels are less elevated, and a cutoff of 0.25 ng/mL seems to be most predictive of a bacterial respiratory tract infection requiring antibiotic therapy.[6, 7, 8] Procalcitonin levels decrease to 0.25 ng/mL as infection resolves, and a decline in procalcitonin level may guide decisions about discontinuation of antibiotic therapy.[5]
The purpose of this systematic review was to synthesize comparative studies examining the use of procalcitonin to guide antibiotic therapy in patients with suspected local or systemic infections in different patient populations. We are aware of 6 previously published systematic reviews evaluating the utility of procalcitonin guidance in the management of infections.[9, 10, 11, 12, 13, 14] Our systematic review included more studies and pooled patients into the most clinically similar groups compared to other systematic reviews.
METHODS
This review is based on a comparative effectiveness review prepared for the Agency for Healthcare Research and Quality's Effective Health Care Program.[15] A standard protocol consistent with the Methods Guide for Effectiveness and Comparative Effectiveness Reviews[16] was followed. A detailed description of the methods is available online (
Study Question
In selected populations of patients with suspected local or systemic infection, what are the effects of using procalcitonin measurement plus clinical criteria for infection to guide initiation, intensification, and/or discontinuation of antibiotic therapy when compared to clinical criteria for infection alone?
Search Strategy
MEDLINE and EMBASE were searched from January 1, 1990 through December 16, 2011, and the Cochrane Controlled Trials register was searched with no date restriction for randomized and nonrandomized comparative studies using the following search terms: procalcitonin AND chronic obstructive pulmonary disease; COPD; critical illness; critically ill; febrile neutropenia; ICU; intensive care; intensive care unit; postoperative complication(s); postoperative infection(s); postsurgical infection(s); sepsis; septic; surgical wound infection; systemic inflammatory response syndrome OR postoperative infection. In addition, a search for systematic reviews was conducted in MEDLINE, the Cochrane Database of Systematic Reviews, and Web sites of the National Institute for Clinical Excellence, the National Guideline Clearinghouse, and the Health Technology Assessment Programme. Gray literature, including databases with regulatory information, clinical trial registries, abstracts and conference papers, grants and federally funded research, and manufacturing information was searched from January 1, 2006 to June 28, 2011.
Study Selection
A single reviewer screened abstracts and selected studies looking at procalcitonin‐guided antibiotic therapy. Second and third reviewers were consulted to screen articles when needed. Studies were included if they fulfilled all of the following criteria: (1) randomized, controlled trial or nonrandomized comparative study; (2) adult and/or pediatric patients with known or suspected local or systemic infection, including critically ill patients with sepsis syndrome or ventilator‐associated pneumonia, adults with respiratory tract infections, neonates with sepsis, children with fever of unknown source, and postoperative patients at risk of infection; (3) interventions included initiation, intensification, and/or discontinuation of antibiotic therapy guided by procalcitonin plus clinical criteria; (4) primary outcomes included antibiotic usage (antibiotic prescription rate, total antibiotic exposure, duration of antibiotic therapy, and days without antibiotic therapy); and (5) secondary outcomes included morbidity (antibiotic adverse events, hospital and/or intensive care unit length of stay), mortality, and quality of life.
Studies with any of the following criteria were excluded: published in non‐English language, not reporting primary data from original research, not a randomized, controlled trial or nonrandomized comparative study, not reporting relevant outcomes.
Data Extraction and Quality Assessment
A single reviewer abstracted data and a second reviewer confirmed accuracy. Disagreements between reviewers were resolved by group discussion among the research team and final quality rating was assigned by consensus adjudication. Data elements were abstracted into the following categories: quality assessment, applicability and clinical diversity assessment, and outcome assessment. Quality of included studies was assessed using the US Preventive Services Task Force framework[17] by at least 2 independent reviewers. Three quality categories were used: good, fair, and poor.
Data Synthesis and Analysis
The decision to incorporate formal data synthesis in this review was made after completing the formal literature search, and the decision to pool studies was based on the specific number of studies with similar questions and outcomes. If a meta‐analysis could be performed, subgroup and sensitivity analyses were based on clinical similarity of available studies and reporting of mean and standard deviation. The pooling method involved inverse variance weighting and a random effects model.
The strength of evidence was graded using the Methods Guide,[16] a system based on the Grading of Recommendations Assessment, Development and Evaluation Working Group.[18] The following domains were addressed: risk of bias, consistency, directness, and precision. The overall strength of evidence was graded as high, moderate, low, or insufficient. The final strength of evidence grading was made by consensus adjudication among the authors.
RESULTS
Of the 2000 studies identified through the literature search, 1986 were excluded and 14 studies[19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32] were included. Search of gray literature yielded 4 published studies.[33, 34, 35, 36] A total of 18 randomized, controlled trials comparing procalcitonin guidance to use of clinical criteria alone to manage antibiotic therapy in patients with infections were included. The PRISMA diagram (Figure 1) depicts the flow of search screening and study selection. We sought, but did not find, nonrandomized comparative studies of populations, comparisons, interventions, and outcomes that were not adequately studied in randomized, controlled trials.

Data were pooled into clinically similar groups that were reviewed separately: (1) adult intensive care unit (ICU) patients, including patients with ventilator‐associated pneumonia; (2) adult patients with respiratory tract infections; (3) neonates with suspected sepsis; (4) children between 1 to 36 months of age with fever of unknown source; and (5) postoperative patients at risk of infection. Tables summarizing study quality and outcome measures with strength of evidence are available online (
| Outcome | Author, Year | N | PCT‐Guided Therapya | Controla | Difference PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ABT Duration, d | Hochreiter, 2009[22] | 110 | 5.9 | 7.9 | 2.0 (2.5 to 1.5) | 0.001 |
| Nobre, 2008[19] | 79 | 66 | 9.5 (ITT), 10 (PP) | 2.6 (5.5 to 0.3), 3.2 (1.1 to5.4) | 0.15, 0.003 | |
| Schroeder, 2009[20] | 27 | 6.6 | 8.3 | 1.7 (2.4 to 1.0) | 0.001 | |
| Stolz, 2009[21] | 101 | 10 (616)b | 15 (1023)b | 5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 10.3 | 13.3 | 3.0 (4.20 to 1.80) | 0.0001 | |
| Days without ABTs, day 28 | Nobre, 2008[19] | 79 | 15.3, 17.4 | 13, 13.6 | 2.3 (5.9 to 1.8), 3.8 (0.1 to 7.5)c | 0.28, 0.04 |
| Stolz, 2009[21] | 101 | 13 (221)b | 9.5 (1.517)b | 3.5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 14.3 | 11.6 | 2.7 (1.4 to 4.1) | 0.001 | |
| Total ABT exposured | Nobre, 2008[19] | 79 | 541 | 644 | 1.1e (0.9 to 1.3), 1.3e (1.1 to 1,5)c | 0.07, 0.0002 |
| 504 | 655 | |||||
| Stolz, 2009[21] | 101 | 1077 | 1341 | |||
| Bouadma, 2010[23]d | 621 | 653 | 812 | 159 (185 to 131) | 0.001 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ABT duration, days | Jensen, 2011[33] | 1200 | 6 (311)b | 4 (310)b | NR | NR |
| Days spent in ICU on 3 ABTs | Jensen, 2011[33] | 1200 | 3570/5447 (65.5%) | 2721/4717 (57.7%) | 7.9% (6.0 to 9.7) | 0.002 |
| Adult patients with respiratory tract infections | ||||||
| ABT duration, da | Schuetz, 2009[2][5] | 1359 | 5.7 | 8.7 | 3.0 | |
| Christ‐Crain, 2004[30] | 243 | 10.9 | 12.8 | 1.9 (3.1 to 0.7) | 0.002 | |
| Kristoffersen, 2009[26] | 210 | 5.1 | 6.8 | 1.7 | ||
| Briel, 2008[27] | 458 | 6.2 | 7.1 | 1.0 (1.7 to 0.4) | 0.05 | |
| Long, 20113[5] | 162 | 5 (36)f | 7 (59)f | 2.0 | 0.001 | |
| Burkhardt, 2010[34] | 550 | 7.8 | 7.7 | 0.1 (0.7 to 0.9) | 0.8 | |
| Christ‐Crain, 2006[29] | 302 | 5.8 | 12.9 | 7.1(8.4 to 5.8) | 0.0001 | |
| Antibiotic prescription rate, % | Schuetz, 2009[2][5] | 1359 | 506/671 (75.4%) | 603/688 (87.6%) | 12.2% (16.3 to 8.1) | 0.05 |
| Christ‐Crain, 2004[30] | 243 | 55/124 (44.4%) | 99/119 (83.2%) | 38.8% (49.9 to 27.8) | 0.0001 | |
| Kristoffersen, 2009[26] | 210 | 88/103 (85.4%) | 85/107 (79.4%) | 6.0% (4.3 to 16.2) | 0.25 | |
| Briel, 2008[27] | 458 | 58/232 (25.0%) | 219/226 (96.9%) | 72% (78 to 66) | 0.05 | |
| Long, 20113[5] | 162 | NR (84.4%) | NR (97.5%) | 13.1% | 0.004 | |
| Stolz, 2007[28] | 208 | 41/102 (40.2%) | 76/106 (71.7%) | 31.5% (44.3 to 18.7) | 0.0001 | |
| Christ‐Crain, 2006[29] | 302 | 128/151 (84.8%) | 149/151 (98.79%) | 13.9% (19.9 to 7.9) | 0.0001 | |
| Burkhardt, 2010[34] | 550 | 84/275 (30.5%) | 89/275 (32.4%) | 1.8% (9.6 to 5.9) | 0.701 | |
| Total ABT exposure | Stolz, 2007[28] | 208 | NR | NR | 31.5% (18.7 to 44.3) | 0.0001 |
| Long, 20113[5] | 162 | NR | NR | NR | ||
| Christ‐Crain, 2006[29] | 302 | 136g | 323g | |||
| Christ‐Crain, 2004[30] | 243 | 332g | 661g | |||
| Neonates with sepsis | ||||||
| ABTs 72 hours, % | Stocker, 2010[31] | All neonates (N=121) | 33/60 (55%) | 50/61 (82%) | 27.0 (42.8 to 11.1) | 0.002 |
| Infection proven/probably (N=21) | 9/9 (100%) | 12/12 (100%) | 0% (0 to 0) | NA | ||
| Infection possible (N=40) | 13/21 (61.9%) | 19/19 (100%) | 38.1 (58.9 to 17.3) | 0.003 | ||
| Infection unlikely (N=60) | 11/30 (36.7%) | 19/30 (63.3%) | 26.6 (51.1 to 2.3) | 0.038 | ||
| ABT duration, h | Stocker, 2010[31] | All neonates (N=121) | 79.1 | 101.5 | 22.4 | 0.012 |
| Infection proven/probably (N=21) | 177.8 | 170.8 | 7 | NSS | ||
| Infection possible (N=40) | 83.4 | 111.5 | 28.1 | 0.001 | ||
| Infection unlikely (N=60) | 46.5 | 67.4 | 20.9 | 0.001 | ||
| Children ages 136 months with fever of unknown source | ||||||
| Antibiotic prescription rate, % | Manzano, 2010[36] | All children (N=384) | 48/192 (25%) | 54/192 (28.0%) | 3.1 (12.0 to 5.7) | 0.49 |
| No SBI or neutropenia (N=312) | 14/158 (9%) | 16/154 (10%) | 1.5 (8.1 to 5.0) | 0.65 | ||
| Adult postoperative patients at risk of infection | ||||||
| ABT duration, d | Chromik, 2006[32] | All patients (N=20) | 5.5 | 9 | 3.5 | 0.27 |
| Outcome | Author, Year | N | PCTa | Controla | Difference, PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ICU LOS, days | Hochreiter, 2009[22] | 110 | 15.5 | 17.7 | 2.2 | 0.046 |
| Nobre, 2008[19] | 79 | 4 | 7 | 4.6 (8.2 to 1.0) | 0.02 | |
| Schroeder, 2009[20] | 27 | 16.4 | 16.7 | 0.3 (5.6 to 5.0) | NSS | |
| Bouadma, 2010[23] | 621 | 15.9 | 14.4 | 1.5 (0.9 to 3.1) | 0.23 | |
| Hospital LOS, days | Nobre, 2008[19] | 79 | 17 | 23.5 | 2.5 (6.5 to 1.5) | 0.85 |
| Stolz, 2009[21] | 101 | 26 (721)b | 26 (16.822.3)b | 0 | 0.15 | |
| Bouadma, 2010[23] | 621 | 26.1 | 26.4 | 0.3 (3.2 to 2.7) | 0.87 | |
| ICU‐free days alive, 128 | Stolz, 2009[21] | 101 | 10 (018)b | 8.5 (018)c | 1.5 | 0.53 |
| SOFA day 28 | Bouadma, 2010[23] | 621 | 1.5 | 0.9 | 0.6 (0.0, 1.1) | 0.037 |
| SOFA score max | Schroeder, 2009[20] | 27 | 7.3 | 8.3 | 8.1 (4.1 to 1.7) | NSS |
| SAPS II score | Hochreiter, 2009[22] | 110 | 40.1 | 40.5 | 0.4 (6.4 to 5.6) | >0.05 |
| Days without MV | Stolz, 2009[21] | 101 | 21 (224)b | 19 (8.522.5)b | 2.0 | 0.46 |
| Bouadma, 2010[23] | 621 | 16.2 | 16.9 | 0.7 (2.4 to 1.1) | 0.47 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ICU LOS, da | Svoboda, 2007[24] | 72 | 16.1 | 19.4 | 3.3 (7.0 to 0.4) | 0.09 |
| Jensen, 2011[33] | 1200 | 6 (312)b | 5 (311)b | 1 | 0.004 | |
| SOFA scorea | Svoboda, 2007[24] | 72 | 7.9 | 9.3 | 1.4 (2.8 to 0.0) | 0.06 |
| Days on MVa | Svoboda, 2007[24] | 72 | 10.3 | 13.9 | 3.6 (7.6 to 0.4) | 0.08 |
| Jensen, 2011[33] | 1200 | 3569 (65.5%) | 2861 (60.7%) | 4.9% (3 to 6.7) | 0.0001 | |
| Percent days in ICU with GFR 60 | Jensen, 2011[33] | 1200 | 2796 (51.3%) | 2187 (46.4%) | 5.0 % (3.0 to 6.9) | 0.0001 |
| Adult patients with respiratory tract infections | ||||||
| Hospital LOS, da | Schuetz, 2009[2][5] | 1359 | 9.4 | 9.2 | 0.2 | |
| Christ‐Crain, 2004[30] | 224 | 10.78.9 | 11.210.6 | 0.5 (3.0 to 2.0) | 0.69 | |
| Kristoffersen, 2009[26] | 210 | 5.9 | 6.7 | 0.8 | 0.22 | |
| Stolz, 2007[28] | 208 | 9 (115)b | 10 (115)b | 1 | 0.96 | |
| Christ‐Crain, 2006[29] | 302 | 12.09.1 | 13.09.0 | 1 (3.0 to 1.0) | 0.34 | |
| ICU admission, % | Schuetz, 2009[2][5] | 1359 | 43/671 (6.4%) | 60/688 (8.7%) | 2.3% (5.2 to 0.4) | 0.12 |
| Christ‐Crain, 2004[30] | 224 | 5/124 (4.0%) | 6/119 (5.0%) | 1.0% (6.2 to 4.2) | 0.71 | |
| Kristoffersen, 2009[26] | 210 | 7/103 (6.8%) | 5/107 (4.7%) | 2.1% (4.2 to 8.4) | 0.51 | |
| Stolz, 2007[28] | 208 | 8/102 (7.8%) | 11/106 (10.4%) | 2.5% (10.3 to 5.3) | 0.53 | |
| Christ‐Crain, 2006[29] | 302 | 20/151 (13.2%) | 21/151 (13.94%) | 0.7% (8.4 to 7.1) | 0.87 | |
| Antibiotic adverse events | Schuetz, 2009[2][5]c | 1359 | 133/671 (19.8%) | 193/688 (28.1%) | 8.2% (12.7 to 3.7) | |
| Briel, 2008[27]d | 458 | 2.34.6 days | 3.66.1 days | 1.1 days (2.1 to 0.1) | 0.05 | |
| Burkhardt, 2010[34]e | 550 | 11 /59 (18.6%) | 16/101 (15.8%) | 2.8% (9.4 to 15.0) | 0.65 | |
| Restricted activity, df | Briel, 2008[27] | 458 | 8.73.9 | 8.63.9 | 0.2 (0.4 to 0.9) | >0.05 |
| Burkhardt, 2010[34] | 550 | 9.1 | 8.8 | 0.25 (0.52 to 1.03) | >0.05 | |
| Neonates with sepsis | ||||||
| Recurrence of infection | Stocker, 2010[31] | 121 | 32% | 39% | 7 | 0.45 |
| Children ages 136 months with fever of unknown source | ||||||
| Hospitalization rate | Manzano, 2010[36] | All children (N=384) | 50/192 (26%) | 48/192 (25%) | 1 (8 to 10) | 0.81 |
| No SBI or neutropenia (N=312) | 16/158 (10%) | 11/154 (7%) | 3 (3 to 10) | 0.34 | ||
| Adult postoperative patients at risk of infection | ||||||
| Hospital LOS, days | Chromik, 2006[32] | 20 | 18 | 30 | 12 | 0.057 |
| Local wound infection, % | Chromik, 2006[32] | 20 | 1/10 | 2/10 | 10 (41.0 to 21.0) | 0.53 |
| Systemic infection, % | Chromik, 2006[32] | 20 | 3/10 | 7/10 | 40.0 (80.2 to 0.2) | 0.07 |
| Sepsis/SIRS, % | Chromik, 2006[32] | 20 | 2/10 | 8/10 | 60.0 (95.1 to 24.9) | 0.007 |
| Mortality | Mortality | Difference | ||||
|---|---|---|---|---|---|---|
| Outcome | Author, Year | N | PCT‐Guided Therapy | Control | PCT‐CTRL (95% CI) | P Value |
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| 28‐day mortality | Nobre, 2008[19] | 79 | 8/39 (20.5%) | 8/40 (20.0%) | 0.5 (17.2 to 18.2), | 0.95 |
| 5/31 (16.1%) | 6/37 (16.2%) | 0.1 (17.7 to 17.5)a | 0.99 | |||
| Stolz, 2009[21] | 101 | 8/51 (15.7%) | 12/50 (24.0%) | 8.3 (23.8 to 7.2) | 0.29 | |
| Bouadma, 2010[23] | 621 | 65/307 (21.2%) | 64/314 (20.4%) | 0.8 (5.6 to 7.2) | 0.81 | |
| 60‐day mortality | Bouadma, 2010[23] | 621 | 92/307 (30.0%) | 82/314 (26.1%) | 3.9 (3.2 to 10.9) | 0.29 |
| In‐hospital mortality | Nobre, 2008[19] | 79 | 9/39 (23.1%) | 9/40 (22.5%) | 0.6 (17.9 to 19.1) | 0.95 |
| 6/31 (19.4%) | 7/37 (18.9%) | 0.4+ (18.3 to 19.2) | 0.96 | |||
| Stolz, 2009[21] | 101 | 10/51 (19.6%) | 14/50 (28.0%) | 8.4, (24.9 to 8.1) | 0.32 | |
| Hochreiter, 2009[22] | 110 | 15/57 (26.3%) | 14/53 (26.4%) | 0.1, (16.6 to 16.4) | 0.99 | |
| Schroeder, 2009[20] | 27 | 3/14 (21.4%) | 3/13 (23.1%) | 1.7, (33.1 to 29.8) | 0.92 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| 28‐day mortality | Svoboda, 2007[24] | 72 | 10/38 (26.3%) | 13/34 (38.2%) | 11.9 (33.4 to 9.6) | 0.28 |
| 28‐day mortality | Jensen, 2011[33] | 1200 | 190/604 (31.5%) | 191/596 (32.0%) | 0.6 (4.7 to 5.9) | 0.83 |
| Adult patients with respiratory tract infections | ||||||
| 6‐month mortality | Stolz, 2007[28] | 208 | 5/102 (4.9%) | 9/106 (8.5%) | 3.6% (10.3 to 3.2%) | 0.30 |
| 6‐week mortality | Christ‐Crain, 2006[29] | 302 | 18/151 (11.9%) | 20/151 (13.2%) | 1.3% (8.8 to 6.2) | 0.73 |
| 28‐day mortality | Christ‐Crain, 2004[30] | 243 | 4/124(3.2%) | 4/119 (3.4%) | 0.1% (4.6 to 4.4) | 0.95 |
| Schuetz, 2009 (30‐day)[25] | 1359 | 34/671(5.1%) | 33/688(4.8%) | 0.3% (2.1 to 2.5) | 0.82 | |
| Briel, 2008[27] | 458 | 0/231(0%) | 1/224 (0.4%) | 0.4% (1.3 to 0.4) | 0.31 | |
| Burkhardt, 2010[34] | 550 | 0/275(0%) | 0/275 (0%) | 0 | ||
| Kristoffersen, 2009[26] | 210 | 2/103(1.9%) | 1/107 (0.9%) | 1.0% (2.2 to 4.2) | 0.54 | |
| Long, 20113[5] | 162 | 0/81 (0%) | 0/81 (0%) | 0 | ||
| Neonates with sepsis | ||||||
| Mortality (in‐hospital) | Stocker, 2010[31] | 121 | 0% | 0% | 0 (0 to 0) | NA |
| Children ages 136 months with fever of unknown source | ||||||
| Mortality | Manzano, 2010[36] | 384 | All children | 0% | 0% | 0 (0 to 0) |
| Adult postoperative patients at risk of infection | ||||||
| Mortality | Chromik, 2006[32] | 20 | 1/10 (10%) | 3/10 (30%) | 20 (54.0 to 14.0) | 0.07 |
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Discontinuation
Five studies[19, 20, 21, 22, 23] (N=938) addressed procalcitonin‐guided discontinuation of antibiotic therapy in adult ICU patients. Four studies conducted superiority analyses for mortality with procalcitonin‐guided therapy, whereas 1 study conducted a noninferiority analysis. Absolute procalcitonin values for discontinuation of antibiotics ranged from 0.25 to 1 ng/mL. Physicians in control groups administered antibiotics according to their standard practice.
Antibiotic Usage
The absolute reduction in duration of antibiotic usage with procalcitonin guidance in these studies ranged from 1.7 to 5 days, and the relative reduction ranged from 21% to 38%. Meta‐analysis of antibiotic duration in adult ICU patients was performed (Figure 2A).

Morbidity
Procalcitonin‐guided antibiotic discontinuation did not increase morbidity, including ICU length of stay (LOS). Meta‐analysis of ICU LOS is displayed in Figure 2B. Limited data on adverse antibiotic events were reported (Table 2).
Mortality
There was no increase in mortality as a result of shorter duration of antibiotic therapy. Meta‐analysis of short‐term mortality (28‐day or in‐hospital mortality) showed a mortality difference of 0.43% favoring procalcitonin‐guided therapy, and a 95% confidence interval (CI) of 6% to 5% (Figure 2C).
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Intensification
Two studies[24, 33] (N=1272) addressed procalcitonin‐guided intensification of antibiotic therapy in adult ICU patients. The Jensen et al. study[33] was a large (N=1200), high‐quality study that used a detailed algorithm for broadening antibiotic therapy in patients with elevated procalcitonin. The Jensen et al. study also educated physicians about empiric therapy and intensification of antibiotic therapy. A second study[24] was too small (N=72) and lacked sufficient details to be informative.
Antibiotic Usage
The Jensen et al. study found a 2‐day increase, or 50% relative increase, in the duration of antibiotic therapy and a 7.9% absolute increase (P=0.002) in the number of days on 3 antibiotics with procalcitonin‐guided intensification.
Morbidity
The Jensen et al. study showed a significant 1‐day increase in ICU LOS (P=0.004) and a significant increase in organ dysfunction. Specifically, patients had a highly statistically significant 5% increase in days on mechanical ventilation (P0.0001) and 5% increase in days with abnormal renal function (P0.0001).
Mortality
The Jensen et al. study was a superiority trial powered to test a 7.5% decrease in 28‐day mortality, but no significant difference in mortality was observed with procalcitonin‐guided intensification (31.5% vs 32.0, P=0.83).
Adult Patients With Respiratory Tract Infections
Eight studies[25, 26, 27, 28, 29, 30, 34, 35] (N=3492) addressed initiation and/or discontinuation of antibiotics in adult patients with acute upper and lower respiratory tract infections, including community‐acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease, and acute bronchitis. Settings included primary care clinics, emergency departments, and hospital wards. Physicians in control groups administered antibiotics according to their own standard practices and/or evidence‐based guidelines. All studies encouraged initiation of antibiotics with procalcitonin levels >0.25 ng/mL, and 4 studies strongly encouraged antibiotics with procalcitonin levels >0.5 ng/mL.
Antibiotic Usage
Procalcitonin guidance reduced antibiotic duration, antibiotic prescription rate, and total antibiotic exposure. Absolute reduction in antibiotic duration ranged from 1 to 7 days, and relative reductions ranged from 13% to 55%. Four of the 8 studies reported sufficient details to be pooled into a meta‐analysis (Figure 3A) with a statistically significant pooled mean difference of 2.35 days favoring procalcitonin (95% CI: 4.38 to 0.33). Procalcitonin guidance also reduced antibiotic prescription rate with absolute reductions ranging from 2% to 7% and relative reductions ranging from 1.8% to 72%. Meta‐analysis of prescription rates from 8 studies (Figure 3B) yielded a statistically significant pooled risk difference of 22% (95% CI: 41% to 4%). Total antibiotic exposure was consistently reduced in the 4 studies reporting this outcome.

Morbidity
Procalcitonin guidance did not increase hospital LOS or ICU admission rates. Meta‐analysis of ICU admission rates from 5 studies (Figure 3C) produced a risk difference of 1%, with a narrow 95% CI (4% to 1%). There was insufficient evidence to judge the effect on days of restricted activity or antibiotic adverse events.
Mortality
Procalcitonin guidance did not increase mortality, and meta‐analysis of 4 studies (Figure 3D) produced a risk difference of 0.3% with a narrow 95% CI (1% to 2%), with no statistical heterogeneity (I2=0%).
Neonates With Sepsis
One study[31] (N=121) evaluated procalcitonin‐guided antibiotic therapy for suspected neonatal sepsis. Neonatal sepsis was suspected on the basis of risk factors and clinical signs and symptoms. Antibiotic initiation or discontinuation was based on a procalcitonin nomogram. Antibiotic therapy in the control group was based on the physician's assessment. The quality of this study was rated good, and strength of evidence was rated moderate for antibiotic usage and insufficient for morbidity and mortality outcomes.
Antibiotic Usage
Duration of antibiotic therapy was decreased by 22.4 hours (P=0.012), a 24% relative reduction, and the proportion of neonates on antibiotics 72 hours was reduced by 27% (P=0.002). The largest reduction in antibiotic duration was seen in the 80% to 85% of neonates who were categorized as having possible or infection or unlikely to have infection.
Morbidity
A statistically insignificant 7% reduction in rate of recurrence of infection was seen with procalcitonin‐guided antibiotic therapy (P=0.45).
Mortality
No in‐hospital deaths occurred in either the procalcitonin or control group.
Children Ages 1 to 36 Months With Fever of Unknown Source
One study[36] (N=384) evaluated procalcitonin‐guided antibiotic therapy for fever of unknown source in children 1 to 36 months of age, but the overall strength of evidence was judged insufficient to draw conclusions.
Antibiotic Usage
A statistically insignificant reduction of 3.1% in antibiotic prescription rate was seen with procalcitonin‐guided antibiotic therapy (P=0.49).
Morbidity
Rate of hospitalization was relatively low, and no significant difference was seen between procalcitonin and control groups.
Mortality
In‐hospital mortality was reported as 0% in both arms.
Adult Postoperative Patients at Risk of Infection
One study[32] (N =250) monitored procalcitonin in consecutive patients after colorectal surgery to identify patients at risk of infection who might benefit from prophylactic antibiotic therapy. Two hundred thirty patients had normal procalcitonin levels. Twenty patients with elevated procalcitonin levels (>1.5 ng/mL) were randomized to receive prophylactic antibiotic therapy with ceftriaxone or no antibiotics. The strength of evidence was judged insufficient to draw conclusions from this study.
Antibiotic Usage
Duration of antibiotic therapy was reduced by 3.5% but was not statistically insignificant (P=0.27).
Morbidity
Procalcitonin guidance reduced the incidence of sepsis/systemic inflammatory response syndrome by 60% (p=0.007). The incidences of local and systemic infection were reduced with procalcitonin guidance but were not statistically significant (10%, P=0.53; and 40%, P=0.07, respectively).
Mortality
Mortality was 20% higher in the control arm but was not statistically significant (P=0.07).
DISCUSSION
Summary of the Main Findings
Diagnosis of sepsis or other serious infections in critically ill patients is challenging because clinical criteria for diagnosis overlap with noninfectious causes of the systemic inflammatory response syndrome. Initiation of antibiotic therapy for presumed sepsis is necessary while diagnostic evaluation is ongoing, because delaying antibiotic therapy is associated with increased mortality.[37, 38, 39] Our review found that procalcitonin guidance significantly reduced antibiotic usage in adult ICU patients by reducing the duration of antibiotic therapy, rather than decreasing the initiation of antibiotics, without increasing morbidity or mortality.
In contrast, the use of procalcitonin as an indicator of need for intensification of antibiotic therapy in adult ICU patients should be discouraged because this approach was associated with increased morbidity. The large, well‐designed study by Jensen[33] showed that antibiotic intensification in response to elevated procalcitonin measurement was associated with increased morbidity: a longer ICU LOS, an increase in days on mechanical ventilation, and an increase in days with abnormal renal function. The authors concluded that the increased morbidity could only be explained by clinical harms of increased exposure to broad‐spectrum antibiotics.
Clinical and microbiological evaluations are neither sensitive nor specific for differentiating bacterial from viral respiratory tract infections. Procalcitonin can guide initiation of antibiotic therapy in adults with suspected bacterial respiratory tract infection. Our review showed that procalcitonin guidance significantly reduced antibiotic usage with respect to antibiotic prescription rate, duration of antibiotic therapy, and total exposure to antibiotic therapy in adult patients with respiratory tract infections.
The role of procalcitonin‐guided therapy in other populations is less clear. One study in postoperative colorectal surgery patients reported that elevated procalcitonin levels may identify patients at risk for infection who benefit from prophylactic antibiotic therapy.[32] Patients with elevated procalcitonin levels who received prophylactic antibiotic therapy had a significant decrease in the incidence and severity of systemic infections, whereas patients with normal procalcitonin levels did not require any additional surgical or medical therapy. Although these findings are promising, more data in postoperative patients are needed.
The utility of procalcitonin in pediatric settings is a significant gap in the present literature. One study[31] in neonates with suspected sepsis showed a significant decrease in the proportion of neonates started on empiric antibiotic therapy and a decrease in the duration of antibiotic therapy with procalcitonin guidance. However, there was insufficient evidence that procalcitonin guidance does not increase morbidity or mortality.
Comparison to Other Systematic Reviews
Six systematic reviews of procalcitonin guidance in the management of patients with infections were published prior to our review.[9, 10, 11, 12, 13, 14] Our systematic review differs from past reviews in the number of studies included and the pooling of studies according to patient population, type and severity of infection, and different uses of procalcitonin measurements, either for initiation, discontinuation, or intensification of antibiotic therapy. Previous systematic reviews included 7 to 14 studies, whereas ours included 18 randomized, controlled trials. One previous review[13] included and pooled the Jensen et al. study[33] with other studies of adult ICU patients. We evaluated the Jensen et al. study separately because it uniquely looked at procalcitonin‐guided antibiotic intensification in adult ICU patients, in contrast to other studies that looked at procalcitonin‐guided antibiotic discontinuation. We addressed pediatric populations separately from adult patients, and recognizing that there are distinct groups within the pediatric population, we separately grouped neonates and children ages 1 to 36 months. Despite these differences, our review and other systematic reviews, we came to similar conclusions: procalcitonin‐guided antibiotic decision making compared to clinical criteria‐guided antibiotic decision making reduces antibiotic usage without increasing morbidity or mortality.
Limitations
An important limitation of this review was the uncertainty about the noninferiority margin for morbidity and mortality in adult ICU patients. Only the Bouadma et al. study[23] did a power analysis and predefined a margin for noninferiority for 28‐ and 60‐day mortality. Meta‐analysis of all 5 ICU studies showed a pooled point estimate of 0.43% in mortality and a 95% CI of 6% to 5% for difference in mortality between procalcitonin‐guided therapy versus standard care. A 10% noninferiority margin for mortality has been recommended by the Infectious Diseases Society of America and American College of Chest Physicians, but there is concern that a 10% margin for mortality may be too high. Presently, 2 large trials are in progress that may yield more precise estimates of mortality in the future.
Differences in reporting of total antibiotic exposure and morbidity outcomes limited our ability to pool data. Total antibiotic exposure is conventionally reported as mean days per 1000 days of follow‐up, but some studies only reported relative or absolute differences. Likewise, morbidity was reported with different severity of illness scales, including Sepsis‐Related Organ Failure Assessment, Simplified Acute Physiology (SAP) II, SAP III, and Acute Physiology and Chronic Health Evaluation II, which limited comparisons across studies.
Research Gaps
We identified gaps in the available literature and opportunities for future research. First, the safety and efficacy of procalcitonin‐guided antibiotic therapy needs to be studied in patient populations excluded from current randomized controlled studies, such as immunocompromised patients and pregnant women. Patients who are immunocompromised or have chronic conditions, such as cystic fibrosis, account for a significant percentage of community‐acquired respiratory tract infections and are often treated empirically.[29, 30] Second, standardized reporting of antibiotic adverse events and emergence of antibiotic resistance is needed. Strategies to reduce antibiotic usage have been associated with reductions in antibiotic adverse events, such as Clostridium difficile colitis and superinfection with multi‐drug resistant Gram‐negative bacteria.[37, 40, 41] Few studies in our review reported allergic reactions or adverse events of antibiotic therapy, [25, 27, 34] and only 1 reported antibiotic resistance.[19] Third, procalcitonin guidance should be compared to other strategies to reduce antibiotic usage, such as structured implementation of practice guidelines and antibiotic stewardship programs.[42] One single‐arm study describes how procalcitonin can be used in antibiotic stewardship programs to decrease the duration of antibiotic therapy,[43] but additional studies are needed. Finally, generalizing results from those studies that were conducted primarily in Europe would depend on similar use of and adherence to study‐based algorithms. Newer observational studies have demonstrated reduced antibiotic usage with implementation of procalcitonin algorithms in real‐life settings in Europe, but algorithm adherence was significantly less in the United States.[44, 45]
In summary, our systematic review found that procalcitonin‐guided antibiotic therapy can significantly reduce antibiotic usage in adult ICU patients without affecting morbidity or mortality. Procalcitonin should not be used to guide intensification of antibiotic therapy in adult ICU patients because this approach may increase morbidity. In adults with respiratory infections, procalcitonin guidance can significantly reduce antibiotic usage without adversely affecting morbidity or mortality. There is insufficient evidence to recommend procalcitonin‐guided antibiotic therapy in neonates with sepsis, children with fever of unknown source, or postoperative patients at risk for infection.
Acknowledgments
Disclosures: This project was funded under contract HHSA 2902007‐10058 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services. The authors of this article are responsible for its content, including any clinical treatment recommendations. No statement in this article should be construed as an official position of AHRQ or of the US Department of Health and Human Services. There are no conflicts of interest reported by any of the authors.
Many serum biomarkers have been identified in recent years with a wide range of potential applications, including diagnosis of local and systemic infections, differentiation of bacterial and fungal infections from viral syndromes or noninfectious conditions, prognostic stratification of patients, and enhanced management of antibiotic therapy. Currently, there are at least 178 serum biomarkers that have potential roles to guide antibiotic therapy, and among these, procalcitonin has been the most extensively studied biomarker.[1, 2]
Procalcitonin is the prohormone precursor of calcitonin that is expressed primarily in C cells of the thyroid gland. Conversion of procalcitonin to calcitonin is inhibited by various cytokines and bacterial endotoxins. Procalcitonin's primary diagnostic utility is thought to be in establishing the presence of bacterial infections, because serum procalcitonin levels rise and fall rapidly in bacterial infections.[3, 4, 5] In healthy individuals, procalcitonin levels are very low. In systemic infections, including sepsis, procalcitonin levels are generally greater than 0.5 to 2 ng/mL, but often reach levels 10 ng/mL, which correlates with severity of illness and a poor prognosis. In patients with respiratory tract infections, procalcitonin levels are less elevated, and a cutoff of 0.25 ng/mL seems to be most predictive of a bacterial respiratory tract infection requiring antibiotic therapy.[6, 7, 8] Procalcitonin levels decrease to 0.25 ng/mL as infection resolves, and a decline in procalcitonin level may guide decisions about discontinuation of antibiotic therapy.[5]
The purpose of this systematic review was to synthesize comparative studies examining the use of procalcitonin to guide antibiotic therapy in patients with suspected local or systemic infections in different patient populations. We are aware of 6 previously published systematic reviews evaluating the utility of procalcitonin guidance in the management of infections.[9, 10, 11, 12, 13, 14] Our systematic review included more studies and pooled patients into the most clinically similar groups compared to other systematic reviews.
METHODS
This review is based on a comparative effectiveness review prepared for the Agency for Healthcare Research and Quality's Effective Health Care Program.[15] A standard protocol consistent with the Methods Guide for Effectiveness and Comparative Effectiveness Reviews[16] was followed. A detailed description of the methods is available online (
Study Question
In selected populations of patients with suspected local or systemic infection, what are the effects of using procalcitonin measurement plus clinical criteria for infection to guide initiation, intensification, and/or discontinuation of antibiotic therapy when compared to clinical criteria for infection alone?
Search Strategy
MEDLINE and EMBASE were searched from January 1, 1990 through December 16, 2011, and the Cochrane Controlled Trials register was searched with no date restriction for randomized and nonrandomized comparative studies using the following search terms: procalcitonin AND chronic obstructive pulmonary disease; COPD; critical illness; critically ill; febrile neutropenia; ICU; intensive care; intensive care unit; postoperative complication(s); postoperative infection(s); postsurgical infection(s); sepsis; septic; surgical wound infection; systemic inflammatory response syndrome OR postoperative infection. In addition, a search for systematic reviews was conducted in MEDLINE, the Cochrane Database of Systematic Reviews, and Web sites of the National Institute for Clinical Excellence, the National Guideline Clearinghouse, and the Health Technology Assessment Programme. Gray literature, including databases with regulatory information, clinical trial registries, abstracts and conference papers, grants and federally funded research, and manufacturing information was searched from January 1, 2006 to June 28, 2011.
Study Selection
A single reviewer screened abstracts and selected studies looking at procalcitonin‐guided antibiotic therapy. Second and third reviewers were consulted to screen articles when needed. Studies were included if they fulfilled all of the following criteria: (1) randomized, controlled trial or nonrandomized comparative study; (2) adult and/or pediatric patients with known or suspected local or systemic infection, including critically ill patients with sepsis syndrome or ventilator‐associated pneumonia, adults with respiratory tract infections, neonates with sepsis, children with fever of unknown source, and postoperative patients at risk of infection; (3) interventions included initiation, intensification, and/or discontinuation of antibiotic therapy guided by procalcitonin plus clinical criteria; (4) primary outcomes included antibiotic usage (antibiotic prescription rate, total antibiotic exposure, duration of antibiotic therapy, and days without antibiotic therapy); and (5) secondary outcomes included morbidity (antibiotic adverse events, hospital and/or intensive care unit length of stay), mortality, and quality of life.
Studies with any of the following criteria were excluded: published in non‐English language, not reporting primary data from original research, not a randomized, controlled trial or nonrandomized comparative study, not reporting relevant outcomes.
Data Extraction and Quality Assessment
A single reviewer abstracted data and a second reviewer confirmed accuracy. Disagreements between reviewers were resolved by group discussion among the research team and final quality rating was assigned by consensus adjudication. Data elements were abstracted into the following categories: quality assessment, applicability and clinical diversity assessment, and outcome assessment. Quality of included studies was assessed using the US Preventive Services Task Force framework[17] by at least 2 independent reviewers. Three quality categories were used: good, fair, and poor.
Data Synthesis and Analysis
The decision to incorporate formal data synthesis in this review was made after completing the formal literature search, and the decision to pool studies was based on the specific number of studies with similar questions and outcomes. If a meta‐analysis could be performed, subgroup and sensitivity analyses were based on clinical similarity of available studies and reporting of mean and standard deviation. The pooling method involved inverse variance weighting and a random effects model.
The strength of evidence was graded using the Methods Guide,[16] a system based on the Grading of Recommendations Assessment, Development and Evaluation Working Group.[18] The following domains were addressed: risk of bias, consistency, directness, and precision. The overall strength of evidence was graded as high, moderate, low, or insufficient. The final strength of evidence grading was made by consensus adjudication among the authors.
RESULTS
Of the 2000 studies identified through the literature search, 1986 were excluded and 14 studies[19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32] were included. Search of gray literature yielded 4 published studies.[33, 34, 35, 36] A total of 18 randomized, controlled trials comparing procalcitonin guidance to use of clinical criteria alone to manage antibiotic therapy in patients with infections were included. The PRISMA diagram (Figure 1) depicts the flow of search screening and study selection. We sought, but did not find, nonrandomized comparative studies of populations, comparisons, interventions, and outcomes that were not adequately studied in randomized, controlled trials.

Data were pooled into clinically similar groups that were reviewed separately: (1) adult intensive care unit (ICU) patients, including patients with ventilator‐associated pneumonia; (2) adult patients with respiratory tract infections; (3) neonates with suspected sepsis; (4) children between 1 to 36 months of age with fever of unknown source; and (5) postoperative patients at risk of infection. Tables summarizing study quality and outcome measures with strength of evidence are available online (
| Outcome | Author, Year | N | PCT‐Guided Therapya | Controla | Difference PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ABT Duration, d | Hochreiter, 2009[22] | 110 | 5.9 | 7.9 | 2.0 (2.5 to 1.5) | 0.001 |
| Nobre, 2008[19] | 79 | 66 | 9.5 (ITT), 10 (PP) | 2.6 (5.5 to 0.3), 3.2 (1.1 to5.4) | 0.15, 0.003 | |
| Schroeder, 2009[20] | 27 | 6.6 | 8.3 | 1.7 (2.4 to 1.0) | 0.001 | |
| Stolz, 2009[21] | 101 | 10 (616)b | 15 (1023)b | 5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 10.3 | 13.3 | 3.0 (4.20 to 1.80) | 0.0001 | |
| Days without ABTs, day 28 | Nobre, 2008[19] | 79 | 15.3, 17.4 | 13, 13.6 | 2.3 (5.9 to 1.8), 3.8 (0.1 to 7.5)c | 0.28, 0.04 |
| Stolz, 2009[21] | 101 | 13 (221)b | 9.5 (1.517)b | 3.5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 14.3 | 11.6 | 2.7 (1.4 to 4.1) | 0.001 | |
| Total ABT exposured | Nobre, 2008[19] | 79 | 541 | 644 | 1.1e (0.9 to 1.3), 1.3e (1.1 to 1,5)c | 0.07, 0.0002 |
| 504 | 655 | |||||
| Stolz, 2009[21] | 101 | 1077 | 1341 | |||
| Bouadma, 2010[23]d | 621 | 653 | 812 | 159 (185 to 131) | 0.001 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ABT duration, days | Jensen, 2011[33] | 1200 | 6 (311)b | 4 (310)b | NR | NR |
| Days spent in ICU on 3 ABTs | Jensen, 2011[33] | 1200 | 3570/5447 (65.5%) | 2721/4717 (57.7%) | 7.9% (6.0 to 9.7) | 0.002 |
| Adult patients with respiratory tract infections | ||||||
| ABT duration, da | Schuetz, 2009[2][5] | 1359 | 5.7 | 8.7 | 3.0 | |
| Christ‐Crain, 2004[30] | 243 | 10.9 | 12.8 | 1.9 (3.1 to 0.7) | 0.002 | |
| Kristoffersen, 2009[26] | 210 | 5.1 | 6.8 | 1.7 | ||
| Briel, 2008[27] | 458 | 6.2 | 7.1 | 1.0 (1.7 to 0.4) | 0.05 | |
| Long, 20113[5] | 162 | 5 (36)f | 7 (59)f | 2.0 | 0.001 | |
| Burkhardt, 2010[34] | 550 | 7.8 | 7.7 | 0.1 (0.7 to 0.9) | 0.8 | |
| Christ‐Crain, 2006[29] | 302 | 5.8 | 12.9 | 7.1(8.4 to 5.8) | 0.0001 | |
| Antibiotic prescription rate, % | Schuetz, 2009[2][5] | 1359 | 506/671 (75.4%) | 603/688 (87.6%) | 12.2% (16.3 to 8.1) | 0.05 |
| Christ‐Crain, 2004[30] | 243 | 55/124 (44.4%) | 99/119 (83.2%) | 38.8% (49.9 to 27.8) | 0.0001 | |
| Kristoffersen, 2009[26] | 210 | 88/103 (85.4%) | 85/107 (79.4%) | 6.0% (4.3 to 16.2) | 0.25 | |
| Briel, 2008[27] | 458 | 58/232 (25.0%) | 219/226 (96.9%) | 72% (78 to 66) | 0.05 | |
| Long, 20113[5] | 162 | NR (84.4%) | NR (97.5%) | 13.1% | 0.004 | |
| Stolz, 2007[28] | 208 | 41/102 (40.2%) | 76/106 (71.7%) | 31.5% (44.3 to 18.7) | 0.0001 | |
| Christ‐Crain, 2006[29] | 302 | 128/151 (84.8%) | 149/151 (98.79%) | 13.9% (19.9 to 7.9) | 0.0001 | |
| Burkhardt, 2010[34] | 550 | 84/275 (30.5%) | 89/275 (32.4%) | 1.8% (9.6 to 5.9) | 0.701 | |
| Total ABT exposure | Stolz, 2007[28] | 208 | NR | NR | 31.5% (18.7 to 44.3) | 0.0001 |
| Long, 20113[5] | 162 | NR | NR | NR | ||
| Christ‐Crain, 2006[29] | 302 | 136g | 323g | |||
| Christ‐Crain, 2004[30] | 243 | 332g | 661g | |||
| Neonates with sepsis | ||||||
| ABTs 72 hours, % | Stocker, 2010[31] | All neonates (N=121) | 33/60 (55%) | 50/61 (82%) | 27.0 (42.8 to 11.1) | 0.002 |
| Infection proven/probably (N=21) | 9/9 (100%) | 12/12 (100%) | 0% (0 to 0) | NA | ||
| Infection possible (N=40) | 13/21 (61.9%) | 19/19 (100%) | 38.1 (58.9 to 17.3) | 0.003 | ||
| Infection unlikely (N=60) | 11/30 (36.7%) | 19/30 (63.3%) | 26.6 (51.1 to 2.3) | 0.038 | ||
| ABT duration, h | Stocker, 2010[31] | All neonates (N=121) | 79.1 | 101.5 | 22.4 | 0.012 |
| Infection proven/probably (N=21) | 177.8 | 170.8 | 7 | NSS | ||
| Infection possible (N=40) | 83.4 | 111.5 | 28.1 | 0.001 | ||
| Infection unlikely (N=60) | 46.5 | 67.4 | 20.9 | 0.001 | ||
| Children ages 136 months with fever of unknown source | ||||||
| Antibiotic prescription rate, % | Manzano, 2010[36] | All children (N=384) | 48/192 (25%) | 54/192 (28.0%) | 3.1 (12.0 to 5.7) | 0.49 |
| No SBI or neutropenia (N=312) | 14/158 (9%) | 16/154 (10%) | 1.5 (8.1 to 5.0) | 0.65 | ||
| Adult postoperative patients at risk of infection | ||||||
| ABT duration, d | Chromik, 2006[32] | All patients (N=20) | 5.5 | 9 | 3.5 | 0.27 |
| Outcome | Author, Year | N | PCTa | Controla | Difference, PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ICU LOS, days | Hochreiter, 2009[22] | 110 | 15.5 | 17.7 | 2.2 | 0.046 |
| Nobre, 2008[19] | 79 | 4 | 7 | 4.6 (8.2 to 1.0) | 0.02 | |
| Schroeder, 2009[20] | 27 | 16.4 | 16.7 | 0.3 (5.6 to 5.0) | NSS | |
| Bouadma, 2010[23] | 621 | 15.9 | 14.4 | 1.5 (0.9 to 3.1) | 0.23 | |
| Hospital LOS, days | Nobre, 2008[19] | 79 | 17 | 23.5 | 2.5 (6.5 to 1.5) | 0.85 |
| Stolz, 2009[21] | 101 | 26 (721)b | 26 (16.822.3)b | 0 | 0.15 | |
| Bouadma, 2010[23] | 621 | 26.1 | 26.4 | 0.3 (3.2 to 2.7) | 0.87 | |
| ICU‐free days alive, 128 | Stolz, 2009[21] | 101 | 10 (018)b | 8.5 (018)c | 1.5 | 0.53 |
| SOFA day 28 | Bouadma, 2010[23] | 621 | 1.5 | 0.9 | 0.6 (0.0, 1.1) | 0.037 |
| SOFA score max | Schroeder, 2009[20] | 27 | 7.3 | 8.3 | 8.1 (4.1 to 1.7) | NSS |
| SAPS II score | Hochreiter, 2009[22] | 110 | 40.1 | 40.5 | 0.4 (6.4 to 5.6) | >0.05 |
| Days without MV | Stolz, 2009[21] | 101 | 21 (224)b | 19 (8.522.5)b | 2.0 | 0.46 |
| Bouadma, 2010[23] | 621 | 16.2 | 16.9 | 0.7 (2.4 to 1.1) | 0.47 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ICU LOS, da | Svoboda, 2007[24] | 72 | 16.1 | 19.4 | 3.3 (7.0 to 0.4) | 0.09 |
| Jensen, 2011[33] | 1200 | 6 (312)b | 5 (311)b | 1 | 0.004 | |
| SOFA scorea | Svoboda, 2007[24] | 72 | 7.9 | 9.3 | 1.4 (2.8 to 0.0) | 0.06 |
| Days on MVa | Svoboda, 2007[24] | 72 | 10.3 | 13.9 | 3.6 (7.6 to 0.4) | 0.08 |
| Jensen, 2011[33] | 1200 | 3569 (65.5%) | 2861 (60.7%) | 4.9% (3 to 6.7) | 0.0001 | |
| Percent days in ICU with GFR 60 | Jensen, 2011[33] | 1200 | 2796 (51.3%) | 2187 (46.4%) | 5.0 % (3.0 to 6.9) | 0.0001 |
| Adult patients with respiratory tract infections | ||||||
| Hospital LOS, da | Schuetz, 2009[2][5] | 1359 | 9.4 | 9.2 | 0.2 | |
| Christ‐Crain, 2004[30] | 224 | 10.78.9 | 11.210.6 | 0.5 (3.0 to 2.0) | 0.69 | |
| Kristoffersen, 2009[26] | 210 | 5.9 | 6.7 | 0.8 | 0.22 | |
| Stolz, 2007[28] | 208 | 9 (115)b | 10 (115)b | 1 | 0.96 | |
| Christ‐Crain, 2006[29] | 302 | 12.09.1 | 13.09.0 | 1 (3.0 to 1.0) | 0.34 | |
| ICU admission, % | Schuetz, 2009[2][5] | 1359 | 43/671 (6.4%) | 60/688 (8.7%) | 2.3% (5.2 to 0.4) | 0.12 |
| Christ‐Crain, 2004[30] | 224 | 5/124 (4.0%) | 6/119 (5.0%) | 1.0% (6.2 to 4.2) | 0.71 | |
| Kristoffersen, 2009[26] | 210 | 7/103 (6.8%) | 5/107 (4.7%) | 2.1% (4.2 to 8.4) | 0.51 | |
| Stolz, 2007[28] | 208 | 8/102 (7.8%) | 11/106 (10.4%) | 2.5% (10.3 to 5.3) | 0.53 | |
| Christ‐Crain, 2006[29] | 302 | 20/151 (13.2%) | 21/151 (13.94%) | 0.7% (8.4 to 7.1) | 0.87 | |
| Antibiotic adverse events | Schuetz, 2009[2][5]c | 1359 | 133/671 (19.8%) | 193/688 (28.1%) | 8.2% (12.7 to 3.7) | |
| Briel, 2008[27]d | 458 | 2.34.6 days | 3.66.1 days | 1.1 days (2.1 to 0.1) | 0.05 | |
| Burkhardt, 2010[34]e | 550 | 11 /59 (18.6%) | 16/101 (15.8%) | 2.8% (9.4 to 15.0) | 0.65 | |
| Restricted activity, df | Briel, 2008[27] | 458 | 8.73.9 | 8.63.9 | 0.2 (0.4 to 0.9) | >0.05 |
| Burkhardt, 2010[34] | 550 | 9.1 | 8.8 | 0.25 (0.52 to 1.03) | >0.05 | |
| Neonates with sepsis | ||||||
| Recurrence of infection | Stocker, 2010[31] | 121 | 32% | 39% | 7 | 0.45 |
| Children ages 136 months with fever of unknown source | ||||||
| Hospitalization rate | Manzano, 2010[36] | All children (N=384) | 50/192 (26%) | 48/192 (25%) | 1 (8 to 10) | 0.81 |
| No SBI or neutropenia (N=312) | 16/158 (10%) | 11/154 (7%) | 3 (3 to 10) | 0.34 | ||
| Adult postoperative patients at risk of infection | ||||||
| Hospital LOS, days | Chromik, 2006[32] | 20 | 18 | 30 | 12 | 0.057 |
| Local wound infection, % | Chromik, 2006[32] | 20 | 1/10 | 2/10 | 10 (41.0 to 21.0) | 0.53 |
| Systemic infection, % | Chromik, 2006[32] | 20 | 3/10 | 7/10 | 40.0 (80.2 to 0.2) | 0.07 |
| Sepsis/SIRS, % | Chromik, 2006[32] | 20 | 2/10 | 8/10 | 60.0 (95.1 to 24.9) | 0.007 |
| Mortality | Mortality | Difference | ||||
|---|---|---|---|---|---|---|
| Outcome | Author, Year | N | PCT‐Guided Therapy | Control | PCT‐CTRL (95% CI) | P Value |
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| 28‐day mortality | Nobre, 2008[19] | 79 | 8/39 (20.5%) | 8/40 (20.0%) | 0.5 (17.2 to 18.2), | 0.95 |
| 5/31 (16.1%) | 6/37 (16.2%) | 0.1 (17.7 to 17.5)a | 0.99 | |||
| Stolz, 2009[21] | 101 | 8/51 (15.7%) | 12/50 (24.0%) | 8.3 (23.8 to 7.2) | 0.29 | |
| Bouadma, 2010[23] | 621 | 65/307 (21.2%) | 64/314 (20.4%) | 0.8 (5.6 to 7.2) | 0.81 | |
| 60‐day mortality | Bouadma, 2010[23] | 621 | 92/307 (30.0%) | 82/314 (26.1%) | 3.9 (3.2 to 10.9) | 0.29 |
| In‐hospital mortality | Nobre, 2008[19] | 79 | 9/39 (23.1%) | 9/40 (22.5%) | 0.6 (17.9 to 19.1) | 0.95 |
| 6/31 (19.4%) | 7/37 (18.9%) | 0.4+ (18.3 to 19.2) | 0.96 | |||
| Stolz, 2009[21] | 101 | 10/51 (19.6%) | 14/50 (28.0%) | 8.4, (24.9 to 8.1) | 0.32 | |
| Hochreiter, 2009[22] | 110 | 15/57 (26.3%) | 14/53 (26.4%) | 0.1, (16.6 to 16.4) | 0.99 | |
| Schroeder, 2009[20] | 27 | 3/14 (21.4%) | 3/13 (23.1%) | 1.7, (33.1 to 29.8) | 0.92 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| 28‐day mortality | Svoboda, 2007[24] | 72 | 10/38 (26.3%) | 13/34 (38.2%) | 11.9 (33.4 to 9.6) | 0.28 |
| 28‐day mortality | Jensen, 2011[33] | 1200 | 190/604 (31.5%) | 191/596 (32.0%) | 0.6 (4.7 to 5.9) | 0.83 |
| Adult patients with respiratory tract infections | ||||||
| 6‐month mortality | Stolz, 2007[28] | 208 | 5/102 (4.9%) | 9/106 (8.5%) | 3.6% (10.3 to 3.2%) | 0.30 |
| 6‐week mortality | Christ‐Crain, 2006[29] | 302 | 18/151 (11.9%) | 20/151 (13.2%) | 1.3% (8.8 to 6.2) | 0.73 |
| 28‐day mortality | Christ‐Crain, 2004[30] | 243 | 4/124(3.2%) | 4/119 (3.4%) | 0.1% (4.6 to 4.4) | 0.95 |
| Schuetz, 2009 (30‐day)[25] | 1359 | 34/671(5.1%) | 33/688(4.8%) | 0.3% (2.1 to 2.5) | 0.82 | |
| Briel, 2008[27] | 458 | 0/231(0%) | 1/224 (0.4%) | 0.4% (1.3 to 0.4) | 0.31 | |
| Burkhardt, 2010[34] | 550 | 0/275(0%) | 0/275 (0%) | 0 | ||
| Kristoffersen, 2009[26] | 210 | 2/103(1.9%) | 1/107 (0.9%) | 1.0% (2.2 to 4.2) | 0.54 | |
| Long, 20113[5] | 162 | 0/81 (0%) | 0/81 (0%) | 0 | ||
| Neonates with sepsis | ||||||
| Mortality (in‐hospital) | Stocker, 2010[31] | 121 | 0% | 0% | 0 (0 to 0) | NA |
| Children ages 136 months with fever of unknown source | ||||||
| Mortality | Manzano, 2010[36] | 384 | All children | 0% | 0% | 0 (0 to 0) |
| Adult postoperative patients at risk of infection | ||||||
| Mortality | Chromik, 2006[32] | 20 | 1/10 (10%) | 3/10 (30%) | 20 (54.0 to 14.0) | 0.07 |
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Discontinuation
Five studies[19, 20, 21, 22, 23] (N=938) addressed procalcitonin‐guided discontinuation of antibiotic therapy in adult ICU patients. Four studies conducted superiority analyses for mortality with procalcitonin‐guided therapy, whereas 1 study conducted a noninferiority analysis. Absolute procalcitonin values for discontinuation of antibiotics ranged from 0.25 to 1 ng/mL. Physicians in control groups administered antibiotics according to their standard practice.
Antibiotic Usage
The absolute reduction in duration of antibiotic usage with procalcitonin guidance in these studies ranged from 1.7 to 5 days, and the relative reduction ranged from 21% to 38%. Meta‐analysis of antibiotic duration in adult ICU patients was performed (Figure 2A).

Morbidity
Procalcitonin‐guided antibiotic discontinuation did not increase morbidity, including ICU length of stay (LOS). Meta‐analysis of ICU LOS is displayed in Figure 2B. Limited data on adverse antibiotic events were reported (Table 2).
Mortality
There was no increase in mortality as a result of shorter duration of antibiotic therapy. Meta‐analysis of short‐term mortality (28‐day or in‐hospital mortality) showed a mortality difference of 0.43% favoring procalcitonin‐guided therapy, and a 95% confidence interval (CI) of 6% to 5% (Figure 2C).
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Intensification
Two studies[24, 33] (N=1272) addressed procalcitonin‐guided intensification of antibiotic therapy in adult ICU patients. The Jensen et al. study[33] was a large (N=1200), high‐quality study that used a detailed algorithm for broadening antibiotic therapy in patients with elevated procalcitonin. The Jensen et al. study also educated physicians about empiric therapy and intensification of antibiotic therapy. A second study[24] was too small (N=72) and lacked sufficient details to be informative.
Antibiotic Usage
The Jensen et al. study found a 2‐day increase, or 50% relative increase, in the duration of antibiotic therapy and a 7.9% absolute increase (P=0.002) in the number of days on 3 antibiotics with procalcitonin‐guided intensification.
Morbidity
The Jensen et al. study showed a significant 1‐day increase in ICU LOS (P=0.004) and a significant increase in organ dysfunction. Specifically, patients had a highly statistically significant 5% increase in days on mechanical ventilation (P0.0001) and 5% increase in days with abnormal renal function (P0.0001).
Mortality
The Jensen et al. study was a superiority trial powered to test a 7.5% decrease in 28‐day mortality, but no significant difference in mortality was observed with procalcitonin‐guided intensification (31.5% vs 32.0, P=0.83).
Adult Patients With Respiratory Tract Infections
Eight studies[25, 26, 27, 28, 29, 30, 34, 35] (N=3492) addressed initiation and/or discontinuation of antibiotics in adult patients with acute upper and lower respiratory tract infections, including community‐acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease, and acute bronchitis. Settings included primary care clinics, emergency departments, and hospital wards. Physicians in control groups administered antibiotics according to their own standard practices and/or evidence‐based guidelines. All studies encouraged initiation of antibiotics with procalcitonin levels >0.25 ng/mL, and 4 studies strongly encouraged antibiotics with procalcitonin levels >0.5 ng/mL.
Antibiotic Usage
Procalcitonin guidance reduced antibiotic duration, antibiotic prescription rate, and total antibiotic exposure. Absolute reduction in antibiotic duration ranged from 1 to 7 days, and relative reductions ranged from 13% to 55%. Four of the 8 studies reported sufficient details to be pooled into a meta‐analysis (Figure 3A) with a statistically significant pooled mean difference of 2.35 days favoring procalcitonin (95% CI: 4.38 to 0.33). Procalcitonin guidance also reduced antibiotic prescription rate with absolute reductions ranging from 2% to 7% and relative reductions ranging from 1.8% to 72%. Meta‐analysis of prescription rates from 8 studies (Figure 3B) yielded a statistically significant pooled risk difference of 22% (95% CI: 41% to 4%). Total antibiotic exposure was consistently reduced in the 4 studies reporting this outcome.

Morbidity
Procalcitonin guidance did not increase hospital LOS or ICU admission rates. Meta‐analysis of ICU admission rates from 5 studies (Figure 3C) produced a risk difference of 1%, with a narrow 95% CI (4% to 1%). There was insufficient evidence to judge the effect on days of restricted activity or antibiotic adverse events.
Mortality
Procalcitonin guidance did not increase mortality, and meta‐analysis of 4 studies (Figure 3D) produced a risk difference of 0.3% with a narrow 95% CI (1% to 2%), with no statistical heterogeneity (I2=0%).
Neonates With Sepsis
One study[31] (N=121) evaluated procalcitonin‐guided antibiotic therapy for suspected neonatal sepsis. Neonatal sepsis was suspected on the basis of risk factors and clinical signs and symptoms. Antibiotic initiation or discontinuation was based on a procalcitonin nomogram. Antibiotic therapy in the control group was based on the physician's assessment. The quality of this study was rated good, and strength of evidence was rated moderate for antibiotic usage and insufficient for morbidity and mortality outcomes.
Antibiotic Usage
Duration of antibiotic therapy was decreased by 22.4 hours (P=0.012), a 24% relative reduction, and the proportion of neonates on antibiotics 72 hours was reduced by 27% (P=0.002). The largest reduction in antibiotic duration was seen in the 80% to 85% of neonates who were categorized as having possible or infection or unlikely to have infection.
Morbidity
A statistically insignificant 7% reduction in rate of recurrence of infection was seen with procalcitonin‐guided antibiotic therapy (P=0.45).
Mortality
No in‐hospital deaths occurred in either the procalcitonin or control group.
Children Ages 1 to 36 Months With Fever of Unknown Source
One study[36] (N=384) evaluated procalcitonin‐guided antibiotic therapy for fever of unknown source in children 1 to 36 months of age, but the overall strength of evidence was judged insufficient to draw conclusions.
Antibiotic Usage
A statistically insignificant reduction of 3.1% in antibiotic prescription rate was seen with procalcitonin‐guided antibiotic therapy (P=0.49).
Morbidity
Rate of hospitalization was relatively low, and no significant difference was seen between procalcitonin and control groups.
Mortality
In‐hospital mortality was reported as 0% in both arms.
Adult Postoperative Patients at Risk of Infection
One study[32] (N =250) monitored procalcitonin in consecutive patients after colorectal surgery to identify patients at risk of infection who might benefit from prophylactic antibiotic therapy. Two hundred thirty patients had normal procalcitonin levels. Twenty patients with elevated procalcitonin levels (>1.5 ng/mL) were randomized to receive prophylactic antibiotic therapy with ceftriaxone or no antibiotics. The strength of evidence was judged insufficient to draw conclusions from this study.
Antibiotic Usage
Duration of antibiotic therapy was reduced by 3.5% but was not statistically insignificant (P=0.27).
Morbidity
Procalcitonin guidance reduced the incidence of sepsis/systemic inflammatory response syndrome by 60% (p=0.007). The incidences of local and systemic infection were reduced with procalcitonin guidance but were not statistically significant (10%, P=0.53; and 40%, P=0.07, respectively).
Mortality
Mortality was 20% higher in the control arm but was not statistically significant (P=0.07).
DISCUSSION
Summary of the Main Findings
Diagnosis of sepsis or other serious infections in critically ill patients is challenging because clinical criteria for diagnosis overlap with noninfectious causes of the systemic inflammatory response syndrome. Initiation of antibiotic therapy for presumed sepsis is necessary while diagnostic evaluation is ongoing, because delaying antibiotic therapy is associated with increased mortality.[37, 38, 39] Our review found that procalcitonin guidance significantly reduced antibiotic usage in adult ICU patients by reducing the duration of antibiotic therapy, rather than decreasing the initiation of antibiotics, without increasing morbidity or mortality.
In contrast, the use of procalcitonin as an indicator of need for intensification of antibiotic therapy in adult ICU patients should be discouraged because this approach was associated with increased morbidity. The large, well‐designed study by Jensen[33] showed that antibiotic intensification in response to elevated procalcitonin measurement was associated with increased morbidity: a longer ICU LOS, an increase in days on mechanical ventilation, and an increase in days with abnormal renal function. The authors concluded that the increased morbidity could only be explained by clinical harms of increased exposure to broad‐spectrum antibiotics.
Clinical and microbiological evaluations are neither sensitive nor specific for differentiating bacterial from viral respiratory tract infections. Procalcitonin can guide initiation of antibiotic therapy in adults with suspected bacterial respiratory tract infection. Our review showed that procalcitonin guidance significantly reduced antibiotic usage with respect to antibiotic prescription rate, duration of antibiotic therapy, and total exposure to antibiotic therapy in adult patients with respiratory tract infections.
The role of procalcitonin‐guided therapy in other populations is less clear. One study in postoperative colorectal surgery patients reported that elevated procalcitonin levels may identify patients at risk for infection who benefit from prophylactic antibiotic therapy.[32] Patients with elevated procalcitonin levels who received prophylactic antibiotic therapy had a significant decrease in the incidence and severity of systemic infections, whereas patients with normal procalcitonin levels did not require any additional surgical or medical therapy. Although these findings are promising, more data in postoperative patients are needed.
The utility of procalcitonin in pediatric settings is a significant gap in the present literature. One study[31] in neonates with suspected sepsis showed a significant decrease in the proportion of neonates started on empiric antibiotic therapy and a decrease in the duration of antibiotic therapy with procalcitonin guidance. However, there was insufficient evidence that procalcitonin guidance does not increase morbidity or mortality.
Comparison to Other Systematic Reviews
Six systematic reviews of procalcitonin guidance in the management of patients with infections were published prior to our review.[9, 10, 11, 12, 13, 14] Our systematic review differs from past reviews in the number of studies included and the pooling of studies according to patient population, type and severity of infection, and different uses of procalcitonin measurements, either for initiation, discontinuation, or intensification of antibiotic therapy. Previous systematic reviews included 7 to 14 studies, whereas ours included 18 randomized, controlled trials. One previous review[13] included and pooled the Jensen et al. study[33] with other studies of adult ICU patients. We evaluated the Jensen et al. study separately because it uniquely looked at procalcitonin‐guided antibiotic intensification in adult ICU patients, in contrast to other studies that looked at procalcitonin‐guided antibiotic discontinuation. We addressed pediatric populations separately from adult patients, and recognizing that there are distinct groups within the pediatric population, we separately grouped neonates and children ages 1 to 36 months. Despite these differences, our review and other systematic reviews, we came to similar conclusions: procalcitonin‐guided antibiotic decision making compared to clinical criteria‐guided antibiotic decision making reduces antibiotic usage without increasing morbidity or mortality.
Limitations
An important limitation of this review was the uncertainty about the noninferiority margin for morbidity and mortality in adult ICU patients. Only the Bouadma et al. study[23] did a power analysis and predefined a margin for noninferiority for 28‐ and 60‐day mortality. Meta‐analysis of all 5 ICU studies showed a pooled point estimate of 0.43% in mortality and a 95% CI of 6% to 5% for difference in mortality between procalcitonin‐guided therapy versus standard care. A 10% noninferiority margin for mortality has been recommended by the Infectious Diseases Society of America and American College of Chest Physicians, but there is concern that a 10% margin for mortality may be too high. Presently, 2 large trials are in progress that may yield more precise estimates of mortality in the future.
Differences in reporting of total antibiotic exposure and morbidity outcomes limited our ability to pool data. Total antibiotic exposure is conventionally reported as mean days per 1000 days of follow‐up, but some studies only reported relative or absolute differences. Likewise, morbidity was reported with different severity of illness scales, including Sepsis‐Related Organ Failure Assessment, Simplified Acute Physiology (SAP) II, SAP III, and Acute Physiology and Chronic Health Evaluation II, which limited comparisons across studies.
Research Gaps
We identified gaps in the available literature and opportunities for future research. First, the safety and efficacy of procalcitonin‐guided antibiotic therapy needs to be studied in patient populations excluded from current randomized controlled studies, such as immunocompromised patients and pregnant women. Patients who are immunocompromised or have chronic conditions, such as cystic fibrosis, account for a significant percentage of community‐acquired respiratory tract infections and are often treated empirically.[29, 30] Second, standardized reporting of antibiotic adverse events and emergence of antibiotic resistance is needed. Strategies to reduce antibiotic usage have been associated with reductions in antibiotic adverse events, such as Clostridium difficile colitis and superinfection with multi‐drug resistant Gram‐negative bacteria.[37, 40, 41] Few studies in our review reported allergic reactions or adverse events of antibiotic therapy, [25, 27, 34] and only 1 reported antibiotic resistance.[19] Third, procalcitonin guidance should be compared to other strategies to reduce antibiotic usage, such as structured implementation of practice guidelines and antibiotic stewardship programs.[42] One single‐arm study describes how procalcitonin can be used in antibiotic stewardship programs to decrease the duration of antibiotic therapy,[43] but additional studies are needed. Finally, generalizing results from those studies that were conducted primarily in Europe would depend on similar use of and adherence to study‐based algorithms. Newer observational studies have demonstrated reduced antibiotic usage with implementation of procalcitonin algorithms in real‐life settings in Europe, but algorithm adherence was significantly less in the United States.[44, 45]
In summary, our systematic review found that procalcitonin‐guided antibiotic therapy can significantly reduce antibiotic usage in adult ICU patients without affecting morbidity or mortality. Procalcitonin should not be used to guide intensification of antibiotic therapy in adult ICU patients because this approach may increase morbidity. In adults with respiratory infections, procalcitonin guidance can significantly reduce antibiotic usage without adversely affecting morbidity or mortality. There is insufficient evidence to recommend procalcitonin‐guided antibiotic therapy in neonates with sepsis, children with fever of unknown source, or postoperative patients at risk for infection.
Acknowledgments
Disclosures: This project was funded under contract HHSA 2902007‐10058 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services. The authors of this article are responsible for its content, including any clinical treatment recommendations. No statement in this article should be construed as an official position of AHRQ or of the US Department of Health and Human Services. There are no conflicts of interest reported by any of the authors.
- , . Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15.
- , . Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298.
- , , . Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888–889.
- , , , et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–1608.
- , , , et al. Procalcitonin kinetics as a prognostic marker of ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48–53.
- , , , , . Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clin Infect Dis. 2004;39(2):206–217.
- , . Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30(3):556–573.
- , , , et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26(3):664–672.
- , , , , . Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection. 2009;37(6):497–507.
- , . Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53(4):379–387.
- , , , , . Procalcitonin‐guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta‐analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229–2241.
- , , , . Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331.
- , , , , , . An ESCIM systematic review and meta‐analysis of procalcitonin‐guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med. 2012;38:940–949.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
- , , , , , . Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under contract no. 290–2007‐10058‐I. Procalcitonin‐guided antibiotic therapy. Comparative effectiveness review No. 78. AHRQ publication no. 12(13)‐EHC124‐EF. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Published Accessed October 2012.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ publication no. 10(11)‐EHC063‐EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , , . Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505.
- , , , et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226.
- , , , et al. Procalcitonin for reduced antibiotic exposure in ventilator‐associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375.
- , , , et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83.
- , , , et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474.
- , , , , . Can procalcitonin help us in timing of re‐intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology. 2007;54(74):359–363.
- , , , et al. Effect of procalcitonin‐based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin Microbiol Infect. 2009;15(5):481–487.
- , , , et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–2007; discussion 2007–2008.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131(1):9–19.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363(9409):600–607.
- , , , , . Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology. 2010;97(2):165–174.
- , , , , , . Pre‐emptive antibiotic treatment vs “standard” treatment in patients with elevated serum procalcitonin levels after elective colorectal surgery: a prospective randomised pilot study. Langenbecks Arch Surg. 2006;391(3):187–194.
- , , , et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–2058.
- , , , et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–607.
- , , , , , . Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community‐acquired pneumonia. Respirology. 2011;16(5):819–824.
- , , , , , . Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. Am J Emerg Med. 2010;28(6):647–653.
- , , , , , . Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia. Crit Care Med. 2001;29(6):1109–1115.
- , , , et al. Diagnostic value of procalcitonin, interleukin‐6, and interleukin‐8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402.
- , , , . Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462–474.
- , , , , . Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24(9):699–706.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- , , , , . Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). Eur J Clin Microbiol Infect Dis. 2011;30(7):853–855.
- , , , et al. Effectiveness and safety of procalcitonin‐guided antibiotic therapy in lower respiratory tract infections in “real life.” Arch Intern Med. 2012;172(9):715–722.
- , , , et al. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post‐study survey. Eur J Clin Microbiol Infect Dis. 2012;29(3):269–277.
- , . Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15.
- , . Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298.
- , , . Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888–889.
- , , , et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–1608.
- , , , et al. Procalcitonin kinetics as a prognostic marker of ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48–53.
- , , , , . Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clin Infect Dis. 2004;39(2):206–217.
- , . Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30(3):556–573.
- , , , et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26(3):664–672.
- , , , , . Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection. 2009;37(6):497–507.
- , . Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53(4):379–387.
- , , , , . Procalcitonin‐guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta‐analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229–2241.
- , , , . Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331.
- , , , , , . An ESCIM systematic review and meta‐analysis of procalcitonin‐guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med. 2012;38:940–949.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
- , , , , , . Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under contract no. 290–2007‐10058‐I. Procalcitonin‐guided antibiotic therapy. Comparative effectiveness review No. 78. AHRQ publication no. 12(13)‐EHC124‐EF. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Published Accessed October 2012.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ publication no. 10(11)‐EHC063‐EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , , . Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505.
- , , , et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226.
- , , , et al. Procalcitonin for reduced antibiotic exposure in ventilator‐associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375.
- , , , et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83.
- , , , et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474.
- , , , , . Can procalcitonin help us in timing of re‐intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology. 2007;54(74):359–363.
- , , , et al. Effect of procalcitonin‐based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin Microbiol Infect. 2009;15(5):481–487.
- , , , et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–2007; discussion 2007–2008.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131(1):9–19.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363(9409):600–607.
- , , , , . Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology. 2010;97(2):165–174.
- , , , , , . Pre‐emptive antibiotic treatment vs “standard” treatment in patients with elevated serum procalcitonin levels after elective colorectal surgery: a prospective randomised pilot study. Langenbecks Arch Surg. 2006;391(3):187–194.
- , , , et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–2058.
- , , , et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–607.
- , , , , , . Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community‐acquired pneumonia. Respirology. 2011;16(5):819–824.
- , , , , , . Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. Am J Emerg Med. 2010;28(6):647–653.
- , , , , , . Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia. Crit Care Med. 2001;29(6):1109–1115.
- , , , et al. Diagnostic value of procalcitonin, interleukin‐6, and interleukin‐8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402.
- , , , . Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462–474.
- , , , , . Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24(9):699–706.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- , , , , . Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). Eur J Clin Microbiol Infect Dis. 2011;30(7):853–855.
- , , , et al. Effectiveness and safety of procalcitonin‐guided antibiotic therapy in lower respiratory tract infections in “real life.” Arch Intern Med. 2012;172(9):715–722.
- , , , et al. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post‐study survey. Eur J Clin Microbiol Infect Dis. 2012;29(3):269–277.
Smartphone Policy for Attending Rounds
Despite the many benefits of smartphones for physicians, there are also potential downsides to utilizing these devices in the patient care setting. Prior research at our hospital found that smartphone use during inpatient attending rounds can distract faculty and residents, and nearly 80% of attendings favored the institution of codes of conduct governing appropriate use of smartphones during rounds.[1] Based on these findings, a policy regulating faculty and resident smartphone use was instituted in February 2012 in the Departments of Medicine and Pediatrics at our hospital.[1]
Although our faculty's enthusiasm for the smartphone policy was clear, residents' attitudes toward this new regulation were unknown. Born in the 1980s, today's residents are members of the millennial generation, who seamlessly integrate technology into their lives.[2, 3, 4, 5] Millennials generally do not perceive their multitasking with technology to be rude or distracting.[2] Having grown up with the Internet, they employ digital tools as an inherent sixth sense,[3] and view their use of technology as the defining characteristic of their generation.[5]
Housestaff feedback was instrumental in shaping the specifics of the smartphone policy.[1] However, given the primacy of technology in the life of the millennial, it is plausible that residents would resent restrictions on their smartphone use. Such resentment could limit a policy's effectiveness, as well as negatively impact resident morale. With increasing discussion about the need to manage personal electronic device use in the patient care setting,[2, 6, 7, 8] we sought to assess residents' attitudes toward our hospital's smartphone policy.
METHODS
A brief survey instrument was designed to increase housestaff awareness of and evaluate their attitudes toward the smartphone policy. In November 2012, the anonymous survey was administered via SurveyMonkey (
The survey provided a summary of the policy: The smartphone code of conduct policy was instituted to minimize distraction during attending rounds. The policy applies to all team members, including faculty, and essentially states that at the start of attending rounds, all phones must be silenced or turned off. These devices are to be used during rounds only for patient care or for urgent personal/family concerns. Any use must be made explicit to the person leading rounds. Residents also received a copy of the complete policy as an attachment to the request email. A copy of this policy is available as an appendix to Katz‐Sidlow et al.[1]
The survey requested information regarding departmental affiliation, and asked whether the resident had prior awareness of the smartphone policy. Residents' attitudes were evaluated by asking for their level of agreement with the following statement: It is a good idea to have clear guidelines and expectations about how team members should use smartphones during attending rounds. This statement was graded on a 4‐point frequency scale (strongly disagree, disagree, agree, or strongly agree). Residents' attitudes were further explored in a follow‐up question: Which statement most closely expresses your feelings? Three options were offered: (1) There should be no guidelines as to how team members should use smartphones during inpatient attending rounds. Every person should decide for him/herself how and when to use the phone during rounds. (2) I agree that a smartphone code of conduct for attending rounds is a good idea, but I suggest modifying the current policy (please use the text box below to explain). (3) I agree with the current smartphone code of conduct policy for attending rounds. A text box was provided for comments.
RESULTS
The overall response rate was 65% (93/142), representing 58% (57/98) of all Department of Medicine residents and 82% (36/44) of all Department of Pediatrics residents. Seventy‐one percent of respondents (57% Department of Medicine; 92% Department of Pediatrics) indicated a prior knowledge of the smartphone policy.
Overall, 82% of respondents agreed or strongly agreed with the statement, It is a good idea to have clear guidelines and expectations about how team members should use smartphones during attending rounds (Figure 1). Residents' responses to the follow‐up question revealed that nearly 60% agreed with the stipulations of the current policy; another 18% believed that a policy is needed, but felt that the current code should be modified. Only one resident provided a modification suggestion, which was to expand the policy to include resident work rounds.
Responses to these 2 questions differed slightly for trainees with an awareness of the preexisting policy as compared to those without prior awareness; however, these differences were not statistically significant.
CONCLUSIONS
Despite concerns that residents would resent policies regulating their use of technology, we found that the majority of residents indicated a desire for, and acceptance of, clear guidelines regarding smartphone use during inpatient rounds. Our findings are in line with prior research suggesting that millennials appreciate a structured work environment and explicit guidance regarding workplace expectations.[2, 3, 4] To minimize distraction and support residents' professionalism, we recommend that training programs develop and implement clear expectations regarding smartphone use in the active patient care setting.
- , , , . Smartphone use during inpatient attending rounds: prevalence, patterns and potential for distraction. J Hosp Med. 2012;7:595–599.
- , . The challenges and opportunities of teaching “generation y.” J Grad Med Educ. 2011;3:458–461.
- , . Millennials and the world of work: an organization and management perspective. J Bus Psychol. 2010;25:211–223.
- , , . Is your residency program ready for generation y? J Surg Educ. 2010;67:108–111.
- Pew Research Center. Millennials: a portrait of generation next. Pew Research Center Web site. February 2010. Available at: http://www.pewsocialtrends.org/files/2010/10/millennials‐confident‐connected‐open‐to‐change.pdf. Accessed May 9, 2013.
- . Spotlight case. Order interrupted by text: multitasking mishap. Agency for Healthcare Research and Quality Web site. December 2011. Available at: http://webmm.ahrq.gov/case.aspx?caseID=257. Accessed May 9, 2013.
- . Training health care professionals to deal with an explosion of electronic distraction. Neurocrit Care. 2013;18:115–117.
- ECRI Institute. Top 10 health technology hazards for 2013. ECRI Institute Web site. Available at: https://www.ecri.org/Documents/Secure/Health_Devices_Top_10_Hazards_2013.pdf. Accessed May 9, 2013.
Despite the many benefits of smartphones for physicians, there are also potential downsides to utilizing these devices in the patient care setting. Prior research at our hospital found that smartphone use during inpatient attending rounds can distract faculty and residents, and nearly 80% of attendings favored the institution of codes of conduct governing appropriate use of smartphones during rounds.[1] Based on these findings, a policy regulating faculty and resident smartphone use was instituted in February 2012 in the Departments of Medicine and Pediatrics at our hospital.[1]
Although our faculty's enthusiasm for the smartphone policy was clear, residents' attitudes toward this new regulation were unknown. Born in the 1980s, today's residents are members of the millennial generation, who seamlessly integrate technology into their lives.[2, 3, 4, 5] Millennials generally do not perceive their multitasking with technology to be rude or distracting.[2] Having grown up with the Internet, they employ digital tools as an inherent sixth sense,[3] and view their use of technology as the defining characteristic of their generation.[5]
Housestaff feedback was instrumental in shaping the specifics of the smartphone policy.[1] However, given the primacy of technology in the life of the millennial, it is plausible that residents would resent restrictions on their smartphone use. Such resentment could limit a policy's effectiveness, as well as negatively impact resident morale. With increasing discussion about the need to manage personal electronic device use in the patient care setting,[2, 6, 7, 8] we sought to assess residents' attitudes toward our hospital's smartphone policy.
METHODS
A brief survey instrument was designed to increase housestaff awareness of and evaluate their attitudes toward the smartphone policy. In November 2012, the anonymous survey was administered via SurveyMonkey (
The survey provided a summary of the policy: The smartphone code of conduct policy was instituted to minimize distraction during attending rounds. The policy applies to all team members, including faculty, and essentially states that at the start of attending rounds, all phones must be silenced or turned off. These devices are to be used during rounds only for patient care or for urgent personal/family concerns. Any use must be made explicit to the person leading rounds. Residents also received a copy of the complete policy as an attachment to the request email. A copy of this policy is available as an appendix to Katz‐Sidlow et al.[1]
The survey requested information regarding departmental affiliation, and asked whether the resident had prior awareness of the smartphone policy. Residents' attitudes were evaluated by asking for their level of agreement with the following statement: It is a good idea to have clear guidelines and expectations about how team members should use smartphones during attending rounds. This statement was graded on a 4‐point frequency scale (strongly disagree, disagree, agree, or strongly agree). Residents' attitudes were further explored in a follow‐up question: Which statement most closely expresses your feelings? Three options were offered: (1) There should be no guidelines as to how team members should use smartphones during inpatient attending rounds. Every person should decide for him/herself how and when to use the phone during rounds. (2) I agree that a smartphone code of conduct for attending rounds is a good idea, but I suggest modifying the current policy (please use the text box below to explain). (3) I agree with the current smartphone code of conduct policy for attending rounds. A text box was provided for comments.
RESULTS
The overall response rate was 65% (93/142), representing 58% (57/98) of all Department of Medicine residents and 82% (36/44) of all Department of Pediatrics residents. Seventy‐one percent of respondents (57% Department of Medicine; 92% Department of Pediatrics) indicated a prior knowledge of the smartphone policy.
Overall, 82% of respondents agreed or strongly agreed with the statement, It is a good idea to have clear guidelines and expectations about how team members should use smartphones during attending rounds (Figure 1). Residents' responses to the follow‐up question revealed that nearly 60% agreed with the stipulations of the current policy; another 18% believed that a policy is needed, but felt that the current code should be modified. Only one resident provided a modification suggestion, which was to expand the policy to include resident work rounds.

Responses to these 2 questions differed slightly for trainees with an awareness of the preexisting policy as compared to those without prior awareness; however, these differences were not statistically significant.
CONCLUSIONS
Despite concerns that residents would resent policies regulating their use of technology, we found that the majority of residents indicated a desire for, and acceptance of, clear guidelines regarding smartphone use during inpatient rounds. Our findings are in line with prior research suggesting that millennials appreciate a structured work environment and explicit guidance regarding workplace expectations.[2, 3, 4] To minimize distraction and support residents' professionalism, we recommend that training programs develop and implement clear expectations regarding smartphone use in the active patient care setting.
Despite the many benefits of smartphones for physicians, there are also potential downsides to utilizing these devices in the patient care setting. Prior research at our hospital found that smartphone use during inpatient attending rounds can distract faculty and residents, and nearly 80% of attendings favored the institution of codes of conduct governing appropriate use of smartphones during rounds.[1] Based on these findings, a policy regulating faculty and resident smartphone use was instituted in February 2012 in the Departments of Medicine and Pediatrics at our hospital.[1]
Although our faculty's enthusiasm for the smartphone policy was clear, residents' attitudes toward this new regulation were unknown. Born in the 1980s, today's residents are members of the millennial generation, who seamlessly integrate technology into their lives.[2, 3, 4, 5] Millennials generally do not perceive their multitasking with technology to be rude or distracting.[2] Having grown up with the Internet, they employ digital tools as an inherent sixth sense,[3] and view their use of technology as the defining characteristic of their generation.[5]
Housestaff feedback was instrumental in shaping the specifics of the smartphone policy.[1] However, given the primacy of technology in the life of the millennial, it is plausible that residents would resent restrictions on their smartphone use. Such resentment could limit a policy's effectiveness, as well as negatively impact resident morale. With increasing discussion about the need to manage personal electronic device use in the patient care setting,[2, 6, 7, 8] we sought to assess residents' attitudes toward our hospital's smartphone policy.
METHODS
A brief survey instrument was designed to increase housestaff awareness of and evaluate their attitudes toward the smartphone policy. In November 2012, the anonymous survey was administered via SurveyMonkey (
The survey provided a summary of the policy: The smartphone code of conduct policy was instituted to minimize distraction during attending rounds. The policy applies to all team members, including faculty, and essentially states that at the start of attending rounds, all phones must be silenced or turned off. These devices are to be used during rounds only for patient care or for urgent personal/family concerns. Any use must be made explicit to the person leading rounds. Residents also received a copy of the complete policy as an attachment to the request email. A copy of this policy is available as an appendix to Katz‐Sidlow et al.[1]
The survey requested information regarding departmental affiliation, and asked whether the resident had prior awareness of the smartphone policy. Residents' attitudes were evaluated by asking for their level of agreement with the following statement: It is a good idea to have clear guidelines and expectations about how team members should use smartphones during attending rounds. This statement was graded on a 4‐point frequency scale (strongly disagree, disagree, agree, or strongly agree). Residents' attitudes were further explored in a follow‐up question: Which statement most closely expresses your feelings? Three options were offered: (1) There should be no guidelines as to how team members should use smartphones during inpatient attending rounds. Every person should decide for him/herself how and when to use the phone during rounds. (2) I agree that a smartphone code of conduct for attending rounds is a good idea, but I suggest modifying the current policy (please use the text box below to explain). (3) I agree with the current smartphone code of conduct policy for attending rounds. A text box was provided for comments.
RESULTS
The overall response rate was 65% (93/142), representing 58% (57/98) of all Department of Medicine residents and 82% (36/44) of all Department of Pediatrics residents. Seventy‐one percent of respondents (57% Department of Medicine; 92% Department of Pediatrics) indicated a prior knowledge of the smartphone policy.
Overall, 82% of respondents agreed or strongly agreed with the statement, It is a good idea to have clear guidelines and expectations about how team members should use smartphones during attending rounds (Figure 1). Residents' responses to the follow‐up question revealed that nearly 60% agreed with the stipulations of the current policy; another 18% believed that a policy is needed, but felt that the current code should be modified. Only one resident provided a modification suggestion, which was to expand the policy to include resident work rounds.

Responses to these 2 questions differed slightly for trainees with an awareness of the preexisting policy as compared to those without prior awareness; however, these differences were not statistically significant.
CONCLUSIONS
Despite concerns that residents would resent policies regulating their use of technology, we found that the majority of residents indicated a desire for, and acceptance of, clear guidelines regarding smartphone use during inpatient rounds. Our findings are in line with prior research suggesting that millennials appreciate a structured work environment and explicit guidance regarding workplace expectations.[2, 3, 4] To minimize distraction and support residents' professionalism, we recommend that training programs develop and implement clear expectations regarding smartphone use in the active patient care setting.
- , , , . Smartphone use during inpatient attending rounds: prevalence, patterns and potential for distraction. J Hosp Med. 2012;7:595–599.
- , . The challenges and opportunities of teaching “generation y.” J Grad Med Educ. 2011;3:458–461.
- , . Millennials and the world of work: an organization and management perspective. J Bus Psychol. 2010;25:211–223.
- , , . Is your residency program ready for generation y? J Surg Educ. 2010;67:108–111.
- Pew Research Center. Millennials: a portrait of generation next. Pew Research Center Web site. February 2010. Available at: http://www.pewsocialtrends.org/files/2010/10/millennials‐confident‐connected‐open‐to‐change.pdf. Accessed May 9, 2013.
- . Spotlight case. Order interrupted by text: multitasking mishap. Agency for Healthcare Research and Quality Web site. December 2011. Available at: http://webmm.ahrq.gov/case.aspx?caseID=257. Accessed May 9, 2013.
- . Training health care professionals to deal with an explosion of electronic distraction. Neurocrit Care. 2013;18:115–117.
- ECRI Institute. Top 10 health technology hazards for 2013. ECRI Institute Web site. Available at: https://www.ecri.org/Documents/Secure/Health_Devices_Top_10_Hazards_2013.pdf. Accessed May 9, 2013.
- , , , . Smartphone use during inpatient attending rounds: prevalence, patterns and potential for distraction. J Hosp Med. 2012;7:595–599.
- , . The challenges and opportunities of teaching “generation y.” J Grad Med Educ. 2011;3:458–461.
- , . Millennials and the world of work: an organization and management perspective. J Bus Psychol. 2010;25:211–223.
- , , . Is your residency program ready for generation y? J Surg Educ. 2010;67:108–111.
- Pew Research Center. Millennials: a portrait of generation next. Pew Research Center Web site. February 2010. Available at: http://www.pewsocialtrends.org/files/2010/10/millennials‐confident‐connected‐open‐to‐change.pdf. Accessed May 9, 2013.
- . Spotlight case. Order interrupted by text: multitasking mishap. Agency for Healthcare Research and Quality Web site. December 2011. Available at: http://webmm.ahrq.gov/case.aspx?caseID=257. Accessed May 9, 2013.
- . Training health care professionals to deal with an explosion of electronic distraction. Neurocrit Care. 2013;18:115–117.
- ECRI Institute. Top 10 health technology hazards for 2013. ECRI Institute Web site. Available at: https://www.ecri.org/Documents/Secure/Health_Devices_Top_10_Hazards_2013.pdf. Accessed May 9, 2013.
Apps for your smart phone
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to [email protected] and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
[email protected] On Twitter @NaseemSMiller
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to [email protected] and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
[email protected] On Twitter @NaseemSMiller
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to [email protected] and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
[email protected] On Twitter @NaseemSMiller
Turn up the tunes in the ICU
Clinical question
Can patient-directed music therapy decrease anxiety and reduce sedative use in the intensive care unit?
Bottom line
Patient-directed music therapy in the intensive care unit (ICU) reduces anxiety in awake, ventilated patients while also decreasing the intensity and frequency of sedative use. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded);
Allocation
Concealed
Setting
Inpatient (ICU only)
Synopsis
These investigators studied the effects of patient-directed music therapy in reducing anxiety and sedative use in the ICU. Patients using ventilatory support for acute respiratory failure who were alert enough to consent and operate a music player were randomized, using concealed allocation, to 1 of 3 groups: (1) the use of headphones to listen to music (n = 126), (2) the use of noise-cancelling headphones to block out ICU noise (n = 122), and (3) usual care (n = 125). Only 5% of patients who were assessed for eligibility actually underwent randomization, as patients who were unable to consent because of confusion or deep sedation were excluded. A music therapist helped patients in group 1 select their preferred music. These patients were then directed and prompted to listen to music via headphones as often as desired. In group 2, patients were encouraged to wear noise-cancelling headphones whenever they wanted to block out ICU noise. Patients in all 3 groups had similar baseline characteristics, including anxiety scores at study entry and intensity and frequency of sedation 24 hours prior to enrollment. There was a wide range of Acute Physiology, Age and Chronic Health Evaluation III (APACHE III) scores, but the mean fell between 62 and 66 in all 3 groups. A research nurse administered a 100-mm anxiety visual analog scale to patients daily when feasible.
Patients in the music therapy group listened to music for an average of 80 minutes per day; those in the noise-cancelling group wore their headphones for 34 minutes per day. After adjusting for APACHE III scores and sedation frequency and intensity, the use of music therapy lowered anxiety scores by 19 mm compared with usual care (relative decrease of 36%; P = .003). The music group also had decreased sedation intensity (P = .05) and frequency (P = .01) over time when compared with usual care after adjustments were made for imbalances. For example, by day 5, patients in the music group received 3 doses per day of sedative medication, while those in the usual care group received 5 doses. The music therapy group also showed reduction in sedation frequency when compared with the noise-cancelling headphones group, but there were no significant differences detected in anxiety scoring or sedation intensity between these 2 groups. The study did not examine ICU length of stay or other clinical outcomes.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Can patient-directed music therapy decrease anxiety and reduce sedative use in the intensive care unit?
Bottom line
Patient-directed music therapy in the intensive care unit (ICU) reduces anxiety in awake, ventilated patients while also decreasing the intensity and frequency of sedative use. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded);
Allocation
Concealed
Setting
Inpatient (ICU only)
Synopsis
These investigators studied the effects of patient-directed music therapy in reducing anxiety and sedative use in the ICU. Patients using ventilatory support for acute respiratory failure who were alert enough to consent and operate a music player were randomized, using concealed allocation, to 1 of 3 groups: (1) the use of headphones to listen to music (n = 126), (2) the use of noise-cancelling headphones to block out ICU noise (n = 122), and (3) usual care (n = 125). Only 5% of patients who were assessed for eligibility actually underwent randomization, as patients who were unable to consent because of confusion or deep sedation were excluded. A music therapist helped patients in group 1 select their preferred music. These patients were then directed and prompted to listen to music via headphones as often as desired. In group 2, patients were encouraged to wear noise-cancelling headphones whenever they wanted to block out ICU noise. Patients in all 3 groups had similar baseline characteristics, including anxiety scores at study entry and intensity and frequency of sedation 24 hours prior to enrollment. There was a wide range of Acute Physiology, Age and Chronic Health Evaluation III (APACHE III) scores, but the mean fell between 62 and 66 in all 3 groups. A research nurse administered a 100-mm anxiety visual analog scale to patients daily when feasible.
Patients in the music therapy group listened to music for an average of 80 minutes per day; those in the noise-cancelling group wore their headphones for 34 minutes per day. After adjusting for APACHE III scores and sedation frequency and intensity, the use of music therapy lowered anxiety scores by 19 mm compared with usual care (relative decrease of 36%; P = .003). The music group also had decreased sedation intensity (P = .05) and frequency (P = .01) over time when compared with usual care after adjustments were made for imbalances. For example, by day 5, patients in the music group received 3 doses per day of sedative medication, while those in the usual care group received 5 doses. The music therapy group also showed reduction in sedation frequency when compared with the noise-cancelling headphones group, but there were no significant differences detected in anxiety scoring or sedation intensity between these 2 groups. The study did not examine ICU length of stay or other clinical outcomes.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Can patient-directed music therapy decrease anxiety and reduce sedative use in the intensive care unit?
Bottom line
Patient-directed music therapy in the intensive care unit (ICU) reduces anxiety in awake, ventilated patients while also decreasing the intensity and frequency of sedative use. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded);
Allocation
Concealed
Setting
Inpatient (ICU only)
Synopsis
These investigators studied the effects of patient-directed music therapy in reducing anxiety and sedative use in the ICU. Patients using ventilatory support for acute respiratory failure who were alert enough to consent and operate a music player were randomized, using concealed allocation, to 1 of 3 groups: (1) the use of headphones to listen to music (n = 126), (2) the use of noise-cancelling headphones to block out ICU noise (n = 122), and (3) usual care (n = 125). Only 5% of patients who were assessed for eligibility actually underwent randomization, as patients who were unable to consent because of confusion or deep sedation were excluded. A music therapist helped patients in group 1 select their preferred music. These patients were then directed and prompted to listen to music via headphones as often as desired. In group 2, patients were encouraged to wear noise-cancelling headphones whenever they wanted to block out ICU noise. Patients in all 3 groups had similar baseline characteristics, including anxiety scores at study entry and intensity and frequency of sedation 24 hours prior to enrollment. There was a wide range of Acute Physiology, Age and Chronic Health Evaluation III (APACHE III) scores, but the mean fell between 62 and 66 in all 3 groups. A research nurse administered a 100-mm anxiety visual analog scale to patients daily when feasible.
Patients in the music therapy group listened to music for an average of 80 minutes per day; those in the noise-cancelling group wore their headphones for 34 minutes per day. After adjusting for APACHE III scores and sedation frequency and intensity, the use of music therapy lowered anxiety scores by 19 mm compared with usual care (relative decrease of 36%; P = .003). The music group also had decreased sedation intensity (P = .05) and frequency (P = .01) over time when compared with usual care after adjustments were made for imbalances. For example, by day 5, patients in the music group received 3 doses per day of sedative medication, while those in the usual care group received 5 doses. The music therapy group also showed reduction in sedation frequency when compared with the noise-cancelling headphones group, but there were no significant differences detected in anxiety scoring or sedation intensity between these 2 groups. The study did not examine ICU length of stay or other clinical outcomes.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
No Mortality Benefit to Rapid Reduction of BP for Intracranial Bleeds (INTERACT2)
Clinical question
Does early intensive reduction of blood pressure improve outcomes in patients with hemorrhagic strokes?
Bottom line
As compared with more conservative management, early intensive lowering of blood pressure (BP) for patients with spontaneous intracranial bleeds does not significantly decrease mortality. However, this approach may result in better functional outcomes and quality of life. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded);
Allocation
(Uncertain)
Setting
Inpatient (ICU only)
Synopsis
Patients who presented with spontaneous intracranial hemorrhage within the previous 6 hours were randomly assigned, using concealed allocation, to receive intensive BP-lowering therapy (n = 1403) or standard BP management according to current guidelines (n = 1436). Patients with structural causes for the bleed, those who were in a deep coma, or those who required early hematoma evacuation were excluded. In the intensive treatment group, intravenous and oral BP medications were used to lower the systolic BP to less than 140 mmHg within 1 hour and maintain it at this level for 1 week. Those in the standard treatment group received BP-lowering agents only if their systolic BP was greater than 180 mmHg. The mean age of the patients in the 2 groups was 64 years and the median presenting Glasgow Coma Scale score was 14. As compared with the standard therapy group, the intensive treatment group started BP-lowering therapy earlier after onset of intracranial hemorrhage (4.0 hours vs 4.5 hours; P < .001) and was more likely to receive intravenous agents (90% vs 43%; P < .001). At 90 days, there was no difference in the primary outcome of death or major disability, defined as a score of 3 to 5 on the modified Rankin scale (0 to 6, where 0 indicates no symptoms and 6 indicates death). Overall, 12% of the patients in each group died. When looking at disability alone, the intensive treatment group had significantly lower modified Rankin scores than the standard therapy group (odds ratio = 0.87; 95% CI, 0.77-1.0; P = .04) and had higher health-related quality of life scores (0.60 vs 0.55; P = .002). As this trial was not masked, the possibility exists that patients in the 2 groups were managed differently beyond just the 2 BP-lowering strategies.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does early intensive reduction of blood pressure improve outcomes in patients with hemorrhagic strokes?
Bottom line
As compared with more conservative management, early intensive lowering of blood pressure (BP) for patients with spontaneous intracranial bleeds does not significantly decrease mortality. However, this approach may result in better functional outcomes and quality of life. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded);
Allocation
(Uncertain)
Setting
Inpatient (ICU only)
Synopsis
Patients who presented with spontaneous intracranial hemorrhage within the previous 6 hours were randomly assigned, using concealed allocation, to receive intensive BP-lowering therapy (n = 1403) or standard BP management according to current guidelines (n = 1436). Patients with structural causes for the bleed, those who were in a deep coma, or those who required early hematoma evacuation were excluded. In the intensive treatment group, intravenous and oral BP medications were used to lower the systolic BP to less than 140 mmHg within 1 hour and maintain it at this level for 1 week. Those in the standard treatment group received BP-lowering agents only if their systolic BP was greater than 180 mmHg. The mean age of the patients in the 2 groups was 64 years and the median presenting Glasgow Coma Scale score was 14. As compared with the standard therapy group, the intensive treatment group started BP-lowering therapy earlier after onset of intracranial hemorrhage (4.0 hours vs 4.5 hours; P < .001) and was more likely to receive intravenous agents (90% vs 43%; P < .001). At 90 days, there was no difference in the primary outcome of death or major disability, defined as a score of 3 to 5 on the modified Rankin scale (0 to 6, where 0 indicates no symptoms and 6 indicates death). Overall, 12% of the patients in each group died. When looking at disability alone, the intensive treatment group had significantly lower modified Rankin scores than the standard therapy group (odds ratio = 0.87; 95% CI, 0.77-1.0; P = .04) and had higher health-related quality of life scores (0.60 vs 0.55; P = .002). As this trial was not masked, the possibility exists that patients in the 2 groups were managed differently beyond just the 2 BP-lowering strategies.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does early intensive reduction of blood pressure improve outcomes in patients with hemorrhagic strokes?
Bottom line
As compared with more conservative management, early intensive lowering of blood pressure (BP) for patients with spontaneous intracranial bleeds does not significantly decrease mortality. However, this approach may result in better functional outcomes and quality of life. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded);
Allocation
(Uncertain)
Setting
Inpatient (ICU only)
Synopsis
Patients who presented with spontaneous intracranial hemorrhage within the previous 6 hours were randomly assigned, using concealed allocation, to receive intensive BP-lowering therapy (n = 1403) or standard BP management according to current guidelines (n = 1436). Patients with structural causes for the bleed, those who were in a deep coma, or those who required early hematoma evacuation were excluded. In the intensive treatment group, intravenous and oral BP medications were used to lower the systolic BP to less than 140 mmHg within 1 hour and maintain it at this level for 1 week. Those in the standard treatment group received BP-lowering agents only if their systolic BP was greater than 180 mmHg. The mean age of the patients in the 2 groups was 64 years and the median presenting Glasgow Coma Scale score was 14. As compared with the standard therapy group, the intensive treatment group started BP-lowering therapy earlier after onset of intracranial hemorrhage (4.0 hours vs 4.5 hours; P < .001) and was more likely to receive intravenous agents (90% vs 43%; P < .001). At 90 days, there was no difference in the primary outcome of death or major disability, defined as a score of 3 to 5 on the modified Rankin scale (0 to 6, where 0 indicates no symptoms and 6 indicates death). Overall, 12% of the patients in each group died. When looking at disability alone, the intensive treatment group had significantly lower modified Rankin scores than the standard therapy group (odds ratio = 0.87; 95% CI, 0.77-1.0; P = .04) and had higher health-related quality of life scores (0.60 vs 0.55; P = .002). As this trial was not masked, the possibility exists that patients in the 2 groups were managed differently beyond just the 2 BP-lowering strategies.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Patient privacy, part 2
We live in an increasingly social and connected world. As such, doctor-patient online communication will become more common. As I noted in my last column, many physicians see online communication as a minefield of potential patient privacy violations, and they are reluctant to use it. Rather than avoid it for fear of committing a privacy violation, I hope all physicians will educate themselves on how to communicate online safely and effectively. To this end, I’m providing some examples of online questions you will likely encounter, with sample responses.
• A nonpatient asks you to make a diagnosis online. As a dermatologist who is active in social media, I often have nonpatients send me photos of their skin conditions requesting a diagnosis.
Sample question: "I’ve attached a photo of a mole on my leg. Does it look OK to you, Doc? Could it be cancerous?"
Sample response: "Thank you for sending me the picture. Without an established doctor-patient relationship, I’m unable to provide a diagnosis. I recommend you make an appointment with your dermatologist. If you don’t have one, here’s a link to the American Academy of Dermatology website where you can enter your ZIP code to find a dermatologist near you."
• A nonpatient asks your opinion about symptoms he is experiencing.
Sample question: "I’ve been experiencing bouts of diarrhea and stomach cramping and think I have celiac disease. I’m going to stop eating gluten. Do you think that’s a good idea?"
Sample response: "I’m sorry you’ve been unwell. Unfortunately, I cannot provide medical advice to you since we don’t have an established doctor-patient relationship. I can tell you, however, that diarrhea and cramping can be symptomatic of many conditions including, but not limited to celiac disease, irritable bowel syndrome, and lactose intolerance. I recommend you make an appointment with your primary care doctor so he or she can help you with your actual diagnosis and treatment."
• A patient of yours asks you a clinical question in an open forum such as Facebook or Twitter.
Sample question: "Hi, Doc. The birth control pills you gave me aren’t helping. I’ve been on them for 2 months, and I’m still having all the symptoms I had originally – mood swings, spotting, difficulty sleeping, and my acne’s not better. I think I need a different pill. Can you prescribe me one?"
Sample response: "I’m sorry to hear that. I’m happy to discuss this with you, but let’s do so privately. Please use our secure office e-mail to contact me, or call me during office hours and we can talk about what’s happening and what to do next. Hope to hear from you soon."
• Someone asks for specific product recommendations.
Sample question: "My doctor wants me to buy the sunscreen he sells in his office. He says it’s better than drug store brands, but it’s expensive. Is he telling the truth?"
Sample response: "I can’t speak specifically to your doctor’s sunscreen. But in general, you should look for a sunscreen that is labeled "broad spectrum," which protects against both UVA and UVB rays and has an SPF of 30-50. Most often, price doesn’t correlate with effectiveness. So, just because a sunscreen is more expensive doesn’t necessarily mean it’s more effective."
• Someone criticizes his current doctor or medical provider.
Sample question: "I’ve been going to my dermatologist for 6 months now and my acne hasn’t gotten any better. He put me on antibiotics and topical creams, and I still have acne. He obviously doesn’t know what he’s doing. Can you tell me what to do?"
Sample reply: "I’m sorry to hear that. I know how frustrating it can be. Acne can be very difficult to treat and can take a long time. Be sure that you’re communicating with your doctor about your situation so he can help you. Also, remember that you can always request a second opinion."
• You want to blog about a patient’s condition. How do you do it without violating the patient’s privacy while ensuring that he cannot be identified? One option is to obtain the patient’s written consent. Another option is to create a composite: Use real facts with fictional patients. For example, your actual patient is a 30-year-old UPS driver with severe hand eczema. You want to write about connections between hand eczema and occupational exposure. You create a fictional patient who is a 40-year-old female mail carrier. You discuss the symptoms of your actual patient in a way that maintains his privacy yet allows you to educate patients online.
Clearly, there are many more scenarios you may encounter online. This is a small sampling to give you some idea of how to respond safely and professionally. If you have specific questions or suggestions, feel free to share them by writing to [email protected].
Dr. Benabio is physician director of innovation at Kaiser Permanente in San Diego. Visit his consumer health blog at thedermblog.com and his health care blog at benabio.com. Connect with him on Twitter @Dermdoc and on Facebook (DermDoc).
We live in an increasingly social and connected world. As such, doctor-patient online communication will become more common. As I noted in my last column, many physicians see online communication as a minefield of potential patient privacy violations, and they are reluctant to use it. Rather than avoid it for fear of committing a privacy violation, I hope all physicians will educate themselves on how to communicate online safely and effectively. To this end, I’m providing some examples of online questions you will likely encounter, with sample responses.
• A nonpatient asks you to make a diagnosis online. As a dermatologist who is active in social media, I often have nonpatients send me photos of their skin conditions requesting a diagnosis.
Sample question: "I’ve attached a photo of a mole on my leg. Does it look OK to you, Doc? Could it be cancerous?"
Sample response: "Thank you for sending me the picture. Without an established doctor-patient relationship, I’m unable to provide a diagnosis. I recommend you make an appointment with your dermatologist. If you don’t have one, here’s a link to the American Academy of Dermatology website where you can enter your ZIP code to find a dermatologist near you."
• A nonpatient asks your opinion about symptoms he is experiencing.
Sample question: "I’ve been experiencing bouts of diarrhea and stomach cramping and think I have celiac disease. I’m going to stop eating gluten. Do you think that’s a good idea?"
Sample response: "I’m sorry you’ve been unwell. Unfortunately, I cannot provide medical advice to you since we don’t have an established doctor-patient relationship. I can tell you, however, that diarrhea and cramping can be symptomatic of many conditions including, but not limited to celiac disease, irritable bowel syndrome, and lactose intolerance. I recommend you make an appointment with your primary care doctor so he or she can help you with your actual diagnosis and treatment."
• A patient of yours asks you a clinical question in an open forum such as Facebook or Twitter.
Sample question: "Hi, Doc. The birth control pills you gave me aren’t helping. I’ve been on them for 2 months, and I’m still having all the symptoms I had originally – mood swings, spotting, difficulty sleeping, and my acne’s not better. I think I need a different pill. Can you prescribe me one?"
Sample response: "I’m sorry to hear that. I’m happy to discuss this with you, but let’s do so privately. Please use our secure office e-mail to contact me, or call me during office hours and we can talk about what’s happening and what to do next. Hope to hear from you soon."
• Someone asks for specific product recommendations.
Sample question: "My doctor wants me to buy the sunscreen he sells in his office. He says it’s better than drug store brands, but it’s expensive. Is he telling the truth?"
Sample response: "I can’t speak specifically to your doctor’s sunscreen. But in general, you should look for a sunscreen that is labeled "broad spectrum," which protects against both UVA and UVB rays and has an SPF of 30-50. Most often, price doesn’t correlate with effectiveness. So, just because a sunscreen is more expensive doesn’t necessarily mean it’s more effective."
• Someone criticizes his current doctor or medical provider.
Sample question: "I’ve been going to my dermatologist for 6 months now and my acne hasn’t gotten any better. He put me on antibiotics and topical creams, and I still have acne. He obviously doesn’t know what he’s doing. Can you tell me what to do?"
Sample reply: "I’m sorry to hear that. I know how frustrating it can be. Acne can be very difficult to treat and can take a long time. Be sure that you’re communicating with your doctor about your situation so he can help you. Also, remember that you can always request a second opinion."
• You want to blog about a patient’s condition. How do you do it without violating the patient’s privacy while ensuring that he cannot be identified? One option is to obtain the patient’s written consent. Another option is to create a composite: Use real facts with fictional patients. For example, your actual patient is a 30-year-old UPS driver with severe hand eczema. You want to write about connections between hand eczema and occupational exposure. You create a fictional patient who is a 40-year-old female mail carrier. You discuss the symptoms of your actual patient in a way that maintains his privacy yet allows you to educate patients online.
Clearly, there are many more scenarios you may encounter online. This is a small sampling to give you some idea of how to respond safely and professionally. If you have specific questions or suggestions, feel free to share them by writing to [email protected].
Dr. Benabio is physician director of innovation at Kaiser Permanente in San Diego. Visit his consumer health blog at thedermblog.com and his health care blog at benabio.com. Connect with him on Twitter @Dermdoc and on Facebook (DermDoc).
We live in an increasingly social and connected world. As such, doctor-patient online communication will become more common. As I noted in my last column, many physicians see online communication as a minefield of potential patient privacy violations, and they are reluctant to use it. Rather than avoid it for fear of committing a privacy violation, I hope all physicians will educate themselves on how to communicate online safely and effectively. To this end, I’m providing some examples of online questions you will likely encounter, with sample responses.
• A nonpatient asks you to make a diagnosis online. As a dermatologist who is active in social media, I often have nonpatients send me photos of their skin conditions requesting a diagnosis.
Sample question: "I’ve attached a photo of a mole on my leg. Does it look OK to you, Doc? Could it be cancerous?"
Sample response: "Thank you for sending me the picture. Without an established doctor-patient relationship, I’m unable to provide a diagnosis. I recommend you make an appointment with your dermatologist. If you don’t have one, here’s a link to the American Academy of Dermatology website where you can enter your ZIP code to find a dermatologist near you."
• A nonpatient asks your opinion about symptoms he is experiencing.
Sample question: "I’ve been experiencing bouts of diarrhea and stomach cramping and think I have celiac disease. I’m going to stop eating gluten. Do you think that’s a good idea?"
Sample response: "I’m sorry you’ve been unwell. Unfortunately, I cannot provide medical advice to you since we don’t have an established doctor-patient relationship. I can tell you, however, that diarrhea and cramping can be symptomatic of many conditions including, but not limited to celiac disease, irritable bowel syndrome, and lactose intolerance. I recommend you make an appointment with your primary care doctor so he or she can help you with your actual diagnosis and treatment."
• A patient of yours asks you a clinical question in an open forum such as Facebook or Twitter.
Sample question: "Hi, Doc. The birth control pills you gave me aren’t helping. I’ve been on them for 2 months, and I’m still having all the symptoms I had originally – mood swings, spotting, difficulty sleeping, and my acne’s not better. I think I need a different pill. Can you prescribe me one?"
Sample response: "I’m sorry to hear that. I’m happy to discuss this with you, but let’s do so privately. Please use our secure office e-mail to contact me, or call me during office hours and we can talk about what’s happening and what to do next. Hope to hear from you soon."
• Someone asks for specific product recommendations.
Sample question: "My doctor wants me to buy the sunscreen he sells in his office. He says it’s better than drug store brands, but it’s expensive. Is he telling the truth?"
Sample response: "I can’t speak specifically to your doctor’s sunscreen. But in general, you should look for a sunscreen that is labeled "broad spectrum," which protects against both UVA and UVB rays and has an SPF of 30-50. Most often, price doesn’t correlate with effectiveness. So, just because a sunscreen is more expensive doesn’t necessarily mean it’s more effective."
• Someone criticizes his current doctor or medical provider.
Sample question: "I’ve been going to my dermatologist for 6 months now and my acne hasn’t gotten any better. He put me on antibiotics and topical creams, and I still have acne. He obviously doesn’t know what he’s doing. Can you tell me what to do?"
Sample reply: "I’m sorry to hear that. I know how frustrating it can be. Acne can be very difficult to treat and can take a long time. Be sure that you’re communicating with your doctor about your situation so he can help you. Also, remember that you can always request a second opinion."
• You want to blog about a patient’s condition. How do you do it without violating the patient’s privacy while ensuring that he cannot be identified? One option is to obtain the patient’s written consent. Another option is to create a composite: Use real facts with fictional patients. For example, your actual patient is a 30-year-old UPS driver with severe hand eczema. You want to write about connections between hand eczema and occupational exposure. You create a fictional patient who is a 40-year-old female mail carrier. You discuss the symptoms of your actual patient in a way that maintains his privacy yet allows you to educate patients online.
Clearly, there are many more scenarios you may encounter online. This is a small sampling to give you some idea of how to respond safely and professionally. If you have specific questions or suggestions, feel free to share them by writing to [email protected].
Dr. Benabio is physician director of innovation at Kaiser Permanente in San Diego. Visit his consumer health blog at thedermblog.com and his health care blog at benabio.com. Connect with him on Twitter @Dermdoc and on Facebook (DermDoc).
Joining forces
Tough economic times and the unpredictable consequences of health care reform are making a growing number of solo practitioners and small private groups very nervous. I’ve been receiving many inquiries about protective options, such as joining a multispecialty group, or merging two or more small practices into larger entities.
If becoming an employee of a large corporation does not appeal to you, a merger can offer significant advantages in stabilization of income and expenses; but careful planning – and a written agreement – is essential.
If you are considering this option, here are some things to think about.
• What is the compensation formula? Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination, so productivity is rewarded but your income doesn’t drop to zero when you take time off.
• Who will be in charge, and what percentage vote will be needed to approve important decisions? Typically, the majority rules; but you may wish to create a list of pivotal moves that will require unanimous approval, such as purchasing expensive equipment, borrowing money, or adding new partners.
• Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same as or different from any of the existing plans. You’ll probably need some legal guidance to ensure that assets from existing plans can be transferred into a new plan without tax issues.
If both practices are incorporated, there are two basic options for combining them. Corporation A can simply absorb corporation B; the latter ceases to exist, and corporation A, the so-called "surviving entity," assumes all assets and liabilities of both old corporations. Corporation B shareholders exchange shares of its stock for shares of corporation A, with adjustments for any inequalities in stock value.
The second option is to start a completely new corporation, which I’ll call corporation C. Corporations A and B dissolve, and distribute their equipment and charts to their shareholders, who then transfer the assets to corporation C.
Option 2 is popular, but I am not a fan. It is billed as an opportunity to start fresh, shielding everyone from exposure to malpractice suits and other liabilities, but the reality is, anyone looking to sue either old corporation will simply sue corporation C as the so-called "successor" corporation, on the grounds that it has assumed responsibility for its predecessors’ liabilities. You also will need new provider numbers, which may impede cash flow for months. Plus, the IRS treats corporate liquidations, even for merger purposes, as sales of assets, and taxes them.
In general, most experts that I’ve talked with favor the outright merger of corporations; it is tax neutral, and while it may theoretically be less satisfactory liability-wise, you can minimize risk by examining financial and legal records, and by identifying any glaring flaws in charting or coding. Your lawyers can add "hold harmless" clauses to the merger agreement, indemnifying each party against the others’ liabilities. This area, especially, is where you need experienced, competent legal advice.
Another common sticking point is known as "equalization." Ideally, each party brings an equal amount of assets to the table, but in the real world that is hardly ever the case. One party may contribute more equipment, for example, and the others are often asked to make up the difference ("equalize") with something else, usually cash.
An alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Non-compete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
Tough economic times and the unpredictable consequences of health care reform are making a growing number of solo practitioners and small private groups very nervous. I’ve been receiving many inquiries about protective options, such as joining a multispecialty group, or merging two or more small practices into larger entities.
If becoming an employee of a large corporation does not appeal to you, a merger can offer significant advantages in stabilization of income and expenses; but careful planning – and a written agreement – is essential.
If you are considering this option, here are some things to think about.
• What is the compensation formula? Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination, so productivity is rewarded but your income doesn’t drop to zero when you take time off.
• Who will be in charge, and what percentage vote will be needed to approve important decisions? Typically, the majority rules; but you may wish to create a list of pivotal moves that will require unanimous approval, such as purchasing expensive equipment, borrowing money, or adding new partners.
• Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same as or different from any of the existing plans. You’ll probably need some legal guidance to ensure that assets from existing plans can be transferred into a new plan without tax issues.
If both practices are incorporated, there are two basic options for combining them. Corporation A can simply absorb corporation B; the latter ceases to exist, and corporation A, the so-called "surviving entity," assumes all assets and liabilities of both old corporations. Corporation B shareholders exchange shares of its stock for shares of corporation A, with adjustments for any inequalities in stock value.
The second option is to start a completely new corporation, which I’ll call corporation C. Corporations A and B dissolve, and distribute their equipment and charts to their shareholders, who then transfer the assets to corporation C.
Option 2 is popular, but I am not a fan. It is billed as an opportunity to start fresh, shielding everyone from exposure to malpractice suits and other liabilities, but the reality is, anyone looking to sue either old corporation will simply sue corporation C as the so-called "successor" corporation, on the grounds that it has assumed responsibility for its predecessors’ liabilities. You also will need new provider numbers, which may impede cash flow for months. Plus, the IRS treats corporate liquidations, even for merger purposes, as sales of assets, and taxes them.
In general, most experts that I’ve talked with favor the outright merger of corporations; it is tax neutral, and while it may theoretically be less satisfactory liability-wise, you can minimize risk by examining financial and legal records, and by identifying any glaring flaws in charting or coding. Your lawyers can add "hold harmless" clauses to the merger agreement, indemnifying each party against the others’ liabilities. This area, especially, is where you need experienced, competent legal advice.
Another common sticking point is known as "equalization." Ideally, each party brings an equal amount of assets to the table, but in the real world that is hardly ever the case. One party may contribute more equipment, for example, and the others are often asked to make up the difference ("equalize") with something else, usually cash.
An alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Non-compete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
Tough economic times and the unpredictable consequences of health care reform are making a growing number of solo practitioners and small private groups very nervous. I’ve been receiving many inquiries about protective options, such as joining a multispecialty group, or merging two or more small practices into larger entities.
If becoming an employee of a large corporation does not appeal to you, a merger can offer significant advantages in stabilization of income and expenses; but careful planning – and a written agreement – is essential.
If you are considering this option, here are some things to think about.
• What is the compensation formula? Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination, so productivity is rewarded but your income doesn’t drop to zero when you take time off.
• Who will be in charge, and what percentage vote will be needed to approve important decisions? Typically, the majority rules; but you may wish to create a list of pivotal moves that will require unanimous approval, such as purchasing expensive equipment, borrowing money, or adding new partners.
• Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same as or different from any of the existing plans. You’ll probably need some legal guidance to ensure that assets from existing plans can be transferred into a new plan without tax issues.
If both practices are incorporated, there are two basic options for combining them. Corporation A can simply absorb corporation B; the latter ceases to exist, and corporation A, the so-called "surviving entity," assumes all assets and liabilities of both old corporations. Corporation B shareholders exchange shares of its stock for shares of corporation A, with adjustments for any inequalities in stock value.
The second option is to start a completely new corporation, which I’ll call corporation C. Corporations A and B dissolve, and distribute their equipment and charts to their shareholders, who then transfer the assets to corporation C.
Option 2 is popular, but I am not a fan. It is billed as an opportunity to start fresh, shielding everyone from exposure to malpractice suits and other liabilities, but the reality is, anyone looking to sue either old corporation will simply sue corporation C as the so-called "successor" corporation, on the grounds that it has assumed responsibility for its predecessors’ liabilities. You also will need new provider numbers, which may impede cash flow for months. Plus, the IRS treats corporate liquidations, even for merger purposes, as sales of assets, and taxes them.
In general, most experts that I’ve talked with favor the outright merger of corporations; it is tax neutral, and while it may theoretically be less satisfactory liability-wise, you can minimize risk by examining financial and legal records, and by identifying any glaring flaws in charting or coding. Your lawyers can add "hold harmless" clauses to the merger agreement, indemnifying each party against the others’ liabilities. This area, especially, is where you need experienced, competent legal advice.
Another common sticking point is known as "equalization." Ideally, each party brings an equal amount of assets to the table, but in the real world that is hardly ever the case. One party may contribute more equipment, for example, and the others are often asked to make up the difference ("equalize") with something else, usually cash.
An alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Non-compete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
From ATLAS to HORIZONTAL: musings on five key trials
The ATLAS trial1
For decades, 5 years of tamoxifen has been the standard of care for the adjuvant therapy of women with estrogen receptor positive breast cancer, although in recent years for postmenopausal women this treatment has been largely replaced with aromatase inhibitors (AIs). Would extending tamoxifen therapy to 10 years provide further benefit or merely increase toxicity? Do the results of the ATLAS trial, in which nearly 13,000 women were recruited and randomized to receive 5 more years of tamoxifen or to stop tamoxifen at 5 years, provide us with a new standard of care for premenopausal women?
Click on the PDF icon at the top of this introduction to read the full article.
The ATLAS trial1
For decades, 5 years of tamoxifen has been the standard of care for the adjuvant therapy of women with estrogen receptor positive breast cancer, although in recent years for postmenopausal women this treatment has been largely replaced with aromatase inhibitors (AIs). Would extending tamoxifen therapy to 10 years provide further benefit or merely increase toxicity? Do the results of the ATLAS trial, in which nearly 13,000 women were recruited and randomized to receive 5 more years of tamoxifen or to stop tamoxifen at 5 years, provide us with a new standard of care for premenopausal women?
Click on the PDF icon at the top of this introduction to read the full article.
The ATLAS trial1
For decades, 5 years of tamoxifen has been the standard of care for the adjuvant therapy of women with estrogen receptor positive breast cancer, although in recent years for postmenopausal women this treatment has been largely replaced with aromatase inhibitors (AIs). Would extending tamoxifen therapy to 10 years provide further benefit or merely increase toxicity? Do the results of the ATLAS trial, in which nearly 13,000 women were recruited and randomized to receive 5 more years of tamoxifen or to stop tamoxifen at 5 years, provide us with a new standard of care for premenopausal women?
Click on the PDF icon at the top of this introduction to read the full article.