User login
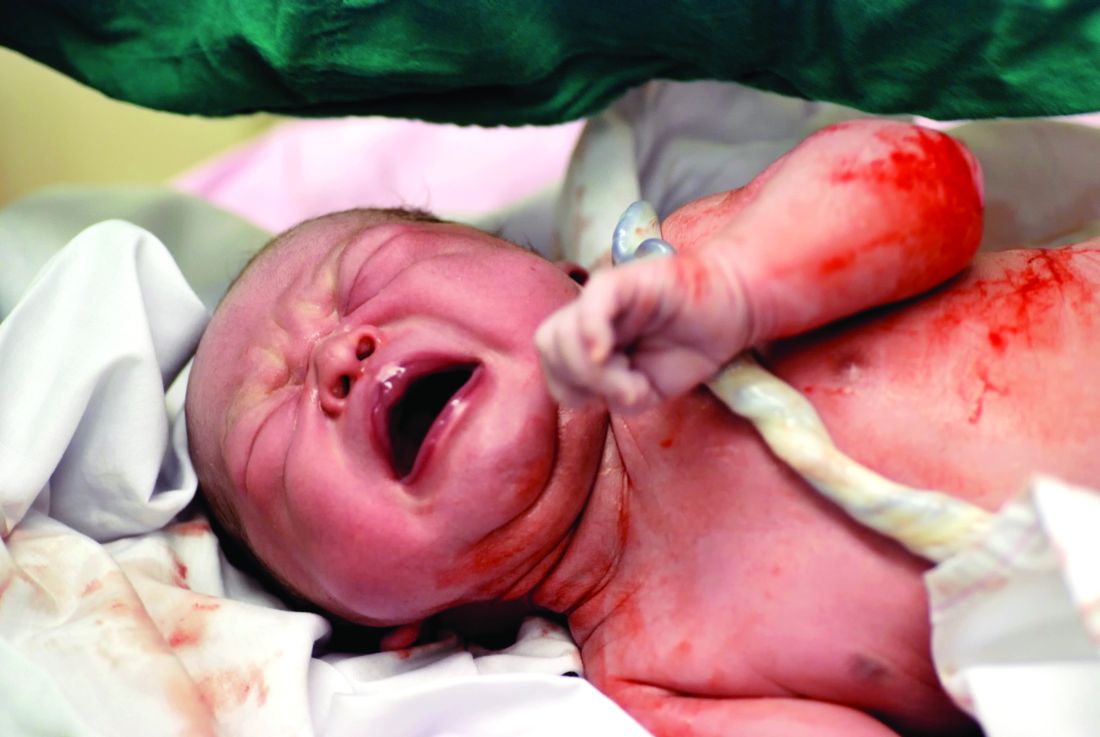
Delayed cord clamping cuts anemia for 1 year
Delaying the clamping of the umbilical cord for 3 minutes after delivery decreased anemia for as long as 8-12 months in a population at high risk for the disorder, according to a report published online Jan. 17 in JAMA Pediatrics.
If early (within 1 minute of birth) cord clamping is avoided after delivery, fetoplacental blood moves into the newborn, augmenting his or her blood volume by 30%-40%. This is known to increase iron stores and prevent iron deficiency for at least 6 months, but until now there has been little evidence of how long this beneficial effect persists, said Ashish KC, MD, PhD, of International Maternal and Child Health, Uppsala (Sweden) University and the United Nations Children’s Fund, Lalitpur, Nepal, and associates.
The primary outcome measure – the hemoglobin level at 8 months of age – was a significant 0.2 g/dL higher after delayed clamping. Also, anemia was significantly less prevalent with delayed cord clamping (73.0% vs. 82.2%). This represents an 11% reduction in the risk of anemia and a 42% reduction in the risk of iron deficiency. “The relative risk for having iron deficiency anemia was 0.58, with a number needed to treat of 7,” Dr. KC and associates said (JAMA Ped. 2017 Jan 17. doi: 10.1001/jamapediatrics.2016.3971).
The benefits of delayed cord clamping persisted at 12 months of age; the mean hemoglobin was 0.3 g/dL higher in the delayed group. Anemia was less prevalent in the delayed clamping group; the relative risk was 0.91, the investigators said.
If this intervention were implemented worldwide, “this could translate to 5 million fewer infants with anemia at 8 months of age, with particular public health significance in South Asia and sub-Saharan Africa, where the prevalence of anemia is highest,” the investigators said. This study was supported by the Midwifery Society of Nepal, the Swedish Society of Medicine, the Little Child’s Foundation, the Swedish Society of Medical Research, and the United Nations Children’s Fund. Dr. KC and associates reported having no relevant financial disclosures.
Delaying the clamping of the umbilical cord for 3 minutes after delivery decreased anemia for as long as 8-12 months in a population at high risk for the disorder, according to a report published online Jan. 17 in JAMA Pediatrics.
If early (within 1 minute of birth) cord clamping is avoided after delivery, fetoplacental blood moves into the newborn, augmenting his or her blood volume by 30%-40%. This is known to increase iron stores and prevent iron deficiency for at least 6 months, but until now there has been little evidence of how long this beneficial effect persists, said Ashish KC, MD, PhD, of International Maternal and Child Health, Uppsala (Sweden) University and the United Nations Children’s Fund, Lalitpur, Nepal, and associates.
The primary outcome measure – the hemoglobin level at 8 months of age – was a significant 0.2 g/dL higher after delayed clamping. Also, anemia was significantly less prevalent with delayed cord clamping (73.0% vs. 82.2%). This represents an 11% reduction in the risk of anemia and a 42% reduction in the risk of iron deficiency. “The relative risk for having iron deficiency anemia was 0.58, with a number needed to treat of 7,” Dr. KC and associates said (JAMA Ped. 2017 Jan 17. doi: 10.1001/jamapediatrics.2016.3971).
The benefits of delayed cord clamping persisted at 12 months of age; the mean hemoglobin was 0.3 g/dL higher in the delayed group. Anemia was less prevalent in the delayed clamping group; the relative risk was 0.91, the investigators said.
If this intervention were implemented worldwide, “this could translate to 5 million fewer infants with anemia at 8 months of age, with particular public health significance in South Asia and sub-Saharan Africa, where the prevalence of anemia is highest,” the investigators said. This study was supported by the Midwifery Society of Nepal, the Swedish Society of Medicine, the Little Child’s Foundation, the Swedish Society of Medical Research, and the United Nations Children’s Fund. Dr. KC and associates reported having no relevant financial disclosures.
Delaying the clamping of the umbilical cord for 3 minutes after delivery decreased anemia for as long as 8-12 months in a population at high risk for the disorder, according to a report published online Jan. 17 in JAMA Pediatrics.
If early (within 1 minute of birth) cord clamping is avoided after delivery, fetoplacental blood moves into the newborn, augmenting his or her blood volume by 30%-40%. This is known to increase iron stores and prevent iron deficiency for at least 6 months, but until now there has been little evidence of how long this beneficial effect persists, said Ashish KC, MD, PhD, of International Maternal and Child Health, Uppsala (Sweden) University and the United Nations Children’s Fund, Lalitpur, Nepal, and associates.
The primary outcome measure – the hemoglobin level at 8 months of age – was a significant 0.2 g/dL higher after delayed clamping. Also, anemia was significantly less prevalent with delayed cord clamping (73.0% vs. 82.2%). This represents an 11% reduction in the risk of anemia and a 42% reduction in the risk of iron deficiency. “The relative risk for having iron deficiency anemia was 0.58, with a number needed to treat of 7,” Dr. KC and associates said (JAMA Ped. 2017 Jan 17. doi: 10.1001/jamapediatrics.2016.3971).
The benefits of delayed cord clamping persisted at 12 months of age; the mean hemoglobin was 0.3 g/dL higher in the delayed group. Anemia was less prevalent in the delayed clamping group; the relative risk was 0.91, the investigators said.
If this intervention were implemented worldwide, “this could translate to 5 million fewer infants with anemia at 8 months of age, with particular public health significance in South Asia and sub-Saharan Africa, where the prevalence of anemia is highest,” the investigators said. This study was supported by the Midwifery Society of Nepal, the Swedish Society of Medicine, the Little Child’s Foundation, the Swedish Society of Medical Research, and the United Nations Children’s Fund. Dr. KC and associates reported having no relevant financial disclosures.
FROM JAMA PEDIATRICS
Key clinical point: Delaying the clamping of the umbilical cord for 3 minutes decreased anemia for as long as 8-12 months in a population at high risk for the disorder.
Major finding: Delayed cord clamping yielded an 11% reduction in the risk of anemia and a 42% reduction in the risk of iron deficiency, compared with early cord clamping.
Data source: A prospective randomized trial involving 540 term and late preterm infants born in Nepal during a 7-week period
Disclosures: This study was supported by the Midwifery Society of Nepal, the Swedish Society of Medicine, the Little Child’s Foundation, the Swedish Society of Medical Research, and the United Nations Children’s Fund. Dr. KC and associates reported having no relevant financial disclosures.
Asthma ruled out in 33% of diagnosed adults
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician.
Major finding: Only 44% of the participants in whom asthma was ruled out had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed.
Data source: A prospective multicenter cohort study involving 613 adults who had been diagnosed as having asthma during the preceding 5 years.
Disclosures: The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Assay testing accurate in distinguishing bacterial from viral respiratory tract infections
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: The assay distinguished between bacterial and viral infections with 86.7% sensitivity, compared with unanimous panel diagnosis, which did so with 87.8% sensitivity.
Data source: A double-blind, multicenter, validation study of 577 children aged 2-60 months from October 2013 through March 2015.
Disclosures: The study was funded by MeMed Diagnostics. The authors reported no relevant financial disclosures.
How to limit radiation in endovascular procedures
CHICAGO – Applying the key principles for limiting radiation exposure for vascular surgeons and staff – not to mention patients – during endovascular procedures involves a thorough understanding of dose metrics as well as risk factors for high-dose interventions, according to recent findings reported at a symposium on vascular surgery sponsored by Northwestern University.
Melissa Kirkwood, MD, of the University of Texas Southwestern Medical Center in Dallas, reported on her institution’s experience with limiting radiation exposure in typically high-dose cases. “What we found was that even though we had a substantial amount of dose in these cases – they included a significant proportion of 5- to 10-Gy and greater than 10-Gy cases – there were still no skin injuries detected in these patients,” she said, referencing retrospective and prospective analyses (J Vasc Surg. 2014;60:742-8; J Vasc Surg. 2015;61:902-6).
Vigilance regarding these principles for vascular surgeons is paramount, Dr. Kirkwood said, noting that the National Council on Radiation Protection and Measurements threshold for substantial radiation dose is 5 Gy or greater. “When you’re doing complex endovascular work, your doses can get that high,” she said.
As a means of measuring dose, Dr. Kirkwood called fluoroscopy time a “universally poor indicator” because in current practice vascular surgeons use digital acquisition mode in addition to fluoroscopy. “The digital acquisitions generate 10-100 times more dose than fluoroscopy, so if you’re only looking at fluoroscopy time, your potentially missing the majority of the dose for that case,” she said.
More applicable dose measures, she said, are kerma area product that measures total radiation beam output from the x-ray tube, which she called “a better reflection of operator exposure,” and reference air kerma (RAK), a measure of the dose at a reference point 15 cm along the beam axis toward the focal spot from the isocenter, which she said is the best approximation for patient peak skin dose exposure. However, the latter does not account for angle of the x-ray tube or patient position, which can vary based on the type of procedure or the patient’s size.
Dr. Kirkwood’s work at UTSW also determined that operator exposure during an endovascular procedure depends on where they stand. “Doubling the distance from the source can decrease the radiation level by a factor of four,” she said. For femoral access in the right groin, the operator is at greatest risk for exposure followed by the assistant when the assistant is standing to the right of the operator. The left brachial access site carries an even higher exposure for the operator, she said.
The table-mounted lead skirt plays a key role in limiting operator exposure, Dr. Kirkwood said. “It can be cumbersome, but it is very important in lowering your lower-body dose,” she said, because it will block radiation scatter coming off the bottom of the table.
At UTSW, the endovascular operators had a tutorial with the staff medical physicist on best practices to limit radiation exposure. “What we found was that we were significantly able to decrease the dose across all cases by simply going over a few principles,” she said.
Among those principles: “Always be aware when you’re on the fluoroscopy pedal and always use the lowest fluoroscopy mode possible,” she said. However, she noted that in difficult-to-visualize cases, a short-duration boost in fluoroscopy level might reduce overall fluoroscopy time and hence limit exposure. To limit digital acquisition mode, the use of fluoroscopic looping can allow for review of images during the procedure with a fraction of the dose that would be needed for a digital acquisition run.
Limiting magnification and using collimation can be complementary, Dr. Kirkwood said. “If you really have to magnify to see the area of interest, make sure you have tight collimation to try to decrease the scatter to you and your colleagues in the OR,” she said.
Dr. Kirkwood noted that raising the angio table as high as is comfortable and decreasing the distance between the source and image detector can limit patient exposure. Operators should avoid steep angulations of the x-ray tube, she said, but when angulations are necessary, operators should stand on the opposite side of the x-ray tube. “The best operating practice if you know you’re going to have a high-dose case with a lot of gantry angulation would be to tightly collimate to the area of interest and minimize the magnification,” she said.
Though not necessarily a principle, keeping up with software advances for imaging devices can also prove valuable for limiting radiation exposure, Dr. Kirkwood said. “It’s important to know about them because if you are purchasing new equipment, they are not necessarily included if you’re institution is looking to hold down costs,” she said.
Dr. Kirkwood had no relevant financial disclosures.
CHICAGO – Applying the key principles for limiting radiation exposure for vascular surgeons and staff – not to mention patients – during endovascular procedures involves a thorough understanding of dose metrics as well as risk factors for high-dose interventions, according to recent findings reported at a symposium on vascular surgery sponsored by Northwestern University.
Melissa Kirkwood, MD, of the University of Texas Southwestern Medical Center in Dallas, reported on her institution’s experience with limiting radiation exposure in typically high-dose cases. “What we found was that even though we had a substantial amount of dose in these cases – they included a significant proportion of 5- to 10-Gy and greater than 10-Gy cases – there were still no skin injuries detected in these patients,” she said, referencing retrospective and prospective analyses (J Vasc Surg. 2014;60:742-8; J Vasc Surg. 2015;61:902-6).
Vigilance regarding these principles for vascular surgeons is paramount, Dr. Kirkwood said, noting that the National Council on Radiation Protection and Measurements threshold for substantial radiation dose is 5 Gy or greater. “When you’re doing complex endovascular work, your doses can get that high,” she said.
As a means of measuring dose, Dr. Kirkwood called fluoroscopy time a “universally poor indicator” because in current practice vascular surgeons use digital acquisition mode in addition to fluoroscopy. “The digital acquisitions generate 10-100 times more dose than fluoroscopy, so if you’re only looking at fluoroscopy time, your potentially missing the majority of the dose for that case,” she said.
More applicable dose measures, she said, are kerma area product that measures total radiation beam output from the x-ray tube, which she called “a better reflection of operator exposure,” and reference air kerma (RAK), a measure of the dose at a reference point 15 cm along the beam axis toward the focal spot from the isocenter, which she said is the best approximation for patient peak skin dose exposure. However, the latter does not account for angle of the x-ray tube or patient position, which can vary based on the type of procedure or the patient’s size.
Dr. Kirkwood’s work at UTSW also determined that operator exposure during an endovascular procedure depends on where they stand. “Doubling the distance from the source can decrease the radiation level by a factor of four,” she said. For femoral access in the right groin, the operator is at greatest risk for exposure followed by the assistant when the assistant is standing to the right of the operator. The left brachial access site carries an even higher exposure for the operator, she said.
The table-mounted lead skirt plays a key role in limiting operator exposure, Dr. Kirkwood said. “It can be cumbersome, but it is very important in lowering your lower-body dose,” she said, because it will block radiation scatter coming off the bottom of the table.
At UTSW, the endovascular operators had a tutorial with the staff medical physicist on best practices to limit radiation exposure. “What we found was that we were significantly able to decrease the dose across all cases by simply going over a few principles,” she said.
Among those principles: “Always be aware when you’re on the fluoroscopy pedal and always use the lowest fluoroscopy mode possible,” she said. However, she noted that in difficult-to-visualize cases, a short-duration boost in fluoroscopy level might reduce overall fluoroscopy time and hence limit exposure. To limit digital acquisition mode, the use of fluoroscopic looping can allow for review of images during the procedure with a fraction of the dose that would be needed for a digital acquisition run.
Limiting magnification and using collimation can be complementary, Dr. Kirkwood said. “If you really have to magnify to see the area of interest, make sure you have tight collimation to try to decrease the scatter to you and your colleagues in the OR,” she said.
Dr. Kirkwood noted that raising the angio table as high as is comfortable and decreasing the distance between the source and image detector can limit patient exposure. Operators should avoid steep angulations of the x-ray tube, she said, but when angulations are necessary, operators should stand on the opposite side of the x-ray tube. “The best operating practice if you know you’re going to have a high-dose case with a lot of gantry angulation would be to tightly collimate to the area of interest and minimize the magnification,” she said.
Though not necessarily a principle, keeping up with software advances for imaging devices can also prove valuable for limiting radiation exposure, Dr. Kirkwood said. “It’s important to know about them because if you are purchasing new equipment, they are not necessarily included if you’re institution is looking to hold down costs,” she said.
Dr. Kirkwood had no relevant financial disclosures.
CHICAGO – Applying the key principles for limiting radiation exposure for vascular surgeons and staff – not to mention patients – during endovascular procedures involves a thorough understanding of dose metrics as well as risk factors for high-dose interventions, according to recent findings reported at a symposium on vascular surgery sponsored by Northwestern University.
Melissa Kirkwood, MD, of the University of Texas Southwestern Medical Center in Dallas, reported on her institution’s experience with limiting radiation exposure in typically high-dose cases. “What we found was that even though we had a substantial amount of dose in these cases – they included a significant proportion of 5- to 10-Gy and greater than 10-Gy cases – there were still no skin injuries detected in these patients,” she said, referencing retrospective and prospective analyses (J Vasc Surg. 2014;60:742-8; J Vasc Surg. 2015;61:902-6).
Vigilance regarding these principles for vascular surgeons is paramount, Dr. Kirkwood said, noting that the National Council on Radiation Protection and Measurements threshold for substantial radiation dose is 5 Gy or greater. “When you’re doing complex endovascular work, your doses can get that high,” she said.
As a means of measuring dose, Dr. Kirkwood called fluoroscopy time a “universally poor indicator” because in current practice vascular surgeons use digital acquisition mode in addition to fluoroscopy. “The digital acquisitions generate 10-100 times more dose than fluoroscopy, so if you’re only looking at fluoroscopy time, your potentially missing the majority of the dose for that case,” she said.
More applicable dose measures, she said, are kerma area product that measures total radiation beam output from the x-ray tube, which she called “a better reflection of operator exposure,” and reference air kerma (RAK), a measure of the dose at a reference point 15 cm along the beam axis toward the focal spot from the isocenter, which she said is the best approximation for patient peak skin dose exposure. However, the latter does not account for angle of the x-ray tube or patient position, which can vary based on the type of procedure or the patient’s size.
Dr. Kirkwood’s work at UTSW also determined that operator exposure during an endovascular procedure depends on where they stand. “Doubling the distance from the source can decrease the radiation level by a factor of four,” she said. For femoral access in the right groin, the operator is at greatest risk for exposure followed by the assistant when the assistant is standing to the right of the operator. The left brachial access site carries an even higher exposure for the operator, she said.
The table-mounted lead skirt plays a key role in limiting operator exposure, Dr. Kirkwood said. “It can be cumbersome, but it is very important in lowering your lower-body dose,” she said, because it will block radiation scatter coming off the bottom of the table.
At UTSW, the endovascular operators had a tutorial with the staff medical physicist on best practices to limit radiation exposure. “What we found was that we were significantly able to decrease the dose across all cases by simply going over a few principles,” she said.
Among those principles: “Always be aware when you’re on the fluoroscopy pedal and always use the lowest fluoroscopy mode possible,” she said. However, she noted that in difficult-to-visualize cases, a short-duration boost in fluoroscopy level might reduce overall fluoroscopy time and hence limit exposure. To limit digital acquisition mode, the use of fluoroscopic looping can allow for review of images during the procedure with a fraction of the dose that would be needed for a digital acquisition run.
Limiting magnification and using collimation can be complementary, Dr. Kirkwood said. “If you really have to magnify to see the area of interest, make sure you have tight collimation to try to decrease the scatter to you and your colleagues in the OR,” she said.
Dr. Kirkwood noted that raising the angio table as high as is comfortable and decreasing the distance between the source and image detector can limit patient exposure. Operators should avoid steep angulations of the x-ray tube, she said, but when angulations are necessary, operators should stand on the opposite side of the x-ray tube. “The best operating practice if you know you’re going to have a high-dose case with a lot of gantry angulation would be to tightly collimate to the area of interest and minimize the magnification,” she said.
Though not necessarily a principle, keeping up with software advances for imaging devices can also prove valuable for limiting radiation exposure, Dr. Kirkwood said. “It’s important to know about them because if you are purchasing new equipment, they are not necessarily included if you’re institution is looking to hold down costs,” she said.
Dr. Kirkwood had no relevant financial disclosures.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: Vascular surgeons can lower their radiation exposure during endovascular procedures by employing key principles like appropriate shielding.
Major finding: Familiarity with dose terminology and metrics, possible radiation-induced injuries, and techniques to lower radiation dosing are keys to limiting radiation exposure.
Data source: Review of literature, including National Council on Radiation and Protection guidelines and National Cancer Institute grades of skin toxicity for radiation dermatitis.
Disclosures: Dr. Kirkwood had no financial relationships to disclose.
Two-thirds of patient advocacy groups receive industry funding
About two-thirds of patient advocacy organizations report receiving funds from for-profit firms, including pharmaceutical, device and biotechnology manufacturers, according to new survey results.
The study, published online Jan. 17 in JAMA Internal Medicine, sought responses from a randomly chosen sample of 439 executives at patient advocacy organizations (PAOs), of whom 66% (n = 289) responded. The median annual revenue for the groups surveyed was nearly $300,000, and 67% (n = 165) reported receiving at least some industry funding. Of those, 12% reported that more than half their annual funding comes from industry sources.
A total of 22 PAO leaders surveyed (8%) reported that they perceived pressure to conform their organizational interests to those of corporate donors (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8443).
The vast majority of respondents (82%) said they found conflicts of interest to be relevant to PAOs, and most reported having a written policy on conflicts. More than half said they viewed their own organizations’ conflict of interest policies as sound.
The findings warrant a broad re-examination of PAO conflict-of-interest policies and disclosures, and a detailed examination of the specific ways in which PAOs are influenced or pressured, wrote Susannah L. Rose, PhD, of the Cleveland Clinic, and her colleagues.
PAOs do not routinely and publicly disclose all their sources of funding on websites, tax returns, or annual reports, and there are media investigations into some groups “regarding their ties to industry and the integrity of their activities,” the investigators wrote.
Dr. Rose and her colleagues recommended that PAOs disclose detailed information about industry funding on their websites and on Open Payments, a government website. The researchers acknowledged that their study relied on self-reported data, and though it was designed to obscure the identity of respondents and their organizations, the possibility of response bias exists.
Harvard University’s Edmond J. Safra Center for Ethics funded the study. One coauthor, Steven Joffe, MD, MPH, disclosed past funding from Genzyme Sanofi. No other conflicts were reported.
Industry funding strengthens and extends the much-needed patient voice in health care, but at what cost? During the EpiPen scandal, the manufacturer-sponsored advocacy groups were largely silent about price gouging. Recently, a drug company–funded “patient advocacy” campaign called “Even the Score” helped win regulatory approval for the thrice rejected controversial female sex drug flibanserin.
Just as the industry funding of clinical trials has been associated with more favorable findings, patient groups also face risks of bias when accepting money from companies seeking to expand markets for their new tests and treatments.
This new work demonstrates an urgent need for patient advocacy organizations to explicitly focus much more on representing the interests of patients and citizens, rather than serving – inadvertently or otherwise – the interests of their industry sponsors. In the meantime, we need much greater transparency about industry funding, including prominently displayed disclosures of dollar amounts and proportions of total funding on group websites, as well as addition of patient advocacy groups to the Open Payments program established by the Sunshine Act, which would mean mandatory disclosure of funding by sponsors.
Ray Moynihan, PhD, and Lisa Bero, PhD, are at the University of Sydney, New South Wales, Australia. Their comments are adapted from an editorial. They reported having no relevant financial disclosures. (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.9179 ).
Industry funding strengthens and extends the much-needed patient voice in health care, but at what cost? During the EpiPen scandal, the manufacturer-sponsored advocacy groups were largely silent about price gouging. Recently, a drug company–funded “patient advocacy” campaign called “Even the Score” helped win regulatory approval for the thrice rejected controversial female sex drug flibanserin.
Just as the industry funding of clinical trials has been associated with more favorable findings, patient groups also face risks of bias when accepting money from companies seeking to expand markets for their new tests and treatments.
This new work demonstrates an urgent need for patient advocacy organizations to explicitly focus much more on representing the interests of patients and citizens, rather than serving – inadvertently or otherwise – the interests of their industry sponsors. In the meantime, we need much greater transparency about industry funding, including prominently displayed disclosures of dollar amounts and proportions of total funding on group websites, as well as addition of patient advocacy groups to the Open Payments program established by the Sunshine Act, which would mean mandatory disclosure of funding by sponsors.
Ray Moynihan, PhD, and Lisa Bero, PhD, are at the University of Sydney, New South Wales, Australia. Their comments are adapted from an editorial. They reported having no relevant financial disclosures. (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.9179 ).
Industry funding strengthens and extends the much-needed patient voice in health care, but at what cost? During the EpiPen scandal, the manufacturer-sponsored advocacy groups were largely silent about price gouging. Recently, a drug company–funded “patient advocacy” campaign called “Even the Score” helped win regulatory approval for the thrice rejected controversial female sex drug flibanserin.
Just as the industry funding of clinical trials has been associated with more favorable findings, patient groups also face risks of bias when accepting money from companies seeking to expand markets for their new tests and treatments.
This new work demonstrates an urgent need for patient advocacy organizations to explicitly focus much more on representing the interests of patients and citizens, rather than serving – inadvertently or otherwise – the interests of their industry sponsors. In the meantime, we need much greater transparency about industry funding, including prominently displayed disclosures of dollar amounts and proportions of total funding on group websites, as well as addition of patient advocacy groups to the Open Payments program established by the Sunshine Act, which would mean mandatory disclosure of funding by sponsors.
Ray Moynihan, PhD, and Lisa Bero, PhD, are at the University of Sydney, New South Wales, Australia. Their comments are adapted from an editorial. They reported having no relevant financial disclosures. (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.9179 ).
About two-thirds of patient advocacy organizations report receiving funds from for-profit firms, including pharmaceutical, device and biotechnology manufacturers, according to new survey results.
The study, published online Jan. 17 in JAMA Internal Medicine, sought responses from a randomly chosen sample of 439 executives at patient advocacy organizations (PAOs), of whom 66% (n = 289) responded. The median annual revenue for the groups surveyed was nearly $300,000, and 67% (n = 165) reported receiving at least some industry funding. Of those, 12% reported that more than half their annual funding comes from industry sources.
A total of 22 PAO leaders surveyed (8%) reported that they perceived pressure to conform their organizational interests to those of corporate donors (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8443).
The vast majority of respondents (82%) said they found conflicts of interest to be relevant to PAOs, and most reported having a written policy on conflicts. More than half said they viewed their own organizations’ conflict of interest policies as sound.
The findings warrant a broad re-examination of PAO conflict-of-interest policies and disclosures, and a detailed examination of the specific ways in which PAOs are influenced or pressured, wrote Susannah L. Rose, PhD, of the Cleveland Clinic, and her colleagues.
PAOs do not routinely and publicly disclose all their sources of funding on websites, tax returns, or annual reports, and there are media investigations into some groups “regarding their ties to industry and the integrity of their activities,” the investigators wrote.
Dr. Rose and her colleagues recommended that PAOs disclose detailed information about industry funding on their websites and on Open Payments, a government website. The researchers acknowledged that their study relied on self-reported data, and though it was designed to obscure the identity of respondents and their organizations, the possibility of response bias exists.
Harvard University’s Edmond J. Safra Center for Ethics funded the study. One coauthor, Steven Joffe, MD, MPH, disclosed past funding from Genzyme Sanofi. No other conflicts were reported.
About two-thirds of patient advocacy organizations report receiving funds from for-profit firms, including pharmaceutical, device and biotechnology manufacturers, according to new survey results.
The study, published online Jan. 17 in JAMA Internal Medicine, sought responses from a randomly chosen sample of 439 executives at patient advocacy organizations (PAOs), of whom 66% (n = 289) responded. The median annual revenue for the groups surveyed was nearly $300,000, and 67% (n = 165) reported receiving at least some industry funding. Of those, 12% reported that more than half their annual funding comes from industry sources.
A total of 22 PAO leaders surveyed (8%) reported that they perceived pressure to conform their organizational interests to those of corporate donors (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8443).
The vast majority of respondents (82%) said they found conflicts of interest to be relevant to PAOs, and most reported having a written policy on conflicts. More than half said they viewed their own organizations’ conflict of interest policies as sound.
The findings warrant a broad re-examination of PAO conflict-of-interest policies and disclosures, and a detailed examination of the specific ways in which PAOs are influenced or pressured, wrote Susannah L. Rose, PhD, of the Cleveland Clinic, and her colleagues.
PAOs do not routinely and publicly disclose all their sources of funding on websites, tax returns, or annual reports, and there are media investigations into some groups “regarding their ties to industry and the integrity of their activities,” the investigators wrote.
Dr. Rose and her colleagues recommended that PAOs disclose detailed information about industry funding on their websites and on Open Payments, a government website. The researchers acknowledged that their study relied on self-reported data, and though it was designed to obscure the identity of respondents and their organizations, the possibility of response bias exists.
Harvard University’s Edmond J. Safra Center for Ethics funded the study. One coauthor, Steven Joffe, MD, MPH, disclosed past funding from Genzyme Sanofi. No other conflicts were reported.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: A total of 67% of PAOs receive industry support, with 12% reporting funds comprising more than half their yearly funding.
Data source: A survey study sent to executives of 439 PAOs chosen at random; the response rate was 66%.
Disclosures: An ethics center at Harvard University sponsored the study; one coauthor reported prior funding from Genzyme Sanofi.
SOFA score may be best to identify sepsis in the ICU
Among critically ill patients admitted to the ICU with a suspected infection, defining sepsis by an increase of 2 or more points in the Sequential Organ Failure Assessment score yielded greater prognostic accuracy for in-hospital mortality, compared with the quick SOFA and the systemic inflammatory response syndrome criteria, results from a large analysis showed.
“Accurate diagnostic criteria and consensus definitions have an important role in adult intensive care medicine, providing tools for research, benchmarking, performance monitoring, and accreditation,” researchers from The Australian and New Zealand Intensive Care Society Centre for Outcomes and Resource Evaluation reported in the Jan. 17, 2017 edition of JAMA. “Seymour and colleagues published data concerning the validity of a 2 or more–point change in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score as a means of identifying sepsis among patients who are critically ill with suspected infection, assuming a SOFA of 0 for patients not known to have preexisting organ dysfunction (JAMA. 2016; 315[8]:762-74). In addition, the concept of the quick SOFA (qSOFA) score was introduced as a possible predictive tool among encounters with suspected infection outside the intensive care unit (ICU). These data were drawn from North American cohorts and a single German cohort and have not been validated externally.”
The mean age of the patients was 63 years, 45% were women, and the most common diagnosis was bacterial pneumonia (18%). Nearly 19% of patients died in the hospital and 56% died or experienced an ICU length of stay of at least 3 days or more. The researchers found that the SOFA score increased by 2 or more points in 90% of patients, while 87% manifested 2 or more SIRS criteria, and 54% had a qSOFA score of 2 or more points. In addition, discrimination of in-hospital mortality was significantly higher using SOFA (area under the receiving operating characteristic curve [AUROC], 0.753), compared with both SIRS criteria (AUROC, 0.589) and qSOFA (0.607); the between-group difference reached a P value of less than .001. Similar results were seen for the composite outcome of in-hospital mortality or an ICU length of stay of 3 days or more, which was higher using SOFA (AUROC, 0.736), compared with both SIRS criteria (AUROC, 0.609) and qSOFA (0.606); the between-group difference also reached a P value of less than .001.
The researchers acknowledged certain limitations of the study, including the fact that SOFA, SIRS criteria, and qSOFA could only be studied for the first 24 hours in the ICU. “Biochemical and physiological values could have come from any time within the first 24 hours of ICU admission and, as a result, could not be more accurately linked to the timing of the diagnosis of infection,” they wrote. “The SOFA score used should be considered a modification of the original because the cardiovascular component was estimated without knowledge of inotrope or vasopressor dose. The incidence of nosocomial infections and of infections in patients admitted with another diagnosis were unknown.”
Three of the seven study authors disclosed that they receive salary support from Monash University in Melbourne. The remainder reported having no financial disclosures.
It is neither surprising that qSOFA did not perform as well as the SOFA score in the ICU, given that this finding was already reported by Seymour et al. in their initial work, nor is it critically important because qSOFA is more likely to be useful outside of the ICU setting.
Thus, the findings ... support the results reported by Seymour et al. that qSOFA is potentially helpful in settings outside the ICU in rapidly identifying patients with suspected infection who have, or will likely develop, sepsis (JAMA. 2016;315[8]:762-74). However, qSOFA still warrants further testing, particularly in lower- and middle-income settings where context (for example, timing of presentation to the hospital among patients with a suspected infection) might vary considerably and such contextual factors might affect predictive validity. In addition, prospective studies may evaluate the utility of qSOFA when used longitudinally, with repeated measurements throughout the hospital stay. Arguably, the highest-quality evidence for validation of any tool to support clinical decision making would come from an analysis to establish whether decisions made with the support of the tool lead to better patient outcomes than those made by clinical judgment alone.
Ultimately, the utility of qSOFA will likely become surpassed if and when highly accurate, rapid diagnostic tests for sepsis emerge. For now, however, outside the ICU in the high-income settings where it has been tested, qSOFA appears a simple, rapid, inexpensive, and valid way to identify – among patients with suspected infection – those at a higher risk of having or developing sepsis.
Francois Lamontagne, MD, David A. Harrison, PhD, and Kathryn M. Rowan, PhD, are affiliated with the Intensive Care National Audit & Research Centre, London. Dr. Lamontagne reported serving as investigator for a study funded by GlaxoSmithKline and E-Motion. These comments are extracted from an editorial that appears in the Jan. 17, 2017 edition of JAMA (317[3]:267-8).
It is neither surprising that qSOFA did not perform as well as the SOFA score in the ICU, given that this finding was already reported by Seymour et al. in their initial work, nor is it critically important because qSOFA is more likely to be useful outside of the ICU setting.
Thus, the findings ... support the results reported by Seymour et al. that qSOFA is potentially helpful in settings outside the ICU in rapidly identifying patients with suspected infection who have, or will likely develop, sepsis (JAMA. 2016;315[8]:762-74). However, qSOFA still warrants further testing, particularly in lower- and middle-income settings where context (for example, timing of presentation to the hospital among patients with a suspected infection) might vary considerably and such contextual factors might affect predictive validity. In addition, prospective studies may evaluate the utility of qSOFA when used longitudinally, with repeated measurements throughout the hospital stay. Arguably, the highest-quality evidence for validation of any tool to support clinical decision making would come from an analysis to establish whether decisions made with the support of the tool lead to better patient outcomes than those made by clinical judgment alone.
Ultimately, the utility of qSOFA will likely become surpassed if and when highly accurate, rapid diagnostic tests for sepsis emerge. For now, however, outside the ICU in the high-income settings where it has been tested, qSOFA appears a simple, rapid, inexpensive, and valid way to identify – among patients with suspected infection – those at a higher risk of having or developing sepsis.
Francois Lamontagne, MD, David A. Harrison, PhD, and Kathryn M. Rowan, PhD, are affiliated with the Intensive Care National Audit & Research Centre, London. Dr. Lamontagne reported serving as investigator for a study funded by GlaxoSmithKline and E-Motion. These comments are extracted from an editorial that appears in the Jan. 17, 2017 edition of JAMA (317[3]:267-8).
It is neither surprising that qSOFA did not perform as well as the SOFA score in the ICU, given that this finding was already reported by Seymour et al. in their initial work, nor is it critically important because qSOFA is more likely to be useful outside of the ICU setting.
Thus, the findings ... support the results reported by Seymour et al. that qSOFA is potentially helpful in settings outside the ICU in rapidly identifying patients with suspected infection who have, or will likely develop, sepsis (JAMA. 2016;315[8]:762-74). However, qSOFA still warrants further testing, particularly in lower- and middle-income settings where context (for example, timing of presentation to the hospital among patients with a suspected infection) might vary considerably and such contextual factors might affect predictive validity. In addition, prospective studies may evaluate the utility of qSOFA when used longitudinally, with repeated measurements throughout the hospital stay. Arguably, the highest-quality evidence for validation of any tool to support clinical decision making would come from an analysis to establish whether decisions made with the support of the tool lead to better patient outcomes than those made by clinical judgment alone.
Ultimately, the utility of qSOFA will likely become surpassed if and when highly accurate, rapid diagnostic tests for sepsis emerge. For now, however, outside the ICU in the high-income settings where it has been tested, qSOFA appears a simple, rapid, inexpensive, and valid way to identify – among patients with suspected infection – those at a higher risk of having or developing sepsis.
Francois Lamontagne, MD, David A. Harrison, PhD, and Kathryn M. Rowan, PhD, are affiliated with the Intensive Care National Audit & Research Centre, London. Dr. Lamontagne reported serving as investigator for a study funded by GlaxoSmithKline and E-Motion. These comments are extracted from an editorial that appears in the Jan. 17, 2017 edition of JAMA (317[3]:267-8).
Among critically ill patients admitted to the ICU with a suspected infection, defining sepsis by an increase of 2 or more points in the Sequential Organ Failure Assessment score yielded greater prognostic accuracy for in-hospital mortality, compared with the quick SOFA and the systemic inflammatory response syndrome criteria, results from a large analysis showed.
“Accurate diagnostic criteria and consensus definitions have an important role in adult intensive care medicine, providing tools for research, benchmarking, performance monitoring, and accreditation,” researchers from The Australian and New Zealand Intensive Care Society Centre for Outcomes and Resource Evaluation reported in the Jan. 17, 2017 edition of JAMA. “Seymour and colleagues published data concerning the validity of a 2 or more–point change in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score as a means of identifying sepsis among patients who are critically ill with suspected infection, assuming a SOFA of 0 for patients not known to have preexisting organ dysfunction (JAMA. 2016; 315[8]:762-74). In addition, the concept of the quick SOFA (qSOFA) score was introduced as a possible predictive tool among encounters with suspected infection outside the intensive care unit (ICU). These data were drawn from North American cohorts and a single German cohort and have not been validated externally.”
The mean age of the patients was 63 years, 45% were women, and the most common diagnosis was bacterial pneumonia (18%). Nearly 19% of patients died in the hospital and 56% died or experienced an ICU length of stay of at least 3 days or more. The researchers found that the SOFA score increased by 2 or more points in 90% of patients, while 87% manifested 2 or more SIRS criteria, and 54% had a qSOFA score of 2 or more points. In addition, discrimination of in-hospital mortality was significantly higher using SOFA (area under the receiving operating characteristic curve [AUROC], 0.753), compared with both SIRS criteria (AUROC, 0.589) and qSOFA (0.607); the between-group difference reached a P value of less than .001. Similar results were seen for the composite outcome of in-hospital mortality or an ICU length of stay of 3 days or more, which was higher using SOFA (AUROC, 0.736), compared with both SIRS criteria (AUROC, 0.609) and qSOFA (0.606); the between-group difference also reached a P value of less than .001.
The researchers acknowledged certain limitations of the study, including the fact that SOFA, SIRS criteria, and qSOFA could only be studied for the first 24 hours in the ICU. “Biochemical and physiological values could have come from any time within the first 24 hours of ICU admission and, as a result, could not be more accurately linked to the timing of the diagnosis of infection,” they wrote. “The SOFA score used should be considered a modification of the original because the cardiovascular component was estimated without knowledge of inotrope or vasopressor dose. The incidence of nosocomial infections and of infections in patients admitted with another diagnosis were unknown.”
Three of the seven study authors disclosed that they receive salary support from Monash University in Melbourne. The remainder reported having no financial disclosures.
Among critically ill patients admitted to the ICU with a suspected infection, defining sepsis by an increase of 2 or more points in the Sequential Organ Failure Assessment score yielded greater prognostic accuracy for in-hospital mortality, compared with the quick SOFA and the systemic inflammatory response syndrome criteria, results from a large analysis showed.
“Accurate diagnostic criteria and consensus definitions have an important role in adult intensive care medicine, providing tools for research, benchmarking, performance monitoring, and accreditation,” researchers from The Australian and New Zealand Intensive Care Society Centre for Outcomes and Resource Evaluation reported in the Jan. 17, 2017 edition of JAMA. “Seymour and colleagues published data concerning the validity of a 2 or more–point change in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score as a means of identifying sepsis among patients who are critically ill with suspected infection, assuming a SOFA of 0 for patients not known to have preexisting organ dysfunction (JAMA. 2016; 315[8]:762-74). In addition, the concept of the quick SOFA (qSOFA) score was introduced as a possible predictive tool among encounters with suspected infection outside the intensive care unit (ICU). These data were drawn from North American cohorts and a single German cohort and have not been validated externally.”
The mean age of the patients was 63 years, 45% were women, and the most common diagnosis was bacterial pneumonia (18%). Nearly 19% of patients died in the hospital and 56% died or experienced an ICU length of stay of at least 3 days or more. The researchers found that the SOFA score increased by 2 or more points in 90% of patients, while 87% manifested 2 or more SIRS criteria, and 54% had a qSOFA score of 2 or more points. In addition, discrimination of in-hospital mortality was significantly higher using SOFA (area under the receiving operating characteristic curve [AUROC], 0.753), compared with both SIRS criteria (AUROC, 0.589) and qSOFA (0.607); the between-group difference reached a P value of less than .001. Similar results were seen for the composite outcome of in-hospital mortality or an ICU length of stay of 3 days or more, which was higher using SOFA (AUROC, 0.736), compared with both SIRS criteria (AUROC, 0.609) and qSOFA (0.606); the between-group difference also reached a P value of less than .001.
The researchers acknowledged certain limitations of the study, including the fact that SOFA, SIRS criteria, and qSOFA could only be studied for the first 24 hours in the ICU. “Biochemical and physiological values could have come from any time within the first 24 hours of ICU admission and, as a result, could not be more accurately linked to the timing of the diagnosis of infection,” they wrote. “The SOFA score used should be considered a modification of the original because the cardiovascular component was estimated without knowledge of inotrope or vasopressor dose. The incidence of nosocomial infections and of infections in patients admitted with another diagnosis were unknown.”
Three of the seven study authors disclosed that they receive salary support from Monash University in Melbourne. The remainder reported having no financial disclosures.
FROM JAMA
Key clinical point:
Major finding: The SOFA score increased by 2 or more points in 90% of patients, while 87% manifested 2 or more SIRS criteria, and 54% had a qSOFA score of 2 or more points.
Data source: A retrospective cohort study of 184,875 patients with an infection-related primary diagnosis who were admitted to 182 ICUs in Australia and New Zealand between 2000 and 2015.
Disclosures: Three of the seven study authors disclosed that they receive salary support from Monash University in Melbourne. The remainder reported having no financial disclosures.
Telmisartan-Induced Lichen Planus Eruption Manifested on Vitiliginous Skin
To the Editor:
A 39-year-old man with a history of hypertension and vitiligo presented with a rapid-onset, generalized, pruritic rash covering the body of 4 weeks’ duration. He reported that the rash progressively worsened after developing mild sunburn. The patient stated that the rash was extremely pruritic with a burning sensation and was tender to touch. He was treated with betamethasone valerate cream 0.1% by an outside physician and an over-the-counter anti-itch lotion with no notable improvement. His only medication was telmisartan-hydrochlorothiazide (HCTZ) for hypertension. He denied any drug allergies.
Physical examination revealed multiple discrete and coalescent planar erythematous papules and plaques involving only the depigmented vitiliginous skin of the forehead, eyelids, and nape of the neck (Figure 1A), and confluent on the lateral aspect of the bilateral forearms (Figure 1B), dorsal aspect of the right hand, and bilateral dorsi of the feet. Wickham striae were noted on the lips (Figure 1C). A clinical diagnosis of lichen planus (LP) was made. The patient initially was prescribed halobetasol propionate ointment 0.05% twice daily. He reported notable relief of pruritus with reduction of overall symptoms and new lesion formation.
A 4-mm punch biopsy was performed on the left forearm. Histopathology revealed LP. Microscopic examination of the hematoxylin and eosin–stained specimen revealed a bandlike lymphohistiocytic infiltrate that extended across the papillary dermis, focally obscuring the dermoepidermal junction where there were vacuolar changes and colloid bodies. The epidermis showed sawtooth rete ridges, wedge-shaped foci of hypergranulosis, and compact hyperkeratosis (Figure 2).
On further questioning during follow-up, the patient revealed that his hypertensive medication was changed from HCTZ, which he had been taking for the last 8 years, to the combination antihypertensive medication telmisartan-HCTZ before the onset of the skin eruption. Due to the temporal relationship between the new medication and onset of the eruption, the clinical impression was highly suspicious for drug-induced eruptive LP with Köbner phenomenon caused by the recent sunburn. Systemic workup for underlying causes of LP was negative. Laboratory tests revealed normal complete blood cell counts. The hepatitis panel included hepatitis A antibodies; hepatitis B surface, e antigen, and core antibodies; hepatitis B surface antigen and e antibodies; hepatitis C antibodies; and antinuclear antibodies, which were all negative.
The patient continued to develop new pruritic papules clinically consistent with LP. He was instructed to return to his primary care physician to change the telmisartan-HCTZ to a different class of antihypertensive medication. His medication was changed to atenolol. The patient also was instructed to continue the halobetasol propionate ointment 0.05% twice daily to the affected areas.
The patient returned for a follow-up visit 1 month later and reported notable improvement in pruritus and near-complete resolution of the LP after discontinuation of telmisartan-HCTZ. He also noted some degree of perifollicular repigmentation of the vitiliginous skin that had been unresponsive to prior therapy (Figure 3).
Lichen planus is a pruritic and inflammatory papulosquamous skin condition that presents as scaly, flat-topped, violaceous, polygonal-shaped papules commonly involving the flexor surface of the arms and legs, oral mucosa, scalp, nails, and genitalia. Clinically, LP can present in various forms including actinic, annular, atrophic, erosive, follicular, hypertrophic, linear, pigmented, and vesicular/bullous types. Koebnerization is common, especially in the linear form of LP. There are no specific laboratory findings or serologic markers seen in LP.
The exact cause of LP remains unknown. Clinical observations and anecdotal evidence have directed the cell-mediated immune response to insulting agents such as medications or contact allergy to metals triggering an abnormal cellular immune response. Various viral agents have been reported including hepatitis C virus, human herpesvirus, herpes simplex virus, and varicella-zoster virus.1-5 Other factors such as seasonal change and the environment may contribute to the development of LP and an increase in the incidence of LP eruption has been observed from January to July throughout the United States.6 Lichen planus also has been associated with other altered immune-related disease such as ulcerative colitis, alopecia areata, vitiligo, dermatomyositis, morphea, lichen sclerosis, and myasthenia gravis.7 Increased levels of emotional stress, particularly related to family members, often is related to the onset or aggravation of symptoms.8,9
Many drug-related LP-like and lichenoid eruptions have been reported with antihypertensive drugs, antimalarial drugs, diuretics, antidepressants, nonsteroidal anti-inflammatory drugs, antimicrobial drugs, and metals. In particular, medications such as captopril, enalapril, labetalol, propranolol, chlorothiazide, HCTZ, methyldopa, chloroquine, hydroxychloroquine, quinacrine, gold salts, penicillamine, and quinidine commonly are reported to induce lichenoid drug eruption.10
Several inflammatory papulosquamous skin conditions should be considered in the differential diagnosis before confirming the diagnosis of LP. It is important to rule out lupus erythematosus, especially if the oral mucosa and scalp are involved. In addition, erosive paraneoplastic pemphigus involving primarily the oral mucosa can resemble oral LP. Nail diseases such as psoriasis, onychomycosis, and alopecia areata should be considered as the differential diagnosis of nail disease. Genital involvement also can be seen in psoriasis and lichen sclerosus.
Treatment of LP is mainly symptomatic because of the benign nature of the disease and the high spontaneous remission rate with varying amount of time. If drugs, dental/metal implants, or underlying viral infections are the identifiable triggering factors of LP, the offending agents should be discontinued or removed. Additionally, topical or systemic treatments can be given depending on the severity of the disease, focusing mainly on symptomatic relief as well as the balance of risks and benefits associated with treatment.
Treatment options include topical and intralesional corticosteroids. Systemic medications such as oral corticosteroids and/or acitretin commonly are used in acute, severe, and disseminated cases, though treatment duration varies depending on the clinical response. Other systemic agents used to treat LP include griseofulvin, metronidazole, sulfasalazine, cyclosporine, and mycophenolate mofetil.
Phototherapy is considered an alternative therapy, especially for recalcitrant LP. UVA1 and narrowband UVB (wavelength, 311 nm) have been reported to effectively treat long-standing and therapy-resistant LP.11 In addition, a small study used the excimer laser (wavelength, 308 nm), which is well tolerated by patients, to treat focal recalcitrant oral lesions with excellent results.12 Photochemotherapy has been used with notable improvement, but the potential of carcinogenicity, especially in patients with Fitzpatrick skin types I and II, has limited its use.13
Our patient developed an unusual extensive LP eruption involving only vitiliginous skin shortly after initiation of the combined antihypertensive medication telmisartan-HCTZ, an angiotensin receptor blocker with a thiazide diuretic. Telmisartan and other angiotensin receptor blockers have not been reported to trigger LP; HCTZ is listed as one of the common drugs causing photosensitivity and LP.14,15 Although it is possible that our patient exhibited a delayed lichenoid drug eruption from the HCTZ, it is noteworthy that he did not experience a single episode of LP during his 8-year history of taking HCTZ. Instead, he developed the LP eruption shortly after the addition of telmisartan to his HCTZ antihypertensive regimen. The temporal relationship led us to direct the patient to the prescribing physician to discontinue telmisartan-HCTZ. After changing his antihypertensive medication to atenolol, the patient presented with improvement within the first month and near-complete resolution 2 months after the discontinuation of telmisartan-HCTZ.
Our patient’s LP lesions only manifested on the skin affected by vitiligo, sparing the normal-pigmented skin. Studies have demonstrated an increased ratio of CD8+ T cells to CD4+ T cells as well as increased intercellular adhesion molecule 1 at the dermal level.10,16 Both vitiligo and LP share some common histopathologic features including highly populated CD8+ T cells and intercellular adhesion molecule 1. In our case, LP was triggered on the vitiliginous skin by telmisartan. Vitiligo in combination with trauma induced by sunburn may represent the trigger that altered the cellular immune response and created the telmisartan-induced LP. As a result, the LP eruption was confined to the vitiliginous skin lesions.
Perifollicular repigmentation was observed in our patient after the LP lesions resolved; the patient’s vitiligo was unresponsive to prior treatment. The inflammatory process occurring in LP may exert and interfere in the underlying autoimmune cytotoxic effect toward the melanocytes and the melanin synthesis. It may be of interest to find out if the inflammatory response of LP has a positive influence on the effect of melanogenesis pathways or on the underlying autoimmune-related inflammatory process in vitiligo. Further studies are needed to investigate the role of immunotherapy targeting specific inflammatory pathways and the impact on the repigmentation in vitiligo.
Acknowledgment—Special thanks to Paul Chu, MD (Port Chester, New York).
- Pilli M, Zerbini A, Vescovi P, et al. Oral lichen planus pathogenesis: a role for the HCV-specific cellular immune response. Hepatology. 2002;36:1446-1452.
- De Vries HJ, van Marle J, Teunissen MB, et al. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br J Dermatol. 2006;154:361-364.
- De Vries HJ, Teunissen MB, Zorgdrager F, et al. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. 2007;299:213-219.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Al-Khenaizan S. Lichen planus occurring after hepatitis B vaccination: a new case. J Am Acad Dermatol. 2001;45:614-615.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Sadr-Ashkevari S. Familial actinic lichen planus: case reports in two brothers. Arch Int Med. 2001;4:204-206.
- Manolache L, Seceleanu-Petrescu D, Benea V. Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437-441.
- Mahood JM. Familial lichen planus. Arch Dermatol. 1983;119:292-294.
- Shimizu M, Higaki Y, Higaki M, et al. The role of granzyme B-expressing CD8-positive T cells in apoptosis of keratinocytes in lichen planus. Arch Dermatol Res. 1997;289:527-532.
- Bécherel PA, Bussel A, Chosidow O, et al. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet. 1998;351:805.
- Trehan M, Taylar CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol. 2004;140:415-420.
- Wackernagel A, Legat FJ, Hofer A, et al. Psoralen plus UVA vs. UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2007;23:15-19.
- Fellner MJ. Lichen planus. Int J Dermatol. 1980;19:71-75.
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345-372.
- Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
To the Editor:
A 39-year-old man with a history of hypertension and vitiligo presented with a rapid-onset, generalized, pruritic rash covering the body of 4 weeks’ duration. He reported that the rash progressively worsened after developing mild sunburn. The patient stated that the rash was extremely pruritic with a burning sensation and was tender to touch. He was treated with betamethasone valerate cream 0.1% by an outside physician and an over-the-counter anti-itch lotion with no notable improvement. His only medication was telmisartan-hydrochlorothiazide (HCTZ) for hypertension. He denied any drug allergies.
Physical examination revealed multiple discrete and coalescent planar erythematous papules and plaques involving only the depigmented vitiliginous skin of the forehead, eyelids, and nape of the neck (Figure 1A), and confluent on the lateral aspect of the bilateral forearms (Figure 1B), dorsal aspect of the right hand, and bilateral dorsi of the feet. Wickham striae were noted on the lips (Figure 1C). A clinical diagnosis of lichen planus (LP) was made. The patient initially was prescribed halobetasol propionate ointment 0.05% twice daily. He reported notable relief of pruritus with reduction of overall symptoms and new lesion formation.

A 4-mm punch biopsy was performed on the left forearm. Histopathology revealed LP. Microscopic examination of the hematoxylin and eosin–stained specimen revealed a bandlike lymphohistiocytic infiltrate that extended across the papillary dermis, focally obscuring the dermoepidermal junction where there were vacuolar changes and colloid bodies. The epidermis showed sawtooth rete ridges, wedge-shaped foci of hypergranulosis, and compact hyperkeratosis (Figure 2).

On further questioning during follow-up, the patient revealed that his hypertensive medication was changed from HCTZ, which he had been taking for the last 8 years, to the combination antihypertensive medication telmisartan-HCTZ before the onset of the skin eruption. Due to the temporal relationship between the new medication and onset of the eruption, the clinical impression was highly suspicious for drug-induced eruptive LP with Köbner phenomenon caused by the recent sunburn. Systemic workup for underlying causes of LP was negative. Laboratory tests revealed normal complete blood cell counts. The hepatitis panel included hepatitis A antibodies; hepatitis B surface, e antigen, and core antibodies; hepatitis B surface antigen and e antibodies; hepatitis C antibodies; and antinuclear antibodies, which were all negative.
The patient continued to develop new pruritic papules clinically consistent with LP. He was instructed to return to his primary care physician to change the telmisartan-HCTZ to a different class of antihypertensive medication. His medication was changed to atenolol. The patient also was instructed to continue the halobetasol propionate ointment 0.05% twice daily to the affected areas.
The patient returned for a follow-up visit 1 month later and reported notable improvement in pruritus and near-complete resolution of the LP after discontinuation of telmisartan-HCTZ. He also noted some degree of perifollicular repigmentation of the vitiliginous skin that had been unresponsive to prior therapy (Figure 3).

Lichen planus is a pruritic and inflammatory papulosquamous skin condition that presents as scaly, flat-topped, violaceous, polygonal-shaped papules commonly involving the flexor surface of the arms and legs, oral mucosa, scalp, nails, and genitalia. Clinically, LP can present in various forms including actinic, annular, atrophic, erosive, follicular, hypertrophic, linear, pigmented, and vesicular/bullous types. Koebnerization is common, especially in the linear form of LP. There are no specific laboratory findings or serologic markers seen in LP.
The exact cause of LP remains unknown. Clinical observations and anecdotal evidence have directed the cell-mediated immune response to insulting agents such as medications or contact allergy to metals triggering an abnormal cellular immune response. Various viral agents have been reported including hepatitis C virus, human herpesvirus, herpes simplex virus, and varicella-zoster virus.1-5 Other factors such as seasonal change and the environment may contribute to the development of LP and an increase in the incidence of LP eruption has been observed from January to July throughout the United States.6 Lichen planus also has been associated with other altered immune-related disease such as ulcerative colitis, alopecia areata, vitiligo, dermatomyositis, morphea, lichen sclerosis, and myasthenia gravis.7 Increased levels of emotional stress, particularly related to family members, often is related to the onset or aggravation of symptoms.8,9
Many drug-related LP-like and lichenoid eruptions have been reported with antihypertensive drugs, antimalarial drugs, diuretics, antidepressants, nonsteroidal anti-inflammatory drugs, antimicrobial drugs, and metals. In particular, medications such as captopril, enalapril, labetalol, propranolol, chlorothiazide, HCTZ, methyldopa, chloroquine, hydroxychloroquine, quinacrine, gold salts, penicillamine, and quinidine commonly are reported to induce lichenoid drug eruption.10
Several inflammatory papulosquamous skin conditions should be considered in the differential diagnosis before confirming the diagnosis of LP. It is important to rule out lupus erythematosus, especially if the oral mucosa and scalp are involved. In addition, erosive paraneoplastic pemphigus involving primarily the oral mucosa can resemble oral LP. Nail diseases such as psoriasis, onychomycosis, and alopecia areata should be considered as the differential diagnosis of nail disease. Genital involvement also can be seen in psoriasis and lichen sclerosus.
Treatment of LP is mainly symptomatic because of the benign nature of the disease and the high spontaneous remission rate with varying amount of time. If drugs, dental/metal implants, or underlying viral infections are the identifiable triggering factors of LP, the offending agents should be discontinued or removed. Additionally, topical or systemic treatments can be given depending on the severity of the disease, focusing mainly on symptomatic relief as well as the balance of risks and benefits associated with treatment.
Treatment options include topical and intralesional corticosteroids. Systemic medications such as oral corticosteroids and/or acitretin commonly are used in acute, severe, and disseminated cases, though treatment duration varies depending on the clinical response. Other systemic agents used to treat LP include griseofulvin, metronidazole, sulfasalazine, cyclosporine, and mycophenolate mofetil.
Phototherapy is considered an alternative therapy, especially for recalcitrant LP. UVA1 and narrowband UVB (wavelength, 311 nm) have been reported to effectively treat long-standing and therapy-resistant LP.11 In addition, a small study used the excimer laser (wavelength, 308 nm), which is well tolerated by patients, to treat focal recalcitrant oral lesions with excellent results.12 Photochemotherapy has been used with notable improvement, but the potential of carcinogenicity, especially in patients with Fitzpatrick skin types I and II, has limited its use.13
Our patient developed an unusual extensive LP eruption involving only vitiliginous skin shortly after initiation of the combined antihypertensive medication telmisartan-HCTZ, an angiotensin receptor blocker with a thiazide diuretic. Telmisartan and other angiotensin receptor blockers have not been reported to trigger LP; HCTZ is listed as one of the common drugs causing photosensitivity and LP.14,15 Although it is possible that our patient exhibited a delayed lichenoid drug eruption from the HCTZ, it is noteworthy that he did not experience a single episode of LP during his 8-year history of taking HCTZ. Instead, he developed the LP eruption shortly after the addition of telmisartan to his HCTZ antihypertensive regimen. The temporal relationship led us to direct the patient to the prescribing physician to discontinue telmisartan-HCTZ. After changing his antihypertensive medication to atenolol, the patient presented with improvement within the first month and near-complete resolution 2 months after the discontinuation of telmisartan-HCTZ.
Our patient’s LP lesions only manifested on the skin affected by vitiligo, sparing the normal-pigmented skin. Studies have demonstrated an increased ratio of CD8+ T cells to CD4+ T cells as well as increased intercellular adhesion molecule 1 at the dermal level.10,16 Both vitiligo and LP share some common histopathologic features including highly populated CD8+ T cells and intercellular adhesion molecule 1. In our case, LP was triggered on the vitiliginous skin by telmisartan. Vitiligo in combination with trauma induced by sunburn may represent the trigger that altered the cellular immune response and created the telmisartan-induced LP. As a result, the LP eruption was confined to the vitiliginous skin lesions.
Perifollicular repigmentation was observed in our patient after the LP lesions resolved; the patient’s vitiligo was unresponsive to prior treatment. The inflammatory process occurring in LP may exert and interfere in the underlying autoimmune cytotoxic effect toward the melanocytes and the melanin synthesis. It may be of interest to find out if the inflammatory response of LP has a positive influence on the effect of melanogenesis pathways or on the underlying autoimmune-related inflammatory process in vitiligo. Further studies are needed to investigate the role of immunotherapy targeting specific inflammatory pathways and the impact on the repigmentation in vitiligo.
Acknowledgment—Special thanks to Paul Chu, MD (Port Chester, New York).
To the Editor:
A 39-year-old man with a history of hypertension and vitiligo presented with a rapid-onset, generalized, pruritic rash covering the body of 4 weeks’ duration. He reported that the rash progressively worsened after developing mild sunburn. The patient stated that the rash was extremely pruritic with a burning sensation and was tender to touch. He was treated with betamethasone valerate cream 0.1% by an outside physician and an over-the-counter anti-itch lotion with no notable improvement. His only medication was telmisartan-hydrochlorothiazide (HCTZ) for hypertension. He denied any drug allergies.
Physical examination revealed multiple discrete and coalescent planar erythematous papules and plaques involving only the depigmented vitiliginous skin of the forehead, eyelids, and nape of the neck (Figure 1A), and confluent on the lateral aspect of the bilateral forearms (Figure 1B), dorsal aspect of the right hand, and bilateral dorsi of the feet. Wickham striae were noted on the lips (Figure 1C). A clinical diagnosis of lichen planus (LP) was made. The patient initially was prescribed halobetasol propionate ointment 0.05% twice daily. He reported notable relief of pruritus with reduction of overall symptoms and new lesion formation.

A 4-mm punch biopsy was performed on the left forearm. Histopathology revealed LP. Microscopic examination of the hematoxylin and eosin–stained specimen revealed a bandlike lymphohistiocytic infiltrate that extended across the papillary dermis, focally obscuring the dermoepidermal junction where there were vacuolar changes and colloid bodies. The epidermis showed sawtooth rete ridges, wedge-shaped foci of hypergranulosis, and compact hyperkeratosis (Figure 2).

On further questioning during follow-up, the patient revealed that his hypertensive medication was changed from HCTZ, which he had been taking for the last 8 years, to the combination antihypertensive medication telmisartan-HCTZ before the onset of the skin eruption. Due to the temporal relationship between the new medication and onset of the eruption, the clinical impression was highly suspicious for drug-induced eruptive LP with Köbner phenomenon caused by the recent sunburn. Systemic workup for underlying causes of LP was negative. Laboratory tests revealed normal complete blood cell counts. The hepatitis panel included hepatitis A antibodies; hepatitis B surface, e antigen, and core antibodies; hepatitis B surface antigen and e antibodies; hepatitis C antibodies; and antinuclear antibodies, which were all negative.
The patient continued to develop new pruritic papules clinically consistent with LP. He was instructed to return to his primary care physician to change the telmisartan-HCTZ to a different class of antihypertensive medication. His medication was changed to atenolol. The patient also was instructed to continue the halobetasol propionate ointment 0.05% twice daily to the affected areas.
The patient returned for a follow-up visit 1 month later and reported notable improvement in pruritus and near-complete resolution of the LP after discontinuation of telmisartan-HCTZ. He also noted some degree of perifollicular repigmentation of the vitiliginous skin that had been unresponsive to prior therapy (Figure 3).

Lichen planus is a pruritic and inflammatory papulosquamous skin condition that presents as scaly, flat-topped, violaceous, polygonal-shaped papules commonly involving the flexor surface of the arms and legs, oral mucosa, scalp, nails, and genitalia. Clinically, LP can present in various forms including actinic, annular, atrophic, erosive, follicular, hypertrophic, linear, pigmented, and vesicular/bullous types. Koebnerization is common, especially in the linear form of LP. There are no specific laboratory findings or serologic markers seen in LP.
The exact cause of LP remains unknown. Clinical observations and anecdotal evidence have directed the cell-mediated immune response to insulting agents such as medications or contact allergy to metals triggering an abnormal cellular immune response. Various viral agents have been reported including hepatitis C virus, human herpesvirus, herpes simplex virus, and varicella-zoster virus.1-5 Other factors such as seasonal change and the environment may contribute to the development of LP and an increase in the incidence of LP eruption has been observed from January to July throughout the United States.6 Lichen planus also has been associated with other altered immune-related disease such as ulcerative colitis, alopecia areata, vitiligo, dermatomyositis, morphea, lichen sclerosis, and myasthenia gravis.7 Increased levels of emotional stress, particularly related to family members, often is related to the onset or aggravation of symptoms.8,9
Many drug-related LP-like and lichenoid eruptions have been reported with antihypertensive drugs, antimalarial drugs, diuretics, antidepressants, nonsteroidal anti-inflammatory drugs, antimicrobial drugs, and metals. In particular, medications such as captopril, enalapril, labetalol, propranolol, chlorothiazide, HCTZ, methyldopa, chloroquine, hydroxychloroquine, quinacrine, gold salts, penicillamine, and quinidine commonly are reported to induce lichenoid drug eruption.10
Several inflammatory papulosquamous skin conditions should be considered in the differential diagnosis before confirming the diagnosis of LP. It is important to rule out lupus erythematosus, especially if the oral mucosa and scalp are involved. In addition, erosive paraneoplastic pemphigus involving primarily the oral mucosa can resemble oral LP. Nail diseases such as psoriasis, onychomycosis, and alopecia areata should be considered as the differential diagnosis of nail disease. Genital involvement also can be seen in psoriasis and lichen sclerosus.
Treatment of LP is mainly symptomatic because of the benign nature of the disease and the high spontaneous remission rate with varying amount of time. If drugs, dental/metal implants, or underlying viral infections are the identifiable triggering factors of LP, the offending agents should be discontinued or removed. Additionally, topical or systemic treatments can be given depending on the severity of the disease, focusing mainly on symptomatic relief as well as the balance of risks and benefits associated with treatment.
Treatment options include topical and intralesional corticosteroids. Systemic medications such as oral corticosteroids and/or acitretin commonly are used in acute, severe, and disseminated cases, though treatment duration varies depending on the clinical response. Other systemic agents used to treat LP include griseofulvin, metronidazole, sulfasalazine, cyclosporine, and mycophenolate mofetil.
Phototherapy is considered an alternative therapy, especially for recalcitrant LP. UVA1 and narrowband UVB (wavelength, 311 nm) have been reported to effectively treat long-standing and therapy-resistant LP.11 In addition, a small study used the excimer laser (wavelength, 308 nm), which is well tolerated by patients, to treat focal recalcitrant oral lesions with excellent results.12 Photochemotherapy has been used with notable improvement, but the potential of carcinogenicity, especially in patients with Fitzpatrick skin types I and II, has limited its use.13
Our patient developed an unusual extensive LP eruption involving only vitiliginous skin shortly after initiation of the combined antihypertensive medication telmisartan-HCTZ, an angiotensin receptor blocker with a thiazide diuretic. Telmisartan and other angiotensin receptor blockers have not been reported to trigger LP; HCTZ is listed as one of the common drugs causing photosensitivity and LP.14,15 Although it is possible that our patient exhibited a delayed lichenoid drug eruption from the HCTZ, it is noteworthy that he did not experience a single episode of LP during his 8-year history of taking HCTZ. Instead, he developed the LP eruption shortly after the addition of telmisartan to his HCTZ antihypertensive regimen. The temporal relationship led us to direct the patient to the prescribing physician to discontinue telmisartan-HCTZ. After changing his antihypertensive medication to atenolol, the patient presented with improvement within the first month and near-complete resolution 2 months after the discontinuation of telmisartan-HCTZ.
Our patient’s LP lesions only manifested on the skin affected by vitiligo, sparing the normal-pigmented skin. Studies have demonstrated an increased ratio of CD8+ T cells to CD4+ T cells as well as increased intercellular adhesion molecule 1 at the dermal level.10,16 Both vitiligo and LP share some common histopathologic features including highly populated CD8+ T cells and intercellular adhesion molecule 1. In our case, LP was triggered on the vitiliginous skin by telmisartan. Vitiligo in combination with trauma induced by sunburn may represent the trigger that altered the cellular immune response and created the telmisartan-induced LP. As a result, the LP eruption was confined to the vitiliginous skin lesions.
Perifollicular repigmentation was observed in our patient after the LP lesions resolved; the patient’s vitiligo was unresponsive to prior treatment. The inflammatory process occurring in LP may exert and interfere in the underlying autoimmune cytotoxic effect toward the melanocytes and the melanin synthesis. It may be of interest to find out if the inflammatory response of LP has a positive influence on the effect of melanogenesis pathways or on the underlying autoimmune-related inflammatory process in vitiligo. Further studies are needed to investigate the role of immunotherapy targeting specific inflammatory pathways and the impact on the repigmentation in vitiligo.
Acknowledgment—Special thanks to Paul Chu, MD (Port Chester, New York).
- Pilli M, Zerbini A, Vescovi P, et al. Oral lichen planus pathogenesis: a role for the HCV-specific cellular immune response. Hepatology. 2002;36:1446-1452.
- De Vries HJ, van Marle J, Teunissen MB, et al. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br J Dermatol. 2006;154:361-364.
- De Vries HJ, Teunissen MB, Zorgdrager F, et al. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. 2007;299:213-219.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Al-Khenaizan S. Lichen planus occurring after hepatitis B vaccination: a new case. J Am Acad Dermatol. 2001;45:614-615.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Sadr-Ashkevari S. Familial actinic lichen planus: case reports in two brothers. Arch Int Med. 2001;4:204-206.
- Manolache L, Seceleanu-Petrescu D, Benea V. Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437-441.
- Mahood JM. Familial lichen planus. Arch Dermatol. 1983;119:292-294.
- Shimizu M, Higaki Y, Higaki M, et al. The role of granzyme B-expressing CD8-positive T cells in apoptosis of keratinocytes in lichen planus. Arch Dermatol Res. 1997;289:527-532.
- Bécherel PA, Bussel A, Chosidow O, et al. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet. 1998;351:805.
- Trehan M, Taylar CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol. 2004;140:415-420.
- Wackernagel A, Legat FJ, Hofer A, et al. Psoralen plus UVA vs. UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2007;23:15-19.
- Fellner MJ. Lichen planus. Int J Dermatol. 1980;19:71-75.
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345-372.
- Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
- Pilli M, Zerbini A, Vescovi P, et al. Oral lichen planus pathogenesis: a role for the HCV-specific cellular immune response. Hepatology. 2002;36:1446-1452.
- De Vries HJ, van Marle J, Teunissen MB, et al. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br J Dermatol. 2006;154:361-364.
- De Vries HJ, Teunissen MB, Zorgdrager F, et al. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. 2007;299:213-219.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Al-Khenaizan S. Lichen planus occurring after hepatitis B vaccination: a new case. J Am Acad Dermatol. 2001;45:614-615.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Sadr-Ashkevari S. Familial actinic lichen planus: case reports in two brothers. Arch Int Med. 2001;4:204-206.
- Manolache L, Seceleanu-Petrescu D, Benea V. Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437-441.
- Mahood JM. Familial lichen planus. Arch Dermatol. 1983;119:292-294.
- Shimizu M, Higaki Y, Higaki M, et al. The role of granzyme B-expressing CD8-positive T cells in apoptosis of keratinocytes in lichen planus. Arch Dermatol Res. 1997;289:527-532.
- Bécherel PA, Bussel A, Chosidow O, et al. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet. 1998;351:805.
- Trehan M, Taylar CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol. 2004;140:415-420.
- Wackernagel A, Legat FJ, Hofer A, et al. Psoralen plus UVA vs. UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2007;23:15-19.
- Fellner MJ. Lichen planus. Int J Dermatol. 1980;19:71-75.
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345-372.
- Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
Practice Points
- Lichen planus (LP) is a T-cell–mediated autoimmune disease that affects the skin and often the mucosa, nails, and scalp.
- The etiology of LP is unknown. It can be induced by a variety of medications and may spread through the isomorphic phenomenon.
- Immune factors play a role in the development of LP, drug-induced LP, and vitiligo.
Bilateral Symmetric Onycholysis of Distal Fingernails
The Diagnosis: Allergic Contact Dermatitis
An allergic contact dermatitis (ACD) to acrylates was suspected and 4 patches were applied to the forearm (the North American Standard Series of the North American Contact Dermatitis Group). The patches were 2-hydroxyethyl methacrylate (2-HEMA) 2.0% permissible exposure limit (peL), ethyl acrylate 0.1% peL, tosylamide formaldehyde resin 10.0% peL, and methyl methacrylate 2.0% peL. A reading at 72 hours was performed and showed a positive reaction to hydroxyethyl methacrylate, ethyl acrylate, and methyl methacrylate, and a negative patch test to tosylamide formaldehyde resin (nail polish)(Figure). The patient was diagnosed with an allergic contact hypersensitivity to the aforementioned acrylates and instructed to avoid artificial nails and acrylate glues. She also was started on oral biotin supplements. On 6-month follow-up the patient had regrowth of all 10 fingernails without brittleness or splitting. She was able to use nail polishes but avoided all acrylic artificial nails and acrylate-containing personal care products.
Acrylate Allergy and Artificial Nails
Acrylates are plastic materials formed by polymerization of acrylic or methacrylic acid monomers and have been cited as a major cause of occupational and nonoccupational contact dermatitis. Contact dermatitis to acrylates in artificial nails was first reported in the 1950s.1,2 Products containing 100% methyl methacrylate monomers in acrylic nails were banned by the US Food and Drug Administration in the early 1970s after receiving a number of complaints.3 However, no regulation prohibits the use of methyl methacrylate monomer in cosmetic products, and various methacrylate and acrylate monomers remain widely used.4 With a growing popularity in artificial nails, it is expected the number of sensitized persons will increase.
Acrylate allergy from sculptured nails concern self-curing resins made from a polymer powder and a liquid monomer solution. Advantages of new UV-cured products include the lack of unpleasant smell and simplified modeling. They also do not require an irritant, such as methacrylic acid, as a bonding agent. Instead, 2-HEMA and 2-hydroxypropyl methacrylate are added. These photobonded nails colloquially are called gel nails (acid free) as opposed to acrylic nails (using methacrylic acid as a primer). It is important to note that the esters of acrylic acid but not the acid itself sensitize patients, and sensitization is not caused by the uncured gel or the monomer solution but by the remaining monomers in the cured plastic nail and the dust filings that are produced during the finishing process.
Clinical Presentation
Symptoms of an ACD to nail acrylates include pruritus and fingertip dermatitis along with nail plate dystrophy. There may be pruritus at the nail base, with subsequent dryness, thickening, and onycholysis. The brittle nails may become split, discolored, and develop paronychia. Inadvertent contact with glue monomers or other acrylate-containing substances may cause eczematous lesions at distant sites. Avoidance of the allergen often results in complete restoration of the normal nail and fingertip within months.
Sensitization
Acrylates and methacrylates are ubiquitous materials used for both industrial and commercial applications. Due to their widespread industrial use, contact allergies to acrylates including 2-HEMA, 2-hydroxypropyl methacrylate, and triethyleneglycol diacrylate (TREGDA) are common. Cross-reaction of these compounds has been observed and is postulated to be due to reaction of the (meth)acrylate carboxyethyl group with the receptors of antigen-presenting cells.5 As a result, an individual with an acrylate allergy sensitized to one allergen often is allergic to its similar compounds and cross-reactors and must avoid the assortment of compounds containing these ingredients, which is important for individuals with occupational sensitization to a particular acrylate who is subsequently susceptible to other acrylate-containing compounds triggering allergic reactions when reexposure occurs in different settings.
Allergens and Occupational Exposure
Acrylates in cosmetic nail products are a source of ACD for not only the customer but also the manicurist.6 The most frequently cited sources of ACD in beauticians are acrylate chemicals.7 However, acrylate compounds are an occupational hazard for a number of other specialists, including dentists and dental technicians, histology technicians, and individuals in the printing industry.8,9 Other individuals may be sensitized to acrylates through their inclusion in adhesives, dental bonding agents, hearing aids, electrocardiogram electrodes, artificial bone cement, and a myriad of other medical and nonmedical applications.4,10-12 For workers who cannot avoid occupational exposure to these allergens, polyvinyl alcohol and multilayer laminate gloves are recommended, as natural rubber latex gloves do not always provide adequate protection from many of these agents.10
Testing for Suspected Acrylate Allergy
Cross-reactivity among acrylates is widely considered in the literature but remains enigmatic and is an important consideration with regard to routine patch test screening.13 In the case of an acrylate allergy to nail products, using 2-HEMA and ethylene glycol dimethacrylate is effective in detecting sensitization by photobonded nails and in patients sensitized by powder liquid products.14 One study showed a patch test panel including 2-HEMA, ethylene glycol dimethacrylate, and TREGDA was effective in identifying the majority of individuals with an allergy to acrylates in nail products and nail technicians.15 Another study has shown the most commonly positive testing allergens to be HEMA, ethyl acrylate, and methyl methacrylate.16 If one is patch testing only one chemical, it appears 2-HEMA is preferred.17 However, broader panels of screening allergens are necessary to achieve an accurate diagnosis. Furthermore, different panels of test allergens have been shown to vary in their ability to detect an acrylate allergy in different occupational exposures.12
The time to patch test read also is important. A standard read at 72 hours is warranted; however, one study showed if only one read at day 3 was done without a subsequent day 7 read, then 25% of TREGDA and 50% of 2-HEMA allergies would have been missed in patients with occupational acrylate allergy.15 Other studies have reported late-appearing and long-lasting test reactions when testing for an acrylate allergy.18,19 Clinicians should be cognizant that an acrylate allergy may be present even if initial screening is negative but the history and clinical picture are suggestive.
- Canizares O. Contact dermatitis due to the acrylic materials used in artificial nails. AMA Arch Derm. 1956;74:141-143.
- Fisher AA, Franks A, Glick H. Allergic sensitization of the skin and nails to acrylic plastic nails. J Allergy. 1957;28:84-88.
- US Food and Drug Administration. Nail care products. http://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm127068.htm. Updated October 26, 2016. Accessed December 27, 2016.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59(5 suppl):S123-S124.
- Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59:320-329.
- Tammaro A, Narcisi A, Abruzzese C, et al. Fingertip dermatitis: occupational acrylate cross reaction. Allergol Int. 2014;63:609-610.
- Kwok C, Money A, Carder M, et al. Cases of occupational dermatitis and asthma in beauticians that were reported to The Health and Occupation Research (THOR) network from 1996 to 2011. Clin Exp Dermatol. 2014;39:590-595.
- Aalto-Korte K, Alanko K, Kuuliala O, et al. Methacrylate and acrylate allergy in dental personnel. Contact Dermatitis. 2007;57:324-330.
- Molina L, Amado A, Mattei PL 4th, et al. Contact dermatitis from acrylics in a histology laboratory assistant. Dermatitis. 2009;20:E11-E12.
- Prasad Hunasehally RY, Hughes TM, Stone NM. Atypical pattern of (meth)acrylate allergic contact dermatitis in dental professionals. Br Dent J. 2012;213:223-224.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes [published online January 27, 2015]. Contact Dermatitis. 2015;73:44-48.
- Sasseville D. Acrylates in contact dermatitis. Dermatitis. 2012;23:6-16.
- Fisher AA. Cross reactions between methyl methacrylate monomer and acrylic monomers presently used in acrylic nail preparations. Contact Dermatitis. 1980;6:345-347.
- Hemmer W, Focke M, Wantke F, et al. Allergic contact dermatitis to artificial fingernails prepared from UV light-cured acrylates. J Am Acad Dermatol. 1996;35(3, pt 1):377-380.
- Teik-Jin Goon A, Bruze M, Zimerson E, et al. Contact allergy to acrylates/methacrylates in the acrylate and nail acrylics series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2007;57:21-27.
- Drucker AM, Pratt MD. Acrylate contact allergy: patient characteristics and evaluation of screening allergens. Dermatitis. 2011;22:98-101.
- Ramos L, Cabral R, Goncalo M. Allergic contact dermatitis caused by acrylates and methacrylates--a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Goon AT, Isaksson M, Zimerson E, et al. Contact allergy to (meth)acrylates in the dental series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2006;55:219-226.
- Isaksson M, Lindberg M, Sundberg K, et al. The development and course of patch-test reactions to 2-hydroxyethyl methacrylate and ethyleneglycol dimethacrylate. Contact Dermatitis. 2005;53:292-297.
The Diagnosis: Allergic Contact Dermatitis
An allergic contact dermatitis (ACD) to acrylates was suspected and 4 patches were applied to the forearm (the North American Standard Series of the North American Contact Dermatitis Group). The patches were 2-hydroxyethyl methacrylate (2-HEMA) 2.0% permissible exposure limit (peL), ethyl acrylate 0.1% peL, tosylamide formaldehyde resin 10.0% peL, and methyl methacrylate 2.0% peL. A reading at 72 hours was performed and showed a positive reaction to hydroxyethyl methacrylate, ethyl acrylate, and methyl methacrylate, and a negative patch test to tosylamide formaldehyde resin (nail polish)(Figure). The patient was diagnosed with an allergic contact hypersensitivity to the aforementioned acrylates and instructed to avoid artificial nails and acrylate glues. She also was started on oral biotin supplements. On 6-month follow-up the patient had regrowth of all 10 fingernails without brittleness or splitting. She was able to use nail polishes but avoided all acrylic artificial nails and acrylate-containing personal care products.

Acrylate Allergy and Artificial Nails
Acrylates are plastic materials formed by polymerization of acrylic or methacrylic acid monomers and have been cited as a major cause of occupational and nonoccupational contact dermatitis. Contact dermatitis to acrylates in artificial nails was first reported in the 1950s.1,2 Products containing 100% methyl methacrylate monomers in acrylic nails were banned by the US Food and Drug Administration in the early 1970s after receiving a number of complaints.3 However, no regulation prohibits the use of methyl methacrylate monomer in cosmetic products, and various methacrylate and acrylate monomers remain widely used.4 With a growing popularity in artificial nails, it is expected the number of sensitized persons will increase.
Acrylate allergy from sculptured nails concern self-curing resins made from a polymer powder and a liquid monomer solution. Advantages of new UV-cured products include the lack of unpleasant smell and simplified modeling. They also do not require an irritant, such as methacrylic acid, as a bonding agent. Instead, 2-HEMA and 2-hydroxypropyl methacrylate are added. These photobonded nails colloquially are called gel nails (acid free) as opposed to acrylic nails (using methacrylic acid as a primer). It is important to note that the esters of acrylic acid but not the acid itself sensitize patients, and sensitization is not caused by the uncured gel or the monomer solution but by the remaining monomers in the cured plastic nail and the dust filings that are produced during the finishing process.
Clinical Presentation
Symptoms of an ACD to nail acrylates include pruritus and fingertip dermatitis along with nail plate dystrophy. There may be pruritus at the nail base, with subsequent dryness, thickening, and onycholysis. The brittle nails may become split, discolored, and develop paronychia. Inadvertent contact with glue monomers or other acrylate-containing substances may cause eczematous lesions at distant sites. Avoidance of the allergen often results in complete restoration of the normal nail and fingertip within months.
Sensitization
Acrylates and methacrylates are ubiquitous materials used for both industrial and commercial applications. Due to their widespread industrial use, contact allergies to acrylates including 2-HEMA, 2-hydroxypropyl methacrylate, and triethyleneglycol diacrylate (TREGDA) are common. Cross-reaction of these compounds has been observed and is postulated to be due to reaction of the (meth)acrylate carboxyethyl group with the receptors of antigen-presenting cells.5 As a result, an individual with an acrylate allergy sensitized to one allergen often is allergic to its similar compounds and cross-reactors and must avoid the assortment of compounds containing these ingredients, which is important for individuals with occupational sensitization to a particular acrylate who is subsequently susceptible to other acrylate-containing compounds triggering allergic reactions when reexposure occurs in different settings.
Allergens and Occupational Exposure
Acrylates in cosmetic nail products are a source of ACD for not only the customer but also the manicurist.6 The most frequently cited sources of ACD in beauticians are acrylate chemicals.7 However, acrylate compounds are an occupational hazard for a number of other specialists, including dentists and dental technicians, histology technicians, and individuals in the printing industry.8,9 Other individuals may be sensitized to acrylates through their inclusion in adhesives, dental bonding agents, hearing aids, electrocardiogram electrodes, artificial bone cement, and a myriad of other medical and nonmedical applications.4,10-12 For workers who cannot avoid occupational exposure to these allergens, polyvinyl alcohol and multilayer laminate gloves are recommended, as natural rubber latex gloves do not always provide adequate protection from many of these agents.10
Testing for Suspected Acrylate Allergy
Cross-reactivity among acrylates is widely considered in the literature but remains enigmatic and is an important consideration with regard to routine patch test screening.13 In the case of an acrylate allergy to nail products, using 2-HEMA and ethylene glycol dimethacrylate is effective in detecting sensitization by photobonded nails and in patients sensitized by powder liquid products.14 One study showed a patch test panel including 2-HEMA, ethylene glycol dimethacrylate, and TREGDA was effective in identifying the majority of individuals with an allergy to acrylates in nail products and nail technicians.15 Another study has shown the most commonly positive testing allergens to be HEMA, ethyl acrylate, and methyl methacrylate.16 If one is patch testing only one chemical, it appears 2-HEMA is preferred.17 However, broader panels of screening allergens are necessary to achieve an accurate diagnosis. Furthermore, different panels of test allergens have been shown to vary in their ability to detect an acrylate allergy in different occupational exposures.12
The time to patch test read also is important. A standard read at 72 hours is warranted; however, one study showed if only one read at day 3 was done without a subsequent day 7 read, then 25% of TREGDA and 50% of 2-HEMA allergies would have been missed in patients with occupational acrylate allergy.15 Other studies have reported late-appearing and long-lasting test reactions when testing for an acrylate allergy.18,19 Clinicians should be cognizant that an acrylate allergy may be present even if initial screening is negative but the history and clinical picture are suggestive.
The Diagnosis: Allergic Contact Dermatitis
An allergic contact dermatitis (ACD) to acrylates was suspected and 4 patches were applied to the forearm (the North American Standard Series of the North American Contact Dermatitis Group). The patches were 2-hydroxyethyl methacrylate (2-HEMA) 2.0% permissible exposure limit (peL), ethyl acrylate 0.1% peL, tosylamide formaldehyde resin 10.0% peL, and methyl methacrylate 2.0% peL. A reading at 72 hours was performed and showed a positive reaction to hydroxyethyl methacrylate, ethyl acrylate, and methyl methacrylate, and a negative patch test to tosylamide formaldehyde resin (nail polish)(Figure). The patient was diagnosed with an allergic contact hypersensitivity to the aforementioned acrylates and instructed to avoid artificial nails and acrylate glues. She also was started on oral biotin supplements. On 6-month follow-up the patient had regrowth of all 10 fingernails without brittleness or splitting. She was able to use nail polishes but avoided all acrylic artificial nails and acrylate-containing personal care products.

Acrylate Allergy and Artificial Nails
Acrylates are plastic materials formed by polymerization of acrylic or methacrylic acid monomers and have been cited as a major cause of occupational and nonoccupational contact dermatitis. Contact dermatitis to acrylates in artificial nails was first reported in the 1950s.1,2 Products containing 100% methyl methacrylate monomers in acrylic nails were banned by the US Food and Drug Administration in the early 1970s after receiving a number of complaints.3 However, no regulation prohibits the use of methyl methacrylate monomer in cosmetic products, and various methacrylate and acrylate monomers remain widely used.4 With a growing popularity in artificial nails, it is expected the number of sensitized persons will increase.
Acrylate allergy from sculptured nails concern self-curing resins made from a polymer powder and a liquid monomer solution. Advantages of new UV-cured products include the lack of unpleasant smell and simplified modeling. They also do not require an irritant, such as methacrylic acid, as a bonding agent. Instead, 2-HEMA and 2-hydroxypropyl methacrylate are added. These photobonded nails colloquially are called gel nails (acid free) as opposed to acrylic nails (using methacrylic acid as a primer). It is important to note that the esters of acrylic acid but not the acid itself sensitize patients, and sensitization is not caused by the uncured gel or the monomer solution but by the remaining monomers in the cured plastic nail and the dust filings that are produced during the finishing process.
Clinical Presentation
Symptoms of an ACD to nail acrylates include pruritus and fingertip dermatitis along with nail plate dystrophy. There may be pruritus at the nail base, with subsequent dryness, thickening, and onycholysis. The brittle nails may become split, discolored, and develop paronychia. Inadvertent contact with glue monomers or other acrylate-containing substances may cause eczematous lesions at distant sites. Avoidance of the allergen often results in complete restoration of the normal nail and fingertip within months.
Sensitization
Acrylates and methacrylates are ubiquitous materials used for both industrial and commercial applications. Due to their widespread industrial use, contact allergies to acrylates including 2-HEMA, 2-hydroxypropyl methacrylate, and triethyleneglycol diacrylate (TREGDA) are common. Cross-reaction of these compounds has been observed and is postulated to be due to reaction of the (meth)acrylate carboxyethyl group with the receptors of antigen-presenting cells.5 As a result, an individual with an acrylate allergy sensitized to one allergen often is allergic to its similar compounds and cross-reactors and must avoid the assortment of compounds containing these ingredients, which is important for individuals with occupational sensitization to a particular acrylate who is subsequently susceptible to other acrylate-containing compounds triggering allergic reactions when reexposure occurs in different settings.
Allergens and Occupational Exposure
Acrylates in cosmetic nail products are a source of ACD for not only the customer but also the manicurist.6 The most frequently cited sources of ACD in beauticians are acrylate chemicals.7 However, acrylate compounds are an occupational hazard for a number of other specialists, including dentists and dental technicians, histology technicians, and individuals in the printing industry.8,9 Other individuals may be sensitized to acrylates through their inclusion in adhesives, dental bonding agents, hearing aids, electrocardiogram electrodes, artificial bone cement, and a myriad of other medical and nonmedical applications.4,10-12 For workers who cannot avoid occupational exposure to these allergens, polyvinyl alcohol and multilayer laminate gloves are recommended, as natural rubber latex gloves do not always provide adequate protection from many of these agents.10
Testing for Suspected Acrylate Allergy
Cross-reactivity among acrylates is widely considered in the literature but remains enigmatic and is an important consideration with regard to routine patch test screening.13 In the case of an acrylate allergy to nail products, using 2-HEMA and ethylene glycol dimethacrylate is effective in detecting sensitization by photobonded nails and in patients sensitized by powder liquid products.14 One study showed a patch test panel including 2-HEMA, ethylene glycol dimethacrylate, and TREGDA was effective in identifying the majority of individuals with an allergy to acrylates in nail products and nail technicians.15 Another study has shown the most commonly positive testing allergens to be HEMA, ethyl acrylate, and methyl methacrylate.16 If one is patch testing only one chemical, it appears 2-HEMA is preferred.17 However, broader panels of screening allergens are necessary to achieve an accurate diagnosis. Furthermore, different panels of test allergens have been shown to vary in their ability to detect an acrylate allergy in different occupational exposures.12
The time to patch test read also is important. A standard read at 72 hours is warranted; however, one study showed if only one read at day 3 was done without a subsequent day 7 read, then 25% of TREGDA and 50% of 2-HEMA allergies would have been missed in patients with occupational acrylate allergy.15 Other studies have reported late-appearing and long-lasting test reactions when testing for an acrylate allergy.18,19 Clinicians should be cognizant that an acrylate allergy may be present even if initial screening is negative but the history and clinical picture are suggestive.
- Canizares O. Contact dermatitis due to the acrylic materials used in artificial nails. AMA Arch Derm. 1956;74:141-143.
- Fisher AA, Franks A, Glick H. Allergic sensitization of the skin and nails to acrylic plastic nails. J Allergy. 1957;28:84-88.
- US Food and Drug Administration. Nail care products. http://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm127068.htm. Updated October 26, 2016. Accessed December 27, 2016.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59(5 suppl):S123-S124.
- Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59:320-329.
- Tammaro A, Narcisi A, Abruzzese C, et al. Fingertip dermatitis: occupational acrylate cross reaction. Allergol Int. 2014;63:609-610.
- Kwok C, Money A, Carder M, et al. Cases of occupational dermatitis and asthma in beauticians that were reported to The Health and Occupation Research (THOR) network from 1996 to 2011. Clin Exp Dermatol. 2014;39:590-595.
- Aalto-Korte K, Alanko K, Kuuliala O, et al. Methacrylate and acrylate allergy in dental personnel. Contact Dermatitis. 2007;57:324-330.
- Molina L, Amado A, Mattei PL 4th, et al. Contact dermatitis from acrylics in a histology laboratory assistant. Dermatitis. 2009;20:E11-E12.
- Prasad Hunasehally RY, Hughes TM, Stone NM. Atypical pattern of (meth)acrylate allergic contact dermatitis in dental professionals. Br Dent J. 2012;213:223-224.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes [published online January 27, 2015]. Contact Dermatitis. 2015;73:44-48.
- Sasseville D. Acrylates in contact dermatitis. Dermatitis. 2012;23:6-16.
- Fisher AA. Cross reactions between methyl methacrylate monomer and acrylic monomers presently used in acrylic nail preparations. Contact Dermatitis. 1980;6:345-347.
- Hemmer W, Focke M, Wantke F, et al. Allergic contact dermatitis to artificial fingernails prepared from UV light-cured acrylates. J Am Acad Dermatol. 1996;35(3, pt 1):377-380.
- Teik-Jin Goon A, Bruze M, Zimerson E, et al. Contact allergy to acrylates/methacrylates in the acrylate and nail acrylics series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2007;57:21-27.
- Drucker AM, Pratt MD. Acrylate contact allergy: patient characteristics and evaluation of screening allergens. Dermatitis. 2011;22:98-101.
- Ramos L, Cabral R, Goncalo M. Allergic contact dermatitis caused by acrylates and methacrylates--a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Goon AT, Isaksson M, Zimerson E, et al. Contact allergy to (meth)acrylates in the dental series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2006;55:219-226.
- Isaksson M, Lindberg M, Sundberg K, et al. The development and course of patch-test reactions to 2-hydroxyethyl methacrylate and ethyleneglycol dimethacrylate. Contact Dermatitis. 2005;53:292-297.
- Canizares O. Contact dermatitis due to the acrylic materials used in artificial nails. AMA Arch Derm. 1956;74:141-143.
- Fisher AA, Franks A, Glick H. Allergic sensitization of the skin and nails to acrylic plastic nails. J Allergy. 1957;28:84-88.
- US Food and Drug Administration. Nail care products. http://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm127068.htm. Updated October 26, 2016. Accessed December 27, 2016.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59(5 suppl):S123-S124.
- Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59:320-329.
- Tammaro A, Narcisi A, Abruzzese C, et al. Fingertip dermatitis: occupational acrylate cross reaction. Allergol Int. 2014;63:609-610.
- Kwok C, Money A, Carder M, et al. Cases of occupational dermatitis and asthma in beauticians that were reported to The Health and Occupation Research (THOR) network from 1996 to 2011. Clin Exp Dermatol. 2014;39:590-595.
- Aalto-Korte K, Alanko K, Kuuliala O, et al. Methacrylate and acrylate allergy in dental personnel. Contact Dermatitis. 2007;57:324-330.
- Molina L, Amado A, Mattei PL 4th, et al. Contact dermatitis from acrylics in a histology laboratory assistant. Dermatitis. 2009;20:E11-E12.
- Prasad Hunasehally RY, Hughes TM, Stone NM. Atypical pattern of (meth)acrylate allergic contact dermatitis in dental professionals. Br Dent J. 2012;213:223-224.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes [published online January 27, 2015]. Contact Dermatitis. 2015;73:44-48.
- Sasseville D. Acrylates in contact dermatitis. Dermatitis. 2012;23:6-16.
- Fisher AA. Cross reactions between methyl methacrylate monomer and acrylic monomers presently used in acrylic nail preparations. Contact Dermatitis. 1980;6:345-347.
- Hemmer W, Focke M, Wantke F, et al. Allergic contact dermatitis to artificial fingernails prepared from UV light-cured acrylates. J Am Acad Dermatol. 1996;35(3, pt 1):377-380.
- Teik-Jin Goon A, Bruze M, Zimerson E, et al. Contact allergy to acrylates/methacrylates in the acrylate and nail acrylics series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2007;57:21-27.
- Drucker AM, Pratt MD. Acrylate contact allergy: patient characteristics and evaluation of screening allergens. Dermatitis. 2011;22:98-101.
- Ramos L, Cabral R, Goncalo M. Allergic contact dermatitis caused by acrylates and methacrylates--a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Goon AT, Isaksson M, Zimerson E, et al. Contact allergy to (meth)acrylates in the dental series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2006;55:219-226.
- Isaksson M, Lindberg M, Sundberg K, et al. The development and course of patch-test reactions to 2-hydroxyethyl methacrylate and ethyleneglycol dimethacrylate. Contact Dermatitis. 2005;53:292-297.
A 28-year-old woman presented with distal onycholysis of all 10 fingernails. The patient started to notice brittleness in the first, second, and third fingernails of the right hand 2 months prior. She had a 10-year history of wearing acrylic nails and reported a history of periungual eczema. On physical examination, all 10 fingernails had distal onycholysis and there was a green discoloration of the first fingernail on the left hand. On blood analysis, thyroid-stimulating hormone and free thyroxine were within reference range. A nail clipping showed onychodystrophy and a negative periodic acid-Schiff stain.
High-risk relatives of MS patients show early signs of disease
Asymptomatic first-degree relatives of multiple sclerosis patients at high risk for developing the disease were significantly more likely to show subclinical signs of MS than were family members at lower risk, in the Genes and Environment in Multiple Sclerosis prospective cohort study. The findings were published online on Jan. 17 in JAMA Neurology.
The Genes and Environment in Multiple Sclerosis (GEMS) project is the first prospective study of populations at risk for MS and is the first detailed cross-sectional examination of higher-risk and lower-risk family members to date, according to investigators led by Zongqi Xia, MD, PhD, of Brigham and Women’s Hospital, Boston. Although the totality of evidence put together through neuroimaging and numerous clinical tests in the study indicate that individuals with the highest risk for MS have higher risk for the disease than do those with the lowest risk, simple vibration threshold testing gave the best results, Dr. Xia and his colleagues reported.
The study involved 100 neurologically asymptomatic adults aged 18-50 years who were first-degree relatives of people with MS who participated in the GEMS project during August 2012 to July 2015. These 100 comprised 41 high-risk participants from the top 10% of a Genetic and Environmental Risk Score (GERS) and 59 low-risk participants from the bottom 10% on the GERS. The GERS included genetic risk factors (HLA alleles and several MS-associated non-HLA genetic variants) and environmental factors, such as smoking status, body mass index, history of infectious mononucleosis and migraine, and vitamin D levels.
However, because 40 of the 41 high-risk individuals were female and 25 of the 59 low-risk individuals were female, the investigators limited the study to the 65 female participants to avoid “attributing any potential difference primarily to the role of sex.”
To help in identifying early signs of MS, the researchers used brain MRI and optical coherence tomography and other measures of neurological function, including the Expanded Disability Status Scale, timed 25-foot walk, Nine-Hole Peg Test, Paced Auditory Serial Addition Test, Symbol Digit Modalities Test, Timed Up and Go, and high-contrast and low-contrast visual acuity (JAMA Neurol. 2017 Jan 17. doi: 10.1001/jamaneurol.2016.5056).
Overall, high-risk women showed more subclinical signs of MS than did low-risk women based on an omnibus test that globally assessed the burden of neurological dysfunction by comparing the overall differences between the two groups when considering all of the measured outcomes (P = .01). However, impaired vibration perception yielded a stronger result; of 47 women (27 high-risk and 20 low-risk) tested in this manner, high-risk women showed significantly reduced vibration perception in the distal lower extremities (P = .008).
One individual in the high-risk group converted to clinically definite MS during the study. Four of the high-risk women had T2-weighted hyperintense lesions that met the 2010 McDonald MRI criteria for dissemination in space, compared with one low-risk woman. The 2016 proposed consensus MRI criteria for MS diagnosis were met by two high-risk women and one low-risk woman. Radiological isolated syndrome occurred in one woman of each group, and there was a single foci of leptominengeal enhancement in three high-risk women and one low-risk woman.
The findings were limited by several factors including the small size, lack of male participants, cross-sectional nature of the study, and the fact that vibration sensitivity thresholds were in the normal range for high-risk and low-risk individuals. However, the researchers wrote that they “plan to confirm the finding of change in vibration sensitivity with a follow-up study,” and they noted that the “study highlights the important need to develop and test more sensitive measures, particularly with biometric devices, to detect subtle subclinical changes early in the disease process.”
The National Institutes of Health and the National Multiple Sclerosis Society supported the study. Some of the authors reported receiving awards from the National Multiple Sclerosis Society, the American Academy of Neurology, and the National Institute of Neurological Disorders and Stroke.
The GEMS study represents the most ambitious effort yet to identify presymptomatic individuals who are at increased risk for MS, and it is a valuable first step toward targeted screening. Even if we cannot yet intervene therapeutically using currently available disease-modifying treatments in presymptomatic stages of MS, the ability to better define high-risk individuals is likely to make active surveillance programs more cost effective. It also provides important information to counsel individuals about lifestyle changes, such as quitting smoking.
The GERS also can likely be further refined with more up-to-date data on the interaction between specific genetic and environmental factors.
Fredrik Piehl, MD, PhD, is a member of the department of neurology at the Karolinska Institutet and Karolinska University Hospital, Stockholm. His comments are derived from an editorial accompanying the report by Dr. Xia and colleagues (JAMA Neurol. 2017 Jan 17. doi: 10.1001/jamaneurol.2016.5126). He disclosed research support, travel grants, and other relationships with Biogen, Genzyme, Novartis, Merck, Roche, Serono, and Teva.
The GEMS study represents the most ambitious effort yet to identify presymptomatic individuals who are at increased risk for MS, and it is a valuable first step toward targeted screening. Even if we cannot yet intervene therapeutically using currently available disease-modifying treatments in presymptomatic stages of MS, the ability to better define high-risk individuals is likely to make active surveillance programs more cost effective. It also provides important information to counsel individuals about lifestyle changes, such as quitting smoking.
The GERS also can likely be further refined with more up-to-date data on the interaction between specific genetic and environmental factors.
Fredrik Piehl, MD, PhD, is a member of the department of neurology at the Karolinska Institutet and Karolinska University Hospital, Stockholm. His comments are derived from an editorial accompanying the report by Dr. Xia and colleagues (JAMA Neurol. 2017 Jan 17. doi: 10.1001/jamaneurol.2016.5126). He disclosed research support, travel grants, and other relationships with Biogen, Genzyme, Novartis, Merck, Roche, Serono, and Teva.
The GEMS study represents the most ambitious effort yet to identify presymptomatic individuals who are at increased risk for MS, and it is a valuable first step toward targeted screening. Even if we cannot yet intervene therapeutically using currently available disease-modifying treatments in presymptomatic stages of MS, the ability to better define high-risk individuals is likely to make active surveillance programs more cost effective. It also provides important information to counsel individuals about lifestyle changes, such as quitting smoking.
The GERS also can likely be further refined with more up-to-date data on the interaction between specific genetic and environmental factors.
Fredrik Piehl, MD, PhD, is a member of the department of neurology at the Karolinska Institutet and Karolinska University Hospital, Stockholm. His comments are derived from an editorial accompanying the report by Dr. Xia and colleagues (JAMA Neurol. 2017 Jan 17. doi: 10.1001/jamaneurol.2016.5126). He disclosed research support, travel grants, and other relationships with Biogen, Genzyme, Novartis, Merck, Roche, Serono, and Teva.
Asymptomatic first-degree relatives of multiple sclerosis patients at high risk for developing the disease were significantly more likely to show subclinical signs of MS than were family members at lower risk, in the Genes and Environment in Multiple Sclerosis prospective cohort study. The findings were published online on Jan. 17 in JAMA Neurology.
The Genes and Environment in Multiple Sclerosis (GEMS) project is the first prospective study of populations at risk for MS and is the first detailed cross-sectional examination of higher-risk and lower-risk family members to date, according to investigators led by Zongqi Xia, MD, PhD, of Brigham and Women’s Hospital, Boston. Although the totality of evidence put together through neuroimaging and numerous clinical tests in the study indicate that individuals with the highest risk for MS have higher risk for the disease than do those with the lowest risk, simple vibration threshold testing gave the best results, Dr. Xia and his colleagues reported.
The study involved 100 neurologically asymptomatic adults aged 18-50 years who were first-degree relatives of people with MS who participated in the GEMS project during August 2012 to July 2015. These 100 comprised 41 high-risk participants from the top 10% of a Genetic and Environmental Risk Score (GERS) and 59 low-risk participants from the bottom 10% on the GERS. The GERS included genetic risk factors (HLA alleles and several MS-associated non-HLA genetic variants) and environmental factors, such as smoking status, body mass index, history of infectious mononucleosis and migraine, and vitamin D levels.
However, because 40 of the 41 high-risk individuals were female and 25 of the 59 low-risk individuals were female, the investigators limited the study to the 65 female participants to avoid “attributing any potential difference primarily to the role of sex.”
To help in identifying early signs of MS, the researchers used brain MRI and optical coherence tomography and other measures of neurological function, including the Expanded Disability Status Scale, timed 25-foot walk, Nine-Hole Peg Test, Paced Auditory Serial Addition Test, Symbol Digit Modalities Test, Timed Up and Go, and high-contrast and low-contrast visual acuity (JAMA Neurol. 2017 Jan 17. doi: 10.1001/jamaneurol.2016.5056).
Overall, high-risk women showed more subclinical signs of MS than did low-risk women based on an omnibus test that globally assessed the burden of neurological dysfunction by comparing the overall differences between the two groups when considering all of the measured outcomes (P = .01). However, impaired vibration perception yielded a stronger result; of 47 women (27 high-risk and 20 low-risk) tested in this manner, high-risk women showed significantly reduced vibration perception in the distal lower extremities (P = .008).
One individual in the high-risk group converted to clinically definite MS during the study. Four of the high-risk women had T2-weighted hyperintense lesions that met the 2010 McDonald MRI criteria for dissemination in space, compared with one low-risk woman. The 2016 proposed consensus MRI criteria for MS diagnosis were met by two high-risk women and one low-risk woman. Radiological isolated syndrome occurred in one woman of each group, and there was a single foci of leptominengeal enhancement in three high-risk women and one low-risk woman.
The findings were limited by several factors including the small size, lack of male participants, cross-sectional nature of the study, and the fact that vibration sensitivity thresholds were in the normal range for high-risk and low-risk individuals. However, the researchers wrote that they “plan to confirm the finding of change in vibration sensitivity with a follow-up study,” and they noted that the “study highlights the important need to develop and test more sensitive measures, particularly with biometric devices, to detect subtle subclinical changes early in the disease process.”
The National Institutes of Health and the National Multiple Sclerosis Society supported the study. Some of the authors reported receiving awards from the National Multiple Sclerosis Society, the American Academy of Neurology, and the National Institute of Neurological Disorders and Stroke.
Asymptomatic first-degree relatives of multiple sclerosis patients at high risk for developing the disease were significantly more likely to show subclinical signs of MS than were family members at lower risk, in the Genes and Environment in Multiple Sclerosis prospective cohort study. The findings were published online on Jan. 17 in JAMA Neurology.
The Genes and Environment in Multiple Sclerosis (GEMS) project is the first prospective study of populations at risk for MS and is the first detailed cross-sectional examination of higher-risk and lower-risk family members to date, according to investigators led by Zongqi Xia, MD, PhD, of Brigham and Women’s Hospital, Boston. Although the totality of evidence put together through neuroimaging and numerous clinical tests in the study indicate that individuals with the highest risk for MS have higher risk for the disease than do those with the lowest risk, simple vibration threshold testing gave the best results, Dr. Xia and his colleagues reported.
The study involved 100 neurologically asymptomatic adults aged 18-50 years who were first-degree relatives of people with MS who participated in the GEMS project during August 2012 to July 2015. These 100 comprised 41 high-risk participants from the top 10% of a Genetic and Environmental Risk Score (GERS) and 59 low-risk participants from the bottom 10% on the GERS. The GERS included genetic risk factors (HLA alleles and several MS-associated non-HLA genetic variants) and environmental factors, such as smoking status, body mass index, history of infectious mononucleosis and migraine, and vitamin D levels.
However, because 40 of the 41 high-risk individuals were female and 25 of the 59 low-risk individuals were female, the investigators limited the study to the 65 female participants to avoid “attributing any potential difference primarily to the role of sex.”
To help in identifying early signs of MS, the researchers used brain MRI and optical coherence tomography and other measures of neurological function, including the Expanded Disability Status Scale, timed 25-foot walk, Nine-Hole Peg Test, Paced Auditory Serial Addition Test, Symbol Digit Modalities Test, Timed Up and Go, and high-contrast and low-contrast visual acuity (JAMA Neurol. 2017 Jan 17. doi: 10.1001/jamaneurol.2016.5056).
Overall, high-risk women showed more subclinical signs of MS than did low-risk women based on an omnibus test that globally assessed the burden of neurological dysfunction by comparing the overall differences between the two groups when considering all of the measured outcomes (P = .01). However, impaired vibration perception yielded a stronger result; of 47 women (27 high-risk and 20 low-risk) tested in this manner, high-risk women showed significantly reduced vibration perception in the distal lower extremities (P = .008).
One individual in the high-risk group converted to clinically definite MS during the study. Four of the high-risk women had T2-weighted hyperintense lesions that met the 2010 McDonald MRI criteria for dissemination in space, compared with one low-risk woman. The 2016 proposed consensus MRI criteria for MS diagnosis were met by two high-risk women and one low-risk woman. Radiological isolated syndrome occurred in one woman of each group, and there was a single foci of leptominengeal enhancement in three high-risk women and one low-risk woman.
The findings were limited by several factors including the small size, lack of male participants, cross-sectional nature of the study, and the fact that vibration sensitivity thresholds were in the normal range for high-risk and low-risk individuals. However, the researchers wrote that they “plan to confirm the finding of change in vibration sensitivity with a follow-up study,” and they noted that the “study highlights the important need to develop and test more sensitive measures, particularly with biometric devices, to detect subtle subclinical changes early in the disease process.”
The National Institutes of Health and the National Multiple Sclerosis Society supported the study. Some of the authors reported receiving awards from the National Multiple Sclerosis Society, the American Academy of Neurology, and the National Institute of Neurological Disorders and Stroke.
FROM JAMA NEUROLOGY
Key clinical point: Higher-risk asymptomatic female family relatives of patients with MS are more likely to have early subclinical manifestations of the disease and deserve further monitoring.
Major finding: Women at high risk for MS scored significantly higher on a composite of measured outcomes (P = .01) and on a vibration sensitivity test (P = .008), compared with lower-risk women.
Data source: A prospective, cross-sectional, cohort study of 65 adult women at risk for MS.
Disclosures: The National Institutes of Health and the National Multiple Sclerosis Society supported the study. Some of the authors reported receiving awards from the National Multiple Sclerosis Society, the American Academy of Neurology, and the National Institute of Neurological Disorders and Stroke.
January Hot Threads in ACS Communities
Want to know what your colleagues are talking about? Here are the top discussion threads in ACS Communities (all from the General Surgery community):
1. A lap appy ain’t always easy ...
3. Privileging
4. Malpractice moles
5. Safe laparoscopic entry
6. Is PID a general surgery problem?
7. Family debriefing after surgery
8. Peer-to-peer review
9. What should I do?
10. CT colonography
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].
Want to know what your colleagues are talking about? Here are the top discussion threads in ACS Communities (all from the General Surgery community):
1. A lap appy ain’t always easy ...
3. Privileging
4. Malpractice moles
5. Safe laparoscopic entry
6. Is PID a general surgery problem?
7. Family debriefing after surgery
8. Peer-to-peer review
9. What should I do?
10. CT colonography
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].
Want to know what your colleagues are talking about? Here are the top discussion threads in ACS Communities (all from the General Surgery community):
1. A lap appy ain’t always easy ...
3. Privileging
4. Malpractice moles
5. Safe laparoscopic entry
6. Is PID a general surgery problem?
7. Family debriefing after surgery
8. Peer-to-peer review
9. What should I do?
10. CT colonography
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].