User login
Uterine fibroids registry looks to provide comparative treatment data
Early findings from a uterine fibroids registry suggest more than half of enrolled women chose an alternative to hysterectomy, underscoring the need to study the efficacy of these uterine-sparing treatment options, said Elizabeth A. Stewart, MD, of the Mayo Clinic, Rochester, Minn., and her associates.
There is very little evidence on the comparative efficacy of uterine fibroid treatment options so the COMPARE-UF (Comparing Options for Management: Patient-Centered Results for Uterine Fibroids) registry was initiated for women who choose procedural therapy for symptomatic uterine fibroids. Data at the nine clinical centers – representing rural and urban populations – are being collected regarding hysterectomy, myomectomy (abdominal, hysteroscopic, vaginal, and laparoscopic/robotic), endometrial ablation, radiofrequency fibroid ablation, uterine artery embolization, magnetic resonance–guided focused ultrasound, and therapeutic use of progestin-releasing intrauterine devices.
A total of 16% of the women were under age 35, and 40% were aged 40 years or younger. “The fact that a sizable proportion of women are under age 40 suggests that with long-term follow-up, data on reproductive outcomes can be obtained as these women seek pregnancy,” Dr. Stewart and her associates noted.
Although African American women make up only 13% of the U.S. population, they constitute 42% of enrollment in COMPARE-UF, the investigators reported in the American Journal of Obstetrics and Gynecology. African American women chose similar or greater numbers of each type of myomectomy and uterine embolization as white women chose.
The study was supported by a grant from the Agency for Healthcare Research and Quality. The registry is supported by AHRQ and the Patient-Centered Outcomes Research Institute. Dr. Stewart reported personal fees from AbbVie, Allergan, Astellas, Pharma, Bayer, Gynesonics, and Myovant Sciences. Several researchers reported grants from pharmaceutical companies outside this study, and the remainder of the investigators reported no relevant financial disclosures.
SOURCE: Stewart EA et al. Am J Obstet Gynecol. 2018 May 8. doi: 10.1016/j.ajog.2018.05.004.
Early findings from a uterine fibroids registry suggest more than half of enrolled women chose an alternative to hysterectomy, underscoring the need to study the efficacy of these uterine-sparing treatment options, said Elizabeth A. Stewart, MD, of the Mayo Clinic, Rochester, Minn., and her associates.
There is very little evidence on the comparative efficacy of uterine fibroid treatment options so the COMPARE-UF (Comparing Options for Management: Patient-Centered Results for Uterine Fibroids) registry was initiated for women who choose procedural therapy for symptomatic uterine fibroids. Data at the nine clinical centers – representing rural and urban populations – are being collected regarding hysterectomy, myomectomy (abdominal, hysteroscopic, vaginal, and laparoscopic/robotic), endometrial ablation, radiofrequency fibroid ablation, uterine artery embolization, magnetic resonance–guided focused ultrasound, and therapeutic use of progestin-releasing intrauterine devices.
A total of 16% of the women were under age 35, and 40% were aged 40 years or younger. “The fact that a sizable proportion of women are under age 40 suggests that with long-term follow-up, data on reproductive outcomes can be obtained as these women seek pregnancy,” Dr. Stewart and her associates noted.
Although African American women make up only 13% of the U.S. population, they constitute 42% of enrollment in COMPARE-UF, the investigators reported in the American Journal of Obstetrics and Gynecology. African American women chose similar or greater numbers of each type of myomectomy and uterine embolization as white women chose.
The study was supported by a grant from the Agency for Healthcare Research and Quality. The registry is supported by AHRQ and the Patient-Centered Outcomes Research Institute. Dr. Stewart reported personal fees from AbbVie, Allergan, Astellas, Pharma, Bayer, Gynesonics, and Myovant Sciences. Several researchers reported grants from pharmaceutical companies outside this study, and the remainder of the investigators reported no relevant financial disclosures.
SOURCE: Stewart EA et al. Am J Obstet Gynecol. 2018 May 8. doi: 10.1016/j.ajog.2018.05.004.
Early findings from a uterine fibroids registry suggest more than half of enrolled women chose an alternative to hysterectomy, underscoring the need to study the efficacy of these uterine-sparing treatment options, said Elizabeth A. Stewart, MD, of the Mayo Clinic, Rochester, Minn., and her associates.
There is very little evidence on the comparative efficacy of uterine fibroid treatment options so the COMPARE-UF (Comparing Options for Management: Patient-Centered Results for Uterine Fibroids) registry was initiated for women who choose procedural therapy for symptomatic uterine fibroids. Data at the nine clinical centers – representing rural and urban populations – are being collected regarding hysterectomy, myomectomy (abdominal, hysteroscopic, vaginal, and laparoscopic/robotic), endometrial ablation, radiofrequency fibroid ablation, uterine artery embolization, magnetic resonance–guided focused ultrasound, and therapeutic use of progestin-releasing intrauterine devices.
A total of 16% of the women were under age 35, and 40% were aged 40 years or younger. “The fact that a sizable proportion of women are under age 40 suggests that with long-term follow-up, data on reproductive outcomes can be obtained as these women seek pregnancy,” Dr. Stewart and her associates noted.
Although African American women make up only 13% of the U.S. population, they constitute 42% of enrollment in COMPARE-UF, the investigators reported in the American Journal of Obstetrics and Gynecology. African American women chose similar or greater numbers of each type of myomectomy and uterine embolization as white women chose.
The study was supported by a grant from the Agency for Healthcare Research and Quality. The registry is supported by AHRQ and the Patient-Centered Outcomes Research Institute. Dr. Stewart reported personal fees from AbbVie, Allergan, Astellas, Pharma, Bayer, Gynesonics, and Myovant Sciences. Several researchers reported grants from pharmaceutical companies outside this study, and the remainder of the investigators reported no relevant financial disclosures.
SOURCE: Stewart EA et al. Am J Obstet Gynecol. 2018 May 8. doi: 10.1016/j.ajog.2018.05.004.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Key clinical point:
Major finding: Of the initial 2,031 women enrolled in the registry, hysterectomy was chosen by 38%, and myomectomies were chosen by 46%.
Study details: Initial results from the COMPARE-UF registry.
Disclosures: The study was supported by a grant from the Agency for Healthcare Research and Quality. Dr. Stewart reported personal fees from AbbVie, Allergan, Astellas, Pharma, Bayer, Gynesonics, and Myovant Sciences. Several researchers reported grants from pharmaceutical companies outside this study, and the remainder of the investigators reported no relevant financial disclosures.
Source: Stewart EA et al. Am J Obstet Gynecol. 2018 May 8. doi: 10.1016/j.ajog.2018.05.004.
Dermatologic complaints prolong hospital stay for hematologic cancer
and are associated with an increased mean length of hospital stay, according to a large retrospective chart review from a major cancer center.
The substantial burden imposed by dermatologic complications in patients with cancer, particularly a hematologic malignancy, highlights the importance of greater collaboration between oncology and dermatology services to mitigate the impact of these events on both quality of life and outcome, wrote Gregory S. Phillips of Memorial Sloan Kettering Cancer Center, New York, and his associates. The study was published in the Journal of the American Academy of Dermatology.
The data that produced these conclusions were drawn from a retrospective chart review of 11,533 cancer patients treated at the center during 2015. Of these, 412 (3.6%) were referred for a dermatology consultation.
Those who received a dermatology consultation were comparable for median age (60 years) and gender (roughly 50:50 male:female), compared with those who did not. However, the odds ratio (OR) for a dermatology consultation was 6.56 among those with a hematologic malignancy compared with those who had a solid tumor. In those with leukemia, the proportion receiving a dermatologic consult was nearly ninefold greater.
Whether or not undertaken in a patient with a hematologic malignancy, dermatologic consults correlated with significantly greater morbidity, as well as mortality. This included a longer median length of stay (11 vs. 5 days; P less than .0001) and a higher in-hospital rate of death (9% vs. 2%; P less than .0001), compared with patients not needing a dermatology consultation.
Of dermatologic consultations in the total study population, the most common were for inflammatory conditions (27%), infections (24%), and drug reactions (17%). Neoplasm was the dermatologic diagnosis in 10% of the total population, but in 13% of those with hematologic malignancies.
Inpatient dermatology consultations were most frequently ordered by the hematology-oncology service, accounting for 44% of the total, followed by the solid tumor oncology service (27%) and the surgery service (15%). Multiple consultations were more likely in patients with leukemia or lymphoma than other forms of cancer.
In the dermatology consult, biopsy was employed for diagnosis in only 18%. As for treatment, 42% received topical therapy alone, and 38% received a systemic therapy. Dermatologic consultations that subsequently involved consultation with another service such as allergy and immunology, were rare, occurring in only 4% of cases.
Furthermore, they suggested that the data support increased attention to dermatologic complications in cancer. Although the impact of consultations on outcome was not evaluated in this study, the authors cited another recent study in which there was a more than 2-day reduction in hospital stay when a dermatology consultation was employed in noncancer patients with an inflammatory skin disease (JAMA Dermatol. 2017 Jun 1;153[6]:523-8). Moreover, they speculated that a prompt resolution of dermatologic complaints in cancer patients has implications for better outcomes if they result in fewer delays in anti-cancer therapy.
The study was partly funded by the a grant from the National Cancer Institute’s Cancer Centers Program. Dr. Lacouture reported financial relationships with AstraZeneca, Adgero Biopharmaceuticals, Berg, Bristol-Myers Squibb, Foamix, Janssen, Legacy Healthcare, NovoCure, and Quintiles. Another author reported ties with Amgen, Roche, Eaisi, and P Value Communications.
SOURCE: Phillips GS et al. J Am Acad Dermatol. 2018 Jun;78(6):1102-9.
and are associated with an increased mean length of hospital stay, according to a large retrospective chart review from a major cancer center.
The substantial burden imposed by dermatologic complications in patients with cancer, particularly a hematologic malignancy, highlights the importance of greater collaboration between oncology and dermatology services to mitigate the impact of these events on both quality of life and outcome, wrote Gregory S. Phillips of Memorial Sloan Kettering Cancer Center, New York, and his associates. The study was published in the Journal of the American Academy of Dermatology.
The data that produced these conclusions were drawn from a retrospective chart review of 11,533 cancer patients treated at the center during 2015. Of these, 412 (3.6%) were referred for a dermatology consultation.
Those who received a dermatology consultation were comparable for median age (60 years) and gender (roughly 50:50 male:female), compared with those who did not. However, the odds ratio (OR) for a dermatology consultation was 6.56 among those with a hematologic malignancy compared with those who had a solid tumor. In those with leukemia, the proportion receiving a dermatologic consult was nearly ninefold greater.
Whether or not undertaken in a patient with a hematologic malignancy, dermatologic consults correlated with significantly greater morbidity, as well as mortality. This included a longer median length of stay (11 vs. 5 days; P less than .0001) and a higher in-hospital rate of death (9% vs. 2%; P less than .0001), compared with patients not needing a dermatology consultation.
Of dermatologic consultations in the total study population, the most common were for inflammatory conditions (27%), infections (24%), and drug reactions (17%). Neoplasm was the dermatologic diagnosis in 10% of the total population, but in 13% of those with hematologic malignancies.
Inpatient dermatology consultations were most frequently ordered by the hematology-oncology service, accounting for 44% of the total, followed by the solid tumor oncology service (27%) and the surgery service (15%). Multiple consultations were more likely in patients with leukemia or lymphoma than other forms of cancer.
In the dermatology consult, biopsy was employed for diagnosis in only 18%. As for treatment, 42% received topical therapy alone, and 38% received a systemic therapy. Dermatologic consultations that subsequently involved consultation with another service such as allergy and immunology, were rare, occurring in only 4% of cases.
Furthermore, they suggested that the data support increased attention to dermatologic complications in cancer. Although the impact of consultations on outcome was not evaluated in this study, the authors cited another recent study in which there was a more than 2-day reduction in hospital stay when a dermatology consultation was employed in noncancer patients with an inflammatory skin disease (JAMA Dermatol. 2017 Jun 1;153[6]:523-8). Moreover, they speculated that a prompt resolution of dermatologic complaints in cancer patients has implications for better outcomes if they result in fewer delays in anti-cancer therapy.
The study was partly funded by the a grant from the National Cancer Institute’s Cancer Centers Program. Dr. Lacouture reported financial relationships with AstraZeneca, Adgero Biopharmaceuticals, Berg, Bristol-Myers Squibb, Foamix, Janssen, Legacy Healthcare, NovoCure, and Quintiles. Another author reported ties with Amgen, Roche, Eaisi, and P Value Communications.
SOURCE: Phillips GS et al. J Am Acad Dermatol. 2018 Jun;78(6):1102-9.
and are associated with an increased mean length of hospital stay, according to a large retrospective chart review from a major cancer center.
The substantial burden imposed by dermatologic complications in patients with cancer, particularly a hematologic malignancy, highlights the importance of greater collaboration between oncology and dermatology services to mitigate the impact of these events on both quality of life and outcome, wrote Gregory S. Phillips of Memorial Sloan Kettering Cancer Center, New York, and his associates. The study was published in the Journal of the American Academy of Dermatology.
The data that produced these conclusions were drawn from a retrospective chart review of 11,533 cancer patients treated at the center during 2015. Of these, 412 (3.6%) were referred for a dermatology consultation.
Those who received a dermatology consultation were comparable for median age (60 years) and gender (roughly 50:50 male:female), compared with those who did not. However, the odds ratio (OR) for a dermatology consultation was 6.56 among those with a hematologic malignancy compared with those who had a solid tumor. In those with leukemia, the proportion receiving a dermatologic consult was nearly ninefold greater.
Whether or not undertaken in a patient with a hematologic malignancy, dermatologic consults correlated with significantly greater morbidity, as well as mortality. This included a longer median length of stay (11 vs. 5 days; P less than .0001) and a higher in-hospital rate of death (9% vs. 2%; P less than .0001), compared with patients not needing a dermatology consultation.
Of dermatologic consultations in the total study population, the most common were for inflammatory conditions (27%), infections (24%), and drug reactions (17%). Neoplasm was the dermatologic diagnosis in 10% of the total population, but in 13% of those with hematologic malignancies.
Inpatient dermatology consultations were most frequently ordered by the hematology-oncology service, accounting for 44% of the total, followed by the solid tumor oncology service (27%) and the surgery service (15%). Multiple consultations were more likely in patients with leukemia or lymphoma than other forms of cancer.
In the dermatology consult, biopsy was employed for diagnosis in only 18%. As for treatment, 42% received topical therapy alone, and 38% received a systemic therapy. Dermatologic consultations that subsequently involved consultation with another service such as allergy and immunology, were rare, occurring in only 4% of cases.
Furthermore, they suggested that the data support increased attention to dermatologic complications in cancer. Although the impact of consultations on outcome was not evaluated in this study, the authors cited another recent study in which there was a more than 2-day reduction in hospital stay when a dermatology consultation was employed in noncancer patients with an inflammatory skin disease (JAMA Dermatol. 2017 Jun 1;153[6]:523-8). Moreover, they speculated that a prompt resolution of dermatologic complaints in cancer patients has implications for better outcomes if they result in fewer delays in anti-cancer therapy.
The study was partly funded by the a grant from the National Cancer Institute’s Cancer Centers Program. Dr. Lacouture reported financial relationships with AstraZeneca, Adgero Biopharmaceuticals, Berg, Bristol-Myers Squibb, Foamix, Janssen, Legacy Healthcare, NovoCure, and Quintiles. Another author reported ties with Amgen, Roche, Eaisi, and P Value Communications.
SOURCE: Phillips GS et al. J Am Acad Dermatol. 2018 Jun;78(6):1102-9.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Dermatologic complications are more common in hematologic than solid tumor cancers and correlate with adverse outcomes.
Major finding: In cancer patients, dermatologic consults are associated with longer hospital stay (11 vs. 5 days) and death (9% vs. 2%).
Study details: Retrospective chart review of inpatient dermatology consultations during 2015.
Disclosures: The study was partly funded by the a grant from the National Cancer Institute’s Cancer Centers Program. Dr. Lacouture reported financial relationships with AstraZeneca, Adgero Biopharmaceuticals, Berg, Bristol-Myers Squibb, Foamix, Janssen, Legacy Healthcare, NovoCure, and Quintiles. Another author reported ties with Amgen, Roche, Eaisi, and P Value Communications.
Source: Phillips et al. J Am Acad Dermatol. 2018;78:1102-9.
MDedge Psychcast: Episode 107
Dr. Worley uses a nautical metaphor to describe the phenomenon and possible ways to combat it.
Dr. Worley uses a nautical metaphor to describe the phenomenon and possible ways to combat it.
Dr. Worley uses a nautical metaphor to describe the phenomenon and possible ways to combat it.
An MRI Analysis of the Pelvis to Determine the Ideal Method for Ultrasound-Guided Bone Marrow Aspiration from the Iliac Crest
ABSTRACT
Use of mesenchymal stem cells from bone marrow has gained significant popularity. The iliac crest has been determined to be an effective site for harvesting mesenchymal stem cells. Review of the literature reveals that multiple techniques are used to harvest bone marrow aspirate from the iliac crest, but the descriptions are based on the experience of various authors as opposed to studied anatomy. A safe, reliable, and reproducible method for aspiration has yet to be studied and described. We hypothesized that there would be an ideal angle and distance for aspiration that would be the safest, most consistent, and most reliable. Using magnetic resonance imaging (MRI), we reviewed 26 total lumbar spine MRI scans (13 males, 13 females) and found that an angle of 24° should be used when entering the most medial aspect of the posterior superior iliac spine (PSIS) and that this angle did not differ between the sexes. The distance that the trocar can advance after entry before hitting the anterior ilium wall varied significantly between males and females, being 7.53 cm in males and 6.74 cm in females. In addition, the size of the PSIS table was significantly different between males and females (1.20 cm and 0.96 cm, respectively). No other significant differences in the measurements gathered were found. Using the data gleaned from this study, we developed an aspiration technique. This method uses ultrasound to determine the location of the PSIS and the entry point on the PSIS. This contrasts with most techniques that use landmark palpation, which is known to be unreliable and inaccurate. The described technique for aspiration from the PSIS is safe, reliable, reproducible, and substantiated by data.
The iliac crest is an effective site for harvesting bone marrow stem cells. It allows for easy access and is superficial in most individuals, allowing for a relatively quick and simple procedure. Use of mesenchymal stem cells (MSCs) for treatment of orthopedic injuries has grown recently. Whereas overall use has increased, review of the literature reveals very few techniques for iliac crest aspiration,1 but these are not based on anatomic relationships or studies. Hernigou and colleagues2,3 attempted to quantitatively evaluate potential “sectors” allowing for safe aspiration using cadaver and computed tomographic reconstruction imaging. We used magnetic resonance imaging (MRI) to analyze aspiration parameters. Owing to the ilium’s anatomy, improper positioning or aspiration technique during aspiration can result in serious injury.2,4-6 We hypothesized that there is an ideal angle and positioning for bone marrow aspiration from the posterior superior iliac spine (PSIS) that is safe, consistent, and reproducible. Although most aspiration techniques use landmark palpation, this is unreliable and inaccurate, especially when compared with ultrasound-guided injections7-16 and procedures.9,12,17-19 We describe our technique using ultrasound to visualize patient anatomy and accurately determine anatomic entry with the trocar.
METHODS
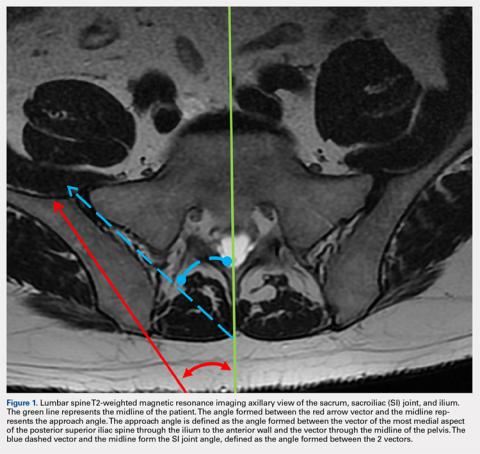
MRI scans of 26 patients (13 males, 13 females) were reviewed to determine average angles and distances. Axial T2-weighted views of the lumbar spine were used in all analyses. The sacroiliac (SI) joint angle was defined as the angle formed between the vector through the midline of the pelvis and the vector that is parallel to the SI joint. The approach angle was defined as the angle formed between the vector of the most medial aspect of the PSIS through the ilium to the anterior wall and the vector through the midline of the pelvis (Figure 1).
Continue to: For the 13 males, the mean SI joint...
RESULTS
The results are reported in the Table.
Table. Measurements of Patients Taken on Axial T2-Weighted Views of Lumbosacral MRI Scansa
Patient | SI Joint Angle (°) | Approach Angle (°) | PSIS Table Width (cm) | PSIS to Anterior Ilium Wall (cm) | Perpendicular Distance PSIS to Anterior Joint (cm) | Post Ilium Wall to SI Joint Width (cm) |
Males | ||||||
1 | 28.80 | 19.50 | 1.24 | 8.80 | 4.16 | 1.52 |
2 | 31.80 | 27.60 | 1.70 | 7.89 | 3.49 | 1.02 |
3 | 33.70 | 27.70 | 1.12 | 8.14 | 3.15 | 1.28 |
4 | 23.70 | 26.40 | 0.95 | 6.66 | 3.22 | 0.65 |
5 | 35.90 | 28.40 | 0.84 | 7.60 | 2.57 | 0.95 |
6 | 33.80 | 29.30 | 1.20 | 7.73 | 2.34 | 0.90 |
7 | 30.30 | 21.20 | 1.36 | 8.44 | 3.95 | 1.18 |
8 | 34.50 | 20.40 | 1.53 | 7.08 | 3.98 | 1.56 |
9 | 28.70 | 24.00 | 1.34 | 8.19 | 3.51 | 1.31 |
10 | 22.40 | 20.10 | 1.37 | 7.30 | 3.87 | 1.28 |
11 | 33.60 | 20.80 | 0.88 | 6.43 | 3.26 | 0.94 |
12 | 48.50 | 31.00 | 1.15 | 6.69 | 2.97 | 1.38 |
13 | 20.20 | 20.90 | 0.94 | 6.95 | 3.79 | 1.05 |
Averages | 31.22 | 24.41 | 1.20 | 7.53 | 3.40 | 1.16 |
Standard Deviation | 7.18 | 4.11 | 0.26 | 0.75 | 0.56 | 0.26 |
Females | ||||||
14 | 22.80 | 23.20 | 1.54 | 7.21 | 3.45 | 1.39 |
15 | 33.30 | 21.40 | 1.09 | 7.26 | 3.57 | 0.98 |
16 | 19.70 | 15.60 | 0.78 | 8.32 | 3.76 | 0.86 |
17 | 17.50 | 15.60 | 0.61 | 7.57 | 3.37 | 1.03 |
18 | 48.20 | 26.60 | 0.94 | 6.62 | 3.16 | 0.71 |
19 | 38.20 | 28.30 | 0.90 | 6.32 | 2.23 | 0.91 |
20 | 44.50 | 31.70 | 0.99 | 6.19 | 3.06 | 0.76 |
21 | 24.10 | 18.00 | 0.92 | 6.99 | 3.23 | 0.71 |
22 | 17.20 | 14.80 | 0.81 | 6.00 | 2.81 | 1.13 |
23 | 42.00 | 38.50 | 1.00 | 5.33 | 2.47 | 1.42 |
24 | 32.00 | 25.50 | 0.98 | 6.01 | 2.79 | 1.21 |
25 | 24.70 | 24.80 | 0.87 | 6.09 | 2.79 | 1.02 |
26 | 19.80 | 22.30 | 1.04 | 7.71 | 2.37 | 1.36 |
Averages | 29.54 | 23.56 | 0.96 | 6.74 | 3.00 | 1.04 |
Standard Deviation | 10.84 | 6.88 | 0.21 | 0.85 | 0.48 | 0.25 |
All patients Averages | 30.38 | 23.98 | 1.08 | 7.14 | 3.20 | 1.10 |
Standard Deviation | 9.05 | 5.57 | 0.26 | 0.88 | 0.55 | 0.26 |
aStatistical significance is denoted as P < .02.
Abbreviations: MRI, magnetic resonance imaging; PSIS, posterior iliac spine; SI, sacroiliac.
For the 13 males, the mean SI joint angle was 31.22° ± 7.18° (range, 20.20° to 48.50°). The mean approach angle was 24.41° ± 4.11° (range, 19.50° to 31.00°). The mean PSIS table width was 1.20 cm ± 0.26 cm (range, 0.84 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.53 cm ± 0.75 cm (range, 6.43 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.40 cm ± 0.56 cm (range, 2.34 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.16 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
For the 13 females, the mean SI joint angle was 29.54° ± 10.84° (range, 17.20° to 48.20°). The mean approach angle was 23.56° ± 6.88° (range, 14.80° to 38.50°). The mean PSIS table width was 0.96 cm ± 0.21 cm (range, 0.61 cm to 1.54 cm). The mean distance from the PSIS to the anterior ilium wall was 6.74 cm ± 0.85 cm (range, 5.33 cm to 8.32 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.00 cm ± 0.48 cm (range, 2.23 cm to 3.76 cm). The mean minimum width of the ilium to the SI joint was 1.04 cm ± 0.25 cm (range, 0.71 cm to 1.42 cm).
For the 26 total patients, the mean SI joint angle was 30.38° ± 9.05° (range, 17.20° to 48.50°). The mean approach angle was 23.98° ± 5.57° (range, 14.80° to 38.50°). The mean PSIS table width was 1.08 cm ± 0.26 cm (range, 0.61 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.14 cm ± 0.88 cm (range, 5.33 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.20 cm ± 0.55 cm (range, 2.23 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.10 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
There was a statistically significant difference between the male and female groups for the maximum distance the trocar can be advanced from the PSIS to the anterior ilium wall (P < .02), and a statistically significant difference for the PSIS table width (P < .02). There were no significant differences between the male and female groups for the approach angle, the SI joint angle, the perpendicular distance from the PSIS to the anterior ilium, and the minimum width of the ilium to the SI joint.
Continue to: The patient is brought to the procedure...
TECHNIQUE: ILIAC CREST (PSIS) BONE MARROW ASPIRATION
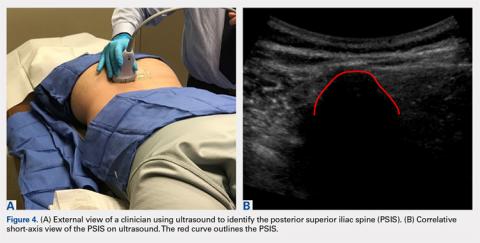
The patient is brought to the procedure room and placed in a prone position. The donor site is prepared and draped in the usual sterile manner. Ultrasound is used to identify the median sacral crest in a short-axis view. The probe is then moved laterally to identify the PSIS (Figures 4A, 4B).
The crosshairs on the ultrasound probe are used to mark the center lines of each plane. The central point marks the location of the PSIS. Alternatively, an in-plane technique can be used to place a spinal needle on the exact entry point on the PSIS. Once the PSIS and entry point are identified, the site is blocked with 10 mL of 0.5% ropivacaine.
Prior to introduction of the trocar, all instrumentation is primed with heparin and syringes are prepped with anticoagulant citrate dextrose solution, solution A. A stab incision is made at the site. The trocar is placed at the entry point, which should be centered in a superior-inferior plane and at the most medial point of the PSIS. Starting with the trocar vertical, the trocar is angled laterally 24° by dropping the hand medially toward the midline. No angulation cephalad or caudad is necessary, but cephalad must be avoided so as not to skive superiorly. This angle, which is recommended for both males and females, allows for the greatest distance the trocar can travel in bone before hitting the anterior ilium wall. A standard deviation of 5.57° is present, which should be considered. Steady pressure should be applied with a slight twisting motion on the PSIS. If advancement of the trocar is too difficult, a mallet or drill can be used to assist in penetration.
With the trocar advanced into the bone 1 cm, the trocar needle is removed while the cannula remains in place. The syringe is attached to the top of the cannula. The syringe plunger is pulled back to aspirate 20 mL of bone marrow. The cannula and syringe assembly are advanced 2 cm farther into the bone to allow for aspiration of a new location within the bone marrow cavity, and 20 mL of bone marrow are again aspirated. This is done a final time, advancing the trocar another 2 cm and aspirating a final 20 mL of bone marrow. The entire process should yield roughly 60 mL of bone marrow from one side. If desired, the same process can be repeated for the contralateral PSIS to yield a total of 120 mL of bone marrow from the 2 sites.
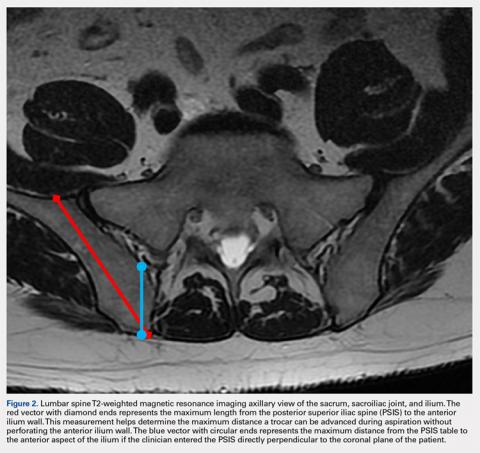
Based on our data, the average distance to the anterior ilium wall was 7 cm, but the shortest distance noted in this study was 5 cm. On the basis of the data presented, this technique allows for safe advancement based on even the shortest measured distance, without fear of puncturing the anterior ilium wall. Perforation could damage the femoral nerve and the internal or external iliac artery or vein that lie anterior to the ilium.
Continue to: We hypothesized that there...
DISCUSSION
We hypothesized that there would be an optimal angle of entry and maximal safe distance the trocar could advance through the ilium when aspirating. Because male and female pelvic anatomy differs, we also hypothesized that there would be differences in distance and size measurements for males and females. Our results supported our hypothesis that there is an ideal approach angle. The results also showed that the maximum distance the trocar can advance and the width of the PSIS table differ significantly between males and females.
Although pelvic anatomy differs between males and females, there should be an ideal entry angle that would allow maximum advancement into the ilium without perforating the anterior wall, which we defined as the approach angle. In our comparison of 26 MRI scans, we found that the approach angle did not differ significantly between the 2 groups (13 males, 13 females). This allows clinicians to enter the PSIS at roughly 24° medial to the parasagittal line, maximizing the space before puncturing into the anterior pelvis in either males or females.
If clinicians were to enter perpendicular to the patient’s PSIS, they would, on average, be able to advance only 3.20 cm before encountering the SI joint. When entering at 24° as we recommend, the average distance increases to 7.14 cm. Although the angle did not differ significantly, there was a significant difference between males and females in the length from the PSIS to the anterior wall, with males having 7.53 cm distance and females 6.74 cm. This is an important measurement because if the anterior ilium wall is punctured, the femoral nerve and the common, internal and external iliac arteries and veins could be damaged, resulting in retroperitoneal hemorrhage.
A fatality in 2001 in the United Kingdom led to a national audit of bone marrow aspiration and biopsies.4-6 Although these procedures were done primarily for patients with cancer, hemorrhagic events were the most frequent and serious events. This audit led to the identification of many risk factors. Bain4-6 conducted reviews of bone marrow aspirations and biopsies in the United Kingdom from 2002 to 2004. Of a total of 53,088 procedures conducted during that time frame, 48 (0.09%) adverse events occurred, with 29 (0.05%) being hemorrhagic events. Although infrequent, hemorrhagic adverse events represent significant morbidity. Reviews such as those conducted by Bain4-6 highlight the importance of a study that helps determine the optimal parameters for aspiration to ensure safety and reliability.
Hernigou and colleagues2,3 conducted studies analyzing different “sectors” in an attempt to develop a safe aspiration technique. They found that obese patients were at higher risk, and some sites of aspiration (sectors 1, 4, 5) had increased risk for perforation and damage to surrounding structures. Their sector 6, which incorporated the entirety of the PSIS table, was considered the safest, most reliable site for trocar introduction.2,3 Hernigou and colleagues,2 in comparing the bone mass of the sectors, also noted that sector 6 has the greatest bone thickness close to the entry point, making it the most favorable site. The PSIS is not just a point; it is more a “table.” The PSIS can be palpated posteriorly, but this is inaccurate and unreliable, particularly in larger individuals. The PSIS table can be identified on ultrasound before introducing the trocar, which is a more reliable method of landmark identification than palpation guidance, just as in ultrasound-guided injections7-16 and procedures.9,12,17-19
Continue to: If the PSIS is not accurately...
If the PSIS is not accurately identified, penetration laterally will result in entering the ilium wing, where it is quite narrow. We found the distance between the posterior ilium wall and the SI joint to be only 1.10 cm wide (Figure 3); we defined this area as the narrow corridor. Superior and lateral entry could damage the superior cluneal nerves coming over the iliac crest, which are located 6 cm lateral to the SI joint. Inferior and lateral entry 6 cm below the PSIS could reach the greater sciatic foramen, damaging the sacral plexus and superior gluteal artery and vein. If the entry slips above the PSIS over the pelvis, the trocar could enter the retroperitoneal space and damage the femoral nerve and common iliac artery and vein, leading to a retroperitoneal hemorrhage.4-6,20
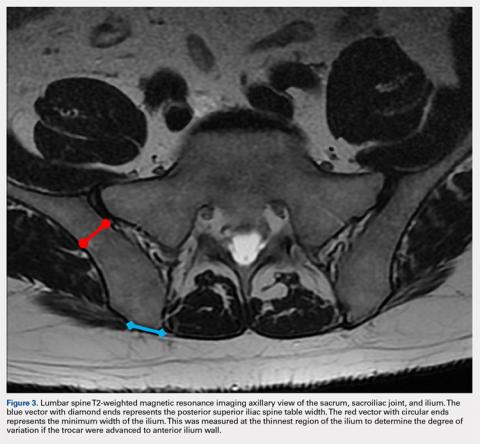
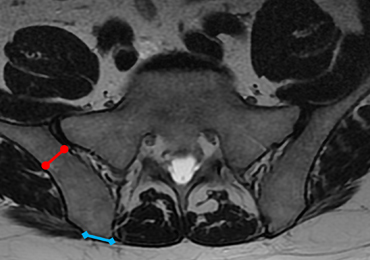
MSCs are found as perivascular cells and lie in the cortices of bones.21 Following the approach angle and directed line from the PSIS to the anterior ilium wall described in this study (Figures 1 and 2), the trocar would pass through the narrow corridor as it advances farther into the ilium. The minimum width of this corridor was measured in this study and, on average, was 1.10 cm wide from cortex to cortex (Figure 3). As the bone marrow is aspirated from this narrow corridor, the clinician is gathering MSCs from both the lateral and medial cortices of the ilium. By aspirating from a greater surface area of the cortices, it is believed that this will increase the total collection of MSCs.
CONCLUSION
Although there are reports in the literature that describe techniques for bone marrow aspiration from the iliac crest, the techniques are very general and vague regarding the ideal angles and methods. Studies have attempted to quantify the safest entry sites for aspiration but have not detailed ideal parameters for collection. Blind aspiration from the iliac crest can have serious implications if adverse events occur, and thus there is a need for a safe and reliable method of aspiration from the iliac crest. Ultrasound guidance to identify anatomy, as opposed to palpation guidance, ensures anatomic placement of the trocar while minimizing the risk of aspiration. Based on the measurements gathered in this study, an optimal angle of entry and safe distance of penetration have been identified. Using our data and relevant literature, we developed a technique for a safe, consistent, and reliable method of bone marrow aspiration out of the iliac crest.
1. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6(2):e441-e445. doi:10.1016/j.eats.2016.10.024.
2. Hernigou J, Alves A, Homma Y, Guissou I, Hernigou P. Anatomy of the ilium for bone marrow aspiration: map of sectors and implication for safe trocar placement. Int Orthop. 2014;38(12):2585-2590. doi:10.1007/s00264-014-2353-7.
3. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384. doi:10.1007/s00264-014-2343-9.
4. Bain BJ. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol. 2005;58(4):406-408. doi:10.1136/jcp.2004.022178.
5. Bain BJ. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol. 2004;26(5):315-318. doi:10.1111/j.1365-2257.2004.00630.x.
6. Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica. 2006;91(9):1293-1294.
7. Berkoff DJ, Miller LE, Block JE. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin Interv Aging. 2012;7:89-95. doi:10.2147/CIA.S29265.
8. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282. doi:10.1016/j.arthro.2005.12.019.
9. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
10. Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84-A(9):1522-1527.
11. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
12. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
13. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
14. Sibbit WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36(9):1892-1902. doi:10.3899/jrheum.090013.
15. Smith J, Brault JS, Rizzo M, Sayeed YA, Finnoff JT. Accuracy of sonographically guided and palpation guided scaphotrapeziotrapezoid joint injections. J Ultrasound Med. 2011;30(11):1509-1515. doi:10.7863/jum.2011.30.11.1509.
16. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
17. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
18. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
19. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Submitted.
20. Jamaludin WFW, Mukari SAM, Wahid SFA. Retroperitoneal hemorrhage associated with bone marrow trephine biopsy. Am J Case Rep. 2013;14:489-493. doi:10.12659/AJCR.889274.
21. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35-42. doi:10.1038/nm.3028.
ABSTRACT
Use of mesenchymal stem cells from bone marrow has gained significant popularity. The iliac crest has been determined to be an effective site for harvesting mesenchymal stem cells. Review of the literature reveals that multiple techniques are used to harvest bone marrow aspirate from the iliac crest, but the descriptions are based on the experience of various authors as opposed to studied anatomy. A safe, reliable, and reproducible method for aspiration has yet to be studied and described. We hypothesized that there would be an ideal angle and distance for aspiration that would be the safest, most consistent, and most reliable. Using magnetic resonance imaging (MRI), we reviewed 26 total lumbar spine MRI scans (13 males, 13 females) and found that an angle of 24° should be used when entering the most medial aspect of the posterior superior iliac spine (PSIS) and that this angle did not differ between the sexes. The distance that the trocar can advance after entry before hitting the anterior ilium wall varied significantly between males and females, being 7.53 cm in males and 6.74 cm in females. In addition, the size of the PSIS table was significantly different between males and females (1.20 cm and 0.96 cm, respectively). No other significant differences in the measurements gathered were found. Using the data gleaned from this study, we developed an aspiration technique. This method uses ultrasound to determine the location of the PSIS and the entry point on the PSIS. This contrasts with most techniques that use landmark palpation, which is known to be unreliable and inaccurate. The described technique for aspiration from the PSIS is safe, reliable, reproducible, and substantiated by data.
The iliac crest is an effective site for harvesting bone marrow stem cells. It allows for easy access and is superficial in most individuals, allowing for a relatively quick and simple procedure. Use of mesenchymal stem cells (MSCs) for treatment of orthopedic injuries has grown recently. Whereas overall use has increased, review of the literature reveals very few techniques for iliac crest aspiration,1 but these are not based on anatomic relationships or studies. Hernigou and colleagues2,3 attempted to quantitatively evaluate potential “sectors” allowing for safe aspiration using cadaver and computed tomographic reconstruction imaging. We used magnetic resonance imaging (MRI) to analyze aspiration parameters. Owing to the ilium’s anatomy, improper positioning or aspiration technique during aspiration can result in serious injury.2,4-6 We hypothesized that there is an ideal angle and positioning for bone marrow aspiration from the posterior superior iliac spine (PSIS) that is safe, consistent, and reproducible. Although most aspiration techniques use landmark palpation, this is unreliable and inaccurate, especially when compared with ultrasound-guided injections7-16 and procedures.9,12,17-19 We describe our technique using ultrasound to visualize patient anatomy and accurately determine anatomic entry with the trocar.
METHODS
MRI scans of 26 patients (13 males, 13 females) were reviewed to determine average angles and distances. Axial T2-weighted views of the lumbar spine were used in all analyses. The sacroiliac (SI) joint angle was defined as the angle formed between the vector through the midline of the pelvis and the vector that is parallel to the SI joint. The approach angle was defined as the angle formed between the vector of the most medial aspect of the PSIS through the ilium to the anterior wall and the vector through the midline of the pelvis (Figure 1).
Continue to: For the 13 males, the mean SI joint...
RESULTS
The results are reported in the Table.
Table. Measurements of Patients Taken on Axial T2-Weighted Views of Lumbosacral MRI Scansa
Patient | SI Joint Angle (°) | Approach Angle (°) | PSIS Table Width (cm) | PSIS to Anterior Ilium Wall (cm) | Perpendicular Distance PSIS to Anterior Joint (cm) | Post Ilium Wall to SI Joint Width (cm) |
Males | ||||||
1 | 28.80 | 19.50 | 1.24 | 8.80 | 4.16 | 1.52 |
2 | 31.80 | 27.60 | 1.70 | 7.89 | 3.49 | 1.02 |
3 | 33.70 | 27.70 | 1.12 | 8.14 | 3.15 | 1.28 |
4 | 23.70 | 26.40 | 0.95 | 6.66 | 3.22 | 0.65 |
5 | 35.90 | 28.40 | 0.84 | 7.60 | 2.57 | 0.95 |
6 | 33.80 | 29.30 | 1.20 | 7.73 | 2.34 | 0.90 |
7 | 30.30 | 21.20 | 1.36 | 8.44 | 3.95 | 1.18 |
8 | 34.50 | 20.40 | 1.53 | 7.08 | 3.98 | 1.56 |
9 | 28.70 | 24.00 | 1.34 | 8.19 | 3.51 | 1.31 |
10 | 22.40 | 20.10 | 1.37 | 7.30 | 3.87 | 1.28 |
11 | 33.60 | 20.80 | 0.88 | 6.43 | 3.26 | 0.94 |
12 | 48.50 | 31.00 | 1.15 | 6.69 | 2.97 | 1.38 |
13 | 20.20 | 20.90 | 0.94 | 6.95 | 3.79 | 1.05 |
Averages | 31.22 | 24.41 | 1.20 | 7.53 | 3.40 | 1.16 |
Standard Deviation | 7.18 | 4.11 | 0.26 | 0.75 | 0.56 | 0.26 |
Females | ||||||
14 | 22.80 | 23.20 | 1.54 | 7.21 | 3.45 | 1.39 |
15 | 33.30 | 21.40 | 1.09 | 7.26 | 3.57 | 0.98 |
16 | 19.70 | 15.60 | 0.78 | 8.32 | 3.76 | 0.86 |
17 | 17.50 | 15.60 | 0.61 | 7.57 | 3.37 | 1.03 |
18 | 48.20 | 26.60 | 0.94 | 6.62 | 3.16 | 0.71 |
19 | 38.20 | 28.30 | 0.90 | 6.32 | 2.23 | 0.91 |
20 | 44.50 | 31.70 | 0.99 | 6.19 | 3.06 | 0.76 |
21 | 24.10 | 18.00 | 0.92 | 6.99 | 3.23 | 0.71 |
22 | 17.20 | 14.80 | 0.81 | 6.00 | 2.81 | 1.13 |
23 | 42.00 | 38.50 | 1.00 | 5.33 | 2.47 | 1.42 |
24 | 32.00 | 25.50 | 0.98 | 6.01 | 2.79 | 1.21 |
25 | 24.70 | 24.80 | 0.87 | 6.09 | 2.79 | 1.02 |
26 | 19.80 | 22.30 | 1.04 | 7.71 | 2.37 | 1.36 |
Averages | 29.54 | 23.56 | 0.96 | 6.74 | 3.00 | 1.04 |
Standard Deviation | 10.84 | 6.88 | 0.21 | 0.85 | 0.48 | 0.25 |
All patients Averages | 30.38 | 23.98 | 1.08 | 7.14 | 3.20 | 1.10 |
Standard Deviation | 9.05 | 5.57 | 0.26 | 0.88 | 0.55 | 0.26 |
aStatistical significance is denoted as P < .02.
Abbreviations: MRI, magnetic resonance imaging; PSIS, posterior iliac spine; SI, sacroiliac.
For the 13 males, the mean SI joint angle was 31.22° ± 7.18° (range, 20.20° to 48.50°). The mean approach angle was 24.41° ± 4.11° (range, 19.50° to 31.00°). The mean PSIS table width was 1.20 cm ± 0.26 cm (range, 0.84 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.53 cm ± 0.75 cm (range, 6.43 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.40 cm ± 0.56 cm (range, 2.34 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.16 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
For the 13 females, the mean SI joint angle was 29.54° ± 10.84° (range, 17.20° to 48.20°). The mean approach angle was 23.56° ± 6.88° (range, 14.80° to 38.50°). The mean PSIS table width was 0.96 cm ± 0.21 cm (range, 0.61 cm to 1.54 cm). The mean distance from the PSIS to the anterior ilium wall was 6.74 cm ± 0.85 cm (range, 5.33 cm to 8.32 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.00 cm ± 0.48 cm (range, 2.23 cm to 3.76 cm). The mean minimum width of the ilium to the SI joint was 1.04 cm ± 0.25 cm (range, 0.71 cm to 1.42 cm).
For the 26 total patients, the mean SI joint angle was 30.38° ± 9.05° (range, 17.20° to 48.50°). The mean approach angle was 23.98° ± 5.57° (range, 14.80° to 38.50°). The mean PSIS table width was 1.08 cm ± 0.26 cm (range, 0.61 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.14 cm ± 0.88 cm (range, 5.33 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.20 cm ± 0.55 cm (range, 2.23 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.10 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
There was a statistically significant difference between the male and female groups for the maximum distance the trocar can be advanced from the PSIS to the anterior ilium wall (P < .02), and a statistically significant difference for the PSIS table width (P < .02). There were no significant differences between the male and female groups for the approach angle, the SI joint angle, the perpendicular distance from the PSIS to the anterior ilium, and the minimum width of the ilium to the SI joint.
Continue to: The patient is brought to the procedure...
TECHNIQUE: ILIAC CREST (PSIS) BONE MARROW ASPIRATION
The patient is brought to the procedure room and placed in a prone position. The donor site is prepared and draped in the usual sterile manner. Ultrasound is used to identify the median sacral crest in a short-axis view. The probe is then moved laterally to identify the PSIS (Figures 4A, 4B).
The crosshairs on the ultrasound probe are used to mark the center lines of each plane. The central point marks the location of the PSIS. Alternatively, an in-plane technique can be used to place a spinal needle on the exact entry point on the PSIS. Once the PSIS and entry point are identified, the site is blocked with 10 mL of 0.5% ropivacaine.
Prior to introduction of the trocar, all instrumentation is primed with heparin and syringes are prepped with anticoagulant citrate dextrose solution, solution A. A stab incision is made at the site. The trocar is placed at the entry point, which should be centered in a superior-inferior plane and at the most medial point of the PSIS. Starting with the trocar vertical, the trocar is angled laterally 24° by dropping the hand medially toward the midline. No angulation cephalad or caudad is necessary, but cephalad must be avoided so as not to skive superiorly. This angle, which is recommended for both males and females, allows for the greatest distance the trocar can travel in bone before hitting the anterior ilium wall. A standard deviation of 5.57° is present, which should be considered. Steady pressure should be applied with a slight twisting motion on the PSIS. If advancement of the trocar is too difficult, a mallet or drill can be used to assist in penetration.
With the trocar advanced into the bone 1 cm, the trocar needle is removed while the cannula remains in place. The syringe is attached to the top of the cannula. The syringe plunger is pulled back to aspirate 20 mL of bone marrow. The cannula and syringe assembly are advanced 2 cm farther into the bone to allow for aspiration of a new location within the bone marrow cavity, and 20 mL of bone marrow are again aspirated. This is done a final time, advancing the trocar another 2 cm and aspirating a final 20 mL of bone marrow. The entire process should yield roughly 60 mL of bone marrow from one side. If desired, the same process can be repeated for the contralateral PSIS to yield a total of 120 mL of bone marrow from the 2 sites.
Based on our data, the average distance to the anterior ilium wall was 7 cm, but the shortest distance noted in this study was 5 cm. On the basis of the data presented, this technique allows for safe advancement based on even the shortest measured distance, without fear of puncturing the anterior ilium wall. Perforation could damage the femoral nerve and the internal or external iliac artery or vein that lie anterior to the ilium.
Continue to: We hypothesized that there...
DISCUSSION
We hypothesized that there would be an optimal angle of entry and maximal safe distance the trocar could advance through the ilium when aspirating. Because male and female pelvic anatomy differs, we also hypothesized that there would be differences in distance and size measurements for males and females. Our results supported our hypothesis that there is an ideal approach angle. The results also showed that the maximum distance the trocar can advance and the width of the PSIS table differ significantly between males and females.
Although pelvic anatomy differs between males and females, there should be an ideal entry angle that would allow maximum advancement into the ilium without perforating the anterior wall, which we defined as the approach angle. In our comparison of 26 MRI scans, we found that the approach angle did not differ significantly between the 2 groups (13 males, 13 females). This allows clinicians to enter the PSIS at roughly 24° medial to the parasagittal line, maximizing the space before puncturing into the anterior pelvis in either males or females.
If clinicians were to enter perpendicular to the patient’s PSIS, they would, on average, be able to advance only 3.20 cm before encountering the SI joint. When entering at 24° as we recommend, the average distance increases to 7.14 cm. Although the angle did not differ significantly, there was a significant difference between males and females in the length from the PSIS to the anterior wall, with males having 7.53 cm distance and females 6.74 cm. This is an important measurement because if the anterior ilium wall is punctured, the femoral nerve and the common, internal and external iliac arteries and veins could be damaged, resulting in retroperitoneal hemorrhage.
A fatality in 2001 in the United Kingdom led to a national audit of bone marrow aspiration and biopsies.4-6 Although these procedures were done primarily for patients with cancer, hemorrhagic events were the most frequent and serious events. This audit led to the identification of many risk factors. Bain4-6 conducted reviews of bone marrow aspirations and biopsies in the United Kingdom from 2002 to 2004. Of a total of 53,088 procedures conducted during that time frame, 48 (0.09%) adverse events occurred, with 29 (0.05%) being hemorrhagic events. Although infrequent, hemorrhagic adverse events represent significant morbidity. Reviews such as those conducted by Bain4-6 highlight the importance of a study that helps determine the optimal parameters for aspiration to ensure safety and reliability.
Hernigou and colleagues2,3 conducted studies analyzing different “sectors” in an attempt to develop a safe aspiration technique. They found that obese patients were at higher risk, and some sites of aspiration (sectors 1, 4, 5) had increased risk for perforation and damage to surrounding structures. Their sector 6, which incorporated the entirety of the PSIS table, was considered the safest, most reliable site for trocar introduction.2,3 Hernigou and colleagues,2 in comparing the bone mass of the sectors, also noted that sector 6 has the greatest bone thickness close to the entry point, making it the most favorable site. The PSIS is not just a point; it is more a “table.” The PSIS can be palpated posteriorly, but this is inaccurate and unreliable, particularly in larger individuals. The PSIS table can be identified on ultrasound before introducing the trocar, which is a more reliable method of landmark identification than palpation guidance, just as in ultrasound-guided injections7-16 and procedures.9,12,17-19
Continue to: If the PSIS is not accurately...
If the PSIS is not accurately identified, penetration laterally will result in entering the ilium wing, where it is quite narrow. We found the distance between the posterior ilium wall and the SI joint to be only 1.10 cm wide (Figure 3); we defined this area as the narrow corridor. Superior and lateral entry could damage the superior cluneal nerves coming over the iliac crest, which are located 6 cm lateral to the SI joint. Inferior and lateral entry 6 cm below the PSIS could reach the greater sciatic foramen, damaging the sacral plexus and superior gluteal artery and vein. If the entry slips above the PSIS over the pelvis, the trocar could enter the retroperitoneal space and damage the femoral nerve and common iliac artery and vein, leading to a retroperitoneal hemorrhage.4-6,20
MSCs are found as perivascular cells and lie in the cortices of bones.21 Following the approach angle and directed line from the PSIS to the anterior ilium wall described in this study (Figures 1 and 2), the trocar would pass through the narrow corridor as it advances farther into the ilium. The minimum width of this corridor was measured in this study and, on average, was 1.10 cm wide from cortex to cortex (Figure 3). As the bone marrow is aspirated from this narrow corridor, the clinician is gathering MSCs from both the lateral and medial cortices of the ilium. By aspirating from a greater surface area of the cortices, it is believed that this will increase the total collection of MSCs.
CONCLUSION
Although there are reports in the literature that describe techniques for bone marrow aspiration from the iliac crest, the techniques are very general and vague regarding the ideal angles and methods. Studies have attempted to quantify the safest entry sites for aspiration but have not detailed ideal parameters for collection. Blind aspiration from the iliac crest can have serious implications if adverse events occur, and thus there is a need for a safe and reliable method of aspiration from the iliac crest. Ultrasound guidance to identify anatomy, as opposed to palpation guidance, ensures anatomic placement of the trocar while minimizing the risk of aspiration. Based on the measurements gathered in this study, an optimal angle of entry and safe distance of penetration have been identified. Using our data and relevant literature, we developed a technique for a safe, consistent, and reliable method of bone marrow aspiration out of the iliac crest.
ABSTRACT
Use of mesenchymal stem cells from bone marrow has gained significant popularity. The iliac crest has been determined to be an effective site for harvesting mesenchymal stem cells. Review of the literature reveals that multiple techniques are used to harvest bone marrow aspirate from the iliac crest, but the descriptions are based on the experience of various authors as opposed to studied anatomy. A safe, reliable, and reproducible method for aspiration has yet to be studied and described. We hypothesized that there would be an ideal angle and distance for aspiration that would be the safest, most consistent, and most reliable. Using magnetic resonance imaging (MRI), we reviewed 26 total lumbar spine MRI scans (13 males, 13 females) and found that an angle of 24° should be used when entering the most medial aspect of the posterior superior iliac spine (PSIS) and that this angle did not differ between the sexes. The distance that the trocar can advance after entry before hitting the anterior ilium wall varied significantly between males and females, being 7.53 cm in males and 6.74 cm in females. In addition, the size of the PSIS table was significantly different between males and females (1.20 cm and 0.96 cm, respectively). No other significant differences in the measurements gathered were found. Using the data gleaned from this study, we developed an aspiration technique. This method uses ultrasound to determine the location of the PSIS and the entry point on the PSIS. This contrasts with most techniques that use landmark palpation, which is known to be unreliable and inaccurate. The described technique for aspiration from the PSIS is safe, reliable, reproducible, and substantiated by data.
The iliac crest is an effective site for harvesting bone marrow stem cells. It allows for easy access and is superficial in most individuals, allowing for a relatively quick and simple procedure. Use of mesenchymal stem cells (MSCs) for treatment of orthopedic injuries has grown recently. Whereas overall use has increased, review of the literature reveals very few techniques for iliac crest aspiration,1 but these are not based on anatomic relationships or studies. Hernigou and colleagues2,3 attempted to quantitatively evaluate potential “sectors” allowing for safe aspiration using cadaver and computed tomographic reconstruction imaging. We used magnetic resonance imaging (MRI) to analyze aspiration parameters. Owing to the ilium’s anatomy, improper positioning or aspiration technique during aspiration can result in serious injury.2,4-6 We hypothesized that there is an ideal angle and positioning for bone marrow aspiration from the posterior superior iliac spine (PSIS) that is safe, consistent, and reproducible. Although most aspiration techniques use landmark palpation, this is unreliable and inaccurate, especially when compared with ultrasound-guided injections7-16 and procedures.9,12,17-19 We describe our technique using ultrasound to visualize patient anatomy and accurately determine anatomic entry with the trocar.
METHODS
MRI scans of 26 patients (13 males, 13 females) were reviewed to determine average angles and distances. Axial T2-weighted views of the lumbar spine were used in all analyses. The sacroiliac (SI) joint angle was defined as the angle formed between the vector through the midline of the pelvis and the vector that is parallel to the SI joint. The approach angle was defined as the angle formed between the vector of the most medial aspect of the PSIS through the ilium to the anterior wall and the vector through the midline of the pelvis (Figure 1).
Continue to: For the 13 males, the mean SI joint...
RESULTS
The results are reported in the Table.
Table. Measurements of Patients Taken on Axial T2-Weighted Views of Lumbosacral MRI Scansa
Patient | SI Joint Angle (°) | Approach Angle (°) | PSIS Table Width (cm) | PSIS to Anterior Ilium Wall (cm) | Perpendicular Distance PSIS to Anterior Joint (cm) | Post Ilium Wall to SI Joint Width (cm) |
Males | ||||||
1 | 28.80 | 19.50 | 1.24 | 8.80 | 4.16 | 1.52 |
2 | 31.80 | 27.60 | 1.70 | 7.89 | 3.49 | 1.02 |
3 | 33.70 | 27.70 | 1.12 | 8.14 | 3.15 | 1.28 |
4 | 23.70 | 26.40 | 0.95 | 6.66 | 3.22 | 0.65 |
5 | 35.90 | 28.40 | 0.84 | 7.60 | 2.57 | 0.95 |
6 | 33.80 | 29.30 | 1.20 | 7.73 | 2.34 | 0.90 |
7 | 30.30 | 21.20 | 1.36 | 8.44 | 3.95 | 1.18 |
8 | 34.50 | 20.40 | 1.53 | 7.08 | 3.98 | 1.56 |
9 | 28.70 | 24.00 | 1.34 | 8.19 | 3.51 | 1.31 |
10 | 22.40 | 20.10 | 1.37 | 7.30 | 3.87 | 1.28 |
11 | 33.60 | 20.80 | 0.88 | 6.43 | 3.26 | 0.94 |
12 | 48.50 | 31.00 | 1.15 | 6.69 | 2.97 | 1.38 |
13 | 20.20 | 20.90 | 0.94 | 6.95 | 3.79 | 1.05 |
Averages | 31.22 | 24.41 | 1.20 | 7.53 | 3.40 | 1.16 |
Standard Deviation | 7.18 | 4.11 | 0.26 | 0.75 | 0.56 | 0.26 |
Females | ||||||
14 | 22.80 | 23.20 | 1.54 | 7.21 | 3.45 | 1.39 |
15 | 33.30 | 21.40 | 1.09 | 7.26 | 3.57 | 0.98 |
16 | 19.70 | 15.60 | 0.78 | 8.32 | 3.76 | 0.86 |
17 | 17.50 | 15.60 | 0.61 | 7.57 | 3.37 | 1.03 |
18 | 48.20 | 26.60 | 0.94 | 6.62 | 3.16 | 0.71 |
19 | 38.20 | 28.30 | 0.90 | 6.32 | 2.23 | 0.91 |
20 | 44.50 | 31.70 | 0.99 | 6.19 | 3.06 | 0.76 |
21 | 24.10 | 18.00 | 0.92 | 6.99 | 3.23 | 0.71 |
22 | 17.20 | 14.80 | 0.81 | 6.00 | 2.81 | 1.13 |
23 | 42.00 | 38.50 | 1.00 | 5.33 | 2.47 | 1.42 |
24 | 32.00 | 25.50 | 0.98 | 6.01 | 2.79 | 1.21 |
25 | 24.70 | 24.80 | 0.87 | 6.09 | 2.79 | 1.02 |
26 | 19.80 | 22.30 | 1.04 | 7.71 | 2.37 | 1.36 |
Averages | 29.54 | 23.56 | 0.96 | 6.74 | 3.00 | 1.04 |
Standard Deviation | 10.84 | 6.88 | 0.21 | 0.85 | 0.48 | 0.25 |
All patients Averages | 30.38 | 23.98 | 1.08 | 7.14 | 3.20 | 1.10 |
Standard Deviation | 9.05 | 5.57 | 0.26 | 0.88 | 0.55 | 0.26 |
aStatistical significance is denoted as P < .02.
Abbreviations: MRI, magnetic resonance imaging; PSIS, posterior iliac spine; SI, sacroiliac.
For the 13 males, the mean SI joint angle was 31.22° ± 7.18° (range, 20.20° to 48.50°). The mean approach angle was 24.41° ± 4.11° (range, 19.50° to 31.00°). The mean PSIS table width was 1.20 cm ± 0.26 cm (range, 0.84 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.53 cm ± 0.75 cm (range, 6.43 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.40 cm ± 0.56 cm (range, 2.34 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.16 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
For the 13 females, the mean SI joint angle was 29.54° ± 10.84° (range, 17.20° to 48.20°). The mean approach angle was 23.56° ± 6.88° (range, 14.80° to 38.50°). The mean PSIS table width was 0.96 cm ± 0.21 cm (range, 0.61 cm to 1.54 cm). The mean distance from the PSIS to the anterior ilium wall was 6.74 cm ± 0.85 cm (range, 5.33 cm to 8.32 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.00 cm ± 0.48 cm (range, 2.23 cm to 3.76 cm). The mean minimum width of the ilium to the SI joint was 1.04 cm ± 0.25 cm (range, 0.71 cm to 1.42 cm).
For the 26 total patients, the mean SI joint angle was 30.38° ± 9.05° (range, 17.20° to 48.50°). The mean approach angle was 23.98° ± 5.57° (range, 14.80° to 38.50°). The mean PSIS table width was 1.08 cm ± 0.26 cm (range, 0.61 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.14 cm ± 0.88 cm (range, 5.33 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.20 cm ± 0.55 cm (range, 2.23 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.10 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
There was a statistically significant difference between the male and female groups for the maximum distance the trocar can be advanced from the PSIS to the anterior ilium wall (P < .02), and a statistically significant difference for the PSIS table width (P < .02). There were no significant differences between the male and female groups for the approach angle, the SI joint angle, the perpendicular distance from the PSIS to the anterior ilium, and the minimum width of the ilium to the SI joint.
Continue to: The patient is brought to the procedure...
TECHNIQUE: ILIAC CREST (PSIS) BONE MARROW ASPIRATION
The patient is brought to the procedure room and placed in a prone position. The donor site is prepared and draped in the usual sterile manner. Ultrasound is used to identify the median sacral crest in a short-axis view. The probe is then moved laterally to identify the PSIS (Figures 4A, 4B).
The crosshairs on the ultrasound probe are used to mark the center lines of each plane. The central point marks the location of the PSIS. Alternatively, an in-plane technique can be used to place a spinal needle on the exact entry point on the PSIS. Once the PSIS and entry point are identified, the site is blocked with 10 mL of 0.5% ropivacaine.
Prior to introduction of the trocar, all instrumentation is primed with heparin and syringes are prepped with anticoagulant citrate dextrose solution, solution A. A stab incision is made at the site. The trocar is placed at the entry point, which should be centered in a superior-inferior plane and at the most medial point of the PSIS. Starting with the trocar vertical, the trocar is angled laterally 24° by dropping the hand medially toward the midline. No angulation cephalad or caudad is necessary, but cephalad must be avoided so as not to skive superiorly. This angle, which is recommended for both males and females, allows for the greatest distance the trocar can travel in bone before hitting the anterior ilium wall. A standard deviation of 5.57° is present, which should be considered. Steady pressure should be applied with a slight twisting motion on the PSIS. If advancement of the trocar is too difficult, a mallet or drill can be used to assist in penetration.
With the trocar advanced into the bone 1 cm, the trocar needle is removed while the cannula remains in place. The syringe is attached to the top of the cannula. The syringe plunger is pulled back to aspirate 20 mL of bone marrow. The cannula and syringe assembly are advanced 2 cm farther into the bone to allow for aspiration of a new location within the bone marrow cavity, and 20 mL of bone marrow are again aspirated. This is done a final time, advancing the trocar another 2 cm and aspirating a final 20 mL of bone marrow. The entire process should yield roughly 60 mL of bone marrow from one side. If desired, the same process can be repeated for the contralateral PSIS to yield a total of 120 mL of bone marrow from the 2 sites.
Based on our data, the average distance to the anterior ilium wall was 7 cm, but the shortest distance noted in this study was 5 cm. On the basis of the data presented, this technique allows for safe advancement based on even the shortest measured distance, without fear of puncturing the anterior ilium wall. Perforation could damage the femoral nerve and the internal or external iliac artery or vein that lie anterior to the ilium.
Continue to: We hypothesized that there...
DISCUSSION
We hypothesized that there would be an optimal angle of entry and maximal safe distance the trocar could advance through the ilium when aspirating. Because male and female pelvic anatomy differs, we also hypothesized that there would be differences in distance and size measurements for males and females. Our results supported our hypothesis that there is an ideal approach angle. The results also showed that the maximum distance the trocar can advance and the width of the PSIS table differ significantly between males and females.
Although pelvic anatomy differs between males and females, there should be an ideal entry angle that would allow maximum advancement into the ilium without perforating the anterior wall, which we defined as the approach angle. In our comparison of 26 MRI scans, we found that the approach angle did not differ significantly between the 2 groups (13 males, 13 females). This allows clinicians to enter the PSIS at roughly 24° medial to the parasagittal line, maximizing the space before puncturing into the anterior pelvis in either males or females.
If clinicians were to enter perpendicular to the patient’s PSIS, they would, on average, be able to advance only 3.20 cm before encountering the SI joint. When entering at 24° as we recommend, the average distance increases to 7.14 cm. Although the angle did not differ significantly, there was a significant difference between males and females in the length from the PSIS to the anterior wall, with males having 7.53 cm distance and females 6.74 cm. This is an important measurement because if the anterior ilium wall is punctured, the femoral nerve and the common, internal and external iliac arteries and veins could be damaged, resulting in retroperitoneal hemorrhage.
A fatality in 2001 in the United Kingdom led to a national audit of bone marrow aspiration and biopsies.4-6 Although these procedures were done primarily for patients with cancer, hemorrhagic events were the most frequent and serious events. This audit led to the identification of many risk factors. Bain4-6 conducted reviews of bone marrow aspirations and biopsies in the United Kingdom from 2002 to 2004. Of a total of 53,088 procedures conducted during that time frame, 48 (0.09%) adverse events occurred, with 29 (0.05%) being hemorrhagic events. Although infrequent, hemorrhagic adverse events represent significant morbidity. Reviews such as those conducted by Bain4-6 highlight the importance of a study that helps determine the optimal parameters for aspiration to ensure safety and reliability.
Hernigou and colleagues2,3 conducted studies analyzing different “sectors” in an attempt to develop a safe aspiration technique. They found that obese patients were at higher risk, and some sites of aspiration (sectors 1, 4, 5) had increased risk for perforation and damage to surrounding structures. Their sector 6, which incorporated the entirety of the PSIS table, was considered the safest, most reliable site for trocar introduction.2,3 Hernigou and colleagues,2 in comparing the bone mass of the sectors, also noted that sector 6 has the greatest bone thickness close to the entry point, making it the most favorable site. The PSIS is not just a point; it is more a “table.” The PSIS can be palpated posteriorly, but this is inaccurate and unreliable, particularly in larger individuals. The PSIS table can be identified on ultrasound before introducing the trocar, which is a more reliable method of landmark identification than palpation guidance, just as in ultrasound-guided injections7-16 and procedures.9,12,17-19
Continue to: If the PSIS is not accurately...
If the PSIS is not accurately identified, penetration laterally will result in entering the ilium wing, where it is quite narrow. We found the distance between the posterior ilium wall and the SI joint to be only 1.10 cm wide (Figure 3); we defined this area as the narrow corridor. Superior and lateral entry could damage the superior cluneal nerves coming over the iliac crest, which are located 6 cm lateral to the SI joint. Inferior and lateral entry 6 cm below the PSIS could reach the greater sciatic foramen, damaging the sacral plexus and superior gluteal artery and vein. If the entry slips above the PSIS over the pelvis, the trocar could enter the retroperitoneal space and damage the femoral nerve and common iliac artery and vein, leading to a retroperitoneal hemorrhage.4-6,20
MSCs are found as perivascular cells and lie in the cortices of bones.21 Following the approach angle and directed line from the PSIS to the anterior ilium wall described in this study (Figures 1 and 2), the trocar would pass through the narrow corridor as it advances farther into the ilium. The minimum width of this corridor was measured in this study and, on average, was 1.10 cm wide from cortex to cortex (Figure 3). As the bone marrow is aspirated from this narrow corridor, the clinician is gathering MSCs from both the lateral and medial cortices of the ilium. By aspirating from a greater surface area of the cortices, it is believed that this will increase the total collection of MSCs.
CONCLUSION
Although there are reports in the literature that describe techniques for bone marrow aspiration from the iliac crest, the techniques are very general and vague regarding the ideal angles and methods. Studies have attempted to quantify the safest entry sites for aspiration but have not detailed ideal parameters for collection. Blind aspiration from the iliac crest can have serious implications if adverse events occur, and thus there is a need for a safe and reliable method of aspiration from the iliac crest. Ultrasound guidance to identify anatomy, as opposed to palpation guidance, ensures anatomic placement of the trocar while minimizing the risk of aspiration. Based on the measurements gathered in this study, an optimal angle of entry and safe distance of penetration have been identified. Using our data and relevant literature, we developed a technique for a safe, consistent, and reliable method of bone marrow aspiration out of the iliac crest.
1. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6(2):e441-e445. doi:10.1016/j.eats.2016.10.024.
2. Hernigou J, Alves A, Homma Y, Guissou I, Hernigou P. Anatomy of the ilium for bone marrow aspiration: map of sectors and implication for safe trocar placement. Int Orthop. 2014;38(12):2585-2590. doi:10.1007/s00264-014-2353-7.
3. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384. doi:10.1007/s00264-014-2343-9.
4. Bain BJ. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol. 2005;58(4):406-408. doi:10.1136/jcp.2004.022178.
5. Bain BJ. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol. 2004;26(5):315-318. doi:10.1111/j.1365-2257.2004.00630.x.
6. Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica. 2006;91(9):1293-1294.
7. Berkoff DJ, Miller LE, Block JE. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin Interv Aging. 2012;7:89-95. doi:10.2147/CIA.S29265.
8. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282. doi:10.1016/j.arthro.2005.12.019.
9. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
10. Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84-A(9):1522-1527.
11. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
12. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
13. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
14. Sibbit WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36(9):1892-1902. doi:10.3899/jrheum.090013.
15. Smith J, Brault JS, Rizzo M, Sayeed YA, Finnoff JT. Accuracy of sonographically guided and palpation guided scaphotrapeziotrapezoid joint injections. J Ultrasound Med. 2011;30(11):1509-1515. doi:10.7863/jum.2011.30.11.1509.
16. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
17. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
18. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
19. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Submitted.
20. Jamaludin WFW, Mukari SAM, Wahid SFA. Retroperitoneal hemorrhage associated with bone marrow trephine biopsy. Am J Case Rep. 2013;14:489-493. doi:10.12659/AJCR.889274.
21. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35-42. doi:10.1038/nm.3028.
1. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6(2):e441-e445. doi:10.1016/j.eats.2016.10.024.
2. Hernigou J, Alves A, Homma Y, Guissou I, Hernigou P. Anatomy of the ilium for bone marrow aspiration: map of sectors and implication for safe trocar placement. Int Orthop. 2014;38(12):2585-2590. doi:10.1007/s00264-014-2353-7.
3. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384. doi:10.1007/s00264-014-2343-9.
4. Bain BJ. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol. 2005;58(4):406-408. doi:10.1136/jcp.2004.022178.
5. Bain BJ. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol. 2004;26(5):315-318. doi:10.1111/j.1365-2257.2004.00630.x.
6. Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica. 2006;91(9):1293-1294.
7. Berkoff DJ, Miller LE, Block JE. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin Interv Aging. 2012;7:89-95. doi:10.2147/CIA.S29265.
8. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282. doi:10.1016/j.arthro.2005.12.019.
9. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
10. Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84-A(9):1522-1527.
11. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
12. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
13. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
14. Sibbit WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36(9):1892-1902. doi:10.3899/jrheum.090013.
15. Smith J, Brault JS, Rizzo M, Sayeed YA, Finnoff JT. Accuracy of sonographically guided and palpation guided scaphotrapeziotrapezoid joint injections. J Ultrasound Med. 2011;30(11):1509-1515. doi:10.7863/jum.2011.30.11.1509.
16. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
17. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
18. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
19. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Submitted.
20. Jamaludin WFW, Mukari SAM, Wahid SFA. Retroperitoneal hemorrhage associated with bone marrow trephine biopsy. Am J Case Rep. 2013;14:489-493. doi:10.12659/AJCR.889274.
21. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35-42. doi:10.1038/nm.3028.
TAKE-HOME POINTS
- There is an ideal angle and distance for optimization of a bone marrow harvest from the iliac crest.
- Ultrasound is a reliable technology that allows clinicians to accurately and consistently identify the PSIS and avoid neurovascular structures.
- This safe, reliable bone marrow aspiration technique can lower the risk of serious potential complications.
- The ideal angle does not differ significantly between sexes, but the safe distance a clinician can advance does.
- The PSIS should be considered a “table” as opposed to a protuberance.
FP can perform the circumcision
FP can perform the circumcision
I enjoyed Dr. Lerner's brief review but was puzzled by the question of who should perform the circumcision. The most obvious and one of the most frequent answers was ignored. It should be done by the family physician who delivered the baby, is caring for him in the nursery, and will be caring for him another couple of decades.
John R. Carroll, MD
Corpus Christi, Texas
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
FP can perform the circumcision
I enjoyed Dr. Lerner's brief review but was puzzled by the question of who should perform the circumcision. The most obvious and one of the most frequent answers was ignored. It should be done by the family physician who delivered the baby, is caring for him in the nursery, and will be caring for him another couple of decades.
John R. Carroll, MD
Corpus Christi, Texas
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
FP can perform the circumcision
I enjoyed Dr. Lerner's brief review but was puzzled by the question of who should perform the circumcision. The most obvious and one of the most frequent answers was ignored. It should be done by the family physician who delivered the baby, is caring for him in the nursery, and will be caring for him another couple of decades.
John R. Carroll, MD
Corpus Christi, Texas
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Does Age of Exposure to Tackle Football Affect CTE Severity?
Younger age of exposure to tackle football is not associated with chronic traumatic encephalopathy (CTE) pathologic severity, Alzheimer’s disease pathology, or Lewy body pathology, according to data published online ahead of print April 30 in Annals of Neurology. Younger age of exposure does appear to predict earlier neurobehavioral symptom onset, however, the authors said.
“These findings suggest that exposure to repetitive head impacts from tackle football as a youth may reduce resiliency to diseases, including, but not limited to, CTE, that affect the brain in later life,” said Michael L. Alosco, PhD, Assistant Professor of Neurology at the the Boston University Alzheimer’s Disease and CTE Center. “This study adds to growing research suggesting that incurring repeated head impacts through tackle football in earlier life can lead to both short-term and long-term effects on the brain.”
Repetitive Head Impacts and Neurodevelopment
Previous research has linked younger age of first exposure to tackle football with smaller thalamic volume in former National Football League players. A recent study of 214 former and amateur football players found that age of first exposure to tackle football—before age 12, in particular—predicted increased odds of self-reported neuropsychiatric and executive impairment.
“Youth exposure to repetitive head impacts may disrupt neurodevelopment to lower the threshold for later clinical dysfunction,” said the researchers.
To examine the effect of age of first exposure to tackle football on CTE pathologic severity and age of neurobehavioral symptom onset in tackle football players with neuropathologically confirmed CTE, Dr. Alosco and colleagues analyzed a sample of 246 amateur and professional tackle football players whose brains had been donated to the Veteran’s Affairs–Boston University–Concussion Legacy Foundation Brain Bank. The researchers interviewed informants to ascertain players’ age of first exposure and age of onset of cognitive, behavioral, or mood symptoms. A total of 211 football players were diagnosed with CTE; 35 did not have CTE. Of the 211 participants with CTE, 126 had CTE only, and the other participants had comorbid neurodegenerative diseases.
Onset of Cognitive, Behavioral, and Mood Symptoms
Of the 211 participants with CTE, 183 developed cognitive and behavioral or mood symptoms prior to death, eight had only cognitive symptoms, 12 had only behavioral or mood symptoms, and seven did not endorse any symptoms examined in the study. Clinical data for one participant were not available.
Among tackle football players with CTE, every one year younger that they began to play tackle football predicted earlier onset of cognitive symptoms by 2.44 years and of behavioral or mood symptoms by 2.50 years. Exposure before age 12 predicted earlier cognitive and behavioral or mood symptom onset by 13.39 years and 13.28 years, respectively.
Secondary subset analyses indicated that younger age of exposure to tackle football was associated with earlier onset of functional impairment in participants who were determined to have had dementia. Researchers observed nearly identical effects in participants with CTE only.
Study limitations include the lack of an appropriate control or comparison group, the researchers noted. In addition, the results may not be generalizable to a broader tackle football population.
“Given the growing public health concerns for participation in tackle football, prospective studies of former tackle football players that include objective clinical assessments are needed to better understand the relationship between youth tackle football exposure and long-term neurobehavioral outcomes,” said the researchers.
“More research on this topic is needed before any clinical recommendations, as well as recommendations on policy or rule changes, can be made,” said Dr. Alosco.
“Boston University and sites across the country are currently conducting longitudinal studies on former football players, which will allow us to begin to study cognition and behavior and mood functioning over time.”
—Erica Tricarico
Suggested Reading
Alosco ML, Mez J, Tripodis Y, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018 Apr 30 [Epub ahead of print].
Younger age of exposure to tackle football is not associated with chronic traumatic encephalopathy (CTE) pathologic severity, Alzheimer’s disease pathology, or Lewy body pathology, according to data published online ahead of print April 30 in Annals of Neurology. Younger age of exposure does appear to predict earlier neurobehavioral symptom onset, however, the authors said.
“These findings suggest that exposure to repetitive head impacts from tackle football as a youth may reduce resiliency to diseases, including, but not limited to, CTE, that affect the brain in later life,” said Michael L. Alosco, PhD, Assistant Professor of Neurology at the the Boston University Alzheimer’s Disease and CTE Center. “This study adds to growing research suggesting that incurring repeated head impacts through tackle football in earlier life can lead to both short-term and long-term effects on the brain.”
Repetitive Head Impacts and Neurodevelopment
Previous research has linked younger age of first exposure to tackle football with smaller thalamic volume in former National Football League players. A recent study of 214 former and amateur football players found that age of first exposure to tackle football—before age 12, in particular—predicted increased odds of self-reported neuropsychiatric and executive impairment.
“Youth exposure to repetitive head impacts may disrupt neurodevelopment to lower the threshold for later clinical dysfunction,” said the researchers.
To examine the effect of age of first exposure to tackle football on CTE pathologic severity and age of neurobehavioral symptom onset in tackle football players with neuropathologically confirmed CTE, Dr. Alosco and colleagues analyzed a sample of 246 amateur and professional tackle football players whose brains had been donated to the Veteran’s Affairs–Boston University–Concussion Legacy Foundation Brain Bank. The researchers interviewed informants to ascertain players’ age of first exposure and age of onset of cognitive, behavioral, or mood symptoms. A total of 211 football players were diagnosed with CTE; 35 did not have CTE. Of the 211 participants with CTE, 126 had CTE only, and the other participants had comorbid neurodegenerative diseases.
Onset of Cognitive, Behavioral, and Mood Symptoms
Of the 211 participants with CTE, 183 developed cognitive and behavioral or mood symptoms prior to death, eight had only cognitive symptoms, 12 had only behavioral or mood symptoms, and seven did not endorse any symptoms examined in the study. Clinical data for one participant were not available.
Among tackle football players with CTE, every one year younger that they began to play tackle football predicted earlier onset of cognitive symptoms by 2.44 years and of behavioral or mood symptoms by 2.50 years. Exposure before age 12 predicted earlier cognitive and behavioral or mood symptom onset by 13.39 years and 13.28 years, respectively.
Secondary subset analyses indicated that younger age of exposure to tackle football was associated with earlier onset of functional impairment in participants who were determined to have had dementia. Researchers observed nearly identical effects in participants with CTE only.
Study limitations include the lack of an appropriate control or comparison group, the researchers noted. In addition, the results may not be generalizable to a broader tackle football population.
“Given the growing public health concerns for participation in tackle football, prospective studies of former tackle football players that include objective clinical assessments are needed to better understand the relationship between youth tackle football exposure and long-term neurobehavioral outcomes,” said the researchers.
“More research on this topic is needed before any clinical recommendations, as well as recommendations on policy or rule changes, can be made,” said Dr. Alosco.
“Boston University and sites across the country are currently conducting longitudinal studies on former football players, which will allow us to begin to study cognition and behavior and mood functioning over time.”
—Erica Tricarico
Suggested Reading
Alosco ML, Mez J, Tripodis Y, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018 Apr 30 [Epub ahead of print].
Younger age of exposure to tackle football is not associated with chronic traumatic encephalopathy (CTE) pathologic severity, Alzheimer’s disease pathology, or Lewy body pathology, according to data published online ahead of print April 30 in Annals of Neurology. Younger age of exposure does appear to predict earlier neurobehavioral symptom onset, however, the authors said.
“These findings suggest that exposure to repetitive head impacts from tackle football as a youth may reduce resiliency to diseases, including, but not limited to, CTE, that affect the brain in later life,” said Michael L. Alosco, PhD, Assistant Professor of Neurology at the the Boston University Alzheimer’s Disease and CTE Center. “This study adds to growing research suggesting that incurring repeated head impacts through tackle football in earlier life can lead to both short-term and long-term effects on the brain.”
Repetitive Head Impacts and Neurodevelopment
Previous research has linked younger age of first exposure to tackle football with smaller thalamic volume in former National Football League players. A recent study of 214 former and amateur football players found that age of first exposure to tackle football—before age 12, in particular—predicted increased odds of self-reported neuropsychiatric and executive impairment.
“Youth exposure to repetitive head impacts may disrupt neurodevelopment to lower the threshold for later clinical dysfunction,” said the researchers.
To examine the effect of age of first exposure to tackle football on CTE pathologic severity and age of neurobehavioral symptom onset in tackle football players with neuropathologically confirmed CTE, Dr. Alosco and colleagues analyzed a sample of 246 amateur and professional tackle football players whose brains had been donated to the Veteran’s Affairs–Boston University–Concussion Legacy Foundation Brain Bank. The researchers interviewed informants to ascertain players’ age of first exposure and age of onset of cognitive, behavioral, or mood symptoms. A total of 211 football players were diagnosed with CTE; 35 did not have CTE. Of the 211 participants with CTE, 126 had CTE only, and the other participants had comorbid neurodegenerative diseases.
Onset of Cognitive, Behavioral, and Mood Symptoms
Of the 211 participants with CTE, 183 developed cognitive and behavioral or mood symptoms prior to death, eight had only cognitive symptoms, 12 had only behavioral or mood symptoms, and seven did not endorse any symptoms examined in the study. Clinical data for one participant were not available.
Among tackle football players with CTE, every one year younger that they began to play tackle football predicted earlier onset of cognitive symptoms by 2.44 years and of behavioral or mood symptoms by 2.50 years. Exposure before age 12 predicted earlier cognitive and behavioral or mood symptom onset by 13.39 years and 13.28 years, respectively.
Secondary subset analyses indicated that younger age of exposure to tackle football was associated with earlier onset of functional impairment in participants who were determined to have had dementia. Researchers observed nearly identical effects in participants with CTE only.
Study limitations include the lack of an appropriate control or comparison group, the researchers noted. In addition, the results may not be generalizable to a broader tackle football population.
“Given the growing public health concerns for participation in tackle football, prospective studies of former tackle football players that include objective clinical assessments are needed to better understand the relationship between youth tackle football exposure and long-term neurobehavioral outcomes,” said the researchers.
“More research on this topic is needed before any clinical recommendations, as well as recommendations on policy or rule changes, can be made,” said Dr. Alosco.
“Boston University and sites across the country are currently conducting longitudinal studies on former football players, which will allow us to begin to study cognition and behavior and mood functioning over time.”
—Erica Tricarico
Suggested Reading
Alosco ML, Mez J, Tripodis Y, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018 Apr 30 [Epub ahead of print].
Tear Proteins May Be Biomarkers for Parkinson’s Disease
LOS ANGELES—Tears may hold diagnostic clues as to whether someone has Parkinson’s disease, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting. “We believe our research is the first to show that tears may be a reliable, inexpensive, and noninvasive biologic marker of Parkinson’s disease,” said study author Mark Floyd Lew, MD, Professor of Clinical Neurology, and Joseph P. Van Der Meulen, MD, Chair in Parkinson’s Disease Research in Honor of Robert J. Pasarow, and Vice Chair, in the Department of Neurology at the Keck School of Medicine of the University of Southern California in Los Angeles.
Nonmotor features of Parkinson’s disease occur years prior to motor dysfunction and represent a well-suited platform to investigate for a possible biomarker. Lacrimal glands are highly innervated by cholinergic neurons, and tear fluid secreted by lacrimal glands is greatly stimulated by cholinergic neurons. The production, packaging, and secretion of specific proteins into tears may be regulated by changes in nerve function to lacrimal glands. According to the researchers, analysis of any alteration in the secretion of proteins into tears may identify a reliable and noninvasive biomarker for Parkinson’s disease.
For the study, tear samples were collected from 55 patients with Parkinson’s disease of varying severity and 27 age- and gender-matched controls without Parkinson’s disease. In addition, tears were analyzed for the levels of four proteins—total alpha synuclein, CC chemokine ligand 2 (CCL-2), DJ-1 (Parkinson’s disease protein 7), and oligomeric alpha synuclein.
The researchers found differences in the levels of a total alpha-synuclein in the tears of patients with Parkinson’s disease, compared with those of controls. Additionally, levels of oligomeric alpha-synuclein, which is alpha-synuclein that has formed aggregates that are implicated in nerve damage in Parkinson’s disease, were also significantly different, compared with controls. It is also possible that the tear gland secretory cells themselves produce these different forms of alpha-synuclein that can be directly secreted into tears, the researchers said.
Total levels of alpha-synuclein were decreased in patients with Parkinson’s disease, with an average of 423 picograms of that protein per milligram (pg/mg) compared with 704 pg/mg in healthy controls. However, levels of oligomeric alpha-synuclein were increased in patients with Parkinson’s disease, with an average of 1.45 nanograms per milligram of tear protein (ng/mg), compared with 0.27 ng/mg in controls. While detectable in tears, neither CCL-2 nor DJ-1 varied between patients with Parkinson’s disease and controls.
“Knowing that something as simple as tears could help neurologists differentiate between people who have Parkinson’s disease and those who do not in a noninvasive manner is exciting,” said Dr. Lew. “And because the Parkinson’s disease process can begin years or decades before symptoms appear, a biologic marker like this could be useful in diagnosing, or even treating, the disease earlier.”
More research needs to be done in larger groups of people to investigate whether these protein changes can be detected in tears in the earliest presymptomatic stages of the disease, said the researchers.
The study was supported by the Michael J. Fox Foundation for Parkinson’s Research and the Plotkin Foundation.
LOS ANGELES—Tears may hold diagnostic clues as to whether someone has Parkinson’s disease, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting. “We believe our research is the first to show that tears may be a reliable, inexpensive, and noninvasive biologic marker of Parkinson’s disease,” said study author Mark Floyd Lew, MD, Professor of Clinical Neurology, and Joseph P. Van Der Meulen, MD, Chair in Parkinson’s Disease Research in Honor of Robert J. Pasarow, and Vice Chair, in the Department of Neurology at the Keck School of Medicine of the University of Southern California in Los Angeles.
Nonmotor features of Parkinson’s disease occur years prior to motor dysfunction and represent a well-suited platform to investigate for a possible biomarker. Lacrimal glands are highly innervated by cholinergic neurons, and tear fluid secreted by lacrimal glands is greatly stimulated by cholinergic neurons. The production, packaging, and secretion of specific proteins into tears may be regulated by changes in nerve function to lacrimal glands. According to the researchers, analysis of any alteration in the secretion of proteins into tears may identify a reliable and noninvasive biomarker for Parkinson’s disease.
For the study, tear samples were collected from 55 patients with Parkinson’s disease of varying severity and 27 age- and gender-matched controls without Parkinson’s disease. In addition, tears were analyzed for the levels of four proteins—total alpha synuclein, CC chemokine ligand 2 (CCL-2), DJ-1 (Parkinson’s disease protein 7), and oligomeric alpha synuclein.
The researchers found differences in the levels of a total alpha-synuclein in the tears of patients with Parkinson’s disease, compared with those of controls. Additionally, levels of oligomeric alpha-synuclein, which is alpha-synuclein that has formed aggregates that are implicated in nerve damage in Parkinson’s disease, were also significantly different, compared with controls. It is also possible that the tear gland secretory cells themselves produce these different forms of alpha-synuclein that can be directly secreted into tears, the researchers said.
Total levels of alpha-synuclein were decreased in patients with Parkinson’s disease, with an average of 423 picograms of that protein per milligram (pg/mg) compared with 704 pg/mg in healthy controls. However, levels of oligomeric alpha-synuclein were increased in patients with Parkinson’s disease, with an average of 1.45 nanograms per milligram of tear protein (ng/mg), compared with 0.27 ng/mg in controls. While detectable in tears, neither CCL-2 nor DJ-1 varied between patients with Parkinson’s disease and controls.
“Knowing that something as simple as tears could help neurologists differentiate between people who have Parkinson’s disease and those who do not in a noninvasive manner is exciting,” said Dr. Lew. “And because the Parkinson’s disease process can begin years or decades before symptoms appear, a biologic marker like this could be useful in diagnosing, or even treating, the disease earlier.”
More research needs to be done in larger groups of people to investigate whether these protein changes can be detected in tears in the earliest presymptomatic stages of the disease, said the researchers.
The study was supported by the Michael J. Fox Foundation for Parkinson’s Research and the Plotkin Foundation.
LOS ANGELES—Tears may hold diagnostic clues as to whether someone has Parkinson’s disease, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting. “We believe our research is the first to show that tears may be a reliable, inexpensive, and noninvasive biologic marker of Parkinson’s disease,” said study author Mark Floyd Lew, MD, Professor of Clinical Neurology, and Joseph P. Van Der Meulen, MD, Chair in Parkinson’s Disease Research in Honor of Robert J. Pasarow, and Vice Chair, in the Department of Neurology at the Keck School of Medicine of the University of Southern California in Los Angeles.
Nonmotor features of Parkinson’s disease occur years prior to motor dysfunction and represent a well-suited platform to investigate for a possible biomarker. Lacrimal glands are highly innervated by cholinergic neurons, and tear fluid secreted by lacrimal glands is greatly stimulated by cholinergic neurons. The production, packaging, and secretion of specific proteins into tears may be regulated by changes in nerve function to lacrimal glands. According to the researchers, analysis of any alteration in the secretion of proteins into tears may identify a reliable and noninvasive biomarker for Parkinson’s disease.
For the study, tear samples were collected from 55 patients with Parkinson’s disease of varying severity and 27 age- and gender-matched controls without Parkinson’s disease. In addition, tears were analyzed for the levels of four proteins—total alpha synuclein, CC chemokine ligand 2 (CCL-2), DJ-1 (Parkinson’s disease protein 7), and oligomeric alpha synuclein.
The researchers found differences in the levels of a total alpha-synuclein in the tears of patients with Parkinson’s disease, compared with those of controls. Additionally, levels of oligomeric alpha-synuclein, which is alpha-synuclein that has formed aggregates that are implicated in nerve damage in Parkinson’s disease, were also significantly different, compared with controls. It is also possible that the tear gland secretory cells themselves produce these different forms of alpha-synuclein that can be directly secreted into tears, the researchers said.
Total levels of alpha-synuclein were decreased in patients with Parkinson’s disease, with an average of 423 picograms of that protein per milligram (pg/mg) compared with 704 pg/mg in healthy controls. However, levels of oligomeric alpha-synuclein were increased in patients with Parkinson’s disease, with an average of 1.45 nanograms per milligram of tear protein (ng/mg), compared with 0.27 ng/mg in controls. While detectable in tears, neither CCL-2 nor DJ-1 varied between patients with Parkinson’s disease and controls.
“Knowing that something as simple as tears could help neurologists differentiate between people who have Parkinson’s disease and those who do not in a noninvasive manner is exciting,” said Dr. Lew. “And because the Parkinson’s disease process can begin years or decades before symptoms appear, a biologic marker like this could be useful in diagnosing, or even treating, the disease earlier.”
More research needs to be done in larger groups of people to investigate whether these protein changes can be detected in tears in the earliest presymptomatic stages of the disease, said the researchers.
The study was supported by the Michael J. Fox Foundation for Parkinson’s Research and the Plotkin Foundation.
Tip for when using phenazophyridine
Tip for when using phenazophyridine
I enjoyed the review by Dr. Iglesia on agents that are used to demonstrate ureteral patency. I like phenazopyridine because it is cheap and readily available. It works great if you remember one thing: drain the bladder first so the orange color stands out in the colorless saline irrigant. Otherwise, the orange-dyed urine obscures the orange ureteral jets.
John H. Sand, MD
Ellensburg, Washington
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Tip for when using phenazophyridine
I enjoyed the review by Dr. Iglesia on agents that are used to demonstrate ureteral patency. I like phenazopyridine because it is cheap and readily available. It works great if you remember one thing: drain the bladder first so the orange color stands out in the colorless saline irrigant. Otherwise, the orange-dyed urine obscures the orange ureteral jets.
John H. Sand, MD
Ellensburg, Washington
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Tip for when using phenazophyridine
I enjoyed the review by Dr. Iglesia on agents that are used to demonstrate ureteral patency. I like phenazopyridine because it is cheap and readily available. It works great if you remember one thing: drain the bladder first so the orange color stands out in the colorless saline irrigant. Otherwise, the orange-dyed urine obscures the orange ureteral jets.
John H. Sand, MD
Ellensburg, Washington
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Emergency gout admission increase is ‘call to arms’
LIVERPOOL, ENGLAND – The rate of emergency hospital admissions for gout in England has seen a 59% increase over the past decade, while that of rheumatoid arthritis has halved, it was reported at the British Society for Rheumatology annual conference.
Over an approximate 10-year period (2006-2017), the incidence rate of unplanned gout admissions increased from 7.9 to 12.5 admissions per 100,000 of the population. This represented an increase from 0.023% to 0.032% of all hospital admissions during the period.
To put that into perspective, unplanned admissions for rheumatoid arthritis (RA), decreased from 8.6 to 4.3 admissions per 100,000 of the population, said Mark D. Russell, MD, a rheumatology registrar at Guy’s and St Thomas’ Hospital in London.
Furthermore, primary care prescriptions for common gout medications have seen a dramatic increase over the same time period in England; allopurinol prescriptions are up 72%, there’s been a 166% increase in colchicine prescriptions, and a 20-fold increase in febuxostat (Uloric) prescriptions since data became available for its in 2010.
“Gout’s very much a treatable condition,” Dr. Russell said, but with 82% of all gout admissions being unplanned, “there’s clearly more to do; this should be a call to arms for rheumatologists to help reduce the in-patient burden of this condition.”
The mean length of hospital stay was estimated at 6.6 days, with the median being 3.2 days. Gout accounted for just under 350,000 hospital bed days from 2006 to 2017, and with the cost of a single gout admission being anything from £850 up to £5,600, it constitutes a significant burden for the country’s National Health Service.
But what can be done? Would a “door-to-needle time campaign” help? So that when patients attend the emergency department they are assessed rapidly and treated accordingly? Dr. Russell queried.
Education could be the key, was the consensus during the discussion following his presentation. Education, and not just of those affected by the condition, but also of the family physicians who seem to have a “knee-jerk reaction” to prescribe medications, and not always appropriately. Even hospital staff may need help in differentiating gout from other emergency presentations, with some admitting patients suspecting infection or wrongly discharging them.
Pharmacist-led gout clinics
Another approach to try to avoid emergency hospital visits could be better management and perhaps setting up specialist gout clinics. Such clinics have already been piloted, and rheumatology pharmacist Jane Whiteman shared her experience of setting up a monthly, pharmacist-led gout clinic in a separate presentation.
Dr. Whiteman, who works at Royal Victoria Hospital, part of the Belfast Health and Social Care Trust in Ireland, presented data on 52 patients who were seen at the clinic between June 2015 and May 2017. The total number of patient visits was 87, with an average of 1.7 visits per patient.
Of 38 patients who were discharged from the clinic, 29 (76%) had met target levels of serum uric acid, which guidelines from the British Society for Rheumatology set at less than 0.3 mmol/L (5 mg/dL) and those from the European League of Rheumatism set at less than 0.36 mmol/L (6 mg/dL).
It is important to reduce serum uric acid levels, Dr. Whiteman explained. “Gout occurs when serum uric acid rises and urate crystals reach their saturation point in the serum and start to crystallize out into the joints and into the tissues,” she said. “It’s a progressive disease that usually starts in one joint, but if the serum uric acid isn’t controlled then it can go on to affect a number of joints,” and cause chronic arthritis, among other potentially serious conditions, and is an independent risk factor for cardiovascular and renal disease.
“Studies across the world have shown that gout is not well looked after; patient adherence to treatment is very poor,” Dr. Whiteman said. “Among all the chronic diseases it has the lowest adherence to treatment,” she added, noting that one study showed just one-fifth of patients remained on gout medication at 1 year.
Patient education is an important part of the clinic’s services, as when people are asymptomatic and between bouts of gout, they perhaps do not realize that they still need to take their medication. Education thus needs to include talking about their diet and lifestyle, providing information on medication, and why it is important to keep their serum uric acid levels in check.
After patients’ initial referral to the clinic, they are followed up by the clinic every month until their serum urate levels are below the 0.3 mmol/L target. They can then be discharged and monitored by their family physician.
Postdischarge, Dr. Whiteman and her colleagues found that 22 (76%) of the 29 patients who had met their target levels of serum uric acid later had serum uric acid tested at least once, showing an average level of 0.29 mmol/L at follow-up. This reassuring result occurred perhaps because of a majority of patients (79%) who were still being prescribed, and presumably taking, urate-lowering therapy. Seven patients did not undergo follow-up serum uric acid testing because they had died (one), were in the hospital (one), were not taking medication because they had made diet or lifestyle changes (three), or had their treatment on hold (two).
“I think the gout clinic has been a success; it has addressed barriers to optimal management through education of the patient,” Dr. Whiteman said.
Both presenters had nothing to disclose.
SOURCE: Russel M et al. Rheumatology. 2018;57[Suppl. 3]:key075.186; Whiteman J, et al. Rheumatology. 2018;57[Suppl. 3]:key075.215.
LIVERPOOL, ENGLAND – The rate of emergency hospital admissions for gout in England has seen a 59% increase over the past decade, while that of rheumatoid arthritis has halved, it was reported at the British Society for Rheumatology annual conference.
Over an approximate 10-year period (2006-2017), the incidence rate of unplanned gout admissions increased from 7.9 to 12.5 admissions per 100,000 of the population. This represented an increase from 0.023% to 0.032% of all hospital admissions during the period.
To put that into perspective, unplanned admissions for rheumatoid arthritis (RA), decreased from 8.6 to 4.3 admissions per 100,000 of the population, said Mark D. Russell, MD, a rheumatology registrar at Guy’s and St Thomas’ Hospital in London.
Furthermore, primary care prescriptions for common gout medications have seen a dramatic increase over the same time period in England; allopurinol prescriptions are up 72%, there’s been a 166% increase in colchicine prescriptions, and a 20-fold increase in febuxostat (Uloric) prescriptions since data became available for its in 2010.
“Gout’s very much a treatable condition,” Dr. Russell said, but with 82% of all gout admissions being unplanned, “there’s clearly more to do; this should be a call to arms for rheumatologists to help reduce the in-patient burden of this condition.”
The mean length of hospital stay was estimated at 6.6 days, with the median being 3.2 days. Gout accounted for just under 350,000 hospital bed days from 2006 to 2017, and with the cost of a single gout admission being anything from £850 up to £5,600, it constitutes a significant burden for the country’s National Health Service.
But what can be done? Would a “door-to-needle time campaign” help? So that when patients attend the emergency department they are assessed rapidly and treated accordingly? Dr. Russell queried.
Education could be the key, was the consensus during the discussion following his presentation. Education, and not just of those affected by the condition, but also of the family physicians who seem to have a “knee-jerk reaction” to prescribe medications, and not always appropriately. Even hospital staff may need help in differentiating gout from other emergency presentations, with some admitting patients suspecting infection or wrongly discharging them.
Pharmacist-led gout clinics
Another approach to try to avoid emergency hospital visits could be better management and perhaps setting up specialist gout clinics. Such clinics have already been piloted, and rheumatology pharmacist Jane Whiteman shared her experience of setting up a monthly, pharmacist-led gout clinic in a separate presentation.
Dr. Whiteman, who works at Royal Victoria Hospital, part of the Belfast Health and Social Care Trust in Ireland, presented data on 52 patients who were seen at the clinic between June 2015 and May 2017. The total number of patient visits was 87, with an average of 1.7 visits per patient.
Of 38 patients who were discharged from the clinic, 29 (76%) had met target levels of serum uric acid, which guidelines from the British Society for Rheumatology set at less than 0.3 mmol/L (5 mg/dL) and those from the European League of Rheumatism set at less than 0.36 mmol/L (6 mg/dL).
It is important to reduce serum uric acid levels, Dr. Whiteman explained. “Gout occurs when serum uric acid rises and urate crystals reach their saturation point in the serum and start to crystallize out into the joints and into the tissues,” she said. “It’s a progressive disease that usually starts in one joint, but if the serum uric acid isn’t controlled then it can go on to affect a number of joints,” and cause chronic arthritis, among other potentially serious conditions, and is an independent risk factor for cardiovascular and renal disease.
“Studies across the world have shown that gout is not well looked after; patient adherence to treatment is very poor,” Dr. Whiteman said. “Among all the chronic diseases it has the lowest adherence to treatment,” she added, noting that one study showed just one-fifth of patients remained on gout medication at 1 year.
Patient education is an important part of the clinic’s services, as when people are asymptomatic and between bouts of gout, they perhaps do not realize that they still need to take their medication. Education thus needs to include talking about their diet and lifestyle, providing information on medication, and why it is important to keep their serum uric acid levels in check.
After patients’ initial referral to the clinic, they are followed up by the clinic every month until their serum urate levels are below the 0.3 mmol/L target. They can then be discharged and monitored by their family physician.
Postdischarge, Dr. Whiteman and her colleagues found that 22 (76%) of the 29 patients who had met their target levels of serum uric acid later had serum uric acid tested at least once, showing an average level of 0.29 mmol/L at follow-up. This reassuring result occurred perhaps because of a majority of patients (79%) who were still being prescribed, and presumably taking, urate-lowering therapy. Seven patients did not undergo follow-up serum uric acid testing because they had died (one), were in the hospital (one), were not taking medication because they had made diet or lifestyle changes (three), or had their treatment on hold (two).
“I think the gout clinic has been a success; it has addressed barriers to optimal management through education of the patient,” Dr. Whiteman said.
Both presenters had nothing to disclose.
SOURCE: Russel M et al. Rheumatology. 2018;57[Suppl. 3]:key075.186; Whiteman J, et al. Rheumatology. 2018;57[Suppl. 3]:key075.215.
LIVERPOOL, ENGLAND – The rate of emergency hospital admissions for gout in England has seen a 59% increase over the past decade, while that of rheumatoid arthritis has halved, it was reported at the British Society for Rheumatology annual conference.
Over an approximate 10-year period (2006-2017), the incidence rate of unplanned gout admissions increased from 7.9 to 12.5 admissions per 100,000 of the population. This represented an increase from 0.023% to 0.032% of all hospital admissions during the period.
To put that into perspective, unplanned admissions for rheumatoid arthritis (RA), decreased from 8.6 to 4.3 admissions per 100,000 of the population, said Mark D. Russell, MD, a rheumatology registrar at Guy’s and St Thomas’ Hospital in London.
Furthermore, primary care prescriptions for common gout medications have seen a dramatic increase over the same time period in England; allopurinol prescriptions are up 72%, there’s been a 166% increase in colchicine prescriptions, and a 20-fold increase in febuxostat (Uloric) prescriptions since data became available for its in 2010.
“Gout’s very much a treatable condition,” Dr. Russell said, but with 82% of all gout admissions being unplanned, “there’s clearly more to do; this should be a call to arms for rheumatologists to help reduce the in-patient burden of this condition.”
The mean length of hospital stay was estimated at 6.6 days, with the median being 3.2 days. Gout accounted for just under 350,000 hospital bed days from 2006 to 2017, and with the cost of a single gout admission being anything from £850 up to £5,600, it constitutes a significant burden for the country’s National Health Service.
But what can be done? Would a “door-to-needle time campaign” help? So that when patients attend the emergency department they are assessed rapidly and treated accordingly? Dr. Russell queried.
Education could be the key, was the consensus during the discussion following his presentation. Education, and not just of those affected by the condition, but also of the family physicians who seem to have a “knee-jerk reaction” to prescribe medications, and not always appropriately. Even hospital staff may need help in differentiating gout from other emergency presentations, with some admitting patients suspecting infection or wrongly discharging them.
Pharmacist-led gout clinics
Another approach to try to avoid emergency hospital visits could be better management and perhaps setting up specialist gout clinics. Such clinics have already been piloted, and rheumatology pharmacist Jane Whiteman shared her experience of setting up a monthly, pharmacist-led gout clinic in a separate presentation.
Dr. Whiteman, who works at Royal Victoria Hospital, part of the Belfast Health and Social Care Trust in Ireland, presented data on 52 patients who were seen at the clinic between June 2015 and May 2017. The total number of patient visits was 87, with an average of 1.7 visits per patient.
Of 38 patients who were discharged from the clinic, 29 (76%) had met target levels of serum uric acid, which guidelines from the British Society for Rheumatology set at less than 0.3 mmol/L (5 mg/dL) and those from the European League of Rheumatism set at less than 0.36 mmol/L (6 mg/dL).
It is important to reduce serum uric acid levels, Dr. Whiteman explained. “Gout occurs when serum uric acid rises and urate crystals reach their saturation point in the serum and start to crystallize out into the joints and into the tissues,” she said. “It’s a progressive disease that usually starts in one joint, but if the serum uric acid isn’t controlled then it can go on to affect a number of joints,” and cause chronic arthritis, among other potentially serious conditions, and is an independent risk factor for cardiovascular and renal disease.
“Studies across the world have shown that gout is not well looked after; patient adherence to treatment is very poor,” Dr. Whiteman said. “Among all the chronic diseases it has the lowest adherence to treatment,” she added, noting that one study showed just one-fifth of patients remained on gout medication at 1 year.
Patient education is an important part of the clinic’s services, as when people are asymptomatic and between bouts of gout, they perhaps do not realize that they still need to take their medication. Education thus needs to include talking about their diet and lifestyle, providing information on medication, and why it is important to keep their serum uric acid levels in check.
After patients’ initial referral to the clinic, they are followed up by the clinic every month until their serum urate levels are below the 0.3 mmol/L target. They can then be discharged and monitored by their family physician.
Postdischarge, Dr. Whiteman and her colleagues found that 22 (76%) of the 29 patients who had met their target levels of serum uric acid later had serum uric acid tested at least once, showing an average level of 0.29 mmol/L at follow-up. This reassuring result occurred perhaps because of a majority of patients (79%) who were still being prescribed, and presumably taking, urate-lowering therapy. Seven patients did not undergo follow-up serum uric acid testing because they had died (one), were in the hospital (one), were not taking medication because they had made diet or lifestyle changes (three), or had their treatment on hold (two).
“I think the gout clinic has been a success; it has addressed barriers to optimal management through education of the patient,” Dr. Whiteman said.
Both presenters had nothing to disclose.
SOURCE: Russel M et al. Rheumatology. 2018;57[Suppl. 3]:key075.186; Whiteman J, et al. Rheumatology. 2018;57[Suppl. 3]:key075.215.
AT RHEUMATOLOGY 2018
Key clinical point:
Major finding: The rate of emergency hospital admissions for gout in England increased by 59%, from 7.9 to 12.5 admissions per 100,000 of the population; 76% of patients attending a pharmacist-led gout clinic achieved target serum uric acid levels.
Study details: An analysis of National Health Service data from April 2006 to March 2017 on hospital admissions for gout and primary care prescription data and a separate study of 52 patients attending a pharmacist-led gout clinic.
Disclosures: Both presenters had nothing to disclose.
Sources: Russell M et al. Rheumatology. 2018;57[Suppl. 3]:key075.186; Whiteman J et al. Rheumatology. 2018;57[Suppl. 3]:key075.215.
Rash goes undetected
Rash goes undetected
As a urogynecologist, in the past 5 years I have had 2 urgent emergency department referrals from 2 towns. The patients had excruciating flank pain and had a negative computed tomography scan and normal pelvic and renal examinations, but no physical exam. They were subsequently found to have shingles!
Bunan Alnaif, MD
Chesapeake, Virginia
Physical exam revealed suspicious mass
Two years ago a regular gynecologic patient of mine came in 2 months early for her Pap test because she was concerned about a pressure in her genital area. I had delivered her 3 children. She was now in her mid-40s. She had visited her usual physician about the problem; she was not physically examined but was advised to see a gastrointestinal specialist, since the pressure caused constipation with discomfort. She then consulted a gastroenterologist, who performed a colonoscopy that was reported as normal. The patient related that she had no pelvic or rectal examination at that time, although it is possible that one could have been done while she was under anesthesia.
She arrived at my office 3 weeks later, and while doing the pelvic and rectal exam, I noted she had a 3- to 4-cm perirectal mass, which I thought was a Bartholin’s tumor. I referred her to a gynecologic oncologist who happened to write a paper on this subject. My diagnosis was wrong—she had a rectal carcinoma, which fortunately was Stage 1.
The patient subsequently has done well. The delay in diagnosis could have been averted if a simple rectal examination had been performed by the first doctor.
James Moran, MD
Santa Monica, California
Case of an almost missed diagnosis
I have many examples of how not performing a physical examination can cause problems, but here is a recent one. This involved a 70-year-old woman who had been seeing only her primary care physician for the past 30 years, with no pelvic examinations done. She had symptoms of vaginal discharge and itch for which she was given multiple courses of antifungals and topical steroids. Finally, she was referred to me. Examination revealed findings of extensive raised, erythematous, hyperkeratotic, macerated lesions throughout the vulva. A punch biopsy revealed severe vulvar dysplasia with areas suspicious for squamous cell carcinoma. I referred the patient to a gynecologic oncologist, who performed a simple vulvectomy. There were extensive foci of vulvar intraepithelial neoplasia 3.
Susan Richman, MD
New Haven, Connecticut
Lack of physical exam leads to tortuous dx course
Here is a story of a patient who must have gone without having a pelvic examination or any evaluation for years. This 83-year-old woman had a previous transvaginal hysterectomy at age 49 for fibroids and bleeding. She is quite healthy and active for her age. She had problems with recurrent urinary tract infection for several years before being referred to a gynecologist. She had emergency room visits and multiple urgent care visits. She saw her primary care physician 3 times in 4 weeks for bladder pain and a sensation of incomplete bladder emptying. She reported that when she got up in the morning, it felt like her urine slowly leaked out for several hours. She was referred to a urologist, who saw her twice and did pelvic ultrasonography and postvoid residual urine testing—without a pelvic exam.
After 2 months of regular visits, an examination by her primary care physician revealed a complete fusion of the labia. Six months after her initial urology visit, the patient had an examination with a plan for cystoscopy, and the urologist ended up doing a “dilation of labial fusion” in the office. The patient’s urinary symptoms were improved slightly, and she had visits to the emergency room or urgent care once monthly for dysuria after dilation of the labia.
At that point she was referred to me. We tried topical estrogen for several months with minimal improvement in symptoms, and I performed a surgical separation of labial fusion in the operating room under monitored anesthesia care. After surgery the patient said that she felt like “I got my life back,” and she never knew how happy she could be to pee in the morning.
Theresa Gipps, MD
Walnut Creek, California
Agrees with importance of clinical exam
I fully agree that clinical examination skill is a dying art. But the American College of Obstetricians and Gynecologists has issued guidelines stating that pelvic examination is not required, especially in asymptomatic women. Another area of concern is hair removal procedures like waxing and laser treatments in the pubic area, and whether these do harm in any way or increase the likelihood of skin problems.
Manju Hotchandani, MD
New Delhi, India
Dr. Barbieri responds
I thank Drs. Alnaif, Moran, Richman, Gipps, and Hotchandani for sharing their comments and important clinical vignettes concerning the primacy of the physical examination with our readers. In clinical practice there are many competing demands on the time of clinicians, but we should strive to preserve time for a good physical examination. If not us, who is going to perform a competent physical examination?
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Rash goes undetected
As a urogynecologist, in the past 5 years I have had 2 urgent emergency department referrals from 2 towns. The patients had excruciating flank pain and had a negative computed tomography scan and normal pelvic and renal examinations, but no physical exam. They were subsequently found to have shingles!
Bunan Alnaif, MD
Chesapeake, Virginia
Physical exam revealed suspicious mass
Two years ago a regular gynecologic patient of mine came in 2 months early for her Pap test because she was concerned about a pressure in her genital area. I had delivered her 3 children. She was now in her mid-40s. She had visited her usual physician about the problem; she was not physically examined but was advised to see a gastrointestinal specialist, since the pressure caused constipation with discomfort. She then consulted a gastroenterologist, who performed a colonoscopy that was reported as normal. The patient related that she had no pelvic or rectal examination at that time, although it is possible that one could have been done while she was under anesthesia.
She arrived at my office 3 weeks later, and while doing the pelvic and rectal exam, I noted she had a 3- to 4-cm perirectal mass, which I thought was a Bartholin’s tumor. I referred her to a gynecologic oncologist who happened to write a paper on this subject. My diagnosis was wrong—she had a rectal carcinoma, which fortunately was Stage 1.
The patient subsequently has done well. The delay in diagnosis could have been averted if a simple rectal examination had been performed by the first doctor.
James Moran, MD
Santa Monica, California
Case of an almost missed diagnosis
I have many examples of how not performing a physical examination can cause problems, but here is a recent one. This involved a 70-year-old woman who had been seeing only her primary care physician for the past 30 years, with no pelvic examinations done. She had symptoms of vaginal discharge and itch for which she was given multiple courses of antifungals and topical steroids. Finally, she was referred to me. Examination revealed findings of extensive raised, erythematous, hyperkeratotic, macerated lesions throughout the vulva. A punch biopsy revealed severe vulvar dysplasia with areas suspicious for squamous cell carcinoma. I referred the patient to a gynecologic oncologist, who performed a simple vulvectomy. There were extensive foci of vulvar intraepithelial neoplasia 3.
Susan Richman, MD
New Haven, Connecticut
Lack of physical exam leads to tortuous dx course
Here is a story of a patient who must have gone without having a pelvic examination or any evaluation for years. This 83-year-old woman had a previous transvaginal hysterectomy at age 49 for fibroids and bleeding. She is quite healthy and active for her age. She had problems with recurrent urinary tract infection for several years before being referred to a gynecologist. She had emergency room visits and multiple urgent care visits. She saw her primary care physician 3 times in 4 weeks for bladder pain and a sensation of incomplete bladder emptying. She reported that when she got up in the morning, it felt like her urine slowly leaked out for several hours. She was referred to a urologist, who saw her twice and did pelvic ultrasonography and postvoid residual urine testing—without a pelvic exam.
After 2 months of regular visits, an examination by her primary care physician revealed a complete fusion of the labia. Six months after her initial urology visit, the patient had an examination with a plan for cystoscopy, and the urologist ended up doing a “dilation of labial fusion” in the office. The patient’s urinary symptoms were improved slightly, and she had visits to the emergency room or urgent care once monthly for dysuria after dilation of the labia.
At that point she was referred to me. We tried topical estrogen for several months with minimal improvement in symptoms, and I performed a surgical separation of labial fusion in the operating room under monitored anesthesia care. After surgery the patient said that she felt like “I got my life back,” and she never knew how happy she could be to pee in the morning.
Theresa Gipps, MD
Walnut Creek, California
Agrees with importance of clinical exam
I fully agree that clinical examination skill is a dying art. But the American College of Obstetricians and Gynecologists has issued guidelines stating that pelvic examination is not required, especially in asymptomatic women. Another area of concern is hair removal procedures like waxing and laser treatments in the pubic area, and whether these do harm in any way or increase the likelihood of skin problems.
Manju Hotchandani, MD
New Delhi, India
Dr. Barbieri responds
I thank Drs. Alnaif, Moran, Richman, Gipps, and Hotchandani for sharing their comments and important clinical vignettes concerning the primacy of the physical examination with our readers. In clinical practice there are many competing demands on the time of clinicians, but we should strive to preserve time for a good physical examination. If not us, who is going to perform a competent physical examination?
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Rash goes undetected
As a urogynecologist, in the past 5 years I have had 2 urgent emergency department referrals from 2 towns. The patients had excruciating flank pain and had a negative computed tomography scan and normal pelvic and renal examinations, but no physical exam. They were subsequently found to have shingles!
Bunan Alnaif, MD
Chesapeake, Virginia
Physical exam revealed suspicious mass
Two years ago a regular gynecologic patient of mine came in 2 months early for her Pap test because she was concerned about a pressure in her genital area. I had delivered her 3 children. She was now in her mid-40s. She had visited her usual physician about the problem; she was not physically examined but was advised to see a gastrointestinal specialist, since the pressure caused constipation with discomfort. She then consulted a gastroenterologist, who performed a colonoscopy that was reported as normal. The patient related that she had no pelvic or rectal examination at that time, although it is possible that one could have been done while she was under anesthesia.
She arrived at my office 3 weeks later, and while doing the pelvic and rectal exam, I noted she had a 3- to 4-cm perirectal mass, which I thought was a Bartholin’s tumor. I referred her to a gynecologic oncologist who happened to write a paper on this subject. My diagnosis was wrong—she had a rectal carcinoma, which fortunately was Stage 1.
The patient subsequently has done well. The delay in diagnosis could have been averted if a simple rectal examination had been performed by the first doctor.
James Moran, MD
Santa Monica, California
Case of an almost missed diagnosis
I have many examples of how not performing a physical examination can cause problems, but here is a recent one. This involved a 70-year-old woman who had been seeing only her primary care physician for the past 30 years, with no pelvic examinations done. She had symptoms of vaginal discharge and itch for which she was given multiple courses of antifungals and topical steroids. Finally, she was referred to me. Examination revealed findings of extensive raised, erythematous, hyperkeratotic, macerated lesions throughout the vulva. A punch biopsy revealed severe vulvar dysplasia with areas suspicious for squamous cell carcinoma. I referred the patient to a gynecologic oncologist, who performed a simple vulvectomy. There were extensive foci of vulvar intraepithelial neoplasia 3.
Susan Richman, MD
New Haven, Connecticut
Lack of physical exam leads to tortuous dx course
Here is a story of a patient who must have gone without having a pelvic examination or any evaluation for years. This 83-year-old woman had a previous transvaginal hysterectomy at age 49 for fibroids and bleeding. She is quite healthy and active for her age. She had problems with recurrent urinary tract infection for several years before being referred to a gynecologist. She had emergency room visits and multiple urgent care visits. She saw her primary care physician 3 times in 4 weeks for bladder pain and a sensation of incomplete bladder emptying. She reported that when she got up in the morning, it felt like her urine slowly leaked out for several hours. She was referred to a urologist, who saw her twice and did pelvic ultrasonography and postvoid residual urine testing—without a pelvic exam.
After 2 months of regular visits, an examination by her primary care physician revealed a complete fusion of the labia. Six months after her initial urology visit, the patient had an examination with a plan for cystoscopy, and the urologist ended up doing a “dilation of labial fusion” in the office. The patient’s urinary symptoms were improved slightly, and she had visits to the emergency room or urgent care once monthly for dysuria after dilation of the labia.
At that point she was referred to me. We tried topical estrogen for several months with minimal improvement in symptoms, and I performed a surgical separation of labial fusion in the operating room under monitored anesthesia care. After surgery the patient said that she felt like “I got my life back,” and she never knew how happy she could be to pee in the morning.
Theresa Gipps, MD
Walnut Creek, California
Agrees with importance of clinical exam
I fully agree that clinical examination skill is a dying art. But the American College of Obstetricians and Gynecologists has issued guidelines stating that pelvic examination is not required, especially in asymptomatic women. Another area of concern is hair removal procedures like waxing and laser treatments in the pubic area, and whether these do harm in any way or increase the likelihood of skin problems.
Manju Hotchandani, MD
New Delhi, India
Dr. Barbieri responds
I thank Drs. Alnaif, Moran, Richman, Gipps, and Hotchandani for sharing their comments and important clinical vignettes concerning the primacy of the physical examination with our readers. In clinical practice there are many competing demands on the time of clinicians, but we should strive to preserve time for a good physical examination. If not us, who is going to perform a competent physical examination?
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.