User login
Fundamentals of laparoscopic surgery (FLS) manual skills exam: Tips and tricks
Patients’ sexual problems: Be proactive, make discussions routine
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
FROM CP/AACP PSYCHIATRY UPDATE
‘Treat youth with gender dysphoria as individuals’
Young people with gender dysphoria should be considered as individuals rather than fall into an age-defined bracket when assessing their understanding to consent to hormone treatment, according to the Tavistock and Portman NHS Foundation Trust, as it awaits the verdict of its recent appeal in London against a High Court ruling.
The High Court ruling, made in December 2020 as reported by this news organization, stated that adolescents with gender dysphoria were unlikely to fully understand the consequences of hormone treatment for gender reassignment and was the result of a case brought by 24-year-old Keira Bell, who transitioned from female to male at the Gender Identity Development Service (GIDS), starting at the age of 16, but later “detransitioned.”
Along with changes made to rules around prescribing puberty blockers and cross-sex hormones to minors with gender dysphoria in countries such as Finland and Sweden, the English ruling signals a more cautious approach to any medical treatment for such children, as detailed in a feature published in April.
However, during the appeal, The Trust argued once more that puberty blockers give children time to “consider options” about their bodies and that the decision (the December ruling) was inconsistent with the law that “entitles children under the age of 16 to make decisions for themselves after being assessed as competent to do so by their doctor.”
Alongside other organizations, the United States–based Endocrine Society submitted written evidence in support of the Tavistock. “The High Court’s decision, if it is allowed to stand, would set a harmful precedent preventing physicians from providing transgender and gender diverse youth with high-quality medical care,” it noted in a statement.
Defending the High Court’s ruling, the lawyer for Ms. Bell said its conclusion was that puberty blockers for gender dysphoria are an “experimental” treatment with a very limited evidence base.
“The judgment of the [High Court] is entirely correct, and there is no proper basis for overturning it,” he asserted.
The 2-day appeal hearing ended on June 24, and a ruling will be made at a later date.
Do children understand the consequences of hormone treatment?
One central aspect of the overall case is the fact that Ms. Bell regrets her decision to transition at age 16, saying she only received three counseling sessions prior to endocrinology referral. And she consequently had a mastectomy at age 20, which she also bitterly regrets.
So a key concern is whether young people fully understand the consequences of taking puberty blockers and therapies that may follow, including cross-sex hormones.
Witness for the appeal Gary Butler, MD, consultant in pediatric and adolescent endocrinology at University College Hospital, London, where children are referred to from GIDS for hormone treatment, said the number of children who go on to cross-sex hormones from puberty blockers is “over 80%.”
But the actual number of children who are referred to endocrinology services (where puberty blockers are initiated) from GIDS is low, at approximately 16%, according to 2019-2020 data, said a GIDS spokesperson.
“Once at the endocrinology service, young people either participate in a group education session, or if under 15 years, an individualized session between the clinician and the patient and family members,” she added. The Trust also maintained that initiation of cross-sex hormones “is separate from the prescription of puberty blockers.”
Since the December ruling, The Trust has put in place multidisciplinary clinical reviews (MDCR) of cases, and in July, NHS England will start implementing an independent multidisciplinary professional review (MDPR) to check that the GIDS has followed due process with each case.
Slow the process down, give appropriate psychotherapy
Stella O’Malley is a psychotherapist who works with transitioners and detransitioners and is a founding member of the International Association of Therapists for Desisters and Detransitioners (IATDD).
Whatever the outcome of the appeal process, Ms. O’Malley said she would like to see the Tavistock slow down and take a broader approach to counseling children before referral to endocrinology services.
In discussing therapy prior to transition, Ms. O’Malley stated that her clients often say they did not explore their inner motivations or other possible reasons for their distress, and the therapy was focused more on when they transition, rather than being sure it was something they wanted to do.
“We need to learn from the mistakes made with people like Keira Bell. , especially when [children are] ... young and especially when they’re traumatized,” Ms. O’Malley said.
“Had they received a more conventional therapy, they might have thought about their decision from different perspectives and in the process acquired more self-awareness, which would have been more beneficial.”
“The ‘affirmative’ approach to gender therapy is too narrow; we need to look at the whole individual. Therapy in other areas would never disregard other, nongender issues such as attention deficit hyperactivity disorder or anxiety [which often co-exist with gender dysphoria] – issues bleed into each other,” Ms. O’Malley pointed out. “We need a more exploratory approach.”
“I’d also like to see other therapists all over the [U.K.] who are perfectly qualified and capable of working with gender actually start working with gender issues,” she said, noting that such an approach might also help reduce the long waiting list at the Tavistock.
The latter had been overwhelmed, and this led to a speeding up of the assessment process, which led to a number of professionals resigning from the service in recent years, saying children were being “fast-tracked” to medical transition.
Fertility and sexual function are complex issues for kids
Also asked to comment was Claire Graham, from Genspect, a group that describes itself as a voice for parents of gender-questioning kids.
She told this news organization that “parents are rightly concerned about their children’s ability to consent to treatments that may lead to infertility and issues surrounding sexual function.” She added that other countries in Europe were changing their approach. “Look to Sweden and Finland, who have both rowed back on puberty blockers and no longer recommend them.”
Ms. Graham, who has worked with children with differences in sexual development, added that it was very difficult for children and young people to understand the life-long implications of decisions made at an early age.
“How can children understand what it is to live with impaired sexual functioning if they have never had sex? Likewise, fertility is a complex issue. Most people do not want to become parents as teenagers, but we understand that this will often change as they grow,” said Ms. Graham.
“Many parents worry that their child is not being considered in the whole [and] that their child’s ability to consent to medical interventions for gender dysphoria is impacted by comorbidities, such as a diagnosis of autism or a history of mental health issues. These children are particularly vulnerable.”
“At Genspect, we hope that the decision from the ... court is upheld,” Ms. Graham concluded.
A version of this article first appeared on Medscape.com.
Young people with gender dysphoria should be considered as individuals rather than fall into an age-defined bracket when assessing their understanding to consent to hormone treatment, according to the Tavistock and Portman NHS Foundation Trust, as it awaits the verdict of its recent appeal in London against a High Court ruling.
The High Court ruling, made in December 2020 as reported by this news organization, stated that adolescents with gender dysphoria were unlikely to fully understand the consequences of hormone treatment for gender reassignment and was the result of a case brought by 24-year-old Keira Bell, who transitioned from female to male at the Gender Identity Development Service (GIDS), starting at the age of 16, but later “detransitioned.”
Along with changes made to rules around prescribing puberty blockers and cross-sex hormones to minors with gender dysphoria in countries such as Finland and Sweden, the English ruling signals a more cautious approach to any medical treatment for such children, as detailed in a feature published in April.
However, during the appeal, The Trust argued once more that puberty blockers give children time to “consider options” about their bodies and that the decision (the December ruling) was inconsistent with the law that “entitles children under the age of 16 to make decisions for themselves after being assessed as competent to do so by their doctor.”
Alongside other organizations, the United States–based Endocrine Society submitted written evidence in support of the Tavistock. “The High Court’s decision, if it is allowed to stand, would set a harmful precedent preventing physicians from providing transgender and gender diverse youth with high-quality medical care,” it noted in a statement.
Defending the High Court’s ruling, the lawyer for Ms. Bell said its conclusion was that puberty blockers for gender dysphoria are an “experimental” treatment with a very limited evidence base.
“The judgment of the [High Court] is entirely correct, and there is no proper basis for overturning it,” he asserted.
The 2-day appeal hearing ended on June 24, and a ruling will be made at a later date.
Do children understand the consequences of hormone treatment?
One central aspect of the overall case is the fact that Ms. Bell regrets her decision to transition at age 16, saying she only received three counseling sessions prior to endocrinology referral. And she consequently had a mastectomy at age 20, which she also bitterly regrets.
So a key concern is whether young people fully understand the consequences of taking puberty blockers and therapies that may follow, including cross-sex hormones.
Witness for the appeal Gary Butler, MD, consultant in pediatric and adolescent endocrinology at University College Hospital, London, where children are referred to from GIDS for hormone treatment, said the number of children who go on to cross-sex hormones from puberty blockers is “over 80%.”
But the actual number of children who are referred to endocrinology services (where puberty blockers are initiated) from GIDS is low, at approximately 16%, according to 2019-2020 data, said a GIDS spokesperson.
“Once at the endocrinology service, young people either participate in a group education session, or if under 15 years, an individualized session between the clinician and the patient and family members,” she added. The Trust also maintained that initiation of cross-sex hormones “is separate from the prescription of puberty blockers.”
Since the December ruling, The Trust has put in place multidisciplinary clinical reviews (MDCR) of cases, and in July, NHS England will start implementing an independent multidisciplinary professional review (MDPR) to check that the GIDS has followed due process with each case.
Slow the process down, give appropriate psychotherapy
Stella O’Malley is a psychotherapist who works with transitioners and detransitioners and is a founding member of the International Association of Therapists for Desisters and Detransitioners (IATDD).
Whatever the outcome of the appeal process, Ms. O’Malley said she would like to see the Tavistock slow down and take a broader approach to counseling children before referral to endocrinology services.
In discussing therapy prior to transition, Ms. O’Malley stated that her clients often say they did not explore their inner motivations or other possible reasons for their distress, and the therapy was focused more on when they transition, rather than being sure it was something they wanted to do.
“We need to learn from the mistakes made with people like Keira Bell. , especially when [children are] ... young and especially when they’re traumatized,” Ms. O’Malley said.
“Had they received a more conventional therapy, they might have thought about their decision from different perspectives and in the process acquired more self-awareness, which would have been more beneficial.”
“The ‘affirmative’ approach to gender therapy is too narrow; we need to look at the whole individual. Therapy in other areas would never disregard other, nongender issues such as attention deficit hyperactivity disorder or anxiety [which often co-exist with gender dysphoria] – issues bleed into each other,” Ms. O’Malley pointed out. “We need a more exploratory approach.”
“I’d also like to see other therapists all over the [U.K.] who are perfectly qualified and capable of working with gender actually start working with gender issues,” she said, noting that such an approach might also help reduce the long waiting list at the Tavistock.
The latter had been overwhelmed, and this led to a speeding up of the assessment process, which led to a number of professionals resigning from the service in recent years, saying children were being “fast-tracked” to medical transition.
Fertility and sexual function are complex issues for kids
Also asked to comment was Claire Graham, from Genspect, a group that describes itself as a voice for parents of gender-questioning kids.
She told this news organization that “parents are rightly concerned about their children’s ability to consent to treatments that may lead to infertility and issues surrounding sexual function.” She added that other countries in Europe were changing their approach. “Look to Sweden and Finland, who have both rowed back on puberty blockers and no longer recommend them.”
Ms. Graham, who has worked with children with differences in sexual development, added that it was very difficult for children and young people to understand the life-long implications of decisions made at an early age.
“How can children understand what it is to live with impaired sexual functioning if they have never had sex? Likewise, fertility is a complex issue. Most people do not want to become parents as teenagers, but we understand that this will often change as they grow,” said Ms. Graham.
“Many parents worry that their child is not being considered in the whole [and] that their child’s ability to consent to medical interventions for gender dysphoria is impacted by comorbidities, such as a diagnosis of autism or a history of mental health issues. These children are particularly vulnerable.”
“At Genspect, we hope that the decision from the ... court is upheld,” Ms. Graham concluded.
A version of this article first appeared on Medscape.com.
Young people with gender dysphoria should be considered as individuals rather than fall into an age-defined bracket when assessing their understanding to consent to hormone treatment, according to the Tavistock and Portman NHS Foundation Trust, as it awaits the verdict of its recent appeal in London against a High Court ruling.
The High Court ruling, made in December 2020 as reported by this news organization, stated that adolescents with gender dysphoria were unlikely to fully understand the consequences of hormone treatment for gender reassignment and was the result of a case brought by 24-year-old Keira Bell, who transitioned from female to male at the Gender Identity Development Service (GIDS), starting at the age of 16, but later “detransitioned.”
Along with changes made to rules around prescribing puberty blockers and cross-sex hormones to minors with gender dysphoria in countries such as Finland and Sweden, the English ruling signals a more cautious approach to any medical treatment for such children, as detailed in a feature published in April.
However, during the appeal, The Trust argued once more that puberty blockers give children time to “consider options” about their bodies and that the decision (the December ruling) was inconsistent with the law that “entitles children under the age of 16 to make decisions for themselves after being assessed as competent to do so by their doctor.”
Alongside other organizations, the United States–based Endocrine Society submitted written evidence in support of the Tavistock. “The High Court’s decision, if it is allowed to stand, would set a harmful precedent preventing physicians from providing transgender and gender diverse youth with high-quality medical care,” it noted in a statement.
Defending the High Court’s ruling, the lawyer for Ms. Bell said its conclusion was that puberty blockers for gender dysphoria are an “experimental” treatment with a very limited evidence base.
“The judgment of the [High Court] is entirely correct, and there is no proper basis for overturning it,” he asserted.
The 2-day appeal hearing ended on June 24, and a ruling will be made at a later date.
Do children understand the consequences of hormone treatment?
One central aspect of the overall case is the fact that Ms. Bell regrets her decision to transition at age 16, saying she only received three counseling sessions prior to endocrinology referral. And she consequently had a mastectomy at age 20, which she also bitterly regrets.
So a key concern is whether young people fully understand the consequences of taking puberty blockers and therapies that may follow, including cross-sex hormones.
Witness for the appeal Gary Butler, MD, consultant in pediatric and adolescent endocrinology at University College Hospital, London, where children are referred to from GIDS for hormone treatment, said the number of children who go on to cross-sex hormones from puberty blockers is “over 80%.”
But the actual number of children who are referred to endocrinology services (where puberty blockers are initiated) from GIDS is low, at approximately 16%, according to 2019-2020 data, said a GIDS spokesperson.
“Once at the endocrinology service, young people either participate in a group education session, or if under 15 years, an individualized session between the clinician and the patient and family members,” she added. The Trust also maintained that initiation of cross-sex hormones “is separate from the prescription of puberty blockers.”
Since the December ruling, The Trust has put in place multidisciplinary clinical reviews (MDCR) of cases, and in July, NHS England will start implementing an independent multidisciplinary professional review (MDPR) to check that the GIDS has followed due process with each case.
Slow the process down, give appropriate psychotherapy
Stella O’Malley is a psychotherapist who works with transitioners and detransitioners and is a founding member of the International Association of Therapists for Desisters and Detransitioners (IATDD).
Whatever the outcome of the appeal process, Ms. O’Malley said she would like to see the Tavistock slow down and take a broader approach to counseling children before referral to endocrinology services.
In discussing therapy prior to transition, Ms. O’Malley stated that her clients often say they did not explore their inner motivations or other possible reasons for their distress, and the therapy was focused more on when they transition, rather than being sure it was something they wanted to do.
“We need to learn from the mistakes made with people like Keira Bell. , especially when [children are] ... young and especially when they’re traumatized,” Ms. O’Malley said.
“Had they received a more conventional therapy, they might have thought about their decision from different perspectives and in the process acquired more self-awareness, which would have been more beneficial.”
“The ‘affirmative’ approach to gender therapy is too narrow; we need to look at the whole individual. Therapy in other areas would never disregard other, nongender issues such as attention deficit hyperactivity disorder or anxiety [which often co-exist with gender dysphoria] – issues bleed into each other,” Ms. O’Malley pointed out. “We need a more exploratory approach.”
“I’d also like to see other therapists all over the [U.K.] who are perfectly qualified and capable of working with gender actually start working with gender issues,” she said, noting that such an approach might also help reduce the long waiting list at the Tavistock.
The latter had been overwhelmed, and this led to a speeding up of the assessment process, which led to a number of professionals resigning from the service in recent years, saying children were being “fast-tracked” to medical transition.
Fertility and sexual function are complex issues for kids
Also asked to comment was Claire Graham, from Genspect, a group that describes itself as a voice for parents of gender-questioning kids.
She told this news organization that “parents are rightly concerned about their children’s ability to consent to treatments that may lead to infertility and issues surrounding sexual function.” She added that other countries in Europe were changing their approach. “Look to Sweden and Finland, who have both rowed back on puberty blockers and no longer recommend them.”
Ms. Graham, who has worked with children with differences in sexual development, added that it was very difficult for children and young people to understand the life-long implications of decisions made at an early age.
“How can children understand what it is to live with impaired sexual functioning if they have never had sex? Likewise, fertility is a complex issue. Most people do not want to become parents as teenagers, but we understand that this will often change as they grow,” said Ms. Graham.
“Many parents worry that their child is not being considered in the whole [and] that their child’s ability to consent to medical interventions for gender dysphoria is impacted by comorbidities, such as a diagnosis of autism or a history of mental health issues. These children are particularly vulnerable.”
“At Genspect, we hope that the decision from the ... court is upheld,” Ms. Graham concluded.
A version of this article first appeared on Medscape.com.
Conservative treatment for spontaneous pneumothorax?
Background: Management of primary spontaneous pneumothorax is usually with the insertion of a chest tube and typically requires hospitalization. This procedure can result in pain, organ injury, bleeding, and infection, and, if unresolved, may require surgery, introducing additional risks and complications. Few data exist from randomized trials comparing conservative versus interventional management.
Study design: Open-label, multicenter, prospective, randomized, noninferiority trial.
Setting: A total of 39 metropolitan and rural hospitals in Australia and New Zealand.
Synopsis: Overall, 316 patients with moderate to large primary spontaneous pneumothorax were randomized (154 to the intervention group and 162 in the conservative group). In the conservative group, 25 patients (15.4%) required eventual intervention for prespecified reasons (uncontrolled pain, chest pain or shortness of breath preventing mobilization, clinical instability, enlarging pneumothorax).
In complete-case analysis, 129 out of 131 (98.5%) patients in the intervention group had resolution within 8 weeks, compared with 118 of 125 (94.4%) in the conservative group (risk difference, –4.1 percentage points; 95% confidence interval, –8.6 to 0.5, P = .02 for noninferiority).
In sensitivity analysis, in which missing data after the 8-week period were imputed as treatment failures, re-expansion occurred in 129 out of 138 (93.5%) patients in the intervention group and 118 out of 143 (82.5%) in the conservative group (risk difference, –11.0 percentage points; 95% CI, –18.4 to –3.5), which is outside the noninferiority margin of –9.0.
Overall, 41 patients in the intervention group and 13 in the conservative group had at least one adverse event.
Bottom line: Missing data limit the ability to make strong conclusions, but this trial suggests that conservative management of primary spontaneous pneumothorax was noninferior to interventional management with lower risk of serious adverse events.
Citation: Brown SG et al. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020; 382:405-15.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Management of primary spontaneous pneumothorax is usually with the insertion of a chest tube and typically requires hospitalization. This procedure can result in pain, organ injury, bleeding, and infection, and, if unresolved, may require surgery, introducing additional risks and complications. Few data exist from randomized trials comparing conservative versus interventional management.
Study design: Open-label, multicenter, prospective, randomized, noninferiority trial.
Setting: A total of 39 metropolitan and rural hospitals in Australia and New Zealand.
Synopsis: Overall, 316 patients with moderate to large primary spontaneous pneumothorax were randomized (154 to the intervention group and 162 in the conservative group). In the conservative group, 25 patients (15.4%) required eventual intervention for prespecified reasons (uncontrolled pain, chest pain or shortness of breath preventing mobilization, clinical instability, enlarging pneumothorax).
In complete-case analysis, 129 out of 131 (98.5%) patients in the intervention group had resolution within 8 weeks, compared with 118 of 125 (94.4%) in the conservative group (risk difference, –4.1 percentage points; 95% confidence interval, –8.6 to 0.5, P = .02 for noninferiority).
In sensitivity analysis, in which missing data after the 8-week period were imputed as treatment failures, re-expansion occurred in 129 out of 138 (93.5%) patients in the intervention group and 118 out of 143 (82.5%) in the conservative group (risk difference, –11.0 percentage points; 95% CI, –18.4 to –3.5), which is outside the noninferiority margin of –9.0.
Overall, 41 patients in the intervention group and 13 in the conservative group had at least one adverse event.
Bottom line: Missing data limit the ability to make strong conclusions, but this trial suggests that conservative management of primary spontaneous pneumothorax was noninferior to interventional management with lower risk of serious adverse events.
Citation: Brown SG et al. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020; 382:405-15.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Management of primary spontaneous pneumothorax is usually with the insertion of a chest tube and typically requires hospitalization. This procedure can result in pain, organ injury, bleeding, and infection, and, if unresolved, may require surgery, introducing additional risks and complications. Few data exist from randomized trials comparing conservative versus interventional management.
Study design: Open-label, multicenter, prospective, randomized, noninferiority trial.
Setting: A total of 39 metropolitan and rural hospitals in Australia and New Zealand.
Synopsis: Overall, 316 patients with moderate to large primary spontaneous pneumothorax were randomized (154 to the intervention group and 162 in the conservative group). In the conservative group, 25 patients (15.4%) required eventual intervention for prespecified reasons (uncontrolled pain, chest pain or shortness of breath preventing mobilization, clinical instability, enlarging pneumothorax).
In complete-case analysis, 129 out of 131 (98.5%) patients in the intervention group had resolution within 8 weeks, compared with 118 of 125 (94.4%) in the conservative group (risk difference, –4.1 percentage points; 95% confidence interval, –8.6 to 0.5, P = .02 for noninferiority).
In sensitivity analysis, in which missing data after the 8-week period were imputed as treatment failures, re-expansion occurred in 129 out of 138 (93.5%) patients in the intervention group and 118 out of 143 (82.5%) in the conservative group (risk difference, –11.0 percentage points; 95% CI, –18.4 to –3.5), which is outside the noninferiority margin of –9.0.
Overall, 41 patients in the intervention group and 13 in the conservative group had at least one adverse event.
Bottom line: Missing data limit the ability to make strong conclusions, but this trial suggests that conservative management of primary spontaneous pneumothorax was noninferior to interventional management with lower risk of serious adverse events.
Citation: Brown SG et al. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020; 382:405-15.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Early-onset CRC associated with longer survival
Individuals diagnosed with primary colorectal cancer (CRC) at less than 50 years of age have better survival outcomes than individuals diagnosed at 51-55 years, based on data from more than 750,000 patients.
This finding emphasizes the importance of early CRC detection in younger individuals, reported lead author En Cheng, MD, PhD, of Yale University, New Haven, Conn., and colleagues.
“Early-onset CRC (i.e., CRC diagnosed at age less than 50 years) has been characterized by unique clinical, genetic, and epigenetic characteristics, and thus it may be associated with different survival from CRC diagnosed among individuals older than 50 years,” the investigators wrote in JAMA Network Open. Previous studies comparing survival times across age groups have yielded inconsistent results.
To gain a better understanding, the investigator conducted a retrospective study using data from the National Cancer Database. Excluding patients with primary CRC who had concomitant diagnosis, history of other malignant tumors, noninvasive adenocarcinoma, or missing data, the final dataset included 769,871 patients. Early-onset CRC was defined by age less than 50 years, whereas later-onset CRC was defined by ages 51-55 years.
“Individuals diagnosed at age 50 years were excluded to minimize an apparent screening detection bias at age 50 years in our population, given that these individuals disproportionately presented with earlier stages,” the investigators wrote.
Initial comparisons across groups revealed several significant differences. Individuals in the early-onset group were more often women (47.3% vs. 43.8%; P < .001), members of races in the “other” category (6.9% vs. 5.9%; P < .001), and Medicaid patients (12.3% vs. 10.3%; P < .001). They were also more likely to be diagnosed with stage IV cancer (27.8% vs 24.1%; P < .001) and have rectal tumors (29.3% vs. 28.7%; P = .004).
In the unadjusted Kaplan-Meier analysis, patients with early-onset CRC had a lower 10-year survival rate (53.6%; 95% CI, 53.2%-54.0% vs. 54.3%; 95% CI, 53.8%-54.8%; P < .001). The fully adjusted model revealed significantly higher survival for early-onset patients, compared with later-onset patients (adjusted hazard ratio, 0.95; 95% CI, 0.93-0.96; P < .001) . This disparity deepened when adjusting only for stage (HR, 0.89; 95% CI, 0.88-0.90; P < .001).
Survival was longest among patients 35-39 years (aHR, 0.88; 95% CI, 0.84-0.92; P < .001), compared with those aged 51-55, and among early-onset individuals with stage I disease (a HR, 0.87; 95% CI, 0.81-0.93; P < .001) or stage II disease (a HR, 0.86; 95% CI, 0.82-0.90; P < .001), compared with those having the same stages of later-onset CRC. No survival advantage was observed among patients diagnosed at age 25 or younger or those with stage III or IV disease.
“Interestingly, hereditary nonpolyposis colorectal cancer, owing to underlying mismatch repair deficiency, is associated with superior survival and is often diagnosed in individuals from ages 35-45 years,” the investigators noted. “In contrast, adenomatous polyposis coli syndrome is more common in individuals who are diagnosed with CRC at age younger than 20 years (10%), compared with those diagnosed at later ages (0.1%), and adenomatous polyposis coli syndrome is not associated with a survival advantage. These high penetrance syndromes could partly account for the relative heterogeneity in survival across ages among individuals with early-onset CRC.”
Cautious about interpretation
Dr. Cheng and colleagues concluded their publication with a disclaimer: “Our finding of a survival advantage associated with early-onset CRC among younger individuals should be interpreted cautiously, given that the advantage had a small magnitude and was heterogeneous by age and stage,” they wrote. “Further study is needed to understand the underlying heterogeneity of survival by age and stage among individuals with early-onset CRC.”
Kirbi L. Yelorda, MD, of Stanford (Calif.) University, and colleagues, had a similar interpretation.
“These results offer support for effectiveness of treatment in patients diagnosed with CRC at younger ages; however, they must be interpreted within the context of epidemiological and biological factors,” Dr. Yelorda and colleagues wrote in an accompanying editorial.
The findings also suggest that the recent reduction in recommended screening age by the U.S. Preventive Services Task Force – from 50 years to 45 years – is warranted, they added, but screening younger patients remains unnecessary.
“While these results do not suggest that screening should start for patients younger than 45 years, they do support the benefit of early detection in young patients,” Dr. Yelorda and colleagues wrote, noting a “fairly low incidence rate” among individuals younger than 45, which is insufficient to justify the risk-to-benefit ratio and increased costs associated with expanded screening.
Important but not surprising
It’s “not surprising” that early-onset patients typically have better survival than later-onset patients, according to Joseph C. Anderson, MD, associate professor at White River Junction Veterans Affairs Medical Center, Hartford, Vt.; Geisel School of Medicine at Dartmouth, Hanover, N.H.; and the University of Connecticut, Farmington.
“They’re younger, have less comorbidities, and can tolerate chemotherapy,” Dr. Anderson said in an interview. “It’s not surprising that people do poorly with later stages. Younger people are no exception.”
Dr. Anderson, who previously coauthored an editorial weighing the pros and cons of earlier screening, noted that earlier screening is needed because of the rising incidence of late-stage diagnoses among younger patients, which, as the study found, are associated with worse outcomes.
Beyond adherence to screening recommendations, Dr. Anderson urged clinicians to be aggressive when doing a workup of CRC symptoms in younger patients, among whom delayed diagnoses are more common.
“We can’t just say it’s something more benign, like hemorrhoids, like we used to,” Dr. Anderson said. “Somebody who’s 30 years old and having rectal bleeding needs to be evaluated promptly – there can’t be a delay.”
The study was supported by the National Institutes of Health and Stand Up To Cancer (grant administered by the American Association for Cancer Research). The investigators disclosed relationships with Evergrande Group, Janssen, Revolution Medicines, and others. One editorialist reported serving as a member of the USPSTF when the guideline for colorectal cancer was developed, and being a coauthor on the guideline. No other disclosures were reported among editorialists. Dr. Anderson reported no relevant conflicts of interest.
Help your patients understand colorectal cancer prevention and screening options by sharing AGA’s patient education from the GI Patient Center: www.gastro.org/CRC.
Individuals diagnosed with primary colorectal cancer (CRC) at less than 50 years of age have better survival outcomes than individuals diagnosed at 51-55 years, based on data from more than 750,000 patients.
This finding emphasizes the importance of early CRC detection in younger individuals, reported lead author En Cheng, MD, PhD, of Yale University, New Haven, Conn., and colleagues.
“Early-onset CRC (i.e., CRC diagnosed at age less than 50 years) has been characterized by unique clinical, genetic, and epigenetic characteristics, and thus it may be associated with different survival from CRC diagnosed among individuals older than 50 years,” the investigators wrote in JAMA Network Open. Previous studies comparing survival times across age groups have yielded inconsistent results.
To gain a better understanding, the investigator conducted a retrospective study using data from the National Cancer Database. Excluding patients with primary CRC who had concomitant diagnosis, history of other malignant tumors, noninvasive adenocarcinoma, or missing data, the final dataset included 769,871 patients. Early-onset CRC was defined by age less than 50 years, whereas later-onset CRC was defined by ages 51-55 years.
“Individuals diagnosed at age 50 years were excluded to minimize an apparent screening detection bias at age 50 years in our population, given that these individuals disproportionately presented with earlier stages,” the investigators wrote.
Initial comparisons across groups revealed several significant differences. Individuals in the early-onset group were more often women (47.3% vs. 43.8%; P < .001), members of races in the “other” category (6.9% vs. 5.9%; P < .001), and Medicaid patients (12.3% vs. 10.3%; P < .001). They were also more likely to be diagnosed with stage IV cancer (27.8% vs 24.1%; P < .001) and have rectal tumors (29.3% vs. 28.7%; P = .004).
In the unadjusted Kaplan-Meier analysis, patients with early-onset CRC had a lower 10-year survival rate (53.6%; 95% CI, 53.2%-54.0% vs. 54.3%; 95% CI, 53.8%-54.8%; P < .001). The fully adjusted model revealed significantly higher survival for early-onset patients, compared with later-onset patients (adjusted hazard ratio, 0.95; 95% CI, 0.93-0.96; P < .001) . This disparity deepened when adjusting only for stage (HR, 0.89; 95% CI, 0.88-0.90; P < .001).
Survival was longest among patients 35-39 years (aHR, 0.88; 95% CI, 0.84-0.92; P < .001), compared with those aged 51-55, and among early-onset individuals with stage I disease (a HR, 0.87; 95% CI, 0.81-0.93; P < .001) or stage II disease (a HR, 0.86; 95% CI, 0.82-0.90; P < .001), compared with those having the same stages of later-onset CRC. No survival advantage was observed among patients diagnosed at age 25 or younger or those with stage III or IV disease.
“Interestingly, hereditary nonpolyposis colorectal cancer, owing to underlying mismatch repair deficiency, is associated with superior survival and is often diagnosed in individuals from ages 35-45 years,” the investigators noted. “In contrast, adenomatous polyposis coli syndrome is more common in individuals who are diagnosed with CRC at age younger than 20 years (10%), compared with those diagnosed at later ages (0.1%), and adenomatous polyposis coli syndrome is not associated with a survival advantage. These high penetrance syndromes could partly account for the relative heterogeneity in survival across ages among individuals with early-onset CRC.”
Cautious about interpretation
Dr. Cheng and colleagues concluded their publication with a disclaimer: “Our finding of a survival advantage associated with early-onset CRC among younger individuals should be interpreted cautiously, given that the advantage had a small magnitude and was heterogeneous by age and stage,” they wrote. “Further study is needed to understand the underlying heterogeneity of survival by age and stage among individuals with early-onset CRC.”
Kirbi L. Yelorda, MD, of Stanford (Calif.) University, and colleagues, had a similar interpretation.
“These results offer support for effectiveness of treatment in patients diagnosed with CRC at younger ages; however, they must be interpreted within the context of epidemiological and biological factors,” Dr. Yelorda and colleagues wrote in an accompanying editorial.
The findings also suggest that the recent reduction in recommended screening age by the U.S. Preventive Services Task Force – from 50 years to 45 years – is warranted, they added, but screening younger patients remains unnecessary.
“While these results do not suggest that screening should start for patients younger than 45 years, they do support the benefit of early detection in young patients,” Dr. Yelorda and colleagues wrote, noting a “fairly low incidence rate” among individuals younger than 45, which is insufficient to justify the risk-to-benefit ratio and increased costs associated with expanded screening.
Important but not surprising
It’s “not surprising” that early-onset patients typically have better survival than later-onset patients, according to Joseph C. Anderson, MD, associate professor at White River Junction Veterans Affairs Medical Center, Hartford, Vt.; Geisel School of Medicine at Dartmouth, Hanover, N.H.; and the University of Connecticut, Farmington.
“They’re younger, have less comorbidities, and can tolerate chemotherapy,” Dr. Anderson said in an interview. “It’s not surprising that people do poorly with later stages. Younger people are no exception.”
Dr. Anderson, who previously coauthored an editorial weighing the pros and cons of earlier screening, noted that earlier screening is needed because of the rising incidence of late-stage diagnoses among younger patients, which, as the study found, are associated with worse outcomes.
Beyond adherence to screening recommendations, Dr. Anderson urged clinicians to be aggressive when doing a workup of CRC symptoms in younger patients, among whom delayed diagnoses are more common.
“We can’t just say it’s something more benign, like hemorrhoids, like we used to,” Dr. Anderson said. “Somebody who’s 30 years old and having rectal bleeding needs to be evaluated promptly – there can’t be a delay.”
The study was supported by the National Institutes of Health and Stand Up To Cancer (grant administered by the American Association for Cancer Research). The investigators disclosed relationships with Evergrande Group, Janssen, Revolution Medicines, and others. One editorialist reported serving as a member of the USPSTF when the guideline for colorectal cancer was developed, and being a coauthor on the guideline. No other disclosures were reported among editorialists. Dr. Anderson reported no relevant conflicts of interest.
Help your patients understand colorectal cancer prevention and screening options by sharing AGA’s patient education from the GI Patient Center: www.gastro.org/CRC.
Individuals diagnosed with primary colorectal cancer (CRC) at less than 50 years of age have better survival outcomes than individuals diagnosed at 51-55 years, based on data from more than 750,000 patients.
This finding emphasizes the importance of early CRC detection in younger individuals, reported lead author En Cheng, MD, PhD, of Yale University, New Haven, Conn., and colleagues.
“Early-onset CRC (i.e., CRC diagnosed at age less than 50 years) has been characterized by unique clinical, genetic, and epigenetic characteristics, and thus it may be associated with different survival from CRC diagnosed among individuals older than 50 years,” the investigators wrote in JAMA Network Open. Previous studies comparing survival times across age groups have yielded inconsistent results.
To gain a better understanding, the investigator conducted a retrospective study using data from the National Cancer Database. Excluding patients with primary CRC who had concomitant diagnosis, history of other malignant tumors, noninvasive adenocarcinoma, or missing data, the final dataset included 769,871 patients. Early-onset CRC was defined by age less than 50 years, whereas later-onset CRC was defined by ages 51-55 years.
“Individuals diagnosed at age 50 years were excluded to minimize an apparent screening detection bias at age 50 years in our population, given that these individuals disproportionately presented with earlier stages,” the investigators wrote.
Initial comparisons across groups revealed several significant differences. Individuals in the early-onset group were more often women (47.3% vs. 43.8%; P < .001), members of races in the “other” category (6.9% vs. 5.9%; P < .001), and Medicaid patients (12.3% vs. 10.3%; P < .001). They were also more likely to be diagnosed with stage IV cancer (27.8% vs 24.1%; P < .001) and have rectal tumors (29.3% vs. 28.7%; P = .004).
In the unadjusted Kaplan-Meier analysis, patients with early-onset CRC had a lower 10-year survival rate (53.6%; 95% CI, 53.2%-54.0% vs. 54.3%; 95% CI, 53.8%-54.8%; P < .001). The fully adjusted model revealed significantly higher survival for early-onset patients, compared with later-onset patients (adjusted hazard ratio, 0.95; 95% CI, 0.93-0.96; P < .001) . This disparity deepened when adjusting only for stage (HR, 0.89; 95% CI, 0.88-0.90; P < .001).
Survival was longest among patients 35-39 years (aHR, 0.88; 95% CI, 0.84-0.92; P < .001), compared with those aged 51-55, and among early-onset individuals with stage I disease (a HR, 0.87; 95% CI, 0.81-0.93; P < .001) or stage II disease (a HR, 0.86; 95% CI, 0.82-0.90; P < .001), compared with those having the same stages of later-onset CRC. No survival advantage was observed among patients diagnosed at age 25 or younger or those with stage III or IV disease.
“Interestingly, hereditary nonpolyposis colorectal cancer, owing to underlying mismatch repair deficiency, is associated with superior survival and is often diagnosed in individuals from ages 35-45 years,” the investigators noted. “In contrast, adenomatous polyposis coli syndrome is more common in individuals who are diagnosed with CRC at age younger than 20 years (10%), compared with those diagnosed at later ages (0.1%), and adenomatous polyposis coli syndrome is not associated with a survival advantage. These high penetrance syndromes could partly account for the relative heterogeneity in survival across ages among individuals with early-onset CRC.”
Cautious about interpretation
Dr. Cheng and colleagues concluded their publication with a disclaimer: “Our finding of a survival advantage associated with early-onset CRC among younger individuals should be interpreted cautiously, given that the advantage had a small magnitude and was heterogeneous by age and stage,” they wrote. “Further study is needed to understand the underlying heterogeneity of survival by age and stage among individuals with early-onset CRC.”
Kirbi L. Yelorda, MD, of Stanford (Calif.) University, and colleagues, had a similar interpretation.
“These results offer support for effectiveness of treatment in patients diagnosed with CRC at younger ages; however, they must be interpreted within the context of epidemiological and biological factors,” Dr. Yelorda and colleagues wrote in an accompanying editorial.
The findings also suggest that the recent reduction in recommended screening age by the U.S. Preventive Services Task Force – from 50 years to 45 years – is warranted, they added, but screening younger patients remains unnecessary.
“While these results do not suggest that screening should start for patients younger than 45 years, they do support the benefit of early detection in young patients,” Dr. Yelorda and colleagues wrote, noting a “fairly low incidence rate” among individuals younger than 45, which is insufficient to justify the risk-to-benefit ratio and increased costs associated with expanded screening.
Important but not surprising
It’s “not surprising” that early-onset patients typically have better survival than later-onset patients, according to Joseph C. Anderson, MD, associate professor at White River Junction Veterans Affairs Medical Center, Hartford, Vt.; Geisel School of Medicine at Dartmouth, Hanover, N.H.; and the University of Connecticut, Farmington.
“They’re younger, have less comorbidities, and can tolerate chemotherapy,” Dr. Anderson said in an interview. “It’s not surprising that people do poorly with later stages. Younger people are no exception.”
Dr. Anderson, who previously coauthored an editorial weighing the pros and cons of earlier screening, noted that earlier screening is needed because of the rising incidence of late-stage diagnoses among younger patients, which, as the study found, are associated with worse outcomes.
Beyond adherence to screening recommendations, Dr. Anderson urged clinicians to be aggressive when doing a workup of CRC symptoms in younger patients, among whom delayed diagnoses are more common.
“We can’t just say it’s something more benign, like hemorrhoids, like we used to,” Dr. Anderson said. “Somebody who’s 30 years old and having rectal bleeding needs to be evaluated promptly – there can’t be a delay.”
The study was supported by the National Institutes of Health and Stand Up To Cancer (grant administered by the American Association for Cancer Research). The investigators disclosed relationships with Evergrande Group, Janssen, Revolution Medicines, and others. One editorialist reported serving as a member of the USPSTF when the guideline for colorectal cancer was developed, and being a coauthor on the guideline. No other disclosures were reported among editorialists. Dr. Anderson reported no relevant conflicts of interest.
Help your patients understand colorectal cancer prevention and screening options by sharing AGA’s patient education from the GI Patient Center: www.gastro.org/CRC.
FROM JAMA NETWORK OPEN
Britney Spears battles conservatorship for mental health protection
In a 24-minute statement, Ms. Spears told the judge overseeing the conservatorship that she wants it to end “without having to be evaluated.” She called the arrangement “abusive” and said she’s been “traumatized” and “in denial” despite the upbeat messages that she’s posted on Instagram during the past year, according to Reuters.
“I just want my life back,” she said. “I’m not here to be anyone’s slave.”
After the statement, the court recessed, and the audio transmission was stopped, Reuters reported. Full transcripts have been published by several news outlets, including this lightly edited version by Variety.
Ms. Spears’ statement came as a shock after years of silence about the conservatorship. Public speculation about the arrangement has resurfaced during the past year because of the #FreeBritney movement on social media, news reports of leaked court documents, and a 2021 documentary that showed she may feel trapped.
During the hearing, Ms. Spears spoke by phone to Los Angeles Superior Court Judge Brenda Penny about the court-approved arrangement that began in 2008 after she had a mental health breakdown. Judge Penny said Ms. Spears would need to submit a petition to the court to ask for the conservatorship to be terminated.
Under the terms of the conservatorship, Ms. Spears would have to demonstrate that she can take responsibility for her personal and financial affairs, Reuters reported. During the hearing, Judge Penny supported Ms. Spears for speaking out.
“I know it took a lot of courage for you to say everything you have to say today,” she said. “I want to let you know that the court does appreciate your coming on the line and sharing how you’re feeling.”
Ms. Spears, 39, said she wanted to get married again and have a baby but that she’s not allowed to go to the doctor to get a contraceptive device removed. She spoke up about her mental health and said doctors had put her on the drug lithium, which made her less able to function. Ms. Spears also said she was forced to perform in the past and is now required to attend numerous therapy sessions each week against her will.
“I’m not happy. I can’t sleep. I’m so angry, it’s insane,” she said. “And I’m depressed. I cry every day.”
Ms. Spears last spoke with the court in May 2019, but the hearing was closed to the public and her testimony was sealed. Ms. Spears recently said she wanted people to hear her thoughts.
“I feel ganged-up on and I feel bullied and I feel left out and alone,” she said. “I deserve to have the same rights as anybody does, by having a child, a family, any of those things, and more so.”
A version of this article first appeared on WebMD.com.
In a 24-minute statement, Ms. Spears told the judge overseeing the conservatorship that she wants it to end “without having to be evaluated.” She called the arrangement “abusive” and said she’s been “traumatized” and “in denial” despite the upbeat messages that she’s posted on Instagram during the past year, according to Reuters.
“I just want my life back,” she said. “I’m not here to be anyone’s slave.”
After the statement, the court recessed, and the audio transmission was stopped, Reuters reported. Full transcripts have been published by several news outlets, including this lightly edited version by Variety.
Ms. Spears’ statement came as a shock after years of silence about the conservatorship. Public speculation about the arrangement has resurfaced during the past year because of the #FreeBritney movement on social media, news reports of leaked court documents, and a 2021 documentary that showed she may feel trapped.
During the hearing, Ms. Spears spoke by phone to Los Angeles Superior Court Judge Brenda Penny about the court-approved arrangement that began in 2008 after she had a mental health breakdown. Judge Penny said Ms. Spears would need to submit a petition to the court to ask for the conservatorship to be terminated.
Under the terms of the conservatorship, Ms. Spears would have to demonstrate that she can take responsibility for her personal and financial affairs, Reuters reported. During the hearing, Judge Penny supported Ms. Spears for speaking out.
“I know it took a lot of courage for you to say everything you have to say today,” she said. “I want to let you know that the court does appreciate your coming on the line and sharing how you’re feeling.”
Ms. Spears, 39, said she wanted to get married again and have a baby but that she’s not allowed to go to the doctor to get a contraceptive device removed. She spoke up about her mental health and said doctors had put her on the drug lithium, which made her less able to function. Ms. Spears also said she was forced to perform in the past and is now required to attend numerous therapy sessions each week against her will.
“I’m not happy. I can’t sleep. I’m so angry, it’s insane,” she said. “And I’m depressed. I cry every day.”
Ms. Spears last spoke with the court in May 2019, but the hearing was closed to the public and her testimony was sealed. Ms. Spears recently said she wanted people to hear her thoughts.
“I feel ganged-up on and I feel bullied and I feel left out and alone,” she said. “I deserve to have the same rights as anybody does, by having a child, a family, any of those things, and more so.”
A version of this article first appeared on WebMD.com.
In a 24-minute statement, Ms. Spears told the judge overseeing the conservatorship that she wants it to end “without having to be evaluated.” She called the arrangement “abusive” and said she’s been “traumatized” and “in denial” despite the upbeat messages that she’s posted on Instagram during the past year, according to Reuters.
“I just want my life back,” she said. “I’m not here to be anyone’s slave.”
After the statement, the court recessed, and the audio transmission was stopped, Reuters reported. Full transcripts have been published by several news outlets, including this lightly edited version by Variety.
Ms. Spears’ statement came as a shock after years of silence about the conservatorship. Public speculation about the arrangement has resurfaced during the past year because of the #FreeBritney movement on social media, news reports of leaked court documents, and a 2021 documentary that showed she may feel trapped.
During the hearing, Ms. Spears spoke by phone to Los Angeles Superior Court Judge Brenda Penny about the court-approved arrangement that began in 2008 after she had a mental health breakdown. Judge Penny said Ms. Spears would need to submit a petition to the court to ask for the conservatorship to be terminated.
Under the terms of the conservatorship, Ms. Spears would have to demonstrate that she can take responsibility for her personal and financial affairs, Reuters reported. During the hearing, Judge Penny supported Ms. Spears for speaking out.
“I know it took a lot of courage for you to say everything you have to say today,” she said. “I want to let you know that the court does appreciate your coming on the line and sharing how you’re feeling.”
Ms. Spears, 39, said she wanted to get married again and have a baby but that she’s not allowed to go to the doctor to get a contraceptive device removed. She spoke up about her mental health and said doctors had put her on the drug lithium, which made her less able to function. Ms. Spears also said she was forced to perform in the past and is now required to attend numerous therapy sessions each week against her will.
“I’m not happy. I can’t sleep. I’m so angry, it’s insane,” she said. “And I’m depressed. I cry every day.”
Ms. Spears last spoke with the court in May 2019, but the hearing was closed to the public and her testimony was sealed. Ms. Spears recently said she wanted people to hear her thoughts.
“I feel ganged-up on and I feel bullied and I feel left out and alone,” she said. “I deserve to have the same rights as anybody does, by having a child, a family, any of those things, and more so.”
A version of this article first appeared on WebMD.com.
Disturbing number of hospital workers still unvaccinated
Tim Oswalt had been in a Fort Worth, Texas, hospital for over a month, receiving treatment for a grapefruit-sized tumor in his chest that was pressing on his heart and lungs. It turned out to be stage 3 non-Hodgkin lymphoma.
Then one day in January, he was moved from his semi-private room to an isolated one with special ventilation. The staff explained he had been infected by the virus that was once again surging in many areas of the country, including Texas.
“How the hell did I catch COVID?” he asked the staff, who now approached him in full moon-suit personal protective equipment (PPE).
The hospital was locked down, and Mr. Oswalt hadn’t had any visitors in weeks. Neither of his two roommates tested positive. He’d been tested for COVID several times over the course of his nearly 5-week stay and was always negative.
“‘Well, you know, it’s easy to [catch it] in a hospital,’” Mr. Oswalt said he was told by hospital staff. “‘We’re having a bad outbreak. So you were just exposed somehow.’”

Officials at John Peter Smith Hospital, where Mr. Oswalt was treated, said they are puzzled by his case. According to their infection prevention team, none of his caregivers tested positive for COVID-19, nor did Mr. Oswalt share space with any other COVID-positive patients. And yet, local media reported a surge in cases among JPS hospital staff in December.
“Infection of any kind is a constant battle within hospitals and one that we all take seriously,” said Rob Stephenson, MD, chief quality officer at JPS Health Network. “Anyone in a vulnerable health condition at the height of the pandemic would have been at greater risk for contracting COVID-19 inside – or even more so, outside – the hospital.”
Mr. Oswalt was diagnosed with COVID in early January. JPS Hospital began vaccinating its health care workers about 2 weeks earlier, so there had not yet been enough time for any of them to develop full protection against catching or spreading the virus.
Today, the hospital said 74% of its staff – 5,300 of 7,200 workers – are now vaccinated.
against the SARS-CoV2 virus.
Refusing vaccinations
In fact, nationwide, 1 in 4 hospital workers who have direct contact with patients had not yet received a single dose of a COVID vaccine by the end of May, according to a WebMD and Medscape Medical News analysis of data collected by the U.S. Department of Health and Human Services (HHS) from 2,500 hospitals across the United States.
Among the nation’s 50 largest hospitals, the percentage of unvaccinated health care workers appears to be even larger, about 1 in 3. Vaccination rates range from a high of 99% at Houston Methodist Hospital, which was the first in the nation to mandate the shots for its workers, to a low between 30% and 40% at some hospitals in Florida.
Memorial Hermann Texas Medical Center in Houston has 1,180 beds and sits less than half a mile from Houston Methodist Hospital. But in terms of worker vaccinations, it is farther away.
Memorial Hermann reported to HHS that about 32% of its 28,000 workers haven’t been inoculated. The hospital’s PR office contests that figure, putting it closer to 25% unvaccinated across their health system. The hospital said it is boosting participation by offering a $300 “shot of hope” bonus to workers who start their vaccination series by the end of June.
Lakeland Regional Medical Center in Lakeland, Fla., reported to HHS that 63% of its health care personnel are still unvaccinated. The hospital did not return a call to verify that number.
To boost vaccination rates, more hospitals are starting to require the shots, after the Equal Employment Opportunity Commission gave its green light to mandates in May.
“It’s a real problem that you have such high levels of unvaccinated individuals in hospitals,” said Lawrence Gostin, JD, director of the O’Neill Institute for National and Global Health Law at Georgetown University, Washington.
“We have to protect our health workforce, and we have to protect our patients. Hospitals should be the safest places in the country, and the only way to make them safe is to have a fully vaccinated workforce,” Mr. Gostin said.
Is the data misleading?
The HHS system designed to amass hospital data was set up quickly, to respond to an emergency. For that reason, experts say the information hasn’t been as carefully collected or vetted as it normally would have been. Some hospitals may have misunderstood how to report their vaccination numbers.
In addition, reporting data on worker vaccinations is voluntary. Only about half of hospitals have chosen to share their numbers. In other cases, like Texas, states have blocked the public release of these statistics.
AdventHealth Orlando, a 1,300-bed hospital in Florida, reported to HHS that 56% of its staff have not started their shots. But spokesman Jeff Grainger said the figures probably overstate the number of unvaccinated workers because the hospital doesn’t always know when people get vaccinated outside of its campus, at a local pharmacy, for example.
For those reasons, the picture of health care worker vaccinations across the country is incomplete.
Where hospitals fall behind
Even if the data are flawed, the vaccination rates from hospitals mirror the general population. A May Gallup poll, for example, found 24% of Americans said they definitely won’t get the vaccine. Another 12% say they plan to get it but are waiting.
The data also align with recent studies. A review of 35 studies by researchers at New Mexico State University that assessed hesitancy in more than 76,000 health care workers around the world found about 23% of them were reluctant to get the shots.
An ongoing monthly survey of more than 1.9 million U.S. Facebook users led by researchers at Carnegie Mellon University, Pittsburgh recently looked at vaccine hesitancy by occupation. It revealed a spectrum of hesitancy among health care workers corresponding to income and education, ranging from a low of 9% among pharmacists to highs of 20%-23% among nursing aides and emergency medical technicians. About 12% of registered nurses and doctors admitted to being hesitant to get a shot.
“Health care workers are not monolithic,” said study author Jagdish Khubchandani, professor of public health sciences at New Mexico State.
“There’s a big divide between males, doctoral degree holders, older people and the younger low-income, low-education frontline, female, health care workers. They are the most hesitant,” he said. Support staff typically outnumbers doctors at hospitals about 3 to 1.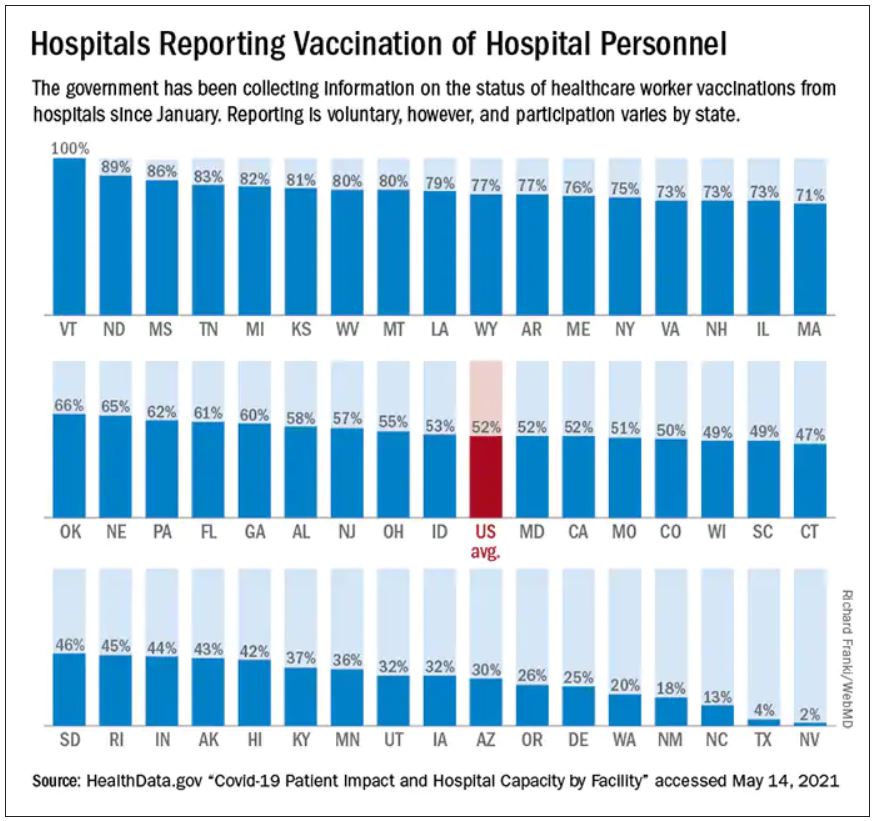
“There is outreach work to be done there,” said Robin Mejia, PhD, director of the Statistics and Human Rights Program at Carnegie Mellon, who is leading the study on Facebook’s survey data. “These are also high-contact professions. These are people who are seeing patients on a regular basis.”
That’s why, when the Centers for Disease Control and Prevention was planning the national vaccine rollout, they prioritized health care workers for the initially scarce first doses. The intent was to protect vulnerable workers and their patients who are at high risk of infection. But the CDC had another reason for putting health care workers first: After they were safely vaccinated, the hope was that they would encourage wary patients to do the same.
Hospitals were supposed to be hubs of education to help build trust within less confident communities. But not all hospitals have risen to that challenge.
Political affiliation seems to be one contributing factor in vaccine hesitancy. Take for example Calhoun, Ga., the seat of Gordon County, where residents voted for Donald Trump over Joe Biden by a 67-point margin in the 2020 general election. Studies have found that Republicans are more likely to decline vaccines than Democrats.
People who live in rural areas are less likely to be vaccinated than those who live in cities, and that’s true in Gordon County too. Vaccinations are lagging in this northwest corner of Georgia where factory jobs in chicken processing plants and carpet manufacturing energize the local economy. Just 24% of Gordon County residents are fully vaccinated, according to the Georgia Department of Public Health.
At AdventHealth Gordon, a 112-bed hospital in Calhoun, just 35% of the 1,723 workers that serve the hospital are at least partially vaccinated, according to data reported to HHS.
‘I am not vaccinated’
One reason some hospital staff say they are resisting COVID vaccination is because it’s so new and not yet fully approved by the FDA.
“I am not vaccinated,” said a social services worker for AdventHealth Gordon who asked that her name not be used because she was unauthorized to speak to this news organization and Georgia Health News (who collaborated on this project). “I just have not felt the need to do that at this time.”
The woman said she doesn’t have a problem with vaccines. She gets the flu shot every year. “I’ve been vaccinated all my life,” she said. But she doesn’t view COVID-19 vaccination in the same way.
“I want to see more testing done,” she said. “It took a long time to get a flu vaccine, and we made a COVID vaccine in 6 months. I want to know, before I start putting something into my body, that the testing is done.”
Staff at her hospital were given the option to be vaccinated or wear a mask. She chose the mask.
Many of her coworkers share her feelings, she said.
Mask expert Linsey Marr, PhD, a professor of civil and environmental engineering at Virginia Tech University, Blacksburg, Va., said N95 masks and vaccines are both highly effective, but the protection from the vaccine is superior because it is continuous.
“It’s hard to wear an N95 at all times. You have to take it off to eat, for example, in a break room in a hospital. I should point out that you can be exposed to the virus in other buildings besides a hospital – restaurants, stores, people’s homes – and because someone can be infected without symptoms, you could easily be around an infected person without knowing it,” she said.
Eventually, staff at AdventHealth Gordon may get a stronger nudge to get the shots. Chief Medical Officer Joseph Joyave, MD, said AdventHealth asks workers to get flu vaccines or provide the hospital with a reason why they won’t. He expects a similar policy will be adopted for COVID vaccines once they are fully licensed by the FDA.
In the meantime, he does not believe that the hospital is putting patients at risk with its low vaccination rate. “We continue to use PPE, masking in all clinical areas, and continue to screen daily all employees and visitors,” he said.
AdventHealth, the 12th largest hospital system in the nation with 49 hospitals, has at least 20 hospitals with vaccination rates lower than 50%, according to HHS data.
Other hospital systems have approached hesitation around the COVID vaccines differently.
When infectious disease experts at Vanderbilt Hospital in Nashville realized early on that many of their workers felt unsure about the vaccines, they set out to provide a wealth of information.
“There was a lot of hesitancy and skepticism,” said William Schaffner, MD, a professor of preventive medicine and infectious disease at Vanderbilt. So the infectious disease division put together a multifaceted program including Q&As, educational sessions, and one-on-one visits with employees “from the custodians all the way up to the C-Suite,” he said.
Today, HHS data shows the hospital is 83% vaccinated. Dr. Schaffner thinks the true number is probably higher, about 90%. “We’re very pleased with that,” he said.
In his experience with flu vaccinations, it was extremely difficult in the first year to get workers to take flu shots. The second year it was easier. By the third year it was humdrum, he said, because it had become a cultural norm.
Dr. Schaffner expects winning people over to the COVID vaccines will follow a similar course, but “we’re not there yet,” he said.
Protecting patients and caregivers
There is no question that health care workers carried a heavy load through the worst months of the pandemic. Many of them worked to the point of exhaustion and burnout. Some were the only conduits between isolated patients and their families, holding hands and mobile phones so distanced loved ones could video chat. Many were left inadequately protected because of shortages of masks, gowns, gloves, and other gear.
An investigation by Kaiser Health News and The Guardian recently revealed that more than 3,600 health care workers died in COVID’s first year in the United States.
Vaccination of health care workers is important to protect these frontline workers and their families who will continue to be at risk of coming into contact with the infection, even as the number of cases falls.
Hesitancy in health care is also dangerous because these clinicians and allied health workers – who may not show any symptoms – can also carry the virus to someone who wouldn’t survive an infection, including patients with organ transplants, those with autoimmune diseases, premature infants, and the elderly.
It is not known how often patients in the United States are infected with COVID in health care settings, but case reports reveal that hospitals are still experiencing outbreaks.
On June 1, Northern Lights A.R. Gould Hospital in Presque Isle, Maine, announced a COVID outbreak on its medical-surgical unit. As of June 22, 13 residents and staff have caught the virus, according to the Maine Centers for Disease Control, which is investigating. Four of the first five staff members to test positive had not been fully vaccinated.
According to HHS data, about 20% of the health care workers at that hospital are still unvaccinated.
Oregon Health & Science University experienced a COVID outbreak connected to the hospital’s cardiovascular care unit from April to mid-May of this year. According to hospital spokesperson Tracy Brawley, a patient visitor brought the infection to campus, where it ultimately spread to 14 others, including “patients, visitors, employees, and learners.”
In a written statement, the hospital said “nearly all” health care workers who tested positive were previously vaccinated and experienced no symptoms or only minor ones. The hospital said it hasn’t identified any onward transmission from health care workers to patients, and also stated: “It is not yet understood how transmission may have occurred between patients, visitors, and health care workers.”
In March, an unvaccinated health care worker in Kentucky carried a SARS-CoV-2 variant back to the nursing home where the person worked. Some 90% of the residents were fully vaccinated. Ultimately, 26 patients were infected; 18 of them were fully vaccinated. And 20 health care workers, four of whom were vaccinated, were infected.
Vaccines slowed the virus down and made infections less severe, but in this fragile population, they couldn’t stop it completely. One resident, who had survived a bout of COVID almost a year earlier, died. According to the CDC’s Morbidity and Mortality Weekly Report, 47% of the workers in that facility were unvaccinated.
In the United Kingdom, statistics collected through that country’s National Health Service also suggest a heavy toll. More than 32,300 patients caught COVID in English hospitals since March 2020. Up to 8,700 of them died, according to a recent analysis by The Guardian. The U.K. government recently made COVID vaccinations mandatory for health care workers.
COVID delays cancer care
When Mr. Oswalt, the Fort Worth, Texas man with non-Hodgkin lymphoma, contracted COVID-19, the virus took down his kidneys first. Toxins were building up in his blood, so doctors prescribed dialysis to support his body and buy his kidneys time to heal.
He was in one of these dialysis treatments when his lungs succumbed.
“Look, I can’t breathe,” he told the nurse who was supervising his treatment. The nurse gestured to an oxygen tank already hanging by his side, and said, “You should be OK.”
But he wasn’t.
“I can’t breathe,” Mr. Oswalt said again. Then the air hunger hit. Mr. Oswalt began gasping and couldn’t stop. Today, his voice breaks when he describes this moment. “A lot of it becomes a blur.”
When Mr. Oswalt, 61, regained consciousness, he was hooked up to a ventilator to ease his breathing.
For days, Mr. Oswalt clung to the edge of life. His wife, Molly, who wasn’t allowed to see him in the hospital, got a call that he might not make it through the night. She made frantic phone calls to her brother and sister and prayed.
Mr. Oswalt was on a ventilator for about a week. His kidneys and lungs healed enough so that he could restart his chemotherapy. He was eventually discharged home on January 22.
The last time he was scanned, the large tumor in his chest had shrunk from the size of a grapefruit to the size of a dime.
But having COVID on top of cancer has had a devastating effect on his life. Before he got sick, Molly said, he couldn’t stay still. He was busy all the time. After spending months in the hospital, his energy was depleted. He couldn’t keep his swimming pool installation business going.
He and Molly had to give up their house in Fort Worth and move in with family in Amarillo. He has had to pause his cancer treatments while doctors wait for his kidneys to heal. Relatives have been raising money on GoFundMe to pay their bills.
Months after moving across the state to Amarillo and hoping for better days, Tim said he got good news this week: He no longer needs dialysis. A new round of tests found no signs of cancer. His white blood cell count is back to normal. His lymph nodes are no longer swollen.
He goes back for another scan in a few weeks, but the doctor told him she isn’t going to recommend any further chemo at this point.
“It was shocking, to tell you the truth. It still is. When I talk about it, I get kind of emotional” about his recovery, he said.
Tim said he was really dreading more chemotherapy. His hair has just started growing back. He can finally taste food again. He wasn’t ready to face more side effects from the treatments, or the COVID – he no longer knows exactly which diagnosis led to his most debilitating symptoms.
He said his ordeal has left him with no patience for health care workers who don’t think they need to be vaccinated.
The way he sees it, it’s no different than the electrical training he had to get before he could wire the lights and pumps in a swimming pool.
“You know, if I don’t certify and keep my license, I can’t work on anything electrical. So, if I’ve made the choice not to go down and take the test and get a license, then I made the choice not to work on electrical stuff,” he said.
He supports the growing number of hospitals that have made vaccination mandatory for their workers.
“They don’t let electricians put people at risk. And they shouldn’t let health care workers for sure,” he said.
A version of this article first appeared on Medscape.com.
Tim Oswalt had been in a Fort Worth, Texas, hospital for over a month, receiving treatment for a grapefruit-sized tumor in his chest that was pressing on his heart and lungs. It turned out to be stage 3 non-Hodgkin lymphoma.
Then one day in January, he was moved from his semi-private room to an isolated one with special ventilation. The staff explained he had been infected by the virus that was once again surging in many areas of the country, including Texas.
“How the hell did I catch COVID?” he asked the staff, who now approached him in full moon-suit personal protective equipment (PPE).
The hospital was locked down, and Mr. Oswalt hadn’t had any visitors in weeks. Neither of his two roommates tested positive. He’d been tested for COVID several times over the course of his nearly 5-week stay and was always negative.
“‘Well, you know, it’s easy to [catch it] in a hospital,’” Mr. Oswalt said he was told by hospital staff. “‘We’re having a bad outbreak. So you were just exposed somehow.’”

Officials at John Peter Smith Hospital, where Mr. Oswalt was treated, said they are puzzled by his case. According to their infection prevention team, none of his caregivers tested positive for COVID-19, nor did Mr. Oswalt share space with any other COVID-positive patients. And yet, local media reported a surge in cases among JPS hospital staff in December.
“Infection of any kind is a constant battle within hospitals and one that we all take seriously,” said Rob Stephenson, MD, chief quality officer at JPS Health Network. “Anyone in a vulnerable health condition at the height of the pandemic would have been at greater risk for contracting COVID-19 inside – or even more so, outside – the hospital.”
Mr. Oswalt was diagnosed with COVID in early January. JPS Hospital began vaccinating its health care workers about 2 weeks earlier, so there had not yet been enough time for any of them to develop full protection against catching or spreading the virus.
Today, the hospital said 74% of its staff – 5,300 of 7,200 workers – are now vaccinated.
against the SARS-CoV2 virus.
Refusing vaccinations
In fact, nationwide, 1 in 4 hospital workers who have direct contact with patients had not yet received a single dose of a COVID vaccine by the end of May, according to a WebMD and Medscape Medical News analysis of data collected by the U.S. Department of Health and Human Services (HHS) from 2,500 hospitals across the United States.
Among the nation’s 50 largest hospitals, the percentage of unvaccinated health care workers appears to be even larger, about 1 in 3. Vaccination rates range from a high of 99% at Houston Methodist Hospital, which was the first in the nation to mandate the shots for its workers, to a low between 30% and 40% at some hospitals in Florida.
Memorial Hermann Texas Medical Center in Houston has 1,180 beds and sits less than half a mile from Houston Methodist Hospital. But in terms of worker vaccinations, it is farther away.
Memorial Hermann reported to HHS that about 32% of its 28,000 workers haven’t been inoculated. The hospital’s PR office contests that figure, putting it closer to 25% unvaccinated across their health system. The hospital said it is boosting participation by offering a $300 “shot of hope” bonus to workers who start their vaccination series by the end of June.
Lakeland Regional Medical Center in Lakeland, Fla., reported to HHS that 63% of its health care personnel are still unvaccinated. The hospital did not return a call to verify that number.
To boost vaccination rates, more hospitals are starting to require the shots, after the Equal Employment Opportunity Commission gave its green light to mandates in May.
“It’s a real problem that you have such high levels of unvaccinated individuals in hospitals,” said Lawrence Gostin, JD, director of the O’Neill Institute for National and Global Health Law at Georgetown University, Washington.
“We have to protect our health workforce, and we have to protect our patients. Hospitals should be the safest places in the country, and the only way to make them safe is to have a fully vaccinated workforce,” Mr. Gostin said.
Is the data misleading?
The HHS system designed to amass hospital data was set up quickly, to respond to an emergency. For that reason, experts say the information hasn’t been as carefully collected or vetted as it normally would have been. Some hospitals may have misunderstood how to report their vaccination numbers.
In addition, reporting data on worker vaccinations is voluntary. Only about half of hospitals have chosen to share their numbers. In other cases, like Texas, states have blocked the public release of these statistics.
AdventHealth Orlando, a 1,300-bed hospital in Florida, reported to HHS that 56% of its staff have not started their shots. But spokesman Jeff Grainger said the figures probably overstate the number of unvaccinated workers because the hospital doesn’t always know when people get vaccinated outside of its campus, at a local pharmacy, for example.
For those reasons, the picture of health care worker vaccinations across the country is incomplete.
Where hospitals fall behind
Even if the data are flawed, the vaccination rates from hospitals mirror the general population. A May Gallup poll, for example, found 24% of Americans said they definitely won’t get the vaccine. Another 12% say they plan to get it but are waiting.
The data also align with recent studies. A review of 35 studies by researchers at New Mexico State University that assessed hesitancy in more than 76,000 health care workers around the world found about 23% of them were reluctant to get the shots.
An ongoing monthly survey of more than 1.9 million U.S. Facebook users led by researchers at Carnegie Mellon University, Pittsburgh recently looked at vaccine hesitancy by occupation. It revealed a spectrum of hesitancy among health care workers corresponding to income and education, ranging from a low of 9% among pharmacists to highs of 20%-23% among nursing aides and emergency medical technicians. About 12% of registered nurses and doctors admitted to being hesitant to get a shot.
“Health care workers are not monolithic,” said study author Jagdish Khubchandani, professor of public health sciences at New Mexico State.
“There’s a big divide between males, doctoral degree holders, older people and the younger low-income, low-education frontline, female, health care workers. They are the most hesitant,” he said. Support staff typically outnumbers doctors at hospitals about 3 to 1.
“There is outreach work to be done there,” said Robin Mejia, PhD, director of the Statistics and Human Rights Program at Carnegie Mellon, who is leading the study on Facebook’s survey data. “These are also high-contact professions. These are people who are seeing patients on a regular basis.”
That’s why, when the Centers for Disease Control and Prevention was planning the national vaccine rollout, they prioritized health care workers for the initially scarce first doses. The intent was to protect vulnerable workers and their patients who are at high risk of infection. But the CDC had another reason for putting health care workers first: After they were safely vaccinated, the hope was that they would encourage wary patients to do the same.
Hospitals were supposed to be hubs of education to help build trust within less confident communities. But not all hospitals have risen to that challenge.
Political affiliation seems to be one contributing factor in vaccine hesitancy. Take for example Calhoun, Ga., the seat of Gordon County, where residents voted for Donald Trump over Joe Biden by a 67-point margin in the 2020 general election. Studies have found that Republicans are more likely to decline vaccines than Democrats.
People who live in rural areas are less likely to be vaccinated than those who live in cities, and that’s true in Gordon County too. Vaccinations are lagging in this northwest corner of Georgia where factory jobs in chicken processing plants and carpet manufacturing energize the local economy. Just 24% of Gordon County residents are fully vaccinated, according to the Georgia Department of Public Health.
At AdventHealth Gordon, a 112-bed hospital in Calhoun, just 35% of the 1,723 workers that serve the hospital are at least partially vaccinated, according to data reported to HHS.
‘I am not vaccinated’
One reason some hospital staff say they are resisting COVID vaccination is because it’s so new and not yet fully approved by the FDA.
“I am not vaccinated,” said a social services worker for AdventHealth Gordon who asked that her name not be used because she was unauthorized to speak to this news organization and Georgia Health News (who collaborated on this project). “I just have not felt the need to do that at this time.”
The woman said she doesn’t have a problem with vaccines. She gets the flu shot every year. “I’ve been vaccinated all my life,” she said. But she doesn’t view COVID-19 vaccination in the same way.
“I want to see more testing done,” she said. “It took a long time to get a flu vaccine, and we made a COVID vaccine in 6 months. I want to know, before I start putting something into my body, that the testing is done.”
Staff at her hospital were given the option to be vaccinated or wear a mask. She chose the mask.
Many of her coworkers share her feelings, she said.
Mask expert Linsey Marr, PhD, a professor of civil and environmental engineering at Virginia Tech University, Blacksburg, Va., said N95 masks and vaccines are both highly effective, but the protection from the vaccine is superior because it is continuous.
“It’s hard to wear an N95 at all times. You have to take it off to eat, for example, in a break room in a hospital. I should point out that you can be exposed to the virus in other buildings besides a hospital – restaurants, stores, people’s homes – and because someone can be infected without symptoms, you could easily be around an infected person without knowing it,” she said.
Eventually, staff at AdventHealth Gordon may get a stronger nudge to get the shots. Chief Medical Officer Joseph Joyave, MD, said AdventHealth asks workers to get flu vaccines or provide the hospital with a reason why they won’t. He expects a similar policy will be adopted for COVID vaccines once they are fully licensed by the FDA.
In the meantime, he does not believe that the hospital is putting patients at risk with its low vaccination rate. “We continue to use PPE, masking in all clinical areas, and continue to screen daily all employees and visitors,” he said.
AdventHealth, the 12th largest hospital system in the nation with 49 hospitals, has at least 20 hospitals with vaccination rates lower than 50%, according to HHS data.
Other hospital systems have approached hesitation around the COVID vaccines differently.
When infectious disease experts at Vanderbilt Hospital in Nashville realized early on that many of their workers felt unsure about the vaccines, they set out to provide a wealth of information.
“There was a lot of hesitancy and skepticism,” said William Schaffner, MD, a professor of preventive medicine and infectious disease at Vanderbilt. So the infectious disease division put together a multifaceted program including Q&As, educational sessions, and one-on-one visits with employees “from the custodians all the way up to the C-Suite,” he said.
Today, HHS data shows the hospital is 83% vaccinated. Dr. Schaffner thinks the true number is probably higher, about 90%. “We’re very pleased with that,” he said.
In his experience with flu vaccinations, it was extremely difficult in the first year to get workers to take flu shots. The second year it was easier. By the third year it was humdrum, he said, because it had become a cultural norm.
Dr. Schaffner expects winning people over to the COVID vaccines will follow a similar course, but “we’re not there yet,” he said.
Protecting patients and caregivers
There is no question that health care workers carried a heavy load through the worst months of the pandemic. Many of them worked to the point of exhaustion and burnout. Some were the only conduits between isolated patients and their families, holding hands and mobile phones so distanced loved ones could video chat. Many were left inadequately protected because of shortages of masks, gowns, gloves, and other gear.
An investigation by Kaiser Health News and The Guardian recently revealed that more than 3,600 health care workers died in COVID’s first year in the United States.
Vaccination of health care workers is important to protect these frontline workers and their families who will continue to be at risk of coming into contact with the infection, even as the number of cases falls.
Hesitancy in health care is also dangerous because these clinicians and allied health workers – who may not show any symptoms – can also carry the virus to someone who wouldn’t survive an infection, including patients with organ transplants, those with autoimmune diseases, premature infants, and the elderly.
It is not known how often patients in the United States are infected with COVID in health care settings, but case reports reveal that hospitals are still experiencing outbreaks.
On June 1, Northern Lights A.R. Gould Hospital in Presque Isle, Maine, announced a COVID outbreak on its medical-surgical unit. As of June 22, 13 residents and staff have caught the virus, according to the Maine Centers for Disease Control, which is investigating. Four of the first five staff members to test positive had not been fully vaccinated.
According to HHS data, about 20% of the health care workers at that hospital are still unvaccinated.
Oregon Health & Science University experienced a COVID outbreak connected to the hospital’s cardiovascular care unit from April to mid-May of this year. According to hospital spokesperson Tracy Brawley, a patient visitor brought the infection to campus, where it ultimately spread to 14 others, including “patients, visitors, employees, and learners.”
In a written statement, the hospital said “nearly all” health care workers who tested positive were previously vaccinated and experienced no symptoms or only minor ones. The hospital said it hasn’t identified any onward transmission from health care workers to patients, and also stated: “It is not yet understood how transmission may have occurred between patients, visitors, and health care workers.”
In March, an unvaccinated health care worker in Kentucky carried a SARS-CoV-2 variant back to the nursing home where the person worked. Some 90% of the residents were fully vaccinated. Ultimately, 26 patients were infected; 18 of them were fully vaccinated. And 20 health care workers, four of whom were vaccinated, were infected.
Vaccines slowed the virus down and made infections less severe, but in this fragile population, they couldn’t stop it completely. One resident, who had survived a bout of COVID almost a year earlier, died. According to the CDC’s Morbidity and Mortality Weekly Report, 47% of the workers in that facility were unvaccinated.
In the United Kingdom, statistics collected through that country’s National Health Service also suggest a heavy toll. More than 32,300 patients caught COVID in English hospitals since March 2020. Up to 8,700 of them died, according to a recent analysis by The Guardian. The U.K. government recently made COVID vaccinations mandatory for health care workers.
COVID delays cancer care
When Mr. Oswalt, the Fort Worth, Texas man with non-Hodgkin lymphoma, contracted COVID-19, the virus took down his kidneys first. Toxins were building up in his blood, so doctors prescribed dialysis to support his body and buy his kidneys time to heal.
He was in one of these dialysis treatments when his lungs succumbed.
“Look, I can’t breathe,” he told the nurse who was supervising his treatment. The nurse gestured to an oxygen tank already hanging by his side, and said, “You should be OK.”
But he wasn’t.
“I can’t breathe,” Mr. Oswalt said again. Then the air hunger hit. Mr. Oswalt began gasping and couldn’t stop. Today, his voice breaks when he describes this moment. “A lot of it becomes a blur.”
When Mr. Oswalt, 61, regained consciousness, he was hooked up to a ventilator to ease his breathing.
For days, Mr. Oswalt clung to the edge of life. His wife, Molly, who wasn’t allowed to see him in the hospital, got a call that he might not make it through the night. She made frantic phone calls to her brother and sister and prayed.
Mr. Oswalt was on a ventilator for about a week. His kidneys and lungs healed enough so that he could restart his chemotherapy. He was eventually discharged home on January 22.
The last time he was scanned, the large tumor in his chest had shrunk from the size of a grapefruit to the size of a dime.
But having COVID on top of cancer has had a devastating effect on his life. Before he got sick, Molly said, he couldn’t stay still. He was busy all the time. After spending months in the hospital, his energy was depleted. He couldn’t keep his swimming pool installation business going.
He and Molly had to give up their house in Fort Worth and move in with family in Amarillo. He has had to pause his cancer treatments while doctors wait for his kidneys to heal. Relatives have been raising money on GoFundMe to pay their bills.
Months after moving across the state to Amarillo and hoping for better days, Tim said he got good news this week: He no longer needs dialysis. A new round of tests found no signs of cancer. His white blood cell count is back to normal. His lymph nodes are no longer swollen.
He goes back for another scan in a few weeks, but the doctor told him she isn’t going to recommend any further chemo at this point.
“It was shocking, to tell you the truth. It still is. When I talk about it, I get kind of emotional” about his recovery, he said.
Tim said he was really dreading more chemotherapy. His hair has just started growing back. He can finally taste food again. He wasn’t ready to face more side effects from the treatments, or the COVID – he no longer knows exactly which diagnosis led to his most debilitating symptoms.
He said his ordeal has left him with no patience for health care workers who don’t think they need to be vaccinated.
The way he sees it, it’s no different than the electrical training he had to get before he could wire the lights and pumps in a swimming pool.
“You know, if I don’t certify and keep my license, I can’t work on anything electrical. So, if I’ve made the choice not to go down and take the test and get a license, then I made the choice not to work on electrical stuff,” he said.
He supports the growing number of hospitals that have made vaccination mandatory for their workers.
“They don’t let electricians put people at risk. And they shouldn’t let health care workers for sure,” he said.
A version of this article first appeared on Medscape.com.
Tim Oswalt had been in a Fort Worth, Texas, hospital for over a month, receiving treatment for a grapefruit-sized tumor in his chest that was pressing on his heart and lungs. It turned out to be stage 3 non-Hodgkin lymphoma.
Then one day in January, he was moved from his semi-private room to an isolated one with special ventilation. The staff explained he had been infected by the virus that was once again surging in many areas of the country, including Texas.
“How the hell did I catch COVID?” he asked the staff, who now approached him in full moon-suit personal protective equipment (PPE).
The hospital was locked down, and Mr. Oswalt hadn’t had any visitors in weeks. Neither of his two roommates tested positive. He’d been tested for COVID several times over the course of his nearly 5-week stay and was always negative.
“‘Well, you know, it’s easy to [catch it] in a hospital,’” Mr. Oswalt said he was told by hospital staff. “‘We’re having a bad outbreak. So you were just exposed somehow.’”

Officials at John Peter Smith Hospital, where Mr. Oswalt was treated, said they are puzzled by his case. According to their infection prevention team, none of his caregivers tested positive for COVID-19, nor did Mr. Oswalt share space with any other COVID-positive patients. And yet, local media reported a surge in cases among JPS hospital staff in December.
“Infection of any kind is a constant battle within hospitals and one that we all take seriously,” said Rob Stephenson, MD, chief quality officer at JPS Health Network. “Anyone in a vulnerable health condition at the height of the pandemic would have been at greater risk for contracting COVID-19 inside – or even more so, outside – the hospital.”
Mr. Oswalt was diagnosed with COVID in early January. JPS Hospital began vaccinating its health care workers about 2 weeks earlier, so there had not yet been enough time for any of them to develop full protection against catching or spreading the virus.
Today, the hospital said 74% of its staff – 5,300 of 7,200 workers – are now vaccinated.
against the SARS-CoV2 virus.
Refusing vaccinations
In fact, nationwide, 1 in 4 hospital workers who have direct contact with patients had not yet received a single dose of a COVID vaccine by the end of May, according to a WebMD and Medscape Medical News analysis of data collected by the U.S. Department of Health and Human Services (HHS) from 2,500 hospitals across the United States.
Among the nation’s 50 largest hospitals, the percentage of unvaccinated health care workers appears to be even larger, about 1 in 3. Vaccination rates range from a high of 99% at Houston Methodist Hospital, which was the first in the nation to mandate the shots for its workers, to a low between 30% and 40% at some hospitals in Florida.
Memorial Hermann Texas Medical Center in Houston has 1,180 beds and sits less than half a mile from Houston Methodist Hospital. But in terms of worker vaccinations, it is farther away.
Memorial Hermann reported to HHS that about 32% of its 28,000 workers haven’t been inoculated. The hospital’s PR office contests that figure, putting it closer to 25% unvaccinated across their health system. The hospital said it is boosting participation by offering a $300 “shot of hope” bonus to workers who start their vaccination series by the end of June.
Lakeland Regional Medical Center in Lakeland, Fla., reported to HHS that 63% of its health care personnel are still unvaccinated. The hospital did not return a call to verify that number.
To boost vaccination rates, more hospitals are starting to require the shots, after the Equal Employment Opportunity Commission gave its green light to mandates in May.
“It’s a real problem that you have such high levels of unvaccinated individuals in hospitals,” said Lawrence Gostin, JD, director of the O’Neill Institute for National and Global Health Law at Georgetown University, Washington.
“We have to protect our health workforce, and we have to protect our patients. Hospitals should be the safest places in the country, and the only way to make them safe is to have a fully vaccinated workforce,” Mr. Gostin said.
Is the data misleading?
The HHS system designed to amass hospital data was set up quickly, to respond to an emergency. For that reason, experts say the information hasn’t been as carefully collected or vetted as it normally would have been. Some hospitals may have misunderstood how to report their vaccination numbers.
In addition, reporting data on worker vaccinations is voluntary. Only about half of hospitals have chosen to share their numbers. In other cases, like Texas, states have blocked the public release of these statistics.
AdventHealth Orlando, a 1,300-bed hospital in Florida, reported to HHS that 56% of its staff have not started their shots. But spokesman Jeff Grainger said the figures probably overstate the number of unvaccinated workers because the hospital doesn’t always know when people get vaccinated outside of its campus, at a local pharmacy, for example.
For those reasons, the picture of health care worker vaccinations across the country is incomplete.
Where hospitals fall behind
Even if the data are flawed, the vaccination rates from hospitals mirror the general population. A May Gallup poll, for example, found 24% of Americans said they definitely won’t get the vaccine. Another 12% say they plan to get it but are waiting.
The data also align with recent studies. A review of 35 studies by researchers at New Mexico State University that assessed hesitancy in more than 76,000 health care workers around the world found about 23% of them were reluctant to get the shots.
An ongoing monthly survey of more than 1.9 million U.S. Facebook users led by researchers at Carnegie Mellon University, Pittsburgh recently looked at vaccine hesitancy by occupation. It revealed a spectrum of hesitancy among health care workers corresponding to income and education, ranging from a low of 9% among pharmacists to highs of 20%-23% among nursing aides and emergency medical technicians. About 12% of registered nurses and doctors admitted to being hesitant to get a shot.
“Health care workers are not monolithic,” said study author Jagdish Khubchandani, professor of public health sciences at New Mexico State.
“There’s a big divide between males, doctoral degree holders, older people and the younger low-income, low-education frontline, female, health care workers. They are the most hesitant,” he said. Support staff typically outnumbers doctors at hospitals about 3 to 1.
“There is outreach work to be done there,” said Robin Mejia, PhD, director of the Statistics and Human Rights Program at Carnegie Mellon, who is leading the study on Facebook’s survey data. “These are also high-contact professions. These are people who are seeing patients on a regular basis.”
That’s why, when the Centers for Disease Control and Prevention was planning the national vaccine rollout, they prioritized health care workers for the initially scarce first doses. The intent was to protect vulnerable workers and their patients who are at high risk of infection. But the CDC had another reason for putting health care workers first: After they were safely vaccinated, the hope was that they would encourage wary patients to do the same.
Hospitals were supposed to be hubs of education to help build trust within less confident communities. But not all hospitals have risen to that challenge.
Political affiliation seems to be one contributing factor in vaccine hesitancy. Take for example Calhoun, Ga., the seat of Gordon County, where residents voted for Donald Trump over Joe Biden by a 67-point margin in the 2020 general election. Studies have found that Republicans are more likely to decline vaccines than Democrats.
People who live in rural areas are less likely to be vaccinated than those who live in cities, and that’s true in Gordon County too. Vaccinations are lagging in this northwest corner of Georgia where factory jobs in chicken processing plants and carpet manufacturing energize the local economy. Just 24% of Gordon County residents are fully vaccinated, according to the Georgia Department of Public Health.
At AdventHealth Gordon, a 112-bed hospital in Calhoun, just 35% of the 1,723 workers that serve the hospital are at least partially vaccinated, according to data reported to HHS.
‘I am not vaccinated’
One reason some hospital staff say they are resisting COVID vaccination is because it’s so new and not yet fully approved by the FDA.
“I am not vaccinated,” said a social services worker for AdventHealth Gordon who asked that her name not be used because she was unauthorized to speak to this news organization and Georgia Health News (who collaborated on this project). “I just have not felt the need to do that at this time.”
The woman said she doesn’t have a problem with vaccines. She gets the flu shot every year. “I’ve been vaccinated all my life,” she said. But she doesn’t view COVID-19 vaccination in the same way.
“I want to see more testing done,” she said. “It took a long time to get a flu vaccine, and we made a COVID vaccine in 6 months. I want to know, before I start putting something into my body, that the testing is done.”
Staff at her hospital were given the option to be vaccinated or wear a mask. She chose the mask.
Many of her coworkers share her feelings, she said.
Mask expert Linsey Marr, PhD, a professor of civil and environmental engineering at Virginia Tech University, Blacksburg, Va., said N95 masks and vaccines are both highly effective, but the protection from the vaccine is superior because it is continuous.
“It’s hard to wear an N95 at all times. You have to take it off to eat, for example, in a break room in a hospital. I should point out that you can be exposed to the virus in other buildings besides a hospital – restaurants, stores, people’s homes – and because someone can be infected without symptoms, you could easily be around an infected person without knowing it,” she said.
Eventually, staff at AdventHealth Gordon may get a stronger nudge to get the shots. Chief Medical Officer Joseph Joyave, MD, said AdventHealth asks workers to get flu vaccines or provide the hospital with a reason why they won’t. He expects a similar policy will be adopted for COVID vaccines once they are fully licensed by the FDA.
In the meantime, he does not believe that the hospital is putting patients at risk with its low vaccination rate. “We continue to use PPE, masking in all clinical areas, and continue to screen daily all employees and visitors,” he said.
AdventHealth, the 12th largest hospital system in the nation with 49 hospitals, has at least 20 hospitals with vaccination rates lower than 50%, according to HHS data.
Other hospital systems have approached hesitation around the COVID vaccines differently.
When infectious disease experts at Vanderbilt Hospital in Nashville realized early on that many of their workers felt unsure about the vaccines, they set out to provide a wealth of information.
“There was a lot of hesitancy and skepticism,” said William Schaffner, MD, a professor of preventive medicine and infectious disease at Vanderbilt. So the infectious disease division put together a multifaceted program including Q&As, educational sessions, and one-on-one visits with employees “from the custodians all the way up to the C-Suite,” he said.
Today, HHS data shows the hospital is 83% vaccinated. Dr. Schaffner thinks the true number is probably higher, about 90%. “We’re very pleased with that,” he said.
In his experience with flu vaccinations, it was extremely difficult in the first year to get workers to take flu shots. The second year it was easier. By the third year it was humdrum, he said, because it had become a cultural norm.
Dr. Schaffner expects winning people over to the COVID vaccines will follow a similar course, but “we’re not there yet,” he said.
Protecting patients and caregivers
There is no question that health care workers carried a heavy load through the worst months of the pandemic. Many of them worked to the point of exhaustion and burnout. Some were the only conduits between isolated patients and their families, holding hands and mobile phones so distanced loved ones could video chat. Many were left inadequately protected because of shortages of masks, gowns, gloves, and other gear.
An investigation by Kaiser Health News and The Guardian recently revealed that more than 3,600 health care workers died in COVID’s first year in the United States.
Vaccination of health care workers is important to protect these frontline workers and their families who will continue to be at risk of coming into contact with the infection, even as the number of cases falls.
Hesitancy in health care is also dangerous because these clinicians and allied health workers – who may not show any symptoms – can also carry the virus to someone who wouldn’t survive an infection, including patients with organ transplants, those with autoimmune diseases, premature infants, and the elderly.
It is not known how often patients in the United States are infected with COVID in health care settings, but case reports reveal that hospitals are still experiencing outbreaks.
On June 1, Northern Lights A.R. Gould Hospital in Presque Isle, Maine, announced a COVID outbreak on its medical-surgical unit. As of June 22, 13 residents and staff have caught the virus, according to the Maine Centers for Disease Control, which is investigating. Four of the first five staff members to test positive had not been fully vaccinated.
According to HHS data, about 20% of the health care workers at that hospital are still unvaccinated.
Oregon Health & Science University experienced a COVID outbreak connected to the hospital’s cardiovascular care unit from April to mid-May of this year. According to hospital spokesperson Tracy Brawley, a patient visitor brought the infection to campus, where it ultimately spread to 14 others, including “patients, visitors, employees, and learners.”
In a written statement, the hospital said “nearly all” health care workers who tested positive were previously vaccinated and experienced no symptoms or only minor ones. The hospital said it hasn’t identified any onward transmission from health care workers to patients, and also stated: “It is not yet understood how transmission may have occurred between patients, visitors, and health care workers.”
In March, an unvaccinated health care worker in Kentucky carried a SARS-CoV-2 variant back to the nursing home where the person worked. Some 90% of the residents were fully vaccinated. Ultimately, 26 patients were infected; 18 of them were fully vaccinated. And 20 health care workers, four of whom were vaccinated, were infected.
Vaccines slowed the virus down and made infections less severe, but in this fragile population, they couldn’t stop it completely. One resident, who had survived a bout of COVID almost a year earlier, died. According to the CDC’s Morbidity and Mortality Weekly Report, 47% of the workers in that facility were unvaccinated.
In the United Kingdom, statistics collected through that country’s National Health Service also suggest a heavy toll. More than 32,300 patients caught COVID in English hospitals since March 2020. Up to 8,700 of them died, according to a recent analysis by The Guardian. The U.K. government recently made COVID vaccinations mandatory for health care workers.
COVID delays cancer care
When Mr. Oswalt, the Fort Worth, Texas man with non-Hodgkin lymphoma, contracted COVID-19, the virus took down his kidneys first. Toxins were building up in his blood, so doctors prescribed dialysis to support his body and buy his kidneys time to heal.
He was in one of these dialysis treatments when his lungs succumbed.
“Look, I can’t breathe,” he told the nurse who was supervising his treatment. The nurse gestured to an oxygen tank already hanging by his side, and said, “You should be OK.”
But he wasn’t.
“I can’t breathe,” Mr. Oswalt said again. Then the air hunger hit. Mr. Oswalt began gasping and couldn’t stop. Today, his voice breaks when he describes this moment. “A lot of it becomes a blur.”
When Mr. Oswalt, 61, regained consciousness, he was hooked up to a ventilator to ease his breathing.
For days, Mr. Oswalt clung to the edge of life. His wife, Molly, who wasn’t allowed to see him in the hospital, got a call that he might not make it through the night. She made frantic phone calls to her brother and sister and prayed.
Mr. Oswalt was on a ventilator for about a week. His kidneys and lungs healed enough so that he could restart his chemotherapy. He was eventually discharged home on January 22.
The last time he was scanned, the large tumor in his chest had shrunk from the size of a grapefruit to the size of a dime.
But having COVID on top of cancer has had a devastating effect on his life. Before he got sick, Molly said, he couldn’t stay still. He was busy all the time. After spending months in the hospital, his energy was depleted. He couldn’t keep his swimming pool installation business going.
He and Molly had to give up their house in Fort Worth and move in with family in Amarillo. He has had to pause his cancer treatments while doctors wait for his kidneys to heal. Relatives have been raising money on GoFundMe to pay their bills.
Months after moving across the state to Amarillo and hoping for better days, Tim said he got good news this week: He no longer needs dialysis. A new round of tests found no signs of cancer. His white blood cell count is back to normal. His lymph nodes are no longer swollen.
He goes back for another scan in a few weeks, but the doctor told him she isn’t going to recommend any further chemo at this point.
“It was shocking, to tell you the truth. It still is. When I talk about it, I get kind of emotional” about his recovery, he said.
Tim said he was really dreading more chemotherapy. His hair has just started growing back. He can finally taste food again. He wasn’t ready to face more side effects from the treatments, or the COVID – he no longer knows exactly which diagnosis led to his most debilitating symptoms.
He said his ordeal has left him with no patience for health care workers who don’t think they need to be vaccinated.
The way he sees it, it’s no different than the electrical training he had to get before he could wire the lights and pumps in a swimming pool.
“You know, if I don’t certify and keep my license, I can’t work on anything electrical. So, if I’ve made the choice not to go down and take the test and get a license, then I made the choice not to work on electrical stuff,” he said.
He supports the growing number of hospitals that have made vaccination mandatory for their workers.
“They don’t let electricians put people at risk. And they shouldn’t let health care workers for sure,” he said.
A version of this article first appeared on Medscape.com.
Semaglutide 2.4 mg ‘likely to usher in a new era’ in obesity treatment
The recently licensed weight-loss drug semaglutide 2.4 mg/week (Wegovy, Novo Nordisk) “is likely to usher in a new era in the medical treatment of obesity,” Lee M. Kaplan, MD, PhD, stated at the annual scientific sessions of the American Diabetes Association, held virtually.
Dr. Kaplan discussed the clinical implications of caring for patients with obesity now that the glucagon-like peptide-1 (GLP-1) receptor agonist is approved in the United States for weight loss.
Weight loss with semaglutide 2.4 mg was twice that achieved with liraglutide 3 mg (Saxenda, Novo Nordisk) – that is, roughly a 10%-15% weight loss at 68 weeks, said Dr. Kaplan, who was not involved in the pivotal STEP clinical trials of the agent.
“I think as we start to see more data come in over the next couple of years,” including from the cardiovascular outcome trial SELECT, he continued, “we’ll be able to use the data to create a nuanced [individualized patient treatment] approach, but we’ll also be able to use our clinical experience, which will grow rapidly over the next few years.”
In the future, semaglutide is likely to be combined with other drugs to provide even greater weight loss, predicts Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital in Boston.
In the meantime, “to be effective, semaglutide needs to be used,” he stressed, while noting that responses to the drug vary by individual, and so this will need to be taken into account.
“Obesity needs to be recognized as a disease in its own right, as well as a risk factor for numerous other diseases, [and] equitable access to obesity treatment needs to be broadened,” he emphasized.
Four pivotal phase 3 trials
As previously reported, four pivotal 68-week, phase 3 clinical trials in the Semaglutide Treatment Effect in People With Obesity (STEP) program tested the safety and efficacy of subcutaneous semaglutide 2.4 mg/week in more than 4,500 adults with overweight or obesity.
The trials have been published in high profile journals – the New England Journal of Medicine (STEP 1), The Lancet (STEP 2), and JAMA (STEP 3 and STEP 4) – said Robert F. Kushner, MD.
“I would encourage all of you to download and read each of these trials on your own,” Dr. Kushner, professor of medicine and medicine education at Northwestern University, Chicago, and coauthor of STEP 1, said before presenting a top-level review of key results.
STEP 1 examined weight management, STEP 3 added a background of intensive behavioral therapy, STEP 4 investigated sustained weight management, and STEP 2 (unlike the others) investigated weight management in patients with type 2 diabetes, he summarized.
In STEP 1, patients who received semaglutide had an average 15% weight loss, and those who stayed on the drug had a 17% weight loss, compared with the 2.4% weight loss in the placebo group.
“One-third of individuals in the trial achieved at least a 20% weight loss or more,” Dr. Kushner said, which is “really phenomenal.”
The results of STEP 3 “suggest that semaglutide with monthly brief lifestyle counseling alone is sufficient to produce a mean weight loss of 15%,” he noted, as adding a low-calorie diet and intensive behavior therapy sped up the initial weight loss but did not increase the final weight loss.
A post hoc analysis of STEP 2 showed “it’s clear that improvement in A1c” is greater with at least a 10% weight loss versus a smaller weight loss, Dr. Kushner said. A1c dropped by 2.2% versus 1.3%, with these two weight losses, respectively.
In STEP 4, after dose escalation to 2.4 mg at 20 weeks, patients had lost 10.6% of their initial weight. At 68 weeks, those who were switched to placebo at 20 weeks had lost 5.4% of their initial weight, whereas those who remained on semaglutide had lost 17.7% of their initial weight.
This shows that “if you remove the drug, the disease starts to come back,” Dr. Kushner pointed out.
Nausea, the most common side effect, occurred in 20% of patients, but was mostly mild or moderate, and gastrointestinal effects including constipation, vomiting, and diarrhea were transient and occurred early in the dose escalation phase.
Large individual variability, combination therapies on horizon
Dr. Kaplan pointed out, however, that “like [with] other antiobesity therapies ... there’s a large patient-to-patient variability.”
A third of patients exhibit more than 20% weight loss, and 10% exhibit more than 30% weight loss – approaching the efficacy of bariatric surgery.
However, nearly 10% of patients without diabetes and upwards of 30% of patients with diabetes will experience less than 5% weight loss, he said.
Therefore, “success or failure in one patient doesn’t predict response in another, and we should always remember that as we treat different patients with these medications,” Dr. Kaplan advised.
A recent phase 1b study suggests that combination therapy with semaglutide and the amylin agonist cagrilintide ups weight loss, as previously reported.
In this short trial with no lifestyle modification, it took 16 weeks for patients to reach full dosing, and at 20 weeks, patients on semaglutide had lost 8% of their initial weight, whereas those on combination therapy had lost 17% of their initial weight.
“There’s hope that, in combination with cagrilintide and probably with several other agents that are still in early development, we’ll be seeing average weight loss that is in the range of that seen with bariatric surgery,” Dr. Kushner said.
Doctors discuss two hypothetical cases
Session moderator Julio Rosenstock, MD, of the University of Texas, Dallas, a coinvestigator in several of the STEP trials, invited Dr. Kaplan and two other panelists to explain how they would manage two hypothetical patients.
Case 1
You have a patient with type 2 diabetes, a body mass index of 32, 33 kg/m2, and an A1c of 7.5% or 8% on metformin. Would you use semaglutide 1 mg (Ozempic, Novo Nordisk) that is indicated for type 2 diabetes, or would you use semaglutide 2.4 mg that is indicated for obesity and risk factors?
“We have the answer to that from STEP 2,” said Melanie J. Davies, MB ChB, MD, professor of diabetes medicine at the University of Leicester, England, who led the STEP 2 trial.
“For some patients, the 1-mg dose, which we use routinely in the clinic, may be reasonable to get good glycemic control for cardiovascular protection and will obviously achieve some weight loss. But if you really want to go for the weight-related comorbidities, then the 2.4-mg dose is what you need,” she said.
“A lot of [clinicians] might say: ‘I’ll see how [the patient goes] with the 1-mg dose, and then maybe if they’re not losing the weight and not getting to glycemic target, then maybe I’ll switch to 2.4 mg,’” said John Wilding, MD, who leads clinical research into obesity, diabetes, and endocrinology at the University of Liverpool, England, and led the STEP 1 trial.
“But the STEP 2 data show very clearly that you get almost the same A1c,” Dr. Rosenstock interjected. “I would go for 2.4 mg. The patient has a BMI of 32, 33 kg/m2. I would hit hard the BMI. We need to change that paradigm.”
“For other diseases we don’t always go to the maximum dose that’s available. We go to the dose that’s necessary to achieve the clinical endpoint that we want,” Dr. Kaplan noted. “I think one of the challenges is going to be to learn how to clinically nuance our therapy the way we do for other diseases.”
“That is the usual thinking,” Dr. Rosenstock agreed. But “with the 2.4-mg dose, one third get a 20% reduction of BMI, and 10% get almost a 30% reduction – and you [aren’t] going to see that with semaglutide 1 mg!”
“That’s true,” Dr. Kaplan conceded. However, a patient with a relatively low BMI of 32, 33 kg/m2 may not need the higher dose, unlike a patient who has a BMI of 45 kg/m2 and diabetes. But we’re going to find that out over the next couple of years, he expects.
Case 2
You have a patient with a BMI of 31 kg/m2 who is newly diagnosed with type 2 diabetes. Why should you start that patient with metformin? Why won’t you start with something that will directly tackle obesity and get the patient to lose 20 pounds and for sure the blood sugar is going to be better?
“I think if I have someone who is really keen to put their diabetes into remission,” Dr. Wilding said, “this would be a fantastic approach because they would have a really high chance of doing that.”
The prediabetes data from STEP showed that “we can put a lot of people from prediabetes back to normal glucose tolerance,” Dr. Wilding noted. “Maybe we can put people with early diabetes back to normal as well. I think that’s a trial that really does need to be done,” he said.
“We’re going to have to figure out the best pathway forward,” Dr. Kaplan observed, noting that multiple stakeholders, including payers, patients, and providers, play a role in the uptake of new obesity drugs.
“Do you think we will see less bariatric surgery with these drugs?” Dr. Rosenstock asked Dr. Kaplan.
“I think you have to remember that of the millions and millions of people with obesity, a very small portion are currently treated with antiobesity medication, and an even smaller portion are getting bariatric surgery,” Dr. Kaplan replied.
“In the United States, 90% of people who get bariatric surgery are self-referred,” he said, so, “I think initially we are not going to see much of a change” in rates of bariatric surgery.
Dr. Rosenstock, Dr. Kaplan, Dr. Wilding, and Dr. Davies disclosed ties with Novo Nordisk and numerous other companies.
A version of this article first appeared on Medscape.com.
The recently licensed weight-loss drug semaglutide 2.4 mg/week (Wegovy, Novo Nordisk) “is likely to usher in a new era in the medical treatment of obesity,” Lee M. Kaplan, MD, PhD, stated at the annual scientific sessions of the American Diabetes Association, held virtually.
Dr. Kaplan discussed the clinical implications of caring for patients with obesity now that the glucagon-like peptide-1 (GLP-1) receptor agonist is approved in the United States for weight loss.
Weight loss with semaglutide 2.4 mg was twice that achieved with liraglutide 3 mg (Saxenda, Novo Nordisk) – that is, roughly a 10%-15% weight loss at 68 weeks, said Dr. Kaplan, who was not involved in the pivotal STEP clinical trials of the agent.
“I think as we start to see more data come in over the next couple of years,” including from the cardiovascular outcome trial SELECT, he continued, “we’ll be able to use the data to create a nuanced [individualized patient treatment] approach, but we’ll also be able to use our clinical experience, which will grow rapidly over the next few years.”
In the future, semaglutide is likely to be combined with other drugs to provide even greater weight loss, predicts Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital in Boston.
In the meantime, “to be effective, semaglutide needs to be used,” he stressed, while noting that responses to the drug vary by individual, and so this will need to be taken into account.
“Obesity needs to be recognized as a disease in its own right, as well as a risk factor for numerous other diseases, [and] equitable access to obesity treatment needs to be broadened,” he emphasized.
Four pivotal phase 3 trials
As previously reported, four pivotal 68-week, phase 3 clinical trials in the Semaglutide Treatment Effect in People With Obesity (STEP) program tested the safety and efficacy of subcutaneous semaglutide 2.4 mg/week in more than 4,500 adults with overweight or obesity.
The trials have been published in high profile journals – the New England Journal of Medicine (STEP 1), The Lancet (STEP 2), and JAMA (STEP 3 and STEP 4) – said Robert F. Kushner, MD.
“I would encourage all of you to download and read each of these trials on your own,” Dr. Kushner, professor of medicine and medicine education at Northwestern University, Chicago, and coauthor of STEP 1, said before presenting a top-level review of key results.
STEP 1 examined weight management, STEP 3 added a background of intensive behavioral therapy, STEP 4 investigated sustained weight management, and STEP 2 (unlike the others) investigated weight management in patients with type 2 diabetes, he summarized.
In STEP 1, patients who received semaglutide had an average 15% weight loss, and those who stayed on the drug had a 17% weight loss, compared with the 2.4% weight loss in the placebo group.
“One-third of individuals in the trial achieved at least a 20% weight loss or more,” Dr. Kushner said, which is “really phenomenal.”
The results of STEP 3 “suggest that semaglutide with monthly brief lifestyle counseling alone is sufficient to produce a mean weight loss of 15%,” he noted, as adding a low-calorie diet and intensive behavior therapy sped up the initial weight loss but did not increase the final weight loss.
A post hoc analysis of STEP 2 showed “it’s clear that improvement in A1c” is greater with at least a 10% weight loss versus a smaller weight loss, Dr. Kushner said. A1c dropped by 2.2% versus 1.3%, with these two weight losses, respectively.
In STEP 4, after dose escalation to 2.4 mg at 20 weeks, patients had lost 10.6% of their initial weight. At 68 weeks, those who were switched to placebo at 20 weeks had lost 5.4% of their initial weight, whereas those who remained on semaglutide had lost 17.7% of their initial weight.
This shows that “if you remove the drug, the disease starts to come back,” Dr. Kushner pointed out.
Nausea, the most common side effect, occurred in 20% of patients, but was mostly mild or moderate, and gastrointestinal effects including constipation, vomiting, and diarrhea were transient and occurred early in the dose escalation phase.
Large individual variability, combination therapies on horizon
Dr. Kaplan pointed out, however, that “like [with] other antiobesity therapies ... there’s a large patient-to-patient variability.”
A third of patients exhibit more than 20% weight loss, and 10% exhibit more than 30% weight loss – approaching the efficacy of bariatric surgery.
However, nearly 10% of patients without diabetes and upwards of 30% of patients with diabetes will experience less than 5% weight loss, he said.
Therefore, “success or failure in one patient doesn’t predict response in another, and we should always remember that as we treat different patients with these medications,” Dr. Kaplan advised.
A recent phase 1b study suggests that combination therapy with semaglutide and the amylin agonist cagrilintide ups weight loss, as previously reported.
In this short trial with no lifestyle modification, it took 16 weeks for patients to reach full dosing, and at 20 weeks, patients on semaglutide had lost 8% of their initial weight, whereas those on combination therapy had lost 17% of their initial weight.
“There’s hope that, in combination with cagrilintide and probably with several other agents that are still in early development, we’ll be seeing average weight loss that is in the range of that seen with bariatric surgery,” Dr. Kushner said.
Doctors discuss two hypothetical cases
Session moderator Julio Rosenstock, MD, of the University of Texas, Dallas, a coinvestigator in several of the STEP trials, invited Dr. Kaplan and two other panelists to explain how they would manage two hypothetical patients.
Case 1
You have a patient with type 2 diabetes, a body mass index of 32, 33 kg/m2, and an A1c of 7.5% or 8% on metformin. Would you use semaglutide 1 mg (Ozempic, Novo Nordisk) that is indicated for type 2 diabetes, or would you use semaglutide 2.4 mg that is indicated for obesity and risk factors?
“We have the answer to that from STEP 2,” said Melanie J. Davies, MB ChB, MD, professor of diabetes medicine at the University of Leicester, England, who led the STEP 2 trial.
“For some patients, the 1-mg dose, which we use routinely in the clinic, may be reasonable to get good glycemic control for cardiovascular protection and will obviously achieve some weight loss. But if you really want to go for the weight-related comorbidities, then the 2.4-mg dose is what you need,” she said.
“A lot of [clinicians] might say: ‘I’ll see how [the patient goes] with the 1-mg dose, and then maybe if they’re not losing the weight and not getting to glycemic target, then maybe I’ll switch to 2.4 mg,’” said John Wilding, MD, who leads clinical research into obesity, diabetes, and endocrinology at the University of Liverpool, England, and led the STEP 1 trial.
“But the STEP 2 data show very clearly that you get almost the same A1c,” Dr. Rosenstock interjected. “I would go for 2.4 mg. The patient has a BMI of 32, 33 kg/m2. I would hit hard the BMI. We need to change that paradigm.”
“For other diseases we don’t always go to the maximum dose that’s available. We go to the dose that’s necessary to achieve the clinical endpoint that we want,” Dr. Kaplan noted. “I think one of the challenges is going to be to learn how to clinically nuance our therapy the way we do for other diseases.”
“That is the usual thinking,” Dr. Rosenstock agreed. But “with the 2.4-mg dose, one third get a 20% reduction of BMI, and 10% get almost a 30% reduction – and you [aren’t] going to see that with semaglutide 1 mg!”
“That’s true,” Dr. Kaplan conceded. However, a patient with a relatively low BMI of 32, 33 kg/m2 may not need the higher dose, unlike a patient who has a BMI of 45 kg/m2 and diabetes. But we’re going to find that out over the next couple of years, he expects.
Case 2
You have a patient with a BMI of 31 kg/m2 who is newly diagnosed with type 2 diabetes. Why should you start that patient with metformin? Why won’t you start with something that will directly tackle obesity and get the patient to lose 20 pounds and for sure the blood sugar is going to be better?
“I think if I have someone who is really keen to put their diabetes into remission,” Dr. Wilding said, “this would be a fantastic approach because they would have a really high chance of doing that.”
The prediabetes data from STEP showed that “we can put a lot of people from prediabetes back to normal glucose tolerance,” Dr. Wilding noted. “Maybe we can put people with early diabetes back to normal as well. I think that’s a trial that really does need to be done,” he said.
“We’re going to have to figure out the best pathway forward,” Dr. Kaplan observed, noting that multiple stakeholders, including payers, patients, and providers, play a role in the uptake of new obesity drugs.
“Do you think we will see less bariatric surgery with these drugs?” Dr. Rosenstock asked Dr. Kaplan.
“I think you have to remember that of the millions and millions of people with obesity, a very small portion are currently treated with antiobesity medication, and an even smaller portion are getting bariatric surgery,” Dr. Kaplan replied.
“In the United States, 90% of people who get bariatric surgery are self-referred,” he said, so, “I think initially we are not going to see much of a change” in rates of bariatric surgery.
Dr. Rosenstock, Dr. Kaplan, Dr. Wilding, and Dr. Davies disclosed ties with Novo Nordisk and numerous other companies.
A version of this article first appeared on Medscape.com.
The recently licensed weight-loss drug semaglutide 2.4 mg/week (Wegovy, Novo Nordisk) “is likely to usher in a new era in the medical treatment of obesity,” Lee M. Kaplan, MD, PhD, stated at the annual scientific sessions of the American Diabetes Association, held virtually.
Dr. Kaplan discussed the clinical implications of caring for patients with obesity now that the glucagon-like peptide-1 (GLP-1) receptor agonist is approved in the United States for weight loss.
Weight loss with semaglutide 2.4 mg was twice that achieved with liraglutide 3 mg (Saxenda, Novo Nordisk) – that is, roughly a 10%-15% weight loss at 68 weeks, said Dr. Kaplan, who was not involved in the pivotal STEP clinical trials of the agent.
“I think as we start to see more data come in over the next couple of years,” including from the cardiovascular outcome trial SELECT, he continued, “we’ll be able to use the data to create a nuanced [individualized patient treatment] approach, but we’ll also be able to use our clinical experience, which will grow rapidly over the next few years.”
In the future, semaglutide is likely to be combined with other drugs to provide even greater weight loss, predicts Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital in Boston.
In the meantime, “to be effective, semaglutide needs to be used,” he stressed, while noting that responses to the drug vary by individual, and so this will need to be taken into account.
“Obesity needs to be recognized as a disease in its own right, as well as a risk factor for numerous other diseases, [and] equitable access to obesity treatment needs to be broadened,” he emphasized.
Four pivotal phase 3 trials
As previously reported, four pivotal 68-week, phase 3 clinical trials in the Semaglutide Treatment Effect in People With Obesity (STEP) program tested the safety and efficacy of subcutaneous semaglutide 2.4 mg/week in more than 4,500 adults with overweight or obesity.
The trials have been published in high profile journals – the New England Journal of Medicine (STEP 1), The Lancet (STEP 2), and JAMA (STEP 3 and STEP 4) – said Robert F. Kushner, MD.
“I would encourage all of you to download and read each of these trials on your own,” Dr. Kushner, professor of medicine and medicine education at Northwestern University, Chicago, and coauthor of STEP 1, said before presenting a top-level review of key results.
STEP 1 examined weight management, STEP 3 added a background of intensive behavioral therapy, STEP 4 investigated sustained weight management, and STEP 2 (unlike the others) investigated weight management in patients with type 2 diabetes, he summarized.
In STEP 1, patients who received semaglutide had an average 15% weight loss, and those who stayed on the drug had a 17% weight loss, compared with the 2.4% weight loss in the placebo group.
“One-third of individuals in the trial achieved at least a 20% weight loss or more,” Dr. Kushner said, which is “really phenomenal.”
The results of STEP 3 “suggest that semaglutide with monthly brief lifestyle counseling alone is sufficient to produce a mean weight loss of 15%,” he noted, as adding a low-calorie diet and intensive behavior therapy sped up the initial weight loss but did not increase the final weight loss.
A post hoc analysis of STEP 2 showed “it’s clear that improvement in A1c” is greater with at least a 10% weight loss versus a smaller weight loss, Dr. Kushner said. A1c dropped by 2.2% versus 1.3%, with these two weight losses, respectively.
In STEP 4, after dose escalation to 2.4 mg at 20 weeks, patients had lost 10.6% of their initial weight. At 68 weeks, those who were switched to placebo at 20 weeks had lost 5.4% of their initial weight, whereas those who remained on semaglutide had lost 17.7% of their initial weight.
This shows that “if you remove the drug, the disease starts to come back,” Dr. Kushner pointed out.
Nausea, the most common side effect, occurred in 20% of patients, but was mostly mild or moderate, and gastrointestinal effects including constipation, vomiting, and diarrhea were transient and occurred early in the dose escalation phase.
Large individual variability, combination therapies on horizon
Dr. Kaplan pointed out, however, that “like [with] other antiobesity therapies ... there’s a large patient-to-patient variability.”
A third of patients exhibit more than 20% weight loss, and 10% exhibit more than 30% weight loss – approaching the efficacy of bariatric surgery.
However, nearly 10% of patients without diabetes and upwards of 30% of patients with diabetes will experience less than 5% weight loss, he said.
Therefore, “success or failure in one patient doesn’t predict response in another, and we should always remember that as we treat different patients with these medications,” Dr. Kaplan advised.
A recent phase 1b study suggests that combination therapy with semaglutide and the amylin agonist cagrilintide ups weight loss, as previously reported.
In this short trial with no lifestyle modification, it took 16 weeks for patients to reach full dosing, and at 20 weeks, patients on semaglutide had lost 8% of their initial weight, whereas those on combination therapy had lost 17% of their initial weight.
“There’s hope that, in combination with cagrilintide and probably with several other agents that are still in early development, we’ll be seeing average weight loss that is in the range of that seen with bariatric surgery,” Dr. Kushner said.
Doctors discuss two hypothetical cases
Session moderator Julio Rosenstock, MD, of the University of Texas, Dallas, a coinvestigator in several of the STEP trials, invited Dr. Kaplan and two other panelists to explain how they would manage two hypothetical patients.
Case 1
You have a patient with type 2 diabetes, a body mass index of 32, 33 kg/m2, and an A1c of 7.5% or 8% on metformin. Would you use semaglutide 1 mg (Ozempic, Novo Nordisk) that is indicated for type 2 diabetes, or would you use semaglutide 2.4 mg that is indicated for obesity and risk factors?
“We have the answer to that from STEP 2,” said Melanie J. Davies, MB ChB, MD, professor of diabetes medicine at the University of Leicester, England, who led the STEP 2 trial.
“For some patients, the 1-mg dose, which we use routinely in the clinic, may be reasonable to get good glycemic control for cardiovascular protection and will obviously achieve some weight loss. But if you really want to go for the weight-related comorbidities, then the 2.4-mg dose is what you need,” she said.
“A lot of [clinicians] might say: ‘I’ll see how [the patient goes] with the 1-mg dose, and then maybe if they’re not losing the weight and not getting to glycemic target, then maybe I’ll switch to 2.4 mg,’” said John Wilding, MD, who leads clinical research into obesity, diabetes, and endocrinology at the University of Liverpool, England, and led the STEP 1 trial.
“But the STEP 2 data show very clearly that you get almost the same A1c,” Dr. Rosenstock interjected. “I would go for 2.4 mg. The patient has a BMI of 32, 33 kg/m2. I would hit hard the BMI. We need to change that paradigm.”
“For other diseases we don’t always go to the maximum dose that’s available. We go to the dose that’s necessary to achieve the clinical endpoint that we want,” Dr. Kaplan noted. “I think one of the challenges is going to be to learn how to clinically nuance our therapy the way we do for other diseases.”
“That is the usual thinking,” Dr. Rosenstock agreed. But “with the 2.4-mg dose, one third get a 20% reduction of BMI, and 10% get almost a 30% reduction – and you [aren’t] going to see that with semaglutide 1 mg!”
“That’s true,” Dr. Kaplan conceded. However, a patient with a relatively low BMI of 32, 33 kg/m2 may not need the higher dose, unlike a patient who has a BMI of 45 kg/m2 and diabetes. But we’re going to find that out over the next couple of years, he expects.
Case 2
You have a patient with a BMI of 31 kg/m2 who is newly diagnosed with type 2 diabetes. Why should you start that patient with metformin? Why won’t you start with something that will directly tackle obesity and get the patient to lose 20 pounds and for sure the blood sugar is going to be better?
“I think if I have someone who is really keen to put their diabetes into remission,” Dr. Wilding said, “this would be a fantastic approach because they would have a really high chance of doing that.”
The prediabetes data from STEP showed that “we can put a lot of people from prediabetes back to normal glucose tolerance,” Dr. Wilding noted. “Maybe we can put people with early diabetes back to normal as well. I think that’s a trial that really does need to be done,” he said.
“We’re going to have to figure out the best pathway forward,” Dr. Kaplan observed, noting that multiple stakeholders, including payers, patients, and providers, play a role in the uptake of new obesity drugs.
“Do you think we will see less bariatric surgery with these drugs?” Dr. Rosenstock asked Dr. Kaplan.
“I think you have to remember that of the millions and millions of people with obesity, a very small portion are currently treated with antiobesity medication, and an even smaller portion are getting bariatric surgery,” Dr. Kaplan replied.
“In the United States, 90% of people who get bariatric surgery are self-referred,” he said, so, “I think initially we are not going to see much of a change” in rates of bariatric surgery.
Dr. Rosenstock, Dr. Kaplan, Dr. Wilding, and Dr. Davies disclosed ties with Novo Nordisk and numerous other companies.
A version of this article first appeared on Medscape.com.
No increase in breast cancer risk with fertility treatments
No link between fertility treatment and an increase in the risk for breast cancer was found in the largest study of the issue to date.
This study “provides the evidence needed to reassure women and couples seeking fertility treatments,” commented senior author Sesh Sunkara, MD, a reproductive medicine specialist at King’s College London in a press release.
With an increasing number of women seeking help to become mothers, the question “is a matter of great importance” and a source of considerable concern among patients, the study authors comment.
This is the largest meta-analysis to date, involving 1.8 million women who were followed for an average of 27 years. The investigators found no link with the use of gonadotropins or clomiphene citrate to increase egg production in fertility cycles.
There has been concern over the years that fertility treatment could stimulate estrogen-sensitive precursor breast cancer cells.
More than 4,000 studies of this issue have been conducted since 1990, and results have been conflicting. The investigators analyzed results from the 20 strongest ones.
The new meta-analysis included nine retrospective studies, five case-control studies, five prospective studies, and one comparative study
The team cautioned that the quality of evidence in even these top 20 studies was “very low” but that such an approach is perhaps the best possible on this issue because a randomized trial among women seeking help to have children would be “ethically challenging.”
In the study, the team compared breast cancer incidence among women who underwent ovarian stimulation with the incidence in both age-matched unexposed women in the general population and unexposed infertile women.
There was no significant increase in the risk for breast cancer among women treated with any ovarian stimulation drug (pooled odds ratio, 1.03; 95% confidence interval, 0.86-1.23, but with substantial heterogeneity between study outcomes).
There was also no increased risk when the analysis was limited to the eight studies in which women were treated with both gonadotropins and clomiphene citrate (pooled OR, 0.92; 95% CI, 0.52-1.60, with substantial heterogeneity).
The authors noted that, among the many study limitations, no distinction was made between physiological dosing for anovulation and supraphysiological dosing for in vitro fertilization cycles. In addition, because the treated women were generally young, the follow-up period fell short of the age at which they’d be most at risk for breast cancer.
Individual patient data were also not available, but 14 studies did adjust for confounders, including weight, race, parity, age at first birth, age at menarche, and family history of breast cancer.
Although the findings are reassuring, “further long-term and detailed studies are now needed to confirm” them, Kotryna Temcinaite, PhD, senior research communications manager at the U.K. charity Breast Cancer Now, said in the press release.
A version of this article first appeared on Medscape.com.
No link between fertility treatment and an increase in the risk for breast cancer was found in the largest study of the issue to date.
This study “provides the evidence needed to reassure women and couples seeking fertility treatments,” commented senior author Sesh Sunkara, MD, a reproductive medicine specialist at King’s College London in a press release.
With an increasing number of women seeking help to become mothers, the question “is a matter of great importance” and a source of considerable concern among patients, the study authors comment.
This is the largest meta-analysis to date, involving 1.8 million women who were followed for an average of 27 years. The investigators found no link with the use of gonadotropins or clomiphene citrate to increase egg production in fertility cycles.
There has been concern over the years that fertility treatment could stimulate estrogen-sensitive precursor breast cancer cells.
More than 4,000 studies of this issue have been conducted since 1990, and results have been conflicting. The investigators analyzed results from the 20 strongest ones.
The new meta-analysis included nine retrospective studies, five case-control studies, five prospective studies, and one comparative study
The team cautioned that the quality of evidence in even these top 20 studies was “very low” but that such an approach is perhaps the best possible on this issue because a randomized trial among women seeking help to have children would be “ethically challenging.”
In the study, the team compared breast cancer incidence among women who underwent ovarian stimulation with the incidence in both age-matched unexposed women in the general population and unexposed infertile women.
There was no significant increase in the risk for breast cancer among women treated with any ovarian stimulation drug (pooled odds ratio, 1.03; 95% confidence interval, 0.86-1.23, but with substantial heterogeneity between study outcomes).
There was also no increased risk when the analysis was limited to the eight studies in which women were treated with both gonadotropins and clomiphene citrate (pooled OR, 0.92; 95% CI, 0.52-1.60, with substantial heterogeneity).
The authors noted that, among the many study limitations, no distinction was made between physiological dosing for anovulation and supraphysiological dosing for in vitro fertilization cycles. In addition, because the treated women were generally young, the follow-up period fell short of the age at which they’d be most at risk for breast cancer.
Individual patient data were also not available, but 14 studies did adjust for confounders, including weight, race, parity, age at first birth, age at menarche, and family history of breast cancer.
Although the findings are reassuring, “further long-term and detailed studies are now needed to confirm” them, Kotryna Temcinaite, PhD, senior research communications manager at the U.K. charity Breast Cancer Now, said in the press release.
A version of this article first appeared on Medscape.com.
No link between fertility treatment and an increase in the risk for breast cancer was found in the largest study of the issue to date.
This study “provides the evidence needed to reassure women and couples seeking fertility treatments,” commented senior author Sesh Sunkara, MD, a reproductive medicine specialist at King’s College London in a press release.
With an increasing number of women seeking help to become mothers, the question “is a matter of great importance” and a source of considerable concern among patients, the study authors comment.
This is the largest meta-analysis to date, involving 1.8 million women who were followed for an average of 27 years. The investigators found no link with the use of gonadotropins or clomiphene citrate to increase egg production in fertility cycles.
There has been concern over the years that fertility treatment could stimulate estrogen-sensitive precursor breast cancer cells.
More than 4,000 studies of this issue have been conducted since 1990, and results have been conflicting. The investigators analyzed results from the 20 strongest ones.
The new meta-analysis included nine retrospective studies, five case-control studies, five prospective studies, and one comparative study
The team cautioned that the quality of evidence in even these top 20 studies was “very low” but that such an approach is perhaps the best possible on this issue because a randomized trial among women seeking help to have children would be “ethically challenging.”
In the study, the team compared breast cancer incidence among women who underwent ovarian stimulation with the incidence in both age-matched unexposed women in the general population and unexposed infertile women.
There was no significant increase in the risk for breast cancer among women treated with any ovarian stimulation drug (pooled odds ratio, 1.03; 95% confidence interval, 0.86-1.23, but with substantial heterogeneity between study outcomes).
There was also no increased risk when the analysis was limited to the eight studies in which women were treated with both gonadotropins and clomiphene citrate (pooled OR, 0.92; 95% CI, 0.52-1.60, with substantial heterogeneity).
The authors noted that, among the many study limitations, no distinction was made between physiological dosing for anovulation and supraphysiological dosing for in vitro fertilization cycles. In addition, because the treated women were generally young, the follow-up period fell short of the age at which they’d be most at risk for breast cancer.
Individual patient data were also not available, but 14 studies did adjust for confounders, including weight, race, parity, age at first birth, age at menarche, and family history of breast cancer.
Although the findings are reassuring, “further long-term and detailed studies are now needed to confirm” them, Kotryna Temcinaite, PhD, senior research communications manager at the U.K. charity Breast Cancer Now, said in the press release.
A version of this article first appeared on Medscape.com.
Racial and economic disparities persist in endometrial cancer care
Women who were Black, Latina, American Indian, or Alaska Native were significantly less likely than White women to receive guidelines-adherent treatment for endometrial cancer, based on data from more than 80,000 women.
The incidence of uterine cancer has increased across all ethnicities in recent decades, and adherence to the National Comprehensive Cancer Network treatment guidelines has been associated with improved survival, wrote Victoria A. Rodriguez, MSW, MPH, of the University of California, Irvine, and colleagues. “To date, however, there are few studies that have looked at endometrial cancer disparities with adherence to National Comprehensive Cancer Network treatment guidelines.”
In a retrospective study published in Obstetrics & Gynecology, the researchers used data from the SEER (Surveillance, Epidemiology, and End Results) database between Jan. 1, 2006, and Dec. 31, 2015. The study population included 83,883 women aged 18 years and older who were diagnosed with their first or only endometrial carcinoma. The primary dependent variable was adherence to the NCCN guidelines for the initial course of treatment, which included a combination of therapies based on cancer subtype and the extent of the disease, the researchers said.
The researchers combined the guidelines and the corresponding data from the SEER database to create “a binary variable representing adherence to [NCCN] guidelines (1 = adherent treatment, 0 = nonadherent treatment).”
Approximately 60% of the total patient population received guidelines-adherent treatment. In a multivariate analysis, Black women, Latina women, and American Indian or Alaska Native women were significantly less likely than White women to receive such treatment (odds ratios, 0.88, 0.92, and 0.82, respectively), controlling for factors including neighborhood socioeconomic status, age, and stage at diagnosis, year of diagnosis, histology, and disease grade. Asian women and Native Hawaiian/Pacific Islander women were significantly more likely to received guidelines-adherent treatment, compared with White women (OR, 1.14 and 1.19, respectively).
The researchers also found a significant gradient in guidelines-adherent treatment based on neighborhood socioeconomic status. Relative to the highest neighborhood socioeconomic status group, women in the lower groups had significantly lower odds of receiving guidelines-adherent treatment, with ORs of 0.89, 0.84, 0.80, and 0.73, respectively, for the high-middle neighborhood socioeconomic group, the middle group, the low-middle group, and the lowest group (P < .001 for all).
“Our study is novel in that it examines neighborhood socioeconomic disparities in the understudied context of treatment adherence for endometrial cancer,” the researchers noted.
The study findings were limited by several factors in including the retrospective design and potential for unmeasured confounding variables not included in SEER, such as hospital and physician characteristics, the researchers said. Also, the SEER data set was limited to only the first course of treatment, and did not include information on patient comorbidities that might affect treatment.
“Future research should qualitatively explore reasons for nonadherent treatment within endometrial cancer and other cancer sites among various racial-ethnic groups and socioeconomic status groups, with special attention to low-income women of color,” the researchers emphasized. More research on the impact of comorbidities on a patient’s ability to receive guidelines-based care should be used to inform whether comorbidities should be part of the NCCN guidelines.
However, the results were strengthened by the large sample size and diverse population, so the findings are generalizable to the overall U.S. population, the researchers said.
“Interventions are needed to ensure that equitable cancer treatment practices are available for all individuals regardless of their racial-ethnic or socioeconomic backgrounds,” they concluded.
Pursue optimal treatment to curb mortality
Even more concerning than the increase in the incidence of endometrial cancer in the United States is the increase in mortality from this disease, said Emma C. Rossi, MD, of the University of North Carolina at Chapel Hill, in an interview.
“Therefore, it is critical that we identify factors which might be contributing to the increasing lethality of this cancer,” she emphasized. “One such potential factor is race, as it has been observed that Black race is associated with an increased risk of death from endometrial cancer. Historically, this was attributed to the more aggressive subtypes of endometrial cancer (such as serous) which have a higher incidence among Black women. However, more recently, population-based studies have identified that this worse prognosis is independent of histologic cell type,” which suggests that something in our health care delivery is contributing to these worse outcomes.
“The present study helps to confirm these concerning associations, shedding some light on contributory factors, in this case, modifiable (adherence to recommended guidelines) and less modifiable (neighborhood socioeconomic environments) [ones],” Dr. Rossi noted. “The guidelines that are established by the NCCN are chosen after they have been shown to be associated with improved outcomes (including either survival or quality of life), and therefore lack of adherence to these outcomes may suggest inferior quality care is being delivered.”
Studies such as this are helpful in exposing the problem of treatment disparity to help identify sources of problems to develop solutions, she added.
The results should inspire clinicians “to feel agency in changing these outcomes, albeit by tackling very difficult social, political, and health system shortfalls,” she said.
Identify barriers to care
Barriers to greater adherence to guidelines-based care include varying definitions of such care, Dr. Rossi said.
“This is particularly true for surgical management of endometrial cancer, which remains controversial with respect to lymph node assessment. Lack of surgical staging with lymph node assessment was considered noncompliant care for this study; however, lymphadenectomy has not specifically, in and of itself, been associated with improved outcomes, and therefore some surgeons argue against performing it routinely,” she explained.
“Lack of access to sophisticated surgical tools and advanced surgical techniques may account for nonguidelines-based care in the patients with early-stage endometrial cancer; however, there are likely other differences in the ability to deliver guideline-concordant care (such as chemotherapy and radiation therapy) for advanced-stage cancers,” Dr. Rossi said. “Patient and provider positive attitudes toward adjuvant therapy, access to transportation, supportive home environments, paid sick leave, well-controlled or minimal comorbidities are all factors which promote the administration of complex adjuvant therapies such as chemotherapy and radiation. In low-resource neighborhoods and minority communities, barriers to these factors may be contributing to nonguidelines-concordant care.”
Dr. Rossi emphasized the need to “dive deeper into these data at individual health-system and provider levels.” For example, research is needed to compare the practice patterns and models of high-performing clinical practices with lower-performing practices in terms of factors such as tumor boards, journal review, peer review, dashboards, and metrics. By doing so, “we can ensure that we are understanding where and why variations in care are occurring,” Dr. Rossi said.
The study was supported in part by the Faculty Mentor Program Fellowship from the University of California, Irvine, graduate division. Ms. Rodriguez was supported in part by a grant from the National Cancer Institute. The researchers had no financial conflicts to disclose. Dr. Rossi had no financial conflicts to disclose.
Women who were Black, Latina, American Indian, or Alaska Native were significantly less likely than White women to receive guidelines-adherent treatment for endometrial cancer, based on data from more than 80,000 women.
The incidence of uterine cancer has increased across all ethnicities in recent decades, and adherence to the National Comprehensive Cancer Network treatment guidelines has been associated with improved survival, wrote Victoria A. Rodriguez, MSW, MPH, of the University of California, Irvine, and colleagues. “To date, however, there are few studies that have looked at endometrial cancer disparities with adherence to National Comprehensive Cancer Network treatment guidelines.”
In a retrospective study published in Obstetrics & Gynecology, the researchers used data from the SEER (Surveillance, Epidemiology, and End Results) database between Jan. 1, 2006, and Dec. 31, 2015. The study population included 83,883 women aged 18 years and older who were diagnosed with their first or only endometrial carcinoma. The primary dependent variable was adherence to the NCCN guidelines for the initial course of treatment, which included a combination of therapies based on cancer subtype and the extent of the disease, the researchers said.
The researchers combined the guidelines and the corresponding data from the SEER database to create “a binary variable representing adherence to [NCCN] guidelines (1 = adherent treatment, 0 = nonadherent treatment).”
Approximately 60% of the total patient population received guidelines-adherent treatment. In a multivariate analysis, Black women, Latina women, and American Indian or Alaska Native women were significantly less likely than White women to receive such treatment (odds ratios, 0.88, 0.92, and 0.82, respectively), controlling for factors including neighborhood socioeconomic status, age, and stage at diagnosis, year of diagnosis, histology, and disease grade. Asian women and Native Hawaiian/Pacific Islander women were significantly more likely to received guidelines-adherent treatment, compared with White women (OR, 1.14 and 1.19, respectively).
The researchers also found a significant gradient in guidelines-adherent treatment based on neighborhood socioeconomic status. Relative to the highest neighborhood socioeconomic status group, women in the lower groups had significantly lower odds of receiving guidelines-adherent treatment, with ORs of 0.89, 0.84, 0.80, and 0.73, respectively, for the high-middle neighborhood socioeconomic group, the middle group, the low-middle group, and the lowest group (P < .001 for all).
“Our study is novel in that it examines neighborhood socioeconomic disparities in the understudied context of treatment adherence for endometrial cancer,” the researchers noted.
The study findings were limited by several factors in including the retrospective design and potential for unmeasured confounding variables not included in SEER, such as hospital and physician characteristics, the researchers said. Also, the SEER data set was limited to only the first course of treatment, and did not include information on patient comorbidities that might affect treatment.
“Future research should qualitatively explore reasons for nonadherent treatment within endometrial cancer and other cancer sites among various racial-ethnic groups and socioeconomic status groups, with special attention to low-income women of color,” the researchers emphasized. More research on the impact of comorbidities on a patient’s ability to receive guidelines-based care should be used to inform whether comorbidities should be part of the NCCN guidelines.
However, the results were strengthened by the large sample size and diverse population, so the findings are generalizable to the overall U.S. population, the researchers said.
“Interventions are needed to ensure that equitable cancer treatment practices are available for all individuals regardless of their racial-ethnic or socioeconomic backgrounds,” they concluded.
Pursue optimal treatment to curb mortality
Even more concerning than the increase in the incidence of endometrial cancer in the United States is the increase in mortality from this disease, said Emma C. Rossi, MD, of the University of North Carolina at Chapel Hill, in an interview.
“Therefore, it is critical that we identify factors which might be contributing to the increasing lethality of this cancer,” she emphasized. “One such potential factor is race, as it has been observed that Black race is associated with an increased risk of death from endometrial cancer. Historically, this was attributed to the more aggressive subtypes of endometrial cancer (such as serous) which have a higher incidence among Black women. However, more recently, population-based studies have identified that this worse prognosis is independent of histologic cell type,” which suggests that something in our health care delivery is contributing to these worse outcomes.
“The present study helps to confirm these concerning associations, shedding some light on contributory factors, in this case, modifiable (adherence to recommended guidelines) and less modifiable (neighborhood socioeconomic environments) [ones],” Dr. Rossi noted. “The guidelines that are established by the NCCN are chosen after they have been shown to be associated with improved outcomes (including either survival or quality of life), and therefore lack of adherence to these outcomes may suggest inferior quality care is being delivered.”
Studies such as this are helpful in exposing the problem of treatment disparity to help identify sources of problems to develop solutions, she added.
The results should inspire clinicians “to feel agency in changing these outcomes, albeit by tackling very difficult social, political, and health system shortfalls,” she said.
Identify barriers to care
Barriers to greater adherence to guidelines-based care include varying definitions of such care, Dr. Rossi said.
“This is particularly true for surgical management of endometrial cancer, which remains controversial with respect to lymph node assessment. Lack of surgical staging with lymph node assessment was considered noncompliant care for this study; however, lymphadenectomy has not specifically, in and of itself, been associated with improved outcomes, and therefore some surgeons argue against performing it routinely,” she explained.
“Lack of access to sophisticated surgical tools and advanced surgical techniques may account for nonguidelines-based care in the patients with early-stage endometrial cancer; however, there are likely other differences in the ability to deliver guideline-concordant care (such as chemotherapy and radiation therapy) for advanced-stage cancers,” Dr. Rossi said. “Patient and provider positive attitudes toward adjuvant therapy, access to transportation, supportive home environments, paid sick leave, well-controlled or minimal comorbidities are all factors which promote the administration of complex adjuvant therapies such as chemotherapy and radiation. In low-resource neighborhoods and minority communities, barriers to these factors may be contributing to nonguidelines-concordant care.”
Dr. Rossi emphasized the need to “dive deeper into these data at individual health-system and provider levels.” For example, research is needed to compare the practice patterns and models of high-performing clinical practices with lower-performing practices in terms of factors such as tumor boards, journal review, peer review, dashboards, and metrics. By doing so, “we can ensure that we are understanding where and why variations in care are occurring,” Dr. Rossi said.
The study was supported in part by the Faculty Mentor Program Fellowship from the University of California, Irvine, graduate division. Ms. Rodriguez was supported in part by a grant from the National Cancer Institute. The researchers had no financial conflicts to disclose. Dr. Rossi had no financial conflicts to disclose.
Women who were Black, Latina, American Indian, or Alaska Native were significantly less likely than White women to receive guidelines-adherent treatment for endometrial cancer, based on data from more than 80,000 women.
The incidence of uterine cancer has increased across all ethnicities in recent decades, and adherence to the National Comprehensive Cancer Network treatment guidelines has been associated with improved survival, wrote Victoria A. Rodriguez, MSW, MPH, of the University of California, Irvine, and colleagues. “To date, however, there are few studies that have looked at endometrial cancer disparities with adherence to National Comprehensive Cancer Network treatment guidelines.”
In a retrospective study published in Obstetrics & Gynecology, the researchers used data from the SEER (Surveillance, Epidemiology, and End Results) database between Jan. 1, 2006, and Dec. 31, 2015. The study population included 83,883 women aged 18 years and older who were diagnosed with their first or only endometrial carcinoma. The primary dependent variable was adherence to the NCCN guidelines for the initial course of treatment, which included a combination of therapies based on cancer subtype and the extent of the disease, the researchers said.
The researchers combined the guidelines and the corresponding data from the SEER database to create “a binary variable representing adherence to [NCCN] guidelines (1 = adherent treatment, 0 = nonadherent treatment).”
Approximately 60% of the total patient population received guidelines-adherent treatment. In a multivariate analysis, Black women, Latina women, and American Indian or Alaska Native women were significantly less likely than White women to receive such treatment (odds ratios, 0.88, 0.92, and 0.82, respectively), controlling for factors including neighborhood socioeconomic status, age, and stage at diagnosis, year of diagnosis, histology, and disease grade. Asian women and Native Hawaiian/Pacific Islander women were significantly more likely to received guidelines-adherent treatment, compared with White women (OR, 1.14 and 1.19, respectively).
The researchers also found a significant gradient in guidelines-adherent treatment based on neighborhood socioeconomic status. Relative to the highest neighborhood socioeconomic status group, women in the lower groups had significantly lower odds of receiving guidelines-adherent treatment, with ORs of 0.89, 0.84, 0.80, and 0.73, respectively, for the high-middle neighborhood socioeconomic group, the middle group, the low-middle group, and the lowest group (P < .001 for all).
“Our study is novel in that it examines neighborhood socioeconomic disparities in the understudied context of treatment adherence for endometrial cancer,” the researchers noted.
The study findings were limited by several factors in including the retrospective design and potential for unmeasured confounding variables not included in SEER, such as hospital and physician characteristics, the researchers said. Also, the SEER data set was limited to only the first course of treatment, and did not include information on patient comorbidities that might affect treatment.
“Future research should qualitatively explore reasons for nonadherent treatment within endometrial cancer and other cancer sites among various racial-ethnic groups and socioeconomic status groups, with special attention to low-income women of color,” the researchers emphasized. More research on the impact of comorbidities on a patient’s ability to receive guidelines-based care should be used to inform whether comorbidities should be part of the NCCN guidelines.
However, the results were strengthened by the large sample size and diverse population, so the findings are generalizable to the overall U.S. population, the researchers said.
“Interventions are needed to ensure that equitable cancer treatment practices are available for all individuals regardless of their racial-ethnic or socioeconomic backgrounds,” they concluded.
Pursue optimal treatment to curb mortality
Even more concerning than the increase in the incidence of endometrial cancer in the United States is the increase in mortality from this disease, said Emma C. Rossi, MD, of the University of North Carolina at Chapel Hill, in an interview.
“Therefore, it is critical that we identify factors which might be contributing to the increasing lethality of this cancer,” she emphasized. “One such potential factor is race, as it has been observed that Black race is associated with an increased risk of death from endometrial cancer. Historically, this was attributed to the more aggressive subtypes of endometrial cancer (such as serous) which have a higher incidence among Black women. However, more recently, population-based studies have identified that this worse prognosis is independent of histologic cell type,” which suggests that something in our health care delivery is contributing to these worse outcomes.
“The present study helps to confirm these concerning associations, shedding some light on contributory factors, in this case, modifiable (adherence to recommended guidelines) and less modifiable (neighborhood socioeconomic environments) [ones],” Dr. Rossi noted. “The guidelines that are established by the NCCN are chosen after they have been shown to be associated with improved outcomes (including either survival or quality of life), and therefore lack of adherence to these outcomes may suggest inferior quality care is being delivered.”
Studies such as this are helpful in exposing the problem of treatment disparity to help identify sources of problems to develop solutions, she added.
The results should inspire clinicians “to feel agency in changing these outcomes, albeit by tackling very difficult social, political, and health system shortfalls,” she said.
Identify barriers to care
Barriers to greater adherence to guidelines-based care include varying definitions of such care, Dr. Rossi said.
“This is particularly true for surgical management of endometrial cancer, which remains controversial with respect to lymph node assessment. Lack of surgical staging with lymph node assessment was considered noncompliant care for this study; however, lymphadenectomy has not specifically, in and of itself, been associated with improved outcomes, and therefore some surgeons argue against performing it routinely,” she explained.
“Lack of access to sophisticated surgical tools and advanced surgical techniques may account for nonguidelines-based care in the patients with early-stage endometrial cancer; however, there are likely other differences in the ability to deliver guideline-concordant care (such as chemotherapy and radiation therapy) for advanced-stage cancers,” Dr. Rossi said. “Patient and provider positive attitudes toward adjuvant therapy, access to transportation, supportive home environments, paid sick leave, well-controlled or minimal comorbidities are all factors which promote the administration of complex adjuvant therapies such as chemotherapy and radiation. In low-resource neighborhoods and minority communities, barriers to these factors may be contributing to nonguidelines-concordant care.”
Dr. Rossi emphasized the need to “dive deeper into these data at individual health-system and provider levels.” For example, research is needed to compare the practice patterns and models of high-performing clinical practices with lower-performing practices in terms of factors such as tumor boards, journal review, peer review, dashboards, and metrics. By doing so, “we can ensure that we are understanding where and why variations in care are occurring,” Dr. Rossi said.
The study was supported in part by the Faculty Mentor Program Fellowship from the University of California, Irvine, graduate division. Ms. Rodriguez was supported in part by a grant from the National Cancer Institute. The researchers had no financial conflicts to disclose. Dr. Rossi had no financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY







