User login
Endoscopic obesity treatments offer alternatives to surgery
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
AT 2022 AGA TECH SUMMIT
Complicated appendicitis during pregnancy: Immediate surgery may be best
Pregnant women who underwent immediate surgery to treat a ruptured or abscessed appendix had lower risk of infectious complications, compared with those whose complicated appendicitis was managed without surgery, according to new research.
Most cases that began with nonoperative management eventually required surgery, and the operative delay was associated with an increased risk of preterm labor, preterm delivery, and abortion.
“Our study findings may help to define the preferred management strategy in complicated appendicitis during pregnancy to be immediate operation,” Kazuhide Matsushima, MD, an assistant professor of clinical surgery at the University of Southern California, Los Angeles, and colleagues wrote.
The retrospective study was published in JAMA Network Open.
While acute appendicitis is relatively rare during pregnancy, it is the most common nonobstetric emergency in pregnant women, Dr. Matsushima said. This condition occurs in an estimated 1 in 700 to 1 in 1,500 pregnancies, and some data suggest that pregnant women are at higher risk for perforation and other forms of complicated appendicitis.
National guidelines support appendectomy as the first-line treatment for pregnant women with acute uncomplicated appendicitis, but there is no clear guidance on the best treatment approach for managing complicated appendicitis in this population, the authors note.
To better understand how surgical and nonoperational interventions affected outcomes, investigators analyzed data from the National Inpatient Sample from January 2003 to September 2015 to identify pregnant women with complicated appendicitis. The condition was defined as “acute appendicitis with generalized peritonitis” and “acute appendicitis with peritoneal abscess.” Patients were excluded if they had complications such as ectopic pregnancy and hydatidiform mole.
Investigators split the patients into three groups: those who underwent immediate operation for complicated appendicitis, those whose appendicitis was successfully managed without surgery, and those in whom nonoperative management of their condition failed, resulting in delayed surgery. Failed nonoperative management was defined as at least 1 day of nonoperative management followed by a laparoscopic or open appendectomy.
Of the 8,087 pregnant women identified during the study with complicated appendicitis, 55.5% underwent immediate appendectomy, 11.8% were successfully treated without surgical intervention, and 32.7% had delayed operations after initial failed nonoperative management. There was no significant difference in preterm delivery, preterm labor, or abortion between the immediate operative and successful nonoperative groups; however, the successful nonoperative group was more than twice as likely to experience premature rupture of membranes (odds ratio, 2.77; P = .03). Patients successfully treated without surgery also were at higher risk for infections such as amniotic infection (OR, 4.35; P < .001), pneumonia (OR, 2.52; P < .001), and sepsis (OR, 1.52; P = .01), compared with patients who underwent immediate operation.
Patients who had delayed surgery were 45% more likely to experience preterm delivery, preterm labor, or abortion (OR, 1.45; P < .001), compared with the immediate surgery group. The delayed surgery group was also at higher risk for antepartum hemorrhage (OR, 1.56; P = .03) and premature rupture of membranes (OR, 3.44; P = .002). They were more than four times as likely to have amniotic infection (OR, 4.74; P < .001), twice as likely to contract pneumonia (OR, 2.01; P < .001), and 58% more likely to develop sepsis (OR, 1.58; P < .001), compared with the immediate surgery group. The researchers calculated that every day surgery was delayed, the risk of preterm delivery, preterm labor, and abortion rose by 23% (OR, 1.23; P < .001).
Delayed surgery and successful nonoperative management were also associated with higher hospital charges and longer hospital stays.
Because this was a retrospective study, there are some limitations to the findings, Dr. Matsushima said, and therefore it should not be used to justify changing standards of care; however, it does give more information on the risks associated with different interventions. “It’s very important to have a discussion with the patient and make a shared decision,” he told this news organization, “because each option has significant risks and benefits.”
Because the data were from a database, he added, the research team was not able to see if outcomes from immediate surgery, nonoperative management, and delayed surgery differed in each trimester.
Kenneth W. Sharp, MD, a professor of surgery at Vanderbilt University Medical Center in Nashville, Tenn., agreed that the study does have limitations, such as lack of information on how complicated appendicitis was identified and diagnosed; however, the study does provide guidance to surgeons in a surgical area with “very sparse literature,” he told this news organization. Dr. Sharp is also a regent from the American College of Surgeons, which arranged the interview.
“Especially with these very complicated patients, it was never clear what to do,” he said. “With the recent studies showing that treatment of appendicitis with antibiotics works for a large number of people, people start extrapolating [those findings] to complicated appendicitis and they start extrapolating it to pregnant women, none of which the studies were meant to show anything about,” he said.
This analysis gives additional information to inform treatment decisions in pregnant women who may be hesitant to undergo this abdominal surgery because of possible complications, like pregnancy loss, he added. “Now, I can say to them that the data would suggest that with your particular complicated appendicitis, we should operate sooner, not later.”
Dr. Matsushima and Dr. Sharp have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women who underwent immediate surgery to treat a ruptured or abscessed appendix had lower risk of infectious complications, compared with those whose complicated appendicitis was managed without surgery, according to new research.
Most cases that began with nonoperative management eventually required surgery, and the operative delay was associated with an increased risk of preterm labor, preterm delivery, and abortion.
“Our study findings may help to define the preferred management strategy in complicated appendicitis during pregnancy to be immediate operation,” Kazuhide Matsushima, MD, an assistant professor of clinical surgery at the University of Southern California, Los Angeles, and colleagues wrote.
The retrospective study was published in JAMA Network Open.
While acute appendicitis is relatively rare during pregnancy, it is the most common nonobstetric emergency in pregnant women, Dr. Matsushima said. This condition occurs in an estimated 1 in 700 to 1 in 1,500 pregnancies, and some data suggest that pregnant women are at higher risk for perforation and other forms of complicated appendicitis.
National guidelines support appendectomy as the first-line treatment for pregnant women with acute uncomplicated appendicitis, but there is no clear guidance on the best treatment approach for managing complicated appendicitis in this population, the authors note.
To better understand how surgical and nonoperational interventions affected outcomes, investigators analyzed data from the National Inpatient Sample from January 2003 to September 2015 to identify pregnant women with complicated appendicitis. The condition was defined as “acute appendicitis with generalized peritonitis” and “acute appendicitis with peritoneal abscess.” Patients were excluded if they had complications such as ectopic pregnancy and hydatidiform mole.
Investigators split the patients into three groups: those who underwent immediate operation for complicated appendicitis, those whose appendicitis was successfully managed without surgery, and those in whom nonoperative management of their condition failed, resulting in delayed surgery. Failed nonoperative management was defined as at least 1 day of nonoperative management followed by a laparoscopic or open appendectomy.
Of the 8,087 pregnant women identified during the study with complicated appendicitis, 55.5% underwent immediate appendectomy, 11.8% were successfully treated without surgical intervention, and 32.7% had delayed operations after initial failed nonoperative management. There was no significant difference in preterm delivery, preterm labor, or abortion between the immediate operative and successful nonoperative groups; however, the successful nonoperative group was more than twice as likely to experience premature rupture of membranes (odds ratio, 2.77; P = .03). Patients successfully treated without surgery also were at higher risk for infections such as amniotic infection (OR, 4.35; P < .001), pneumonia (OR, 2.52; P < .001), and sepsis (OR, 1.52; P = .01), compared with patients who underwent immediate operation.
Patients who had delayed surgery were 45% more likely to experience preterm delivery, preterm labor, or abortion (OR, 1.45; P < .001), compared with the immediate surgery group. The delayed surgery group was also at higher risk for antepartum hemorrhage (OR, 1.56; P = .03) and premature rupture of membranes (OR, 3.44; P = .002). They were more than four times as likely to have amniotic infection (OR, 4.74; P < .001), twice as likely to contract pneumonia (OR, 2.01; P < .001), and 58% more likely to develop sepsis (OR, 1.58; P < .001), compared with the immediate surgery group. The researchers calculated that every day surgery was delayed, the risk of preterm delivery, preterm labor, and abortion rose by 23% (OR, 1.23; P < .001).
Delayed surgery and successful nonoperative management were also associated with higher hospital charges and longer hospital stays.
Because this was a retrospective study, there are some limitations to the findings, Dr. Matsushima said, and therefore it should not be used to justify changing standards of care; however, it does give more information on the risks associated with different interventions. “It’s very important to have a discussion with the patient and make a shared decision,” he told this news organization, “because each option has significant risks and benefits.”
Because the data were from a database, he added, the research team was not able to see if outcomes from immediate surgery, nonoperative management, and delayed surgery differed in each trimester.
Kenneth W. Sharp, MD, a professor of surgery at Vanderbilt University Medical Center in Nashville, Tenn., agreed that the study does have limitations, such as lack of information on how complicated appendicitis was identified and diagnosed; however, the study does provide guidance to surgeons in a surgical area with “very sparse literature,” he told this news organization. Dr. Sharp is also a regent from the American College of Surgeons, which arranged the interview.
“Especially with these very complicated patients, it was never clear what to do,” he said. “With the recent studies showing that treatment of appendicitis with antibiotics works for a large number of people, people start extrapolating [those findings] to complicated appendicitis and they start extrapolating it to pregnant women, none of which the studies were meant to show anything about,” he said.
This analysis gives additional information to inform treatment decisions in pregnant women who may be hesitant to undergo this abdominal surgery because of possible complications, like pregnancy loss, he added. “Now, I can say to them that the data would suggest that with your particular complicated appendicitis, we should operate sooner, not later.”
Dr. Matsushima and Dr. Sharp have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women who underwent immediate surgery to treat a ruptured or abscessed appendix had lower risk of infectious complications, compared with those whose complicated appendicitis was managed without surgery, according to new research.
Most cases that began with nonoperative management eventually required surgery, and the operative delay was associated with an increased risk of preterm labor, preterm delivery, and abortion.
“Our study findings may help to define the preferred management strategy in complicated appendicitis during pregnancy to be immediate operation,” Kazuhide Matsushima, MD, an assistant professor of clinical surgery at the University of Southern California, Los Angeles, and colleagues wrote.
The retrospective study was published in JAMA Network Open.
While acute appendicitis is relatively rare during pregnancy, it is the most common nonobstetric emergency in pregnant women, Dr. Matsushima said. This condition occurs in an estimated 1 in 700 to 1 in 1,500 pregnancies, and some data suggest that pregnant women are at higher risk for perforation and other forms of complicated appendicitis.
National guidelines support appendectomy as the first-line treatment for pregnant women with acute uncomplicated appendicitis, but there is no clear guidance on the best treatment approach for managing complicated appendicitis in this population, the authors note.
To better understand how surgical and nonoperational interventions affected outcomes, investigators analyzed data from the National Inpatient Sample from January 2003 to September 2015 to identify pregnant women with complicated appendicitis. The condition was defined as “acute appendicitis with generalized peritonitis” and “acute appendicitis with peritoneal abscess.” Patients were excluded if they had complications such as ectopic pregnancy and hydatidiform mole.
Investigators split the patients into three groups: those who underwent immediate operation for complicated appendicitis, those whose appendicitis was successfully managed without surgery, and those in whom nonoperative management of their condition failed, resulting in delayed surgery. Failed nonoperative management was defined as at least 1 day of nonoperative management followed by a laparoscopic or open appendectomy.
Of the 8,087 pregnant women identified during the study with complicated appendicitis, 55.5% underwent immediate appendectomy, 11.8% were successfully treated without surgical intervention, and 32.7% had delayed operations after initial failed nonoperative management. There was no significant difference in preterm delivery, preterm labor, or abortion between the immediate operative and successful nonoperative groups; however, the successful nonoperative group was more than twice as likely to experience premature rupture of membranes (odds ratio, 2.77; P = .03). Patients successfully treated without surgery also were at higher risk for infections such as amniotic infection (OR, 4.35; P < .001), pneumonia (OR, 2.52; P < .001), and sepsis (OR, 1.52; P = .01), compared with patients who underwent immediate operation.
Patients who had delayed surgery were 45% more likely to experience preterm delivery, preterm labor, or abortion (OR, 1.45; P < .001), compared with the immediate surgery group. The delayed surgery group was also at higher risk for antepartum hemorrhage (OR, 1.56; P = .03) and premature rupture of membranes (OR, 3.44; P = .002). They were more than four times as likely to have amniotic infection (OR, 4.74; P < .001), twice as likely to contract pneumonia (OR, 2.01; P < .001), and 58% more likely to develop sepsis (OR, 1.58; P < .001), compared with the immediate surgery group. The researchers calculated that every day surgery was delayed, the risk of preterm delivery, preterm labor, and abortion rose by 23% (OR, 1.23; P < .001).
Delayed surgery and successful nonoperative management were also associated with higher hospital charges and longer hospital stays.
Because this was a retrospective study, there are some limitations to the findings, Dr. Matsushima said, and therefore it should not be used to justify changing standards of care; however, it does give more information on the risks associated with different interventions. “It’s very important to have a discussion with the patient and make a shared decision,” he told this news organization, “because each option has significant risks and benefits.”
Because the data were from a database, he added, the research team was not able to see if outcomes from immediate surgery, nonoperative management, and delayed surgery differed in each trimester.
Kenneth W. Sharp, MD, a professor of surgery at Vanderbilt University Medical Center in Nashville, Tenn., agreed that the study does have limitations, such as lack of information on how complicated appendicitis was identified and diagnosed; however, the study does provide guidance to surgeons in a surgical area with “very sparse literature,” he told this news organization. Dr. Sharp is also a regent from the American College of Surgeons, which arranged the interview.
“Especially with these very complicated patients, it was never clear what to do,” he said. “With the recent studies showing that treatment of appendicitis with antibiotics works for a large number of people, people start extrapolating [those findings] to complicated appendicitis and they start extrapolating it to pregnant women, none of which the studies were meant to show anything about,” he said.
This analysis gives additional information to inform treatment decisions in pregnant women who may be hesitant to undergo this abdominal surgery because of possible complications, like pregnancy loss, he added. “Now, I can say to them that the data would suggest that with your particular complicated appendicitis, we should operate sooner, not later.”
Dr. Matsushima and Dr. Sharp have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Granuloma Faciale in Woman With Levamisole-Induced Vasculitis
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
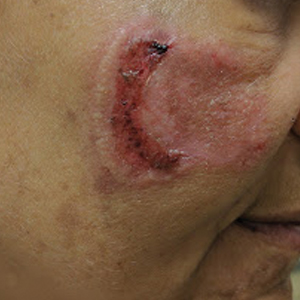
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.
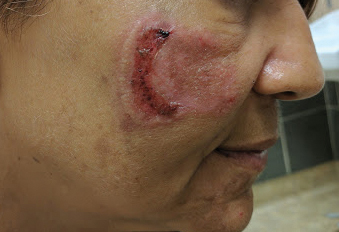
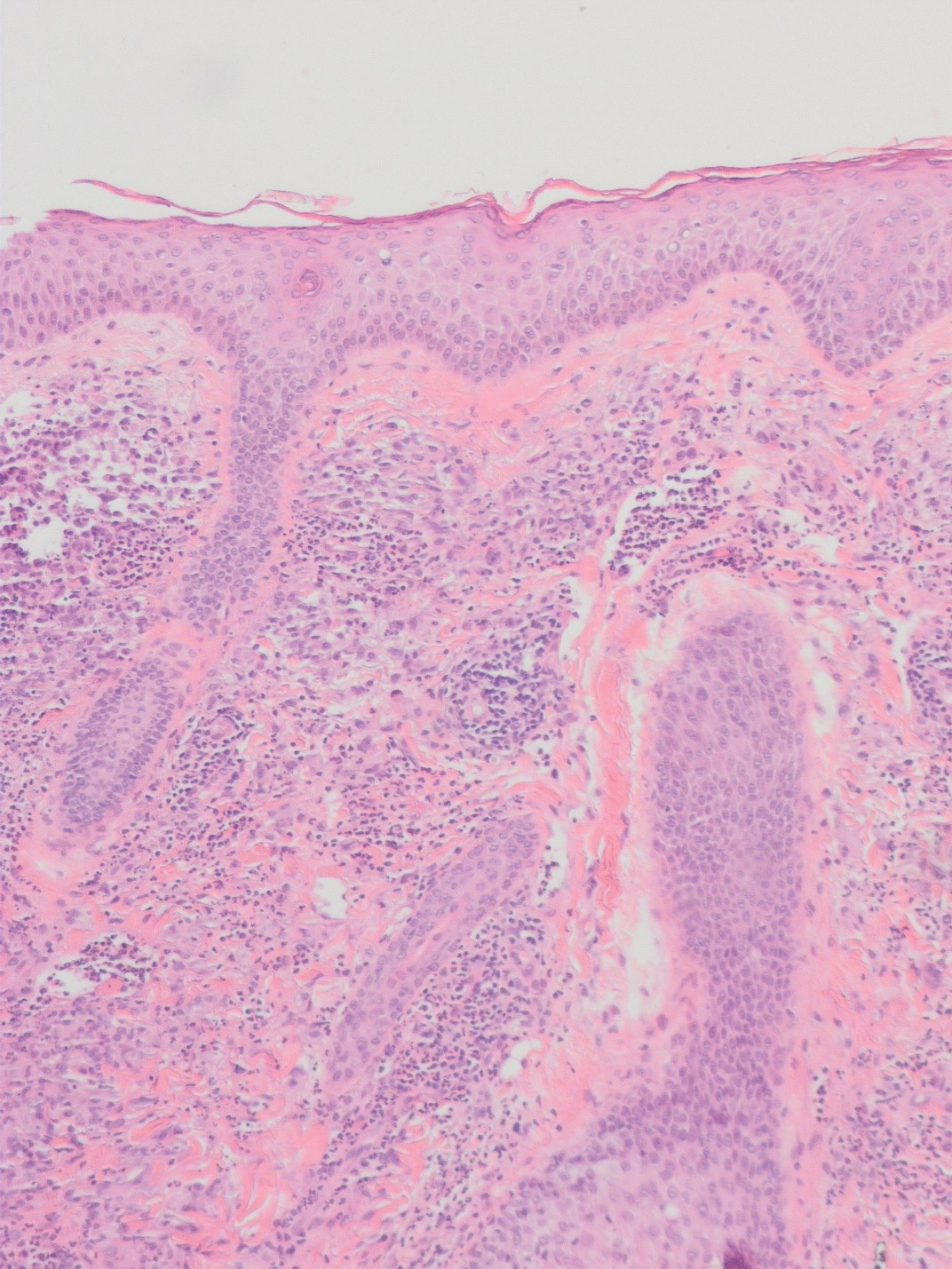
There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.
Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.

There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.

Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.

There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.

Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
Practice Points
- Granuloma faciale is a benign dermal process presenting with a red-brown plaque on the face of adults that typically is not ulcerated unless physically manipulated.
- Skin biopsy often is required for correct diagnosis.
- Granuloma faciale does not resolve spontaneously and tends to be chronic.
Necrosis of the Ear Following Skin Cancer Resection
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
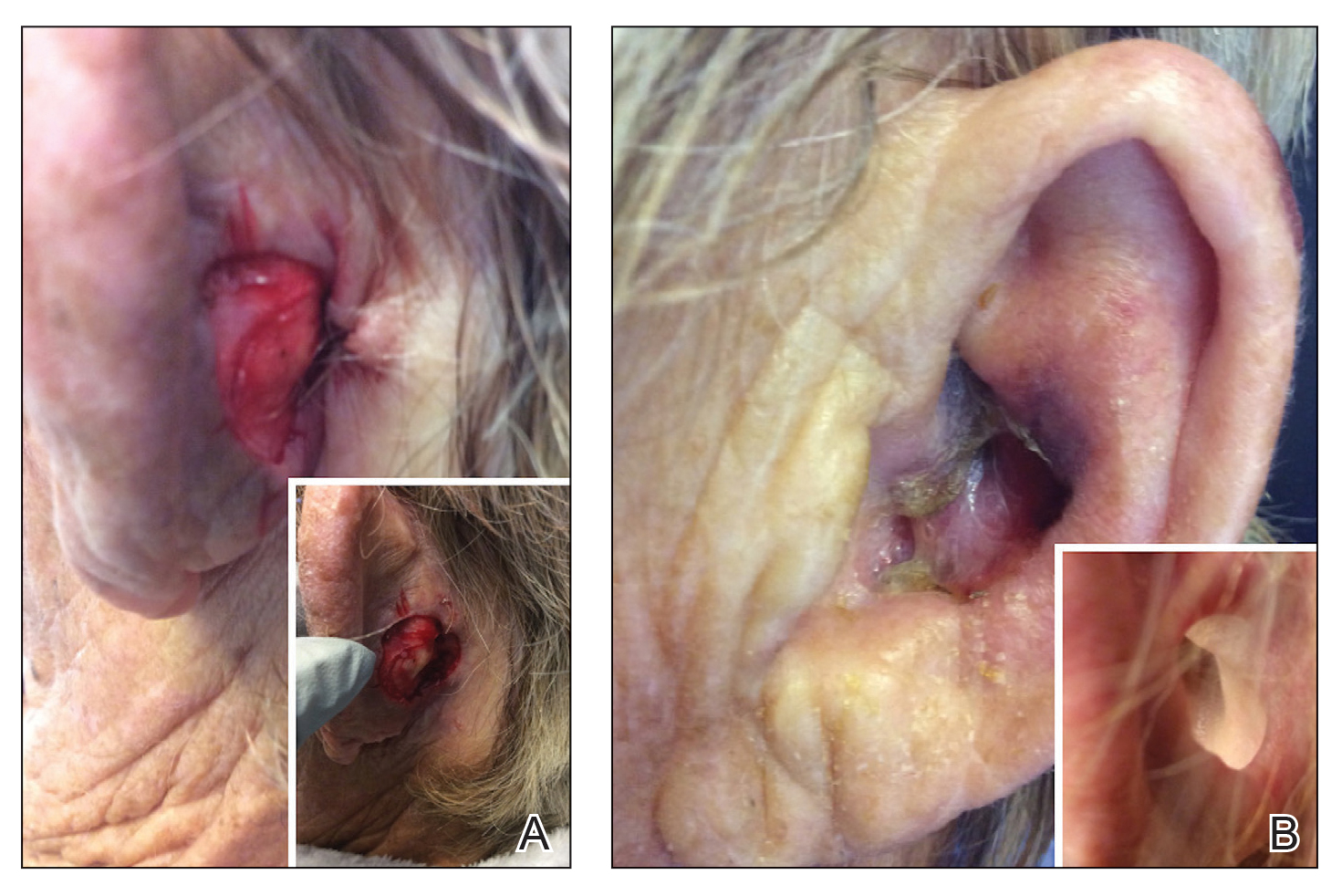
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.
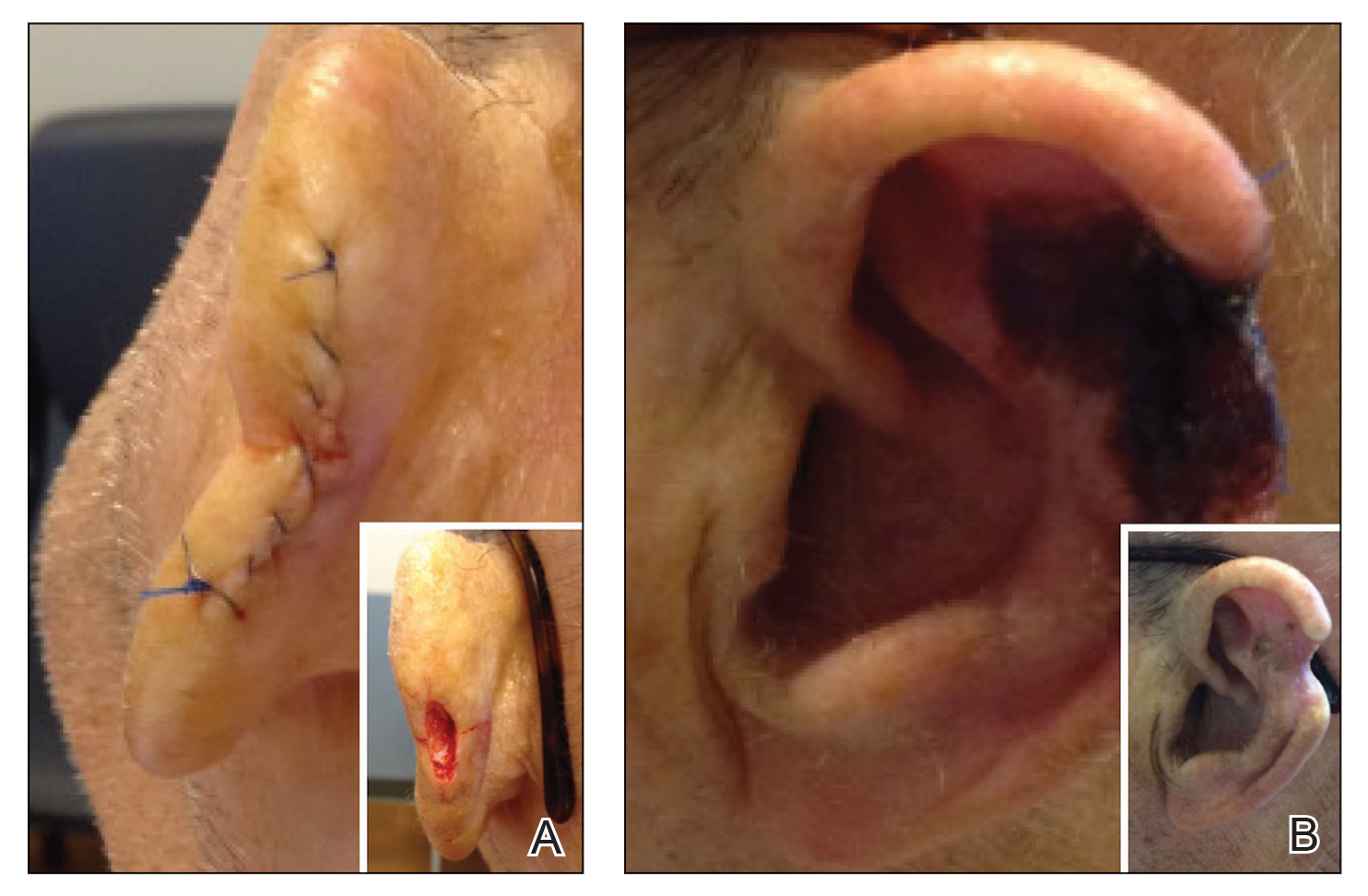
Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
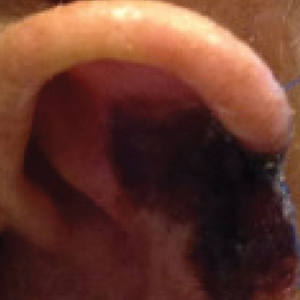
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.
Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).
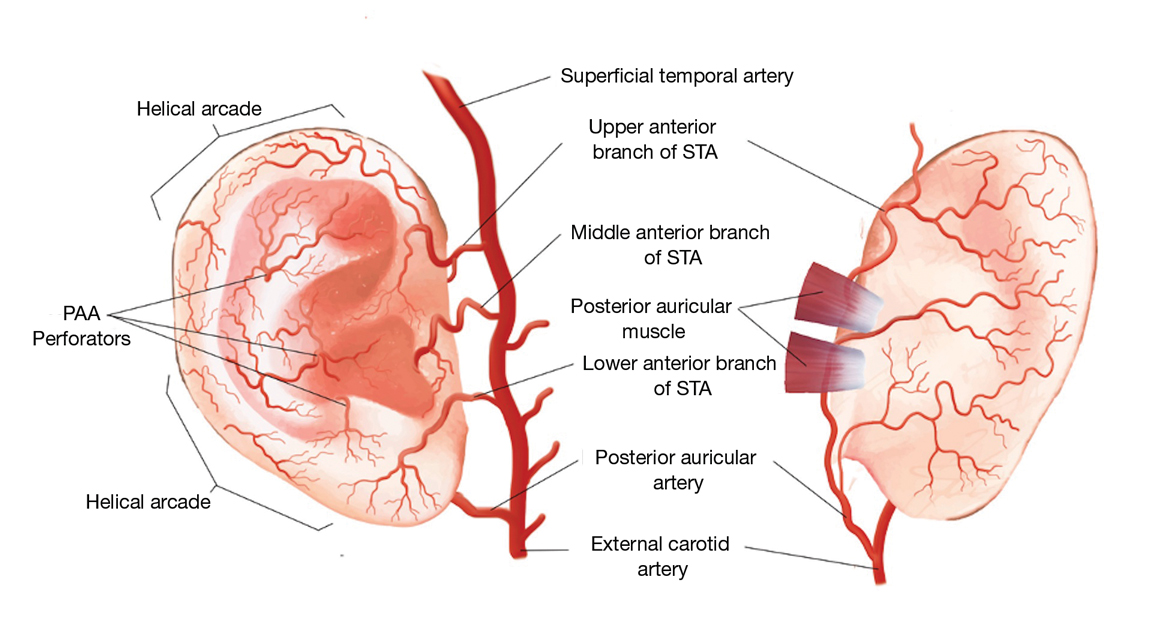
The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.

Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.

Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).

The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.

Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.

Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).

The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
Practice Points
- The auricular vasculature supply is complex and forms several anastomoses and arcades, making it susceptible to vascular compromise.
- Damage to the auricular helical arcades or perforating branches can result in postoperative tissue necrosis.
- Clinicians should pay special attention during operative planning for Mohs micrographic surgery to account for the external ear vascular arcades and, when possible, should choose the least invasive treatment and closure options available.
Gastric cancer: Robotic gastrectomy has superior outcomes in patients with obesity
Key clinical point: In patients with early-stage gastric cancer and visceral obesity, robotic gastrectomy (RG) is associated with lower incidence of postoperative complications and improved survival vs. laparoscopic gastrectomy (LG).
Major finding: In patients with high visceral fat area (VFA), RG vs. LG was associated with lower incidences of all complications (2.7% vs. 8.2%; P = .019) and intra-abdominal infectious complications (2.0% vs. 6.6%; P = .048). LG was an independent risk factor for overall (odds ratio [OR] 3.281; P = .012) and intra-abdominal infectious (OR 3.462; P = .021) complications in patients with high VFA. The overall survival in patients with high VFA was significantly higher with RG vs. LG (P = .045).
Study details: This article reports a retrospective study of 1306 patients with clinical stage I-II gastric cancer who underwent minimally invasive gastrectomy between 2012 and 2020.
Disclosures: No funding source was identified for this study. The authors received personal fees outside this work.
Source: Hikage M et al. Advantages of a robotic approach compared with laparoscopy gastrectomy for patients with high visceral fat area. Surg Endosc. 2022 (Mar 16). Doi: 10.1007/s00464-022-09178-x
Key clinical point: In patients with early-stage gastric cancer and visceral obesity, robotic gastrectomy (RG) is associated with lower incidence of postoperative complications and improved survival vs. laparoscopic gastrectomy (LG).
Major finding: In patients with high visceral fat area (VFA), RG vs. LG was associated with lower incidences of all complications (2.7% vs. 8.2%; P = .019) and intra-abdominal infectious complications (2.0% vs. 6.6%; P = .048). LG was an independent risk factor for overall (odds ratio [OR] 3.281; P = .012) and intra-abdominal infectious (OR 3.462; P = .021) complications in patients with high VFA. The overall survival in patients with high VFA was significantly higher with RG vs. LG (P = .045).
Study details: This article reports a retrospective study of 1306 patients with clinical stage I-II gastric cancer who underwent minimally invasive gastrectomy between 2012 and 2020.
Disclosures: No funding source was identified for this study. The authors received personal fees outside this work.
Source: Hikage M et al. Advantages of a robotic approach compared with laparoscopy gastrectomy for patients with high visceral fat area. Surg Endosc. 2022 (Mar 16). Doi: 10.1007/s00464-022-09178-x
Key clinical point: In patients with early-stage gastric cancer and visceral obesity, robotic gastrectomy (RG) is associated with lower incidence of postoperative complications and improved survival vs. laparoscopic gastrectomy (LG).
Major finding: In patients with high visceral fat area (VFA), RG vs. LG was associated with lower incidences of all complications (2.7% vs. 8.2%; P = .019) and intra-abdominal infectious complications (2.0% vs. 6.6%; P = .048). LG was an independent risk factor for overall (odds ratio [OR] 3.281; P = .012) and intra-abdominal infectious (OR 3.462; P = .021) complications in patients with high VFA. The overall survival in patients with high VFA was significantly higher with RG vs. LG (P = .045).
Study details: This article reports a retrospective study of 1306 patients with clinical stage I-II gastric cancer who underwent minimally invasive gastrectomy between 2012 and 2020.
Disclosures: No funding source was identified for this study. The authors received personal fees outside this work.
Source: Hikage M et al. Advantages of a robotic approach compared with laparoscopy gastrectomy for patients with high visceral fat area. Surg Endosc. 2022 (Mar 16). Doi: 10.1007/s00464-022-09178-x
Gastric cancer: LNR can help select patients for adjuvant chemotherapy
Key clinical point: Adjuvant chemotherapy (ACT) after neoadjuvant chemotherapy (NACT) and surgery significantly improved survival in patients with locally advanced gastric cancer (LAGC) with a lymph node ratio (LNR) of ≥9%.
Major finding: At 3 years, the overall survival (OS) was significantly higher in patients who received ACT (60.1% vs. 49.3%; P = .02) vs. those who did not. ACT was associated with significantly improved OS at 3 years in patients with higher (≥9%) LNR (46.6% vs. 21.7%; P < .001). ACT improved OS at 3 years in patients with an LNR of ≥9% in an external validation cohort (53.0% vs. 26.3%; P = .04).
Study details: This article reports on a multicenter retrospective cohort study including 353 patients with LAGC undergoing curative-intent gastrectomy after NACT and an external validation cohort of 109 patients.
Disclosures: No funding source was identified for this study. The authors declared no conflicts of interest.
Source: Lin J-X et al. Association of adjuvant chemotherapy with overall survival among patients with locally advanced gastric cancer after neoadjuvant chemotherapy. JAMA Netw Open. 2022;5(4):e225557 (Apr 1). Doi: 10.1001/jamanetworkopen.2022.5557
Key clinical point: Adjuvant chemotherapy (ACT) after neoadjuvant chemotherapy (NACT) and surgery significantly improved survival in patients with locally advanced gastric cancer (LAGC) with a lymph node ratio (LNR) of ≥9%.
Major finding: At 3 years, the overall survival (OS) was significantly higher in patients who received ACT (60.1% vs. 49.3%; P = .02) vs. those who did not. ACT was associated with significantly improved OS at 3 years in patients with higher (≥9%) LNR (46.6% vs. 21.7%; P < .001). ACT improved OS at 3 years in patients with an LNR of ≥9% in an external validation cohort (53.0% vs. 26.3%; P = .04).
Study details: This article reports on a multicenter retrospective cohort study including 353 patients with LAGC undergoing curative-intent gastrectomy after NACT and an external validation cohort of 109 patients.
Disclosures: No funding source was identified for this study. The authors declared no conflicts of interest.
Source: Lin J-X et al. Association of adjuvant chemotherapy with overall survival among patients with locally advanced gastric cancer after neoadjuvant chemotherapy. JAMA Netw Open. 2022;5(4):e225557 (Apr 1). Doi: 10.1001/jamanetworkopen.2022.5557
Key clinical point: Adjuvant chemotherapy (ACT) after neoadjuvant chemotherapy (NACT) and surgery significantly improved survival in patients with locally advanced gastric cancer (LAGC) with a lymph node ratio (LNR) of ≥9%.
Major finding: At 3 years, the overall survival (OS) was significantly higher in patients who received ACT (60.1% vs. 49.3%; P = .02) vs. those who did not. ACT was associated with significantly improved OS at 3 years in patients with higher (≥9%) LNR (46.6% vs. 21.7%; P < .001). ACT improved OS at 3 years in patients with an LNR of ≥9% in an external validation cohort (53.0% vs. 26.3%; P = .04).
Study details: This article reports on a multicenter retrospective cohort study including 353 patients with LAGC undergoing curative-intent gastrectomy after NACT and an external validation cohort of 109 patients.
Disclosures: No funding source was identified for this study. The authors declared no conflicts of interest.
Source: Lin J-X et al. Association of adjuvant chemotherapy with overall survival among patients with locally advanced gastric cancer after neoadjuvant chemotherapy. JAMA Netw Open. 2022;5(4):e225557 (Apr 1). Doi: 10.1001/jamanetworkopen.2022.5557
Salt intake is associated with higher risk for gastric cancer
Key clinical point: Salty taste preference, always using table salt, and a greater high-salt and salt-preserved foods intake are associated with a higher risk for gastric cancer.
Major finding: The risk for gastric cancer was higher with salty vs. tasteless taste preference (adjusted odds ratio [aOR] 1.59; 95% CI 1.25-2.03), always vs. never using table salt (aOR 1.33; 95% CI 1.16-1.54), and the highest vs. lowest tertile of high-salt and salt-preserved foods intake (aOR 1.24; 95% CI 1.01-1.51). There was no significant association for the highest vs. lowest tertile of total sodium intake (aOR 1.08; 95% CI 0.82-1.43).
Study details: This study was an individual participant data meta-analysis of 25 studies including 10,283 patients with gastric cancer and 24,643 control individuals.
Disclosures: This study was supported by Portuguese Ministry of Science, Technology, and Higher Education; Instituto de Saúde Pública da Universidade do Porto; Associazione Italiana per la Ricerca sul Cancro; and others. The authors declared no conflicts of interest.
Source: Morais S et al. Salt intake and gastric cancer: A pooled analysis within the Stomach cancer Pooling (StoP) Project. Cancer Causes Control. 2022;33:779-791 (Mar 19). Doi: 10.1007/s10552-022-01565-y
Key clinical point: Salty taste preference, always using table salt, and a greater high-salt and salt-preserved foods intake are associated with a higher risk for gastric cancer.
Major finding: The risk for gastric cancer was higher with salty vs. tasteless taste preference (adjusted odds ratio [aOR] 1.59; 95% CI 1.25-2.03), always vs. never using table salt (aOR 1.33; 95% CI 1.16-1.54), and the highest vs. lowest tertile of high-salt and salt-preserved foods intake (aOR 1.24; 95% CI 1.01-1.51). There was no significant association for the highest vs. lowest tertile of total sodium intake (aOR 1.08; 95% CI 0.82-1.43).
Study details: This study was an individual participant data meta-analysis of 25 studies including 10,283 patients with gastric cancer and 24,643 control individuals.
Disclosures: This study was supported by Portuguese Ministry of Science, Technology, and Higher Education; Instituto de Saúde Pública da Universidade do Porto; Associazione Italiana per la Ricerca sul Cancro; and others. The authors declared no conflicts of interest.
Source: Morais S et al. Salt intake and gastric cancer: A pooled analysis within the Stomach cancer Pooling (StoP) Project. Cancer Causes Control. 2022;33:779-791 (Mar 19). Doi: 10.1007/s10552-022-01565-y
Key clinical point: Salty taste preference, always using table salt, and a greater high-salt and salt-preserved foods intake are associated with a higher risk for gastric cancer.
Major finding: The risk for gastric cancer was higher with salty vs. tasteless taste preference (adjusted odds ratio [aOR] 1.59; 95% CI 1.25-2.03), always vs. never using table salt (aOR 1.33; 95% CI 1.16-1.54), and the highest vs. lowest tertile of high-salt and salt-preserved foods intake (aOR 1.24; 95% CI 1.01-1.51). There was no significant association for the highest vs. lowest tertile of total sodium intake (aOR 1.08; 95% CI 0.82-1.43).
Study details: This study was an individual participant data meta-analysis of 25 studies including 10,283 patients with gastric cancer and 24,643 control individuals.
Disclosures: This study was supported by Portuguese Ministry of Science, Technology, and Higher Education; Instituto de Saúde Pública da Universidade do Porto; Associazione Italiana per la Ricerca sul Cancro; and others. The authors declared no conflicts of interest.
Source: Morais S et al. Salt intake and gastric cancer: A pooled analysis within the Stomach cancer Pooling (StoP) Project. Cancer Causes Control. 2022;33:779-791 (Mar 19). Doi: 10.1007/s10552-022-01565-y
Cimetropium bromide use during endoscopy improves gastric neoplasm detection rate
Key clinical point: The use vs. nonuse of cimetropium bromide as premedication is associated with higher gastric neoplasm detection rate during esophagogastroduodenoscopy (EGD) screening.
Major finding: In 41,670 matched participants, the gastric neoplasm detection rate was significantly higher in the cimetropium bromide vs. control group (0.30% vs. 0.19%; P = .02). Cimetropium bromide use vs. nonuse was associated with a significantly higher combined detection rate of dysplasia and early gastric cancer (0.27% vs. 0.17%; P = .02). Cimetropium bromide was associated with higher odds for the detection of gastric neoplasms (odds ratio 1.42; P = .03).
Study details: This article reports a propensity score-matched retrospective cohort study of 67,683 participants who received EGD screening with cimetropium bromide (41.8%) or without (control group; 58.2%).
Disclosures: This study was supported by the Ministry of Education, Science, and Technology, Korea; and Myongji Hospital. The authors declared no competing interests.
Source: Kim SY et al. Assessment of cimetropium bromide use for the detection of gastric neoplasms during esophagogastroduodenoscopy. JAMA Netw Open. 2022;5(3):e223827 (Mar 23). Doi: 10.1001/jamanetworkopen.2022.3827
Key clinical point: The use vs. nonuse of cimetropium bromide as premedication is associated with higher gastric neoplasm detection rate during esophagogastroduodenoscopy (EGD) screening.
Major finding: In 41,670 matched participants, the gastric neoplasm detection rate was significantly higher in the cimetropium bromide vs. control group (0.30% vs. 0.19%; P = .02). Cimetropium bromide use vs. nonuse was associated with a significantly higher combined detection rate of dysplasia and early gastric cancer (0.27% vs. 0.17%; P = .02). Cimetropium bromide was associated with higher odds for the detection of gastric neoplasms (odds ratio 1.42; P = .03).
Study details: This article reports a propensity score-matched retrospective cohort study of 67,683 participants who received EGD screening with cimetropium bromide (41.8%) or without (control group; 58.2%).
Disclosures: This study was supported by the Ministry of Education, Science, and Technology, Korea; and Myongji Hospital. The authors declared no competing interests.
Source: Kim SY et al. Assessment of cimetropium bromide use for the detection of gastric neoplasms during esophagogastroduodenoscopy. JAMA Netw Open. 2022;5(3):e223827 (Mar 23). Doi: 10.1001/jamanetworkopen.2022.3827
Key clinical point: The use vs. nonuse of cimetropium bromide as premedication is associated with higher gastric neoplasm detection rate during esophagogastroduodenoscopy (EGD) screening.
Major finding: In 41,670 matched participants, the gastric neoplasm detection rate was significantly higher in the cimetropium bromide vs. control group (0.30% vs. 0.19%; P = .02). Cimetropium bromide use vs. nonuse was associated with a significantly higher combined detection rate of dysplasia and early gastric cancer (0.27% vs. 0.17%; P = .02). Cimetropium bromide was associated with higher odds for the detection of gastric neoplasms (odds ratio 1.42; P = .03).
Study details: This article reports a propensity score-matched retrospective cohort study of 67,683 participants who received EGD screening with cimetropium bromide (41.8%) or without (control group; 58.2%).
Disclosures: This study was supported by the Ministry of Education, Science, and Technology, Korea; and Myongji Hospital. The authors declared no competing interests.
Source: Kim SY et al. Assessment of cimetropium bromide use for the detection of gastric neoplasms during esophagogastroduodenoscopy. JAMA Netw Open. 2022;5(3):e223827 (Mar 23). Doi: 10.1001/jamanetworkopen.2022.3827
Gastric cancer: Modified Glasgow prognostic score predicts sarcopenia
Key clinical point: Modified Glasgow prognostic score (mGPS) predicts sarcopenia and is a strong prognostic factor for overall survival in patients with advanced gastric cancer or esophagogastric junction cancer undergoing first-line treatment.
Major finding: Baseline mGPS was significantly correlated with baseline mean muscle attenuation (P < .0001). The mGPS was a strong prognostic factor for overall survival (P < .0001) and progression-free survival (P < .001).
Study details: Computed tomography was performed in 509 patients with advanced gastric or esophagogastric junction cancer from the phase 3 EXPAND trial who received first-line platinum-fluoropyrimidine chemotherapy.
Disclosures: No funding source was identified for this study. The authors received personal fees and research grants outside this work.
Source: Hacker UT et al. Modified Glasgow prognostic score (mGPS) is correlated with sarcopenia and dominates the prognostic role of baseline body composition parameters in advanced gastric and esophagogastric junction cancer patients undergoing first-line treatment from the phase III EXPAND trial. Ann Oncol. 2022 (Apr 4). Doi: 10.1016/j.annonc.2022.03.274
Key clinical point: Modified Glasgow prognostic score (mGPS) predicts sarcopenia and is a strong prognostic factor for overall survival in patients with advanced gastric cancer or esophagogastric junction cancer undergoing first-line treatment.
Major finding: Baseline mGPS was significantly correlated with baseline mean muscle attenuation (P < .0001). The mGPS was a strong prognostic factor for overall survival (P < .0001) and progression-free survival (P < .001).
Study details: Computed tomography was performed in 509 patients with advanced gastric or esophagogastric junction cancer from the phase 3 EXPAND trial who received first-line platinum-fluoropyrimidine chemotherapy.
Disclosures: No funding source was identified for this study. The authors received personal fees and research grants outside this work.
Source: Hacker UT et al. Modified Glasgow prognostic score (mGPS) is correlated with sarcopenia and dominates the prognostic role of baseline body composition parameters in advanced gastric and esophagogastric junction cancer patients undergoing first-line treatment from the phase III EXPAND trial. Ann Oncol. 2022 (Apr 4). Doi: 10.1016/j.annonc.2022.03.274
Key clinical point: Modified Glasgow prognostic score (mGPS) predicts sarcopenia and is a strong prognostic factor for overall survival in patients with advanced gastric cancer or esophagogastric junction cancer undergoing first-line treatment.
Major finding: Baseline mGPS was significantly correlated with baseline mean muscle attenuation (P < .0001). The mGPS was a strong prognostic factor for overall survival (P < .0001) and progression-free survival (P < .001).
Study details: Computed tomography was performed in 509 patients with advanced gastric or esophagogastric junction cancer from the phase 3 EXPAND trial who received first-line platinum-fluoropyrimidine chemotherapy.
Disclosures: No funding source was identified for this study. The authors received personal fees and research grants outside this work.
Source: Hacker UT et al. Modified Glasgow prognostic score (mGPS) is correlated with sarcopenia and dominates the prognostic role of baseline body composition parameters in advanced gastric and esophagogastric junction cancer patients undergoing first-line treatment from the phase III EXPAND trial. Ann Oncol. 2022 (Apr 4). Doi: 10.1016/j.annonc.2022.03.274
Early gastric cancer: Undifferentiated-predominant mixed-type is more aggressive
Key clinical point: Patients with undifferentiated-predominant mixed-type (MU) early-stage gastric cancer (EGC) had a higher risk for submucosal invasion and lymph node metastasis vs. pure undifferentiated-type (PU) EGC.
Major finding: Patients with MU vs. PU EGC had a significantly higher risk for submucosal invasion (odds ratio [OR] 2.19; 95% CI 1.90-2.52) and lymph node metastasis (OR 2.28; 95% CI 1.72-3.03). There was no difference in the risk for lymphovascular invasion in patients with MU vs. PU EGC (OR 1.81; 95% CI 0.84-3.87).
Study details: This article was a meta-analysis of 12 observational studies including 5644 patients with early gastric cancer.
Disclosures: This study was sponsored by the International Science and Technology Cooperation Fund of Changzhou, China, Young Medical Talents of Jiangsu Province, Changzhou Special Fund for Introducing Foreign Talents, and others. The authors declared no competing interests.
Source: Yang P et al. Undifferentiated-predominant mixed-type early gastric cancer is more aggressive than pure undifferentiated type: a systematic review and meta-analysis. BMJ Open. 2022;12:e054473 (Apr 7). Doi: 10.1136/bmjopen-2021-054473
Key clinical point: Patients with undifferentiated-predominant mixed-type (MU) early-stage gastric cancer (EGC) had a higher risk for submucosal invasion and lymph node metastasis vs. pure undifferentiated-type (PU) EGC.
Major finding: Patients with MU vs. PU EGC had a significantly higher risk for submucosal invasion (odds ratio [OR] 2.19; 95% CI 1.90-2.52) and lymph node metastasis (OR 2.28; 95% CI 1.72-3.03). There was no difference in the risk for lymphovascular invasion in patients with MU vs. PU EGC (OR 1.81; 95% CI 0.84-3.87).
Study details: This article was a meta-analysis of 12 observational studies including 5644 patients with early gastric cancer.
Disclosures: This study was sponsored by the International Science and Technology Cooperation Fund of Changzhou, China, Young Medical Talents of Jiangsu Province, Changzhou Special Fund for Introducing Foreign Talents, and others. The authors declared no competing interests.
Source: Yang P et al. Undifferentiated-predominant mixed-type early gastric cancer is more aggressive than pure undifferentiated type: a systematic review and meta-analysis. BMJ Open. 2022;12:e054473 (Apr 7). Doi: 10.1136/bmjopen-2021-054473
Key clinical point: Patients with undifferentiated-predominant mixed-type (MU) early-stage gastric cancer (EGC) had a higher risk for submucosal invasion and lymph node metastasis vs. pure undifferentiated-type (PU) EGC.
Major finding: Patients with MU vs. PU EGC had a significantly higher risk for submucosal invasion (odds ratio [OR] 2.19; 95% CI 1.90-2.52) and lymph node metastasis (OR 2.28; 95% CI 1.72-3.03). There was no difference in the risk for lymphovascular invasion in patients with MU vs. PU EGC (OR 1.81; 95% CI 0.84-3.87).
Study details: This article was a meta-analysis of 12 observational studies including 5644 patients with early gastric cancer.
Disclosures: This study was sponsored by the International Science and Technology Cooperation Fund of Changzhou, China, Young Medical Talents of Jiangsu Province, Changzhou Special Fund for Introducing Foreign Talents, and others. The authors declared no competing interests.
Source: Yang P et al. Undifferentiated-predominant mixed-type early gastric cancer is more aggressive than pure undifferentiated type: a systematic review and meta-analysis. BMJ Open. 2022;12:e054473 (Apr 7). Doi: 10.1136/bmjopen-2021-054473