User login
USPSTF final recommendation on aspirin for primary CV prevention
The U.S. Preventive Services Task Force has published a final recommendation statement on aspirin use to prevent cardiovascular disease.
For people aged 40-59 years, the USPSTF suggests that aspirin could be considered in those at increased risk of cardiovascular disease (10-year risk of 10% or greater) but that the decision should be individualized.
It notes that in the 40-59 age group, evidence indicates that the net benefit of aspirin use is small, and that persons who are not at increased risk for bleeding are more likely to benefit.
It adds that these recommendations apply only to people who do not have a history of cardiovascular disease and are not already taking daily aspirin.
The USPSTF statement was published online in the Journal of the American Medical Association. It is accompanied by an evidence review, a modeling study, a patient page, and an editorial.
A draft version of the recommendation statement, evidence review, and modeling report were previously available for public comment. The final recommendation statement is consistent with the draft version.
The task force concludes that there is adequate evidence that low-dose aspirin has a small benefit to reduce risk for cardiovascular events (nonfatal myocardial infarction and stroke) in adults 40 years or older who have no history of cardiovascular disease but are at increased cardiovascular risk.
Evidence shows that the absolute magnitude of benefit increases with increasing 10-year cardiovascular risk and that the magnitude of the lifetime benefits is greater when aspirin is initiated at a younger age.
But it adds that there is also adequate evidence that aspirin use in adults increases the risk for gastrointestinal bleeding, intracranial bleeding, and hemorrhagic stroke. The USPSTF determined that the magnitude of the harms is small overall but increases in older age groups, particularly in adults older than 60 years.
For patients who are eligible and choose to start taking aspirin, the benefits become smaller with advancing age, and data suggest that clinicians and patients should consider stopping aspirin use around age 75 years, the statement advises.
It also says that evidence is unclear whether aspirin use reduces the risk of colorectal cancer incidence or mortality.
USPSTF vice chair Michael Barry, MD, director of the Informed Medical Decisions Program in the Health Decision Sciences Center at Massachusetts General Hospital, Boston, told this news organization that these recommendations apply only to patients not taking aspirin already and who have no evidence of existing cardiovascular disease.
“In adults aged 60 or over we do not recommend starting aspirin for primary prevention. That is because in this age group the risk of bleeding outweighs the cardiovascular benefit,” he said.
“For adults aged 40-59 years with a greater than 10% predicted risk of cardiovascular disease, there appears to be a net benefit from taking aspirin, but this net benefit is relatively small and will vary with other factors such as magnitude of cardiovascular and bleeding risk. People should talk to their physician about these factors and whether to take aspirin or not,” he added.
Dr. Barry noted that these recommendations do not apply to people who are already taking aspirin for primary prevention. “These people need to talk to their physicians about whether they should continue. They need to review the reasons why they started aspirin in the first place, and they need to have their bleeding risk evaluated. Someone who has taken aspirin long term without any bleeding complications has a lower risk of future bleeding complications,” he said.
The task force recommends an aspirin dose of 81 mg daily for those people deciding to take aspirin for primary prevention.
“There is an abundance of evidence that less than 100 mg a day is enough. The lower the dose the lower the bleeding risk. So, the most convenient dose is the widely available 81-mg baby aspirin tablet,” Dr. Barry noted. “While enteric coated products are meant to reduce gastric irritation, the data do not show any difference in bleeding risk between various aspirin formulations,” he added.
Dr. Barry pointed out that aspirin is just one tool for reducing cardiovascular risk.
“People can reduce their risk significantly in many other ways including taking regular exercise, eating a healthy diet, controlling blood pressure and diabetes, and taking statins if they are at increased cardiovascular risk.”
He noted that recent trials have suggested that aspirin has only a marginal value over and above all these other factors. And the risk reduction with aspirin is smaller than with some other interventions.
“For example, aspirin is associated with a 12% reduction in MI whereas statins are associated with a 25%-30% reduction. Statins are a more powerful tool in reducing cardiovascular risk than aspirin, so perhaps people should consider taking statins first. The benefit of aspirin may be smaller in individuals already taking a statin, and clinicians need to think about the big picture,” Dr. Barry said.
He explained that physicians need to evaluate the cardiovascular and bleeding risk in each individual patient. “While there are widely available tools to estimate cardiovascular risk, there are no easy tools yet available to evaluate bleeding risk, so physicians need to consider clinical factors such as history of peptic ulcers.”
He suggests for the many people who have an average bleeding risk, then personal preference may come into play. “In the 40-59 age group, the benefits and harms of aspirin are pretty well-balanced. For the average person we think there may be a small net benefit, but this is small enough for personal preference to be considered as well.”
Pendulum swinging away from aspirin use
In an editorial accompanying publication of the task force statement in JAMA, Allan S. Brett, MD, clinical professor of internal medicine at the University of Colorado at Denver, Aurora, explains that the USPSTF recommendations on aspirin use for primary prevention of cardiovascular disease have changed numerous times over the past 30 years, with the last update in 2016 narrowing the eligible population.
In the new recommendation statement, “the pendulum has swung further away from aspirin prophylaxis for primary prevention: The guideline does not recommend routine preventive aspirin for anyone,” Dr. Brett notes.
He points out that an important development between the 2016 and current version was the publication in 2018 of three large placebo-controlled randomized clinical trials of primary prevention with aspirin – ARRIVE, ASPREE and ASCEND – which taken together “cast doubt about net benefit for aspirin prophylaxis in current practice.”
Asked how physicians should go about “individualizing” the decision on the use of aspirin in the 40-59 age group at increased cardiovascular risk, Dr. Brett suggests that some patents will have a general philosophy of medical care of “don’t prescribe medication for me unless there is strong evidence to support it,” while others may favor preventive interventions even in borderline cases.
But he notes that many patients have no strong general preferences and often ask a trusted clinician to decide for them. “For such patients, the best approach is for clinicians to be knowledgeable about the data on primary prevention with aspirin. Close reading of the new USPSTF guideline and its companion evidence review, and becoming familiar with the three more recent aspirin trials, is a good way to prepare for these clinical encounters,” he concludes.
A cardiologist’s view
Commenting on the task force statement for this news organization, Andrew Freeman, MD, a cardiologist at National Jewish Health, Denver, noted that cardiology societies are already making similar recommendations on aspirin use in primary prevention. “The American College of Cardiology prevention guidelines have been giving similar advice for a couple of years now. It takes a few years for professional societies to catch up with each other,” he said.
“Over the last few years, it has become obvious that the benefit of aspirin is not really very positive until a patient has had a cardiovascular event. In primary prevention, it doesn’t become beneficial unless they are at quite a high risk of having an event,” Dr. Freeman noted.
“In general, most cardiologists are now telling people that, despite what they may have been told in the past, they don’t need to be on aspirin unless they have had a cardiovascular event,” he added. “Our understanding has changed over the years and the weight of evidence has now become clear that the risk of bleeding is not insignificant.”
Dr. Freeman agreed with the shared decision-making advocated for patients in the 40-59 age group. “If a patient is particularly worried about a family history of heart disease, taking aspirin may make some sense, but for most people who have not had a cardiovascular event, the net benefit is very low and gets lower with age as the bleeding risk increases,” he said.
The USPSTF is an independent, voluntary body. The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force has published a final recommendation statement on aspirin use to prevent cardiovascular disease.
For people aged 40-59 years, the USPSTF suggests that aspirin could be considered in those at increased risk of cardiovascular disease (10-year risk of 10% or greater) but that the decision should be individualized.
It notes that in the 40-59 age group, evidence indicates that the net benefit of aspirin use is small, and that persons who are not at increased risk for bleeding are more likely to benefit.
It adds that these recommendations apply only to people who do not have a history of cardiovascular disease and are not already taking daily aspirin.
The USPSTF statement was published online in the Journal of the American Medical Association. It is accompanied by an evidence review, a modeling study, a patient page, and an editorial.
A draft version of the recommendation statement, evidence review, and modeling report were previously available for public comment. The final recommendation statement is consistent with the draft version.
The task force concludes that there is adequate evidence that low-dose aspirin has a small benefit to reduce risk for cardiovascular events (nonfatal myocardial infarction and stroke) in adults 40 years or older who have no history of cardiovascular disease but are at increased cardiovascular risk.
Evidence shows that the absolute magnitude of benefit increases with increasing 10-year cardiovascular risk and that the magnitude of the lifetime benefits is greater when aspirin is initiated at a younger age.
But it adds that there is also adequate evidence that aspirin use in adults increases the risk for gastrointestinal bleeding, intracranial bleeding, and hemorrhagic stroke. The USPSTF determined that the magnitude of the harms is small overall but increases in older age groups, particularly in adults older than 60 years.
For patients who are eligible and choose to start taking aspirin, the benefits become smaller with advancing age, and data suggest that clinicians and patients should consider stopping aspirin use around age 75 years, the statement advises.
It also says that evidence is unclear whether aspirin use reduces the risk of colorectal cancer incidence or mortality.
USPSTF vice chair Michael Barry, MD, director of the Informed Medical Decisions Program in the Health Decision Sciences Center at Massachusetts General Hospital, Boston, told this news organization that these recommendations apply only to patients not taking aspirin already and who have no evidence of existing cardiovascular disease.
“In adults aged 60 or over we do not recommend starting aspirin for primary prevention. That is because in this age group the risk of bleeding outweighs the cardiovascular benefit,” he said.
“For adults aged 40-59 years with a greater than 10% predicted risk of cardiovascular disease, there appears to be a net benefit from taking aspirin, but this net benefit is relatively small and will vary with other factors such as magnitude of cardiovascular and bleeding risk. People should talk to their physician about these factors and whether to take aspirin or not,” he added.
Dr. Barry noted that these recommendations do not apply to people who are already taking aspirin for primary prevention. “These people need to talk to their physicians about whether they should continue. They need to review the reasons why they started aspirin in the first place, and they need to have their bleeding risk evaluated. Someone who has taken aspirin long term without any bleeding complications has a lower risk of future bleeding complications,” he said.
The task force recommends an aspirin dose of 81 mg daily for those people deciding to take aspirin for primary prevention.
“There is an abundance of evidence that less than 100 mg a day is enough. The lower the dose the lower the bleeding risk. So, the most convenient dose is the widely available 81-mg baby aspirin tablet,” Dr. Barry noted. “While enteric coated products are meant to reduce gastric irritation, the data do not show any difference in bleeding risk between various aspirin formulations,” he added.
Dr. Barry pointed out that aspirin is just one tool for reducing cardiovascular risk.
“People can reduce their risk significantly in many other ways including taking regular exercise, eating a healthy diet, controlling blood pressure and diabetes, and taking statins if they are at increased cardiovascular risk.”
He noted that recent trials have suggested that aspirin has only a marginal value over and above all these other factors. And the risk reduction with aspirin is smaller than with some other interventions.
“For example, aspirin is associated with a 12% reduction in MI whereas statins are associated with a 25%-30% reduction. Statins are a more powerful tool in reducing cardiovascular risk than aspirin, so perhaps people should consider taking statins first. The benefit of aspirin may be smaller in individuals already taking a statin, and clinicians need to think about the big picture,” Dr. Barry said.
He explained that physicians need to evaluate the cardiovascular and bleeding risk in each individual patient. “While there are widely available tools to estimate cardiovascular risk, there are no easy tools yet available to evaluate bleeding risk, so physicians need to consider clinical factors such as history of peptic ulcers.”
He suggests for the many people who have an average bleeding risk, then personal preference may come into play. “In the 40-59 age group, the benefits and harms of aspirin are pretty well-balanced. For the average person we think there may be a small net benefit, but this is small enough for personal preference to be considered as well.”
Pendulum swinging away from aspirin use
In an editorial accompanying publication of the task force statement in JAMA, Allan S. Brett, MD, clinical professor of internal medicine at the University of Colorado at Denver, Aurora, explains that the USPSTF recommendations on aspirin use for primary prevention of cardiovascular disease have changed numerous times over the past 30 years, with the last update in 2016 narrowing the eligible population.
In the new recommendation statement, “the pendulum has swung further away from aspirin prophylaxis for primary prevention: The guideline does not recommend routine preventive aspirin for anyone,” Dr. Brett notes.
He points out that an important development between the 2016 and current version was the publication in 2018 of three large placebo-controlled randomized clinical trials of primary prevention with aspirin – ARRIVE, ASPREE and ASCEND – which taken together “cast doubt about net benefit for aspirin prophylaxis in current practice.”
Asked how physicians should go about “individualizing” the decision on the use of aspirin in the 40-59 age group at increased cardiovascular risk, Dr. Brett suggests that some patents will have a general philosophy of medical care of “don’t prescribe medication for me unless there is strong evidence to support it,” while others may favor preventive interventions even in borderline cases.
But he notes that many patients have no strong general preferences and often ask a trusted clinician to decide for them. “For such patients, the best approach is for clinicians to be knowledgeable about the data on primary prevention with aspirin. Close reading of the new USPSTF guideline and its companion evidence review, and becoming familiar with the three more recent aspirin trials, is a good way to prepare for these clinical encounters,” he concludes.
A cardiologist’s view
Commenting on the task force statement for this news organization, Andrew Freeman, MD, a cardiologist at National Jewish Health, Denver, noted that cardiology societies are already making similar recommendations on aspirin use in primary prevention. “The American College of Cardiology prevention guidelines have been giving similar advice for a couple of years now. It takes a few years for professional societies to catch up with each other,” he said.
“Over the last few years, it has become obvious that the benefit of aspirin is not really very positive until a patient has had a cardiovascular event. In primary prevention, it doesn’t become beneficial unless they are at quite a high risk of having an event,” Dr. Freeman noted.
“In general, most cardiologists are now telling people that, despite what they may have been told in the past, they don’t need to be on aspirin unless they have had a cardiovascular event,” he added. “Our understanding has changed over the years and the weight of evidence has now become clear that the risk of bleeding is not insignificant.”
Dr. Freeman agreed with the shared decision-making advocated for patients in the 40-59 age group. “If a patient is particularly worried about a family history of heart disease, taking aspirin may make some sense, but for most people who have not had a cardiovascular event, the net benefit is very low and gets lower with age as the bleeding risk increases,” he said.
The USPSTF is an independent, voluntary body. The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force has published a final recommendation statement on aspirin use to prevent cardiovascular disease.
For people aged 40-59 years, the USPSTF suggests that aspirin could be considered in those at increased risk of cardiovascular disease (10-year risk of 10% or greater) but that the decision should be individualized.
It notes that in the 40-59 age group, evidence indicates that the net benefit of aspirin use is small, and that persons who are not at increased risk for bleeding are more likely to benefit.
It adds that these recommendations apply only to people who do not have a history of cardiovascular disease and are not already taking daily aspirin.
The USPSTF statement was published online in the Journal of the American Medical Association. It is accompanied by an evidence review, a modeling study, a patient page, and an editorial.
A draft version of the recommendation statement, evidence review, and modeling report were previously available for public comment. The final recommendation statement is consistent with the draft version.
The task force concludes that there is adequate evidence that low-dose aspirin has a small benefit to reduce risk for cardiovascular events (nonfatal myocardial infarction and stroke) in adults 40 years or older who have no history of cardiovascular disease but are at increased cardiovascular risk.
Evidence shows that the absolute magnitude of benefit increases with increasing 10-year cardiovascular risk and that the magnitude of the lifetime benefits is greater when aspirin is initiated at a younger age.
But it adds that there is also adequate evidence that aspirin use in adults increases the risk for gastrointestinal bleeding, intracranial bleeding, and hemorrhagic stroke. The USPSTF determined that the magnitude of the harms is small overall but increases in older age groups, particularly in adults older than 60 years.
For patients who are eligible and choose to start taking aspirin, the benefits become smaller with advancing age, and data suggest that clinicians and patients should consider stopping aspirin use around age 75 years, the statement advises.
It also says that evidence is unclear whether aspirin use reduces the risk of colorectal cancer incidence or mortality.
USPSTF vice chair Michael Barry, MD, director of the Informed Medical Decisions Program in the Health Decision Sciences Center at Massachusetts General Hospital, Boston, told this news organization that these recommendations apply only to patients not taking aspirin already and who have no evidence of existing cardiovascular disease.
“In adults aged 60 or over we do not recommend starting aspirin for primary prevention. That is because in this age group the risk of bleeding outweighs the cardiovascular benefit,” he said.
“For adults aged 40-59 years with a greater than 10% predicted risk of cardiovascular disease, there appears to be a net benefit from taking aspirin, but this net benefit is relatively small and will vary with other factors such as magnitude of cardiovascular and bleeding risk. People should talk to their physician about these factors and whether to take aspirin or not,” he added.
Dr. Barry noted that these recommendations do not apply to people who are already taking aspirin for primary prevention. “These people need to talk to their physicians about whether they should continue. They need to review the reasons why they started aspirin in the first place, and they need to have their bleeding risk evaluated. Someone who has taken aspirin long term without any bleeding complications has a lower risk of future bleeding complications,” he said.
The task force recommends an aspirin dose of 81 mg daily for those people deciding to take aspirin for primary prevention.
“There is an abundance of evidence that less than 100 mg a day is enough. The lower the dose the lower the bleeding risk. So, the most convenient dose is the widely available 81-mg baby aspirin tablet,” Dr. Barry noted. “While enteric coated products are meant to reduce gastric irritation, the data do not show any difference in bleeding risk between various aspirin formulations,” he added.
Dr. Barry pointed out that aspirin is just one tool for reducing cardiovascular risk.
“People can reduce their risk significantly in many other ways including taking regular exercise, eating a healthy diet, controlling blood pressure and diabetes, and taking statins if they are at increased cardiovascular risk.”
He noted that recent trials have suggested that aspirin has only a marginal value over and above all these other factors. And the risk reduction with aspirin is smaller than with some other interventions.
“For example, aspirin is associated with a 12% reduction in MI whereas statins are associated with a 25%-30% reduction. Statins are a more powerful tool in reducing cardiovascular risk than aspirin, so perhaps people should consider taking statins first. The benefit of aspirin may be smaller in individuals already taking a statin, and clinicians need to think about the big picture,” Dr. Barry said.
He explained that physicians need to evaluate the cardiovascular and bleeding risk in each individual patient. “While there are widely available tools to estimate cardiovascular risk, there are no easy tools yet available to evaluate bleeding risk, so physicians need to consider clinical factors such as history of peptic ulcers.”
He suggests for the many people who have an average bleeding risk, then personal preference may come into play. “In the 40-59 age group, the benefits and harms of aspirin are pretty well-balanced. For the average person we think there may be a small net benefit, but this is small enough for personal preference to be considered as well.”
Pendulum swinging away from aspirin use
In an editorial accompanying publication of the task force statement in JAMA, Allan S. Brett, MD, clinical professor of internal medicine at the University of Colorado at Denver, Aurora, explains that the USPSTF recommendations on aspirin use for primary prevention of cardiovascular disease have changed numerous times over the past 30 years, with the last update in 2016 narrowing the eligible population.
In the new recommendation statement, “the pendulum has swung further away from aspirin prophylaxis for primary prevention: The guideline does not recommend routine preventive aspirin for anyone,” Dr. Brett notes.
He points out that an important development between the 2016 and current version was the publication in 2018 of three large placebo-controlled randomized clinical trials of primary prevention with aspirin – ARRIVE, ASPREE and ASCEND – which taken together “cast doubt about net benefit for aspirin prophylaxis in current practice.”
Asked how physicians should go about “individualizing” the decision on the use of aspirin in the 40-59 age group at increased cardiovascular risk, Dr. Brett suggests that some patents will have a general philosophy of medical care of “don’t prescribe medication for me unless there is strong evidence to support it,” while others may favor preventive interventions even in borderline cases.
But he notes that many patients have no strong general preferences and often ask a trusted clinician to decide for them. “For such patients, the best approach is for clinicians to be knowledgeable about the data on primary prevention with aspirin. Close reading of the new USPSTF guideline and its companion evidence review, and becoming familiar with the three more recent aspirin trials, is a good way to prepare for these clinical encounters,” he concludes.
A cardiologist’s view
Commenting on the task force statement for this news organization, Andrew Freeman, MD, a cardiologist at National Jewish Health, Denver, noted that cardiology societies are already making similar recommendations on aspirin use in primary prevention. “The American College of Cardiology prevention guidelines have been giving similar advice for a couple of years now. It takes a few years for professional societies to catch up with each other,” he said.
“Over the last few years, it has become obvious that the benefit of aspirin is not really very positive until a patient has had a cardiovascular event. In primary prevention, it doesn’t become beneficial unless they are at quite a high risk of having an event,” Dr. Freeman noted.
“In general, most cardiologists are now telling people that, despite what they may have been told in the past, they don’t need to be on aspirin unless they have had a cardiovascular event,” he added. “Our understanding has changed over the years and the weight of evidence has now become clear that the risk of bleeding is not insignificant.”
Dr. Freeman agreed with the shared decision-making advocated for patients in the 40-59 age group. “If a patient is particularly worried about a family history of heart disease, taking aspirin may make some sense, but for most people who have not had a cardiovascular event, the net benefit is very low and gets lower with age as the bleeding risk increases,” he said.
The USPSTF is an independent, voluntary body. The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
A version of this article first appeared on Medscape.com.
FROM JAMA
Shortage of ICU beds did not drive COVID-19 deaths
Contrary to popular belief, no association appeared between the number of intensive care unit beds and COVID-19 deaths, based on a review of data from all 50 states between March 1, 2020, and June 30, 2021.
One of the reasons for poor patient outcomes in the early months of the COVID-19 pandemic was the presumed scarcity of ICU beds, Omar Haider, MD, of Houston Methodist Hospital, and colleagues said. “We hypothesized that the states having a lower number of ICU beds had more COVID-related deaths when compared to the states that had a higher number of ICU beds,” they wrote in an abstract presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
According to the researchers, the total number of ICU beds in the United States is approximately 85,000. Hawaii has the highest number of beds per 10,000 persons, and the District of Columbia has the lowest (6.0 vs. 1.6).
The researchers collected data on ICU bed totals from the Kaiser Family Foundation. Statistics on COVID-19 deaths were obtained from The New York Times database, which provided real-time information collected from the Department of Health & Human Services, the Centers for Disease Control and Prevention, and the Census Bureau.
The researchers used the Pearson Correlation Coefficient to compare ICU beds and COVID deaths per 10,000 persons in each state. The R value was 0.29, which indicates no inverse correlation. “Our value of R2, the coefficient of determination, was 0.0858,” they added. They confirmed the results using the Spearman’s Rho, which yielded an rs of 0.3, also a sign of no inverse correlation. No correlation was found between low numbers of ICU beds and high numbers of COVID-19 deaths for any states.
The study findings were limited by several factors, including the lack of standardized reporting timelines across states, differences in state-based vaccination rates, the emergence of the Delta variant during the study period, and time-lag in contemporaneous database updates, the researchers noted.
However, the results suggest that physical ICU beds do not play a role in determining the number of COVID-related deaths. Instead, “other constraints such as less staffing, lack of medical supplies (ventilators and [personal protective equipment]) should be evaluated for potential implications on poor patients’ outcomes,” they concluded.
Pandemic challenges can inform future plans
“As the health care system emerges from the effects of the pandemic, it is important to understand the factors that contributed to adverse outcomes to better prepare for future challenges and improve the delivery of care,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The findings are not surprising considering what is known about the multitude of factors that determine outcomes for our patients from medical comorbidities, and social determinants of health to upstream structural factors such as systemic inequities and generational trauma,” said Dr. Pal, who was not involved with the study. “Thus, a simple correlation of the number of ICU beds to COVID-19 outcomes is not likely to capture the interplay of all these factors.”
The challenges of the pandemic offer insights to inform future planning, said Dr. Pal.
“In my opinion, a key factor to understand and address would be employee wellness for health care workers,” he said. “The problem of burnout leading to health care workers leaving the workforce has exacerbated the already acute shortages in personnel in recent years.
“In the long term, it may be prudent to reconsider the approach to health by increasing support for preventative and primary care, addressing social factors such as education, nutrition, and housing, to mitigate preventable aspects of diseases.”
Further research is needed to examine the multitude of factors associated with the pandemic, and their interplay, said Dr. Pal. The goals of such research “would be needed to develop a deeper understanding of the factors that contributed to mortality in COVID-19 and the disparities with this across different subpopulations.”
The study received no outside funding. The researchers and Dr. Pal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to popular belief, no association appeared between the number of intensive care unit beds and COVID-19 deaths, based on a review of data from all 50 states between March 1, 2020, and June 30, 2021.
One of the reasons for poor patient outcomes in the early months of the COVID-19 pandemic was the presumed scarcity of ICU beds, Omar Haider, MD, of Houston Methodist Hospital, and colleagues said. “We hypothesized that the states having a lower number of ICU beds had more COVID-related deaths when compared to the states that had a higher number of ICU beds,” they wrote in an abstract presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
According to the researchers, the total number of ICU beds in the United States is approximately 85,000. Hawaii has the highest number of beds per 10,000 persons, and the District of Columbia has the lowest (6.0 vs. 1.6).
The researchers collected data on ICU bed totals from the Kaiser Family Foundation. Statistics on COVID-19 deaths were obtained from The New York Times database, which provided real-time information collected from the Department of Health & Human Services, the Centers for Disease Control and Prevention, and the Census Bureau.
The researchers used the Pearson Correlation Coefficient to compare ICU beds and COVID deaths per 10,000 persons in each state. The R value was 0.29, which indicates no inverse correlation. “Our value of R2, the coefficient of determination, was 0.0858,” they added. They confirmed the results using the Spearman’s Rho, which yielded an rs of 0.3, also a sign of no inverse correlation. No correlation was found between low numbers of ICU beds and high numbers of COVID-19 deaths for any states.
The study findings were limited by several factors, including the lack of standardized reporting timelines across states, differences in state-based vaccination rates, the emergence of the Delta variant during the study period, and time-lag in contemporaneous database updates, the researchers noted.
However, the results suggest that physical ICU beds do not play a role in determining the number of COVID-related deaths. Instead, “other constraints such as less staffing, lack of medical supplies (ventilators and [personal protective equipment]) should be evaluated for potential implications on poor patients’ outcomes,” they concluded.
Pandemic challenges can inform future plans
“As the health care system emerges from the effects of the pandemic, it is important to understand the factors that contributed to adverse outcomes to better prepare for future challenges and improve the delivery of care,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The findings are not surprising considering what is known about the multitude of factors that determine outcomes for our patients from medical comorbidities, and social determinants of health to upstream structural factors such as systemic inequities and generational trauma,” said Dr. Pal, who was not involved with the study. “Thus, a simple correlation of the number of ICU beds to COVID-19 outcomes is not likely to capture the interplay of all these factors.”
The challenges of the pandemic offer insights to inform future planning, said Dr. Pal.
“In my opinion, a key factor to understand and address would be employee wellness for health care workers,” he said. “The problem of burnout leading to health care workers leaving the workforce has exacerbated the already acute shortages in personnel in recent years.
“In the long term, it may be prudent to reconsider the approach to health by increasing support for preventative and primary care, addressing social factors such as education, nutrition, and housing, to mitigate preventable aspects of diseases.”
Further research is needed to examine the multitude of factors associated with the pandemic, and their interplay, said Dr. Pal. The goals of such research “would be needed to develop a deeper understanding of the factors that contributed to mortality in COVID-19 and the disparities with this across different subpopulations.”
The study received no outside funding. The researchers and Dr. Pal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to popular belief, no association appeared between the number of intensive care unit beds and COVID-19 deaths, based on a review of data from all 50 states between March 1, 2020, and June 30, 2021.
One of the reasons for poor patient outcomes in the early months of the COVID-19 pandemic was the presumed scarcity of ICU beds, Omar Haider, MD, of Houston Methodist Hospital, and colleagues said. “We hypothesized that the states having a lower number of ICU beds had more COVID-related deaths when compared to the states that had a higher number of ICU beds,” they wrote in an abstract presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
According to the researchers, the total number of ICU beds in the United States is approximately 85,000. Hawaii has the highest number of beds per 10,000 persons, and the District of Columbia has the lowest (6.0 vs. 1.6).
The researchers collected data on ICU bed totals from the Kaiser Family Foundation. Statistics on COVID-19 deaths were obtained from The New York Times database, which provided real-time information collected from the Department of Health & Human Services, the Centers for Disease Control and Prevention, and the Census Bureau.
The researchers used the Pearson Correlation Coefficient to compare ICU beds and COVID deaths per 10,000 persons in each state. The R value was 0.29, which indicates no inverse correlation. “Our value of R2, the coefficient of determination, was 0.0858,” they added. They confirmed the results using the Spearman’s Rho, which yielded an rs of 0.3, also a sign of no inverse correlation. No correlation was found between low numbers of ICU beds and high numbers of COVID-19 deaths for any states.
The study findings were limited by several factors, including the lack of standardized reporting timelines across states, differences in state-based vaccination rates, the emergence of the Delta variant during the study period, and time-lag in contemporaneous database updates, the researchers noted.
However, the results suggest that physical ICU beds do not play a role in determining the number of COVID-related deaths. Instead, “other constraints such as less staffing, lack of medical supplies (ventilators and [personal protective equipment]) should be evaluated for potential implications on poor patients’ outcomes,” they concluded.
Pandemic challenges can inform future plans
“As the health care system emerges from the effects of the pandemic, it is important to understand the factors that contributed to adverse outcomes to better prepare for future challenges and improve the delivery of care,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The findings are not surprising considering what is known about the multitude of factors that determine outcomes for our patients from medical comorbidities, and social determinants of health to upstream structural factors such as systemic inequities and generational trauma,” said Dr. Pal, who was not involved with the study. “Thus, a simple correlation of the number of ICU beds to COVID-19 outcomes is not likely to capture the interplay of all these factors.”
The challenges of the pandemic offer insights to inform future planning, said Dr. Pal.
“In my opinion, a key factor to understand and address would be employee wellness for health care workers,” he said. “The problem of burnout leading to health care workers leaving the workforce has exacerbated the already acute shortages in personnel in recent years.
“In the long term, it may be prudent to reconsider the approach to health by increasing support for preventative and primary care, addressing social factors such as education, nutrition, and housing, to mitigate preventable aspects of diseases.”
Further research is needed to examine the multitude of factors associated with the pandemic, and their interplay, said Dr. Pal. The goals of such research “would be needed to develop a deeper understanding of the factors that contributed to mortality in COVID-19 and the disparities with this across different subpopulations.”
The study received no outside funding. The researchers and Dr. Pal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Virtual reality therapy promising for agoraphobia
The cognitive-behavioral therapy–based treatment was particularly effective for patients with the highest level of avoidance of everyday situations.
“Virtual reality is an inherently therapeutic medium which could be extremely useful in mental health services,” study investigator Daniel Freeman, PhD, DClinPsy, professor of clinical psychology, University of Oxford (England), told this news organization. “This intervention is coming; the question really is when.”
The study was published online in The Lancet Psychiatry.
Real-world feel
Immersive VR involves interactive three-dimensional computer-generated environments that produce the sensation of being in the real world.
For patients with psychosis, dealing with the real world can be an anxious experience, particularly if they have verbal or auditory hallucinations.
Some may develop agoraphobia and start to avoid places or situations. A virtual environment allows patients to practice dealing with situations that make them anxious or uncomfortable and to learn to reengage in everyday situations.
The study included 346 patients diagnosed with schizophrenia or a related disorder. The mean age of the patients was 37.2 years (67% were men, 85% were White). Most were single and unemployed. All were receiving treatment for psychosis and had difficulty going out because of anxiety.
The researchers randomly assigned 174 participants to an automated VR cognitive therapy intervention (gameChange) plus usual care and 172 to usual care alone. Trial assessors were blinded to group allocation.
The gameChange intervention was delivered in six sessions that were conducted over a 6-week period. Each session involved 30 minutes of VR.
A session begins when participants enter the virtual therapist’s office. They are met by a coach who guides them through the therapy. They can choose from among six VR social situations. These include a cafe, a general practice waiting room, a pub, a bus, opening the front door of their home onto the street, or entering a small local shop.
Each scenario has five levels of difficulty that are based on the number and proximity of people in the social situation and the degree of social interaction. Users can work their way through these various levels.
The virtual sessions took place in patients’ homes in about 50% of cases; the remainder were conducted in the clinic. A mental health worker was in the room during the therapy.
Between virtual sessions, participants were encouraged to apply what they learned in the real world, for example, by spending time in a pub.
Usual care typically included regular visits from a community mental health worker and occasional outpatient appointments with a psychiatrist.
Widely applicable?
The primary outcome was the eight-item Oxford Agoraphobic Avoidance Scale (O-AS) questionnaire. This scale assesses distress and avoidance related to performing increasingly difficult everyday tasks.
The researchers assessed patients at baseline, at the conclusion of the 6-week treatment, and at 26 weeks.
Compared with the group that received usual care alone, the VR therapy group demonstrated a significant reduction in both agoraphobic avoidance (O-AS adjusted mean difference, -0.47; 95% confidence interval [CI], –0.88 to –0.06; Cohen’s d, –0.18; P = .026) and distress (–4.33; 95% CI, –7.78 to –0.87; Cohen’s d, –0.26; P = .014) at 6 weeks.
This translates to being able to do about 1.5 more activities on the O-AS, such as going to a shopping center alone, said Dr. Freeman.
Further analyses showed that VR therapy was especially effective for patients with severe agoraphobia. On average, these patients could complete two more O-AS activities at 26 weeks, said Dr. Freeman.
The authors believe the intervention worked by reducing defense behaviors, such as avoiding eye contact and fearful thoughts.
There was no significant difference in occurrence of adverse events between the study groups. These events, which were mild, transient, and did not affect the outcome, included side effects such as claustrophobia when using headsets.
The intervention would likely work for patients with agoraphobia who do not have psychosis, said Dr. Freeman. “Agoraphobia is often the final common pathway in lots of mental health conditions.”
Automated VR not only addresses the problem of patients being too afraid to leave home for in-person treatment but may also help address the shortage of trained mental health care providers.
The intervention is available at pilot implementation sites in the United Kingdom and a few sites in the United States, he said.
‘Cool, interesting’
Commenting on the research, Arash Javanbakht, MD, associate professor (clinical scholar), Wayne State University, Detroit, described the study as “cool and interesting.”
However, he said, the findings were not surprising, because exposure therapy has proved effective in treating phobias. Because of the significant lack of access to exposure therapy providers, “the more mechanized, the more automated therapies that can be easily used, the better,” he said.
He noted the VR therapy did not require a high level of training; the study used peer support staff who sat next to those using the technology.
He also liked the fact that the intervention “focused on things that in reality impair a person’s life,” for example, not being able to go to the grocery store.
However, he wondered why the investigators studied VR for patients with psychosis and agoraphobia and not for those with just agoraphobia.
In addition, he noted that the treatment’s efficacy was partly due to having someone next to the participants offering support, which the control group didn’t have.
Dr. Javanbakht has researched augmented therapy (AR) for delivering exposure therapy. This technology, which mixes virtually created objects with reality and allows users to move around their real environment, is newer and more advanced than VR but is more complicated, he said.
He explained that AR is more appropriate for delivering exposure therapy in certain situations.
“The basis of exposure therapy is ‘extinction learning’ – exposing a person to a fear cue over and over again until the fear response is extinguished,” and extinction learning is “context dependent,” said Dr. Javanbakht.
“VR is good when you need to create the whole context and environment, and AR is good when you need to focus on specific objects or cues in the environment,” for example, spiders or snakes, he said.
The study was funded by the National Institute of Health Research. Dr. Freeman is a founder and a non-executive director of Oxford VR, which will commercialize the therapy. He holds equity in and receives personal payments from Oxford VR; holds a contract for his university team to advise Oxford VR on treatment development; and reports grants from the National Institute for Health Research, the Medical Research Council, and the International Foundation. Dr. Javanbakht has a patent for an AR exposure therapy.
A version of this article first appeared on Medscape.com.
The cognitive-behavioral therapy–based treatment was particularly effective for patients with the highest level of avoidance of everyday situations.
“Virtual reality is an inherently therapeutic medium which could be extremely useful in mental health services,” study investigator Daniel Freeman, PhD, DClinPsy, professor of clinical psychology, University of Oxford (England), told this news organization. “This intervention is coming; the question really is when.”
The study was published online in The Lancet Psychiatry.
Real-world feel
Immersive VR involves interactive three-dimensional computer-generated environments that produce the sensation of being in the real world.
For patients with psychosis, dealing with the real world can be an anxious experience, particularly if they have verbal or auditory hallucinations.
Some may develop agoraphobia and start to avoid places or situations. A virtual environment allows patients to practice dealing with situations that make them anxious or uncomfortable and to learn to reengage in everyday situations.
The study included 346 patients diagnosed with schizophrenia or a related disorder. The mean age of the patients was 37.2 years (67% were men, 85% were White). Most were single and unemployed. All were receiving treatment for psychosis and had difficulty going out because of anxiety.
The researchers randomly assigned 174 participants to an automated VR cognitive therapy intervention (gameChange) plus usual care and 172 to usual care alone. Trial assessors were blinded to group allocation.
The gameChange intervention was delivered in six sessions that were conducted over a 6-week period. Each session involved 30 minutes of VR.
A session begins when participants enter the virtual therapist’s office. They are met by a coach who guides them through the therapy. They can choose from among six VR social situations. These include a cafe, a general practice waiting room, a pub, a bus, opening the front door of their home onto the street, or entering a small local shop.
Each scenario has five levels of difficulty that are based on the number and proximity of people in the social situation and the degree of social interaction. Users can work their way through these various levels.
The virtual sessions took place in patients’ homes in about 50% of cases; the remainder were conducted in the clinic. A mental health worker was in the room during the therapy.
Between virtual sessions, participants were encouraged to apply what they learned in the real world, for example, by spending time in a pub.
Usual care typically included regular visits from a community mental health worker and occasional outpatient appointments with a psychiatrist.
Widely applicable?
The primary outcome was the eight-item Oxford Agoraphobic Avoidance Scale (O-AS) questionnaire. This scale assesses distress and avoidance related to performing increasingly difficult everyday tasks.
The researchers assessed patients at baseline, at the conclusion of the 6-week treatment, and at 26 weeks.
Compared with the group that received usual care alone, the VR therapy group demonstrated a significant reduction in both agoraphobic avoidance (O-AS adjusted mean difference, -0.47; 95% confidence interval [CI], –0.88 to –0.06; Cohen’s d, –0.18; P = .026) and distress (–4.33; 95% CI, –7.78 to –0.87; Cohen’s d, –0.26; P = .014) at 6 weeks.
This translates to being able to do about 1.5 more activities on the O-AS, such as going to a shopping center alone, said Dr. Freeman.
Further analyses showed that VR therapy was especially effective for patients with severe agoraphobia. On average, these patients could complete two more O-AS activities at 26 weeks, said Dr. Freeman.
The authors believe the intervention worked by reducing defense behaviors, such as avoiding eye contact and fearful thoughts.
There was no significant difference in occurrence of adverse events between the study groups. These events, which were mild, transient, and did not affect the outcome, included side effects such as claustrophobia when using headsets.
The intervention would likely work for patients with agoraphobia who do not have psychosis, said Dr. Freeman. “Agoraphobia is often the final common pathway in lots of mental health conditions.”
Automated VR not only addresses the problem of patients being too afraid to leave home for in-person treatment but may also help address the shortage of trained mental health care providers.
The intervention is available at pilot implementation sites in the United Kingdom and a few sites in the United States, he said.
‘Cool, interesting’
Commenting on the research, Arash Javanbakht, MD, associate professor (clinical scholar), Wayne State University, Detroit, described the study as “cool and interesting.”
However, he said, the findings were not surprising, because exposure therapy has proved effective in treating phobias. Because of the significant lack of access to exposure therapy providers, “the more mechanized, the more automated therapies that can be easily used, the better,” he said.
He noted the VR therapy did not require a high level of training; the study used peer support staff who sat next to those using the technology.
He also liked the fact that the intervention “focused on things that in reality impair a person’s life,” for example, not being able to go to the grocery store.
However, he wondered why the investigators studied VR for patients with psychosis and agoraphobia and not for those with just agoraphobia.
In addition, he noted that the treatment’s efficacy was partly due to having someone next to the participants offering support, which the control group didn’t have.
Dr. Javanbakht has researched augmented therapy (AR) for delivering exposure therapy. This technology, which mixes virtually created objects with reality and allows users to move around their real environment, is newer and more advanced than VR but is more complicated, he said.
He explained that AR is more appropriate for delivering exposure therapy in certain situations.
“The basis of exposure therapy is ‘extinction learning’ – exposing a person to a fear cue over and over again until the fear response is extinguished,” and extinction learning is “context dependent,” said Dr. Javanbakht.
“VR is good when you need to create the whole context and environment, and AR is good when you need to focus on specific objects or cues in the environment,” for example, spiders or snakes, he said.
The study was funded by the National Institute of Health Research. Dr. Freeman is a founder and a non-executive director of Oxford VR, which will commercialize the therapy. He holds equity in and receives personal payments from Oxford VR; holds a contract for his university team to advise Oxford VR on treatment development; and reports grants from the National Institute for Health Research, the Medical Research Council, and the International Foundation. Dr. Javanbakht has a patent for an AR exposure therapy.
A version of this article first appeared on Medscape.com.
The cognitive-behavioral therapy–based treatment was particularly effective for patients with the highest level of avoidance of everyday situations.
“Virtual reality is an inherently therapeutic medium which could be extremely useful in mental health services,” study investigator Daniel Freeman, PhD, DClinPsy, professor of clinical psychology, University of Oxford (England), told this news organization. “This intervention is coming; the question really is when.”
The study was published online in The Lancet Psychiatry.
Real-world feel
Immersive VR involves interactive three-dimensional computer-generated environments that produce the sensation of being in the real world.
For patients with psychosis, dealing with the real world can be an anxious experience, particularly if they have verbal or auditory hallucinations.
Some may develop agoraphobia and start to avoid places or situations. A virtual environment allows patients to practice dealing with situations that make them anxious or uncomfortable and to learn to reengage in everyday situations.
The study included 346 patients diagnosed with schizophrenia or a related disorder. The mean age of the patients was 37.2 years (67% were men, 85% were White). Most were single and unemployed. All were receiving treatment for psychosis and had difficulty going out because of anxiety.
The researchers randomly assigned 174 participants to an automated VR cognitive therapy intervention (gameChange) plus usual care and 172 to usual care alone. Trial assessors were blinded to group allocation.
The gameChange intervention was delivered in six sessions that were conducted over a 6-week period. Each session involved 30 minutes of VR.
A session begins when participants enter the virtual therapist’s office. They are met by a coach who guides them through the therapy. They can choose from among six VR social situations. These include a cafe, a general practice waiting room, a pub, a bus, opening the front door of their home onto the street, or entering a small local shop.
Each scenario has five levels of difficulty that are based on the number and proximity of people in the social situation and the degree of social interaction. Users can work their way through these various levels.
The virtual sessions took place in patients’ homes in about 50% of cases; the remainder were conducted in the clinic. A mental health worker was in the room during the therapy.
Between virtual sessions, participants were encouraged to apply what they learned in the real world, for example, by spending time in a pub.
Usual care typically included regular visits from a community mental health worker and occasional outpatient appointments with a psychiatrist.
Widely applicable?
The primary outcome was the eight-item Oxford Agoraphobic Avoidance Scale (O-AS) questionnaire. This scale assesses distress and avoidance related to performing increasingly difficult everyday tasks.
The researchers assessed patients at baseline, at the conclusion of the 6-week treatment, and at 26 weeks.
Compared with the group that received usual care alone, the VR therapy group demonstrated a significant reduction in both agoraphobic avoidance (O-AS adjusted mean difference, -0.47; 95% confidence interval [CI], –0.88 to –0.06; Cohen’s d, –0.18; P = .026) and distress (–4.33; 95% CI, –7.78 to –0.87; Cohen’s d, –0.26; P = .014) at 6 weeks.
This translates to being able to do about 1.5 more activities on the O-AS, such as going to a shopping center alone, said Dr. Freeman.
Further analyses showed that VR therapy was especially effective for patients with severe agoraphobia. On average, these patients could complete two more O-AS activities at 26 weeks, said Dr. Freeman.
The authors believe the intervention worked by reducing defense behaviors, such as avoiding eye contact and fearful thoughts.
There was no significant difference in occurrence of adverse events between the study groups. These events, which were mild, transient, and did not affect the outcome, included side effects such as claustrophobia when using headsets.
The intervention would likely work for patients with agoraphobia who do not have psychosis, said Dr. Freeman. “Agoraphobia is often the final common pathway in lots of mental health conditions.”
Automated VR not only addresses the problem of patients being too afraid to leave home for in-person treatment but may also help address the shortage of trained mental health care providers.
The intervention is available at pilot implementation sites in the United Kingdom and a few sites in the United States, he said.
‘Cool, interesting’
Commenting on the research, Arash Javanbakht, MD, associate professor (clinical scholar), Wayne State University, Detroit, described the study as “cool and interesting.”
However, he said, the findings were not surprising, because exposure therapy has proved effective in treating phobias. Because of the significant lack of access to exposure therapy providers, “the more mechanized, the more automated therapies that can be easily used, the better,” he said.
He noted the VR therapy did not require a high level of training; the study used peer support staff who sat next to those using the technology.
He also liked the fact that the intervention “focused on things that in reality impair a person’s life,” for example, not being able to go to the grocery store.
However, he wondered why the investigators studied VR for patients with psychosis and agoraphobia and not for those with just agoraphobia.
In addition, he noted that the treatment’s efficacy was partly due to having someone next to the participants offering support, which the control group didn’t have.
Dr. Javanbakht has researched augmented therapy (AR) for delivering exposure therapy. This technology, which mixes virtually created objects with reality and allows users to move around their real environment, is newer and more advanced than VR but is more complicated, he said.
He explained that AR is more appropriate for delivering exposure therapy in certain situations.
“The basis of exposure therapy is ‘extinction learning’ – exposing a person to a fear cue over and over again until the fear response is extinguished,” and extinction learning is “context dependent,” said Dr. Javanbakht.
“VR is good when you need to create the whole context and environment, and AR is good when you need to focus on specific objects or cues in the environment,” for example, spiders or snakes, he said.
The study was funded by the National Institute of Health Research. Dr. Freeman is a founder and a non-executive director of Oxford VR, which will commercialize the therapy. He holds equity in and receives personal payments from Oxford VR; holds a contract for his university team to advise Oxford VR on treatment development; and reports grants from the National Institute for Health Research, the Medical Research Council, and the International Foundation. Dr. Javanbakht has a patent for an AR exposure therapy.
A version of this article first appeared on Medscape.com.
Antidepressant study yields controversial findings
Researchers who conducted the study admit this finding was unexpected, and outside experts say no firm conclusions can be drawn from the research.
“Of course we were surprised by the results,” first author Omar Almohammed, PharmD, PhD, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia, told this news organization.
“We were expecting to see some positive impact with the use of antidepressant medications on the HRQoL measures when we compared these patients to patients that did not use antidepressant medications,” Dr. Almohammed said.
The study was published online in PLOS ONE.
Controversial impact on quality of life
Depression is known to harm HRQoL. Despite evidence that antidepressants improve depressed mood, their effect on patients’ overall well-being and HRQoL remains controversial.
The researchers examined the effect of antidepressants on HRQoL in adults with depression using 11 years of data from the U.S. Medical Expenditures Panel Survey (MEPS), a large longitudinal survey that tracks health service use in the United States. HRQoL was measured using the 12-item Short Form Health Survey (SF-12).
On average, about 17.5 million adults were diagnosed with depression each year during the study period (2005-2016). More than half (57.6%) of these patients were treated with antidepressants.
Patients with depression had an average age of 48.3 years. Women made up more than two-thirds of the total sample (68%), and more women than men received antidepressants (61% vs. 52%).
Compared with no antidepressant use, antidepressant use was associated with some improvement on the mental, but not physical, component of the SF-12, the researchers report.
However, difference-in-differences (D-I-D) univariate analysis showed no significant difference between adults using and not using antidepressants in the SF-12 physical (-0.35 vs. -0.34; P = .9,595) or mental component (1.28 vs. 1.13; P = .6,405).
“The multivariate D-I-D analyses ensured the robustness of these results,” the researchers note.
The change in HRQoL observed in patients using antidepressants was not significantly different from that seen among peers not using these drugs, the researchers report.
“We are not saying that antidepressant medications are not helpful at all; HRQoL is only one of many measures intended to assess health outcomes,” Dr. Almohammed told this news organization.
“Based on our research design and data, we can only say that patients who used antidepressant medications did not experience better change in terms of HRQoL compared to patients who did not use antidepressant medications,” he said.
“These patients may have had some improvement on other clinical outcome measures, but that clinical improvement did not have a significant positive impact on HRQoL,” he noted.
“We still recommend that patients continue using their antidepressant medications, but they may want to ask their doctors to provide them with other nonpharmacologic interventions as this may have additional impact on their HRQoL,” Dr. Almohammed said.
Further research is needed to address a “gap in knowledge” about the impact of nondrug interventions – alone or in combination with antidepressant medications – on patients’ HRQoL, Dr. Almohammed added.
Experts weigh in
Several experts weighed in on the study in a statement from the British nonprofit Science Media Center.
Gemma Lewis, PhD, with University College London (UCL), noted that “clinical trials with experimental designs have found that antidepressants improve mental health-related quality of life.”
“In this study, the people who received antidepressants had worse quality of life, and are likely to have been more severely depressed, than those who did not. This type of bias is difficult to eliminate in a naturalistic study like this, which does not involve an experimental design,” Dr. Lewis commented.
Eduard Vieta, PhD, with University of Barcelona, noted the “inability to control for severity of depression between the two different groups is a crucial flaw, and therefore, there is little we can learn from this data.”
Echoing Dr. Vieta, David Curtis, MBBS, MD, PhD, with UCL Genetics Institute, said, “One might well assume that the people who were taking antidepressants had been more severely depressed than those who were not.”
“From this point of view, one could argue that it seems that the antidepressants were effective and that with their use people who had presented with more severe depression did not have markedly reduced quality of life,” Dr. Curtis said.
“However, the reality is that this kind of observational study tells us nothing about causation. For that, clinical trials are required, and numerous such trials have demonstrated that, on average, antidepressants are effective in terms of treating depressive illness and in improving the quality of life of patients with significant depression,” he added.
Michael Sharpe, MD, with University of Oxford, said the study highlights the importance of measuring the long-term outcomes of treatments for depression. “However, this study has no clear implication for the care of patients with depression and certainly should not discourage patients who may benefit from taking these drugs.”
Livia de Picker, MD, PhD, with University of Antwerp, Belgium, said, “What these data do point towards is the persistent treatment gap for depression in the United States, with only 57.6% of patients with major depressive disorder receiving treatment with antidepressants over a 2-year follow-up.”
Funding for the study was provided by King Saud University, Riyadh, Saudi Arabia. Dr. Almohammed, Dr. de Picker, Dr. Curtis, Dr. Lewis, and Dr. Sharpe have disclosed no relevant financial relationships. Dr. Vieta has participated in clinical trials of antidepressants and advisory boards for Angelini, Biogen, Janssen, and Lundbeck.
A version of this article first appeared on Medscape.com.
Researchers who conducted the study admit this finding was unexpected, and outside experts say no firm conclusions can be drawn from the research.
“Of course we were surprised by the results,” first author Omar Almohammed, PharmD, PhD, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia, told this news organization.
“We were expecting to see some positive impact with the use of antidepressant medications on the HRQoL measures when we compared these patients to patients that did not use antidepressant medications,” Dr. Almohammed said.
The study was published online in PLOS ONE.
Controversial impact on quality of life
Depression is known to harm HRQoL. Despite evidence that antidepressants improve depressed mood, their effect on patients’ overall well-being and HRQoL remains controversial.
The researchers examined the effect of antidepressants on HRQoL in adults with depression using 11 years of data from the U.S. Medical Expenditures Panel Survey (MEPS), a large longitudinal survey that tracks health service use in the United States. HRQoL was measured using the 12-item Short Form Health Survey (SF-12).
On average, about 17.5 million adults were diagnosed with depression each year during the study period (2005-2016). More than half (57.6%) of these patients were treated with antidepressants.
Patients with depression had an average age of 48.3 years. Women made up more than two-thirds of the total sample (68%), and more women than men received antidepressants (61% vs. 52%).
Compared with no antidepressant use, antidepressant use was associated with some improvement on the mental, but not physical, component of the SF-12, the researchers report.
However, difference-in-differences (D-I-D) univariate analysis showed no significant difference between adults using and not using antidepressants in the SF-12 physical (-0.35 vs. -0.34; P = .9,595) or mental component (1.28 vs. 1.13; P = .6,405).
“The multivariate D-I-D analyses ensured the robustness of these results,” the researchers note.
The change in HRQoL observed in patients using antidepressants was not significantly different from that seen among peers not using these drugs, the researchers report.
“We are not saying that antidepressant medications are not helpful at all; HRQoL is only one of many measures intended to assess health outcomes,” Dr. Almohammed told this news organization.
“Based on our research design and data, we can only say that patients who used antidepressant medications did not experience better change in terms of HRQoL compared to patients who did not use antidepressant medications,” he said.
“These patients may have had some improvement on other clinical outcome measures, but that clinical improvement did not have a significant positive impact on HRQoL,” he noted.
“We still recommend that patients continue using their antidepressant medications, but they may want to ask their doctors to provide them with other nonpharmacologic interventions as this may have additional impact on their HRQoL,” Dr. Almohammed said.
Further research is needed to address a “gap in knowledge” about the impact of nondrug interventions – alone or in combination with antidepressant medications – on patients’ HRQoL, Dr. Almohammed added.
Experts weigh in
Several experts weighed in on the study in a statement from the British nonprofit Science Media Center.
Gemma Lewis, PhD, with University College London (UCL), noted that “clinical trials with experimental designs have found that antidepressants improve mental health-related quality of life.”
“In this study, the people who received antidepressants had worse quality of life, and are likely to have been more severely depressed, than those who did not. This type of bias is difficult to eliminate in a naturalistic study like this, which does not involve an experimental design,” Dr. Lewis commented.
Eduard Vieta, PhD, with University of Barcelona, noted the “inability to control for severity of depression between the two different groups is a crucial flaw, and therefore, there is little we can learn from this data.”
Echoing Dr. Vieta, David Curtis, MBBS, MD, PhD, with UCL Genetics Institute, said, “One might well assume that the people who were taking antidepressants had been more severely depressed than those who were not.”
“From this point of view, one could argue that it seems that the antidepressants were effective and that with their use people who had presented with more severe depression did not have markedly reduced quality of life,” Dr. Curtis said.
“However, the reality is that this kind of observational study tells us nothing about causation. For that, clinical trials are required, and numerous such trials have demonstrated that, on average, antidepressants are effective in terms of treating depressive illness and in improving the quality of life of patients with significant depression,” he added.
Michael Sharpe, MD, with University of Oxford, said the study highlights the importance of measuring the long-term outcomes of treatments for depression. “However, this study has no clear implication for the care of patients with depression and certainly should not discourage patients who may benefit from taking these drugs.”
Livia de Picker, MD, PhD, with University of Antwerp, Belgium, said, “What these data do point towards is the persistent treatment gap for depression in the United States, with only 57.6% of patients with major depressive disorder receiving treatment with antidepressants over a 2-year follow-up.”
Funding for the study was provided by King Saud University, Riyadh, Saudi Arabia. Dr. Almohammed, Dr. de Picker, Dr. Curtis, Dr. Lewis, and Dr. Sharpe have disclosed no relevant financial relationships. Dr. Vieta has participated in clinical trials of antidepressants and advisory boards for Angelini, Biogen, Janssen, and Lundbeck.
A version of this article first appeared on Medscape.com.
Researchers who conducted the study admit this finding was unexpected, and outside experts say no firm conclusions can be drawn from the research.
“Of course we were surprised by the results,” first author Omar Almohammed, PharmD, PhD, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia, told this news organization.
“We were expecting to see some positive impact with the use of antidepressant medications on the HRQoL measures when we compared these patients to patients that did not use antidepressant medications,” Dr. Almohammed said.
The study was published online in PLOS ONE.
Controversial impact on quality of life
Depression is known to harm HRQoL. Despite evidence that antidepressants improve depressed mood, their effect on patients’ overall well-being and HRQoL remains controversial.
The researchers examined the effect of antidepressants on HRQoL in adults with depression using 11 years of data from the U.S. Medical Expenditures Panel Survey (MEPS), a large longitudinal survey that tracks health service use in the United States. HRQoL was measured using the 12-item Short Form Health Survey (SF-12).
On average, about 17.5 million adults were diagnosed with depression each year during the study period (2005-2016). More than half (57.6%) of these patients were treated with antidepressants.
Patients with depression had an average age of 48.3 years. Women made up more than two-thirds of the total sample (68%), and more women than men received antidepressants (61% vs. 52%).
Compared with no antidepressant use, antidepressant use was associated with some improvement on the mental, but not physical, component of the SF-12, the researchers report.
However, difference-in-differences (D-I-D) univariate analysis showed no significant difference between adults using and not using antidepressants in the SF-12 physical (-0.35 vs. -0.34; P = .9,595) or mental component (1.28 vs. 1.13; P = .6,405).
“The multivariate D-I-D analyses ensured the robustness of these results,” the researchers note.
The change in HRQoL observed in patients using antidepressants was not significantly different from that seen among peers not using these drugs, the researchers report.
“We are not saying that antidepressant medications are not helpful at all; HRQoL is only one of many measures intended to assess health outcomes,” Dr. Almohammed told this news organization.
“Based on our research design and data, we can only say that patients who used antidepressant medications did not experience better change in terms of HRQoL compared to patients who did not use antidepressant medications,” he said.
“These patients may have had some improvement on other clinical outcome measures, but that clinical improvement did not have a significant positive impact on HRQoL,” he noted.
“We still recommend that patients continue using their antidepressant medications, but they may want to ask their doctors to provide them with other nonpharmacologic interventions as this may have additional impact on their HRQoL,” Dr. Almohammed said.
Further research is needed to address a “gap in knowledge” about the impact of nondrug interventions – alone or in combination with antidepressant medications – on patients’ HRQoL, Dr. Almohammed added.
Experts weigh in
Several experts weighed in on the study in a statement from the British nonprofit Science Media Center.
Gemma Lewis, PhD, with University College London (UCL), noted that “clinical trials with experimental designs have found that antidepressants improve mental health-related quality of life.”
“In this study, the people who received antidepressants had worse quality of life, and are likely to have been more severely depressed, than those who did not. This type of bias is difficult to eliminate in a naturalistic study like this, which does not involve an experimental design,” Dr. Lewis commented.
Eduard Vieta, PhD, with University of Barcelona, noted the “inability to control for severity of depression between the two different groups is a crucial flaw, and therefore, there is little we can learn from this data.”
Echoing Dr. Vieta, David Curtis, MBBS, MD, PhD, with UCL Genetics Institute, said, “One might well assume that the people who were taking antidepressants had been more severely depressed than those who were not.”
“From this point of view, one could argue that it seems that the antidepressants were effective and that with their use people who had presented with more severe depression did not have markedly reduced quality of life,” Dr. Curtis said.
“However, the reality is that this kind of observational study tells us nothing about causation. For that, clinical trials are required, and numerous such trials have demonstrated that, on average, antidepressants are effective in terms of treating depressive illness and in improving the quality of life of patients with significant depression,” he added.
Michael Sharpe, MD, with University of Oxford, said the study highlights the importance of measuring the long-term outcomes of treatments for depression. “However, this study has no clear implication for the care of patients with depression and certainly should not discourage patients who may benefit from taking these drugs.”
Livia de Picker, MD, PhD, with University of Antwerp, Belgium, said, “What these data do point towards is the persistent treatment gap for depression in the United States, with only 57.6% of patients with major depressive disorder receiving treatment with antidepressants over a 2-year follow-up.”
Funding for the study was provided by King Saud University, Riyadh, Saudi Arabia. Dr. Almohammed, Dr. de Picker, Dr. Curtis, Dr. Lewis, and Dr. Sharpe have disclosed no relevant financial relationships. Dr. Vieta has participated in clinical trials of antidepressants and advisory boards for Angelini, Biogen, Janssen, and Lundbeck.
A version of this article first appeared on Medscape.com.
How can we help refugees with PTSD?
This article was originally published in Italian on Univadis .
The arrival of Ukrainian refugees in Western Europe, and especially the arrival of women and children coming from the cities most affected by the attacks, has made local medical services the first point of contact for the diagnosis and care of the psychological effects of war.
Many studies demonstrate the high prevalence of posttraumatic stress disorder (PTSD), depression, and anxiety among refugees. For example, a study from 2019 on refugee mental health offers a disturbing epidemiologic insight: Ten percent of refugees escaping conflict in Nepal showed signs of PTSD, 27.5% suffered from depression, and 22.9% suffered from anxiety. The rate of depression surpasses 90% in all studies carried out on survivors of torture.
Official guidelines
Posttraumatic stress is a form of mental disorder that manifests after experiencing highly traumatic events. Defined and studied in the United States, especially in veterans of the Vietnam War, and subsequently reviewed in relation to more recent conflicts, PTSD may appear in people of all ages. It also can occur in family members, witnesses, and aid workers involved in traumatic events. PTSD may come from repeated exposure to episodes of violence and degradation.
Because PTSD is a complex mental disorder derived from multiple factors, both personal and environmental, a diagnosis is never straightforward. It is generally indicated as “a condition of acute stress which manifests after exposure to a traumatic event.”
Among the most common symptoms of war-related trauma, according to experts, are the onset of flashbacks of troublesome memories, intrusive trauma-related thoughts, panic attacks, insomnia and night terrors, and social avoidance. In children, elements of regression can be observed, such as a need to sleep next to their parents.
Research performed directly on different areas of the brain has demonstrated that people affected by PTSD produce abnormal levels of hormones that are involved in responding to stress and fear. The area of the brain responsible for this response is the amygdala, which activates during moments of fear and produces natural pain-relieving molecules. In people with PTSD, the production of these molecules can carry on long after the event is over, causing a change in emotional state. Furthermore, levels of neurotransmitters that reach the hippocampus are also altered, which influences memory and learning capability. These same alterations concerning neurotransmitter levels are the basis for sudden flashbacks.
People with PTSD are subject to a change in blood flow to the brain and structural changes to brain tissue.
Social factors
“A factor that worsens the mental condition of Ukrainian refugees is the speed in which they have passed from a normal life, similar to that of many other Western countries, to a state of war, death, and injury,” writes Arash Javanbakht, MD, associate professor of psychiatry at Wayne State University in Detroit, and an expert in PTSD in war refugees.
“Furthermore, they are experiencing an awful feeling of injustice, as the democracy and freedom that they have fought so hard to have has been put at risk, and they don’t feel sufficiently supported by their allies.”
As of this writing, the World Health Organization has estimated that there are 3.6 million Ukrainian refugees. This population already has experienced war and its psychological consequences. A study from 2019 assessed the prevalence of PTSD (27%) and depression (21%) among the 1.5 million Ukrainians who had to leave their homes after the first Russian invasion in 2014 and the rebellion of predominantly Russian regions.
Children are particularly at risk of developing PTSD, as seen from studies conducted on Syrian refugees. They have a roughly 70% chance of developing separation anxiety, a condition that many workers and volunteers have experienced firsthand recently. Some children do not accept being separated from their parents even to allow them to go to the bathroom or wash themselves, which also aggravates stress levels among adults. Infant trauma increases the risk of developing physical or mental disorders during adulthood, including depression, chronic pain, heart disorders, and diabetes.
War-related trauma entails transmissible epigenetic alterations, as shown by studies on the transmissibility of trauma on a biological level.
Diagnosis and treatment
People with PTSD have difficulty controlling their emotions, resulting in irritability, sudden rage or emotional confusion, depression and anxiety, and insomnia. They also are determined to avoid any actions that remind them of the traumatic event. Another common symptom is a sense of shame, as a result of having survived or not having been able to save others.
Physical symptoms include chest pain, dizziness, gastrointestinal problems, migraine, and a weakened immune system. A diagnosis of PTSD can be made when, in accordance with the National Institute of Mental Health (NIMH), the patient presents with the characteristic symptoms for more than a month after the event that caused the symptoms occurred.
The NIMH highlights that a diagnosis cannot always be made in a systematic way. In many cases, patients with PTSD are treated for the physical symptoms only, without any consideration for the overall picture.
The American Psychiatric Association (APA) has created a detailed list of PTSD symptoms. According to the APA, these symptoms usually appear within 3 months of the trauma, even if stress may appear later. Symptoms are arranged into the following three well-defined categories:
- Intrusive memories. People with PTSD suffer from sudden, vivid memories that are accompanied by painful emotions and a feeling of reliving the trauma. At times, this experience is so strong that the person involved feels as though the traumatic event is repeating itself.
- Avoidance and numbing. The individual seeks to avoid contact with anyone or anything that brings back memories of the trauma. Initially, the person experiences an emotional state of disinterest and detachment, reducing the capacity for emotional interaction and resulting in participation only in simple, routine activities. A lack of emotional processing causes an accumulation of anxiety and tension, which can become a chronic condition, leading to depression. At the same time, people frequently experience a sense of shame.
- Hyperarousal and hypervigilance. People behave as if they are constantly threatened. They react in a sudden, violent way, are unable to concentrate, and have problems with their memory. At times, they use alcohol or other drugs to alleviate pain. People with PTSD may lose control of their lives and therefore be at risk for suicidal behavior.
Why do some people pass unscathed through traumatic situations, whereas others carry the scars forever? There is a correlation with the severity of the trauma, but also with biological and genetic factors, as well as with previous experiences that contribute to increasing an individual’s resilience. Another key element is the rapid and effective treatment of symptoms, which also relates to personal and financial security.
It is not a coincidence that the first guidelines that clinicians follow when treating a traumatized patient aren’t strictly medical. It is necessary to guarantee the financial security of a refugee, but also the security of the few valuable items they have with them (such as keepsakes and pets). Clinicians are advised to facilitate contact with any of the patient’s family members located elsewhere whenever possible. It is appropriate to use relaxation techniques that are compatible with the patient’s cultural approach. Clinicians also check for the most common conditions in the refugee’s population of origin. It is advisable constantly to check for trauma-related symptoms and to listen to the patient’s story. Caregivers should be allowed to stay close to their children and should be provided sufficient information, but not an overwhelming amount.
There isn’t a consensus on how to treat people with PTSD. The possibility that PTSD can be resolved even without specific treatments has not been excluded, if the affected person is cared for and helped within a family and community setting, and if the person’s personal condition allows for this. However, in general, some form of treatment is beneficial before symptoms become chronic.
Pharmacologic and psychological treatment may be implemented. For the latter, the NIMH and the APA suggest that good results can be obtained from cognitive behavioral therapy, where the patient learns to manage his or her anxiety and depression and amend dangerous behaviors, such as the dismissal of his or her own emotions. According to these organizations, group therapy and other forms of psychotherapy have provided good results. The indicated duration of treatment is generally 6-12 weeks, even if this duration strongly depends on the individual’s condition, with subsequent periodic follow-ups. The involvement of the patient’s family and community is important.
The National Center for PTSD in Washington (run by the U.S. Department of Veterans Affairs) has highlighted the importance of a detailed case-by-case assessment to put in place a precise therapy plan. If patients should continue to find themselves in a state of crisis, for example during a war or in cases of domestic violence, working toward removing the cause of stress is first necessary before beginning treatment.
An important aspect is making the victim aware of the disorder. Treatment should therefore begin after the patient and family have been informed about the possibility of PTSD and the way in which it develops. Recognizing the symptoms over the following weeks and working quickly to manage and treat them significantly affects treatment success.
A version of this article first appeared on Medscape.com.
This article was originally published in Italian on Univadis .
The arrival of Ukrainian refugees in Western Europe, and especially the arrival of women and children coming from the cities most affected by the attacks, has made local medical services the first point of contact for the diagnosis and care of the psychological effects of war.
Many studies demonstrate the high prevalence of posttraumatic stress disorder (PTSD), depression, and anxiety among refugees. For example, a study from 2019 on refugee mental health offers a disturbing epidemiologic insight: Ten percent of refugees escaping conflict in Nepal showed signs of PTSD, 27.5% suffered from depression, and 22.9% suffered from anxiety. The rate of depression surpasses 90% in all studies carried out on survivors of torture.
Official guidelines
Posttraumatic stress is a form of mental disorder that manifests after experiencing highly traumatic events. Defined and studied in the United States, especially in veterans of the Vietnam War, and subsequently reviewed in relation to more recent conflicts, PTSD may appear in people of all ages. It also can occur in family members, witnesses, and aid workers involved in traumatic events. PTSD may come from repeated exposure to episodes of violence and degradation.
Because PTSD is a complex mental disorder derived from multiple factors, both personal and environmental, a diagnosis is never straightforward. It is generally indicated as “a condition of acute stress which manifests after exposure to a traumatic event.”
Among the most common symptoms of war-related trauma, according to experts, are the onset of flashbacks of troublesome memories, intrusive trauma-related thoughts, panic attacks, insomnia and night terrors, and social avoidance. In children, elements of regression can be observed, such as a need to sleep next to their parents.
Research performed directly on different areas of the brain has demonstrated that people affected by PTSD produce abnormal levels of hormones that are involved in responding to stress and fear. The area of the brain responsible for this response is the amygdala, which activates during moments of fear and produces natural pain-relieving molecules. In people with PTSD, the production of these molecules can carry on long after the event is over, causing a change in emotional state. Furthermore, levels of neurotransmitters that reach the hippocampus are also altered, which influences memory and learning capability. These same alterations concerning neurotransmitter levels are the basis for sudden flashbacks.
People with PTSD are subject to a change in blood flow to the brain and structural changes to brain tissue.
Social factors
“A factor that worsens the mental condition of Ukrainian refugees is the speed in which they have passed from a normal life, similar to that of many other Western countries, to a state of war, death, and injury,” writes Arash Javanbakht, MD, associate professor of psychiatry at Wayne State University in Detroit, and an expert in PTSD in war refugees.
“Furthermore, they are experiencing an awful feeling of injustice, as the democracy and freedom that they have fought so hard to have has been put at risk, and they don’t feel sufficiently supported by their allies.”
As of this writing, the World Health Organization has estimated that there are 3.6 million Ukrainian refugees. This population already has experienced war and its psychological consequences. A study from 2019 assessed the prevalence of PTSD (27%) and depression (21%) among the 1.5 million Ukrainians who had to leave their homes after the first Russian invasion in 2014 and the rebellion of predominantly Russian regions.
Children are particularly at risk of developing PTSD, as seen from studies conducted on Syrian refugees. They have a roughly 70% chance of developing separation anxiety, a condition that many workers and volunteers have experienced firsthand recently. Some children do not accept being separated from their parents even to allow them to go to the bathroom or wash themselves, which also aggravates stress levels among adults. Infant trauma increases the risk of developing physical or mental disorders during adulthood, including depression, chronic pain, heart disorders, and diabetes.
War-related trauma entails transmissible epigenetic alterations, as shown by studies on the transmissibility of trauma on a biological level.
Diagnosis and treatment
People with PTSD have difficulty controlling their emotions, resulting in irritability, sudden rage or emotional confusion, depression and anxiety, and insomnia. They also are determined to avoid any actions that remind them of the traumatic event. Another common symptom is a sense of shame, as a result of having survived or not having been able to save others.
Physical symptoms include chest pain, dizziness, gastrointestinal problems, migraine, and a weakened immune system. A diagnosis of PTSD can be made when, in accordance with the National Institute of Mental Health (NIMH), the patient presents with the characteristic symptoms for more than a month after the event that caused the symptoms occurred.
The NIMH highlights that a diagnosis cannot always be made in a systematic way. In many cases, patients with PTSD are treated for the physical symptoms only, without any consideration for the overall picture.
The American Psychiatric Association (APA) has created a detailed list of PTSD symptoms. According to the APA, these symptoms usually appear within 3 months of the trauma, even if stress may appear later. Symptoms are arranged into the following three well-defined categories:
- Intrusive memories. People with PTSD suffer from sudden, vivid memories that are accompanied by painful emotions and a feeling of reliving the trauma. At times, this experience is so strong that the person involved feels as though the traumatic event is repeating itself.
- Avoidance and numbing. The individual seeks to avoid contact with anyone or anything that brings back memories of the trauma. Initially, the person experiences an emotional state of disinterest and detachment, reducing the capacity for emotional interaction and resulting in participation only in simple, routine activities. A lack of emotional processing causes an accumulation of anxiety and tension, which can become a chronic condition, leading to depression. At the same time, people frequently experience a sense of shame.
- Hyperarousal and hypervigilance. People behave as if they are constantly threatened. They react in a sudden, violent way, are unable to concentrate, and have problems with their memory. At times, they use alcohol or other drugs to alleviate pain. People with PTSD may lose control of their lives and therefore be at risk for suicidal behavior.
Why do some people pass unscathed through traumatic situations, whereas others carry the scars forever? There is a correlation with the severity of the trauma, but also with biological and genetic factors, as well as with previous experiences that contribute to increasing an individual’s resilience. Another key element is the rapid and effective treatment of symptoms, which also relates to personal and financial security.
It is not a coincidence that the first guidelines that clinicians follow when treating a traumatized patient aren’t strictly medical. It is necessary to guarantee the financial security of a refugee, but also the security of the few valuable items they have with them (such as keepsakes and pets). Clinicians are advised to facilitate contact with any of the patient’s family members located elsewhere whenever possible. It is appropriate to use relaxation techniques that are compatible with the patient’s cultural approach. Clinicians also check for the most common conditions in the refugee’s population of origin. It is advisable constantly to check for trauma-related symptoms and to listen to the patient’s story. Caregivers should be allowed to stay close to their children and should be provided sufficient information, but not an overwhelming amount.
There isn’t a consensus on how to treat people with PTSD. The possibility that PTSD can be resolved even without specific treatments has not been excluded, if the affected person is cared for and helped within a family and community setting, and if the person’s personal condition allows for this. However, in general, some form of treatment is beneficial before symptoms become chronic.
Pharmacologic and psychological treatment may be implemented. For the latter, the NIMH and the APA suggest that good results can be obtained from cognitive behavioral therapy, where the patient learns to manage his or her anxiety and depression and amend dangerous behaviors, such as the dismissal of his or her own emotions. According to these organizations, group therapy and other forms of psychotherapy have provided good results. The indicated duration of treatment is generally 6-12 weeks, even if this duration strongly depends on the individual’s condition, with subsequent periodic follow-ups. The involvement of the patient’s family and community is important.
The National Center for PTSD in Washington (run by the U.S. Department of Veterans Affairs) has highlighted the importance of a detailed case-by-case assessment to put in place a precise therapy plan. If patients should continue to find themselves in a state of crisis, for example during a war or in cases of domestic violence, working toward removing the cause of stress is first necessary before beginning treatment.
An important aspect is making the victim aware of the disorder. Treatment should therefore begin after the patient and family have been informed about the possibility of PTSD and the way in which it develops. Recognizing the symptoms over the following weeks and working quickly to manage and treat them significantly affects treatment success.
A version of this article first appeared on Medscape.com.
This article was originally published in Italian on Univadis .
The arrival of Ukrainian refugees in Western Europe, and especially the arrival of women and children coming from the cities most affected by the attacks, has made local medical services the first point of contact for the diagnosis and care of the psychological effects of war.
Many studies demonstrate the high prevalence of posttraumatic stress disorder (PTSD), depression, and anxiety among refugees. For example, a study from 2019 on refugee mental health offers a disturbing epidemiologic insight: Ten percent of refugees escaping conflict in Nepal showed signs of PTSD, 27.5% suffered from depression, and 22.9% suffered from anxiety. The rate of depression surpasses 90% in all studies carried out on survivors of torture.
Official guidelines
Posttraumatic stress is a form of mental disorder that manifests after experiencing highly traumatic events. Defined and studied in the United States, especially in veterans of the Vietnam War, and subsequently reviewed in relation to more recent conflicts, PTSD may appear in people of all ages. It also can occur in family members, witnesses, and aid workers involved in traumatic events. PTSD may come from repeated exposure to episodes of violence and degradation.
Because PTSD is a complex mental disorder derived from multiple factors, both personal and environmental, a diagnosis is never straightforward. It is generally indicated as “a condition of acute stress which manifests after exposure to a traumatic event.”
Among the most common symptoms of war-related trauma, according to experts, are the onset of flashbacks of troublesome memories, intrusive trauma-related thoughts, panic attacks, insomnia and night terrors, and social avoidance. In children, elements of regression can be observed, such as a need to sleep next to their parents.
Research performed directly on different areas of the brain has demonstrated that people affected by PTSD produce abnormal levels of hormones that are involved in responding to stress and fear. The area of the brain responsible for this response is the amygdala, which activates during moments of fear and produces natural pain-relieving molecules. In people with PTSD, the production of these molecules can carry on long after the event is over, causing a change in emotional state. Furthermore, levels of neurotransmitters that reach the hippocampus are also altered, which influences memory and learning capability. These same alterations concerning neurotransmitter levels are the basis for sudden flashbacks.
People with PTSD are subject to a change in blood flow to the brain and structural changes to brain tissue.
Social factors
“A factor that worsens the mental condition of Ukrainian refugees is the speed in which they have passed from a normal life, similar to that of many other Western countries, to a state of war, death, and injury,” writes Arash Javanbakht, MD, associate professor of psychiatry at Wayne State University in Detroit, and an expert in PTSD in war refugees.
“Furthermore, they are experiencing an awful feeling of injustice, as the democracy and freedom that they have fought so hard to have has been put at risk, and they don’t feel sufficiently supported by their allies.”
As of this writing, the World Health Organization has estimated that there are 3.6 million Ukrainian refugees. This population already has experienced war and its psychological consequences. A study from 2019 assessed the prevalence of PTSD (27%) and depression (21%) among the 1.5 million Ukrainians who had to leave their homes after the first Russian invasion in 2014 and the rebellion of predominantly Russian regions.
Children are particularly at risk of developing PTSD, as seen from studies conducted on Syrian refugees. They have a roughly 70% chance of developing separation anxiety, a condition that many workers and volunteers have experienced firsthand recently. Some children do not accept being separated from their parents even to allow them to go to the bathroom or wash themselves, which also aggravates stress levels among adults. Infant trauma increases the risk of developing physical or mental disorders during adulthood, including depression, chronic pain, heart disorders, and diabetes.
War-related trauma entails transmissible epigenetic alterations, as shown by studies on the transmissibility of trauma on a biological level.
Diagnosis and treatment
People with PTSD have difficulty controlling their emotions, resulting in irritability, sudden rage or emotional confusion, depression and anxiety, and insomnia. They also are determined to avoid any actions that remind them of the traumatic event. Another common symptom is a sense of shame, as a result of having survived or not having been able to save others.
Physical symptoms include chest pain, dizziness, gastrointestinal problems, migraine, and a weakened immune system. A diagnosis of PTSD can be made when, in accordance with the National Institute of Mental Health (NIMH), the patient presents with the characteristic symptoms for more than a month after the event that caused the symptoms occurred.
The NIMH highlights that a diagnosis cannot always be made in a systematic way. In many cases, patients with PTSD are treated for the physical symptoms only, without any consideration for the overall picture.
The American Psychiatric Association (APA) has created a detailed list of PTSD symptoms. According to the APA, these symptoms usually appear within 3 months of the trauma, even if stress may appear later. Symptoms are arranged into the following three well-defined categories:
- Intrusive memories. People with PTSD suffer from sudden, vivid memories that are accompanied by painful emotions and a feeling of reliving the trauma. At times, this experience is so strong that the person involved feels as though the traumatic event is repeating itself.
- Avoidance and numbing. The individual seeks to avoid contact with anyone or anything that brings back memories of the trauma. Initially, the person experiences an emotional state of disinterest and detachment, reducing the capacity for emotional interaction and resulting in participation only in simple, routine activities. A lack of emotional processing causes an accumulation of anxiety and tension, which can become a chronic condition, leading to depression. At the same time, people frequently experience a sense of shame.
- Hyperarousal and hypervigilance. People behave as if they are constantly threatened. They react in a sudden, violent way, are unable to concentrate, and have problems with their memory. At times, they use alcohol or other drugs to alleviate pain. People with PTSD may lose control of their lives and therefore be at risk for suicidal behavior.
Why do some people pass unscathed through traumatic situations, whereas others carry the scars forever? There is a correlation with the severity of the trauma, but also with biological and genetic factors, as well as with previous experiences that contribute to increasing an individual’s resilience. Another key element is the rapid and effective treatment of symptoms, which also relates to personal and financial security.
It is not a coincidence that the first guidelines that clinicians follow when treating a traumatized patient aren’t strictly medical. It is necessary to guarantee the financial security of a refugee, but also the security of the few valuable items they have with them (such as keepsakes and pets). Clinicians are advised to facilitate contact with any of the patient’s family members located elsewhere whenever possible. It is appropriate to use relaxation techniques that are compatible with the patient’s cultural approach. Clinicians also check for the most common conditions in the refugee’s population of origin. It is advisable constantly to check for trauma-related symptoms and to listen to the patient’s story. Caregivers should be allowed to stay close to their children and should be provided sufficient information, but not an overwhelming amount.
There isn’t a consensus on how to treat people with PTSD. The possibility that PTSD can be resolved even without specific treatments has not been excluded, if the affected person is cared for and helped within a family and community setting, and if the person’s personal condition allows for this. However, in general, some form of treatment is beneficial before symptoms become chronic.
Pharmacologic and psychological treatment may be implemented. For the latter, the NIMH and the APA suggest that good results can be obtained from cognitive behavioral therapy, where the patient learns to manage his or her anxiety and depression and amend dangerous behaviors, such as the dismissal of his or her own emotions. According to these organizations, group therapy and other forms of psychotherapy have provided good results. The indicated duration of treatment is generally 6-12 weeks, even if this duration strongly depends on the individual’s condition, with subsequent periodic follow-ups. The involvement of the patient’s family and community is important.
The National Center for PTSD in Washington (run by the U.S. Department of Veterans Affairs) has highlighted the importance of a detailed case-by-case assessment to put in place a precise therapy plan. If patients should continue to find themselves in a state of crisis, for example during a war or in cases of domestic violence, working toward removing the cause of stress is first necessary before beginning treatment.
An important aspect is making the victim aware of the disorder. Treatment should therefore begin after the patient and family have been informed about the possibility of PTSD and the way in which it develops. Recognizing the symptoms over the following weeks and working quickly to manage and treat them significantly affects treatment success.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases rise again, but more slowly
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.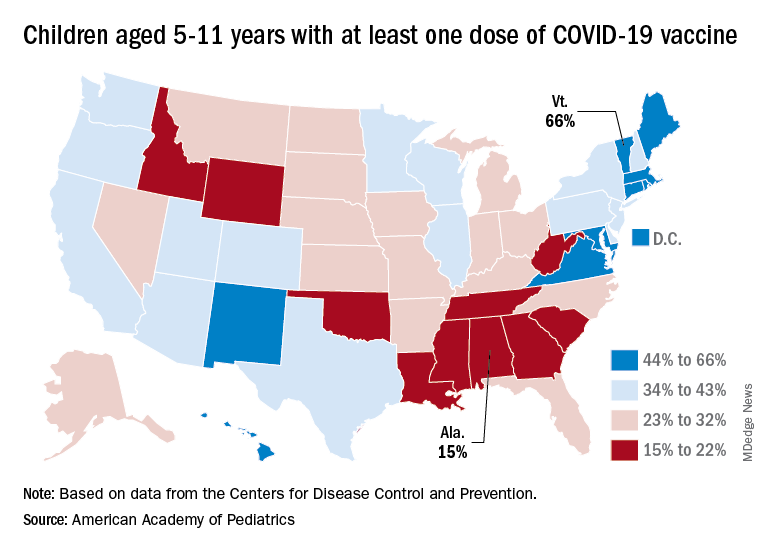
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
Polypharmacy common among patients aged 65 or older with HIV
People aged 65 or older with human immunodeficiency virus (HIV) receive significantly more nonantiretroviral therapy (non-ART) medications, compared with patients with HIV who are between ages 50 and 64, according to a new study.
Moreover, in a sample of more than 900 patients with HIV, about 60% were taking at least one potentially inappropriate medication (PIM).
“Clinicians looking after persons living with HIV need to provide medication reconciliation with prioritization of medications based on the patients’ wishes and patients’ goals and life expectancy,” lead author Jacqueline McMillan, MD, clinical assistant professor of geriatric medicine at the University of Calgary (Alt.) told this news organization.
The findings were published online in the Canadian Journal of General Internal Medicine.
Examining the pill burden
A geriatrician by training and a clinical researcher with an interest in aging in patients with HIV, Dr. McMillan said she began to observe that many older adults with HIV were on polypharmacy. “There are many other things that aging people with HIV experience, such as frailty, falls, cognitive impairment, medication nonadherence, and mortality, but in this study, we focused just on the polypharmacy,” said Dr. McMillan.
Her aim was to see if there was a way to improve the pill burden in these older adults.
“Do they need to be on all of these medications? Is there anything that we were overprescribing that they no longer needed, or possibly not prescribing and undertreating people because they were older? I wanted to have a better sense that the medications we were prescribing were appropriate and that we minimized the pill burden for older adults,” Dr. McMillan said.
Persons with HIV are at a particularly increased risk of polypharmacy and potential drug-drug interactions because they need antiretroviral therapy medications and medications to treat comorbidities.
“Certainly, when the ARTs were first discovered, sometimes that regimen required several pills a day, but as time has gone on and our retrovirals have gotten better, some of those requirements have narrowed down to one-pill-a-day regimens. We are now replacing that pill burden with non-HIV drugs,” said Dr. McMillan.
The researchers obtained medication reconciliation data for 951 persons with HIV aged 50 or older as of Feb. 1, 2020. The study population was receiving HIV care through the Southern Alberta HIV Clinic in Calgary. The researchers defined polypharmacy as taking five or more non-ART drugs. They defined PIMs according to the 2019 Beers criteria.
In their analysis, the researchers compared patients aged 65 or older with patients aged 50-64, as well as patients with shorter (< 10 years) and longer (> 10 years) duration of HIV infection.
PIM use common
The population’s mean age was 59 years, and 82% were men. The mean time since HIV diagnosis was 17.8 years, and the median time was 17 years. Most (80%) of the patients were aged 50-64 years, and 20% were 65 and older.
The researchers collected sociodemographic, clinical, medication, and laboratory data for all patients at each clinical visit.
The mean number of non-ART medications was 6.7 for the population. Patients aged 65 years or older were taking significantly more non-ART medications than patients aged 50-64 (8.4 vs. 6.3; P < .001).
Similarly, those living with HIV for more than 10 years were taking significantly more non-ART medications (mean, 6.9) than those living with HIV for 10 or fewer years (mean 6.1; P = .0168).
In all, almost 60% of patients were taking at least one PIM. The mean number of PIMs per patient was 1.6.
Patients living with diagnosed HIV infection for more than 10 years were at greater risk of PIMs (1.6 PIMs) than those with shorter duration of HIV diagnosis (1.4 PIMs; P = .06).
Dr. McMillan says she hopes her study reminds clinicians to review patients’ medications at each visit and ensure they are neither over- nor underprescribing.
“From my perspective as a geriatrician, I hope that we do more dedicated medication reconciliation to actually make sure we know what people are taking,” she said. She asks patients to bring all their medications to the office so that they can review which ones match their diagnoses.
“I want to do more patient-centered personalized care for older adults, with a focus on people who are frail and who may have a limited life expectancy, so that we don’t have someone with a short life expectancy still taking 15 medications a day,” said Dr. McMillan.
‘Carefully document medications’
“This study identifies potentially inappropriate medication use in a group of older people living with HIV who are particularly vulnerable to it at an earlier age because of their medical complexity or frailty than perhaps healthy older adults,” Adrian Wagg, MD, professor of healthy aging in the department of medicine at the University of Alberta, Edmonton, told this news organization.
The study emphasizes the importance of careful documentation of medications that the patient is taking at every clinical visit, he said.
“Make sure you carefully document medications which are taken whenever you see the individual. Also try to limit the number of prescribers, because we know multiple prescribers are associated with greater likelihood of inappropriate prescribing,” Dr. Wagg said.
The move to wean patients from inappropriate medications is gaining momentum, he added.
“There is a huge movement now around actively deprescribing medications which are either no longer indicated or potentially of little benefit, given remaining life expectancy,” said Dr. Wagg. Drugs such as proton pump inhibitors, hypnotics, unrequired antidepressants, and benzodiazepines are the first targets for elimination, he concluded.
The study was funded by the University of Calgary. Dr. McMillan and Dr. Wagg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People aged 65 or older with human immunodeficiency virus (HIV) receive significantly more nonantiretroviral therapy (non-ART) medications, compared with patients with HIV who are between ages 50 and 64, according to a new study.
Moreover, in a sample of more than 900 patients with HIV, about 60% were taking at least one potentially inappropriate medication (PIM).
“Clinicians looking after persons living with HIV need to provide medication reconciliation with prioritization of medications based on the patients’ wishes and patients’ goals and life expectancy,” lead author Jacqueline McMillan, MD, clinical assistant professor of geriatric medicine at the University of Calgary (Alt.) told this news organization.
The findings were published online in the Canadian Journal of General Internal Medicine.
Examining the pill burden
A geriatrician by training and a clinical researcher with an interest in aging in patients with HIV, Dr. McMillan said she began to observe that many older adults with HIV were on polypharmacy. “There are many other things that aging people with HIV experience, such as frailty, falls, cognitive impairment, medication nonadherence, and mortality, but in this study, we focused just on the polypharmacy,” said Dr. McMillan.
Her aim was to see if there was a way to improve the pill burden in these older adults.
“Do they need to be on all of these medications? Is there anything that we were overprescribing that they no longer needed, or possibly not prescribing and undertreating people because they were older? I wanted to have a better sense that the medications we were prescribing were appropriate and that we minimized the pill burden for older adults,” Dr. McMillan said.
Persons with HIV are at a particularly increased risk of polypharmacy and potential drug-drug interactions because they need antiretroviral therapy medications and medications to treat comorbidities.
“Certainly, when the ARTs were first discovered, sometimes that regimen required several pills a day, but as time has gone on and our retrovirals have gotten better, some of those requirements have narrowed down to one-pill-a-day regimens. We are now replacing that pill burden with non-HIV drugs,” said Dr. McMillan.
The researchers obtained medication reconciliation data for 951 persons with HIV aged 50 or older as of Feb. 1, 2020. The study population was receiving HIV care through the Southern Alberta HIV Clinic in Calgary. The researchers defined polypharmacy as taking five or more non-ART drugs. They defined PIMs according to the 2019 Beers criteria.
In their analysis, the researchers compared patients aged 65 or older with patients aged 50-64, as well as patients with shorter (< 10 years) and longer (> 10 years) duration of HIV infection.
PIM use common
The population’s mean age was 59 years, and 82% were men. The mean time since HIV diagnosis was 17.8 years, and the median time was 17 years. Most (80%) of the patients were aged 50-64 years, and 20% were 65 and older.
The researchers collected sociodemographic, clinical, medication, and laboratory data for all patients at each clinical visit.
The mean number of non-ART medications was 6.7 for the population. Patients aged 65 years or older were taking significantly more non-ART medications than patients aged 50-64 (8.4 vs. 6.3; P < .001).
Similarly, those living with HIV for more than 10 years were taking significantly more non-ART medications (mean, 6.9) than those living with HIV for 10 or fewer years (mean 6.1; P = .0168).
In all, almost 60% of patients were taking at least one PIM. The mean number of PIMs per patient was 1.6.
Patients living with diagnosed HIV infection for more than 10 years were at greater risk of PIMs (1.6 PIMs) than those with shorter duration of HIV diagnosis (1.4 PIMs; P = .06).
Dr. McMillan says she hopes her study reminds clinicians to review patients’ medications at each visit and ensure they are neither over- nor underprescribing.
“From my perspective as a geriatrician, I hope that we do more dedicated medication reconciliation to actually make sure we know what people are taking,” she said. She asks patients to bring all their medications to the office so that they can review which ones match their diagnoses.
“I want to do more patient-centered personalized care for older adults, with a focus on people who are frail and who may have a limited life expectancy, so that we don’t have someone with a short life expectancy still taking 15 medications a day,” said Dr. McMillan.
‘Carefully document medications’
“This study identifies potentially inappropriate medication use in a group of older people living with HIV who are particularly vulnerable to it at an earlier age because of their medical complexity or frailty than perhaps healthy older adults,” Adrian Wagg, MD, professor of healthy aging in the department of medicine at the University of Alberta, Edmonton, told this news organization.
The study emphasizes the importance of careful documentation of medications that the patient is taking at every clinical visit, he said.
“Make sure you carefully document medications which are taken whenever you see the individual. Also try to limit the number of prescribers, because we know multiple prescribers are associated with greater likelihood of inappropriate prescribing,” Dr. Wagg said.
The move to wean patients from inappropriate medications is gaining momentum, he added.
“There is a huge movement now around actively deprescribing medications which are either no longer indicated or potentially of little benefit, given remaining life expectancy,” said Dr. Wagg. Drugs such as proton pump inhibitors, hypnotics, unrequired antidepressants, and benzodiazepines are the first targets for elimination, he concluded.
The study was funded by the University of Calgary. Dr. McMillan and Dr. Wagg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People aged 65 or older with human immunodeficiency virus (HIV) receive significantly more nonantiretroviral therapy (non-ART) medications, compared with patients with HIV who are between ages 50 and 64, according to a new study.
Moreover, in a sample of more than 900 patients with HIV, about 60% were taking at least one potentially inappropriate medication (PIM).
“Clinicians looking after persons living with HIV need to provide medication reconciliation with prioritization of medications based on the patients’ wishes and patients’ goals and life expectancy,” lead author Jacqueline McMillan, MD, clinical assistant professor of geriatric medicine at the University of Calgary (Alt.) told this news organization.
The findings were published online in the Canadian Journal of General Internal Medicine.
Examining the pill burden
A geriatrician by training and a clinical researcher with an interest in aging in patients with HIV, Dr. McMillan said she began to observe that many older adults with HIV were on polypharmacy. “There are many other things that aging people with HIV experience, such as frailty, falls, cognitive impairment, medication nonadherence, and mortality, but in this study, we focused just on the polypharmacy,” said Dr. McMillan.
Her aim was to see if there was a way to improve the pill burden in these older adults.
“Do they need to be on all of these medications? Is there anything that we were overprescribing that they no longer needed, or possibly not prescribing and undertreating people because they were older? I wanted to have a better sense that the medications we were prescribing were appropriate and that we minimized the pill burden for older adults,” Dr. McMillan said.
Persons with HIV are at a particularly increased risk of polypharmacy and potential drug-drug interactions because they need antiretroviral therapy medications and medications to treat comorbidities.
“Certainly, when the ARTs were first discovered, sometimes that regimen required several pills a day, but as time has gone on and our retrovirals have gotten better, some of those requirements have narrowed down to one-pill-a-day regimens. We are now replacing that pill burden with non-HIV drugs,” said Dr. McMillan.
The researchers obtained medication reconciliation data for 951 persons with HIV aged 50 or older as of Feb. 1, 2020. The study population was receiving HIV care through the Southern Alberta HIV Clinic in Calgary. The researchers defined polypharmacy as taking five or more non-ART drugs. They defined PIMs according to the 2019 Beers criteria.
In their analysis, the researchers compared patients aged 65 or older with patients aged 50-64, as well as patients with shorter (< 10 years) and longer (> 10 years) duration of HIV infection.
PIM use common
The population’s mean age was 59 years, and 82% were men. The mean time since HIV diagnosis was 17.8 years, and the median time was 17 years. Most (80%) of the patients were aged 50-64 years, and 20% were 65 and older.
The researchers collected sociodemographic, clinical, medication, and laboratory data for all patients at each clinical visit.
The mean number of non-ART medications was 6.7 for the population. Patients aged 65 years or older were taking significantly more non-ART medications than patients aged 50-64 (8.4 vs. 6.3; P < .001).
Similarly, those living with HIV for more than 10 years were taking significantly more non-ART medications (mean, 6.9) than those living with HIV for 10 or fewer years (mean 6.1; P = .0168).
In all, almost 60% of patients were taking at least one PIM. The mean number of PIMs per patient was 1.6.
Patients living with diagnosed HIV infection for more than 10 years were at greater risk of PIMs (1.6 PIMs) than those with shorter duration of HIV diagnosis (1.4 PIMs; P = .06).
Dr. McMillan says she hopes her study reminds clinicians to review patients’ medications at each visit and ensure they are neither over- nor underprescribing.
“From my perspective as a geriatrician, I hope that we do more dedicated medication reconciliation to actually make sure we know what people are taking,” she said. She asks patients to bring all their medications to the office so that they can review which ones match their diagnoses.
“I want to do more patient-centered personalized care for older adults, with a focus on people who are frail and who may have a limited life expectancy, so that we don’t have someone with a short life expectancy still taking 15 medications a day,” said Dr. McMillan.
‘Carefully document medications’
“This study identifies potentially inappropriate medication use in a group of older people living with HIV who are particularly vulnerable to it at an earlier age because of their medical complexity or frailty than perhaps healthy older adults,” Adrian Wagg, MD, professor of healthy aging in the department of medicine at the University of Alberta, Edmonton, told this news organization.
The study emphasizes the importance of careful documentation of medications that the patient is taking at every clinical visit, he said.
“Make sure you carefully document medications which are taken whenever you see the individual. Also try to limit the number of prescribers, because we know multiple prescribers are associated with greater likelihood of inappropriate prescribing,” Dr. Wagg said.
The move to wean patients from inappropriate medications is gaining momentum, he added.
“There is a huge movement now around actively deprescribing medications which are either no longer indicated or potentially of little benefit, given remaining life expectancy,” said Dr. Wagg. Drugs such as proton pump inhibitors, hypnotics, unrequired antidepressants, and benzodiazepines are the first targets for elimination, he concluded.
The study was funded by the University of Calgary. Dr. McMillan and Dr. Wagg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF GENERAL INTERNAL MEDICINE
Harlequin Syndrome: Discovery of an Ancient Schwannoma
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.
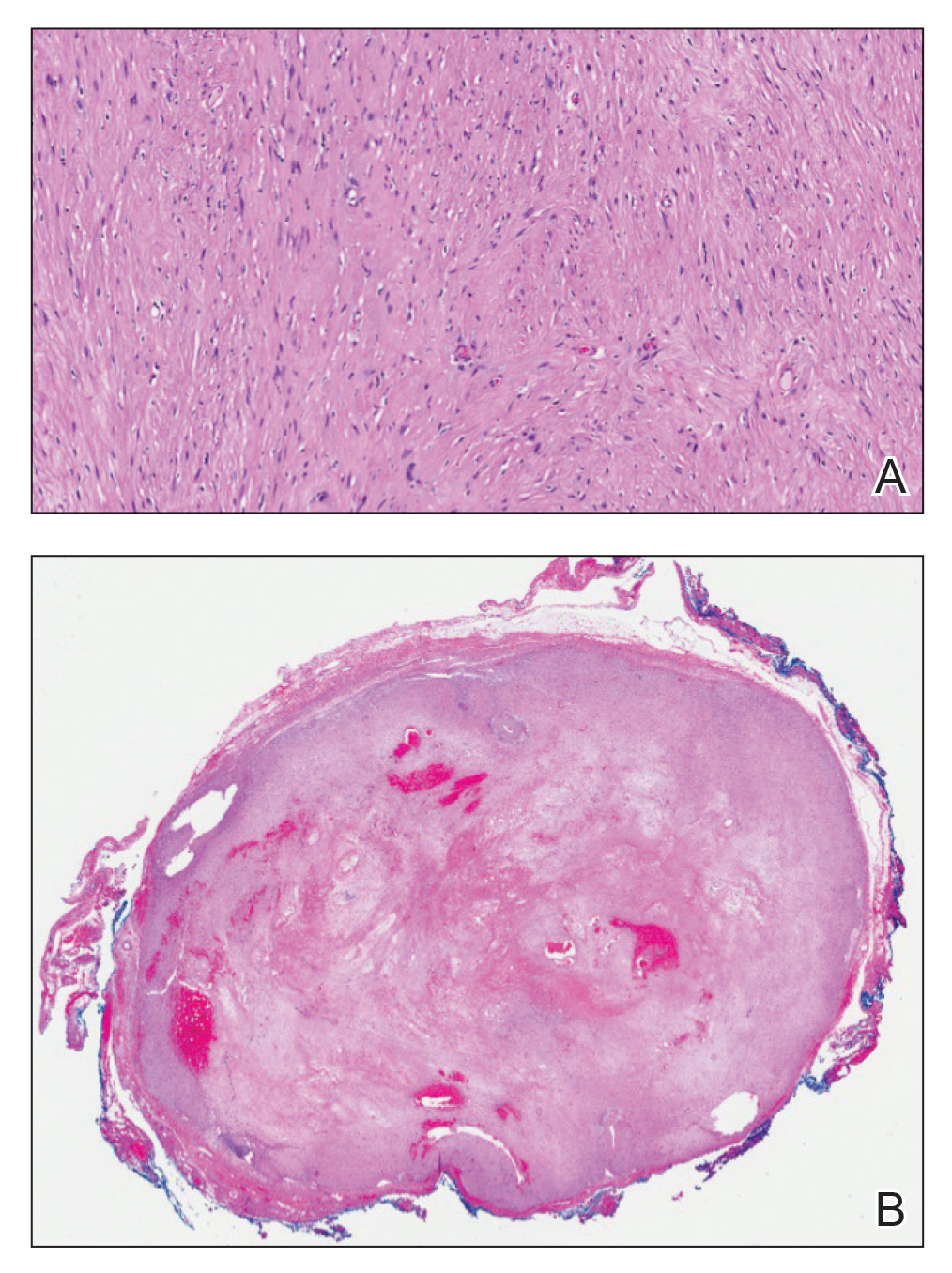
Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.
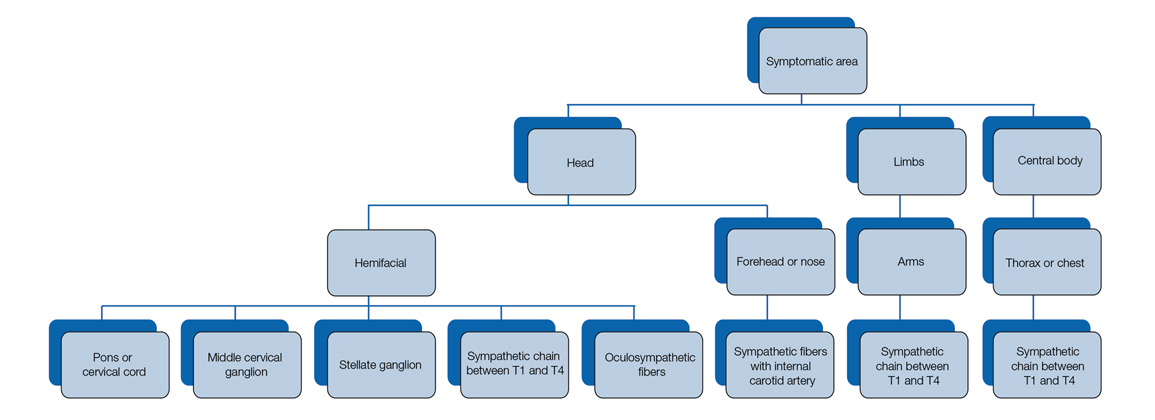
Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.

Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.

Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11

To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.

Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.

Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11

- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
Practice Points
- Harlequin syndrome is a rare disorder of the sympathetic nervous system that is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.
- Secondary causes can be from schwannomas in the cervical chain ganglion.
TV time related to poor eating in toddlers
Toddlers who watched more TV were significantly more likely than those who watched less TV to consume sugar-sweetened drinks and junk foods, based on data from 529 children.
Previous research had shown an association between screen time and poor diet, but most have involved school-aged children; the relationship in toddlers has not been well studied, Melissa R. Lutz, MD, of Johns Hopkins University, Baltimore, said in a presentation at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics currently recommends no digital media for children younger than 18-24 months, and an hour or less daily for children aged 2-5 years.
To examine the association between TV time and dietary practices in 2-year-olds, the researchers conducted a secondary analysis of data from 529 children who presented for their 2-year-old well-child visit at a single center. The study population was 52% Latino/Hispanic and 30% non-Latino/Hispanic Black, and 69% had an annual household income less than $20,000. The median time spent watching TV daily was 42 minutes. The data were taken from participants in the Greenlight Intervention Study, a randomized trial of an obesity prevention program at four academic pediatric primary care clinics in the United States.
Daily screen time and dietary practices were based on parent reports, and included daily volume of juice, daily counts of fruits and vegetables, daily count of junk foods such as chips, ice cream, French fries, and fast food, and consumption of sugar-sweetened beverages. The cross-sectional analysis controlled for race/ethnicity, Women, Infants, and Children Program benefits, number of children at home, caregiver education level, and family income.
In adjusted analysis, more than an hour of TV time was significantly associated with junk food intake, with odds ratios of 1.12 for 90 minutes and 1.25 for 120 minutes (P < .05 for both). Similar associations were seen for TV times of 90 minutes and 120 minutes and intake of fast food and sugar-sweetened beverages.
Additionally, the researchers found that toddlers who watched TV during mealtimes were more than twice as likely to consume sugar-sweetened beverages (OR, 2.74), junk food (OR, 2.72), fast food (OR, 2.09), and only about half as likely to consume fruits and vegetables (OR, 0.62).
The study findings were limited by several factors including the cross-sectional design, the reliance on caregiver self-reports, potential for residual confounding, and the low average screen time, Dr. Lutz noted.
However, the results suggest that “increased screen TV time and mealtime TV were both associated with poor dietary practices in 2-year-old children,” she said.
Future research should include analysis of passive screen time, as well as the relationship between screen time and diet with other digital devices beyond TV, she added.
COVID drove screen time higher
The current study is especially important at this time because of the increased screen exposure for many young children in the wake of the ongoing pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview. “Screen time use is up even more than before [the pandemic], and this study is a reminder to ask parents of young children about screen time and dietary history.”
Dr. Kinsella said she was not surprised by the study findings. In her practice, “I see families with more screen time use in general who also are more likely to have juice and junk food available. If kids had no access to screens, I believe they would still have access to unhealthy foods. I believe more research is needed into why screen time is so high in some families.”
The study received funding from NIH. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Toddlers who watched more TV were significantly more likely than those who watched less TV to consume sugar-sweetened drinks and junk foods, based on data from 529 children.
Previous research had shown an association between screen time and poor diet, but most have involved school-aged children; the relationship in toddlers has not been well studied, Melissa R. Lutz, MD, of Johns Hopkins University, Baltimore, said in a presentation at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics currently recommends no digital media for children younger than 18-24 months, and an hour or less daily for children aged 2-5 years.
To examine the association between TV time and dietary practices in 2-year-olds, the researchers conducted a secondary analysis of data from 529 children who presented for their 2-year-old well-child visit at a single center. The study population was 52% Latino/Hispanic and 30% non-Latino/Hispanic Black, and 69% had an annual household income less than $20,000. The median time spent watching TV daily was 42 minutes. The data were taken from participants in the Greenlight Intervention Study, a randomized trial of an obesity prevention program at four academic pediatric primary care clinics in the United States.
Daily screen time and dietary practices were based on parent reports, and included daily volume of juice, daily counts of fruits and vegetables, daily count of junk foods such as chips, ice cream, French fries, and fast food, and consumption of sugar-sweetened beverages. The cross-sectional analysis controlled for race/ethnicity, Women, Infants, and Children Program benefits, number of children at home, caregiver education level, and family income.
In adjusted analysis, more than an hour of TV time was significantly associated with junk food intake, with odds ratios of 1.12 for 90 minutes and 1.25 for 120 minutes (P < .05 for both). Similar associations were seen for TV times of 90 minutes and 120 minutes and intake of fast food and sugar-sweetened beverages.
Additionally, the researchers found that toddlers who watched TV during mealtimes were more than twice as likely to consume sugar-sweetened beverages (OR, 2.74), junk food (OR, 2.72), fast food (OR, 2.09), and only about half as likely to consume fruits and vegetables (OR, 0.62).
The study findings were limited by several factors including the cross-sectional design, the reliance on caregiver self-reports, potential for residual confounding, and the low average screen time, Dr. Lutz noted.
However, the results suggest that “increased screen TV time and mealtime TV were both associated with poor dietary practices in 2-year-old children,” she said.
Future research should include analysis of passive screen time, as well as the relationship between screen time and diet with other digital devices beyond TV, she added.
COVID drove screen time higher
The current study is especially important at this time because of the increased screen exposure for many young children in the wake of the ongoing pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview. “Screen time use is up even more than before [the pandemic], and this study is a reminder to ask parents of young children about screen time and dietary history.”
Dr. Kinsella said she was not surprised by the study findings. In her practice, “I see families with more screen time use in general who also are more likely to have juice and junk food available. If kids had no access to screens, I believe they would still have access to unhealthy foods. I believe more research is needed into why screen time is so high in some families.”
The study received funding from NIH. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Toddlers who watched more TV were significantly more likely than those who watched less TV to consume sugar-sweetened drinks and junk foods, based on data from 529 children.
Previous research had shown an association between screen time and poor diet, but most have involved school-aged children; the relationship in toddlers has not been well studied, Melissa R. Lutz, MD, of Johns Hopkins University, Baltimore, said in a presentation at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics currently recommends no digital media for children younger than 18-24 months, and an hour or less daily for children aged 2-5 years.
To examine the association between TV time and dietary practices in 2-year-olds, the researchers conducted a secondary analysis of data from 529 children who presented for their 2-year-old well-child visit at a single center. The study population was 52% Latino/Hispanic and 30% non-Latino/Hispanic Black, and 69% had an annual household income less than $20,000. The median time spent watching TV daily was 42 minutes. The data were taken from participants in the Greenlight Intervention Study, a randomized trial of an obesity prevention program at four academic pediatric primary care clinics in the United States.
Daily screen time and dietary practices were based on parent reports, and included daily volume of juice, daily counts of fruits and vegetables, daily count of junk foods such as chips, ice cream, French fries, and fast food, and consumption of sugar-sweetened beverages. The cross-sectional analysis controlled for race/ethnicity, Women, Infants, and Children Program benefits, number of children at home, caregiver education level, and family income.
In adjusted analysis, more than an hour of TV time was significantly associated with junk food intake, with odds ratios of 1.12 for 90 minutes and 1.25 for 120 minutes (P < .05 for both). Similar associations were seen for TV times of 90 minutes and 120 minutes and intake of fast food and sugar-sweetened beverages.
Additionally, the researchers found that toddlers who watched TV during mealtimes were more than twice as likely to consume sugar-sweetened beverages (OR, 2.74), junk food (OR, 2.72), fast food (OR, 2.09), and only about half as likely to consume fruits and vegetables (OR, 0.62).
The study findings were limited by several factors including the cross-sectional design, the reliance on caregiver self-reports, potential for residual confounding, and the low average screen time, Dr. Lutz noted.
However, the results suggest that “increased screen TV time and mealtime TV were both associated with poor dietary practices in 2-year-old children,” she said.
Future research should include analysis of passive screen time, as well as the relationship between screen time and diet with other digital devices beyond TV, she added.
COVID drove screen time higher
The current study is especially important at this time because of the increased screen exposure for many young children in the wake of the ongoing pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview. “Screen time use is up even more than before [the pandemic], and this study is a reminder to ask parents of young children about screen time and dietary history.”
Dr. Kinsella said she was not surprised by the study findings. In her practice, “I see families with more screen time use in general who also are more likely to have juice and junk food available. If kids had no access to screens, I believe they would still have access to unhealthy foods. I believe more research is needed into why screen time is so high in some families.”
The study received funding from NIH. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM PAS 2022
Review of new drugs that may be used during pregnancy
In 2021, the Food and Drug Administration approved 50 new drugs, but 24 will not be described here because they would probably not be used in pregnancy. The 24 are Aduhelm (aducanumab) to treat Alzheimer’s disease; Azstarys (serdexmethylphenidate and dexmethylphenidate), a combination CNS stimulant indicated for the treatment of ADHD; Cabenuva (cabotegravir and rilpivirine) to treat HIV; Voxzogo (vosoritide) for children with achondroplasia and open epiphyses; Qelbree (viloxazine) used in children aged 6-17 years to treat ADHD; and Pylarify (piflufolastat) for prostate cancer. Other anticancer drugs that will not be covered are Cosela (trilaciclib), Cytalux (pafolacianine), Exkivity (mobocertinib); Fotivda (tivozanib), Jemperli (dostarlimab-gxly), Lumakras (sotorasib), Pepaxto (melphalan flufenamide), Rybrevant (amivantamab-vmjw), Rylaze (asparaginase erwinia chrysanthemi), Scemblix (asciminib), Tepmetko (tepotinib), Tivdak (tisotumab vedotin-tftv), Truseltiq (infigratinib), Ukoniq (umbralisib), and Zynlonta (loncastuximab tesirine-lpyl).
Skytrofa (lonapegsomatropin-tcgd) will not be described below because it is indicated to treat short stature and is unlikely to be used in pregnancy. Nextstellis (drospirenone and estetrol) is used to prevent pregnancy.
Typically, for new drugs there will be no published reports describing their use in pregnant women. That information will come much later. In the sections below, the indications, effects on pregnant animals, and the potential for harm of a fetus/embryo are described. However, the relevance of animal data to human pregnancies is not great.
Adbry (tralokinumab) (molecular weight [MW], 147 kilodaltons), is indicated for the treatment of moderate to severe atopic dermatitis in adult patients whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. The drug did not harm fetal monkeys at doses that were 10 times the maximum recommended human dose.
Besremi (ropeginterferon alfa-2b-njft) (MW, 60 kDa) is an interferon alfa-2b indicated for the treatment of adults with polycythemia vera. It is given by subcutaneous injection every 2 weeks. Animal studies assessing reproductive toxicity have not been conducted. The manufacturer states that the drug may cause fetal harm and should be assumed to have abortifacient potential.
Brexafemme (ibrexafungerp) (MW, 922) is indicated for the treatment of vulvovaginal candidiasis. The drug was teratogenic in pregnant rabbits but not in pregnant rats. The manufacturer recommends females with reproductive potential should use effective contraception during treatment and for 4 days after the final dose.
Bylvay (odevixibat) (MW unknown) is indicated for the treatment of pruritus in patients aged 3 months and older. There are no human data regarding its use in pregnant women. The drug was teratogenic in pregnant rabbits. Although there are no data, the drug has low absorption following oral administration and breastfeeding is not expected to result in exposure of the infant.
Empaveli (pegcetacoplan) (MW, 44 kDa) is used to treat paroxysmal nocturnal hemoglobinuria. When the drug was given to pregnant cynomolgus monkeys there was an increase in abortions and stillbirths.
Evkeeza (evinacumab-dgnb) (MW, 146k) is used to treat homozygous familial hypercholesterolemia. The drug was teratogenic in rabbits but not rats.
Fexinidazole (MW not specified) is indicated to treat human African trypanosomiasis caused by the parasite Trypanosoma brucei gambiense. Additional information not available.
Kerendia (finerenone) (MW, 378), is indicated to reduce the risk of kidney and heart complications in chronic kidney disease associated with type 2 diabetes. The drug was teratogenic in rats.
Korsuva (difelikefalin) (MW, 679) is a kappa opioid–receptor agonist indicated for the treatment of moderate to severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis. No adverse effects were observed in pregnant rats and rabbits. The limited human data on use of Korsuva in pregnant women are not sufficient to evaluate a drug associated risk for major birth defects or miscarriage.
Leqvio (inclisiran) (MW, 17,285) is indicated to treat heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease as an add-on therapy. The drug was not teratogenic in rats and rabbits.
Livmarli (maralixibat) (MW, 710) is indicated for the treatment of cholestatic pruritus associated with Alagille syndrome. Because systemic absorption is low, the recommended clinical dose is not expected to result in measurable fetal exposure. No effects on fetal rats were observed.
Livtencity (maribavir) (MW, 376) is used to treat posttransplant cytomegalovirus infection that has not responded to other treatment. Embryo/fetal survival was reduced in rats but not in rabbits at doses less then the human dose.
Lupkynis (voclosporin) (MW, 1,215) is used to treat nephritis. Avoid use of Lupkynis in pregnant women because of the alcohol content of the drug formulation. The drug was embryocidal and feticidal in rats and rabbits but with no treatment-related fetal malformations or variations.
Lybalvi (olanzapine and samidorphan) (MW, 312 and 505) is a combination drug used to treat schizophrenia and bipolar disorder. It was fetal toxic in pregnant rats and rabbits but with no evidence of malformations. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including this drug, during pregnancy. Health care providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit the Reproductive Psychiatry Resource and Information Center of the MGH Center for Women’s Mental Health.
Nexviazyme (avalglucosidase alfa-ngpt) (MW, 124k) is a hydrolytic lysosomal glycogen-specific enzyme indicated for the treatment of patients aged 1 year and older with late-onset Pompe disease. The drug was not teratogenic in mice and rabbits.
Nulibry (fosdenopterin) (MW, 480) is used to reduce the risk of mortality in molybdenum cofactor deficiency type A. Studies have not been conducted in pregnant animals.
Ponvory (ponesimod) (MW, 461) is used to treat relapsing forms of multiple sclerosis. The drug caused severe adverse effects in pregnant rats and rabbits.
Qulipta (atogepant) (MW, 604) is indicated to prevent episodic migraines. It is embryo/fetal toxic in rats and rabbits.
Saphnelo (anifrolumab-fnia) (MW, 148k) is used to treat moderate to severe systemic lupus erythematosus along with standard therapy. In pregnant cynomolgus monkeys, there was no evidence of embryotoxicity or fetal malformations with exposures up to approximately 28 times the exposure at the maximum recommended human dose.
Tavneos (avacopan) (MW, 582) is indicated to treat severe active antineutrophil cytoplasmic autoantibody–associated vasculitis in combination with standard therapy including glucocorticoids. There appears to be an increased risk for hepatotoxicity. The drug caused no defects in hamsters and rabbits, but in rabbits there was an increase in abortions.
Tezspire (tezepelumab-ekko) (MW, 147k) is indicated to treat severe asthma as an add-on maintenance therapy. No adverse fetal effects were observed in pregnant cynomolgus monkeys.
Verquvo (vericiguat) (MW, 426) is used to mitigate the risk of cardiovascular death and hospitalization for chronic heart failure. The drug was teratogenic in pregnant rabbits but not rats.
Vyvgart (efgartigimod alfa-fcab) (MW, 54k) is indicated to treat generalized myasthenia gravis. The drug did not cause birth defects in rats and rabbits.
Welireg (belzutifan) (MW, 383) is used to treat von Hippel–Lindau disease. In pregnant rats, the drug caused embryo-fetal lethality, reduced fetal body weight, and caused fetal skeletal malformations at maternal exposures of at least 0.2 times the human exposures.
Zegalogue (dasiglucagon) (MW, 3,382) is used to treat severe hypoglycemia. The drug did not cause birth defects in pregnant rats and rabbits.
Breastfeeding
It is not known if the above drugs will be in breast milk, but the safest course for an infant is to not breast feed if the mother is taking any of the above drugs.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
In 2021, the Food and Drug Administration approved 50 new drugs, but 24 will not be described here because they would probably not be used in pregnancy. The 24 are Aduhelm (aducanumab) to treat Alzheimer’s disease; Azstarys (serdexmethylphenidate and dexmethylphenidate), a combination CNS stimulant indicated for the treatment of ADHD; Cabenuva (cabotegravir and rilpivirine) to treat HIV; Voxzogo (vosoritide) for children with achondroplasia and open epiphyses; Qelbree (viloxazine) used in children aged 6-17 years to treat ADHD; and Pylarify (piflufolastat) for prostate cancer. Other anticancer drugs that will not be covered are Cosela (trilaciclib), Cytalux (pafolacianine), Exkivity (mobocertinib); Fotivda (tivozanib), Jemperli (dostarlimab-gxly), Lumakras (sotorasib), Pepaxto (melphalan flufenamide), Rybrevant (amivantamab-vmjw), Rylaze (asparaginase erwinia chrysanthemi), Scemblix (asciminib), Tepmetko (tepotinib), Tivdak (tisotumab vedotin-tftv), Truseltiq (infigratinib), Ukoniq (umbralisib), and Zynlonta (loncastuximab tesirine-lpyl).
Skytrofa (lonapegsomatropin-tcgd) will not be described below because it is indicated to treat short stature and is unlikely to be used in pregnancy. Nextstellis (drospirenone and estetrol) is used to prevent pregnancy.
Typically, for new drugs there will be no published reports describing their use in pregnant women. That information will come much later. In the sections below, the indications, effects on pregnant animals, and the potential for harm of a fetus/embryo are described. However, the relevance of animal data to human pregnancies is not great.
Adbry (tralokinumab) (molecular weight [MW], 147 kilodaltons), is indicated for the treatment of moderate to severe atopic dermatitis in adult patients whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. The drug did not harm fetal monkeys at doses that were 10 times the maximum recommended human dose.
Besremi (ropeginterferon alfa-2b-njft) (MW, 60 kDa) is an interferon alfa-2b indicated for the treatment of adults with polycythemia vera. It is given by subcutaneous injection every 2 weeks. Animal studies assessing reproductive toxicity have not been conducted. The manufacturer states that the drug may cause fetal harm and should be assumed to have abortifacient potential.
Brexafemme (ibrexafungerp) (MW, 922) is indicated for the treatment of vulvovaginal candidiasis. The drug was teratogenic in pregnant rabbits but not in pregnant rats. The manufacturer recommends females with reproductive potential should use effective contraception during treatment and for 4 days after the final dose.
Bylvay (odevixibat) (MW unknown) is indicated for the treatment of pruritus in patients aged 3 months and older. There are no human data regarding its use in pregnant women. The drug was teratogenic in pregnant rabbits. Although there are no data, the drug has low absorption following oral administration and breastfeeding is not expected to result in exposure of the infant.
Empaveli (pegcetacoplan) (MW, 44 kDa) is used to treat paroxysmal nocturnal hemoglobinuria. When the drug was given to pregnant cynomolgus monkeys there was an increase in abortions and stillbirths.
Evkeeza (evinacumab-dgnb) (MW, 146k) is used to treat homozygous familial hypercholesterolemia. The drug was teratogenic in rabbits but not rats.
Fexinidazole (MW not specified) is indicated to treat human African trypanosomiasis caused by the parasite Trypanosoma brucei gambiense. Additional information not available.
Kerendia (finerenone) (MW, 378), is indicated to reduce the risk of kidney and heart complications in chronic kidney disease associated with type 2 diabetes. The drug was teratogenic in rats.
Korsuva (difelikefalin) (MW, 679) is a kappa opioid–receptor agonist indicated for the treatment of moderate to severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis. No adverse effects were observed in pregnant rats and rabbits. The limited human data on use of Korsuva in pregnant women are not sufficient to evaluate a drug associated risk for major birth defects or miscarriage.
Leqvio (inclisiran) (MW, 17,285) is indicated to treat heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease as an add-on therapy. The drug was not teratogenic in rats and rabbits.
Livmarli (maralixibat) (MW, 710) is indicated for the treatment of cholestatic pruritus associated with Alagille syndrome. Because systemic absorption is low, the recommended clinical dose is not expected to result in measurable fetal exposure. No effects on fetal rats were observed.
Livtencity (maribavir) (MW, 376) is used to treat posttransplant cytomegalovirus infection that has not responded to other treatment. Embryo/fetal survival was reduced in rats but not in rabbits at doses less then the human dose.
Lupkynis (voclosporin) (MW, 1,215) is used to treat nephritis. Avoid use of Lupkynis in pregnant women because of the alcohol content of the drug formulation. The drug was embryocidal and feticidal in rats and rabbits but with no treatment-related fetal malformations or variations.
Lybalvi (olanzapine and samidorphan) (MW, 312 and 505) is a combination drug used to treat schizophrenia and bipolar disorder. It was fetal toxic in pregnant rats and rabbits but with no evidence of malformations. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including this drug, during pregnancy. Health care providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit the Reproductive Psychiatry Resource and Information Center of the MGH Center for Women’s Mental Health.
Nexviazyme (avalglucosidase alfa-ngpt) (MW, 124k) is a hydrolytic lysosomal glycogen-specific enzyme indicated for the treatment of patients aged 1 year and older with late-onset Pompe disease. The drug was not teratogenic in mice and rabbits.
Nulibry (fosdenopterin) (MW, 480) is used to reduce the risk of mortality in molybdenum cofactor deficiency type A. Studies have not been conducted in pregnant animals.
Ponvory (ponesimod) (MW, 461) is used to treat relapsing forms of multiple sclerosis. The drug caused severe adverse effects in pregnant rats and rabbits.
Qulipta (atogepant) (MW, 604) is indicated to prevent episodic migraines. It is embryo/fetal toxic in rats and rabbits.
Saphnelo (anifrolumab-fnia) (MW, 148k) is used to treat moderate to severe systemic lupus erythematosus along with standard therapy. In pregnant cynomolgus monkeys, there was no evidence of embryotoxicity or fetal malformations with exposures up to approximately 28 times the exposure at the maximum recommended human dose.
Tavneos (avacopan) (MW, 582) is indicated to treat severe active antineutrophil cytoplasmic autoantibody–associated vasculitis in combination with standard therapy including glucocorticoids. There appears to be an increased risk for hepatotoxicity. The drug caused no defects in hamsters and rabbits, but in rabbits there was an increase in abortions.
Tezspire (tezepelumab-ekko) (MW, 147k) is indicated to treat severe asthma as an add-on maintenance therapy. No adverse fetal effects were observed in pregnant cynomolgus monkeys.
Verquvo (vericiguat) (MW, 426) is used to mitigate the risk of cardiovascular death and hospitalization for chronic heart failure. The drug was teratogenic in pregnant rabbits but not rats.
Vyvgart (efgartigimod alfa-fcab) (MW, 54k) is indicated to treat generalized myasthenia gravis. The drug did not cause birth defects in rats and rabbits.
Welireg (belzutifan) (MW, 383) is used to treat von Hippel–Lindau disease. In pregnant rats, the drug caused embryo-fetal lethality, reduced fetal body weight, and caused fetal skeletal malformations at maternal exposures of at least 0.2 times the human exposures.
Zegalogue (dasiglucagon) (MW, 3,382) is used to treat severe hypoglycemia. The drug did not cause birth defects in pregnant rats and rabbits.
Breastfeeding
It is not known if the above drugs will be in breast milk, but the safest course for an infant is to not breast feed if the mother is taking any of the above drugs.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
In 2021, the Food and Drug Administration approved 50 new drugs, but 24 will not be described here because they would probably not be used in pregnancy. The 24 are Aduhelm (aducanumab) to treat Alzheimer’s disease; Azstarys (serdexmethylphenidate and dexmethylphenidate), a combination CNS stimulant indicated for the treatment of ADHD; Cabenuva (cabotegravir and rilpivirine) to treat HIV; Voxzogo (vosoritide) for children with achondroplasia and open epiphyses; Qelbree (viloxazine) used in children aged 6-17 years to treat ADHD; and Pylarify (piflufolastat) for prostate cancer. Other anticancer drugs that will not be covered are Cosela (trilaciclib), Cytalux (pafolacianine), Exkivity (mobocertinib); Fotivda (tivozanib), Jemperli (dostarlimab-gxly), Lumakras (sotorasib), Pepaxto (melphalan flufenamide), Rybrevant (amivantamab-vmjw), Rylaze (asparaginase erwinia chrysanthemi), Scemblix (asciminib), Tepmetko (tepotinib), Tivdak (tisotumab vedotin-tftv), Truseltiq (infigratinib), Ukoniq (umbralisib), and Zynlonta (loncastuximab tesirine-lpyl).
Skytrofa (lonapegsomatropin-tcgd) will not be described below because it is indicated to treat short stature and is unlikely to be used in pregnancy. Nextstellis (drospirenone and estetrol) is used to prevent pregnancy.
Typically, for new drugs there will be no published reports describing their use in pregnant women. That information will come much later. In the sections below, the indications, effects on pregnant animals, and the potential for harm of a fetus/embryo are described. However, the relevance of animal data to human pregnancies is not great.
Adbry (tralokinumab) (molecular weight [MW], 147 kilodaltons), is indicated for the treatment of moderate to severe atopic dermatitis in adult patients whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. The drug did not harm fetal monkeys at doses that were 10 times the maximum recommended human dose.
Besremi (ropeginterferon alfa-2b-njft) (MW, 60 kDa) is an interferon alfa-2b indicated for the treatment of adults with polycythemia vera. It is given by subcutaneous injection every 2 weeks. Animal studies assessing reproductive toxicity have not been conducted. The manufacturer states that the drug may cause fetal harm and should be assumed to have abortifacient potential.
Brexafemme (ibrexafungerp) (MW, 922) is indicated for the treatment of vulvovaginal candidiasis. The drug was teratogenic in pregnant rabbits but not in pregnant rats. The manufacturer recommends females with reproductive potential should use effective contraception during treatment and for 4 days after the final dose.
Bylvay (odevixibat) (MW unknown) is indicated for the treatment of pruritus in patients aged 3 months and older. There are no human data regarding its use in pregnant women. The drug was teratogenic in pregnant rabbits. Although there are no data, the drug has low absorption following oral administration and breastfeeding is not expected to result in exposure of the infant.
Empaveli (pegcetacoplan) (MW, 44 kDa) is used to treat paroxysmal nocturnal hemoglobinuria. When the drug was given to pregnant cynomolgus monkeys there was an increase in abortions and stillbirths.
Evkeeza (evinacumab-dgnb) (MW, 146k) is used to treat homozygous familial hypercholesterolemia. The drug was teratogenic in rabbits but not rats.
Fexinidazole (MW not specified) is indicated to treat human African trypanosomiasis caused by the parasite Trypanosoma brucei gambiense. Additional information not available.
Kerendia (finerenone) (MW, 378), is indicated to reduce the risk of kidney and heart complications in chronic kidney disease associated with type 2 diabetes. The drug was teratogenic in rats.
Korsuva (difelikefalin) (MW, 679) is a kappa opioid–receptor agonist indicated for the treatment of moderate to severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis. No adverse effects were observed in pregnant rats and rabbits. The limited human data on use of Korsuva in pregnant women are not sufficient to evaluate a drug associated risk for major birth defects or miscarriage.
Leqvio (inclisiran) (MW, 17,285) is indicated to treat heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease as an add-on therapy. The drug was not teratogenic in rats and rabbits.
Livmarli (maralixibat) (MW, 710) is indicated for the treatment of cholestatic pruritus associated with Alagille syndrome. Because systemic absorption is low, the recommended clinical dose is not expected to result in measurable fetal exposure. No effects on fetal rats were observed.
Livtencity (maribavir) (MW, 376) is used to treat posttransplant cytomegalovirus infection that has not responded to other treatment. Embryo/fetal survival was reduced in rats but not in rabbits at doses less then the human dose.
Lupkynis (voclosporin) (MW, 1,215) is used to treat nephritis. Avoid use of Lupkynis in pregnant women because of the alcohol content of the drug formulation. The drug was embryocidal and feticidal in rats and rabbits but with no treatment-related fetal malformations or variations.
Lybalvi (olanzapine and samidorphan) (MW, 312 and 505) is a combination drug used to treat schizophrenia and bipolar disorder. It was fetal toxic in pregnant rats and rabbits but with no evidence of malformations. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including this drug, during pregnancy. Health care providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit the Reproductive Psychiatry Resource and Information Center of the MGH Center for Women’s Mental Health.
Nexviazyme (avalglucosidase alfa-ngpt) (MW, 124k) is a hydrolytic lysosomal glycogen-specific enzyme indicated for the treatment of patients aged 1 year and older with late-onset Pompe disease. The drug was not teratogenic in mice and rabbits.
Nulibry (fosdenopterin) (MW, 480) is used to reduce the risk of mortality in molybdenum cofactor deficiency type A. Studies have not been conducted in pregnant animals.
Ponvory (ponesimod) (MW, 461) is used to treat relapsing forms of multiple sclerosis. The drug caused severe adverse effects in pregnant rats and rabbits.
Qulipta (atogepant) (MW, 604) is indicated to prevent episodic migraines. It is embryo/fetal toxic in rats and rabbits.
Saphnelo (anifrolumab-fnia) (MW, 148k) is used to treat moderate to severe systemic lupus erythematosus along with standard therapy. In pregnant cynomolgus monkeys, there was no evidence of embryotoxicity or fetal malformations with exposures up to approximately 28 times the exposure at the maximum recommended human dose.
Tavneos (avacopan) (MW, 582) is indicated to treat severe active antineutrophil cytoplasmic autoantibody–associated vasculitis in combination with standard therapy including glucocorticoids. There appears to be an increased risk for hepatotoxicity. The drug caused no defects in hamsters and rabbits, but in rabbits there was an increase in abortions.
Tezspire (tezepelumab-ekko) (MW, 147k) is indicated to treat severe asthma as an add-on maintenance therapy. No adverse fetal effects were observed in pregnant cynomolgus monkeys.
Verquvo (vericiguat) (MW, 426) is used to mitigate the risk of cardiovascular death and hospitalization for chronic heart failure. The drug was teratogenic in pregnant rabbits but not rats.
Vyvgart (efgartigimod alfa-fcab) (MW, 54k) is indicated to treat generalized myasthenia gravis. The drug did not cause birth defects in rats and rabbits.
Welireg (belzutifan) (MW, 383) is used to treat von Hippel–Lindau disease. In pregnant rats, the drug caused embryo-fetal lethality, reduced fetal body weight, and caused fetal skeletal malformations at maternal exposures of at least 0.2 times the human exposures.
Zegalogue (dasiglucagon) (MW, 3,382) is used to treat severe hypoglycemia. The drug did not cause birth defects in pregnant rats and rabbits.
Breastfeeding
It is not known if the above drugs will be in breast milk, but the safest course for an infant is to not breast feed if the mother is taking any of the above drugs.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].