User login
The Official Newspaper of the American Association for Thoracic Surgery
2016 AATS Scientific Achievement Award Honors Tirone E. David
Tirone E. David of the University of Toronto was presented with the 2016 AATS Scientific Achievement Award during the Annual Meeting Plenary Session on May 16th.
Dr. David (above left) with his award is shown with Dr. Irving Kron.
The award is the Association’s highest scientific recognition. Created in 1994, it recognizes physicians who have made extraordinary scientific contributions to the CT surgery field.
The honor acknowledges David’s pioneering work in CT surgery and his service as 85th AATS President (2004-2005). During an illustrious career, his innovation, passion and dedication to CT surgery has impacted hundreds of trainees and peers. The David operation revolutionized the treatment of aortic valve disease and resulted in substantial advances in patient care quality worldwide.
Tirone E. David of the University of Toronto was presented with the 2016 AATS Scientific Achievement Award during the Annual Meeting Plenary Session on May 16th.
Dr. David (above left) with his award is shown with Dr. Irving Kron.
The award is the Association’s highest scientific recognition. Created in 1994, it recognizes physicians who have made extraordinary scientific contributions to the CT surgery field.
The honor acknowledges David’s pioneering work in CT surgery and his service as 85th AATS President (2004-2005). During an illustrious career, his innovation, passion and dedication to CT surgery has impacted hundreds of trainees and peers. The David operation revolutionized the treatment of aortic valve disease and resulted in substantial advances in patient care quality worldwide.
Tirone E. David of the University of Toronto was presented with the 2016 AATS Scientific Achievement Award during the Annual Meeting Plenary Session on May 16th.
Dr. David (above left) with his award is shown with Dr. Irving Kron.
The award is the Association’s highest scientific recognition. Created in 1994, it recognizes physicians who have made extraordinary scientific contributions to the CT surgery field.
The honor acknowledges David’s pioneering work in CT surgery and his service as 85th AATS President (2004-2005). During an illustrious career, his innovation, passion and dedication to CT surgery has impacted hundreds of trainees and peers. The David operation revolutionized the treatment of aortic valve disease and resulted in substantial advances in patient care quality worldwide.
Meet the Newest Active AATS Members
At the Annual Meeting, 55 surgeons were elected as active AATS members:
George M. Alfieris (Rochester, NY)
Denis Bouchard (Montréal, Canada)
Ross M. Bremner (Phoenix, AZ)
Christian P. Brizard (Parkville, Australia)
Manuel Castella (Barcelona, Spain)
Renzo Cecere (Montréal, Canada)
Paul J. Chai (New York, NY)
Toyofumi F. Chen-Yoshikawa (Kyoto, Japan)
Francisco D.A. Costa (Curitiba, Brazil)
Philippe Demers (Montréal, Canada)
Benoit de Varennes (Montréal, Canada)
Roberto Di Bartolomeo (Bologna, Italy)
Nianguo Dong (Wuhan, China)
John R. Doty (Murray, UT)
Sitaram M. Emani (Boston, MA)
Jose I. Fragata (Lisbon, Portugal)
James J. Gangemi (Charlottesville, VA)
Isaac George (New York, NY)
Sebastien Gilbert (Ottawa, Canada)
Diego Gonzalez Rivas (Coruña, Spain)
Jie He (Beijing, China)
Tain-Yen Hsia (London, United Kingdom)
Aditya K. Kaza (Boston, MA)
Michael S. Kent (Boston, MA)
Zain I. Khalpey (Tucson, AZ)
Ahmet Kilic (Columbus, OH)
Joo Hyun Kim (Seoul, Republic of Korea)
Takushi Kohmoto (Madison,WI)
Buu-Khanh Lam (Ottawa, Canada)
Joseph Lamelas (Miami Beach, FL)
Hui Li (Beijing, China)
Brian E. Louie (Seattle, WA)
Giovanni Battista Luciani (Verona, Italy)
Shari L. Meyerson (Chicago, IL)
Siamak Mohammadi (Québec City, Canada)
Katie S. Nason (Pittsburgh, PA)
Shigeyuki Ozaki (Tokyo, Japan)
Amit N. Patel (Salt Lake City, UT)
Michel Pellerin (Montréal, Canada)
Mark D. Peterson (Toronto, Canada)
Eyal E. Porat (Houston, TX)
Michael F. Reed (Hershey, PA)
Kisaburo Sakamoto (Shizuoka, Japan)
Arash Salemi (New York, NY)
Norihiko Shiiya (Hamamatsu, Japan)
Hiroo Takayama (New York, NY)
Sachin Talwar (New Delhi, India)
Tomasz Timek (Grand Rapids, MI)
Joseph W. Turek (Iowa City, IA)
Pierre Voisine (Quebec, Canada)
Benny Weksler (Memphis, TN)
Grayson H. Wheatley (Philadelphia, PA)
Ronald K. Woods (Milwaukee, WI)
Hitoshi Yaku (Kyoto, Japan)
Tae-Jin Yun (Seoul, Republic of Korea)
At the Annual Meeting, 55 surgeons were elected as active AATS members:
George M. Alfieris (Rochester, NY)
Denis Bouchard (Montréal, Canada)
Ross M. Bremner (Phoenix, AZ)
Christian P. Brizard (Parkville, Australia)
Manuel Castella (Barcelona, Spain)
Renzo Cecere (Montréal, Canada)
Paul J. Chai (New York, NY)
Toyofumi F. Chen-Yoshikawa (Kyoto, Japan)
Francisco D.A. Costa (Curitiba, Brazil)
Philippe Demers (Montréal, Canada)
Benoit de Varennes (Montréal, Canada)
Roberto Di Bartolomeo (Bologna, Italy)
Nianguo Dong (Wuhan, China)
John R. Doty (Murray, UT)
Sitaram M. Emani (Boston, MA)
Jose I. Fragata (Lisbon, Portugal)
James J. Gangemi (Charlottesville, VA)
Isaac George (New York, NY)
Sebastien Gilbert (Ottawa, Canada)
Diego Gonzalez Rivas (Coruña, Spain)
Jie He (Beijing, China)
Tain-Yen Hsia (London, United Kingdom)
Aditya K. Kaza (Boston, MA)
Michael S. Kent (Boston, MA)
Zain I. Khalpey (Tucson, AZ)
Ahmet Kilic (Columbus, OH)
Joo Hyun Kim (Seoul, Republic of Korea)
Takushi Kohmoto (Madison,WI)
Buu-Khanh Lam (Ottawa, Canada)
Joseph Lamelas (Miami Beach, FL)
Hui Li (Beijing, China)
Brian E. Louie (Seattle, WA)
Giovanni Battista Luciani (Verona, Italy)
Shari L. Meyerson (Chicago, IL)
Siamak Mohammadi (Québec City, Canada)
Katie S. Nason (Pittsburgh, PA)
Shigeyuki Ozaki (Tokyo, Japan)
Amit N. Patel (Salt Lake City, UT)
Michel Pellerin (Montréal, Canada)
Mark D. Peterson (Toronto, Canada)
Eyal E. Porat (Houston, TX)
Michael F. Reed (Hershey, PA)
Kisaburo Sakamoto (Shizuoka, Japan)
Arash Salemi (New York, NY)
Norihiko Shiiya (Hamamatsu, Japan)
Hiroo Takayama (New York, NY)
Sachin Talwar (New Delhi, India)
Tomasz Timek (Grand Rapids, MI)
Joseph W. Turek (Iowa City, IA)
Pierre Voisine (Quebec, Canada)
Benny Weksler (Memphis, TN)
Grayson H. Wheatley (Philadelphia, PA)
Ronald K. Woods (Milwaukee, WI)
Hitoshi Yaku (Kyoto, Japan)
Tae-Jin Yun (Seoul, Republic of Korea)
At the Annual Meeting, 55 surgeons were elected as active AATS members:
George M. Alfieris (Rochester, NY)
Denis Bouchard (Montréal, Canada)
Ross M. Bremner (Phoenix, AZ)
Christian P. Brizard (Parkville, Australia)
Manuel Castella (Barcelona, Spain)
Renzo Cecere (Montréal, Canada)
Paul J. Chai (New York, NY)
Toyofumi F. Chen-Yoshikawa (Kyoto, Japan)
Francisco D.A. Costa (Curitiba, Brazil)
Philippe Demers (Montréal, Canada)
Benoit de Varennes (Montréal, Canada)
Roberto Di Bartolomeo (Bologna, Italy)
Nianguo Dong (Wuhan, China)
John R. Doty (Murray, UT)
Sitaram M. Emani (Boston, MA)
Jose I. Fragata (Lisbon, Portugal)
James J. Gangemi (Charlottesville, VA)
Isaac George (New York, NY)
Sebastien Gilbert (Ottawa, Canada)
Diego Gonzalez Rivas (Coruña, Spain)
Jie He (Beijing, China)
Tain-Yen Hsia (London, United Kingdom)
Aditya K. Kaza (Boston, MA)
Michael S. Kent (Boston, MA)
Zain I. Khalpey (Tucson, AZ)
Ahmet Kilic (Columbus, OH)
Joo Hyun Kim (Seoul, Republic of Korea)
Takushi Kohmoto (Madison,WI)
Buu-Khanh Lam (Ottawa, Canada)
Joseph Lamelas (Miami Beach, FL)
Hui Li (Beijing, China)
Brian E. Louie (Seattle, WA)
Giovanni Battista Luciani (Verona, Italy)
Shari L. Meyerson (Chicago, IL)
Siamak Mohammadi (Québec City, Canada)
Katie S. Nason (Pittsburgh, PA)
Shigeyuki Ozaki (Tokyo, Japan)
Amit N. Patel (Salt Lake City, UT)
Michel Pellerin (Montréal, Canada)
Mark D. Peterson (Toronto, Canada)
Eyal E. Porat (Houston, TX)
Michael F. Reed (Hershey, PA)
Kisaburo Sakamoto (Shizuoka, Japan)
Arash Salemi (New York, NY)
Norihiko Shiiya (Hamamatsu, Japan)
Hiroo Takayama (New York, NY)
Sachin Talwar (New Delhi, India)
Tomasz Timek (Grand Rapids, MI)
Joseph W. Turek (Iowa City, IA)
Pierre Voisine (Quebec, Canada)
Benny Weksler (Memphis, TN)
Grayson H. Wheatley (Philadelphia, PA)
Ronald K. Woods (Milwaukee, WI)
Hitoshi Yaku (Kyoto, Japan)
Tae-Jin Yun (Seoul, Republic of Korea)
Permanent pacemaker in TAVR: Earlier implantation costs much less
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
AT EUROPCR 2016
Key clinical point: The incremental cost of permanent pacemaker implantation more than 24 hours after transcatheter aortic valve replacement is almost twice as great as if the pacemaker goes in within 24 hours.
Major finding: The mean cost of hospitalization for transcatheter aortic valve replacement without permanent pacemaker implication in Medicare patients in 2014 was $63,136, compared with $80,441 if they needed a pacemaker.
Data source: This was a retrospective study of the health care costs and lengths of stay for all 12,148 hospitalizations for transcatheter aortic valve replacement in the Medicare inpatient database for 2014.
Disclosures: The presenter is an employee of Edwards Lifesciences, which funded the study.
Minimalist TAVR without on-site surgery under study
PARIS – The buzzword in transcatheter aortic valve replacement today is “minimalist.” The search is on for ways in which to safely simplify the procedure to reduce the current unsustainably high cost and improve the patient experience.
Among the elements typically involved in minimalist TAVR are performance of the procedure in the cardiac catheterization laboratory via transfemoral access rather than in the costlier operating room, use of conscious sedation rather than general anesthesia, transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients. But these are tepid measures compared with what’s under study in Germany.
The German health care system is engaged in a study of what has to be the ultimate in minimalist TAVR: doing it in hospitals without on-site cardiac surgery. And the short-term results in more than 1,300 German patients treated in such a setting look every bit as good as in patients whose procedure took place in hospitals more conventionally equipped with both cardiology and cardiothoracic surgery departments, Holger Eggebrecht, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Lack of a cardiac surgery department on site should not be regarded as a contraindication for TAVR,” concluded Dr. Eggebrecht of Agaplesion Bethanien Hospital in Frankfurt, Germany.
Of course, it is deemed an absolute contraindication to TAVR both in the current European Society of Cardiology and U.S. guidelines. But that position was developed in an earlier era when procedural safety had not yet been established. It was based on the expert consensus opinion of physicians drawn mostly from large tertiary centers with both cardiology and cardiac surgery on site. And this absolute contraindication was never supported by any data, he said.
In the United States, arguably the most litigious nation in the world, it’s virtually unthinkable – at least for now – to perform TAVR without a cardiothoracic surgeon on site in the event bailout emergency cardiac surgery should become necessary. But Germany, which boasts universal health care coverage at an affordable cost, operates differently. Indeed, in Dr. Eggebrecht’s study of all 17,919 transfemoral TAVR procedures performed in Germany during 2013 and 2014, fully 22 of the 77 hospitals where the procedures took place had no on-site cardiac surgery department.
He presented a comparison of in-hospital outcomes in the 1,332 German patients (7.4%) whose TAVR took place in hospitals without a cardiac surgery department and the 16,587 patients treated in hospitals with both cardiology and cardiac surgery departments. The data came from the prospective German Quality Assurance Registry on Aortic Valve Replacement, which records in extensive detail all TAVR and surgical replacements in the country. Participation in the registry is mandatory.
The main study finding: Even though patients at no–cardiac surgery hospitals were older, had more comorbid conditions, and were at higher predicted perioperative risk of mortality, the rates of intraprocedural complications, in-hospital strokes, and mortality were similar in the two groups.
Moreover, when Dr. Eggebrecht and coinvestigators performed a case-control substudy involving 555 patient pairs extensively matched on more than a dozen variables, including a requirement for identical scores on validated risk prediction tools for estimating in-hospital mortality, the results were the same as in the full study population.
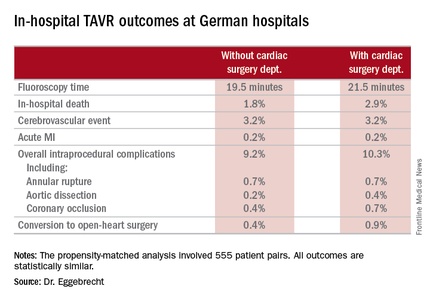
The key to the high-quality TAVR outcomes documented at hospitals without a cardiac surgery department, Dr. Eggebrecht emphasized, is that in Germany TAVR can be performed only at hospitals where a contractually obligated heart team has signed off on the procedure. At hospitals without a cardiac surgery department, this heart team is composed of in-house cardiologists and visiting cardiac surgeons from collaborating hospitals. In TAVRs at some of these hospitals, a collaborating cardiac surgeon is present for the procedure and brings along a heart-lung machine which is primed and ready to go, if needed, as was the case in just 0.4% of the 1,332 TAVRs. At others, the surgeon is off site.
“I would think our data show that close cooperation within the heart team is the key to successful outcomes, not having a cardiac surgeon on site,” he concluded.
Audience member Volkmar Falk, MD, strode briskly to a microphone and made no effort to hide his incredulity at this project.
“What is the real advantage in not having a surgeon present? I don’t get it,” declared Dr. Falk, professor and director of the department of cardiovascular and vascular surgery at Charité University Hospital in Berlin.
“For a surgeon, this is all quite difficult to understand,” he continued. “If a surgeon has to come to a TAVR rescue from 10 km away, I don’t know how well that works. And if surgeons have to travel with all their equipment in order to be on site, I think this is a logistical nightmare and shouldn’t be done at all.
“All of the studies, all the clinical trials we always discuss, have been done in the setting of hospitals where the procedure was done together with a surgeon on site. That’s why we have these excellent results,” Dr. Falk added.
Dr. Eggebrecht replied, “We’re having a scientific discussion here, and I think our data clearly show that you can construct a successful heart team even though you don’t have a cardiac surgery department on site.”
An Israeli cardiologist in the audience said a similar effort is underway in his country to open up TAVR to hospitals without an on-site cardiac surgeon. His objection is that, at least in Israel, a hospital with no on-site cardiac surgery is a marker for a TAVR center that is low volume, is late to embrace TAVR, and has cardiologists who are probably still early on the procedural learning curve.
Dr. Eggebrecht said that this is not the case in Germany, where some of the most experienced TAVR operators work at sites without a cardiac surgery department.
He reported that his study was partially funded by the German Cardiac Society, and he had no financial conflicts.
Simultaneously with his presentation, the study was published online (Eur Heart J. 2016 May 17. pii: ehw190).
PARIS – The buzzword in transcatheter aortic valve replacement today is “minimalist.” The search is on for ways in which to safely simplify the procedure to reduce the current unsustainably high cost and improve the patient experience.
Among the elements typically involved in minimalist TAVR are performance of the procedure in the cardiac catheterization laboratory via transfemoral access rather than in the costlier operating room, use of conscious sedation rather than general anesthesia, transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients. But these are tepid measures compared with what’s under study in Germany.
The German health care system is engaged in a study of what has to be the ultimate in minimalist TAVR: doing it in hospitals without on-site cardiac surgery. And the short-term results in more than 1,300 German patients treated in such a setting look every bit as good as in patients whose procedure took place in hospitals more conventionally equipped with both cardiology and cardiothoracic surgery departments, Holger Eggebrecht, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Lack of a cardiac surgery department on site should not be regarded as a contraindication for TAVR,” concluded Dr. Eggebrecht of Agaplesion Bethanien Hospital in Frankfurt, Germany.
Of course, it is deemed an absolute contraindication to TAVR both in the current European Society of Cardiology and U.S. guidelines. But that position was developed in an earlier era when procedural safety had not yet been established. It was based on the expert consensus opinion of physicians drawn mostly from large tertiary centers with both cardiology and cardiac surgery on site. And this absolute contraindication was never supported by any data, he said.
In the United States, arguably the most litigious nation in the world, it’s virtually unthinkable – at least for now – to perform TAVR without a cardiothoracic surgeon on site in the event bailout emergency cardiac surgery should become necessary. But Germany, which boasts universal health care coverage at an affordable cost, operates differently. Indeed, in Dr. Eggebrecht’s study of all 17,919 transfemoral TAVR procedures performed in Germany during 2013 and 2014, fully 22 of the 77 hospitals where the procedures took place had no on-site cardiac surgery department.
He presented a comparison of in-hospital outcomes in the 1,332 German patients (7.4%) whose TAVR took place in hospitals without a cardiac surgery department and the 16,587 patients treated in hospitals with both cardiology and cardiac surgery departments. The data came from the prospective German Quality Assurance Registry on Aortic Valve Replacement, which records in extensive detail all TAVR and surgical replacements in the country. Participation in the registry is mandatory.
The main study finding: Even though patients at no–cardiac surgery hospitals were older, had more comorbid conditions, and were at higher predicted perioperative risk of mortality, the rates of intraprocedural complications, in-hospital strokes, and mortality were similar in the two groups.
Moreover, when Dr. Eggebrecht and coinvestigators performed a case-control substudy involving 555 patient pairs extensively matched on more than a dozen variables, including a requirement for identical scores on validated risk prediction tools for estimating in-hospital mortality, the results were the same as in the full study population.

The key to the high-quality TAVR outcomes documented at hospitals without a cardiac surgery department, Dr. Eggebrecht emphasized, is that in Germany TAVR can be performed only at hospitals where a contractually obligated heart team has signed off on the procedure. At hospitals without a cardiac surgery department, this heart team is composed of in-house cardiologists and visiting cardiac surgeons from collaborating hospitals. In TAVRs at some of these hospitals, a collaborating cardiac surgeon is present for the procedure and brings along a heart-lung machine which is primed and ready to go, if needed, as was the case in just 0.4% of the 1,332 TAVRs. At others, the surgeon is off site.
“I would think our data show that close cooperation within the heart team is the key to successful outcomes, not having a cardiac surgeon on site,” he concluded.
Audience member Volkmar Falk, MD, strode briskly to a microphone and made no effort to hide his incredulity at this project.
“What is the real advantage in not having a surgeon present? I don’t get it,” declared Dr. Falk, professor and director of the department of cardiovascular and vascular surgery at Charité University Hospital in Berlin.
“For a surgeon, this is all quite difficult to understand,” he continued. “If a surgeon has to come to a TAVR rescue from 10 km away, I don’t know how well that works. And if surgeons have to travel with all their equipment in order to be on site, I think this is a logistical nightmare and shouldn’t be done at all.
“All of the studies, all the clinical trials we always discuss, have been done in the setting of hospitals where the procedure was done together with a surgeon on site. That’s why we have these excellent results,” Dr. Falk added.
Dr. Eggebrecht replied, “We’re having a scientific discussion here, and I think our data clearly show that you can construct a successful heart team even though you don’t have a cardiac surgery department on site.”
An Israeli cardiologist in the audience said a similar effort is underway in his country to open up TAVR to hospitals without an on-site cardiac surgeon. His objection is that, at least in Israel, a hospital with no on-site cardiac surgery is a marker for a TAVR center that is low volume, is late to embrace TAVR, and has cardiologists who are probably still early on the procedural learning curve.
Dr. Eggebrecht said that this is not the case in Germany, where some of the most experienced TAVR operators work at sites without a cardiac surgery department.
He reported that his study was partially funded by the German Cardiac Society, and he had no financial conflicts.
Simultaneously with his presentation, the study was published online (Eur Heart J. 2016 May 17. pii: ehw190).
PARIS – The buzzword in transcatheter aortic valve replacement today is “minimalist.” The search is on for ways in which to safely simplify the procedure to reduce the current unsustainably high cost and improve the patient experience.
Among the elements typically involved in minimalist TAVR are performance of the procedure in the cardiac catheterization laboratory via transfemoral access rather than in the costlier operating room, use of conscious sedation rather than general anesthesia, transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients. But these are tepid measures compared with what’s under study in Germany.
The German health care system is engaged in a study of what has to be the ultimate in minimalist TAVR: doing it in hospitals without on-site cardiac surgery. And the short-term results in more than 1,300 German patients treated in such a setting look every bit as good as in patients whose procedure took place in hospitals more conventionally equipped with both cardiology and cardiothoracic surgery departments, Holger Eggebrecht, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Lack of a cardiac surgery department on site should not be regarded as a contraindication for TAVR,” concluded Dr. Eggebrecht of Agaplesion Bethanien Hospital in Frankfurt, Germany.
Of course, it is deemed an absolute contraindication to TAVR both in the current European Society of Cardiology and U.S. guidelines. But that position was developed in an earlier era when procedural safety had not yet been established. It was based on the expert consensus opinion of physicians drawn mostly from large tertiary centers with both cardiology and cardiac surgery on site. And this absolute contraindication was never supported by any data, he said.
In the United States, arguably the most litigious nation in the world, it’s virtually unthinkable – at least for now – to perform TAVR without a cardiothoracic surgeon on site in the event bailout emergency cardiac surgery should become necessary. But Germany, which boasts universal health care coverage at an affordable cost, operates differently. Indeed, in Dr. Eggebrecht’s study of all 17,919 transfemoral TAVR procedures performed in Germany during 2013 and 2014, fully 22 of the 77 hospitals where the procedures took place had no on-site cardiac surgery department.
He presented a comparison of in-hospital outcomes in the 1,332 German patients (7.4%) whose TAVR took place in hospitals without a cardiac surgery department and the 16,587 patients treated in hospitals with both cardiology and cardiac surgery departments. The data came from the prospective German Quality Assurance Registry on Aortic Valve Replacement, which records in extensive detail all TAVR and surgical replacements in the country. Participation in the registry is mandatory.
The main study finding: Even though patients at no–cardiac surgery hospitals were older, had more comorbid conditions, and were at higher predicted perioperative risk of mortality, the rates of intraprocedural complications, in-hospital strokes, and mortality were similar in the two groups.
Moreover, when Dr. Eggebrecht and coinvestigators performed a case-control substudy involving 555 patient pairs extensively matched on more than a dozen variables, including a requirement for identical scores on validated risk prediction tools for estimating in-hospital mortality, the results were the same as in the full study population.

The key to the high-quality TAVR outcomes documented at hospitals without a cardiac surgery department, Dr. Eggebrecht emphasized, is that in Germany TAVR can be performed only at hospitals where a contractually obligated heart team has signed off on the procedure. At hospitals without a cardiac surgery department, this heart team is composed of in-house cardiologists and visiting cardiac surgeons from collaborating hospitals. In TAVRs at some of these hospitals, a collaborating cardiac surgeon is present for the procedure and brings along a heart-lung machine which is primed and ready to go, if needed, as was the case in just 0.4% of the 1,332 TAVRs. At others, the surgeon is off site.
“I would think our data show that close cooperation within the heart team is the key to successful outcomes, not having a cardiac surgeon on site,” he concluded.
Audience member Volkmar Falk, MD, strode briskly to a microphone and made no effort to hide his incredulity at this project.
“What is the real advantage in not having a surgeon present? I don’t get it,” declared Dr. Falk, professor and director of the department of cardiovascular and vascular surgery at Charité University Hospital in Berlin.
“For a surgeon, this is all quite difficult to understand,” he continued. “If a surgeon has to come to a TAVR rescue from 10 km away, I don’t know how well that works. And if surgeons have to travel with all their equipment in order to be on site, I think this is a logistical nightmare and shouldn’t be done at all.
“All of the studies, all the clinical trials we always discuss, have been done in the setting of hospitals where the procedure was done together with a surgeon on site. That’s why we have these excellent results,” Dr. Falk added.
Dr. Eggebrecht replied, “We’re having a scientific discussion here, and I think our data clearly show that you can construct a successful heart team even though you don’t have a cardiac surgery department on site.”
An Israeli cardiologist in the audience said a similar effort is underway in his country to open up TAVR to hospitals without an on-site cardiac surgeon. His objection is that, at least in Israel, a hospital with no on-site cardiac surgery is a marker for a TAVR center that is low volume, is late to embrace TAVR, and has cardiologists who are probably still early on the procedural learning curve.
Dr. Eggebrecht said that this is not the case in Germany, where some of the most experienced TAVR operators work at sites without a cardiac surgery department.
He reported that his study was partially funded by the German Cardiac Society, and he had no financial conflicts.
Simultaneously with his presentation, the study was published online (Eur Heart J. 2016 May 17. pii: ehw190).
AT EUROPCR 2016
Key clinical point: TAVR can be performed in hospitals safely without a cardiac surgery department.
Major finding: In-hospital mortality occurred in 3.8% of patients who underwent TAVR at hospitals without on-site cardiac surgery and in 4.2% of patients whose procedure was done at hospitals with a cardiac surgery department.
Data source: An analysis of in-hospital outcomes of all 17,919 patients who underwent TAVR in Germany during 2013 and 2014, including 1,332 whose procedures took place at one of the 22 hospitals with no on-site cardiac surgery department.
Disclosures: The study was partially funded by the German Cardiac Society. The presenter reported having no financial conflicts.
Early results positive for nivolumab as first-line therapy in advanced NSCLC
The PD-1 immune checkpoint inhibitor nivolumab may be safe and effective as a first-line therapy in adult patients with non–small cell lung cancer (NSCLC), according to the results of the phase I CheckMate 012 trial.
Of 52 adult patients with advanced NSCLC who received nivolumab, 19% experienced grade three or four adverse events, and the overall response rate was 23% with four ongoing complete responses, reported Scott Gettinger, MD, of the Yale Cancer Center, New Haven, Conn., and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9929).
In the study cohort, 94% had stage IV NSCLC, 79% were former or current smokers, and 65% had received either radiotherapy, adjuvant or neoadjuvant chemotherapy.
Treatment-related adverse events were reported in 71% of patients, the most common being fatigue (29%), rash (19%), and nausea (14%). Grade 3 or 4 adverse events including rash, cardiac failure, and lung infection occurred in 10 patients (19%). There were no treatment-related deaths, but adverse events led to the discontinuation of the drug treatment in six patients.
Responses to nivolumab (overall response rate, 23%) were durable with duration of responses ranging from 4.2 to 25.8 months. In addition, the median overall survival was 19.4 months, median progression-free survival was 3.5 months, and the 24-week progression-free survival rate was 31% (95% confidence interval, 28%-60%).
Forty-six patients had tumor specimens evaluable for PD-L1 expression. Clinical activity was observed regardless of PD-L1 expression, the investigators reported. However, the overall response rate was higher in patients with tumors that expressed PD-L1, compared with non-PD-L1-expressing tumors.
All investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock in multiple companies including Bristol-Myers Squibb, which funded the study.
On Twitter @jessnicolecraig
The PD-1 immune checkpoint inhibitor nivolumab may be safe and effective as a first-line therapy in adult patients with non–small cell lung cancer (NSCLC), according to the results of the phase I CheckMate 012 trial.
Of 52 adult patients with advanced NSCLC who received nivolumab, 19% experienced grade three or four adverse events, and the overall response rate was 23% with four ongoing complete responses, reported Scott Gettinger, MD, of the Yale Cancer Center, New Haven, Conn., and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9929).
In the study cohort, 94% had stage IV NSCLC, 79% were former or current smokers, and 65% had received either radiotherapy, adjuvant or neoadjuvant chemotherapy.
Treatment-related adverse events were reported in 71% of patients, the most common being fatigue (29%), rash (19%), and nausea (14%). Grade 3 or 4 adverse events including rash, cardiac failure, and lung infection occurred in 10 patients (19%). There were no treatment-related deaths, but adverse events led to the discontinuation of the drug treatment in six patients.
Responses to nivolumab (overall response rate, 23%) were durable with duration of responses ranging from 4.2 to 25.8 months. In addition, the median overall survival was 19.4 months, median progression-free survival was 3.5 months, and the 24-week progression-free survival rate was 31% (95% confidence interval, 28%-60%).
Forty-six patients had tumor specimens evaluable for PD-L1 expression. Clinical activity was observed regardless of PD-L1 expression, the investigators reported. However, the overall response rate was higher in patients with tumors that expressed PD-L1, compared with non-PD-L1-expressing tumors.
All investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock in multiple companies including Bristol-Myers Squibb, which funded the study.
On Twitter @jessnicolecraig
The PD-1 immune checkpoint inhibitor nivolumab may be safe and effective as a first-line therapy in adult patients with non–small cell lung cancer (NSCLC), according to the results of the phase I CheckMate 012 trial.
Of 52 adult patients with advanced NSCLC who received nivolumab, 19% experienced grade three or four adverse events, and the overall response rate was 23% with four ongoing complete responses, reported Scott Gettinger, MD, of the Yale Cancer Center, New Haven, Conn., and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9929).
In the study cohort, 94% had stage IV NSCLC, 79% were former or current smokers, and 65% had received either radiotherapy, adjuvant or neoadjuvant chemotherapy.
Treatment-related adverse events were reported in 71% of patients, the most common being fatigue (29%), rash (19%), and nausea (14%). Grade 3 or 4 adverse events including rash, cardiac failure, and lung infection occurred in 10 patients (19%). There were no treatment-related deaths, but adverse events led to the discontinuation of the drug treatment in six patients.
Responses to nivolumab (overall response rate, 23%) were durable with duration of responses ranging from 4.2 to 25.8 months. In addition, the median overall survival was 19.4 months, median progression-free survival was 3.5 months, and the 24-week progression-free survival rate was 31% (95% confidence interval, 28%-60%).
Forty-six patients had tumor specimens evaluable for PD-L1 expression. Clinical activity was observed regardless of PD-L1 expression, the investigators reported. However, the overall response rate was higher in patients with tumors that expressed PD-L1, compared with non-PD-L1-expressing tumors.
All investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock in multiple companies including Bristol-Myers Squibb, which funded the study.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A phase I trial indicates that nivolumab is safe and shows durable activity as a first-line therapy in treating patients with advanced NSCLC.
Major finding: Grade 3 or 4 adverse events were reported in 19% of patients. The overall response rate was 23% with four ongoing complete responses.
Data source: A phase I trial of 52 patients with stage 3 or 4 non–small-cell lung cancer.
Disclosures: All investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock interest in multiple companies including Bristol-Myers Squibb, which funded the study.
Tool kit improves communication after an adverse event
The Communication and Optimal Resolution tool kit, offered by the Agency for Healthcare Research and Quality, helps hospitals, health systems, and clinicians respond to patients who are harmed by the care they receive, Andy Bindman, MD, AHRQ director, said in a blog post.
The tool kit is a new addition to AHRQ’s suite of patient safety tools and training materials.
Poor communication can lead to life-and-death mistakes, which can then become legal issues, said Dr. Bindman. The Communication and Optimal Resolution (CANDOR) tool kit uses time as a key factor by disclosing harm to patients and families as soon as it happens.
“It has been estimated that medical errors are the third-leading cause of death in the United States and that the majority of clinicians have experience with a medical error that resulted in harm to a patient. Often these ‘mistakes’ are not the result of poorly trained individuals but the result of the faulty systems we sometimes work in,” Dr. Bindman noted.
“It is also important for us to engage with colleagues to reflect on the mistake and explore the root causes of how it happened. This helps us to learn from the situation and take steps to minimize the chances of a similar mistake happening again to another patient,” he said.
Funding for CANDOR was provided by the AHRQ’s $23 million Patient Safety and Medical Liability grant initiative, launched in 2009. The initiative is the largest federal investment in research linking improved patient safety to reduced medical liability, according to Dr. Bindman.
The Communication and Optimal Resolution tool kit, offered by the Agency for Healthcare Research and Quality, helps hospitals, health systems, and clinicians respond to patients who are harmed by the care they receive, Andy Bindman, MD, AHRQ director, said in a blog post.
The tool kit is a new addition to AHRQ’s suite of patient safety tools and training materials.
Poor communication can lead to life-and-death mistakes, which can then become legal issues, said Dr. Bindman. The Communication and Optimal Resolution (CANDOR) tool kit uses time as a key factor by disclosing harm to patients and families as soon as it happens.
“It has been estimated that medical errors are the third-leading cause of death in the United States and that the majority of clinicians have experience with a medical error that resulted in harm to a patient. Often these ‘mistakes’ are not the result of poorly trained individuals but the result of the faulty systems we sometimes work in,” Dr. Bindman noted.
“It is also important for us to engage with colleagues to reflect on the mistake and explore the root causes of how it happened. This helps us to learn from the situation and take steps to minimize the chances of a similar mistake happening again to another patient,” he said.
Funding for CANDOR was provided by the AHRQ’s $23 million Patient Safety and Medical Liability grant initiative, launched in 2009. The initiative is the largest federal investment in research linking improved patient safety to reduced medical liability, according to Dr. Bindman.
The Communication and Optimal Resolution tool kit, offered by the Agency for Healthcare Research and Quality, helps hospitals, health systems, and clinicians respond to patients who are harmed by the care they receive, Andy Bindman, MD, AHRQ director, said in a blog post.
The tool kit is a new addition to AHRQ’s suite of patient safety tools and training materials.
Poor communication can lead to life-and-death mistakes, which can then become legal issues, said Dr. Bindman. The Communication and Optimal Resolution (CANDOR) tool kit uses time as a key factor by disclosing harm to patients and families as soon as it happens.
“It has been estimated that medical errors are the third-leading cause of death in the United States and that the majority of clinicians have experience with a medical error that resulted in harm to a patient. Often these ‘mistakes’ are not the result of poorly trained individuals but the result of the faulty systems we sometimes work in,” Dr. Bindman noted.
“It is also important for us to engage with colleagues to reflect on the mistake and explore the root causes of how it happened. This helps us to learn from the situation and take steps to minimize the chances of a similar mistake happening again to another patient,” he said.
Funding for CANDOR was provided by the AHRQ’s $23 million Patient Safety and Medical Liability grant initiative, launched in 2009. The initiative is the largest federal investment in research linking improved patient safety to reduced medical liability, according to Dr. Bindman.
FDA panel narrowly endorses empagliflozin’s cardiovascular mortality benefit
ROCKVILLE, MD. – In a 12-11 vote, a Food and Drug Administration advisory panel just barely came down in favor of the agency adding a new labeling entry to the already-approved diabetes drug empagliflozin (Jardiance) that would say the drug reduces cardiovascular mortality.
While several members of the panel wished the FDA’s staff good luck in weighing both the evidence and the advisory committee’s closely split endorsement when deciding whether to grant this unprecedented labeling to a diabetes drug, the fact that a majority of panelists favored this course marked a watershed moment in the development of new agents for treating hyperglycemia.
“It’s the first time we have evidence that a diabetes drug can reduce cardiovascular risk. That’s never been seen before, and it’s huge,” said Marvin A. Konstam, MD, a temporary member of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) and chief physician executive of the cardiovascular center at Tufts Medical Center in Boston. “The question is whether or not this effect is real, and the vote was 50-50, but I think there is a good chance it’s real, and, if so, it’s a game changer,” Dr. Konstam said in an interview. He voted in favor of the new labeling, and, like many of his colleagues on the panel, he admitted to agonizing over the decision during the postvote comment period.
What he and the other committee members struggled with was a remarkably strong effect by empagliflozin on reducing cardiovascular mortality by a relative 38%, compared with placebo, in more than 7,000 patients with type 2 diabetes selected for their high cardiovascular disease risk. The major sticking point was that the study enrolled patients into a randomized, placebo-controlled trial that was primarily designed to test the drug’s cardiovascular safety and not its efficacy, and where cardiovascular death was not even a prespecified secondary endpoint.
“It’s very hard to go from safety to superiority in one study,” said Peter W.F. Wilson, MD, who voted against the added indication. Like many panel members who voted no, Dr. Wilson said that any claim to preventing cardiovascular mortality with empagliflozin should meet the standard FDA requirement to have consistent results from at least two studies. “This is the first drug in its class [the sodium-glucose cotransporter 2 inhibitors], and we should have a high bar for the quality of the evidence,” said Dr. Wilson, professor of medicine and public health at Emory University in Atlanta.
“There is substantial evidence [to support the mortality claim], but not yet to the extent to put it on the label,” said another voter on the no side, Judith Fradkin, MD, also a temporary committee member and director of the division of diabetes, endocrinology and metabolic diseases at the National Institutes of Health. The data collected so far in favor of the mortality claim “are very compelling, but what I couldn’t get past is my long-standing belief that a positive result to a study’s secondary outcome is hypothesis generating. We need a second study to put this on the label,” Dr. Fradkin said.
That dramatic and highly meaningful clinical effect of empagliflozin on cardiovascular mortality jumped out at the investigators who ran the EMPA-REG OUTCOME trial as well as to many others from the moment the results had their unveiling less than a year ago, at the annual meeting of the European Association for the Study of Diabetes in Stockholm, and in a concurrently published article (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The primary efficacy endpoint placed into the EMPA-REG OUTCOME safety trial as the study developed following the 2008 FDA mandate for cardiovascular safety trials for all new hypoglycemic drugs was a three-part, combined-outcome endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke. Although this primary, combined endpoint had a statistically significant but much more modest benefit with a 14% relative risk reduction, compared with placebo, “the benefit was all driven by the reduction in cardiovascular death that had an astonishing P value of less than .0001 with no suggestion of benefit or risk for MI or stroke,” said Stuart Pocock, PhD, professor of medical statistics at the London School of Hygiene and Tropical Medicine who appeared before the committee as a consultant brought in by the applicant, Boehringer Ingelheim.
The total 309 cardiovascular deaths seen during the study that led to this finding provided more data than most cardiovascular trials, and while in some respects, the cardiovascular benefit seemed “too good to be true,” it also turned out that “the data were so strong that they overwhelm skepticism,” Dr. Pocock told the panel while presenting some advanced statistical test results to prove this assertion. The trial results showed “overwhelming evidence of benefit, beyond a reasonable doubt,” and while cardiovascular death was just one part of the efficacy endpoint, “mortality merits special attention,” he said. The statistical analyses also showed an equally robust 32% relative risk reduction in all-cause mortality, and both the cardiovascular and all-cause death benefits seen in EMPA-REG OUTCOME were consistent across both dosages of empagliflozin tested in the study (10 mg and 25 mg daily) and across the sensitivity analyses applied by the investigators.
“These are convincing data, but I’m not comfortable enough that these robust data would be reproduced in a second trial,” said panel chair Robert J. Smith, MD, who voted against the indication.
An additional limitation acting against the proposed new labeling, according to several panel members, is that the mechanism by which empagliflozin might exert protection against cardiovascular death remains unknown, with no suggestion in the trial results that it acts by protecting patients against ischemic disease.
Current opinion also splits among clinicians on how empagliflozin, which has had FDA approval since 2014 as an option for treating type 2 diabetes, should be used in routine practice to treat diabetes patients with high cardiovascular risk who match those enrolled in the EMPA-REG OUTCOME trial. Dr. Smith urged a cautious approach.
“I think it’s important [for prescribers] to wait to hear from the FDA. If the cardiovascular mortality benefit was proven, then it would be an important option given the magnitude of cardiovascular disease and death as a consequence of type 2 diabetes. But people should be cautious in drawing their own interpretations of the data,” Dr. Smith, professor of medicine at Brown University in Providence, R.I., said in an interview. For the time being, metformin remains the top oral drug for most of these patients because of its proven effectiveness and low cost, he added.
But others have already been active in prescribing empagliflozin to at-risk patients with type 2 diabetes based on last year’s EMPA-REG OUTCOME report.
“I am using it in addition to metformin and aggressive lifestyle changes in patients with established cardiovascular disease and uncontrolled type 2 diabetes,” commented Alison L. Bailey, MD, a cardiologist at the Erlanger Health System and University of Tennessee in Chattanooga. “A patient’s health insurance status must be taken into account as empagliflozin can be a significant financial burden, but if all other things are equal and cost is not prohibitive, I am definitely using this in my patients with type 2 diabetes and cardiovascular disease. I think there are enough data to warrant its use first line in patients who can get the drug without a financial burden,” she said in an interview.
Dr. Konstam cautioned that “just because empagliflozin may have a cardiovascular effect does not make it a cardiovascular drug. As a cardiologist I am not comfortable prescribing this drug. When it comes to diabetes management ,you need to take many things into consideration, most notably blood sugar and hemoglobin A1c,” which are usually best managed by a diabetologist or experienced primary care physician, he said.
Dr. Konstam, Dr. Wilson, Dr. Fradkin, and Dr. Smith had no relevant financial disclosures. Dr. Pocock is a consultant to Boehringer Ingelheim. Dr. Bailey has received research grants from CSL Behring.
On Twitter@mitchelzoler
ROCKVILLE, MD. – In a 12-11 vote, a Food and Drug Administration advisory panel just barely came down in favor of the agency adding a new labeling entry to the already-approved diabetes drug empagliflozin (Jardiance) that would say the drug reduces cardiovascular mortality.
While several members of the panel wished the FDA’s staff good luck in weighing both the evidence and the advisory committee’s closely split endorsement when deciding whether to grant this unprecedented labeling to a diabetes drug, the fact that a majority of panelists favored this course marked a watershed moment in the development of new agents for treating hyperglycemia.
“It’s the first time we have evidence that a diabetes drug can reduce cardiovascular risk. That’s never been seen before, and it’s huge,” said Marvin A. Konstam, MD, a temporary member of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) and chief physician executive of the cardiovascular center at Tufts Medical Center in Boston. “The question is whether or not this effect is real, and the vote was 50-50, but I think there is a good chance it’s real, and, if so, it’s a game changer,” Dr. Konstam said in an interview. He voted in favor of the new labeling, and, like many of his colleagues on the panel, he admitted to agonizing over the decision during the postvote comment period.
What he and the other committee members struggled with was a remarkably strong effect by empagliflozin on reducing cardiovascular mortality by a relative 38%, compared with placebo, in more than 7,000 patients with type 2 diabetes selected for their high cardiovascular disease risk. The major sticking point was that the study enrolled patients into a randomized, placebo-controlled trial that was primarily designed to test the drug’s cardiovascular safety and not its efficacy, and where cardiovascular death was not even a prespecified secondary endpoint.
“It’s very hard to go from safety to superiority in one study,” said Peter W.F. Wilson, MD, who voted against the added indication. Like many panel members who voted no, Dr. Wilson said that any claim to preventing cardiovascular mortality with empagliflozin should meet the standard FDA requirement to have consistent results from at least two studies. “This is the first drug in its class [the sodium-glucose cotransporter 2 inhibitors], and we should have a high bar for the quality of the evidence,” said Dr. Wilson, professor of medicine and public health at Emory University in Atlanta.
“There is substantial evidence [to support the mortality claim], but not yet to the extent to put it on the label,” said another voter on the no side, Judith Fradkin, MD, also a temporary committee member and director of the division of diabetes, endocrinology and metabolic diseases at the National Institutes of Health. The data collected so far in favor of the mortality claim “are very compelling, but what I couldn’t get past is my long-standing belief that a positive result to a study’s secondary outcome is hypothesis generating. We need a second study to put this on the label,” Dr. Fradkin said.
That dramatic and highly meaningful clinical effect of empagliflozin on cardiovascular mortality jumped out at the investigators who ran the EMPA-REG OUTCOME trial as well as to many others from the moment the results had their unveiling less than a year ago, at the annual meeting of the European Association for the Study of Diabetes in Stockholm, and in a concurrently published article (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The primary efficacy endpoint placed into the EMPA-REG OUTCOME safety trial as the study developed following the 2008 FDA mandate for cardiovascular safety trials for all new hypoglycemic drugs was a three-part, combined-outcome endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke. Although this primary, combined endpoint had a statistically significant but much more modest benefit with a 14% relative risk reduction, compared with placebo, “the benefit was all driven by the reduction in cardiovascular death that had an astonishing P value of less than .0001 with no suggestion of benefit or risk for MI or stroke,” said Stuart Pocock, PhD, professor of medical statistics at the London School of Hygiene and Tropical Medicine who appeared before the committee as a consultant brought in by the applicant, Boehringer Ingelheim.
The total 309 cardiovascular deaths seen during the study that led to this finding provided more data than most cardiovascular trials, and while in some respects, the cardiovascular benefit seemed “too good to be true,” it also turned out that “the data were so strong that they overwhelm skepticism,” Dr. Pocock told the panel while presenting some advanced statistical test results to prove this assertion. The trial results showed “overwhelming evidence of benefit, beyond a reasonable doubt,” and while cardiovascular death was just one part of the efficacy endpoint, “mortality merits special attention,” he said. The statistical analyses also showed an equally robust 32% relative risk reduction in all-cause mortality, and both the cardiovascular and all-cause death benefits seen in EMPA-REG OUTCOME were consistent across both dosages of empagliflozin tested in the study (10 mg and 25 mg daily) and across the sensitivity analyses applied by the investigators.
“These are convincing data, but I’m not comfortable enough that these robust data would be reproduced in a second trial,” said panel chair Robert J. Smith, MD, who voted against the indication.
An additional limitation acting against the proposed new labeling, according to several panel members, is that the mechanism by which empagliflozin might exert protection against cardiovascular death remains unknown, with no suggestion in the trial results that it acts by protecting patients against ischemic disease.
Current opinion also splits among clinicians on how empagliflozin, which has had FDA approval since 2014 as an option for treating type 2 diabetes, should be used in routine practice to treat diabetes patients with high cardiovascular risk who match those enrolled in the EMPA-REG OUTCOME trial. Dr. Smith urged a cautious approach.
“I think it’s important [for prescribers] to wait to hear from the FDA. If the cardiovascular mortality benefit was proven, then it would be an important option given the magnitude of cardiovascular disease and death as a consequence of type 2 diabetes. But people should be cautious in drawing their own interpretations of the data,” Dr. Smith, professor of medicine at Brown University in Providence, R.I., said in an interview. For the time being, metformin remains the top oral drug for most of these patients because of its proven effectiveness and low cost, he added.
But others have already been active in prescribing empagliflozin to at-risk patients with type 2 diabetes based on last year’s EMPA-REG OUTCOME report.
“I am using it in addition to metformin and aggressive lifestyle changes in patients with established cardiovascular disease and uncontrolled type 2 diabetes,” commented Alison L. Bailey, MD, a cardiologist at the Erlanger Health System and University of Tennessee in Chattanooga. “A patient’s health insurance status must be taken into account as empagliflozin can be a significant financial burden, but if all other things are equal and cost is not prohibitive, I am definitely using this in my patients with type 2 diabetes and cardiovascular disease. I think there are enough data to warrant its use first line in patients who can get the drug without a financial burden,” she said in an interview.
Dr. Konstam cautioned that “just because empagliflozin may have a cardiovascular effect does not make it a cardiovascular drug. As a cardiologist I am not comfortable prescribing this drug. When it comes to diabetes management ,you need to take many things into consideration, most notably blood sugar and hemoglobin A1c,” which are usually best managed by a diabetologist or experienced primary care physician, he said.
Dr. Konstam, Dr. Wilson, Dr. Fradkin, and Dr. Smith had no relevant financial disclosures. Dr. Pocock is a consultant to Boehringer Ingelheim. Dr. Bailey has received research grants from CSL Behring.
On Twitter@mitchelzoler
ROCKVILLE, MD. – In a 12-11 vote, a Food and Drug Administration advisory panel just barely came down in favor of the agency adding a new labeling entry to the already-approved diabetes drug empagliflozin (Jardiance) that would say the drug reduces cardiovascular mortality.
While several members of the panel wished the FDA’s staff good luck in weighing both the evidence and the advisory committee’s closely split endorsement when deciding whether to grant this unprecedented labeling to a diabetes drug, the fact that a majority of panelists favored this course marked a watershed moment in the development of new agents for treating hyperglycemia.
“It’s the first time we have evidence that a diabetes drug can reduce cardiovascular risk. That’s never been seen before, and it’s huge,” said Marvin A. Konstam, MD, a temporary member of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) and chief physician executive of the cardiovascular center at Tufts Medical Center in Boston. “The question is whether or not this effect is real, and the vote was 50-50, but I think there is a good chance it’s real, and, if so, it’s a game changer,” Dr. Konstam said in an interview. He voted in favor of the new labeling, and, like many of his colleagues on the panel, he admitted to agonizing over the decision during the postvote comment period.
What he and the other committee members struggled with was a remarkably strong effect by empagliflozin on reducing cardiovascular mortality by a relative 38%, compared with placebo, in more than 7,000 patients with type 2 diabetes selected for their high cardiovascular disease risk. The major sticking point was that the study enrolled patients into a randomized, placebo-controlled trial that was primarily designed to test the drug’s cardiovascular safety and not its efficacy, and where cardiovascular death was not even a prespecified secondary endpoint.
“It’s very hard to go from safety to superiority in one study,” said Peter W.F. Wilson, MD, who voted against the added indication. Like many panel members who voted no, Dr. Wilson said that any claim to preventing cardiovascular mortality with empagliflozin should meet the standard FDA requirement to have consistent results from at least two studies. “This is the first drug in its class [the sodium-glucose cotransporter 2 inhibitors], and we should have a high bar for the quality of the evidence,” said Dr. Wilson, professor of medicine and public health at Emory University in Atlanta.
“There is substantial evidence [to support the mortality claim], but not yet to the extent to put it on the label,” said another voter on the no side, Judith Fradkin, MD, also a temporary committee member and director of the division of diabetes, endocrinology and metabolic diseases at the National Institutes of Health. The data collected so far in favor of the mortality claim “are very compelling, but what I couldn’t get past is my long-standing belief that a positive result to a study’s secondary outcome is hypothesis generating. We need a second study to put this on the label,” Dr. Fradkin said.
That dramatic and highly meaningful clinical effect of empagliflozin on cardiovascular mortality jumped out at the investigators who ran the EMPA-REG OUTCOME trial as well as to many others from the moment the results had their unveiling less than a year ago, at the annual meeting of the European Association for the Study of Diabetes in Stockholm, and in a concurrently published article (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The primary efficacy endpoint placed into the EMPA-REG OUTCOME safety trial as the study developed following the 2008 FDA mandate for cardiovascular safety trials for all new hypoglycemic drugs was a three-part, combined-outcome endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke. Although this primary, combined endpoint had a statistically significant but much more modest benefit with a 14% relative risk reduction, compared with placebo, “the benefit was all driven by the reduction in cardiovascular death that had an astonishing P value of less than .0001 with no suggestion of benefit or risk for MI or stroke,” said Stuart Pocock, PhD, professor of medical statistics at the London School of Hygiene and Tropical Medicine who appeared before the committee as a consultant brought in by the applicant, Boehringer Ingelheim.
The total 309 cardiovascular deaths seen during the study that led to this finding provided more data than most cardiovascular trials, and while in some respects, the cardiovascular benefit seemed “too good to be true,” it also turned out that “the data were so strong that they overwhelm skepticism,” Dr. Pocock told the panel while presenting some advanced statistical test results to prove this assertion. The trial results showed “overwhelming evidence of benefit, beyond a reasonable doubt,” and while cardiovascular death was just one part of the efficacy endpoint, “mortality merits special attention,” he said. The statistical analyses also showed an equally robust 32% relative risk reduction in all-cause mortality, and both the cardiovascular and all-cause death benefits seen in EMPA-REG OUTCOME were consistent across both dosages of empagliflozin tested in the study (10 mg and 25 mg daily) and across the sensitivity analyses applied by the investigators.
“These are convincing data, but I’m not comfortable enough that these robust data would be reproduced in a second trial,” said panel chair Robert J. Smith, MD, who voted against the indication.
An additional limitation acting against the proposed new labeling, according to several panel members, is that the mechanism by which empagliflozin might exert protection against cardiovascular death remains unknown, with no suggestion in the trial results that it acts by protecting patients against ischemic disease.
Current opinion also splits among clinicians on how empagliflozin, which has had FDA approval since 2014 as an option for treating type 2 diabetes, should be used in routine practice to treat diabetes patients with high cardiovascular risk who match those enrolled in the EMPA-REG OUTCOME trial. Dr. Smith urged a cautious approach.
“I think it’s important [for prescribers] to wait to hear from the FDA. If the cardiovascular mortality benefit was proven, then it would be an important option given the magnitude of cardiovascular disease and death as a consequence of type 2 diabetes. But people should be cautious in drawing their own interpretations of the data,” Dr. Smith, professor of medicine at Brown University in Providence, R.I., said in an interview. For the time being, metformin remains the top oral drug for most of these patients because of its proven effectiveness and low cost, he added.
But others have already been active in prescribing empagliflozin to at-risk patients with type 2 diabetes based on last year’s EMPA-REG OUTCOME report.
“I am using it in addition to metformin and aggressive lifestyle changes in patients with established cardiovascular disease and uncontrolled type 2 diabetes,” commented Alison L. Bailey, MD, a cardiologist at the Erlanger Health System and University of Tennessee in Chattanooga. “A patient’s health insurance status must be taken into account as empagliflozin can be a significant financial burden, but if all other things are equal and cost is not prohibitive, I am definitely using this in my patients with type 2 diabetes and cardiovascular disease. I think there are enough data to warrant its use first line in patients who can get the drug without a financial burden,” she said in an interview.
Dr. Konstam cautioned that “just because empagliflozin may have a cardiovascular effect does not make it a cardiovascular drug. As a cardiologist I am not comfortable prescribing this drug. When it comes to diabetes management ,you need to take many things into consideration, most notably blood sugar and hemoglobin A1c,” which are usually best managed by a diabetologist or experienced primary care physician, he said.
Dr. Konstam, Dr. Wilson, Dr. Fradkin, and Dr. Smith had no relevant financial disclosures. Dr. Pocock is a consultant to Boehringer Ingelheim. Dr. Bailey has received research grants from CSL Behring.
On Twitter@mitchelzoler
AT AN FDA EMDAC MEETING
CABG tops PCI for nondiabetic patients with multivessel CAD
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Coronary artery bypass graft is superior to percutaneous coronary intervention for nondiabetic patients with multivessel coronary artery disease.
Major finding: All-cause mortality rates were 6% for CABG and 9.3% for PCI (HR, 0.65; P = .039).
Data source: A pooled analysis of 1,275 nondiabetic patients with two- or three-vessel CAD from the SYNTAX and BEST trials.
Disclosures: The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
Resident transitions increase inpatients’ risk of death
SAN FRANCISCO – Hospitalized patients who have a change in the medical residents responsible for their care are more likely to die, finds a retrospective cohort study of roughly a quarter million discharges from Veterans Affairs medical centers.
A monthly change in resident care was associated with 9%-20% higher adjusted odds of death during the hospital stay and after discharge, investigators reported in a poster discussion session and press briefing at an international conference of the American Thoracic Society. Analyses suggested that such transitions accounted for 718 additional deaths in the hospital alone during the 6-year study period.
“These are very strong findings,” said Dr. Joshua L. Denson, a fellow in the divisions of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The study results represent an important initial step in bringing the problem to light, he said. “Handoffs shift to shift have been looked at, but not this end-of-month, more permanent switching, which I think is a much more substantial transition in care.”
The factors driving the increased mortality are unclear, according to Dr. Denson; however, “when you go on to a new service [as a resident] ... you are now responsible for 20 new people all of a sudden that night.” Therefore, these transitions can be a hectic time characterized by reduced communication and inefficient discharges. In addition, the incoming residents lack familiarity with their new patients’ particulars.
“The handoffs are definitely not preventable, so this is something that has to be dealt with,” he maintained. The study’s findings hint at several possible areas for improvement.
None of the 10 residency programs surveyed provided formal education for monthly resident handoffs, focusing instead on handoffs at shift changes, and most programs lacked a standard procedure, with just one requiring that the handoff be done in person. The programs also varied greatly in their staggering of handoffs – separating transitions of interns (first-year residents) and higher-level residents by at least a few days – to minimize impact.
Despite the absence of outcomes data in this area, some hospitals are forging ahead with their own interventions intended to smooth care transitions, Dr. Denson reported. “In at least two hospitals that I’ve worked in, they are implementing what is called a warm handoff,” he explained. “Basically, a resident from the prior rotation comes the next day and rounds with the new team so he can tell them, ‘Oh, this guy looks a little worse today, you may want to watch him,’ or ‘He looks a little better.’ ”
In the study, conducted while Dr. Denson was chief resident at the NYU School of Medicine, he and his colleagues analyzed data from 10 university-affiliated Veterans Affairs hospitals and internal medicine residency programs that provided their residents’ schedules. Analyses were based on a total of 230,701 discharges of adult medical patients between July 2008 and June 2014.
Hospitalized patients were categorized as having a transition in resident care if they were admitted before the date of an end-of-month house staff transition in care and were discharged in the week after it.
In unadjusted analyses, patients who had a transition of care – whether of intern only, resident only, or both – had significantly higher odds of inpatient mortality and of 30-day mortality and 90-day postdischarge mortality, compared with counterparts who did not have the corresponding transition of care.
In adjusted analyses, patients who had an intern transition still had higher odds of in-hospital mortality (odds ratio, 1.14). In addition, patients had persistently elevated odds of 30-day mortality and 90-day postdischarge mortality if they had an intern transition (odds ratios, 1.20 and 1.17, respectively), a resident transition (1.15 and 1.14), or both (1.10 and 1.09).
The findings “suggest possibly a level-of-training effect to these transitions, as it’s the most inexperienced people that have the higher rate of mortality,” noted Dr. Denson, who disclosed that he had no relevant conflicts of interest. “Interns, being the first-years, tend to carry the bulk of the work in most hospitals, which is an interesting paradigm in our organization. And that may be a good explanation for why we are seeing this.”
SAN FRANCISCO – Hospitalized patients who have a change in the medical residents responsible for their care are more likely to die, finds a retrospective cohort study of roughly a quarter million discharges from Veterans Affairs medical centers.
A monthly change in resident care was associated with 9%-20% higher adjusted odds of death during the hospital stay and after discharge, investigators reported in a poster discussion session and press briefing at an international conference of the American Thoracic Society. Analyses suggested that such transitions accounted for 718 additional deaths in the hospital alone during the 6-year study period.
“These are very strong findings,” said Dr. Joshua L. Denson, a fellow in the divisions of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The study results represent an important initial step in bringing the problem to light, he said. “Handoffs shift to shift have been looked at, but not this end-of-month, more permanent switching, which I think is a much more substantial transition in care.”
The factors driving the increased mortality are unclear, according to Dr. Denson; however, “when you go on to a new service [as a resident] ... you are now responsible for 20 new people all of a sudden that night.” Therefore, these transitions can be a hectic time characterized by reduced communication and inefficient discharges. In addition, the incoming residents lack familiarity with their new patients’ particulars.
“The handoffs are definitely not preventable, so this is something that has to be dealt with,” he maintained. The study’s findings hint at several possible areas for improvement.
None of the 10 residency programs surveyed provided formal education for monthly resident handoffs, focusing instead on handoffs at shift changes, and most programs lacked a standard procedure, with just one requiring that the handoff be done in person. The programs also varied greatly in their staggering of handoffs – separating transitions of interns (first-year residents) and higher-level residents by at least a few days – to minimize impact.
Despite the absence of outcomes data in this area, some hospitals are forging ahead with their own interventions intended to smooth care transitions, Dr. Denson reported. “In at least two hospitals that I’ve worked in, they are implementing what is called a warm handoff,” he explained. “Basically, a resident from the prior rotation comes the next day and rounds with the new team so he can tell them, ‘Oh, this guy looks a little worse today, you may want to watch him,’ or ‘He looks a little better.’ ”
In the study, conducted while Dr. Denson was chief resident at the NYU School of Medicine, he and his colleagues analyzed data from 10 university-affiliated Veterans Affairs hospitals and internal medicine residency programs that provided their residents’ schedules. Analyses were based on a total of 230,701 discharges of adult medical patients between July 2008 and June 2014.
Hospitalized patients were categorized as having a transition in resident care if they were admitted before the date of an end-of-month house staff transition in care and were discharged in the week after it.
In unadjusted analyses, patients who had a transition of care – whether of intern only, resident only, or both – had significantly higher odds of inpatient mortality and of 30-day mortality and 90-day postdischarge mortality, compared with counterparts who did not have the corresponding transition of care.
In adjusted analyses, patients who had an intern transition still had higher odds of in-hospital mortality (odds ratio, 1.14). In addition, patients had persistently elevated odds of 30-day mortality and 90-day postdischarge mortality if they had an intern transition (odds ratios, 1.20 and 1.17, respectively), a resident transition (1.15 and 1.14), or both (1.10 and 1.09).
The findings “suggest possibly a level-of-training effect to these transitions, as it’s the most inexperienced people that have the higher rate of mortality,” noted Dr. Denson, who disclosed that he had no relevant conflicts of interest. “Interns, being the first-years, tend to carry the bulk of the work in most hospitals, which is an interesting paradigm in our organization. And that may be a good explanation for why we are seeing this.”
SAN FRANCISCO – Hospitalized patients who have a change in the medical residents responsible for their care are more likely to die, finds a retrospective cohort study of roughly a quarter million discharges from Veterans Affairs medical centers.
A monthly change in resident care was associated with 9%-20% higher adjusted odds of death during the hospital stay and after discharge, investigators reported in a poster discussion session and press briefing at an international conference of the American Thoracic Society. Analyses suggested that such transitions accounted for 718 additional deaths in the hospital alone during the 6-year study period.
“These are very strong findings,” said Dr. Joshua L. Denson, a fellow in the divisions of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The study results represent an important initial step in bringing the problem to light, he said. “Handoffs shift to shift have been looked at, but not this end-of-month, more permanent switching, which I think is a much more substantial transition in care.”
The factors driving the increased mortality are unclear, according to Dr. Denson; however, “when you go on to a new service [as a resident] ... you are now responsible for 20 new people all of a sudden that night.” Therefore, these transitions can be a hectic time characterized by reduced communication and inefficient discharges. In addition, the incoming residents lack familiarity with their new patients’ particulars.
“The handoffs are definitely not preventable, so this is something that has to be dealt with,” he maintained. The study’s findings hint at several possible areas for improvement.
None of the 10 residency programs surveyed provided formal education for monthly resident handoffs, focusing instead on handoffs at shift changes, and most programs lacked a standard procedure, with just one requiring that the handoff be done in person. The programs also varied greatly in their staggering of handoffs – separating transitions of interns (first-year residents) and higher-level residents by at least a few days – to minimize impact.
Despite the absence of outcomes data in this area, some hospitals are forging ahead with their own interventions intended to smooth care transitions, Dr. Denson reported. “In at least two hospitals that I’ve worked in, they are implementing what is called a warm handoff,” he explained. “Basically, a resident from the prior rotation comes the next day and rounds with the new team so he can tell them, ‘Oh, this guy looks a little worse today, you may want to watch him,’ or ‘He looks a little better.’ ”
In the study, conducted while Dr. Denson was chief resident at the NYU School of Medicine, he and his colleagues analyzed data from 10 university-affiliated Veterans Affairs hospitals and internal medicine residency programs that provided their residents’ schedules. Analyses were based on a total of 230,701 discharges of adult medical patients between July 2008 and June 2014.
Hospitalized patients were categorized as having a transition in resident care if they were admitted before the date of an end-of-month house staff transition in care and were discharged in the week after it.
In unadjusted analyses, patients who had a transition of care – whether of intern only, resident only, or both – had significantly higher odds of inpatient mortality and of 30-day mortality and 90-day postdischarge mortality, compared with counterparts who did not have the corresponding transition of care.
In adjusted analyses, patients who had an intern transition still had higher odds of in-hospital mortality (odds ratio, 1.14). In addition, patients had persistently elevated odds of 30-day mortality and 90-day postdischarge mortality if they had an intern transition (odds ratios, 1.20 and 1.17, respectively), a resident transition (1.15 and 1.14), or both (1.10 and 1.09).
The findings “suggest possibly a level-of-training effect to these transitions, as it’s the most inexperienced people that have the higher rate of mortality,” noted Dr. Denson, who disclosed that he had no relevant conflicts of interest. “Interns, being the first-years, tend to carry the bulk of the work in most hospitals, which is an interesting paradigm in our organization. And that may be a good explanation for why we are seeing this.”
AT ATS 2016
Key clinical point: The risk of death for hospitalized patients rises when their care is handed off from one resident to another.
Major finding: Patients who had a resident transition in care during their stay had 9%-20% higher adjusted odds of death.
Data source: A multicenter retrospective cohort study of 230,701 discharges of adult medical patients from Veterans Affairs medical centers.
Disclosures: Dr. Denson disclosed that he had no relevant conflicts of interest.
IVUS has role for annular sizing in TAVR
PARIS – Intravascular ultrasound can reliably be used in lieu of multidetector computerized tomography for the key task of annular sizing in patients undergoing transcatheter aortic valve replacement, Dr. Diaa Hakim declared at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Multidetector CT (MDCT) is considered the standard imaging method for this purpose. But the requirement for contrast media makes MDCT problematic for patients with chronic kidney disease, who can easily be driven into acute kidney injury through exposure to this material.
Moreover, renal failure is common among patients with a failing native aortic valve. Interventionalists who perform transaortic valve replacement (TAVR) are encountering renal failure more and more frequently as the nonsurgical treatment takes off in popularity. An alternative imaging method is sorely needed, observed Dr. Hakim of the University of Alabama at Birmingham.
Unlike MDCT, intravascular ultrasound (IVUS) doesn’t require contrast. And in Dr. Hakim’s head-to-head comparative trial conducted in 50 consecutive TAVR patients who underwent annular sizing by both methods, there were no significant differences between the two in measurements of maximum and minimum annular diameter, mean annular diameter, or annular area.
The decision as to the size of the replacement aortic valve was based upon MDCT, which was performed first. Then came IVUS carried out with a Boston Science Atlantis PV Peripheral IVUS catheter at 8-French and 15 Hz. The catheter was advanced over the guidewire, then pullback imaging was obtained automatically from the left ventricular outflow tract to the aortic root. The IVUS measurements were made at the level of basal attachment of the aortic valve cusps, which was quite close to the same point as the MDCT measurements.
Post TAVR, 37 of the 50 patients had no or trivial paravalvular regurgitation. Six patients developed acute kidney injury.
Asked if he believes IVUS now enables operators to routinely skip MDCT for TAVR patients, Dr. Hakim replied, “Not for the moment.” In patients with chronic kidney disease, yes, but in order for IVUS for annular sizing to expand beyond that population it will be necessary for device makers to develop an IVUS catheter with better visualization, a device designed specifically to see all the details of the aortic valve and annulus. He noted that the Atlantis PV Peripheral IVUS catheter employed in his study was designed for the aorta, not the aortic valve. It doesn’t provide optimal imaging of the valve cusps, nor can it measure paravalvular regurgitation after valve implantation.
How much time does IVUS for annular sizing add to the TAVR procedure? “Five minutes, no more,” according to Dr. Hakim.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
PARIS – Intravascular ultrasound can reliably be used in lieu of multidetector computerized tomography for the key task of annular sizing in patients undergoing transcatheter aortic valve replacement, Dr. Diaa Hakim declared at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Multidetector CT (MDCT) is considered the standard imaging method for this purpose. But the requirement for contrast media makes MDCT problematic for patients with chronic kidney disease, who can easily be driven into acute kidney injury through exposure to this material.
Moreover, renal failure is common among patients with a failing native aortic valve. Interventionalists who perform transaortic valve replacement (TAVR) are encountering renal failure more and more frequently as the nonsurgical treatment takes off in popularity. An alternative imaging method is sorely needed, observed Dr. Hakim of the University of Alabama at Birmingham.
Unlike MDCT, intravascular ultrasound (IVUS) doesn’t require contrast. And in Dr. Hakim’s head-to-head comparative trial conducted in 50 consecutive TAVR patients who underwent annular sizing by both methods, there were no significant differences between the two in measurements of maximum and minimum annular diameter, mean annular diameter, or annular area.
The decision as to the size of the replacement aortic valve was based upon MDCT, which was performed first. Then came IVUS carried out with a Boston Science Atlantis PV Peripheral IVUS catheter at 8-French and 15 Hz. The catheter was advanced over the guidewire, then pullback imaging was obtained automatically from the left ventricular outflow tract to the aortic root. The IVUS measurements were made at the level of basal attachment of the aortic valve cusps, which was quite close to the same point as the MDCT measurements.
Post TAVR, 37 of the 50 patients had no or trivial paravalvular regurgitation. Six patients developed acute kidney injury.
Asked if he believes IVUS now enables operators to routinely skip MDCT for TAVR patients, Dr. Hakim replied, “Not for the moment.” In patients with chronic kidney disease, yes, but in order for IVUS for annular sizing to expand beyond that population it will be necessary for device makers to develop an IVUS catheter with better visualization, a device designed specifically to see all the details of the aortic valve and annulus. He noted that the Atlantis PV Peripheral IVUS catheter employed in his study was designed for the aorta, not the aortic valve. It doesn’t provide optimal imaging of the valve cusps, nor can it measure paravalvular regurgitation after valve implantation.
How much time does IVUS for annular sizing add to the TAVR procedure? “Five minutes, no more,” according to Dr. Hakim.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
PARIS – Intravascular ultrasound can reliably be used in lieu of multidetector computerized tomography for the key task of annular sizing in patients undergoing transcatheter aortic valve replacement, Dr. Diaa Hakim declared at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Multidetector CT (MDCT) is considered the standard imaging method for this purpose. But the requirement for contrast media makes MDCT problematic for patients with chronic kidney disease, who can easily be driven into acute kidney injury through exposure to this material.
Moreover, renal failure is common among patients with a failing native aortic valve. Interventionalists who perform transaortic valve replacement (TAVR) are encountering renal failure more and more frequently as the nonsurgical treatment takes off in popularity. An alternative imaging method is sorely needed, observed Dr. Hakim of the University of Alabama at Birmingham.
Unlike MDCT, intravascular ultrasound (IVUS) doesn’t require contrast. And in Dr. Hakim’s head-to-head comparative trial conducted in 50 consecutive TAVR patients who underwent annular sizing by both methods, there were no significant differences between the two in measurements of maximum and minimum annular diameter, mean annular diameter, or annular area.
The decision as to the size of the replacement aortic valve was based upon MDCT, which was performed first. Then came IVUS carried out with a Boston Science Atlantis PV Peripheral IVUS catheter at 8-French and 15 Hz. The catheter was advanced over the guidewire, then pullback imaging was obtained automatically from the left ventricular outflow tract to the aortic root. The IVUS measurements were made at the level of basal attachment of the aortic valve cusps, which was quite close to the same point as the MDCT measurements.
Post TAVR, 37 of the 50 patients had no or trivial paravalvular regurgitation. Six patients developed acute kidney injury.
Asked if he believes IVUS now enables operators to routinely skip MDCT for TAVR patients, Dr. Hakim replied, “Not for the moment.” In patients with chronic kidney disease, yes, but in order for IVUS for annular sizing to expand beyond that population it will be necessary for device makers to develop an IVUS catheter with better visualization, a device designed specifically to see all the details of the aortic valve and annulus. He noted that the Atlantis PV Peripheral IVUS catheter employed in his study was designed for the aorta, not the aortic valve. It doesn’t provide optimal imaging of the valve cusps, nor can it measure paravalvular regurgitation after valve implantation.
How much time does IVUS for annular sizing add to the TAVR procedure? “Five minutes, no more,” according to Dr. Hakim.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
AT EUROPCR 2016
Key clinical point: Intravascular ultrasound is a reliable alternative to multidetector CT for annular sizing in TAVR patients with chronic kidney disease for whom contrast media could be a problem.
Major finding: Measurements of aortic annulus maximum and minimum diameter, mean annular diameter, and annular area didn’t differ significantly whether measured by multidetector CT or contrast-free intravascular ultrasound.
Data source: This head-to-head study included 50 consecutive TAVR patients who underwent annular sizing by both CT and intravascular ultrasound.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.