User login
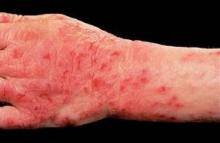
Alitretinoin for Hand Eczema Slashes Sick Days in Half
LISBON – Physicians outside the United States are turning cartwheels over the outcomes they're obtaining with oral alitretinoin for chronic hand eczema.
New data from the postmarketing observational TOCCATA trial demonstrate that oral alitretinoin reduced eczema-related work absences by 50% in German workers, Dr. Thomas Diepgen reported at the annual congress of the European Academy of Dermatology and Venereology.
The TOCCATA study involved 680 German patients with chronic hand eczema rated as severe in two-thirds of participants and moderate in the remainder. TOCCATA was designed to determine whether alitretinoin’s performance in real-world clinical practice is similar to that in the pivotal 1,032-patient randomized BACH (Benefits of Alitretinoin in Chronic Hand Eczema) trial, which resulted in European approval of the novel retinoid (Br. J. Dermatol. 2008;158:808-17).
TOCCATA participants received either 10 mg or 30 mg of alitretinoin per day at their physician’s discretion for up to 24 weeks, with follow-up evaluations at 4-week intervals. Treatment outcomes in TOCCATA proved comparable to those in the BACH trial: a 56.7% rating of clear or almost clear by Physician Global Assessment, as compared with 47.7% in BACH.
As in BACH, the greatest response in TOCCATA was in patients with the hyperkeratotic rhagadiform form of hand eczema, with a 59.2% rate of clear or almost clear. But all the morphologic types showed a favorable response: a 47.9% clear or almost clear rate in dyshidrosiform hand eczema, and a 52.2% rate in fingertip eczema.
In TOCCATA, 522 participants were employed, mostly as steel or other metal workers, or in food processing. At baseline, 80 reported a work absence because of their chronic hand eczema within the previous 4 weeks. By week 8, this number had dropped to 53 workers. At weeks 12 and 16 it was 43 workers, at week 20 it was 41, and by week 24 only 37 TOCCATA participants had missed work during the previous 4 weeks, according to Dr. Diepgen, director of the department of social medicine, occupational, and environmental dermatology at the University of Heidelberg (Germany).
Not only was the number of workers taking sick leave reduced by slightly more than half, but total sick days were even more dramatically impacted. At baseline, workers who had taken hand eczema-related sick leave within the prior 4 weeks had missed an average of 17.6 work days during that period. By week 8, that figure had dropped to an average 10.7 days of absence in the last 4 weeks. At week 24, it was 7.6 days, meaning that after 6 months of daily alitretinoin workers who were off the job because of their hand eczema were taking 57% fewer sick days.
The reduction in work absenteeism in TOCCATA was paralleled by the clinical improvement. It took about 4 weeks of alitretinoin therapy to see a significant impact on Physician Global Assessment ratings. The bulk of the clinical improvement was achieved by week 12, Dr. Diepgen noted.
The most common adverse events in TOCCATA were mild to moderate mucocutaneous reactions, seen in about 10% of patients, which is a lower rate than reported with other retinoids. Headache occurred in 8% of patients, and flushing in 1%.
Alitretinoin is a potent teratogen, like other retinoids. However, its relatively short half-life of 2-10 hours means that women of childbearing potential must continue on contraception for 1 month posttreatment, as compared to 3 years with acitretin.
Asked how much oral alitretinoin costs, Dr. Diepgen said it’s about 600 euros (US $811) per month in Germany. But he added that in another study, he and his coworkers calculated that the yearly cost of severe chronic hand eczema was about 9,000 euros (US $12,170), mostly from sick leave and resultant lost workplace productivity. And that’s a conservative figure, he added; the indirect costs of severe hand eczema run nearly twice as high in expertly skilled, higher-paid workers.
The U.K. National Institute for Health and Clinical Excellence guidelines recommend oral 9-cis-retinoic acid as first-line therapy for patients who fail to respond to potent topical corticosteroids, as do Italian and Canadian guidelines and a German dermatology association. A phase III clinical trial (HANDEL) is currently underway in the United States.
Dr. Diepgen is an adviser to Basilea Pharmaceutica, which markets oral alitretinoin.
LISBON – Physicians outside the United States are turning cartwheels over the outcomes they're obtaining with oral alitretinoin for chronic hand eczema.
New data from the postmarketing observational TOCCATA trial demonstrate that oral alitretinoin reduced eczema-related work absences by 50% in German workers, Dr. Thomas Diepgen reported at the annual congress of the European Academy of Dermatology and Venereology.
The TOCCATA study involved 680 German patients with chronic hand eczema rated as severe in two-thirds of participants and moderate in the remainder. TOCCATA was designed to determine whether alitretinoin’s performance in real-world clinical practice is similar to that in the pivotal 1,032-patient randomized BACH (Benefits of Alitretinoin in Chronic Hand Eczema) trial, which resulted in European approval of the novel retinoid (Br. J. Dermatol. 2008;158:808-17).
TOCCATA participants received either 10 mg or 30 mg of alitretinoin per day at their physician’s discretion for up to 24 weeks, with follow-up evaluations at 4-week intervals. Treatment outcomes in TOCCATA proved comparable to those in the BACH trial: a 56.7% rating of clear or almost clear by Physician Global Assessment, as compared with 47.7% in BACH.
As in BACH, the greatest response in TOCCATA was in patients with the hyperkeratotic rhagadiform form of hand eczema, with a 59.2% rate of clear or almost clear. But all the morphologic types showed a favorable response: a 47.9% clear or almost clear rate in dyshidrosiform hand eczema, and a 52.2% rate in fingertip eczema.
In TOCCATA, 522 participants were employed, mostly as steel or other metal workers, or in food processing. At baseline, 80 reported a work absence because of their chronic hand eczema within the previous 4 weeks. By week 8, this number had dropped to 53 workers. At weeks 12 and 16 it was 43 workers, at week 20 it was 41, and by week 24 only 37 TOCCATA participants had missed work during the previous 4 weeks, according to Dr. Diepgen, director of the department of social medicine, occupational, and environmental dermatology at the University of Heidelberg (Germany).
Not only was the number of workers taking sick leave reduced by slightly more than half, but total sick days were even more dramatically impacted. At baseline, workers who had taken hand eczema-related sick leave within the prior 4 weeks had missed an average of 17.6 work days during that period. By week 8, that figure had dropped to an average 10.7 days of absence in the last 4 weeks. At week 24, it was 7.6 days, meaning that after 6 months of daily alitretinoin workers who were off the job because of their hand eczema were taking 57% fewer sick days.
The reduction in work absenteeism in TOCCATA was paralleled by the clinical improvement. It took about 4 weeks of alitretinoin therapy to see a significant impact on Physician Global Assessment ratings. The bulk of the clinical improvement was achieved by week 12, Dr. Diepgen noted.
The most common adverse events in TOCCATA were mild to moderate mucocutaneous reactions, seen in about 10% of patients, which is a lower rate than reported with other retinoids. Headache occurred in 8% of patients, and flushing in 1%.
Alitretinoin is a potent teratogen, like other retinoids. However, its relatively short half-life of 2-10 hours means that women of childbearing potential must continue on contraception for 1 month posttreatment, as compared to 3 years with acitretin.
Asked how much oral alitretinoin costs, Dr. Diepgen said it’s about 600 euros (US $811) per month in Germany. But he added that in another study, he and his coworkers calculated that the yearly cost of severe chronic hand eczema was about 9,000 euros (US $12,170), mostly from sick leave and resultant lost workplace productivity. And that’s a conservative figure, he added; the indirect costs of severe hand eczema run nearly twice as high in expertly skilled, higher-paid workers.
The U.K. National Institute for Health and Clinical Excellence guidelines recommend oral 9-cis-retinoic acid as first-line therapy for patients who fail to respond to potent topical corticosteroids, as do Italian and Canadian guidelines and a German dermatology association. A phase III clinical trial (HANDEL) is currently underway in the United States.
Dr. Diepgen is an adviser to Basilea Pharmaceutica, which markets oral alitretinoin.
LISBON – Physicians outside the United States are turning cartwheels over the outcomes they're obtaining with oral alitretinoin for chronic hand eczema.
New data from the postmarketing observational TOCCATA trial demonstrate that oral alitretinoin reduced eczema-related work absences by 50% in German workers, Dr. Thomas Diepgen reported at the annual congress of the European Academy of Dermatology and Venereology.
The TOCCATA study involved 680 German patients with chronic hand eczema rated as severe in two-thirds of participants and moderate in the remainder. TOCCATA was designed to determine whether alitretinoin’s performance in real-world clinical practice is similar to that in the pivotal 1,032-patient randomized BACH (Benefits of Alitretinoin in Chronic Hand Eczema) trial, which resulted in European approval of the novel retinoid (Br. J. Dermatol. 2008;158:808-17).
TOCCATA participants received either 10 mg or 30 mg of alitretinoin per day at their physician’s discretion for up to 24 weeks, with follow-up evaluations at 4-week intervals. Treatment outcomes in TOCCATA proved comparable to those in the BACH trial: a 56.7% rating of clear or almost clear by Physician Global Assessment, as compared with 47.7% in BACH.
As in BACH, the greatest response in TOCCATA was in patients with the hyperkeratotic rhagadiform form of hand eczema, with a 59.2% rate of clear or almost clear. But all the morphologic types showed a favorable response: a 47.9% clear or almost clear rate in dyshidrosiform hand eczema, and a 52.2% rate in fingertip eczema.
In TOCCATA, 522 participants were employed, mostly as steel or other metal workers, or in food processing. At baseline, 80 reported a work absence because of their chronic hand eczema within the previous 4 weeks. By week 8, this number had dropped to 53 workers. At weeks 12 and 16 it was 43 workers, at week 20 it was 41, and by week 24 only 37 TOCCATA participants had missed work during the previous 4 weeks, according to Dr. Diepgen, director of the department of social medicine, occupational, and environmental dermatology at the University of Heidelberg (Germany).
Not only was the number of workers taking sick leave reduced by slightly more than half, but total sick days were even more dramatically impacted. At baseline, workers who had taken hand eczema-related sick leave within the prior 4 weeks had missed an average of 17.6 work days during that period. By week 8, that figure had dropped to an average 10.7 days of absence in the last 4 weeks. At week 24, it was 7.6 days, meaning that after 6 months of daily alitretinoin workers who were off the job because of their hand eczema were taking 57% fewer sick days.
The reduction in work absenteeism in TOCCATA was paralleled by the clinical improvement. It took about 4 weeks of alitretinoin therapy to see a significant impact on Physician Global Assessment ratings. The bulk of the clinical improvement was achieved by week 12, Dr. Diepgen noted.
The most common adverse events in TOCCATA were mild to moderate mucocutaneous reactions, seen in about 10% of patients, which is a lower rate than reported with other retinoids. Headache occurred in 8% of patients, and flushing in 1%.
Alitretinoin is a potent teratogen, like other retinoids. However, its relatively short half-life of 2-10 hours means that women of childbearing potential must continue on contraception for 1 month posttreatment, as compared to 3 years with acitretin.
Asked how much oral alitretinoin costs, Dr. Diepgen said it’s about 600 euros (US $811) per month in Germany. But he added that in another study, he and his coworkers calculated that the yearly cost of severe chronic hand eczema was about 9,000 euros (US $12,170), mostly from sick leave and resultant lost workplace productivity. And that’s a conservative figure, he added; the indirect costs of severe hand eczema run nearly twice as high in expertly skilled, higher-paid workers.
The U.K. National Institute for Health and Clinical Excellence guidelines recommend oral 9-cis-retinoic acid as first-line therapy for patients who fail to respond to potent topical corticosteroids, as do Italian and Canadian guidelines and a German dermatology association. A phase III clinical trial (HANDEL) is currently underway in the United States.
Dr. Diepgen is an adviser to Basilea Pharmaceutica, which markets oral alitretinoin.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: The number of German workers who took sick leave for their chronic hand eczema was reduced by half after they went on daily therapy with oral alliteration.
Data Source: The observational 680-patient TOCCATA study.
Disclosures: The study was funded by Basilea Pharmaceuticals, which markets oral alitretinoin in Europe and Canada. Dr. Diepgen is an adviser to the company.
Ustekinumab Re-treatment Delivers Excellent Results
LISBON – Roughly 85% of psoriasis patients re-treated with ustekinumab after withdrawing from an earlier successful course of therapy will experience an excellent response in round two.
This benefit was consistently seen in secondary analyses of two separate phase III clinical trials. This finding provides information that is valuable in clinical practice, Dr. Christopher E. Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
Psoriasis patients often have to halt effective biologic therapy for a variety of reasons – pregnancy, elective major surgery, vaccinations, or just to enjoy a welcome break from potent therapy while their disease has cleared – but not all biologics retain their efficacy upon re-treatment.
Ustekinumab (Stelara) now joins etanercept (Enbrel) and alefacept (Amevive) on the list of biologic therapies that are able to recapture their clinical response upon re-treatment, said Dr. Griffiths, professor of dermatology at the University of Manchester (England).
He reported on 260 ustekinumab-treated patients in the phase-III PHOENIX I trial and 375 in the ACCEPT trial, all of whom attained at least a 75% improvement, compared with baseline, in Psoriasis Area and Severity Index (PASI) scores, and a Physician Global Assessment (PGA) score of 0-2, meaning clear, minimal, or mild disease. The study patients were withdrawn from the biologic at week 40 in PHOENIX I and at week 12 in ACCEPT.
The PHOENIX I participants began re-treatment after losing at least 50% of the improvement obtained during the initial course of therapy. At week 12 of re-treatment, 84% of patients from PHOENIX I who were on the ustekinumab 45 mg dose and 85% on ustekinumab 90 mg achieved a PASI 75 response. The median improvement in PASI scores, compared with when they commenced re-treatment, was 89%-90%.
ACCEPT participants entered re-treatment after their PGA score, which was 0-2 upon treatment withdrawal, regressed to greater than 2.
Among ACCEPT participants undergoing re-treatment with ustekinumab 45 mg after earlier ustekinumab withdrawal, 85% were rated as having a 0-2 score on PGA at week 12. So were 89% on ustekinumab 90 mg. The median improvement in PASI scores from baseline was 85% in the ustekinumab 45 mg group and 90% in the ustekinumab 90 mg arm.
Rates of overall adverse events, serious adverse events, and infections were similar in the initial round of treatment and in re-treatment. No new safety signals arose during 12 weeks of re-treatment. Five patients being re-treated with ustekinumab 45 mg and two on ustekinumab 90 mg developed anti-ustekinumab antibodies; their response to re-treatment was significantly less robust than in patients without antibodies, Dr. Griffiths noted.
The study was funded by Centocor, the manufacturer of ustekinumab. Dr. Griffiths is an advisor to the company.
LISBON – Roughly 85% of psoriasis patients re-treated with ustekinumab after withdrawing from an earlier successful course of therapy will experience an excellent response in round two.
This benefit was consistently seen in secondary analyses of two separate phase III clinical trials. This finding provides information that is valuable in clinical practice, Dr. Christopher E. Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
Psoriasis patients often have to halt effective biologic therapy for a variety of reasons – pregnancy, elective major surgery, vaccinations, or just to enjoy a welcome break from potent therapy while their disease has cleared – but not all biologics retain their efficacy upon re-treatment.
Ustekinumab (Stelara) now joins etanercept (Enbrel) and alefacept (Amevive) on the list of biologic therapies that are able to recapture their clinical response upon re-treatment, said Dr. Griffiths, professor of dermatology at the University of Manchester (England).
He reported on 260 ustekinumab-treated patients in the phase-III PHOENIX I trial and 375 in the ACCEPT trial, all of whom attained at least a 75% improvement, compared with baseline, in Psoriasis Area and Severity Index (PASI) scores, and a Physician Global Assessment (PGA) score of 0-2, meaning clear, minimal, or mild disease. The study patients were withdrawn from the biologic at week 40 in PHOENIX I and at week 12 in ACCEPT.
The PHOENIX I participants began re-treatment after losing at least 50% of the improvement obtained during the initial course of therapy. At week 12 of re-treatment, 84% of patients from PHOENIX I who were on the ustekinumab 45 mg dose and 85% on ustekinumab 90 mg achieved a PASI 75 response. The median improvement in PASI scores, compared with when they commenced re-treatment, was 89%-90%.
ACCEPT participants entered re-treatment after their PGA score, which was 0-2 upon treatment withdrawal, regressed to greater than 2.
Among ACCEPT participants undergoing re-treatment with ustekinumab 45 mg after earlier ustekinumab withdrawal, 85% were rated as having a 0-2 score on PGA at week 12. So were 89% on ustekinumab 90 mg. The median improvement in PASI scores from baseline was 85% in the ustekinumab 45 mg group and 90% in the ustekinumab 90 mg arm.
Rates of overall adverse events, serious adverse events, and infections were similar in the initial round of treatment and in re-treatment. No new safety signals arose during 12 weeks of re-treatment. Five patients being re-treated with ustekinumab 45 mg and two on ustekinumab 90 mg developed anti-ustekinumab antibodies; their response to re-treatment was significantly less robust than in patients without antibodies, Dr. Griffiths noted.
The study was funded by Centocor, the manufacturer of ustekinumab. Dr. Griffiths is an advisor to the company.
LISBON – Roughly 85% of psoriasis patients re-treated with ustekinumab after withdrawing from an earlier successful course of therapy will experience an excellent response in round two.
This benefit was consistently seen in secondary analyses of two separate phase III clinical trials. This finding provides information that is valuable in clinical practice, Dr. Christopher E. Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
Psoriasis patients often have to halt effective biologic therapy for a variety of reasons – pregnancy, elective major surgery, vaccinations, or just to enjoy a welcome break from potent therapy while their disease has cleared – but not all biologics retain their efficacy upon re-treatment.
Ustekinumab (Stelara) now joins etanercept (Enbrel) and alefacept (Amevive) on the list of biologic therapies that are able to recapture their clinical response upon re-treatment, said Dr. Griffiths, professor of dermatology at the University of Manchester (England).
He reported on 260 ustekinumab-treated patients in the phase-III PHOENIX I trial and 375 in the ACCEPT trial, all of whom attained at least a 75% improvement, compared with baseline, in Psoriasis Area and Severity Index (PASI) scores, and a Physician Global Assessment (PGA) score of 0-2, meaning clear, minimal, or mild disease. The study patients were withdrawn from the biologic at week 40 in PHOENIX I and at week 12 in ACCEPT.
The PHOENIX I participants began re-treatment after losing at least 50% of the improvement obtained during the initial course of therapy. At week 12 of re-treatment, 84% of patients from PHOENIX I who were on the ustekinumab 45 mg dose and 85% on ustekinumab 90 mg achieved a PASI 75 response. The median improvement in PASI scores, compared with when they commenced re-treatment, was 89%-90%.
ACCEPT participants entered re-treatment after their PGA score, which was 0-2 upon treatment withdrawal, regressed to greater than 2.
Among ACCEPT participants undergoing re-treatment with ustekinumab 45 mg after earlier ustekinumab withdrawal, 85% were rated as having a 0-2 score on PGA at week 12. So were 89% on ustekinumab 90 mg. The median improvement in PASI scores from baseline was 85% in the ustekinumab 45 mg group and 90% in the ustekinumab 90 mg arm.
Rates of overall adverse events, serious adverse events, and infections were similar in the initial round of treatment and in re-treatment. No new safety signals arose during 12 weeks of re-treatment. Five patients being re-treated with ustekinumab 45 mg and two on ustekinumab 90 mg developed anti-ustekinumab antibodies; their response to re-treatment was significantly less robust than in patients without antibodies, Dr. Griffiths noted.
The study was funded by Centocor, the manufacturer of ustekinumab. Dr. Griffiths is an advisor to the company.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: At week 12 of re-treatment, 84% of patients from PHOENIX I who were on the ustekinumab 45 mg dose and 85% on ustekinumab 90 mg achieved a PASI 75 response.
Data Source: Secondary analysis of the phase III PHOENIX 1 and ACCEPT randomized trials.
Disclosures: The study was funded by Centocor, manufacturer of ustekinumab. Dr. Griffiths is an advisor to the company.
Noninflammatory Acne Lesions Scar, Too
LISBON – Contrary to conventional wisdom, atrophic acne scars may arise from what was clinically normal skin 3 months earlier.
Inflammatory acne lesions clearly play a major role in atrophic scarring, but scars can arise from erythematous macules and closed comedones as well.
"And it’s also important to emphasize that we’re able to identify atrophic scars arising from clinically normal skin," Dr. Sewon Kang said at the annual Congress of the European Academy of Dermatology and Venereology, during a session sponsored by Galderma.
His study of the natural history of acne over a 12-week time frame used computer-assisted spatial alignment and serial high-definition digital photographs to track new lesions and atrophic scarring in 25 subjects with untreated mild to moderate facial acne. Participants were formally assessed every 2 weeks.
Closed comedones accounted for 37% of all lesions, followed by erythematous macules at 26%, inflammatory papules at 15%, open comedones at 12%, with pustules and nodules accounting for the rest.
"Both non- inflammatory as well as inflammatory acne lesions need to be addressed in order to prevent the most terrible sequelae of acne: the formation of scars."
At 12 weeks, a total of 219 inflammatory lesions were present: 176 papules, 35 pustules, and 8 nodules. Working backward via the serial tracking system, Dr. Kang and coworkers determined that 41% of the inflammatory lesions were preceded by closed comedones, 13% by open comedones, 12% from erythematous macules, and 6% from ice pick scars. Importantly, 28% of inflammatory lesions arose from clinically normal-appearing skin, said Dr. Kang, professor and chair of the department of dermatology at Johns Hopkins University, Baltimore.
Also present at 12 weeks were a total of 104 atrophic scars as agreed upon by at least two of the three independent examining dermatologists. Nearly 70% were ice pick scars, 30% were boxcar acne scars, and 2% were rolling scars.
Of note, 23 of the 25 study participants had one or more acne scars at 3 months of follow-up.
In all, 30% of the scars were already present at baseline. Another 20% arose from inflamed papules or pustules or from closed comedones. But fully half of the scars arose from noninflammatory lesions.
The clinical implication of these findings is that aggressive treatment should be prescribed from the outset in acne patients – even in those with mild disease – to prevent acne scarring.
"Both noninflammatory as well as inflammatory acne lesions need to be addressed in order to prevent the most terrible sequelae of acne: the formation of scars," said Dr. Kang.
Based upon these findings, he said he favors multimodal acne therapy with a retinoid, an antimicrobial agent, and benzoyl peroxide.
Dr. Kang is a paid speaker for Galderma.
LISBON – Contrary to conventional wisdom, atrophic acne scars may arise from what was clinically normal skin 3 months earlier.
Inflammatory acne lesions clearly play a major role in atrophic scarring, but scars can arise from erythematous macules and closed comedones as well.
"And it’s also important to emphasize that we’re able to identify atrophic scars arising from clinically normal skin," Dr. Sewon Kang said at the annual Congress of the European Academy of Dermatology and Venereology, during a session sponsored by Galderma.
His study of the natural history of acne over a 12-week time frame used computer-assisted spatial alignment and serial high-definition digital photographs to track new lesions and atrophic scarring in 25 subjects with untreated mild to moderate facial acne. Participants were formally assessed every 2 weeks.
Closed comedones accounted for 37% of all lesions, followed by erythematous macules at 26%, inflammatory papules at 15%, open comedones at 12%, with pustules and nodules accounting for the rest.
"Both non- inflammatory as well as inflammatory acne lesions need to be addressed in order to prevent the most terrible sequelae of acne: the formation of scars."
At 12 weeks, a total of 219 inflammatory lesions were present: 176 papules, 35 pustules, and 8 nodules. Working backward via the serial tracking system, Dr. Kang and coworkers determined that 41% of the inflammatory lesions were preceded by closed comedones, 13% by open comedones, 12% from erythematous macules, and 6% from ice pick scars. Importantly, 28% of inflammatory lesions arose from clinically normal-appearing skin, said Dr. Kang, professor and chair of the department of dermatology at Johns Hopkins University, Baltimore.
Also present at 12 weeks were a total of 104 atrophic scars as agreed upon by at least two of the three independent examining dermatologists. Nearly 70% were ice pick scars, 30% were boxcar acne scars, and 2% were rolling scars.
Of note, 23 of the 25 study participants had one or more acne scars at 3 months of follow-up.
In all, 30% of the scars were already present at baseline. Another 20% arose from inflamed papules or pustules or from closed comedones. But fully half of the scars arose from noninflammatory lesions.
The clinical implication of these findings is that aggressive treatment should be prescribed from the outset in acne patients – even in those with mild disease – to prevent acne scarring.
"Both noninflammatory as well as inflammatory acne lesions need to be addressed in order to prevent the most terrible sequelae of acne: the formation of scars," said Dr. Kang.
Based upon these findings, he said he favors multimodal acne therapy with a retinoid, an antimicrobial agent, and benzoyl peroxide.
Dr. Kang is a paid speaker for Galderma.
LISBON – Contrary to conventional wisdom, atrophic acne scars may arise from what was clinically normal skin 3 months earlier.
Inflammatory acne lesions clearly play a major role in atrophic scarring, but scars can arise from erythematous macules and closed comedones as well.
"And it’s also important to emphasize that we’re able to identify atrophic scars arising from clinically normal skin," Dr. Sewon Kang said at the annual Congress of the European Academy of Dermatology and Venereology, during a session sponsored by Galderma.
His study of the natural history of acne over a 12-week time frame used computer-assisted spatial alignment and serial high-definition digital photographs to track new lesions and atrophic scarring in 25 subjects with untreated mild to moderate facial acne. Participants were formally assessed every 2 weeks.
Closed comedones accounted for 37% of all lesions, followed by erythematous macules at 26%, inflammatory papules at 15%, open comedones at 12%, with pustules and nodules accounting for the rest.
"Both non- inflammatory as well as inflammatory acne lesions need to be addressed in order to prevent the most terrible sequelae of acne: the formation of scars."
At 12 weeks, a total of 219 inflammatory lesions were present: 176 papules, 35 pustules, and 8 nodules. Working backward via the serial tracking system, Dr. Kang and coworkers determined that 41% of the inflammatory lesions were preceded by closed comedones, 13% by open comedones, 12% from erythematous macules, and 6% from ice pick scars. Importantly, 28% of inflammatory lesions arose from clinically normal-appearing skin, said Dr. Kang, professor and chair of the department of dermatology at Johns Hopkins University, Baltimore.
Also present at 12 weeks were a total of 104 atrophic scars as agreed upon by at least two of the three independent examining dermatologists. Nearly 70% were ice pick scars, 30% were boxcar acne scars, and 2% were rolling scars.
Of note, 23 of the 25 study participants had one or more acne scars at 3 months of follow-up.
In all, 30% of the scars were already present at baseline. Another 20% arose from inflamed papules or pustules or from closed comedones. But fully half of the scars arose from noninflammatory lesions.
The clinical implication of these findings is that aggressive treatment should be prescribed from the outset in acne patients – even in those with mild disease – to prevent acne scarring.
"Both noninflammatory as well as inflammatory acne lesions need to be addressed in order to prevent the most terrible sequelae of acne: the formation of scars," said Dr. Kang.
Based upon these findings, he said he favors multimodal acne therapy with a retinoid, an antimicrobial agent, and benzoyl peroxide.
Dr. Kang is a paid speaker for Galderma.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Half of the scars in a study of 25 patients arose from noninflammatory lesions.
Data Source: A prospective study of the natural history of acne using novel computer-assisted spatial alignment and serial high-definition digital photos to track lesions over 12 weeks.
Disclosures: The presentation was sponsored by Galderma, for which Dr. Kang is a paid speaker.
Topical Treatment for Genodermatosis Called 'Genius'
LISBON – A new topical approach to the treatment of the cutaneous abnormalities of a rare inherited disorder of lipid metabolism is drawing rave reviews from other genodermatosis experts.
"It’s a simple idea, but I think it’s a genius approach," Dr. Thomas Schwarz said at the annual congress of the European Academy of Dermatology and Venereology.
He was referring to a recent report by Dr. Amy S. Paller and her coworkers in which they described their successful reversal of the skin manifestations of congenital hemidysplasia with ichthyosiform erythroderma and limb defects (CHILD) syndrome in two patients having the X-linked dominant disorder of distal cholesterol metabolism.
The group, led by Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago, accomplished this by determining that the dry, ichthyosiform appearance of the skin in patients with CHILD syndrome is from a deficiency of cholesterol in the skin, with resultant accumulation of toxic metabolites.
"It’s a simple idea, but I think it’s a genius approach."
They opted to address this not through gene therapy, but via topical therapy with lovastatin and cholesterol. Topical lovastatin was used to block the aberrant mevalonate pathway, thereby curbing production of the toxic metabolic intermediates, and topical cholesterol was used to replace the missing lipid in the skin.
The result was clearance of the skin lesions by 3 months. This was accompanied by ultrastructural evidence indicative of normalization of epidermal structure and lipid secretion in affected skin (J. Invest. Dermatol. 2011;131:2242-8).
The brilliance of this approach lies in the fact that Dr. Paller and her coworkers were able to bypass gene therapy, said Dr. Schwarz. Gene therapy is the focus of much of the research effort aimed at developing new therapies for genodermatoses, but it is proving technically daunting and costly, and progress has been slow.
"They’ve shown you don’t need to correct the gene defect; you can just supplement what the gene is not doing. I think this is very exciting. It’s going to have a significant impact, because there are many other genodermatoses with distal defects in cholesterol metabolism," said Dr. Schwarz, director of the department of dermatology, allergy, and venereology at the University Hospital in Kiel, Germany.
Other genodermatoses where a similar therapeutic approach deserves study are X-linkedrecessive ichthyosis, Sjögren-Larsson syndrome, Conradi-Hünermann-Happle syndrome, Refsum disease, and Chanarin-Dorfman syndrome, he added.
Dr. Schwarz said he had no relevant financial disclosures.
LISBON – A new topical approach to the treatment of the cutaneous abnormalities of a rare inherited disorder of lipid metabolism is drawing rave reviews from other genodermatosis experts.
"It’s a simple idea, but I think it’s a genius approach," Dr. Thomas Schwarz said at the annual congress of the European Academy of Dermatology and Venereology.
He was referring to a recent report by Dr. Amy S. Paller and her coworkers in which they described their successful reversal of the skin manifestations of congenital hemidysplasia with ichthyosiform erythroderma and limb defects (CHILD) syndrome in two patients having the X-linked dominant disorder of distal cholesterol metabolism.
The group, led by Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago, accomplished this by determining that the dry, ichthyosiform appearance of the skin in patients with CHILD syndrome is from a deficiency of cholesterol in the skin, with resultant accumulation of toxic metabolites.
"It’s a simple idea, but I think it’s a genius approach."
They opted to address this not through gene therapy, but via topical therapy with lovastatin and cholesterol. Topical lovastatin was used to block the aberrant mevalonate pathway, thereby curbing production of the toxic metabolic intermediates, and topical cholesterol was used to replace the missing lipid in the skin.
The result was clearance of the skin lesions by 3 months. This was accompanied by ultrastructural evidence indicative of normalization of epidermal structure and lipid secretion in affected skin (J. Invest. Dermatol. 2011;131:2242-8).
The brilliance of this approach lies in the fact that Dr. Paller and her coworkers were able to bypass gene therapy, said Dr. Schwarz. Gene therapy is the focus of much of the research effort aimed at developing new therapies for genodermatoses, but it is proving technically daunting and costly, and progress has been slow.
"They’ve shown you don’t need to correct the gene defect; you can just supplement what the gene is not doing. I think this is very exciting. It’s going to have a significant impact, because there are many other genodermatoses with distal defects in cholesterol metabolism," said Dr. Schwarz, director of the department of dermatology, allergy, and venereology at the University Hospital in Kiel, Germany.
Other genodermatoses where a similar therapeutic approach deserves study are X-linkedrecessive ichthyosis, Sjögren-Larsson syndrome, Conradi-Hünermann-Happle syndrome, Refsum disease, and Chanarin-Dorfman syndrome, he added.
Dr. Schwarz said he had no relevant financial disclosures.
LISBON – A new topical approach to the treatment of the cutaneous abnormalities of a rare inherited disorder of lipid metabolism is drawing rave reviews from other genodermatosis experts.
"It’s a simple idea, but I think it’s a genius approach," Dr. Thomas Schwarz said at the annual congress of the European Academy of Dermatology and Venereology.
He was referring to a recent report by Dr. Amy S. Paller and her coworkers in which they described their successful reversal of the skin manifestations of congenital hemidysplasia with ichthyosiform erythroderma and limb defects (CHILD) syndrome in two patients having the X-linked dominant disorder of distal cholesterol metabolism.
The group, led by Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago, accomplished this by determining that the dry, ichthyosiform appearance of the skin in patients with CHILD syndrome is from a deficiency of cholesterol in the skin, with resultant accumulation of toxic metabolites.
"It’s a simple idea, but I think it’s a genius approach."
They opted to address this not through gene therapy, but via topical therapy with lovastatin and cholesterol. Topical lovastatin was used to block the aberrant mevalonate pathway, thereby curbing production of the toxic metabolic intermediates, and topical cholesterol was used to replace the missing lipid in the skin.
The result was clearance of the skin lesions by 3 months. This was accompanied by ultrastructural evidence indicative of normalization of epidermal structure and lipid secretion in affected skin (J. Invest. Dermatol. 2011;131:2242-8).
The brilliance of this approach lies in the fact that Dr. Paller and her coworkers were able to bypass gene therapy, said Dr. Schwarz. Gene therapy is the focus of much of the research effort aimed at developing new therapies for genodermatoses, but it is proving technically daunting and costly, and progress has been slow.
"They’ve shown you don’t need to correct the gene defect; you can just supplement what the gene is not doing. I think this is very exciting. It’s going to have a significant impact, because there are many other genodermatoses with distal defects in cholesterol metabolism," said Dr. Schwarz, director of the department of dermatology, allergy, and venereology at the University Hospital in Kiel, Germany.
Other genodermatoses where a similar therapeutic approach deserves study are X-linkedrecessive ichthyosis, Sjögren-Larsson syndrome, Conradi-Hünermann-Happle syndrome, Refsum disease, and Chanarin-Dorfman syndrome, he added.
Dr. Schwarz said he had no relevant financial disclosures.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: The cutaneous abnormalities of CHILD syndrome were reversed by topical therapy with lovastatin and cholesterol.
Data Source: A published report on two treated patients.
Disclosures: Dr. Schwarz said he had no relevant financial disclosures.
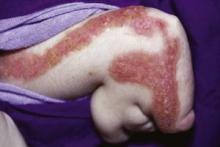
Going Green Can Turn Baby Bottoms Red
LISBON- The growing popularity of environmentally friendly reusable diapers made of cotton and bamboo is leading to increased cases of a spectrum of infant diaper dermatitis dubbed "irritant napkin papulonodules," according to Dr. Annabel Maruani.
"We believe and warn that the increasing use of reusable diapers, linked to the ecorevolution, is responsible for the reemergence of irritant napkin papulonodules. Recognizing these groups of patients is crucial in relation to management and counseling," she said at the annual congress of the European Academy of Dermatology and Venereology in Lisbon.
Dr. Maruani reported on five infants with papulonodular lesions in convex skin areas covered by reusable diapers. All cases resolved within 1 month after the reusable diapers were replaced by disposable ones, which appear to be considerably more absorbent, according to Dr. Maruani of the University of Tours (France).
One patient presented with typical Sevestre-Jacquet erosive diaper dermatitis. The other infants presented with flesh-colored umbilicated papules and/or nodules with little to no erythema. One of the children had lesions of granuloma gluteale, while the others had pseudo-verrucous papules.
Triggering factors were identified in three patients: one who had developed diarrhea, worsening the moisture problem, and two others with underfeeding, resulting in a lack of weight gain and diminished ability of the skin to deal with insults, she said.
No financial disclosures were reported.
LISBON- The growing popularity of environmentally friendly reusable diapers made of cotton and bamboo is leading to increased cases of a spectrum of infant diaper dermatitis dubbed "irritant napkin papulonodules," according to Dr. Annabel Maruani.
"We believe and warn that the increasing use of reusable diapers, linked to the ecorevolution, is responsible for the reemergence of irritant napkin papulonodules. Recognizing these groups of patients is crucial in relation to management and counseling," she said at the annual congress of the European Academy of Dermatology and Venereology in Lisbon.
Dr. Maruani reported on five infants with papulonodular lesions in convex skin areas covered by reusable diapers. All cases resolved within 1 month after the reusable diapers were replaced by disposable ones, which appear to be considerably more absorbent, according to Dr. Maruani of the University of Tours (France).
One patient presented with typical Sevestre-Jacquet erosive diaper dermatitis. The other infants presented with flesh-colored umbilicated papules and/or nodules with little to no erythema. One of the children had lesions of granuloma gluteale, while the others had pseudo-verrucous papules.
Triggering factors were identified in three patients: one who had developed diarrhea, worsening the moisture problem, and two others with underfeeding, resulting in a lack of weight gain and diminished ability of the skin to deal with insults, she said.
No financial disclosures were reported.
LISBON- The growing popularity of environmentally friendly reusable diapers made of cotton and bamboo is leading to increased cases of a spectrum of infant diaper dermatitis dubbed "irritant napkin papulonodules," according to Dr. Annabel Maruani.
"We believe and warn that the increasing use of reusable diapers, linked to the ecorevolution, is responsible for the reemergence of irritant napkin papulonodules. Recognizing these groups of patients is crucial in relation to management and counseling," she said at the annual congress of the European Academy of Dermatology and Venereology in Lisbon.
Dr. Maruani reported on five infants with papulonodular lesions in convex skin areas covered by reusable diapers. All cases resolved within 1 month after the reusable diapers were replaced by disposable ones, which appear to be considerably more absorbent, according to Dr. Maruani of the University of Tours (France).
One patient presented with typical Sevestre-Jacquet erosive diaper dermatitis. The other infants presented with flesh-colored umbilicated papules and/or nodules with little to no erythema. One of the children had lesions of granuloma gluteale, while the others had pseudo-verrucous papules.
Triggering factors were identified in three patients: one who had developed diarrhea, worsening the moisture problem, and two others with underfeeding, resulting in a lack of weight gain and diminished ability of the skin to deal with insults, she said.
No financial disclosures were reported.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
When Vitiligo and Psoriasis Coexist, Keep Looking
LISBON – Comorbid vitiligo and psoriasis is a red flag signaling the need to dig deeper looking for potentially associated diseases, including cardiovascular, autoimmune, or psychiatric diagnoses.
A study of 154 Italian vitiligo patients showed that a family history of cardiovascular disease was present in none of 54 participants with vitiligo alone compared to one-third of the 19 patients with both vitiligo and psoriasis, Dr. Silvia Moretti reported at the annual congress of the European Academy of Dermatology and Venereology.
Subjects with strong genetic loading for either or both of the skin diseases also tended to have an increased prevalence of a positive family history of cardiovascular disease, although this trend did not reach statistical significance. For example, a family history of cardiovascular disease was present in 10% of 52 patients with vitiligo and a family history of vitiligo, 13% of 15 patients with vitiligo as well as a family history of both vitiligo and psoriasis, and 18% of the 16 patients with vitiligo and a family history of psoriasis, according to Dr. Moretti of the University of Florence.
Similarly, a strong family history of hypertension was present in 4% of vitiligo patients, 8% of those with both vitiligo and a family history of vitiligo, 13% with vitiligo and a family history of both psoriasis and vitiligo, 25% of vitiligo patients with a family history of psoriasis, and 26% of patients carrying diagnoses of both vitiligo and psoriasis but no family history of either dermatologic disease. In only the group with both vitiligo and psoriasis was a family history of hypertension significantly more common than in patients with vitiligo alone.
The investigators also checked for a grab bag of comorbid, nondermatologic diseases they suspect might be genetically linked to vitiligo and psoriasis, including lupus, celiac disease, megaloblastic anemia, and allergic rhinitis. Vitiligo patients with a family history of both vitiligo and psoriasis had a 47% prevalence of one or more of these conditions, significantly greater than in the other subgroups, where the prevalence ranged from 11% to 37%. In patients with comorbid vitiligo and psoriasis but no family history of either skin disease, the rate was 21%.
The prevalence of a diagnosed psychiatric disorder was zero in patients with vitiligo with or without a family history of vitiligo, 7% in those with vitiligo and a family history of both vitiligo and psoriasis, 6% in those with vitiligo and a family history of psoriasis, and 5% in patients with comorbid vitiligo and psoriasis.
Seventy-two percent of patients with both vitiligo and psoriasis were Fitzpatrick phototype 2 and the rest were phototype 3. Seventy-nine percent of those with vitiligo and a family history of both psoriasis and vitiligo were phototype 2, as were 56% of vitiligo patients with a family history of psoriasis.
In contrast, 60% of patients with only vitiligo were phototype 3 and 40% were phototype 2. The same 60/40 split held for patients with vitiligo and a family history of vitiligo.
Dr. Moretti declared having no financial conflicts of interest.
LISBON – Comorbid vitiligo and psoriasis is a red flag signaling the need to dig deeper looking for potentially associated diseases, including cardiovascular, autoimmune, or psychiatric diagnoses.
A study of 154 Italian vitiligo patients showed that a family history of cardiovascular disease was present in none of 54 participants with vitiligo alone compared to one-third of the 19 patients with both vitiligo and psoriasis, Dr. Silvia Moretti reported at the annual congress of the European Academy of Dermatology and Venereology.
Subjects with strong genetic loading for either or both of the skin diseases also tended to have an increased prevalence of a positive family history of cardiovascular disease, although this trend did not reach statistical significance. For example, a family history of cardiovascular disease was present in 10% of 52 patients with vitiligo and a family history of vitiligo, 13% of 15 patients with vitiligo as well as a family history of both vitiligo and psoriasis, and 18% of the 16 patients with vitiligo and a family history of psoriasis, according to Dr. Moretti of the University of Florence.
Similarly, a strong family history of hypertension was present in 4% of vitiligo patients, 8% of those with both vitiligo and a family history of vitiligo, 13% with vitiligo and a family history of both psoriasis and vitiligo, 25% of vitiligo patients with a family history of psoriasis, and 26% of patients carrying diagnoses of both vitiligo and psoriasis but no family history of either dermatologic disease. In only the group with both vitiligo and psoriasis was a family history of hypertension significantly more common than in patients with vitiligo alone.
The investigators also checked for a grab bag of comorbid, nondermatologic diseases they suspect might be genetically linked to vitiligo and psoriasis, including lupus, celiac disease, megaloblastic anemia, and allergic rhinitis. Vitiligo patients with a family history of both vitiligo and psoriasis had a 47% prevalence of one or more of these conditions, significantly greater than in the other subgroups, where the prevalence ranged from 11% to 37%. In patients with comorbid vitiligo and psoriasis but no family history of either skin disease, the rate was 21%.
The prevalence of a diagnosed psychiatric disorder was zero in patients with vitiligo with or without a family history of vitiligo, 7% in those with vitiligo and a family history of both vitiligo and psoriasis, 6% in those with vitiligo and a family history of psoriasis, and 5% in patients with comorbid vitiligo and psoriasis.
Seventy-two percent of patients with both vitiligo and psoriasis were Fitzpatrick phototype 2 and the rest were phototype 3. Seventy-nine percent of those with vitiligo and a family history of both psoriasis and vitiligo were phototype 2, as were 56% of vitiligo patients with a family history of psoriasis.
In contrast, 60% of patients with only vitiligo were phototype 3 and 40% were phototype 2. The same 60/40 split held for patients with vitiligo and a family history of vitiligo.
Dr. Moretti declared having no financial conflicts of interest.
LISBON – Comorbid vitiligo and psoriasis is a red flag signaling the need to dig deeper looking for potentially associated diseases, including cardiovascular, autoimmune, or psychiatric diagnoses.
A study of 154 Italian vitiligo patients showed that a family history of cardiovascular disease was present in none of 54 participants with vitiligo alone compared to one-third of the 19 patients with both vitiligo and psoriasis, Dr. Silvia Moretti reported at the annual congress of the European Academy of Dermatology and Venereology.
Subjects with strong genetic loading for either or both of the skin diseases also tended to have an increased prevalence of a positive family history of cardiovascular disease, although this trend did not reach statistical significance. For example, a family history of cardiovascular disease was present in 10% of 52 patients with vitiligo and a family history of vitiligo, 13% of 15 patients with vitiligo as well as a family history of both vitiligo and psoriasis, and 18% of the 16 patients with vitiligo and a family history of psoriasis, according to Dr. Moretti of the University of Florence.
Similarly, a strong family history of hypertension was present in 4% of vitiligo patients, 8% of those with both vitiligo and a family history of vitiligo, 13% with vitiligo and a family history of both psoriasis and vitiligo, 25% of vitiligo patients with a family history of psoriasis, and 26% of patients carrying diagnoses of both vitiligo and psoriasis but no family history of either dermatologic disease. In only the group with both vitiligo and psoriasis was a family history of hypertension significantly more common than in patients with vitiligo alone.
The investigators also checked for a grab bag of comorbid, nondermatologic diseases they suspect might be genetically linked to vitiligo and psoriasis, including lupus, celiac disease, megaloblastic anemia, and allergic rhinitis. Vitiligo patients with a family history of both vitiligo and psoriasis had a 47% prevalence of one or more of these conditions, significantly greater than in the other subgroups, where the prevalence ranged from 11% to 37%. In patients with comorbid vitiligo and psoriasis but no family history of either skin disease, the rate was 21%.
The prevalence of a diagnosed psychiatric disorder was zero in patients with vitiligo with or without a family history of vitiligo, 7% in those with vitiligo and a family history of both vitiligo and psoriasis, 6% in those with vitiligo and a family history of psoriasis, and 5% in patients with comorbid vitiligo and psoriasis.
Seventy-two percent of patients with both vitiligo and psoriasis were Fitzpatrick phototype 2 and the rest were phototype 3. Seventy-nine percent of those with vitiligo and a family history of both psoriasis and vitiligo were phototype 2, as were 56% of vitiligo patients with a family history of psoriasis.
In contrast, 60% of patients with only vitiligo were phototype 3 and 40% were phototype 2. The same 60/40 split held for patients with vitiligo and a family history of vitiligo.
Dr. Moretti declared having no financial conflicts of interest.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: A family history of cardiovascular disease was present in none of 54 participants with vitiligo alone compared with one-third of the 19 patients with both vitiligo and psoriasis.
Data Source: A study of 154 vitiligo patients and their families.
Disclosures: No financial conflicts were reported.
Aggressive Nonmelanoma Skin Cancers Often Misdiagnosed
LISBON – Two of 10 nonmelanoma skin cancers are misdiagnosed as being of a nonaggressive tumor subtype at initial biopsy, according to Dr. Nathalie Zeitouni.
This raises concern that a substantial number of biopsied squamous and basal cell carcinomas are being treated suboptimally, Dr. Zeitouni said at the congress.
She presented a consecutive series of 513 patients referred for Mohs micrographic surgery for biopsy-proven BCC or SCC. Based upon routine Mohs intraoperative evaluation of all histologic tumor layers, 21.1% of the cancers were of aggressive subtypes that went undiagnosed on initial biopsy.
Aggressive subtypes of nonmelanoma skin cancer include basosquamous carcinoma, invasive SCC, and morpheaform, infiltrating, keratinizing, and micronodular BCC. Nonaggressive subtypes include follicular, nodular, adenoid cystic, and superficial BCC, as well as SCC in situ, according to Dr. Zeitouni, chief of dermatologic surgery at the Roswell Park Cancer Institute, Buffalo, N.Y.
In only 51% of cases was there concordance between the preoperative and the definitive intraoperative diagnosis of a nonmelanoma skin cancer as being of an aggressive or nonaggressive subtype.
In 21% of cases the intraoperative evaluation showed no residual tumor present, only scar. In 5.5% of cases, intraoperative histologic tumor layer evaluation resulted in downgrading of the nonmelanoma skin cancer from an aggressive to a nonaggressive subtype.
Dr. Zeitouni stressed that dermatologists need to have a low threshold for suspecting that a nonmelanoma skin cancer is of an undiagnosed aggressive subtype. If the lesion is clinically atypical or it responds poorly to standard excision or simple destructive measures, that possibility becomes distinctly more likely. Aggressive subtypes, she added, are best managed by Mohs surgery.
She said she had no relevant financial disclosures.
LISBON – Two of 10 nonmelanoma skin cancers are misdiagnosed as being of a nonaggressive tumor subtype at initial biopsy, according to Dr. Nathalie Zeitouni.
This raises concern that a substantial number of biopsied squamous and basal cell carcinomas are being treated suboptimally, Dr. Zeitouni said at the congress.
She presented a consecutive series of 513 patients referred for Mohs micrographic surgery for biopsy-proven BCC or SCC. Based upon routine Mohs intraoperative evaluation of all histologic tumor layers, 21.1% of the cancers were of aggressive subtypes that went undiagnosed on initial biopsy.
Aggressive subtypes of nonmelanoma skin cancer include basosquamous carcinoma, invasive SCC, and morpheaform, infiltrating, keratinizing, and micronodular BCC. Nonaggressive subtypes include follicular, nodular, adenoid cystic, and superficial BCC, as well as SCC in situ, according to Dr. Zeitouni, chief of dermatologic surgery at the Roswell Park Cancer Institute, Buffalo, N.Y.
In only 51% of cases was there concordance between the preoperative and the definitive intraoperative diagnosis of a nonmelanoma skin cancer as being of an aggressive or nonaggressive subtype.
In 21% of cases the intraoperative evaluation showed no residual tumor present, only scar. In 5.5% of cases, intraoperative histologic tumor layer evaluation resulted in downgrading of the nonmelanoma skin cancer from an aggressive to a nonaggressive subtype.
Dr. Zeitouni stressed that dermatologists need to have a low threshold for suspecting that a nonmelanoma skin cancer is of an undiagnosed aggressive subtype. If the lesion is clinically atypical or it responds poorly to standard excision or simple destructive measures, that possibility becomes distinctly more likely. Aggressive subtypes, she added, are best managed by Mohs surgery.
She said she had no relevant financial disclosures.
LISBON – Two of 10 nonmelanoma skin cancers are misdiagnosed as being of a nonaggressive tumor subtype at initial biopsy, according to Dr. Nathalie Zeitouni.
This raises concern that a substantial number of biopsied squamous and basal cell carcinomas are being treated suboptimally, Dr. Zeitouni said at the congress.
She presented a consecutive series of 513 patients referred for Mohs micrographic surgery for biopsy-proven BCC or SCC. Based upon routine Mohs intraoperative evaluation of all histologic tumor layers, 21.1% of the cancers were of aggressive subtypes that went undiagnosed on initial biopsy.
Aggressive subtypes of nonmelanoma skin cancer include basosquamous carcinoma, invasive SCC, and morpheaform, infiltrating, keratinizing, and micronodular BCC. Nonaggressive subtypes include follicular, nodular, adenoid cystic, and superficial BCC, as well as SCC in situ, according to Dr. Zeitouni, chief of dermatologic surgery at the Roswell Park Cancer Institute, Buffalo, N.Y.
In only 51% of cases was there concordance between the preoperative and the definitive intraoperative diagnosis of a nonmelanoma skin cancer as being of an aggressive or nonaggressive subtype.
In 21% of cases the intraoperative evaluation showed no residual tumor present, only scar. In 5.5% of cases, intraoperative histologic tumor layer evaluation resulted in downgrading of the nonmelanoma skin cancer from an aggressive to a nonaggressive subtype.
Dr. Zeitouni stressed that dermatologists need to have a low threshold for suspecting that a nonmelanoma skin cancer is of an undiagnosed aggressive subtype. If the lesion is clinically atypical or it responds poorly to standard excision or simple destructive measures, that possibility becomes distinctly more likely. Aggressive subtypes, she added, are best managed by Mohs surgery.
She said she had no relevant financial disclosures.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: In only 51% of cases was there concordance between the preoperative and the definitive intraoperative diagnosis of a nonmelanoma skin cancer as being of an aggressive or nonaggressive subtype.
Data Source: A retrospective analysis of 513 consecutive patients with biopsy-proven basal or squamous cell carcinoma treated with Mohs micrographic surgery.
Disclosures: Dr. Zeitouni reported having no relevant financial disclosures.
Atopy May Protect Against Skin Cancer
LISBON – In the latest installment in an ongoing debate, dermatologist Thomas Kornek reported that atopic dermatitis was found to provide protection against skin cancer in patients in a large German study.
Standardized, onsite, dermatologist-conducted skin screenings of 90,880 employees in more than 300 German businesses demonstrated that the prevalence of nonmelanoma skin cancer among the 1.3% of workers with atopic dermatitis was less than half of that in coworkers without atopic dermatitis, Dr. Kornek reported at the congress.
Moreover, even though the subgroup with atopic dermatitis had significantly higher rates of well-established risk factors for melanoma, their actual prevalence of the malignancy was identical to that in workers without atopic dermatitis, implying a protective effect against melanoma as well, added Dr. Kornek of the University Medical Center Hamburg, Germany.
Participants in the workplace skin screening program averaged 43 years of age, and 53% were men. The prevalence of clinically diagnosed nonmelanoma skin cancer among patients with atopic dermatitis was 0.4%, compared with 0.9% in participants without atopy (P less than .05).
Premalignant skin lesions were identified in 1.7% of workers with atopic dermatitis and 2.1% of controls, a nonsignificant difference.
Melanomas were detected in 0.2% of participants with or without atopic dermatitis. Yet the prevalences of Fitzpatrick skin type I, more than 40 nevi, and a history of severe sunburns in childhood – all of which are risk factors for melanoma – were significantly greater in the atopic dermatitis subgroup, which in theory should have translated into more melanomas, said Dr. Kornek.
Two warring schools of thought have clashed with regard to the relationship between atopic dermatitis and cancer. One holds that the hyperreactive immune system that defines atopy in the form of asthma, hay fever, or atopic dermatitis ought to protect against carcinoma. The other school holds that the chronic immune stimulation present in individuals with atopic dermatitis ought to result in elevated risk. An additional consideration in patients with atopic dermatitis is that local and systemic immunotherapy could potentially boost the risk of developing skin cancer, noted Dr. Kornek.
Each side can point to supporting epidemiologic studies. For example, researchers in Sweden found that the risk of developing melanoma in 6,280 atopic dermatitis patients followed for more than 230,000 person-years was half that in the general Swedish population (J. Eur. Acad. Dermatol. Venereol. 2008;22:1423-8).
Dermatologists in Stockholm used the Swedish Cancer Registry to determine that a large cohort of atopic patients had no increased risk of nonmelanoma skin cancer, lymphoma, or cancer of the lung, pancreas, or cervix (Allergy 2005;60:1116-20).
Another group of investigators in Sweden reported modest but significantly increased risks of cancer of the pancreas, esophagus, lung, and brain, as well as lymphoma, in 15,666 patients earlier hospitalized for atopic dermatitis. There was a nonsignificant trend for more nonmelanoma skin cancers in the group, but no increase in melanoma (Arch. Dermatol. 2005;141:1123-7).
A literature review by dermatologists in Germany found mixed results regarding a possible association between atopy and cancer, although the investigators summed up the findings by noting that "the emerging picture from most of the currently available epidemiological data indicates that atopic disease is associated with a reduced risk of cancer" (Allergy 2005;60:1098-11).
Dr. Kornek speculated that one possible explanation for the reduced risk of skin cancer noted in the German workplace study is because individuals with atopic dermatitis are more aware of their skin and more alert to the rise of abnormal lesions than nonatopic persons.
He declared having no financial conflicts of interest.
LISBON – In the latest installment in an ongoing debate, dermatologist Thomas Kornek reported that atopic dermatitis was found to provide protection against skin cancer in patients in a large German study.
Standardized, onsite, dermatologist-conducted skin screenings of 90,880 employees in more than 300 German businesses demonstrated that the prevalence of nonmelanoma skin cancer among the 1.3% of workers with atopic dermatitis was less than half of that in coworkers without atopic dermatitis, Dr. Kornek reported at the congress.
Moreover, even though the subgroup with atopic dermatitis had significantly higher rates of well-established risk factors for melanoma, their actual prevalence of the malignancy was identical to that in workers without atopic dermatitis, implying a protective effect against melanoma as well, added Dr. Kornek of the University Medical Center Hamburg, Germany.
Participants in the workplace skin screening program averaged 43 years of age, and 53% were men. The prevalence of clinically diagnosed nonmelanoma skin cancer among patients with atopic dermatitis was 0.4%, compared with 0.9% in participants without atopy (P less than .05).
Premalignant skin lesions were identified in 1.7% of workers with atopic dermatitis and 2.1% of controls, a nonsignificant difference.
Melanomas were detected in 0.2% of participants with or without atopic dermatitis. Yet the prevalences of Fitzpatrick skin type I, more than 40 nevi, and a history of severe sunburns in childhood – all of which are risk factors for melanoma – were significantly greater in the atopic dermatitis subgroup, which in theory should have translated into more melanomas, said Dr. Kornek.
Two warring schools of thought have clashed with regard to the relationship between atopic dermatitis and cancer. One holds that the hyperreactive immune system that defines atopy in the form of asthma, hay fever, or atopic dermatitis ought to protect against carcinoma. The other school holds that the chronic immune stimulation present in individuals with atopic dermatitis ought to result in elevated risk. An additional consideration in patients with atopic dermatitis is that local and systemic immunotherapy could potentially boost the risk of developing skin cancer, noted Dr. Kornek.
Each side can point to supporting epidemiologic studies. For example, researchers in Sweden found that the risk of developing melanoma in 6,280 atopic dermatitis patients followed for more than 230,000 person-years was half that in the general Swedish population (J. Eur. Acad. Dermatol. Venereol. 2008;22:1423-8).
Dermatologists in Stockholm used the Swedish Cancer Registry to determine that a large cohort of atopic patients had no increased risk of nonmelanoma skin cancer, lymphoma, or cancer of the lung, pancreas, or cervix (Allergy 2005;60:1116-20).
Another group of investigators in Sweden reported modest but significantly increased risks of cancer of the pancreas, esophagus, lung, and brain, as well as lymphoma, in 15,666 patients earlier hospitalized for atopic dermatitis. There was a nonsignificant trend for more nonmelanoma skin cancers in the group, but no increase in melanoma (Arch. Dermatol. 2005;141:1123-7).
A literature review by dermatologists in Germany found mixed results regarding a possible association between atopy and cancer, although the investigators summed up the findings by noting that "the emerging picture from most of the currently available epidemiological data indicates that atopic disease is associated with a reduced risk of cancer" (Allergy 2005;60:1098-11).
Dr. Kornek speculated that one possible explanation for the reduced risk of skin cancer noted in the German workplace study is because individuals with atopic dermatitis are more aware of their skin and more alert to the rise of abnormal lesions than nonatopic persons.
He declared having no financial conflicts of interest.
LISBON – In the latest installment in an ongoing debate, dermatologist Thomas Kornek reported that atopic dermatitis was found to provide protection against skin cancer in patients in a large German study.
Standardized, onsite, dermatologist-conducted skin screenings of 90,880 employees in more than 300 German businesses demonstrated that the prevalence of nonmelanoma skin cancer among the 1.3% of workers with atopic dermatitis was less than half of that in coworkers without atopic dermatitis, Dr. Kornek reported at the congress.
Moreover, even though the subgroup with atopic dermatitis had significantly higher rates of well-established risk factors for melanoma, their actual prevalence of the malignancy was identical to that in workers without atopic dermatitis, implying a protective effect against melanoma as well, added Dr. Kornek of the University Medical Center Hamburg, Germany.
Participants in the workplace skin screening program averaged 43 years of age, and 53% were men. The prevalence of clinically diagnosed nonmelanoma skin cancer among patients with atopic dermatitis was 0.4%, compared with 0.9% in participants without atopy (P less than .05).
Premalignant skin lesions were identified in 1.7% of workers with atopic dermatitis and 2.1% of controls, a nonsignificant difference.
Melanomas were detected in 0.2% of participants with or without atopic dermatitis. Yet the prevalences of Fitzpatrick skin type I, more than 40 nevi, and a history of severe sunburns in childhood – all of which are risk factors for melanoma – were significantly greater in the atopic dermatitis subgroup, which in theory should have translated into more melanomas, said Dr. Kornek.
Two warring schools of thought have clashed with regard to the relationship between atopic dermatitis and cancer. One holds that the hyperreactive immune system that defines atopy in the form of asthma, hay fever, or atopic dermatitis ought to protect against carcinoma. The other school holds that the chronic immune stimulation present in individuals with atopic dermatitis ought to result in elevated risk. An additional consideration in patients with atopic dermatitis is that local and systemic immunotherapy could potentially boost the risk of developing skin cancer, noted Dr. Kornek.
Each side can point to supporting epidemiologic studies. For example, researchers in Sweden found that the risk of developing melanoma in 6,280 atopic dermatitis patients followed for more than 230,000 person-years was half that in the general Swedish population (J. Eur. Acad. Dermatol. Venereol. 2008;22:1423-8).
Dermatologists in Stockholm used the Swedish Cancer Registry to determine that a large cohort of atopic patients had no increased risk of nonmelanoma skin cancer, lymphoma, or cancer of the lung, pancreas, or cervix (Allergy 2005;60:1116-20).
Another group of investigators in Sweden reported modest but significantly increased risks of cancer of the pancreas, esophagus, lung, and brain, as well as lymphoma, in 15,666 patients earlier hospitalized for atopic dermatitis. There was a nonsignificant trend for more nonmelanoma skin cancers in the group, but no increase in melanoma (Arch. Dermatol. 2005;141:1123-7).
A literature review by dermatologists in Germany found mixed results regarding a possible association between atopy and cancer, although the investigators summed up the findings by noting that "the emerging picture from most of the currently available epidemiological data indicates that atopic disease is associated with a reduced risk of cancer" (Allergy 2005;60:1098-11).
Dr. Kornek speculated that one possible explanation for the reduced risk of skin cancer noted in the German workplace study is because individuals with atopic dermatitis are more aware of their skin and more alert to the rise of abnormal lesions than nonatopic persons.
He declared having no financial conflicts of interest.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: German workers with atopic dermatitis had a 56% lower prevalence of nonmelanoma skin cancer than their coworkers without atopy.
Data Source: A dermatologist-conducted skin screening program involving nearly 91,000 employees at more than 300 German companies.
Disclosures: Dr. Kornek had no conflicts of interest.
Adding Methotrexate to Etanercept Boosts Psoriasis Efficacy
LISBON – Adding methotrexate to etanercept in patients with moderate to severe plaque psoriasis brings a bump in efficacy with no increase in serious adverse events, according to a large, double-blind, randomized clinical trial.
The multicenter trial included 478 patients on etanercept (Enbrel) at 50 mg twice weekly for 12 weeks, followed by once-weekly treatment thereafter. Participants were randomized to methotrexate or placebo from the outset. Methotrexate was dosed at 7.5 mg/week for the first 2 weeks, 10 mg/week for the next 2 weeks, and 15 mg/week after that.
The primary study end point was the proportion of patients attaining a PASI 75 response – that is, at least a 75% improvement over baseline scores on the Psoriasis Area and Severity Index – at week 24. The PASI 75 rate was 77.3% with dual therapy, significantly better than the 60.3% rate with etanercept alone, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
These results with combination therapy are in line with the enhanced efficacy seen with ustekinumab (Stelara) and other biologics targeting interleukin-23/Th17. But the combination therapy relies upon familiar medications that have been around a long time and have well-understood safety profiles, she noted.
The 24-week PASI 90 rate was also markedly better with dual therapy: 53.8%, compared with 34.2%. Seventy-two percent of patients on methotrexate plus etanercept were rated clear or almost clear on Physician’s Global Assessment, as were 53% on etanercept alone, according to Dr. Gottlieb, professor and chair of dermatology at Tufts Medical Center, Boston.
Adding methotrexate accelerated the time course of improvement. By week 12, 70% of patients on methotrexate plus etanercept had achieved a PASI 75 response, compared with 54% of those on etanercept alone.
Three serious adverse events occurred in each study arm. In the dual-therapy arm, there was one case each of bacterial pneumonia, lumbar spinal stenosis, and synovial cyst. In the etanercept-only arm, there was a case of acute MI, cholecystitis, and asthma.
Dr. Gottlieb disclosed that she serves as a consultant to Amgen, which sponsored the trial. In addition, she is a consultant and/or advisory board member for numerous other pharmaceutical companies.
LISBON – Adding methotrexate to etanercept in patients with moderate to severe plaque psoriasis brings a bump in efficacy with no increase in serious adverse events, according to a large, double-blind, randomized clinical trial.
The multicenter trial included 478 patients on etanercept (Enbrel) at 50 mg twice weekly for 12 weeks, followed by once-weekly treatment thereafter. Participants were randomized to methotrexate or placebo from the outset. Methotrexate was dosed at 7.5 mg/week for the first 2 weeks, 10 mg/week for the next 2 weeks, and 15 mg/week after that.
The primary study end point was the proportion of patients attaining a PASI 75 response – that is, at least a 75% improvement over baseline scores on the Psoriasis Area and Severity Index – at week 24. The PASI 75 rate was 77.3% with dual therapy, significantly better than the 60.3% rate with etanercept alone, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
These results with combination therapy are in line with the enhanced efficacy seen with ustekinumab (Stelara) and other biologics targeting interleukin-23/Th17. But the combination therapy relies upon familiar medications that have been around a long time and have well-understood safety profiles, she noted.
The 24-week PASI 90 rate was also markedly better with dual therapy: 53.8%, compared with 34.2%. Seventy-two percent of patients on methotrexate plus etanercept were rated clear or almost clear on Physician’s Global Assessment, as were 53% on etanercept alone, according to Dr. Gottlieb, professor and chair of dermatology at Tufts Medical Center, Boston.
Adding methotrexate accelerated the time course of improvement. By week 12, 70% of patients on methotrexate plus etanercept had achieved a PASI 75 response, compared with 54% of those on etanercept alone.
Three serious adverse events occurred in each study arm. In the dual-therapy arm, there was one case each of bacterial pneumonia, lumbar spinal stenosis, and synovial cyst. In the etanercept-only arm, there was a case of acute MI, cholecystitis, and asthma.
Dr. Gottlieb disclosed that she serves as a consultant to Amgen, which sponsored the trial. In addition, she is a consultant and/or advisory board member for numerous other pharmaceutical companies.
LISBON – Adding methotrexate to etanercept in patients with moderate to severe plaque psoriasis brings a bump in efficacy with no increase in serious adverse events, according to a large, double-blind, randomized clinical trial.
The multicenter trial included 478 patients on etanercept (Enbrel) at 50 mg twice weekly for 12 weeks, followed by once-weekly treatment thereafter. Participants were randomized to methotrexate or placebo from the outset. Methotrexate was dosed at 7.5 mg/week for the first 2 weeks, 10 mg/week for the next 2 weeks, and 15 mg/week after that.
The primary study end point was the proportion of patients attaining a PASI 75 response – that is, at least a 75% improvement over baseline scores on the Psoriasis Area and Severity Index – at week 24. The PASI 75 rate was 77.3% with dual therapy, significantly better than the 60.3% rate with etanercept alone, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
These results with combination therapy are in line with the enhanced efficacy seen with ustekinumab (Stelara) and other biologics targeting interleukin-23/Th17. But the combination therapy relies upon familiar medications that have been around a long time and have well-understood safety profiles, she noted.
The 24-week PASI 90 rate was also markedly better with dual therapy: 53.8%, compared with 34.2%. Seventy-two percent of patients on methotrexate plus etanercept were rated clear or almost clear on Physician’s Global Assessment, as were 53% on etanercept alone, according to Dr. Gottlieb, professor and chair of dermatology at Tufts Medical Center, Boston.
Adding methotrexate accelerated the time course of improvement. By week 12, 70% of patients on methotrexate plus etanercept had achieved a PASI 75 response, compared with 54% of those on etanercept alone.
Three serious adverse events occurred in each study arm. In the dual-therapy arm, there was one case each of bacterial pneumonia, lumbar spinal stenosis, and synovial cyst. In the etanercept-only arm, there was a case of acute MI, cholecystitis, and asthma.
Dr. Gottlieb disclosed that she serves as a consultant to Amgen, which sponsored the trial. In addition, she is a consultant and/or advisory board member for numerous other pharmaceutical companies.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Adding methotrexate to etanercept resulted in a 77% PASI 75 response rate at 24 weeks, compared with 60% with etanercept alone.
Data Source: A double-blind, randomized, placebo-controlled clinical trial involving 478 patients with moderate to severe plaque psoriasis.
Disclosures: Dr. Gottlieb disclosed that she serves as a consultant to Amgen, which sponsored the trial. In addition, she is a consultant and/or advisory board member for numerous other pharmaceutical companies.
Overweight Female Teens More Likely to Have Acne
LISBON – Overweight or obesity is independently associated with a twofold increased prevalence of moderate to severe acne in adolescent girls, according to a large population-based Norwegian study.
No such association was apparent in adolescent boys, Dr. Jon A. Halvorsen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional questionnaire study of 3,774 Oslo teenagers aged 18-19 years. He and his coworkers undertook the study because the relationship between acne and body mass index has seldom been examined. The only prior study addressing the issue dates back to 1956; it found no association between acne and overweight in a group of young male soldiers (Br. Med. J. 1956;1:1268-70).
In the new Oslo study, overweight or obesity marked by a body mass index (BMI) of at least 25 kg/m2 was present in 9.5% of girls and 15.4% of boys. Self-reported moderate or severe acne, as defined using validated criteria, was present in 13.1% of girls and 14% of boys.
In the multivariate logistic regression analysis adjusted for mental health issues, sociodemographic factors, and diet, the prevalence of moderate or severe acne in overweight or obese girls was 18.5%, compared with less than 12% in the normal-weight comparison group of girls with a BMI of 18.5 to less than 23, reported Dr. Halvorsen of the University of Oslo. Girls with a BMI of 23 to less than 25 were 30% more likely than the comparison group to have significant acne, a trend that didn’t reach significance.
The situation was quite different in boys. The highest prevalence of moderate or severe acne (18%) occurred in underweight boys having a BMI less than 18.5. Normal-weight boys had less than a 12% prevalence of significant acne, while in overweight or obese boys the prevalence was slightly greater than 13%.
Dr. Halvorsen stressed that since these findings come from a cross-sectional study, they don’t establish a causal relationship between acne and BMI. But the data do raise questions worthy of further investigation.
"Can overweight cause acne? How important is inflammation in the pathogenesis of acne? How important are hormonal factors in adolescent acne? What about physical activity: Is it related to acne prevalence? How important are lifestyle factors?" he asked.
A lack of hormonal measurements is an important limitation of the Norwegian study. It’s an issue because polycystic ovary syndrome is known to be a risk factor for both overweight and acne, he noted.
The dietary variables incorporated in the analysis included rates of consumption of soft drinks, fatty junk foods, and raw vegetables. An audience member observed that milk consumption has previously been linked to acne; however, milk intake wasn’t recorded in the study.
Dr. Halvorsen reported having no financial conflicts of interest.
LISBON – Overweight or obesity is independently associated with a twofold increased prevalence of moderate to severe acne in adolescent girls, according to a large population-based Norwegian study.
No such association was apparent in adolescent boys, Dr. Jon A. Halvorsen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional questionnaire study of 3,774 Oslo teenagers aged 18-19 years. He and his coworkers undertook the study because the relationship between acne and body mass index has seldom been examined. The only prior study addressing the issue dates back to 1956; it found no association between acne and overweight in a group of young male soldiers (Br. Med. J. 1956;1:1268-70).
In the new Oslo study, overweight or obesity marked by a body mass index (BMI) of at least 25 kg/m2 was present in 9.5% of girls and 15.4% of boys. Self-reported moderate or severe acne, as defined using validated criteria, was present in 13.1% of girls and 14% of boys.
In the multivariate logistic regression analysis adjusted for mental health issues, sociodemographic factors, and diet, the prevalence of moderate or severe acne in overweight or obese girls was 18.5%, compared with less than 12% in the normal-weight comparison group of girls with a BMI of 18.5 to less than 23, reported Dr. Halvorsen of the University of Oslo. Girls with a BMI of 23 to less than 25 were 30% more likely than the comparison group to have significant acne, a trend that didn’t reach significance.
The situation was quite different in boys. The highest prevalence of moderate or severe acne (18%) occurred in underweight boys having a BMI less than 18.5. Normal-weight boys had less than a 12% prevalence of significant acne, while in overweight or obese boys the prevalence was slightly greater than 13%.
Dr. Halvorsen stressed that since these findings come from a cross-sectional study, they don’t establish a causal relationship between acne and BMI. But the data do raise questions worthy of further investigation.
"Can overweight cause acne? How important is inflammation in the pathogenesis of acne? How important are hormonal factors in adolescent acne? What about physical activity: Is it related to acne prevalence? How important are lifestyle factors?" he asked.
A lack of hormonal measurements is an important limitation of the Norwegian study. It’s an issue because polycystic ovary syndrome is known to be a risk factor for both overweight and acne, he noted.
The dietary variables incorporated in the analysis included rates of consumption of soft drinks, fatty junk foods, and raw vegetables. An audience member observed that milk consumption has previously been linked to acne; however, milk intake wasn’t recorded in the study.
Dr. Halvorsen reported having no financial conflicts of interest.
LISBON – Overweight or obesity is independently associated with a twofold increased prevalence of moderate to severe acne in adolescent girls, according to a large population-based Norwegian study.
No such association was apparent in adolescent boys, Dr. Jon A. Halvorsen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional questionnaire study of 3,774 Oslo teenagers aged 18-19 years. He and his coworkers undertook the study because the relationship between acne and body mass index has seldom been examined. The only prior study addressing the issue dates back to 1956; it found no association between acne and overweight in a group of young male soldiers (Br. Med. J. 1956;1:1268-70).
In the new Oslo study, overweight or obesity marked by a body mass index (BMI) of at least 25 kg/m2 was present in 9.5% of girls and 15.4% of boys. Self-reported moderate or severe acne, as defined using validated criteria, was present in 13.1% of girls and 14% of boys.
In the multivariate logistic regression analysis adjusted for mental health issues, sociodemographic factors, and diet, the prevalence of moderate or severe acne in overweight or obese girls was 18.5%, compared with less than 12% in the normal-weight comparison group of girls with a BMI of 18.5 to less than 23, reported Dr. Halvorsen of the University of Oslo. Girls with a BMI of 23 to less than 25 were 30% more likely than the comparison group to have significant acne, a trend that didn’t reach significance.
The situation was quite different in boys. The highest prevalence of moderate or severe acne (18%) occurred in underweight boys having a BMI less than 18.5. Normal-weight boys had less than a 12% prevalence of significant acne, while in overweight or obese boys the prevalence was slightly greater than 13%.
Dr. Halvorsen stressed that since these findings come from a cross-sectional study, they don’t establish a causal relationship between acne and BMI. But the data do raise questions worthy of further investigation.
"Can overweight cause acne? How important is inflammation in the pathogenesis of acne? How important are hormonal factors in adolescent acne? What about physical activity: Is it related to acne prevalence? How important are lifestyle factors?" he asked.
A lack of hormonal measurements is an important limitation of the Norwegian study. It’s an issue because polycystic ovary syndrome is known to be a risk factor for both overweight and acne, he noted.
The dietary variables incorporated in the analysis included rates of consumption of soft drinks, fatty junk foods, and raw vegetables. An audience member observed that milk consumption has previously been linked to acne; however, milk intake wasn’t recorded in the study.
Dr. Halvorsen reported having no financial conflicts of interest.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: The prevalence of moderate or severe acne in overweight or obese girls was 18.5%, compared with less than 12% in the normal-weight comparison group of girls.
Data Source: A population-based, cross-sectional, questionnaire study of 3,774 patients aged 18-19 years.
Disclosures: Dr. Halvorsen reported having no financial conflicts of interest.