User login
Research refining radiomic features for lung cancer screening
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
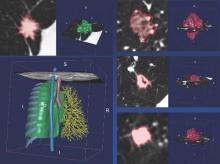
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
AT AN AACR/IASLC JOINT CONFERENCE
Key clinical point: Non-invasive radiomics is showing potential for reducing false positives by differentiating between benign and cancerous lung nodules and quantitatively predicting future risk of lung cancer incidence.
Major finding: The receiver operating characteristic area under the curve for the risk prediction model was 0.92, with 75% sensitivity and 89% specificity.
Data source: Post hoc case-control analysis in 588 persons at high risk for lung cancer in the National Lung Screening Trial.
Disclosures: Dr. Schabath reported having no relevant conflicts of interest. Dr. Gillies reported serving as a speaker for HealthMyne.
Deciphering lung cancer biomarkers shrouded in tobacco smoke
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
AT AN AACR/IASLC JOINT CONFERENCE