User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Study confirms it’s possible to catch COVID-19 twice
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Research examines links between ‘long COVID’ and ME/CFS
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FDA clears first brain stimulation device to help smokers quit
The Food and Drug Administration has granted marketing approval for the BrainsWay deep transcranial magnetic stimulation (TMS) system to help adult smokers kick tobacco.
in a press release.
As previously reported, the system has already been approved by the FDA as a treatment for patients suffering from obsessive-compulsive disorder and major depressive disorder.
The BrainsWay deep TMS system with H4-coil is designed to target addiction-related brain circuits.
It was evaluated as an aid to short-term smoking cessation in a prospective, double-blind, randomized, sham-controlled, multicenter study that involved 262 adults who had a history of smoking an average of more than 26 years and had attempted to quit multiple times but failed.
Active and sham treatments were performed daily 5 days a week for 3 weeks, followed by an additional three sessions once weekly for 3 weeks, for a total of 18 sessions over 6 weeks.
In the full intention-to-treat population (all 262 participants), the 4-week continuous quit rate (CQR, the primary endpoint) was higher in the active deep TMS group than in the sham TMS group (17.1% vs. 7.9%; P = .0238).
Among participants who completed the study, that is, those who underwent treatment for 4 weeks, who kept daily records, and for whom confirmatory urine samples were available, the CQR was 28.4% in the active deep TMS group, compared with 11.7% in the sham treatment group (P = .0063).
The average number of cigarettes smoked per day, as determined on the basis of daily records (secondary endpoint), was statistically significantly lower in the active deep TMS group, compared with the sham treatment group (P = .0311).
No patient suffered a seizure. The most common adverse event was headache, for which there was no statistical difference between the active and sham treatment groups. Other side effects included application site discomfort, back pain, muscle twitching, and discomfort.
“This FDA clearance represents a significant milestone for BrainsWay and our deep TMS platform technology,” Christopher von Jako, PhD, president and CEO of the company, said in the release.
“While other therapies are currently available, a substantial medical need continues to exist for treatments that can increase the continuous quit rate among smokers,” Dr. von Jako noted.
“Based on the compelling data from our large, randomized pivotal study of 262 subjects, we are confident that our deep TMS technology can play an important role in treating cigarette smokers who seek to quit,” he added.
The company plans a “controlled” U.S. market release of the system for this indication early next year.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has granted marketing approval for the BrainsWay deep transcranial magnetic stimulation (TMS) system to help adult smokers kick tobacco.
in a press release.
As previously reported, the system has already been approved by the FDA as a treatment for patients suffering from obsessive-compulsive disorder and major depressive disorder.
The BrainsWay deep TMS system with H4-coil is designed to target addiction-related brain circuits.
It was evaluated as an aid to short-term smoking cessation in a prospective, double-blind, randomized, sham-controlled, multicenter study that involved 262 adults who had a history of smoking an average of more than 26 years and had attempted to quit multiple times but failed.
Active and sham treatments were performed daily 5 days a week for 3 weeks, followed by an additional three sessions once weekly for 3 weeks, for a total of 18 sessions over 6 weeks.
In the full intention-to-treat population (all 262 participants), the 4-week continuous quit rate (CQR, the primary endpoint) was higher in the active deep TMS group than in the sham TMS group (17.1% vs. 7.9%; P = .0238).
Among participants who completed the study, that is, those who underwent treatment for 4 weeks, who kept daily records, and for whom confirmatory urine samples were available, the CQR was 28.4% in the active deep TMS group, compared with 11.7% in the sham treatment group (P = .0063).
The average number of cigarettes smoked per day, as determined on the basis of daily records (secondary endpoint), was statistically significantly lower in the active deep TMS group, compared with the sham treatment group (P = .0311).
No patient suffered a seizure. The most common adverse event was headache, for which there was no statistical difference between the active and sham treatment groups. Other side effects included application site discomfort, back pain, muscle twitching, and discomfort.
“This FDA clearance represents a significant milestone for BrainsWay and our deep TMS platform technology,” Christopher von Jako, PhD, president and CEO of the company, said in the release.
“While other therapies are currently available, a substantial medical need continues to exist for treatments that can increase the continuous quit rate among smokers,” Dr. von Jako noted.
“Based on the compelling data from our large, randomized pivotal study of 262 subjects, we are confident that our deep TMS technology can play an important role in treating cigarette smokers who seek to quit,” he added.
The company plans a “controlled” U.S. market release of the system for this indication early next year.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has granted marketing approval for the BrainsWay deep transcranial magnetic stimulation (TMS) system to help adult smokers kick tobacco.
in a press release.
As previously reported, the system has already been approved by the FDA as a treatment for patients suffering from obsessive-compulsive disorder and major depressive disorder.
The BrainsWay deep TMS system with H4-coil is designed to target addiction-related brain circuits.
It was evaluated as an aid to short-term smoking cessation in a prospective, double-blind, randomized, sham-controlled, multicenter study that involved 262 adults who had a history of smoking an average of more than 26 years and had attempted to quit multiple times but failed.
Active and sham treatments were performed daily 5 days a week for 3 weeks, followed by an additional three sessions once weekly for 3 weeks, for a total of 18 sessions over 6 weeks.
In the full intention-to-treat population (all 262 participants), the 4-week continuous quit rate (CQR, the primary endpoint) was higher in the active deep TMS group than in the sham TMS group (17.1% vs. 7.9%; P = .0238).
Among participants who completed the study, that is, those who underwent treatment for 4 weeks, who kept daily records, and for whom confirmatory urine samples were available, the CQR was 28.4% in the active deep TMS group, compared with 11.7% in the sham treatment group (P = .0063).
The average number of cigarettes smoked per day, as determined on the basis of daily records (secondary endpoint), was statistically significantly lower in the active deep TMS group, compared with the sham treatment group (P = .0311).
No patient suffered a seizure. The most common adverse event was headache, for which there was no statistical difference between the active and sham treatment groups. Other side effects included application site discomfort, back pain, muscle twitching, and discomfort.
“This FDA clearance represents a significant milestone for BrainsWay and our deep TMS platform technology,” Christopher von Jako, PhD, president and CEO of the company, said in the release.
“While other therapies are currently available, a substantial medical need continues to exist for treatments that can increase the continuous quit rate among smokers,” Dr. von Jako noted.
“Based on the compelling data from our large, randomized pivotal study of 262 subjects, we are confident that our deep TMS technology can play an important role in treating cigarette smokers who seek to quit,” he added.
The company plans a “controlled” U.S. market release of the system for this indication early next year.
A version of this article originally appeared on Medscape.com.
FDA authorizes convalescent plasma for COVID-19
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
FDA updates hydrochlorothiazide label to include nonmelanoma skin cancer risk
and undergo regular skin cancer screening, according to updates to the medication’s label.
The skin cancer risk is small, however, and patients should continue taking HCTZ, a commonly used diuretic and antihypertensive drug, unless their doctor says otherwise, according to a U.S. Food and Drug Administration announcement about the labeling changes, which the agency approved on Aug. 20.
HCTZ, first approved in 1959, is associated with photosensitivity. Researchers identified a relationship between HCTZ and nonmelanoma skin cancer in postmarketing studies. Investigators have described dose-response patterns for basal cell carcinoma and squamous cell carcinoma (SCC).
An FDA analysis found that the risk mostly was increased for SCC. The drug was associated with approximately one additional case of SCC per 16,000 patients per year. For white patients who received a cumulative dose of 50,000 mg or more, the risk was greater. In this patient population, HCTZ was associated with about one additional case of SCC per 6,700 patients per year, according to the label.
Reliably estimating the frequency of nonmelanoma skin cancer and establishing a causal relationship to drug exposure is not possible with the available postmarketing data, the label notes
“Treatment for nonmelanoma skin cancer is typically local and successful, with very low rates of death,” the FDA said. “Meanwhile, the risks of uncontrolled blood pressure can be severe and include life-threatening heart attacks or stroke. Given this information, patients should continue to use HCTZ and take protective skin care measures to reduce their risk of nonmelanoma skin cancer, unless directed otherwise from their health care provider.”
Patients can reduce sun exposure by using broad-spectrum sunscreens with a sun protection factor value of at least 15, limiting time in the sun, and wearing protective clothing, the agency advised.
and undergo regular skin cancer screening, according to updates to the medication’s label.
The skin cancer risk is small, however, and patients should continue taking HCTZ, a commonly used diuretic and antihypertensive drug, unless their doctor says otherwise, according to a U.S. Food and Drug Administration announcement about the labeling changes, which the agency approved on Aug. 20.
HCTZ, first approved in 1959, is associated with photosensitivity. Researchers identified a relationship between HCTZ and nonmelanoma skin cancer in postmarketing studies. Investigators have described dose-response patterns for basal cell carcinoma and squamous cell carcinoma (SCC).
An FDA analysis found that the risk mostly was increased for SCC. The drug was associated with approximately one additional case of SCC per 16,000 patients per year. For white patients who received a cumulative dose of 50,000 mg or more, the risk was greater. In this patient population, HCTZ was associated with about one additional case of SCC per 6,700 patients per year, according to the label.
Reliably estimating the frequency of nonmelanoma skin cancer and establishing a causal relationship to drug exposure is not possible with the available postmarketing data, the label notes
“Treatment for nonmelanoma skin cancer is typically local and successful, with very low rates of death,” the FDA said. “Meanwhile, the risks of uncontrolled blood pressure can be severe and include life-threatening heart attacks or stroke. Given this information, patients should continue to use HCTZ and take protective skin care measures to reduce their risk of nonmelanoma skin cancer, unless directed otherwise from their health care provider.”
Patients can reduce sun exposure by using broad-spectrum sunscreens with a sun protection factor value of at least 15, limiting time in the sun, and wearing protective clothing, the agency advised.
and undergo regular skin cancer screening, according to updates to the medication’s label.
The skin cancer risk is small, however, and patients should continue taking HCTZ, a commonly used diuretic and antihypertensive drug, unless their doctor says otherwise, according to a U.S. Food and Drug Administration announcement about the labeling changes, which the agency approved on Aug. 20.
HCTZ, first approved in 1959, is associated with photosensitivity. Researchers identified a relationship between HCTZ and nonmelanoma skin cancer in postmarketing studies. Investigators have described dose-response patterns for basal cell carcinoma and squamous cell carcinoma (SCC).
An FDA analysis found that the risk mostly was increased for SCC. The drug was associated with approximately one additional case of SCC per 16,000 patients per year. For white patients who received a cumulative dose of 50,000 mg or more, the risk was greater. In this patient population, HCTZ was associated with about one additional case of SCC per 6,700 patients per year, according to the label.
Reliably estimating the frequency of nonmelanoma skin cancer and establishing a causal relationship to drug exposure is not possible with the available postmarketing data, the label notes
“Treatment for nonmelanoma skin cancer is typically local and successful, with very low rates of death,” the FDA said. “Meanwhile, the risks of uncontrolled blood pressure can be severe and include life-threatening heart attacks or stroke. Given this information, patients should continue to use HCTZ and take protective skin care measures to reduce their risk of nonmelanoma skin cancer, unless directed otherwise from their health care provider.”
Patients can reduce sun exposure by using broad-spectrum sunscreens with a sun protection factor value of at least 15, limiting time in the sun, and wearing protective clothing, the agency advised.
‘The pandemic within the pandemic’
The coronavirus has infected millions of Americans and killed over 174,000. But could it be worse? Maybe.
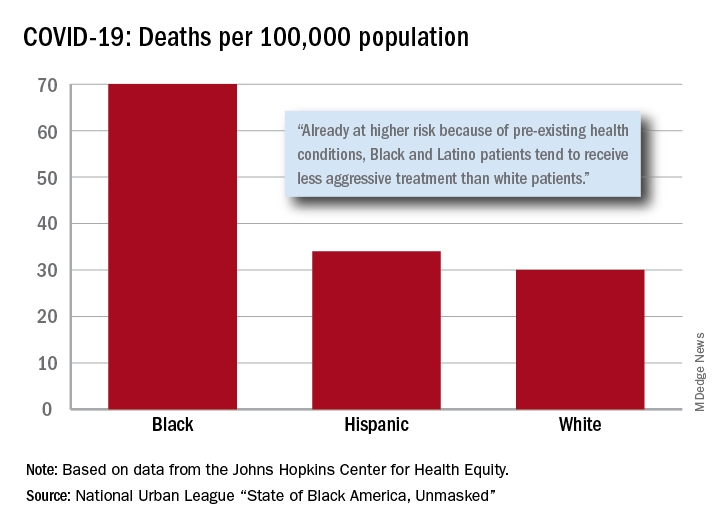
“Racism is the pandemic within the pandemic,” Marc H. Morial, president and CEO of the National Urban League, said in the 2020 “State of Black America, Unmasked” report.
“Black people with COVID-19 symptoms in February and March were less likely to get tested or treated than white patients,” he wrote.
After less testing and less treatment, the next step seems inevitable. The death rate from COVID-19 is 70 per 100,000 population among Black Americans, compared with 30 per 100,000 for Whites and 34 per 100,000 for Hispanics, the league said based on data from the Johns Hopkins Center for Health Equity.
Black and Hispanic patients with COVID-19 are more likely to have preexisting health conditions, but they “tend to receive less aggressive treatment than white patients,” the report noted. The lower death rate among Hispanics may be explained by the Black population’s greater age, although Hispanic Americans have a higher infection rate (73 per 10,000) than Blacks (62 per 10,000) or Whites (23 per 10,000).
Another possible explanation for the differences in infection rates: Blacks and Hispanics are less able to work at home because they “are overrepresented in low-wage jobs that offer the least flexibility and increase their risk of exposure to the coronavirus,” the league said.
Hispanics and Blacks also are more likely to be uninsured than Whites – 19.5% and 11.5%, respectively, vs. 7.5% – so “they tend to delay seeking treatment and are sicker than white patients when they finally do,” the league said. That may account for their much higher COVID-19 hospitalization rates: 213 per 100,000 for Blacks, 205 for Hispanics, and 46 for Whites.
“The silver lining during these dark times is that this pandemic has revealed our shared vulnerability and our interconnectedness. Many people are beginning to see that when others don’t have the opportunity to be healthy, it puts all of us at risk,” Lisa Cooper, MD, James F. Fries Professor of Medicine and Bloomberg Distinguished Professor in Health Equity at Johns Hopkins University, Baltimore, wrote in an essay accompanying the report.
The coronavirus has infected millions of Americans and killed over 174,000. But could it be worse? Maybe.

“Racism is the pandemic within the pandemic,” Marc H. Morial, president and CEO of the National Urban League, said in the 2020 “State of Black America, Unmasked” report.
“Black people with COVID-19 symptoms in February and March were less likely to get tested or treated than white patients,” he wrote.
After less testing and less treatment, the next step seems inevitable. The death rate from COVID-19 is 70 per 100,000 population among Black Americans, compared with 30 per 100,000 for Whites and 34 per 100,000 for Hispanics, the league said based on data from the Johns Hopkins Center for Health Equity.
Black and Hispanic patients with COVID-19 are more likely to have preexisting health conditions, but they “tend to receive less aggressive treatment than white patients,” the report noted. The lower death rate among Hispanics may be explained by the Black population’s greater age, although Hispanic Americans have a higher infection rate (73 per 10,000) than Blacks (62 per 10,000) or Whites (23 per 10,000).
Another possible explanation for the differences in infection rates: Blacks and Hispanics are less able to work at home because they “are overrepresented in low-wage jobs that offer the least flexibility and increase their risk of exposure to the coronavirus,” the league said.
Hispanics and Blacks also are more likely to be uninsured than Whites – 19.5% and 11.5%, respectively, vs. 7.5% – so “they tend to delay seeking treatment and are sicker than white patients when they finally do,” the league said. That may account for their much higher COVID-19 hospitalization rates: 213 per 100,000 for Blacks, 205 for Hispanics, and 46 for Whites.
“The silver lining during these dark times is that this pandemic has revealed our shared vulnerability and our interconnectedness. Many people are beginning to see that when others don’t have the opportunity to be healthy, it puts all of us at risk,” Lisa Cooper, MD, James F. Fries Professor of Medicine and Bloomberg Distinguished Professor in Health Equity at Johns Hopkins University, Baltimore, wrote in an essay accompanying the report.
The coronavirus has infected millions of Americans and killed over 174,000. But could it be worse? Maybe.

“Racism is the pandemic within the pandemic,” Marc H. Morial, president and CEO of the National Urban League, said in the 2020 “State of Black America, Unmasked” report.
“Black people with COVID-19 symptoms in February and March were less likely to get tested or treated than white patients,” he wrote.
After less testing and less treatment, the next step seems inevitable. The death rate from COVID-19 is 70 per 100,000 population among Black Americans, compared with 30 per 100,000 for Whites and 34 per 100,000 for Hispanics, the league said based on data from the Johns Hopkins Center for Health Equity.
Black and Hispanic patients with COVID-19 are more likely to have preexisting health conditions, but they “tend to receive less aggressive treatment than white patients,” the report noted. The lower death rate among Hispanics may be explained by the Black population’s greater age, although Hispanic Americans have a higher infection rate (73 per 10,000) than Blacks (62 per 10,000) or Whites (23 per 10,000).
Another possible explanation for the differences in infection rates: Blacks and Hispanics are less able to work at home because they “are overrepresented in low-wage jobs that offer the least flexibility and increase their risk of exposure to the coronavirus,” the league said.
Hispanics and Blacks also are more likely to be uninsured than Whites – 19.5% and 11.5%, respectively, vs. 7.5% – so “they tend to delay seeking treatment and are sicker than white patients when they finally do,” the league said. That may account for their much higher COVID-19 hospitalization rates: 213 per 100,000 for Blacks, 205 for Hispanics, and 46 for Whites.
“The silver lining during these dark times is that this pandemic has revealed our shared vulnerability and our interconnectedness. Many people are beginning to see that when others don’t have the opportunity to be healthy, it puts all of us at risk,” Lisa Cooper, MD, James F. Fries Professor of Medicine and Bloomberg Distinguished Professor in Health Equity at Johns Hopkins University, Baltimore, wrote in an essay accompanying the report.
Patient visits post COVID-19
Has telemedicine found its footing?
When Alexander Graham Bell invented the telephone, he accomplished something that many telegraph devotees never thought possible: the synchronous, bidirectional transmission of voice over electrical lines.
This was an incredible milestone in the advancement of mankind and enabled true revolutions in commerce, scientific collaboration, and human interaction. But Mr. Bell knew his invention didn’t represent the final advancement in telecommunication; he was quite prescient in imagining a day when individuals could see each other while speaking on the phone.
Many years later, what was once only a dream is now commonplace, and children growing up today can’t imagine a world where apps such as FaceTime and Skype don’t exist. Until recently, however, the medical community has been slow to adopt the idea of video interactions. This has dramatically changed because of the pandemic and the need for social distancing. It appears that telemedicine has found its footing, but whether it will remain popular once patients feel safe going to see their doctors in person again remains to be seen. This month, we’ll examine a few key issues that will determine the future of virtual medical visits.
Collect calling
The pandemic has wrought both human and economic casualties. With fear, job loss, and regulations leading to decreased spending, many large and small businesses have been and will continue to be unable to survive. Companies, including Brooks Brothers, Hertz, Lord and Taylor, GNC, and J.C. Penney, have declared bankruptcy.1 Medical practices and hospitals have taken cuts to their bottom line, and we’ve heard of many physician groups that have had to enact substantial salary cuts or even lay off providers – something previously unheard of. Recent months have demonstrated the health care community’s commitment to put patients first, but we simply cannot survive if we aren’t adequately reimbursed. Traditionally, this has been a significant roadblock toward the widespread adoption of telemedicine.
In most cases, these visits were not reimbursed at all. Thankfully, shortly after the coronavirus hit our shores, Medicare and Medicaid changed their policies, offering equal payment for video and in-person patient encounters. Most private insurers have followed suit, but the commitment to this payment parity appears – thus far – to be temporary. It is unclear that the financial support of telemedicine will continue post COVID-19, and this has many physicians feeling uncomfortable. In the meantime, many patients have come to prefer virtual visits, appreciating the convenience and efficiency.
Physicians don’t always have the same experience. Telemedicine can be technically challenging and take just as much – or sometimes more – time to navigate and document. Unless they are reimbursed equitably, providers will be forced to limit their use of virtual visits or not offer them at all. This leads to another issue: reliability.
‘Can you hear me now?’
Over the past several months, we have had the opportunity to use telemedicine firsthand and have spoken to many other physicians and patients about their experiences with it. The reports are all quite consistent: Most have had generally positive things to say. Still, some common concerns emerge when diving a bit deeper. Most notably are complaints about usability and reliability of the software.
While there are large telemedicine companies that have developed world-class cross-platform products, many in use today are proprietary and EHR dependent. As a result, the quality varies widely. Many EHR vendors were caught completely off guard by the sudden demand for telemedicine and are playing catch-up as they develop their own virtual visit platforms. While these vendor-developed platforms promise tight integration with patient records, some have significant shortcomings in stability when taxed under high utilization, including choppy video and garbled voice. This simply won’t do if telemedicine is to survive. It is incumbent on software developers and health care providers to invest in high-quality, reliable platforms on which to build their virtual visit offerings. This will ensure a more rapid adoption and the “staying power” of the new technology.
Dialing ‘0’ for the operator
Once seen as a “novelty” offered by only a small number of medical providers, virtual visits now represent a significant and ever-increasing percentage of patient encounters. The technology therefore must be easy to use. Given confidentiality and documentation requirements, along with the broad variety of available computing platforms and devices (e.g., PC, Mac, iOS, and Android), the process is often far from problem free. Patients may need help downloading apps, setting up webcams, or registering for the service. Providers may face issues with Internet connectivity or EHR-related delays.
It is critical that help be available to make the connection seamless and the experience a positive one. We are fortunate to work for a health care institution that has made this a priority, dedicating a team of individuals to provide real-time support to patients and clinicians. Small independent practices may not have this luxury, but we would encourage all providers to engage with their telemedicine or EHR vendors to determine what resources are available when problems arise, as they undoubtedly will.
Answering the call
Like the invention of the telephone, the advent of telemedicine is another milestone on the journey toward better communication with our patients, and it appears to be here to stay. Virtual visits won’t completely replace in-person care, nor minimize the benefit of human interaction, but they will continue to play an important role in the care continuum. By addressing the above concerns, we’ll lay a solid foundation for success and create a positive experience for physicians and patients alike.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Reference
1. A running list of companies that have filed for bankruptcy during the coronavirus pandemic. Fortune.
Has telemedicine found its footing?
Has telemedicine found its footing?
When Alexander Graham Bell invented the telephone, he accomplished something that many telegraph devotees never thought possible: the synchronous, bidirectional transmission of voice over electrical lines.
This was an incredible milestone in the advancement of mankind and enabled true revolutions in commerce, scientific collaboration, and human interaction. But Mr. Bell knew his invention didn’t represent the final advancement in telecommunication; he was quite prescient in imagining a day when individuals could see each other while speaking on the phone.
Many years later, what was once only a dream is now commonplace, and children growing up today can’t imagine a world where apps such as FaceTime and Skype don’t exist. Until recently, however, the medical community has been slow to adopt the idea of video interactions. This has dramatically changed because of the pandemic and the need for social distancing. It appears that telemedicine has found its footing, but whether it will remain popular once patients feel safe going to see their doctors in person again remains to be seen. This month, we’ll examine a few key issues that will determine the future of virtual medical visits.
Collect calling
The pandemic has wrought both human and economic casualties. With fear, job loss, and regulations leading to decreased spending, many large and small businesses have been and will continue to be unable to survive. Companies, including Brooks Brothers, Hertz, Lord and Taylor, GNC, and J.C. Penney, have declared bankruptcy.1 Medical practices and hospitals have taken cuts to their bottom line, and we’ve heard of many physician groups that have had to enact substantial salary cuts or even lay off providers – something previously unheard of. Recent months have demonstrated the health care community’s commitment to put patients first, but we simply cannot survive if we aren’t adequately reimbursed. Traditionally, this has been a significant roadblock toward the widespread adoption of telemedicine.
In most cases, these visits were not reimbursed at all. Thankfully, shortly after the coronavirus hit our shores, Medicare and Medicaid changed their policies, offering equal payment for video and in-person patient encounters. Most private insurers have followed suit, but the commitment to this payment parity appears – thus far – to be temporary. It is unclear that the financial support of telemedicine will continue post COVID-19, and this has many physicians feeling uncomfortable. In the meantime, many patients have come to prefer virtual visits, appreciating the convenience and efficiency.
Physicians don’t always have the same experience. Telemedicine can be technically challenging and take just as much – or sometimes more – time to navigate and document. Unless they are reimbursed equitably, providers will be forced to limit their use of virtual visits or not offer them at all. This leads to another issue: reliability.
‘Can you hear me now?’
Over the past several months, we have had the opportunity to use telemedicine firsthand and have spoken to many other physicians and patients about their experiences with it. The reports are all quite consistent: Most have had generally positive things to say. Still, some common concerns emerge when diving a bit deeper. Most notably are complaints about usability and reliability of the software.
While there are large telemedicine companies that have developed world-class cross-platform products, many in use today are proprietary and EHR dependent. As a result, the quality varies widely. Many EHR vendors were caught completely off guard by the sudden demand for telemedicine and are playing catch-up as they develop their own virtual visit platforms. While these vendor-developed platforms promise tight integration with patient records, some have significant shortcomings in stability when taxed under high utilization, including choppy video and garbled voice. This simply won’t do if telemedicine is to survive. It is incumbent on software developers and health care providers to invest in high-quality, reliable platforms on which to build their virtual visit offerings. This will ensure a more rapid adoption and the “staying power” of the new technology.
Dialing ‘0’ for the operator
Once seen as a “novelty” offered by only a small number of medical providers, virtual visits now represent a significant and ever-increasing percentage of patient encounters. The technology therefore must be easy to use. Given confidentiality and documentation requirements, along with the broad variety of available computing platforms and devices (e.g., PC, Mac, iOS, and Android), the process is often far from problem free. Patients may need help downloading apps, setting up webcams, or registering for the service. Providers may face issues with Internet connectivity or EHR-related delays.
It is critical that help be available to make the connection seamless and the experience a positive one. We are fortunate to work for a health care institution that has made this a priority, dedicating a team of individuals to provide real-time support to patients and clinicians. Small independent practices may not have this luxury, but we would encourage all providers to engage with their telemedicine or EHR vendors to determine what resources are available when problems arise, as they undoubtedly will.
Answering the call
Like the invention of the telephone, the advent of telemedicine is another milestone on the journey toward better communication with our patients, and it appears to be here to stay. Virtual visits won’t completely replace in-person care, nor minimize the benefit of human interaction, but they will continue to play an important role in the care continuum. By addressing the above concerns, we’ll lay a solid foundation for success and create a positive experience for physicians and patients alike.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Reference
1. A running list of companies that have filed for bankruptcy during the coronavirus pandemic. Fortune.
When Alexander Graham Bell invented the telephone, he accomplished something that many telegraph devotees never thought possible: the synchronous, bidirectional transmission of voice over electrical lines.
This was an incredible milestone in the advancement of mankind and enabled true revolutions in commerce, scientific collaboration, and human interaction. But Mr. Bell knew his invention didn’t represent the final advancement in telecommunication; he was quite prescient in imagining a day when individuals could see each other while speaking on the phone.
Many years later, what was once only a dream is now commonplace, and children growing up today can’t imagine a world where apps such as FaceTime and Skype don’t exist. Until recently, however, the medical community has been slow to adopt the idea of video interactions. This has dramatically changed because of the pandemic and the need for social distancing. It appears that telemedicine has found its footing, but whether it will remain popular once patients feel safe going to see their doctors in person again remains to be seen. This month, we’ll examine a few key issues that will determine the future of virtual medical visits.
Collect calling
The pandemic has wrought both human and economic casualties. With fear, job loss, and regulations leading to decreased spending, many large and small businesses have been and will continue to be unable to survive. Companies, including Brooks Brothers, Hertz, Lord and Taylor, GNC, and J.C. Penney, have declared bankruptcy.1 Medical practices and hospitals have taken cuts to their bottom line, and we’ve heard of many physician groups that have had to enact substantial salary cuts or even lay off providers – something previously unheard of. Recent months have demonstrated the health care community’s commitment to put patients first, but we simply cannot survive if we aren’t adequately reimbursed. Traditionally, this has been a significant roadblock toward the widespread adoption of telemedicine.
In most cases, these visits were not reimbursed at all. Thankfully, shortly after the coronavirus hit our shores, Medicare and Medicaid changed their policies, offering equal payment for video and in-person patient encounters. Most private insurers have followed suit, but the commitment to this payment parity appears – thus far – to be temporary. It is unclear that the financial support of telemedicine will continue post COVID-19, and this has many physicians feeling uncomfortable. In the meantime, many patients have come to prefer virtual visits, appreciating the convenience and efficiency.
Physicians don’t always have the same experience. Telemedicine can be technically challenging and take just as much – or sometimes more – time to navigate and document. Unless they are reimbursed equitably, providers will be forced to limit their use of virtual visits or not offer them at all. This leads to another issue: reliability.
‘Can you hear me now?’
Over the past several months, we have had the opportunity to use telemedicine firsthand and have spoken to many other physicians and patients about their experiences with it. The reports are all quite consistent: Most have had generally positive things to say. Still, some common concerns emerge when diving a bit deeper. Most notably are complaints about usability and reliability of the software.
While there are large telemedicine companies that have developed world-class cross-platform products, many in use today are proprietary and EHR dependent. As a result, the quality varies widely. Many EHR vendors were caught completely off guard by the sudden demand for telemedicine and are playing catch-up as they develop their own virtual visit platforms. While these vendor-developed platforms promise tight integration with patient records, some have significant shortcomings in stability when taxed under high utilization, including choppy video and garbled voice. This simply won’t do if telemedicine is to survive. It is incumbent on software developers and health care providers to invest in high-quality, reliable platforms on which to build their virtual visit offerings. This will ensure a more rapid adoption and the “staying power” of the new technology.
Dialing ‘0’ for the operator
Once seen as a “novelty” offered by only a small number of medical providers, virtual visits now represent a significant and ever-increasing percentage of patient encounters. The technology therefore must be easy to use. Given confidentiality and documentation requirements, along with the broad variety of available computing platforms and devices (e.g., PC, Mac, iOS, and Android), the process is often far from problem free. Patients may need help downloading apps, setting up webcams, or registering for the service. Providers may face issues with Internet connectivity or EHR-related delays.
It is critical that help be available to make the connection seamless and the experience a positive one. We are fortunate to work for a health care institution that has made this a priority, dedicating a team of individuals to provide real-time support to patients and clinicians. Small independent practices may not have this luxury, but we would encourage all providers to engage with their telemedicine or EHR vendors to determine what resources are available when problems arise, as they undoubtedly will.
Answering the call
Like the invention of the telephone, the advent of telemedicine is another milestone on the journey toward better communication with our patients, and it appears to be here to stay. Virtual visits won’t completely replace in-person care, nor minimize the benefit of human interaction, but they will continue to play an important role in the care continuum. By addressing the above concerns, we’ll lay a solid foundation for success and create a positive experience for physicians and patients alike.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Reference
1. A running list of companies that have filed for bankruptcy during the coronavirus pandemic. Fortune.
Swab, spit, stay home? College coronavirus testing plans are all over the map
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.

What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.

What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.

What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
COVID-19 linked to development of myasthenia gravis
Myasthenia gravis should be added to the growing list of potential neurological sequelae associated with COVID-19, new research suggests. Clinicians from Italy have described what they believe are
“I think it is possible that there could be many more cases,” said lead author Domenico Restivo, MD, of the Garibaldi Hospital, Catania, Italy. “In fact, myasthenia gravis could be underestimated especially in the course of COVID-19 infection in which a specific muscular weakness is frequently present. For this reason, this association is easy to miss if not top of mind,” Dr. Restivo said.
None of the three patients had previous neurologic or autoimmune disorders. In all three cases, symptoms of myasthenia gravis appeared within 5-7 days after onset of fever caused by SARS-CoV-2 infection. The time from presumed SARS-CoV-2 infection to myasthenia gravis symptoms “is consistent with the time from infection to symptoms in other neurologic disorders triggered by infections,” the investigators reported.
The findings were published online August 10 in Annals of Internal Medicine.
First patients
The first patient described in the report was a 64-year-old man who had a fever as high as 39° C (102.2° F) for 4 days. Five days after fever onset, he developed diplopia and muscle fatigue. The patient’s neurologic examination was “unremarkable.” Computed tomography (CT) of the thorax excluded thymoma, and findings on chest radiograph were normal. He tested positive for SARS-CoV-2 on nasopharyngeal swab and real-time reverse transcriptase polymerase chain reaction (RT-PCR).
The patient’s symptoms led the investigators to suspect myasthenia gravis. Repetitive stimulation of the patient’s facial nerve showed a 57% decrement, confirming involvement of the postsynaptic neuromuscular junction. The concentration of AChR antibodies in serum was also elevated (22.8 pmol/L; reference range, <0.4 pmol/L). The patient was treated with pyridostigmine bromide and prednisone and had a response “typical for someone with myasthenia gravis,” the researchers wrote.
The second patient was a 68-year-old man who had a fever as high as 38.8° C (101.8° F) for 7 days. On day 7, he developed muscle fatigue, diplopia, and dysphagia. Findings of a chest CT and neurologic exam were normal. Nasopharyngeal swab and RT-PCR testing for COVID-19 were positive. As with the first patient, myasthenia gravis was suspected because of the patient’s symptoms. Repetitive nerve stimulation revealed a postsynaptic deficit of neuromuscular transmission of the facial (52%) and ulnar (21%) nerves. His serum AChR antibody level was elevated (27.6 pmol/L). The patient improved after one cycle of intravenous immunoglobulin treatment.
Possible mechanisms
The third patient was a 71-year-old woman with cough and a fever up to 38.6° C (101.5° F) for 6 days. She initially tested negative for SARS-CoV-2 on nasopharyngeal swab and RT-PCR. Five days after her symptoms began, she developed bilateral ocular ptosis, diplopia, and hypophonia. CT of the thorax excluded thymoma but showed bilateral interstitial pneumonia. On day 6, she developed dysphagia and respiratory failure, and was transferred to the ICU where she received mechanical ventilation.
Repetitive nerve stimulation revealed a postsynaptic deficit of neuromuscular transmission of the ulnar nerve (56%), and her serum AChR antibody level was elevated (35.6 pmol/L). Five days later, a second nasopharyngeal swab test for SARS-CoV-2 was positive. The patient improved following plasmapheresis treatment and was successfully extubated.
The investigators noted that this patient received hydroxychloroquine the day after the onset of neurologic symptoms, but the drug was withdrawn a day later, so they do not believe that it caused the symptoms of myasthenia gravis.
The observations in these three patients are “consistent with reports of other infections that induce autoimmune disorders, as well as with the growing evidence of other neurologic disorders with presumed autoimmune mechanisms after COVID-19 onset,” the researchers wrote.
They offered several possible explanations for the link between COVID-19 and myasthenia gravis. “Antibodies that are directed against SARS-CoV-2 proteins may cross-react with AChR subunits, because the virus has epitopes that are similar to components of the neuromuscular junction; this is known to occur in other neurologic autoimmune disorders after infection. Alternatively, COVID-19 infection may break immunologic self-tolerance,” the investigators wrote.
“The main message for clinicians is that myasthenia gravis, as well as other neurological disorders associated with autoimmunity, could occur in the course of SARS-CoV-2 infection,” Dr. Restivo said. Prompt recognition of the disease “could lead to a drug treatment that limits its evolution as quickly as possible,” he added.
An “unmasking”
Commenting on the findings, Anthony Geraci, MD, director of neuromuscular medicine, Northwell Health, Great Neck, N.Y., said these case reports of myasthenia gravis after SARS-CoV-2 infection are “not unique or novel as there has been a long understanding that seropositive [AChR antibody-positive] myasthenia gravis can and is frequently ‘unmasked’ in the setting” of several viral and bacterial infections.
“Antibodies in myasthenia gravis are of a type that take several weeks to develop to measurable levels as in the reported cases by Restivo et al., giving strong support to the notion that subclinical myasthenia gravis can be immunologically upregulated in the setting of viral infection and this is a far more likely explanation of the observed association reported,” added Dr. Geraci, who was not involved with the research.
He noted that, at his institution, “we have also observed ocular myasthenia gravis emerge in patients with SARS-CoV-2 infection, with similar double vision and lid droop, as we have seen similarly in patients with Zika, West Nile, and other viral infections, as well as a multiplicity of bacterial infections.”
“Most of our observed patients have responded to treatment much the same as reported by the three cases from Restivo and colleagues,” Dr.Geraci reported.
The authors of the study disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Myasthenia gravis should be added to the growing list of potential neurological sequelae associated with COVID-19, new research suggests. Clinicians from Italy have described what they believe are
“I think it is possible that there could be many more cases,” said lead author Domenico Restivo, MD, of the Garibaldi Hospital, Catania, Italy. “In fact, myasthenia gravis could be underestimated especially in the course of COVID-19 infection in which a specific muscular weakness is frequently present. For this reason, this association is easy to miss if not top of mind,” Dr. Restivo said.
None of the three patients had previous neurologic or autoimmune disorders. In all three cases, symptoms of myasthenia gravis appeared within 5-7 days after onset of fever caused by SARS-CoV-2 infection. The time from presumed SARS-CoV-2 infection to myasthenia gravis symptoms “is consistent with the time from infection to symptoms in other neurologic disorders triggered by infections,” the investigators reported.
The findings were published online August 10 in Annals of Internal Medicine.
First patients
The first patient described in the report was a 64-year-old man who had a fever as high as 39° C (102.2° F) for 4 days. Five days after fever onset, he developed diplopia and muscle fatigue. The patient’s neurologic examination was “unremarkable.” Computed tomography (CT) of the thorax excluded thymoma, and findings on chest radiograph were normal. He tested positive for SARS-CoV-2 on nasopharyngeal swab and real-time reverse transcriptase polymerase chain reaction (RT-PCR).
The patient’s symptoms led the investigators to suspect myasthenia gravis. Repetitive stimulation of the patient’s facial nerve showed a 57% decrement, confirming involvement of the postsynaptic neuromuscular junction. The concentration of AChR antibodies in serum was also elevated (22.8 pmol/L; reference range, <0.4 pmol/L). The patient was treated with pyridostigmine bromide and prednisone and had a response “typical for someone with myasthenia gravis,” the researchers wrote.
The second patient was a 68-year-old man who had a fever as high as 38.8° C (101.8° F) for 7 days. On day 7, he developed muscle fatigue, diplopia, and dysphagia. Findings of a chest CT and neurologic exam were normal. Nasopharyngeal swab and RT-PCR testing for COVID-19 were positive. As with the first patient, myasthenia gravis was suspected because of the patient’s symptoms. Repetitive nerve stimulation revealed a postsynaptic deficit of neuromuscular transmission of the facial (52%) and ulnar (21%) nerves. His serum AChR antibody level was elevated (27.6 pmol/L). The patient improved after one cycle of intravenous immunoglobulin treatment.
Possible mechanisms
The third patient was a 71-year-old woman with cough and a fever up to 38.6° C (101.5° F) for 6 days. She initially tested negative for SARS-CoV-2 on nasopharyngeal swab and RT-PCR. Five days after her symptoms began, she developed bilateral ocular ptosis, diplopia, and hypophonia. CT of the thorax excluded thymoma but showed bilateral interstitial pneumonia. On day 6, she developed dysphagia and respiratory failure, and was transferred to the ICU where she received mechanical ventilation.
Repetitive nerve stimulation revealed a postsynaptic deficit of neuromuscular transmission of the ulnar nerve (56%), and her serum AChR antibody level was elevated (35.6 pmol/L). Five days later, a second nasopharyngeal swab test for SARS-CoV-2 was positive. The patient improved following plasmapheresis treatment and was successfully extubated.
The investigators noted that this patient received hydroxychloroquine the day after the onset of neurologic symptoms, but the drug was withdrawn a day later, so they do not believe that it caused the symptoms of myasthenia gravis.
The observations in these three patients are “consistent with reports of other infections that induce autoimmune disorders, as well as with the growing evidence of other neurologic disorders with presumed autoimmune mechanisms after COVID-19 onset,” the researchers wrote.
They offered several possible explanations for the link between COVID-19 and myasthenia gravis. “Antibodies that are directed against SARS-CoV-2 proteins may cross-react with AChR subunits, because the virus has epitopes that are similar to components of the neuromuscular junction; this is known to occur in other neurologic autoimmune disorders after infection. Alternatively, COVID-19 infection may break immunologic self-tolerance,” the investigators wrote.
“The main message for clinicians is that myasthenia gravis, as well as other neurological disorders associated with autoimmunity, could occur in the course of SARS-CoV-2 infection,” Dr. Restivo said. Prompt recognition of the disease “could lead to a drug treatment that limits its evolution as quickly as possible,” he added.
An “unmasking”
Commenting on the findings, Anthony Geraci, MD, director of neuromuscular medicine, Northwell Health, Great Neck, N.Y., said these case reports of myasthenia gravis after SARS-CoV-2 infection are “not unique or novel as there has been a long understanding that seropositive [AChR antibody-positive] myasthenia gravis can and is frequently ‘unmasked’ in the setting” of several viral and bacterial infections.
“Antibodies in myasthenia gravis are of a type that take several weeks to develop to measurable levels as in the reported cases by Restivo et al., giving strong support to the notion that subclinical myasthenia gravis can be immunologically upregulated in the setting of viral infection and this is a far more likely explanation of the observed association reported,” added Dr. Geraci, who was not involved with the research.
He noted that, at his institution, “we have also observed ocular myasthenia gravis emerge in patients with SARS-CoV-2 infection, with similar double vision and lid droop, as we have seen similarly in patients with Zika, West Nile, and other viral infections, as well as a multiplicity of bacterial infections.”
“Most of our observed patients have responded to treatment much the same as reported by the three cases from Restivo and colleagues,” Dr.Geraci reported.
The authors of the study disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Myasthenia gravis should be added to the growing list of potential neurological sequelae associated with COVID-19, new research suggests. Clinicians from Italy have described what they believe are
“I think it is possible that there could be many more cases,” said lead author Domenico Restivo, MD, of the Garibaldi Hospital, Catania, Italy. “In fact, myasthenia gravis could be underestimated especially in the course of COVID-19 infection in which a specific muscular weakness is frequently present. For this reason, this association is easy to miss if not top of mind,” Dr. Restivo said.
None of the three patients had previous neurologic or autoimmune disorders. In all three cases, symptoms of myasthenia gravis appeared within 5-7 days after onset of fever caused by SARS-CoV-2 infection. The time from presumed SARS-CoV-2 infection to myasthenia gravis symptoms “is consistent with the time from infection to symptoms in other neurologic disorders triggered by infections,” the investigators reported.
The findings were published online August 10 in Annals of Internal Medicine.
First patients
The first patient described in the report was a 64-year-old man who had a fever as high as 39° C (102.2° F) for 4 days. Five days after fever onset, he developed diplopia and muscle fatigue. The patient’s neurologic examination was “unremarkable.” Computed tomography (CT) of the thorax excluded thymoma, and findings on chest radiograph were normal. He tested positive for SARS-CoV-2 on nasopharyngeal swab and real-time reverse transcriptase polymerase chain reaction (RT-PCR).
The patient’s symptoms led the investigators to suspect myasthenia gravis. Repetitive stimulation of the patient’s facial nerve showed a 57% decrement, confirming involvement of the postsynaptic neuromuscular junction. The concentration of AChR antibodies in serum was also elevated (22.8 pmol/L; reference range, <0.4 pmol/L). The patient was treated with pyridostigmine bromide and prednisone and had a response “typical for someone with myasthenia gravis,” the researchers wrote.
The second patient was a 68-year-old man who had a fever as high as 38.8° C (101.8° F) for 7 days. On day 7, he developed muscle fatigue, diplopia, and dysphagia. Findings of a chest CT and neurologic exam were normal. Nasopharyngeal swab and RT-PCR testing for COVID-19 were positive. As with the first patient, myasthenia gravis was suspected because of the patient’s symptoms. Repetitive nerve stimulation revealed a postsynaptic deficit of neuromuscular transmission of the facial (52%) and ulnar (21%) nerves. His serum AChR antibody level was elevated (27.6 pmol/L). The patient improved after one cycle of intravenous immunoglobulin treatment.
Possible mechanisms
The third patient was a 71-year-old woman with cough and a fever up to 38.6° C (101.5° F) for 6 days. She initially tested negative for SARS-CoV-2 on nasopharyngeal swab and RT-PCR. Five days after her symptoms began, she developed bilateral ocular ptosis, diplopia, and hypophonia. CT of the thorax excluded thymoma but showed bilateral interstitial pneumonia. On day 6, she developed dysphagia and respiratory failure, and was transferred to the ICU where she received mechanical ventilation.
Repetitive nerve stimulation revealed a postsynaptic deficit of neuromuscular transmission of the ulnar nerve (56%), and her serum AChR antibody level was elevated (35.6 pmol/L). Five days later, a second nasopharyngeal swab test for SARS-CoV-2 was positive. The patient improved following plasmapheresis treatment and was successfully extubated.
The investigators noted that this patient received hydroxychloroquine the day after the onset of neurologic symptoms, but the drug was withdrawn a day later, so they do not believe that it caused the symptoms of myasthenia gravis.
The observations in these three patients are “consistent with reports of other infections that induce autoimmune disorders, as well as with the growing evidence of other neurologic disorders with presumed autoimmune mechanisms after COVID-19 onset,” the researchers wrote.
They offered several possible explanations for the link between COVID-19 and myasthenia gravis. “Antibodies that are directed against SARS-CoV-2 proteins may cross-react with AChR subunits, because the virus has epitopes that are similar to components of the neuromuscular junction; this is known to occur in other neurologic autoimmune disorders after infection. Alternatively, COVID-19 infection may break immunologic self-tolerance,” the investigators wrote.
“The main message for clinicians is that myasthenia gravis, as well as other neurological disorders associated with autoimmunity, could occur in the course of SARS-CoV-2 infection,” Dr. Restivo said. Prompt recognition of the disease “could lead to a drug treatment that limits its evolution as quickly as possible,” he added.
An “unmasking”
Commenting on the findings, Anthony Geraci, MD, director of neuromuscular medicine, Northwell Health, Great Neck, N.Y., said these case reports of myasthenia gravis after SARS-CoV-2 infection are “not unique or novel as there has been a long understanding that seropositive [AChR antibody-positive] myasthenia gravis can and is frequently ‘unmasked’ in the setting” of several viral and bacterial infections.
“Antibodies in myasthenia gravis are of a type that take several weeks to develop to measurable levels as in the reported cases by Restivo et al., giving strong support to the notion that subclinical myasthenia gravis can be immunologically upregulated in the setting of viral infection and this is a far more likely explanation of the observed association reported,” added Dr. Geraci, who was not involved with the research.
He noted that, at his institution, “we have also observed ocular myasthenia gravis emerge in patients with SARS-CoV-2 infection, with similar double vision and lid droop, as we have seen similarly in patients with Zika, West Nile, and other viral infections, as well as a multiplicity of bacterial infections.”
“Most of our observed patients have responded to treatment much the same as reported by the three cases from Restivo and colleagues,” Dr.Geraci reported.
The authors of the study disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Hospitalists confront administrative, financial challenges of COVID-19 crisis
Hospitalists nationwide have put in longer hours, played new clinical roles, and stretched beyond their medical specialty and comfort level to meet their hospital’s COVID-19 care demands. Can they expect some kind of financial recognition – perhaps in the form of “hazard pay” for going above and beyond – even though their institutions are experiencing negative financial fallout from the crisis?
Hospitals in regions experiencing a COVID-19 surge have limited elective procedures, discouraged non–COVID-19 admissions, and essentially entered crisis management mode. Other facilities in less hard-hit communities are also standing by, with reduced hospital census, smaller caseloads and less work to do, while trying to prepare their bottom lines for lower demand.
“This crisis has put most hospitals in financial jeopardy and that is likely to trickle down to all employees – including hospitalists,” said Ron Greeno, MD, FCCP, MHM, a past president of SHM and the society’s current senior advisor for government affairs. “But it’s not like hospitals could or would forgo an effective hospitalist program today. Hospitalists will be important players in defining the hospital’s future direction post crisis.”
That doesn’t mean tighter financials, caps on annual salary increases, or higher productivity expectations won’t be part of future conversations between hospital administrators and their hospitalists, Dr. Greeno said. Administrators are starting to look ahead to the post–COVID-19 era even as numbers of cases and rates of growth continue to rise in various regions, and Dr. Greeno sees a lot of uncertainty ahead.
Even prior to the crisis, he noted, hospital margins had been falling, while the cost of labor, including hospitalist labor, was going up. That was pointing toward an inevitable collision, which has only intensified with the new financial crisis facing hospitals – created by SARS-CoV-2 and by policies such as shutting down elective surgeries in anticipation of a COVID-19 patient surge that, for some institutions, may never come.
Brian Harte, MD, MHM, president of Cleveland Clinic Akron General and a past president of SHM, said that the Cleveland Clinic system has been planning since January its response to the coming crisis. “Governor Mike DeWine and the state Department of Health led the way in flattening the curve in Ohio. We engaged our hospitalists in brainstorming solutions. They have been excellent partners,” he said.
Approaching the crisis with a sense of urgency from the outset, the Cleveland Clinic built a COVID-19 surge team and incident command structure, with nursing, infectious diseases, critical care and hospital medicine represented. “We used that time to get ready for what was coming. We worked on streamlining consultant work flows.”
But utilization numbers are off in almost every service line, Dr. Harte said. “It has forced us to look at things we’ve always talked about, including greater use of telemedicine and exploring other ways of caring for patients, such as increased use of evening hours.”
Cleveland Clinic contracts with Sound Physicians of Tacoma, Wash., for its hospitalist coverage. “We have an excellent working relationship with Sound at the local, regional, and national levels, with common goals for quality and utilization. We tried to involve our hospitalists as early as possible in planning. We needed them to step in and role model and lead the way,” Dr. Harte said, for everybody’s anxiety levels.
“We’re still in the process of understanding the long-term financial impact of the epidemic,” Dr. Harte added. “But at this point I see no reason to think our relationship with our hospitalists needs to change. We’re the stewards of long-term finances. We’ll need to keep a close eye on this. But we’re committed to working through this together.”
Hazard pay for frontline health care workers was included in the COVID-19 relief package assembled in mid-May by Democrats in the House of Representatives. The $3 trillion HEROES Act includes $200 billion to award hazard pay to essential workers, including those in the health field, but Senate Majority Leader Mitch McConnell (R-Ky.) declared the legislation “dead on arrival” in the Senate.
Supplementary hazard payments made by hospitals to their hospitalists as a reward for sacrifices they made in the crisis is an interesting question, Dr. Greeno noted, and it’s definitely on the table at some hospitals. “But I think it is going to be a tough ask in these times.”
Dr. Harte said he has not offered nor been asked about hazard pay for hospitalists. Cleveland Clinic Akron General made a strategic decision that hazard pay was not going to be part of its response to the pandemic. Other hospital administrators interviewed for this article concur.
Hospitals respond to the fiscal crisis
Hospitals in other parts of the country also report significant fiscal fallout from the COVID-19 crisis, with predictions that 100 or more hospitals may be forced to close. Jeff Dye, president of the New Mexico Hospital Association, told the Albuquerque Journal on May 1 that hospitals in his state have been squeezed on all sides by increased costs, patients delaying routine care, and public health orders restricting elective surgeries. New Mexico hospitals, especially in rural areas, face incredible financial strain.
The University of Virginia Medical Center, Charlottesville, recently announced 20% reductions in total compensation for its providers through July 31, along with suspension of retirement contributions. Those changes won’t affect team members caring for COVID-19 patients. And the Spectrum Health Medical Group of 15 hospitals in western Michigan, according to Michigan Public Radio, told its doctors they either needed to sign “contract addendums” giving the system more control over their hours – or face a 25% pay cut, or worse.
Cheyenne (Wyo.) Regional Medical Center issued a statement April 24 that it expected losses of $10 million for the month of April. “CRMC, like every other hospital in Wyoming, is certainly feeling the financial impact that COVID-19 is having,” CEO Tim Thornell told the Cowboy State Daily on April 24. That includes a 30% reduction in inpatient care and 50% reduction in outpatient care, while the hospital has only had a handful of COVID-19 patients at any time. Capital projects are now on hold, overtime is limited, and a hiring freeze is in effect.
“We’re certainly prepared for a larger surge, which hasn’t come yet,” Mr. Thornell said in an interview. CRMC’s ICU was split to create a nine-bed dedicated COVID-19 unit. Intensivists see most of the critical care patients, while the hospital’s 15 directly-employed hospitalists are treating all of the non-ICU COVID-19 patients. “Among themselves, the hospitalists volunteered who would work on the unit. We’ve been fortunate enough to have enough volunteers and enough PPE [personal protective equipment],” he said.
Preparing for the COVID-19 pandemic has strengthened the medical center’s relationship with its hospitalists, Mr. Thornell explained. “Hospitalists are key to our operations, involved in so much that happens here. We’re trying to staff to volume with decreased utilization. We’ve scaled back, which only makes fiscal sense. Now, how do we reinfuse patients back into the mix? Our hospitalists are paid by the number of shifts, and as you distribute shift reductions over 15 providers, it shouldn’t be an intolerable burden.” But two open hospitalist positions have not been filled, he noted.
CRMC is trying to approach these changes with a Lean perspective, Mr. Thornell said. “We had already adopted a Lean program, but this has been a chance to go through a life-altering circumstance using the tools of Lean planning and applying them instantaneously.”
Providers step up
At Emory Healthcare in Atlanta, a major center for COVID-19 cases, communication has been essential in the crisis, said Bryce Gartland, MD, SFHM, Emory’s hospital group president and cochief of clinical operations. “Our group was prepared for a significant influx of patients. Like every other institution, we made the decision to postpone elective care, with a resulting plummet in volume,” he said.
As COVID-19 patients entered the Emory system, frontline hospitalists stepped up to care for those patients. “We’ve had ample providers in terms of clinical care. We guaranteed our physicians’ base compensation. They have flexed teams up and down as needed.” Advanced practice professionals also stepped up to bridge gaps.
With regard to the return of volumes of non–COVID-19 patients, the jury’s still out, Dr. Gartland said. “None of us has a crystal ball, and there are tremendous variables and decision points that will have significant impact. We have started to see numbers of time-sensitive and essential cases increase as of the first week of May.”
What lies ahead will likely include some rightsizing to future volumes. On top of that, the broader economic pressures on hospitals from high rates of unemployment, uninsured patients, bad debt, and charity care will push health care systems to significantly address costs and infrastructure, he said. “We’re still early in planning, and striving to maintain flexibility and nimbleness, given the uncertainties to this early understanding of our new normal. No hospital is immune from the financial impact. We’ll see and hear about more of these conversations in the months ahead.”
But the experience has also generated some positives, Dr. Gartland noted. “Things like telehealth, which we’ve been talking about for years but previously faced barriers to widespread adoption.” Now with COVID-19, the federal government issued waivers, and barriers – both internal and external – came down. “With telehealth, what will the role and deployment of hospitalists look like in this new model? How will traditional productivity expectations change, or the numbers and types of providers? This will make the relationship and partnership between hospitalist groups and hospital administrators ever more important as we consider the evolution toward new care models.”
Dr. Gartland said that “one of the great things about hospital medicine as a field is its flexibility and adaptability. Where there have been gaps, hospitalists were quick to step in. As long as hospital medicine continues to embrace those kinds of behaviors, it will be successful.” But if the conversation with hospitals is just about money, it will be harder, he acknowledged. “Where there is this kind of disruption in our usual way of doing things, there are also tremendous opportunities for care model innovation. I would encourage hospitalist groups to try to be true value partners.”
Command center mode
Like other physicians in hospital C-suites, Chad Whelan MD, FACP, SFHM, chief executive officer of Banner–University Medicine in Tucson, Ariz., led his two hospitals into command center mode when the crisis hit, planning for a surge of COVID-19 cases that could overwhelm hospital capacity.
“In terms of our hospitalists, we leaned in to them hard in the beginning, preparing them to supervise other physicians who came in to help if needed,” he said. “Our [non–COVID-19] census is down, revenues are down, and the implications are enormous – like nothing we’ve ever seen before.”
“We’re fortunate that we’re part of the Banner health system. We made a decision that we would essentially keep our physicians financially protected through this crisis,” Dr. Whelan said. “In return, we called on them to step up and be on the front lines and to put in enormous hours for planning. We asked them to consider: How could you contribute if the surge comes?”
He affirmed that hospital medicine has been a major part of his medical center’s planning and implementation. “I’ve been overwhelmed by the degree to which the entire delivery team has rallied around the pandemic, with everybody saying they want to keep people safe and be part of the solution. We have always had hospitalist leaders at the table as we’ve planned our response and as decisions were made,” said Dr. Whelan, a practicing hospitalist and teaching service attending since 2000 until he assumed his current executive position in Arizona 18 months ago.
“While we have kept people whole during the immediate crisis, we have acknowledged that we don’t know what our recovery will look like. What if [non–COVID-19] volume doesn’t return? That keeps me awake at night,” he said. “I have talked to our physician leadership in hospital medicine and more broadly. We need to ask ourselves many questions, including: do we have the right levels of staffing? Is this the time to consider alternate models of staffing, for example, advanced practice providers? And does the compensation plan need adjustments?”
Dr. Whelan thinks that the COVID-19 crisis is an opportunity for hospital medicine to more rapidly explore different models and to ask what additional value hospitalists can bring to the care model. “For example, what would it mean to redefine the hospitalist’s scope of practice as an acute medicine specialist, not defined by the hospital’s four walls?” he noted.
“One of the reasons our smaller hospital reached capacity with COVID-19 patients was the skilled nursing facility located a few hundred feet away that turned into a hot spot. If we had imported the hospital medicine model virtually into that SNF early on, could there have been a different scenario? Have we thought through what that would have even looked like?” Dr. Whelan asked.
He challenges the hospital medicine field, once it gets to the other side of this crisis, to not fall back on old way of doing things. “Instead, let’s use this time to create a better model today,” he said. “That’s what we’re trying to do at a system level at Banner, with our hospital medicine groups partnering with the hospital. I want to see our hospitalists create and thrive in that new model.”
Hospitalists nationwide have put in longer hours, played new clinical roles, and stretched beyond their medical specialty and comfort level to meet their hospital’s COVID-19 care demands. Can they expect some kind of financial recognition – perhaps in the form of “hazard pay” for going above and beyond – even though their institutions are experiencing negative financial fallout from the crisis?
Hospitals in regions experiencing a COVID-19 surge have limited elective procedures, discouraged non–COVID-19 admissions, and essentially entered crisis management mode. Other facilities in less hard-hit communities are also standing by, with reduced hospital census, smaller caseloads and less work to do, while trying to prepare their bottom lines for lower demand.
“This crisis has put most hospitals in financial jeopardy and that is likely to trickle down to all employees – including hospitalists,” said Ron Greeno, MD, FCCP, MHM, a past president of SHM and the society’s current senior advisor for government affairs. “But it’s not like hospitals could or would forgo an effective hospitalist program today. Hospitalists will be important players in defining the hospital’s future direction post crisis.”
That doesn’t mean tighter financials, caps on annual salary increases, or higher productivity expectations won’t be part of future conversations between hospital administrators and their hospitalists, Dr. Greeno said. Administrators are starting to look ahead to the post–COVID-19 era even as numbers of cases and rates of growth continue to rise in various regions, and Dr. Greeno sees a lot of uncertainty ahead.
Even prior to the crisis, he noted, hospital margins had been falling, while the cost of labor, including hospitalist labor, was going up. That was pointing toward an inevitable collision, which has only intensified with the new financial crisis facing hospitals – created by SARS-CoV-2 and by policies such as shutting down elective surgeries in anticipation of a COVID-19 patient surge that, for some institutions, may never come.
Brian Harte, MD, MHM, president of Cleveland Clinic Akron General and a past president of SHM, said that the Cleveland Clinic system has been planning since January its response to the coming crisis. “Governor Mike DeWine and the state Department of Health led the way in flattening the curve in Ohio. We engaged our hospitalists in brainstorming solutions. They have been excellent partners,” he said.
Approaching the crisis with a sense of urgency from the outset, the Cleveland Clinic built a COVID-19 surge team and incident command structure, with nursing, infectious diseases, critical care and hospital medicine represented. “We used that time to get ready for what was coming. We worked on streamlining consultant work flows.”
But utilization numbers are off in almost every service line, Dr. Harte said. “It has forced us to look at things we’ve always talked about, including greater use of telemedicine and exploring other ways of caring for patients, such as increased use of evening hours.”
Cleveland Clinic contracts with Sound Physicians of Tacoma, Wash., for its hospitalist coverage. “We have an excellent working relationship with Sound at the local, regional, and national levels, with common goals for quality and utilization. We tried to involve our hospitalists as early as possible in planning. We needed them to step in and role model and lead the way,” Dr. Harte said, for everybody’s anxiety levels.
“We’re still in the process of understanding the long-term financial impact of the epidemic,” Dr. Harte added. “But at this point I see no reason to think our relationship with our hospitalists needs to change. We’re the stewards of long-term finances. We’ll need to keep a close eye on this. But we’re committed to working through this together.”
Hazard pay for frontline health care workers was included in the COVID-19 relief package assembled in mid-May by Democrats in the House of Representatives. The $3 trillion HEROES Act includes $200 billion to award hazard pay to essential workers, including those in the health field, but Senate Majority Leader Mitch McConnell (R-Ky.) declared the legislation “dead on arrival” in the Senate.
Supplementary hazard payments made by hospitals to their hospitalists as a reward for sacrifices they made in the crisis is an interesting question, Dr. Greeno noted, and it’s definitely on the table at some hospitals. “But I think it is going to be a tough ask in these times.”
Dr. Harte said he has not offered nor been asked about hazard pay for hospitalists. Cleveland Clinic Akron General made a strategic decision that hazard pay was not going to be part of its response to the pandemic. Other hospital administrators interviewed for this article concur.
Hospitals respond to the fiscal crisis
Hospitals in other parts of the country also report significant fiscal fallout from the COVID-19 crisis, with predictions that 100 or more hospitals may be forced to close. Jeff Dye, president of the New Mexico Hospital Association, told the Albuquerque Journal on May 1 that hospitals in his state have been squeezed on all sides by increased costs, patients delaying routine care, and public health orders restricting elective surgeries. New Mexico hospitals, especially in rural areas, face incredible financial strain.
The University of Virginia Medical Center, Charlottesville, recently announced 20% reductions in total compensation for its providers through July 31, along with suspension of retirement contributions. Those changes won’t affect team members caring for COVID-19 patients. And the Spectrum Health Medical Group of 15 hospitals in western Michigan, according to Michigan Public Radio, told its doctors they either needed to sign “contract addendums” giving the system more control over their hours – or face a 25% pay cut, or worse.
Cheyenne (Wyo.) Regional Medical Center issued a statement April 24 that it expected losses of $10 million for the month of April. “CRMC, like every other hospital in Wyoming, is certainly feeling the financial impact that COVID-19 is having,” CEO Tim Thornell told the Cowboy State Daily on April 24. That includes a 30% reduction in inpatient care and 50% reduction in outpatient care, while the hospital has only had a handful of COVID-19 patients at any time. Capital projects are now on hold, overtime is limited, and a hiring freeze is in effect.
“We’re certainly prepared for a larger surge, which hasn’t come yet,” Mr. Thornell said in an interview. CRMC’s ICU was split to create a nine-bed dedicated COVID-19 unit. Intensivists see most of the critical care patients, while the hospital’s 15 directly-employed hospitalists are treating all of the non-ICU COVID-19 patients. “Among themselves, the hospitalists volunteered who would work on the unit. We’ve been fortunate enough to have enough volunteers and enough PPE [personal protective equipment],” he said.
Preparing for the COVID-19 pandemic has strengthened the medical center’s relationship with its hospitalists, Mr. Thornell explained. “Hospitalists are key to our operations, involved in so much that happens here. We’re trying to staff to volume with decreased utilization. We’ve scaled back, which only makes fiscal sense. Now, how do we reinfuse patients back into the mix? Our hospitalists are paid by the number of shifts, and as you distribute shift reductions over 15 providers, it shouldn’t be an intolerable burden.” But two open hospitalist positions have not been filled, he noted.
CRMC is trying to approach these changes with a Lean perspective, Mr. Thornell said. “We had already adopted a Lean program, but this has been a chance to go through a life-altering circumstance using the tools of Lean planning and applying them instantaneously.”
Providers step up
At Emory Healthcare in Atlanta, a major center for COVID-19 cases, communication has been essential in the crisis, said Bryce Gartland, MD, SFHM, Emory’s hospital group president and cochief of clinical operations. “Our group was prepared for a significant influx of patients. Like every other institution, we made the decision to postpone elective care, with a resulting plummet in volume,” he said.
As COVID-19 patients entered the Emory system, frontline hospitalists stepped up to care for those patients. “We’ve had ample providers in terms of clinical care. We guaranteed our physicians’ base compensation. They have flexed teams up and down as needed.” Advanced practice professionals also stepped up to bridge gaps.
With regard to the return of volumes of non–COVID-19 patients, the jury’s still out, Dr. Gartland said. “None of us has a crystal ball, and there are tremendous variables and decision points that will have significant impact. We have started to see numbers of time-sensitive and essential cases increase as of the first week of May.”
What lies ahead will likely include some rightsizing to future volumes. On top of that, the broader economic pressures on hospitals from high rates of unemployment, uninsured patients, bad debt, and charity care will push health care systems to significantly address costs and infrastructure, he said. “We’re still early in planning, and striving to maintain flexibility and nimbleness, given the uncertainties to this early understanding of our new normal. No hospital is immune from the financial impact. We’ll see and hear about more of these conversations in the months ahead.”
But the experience has also generated some positives, Dr. Gartland noted. “Things like telehealth, which we’ve been talking about for years but previously faced barriers to widespread adoption.” Now with COVID-19, the federal government issued waivers, and barriers – both internal and external – came down. “With telehealth, what will the role and deployment of hospitalists look like in this new model? How will traditional productivity expectations change, or the numbers and types of providers? This will make the relationship and partnership between hospitalist groups and hospital administrators ever more important as we consider the evolution toward new care models.”
Dr. Gartland said that “one of the great things about hospital medicine as a field is its flexibility and adaptability. Where there have been gaps, hospitalists were quick to step in. As long as hospital medicine continues to embrace those kinds of behaviors, it will be successful.” But if the conversation with hospitals is just about money, it will be harder, he acknowledged. “Where there is this kind of disruption in our usual way of doing things, there are also tremendous opportunities for care model innovation. I would encourage hospitalist groups to try to be true value partners.”
Command center mode
Like other physicians in hospital C-suites, Chad Whelan MD, FACP, SFHM, chief executive officer of Banner–University Medicine in Tucson, Ariz., led his two hospitals into command center mode when the crisis hit, planning for a surge of COVID-19 cases that could overwhelm hospital capacity.
“In terms of our hospitalists, we leaned in to them hard in the beginning, preparing them to supervise other physicians who came in to help if needed,” he said. “Our [non–COVID-19] census is down, revenues are down, and the implications are enormous – like nothing we’ve ever seen before.”
“We’re fortunate that we’re part of the Banner health system. We made a decision that we would essentially keep our physicians financially protected through this crisis,” Dr. Whelan said. “In return, we called on them to step up and be on the front lines and to put in enormous hours for planning. We asked them to consider: How could you contribute if the surge comes?”
He affirmed that hospital medicine has been a major part of his medical center’s planning and implementation. “I’ve been overwhelmed by the degree to which the entire delivery team has rallied around the pandemic, with everybody saying they want to keep people safe and be part of the solution. We have always had hospitalist leaders at the table as we’ve planned our response and as decisions were made,” said Dr. Whelan, a practicing hospitalist and teaching service attending since 2000 until he assumed his current executive position in Arizona 18 months ago.
“While we have kept people whole during the immediate crisis, we have acknowledged that we don’t know what our recovery will look like. What if [non–COVID-19] volume doesn’t return? That keeps me awake at night,” he said. “I have talked to our physician leadership in hospital medicine and more broadly. We need to ask ourselves many questions, including: do we have the right levels of staffing? Is this the time to consider alternate models of staffing, for example, advanced practice providers? And does the compensation plan need adjustments?”
Dr. Whelan thinks that the COVID-19 crisis is an opportunity for hospital medicine to more rapidly explore different models and to ask what additional value hospitalists can bring to the care model. “For example, what would it mean to redefine the hospitalist’s scope of practice as an acute medicine specialist, not defined by the hospital’s four walls?” he noted.
“One of the reasons our smaller hospital reached capacity with COVID-19 patients was the skilled nursing facility located a few hundred feet away that turned into a hot spot. If we had imported the hospital medicine model virtually into that SNF early on, could there have been a different scenario? Have we thought through what that would have even looked like?” Dr. Whelan asked.
He challenges the hospital medicine field, once it gets to the other side of this crisis, to not fall back on old way of doing things. “Instead, let’s use this time to create a better model today,” he said. “That’s what we’re trying to do at a system level at Banner, with our hospital medicine groups partnering with the hospital. I want to see our hospitalists create and thrive in that new model.”
Hospitalists nationwide have put in longer hours, played new clinical roles, and stretched beyond their medical specialty and comfort level to meet their hospital’s COVID-19 care demands. Can they expect some kind of financial recognition – perhaps in the form of “hazard pay” for going above and beyond – even though their institutions are experiencing negative financial fallout from the crisis?
Hospitals in regions experiencing a COVID-19 surge have limited elective procedures, discouraged non–COVID-19 admissions, and essentially entered crisis management mode. Other facilities in less hard-hit communities are also standing by, with reduced hospital census, smaller caseloads and less work to do, while trying to prepare their bottom lines for lower demand.
“This crisis has put most hospitals in financial jeopardy and that is likely to trickle down to all employees – including hospitalists,” said Ron Greeno, MD, FCCP, MHM, a past president of SHM and the society’s current senior advisor for government affairs. “But it’s not like hospitals could or would forgo an effective hospitalist program today. Hospitalists will be important players in defining the hospital’s future direction post crisis.”
That doesn’t mean tighter financials, caps on annual salary increases, or higher productivity expectations won’t be part of future conversations between hospital administrators and their hospitalists, Dr. Greeno said. Administrators are starting to look ahead to the post–COVID-19 era even as numbers of cases and rates of growth continue to rise in various regions, and Dr. Greeno sees a lot of uncertainty ahead.
Even prior to the crisis, he noted, hospital margins had been falling, while the cost of labor, including hospitalist labor, was going up. That was pointing toward an inevitable collision, which has only intensified with the new financial crisis facing hospitals – created by SARS-CoV-2 and by policies such as shutting down elective surgeries in anticipation of a COVID-19 patient surge that, for some institutions, may never come.
Brian Harte, MD, MHM, president of Cleveland Clinic Akron General and a past president of SHM, said that the Cleveland Clinic system has been planning since January its response to the coming crisis. “Governor Mike DeWine and the state Department of Health led the way in flattening the curve in Ohio. We engaged our hospitalists in brainstorming solutions. They have been excellent partners,” he said.
Approaching the crisis with a sense of urgency from the outset, the Cleveland Clinic built a COVID-19 surge team and incident command structure, with nursing, infectious diseases, critical care and hospital medicine represented. “We used that time to get ready for what was coming. We worked on streamlining consultant work flows.”
But utilization numbers are off in almost every service line, Dr. Harte said. “It has forced us to look at things we’ve always talked about, including greater use of telemedicine and exploring other ways of caring for patients, such as increased use of evening hours.”
Cleveland Clinic contracts with Sound Physicians of Tacoma, Wash., for its hospitalist coverage. “We have an excellent working relationship with Sound at the local, regional, and national levels, with common goals for quality and utilization. We tried to involve our hospitalists as early as possible in planning. We needed them to step in and role model and lead the way,” Dr. Harte said, for everybody’s anxiety levels.
“We’re still in the process of understanding the long-term financial impact of the epidemic,” Dr. Harte added. “But at this point I see no reason to think our relationship with our hospitalists needs to change. We’re the stewards of long-term finances. We’ll need to keep a close eye on this. But we’re committed to working through this together.”
Hazard pay for frontline health care workers was included in the COVID-19 relief package assembled in mid-May by Democrats in the House of Representatives. The $3 trillion HEROES Act includes $200 billion to award hazard pay to essential workers, including those in the health field, but Senate Majority Leader Mitch McConnell (R-Ky.) declared the legislation “dead on arrival” in the Senate.
Supplementary hazard payments made by hospitals to their hospitalists as a reward for sacrifices they made in the crisis is an interesting question, Dr. Greeno noted, and it’s definitely on the table at some hospitals. “But I think it is going to be a tough ask in these times.”
Dr. Harte said he has not offered nor been asked about hazard pay for hospitalists. Cleveland Clinic Akron General made a strategic decision that hazard pay was not going to be part of its response to the pandemic. Other hospital administrators interviewed for this article concur.
Hospitals respond to the fiscal crisis
Hospitals in other parts of the country also report significant fiscal fallout from the COVID-19 crisis, with predictions that 100 or more hospitals may be forced to close. Jeff Dye, president of the New Mexico Hospital Association, told the Albuquerque Journal on May 1 that hospitals in his state have been squeezed on all sides by increased costs, patients delaying routine care, and public health orders restricting elective surgeries. New Mexico hospitals, especially in rural areas, face incredible financial strain.
The University of Virginia Medical Center, Charlottesville, recently announced 20% reductions in total compensation for its providers through July 31, along with suspension of retirement contributions. Those changes won’t affect team members caring for COVID-19 patients. And the Spectrum Health Medical Group of 15 hospitals in western Michigan, according to Michigan Public Radio, told its doctors they either needed to sign “contract addendums” giving the system more control over their hours – or face a 25% pay cut, or worse.
Cheyenne (Wyo.) Regional Medical Center issued a statement April 24 that it expected losses of $10 million for the month of April. “CRMC, like every other hospital in Wyoming, is certainly feeling the financial impact that COVID-19 is having,” CEO Tim Thornell told the Cowboy State Daily on April 24. That includes a 30% reduction in inpatient care and 50% reduction in outpatient care, while the hospital has only had a handful of COVID-19 patients at any time. Capital projects are now on hold, overtime is limited, and a hiring freeze is in effect.
“We’re certainly prepared for a larger surge, which hasn’t come yet,” Mr. Thornell said in an interview. CRMC’s ICU was split to create a nine-bed dedicated COVID-19 unit. Intensivists see most of the critical care patients, while the hospital’s 15 directly-employed hospitalists are treating all of the non-ICU COVID-19 patients. “Among themselves, the hospitalists volunteered who would work on the unit. We’ve been fortunate enough to have enough volunteers and enough PPE [personal protective equipment],” he said.
Preparing for the COVID-19 pandemic has strengthened the medical center’s relationship with its hospitalists, Mr. Thornell explained. “Hospitalists are key to our operations, involved in so much that happens here. We’re trying to staff to volume with decreased utilization. We’ve scaled back, which only makes fiscal sense. Now, how do we reinfuse patients back into the mix? Our hospitalists are paid by the number of shifts, and as you distribute shift reductions over 15 providers, it shouldn’t be an intolerable burden.” But two open hospitalist positions have not been filled, he noted.
CRMC is trying to approach these changes with a Lean perspective, Mr. Thornell said. “We had already adopted a Lean program, but this has been a chance to go through a life-altering circumstance using the tools of Lean planning and applying them instantaneously.”
Providers step up
At Emory Healthcare in Atlanta, a major center for COVID-19 cases, communication has been essential in the crisis, said Bryce Gartland, MD, SFHM, Emory’s hospital group president and cochief of clinical operations. “Our group was prepared for a significant influx of patients. Like every other institution, we made the decision to postpone elective care, with a resulting plummet in volume,” he said.
As COVID-19 patients entered the Emory system, frontline hospitalists stepped up to care for those patients. “We’ve had ample providers in terms of clinical care. We guaranteed our physicians’ base compensation. They have flexed teams up and down as needed.” Advanced practice professionals also stepped up to bridge gaps.
With regard to the return of volumes of non–COVID-19 patients, the jury’s still out, Dr. Gartland said. “None of us has a crystal ball, and there are tremendous variables and decision points that will have significant impact. We have started to see numbers of time-sensitive and essential cases increase as of the first week of May.”
What lies ahead will likely include some rightsizing to future volumes. On top of that, the broader economic pressures on hospitals from high rates of unemployment, uninsured patients, bad debt, and charity care will push health care systems to significantly address costs and infrastructure, he said. “We’re still early in planning, and striving to maintain flexibility and nimbleness, given the uncertainties to this early understanding of our new normal. No hospital is immune from the financial impact. We’ll see and hear about more of these conversations in the months ahead.”
But the experience has also generated some positives, Dr. Gartland noted. “Things like telehealth, which we’ve been talking about for years but previously faced barriers to widespread adoption.” Now with COVID-19, the federal government issued waivers, and barriers – both internal and external – came down. “With telehealth, what will the role and deployment of hospitalists look like in this new model? How will traditional productivity expectations change, or the numbers and types of providers? This will make the relationship and partnership between hospitalist groups and hospital administrators ever more important as we consider the evolution toward new care models.”
Dr. Gartland said that “one of the great things about hospital medicine as a field is its flexibility and adaptability. Where there have been gaps, hospitalists were quick to step in. As long as hospital medicine continues to embrace those kinds of behaviors, it will be successful.” But if the conversation with hospitals is just about money, it will be harder, he acknowledged. “Where there is this kind of disruption in our usual way of doing things, there are also tremendous opportunities for care model innovation. I would encourage hospitalist groups to try to be true value partners.”
Command center mode
Like other physicians in hospital C-suites, Chad Whelan MD, FACP, SFHM, chief executive officer of Banner–University Medicine in Tucson, Ariz., led his two hospitals into command center mode when the crisis hit, planning for a surge of COVID-19 cases that could overwhelm hospital capacity.
“In terms of our hospitalists, we leaned in to them hard in the beginning, preparing them to supervise other physicians who came in to help if needed,” he said. “Our [non–COVID-19] census is down, revenues are down, and the implications are enormous – like nothing we’ve ever seen before.”
“We’re fortunate that we’re part of the Banner health system. We made a decision that we would essentially keep our physicians financially protected through this crisis,” Dr. Whelan said. “In return, we called on them to step up and be on the front lines and to put in enormous hours for planning. We asked them to consider: How could you contribute if the surge comes?”
He affirmed that hospital medicine has been a major part of his medical center’s planning and implementation. “I’ve been overwhelmed by the degree to which the entire delivery team has rallied around the pandemic, with everybody saying they want to keep people safe and be part of the solution. We have always had hospitalist leaders at the table as we’ve planned our response and as decisions were made,” said Dr. Whelan, a practicing hospitalist and teaching service attending since 2000 until he assumed his current executive position in Arizona 18 months ago.
“While we have kept people whole during the immediate crisis, we have acknowledged that we don’t know what our recovery will look like. What if [non–COVID-19] volume doesn’t return? That keeps me awake at night,” he said. “I have talked to our physician leadership in hospital medicine and more broadly. We need to ask ourselves many questions, including: do we have the right levels of staffing? Is this the time to consider alternate models of staffing, for example, advanced practice providers? And does the compensation plan need adjustments?”
Dr. Whelan thinks that the COVID-19 crisis is an opportunity for hospital medicine to more rapidly explore different models and to ask what additional value hospitalists can bring to the care model. “For example, what would it mean to redefine the hospitalist’s scope of practice as an acute medicine specialist, not defined by the hospital’s four walls?” he noted.
“One of the reasons our smaller hospital reached capacity with COVID-19 patients was the skilled nursing facility located a few hundred feet away that turned into a hot spot. If we had imported the hospital medicine model virtually into that SNF early on, could there have been a different scenario? Have we thought through what that would have even looked like?” Dr. Whelan asked.
He challenges the hospital medicine field, once it gets to the other side of this crisis, to not fall back on old way of doing things. “Instead, let’s use this time to create a better model today,” he said. “That’s what we’re trying to do at a system level at Banner, with our hospital medicine groups partnering with the hospital. I want to see our hospitalists create and thrive in that new model.”







