User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
HHS, Surgeon General urge action on maternal health
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.
Cost is the main hurdle to broad use of caplacizumab for TTP
As hematologists debated the role of the anti–von Willebrand factor agent caplacizumab for acquired thrombotic thrombocytopenic purpura (TTP), an investigator on the phase 3 trial that led to its approval had a message.
,” said hematologist Spero Cataland, MD, of the department of internal medicine at Ohio State University in Columbus.
If cost is going to be a factor, and it “has to be in our world these days, it’s more of a discussion,” he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The HERCULES trial Dr. Cataland helped conduct found a median time to platelet count normalization of 2.69 days when caplacizumab was started during plasma exchange versus 2.88 days for placebo; 12% of patients had a TTP recurrence while they continued caplacizumab for 30 days past their last exchange and were followed for an additional 28 days versus 38% randomized to placebo. Caplacizumab subjects needed an average of 5.8 days of plasma exchange versus 9.4 days in the placebo arm (N Engl J Med. 2019 Jan 24;380(4):335-46).
Based on the results, the Food and Drug Administration approved the agent for acquired TTP in combination with plasma exchange and immunosuppressives in Feb. 2019 for 30 days beyond the last plasma exchange, with up to 28 additional days if ADAMTS13 activity remains suppressed. Labeling notes a risk of severe bleeding.
“The data on refractory disease and mortality aren’t quite there yet, but there’s a suggestion [caplacizumab] might impact that as well,” Dr. Cataland said. In its recent TTP guidelines, the International Society on Thrombosis and Haemostasis gave the agent only a conditional recommendation, in part because it’s backed up only by HERCULES and a phase 2 trial.
Also, the group noted that in the phase 2 study caplacizumab patients had a clinically and statistically significant increase in the number of relapses at 12 months: 31% versus 8% placebo. “Caplacizumab may leave patients prone to experience a later recurrence owing to the unresolved ADAMTS13 deficiency and inhibitors,” Dr. Cataland said.
“We do see some early recurrence” when caplacizumab is stopped, suggesting that when the agent’s “protective effect is removed, the risk is still there,” said Dr. Cataland, who was also an author on the ISTH guidelines, as well as the phase 2 trial.
It raises the question of how long patients should be kept on caplacizumab. There are few data on the issue, “but the consensus has been to stop caplacizumab when two consecutive ADAMTS13 measurements show 20% or greater activity,” or perhaps with one reading above 20% in a patient trending in the right direction. “With a bleeding complication, you might stop it sooner,” he said.
Dr. Cataland anticipates TTP management will eventually move away from plasma exchange to more directed therapies, including caplacizumab and perhaps recombinant ADAMTS13, which is in development.
There have been a few reports of TTP patients who refuse plasma exchange on religious grounds being successfully treated with caplacizumab. Dr. Cataland also noted a patient of his with relapsing TTP who didn’t want to be admitted yet again for plasma exchange and steroids at the start of a new episode.
“We managed her with caplacizumab and rituximab, and in a couple weeks she had recovered her ADAMTS13 activity and was able to stop the caplacizumab.” She was a motivated, knowledgeable person, “someone I trusted, so I was comfortable with the approach. I think that may be where we are headed in the future, hopefully,” he said.
Dr. Cataland disclosed research funding and consulting fees from Alexion, caplacizumab’s maker, Sanofi Genzyme, and Takeda,. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
As hematologists debated the role of the anti–von Willebrand factor agent caplacizumab for acquired thrombotic thrombocytopenic purpura (TTP), an investigator on the phase 3 trial that led to its approval had a message.
,” said hematologist Spero Cataland, MD, of the department of internal medicine at Ohio State University in Columbus.
If cost is going to be a factor, and it “has to be in our world these days, it’s more of a discussion,” he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The HERCULES trial Dr. Cataland helped conduct found a median time to platelet count normalization of 2.69 days when caplacizumab was started during plasma exchange versus 2.88 days for placebo; 12% of patients had a TTP recurrence while they continued caplacizumab for 30 days past their last exchange and were followed for an additional 28 days versus 38% randomized to placebo. Caplacizumab subjects needed an average of 5.8 days of plasma exchange versus 9.4 days in the placebo arm (N Engl J Med. 2019 Jan 24;380(4):335-46).
Based on the results, the Food and Drug Administration approved the agent for acquired TTP in combination with plasma exchange and immunosuppressives in Feb. 2019 for 30 days beyond the last plasma exchange, with up to 28 additional days if ADAMTS13 activity remains suppressed. Labeling notes a risk of severe bleeding.
“The data on refractory disease and mortality aren’t quite there yet, but there’s a suggestion [caplacizumab] might impact that as well,” Dr. Cataland said. In its recent TTP guidelines, the International Society on Thrombosis and Haemostasis gave the agent only a conditional recommendation, in part because it’s backed up only by HERCULES and a phase 2 trial.
Also, the group noted that in the phase 2 study caplacizumab patients had a clinically and statistically significant increase in the number of relapses at 12 months: 31% versus 8% placebo. “Caplacizumab may leave patients prone to experience a later recurrence owing to the unresolved ADAMTS13 deficiency and inhibitors,” Dr. Cataland said.
“We do see some early recurrence” when caplacizumab is stopped, suggesting that when the agent’s “protective effect is removed, the risk is still there,” said Dr. Cataland, who was also an author on the ISTH guidelines, as well as the phase 2 trial.
It raises the question of how long patients should be kept on caplacizumab. There are few data on the issue, “but the consensus has been to stop caplacizumab when two consecutive ADAMTS13 measurements show 20% or greater activity,” or perhaps with one reading above 20% in a patient trending in the right direction. “With a bleeding complication, you might stop it sooner,” he said.
Dr. Cataland anticipates TTP management will eventually move away from plasma exchange to more directed therapies, including caplacizumab and perhaps recombinant ADAMTS13, which is in development.
There have been a few reports of TTP patients who refuse plasma exchange on religious grounds being successfully treated with caplacizumab. Dr. Cataland also noted a patient of his with relapsing TTP who didn’t want to be admitted yet again for plasma exchange and steroids at the start of a new episode.
“We managed her with caplacizumab and rituximab, and in a couple weeks she had recovered her ADAMTS13 activity and was able to stop the caplacizumab.” She was a motivated, knowledgeable person, “someone I trusted, so I was comfortable with the approach. I think that may be where we are headed in the future, hopefully,” he said.
Dr. Cataland disclosed research funding and consulting fees from Alexion, caplacizumab’s maker, Sanofi Genzyme, and Takeda,. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
As hematologists debated the role of the anti–von Willebrand factor agent caplacizumab for acquired thrombotic thrombocytopenic purpura (TTP), an investigator on the phase 3 trial that led to its approval had a message.
,” said hematologist Spero Cataland, MD, of the department of internal medicine at Ohio State University in Columbus.
If cost is going to be a factor, and it “has to be in our world these days, it’s more of a discussion,” he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The HERCULES trial Dr. Cataland helped conduct found a median time to platelet count normalization of 2.69 days when caplacizumab was started during plasma exchange versus 2.88 days for placebo; 12% of patients had a TTP recurrence while they continued caplacizumab for 30 days past their last exchange and were followed for an additional 28 days versus 38% randomized to placebo. Caplacizumab subjects needed an average of 5.8 days of plasma exchange versus 9.4 days in the placebo arm (N Engl J Med. 2019 Jan 24;380(4):335-46).
Based on the results, the Food and Drug Administration approved the agent for acquired TTP in combination with plasma exchange and immunosuppressives in Feb. 2019 for 30 days beyond the last plasma exchange, with up to 28 additional days if ADAMTS13 activity remains suppressed. Labeling notes a risk of severe bleeding.
“The data on refractory disease and mortality aren’t quite there yet, but there’s a suggestion [caplacizumab] might impact that as well,” Dr. Cataland said. In its recent TTP guidelines, the International Society on Thrombosis and Haemostasis gave the agent only a conditional recommendation, in part because it’s backed up only by HERCULES and a phase 2 trial.
Also, the group noted that in the phase 2 study caplacizumab patients had a clinically and statistically significant increase in the number of relapses at 12 months: 31% versus 8% placebo. “Caplacizumab may leave patients prone to experience a later recurrence owing to the unresolved ADAMTS13 deficiency and inhibitors,” Dr. Cataland said.
“We do see some early recurrence” when caplacizumab is stopped, suggesting that when the agent’s “protective effect is removed, the risk is still there,” said Dr. Cataland, who was also an author on the ISTH guidelines, as well as the phase 2 trial.
It raises the question of how long patients should be kept on caplacizumab. There are few data on the issue, “but the consensus has been to stop caplacizumab when two consecutive ADAMTS13 measurements show 20% or greater activity,” or perhaps with one reading above 20% in a patient trending in the right direction. “With a bleeding complication, you might stop it sooner,” he said.
Dr. Cataland anticipates TTP management will eventually move away from plasma exchange to more directed therapies, including caplacizumab and perhaps recombinant ADAMTS13, which is in development.
There have been a few reports of TTP patients who refuse plasma exchange on religious grounds being successfully treated with caplacizumab. Dr. Cataland also noted a patient of his with relapsing TTP who didn’t want to be admitted yet again for plasma exchange and steroids at the start of a new episode.
“We managed her with caplacizumab and rituximab, and in a couple weeks she had recovered her ADAMTS13 activity and was able to stop the caplacizumab.” She was a motivated, knowledgeable person, “someone I trusted, so I was comfortable with the approach. I think that may be where we are headed in the future, hopefully,” he said.
Dr. Cataland disclosed research funding and consulting fees from Alexion, caplacizumab’s maker, Sanofi Genzyme, and Takeda,. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
FROM 2020 UNNH
No benefit from tranexamic acid prophylaxis in blood cancers
Despite being routinely used in clinical settings, prophylactic use of tranexamic acid, an antifibrinolytic agent administered with platelet transfusions, did not reduce bleeding among patients with blood cancers and severe thrombocytopenia, according to a new study.
The study compared tranexamic acid to placebo and found no significant differences in terms of the number of bleeding events, the number of red blood cell transfusions, or the number of platelet transfusions that were required.
However, the rate of occlusions in the central venous line was significantly higher for patients in the tranexamic acid group, although there was no difference between groups for other types of thrombotic events.
The findings were presented at the annual meeting of the American Society of Hematology, which was held online.
The study was highlighted as potentially practice changing at a press preview webinar by ASH Secretary Robert Brodsky, MD.
“They found absolutely no difference in bleeding or need for transfusion,” said Brodsky. “What they did find was more catheter-associated blood clots in the tranexamic acid group. This is a practice changer in that it probably should not be given prophylactically to patients with thrombocytopenia.”
Senior author Terry B. Gernsheimer, MD, of the University of Washington, Seattle, noted that tranexamic acid has been found to be effective in the treatment of bleeding related to childbirth, surgery, and inherited blood disorders.
It is also used for patients with blood cancers and severe thrombocytopenia. There is little evidence to support this use, which is why the researchers decided to investigate.
“Clearly patients with low platelet counts and blood cancers have a different kind of bleeding than the bleeding experienced by patients who have suffered some kind of trauma or surgery,” Dr. Gernsheimer said in a statement.
“Their bleeding likely is due to endothelial damage – damage to the lining of blood vessels – that tranexamic acid would not treat,” she added.
“To prevent bleeding in these patients, we may need to look at ways to speed the healing of the endothelium that occurs with chemotherapy, radiation, and graft-vs-host disease in patients receiving a transplant,” Dr. Gernsheimer commented.
Temper enthusiasm
“Overall, I think these results will temper enthusiasm for using tranexamic acid in this setting,” said Mitul Gandhi, MD, a medical oncologist with Virginia Cancer Specialists, who was approached for comment.
These data do not support the routine use of prophylactic tranexamic acid in chemotherapy-induced thrombocytopenia for patients with platelet counts lower than 30,000/μL, he added.
“The primary objective was not met, and there was an observed increased rate of catheter-associated thrombosis,” he said. “Continued use of judicious transfusion support and correction of a concomitant coagulopathy remains the main clinical approach to these patients.”
Dr. Gandhi commented that tranexamic acid “remains a potentially useful adjunct agent in certain cases of recalcitrant bleeding related to thrombocytopenia or coagulopathy.
“While there is no uniform scenario, it is typically reserved on a case-by-case basis after addressing vascular defects, utilization of platelet, fresh frozen plasma, cryoprecipitate transfusions, vitamin K repletion, and of course excluding any antiplatelet or anticoagulant therapy,» he told this news organization. “For persistent bleeding in spite of all corrective measures or hemorrhage into noncompressible vascular beds, such as with intracranial bleeds, antifibrinolytic therapy may assist in mitigating further blood loss.”
However, this has to be balanced with the potential increased risk for thrombosis after correction of the hemostatic insult.
At present, tranexamic acid “only has an FDA indication for uterine bleeding, but it is frequently used in trauma settings and obstetrical emergencies,” said Douglas Tremblay, MD, an internist at the Icahn School of Medicine at Mount Sinai, New York, who was also approached for comment.
“There is evidence from prior studies that were done 20 or 30 years ago that it may help in this setting, so it is used in some institutions, although we don’t give it prophylactically for patients with a hematologic malignancy.”
Although this was a negative study, Dr. Tremblay pointed out that one thing that may come out of it is that there may be subgroups who can benefit from the prophylactic use of tranexamic acid. “There is very wide inclusion criteria for the study – any type of hematologic malignancy in patients undergoing chemotherapy or stem cell transplant,” he said in an interview. “Even among chemotherapy and transplant patients, there are different risks for bleeding.”
For example, patients undergoing induction chemotherapy for acute myeloid leukemia are at an increased risk of bleeding in comparison with patients with other hematologic malignancies, and those undergoing allogeneic transplant are at an increased risk of bleeding in comparison with patients undergoing autologous transplant. “So while its unclear if a subgroup may benefit from this strategy, lumped together, it doesn’t appear it is of any benefit and potentially harmful, in terms of line occlusions,” he said. “While that may seem to be a nuisance, it can delay chemotherapy or supportive infusions, and that can be a big deal.”
No evidence of benefit
Dr. Gernsheimer and colleagues conducted the American Trial Using Tranexamic Acid in Thrombocytopenia (A-TREAT), which evaluated the effects of prophylactic tranexamic acid as an adjunct to routine transfusion therapy on bleeding and transfusion requirements.
A total of 330 patients were randomly assigned to receive either tranexamic acid 1,000 mg IV or 1,300 mg or placebo. Randomization was stratified by site and therapy: chemotherapy, allogeneic transplant, or autologous transplant. It was anticipated that all patients had hypoproliferative thrombocytopenia (expected platelet count, 10,000/µL for at least 5 days).
Treatment continued for 30 days or platelet count recovery (>30,000/µL), diagnosis of thrombosis or veno-occlusive disease, recurrent line occlusion, visible hematuria, or physician or patient request.
The primary endpoint of the study was the proportion of patients with bleeding of World Health Organization grade 2 or above over 30 days after beginning therapy. Secondary endpoints included the number of transfusions and the number of days alive without WHO grade 2+ bleeding during the first 30 days post activation of study drug.
The time to first WHO 2+ bleeding was “remarkably similar” between the tranexamic acid groups and the placebo group, said Dr. Gernsheimer.
In the cohort as a whole, 48.8% in the placebo group experienced a grade 2+ bleed vs. 45.4% in the tranexamic group (odds ratio, 0.86).
Similar results were observed across subgroups: allogeneic transplant, 57.3% vs. 58.8% (OR, 0.94); autologous transplant, 19.9% vs. 24.7% ( OR, 0.71); or chemotherapy, 48% vs. 52.1% (OR, 0.84).
There were no significant differences in mean number of transfusions (difference, 0.1; 95% confidence interval, –1.9 to 2) or days alive without grade 2 or higher bleeding (difference, 0.1; 95% CI, –1.4 to 1.5).
“A post hoc analysis of WHO 3+ bleeding showed these events to be rare and without any improvement with tranexamic acid,” she said.
A higher percentage of patients in the tranexamic acid group experienced thrombotic events (19.5% vs. 11%). “But importantly, in both groups, it was primarily due to central line occlusions without an associated thrombus,” said Dr. Gernsheimer. “This was statistically significant.”
Fewer non–catheter related thrombotic events occurred in the tranexamic acid group (3.7% vs. 5.5%), but the difference was not statistically significant.
There was also no significant difference between groups in veno-occlusive disease after 30 days (1.8% vs. 1.2%) or all-cause mortality at 30 days (2.4% vs. 3%) or 100 days (11.5% vs. 11.5%). No deaths associated with thrombosis had occurred in either group at 120 days.
The study was supported by the University of Washington and the National Heart, Lung, and Blood Institute. Dr. Gernsheimer has relationships with Amgen, Cellphire, Dova Pharmaceuticals, Novartis, Principia, Rigel, Sanofi, and Vertex. Dr. Tremblay and Dr. Gandhi have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Despite being routinely used in clinical settings, prophylactic use of tranexamic acid, an antifibrinolytic agent administered with platelet transfusions, did not reduce bleeding among patients with blood cancers and severe thrombocytopenia, according to a new study.
The study compared tranexamic acid to placebo and found no significant differences in terms of the number of bleeding events, the number of red blood cell transfusions, or the number of platelet transfusions that were required.
However, the rate of occlusions in the central venous line was significantly higher for patients in the tranexamic acid group, although there was no difference between groups for other types of thrombotic events.
The findings were presented at the annual meeting of the American Society of Hematology, which was held online.
The study was highlighted as potentially practice changing at a press preview webinar by ASH Secretary Robert Brodsky, MD.
“They found absolutely no difference in bleeding or need for transfusion,” said Brodsky. “What they did find was more catheter-associated blood clots in the tranexamic acid group. This is a practice changer in that it probably should not be given prophylactically to patients with thrombocytopenia.”
Senior author Terry B. Gernsheimer, MD, of the University of Washington, Seattle, noted that tranexamic acid has been found to be effective in the treatment of bleeding related to childbirth, surgery, and inherited blood disorders.
It is also used for patients with blood cancers and severe thrombocytopenia. There is little evidence to support this use, which is why the researchers decided to investigate.
“Clearly patients with low platelet counts and blood cancers have a different kind of bleeding than the bleeding experienced by patients who have suffered some kind of trauma or surgery,” Dr. Gernsheimer said in a statement.
“Their bleeding likely is due to endothelial damage – damage to the lining of blood vessels – that tranexamic acid would not treat,” she added.
“To prevent bleeding in these patients, we may need to look at ways to speed the healing of the endothelium that occurs with chemotherapy, radiation, and graft-vs-host disease in patients receiving a transplant,” Dr. Gernsheimer commented.
Temper enthusiasm
“Overall, I think these results will temper enthusiasm for using tranexamic acid in this setting,” said Mitul Gandhi, MD, a medical oncologist with Virginia Cancer Specialists, who was approached for comment.
These data do not support the routine use of prophylactic tranexamic acid in chemotherapy-induced thrombocytopenia for patients with platelet counts lower than 30,000/μL, he added.
“The primary objective was not met, and there was an observed increased rate of catheter-associated thrombosis,” he said. “Continued use of judicious transfusion support and correction of a concomitant coagulopathy remains the main clinical approach to these patients.”
Dr. Gandhi commented that tranexamic acid “remains a potentially useful adjunct agent in certain cases of recalcitrant bleeding related to thrombocytopenia or coagulopathy.
“While there is no uniform scenario, it is typically reserved on a case-by-case basis after addressing vascular defects, utilization of platelet, fresh frozen plasma, cryoprecipitate transfusions, vitamin K repletion, and of course excluding any antiplatelet or anticoagulant therapy,» he told this news organization. “For persistent bleeding in spite of all corrective measures or hemorrhage into noncompressible vascular beds, such as with intracranial bleeds, antifibrinolytic therapy may assist in mitigating further blood loss.”
However, this has to be balanced with the potential increased risk for thrombosis after correction of the hemostatic insult.
At present, tranexamic acid “only has an FDA indication for uterine bleeding, but it is frequently used in trauma settings and obstetrical emergencies,” said Douglas Tremblay, MD, an internist at the Icahn School of Medicine at Mount Sinai, New York, who was also approached for comment.
“There is evidence from prior studies that were done 20 or 30 years ago that it may help in this setting, so it is used in some institutions, although we don’t give it prophylactically for patients with a hematologic malignancy.”
Although this was a negative study, Dr. Tremblay pointed out that one thing that may come out of it is that there may be subgroups who can benefit from the prophylactic use of tranexamic acid. “There is very wide inclusion criteria for the study – any type of hematologic malignancy in patients undergoing chemotherapy or stem cell transplant,” he said in an interview. “Even among chemotherapy and transplant patients, there are different risks for bleeding.”
For example, patients undergoing induction chemotherapy for acute myeloid leukemia are at an increased risk of bleeding in comparison with patients with other hematologic malignancies, and those undergoing allogeneic transplant are at an increased risk of bleeding in comparison with patients undergoing autologous transplant. “So while its unclear if a subgroup may benefit from this strategy, lumped together, it doesn’t appear it is of any benefit and potentially harmful, in terms of line occlusions,” he said. “While that may seem to be a nuisance, it can delay chemotherapy or supportive infusions, and that can be a big deal.”
No evidence of benefit
Dr. Gernsheimer and colleagues conducted the American Trial Using Tranexamic Acid in Thrombocytopenia (A-TREAT), which evaluated the effects of prophylactic tranexamic acid as an adjunct to routine transfusion therapy on bleeding and transfusion requirements.
A total of 330 patients were randomly assigned to receive either tranexamic acid 1,000 mg IV or 1,300 mg or placebo. Randomization was stratified by site and therapy: chemotherapy, allogeneic transplant, or autologous transplant. It was anticipated that all patients had hypoproliferative thrombocytopenia (expected platelet count, 10,000/µL for at least 5 days).
Treatment continued for 30 days or platelet count recovery (>30,000/µL), diagnosis of thrombosis or veno-occlusive disease, recurrent line occlusion, visible hematuria, or physician or patient request.
The primary endpoint of the study was the proportion of patients with bleeding of World Health Organization grade 2 or above over 30 days after beginning therapy. Secondary endpoints included the number of transfusions and the number of days alive without WHO grade 2+ bleeding during the first 30 days post activation of study drug.
The time to first WHO 2+ bleeding was “remarkably similar” between the tranexamic acid groups and the placebo group, said Dr. Gernsheimer.
In the cohort as a whole, 48.8% in the placebo group experienced a grade 2+ bleed vs. 45.4% in the tranexamic group (odds ratio, 0.86).
Similar results were observed across subgroups: allogeneic transplant, 57.3% vs. 58.8% (OR, 0.94); autologous transplant, 19.9% vs. 24.7% ( OR, 0.71); or chemotherapy, 48% vs. 52.1% (OR, 0.84).
There were no significant differences in mean number of transfusions (difference, 0.1; 95% confidence interval, –1.9 to 2) or days alive without grade 2 or higher bleeding (difference, 0.1; 95% CI, –1.4 to 1.5).
“A post hoc analysis of WHO 3+ bleeding showed these events to be rare and without any improvement with tranexamic acid,” she said.
A higher percentage of patients in the tranexamic acid group experienced thrombotic events (19.5% vs. 11%). “But importantly, in both groups, it was primarily due to central line occlusions without an associated thrombus,” said Dr. Gernsheimer. “This was statistically significant.”
Fewer non–catheter related thrombotic events occurred in the tranexamic acid group (3.7% vs. 5.5%), but the difference was not statistically significant.
There was also no significant difference between groups in veno-occlusive disease after 30 days (1.8% vs. 1.2%) or all-cause mortality at 30 days (2.4% vs. 3%) or 100 days (11.5% vs. 11.5%). No deaths associated with thrombosis had occurred in either group at 120 days.
The study was supported by the University of Washington and the National Heart, Lung, and Blood Institute. Dr. Gernsheimer has relationships with Amgen, Cellphire, Dova Pharmaceuticals, Novartis, Principia, Rigel, Sanofi, and Vertex. Dr. Tremblay and Dr. Gandhi have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Despite being routinely used in clinical settings, prophylactic use of tranexamic acid, an antifibrinolytic agent administered with platelet transfusions, did not reduce bleeding among patients with blood cancers and severe thrombocytopenia, according to a new study.
The study compared tranexamic acid to placebo and found no significant differences in terms of the number of bleeding events, the number of red blood cell transfusions, or the number of platelet transfusions that were required.
However, the rate of occlusions in the central venous line was significantly higher for patients in the tranexamic acid group, although there was no difference between groups for other types of thrombotic events.
The findings were presented at the annual meeting of the American Society of Hematology, which was held online.
The study was highlighted as potentially practice changing at a press preview webinar by ASH Secretary Robert Brodsky, MD.
“They found absolutely no difference in bleeding or need for transfusion,” said Brodsky. “What they did find was more catheter-associated blood clots in the tranexamic acid group. This is a practice changer in that it probably should not be given prophylactically to patients with thrombocytopenia.”
Senior author Terry B. Gernsheimer, MD, of the University of Washington, Seattle, noted that tranexamic acid has been found to be effective in the treatment of bleeding related to childbirth, surgery, and inherited blood disorders.
It is also used for patients with blood cancers and severe thrombocytopenia. There is little evidence to support this use, which is why the researchers decided to investigate.
“Clearly patients with low platelet counts and blood cancers have a different kind of bleeding than the bleeding experienced by patients who have suffered some kind of trauma or surgery,” Dr. Gernsheimer said in a statement.
“Their bleeding likely is due to endothelial damage – damage to the lining of blood vessels – that tranexamic acid would not treat,” she added.
“To prevent bleeding in these patients, we may need to look at ways to speed the healing of the endothelium that occurs with chemotherapy, radiation, and graft-vs-host disease in patients receiving a transplant,” Dr. Gernsheimer commented.
Temper enthusiasm
“Overall, I think these results will temper enthusiasm for using tranexamic acid in this setting,” said Mitul Gandhi, MD, a medical oncologist with Virginia Cancer Specialists, who was approached for comment.
These data do not support the routine use of prophylactic tranexamic acid in chemotherapy-induced thrombocytopenia for patients with platelet counts lower than 30,000/μL, he added.
“The primary objective was not met, and there was an observed increased rate of catheter-associated thrombosis,” he said. “Continued use of judicious transfusion support and correction of a concomitant coagulopathy remains the main clinical approach to these patients.”
Dr. Gandhi commented that tranexamic acid “remains a potentially useful adjunct agent in certain cases of recalcitrant bleeding related to thrombocytopenia or coagulopathy.
“While there is no uniform scenario, it is typically reserved on a case-by-case basis after addressing vascular defects, utilization of platelet, fresh frozen plasma, cryoprecipitate transfusions, vitamin K repletion, and of course excluding any antiplatelet or anticoagulant therapy,» he told this news organization. “For persistent bleeding in spite of all corrective measures or hemorrhage into noncompressible vascular beds, such as with intracranial bleeds, antifibrinolytic therapy may assist in mitigating further blood loss.”
However, this has to be balanced with the potential increased risk for thrombosis after correction of the hemostatic insult.
At present, tranexamic acid “only has an FDA indication for uterine bleeding, but it is frequently used in trauma settings and obstetrical emergencies,” said Douglas Tremblay, MD, an internist at the Icahn School of Medicine at Mount Sinai, New York, who was also approached for comment.
“There is evidence from prior studies that were done 20 or 30 years ago that it may help in this setting, so it is used in some institutions, although we don’t give it prophylactically for patients with a hematologic malignancy.”
Although this was a negative study, Dr. Tremblay pointed out that one thing that may come out of it is that there may be subgroups who can benefit from the prophylactic use of tranexamic acid. “There is very wide inclusion criteria for the study – any type of hematologic malignancy in patients undergoing chemotherapy or stem cell transplant,” he said in an interview. “Even among chemotherapy and transplant patients, there are different risks for bleeding.”
For example, patients undergoing induction chemotherapy for acute myeloid leukemia are at an increased risk of bleeding in comparison with patients with other hematologic malignancies, and those undergoing allogeneic transplant are at an increased risk of bleeding in comparison with patients undergoing autologous transplant. “So while its unclear if a subgroup may benefit from this strategy, lumped together, it doesn’t appear it is of any benefit and potentially harmful, in terms of line occlusions,” he said. “While that may seem to be a nuisance, it can delay chemotherapy or supportive infusions, and that can be a big deal.”
No evidence of benefit
Dr. Gernsheimer and colleagues conducted the American Trial Using Tranexamic Acid in Thrombocytopenia (A-TREAT), which evaluated the effects of prophylactic tranexamic acid as an adjunct to routine transfusion therapy on bleeding and transfusion requirements.
A total of 330 patients were randomly assigned to receive either tranexamic acid 1,000 mg IV or 1,300 mg or placebo. Randomization was stratified by site and therapy: chemotherapy, allogeneic transplant, or autologous transplant. It was anticipated that all patients had hypoproliferative thrombocytopenia (expected platelet count, 10,000/µL for at least 5 days).
Treatment continued for 30 days or platelet count recovery (>30,000/µL), diagnosis of thrombosis or veno-occlusive disease, recurrent line occlusion, visible hematuria, or physician or patient request.
The primary endpoint of the study was the proportion of patients with bleeding of World Health Organization grade 2 or above over 30 days after beginning therapy. Secondary endpoints included the number of transfusions and the number of days alive without WHO grade 2+ bleeding during the first 30 days post activation of study drug.
The time to first WHO 2+ bleeding was “remarkably similar” between the tranexamic acid groups and the placebo group, said Dr. Gernsheimer.
In the cohort as a whole, 48.8% in the placebo group experienced a grade 2+ bleed vs. 45.4% in the tranexamic group (odds ratio, 0.86).
Similar results were observed across subgroups: allogeneic transplant, 57.3% vs. 58.8% (OR, 0.94); autologous transplant, 19.9% vs. 24.7% ( OR, 0.71); or chemotherapy, 48% vs. 52.1% (OR, 0.84).
There were no significant differences in mean number of transfusions (difference, 0.1; 95% confidence interval, –1.9 to 2) or days alive without grade 2 or higher bleeding (difference, 0.1; 95% CI, –1.4 to 1.5).
“A post hoc analysis of WHO 3+ bleeding showed these events to be rare and without any improvement with tranexamic acid,” she said.
A higher percentage of patients in the tranexamic acid group experienced thrombotic events (19.5% vs. 11%). “But importantly, in both groups, it was primarily due to central line occlusions without an associated thrombus,” said Dr. Gernsheimer. “This was statistically significant.”
Fewer non–catheter related thrombotic events occurred in the tranexamic acid group (3.7% vs. 5.5%), but the difference was not statistically significant.
There was also no significant difference between groups in veno-occlusive disease after 30 days (1.8% vs. 1.2%) or all-cause mortality at 30 days (2.4% vs. 3%) or 100 days (11.5% vs. 11.5%). No deaths associated with thrombosis had occurred in either group at 120 days.
The study was supported by the University of Washington and the National Heart, Lung, and Blood Institute. Dr. Gernsheimer has relationships with Amgen, Cellphire, Dova Pharmaceuticals, Novartis, Principia, Rigel, Sanofi, and Vertex. Dr. Tremblay and Dr. Gandhi have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Childhood Hodgkin survivors have neurocognitive impairment
More than 2 decades on, adult survivors of childhood Hodgkin lymphoma report significantly more neurocognitive impairment than their siblings, but the differences may be related to risk factors in adulthood rather than to treatment in childhood, investigators say.
Among adults with a history of childhood Hodgkin lymphoma and their siblings as controls, the survivors reported significantly worse functioning than their brothers or sisters in four domains of neurocognitive functioning.
In multivariate analysis, however, while sex, race, activity level and smoking status were all significant predictors for worse neurocognitive impairment, there were no significant associations between chemotherapy drugs or chest radiation and neurocognitive impairment, said Annalynn M. Williams, PhD, from St. Jude Children’s Research Hospital in Memphis.
“Hodgkin lymphoma is the most common cancer diagnosed in adolescents, and for many years we’ve had high cure rates, resulting in a growing population of survivors who are now, unfortunately, at an increased risk for cardiovascular, respiratory, endocrine and neurologic late morbidity. The neurocognitive morbidity in this population, however, is unknown,” she said in oral abstract presented at the annual meeting of the American Society of Hematology.
Survivors and sibs
To better characterize the potential late neurocognitive effects of intensive Hodgkin lymphoma therapy in childhood, Dr. Williams and colleagues polled survivors of childhood Hodgkin lymphoma and randomly selected sibling controls who were participants in the Childhood Cancer Survivor Study (CCSS).
Participants were asked to complete questionnaires regarding four domains of neurocognitive impairment: task efficiency, emotional regulation, organization, and memory. The investigators defined impairment in each domain as a score lower than that of the 90th percentile of community controls from the St. Jude Lifetime Cohort.
A total of 1,564 survivors and 725 controls completed the questionnaires and were included in the study.
The median age at follow-up was slightly higher among survivors, at 37 versus 32 years. The median age at diagnosis was 14, and the median time since diagnosis was 23 years.
In all, 10.8% of survivors reported impaired task efficiency, compared with 7.7% of controls. Problems with emotional regulation were reported by 16.6% of survivors versus 11.5% of siblings, and difficulties with organization and memory were reported by 12.1% versus10.3%, and 8.1% versus 5.7%, respectively.
In a model adjusted for age, sex, and race, the relative risks for neurocognitive impairment among survivors versus siblings, were as follows: task efficiency (RR,1.37); emotional regulation (RR, 1.56); organization (RR, 1.32); memory (RR, 1.72) (all significant by confidence interval).
In a model adjusted for sex, race, smoking status, exercise, age, time since diagnosis, and treatment exposures, risk factors for neurocognitive impairment among survivors included female versus male sex (significant for emotional regulation and memory deficits); non-White versus White (significant for task efficiency); former smoker versus never (significant for all domains except organization); current smoker versus never (significant for task efficiency and emotional regulation); and meeting Centers for Disease Control and Prevention exercise criteria versus not (negatively significant for task efficiency and organization); (P < .05 for all above comparisons).
However, in a model adjusted for relapse, second malignancy, treatment exposures, age, sex, race, time since diagnosis, smoking status and physical activity, only relapse or second malignancy – surrogates for additional treatment exposures – were significantly associated with neurocognitive impairment, and then only in the domain of task efficiency.
Chronic conditions significantly associated with risk for impairment included cardiovascular disease (significant across all domains), respiratory comorbidities (significant for task efficiency), endocrine disorders (significant for task efficiency), and neurologic disorders (significant in all domains except organization).
“While these analyses give us a sense of the presence of neurocognitive impairment in a large sample of Hodgkin lymphoma survivors from across the U.S., these analyses are limited by the self-reported nature of the data,” Dr. Williams acknowledged.
“Because survivors self-report impairments, these likely represent overt, symptomatic neurocognitive impairments. Many more survivors may experience more subtle neurocognitive impairments, and additional research with objective measures of both chronic health conditions and neurocognitive functioning are warranted,” she added.
Smoking gun?
In the question-and-answer session following the presentation, session comoderator Pallawi Torka, MD, from Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., who was not involved in the research, commented that the finding regarding a link between current and former smoking as risk factors for neurocognitive impairment was “intriguing.”
“Do you think that smoking is a cause or an effect of having that impairment in childhood survivors of Hodgkin lymphoma?” she asked.
“That’s a great question, and actually one we have spent a great deal of time discussing, and we’re still trying to tease that apart. We’re still not really sure where that association is coming from,” Dr. Williams replied.
She noted that, in a different sample of CCSS participants from whom biospecimens were collected, the investigators plan to see whether smoking drives inflammation and oxidative stress mechanisms that may be contributing to neurocognitive impairment, or whether smoking is a coping mechanism related to anxiety and depression, which have also been seen in survivors.
Kara Kelly, MD, a pediatric oncologist at Roswell Park, commented that some survivors report symptoms of cognitive dysfunction shortly after treatment, and asked whether there might be a relationship to Hodgkin-specific factors such as B symptoms, in which cytokine-mediated inflammation may play a role.
Dr. Williams said that, “unfortunately, in CCSS these survivors had to be at least 5 years from diagnosis, but in many cases were recruited years after their diagnosis and treatment, so we don’t have data on B symptoms.”
The CCSS is funded by the National Cancer Institute. Dr. Williams, Dr. Palawi, and Dr. Kelly all reported no relevant conflicts of interest to disclose.
SOURCE: Williams AM et al. ASH 2020, Abstract 370.
More than 2 decades on, adult survivors of childhood Hodgkin lymphoma report significantly more neurocognitive impairment than their siblings, but the differences may be related to risk factors in adulthood rather than to treatment in childhood, investigators say.
Among adults with a history of childhood Hodgkin lymphoma and their siblings as controls, the survivors reported significantly worse functioning than their brothers or sisters in four domains of neurocognitive functioning.
In multivariate analysis, however, while sex, race, activity level and smoking status were all significant predictors for worse neurocognitive impairment, there were no significant associations between chemotherapy drugs or chest radiation and neurocognitive impairment, said Annalynn M. Williams, PhD, from St. Jude Children’s Research Hospital in Memphis.
“Hodgkin lymphoma is the most common cancer diagnosed in adolescents, and for many years we’ve had high cure rates, resulting in a growing population of survivors who are now, unfortunately, at an increased risk for cardiovascular, respiratory, endocrine and neurologic late morbidity. The neurocognitive morbidity in this population, however, is unknown,” she said in oral abstract presented at the annual meeting of the American Society of Hematology.
Survivors and sibs
To better characterize the potential late neurocognitive effects of intensive Hodgkin lymphoma therapy in childhood, Dr. Williams and colleagues polled survivors of childhood Hodgkin lymphoma and randomly selected sibling controls who were participants in the Childhood Cancer Survivor Study (CCSS).
Participants were asked to complete questionnaires regarding four domains of neurocognitive impairment: task efficiency, emotional regulation, organization, and memory. The investigators defined impairment in each domain as a score lower than that of the 90th percentile of community controls from the St. Jude Lifetime Cohort.
A total of 1,564 survivors and 725 controls completed the questionnaires and were included in the study.
The median age at follow-up was slightly higher among survivors, at 37 versus 32 years. The median age at diagnosis was 14, and the median time since diagnosis was 23 years.
In all, 10.8% of survivors reported impaired task efficiency, compared with 7.7% of controls. Problems with emotional regulation were reported by 16.6% of survivors versus 11.5% of siblings, and difficulties with organization and memory were reported by 12.1% versus10.3%, and 8.1% versus 5.7%, respectively.
In a model adjusted for age, sex, and race, the relative risks for neurocognitive impairment among survivors versus siblings, were as follows: task efficiency (RR,1.37); emotional regulation (RR, 1.56); organization (RR, 1.32); memory (RR, 1.72) (all significant by confidence interval).
In a model adjusted for sex, race, smoking status, exercise, age, time since diagnosis, and treatment exposures, risk factors for neurocognitive impairment among survivors included female versus male sex (significant for emotional regulation and memory deficits); non-White versus White (significant for task efficiency); former smoker versus never (significant for all domains except organization); current smoker versus never (significant for task efficiency and emotional regulation); and meeting Centers for Disease Control and Prevention exercise criteria versus not (negatively significant for task efficiency and organization); (P < .05 for all above comparisons).
However, in a model adjusted for relapse, second malignancy, treatment exposures, age, sex, race, time since diagnosis, smoking status and physical activity, only relapse or second malignancy – surrogates for additional treatment exposures – were significantly associated with neurocognitive impairment, and then only in the domain of task efficiency.
Chronic conditions significantly associated with risk for impairment included cardiovascular disease (significant across all domains), respiratory comorbidities (significant for task efficiency), endocrine disorders (significant for task efficiency), and neurologic disorders (significant in all domains except organization).
“While these analyses give us a sense of the presence of neurocognitive impairment in a large sample of Hodgkin lymphoma survivors from across the U.S., these analyses are limited by the self-reported nature of the data,” Dr. Williams acknowledged.
“Because survivors self-report impairments, these likely represent overt, symptomatic neurocognitive impairments. Many more survivors may experience more subtle neurocognitive impairments, and additional research with objective measures of both chronic health conditions and neurocognitive functioning are warranted,” she added.
Smoking gun?
In the question-and-answer session following the presentation, session comoderator Pallawi Torka, MD, from Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., who was not involved in the research, commented that the finding regarding a link between current and former smoking as risk factors for neurocognitive impairment was “intriguing.”
“Do you think that smoking is a cause or an effect of having that impairment in childhood survivors of Hodgkin lymphoma?” she asked.
“That’s a great question, and actually one we have spent a great deal of time discussing, and we’re still trying to tease that apart. We’re still not really sure where that association is coming from,” Dr. Williams replied.
She noted that, in a different sample of CCSS participants from whom biospecimens were collected, the investigators plan to see whether smoking drives inflammation and oxidative stress mechanisms that may be contributing to neurocognitive impairment, or whether smoking is a coping mechanism related to anxiety and depression, which have also been seen in survivors.
Kara Kelly, MD, a pediatric oncologist at Roswell Park, commented that some survivors report symptoms of cognitive dysfunction shortly after treatment, and asked whether there might be a relationship to Hodgkin-specific factors such as B symptoms, in which cytokine-mediated inflammation may play a role.
Dr. Williams said that, “unfortunately, in CCSS these survivors had to be at least 5 years from diagnosis, but in many cases were recruited years after their diagnosis and treatment, so we don’t have data on B symptoms.”
The CCSS is funded by the National Cancer Institute. Dr. Williams, Dr. Palawi, and Dr. Kelly all reported no relevant conflicts of interest to disclose.
SOURCE: Williams AM et al. ASH 2020, Abstract 370.
More than 2 decades on, adult survivors of childhood Hodgkin lymphoma report significantly more neurocognitive impairment than their siblings, but the differences may be related to risk factors in adulthood rather than to treatment in childhood, investigators say.
Among adults with a history of childhood Hodgkin lymphoma and their siblings as controls, the survivors reported significantly worse functioning than their brothers or sisters in four domains of neurocognitive functioning.
In multivariate analysis, however, while sex, race, activity level and smoking status were all significant predictors for worse neurocognitive impairment, there were no significant associations between chemotherapy drugs or chest radiation and neurocognitive impairment, said Annalynn M. Williams, PhD, from St. Jude Children’s Research Hospital in Memphis.
“Hodgkin lymphoma is the most common cancer diagnosed in adolescents, and for many years we’ve had high cure rates, resulting in a growing population of survivors who are now, unfortunately, at an increased risk for cardiovascular, respiratory, endocrine and neurologic late morbidity. The neurocognitive morbidity in this population, however, is unknown,” she said in oral abstract presented at the annual meeting of the American Society of Hematology.
Survivors and sibs
To better characterize the potential late neurocognitive effects of intensive Hodgkin lymphoma therapy in childhood, Dr. Williams and colleagues polled survivors of childhood Hodgkin lymphoma and randomly selected sibling controls who were participants in the Childhood Cancer Survivor Study (CCSS).
Participants were asked to complete questionnaires regarding four domains of neurocognitive impairment: task efficiency, emotional regulation, organization, and memory. The investigators defined impairment in each domain as a score lower than that of the 90th percentile of community controls from the St. Jude Lifetime Cohort.
A total of 1,564 survivors and 725 controls completed the questionnaires and were included in the study.
The median age at follow-up was slightly higher among survivors, at 37 versus 32 years. The median age at diagnosis was 14, and the median time since diagnosis was 23 years.
In all, 10.8% of survivors reported impaired task efficiency, compared with 7.7% of controls. Problems with emotional regulation were reported by 16.6% of survivors versus 11.5% of siblings, and difficulties with organization and memory were reported by 12.1% versus10.3%, and 8.1% versus 5.7%, respectively.
In a model adjusted for age, sex, and race, the relative risks for neurocognitive impairment among survivors versus siblings, were as follows: task efficiency (RR,1.37); emotional regulation (RR, 1.56); organization (RR, 1.32); memory (RR, 1.72) (all significant by confidence interval).
In a model adjusted for sex, race, smoking status, exercise, age, time since diagnosis, and treatment exposures, risk factors for neurocognitive impairment among survivors included female versus male sex (significant for emotional regulation and memory deficits); non-White versus White (significant for task efficiency); former smoker versus never (significant for all domains except organization); current smoker versus never (significant for task efficiency and emotional regulation); and meeting Centers for Disease Control and Prevention exercise criteria versus not (negatively significant for task efficiency and organization); (P < .05 for all above comparisons).
However, in a model adjusted for relapse, second malignancy, treatment exposures, age, sex, race, time since diagnosis, smoking status and physical activity, only relapse or second malignancy – surrogates for additional treatment exposures – were significantly associated with neurocognitive impairment, and then only in the domain of task efficiency.
Chronic conditions significantly associated with risk for impairment included cardiovascular disease (significant across all domains), respiratory comorbidities (significant for task efficiency), endocrine disorders (significant for task efficiency), and neurologic disorders (significant in all domains except organization).
“While these analyses give us a sense of the presence of neurocognitive impairment in a large sample of Hodgkin lymphoma survivors from across the U.S., these analyses are limited by the self-reported nature of the data,” Dr. Williams acknowledged.
“Because survivors self-report impairments, these likely represent overt, symptomatic neurocognitive impairments. Many more survivors may experience more subtle neurocognitive impairments, and additional research with objective measures of both chronic health conditions and neurocognitive functioning are warranted,” she added.
Smoking gun?
In the question-and-answer session following the presentation, session comoderator Pallawi Torka, MD, from Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., who was not involved in the research, commented that the finding regarding a link between current and former smoking as risk factors for neurocognitive impairment was “intriguing.”
“Do you think that smoking is a cause or an effect of having that impairment in childhood survivors of Hodgkin lymphoma?” she asked.
“That’s a great question, and actually one we have spent a great deal of time discussing, and we’re still trying to tease that apart. We’re still not really sure where that association is coming from,” Dr. Williams replied.
She noted that, in a different sample of CCSS participants from whom biospecimens were collected, the investigators plan to see whether smoking drives inflammation and oxidative stress mechanisms that may be contributing to neurocognitive impairment, or whether smoking is a coping mechanism related to anxiety and depression, which have also been seen in survivors.
Kara Kelly, MD, a pediatric oncologist at Roswell Park, commented that some survivors report symptoms of cognitive dysfunction shortly after treatment, and asked whether there might be a relationship to Hodgkin-specific factors such as B symptoms, in which cytokine-mediated inflammation may play a role.
Dr. Williams said that, “unfortunately, in CCSS these survivors had to be at least 5 years from diagnosis, but in many cases were recruited years after their diagnosis and treatment, so we don’t have data on B symptoms.”
The CCSS is funded by the National Cancer Institute. Dr. Williams, Dr. Palawi, and Dr. Kelly all reported no relevant conflicts of interest to disclose.
SOURCE: Williams AM et al. ASH 2020, Abstract 370.
FROM ASH 2020
PPE shortage crisis continues at most hospitals, survey shows
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
Addressing Maternal Mortality Through Education: The Mommies Methadone Program
From the UT Health Long School of Medicine San Antonio, Texas.
Abstract
Objective: To educate pregnant patients with opioid use disorder (OUD) about the effects of opioids in order to improve understanding and help achieve sustained abstinence.
Methods: The Center for Health Care Services and University Hospital System (UHS) in San Antonio, TX, jointly o
Results: Of 68 women enrolled in the program, 33 completed both the pre-survey and the post-survey (48.5%). Nearly half (48%) were very motivated to quit before pregnancy, but 85% were very motivated to quit once pregnant. All participants said learning more about the effects of opiates would increase motivation for sobriety. Prior to the educational intervention, 39% of participants knew it was safe to breastfeed on methadone, which improved to 97% in the post-survey, and 76% incorrectly thought they would be reported to authorities by their health care providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so.
Conclusion: Pregnancy and education about opioids increased patients’ motivation to quit. Patients also advanced their breastfeeding knowledge and learned about patient-provider confidentiality. Our greatest challenge was participant follow-up; however, this improved with the help of a full-time Mommies Program nurse. Our future aim is to increase project awareness and extend the educational research.
Keywords: pregnancy; addiction; opioids; OUD; counseling.
In 2012 more than 259 million prescriptions for opioids were written in the United States, which was a 200% increase since 1998.1 Since the early 2000s, admissions to opioid substance abuse programs and the death rate from opioids have quadrupled.2-4 Specifically, the rate of heroin use increased more than 300% from 2010 to 2014.5 Opioid use in pregnancy has also escalated in recent years, with a 3- to 4-fold increase from 2000 to 2009 and with 4 in 1000 deliveries being complicated by opioid use disorder (OUD) in 2011.6-8
Between 2000 and 2014, the maternal mortality rate in the United States increased 24%, making it the only industrialized nation with a maternal mortality rate that is rising rather than falling.9 The Texas Maternal Mortality and Morbidity Task Force found that between 2012 and 2015 drug overdose was the leading cause of maternal death in the period from delivery to 365 days postpartum, and it has increased dramatically since 2010.10,11
In addition, maternal mortality reviews in several states have identified substance use as a major risk factor for pregnancy-associated deaths.12,13 In Texas between 2012 and 2015, opioids were found in 58% of maternal drug overdoses.10 In 2007, 22.8% of women who were enrolled in Medicaid programs in 46 states filled an opioid prescription during pregnancy.14 Additionally, the rising prevalence of opioid use in pregnancy has led to a sharp increase in neonatal abstinence syndrome (NAS), rising from 1.5 cases per 1000 hospital births in 1999 to 6.0 per 1000 hospital births in 2013.15 Unsurprisingly, states with the highest rates of opioid prescribing also have the highest rates of NAS.16
Methadone combined with counseling and behavioral therapy has been the standard of care for the treatment of OUD in pregnancy since the 1970s. Methadone treatment prevents opioid withdrawal symptoms and increases adherence to prenatal care.17 One of the largest methadone treatment clinics in the San Antonio, TX, area is the Center for Health Care Services (CHCS). University Health System in San Antonio (UHS) has established a clinic called The Mommies Program, where mothers addicted to opioids can receive prenatal care by a dedicated physician, registered nurse, and a certified nurse midwife, who work in collaboration with the CHCS methadone clinic. Pregnant patients with OUD in pregnancy are concurrently enrolled in the Mommies Program and receive prenatal care through UHS and methadone treatment and counseling through CHCS. The continuity effort aims to increase prenatal care rates and adherence to methadone treatment.
Once mothers are off illicit opioids and on methadone, it is essential to discuss breastfeeding with them, as many mothers addicted to illicit opioids may have been told that they should not be breastfeeding. However, breastfeeding should be encouraged in women who are stable on methadone if they are not using illicit drugs and do not have other contraindications, regardless of maternal methadone dose, since the transfer of methadone into breast milk is minimal.18-20 Breastfeeding is beneficial in women taking methadone and has been associated with decreased severity of NAS symptoms, decreased need for pharmacotherapy, and a shorter hospital stay for the baby.21 In addition, breastfeeding contributes to the development of an attachment between mother and infant, while also providing the infant with natural immunity. Women should be counseled about the need to stop breastfeeding in the event of a relapse.22
Finally, the postpartum period represents a time of increased stressors, such as loss of sleep, child protective services involvement, and frustration with constant demands from new baby. For mothers with addiction, this is an especially sensitive time, as the stressors may be exacerbated by their new sobriety and a sudden end to the motivation they experienced from pregnancy.23 Therefore, early and frequent postpartum care with methadone dose evaluation is essential in order to decrease drug relapse and screen for postpartum depression in detail, since patients with a history of drug use are at increased risk of postpartum depression.
In 2017 medical students at UT Health Long School of Medicine in San Antonio created a project to educate women about OUD in pregnancy and provide motivational incentives for sustained abstinence; this project has continued each year since. Students provide education about methadone treatment and the dangers of using illicit opioids during and after pregnancy. Students especially focus on educating patients on the key problem areas in the literature, such as overdose, NAS, breastfeeding, postpartum substance use, and postpartum depression.
Methods
From October 2018 to February 2020, a total of 15 medical students volunteered between 1 and 20 times at the Mommies Program clinic, which was held once or twice per week from 8
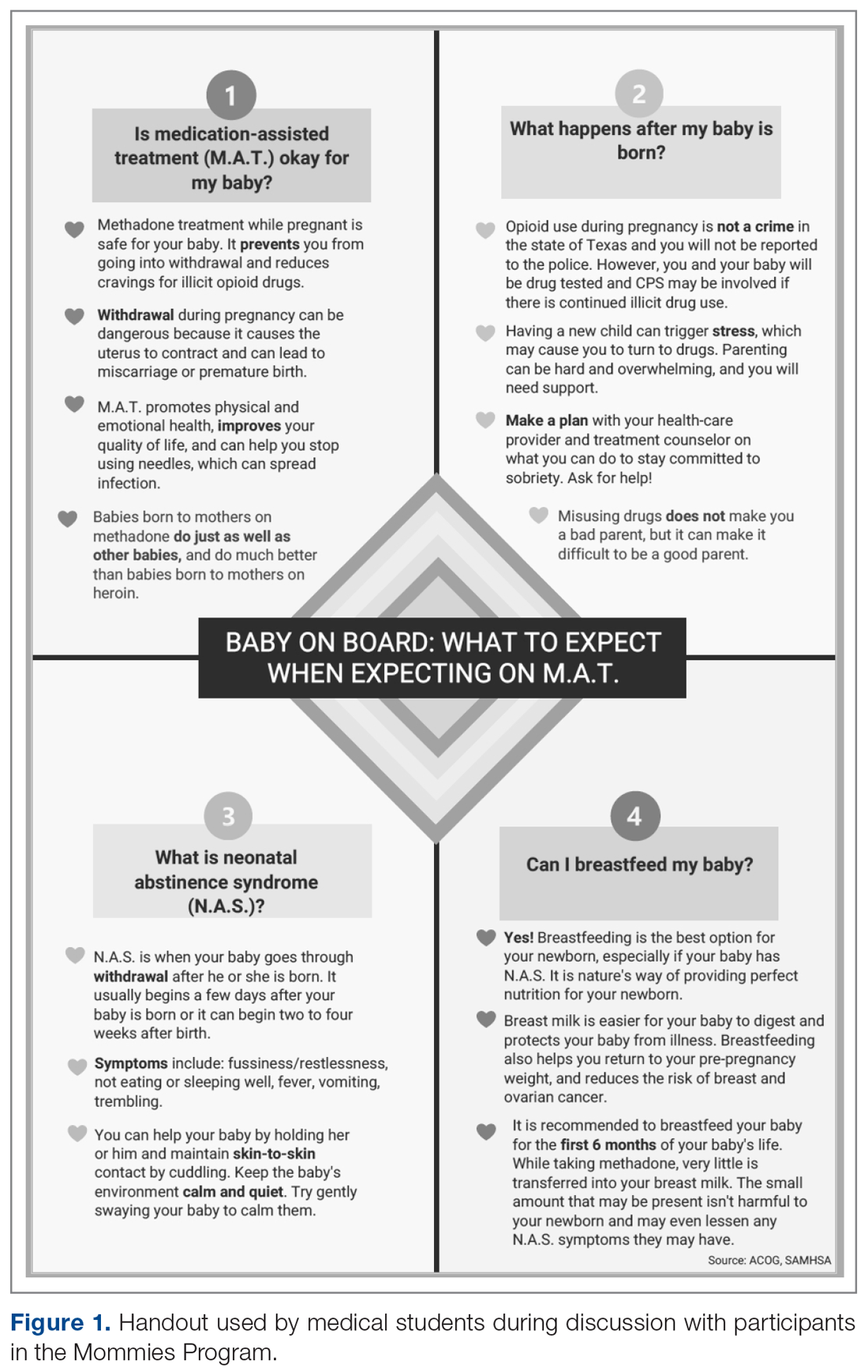
The only inclusion criteria for participating in the educational intervention and survey was participants had to be 18 years of age or older and enrolled in the Mommies Program. Patients who met the inclusion criteria and agreed to participate completed a pre-survey administered by the students during the patient’s initial prenatal visit (Figure 2). This survey collected baseline information about the patient’s history with opioid use and their current knowledge of methadone treatment, NAS, legal aspects of drug use disclosure, and drug testing prior to the education portion of the encounter. After the pre-survey was administered, students spent 30 minutes reviewing the correct answers of the survey with the patients by utilizing the standardized handout to help patients understand details of methadone and opioid use in pregnancy (Figure 1). The post-survey was administered by a student once patients entered the third trimester to assess whether the education session increased patients’ knowledge of these topics.

At the time patients completed the post-survey, they received a Baby Bag as well as education regarding each item in the bag. The aim of distributing Baby Bags was to relieve some possible postnatal stressors and educate the patients about infant care. Items included in the bag were diapers, wipes, bottles, clothes, and swaddles. Prenatal vitamins were added in January 2020, as many patients struggle to afford vitamins if they are not currently covered by Medicaid or have other barriers. The Baby Bag items were purchased through a Community Service Learning grant through UT Health San Antonio.
Results
Of 68 women enrolled in the Mommies Program during the intervention period, 33 completed the pre-survey and the post-survey (48.5%). Even though all patients enrolled in the program met the inclusion criteria, patients were not included in the educational program for multiple reasons, including refusal to participate, poor clinic follow-up, or lack of students to collect surveys. However, all patients who completed the pre-survey did complete the post-survey. In the pre-survey, only 39% of participants knew it was safe to breastfeed while on methadone. In the post-survey, 97% knew it safe to breastfeed. Nearly half (48%) reported being very motivated to quit opioids before pregnancy, but 85% were very motivated to quit once pregnant. In the pre-survey, 76% incorrectly thought they would be reported to authorities by their health providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so. Also, all participants said learning more about the effects of opiates would increase motivation for sobriety.
Discussion
Questions assessed during the educational surveys revolved around patients’ knowledge of the intricacies, legally and physiologically, of methadone treatment for OUD, as well as beneficial aspects for patients and future child health, such as breastfeeding and motivation to quit and stay sober.
It was clear that there was a lack of knowledge and education about breastfeeding, as only 39% of the participants thought that it was safe to breastfeed while on methadone in the pre-survey; in the post survey, this improved to 97%. Students spent a large portion of the educational time going over the safety of breastfeeding for patients on methadone and the many benefits to mother and baby. Students also reviewed breastfeeding with patients every time patients came in for a visit and debunked any falsehoods about the negatives of breastfeeding while on methadone. This is another testament to the benefits of reinforcement around patient education.
The area of trust between provider and patient is essential in all provider-patient relationships. However, in the area of addiction, a trusting bond is especially important, as patients must feel confident and comfortable to disclose every aspect of their lives so the provider can give the best care. It was clear from our initial data that many patients did not feel this trust or understand the legal aspects regarding the provider-patient relationship in the terms of drug use, as the pre-survey shows 76% of patients originally thought they would be reported to authorities if they told their provider they used illegal drugs during pregnancy. This was an enormous issue in the clinic and something that needed to be addressed because, based on these data, we feared many patients would not be honest about using illegal drugs to supplement their methadone if they believed they would be reported to the authorities or even jailed. The medical student education team continually assured patients that their honesty about illegal drug use during pregnancy would not be revealed to the authorities, and also made it clear to patients that it was essential they were honest about illegal drug use so the optimal care could be provided by the team. These discussions were successful, as the post-survey showed that 100% of patients knew they would not be reported to the authorities if they used illegal drugs during the pregnancy. This showed an increase in knowledge, but also suggested an increase in confidence in the provider-patient relationship by patients, which we speculate allowed for a better patient experience, better patient outcomes, and less emotional stress for the patient and provider.
Last, we wanted to study and address the motivation to quit using drugs and stay sober through learning about the effects of opiates and how this motivation was related to pregnancy. A study by Mitchell et al makes clear that pregnancy is a motivation to seek treatment for drug use and to quit,24 and our survey data support these findings, with 48% of patients motivated to quit before they were pregnant and 85% motivated to quit once they knew they were pregnant. In addition, all patients attested on the pre- and post-survey that learning more about opioids would increase their motivation for sobriety. Therefore, we believe education about the use of opioids and other drugs is a strong motivation towards sobriety and should be further studied in methadone treatment and other drugs as well.
We will continue to focus on sobriety postpartum by using the education in pregnancy as a springboard to further postpartum education, as education seems to be very beneficial to future sobriety. In the future, we believe extending the educational program past pregnancy and discussing opioid use and addiction with patients at multiple follow-up visits will be beneficial to patients’ sobriety.
We faced 2 main challenges in implementing this intervention and survey: patients would often miss multiple appointments during their third trimester or would not attend their postpartum visit if they only had 1 prenatal visit; and many clinic sessions had low student attendance because students often had many other responsibilities in medical school and there were only 15 volunteers over the study time. These challenges decreased our post-survey completion rate. However, there has been improvement in follow-up as the project has continued. The Mommies Program now has a full-time registered nurse, and a larger number of medical student teachers have volunteered to attend the clinic. In the future, we aim to increase awareness of our project and the benefits of participation, expand advertising at our medical school to increase student participation, and increase follow-up education in the postpartum period.
Another future direction is to include local, free doula services, which are offered through Catholic Charities in San Antonio. Doulas provide antepartum, intrapartum, and postpartum services, which we believe will help our patients through advocacy and support for sobriety during this emotional and stressful time.
Conclusion
Counseled participants were receptive to learning about the effects of OUD and methadone on themselves and their newborn. Participants unanimously stated that learning more about OUD increased their motivation for sobriety. It was also clear that the increased motivation to be sober during pregnancy, as compared to before pregnancy, is an opportunity to help these women take steps to get sober. Patients also advanced their breastfeeding knowledge, as we helped debunk falsehoods surrounding breastfeeding while on methadone, and we anticipate this will lead to greater breastfeeding rates for our patients on methadone, although this was not specifically studied. Finally, patients learned about patient-provider confidentiality, which allowed for more open and clear communication with patients so they could be cared for to the greatest degree and trust could remain paramount.
Drug use is a common problem in the health care system, and exposure to patients with addiction is important for medical students in training. We believe that attending the Mommies Program allowed medical students to gain exposure and skills to better help patients with OUD.
Corresponding author: Nicholas Stansbury, MD, [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Opioid painkiller prescribing: where you live makes a difference. CDC website. www.cdc.gov/vitalsigns/opioid-prescribing. Accessed October 28, 2020.
2. Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville (MD): SAMHSA; 2013. www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. Accessed October 28, 2020.
3. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154-63.
4. National Center for Health Statistics. NCHS data on drug-poisoning deaths. NCHS Factsheet. https://www.cdc.gov/nchs/data/factsheets/factsheet-drug-poisoning-H.pdf. Accessed October 28, 2020.
5. National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. Bethesda (MD): NIDA; 2014. www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Accessed October 28, 2020.
6. Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SAMHSA, 2011 Contract No.: HHS Publication no. (SMA) 11–4658.
7. Maeda A, Bateman BT, Clancy CR, et al. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158-1165.
8. Whiteman VE, Salemi JL, Mogos MF, et al. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:1-8
9. Pregnancy Mortality Surveillance System. www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm#trends. Accessed February 4, 2020.
10. Macdorman MF, Declercq E, Cabral H, Morton C. Recent increases in the U.S. maternal mortality rate. Obstet Gynecol. 2016;128:447-455.
11. Texas Health and Human Services. Maternal Mortality and Morbidity Task Force and Department of State Health Services Joint Biennial Report, September 2018. www.dshs.texas.gov/legislative/2018-Reports/MMMTFJointReport2018.pdf
12. Virginia Department of Health. Pregnancy-associated deaths from drug overdose in Virginia, 1999-2007: a report from the Virginia Maternal Mortality Review Team. Richmond, VA: VDH; 2015. www.vdh.virginia.gov/content/uploads/sites/18/2016/04/Final-Pregnancy-Associated-Deaths-Due-to-Drug-Overdose.pdf. Accessed October 28, 2020.
13. Maryland Department of Health and Mental Hygiene. Maryland maternal mortality review 2016 annual report. Baltimore: DHMH; 2016. https://phpa.health.maryland.gov/Documents/Maryland-Maternal-Mortality-Review-2016-Report.pdf. Accessed October 28, 2020.
14. Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997-1002.
15. Reddy UM, Davis JM, Ren Z, et al. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes. Obstet Gynecol Survey. 2017;72:703-705.
16. Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:650-655.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction during pregnancy. In: Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series, No. 43. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005:211-224.
18. Wojnar-Horton RE, Kristensen JH, Yapp P, et al. Methadone distribution and excretion into breast milk of clients in a methadone maintenance programme. Br J Clin Pharmacol. 1997;44:543-547.
19. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10:135-141.
20. Sachs HC. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Committee on Drugs. Pediatrics. 2013;132:e796-809.
21. Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract. 2014;9:19.
22. Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. Obstet Gynecol. 2017;130:488-489.
23. Gopman S. Prenatal and postpartum care of women with substance use disorders. Obstet Gynecol Clin North Am. 2014;41:213-228.
24. Mitchell M, Severtson S, Latimer W. Pregnancy and race/ethnicity as predictors of motivation for drug treatment. Am J Drug Alcohol Abuse. 2008;34:397-404.
From the UT Health Long School of Medicine San Antonio, Texas.
Abstract
Objective: To educate pregnant patients with opioid use disorder (OUD) about the effects of opioids in order to improve understanding and help achieve sustained abstinence.
Methods: The Center for Health Care Services and University Hospital System (UHS) in San Antonio, TX, jointly o
Results: Of 68 women enrolled in the program, 33 completed both the pre-survey and the post-survey (48.5%). Nearly half (48%) were very motivated to quit before pregnancy, but 85% were very motivated to quit once pregnant. All participants said learning more about the effects of opiates would increase motivation for sobriety. Prior to the educational intervention, 39% of participants knew it was safe to breastfeed on methadone, which improved to 97% in the post-survey, and 76% incorrectly thought they would be reported to authorities by their health care providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so.
Conclusion: Pregnancy and education about opioids increased patients’ motivation to quit. Patients also advanced their breastfeeding knowledge and learned about patient-provider confidentiality. Our greatest challenge was participant follow-up; however, this improved with the help of a full-time Mommies Program nurse. Our future aim is to increase project awareness and extend the educational research.
Keywords: pregnancy; addiction; opioids; OUD; counseling.
In 2012 more than 259 million prescriptions for opioids were written in the United States, which was a 200% increase since 1998.1 Since the early 2000s, admissions to opioid substance abuse programs and the death rate from opioids have quadrupled.2-4 Specifically, the rate of heroin use increased more than 300% from 2010 to 2014.5 Opioid use in pregnancy has also escalated in recent years, with a 3- to 4-fold increase from 2000 to 2009 and with 4 in 1000 deliveries being complicated by opioid use disorder (OUD) in 2011.6-8
Between 2000 and 2014, the maternal mortality rate in the United States increased 24%, making it the only industrialized nation with a maternal mortality rate that is rising rather than falling.9 The Texas Maternal Mortality and Morbidity Task Force found that between 2012 and 2015 drug overdose was the leading cause of maternal death in the period from delivery to 365 days postpartum, and it has increased dramatically since 2010.10,11
In addition, maternal mortality reviews in several states have identified substance use as a major risk factor for pregnancy-associated deaths.12,13 In Texas between 2012 and 2015, opioids were found in 58% of maternal drug overdoses.10 In 2007, 22.8% of women who were enrolled in Medicaid programs in 46 states filled an opioid prescription during pregnancy.14 Additionally, the rising prevalence of opioid use in pregnancy has led to a sharp increase in neonatal abstinence syndrome (NAS), rising from 1.5 cases per 1000 hospital births in 1999 to 6.0 per 1000 hospital births in 2013.15 Unsurprisingly, states with the highest rates of opioid prescribing also have the highest rates of NAS.16
Methadone combined with counseling and behavioral therapy has been the standard of care for the treatment of OUD in pregnancy since the 1970s. Methadone treatment prevents opioid withdrawal symptoms and increases adherence to prenatal care.17 One of the largest methadone treatment clinics in the San Antonio, TX, area is the Center for Health Care Services (CHCS). University Health System in San Antonio (UHS) has established a clinic called The Mommies Program, where mothers addicted to opioids can receive prenatal care by a dedicated physician, registered nurse, and a certified nurse midwife, who work in collaboration with the CHCS methadone clinic. Pregnant patients with OUD in pregnancy are concurrently enrolled in the Mommies Program and receive prenatal care through UHS and methadone treatment and counseling through CHCS. The continuity effort aims to increase prenatal care rates and adherence to methadone treatment.
Once mothers are off illicit opioids and on methadone, it is essential to discuss breastfeeding with them, as many mothers addicted to illicit opioids may have been told that they should not be breastfeeding. However, breastfeeding should be encouraged in women who are stable on methadone if they are not using illicit drugs and do not have other contraindications, regardless of maternal methadone dose, since the transfer of methadone into breast milk is minimal.18-20 Breastfeeding is beneficial in women taking methadone and has been associated with decreased severity of NAS symptoms, decreased need for pharmacotherapy, and a shorter hospital stay for the baby.21 In addition, breastfeeding contributes to the development of an attachment between mother and infant, while also providing the infant with natural immunity. Women should be counseled about the need to stop breastfeeding in the event of a relapse.22
Finally, the postpartum period represents a time of increased stressors, such as loss of sleep, child protective services involvement, and frustration with constant demands from new baby. For mothers with addiction, this is an especially sensitive time, as the stressors may be exacerbated by their new sobriety and a sudden end to the motivation they experienced from pregnancy.23 Therefore, early and frequent postpartum care with methadone dose evaluation is essential in order to decrease drug relapse and screen for postpartum depression in detail, since patients with a history of drug use are at increased risk of postpartum depression.
In 2017 medical students at UT Health Long School of Medicine in San Antonio created a project to educate women about OUD in pregnancy and provide motivational incentives for sustained abstinence; this project has continued each year since. Students provide education about methadone treatment and the dangers of using illicit opioids during and after pregnancy. Students especially focus on educating patients on the key problem areas in the literature, such as overdose, NAS, breastfeeding, postpartum substance use, and postpartum depression.
Methods
From October 2018 to February 2020, a total of 15 medical students volunteered between 1 and 20 times at the Mommies Program clinic, which was held once or twice per week from 8

The only inclusion criteria for participating in the educational intervention and survey was participants had to be 18 years of age or older and enrolled in the Mommies Program. Patients who met the inclusion criteria and agreed to participate completed a pre-survey administered by the students during the patient’s initial prenatal visit (Figure 2). This survey collected baseline information about the patient’s history with opioid use and their current knowledge of methadone treatment, NAS, legal aspects of drug use disclosure, and drug testing prior to the education portion of the encounter. After the pre-survey was administered, students spent 30 minutes reviewing the correct answers of the survey with the patients by utilizing the standardized handout to help patients understand details of methadone and opioid use in pregnancy (Figure 1). The post-survey was administered by a student once patients entered the third trimester to assess whether the education session increased patients’ knowledge of these topics.

At the time patients completed the post-survey, they received a Baby Bag as well as education regarding each item in the bag. The aim of distributing Baby Bags was to relieve some possible postnatal stressors and educate the patients about infant care. Items included in the bag were diapers, wipes, bottles, clothes, and swaddles. Prenatal vitamins were added in January 2020, as many patients struggle to afford vitamins if they are not currently covered by Medicaid or have other barriers. The Baby Bag items were purchased through a Community Service Learning grant through UT Health San Antonio.
Results
Of 68 women enrolled in the Mommies Program during the intervention period, 33 completed the pre-survey and the post-survey (48.5%). Even though all patients enrolled in the program met the inclusion criteria, patients were not included in the educational program for multiple reasons, including refusal to participate, poor clinic follow-up, or lack of students to collect surveys. However, all patients who completed the pre-survey did complete the post-survey. In the pre-survey, only 39% of participants knew it was safe to breastfeed while on methadone. In the post-survey, 97% knew it safe to breastfeed. Nearly half (48%) reported being very motivated to quit opioids before pregnancy, but 85% were very motivated to quit once pregnant. In the pre-survey, 76% incorrectly thought they would be reported to authorities by their health providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so. Also, all participants said learning more about the effects of opiates would increase motivation for sobriety.
Discussion
Questions assessed during the educational surveys revolved around patients’ knowledge of the intricacies, legally and physiologically, of methadone treatment for OUD, as well as beneficial aspects for patients and future child health, such as breastfeeding and motivation to quit and stay sober.
It was clear that there was a lack of knowledge and education about breastfeeding, as only 39% of the participants thought that it was safe to breastfeed while on methadone in the pre-survey; in the post survey, this improved to 97%. Students spent a large portion of the educational time going over the safety of breastfeeding for patients on methadone and the many benefits to mother and baby. Students also reviewed breastfeeding with patients every time patients came in for a visit and debunked any falsehoods about the negatives of breastfeeding while on methadone. This is another testament to the benefits of reinforcement around patient education.
The area of trust between provider and patient is essential in all provider-patient relationships. However, in the area of addiction, a trusting bond is especially important, as patients must feel confident and comfortable to disclose every aspect of their lives so the provider can give the best care. It was clear from our initial data that many patients did not feel this trust or understand the legal aspects regarding the provider-patient relationship in the terms of drug use, as the pre-survey shows 76% of patients originally thought they would be reported to authorities if they told their provider they used illegal drugs during pregnancy. This was an enormous issue in the clinic and something that needed to be addressed because, based on these data, we feared many patients would not be honest about using illegal drugs to supplement their methadone if they believed they would be reported to the authorities or even jailed. The medical student education team continually assured patients that their honesty about illegal drug use during pregnancy would not be revealed to the authorities, and also made it clear to patients that it was essential they were honest about illegal drug use so the optimal care could be provided by the team. These discussions were successful, as the post-survey showed that 100% of patients knew they would not be reported to the authorities if they used illegal drugs during the pregnancy. This showed an increase in knowledge, but also suggested an increase in confidence in the provider-patient relationship by patients, which we speculate allowed for a better patient experience, better patient outcomes, and less emotional stress for the patient and provider.
Last, we wanted to study and address the motivation to quit using drugs and stay sober through learning about the effects of opiates and how this motivation was related to pregnancy. A study by Mitchell et al makes clear that pregnancy is a motivation to seek treatment for drug use and to quit,24 and our survey data support these findings, with 48% of patients motivated to quit before they were pregnant and 85% motivated to quit once they knew they were pregnant. In addition, all patients attested on the pre- and post-survey that learning more about opioids would increase their motivation for sobriety. Therefore, we believe education about the use of opioids and other drugs is a strong motivation towards sobriety and should be further studied in methadone treatment and other drugs as well.
We will continue to focus on sobriety postpartum by using the education in pregnancy as a springboard to further postpartum education, as education seems to be very beneficial to future sobriety. In the future, we believe extending the educational program past pregnancy and discussing opioid use and addiction with patients at multiple follow-up visits will be beneficial to patients’ sobriety.
We faced 2 main challenges in implementing this intervention and survey: patients would often miss multiple appointments during their third trimester or would not attend their postpartum visit if they only had 1 prenatal visit; and many clinic sessions had low student attendance because students often had many other responsibilities in medical school and there were only 15 volunteers over the study time. These challenges decreased our post-survey completion rate. However, there has been improvement in follow-up as the project has continued. The Mommies Program now has a full-time registered nurse, and a larger number of medical student teachers have volunteered to attend the clinic. In the future, we aim to increase awareness of our project and the benefits of participation, expand advertising at our medical school to increase student participation, and increase follow-up education in the postpartum period.
Another future direction is to include local, free doula services, which are offered through Catholic Charities in San Antonio. Doulas provide antepartum, intrapartum, and postpartum services, which we believe will help our patients through advocacy and support for sobriety during this emotional and stressful time.
Conclusion
Counseled participants were receptive to learning about the effects of OUD and methadone on themselves and their newborn. Participants unanimously stated that learning more about OUD increased their motivation for sobriety. It was also clear that the increased motivation to be sober during pregnancy, as compared to before pregnancy, is an opportunity to help these women take steps to get sober. Patients also advanced their breastfeeding knowledge, as we helped debunk falsehoods surrounding breastfeeding while on methadone, and we anticipate this will lead to greater breastfeeding rates for our patients on methadone, although this was not specifically studied. Finally, patients learned about patient-provider confidentiality, which allowed for more open and clear communication with patients so they could be cared for to the greatest degree and trust could remain paramount.
Drug use is a common problem in the health care system, and exposure to patients with addiction is important for medical students in training. We believe that attending the Mommies Program allowed medical students to gain exposure and skills to better help patients with OUD.
Corresponding author: Nicholas Stansbury, MD, [email protected].
Financial disclosures: None.
From the UT Health Long School of Medicine San Antonio, Texas.
Abstract
Objective: To educate pregnant patients with opioid use disorder (OUD) about the effects of opioids in order to improve understanding and help achieve sustained abstinence.
Methods: The Center for Health Care Services and University Hospital System (UHS) in San Antonio, TX, jointly o
Results: Of 68 women enrolled in the program, 33 completed both the pre-survey and the post-survey (48.5%). Nearly half (48%) were very motivated to quit before pregnancy, but 85% were very motivated to quit once pregnant. All participants said learning more about the effects of opiates would increase motivation for sobriety. Prior to the educational intervention, 39% of participants knew it was safe to breastfeed on methadone, which improved to 97% in the post-survey, and 76% incorrectly thought they would be reported to authorities by their health care providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so.
Conclusion: Pregnancy and education about opioids increased patients’ motivation to quit. Patients also advanced their breastfeeding knowledge and learned about patient-provider confidentiality. Our greatest challenge was participant follow-up; however, this improved with the help of a full-time Mommies Program nurse. Our future aim is to increase project awareness and extend the educational research.
Keywords: pregnancy; addiction; opioids; OUD; counseling.
In 2012 more than 259 million prescriptions for opioids were written in the United States, which was a 200% increase since 1998.1 Since the early 2000s, admissions to opioid substance abuse programs and the death rate from opioids have quadrupled.2-4 Specifically, the rate of heroin use increased more than 300% from 2010 to 2014.5 Opioid use in pregnancy has also escalated in recent years, with a 3- to 4-fold increase from 2000 to 2009 and with 4 in 1000 deliveries being complicated by opioid use disorder (OUD) in 2011.6-8
Between 2000 and 2014, the maternal mortality rate in the United States increased 24%, making it the only industrialized nation with a maternal mortality rate that is rising rather than falling.9 The Texas Maternal Mortality and Morbidity Task Force found that between 2012 and 2015 drug overdose was the leading cause of maternal death in the period from delivery to 365 days postpartum, and it has increased dramatically since 2010.10,11
In addition, maternal mortality reviews in several states have identified substance use as a major risk factor for pregnancy-associated deaths.12,13 In Texas between 2012 and 2015, opioids were found in 58% of maternal drug overdoses.10 In 2007, 22.8% of women who were enrolled in Medicaid programs in 46 states filled an opioid prescription during pregnancy.14 Additionally, the rising prevalence of opioid use in pregnancy has led to a sharp increase in neonatal abstinence syndrome (NAS), rising from 1.5 cases per 1000 hospital births in 1999 to 6.0 per 1000 hospital births in 2013.15 Unsurprisingly, states with the highest rates of opioid prescribing also have the highest rates of NAS.16
Methadone combined with counseling and behavioral therapy has been the standard of care for the treatment of OUD in pregnancy since the 1970s. Methadone treatment prevents opioid withdrawal symptoms and increases adherence to prenatal care.17 One of the largest methadone treatment clinics in the San Antonio, TX, area is the Center for Health Care Services (CHCS). University Health System in San Antonio (UHS) has established a clinic called The Mommies Program, where mothers addicted to opioids can receive prenatal care by a dedicated physician, registered nurse, and a certified nurse midwife, who work in collaboration with the CHCS methadone clinic. Pregnant patients with OUD in pregnancy are concurrently enrolled in the Mommies Program and receive prenatal care through UHS and methadone treatment and counseling through CHCS. The continuity effort aims to increase prenatal care rates and adherence to methadone treatment.
Once mothers are off illicit opioids and on methadone, it is essential to discuss breastfeeding with them, as many mothers addicted to illicit opioids may have been told that they should not be breastfeeding. However, breastfeeding should be encouraged in women who are stable on methadone if they are not using illicit drugs and do not have other contraindications, regardless of maternal methadone dose, since the transfer of methadone into breast milk is minimal.18-20 Breastfeeding is beneficial in women taking methadone and has been associated with decreased severity of NAS symptoms, decreased need for pharmacotherapy, and a shorter hospital stay for the baby.21 In addition, breastfeeding contributes to the development of an attachment between mother and infant, while also providing the infant with natural immunity. Women should be counseled about the need to stop breastfeeding in the event of a relapse.22
Finally, the postpartum period represents a time of increased stressors, such as loss of sleep, child protective services involvement, and frustration with constant demands from new baby. For mothers with addiction, this is an especially sensitive time, as the stressors may be exacerbated by their new sobriety and a sudden end to the motivation they experienced from pregnancy.23 Therefore, early and frequent postpartum care with methadone dose evaluation is essential in order to decrease drug relapse and screen for postpartum depression in detail, since patients with a history of drug use are at increased risk of postpartum depression.
In 2017 medical students at UT Health Long School of Medicine in San Antonio created a project to educate women about OUD in pregnancy and provide motivational incentives for sustained abstinence; this project has continued each year since. Students provide education about methadone treatment and the dangers of using illicit opioids during and after pregnancy. Students especially focus on educating patients on the key problem areas in the literature, such as overdose, NAS, breastfeeding, postpartum substance use, and postpartum depression.
Methods
From October 2018 to February 2020, a total of 15 medical students volunteered between 1 and 20 times at the Mommies Program clinic, which was held once or twice per week from 8

The only inclusion criteria for participating in the educational intervention and survey was participants had to be 18 years of age or older and enrolled in the Mommies Program. Patients who met the inclusion criteria and agreed to participate completed a pre-survey administered by the students during the patient’s initial prenatal visit (Figure 2). This survey collected baseline information about the patient’s history with opioid use and their current knowledge of methadone treatment, NAS, legal aspects of drug use disclosure, and drug testing prior to the education portion of the encounter. After the pre-survey was administered, students spent 30 minutes reviewing the correct answers of the survey with the patients by utilizing the standardized handout to help patients understand details of methadone and opioid use in pregnancy (Figure 1). The post-survey was administered by a student once patients entered the third trimester to assess whether the education session increased patients’ knowledge of these topics.

At the time patients completed the post-survey, they received a Baby Bag as well as education regarding each item in the bag. The aim of distributing Baby Bags was to relieve some possible postnatal stressors and educate the patients about infant care. Items included in the bag were diapers, wipes, bottles, clothes, and swaddles. Prenatal vitamins were added in January 2020, as many patients struggle to afford vitamins if they are not currently covered by Medicaid or have other barriers. The Baby Bag items were purchased through a Community Service Learning grant through UT Health San Antonio.
Results
Of 68 women enrolled in the Mommies Program during the intervention period, 33 completed the pre-survey and the post-survey (48.5%). Even though all patients enrolled in the program met the inclusion criteria, patients were not included in the educational program for multiple reasons, including refusal to participate, poor clinic follow-up, or lack of students to collect surveys. However, all patients who completed the pre-survey did complete the post-survey. In the pre-survey, only 39% of participants knew it was safe to breastfeed while on methadone. In the post-survey, 97% knew it safe to breastfeed. Nearly half (48%) reported being very motivated to quit opioids before pregnancy, but 85% were very motivated to quit once pregnant. In the pre-survey, 76% incorrectly thought they would be reported to authorities by their health providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so. Also, all participants said learning more about the effects of opiates would increase motivation for sobriety.
Discussion
Questions assessed during the educational surveys revolved around patients’ knowledge of the intricacies, legally and physiologically, of methadone treatment for OUD, as well as beneficial aspects for patients and future child health, such as breastfeeding and motivation to quit and stay sober.
It was clear that there was a lack of knowledge and education about breastfeeding, as only 39% of the participants thought that it was safe to breastfeed while on methadone in the pre-survey; in the post survey, this improved to 97%. Students spent a large portion of the educational time going over the safety of breastfeeding for patients on methadone and the many benefits to mother and baby. Students also reviewed breastfeeding with patients every time patients came in for a visit and debunked any falsehoods about the negatives of breastfeeding while on methadone. This is another testament to the benefits of reinforcement around patient education.
The area of trust between provider and patient is essential in all provider-patient relationships. However, in the area of addiction, a trusting bond is especially important, as patients must feel confident and comfortable to disclose every aspect of their lives so the provider can give the best care. It was clear from our initial data that many patients did not feel this trust or understand the legal aspects regarding the provider-patient relationship in the terms of drug use, as the pre-survey shows 76% of patients originally thought they would be reported to authorities if they told their provider they used illegal drugs during pregnancy. This was an enormous issue in the clinic and something that needed to be addressed because, based on these data, we feared many patients would not be honest about using illegal drugs to supplement their methadone if they believed they would be reported to the authorities or even jailed. The medical student education team continually assured patients that their honesty about illegal drug use during pregnancy would not be revealed to the authorities, and also made it clear to patients that it was essential they were honest about illegal drug use so the optimal care could be provided by the team. These discussions were successful, as the post-survey showed that 100% of patients knew they would not be reported to the authorities if they used illegal drugs during the pregnancy. This showed an increase in knowledge, but also suggested an increase in confidence in the provider-patient relationship by patients, which we speculate allowed for a better patient experience, better patient outcomes, and less emotional stress for the patient and provider.
Last, we wanted to study and address the motivation to quit using drugs and stay sober through learning about the effects of opiates and how this motivation was related to pregnancy. A study by Mitchell et al makes clear that pregnancy is a motivation to seek treatment for drug use and to quit,24 and our survey data support these findings, with 48% of patients motivated to quit before they were pregnant and 85% motivated to quit once they knew they were pregnant. In addition, all patients attested on the pre- and post-survey that learning more about opioids would increase their motivation for sobriety. Therefore, we believe education about the use of opioids and other drugs is a strong motivation towards sobriety and should be further studied in methadone treatment and other drugs as well.
We will continue to focus on sobriety postpartum by using the education in pregnancy as a springboard to further postpartum education, as education seems to be very beneficial to future sobriety. In the future, we believe extending the educational program past pregnancy and discussing opioid use and addiction with patients at multiple follow-up visits will be beneficial to patients’ sobriety.
We faced 2 main challenges in implementing this intervention and survey: patients would often miss multiple appointments during their third trimester or would not attend their postpartum visit if they only had 1 prenatal visit; and many clinic sessions had low student attendance because students often had many other responsibilities in medical school and there were only 15 volunteers over the study time. These challenges decreased our post-survey completion rate. However, there has been improvement in follow-up as the project has continued. The Mommies Program now has a full-time registered nurse, and a larger number of medical student teachers have volunteered to attend the clinic. In the future, we aim to increase awareness of our project and the benefits of participation, expand advertising at our medical school to increase student participation, and increase follow-up education in the postpartum period.
Another future direction is to include local, free doula services, which are offered through Catholic Charities in San Antonio. Doulas provide antepartum, intrapartum, and postpartum services, which we believe will help our patients through advocacy and support for sobriety during this emotional and stressful time.
Conclusion
Counseled participants were receptive to learning about the effects of OUD and methadone on themselves and their newborn. Participants unanimously stated that learning more about OUD increased their motivation for sobriety. It was also clear that the increased motivation to be sober during pregnancy, as compared to before pregnancy, is an opportunity to help these women take steps to get sober. Patients also advanced their breastfeeding knowledge, as we helped debunk falsehoods surrounding breastfeeding while on methadone, and we anticipate this will lead to greater breastfeeding rates for our patients on methadone, although this was not specifically studied. Finally, patients learned about patient-provider confidentiality, which allowed for more open and clear communication with patients so they could be cared for to the greatest degree and trust could remain paramount.
Drug use is a common problem in the health care system, and exposure to patients with addiction is important for medical students in training. We believe that attending the Mommies Program allowed medical students to gain exposure and skills to better help patients with OUD.
Corresponding author: Nicholas Stansbury, MD, [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Opioid painkiller prescribing: where you live makes a difference. CDC website. www.cdc.gov/vitalsigns/opioid-prescribing. Accessed October 28, 2020.
2. Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville (MD): SAMHSA; 2013. www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. Accessed October 28, 2020.
3. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154-63.
4. National Center for Health Statistics. NCHS data on drug-poisoning deaths. NCHS Factsheet. https://www.cdc.gov/nchs/data/factsheets/factsheet-drug-poisoning-H.pdf. Accessed October 28, 2020.
5. National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. Bethesda (MD): NIDA; 2014. www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Accessed October 28, 2020.
6. Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SAMHSA, 2011 Contract No.: HHS Publication no. (SMA) 11–4658.
7. Maeda A, Bateman BT, Clancy CR, et al. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158-1165.
8. Whiteman VE, Salemi JL, Mogos MF, et al. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:1-8
9. Pregnancy Mortality Surveillance System. www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm#trends. Accessed February 4, 2020.
10. Macdorman MF, Declercq E, Cabral H, Morton C. Recent increases in the U.S. maternal mortality rate. Obstet Gynecol. 2016;128:447-455.
11. Texas Health and Human Services. Maternal Mortality and Morbidity Task Force and Department of State Health Services Joint Biennial Report, September 2018. www.dshs.texas.gov/legislative/2018-Reports/MMMTFJointReport2018.pdf
12. Virginia Department of Health. Pregnancy-associated deaths from drug overdose in Virginia, 1999-2007: a report from the Virginia Maternal Mortality Review Team. Richmond, VA: VDH; 2015. www.vdh.virginia.gov/content/uploads/sites/18/2016/04/Final-Pregnancy-Associated-Deaths-Due-to-Drug-Overdose.pdf. Accessed October 28, 2020.
13. Maryland Department of Health and Mental Hygiene. Maryland maternal mortality review 2016 annual report. Baltimore: DHMH; 2016. https://phpa.health.maryland.gov/Documents/Maryland-Maternal-Mortality-Review-2016-Report.pdf. Accessed October 28, 2020.
14. Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997-1002.
15. Reddy UM, Davis JM, Ren Z, et al. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes. Obstet Gynecol Survey. 2017;72:703-705.
16. Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:650-655.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction during pregnancy. In: Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series, No. 43. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005:211-224.
18. Wojnar-Horton RE, Kristensen JH, Yapp P, et al. Methadone distribution and excretion into breast milk of clients in a methadone maintenance programme. Br J Clin Pharmacol. 1997;44:543-547.
19. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10:135-141.
20. Sachs HC. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Committee on Drugs. Pediatrics. 2013;132:e796-809.
21. Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract. 2014;9:19.
22. Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. Obstet Gynecol. 2017;130:488-489.
23. Gopman S. Prenatal and postpartum care of women with substance use disorders. Obstet Gynecol Clin North Am. 2014;41:213-228.
24. Mitchell M, Severtson S, Latimer W. Pregnancy and race/ethnicity as predictors of motivation for drug treatment. Am J Drug Alcohol Abuse. 2008;34:397-404.
1. Centers for Disease Control and Prevention. Opioid painkiller prescribing: where you live makes a difference. CDC website. www.cdc.gov/vitalsigns/opioid-prescribing. Accessed October 28, 2020.
2. Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville (MD): SAMHSA; 2013. www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. Accessed October 28, 2020.
3. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154-63.
4. National Center for Health Statistics. NCHS data on drug-poisoning deaths. NCHS Factsheet. https://www.cdc.gov/nchs/data/factsheets/factsheet-drug-poisoning-H.pdf. Accessed October 28, 2020.
5. National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. Bethesda (MD): NIDA; 2014. www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Accessed October 28, 2020.
6. Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SAMHSA, 2011 Contract No.: HHS Publication no. (SMA) 11–4658.
7. Maeda A, Bateman BT, Clancy CR, et al. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158-1165.
8. Whiteman VE, Salemi JL, Mogos MF, et al. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:1-8
9. Pregnancy Mortality Surveillance System. www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm#trends. Accessed February 4, 2020.
10. Macdorman MF, Declercq E, Cabral H, Morton C. Recent increases in the U.S. maternal mortality rate. Obstet Gynecol. 2016;128:447-455.
11. Texas Health and Human Services. Maternal Mortality and Morbidity Task Force and Department of State Health Services Joint Biennial Report, September 2018. www.dshs.texas.gov/legislative/2018-Reports/MMMTFJointReport2018.pdf
12. Virginia Department of Health. Pregnancy-associated deaths from drug overdose in Virginia, 1999-2007: a report from the Virginia Maternal Mortality Review Team. Richmond, VA: VDH; 2015. www.vdh.virginia.gov/content/uploads/sites/18/2016/04/Final-Pregnancy-Associated-Deaths-Due-to-Drug-Overdose.pdf. Accessed October 28, 2020.
13. Maryland Department of Health and Mental Hygiene. Maryland maternal mortality review 2016 annual report. Baltimore: DHMH; 2016. https://phpa.health.maryland.gov/Documents/Maryland-Maternal-Mortality-Review-2016-Report.pdf. Accessed October 28, 2020.
14. Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997-1002.
15. Reddy UM, Davis JM, Ren Z, et al. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes. Obstet Gynecol Survey. 2017;72:703-705.
16. Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:650-655.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction during pregnancy. In: Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series, No. 43. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005:211-224.
18. Wojnar-Horton RE, Kristensen JH, Yapp P, et al. Methadone distribution and excretion into breast milk of clients in a methadone maintenance programme. Br J Clin Pharmacol. 1997;44:543-547.
19. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10:135-141.
20. Sachs HC. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Committee on Drugs. Pediatrics. 2013;132:e796-809.
21. Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract. 2014;9:19.
22. Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. Obstet Gynecol. 2017;130:488-489.
23. Gopman S. Prenatal and postpartum care of women with substance use disorders. Obstet Gynecol Clin North Am. 2014;41:213-228.
24. Mitchell M, Severtson S, Latimer W. Pregnancy and race/ethnicity as predictors of motivation for drug treatment. Am J Drug Alcohol Abuse. 2008;34:397-404.
SABCS 2020: What’s hot, including a major chemotherapy trial
That’s the word from Virginia Kaklamani, MD, from the University of Texas Health Sciences Center San Antonio. Dr. Kaklamani, a professor of medicine in the division of hematology/oncology, is codirector of the meeting that runs online Dec. 8-11.
If the new trial sounds familiar, that’s because it’s a lot like the TAILORx trial, the results of which were first presented in 2018 and have changed practice in women with early-stage disease and no lymph node involvement.
“This is the lymph-node positive TAILORx. It’s extremely important,” Dr. Kaklamani said in an interview, adding that both trials involved women with hormone receptor (HR)–positive, HER2-negative disease.
If the RxPONDER trial shows similar outcomes between women randomized to adjuvant endocrine therapy alone versus endocrine therapy plus chemotherapy, then clinicians “can potentially avoid giving chemotherapy to a large number of women who are currently receiving it,” she explained.
Because women with nodal involvement (one to three positive axillary nodes) are at a higher risk of recurrence, RxPONDER may provide needed insight on the management of these types of breast cancers, Dr. Kaklamani suggested.
Both trials have used the 21-tumor gene expression assay (Oncotype Dx) to determine recurrence-risk status.
Dr. Kaklamani also spotlighted the phase 3 CONTESSA trial (abstract GS4-01) in 600+ patients with locally advanced or metastatic breast cancer that is HR positive and HER2 negative and has been previously treated with a taxane.
The trial features an experimental oral taxane, tesetaxel. The primary objective is to compare the efficacy of tesetaxel plus a reduced dose of capecitabine (Xeloda) versus the approved dose of capecitabine alone. Presented data will include progression-free survival results, indicating about a 3-month PFS advantage with tesetaxel, which is taken once every 3 weeks.
“Oral drugs are convenient for patients and, despite limitations, they are, all in all, a revolution in cancer treatment,” noted Dr. Kaklamani, adding that they beneficially eliminate the need for time-consuming infusions and related clinic visits as well as drug ports.
It will be interesting to see what Steven Vogl, MD, a private practitioner in New Yorky, has to say about CONTESSA and the rest of the SABCS.
He is usually a commentator from the meeting floor, whose self-introduction, “Vogl, New York,” is well known to perennial meeting attendees, according to a profile piece published some years ago.
This year the medical oncologist will also serve as the chair of the “View from the Trenches” session, which is devoted to summarizing the meeting’s most important findings for everyday practitioners.
A number of years ago, Dr. Vogl proposed the idea of this where-the-rubber-meets-the-road session to SABCS meeting planners, which they then adopted. This year, Dr. Kaklamani invited Dr. Vogl to run the session and he accepted.
Dr. Vogl is a “really smart guy who is always right on” with his comments and questions, and he will be the first-ever independent, community-based oncologist to chair a meeting session, said Dr. Kaklamani.
Other hot topics
Another hot topic featured at the meeting will be the use of CDK4/6 inhibitors in the adjuvant treatment of HR-positive and HER2-negative disease that has a high risk of recurrence, Dr. Kaklamani said. New data from two trials, monarchE and PENELOPE-B, will be presented.
First, there will be an update from the monarchE trial (abstract GS1-01). The first results from this trial were reported in September at the European Society for Medical Oncology Virtual Congress 2020. They showed that adding abemaciclib (Verzenio) to endocrine therapy reduced the risk of early recurrence. The positive outcome represented the first treatment improvement in this high-risk setting in more than 20 years, according to experts.
A similar trial, PENELOPE-B (abstract GS1-02), looks at palbociclib (Ibrance) in a somewhat different population – those patients with high relapse risk after neoadjuvant chemotherapy. “These are even higher risk ER+ patients [than those in monarchE], which is why they received chemotherapy before surgery,” commented Dr. Kaklamani.
In triple-negative disease, there will be overall survival (OS) results from the phase 3 KEYNOTE-355 study (abstract GS3-01) of pembrolizumab (Keytruda) versus placebo (plus chemotherapy for all patients) as first-line therapy for locally recurrent inoperable or metastatic triple-negative breast cancer. “It’s potentially a huge deal,” said Dr. Kaklamani about the OS data, if they are statistically significant.
A meta-analysis (abstract GS4-08) of data on circulating tumor cells (CTCs), which are shed from the primary tumor into the bloodstream, may point to their value as a tool to determine whether or not a breast cancer treatment is effective. “CTCs allow you to assess how a treatment is doing before you do scans, which typically occur 3 months or so later,” explained Dr. Kaklamani.
CTC results can be assessed in 3-4 weeks and allow clinicians to change treatments if CTC volume increases. However, a previous study of CTCs did not show a clinical benefit with the tool among patients treated mainly with chemotherapies. What’s different about the new study, which is from an international group of investigators, is in the treatments patients with metastatic breast cancer received. “This study is from a different era – with targeted therapies,” said Dr. Kaklamani.
In the new study, changes in CTC levels (with a reduction being a good result) between baseline (pretreatment) and follow-up were analyzed to determine whether they were associated with overall survival.
COVID sessions
On the meeting’s first day, SABCS will feature a special session on COVID-19 and breast cancer. The meeting organizers sought to separate the wheat from the chaff in this subject, as much has already been written, published, or presented.
“We received a lot of abstracts on COVID that were studies that were poorly done. We tried to tease through them and select the well-researched ones,” acknowledged Dr. Kaklamani.
The organizers included two patient advocates who have had COVID-19, including during treatment for breast cancer, as participants in the meeting session. The session will also feature global perspectives, with presenters from Brazil, Italy, and the Netherlands.
Plenary lectures
The meeting’s two plenary lectures will focus, respectively, on the increasingly used clinical approach of neoadjuvant therapy in breast cancer, and research in the time of a pandemic.
Elizabeth Mittendorf, MD, PhD, a surgical oncologist and director of the Breast lmmuno-Oncology program and co-director of the Breast Cancer Clinical Research Program at the Dana-Farber/Brigham and Women’s Cancer Center, Boston, will present “Local regional management following neoadjuvant therapy: Minding the knowledge gaps.”
Ned Sharpless, MD, director of the National Cancer Institute, will present “Advancing cancer research during challenging times.”
Dr. Kaklamani disclosed recieving consulting fees with Amgen, Eisai, Puma, Celldex, AstraZeneca, and Athenex; receiving fees for non-CME services received directly from commercial interest or their agents from Pfizer, Celgene, Genentech, Genomic Health, Puma, Eisai, and Novartis; and contracted research with Eisai.
This article first appeared on Medscape.com.
That’s the word from Virginia Kaklamani, MD, from the University of Texas Health Sciences Center San Antonio. Dr. Kaklamani, a professor of medicine in the division of hematology/oncology, is codirector of the meeting that runs online Dec. 8-11.
If the new trial sounds familiar, that’s because it’s a lot like the TAILORx trial, the results of which were first presented in 2018 and have changed practice in women with early-stage disease and no lymph node involvement.
“This is the lymph-node positive TAILORx. It’s extremely important,” Dr. Kaklamani said in an interview, adding that both trials involved women with hormone receptor (HR)–positive, HER2-negative disease.
If the RxPONDER trial shows similar outcomes between women randomized to adjuvant endocrine therapy alone versus endocrine therapy plus chemotherapy, then clinicians “can potentially avoid giving chemotherapy to a large number of women who are currently receiving it,” she explained.
Because women with nodal involvement (one to three positive axillary nodes) are at a higher risk of recurrence, RxPONDER may provide needed insight on the management of these types of breast cancers, Dr. Kaklamani suggested.
Both trials have used the 21-tumor gene expression assay (Oncotype Dx) to determine recurrence-risk status.
Dr. Kaklamani also spotlighted the phase 3 CONTESSA trial (abstract GS4-01) in 600+ patients with locally advanced or metastatic breast cancer that is HR positive and HER2 negative and has been previously treated with a taxane.
The trial features an experimental oral taxane, tesetaxel. The primary objective is to compare the efficacy of tesetaxel plus a reduced dose of capecitabine (Xeloda) versus the approved dose of capecitabine alone. Presented data will include progression-free survival results, indicating about a 3-month PFS advantage with tesetaxel, which is taken once every 3 weeks.
“Oral drugs are convenient for patients and, despite limitations, they are, all in all, a revolution in cancer treatment,” noted Dr. Kaklamani, adding that they beneficially eliminate the need for time-consuming infusions and related clinic visits as well as drug ports.
It will be interesting to see what Steven Vogl, MD, a private practitioner in New Yorky, has to say about CONTESSA and the rest of the SABCS.
He is usually a commentator from the meeting floor, whose self-introduction, “Vogl, New York,” is well known to perennial meeting attendees, according to a profile piece published some years ago.
This year the medical oncologist will also serve as the chair of the “View from the Trenches” session, which is devoted to summarizing the meeting’s most important findings for everyday practitioners.
A number of years ago, Dr. Vogl proposed the idea of this where-the-rubber-meets-the-road session to SABCS meeting planners, which they then adopted. This year, Dr. Kaklamani invited Dr. Vogl to run the session and he accepted.
Dr. Vogl is a “really smart guy who is always right on” with his comments and questions, and he will be the first-ever independent, community-based oncologist to chair a meeting session, said Dr. Kaklamani.
Other hot topics
Another hot topic featured at the meeting will be the use of CDK4/6 inhibitors in the adjuvant treatment of HR-positive and HER2-negative disease that has a high risk of recurrence, Dr. Kaklamani said. New data from two trials, monarchE and PENELOPE-B, will be presented.
First, there will be an update from the monarchE trial (abstract GS1-01). The first results from this trial were reported in September at the European Society for Medical Oncology Virtual Congress 2020. They showed that adding abemaciclib (Verzenio) to endocrine therapy reduced the risk of early recurrence. The positive outcome represented the first treatment improvement in this high-risk setting in more than 20 years, according to experts.
A similar trial, PENELOPE-B (abstract GS1-02), looks at palbociclib (Ibrance) in a somewhat different population – those patients with high relapse risk after neoadjuvant chemotherapy. “These are even higher risk ER+ patients [than those in monarchE], which is why they received chemotherapy before surgery,” commented Dr. Kaklamani.
In triple-negative disease, there will be overall survival (OS) results from the phase 3 KEYNOTE-355 study (abstract GS3-01) of pembrolizumab (Keytruda) versus placebo (plus chemotherapy for all patients) as first-line therapy for locally recurrent inoperable or metastatic triple-negative breast cancer. “It’s potentially a huge deal,” said Dr. Kaklamani about the OS data, if they are statistically significant.
A meta-analysis (abstract GS4-08) of data on circulating tumor cells (CTCs), which are shed from the primary tumor into the bloodstream, may point to their value as a tool to determine whether or not a breast cancer treatment is effective. “CTCs allow you to assess how a treatment is doing before you do scans, which typically occur 3 months or so later,” explained Dr. Kaklamani.
CTC results can be assessed in 3-4 weeks and allow clinicians to change treatments if CTC volume increases. However, a previous study of CTCs did not show a clinical benefit with the tool among patients treated mainly with chemotherapies. What’s different about the new study, which is from an international group of investigators, is in the treatments patients with metastatic breast cancer received. “This study is from a different era – with targeted therapies,” said Dr. Kaklamani.
In the new study, changes in CTC levels (with a reduction being a good result) between baseline (pretreatment) and follow-up were analyzed to determine whether they were associated with overall survival.
COVID sessions
On the meeting’s first day, SABCS will feature a special session on COVID-19 and breast cancer. The meeting organizers sought to separate the wheat from the chaff in this subject, as much has already been written, published, or presented.
“We received a lot of abstracts on COVID that were studies that were poorly done. We tried to tease through them and select the well-researched ones,” acknowledged Dr. Kaklamani.
The organizers included two patient advocates who have had COVID-19, including during treatment for breast cancer, as participants in the meeting session. The session will also feature global perspectives, with presenters from Brazil, Italy, and the Netherlands.
Plenary lectures
The meeting’s two plenary lectures will focus, respectively, on the increasingly used clinical approach of neoadjuvant therapy in breast cancer, and research in the time of a pandemic.
Elizabeth Mittendorf, MD, PhD, a surgical oncologist and director of the Breast lmmuno-Oncology program and co-director of the Breast Cancer Clinical Research Program at the Dana-Farber/Brigham and Women’s Cancer Center, Boston, will present “Local regional management following neoadjuvant therapy: Minding the knowledge gaps.”
Ned Sharpless, MD, director of the National Cancer Institute, will present “Advancing cancer research during challenging times.”
Dr. Kaklamani disclosed recieving consulting fees with Amgen, Eisai, Puma, Celldex, AstraZeneca, and Athenex; receiving fees for non-CME services received directly from commercial interest or their agents from Pfizer, Celgene, Genentech, Genomic Health, Puma, Eisai, and Novartis; and contracted research with Eisai.
This article first appeared on Medscape.com.
That’s the word from Virginia Kaklamani, MD, from the University of Texas Health Sciences Center San Antonio. Dr. Kaklamani, a professor of medicine in the division of hematology/oncology, is codirector of the meeting that runs online Dec. 8-11.
If the new trial sounds familiar, that’s because it’s a lot like the TAILORx trial, the results of which were first presented in 2018 and have changed practice in women with early-stage disease and no lymph node involvement.
“This is the lymph-node positive TAILORx. It’s extremely important,” Dr. Kaklamani said in an interview, adding that both trials involved women with hormone receptor (HR)–positive, HER2-negative disease.
If the RxPONDER trial shows similar outcomes between women randomized to adjuvant endocrine therapy alone versus endocrine therapy plus chemotherapy, then clinicians “can potentially avoid giving chemotherapy to a large number of women who are currently receiving it,” she explained.
Because women with nodal involvement (one to three positive axillary nodes) are at a higher risk of recurrence, RxPONDER may provide needed insight on the management of these types of breast cancers, Dr. Kaklamani suggested.
Both trials have used the 21-tumor gene expression assay (Oncotype Dx) to determine recurrence-risk status.
Dr. Kaklamani also spotlighted the phase 3 CONTESSA trial (abstract GS4-01) in 600+ patients with locally advanced or metastatic breast cancer that is HR positive and HER2 negative and has been previously treated with a taxane.
The trial features an experimental oral taxane, tesetaxel. The primary objective is to compare the efficacy of tesetaxel plus a reduced dose of capecitabine (Xeloda) versus the approved dose of capecitabine alone. Presented data will include progression-free survival results, indicating about a 3-month PFS advantage with tesetaxel, which is taken once every 3 weeks.
“Oral drugs are convenient for patients and, despite limitations, they are, all in all, a revolution in cancer treatment,” noted Dr. Kaklamani, adding that they beneficially eliminate the need for time-consuming infusions and related clinic visits as well as drug ports.
It will be interesting to see what Steven Vogl, MD, a private practitioner in New Yorky, has to say about CONTESSA and the rest of the SABCS.
He is usually a commentator from the meeting floor, whose self-introduction, “Vogl, New York,” is well known to perennial meeting attendees, according to a profile piece published some years ago.
This year the medical oncologist will also serve as the chair of the “View from the Trenches” session, which is devoted to summarizing the meeting’s most important findings for everyday practitioners.
A number of years ago, Dr. Vogl proposed the idea of this where-the-rubber-meets-the-road session to SABCS meeting planners, which they then adopted. This year, Dr. Kaklamani invited Dr. Vogl to run the session and he accepted.
Dr. Vogl is a “really smart guy who is always right on” with his comments and questions, and he will be the first-ever independent, community-based oncologist to chair a meeting session, said Dr. Kaklamani.
Other hot topics
Another hot topic featured at the meeting will be the use of CDK4/6 inhibitors in the adjuvant treatment of HR-positive and HER2-negative disease that has a high risk of recurrence, Dr. Kaklamani said. New data from two trials, monarchE and PENELOPE-B, will be presented.
First, there will be an update from the monarchE trial (abstract GS1-01). The first results from this trial were reported in September at the European Society for Medical Oncology Virtual Congress 2020. They showed that adding abemaciclib (Verzenio) to endocrine therapy reduced the risk of early recurrence. The positive outcome represented the first treatment improvement in this high-risk setting in more than 20 years, according to experts.
A similar trial, PENELOPE-B (abstract GS1-02), looks at palbociclib (Ibrance) in a somewhat different population – those patients with high relapse risk after neoadjuvant chemotherapy. “These are even higher risk ER+ patients [than those in monarchE], which is why they received chemotherapy before surgery,” commented Dr. Kaklamani.
In triple-negative disease, there will be overall survival (OS) results from the phase 3 KEYNOTE-355 study (abstract GS3-01) of pembrolizumab (Keytruda) versus placebo (plus chemotherapy for all patients) as first-line therapy for locally recurrent inoperable or metastatic triple-negative breast cancer. “It’s potentially a huge deal,” said Dr. Kaklamani about the OS data, if they are statistically significant.
A meta-analysis (abstract GS4-08) of data on circulating tumor cells (CTCs), which are shed from the primary tumor into the bloodstream, may point to their value as a tool to determine whether or not a breast cancer treatment is effective. “CTCs allow you to assess how a treatment is doing before you do scans, which typically occur 3 months or so later,” explained Dr. Kaklamani.
CTC results can be assessed in 3-4 weeks and allow clinicians to change treatments if CTC volume increases. However, a previous study of CTCs did not show a clinical benefit with the tool among patients treated mainly with chemotherapies. What’s different about the new study, which is from an international group of investigators, is in the treatments patients with metastatic breast cancer received. “This study is from a different era – with targeted therapies,” said Dr. Kaklamani.
In the new study, changes in CTC levels (with a reduction being a good result) between baseline (pretreatment) and follow-up were analyzed to determine whether they were associated with overall survival.
COVID sessions
On the meeting’s first day, SABCS will feature a special session on COVID-19 and breast cancer. The meeting organizers sought to separate the wheat from the chaff in this subject, as much has already been written, published, or presented.
“We received a lot of abstracts on COVID that were studies that were poorly done. We tried to tease through them and select the well-researched ones,” acknowledged Dr. Kaklamani.
The organizers included two patient advocates who have had COVID-19, including during treatment for breast cancer, as participants in the meeting session. The session will also feature global perspectives, with presenters from Brazil, Italy, and the Netherlands.
Plenary lectures
The meeting’s two plenary lectures will focus, respectively, on the increasingly used clinical approach of neoadjuvant therapy in breast cancer, and research in the time of a pandemic.
Elizabeth Mittendorf, MD, PhD, a surgical oncologist and director of the Breast lmmuno-Oncology program and co-director of the Breast Cancer Clinical Research Program at the Dana-Farber/Brigham and Women’s Cancer Center, Boston, will present “Local regional management following neoadjuvant therapy: Minding the knowledge gaps.”
Ned Sharpless, MD, director of the National Cancer Institute, will present “Advancing cancer research during challenging times.”
Dr. Kaklamani disclosed recieving consulting fees with Amgen, Eisai, Puma, Celldex, AstraZeneca, and Athenex; receiving fees for non-CME services received directly from commercial interest or their agents from Pfizer, Celgene, Genentech, Genomic Health, Puma, Eisai, and Novartis; and contracted research with Eisai.
This article first appeared on Medscape.com.
FROM SABCS 2020
Fixed duration ibrutinib/venetoclax appears feasible for some CLL/SLL patients
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
FROM ASH 2020
Key clinical point: A favorable 1-year DFS in patients after 12 cycles of ibrutinib plus venetoclax in the MRD cohort of the phase 2 CAPTIVATE trial supports fixed-duration treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma.
Major finding: One-year DFS after randomization to placebo or ibrutinib in the confirmed undetectable MRD group was 95.3% in the placebo group and 100.0 percent in the ibrutinib group (P = .1475).
Study details: The phase 2 CAPTIVATE study included 164 patients with previously untreated active chronic lymphocytic leukemia/small lymphocytic lymphoma requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
Disclosures: Dr. Wierda disclosed consultancy and research funding with multiple pharmaceutical companies.
Source: William G. Wierda, MD, PhD. ASH 2020 Abstract 123.
Durable responses with anti-BCMA CAR T-cell for multiple myeloma
For patients with heavily-pretreated multiple myeloma, the early and deep responses seen with the novel chimeric antigen receptor T-cell (CAR T-cell) construct ciltacabtagene autoleucel (cilta-cel) have also been durable, according to investigators in the CARTITUDE-1 trial.
Among 97 patients with multiple myeloma that had progressed on three or more prior lines of therapy or following treatment with at least two lines of therapy with a proteasome inhibitor and immunomodulating agent, the overall response rate (ORR) was 96.9%, with a median duration of response not reached after a median of 12.4 months of follow-up, reported Deepu Madduri, MD of Mount Sinai Medical Center in New York, and colleagues.
“We saw how heavily pretreated these patients were, and to see a one-time treatment get these kind of response rates is quite exceptional. What’s even more impressive is that 72% of these patients were still maintaining their response at the time of data cutoff,“ she said in an oral abstract presented during the virtual American Society of Hematology annual meeting.
Cilta-cel is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
As previously reported, the same CAR T-cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma.
Ciltacabtagene autoleucel was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration in December 2019, a priority medicines (PRIME) designation by the European Medicines Agency in April 2019, and breakthrough designation in China in September 2020.
At the 2019 ASH annual meeting, Dr. Madduri reported phase 1b results from the trial, which showed that for 29 patients with heavily pretreated, relapsed/refractory multiple myeloma, the ORR at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression.
Combined data
For the 2020 ASH annual meeting, Dr. Madduri reported combined results from phases 1b and 2 of the CARTITUDE-1 study.
The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least three prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug, and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T-cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
Of the 101 patients who underwent lymphodepletion, 97 (29 in phase 1b and 68 in phase 2) were treated with cilta-cel. Five of the patients in phase 1b and nine in phase 2 died on study, five of whom succumbed to progressive disease, and three due to adverse events unrelated to treatment. The remaining six patients died from treatment-related causes, including two patients from sepsis or septic shock, and one each from the cytokine release syndrome (CRS)/hemophagocytic lymphohistiocytosis (HLH), lung abscess, respiratory failure, and neurotoxicity.
At the time of data cutoff, 83 patients remained on study.
High ORR
The ORR was 96.9% (94 of 97 patients), comprising 67% stringent complete responses (sCR), 25.8% very good partial responses (VGPR), and 4.1% partial responses (PR).
Among 57 patients evaluable for minimal residual disease (MRD), 53 (93%) were MRD negative. Of this group, 49 had a VGPR or better.
The median time to first response was 1 month (range 0.9 to 8.5 months). At the time of data cutoff 70 patients had an ongoing response.
Among patients followed for a minimum of 6 months, most had cilta-cel CAR T-cells below the level of quantification (2 cells per microliter) in peripheral blood.
At a median follow-up of 12.4 months, 12-month overall progression-free survival rate was 76%, with the median PFS not reached. The 12-month overall survival rate was 88.5%, with the median OS not reached.
Safety data
All patients had at least one hematologic adverse event, 96 of which were grade 3 or 4 in severity. The events include neutropenia, anemia, thrombocytopenia, leukopenia, and lymphopenia. The median time to recovery was 2 weeks for grade 3 or 4 neutropenia and 4 weeks for thrombocytopenia.
Infections of any grade occurred in 57.7% of patients, including grade 3/4 pneumonia in 8.2% and grade 3/4 sepsis in 4.1%.
Grade 3 or 4 nonhematologic toxicities were uncommon, Dr. Madduri noted.
CRS of any grade occurred in 92 patients, but only 4 had grade 3 or 4 CRS.
Neurotoxicities occurred in 20 patients, of whom 10 had grade 3 or 4 neurotoxicity.
Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 16 patients, with 2 having grade 3 or greater ICANS. Other neurotoxicities of any grade, many which overlapped with ICANS, occurred in 12 patients, with 9 having grade 3 or 4 neurotoxicity.
The median time to ICANS onset was 8 days, with a median time to recovery of 4 days. Other neurotoxicities took longer to manifest and disappear, however, with a median time to onset of 27 days, and median time to recovery of 75 days.
Neurotoxicity mechanism questioned
In the question-and-answer session following her presentation, an audience member asked whether the investigators had any insights into the mechanism underlying the non-ICANS neurotoxicities they saw.
“We saw no clear etiology in the other neurotoxicities, but we saw that maybe there could be some mild associations with high tumor burden, prior CRS, ICANS, or even the higher expansion and persistence of these cells,” Dr. Madduri replied.
She noted that subsequent to these findings, the investigators have implemented mitigation strategies including allowing patients to have more bridging chemotherapy, more aggressive steroid use for early ICANS, and extensive monitoring.
Eric Smith, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said that the non-ICANS neurotoxicity profile of cilta-cel was different from that seen in other CAR T-cell trials, and asked how it compared to that of bi-specific BCMA/CD3 CAR T constructs.
“We did see some nerve palsies and peripheral motor neuropathy, but it wasn’t that many patients, and it’s really hard to compare what happened here with the bi-specifics, as every product is very different,” she said.
The study was sponsored by Janssen Research & Development and Legend Biotech. Dr. Madduri disclosed honoraria, consultancy, and speakers bureau activities for those companies and others.
SOURCE: Madduri D et al. ASH 2020. Abstract 177.
For patients with heavily-pretreated multiple myeloma, the early and deep responses seen with the novel chimeric antigen receptor T-cell (CAR T-cell) construct ciltacabtagene autoleucel (cilta-cel) have also been durable, according to investigators in the CARTITUDE-1 trial.
Among 97 patients with multiple myeloma that had progressed on three or more prior lines of therapy or following treatment with at least two lines of therapy with a proteasome inhibitor and immunomodulating agent, the overall response rate (ORR) was 96.9%, with a median duration of response not reached after a median of 12.4 months of follow-up, reported Deepu Madduri, MD of Mount Sinai Medical Center in New York, and colleagues.
“We saw how heavily pretreated these patients were, and to see a one-time treatment get these kind of response rates is quite exceptional. What’s even more impressive is that 72% of these patients were still maintaining their response at the time of data cutoff,“ she said in an oral abstract presented during the virtual American Society of Hematology annual meeting.
Cilta-cel is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
As previously reported, the same CAR T-cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma.
Ciltacabtagene autoleucel was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration in December 2019, a priority medicines (PRIME) designation by the European Medicines Agency in April 2019, and breakthrough designation in China in September 2020.
At the 2019 ASH annual meeting, Dr. Madduri reported phase 1b results from the trial, which showed that for 29 patients with heavily pretreated, relapsed/refractory multiple myeloma, the ORR at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression.
Combined data
For the 2020 ASH annual meeting, Dr. Madduri reported combined results from phases 1b and 2 of the CARTITUDE-1 study.
The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least three prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug, and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T-cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
Of the 101 patients who underwent lymphodepletion, 97 (29 in phase 1b and 68 in phase 2) were treated with cilta-cel. Five of the patients in phase 1b and nine in phase 2 died on study, five of whom succumbed to progressive disease, and three due to adverse events unrelated to treatment. The remaining six patients died from treatment-related causes, including two patients from sepsis or septic shock, and one each from the cytokine release syndrome (CRS)/hemophagocytic lymphohistiocytosis (HLH), lung abscess, respiratory failure, and neurotoxicity.
At the time of data cutoff, 83 patients remained on study.
High ORR
The ORR was 96.9% (94 of 97 patients), comprising 67% stringent complete responses (sCR), 25.8% very good partial responses (VGPR), and 4.1% partial responses (PR).
Among 57 patients evaluable for minimal residual disease (MRD), 53 (93%) were MRD negative. Of this group, 49 had a VGPR or better.
The median time to first response was 1 month (range 0.9 to 8.5 months). At the time of data cutoff 70 patients had an ongoing response.
Among patients followed for a minimum of 6 months, most had cilta-cel CAR T-cells below the level of quantification (2 cells per microliter) in peripheral blood.
At a median follow-up of 12.4 months, 12-month overall progression-free survival rate was 76%, with the median PFS not reached. The 12-month overall survival rate was 88.5%, with the median OS not reached.
Safety data
All patients had at least one hematologic adverse event, 96 of which were grade 3 or 4 in severity. The events include neutropenia, anemia, thrombocytopenia, leukopenia, and lymphopenia. The median time to recovery was 2 weeks for grade 3 or 4 neutropenia and 4 weeks for thrombocytopenia.
Infections of any grade occurred in 57.7% of patients, including grade 3/4 pneumonia in 8.2% and grade 3/4 sepsis in 4.1%.
Grade 3 or 4 nonhematologic toxicities were uncommon, Dr. Madduri noted.
CRS of any grade occurred in 92 patients, but only 4 had grade 3 or 4 CRS.
Neurotoxicities occurred in 20 patients, of whom 10 had grade 3 or 4 neurotoxicity.
Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 16 patients, with 2 having grade 3 or greater ICANS. Other neurotoxicities of any grade, many which overlapped with ICANS, occurred in 12 patients, with 9 having grade 3 or 4 neurotoxicity.
The median time to ICANS onset was 8 days, with a median time to recovery of 4 days. Other neurotoxicities took longer to manifest and disappear, however, with a median time to onset of 27 days, and median time to recovery of 75 days.
Neurotoxicity mechanism questioned
In the question-and-answer session following her presentation, an audience member asked whether the investigators had any insights into the mechanism underlying the non-ICANS neurotoxicities they saw.
“We saw no clear etiology in the other neurotoxicities, but we saw that maybe there could be some mild associations with high tumor burden, prior CRS, ICANS, or even the higher expansion and persistence of these cells,” Dr. Madduri replied.
She noted that subsequent to these findings, the investigators have implemented mitigation strategies including allowing patients to have more bridging chemotherapy, more aggressive steroid use for early ICANS, and extensive monitoring.
Eric Smith, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said that the non-ICANS neurotoxicity profile of cilta-cel was different from that seen in other CAR T-cell trials, and asked how it compared to that of bi-specific BCMA/CD3 CAR T constructs.
“We did see some nerve palsies and peripheral motor neuropathy, but it wasn’t that many patients, and it’s really hard to compare what happened here with the bi-specifics, as every product is very different,” she said.
The study was sponsored by Janssen Research & Development and Legend Biotech. Dr. Madduri disclosed honoraria, consultancy, and speakers bureau activities for those companies and others.
SOURCE: Madduri D et al. ASH 2020. Abstract 177.
For patients with heavily-pretreated multiple myeloma, the early and deep responses seen with the novel chimeric antigen receptor T-cell (CAR T-cell) construct ciltacabtagene autoleucel (cilta-cel) have also been durable, according to investigators in the CARTITUDE-1 trial.
Among 97 patients with multiple myeloma that had progressed on three or more prior lines of therapy or following treatment with at least two lines of therapy with a proteasome inhibitor and immunomodulating agent, the overall response rate (ORR) was 96.9%, with a median duration of response not reached after a median of 12.4 months of follow-up, reported Deepu Madduri, MD of Mount Sinai Medical Center in New York, and colleagues.
“We saw how heavily pretreated these patients were, and to see a one-time treatment get these kind of response rates is quite exceptional. What’s even more impressive is that 72% of these patients were still maintaining their response at the time of data cutoff,“ she said in an oral abstract presented during the virtual American Society of Hematology annual meeting.
Cilta-cel is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
As previously reported, the same CAR T-cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma.
Ciltacabtagene autoleucel was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration in December 2019, a priority medicines (PRIME) designation by the European Medicines Agency in April 2019, and breakthrough designation in China in September 2020.
At the 2019 ASH annual meeting, Dr. Madduri reported phase 1b results from the trial, which showed that for 29 patients with heavily pretreated, relapsed/refractory multiple myeloma, the ORR at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression.
Combined data
For the 2020 ASH annual meeting, Dr. Madduri reported combined results from phases 1b and 2 of the CARTITUDE-1 study.
The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least three prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug, and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T-cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
Of the 101 patients who underwent lymphodepletion, 97 (29 in phase 1b and 68 in phase 2) were treated with cilta-cel. Five of the patients in phase 1b and nine in phase 2 died on study, five of whom succumbed to progressive disease, and three due to adverse events unrelated to treatment. The remaining six patients died from treatment-related causes, including two patients from sepsis or septic shock, and one each from the cytokine release syndrome (CRS)/hemophagocytic lymphohistiocytosis (HLH), lung abscess, respiratory failure, and neurotoxicity.
At the time of data cutoff, 83 patients remained on study.
High ORR
The ORR was 96.9% (94 of 97 patients), comprising 67% stringent complete responses (sCR), 25.8% very good partial responses (VGPR), and 4.1% partial responses (PR).
Among 57 patients evaluable for minimal residual disease (MRD), 53 (93%) were MRD negative. Of this group, 49 had a VGPR or better.
The median time to first response was 1 month (range 0.9 to 8.5 months). At the time of data cutoff 70 patients had an ongoing response.
Among patients followed for a minimum of 6 months, most had cilta-cel CAR T-cells below the level of quantification (2 cells per microliter) in peripheral blood.
At a median follow-up of 12.4 months, 12-month overall progression-free survival rate was 76%, with the median PFS not reached. The 12-month overall survival rate was 88.5%, with the median OS not reached.
Safety data
All patients had at least one hematologic adverse event, 96 of which were grade 3 or 4 in severity. The events include neutropenia, anemia, thrombocytopenia, leukopenia, and lymphopenia. The median time to recovery was 2 weeks for grade 3 or 4 neutropenia and 4 weeks for thrombocytopenia.
Infections of any grade occurred in 57.7% of patients, including grade 3/4 pneumonia in 8.2% and grade 3/4 sepsis in 4.1%.
Grade 3 or 4 nonhematologic toxicities were uncommon, Dr. Madduri noted.
CRS of any grade occurred in 92 patients, but only 4 had grade 3 or 4 CRS.
Neurotoxicities occurred in 20 patients, of whom 10 had grade 3 or 4 neurotoxicity.
Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 16 patients, with 2 having grade 3 or greater ICANS. Other neurotoxicities of any grade, many which overlapped with ICANS, occurred in 12 patients, with 9 having grade 3 or 4 neurotoxicity.
The median time to ICANS onset was 8 days, with a median time to recovery of 4 days. Other neurotoxicities took longer to manifest and disappear, however, with a median time to onset of 27 days, and median time to recovery of 75 days.
Neurotoxicity mechanism questioned
In the question-and-answer session following her presentation, an audience member asked whether the investigators had any insights into the mechanism underlying the non-ICANS neurotoxicities they saw.
“We saw no clear etiology in the other neurotoxicities, but we saw that maybe there could be some mild associations with high tumor burden, prior CRS, ICANS, or even the higher expansion and persistence of these cells,” Dr. Madduri replied.
She noted that subsequent to these findings, the investigators have implemented mitigation strategies including allowing patients to have more bridging chemotherapy, more aggressive steroid use for early ICANS, and extensive monitoring.
Eric Smith, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said that the non-ICANS neurotoxicity profile of cilta-cel was different from that seen in other CAR T-cell trials, and asked how it compared to that of bi-specific BCMA/CD3 CAR T constructs.
“We did see some nerve palsies and peripheral motor neuropathy, but it wasn’t that many patients, and it’s really hard to compare what happened here with the bi-specifics, as every product is very different,” she said.
The study was sponsored by Janssen Research & Development and Legend Biotech. Dr. Madduri disclosed honoraria, consultancy, and speakers bureau activities for those companies and others.
SOURCE: Madduri D et al. ASH 2020. Abstract 177.
FROM ASH 2020
Allogeneic transplant leads to durable remissions in T-cell lymphomas
, results of a large retrospective observational study suggest.
Five-year progression-free survival (PFS) approached 40% and 5-year overall survival (OS) was over 50% in the study, which according to an investigator is the largest-ever reported patient series of allogeneic stem cell transplantation in T-cell lymphomas.
“We believe that eligible patients with relapsed/refractory T-cell lymphomas should be considered for consultation for allogeneic transplant by an expert clinician,” said investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
“These decisions should occur on a patient by patient level – but it’s important to consider this,” Dr. Mehta-Shah said at the annual meeting of the American Society of Hematology, held virtually this year.
Notably, patients with cutaneous T-cell lymphoma (CTCL) had a higher rate of relapse yet similar overall survival (OS) compared to patients with common peripheral T-cell lymphoma (PTCL) subtypes, according to Dr. Mehta-Shah.
Among PTCL subtypes, there was a trend toward improved PFS and OS for angioimmunoblastic T-cell lymphoma (AITL), compared with PTCL not otherwise specified (PTCL-NOS) and anaplastic large-cell lymphoma (ALCL), she added.
Catherine M. Diefenbach, MD, director of the clinical lymphoma program at NYU Langone’s Perlmutter Cancer Center, said the results of this retrospective study need to considered in light of the treatment-related risks associated with allogeneic transplantation.
Treatment-related mortality in the study ranged from about 8% to 24%, depending on the donor type, while acute and chronic graft-versus-host-disease (GvHD) was seen in more than 40% of patients, the reported data show.
“If I have a relapsed patient with AITL, I would look to this data and say that patients with AITL appear in a retrospective study to have a strong benefit,” Dr. Diefenbach said in an interview.
“For the other patients, you would describe both potential benefits and also discuss the treatment-associated risks – both the chronic GvHD and transplant-related mortality – and you’d have to balance the risk with the benefits for each individual case,” Dr. Diefenbach added.
The retrospective analysis by Dr. Mehta-Shah and colleagues included 508 consecutive T-cell lymphoma patients receiving allogeneic transplants at 12 academic centers between 2000 and 2019. The most common subtypes were PTCL-NOS in 26%, AITL in 16%, CTCL in 13%, and hepatosplenic T-cell lymphoma (HSTCL) in 7%. About 40% had a matched related donor (MRD) and 39% had a matched unrelated donor (MUD). The conditioning regimen was myeloablative in about a third of patients and nonmyeloablative in two-thirds.
At 5 years, PFS was 39.4% and OS was 50.8% for the overall study cohort, Dr. Mehta-Shah reported, noting that the median time from relapse to death post allogeneic transplant was 10.2 months.
Patients in complete remission at the time of transplant fared better than others, with a median PFS of 44.6 months vs. 8.5 months for those in partial remission, 21.0 months in those with stable disease, and 3.5 months for those with progressive disease at time of transplant, data show.
Patients with common PTCL subtypes had better PFS compared to patients with CTCL, yet OS was similar, according to the investigator. At 5 years, PFS was 43.7% and 18.6%, respectively, for PTCL and CTCL, while OS was 53.1% and 44.0%, respectively.
There was a trend toward improved outcomes for AITL relative to PTCL-NOS and ALCL, with a median PFS of 51.4 months for AITL versus 18.3 months those other subtypes. Similarly, median OS was not reached for AITL versus 73.1 months in the other subtypes.
Treatment-related mortality was lowest for patients with MRDs, or 8.2% at 12 months, Dr. Mehta-Shah reported, while patients with MUDs, mismatched donors, or haploidentical donors had treatment-related mortality of 13% to 16% at 12 months, and those with cord blood donors had treatment-related mortality of nearly 24% at 12 months.
Acute GvHD was observed in 46% of patients and chronic GvHD was seen in nearly 41%, the investigator added.
While these findings are important to consider in individual patient consultations, the study is nevertheless subject to limitations including patient selection and referral bias, according to Dr. Mehta-Shah.
“This was a retrospective analysis of patients who underwent transplant,” she said in a question-and-answer period. “Of course, that is heavily biased by who got to a transplant center, who was well enough to achieve transplant, and who had a donor or donor options, as well as their overall health and depth of remission,” the researcher said.
“I think this just represents what we could tell patients about what may happen to them once they embark on a transplant,” she added, “but really, there would be more prospective work needed to be done for what happens to patients overarching, and how many of them even get to a transplant consultation.”
Further studies should be done to develop predictive tools or biomarkers to determine who benefits from an allogeneic transplant, if there are predictors of relapse following allogeneic transplant, and what are the mechanisms of relapse following allogeneic transplant, according to Dr. Mehta-Shah.
Dr. Mehta-Shah reported research funding from Bristol Myers-Squibb, Celgene, Verastem, Corvus, Innate Pharmaceuticals, and Genentech/Roche. She reported consultancy with Kyowa Hakko Kirin, C4 Therapeutics, and Karyopharm Therapeutics.
SOURCE: Mehta-Shah N et al. ASH 2020, Abstract 41.
, results of a large retrospective observational study suggest.
Five-year progression-free survival (PFS) approached 40% and 5-year overall survival (OS) was over 50% in the study, which according to an investigator is the largest-ever reported patient series of allogeneic stem cell transplantation in T-cell lymphomas.
“We believe that eligible patients with relapsed/refractory T-cell lymphomas should be considered for consultation for allogeneic transplant by an expert clinician,” said investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
“These decisions should occur on a patient by patient level – but it’s important to consider this,” Dr. Mehta-Shah said at the annual meeting of the American Society of Hematology, held virtually this year.
Notably, patients with cutaneous T-cell lymphoma (CTCL) had a higher rate of relapse yet similar overall survival (OS) compared to patients with common peripheral T-cell lymphoma (PTCL) subtypes, according to Dr. Mehta-Shah.
Among PTCL subtypes, there was a trend toward improved PFS and OS for angioimmunoblastic T-cell lymphoma (AITL), compared with PTCL not otherwise specified (PTCL-NOS) and anaplastic large-cell lymphoma (ALCL), she added.
Catherine M. Diefenbach, MD, director of the clinical lymphoma program at NYU Langone’s Perlmutter Cancer Center, said the results of this retrospective study need to considered in light of the treatment-related risks associated with allogeneic transplantation.
Treatment-related mortality in the study ranged from about 8% to 24%, depending on the donor type, while acute and chronic graft-versus-host-disease (GvHD) was seen in more than 40% of patients, the reported data show.
“If I have a relapsed patient with AITL, I would look to this data and say that patients with AITL appear in a retrospective study to have a strong benefit,” Dr. Diefenbach said in an interview.
“For the other patients, you would describe both potential benefits and also discuss the treatment-associated risks – both the chronic GvHD and transplant-related mortality – and you’d have to balance the risk with the benefits for each individual case,” Dr. Diefenbach added.
The retrospective analysis by Dr. Mehta-Shah and colleagues included 508 consecutive T-cell lymphoma patients receiving allogeneic transplants at 12 academic centers between 2000 and 2019. The most common subtypes were PTCL-NOS in 26%, AITL in 16%, CTCL in 13%, and hepatosplenic T-cell lymphoma (HSTCL) in 7%. About 40% had a matched related donor (MRD) and 39% had a matched unrelated donor (MUD). The conditioning regimen was myeloablative in about a third of patients and nonmyeloablative in two-thirds.
At 5 years, PFS was 39.4% and OS was 50.8% for the overall study cohort, Dr. Mehta-Shah reported, noting that the median time from relapse to death post allogeneic transplant was 10.2 months.
Patients in complete remission at the time of transplant fared better than others, with a median PFS of 44.6 months vs. 8.5 months for those in partial remission, 21.0 months in those with stable disease, and 3.5 months for those with progressive disease at time of transplant, data show.
Patients with common PTCL subtypes had better PFS compared to patients with CTCL, yet OS was similar, according to the investigator. At 5 years, PFS was 43.7% and 18.6%, respectively, for PTCL and CTCL, while OS was 53.1% and 44.0%, respectively.
There was a trend toward improved outcomes for AITL relative to PTCL-NOS and ALCL, with a median PFS of 51.4 months for AITL versus 18.3 months those other subtypes. Similarly, median OS was not reached for AITL versus 73.1 months in the other subtypes.
Treatment-related mortality was lowest for patients with MRDs, or 8.2% at 12 months, Dr. Mehta-Shah reported, while patients with MUDs, mismatched donors, or haploidentical donors had treatment-related mortality of 13% to 16% at 12 months, and those with cord blood donors had treatment-related mortality of nearly 24% at 12 months.
Acute GvHD was observed in 46% of patients and chronic GvHD was seen in nearly 41%, the investigator added.
While these findings are important to consider in individual patient consultations, the study is nevertheless subject to limitations including patient selection and referral bias, according to Dr. Mehta-Shah.
“This was a retrospective analysis of patients who underwent transplant,” she said in a question-and-answer period. “Of course, that is heavily biased by who got to a transplant center, who was well enough to achieve transplant, and who had a donor or donor options, as well as their overall health and depth of remission,” the researcher said.
“I think this just represents what we could tell patients about what may happen to them once they embark on a transplant,” she added, “but really, there would be more prospective work needed to be done for what happens to patients overarching, and how many of them even get to a transplant consultation.”
Further studies should be done to develop predictive tools or biomarkers to determine who benefits from an allogeneic transplant, if there are predictors of relapse following allogeneic transplant, and what are the mechanisms of relapse following allogeneic transplant, according to Dr. Mehta-Shah.
Dr. Mehta-Shah reported research funding from Bristol Myers-Squibb, Celgene, Verastem, Corvus, Innate Pharmaceuticals, and Genentech/Roche. She reported consultancy with Kyowa Hakko Kirin, C4 Therapeutics, and Karyopharm Therapeutics.
SOURCE: Mehta-Shah N et al. ASH 2020, Abstract 41.
, results of a large retrospective observational study suggest.
Five-year progression-free survival (PFS) approached 40% and 5-year overall survival (OS) was over 50% in the study, which according to an investigator is the largest-ever reported patient series of allogeneic stem cell transplantation in T-cell lymphomas.
“We believe that eligible patients with relapsed/refractory T-cell lymphomas should be considered for consultation for allogeneic transplant by an expert clinician,” said investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
“These decisions should occur on a patient by patient level – but it’s important to consider this,” Dr. Mehta-Shah said at the annual meeting of the American Society of Hematology, held virtually this year.
Notably, patients with cutaneous T-cell lymphoma (CTCL) had a higher rate of relapse yet similar overall survival (OS) compared to patients with common peripheral T-cell lymphoma (PTCL) subtypes, according to Dr. Mehta-Shah.
Among PTCL subtypes, there was a trend toward improved PFS and OS for angioimmunoblastic T-cell lymphoma (AITL), compared with PTCL not otherwise specified (PTCL-NOS) and anaplastic large-cell lymphoma (ALCL), she added.
Catherine M. Diefenbach, MD, director of the clinical lymphoma program at NYU Langone’s Perlmutter Cancer Center, said the results of this retrospective study need to considered in light of the treatment-related risks associated with allogeneic transplantation.
Treatment-related mortality in the study ranged from about 8% to 24%, depending on the donor type, while acute and chronic graft-versus-host-disease (GvHD) was seen in more than 40% of patients, the reported data show.
“If I have a relapsed patient with AITL, I would look to this data and say that patients with AITL appear in a retrospective study to have a strong benefit,” Dr. Diefenbach said in an interview.
“For the other patients, you would describe both potential benefits and also discuss the treatment-associated risks – both the chronic GvHD and transplant-related mortality – and you’d have to balance the risk with the benefits for each individual case,” Dr. Diefenbach added.
The retrospective analysis by Dr. Mehta-Shah and colleagues included 508 consecutive T-cell lymphoma patients receiving allogeneic transplants at 12 academic centers between 2000 and 2019. The most common subtypes were PTCL-NOS in 26%, AITL in 16%, CTCL in 13%, and hepatosplenic T-cell lymphoma (HSTCL) in 7%. About 40% had a matched related donor (MRD) and 39% had a matched unrelated donor (MUD). The conditioning regimen was myeloablative in about a third of patients and nonmyeloablative in two-thirds.
At 5 years, PFS was 39.4% and OS was 50.8% for the overall study cohort, Dr. Mehta-Shah reported, noting that the median time from relapse to death post allogeneic transplant was 10.2 months.
Patients in complete remission at the time of transplant fared better than others, with a median PFS of 44.6 months vs. 8.5 months for those in partial remission, 21.0 months in those with stable disease, and 3.5 months for those with progressive disease at time of transplant, data show.
Patients with common PTCL subtypes had better PFS compared to patients with CTCL, yet OS was similar, according to the investigator. At 5 years, PFS was 43.7% and 18.6%, respectively, for PTCL and CTCL, while OS was 53.1% and 44.0%, respectively.
There was a trend toward improved outcomes for AITL relative to PTCL-NOS and ALCL, with a median PFS of 51.4 months for AITL versus 18.3 months those other subtypes. Similarly, median OS was not reached for AITL versus 73.1 months in the other subtypes.
Treatment-related mortality was lowest for patients with MRDs, or 8.2% at 12 months, Dr. Mehta-Shah reported, while patients with MUDs, mismatched donors, or haploidentical donors had treatment-related mortality of 13% to 16% at 12 months, and those with cord blood donors had treatment-related mortality of nearly 24% at 12 months.
Acute GvHD was observed in 46% of patients and chronic GvHD was seen in nearly 41%, the investigator added.
While these findings are important to consider in individual patient consultations, the study is nevertheless subject to limitations including patient selection and referral bias, according to Dr. Mehta-Shah.
“This was a retrospective analysis of patients who underwent transplant,” she said in a question-and-answer period. “Of course, that is heavily biased by who got to a transplant center, who was well enough to achieve transplant, and who had a donor or donor options, as well as their overall health and depth of remission,” the researcher said.
“I think this just represents what we could tell patients about what may happen to them once they embark on a transplant,” she added, “but really, there would be more prospective work needed to be done for what happens to patients overarching, and how many of them even get to a transplant consultation.”
Further studies should be done to develop predictive tools or biomarkers to determine who benefits from an allogeneic transplant, if there are predictors of relapse following allogeneic transplant, and what are the mechanisms of relapse following allogeneic transplant, according to Dr. Mehta-Shah.
Dr. Mehta-Shah reported research funding from Bristol Myers-Squibb, Celgene, Verastem, Corvus, Innate Pharmaceuticals, and Genentech/Roche. She reported consultancy with Kyowa Hakko Kirin, C4 Therapeutics, and Karyopharm Therapeutics.
SOURCE: Mehta-Shah N et al. ASH 2020, Abstract 41.
FROM ASH 2020