User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Infographic: Is your compensation rising as fast as your peers?
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.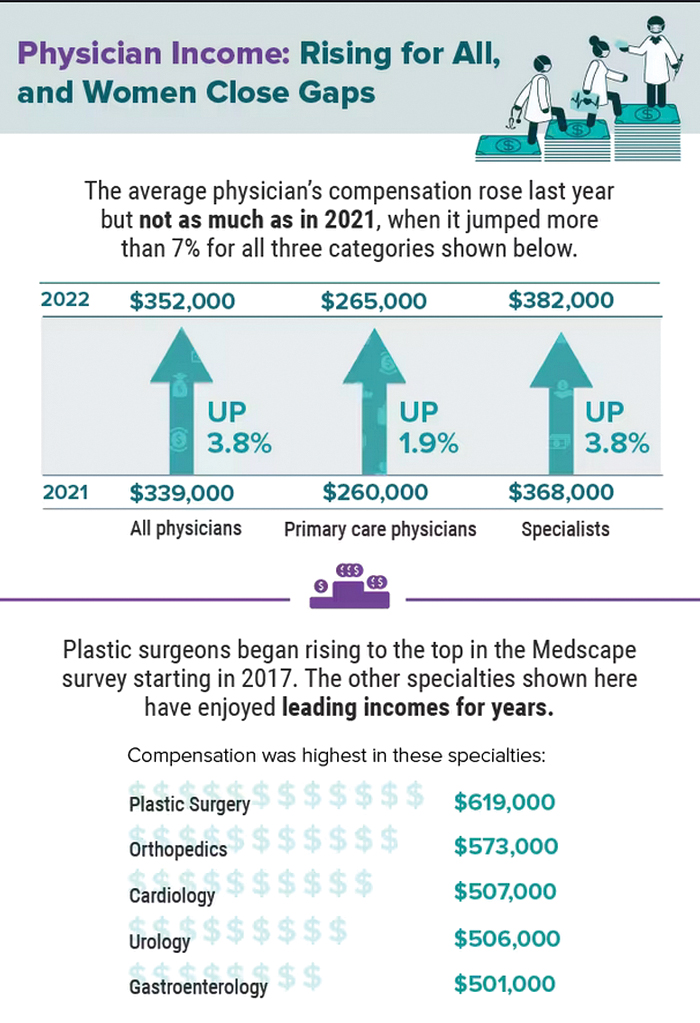
A version of this article first appeared on Medscape.com.
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.

A version of this article first appeared on Medscape.com.
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.

A version of this article first appeared on Medscape.com.
A baby stops breathing at a grocery store – An ICU nurse steps in
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
Green Mediterranean diet may relieve aortic stiffness
A green adaptation to the traditional Mediterranean diet improves proximal aortic stiffness (PAS), a distinct marker of vascular aging and increased cardiovascular risk, according to an exploratory post hoc analysis of the DIRECT-PLUS randomized clinical trial.
The green Mediterranean diet is distinct from the traditional Mediterranean diet because of its more abundant dietary polyphenols, from green tea and a Wolffia globosa (Mankai) plant green shake, and lower intake of red or processed meat.
Independent of weight loss, the modified green Mediterranean diet regressed PAS by 15%, the traditional Mediterranean diet by 7.3%, and the healthy dietary guideline–based diet by 4.8%, the study team observed.
“The DIRECT-PLUS trial research team was the first to introduce the concept of the green-Mediterranean/high polyphenols diet,” lead researcher Iris Shai, RD, PhD, with Ben-Gurion University of the Negev, Be’er-Sheva, Israel, told this news organization.
This diet promoted “dramatic proximal aortic de-stiffening” as assessed by MRI over 18 months in roughly 300 participants with abdominal obesity/dyslipidemia. “To date, no dietary strategies have been shown to impact vascular aging physiology,” Dr. Shai said.
The analysis was published online in the Journal of the American College of Cardiology.
Not all healthy diets are equal
Of the 294 participants, 281 had valid PAS measurements at baseline. The baseline PAS (6.1 m/s) was similar across intervention groups (P = .20). Increased PAS was associated with aging, hypertension, dyslipidemia, diabetes, and visceral adiposity (P < .05).
After 18 months’ intervention (retention rate 89.8%), all diet groups showed significant PAS reductions: –0.05 m/s with the standard healthy diet (4.8%), –0.08 m/s with the traditional Mediterranean diet (7.3%) and –0.15 the green Mediterranean diet (15%).
In the multivariable model, the green Mediterranean dieters had greater PAS reduction than did the healthy-diet and Mediterranean dieters (P = .003 and P = .032, respectively).
The researchers caution that DIRECT-PLUS had multiple endpoints and this exploratory post hoc analysis might be sensitive to type I statistical error and should be considered “hypothesis-generating.”
High-quality study, believable results
Reached for comment on the study, Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart in New York, said, “There is not a lot of high-quality research on diet, and I would call this high-quality research in as much as they used randomization which most dietary studies don’t do.
“The greener Mediterranean diet seemed to be the best one on the surrogate marker of MRI-defined aortic stiffness,” Dr. Bhatt, professor of cardiovascular medicine, Icahn School of Medicine at Mount Sinai, who wasn’t involved in the study, told this news organization.
“It makes sense that a diet that has more green in it, more polyphenols, would be healthier. This has been shown in some other studies, that these plant-based polyphenols might have various cardiovascular protective aspects to them,” Dr. Bhatt said.
Overall, he said the results are “quite believable, with the caveat that it would be nice to see the results reproduced in a more diverse and larger sample.”
“There is emerging evidence that diets that are higher in fresh fruits and vegetables and whole grains and lower in overall caloric intake, in general, seem to be good diets to reduce cardiovascular risk factors and maybe even reduce actual cardiovascular risk,” Dr. Bhatt added.
The study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), the Rosetrees Trust, Israel Ministry of Health, Israel Ministry of Science and Technology, and the California Walnuts Commission. Dr. Shai and Dr. Bhatt have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A green adaptation to the traditional Mediterranean diet improves proximal aortic stiffness (PAS), a distinct marker of vascular aging and increased cardiovascular risk, according to an exploratory post hoc analysis of the DIRECT-PLUS randomized clinical trial.
The green Mediterranean diet is distinct from the traditional Mediterranean diet because of its more abundant dietary polyphenols, from green tea and a Wolffia globosa (Mankai) plant green shake, and lower intake of red or processed meat.
Independent of weight loss, the modified green Mediterranean diet regressed PAS by 15%, the traditional Mediterranean diet by 7.3%, and the healthy dietary guideline–based diet by 4.8%, the study team observed.
“The DIRECT-PLUS trial research team was the first to introduce the concept of the green-Mediterranean/high polyphenols diet,” lead researcher Iris Shai, RD, PhD, with Ben-Gurion University of the Negev, Be’er-Sheva, Israel, told this news organization.
This diet promoted “dramatic proximal aortic de-stiffening” as assessed by MRI over 18 months in roughly 300 participants with abdominal obesity/dyslipidemia. “To date, no dietary strategies have been shown to impact vascular aging physiology,” Dr. Shai said.
The analysis was published online in the Journal of the American College of Cardiology.
Not all healthy diets are equal
Of the 294 participants, 281 had valid PAS measurements at baseline. The baseline PAS (6.1 m/s) was similar across intervention groups (P = .20). Increased PAS was associated with aging, hypertension, dyslipidemia, diabetes, and visceral adiposity (P < .05).
After 18 months’ intervention (retention rate 89.8%), all diet groups showed significant PAS reductions: –0.05 m/s with the standard healthy diet (4.8%), –0.08 m/s with the traditional Mediterranean diet (7.3%) and –0.15 the green Mediterranean diet (15%).
In the multivariable model, the green Mediterranean dieters had greater PAS reduction than did the healthy-diet and Mediterranean dieters (P = .003 and P = .032, respectively).
The researchers caution that DIRECT-PLUS had multiple endpoints and this exploratory post hoc analysis might be sensitive to type I statistical error and should be considered “hypothesis-generating.”
High-quality study, believable results
Reached for comment on the study, Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart in New York, said, “There is not a lot of high-quality research on diet, and I would call this high-quality research in as much as they used randomization which most dietary studies don’t do.
“The greener Mediterranean diet seemed to be the best one on the surrogate marker of MRI-defined aortic stiffness,” Dr. Bhatt, professor of cardiovascular medicine, Icahn School of Medicine at Mount Sinai, who wasn’t involved in the study, told this news organization.
“It makes sense that a diet that has more green in it, more polyphenols, would be healthier. This has been shown in some other studies, that these plant-based polyphenols might have various cardiovascular protective aspects to them,” Dr. Bhatt said.
Overall, he said the results are “quite believable, with the caveat that it would be nice to see the results reproduced in a more diverse and larger sample.”
“There is emerging evidence that diets that are higher in fresh fruits and vegetables and whole grains and lower in overall caloric intake, in general, seem to be good diets to reduce cardiovascular risk factors and maybe even reduce actual cardiovascular risk,” Dr. Bhatt added.
The study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), the Rosetrees Trust, Israel Ministry of Health, Israel Ministry of Science and Technology, and the California Walnuts Commission. Dr. Shai and Dr. Bhatt have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A green adaptation to the traditional Mediterranean diet improves proximal aortic stiffness (PAS), a distinct marker of vascular aging and increased cardiovascular risk, according to an exploratory post hoc analysis of the DIRECT-PLUS randomized clinical trial.
The green Mediterranean diet is distinct from the traditional Mediterranean diet because of its more abundant dietary polyphenols, from green tea and a Wolffia globosa (Mankai) plant green shake, and lower intake of red or processed meat.
Independent of weight loss, the modified green Mediterranean diet regressed PAS by 15%, the traditional Mediterranean diet by 7.3%, and the healthy dietary guideline–based diet by 4.8%, the study team observed.
“The DIRECT-PLUS trial research team was the first to introduce the concept of the green-Mediterranean/high polyphenols diet,” lead researcher Iris Shai, RD, PhD, with Ben-Gurion University of the Negev, Be’er-Sheva, Israel, told this news organization.
This diet promoted “dramatic proximal aortic de-stiffening” as assessed by MRI over 18 months in roughly 300 participants with abdominal obesity/dyslipidemia. “To date, no dietary strategies have been shown to impact vascular aging physiology,” Dr. Shai said.
The analysis was published online in the Journal of the American College of Cardiology.
Not all healthy diets are equal
Of the 294 participants, 281 had valid PAS measurements at baseline. The baseline PAS (6.1 m/s) was similar across intervention groups (P = .20). Increased PAS was associated with aging, hypertension, dyslipidemia, diabetes, and visceral adiposity (P < .05).
After 18 months’ intervention (retention rate 89.8%), all diet groups showed significant PAS reductions: –0.05 m/s with the standard healthy diet (4.8%), –0.08 m/s with the traditional Mediterranean diet (7.3%) and –0.15 the green Mediterranean diet (15%).
In the multivariable model, the green Mediterranean dieters had greater PAS reduction than did the healthy-diet and Mediterranean dieters (P = .003 and P = .032, respectively).
The researchers caution that DIRECT-PLUS had multiple endpoints and this exploratory post hoc analysis might be sensitive to type I statistical error and should be considered “hypothesis-generating.”
High-quality study, believable results
Reached for comment on the study, Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart in New York, said, “There is not a lot of high-quality research on diet, and I would call this high-quality research in as much as they used randomization which most dietary studies don’t do.
“The greener Mediterranean diet seemed to be the best one on the surrogate marker of MRI-defined aortic stiffness,” Dr. Bhatt, professor of cardiovascular medicine, Icahn School of Medicine at Mount Sinai, who wasn’t involved in the study, told this news organization.
“It makes sense that a diet that has more green in it, more polyphenols, would be healthier. This has been shown in some other studies, that these plant-based polyphenols might have various cardiovascular protective aspects to them,” Dr. Bhatt said.
Overall, he said the results are “quite believable, with the caveat that it would be nice to see the results reproduced in a more diverse and larger sample.”
“There is emerging evidence that diets that are higher in fresh fruits and vegetables and whole grains and lower in overall caloric intake, in general, seem to be good diets to reduce cardiovascular risk factors and maybe even reduce actual cardiovascular risk,” Dr. Bhatt added.
The study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), the Rosetrees Trust, Israel Ministry of Health, Israel Ministry of Science and Technology, and the California Walnuts Commission. Dr. Shai and Dr. Bhatt have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea linked to early cognitive decline
In a pilot study out of King’s College London, participants with severe OSA experienced worse executive functioning as well as social and emotional recognition versus healthy controls.
Major risk factors for OSA include obesity, high blood pressure, smoking, high cholesterol, and being middle-aged or older. Because some researchers have hypothesized that cognitive deficits could be driven by such comorbidities, the study investigators recruited middle-aged men with no medical comorbidities.
“Traditionally, we were more concerned with sleep apnea’s metabolic and cardiovascular comorbidities, and indeed, when cognitive deficits were demonstrated, most were attributed to them, and yet, our patients and their partners/families commonly tell us differently,” lead investigator Ivana Rosenzweig, MD, PhD, of King’s College London, who is also a consultant in sleep medicine and neuropsychiatry at Guy’s and St Thomas’ Hospital, London, said in an interview.
“Our findings provide a very important first step towards challenging the long-standing dogma that sleep apnea has little to do with the brain – apart from causing sleepiness – and that it is a predominantly nonneuro/psychiatric illness,” added Dr. Rosenzweig.
The findings were published online in Frontiers in Sleep.
Brain changes
The researchers wanted to understand how OSA may be linked to cognitive decline in the absence of cardiovascular and metabolic conditions.
To accomplish this, the investigators studied 27 men between the ages of 35 and 70 with a new diagnosis of mild to severe OSA without any comorbidities (16 with mild OSA and 11 with severe OSA). They also studied a control group of seven men matched for age, body mass index, and education level.
The team tested participants’ cognitive performance using the Cambridge Neuropsychological Test Automated Battery and found that the most significant deficits for the OSA group, compared with controls, were in areas of visual matching ability (P < .0001), short-term visual recognition memory, nonverbal patterns, executive functioning and attentional set-shifting (P < .001), psychomotor functioning, and social cognition and emotional recognition (P < .05).
On the latter two tests, impaired participants were less likely to accurately identify the emotion on computer-generated faces. Those with mild OSA performed better than those with severe OSA on these tasks, but rarely worse than controls.
Dr. Rosenzweig noted that the findings were one-of-a-kind because of the recruitment of patients with OSA who were otherwise healthy and nonobese, “something one rarely sees in the sleep clinic, where we commonly encounter patients with already developed comorbidities.
“In order to truly revolutionize the treatment for our patients, it is important to understand how much the accompanying comorbidities, such as systemic hypertension, obesity, diabetes, hyperlipidemia, and other various serious cardiovascular and metabolic diseases and how much the illness itself may shape the demonstrated cognitive deficits,” she said.
She also said that “it is widely agreed that medical problems in middle age may predispose to increased prevalence of dementia in later years.
Moreover, the very link between sleep apnea and Alzheimer’s, vascular and mixed dementia is increasingly demonstrated,” said Dr. Rosenzweig.
Although women typically have a lower prevalence of OSA than men, Dr. Rosenzweig said women were not included in the study “because we are too complex. As a lifelong feminist it pains me to say this, but to get any authoritative answer on our physiology, we need decent funding in place so that we can take into account all the intricacies of the changes of our sleep, physiology, and metabolism.
“While there is always lots of noise about how important it is to answer these questions, there are only very limited funds available for the sleep research,” she added.
Dr. Rosenzweig’s future research will focus on the potential link between OSA and neuroinflammation.
In a comment, Liza Ashbrook, MD, associate professor of neurology at the University of California, San Francisco, said the findings “add to the growing list of negative health consequences associated with sleep apnea.”
She said that, if the cognitive changes found in the study are, in fact, caused by OSA, it is unclear whether they are the beginning of long-term cognitive changes or a symptom of fragmented sleep that may be reversible.
Dr. Ashbrook said she would be interested in seeing research on understanding the effect of OSA treatment on the affected cognitive domains.
The study was funded by the Wellcome Trust. No relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
In a pilot study out of King’s College London, participants with severe OSA experienced worse executive functioning as well as social and emotional recognition versus healthy controls.
Major risk factors for OSA include obesity, high blood pressure, smoking, high cholesterol, and being middle-aged or older. Because some researchers have hypothesized that cognitive deficits could be driven by such comorbidities, the study investigators recruited middle-aged men with no medical comorbidities.
“Traditionally, we were more concerned with sleep apnea’s metabolic and cardiovascular comorbidities, and indeed, when cognitive deficits were demonstrated, most were attributed to them, and yet, our patients and their partners/families commonly tell us differently,” lead investigator Ivana Rosenzweig, MD, PhD, of King’s College London, who is also a consultant in sleep medicine and neuropsychiatry at Guy’s and St Thomas’ Hospital, London, said in an interview.
“Our findings provide a very important first step towards challenging the long-standing dogma that sleep apnea has little to do with the brain – apart from causing sleepiness – and that it is a predominantly nonneuro/psychiatric illness,” added Dr. Rosenzweig.
The findings were published online in Frontiers in Sleep.
Brain changes
The researchers wanted to understand how OSA may be linked to cognitive decline in the absence of cardiovascular and metabolic conditions.
To accomplish this, the investigators studied 27 men between the ages of 35 and 70 with a new diagnosis of mild to severe OSA without any comorbidities (16 with mild OSA and 11 with severe OSA). They also studied a control group of seven men matched for age, body mass index, and education level.
The team tested participants’ cognitive performance using the Cambridge Neuropsychological Test Automated Battery and found that the most significant deficits for the OSA group, compared with controls, were in areas of visual matching ability (P < .0001), short-term visual recognition memory, nonverbal patterns, executive functioning and attentional set-shifting (P < .001), psychomotor functioning, and social cognition and emotional recognition (P < .05).
On the latter two tests, impaired participants were less likely to accurately identify the emotion on computer-generated faces. Those with mild OSA performed better than those with severe OSA on these tasks, but rarely worse than controls.
Dr. Rosenzweig noted that the findings were one-of-a-kind because of the recruitment of patients with OSA who were otherwise healthy and nonobese, “something one rarely sees in the sleep clinic, where we commonly encounter patients with already developed comorbidities.
“In order to truly revolutionize the treatment for our patients, it is important to understand how much the accompanying comorbidities, such as systemic hypertension, obesity, diabetes, hyperlipidemia, and other various serious cardiovascular and metabolic diseases and how much the illness itself may shape the demonstrated cognitive deficits,” she said.
She also said that “it is widely agreed that medical problems in middle age may predispose to increased prevalence of dementia in later years.
Moreover, the very link between sleep apnea and Alzheimer’s, vascular and mixed dementia is increasingly demonstrated,” said Dr. Rosenzweig.
Although women typically have a lower prevalence of OSA than men, Dr. Rosenzweig said women were not included in the study “because we are too complex. As a lifelong feminist it pains me to say this, but to get any authoritative answer on our physiology, we need decent funding in place so that we can take into account all the intricacies of the changes of our sleep, physiology, and metabolism.
“While there is always lots of noise about how important it is to answer these questions, there are only very limited funds available for the sleep research,” she added.
Dr. Rosenzweig’s future research will focus on the potential link between OSA and neuroinflammation.
In a comment, Liza Ashbrook, MD, associate professor of neurology at the University of California, San Francisco, said the findings “add to the growing list of negative health consequences associated with sleep apnea.”
She said that, if the cognitive changes found in the study are, in fact, caused by OSA, it is unclear whether they are the beginning of long-term cognitive changes or a symptom of fragmented sleep that may be reversible.
Dr. Ashbrook said she would be interested in seeing research on understanding the effect of OSA treatment on the affected cognitive domains.
The study was funded by the Wellcome Trust. No relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
In a pilot study out of King’s College London, participants with severe OSA experienced worse executive functioning as well as social and emotional recognition versus healthy controls.
Major risk factors for OSA include obesity, high blood pressure, smoking, high cholesterol, and being middle-aged or older. Because some researchers have hypothesized that cognitive deficits could be driven by such comorbidities, the study investigators recruited middle-aged men with no medical comorbidities.
“Traditionally, we were more concerned with sleep apnea’s metabolic and cardiovascular comorbidities, and indeed, when cognitive deficits were demonstrated, most were attributed to them, and yet, our patients and their partners/families commonly tell us differently,” lead investigator Ivana Rosenzweig, MD, PhD, of King’s College London, who is also a consultant in sleep medicine and neuropsychiatry at Guy’s and St Thomas’ Hospital, London, said in an interview.
“Our findings provide a very important first step towards challenging the long-standing dogma that sleep apnea has little to do with the brain – apart from causing sleepiness – and that it is a predominantly nonneuro/psychiatric illness,” added Dr. Rosenzweig.
The findings were published online in Frontiers in Sleep.
Brain changes
The researchers wanted to understand how OSA may be linked to cognitive decline in the absence of cardiovascular and metabolic conditions.
To accomplish this, the investigators studied 27 men between the ages of 35 and 70 with a new diagnosis of mild to severe OSA without any comorbidities (16 with mild OSA and 11 with severe OSA). They also studied a control group of seven men matched for age, body mass index, and education level.
The team tested participants’ cognitive performance using the Cambridge Neuropsychological Test Automated Battery and found that the most significant deficits for the OSA group, compared with controls, were in areas of visual matching ability (P < .0001), short-term visual recognition memory, nonverbal patterns, executive functioning and attentional set-shifting (P < .001), psychomotor functioning, and social cognition and emotional recognition (P < .05).
On the latter two tests, impaired participants were less likely to accurately identify the emotion on computer-generated faces. Those with mild OSA performed better than those with severe OSA on these tasks, but rarely worse than controls.
Dr. Rosenzweig noted that the findings were one-of-a-kind because of the recruitment of patients with OSA who were otherwise healthy and nonobese, “something one rarely sees in the sleep clinic, where we commonly encounter patients with already developed comorbidities.
“In order to truly revolutionize the treatment for our patients, it is important to understand how much the accompanying comorbidities, such as systemic hypertension, obesity, diabetes, hyperlipidemia, and other various serious cardiovascular and metabolic diseases and how much the illness itself may shape the demonstrated cognitive deficits,” she said.
She also said that “it is widely agreed that medical problems in middle age may predispose to increased prevalence of dementia in later years.
Moreover, the very link between sleep apnea and Alzheimer’s, vascular and mixed dementia is increasingly demonstrated,” said Dr. Rosenzweig.
Although women typically have a lower prevalence of OSA than men, Dr. Rosenzweig said women were not included in the study “because we are too complex. As a lifelong feminist it pains me to say this, but to get any authoritative answer on our physiology, we need decent funding in place so that we can take into account all the intricacies of the changes of our sleep, physiology, and metabolism.
“While there is always lots of noise about how important it is to answer these questions, there are only very limited funds available for the sleep research,” she added.
Dr. Rosenzweig’s future research will focus on the potential link between OSA and neuroinflammation.
In a comment, Liza Ashbrook, MD, associate professor of neurology at the University of California, San Francisco, said the findings “add to the growing list of negative health consequences associated with sleep apnea.”
She said that, if the cognitive changes found in the study are, in fact, caused by OSA, it is unclear whether they are the beginning of long-term cognitive changes or a symptom of fragmented sleep that may be reversible.
Dr. Ashbrook said she would be interested in seeing research on understanding the effect of OSA treatment on the affected cognitive domains.
The study was funded by the Wellcome Trust. No relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
FROM FRONTIERS IN SLEEP
Physicians may retire en masse soon. What does that mean for medicine?
The double whammy of pandemic burnout and the aging of baby boomer physicians has, indeed, the makings of some scary headlines. A recent survey by Elsevier Health predicts that up to 75% of health care workers will leave the profession by 2025. And a 2020 study conducted by the Association of American Medical Colleges (AAMC) projected a shortfall of up to 139,000 physicians by 2033.
“We’ve paid a lot of attention to physician retirement,” says Michael Dill, AAMC’s director of workforce studies. “It’s a significant concern in terms of whether we have an adequate supply of physicians in the U.S. to meet our nation’s medical care needs. Anyone who thinks otherwise is incorrect.”
To Mr. Dill,
“The physician workforce as a whole is aging,” he said. “Close to a quarter of the physicians in the U.S. are 65 and over. So, you don’t need any extraordinary events driving retirement in order for retirement to be a real phenomenon of which we should all be concerned.”
And, although Mr. Dill said there aren’t any data to suggest that doctors in rural or urban areas are retiring faster than in the suburbs, that doesn’t mean retirement will have the same impact depending on where patients live.
“If you live in a rural area with one small practice in town and that physician retires, there goes the entirety of the physician supply,” he said. “In a major metro area, that’s not as big a deal.”
Why younger doctors are fast-tracking retirement
Fernando Mendoza, MD, 54, a pediatric emergency department physician in Miami, worries that physicians are getting so bogged down by paperwork that this may lead to even more doctors, at younger ages, leaving the profession.
“I love taking care of kids, but there’s going to be a cost to doing your work when you’re spending as much time as we need to spend on charts, pharmacy requests, and making sure all of the Medicare and Medicaid compliance issues are worked out.”
These stressors may compel some younger doctors to consider carving out a second career or fast-track younger physicians toward retirement.
“A medical degree carries a lot of weight, which helps when pivoting,” said Dr. Mendoza, who launched Scrivas, a Miami-based medical scribe agency, to help reduce the paperwork workload for physicians. “It might be that a doctor wants to get involved in the acquisition of medical equipment, or maybe they can focus on their investments. Either way, by leaving medicine, they’re not dealing with the hassle and churn-and-burn of seeing patients.”
What this means for patients
The time is now to stem the upcoming tide of retirement, said Mr. Dill. But the challenges remain daunting. For starters, the country needs more physicians trained now – but it will take years to replace those baby boomer doctors ready to hang up their white coats.
The medical profession also needs to find ways to support physicians who spend their days juggling an endless array of responsibilities, he said.
The AAMC study found that patients already feel the physician shortfall. Their public opinion research in 2019 said 35% of patients had trouble finding a physician over the past 2 or 3 years, up 10 percentage points since they asked the question in 2015.
Moreover, according to the report, the over-65 population is expected to grow by 45.1%, leaving a specialty care gap because older people generally have more complicated health cases that require specialists. In addition, physician burnout may lead more physicians under 65 to retire much earlier than expected.
Changes in how medicine is practiced, telemedicine care, and medical education – such as disruption of classes or clinical rotations, regulatory changes, and a lack of interest in certain specialties – could also be affected by a mass physician retirement.
What can we do about mass retirement?
The AAMC reports in “The Complexities of Physician Supply and Demand: Projections From 2019 to 2034” that federally funded GME support is in the works to train 15,000 physicians per year, with 3,000 new residency slots added per year over 5 years. The proposed model will add 3,750 new physicians each year beginning in 2026.
Other efforts include increasing use of APRNs and PAs, whose population is estimated to more than double by 2034, improve population health through preventive care, increase equity in health outcomes, and improve access and affordable care.
Removing licensing barriers for immigrant doctors can also help alleviate the shortage.
“We need to find better ways to leverage the entirety of the health care team so that not as much falls on physicians,” Mr. Dill said. “It’s also imperative that we focus on ways to support physician wellness and allow physicians to remain active in the field, but at a reduced rate.”
That’s precisely what Marie Brown, MD, director of practice redesign at the American Medical Association, is seeing nationwide. Cutting back their hours is not only trending, but it’s also helping doctors cope with burnout.
“We’re seeing physicians take a 20% or more cut in salary in order to decrease their burden,” she said. “They’ll spend 4 days on clinical time with patients so that on that fifth ‘day off,’ they’re doing the paperwork and documentation they need to do so they don’t compromise care on the other 4 days of the week.”
And this may only be a Band-Aid solution, she fears.
“If a physician is spending 3 hours a day doing unnecessary work that could be done by another team member, that’s contributing to burnout,” Dr. Brown said. “It’s no surprise that they’ll want to escape and retire if they’re in a financial situation to do so.”
“I advocate negotiating within your organization so you’re doing more of what you like, such as mentoring or running a residency, and less of what you don’t, while cutting back from full-time to something less than full-time while maintaining benefits,” said Joel Greenwald, MD, a certified financial planner in Minneapolis, who specializes in helping physicians manage their financial affairs.
“Falling into the ‘like less’ bucket are usually things like working weekends and taking calls,” he said.
“This benefits everyone on a large scale because those doctors who find things they enjoy are generally working to a later age but working less hard,” he said. “Remaining comfortably and happily gainfully employed for a longer period, even if you’re not working full-time, has a very powerful effect on your financial planning, and you’ll avoid the risk of running out of money.”
A version of this article first appeared on Medscape.com.
The double whammy of pandemic burnout and the aging of baby boomer physicians has, indeed, the makings of some scary headlines. A recent survey by Elsevier Health predicts that up to 75% of health care workers will leave the profession by 2025. And a 2020 study conducted by the Association of American Medical Colleges (AAMC) projected a shortfall of up to 139,000 physicians by 2033.
“We’ve paid a lot of attention to physician retirement,” says Michael Dill, AAMC’s director of workforce studies. “It’s a significant concern in terms of whether we have an adequate supply of physicians in the U.S. to meet our nation’s medical care needs. Anyone who thinks otherwise is incorrect.”
To Mr. Dill,
“The physician workforce as a whole is aging,” he said. “Close to a quarter of the physicians in the U.S. are 65 and over. So, you don’t need any extraordinary events driving retirement in order for retirement to be a real phenomenon of which we should all be concerned.”
And, although Mr. Dill said there aren’t any data to suggest that doctors in rural or urban areas are retiring faster than in the suburbs, that doesn’t mean retirement will have the same impact depending on where patients live.
“If you live in a rural area with one small practice in town and that physician retires, there goes the entirety of the physician supply,” he said. “In a major metro area, that’s not as big a deal.”
Why younger doctors are fast-tracking retirement
Fernando Mendoza, MD, 54, a pediatric emergency department physician in Miami, worries that physicians are getting so bogged down by paperwork that this may lead to even more doctors, at younger ages, leaving the profession.
“I love taking care of kids, but there’s going to be a cost to doing your work when you’re spending as much time as we need to spend on charts, pharmacy requests, and making sure all of the Medicare and Medicaid compliance issues are worked out.”
These stressors may compel some younger doctors to consider carving out a second career or fast-track younger physicians toward retirement.
“A medical degree carries a lot of weight, which helps when pivoting,” said Dr. Mendoza, who launched Scrivas, a Miami-based medical scribe agency, to help reduce the paperwork workload for physicians. “It might be that a doctor wants to get involved in the acquisition of medical equipment, or maybe they can focus on their investments. Either way, by leaving medicine, they’re not dealing with the hassle and churn-and-burn of seeing patients.”
What this means for patients
The time is now to stem the upcoming tide of retirement, said Mr. Dill. But the challenges remain daunting. For starters, the country needs more physicians trained now – but it will take years to replace those baby boomer doctors ready to hang up their white coats.
The medical profession also needs to find ways to support physicians who spend their days juggling an endless array of responsibilities, he said.
The AAMC study found that patients already feel the physician shortfall. Their public opinion research in 2019 said 35% of patients had trouble finding a physician over the past 2 or 3 years, up 10 percentage points since they asked the question in 2015.
Moreover, according to the report, the over-65 population is expected to grow by 45.1%, leaving a specialty care gap because older people generally have more complicated health cases that require specialists. In addition, physician burnout may lead more physicians under 65 to retire much earlier than expected.
Changes in how medicine is practiced, telemedicine care, and medical education – such as disruption of classes or clinical rotations, regulatory changes, and a lack of interest in certain specialties – could also be affected by a mass physician retirement.
What can we do about mass retirement?
The AAMC reports in “The Complexities of Physician Supply and Demand: Projections From 2019 to 2034” that federally funded GME support is in the works to train 15,000 physicians per year, with 3,000 new residency slots added per year over 5 years. The proposed model will add 3,750 new physicians each year beginning in 2026.
Other efforts include increasing use of APRNs and PAs, whose population is estimated to more than double by 2034, improve population health through preventive care, increase equity in health outcomes, and improve access and affordable care.
Removing licensing barriers for immigrant doctors can also help alleviate the shortage.
“We need to find better ways to leverage the entirety of the health care team so that not as much falls on physicians,” Mr. Dill said. “It’s also imperative that we focus on ways to support physician wellness and allow physicians to remain active in the field, but at a reduced rate.”
That’s precisely what Marie Brown, MD, director of practice redesign at the American Medical Association, is seeing nationwide. Cutting back their hours is not only trending, but it’s also helping doctors cope with burnout.
“We’re seeing physicians take a 20% or more cut in salary in order to decrease their burden,” she said. “They’ll spend 4 days on clinical time with patients so that on that fifth ‘day off,’ they’re doing the paperwork and documentation they need to do so they don’t compromise care on the other 4 days of the week.”
And this may only be a Band-Aid solution, she fears.
“If a physician is spending 3 hours a day doing unnecessary work that could be done by another team member, that’s contributing to burnout,” Dr. Brown said. “It’s no surprise that they’ll want to escape and retire if they’re in a financial situation to do so.”
“I advocate negotiating within your organization so you’re doing more of what you like, such as mentoring or running a residency, and less of what you don’t, while cutting back from full-time to something less than full-time while maintaining benefits,” said Joel Greenwald, MD, a certified financial planner in Minneapolis, who specializes in helping physicians manage their financial affairs.
“Falling into the ‘like less’ bucket are usually things like working weekends and taking calls,” he said.
“This benefits everyone on a large scale because those doctors who find things they enjoy are generally working to a later age but working less hard,” he said. “Remaining comfortably and happily gainfully employed for a longer period, even if you’re not working full-time, has a very powerful effect on your financial planning, and you’ll avoid the risk of running out of money.”
A version of this article first appeared on Medscape.com.
The double whammy of pandemic burnout and the aging of baby boomer physicians has, indeed, the makings of some scary headlines. A recent survey by Elsevier Health predicts that up to 75% of health care workers will leave the profession by 2025. And a 2020 study conducted by the Association of American Medical Colleges (AAMC) projected a shortfall of up to 139,000 physicians by 2033.
“We’ve paid a lot of attention to physician retirement,” says Michael Dill, AAMC’s director of workforce studies. “It’s a significant concern in terms of whether we have an adequate supply of physicians in the U.S. to meet our nation’s medical care needs. Anyone who thinks otherwise is incorrect.”
To Mr. Dill,
“The physician workforce as a whole is aging,” he said. “Close to a quarter of the physicians in the U.S. are 65 and over. So, you don’t need any extraordinary events driving retirement in order for retirement to be a real phenomenon of which we should all be concerned.”
And, although Mr. Dill said there aren’t any data to suggest that doctors in rural or urban areas are retiring faster than in the suburbs, that doesn’t mean retirement will have the same impact depending on where patients live.
“If you live in a rural area with one small practice in town and that physician retires, there goes the entirety of the physician supply,” he said. “In a major metro area, that’s not as big a deal.”
Why younger doctors are fast-tracking retirement
Fernando Mendoza, MD, 54, a pediatric emergency department physician in Miami, worries that physicians are getting so bogged down by paperwork that this may lead to even more doctors, at younger ages, leaving the profession.
“I love taking care of kids, but there’s going to be a cost to doing your work when you’re spending as much time as we need to spend on charts, pharmacy requests, and making sure all of the Medicare and Medicaid compliance issues are worked out.”
These stressors may compel some younger doctors to consider carving out a second career or fast-track younger physicians toward retirement.
“A medical degree carries a lot of weight, which helps when pivoting,” said Dr. Mendoza, who launched Scrivas, a Miami-based medical scribe agency, to help reduce the paperwork workload for physicians. “It might be that a doctor wants to get involved in the acquisition of medical equipment, or maybe they can focus on their investments. Either way, by leaving medicine, they’re not dealing with the hassle and churn-and-burn of seeing patients.”
What this means for patients
The time is now to stem the upcoming tide of retirement, said Mr. Dill. But the challenges remain daunting. For starters, the country needs more physicians trained now – but it will take years to replace those baby boomer doctors ready to hang up their white coats.
The medical profession also needs to find ways to support physicians who spend their days juggling an endless array of responsibilities, he said.
The AAMC study found that patients already feel the physician shortfall. Their public opinion research in 2019 said 35% of patients had trouble finding a physician over the past 2 or 3 years, up 10 percentage points since they asked the question in 2015.
Moreover, according to the report, the over-65 population is expected to grow by 45.1%, leaving a specialty care gap because older people generally have more complicated health cases that require specialists. In addition, physician burnout may lead more physicians under 65 to retire much earlier than expected.
Changes in how medicine is practiced, telemedicine care, and medical education – such as disruption of classes or clinical rotations, regulatory changes, and a lack of interest in certain specialties – could also be affected by a mass physician retirement.
What can we do about mass retirement?
The AAMC reports in “The Complexities of Physician Supply and Demand: Projections From 2019 to 2034” that federally funded GME support is in the works to train 15,000 physicians per year, with 3,000 new residency slots added per year over 5 years. The proposed model will add 3,750 new physicians each year beginning in 2026.
Other efforts include increasing use of APRNs and PAs, whose population is estimated to more than double by 2034, improve population health through preventive care, increase equity in health outcomes, and improve access and affordable care.
Removing licensing barriers for immigrant doctors can also help alleviate the shortage.
“We need to find better ways to leverage the entirety of the health care team so that not as much falls on physicians,” Mr. Dill said. “It’s also imperative that we focus on ways to support physician wellness and allow physicians to remain active in the field, but at a reduced rate.”
That’s precisely what Marie Brown, MD, director of practice redesign at the American Medical Association, is seeing nationwide. Cutting back their hours is not only trending, but it’s also helping doctors cope with burnout.
“We’re seeing physicians take a 20% or more cut in salary in order to decrease their burden,” she said. “They’ll spend 4 days on clinical time with patients so that on that fifth ‘day off,’ they’re doing the paperwork and documentation they need to do so they don’t compromise care on the other 4 days of the week.”
And this may only be a Band-Aid solution, she fears.
“If a physician is spending 3 hours a day doing unnecessary work that could be done by another team member, that’s contributing to burnout,” Dr. Brown said. “It’s no surprise that they’ll want to escape and retire if they’re in a financial situation to do so.”
“I advocate negotiating within your organization so you’re doing more of what you like, such as mentoring or running a residency, and less of what you don’t, while cutting back from full-time to something less than full-time while maintaining benefits,” said Joel Greenwald, MD, a certified financial planner in Minneapolis, who specializes in helping physicians manage their financial affairs.
“Falling into the ‘like less’ bucket are usually things like working weekends and taking calls,” he said.
“This benefits everyone on a large scale because those doctors who find things they enjoy are generally working to a later age but working less hard,” he said. “Remaining comfortably and happily gainfully employed for a longer period, even if you’re not working full-time, has a very powerful effect on your financial planning, and you’ll avoid the risk of running out of money.”
A version of this article first appeared on Medscape.com.
Physician compensation continues to climb amid postpandemic change
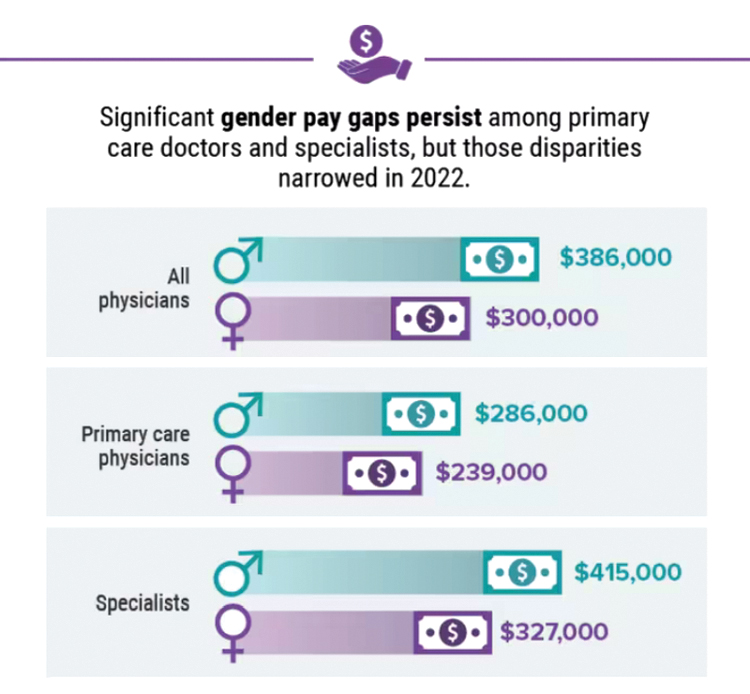
In addition, gender-based pay disparity among primary care physicians shrank, and the number of physicians who declined to take new Medicare patients rose.
The annual report is based on a survey of more than 10,000 physicians in over 29 specialties who answered questions about their income, workload, challenges, and level of satisfaction.
Average compensation across specialties rose to $352,000 – up nearly 17% from the 2018 average of $299,000. Fallout from the COVID-19 public health emergency continued to affect both physician compensation and job satisfaction, including Medicare reimbursements and staffing shortages due to burnout or retirement.
“Many physicians reevaluated what drove them to be a physician,” says Marc Adam, a recruiter at MASC Medical, a Florida physician recruiting firm.
Adam cites telehealth as an example. “An overwhelming majority of physicians prefer telehealth because of the convenience, but some really did not want to do it long term. They miss the patient interaction.”
The report also revealed that the gender-based pay gap in primary physicians fell, with men earning 19% more – down from 25% more in recent years. Among specialists, the gender gap was 27% on average, down from 31% last year. One reason may be an increase in compensation transparency, which Mr. Adam says should be the norm.
Income increases will likely continue, owing in large part to the growing disparity between physician supply and demand.
The projected physician shortage is expected to grow to 124,000 by 2034, according to the American Association of Medical Colleges. Federal lawmakers are considering passing the Resident Physician Shortage Reduction Act of 2023, which would add 14,000 Medicare-funded residency positions to help alleviate shortages.
Patient needs, Medicare rules continue to shift
Specialties with the biggest increases in compensation include oncology, anesthesiology, gastroenterology, radiology, critical care, and urology. Many procedure-related specialties saw more volume post pandemic.
Some respondents identified Medicare cuts and low reimbursement rates as a factor in tamping down compensation hikes. The number of physicians who expect to continue to take new Medicare patients is 65%, down from 71% 5 years ago.
For example, Medicare reimbursements for telehealth are expected to scale down in May, when the COVID-19 Public Health Emergency, which expanded telehealth services for Medicare patients, winds down.
“Telehealth will still exist,” says Mr. Adam, “but certain requirements will shape it going forward.”
Medicare isn’t viewed negatively across the board, however. Florida is among the top-earning states for physicians – along with Indiana, Connecticut, and Missouri. One reason is Florida’s unique health care environment, explains Mr. Adam, whose Florida-based firm places physicians nationwide.
“Florida is very progressive in terms of health care. For one thing, we have a large aging population and a large Medicare population.” Several growing organizations that focus on quality-based care are based in Florida, including ChenMed and Cano Health. Add to that the fact that owners of Florida’s health care organizations don’t have to be physicians, he explains, and the stage is set for experimentation.
“Being able to segment tasks frees up physicians to be more focused on medicine and provide better care while other people focus on the business and innovation.”
If Florida’s high compensation ranking continues, it may help employers there fulfill a growing need. The state is among those expected to experience the largest physician shortages in 2030, along with California, Texas, Arizona, and Georgia.
Side gigs up, satisfaction (slightly) down
In general, physicians aren’t fazed by these challenges. Many reported taking side gigs, some for additional income. Even so, 73% say they would still choose medicine, and more than 90% of physicians in 10 specialties would choose their specialty again. Still, burnout and stressors have led some to stop practicing altogether.
More and more organizations are hiring “travel physicians,” Mr. Adam says, and more physicians are choosing to take contract work (“locum tenens”) and practice in many different regions. Contract physicians typically help meet patient demand or provide coverage during the hiring process as well as while staff are on vacation or maternity leave.
Says Mr. Adam, “There’s no security, but there’s higher income and more flexibility.”
According to CHG Healthcare, locum tenens staffing is rising – approximately 7% of U.S. physicians (around 50,000) filled assignments in 2022, up 88% from 2015. In 2022, 56% of locum tenens employers reported a reduction in staff burnout, up from 30% in 2020.
The report indicates that more than half of physicians are satisfied with their income, down slightly from 55% 5 years ago (prepandemic). Physicians in some of the lower-paying specialties are among those most satisfied with their income. It’s not very surprising to Mr. Adam: “Higher earners generally suffer the most from burnout.
“They’re overworked, they have the largest number of patients, and they’re performing in high-stress situations doing challenging procedures on a daily basis – and they probably have worse work-life balance.” These physicians know going in that they need to be paid more to deal with such burdens. “That’s the feedback I get when I speak to high earners,” says Mr. Adam.
“The experienced ones are very clear about their [compensation] expectations.”
A version of this article first appeared on Medscape.com.
In addition, gender-based pay disparity among primary care physicians shrank, and the number of physicians who declined to take new Medicare patients rose.
The annual report is based on a survey of more than 10,000 physicians in over 29 specialties who answered questions about their income, workload, challenges, and level of satisfaction.
Average compensation across specialties rose to $352,000 – up nearly 17% from the 2018 average of $299,000. Fallout from the COVID-19 public health emergency continued to affect both physician compensation and job satisfaction, including Medicare reimbursements and staffing shortages due to burnout or retirement.
“Many physicians reevaluated what drove them to be a physician,” says Marc Adam, a recruiter at MASC Medical, a Florida physician recruiting firm.
Adam cites telehealth as an example. “An overwhelming majority of physicians prefer telehealth because of the convenience, but some really did not want to do it long term. They miss the patient interaction.”
The report also revealed that the gender-based pay gap in primary physicians fell, with men earning 19% more – down from 25% more in recent years. Among specialists, the gender gap was 27% on average, down from 31% last year. One reason may be an increase in compensation transparency, which Mr. Adam says should be the norm.
Income increases will likely continue, owing in large part to the growing disparity between physician supply and demand.
The projected physician shortage is expected to grow to 124,000 by 2034, according to the American Association of Medical Colleges. Federal lawmakers are considering passing the Resident Physician Shortage Reduction Act of 2023, which would add 14,000 Medicare-funded residency positions to help alleviate shortages.
Patient needs, Medicare rules continue to shift
Specialties with the biggest increases in compensation include oncology, anesthesiology, gastroenterology, radiology, critical care, and urology. Many procedure-related specialties saw more volume post pandemic.
Some respondents identified Medicare cuts and low reimbursement rates as a factor in tamping down compensation hikes. The number of physicians who expect to continue to take new Medicare patients is 65%, down from 71% 5 years ago.
For example, Medicare reimbursements for telehealth are expected to scale down in May, when the COVID-19 Public Health Emergency, which expanded telehealth services for Medicare patients, winds down.
“Telehealth will still exist,” says Mr. Adam, “but certain requirements will shape it going forward.”
Medicare isn’t viewed negatively across the board, however. Florida is among the top-earning states for physicians – along with Indiana, Connecticut, and Missouri. One reason is Florida’s unique health care environment, explains Mr. Adam, whose Florida-based firm places physicians nationwide.
“Florida is very progressive in terms of health care. For one thing, we have a large aging population and a large Medicare population.” Several growing organizations that focus on quality-based care are based in Florida, including ChenMed and Cano Health. Add to that the fact that owners of Florida’s health care organizations don’t have to be physicians, he explains, and the stage is set for experimentation.
“Being able to segment tasks frees up physicians to be more focused on medicine and provide better care while other people focus on the business and innovation.”
If Florida’s high compensation ranking continues, it may help employers there fulfill a growing need. The state is among those expected to experience the largest physician shortages in 2030, along with California, Texas, Arizona, and Georgia.
Side gigs up, satisfaction (slightly) down
In general, physicians aren’t fazed by these challenges. Many reported taking side gigs, some for additional income. Even so, 73% say they would still choose medicine, and more than 90% of physicians in 10 specialties would choose their specialty again. Still, burnout and stressors have led some to stop practicing altogether.
More and more organizations are hiring “travel physicians,” Mr. Adam says, and more physicians are choosing to take contract work (“locum tenens”) and practice in many different regions. Contract physicians typically help meet patient demand or provide coverage during the hiring process as well as while staff are on vacation or maternity leave.
Says Mr. Adam, “There’s no security, but there’s higher income and more flexibility.”
According to CHG Healthcare, locum tenens staffing is rising – approximately 7% of U.S. physicians (around 50,000) filled assignments in 2022, up 88% from 2015. In 2022, 56% of locum tenens employers reported a reduction in staff burnout, up from 30% in 2020.
The report indicates that more than half of physicians are satisfied with their income, down slightly from 55% 5 years ago (prepandemic). Physicians in some of the lower-paying specialties are among those most satisfied with their income. It’s not very surprising to Mr. Adam: “Higher earners generally suffer the most from burnout.
“They’re overworked, they have the largest number of patients, and they’re performing in high-stress situations doing challenging procedures on a daily basis – and they probably have worse work-life balance.” These physicians know going in that they need to be paid more to deal with such burdens. “That’s the feedback I get when I speak to high earners,” says Mr. Adam.
“The experienced ones are very clear about their [compensation] expectations.”
A version of this article first appeared on Medscape.com.
In addition, gender-based pay disparity among primary care physicians shrank, and the number of physicians who declined to take new Medicare patients rose.
The annual report is based on a survey of more than 10,000 physicians in over 29 specialties who answered questions about their income, workload, challenges, and level of satisfaction.
Average compensation across specialties rose to $352,000 – up nearly 17% from the 2018 average of $299,000. Fallout from the COVID-19 public health emergency continued to affect both physician compensation and job satisfaction, including Medicare reimbursements and staffing shortages due to burnout or retirement.
“Many physicians reevaluated what drove them to be a physician,” says Marc Adam, a recruiter at MASC Medical, a Florida physician recruiting firm.
Adam cites telehealth as an example. “An overwhelming majority of physicians prefer telehealth because of the convenience, but some really did not want to do it long term. They miss the patient interaction.”
The report also revealed that the gender-based pay gap in primary physicians fell, with men earning 19% more – down from 25% more in recent years. Among specialists, the gender gap was 27% on average, down from 31% last year. One reason may be an increase in compensation transparency, which Mr. Adam says should be the norm.
Income increases will likely continue, owing in large part to the growing disparity between physician supply and demand.
The projected physician shortage is expected to grow to 124,000 by 2034, according to the American Association of Medical Colleges. Federal lawmakers are considering passing the Resident Physician Shortage Reduction Act of 2023, which would add 14,000 Medicare-funded residency positions to help alleviate shortages.
Patient needs, Medicare rules continue to shift
Specialties with the biggest increases in compensation include oncology, anesthesiology, gastroenterology, radiology, critical care, and urology. Many procedure-related specialties saw more volume post pandemic.
Some respondents identified Medicare cuts and low reimbursement rates as a factor in tamping down compensation hikes. The number of physicians who expect to continue to take new Medicare patients is 65%, down from 71% 5 years ago.
For example, Medicare reimbursements for telehealth are expected to scale down in May, when the COVID-19 Public Health Emergency, which expanded telehealth services for Medicare patients, winds down.
“Telehealth will still exist,” says Mr. Adam, “but certain requirements will shape it going forward.”
Medicare isn’t viewed negatively across the board, however. Florida is among the top-earning states for physicians – along with Indiana, Connecticut, and Missouri. One reason is Florida’s unique health care environment, explains Mr. Adam, whose Florida-based firm places physicians nationwide.
“Florida is very progressive in terms of health care. For one thing, we have a large aging population and a large Medicare population.” Several growing organizations that focus on quality-based care are based in Florida, including ChenMed and Cano Health. Add to that the fact that owners of Florida’s health care organizations don’t have to be physicians, he explains, and the stage is set for experimentation.
“Being able to segment tasks frees up physicians to be more focused on medicine and provide better care while other people focus on the business and innovation.”
If Florida’s high compensation ranking continues, it may help employers there fulfill a growing need. The state is among those expected to experience the largest physician shortages in 2030, along with California, Texas, Arizona, and Georgia.
Side gigs up, satisfaction (slightly) down
In general, physicians aren’t fazed by these challenges. Many reported taking side gigs, some for additional income. Even so, 73% say they would still choose medicine, and more than 90% of physicians in 10 specialties would choose their specialty again. Still, burnout and stressors have led some to stop practicing altogether.
More and more organizations are hiring “travel physicians,” Mr. Adam says, and more physicians are choosing to take contract work (“locum tenens”) and practice in many different regions. Contract physicians typically help meet patient demand or provide coverage during the hiring process as well as while staff are on vacation or maternity leave.
Says Mr. Adam, “There’s no security, but there’s higher income and more flexibility.”
According to CHG Healthcare, locum tenens staffing is rising – approximately 7% of U.S. physicians (around 50,000) filled assignments in 2022, up 88% from 2015. In 2022, 56% of locum tenens employers reported a reduction in staff burnout, up from 30% in 2020.
The report indicates that more than half of physicians are satisfied with their income, down slightly from 55% 5 years ago (prepandemic). Physicians in some of the lower-paying specialties are among those most satisfied with their income. It’s not very surprising to Mr. Adam: “Higher earners generally suffer the most from burnout.
“They’re overworked, they have the largest number of patients, and they’re performing in high-stress situations doing challenging procedures on a daily basis – and they probably have worse work-life balance.” These physicians know going in that they need to be paid more to deal with such burdens. “That’s the feedback I get when I speak to high earners,” says Mr. Adam.
“The experienced ones are very clear about their [compensation] expectations.”
A version of this article first appeared on Medscape.com.
Forgotten but not gone: EVALI epidemic continues
Rashelle Bernal vaped and ended up in an induced coma for a week. She was one of almost 3,000 people who were hospitalized during 2019 and early 2020 with severe lung damage from vaping and became part of what is now known as the epidemic of e-cigarette, or vaping, product use–associated lung injury (EVALI).
For many, the EVALI epidemic is a distant, pre-COVID memory.
But the vaping-related injuries are still happening. And for Ms. Bernal, the aftermath is her reality. Her pulmonologist from that time described the harm from the vape ingredients as an oil spill in her lungs. Eventually, the toxins would probably clear. But she will likely wrestle with the injuries for a very long time.
More than 3 years later, she frequently finds herself in the emergency department.
“If I get sick, if there’s anything that irritates my lungs – it could be something as simple as pollen in the air – it will cause me to get like a bacterial infection or other issues, and I can’t breathe,” Ms. Ms. Bernal, now 30, said in a recent interview. “I get really winded, to the point where I’ll walk up the stairs and I feel like I just ran a mile.”
In 2019 and 2020, a media firestorm erupted as hospitals notified the public of outbreaks of vaping-related lung injuries. News headlines reported e-cigarettes were killing teens from Texas to the Bronx. Investigators at the U.S. Centers for Disease Control and Prevention tracked most of the cases to vitamin E acetate, an additive in illicit cannabis vaping products intended to promote the metabolism of tetrahydrocannabinol (THC). The agency stopped tracking EVALI in February 2020.
But 2 months later, in April 2020, the agency’s National Center for Health Statistics implemented a diagnostic code, U07.0, for health care professionals in the United States to diagnose EVALI for the first time. The code is also used for lung damage related to use of electronic cigarettes and “dabbing” – a method of inhaling cannabis. Damage could include inflammation of the lungs, pulmonary hemorrhage, and eosinophilic pneumonia.
The incidence of these diagnoses appears to have risen sharply since 2020. In the last three months of 2020, a total of 11,300 medical claims included the U07.0 code. That figure rose to 22,000 in 2021 and hit 31,600 in 2022, according to data compiled for and provided to Medscape by Komodo Health, a health care technology company that holds a database of more than 330 million U.S. patients from Medicare, Medicaid, and commercial insurers’ medical, pharmacy, and laboratory claims.
Harm from vaping, including EVALI, has continued.
said Usha Periyanayagam, MD, MPH, head of clinic product and real-world evidence for Komodo and a former emergency medicine physician.
Where it started
Devika Rao, MD, a pediatric pulmonology specialist at UT Southwestern Medical Center, Dallas, has cared for most of her EVALI patients in the hospital, with the most recent case in early 2023. But in January, for the first time, she saw an EVALI patient in an outpatient clinic. The person had not been admitted to the hospital – like most were pre-pandemic. And like most who were seen during the pandemic, this patient had milder symptoms, not requiring intubation or take-home oxygen.
In 2019 and the beginning of 2020, many EVALI patients who were eventually hospitalized first sought help at urgent care centers or with primary care doctors and were presumed to have pneumonia or gastroenteritis and sent home.
“But they got worse, and they would present to our emergency room; their chest X-rays and CT scans showed extensive lung disease,” Dr. Rao said, adding that the damage was striking among patients all under age 18. “They were short of breath. Their oxygen levels were low. They had diminished lung function. And they had a lot of GI issues like abdominal pain and weight loss from nausea and vomiting.”
“These overwhelming inflammatory reactions that we see with EVALI,” said Karen M. Wilson, MD, MPH, a pediatric hospitalist at the University of Rochester (N.Y.) Medical Center and a tobacco use researcher. “You might find some microvascular changes with normal inhaling of smoke or aerosol, but you’re not going to find macro changes like we see with the EVALI.”
In late 2019, images of the CT scans of patients with EVALI were published, grabbing the attention of Arun Kannappan, MD, an assistant professor of pulmonary sciences and critical care at the University of Colorado Anschutz School of Medicine, Aurora. Dr. Kannappan knew a patient with such severe lung damage could develop acute respiratory distress syndrome, which means a patient would be put on a ventilator because their inflamed lungs could not oxygenate blood.
“That confers within somewhere between 30% to 50% chance of dying; it made all of the pulmonary specialists really turn their heads to make sure that we keep a lookout for it,” said Dr. Kannappan.
CT scans of lungs proved to be a critical diagnostic tool for doctors. Most of the images from patients showed acute inflammation and diffuse lung damage. Ehab Ali, MD, a critical care and pulmonary disease medicine specialist in Louisville, Ky., said the damage was often spread across both lungs in many areas and appeared opaque and hazy, known as “ground glass.” COVID-19, meanwhile, appeared differently in lung scans, often with damage that was more isolated.
But many diseases carry a “ground glass” appearance, with many potential causes, like infections, cigarette smoke, or an autoimmune condition.
“Before you even talk to the patient, you can immediately put it in your mind that ‘I’m going to ask this patient if they vape,’ when I see the distribution of ground glass appearance,” Dr. Ali said.
Dr. Ali said other factors, like the age of the patient – about three-quarters of EVALI patients are under age 34, according to the CDC – would spur him to ask about vaping. But because so many patients were young, discerning vape usage wasn’t always easy.
“When you’re talking to teenagers, if you ask them upon admission, with the parents in the room, they’re going say ‘no,’ ” said Rachel Boykan, MD, a pediatric hospitalist at Stony Brook (N.Y.) Children’s. She added that her hospital is still seeing cases.
Dr. Rao said it often takes two to three people asking a patient about any vape usage before they confess.
Ms. Bernal, who was 27 at the time of her hospital admission for EVALI, said she bought vapes with THC at a retail shop in California. She’d been a traditional marijuana smoker, using the leaf product, but switched when someone told her it was healthier to vape THC than inhale smoke from burned marijuana leaves into her lungs. “I thought this was safe.”
Dr. Rao and her colleagues recently published a study of 41 teenage patients with EVALI who were seen at Children’s Medical Center Dallas between December 2018 and July 2021. All but one reported using e-cigarettes containing THC, and the CDC in its most recent report from February 2020 said about 80% of patients had used vapes containing THC.
The CDC also found that vitamin E acetate, an oily substance that allows THC to travel from the lungs to the brain quickly and an ingredient used in the food and cosmetics industries, was found in many of the lungs of EVALI patients, though not all.
The aftermath
The outcomes of the thousands of patients who had EVALI – and those who may still be developing it – are largely untracked.
Bonnie Halpern-Felsher, PhD, director at the Stanford (Calif.) Reach Lab that bears her name and a researcher on tobacco in youth, said she and many of her colleagues are frustrated that the CDC is not continuing to collect data on EVALI.
“I know a lot of colleagues who’ve said that they’re still seeing EVALI, but because of COVID-19 they stopped collecting the data. And that’s been very frustrating because it’s hard to say whether the kinds of lung issues you’re having are related to e-cigarettes, generally, or EVALI,” Dr. Halpern-Felsher said.
Researchers and doctors affiliated with the American Thoracic Society published a report with solutions on how to better track EVALI. They recommended that a national case registry and biorepository be created.
Doctors also worry that many cases were missed. Dr. Boykan said that while protocol dictated nurses and other clinicians ask about a history of vaping – a key part of EVALI diagnosis – many did not. Dr. Ali, the Louisville critical care physician, said EVALI symptoms of nausea, cough, and fever are associated with viral infections.
“I’m sure that some of these cases might be discharged from the emergency room as a virus,” Dr. Ali said. “Most of the time patients would get prescribed steroids for viral infections, which may help EVALI patients even though it’s never been studied.”
Dr. Rao also said the treatment regimen at Children’s MC Dallas, which included high doses of intravenous steroids, seemed to help. But the best management approach for treatment, or long-term follow up care, has not been studied.
The report in the Annals of the American Thoracic Society said prospective studies are showing that a significant portion of patients with EVALI experience prolonged respiratory issues and cognitive and mood impairment. Dr. Rao said a common thread for many of her EVALI patients has been significant stress in their lives with school or family, which led them to vape in an attempt to reduce stress.
That was certainly the case for Ms. Bernal before her hospital admission. She had recently moved across the country for her husband’s job, was trying to buy a house, and had spent months in a hotel with three children. She vaped to cope.
But she said her mental and cognitive health has worsened. Back in Louisville, she saw a neurologist, who told her that her brain had shrunk, she said. She hasn’t found a new neurologist in Portland, Ore., where her family moved a year after the EVALI episode.
But she often finds herself overwhelmed and overstimulated with tasks that she said she never had problems with before. She tears up while talking about the newfound limitations. She struggled to find a primary care physician who could medically manage her mental health and a counselor who can understand what she’s been through with EVALI.
But, “a lot of doctors aren’t educated in it, and they don’t know how to respond or they don’t know what to do,” Ms. Bernal said. “And that makes me feel like, I guess, what I had wasn’t important.”
Ms. Bernal does have a new pulmonologist and is going in for a round of pulmonary tests soon because she often finds herself unable to breathe while completing simple tasks. She is tired of rushing to the ER. She wants answers, or some kind of treatment to help her feel normal again.
“I feel like this is my fault,” Ms. Bernal said. “Had I not smoked, I would be fine, and that’s hard to live with. Every day. Telling yourself, ‘It’s your fault.’ It’s been how many years now? And I still haven’t found peace yet. I don’t know if ever will.”
A version of this article first appeared on Medscape.com.
Rashelle Bernal vaped and ended up in an induced coma for a week. She was one of almost 3,000 people who were hospitalized during 2019 and early 2020 with severe lung damage from vaping and became part of what is now known as the epidemic of e-cigarette, or vaping, product use–associated lung injury (EVALI).
For many, the EVALI epidemic is a distant, pre-COVID memory.
But the vaping-related injuries are still happening. And for Ms. Bernal, the aftermath is her reality. Her pulmonologist from that time described the harm from the vape ingredients as an oil spill in her lungs. Eventually, the toxins would probably clear. But she will likely wrestle with the injuries for a very long time.
More than 3 years later, she frequently finds herself in the emergency department.
“If I get sick, if there’s anything that irritates my lungs – it could be something as simple as pollen in the air – it will cause me to get like a bacterial infection or other issues, and I can’t breathe,” Ms. Ms. Bernal, now 30, said in a recent interview. “I get really winded, to the point where I’ll walk up the stairs and I feel like I just ran a mile.”
In 2019 and 2020, a media firestorm erupted as hospitals notified the public of outbreaks of vaping-related lung injuries. News headlines reported e-cigarettes were killing teens from Texas to the Bronx. Investigators at the U.S. Centers for Disease Control and Prevention tracked most of the cases to vitamin E acetate, an additive in illicit cannabis vaping products intended to promote the metabolism of tetrahydrocannabinol (THC). The agency stopped tracking EVALI in February 2020.
But 2 months later, in April 2020, the agency’s National Center for Health Statistics implemented a diagnostic code, U07.0, for health care professionals in the United States to diagnose EVALI for the first time. The code is also used for lung damage related to use of electronic cigarettes and “dabbing” – a method of inhaling cannabis. Damage could include inflammation of the lungs, pulmonary hemorrhage, and eosinophilic pneumonia.
The incidence of these diagnoses appears to have risen sharply since 2020. In the last three months of 2020, a total of 11,300 medical claims included the U07.0 code. That figure rose to 22,000 in 2021 and hit 31,600 in 2022, according to data compiled for and provided to Medscape by Komodo Health, a health care technology company that holds a database of more than 330 million U.S. patients from Medicare, Medicaid, and commercial insurers’ medical, pharmacy, and laboratory claims.
Harm from vaping, including EVALI, has continued.
said Usha Periyanayagam, MD, MPH, head of clinic product and real-world evidence for Komodo and a former emergency medicine physician.
Where it started
Devika Rao, MD, a pediatric pulmonology specialist at UT Southwestern Medical Center, Dallas, has cared for most of her EVALI patients in the hospital, with the most recent case in early 2023. But in January, for the first time, she saw an EVALI patient in an outpatient clinic. The person had not been admitted to the hospital – like most were pre-pandemic. And like most who were seen during the pandemic, this patient had milder symptoms, not requiring intubation or take-home oxygen.
In 2019 and the beginning of 2020, many EVALI patients who were eventually hospitalized first sought help at urgent care centers or with primary care doctors and were presumed to have pneumonia or gastroenteritis and sent home.
“But they got worse, and they would present to our emergency room; their chest X-rays and CT scans showed extensive lung disease,” Dr. Rao said, adding that the damage was striking among patients all under age 18. “They were short of breath. Their oxygen levels were low. They had diminished lung function. And they had a lot of GI issues like abdominal pain and weight loss from nausea and vomiting.”
“These overwhelming inflammatory reactions that we see with EVALI,” said Karen M. Wilson, MD, MPH, a pediatric hospitalist at the University of Rochester (N.Y.) Medical Center and a tobacco use researcher. “You might find some microvascular changes with normal inhaling of smoke or aerosol, but you’re not going to find macro changes like we see with the EVALI.”
In late 2019, images of the CT scans of patients with EVALI were published, grabbing the attention of Arun Kannappan, MD, an assistant professor of pulmonary sciences and critical care at the University of Colorado Anschutz School of Medicine, Aurora. Dr. Kannappan knew a patient with such severe lung damage could develop acute respiratory distress syndrome, which means a patient would be put on a ventilator because their inflamed lungs could not oxygenate blood.
“That confers within somewhere between 30% to 50% chance of dying; it made all of the pulmonary specialists really turn their heads to make sure that we keep a lookout for it,” said Dr. Kannappan.
CT scans of lungs proved to be a critical diagnostic tool for doctors. Most of the images from patients showed acute inflammation and diffuse lung damage. Ehab Ali, MD, a critical care and pulmonary disease medicine specialist in Louisville, Ky., said the damage was often spread across both lungs in many areas and appeared opaque and hazy, known as “ground glass.” COVID-19, meanwhile, appeared differently in lung scans, often with damage that was more isolated.
But many diseases carry a “ground glass” appearance, with many potential causes, like infections, cigarette smoke, or an autoimmune condition.
“Before you even talk to the patient, you can immediately put it in your mind that ‘I’m going to ask this patient if they vape,’ when I see the distribution of ground glass appearance,” Dr. Ali said.
Dr. Ali said other factors, like the age of the patient – about three-quarters of EVALI patients are under age 34, according to the CDC – would spur him to ask about vaping. But because so many patients were young, discerning vape usage wasn’t always easy.
“When you’re talking to teenagers, if you ask them upon admission, with the parents in the room, they’re going say ‘no,’ ” said Rachel Boykan, MD, a pediatric hospitalist at Stony Brook (N.Y.) Children’s. She added that her hospital is still seeing cases.
Dr. Rao said it often takes two to three people asking a patient about any vape usage before they confess.
Ms. Bernal, who was 27 at the time of her hospital admission for EVALI, said she bought vapes with THC at a retail shop in California. She’d been a traditional marijuana smoker, using the leaf product, but switched when someone told her it was healthier to vape THC than inhale smoke from burned marijuana leaves into her lungs. “I thought this was safe.”
Dr. Rao and her colleagues recently published a study of 41 teenage patients with EVALI who were seen at Children’s Medical Center Dallas between December 2018 and July 2021. All but one reported using e-cigarettes containing THC, and the CDC in its most recent report from February 2020 said about 80% of patients had used vapes containing THC.
The CDC also found that vitamin E acetate, an oily substance that allows THC to travel from the lungs to the brain quickly and an ingredient used in the food and cosmetics industries, was found in many of the lungs of EVALI patients, though not all.
The aftermath
The outcomes of the thousands of patients who had EVALI – and those who may still be developing it – are largely untracked.
Bonnie Halpern-Felsher, PhD, director at the Stanford (Calif.) Reach Lab that bears her name and a researcher on tobacco in youth, said she and many of her colleagues are frustrated that the CDC is not continuing to collect data on EVALI.
“I know a lot of colleagues who’ve said that they’re still seeing EVALI, but because of COVID-19 they stopped collecting the data. And that’s been very frustrating because it’s hard to say whether the kinds of lung issues you’re having are related to e-cigarettes, generally, or EVALI,” Dr. Halpern-Felsher said.
Researchers and doctors affiliated with the American Thoracic Society published a report with solutions on how to better track EVALI. They recommended that a national case registry and biorepository be created.
Doctors also worry that many cases were missed. Dr. Boykan said that while protocol dictated nurses and other clinicians ask about a history of vaping – a key part of EVALI diagnosis – many did not. Dr. Ali, the Louisville critical care physician, said EVALI symptoms of nausea, cough, and fever are associated with viral infections.
“I’m sure that some of these cases might be discharged from the emergency room as a virus,” Dr. Ali said. “Most of the time patients would get prescribed steroids for viral infections, which may help EVALI patients even though it’s never been studied.”
Dr. Rao also said the treatment regimen at Children’s MC Dallas, which included high doses of intravenous steroids, seemed to help. But the best management approach for treatment, or long-term follow up care, has not been studied.
The report in the Annals of the American Thoracic Society said prospective studies are showing that a significant portion of patients with EVALI experience prolonged respiratory issues and cognitive and mood impairment. Dr. Rao said a common thread for many of her EVALI patients has been significant stress in their lives with school or family, which led them to vape in an attempt to reduce stress.
That was certainly the case for Ms. Bernal before her hospital admission. She had recently moved across the country for her husband’s job, was trying to buy a house, and had spent months in a hotel with three children. She vaped to cope.
But she said her mental and cognitive health has worsened. Back in Louisville, she saw a neurologist, who told her that her brain had shrunk, she said. She hasn’t found a new neurologist in Portland, Ore., where her family moved a year after the EVALI episode.
But she often finds herself overwhelmed and overstimulated with tasks that she said she never had problems with before. She tears up while talking about the newfound limitations. She struggled to find a primary care physician who could medically manage her mental health and a counselor who can understand what she’s been through with EVALI.
But, “a lot of doctors aren’t educated in it, and they don’t know how to respond or they don’t know what to do,” Ms. Bernal said. “And that makes me feel like, I guess, what I had wasn’t important.”
Ms. Bernal does have a new pulmonologist and is going in for a round of pulmonary tests soon because she often finds herself unable to breathe while completing simple tasks. She is tired of rushing to the ER. She wants answers, or some kind of treatment to help her feel normal again.
“I feel like this is my fault,” Ms. Bernal said. “Had I not smoked, I would be fine, and that’s hard to live with. Every day. Telling yourself, ‘It’s your fault.’ It’s been how many years now? And I still haven’t found peace yet. I don’t know if ever will.”
A version of this article first appeared on Medscape.com.
Rashelle Bernal vaped and ended up in an induced coma for a week. She was one of almost 3,000 people who were hospitalized during 2019 and early 2020 with severe lung damage from vaping and became part of what is now known as the epidemic of e-cigarette, or vaping, product use–associated lung injury (EVALI).
For many, the EVALI epidemic is a distant, pre-COVID memory.
But the vaping-related injuries are still happening. And for Ms. Bernal, the aftermath is her reality. Her pulmonologist from that time described the harm from the vape ingredients as an oil spill in her lungs. Eventually, the toxins would probably clear. But she will likely wrestle with the injuries for a very long time.
More than 3 years later, she frequently finds herself in the emergency department.
“If I get sick, if there’s anything that irritates my lungs – it could be something as simple as pollen in the air – it will cause me to get like a bacterial infection or other issues, and I can’t breathe,” Ms. Ms. Bernal, now 30, said in a recent interview. “I get really winded, to the point where I’ll walk up the stairs and I feel like I just ran a mile.”
In 2019 and 2020, a media firestorm erupted as hospitals notified the public of outbreaks of vaping-related lung injuries. News headlines reported e-cigarettes were killing teens from Texas to the Bronx. Investigators at the U.S. Centers for Disease Control and Prevention tracked most of the cases to vitamin E acetate, an additive in illicit cannabis vaping products intended to promote the metabolism of tetrahydrocannabinol (THC). The agency stopped tracking EVALI in February 2020.
But 2 months later, in April 2020, the agency’s National Center for Health Statistics implemented a diagnostic code, U07.0, for health care professionals in the United States to diagnose EVALI for the first time. The code is also used for lung damage related to use of electronic cigarettes and “dabbing” – a method of inhaling cannabis. Damage could include inflammation of the lungs, pulmonary hemorrhage, and eosinophilic pneumonia.
The incidence of these diagnoses appears to have risen sharply since 2020. In the last three months of 2020, a total of 11,300 medical claims included the U07.0 code. That figure rose to 22,000 in 2021 and hit 31,600 in 2022, according to data compiled for and provided to Medscape by Komodo Health, a health care technology company that holds a database of more than 330 million U.S. patients from Medicare, Medicaid, and commercial insurers’ medical, pharmacy, and laboratory claims.
Harm from vaping, including EVALI, has continued.
said Usha Periyanayagam, MD, MPH, head of clinic product and real-world evidence for Komodo and a former emergency medicine physician.
Where it started
Devika Rao, MD, a pediatric pulmonology specialist at UT Southwestern Medical Center, Dallas, has cared for most of her EVALI patients in the hospital, with the most recent case in early 2023. But in January, for the first time, she saw an EVALI patient in an outpatient clinic. The person had not been admitted to the hospital – like most were pre-pandemic. And like most who were seen during the pandemic, this patient had milder symptoms, not requiring intubation or take-home oxygen.
In 2019 and the beginning of 2020, many EVALI patients who were eventually hospitalized first sought help at urgent care centers or with primary care doctors and were presumed to have pneumonia or gastroenteritis and sent home.
“But they got worse, and they would present to our emergency room; their chest X-rays and CT scans showed extensive lung disease,” Dr. Rao said, adding that the damage was striking among patients all under age 18. “They were short of breath. Their oxygen levels were low. They had diminished lung function. And they had a lot of GI issues like abdominal pain and weight loss from nausea and vomiting.”
“These overwhelming inflammatory reactions that we see with EVALI,” said Karen M. Wilson, MD, MPH, a pediatric hospitalist at the University of Rochester (N.Y.) Medical Center and a tobacco use researcher. “You might find some microvascular changes with normal inhaling of smoke or aerosol, but you’re not going to find macro changes like we see with the EVALI.”
In late 2019, images of the CT scans of patients with EVALI were published, grabbing the attention of Arun Kannappan, MD, an assistant professor of pulmonary sciences and critical care at the University of Colorado Anschutz School of Medicine, Aurora. Dr. Kannappan knew a patient with such severe lung damage could develop acute respiratory distress syndrome, which means a patient would be put on a ventilator because their inflamed lungs could not oxygenate blood.
“That confers within somewhere between 30% to 50% chance of dying; it made all of the pulmonary specialists really turn their heads to make sure that we keep a lookout for it,” said Dr. Kannappan.
CT scans of lungs proved to be a critical diagnostic tool for doctors. Most of the images from patients showed acute inflammation and diffuse lung damage. Ehab Ali, MD, a critical care and pulmonary disease medicine specialist in Louisville, Ky., said the damage was often spread across both lungs in many areas and appeared opaque and hazy, known as “ground glass.” COVID-19, meanwhile, appeared differently in lung scans, often with damage that was more isolated.
But many diseases carry a “ground glass” appearance, with many potential causes, like infections, cigarette smoke, or an autoimmune condition.
“Before you even talk to the patient, you can immediately put it in your mind that ‘I’m going to ask this patient if they vape,’ when I see the distribution of ground glass appearance,” Dr. Ali said.
Dr. Ali said other factors, like the age of the patient – about three-quarters of EVALI patients are under age 34, according to the CDC – would spur him to ask about vaping. But because so many patients were young, discerning vape usage wasn’t always easy.
“When you’re talking to teenagers, if you ask them upon admission, with the parents in the room, they’re going say ‘no,’ ” said Rachel Boykan, MD, a pediatric hospitalist at Stony Brook (N.Y.) Children’s. She added that her hospital is still seeing cases.
Dr. Rao said it often takes two to three people asking a patient about any vape usage before they confess.
Ms. Bernal, who was 27 at the time of her hospital admission for EVALI, said she bought vapes with THC at a retail shop in California. She’d been a traditional marijuana smoker, using the leaf product, but switched when someone told her it was healthier to vape THC than inhale smoke from burned marijuana leaves into her lungs. “I thought this was safe.”
Dr. Rao and her colleagues recently published a study of 41 teenage patients with EVALI who were seen at Children’s Medical Center Dallas between December 2018 and July 2021. All but one reported using e-cigarettes containing THC, and the CDC in its most recent report from February 2020 said about 80% of patients had used vapes containing THC.
The CDC also found that vitamin E acetate, an oily substance that allows THC to travel from the lungs to the brain quickly and an ingredient used in the food and cosmetics industries, was found in many of the lungs of EVALI patients, though not all.
The aftermath
The outcomes of the thousands of patients who had EVALI – and those who may still be developing it – are largely untracked.
Bonnie Halpern-Felsher, PhD, director at the Stanford (Calif.) Reach Lab that bears her name and a researcher on tobacco in youth, said she and many of her colleagues are frustrated that the CDC is not continuing to collect data on EVALI.
“I know a lot of colleagues who’ve said that they’re still seeing EVALI, but because of COVID-19 they stopped collecting the data. And that’s been very frustrating because it’s hard to say whether the kinds of lung issues you’re having are related to e-cigarettes, generally, or EVALI,” Dr. Halpern-Felsher said.
Researchers and doctors affiliated with the American Thoracic Society published a report with solutions on how to better track EVALI. They recommended that a national case registry and biorepository be created.
Doctors also worry that many cases were missed. Dr. Boykan said that while protocol dictated nurses and other clinicians ask about a history of vaping – a key part of EVALI diagnosis – many did not. Dr. Ali, the Louisville critical care physician, said EVALI symptoms of nausea, cough, and fever are associated with viral infections.
“I’m sure that some of these cases might be discharged from the emergency room as a virus,” Dr. Ali said. “Most of the time patients would get prescribed steroids for viral infections, which may help EVALI patients even though it’s never been studied.”
Dr. Rao also said the treatment regimen at Children’s MC Dallas, which included high doses of intravenous steroids, seemed to help. But the best management approach for treatment, or long-term follow up care, has not been studied.
The report in the Annals of the American Thoracic Society said prospective studies are showing that a significant portion of patients with EVALI experience prolonged respiratory issues and cognitive and mood impairment. Dr. Rao said a common thread for many of her EVALI patients has been significant stress in their lives with school or family, which led them to vape in an attempt to reduce stress.
That was certainly the case for Ms. Bernal before her hospital admission. She had recently moved across the country for her husband’s job, was trying to buy a house, and had spent months in a hotel with three children. She vaped to cope.
But she said her mental and cognitive health has worsened. Back in Louisville, she saw a neurologist, who told her that her brain had shrunk, she said. She hasn’t found a new neurologist in Portland, Ore., where her family moved a year after the EVALI episode.
But she often finds herself overwhelmed and overstimulated with tasks that she said she never had problems with before. She tears up while talking about the newfound limitations. She struggled to find a primary care physician who could medically manage her mental health and a counselor who can understand what she’s been through with EVALI.
But, “a lot of doctors aren’t educated in it, and they don’t know how to respond or they don’t know what to do,” Ms. Bernal said. “And that makes me feel like, I guess, what I had wasn’t important.”
Ms. Bernal does have a new pulmonologist and is going in for a round of pulmonary tests soon because she often finds herself unable to breathe while completing simple tasks. She is tired of rushing to the ER. She wants answers, or some kind of treatment to help her feel normal again.
“I feel like this is my fault,” Ms. Bernal said. “Had I not smoked, I would be fine, and that’s hard to live with. Every day. Telling yourself, ‘It’s your fault.’ It’s been how many years now? And I still haven’t found peace yet. I don’t know if ever will.”
A version of this article first appeared on Medscape.com.
Sleep disturbances linked to post-COVID dyspnea
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
FROM ECCMID 2023
USPSTF releases updated recommendations on skin cancer screening
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
Intermittent fasting plus early eating may prevent type 2 diabetes
, indicate the results of a randomized controlled trial.
The study involved more than 200 individuals randomized to one of three groups: eat only in the morning (from 8:00 a.m. to noon) followed by 20 hours of fasting 3 days per week and eat as desired on the other days; daily calorie restriction to 70% of requirements; or standard weight loss advice.
The IF plus early time-restricted eating intervention was associated with a significant improvement in a key measure of glucose control versus calorie restriction at 6 months, while both interventions were linked to benefits in terms of cardiovascular risk markers and body composition, compared with the standard weight loss advice.
However, the research, published in Nature Medicine, showed that the additional benefit of IF plus early time-restricted eating did not persist, and less than half of participants were still following the plan at 18 months, compared with almost 80% of those in the calorie-restriction group.
“Following a time-restricted, IF diet could help lower the chances of developing type 2 diabetes,” senior author Leonie K. Heilbronn, PhD, University of Adelaide, South Australia, said in a press release.
This is “the largest study in the world to date, and the first powered to assess how the body processes and uses glucose after eating a meal,” with the latter being a better indicator of diabetes risk than a fasting glucose test, added first author Xiao Tong Teong, a PhD student, also at the University of Adelaide.
“The results of this study add to the growing body of evidence to indicate that meal timing and fasting advice extends the health benefits of a restricted-calorie diet, independently from weight loss, and this may be influential in clinical practice,” Ms. Teong added.
Adherence difficult to IF plus early time-restricted eating
Asked to comment, Krista Varady, PhD, said that the study design “would have been stronger if the time-restricted eating and IF interventions were separated” and compared.
“Time-restricted eating has been shown to naturally reduce calorie intake by 300-500 kcal/day,” she said in an interview, “so I’m not sure why the investigators chose to combine [it] with IF. It ... defeats the point of time-restricted eating.”
Dr. Varady, who recently coauthored a review of the clinical application of IF for weight loss, also doubted whether individuals would adhere to combined early time-restricted eating and IF. “In all honesty, I don’t think anyone would follow this diet for very long,” she said.
She added that the feasibility of this particular approach is “very questionable. In general, people don’t like diets that require them to skip dinner with family/friends on multiple days of the week,” explained Dr. Varady, professor of nutrition at the University of Illinois, Chicago. “These regimens make social eating very difficult, which results in high attrition.
“Indeed, evidence from a recent large-scale observational study of nearly 800,000 adults shows that Americans who engage in time-restricted eating placed their eating window in the afternoon or evening,” she noted.
Dr. Varady therefore suggested that future trials should test “more feasible time-restricted eating approaches,” such as those with later eating windows and without “vigilant calorie monitoring.”
“These types of diets are much easier to follow and are more likely to produce lasting weight and glycemic control in people with obesity and prediabetes,” she observed.
A novel way to cut calories?
The Australian authors say there is growing interest in extending the established health benefits of calorie restriction through new approaches such as timing of meals and prolonged fasting, with IF – defined as fasting interspersed with days of ad libitum eating – gaining in popularity as an alternative to simple calorie restriction.
Time-restricted eating, which emphasizes shorter daily eating windows in alignment with circadian rhythms, has also become popular in recent years, although the authors acknowledge that current evidence suggests any benefits over calorie restriction alone in terms of body composition, blood lipids, or glucose parameters are small.
To examine the combination of IF plus early time-restricted eating, in the DIRECT trial, the team recruited individuals aged 35-75 years who had a score of at least 12 on the Australian Type 2 Diabetes Risk Assessment Tool but did not have a diagnosis of diabetes and had stable weight for more than 6 months prior to study entry.
The participants were randomized to one of three groups:
- IF plus early time-restricted eating, which allowed consumption of 30% of calculated baseline energy requirements between 8:00 a.m. and midday, followed by a 20-hour fast from midday on 3 nonconsecutive days per week. They consumed their regular diet on nonfasting days.
- Calorie restriction, where they consumed 70% of daily calculated baseline energy requirements each day and were given rotating menu plans, but no specific mealtimes.
- Standard care, where they were given a booklet on current guidelines, with no counseling or meal replacement.
There were clinic visits every 2 weeks for the first 6 months of follow-up, and then monthly visits for 12 months. The two intervention groups had one-on-one diet counseling for the first 6 months. All groups were instructed to maintain their usual physical activity levels.
A total of 209 individuals were enrolled between Sept. 26, 2018, and May 4, 2020. Their mean age was 58 years, and 57% were women. Mean body mass index was 34.8 kg/m2.
In all, 40.7% of participants were allocated to IF plus early time-restricted eating, 39.7% to calorie restriction, and the remaining 19.6% to standard care.
The results showed that IF plus early time-restricted eating was associated with a significantly greater improvement in the primary outcome of postprandial glucose area under the curve (AUC) at month 6 compared with calorie restriction, at –10.1 mg/dL/min versus –3.6 mg/dL/min (P = .03).
“To our knowledge, no [prior] studies have been powered for postprandial assessments of glycemia, which are better indicators of diabetes risk than fasting assessment,” the authors underlined.
IF plus early time-restricted eating was also associated with greater reductions in postprandial insulin AUC versus calorie restriction at 6 months (P = .04). However, the differences between the IF plus early time-restricted eating and calorie restriction groups for postmeal insulin did not remain significant at 18 months of follow-up.
Both IF plus early time-restricted eating and calorie restriction were associated with greater reductions in A1c levels at 6 months versus standard care, but there was no significant difference between the two active interventions (P = .46).
Both interventions were also associated with improvements in markers of cardiovascular risk versus standard care, such as systolic blood pressure at 2 months, diastolic blood pressure at 6 months, and fasting triglycerides at both time points, with no significant differences between the two intervention groups.
IF plus early time-restricted eating and calorie restriction were also both associated with greater reductions in BMI and fat mass in the first 6 months, as well as in waist circumference.
Calorie restriction easier to stick to, less likely to cause fatigue
When offered the chance to modify their diet plan at 6 months, 46% of participants in the IF plus early time-restricted eating group said they would maintain 3 days of restrictions per week, while 51% chose to reduce the restrictions to 2 days per week.
In contrast, 97% of those who completed the calorie-restriction plan indicated they would continue with their current diet plan.
At 18 months, 42% of participants in the IF plus early time-restricted eating group said they still undertook 2-3 days of restrictions per week, while 78% of those assigned to calorie restriction reported that they followed a calorie-restricted diet.
Fatigue was more common with IF plus early time-restricted eating, reported by 56% of participants versus 37% of those following calorie restriction, and 35% of those in the standard care group at 6 months. Headaches and constipation were more common in the intervention groups than with standard care.
The study was supported by a National Health and Medical Research Council Project Grant, an Australian Government Research Training Program Scholarship from the University of Adelaide, and a Diabetes Australia Research Program Grant.
No relevant financial relationships were declared.
A version of this article originally appeared on Medscape.com.
, indicate the results of a randomized controlled trial.
The study involved more than 200 individuals randomized to one of three groups: eat only in the morning (from 8:00 a.m. to noon) followed by 20 hours of fasting 3 days per week and eat as desired on the other days; daily calorie restriction to 70% of requirements; or standard weight loss advice.
The IF plus early time-restricted eating intervention was associated with a significant improvement in a key measure of glucose control versus calorie restriction at 6 months, while both interventions were linked to benefits in terms of cardiovascular risk markers and body composition, compared with the standard weight loss advice.
However, the research, published in Nature Medicine, showed that the additional benefit of IF plus early time-restricted eating did not persist, and less than half of participants were still following the plan at 18 months, compared with almost 80% of those in the calorie-restriction group.
“Following a time-restricted, IF diet could help lower the chances of developing type 2 diabetes,” senior author Leonie K. Heilbronn, PhD, University of Adelaide, South Australia, said in a press release.
This is “the largest study in the world to date, and the first powered to assess how the body processes and uses glucose after eating a meal,” with the latter being a better indicator of diabetes risk than a fasting glucose test, added first author Xiao Tong Teong, a PhD student, also at the University of Adelaide.
“The results of this study add to the growing body of evidence to indicate that meal timing and fasting advice extends the health benefits of a restricted-calorie diet, independently from weight loss, and this may be influential in clinical practice,” Ms. Teong added.
Adherence difficult to IF plus early time-restricted eating
Asked to comment, Krista Varady, PhD, said that the study design “would have been stronger if the time-restricted eating and IF interventions were separated” and compared.
“Time-restricted eating has been shown to naturally reduce calorie intake by 300-500 kcal/day,” she said in an interview, “so I’m not sure why the investigators chose to combine [it] with IF. It ... defeats the point of time-restricted eating.”
Dr. Varady, who recently coauthored a review of the clinical application of IF for weight loss, also doubted whether individuals would adhere to combined early time-restricted eating and IF. “In all honesty, I don’t think anyone would follow this diet for very long,” she said.
She added that the feasibility of this particular approach is “very questionable. In general, people don’t like diets that require them to skip dinner with family/friends on multiple days of the week,” explained Dr. Varady, professor of nutrition at the University of Illinois, Chicago. “These regimens make social eating very difficult, which results in high attrition.
“Indeed, evidence from a recent large-scale observational study of nearly 800,000 adults shows that Americans who engage in time-restricted eating placed their eating window in the afternoon or evening,” she noted.
Dr. Varady therefore suggested that future trials should test “more feasible time-restricted eating approaches,” such as those with later eating windows and without “vigilant calorie monitoring.”
“These types of diets are much easier to follow and are more likely to produce lasting weight and glycemic control in people with obesity and prediabetes,” she observed.
A novel way to cut calories?
The Australian authors say there is growing interest in extending the established health benefits of calorie restriction through new approaches such as timing of meals and prolonged fasting, with IF – defined as fasting interspersed with days of ad libitum eating – gaining in popularity as an alternative to simple calorie restriction.
Time-restricted eating, which emphasizes shorter daily eating windows in alignment with circadian rhythms, has also become popular in recent years, although the authors acknowledge that current evidence suggests any benefits over calorie restriction alone in terms of body composition, blood lipids, or glucose parameters are small.
To examine the combination of IF plus early time-restricted eating, in the DIRECT trial, the team recruited individuals aged 35-75 years who had a score of at least 12 on the Australian Type 2 Diabetes Risk Assessment Tool but did not have a diagnosis of diabetes and had stable weight for more than 6 months prior to study entry.
The participants were randomized to one of three groups:
- IF plus early time-restricted eating, which allowed consumption of 30% of calculated baseline energy requirements between 8:00 a.m. and midday, followed by a 20-hour fast from midday on 3 nonconsecutive days per week. They consumed their regular diet on nonfasting days.
- Calorie restriction, where they consumed 70% of daily calculated baseline energy requirements each day and were given rotating menu plans, but no specific mealtimes.
- Standard care, where they were given a booklet on current guidelines, with no counseling or meal replacement.
There were clinic visits every 2 weeks for the first 6 months of follow-up, and then monthly visits for 12 months. The two intervention groups had one-on-one diet counseling for the first 6 months. All groups were instructed to maintain their usual physical activity levels.
A total of 209 individuals were enrolled between Sept. 26, 2018, and May 4, 2020. Their mean age was 58 years, and 57% were women. Mean body mass index was 34.8 kg/m2.
In all, 40.7% of participants were allocated to IF plus early time-restricted eating, 39.7% to calorie restriction, and the remaining 19.6% to standard care.
The results showed that IF plus early time-restricted eating was associated with a significantly greater improvement in the primary outcome of postprandial glucose area under the curve (AUC) at month 6 compared with calorie restriction, at –10.1 mg/dL/min versus –3.6 mg/dL/min (P = .03).
“To our knowledge, no [prior] studies have been powered for postprandial assessments of glycemia, which are better indicators of diabetes risk than fasting assessment,” the authors underlined.
IF plus early time-restricted eating was also associated with greater reductions in postprandial insulin AUC versus calorie restriction at 6 months (P = .04). However, the differences between the IF plus early time-restricted eating and calorie restriction groups for postmeal insulin did not remain significant at 18 months of follow-up.
Both IF plus early time-restricted eating and calorie restriction were associated with greater reductions in A1c levels at 6 months versus standard care, but there was no significant difference between the two active interventions (P = .46).
Both interventions were also associated with improvements in markers of cardiovascular risk versus standard care, such as systolic blood pressure at 2 months, diastolic blood pressure at 6 months, and fasting triglycerides at both time points, with no significant differences between the two intervention groups.
IF plus early time-restricted eating and calorie restriction were also both associated with greater reductions in BMI and fat mass in the first 6 months, as well as in waist circumference.
Calorie restriction easier to stick to, less likely to cause fatigue
When offered the chance to modify their diet plan at 6 months, 46% of participants in the IF plus early time-restricted eating group said they would maintain 3 days of restrictions per week, while 51% chose to reduce the restrictions to 2 days per week.
In contrast, 97% of those who completed the calorie-restriction plan indicated they would continue with their current diet plan.
At 18 months, 42% of participants in the IF plus early time-restricted eating group said they still undertook 2-3 days of restrictions per week, while 78% of those assigned to calorie restriction reported that they followed a calorie-restricted diet.
Fatigue was more common with IF plus early time-restricted eating, reported by 56% of participants versus 37% of those following calorie restriction, and 35% of those in the standard care group at 6 months. Headaches and constipation were more common in the intervention groups than with standard care.
The study was supported by a National Health and Medical Research Council Project Grant, an Australian Government Research Training Program Scholarship from the University of Adelaide, and a Diabetes Australia Research Program Grant.
No relevant financial relationships were declared.
A version of this article originally appeared on Medscape.com.
, indicate the results of a randomized controlled trial.
The study involved more than 200 individuals randomized to one of three groups: eat only in the morning (from 8:00 a.m. to noon) followed by 20 hours of fasting 3 days per week and eat as desired on the other days; daily calorie restriction to 70% of requirements; or standard weight loss advice.
The IF plus early time-restricted eating intervention was associated with a significant improvement in a key measure of glucose control versus calorie restriction at 6 months, while both interventions were linked to benefits in terms of cardiovascular risk markers and body composition, compared with the standard weight loss advice.
However, the research, published in Nature Medicine, showed that the additional benefit of IF plus early time-restricted eating did not persist, and less than half of participants were still following the plan at 18 months, compared with almost 80% of those in the calorie-restriction group.
“Following a time-restricted, IF diet could help lower the chances of developing type 2 diabetes,” senior author Leonie K. Heilbronn, PhD, University of Adelaide, South Australia, said in a press release.
This is “the largest study in the world to date, and the first powered to assess how the body processes and uses glucose after eating a meal,” with the latter being a better indicator of diabetes risk than a fasting glucose test, added first author Xiao Tong Teong, a PhD student, also at the University of Adelaide.
“The results of this study add to the growing body of evidence to indicate that meal timing and fasting advice extends the health benefits of a restricted-calorie diet, independently from weight loss, and this may be influential in clinical practice,” Ms. Teong added.
Adherence difficult to IF plus early time-restricted eating
Asked to comment, Krista Varady, PhD, said that the study design “would have been stronger if the time-restricted eating and IF interventions were separated” and compared.
“Time-restricted eating has been shown to naturally reduce calorie intake by 300-500 kcal/day,” she said in an interview, “so I’m not sure why the investigators chose to combine [it] with IF. It ... defeats the point of time-restricted eating.”
Dr. Varady, who recently coauthored a review of the clinical application of IF for weight loss, also doubted whether individuals would adhere to combined early time-restricted eating and IF. “In all honesty, I don’t think anyone would follow this diet for very long,” she said.
She added that the feasibility of this particular approach is “very questionable. In general, people don’t like diets that require them to skip dinner with family/friends on multiple days of the week,” explained Dr. Varady, professor of nutrition at the University of Illinois, Chicago. “These regimens make social eating very difficult, which results in high attrition.
“Indeed, evidence from a recent large-scale observational study of nearly 800,000 adults shows that Americans who engage in time-restricted eating placed their eating window in the afternoon or evening,” she noted.
Dr. Varady therefore suggested that future trials should test “more feasible time-restricted eating approaches,” such as those with later eating windows and without “vigilant calorie monitoring.”
“These types of diets are much easier to follow and are more likely to produce lasting weight and glycemic control in people with obesity and prediabetes,” she observed.
A novel way to cut calories?
The Australian authors say there is growing interest in extending the established health benefits of calorie restriction through new approaches such as timing of meals and prolonged fasting, with IF – defined as fasting interspersed with days of ad libitum eating – gaining in popularity as an alternative to simple calorie restriction.
Time-restricted eating, which emphasizes shorter daily eating windows in alignment with circadian rhythms, has also become popular in recent years, although the authors acknowledge that current evidence suggests any benefits over calorie restriction alone in terms of body composition, blood lipids, or glucose parameters are small.
To examine the combination of IF plus early time-restricted eating, in the DIRECT trial, the team recruited individuals aged 35-75 years who had a score of at least 12 on the Australian Type 2 Diabetes Risk Assessment Tool but did not have a diagnosis of diabetes and had stable weight for more than 6 months prior to study entry.
The participants were randomized to one of three groups:
- IF plus early time-restricted eating, which allowed consumption of 30% of calculated baseline energy requirements between 8:00 a.m. and midday, followed by a 20-hour fast from midday on 3 nonconsecutive days per week. They consumed their regular diet on nonfasting days.
- Calorie restriction, where they consumed 70% of daily calculated baseline energy requirements each day and were given rotating menu plans, but no specific mealtimes.
- Standard care, where they were given a booklet on current guidelines, with no counseling or meal replacement.
There were clinic visits every 2 weeks for the first 6 months of follow-up, and then monthly visits for 12 months. The two intervention groups had one-on-one diet counseling for the first 6 months. All groups were instructed to maintain their usual physical activity levels.
A total of 209 individuals were enrolled between Sept. 26, 2018, and May 4, 2020. Their mean age was 58 years, and 57% were women. Mean body mass index was 34.8 kg/m2.
In all, 40.7% of participants were allocated to IF plus early time-restricted eating, 39.7% to calorie restriction, and the remaining 19.6% to standard care.
The results showed that IF plus early time-restricted eating was associated with a significantly greater improvement in the primary outcome of postprandial glucose area under the curve (AUC) at month 6 compared with calorie restriction, at –10.1 mg/dL/min versus –3.6 mg/dL/min (P = .03).
“To our knowledge, no [prior] studies have been powered for postprandial assessments of glycemia, which are better indicators of diabetes risk than fasting assessment,” the authors underlined.
IF plus early time-restricted eating was also associated with greater reductions in postprandial insulin AUC versus calorie restriction at 6 months (P = .04). However, the differences between the IF plus early time-restricted eating and calorie restriction groups for postmeal insulin did not remain significant at 18 months of follow-up.
Both IF plus early time-restricted eating and calorie restriction were associated with greater reductions in A1c levels at 6 months versus standard care, but there was no significant difference between the two active interventions (P = .46).
Both interventions were also associated with improvements in markers of cardiovascular risk versus standard care, such as systolic blood pressure at 2 months, diastolic blood pressure at 6 months, and fasting triglycerides at both time points, with no significant differences between the two intervention groups.
IF plus early time-restricted eating and calorie restriction were also both associated with greater reductions in BMI and fat mass in the first 6 months, as well as in waist circumference.
Calorie restriction easier to stick to, less likely to cause fatigue
When offered the chance to modify their diet plan at 6 months, 46% of participants in the IF plus early time-restricted eating group said they would maintain 3 days of restrictions per week, while 51% chose to reduce the restrictions to 2 days per week.
In contrast, 97% of those who completed the calorie-restriction plan indicated they would continue with their current diet plan.
At 18 months, 42% of participants in the IF plus early time-restricted eating group said they still undertook 2-3 days of restrictions per week, while 78% of those assigned to calorie restriction reported that they followed a calorie-restricted diet.
Fatigue was more common with IF plus early time-restricted eating, reported by 56% of participants versus 37% of those following calorie restriction, and 35% of those in the standard care group at 6 months. Headaches and constipation were more common in the intervention groups than with standard care.
The study was supported by a National Health and Medical Research Council Project Grant, an Australian Government Research Training Program Scholarship from the University of Adelaide, and a Diabetes Australia Research Program Grant.
No relevant financial relationships were declared.
A version of this article originally appeared on Medscape.com.
FROM NATURE MEDICINE




