User login
Painful Retiform Purpura in a Peritoneal Dialysis Patient
The Diagnosis: Calcific Uremic Arteriolopathy
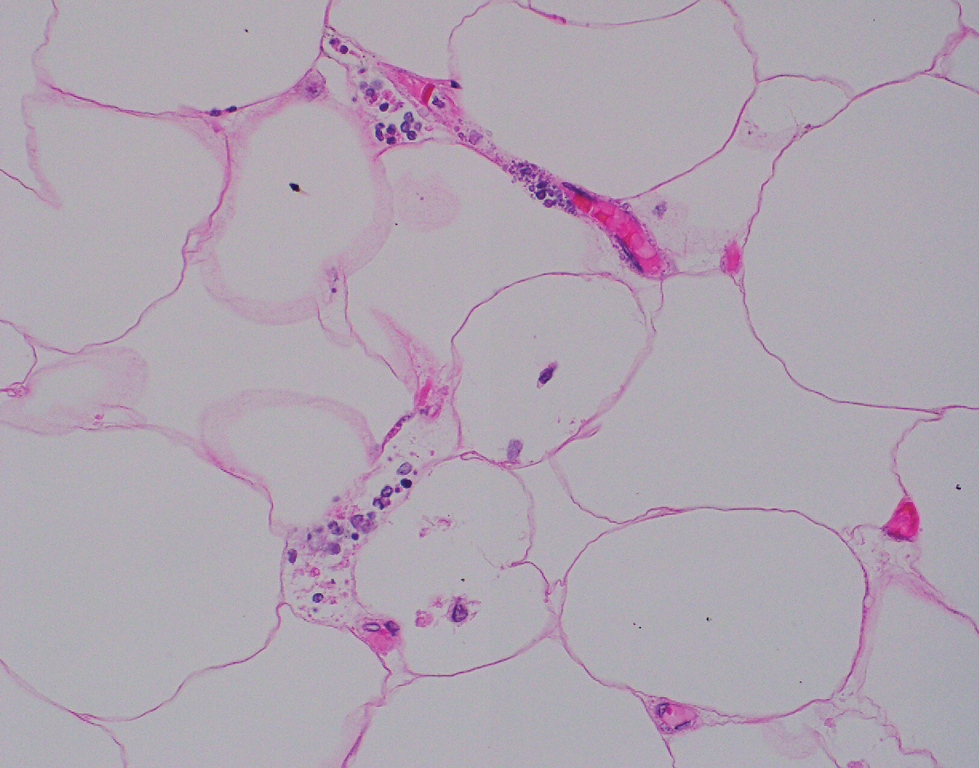
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.
Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
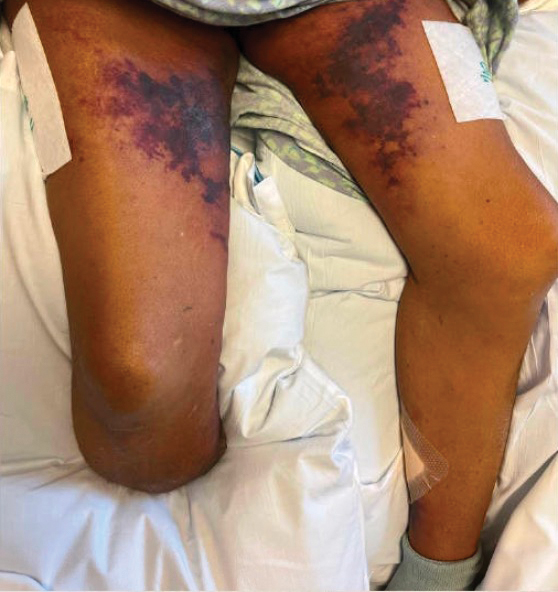
A 72-year-old woman presented to the emergency department with concerns of confusion and lethargy during a session of peritoneal dialysis, which she had been receiving for the last 2 years for end-stage renal disease. She had a history of type 2 diabetes mellitus, diabetic retinopathy, hypertension, coronary artery disease, and peripheral vascular disease preceding a recent right below-knee amputation. A review of systems was positive for a rash on the thighs of several weeks’ duration that was preceded by several days of burning pain in the same distribution. Physical examination revealed retiform purpura with irregular contours and interspersed white stellate patterns scattered across the superomedial thighs, right lower back, and left lower abdomen. An initial laboratory workup revealed an elevated creatinine level of 5.03 mg/dL (reference range, 0.6–1.1 mg/dL; baseline level, 3.0 mg/dL) and mild leukocytosis (12.5 cells/mm3 [reference range, 4.5–11.0 cells/mm3]). Dermatology was consulted, and a 4-mm punch biopsy was obtained from the left medial thigh. Nephrology, infectious disease, and wound care consultations also were placed.
Prednisolone May Improve MOH Withdrawal
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
FROM HEADACHE
OTC Topical Scar Products May Contain Allergens, Study Finds
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
High Rate of Dementia Among Attendees in Adult Day Service Centers
About one-quarter of all adult day services center (ADSC) participants have dementia, and the prevalence of dementia in ADSCs that specialize in the disorder is more than 40%, a new US National Health Statistics Report revealed.
ADSCs are a growing sector of the US home- and community-based long-term care delivery system, providing daytime services to adults with disabilities who often have multiple chronic conditions, including various types of dementia, according to report authors Priyanka Singha, MPH, and colleagues at the US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics in Bethesda, Maryland.
Dementia often leads to the transition to receiving long-term care services, such as nursing home care. Delaying institutionalization is a primary goal of ADSCs, so they also try to meet the needs of a growing population of community-dwelling adults with dementia.
Survey responses from 1800 ADSCs across the United States showed that overall, 42.2% of participants had dementia in ADSCs specializing in dementia care, while 22.7% of participants in nonspecialized ADSCs also had dementia.
Dementia was more prevalent in the Midwest and West, where nearly one half of participants in specialized centers had dementia.
Nevertheless, the overall prevalence of dementia in ADSCs was similar across US regions, with a slightly lower percentage in the West.
Positive Outcomes
The new report used data from the ADSC component of the 2020 National Post-acute and Long-term Care Study collected from January 2020 through mid-July 2021. About 1800 ADSCs from a census of 5500 ADSCs were included and weighted to be nationally representative.
The authors compared dementia prevalence among participants in ADSCs that provide specialized care for dementia with other ADSCs by census region, metropolitan statistical area (MSA) status, chain affiliation, and ownership type.
MSA is a core urban area population of 50,000 or more. ADSCs that specialize in dementia care have specially trained staff, activities, and facilities. They offer social activities, including art and music therapy, dementia-appropriate games, and group exercises, as well as respite care for unpaid caregivers. The survey found that 14% of ADSCs reported specializing in dementia.
The investigators also found that the percentage of ADSC participants with dementia, regardless of center specialization, was higher in the Midwest (32.1%), Northeast (28.5%), and South (24.5%) than in the West (21.1%).
The percentage of participants with dementia in specialized centers was higher in the Midwest (49.5%) and West (48.8%) than in the Northeast (31.9%) and in nonchain centers (50.5%) than in chain-affiliated centers (30.4%).
In addition, the percentage of participants with dementia, regardless of specialization, was higher in nonchain ADSCs (25%) than in chain-affiliated centers (20.1%). In addition, the percentage of participants with dementia in nonspecialized centers was higher in nonchain centers (25%) than in chain-affiliated centers (20.1%).
Finally, the research revealed that the percentage of participants with dementia, regardless of specialization, was higher in nonprofit ADSCs (28.7%) than for-profit centers (21%).
“These findings indicate that ADSCs in MSAs, nonprofit organizations, and nonchain centers provide services to a higher proportion of participants with dementia, particularly among centers that specialize in dementia care,” the investigators wrote.
Whereas “caregivers manage prescription medications, help with activities of daily living, and offer nutritional diets, exercise, and social engagement, ADSCs play a role in providing this type of care for people with dementia while also offering respite for their unpaid caregivers,” they noted.
Overall, they concluded that ADSCs provide positive outcomes for both family caregivers and people with dementia.
They noted that the study’s limitations include the use of cross-sectional data, which cannot show effectiveness for participants receiving care in specialized centers or be used to analyze relationships between other participant-level sociodemographic or health characteristics and specialized dementia care.
A version of this article appeared on Medscape.com.
About one-quarter of all adult day services center (ADSC) participants have dementia, and the prevalence of dementia in ADSCs that specialize in the disorder is more than 40%, a new US National Health Statistics Report revealed.
ADSCs are a growing sector of the US home- and community-based long-term care delivery system, providing daytime services to adults with disabilities who often have multiple chronic conditions, including various types of dementia, according to report authors Priyanka Singha, MPH, and colleagues at the US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics in Bethesda, Maryland.
Dementia often leads to the transition to receiving long-term care services, such as nursing home care. Delaying institutionalization is a primary goal of ADSCs, so they also try to meet the needs of a growing population of community-dwelling adults with dementia.
Survey responses from 1800 ADSCs across the United States showed that overall, 42.2% of participants had dementia in ADSCs specializing in dementia care, while 22.7% of participants in nonspecialized ADSCs also had dementia.
Dementia was more prevalent in the Midwest and West, where nearly one half of participants in specialized centers had dementia.
Nevertheless, the overall prevalence of dementia in ADSCs was similar across US regions, with a slightly lower percentage in the West.
Positive Outcomes
The new report used data from the ADSC component of the 2020 National Post-acute and Long-term Care Study collected from January 2020 through mid-July 2021. About 1800 ADSCs from a census of 5500 ADSCs were included and weighted to be nationally representative.
The authors compared dementia prevalence among participants in ADSCs that provide specialized care for dementia with other ADSCs by census region, metropolitan statistical area (MSA) status, chain affiliation, and ownership type.
MSA is a core urban area population of 50,000 or more. ADSCs that specialize in dementia care have specially trained staff, activities, and facilities. They offer social activities, including art and music therapy, dementia-appropriate games, and group exercises, as well as respite care for unpaid caregivers. The survey found that 14% of ADSCs reported specializing in dementia.
The investigators also found that the percentage of ADSC participants with dementia, regardless of center specialization, was higher in the Midwest (32.1%), Northeast (28.5%), and South (24.5%) than in the West (21.1%).
The percentage of participants with dementia in specialized centers was higher in the Midwest (49.5%) and West (48.8%) than in the Northeast (31.9%) and in nonchain centers (50.5%) than in chain-affiliated centers (30.4%).
In addition, the percentage of participants with dementia, regardless of specialization, was higher in nonchain ADSCs (25%) than in chain-affiliated centers (20.1%). In addition, the percentage of participants with dementia in nonspecialized centers was higher in nonchain centers (25%) than in chain-affiliated centers (20.1%).
Finally, the research revealed that the percentage of participants with dementia, regardless of specialization, was higher in nonprofit ADSCs (28.7%) than for-profit centers (21%).
“These findings indicate that ADSCs in MSAs, nonprofit organizations, and nonchain centers provide services to a higher proportion of participants with dementia, particularly among centers that specialize in dementia care,” the investigators wrote.
Whereas “caregivers manage prescription medications, help with activities of daily living, and offer nutritional diets, exercise, and social engagement, ADSCs play a role in providing this type of care for people with dementia while also offering respite for their unpaid caregivers,” they noted.
Overall, they concluded that ADSCs provide positive outcomes for both family caregivers and people with dementia.
They noted that the study’s limitations include the use of cross-sectional data, which cannot show effectiveness for participants receiving care in specialized centers or be used to analyze relationships between other participant-level sociodemographic or health characteristics and specialized dementia care.
A version of this article appeared on Medscape.com.
About one-quarter of all adult day services center (ADSC) participants have dementia, and the prevalence of dementia in ADSCs that specialize in the disorder is more than 40%, a new US National Health Statistics Report revealed.
ADSCs are a growing sector of the US home- and community-based long-term care delivery system, providing daytime services to adults with disabilities who often have multiple chronic conditions, including various types of dementia, according to report authors Priyanka Singha, MPH, and colleagues at the US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics in Bethesda, Maryland.
Dementia often leads to the transition to receiving long-term care services, such as nursing home care. Delaying institutionalization is a primary goal of ADSCs, so they also try to meet the needs of a growing population of community-dwelling adults with dementia.
Survey responses from 1800 ADSCs across the United States showed that overall, 42.2% of participants had dementia in ADSCs specializing in dementia care, while 22.7% of participants in nonspecialized ADSCs also had dementia.
Dementia was more prevalent in the Midwest and West, where nearly one half of participants in specialized centers had dementia.
Nevertheless, the overall prevalence of dementia in ADSCs was similar across US regions, with a slightly lower percentage in the West.
Positive Outcomes
The new report used data from the ADSC component of the 2020 National Post-acute and Long-term Care Study collected from January 2020 through mid-July 2021. About 1800 ADSCs from a census of 5500 ADSCs were included and weighted to be nationally representative.
The authors compared dementia prevalence among participants in ADSCs that provide specialized care for dementia with other ADSCs by census region, metropolitan statistical area (MSA) status, chain affiliation, and ownership type.
MSA is a core urban area population of 50,000 or more. ADSCs that specialize in dementia care have specially trained staff, activities, and facilities. They offer social activities, including art and music therapy, dementia-appropriate games, and group exercises, as well as respite care for unpaid caregivers. The survey found that 14% of ADSCs reported specializing in dementia.
The investigators also found that the percentage of ADSC participants with dementia, regardless of center specialization, was higher in the Midwest (32.1%), Northeast (28.5%), and South (24.5%) than in the West (21.1%).
The percentage of participants with dementia in specialized centers was higher in the Midwest (49.5%) and West (48.8%) than in the Northeast (31.9%) and in nonchain centers (50.5%) than in chain-affiliated centers (30.4%).
In addition, the percentage of participants with dementia, regardless of specialization, was higher in nonchain ADSCs (25%) than in chain-affiliated centers (20.1%). In addition, the percentage of participants with dementia in nonspecialized centers was higher in nonchain centers (25%) than in chain-affiliated centers (20.1%).
Finally, the research revealed that the percentage of participants with dementia, regardless of specialization, was higher in nonprofit ADSCs (28.7%) than for-profit centers (21%).
“These findings indicate that ADSCs in MSAs, nonprofit organizations, and nonchain centers provide services to a higher proportion of participants with dementia, particularly among centers that specialize in dementia care,” the investigators wrote.
Whereas “caregivers manage prescription medications, help with activities of daily living, and offer nutritional diets, exercise, and social engagement, ADSCs play a role in providing this type of care for people with dementia while also offering respite for their unpaid caregivers,” they noted.
Overall, they concluded that ADSCs provide positive outcomes for both family caregivers and people with dementia.
They noted that the study’s limitations include the use of cross-sectional data, which cannot show effectiveness for participants receiving care in specialized centers or be used to analyze relationships between other participant-level sociodemographic or health characteristics and specialized dementia care.
A version of this article appeared on Medscape.com.
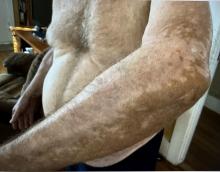
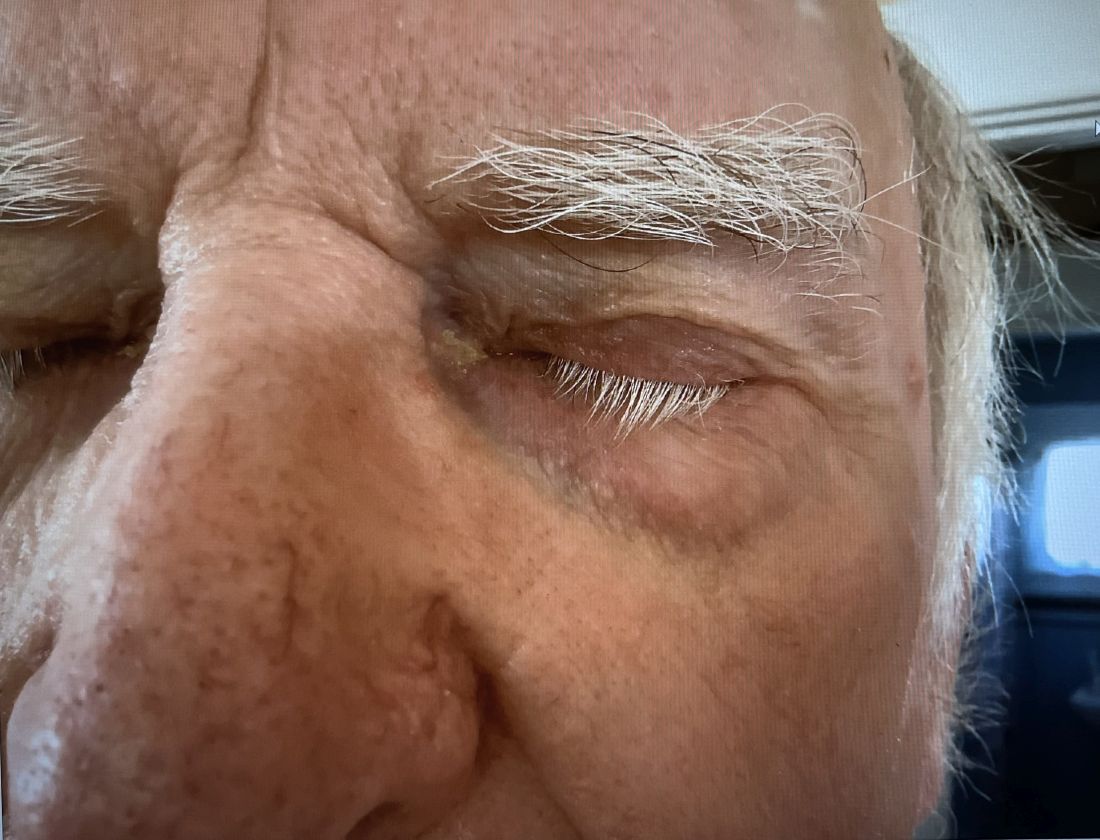
A 74-year-old White male presented with a 1-year history of depigmented patches on the hands, arms, and face, as well as white eyelashes and eyebrows
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
Switching From IV to Oral Antibiotics Safe for Patients, Study Shows
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
Higher Dose of Naloxone Has No Impact on Overdose Survival; Increases Withdrawal Symptoms
TOPLINE:
A new report from the US Centers for Disease Control and Prevention (CDC) showed that administering an 8 mg dose of intranasal naloxone does not increase the odds of surviving an opioid overdose; a higher dose than the usual 4 mg may result in a greater risk for onset of opioid withdrawal symptoms.
METHODOLOGY:
- The Morbidity and Mortality Weekly Report from the CDC presents data from a New York State Department of Health initiative.
- New York State Police troops administered either 8-mg or 4-mg doses of intranasal naloxone in response to suspected opiate overdose cases between March 2022 and August 2023.
- People who had died before the administration of the naloxone were excluded from the study.
- A total of 354 people were included in the study, 101 of whom received an 8-mg dose, while the others received the usual 4-mg dosage.
- Police officers documented the behavior and symptoms of people after receiving each dose, which could have included vomiting, disorientation, refusal to be transported to an emergency department, lethargy, and anger or combativeness.
TAKEAWAY:
- Survival rates were nearly identical regardless of intranasal naloxone dosage: 99% of people who received 8 mg compared with 99.2% of those who received 4 mg of the drug.
- Opioid withdrawal signs, including vomiting, were more prevalent among 8 mg naloxone recipients (37.6%) than among 4 mg recipients (19.4%) (risk ratio [RR], 2.51; P < .001).
- Police officers documented that people who received 8 mg were more frequently displayed anger or combativeness after revival than those who received the lower dose (RR, 1.42; P = .37).
IN PRACTICE:
The study “suggests that there are no benefits to law enforcement administration of higher-dose naloxone ... even in light of the increased prevalence of synthetic opioids, including fentanyl, in the drug supply.”
SOURCE:
Emily R. Payne, MSPH, of the New York State Department of Health, was the lead author of the study published in the Morbidity and Mortality Weekly Report on February 8, 2024.
LIMITATIONS:
The sample size of people receiving 8-mg doses was not equal to that of those receiving the usual dosage. Medical professionals did not report on the symptoms and behavior of people after receiving naloxone, law enforcement workers did, and may not have accurately captured what was occurring. In addition, researchers lacked complete data on the substances people used before an overdose, and the results may only be generalizable to New York State.
DISCLOSURES:
Study author Sharon Stancliff reported institutional support from the New York State Stewardship Funding Harm Reduction. No other potential conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.
TOPLINE:
A new report from the US Centers for Disease Control and Prevention (CDC) showed that administering an 8 mg dose of intranasal naloxone does not increase the odds of surviving an opioid overdose; a higher dose than the usual 4 mg may result in a greater risk for onset of opioid withdrawal symptoms.
METHODOLOGY:
- The Morbidity and Mortality Weekly Report from the CDC presents data from a New York State Department of Health initiative.
- New York State Police troops administered either 8-mg or 4-mg doses of intranasal naloxone in response to suspected opiate overdose cases between March 2022 and August 2023.
- People who had died before the administration of the naloxone were excluded from the study.
- A total of 354 people were included in the study, 101 of whom received an 8-mg dose, while the others received the usual 4-mg dosage.
- Police officers documented the behavior and symptoms of people after receiving each dose, which could have included vomiting, disorientation, refusal to be transported to an emergency department, lethargy, and anger or combativeness.
TAKEAWAY:
- Survival rates were nearly identical regardless of intranasal naloxone dosage: 99% of people who received 8 mg compared with 99.2% of those who received 4 mg of the drug.
- Opioid withdrawal signs, including vomiting, were more prevalent among 8 mg naloxone recipients (37.6%) than among 4 mg recipients (19.4%) (risk ratio [RR], 2.51; P < .001).
- Police officers documented that people who received 8 mg were more frequently displayed anger or combativeness after revival than those who received the lower dose (RR, 1.42; P = .37).
IN PRACTICE:
The study “suggests that there are no benefits to law enforcement administration of higher-dose naloxone ... even in light of the increased prevalence of synthetic opioids, including fentanyl, in the drug supply.”
SOURCE:
Emily R. Payne, MSPH, of the New York State Department of Health, was the lead author of the study published in the Morbidity and Mortality Weekly Report on February 8, 2024.
LIMITATIONS:
The sample size of people receiving 8-mg doses was not equal to that of those receiving the usual dosage. Medical professionals did not report on the symptoms and behavior of people after receiving naloxone, law enforcement workers did, and may not have accurately captured what was occurring. In addition, researchers lacked complete data on the substances people used before an overdose, and the results may only be generalizable to New York State.
DISCLOSURES:
Study author Sharon Stancliff reported institutional support from the New York State Stewardship Funding Harm Reduction. No other potential conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.
TOPLINE:
A new report from the US Centers for Disease Control and Prevention (CDC) showed that administering an 8 mg dose of intranasal naloxone does not increase the odds of surviving an opioid overdose; a higher dose than the usual 4 mg may result in a greater risk for onset of opioid withdrawal symptoms.
METHODOLOGY:
- The Morbidity and Mortality Weekly Report from the CDC presents data from a New York State Department of Health initiative.
- New York State Police troops administered either 8-mg or 4-mg doses of intranasal naloxone in response to suspected opiate overdose cases between March 2022 and August 2023.
- People who had died before the administration of the naloxone were excluded from the study.
- A total of 354 people were included in the study, 101 of whom received an 8-mg dose, while the others received the usual 4-mg dosage.
- Police officers documented the behavior and symptoms of people after receiving each dose, which could have included vomiting, disorientation, refusal to be transported to an emergency department, lethargy, and anger or combativeness.
TAKEAWAY:
- Survival rates were nearly identical regardless of intranasal naloxone dosage: 99% of people who received 8 mg compared with 99.2% of those who received 4 mg of the drug.
- Opioid withdrawal signs, including vomiting, were more prevalent among 8 mg naloxone recipients (37.6%) than among 4 mg recipients (19.4%) (risk ratio [RR], 2.51; P < .001).
- Police officers documented that people who received 8 mg were more frequently displayed anger or combativeness after revival than those who received the lower dose (RR, 1.42; P = .37).
IN PRACTICE:
The study “suggests that there are no benefits to law enforcement administration of higher-dose naloxone ... even in light of the increased prevalence of synthetic opioids, including fentanyl, in the drug supply.”
SOURCE:
Emily R. Payne, MSPH, of the New York State Department of Health, was the lead author of the study published in the Morbidity and Mortality Weekly Report on February 8, 2024.
LIMITATIONS:
The sample size of people receiving 8-mg doses was not equal to that of those receiving the usual dosage. Medical professionals did not report on the symptoms and behavior of people after receiving naloxone, law enforcement workers did, and may not have accurately captured what was occurring. In addition, researchers lacked complete data on the substances people used before an overdose, and the results may only be generalizable to New York State.
DISCLOSURES:
Study author Sharon Stancliff reported institutional support from the New York State Stewardship Funding Harm Reduction. No other potential conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.
When Babies ‘Stop Breathing,’ Who Needs Admission and a Workup?
Many infants have experienced an episode of apnea, defined as a pause in respiration of 20 seconds or more. Most episodes remain unexplained, and no underlying cause can be found. Historically, these were referred to as “near-miss SIDS,” episodes, but that label suggested that all of these events would have ended in death had someone not intervened. New descriptive terminology was needed.
In the mid-1980s, the term “apparent life-threatening event” (ALTE) was adopted. But that term, too, was an overstatement, because although scary for parents, these brief apnea episodes were not, in most cases, truly life-threatening.
In 2013, authors of a systematic review coined the term “brief resolved unexplained event” (BRUE). This review also addressed the history and physical exam features associated with risk for a subsequent episode. It was felt that hospitalization and testing might be warranted if certain infants could be identified as high risk for recurrence.
What Is Considered a BRUE?
In the current working definition of BRUE, the child must be < 1 year old. The episode must be a sudden, brief, and resolved, with one or more of these characteristics:
- Cyanosis or pallor (but not turning red)
- A change in breathing (absent, decreased, or irregular)
- A change in tone (hypertonia or hypotonia)
- A change in responsiveness.
Furthermore, to qualify as a BRUE, no explanation can be found for the event based on the history and physical examination but before any laboratory testing is done. The definition also excludes children with known potential explanatory diagnoses (such as gastroesophageal reflux or bronchiolitis) and those who are otherwise symptomatically ill at the time of the event.
Decision to Admit and Recurrence Risk
An apnea event in an otherwise healthy infant, regardless of what it’s called, puts providers and parents in a difficult position. Should the infant be hospitalized for further monitoring and potentially more invasive testing to determine the cause of the episode? And what are the chances that the episode will be repeated?
A clinical practice guideline (CPG) for BRUE, widely adopted in 2016, resulted in significant reductions in healthcare utilization. The CPG attempted to identify low-risk infants who could safely be discharged from the emergency department. Although the CPG improved outcomes, experts acknowledged that an underlying problem was not likely to be identified even among infants deemed high risk, and these infants would be hospitalized unnecessarily.
Available data were simply insufficient to support this decision. So, with the goal of identifying factors that could help predict recurrent BRUE risk, a 15-hospital collaborative study was undertaken, followed by the development and validation of a clinical decision rule for predicting the risk for a serious underlying diagnosis or event recurrence among infants presenting with BRUE.
Here’s what we learned from more than 3000 cases of BRUE.
First, it turns out that it’s not easy to determine whether an infant is at low or high risk for recurrence of BRUE. Initially, 91.5% of patients enrolled in the study would have been labeled high risk.
Furthermore, a BRUE recurred in 14.3% of the cohort, and 4.8% of high-risk infants were found to have a serious undiagnosed condition. Seizures, airway anomalies, and gastroesophageal reflux were the top three causes of BRUE, but the spectrum of underlying pathology was quite considerable.
The problem was that 4.6% of the entire cohort were found to have a serious underlying condition, nearly identical to the proportion of high-risk infants with these conditions. This prompted the question of whether simply labeling infants “high risk” was really appropriate any longer.
Revised BRUE Management
Although it hasn’t been possible to group infants neatly in low and high-risk categories, the data from that large cohort led to the development of the BRUE 2.0 criteria, which enabled more focused risk assessment of an infant who experienced a BRUE. With an app on MDCalc, these criteria allow providers to ascertain, and show families, a visual representation of their infant’s individualized risk for a subsequent BRUE and of having a serious underlying condition.
The cohort study also identified red flags from the history or physical exam of infants who experienced a BRUE: weight loss, failure to thrive, or a history of feeding problems. Exam findings such as a bulging fontanelle, forceful or bilious emesis, and evidence of gastrointestinal (GI) bleeding suggest a medical diagnosis rather than a BRUE. If GI-related causes are high on the differential, a feeding evaluation can be helpful. A feeding evaluation can be done in the outpatient setting and does not require hospitalization.
For suspicion of an underlying neurological condition (such as seizures), experts recommend obtaining a short EEG, which is highly sensitive for detecting infantile spasms and encephalopathy. They recommend reserving MRI for infants with abnormalities on EEG or physical exam. Metabolic or genetic testing should be done only if the infant looks ill, because most patients with genetic or inborn errors of metabolism will continue to have symptoms as they become older.
The approach to BRUE has moved into the realm of shared decision-making with families. The likelihood of identifying a serious diagnosis is low for most of these children. And unfortunately, no single test can diagnose the full spectrum of potential explanatory diagnoses. For example, data from 2023 demonstrate that only 1.1% of lab tests following a BRUE contributed to a diagnosis, and most of the time that was a positive viral test. Similarly, imaging was helpful in only 1.5% of cases. So, explaining the evidence and deciding along with parents what is reasonable to do (or not do) is the current state of affairs.
My Take
As I reflect back on two and a half decades of caring for these patients, I believe that recent data have helped us a great deal. We do less testing and admit fewer infants to the hospital than we did 20 years ago, and that’s a good thing. Nevertheless, looking for a few red flags, having a high index of suspicion when the clinical exam is abnormal, and engaging in shared decision-making with families can help make the caring for these challenging patients more bearable and lead to better outcomes for all involved.
Dr. Basco is Professor, Department of Pediatrics, Medical University of South Carolina (MUSC); Director, Division of General Pediatrics, Department of Pediatrics, MUSC Children’s Hospital, Charleston, South Carolina. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many infants have experienced an episode of apnea, defined as a pause in respiration of 20 seconds or more. Most episodes remain unexplained, and no underlying cause can be found. Historically, these were referred to as “near-miss SIDS,” episodes, but that label suggested that all of these events would have ended in death had someone not intervened. New descriptive terminology was needed.
In the mid-1980s, the term “apparent life-threatening event” (ALTE) was adopted. But that term, too, was an overstatement, because although scary for parents, these brief apnea episodes were not, in most cases, truly life-threatening.
In 2013, authors of a systematic review coined the term “brief resolved unexplained event” (BRUE). This review also addressed the history and physical exam features associated with risk for a subsequent episode. It was felt that hospitalization and testing might be warranted if certain infants could be identified as high risk for recurrence.
What Is Considered a BRUE?
In the current working definition of BRUE, the child must be < 1 year old. The episode must be a sudden, brief, and resolved, with one or more of these characteristics:
- Cyanosis or pallor (but not turning red)
- A change in breathing (absent, decreased, or irregular)
- A change in tone (hypertonia or hypotonia)
- A change in responsiveness.
Furthermore, to qualify as a BRUE, no explanation can be found for the event based on the history and physical examination but before any laboratory testing is done. The definition also excludes children with known potential explanatory diagnoses (such as gastroesophageal reflux or bronchiolitis) and those who are otherwise symptomatically ill at the time of the event.
Decision to Admit and Recurrence Risk
An apnea event in an otherwise healthy infant, regardless of what it’s called, puts providers and parents in a difficult position. Should the infant be hospitalized for further monitoring and potentially more invasive testing to determine the cause of the episode? And what are the chances that the episode will be repeated?
A clinical practice guideline (CPG) for BRUE, widely adopted in 2016, resulted in significant reductions in healthcare utilization. The CPG attempted to identify low-risk infants who could safely be discharged from the emergency department. Although the CPG improved outcomes, experts acknowledged that an underlying problem was not likely to be identified even among infants deemed high risk, and these infants would be hospitalized unnecessarily.
Available data were simply insufficient to support this decision. So, with the goal of identifying factors that could help predict recurrent BRUE risk, a 15-hospital collaborative study was undertaken, followed by the development and validation of a clinical decision rule for predicting the risk for a serious underlying diagnosis or event recurrence among infants presenting with BRUE.
Here’s what we learned from more than 3000 cases of BRUE.
First, it turns out that it’s not easy to determine whether an infant is at low or high risk for recurrence of BRUE. Initially, 91.5% of patients enrolled in the study would have been labeled high risk.
Furthermore, a BRUE recurred in 14.3% of the cohort, and 4.8% of high-risk infants were found to have a serious undiagnosed condition. Seizures, airway anomalies, and gastroesophageal reflux were the top three causes of BRUE, but the spectrum of underlying pathology was quite considerable.
The problem was that 4.6% of the entire cohort were found to have a serious underlying condition, nearly identical to the proportion of high-risk infants with these conditions. This prompted the question of whether simply labeling infants “high risk” was really appropriate any longer.
Revised BRUE Management
Although it hasn’t been possible to group infants neatly in low and high-risk categories, the data from that large cohort led to the development of the BRUE 2.0 criteria, which enabled more focused risk assessment of an infant who experienced a BRUE. With an app on MDCalc, these criteria allow providers to ascertain, and show families, a visual representation of their infant’s individualized risk for a subsequent BRUE and of having a serious underlying condition.
The cohort study also identified red flags from the history or physical exam of infants who experienced a BRUE: weight loss, failure to thrive, or a history of feeding problems. Exam findings such as a bulging fontanelle, forceful or bilious emesis, and evidence of gastrointestinal (GI) bleeding suggest a medical diagnosis rather than a BRUE. If GI-related causes are high on the differential, a feeding evaluation can be helpful. A feeding evaluation can be done in the outpatient setting and does not require hospitalization.
For suspicion of an underlying neurological condition (such as seizures), experts recommend obtaining a short EEG, which is highly sensitive for detecting infantile spasms and encephalopathy. They recommend reserving MRI for infants with abnormalities on EEG or physical exam. Metabolic or genetic testing should be done only if the infant looks ill, because most patients with genetic or inborn errors of metabolism will continue to have symptoms as they become older.
The approach to BRUE has moved into the realm of shared decision-making with families. The likelihood of identifying a serious diagnosis is low for most of these children. And unfortunately, no single test can diagnose the full spectrum of potential explanatory diagnoses. For example, data from 2023 demonstrate that only 1.1% of lab tests following a BRUE contributed to a diagnosis, and most of the time that was a positive viral test. Similarly, imaging was helpful in only 1.5% of cases. So, explaining the evidence and deciding along with parents what is reasonable to do (or not do) is the current state of affairs.
My Take
As I reflect back on two and a half decades of caring for these patients, I believe that recent data have helped us a great deal. We do less testing and admit fewer infants to the hospital than we did 20 years ago, and that’s a good thing. Nevertheless, looking for a few red flags, having a high index of suspicion when the clinical exam is abnormal, and engaging in shared decision-making with families can help make the caring for these challenging patients more bearable and lead to better outcomes for all involved.
Dr. Basco is Professor, Department of Pediatrics, Medical University of South Carolina (MUSC); Director, Division of General Pediatrics, Department of Pediatrics, MUSC Children’s Hospital, Charleston, South Carolina. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many infants have experienced an episode of apnea, defined as a pause in respiration of 20 seconds or more. Most episodes remain unexplained, and no underlying cause can be found. Historically, these were referred to as “near-miss SIDS,” episodes, but that label suggested that all of these events would have ended in death had someone not intervened. New descriptive terminology was needed.
In the mid-1980s, the term “apparent life-threatening event” (ALTE) was adopted. But that term, too, was an overstatement, because although scary for parents, these brief apnea episodes were not, in most cases, truly life-threatening.
In 2013, authors of a systematic review coined the term “brief resolved unexplained event” (BRUE). This review also addressed the history and physical exam features associated with risk for a subsequent episode. It was felt that hospitalization and testing might be warranted if certain infants could be identified as high risk for recurrence.
What Is Considered a BRUE?
In the current working definition of BRUE, the child must be < 1 year old. The episode must be a sudden, brief, and resolved, with one or more of these characteristics:
- Cyanosis or pallor (but not turning red)
- A change in breathing (absent, decreased, or irregular)
- A change in tone (hypertonia or hypotonia)
- A change in responsiveness.
Furthermore, to qualify as a BRUE, no explanation can be found for the event based on the history and physical examination but before any laboratory testing is done. The definition also excludes children with known potential explanatory diagnoses (such as gastroesophageal reflux or bronchiolitis) and those who are otherwise symptomatically ill at the time of the event.
Decision to Admit and Recurrence Risk
An apnea event in an otherwise healthy infant, regardless of what it’s called, puts providers and parents in a difficult position. Should the infant be hospitalized for further monitoring and potentially more invasive testing to determine the cause of the episode? And what are the chances that the episode will be repeated?
A clinical practice guideline (CPG) for BRUE, widely adopted in 2016, resulted in significant reductions in healthcare utilization. The CPG attempted to identify low-risk infants who could safely be discharged from the emergency department. Although the CPG improved outcomes, experts acknowledged that an underlying problem was not likely to be identified even among infants deemed high risk, and these infants would be hospitalized unnecessarily.
Available data were simply insufficient to support this decision. So, with the goal of identifying factors that could help predict recurrent BRUE risk, a 15-hospital collaborative study was undertaken, followed by the development and validation of a clinical decision rule for predicting the risk for a serious underlying diagnosis or event recurrence among infants presenting with BRUE.
Here’s what we learned from more than 3000 cases of BRUE.
First, it turns out that it’s not easy to determine whether an infant is at low or high risk for recurrence of BRUE. Initially, 91.5% of patients enrolled in the study would have been labeled high risk.
Furthermore, a BRUE recurred in 14.3% of the cohort, and 4.8% of high-risk infants were found to have a serious undiagnosed condition. Seizures, airway anomalies, and gastroesophageal reflux were the top three causes of BRUE, but the spectrum of underlying pathology was quite considerable.
The problem was that 4.6% of the entire cohort were found to have a serious underlying condition, nearly identical to the proportion of high-risk infants with these conditions. This prompted the question of whether simply labeling infants “high risk” was really appropriate any longer.
Revised BRUE Management
Although it hasn’t been possible to group infants neatly in low and high-risk categories, the data from that large cohort led to the development of the BRUE 2.0 criteria, which enabled more focused risk assessment of an infant who experienced a BRUE. With an app on MDCalc, these criteria allow providers to ascertain, and show families, a visual representation of their infant’s individualized risk for a subsequent BRUE and of having a serious underlying condition.
The cohort study also identified red flags from the history or physical exam of infants who experienced a BRUE: weight loss, failure to thrive, or a history of feeding problems. Exam findings such as a bulging fontanelle, forceful or bilious emesis, and evidence of gastrointestinal (GI) bleeding suggest a medical diagnosis rather than a BRUE. If GI-related causes are high on the differential, a feeding evaluation can be helpful. A feeding evaluation can be done in the outpatient setting and does not require hospitalization.
For suspicion of an underlying neurological condition (such as seizures), experts recommend obtaining a short EEG, which is highly sensitive for detecting infantile spasms and encephalopathy. They recommend reserving MRI for infants with abnormalities on EEG or physical exam. Metabolic or genetic testing should be done only if the infant looks ill, because most patients with genetic or inborn errors of metabolism will continue to have symptoms as they become older.
The approach to BRUE has moved into the realm of shared decision-making with families. The likelihood of identifying a serious diagnosis is low for most of these children. And unfortunately, no single test can diagnose the full spectrum of potential explanatory diagnoses. For example, data from 2023 demonstrate that only 1.1% of lab tests following a BRUE contributed to a diagnosis, and most of the time that was a positive viral test. Similarly, imaging was helpful in only 1.5% of cases. So, explaining the evidence and deciding along with parents what is reasonable to do (or not do) is the current state of affairs.
My Take
As I reflect back on two and a half decades of caring for these patients, I believe that recent data have helped us a great deal. We do less testing and admit fewer infants to the hospital than we did 20 years ago, and that’s a good thing. Nevertheless, looking for a few red flags, having a high index of suspicion when the clinical exam is abnormal, and engaging in shared decision-making with families can help make the caring for these challenging patients more bearable and lead to better outcomes for all involved.
Dr. Basco is Professor, Department of Pediatrics, Medical University of South Carolina (MUSC); Director, Division of General Pediatrics, Department of Pediatrics, MUSC Children’s Hospital, Charleston, South Carolina. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tirofiban Reduces Early Neurologic Deterioration After Stroke
Intravenous (IV) administration of the antiplatelet agent tirofiban for 72 hours was associated with a reduction in early neurologic deterioration compared with oral aspirin therapy in patients with acute ischemic stroke, in the randomized TREND trial.
The results were presented at the International Stroke Conference 2024, held on February 7-9 in Phoenix, Arizona.
Lead author Zhao Wenbo, MD, Xuanwu Hospital, Beijing, China, noted that neurologic deterioration, characterized by a sudden onset and quick peak of neurologic deficits, is a common phenomenon in acute ischemic stroke and is strongly associated with poor clinical outcomes.
Ischemic stroke progression is the main cause of neurologic deterioration, especially during the first few days after onset, Dr. Wenbo said. , but administering oral antiplatelet agents can be difficult because of dysphagia, he reported.
The TREND trial was conducted to investigate whether IV tirofiban could prevent early neurologic deterioration without increasing the risk for symptomatic intracerebral hemorrhage in acute ischemic stroke.
The study included 426 patients with acute ischemic stroke within 24 hours of symptom onset who had a neurologic deficit attributed to focal cerebral ischemia and a National Institutes of Health Stroke Scale (NIHSS) score between 4 and 20 points and who were not treated with thrombolysis or endovascular thrombectomy. Patients with cardioembolic stroke were also excluded.
Patients were a median of 10-12 hours from symptom onset and had a baseline NIHSS score of 5.
They were randomized to IV tirofiban or oral aspirin for 72 hours. All patients were then continued on oral antiplatelet therapy.
The primary efficacy outcome was neurologic deterioration within 72 hours after randomization, defined as an increase in NIHSS score of 4 points or more.
This occurred in nine patients (4.2%) in the tirofiban group vs 28 (13.2%) in the control group (relative risk, 0.32; 95% CI, 0.15-0.66; P = .002).
A consistent benefit of IV tirofiban was seen across all subgroups.
The secondary endpoint of neurologic deterioration within 72 hours after randomization, defined as an increase of NIHSS score of 2 points or more, was also significantly reduced. This occurred in 11.7% of the tirofiban group vs 23.6% of the aspirin group (RR, 0.49; 95% CI, 0.32-0.75; P = .001).
An excellent outcome on the modified Rankin Scale (mRS) disability score (mRS, 0-1) at 90 days was seen in 75% of tirofiban vs 68% of aspirin patients, a nonsignificant difference.
A good outcome (mRS, 0-2) occurred in 89% of tirofiban vs 86% of aspirin patients, again a nonsignificant difference.
There were no symptomatic intracerebral hemorrhages within 72 hours after randomization (the primary safety endpoint) in either group, and the incidence of systemic bleeding also did not differ significantly between the groups.
Dr. Wenbo concluded that further randomized clinical trials are needed to determine the efficacy of tirofiban on functional outcomes.
‘Promising Results’
Commenting on the study for this news organization, conference chair, Tudor Jovin, MD, Cooper Medical School of Rowan University, Camden, New Jersey, and vice-chair, Lauren Sansing, MD, Yale School of Medicine, New Haven, Connecticut, both said they thought the results were promising.
“This study didn’t show any long-term outcome benefit, but this was a smaller study, and the results need to be replicated in a larger study with sufficient power to look at longer-term outcomes,” Sansing noted. “But we don’t have anything better than aspirin at present for these patients, so it’s exciting that there may be something in the pipeline for this group.”
Dr. Jovin pointed out that the TREND trial selected patients on the cause of their stroke, in line with the practice of precision medicine.
“By excluding patients who received thrombolysis or thrombectomy and those who had cardioembolic strokes, we are left with a population who we don’t have many treatment options for,” he said. “These are patients with smaller or moderate strokes who may arrive too late for thrombolysis. It would be great to be able to do something more than just aspirin for these patients.”
Dr. Jovin noted that the study was underpowered to show long-term benefits, but there were some promising trends.
“It stands to reason that if neurologic function does not get worse in the early hours and days after stroke, then the long-term outcomes are likely to be better,” he noted. “But this needs to be confirmed in larger trials.”
Interestingly, another study, the MOST trial, also presented at the ISC-24 meeting, showed no benefit with the IV antithrombotic agents argatroban or eptifibatide on 90-day functional outcomes when added to thrombolysis in acute ischemic stroke.
Dr. Jovin pointed out that the MOST and TREND trials included different populations of patients — the MOST patients received thrombolysis, while the TREND patients did not. And in the MOST trial, about half the patients had a large vessel occlusion and underwent thrombectomy, whereas these patients were excluded in TREND.
Dr. Sansing added that patients in the TREND trial may have had small vessel disease or other atherosclerotic disease, or strokes due to the narrowing of vessels or due to an unknown cause. They were also given 3 days of IV tirofiban, whereas the duration of antithrombotic treatment in MOST was shorter.
The TREND study was funded by the National Key Research and Development Program of China, the National Science Foundation of Beijing Municipality, and the Beijing Municipal Science and Technology Commission.
A version of this article appeared on Medscape.com.
Intravenous (IV) administration of the antiplatelet agent tirofiban for 72 hours was associated with a reduction in early neurologic deterioration compared with oral aspirin therapy in patients with acute ischemic stroke, in the randomized TREND trial.
The results were presented at the International Stroke Conference 2024, held on February 7-9 in Phoenix, Arizona.
Lead author Zhao Wenbo, MD, Xuanwu Hospital, Beijing, China, noted that neurologic deterioration, characterized by a sudden onset and quick peak of neurologic deficits, is a common phenomenon in acute ischemic stroke and is strongly associated with poor clinical outcomes.
Ischemic stroke progression is the main cause of neurologic deterioration, especially during the first few days after onset, Dr. Wenbo said. , but administering oral antiplatelet agents can be difficult because of dysphagia, he reported.
The TREND trial was conducted to investigate whether IV tirofiban could prevent early neurologic deterioration without increasing the risk for symptomatic intracerebral hemorrhage in acute ischemic stroke.
The study included 426 patients with acute ischemic stroke within 24 hours of symptom onset who had a neurologic deficit attributed to focal cerebral ischemia and a National Institutes of Health Stroke Scale (NIHSS) score between 4 and 20 points and who were not treated with thrombolysis or endovascular thrombectomy. Patients with cardioembolic stroke were also excluded.
Patients were a median of 10-12 hours from symptom onset and had a baseline NIHSS score of 5.
They were randomized to IV tirofiban or oral aspirin for 72 hours. All patients were then continued on oral antiplatelet therapy.
The primary efficacy outcome was neurologic deterioration within 72 hours after randomization, defined as an increase in NIHSS score of 4 points or more.
This occurred in nine patients (4.2%) in the tirofiban group vs 28 (13.2%) in the control group (relative risk, 0.32; 95% CI, 0.15-0.66; P = .002).
A consistent benefit of IV tirofiban was seen across all subgroups.
The secondary endpoint of neurologic deterioration within 72 hours after randomization, defined as an increase of NIHSS score of 2 points or more, was also significantly reduced. This occurred in 11.7% of the tirofiban group vs 23.6% of the aspirin group (RR, 0.49; 95% CI, 0.32-0.75; P = .001).
An excellent outcome on the modified Rankin Scale (mRS) disability score (mRS, 0-1) at 90 days was seen in 75% of tirofiban vs 68% of aspirin patients, a nonsignificant difference.
A good outcome (mRS, 0-2) occurred in 89% of tirofiban vs 86% of aspirin patients, again a nonsignificant difference.
There were no symptomatic intracerebral hemorrhages within 72 hours after randomization (the primary safety endpoint) in either group, and the incidence of systemic bleeding also did not differ significantly between the groups.
Dr. Wenbo concluded that further randomized clinical trials are needed to determine the efficacy of tirofiban on functional outcomes.
‘Promising Results’
Commenting on the study for this news organization, conference chair, Tudor Jovin, MD, Cooper Medical School of Rowan University, Camden, New Jersey, and vice-chair, Lauren Sansing, MD, Yale School of Medicine, New Haven, Connecticut, both said they thought the results were promising.
“This study didn’t show any long-term outcome benefit, but this was a smaller study, and the results need to be replicated in a larger study with sufficient power to look at longer-term outcomes,” Sansing noted. “But we don’t have anything better than aspirin at present for these patients, so it’s exciting that there may be something in the pipeline for this group.”
Dr. Jovin pointed out that the TREND trial selected patients on the cause of their stroke, in line with the practice of precision medicine.
“By excluding patients who received thrombolysis or thrombectomy and those who had cardioembolic strokes, we are left with a population who we don’t have many treatment options for,” he said. “These are patients with smaller or moderate strokes who may arrive too late for thrombolysis. It would be great to be able to do something more than just aspirin for these patients.”
Dr. Jovin noted that the study was underpowered to show long-term benefits, but there were some promising trends.
“It stands to reason that if neurologic function does not get worse in the early hours and days after stroke, then the long-term outcomes are likely to be better,” he noted. “But this needs to be confirmed in larger trials.”
Interestingly, another study, the MOST trial, also presented at the ISC-24 meeting, showed no benefit with the IV antithrombotic agents argatroban or eptifibatide on 90-day functional outcomes when added to thrombolysis in acute ischemic stroke.
Dr. Jovin pointed out that the MOST and TREND trials included different populations of patients — the MOST patients received thrombolysis, while the TREND patients did not. And in the MOST trial, about half the patients had a large vessel occlusion and underwent thrombectomy, whereas these patients were excluded in TREND.
Dr. Sansing added that patients in the TREND trial may have had small vessel disease or other atherosclerotic disease, or strokes due to the narrowing of vessels or due to an unknown cause. They were also given 3 days of IV tirofiban, whereas the duration of antithrombotic treatment in MOST was shorter.
The TREND study was funded by the National Key Research and Development Program of China, the National Science Foundation of Beijing Municipality, and the Beijing Municipal Science and Technology Commission.
A version of this article appeared on Medscape.com.
Intravenous (IV) administration of the antiplatelet agent tirofiban for 72 hours was associated with a reduction in early neurologic deterioration compared with oral aspirin therapy in patients with acute ischemic stroke, in the randomized TREND trial.
The results were presented at the International Stroke Conference 2024, held on February 7-9 in Phoenix, Arizona.
Lead author Zhao Wenbo, MD, Xuanwu Hospital, Beijing, China, noted that neurologic deterioration, characterized by a sudden onset and quick peak of neurologic deficits, is a common phenomenon in acute ischemic stroke and is strongly associated with poor clinical outcomes.
Ischemic stroke progression is the main cause of neurologic deterioration, especially during the first few days after onset, Dr. Wenbo said. , but administering oral antiplatelet agents can be difficult because of dysphagia, he reported.
The TREND trial was conducted to investigate whether IV tirofiban could prevent early neurologic deterioration without increasing the risk for symptomatic intracerebral hemorrhage in acute ischemic stroke.
The study included 426 patients with acute ischemic stroke within 24 hours of symptom onset who had a neurologic deficit attributed to focal cerebral ischemia and a National Institutes of Health Stroke Scale (NIHSS) score between 4 and 20 points and who were not treated with thrombolysis or endovascular thrombectomy. Patients with cardioembolic stroke were also excluded.
Patients were a median of 10-12 hours from symptom onset and had a baseline NIHSS score of 5.
They were randomized to IV tirofiban or oral aspirin for 72 hours. All patients were then continued on oral antiplatelet therapy.
The primary efficacy outcome was neurologic deterioration within 72 hours after randomization, defined as an increase in NIHSS score of 4 points or more.
This occurred in nine patients (4.2%) in the tirofiban group vs 28 (13.2%) in the control group (relative risk, 0.32; 95% CI, 0.15-0.66; P = .002).
A consistent benefit of IV tirofiban was seen across all subgroups.
The secondary endpoint of neurologic deterioration within 72 hours after randomization, defined as an increase of NIHSS score of 2 points or more, was also significantly reduced. This occurred in 11.7% of the tirofiban group vs 23.6% of the aspirin group (RR, 0.49; 95% CI, 0.32-0.75; P = .001).
An excellent outcome on the modified Rankin Scale (mRS) disability score (mRS, 0-1) at 90 days was seen in 75% of tirofiban vs 68% of aspirin patients, a nonsignificant difference.
A good outcome (mRS, 0-2) occurred in 89% of tirofiban vs 86% of aspirin patients, again a nonsignificant difference.
There were no symptomatic intracerebral hemorrhages within 72 hours after randomization (the primary safety endpoint) in either group, and the incidence of systemic bleeding also did not differ significantly between the groups.
Dr. Wenbo concluded that further randomized clinical trials are needed to determine the efficacy of tirofiban on functional outcomes.
‘Promising Results’
Commenting on the study for this news organization, conference chair, Tudor Jovin, MD, Cooper Medical School of Rowan University, Camden, New Jersey, and vice-chair, Lauren Sansing, MD, Yale School of Medicine, New Haven, Connecticut, both said they thought the results were promising.
“This study didn’t show any long-term outcome benefit, but this was a smaller study, and the results need to be replicated in a larger study with sufficient power to look at longer-term outcomes,” Sansing noted. “But we don’t have anything better than aspirin at present for these patients, so it’s exciting that there may be something in the pipeline for this group.”
Dr. Jovin pointed out that the TREND trial selected patients on the cause of their stroke, in line with the practice of precision medicine.
“By excluding patients who received thrombolysis or thrombectomy and those who had cardioembolic strokes, we are left with a population who we don’t have many treatment options for,” he said. “These are patients with smaller or moderate strokes who may arrive too late for thrombolysis. It would be great to be able to do something more than just aspirin for these patients.”
Dr. Jovin noted that the study was underpowered to show long-term benefits, but there were some promising trends.
“It stands to reason that if neurologic function does not get worse in the early hours and days after stroke, then the long-term outcomes are likely to be better,” he noted. “But this needs to be confirmed in larger trials.”
Interestingly, another study, the MOST trial, also presented at the ISC-24 meeting, showed no benefit with the IV antithrombotic agents argatroban or eptifibatide on 90-day functional outcomes when added to thrombolysis in acute ischemic stroke.
Dr. Jovin pointed out that the MOST and TREND trials included different populations of patients — the MOST patients received thrombolysis, while the TREND patients did not. And in the MOST trial, about half the patients had a large vessel occlusion and underwent thrombectomy, whereas these patients were excluded in TREND.
Dr. Sansing added that patients in the TREND trial may have had small vessel disease or other atherosclerotic disease, or strokes due to the narrowing of vessels or due to an unknown cause. They were also given 3 days of IV tirofiban, whereas the duration of antithrombotic treatment in MOST was shorter.
The TREND study was funded by the National Key Research and Development Program of China, the National Science Foundation of Beijing Municipality, and the Beijing Municipal Science and Technology Commission.
A version of this article appeared on Medscape.com.
Study Suggests Mind-Body Benefits of GLP-1s
, according to an analysis of millions of people’s health records.
The findings were published this week by researchers from the electronic health record company Epic. Researchers looked for new diagnoses of depression or anxiety among people who started taking drugs from a class called GLP-1 agonists that can help manage blood sugar or treat obesity by mimicking hormone levels in the body that can affect appetite and blood sugar. Many people who take the drugs experience significant weight loss.
The researchers found that people with diabetes who started taking most versions of GLP-1 agonists were between 11% and 65% less likely to be newly diagnosed with depression than people with diabetes who didn’t take one of the drugs. The greatest reduction in likelihood of a new depression diagnosis was observed among people taking tirzepatide, which is sold under the brand names Mounjaro and Zepbound.
A reduced likelihood of being diagnosed with anxiety was also observed among people with diabetes after they started taking a GLP-1 agonist, compared to people with diabetes who didn’t take one of the drugs. Again, tirzepatide showed the greatest reduction in odds, with people taking that drug experiencing a 60% reduced likelihood of being newly diagnosed with anxiety.
Similar reductions in the likelihood of new depression or anxiety diagnoses were observed among people who didn’t have diabetes but were taking GLP-1 agonists, such as for weight loss.
The mind-body connection has been well established by research.
“Thoughts, feelings, beliefs, and attitudes can affect how healthy your body is,” according to a summary from the CDC about the connection between diabetes and depression. “Untreated mental health issues can make diabetes worse, and problems with diabetes can make mental health issues worse. But fortunately if one gets better, the other tends to get better, too.”
This latest analysis included the drugs dulaglutide, exenatide, liraglutide, semaglutide, and tirzepatide. The medicines, used for weight loss or to manage diabetes, include the brand names Byetta, Ozempic, Mounjaro, Trulicity, Wegovy, and Zepbound. The researchers also looked for links between depression or anxiety diagnoses among people taking liraglutide (sold under brand names Saxenda and Victoza), but found that there was little to no change in the likelihood of being diagnosed with depression or anxiety after starting liraglutide.
The findings are timely as regulators in the U.S. and Europe are investigating reports of suicidal thoughts among people using the drugs. In January, the FDA announced that a preliminary investigation showed no increased risk of suicidal thoughts or actions, but the agency could not “definitively rule out that a small risk may exist; therefore, FDA is continuing to look into this issue.”
This latest analysis from Epic Research only looked at health records, was not published in a peer-reviewed journal, nor could it establish a definitive role the medications may have played in whether or not someone was diagnosed with depression or anxiety. It’s unknown whether people in the study had symptoms of depression or anxiety before starting the medications.
“These results show that these medications may serve a dual purpose for patients, but we do not understand them well enough yet to say these medications should be given as a treatment for anxiety or depression outside of diabetes or weight management,” Kersten Bartelt, a researcher employed by Epic, told ABC News.
A version of this article appeared on WebMD.com.
, according to an analysis of millions of people’s health records.
The findings were published this week by researchers from the electronic health record company Epic. Researchers looked for new diagnoses of depression or anxiety among people who started taking drugs from a class called GLP-1 agonists that can help manage blood sugar or treat obesity by mimicking hormone levels in the body that can affect appetite and blood sugar. Many people who take the drugs experience significant weight loss.
The researchers found that people with diabetes who started taking most versions of GLP-1 agonists were between 11% and 65% less likely to be newly diagnosed with depression than people with diabetes who didn’t take one of the drugs. The greatest reduction in likelihood of a new depression diagnosis was observed among people taking tirzepatide, which is sold under the brand names Mounjaro and Zepbound.
A reduced likelihood of being diagnosed with anxiety was also observed among people with diabetes after they started taking a GLP-1 agonist, compared to people with diabetes who didn’t take one of the drugs. Again, tirzepatide showed the greatest reduction in odds, with people taking that drug experiencing a 60% reduced likelihood of being newly diagnosed with anxiety.
Similar reductions in the likelihood of new depression or anxiety diagnoses were observed among people who didn’t have diabetes but were taking GLP-1 agonists, such as for weight loss.
The mind-body connection has been well established by research.
“Thoughts, feelings, beliefs, and attitudes can affect how healthy your body is,” according to a summary from the CDC about the connection between diabetes and depression. “Untreated mental health issues can make diabetes worse, and problems with diabetes can make mental health issues worse. But fortunately if one gets better, the other tends to get better, too.”
This latest analysis included the drugs dulaglutide, exenatide, liraglutide, semaglutide, and tirzepatide. The medicines, used for weight loss or to manage diabetes, include the brand names Byetta, Ozempic, Mounjaro, Trulicity, Wegovy, and Zepbound. The researchers also looked for links between depression or anxiety diagnoses among people taking liraglutide (sold under brand names Saxenda and Victoza), but found that there was little to no change in the likelihood of being diagnosed with depression or anxiety after starting liraglutide.
The findings are timely as regulators in the U.S. and Europe are investigating reports of suicidal thoughts among people using the drugs. In January, the FDA announced that a preliminary investigation showed no increased risk of suicidal thoughts or actions, but the agency could not “definitively rule out that a small risk may exist; therefore, FDA is continuing to look into this issue.”
This latest analysis from Epic Research only looked at health records, was not published in a peer-reviewed journal, nor could it establish a definitive role the medications may have played in whether or not someone was diagnosed with depression or anxiety. It’s unknown whether people in the study had symptoms of depression or anxiety before starting the medications.
“These results show that these medications may serve a dual purpose for patients, but we do not understand them well enough yet to say these medications should be given as a treatment for anxiety or depression outside of diabetes or weight management,” Kersten Bartelt, a researcher employed by Epic, told ABC News.
A version of this article appeared on WebMD.com.
, according to an analysis of millions of people’s health records.
The findings were published this week by researchers from the electronic health record company Epic. Researchers looked for new diagnoses of depression or anxiety among people who started taking drugs from a class called GLP-1 agonists that can help manage blood sugar or treat obesity by mimicking hormone levels in the body that can affect appetite and blood sugar. Many people who take the drugs experience significant weight loss.
The researchers found that people with diabetes who started taking most versions of GLP-1 agonists were between 11% and 65% less likely to be newly diagnosed with depression than people with diabetes who didn’t take one of the drugs. The greatest reduction in likelihood of a new depression diagnosis was observed among people taking tirzepatide, which is sold under the brand names Mounjaro and Zepbound.
A reduced likelihood of being diagnosed with anxiety was also observed among people with diabetes after they started taking a GLP-1 agonist, compared to people with diabetes who didn’t take one of the drugs. Again, tirzepatide showed the greatest reduction in odds, with people taking that drug experiencing a 60% reduced likelihood of being newly diagnosed with anxiety.
Similar reductions in the likelihood of new depression or anxiety diagnoses were observed among people who didn’t have diabetes but were taking GLP-1 agonists, such as for weight loss.
The mind-body connection has been well established by research.
“Thoughts, feelings, beliefs, and attitudes can affect how healthy your body is,” according to a summary from the CDC about the connection between diabetes and depression. “Untreated mental health issues can make diabetes worse, and problems with diabetes can make mental health issues worse. But fortunately if one gets better, the other tends to get better, too.”
This latest analysis included the drugs dulaglutide, exenatide, liraglutide, semaglutide, and tirzepatide. The medicines, used for weight loss or to manage diabetes, include the brand names Byetta, Ozempic, Mounjaro, Trulicity, Wegovy, and Zepbound. The researchers also looked for links between depression or anxiety diagnoses among people taking liraglutide (sold under brand names Saxenda and Victoza), but found that there was little to no change in the likelihood of being diagnosed with depression or anxiety after starting liraglutide.
The findings are timely as regulators in the U.S. and Europe are investigating reports of suicidal thoughts among people using the drugs. In January, the FDA announced that a preliminary investigation showed no increased risk of suicidal thoughts or actions, but the agency could not “definitively rule out that a small risk may exist; therefore, FDA is continuing to look into this issue.”
This latest analysis from Epic Research only looked at health records, was not published in a peer-reviewed journal, nor could it establish a definitive role the medications may have played in whether or not someone was diagnosed with depression or anxiety. It’s unknown whether people in the study had symptoms of depression or anxiety before starting the medications.
“These results show that these medications may serve a dual purpose for patients, but we do not understand them well enough yet to say these medications should be given as a treatment for anxiety or depression outside of diabetes or weight management,” Kersten Bartelt, a researcher employed by Epic, told ABC News.
A version of this article appeared on WebMD.com.