User login
What Is the Long-Term Mortality Risk for Men With HR+ Breast Cancer?
Breast cancer-specific mortality risk in men with hormone receptor–positive breast cancer persists for at least 20 years, but patterns of breast cancer–specific mortality (BCSM) are distinct from those in women, a new study finds.
Previous studies in women with hormone receptor–positive (HR+) breast cancer have shown a risk of distant recurrence and death for at least 20 years after diagnosis, but data for men are limited, wrote Julieta Leone, MD, of the Dana Farber Cancer Institute, Boston, and colleagues.
What is Known About Hormone Receptor–Positive Breast Cancer Mortality in Men vs. Women?
Invasive breast cancer in men is rare and consequently understudied, the researchers wrote. Previous studies of BCSM in men with more than 5 years’ follow-up consist mainly of case series at single institutions, the researchers wrote in JAMA Oncology (2024 Feb 29. doi: 10.1001/jamaoncol.2023.7194).
“We believed it would be important to study this issue to help inform the management of men with breast cancer,” corresponding author, José P. Leone, MD, said in an interview.
In 2021, Dr. J.P. Leone and colleagues published a study in Breast Cancer Research and Treatment that examined the 20-year risk of BCSM in women that included more than 36,000 individuals who had survived for 5 years, with a median of 14 years’ follow-up.
In that study of women, the BCSM risk at 20 years was significantly higher for those with HR-negative tumors, but the risk was still elevated for both types. Patients with stage IIIC HR-positive disease had four times the risk of BCSM over 20 years and those with stage IIIC HR-negative disease had seven times the risk of BCSM over 20 years compared with the risk of death not related to breast cancer, the researchers wrote.
Another study of nearly 2,400 men with breast cancer (mainly HR+) by Dr. J.P. Leone and colleagues showed that cancer stage, tumor subtype, and race were associated with overall survival and breast cancer-specific survival.
What Does the New Study Add?
The current study included 2,836 men diagnosed with stage I to stage III HR+ breast cancer between 1990 and 2008, using data from the Surveillance, Epidemiology, and End Results (SEER) database.
“We found that in men with breast cancer, the risk of breast cancer mortality persists for at least 20 years and that [the risk] depends on traditional clinicopathologic factors, such as age, tumor size, nodal status and tumor grade,” Dr. J.P. Leone said in an interview.
“The prolonged risk [of breast-cancer specific mortality] over 20 years that we observed in our study is similar to that previously reported in women; however, the kinetics of the risk over the 20-year period appears different in men,” he emphasized.
The men in the study, especially those with a higher stage of disease, appeared to have a bimodal distribution of the BRCA mortality risk, he said.
Two peaks were identified, he explained; an early peak in mortality risk at approximately 4 years from diagnosis and another at approximately 11 years after diagnosis.
Although women with breast cancer had a prolonged risk of breast cancer mortality, “the kinetics of the risk does not include 2 peaks, even in women with higher stage of disease.” In women with higher stage breast cancer, the peak mortality risk occurs approximately 5 years after diagnosis, he said.
The reasons for the later peak in men remain unclear, the researchers wrote in the study, but possible explanations include nonadherence to endocrine therapy, differences in tumor biologic factors, and differences in the tumor microenvironment between men and women, they noted, in the discussion section.
What Drives the Risk?
Key factors for breast cancer-specific mortality were age, tumor stage, and tumor grade.
The cumulative 20-year risk of BCSM in the current study was 12.4%, 26.2%, and 46.0% for stage I, II, and III, respectively. The adjusted BCSM risk was increased in patients younger than 50 years, those with grade II or III/IV tumors, and those with stage II or III disease.
What Are the Limitations?
The current study by Leone and colleagues was limited by the relatively small subgroup sample of men with stage III and N3 disease, lack of data on the use of systemic therapies, and lack of data on human epidermal growth factor receptor 2 gene (ERBB2), the researchers wrote. However, the long-term follow-up strengthened the results, and the study is the first known to assess 20-year BCSM risk in men with nonmetastatic HR+ breast cancer.
What Do Oncologists Need to Know About the Study?
The study findings indicate that the risk of breast cancer mortality persists for 20 years in men with hormone receptor–positive breast cancer, Dr. J.P. Leone said in an interview. As in women, the risk depends on traditional clinicopathologic factors, he noted.
“However, the kinetics of that risk appears to be different between men and women. In order to reduce the breast cancer mortality risk in men with hormone receptor–positive breast cancer, it will be important for men to consider the benefits of the treatment options that may be indicated for their specific situation,” he said.
“I think early detection is also very important,” he emphasized. To that end, increased awareness of the possibility for breast cancer in men, as well as prompt intervention when breast cancer is suspected, will help to improve early detection when the risk of breast cancer mortality is lower, he added.
What Are the Next Steps for Research?
“I think our study underscores the need for additional research to improve our adjuvant therapy options in both men and women with hormone receptor-positive breast cancer, to reduce the risk of long-term mortality,” he said.
The study received no outside funding. Lead author Julieta Leone had no financial conflicts to disclose. Dr. José P. Leone disclosed receiving institutional grants from Kazia Therapeutics and Seagen unrelated to the current study.
Breast cancer-specific mortality risk in men with hormone receptor–positive breast cancer persists for at least 20 years, but patterns of breast cancer–specific mortality (BCSM) are distinct from those in women, a new study finds.
Previous studies in women with hormone receptor–positive (HR+) breast cancer have shown a risk of distant recurrence and death for at least 20 years after diagnosis, but data for men are limited, wrote Julieta Leone, MD, of the Dana Farber Cancer Institute, Boston, and colleagues.
What is Known About Hormone Receptor–Positive Breast Cancer Mortality in Men vs. Women?
Invasive breast cancer in men is rare and consequently understudied, the researchers wrote. Previous studies of BCSM in men with more than 5 years’ follow-up consist mainly of case series at single institutions, the researchers wrote in JAMA Oncology (2024 Feb 29. doi: 10.1001/jamaoncol.2023.7194).
“We believed it would be important to study this issue to help inform the management of men with breast cancer,” corresponding author, José P. Leone, MD, said in an interview.
In 2021, Dr. J.P. Leone and colleagues published a study in Breast Cancer Research and Treatment that examined the 20-year risk of BCSM in women that included more than 36,000 individuals who had survived for 5 years, with a median of 14 years’ follow-up.
In that study of women, the BCSM risk at 20 years was significantly higher for those with HR-negative tumors, but the risk was still elevated for both types. Patients with stage IIIC HR-positive disease had four times the risk of BCSM over 20 years and those with stage IIIC HR-negative disease had seven times the risk of BCSM over 20 years compared with the risk of death not related to breast cancer, the researchers wrote.
Another study of nearly 2,400 men with breast cancer (mainly HR+) by Dr. J.P. Leone and colleagues showed that cancer stage, tumor subtype, and race were associated with overall survival and breast cancer-specific survival.
What Does the New Study Add?
The current study included 2,836 men diagnosed with stage I to stage III HR+ breast cancer between 1990 and 2008, using data from the Surveillance, Epidemiology, and End Results (SEER) database.
“We found that in men with breast cancer, the risk of breast cancer mortality persists for at least 20 years and that [the risk] depends on traditional clinicopathologic factors, such as age, tumor size, nodal status and tumor grade,” Dr. J.P. Leone said in an interview.
“The prolonged risk [of breast-cancer specific mortality] over 20 years that we observed in our study is similar to that previously reported in women; however, the kinetics of the risk over the 20-year period appears different in men,” he emphasized.
The men in the study, especially those with a higher stage of disease, appeared to have a bimodal distribution of the BRCA mortality risk, he said.
Two peaks were identified, he explained; an early peak in mortality risk at approximately 4 years from diagnosis and another at approximately 11 years after diagnosis.
Although women with breast cancer had a prolonged risk of breast cancer mortality, “the kinetics of the risk does not include 2 peaks, even in women with higher stage of disease.” In women with higher stage breast cancer, the peak mortality risk occurs approximately 5 years after diagnosis, he said.
The reasons for the later peak in men remain unclear, the researchers wrote in the study, but possible explanations include nonadherence to endocrine therapy, differences in tumor biologic factors, and differences in the tumor microenvironment between men and women, they noted, in the discussion section.
What Drives the Risk?
Key factors for breast cancer-specific mortality were age, tumor stage, and tumor grade.
The cumulative 20-year risk of BCSM in the current study was 12.4%, 26.2%, and 46.0% for stage I, II, and III, respectively. The adjusted BCSM risk was increased in patients younger than 50 years, those with grade II or III/IV tumors, and those with stage II or III disease.
What Are the Limitations?
The current study by Leone and colleagues was limited by the relatively small subgroup sample of men with stage III and N3 disease, lack of data on the use of systemic therapies, and lack of data on human epidermal growth factor receptor 2 gene (ERBB2), the researchers wrote. However, the long-term follow-up strengthened the results, and the study is the first known to assess 20-year BCSM risk in men with nonmetastatic HR+ breast cancer.
What Do Oncologists Need to Know About the Study?
The study findings indicate that the risk of breast cancer mortality persists for 20 years in men with hormone receptor–positive breast cancer, Dr. J.P. Leone said in an interview. As in women, the risk depends on traditional clinicopathologic factors, he noted.
“However, the kinetics of that risk appears to be different between men and women. In order to reduce the breast cancer mortality risk in men with hormone receptor–positive breast cancer, it will be important for men to consider the benefits of the treatment options that may be indicated for their specific situation,” he said.
“I think early detection is also very important,” he emphasized. To that end, increased awareness of the possibility for breast cancer in men, as well as prompt intervention when breast cancer is suspected, will help to improve early detection when the risk of breast cancer mortality is lower, he added.
What Are the Next Steps for Research?
“I think our study underscores the need for additional research to improve our adjuvant therapy options in both men and women with hormone receptor-positive breast cancer, to reduce the risk of long-term mortality,” he said.
The study received no outside funding. Lead author Julieta Leone had no financial conflicts to disclose. Dr. José P. Leone disclosed receiving institutional grants from Kazia Therapeutics and Seagen unrelated to the current study.
Breast cancer-specific mortality risk in men with hormone receptor–positive breast cancer persists for at least 20 years, but patterns of breast cancer–specific mortality (BCSM) are distinct from those in women, a new study finds.
Previous studies in women with hormone receptor–positive (HR+) breast cancer have shown a risk of distant recurrence and death for at least 20 years after diagnosis, but data for men are limited, wrote Julieta Leone, MD, of the Dana Farber Cancer Institute, Boston, and colleagues.
What is Known About Hormone Receptor–Positive Breast Cancer Mortality in Men vs. Women?
Invasive breast cancer in men is rare and consequently understudied, the researchers wrote. Previous studies of BCSM in men with more than 5 years’ follow-up consist mainly of case series at single institutions, the researchers wrote in JAMA Oncology (2024 Feb 29. doi: 10.1001/jamaoncol.2023.7194).
“We believed it would be important to study this issue to help inform the management of men with breast cancer,” corresponding author, José P. Leone, MD, said in an interview.
In 2021, Dr. J.P. Leone and colleagues published a study in Breast Cancer Research and Treatment that examined the 20-year risk of BCSM in women that included more than 36,000 individuals who had survived for 5 years, with a median of 14 years’ follow-up.
In that study of women, the BCSM risk at 20 years was significantly higher for those with HR-negative tumors, but the risk was still elevated for both types. Patients with stage IIIC HR-positive disease had four times the risk of BCSM over 20 years and those with stage IIIC HR-negative disease had seven times the risk of BCSM over 20 years compared with the risk of death not related to breast cancer, the researchers wrote.
Another study of nearly 2,400 men with breast cancer (mainly HR+) by Dr. J.P. Leone and colleagues showed that cancer stage, tumor subtype, and race were associated with overall survival and breast cancer-specific survival.
What Does the New Study Add?
The current study included 2,836 men diagnosed with stage I to stage III HR+ breast cancer between 1990 and 2008, using data from the Surveillance, Epidemiology, and End Results (SEER) database.
“We found that in men with breast cancer, the risk of breast cancer mortality persists for at least 20 years and that [the risk] depends on traditional clinicopathologic factors, such as age, tumor size, nodal status and tumor grade,” Dr. J.P. Leone said in an interview.
“The prolonged risk [of breast-cancer specific mortality] over 20 years that we observed in our study is similar to that previously reported in women; however, the kinetics of the risk over the 20-year period appears different in men,” he emphasized.
The men in the study, especially those with a higher stage of disease, appeared to have a bimodal distribution of the BRCA mortality risk, he said.
Two peaks were identified, he explained; an early peak in mortality risk at approximately 4 years from diagnosis and another at approximately 11 years after diagnosis.
Although women with breast cancer had a prolonged risk of breast cancer mortality, “the kinetics of the risk does not include 2 peaks, even in women with higher stage of disease.” In women with higher stage breast cancer, the peak mortality risk occurs approximately 5 years after diagnosis, he said.
The reasons for the later peak in men remain unclear, the researchers wrote in the study, but possible explanations include nonadherence to endocrine therapy, differences in tumor biologic factors, and differences in the tumor microenvironment between men and women, they noted, in the discussion section.
What Drives the Risk?
Key factors for breast cancer-specific mortality were age, tumor stage, and tumor grade.
The cumulative 20-year risk of BCSM in the current study was 12.4%, 26.2%, and 46.0% for stage I, II, and III, respectively. The adjusted BCSM risk was increased in patients younger than 50 years, those with grade II or III/IV tumors, and those with stage II or III disease.
What Are the Limitations?
The current study by Leone and colleagues was limited by the relatively small subgroup sample of men with stage III and N3 disease, lack of data on the use of systemic therapies, and lack of data on human epidermal growth factor receptor 2 gene (ERBB2), the researchers wrote. However, the long-term follow-up strengthened the results, and the study is the first known to assess 20-year BCSM risk in men with nonmetastatic HR+ breast cancer.
What Do Oncologists Need to Know About the Study?
The study findings indicate that the risk of breast cancer mortality persists for 20 years in men with hormone receptor–positive breast cancer, Dr. J.P. Leone said in an interview. As in women, the risk depends on traditional clinicopathologic factors, he noted.
“However, the kinetics of that risk appears to be different between men and women. In order to reduce the breast cancer mortality risk in men with hormone receptor–positive breast cancer, it will be important for men to consider the benefits of the treatment options that may be indicated for their specific situation,” he said.
“I think early detection is also very important,” he emphasized. To that end, increased awareness of the possibility for breast cancer in men, as well as prompt intervention when breast cancer is suspected, will help to improve early detection when the risk of breast cancer mortality is lower, he added.
What Are the Next Steps for Research?
“I think our study underscores the need for additional research to improve our adjuvant therapy options in both men and women with hormone receptor-positive breast cancer, to reduce the risk of long-term mortality,” he said.
The study received no outside funding. Lead author Julieta Leone had no financial conflicts to disclose. Dr. José P. Leone disclosed receiving institutional grants from Kazia Therapeutics and Seagen unrelated to the current study.
FROM JAMA ONCOLOGY
Hospital Mergers in 2024: Five Things to Know
Hospital mergers and acquisitions continue to garner intense scrutiny from lawmakers, with pressure likely to hold steady following the recent announcement of new antitrust guidelines and state and federal investigations into potential healthcare monopolies.
In December, the US Department of Justice (DOJ) and the Federal Trade Commission (FTC) released updated guidelines outlining the factors they consider when determining if a merger illegally monopolizes a local healthcare market or jeopardizes access to critical healthcare services.
Last week, the DOJ also announced a UnitedHealth Group antitrust probe, just months after the healthcare conglomerate’s workforce numbers indicated it is now affiliated with or employs 10% of the US physician workforce.
While the impact of the latest guidelines is yet to be seen, concerns over healthcare market consolidation are not new. Over the past two decades, mergers have attracted attention for contributing to a decline in independent hospitals, said Rachel M. Werner, MD, PhD, executive director of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, Pennsylvania.
“At this point, most hospitals are operating in a pretty concentrated market,” she said.
Here are five things to know about the current state of hospital mergers.
1. Record-Breaking Merger Enforcements
The DOJ and FTC reported the highest level of enforcement activity in over 20 years in fiscal year 2022 — the latest available data. Together, the agencies filed 50 merger enforcement actions and brought a record-breaking number of merger enforcement challenges, resulting in 11 approved actions, the restructuring or abandonment of seven mergers, and six business deals entering litigation.
Included in those statistics was a proposed merger between the two largest health systems in Rhode Island, Lifespan and Care New England Health System, which was abandoned after the FTC and the state Attorney General took steps to block it. the HCA branch in Utah Healthcare abandoned plans for to acquire five Salt Lake City area hospitals from competitor Steward Health Care System, as did RWJBarnabas Health after exploring a merger with Saint Peter's Healthcare System in New Jersey.
2. New Antitrust Guidelines Consider Labor Market
The new guidelines notably focus on labor competition, said Jody Boudreault, JD, attorney and chair of the Antitrust Life Sciences and Healthcare Group at Baker Botts law firm in Washington, DC. Health professionals typically have more employment opportunities in an urban area, unless hindered by restrictive noncompete agreements, and fewer options in rural settings.
In the Lifespan merger that fell through, Ms. Boudreault said that the newly created hospital system would have employed two thirds of Rhode Island's full-time nurses, limiting opportunities for local employment elsewhere.
“Going forward, I would expect federal authorities to review not only the competitive impact of the hospitals merging but also the competitive impact of the physician, and especially nursing, workforce,” she said.
FTC Chair Lina M. Khan noted similar labor market concerns.
In a statement to Congress, she said that hospital consolidation reduces options for employees, who fear “being blacklisted from further hiring in a system that controls many of the hospitals in the area” and “makes workers afraid to file complaints, organize their workplace, or leave before the end of a contract.”
3. Mergers Can Drive Care Costs Higher
When hospital markets become less competitive, the cost of care often increases. In Indiana, inpatient prices rose 13% in hospitals that merged. Another study found that prices at monopoly hospitals are 12% higher than in markets with four or more rivals. Even cross-market mergers, when hospitals in different geographic locations combine, can drive prices higher.
Dr. Werner told this news organization that more significant price hikes of 20-30% aren’t unheard of, with reimbursements by some commercial insurance companies rising as much as 50%. “That’s the direct price that the insurers pay, but the burden of those higher prices ultimately falls on patients through higher premiums,” she said.
Still, the American Hospital Association (AHA) says that mergers and acquisitions can significantly lower annual operating expenses per admission and reduce inpatient readmission rates and mortality measures. In comments to the FTC, the AHA stated that mergers could provide a lifeline for rural and community hospitals struggling with shrinking payer reimbursement and rising labor and supply costs. The business arrangements also could ensure these communities maintain continuity of care.
Although a cross-market merger may initially benefit cash-strapped rural hospitals, Dr. Werner urged caution.
“In the long run, it’s not clear that it is good for patients because we start to see decreased access to some types of service, like labor and delivery, which are services needed in rural markets,” she said.
4. Mergers to Watch in 2024
Ms. Boudreault, who represented RWJBarnabas in the abandoned Saint Peter’s transaction, says the courts widely accepted the old merger guidelines, and it will take time to see how the new measures are interpreted. “The guidelines don’t yet have the force of law, but they can be persuasive to a court.”
Looking ahead, she is watching how Steward Health Care navigates its impending financial collapse. The nation’s largest private for-profit health system was previously owned by private equity firm Cerberus Capital Management and includes nine Massachusetts hospitals plus entities in at least seven other states.
Ms. Boudreault also plans to monitor Jefferson Health’s intent to merge with Lehigh Valley Health Network. “It’s a pretty big deal because they would become a 30-hospital system,” said Ms. Boudreault. The newly formed network would become the largest employer in Philadelphia.
5. Merger and Acquisition Reversals Unlikely
Dr. Werner said that mergers and acquisitions are complicated business moves that are nearly impossible to undo once approved, so it makes sense for agencies to continue to evaluate them closely.
“The costs of healthcare are borne by us as a society,” she said. “We’re going to have to live with the ill effects of a consolidated market once we let hospitals merge, so they deserve additional scrutiny.”
A version of this article appeared on Medscape.com.
Hospital mergers and acquisitions continue to garner intense scrutiny from lawmakers, with pressure likely to hold steady following the recent announcement of new antitrust guidelines and state and federal investigations into potential healthcare monopolies.
In December, the US Department of Justice (DOJ) and the Federal Trade Commission (FTC) released updated guidelines outlining the factors they consider when determining if a merger illegally monopolizes a local healthcare market or jeopardizes access to critical healthcare services.
Last week, the DOJ also announced a UnitedHealth Group antitrust probe, just months after the healthcare conglomerate’s workforce numbers indicated it is now affiliated with or employs 10% of the US physician workforce.
While the impact of the latest guidelines is yet to be seen, concerns over healthcare market consolidation are not new. Over the past two decades, mergers have attracted attention for contributing to a decline in independent hospitals, said Rachel M. Werner, MD, PhD, executive director of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, Pennsylvania.
“At this point, most hospitals are operating in a pretty concentrated market,” she said.
Here are five things to know about the current state of hospital mergers.
1. Record-Breaking Merger Enforcements
The DOJ and FTC reported the highest level of enforcement activity in over 20 years in fiscal year 2022 — the latest available data. Together, the agencies filed 50 merger enforcement actions and brought a record-breaking number of merger enforcement challenges, resulting in 11 approved actions, the restructuring or abandonment of seven mergers, and six business deals entering litigation.
Included in those statistics was a proposed merger between the two largest health systems in Rhode Island, Lifespan and Care New England Health System, which was abandoned after the FTC and the state Attorney General took steps to block it. the HCA branch in Utah Healthcare abandoned plans for to acquire five Salt Lake City area hospitals from competitor Steward Health Care System, as did RWJBarnabas Health after exploring a merger with Saint Peter's Healthcare System in New Jersey.
2. New Antitrust Guidelines Consider Labor Market
The new guidelines notably focus on labor competition, said Jody Boudreault, JD, attorney and chair of the Antitrust Life Sciences and Healthcare Group at Baker Botts law firm in Washington, DC. Health professionals typically have more employment opportunities in an urban area, unless hindered by restrictive noncompete agreements, and fewer options in rural settings.
In the Lifespan merger that fell through, Ms. Boudreault said that the newly created hospital system would have employed two thirds of Rhode Island's full-time nurses, limiting opportunities for local employment elsewhere.
“Going forward, I would expect federal authorities to review not only the competitive impact of the hospitals merging but also the competitive impact of the physician, and especially nursing, workforce,” she said.
FTC Chair Lina M. Khan noted similar labor market concerns.
In a statement to Congress, she said that hospital consolidation reduces options for employees, who fear “being blacklisted from further hiring in a system that controls many of the hospitals in the area” and “makes workers afraid to file complaints, organize their workplace, or leave before the end of a contract.”
3. Mergers Can Drive Care Costs Higher
When hospital markets become less competitive, the cost of care often increases. In Indiana, inpatient prices rose 13% in hospitals that merged. Another study found that prices at monopoly hospitals are 12% higher than in markets with four or more rivals. Even cross-market mergers, when hospitals in different geographic locations combine, can drive prices higher.
Dr. Werner told this news organization that more significant price hikes of 20-30% aren’t unheard of, with reimbursements by some commercial insurance companies rising as much as 50%. “That’s the direct price that the insurers pay, but the burden of those higher prices ultimately falls on patients through higher premiums,” she said.
Still, the American Hospital Association (AHA) says that mergers and acquisitions can significantly lower annual operating expenses per admission and reduce inpatient readmission rates and mortality measures. In comments to the FTC, the AHA stated that mergers could provide a lifeline for rural and community hospitals struggling with shrinking payer reimbursement and rising labor and supply costs. The business arrangements also could ensure these communities maintain continuity of care.
Although a cross-market merger may initially benefit cash-strapped rural hospitals, Dr. Werner urged caution.
“In the long run, it’s not clear that it is good for patients because we start to see decreased access to some types of service, like labor and delivery, which are services needed in rural markets,” she said.
4. Mergers to Watch in 2024
Ms. Boudreault, who represented RWJBarnabas in the abandoned Saint Peter’s transaction, says the courts widely accepted the old merger guidelines, and it will take time to see how the new measures are interpreted. “The guidelines don’t yet have the force of law, but they can be persuasive to a court.”
Looking ahead, she is watching how Steward Health Care navigates its impending financial collapse. The nation’s largest private for-profit health system was previously owned by private equity firm Cerberus Capital Management and includes nine Massachusetts hospitals plus entities in at least seven other states.
Ms. Boudreault also plans to monitor Jefferson Health’s intent to merge with Lehigh Valley Health Network. “It’s a pretty big deal because they would become a 30-hospital system,” said Ms. Boudreault. The newly formed network would become the largest employer in Philadelphia.
5. Merger and Acquisition Reversals Unlikely
Dr. Werner said that mergers and acquisitions are complicated business moves that are nearly impossible to undo once approved, so it makes sense for agencies to continue to evaluate them closely.
“The costs of healthcare are borne by us as a society,” she said. “We’re going to have to live with the ill effects of a consolidated market once we let hospitals merge, so they deserve additional scrutiny.”
A version of this article appeared on Medscape.com.
Hospital mergers and acquisitions continue to garner intense scrutiny from lawmakers, with pressure likely to hold steady following the recent announcement of new antitrust guidelines and state and federal investigations into potential healthcare monopolies.
In December, the US Department of Justice (DOJ) and the Federal Trade Commission (FTC) released updated guidelines outlining the factors they consider when determining if a merger illegally monopolizes a local healthcare market or jeopardizes access to critical healthcare services.
Last week, the DOJ also announced a UnitedHealth Group antitrust probe, just months after the healthcare conglomerate’s workforce numbers indicated it is now affiliated with or employs 10% of the US physician workforce.
While the impact of the latest guidelines is yet to be seen, concerns over healthcare market consolidation are not new. Over the past two decades, mergers have attracted attention for contributing to a decline in independent hospitals, said Rachel M. Werner, MD, PhD, executive director of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, Pennsylvania.
“At this point, most hospitals are operating in a pretty concentrated market,” she said.
Here are five things to know about the current state of hospital mergers.
1. Record-Breaking Merger Enforcements
The DOJ and FTC reported the highest level of enforcement activity in over 20 years in fiscal year 2022 — the latest available data. Together, the agencies filed 50 merger enforcement actions and brought a record-breaking number of merger enforcement challenges, resulting in 11 approved actions, the restructuring or abandonment of seven mergers, and six business deals entering litigation.
Included in those statistics was a proposed merger between the two largest health systems in Rhode Island, Lifespan and Care New England Health System, which was abandoned after the FTC and the state Attorney General took steps to block it. the HCA branch in Utah Healthcare abandoned plans for to acquire five Salt Lake City area hospitals from competitor Steward Health Care System, as did RWJBarnabas Health after exploring a merger with Saint Peter's Healthcare System in New Jersey.
2. New Antitrust Guidelines Consider Labor Market
The new guidelines notably focus on labor competition, said Jody Boudreault, JD, attorney and chair of the Antitrust Life Sciences and Healthcare Group at Baker Botts law firm in Washington, DC. Health professionals typically have more employment opportunities in an urban area, unless hindered by restrictive noncompete agreements, and fewer options in rural settings.
In the Lifespan merger that fell through, Ms. Boudreault said that the newly created hospital system would have employed two thirds of Rhode Island's full-time nurses, limiting opportunities for local employment elsewhere.
“Going forward, I would expect federal authorities to review not only the competitive impact of the hospitals merging but also the competitive impact of the physician, and especially nursing, workforce,” she said.
FTC Chair Lina M. Khan noted similar labor market concerns.
In a statement to Congress, she said that hospital consolidation reduces options for employees, who fear “being blacklisted from further hiring in a system that controls many of the hospitals in the area” and “makes workers afraid to file complaints, organize their workplace, or leave before the end of a contract.”
3. Mergers Can Drive Care Costs Higher
When hospital markets become less competitive, the cost of care often increases. In Indiana, inpatient prices rose 13% in hospitals that merged. Another study found that prices at monopoly hospitals are 12% higher than in markets with four or more rivals. Even cross-market mergers, when hospitals in different geographic locations combine, can drive prices higher.
Dr. Werner told this news organization that more significant price hikes of 20-30% aren’t unheard of, with reimbursements by some commercial insurance companies rising as much as 50%. “That’s the direct price that the insurers pay, but the burden of those higher prices ultimately falls on patients through higher premiums,” she said.
Still, the American Hospital Association (AHA) says that mergers and acquisitions can significantly lower annual operating expenses per admission and reduce inpatient readmission rates and mortality measures. In comments to the FTC, the AHA stated that mergers could provide a lifeline for rural and community hospitals struggling with shrinking payer reimbursement and rising labor and supply costs. The business arrangements also could ensure these communities maintain continuity of care.
Although a cross-market merger may initially benefit cash-strapped rural hospitals, Dr. Werner urged caution.
“In the long run, it’s not clear that it is good for patients because we start to see decreased access to some types of service, like labor and delivery, which are services needed in rural markets,” she said.
4. Mergers to Watch in 2024
Ms. Boudreault, who represented RWJBarnabas in the abandoned Saint Peter’s transaction, says the courts widely accepted the old merger guidelines, and it will take time to see how the new measures are interpreted. “The guidelines don’t yet have the force of law, but they can be persuasive to a court.”
Looking ahead, she is watching how Steward Health Care navigates its impending financial collapse. The nation’s largest private for-profit health system was previously owned by private equity firm Cerberus Capital Management and includes nine Massachusetts hospitals plus entities in at least seven other states.
Ms. Boudreault also plans to monitor Jefferson Health’s intent to merge with Lehigh Valley Health Network. “It’s a pretty big deal because they would become a 30-hospital system,” said Ms. Boudreault. The newly formed network would become the largest employer in Philadelphia.
5. Merger and Acquisition Reversals Unlikely
Dr. Werner said that mergers and acquisitions are complicated business moves that are nearly impossible to undo once approved, so it makes sense for agencies to continue to evaluate them closely.
“The costs of healthcare are borne by us as a society,” she said. “We’re going to have to live with the ill effects of a consolidated market once we let hospitals merge, so they deserve additional scrutiny.”
A version of this article appeared on Medscape.com.
Is Adrenal Fatigue a Real Condition?
While TikTok overflows with images of influencers making “adrenal cocktails” to combat what they call adrenal fatigue, the Endocrine Society says “no scientific proof exists to support adrenal fatigue as a true medical condition.”
Even before influencers began touting it on social media, a 2016 systematic review concluded that there is “no substantiation that adrenal fatigue” is an actual medical condition. Therefore, adrenal fatigue is still a myth.
Lynette Nieman, MD, Senior Investigator and Chief of the Endocrinology Consultation Service at the National Institutes of Health Clinical Center, Bethesda, Maryland, concurs.
“There is no scientific evidence that adrenal fatigue exists or causes [general] fatigue, depression, or the many common symptoms that are said to result from this condition,” she told this news organization via email.
Still, the term has gained currency among not only social media influencers who blame it for everything from cortisol surges to estrogen imbalances but also functional and integrative medical practitioners as an explanation for chronic dysfunction related to stress.
Adrenal Fatigue, Burnout, or Adrenal Insufficiency?
Rather than “adrenal fatigue,” Marcelo Campos, MD, a primary care doctor at Atrius Health, said he prefers the medical term “burnout.”
Use of “burnout” shifts attention to the brain’s role in stress-related chronic dysfunction rather than the adrenal glands, said Dr. Campos, who also teaches at Harvard Medical School, Cambridge, Massachusetts.
More specifically still, the focuses might shift to the stress-response via the hypothalamic-pituitary-adrenocortical axis and its role in reducing levels of these cortisol and dehydroepiandrosterone sulfate.
He points out that part of the reason for the misuse of the term adrenal fatigue arises from the fact that burnout is often only associated with work stress.
“Recently, the ICD-11 [International Classification of Diseases-11] recognized burnout as a disease but focused only on work stress as a cause. The truth is that people can be burned out for many other reasons,” said Dr. Campos.
The Endocrine Society notes on their webpage dedicated to the topic that “adrenal fatigue” as a term, relates to long-term mental, emotional, or physical stress.
“The problem is not the adrenals — it is the exposure to stress in the brain. The brain — only one organ — is responsible for 40% of energy consumption in the body. As you can imagine, if you are under constant stress, you run out of gas very quickly and cannot function well,” he explained.
Adrenal fatigue theory suggests that, under stress, the adrenal glands produce too many short bursts of cortisol resulting in overall reduced cortisol levels and a feeling of being drained.
“As with many other psychiatric diseases, we do not have a way to measure biomarkers in the brain. The testing for cortisol does not work because it fluctuates too much from time to time. So, it is not reliable or reproducible,” Dr. Campos said.
This leads to the ongoing question of the best way to test and diagnose adrenal fatigue, whether it should be via blood, urine and/or saliva. And even if that is determined, there are still questions about the best time to test, how often, what the normal ranges are and how reliable the tests are.
While adrenal fatigue is not a recognized condition, adrenal insufficiency is medically recognized, resulting from an inability of the adrenal glands to make the life-essential hormones aldosterone and/or cortisol, with symptoms that include fatigue, belly pain, nausea, vomiting, diarrhea, and joint aches.
“Adrenal cocktails are not an effective treatment for adrenal insufficiency because they do not replace the missing hormones,” Dr. Nieman stated, pointing out that anyone with symptoms of adrenal insufficiency needs to see an endocrinologist.
Pratibha Rao, MD, MPH, an endocrinologist at the Cleveland Clinic, Ohio, and medical director of the Adrenal Center at Cleveland Clinic, agreed, advising that if people continue to feel exhausted beyond their normal exertion, then they should get checked for signs of adrenal insufficiency.
“In primary adrenal insufficiency, you can actually start seeing darkening of the gums and of the skin on the palms of the hands or the soles of your feet. Sometimes people can feel dizzy or experience some loss of consciousness,” she said. “If it’s sudden and severe, you may crave salt or have extreme heat or cold intolerance.”
Recognizing and Managing Patient Frustration
The lack of formal diagnostic criteria and medical evidence, however, doesn’t mean that such symptoms as fatigue and depression don’t present, often causing significant distress for patients. While the symptoms might not be associated with the adrenal glands, they still need addressing — but how that is done is, in essence, a bone of contention.
Dr. Rao empathizes with the situation that many people, often young women, find themselves in.
“Patients are frustrated. They’ve gone to multiple doctors across the country, and they feel convinced they have adrenal fatigue, but no medical doctor has endorsed it. They end up coming to us with a cry that has so often gone unanswered.”
This issue also highlights that there are millions of people experiencing mental, emotional, and physical distress of unknown cause who seek help, many of whom believe it is related to their adrenal gland function.
But rather than turning to a social media cure, Dr. Rao stresses that people would benefit more from paying greater attention to following a healthy lifestyle than regularly consuming sugar-rich drinks claimed to offer a solution. Adrenal cocktails are energy-rich, frothy blends of orange juice, coconut milk, cream of tartar, and Himalayan salt.
“We truly are what we eat, and we are what we think,” she noted.
The body is a miraculous machine, but “we forget that it does need maintenance,” Dr. Rao said. “Up to age 30, the body is so forgiving with drugs, alcohol, or whatever insult we do to it, but after the third decade, slowly every cell starts to degenerate instead of growing. We start to see the ill or beneficial effects of lifestyle habits.”
“We insult the body, and then we say, ‘oh, I have fatigue’ and seek a quick fix,” she added. “Everyone wants instant gratification.”
Dr. Rao cautioned that adrenal cocktails could be dangerous for someone who has other medical conditions.
“If someone has kidney disease, uncontrolled hypertension, or diabetes, for example, then adrenal cocktails are definitely not safe,” Dr. Rao said. “Loading up with potassium and sodium, which is found in high quantities in adrenal cocktails, will actually worsen any kidney damage, while consuming so much sugar will cause an unregulated rise in blood sugar and further damage in someone with diabetes.”
Dr. Rao also stressed that nonprofessional advice given on social media could take patient people down the wrong path with associated danger.
A version of this article appeared on Medscape.com.
While TikTok overflows with images of influencers making “adrenal cocktails” to combat what they call adrenal fatigue, the Endocrine Society says “no scientific proof exists to support adrenal fatigue as a true medical condition.”
Even before influencers began touting it on social media, a 2016 systematic review concluded that there is “no substantiation that adrenal fatigue” is an actual medical condition. Therefore, adrenal fatigue is still a myth.
Lynette Nieman, MD, Senior Investigator and Chief of the Endocrinology Consultation Service at the National Institutes of Health Clinical Center, Bethesda, Maryland, concurs.
“There is no scientific evidence that adrenal fatigue exists or causes [general] fatigue, depression, or the many common symptoms that are said to result from this condition,” she told this news organization via email.
Still, the term has gained currency among not only social media influencers who blame it for everything from cortisol surges to estrogen imbalances but also functional and integrative medical practitioners as an explanation for chronic dysfunction related to stress.
Adrenal Fatigue, Burnout, or Adrenal Insufficiency?
Rather than “adrenal fatigue,” Marcelo Campos, MD, a primary care doctor at Atrius Health, said he prefers the medical term “burnout.”
Use of “burnout” shifts attention to the brain’s role in stress-related chronic dysfunction rather than the adrenal glands, said Dr. Campos, who also teaches at Harvard Medical School, Cambridge, Massachusetts.
More specifically still, the focuses might shift to the stress-response via the hypothalamic-pituitary-adrenocortical axis and its role in reducing levels of these cortisol and dehydroepiandrosterone sulfate.
He points out that part of the reason for the misuse of the term adrenal fatigue arises from the fact that burnout is often only associated with work stress.
“Recently, the ICD-11 [International Classification of Diseases-11] recognized burnout as a disease but focused only on work stress as a cause. The truth is that people can be burned out for many other reasons,” said Dr. Campos.
The Endocrine Society notes on their webpage dedicated to the topic that “adrenal fatigue” as a term, relates to long-term mental, emotional, or physical stress.
“The problem is not the adrenals — it is the exposure to stress in the brain. The brain — only one organ — is responsible for 40% of energy consumption in the body. As you can imagine, if you are under constant stress, you run out of gas very quickly and cannot function well,” he explained.
Adrenal fatigue theory suggests that, under stress, the adrenal glands produce too many short bursts of cortisol resulting in overall reduced cortisol levels and a feeling of being drained.
“As with many other psychiatric diseases, we do not have a way to measure biomarkers in the brain. The testing for cortisol does not work because it fluctuates too much from time to time. So, it is not reliable or reproducible,” Dr. Campos said.
This leads to the ongoing question of the best way to test and diagnose adrenal fatigue, whether it should be via blood, urine and/or saliva. And even if that is determined, there are still questions about the best time to test, how often, what the normal ranges are and how reliable the tests are.
While adrenal fatigue is not a recognized condition, adrenal insufficiency is medically recognized, resulting from an inability of the adrenal glands to make the life-essential hormones aldosterone and/or cortisol, with symptoms that include fatigue, belly pain, nausea, vomiting, diarrhea, and joint aches.
“Adrenal cocktails are not an effective treatment for adrenal insufficiency because they do not replace the missing hormones,” Dr. Nieman stated, pointing out that anyone with symptoms of adrenal insufficiency needs to see an endocrinologist.
Pratibha Rao, MD, MPH, an endocrinologist at the Cleveland Clinic, Ohio, and medical director of the Adrenal Center at Cleveland Clinic, agreed, advising that if people continue to feel exhausted beyond their normal exertion, then they should get checked for signs of adrenal insufficiency.
“In primary adrenal insufficiency, you can actually start seeing darkening of the gums and of the skin on the palms of the hands or the soles of your feet. Sometimes people can feel dizzy or experience some loss of consciousness,” she said. “If it’s sudden and severe, you may crave salt or have extreme heat or cold intolerance.”
Recognizing and Managing Patient Frustration
The lack of formal diagnostic criteria and medical evidence, however, doesn’t mean that such symptoms as fatigue and depression don’t present, often causing significant distress for patients. While the symptoms might not be associated with the adrenal glands, they still need addressing — but how that is done is, in essence, a bone of contention.
Dr. Rao empathizes with the situation that many people, often young women, find themselves in.
“Patients are frustrated. They’ve gone to multiple doctors across the country, and they feel convinced they have adrenal fatigue, but no medical doctor has endorsed it. They end up coming to us with a cry that has so often gone unanswered.”
This issue also highlights that there are millions of people experiencing mental, emotional, and physical distress of unknown cause who seek help, many of whom believe it is related to their adrenal gland function.
But rather than turning to a social media cure, Dr. Rao stresses that people would benefit more from paying greater attention to following a healthy lifestyle than regularly consuming sugar-rich drinks claimed to offer a solution. Adrenal cocktails are energy-rich, frothy blends of orange juice, coconut milk, cream of tartar, and Himalayan salt.
“We truly are what we eat, and we are what we think,” she noted.
The body is a miraculous machine, but “we forget that it does need maintenance,” Dr. Rao said. “Up to age 30, the body is so forgiving with drugs, alcohol, or whatever insult we do to it, but after the third decade, slowly every cell starts to degenerate instead of growing. We start to see the ill or beneficial effects of lifestyle habits.”
“We insult the body, and then we say, ‘oh, I have fatigue’ and seek a quick fix,” she added. “Everyone wants instant gratification.”
Dr. Rao cautioned that adrenal cocktails could be dangerous for someone who has other medical conditions.
“If someone has kidney disease, uncontrolled hypertension, or diabetes, for example, then adrenal cocktails are definitely not safe,” Dr. Rao said. “Loading up with potassium and sodium, which is found in high quantities in adrenal cocktails, will actually worsen any kidney damage, while consuming so much sugar will cause an unregulated rise in blood sugar and further damage in someone with diabetes.”
Dr. Rao also stressed that nonprofessional advice given on social media could take patient people down the wrong path with associated danger.
A version of this article appeared on Medscape.com.
While TikTok overflows with images of influencers making “adrenal cocktails” to combat what they call adrenal fatigue, the Endocrine Society says “no scientific proof exists to support adrenal fatigue as a true medical condition.”
Even before influencers began touting it on social media, a 2016 systematic review concluded that there is “no substantiation that adrenal fatigue” is an actual medical condition. Therefore, adrenal fatigue is still a myth.
Lynette Nieman, MD, Senior Investigator and Chief of the Endocrinology Consultation Service at the National Institutes of Health Clinical Center, Bethesda, Maryland, concurs.
“There is no scientific evidence that adrenal fatigue exists or causes [general] fatigue, depression, or the many common symptoms that are said to result from this condition,” she told this news organization via email.
Still, the term has gained currency among not only social media influencers who blame it for everything from cortisol surges to estrogen imbalances but also functional and integrative medical practitioners as an explanation for chronic dysfunction related to stress.
Adrenal Fatigue, Burnout, or Adrenal Insufficiency?
Rather than “adrenal fatigue,” Marcelo Campos, MD, a primary care doctor at Atrius Health, said he prefers the medical term “burnout.”
Use of “burnout” shifts attention to the brain’s role in stress-related chronic dysfunction rather than the adrenal glands, said Dr. Campos, who also teaches at Harvard Medical School, Cambridge, Massachusetts.
More specifically still, the focuses might shift to the stress-response via the hypothalamic-pituitary-adrenocortical axis and its role in reducing levels of these cortisol and dehydroepiandrosterone sulfate.
He points out that part of the reason for the misuse of the term adrenal fatigue arises from the fact that burnout is often only associated with work stress.
“Recently, the ICD-11 [International Classification of Diseases-11] recognized burnout as a disease but focused only on work stress as a cause. The truth is that people can be burned out for many other reasons,” said Dr. Campos.
The Endocrine Society notes on their webpage dedicated to the topic that “adrenal fatigue” as a term, relates to long-term mental, emotional, or physical stress.
“The problem is not the adrenals — it is the exposure to stress in the brain. The brain — only one organ — is responsible for 40% of energy consumption in the body. As you can imagine, if you are under constant stress, you run out of gas very quickly and cannot function well,” he explained.
Adrenal fatigue theory suggests that, under stress, the adrenal glands produce too many short bursts of cortisol resulting in overall reduced cortisol levels and a feeling of being drained.
“As with many other psychiatric diseases, we do not have a way to measure biomarkers in the brain. The testing for cortisol does not work because it fluctuates too much from time to time. So, it is not reliable or reproducible,” Dr. Campos said.
This leads to the ongoing question of the best way to test and diagnose adrenal fatigue, whether it should be via blood, urine and/or saliva. And even if that is determined, there are still questions about the best time to test, how often, what the normal ranges are and how reliable the tests are.
While adrenal fatigue is not a recognized condition, adrenal insufficiency is medically recognized, resulting from an inability of the adrenal glands to make the life-essential hormones aldosterone and/or cortisol, with symptoms that include fatigue, belly pain, nausea, vomiting, diarrhea, and joint aches.
“Adrenal cocktails are not an effective treatment for adrenal insufficiency because they do not replace the missing hormones,” Dr. Nieman stated, pointing out that anyone with symptoms of adrenal insufficiency needs to see an endocrinologist.
Pratibha Rao, MD, MPH, an endocrinologist at the Cleveland Clinic, Ohio, and medical director of the Adrenal Center at Cleveland Clinic, agreed, advising that if people continue to feel exhausted beyond their normal exertion, then they should get checked for signs of adrenal insufficiency.
“In primary adrenal insufficiency, you can actually start seeing darkening of the gums and of the skin on the palms of the hands or the soles of your feet. Sometimes people can feel dizzy or experience some loss of consciousness,” she said. “If it’s sudden and severe, you may crave salt or have extreme heat or cold intolerance.”
Recognizing and Managing Patient Frustration
The lack of formal diagnostic criteria and medical evidence, however, doesn’t mean that such symptoms as fatigue and depression don’t present, often causing significant distress for patients. While the symptoms might not be associated with the adrenal glands, they still need addressing — but how that is done is, in essence, a bone of contention.
Dr. Rao empathizes with the situation that many people, often young women, find themselves in.
“Patients are frustrated. They’ve gone to multiple doctors across the country, and they feel convinced they have adrenal fatigue, but no medical doctor has endorsed it. They end up coming to us with a cry that has so often gone unanswered.”
This issue also highlights that there are millions of people experiencing mental, emotional, and physical distress of unknown cause who seek help, many of whom believe it is related to their adrenal gland function.
But rather than turning to a social media cure, Dr. Rao stresses that people would benefit more from paying greater attention to following a healthy lifestyle than regularly consuming sugar-rich drinks claimed to offer a solution. Adrenal cocktails are energy-rich, frothy blends of orange juice, coconut milk, cream of tartar, and Himalayan salt.
“We truly are what we eat, and we are what we think,” she noted.
The body is a miraculous machine, but “we forget that it does need maintenance,” Dr. Rao said. “Up to age 30, the body is so forgiving with drugs, alcohol, or whatever insult we do to it, but after the third decade, slowly every cell starts to degenerate instead of growing. We start to see the ill or beneficial effects of lifestyle habits.”
“We insult the body, and then we say, ‘oh, I have fatigue’ and seek a quick fix,” she added. “Everyone wants instant gratification.”
Dr. Rao cautioned that adrenal cocktails could be dangerous for someone who has other medical conditions.
“If someone has kidney disease, uncontrolled hypertension, or diabetes, for example, then adrenal cocktails are definitely not safe,” Dr. Rao said. “Loading up with potassium and sodium, which is found in high quantities in adrenal cocktails, will actually worsen any kidney damage, while consuming so much sugar will cause an unregulated rise in blood sugar and further damage in someone with diabetes.”
Dr. Rao also stressed that nonprofessional advice given on social media could take patient people down the wrong path with associated danger.
A version of this article appeared on Medscape.com.
The Small Office Vibe
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Primary Care Physician’s Next Frontier: Palliative Care
Jason Black, MD, trained in family medicine, worked for Kaiser Permanente, and subsequently completed a fellowship in geriatrics. Today, he treats frail elderly patients, mostly residents of nursing homes and assisted living facilities or living in their own homes, for Gilchrist, a hospice and palliative care organization serving Baltimore and central Maryland.
“I’m practicing family medicine to the extent that I’m treating the family unit, including the anxieties of the adult children, and finding solutions for the parents,” said Dr. Black, one of Gilchrist’s 62 employed providers, one third of whom are physicians. One of his most important roles is medication reconciliation and deprescribing.
Palliative care, a medical specialty that focuses on clarifying the treatment goals of seriously ill patients, helping with end-of-life planning, and emphasizing pain and symptom management, has been growing in recent years. Already well-established in most US hospitals, it is expanding in community settings, often as an extension of hospice programs.
Now, by adding primary care physicians and practices to their service mix, palliative care groups are better meeting the needs of a neglected — and costly — population of frail elders. In doing so, they also are better able to find a niche in the rapidly evolving alphabet soup of value-based care and its varieties of shared savings for providers who post positive outcomes.
Most patients Dr. Black sees find it difficult to visit their doctors in a clinic setting, although they face a variety of medical needs and chronic conditions of aging. They may have a prognosis of several years to live and, thus, do not qualify for hospice care. To him, a palliative approach offers the satisfaction of focusing on what is most important to patients at this difficult time in their lives, rather than predetermined clinical metrics like blood pressure or blood glucose. “It takes a lot of work, but it feels important and rewarding,” he said.
A recent survey of community-dwelling older adults in Ontario, Canada, found most patients fail to receive this treatment homes in the final 3 months of life.
Continuums of Patients and Models
Gilchrist started as a nonprofit, hospital-affiliated hospice program in 1994 and in 2000 took on the management of a geriatric medicine practice for its parent, Greater Baltimore Medical Center. Today, its physicians and nurse practitioners see a range of patients in geriatric primary care, palliative medicine, and hospice, according to its chief medical officer, Mark J. Gloth, DO.
“As people progress in their disease, their location — where they call home — may change as well,” Dr. Gloth said. “We offer a continuum of care in order to not lose people through those transitions. That’s the core of our mission — making sure we are there to escort people through the difficult moments in their lives.”
Models for value-based care encompass accountable care organizations (ACOs), including the ACO REACH (Realizing Equity, Access, and Community Health) high-needs model for traditional Medicare patients, and Medicare Shared Savings Programs for fee-for-service beneficiaries. These value-based models offer a variety of opportunities for the palliative care organization to share in savings resulting from keeping the patient out of the hospital or emergency room and other quality and cost benchmarks.
Coming Together to Meet Needs
Gilchrist is one of nine hospice and palliative organizations that have joined to form their own multistate ACO, Responsive Care Solutions, focused on the clinical needs of frail elderly Medicare beneficiaries. Hospice of the Valley in Phoenix has Geriatric Solutions, a frail elder physician practice. And Capital Caring Health, a hospice and palliative care agency serving metro Washington, DC, has deployed several physicians and nurse practitioners on the road doing primary care at home, said Heidi Young, MD, its Primary Care at Home Lead Physician.
“Five years ago, we started our primary care practice under the umbrella of a 40-year-old hospice organization because we thought we needed to prepare for the changes that are coming to the hospice model,” Dr. Young said. “The thought was that we’re not just a hospice organization; we’re an advanced illness organization. We will come to your home, whatever that is, and provide your primary care.”
The greatest potential gains for a hospice organization are from assuming 100% risk for a large population of patients, keeping them out of the hospital to lower the costs of their care, then reaping those gains under a value-based profit-sharing model, Dr. Young said.
“Our program is still new and working toward getting more patients aligned into value-based models,” she said. “It’s a work in progress.”
A Foot in Each World
Agencies like Capital Caring and Gilchrist derive the largest share of their physician income from billing Medicare Part B and other insurers per visit. But that billing is not enough to break even on physician services.
With hopes for a value-based future, Gilchrist also gets grants from elder-facing charitable foundations to cover up to 40% of the costs of its home-based primary care, according to its president, Catherine Hamel. Hospice care continues to be paid on a per-diem basis by Medicare for eligible terminally ill patients, including Medicare Advantage patients, although the Centers for Medicare & Medicaid Services is reportedly considering new models for the hospice benefit.
The National Partnership for Healthcare and Hospice Innovation (NPHI) is a trade group representing more than 100 nonprofit, hospice-based organizations participating in palliative care and value-based care.
For a hospice to be successful in the evolving post–acute care/end-of-life care landscape, it can no longer rely solely on its hospice line of business, no matter how high-quality, said Ethan McChesney, policy director for the Washington, DC-based nonprofit.
NPHI members have developed their own palliative care programs, and perhaps, a quarter have primary care at home practices, Mr. McChesney said. Some of them acquired existing primary care practices in their service area with which they already had relationships; others created their own.
For hospice organizations building a continuum of services for the seriously ill, adding a primary care at home practice is a natural fit, he said. “You can provide all the services you would as a traditional primary care practice while you have the opportunity to establish long-term relationships with patients and their caregivers that lend themselves to palliative care referrals and then hospice referrals downstream [when the patient becomes eligible for hospice care].” Often, this primary medical care is a mix of in-person and telehealth.
Cameron Muir, MD, NPHI’s chief innovation officer, noted that the hamster wheel for primary care doctors has been spinning faster and faster, with reimbursement going down and costs going up.
But with home-based primary care for frail elders under value-based models, Dr. Muir said, the clinician is paid not for making more visits but for taking great care of the patient: “And I’m actually saving Medicare money and getting credit for the hospitalizations that were avoided.”
A version of this article appeared on Medscape.com.
Jason Black, MD, trained in family medicine, worked for Kaiser Permanente, and subsequently completed a fellowship in geriatrics. Today, he treats frail elderly patients, mostly residents of nursing homes and assisted living facilities or living in their own homes, for Gilchrist, a hospice and palliative care organization serving Baltimore and central Maryland.
“I’m practicing family medicine to the extent that I’m treating the family unit, including the anxieties of the adult children, and finding solutions for the parents,” said Dr. Black, one of Gilchrist’s 62 employed providers, one third of whom are physicians. One of his most important roles is medication reconciliation and deprescribing.
Palliative care, a medical specialty that focuses on clarifying the treatment goals of seriously ill patients, helping with end-of-life planning, and emphasizing pain and symptom management, has been growing in recent years. Already well-established in most US hospitals, it is expanding in community settings, often as an extension of hospice programs.
Now, by adding primary care physicians and practices to their service mix, palliative care groups are better meeting the needs of a neglected — and costly — population of frail elders. In doing so, they also are better able to find a niche in the rapidly evolving alphabet soup of value-based care and its varieties of shared savings for providers who post positive outcomes.
Most patients Dr. Black sees find it difficult to visit their doctors in a clinic setting, although they face a variety of medical needs and chronic conditions of aging. They may have a prognosis of several years to live and, thus, do not qualify for hospice care. To him, a palliative approach offers the satisfaction of focusing on what is most important to patients at this difficult time in their lives, rather than predetermined clinical metrics like blood pressure or blood glucose. “It takes a lot of work, but it feels important and rewarding,” he said.
A recent survey of community-dwelling older adults in Ontario, Canada, found most patients fail to receive this treatment homes in the final 3 months of life.
Continuums of Patients and Models
Gilchrist started as a nonprofit, hospital-affiliated hospice program in 1994 and in 2000 took on the management of a geriatric medicine practice for its parent, Greater Baltimore Medical Center. Today, its physicians and nurse practitioners see a range of patients in geriatric primary care, palliative medicine, and hospice, according to its chief medical officer, Mark J. Gloth, DO.
“As people progress in their disease, their location — where they call home — may change as well,” Dr. Gloth said. “We offer a continuum of care in order to not lose people through those transitions. That’s the core of our mission — making sure we are there to escort people through the difficult moments in their lives.”
Models for value-based care encompass accountable care organizations (ACOs), including the ACO REACH (Realizing Equity, Access, and Community Health) high-needs model for traditional Medicare patients, and Medicare Shared Savings Programs for fee-for-service beneficiaries. These value-based models offer a variety of opportunities for the palliative care organization to share in savings resulting from keeping the patient out of the hospital or emergency room and other quality and cost benchmarks.
Coming Together to Meet Needs
Gilchrist is one of nine hospice and palliative organizations that have joined to form their own multistate ACO, Responsive Care Solutions, focused on the clinical needs of frail elderly Medicare beneficiaries. Hospice of the Valley in Phoenix has Geriatric Solutions, a frail elder physician practice. And Capital Caring Health, a hospice and palliative care agency serving metro Washington, DC, has deployed several physicians and nurse practitioners on the road doing primary care at home, said Heidi Young, MD, its Primary Care at Home Lead Physician.
“Five years ago, we started our primary care practice under the umbrella of a 40-year-old hospice organization because we thought we needed to prepare for the changes that are coming to the hospice model,” Dr. Young said. “The thought was that we’re not just a hospice organization; we’re an advanced illness organization. We will come to your home, whatever that is, and provide your primary care.”
The greatest potential gains for a hospice organization are from assuming 100% risk for a large population of patients, keeping them out of the hospital to lower the costs of their care, then reaping those gains under a value-based profit-sharing model, Dr. Young said.
“Our program is still new and working toward getting more patients aligned into value-based models,” she said. “It’s a work in progress.”
A Foot in Each World
Agencies like Capital Caring and Gilchrist derive the largest share of their physician income from billing Medicare Part B and other insurers per visit. But that billing is not enough to break even on physician services.
With hopes for a value-based future, Gilchrist also gets grants from elder-facing charitable foundations to cover up to 40% of the costs of its home-based primary care, according to its president, Catherine Hamel. Hospice care continues to be paid on a per-diem basis by Medicare for eligible terminally ill patients, including Medicare Advantage patients, although the Centers for Medicare & Medicaid Services is reportedly considering new models for the hospice benefit.
The National Partnership for Healthcare and Hospice Innovation (NPHI) is a trade group representing more than 100 nonprofit, hospice-based organizations participating in palliative care and value-based care.
For a hospice to be successful in the evolving post–acute care/end-of-life care landscape, it can no longer rely solely on its hospice line of business, no matter how high-quality, said Ethan McChesney, policy director for the Washington, DC-based nonprofit.
NPHI members have developed their own palliative care programs, and perhaps, a quarter have primary care at home practices, Mr. McChesney said. Some of them acquired existing primary care practices in their service area with which they already had relationships; others created their own.
For hospice organizations building a continuum of services for the seriously ill, adding a primary care at home practice is a natural fit, he said. “You can provide all the services you would as a traditional primary care practice while you have the opportunity to establish long-term relationships with patients and their caregivers that lend themselves to palliative care referrals and then hospice referrals downstream [when the patient becomes eligible for hospice care].” Often, this primary medical care is a mix of in-person and telehealth.
Cameron Muir, MD, NPHI’s chief innovation officer, noted that the hamster wheel for primary care doctors has been spinning faster and faster, with reimbursement going down and costs going up.
But with home-based primary care for frail elders under value-based models, Dr. Muir said, the clinician is paid not for making more visits but for taking great care of the patient: “And I’m actually saving Medicare money and getting credit for the hospitalizations that were avoided.”
A version of this article appeared on Medscape.com.
Jason Black, MD, trained in family medicine, worked for Kaiser Permanente, and subsequently completed a fellowship in geriatrics. Today, he treats frail elderly patients, mostly residents of nursing homes and assisted living facilities or living in their own homes, for Gilchrist, a hospice and palliative care organization serving Baltimore and central Maryland.
“I’m practicing family medicine to the extent that I’m treating the family unit, including the anxieties of the adult children, and finding solutions for the parents,” said Dr. Black, one of Gilchrist’s 62 employed providers, one third of whom are physicians. One of his most important roles is medication reconciliation and deprescribing.
Palliative care, a medical specialty that focuses on clarifying the treatment goals of seriously ill patients, helping with end-of-life planning, and emphasizing pain and symptom management, has been growing in recent years. Already well-established in most US hospitals, it is expanding in community settings, often as an extension of hospice programs.
Now, by adding primary care physicians and practices to their service mix, palliative care groups are better meeting the needs of a neglected — and costly — population of frail elders. In doing so, they also are better able to find a niche in the rapidly evolving alphabet soup of value-based care and its varieties of shared savings for providers who post positive outcomes.
Most patients Dr. Black sees find it difficult to visit their doctors in a clinic setting, although they face a variety of medical needs and chronic conditions of aging. They may have a prognosis of several years to live and, thus, do not qualify for hospice care. To him, a palliative approach offers the satisfaction of focusing on what is most important to patients at this difficult time in their lives, rather than predetermined clinical metrics like blood pressure or blood glucose. “It takes a lot of work, but it feels important and rewarding,” he said.
A recent survey of community-dwelling older adults in Ontario, Canada, found most patients fail to receive this treatment homes in the final 3 months of life.
Continuums of Patients and Models
Gilchrist started as a nonprofit, hospital-affiliated hospice program in 1994 and in 2000 took on the management of a geriatric medicine practice for its parent, Greater Baltimore Medical Center. Today, its physicians and nurse practitioners see a range of patients in geriatric primary care, palliative medicine, and hospice, according to its chief medical officer, Mark J. Gloth, DO.
“As people progress in their disease, their location — where they call home — may change as well,” Dr. Gloth said. “We offer a continuum of care in order to not lose people through those transitions. That’s the core of our mission — making sure we are there to escort people through the difficult moments in their lives.”
Models for value-based care encompass accountable care organizations (ACOs), including the ACO REACH (Realizing Equity, Access, and Community Health) high-needs model for traditional Medicare patients, and Medicare Shared Savings Programs for fee-for-service beneficiaries. These value-based models offer a variety of opportunities for the palliative care organization to share in savings resulting from keeping the patient out of the hospital or emergency room and other quality and cost benchmarks.
Coming Together to Meet Needs
Gilchrist is one of nine hospice and palliative organizations that have joined to form their own multistate ACO, Responsive Care Solutions, focused on the clinical needs of frail elderly Medicare beneficiaries. Hospice of the Valley in Phoenix has Geriatric Solutions, a frail elder physician practice. And Capital Caring Health, a hospice and palliative care agency serving metro Washington, DC, has deployed several physicians and nurse practitioners on the road doing primary care at home, said Heidi Young, MD, its Primary Care at Home Lead Physician.
“Five years ago, we started our primary care practice under the umbrella of a 40-year-old hospice organization because we thought we needed to prepare for the changes that are coming to the hospice model,” Dr. Young said. “The thought was that we’re not just a hospice organization; we’re an advanced illness organization. We will come to your home, whatever that is, and provide your primary care.”
The greatest potential gains for a hospice organization are from assuming 100% risk for a large population of patients, keeping them out of the hospital to lower the costs of their care, then reaping those gains under a value-based profit-sharing model, Dr. Young said.
“Our program is still new and working toward getting more patients aligned into value-based models,” she said. “It’s a work in progress.”
A Foot in Each World
Agencies like Capital Caring and Gilchrist derive the largest share of their physician income from billing Medicare Part B and other insurers per visit. But that billing is not enough to break even on physician services.
With hopes for a value-based future, Gilchrist also gets grants from elder-facing charitable foundations to cover up to 40% of the costs of its home-based primary care, according to its president, Catherine Hamel. Hospice care continues to be paid on a per-diem basis by Medicare for eligible terminally ill patients, including Medicare Advantage patients, although the Centers for Medicare & Medicaid Services is reportedly considering new models for the hospice benefit.
The National Partnership for Healthcare and Hospice Innovation (NPHI) is a trade group representing more than 100 nonprofit, hospice-based organizations participating in palliative care and value-based care.
For a hospice to be successful in the evolving post–acute care/end-of-life care landscape, it can no longer rely solely on its hospice line of business, no matter how high-quality, said Ethan McChesney, policy director for the Washington, DC-based nonprofit.
NPHI members have developed their own palliative care programs, and perhaps, a quarter have primary care at home practices, Mr. McChesney said. Some of them acquired existing primary care practices in their service area with which they already had relationships; others created their own.
For hospice organizations building a continuum of services for the seriously ill, adding a primary care at home practice is a natural fit, he said. “You can provide all the services you would as a traditional primary care practice while you have the opportunity to establish long-term relationships with patients and their caregivers that lend themselves to palliative care referrals and then hospice referrals downstream [when the patient becomes eligible for hospice care].” Often, this primary medical care is a mix of in-person and telehealth.
Cameron Muir, MD, NPHI’s chief innovation officer, noted that the hamster wheel for primary care doctors has been spinning faster and faster, with reimbursement going down and costs going up.
But with home-based primary care for frail elders under value-based models, Dr. Muir said, the clinician is paid not for making more visits but for taking great care of the patient: “And I’m actually saving Medicare money and getting credit for the hospitalizations that were avoided.”
A version of this article appeared on Medscape.com.
Bartonella henselae Infection May Occasionally Distract Immune Control of Latent Human Herpesviruses
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
Hemorrhagic Crescent Sign in Pseudocellulitis
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.
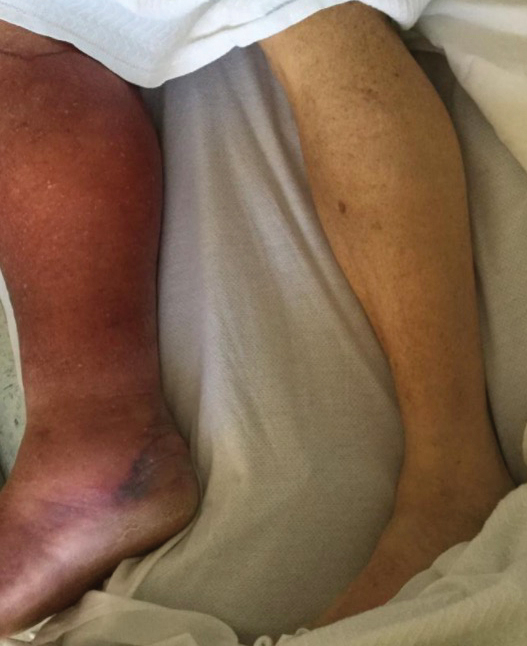
This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.

This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.

This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
Practice Points
- Pseudocellulitis is common in patients presenting with cellulitislike symptoms.
- A hemorrhagic crescent at the medial malleolus should lead to the suspicion on bursa or joint pathology as a cause of pseudocellulitis.
Poorly Controlled Asthma Equal to Greenhouse Gases From More Than 124,000 Homes
Asthma is not well controlled in about half of patients with the disease in the UK and Europe, increasing the risk of hospital admission and severe illness, and increasing healthcare costs.
Now, the authors of a new study have reported that poorly controlled asthma is also associated with a higher carbon footprint, eight times higher than that of well-controlled asthma and equivalent to the greenhouse gas emissions produced by more than 124,000 homes each year in the UK.
The study was published in the journal Thorax and is part of the Healthcare-Based Environmental Cost of Treatment (CARBON) programme, which aims to provide a broader understanding of the carbon footprint associated with respiratory care.
John Bell, BMBCh, medical director of BioPharmaceuticals Medical, AstraZeneca, and co-author of the study, said that he was surprised by the scale to which poorly controlled asthma contributed to the overall carbon footprint of asthma care. “This suggests that suboptimal asthma care is not just a public health issue, but also one which has environmental consequences,” he said.
Improving the care of asthma patients could help the NHS meet its net zero target, the authors suggested.
SABA – Largest Contributor to Asthma-Related Greenhouse Gases
Healthcare is a major contributor to greenhouse gas emissions. In 2020, the NHS set an ambitious target of reducing its carbon footprint by 80% over the next 15 years, with the aim of reaching net zero by 2045.
To estimate the environmental footprint of asthma care in the UK, the researchers retrospectively analyzed anonymized data of 236,506 people with asthma submitted to the Clinical Practice Research Datalink between 2008 and 2019.
Greenhouse gas (GHG) emissions, measured as carbon dioxide equivalent (CO2e), were then estimated for asthma-related medication use, healthcare resource utilization, and severe exacerbations.
Well-controlled asthma was considered as having no episodes of severe worsening symptoms and fewer than three prescriptions of short-acting beta-agonists (SABAs) reliever inhalers in a year. Poorly controlled asthma included three or more SABA canister prescriptions or one or more episodes of severe worsening symptoms in a year.
Almost one in two patients with asthma (47.3%) were categorized as being poorly controlled.
The researchers estimated the overall carbon footprint attributed to asthma care when scaled to the entire UK asthma population was 750,540 tonnes CO2e/year, with poorly controlled asthma contributing to excess GHG emissions of 303,874 tonnes CO2e/year.
“Poorly controlled asthma generated three-fold higher greenhouse gas emissions per capita compared with well-controlled asthma, when taking into account GHG emissions related to all aspects of asthma care, including routine prescribing and management,” Dr. Bell explained. It also generated eight-fold higher excess per capita carbon footprint compared to well-controlled asthma.
SABA relievers represented the largest contributors to per capita asthma-related GHG emissions, accounting for more than 60% of overall GHG emissions and more than 90% of excess GHG emissions. The remainder was mostly due to healthcare resource utilization, such as GP and hospital visits, required to treat severe worsening symptoms.
The researchers acknowledged various limitations to their findings, including that the study results were largely descriptive in nature. And factors other than the level of asthma symptom control, such as prescribing patterns, may also have contributed to high SABA use.
Couple Optimized Patient Outcomes With Environmental Targets
With inappropriate SABA use having emerged as the single largest contributor to asthma care-related GHG emissions, improving this care could achieve substantial carbon emissions savings and help the NHS meet its net zero target, the authors explained.
This improvement could include the adoption of the Global Initiative for Asthma (GINA) treatment strategies that, since 2019, no longer recommends that SABAs are used alone as the preferred reliever for acute asthma symptoms, the authors wrote.
However, the National Institute for Health and Care Excellence (NICE) asthma guidelines still recommend SABA alone as a reliever therapy.
On the other hand, the Primary Care Respiratory Society (PCRS) highlights on its website that the Medicines and Healthcare Products Regulatory Agency (MHRA) had approved the use of a dual (inhaled corticosteroid/formoterol) combination treatment to be used as a reliever therapy for people aged 12 and over.
“In the UK, this new therapy option does not yet sit within an approved national guideline as NICE last updated its treatment pathway in 2020. We await a new national asthma guideline but do not anticipate this new joint approach between NICE, BTS [British Thoracic Society], and SIGN [Scottish Intercollegiate Guidelines Network] to publish until 2024,” the society wrote.
Dr. Bell explained that the carbon footprint of asthma care increased with higher socio-economic deprivation. “Thus, targeting suboptimal care to areas of higher deprivation could help improve patient outcomes and address health inequities, with the additional benefit of reducing the overall carbon footprint of asthma care,” he said.
This coupling of optimized patient outcomes with environmental targets to decrease GHG emissions could be extended to other chronic progressive diseases, particularly those associated with multi-morbidities, the authors wrote.
Dr. Andy Whittamore, MBBS, clinical lead at Asthma + Lung UK, who was not involved in the research, said: “This study highlights that high levels of uncontrolled asthma not only put thousands of people at risk of life-threatening asthma attacks, but also have a detrimental effect on the environment. It’s important to point out that people shouldn’t stop taking their inhalers because they are worried about the environment. The best thing for the environment is to keep your asthma under control,” he emphasized.
Please refer to the study for full study author disclosures.Dr. Hicks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape UK.
Asthma is not well controlled in about half of patients with the disease in the UK and Europe, increasing the risk of hospital admission and severe illness, and increasing healthcare costs.
Now, the authors of a new study have reported that poorly controlled asthma is also associated with a higher carbon footprint, eight times higher than that of well-controlled asthma and equivalent to the greenhouse gas emissions produced by more than 124,000 homes each year in the UK.
The study was published in the journal Thorax and is part of the Healthcare-Based Environmental Cost of Treatment (CARBON) programme, which aims to provide a broader understanding of the carbon footprint associated with respiratory care.
John Bell, BMBCh, medical director of BioPharmaceuticals Medical, AstraZeneca, and co-author of the study, said that he was surprised by the scale to which poorly controlled asthma contributed to the overall carbon footprint of asthma care. “This suggests that suboptimal asthma care is not just a public health issue, but also one which has environmental consequences,” he said.
Improving the care of asthma patients could help the NHS meet its net zero target, the authors suggested.
SABA – Largest Contributor to Asthma-Related Greenhouse Gases
Healthcare is a major contributor to greenhouse gas emissions. In 2020, the NHS set an ambitious target of reducing its carbon footprint by 80% over the next 15 years, with the aim of reaching net zero by 2045.
To estimate the environmental footprint of asthma care in the UK, the researchers retrospectively analyzed anonymized data of 236,506 people with asthma submitted to the Clinical Practice Research Datalink between 2008 and 2019.
Greenhouse gas (GHG) emissions, measured as carbon dioxide equivalent (CO2e), were then estimated for asthma-related medication use, healthcare resource utilization, and severe exacerbations.
Well-controlled asthma was considered as having no episodes of severe worsening symptoms and fewer than three prescriptions of short-acting beta-agonists (SABAs) reliever inhalers in a year. Poorly controlled asthma included three or more SABA canister prescriptions or one or more episodes of severe worsening symptoms in a year.
Almost one in two patients with asthma (47.3%) were categorized as being poorly controlled.
The researchers estimated the overall carbon footprint attributed to asthma care when scaled to the entire UK asthma population was 750,540 tonnes CO2e/year, with poorly controlled asthma contributing to excess GHG emissions of 303,874 tonnes CO2e/year.
“Poorly controlled asthma generated three-fold higher greenhouse gas emissions per capita compared with well-controlled asthma, when taking into account GHG emissions related to all aspects of asthma care, including routine prescribing and management,” Dr. Bell explained. It also generated eight-fold higher excess per capita carbon footprint compared to well-controlled asthma.
SABA relievers represented the largest contributors to per capita asthma-related GHG emissions, accounting for more than 60% of overall GHG emissions and more than 90% of excess GHG emissions. The remainder was mostly due to healthcare resource utilization, such as GP and hospital visits, required to treat severe worsening symptoms.
The researchers acknowledged various limitations to their findings, including that the study results were largely descriptive in nature. And factors other than the level of asthma symptom control, such as prescribing patterns, may also have contributed to high SABA use.
Couple Optimized Patient Outcomes With Environmental Targets
With inappropriate SABA use having emerged as the single largest contributor to asthma care-related GHG emissions, improving this care could achieve substantial carbon emissions savings and help the NHS meet its net zero target, the authors explained.
This improvement could include the adoption of the Global Initiative for Asthma (GINA) treatment strategies that, since 2019, no longer recommends that SABAs are used alone as the preferred reliever for acute asthma symptoms, the authors wrote.
However, the National Institute for Health and Care Excellence (NICE) asthma guidelines still recommend SABA alone as a reliever therapy.
On the other hand, the Primary Care Respiratory Society (PCRS) highlights on its website that the Medicines and Healthcare Products Regulatory Agency (MHRA) had approved the use of a dual (inhaled corticosteroid/formoterol) combination treatment to be used as a reliever therapy for people aged 12 and over.
“In the UK, this new therapy option does not yet sit within an approved national guideline as NICE last updated its treatment pathway in 2020. We await a new national asthma guideline but do not anticipate this new joint approach between NICE, BTS [British Thoracic Society], and SIGN [Scottish Intercollegiate Guidelines Network] to publish until 2024,” the society wrote.
Dr. Bell explained that the carbon footprint of asthma care increased with higher socio-economic deprivation. “Thus, targeting suboptimal care to areas of higher deprivation could help improve patient outcomes and address health inequities, with the additional benefit of reducing the overall carbon footprint of asthma care,” he said.
This coupling of optimized patient outcomes with environmental targets to decrease GHG emissions could be extended to other chronic progressive diseases, particularly those associated with multi-morbidities, the authors wrote.
Dr. Andy Whittamore, MBBS, clinical lead at Asthma + Lung UK, who was not involved in the research, said: “This study highlights that high levels of uncontrolled asthma not only put thousands of people at risk of life-threatening asthma attacks, but also have a detrimental effect on the environment. It’s important to point out that people shouldn’t stop taking their inhalers because they are worried about the environment. The best thing for the environment is to keep your asthma under control,” he emphasized.
Please refer to the study for full study author disclosures.Dr. Hicks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape UK.
Asthma is not well controlled in about half of patients with the disease in the UK and Europe, increasing the risk of hospital admission and severe illness, and increasing healthcare costs.
Now, the authors of a new study have reported that poorly controlled asthma is also associated with a higher carbon footprint, eight times higher than that of well-controlled asthma and equivalent to the greenhouse gas emissions produced by more than 124,000 homes each year in the UK.
The study was published in the journal Thorax and is part of the Healthcare-Based Environmental Cost of Treatment (CARBON) programme, which aims to provide a broader understanding of the carbon footprint associated with respiratory care.
John Bell, BMBCh, medical director of BioPharmaceuticals Medical, AstraZeneca, and co-author of the study, said that he was surprised by the scale to which poorly controlled asthma contributed to the overall carbon footprint of asthma care. “This suggests that suboptimal asthma care is not just a public health issue, but also one which has environmental consequences,” he said.
Improving the care of asthma patients could help the NHS meet its net zero target, the authors suggested.
SABA – Largest Contributor to Asthma-Related Greenhouse Gases
Healthcare is a major contributor to greenhouse gas emissions. In 2020, the NHS set an ambitious target of reducing its carbon footprint by 80% over the next 15 years, with the aim of reaching net zero by 2045.
To estimate the environmental footprint of asthma care in the UK, the researchers retrospectively analyzed anonymized data of 236,506 people with asthma submitted to the Clinical Practice Research Datalink between 2008 and 2019.
Greenhouse gas (GHG) emissions, measured as carbon dioxide equivalent (CO2e), were then estimated for asthma-related medication use, healthcare resource utilization, and severe exacerbations.
Well-controlled asthma was considered as having no episodes of severe worsening symptoms and fewer than three prescriptions of short-acting beta-agonists (SABAs) reliever inhalers in a year. Poorly controlled asthma included three or more SABA canister prescriptions or one or more episodes of severe worsening symptoms in a year.
Almost one in two patients with asthma (47.3%) were categorized as being poorly controlled.
The researchers estimated the overall carbon footprint attributed to asthma care when scaled to the entire UK asthma population was 750,540 tonnes CO2e/year, with poorly controlled asthma contributing to excess GHG emissions of 303,874 tonnes CO2e/year.
“Poorly controlled asthma generated three-fold higher greenhouse gas emissions per capita compared with well-controlled asthma, when taking into account GHG emissions related to all aspects of asthma care, including routine prescribing and management,” Dr. Bell explained. It also generated eight-fold higher excess per capita carbon footprint compared to well-controlled asthma.
SABA relievers represented the largest contributors to per capita asthma-related GHG emissions, accounting for more than 60% of overall GHG emissions and more than 90% of excess GHG emissions. The remainder was mostly due to healthcare resource utilization, such as GP and hospital visits, required to treat severe worsening symptoms.
The researchers acknowledged various limitations to their findings, including that the study results were largely descriptive in nature. And factors other than the level of asthma symptom control, such as prescribing patterns, may also have contributed to high SABA use.
Couple Optimized Patient Outcomes With Environmental Targets
With inappropriate SABA use having emerged as the single largest contributor to asthma care-related GHG emissions, improving this care could achieve substantial carbon emissions savings and help the NHS meet its net zero target, the authors explained.
This improvement could include the adoption of the Global Initiative for Asthma (GINA) treatment strategies that, since 2019, no longer recommends that SABAs are used alone as the preferred reliever for acute asthma symptoms, the authors wrote.
However, the National Institute for Health and Care Excellence (NICE) asthma guidelines still recommend SABA alone as a reliever therapy.
On the other hand, the Primary Care Respiratory Society (PCRS) highlights on its website that the Medicines and Healthcare Products Regulatory Agency (MHRA) had approved the use of a dual (inhaled corticosteroid/formoterol) combination treatment to be used as a reliever therapy for people aged 12 and over.
“In the UK, this new therapy option does not yet sit within an approved national guideline as NICE last updated its treatment pathway in 2020. We await a new national asthma guideline but do not anticipate this new joint approach between NICE, BTS [British Thoracic Society], and SIGN [Scottish Intercollegiate Guidelines Network] to publish until 2024,” the society wrote.
Dr. Bell explained that the carbon footprint of asthma care increased with higher socio-economic deprivation. “Thus, targeting suboptimal care to areas of higher deprivation could help improve patient outcomes and address health inequities, with the additional benefit of reducing the overall carbon footprint of asthma care,” he said.
This coupling of optimized patient outcomes with environmental targets to decrease GHG emissions could be extended to other chronic progressive diseases, particularly those associated with multi-morbidities, the authors wrote.
Dr. Andy Whittamore, MBBS, clinical lead at Asthma + Lung UK, who was not involved in the research, said: “This study highlights that high levels of uncontrolled asthma not only put thousands of people at risk of life-threatening asthma attacks, but also have a detrimental effect on the environment. It’s important to point out that people shouldn’t stop taking their inhalers because they are worried about the environment. The best thing for the environment is to keep your asthma under control,” he emphasized.
Please refer to the study for full study author disclosures.Dr. Hicks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape UK.
FROM THORAX
Midwife’s Fake Vaccinations Deserve Harsh Punishment: Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan, at the Division of Medical Ethics at New York University’s Grossman School of Medicine.
Very recently, a homeopathic midwife in New York was fined $300,000 for giving out phony injections for kids who were looking to get immunized in order to go to school. She gave pellets, which are sometimes called nosodes, I believe, with homeopathic ingredients, meaning next to nothing in them, and then basically certified that these children — and there were over 1500 of them — were compliant with New York State requirements to be vaccinated to go to school.
However, homeopathy is straight-up bunk. We have seen it again and again discredited as just something that doesn’t work. It has a tradition, but it’s basically nonsense. It certainly doesn’t work as a way to vaccinate anybody.
This midwife basically lied and gave phony certification to the parents of these kids. I’m not talking about the COVID-19 vaccine. I’m talking measles, mumps, rubella, flu, and polio — the childhood immunization schedule. For whatever reason, they put their faith in her and she went along with this fraud.
I think the fine is appropriate, but I think she should be penalized further. Why? When you send 1500 kids to school, mostly in Long Island, New York, but to schools all over the place, you are setting up conditions to bring back epidemic diseases like measles.
We’re already seeing measles outbreaks. At least five states have them. There’s a significant measles outbreak in Philadelphia. Although I can’t say for sure, I believe those outbreaks are directly linked to parents, post–COVID-19, becoming vaccine hesitant and either not vaccinating and lying or going to alternative practitioners like this midwife and claiming that they have been vaccinated.
You’re doing harm not only to the children who you allow to go to school under phony pretenses, but also you’re putting their classmates at risk. We all know that measles is very, very contagious. You’re risking the return of a disease that leads to hospitalization and sometimes even death. That is basically unconscionable.
I think her license should be taken away and she should not be practicing anymore. I believe that anyone who is involved in this kind of phony, dangerous, fraudulent practice ought to be severely punished.
Pre–COVID-19, we had just about gotten rid of measles and mumps. We didn’t see these diseases. Sometimes parents got a bit lazy in childhood vaccination basically because we had used immunization to get rid of the diseases.
Going to alternative healers and allowing people to get away with fraudulent nonsense risks bringing back disabling and deadly killers is not fair to you, me, and other people who are put at risk. It’s not fair to the kids who go to school with other kids who they think are vaccinated but aren’t.
I’m Art Caplan, at the Division of Medical Ethics at the New York University Grossman School of Medicine. Thanks for watching.
Arthur L. Caplan, PhD, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan, at the Division of Medical Ethics at New York University’s Grossman School of Medicine.
Very recently, a homeopathic midwife in New York was fined $300,000 for giving out phony injections for kids who were looking to get immunized in order to go to school. She gave pellets, which are sometimes called nosodes, I believe, with homeopathic ingredients, meaning next to nothing in them, and then basically certified that these children — and there were over 1500 of them — were compliant with New York State requirements to be vaccinated to go to school.
However, homeopathy is straight-up bunk. We have seen it again and again discredited as just something that doesn’t work. It has a tradition, but it’s basically nonsense. It certainly doesn’t work as a way to vaccinate anybody.
This midwife basically lied and gave phony certification to the parents of these kids. I’m not talking about the COVID-19 vaccine. I’m talking measles, mumps, rubella, flu, and polio — the childhood immunization schedule. For whatever reason, they put their faith in her and she went along with this fraud.
I think the fine is appropriate, but I think she should be penalized further. Why? When you send 1500 kids to school, mostly in Long Island, New York, but to schools all over the place, you are setting up conditions to bring back epidemic diseases like measles.
We’re already seeing measles outbreaks. At least five states have them. There’s a significant measles outbreak in Philadelphia. Although I can’t say for sure, I believe those outbreaks are directly linked to parents, post–COVID-19, becoming vaccine hesitant and either not vaccinating and lying or going to alternative practitioners like this midwife and claiming that they have been vaccinated.
You’re doing harm not only to the children who you allow to go to school under phony pretenses, but also you’re putting their classmates at risk. We all know that measles is very, very contagious. You’re risking the return of a disease that leads to hospitalization and sometimes even death. That is basically unconscionable.
I think her license should be taken away and she should not be practicing anymore. I believe that anyone who is involved in this kind of phony, dangerous, fraudulent practice ought to be severely punished.
Pre–COVID-19, we had just about gotten rid of measles and mumps. We didn’t see these diseases. Sometimes parents got a bit lazy in childhood vaccination basically because we had used immunization to get rid of the diseases.
Going to alternative healers and allowing people to get away with fraudulent nonsense risks bringing back disabling and deadly killers is not fair to you, me, and other people who are put at risk. It’s not fair to the kids who go to school with other kids who they think are vaccinated but aren’t.
I’m Art Caplan, at the Division of Medical Ethics at the New York University Grossman School of Medicine. Thanks for watching.
Arthur L. Caplan, PhD, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan, at the Division of Medical Ethics at New York University’s Grossman School of Medicine.
Very recently, a homeopathic midwife in New York was fined $300,000 for giving out phony injections for kids who were looking to get immunized in order to go to school. She gave pellets, which are sometimes called nosodes, I believe, with homeopathic ingredients, meaning next to nothing in them, and then basically certified that these children — and there were over 1500 of them — were compliant with New York State requirements to be vaccinated to go to school.
However, homeopathy is straight-up bunk. We have seen it again and again discredited as just something that doesn’t work. It has a tradition, but it’s basically nonsense. It certainly doesn’t work as a way to vaccinate anybody.
This midwife basically lied and gave phony certification to the parents of these kids. I’m not talking about the COVID-19 vaccine. I’m talking measles, mumps, rubella, flu, and polio — the childhood immunization schedule. For whatever reason, they put their faith in her and she went along with this fraud.
I think the fine is appropriate, but I think she should be penalized further. Why? When you send 1500 kids to school, mostly in Long Island, New York, but to schools all over the place, you are setting up conditions to bring back epidemic diseases like measles.
We’re already seeing measles outbreaks. At least five states have them. There’s a significant measles outbreak in Philadelphia. Although I can’t say for sure, I believe those outbreaks are directly linked to parents, post–COVID-19, becoming vaccine hesitant and either not vaccinating and lying or going to alternative practitioners like this midwife and claiming that they have been vaccinated.
You’re doing harm not only to the children who you allow to go to school under phony pretenses, but also you’re putting their classmates at risk. We all know that measles is very, very contagious. You’re risking the return of a disease that leads to hospitalization and sometimes even death. That is basically unconscionable.
I think her license should be taken away and she should not be practicing anymore. I believe that anyone who is involved in this kind of phony, dangerous, fraudulent practice ought to be severely punished.
Pre–COVID-19, we had just about gotten rid of measles and mumps. We didn’t see these diseases. Sometimes parents got a bit lazy in childhood vaccination basically because we had used immunization to get rid of the diseases.
Going to alternative healers and allowing people to get away with fraudulent nonsense risks bringing back disabling and deadly killers is not fair to you, me, and other people who are put at risk. It’s not fair to the kids who go to school with other kids who they think are vaccinated but aren’t.
I’m Art Caplan, at the Division of Medical Ethics at the New York University Grossman School of Medicine. Thanks for watching.
Arthur L. Caplan, PhD, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Another Neurotoxin for Frown Lines Enters the Market
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.