User login
Blisters during pregnancy—just with the second husband
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
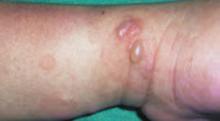
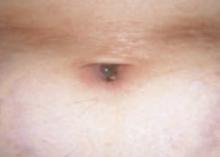
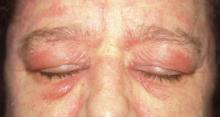
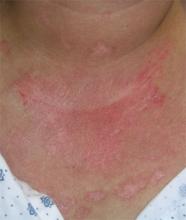
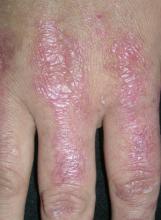
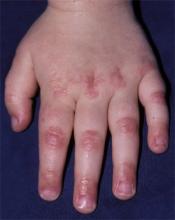
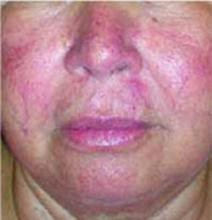
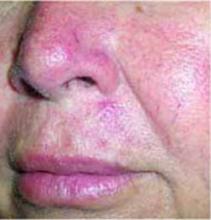
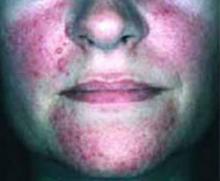
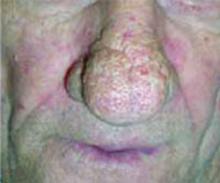
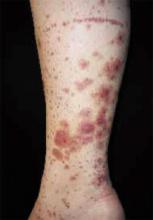
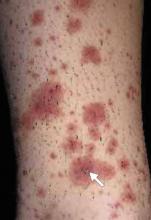
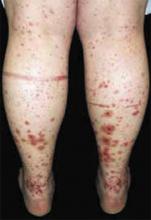
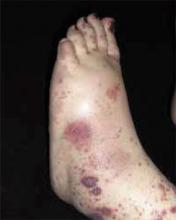
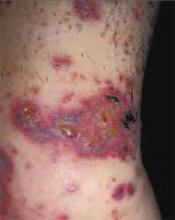
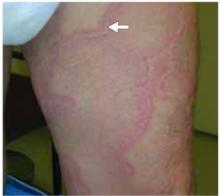
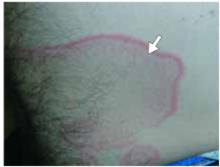
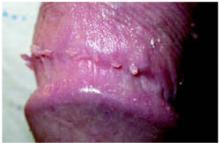
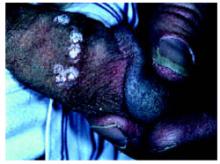
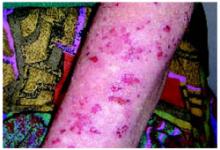
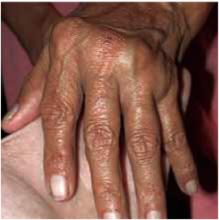
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
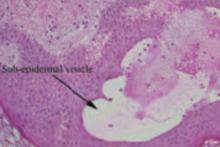
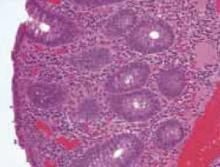
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
Itching and rash in a boy and his grandmother
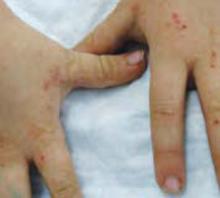
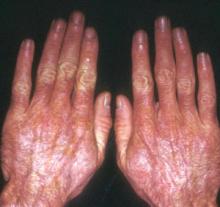
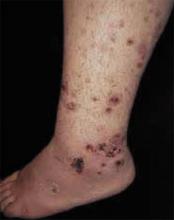
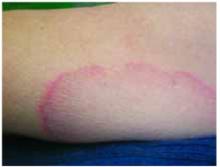
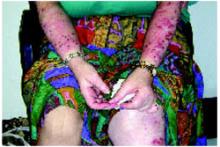
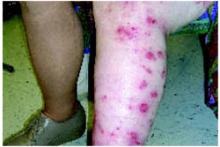
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
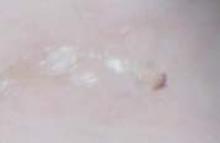
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
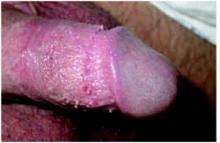
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
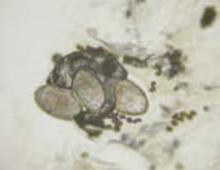
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: [email protected]
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: [email protected]
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: [email protected]
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.
Skin rash and muscle weakness
A 48-year-old Hispanic woman came to the clinic as a new patient—her chief complaint was a rash that appeared on her face 3 months before and had recently spread to her chest and hands (FIGURES 1-3). It itched occasionally and seemed to worsen after exposure to the sun.
She also said that for the last month she had been feeling very weak—she had difficulty rising from a seated position and walking up the stairs to her apartment. She also felt as if her arms were heavy, making it difficult for her to brush and dry her hair in the morning.
The patient was otherwise healthy with no known medical conditions, and she was not taking any medications. Her family history was noncontributory.
A musculoskeletal examination showed the following:
Upper extremities:
- 4/5 strength shoulder abduction, internal and external rotation
- 5/5 strength biceps, triceps, wrist extension/flexion, grip
- 2+ biceps and triceps deep tendon reflexes bilaterally
Lower extremities:
- 4/5 strength hip flexors, quadriceps, hamstrings
- 5/5 dorsiflexion/plantar flexion
- 2+ patellar and ankle deep tendon reflexes bilaterally.
FIGURE 1
Facial rash and swelling
FIGURE 2
Rash spreading to chest
FIGURE 3
Plaques on knuckles
What is your diagnosis?
What diagnostic tests would you order for confirmation?
Diagnosis: Dermatomyositis
Dermatomyositis is a systemic disease classified as a type of idiopathic inflammatory myopathy. Dermatomyositis is a rare disease but one that may present initially to the family physician. It affects people at any age but is more commonly seen among children or adults aged >40 years.
Dermatomyositis involves the skin as well as skeletal muscle. Its cause is unknown; however, among those aged >50 years, malignancy may be an underlying cause. Cancers most commonly associated with dermatomyositis include those of the breast, ovary, lung, and gastrointestinal tract.
Skin manifestations may precede, follow, or present simultaneously with muscle involvement. Patients often complain of having difficulty ascending stairs, rising from a seated position, and performing overhead activities such as combing their hair. Patients may or may not have muscle tenderness and atrophy. Patients can have cutaneous involvement for more than a year before developing muscle weakness.1
The dermatologic signs of dermatomyositis to watch for:
- Periorbital heliotrope erythema, usually associated with edema (FIGURE 1).
- Gottron’s papules—smooth, purple to red papules located over the knuckles, on the sides of the fingers, and sometimes on the elbows and knees. For adults, it is not uncommon to have plaques over the knuckles as opposed to the classic Gottron’s papules (FIGURE 3). In juvenile-onset dermatomyositis, distinct papules are much more evident upon presentation (FIGURE 4). Note that systemic lupus erythematosus (SLE) can present with a rash on the dorsum of the hands, but the rash spares the skin over the metacarpophalangeal and interphalangeal joints and affects the skin between the joints (FIGURE 5).
- Violaceous papular dermatitis with scale—may occur in localized areas, such as elbows and knees, or be diffusely distributed, starting off as a patchy erythema that coalesces and becomes slightly raised with scale. It tends to be confined to sun-exposed areas and worsens after sun exposure (FIGURE 2).
- Periungual erythema and telangiectasia—“moth-eaten” cuticles, a characteristic seen in other connective tissue diseases (FIGURE 6).1
FIGURE 4
Papules on knuckles
FIGURE 5
Sparing interphalangeal joints
FIGURE 6
Cuticles with erythema
Differential diagnosis
Seborrheic dermatitis—white or yellow, greasy scales on an erythematous base with distribution on scalp, nasolabial folds and chest.
Atopic dermatitis—chronic history of pruritic papules or plaques with scale localized to flexural areas or may be generalized; lichenification may be seen.
Contact dermatitis—papules and vesicles that correspond to contact with allergen.
Polymorphous light eruption—clusters of erythematous, pruritic papules or vesicles occurring most frequently on the neck, anterior chest, arms, and forearms following sun exposure; most common among women in their twenties.
Lichen planus—pruritic, purple, polygonal papules (4 Ps) that may involve hair, nails, and mucous membranes in addition to the skin; more common among women, with onset between 30 to 60 years of age; may last months to years.
Psoriasis—well-demarcated papules and plaques on an erythematous base with thick, silvery scale; characteristically found on elbows, knees, scalp, nails, and genitalia.
Steroid myopathy—A side effect of systemic steroids, usually seen 4 to 6 weeks after beginning of treatment.
Dermatomyositis-like reaction—onset of similar skin findings with initiation of the following medications and improvement with discontinuation: penicillamine, nonsteroidal anti-inflammatory drugs, and carbamazepine.
Overlap syndrome—The term “overlap” denotes that certain signs are seen in both dermatomyositis and other connective tissue diseases such as scleroderma, rheumatoid arthritis, and lupus erythematosus. Scleroderma and dermatomyositis are the most commonly associated conditions and have been termed sclerodermatomyositis or mixed connective disease. In mixed connective tissue disease, features of SLE, scleroderma, and polymyositis are evident such as malar rash, alopecia, Raynaud’s phenomenon, waxy-appearing skin, and proximal muscle weakness.1,2
Diagnostic tests: Muscle enzymes, EMG, biopsy
The diagnosis of dermatomyositis is confirmed by 3 laboratory tests: elevated muscle enzyme levels, electromyography, and muscle biopsy. A punch biopsy is helpful in differentiating dermatomyositis from other papulosquamous diseases such as lichen planus and psoriasis, but be careful as the histology of dermatomyositis is indistinguishable from cutaneous lupus erythematosus.
During the acute active phase, the following serum muscle enzymes may be elevated: creatine kinase (CK), lactate dehydrogenase (LDH), alanine aminotransferase (ALT or SGPT), aspartate aminotransferase (AST or SGOT), and aldolase. CK is elevated among 65% of patients and is most specific for muscle disease.3 Only one of the aforementioned enzymes may be elevated, so it is necessary to measure them all.
Measuring antibodies such as antinuclear antibody (ANA), Jo-1, SSA (Ro), SSB (La) supports the diagnosis if positive but dermatomyositis cannot be diagnosed solely on positive titers. It is not necessary to obtain an electromyograph or muscle biopsy for a patient with the characteristic skin findings and evidence of elevated muscle enzymes, as the diagnosis of dermatomyositis can be made with confidence. For a patient in whom the presentation is not as straightforward, it may be useful to obtain the electromyograph and muscle biopsy.
Management: Corticosteroids, watch for malignancy
Oral corticosteroids are the treatment of choice (strength of recommendation [SOR]: B).1,4 Prednisone 0.5 to 1.0 mg/kg body weight per day has been recommended until muscle enzyme levels trend toward normal limits, at which time you can taper the dose. Steroid myopathy is a potential side effect of this treatment regimen; it may occur 4 to 6 weeks after therapy starts.
Several steroid-sparing agents such as methotrexate and cyclosporine are being prescribed by clinicians for dermomyositis but with little published evidence to support effectiveness. Methotrexate is an option for those who do not respond to prednisone or are in need of a steroid-sparing agent secondary to side effects (SOR: C).2,3 A suggested regimen starts with 7.5 to 10 mg/wk, then increases the dose to 2.5 mg/wk until reaching a total dose of 25 mg/wk. The dose of prednisone should be decreased as the methotrexate dose increases. Azathioprine is another option to consider along with methotrexate, but choosing one agent over another or a combination of 2 agents remains empirical. With any of the immunosuppressants or immunomodulatory agents, it is important to look at their side-effect profiles and monitor the patient accordingly.
After initiating treatment, look for evidence of malignancy among those patients older than 50 years so as not to miss an underlying cancer as the cause for their dermatomyositis. For women without any risk factors, a complete annual physical exam—including pelvic, breast, and rectal exam—is sufficient.2 It is not necessary to order expensive radiological studies blindly searching for malignancy, especially more than 2 years after the diagnosis is made. The greatest risk of malignancy occurs during the first year after diagnosis with a six-fold increase.1 The risk drops during the second year and a patient’s risk for malignancy is comparable to the normal population in the years following. A mammogram and colonoscopy might be indicated after considering the patient’s age and family history.
The patient’s treatment and outcome
The patient was started on 60 mg of oral prednisone, taken in a single daily dose. She also began physical therapy twice a week in order to prevent muscle atrophy and maximize function. She took the prednisone for 1 month, at which time her creatine kinase level was trending towards normal. We then began slowly tapering the prednisone over the next 6 months.
She reported improvement in her strength 3 months after starting the systemic steroids. Little improvement was seen in the patient’s skin while on systemic steroids, but after prescribing 0.1% triamcinolone ointment, recommending a broad-spectrum sunscreen, and limiting sun exposure, the patient reported less erythema and edema.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia: Mosby; 2004.
2. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-982.
3. Woff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
4. Choy EHS, Hoogendijk JE, Lecky B, Winer JB. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2005;Issue 3:CD003643.
A 48-year-old Hispanic woman came to the clinic as a new patient—her chief complaint was a rash that appeared on her face 3 months before and had recently spread to her chest and hands (FIGURES 1-3). It itched occasionally and seemed to worsen after exposure to the sun.
She also said that for the last month she had been feeling very weak—she had difficulty rising from a seated position and walking up the stairs to her apartment. She also felt as if her arms were heavy, making it difficult for her to brush and dry her hair in the morning.
The patient was otherwise healthy with no known medical conditions, and she was not taking any medications. Her family history was noncontributory.
A musculoskeletal examination showed the following:
Upper extremities:
- 4/5 strength shoulder abduction, internal and external rotation
- 5/5 strength biceps, triceps, wrist extension/flexion, grip
- 2+ biceps and triceps deep tendon reflexes bilaterally
Lower extremities:
- 4/5 strength hip flexors, quadriceps, hamstrings
- 5/5 dorsiflexion/plantar flexion
- 2+ patellar and ankle deep tendon reflexes bilaterally.
FIGURE 1
Facial rash and swelling
FIGURE 2
Rash spreading to chest
FIGURE 3
Plaques on knuckles
What is your diagnosis?
What diagnostic tests would you order for confirmation?
Diagnosis: Dermatomyositis
Dermatomyositis is a systemic disease classified as a type of idiopathic inflammatory myopathy. Dermatomyositis is a rare disease but one that may present initially to the family physician. It affects people at any age but is more commonly seen among children or adults aged >40 years.
Dermatomyositis involves the skin as well as skeletal muscle. Its cause is unknown; however, among those aged >50 years, malignancy may be an underlying cause. Cancers most commonly associated with dermatomyositis include those of the breast, ovary, lung, and gastrointestinal tract.
Skin manifestations may precede, follow, or present simultaneously with muscle involvement. Patients often complain of having difficulty ascending stairs, rising from a seated position, and performing overhead activities such as combing their hair. Patients may or may not have muscle tenderness and atrophy. Patients can have cutaneous involvement for more than a year before developing muscle weakness.1
The dermatologic signs of dermatomyositis to watch for:
- Periorbital heliotrope erythema, usually associated with edema (FIGURE 1).
- Gottron’s papules—smooth, purple to red papules located over the knuckles, on the sides of the fingers, and sometimes on the elbows and knees. For adults, it is not uncommon to have plaques over the knuckles as opposed to the classic Gottron’s papules (FIGURE 3). In juvenile-onset dermatomyositis, distinct papules are much more evident upon presentation (FIGURE 4). Note that systemic lupus erythematosus (SLE) can present with a rash on the dorsum of the hands, but the rash spares the skin over the metacarpophalangeal and interphalangeal joints and affects the skin between the joints (FIGURE 5).
- Violaceous papular dermatitis with scale—may occur in localized areas, such as elbows and knees, or be diffusely distributed, starting off as a patchy erythema that coalesces and becomes slightly raised with scale. It tends to be confined to sun-exposed areas and worsens after sun exposure (FIGURE 2).
- Periungual erythema and telangiectasia—“moth-eaten” cuticles, a characteristic seen in other connective tissue diseases (FIGURE 6).1
FIGURE 4
Papules on knuckles
FIGURE 5
Sparing interphalangeal joints
FIGURE 6
Cuticles with erythema
Differential diagnosis
Seborrheic dermatitis—white or yellow, greasy scales on an erythematous base with distribution on scalp, nasolabial folds and chest.
Atopic dermatitis—chronic history of pruritic papules or plaques with scale localized to flexural areas or may be generalized; lichenification may be seen.
Contact dermatitis—papules and vesicles that correspond to contact with allergen.
Polymorphous light eruption—clusters of erythematous, pruritic papules or vesicles occurring most frequently on the neck, anterior chest, arms, and forearms following sun exposure; most common among women in their twenties.
Lichen planus—pruritic, purple, polygonal papules (4 Ps) that may involve hair, nails, and mucous membranes in addition to the skin; more common among women, with onset between 30 to 60 years of age; may last months to years.
Psoriasis—well-demarcated papules and plaques on an erythematous base with thick, silvery scale; characteristically found on elbows, knees, scalp, nails, and genitalia.
Steroid myopathy—A side effect of systemic steroids, usually seen 4 to 6 weeks after beginning of treatment.
Dermatomyositis-like reaction—onset of similar skin findings with initiation of the following medications and improvement with discontinuation: penicillamine, nonsteroidal anti-inflammatory drugs, and carbamazepine.
Overlap syndrome—The term “overlap” denotes that certain signs are seen in both dermatomyositis and other connective tissue diseases such as scleroderma, rheumatoid arthritis, and lupus erythematosus. Scleroderma and dermatomyositis are the most commonly associated conditions and have been termed sclerodermatomyositis or mixed connective disease. In mixed connective tissue disease, features of SLE, scleroderma, and polymyositis are evident such as malar rash, alopecia, Raynaud’s phenomenon, waxy-appearing skin, and proximal muscle weakness.1,2
Diagnostic tests: Muscle enzymes, EMG, biopsy
The diagnosis of dermatomyositis is confirmed by 3 laboratory tests: elevated muscle enzyme levels, electromyography, and muscle biopsy. A punch biopsy is helpful in differentiating dermatomyositis from other papulosquamous diseases such as lichen planus and psoriasis, but be careful as the histology of dermatomyositis is indistinguishable from cutaneous lupus erythematosus.
During the acute active phase, the following serum muscle enzymes may be elevated: creatine kinase (CK), lactate dehydrogenase (LDH), alanine aminotransferase (ALT or SGPT), aspartate aminotransferase (AST or SGOT), and aldolase. CK is elevated among 65% of patients and is most specific for muscle disease.3 Only one of the aforementioned enzymes may be elevated, so it is necessary to measure them all.
Measuring antibodies such as antinuclear antibody (ANA), Jo-1, SSA (Ro), SSB (La) supports the diagnosis if positive but dermatomyositis cannot be diagnosed solely on positive titers. It is not necessary to obtain an electromyograph or muscle biopsy for a patient with the characteristic skin findings and evidence of elevated muscle enzymes, as the diagnosis of dermatomyositis can be made with confidence. For a patient in whom the presentation is not as straightforward, it may be useful to obtain the electromyograph and muscle biopsy.
Management: Corticosteroids, watch for malignancy
Oral corticosteroids are the treatment of choice (strength of recommendation [SOR]: B).1,4 Prednisone 0.5 to 1.0 mg/kg body weight per day has been recommended until muscle enzyme levels trend toward normal limits, at which time you can taper the dose. Steroid myopathy is a potential side effect of this treatment regimen; it may occur 4 to 6 weeks after therapy starts.
Several steroid-sparing agents such as methotrexate and cyclosporine are being prescribed by clinicians for dermomyositis but with little published evidence to support effectiveness. Methotrexate is an option for those who do not respond to prednisone or are in need of a steroid-sparing agent secondary to side effects (SOR: C).2,3 A suggested regimen starts with 7.5 to 10 mg/wk, then increases the dose to 2.5 mg/wk until reaching a total dose of 25 mg/wk. The dose of prednisone should be decreased as the methotrexate dose increases. Azathioprine is another option to consider along with methotrexate, but choosing one agent over another or a combination of 2 agents remains empirical. With any of the immunosuppressants or immunomodulatory agents, it is important to look at their side-effect profiles and monitor the patient accordingly.
After initiating treatment, look for evidence of malignancy among those patients older than 50 years so as not to miss an underlying cancer as the cause for their dermatomyositis. For women without any risk factors, a complete annual physical exam—including pelvic, breast, and rectal exam—is sufficient.2 It is not necessary to order expensive radiological studies blindly searching for malignancy, especially more than 2 years after the diagnosis is made. The greatest risk of malignancy occurs during the first year after diagnosis with a six-fold increase.1 The risk drops during the second year and a patient’s risk for malignancy is comparable to the normal population in the years following. A mammogram and colonoscopy might be indicated after considering the patient’s age and family history.
The patient’s treatment and outcome
The patient was started on 60 mg of oral prednisone, taken in a single daily dose. She also began physical therapy twice a week in order to prevent muscle atrophy and maximize function. She took the prednisone for 1 month, at which time her creatine kinase level was trending towards normal. We then began slowly tapering the prednisone over the next 6 months.
She reported improvement in her strength 3 months after starting the systemic steroids. Little improvement was seen in the patient’s skin while on systemic steroids, but after prescribing 0.1% triamcinolone ointment, recommending a broad-spectrum sunscreen, and limiting sun exposure, the patient reported less erythema and edema.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 48-year-old Hispanic woman came to the clinic as a new patient—her chief complaint was a rash that appeared on her face 3 months before and had recently spread to her chest and hands (FIGURES 1-3). It itched occasionally and seemed to worsen after exposure to the sun.
She also said that for the last month she had been feeling very weak—she had difficulty rising from a seated position and walking up the stairs to her apartment. She also felt as if her arms were heavy, making it difficult for her to brush and dry her hair in the morning.
The patient was otherwise healthy with no known medical conditions, and she was not taking any medications. Her family history was noncontributory.
A musculoskeletal examination showed the following:
Upper extremities:
- 4/5 strength shoulder abduction, internal and external rotation
- 5/5 strength biceps, triceps, wrist extension/flexion, grip
- 2+ biceps and triceps deep tendon reflexes bilaterally
Lower extremities:
- 4/5 strength hip flexors, quadriceps, hamstrings
- 5/5 dorsiflexion/plantar flexion
- 2+ patellar and ankle deep tendon reflexes bilaterally.
FIGURE 1
Facial rash and swelling
FIGURE 2
Rash spreading to chest
FIGURE 3
Plaques on knuckles
What is your diagnosis?
What diagnostic tests would you order for confirmation?
Diagnosis: Dermatomyositis
Dermatomyositis is a systemic disease classified as a type of idiopathic inflammatory myopathy. Dermatomyositis is a rare disease but one that may present initially to the family physician. It affects people at any age but is more commonly seen among children or adults aged >40 years.
Dermatomyositis involves the skin as well as skeletal muscle. Its cause is unknown; however, among those aged >50 years, malignancy may be an underlying cause. Cancers most commonly associated with dermatomyositis include those of the breast, ovary, lung, and gastrointestinal tract.
Skin manifestations may precede, follow, or present simultaneously with muscle involvement. Patients often complain of having difficulty ascending stairs, rising from a seated position, and performing overhead activities such as combing their hair. Patients may or may not have muscle tenderness and atrophy. Patients can have cutaneous involvement for more than a year before developing muscle weakness.1
The dermatologic signs of dermatomyositis to watch for:
- Periorbital heliotrope erythema, usually associated with edema (FIGURE 1).
- Gottron’s papules—smooth, purple to red papules located over the knuckles, on the sides of the fingers, and sometimes on the elbows and knees. For adults, it is not uncommon to have plaques over the knuckles as opposed to the classic Gottron’s papules (FIGURE 3). In juvenile-onset dermatomyositis, distinct papules are much more evident upon presentation (FIGURE 4). Note that systemic lupus erythematosus (SLE) can present with a rash on the dorsum of the hands, but the rash spares the skin over the metacarpophalangeal and interphalangeal joints and affects the skin between the joints (FIGURE 5).
- Violaceous papular dermatitis with scale—may occur in localized areas, such as elbows and knees, or be diffusely distributed, starting off as a patchy erythema that coalesces and becomes slightly raised with scale. It tends to be confined to sun-exposed areas and worsens after sun exposure (FIGURE 2).
- Periungual erythema and telangiectasia—“moth-eaten” cuticles, a characteristic seen in other connective tissue diseases (FIGURE 6).1
FIGURE 4
Papules on knuckles
FIGURE 5
Sparing interphalangeal joints
FIGURE 6
Cuticles with erythema
Differential diagnosis
Seborrheic dermatitis—white or yellow, greasy scales on an erythematous base with distribution on scalp, nasolabial folds and chest.
Atopic dermatitis—chronic history of pruritic papules or plaques with scale localized to flexural areas or may be generalized; lichenification may be seen.
Contact dermatitis—papules and vesicles that correspond to contact with allergen.
Polymorphous light eruption—clusters of erythematous, pruritic papules or vesicles occurring most frequently on the neck, anterior chest, arms, and forearms following sun exposure; most common among women in their twenties.
Lichen planus—pruritic, purple, polygonal papules (4 Ps) that may involve hair, nails, and mucous membranes in addition to the skin; more common among women, with onset between 30 to 60 years of age; may last months to years.
Psoriasis—well-demarcated papules and plaques on an erythematous base with thick, silvery scale; characteristically found on elbows, knees, scalp, nails, and genitalia.
Steroid myopathy—A side effect of systemic steroids, usually seen 4 to 6 weeks after beginning of treatment.
Dermatomyositis-like reaction—onset of similar skin findings with initiation of the following medications and improvement with discontinuation: penicillamine, nonsteroidal anti-inflammatory drugs, and carbamazepine.
Overlap syndrome—The term “overlap” denotes that certain signs are seen in both dermatomyositis and other connective tissue diseases such as scleroderma, rheumatoid arthritis, and lupus erythematosus. Scleroderma and dermatomyositis are the most commonly associated conditions and have been termed sclerodermatomyositis or mixed connective disease. In mixed connective tissue disease, features of SLE, scleroderma, and polymyositis are evident such as malar rash, alopecia, Raynaud’s phenomenon, waxy-appearing skin, and proximal muscle weakness.1,2
Diagnostic tests: Muscle enzymes, EMG, biopsy
The diagnosis of dermatomyositis is confirmed by 3 laboratory tests: elevated muscle enzyme levels, electromyography, and muscle biopsy. A punch biopsy is helpful in differentiating dermatomyositis from other papulosquamous diseases such as lichen planus and psoriasis, but be careful as the histology of dermatomyositis is indistinguishable from cutaneous lupus erythematosus.
During the acute active phase, the following serum muscle enzymes may be elevated: creatine kinase (CK), lactate dehydrogenase (LDH), alanine aminotransferase (ALT or SGPT), aspartate aminotransferase (AST or SGOT), and aldolase. CK is elevated among 65% of patients and is most specific for muscle disease.3 Only one of the aforementioned enzymes may be elevated, so it is necessary to measure them all.
Measuring antibodies such as antinuclear antibody (ANA), Jo-1, SSA (Ro), SSB (La) supports the diagnosis if positive but dermatomyositis cannot be diagnosed solely on positive titers. It is not necessary to obtain an electromyograph or muscle biopsy for a patient with the characteristic skin findings and evidence of elevated muscle enzymes, as the diagnosis of dermatomyositis can be made with confidence. For a patient in whom the presentation is not as straightforward, it may be useful to obtain the electromyograph and muscle biopsy.
Management: Corticosteroids, watch for malignancy
Oral corticosteroids are the treatment of choice (strength of recommendation [SOR]: B).1,4 Prednisone 0.5 to 1.0 mg/kg body weight per day has been recommended until muscle enzyme levels trend toward normal limits, at which time you can taper the dose. Steroid myopathy is a potential side effect of this treatment regimen; it may occur 4 to 6 weeks after therapy starts.
Several steroid-sparing agents such as methotrexate and cyclosporine are being prescribed by clinicians for dermomyositis but with little published evidence to support effectiveness. Methotrexate is an option for those who do not respond to prednisone or are in need of a steroid-sparing agent secondary to side effects (SOR: C).2,3 A suggested regimen starts with 7.5 to 10 mg/wk, then increases the dose to 2.5 mg/wk until reaching a total dose of 25 mg/wk. The dose of prednisone should be decreased as the methotrexate dose increases. Azathioprine is another option to consider along with methotrexate, but choosing one agent over another or a combination of 2 agents remains empirical. With any of the immunosuppressants or immunomodulatory agents, it is important to look at their side-effect profiles and monitor the patient accordingly.
After initiating treatment, look for evidence of malignancy among those patients older than 50 years so as not to miss an underlying cancer as the cause for their dermatomyositis. For women without any risk factors, a complete annual physical exam—including pelvic, breast, and rectal exam—is sufficient.2 It is not necessary to order expensive radiological studies blindly searching for malignancy, especially more than 2 years after the diagnosis is made. The greatest risk of malignancy occurs during the first year after diagnosis with a six-fold increase.1 The risk drops during the second year and a patient’s risk for malignancy is comparable to the normal population in the years following. A mammogram and colonoscopy might be indicated after considering the patient’s age and family history.
The patient’s treatment and outcome
The patient was started on 60 mg of oral prednisone, taken in a single daily dose. She also began physical therapy twice a week in order to prevent muscle atrophy and maximize function. She took the prednisone for 1 month, at which time her creatine kinase level was trending towards normal. We then began slowly tapering the prednisone over the next 6 months.
She reported improvement in her strength 3 months after starting the systemic steroids. Little improvement was seen in the patient’s skin while on systemic steroids, but after prescribing 0.1% triamcinolone ointment, recommending a broad-spectrum sunscreen, and limiting sun exposure, the patient reported less erythema and edema.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia: Mosby; 2004.
2. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-982.
3. Woff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
4. Choy EHS, Hoogendijk JE, Lecky B, Winer JB. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2005;Issue 3:CD003643.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia: Mosby; 2004.
2. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-982.
3. Woff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
4. Choy EHS, Hoogendijk JE, Lecky B, Winer JB. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2005;Issue 3:CD003643.
Red facial rash with “granitos”
A 56-year-old Hispanic woman came to the office distraught about the red rash on her face. The rash has been on her cheeks and nose for 5 years, but over the past 2 weeks she has developed red, mildly pruritic, and very tender papules that she calls granitos (the Spanish term for pimples). Foods do not make the rash better or worse, but it gets redder with sun exposure. She had not tried any lotions or sought medical attention until now, when she noticed that the papules were increasing in number but not size.
A few years ago she first noticed small blood vessels becoming more prominent, especially on her cheeks. The lesions have not bled or ulcerated. Her health has been generally good within the past year, with no recent infections. Review of systems indicated no changes in vision, upper respiratory illness, or systemic conditions. She is being treated for type 2 diabetes and hypertension. She does not have a family history of the same rash. FIGURES 1 AND 2 show erythema and telangiectasias distributed symmetrically on both cheeks and nose. A cluster of smooth papules were seen under the nose. No scales were noted.
FIGURE 1
Facial rash
FIGURE 2
Close-up
What is the diagnosis?
How would you treat this condition?
Diagnosis: Rosacea
The patient has rosacea. Rosacea is a common inflammatory skin condition characterized by erythema, edema, papules, pustules, or telangiectasias, most notably found on the concave portions of the cheeks, forehead, chin, and nose.1 Its peak incidence is between the ages of 30 and 50 years.
Because of the marked flushing due to inflammation and capillary hyperreactivity and dilation, the rash has brought a certain degree of social embarrassment for some patients. Moreover, some equate rosacea with excessive alcohol consumption, but this is simply not true.
Although rosacea is a chronic disorder, it does alternate between periods of flare-up and remission. Flares may be triggered by stress, sun exposure, heat, hot drinks and spicy foods, alcohol, exercise, wind, and hot baths. In 50% of all rosacea patients, there are some ocular symptoms, ranging from mild dryness and grittiness to blepharitis, conjunctivitis, and even keratitis.2
Differential diagnosis
Although the differential diagnosis of rosacea is wide—acne, folliculitis, sarcoid, seborrheic dermatitis, and systemic lupus erythematosus [SLE]—there are unique characteristics that can help distinguish these from rosacea.
For example, the age of onset for rosacea tends to be 30 to 50 years, much later than the onset for acne vulgaris. Similarly, in acne vulgaris comedones are often present, but they are absent in rosacea.
Seborrheic dermatitis tends to produce scales while rosacea does not. Moreover, while both seborrheic dermatitis and rosacea can affect the central facial area, papules and telangiectasias are absent in the former while present with the latter.
SLE can be scarring, does not produce papules or pustules, and it spares the nasolabial folds and nose. Whereas rosacea has a more central face distribution, folliculitis typically presents with pustules visibly surrounding hair follicles and has associated tenderness to palpation and sometimes severe pruritus.
Causes and pathophysiology of rosacea
Although the cause of rosacea is unknown, its mechanism is understood to be nonspecific inflammation followed by dilation around follicles and hyperreactive capillaries. Oftentimes these dilated capillaries present as telangiectasias, which collectively exacerbate the red flushing. As the disorder progresses, diffuse hypertrophy of the connective tissue and sebaceous glands ensues.
Rosacea is more common in women than men (FIGURE 3). Men are typically more prone to the extreme forms of hyperplasia, which causes rhinophymatous rosacea (FIGURE 4)—eg,W C Fields’s nose.
Alcohol may accentuate erythema, but does not cause the disease. Sun exposure may precipitate an acute rosacea flare, but flare-ups can happen without sun exposure.
A significant increase in the hair follicle mite Demadex folliculorum is sometimes found in rosacea. It is theorized that these mites play a role because they incite an inflammatory or allergic reaction by mechanical blockage of follicles.
FIGURE 3
Papulopustular rosacea
FIGURE 4
Rhinophymatous rosacea
Stages of rosacea
There are 4 stages or subtypes of rosacea.
- Subtype 1: Erythematotelangiectatic rosacea. This stage is characterized by frequent mild to severe blushing with trace persistent central facial erythema in an individual.
- Subtype 2: Papulopustular rosacea (seen in FIGURES 1 , 2, AND 3). This is a highly vascular stage that involves longer periods of flushing than the first stage—often lasting from days to weeks. Minute telangiectasias and papules start to form by this stage, and some patients begin having very mild ocular complaints such as ocular grittiness or conjunctivitis.
Subtype 3: Phymatous rosacea. The third stage is also called the rhinophymatous stage. It is characterized by deepening shades of erythema and more papules and pustules. In the chronic forms of rosacea, hyperplasia of the sebaceous glands occurs, which forms a thickened confluent plaque of erythema at the tip of the nose known as rhinophyma. This hyperplasia can cause significant disfigurement to the forehead, eyelids, chin, and nose. The nasal disfiguration is seen more commonly in men than women (FIGURE 4).
Subtype 4: Ocular rosacea.The final or fourth stage is an advanced variation of rosacea that is characterized by impressive, severe flushing with persistent telangiectasias, papules, and pustules. At this point, more severe forms of conjunctivitis and blepharitis are more fullblown. The patient may complain of watery eyes, a foreign body sensation, burning, dryness, vision changes, and lid or periocular erythema.3
Diagnosis and treatment
Diagnostic tests
The diagnosis of rosacea is a clinical one. There is no confirmatory laboratory test. Biopsy is warranted only to rule out alternative diagnoses, since histopathological findings are not diagnostic. Scrapings may reveal Demadex folliculorum infection.1
Treatment: Target the inflammation It is important to reassure patients about the benign nature of the disorder as well as explain that its cause is unknown. It may be useful to direct patients to information, such as web sites like those of the National Rosacea Society (www.rosacea.org). Advise patients to keep a daily diary to identify precipitating factors. These can include hot and humid weather, alcohol, hot beverages, spicy foods, and large hot meals. Suggest daily application of sunscreen, which protects against UVA and UVB rays.
Depending on the severity of the skin rosacea, the first-line treatment is an oral antibiotic (tetracycline or erythromycin) and/or topical metronidazole (0.75%–1.0%) twice daily. These antibiotics target the inflammation because rosacea is not a true infection. There is concern in the medical literature about how long-term use of antibiotics can promote drug resistance. Wolf et al4 proposed that once a patient’s lesions have improved after treatment with full-dose antibiotics, the clinician can consider switching to a lower dose and adding a topical agent such as metronidazole for maintenance.4 Studies have shown that 1.0% and 0.75% cream are equally effective.
TABLERosacea treatments
| TREATMENT | DELIVERY | ODDS RATIO* (MEDICATION VS PLACEBO) |
|---|---|---|
| Azelaic acid | Topical | 2.45 |
| Metronidazole | Topical | 5.96 |
| Tetracycline | Oral | 6.06 |
| * Larger number indicates a more effective response to treatment. | ||
Another useful therapy is topical azelaic acid. While the evidence for scabicides in rosacea is limited, some clinicians use these medications for rosacea that is refractory to antibiotics. This is based on the idea that the Demadex mite is a causative agent in rosacea. If a patient has severe papulopustular disease refractory to antibiotics and topical treatments, the physician can start an oral isotretinoin regimen at a low dose of 0.1 to 0.5 mg/kg. Pulse-dye laser and electrosurgery can be used to treat the telangiectasias associated with rosacea.
A systematic review by Van Zuuren et al5 examined the efficacy of metronidazole, tetracycline, and azelaic acid in treating rosacea. Twenty-nine randomized controlled trials were found. Pooled data from 2 of the trials involving 174 participants indicated that, according to the participants, topical metronidazole was more effective than placebo (odds ratio [OR]=5.96; 95% confidence interval [CI], 2.95–12.06).
There was a definite improvement in the azelaic acid group; the rates of treatment success were approximately 70 to 80% versus 50% to 55% (OR=2.45; 95% CI, 1.82–3.28). Data pooled from 3 studies of oral tetracycline vs placebo involving 152 participants showed that, according to physicians, tetracycline was more effective than placebo (OR=6.06; 95% CI, 2.96–12.42).
Maintenance therapy
Because relapse occurs within weeks in about 25% of patients after the cessation of systemic therapy, topical therapy is usually used in an effort to maintain remission.6 The required duration of maintenance therapy is unknown, but a period of 6 months is generally advised. After this time, some patients report that they can keep their skin free of papulopustular lesions with topical therapy applied on alternate days or twice weekly, whereas others require repeated courses of systemic medication. After a few years, the disease may disappear spontaneously.
Patient management
Our patient was counseled about her rosacea and given the web site address for the National Rosacea Organization. She was advised to avoid sun exposure as much as possible and to use sunscreen and a hat to protect her face. She was prescribed tetracycline 500 mg and topical metronidazole to be used twice daily. Follow-up was set for 1 month. At that time, patient will be offered the option of electrocoagulation of her most prominent telangiectasias.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Wolf K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
2. Randleman J, Loft E. Ocular rosacea. eMedicine website. Available at: www.emedicine.com/oph/topic115.htm. Accessed on August 2, 2005.
3. National Rosacea Organization Website. Available at rosacea.org/grading/gradingsystem.pdf. Accessed on August 2, 2005.
4. Wolf J, Parkerson R. Preventing antibiotic resistance in the treatment of rosacea. Fam Pract Recertification 2005;27:50-55.
5. Van Zuuren E, Graber M, Hollis S, Chaudhry M, Gupta A, Gover M. Interventions for rosacea. Cochrane Database Syst Rev 2005;(3):CD003262.-
6. Powell F. Rosacea. N Engl J Med 2005;352:793-803.
A 56-year-old Hispanic woman came to the office distraught about the red rash on her face. The rash has been on her cheeks and nose for 5 years, but over the past 2 weeks she has developed red, mildly pruritic, and very tender papules that she calls granitos (the Spanish term for pimples). Foods do not make the rash better or worse, but it gets redder with sun exposure. She had not tried any lotions or sought medical attention until now, when she noticed that the papules were increasing in number but not size.
A few years ago she first noticed small blood vessels becoming more prominent, especially on her cheeks. The lesions have not bled or ulcerated. Her health has been generally good within the past year, with no recent infections. Review of systems indicated no changes in vision, upper respiratory illness, or systemic conditions. She is being treated for type 2 diabetes and hypertension. She does not have a family history of the same rash. FIGURES 1 AND 2 show erythema and telangiectasias distributed symmetrically on both cheeks and nose. A cluster of smooth papules were seen under the nose. No scales were noted.
FIGURE 1
Facial rash
FIGURE 2
Close-up
What is the diagnosis?
How would you treat this condition?
Diagnosis: Rosacea
The patient has rosacea. Rosacea is a common inflammatory skin condition characterized by erythema, edema, papules, pustules, or telangiectasias, most notably found on the concave portions of the cheeks, forehead, chin, and nose.1 Its peak incidence is between the ages of 30 and 50 years.
Because of the marked flushing due to inflammation and capillary hyperreactivity and dilation, the rash has brought a certain degree of social embarrassment for some patients. Moreover, some equate rosacea with excessive alcohol consumption, but this is simply not true.
Although rosacea is a chronic disorder, it does alternate between periods of flare-up and remission. Flares may be triggered by stress, sun exposure, heat, hot drinks and spicy foods, alcohol, exercise, wind, and hot baths. In 50% of all rosacea patients, there are some ocular symptoms, ranging from mild dryness and grittiness to blepharitis, conjunctivitis, and even keratitis.2
Differential diagnosis
Although the differential diagnosis of rosacea is wide—acne, folliculitis, sarcoid, seborrheic dermatitis, and systemic lupus erythematosus [SLE]—there are unique characteristics that can help distinguish these from rosacea.
For example, the age of onset for rosacea tends to be 30 to 50 years, much later than the onset for acne vulgaris. Similarly, in acne vulgaris comedones are often present, but they are absent in rosacea.
Seborrheic dermatitis tends to produce scales while rosacea does not. Moreover, while both seborrheic dermatitis and rosacea can affect the central facial area, papules and telangiectasias are absent in the former while present with the latter.
SLE can be scarring, does not produce papules or pustules, and it spares the nasolabial folds and nose. Whereas rosacea has a more central face distribution, folliculitis typically presents with pustules visibly surrounding hair follicles and has associated tenderness to palpation and sometimes severe pruritus.
Causes and pathophysiology of rosacea
Although the cause of rosacea is unknown, its mechanism is understood to be nonspecific inflammation followed by dilation around follicles and hyperreactive capillaries. Oftentimes these dilated capillaries present as telangiectasias, which collectively exacerbate the red flushing. As the disorder progresses, diffuse hypertrophy of the connective tissue and sebaceous glands ensues.
Rosacea is more common in women than men (FIGURE 3). Men are typically more prone to the extreme forms of hyperplasia, which causes rhinophymatous rosacea (FIGURE 4)—eg,W C Fields’s nose.
Alcohol may accentuate erythema, but does not cause the disease. Sun exposure may precipitate an acute rosacea flare, but flare-ups can happen without sun exposure.
A significant increase in the hair follicle mite Demadex folliculorum is sometimes found in rosacea. It is theorized that these mites play a role because they incite an inflammatory or allergic reaction by mechanical blockage of follicles.
FIGURE 3
Papulopustular rosacea
FIGURE 4
Rhinophymatous rosacea
Stages of rosacea
There are 4 stages or subtypes of rosacea.
- Subtype 1: Erythematotelangiectatic rosacea. This stage is characterized by frequent mild to severe blushing with trace persistent central facial erythema in an individual.
- Subtype 2: Papulopustular rosacea (seen in FIGURES 1 , 2, AND 3). This is a highly vascular stage that involves longer periods of flushing than the first stage—often lasting from days to weeks. Minute telangiectasias and papules start to form by this stage, and some patients begin having very mild ocular complaints such as ocular grittiness or conjunctivitis.
Subtype 3: Phymatous rosacea. The third stage is also called the rhinophymatous stage. It is characterized by deepening shades of erythema and more papules and pustules. In the chronic forms of rosacea, hyperplasia of the sebaceous glands occurs, which forms a thickened confluent plaque of erythema at the tip of the nose known as rhinophyma. This hyperplasia can cause significant disfigurement to the forehead, eyelids, chin, and nose. The nasal disfiguration is seen more commonly in men than women (FIGURE 4).
Subtype 4: Ocular rosacea.The final or fourth stage is an advanced variation of rosacea that is characterized by impressive, severe flushing with persistent telangiectasias, papules, and pustules. At this point, more severe forms of conjunctivitis and blepharitis are more fullblown. The patient may complain of watery eyes, a foreign body sensation, burning, dryness, vision changes, and lid or periocular erythema.3
Diagnosis and treatment
Diagnostic tests
The diagnosis of rosacea is a clinical one. There is no confirmatory laboratory test. Biopsy is warranted only to rule out alternative diagnoses, since histopathological findings are not diagnostic. Scrapings may reveal Demadex folliculorum infection.1
Treatment: Target the inflammation It is important to reassure patients about the benign nature of the disorder as well as explain that its cause is unknown. It may be useful to direct patients to information, such as web sites like those of the National Rosacea Society (www.rosacea.org). Advise patients to keep a daily diary to identify precipitating factors. These can include hot and humid weather, alcohol, hot beverages, spicy foods, and large hot meals. Suggest daily application of sunscreen, which protects against UVA and UVB rays.
Depending on the severity of the skin rosacea, the first-line treatment is an oral antibiotic (tetracycline or erythromycin) and/or topical metronidazole (0.75%–1.0%) twice daily. These antibiotics target the inflammation because rosacea is not a true infection. There is concern in the medical literature about how long-term use of antibiotics can promote drug resistance. Wolf et al4 proposed that once a patient’s lesions have improved after treatment with full-dose antibiotics, the clinician can consider switching to a lower dose and adding a topical agent such as metronidazole for maintenance.4 Studies have shown that 1.0% and 0.75% cream are equally effective.
TABLERosacea treatments
| TREATMENT | DELIVERY | ODDS RATIO* (MEDICATION VS PLACEBO) |
|---|---|---|
| Azelaic acid | Topical | 2.45 |
| Metronidazole | Topical | 5.96 |
| Tetracycline | Oral | 6.06 |
| * Larger number indicates a more effective response to treatment. | ||
Another useful therapy is topical azelaic acid. While the evidence for scabicides in rosacea is limited, some clinicians use these medications for rosacea that is refractory to antibiotics. This is based on the idea that the Demadex mite is a causative agent in rosacea. If a patient has severe papulopustular disease refractory to antibiotics and topical treatments, the physician can start an oral isotretinoin regimen at a low dose of 0.1 to 0.5 mg/kg. Pulse-dye laser and electrosurgery can be used to treat the telangiectasias associated with rosacea.
A systematic review by Van Zuuren et al5 examined the efficacy of metronidazole, tetracycline, and azelaic acid in treating rosacea. Twenty-nine randomized controlled trials were found. Pooled data from 2 of the trials involving 174 participants indicated that, according to the participants, topical metronidazole was more effective than placebo (odds ratio [OR]=5.96; 95% confidence interval [CI], 2.95–12.06).
There was a definite improvement in the azelaic acid group; the rates of treatment success were approximately 70 to 80% versus 50% to 55% (OR=2.45; 95% CI, 1.82–3.28). Data pooled from 3 studies of oral tetracycline vs placebo involving 152 participants showed that, according to physicians, tetracycline was more effective than placebo (OR=6.06; 95% CI, 2.96–12.42).
Maintenance therapy
Because relapse occurs within weeks in about 25% of patients after the cessation of systemic therapy, topical therapy is usually used in an effort to maintain remission.6 The required duration of maintenance therapy is unknown, but a period of 6 months is generally advised. After this time, some patients report that they can keep their skin free of papulopustular lesions with topical therapy applied on alternate days or twice weekly, whereas others require repeated courses of systemic medication. After a few years, the disease may disappear spontaneously.
Patient management
Our patient was counseled about her rosacea and given the web site address for the National Rosacea Organization. She was advised to avoid sun exposure as much as possible and to use sunscreen and a hat to protect her face. She was prescribed tetracycline 500 mg and topical metronidazole to be used twice daily. Follow-up was set for 1 month. At that time, patient will be offered the option of electrocoagulation of her most prominent telangiectasias.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 56-year-old Hispanic woman came to the office distraught about the red rash on her face. The rash has been on her cheeks and nose for 5 years, but over the past 2 weeks she has developed red, mildly pruritic, and very tender papules that she calls granitos (the Spanish term for pimples). Foods do not make the rash better or worse, but it gets redder with sun exposure. She had not tried any lotions or sought medical attention until now, when she noticed that the papules were increasing in number but not size.
A few years ago she first noticed small blood vessels becoming more prominent, especially on her cheeks. The lesions have not bled or ulcerated. Her health has been generally good within the past year, with no recent infections. Review of systems indicated no changes in vision, upper respiratory illness, or systemic conditions. She is being treated for type 2 diabetes and hypertension. She does not have a family history of the same rash. FIGURES 1 AND 2 show erythema and telangiectasias distributed symmetrically on both cheeks and nose. A cluster of smooth papules were seen under the nose. No scales were noted.
FIGURE 1
Facial rash
FIGURE 2
Close-up
What is the diagnosis?
How would you treat this condition?
Diagnosis: Rosacea
The patient has rosacea. Rosacea is a common inflammatory skin condition characterized by erythema, edema, papules, pustules, or telangiectasias, most notably found on the concave portions of the cheeks, forehead, chin, and nose.1 Its peak incidence is between the ages of 30 and 50 years.
Because of the marked flushing due to inflammation and capillary hyperreactivity and dilation, the rash has brought a certain degree of social embarrassment for some patients. Moreover, some equate rosacea with excessive alcohol consumption, but this is simply not true.
Although rosacea is a chronic disorder, it does alternate between periods of flare-up and remission. Flares may be triggered by stress, sun exposure, heat, hot drinks and spicy foods, alcohol, exercise, wind, and hot baths. In 50% of all rosacea patients, there are some ocular symptoms, ranging from mild dryness and grittiness to blepharitis, conjunctivitis, and even keratitis.2
Differential diagnosis
Although the differential diagnosis of rosacea is wide—acne, folliculitis, sarcoid, seborrheic dermatitis, and systemic lupus erythematosus [SLE]—there are unique characteristics that can help distinguish these from rosacea.
For example, the age of onset for rosacea tends to be 30 to 50 years, much later than the onset for acne vulgaris. Similarly, in acne vulgaris comedones are often present, but they are absent in rosacea.
Seborrheic dermatitis tends to produce scales while rosacea does not. Moreover, while both seborrheic dermatitis and rosacea can affect the central facial area, papules and telangiectasias are absent in the former while present with the latter.
SLE can be scarring, does not produce papules or pustules, and it spares the nasolabial folds and nose. Whereas rosacea has a more central face distribution, folliculitis typically presents with pustules visibly surrounding hair follicles and has associated tenderness to palpation and sometimes severe pruritus.
Causes and pathophysiology of rosacea
Although the cause of rosacea is unknown, its mechanism is understood to be nonspecific inflammation followed by dilation around follicles and hyperreactive capillaries. Oftentimes these dilated capillaries present as telangiectasias, which collectively exacerbate the red flushing. As the disorder progresses, diffuse hypertrophy of the connective tissue and sebaceous glands ensues.
Rosacea is more common in women than men (FIGURE 3). Men are typically more prone to the extreme forms of hyperplasia, which causes rhinophymatous rosacea (FIGURE 4)—eg,W C Fields’s nose.
Alcohol may accentuate erythema, but does not cause the disease. Sun exposure may precipitate an acute rosacea flare, but flare-ups can happen without sun exposure.
A significant increase in the hair follicle mite Demadex folliculorum is sometimes found in rosacea. It is theorized that these mites play a role because they incite an inflammatory or allergic reaction by mechanical blockage of follicles.
FIGURE 3
Papulopustular rosacea
FIGURE 4
Rhinophymatous rosacea
Stages of rosacea
There are 4 stages or subtypes of rosacea.
- Subtype 1: Erythematotelangiectatic rosacea. This stage is characterized by frequent mild to severe blushing with trace persistent central facial erythema in an individual.
- Subtype 2: Papulopustular rosacea (seen in FIGURES 1 , 2, AND 3). This is a highly vascular stage that involves longer periods of flushing than the first stage—often lasting from days to weeks. Minute telangiectasias and papules start to form by this stage, and some patients begin having very mild ocular complaints such as ocular grittiness or conjunctivitis.
Subtype 3: Phymatous rosacea. The third stage is also called the rhinophymatous stage. It is characterized by deepening shades of erythema and more papules and pustules. In the chronic forms of rosacea, hyperplasia of the sebaceous glands occurs, which forms a thickened confluent plaque of erythema at the tip of the nose known as rhinophyma. This hyperplasia can cause significant disfigurement to the forehead, eyelids, chin, and nose. The nasal disfiguration is seen more commonly in men than women (FIGURE 4).
Subtype 4: Ocular rosacea.The final or fourth stage is an advanced variation of rosacea that is characterized by impressive, severe flushing with persistent telangiectasias, papules, and pustules. At this point, more severe forms of conjunctivitis and blepharitis are more fullblown. The patient may complain of watery eyes, a foreign body sensation, burning, dryness, vision changes, and lid or periocular erythema.3
Diagnosis and treatment
Diagnostic tests
The diagnosis of rosacea is a clinical one. There is no confirmatory laboratory test. Biopsy is warranted only to rule out alternative diagnoses, since histopathological findings are not diagnostic. Scrapings may reveal Demadex folliculorum infection.1
Treatment: Target the inflammation It is important to reassure patients about the benign nature of the disorder as well as explain that its cause is unknown. It may be useful to direct patients to information, such as web sites like those of the National Rosacea Society (www.rosacea.org). Advise patients to keep a daily diary to identify precipitating factors. These can include hot and humid weather, alcohol, hot beverages, spicy foods, and large hot meals. Suggest daily application of sunscreen, which protects against UVA and UVB rays.
Depending on the severity of the skin rosacea, the first-line treatment is an oral antibiotic (tetracycline or erythromycin) and/or topical metronidazole (0.75%–1.0%) twice daily. These antibiotics target the inflammation because rosacea is not a true infection. There is concern in the medical literature about how long-term use of antibiotics can promote drug resistance. Wolf et al4 proposed that once a patient’s lesions have improved after treatment with full-dose antibiotics, the clinician can consider switching to a lower dose and adding a topical agent such as metronidazole for maintenance.4 Studies have shown that 1.0% and 0.75% cream are equally effective.
TABLERosacea treatments
| TREATMENT | DELIVERY | ODDS RATIO* (MEDICATION VS PLACEBO) |
|---|---|---|
| Azelaic acid | Topical | 2.45 |
| Metronidazole | Topical | 5.96 |
| Tetracycline | Oral | 6.06 |
| * Larger number indicates a more effective response to treatment. | ||
Another useful therapy is topical azelaic acid. While the evidence for scabicides in rosacea is limited, some clinicians use these medications for rosacea that is refractory to antibiotics. This is based on the idea that the Demadex mite is a causative agent in rosacea. If a patient has severe papulopustular disease refractory to antibiotics and topical treatments, the physician can start an oral isotretinoin regimen at a low dose of 0.1 to 0.5 mg/kg. Pulse-dye laser and electrosurgery can be used to treat the telangiectasias associated with rosacea.
A systematic review by Van Zuuren et al5 examined the efficacy of metronidazole, tetracycline, and azelaic acid in treating rosacea. Twenty-nine randomized controlled trials were found. Pooled data from 2 of the trials involving 174 participants indicated that, according to the participants, topical metronidazole was more effective than placebo (odds ratio [OR]=5.96; 95% confidence interval [CI], 2.95–12.06).
There was a definite improvement in the azelaic acid group; the rates of treatment success were approximately 70 to 80% versus 50% to 55% (OR=2.45; 95% CI, 1.82–3.28). Data pooled from 3 studies of oral tetracycline vs placebo involving 152 participants showed that, according to physicians, tetracycline was more effective than placebo (OR=6.06; 95% CI, 2.96–12.42).
Maintenance therapy
Because relapse occurs within weeks in about 25% of patients after the cessation of systemic therapy, topical therapy is usually used in an effort to maintain remission.6 The required duration of maintenance therapy is unknown, but a period of 6 months is generally advised. After this time, some patients report that they can keep their skin free of papulopustular lesions with topical therapy applied on alternate days or twice weekly, whereas others require repeated courses of systemic medication. After a few years, the disease may disappear spontaneously.
Patient management
Our patient was counseled about her rosacea and given the web site address for the National Rosacea Organization. She was advised to avoid sun exposure as much as possible and to use sunscreen and a hat to protect her face. She was prescribed tetracycline 500 mg and topical metronidazole to be used twice daily. Follow-up was set for 1 month. At that time, patient will be offered the option of electrocoagulation of her most prominent telangiectasias.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Wolf K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
2. Randleman J, Loft E. Ocular rosacea. eMedicine website. Available at: www.emedicine.com/oph/topic115.htm. Accessed on August 2, 2005.
3. National Rosacea Organization Website. Available at rosacea.org/grading/gradingsystem.pdf. Accessed on August 2, 2005.
4. Wolf J, Parkerson R. Preventing antibiotic resistance in the treatment of rosacea. Fam Pract Recertification 2005;27:50-55.
5. Van Zuuren E, Graber M, Hollis S, Chaudhry M, Gupta A, Gover M. Interventions for rosacea. Cochrane Database Syst Rev 2005;(3):CD003262.-
6. Powell F. Rosacea. N Engl J Med 2005;352:793-803.
1. Wolf K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
2. Randleman J, Loft E. Ocular rosacea. eMedicine website. Available at: www.emedicine.com/oph/topic115.htm. Accessed on August 2, 2005.
3. National Rosacea Organization Website. Available at rosacea.org/grading/gradingsystem.pdf. Accessed on August 2, 2005.
4. Wolf J, Parkerson R. Preventing antibiotic resistance in the treatment of rosacea. Fam Pract Recertification 2005;27:50-55.
5. Van Zuuren E, Graber M, Hollis S, Chaudhry M, Gupta A, Gover M. Interventions for rosacea. Cochrane Database Syst Rev 2005;(3):CD003262.-
6. Powell F. Rosacea. N Engl J Med 2005;352:793-803.
Abdominal pain in a pregnant woman
A 24-year-old woman, pregnant with a fetus at 22 weeks gestational age, came to the OB triage area with abdominal pain, nausea, and vomiting. She described a sharp pain that began the night before, starting at the umbilicus and radiating toward her right side; she rated it 7 out of 10.
The patient said there had been no contractions, vaginal bleeding or fluid leaking, or dysuria. She reported having GERD at times. She experienced chills the day before, but no fever. She had similar pain 1 month before that resolved spontaneously, and for which a cause was never determined. She had nothing significant in her medical history; family history was noncontributory.
On examination, she was afebrile, normotensive, and in no apparent distress. Her heart and lungs were normal. Her abdomen was soft and gravid with a fundal height of 22 cm. Bowel sounds were present in all 4 quadrants. Fetal heart tones were normal, and there was no indication of contractions. Her abdomen was diffusely tender, with significant tenderness to deep palpation in the right upper quadrant at first. There was no rebound or guarding. The psoas sign was negative. The obturator sign was positive, with increased pain 4 out of 10 in the right lower quadrant. There were no abdominal masses. Digital rectal examination revealed no rectal masses, and a guaiac stool test result was negative. A few hours later, the tenderness seemed to move toward the right lower quadrant (FIGURES 1 AND 2).
What is the most likely diagnosis?
How do the ultrasound images help you make the diagnosis?
FIGURE 1
Ultrasound of RLQ
FIGURE 2
A second ultrasound of RLQ
Differential diagnosis
The differential diagnosis of abdominal pain in a gravid patient includes placental abruption, cholecystitis, pancreatitis, appendicitis, intussusception, pyelonephritis, round ligament syndrome, hydronephrosis, ovarian torsion, uterine fibroid degeneration, ovarian cysts or tumors, intra-abdominal and rectus muscle abscesses, and Crohn’s disease with diffuse peritoneal inflammation. Given the location of the pain and the lack of vaginal bleeding, the most likely diagnoses are cholecystitis and appendicitis.
Making the diagnosis
We performed several laboratory analyses, including a complete blood count, chemistry panel (including electrolytes and liver function studies), amylase, lipase, and a urinalysis. The test results were all normal. She had a white blood cell count of 15,000/μL, which can be normal in pregnancy. The initial evaluating physician had obtained a right upper quadrant ultrasound, which showed no gallstones or bilateral hydronephrosis; unfortunately, no attempt was made to visualize the right lower quadrant or appendix at that time.
In light of the physical exam findings and the absence of gallstones, the patient was admitted to rule out appendicitis. The surgery team at the university hospital was consulted. They requested a computed tomography (CT) scan of the abdomen with and without contrast. To avoid the risk of radiation to the fetus, the family medicine team spoke with Radiology to obtain another ultrasound.
The ultrasound showed an enlarged and inflamed appendix with a transverse diameter of 13 mm (normal is <6 mm) (FIGURES 3 AND 4).1 A graded compression technique was used to assess the appendix. This involves using pressure of the ultrasound probe starting above the area of tenderness and working toward the tender area while scanning for the appendix. This showed obvious peristalsis in the cecum and no movement within the appendix, indicating obstruction or inflammation.
FIGURE 3
Appendix: Longitudinal view
FIGURE 4
Appendix: Transverse view
Patient management and outcome
An open appendectomy was performed. The appendix was inflamed and enlarged as suspected. The histology showed neutrophilic infiltration of mucosa, muscle, and serosa (FIGURE 5). Postoperatively, the patient recovered in Labor and Delivery to monitor for possible preterm labor. She did not develop any signs or symptoms of preterm labor, and was transferred to a regular antepartum floor after being observed for 6 hours.
She did well during her hospitalization, and was sent home on post-op day 2. Her abdominal pain had resolved, and she had very little post-op tenderness.
FIGURE 5
Histology
Discussion: Appendicitis in pregnancy
Acute appendicitis is the most common condition requiring surgery during pregnancy.2 Suspected appendicitis accounts for nearly two thirds of all nonobstetric exploratory laparotomies performed during pregnancy; most cases occur in the second and third trimesters.
The incidence of appendicitis is 0.4 to 1.4 per 1000 pregnancies.2 Although the incidence of appendicitis in not increased during pregnancy, rupture of the appendix occurs 2 to 3 times more frequently in pregnancy secondary to delays in diagnosis and operation. Maternal and perinatal mortality and morbidity rates are greatly increased when appendicitis is complicated by peritonitis.
A difficult diagnosis
Diagnosis is difficult because many symptoms are considered to be normal during pregnancy. Many times, pain in the right lower quadrant of the abdomen may be attributed to round ligament pain or urinary tract infection. After the first trimester, the appendix is gradually displaced above McBurney’s point, with horizontal rotation of its base. This upward displacement occurs until the eighth month of gestation, when more than 90% of appendices lie above the iliac crest, and 80% rotate upward and toward the right subcostal area.2,3
The most consistent clinical symptom encountered in pregnant women with appendicitis is vague right-sided abdominal pain.2 Depending on the gestation, muscle guarding and rebound tenderness may or may not be present. Nausea, vomiting, and anorexia are usually present as in the nonpregnant patient. Twenty five percent of pregnant patients with appendicitis are afebrile, as our patient was.2,4
The leukocytosis of pregnancy makes it difficult to determine if there is an infection. Not all pregnant patients with appendicitis will have a white blood cell count greater than 16,000/μL, but approximately 75% of them will have a left shift in the differential.2 A urinalysis may reveal pyuria and hematuria and can mislead the physician to explain the symptoms as pyelonephritis.2
Treatment: Appendectomy, antibiotics if needed
Treatment of nonperforated acute appendicitis in pregnancy is appendectomy. In the first trimester, a laparoscopic appendectomy may be performed.2 Intravenous antibiotics are indicated with perforation, peritonitis or abscess formation.2,5
Tocolysis is unnecessary in uncomplicated appendicitis, but may be indicated if the patient goes into labor after surgery. In the late third trimester, with perforation or peritonitis, a cesarean section is indicated.
Evaluation is imperative
Fetal loss may occur in association with preterm labor and delivery or with generalized peritonitis and sepsis, and occurs only rarely in uncomplicated appendicitis. Fetal loss appears to be more closely associated with severity of appendicitis than with surgical intervention.2,5,6
Imaging test characteristics: Is sonography enough?
Thus, it is imperative that any pregnant patient that comes in to the hospital or clinic with abdominal pain be evaluated for appendicitis. Ultrasound was a valuable diagnostic tool in this case and saved both the patient and developing fetus the radiation exposure of a CT scan. Ultrasound has a high specificity for diagnosing appendicitis if the appendix is visualized with abnormal findings. However, the sensitivity is not as high as CT, and failure to visualize the appendix adequately would have required a decision between appendectomy on clinical grounds only or going through with the CT scan.
The sensitivity, specificity, and positive and negative predictive values for ultrasonography and CT scans in the diagnosis of appendicitis are given in the TABLE (level of evidence [LOE]=1a).7
In a prospective study of patients with clinical signs and symptoms of acute appendicitis using a graded compression technique of ultrasonography, sonographic testing was as accurate as the focused unenhanced single-detector helical CT. The primary sonographic criterion for diagnosing acute appendicitis was an incompressible appendix with a transverse outer diameter of 6 mm or larger, as seen in this patient. The sensitivity of CT and sonography was 76% and 79%, respectively; the specificity was 83% and 78%; the accuracy was 78% and 78%; the positive predictive value was 90% and 87%; and the negative predictive value was 64% and 65% (LOE=2a).8
In conclusion, it is reasonable to use graded compression ultrasonography in a pregnant woman with suspected appendicitis. If the suspicion for appendicitis is high, a negative result may still need further evaluation with a CT or ultimately lead to abdominal surgery despite negative imaging studies.
TABLEUltrasound and CT in the diagnosis of appendicitis
| TEST | SN | SP | LR+ | LR– |
|---|---|---|---|---|
| Ultrasound | 0.86 (0.83–0.88) | 0.81 (0.78–0.84) | 5.8 (3.5–9.5) | .019 (0.13–0.27) |
| CT | 0.94 (0.91–0.95) | 0.95 (0.93–0.96) | 13.3 (9.9–17.9) | 0.09 (0.07–0.12) |
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; CT, computed tomography. | ||||
| Source: Teresawa et al, Ann Intern Med 2004.7 | ||||
Corresponding Author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Hansen GC, Toot PJ, Lynch CO. Subtle ultrasound signs of appendicitis in pregnancy. A case report. J Reprod Med 1993;3:223-224.
2. Tamir IL, Bongard FS, Klein SR. Acute appendicitis in the pregnant patient. Am J Surg 1990;160:571-576.
3. Hodjati H, Kazerooni T. Location of the appendix in the gravid patient: a re-evaluation of the established concept. Int J Gynecol Obstet 2003;81:245-247.
4. Morad JDO, Elliott JP, Lisboa L. Appendicitis in pregnancy:new information that contradicts long held clinical beliefs. Am J Obstet Gynecol 2002;182:1027-1029.
5. Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand 1999;78:758-762.
6. Thurnau GR, Hales KA. Appendicitis in pregnancy. Female Patient 1992;17:81.
7. Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med 2004;141:537-546.
8. Poortman P, Lohle PN, Schoemaker CM, et al. Comparison of CT and sonography in the diagnosis of acute appendicitis: a blinded prospective study. AJR Am J Roentgenol 2003;181:1355-1359.
A 24-year-old woman, pregnant with a fetus at 22 weeks gestational age, came to the OB triage area with abdominal pain, nausea, and vomiting. She described a sharp pain that began the night before, starting at the umbilicus and radiating toward her right side; she rated it 7 out of 10.
The patient said there had been no contractions, vaginal bleeding or fluid leaking, or dysuria. She reported having GERD at times. She experienced chills the day before, but no fever. She had similar pain 1 month before that resolved spontaneously, and for which a cause was never determined. She had nothing significant in her medical history; family history was noncontributory.
On examination, she was afebrile, normotensive, and in no apparent distress. Her heart and lungs were normal. Her abdomen was soft and gravid with a fundal height of 22 cm. Bowel sounds were present in all 4 quadrants. Fetal heart tones were normal, and there was no indication of contractions. Her abdomen was diffusely tender, with significant tenderness to deep palpation in the right upper quadrant at first. There was no rebound or guarding. The psoas sign was negative. The obturator sign was positive, with increased pain 4 out of 10 in the right lower quadrant. There were no abdominal masses. Digital rectal examination revealed no rectal masses, and a guaiac stool test result was negative. A few hours later, the tenderness seemed to move toward the right lower quadrant (FIGURES 1 AND 2).
What is the most likely diagnosis?
How do the ultrasound images help you make the diagnosis?
FIGURE 1
Ultrasound of RLQ
FIGURE 2
A second ultrasound of RLQ
Differential diagnosis
The differential diagnosis of abdominal pain in a gravid patient includes placental abruption, cholecystitis, pancreatitis, appendicitis, intussusception, pyelonephritis, round ligament syndrome, hydronephrosis, ovarian torsion, uterine fibroid degeneration, ovarian cysts or tumors, intra-abdominal and rectus muscle abscesses, and Crohn’s disease with diffuse peritoneal inflammation. Given the location of the pain and the lack of vaginal bleeding, the most likely diagnoses are cholecystitis and appendicitis.
Making the diagnosis
We performed several laboratory analyses, including a complete blood count, chemistry panel (including electrolytes and liver function studies), amylase, lipase, and a urinalysis. The test results were all normal. She had a white blood cell count of 15,000/μL, which can be normal in pregnancy. The initial evaluating physician had obtained a right upper quadrant ultrasound, which showed no gallstones or bilateral hydronephrosis; unfortunately, no attempt was made to visualize the right lower quadrant or appendix at that time.
In light of the physical exam findings and the absence of gallstones, the patient was admitted to rule out appendicitis. The surgery team at the university hospital was consulted. They requested a computed tomography (CT) scan of the abdomen with and without contrast. To avoid the risk of radiation to the fetus, the family medicine team spoke with Radiology to obtain another ultrasound.
The ultrasound showed an enlarged and inflamed appendix with a transverse diameter of 13 mm (normal is <6 mm) (FIGURES 3 AND 4).1 A graded compression technique was used to assess the appendix. This involves using pressure of the ultrasound probe starting above the area of tenderness and working toward the tender area while scanning for the appendix. This showed obvious peristalsis in the cecum and no movement within the appendix, indicating obstruction or inflammation.
FIGURE 3
Appendix: Longitudinal view
FIGURE 4
Appendix: Transverse view
Patient management and outcome
An open appendectomy was performed. The appendix was inflamed and enlarged as suspected. The histology showed neutrophilic infiltration of mucosa, muscle, and serosa (FIGURE 5). Postoperatively, the patient recovered in Labor and Delivery to monitor for possible preterm labor. She did not develop any signs or symptoms of preterm labor, and was transferred to a regular antepartum floor after being observed for 6 hours.
She did well during her hospitalization, and was sent home on post-op day 2. Her abdominal pain had resolved, and she had very little post-op tenderness.
FIGURE 5
Histology
Discussion: Appendicitis in pregnancy
Acute appendicitis is the most common condition requiring surgery during pregnancy.2 Suspected appendicitis accounts for nearly two thirds of all nonobstetric exploratory laparotomies performed during pregnancy; most cases occur in the second and third trimesters.
The incidence of appendicitis is 0.4 to 1.4 per 1000 pregnancies.2 Although the incidence of appendicitis in not increased during pregnancy, rupture of the appendix occurs 2 to 3 times more frequently in pregnancy secondary to delays in diagnosis and operation. Maternal and perinatal mortality and morbidity rates are greatly increased when appendicitis is complicated by peritonitis.
A difficult diagnosis
Diagnosis is difficult because many symptoms are considered to be normal during pregnancy. Many times, pain in the right lower quadrant of the abdomen may be attributed to round ligament pain or urinary tract infection. After the first trimester, the appendix is gradually displaced above McBurney’s point, with horizontal rotation of its base. This upward displacement occurs until the eighth month of gestation, when more than 90% of appendices lie above the iliac crest, and 80% rotate upward and toward the right subcostal area.2,3
The most consistent clinical symptom encountered in pregnant women with appendicitis is vague right-sided abdominal pain.2 Depending on the gestation, muscle guarding and rebound tenderness may or may not be present. Nausea, vomiting, and anorexia are usually present as in the nonpregnant patient. Twenty five percent of pregnant patients with appendicitis are afebrile, as our patient was.2,4
The leukocytosis of pregnancy makes it difficult to determine if there is an infection. Not all pregnant patients with appendicitis will have a white blood cell count greater than 16,000/μL, but approximately 75% of them will have a left shift in the differential.2 A urinalysis may reveal pyuria and hematuria and can mislead the physician to explain the symptoms as pyelonephritis.2
Treatment: Appendectomy, antibiotics if needed
Treatment of nonperforated acute appendicitis in pregnancy is appendectomy. In the first trimester, a laparoscopic appendectomy may be performed.2 Intravenous antibiotics are indicated with perforation, peritonitis or abscess formation.2,5
Tocolysis is unnecessary in uncomplicated appendicitis, but may be indicated if the patient goes into labor after surgery. In the late third trimester, with perforation or peritonitis, a cesarean section is indicated.
Evaluation is imperative
Fetal loss may occur in association with preterm labor and delivery or with generalized peritonitis and sepsis, and occurs only rarely in uncomplicated appendicitis. Fetal loss appears to be more closely associated with severity of appendicitis than with surgical intervention.2,5,6
Imaging test characteristics: Is sonography enough?
Thus, it is imperative that any pregnant patient that comes in to the hospital or clinic with abdominal pain be evaluated for appendicitis. Ultrasound was a valuable diagnostic tool in this case and saved both the patient and developing fetus the radiation exposure of a CT scan. Ultrasound has a high specificity for diagnosing appendicitis if the appendix is visualized with abnormal findings. However, the sensitivity is not as high as CT, and failure to visualize the appendix adequately would have required a decision between appendectomy on clinical grounds only or going through with the CT scan.
The sensitivity, specificity, and positive and negative predictive values for ultrasonography and CT scans in the diagnosis of appendicitis are given in the TABLE (level of evidence [LOE]=1a).7
In a prospective study of patients with clinical signs and symptoms of acute appendicitis using a graded compression technique of ultrasonography, sonographic testing was as accurate as the focused unenhanced single-detector helical CT. The primary sonographic criterion for diagnosing acute appendicitis was an incompressible appendix with a transverse outer diameter of 6 mm or larger, as seen in this patient. The sensitivity of CT and sonography was 76% and 79%, respectively; the specificity was 83% and 78%; the accuracy was 78% and 78%; the positive predictive value was 90% and 87%; and the negative predictive value was 64% and 65% (LOE=2a).8
In conclusion, it is reasonable to use graded compression ultrasonography in a pregnant woman with suspected appendicitis. If the suspicion for appendicitis is high, a negative result may still need further evaluation with a CT or ultimately lead to abdominal surgery despite negative imaging studies.
TABLEUltrasound and CT in the diagnosis of appendicitis
| TEST | SN | SP | LR+ | LR– |
|---|---|---|---|---|
| Ultrasound | 0.86 (0.83–0.88) | 0.81 (0.78–0.84) | 5.8 (3.5–9.5) | .019 (0.13–0.27) |
| CT | 0.94 (0.91–0.95) | 0.95 (0.93–0.96) | 13.3 (9.9–17.9) | 0.09 (0.07–0.12) |
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; CT, computed tomography. | ||||
| Source: Teresawa et al, Ann Intern Med 2004.7 | ||||
Corresponding Author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 24-year-old woman, pregnant with a fetus at 22 weeks gestational age, came to the OB triage area with abdominal pain, nausea, and vomiting. She described a sharp pain that began the night before, starting at the umbilicus and radiating toward her right side; she rated it 7 out of 10.
The patient said there had been no contractions, vaginal bleeding or fluid leaking, or dysuria. She reported having GERD at times. She experienced chills the day before, but no fever. She had similar pain 1 month before that resolved spontaneously, and for which a cause was never determined. She had nothing significant in her medical history; family history was noncontributory.
On examination, she was afebrile, normotensive, and in no apparent distress. Her heart and lungs were normal. Her abdomen was soft and gravid with a fundal height of 22 cm. Bowel sounds were present in all 4 quadrants. Fetal heart tones were normal, and there was no indication of contractions. Her abdomen was diffusely tender, with significant tenderness to deep palpation in the right upper quadrant at first. There was no rebound or guarding. The psoas sign was negative. The obturator sign was positive, with increased pain 4 out of 10 in the right lower quadrant. There were no abdominal masses. Digital rectal examination revealed no rectal masses, and a guaiac stool test result was negative. A few hours later, the tenderness seemed to move toward the right lower quadrant (FIGURES 1 AND 2).
What is the most likely diagnosis?
How do the ultrasound images help you make the diagnosis?
FIGURE 1
Ultrasound of RLQ
FIGURE 2
A second ultrasound of RLQ
Differential diagnosis
The differential diagnosis of abdominal pain in a gravid patient includes placental abruption, cholecystitis, pancreatitis, appendicitis, intussusception, pyelonephritis, round ligament syndrome, hydronephrosis, ovarian torsion, uterine fibroid degeneration, ovarian cysts or tumors, intra-abdominal and rectus muscle abscesses, and Crohn’s disease with diffuse peritoneal inflammation. Given the location of the pain and the lack of vaginal bleeding, the most likely diagnoses are cholecystitis and appendicitis.
Making the diagnosis
We performed several laboratory analyses, including a complete blood count, chemistry panel (including electrolytes and liver function studies), amylase, lipase, and a urinalysis. The test results were all normal. She had a white blood cell count of 15,000/μL, which can be normal in pregnancy. The initial evaluating physician had obtained a right upper quadrant ultrasound, which showed no gallstones or bilateral hydronephrosis; unfortunately, no attempt was made to visualize the right lower quadrant or appendix at that time.
In light of the physical exam findings and the absence of gallstones, the patient was admitted to rule out appendicitis. The surgery team at the university hospital was consulted. They requested a computed tomography (CT) scan of the abdomen with and without contrast. To avoid the risk of radiation to the fetus, the family medicine team spoke with Radiology to obtain another ultrasound.
The ultrasound showed an enlarged and inflamed appendix with a transverse diameter of 13 mm (normal is <6 mm) (FIGURES 3 AND 4).1 A graded compression technique was used to assess the appendix. This involves using pressure of the ultrasound probe starting above the area of tenderness and working toward the tender area while scanning for the appendix. This showed obvious peristalsis in the cecum and no movement within the appendix, indicating obstruction or inflammation.
FIGURE 3
Appendix: Longitudinal view
FIGURE 4
Appendix: Transverse view
Patient management and outcome
An open appendectomy was performed. The appendix was inflamed and enlarged as suspected. The histology showed neutrophilic infiltration of mucosa, muscle, and serosa (FIGURE 5). Postoperatively, the patient recovered in Labor and Delivery to monitor for possible preterm labor. She did not develop any signs or symptoms of preterm labor, and was transferred to a regular antepartum floor after being observed for 6 hours.
She did well during her hospitalization, and was sent home on post-op day 2. Her abdominal pain had resolved, and she had very little post-op tenderness.
FIGURE 5
Histology
Discussion: Appendicitis in pregnancy
Acute appendicitis is the most common condition requiring surgery during pregnancy.2 Suspected appendicitis accounts for nearly two thirds of all nonobstetric exploratory laparotomies performed during pregnancy; most cases occur in the second and third trimesters.
The incidence of appendicitis is 0.4 to 1.4 per 1000 pregnancies.2 Although the incidence of appendicitis in not increased during pregnancy, rupture of the appendix occurs 2 to 3 times more frequently in pregnancy secondary to delays in diagnosis and operation. Maternal and perinatal mortality and morbidity rates are greatly increased when appendicitis is complicated by peritonitis.
A difficult diagnosis
Diagnosis is difficult because many symptoms are considered to be normal during pregnancy. Many times, pain in the right lower quadrant of the abdomen may be attributed to round ligament pain or urinary tract infection. After the first trimester, the appendix is gradually displaced above McBurney’s point, with horizontal rotation of its base. This upward displacement occurs until the eighth month of gestation, when more than 90% of appendices lie above the iliac crest, and 80% rotate upward and toward the right subcostal area.2,3
The most consistent clinical symptom encountered in pregnant women with appendicitis is vague right-sided abdominal pain.2 Depending on the gestation, muscle guarding and rebound tenderness may or may not be present. Nausea, vomiting, and anorexia are usually present as in the nonpregnant patient. Twenty five percent of pregnant patients with appendicitis are afebrile, as our patient was.2,4
The leukocytosis of pregnancy makes it difficult to determine if there is an infection. Not all pregnant patients with appendicitis will have a white blood cell count greater than 16,000/μL, but approximately 75% of them will have a left shift in the differential.2 A urinalysis may reveal pyuria and hematuria and can mislead the physician to explain the symptoms as pyelonephritis.2
Treatment: Appendectomy, antibiotics if needed
Treatment of nonperforated acute appendicitis in pregnancy is appendectomy. In the first trimester, a laparoscopic appendectomy may be performed.2 Intravenous antibiotics are indicated with perforation, peritonitis or abscess formation.2,5
Tocolysis is unnecessary in uncomplicated appendicitis, but may be indicated if the patient goes into labor after surgery. In the late third trimester, with perforation or peritonitis, a cesarean section is indicated.
Evaluation is imperative
Fetal loss may occur in association with preterm labor and delivery or with generalized peritonitis and sepsis, and occurs only rarely in uncomplicated appendicitis. Fetal loss appears to be more closely associated with severity of appendicitis than with surgical intervention.2,5,6
Imaging test characteristics: Is sonography enough?
Thus, it is imperative that any pregnant patient that comes in to the hospital or clinic with abdominal pain be evaluated for appendicitis. Ultrasound was a valuable diagnostic tool in this case and saved both the patient and developing fetus the radiation exposure of a CT scan. Ultrasound has a high specificity for diagnosing appendicitis if the appendix is visualized with abnormal findings. However, the sensitivity is not as high as CT, and failure to visualize the appendix adequately would have required a decision between appendectomy on clinical grounds only or going through with the CT scan.
The sensitivity, specificity, and positive and negative predictive values for ultrasonography and CT scans in the diagnosis of appendicitis are given in the TABLE (level of evidence [LOE]=1a).7
In a prospective study of patients with clinical signs and symptoms of acute appendicitis using a graded compression technique of ultrasonography, sonographic testing was as accurate as the focused unenhanced single-detector helical CT. The primary sonographic criterion for diagnosing acute appendicitis was an incompressible appendix with a transverse outer diameter of 6 mm or larger, as seen in this patient. The sensitivity of CT and sonography was 76% and 79%, respectively; the specificity was 83% and 78%; the accuracy was 78% and 78%; the positive predictive value was 90% and 87%; and the negative predictive value was 64% and 65% (LOE=2a).8
In conclusion, it is reasonable to use graded compression ultrasonography in a pregnant woman with suspected appendicitis. If the suspicion for appendicitis is high, a negative result may still need further evaluation with a CT or ultimately lead to abdominal surgery despite negative imaging studies.
TABLEUltrasound and CT in the diagnosis of appendicitis
| TEST | SN | SP | LR+ | LR– |
|---|---|---|---|---|
| Ultrasound | 0.86 (0.83–0.88) | 0.81 (0.78–0.84) | 5.8 (3.5–9.5) | .019 (0.13–0.27) |
| CT | 0.94 (0.91–0.95) | 0.95 (0.93–0.96) | 13.3 (9.9–17.9) | 0.09 (0.07–0.12) |
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; CT, computed tomography. | ||||
| Source: Teresawa et al, Ann Intern Med 2004.7 | ||||
Corresponding Author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Hansen GC, Toot PJ, Lynch CO. Subtle ultrasound signs of appendicitis in pregnancy. A case report. J Reprod Med 1993;3:223-224.
2. Tamir IL, Bongard FS, Klein SR. Acute appendicitis in the pregnant patient. Am J Surg 1990;160:571-576.
3. Hodjati H, Kazerooni T. Location of the appendix in the gravid patient: a re-evaluation of the established concept. Int J Gynecol Obstet 2003;81:245-247.
4. Morad JDO, Elliott JP, Lisboa L. Appendicitis in pregnancy:new information that contradicts long held clinical beliefs. Am J Obstet Gynecol 2002;182:1027-1029.
5. Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand 1999;78:758-762.
6. Thurnau GR, Hales KA. Appendicitis in pregnancy. Female Patient 1992;17:81.
7. Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med 2004;141:537-546.
8. Poortman P, Lohle PN, Schoemaker CM, et al. Comparison of CT and sonography in the diagnosis of acute appendicitis: a blinded prospective study. AJR Am J Roentgenol 2003;181:1355-1359.
1. Hansen GC, Toot PJ, Lynch CO. Subtle ultrasound signs of appendicitis in pregnancy. A case report. J Reprod Med 1993;3:223-224.
2. Tamir IL, Bongard FS, Klein SR. Acute appendicitis in the pregnant patient. Am J Surg 1990;160:571-576.
3. Hodjati H, Kazerooni T. Location of the appendix in the gravid patient: a re-evaluation of the established concept. Int J Gynecol Obstet 2003;81:245-247.
4. Morad JDO, Elliott JP, Lisboa L. Appendicitis in pregnancy:new information that contradicts long held clinical beliefs. Am J Obstet Gynecol 2002;182:1027-1029.
5. Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand 1999;78:758-762.
6. Thurnau GR, Hales KA. Appendicitis in pregnancy. Female Patient 1992;17:81.
7. Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med 2004;141:537-546.
8. Poortman P, Lohle PN, Schoemaker CM, et al. Comparison of CT and sonography in the diagnosis of acute appendicitis: a blinded prospective study. AJR Am J Roentgenol 2003;181:1355-1359.
Palpable purpura and a visible sock line
A 21-year-old woman came to the clinic, frightened by a painful purpuric rash on her lower extremities (FIGURES 1 AND 2). The lesions appeared suddenly 3 days before, with no prior similar episodes.
The pain, and some swelling that happened when she stood, had finally driven her to take some time off from her job and seek medical advice. She was diagnosed with a case of pharyngitis earlier that week; due to multiple drug allergies, she was prescribed a course of clindamycin. She had not experienced any nausea or vomiting, fever, abdominal cramping, or gross hematuria.
On examination, the patient was friendly and good-humored, although she was concerned about her rash and visibly uncomfortable. She was walking with the aid of a borrowed cane, but her lesions were no longer tender to palpation.
The rash consisted mainly of purpuric papules almost entirely limited to her legs, although some isolated lesions were on her back as well. The papules were concentrated around her distal lower extremities, with a clear line of lesions encircling her calves bilaterally where her knee-high socks had applied pressure for the last 2 days (FIGURE 3) Mild edema was noted, but the rest of her physical exam was normal. By dip-stick, the patient had blood in her urine but no protein.
FIGURE 1
Purpura on the lower leg
FIGURE 2
Close-up
FIGURE 3
Visible sock lines
What is the diagnosis?
What is the treatment for this condition?
Diagnosis: Henoch-Schönlein purpura
This patient has Henoch-Schönlein purpura (HSP), a systemic vasculitis, secondary to hypersensitivity, occurring most commonly in children and young adults. Its classic triad includes palpable purpura, abdominal pain or renal involvement, and arthritis.1
Purpura is typically nonblanching, as it represents extravasation of blood into skin, 2 but lesions may also include urticarial wheals or occasional target lesions. As seen in this patient, the lower extremities and buttocks are typically affected. This rash is pressure- and gravity-dependent and pressure lines may develop, causing “sock lines” such as those seen on this young woman’s calves.1,2 Arthritis also tends to affect large joints in lower extremities more severely than upper extremities, and ankles and knees may be swollen and tender, as observed in our patient.
Abdominal pain, not present in this case, develops in up to 65% of cases, and may be accompanied by vomiting, hematemesis, or blood in stools.
Renal involvement is by far the most long-reaching and potentially serious complication of HSP, particularly in adults. Although only 1% of all HSP patients may develop end-stage renal failure, it is estimated that in adults, this number may be as high as 11%.3,4 The young woman in question presented with microscopic hematuria initially, which had resolved by the time of her next visit, 4 days later. A worse prognosis is associated with those patients who exhibit both nephritic and nephrotic features, with hematuria and proteinuria, where clinical remission has been shown to present only in 20% of these patients. Finally, as renal involvement may develop up to years after initial diagnosis, follow-up is crucial to monitor renal function.4
Cause is unknown
The cause of HSP is unknown, although it is thought to be secondary to deposition of immune complexes, and has been associated with vaccinations, viral infections, allergens in foods, drug reactions, exposure to cold, and bacterial respiratory infections, particularly involving group A streptococci.1,2 This patient had been diagnosed with streptococcal pharyngitis and had been given a course of clindamycin due to her numerous drug allergies. Although the precise cause of her HSP is not definite, drugs have been shown to cause approximately 10% of acute cutaneous vasculitis cases, and she had never before been given clindamycin, possibly pointing to the origin of her disease.5
Differential diagnoses
Differential diagnoses for HSP depend on the level of specificity with which the triad presents. When symptoms are not typical, abdominal cramping alone may be confused with acute abdomen. Rash may be predominantly in the form of target lesions, and can be mistaken for erythema multiforme, particularly after a suspected drug reaction. Rheumatoid arthritis or systemic lupus erythematosus may be suspected with arthralgias. Idiopathic thrombocytopenic purpura can be distinguished from HSP by platelet count. Trauma or meningococcal septicemia may also be mistaken for the correct diagnosis in the presence of an atypical rash with few accompanying symptoms.
Treatment: Steroids, NSAIDs, and careful monitoring of renal function
Corticosteroids may be administered for severe abdominal symptoms, but they have not been shown to be useful in the absence of such symptoms. Conservative management with NSAIDs is the treatment of choice, and careful monitoring of renal function, even in the absence of renal involvement.1,3,5
Outcome and discussion
Due to the presence of the classic HSP triad in our patient, we deemed a biopsy necessary, and managed the patient with ibupro-fen. One week later, her rash had receded and her microscopic hematuria was gone, but the vasculitis had led to lower-extremity edema. The patient’s feet were painfully swollen, and where the patient had scratched a lesion, her foot exhibited honey crusting and warmth, indicating a probable infection (FIGURES 4 AND 5). Her feet and ankles were so swollen and painful that she had difficulty walking. She was given cephalexin 500 mg 4 times daily for her infection, and was also restarted on oral prednisone at 60 mg daily until she could see the rheumatologist 2 days later. She was also issued crutches.
The rheumatologist agreed that this was HSP and told her that she would need to continue the prednisone for 2 months due to the severity of her cutaneous vasculitis. Within 5 days, the patient’s rash had diminished significantly. She was able to walk with less pain, as her ankle swelling decreased (FIGURE 6).
FIGURE 4
Swollen ankle
FIGURE 5
Probable infection
FIGURE 6
The same patient after 5 days
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Kraft DM, McKee D, Scott C. Henoch-Schonlein purpura: a review. Am Fam Physician 1998;58:405-8,411.-
2. Leung AKC, Chan KW. Evaluating the child with purpura. Am Fam Physician 2001;64:419-428.
3. Usatine RP. A 4-year-old girl with a rash and joint pain. West J Med 1999;171:116-117.
4. Pillebout E, Thervet E, Hill G, et al. Henoch-Schonlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002;13:1271-1278.
5. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272-1285.
A 21-year-old woman came to the clinic, frightened by a painful purpuric rash on her lower extremities (FIGURES 1 AND 2). The lesions appeared suddenly 3 days before, with no prior similar episodes.
The pain, and some swelling that happened when she stood, had finally driven her to take some time off from her job and seek medical advice. She was diagnosed with a case of pharyngitis earlier that week; due to multiple drug allergies, she was prescribed a course of clindamycin. She had not experienced any nausea or vomiting, fever, abdominal cramping, or gross hematuria.
On examination, the patient was friendly and good-humored, although she was concerned about her rash and visibly uncomfortable. She was walking with the aid of a borrowed cane, but her lesions were no longer tender to palpation.
The rash consisted mainly of purpuric papules almost entirely limited to her legs, although some isolated lesions were on her back as well. The papules were concentrated around her distal lower extremities, with a clear line of lesions encircling her calves bilaterally where her knee-high socks had applied pressure for the last 2 days (FIGURE 3) Mild edema was noted, but the rest of her physical exam was normal. By dip-stick, the patient had blood in her urine but no protein.
FIGURE 1
Purpura on the lower leg
FIGURE 2
Close-up
FIGURE 3
Visible sock lines
What is the diagnosis?
What is the treatment for this condition?
Diagnosis: Henoch-Schönlein purpura
This patient has Henoch-Schönlein purpura (HSP), a systemic vasculitis, secondary to hypersensitivity, occurring most commonly in children and young adults. Its classic triad includes palpable purpura, abdominal pain or renal involvement, and arthritis.1
Purpura is typically nonblanching, as it represents extravasation of blood into skin, 2 but lesions may also include urticarial wheals or occasional target lesions. As seen in this patient, the lower extremities and buttocks are typically affected. This rash is pressure- and gravity-dependent and pressure lines may develop, causing “sock lines” such as those seen on this young woman’s calves.1,2 Arthritis also tends to affect large joints in lower extremities more severely than upper extremities, and ankles and knees may be swollen and tender, as observed in our patient.
Abdominal pain, not present in this case, develops in up to 65% of cases, and may be accompanied by vomiting, hematemesis, or blood in stools.
Renal involvement is by far the most long-reaching and potentially serious complication of HSP, particularly in adults. Although only 1% of all HSP patients may develop end-stage renal failure, it is estimated that in adults, this number may be as high as 11%.3,4 The young woman in question presented with microscopic hematuria initially, which had resolved by the time of her next visit, 4 days later. A worse prognosis is associated with those patients who exhibit both nephritic and nephrotic features, with hematuria and proteinuria, where clinical remission has been shown to present only in 20% of these patients. Finally, as renal involvement may develop up to years after initial diagnosis, follow-up is crucial to monitor renal function.4
Cause is unknown
The cause of HSP is unknown, although it is thought to be secondary to deposition of immune complexes, and has been associated with vaccinations, viral infections, allergens in foods, drug reactions, exposure to cold, and bacterial respiratory infections, particularly involving group A streptococci.1,2 This patient had been diagnosed with streptococcal pharyngitis and had been given a course of clindamycin due to her numerous drug allergies. Although the precise cause of her HSP is not definite, drugs have been shown to cause approximately 10% of acute cutaneous vasculitis cases, and she had never before been given clindamycin, possibly pointing to the origin of her disease.5
Differential diagnoses
Differential diagnoses for HSP depend on the level of specificity with which the triad presents. When symptoms are not typical, abdominal cramping alone may be confused with acute abdomen. Rash may be predominantly in the form of target lesions, and can be mistaken for erythema multiforme, particularly after a suspected drug reaction. Rheumatoid arthritis or systemic lupus erythematosus may be suspected with arthralgias. Idiopathic thrombocytopenic purpura can be distinguished from HSP by platelet count. Trauma or meningococcal septicemia may also be mistaken for the correct diagnosis in the presence of an atypical rash with few accompanying symptoms.
Treatment: Steroids, NSAIDs, and careful monitoring of renal function
Corticosteroids may be administered for severe abdominal symptoms, but they have not been shown to be useful in the absence of such symptoms. Conservative management with NSAIDs is the treatment of choice, and careful monitoring of renal function, even in the absence of renal involvement.1,3,5
Outcome and discussion
Due to the presence of the classic HSP triad in our patient, we deemed a biopsy necessary, and managed the patient with ibupro-fen. One week later, her rash had receded and her microscopic hematuria was gone, but the vasculitis had led to lower-extremity edema. The patient’s feet were painfully swollen, and where the patient had scratched a lesion, her foot exhibited honey crusting and warmth, indicating a probable infection (FIGURES 4 AND 5). Her feet and ankles were so swollen and painful that she had difficulty walking. She was given cephalexin 500 mg 4 times daily for her infection, and was also restarted on oral prednisone at 60 mg daily until she could see the rheumatologist 2 days later. She was also issued crutches.
The rheumatologist agreed that this was HSP and told her that she would need to continue the prednisone for 2 months due to the severity of her cutaneous vasculitis. Within 5 days, the patient’s rash had diminished significantly. She was able to walk with less pain, as her ankle swelling decreased (FIGURE 6).
FIGURE 4
Swollen ankle
FIGURE 5
Probable infection
FIGURE 6
The same patient after 5 days
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 21-year-old woman came to the clinic, frightened by a painful purpuric rash on her lower extremities (FIGURES 1 AND 2). The lesions appeared suddenly 3 days before, with no prior similar episodes.
The pain, and some swelling that happened when she stood, had finally driven her to take some time off from her job and seek medical advice. She was diagnosed with a case of pharyngitis earlier that week; due to multiple drug allergies, she was prescribed a course of clindamycin. She had not experienced any nausea or vomiting, fever, abdominal cramping, or gross hematuria.
On examination, the patient was friendly and good-humored, although she was concerned about her rash and visibly uncomfortable. She was walking with the aid of a borrowed cane, but her lesions were no longer tender to palpation.
The rash consisted mainly of purpuric papules almost entirely limited to her legs, although some isolated lesions were on her back as well. The papules were concentrated around her distal lower extremities, with a clear line of lesions encircling her calves bilaterally where her knee-high socks had applied pressure for the last 2 days (FIGURE 3) Mild edema was noted, but the rest of her physical exam was normal. By dip-stick, the patient had blood in her urine but no protein.
FIGURE 1
Purpura on the lower leg
FIGURE 2
Close-up
FIGURE 3
Visible sock lines
What is the diagnosis?
What is the treatment for this condition?
Diagnosis: Henoch-Schönlein purpura
This patient has Henoch-Schönlein purpura (HSP), a systemic vasculitis, secondary to hypersensitivity, occurring most commonly in children and young adults. Its classic triad includes palpable purpura, abdominal pain or renal involvement, and arthritis.1
Purpura is typically nonblanching, as it represents extravasation of blood into skin, 2 but lesions may also include urticarial wheals or occasional target lesions. As seen in this patient, the lower extremities and buttocks are typically affected. This rash is pressure- and gravity-dependent and pressure lines may develop, causing “sock lines” such as those seen on this young woman’s calves.1,2 Arthritis also tends to affect large joints in lower extremities more severely than upper extremities, and ankles and knees may be swollen and tender, as observed in our patient.
Abdominal pain, not present in this case, develops in up to 65% of cases, and may be accompanied by vomiting, hematemesis, or blood in stools.
Renal involvement is by far the most long-reaching and potentially serious complication of HSP, particularly in adults. Although only 1% of all HSP patients may develop end-stage renal failure, it is estimated that in adults, this number may be as high as 11%.3,4 The young woman in question presented with microscopic hematuria initially, which had resolved by the time of her next visit, 4 days later. A worse prognosis is associated with those patients who exhibit both nephritic and nephrotic features, with hematuria and proteinuria, where clinical remission has been shown to present only in 20% of these patients. Finally, as renal involvement may develop up to years after initial diagnosis, follow-up is crucial to monitor renal function.4
Cause is unknown
The cause of HSP is unknown, although it is thought to be secondary to deposition of immune complexes, and has been associated with vaccinations, viral infections, allergens in foods, drug reactions, exposure to cold, and bacterial respiratory infections, particularly involving group A streptococci.1,2 This patient had been diagnosed with streptococcal pharyngitis and had been given a course of clindamycin due to her numerous drug allergies. Although the precise cause of her HSP is not definite, drugs have been shown to cause approximately 10% of acute cutaneous vasculitis cases, and she had never before been given clindamycin, possibly pointing to the origin of her disease.5
Differential diagnoses
Differential diagnoses for HSP depend on the level of specificity with which the triad presents. When symptoms are not typical, abdominal cramping alone may be confused with acute abdomen. Rash may be predominantly in the form of target lesions, and can be mistaken for erythema multiforme, particularly after a suspected drug reaction. Rheumatoid arthritis or systemic lupus erythematosus may be suspected with arthralgias. Idiopathic thrombocytopenic purpura can be distinguished from HSP by platelet count. Trauma or meningococcal septicemia may also be mistaken for the correct diagnosis in the presence of an atypical rash with few accompanying symptoms.
Treatment: Steroids, NSAIDs, and careful monitoring of renal function
Corticosteroids may be administered for severe abdominal symptoms, but they have not been shown to be useful in the absence of such symptoms. Conservative management with NSAIDs is the treatment of choice, and careful monitoring of renal function, even in the absence of renal involvement.1,3,5
Outcome and discussion
Due to the presence of the classic HSP triad in our patient, we deemed a biopsy necessary, and managed the patient with ibupro-fen. One week later, her rash had receded and her microscopic hematuria was gone, but the vasculitis had led to lower-extremity edema. The patient’s feet were painfully swollen, and where the patient had scratched a lesion, her foot exhibited honey crusting and warmth, indicating a probable infection (FIGURES 4 AND 5). Her feet and ankles were so swollen and painful that she had difficulty walking. She was given cephalexin 500 mg 4 times daily for her infection, and was also restarted on oral prednisone at 60 mg daily until she could see the rheumatologist 2 days later. She was also issued crutches.
The rheumatologist agreed that this was HSP and told her that she would need to continue the prednisone for 2 months due to the severity of her cutaneous vasculitis. Within 5 days, the patient’s rash had diminished significantly. She was able to walk with less pain, as her ankle swelling decreased (FIGURE 6).
FIGURE 4
Swollen ankle
FIGURE 5
Probable infection
FIGURE 6
The same patient after 5 days
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Kraft DM, McKee D, Scott C. Henoch-Schonlein purpura: a review. Am Fam Physician 1998;58:405-8,411.-
2. Leung AKC, Chan KW. Evaluating the child with purpura. Am Fam Physician 2001;64:419-428.
3. Usatine RP. A 4-year-old girl with a rash and joint pain. West J Med 1999;171:116-117.
4. Pillebout E, Thervet E, Hill G, et al. Henoch-Schonlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002;13:1271-1278.
5. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272-1285.
1. Kraft DM, McKee D, Scott C. Henoch-Schonlein purpura: a review. Am Fam Physician 1998;58:405-8,411.-
2. Leung AKC, Chan KW. Evaluating the child with purpura. Am Fam Physician 2001;64:419-428.
3. Usatine RP. A 4-year-old girl with a rash and joint pain. West J Med 1999;171:116-117.
4. Pillebout E, Thervet E, Hill G, et al. Henoch-Schonlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002;13:1271-1278.
5. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272-1285.
Persistent itchy pink rings
A 57-year-old retired farm worker came into the clinic with itchy pink rings all over his abdomen, legs, and arms. He said that the rash began 13 years ago, soon after he had retired from working in the fields. He said he had been exposed to pesticide chemicals but no other toxins. He’d never received a diagnosis of atopy or “sensitive skin.” He had sun damage on his lower arms, but none on the areas where the rash appeared.
Over the years, he had tried over-the-counter topical steroids, antifungals, and oral antihistamines, all with minimal relief. Furthermore, the lesions did not respond to 2 courses of oral antifungal medications. He said, “some days it itches more than others, but I just deal with it.” The patient was otherwise healthy and on no medications. He had been treated for active tuberculosis in the past but had no symptoms at this time. Out of his frustration, he recently began using paint thinner on the lesions. He thought the paint thinner “dried the rash out” and decreased the itching.
The distribution of the lesions can be seen in Figure 1. Erythematous rings can be seen on the abdomen and legs. These vary in size and shape. Figure 2 is a close-up of the left thigh showing the scale. There was no blanching erythema, no induration, no warmth, and no tenderness to palpation.
FIGURE 1
Rings on the trunk and legs
FIGURE 2
Close-up of thigh
What is the Diagnosis?
Diagnosis: Erythema Annulare Centrifugum
This patient has a rare skin condition called erythema annulare centrifugum (EAC). The primary lesion begins as a group of pink/erythematous papules that spread peripherally as they clear centrally, forming a “trailing scale” (Figure 3) They can enlarge at a rate of 2 to 5 mm/d, producing a plaque or patch of annular, arcuate, or polycyclic shape.1 The margin is indurated and can vary in width from 4 to 6 mm. Vesicles may also be present. Distribution is usually on the thighs and legs but can also appear on the upper extremities (Figure 4), trunk, or face.
Differential diagnosis
Other causes of similar erythematous itchy rings to consider include pityriasis rosea, tinea corporis, psoriasis, nummular eczema, atopic dermatitis, drug reaction, and erythema migrans. The physical exam is the most effective method to differentiate between the above diagnoses—specifically, the distribution and morphology of the lesions. The natural history of the lesions can further narrow the diagnosis.
Pityriasis rosea has erythematous patches distributed on the trunk and extremities. These patches have a distinctive collarette border. The “herald patch,” the first patch to appear, is often oval and about 1 to 3 inches in diameter. Lesions classically follow skin lines to create a “Christmas tree” pattern on the back. Unlike EAC, pityriasis only lasts 6 to 8 weeks.
Tinea corporis (ringworm) is a fungal infection that is often a single scaly annular patch. Like EAC, it can be erythematous and is distributed anywhere on the body. When it presents as multiple lesions, it more closely resembles EAC. Unlike EAC, however, it should show branched hyphae with septae on a KOH prep and fungal culture would show growth of dermatophytes. Furthermore, it responds well to 4 to 6 weeks of antifungal treatment.
Psoriasis may present as plaques on the extensor surfaces of extremities. “Guttate” psoriasis (meaning “like water drops”) appears like small drops on the back and abdomen. Psoriatic plaques do not have central clearing or the trailing scale characteristic of EAC. Psoriatic plaques are often more responsive to steroids than EAC.
FIGURE 3
“Trailing scale”on abdomen
FIGURE 4
Ring on inner arm
Epidemiology and Pathophysiology
EAC is an uncommon inflammatory skin condition of unknown cause. Histologically, it resembles pityriasis rosea, having a superficial perivascular lymphocytic infiltrate with moderate epidermal acanthosis.1,2 The primary lesions spread peripherally and clear centrally at a maximum rate of 2 to 5 mm/d. Pruritus is common but not always present.1
Although the cause and exact pathogenesis are unknown, EAC may in some cases be triggered by drugs, bacterial infections (like tuberculosis), fungi, viruses, parasites, autoimmune diseases, neoplasms, liver diseases, and dysproteinemias.1,3 Other hypotheses suggest that it may be a type IV hypersensitivity reaction.1 The mean duration is 11 months, but it can vary from as little as 4 weeks to 34 years.1
Diagnostic Tests
No specific tests are needed to make the diagnosis of EAC. However, it is a good idea to do a KOH prep to rule out tinea corporis or candidiasis. This technique has a sensitivity of 88% and a specificity of 95% for tinea.4 Thus, a positive result is sufficient for diagnosis. Fungal culture may be useful if you believe that the diagnosis is truly fungal but the KOH result is negative.
If physical exam leads to suspicion of underlying disease, it is important to order the appropriate diagnostic lab tests. A few case reports have described EAC as the presenting sign in patients diagnosed with cancer approximately 2 years later.1
Treatment
It is helpful if you can find an underlying disease process because most cases of EAC clear as the underlying disease is treated. Review the current drugs and consider replacing any drug reported to have been associated with EAC: chloroquine, hydroxychloroquine, estrogen, cimetidine, penicillin, salicylates, piroxicam, hydrochlorothiazide, and amitriptyline.1
If you have minimal suspicion for an underlying disease process and no causative drug agent is found, most cases of EAC require no treatment and resolve spontaneously.1 However, if pruritus is significant, treat symptomatically. One option is to start with a topical high-potency steroid ointment twice daily. Use this regimen for 2 weeks, then assess response. If the response is good, you may continue a mid-potency steroid as needed or pulse-dosing of a high-potency steroid on the weekends. The goal is to minimize the itching while also minimizing the side effects associated with long-term steroid treatment. Treat as needed and taper when possible. Steroids may cause resolution of lesions but do not prevent recurrence.
A second option is topical calcipotriol, a topical vitamin D derivative. A recent case report has shown this to be beneficial,2 but this is an off-label indication. To date, no randomized clinical trials have been done to demonstrate the efficacy of any other treatment.
Patient Outcome
A KOH prep and a fungal culture were obtained to confidently rule out tinea. Both results were negative. We decided to order a chest x-ray because of his past history of active tuberculosis; the test result was negative. To investigate for any underlying diseases, we ordered a complete blood count, chemistry panel, and erythrocyte sedimentation rate, which all showed normal results.
We explained the diagnosis of EAC as a chronic condition of unknown cause. Since steroids did not provide any relief for him in the past, we offered the option of using calcipotriol ointment, even though the evidence is only based on 1 case report.2 He chose to try the calcipotriol. We warned the patient to not use paint thinner because inhalation and skin exposure are harmful. The patient agreed to stop using it.
Corresponding author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Willard RJ, Montemarano AD. Erythema annulare centrifugum. eMedicine website. Available at: http://www.emedicine.com/derm/topic131.htm.
2. Gniadecki R. Case report: Calcipotriol for erythema annulare centrifugum. 2002 British Association of Dermatologists.British Journal of Dermatology 2002;146:317-319.
3. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol 2003;25:451-462.
4. Bergus, GR, Johnson JS. Superficial tinea infections. Am Fam Physician 1993;48:259-268.
A 57-year-old retired farm worker came into the clinic with itchy pink rings all over his abdomen, legs, and arms. He said that the rash began 13 years ago, soon after he had retired from working in the fields. He said he had been exposed to pesticide chemicals but no other toxins. He’d never received a diagnosis of atopy or “sensitive skin.” He had sun damage on his lower arms, but none on the areas where the rash appeared.
Over the years, he had tried over-the-counter topical steroids, antifungals, and oral antihistamines, all with minimal relief. Furthermore, the lesions did not respond to 2 courses of oral antifungal medications. He said, “some days it itches more than others, but I just deal with it.” The patient was otherwise healthy and on no medications. He had been treated for active tuberculosis in the past but had no symptoms at this time. Out of his frustration, he recently began using paint thinner on the lesions. He thought the paint thinner “dried the rash out” and decreased the itching.
The distribution of the lesions can be seen in Figure 1. Erythematous rings can be seen on the abdomen and legs. These vary in size and shape. Figure 2 is a close-up of the left thigh showing the scale. There was no blanching erythema, no induration, no warmth, and no tenderness to palpation.
FIGURE 1
Rings on the trunk and legs
FIGURE 2
Close-up of thigh
What is the Diagnosis?
Diagnosis: Erythema Annulare Centrifugum
This patient has a rare skin condition called erythema annulare centrifugum (EAC). The primary lesion begins as a group of pink/erythematous papules that spread peripherally as they clear centrally, forming a “trailing scale” (Figure 3) They can enlarge at a rate of 2 to 5 mm/d, producing a plaque or patch of annular, arcuate, or polycyclic shape.1 The margin is indurated and can vary in width from 4 to 6 mm. Vesicles may also be present. Distribution is usually on the thighs and legs but can also appear on the upper extremities (Figure 4), trunk, or face.
Differential diagnosis
Other causes of similar erythematous itchy rings to consider include pityriasis rosea, tinea corporis, psoriasis, nummular eczema, atopic dermatitis, drug reaction, and erythema migrans. The physical exam is the most effective method to differentiate between the above diagnoses—specifically, the distribution and morphology of the lesions. The natural history of the lesions can further narrow the diagnosis.
Pityriasis rosea has erythematous patches distributed on the trunk and extremities. These patches have a distinctive collarette border. The “herald patch,” the first patch to appear, is often oval and about 1 to 3 inches in diameter. Lesions classically follow skin lines to create a “Christmas tree” pattern on the back. Unlike EAC, pityriasis only lasts 6 to 8 weeks.
Tinea corporis (ringworm) is a fungal infection that is often a single scaly annular patch. Like EAC, it can be erythematous and is distributed anywhere on the body. When it presents as multiple lesions, it more closely resembles EAC. Unlike EAC, however, it should show branched hyphae with septae on a KOH prep and fungal culture would show growth of dermatophytes. Furthermore, it responds well to 4 to 6 weeks of antifungal treatment.
Psoriasis may present as plaques on the extensor surfaces of extremities. “Guttate” psoriasis (meaning “like water drops”) appears like small drops on the back and abdomen. Psoriatic plaques do not have central clearing or the trailing scale characteristic of EAC. Psoriatic plaques are often more responsive to steroids than EAC.
FIGURE 3
“Trailing scale”on abdomen
FIGURE 4
Ring on inner arm
Epidemiology and Pathophysiology
EAC is an uncommon inflammatory skin condition of unknown cause. Histologically, it resembles pityriasis rosea, having a superficial perivascular lymphocytic infiltrate with moderate epidermal acanthosis.1,2 The primary lesions spread peripherally and clear centrally at a maximum rate of 2 to 5 mm/d. Pruritus is common but not always present.1
Although the cause and exact pathogenesis are unknown, EAC may in some cases be triggered by drugs, bacterial infections (like tuberculosis), fungi, viruses, parasites, autoimmune diseases, neoplasms, liver diseases, and dysproteinemias.1,3 Other hypotheses suggest that it may be a type IV hypersensitivity reaction.1 The mean duration is 11 months, but it can vary from as little as 4 weeks to 34 years.1
Diagnostic Tests
No specific tests are needed to make the diagnosis of EAC. However, it is a good idea to do a KOH prep to rule out tinea corporis or candidiasis. This technique has a sensitivity of 88% and a specificity of 95% for tinea.4 Thus, a positive result is sufficient for diagnosis. Fungal culture may be useful if you believe that the diagnosis is truly fungal but the KOH result is negative.
If physical exam leads to suspicion of underlying disease, it is important to order the appropriate diagnostic lab tests. A few case reports have described EAC as the presenting sign in patients diagnosed with cancer approximately 2 years later.1
Treatment
It is helpful if you can find an underlying disease process because most cases of EAC clear as the underlying disease is treated. Review the current drugs and consider replacing any drug reported to have been associated with EAC: chloroquine, hydroxychloroquine, estrogen, cimetidine, penicillin, salicylates, piroxicam, hydrochlorothiazide, and amitriptyline.1
If you have minimal suspicion for an underlying disease process and no causative drug agent is found, most cases of EAC require no treatment and resolve spontaneously.1 However, if pruritus is significant, treat symptomatically. One option is to start with a topical high-potency steroid ointment twice daily. Use this regimen for 2 weeks, then assess response. If the response is good, you may continue a mid-potency steroid as needed or pulse-dosing of a high-potency steroid on the weekends. The goal is to minimize the itching while also minimizing the side effects associated with long-term steroid treatment. Treat as needed and taper when possible. Steroids may cause resolution of lesions but do not prevent recurrence.
A second option is topical calcipotriol, a topical vitamin D derivative. A recent case report has shown this to be beneficial,2 but this is an off-label indication. To date, no randomized clinical trials have been done to demonstrate the efficacy of any other treatment.
Patient Outcome
A KOH prep and a fungal culture were obtained to confidently rule out tinea. Both results were negative. We decided to order a chest x-ray because of his past history of active tuberculosis; the test result was negative. To investigate for any underlying diseases, we ordered a complete blood count, chemistry panel, and erythrocyte sedimentation rate, which all showed normal results.
We explained the diagnosis of EAC as a chronic condition of unknown cause. Since steroids did not provide any relief for him in the past, we offered the option of using calcipotriol ointment, even though the evidence is only based on 1 case report.2 He chose to try the calcipotriol. We warned the patient to not use paint thinner because inhalation and skin exposure are harmful. The patient agreed to stop using it.
Corresponding author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
A 57-year-old retired farm worker came into the clinic with itchy pink rings all over his abdomen, legs, and arms. He said that the rash began 13 years ago, soon after he had retired from working in the fields. He said he had been exposed to pesticide chemicals but no other toxins. He’d never received a diagnosis of atopy or “sensitive skin.” He had sun damage on his lower arms, but none on the areas where the rash appeared.
Over the years, he had tried over-the-counter topical steroids, antifungals, and oral antihistamines, all with minimal relief. Furthermore, the lesions did not respond to 2 courses of oral antifungal medications. He said, “some days it itches more than others, but I just deal with it.” The patient was otherwise healthy and on no medications. He had been treated for active tuberculosis in the past but had no symptoms at this time. Out of his frustration, he recently began using paint thinner on the lesions. He thought the paint thinner “dried the rash out” and decreased the itching.
The distribution of the lesions can be seen in Figure 1. Erythematous rings can be seen on the abdomen and legs. These vary in size and shape. Figure 2 is a close-up of the left thigh showing the scale. There was no blanching erythema, no induration, no warmth, and no tenderness to palpation.
FIGURE 1
Rings on the trunk and legs
FIGURE 2
Close-up of thigh
What is the Diagnosis?
Diagnosis: Erythema Annulare Centrifugum
This patient has a rare skin condition called erythema annulare centrifugum (EAC). The primary lesion begins as a group of pink/erythematous papules that spread peripherally as they clear centrally, forming a “trailing scale” (Figure 3) They can enlarge at a rate of 2 to 5 mm/d, producing a plaque or patch of annular, arcuate, or polycyclic shape.1 The margin is indurated and can vary in width from 4 to 6 mm. Vesicles may also be present. Distribution is usually on the thighs and legs but can also appear on the upper extremities (Figure 4), trunk, or face.
Differential diagnosis
Other causes of similar erythematous itchy rings to consider include pityriasis rosea, tinea corporis, psoriasis, nummular eczema, atopic dermatitis, drug reaction, and erythema migrans. The physical exam is the most effective method to differentiate between the above diagnoses—specifically, the distribution and morphology of the lesions. The natural history of the lesions can further narrow the diagnosis.
Pityriasis rosea has erythematous patches distributed on the trunk and extremities. These patches have a distinctive collarette border. The “herald patch,” the first patch to appear, is often oval and about 1 to 3 inches in diameter. Lesions classically follow skin lines to create a “Christmas tree” pattern on the back. Unlike EAC, pityriasis only lasts 6 to 8 weeks.
Tinea corporis (ringworm) is a fungal infection that is often a single scaly annular patch. Like EAC, it can be erythematous and is distributed anywhere on the body. When it presents as multiple lesions, it more closely resembles EAC. Unlike EAC, however, it should show branched hyphae with septae on a KOH prep and fungal culture would show growth of dermatophytes. Furthermore, it responds well to 4 to 6 weeks of antifungal treatment.
Psoriasis may present as plaques on the extensor surfaces of extremities. “Guttate” psoriasis (meaning “like water drops”) appears like small drops on the back and abdomen. Psoriatic plaques do not have central clearing or the trailing scale characteristic of EAC. Psoriatic plaques are often more responsive to steroids than EAC.
FIGURE 3
“Trailing scale”on abdomen
FIGURE 4
Ring on inner arm
Epidemiology and Pathophysiology
EAC is an uncommon inflammatory skin condition of unknown cause. Histologically, it resembles pityriasis rosea, having a superficial perivascular lymphocytic infiltrate with moderate epidermal acanthosis.1,2 The primary lesions spread peripherally and clear centrally at a maximum rate of 2 to 5 mm/d. Pruritus is common but not always present.1
Although the cause and exact pathogenesis are unknown, EAC may in some cases be triggered by drugs, bacterial infections (like tuberculosis), fungi, viruses, parasites, autoimmune diseases, neoplasms, liver diseases, and dysproteinemias.1,3 Other hypotheses suggest that it may be a type IV hypersensitivity reaction.1 The mean duration is 11 months, but it can vary from as little as 4 weeks to 34 years.1
Diagnostic Tests
No specific tests are needed to make the diagnosis of EAC. However, it is a good idea to do a KOH prep to rule out tinea corporis or candidiasis. This technique has a sensitivity of 88% and a specificity of 95% for tinea.4 Thus, a positive result is sufficient for diagnosis. Fungal culture may be useful if you believe that the diagnosis is truly fungal but the KOH result is negative.
If physical exam leads to suspicion of underlying disease, it is important to order the appropriate diagnostic lab tests. A few case reports have described EAC as the presenting sign in patients diagnosed with cancer approximately 2 years later.1
Treatment
It is helpful if you can find an underlying disease process because most cases of EAC clear as the underlying disease is treated. Review the current drugs and consider replacing any drug reported to have been associated with EAC: chloroquine, hydroxychloroquine, estrogen, cimetidine, penicillin, salicylates, piroxicam, hydrochlorothiazide, and amitriptyline.1
If you have minimal suspicion for an underlying disease process and no causative drug agent is found, most cases of EAC require no treatment and resolve spontaneously.1 However, if pruritus is significant, treat symptomatically. One option is to start with a topical high-potency steroid ointment twice daily. Use this regimen for 2 weeks, then assess response. If the response is good, you may continue a mid-potency steroid as needed or pulse-dosing of a high-potency steroid on the weekends. The goal is to minimize the itching while also minimizing the side effects associated with long-term steroid treatment. Treat as needed and taper when possible. Steroids may cause resolution of lesions but do not prevent recurrence.
A second option is topical calcipotriol, a topical vitamin D derivative. A recent case report has shown this to be beneficial,2 but this is an off-label indication. To date, no randomized clinical trials have been done to demonstrate the efficacy of any other treatment.
Patient Outcome
A KOH prep and a fungal culture were obtained to confidently rule out tinea. Both results were negative. We decided to order a chest x-ray because of his past history of active tuberculosis; the test result was negative. To investigate for any underlying diseases, we ordered a complete blood count, chemistry panel, and erythrocyte sedimentation rate, which all showed normal results.
We explained the diagnosis of EAC as a chronic condition of unknown cause. Since steroids did not provide any relief for him in the past, we offered the option of using calcipotriol ointment, even though the evidence is only based on 1 case report.2 He chose to try the calcipotriol. We warned the patient to not use paint thinner because inhalation and skin exposure are harmful. The patient agreed to stop using it.
Corresponding author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Willard RJ, Montemarano AD. Erythema annulare centrifugum. eMedicine website. Available at: http://www.emedicine.com/derm/topic131.htm.
2. Gniadecki R. Case report: Calcipotriol for erythema annulare centrifugum. 2002 British Association of Dermatologists.British Journal of Dermatology 2002;146:317-319.
3. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol 2003;25:451-462.
4. Bergus, GR, Johnson JS. Superficial tinea infections. Am Fam Physician 1993;48:259-268.
1. Willard RJ, Montemarano AD. Erythema annulare centrifugum. eMedicine website. Available at: http://www.emedicine.com/derm/topic131.htm.
2. Gniadecki R. Case report: Calcipotriol for erythema annulare centrifugum. 2002 British Association of Dermatologists.British Journal of Dermatology 2002;146:317-319.
3. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol 2003;25:451-462.
4. Bergus, GR, Johnson JS. Superficial tinea infections. Am Fam Physician 1993;48:259-268.
Pearly penile lesions
A 22-year-old man came into the office concerned he may have warts on his penis. He believed the warts appeared about 3 months ago. He was single and did not have a sexual partner. He had been dating a woman for 1 year until he graduated from college 4 months ago. His sexual history was serial monogamy with 5 lifetime female sexual partners.
After some hesitation, he noted he slept with a woman one night following a graduation party. He admitted that they were both drunk and that he did not use a condom. He asked if this was how the condition could have developed.
He denied any history of sexually transmitted diseases (STDs) and the result of an HIV test was negative when he donated blood last year. He did not have urethral discharge or burning on urination. The patient had no other symptoms and no chronic illnesses. He generally had a healthy lifestyle, without drug and tobacco use. He said he used to drink at college parties, but rarely had a drink since starting work full-time.
On physical exam, the patient had no fever or lymphadenopathy. A genital exam (Figure 1) with the foreskin retracted revealed skin-colored papules on the shaft of the penis that were somewhat verrucous. The papules seemed to make a ring around the shaft just proximal to the corona of the glans. Closer inspection showed smaller pearly papules surrounding the glans on the corona (Figure 2).
FIGURE 1
Large verrucuous papules
FIGURE 2
Detail of the smaller papules
Are The Larger Verrucous Papules Really Warts?
What Are The Smaller Papules And do they need treatment?
Diagnosis: Condyloma Acuminata
The larger verrucous papules are genital warts, also known as condyloma acuminata. These are caused by the human papillomavirus (HPV) and are sexually transmitted. The patient most likely acquired these from his last unprotected sexual encounter, but he may have been infected earlier and the warts just became visible in the last 3 months. The differential diagnosis includes condyloma lata, the flat warts of secondary syphilis, but these are much less common.
The small pearly papules on the corona are not warts but a variation of the normal male anatomy, called pearly penile papules. The reason to recognize these papules is to reassure worried men that they are normal and to avoid performing any invasive treatments to remove them.
Laboratory Examination
All patients with any sexually transmitted disease should be tested for syphilis and HIV regardless of other risk factors.1 In this case, testing for syphilis with either a rapid plasma reagin (RPR) or VDRL will also be helpful to rule out condyloma lata.
These genital warts do not need to be biopsied to make the diagnosis. No data support the use of type-specific HPV nucleic acid tests in the routine diagnosis or management of visible genital warts.1
Treatment: Removal With Medication Or Surgery
The primary goal of treating visible genital warts is the removal of symptomatic warts.1 Treatment can induce wart-free periods. Available therapies for genital warts may reduce, but probably do not eradicate, infectivity.1 No evidence suggests any available treatment is superior to another, and no single treatment is ideal for all patients or all warts. The natural history of genital warts is benign, and the types of HPV that usually cause external genital warts (HPV 6 and 11) are not associated with cancer.
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines1 for STDs recommend the following options. Cost data are given in the Table.
TABLE
Self-administered medications for HPV
| Medication | Method | Cost |
|---|---|---|
| Podofilox 0.5% solution or gel (podophyllotoxin) | Apply twice daily for 3 days, then off 4 days. May repeat cycle total of 4 times | Gel 0.5%, one 3.5-g tube:$164 Solution 0.5% one 3.5-g tube:$121* |
| Imiquimod 5% cream | Apply nightly 3 times per week. | 12 packets:$159* (enough for 4 weeks of therapy if 1 packet is used per application;using a packet for more than 1 day is possible) |
| Wash off after 6 to 10 hours. | ||
| May use up to 16 weeks. | ||
| * Prices from ePocrates,accessed on October 3,2004. | ||
Patient-applied treatments
- Podofilox 0.5% solution or gel. Patients should apply podofilox solution with a cotton swab, or podofilox gel with a finger, to visible genital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle may be repeated, as necessary, for up to 4 cycles. The health care provider may apply the initial treatment to demonstrate the proper application technique and identify which warts should be treated.
- Imiquimod 5% cream. Patients should apply imiquimod cream once daily at bedtime, 3 times a week for up to 16 weeks. The treatment area should be washed with soap and water 6 to 10 hours after the application.
Provider-administered treatments
- Cryotherapy with liquid nitrogen or cryoprobe. Repeat applications every 1 to 2 weeks.
- Podophyllin resin 10% to 25% in a compound tincture of benzoin. A small amount should be applied to each wart and allowed to air-dry. The treatment can be repeated weekly, if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, some specialists recommend that application be limited to ≤0.5 mL of podophyllin or an area of <10 cm2 of warts per session. Some specialists suggest the preparation should be thoroughly washed off 1 to 4 hours after application to reduce local irritation. The safety of podophyllin during pregnancy has not been established.
- Trichloroacetic acid (TCA): a small amount should be applied only to warts and allowed to dry, at which time a white “frosting” develops (Figure 3). This treatment can be repeated weekly, if necessary.
- Surgical removal either by tangential scissor excision, tangential shave excision, curettage, or electrosurgery.
FIGURE 3
Treatment of genital warts
Keratinized vs nonkeratinized warts
In choosing the type of therapy for a patient, it is helpful to note that the soft, nonkeratinized warts respond well to the various forms of podophyllin and trichloroacetic acid. The more keratinized lesions, however, respond better to physical ablative methods such as cryotherapy, excision, or electrocautery. Imiquimod appears to work well for both types of lesions but is more effective for the nonkeratinized warts.
The softer, nonkeratinized warts are found more often on softer mucosa around the anus, under the foreskin, and around the female introitus. The firmer, more keratinized warts are found more often on more keratinized skin such as on the shaft of the penis.
Most options yield inadequate results
The reason for so many treatment options is in part due to the therapeutic inadequacy of any one of them. Cure rates are far less than optimal, and relapse rates are disappointing. While we have reasonable treatment guidelines from the CDC, studies on many of the treatment options are limited. There are few head-to-head studies of one method vs another.
One double-blind, randomized, multicenter, vehicle-controlled study2 demonstrated that 0.5% podofilox gel was significantly better than vehicle gel for successfully eliminating and reducing the number and size of anogenital warts. In the intention-to-treat population, 37% treated with podofilox gel had complete clearing of the treated areas, compared with 2% who had clearing of warts with the vehicle gel after 4 weeks (P<.001; number needed to treat=3) (level of evidence [LOE]: 1b).
The best systematic review of genital wart treatment was published in 2004 in the International Journal of STD & AIDS.3 Imiquimod has proven effective in many studies, including 3 randomized controlled trials in which clearance rates were 37% to 52% after 8 to 16 weeks of treatment (LOE=1a).3 One study using imiquimod found that clearance rates were twofold higher for women than for men (72% and 33%, respectively).4 This is probably because genital warts in females are less keratinized than warts on the usual site in males, the penile shaft. Similarly, clearance rates with imiquimod seem to be higher in uncircumcised men (62%) than circumcised men (33%), probably related to the degree of keratinization.3
Recurrence rates for sole therapy with imiquimod (9%–19%) are substantially lower than for most other genital wart treatments, including podophyllotoxin.3 Imiquimod may even be effective in reducing wart recurrence rates when used as an adjunct to surgical treatment.3
The Patient’s Treatment: Cryotherapy, Imiquimod Cream
The treatment options were discussed with the patient. The patient decided to have cryotherapy performed in the office and was given a prescription for imiquimod cream to be used on any remaining warts. The normal pearly penile papules on the corona were left alone and the patient tolerated the cryotherapy to the real warts with an acceptable level of temporary discomfort.
- Imiquimod • Aldara
- Podofilox • Condylox
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6):1-78.Available online at: http://www.cdc.gov/std/treatment/. (Note: The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at the following website: www.cdcnpin.org/scripts/std/pda.asp.)
2. Tyring S, Edwards L, Cherry LK, et al. Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts. Arch Dermatol 1998;134:33-38.
3. Maw R. Critical appraisal of commonly used treatment for genital warts. Int J STD AIDS 2004;15:357-364.
4. Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis 2003;Feb;30(2):124-8.
A 22-year-old man came into the office concerned he may have warts on his penis. He believed the warts appeared about 3 months ago. He was single and did not have a sexual partner. He had been dating a woman for 1 year until he graduated from college 4 months ago. His sexual history was serial monogamy with 5 lifetime female sexual partners.
After some hesitation, he noted he slept with a woman one night following a graduation party. He admitted that they were both drunk and that he did not use a condom. He asked if this was how the condition could have developed.
He denied any history of sexually transmitted diseases (STDs) and the result of an HIV test was negative when he donated blood last year. He did not have urethral discharge or burning on urination. The patient had no other symptoms and no chronic illnesses. He generally had a healthy lifestyle, without drug and tobacco use. He said he used to drink at college parties, but rarely had a drink since starting work full-time.
On physical exam, the patient had no fever or lymphadenopathy. A genital exam (Figure 1) with the foreskin retracted revealed skin-colored papules on the shaft of the penis that were somewhat verrucous. The papules seemed to make a ring around the shaft just proximal to the corona of the glans. Closer inspection showed smaller pearly papules surrounding the glans on the corona (Figure 2).
FIGURE 1
Large verrucuous papules
FIGURE 2
Detail of the smaller papules
Are The Larger Verrucous Papules Really Warts?
What Are The Smaller Papules And do they need treatment?
Diagnosis: Condyloma Acuminata
The larger verrucous papules are genital warts, also known as condyloma acuminata. These are caused by the human papillomavirus (HPV) and are sexually transmitted. The patient most likely acquired these from his last unprotected sexual encounter, but he may have been infected earlier and the warts just became visible in the last 3 months. The differential diagnosis includes condyloma lata, the flat warts of secondary syphilis, but these are much less common.
The small pearly papules on the corona are not warts but a variation of the normal male anatomy, called pearly penile papules. The reason to recognize these papules is to reassure worried men that they are normal and to avoid performing any invasive treatments to remove them.
Laboratory Examination
All patients with any sexually transmitted disease should be tested for syphilis and HIV regardless of other risk factors.1 In this case, testing for syphilis with either a rapid plasma reagin (RPR) or VDRL will also be helpful to rule out condyloma lata.
These genital warts do not need to be biopsied to make the diagnosis. No data support the use of type-specific HPV nucleic acid tests in the routine diagnosis or management of visible genital warts.1
Treatment: Removal With Medication Or Surgery
The primary goal of treating visible genital warts is the removal of symptomatic warts.1 Treatment can induce wart-free periods. Available therapies for genital warts may reduce, but probably do not eradicate, infectivity.1 No evidence suggests any available treatment is superior to another, and no single treatment is ideal for all patients or all warts. The natural history of genital warts is benign, and the types of HPV that usually cause external genital warts (HPV 6 and 11) are not associated with cancer.
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines1 for STDs recommend the following options. Cost data are given in the Table.
TABLE
Self-administered medications for HPV
| Medication | Method | Cost |
|---|---|---|
| Podofilox 0.5% solution or gel (podophyllotoxin) | Apply twice daily for 3 days, then off 4 days. May repeat cycle total of 4 times | Gel 0.5%, one 3.5-g tube:$164 Solution 0.5% one 3.5-g tube:$121* |
| Imiquimod 5% cream | Apply nightly 3 times per week. | 12 packets:$159* (enough for 4 weeks of therapy if 1 packet is used per application;using a packet for more than 1 day is possible) |
| Wash off after 6 to 10 hours. | ||
| May use up to 16 weeks. | ||
| * Prices from ePocrates,accessed on October 3,2004. | ||
Patient-applied treatments
- Podofilox 0.5% solution or gel. Patients should apply podofilox solution with a cotton swab, or podofilox gel with a finger, to visible genital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle may be repeated, as necessary, for up to 4 cycles. The health care provider may apply the initial treatment to demonstrate the proper application technique and identify which warts should be treated.
- Imiquimod 5% cream. Patients should apply imiquimod cream once daily at bedtime, 3 times a week for up to 16 weeks. The treatment area should be washed with soap and water 6 to 10 hours after the application.
Provider-administered treatments
- Cryotherapy with liquid nitrogen or cryoprobe. Repeat applications every 1 to 2 weeks.
- Podophyllin resin 10% to 25% in a compound tincture of benzoin. A small amount should be applied to each wart and allowed to air-dry. The treatment can be repeated weekly, if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, some specialists recommend that application be limited to ≤0.5 mL of podophyllin or an area of <10 cm2 of warts per session. Some specialists suggest the preparation should be thoroughly washed off 1 to 4 hours after application to reduce local irritation. The safety of podophyllin during pregnancy has not been established.
- Trichloroacetic acid (TCA): a small amount should be applied only to warts and allowed to dry, at which time a white “frosting” develops (Figure 3). This treatment can be repeated weekly, if necessary.
- Surgical removal either by tangential scissor excision, tangential shave excision, curettage, or electrosurgery.
FIGURE 3
Treatment of genital warts
Keratinized vs nonkeratinized warts
In choosing the type of therapy for a patient, it is helpful to note that the soft, nonkeratinized warts respond well to the various forms of podophyllin and trichloroacetic acid. The more keratinized lesions, however, respond better to physical ablative methods such as cryotherapy, excision, or electrocautery. Imiquimod appears to work well for both types of lesions but is more effective for the nonkeratinized warts.
The softer, nonkeratinized warts are found more often on softer mucosa around the anus, under the foreskin, and around the female introitus. The firmer, more keratinized warts are found more often on more keratinized skin such as on the shaft of the penis.
Most options yield inadequate results
The reason for so many treatment options is in part due to the therapeutic inadequacy of any one of them. Cure rates are far less than optimal, and relapse rates are disappointing. While we have reasonable treatment guidelines from the CDC, studies on many of the treatment options are limited. There are few head-to-head studies of one method vs another.
One double-blind, randomized, multicenter, vehicle-controlled study2 demonstrated that 0.5% podofilox gel was significantly better than vehicle gel for successfully eliminating and reducing the number and size of anogenital warts. In the intention-to-treat population, 37% treated with podofilox gel had complete clearing of the treated areas, compared with 2% who had clearing of warts with the vehicle gel after 4 weeks (P<.001; number needed to treat=3) (level of evidence [LOE]: 1b).
The best systematic review of genital wart treatment was published in 2004 in the International Journal of STD & AIDS.3 Imiquimod has proven effective in many studies, including 3 randomized controlled trials in which clearance rates were 37% to 52% after 8 to 16 weeks of treatment (LOE=1a).3 One study using imiquimod found that clearance rates were twofold higher for women than for men (72% and 33%, respectively).4 This is probably because genital warts in females are less keratinized than warts on the usual site in males, the penile shaft. Similarly, clearance rates with imiquimod seem to be higher in uncircumcised men (62%) than circumcised men (33%), probably related to the degree of keratinization.3
Recurrence rates for sole therapy with imiquimod (9%–19%) are substantially lower than for most other genital wart treatments, including podophyllotoxin.3 Imiquimod may even be effective in reducing wart recurrence rates when used as an adjunct to surgical treatment.3
The Patient’s Treatment: Cryotherapy, Imiquimod Cream
The treatment options were discussed with the patient. The patient decided to have cryotherapy performed in the office and was given a prescription for imiquimod cream to be used on any remaining warts. The normal pearly penile papules on the corona were left alone and the patient tolerated the cryotherapy to the real warts with an acceptable level of temporary discomfort.
- Imiquimod • Aldara
- Podofilox • Condylox
A 22-year-old man came into the office concerned he may have warts on his penis. He believed the warts appeared about 3 months ago. He was single and did not have a sexual partner. He had been dating a woman for 1 year until he graduated from college 4 months ago. His sexual history was serial monogamy with 5 lifetime female sexual partners.
After some hesitation, he noted he slept with a woman one night following a graduation party. He admitted that they were both drunk and that he did not use a condom. He asked if this was how the condition could have developed.
He denied any history of sexually transmitted diseases (STDs) and the result of an HIV test was negative when he donated blood last year. He did not have urethral discharge or burning on urination. The patient had no other symptoms and no chronic illnesses. He generally had a healthy lifestyle, without drug and tobacco use. He said he used to drink at college parties, but rarely had a drink since starting work full-time.
On physical exam, the patient had no fever or lymphadenopathy. A genital exam (Figure 1) with the foreskin retracted revealed skin-colored papules on the shaft of the penis that were somewhat verrucous. The papules seemed to make a ring around the shaft just proximal to the corona of the glans. Closer inspection showed smaller pearly papules surrounding the glans on the corona (Figure 2).
FIGURE 1
Large verrucuous papules
FIGURE 2
Detail of the smaller papules
Are The Larger Verrucous Papules Really Warts?
What Are The Smaller Papules And do they need treatment?
Diagnosis: Condyloma Acuminata
The larger verrucous papules are genital warts, also known as condyloma acuminata. These are caused by the human papillomavirus (HPV) and are sexually transmitted. The patient most likely acquired these from his last unprotected sexual encounter, but he may have been infected earlier and the warts just became visible in the last 3 months. The differential diagnosis includes condyloma lata, the flat warts of secondary syphilis, but these are much less common.
The small pearly papules on the corona are not warts but a variation of the normal male anatomy, called pearly penile papules. The reason to recognize these papules is to reassure worried men that they are normal and to avoid performing any invasive treatments to remove them.
Laboratory Examination
All patients with any sexually transmitted disease should be tested for syphilis and HIV regardless of other risk factors.1 In this case, testing for syphilis with either a rapid plasma reagin (RPR) or VDRL will also be helpful to rule out condyloma lata.
These genital warts do not need to be biopsied to make the diagnosis. No data support the use of type-specific HPV nucleic acid tests in the routine diagnosis or management of visible genital warts.1
Treatment: Removal With Medication Or Surgery
The primary goal of treating visible genital warts is the removal of symptomatic warts.1 Treatment can induce wart-free periods. Available therapies for genital warts may reduce, but probably do not eradicate, infectivity.1 No evidence suggests any available treatment is superior to another, and no single treatment is ideal for all patients or all warts. The natural history of genital warts is benign, and the types of HPV that usually cause external genital warts (HPV 6 and 11) are not associated with cancer.
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines1 for STDs recommend the following options. Cost data are given in the Table.
TABLE
Self-administered medications for HPV
| Medication | Method | Cost |
|---|---|---|
| Podofilox 0.5% solution or gel (podophyllotoxin) | Apply twice daily for 3 days, then off 4 days. May repeat cycle total of 4 times | Gel 0.5%, one 3.5-g tube:$164 Solution 0.5% one 3.5-g tube:$121* |
| Imiquimod 5% cream | Apply nightly 3 times per week. | 12 packets:$159* (enough for 4 weeks of therapy if 1 packet is used per application;using a packet for more than 1 day is possible) |
| Wash off after 6 to 10 hours. | ||
| May use up to 16 weeks. | ||
| * Prices from ePocrates,accessed on October 3,2004. | ||
Patient-applied treatments
- Podofilox 0.5% solution or gel. Patients should apply podofilox solution with a cotton swab, or podofilox gel with a finger, to visible genital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle may be repeated, as necessary, for up to 4 cycles. The health care provider may apply the initial treatment to demonstrate the proper application technique and identify which warts should be treated.
- Imiquimod 5% cream. Patients should apply imiquimod cream once daily at bedtime, 3 times a week for up to 16 weeks. The treatment area should be washed with soap and water 6 to 10 hours after the application.
Provider-administered treatments
- Cryotherapy with liquid nitrogen or cryoprobe. Repeat applications every 1 to 2 weeks.
- Podophyllin resin 10% to 25% in a compound tincture of benzoin. A small amount should be applied to each wart and allowed to air-dry. The treatment can be repeated weekly, if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, some specialists recommend that application be limited to ≤0.5 mL of podophyllin or an area of <10 cm2 of warts per session. Some specialists suggest the preparation should be thoroughly washed off 1 to 4 hours after application to reduce local irritation. The safety of podophyllin during pregnancy has not been established.
- Trichloroacetic acid (TCA): a small amount should be applied only to warts and allowed to dry, at which time a white “frosting” develops (Figure 3). This treatment can be repeated weekly, if necessary.
- Surgical removal either by tangential scissor excision, tangential shave excision, curettage, or electrosurgery.
FIGURE 3
Treatment of genital warts
Keratinized vs nonkeratinized warts
In choosing the type of therapy for a patient, it is helpful to note that the soft, nonkeratinized warts respond well to the various forms of podophyllin and trichloroacetic acid. The more keratinized lesions, however, respond better to physical ablative methods such as cryotherapy, excision, or electrocautery. Imiquimod appears to work well for both types of lesions but is more effective for the nonkeratinized warts.
The softer, nonkeratinized warts are found more often on softer mucosa around the anus, under the foreskin, and around the female introitus. The firmer, more keratinized warts are found more often on more keratinized skin such as on the shaft of the penis.
Most options yield inadequate results
The reason for so many treatment options is in part due to the therapeutic inadequacy of any one of them. Cure rates are far less than optimal, and relapse rates are disappointing. While we have reasonable treatment guidelines from the CDC, studies on many of the treatment options are limited. There are few head-to-head studies of one method vs another.
One double-blind, randomized, multicenter, vehicle-controlled study2 demonstrated that 0.5% podofilox gel was significantly better than vehicle gel for successfully eliminating and reducing the number and size of anogenital warts. In the intention-to-treat population, 37% treated with podofilox gel had complete clearing of the treated areas, compared with 2% who had clearing of warts with the vehicle gel after 4 weeks (P<.001; number needed to treat=3) (level of evidence [LOE]: 1b).
The best systematic review of genital wart treatment was published in 2004 in the International Journal of STD & AIDS.3 Imiquimod has proven effective in many studies, including 3 randomized controlled trials in which clearance rates were 37% to 52% after 8 to 16 weeks of treatment (LOE=1a).3 One study using imiquimod found that clearance rates were twofold higher for women than for men (72% and 33%, respectively).4 This is probably because genital warts in females are less keratinized than warts on the usual site in males, the penile shaft. Similarly, clearance rates with imiquimod seem to be higher in uncircumcised men (62%) than circumcised men (33%), probably related to the degree of keratinization.3
Recurrence rates for sole therapy with imiquimod (9%–19%) are substantially lower than for most other genital wart treatments, including podophyllotoxin.3 Imiquimod may even be effective in reducing wart recurrence rates when used as an adjunct to surgical treatment.3
The Patient’s Treatment: Cryotherapy, Imiquimod Cream
The treatment options were discussed with the patient. The patient decided to have cryotherapy performed in the office and was given a prescription for imiquimod cream to be used on any remaining warts. The normal pearly penile papules on the corona were left alone and the patient tolerated the cryotherapy to the real warts with an acceptable level of temporary discomfort.
- Imiquimod • Aldara
- Podofilox • Condylox
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6):1-78.Available online at: http://www.cdc.gov/std/treatment/. (Note: The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at the following website: www.cdcnpin.org/scripts/std/pda.asp.)
2. Tyring S, Edwards L, Cherry LK, et al. Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts. Arch Dermatol 1998;134:33-38.
3. Maw R. Critical appraisal of commonly used treatment for genital warts. Int J STD AIDS 2004;15:357-364.
4. Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis 2003;Feb;30(2):124-8.
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6):1-78.Available online at: http://www.cdc.gov/std/treatment/. (Note: The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at the following website: www.cdcnpin.org/scripts/std/pda.asp.)
2. Tyring S, Edwards L, Cherry LK, et al. Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts. Arch Dermatol 1998;134:33-38.
3. Maw R. Critical appraisal of commonly used treatment for genital warts. Int J STD AIDS 2004;15:357-364.
4. Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis 2003;Feb;30(2):124-8.
Excoriations and ulcers on the arms and legs
A 55-year-old woman came to the clinic complaining of severe itching on her arms and legs. Although she itched throughout the day, it became intolerable at night, disrupting her sleep. She would sometimes scratch her arms and legs until exhaustion but could find no relief. Being outside in warm and sunny weather aggravated the problem. She had used moisturizers, emollients, and topical corticosteroids, but they only alleviated the itching temporarily. The itching began 10 months earlier, just after she finalized the divorce from her husband of 20 years.
Examination of the skin revealed numerous excoriations with ulcerations and xerosis on the arms and left leg (Figure 1 and 2). The excoriations were located extensively from the dorsum of her left foot to above the knee and bilaterally from the wrist to above the elbow. They also showed signs of infection. The patient admitted they were self-inflicted. The patient’s right leg had been amputated 5 years before after a car accident, and she wore a prosthetic leg. Examination of other areas showed nothing remarkable.
The patient readily admitted to a great deal of psychological distress. She described feeling depressed since her divorce. She has had difficulty securing a full-time job and has high anxiety about being able to pay her rent and bills.
FIGURE 1
Excoriations on the arms…
FIGURE 2
…and the left leg
WHAT IS THE DIAGNOSIS?
WHAT IS THE TREATMENT STRATEGY?
DIFFERENTIAL DIAGNOSIS: PSYCHODERMATOLOGIC DISORDER
The patient’s history and physical examination points to a psychodermatologic disorder. Psycho-dermatologic disorders are conditions involving an interaction between the mind and skin and are classified as:3
- Psychophysiologic disorders:skin disorders worsened by emotional stress
- Primary psychiatric disorders:usually caused by psychological conditions with self-induced skin damage
- Secondary psychiatric disorders: psychological problems developed as a consequence of a disfiguring skin disorder, which negatively effects self-esteem and body image.3
This patient’s excoriations and ulcers are due to self-mutilation. The differential diagnosis includes psychogenic parasitosis, factitial dermatitis, and neurotic excoriations. These 3 skin conditions are primary psychiatric symptoms, and proper diagnosis revolves around being able to assess the dermatologic features and associated psychological disorder.2
Psychogenic parasitosis
Also known as delusional parasitosis, psychogenic parasitosis is a psychodermatologic disorder in which patients believe they are infested with parasites. Patients with this disorder report seeing or feeling parasites on their skin, and they damage their skin in an attempt to remove them. Patients create excoriations and ulcers on easily reached areas, usually the ears, eyes, nose, and extremities. They often present with what is termed the “matchbox sign,” in which they bring containers filled with “small bits of excoriated skin, dried blood, debris, or insect parts as proof of infestation.”2
Women over the age of 50 years are more often affected with psychogenic parasitosis, and the disorder is associated with anxiety, depression, and hypochondriasis.
Factitial dermatitis
Factitial dermatitis, also known as dermatitis artefacta, is a psychodermatologic disorder in which patients damage their skin but deny their self-involvement. This disorder encompasses a wide range of lesions, including blisters, cuts, ulcers, and burns. Patients often are unable to describe how the lesions evolved. Lesions exhibit bizarre patterns not characteristic of any disease.
Young adults and adolescents are more commonly affected, and it is 4 times more common among women than men. Psychological disorders involved with factitial dermatitis include personality disorders and posttraumatic stress disorder.2
Neurotic excoriations
Neurotic excoriations, sometimes referred to as neurodermatitis, are a result of a psychodermatologic disorder in which patients inflict excoriations and ulcers on their skin and admit to their involvement.3 The condition is characterized by excoriations similar in size and shape that are localized on areas easily reached by the patient, such as the arms, legs, and upper back.
The lesions may present in various stages, varying from dugout ulcers to ulcers covered with crusts and surrounded by erythema to areas receding into depressed scars. These lesions are a result of repetitive scratching and digging by the patient, usually without an underlying physical pathology but sometimes initiated by pruritus.1,2
Studies show the condition primarily affects women, with a mean onset between the ages of 30 to 45 years.1 Common psychiatric problems associated with neurotic excoriations include significant social stress, depression, anxiety, and obsessive-compulsive disorder.
Diagnosis: Neurotic excoriations due to depression and stress
The patient’s history and physical examination suggests a diagnosis of neurotic excoriations due to the characteristics of the excoriations and ulcers on her arms and leg, her admission of their self-inflicted nature, and the associated depression and psychosocial stress.
Laboratory tests
Although there are no available laboratory tests to confirm a positive finding of neurotic excoriations, tests could be performed to disprove any systemic causes of pruritus and the resulting dermatological damage.5 These tests include complete blood count with differential, chemistry profile, determination of thyroid-stimulating hormone levels, fasting plasma glucose level, and skin biopsies.1,
Laboratory tests for systemic causes of pruritus should be based on the patient’s physical and history, avoiding a broad approach.
Treatment: address the skin and the psyche
The treatment of neurotic excoriations requires a dual approach, addressing both the dermatological problems and the underlying psychological disorder.3 Supportive dermatologic care is necessary to avoid secondary complications and to ensure that the patient feels supported.3
Dermatologic care
Topical steroids can be helpful to decrease the pruritus and inflammation. The steroid strength should be chosen based upon the severity of the lesions and the thickness of the skin. Steroid ointments are preferred to creams when there are skin ulcers and deep excoriations.
If there is significant crusting or exudate, there is probably a secondary bacterial infection and oral antibiotics are indicated ( Figure 3). A first-generation cephalosporin or dicloxacillin should provide adequate coverage against the most likely organisms, Staphylococcus aureus and Streptococcus pyogenes.
FIGURE 3
Detail of lesions on the arm
Psychological care
The selective serotonin reuptake inhibitors produce a strong antipruritic response in patients with neurotic excoriations. Doxepin is a tricyclic antidepressant with one of the most powerful antihistamines for pruritus. When prescribing doxepin, limit the amount of medication dispensed at one time to minimize the risk of suicide.3 The patient should trim the fingernails to reduce the amount of damage caused by scratching and digging.
Treating the underlying psychological disorder requires supportive and empathic counseling. It may be necessary to collaborate with other mental health specialists. Management options include psychotropic medication, stress management courses, and referral to a psychiatrist. Patients with psychodermatologic disorders frequently resist referral to mental health professionals.3 If this is the case, family physicians are well positioned to help patients with psychological problems.
Alternative courses of treatment include hypnosis to disrupt the itch-scratch cycle, and acupuncture and supportive therapy to reduce underlying stressors.1
Patient’s outcome
By showing support and concern for the patient’s health during the visit, the physician strengthened the relationship with the patient, who felt comfortable disclosing many of her concerns and troubles. She received prescriptions for cephalexin 500 mg orally 3 times daily, 0.1% triamcinolone ointment to be applied twice daily, and doxepin 25 mg once nightly. She also received a referral for counseling.
At first she refused to trim her fingernails, but as she began to see that her ulcers were self-inflicted, she reconsidered and agreed. In fact, she asked to borrow our nail clippers before leaving the office.
- Cephalexin • Biocef, Keflex, Keftab
- Dicloxacillin • Dycill, Dynapen, Pathocil
- Doxepin • Adapin, Sinequan
Corresponding author
Richard P. Usatine, MD, University of Texas Health Sceicnes Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900.
Note: A handout developed by the American Academy of Family Physicians for patients with neurotic excoriations is available to print or photocopy at the following website: www.aafp.org/afp/20011215/neurph.html.
1. Cyr PR, Dreher GK. Neurotic excoriations. Am Fam Physician 2001;64:1981-1984.
2. Habif TP. Clinical Dermatology.4th ed. New York: Mosby; 2004.
3. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician 2001;64:1873-1878.
4. Moses S. Pruritic condition. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER258.htm. Accessed July 23, 2004.
5. Moses S. Pruritus causes. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER259.htm. Accessed July 23, 2004.
A 55-year-old woman came to the clinic complaining of severe itching on her arms and legs. Although she itched throughout the day, it became intolerable at night, disrupting her sleep. She would sometimes scratch her arms and legs until exhaustion but could find no relief. Being outside in warm and sunny weather aggravated the problem. She had used moisturizers, emollients, and topical corticosteroids, but they only alleviated the itching temporarily. The itching began 10 months earlier, just after she finalized the divorce from her husband of 20 years.
Examination of the skin revealed numerous excoriations with ulcerations and xerosis on the arms and left leg (Figure 1 and 2). The excoriations were located extensively from the dorsum of her left foot to above the knee and bilaterally from the wrist to above the elbow. They also showed signs of infection. The patient admitted they were self-inflicted. The patient’s right leg had been amputated 5 years before after a car accident, and she wore a prosthetic leg. Examination of other areas showed nothing remarkable.
The patient readily admitted to a great deal of psychological distress. She described feeling depressed since her divorce. She has had difficulty securing a full-time job and has high anxiety about being able to pay her rent and bills.
FIGURE 1
Excoriations on the arms…
FIGURE 2
…and the left leg
WHAT IS THE DIAGNOSIS?
WHAT IS THE TREATMENT STRATEGY?
DIFFERENTIAL DIAGNOSIS: PSYCHODERMATOLOGIC DISORDER
The patient’s history and physical examination points to a psychodermatologic disorder. Psycho-dermatologic disorders are conditions involving an interaction between the mind and skin and are classified as:3
- Psychophysiologic disorders:skin disorders worsened by emotional stress
- Primary psychiatric disorders:usually caused by psychological conditions with self-induced skin damage
- Secondary psychiatric disorders: psychological problems developed as a consequence of a disfiguring skin disorder, which negatively effects self-esteem and body image.3
This patient’s excoriations and ulcers are due to self-mutilation. The differential diagnosis includes psychogenic parasitosis, factitial dermatitis, and neurotic excoriations. These 3 skin conditions are primary psychiatric symptoms, and proper diagnosis revolves around being able to assess the dermatologic features and associated psychological disorder.2
Psychogenic parasitosis
Also known as delusional parasitosis, psychogenic parasitosis is a psychodermatologic disorder in which patients believe they are infested with parasites. Patients with this disorder report seeing or feeling parasites on their skin, and they damage their skin in an attempt to remove them. Patients create excoriations and ulcers on easily reached areas, usually the ears, eyes, nose, and extremities. They often present with what is termed the “matchbox sign,” in which they bring containers filled with “small bits of excoriated skin, dried blood, debris, or insect parts as proof of infestation.”2
Women over the age of 50 years are more often affected with psychogenic parasitosis, and the disorder is associated with anxiety, depression, and hypochondriasis.
Factitial dermatitis
Factitial dermatitis, also known as dermatitis artefacta, is a psychodermatologic disorder in which patients damage their skin but deny their self-involvement. This disorder encompasses a wide range of lesions, including blisters, cuts, ulcers, and burns. Patients often are unable to describe how the lesions evolved. Lesions exhibit bizarre patterns not characteristic of any disease.
Young adults and adolescents are more commonly affected, and it is 4 times more common among women than men. Psychological disorders involved with factitial dermatitis include personality disorders and posttraumatic stress disorder.2
Neurotic excoriations
Neurotic excoriations, sometimes referred to as neurodermatitis, are a result of a psychodermatologic disorder in which patients inflict excoriations and ulcers on their skin and admit to their involvement.3 The condition is characterized by excoriations similar in size and shape that are localized on areas easily reached by the patient, such as the arms, legs, and upper back.
The lesions may present in various stages, varying from dugout ulcers to ulcers covered with crusts and surrounded by erythema to areas receding into depressed scars. These lesions are a result of repetitive scratching and digging by the patient, usually without an underlying physical pathology but sometimes initiated by pruritus.1,2
Studies show the condition primarily affects women, with a mean onset between the ages of 30 to 45 years.1 Common psychiatric problems associated with neurotic excoriations include significant social stress, depression, anxiety, and obsessive-compulsive disorder.
Diagnosis: Neurotic excoriations due to depression and stress
The patient’s history and physical examination suggests a diagnosis of neurotic excoriations due to the characteristics of the excoriations and ulcers on her arms and leg, her admission of their self-inflicted nature, and the associated depression and psychosocial stress.
Laboratory tests
Although there are no available laboratory tests to confirm a positive finding of neurotic excoriations, tests could be performed to disprove any systemic causes of pruritus and the resulting dermatological damage.5 These tests include complete blood count with differential, chemistry profile, determination of thyroid-stimulating hormone levels, fasting plasma glucose level, and skin biopsies.1,
Laboratory tests for systemic causes of pruritus should be based on the patient’s physical and history, avoiding a broad approach.
Treatment: address the skin and the psyche
The treatment of neurotic excoriations requires a dual approach, addressing both the dermatological problems and the underlying psychological disorder.3 Supportive dermatologic care is necessary to avoid secondary complications and to ensure that the patient feels supported.3
Dermatologic care
Topical steroids can be helpful to decrease the pruritus and inflammation. The steroid strength should be chosen based upon the severity of the lesions and the thickness of the skin. Steroid ointments are preferred to creams when there are skin ulcers and deep excoriations.
If there is significant crusting or exudate, there is probably a secondary bacterial infection and oral antibiotics are indicated ( Figure 3). A first-generation cephalosporin or dicloxacillin should provide adequate coverage against the most likely organisms, Staphylococcus aureus and Streptococcus pyogenes.
FIGURE 3
Detail of lesions on the arm
Psychological care
The selective serotonin reuptake inhibitors produce a strong antipruritic response in patients with neurotic excoriations. Doxepin is a tricyclic antidepressant with one of the most powerful antihistamines for pruritus. When prescribing doxepin, limit the amount of medication dispensed at one time to minimize the risk of suicide.3 The patient should trim the fingernails to reduce the amount of damage caused by scratching and digging.
Treating the underlying psychological disorder requires supportive and empathic counseling. It may be necessary to collaborate with other mental health specialists. Management options include psychotropic medication, stress management courses, and referral to a psychiatrist. Patients with psychodermatologic disorders frequently resist referral to mental health professionals.3 If this is the case, family physicians are well positioned to help patients with psychological problems.
Alternative courses of treatment include hypnosis to disrupt the itch-scratch cycle, and acupuncture and supportive therapy to reduce underlying stressors.1
Patient’s outcome
By showing support and concern for the patient’s health during the visit, the physician strengthened the relationship with the patient, who felt comfortable disclosing many of her concerns and troubles. She received prescriptions for cephalexin 500 mg orally 3 times daily, 0.1% triamcinolone ointment to be applied twice daily, and doxepin 25 mg once nightly. She also received a referral for counseling.
At first she refused to trim her fingernails, but as she began to see that her ulcers were self-inflicted, she reconsidered and agreed. In fact, she asked to borrow our nail clippers before leaving the office.
- Cephalexin • Biocef, Keflex, Keftab
- Dicloxacillin • Dycill, Dynapen, Pathocil
- Doxepin • Adapin, Sinequan
Corresponding author
Richard P. Usatine, MD, University of Texas Health Sceicnes Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900.
Note: A handout developed by the American Academy of Family Physicians for patients with neurotic excoriations is available to print or photocopy at the following website: www.aafp.org/afp/20011215/neurph.html.
A 55-year-old woman came to the clinic complaining of severe itching on her arms and legs. Although she itched throughout the day, it became intolerable at night, disrupting her sleep. She would sometimes scratch her arms and legs until exhaustion but could find no relief. Being outside in warm and sunny weather aggravated the problem. She had used moisturizers, emollients, and topical corticosteroids, but they only alleviated the itching temporarily. The itching began 10 months earlier, just after she finalized the divorce from her husband of 20 years.
Examination of the skin revealed numerous excoriations with ulcerations and xerosis on the arms and left leg (Figure 1 and 2). The excoriations were located extensively from the dorsum of her left foot to above the knee and bilaterally from the wrist to above the elbow. They also showed signs of infection. The patient admitted they were self-inflicted. The patient’s right leg had been amputated 5 years before after a car accident, and she wore a prosthetic leg. Examination of other areas showed nothing remarkable.
The patient readily admitted to a great deal of psychological distress. She described feeling depressed since her divorce. She has had difficulty securing a full-time job and has high anxiety about being able to pay her rent and bills.
FIGURE 1
Excoriations on the arms…
FIGURE 2
…and the left leg
WHAT IS THE DIAGNOSIS?
WHAT IS THE TREATMENT STRATEGY?
DIFFERENTIAL DIAGNOSIS: PSYCHODERMATOLOGIC DISORDER
The patient’s history and physical examination points to a psychodermatologic disorder. Psycho-dermatologic disorders are conditions involving an interaction between the mind and skin and are classified as:3
- Psychophysiologic disorders:skin disorders worsened by emotional stress
- Primary psychiatric disorders:usually caused by psychological conditions with self-induced skin damage
- Secondary psychiatric disorders: psychological problems developed as a consequence of a disfiguring skin disorder, which negatively effects self-esteem and body image.3
This patient’s excoriations and ulcers are due to self-mutilation. The differential diagnosis includes psychogenic parasitosis, factitial dermatitis, and neurotic excoriations. These 3 skin conditions are primary psychiatric symptoms, and proper diagnosis revolves around being able to assess the dermatologic features and associated psychological disorder.2
Psychogenic parasitosis
Also known as delusional parasitosis, psychogenic parasitosis is a psychodermatologic disorder in which patients believe they are infested with parasites. Patients with this disorder report seeing or feeling parasites on their skin, and they damage their skin in an attempt to remove them. Patients create excoriations and ulcers on easily reached areas, usually the ears, eyes, nose, and extremities. They often present with what is termed the “matchbox sign,” in which they bring containers filled with “small bits of excoriated skin, dried blood, debris, or insect parts as proof of infestation.”2
Women over the age of 50 years are more often affected with psychogenic parasitosis, and the disorder is associated with anxiety, depression, and hypochondriasis.
Factitial dermatitis
Factitial dermatitis, also known as dermatitis artefacta, is a psychodermatologic disorder in which patients damage their skin but deny their self-involvement. This disorder encompasses a wide range of lesions, including blisters, cuts, ulcers, and burns. Patients often are unable to describe how the lesions evolved. Lesions exhibit bizarre patterns not characteristic of any disease.
Young adults and adolescents are more commonly affected, and it is 4 times more common among women than men. Psychological disorders involved with factitial dermatitis include personality disorders and posttraumatic stress disorder.2
Neurotic excoriations
Neurotic excoriations, sometimes referred to as neurodermatitis, are a result of a psychodermatologic disorder in which patients inflict excoriations and ulcers on their skin and admit to their involvement.3 The condition is characterized by excoriations similar in size and shape that are localized on areas easily reached by the patient, such as the arms, legs, and upper back.
The lesions may present in various stages, varying from dugout ulcers to ulcers covered with crusts and surrounded by erythema to areas receding into depressed scars. These lesions are a result of repetitive scratching and digging by the patient, usually without an underlying physical pathology but sometimes initiated by pruritus.1,2
Studies show the condition primarily affects women, with a mean onset between the ages of 30 to 45 years.1 Common psychiatric problems associated with neurotic excoriations include significant social stress, depression, anxiety, and obsessive-compulsive disorder.
Diagnosis: Neurotic excoriations due to depression and stress
The patient’s history and physical examination suggests a diagnosis of neurotic excoriations due to the characteristics of the excoriations and ulcers on her arms and leg, her admission of their self-inflicted nature, and the associated depression and psychosocial stress.
Laboratory tests
Although there are no available laboratory tests to confirm a positive finding of neurotic excoriations, tests could be performed to disprove any systemic causes of pruritus and the resulting dermatological damage.5 These tests include complete blood count with differential, chemistry profile, determination of thyroid-stimulating hormone levels, fasting plasma glucose level, and skin biopsies.1,
Laboratory tests for systemic causes of pruritus should be based on the patient’s physical and history, avoiding a broad approach.
Treatment: address the skin and the psyche
The treatment of neurotic excoriations requires a dual approach, addressing both the dermatological problems and the underlying psychological disorder.3 Supportive dermatologic care is necessary to avoid secondary complications and to ensure that the patient feels supported.3
Dermatologic care
Topical steroids can be helpful to decrease the pruritus and inflammation. The steroid strength should be chosen based upon the severity of the lesions and the thickness of the skin. Steroid ointments are preferred to creams when there are skin ulcers and deep excoriations.
If there is significant crusting or exudate, there is probably a secondary bacterial infection and oral antibiotics are indicated ( Figure 3). A first-generation cephalosporin or dicloxacillin should provide adequate coverage against the most likely organisms, Staphylococcus aureus and Streptococcus pyogenes.
FIGURE 3
Detail of lesions on the arm
Psychological care
The selective serotonin reuptake inhibitors produce a strong antipruritic response in patients with neurotic excoriations. Doxepin is a tricyclic antidepressant with one of the most powerful antihistamines for pruritus. When prescribing doxepin, limit the amount of medication dispensed at one time to minimize the risk of suicide.3 The patient should trim the fingernails to reduce the amount of damage caused by scratching and digging.
Treating the underlying psychological disorder requires supportive and empathic counseling. It may be necessary to collaborate with other mental health specialists. Management options include psychotropic medication, stress management courses, and referral to a psychiatrist. Patients with psychodermatologic disorders frequently resist referral to mental health professionals.3 If this is the case, family physicians are well positioned to help patients with psychological problems.
Alternative courses of treatment include hypnosis to disrupt the itch-scratch cycle, and acupuncture and supportive therapy to reduce underlying stressors.1
Patient’s outcome
By showing support and concern for the patient’s health during the visit, the physician strengthened the relationship with the patient, who felt comfortable disclosing many of her concerns and troubles. She received prescriptions for cephalexin 500 mg orally 3 times daily, 0.1% triamcinolone ointment to be applied twice daily, and doxepin 25 mg once nightly. She also received a referral for counseling.
At first she refused to trim her fingernails, but as she began to see that her ulcers were self-inflicted, she reconsidered and agreed. In fact, she asked to borrow our nail clippers before leaving the office.
- Cephalexin • Biocef, Keflex, Keftab
- Dicloxacillin • Dycill, Dynapen, Pathocil
- Doxepin • Adapin, Sinequan
Corresponding author
Richard P. Usatine, MD, University of Texas Health Sceicnes Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900.
Note: A handout developed by the American Academy of Family Physicians for patients with neurotic excoriations is available to print or photocopy at the following website: www.aafp.org/afp/20011215/neurph.html.
1. Cyr PR, Dreher GK. Neurotic excoriations. Am Fam Physician 2001;64:1981-1984.
2. Habif TP. Clinical Dermatology.4th ed. New York: Mosby; 2004.
3. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician 2001;64:1873-1878.
4. Moses S. Pruritic condition. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER258.htm. Accessed July 23, 2004.
5. Moses S. Pruritus causes. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER259.htm. Accessed July 23, 2004.
1. Cyr PR, Dreher GK. Neurotic excoriations. Am Fam Physician 2001;64:1981-1984.
2. Habif TP. Clinical Dermatology.4th ed. New York: Mosby; 2004.
3. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician 2001;64:1873-1878.
4. Moses S. Pruritic condition. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER258.htm. Accessed July 23, 2004.
5. Moses S. Pruritus causes. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER259.htm. Accessed July 23, 2004.
Painful and swollen hands
A 67-year-old woman came to the office with pain in her hands. She had just arrived from Panama to live with her son. She has had this pain for decades: she refers to it as artritisin Spanish but does not know what type of arthritis. Aspirin had helped in the past, but lately she had not been getting enough relief from it. Her hands feel stiff in the morning for at least 1 hour, which interferes with cooking and sewing.
Her hands showed signs of joint swelling and deformities (Figure 1). Her swollen joints felt warm. She also had knee pain.
FIGURE 1
Painful,swollen hands
What type of arthritis does she have?
Are any diagnostic tests necessary?
What are the best treatments available?
That same day, another patient was seen with painful hands (Figure 2). What type of arthritis does she have, and how does it differ from the condition of the patient in Figure 1 ?
FIGURE 2
Another patient with hand pain
The obvious ulnar deviation of her fingers and the swelling of the metacarpophalangeal (MCP) joints (Figure 1) are strongly indicative of rheumatoid arthritis. The patient in Figure 2 has swelling and deformities in her proximal inter-phalangeal (PIP) and distal interphalangeal (DIP) joints, indicating that she most likely has osteoarthritis. The swelling of the PIP joints is called Bouchard’s nodes; the swelling of the DIP joints is called Heberden’s nodes.
Differential diagnosis: types of arthritis
The first decision point in diagnosing chronic (>6 weeks) polyarticular joint pain is distinguishing between inflammatory and noninflammatory arthritis. Key features of inflammatory arthritis are stiffness in the morning or after inactivity, and visible joint swelling. The differential diagnosis of inflammatory arthritis includes rheumatoid arthritis, psoriatic arthritis, seronegative spondyloarthropathies, and systemic lupus erythematosus (SLE).
After the age of 50, maturity-onset seronegative synovitis syndrome and crystal-induced synovitis should also be considered.1 Although osteoarthritis is considered noninflammatory, inflammation of the joint tissue occurs occasionally due to the joint’s degenerative loss of cartilage and bony overgrowth.
It is critical to identify rheumatoid arthritis early, as prompt intervention can delay disease progression, and reduce the substantial morbidity and mortality of rheumatoid arthritis.2 A diagnosis of rheumatoid arthritis requires 4 of the following: morning stiffness; arthritis in 3 or more joints; arthritis in the wrist, MCP joints, or PIP joints; symmetric arthritis; rheumatoid nodules; positive rheumatoid factor; radiographic changes.1 This patient has the morning stiffness, arthritis in more than 3 joints, symmetric arthritis, and rheumatoid nodules on her feet (Figure 3).
The most common noninflammatory arthritis is osteoarthritis, which affects 21 million Americans.2 The weight-bearing joints are usually affected, and damage may occur because of trauma or repetitive impact. When the hands are involved, the DIP and PIP joints are more likely to be involved than the MCP joints.
FIGURE 3
Rheumatoid nodules on feet
Diagnostic tests can differentiate between causes
Laboratory tests can help differentiate between conditions causing inflammatory arthritis. Rheumatoid factor is positive in 70% of patients with rheumatoid arthritis, and the antinuclear antibody is invariably positive for patients with SLE. Maturity-onset seronegative synovitis has negative rheumatoid factor and antinuclear antibody tests with marked elevation in erythrocyte sedimentation rate. Polyarticular gout may have increased serum uric acid, and is best diagnosed by demonstrating crystals in the joint fluid.
Laboratory tests are not helpful in diagnosing psoriatic arthritis, seronegative spondylo-arthropathies, and osteoarthritis with inflammation.1 Radiographic studies may show joint erosions in patients with active rheumatoid arthritis for more than a year, and typical osteophytes and joint-space narrowing in osteoarthritis.1
Radiographs are not necessary for the diagnosis of this patient but may help management, especially if hand surgery is going to be considered. In this case, the radiographs showed joint erosions and unequivocal juxta-auricular osteopenia.
Management: decrease pain, optimize mobility
The management of osteoarthritis and rheumatoid arthritis is different. However, in all types of arthritis the goals of therapy are to decrease pain, optimize mobility, and maximize quality of life. In rheumatoid arthritis, another goal is to slow the progression of the disease with disease-modifying antirheumatic drugs.
Osteoarthritis. First-line therapy for osteo arthritis includes exercise, weight loss (if indicated), and acetaminophen in scheduled doses up to 1000 mg 4 times a day. A recent Cochrane Review concluded that acetaminophen is clearly superior to placebo, but slightly less efficacious than nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief in osteoarthritis (level of evidence [LOE]: 1a). Acetaminophen and NSAIDs were equivalent in improving function. This evidence supports the use of acetaminophen first, reserving NSAIDs for those who do not respond.3 Adding NSAIDs may improve pain relief, but carries an increased risk of gastrointestinal ulcerations or bleeding.
A cyclo-oxygenase-2 (COX-2) inhibitor may be preferred to NSAIDs for patients at high risk for gastrointestinal complications. Other treatments include topical analgesics, glucosamine, and chondroitin. Large clinical trials for glucosamine and chondroitin are ongoing.
Rheumatoid arthritis. In rheumatoid arthritis, the recommendation from the American College of Rheumatology is for early, aggressive intervention with disease-modifying antirheumatic drugs, often within a few months of the onset of the disease.4 Unfortunately, the patient in Figure 1 has had rheumatoid arthritis for decades. Patients with rheumatoid arthritis also benefit from exercise and physical therapy.
Other treatments include low-dose corticosteroids and NSAIDs or COX-2 inhibitors. COX-2 inhibitors have similar efficacy to NSAIDs, with a lower risk of gastrointestinal complications (LOE: 1a).5,6 COX-2 inhibitors should be considered in place of NSAIDs for patients with rheumatoid arthritis, as these patients are almost twice as likely to suffer from serious gastrointestinal complications as the general population.2 COX-2 inhibitors, however, are much more expensive than NSAIDs, which limits their use.
Patient’s outcome
The patient was started on an anti-inflammatory medication and referred to a rheumatologist for consideration of a disease-modifying antirheumatic drug.
Acknowledgments
The authors would like to acknowledge Michael Fischbach, MD, in the Rheumatology Department of the University of Texas Health Science Center for his contribution to this article.
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas HealthSciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd CurlDrive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Klinkhoff A. Rheumatology: 5. Diagnosis and management of inflammatory polyarthritis. CMAJ 2000;162:1833-1838
2. Kuritzky L, Weaver A. Advances in rheumatology: coxibs and beyond. J Pain Symptom Manage 2003;25(2 Suppl):S6-S20.
3. Towheed TE, Judd MJ, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2003;(2):CD004257-
4. Guidelines for the Management of Rheumatoid Arthritis from the American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Arthritis Rheum 2002;46:328-346.
5. Garner S, Fidan D, Frankish R, et al. Rofecoxib for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2002;(3):CD003685.-
6. Garner S, Fidan D, Frankish R, et al. Celecoxib for rheumatoid arthritis. Cochrane Database Syst Rev 2002;(4):CD003831.
A 67-year-old woman came to the office with pain in her hands. She had just arrived from Panama to live with her son. She has had this pain for decades: she refers to it as artritisin Spanish but does not know what type of arthritis. Aspirin had helped in the past, but lately she had not been getting enough relief from it. Her hands feel stiff in the morning for at least 1 hour, which interferes with cooking and sewing.
Her hands showed signs of joint swelling and deformities (Figure 1). Her swollen joints felt warm. She also had knee pain.
FIGURE 1
Painful,swollen hands
What type of arthritis does she have?
Are any diagnostic tests necessary?
What are the best treatments available?
That same day, another patient was seen with painful hands (Figure 2). What type of arthritis does she have, and how does it differ from the condition of the patient in Figure 1 ?
FIGURE 2
Another patient with hand pain
The obvious ulnar deviation of her fingers and the swelling of the metacarpophalangeal (MCP) joints (Figure 1) are strongly indicative of rheumatoid arthritis. The patient in Figure 2 has swelling and deformities in her proximal inter-phalangeal (PIP) and distal interphalangeal (DIP) joints, indicating that she most likely has osteoarthritis. The swelling of the PIP joints is called Bouchard’s nodes; the swelling of the DIP joints is called Heberden’s nodes.
Differential diagnosis: types of arthritis
The first decision point in diagnosing chronic (>6 weeks) polyarticular joint pain is distinguishing between inflammatory and noninflammatory arthritis. Key features of inflammatory arthritis are stiffness in the morning or after inactivity, and visible joint swelling. The differential diagnosis of inflammatory arthritis includes rheumatoid arthritis, psoriatic arthritis, seronegative spondyloarthropathies, and systemic lupus erythematosus (SLE).
After the age of 50, maturity-onset seronegative synovitis syndrome and crystal-induced synovitis should also be considered.1 Although osteoarthritis is considered noninflammatory, inflammation of the joint tissue occurs occasionally due to the joint’s degenerative loss of cartilage and bony overgrowth.
It is critical to identify rheumatoid arthritis early, as prompt intervention can delay disease progression, and reduce the substantial morbidity and mortality of rheumatoid arthritis.2 A diagnosis of rheumatoid arthritis requires 4 of the following: morning stiffness; arthritis in 3 or more joints; arthritis in the wrist, MCP joints, or PIP joints; symmetric arthritis; rheumatoid nodules; positive rheumatoid factor; radiographic changes.1 This patient has the morning stiffness, arthritis in more than 3 joints, symmetric arthritis, and rheumatoid nodules on her feet (Figure 3).
The most common noninflammatory arthritis is osteoarthritis, which affects 21 million Americans.2 The weight-bearing joints are usually affected, and damage may occur because of trauma or repetitive impact. When the hands are involved, the DIP and PIP joints are more likely to be involved than the MCP joints.
FIGURE 3
Rheumatoid nodules on feet
Diagnostic tests can differentiate between causes
Laboratory tests can help differentiate between conditions causing inflammatory arthritis. Rheumatoid factor is positive in 70% of patients with rheumatoid arthritis, and the antinuclear antibody is invariably positive for patients with SLE. Maturity-onset seronegative synovitis has negative rheumatoid factor and antinuclear antibody tests with marked elevation in erythrocyte sedimentation rate. Polyarticular gout may have increased serum uric acid, and is best diagnosed by demonstrating crystals in the joint fluid.
Laboratory tests are not helpful in diagnosing psoriatic arthritis, seronegative spondylo-arthropathies, and osteoarthritis with inflammation.1 Radiographic studies may show joint erosions in patients with active rheumatoid arthritis for more than a year, and typical osteophytes and joint-space narrowing in osteoarthritis.1
Radiographs are not necessary for the diagnosis of this patient but may help management, especially if hand surgery is going to be considered. In this case, the radiographs showed joint erosions and unequivocal juxta-auricular osteopenia.
Management: decrease pain, optimize mobility
The management of osteoarthritis and rheumatoid arthritis is different. However, in all types of arthritis the goals of therapy are to decrease pain, optimize mobility, and maximize quality of life. In rheumatoid arthritis, another goal is to slow the progression of the disease with disease-modifying antirheumatic drugs.
Osteoarthritis. First-line therapy for osteo arthritis includes exercise, weight loss (if indicated), and acetaminophen in scheduled doses up to 1000 mg 4 times a day. A recent Cochrane Review concluded that acetaminophen is clearly superior to placebo, but slightly less efficacious than nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief in osteoarthritis (level of evidence [LOE]: 1a). Acetaminophen and NSAIDs were equivalent in improving function. This evidence supports the use of acetaminophen first, reserving NSAIDs for those who do not respond.3 Adding NSAIDs may improve pain relief, but carries an increased risk of gastrointestinal ulcerations or bleeding.
A cyclo-oxygenase-2 (COX-2) inhibitor may be preferred to NSAIDs for patients at high risk for gastrointestinal complications. Other treatments include topical analgesics, glucosamine, and chondroitin. Large clinical trials for glucosamine and chondroitin are ongoing.
Rheumatoid arthritis. In rheumatoid arthritis, the recommendation from the American College of Rheumatology is for early, aggressive intervention with disease-modifying antirheumatic drugs, often within a few months of the onset of the disease.4 Unfortunately, the patient in Figure 1 has had rheumatoid arthritis for decades. Patients with rheumatoid arthritis also benefit from exercise and physical therapy.
Other treatments include low-dose corticosteroids and NSAIDs or COX-2 inhibitors. COX-2 inhibitors have similar efficacy to NSAIDs, with a lower risk of gastrointestinal complications (LOE: 1a).5,6 COX-2 inhibitors should be considered in place of NSAIDs for patients with rheumatoid arthritis, as these patients are almost twice as likely to suffer from serious gastrointestinal complications as the general population.2 COX-2 inhibitors, however, are much more expensive than NSAIDs, which limits their use.
Patient’s outcome
The patient was started on an anti-inflammatory medication and referred to a rheumatologist for consideration of a disease-modifying antirheumatic drug.
Acknowledgments
The authors would like to acknowledge Michael Fischbach, MD, in the Rheumatology Department of the University of Texas Health Science Center for his contribution to this article.
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas HealthSciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd CurlDrive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 67-year-old woman came to the office with pain in her hands. She had just arrived from Panama to live with her son. She has had this pain for decades: she refers to it as artritisin Spanish but does not know what type of arthritis. Aspirin had helped in the past, but lately she had not been getting enough relief from it. Her hands feel stiff in the morning for at least 1 hour, which interferes with cooking and sewing.
Her hands showed signs of joint swelling and deformities (Figure 1). Her swollen joints felt warm. She also had knee pain.
FIGURE 1
Painful,swollen hands
What type of arthritis does she have?
Are any diagnostic tests necessary?
What are the best treatments available?
That same day, another patient was seen with painful hands (Figure 2). What type of arthritis does she have, and how does it differ from the condition of the patient in Figure 1 ?
FIGURE 2
Another patient with hand pain
The obvious ulnar deviation of her fingers and the swelling of the metacarpophalangeal (MCP) joints (Figure 1) are strongly indicative of rheumatoid arthritis. The patient in Figure 2 has swelling and deformities in her proximal inter-phalangeal (PIP) and distal interphalangeal (DIP) joints, indicating that she most likely has osteoarthritis. The swelling of the PIP joints is called Bouchard’s nodes; the swelling of the DIP joints is called Heberden’s nodes.
Differential diagnosis: types of arthritis
The first decision point in diagnosing chronic (>6 weeks) polyarticular joint pain is distinguishing between inflammatory and noninflammatory arthritis. Key features of inflammatory arthritis are stiffness in the morning or after inactivity, and visible joint swelling. The differential diagnosis of inflammatory arthritis includes rheumatoid arthritis, psoriatic arthritis, seronegative spondyloarthropathies, and systemic lupus erythematosus (SLE).
After the age of 50, maturity-onset seronegative synovitis syndrome and crystal-induced synovitis should also be considered.1 Although osteoarthritis is considered noninflammatory, inflammation of the joint tissue occurs occasionally due to the joint’s degenerative loss of cartilage and bony overgrowth.
It is critical to identify rheumatoid arthritis early, as prompt intervention can delay disease progression, and reduce the substantial morbidity and mortality of rheumatoid arthritis.2 A diagnosis of rheumatoid arthritis requires 4 of the following: morning stiffness; arthritis in 3 or more joints; arthritis in the wrist, MCP joints, or PIP joints; symmetric arthritis; rheumatoid nodules; positive rheumatoid factor; radiographic changes.1 This patient has the morning stiffness, arthritis in more than 3 joints, symmetric arthritis, and rheumatoid nodules on her feet (Figure 3).
The most common noninflammatory arthritis is osteoarthritis, which affects 21 million Americans.2 The weight-bearing joints are usually affected, and damage may occur because of trauma or repetitive impact. When the hands are involved, the DIP and PIP joints are more likely to be involved than the MCP joints.
FIGURE 3
Rheumatoid nodules on feet
Diagnostic tests can differentiate between causes
Laboratory tests can help differentiate between conditions causing inflammatory arthritis. Rheumatoid factor is positive in 70% of patients with rheumatoid arthritis, and the antinuclear antibody is invariably positive for patients with SLE. Maturity-onset seronegative synovitis has negative rheumatoid factor and antinuclear antibody tests with marked elevation in erythrocyte sedimentation rate. Polyarticular gout may have increased serum uric acid, and is best diagnosed by demonstrating crystals in the joint fluid.
Laboratory tests are not helpful in diagnosing psoriatic arthritis, seronegative spondylo-arthropathies, and osteoarthritis with inflammation.1 Radiographic studies may show joint erosions in patients with active rheumatoid arthritis for more than a year, and typical osteophytes and joint-space narrowing in osteoarthritis.1
Radiographs are not necessary for the diagnosis of this patient but may help management, especially if hand surgery is going to be considered. In this case, the radiographs showed joint erosions and unequivocal juxta-auricular osteopenia.
Management: decrease pain, optimize mobility
The management of osteoarthritis and rheumatoid arthritis is different. However, in all types of arthritis the goals of therapy are to decrease pain, optimize mobility, and maximize quality of life. In rheumatoid arthritis, another goal is to slow the progression of the disease with disease-modifying antirheumatic drugs.
Osteoarthritis. First-line therapy for osteo arthritis includes exercise, weight loss (if indicated), and acetaminophen in scheduled doses up to 1000 mg 4 times a day. A recent Cochrane Review concluded that acetaminophen is clearly superior to placebo, but slightly less efficacious than nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief in osteoarthritis (level of evidence [LOE]: 1a). Acetaminophen and NSAIDs were equivalent in improving function. This evidence supports the use of acetaminophen first, reserving NSAIDs for those who do not respond.3 Adding NSAIDs may improve pain relief, but carries an increased risk of gastrointestinal ulcerations or bleeding.
A cyclo-oxygenase-2 (COX-2) inhibitor may be preferred to NSAIDs for patients at high risk for gastrointestinal complications. Other treatments include topical analgesics, glucosamine, and chondroitin. Large clinical trials for glucosamine and chondroitin are ongoing.
Rheumatoid arthritis. In rheumatoid arthritis, the recommendation from the American College of Rheumatology is for early, aggressive intervention with disease-modifying antirheumatic drugs, often within a few months of the onset of the disease.4 Unfortunately, the patient in Figure 1 has had rheumatoid arthritis for decades. Patients with rheumatoid arthritis also benefit from exercise and physical therapy.
Other treatments include low-dose corticosteroids and NSAIDs or COX-2 inhibitors. COX-2 inhibitors have similar efficacy to NSAIDs, with a lower risk of gastrointestinal complications (LOE: 1a).5,6 COX-2 inhibitors should be considered in place of NSAIDs for patients with rheumatoid arthritis, as these patients are almost twice as likely to suffer from serious gastrointestinal complications as the general population.2 COX-2 inhibitors, however, are much more expensive than NSAIDs, which limits their use.
Patient’s outcome
The patient was started on an anti-inflammatory medication and referred to a rheumatologist for consideration of a disease-modifying antirheumatic drug.
Acknowledgments
The authors would like to acknowledge Michael Fischbach, MD, in the Rheumatology Department of the University of Texas Health Science Center for his contribution to this article.
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas HealthSciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd CurlDrive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Klinkhoff A. Rheumatology: 5. Diagnosis and management of inflammatory polyarthritis. CMAJ 2000;162:1833-1838
2. Kuritzky L, Weaver A. Advances in rheumatology: coxibs and beyond. J Pain Symptom Manage 2003;25(2 Suppl):S6-S20.
3. Towheed TE, Judd MJ, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2003;(2):CD004257-
4. Guidelines for the Management of Rheumatoid Arthritis from the American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Arthritis Rheum 2002;46:328-346.
5. Garner S, Fidan D, Frankish R, et al. Rofecoxib for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2002;(3):CD003685.-
6. Garner S, Fidan D, Frankish R, et al. Celecoxib for rheumatoid arthritis. Cochrane Database Syst Rev 2002;(4):CD003831.
1. Klinkhoff A. Rheumatology: 5. Diagnosis and management of inflammatory polyarthritis. CMAJ 2000;162:1833-1838
2. Kuritzky L, Weaver A. Advances in rheumatology: coxibs and beyond. J Pain Symptom Manage 2003;25(2 Suppl):S6-S20.
3. Towheed TE, Judd MJ, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2003;(2):CD004257-
4. Guidelines for the Management of Rheumatoid Arthritis from the American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Arthritis Rheum 2002;46:328-346.
5. Garner S, Fidan D, Frankish R, et al. Rofecoxib for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2002;(3):CD003685.-
6. Garner S, Fidan D, Frankish R, et al. Celecoxib for rheumatoid arthritis. Cochrane Database Syst Rev 2002;(4):CD003831.