User login
-
World Cancer Day survey exposes ‘glaring inequities’
The first international public survey on cancer perceptions and attitudes in a decade shows that, in spite of progress, low socioeconomic status and lack of education continue to jeopardize the health of the world’s most vulnerable populations.
The survey was commissioned by the Union for International Cancer Control (UICC) to mark the 20th anniversary of World Cancer Day on Feb. 4, 2020.
The survey, which was conducted by Ipsos, was taken by more than 15,000 people in 20 countries. It shows that people of lower socioeconomic status are less likely than those in higher-income households to recognize the risk factors for cancer or to make lifestyle changes. With the exception of tobacco use, people with low educational attainment also showed less cancer awareness and were less likely to engage in preventive behaviors than those with a university degree.
It is “unacceptable that millions of people have a greater chance of developing cancer in their lifetime because they are simply not aware of the cancer risks to avoid and the healthy behaviors to adopt – information that many of us take for granted. And this is true around the world,” Cary Adams, MBA, CEO of the UICC, commented in a statement.
The survey was conducted from Oct. 25 to Nov., 2019, and included 15,427 participants from Australia, Bolivia, Brazil, Canada, China, France, Germany, Great Britain, India, Israel, Japan, Kenya, Mexico, the Philippines, Saudi Arabia, South Africa, Spain, Sweden, Turkey, and the United States.
The vast majority of those surveyed – 87% – said they were aware of the major risk factors for cancer, while only 6% said they were not.
The cancer risk factors that were most recognized were tobacco use (63%), ultraviolet light exposure (54%), and exposure to secondhand tobacco smoke (50%).
The cancer risks that were least recognized included being overweight (29%), a lack of exercise (28%), and exposure to certain viruses or bacteria (28%).
The difference in awareness across the social spectrum was striking. “Emerging from the survey are the apparent and glaring inequities faced by socioeconomically disadvantaged groups,” the authors said.
“Much more must be done to ensure that everyone has an equal chance to reduce their risk of preventable cancer,” commented Sonali Johnson, PhD, head of knowledge, advocacy, and policy at the UICC in Geneva, Switzerland.
“We’ve seen in the results that those surveyed with a lower education and those on lower incomes appear less aware of the main risk factors associated with cancer and thus are less likely to proactively take the steps needed to reduce their cancer risk as compared to those from a high income household or those with a university education,” Dr. Johnson said in an interview.
Does increased cancer awareness translate into behavioral change for the better? This question can only be answered by more research, the survey authors said. They reported that 7 of 10 survey respondents (69%) said they had made a behavioral change to reduce their cancer risk within the past 12 months. Most said they were eating more healthfully.
Slightly fewer than one-quarter reported that they had not taken any preventive measures in the past year.
When it comes to raising cancer awareness, World Cancer Day is “a powerful tool to remind every person that they can play a crucial role in reducing the impact of cancer,” said Dr. Johnson.
Health care providers are “crucial”
Reacting to the findings of the survey, the European Society for Medical Oncology (ESMO) emphasized the key role that physicians play in cancer prevention.
“Research speaks very clearly for prevention,” said ESMO President Solange Peters, MD, PhD. “With the number of cancer cases expected to rise to 29.5 million by 2040, we must act now. ESMO is committed to educating doctors on all aspects of cancer control, which should begin well before a cancer diagnosis.
“In the face of this emergency, which is rendered even more salient by the results of the report, we must work to enlarge the basis of doctors who are properly educated and trained in key prevention measures,” Dr. Peters added. “General practitioners and organ specialists are in the front line to guide and support patients on their quest for healthy lifestyles and reliable ways to detect cancer early.”
In a comment, Dr. Johnson acknowledged the role physicians play in health promotion and informing patients about noncommunicable disease risks, including those related to cancer. However, she emphasized that nurses, pharmacists, community health workers, midwives, and other health care providers who deliver primary care “are crucial around the world to imparting health information and offering services.”
Frontline health care workers can assess patients’ cancer knowledge and health literacy, determine the barriers to health care, and assess “how best to engage with people across the life course,” Dr. Johnson explained. “Rather than just focusing on physicians, we must work with all those involved in primary care, especially as primary care services are scaled up to achieve universal health coverage.”
Call on governments to do more
The authors noted that, although there is wide awareness of the cancer risks from tobacco use, adults younger than 35 years were less likely than those older than 50 to identify tobacco as a cancer risk factor. They described this finding as “most concerning” and said it “underscores the ongoing need to raise awareness about cancer risk factors in every new generation.”
Almost 60% of survey respondents, regardless of age, education, or income, expressed concern about being diagnosed with cancer in the future or having cancer recur.
In Kenya, where the death toll from cancer rose 30% from 2014 to 2018, people appeared to be the most worried about cancer, with four of five survey respondents (82%) expressing concern.
Survey respondents from Saudi Arabia appeared the least concerned, with one of three people saying they were worried.
Notably, 84% of survey respondents said that governments should be doing more to increase cancer prevention and awareness; 33% demanded that governments improve the affordability of cancer care.
“It is understandable that people turn to their governments for support,” Dr. Johnson commented. “Affordability is a big challenge for low-income settings.”
Data from the World Health Organization show that, for every U.S. $1 invested in low- and middle-income countries, the return is U.S. $3.20, Johnson pointed out. “We really need to convince decision makers ... and see the right investments being made. It is important to ensure that the health system strengthening takes place in tandem with prevention services.”
Governments have begun making commitments to tackle noncommunicable diseases and cancer, Dr. Johnson commented. He highlighted the WHO’s Global Action Plan for Healthy Lives and Well-being for All and the updated cancer resolution adopted at the 2017 World Health Assembly.
“Education, training, and awareness-raising efforts need to be backed by strong and progressive health policies that prioritize prevention and help reduce the consumption of known cancer-causing products such as tobacco, sugary food, and beverages,” she said. “Countries should also invest proactively in national cancer control planning and the establishment of population-based registries to ensure the most effective resource allocation that benefits all groups.”
Up-to-date information on cancer risks and cancer prevention must be delivered to the public in ways that are accessible to those in lower socioeconomic groups, Dr. Johnson added. “Awareness needs to be raised continuously with each new generation,” she noted.
The UICC has relationships with Astellas, Daiichi Sankyo, Diaceutics, MSD Inventing for Life, Bristol-Myers Squibb, CUBEBIO, the Icon Group, Roche, and Sanofi.
This article first appeared on Medscape.com.
The first international public survey on cancer perceptions and attitudes in a decade shows that, in spite of progress, low socioeconomic status and lack of education continue to jeopardize the health of the world’s most vulnerable populations.
The survey was commissioned by the Union for International Cancer Control (UICC) to mark the 20th anniversary of World Cancer Day on Feb. 4, 2020.
The survey, which was conducted by Ipsos, was taken by more than 15,000 people in 20 countries. It shows that people of lower socioeconomic status are less likely than those in higher-income households to recognize the risk factors for cancer or to make lifestyle changes. With the exception of tobacco use, people with low educational attainment also showed less cancer awareness and were less likely to engage in preventive behaviors than those with a university degree.
It is “unacceptable that millions of people have a greater chance of developing cancer in their lifetime because they are simply not aware of the cancer risks to avoid and the healthy behaviors to adopt – information that many of us take for granted. And this is true around the world,” Cary Adams, MBA, CEO of the UICC, commented in a statement.
The survey was conducted from Oct. 25 to Nov., 2019, and included 15,427 participants from Australia, Bolivia, Brazil, Canada, China, France, Germany, Great Britain, India, Israel, Japan, Kenya, Mexico, the Philippines, Saudi Arabia, South Africa, Spain, Sweden, Turkey, and the United States.
The vast majority of those surveyed – 87% – said they were aware of the major risk factors for cancer, while only 6% said they were not.
The cancer risk factors that were most recognized were tobacco use (63%), ultraviolet light exposure (54%), and exposure to secondhand tobacco smoke (50%).
The cancer risks that were least recognized included being overweight (29%), a lack of exercise (28%), and exposure to certain viruses or bacteria (28%).
The difference in awareness across the social spectrum was striking. “Emerging from the survey are the apparent and glaring inequities faced by socioeconomically disadvantaged groups,” the authors said.
“Much more must be done to ensure that everyone has an equal chance to reduce their risk of preventable cancer,” commented Sonali Johnson, PhD, head of knowledge, advocacy, and policy at the UICC in Geneva, Switzerland.
“We’ve seen in the results that those surveyed with a lower education and those on lower incomes appear less aware of the main risk factors associated with cancer and thus are less likely to proactively take the steps needed to reduce their cancer risk as compared to those from a high income household or those with a university education,” Dr. Johnson said in an interview.
Does increased cancer awareness translate into behavioral change for the better? This question can only be answered by more research, the survey authors said. They reported that 7 of 10 survey respondents (69%) said they had made a behavioral change to reduce their cancer risk within the past 12 months. Most said they were eating more healthfully.
Slightly fewer than one-quarter reported that they had not taken any preventive measures in the past year.
When it comes to raising cancer awareness, World Cancer Day is “a powerful tool to remind every person that they can play a crucial role in reducing the impact of cancer,” said Dr. Johnson.
Health care providers are “crucial”
Reacting to the findings of the survey, the European Society for Medical Oncology (ESMO) emphasized the key role that physicians play in cancer prevention.
“Research speaks very clearly for prevention,” said ESMO President Solange Peters, MD, PhD. “With the number of cancer cases expected to rise to 29.5 million by 2040, we must act now. ESMO is committed to educating doctors on all aspects of cancer control, which should begin well before a cancer diagnosis.
“In the face of this emergency, which is rendered even more salient by the results of the report, we must work to enlarge the basis of doctors who are properly educated and trained in key prevention measures,” Dr. Peters added. “General practitioners and organ specialists are in the front line to guide and support patients on their quest for healthy lifestyles and reliable ways to detect cancer early.”
In a comment, Dr. Johnson acknowledged the role physicians play in health promotion and informing patients about noncommunicable disease risks, including those related to cancer. However, she emphasized that nurses, pharmacists, community health workers, midwives, and other health care providers who deliver primary care “are crucial around the world to imparting health information and offering services.”
Frontline health care workers can assess patients’ cancer knowledge and health literacy, determine the barriers to health care, and assess “how best to engage with people across the life course,” Dr. Johnson explained. “Rather than just focusing on physicians, we must work with all those involved in primary care, especially as primary care services are scaled up to achieve universal health coverage.”
Call on governments to do more
The authors noted that, although there is wide awareness of the cancer risks from tobacco use, adults younger than 35 years were less likely than those older than 50 to identify tobacco as a cancer risk factor. They described this finding as “most concerning” and said it “underscores the ongoing need to raise awareness about cancer risk factors in every new generation.”
Almost 60% of survey respondents, regardless of age, education, or income, expressed concern about being diagnosed with cancer in the future or having cancer recur.
In Kenya, where the death toll from cancer rose 30% from 2014 to 2018, people appeared to be the most worried about cancer, with four of five survey respondents (82%) expressing concern.
Survey respondents from Saudi Arabia appeared the least concerned, with one of three people saying they were worried.
Notably, 84% of survey respondents said that governments should be doing more to increase cancer prevention and awareness; 33% demanded that governments improve the affordability of cancer care.
“It is understandable that people turn to their governments for support,” Dr. Johnson commented. “Affordability is a big challenge for low-income settings.”
Data from the World Health Organization show that, for every U.S. $1 invested in low- and middle-income countries, the return is U.S. $3.20, Johnson pointed out. “We really need to convince decision makers ... and see the right investments being made. It is important to ensure that the health system strengthening takes place in tandem with prevention services.”
Governments have begun making commitments to tackle noncommunicable diseases and cancer, Dr. Johnson commented. He highlighted the WHO’s Global Action Plan for Healthy Lives and Well-being for All and the updated cancer resolution adopted at the 2017 World Health Assembly.
“Education, training, and awareness-raising efforts need to be backed by strong and progressive health policies that prioritize prevention and help reduce the consumption of known cancer-causing products such as tobacco, sugary food, and beverages,” she said. “Countries should also invest proactively in national cancer control planning and the establishment of population-based registries to ensure the most effective resource allocation that benefits all groups.”
Up-to-date information on cancer risks and cancer prevention must be delivered to the public in ways that are accessible to those in lower socioeconomic groups, Dr. Johnson added. “Awareness needs to be raised continuously with each new generation,” she noted.
The UICC has relationships with Astellas, Daiichi Sankyo, Diaceutics, MSD Inventing for Life, Bristol-Myers Squibb, CUBEBIO, the Icon Group, Roche, and Sanofi.
This article first appeared on Medscape.com.
The first international public survey on cancer perceptions and attitudes in a decade shows that, in spite of progress, low socioeconomic status and lack of education continue to jeopardize the health of the world’s most vulnerable populations.
The survey was commissioned by the Union for International Cancer Control (UICC) to mark the 20th anniversary of World Cancer Day on Feb. 4, 2020.
The survey, which was conducted by Ipsos, was taken by more than 15,000 people in 20 countries. It shows that people of lower socioeconomic status are less likely than those in higher-income households to recognize the risk factors for cancer or to make lifestyle changes. With the exception of tobacco use, people with low educational attainment also showed less cancer awareness and were less likely to engage in preventive behaviors than those with a university degree.
It is “unacceptable that millions of people have a greater chance of developing cancer in their lifetime because they are simply not aware of the cancer risks to avoid and the healthy behaviors to adopt – information that many of us take for granted. And this is true around the world,” Cary Adams, MBA, CEO of the UICC, commented in a statement.
The survey was conducted from Oct. 25 to Nov., 2019, and included 15,427 participants from Australia, Bolivia, Brazil, Canada, China, France, Germany, Great Britain, India, Israel, Japan, Kenya, Mexico, the Philippines, Saudi Arabia, South Africa, Spain, Sweden, Turkey, and the United States.
The vast majority of those surveyed – 87% – said they were aware of the major risk factors for cancer, while only 6% said they were not.
The cancer risk factors that were most recognized were tobacco use (63%), ultraviolet light exposure (54%), and exposure to secondhand tobacco smoke (50%).
The cancer risks that were least recognized included being overweight (29%), a lack of exercise (28%), and exposure to certain viruses or bacteria (28%).
The difference in awareness across the social spectrum was striking. “Emerging from the survey are the apparent and glaring inequities faced by socioeconomically disadvantaged groups,” the authors said.
“Much more must be done to ensure that everyone has an equal chance to reduce their risk of preventable cancer,” commented Sonali Johnson, PhD, head of knowledge, advocacy, and policy at the UICC in Geneva, Switzerland.
“We’ve seen in the results that those surveyed with a lower education and those on lower incomes appear less aware of the main risk factors associated with cancer and thus are less likely to proactively take the steps needed to reduce their cancer risk as compared to those from a high income household or those with a university education,” Dr. Johnson said in an interview.
Does increased cancer awareness translate into behavioral change for the better? This question can only be answered by more research, the survey authors said. They reported that 7 of 10 survey respondents (69%) said they had made a behavioral change to reduce their cancer risk within the past 12 months. Most said they were eating more healthfully.
Slightly fewer than one-quarter reported that they had not taken any preventive measures in the past year.
When it comes to raising cancer awareness, World Cancer Day is “a powerful tool to remind every person that they can play a crucial role in reducing the impact of cancer,” said Dr. Johnson.
Health care providers are “crucial”
Reacting to the findings of the survey, the European Society for Medical Oncology (ESMO) emphasized the key role that physicians play in cancer prevention.
“Research speaks very clearly for prevention,” said ESMO President Solange Peters, MD, PhD. “With the number of cancer cases expected to rise to 29.5 million by 2040, we must act now. ESMO is committed to educating doctors on all aspects of cancer control, which should begin well before a cancer diagnosis.
“In the face of this emergency, which is rendered even more salient by the results of the report, we must work to enlarge the basis of doctors who are properly educated and trained in key prevention measures,” Dr. Peters added. “General practitioners and organ specialists are in the front line to guide and support patients on their quest for healthy lifestyles and reliable ways to detect cancer early.”
In a comment, Dr. Johnson acknowledged the role physicians play in health promotion and informing patients about noncommunicable disease risks, including those related to cancer. However, she emphasized that nurses, pharmacists, community health workers, midwives, and other health care providers who deliver primary care “are crucial around the world to imparting health information and offering services.”
Frontline health care workers can assess patients’ cancer knowledge and health literacy, determine the barriers to health care, and assess “how best to engage with people across the life course,” Dr. Johnson explained. “Rather than just focusing on physicians, we must work with all those involved in primary care, especially as primary care services are scaled up to achieve universal health coverage.”
Call on governments to do more
The authors noted that, although there is wide awareness of the cancer risks from tobacco use, adults younger than 35 years were less likely than those older than 50 to identify tobacco as a cancer risk factor. They described this finding as “most concerning” and said it “underscores the ongoing need to raise awareness about cancer risk factors in every new generation.”
Almost 60% of survey respondents, regardless of age, education, or income, expressed concern about being diagnosed with cancer in the future or having cancer recur.
In Kenya, where the death toll from cancer rose 30% from 2014 to 2018, people appeared to be the most worried about cancer, with four of five survey respondents (82%) expressing concern.
Survey respondents from Saudi Arabia appeared the least concerned, with one of three people saying they were worried.
Notably, 84% of survey respondents said that governments should be doing more to increase cancer prevention and awareness; 33% demanded that governments improve the affordability of cancer care.
“It is understandable that people turn to their governments for support,” Dr. Johnson commented. “Affordability is a big challenge for low-income settings.”
Data from the World Health Organization show that, for every U.S. $1 invested in low- and middle-income countries, the return is U.S. $3.20, Johnson pointed out. “We really need to convince decision makers ... and see the right investments being made. It is important to ensure that the health system strengthening takes place in tandem with prevention services.”
Governments have begun making commitments to tackle noncommunicable diseases and cancer, Dr. Johnson commented. He highlighted the WHO’s Global Action Plan for Healthy Lives and Well-being for All and the updated cancer resolution adopted at the 2017 World Health Assembly.
“Education, training, and awareness-raising efforts need to be backed by strong and progressive health policies that prioritize prevention and help reduce the consumption of known cancer-causing products such as tobacco, sugary food, and beverages,” she said. “Countries should also invest proactively in national cancer control planning and the establishment of population-based registries to ensure the most effective resource allocation that benefits all groups.”
Up-to-date information on cancer risks and cancer prevention must be delivered to the public in ways that are accessible to those in lower socioeconomic groups, Dr. Johnson added. “Awareness needs to be raised continuously with each new generation,” she noted.
The UICC has relationships with Astellas, Daiichi Sankyo, Diaceutics, MSD Inventing for Life, Bristol-Myers Squibb, CUBEBIO, the Icon Group, Roche, and Sanofi.
This article first appeared on Medscape.com.
Trump takes on multiple health topics in State of the Union
President Donald J. Trump took on multiple health care issues in his State of the Union address, imploring Congress to avoid the “socialism” of Medicare-for-all, to pass legislation banning late-term abortions, and to protect insurance coverage for preexisting conditions while joining together to reduce rising drug prices.
Mr. Trump said his administration has already been “taking on the big pharmaceutical companies,” claiming that, in 2019, “for the first time in 51 years, the cost of prescription drugs actually went down.”
That statement was called “misleading” by the New York Times because such efforts have excluded some high-cost drugs, and prices had risen by the end of the year, the publication noted in a fact-check of the president’s speech.
A survey issued in December 2019 found that the United States pays the highest prices in the world for pharmaceuticals, as reported by Medscape Medical News.
But the president did throw down a gauntlet for Congress. “Working together, the Congress can reduce drug prices substantially from current levels,” he said, stating that he had been “speaking to Sen. Chuck Grassley of Iowa and others in the Congress in order to get something on drug pricing done, and done properly.
“Get a bill to my desk, and I will sign it into law without delay,” Mr. Trump said.
A group of House Democrats then stood up in the chamber and loudly chanted, “HR3, HR3,” referring to the Lower Drug Costs Now Act, which the House passed in December 2019.
The bill would give the Department of Health & Human Services the power to negotiate directly with drug companies on up to 250 drugs per year, in particular, the highest-costing and most-utilized drugs.
The Senate has not taken up the legislation, but Sen. Grassley (R) and Sen. Ron Wyden (D-Ore.) introduced a similar bill, the Prescription Drug Pricing Reduction Act. It has been approved by the Senate Finance Committee but has not been moved to the Senate floor.
“I appreciate President Trump recognizing the work we’re doing to lower prescription drug prices,” Sen. Grassley said in a statement after the State of the Union. “Iowans and Americans across the country are demanding reforms that lower sky-high drug costs. A recent poll showed 70% of Americans want Congress to make lowering drug prices its top priority.”
Rep. Greg Walden (R-Ore.), the ranking Republican on the House Energy and Commerce Committee, said he believed Trump was committed to lowering drug costs. “I’ve never seen a president lean in further than President Donald Trump on lowering health care costs,” said Rep. Walden in a statement after the speech.
Trump touted his price transparency rule, which he said would go into effect next January, as a key way to cut health care costs.
Preexisting conditions
The president said that since he’d taken office, insurance had become more affordable and that the quality of health care had improved. He also said that he was making what he called an “iron-clad pledge” to American families.
“We will always protect patients with preexisting conditions – that is a guarantee,” Mr. Trump said.
In a press conference before the speech, Speaker of the House Nancy Pelosi (D-Calif.) took issue with that pledge. “The president swears that he supports protections for people with preexisting conditions, but right now, he is fighting in federal court to eliminate these lifesaving protections and every last protection and benefit of the Affordable Care Act,” she said.
During the speech, Rep. G. K. Butterfield (D-N.C.) tweeted “#FactCheck: Claiming to protect Americans with preexisting conditions, Trump and his administration have repeatedly sought to undermine protections offered by the ACA through executive orders and the courts. He is seeking to strike down the law and its protections entirely.”
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, pointed out in a tweet that insurance plans that Trump touted as “affordable alternatives” are in fact missing those protections.
“Ironically, the cheaper health insurance plans that President Trump has expanded are short-term plans that don’t cover preexisting conditions,” Mr. Levitt said.
Socialist takeover
Mr. Trump condemned the Medicare-for-all proposals that have been introduced in Congress and that are being backed in whole or in part by all of the Democratic candidates for president.
“As we work to improve Americans’ health care, there are those who want to take away your health care, take away your doctor, and abolish private insurance entirely,” said Mr. Trump.
He said that 132 members of Congress “have endorsed legislation to impose a socialist takeover of our health care system, wiping out the private health insurance plans of 180 million Americans.”
Added Mr. Trump: “We will never let socialism destroy American health care!”
Medicare-for-all has waxed and waned in popularity among voters, with generally more Democrats than Republicans favoring a single-payer system, with or without a public option.
Preliminary exit polls in Iowa that were conducted during Monday’s caucus found that 57% of Iowa Democratic caucus-goers supported a single-payer plan; 38% opposed such a plan, according to the Washington Post.
Opioids, the coronavirus, and abortion
In some of his final remarks on health care, Mr. Trump cited progress in the opioid crisis, noting that, in 2019, drug overdose deaths declined for the first time in 30 years.
He said that his administration was coordinating with the Chinese government regarding the coronavirus outbreak and noted the launch of initiatives to improve care for people with kidney disease, Alzheimer’s, and mental health problems.
Mr. Trump repeated his 2019 State of the Union claim that the government would help end AIDS in America by the end of the decade.
The president also announced that he was asking Congress for “an additional $50 million” to fund neonatal research. He followed that up with a plea about abortion.
“I am calling upon the members of Congress here tonight to pass legislation finally banning the late-term abortion of babies,” he said.
Insulin costs?
In the days before the speech, some news outlets had reported that Mr. Trump and the HHS were working on a plan to lower insulin prices for Medicare beneficiaries, and there were suggestions it would come up in the speech.
At least 13 members of Congress invited people advocating for lower insulin costs as their guests for the State of the Union, Stat reported. Rep. Pelosi invited twins from San Francisco with type 1 diabetes as her guests.
But Mr. Trump never mentioned insulin in his speech.
This article first appeared on Medscape.com.
President Donald J. Trump took on multiple health care issues in his State of the Union address, imploring Congress to avoid the “socialism” of Medicare-for-all, to pass legislation banning late-term abortions, and to protect insurance coverage for preexisting conditions while joining together to reduce rising drug prices.
Mr. Trump said his administration has already been “taking on the big pharmaceutical companies,” claiming that, in 2019, “for the first time in 51 years, the cost of prescription drugs actually went down.”
That statement was called “misleading” by the New York Times because such efforts have excluded some high-cost drugs, and prices had risen by the end of the year, the publication noted in a fact-check of the president’s speech.
A survey issued in December 2019 found that the United States pays the highest prices in the world for pharmaceuticals, as reported by Medscape Medical News.
But the president did throw down a gauntlet for Congress. “Working together, the Congress can reduce drug prices substantially from current levels,” he said, stating that he had been “speaking to Sen. Chuck Grassley of Iowa and others in the Congress in order to get something on drug pricing done, and done properly.
“Get a bill to my desk, and I will sign it into law without delay,” Mr. Trump said.
A group of House Democrats then stood up in the chamber and loudly chanted, “HR3, HR3,” referring to the Lower Drug Costs Now Act, which the House passed in December 2019.
The bill would give the Department of Health & Human Services the power to negotiate directly with drug companies on up to 250 drugs per year, in particular, the highest-costing and most-utilized drugs.
The Senate has not taken up the legislation, but Sen. Grassley (R) and Sen. Ron Wyden (D-Ore.) introduced a similar bill, the Prescription Drug Pricing Reduction Act. It has been approved by the Senate Finance Committee but has not been moved to the Senate floor.
“I appreciate President Trump recognizing the work we’re doing to lower prescription drug prices,” Sen. Grassley said in a statement after the State of the Union. “Iowans and Americans across the country are demanding reforms that lower sky-high drug costs. A recent poll showed 70% of Americans want Congress to make lowering drug prices its top priority.”
Rep. Greg Walden (R-Ore.), the ranking Republican on the House Energy and Commerce Committee, said he believed Trump was committed to lowering drug costs. “I’ve never seen a president lean in further than President Donald Trump on lowering health care costs,” said Rep. Walden in a statement after the speech.
Trump touted his price transparency rule, which he said would go into effect next January, as a key way to cut health care costs.
Preexisting conditions
The president said that since he’d taken office, insurance had become more affordable and that the quality of health care had improved. He also said that he was making what he called an “iron-clad pledge” to American families.
“We will always protect patients with preexisting conditions – that is a guarantee,” Mr. Trump said.
In a press conference before the speech, Speaker of the House Nancy Pelosi (D-Calif.) took issue with that pledge. “The president swears that he supports protections for people with preexisting conditions, but right now, he is fighting in federal court to eliminate these lifesaving protections and every last protection and benefit of the Affordable Care Act,” she said.
During the speech, Rep. G. K. Butterfield (D-N.C.) tweeted “#FactCheck: Claiming to protect Americans with preexisting conditions, Trump and his administration have repeatedly sought to undermine protections offered by the ACA through executive orders and the courts. He is seeking to strike down the law and its protections entirely.”
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, pointed out in a tweet that insurance plans that Trump touted as “affordable alternatives” are in fact missing those protections.
“Ironically, the cheaper health insurance plans that President Trump has expanded are short-term plans that don’t cover preexisting conditions,” Mr. Levitt said.
Socialist takeover
Mr. Trump condemned the Medicare-for-all proposals that have been introduced in Congress and that are being backed in whole or in part by all of the Democratic candidates for president.
“As we work to improve Americans’ health care, there are those who want to take away your health care, take away your doctor, and abolish private insurance entirely,” said Mr. Trump.
He said that 132 members of Congress “have endorsed legislation to impose a socialist takeover of our health care system, wiping out the private health insurance plans of 180 million Americans.”
Added Mr. Trump: “We will never let socialism destroy American health care!”
Medicare-for-all has waxed and waned in popularity among voters, with generally more Democrats than Republicans favoring a single-payer system, with or without a public option.
Preliminary exit polls in Iowa that were conducted during Monday’s caucus found that 57% of Iowa Democratic caucus-goers supported a single-payer plan; 38% opposed such a plan, according to the Washington Post.
Opioids, the coronavirus, and abortion
In some of his final remarks on health care, Mr. Trump cited progress in the opioid crisis, noting that, in 2019, drug overdose deaths declined for the first time in 30 years.
He said that his administration was coordinating with the Chinese government regarding the coronavirus outbreak and noted the launch of initiatives to improve care for people with kidney disease, Alzheimer’s, and mental health problems.
Mr. Trump repeated his 2019 State of the Union claim that the government would help end AIDS in America by the end of the decade.
The president also announced that he was asking Congress for “an additional $50 million” to fund neonatal research. He followed that up with a plea about abortion.
“I am calling upon the members of Congress here tonight to pass legislation finally banning the late-term abortion of babies,” he said.
Insulin costs?
In the days before the speech, some news outlets had reported that Mr. Trump and the HHS were working on a plan to lower insulin prices for Medicare beneficiaries, and there were suggestions it would come up in the speech.
At least 13 members of Congress invited people advocating for lower insulin costs as their guests for the State of the Union, Stat reported. Rep. Pelosi invited twins from San Francisco with type 1 diabetes as her guests.
But Mr. Trump never mentioned insulin in his speech.
This article first appeared on Medscape.com.
President Donald J. Trump took on multiple health care issues in his State of the Union address, imploring Congress to avoid the “socialism” of Medicare-for-all, to pass legislation banning late-term abortions, and to protect insurance coverage for preexisting conditions while joining together to reduce rising drug prices.
Mr. Trump said his administration has already been “taking on the big pharmaceutical companies,” claiming that, in 2019, “for the first time in 51 years, the cost of prescription drugs actually went down.”
That statement was called “misleading” by the New York Times because such efforts have excluded some high-cost drugs, and prices had risen by the end of the year, the publication noted in a fact-check of the president’s speech.
A survey issued in December 2019 found that the United States pays the highest prices in the world for pharmaceuticals, as reported by Medscape Medical News.
But the president did throw down a gauntlet for Congress. “Working together, the Congress can reduce drug prices substantially from current levels,” he said, stating that he had been “speaking to Sen. Chuck Grassley of Iowa and others in the Congress in order to get something on drug pricing done, and done properly.
“Get a bill to my desk, and I will sign it into law without delay,” Mr. Trump said.
A group of House Democrats then stood up in the chamber and loudly chanted, “HR3, HR3,” referring to the Lower Drug Costs Now Act, which the House passed in December 2019.
The bill would give the Department of Health & Human Services the power to negotiate directly with drug companies on up to 250 drugs per year, in particular, the highest-costing and most-utilized drugs.
The Senate has not taken up the legislation, but Sen. Grassley (R) and Sen. Ron Wyden (D-Ore.) introduced a similar bill, the Prescription Drug Pricing Reduction Act. It has been approved by the Senate Finance Committee but has not been moved to the Senate floor.
“I appreciate President Trump recognizing the work we’re doing to lower prescription drug prices,” Sen. Grassley said in a statement after the State of the Union. “Iowans and Americans across the country are demanding reforms that lower sky-high drug costs. A recent poll showed 70% of Americans want Congress to make lowering drug prices its top priority.”
Rep. Greg Walden (R-Ore.), the ranking Republican on the House Energy and Commerce Committee, said he believed Trump was committed to lowering drug costs. “I’ve never seen a president lean in further than President Donald Trump on lowering health care costs,” said Rep. Walden in a statement after the speech.
Trump touted his price transparency rule, which he said would go into effect next January, as a key way to cut health care costs.
Preexisting conditions
The president said that since he’d taken office, insurance had become more affordable and that the quality of health care had improved. He also said that he was making what he called an “iron-clad pledge” to American families.
“We will always protect patients with preexisting conditions – that is a guarantee,” Mr. Trump said.
In a press conference before the speech, Speaker of the House Nancy Pelosi (D-Calif.) took issue with that pledge. “The president swears that he supports protections for people with preexisting conditions, but right now, he is fighting in federal court to eliminate these lifesaving protections and every last protection and benefit of the Affordable Care Act,” she said.
During the speech, Rep. G. K. Butterfield (D-N.C.) tweeted “#FactCheck: Claiming to protect Americans with preexisting conditions, Trump and his administration have repeatedly sought to undermine protections offered by the ACA through executive orders and the courts. He is seeking to strike down the law and its protections entirely.”
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, pointed out in a tweet that insurance plans that Trump touted as “affordable alternatives” are in fact missing those protections.
“Ironically, the cheaper health insurance plans that President Trump has expanded are short-term plans that don’t cover preexisting conditions,” Mr. Levitt said.
Socialist takeover
Mr. Trump condemned the Medicare-for-all proposals that have been introduced in Congress and that are being backed in whole or in part by all of the Democratic candidates for president.
“As we work to improve Americans’ health care, there are those who want to take away your health care, take away your doctor, and abolish private insurance entirely,” said Mr. Trump.
He said that 132 members of Congress “have endorsed legislation to impose a socialist takeover of our health care system, wiping out the private health insurance plans of 180 million Americans.”
Added Mr. Trump: “We will never let socialism destroy American health care!”
Medicare-for-all has waxed and waned in popularity among voters, with generally more Democrats than Republicans favoring a single-payer system, with or without a public option.
Preliminary exit polls in Iowa that were conducted during Monday’s caucus found that 57% of Iowa Democratic caucus-goers supported a single-payer plan; 38% opposed such a plan, according to the Washington Post.
Opioids, the coronavirus, and abortion
In some of his final remarks on health care, Mr. Trump cited progress in the opioid crisis, noting that, in 2019, drug overdose deaths declined for the first time in 30 years.
He said that his administration was coordinating with the Chinese government regarding the coronavirus outbreak and noted the launch of initiatives to improve care for people with kidney disease, Alzheimer’s, and mental health problems.
Mr. Trump repeated his 2019 State of the Union claim that the government would help end AIDS in America by the end of the decade.
The president also announced that he was asking Congress for “an additional $50 million” to fund neonatal research. He followed that up with a plea about abortion.
“I am calling upon the members of Congress here tonight to pass legislation finally banning the late-term abortion of babies,” he said.
Insulin costs?
In the days before the speech, some news outlets had reported that Mr. Trump and the HHS were working on a plan to lower insulin prices for Medicare beneficiaries, and there were suggestions it would come up in the speech.
At least 13 members of Congress invited people advocating for lower insulin costs as their guests for the State of the Union, Stat reported. Rep. Pelosi invited twins from San Francisco with type 1 diabetes as her guests.
But Mr. Trump never mentioned insulin in his speech.
This article first appeared on Medscape.com.
U.S. cancer centers embroiled in Chinese research thefts
Academic cancer centers around the United States continue to get caught up in an ever-evolving investigation into researchers – American and Chinese – who did not disclose payments from or the work they did for Chinese institutions while simultaneously accepting taxpayer money through U.S. government grants.
The U.S. Federal Bureau of Investigation has been ferreting out researchers it says have acted illegally.
On Jan. 28, the agency arrested Charles Lieber, a chemist from Harvard University, Cambridge, Mass., and also unveiled charges against Zheng Zaosong, a cancer researcher who is in the United States on a Harvard-sponsored visa.
The FBI said Mr. Zheng, who worked at the Harvard-affiliated Beth Israel Deaconess Medical Center, Boston, tried to smuggle 21 vials of biological material and research to China. Mr. Zheng was arrested in December at Boston’s Logan Airport. He admitted he planned to conduct and publish research in China using the stolen samples, said the FBI.
“All of the individuals charged today were either directly or indirectly working for the Chinese government, at our country’s expense,” said the agent in charge of the FBI’s Boston office, Joseph R. Bonavolonta.
Sen. Charles Grassley (R-IA), who has been pushing for more government action against foreign theft of U.S. research, said in a statement, “I’m glad the FBI appears to be taking foreign threats to taxpayer-funded research seriously, but I fear that this case is only the tip of the iceberg.”
The FBI said it is investigating China-related cases in all 50 states.
Ross McKinney, MD, the chief scientific officer at the Association of American Medical Colleges (AAMC), said he is aware of some 200 investigations, not all of which are cancer related, at 70-75 institutions.
“It’s a very ubiquitous problem,” Dr. McKinney said in an interview.
He also pointed out that some 6,000 National Institutes of Health–funded principal investigators are of Asian background. “So that 200 is a pretty small proportion,” said Dr. McKinney.
The NIH warned some 10,000 institutions in August 2018 that it had uncovered Chinese manipulation of peer review and a lack of disclosure of work for Chinese institutions. It urged the institutions to report irregularities.
For universities, “the trouble is sorting out who is the violator from who is not,” said Dr. McKinney. He noted that they are not set up to investigate whether someone has a laboratory in China.
“The fact that the Chinese government exploited the fact that universities are typically fairly trusting is extremely disappointing,” he said.
Moffitt story still unfolding
The most serious allegations have been leveled against six former employees of the Moffitt Cancer Center and Research Institute in Tampa, Florida.
In December 2019, Moffitt announced that the six – including President and CEO Alan List, MD, and the center director, Thomas Sellers, PhD – had left Moffitt as a result of “violations of conflict of interest rules through their work in China.”
New details have emerged, thanks to a new investigative report from a committee of the Florida House of Representatives.
The report said that Sheng Wei, a naturalized U.S. citizen who had worked at Moffitt since 2008 – when Moffitt began its affiliation with the Tianjin Medical University Cancer Institute and Hospital – was instrumental in recruiting top executives into the Thousand Talents program, which Wei had joined in 2010, according to the report. These executives included Dr. List, Dr. Sellers, and also Daniel Sullivan, head of Moffitt’s clinical science program, and cancer biologist Pearlie Epling-Burnette, it noted.
Begun in 2008, China’s Thousand Talents Plan gave salaries, funding, laboratory space, and other incentives to researchers who promised to bring U.S.-gained knowledge and research to China.
All information about this program has been removed from the Internet, but the program may still be active, Dr. McKinney commented.
According to the report, Dr. List pledged to work for the Tianjin cancer center 9 months a year for $71,000 annually. He was appointed head of the hematology department ($85,300 a year) in 2016. He opened a bank account in China to receive that salary and other Thousand Talents payments, the report found. The report notes that the exact amount Dr. List was paid is still not known.
Initially, Dr. Sellers, who was the principal investigator for Moffitt’s National Cancer Institute core grant, said he had not been involved in the Thousand Talents program. He later admitted that he had pledged to work in China 2 months a year for the program and that he’d opened a Chinese bank account and had deposited at least $35,000 into the account, the report notes.
The others pledged to work for the Thousand Talents program and also opened bank accounts in China and received money in those accounts.
Another Moffitt employee, Howard McLeod, MD, had worked for Thousand Talents before he joined Moffitt but did not disclose his China work. Dr. McLeod also supervised and had a close relationship with another researcher, Yijing (Bob) He, MD, who was employed by Moffitt but who lived in China, unbeknownst to Moffitt. “Dr. He appears to have functioned as an agent of Dr. McLeod in China,” said the report.
The report concluded that “none of the Moffitt faculty who were Talents program participants properly or timely disclosed their Talents program involvement to Moffitt, and none disclosed the full extent of their Talents program activities prior to Moffitt’s internal investigation.”
No charges have been filed against any of the former Moffitt employees.
However, the Cancer Letter has reported that Dr. Sellers is claiming he was not involved in the program and that he is preparing to sue Moffitt.
AAMC’s Dr. McKinney notes that it is illegal for researchers to take U.S. government grant money and pledge a certain amount of time but not deliver on that commitment because they are working for someone else – in this case, China. They also lied about not having any other research support, which is also illegal, he said.
The researchers received Chinese money and deposited it in Chinese accounts, which was never reported to the U.S. Internal Revenue Service.
“One of the hallmarks of the Chinese recruitment program was that people were instructed to not tell their normal U.S. host institution and not tell any U.S. government agency about their relationship with China,” Dr. McKinney said. “It was creating a culture where dishonesty in this situation was norm,” he added.
The lack of honesty brings up bigger questions for the field, he said. “Once you start lying about one thing, do you lie about your science, too?”
Lack of oversight?
Dr. McKinney said the NIH, as well as universities and hospitals, had a long and trusting relationship with China and should not be blamed for falling prey to the Chinese government’s concerted effort to steal intellectual property.
But some government watchdog groups have chided the NIH for lax oversight. In February 2019, the federal Health & Human Services’ Office of Inspector General found that “NIH has not assessed the risks to national security when permitting data access to foreign [principal investigators].”
Federal investigators have said that Thousand Talents has been one of the biggest threats.
The U.S. Senate Permanent Subcommittee on Investigations reported in November 2019 that “the federal government’s grant-making agencies did little to prevent this from happening, nor did the FBI and other federal agencies develop a coordinated response to mitigate the threat.”
The NIH invests $31 billion a year in medical research through 50,000 competitive grants to more than 300,000 researchers, according to that report. Even after uncovering grant fraud and peer-review manipulation that benefited China, “significant gaps in NIH’s grant integrity process remain,” the report states. Site visits by the NIH’s Division of Grants Compliance and Oversight dropped from 28 in 2012 to just 3 in 2018, the report noted.
Widening dragnet
In April 2019, Science reported that the NIH identified five researchers at MD Anderson Cancer Center in Houston who had failed to disclose their ties to Chinese enterprises and who had failed to keep peer review confidential.
Two resigned before they could be fired, one was fired, another eventually left the institution, and the fifth was found to have not willfully engaged in subterfuge.
Just a month later, Emory University in Atlanta announced that it had fired a husband and wife research team. The neuroscientists were known for their studies of Huntington disease. Both were U.S. citizens and had worked at Emory for more than 2 decades, according to the Science report.
The Moffitt situation led to the Florida legislature’s investigation, and also prompted some soul searching. The Tampa Bay Times reported that U.S. Senator Rick Scott (R-FL) asked state universities to provide information on what they are doing to stop foreign influence. The University of Florida then acknowledged that four faculty members resigned or were terminated because of ties to a foreign recruitment program.
This article first appeared on Medscape.com.
Academic cancer centers around the United States continue to get caught up in an ever-evolving investigation into researchers – American and Chinese – who did not disclose payments from or the work they did for Chinese institutions while simultaneously accepting taxpayer money through U.S. government grants.
The U.S. Federal Bureau of Investigation has been ferreting out researchers it says have acted illegally.
On Jan. 28, the agency arrested Charles Lieber, a chemist from Harvard University, Cambridge, Mass., and also unveiled charges against Zheng Zaosong, a cancer researcher who is in the United States on a Harvard-sponsored visa.
The FBI said Mr. Zheng, who worked at the Harvard-affiliated Beth Israel Deaconess Medical Center, Boston, tried to smuggle 21 vials of biological material and research to China. Mr. Zheng was arrested in December at Boston’s Logan Airport. He admitted he planned to conduct and publish research in China using the stolen samples, said the FBI.
“All of the individuals charged today were either directly or indirectly working for the Chinese government, at our country’s expense,” said the agent in charge of the FBI’s Boston office, Joseph R. Bonavolonta.
Sen. Charles Grassley (R-IA), who has been pushing for more government action against foreign theft of U.S. research, said in a statement, “I’m glad the FBI appears to be taking foreign threats to taxpayer-funded research seriously, but I fear that this case is only the tip of the iceberg.”
The FBI said it is investigating China-related cases in all 50 states.
Ross McKinney, MD, the chief scientific officer at the Association of American Medical Colleges (AAMC), said he is aware of some 200 investigations, not all of which are cancer related, at 70-75 institutions.
“It’s a very ubiquitous problem,” Dr. McKinney said in an interview.
He also pointed out that some 6,000 National Institutes of Health–funded principal investigators are of Asian background. “So that 200 is a pretty small proportion,” said Dr. McKinney.
The NIH warned some 10,000 institutions in August 2018 that it had uncovered Chinese manipulation of peer review and a lack of disclosure of work for Chinese institutions. It urged the institutions to report irregularities.
For universities, “the trouble is sorting out who is the violator from who is not,” said Dr. McKinney. He noted that they are not set up to investigate whether someone has a laboratory in China.
“The fact that the Chinese government exploited the fact that universities are typically fairly trusting is extremely disappointing,” he said.
Moffitt story still unfolding
The most serious allegations have been leveled against six former employees of the Moffitt Cancer Center and Research Institute in Tampa, Florida.
In December 2019, Moffitt announced that the six – including President and CEO Alan List, MD, and the center director, Thomas Sellers, PhD – had left Moffitt as a result of “violations of conflict of interest rules through their work in China.”
New details have emerged, thanks to a new investigative report from a committee of the Florida House of Representatives.
The report said that Sheng Wei, a naturalized U.S. citizen who had worked at Moffitt since 2008 – when Moffitt began its affiliation with the Tianjin Medical University Cancer Institute and Hospital – was instrumental in recruiting top executives into the Thousand Talents program, which Wei had joined in 2010, according to the report. These executives included Dr. List, Dr. Sellers, and also Daniel Sullivan, head of Moffitt’s clinical science program, and cancer biologist Pearlie Epling-Burnette, it noted.
Begun in 2008, China’s Thousand Talents Plan gave salaries, funding, laboratory space, and other incentives to researchers who promised to bring U.S.-gained knowledge and research to China.
All information about this program has been removed from the Internet, but the program may still be active, Dr. McKinney commented.
According to the report, Dr. List pledged to work for the Tianjin cancer center 9 months a year for $71,000 annually. He was appointed head of the hematology department ($85,300 a year) in 2016. He opened a bank account in China to receive that salary and other Thousand Talents payments, the report found. The report notes that the exact amount Dr. List was paid is still not known.
Initially, Dr. Sellers, who was the principal investigator for Moffitt’s National Cancer Institute core grant, said he had not been involved in the Thousand Talents program. He later admitted that he had pledged to work in China 2 months a year for the program and that he’d opened a Chinese bank account and had deposited at least $35,000 into the account, the report notes.
The others pledged to work for the Thousand Talents program and also opened bank accounts in China and received money in those accounts.
Another Moffitt employee, Howard McLeod, MD, had worked for Thousand Talents before he joined Moffitt but did not disclose his China work. Dr. McLeod also supervised and had a close relationship with another researcher, Yijing (Bob) He, MD, who was employed by Moffitt but who lived in China, unbeknownst to Moffitt. “Dr. He appears to have functioned as an agent of Dr. McLeod in China,” said the report.
The report concluded that “none of the Moffitt faculty who were Talents program participants properly or timely disclosed their Talents program involvement to Moffitt, and none disclosed the full extent of their Talents program activities prior to Moffitt’s internal investigation.”
No charges have been filed against any of the former Moffitt employees.
However, the Cancer Letter has reported that Dr. Sellers is claiming he was not involved in the program and that he is preparing to sue Moffitt.
AAMC’s Dr. McKinney notes that it is illegal for researchers to take U.S. government grant money and pledge a certain amount of time but not deliver on that commitment because they are working for someone else – in this case, China. They also lied about not having any other research support, which is also illegal, he said.
The researchers received Chinese money and deposited it in Chinese accounts, which was never reported to the U.S. Internal Revenue Service.
“One of the hallmarks of the Chinese recruitment program was that people were instructed to not tell their normal U.S. host institution and not tell any U.S. government agency about their relationship with China,” Dr. McKinney said. “It was creating a culture where dishonesty in this situation was norm,” he added.
The lack of honesty brings up bigger questions for the field, he said. “Once you start lying about one thing, do you lie about your science, too?”
Lack of oversight?
Dr. McKinney said the NIH, as well as universities and hospitals, had a long and trusting relationship with China and should not be blamed for falling prey to the Chinese government’s concerted effort to steal intellectual property.
But some government watchdog groups have chided the NIH for lax oversight. In February 2019, the federal Health & Human Services’ Office of Inspector General found that “NIH has not assessed the risks to national security when permitting data access to foreign [principal investigators].”
Federal investigators have said that Thousand Talents has been one of the biggest threats.
The U.S. Senate Permanent Subcommittee on Investigations reported in November 2019 that “the federal government’s grant-making agencies did little to prevent this from happening, nor did the FBI and other federal agencies develop a coordinated response to mitigate the threat.”
The NIH invests $31 billion a year in medical research through 50,000 competitive grants to more than 300,000 researchers, according to that report. Even after uncovering grant fraud and peer-review manipulation that benefited China, “significant gaps in NIH’s grant integrity process remain,” the report states. Site visits by the NIH’s Division of Grants Compliance and Oversight dropped from 28 in 2012 to just 3 in 2018, the report noted.
Widening dragnet
In April 2019, Science reported that the NIH identified five researchers at MD Anderson Cancer Center in Houston who had failed to disclose their ties to Chinese enterprises and who had failed to keep peer review confidential.
Two resigned before they could be fired, one was fired, another eventually left the institution, and the fifth was found to have not willfully engaged in subterfuge.
Just a month later, Emory University in Atlanta announced that it had fired a husband and wife research team. The neuroscientists were known for their studies of Huntington disease. Both were U.S. citizens and had worked at Emory for more than 2 decades, according to the Science report.
The Moffitt situation led to the Florida legislature’s investigation, and also prompted some soul searching. The Tampa Bay Times reported that U.S. Senator Rick Scott (R-FL) asked state universities to provide information on what they are doing to stop foreign influence. The University of Florida then acknowledged that four faculty members resigned or were terminated because of ties to a foreign recruitment program.
This article first appeared on Medscape.com.
Academic cancer centers around the United States continue to get caught up in an ever-evolving investigation into researchers – American and Chinese – who did not disclose payments from or the work they did for Chinese institutions while simultaneously accepting taxpayer money through U.S. government grants.
The U.S. Federal Bureau of Investigation has been ferreting out researchers it says have acted illegally.
On Jan. 28, the agency arrested Charles Lieber, a chemist from Harvard University, Cambridge, Mass., and also unveiled charges against Zheng Zaosong, a cancer researcher who is in the United States on a Harvard-sponsored visa.
The FBI said Mr. Zheng, who worked at the Harvard-affiliated Beth Israel Deaconess Medical Center, Boston, tried to smuggle 21 vials of biological material and research to China. Mr. Zheng was arrested in December at Boston’s Logan Airport. He admitted he planned to conduct and publish research in China using the stolen samples, said the FBI.
“All of the individuals charged today were either directly or indirectly working for the Chinese government, at our country’s expense,” said the agent in charge of the FBI’s Boston office, Joseph R. Bonavolonta.
Sen. Charles Grassley (R-IA), who has been pushing for more government action against foreign theft of U.S. research, said in a statement, “I’m glad the FBI appears to be taking foreign threats to taxpayer-funded research seriously, but I fear that this case is only the tip of the iceberg.”
The FBI said it is investigating China-related cases in all 50 states.
Ross McKinney, MD, the chief scientific officer at the Association of American Medical Colleges (AAMC), said he is aware of some 200 investigations, not all of which are cancer related, at 70-75 institutions.
“It’s a very ubiquitous problem,” Dr. McKinney said in an interview.
He also pointed out that some 6,000 National Institutes of Health–funded principal investigators are of Asian background. “So that 200 is a pretty small proportion,” said Dr. McKinney.
The NIH warned some 10,000 institutions in August 2018 that it had uncovered Chinese manipulation of peer review and a lack of disclosure of work for Chinese institutions. It urged the institutions to report irregularities.
For universities, “the trouble is sorting out who is the violator from who is not,” said Dr. McKinney. He noted that they are not set up to investigate whether someone has a laboratory in China.
“The fact that the Chinese government exploited the fact that universities are typically fairly trusting is extremely disappointing,” he said.
Moffitt story still unfolding
The most serious allegations have been leveled against six former employees of the Moffitt Cancer Center and Research Institute in Tampa, Florida.
In December 2019, Moffitt announced that the six – including President and CEO Alan List, MD, and the center director, Thomas Sellers, PhD – had left Moffitt as a result of “violations of conflict of interest rules through their work in China.”
New details have emerged, thanks to a new investigative report from a committee of the Florida House of Representatives.
The report said that Sheng Wei, a naturalized U.S. citizen who had worked at Moffitt since 2008 – when Moffitt began its affiliation with the Tianjin Medical University Cancer Institute and Hospital – was instrumental in recruiting top executives into the Thousand Talents program, which Wei had joined in 2010, according to the report. These executives included Dr. List, Dr. Sellers, and also Daniel Sullivan, head of Moffitt’s clinical science program, and cancer biologist Pearlie Epling-Burnette, it noted.
Begun in 2008, China’s Thousand Talents Plan gave salaries, funding, laboratory space, and other incentives to researchers who promised to bring U.S.-gained knowledge and research to China.
All information about this program has been removed from the Internet, but the program may still be active, Dr. McKinney commented.
According to the report, Dr. List pledged to work for the Tianjin cancer center 9 months a year for $71,000 annually. He was appointed head of the hematology department ($85,300 a year) in 2016. He opened a bank account in China to receive that salary and other Thousand Talents payments, the report found. The report notes that the exact amount Dr. List was paid is still not known.
Initially, Dr. Sellers, who was the principal investigator for Moffitt’s National Cancer Institute core grant, said he had not been involved in the Thousand Talents program. He later admitted that he had pledged to work in China 2 months a year for the program and that he’d opened a Chinese bank account and had deposited at least $35,000 into the account, the report notes.
The others pledged to work for the Thousand Talents program and also opened bank accounts in China and received money in those accounts.
Another Moffitt employee, Howard McLeod, MD, had worked for Thousand Talents before he joined Moffitt but did not disclose his China work. Dr. McLeod also supervised and had a close relationship with another researcher, Yijing (Bob) He, MD, who was employed by Moffitt but who lived in China, unbeknownst to Moffitt. “Dr. He appears to have functioned as an agent of Dr. McLeod in China,” said the report.
The report concluded that “none of the Moffitt faculty who were Talents program participants properly or timely disclosed their Talents program involvement to Moffitt, and none disclosed the full extent of their Talents program activities prior to Moffitt’s internal investigation.”
No charges have been filed against any of the former Moffitt employees.
However, the Cancer Letter has reported that Dr. Sellers is claiming he was not involved in the program and that he is preparing to sue Moffitt.
AAMC’s Dr. McKinney notes that it is illegal for researchers to take U.S. government grant money and pledge a certain amount of time but not deliver on that commitment because they are working for someone else – in this case, China. They also lied about not having any other research support, which is also illegal, he said.
The researchers received Chinese money and deposited it in Chinese accounts, which was never reported to the U.S. Internal Revenue Service.
“One of the hallmarks of the Chinese recruitment program was that people were instructed to not tell their normal U.S. host institution and not tell any U.S. government agency about their relationship with China,” Dr. McKinney said. “It was creating a culture where dishonesty in this situation was norm,” he added.
The lack of honesty brings up bigger questions for the field, he said. “Once you start lying about one thing, do you lie about your science, too?”
Lack of oversight?
Dr. McKinney said the NIH, as well as universities and hospitals, had a long and trusting relationship with China and should not be blamed for falling prey to the Chinese government’s concerted effort to steal intellectual property.
But some government watchdog groups have chided the NIH for lax oversight. In February 2019, the federal Health & Human Services’ Office of Inspector General found that “NIH has not assessed the risks to national security when permitting data access to foreign [principal investigators].”
Federal investigators have said that Thousand Talents has been one of the biggest threats.
The U.S. Senate Permanent Subcommittee on Investigations reported in November 2019 that “the federal government’s grant-making agencies did little to prevent this from happening, nor did the FBI and other federal agencies develop a coordinated response to mitigate the threat.”
The NIH invests $31 billion a year in medical research through 50,000 competitive grants to more than 300,000 researchers, according to that report. Even after uncovering grant fraud and peer-review manipulation that benefited China, “significant gaps in NIH’s grant integrity process remain,” the report states. Site visits by the NIH’s Division of Grants Compliance and Oversight dropped from 28 in 2012 to just 3 in 2018, the report noted.
Widening dragnet
In April 2019, Science reported that the NIH identified five researchers at MD Anderson Cancer Center in Houston who had failed to disclose their ties to Chinese enterprises and who had failed to keep peer review confidential.
Two resigned before they could be fired, one was fired, another eventually left the institution, and the fifth was found to have not willfully engaged in subterfuge.
Just a month later, Emory University in Atlanta announced that it had fired a husband and wife research team. The neuroscientists were known for their studies of Huntington disease. Both were U.S. citizens and had worked at Emory for more than 2 decades, according to the Science report.
The Moffitt situation led to the Florida legislature’s investigation, and also prompted some soul searching. The Tampa Bay Times reported that U.S. Senator Rick Scott (R-FL) asked state universities to provide information on what they are doing to stop foreign influence. The University of Florida then acknowledged that four faculty members resigned or were terminated because of ties to a foreign recruitment program.
This article first appeared on Medscape.com.
Physician groups push back on Medicaid block grant plan
It took less than a day for physician groups to start pushing back at the Centers for Medicare & Medicaid Services over its new Medicaid block grant plan, which was introduced on Jan. 30.
Dubbed “Healthy Adult Opportunity,” the agency is offering all states the chance to participate in a block grant program through the 1115 waiver process.
According to a fact sheet issued by the agency, the program will focus on “adults under age 65 who are not eligible for Medicaid on the basis of disability or their need for long term care services and supports, and who are not eligible under a state plan. Other very low-income parents, children, pregnant women, elderly adults, and people eligible on the basis of a disability will not be directly affected – except from the improvement that results from states reinvesting savings into strengthening their overall programs.”
States will be operating within a defined budget when participating in the program and expenditures exceeding that defined budget will not be eligible for additional federal funding. Budgets will be based on a state’s historic costs, as well as national and regional trends, and will be tied to inflation with the potential to have adjustments made for extraordinary events. States can set their baseline using the prior year’s total spending or a per-enrollee spending model.
A Jan. 30 letter to state Medicaid directors notes that states participating in the program “will be granted extensive flexibility to test alternative approaches to implementing their Medicaid programs, including the ability to make many ongoing program adjustments without the need for demonstration or state plan amendments that require prior approval.”
Among the activities states can engage in under this plan are adjusting cost-sharing requirements, adopting a closed formulary, and applying additional conditions of eligibility. Requests, if approved, will be approved for a 5-year initial period, with a renewal option of up to 10 years.
But physician groups are not seeing a benefit with this new block grant program.
“Moving to a block grant system will likely limit the ability of Medicaid patients to receive preventive and needed medical care from their family physicians, and it will only increase the health disparities that exist in these communities, worsen overall health outcomes, and ultimately increase costs,” Gary LeRoy, MD, president of the American Academy of Family Physicians, said in a statement.
The American Medical Association concurred.
“The AMA opposes caps on federal Medicaid funding, such as block grants, because they would increase the number of uninsured and undermine Medicaid’s role as an indispensable safety net,” Patrice Harris, MD, the AMA’s president, said in a statement. “The AMA supports flexibility in Medicaid and encourages CMS to work with states to develop and test new Medicaid models that best meet the needs and priorities of low-income patients. While encouraging flexibility, the AMA is mindful that expanding Medicaid has been a literal lifesaver for low-income patients. We need to find ways to build on this success. We look forward to reviewing the proposal in detail.”
Officials at the American College of Obstetricians and Gynecologists said the changes have the potential to harm women and children’s health, as well as negatively impact physician reimbursement and ultimately access to care.
“Limits on the federal contribution to the Medicaid program would negatively impact patients by forcing states to reduce the number of people who are eligible for Medicaid coverage, eliminate covered services, and increase beneficiary cost-sharing,” ACOG President Ted Anderson, MD, said in a statement. “ACOG is also concerned that this block grant opportunity could lower physician reimbursement for certain services, forcing providers out of the program and jeopardizing patients’ ability to access health care services. Given our nation’s stark rates of maternal mortality and severe maternal morbidity, we are alarmed by the Administration’s willingness to weaken physician payment in Medicaid.”
It took less than a day for physician groups to start pushing back at the Centers for Medicare & Medicaid Services over its new Medicaid block grant plan, which was introduced on Jan. 30.
Dubbed “Healthy Adult Opportunity,” the agency is offering all states the chance to participate in a block grant program through the 1115 waiver process.
According to a fact sheet issued by the agency, the program will focus on “adults under age 65 who are not eligible for Medicaid on the basis of disability or their need for long term care services and supports, and who are not eligible under a state plan. Other very low-income parents, children, pregnant women, elderly adults, and people eligible on the basis of a disability will not be directly affected – except from the improvement that results from states reinvesting savings into strengthening their overall programs.”
States will be operating within a defined budget when participating in the program and expenditures exceeding that defined budget will not be eligible for additional federal funding. Budgets will be based on a state’s historic costs, as well as national and regional trends, and will be tied to inflation with the potential to have adjustments made for extraordinary events. States can set their baseline using the prior year’s total spending or a per-enrollee spending model.
A Jan. 30 letter to state Medicaid directors notes that states participating in the program “will be granted extensive flexibility to test alternative approaches to implementing their Medicaid programs, including the ability to make many ongoing program adjustments without the need for demonstration or state plan amendments that require prior approval.”
Among the activities states can engage in under this plan are adjusting cost-sharing requirements, adopting a closed formulary, and applying additional conditions of eligibility. Requests, if approved, will be approved for a 5-year initial period, with a renewal option of up to 10 years.
But physician groups are not seeing a benefit with this new block grant program.
“Moving to a block grant system will likely limit the ability of Medicaid patients to receive preventive and needed medical care from their family physicians, and it will only increase the health disparities that exist in these communities, worsen overall health outcomes, and ultimately increase costs,” Gary LeRoy, MD, president of the American Academy of Family Physicians, said in a statement.
The American Medical Association concurred.
“The AMA opposes caps on federal Medicaid funding, such as block grants, because they would increase the number of uninsured and undermine Medicaid’s role as an indispensable safety net,” Patrice Harris, MD, the AMA’s president, said in a statement. “The AMA supports flexibility in Medicaid and encourages CMS to work with states to develop and test new Medicaid models that best meet the needs and priorities of low-income patients. While encouraging flexibility, the AMA is mindful that expanding Medicaid has been a literal lifesaver for low-income patients. We need to find ways to build on this success. We look forward to reviewing the proposal in detail.”
Officials at the American College of Obstetricians and Gynecologists said the changes have the potential to harm women and children’s health, as well as negatively impact physician reimbursement and ultimately access to care.
“Limits on the federal contribution to the Medicaid program would negatively impact patients by forcing states to reduce the number of people who are eligible for Medicaid coverage, eliminate covered services, and increase beneficiary cost-sharing,” ACOG President Ted Anderson, MD, said in a statement. “ACOG is also concerned that this block grant opportunity could lower physician reimbursement for certain services, forcing providers out of the program and jeopardizing patients’ ability to access health care services. Given our nation’s stark rates of maternal mortality and severe maternal morbidity, we are alarmed by the Administration’s willingness to weaken physician payment in Medicaid.”
It took less than a day for physician groups to start pushing back at the Centers for Medicare & Medicaid Services over its new Medicaid block grant plan, which was introduced on Jan. 30.
Dubbed “Healthy Adult Opportunity,” the agency is offering all states the chance to participate in a block grant program through the 1115 waiver process.
According to a fact sheet issued by the agency, the program will focus on “adults under age 65 who are not eligible for Medicaid on the basis of disability or their need for long term care services and supports, and who are not eligible under a state plan. Other very low-income parents, children, pregnant women, elderly adults, and people eligible on the basis of a disability will not be directly affected – except from the improvement that results from states reinvesting savings into strengthening their overall programs.”
States will be operating within a defined budget when participating in the program and expenditures exceeding that defined budget will not be eligible for additional federal funding. Budgets will be based on a state’s historic costs, as well as national and regional trends, and will be tied to inflation with the potential to have adjustments made for extraordinary events. States can set their baseline using the prior year’s total spending or a per-enrollee spending model.
A Jan. 30 letter to state Medicaid directors notes that states participating in the program “will be granted extensive flexibility to test alternative approaches to implementing their Medicaid programs, including the ability to make many ongoing program adjustments without the need for demonstration or state plan amendments that require prior approval.”
Among the activities states can engage in under this plan are adjusting cost-sharing requirements, adopting a closed formulary, and applying additional conditions of eligibility. Requests, if approved, will be approved for a 5-year initial period, with a renewal option of up to 10 years.
But physician groups are not seeing a benefit with this new block grant program.
“Moving to a block grant system will likely limit the ability of Medicaid patients to receive preventive and needed medical care from their family physicians, and it will only increase the health disparities that exist in these communities, worsen overall health outcomes, and ultimately increase costs,” Gary LeRoy, MD, president of the American Academy of Family Physicians, said in a statement.
The American Medical Association concurred.
“The AMA opposes caps on federal Medicaid funding, such as block grants, because they would increase the number of uninsured and undermine Medicaid’s role as an indispensable safety net,” Patrice Harris, MD, the AMA’s president, said in a statement. “The AMA supports flexibility in Medicaid and encourages CMS to work with states to develop and test new Medicaid models that best meet the needs and priorities of low-income patients. While encouraging flexibility, the AMA is mindful that expanding Medicaid has been a literal lifesaver for low-income patients. We need to find ways to build on this success. We look forward to reviewing the proposal in detail.”
Officials at the American College of Obstetricians and Gynecologists said the changes have the potential to harm women and children’s health, as well as negatively impact physician reimbursement and ultimately access to care.
“Limits on the federal contribution to the Medicaid program would negatively impact patients by forcing states to reduce the number of people who are eligible for Medicaid coverage, eliminate covered services, and increase beneficiary cost-sharing,” ACOG President Ted Anderson, MD, said in a statement. “ACOG is also concerned that this block grant opportunity could lower physician reimbursement for certain services, forcing providers out of the program and jeopardizing patients’ ability to access health care services. Given our nation’s stark rates of maternal mortality and severe maternal morbidity, we are alarmed by the Administration’s willingness to weaken physician payment in Medicaid.”
Deferiprone noninferior to deferoxamine for iron overload in SCD, rare anemias
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
REPORTING FROM ASH 2019
Novel mutations contribute to progression of venetoclax-treated CLL
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
FROM BLOOD
ECHELON-1 update: A+AVD bests ABVD in Hodgkin lymphoma
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
FROM BLOOD
Occult HCV infection is correlated to unfavorable genotypes in hemophilia patients
The presence of occult hepatitis C virus infection is determined by finding HCV RNA in the liver and peripheral blood mononuclear cells, with no HCV RNA in the serum. Researchers have shown that the presence of occult HCV infection (OCI) was correlated with unfavorable polymorphisms near interferon lambda-3/4 (IFNL3/4), which has been associated with spontaneous HCV clearance.
This study was conducted to assess the frequency of OCI in 450 hemophilia patients in Iran with negative HCV markers, and to evaluate the association of three IFNL3 single nucleotide polymorphisms (rs8099917, rs12979860, and rs12980275) and the IFNL4 ss469415590 SNP with OCI positivity.
The estimated OCI rate was 10.2%. Among the 46 OCI patients, 56.5%, 23.9%, and 19.6% were infected with HCV-1b, HCV-1a, and HCV-3a, respectively. The researchers found that, compared with patients without OCI, unfavorable IFNL3 rs12979860, IFNL3 rs8099917, IFNL3 rs12980275, and IFNL4 ss469415590 genotypes were more frequently found in OCI patients. Multivariate analysis showed that ALT, cholesterol, triglyceride, as well as the aforementioned unfavorable interferon SNP geneotypes were associated with OCI positivity.
“10.2% of anti-HCV seronegative Iranian patients with hemophilia had OCI in our study; therefore, risk of this infection should be taken into consideration. We also showed that patients with unfavorable IFNL3 SNPs and IFNL4 ss469415590 genotypes were exposed to a higher risk of OCI, compared to hemophilia patients with other genotypes,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Nafari AH et al. Infect Genet Evol. 2019 Dec 13. doi: 10.1016/j.meegid.2019.104144.
The presence of occult hepatitis C virus infection is determined by finding HCV RNA in the liver and peripheral blood mononuclear cells, with no HCV RNA in the serum. Researchers have shown that the presence of occult HCV infection (OCI) was correlated with unfavorable polymorphisms near interferon lambda-3/4 (IFNL3/4), which has been associated with spontaneous HCV clearance.
This study was conducted to assess the frequency of OCI in 450 hemophilia patients in Iran with negative HCV markers, and to evaluate the association of three IFNL3 single nucleotide polymorphisms (rs8099917, rs12979860, and rs12980275) and the IFNL4 ss469415590 SNP with OCI positivity.
The estimated OCI rate was 10.2%. Among the 46 OCI patients, 56.5%, 23.9%, and 19.6% were infected with HCV-1b, HCV-1a, and HCV-3a, respectively. The researchers found that, compared with patients without OCI, unfavorable IFNL3 rs12979860, IFNL3 rs8099917, IFNL3 rs12980275, and IFNL4 ss469415590 genotypes were more frequently found in OCI patients. Multivariate analysis showed that ALT, cholesterol, triglyceride, as well as the aforementioned unfavorable interferon SNP geneotypes were associated with OCI positivity.
“10.2% of anti-HCV seronegative Iranian patients with hemophilia had OCI in our study; therefore, risk of this infection should be taken into consideration. We also showed that patients with unfavorable IFNL3 SNPs and IFNL4 ss469415590 genotypes were exposed to a higher risk of OCI, compared to hemophilia patients with other genotypes,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Nafari AH et al. Infect Genet Evol. 2019 Dec 13. doi: 10.1016/j.meegid.2019.104144.
The presence of occult hepatitis C virus infection is determined by finding HCV RNA in the liver and peripheral blood mononuclear cells, with no HCV RNA in the serum. Researchers have shown that the presence of occult HCV infection (OCI) was correlated with unfavorable polymorphisms near interferon lambda-3/4 (IFNL3/4), which has been associated with spontaneous HCV clearance.
This study was conducted to assess the frequency of OCI in 450 hemophilia patients in Iran with negative HCV markers, and to evaluate the association of three IFNL3 single nucleotide polymorphisms (rs8099917, rs12979860, and rs12980275) and the IFNL4 ss469415590 SNP with OCI positivity.
The estimated OCI rate was 10.2%. Among the 46 OCI patients, 56.5%, 23.9%, and 19.6% were infected with HCV-1b, HCV-1a, and HCV-3a, respectively. The researchers found that, compared with patients without OCI, unfavorable IFNL3 rs12979860, IFNL3 rs8099917, IFNL3 rs12980275, and IFNL4 ss469415590 genotypes were more frequently found in OCI patients. Multivariate analysis showed that ALT, cholesterol, triglyceride, as well as the aforementioned unfavorable interferon SNP geneotypes were associated with OCI positivity.
“10.2% of anti-HCV seronegative Iranian patients with hemophilia had OCI in our study; therefore, risk of this infection should be taken into consideration. We also showed that patients with unfavorable IFNL3 SNPs and IFNL4 ss469415590 genotypes were exposed to a higher risk of OCI, compared to hemophilia patients with other genotypes,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Nafari AH et al. Infect Genet Evol. 2019 Dec 13. doi: 10.1016/j.meegid.2019.104144.
FROM INFECTION, GENETICS AND EVOLUTION
Docs weigh pulling out of MIPS over paltry payments
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Costs are keeping Americans out of the doctor’s office
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.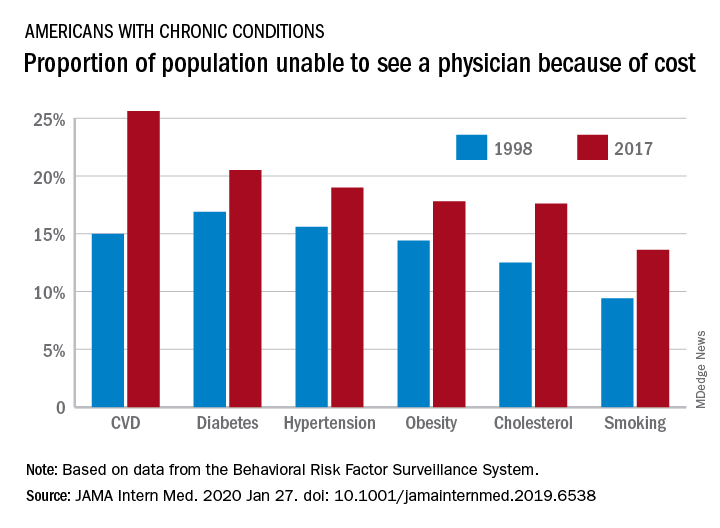
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
FROM JAMA INTERNAL MEDICINE