User login
-
ACP outlines guide for COVID-19 telehealth coding, billing
and for handling clinician and staff absences due to illness or quarantine during the COVID-19 pandemic.
It strongly encourages practices to use telehealth, whenever possible, to mitigate exposure of patients who are sick or at risk because of other underlying conditions and to protect health care workers and the community from the spread of the disease.
The national organization of internists also recommends in the guidance that practices establish protocols and procedures for use by clinicians and all other staff in light of the pandemic.
The billing and coding tips are being offered to help practices deal with the rapidly changing situation surrounding the COVID-19 emergency, according to a statement from the ACP.
The coding-related guidance incorporates changes to a number of telehealth rules for Medicare beneficiaries, announced by the Centers for Medicare and Medicaid Services on March 17.
“Now in a full state of emergency, many Medicare restrictions related to telehealth have been lifted. Patients can be at home, and non-HIPAA compliant technology is allowed. There is no cost sharing for COVID-19 testing. In addition, to encourage use by patients, Medicare is allowing practices to waive cost sharing (copays and deductibles) for all telehealth services,” the organization said in the guidance. It notes, however, that the CMS does not currently reimburse for telephone calls.
The guidance includes details of the new ICD-10 codes, and stresses the importance of using the appropriate codes, given that some service cost-sharing has been waived for COVID-19 testing and treatment.
There is detailed coding guidance for virtual check-in, online evaluation and management, remote monitoring, originating site, and allowed technology and services.
In regard to clinician and staff absence due to illness or quarantine, the ACP says “practices may need to review emergency plans related to telework and to employee and clinician absence.” Among its recommendations are that practices and employers consider temporary adjustments to compensation formulas to accommodate those clinicians who experience a loss of income because they are paid based on production.
The organization emphasizes that, given the rapidly changing availability of testing for COVID-19, practices should contact their local health departments, hospitals, reference labs, or state health authorities to determine the status of their access to testing.
The full list of the ACP’s tips are available here.
Any new guidance for physicians will be posted on the ACP’s COVID-19 resource page.
and for handling clinician and staff absences due to illness or quarantine during the COVID-19 pandemic.
It strongly encourages practices to use telehealth, whenever possible, to mitigate exposure of patients who are sick or at risk because of other underlying conditions and to protect health care workers and the community from the spread of the disease.
The national organization of internists also recommends in the guidance that practices establish protocols and procedures for use by clinicians and all other staff in light of the pandemic.
The billing and coding tips are being offered to help practices deal with the rapidly changing situation surrounding the COVID-19 emergency, according to a statement from the ACP.
The coding-related guidance incorporates changes to a number of telehealth rules for Medicare beneficiaries, announced by the Centers for Medicare and Medicaid Services on March 17.
“Now in a full state of emergency, many Medicare restrictions related to telehealth have been lifted. Patients can be at home, and non-HIPAA compliant technology is allowed. There is no cost sharing for COVID-19 testing. In addition, to encourage use by patients, Medicare is allowing practices to waive cost sharing (copays and deductibles) for all telehealth services,” the organization said in the guidance. It notes, however, that the CMS does not currently reimburse for telephone calls.
The guidance includes details of the new ICD-10 codes, and stresses the importance of using the appropriate codes, given that some service cost-sharing has been waived for COVID-19 testing and treatment.
There is detailed coding guidance for virtual check-in, online evaluation and management, remote monitoring, originating site, and allowed technology and services.
In regard to clinician and staff absence due to illness or quarantine, the ACP says “practices may need to review emergency plans related to telework and to employee and clinician absence.” Among its recommendations are that practices and employers consider temporary adjustments to compensation formulas to accommodate those clinicians who experience a loss of income because they are paid based on production.
The organization emphasizes that, given the rapidly changing availability of testing for COVID-19, practices should contact their local health departments, hospitals, reference labs, or state health authorities to determine the status of their access to testing.
The full list of the ACP’s tips are available here.
Any new guidance for physicians will be posted on the ACP’s COVID-19 resource page.
and for handling clinician and staff absences due to illness or quarantine during the COVID-19 pandemic.
It strongly encourages practices to use telehealth, whenever possible, to mitigate exposure of patients who are sick or at risk because of other underlying conditions and to protect health care workers and the community from the spread of the disease.
The national organization of internists also recommends in the guidance that practices establish protocols and procedures for use by clinicians and all other staff in light of the pandemic.
The billing and coding tips are being offered to help practices deal with the rapidly changing situation surrounding the COVID-19 emergency, according to a statement from the ACP.
The coding-related guidance incorporates changes to a number of telehealth rules for Medicare beneficiaries, announced by the Centers for Medicare and Medicaid Services on March 17.
“Now in a full state of emergency, many Medicare restrictions related to telehealth have been lifted. Patients can be at home, and non-HIPAA compliant technology is allowed. There is no cost sharing for COVID-19 testing. In addition, to encourage use by patients, Medicare is allowing practices to waive cost sharing (copays and deductibles) for all telehealth services,” the organization said in the guidance. It notes, however, that the CMS does not currently reimburse for telephone calls.
The guidance includes details of the new ICD-10 codes, and stresses the importance of using the appropriate codes, given that some service cost-sharing has been waived for COVID-19 testing and treatment.
There is detailed coding guidance for virtual check-in, online evaluation and management, remote monitoring, originating site, and allowed technology and services.
In regard to clinician and staff absence due to illness or quarantine, the ACP says “practices may need to review emergency plans related to telework and to employee and clinician absence.” Among its recommendations are that practices and employers consider temporary adjustments to compensation formulas to accommodate those clinicians who experience a loss of income because they are paid based on production.
The organization emphasizes that, given the rapidly changing availability of testing for COVID-19, practices should contact their local health departments, hospitals, reference labs, or state health authorities to determine the status of their access to testing.
The full list of the ACP’s tips are available here.
Any new guidance for physicians will be posted on the ACP’s COVID-19 resource page.
Clinicians petition government for national quarantine
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19: U.S. cardiology groups reaffirm continued use of RAAS-active drugs
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
COVID-19 guidance for children’s health care providers
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
CME in the time of COVID-19
As the COVID-19 pandemic spreads, it now seems like the norm is that large medical conferences are being canceled.
The American Psychiatric Association (APA) canceled its 2020 annual meeting, which was scheduled for late April. The cancellation disappointed many, because we will miss out on the camaraderie and professional invigoration that comes from gathering with psychiatrists and other mental health professionals from across the United States and around the world. After the APA’s decision was announced, the White House released guidelines advising Americans to avoid social gatherings of 10 or more people.
On a practical level, many psychiatrists will not be able to earn up to 35 continuing medical education credits (CME) from attending the meeting and fulfilling the administrative requirements to obtain a CME certificate. Not only have meetings been canceled, but events many other clinicians count on for CME, such as journal clubs and department grand rounds, have been canceled until they can be moved to a virtual space.
The CME requirements for state medical licenses vary widely. On average, most states require at least 25 credits per year or 60 to 100 credits every 2 years, and the American Board of Psychiatry and Neurology requires diplomates to complete an average of 30 specialty and/or subspecialty CME credits per year, averaged over 3 years. Usually, annual medical conferences would be a great way to get an infusion of CME credits, brush up on cutting-edge treatments, and review the basics.
On top of everything else we have to worry about with COVID-19, getting enough CME credits has been added to the list for many psychiatrists and mental health clinicians. As our schedules and daily lives are disrupted, it’s important to find relief in routine activities that are not affected by social distancing and fears of isolation and quarantine. A routine activity to lean into might include learning or practicing a skill that we enjoy, such as psychiatry (hopefully!) and the practice of medicine. The CME could be focused on a psychiatric topic or perhaps learning about the specifics of COVID-19 or brushing up on medical knowledge that might be a bit rusty after many years of practicing solely psychiatry.
As you start to gather CME credits online, it’s helpful to sign up for a service that stores your CME credits and helps you keep track of the number. When it comes time to renew your medical license or apply for maintenance of certification (MOC), who wants to be the person searching through their email for PDFs of CME certificates or taking pictures or scanning paper certificates? The APA has a section under education and MOC to track certificates earned by watching online modules from its “Learning Center.” The website also allows users to upload external certificates. The American Medical Association offers a similar service on its “Ed Hub,” in which users can log in to watch, listen, or download articles to earn CME credits after finishing the associated quiz. Medscape, in the CME and Education section, also offers an easy-to-use CME dashboard, in which clinicians can filter by their specialty, topic, duration of learning activity – ranging from 0.25 to 3 CME credits. Clinicians also can track their credits as they complete activities.
If you’re someone who’s having trouble focusing on anything besides COVID-19, there are COVID-19-specific CME activities that are available and can help psychiatrists feel comfortable talking with patients, family, and their institutions about the risks of COVID-19. The AMA Ed Hub has a featured 8-credit CME course about the novel coronavirus with updates about diagnosis, treatment, and public health strategies.
For the psychiatrists who may have procrastinated in-depth learning about the opioid crisis or getting their buprenorphine waivers, AMA Ed Hub offers a 42-credit course about opioids and pain management covering guidelines, research, and treatment.
For fun refreshers on general medicine, the New England Journal of Medicine offers up to 20 online CME exams based on quizzes from interesting clinical cases ranging from “regular” medicine to rare clinical scenarios. The APA Learning Center has an easy-to-use search function allowing users to select content from more than 200 modules covering a wide range of general topics; from reviewing recent treatment guidelines to specialized psychiatric topics such as geriatric bipolar disorder. A psychiatrist who has been quickly pushed to telepsychiatry because of the current pandemic could use the APA Learning Center to find educational modules about risk management in telepsychiatry or learn the special considerations of using telepsychiatry to treat patients with serious mental illness.
Using podcasts to earn CME is becoming increasingly common, with such as outlets as JAMA Networks offering podcasts in many specialties in which subscribers can take a quiz through the JAMA app and obtain CME credits.
As our clinical boundaries as psychiatrists are pushed by an ever-changing public health situation, now is the time to earn CME focused on new topics to meet the demands placed on health care workers at the front lines of clinical care.
If the COVID-19 pandemic reaches the number of cases predicted by public health officials, our health care system is going to be under extreme stress. All specialties face the threat of losing part of their working capacity as clinicians get sick with the virus, or as they stay home because of exposure or to take care of a loved one. CME can be a way to empower ourselves by staying current on the cutting edge of our specialties, but also brushing up on the medicine that we may be asked to practice in a time of great need.
Dr. Posada is consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va. She also is associate producer of the MDedge Psychcast. Dr. Posada has no disclosures.
As the COVID-19 pandemic spreads, it now seems like the norm is that large medical conferences are being canceled.
The American Psychiatric Association (APA) canceled its 2020 annual meeting, which was scheduled for late April. The cancellation disappointed many, because we will miss out on the camaraderie and professional invigoration that comes from gathering with psychiatrists and other mental health professionals from across the United States and around the world. After the APA’s decision was announced, the White House released guidelines advising Americans to avoid social gatherings of 10 or more people.
On a practical level, many psychiatrists will not be able to earn up to 35 continuing medical education credits (CME) from attending the meeting and fulfilling the administrative requirements to obtain a CME certificate. Not only have meetings been canceled, but events many other clinicians count on for CME, such as journal clubs and department grand rounds, have been canceled until they can be moved to a virtual space.
The CME requirements for state medical licenses vary widely. On average, most states require at least 25 credits per year or 60 to 100 credits every 2 years, and the American Board of Psychiatry and Neurology requires diplomates to complete an average of 30 specialty and/or subspecialty CME credits per year, averaged over 3 years. Usually, annual medical conferences would be a great way to get an infusion of CME credits, brush up on cutting-edge treatments, and review the basics.
On top of everything else we have to worry about with COVID-19, getting enough CME credits has been added to the list for many psychiatrists and mental health clinicians. As our schedules and daily lives are disrupted, it’s important to find relief in routine activities that are not affected by social distancing and fears of isolation and quarantine. A routine activity to lean into might include learning or practicing a skill that we enjoy, such as psychiatry (hopefully!) and the practice of medicine. The CME could be focused on a psychiatric topic or perhaps learning about the specifics of COVID-19 or brushing up on medical knowledge that might be a bit rusty after many years of practicing solely psychiatry.
As you start to gather CME credits online, it’s helpful to sign up for a service that stores your CME credits and helps you keep track of the number. When it comes time to renew your medical license or apply for maintenance of certification (MOC), who wants to be the person searching through their email for PDFs of CME certificates or taking pictures or scanning paper certificates? The APA has a section under education and MOC to track certificates earned by watching online modules from its “Learning Center.” The website also allows users to upload external certificates. The American Medical Association offers a similar service on its “Ed Hub,” in which users can log in to watch, listen, or download articles to earn CME credits after finishing the associated quiz. Medscape, in the CME and Education section, also offers an easy-to-use CME dashboard, in which clinicians can filter by their specialty, topic, duration of learning activity – ranging from 0.25 to 3 CME credits. Clinicians also can track their credits as they complete activities.
If you’re someone who’s having trouble focusing on anything besides COVID-19, there are COVID-19-specific CME activities that are available and can help psychiatrists feel comfortable talking with patients, family, and their institutions about the risks of COVID-19. The AMA Ed Hub has a featured 8-credit CME course about the novel coronavirus with updates about diagnosis, treatment, and public health strategies.
For the psychiatrists who may have procrastinated in-depth learning about the opioid crisis or getting their buprenorphine waivers, AMA Ed Hub offers a 42-credit course about opioids and pain management covering guidelines, research, and treatment.
For fun refreshers on general medicine, the New England Journal of Medicine offers up to 20 online CME exams based on quizzes from interesting clinical cases ranging from “regular” medicine to rare clinical scenarios. The APA Learning Center has an easy-to-use search function allowing users to select content from more than 200 modules covering a wide range of general topics; from reviewing recent treatment guidelines to specialized psychiatric topics such as geriatric bipolar disorder. A psychiatrist who has been quickly pushed to telepsychiatry because of the current pandemic could use the APA Learning Center to find educational modules about risk management in telepsychiatry or learn the special considerations of using telepsychiatry to treat patients with serious mental illness.
Using podcasts to earn CME is becoming increasingly common, with such as outlets as JAMA Networks offering podcasts in many specialties in which subscribers can take a quiz through the JAMA app and obtain CME credits.
As our clinical boundaries as psychiatrists are pushed by an ever-changing public health situation, now is the time to earn CME focused on new topics to meet the demands placed on health care workers at the front lines of clinical care.
If the COVID-19 pandemic reaches the number of cases predicted by public health officials, our health care system is going to be under extreme stress. All specialties face the threat of losing part of their working capacity as clinicians get sick with the virus, or as they stay home because of exposure or to take care of a loved one. CME can be a way to empower ourselves by staying current on the cutting edge of our specialties, but also brushing up on the medicine that we may be asked to practice in a time of great need.
Dr. Posada is consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va. She also is associate producer of the MDedge Psychcast. Dr. Posada has no disclosures.
As the COVID-19 pandemic spreads, it now seems like the norm is that large medical conferences are being canceled.
The American Psychiatric Association (APA) canceled its 2020 annual meeting, which was scheduled for late April. The cancellation disappointed many, because we will miss out on the camaraderie and professional invigoration that comes from gathering with psychiatrists and other mental health professionals from across the United States and around the world. After the APA’s decision was announced, the White House released guidelines advising Americans to avoid social gatherings of 10 or more people.
On a practical level, many psychiatrists will not be able to earn up to 35 continuing medical education credits (CME) from attending the meeting and fulfilling the administrative requirements to obtain a CME certificate. Not only have meetings been canceled, but events many other clinicians count on for CME, such as journal clubs and department grand rounds, have been canceled until they can be moved to a virtual space.
The CME requirements for state medical licenses vary widely. On average, most states require at least 25 credits per year or 60 to 100 credits every 2 years, and the American Board of Psychiatry and Neurology requires diplomates to complete an average of 30 specialty and/or subspecialty CME credits per year, averaged over 3 years. Usually, annual medical conferences would be a great way to get an infusion of CME credits, brush up on cutting-edge treatments, and review the basics.
On top of everything else we have to worry about with COVID-19, getting enough CME credits has been added to the list for many psychiatrists and mental health clinicians. As our schedules and daily lives are disrupted, it’s important to find relief in routine activities that are not affected by social distancing and fears of isolation and quarantine. A routine activity to lean into might include learning or practicing a skill that we enjoy, such as psychiatry (hopefully!) and the practice of medicine. The CME could be focused on a psychiatric topic or perhaps learning about the specifics of COVID-19 or brushing up on medical knowledge that might be a bit rusty after many years of practicing solely psychiatry.
As you start to gather CME credits online, it’s helpful to sign up for a service that stores your CME credits and helps you keep track of the number. When it comes time to renew your medical license or apply for maintenance of certification (MOC), who wants to be the person searching through their email for PDFs of CME certificates or taking pictures or scanning paper certificates? The APA has a section under education and MOC to track certificates earned by watching online modules from its “Learning Center.” The website also allows users to upload external certificates. The American Medical Association offers a similar service on its “Ed Hub,” in which users can log in to watch, listen, or download articles to earn CME credits after finishing the associated quiz. Medscape, in the CME and Education section, also offers an easy-to-use CME dashboard, in which clinicians can filter by their specialty, topic, duration of learning activity – ranging from 0.25 to 3 CME credits. Clinicians also can track their credits as they complete activities.
If you’re someone who’s having trouble focusing on anything besides COVID-19, there are COVID-19-specific CME activities that are available and can help psychiatrists feel comfortable talking with patients, family, and their institutions about the risks of COVID-19. The AMA Ed Hub has a featured 8-credit CME course about the novel coronavirus with updates about diagnosis, treatment, and public health strategies.
For the psychiatrists who may have procrastinated in-depth learning about the opioid crisis or getting their buprenorphine waivers, AMA Ed Hub offers a 42-credit course about opioids and pain management covering guidelines, research, and treatment.
For fun refreshers on general medicine, the New England Journal of Medicine offers up to 20 online CME exams based on quizzes from interesting clinical cases ranging from “regular” medicine to rare clinical scenarios. The APA Learning Center has an easy-to-use search function allowing users to select content from more than 200 modules covering a wide range of general topics; from reviewing recent treatment guidelines to specialized psychiatric topics such as geriatric bipolar disorder. A psychiatrist who has been quickly pushed to telepsychiatry because of the current pandemic could use the APA Learning Center to find educational modules about risk management in telepsychiatry or learn the special considerations of using telepsychiatry to treat patients with serious mental illness.
Using podcasts to earn CME is becoming increasingly common, with such as outlets as JAMA Networks offering podcasts in many specialties in which subscribers can take a quiz through the JAMA app and obtain CME credits.
As our clinical boundaries as psychiatrists are pushed by an ever-changing public health situation, now is the time to earn CME focused on new topics to meet the demands placed on health care workers at the front lines of clinical care.
If the COVID-19 pandemic reaches the number of cases predicted by public health officials, our health care system is going to be under extreme stress. All specialties face the threat of losing part of their working capacity as clinicians get sick with the virus, or as they stay home because of exposure or to take care of a loved one. CME can be a way to empower ourselves by staying current on the cutting edge of our specialties, but also brushing up on the medicine that we may be asked to practice in a time of great need.
Dr. Posada is consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va. She also is associate producer of the MDedge Psychcast. Dr. Posada has no disclosures.
White House expands Medicare telehealth services amid COVID-19
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“Medicare beneficiaries across the nation, no matter where they live, will now be able to receive a wide range of services via telehealth without ever having to leave home,” CMS Administrator Seema Verma said during a March 17 White House press briefing on administration actions to contain the spread of COVID-19. “These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more.”
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using the telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health and Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states. CMS also said it will not conduct audits to ensure that an established relationship exists between the provider and the patient – a prior requirement for telehealth billing – during this public health emergency.
Billing for virtual check-ins, which are essentially brief conversations that may not require a full visit to the physician office, needs an established relationship between the practice and the patient. Likewise, for e-visits, which include non–face-to-face communications through online patient portals, billing can occur only when there is an established patient relationship.
Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She noted this was all a part of mitigation efforts to limit the spread of COVID-19.
“As we are encouraging Americans to stay home whenever possible, we don’t want our Medicare policies getting in the way,” she said, adding that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“Medicare beneficiaries across the nation, no matter where they live, will now be able to receive a wide range of services via telehealth without ever having to leave home,” CMS Administrator Seema Verma said during a March 17 White House press briefing on administration actions to contain the spread of COVID-19. “These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more.”
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using the telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health and Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states. CMS also said it will not conduct audits to ensure that an established relationship exists between the provider and the patient – a prior requirement for telehealth billing – during this public health emergency.
Billing for virtual check-ins, which are essentially brief conversations that may not require a full visit to the physician office, needs an established relationship between the practice and the patient. Likewise, for e-visits, which include non–face-to-face communications through online patient portals, billing can occur only when there is an established patient relationship.
Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She noted this was all a part of mitigation efforts to limit the spread of COVID-19.
“As we are encouraging Americans to stay home whenever possible, we don’t want our Medicare policies getting in the way,” she said, adding that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“Medicare beneficiaries across the nation, no matter where they live, will now be able to receive a wide range of services via telehealth without ever having to leave home,” CMS Administrator Seema Verma said during a March 17 White House press briefing on administration actions to contain the spread of COVID-19. “These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more.”
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using the telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health and Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states. CMS also said it will not conduct audits to ensure that an established relationship exists between the provider and the patient – a prior requirement for telehealth billing – during this public health emergency.
Billing for virtual check-ins, which are essentially brief conversations that may not require a full visit to the physician office, needs an established relationship between the practice and the patient. Likewise, for e-visits, which include non–face-to-face communications through online patient portals, billing can occur only when there is an established patient relationship.
Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She noted this was all a part of mitigation efforts to limit the spread of COVID-19.
“As we are encouraging Americans to stay home whenever possible, we don’t want our Medicare policies getting in the way,” she said, adding that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
Coronavirus stays in aerosols for hours, on surfaces for days
according to a new study.
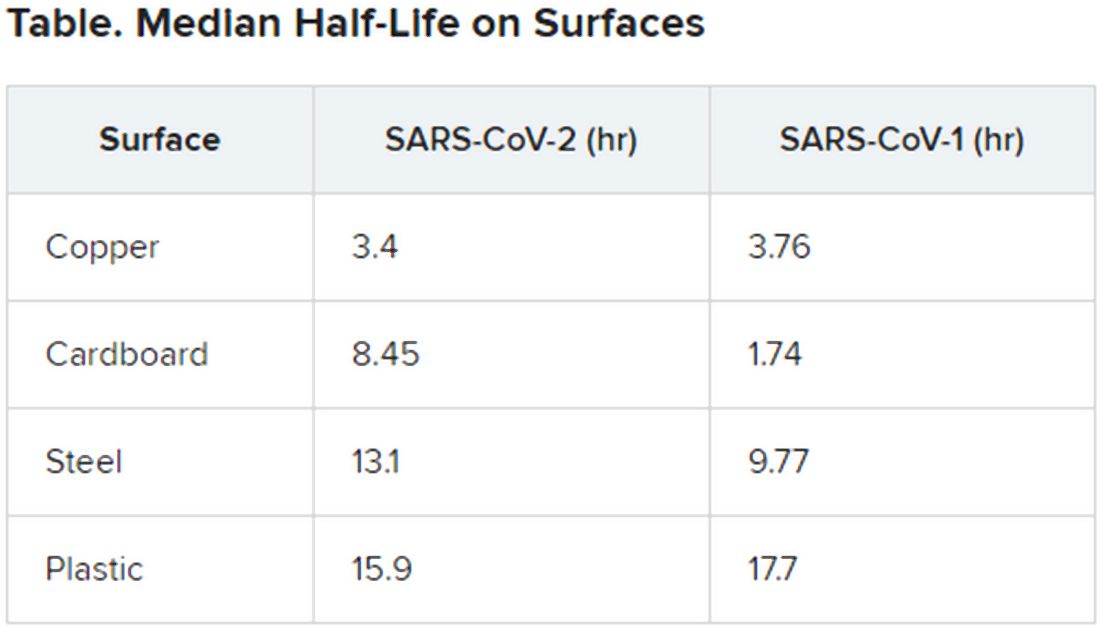
The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Follicular lymphoma treatment can benefit patients 80 years and older
Follicular lymphoma (FL) treatment was associated with improved survival among patients diagnosed with FL at aged 80 years and older, according to the results of a large, retrospective database cohort study.
Researchers used the linked Surveillance, Epidemiology, and End Results–Medicare dataset to identify patients 80 years of age and older, diagnosed with FL between 2000 and 2013, identifying FL-directed treatments based on published guidelines. They used a propensity-score matched sample to compare treated and untreated patient cohorts who had similar observed characteristics.
They assessed 3,705 older patients with FL and a mean age of 84 years). Over a median follow-up of 2.9 years, 68% of the sample received FL-directed therapy and the most common regimen was rituximab monotherapy, which 21% (768 patients) received.
The median overall survival for the treated group was 4.31 years (95% confidence interval [CI], 4.00-4.61) vs. 2.86 years (95% CI, 2.59-3.16) for the untreated group, according to the report, published in the Journal of Geriatric Oncology.
The 3-year restricted mean survival time for the treated group was 2.36 years (95% CI, 2.30-2.41), vs. 2.05 years (95% CI, 1.98-2.11) for the untreated group. Treatment was associated with a 23% reduction in the hazard of death (HR: 0.77; P < .001).
Multivariable analysis showed that older age and a Charlson Comorbidity Index score of two or higher, and a proxy indicator for poor performance status, were inversely associated with receiving treatment. “These factors are expected to discourage physicians from providing FL-directed therapy,” the researchers suggested.
“We observed that, in a cohort of FL patients aged 80 years or older, FL-directed therapy was associated with an overall survival benefit, which persisted in important subgroups,” the researchers concluded.
Financial support for this study was provided by Bayer. One of the authors is employed by Bayer.
SOURCE: Albarmawi H et al. J Geriatric Oncol. 2020;11:55-61.
Follicular lymphoma (FL) treatment was associated with improved survival among patients diagnosed with FL at aged 80 years and older, according to the results of a large, retrospective database cohort study.
Researchers used the linked Surveillance, Epidemiology, and End Results–Medicare dataset to identify patients 80 years of age and older, diagnosed with FL between 2000 and 2013, identifying FL-directed treatments based on published guidelines. They used a propensity-score matched sample to compare treated and untreated patient cohorts who had similar observed characteristics.
They assessed 3,705 older patients with FL and a mean age of 84 years). Over a median follow-up of 2.9 years, 68% of the sample received FL-directed therapy and the most common regimen was rituximab monotherapy, which 21% (768 patients) received.
The median overall survival for the treated group was 4.31 years (95% confidence interval [CI], 4.00-4.61) vs. 2.86 years (95% CI, 2.59-3.16) for the untreated group, according to the report, published in the Journal of Geriatric Oncology.
The 3-year restricted mean survival time for the treated group was 2.36 years (95% CI, 2.30-2.41), vs. 2.05 years (95% CI, 1.98-2.11) for the untreated group. Treatment was associated with a 23% reduction in the hazard of death (HR: 0.77; P < .001).
Multivariable analysis showed that older age and a Charlson Comorbidity Index score of two or higher, and a proxy indicator for poor performance status, were inversely associated with receiving treatment. “These factors are expected to discourage physicians from providing FL-directed therapy,” the researchers suggested.
“We observed that, in a cohort of FL patients aged 80 years or older, FL-directed therapy was associated with an overall survival benefit, which persisted in important subgroups,” the researchers concluded.
Financial support for this study was provided by Bayer. One of the authors is employed by Bayer.
SOURCE: Albarmawi H et al. J Geriatric Oncol. 2020;11:55-61.
Follicular lymphoma (FL) treatment was associated with improved survival among patients diagnosed with FL at aged 80 years and older, according to the results of a large, retrospective database cohort study.
Researchers used the linked Surveillance, Epidemiology, and End Results–Medicare dataset to identify patients 80 years of age and older, diagnosed with FL between 2000 and 2013, identifying FL-directed treatments based on published guidelines. They used a propensity-score matched sample to compare treated and untreated patient cohorts who had similar observed characteristics.
They assessed 3,705 older patients with FL and a mean age of 84 years). Over a median follow-up of 2.9 years, 68% of the sample received FL-directed therapy and the most common regimen was rituximab monotherapy, which 21% (768 patients) received.
The median overall survival for the treated group was 4.31 years (95% confidence interval [CI], 4.00-4.61) vs. 2.86 years (95% CI, 2.59-3.16) for the untreated group, according to the report, published in the Journal of Geriatric Oncology.
The 3-year restricted mean survival time for the treated group was 2.36 years (95% CI, 2.30-2.41), vs. 2.05 years (95% CI, 1.98-2.11) for the untreated group. Treatment was associated with a 23% reduction in the hazard of death (HR: 0.77; P < .001).
Multivariable analysis showed that older age and a Charlson Comorbidity Index score of two or higher, and a proxy indicator for poor performance status, were inversely associated with receiving treatment. “These factors are expected to discourage physicians from providing FL-directed therapy,” the researchers suggested.
“We observed that, in a cohort of FL patients aged 80 years or older, FL-directed therapy was associated with an overall survival benefit, which persisted in important subgroups,” the researchers concluded.
Financial support for this study was provided by Bayer. One of the authors is employed by Bayer.
SOURCE: Albarmawi H et al. J Geriatric Oncol. 2020;11:55-61.
FROM THE JOURNAL OF GERIATRIC ONCOLOGY
COVID-19: ASTCT provides interim guidelines for transplantation
The American Society for Transplantation and Cellular Therapy (ASTCT) has released interim guidelines for the care of hematopoietic cell transplantation (HCT) and cellular therapy patients in the light of the global SARS-CoV-2 pandemic.
The guidelines, summarized briefly below, focus on diagnostic and treatment considerations, evaluation of patients prior to initializing HCT and cellular therapy, and cell donor evaluation. Much of the guideline relies upon recommendations developed by the European Society for Blood and Marrow Transplantation (ESBMT). These guidelines were updated on March 16.
The ASTCT document focuses on patient-treatment specifics and does not cover specific infection-prevention policies and procedures, instead suggesting that local and institutional guidelines, such as those from the Centers for Disease Control and Prevention, should be followed. They did recommend that, in the local presence of COVID-19, “clinic visits that are not critical should be either deferred or substituted with telemedicine visits if deemed appropriate and feasible.”
Diagnostic considerations
In any patient with upper or lower respiratory symptoms, obtain polymerase chain reaction (PCR) testing for SARS-CoV-2, where possible, in addition to other respiratory virus PCR testing from any respiratory sample obtained, following CDC recommendations for sample collection and processing, which are continuously being updated on the CDC website.
These recommendations include nasal sampling, rather than oral sampling, and the discouraging of nasal washes where avoidable. If nasal washing is performed, it should be done with appropriate personal protective equipment as described by the CDC. The CDC has also provided additional infection prevention and control information for known and suspected COVID-19 patients in health care settings.
In patients positive for SARS-CoV-2 in an upper respiratory tract sample, chest imaging should be considered.
Preliminary reports suggest that there may be a discrepancy between upper- and lower-tract specimen positivity. Therefore, even when SARS-CoV-2 is not detected in an upper respiratory sample, the ASTCT recommends that chest imaging should be considered for lower respiratory tract infection when clinical symptoms of lower respiratory tract infection are present, including shortness of breath, hypoxia, and tachypnea.
With regard to routine bronchoalveolar lavage, the ASTCT recommends against it if a patient tests positive for SARS-CoV-2 given the risk of transmission among health care workers. The exception is in the case of suspected coinfection based on abnormal chest imaging and in patients for whom it is clinically indicated (for example, those receiving invasive mechanical ventilation). In addition to testing bronchoalveolar lavage samples for SARS-CoV-2, “copathogens should be evaluated and treated.”
Treatment considerations
“At this point no recommendations can be made on specific therapies due to limited data and unknown risk versus benefit; additional recommendations will be forthcoming. Even less data is available for pediatric patients. Treatment for viral, bacterial, and fungal copathogens should be optimized,” according to the ASTCT.
However, the society lists several therapies currently under consideration, which may be available through compassionate-use programs and are being investigated in current clinical trials in several countries, “including lopinavir/ritonavir, ribavirin, hydroxychloroquine, darunavir/cobicistat, and interferons-alpha and -beta.” Remdesivir, in particular, is being evaluated in a National Institutes of Health–sponsored, placebo-controlled clinical trial (NCT04280705).
In case of known or suspected COVID-19 with normal imaging and no or mild symptoms, no therapy is recommended. However, if symptoms progress or imaging is abnormal, an infectious disease specialist or department should be consulted, according to the ASTCT.
Evaluation prior to HCT or cellular therapy
“There is sufficient concern that COVID-19 could have a significant impact on posttransplant or posttherapy outcomes,” according to the guidelines, and the ASTCT provided the following recommendations to be considered in known or suspected COVID-19 patients. In particular, practitioners need to weigh the risk of delaying or altering therapy plans with the risk of progression of underlying disease.
If SARS-CoV-2 is detected in a respiratory specimen, HCT or cellular therapy procedures should be deferred. Therapy should also be deferred in HCT and cellular therapy candidates with close contact with a person infected with SARS-CoV-2 and in those patients who have traveled to a high-risk area or had close contact with a person traveling from an area at high risk for COVID-19.
In the case of a patient in a community with widespread disease, “all HCT and cellular therapy candidates should undergo screening for SARS-CoV-2 infection by PCR in respiratory specimens at the time of initial evaluation and 2 days prior to conditioning/lymphodepletion, regardless of the presence of symptoms, if testing is available.”
Procedures to be deferred include peripheral blood stem cell mobilization, bone marrow harvest, T-cell collections, and conditioning/lymphodepletion. These should not be performed for at least 14 days (preferably 21 days) from the day of last contact, according to the ASTCT. Two consecutive negative PCR tests each approximately 1 week apart (deferral for 14 days minimum), should be obtained, if available.
In areas with high community spread, the guidelines also state that “interim treatment and/or longer deferral of definite therapy should be considered when feasible (for example, multiple myeloma, germ cell tumors, consolidative transplants).”
Similar considerations should be afforded to potential cellular donors. Donors with SARS-CoV-2 detected in a respiratory sample are considered ineligible. Those meeting exposure criteria for patients, as listed above, should be excluded from donation for at least 28 days. “In individual circumstances, a donor may be considered eligible if respiratory samples are negative for SARS-CoV-2 by PCR and donor is asymptomatic. Donor should be closely monitored for COVID-19.”
In the case of unrelated donors, the ASTCT recommends referral to the National Marrow Donor Program (NMDP) guidelines for updated guidance, but points out that, according to the NMDP, the Food and Drug Administration reports that there have been no reported or suspected cases of transfusion-transmitted COVID-19 to date and that “no cases of transfusion-transmission were ever reported for the other two coronaviruses that emerged during the past 2 decades [SARS, the severe acute respiratory syndrome coronavirus, and MERS-CoV, which causes Mideast respiratory syndrome].”
In the updated ESBMT guidelines, this recommendation was made in reference to the greater spread of COVID-19: “It is therefore strongly recommended to have secured stem cell product access by freezing the product before start of conditioning and, in situations when this is not possible, to have an alternative donor as a backup. For low-risk patients, it is recommended to postpone the start of the transplant procedure if deemed to be safe to do so. This includes both allogeneic and autologous transplant procedures.”
In a recent webinar, Pavan Reddy, MD, of the University of Michigan, Ann Arbor, and ASTCT President; Alpana Waghmare, MD, of the Fred Hutchinson Cancer Research Center, Seattle; and Roy Chemaly, MD, of the MD Anderson Cancer Center, Houston, and chair of the ASTCT Transplant Infectious Disease Special Interest Group, discussed the guidelines and provided some updated information.
Dr. Reddy stated that, at the University of Michigan, they were delaying all nonurgent transplants, largely for myeloma, and are postponing even allotransplants. “The transplants we are not delaying are the high-risk AMLs … and in cases where we truly cannot delay transplants because of patient condition or, in some cases, the donor situation.”
Dr. Chemaly and Dr. Waghmare both agreed that their centers were following a similar approach.
With regard to patient testing, all three institution have recently moved to testing everyone a few days before transplant regardless of symptoms.
They also pointed out that essentially all clinical trials were being put on hold during the crisis, except for those few where patients would be put in danger if the trial were interrupted.
The guidelines discuss in depth the rationale, toxicity, and dosages for use of select agents, including remdesivir, chloroquine/hydroxychloroquine, ribavirin, and tocilizumab. There was some concern expressed about shortages developing in these drugs, which serve a number of other patient communities, in particular the possibility of a tocilizumab shortage was of concern.
Steroids and intravenous immunoglobulins are not are not recommended, according to the guidelines, which also stated that adjunctive therapies such as antibiotics should be considered.
Dr. Chemaly, Dr. Reddy, and Dr. Waghmare did not provide disclosure in the webinar.
The ASTCT recommends following the World Health Organization and CDC COVID-19 pages for continued updates and information on other aspects of the pandemic.
This article was updated 3/26/20.
SOURCE: ASTCT Response to COVID-19. 2020. www.astct.org/connect/astct-response-to-covid-19.
There is emerging data regarding coinfection of SARS-CoV-2 with other viruses including infleunza. Immunocompromised hosts, especially transplantation and cellular therapy (TCT) recipients, are known to frequently have more than one pathogen present, especially in pulmonary infections. As the community spread increases, it would be reasonable to obtain concomitant testing for respiratory viruses along with SARS-CoV-2 as recommended. In addition, viral infection can cause secondary bacterial and fungal infections (especially Aspergillus). In the presence of SARS-CoV-2, where it is recommended to avoid bronchoalveolar lavage, we have to keep a high clinical suspicion based on patients’ risk factors.
Acute Respiratory Distress Syndrome (ARDS) caused by an intense inflammatory response is the main cause of death in COVID-19. Early reports on the use of tocilizumab (an IL-6 receptor blocker) for ARDS to block cytokine mediated injury to the lung should be a consideration early in the course of COVID-19 pneumonitis, especially in setting of high risk for ARDS mortality.
We are considering other IL-6–blocking agents like siltuximab in case of a shortage of tocilizumab while centers scramble to get these agents. It is important to note that any such usages for COVID-19 would be considered off-label.
TCT candidates should of course be practicing social distancing in days leading to transplant to reduce their risk of exposure regardless of state or federal recommendations. Household members of TCT candidates should practice similar caution because transmission has been reported by asymptomatic individuals.
Zainab Shahid, MD, is the medical director of Bone Marrow Transplant Infectious Diseases at the Levine Cancer Institute/Atrium Health and a clinical associate professor of medicine at University of North Carolina at Chapel Hill. She reported that she had no relevant disclosures.
There is emerging data regarding coinfection of SARS-CoV-2 with other viruses including infleunza. Immunocompromised hosts, especially transplantation and cellular therapy (TCT) recipients, are known to frequently have more than one pathogen present, especially in pulmonary infections. As the community spread increases, it would be reasonable to obtain concomitant testing for respiratory viruses along with SARS-CoV-2 as recommended. In addition, viral infection can cause secondary bacterial and fungal infections (especially Aspergillus). In the presence of SARS-CoV-2, where it is recommended to avoid bronchoalveolar lavage, we have to keep a high clinical suspicion based on patients’ risk factors.
Acute Respiratory Distress Syndrome (ARDS) caused by an intense inflammatory response is the main cause of death in COVID-19. Early reports on the use of tocilizumab (an IL-6 receptor blocker) for ARDS to block cytokine mediated injury to the lung should be a consideration early in the course of COVID-19 pneumonitis, especially in setting of high risk for ARDS mortality.
We are considering other IL-6–blocking agents like siltuximab in case of a shortage of tocilizumab while centers scramble to get these agents. It is important to note that any such usages for COVID-19 would be considered off-label.
TCT candidates should of course be practicing social distancing in days leading to transplant to reduce their risk of exposure regardless of state or federal recommendations. Household members of TCT candidates should practice similar caution because transmission has been reported by asymptomatic individuals.
Zainab Shahid, MD, is the medical director of Bone Marrow Transplant Infectious Diseases at the Levine Cancer Institute/Atrium Health and a clinical associate professor of medicine at University of North Carolina at Chapel Hill. She reported that she had no relevant disclosures.
There is emerging data regarding coinfection of SARS-CoV-2 with other viruses including infleunza. Immunocompromised hosts, especially transplantation and cellular therapy (TCT) recipients, are known to frequently have more than one pathogen present, especially in pulmonary infections. As the community spread increases, it would be reasonable to obtain concomitant testing for respiratory viruses along with SARS-CoV-2 as recommended. In addition, viral infection can cause secondary bacterial and fungal infections (especially Aspergillus). In the presence of SARS-CoV-2, where it is recommended to avoid bronchoalveolar lavage, we have to keep a high clinical suspicion based on patients’ risk factors.
Acute Respiratory Distress Syndrome (ARDS) caused by an intense inflammatory response is the main cause of death in COVID-19. Early reports on the use of tocilizumab (an IL-6 receptor blocker) for ARDS to block cytokine mediated injury to the lung should be a consideration early in the course of COVID-19 pneumonitis, especially in setting of high risk for ARDS mortality.
We are considering other IL-6–blocking agents like siltuximab in case of a shortage of tocilizumab while centers scramble to get these agents. It is important to note that any such usages for COVID-19 would be considered off-label.
TCT candidates should of course be practicing social distancing in days leading to transplant to reduce their risk of exposure regardless of state or federal recommendations. Household members of TCT candidates should practice similar caution because transmission has been reported by asymptomatic individuals.
Zainab Shahid, MD, is the medical director of Bone Marrow Transplant Infectious Diseases at the Levine Cancer Institute/Atrium Health and a clinical associate professor of medicine at University of North Carolina at Chapel Hill. She reported that she had no relevant disclosures.
The American Society for Transplantation and Cellular Therapy (ASTCT) has released interim guidelines for the care of hematopoietic cell transplantation (HCT) and cellular therapy patients in the light of the global SARS-CoV-2 pandemic.
The guidelines, summarized briefly below, focus on diagnostic and treatment considerations, evaluation of patients prior to initializing HCT and cellular therapy, and cell donor evaluation. Much of the guideline relies upon recommendations developed by the European Society for Blood and Marrow Transplantation (ESBMT). These guidelines were updated on March 16.
The ASTCT document focuses on patient-treatment specifics and does not cover specific infection-prevention policies and procedures, instead suggesting that local and institutional guidelines, such as those from the Centers for Disease Control and Prevention, should be followed. They did recommend that, in the local presence of COVID-19, “clinic visits that are not critical should be either deferred or substituted with telemedicine visits if deemed appropriate and feasible.”
Diagnostic considerations
In any patient with upper or lower respiratory symptoms, obtain polymerase chain reaction (PCR) testing for SARS-CoV-2, where possible, in addition to other respiratory virus PCR testing from any respiratory sample obtained, following CDC recommendations for sample collection and processing, which are continuously being updated on the CDC website.
These recommendations include nasal sampling, rather than oral sampling, and the discouraging of nasal washes where avoidable. If nasal washing is performed, it should be done with appropriate personal protective equipment as described by the CDC. The CDC has also provided additional infection prevention and control information for known and suspected COVID-19 patients in health care settings.
In patients positive for SARS-CoV-2 in an upper respiratory tract sample, chest imaging should be considered.
Preliminary reports suggest that there may be a discrepancy between upper- and lower-tract specimen positivity. Therefore, even when SARS-CoV-2 is not detected in an upper respiratory sample, the ASTCT recommends that chest imaging should be considered for lower respiratory tract infection when clinical symptoms of lower respiratory tract infection are present, including shortness of breath, hypoxia, and tachypnea.
With regard to routine bronchoalveolar lavage, the ASTCT recommends against it if a patient tests positive for SARS-CoV-2 given the risk of transmission among health care workers. The exception is in the case of suspected coinfection based on abnormal chest imaging and in patients for whom it is clinically indicated (for example, those receiving invasive mechanical ventilation). In addition to testing bronchoalveolar lavage samples for SARS-CoV-2, “copathogens should be evaluated and treated.”
Treatment considerations
“At this point no recommendations can be made on specific therapies due to limited data and unknown risk versus benefit; additional recommendations will be forthcoming. Even less data is available for pediatric patients. Treatment for viral, bacterial, and fungal copathogens should be optimized,” according to the ASTCT.
However, the society lists several therapies currently under consideration, which may be available through compassionate-use programs and are being investigated in current clinical trials in several countries, “including lopinavir/ritonavir, ribavirin, hydroxychloroquine, darunavir/cobicistat, and interferons-alpha and -beta.” Remdesivir, in particular, is being evaluated in a National Institutes of Health–sponsored, placebo-controlled clinical trial (NCT04280705).
In case of known or suspected COVID-19 with normal imaging and no or mild symptoms, no therapy is recommended. However, if symptoms progress or imaging is abnormal, an infectious disease specialist or department should be consulted, according to the ASTCT.
Evaluation prior to HCT or cellular therapy
“There is sufficient concern that COVID-19 could have a significant impact on posttransplant or posttherapy outcomes,” according to the guidelines, and the ASTCT provided the following recommendations to be considered in known or suspected COVID-19 patients. In particular, practitioners need to weigh the risk of delaying or altering therapy plans with the risk of progression of underlying disease.
If SARS-CoV-2 is detected in a respiratory specimen, HCT or cellular therapy procedures should be deferred. Therapy should also be deferred in HCT and cellular therapy candidates with close contact with a person infected with SARS-CoV-2 and in those patients who have traveled to a high-risk area or had close contact with a person traveling from an area at high risk for COVID-19.
In the case of a patient in a community with widespread disease, “all HCT and cellular therapy candidates should undergo screening for SARS-CoV-2 infection by PCR in respiratory specimens at the time of initial evaluation and 2 days prior to conditioning/lymphodepletion, regardless of the presence of symptoms, if testing is available.”
Procedures to be deferred include peripheral blood stem cell mobilization, bone marrow harvest, T-cell collections, and conditioning/lymphodepletion. These should not be performed for at least 14 days (preferably 21 days) from the day of last contact, according to the ASTCT. Two consecutive negative PCR tests each approximately 1 week apart (deferral for 14 days minimum), should be obtained, if available.
In areas with high community spread, the guidelines also state that “interim treatment and/or longer deferral of definite therapy should be considered when feasible (for example, multiple myeloma, germ cell tumors, consolidative transplants).”
Similar considerations should be afforded to potential cellular donors. Donors with SARS-CoV-2 detected in a respiratory sample are considered ineligible. Those meeting exposure criteria for patients, as listed above, should be excluded from donation for at least 28 days. “In individual circumstances, a donor may be considered eligible if respiratory samples are negative for SARS-CoV-2 by PCR and donor is asymptomatic. Donor should be closely monitored for COVID-19.”
In the case of unrelated donors, the ASTCT recommends referral to the National Marrow Donor Program (NMDP) guidelines for updated guidance, but points out that, according to the NMDP, the Food and Drug Administration reports that there have been no reported or suspected cases of transfusion-transmitted COVID-19 to date and that “no cases of transfusion-transmission were ever reported for the other two coronaviruses that emerged during the past 2 decades [SARS, the severe acute respiratory syndrome coronavirus, and MERS-CoV, which causes Mideast respiratory syndrome].”
In the updated ESBMT guidelines, this recommendation was made in reference to the greater spread of COVID-19: “It is therefore strongly recommended to have secured stem cell product access by freezing the product before start of conditioning and, in situations when this is not possible, to have an alternative donor as a backup. For low-risk patients, it is recommended to postpone the start of the transplant procedure if deemed to be safe to do so. This includes both allogeneic and autologous transplant procedures.”
In a recent webinar, Pavan Reddy, MD, of the University of Michigan, Ann Arbor, and ASTCT President; Alpana Waghmare, MD, of the Fred Hutchinson Cancer Research Center, Seattle; and Roy Chemaly, MD, of the MD Anderson Cancer Center, Houston, and chair of the ASTCT Transplant Infectious Disease Special Interest Group, discussed the guidelines and provided some updated information.
Dr. Reddy stated that, at the University of Michigan, they were delaying all nonurgent transplants, largely for myeloma, and are postponing even allotransplants. “The transplants we are not delaying are the high-risk AMLs … and in cases where we truly cannot delay transplants because of patient condition or, in some cases, the donor situation.”
Dr. Chemaly and Dr. Waghmare both agreed that their centers were following a similar approach.
With regard to patient testing, all three institution have recently moved to testing everyone a few days before transplant regardless of symptoms.
They also pointed out that essentially all clinical trials were being put on hold during the crisis, except for those few where patients would be put in danger if the trial were interrupted.
The guidelines discuss in depth the rationale, toxicity, and dosages for use of select agents, including remdesivir, chloroquine/hydroxychloroquine, ribavirin, and tocilizumab. There was some concern expressed about shortages developing in these drugs, which serve a number of other patient communities, in particular the possibility of a tocilizumab shortage was of concern.
Steroids and intravenous immunoglobulins are not are not recommended, according to the guidelines, which also stated that adjunctive therapies such as antibiotics should be considered.
Dr. Chemaly, Dr. Reddy, and Dr. Waghmare did not provide disclosure in the webinar.
The ASTCT recommends following the World Health Organization and CDC COVID-19 pages for continued updates and information on other aspects of the pandemic.
This article was updated 3/26/20.
SOURCE: ASTCT Response to COVID-19. 2020. www.astct.org/connect/astct-response-to-covid-19.
The American Society for Transplantation and Cellular Therapy (ASTCT) has released interim guidelines for the care of hematopoietic cell transplantation (HCT) and cellular therapy patients in the light of the global SARS-CoV-2 pandemic.
The guidelines, summarized briefly below, focus on diagnostic and treatment considerations, evaluation of patients prior to initializing HCT and cellular therapy, and cell donor evaluation. Much of the guideline relies upon recommendations developed by the European Society for Blood and Marrow Transplantation (ESBMT). These guidelines were updated on March 16.
The ASTCT document focuses on patient-treatment specifics and does not cover specific infection-prevention policies and procedures, instead suggesting that local and institutional guidelines, such as those from the Centers for Disease Control and Prevention, should be followed. They did recommend that, in the local presence of COVID-19, “clinic visits that are not critical should be either deferred or substituted with telemedicine visits if deemed appropriate and feasible.”
Diagnostic considerations
In any patient with upper or lower respiratory symptoms, obtain polymerase chain reaction (PCR) testing for SARS-CoV-2, where possible, in addition to other respiratory virus PCR testing from any respiratory sample obtained, following CDC recommendations for sample collection and processing, which are continuously being updated on the CDC website.
These recommendations include nasal sampling, rather than oral sampling, and the discouraging of nasal washes where avoidable. If nasal washing is performed, it should be done with appropriate personal protective equipment as described by the CDC. The CDC has also provided additional infection prevention and control information for known and suspected COVID-19 patients in health care settings.
In patients positive for SARS-CoV-2 in an upper respiratory tract sample, chest imaging should be considered.
Preliminary reports suggest that there may be a discrepancy between upper- and lower-tract specimen positivity. Therefore, even when SARS-CoV-2 is not detected in an upper respiratory sample, the ASTCT recommends that chest imaging should be considered for lower respiratory tract infection when clinical symptoms of lower respiratory tract infection are present, including shortness of breath, hypoxia, and tachypnea.
With regard to routine bronchoalveolar lavage, the ASTCT recommends against it if a patient tests positive for SARS-CoV-2 given the risk of transmission among health care workers. The exception is in the case of suspected coinfection based on abnormal chest imaging and in patients for whom it is clinically indicated (for example, those receiving invasive mechanical ventilation). In addition to testing bronchoalveolar lavage samples for SARS-CoV-2, “copathogens should be evaluated and treated.”
Treatment considerations
“At this point no recommendations can be made on specific therapies due to limited data and unknown risk versus benefit; additional recommendations will be forthcoming. Even less data is available for pediatric patients. Treatment for viral, bacterial, and fungal copathogens should be optimized,” according to the ASTCT.
However, the society lists several therapies currently under consideration, which may be available through compassionate-use programs and are being investigated in current clinical trials in several countries, “including lopinavir/ritonavir, ribavirin, hydroxychloroquine, darunavir/cobicistat, and interferons-alpha and -beta.” Remdesivir, in particular, is being evaluated in a National Institutes of Health–sponsored, placebo-controlled clinical trial (NCT04280705).
In case of known or suspected COVID-19 with normal imaging and no or mild symptoms, no therapy is recommended. However, if symptoms progress or imaging is abnormal, an infectious disease specialist or department should be consulted, according to the ASTCT.
Evaluation prior to HCT or cellular therapy
“There is sufficient concern that COVID-19 could have a significant impact on posttransplant or posttherapy outcomes,” according to the guidelines, and the ASTCT provided the following recommendations to be considered in known or suspected COVID-19 patients. In particular, practitioners need to weigh the risk of delaying or altering therapy plans with the risk of progression of underlying disease.
If SARS-CoV-2 is detected in a respiratory specimen, HCT or cellular therapy procedures should be deferred. Therapy should also be deferred in HCT and cellular therapy candidates with close contact with a person infected with SARS-CoV-2 and in those patients who have traveled to a high-risk area or had close contact with a person traveling from an area at high risk for COVID-19.
In the case of a patient in a community with widespread disease, “all HCT and cellular therapy candidates should undergo screening for SARS-CoV-2 infection by PCR in respiratory specimens at the time of initial evaluation and 2 days prior to conditioning/lymphodepletion, regardless of the presence of symptoms, if testing is available.”
Procedures to be deferred include peripheral blood stem cell mobilization, bone marrow harvest, T-cell collections, and conditioning/lymphodepletion. These should not be performed for at least 14 days (preferably 21 days) from the day of last contact, according to the ASTCT. Two consecutive negative PCR tests each approximately 1 week apart (deferral for 14 days minimum), should be obtained, if available.
In areas with high community spread, the guidelines also state that “interim treatment and/or longer deferral of definite therapy should be considered when feasible (for example, multiple myeloma, germ cell tumors, consolidative transplants).”
Similar considerations should be afforded to potential cellular donors. Donors with SARS-CoV-2 detected in a respiratory sample are considered ineligible. Those meeting exposure criteria for patients, as listed above, should be excluded from donation for at least 28 days. “In individual circumstances, a donor may be considered eligible if respiratory samples are negative for SARS-CoV-2 by PCR and donor is asymptomatic. Donor should be closely monitored for COVID-19.”
In the case of unrelated donors, the ASTCT recommends referral to the National Marrow Donor Program (NMDP) guidelines for updated guidance, but points out that, according to the NMDP, the Food and Drug Administration reports that there have been no reported or suspected cases of transfusion-transmitted COVID-19 to date and that “no cases of transfusion-transmission were ever reported for the other two coronaviruses that emerged during the past 2 decades [SARS, the severe acute respiratory syndrome coronavirus, and MERS-CoV, which causes Mideast respiratory syndrome].”
In the updated ESBMT guidelines, this recommendation was made in reference to the greater spread of COVID-19: “It is therefore strongly recommended to have secured stem cell product access by freezing the product before start of conditioning and, in situations when this is not possible, to have an alternative donor as a backup. For low-risk patients, it is recommended to postpone the start of the transplant procedure if deemed to be safe to do so. This includes both allogeneic and autologous transplant procedures.”
In a recent webinar, Pavan Reddy, MD, of the University of Michigan, Ann Arbor, and ASTCT President; Alpana Waghmare, MD, of the Fred Hutchinson Cancer Research Center, Seattle; and Roy Chemaly, MD, of the MD Anderson Cancer Center, Houston, and chair of the ASTCT Transplant Infectious Disease Special Interest Group, discussed the guidelines and provided some updated information.
Dr. Reddy stated that, at the University of Michigan, they were delaying all nonurgent transplants, largely for myeloma, and are postponing even allotransplants. “The transplants we are not delaying are the high-risk AMLs … and in cases where we truly cannot delay transplants because of patient condition or, in some cases, the donor situation.”
Dr. Chemaly and Dr. Waghmare both agreed that their centers were following a similar approach.
With regard to patient testing, all three institution have recently moved to testing everyone a few days before transplant regardless of symptoms.
They also pointed out that essentially all clinical trials were being put on hold during the crisis, except for those few where patients would be put in danger if the trial were interrupted.
The guidelines discuss in depth the rationale, toxicity, and dosages for use of select agents, including remdesivir, chloroquine/hydroxychloroquine, ribavirin, and tocilizumab. There was some concern expressed about shortages developing in these drugs, which serve a number of other patient communities, in particular the possibility of a tocilizumab shortage was of concern.
Steroids and intravenous immunoglobulins are not are not recommended, according to the guidelines, which also stated that adjunctive therapies such as antibiotics should be considered.
Dr. Chemaly, Dr. Reddy, and Dr. Waghmare did not provide disclosure in the webinar.
The ASTCT recommends following the World Health Organization and CDC COVID-19 pages for continued updates and information on other aspects of the pandemic.
This article was updated 3/26/20.
SOURCE: ASTCT Response to COVID-19. 2020. www.astct.org/connect/astct-response-to-covid-19.
Second transplant a good salvage option for children with ALL, AML, or MDS
ORLANDO – A second hematopoietic stem cell transplant can be a successful salvage therapy for a child who has experienced a relapse following a first allogeneic transplant, investigators report.
A retrospective study of 221 children who experienced a relapse after a first hematopoietic stem cell transplant (HSCT) showed that 3-year overall survival (OS) was six times higher among those who had second HSCT, compared with those who did not, reported Akshay Sharma, MBBS, from St. Jude’s Children’s Research Hospital in Memphis.
“We found that factors that are typically associated with poor outcomes after transplant such as disease status at the time of first transplantation – being in remission or not, type of transplant – myeloablative or reduced intensity, and choice of donor were generally not significantly predictive of outcomes following posttransplant relapse in our multivariable model,” he said at the Transplantation and Cellular Therapy Meetings.
Relapse is the most common cause of death after HSCT, and 20%-30% of children who undergo allogeneic HSCT will experience a relapse.
To study this issue, Dr. Sharma and colleagues took a retrospective look at 703 patients 21 and younger who received a first alloHSCT at St. Jude’s for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) or myelodysplastic syndrome from 1990 through 2018. Of this cohort, 211 patients (31%) experienced a relapse after transplant.
There were no significant differences between patients who had a relapse and those who did not in sex, race, conditioning-regimen intensity, performance status, donor type, or graft type (peripheral blood stem cell, umbilical cord blood, bone marrow), or in the incidence of acute graft-versus-host disease GVHD.
The investigators found that, as expected, outcomes were poor for patients who experienced a posttransplant relapse, with 3-year overall survival from relapse for the 221 patients of just 10%.
In multivariable analysis controlling for sex, disease status at the time of first transplant, interval from first transplant to relapse, management after relapse, chronic GVHD and year of relapse, factors significantly associated with worse overall survival were relapse within 6 months of transplant vs. later than 6 months (hazard ratio, 4.6; P < .001) and decade of transplant (HR, 2.6 for 1990-2000 and 1.6 for 2001-2010 vs. 2011-2018; P < .001).
In contrast, both second HSCT and donor lymphocyte infusion were associated with better overall survival, compared with postrelapse chemotherapy or supportive care (HR, 0.04 and 0.6, respectively; P < .001 for both comparisons).
A longer interval from first transplant to relapse was the strongest predictor of long-term survival, Dr. Sharma said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Among the 221 patients who had a relapse, 61 (28%) had a second HSCT, 28 (13%) received only donor lymphocyte infusions, without second transplant, and 132 (62%) received either chemotherapy or supportive care.
The 3-year overall survival rate for patients who received a second transplant was 28%, compared with 4% for those who did not have a repeat HSCT. The most important independent predictors for getting a second transplant were longer time to relapse after first transplant, first transplantation from a matched sibling donor instead of from a haploidentical donor, some degree of acute GVHD, and decade of first transplant (current decade vs. earlier decades).
The investigators also looked at guideline recommendations from both the American Society of Hematology and UK National Health Service regarding second allogenenic transplant after relapse.
ASH guidelines say that “patients with chemo-sensitive disease in remission who had a long initial remission (> 6-12 months) after first transplant and who never developed any GVHD” are most likely to benefit from a second transplant.
NHS guidelines say that a second transplant can be considered for patients who experience relapse more than 12 months after first alloHSCT. But as Dr. Sharma and colleagues discovered, patients who had a second transplant had better overall survival regardless of time from first to second transplant, compared with patients who had later relapses but no second transplant.
“With these data in mind, we submit that these ASH and NHS guidelines, which are based on older data from the 1900s and 2000 and are mostly based on adult data and do not include much pediatric data should be reconsidered, at least in the context of pediatric patients as we approach them,” he said.
Jaap-Jan Boelens, MD, PhD, a pediatric transplant specialist at Memorial Sloan Kettering Cancer Center in New York City, who was not involved in the study, said that the use of second transplant as salvage therapy is becoming more common at his center.
“But there is a little nuance,” he said in an interview. “If someone relapses a month after transplant, let’s say, it doesn’t make sense to go for another allo transplant. But if the interval between transplant is longer, more than half a year, we usually consider going for a second allo transplant.”
The decision to attempt a second transplant may also hinge on the disease the patient is being treated for, and on the depth of remission prior to relapse, he said.
Reggie E. Duerst, MD, director of the Stem Cell Transplant Program at Ann & Robert H. Lurie Children’s Hospital of Chicago, said that trying to replicate the conditions of the first transplant may not work.
“It’s also a function of who was the donor for the first transplant – was it a matched sibling? And then for the second one do you go to an unrelated donor, or a half-matched relative, banking on the fact that the donor’s immune system for the second transplant is going to be able to mediate some kind of graft-versus-leukemia effect that wasn’t there the first time around?” he said.
Dr. Duerst, who was not involved in the study, noted that, for some patients with lymphoid malignancies, chimeric antigen receptor (CAR) T-cell therapy may be a more effective salvage strategy than second transplant, but added that it’s still too soon to know which strategy will be more effective.
The study was supported by St. Jude’s, the American Society of Hematology, and the American Society for Transplantation and Cellular Therapy. Dr. Sharma, Dr. Boelens, and Dr. Duerst reported having no relevant disclosures.
SOURCE: Sharma A et al. TCT 2020, Abstract 116.
ORLANDO – A second hematopoietic stem cell transplant can be a successful salvage therapy for a child who has experienced a relapse following a first allogeneic transplant, investigators report.
A retrospective study of 221 children who experienced a relapse after a first hematopoietic stem cell transplant (HSCT) showed that 3-year overall survival (OS) was six times higher among those who had second HSCT, compared with those who did not, reported Akshay Sharma, MBBS, from St. Jude’s Children’s Research Hospital in Memphis.
“We found that factors that are typically associated with poor outcomes after transplant such as disease status at the time of first transplantation – being in remission or not, type of transplant – myeloablative or reduced intensity, and choice of donor were generally not significantly predictive of outcomes following posttransplant relapse in our multivariable model,” he said at the Transplantation and Cellular Therapy Meetings.
Relapse is the most common cause of death after HSCT, and 20%-30% of children who undergo allogeneic HSCT will experience a relapse.
To study this issue, Dr. Sharma and colleagues took a retrospective look at 703 patients 21 and younger who received a first alloHSCT at St. Jude’s for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) or myelodysplastic syndrome from 1990 through 2018. Of this cohort, 211 patients (31%) experienced a relapse after transplant.
There were no significant differences between patients who had a relapse and those who did not in sex, race, conditioning-regimen intensity, performance status, donor type, or graft type (peripheral blood stem cell, umbilical cord blood, bone marrow), or in the incidence of acute graft-versus-host disease GVHD.
The investigators found that, as expected, outcomes were poor for patients who experienced a posttransplant relapse, with 3-year overall survival from relapse for the 221 patients of just 10%.
In multivariable analysis controlling for sex, disease status at the time of first transplant, interval from first transplant to relapse, management after relapse, chronic GVHD and year of relapse, factors significantly associated with worse overall survival were relapse within 6 months of transplant vs. later than 6 months (hazard ratio, 4.6; P < .001) and decade of transplant (HR, 2.6 for 1990-2000 and 1.6 for 2001-2010 vs. 2011-2018; P < .001).
In contrast, both second HSCT and donor lymphocyte infusion were associated with better overall survival, compared with postrelapse chemotherapy or supportive care (HR, 0.04 and 0.6, respectively; P < .001 for both comparisons).
A longer interval from first transplant to relapse was the strongest predictor of long-term survival, Dr. Sharma said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Among the 221 patients who had a relapse, 61 (28%) had a second HSCT, 28 (13%) received only donor lymphocyte infusions, without second transplant, and 132 (62%) received either chemotherapy or supportive care.
The 3-year overall survival rate for patients who received a second transplant was 28%, compared with 4% for those who did not have a repeat HSCT. The most important independent predictors for getting a second transplant were longer time to relapse after first transplant, first transplantation from a matched sibling donor instead of from a haploidentical donor, some degree of acute GVHD, and decade of first transplant (current decade vs. earlier decades).
The investigators also looked at guideline recommendations from both the American Society of Hematology and UK National Health Service regarding second allogenenic transplant after relapse.
ASH guidelines say that “patients with chemo-sensitive disease in remission who had a long initial remission (> 6-12 months) after first transplant and who never developed any GVHD” are most likely to benefit from a second transplant.
NHS guidelines say that a second transplant can be considered for patients who experience relapse more than 12 months after first alloHSCT. But as Dr. Sharma and colleagues discovered, patients who had a second transplant had better overall survival regardless of time from first to second transplant, compared with patients who had later relapses but no second transplant.
“With these data in mind, we submit that these ASH and NHS guidelines, which are based on older data from the 1900s and 2000 and are mostly based on adult data and do not include much pediatric data should be reconsidered, at least in the context of pediatric patients as we approach them,” he said.
Jaap-Jan Boelens, MD, PhD, a pediatric transplant specialist at Memorial Sloan Kettering Cancer Center in New York City, who was not involved in the study, said that the use of second transplant as salvage therapy is becoming more common at his center.
“But there is a little nuance,” he said in an interview. “If someone relapses a month after transplant, let’s say, it doesn’t make sense to go for another allo transplant. But if the interval between transplant is longer, more than half a year, we usually consider going for a second allo transplant.”
The decision to attempt a second transplant may also hinge on the disease the patient is being treated for, and on the depth of remission prior to relapse, he said.
Reggie E. Duerst, MD, director of the Stem Cell Transplant Program at Ann & Robert H. Lurie Children’s Hospital of Chicago, said that trying to replicate the conditions of the first transplant may not work.
“It’s also a function of who was the donor for the first transplant – was it a matched sibling? And then for the second one do you go to an unrelated donor, or a half-matched relative, banking on the fact that the donor’s immune system for the second transplant is going to be able to mediate some kind of graft-versus-leukemia effect that wasn’t there the first time around?” he said.
Dr. Duerst, who was not involved in the study, noted that, for some patients with lymphoid malignancies, chimeric antigen receptor (CAR) T-cell therapy may be a more effective salvage strategy than second transplant, but added that it’s still too soon to know which strategy will be more effective.
The study was supported by St. Jude’s, the American Society of Hematology, and the American Society for Transplantation and Cellular Therapy. Dr. Sharma, Dr. Boelens, and Dr. Duerst reported having no relevant disclosures.
SOURCE: Sharma A et al. TCT 2020, Abstract 116.
ORLANDO – A second hematopoietic stem cell transplant can be a successful salvage therapy for a child who has experienced a relapse following a first allogeneic transplant, investigators report.
A retrospective study of 221 children who experienced a relapse after a first hematopoietic stem cell transplant (HSCT) showed that 3-year overall survival (OS) was six times higher among those who had second HSCT, compared with those who did not, reported Akshay Sharma, MBBS, from St. Jude’s Children’s Research Hospital in Memphis.
“We found that factors that are typically associated with poor outcomes after transplant such as disease status at the time of first transplantation – being in remission or not, type of transplant – myeloablative or reduced intensity, and choice of donor were generally not significantly predictive of outcomes following posttransplant relapse in our multivariable model,” he said at the Transplantation and Cellular Therapy Meetings.
Relapse is the most common cause of death after HSCT, and 20%-30% of children who undergo allogeneic HSCT will experience a relapse.
To study this issue, Dr. Sharma and colleagues took a retrospective look at 703 patients 21 and younger who received a first alloHSCT at St. Jude’s for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) or myelodysplastic syndrome from 1990 through 2018. Of this cohort, 211 patients (31%) experienced a relapse after transplant.
There were no significant differences between patients who had a relapse and those who did not in sex, race, conditioning-regimen intensity, performance status, donor type, or graft type (peripheral blood stem cell, umbilical cord blood, bone marrow), or in the incidence of acute graft-versus-host disease GVHD.
The investigators found that, as expected, outcomes were poor for patients who experienced a posttransplant relapse, with 3-year overall survival from relapse for the 221 patients of just 10%.
In multivariable analysis controlling for sex, disease status at the time of first transplant, interval from first transplant to relapse, management after relapse, chronic GVHD and year of relapse, factors significantly associated with worse overall survival were relapse within 6 months of transplant vs. later than 6 months (hazard ratio, 4.6; P < .001) and decade of transplant (HR, 2.6 for 1990-2000 and 1.6 for 2001-2010 vs. 2011-2018; P < .001).
In contrast, both second HSCT and donor lymphocyte infusion were associated with better overall survival, compared with postrelapse chemotherapy or supportive care (HR, 0.04 and 0.6, respectively; P < .001 for both comparisons).
A longer interval from first transplant to relapse was the strongest predictor of long-term survival, Dr. Sharma said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Among the 221 patients who had a relapse, 61 (28%) had a second HSCT, 28 (13%) received only donor lymphocyte infusions, without second transplant, and 132 (62%) received either chemotherapy or supportive care.
The 3-year overall survival rate for patients who received a second transplant was 28%, compared with 4% for those who did not have a repeat HSCT. The most important independent predictors for getting a second transplant were longer time to relapse after first transplant, first transplantation from a matched sibling donor instead of from a haploidentical donor, some degree of acute GVHD, and decade of first transplant (current decade vs. earlier decades).
The investigators also looked at guideline recommendations from both the American Society of Hematology and UK National Health Service regarding second allogenenic transplant after relapse.
ASH guidelines say that “patients with chemo-sensitive disease in remission who had a long initial remission (> 6-12 months) after first transplant and who never developed any GVHD” are most likely to benefit from a second transplant.
NHS guidelines say that a second transplant can be considered for patients who experience relapse more than 12 months after first alloHSCT. But as Dr. Sharma and colleagues discovered, patients who had a second transplant had better overall survival regardless of time from first to second transplant, compared with patients who had later relapses but no second transplant.
“With these data in mind, we submit that these ASH and NHS guidelines, which are based on older data from the 1900s and 2000 and are mostly based on adult data and do not include much pediatric data should be reconsidered, at least in the context of pediatric patients as we approach them,” he said.
Jaap-Jan Boelens, MD, PhD, a pediatric transplant specialist at Memorial Sloan Kettering Cancer Center in New York City, who was not involved in the study, said that the use of second transplant as salvage therapy is becoming more common at his center.
“But there is a little nuance,” he said in an interview. “If someone relapses a month after transplant, let’s say, it doesn’t make sense to go for another allo transplant. But if the interval between transplant is longer, more than half a year, we usually consider going for a second allo transplant.”
The decision to attempt a second transplant may also hinge on the disease the patient is being treated for, and on the depth of remission prior to relapse, he said.
Reggie E. Duerst, MD, director of the Stem Cell Transplant Program at Ann & Robert H. Lurie Children’s Hospital of Chicago, said that trying to replicate the conditions of the first transplant may not work.
“It’s also a function of who was the donor for the first transplant – was it a matched sibling? And then for the second one do you go to an unrelated donor, or a half-matched relative, banking on the fact that the donor’s immune system for the second transplant is going to be able to mediate some kind of graft-versus-leukemia effect that wasn’t there the first time around?” he said.
Dr. Duerst, who was not involved in the study, noted that, for some patients with lymphoid malignancies, chimeric antigen receptor (CAR) T-cell therapy may be a more effective salvage strategy than second transplant, but added that it’s still too soon to know which strategy will be more effective.
The study was supported by St. Jude’s, the American Society of Hematology, and the American Society for Transplantation and Cellular Therapy. Dr. Sharma, Dr. Boelens, and Dr. Duerst reported having no relevant disclosures.
SOURCE: Sharma A et al. TCT 2020, Abstract 116.
REPORTING FROM TCT 2020












