User login
-
FDA warns of higher death risk with Pepaxto in multiple myeloma
participating in the ongoing OCEAN clinical trial.
The drug was granted accelerated approval in February 2021 for use in combination with dexamethasone in the treatment of adults with relapsed or refractory multiple myeloma who had received at least four prior lines of therapy and whose disease was refractory to at least one proteasome inhibitor, one immunomodulator, and one CD38-directed monoclonal antibody.
As a condition of the accelerated approval, the manufacturer, Oncopeptides, was required to conduct a confirmatory clinical trial and launched the OCEAN trial.
Enrollment in OCEAN as well as other ongoing trials of the drug have now been halted, according to the FDA alert.
The warning comes in the wake of OCEAN trial findings showing worse survival among patients in the experimental group, who were receiving melphalan plus low-dose dexamethasone, compared with patients in the control group, who were receiving pomalidomide plus low-dose dexamethasone (hazard ratio for overall survival, 1.104). Median overall survival in the treatment and control groups was 19.7 and 25.0 months, respectively.
Health care professionals should “review patients’ progress on Pepaxto and discuss the risks of continued administration with each patient in the context of other treatments,” and patients currently receiving the drug should discuss the risks and benefits with their health care professional, the FDA advises. “Patients receiving clinical benefit from Pepaxto may continue treatment in the OCEAN trial provided they are informed of the risks and sign a revised written informed consent.”
The FDA also hinted at “a future public meeting to discuss the safety findings and explore the continued marketing of Pepaxto,” which has a price tag of $19,000 per treatment course.
Accelerated approval data
Melphalan flufenamide was initially evaluated in combination with low-dose dexamethasone in the multicenter, single-arm HORIZON trial of adults with relapsed or refractory multiple myeloma who received at least four prior lines of therapy and whose disease was refractory to at least one proteasome inhibitor, one immunomodulator, and one CD38-directed monoclonal antibody.
Patients received melphalan flufenamide at a dose of 40 mg intravenously on day 1 along with oral dexamethasone at a dose of 40 mg (or 20 mg for those over age 75 years) on days 1, 8, 15, and 22 of each 28-day cycle until disease progression or unacceptable toxicity.
The most common adverse reactions, occurring in at least 20% of patients, were fatigue, nausea, diarrhea, pyrexia, and respiratory tract infection. The most common laboratory abnormalities, occurring in at least 50% of patients, were decreased leukocytes, platelets, lymphocytes, neutrophils, and hemoglobin, and increased creatinine.
Accelerated approval was granted after the HORIZON trial showed an overall response rate of 23.7% and median duration of response of 4.2 months. The application by Oncopeptides received priority review and orphan drug status.
Confirmatory trial data
The confirmatory OCEAN trial compared melphalan flufenamide plus low-dose dexamethasone to pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma following 2-4 lines of therapy and in patients who were resistant to lenalidomide in the last line of therapy.
The FDA conducted an efficacy and safety evaluation of the OCEAN trial using a data cutoff date of February 3, 2021. At a median follow-up of 19.1 months, 117 of 246 patients (48%) in the melphalan flufenamide group had died, compared with 108 of 249 patients (43%) in the pomalidomide control group.
“Patient safety is paramount to Oncopeptides,” the company said in a press statement, which also notes that “dialogue with the FDA” is ongoing and that updated information will be provided as it becomes available.
The company plans to submit complete data from the OCEAN study to the International Myeloma Workshop meeting in Vienna being held September 8-11, 2021.
Health care professionals and patients should report adverse events or quality issues experienced with melphalan flufenamide or any other medication to the FDA MedWatch Adverse Event Reporting program, either online or by downloading and completing a reporting form and submitting via fax at 1-800-FDA-0178.
A version of this article first appeared on Medscape.com.
participating in the ongoing OCEAN clinical trial.
The drug was granted accelerated approval in February 2021 for use in combination with dexamethasone in the treatment of adults with relapsed or refractory multiple myeloma who had received at least four prior lines of therapy and whose disease was refractory to at least one proteasome inhibitor, one immunomodulator, and one CD38-directed monoclonal antibody.
As a condition of the accelerated approval, the manufacturer, Oncopeptides, was required to conduct a confirmatory clinical trial and launched the OCEAN trial.
Enrollment in OCEAN as well as other ongoing trials of the drug have now been halted, according to the FDA alert.
The warning comes in the wake of OCEAN trial findings showing worse survival among patients in the experimental group, who were receiving melphalan plus low-dose dexamethasone, compared with patients in the control group, who were receiving pomalidomide plus low-dose dexamethasone (hazard ratio for overall survival, 1.104). Median overall survival in the treatment and control groups was 19.7 and 25.0 months, respectively.
Health care professionals should “review patients’ progress on Pepaxto and discuss the risks of continued administration with each patient in the context of other treatments,” and patients currently receiving the drug should discuss the risks and benefits with their health care professional, the FDA advises. “Patients receiving clinical benefit from Pepaxto may continue treatment in the OCEAN trial provided they are informed of the risks and sign a revised written informed consent.”
The FDA also hinted at “a future public meeting to discuss the safety findings and explore the continued marketing of Pepaxto,” which has a price tag of $19,000 per treatment course.
Accelerated approval data
Melphalan flufenamide was initially evaluated in combination with low-dose dexamethasone in the multicenter, single-arm HORIZON trial of adults with relapsed or refractory multiple myeloma who received at least four prior lines of therapy and whose disease was refractory to at least one proteasome inhibitor, one immunomodulator, and one CD38-directed monoclonal antibody.
Patients received melphalan flufenamide at a dose of 40 mg intravenously on day 1 along with oral dexamethasone at a dose of 40 mg (or 20 mg for those over age 75 years) on days 1, 8, 15, and 22 of each 28-day cycle until disease progression or unacceptable toxicity.
The most common adverse reactions, occurring in at least 20% of patients, were fatigue, nausea, diarrhea, pyrexia, and respiratory tract infection. The most common laboratory abnormalities, occurring in at least 50% of patients, were decreased leukocytes, platelets, lymphocytes, neutrophils, and hemoglobin, and increased creatinine.
Accelerated approval was granted after the HORIZON trial showed an overall response rate of 23.7% and median duration of response of 4.2 months. The application by Oncopeptides received priority review and orphan drug status.
Confirmatory trial data
The confirmatory OCEAN trial compared melphalan flufenamide plus low-dose dexamethasone to pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma following 2-4 lines of therapy and in patients who were resistant to lenalidomide in the last line of therapy.
The FDA conducted an efficacy and safety evaluation of the OCEAN trial using a data cutoff date of February 3, 2021. At a median follow-up of 19.1 months, 117 of 246 patients (48%) in the melphalan flufenamide group had died, compared with 108 of 249 patients (43%) in the pomalidomide control group.
“Patient safety is paramount to Oncopeptides,” the company said in a press statement, which also notes that “dialogue with the FDA” is ongoing and that updated information will be provided as it becomes available.
The company plans to submit complete data from the OCEAN study to the International Myeloma Workshop meeting in Vienna being held September 8-11, 2021.
Health care professionals and patients should report adverse events or quality issues experienced with melphalan flufenamide or any other medication to the FDA MedWatch Adverse Event Reporting program, either online or by downloading and completing a reporting form and submitting via fax at 1-800-FDA-0178.
A version of this article first appeared on Medscape.com.
participating in the ongoing OCEAN clinical trial.
The drug was granted accelerated approval in February 2021 for use in combination with dexamethasone in the treatment of adults with relapsed or refractory multiple myeloma who had received at least four prior lines of therapy and whose disease was refractory to at least one proteasome inhibitor, one immunomodulator, and one CD38-directed monoclonal antibody.
As a condition of the accelerated approval, the manufacturer, Oncopeptides, was required to conduct a confirmatory clinical trial and launched the OCEAN trial.
Enrollment in OCEAN as well as other ongoing trials of the drug have now been halted, according to the FDA alert.
The warning comes in the wake of OCEAN trial findings showing worse survival among patients in the experimental group, who were receiving melphalan plus low-dose dexamethasone, compared with patients in the control group, who were receiving pomalidomide plus low-dose dexamethasone (hazard ratio for overall survival, 1.104). Median overall survival in the treatment and control groups was 19.7 and 25.0 months, respectively.
Health care professionals should “review patients’ progress on Pepaxto and discuss the risks of continued administration with each patient in the context of other treatments,” and patients currently receiving the drug should discuss the risks and benefits with their health care professional, the FDA advises. “Patients receiving clinical benefit from Pepaxto may continue treatment in the OCEAN trial provided they are informed of the risks and sign a revised written informed consent.”
The FDA also hinted at “a future public meeting to discuss the safety findings and explore the continued marketing of Pepaxto,” which has a price tag of $19,000 per treatment course.
Accelerated approval data
Melphalan flufenamide was initially evaluated in combination with low-dose dexamethasone in the multicenter, single-arm HORIZON trial of adults with relapsed or refractory multiple myeloma who received at least four prior lines of therapy and whose disease was refractory to at least one proteasome inhibitor, one immunomodulator, and one CD38-directed monoclonal antibody.
Patients received melphalan flufenamide at a dose of 40 mg intravenously on day 1 along with oral dexamethasone at a dose of 40 mg (or 20 mg for those over age 75 years) on days 1, 8, 15, and 22 of each 28-day cycle until disease progression or unacceptable toxicity.
The most common adverse reactions, occurring in at least 20% of patients, were fatigue, nausea, diarrhea, pyrexia, and respiratory tract infection. The most common laboratory abnormalities, occurring in at least 50% of patients, were decreased leukocytes, platelets, lymphocytes, neutrophils, and hemoglobin, and increased creatinine.
Accelerated approval was granted after the HORIZON trial showed an overall response rate of 23.7% and median duration of response of 4.2 months. The application by Oncopeptides received priority review and orphan drug status.
Confirmatory trial data
The confirmatory OCEAN trial compared melphalan flufenamide plus low-dose dexamethasone to pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma following 2-4 lines of therapy and in patients who were resistant to lenalidomide in the last line of therapy.
The FDA conducted an efficacy and safety evaluation of the OCEAN trial using a data cutoff date of February 3, 2021. At a median follow-up of 19.1 months, 117 of 246 patients (48%) in the melphalan flufenamide group had died, compared with 108 of 249 patients (43%) in the pomalidomide control group.
“Patient safety is paramount to Oncopeptides,” the company said in a press statement, which also notes that “dialogue with the FDA” is ongoing and that updated information will be provided as it becomes available.
The company plans to submit complete data from the OCEAN study to the International Myeloma Workshop meeting in Vienna being held September 8-11, 2021.
Health care professionals and patients should report adverse events or quality issues experienced with melphalan flufenamide or any other medication to the FDA MedWatch Adverse Event Reporting program, either online or by downloading and completing a reporting form and submitting via fax at 1-800-FDA-0178.
A version of this article first appeared on Medscape.com.
Many pandemic-driven changes to cancer clinical trials should remain
Many of the changes to cancer clinical trials forced through by the COVID-19 pandemic should remain, as they have made trials “more patient centered and efficient,” according to a group of thought leaders in oncology.
Among the potential improvements were more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessment of adverse events, and streamlined data collection.
These changes should be implemented on a permanent basis, the group argues in a commentary published online July 21, 2021, in Cancer Discovery, a journal of the American Association for Cancer Research.
“The ability to distribute oral investigational drugs by mail to patients at their home has probably been the single most impactful change to clinical trial conduct, linked with virtual visits with patients to assess side effects and symptoms,” commented lead author Keith Flaherty, MD, who is director of clinical research at Massachusetts General Hospital, a professor at Harvard Medical School, Boston, and a member of the AACR board of directors.
“This has made it more feasible for patients for whom participation in clinical trials poses a disruption of their ability to work or provide care for family members to participate in trials,” he added in a press statement issued by the AACR.
Pandemic halted many clinical trials
A survey of cancer programs in early 2020 showed that nearly 60% halted screening and/or enrollment for at least some trials because of COVID-19.
“In the spring of 2020, clinical trial conduct halted and then restarted focusing on the bare minimum procedures that first allowed patients continued access to their experimental therapies, and then allowed clinical trial sites and sponsors to collect information on the effects of the therapies,” the authors said.
“The COVID-19–induced changes to clinical trials were a big challenge, probably the largest change in clinical trial conduct since the start of modern oncology clinical testing,” they commented.
“But it also represents an opportunity to rethink the key aspects of clinical trial conduct that are strictly necessary to reach the goal of testing the effectiveness of cancer therapies, and which others are dispensable or provide only minor additional contributions,” they added.
As previously reported at the time by this news organization, efforts to find alternative approaches to conducting trials amid the pandemic led to the emergence of a few “silver linings.”
Key adaptations made to clinical trials and highlighted by the authors include:
- Uptake of remote consenting and telemedicine
- Use of alternative laboratories and imaging centers
- Delivery or administration of investigational drugs at patients’ homes or local clinics
- Commercial attainment of study drugs already approved for other indications
Indeed, the restrictions encountered during the pandemic underscore the importance of designing patient-centered trials versus study site–centered trials, added Antoni Ribas, MD, commentary coauthor and immediate past president of the AACR.
Many of the changes implemented during the pandemic could help increase access for patients living in underserved communities who are underrepresented in clinical trials, he explained.
Harnessing the lessons learned
The authors also recommended the following additional adaptations, which they believe will enhance efficiency and further expand access to clinical trials:
- Incorporating patient-reported outcomes and alternative endpoints in efficacy assessments
- Aiming for 100% remote drug infusions and monitoring
- Increasing funding for clinical trials conducted in underserved communities
- Expanding clinical trial eligibility to include patients with a wide range of comorbidities
- Reducing collection of low-grade adverse events and allowing minor protocol deviations
The group’s recommendations are based on discussions by the AACR COVID-19 and Cancer Task Force, in which they participated.
The American Society of Clinical Oncology is also working to leverage pandemic-related lessons to streamline care and trial planning.
ASCO’s “Road to Recovery” recommendations, published in December 2020, aim to “ensure lessons learned from the COVID-19 experience are used to craft a more equitable, accessible, and efficient clinical research system that protects patient safety, ensures scientific integrity, and maintains data quality,” the authors explained.
Dr. Flaherty and colleagues further underscore the importance of focusing on improvements going forward.
“Guided by lessons learned, many of the remote assessments and trial efficiencies deployed during the pandemic can be preserved and improved upon. We strongly encourage use of these streamlined procedures where appropriate in future prospectively designed cancer clinical trials,” they wrote.
Dr. Flaherty reported receiving personal fees from numerous pharmaceutical companies. Dr. Ribas reported receiving grants from Agilent and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Many of the changes to cancer clinical trials forced through by the COVID-19 pandemic should remain, as they have made trials “more patient centered and efficient,” according to a group of thought leaders in oncology.
Among the potential improvements were more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessment of adverse events, and streamlined data collection.
These changes should be implemented on a permanent basis, the group argues in a commentary published online July 21, 2021, in Cancer Discovery, a journal of the American Association for Cancer Research.
“The ability to distribute oral investigational drugs by mail to patients at their home has probably been the single most impactful change to clinical trial conduct, linked with virtual visits with patients to assess side effects and symptoms,” commented lead author Keith Flaherty, MD, who is director of clinical research at Massachusetts General Hospital, a professor at Harvard Medical School, Boston, and a member of the AACR board of directors.
“This has made it more feasible for patients for whom participation in clinical trials poses a disruption of their ability to work or provide care for family members to participate in trials,” he added in a press statement issued by the AACR.
Pandemic halted many clinical trials
A survey of cancer programs in early 2020 showed that nearly 60% halted screening and/or enrollment for at least some trials because of COVID-19.
“In the spring of 2020, clinical trial conduct halted and then restarted focusing on the bare minimum procedures that first allowed patients continued access to their experimental therapies, and then allowed clinical trial sites and sponsors to collect information on the effects of the therapies,” the authors said.
“The COVID-19–induced changes to clinical trials were a big challenge, probably the largest change in clinical trial conduct since the start of modern oncology clinical testing,” they commented.
“But it also represents an opportunity to rethink the key aspects of clinical trial conduct that are strictly necessary to reach the goal of testing the effectiveness of cancer therapies, and which others are dispensable or provide only minor additional contributions,” they added.
As previously reported at the time by this news organization, efforts to find alternative approaches to conducting trials amid the pandemic led to the emergence of a few “silver linings.”
Key adaptations made to clinical trials and highlighted by the authors include:
- Uptake of remote consenting and telemedicine
- Use of alternative laboratories and imaging centers
- Delivery or administration of investigational drugs at patients’ homes or local clinics
- Commercial attainment of study drugs already approved for other indications
Indeed, the restrictions encountered during the pandemic underscore the importance of designing patient-centered trials versus study site–centered trials, added Antoni Ribas, MD, commentary coauthor and immediate past president of the AACR.
Many of the changes implemented during the pandemic could help increase access for patients living in underserved communities who are underrepresented in clinical trials, he explained.
Harnessing the lessons learned
The authors also recommended the following additional adaptations, which they believe will enhance efficiency and further expand access to clinical trials:
- Incorporating patient-reported outcomes and alternative endpoints in efficacy assessments
- Aiming for 100% remote drug infusions and monitoring
- Increasing funding for clinical trials conducted in underserved communities
- Expanding clinical trial eligibility to include patients with a wide range of comorbidities
- Reducing collection of low-grade adverse events and allowing minor protocol deviations
The group’s recommendations are based on discussions by the AACR COVID-19 and Cancer Task Force, in which they participated.
The American Society of Clinical Oncology is also working to leverage pandemic-related lessons to streamline care and trial planning.
ASCO’s “Road to Recovery” recommendations, published in December 2020, aim to “ensure lessons learned from the COVID-19 experience are used to craft a more equitable, accessible, and efficient clinical research system that protects patient safety, ensures scientific integrity, and maintains data quality,” the authors explained.
Dr. Flaherty and colleagues further underscore the importance of focusing on improvements going forward.
“Guided by lessons learned, many of the remote assessments and trial efficiencies deployed during the pandemic can be preserved and improved upon. We strongly encourage use of these streamlined procedures where appropriate in future prospectively designed cancer clinical trials,” they wrote.
Dr. Flaherty reported receiving personal fees from numerous pharmaceutical companies. Dr. Ribas reported receiving grants from Agilent and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Many of the changes to cancer clinical trials forced through by the COVID-19 pandemic should remain, as they have made trials “more patient centered and efficient,” according to a group of thought leaders in oncology.
Among the potential improvements were more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessment of adverse events, and streamlined data collection.
These changes should be implemented on a permanent basis, the group argues in a commentary published online July 21, 2021, in Cancer Discovery, a journal of the American Association for Cancer Research.
“The ability to distribute oral investigational drugs by mail to patients at their home has probably been the single most impactful change to clinical trial conduct, linked with virtual visits with patients to assess side effects and symptoms,” commented lead author Keith Flaherty, MD, who is director of clinical research at Massachusetts General Hospital, a professor at Harvard Medical School, Boston, and a member of the AACR board of directors.
“This has made it more feasible for patients for whom participation in clinical trials poses a disruption of their ability to work or provide care for family members to participate in trials,” he added in a press statement issued by the AACR.
Pandemic halted many clinical trials
A survey of cancer programs in early 2020 showed that nearly 60% halted screening and/or enrollment for at least some trials because of COVID-19.
“In the spring of 2020, clinical trial conduct halted and then restarted focusing on the bare minimum procedures that first allowed patients continued access to their experimental therapies, and then allowed clinical trial sites and sponsors to collect information on the effects of the therapies,” the authors said.
“The COVID-19–induced changes to clinical trials were a big challenge, probably the largest change in clinical trial conduct since the start of modern oncology clinical testing,” they commented.
“But it also represents an opportunity to rethink the key aspects of clinical trial conduct that are strictly necessary to reach the goal of testing the effectiveness of cancer therapies, and which others are dispensable or provide only minor additional contributions,” they added.
As previously reported at the time by this news organization, efforts to find alternative approaches to conducting trials amid the pandemic led to the emergence of a few “silver linings.”
Key adaptations made to clinical trials and highlighted by the authors include:
- Uptake of remote consenting and telemedicine
- Use of alternative laboratories and imaging centers
- Delivery or administration of investigational drugs at patients’ homes or local clinics
- Commercial attainment of study drugs already approved for other indications
Indeed, the restrictions encountered during the pandemic underscore the importance of designing patient-centered trials versus study site–centered trials, added Antoni Ribas, MD, commentary coauthor and immediate past president of the AACR.
Many of the changes implemented during the pandemic could help increase access for patients living in underserved communities who are underrepresented in clinical trials, he explained.
Harnessing the lessons learned
The authors also recommended the following additional adaptations, which they believe will enhance efficiency and further expand access to clinical trials:
- Incorporating patient-reported outcomes and alternative endpoints in efficacy assessments
- Aiming for 100% remote drug infusions and monitoring
- Increasing funding for clinical trials conducted in underserved communities
- Expanding clinical trial eligibility to include patients with a wide range of comorbidities
- Reducing collection of low-grade adverse events and allowing minor protocol deviations
The group’s recommendations are based on discussions by the AACR COVID-19 and Cancer Task Force, in which they participated.
The American Society of Clinical Oncology is also working to leverage pandemic-related lessons to streamline care and trial planning.
ASCO’s “Road to Recovery” recommendations, published in December 2020, aim to “ensure lessons learned from the COVID-19 experience are used to craft a more equitable, accessible, and efficient clinical research system that protects patient safety, ensures scientific integrity, and maintains data quality,” the authors explained.
Dr. Flaherty and colleagues further underscore the importance of focusing on improvements going forward.
“Guided by lessons learned, many of the remote assessments and trial efficiencies deployed during the pandemic can be preserved and improved upon. We strongly encourage use of these streamlined procedures where appropriate in future prospectively designed cancer clinical trials,” they wrote.
Dr. Flaherty reported receiving personal fees from numerous pharmaceutical companies. Dr. Ribas reported receiving grants from Agilent and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Are you at legal risk for speaking at conferences?
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
Direct oral anticoagulants: Competition brought no cost relief
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.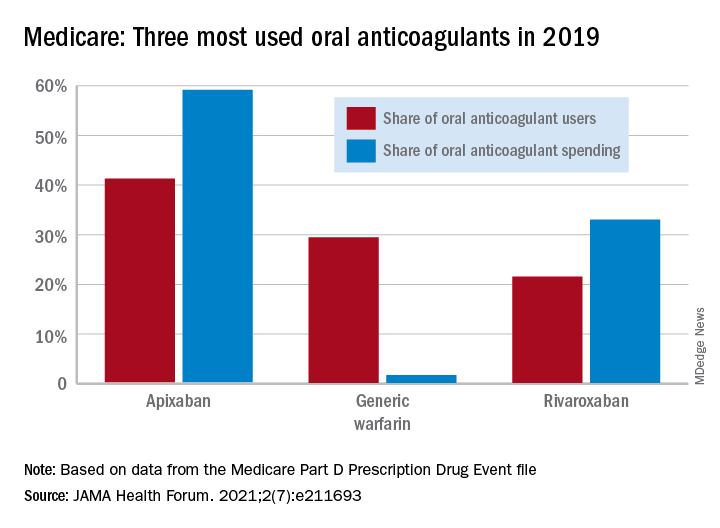
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
FROM JAMA HEALTH FORUM
Mayo, Cleveland Clinics top latest U.S. News & World Report hospital rankings
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
AMA, 55 other groups urge health care vax mandate
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
A clarion call for regulating PBMs: Health care groups, states push back on legal challenges
Mark Nelson, PharmD, recalls the anguish when a major pharmacy benefit manager (PBM) moved all veteran patients with prostate cancer at his facility from an effective medication to a pricier alternative therapy. “All of these patients were stable on their therapy and were extremely distraught about their medications being changed,” said Dr. Nelson, CEO of Northwest Medical Specialties in Washington State. While there was no clinical reason to change the medication, “our oncologists had no choice other than to comply,” he said.
It’s unclear why a PBM would switch to a more expensive medication that has no additional clinical benefit, he continued. “Why upset so many veterans? For what reason? We were not given a reason despite our very vocal protest.”
Angus B. Worthing, MD, sees these scenarios unfold every day in his rheumatology practice in the Washington, D.C., area. “In my clinic with 25 doctors, we have three full-time people that only handle PBMs,” he said in an interview. He and others in the medical community, as well as many states, have been pushing back on what they see as efforts by PBMs to raise drug prices and collect the profits at the expense of patients.
PCMA’s challenges against PBM law
The Pharmaceutical Care Management Association (PCMA), a trade group that represents PBMs, has sued at least a half dozen states on their ability to regulate PBMs. However, a landmark case in late 2020 (Pharmaceutical Care Management Association v. Rutledge) set a new precedent. Reversing a lower appeals court decision, the Supreme Court unanimously ruled in favor of allowing states to put in place fair regulation of these entities.
Dr. Worthing and others hope that the medical community and states can leverage this ruling in another lawsuit PCMA brought against North Dakota (PCMA v. Wehbi). PCMA filed this lawsuit in 2017, which challenges two statutes on PBM regulation. The group has issued similar legal challenges in Maine, the District of Columbia, Iowa, Oklahoma, and Arkansas with the Rutledge case.
“PBMs have become massive profit centers while (ironically) increasing patients’ out-of-pocket costs, interfering with doctor-patient relationships, and impairing patient access to appropriate treatment,” according to an amicus brief filed by The Alliance for Transparent & Affordable Prescriptions (ATAP), the Community Oncology Alliance (COA), and American Pharmacies, supporting North Dakota in the Wehbi case.
This is to ensure the case represents the voices of physicians, patients, nurses, and other stakeholders, and underscores PBM abuses, said Dr. Worthing, vice president of ATAP. He also serves as the American College of Rheumatology’s representative on ATAP’s Executive Committee.
PCMA did not respond to requests for comment. Its CEO and president, J.C. Scott, emphasizes that PBMs have a long track record of reducing drug costs for patients and plan sponsors. In 2021, PCMA released 21 policy solutions, a set of industry principles and a three-part policy platform, all with an aim to bring down costs and increase access to pharmaceutical care, according to the organization.
PCMA estimates that the strategies in its platform (updating Medicare Part D, accelerating value-based care, and eliminating anticompetitive ‘pay for delay’ agreements) would save the federal government a maximum of $398.7 billion over 10 years.
According to Wendy Hemmen, senior director with Texas Oncology in Dallas, PBMs do their own unique calculations to arrive at their cost reductions. “Essentially in a PBM, they use things that make their story. Numbers reported to plan sponsors and to the public are not audited and are usually in terms of percentages or a per member per month. Data points are moved around, dropped, or reclassified to make the story that the PBM needs to tell,” Ms. Hemmen said.
Amicus briefs dispute ERISA connection
North Dakota legislation prohibits PBMs from charging copays to patients that exceed the cost of a drug. It also prohibits gag clause provisions that restrict what pharmacists may discuss with patients. PBMs may charge fees based on performance metrics, but they must use nationally recognized metrics. Fees must be disclosed at the point of sale.
In its legal challenges, PCMA has asserted that state laws violate the preemption clause in the Employee Retirement Income Security Act (ERISA). “Federal preemption allows employers flexibility to administer innovative benefit plans in an environment of increasing health care costs. The court’s decision in Rutledge v. PCMA will either uphold or threaten these federal protections,” PCMA asserted in a statement issued in March 2020.
ATAP’s amicus brief, and another one filed by 34 attorneys general that supports the North Dakota statute to regulate PBMs, counter that this isn’t the case.
“First, PBM regulation (in its common and standard form) does not reference ERISA itself. These laws leave all plans on equal footing; they do not single out ERISA plans for preferred or disfavored coverage, and they do not change the playing field for ERISA plans alone ... Second, PBM regulation does not have any prohibited connection with ERISA plans,” noted authors in the ATAP brief.
PCMA has also included Medicare preemption in its arguments against PBM regulation. This is meritless, wrote the state attorneys general. “Medicare preempts state laws only if a Medicare ‘standard’ particularly addresses the subject of state regulation. Because the challenged North Dakota laws do not dictate plan benefits or conflict with a Medicare standard, they are not preempted.”
The auctioning of medications
PBMs in theory could use their market power to drive down costs by extracting discounts from drug makers and pharmacies. In reality, they retain any price concessions and discounts for themselves, ATAP’s brief continued.
A system that PBMs have put into place, called step therapy, is essentially an auction for the preferred spot that will be authorized and covered, Dr. Worthing explained.
PBMs create formularies through this auction. The highest rebate to the PBM earns the top spot in the auction and becomes the preferred drug. “That highest bid gets paid for by passing the cost along to patients and insurance plans, and PBMs pocket the profits. This provides an incentive for pharmaceutical manufacturers to raise prices,” he said.
Dr. Worthing has seen these practices trickle down and affect his patients. “Frequently, the medication I prescribe based on what’s best for the patient based on their disease activity, values, and medical history is often not covered because a different drug or portfolio of drugs has earned the top spot in step therapy. This is an extremely frustrating and cumbersome process that not only delays access to treatments but also provides an incentive for higher drug prices,” he said.
There are other ways in which PBMs get in the way of care, said Ms. Hemmen, whose facility serves complex-care oncology patients.
“PBMs force scripts out of higher-quality pharmacies that preserve unfragmented care. They incentivize plan sponsors to put programs into place that take away patient choice, fragment care, and drive scripts to their own owned pharmacies,” she said.
Rutledge case sets precedent
In the Rutledge case, PCMA had challenged an Arkansas law that forbid PBMs from paying local pharmacies at a lower rate than what the pharmacies were reimbursed to fill prescriptions. Although the 8th Circuit Court of Appeals agreed with PCMA, the Supreme Court ruled in favor of Arkansas in late 2020.
The appeals court also backed PCMA in PCMA v. Wehbi. However, the Supreme Court vacated this decision and remanded it back to the appeals court, asking for a reconsideration in wake of the outcome in Rutledge v. PCMA.
PCMA has argued that Rutledge was a narrow decision, limited to state laws that regulate PBM reimbursements, and that Rutledge has no bearing on North Dakota law.
While it’s unfortunate that PCMA is trying to delay implementation of sensible regulations, “a lot of us are happy that this issue is coming to light,” Dr. Worthing said. “As a rheumatologist and health policy advocate, exposing drug middlemen is the most important bipartisan issue in the country today because it gets at the core of making sure that sick people get access to the medications they need and reducing the budget of insurance carriers, hospitals, and the federal budget.”
The ATAP brief noted that 28 state attorneys general have filed suit against PBMs, “securing settlements compelling PBMs to correct deceptive trade practices.”
Many people at the state and local level were waiting for the Supreme Court to decide on Rutledge before enacting legislation and sensible regulations, and now they can go ahead and do it, said Dr. Worthing. “I expect to see this across the country as states look at budgets, and as patients bring personal stories to light. We look forward to states passing these kinds of laws to regulate PBMs.”
The ACR doesn’t anticipate a ruling in the Wehbi case until the spring of 2022.
Recent laws passed around PBMs and the pharmacy benefit are a good first step in holding PBMs accountable for quality of care and honoring patient choice, Ms. Hemmen said. The laws also begin to address the fiscal manipulations PBMs use to gain advantage and direct scripts to their own coffers, she added. However, this may not have enough teeth. “These state laws are coming from a provider perspective, and they don’t anticipate what PBMs will do in response. The PBMs are going to work around it.”
Mark Nelson, PharmD, recalls the anguish when a major pharmacy benefit manager (PBM) moved all veteran patients with prostate cancer at his facility from an effective medication to a pricier alternative therapy. “All of these patients were stable on their therapy and were extremely distraught about their medications being changed,” said Dr. Nelson, CEO of Northwest Medical Specialties in Washington State. While there was no clinical reason to change the medication, “our oncologists had no choice other than to comply,” he said.
It’s unclear why a PBM would switch to a more expensive medication that has no additional clinical benefit, he continued. “Why upset so many veterans? For what reason? We were not given a reason despite our very vocal protest.”
Angus B. Worthing, MD, sees these scenarios unfold every day in his rheumatology practice in the Washington, D.C., area. “In my clinic with 25 doctors, we have three full-time people that only handle PBMs,” he said in an interview. He and others in the medical community, as well as many states, have been pushing back on what they see as efforts by PBMs to raise drug prices and collect the profits at the expense of patients.
PCMA’s challenges against PBM law
The Pharmaceutical Care Management Association (PCMA), a trade group that represents PBMs, has sued at least a half dozen states on their ability to regulate PBMs. However, a landmark case in late 2020 (Pharmaceutical Care Management Association v. Rutledge) set a new precedent. Reversing a lower appeals court decision, the Supreme Court unanimously ruled in favor of allowing states to put in place fair regulation of these entities.
Dr. Worthing and others hope that the medical community and states can leverage this ruling in another lawsuit PCMA brought against North Dakota (PCMA v. Wehbi). PCMA filed this lawsuit in 2017, which challenges two statutes on PBM regulation. The group has issued similar legal challenges in Maine, the District of Columbia, Iowa, Oklahoma, and Arkansas with the Rutledge case.
“PBMs have become massive profit centers while (ironically) increasing patients’ out-of-pocket costs, interfering with doctor-patient relationships, and impairing patient access to appropriate treatment,” according to an amicus brief filed by The Alliance for Transparent & Affordable Prescriptions (ATAP), the Community Oncology Alliance (COA), and American Pharmacies, supporting North Dakota in the Wehbi case.
This is to ensure the case represents the voices of physicians, patients, nurses, and other stakeholders, and underscores PBM abuses, said Dr. Worthing, vice president of ATAP. He also serves as the American College of Rheumatology’s representative on ATAP’s Executive Committee.
PCMA did not respond to requests for comment. Its CEO and president, J.C. Scott, emphasizes that PBMs have a long track record of reducing drug costs for patients and plan sponsors. In 2021, PCMA released 21 policy solutions, a set of industry principles and a three-part policy platform, all with an aim to bring down costs and increase access to pharmaceutical care, according to the organization.
PCMA estimates that the strategies in its platform (updating Medicare Part D, accelerating value-based care, and eliminating anticompetitive ‘pay for delay’ agreements) would save the federal government a maximum of $398.7 billion over 10 years.
According to Wendy Hemmen, senior director with Texas Oncology in Dallas, PBMs do their own unique calculations to arrive at their cost reductions. “Essentially in a PBM, they use things that make their story. Numbers reported to plan sponsors and to the public are not audited and are usually in terms of percentages or a per member per month. Data points are moved around, dropped, or reclassified to make the story that the PBM needs to tell,” Ms. Hemmen said.
Amicus briefs dispute ERISA connection
North Dakota legislation prohibits PBMs from charging copays to patients that exceed the cost of a drug. It also prohibits gag clause provisions that restrict what pharmacists may discuss with patients. PBMs may charge fees based on performance metrics, but they must use nationally recognized metrics. Fees must be disclosed at the point of sale.
In its legal challenges, PCMA has asserted that state laws violate the preemption clause in the Employee Retirement Income Security Act (ERISA). “Federal preemption allows employers flexibility to administer innovative benefit plans in an environment of increasing health care costs. The court’s decision in Rutledge v. PCMA will either uphold or threaten these federal protections,” PCMA asserted in a statement issued in March 2020.
ATAP’s amicus brief, and another one filed by 34 attorneys general that supports the North Dakota statute to regulate PBMs, counter that this isn’t the case.
“First, PBM regulation (in its common and standard form) does not reference ERISA itself. These laws leave all plans on equal footing; they do not single out ERISA plans for preferred or disfavored coverage, and they do not change the playing field for ERISA plans alone ... Second, PBM regulation does not have any prohibited connection with ERISA plans,” noted authors in the ATAP brief.
PCMA has also included Medicare preemption in its arguments against PBM regulation. This is meritless, wrote the state attorneys general. “Medicare preempts state laws only if a Medicare ‘standard’ particularly addresses the subject of state regulation. Because the challenged North Dakota laws do not dictate plan benefits or conflict with a Medicare standard, they are not preempted.”
The auctioning of medications
PBMs in theory could use their market power to drive down costs by extracting discounts from drug makers and pharmacies. In reality, they retain any price concessions and discounts for themselves, ATAP’s brief continued.
A system that PBMs have put into place, called step therapy, is essentially an auction for the preferred spot that will be authorized and covered, Dr. Worthing explained.
PBMs create formularies through this auction. The highest rebate to the PBM earns the top spot in the auction and becomes the preferred drug. “That highest bid gets paid for by passing the cost along to patients and insurance plans, and PBMs pocket the profits. This provides an incentive for pharmaceutical manufacturers to raise prices,” he said.
Dr. Worthing has seen these practices trickle down and affect his patients. “Frequently, the medication I prescribe based on what’s best for the patient based on their disease activity, values, and medical history is often not covered because a different drug or portfolio of drugs has earned the top spot in step therapy. This is an extremely frustrating and cumbersome process that not only delays access to treatments but also provides an incentive for higher drug prices,” he said.
There are other ways in which PBMs get in the way of care, said Ms. Hemmen, whose facility serves complex-care oncology patients.
“PBMs force scripts out of higher-quality pharmacies that preserve unfragmented care. They incentivize plan sponsors to put programs into place that take away patient choice, fragment care, and drive scripts to their own owned pharmacies,” she said.
Rutledge case sets precedent
In the Rutledge case, PCMA had challenged an Arkansas law that forbid PBMs from paying local pharmacies at a lower rate than what the pharmacies were reimbursed to fill prescriptions. Although the 8th Circuit Court of Appeals agreed with PCMA, the Supreme Court ruled in favor of Arkansas in late 2020.
The appeals court also backed PCMA in PCMA v. Wehbi. However, the Supreme Court vacated this decision and remanded it back to the appeals court, asking for a reconsideration in wake of the outcome in Rutledge v. PCMA.
PCMA has argued that Rutledge was a narrow decision, limited to state laws that regulate PBM reimbursements, and that Rutledge has no bearing on North Dakota law.
While it’s unfortunate that PCMA is trying to delay implementation of sensible regulations, “a lot of us are happy that this issue is coming to light,” Dr. Worthing said. “As a rheumatologist and health policy advocate, exposing drug middlemen is the most important bipartisan issue in the country today because it gets at the core of making sure that sick people get access to the medications they need and reducing the budget of insurance carriers, hospitals, and the federal budget.”
The ATAP brief noted that 28 state attorneys general have filed suit against PBMs, “securing settlements compelling PBMs to correct deceptive trade practices.”
Many people at the state and local level were waiting for the Supreme Court to decide on Rutledge before enacting legislation and sensible regulations, and now they can go ahead and do it, said Dr. Worthing. “I expect to see this across the country as states look at budgets, and as patients bring personal stories to light. We look forward to states passing these kinds of laws to regulate PBMs.”
The ACR doesn’t anticipate a ruling in the Wehbi case until the spring of 2022.
Recent laws passed around PBMs and the pharmacy benefit are a good first step in holding PBMs accountable for quality of care and honoring patient choice, Ms. Hemmen said. The laws also begin to address the fiscal manipulations PBMs use to gain advantage and direct scripts to their own coffers, she added. However, this may not have enough teeth. “These state laws are coming from a provider perspective, and they don’t anticipate what PBMs will do in response. The PBMs are going to work around it.”
Mark Nelson, PharmD, recalls the anguish when a major pharmacy benefit manager (PBM) moved all veteran patients with prostate cancer at his facility from an effective medication to a pricier alternative therapy. “All of these patients were stable on their therapy and were extremely distraught about their medications being changed,” said Dr. Nelson, CEO of Northwest Medical Specialties in Washington State. While there was no clinical reason to change the medication, “our oncologists had no choice other than to comply,” he said.
It’s unclear why a PBM would switch to a more expensive medication that has no additional clinical benefit, he continued. “Why upset so many veterans? For what reason? We were not given a reason despite our very vocal protest.”
Angus B. Worthing, MD, sees these scenarios unfold every day in his rheumatology practice in the Washington, D.C., area. “In my clinic with 25 doctors, we have three full-time people that only handle PBMs,” he said in an interview. He and others in the medical community, as well as many states, have been pushing back on what they see as efforts by PBMs to raise drug prices and collect the profits at the expense of patients.
PCMA’s challenges against PBM law
The Pharmaceutical Care Management Association (PCMA), a trade group that represents PBMs, has sued at least a half dozen states on their ability to regulate PBMs. However, a landmark case in late 2020 (Pharmaceutical Care Management Association v. Rutledge) set a new precedent. Reversing a lower appeals court decision, the Supreme Court unanimously ruled in favor of allowing states to put in place fair regulation of these entities.
Dr. Worthing and others hope that the medical community and states can leverage this ruling in another lawsuit PCMA brought against North Dakota (PCMA v. Wehbi). PCMA filed this lawsuit in 2017, which challenges two statutes on PBM regulation. The group has issued similar legal challenges in Maine, the District of Columbia, Iowa, Oklahoma, and Arkansas with the Rutledge case.
“PBMs have become massive profit centers while (ironically) increasing patients’ out-of-pocket costs, interfering with doctor-patient relationships, and impairing patient access to appropriate treatment,” according to an amicus brief filed by The Alliance for Transparent & Affordable Prescriptions (ATAP), the Community Oncology Alliance (COA), and American Pharmacies, supporting North Dakota in the Wehbi case.
This is to ensure the case represents the voices of physicians, patients, nurses, and other stakeholders, and underscores PBM abuses, said Dr. Worthing, vice president of ATAP. He also serves as the American College of Rheumatology’s representative on ATAP’s Executive Committee.
PCMA did not respond to requests for comment. Its CEO and president, J.C. Scott, emphasizes that PBMs have a long track record of reducing drug costs for patients and plan sponsors. In 2021, PCMA released 21 policy solutions, a set of industry principles and a three-part policy platform, all with an aim to bring down costs and increase access to pharmaceutical care, according to the organization.
PCMA estimates that the strategies in its platform (updating Medicare Part D, accelerating value-based care, and eliminating anticompetitive ‘pay for delay’ agreements) would save the federal government a maximum of $398.7 billion over 10 years.
According to Wendy Hemmen, senior director with Texas Oncology in Dallas, PBMs do their own unique calculations to arrive at their cost reductions. “Essentially in a PBM, they use things that make their story. Numbers reported to plan sponsors and to the public are not audited and are usually in terms of percentages or a per member per month. Data points are moved around, dropped, or reclassified to make the story that the PBM needs to tell,” Ms. Hemmen said.
Amicus briefs dispute ERISA connection
North Dakota legislation prohibits PBMs from charging copays to patients that exceed the cost of a drug. It also prohibits gag clause provisions that restrict what pharmacists may discuss with patients. PBMs may charge fees based on performance metrics, but they must use nationally recognized metrics. Fees must be disclosed at the point of sale.
In its legal challenges, PCMA has asserted that state laws violate the preemption clause in the Employee Retirement Income Security Act (ERISA). “Federal preemption allows employers flexibility to administer innovative benefit plans in an environment of increasing health care costs. The court’s decision in Rutledge v. PCMA will either uphold or threaten these federal protections,” PCMA asserted in a statement issued in March 2020.
ATAP’s amicus brief, and another one filed by 34 attorneys general that supports the North Dakota statute to regulate PBMs, counter that this isn’t the case.
“First, PBM regulation (in its common and standard form) does not reference ERISA itself. These laws leave all plans on equal footing; they do not single out ERISA plans for preferred or disfavored coverage, and they do not change the playing field for ERISA plans alone ... Second, PBM regulation does not have any prohibited connection with ERISA plans,” noted authors in the ATAP brief.
PCMA has also included Medicare preemption in its arguments against PBM regulation. This is meritless, wrote the state attorneys general. “Medicare preempts state laws only if a Medicare ‘standard’ particularly addresses the subject of state regulation. Because the challenged North Dakota laws do not dictate plan benefits or conflict with a Medicare standard, they are not preempted.”
The auctioning of medications
PBMs in theory could use their market power to drive down costs by extracting discounts from drug makers and pharmacies. In reality, they retain any price concessions and discounts for themselves, ATAP’s brief continued.
A system that PBMs have put into place, called step therapy, is essentially an auction for the preferred spot that will be authorized and covered, Dr. Worthing explained.
PBMs create formularies through this auction. The highest rebate to the PBM earns the top spot in the auction and becomes the preferred drug. “That highest bid gets paid for by passing the cost along to patients and insurance plans, and PBMs pocket the profits. This provides an incentive for pharmaceutical manufacturers to raise prices,” he said.
Dr. Worthing has seen these practices trickle down and affect his patients. “Frequently, the medication I prescribe based on what’s best for the patient based on their disease activity, values, and medical history is often not covered because a different drug or portfolio of drugs has earned the top spot in step therapy. This is an extremely frustrating and cumbersome process that not only delays access to treatments but also provides an incentive for higher drug prices,” he said.
There are other ways in which PBMs get in the way of care, said Ms. Hemmen, whose facility serves complex-care oncology patients.
“PBMs force scripts out of higher-quality pharmacies that preserve unfragmented care. They incentivize plan sponsors to put programs into place that take away patient choice, fragment care, and drive scripts to their own owned pharmacies,” she said.
Rutledge case sets precedent
In the Rutledge case, PCMA had challenged an Arkansas law that forbid PBMs from paying local pharmacies at a lower rate than what the pharmacies were reimbursed to fill prescriptions. Although the 8th Circuit Court of Appeals agreed with PCMA, the Supreme Court ruled in favor of Arkansas in late 2020.
The appeals court also backed PCMA in PCMA v. Wehbi. However, the Supreme Court vacated this decision and remanded it back to the appeals court, asking for a reconsideration in wake of the outcome in Rutledge v. PCMA.
PCMA has argued that Rutledge was a narrow decision, limited to state laws that regulate PBM reimbursements, and that Rutledge has no bearing on North Dakota law.
While it’s unfortunate that PCMA is trying to delay implementation of sensible regulations, “a lot of us are happy that this issue is coming to light,” Dr. Worthing said. “As a rheumatologist and health policy advocate, exposing drug middlemen is the most important bipartisan issue in the country today because it gets at the core of making sure that sick people get access to the medications they need and reducing the budget of insurance carriers, hospitals, and the federal budget.”
The ATAP brief noted that 28 state attorneys general have filed suit against PBMs, “securing settlements compelling PBMs to correct deceptive trade practices.”
Many people at the state and local level were waiting for the Supreme Court to decide on Rutledge before enacting legislation and sensible regulations, and now they can go ahead and do it, said Dr. Worthing. “I expect to see this across the country as states look at budgets, and as patients bring personal stories to light. We look forward to states passing these kinds of laws to regulate PBMs.”
The ACR doesn’t anticipate a ruling in the Wehbi case until the spring of 2022.
Recent laws passed around PBMs and the pharmacy benefit are a good first step in holding PBMs accountable for quality of care and honoring patient choice, Ms. Hemmen said. The laws also begin to address the fiscal manipulations PBMs use to gain advantage and direct scripts to their own coffers, she added. However, this may not have enough teeth. “These state laws are coming from a provider perspective, and they don’t anticipate what PBMs will do in response. The PBMs are going to work around it.”
Lack of after care leaves cancer patients in ‘survivorship abyss’
Although prostate cancer is the second most common cancer in men worldwide, it is not a lethal cancer: Improvements in early detection and treatment have boosted the 10-year relative survival rate to 98%.
But for many men, life after treatment is an ongoing struggle.
One of the first qualitative survey studies of long-term prostate cancer survivorship in Australia found that many survivors are living with adverse effects such as urinary incontinence and sexual dysfunction and that they continue to have heightened feelings of distress.
Many of the patients surveyed said that they had not received follow-up care and felt they had been “abandoned” by their health care professionals.
One man called it “the survivorship abyss.”
“As the prevalence of prostate cancer survivors continues to grow globally, the absence of integrated shared survivorship care models into clinical practice, such as survivorship care guidelines/plans and/or interventions, will perpetuate the sense of abandonment and the overwhelming burden of care of men lost in the PC [prostate cancer] ‘survivorship abyss,’” say the authors.
The report was published online in Psycho-Oncology.
“The good news is that more men than ever before are surviving prostate cancer,” commented lead author Carolyn G. Mazariego, PhD, a research fellow at the Daffodil Center of the Cancer Council New South Wales and the University of Sydney.
“As our population grows and ages, an increasing number of men are facing these survivorship challenges, and the need for clear survivorship care guidelines becomes increasingly vital,” Dr. Mazariego told this news organization.
Details of the survey findings
To find participants for their study, the team drew upon a cohort of 578 men who were included in the 15-year follow-up phase of the longitudinal New South Wales Prostate Cancer Care and Outcomes Study (PCOS).
The researchers interviewed 37 men for the study. The majority (88.6%) had been diagnosed with localized disease, and just over half (54%) had undergone radical prostatectomy as their primary treatment.
Some expressed regret over having had surgery. One respondent, a 15-year survivor, commented: “I really didn’t know how intrusive [surgery] was as far as losing the length of my penis and its functions. To this day, I sometimes can’t believe it’s happened. It’s devastating.”
The survey also found that most survivors viewed active treatment as the only way to avoid death.
In hindsight, some questioned whether radical treatment was necessary and whether they might have been better served by active surveillance.
Many said they were never given a chance to discuss sexual dysfunction, and others said their questions sparked an awkward, limited conversation. Many said they suffered in silence.
“We know that there is a sort of ‘cycle of silence’ between patients and health care providers about sexual issues, and that was particularly true for the men we spoke to,” Dr. Mazariego said. “This cycle of silence can stem from confusion and ambiguity as to who men should speak with regarding these ongoing issues.”
“It’s just like being part of a secret society,” said one survivor. “Like you don’t know about it until you’re in it ... I don’t think people want to know if they have it [prostate cancer] or want it known. It’s all hush hush.”
Prostate cancer patients often feel uncomfortable talking about sexual function, Dr. Mazariego pointed out. “Many men instead internalized these thoughts or concerns and just ‘got on’ with life.”
One survey participant said: “Well, you just gotta deal with it [issues relating to prostate cancer]. Just got to try and wipe out the thoughts, that’s all you can do. Suck it up and carry on.”
Discussions must be part of standard of care
Dr. Mazariego emphasized that for prostate cancer survivorship to improve, conversations about the psychosocial needs of patients must be normalized and made routine. Discussions about post-treatment side effects that cause functional impairments and are stressful to relationships must be embedded into the standards of care, she added. This will allow physicians to use these kinds of conversations “as a springboard for referrals to appropriate care.”
“Patients of all ages will have concerns related to sex and sexual function, whether you ask about them or not,” said Brad Zebrack, PhD, MSW, MPH, professor at the University of Michigan School of Social Work, Ann Arbor, who was approached for comment.
At the very least, physicians should be prepared to “acknowledge and describe possible effects of therapy on sex and sexual function and follow up by describing available supports for education and either individual or couples counseling,” said Dr. Zebrack, who is also a member of the health behavior and outcomes research program at the Rogel Cancer Center.
Physicians can also refer patients to evidence-based resources and toolkits, he said. He noted that Will2Love offers self-help programs for cancer-related sexual problems and is “an outstanding resource.”
Distress screening needed
Universal psychosocial distress screening is needed to identify men with prostate cancer who have high levels of distress, argues Jeff Dunn, AO, PhD, who is CEO of the Prostate Cancer Foundation of Australia (PCFA), in Sydney.
Without this, men will not seek help for their unmet needs, Dr. Dunn noted in a 2019 editorial in the European Journal of Cancer Care.
The unmet psychosocial needs of prostate cancer patients and their partners “are highly prevalent,” said Dr. Dunn and co-author Suzanne K. Chambers, AO, PhD, dean of the Faculty of Health at the University of Technology Sydney. “...[F]or many prostate cancer survivors, the physical, social, psychological and relationship challenges will be long term, if not lifelong,” they write.
Even in Australia, where 1 in every 6 men are expected to be diagnosed with prostate cancer by age 85, efforts to improve survivorship care have not produced the desired results, they note.
In 2019, the PCFA published a monograph in which the organization recommended routine distress screenings and referrals to evidence-based psychosocial care for prostate cancer survivors. The foundation predicted that it would be “a game-changer for every Australian man impacted by the disease.”
“Yet, we still do not have a national survivorship care plan for prostate cancer survivors in Australia,” Dr. Mazariego said.
Left untreated, the psychosocial effects of prostate cancer “are considerable and certainly factors that should be considered as a threat to well-being,” she emphasized.
“Certainly, accumulation of unmet psychosocial needs increases risks for depression and suicide, which is true in both cancer and noncancer populations,” said Dr. Zebrack.
Data back this up. One study of patients with prostate cancer found that after diagnosis, 1 in 4 experienced anxiety, and 1 in 5 experienced depression. Another study found that the risk for suicide was higher among men with prostate cancer during the first year after diagnosis than among men with other solid-organ malignancies.
More recently, a 2018 population-based Australian cohort study of men in New South Wales revealed that the risk for death by suicide was 70% higher among men who had been diagnosed with prostate cancer compared with men in the general population. This risk was higher within the first year following diagnosis and was higher in men with nonlocalized disease, those who were single, those who were living in a major city, and those who were unmarried.
Dr. Mazariego and the team reporting the survey found that men who received supportive care had a greater chance of reconciling living with functional impairments. “Receiving adequate survivorship care and trusting patient-clinician relationships appeared to be associated with greater resilience and positivity in the men’s acceptance of cancer-related, long-term challenges and personal limitations,” they note.
Developing postcancer identity
Survivors also appeared to have developed a post-cancer identity that was more invested in personal relationships or in taking a more active role in their own health.
“Treating my prostate cancer gave me a second chance,” said one survivor. “Yeah, I’m a survivor, but more importantly, I’m a loving husband now. I know I wasn’t as giving back then.”
Another man admitted that before his diagnosis, he never went to the doctor. “I go much more often now,” he said. “You need to check up on yourself, at least once a year. Could have caught the cancer earlier if I was doing that.”
These comments appear to be borne out by data released last year by the American Cancer Society (ACS), which found that the number of number of cancer-related suicides in the United States was on the decline, as reported by this news organization.
Some of the biggest decreases occurred in men with prostate cancer, although prostate cancer was associated with 15% of cancer-related suicides. Only lung cancer was associated with more cancer-related suicides, at 18%.
A factor in the decrease in cancer-related suicides was likely to be an increase in the use of supportive care services, the ACS researchers commented.
“Although no causal relationship can be established, our findings suggest an evolving role of psycho-oncology care and palliative and hospice care given the promotion and increased utilization of these services among cancer patients during this period,” they commented.
The study was funded by the Cancer Institute NSW. Dr. Mazariego and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although prostate cancer is the second most common cancer in men worldwide, it is not a lethal cancer: Improvements in early detection and treatment have boosted the 10-year relative survival rate to 98%.
But for many men, life after treatment is an ongoing struggle.
One of the first qualitative survey studies of long-term prostate cancer survivorship in Australia found that many survivors are living with adverse effects such as urinary incontinence and sexual dysfunction and that they continue to have heightened feelings of distress.
Many of the patients surveyed said that they had not received follow-up care and felt they had been “abandoned” by their health care professionals.
One man called it “the survivorship abyss.”
“As the prevalence of prostate cancer survivors continues to grow globally, the absence of integrated shared survivorship care models into clinical practice, such as survivorship care guidelines/plans and/or interventions, will perpetuate the sense of abandonment and the overwhelming burden of care of men lost in the PC [prostate cancer] ‘survivorship abyss,’” say the authors.
The report was published online in Psycho-Oncology.
“The good news is that more men than ever before are surviving prostate cancer,” commented lead author Carolyn G. Mazariego, PhD, a research fellow at the Daffodil Center of the Cancer Council New South Wales and the University of Sydney.
“As our population grows and ages, an increasing number of men are facing these survivorship challenges, and the need for clear survivorship care guidelines becomes increasingly vital,” Dr. Mazariego told this news organization.
Details of the survey findings
To find participants for their study, the team drew upon a cohort of 578 men who were included in the 15-year follow-up phase of the longitudinal New South Wales Prostate Cancer Care and Outcomes Study (PCOS).
The researchers interviewed 37 men for the study. The majority (88.6%) had been diagnosed with localized disease, and just over half (54%) had undergone radical prostatectomy as their primary treatment.
Some expressed regret over having had surgery. One respondent, a 15-year survivor, commented: “I really didn’t know how intrusive [surgery] was as far as losing the length of my penis and its functions. To this day, I sometimes can’t believe it’s happened. It’s devastating.”
The survey also found that most survivors viewed active treatment as the only way to avoid death.
In hindsight, some questioned whether radical treatment was necessary and whether they might have been better served by active surveillance.
Many said they were never given a chance to discuss sexual dysfunction, and others said their questions sparked an awkward, limited conversation. Many said they suffered in silence.
“We know that there is a sort of ‘cycle of silence’ between patients and health care providers about sexual issues, and that was particularly true for the men we spoke to,” Dr. Mazariego said. “This cycle of silence can stem from confusion and ambiguity as to who men should speak with regarding these ongoing issues.”
“It’s just like being part of a secret society,” said one survivor. “Like you don’t know about it until you’re in it ... I don’t think people want to know if they have it [prostate cancer] or want it known. It’s all hush hush.”
Prostate cancer patients often feel uncomfortable talking about sexual function, Dr. Mazariego pointed out. “Many men instead internalized these thoughts or concerns and just ‘got on’ with life.”
One survey participant said: “Well, you just gotta deal with it [issues relating to prostate cancer]. Just got to try and wipe out the thoughts, that’s all you can do. Suck it up and carry on.”
Discussions must be part of standard of care
Dr. Mazariego emphasized that for prostate cancer survivorship to improve, conversations about the psychosocial needs of patients must be normalized and made routine. Discussions about post-treatment side effects that cause functional impairments and are stressful to relationships must be embedded into the standards of care, she added. This will allow physicians to use these kinds of conversations “as a springboard for referrals to appropriate care.”
“Patients of all ages will have concerns related to sex and sexual function, whether you ask about them or not,” said Brad Zebrack, PhD, MSW, MPH, professor at the University of Michigan School of Social Work, Ann Arbor, who was approached for comment.
At the very least, physicians should be prepared to “acknowledge and describe possible effects of therapy on sex and sexual function and follow up by describing available supports for education and either individual or couples counseling,” said Dr. Zebrack, who is also a member of the health behavior and outcomes research program at the Rogel Cancer Center.
Physicians can also refer patients to evidence-based resources and toolkits, he said. He noted that Will2Love offers self-help programs for cancer-related sexual problems and is “an outstanding resource.”
Distress screening needed
Universal psychosocial distress screening is needed to identify men with prostate cancer who have high levels of distress, argues Jeff Dunn, AO, PhD, who is CEO of the Prostate Cancer Foundation of Australia (PCFA), in Sydney.
Without this, men will not seek help for their unmet needs, Dr. Dunn noted in a 2019 editorial in the European Journal of Cancer Care.
The unmet psychosocial needs of prostate cancer patients and their partners “are highly prevalent,” said Dr. Dunn and co-author Suzanne K. Chambers, AO, PhD, dean of the Faculty of Health at the University of Technology Sydney. “...[F]or many prostate cancer survivors, the physical, social, psychological and relationship challenges will be long term, if not lifelong,” they write.
Even in Australia, where 1 in every 6 men are expected to be diagnosed with prostate cancer by age 85, efforts to improve survivorship care have not produced the desired results, they note.
In 2019, the PCFA published a monograph in which the organization recommended routine distress screenings and referrals to evidence-based psychosocial care for prostate cancer survivors. The foundation predicted that it would be “a game-changer for every Australian man impacted by the disease.”
“Yet, we still do not have a national survivorship care plan for prostate cancer survivors in Australia,” Dr. Mazariego said.
Left untreated, the psychosocial effects of prostate cancer “are considerable and certainly factors that should be considered as a threat to well-being,” she emphasized.
“Certainly, accumulation of unmet psychosocial needs increases risks for depression and suicide, which is true in both cancer and noncancer populations,” said Dr. Zebrack.
Data back this up. One study of patients with prostate cancer found that after diagnosis, 1 in 4 experienced anxiety, and 1 in 5 experienced depression. Another study found that the risk for suicide was higher among men with prostate cancer during the first year after diagnosis than among men with other solid-organ malignancies.
More recently, a 2018 population-based Australian cohort study of men in New South Wales revealed that the risk for death by suicide was 70% higher among men who had been diagnosed with prostate cancer compared with men in the general population. This risk was higher within the first year following diagnosis and was higher in men with nonlocalized disease, those who were single, those who were living in a major city, and those who were unmarried.
Dr. Mazariego and the team reporting the survey found that men who received supportive care had a greater chance of reconciling living with functional impairments. “Receiving adequate survivorship care and trusting patient-clinician relationships appeared to be associated with greater resilience and positivity in the men’s acceptance of cancer-related, long-term challenges and personal limitations,” they note.
Developing postcancer identity
Survivors also appeared to have developed a post-cancer identity that was more invested in personal relationships or in taking a more active role in their own health.
“Treating my prostate cancer gave me a second chance,” said one survivor. “Yeah, I’m a survivor, but more importantly, I’m a loving husband now. I know I wasn’t as giving back then.”
Another man admitted that before his diagnosis, he never went to the doctor. “I go much more often now,” he said. “You need to check up on yourself, at least once a year. Could have caught the cancer earlier if I was doing that.”
These comments appear to be borne out by data released last year by the American Cancer Society (ACS), which found that the number of number of cancer-related suicides in the United States was on the decline, as reported by this news organization.
Some of the biggest decreases occurred in men with prostate cancer, although prostate cancer was associated with 15% of cancer-related suicides. Only lung cancer was associated with more cancer-related suicides, at 18%.
A factor in the decrease in cancer-related suicides was likely to be an increase in the use of supportive care services, the ACS researchers commented.
“Although no causal relationship can be established, our findings suggest an evolving role of psycho-oncology care and palliative and hospice care given the promotion and increased utilization of these services among cancer patients during this period,” they commented.
The study was funded by the Cancer Institute NSW. Dr. Mazariego and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although prostate cancer is the second most common cancer in men worldwide, it is not a lethal cancer: Improvements in early detection and treatment have boosted the 10-year relative survival rate to 98%.
But for many men, life after treatment is an ongoing struggle.
One of the first qualitative survey studies of long-term prostate cancer survivorship in Australia found that many survivors are living with adverse effects such as urinary incontinence and sexual dysfunction and that they continue to have heightened feelings of distress.
Many of the patients surveyed said that they had not received follow-up care and felt they had been “abandoned” by their health care professionals.
One man called it “the survivorship abyss.”
“As the prevalence of prostate cancer survivors continues to grow globally, the absence of integrated shared survivorship care models into clinical practice, such as survivorship care guidelines/plans and/or interventions, will perpetuate the sense of abandonment and the overwhelming burden of care of men lost in the PC [prostate cancer] ‘survivorship abyss,’” say the authors.
The report was published online in Psycho-Oncology.
“The good news is that more men than ever before are surviving prostate cancer,” commented lead author Carolyn G. Mazariego, PhD, a research fellow at the Daffodil Center of the Cancer Council New South Wales and the University of Sydney.
“As our population grows and ages, an increasing number of men are facing these survivorship challenges, and the need for clear survivorship care guidelines becomes increasingly vital,” Dr. Mazariego told this news organization.
Details of the survey findings
To find participants for their study, the team drew upon a cohort of 578 men who were included in the 15-year follow-up phase of the longitudinal New South Wales Prostate Cancer Care and Outcomes Study (PCOS).
The researchers interviewed 37 men for the study. The majority (88.6%) had been diagnosed with localized disease, and just over half (54%) had undergone radical prostatectomy as their primary treatment.
Some expressed regret over having had surgery. One respondent, a 15-year survivor, commented: “I really didn’t know how intrusive [surgery] was as far as losing the length of my penis and its functions. To this day, I sometimes can’t believe it’s happened. It’s devastating.”
The survey also found that most survivors viewed active treatment as the only way to avoid death.
In hindsight, some questioned whether radical treatment was necessary and whether they might have been better served by active surveillance.
Many said they were never given a chance to discuss sexual dysfunction, and others said their questions sparked an awkward, limited conversation. Many said they suffered in silence.
“We know that there is a sort of ‘cycle of silence’ between patients and health care providers about sexual issues, and that was particularly true for the men we spoke to,” Dr. Mazariego said. “This cycle of silence can stem from confusion and ambiguity as to who men should speak with regarding these ongoing issues.”
“It’s just like being part of a secret society,” said one survivor. “Like you don’t know about it until you’re in it ... I don’t think people want to know if they have it [prostate cancer] or want it known. It’s all hush hush.”
Prostate cancer patients often feel uncomfortable talking about sexual function, Dr. Mazariego pointed out. “Many men instead internalized these thoughts or concerns and just ‘got on’ with life.”
One survey participant said: “Well, you just gotta deal with it [issues relating to prostate cancer]. Just got to try and wipe out the thoughts, that’s all you can do. Suck it up and carry on.”
Discussions must be part of standard of care
Dr. Mazariego emphasized that for prostate cancer survivorship to improve, conversations about the psychosocial needs of patients must be normalized and made routine. Discussions about post-treatment side effects that cause functional impairments and are stressful to relationships must be embedded into the standards of care, she added. This will allow physicians to use these kinds of conversations “as a springboard for referrals to appropriate care.”
“Patients of all ages will have concerns related to sex and sexual function, whether you ask about them or not,” said Brad Zebrack, PhD, MSW, MPH, professor at the University of Michigan School of Social Work, Ann Arbor, who was approached for comment.
At the very least, physicians should be prepared to “acknowledge and describe possible effects of therapy on sex and sexual function and follow up by describing available supports for education and either individual or couples counseling,” said Dr. Zebrack, who is also a member of the health behavior and outcomes research program at the Rogel Cancer Center.
Physicians can also refer patients to evidence-based resources and toolkits, he said. He noted that Will2Love offers self-help programs for cancer-related sexual problems and is “an outstanding resource.”
Distress screening needed
Universal psychosocial distress screening is needed to identify men with prostate cancer who have high levels of distress, argues Jeff Dunn, AO, PhD, who is CEO of the Prostate Cancer Foundation of Australia (PCFA), in Sydney.
Without this, men will not seek help for their unmet needs, Dr. Dunn noted in a 2019 editorial in the European Journal of Cancer Care.
The unmet psychosocial needs of prostate cancer patients and their partners “are highly prevalent,” said Dr. Dunn and co-author Suzanne K. Chambers, AO, PhD, dean of the Faculty of Health at the University of Technology Sydney. “...[F]or many prostate cancer survivors, the physical, social, psychological and relationship challenges will be long term, if not lifelong,” they write.
Even in Australia, where 1 in every 6 men are expected to be diagnosed with prostate cancer by age 85, efforts to improve survivorship care have not produced the desired results, they note.
In 2019, the PCFA published a monograph in which the organization recommended routine distress screenings and referrals to evidence-based psychosocial care for prostate cancer survivors. The foundation predicted that it would be “a game-changer for every Australian man impacted by the disease.”
“Yet, we still do not have a national survivorship care plan for prostate cancer survivors in Australia,” Dr. Mazariego said.
Left untreated, the psychosocial effects of prostate cancer “are considerable and certainly factors that should be considered as a threat to well-being,” she emphasized.
“Certainly, accumulation of unmet psychosocial needs increases risks for depression and suicide, which is true in both cancer and noncancer populations,” said Dr. Zebrack.
Data back this up. One study of patients with prostate cancer found that after diagnosis, 1 in 4 experienced anxiety, and 1 in 5 experienced depression. Another study found that the risk for suicide was higher among men with prostate cancer during the first year after diagnosis than among men with other solid-organ malignancies.
More recently, a 2018 population-based Australian cohort study of men in New South Wales revealed that the risk for death by suicide was 70% higher among men who had been diagnosed with prostate cancer compared with men in the general population. This risk was higher within the first year following diagnosis and was higher in men with nonlocalized disease, those who were single, those who were living in a major city, and those who were unmarried.
Dr. Mazariego and the team reporting the survey found that men who received supportive care had a greater chance of reconciling living with functional impairments. “Receiving adequate survivorship care and trusting patient-clinician relationships appeared to be associated with greater resilience and positivity in the men’s acceptance of cancer-related, long-term challenges and personal limitations,” they note.
Developing postcancer identity
Survivors also appeared to have developed a post-cancer identity that was more invested in personal relationships or in taking a more active role in their own health.
“Treating my prostate cancer gave me a second chance,” said one survivor. “Yeah, I’m a survivor, but more importantly, I’m a loving husband now. I know I wasn’t as giving back then.”
Another man admitted that before his diagnosis, he never went to the doctor. “I go much more often now,” he said. “You need to check up on yourself, at least once a year. Could have caught the cancer earlier if I was doing that.”
These comments appear to be borne out by data released last year by the American Cancer Society (ACS), which found that the number of number of cancer-related suicides in the United States was on the decline, as reported by this news organization.
Some of the biggest decreases occurred in men with prostate cancer, although prostate cancer was associated with 15% of cancer-related suicides. Only lung cancer was associated with more cancer-related suicides, at 18%.
A factor in the decrease in cancer-related suicides was likely to be an increase in the use of supportive care services, the ACS researchers commented.
“Although no causal relationship can be established, our findings suggest an evolving role of psycho-oncology care and palliative and hospice care given the promotion and increased utilization of these services among cancer patients during this period,” they commented.
The study was funded by the Cancer Institute NSW. Dr. Mazariego and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hematologic cancer increases risk of delivery complications
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
Lucid abductions and Candy Crush addiction
I dream of alien abductions
There he goes! It’s lunchtime and your colleague Tom is going on and on again about that time he was abducted by aliens. It sounds ridiculous, but he does make some convincing arguments. Tom thinks it was real, but could it have all just been in his head?
Lucid dreaming may help explain alleged alien abductions. During a lucid dream, people know that they’re dreaming, and can also have some control over how the dreams play out. During some dream states, a person can feel intense sensations, such as terror and paralysis, so it’s no wonder these dreams feel so real.
In a recent study, scientists encouraged 152 participants who had self-identified as lucid dreamers to dream about aliens. Many (75%) of the participants were able to dream about alien encounters, and 15% “achieved relatively realistic experiences,” the investigators reported.
So cut Tom some slack. He’s not crazy, he might just have lucid dreaming privileges. Tell him he should dream about something more fun, like a vacation in the Bahamas.
Follow your heart: Drink more coffee
It seems like the world is divided into coffee drinkers and non–coffee drinkers. Then there’s decaf and regular drinkers. Whichever camp you fall into, know this: The widespread belief that caffeine consumption has an effect on your heart is all beans.
In what is the largest investigation of its kind, researchers from the University of California, San Francisco, looked into whether drinking caffeinated coffee was linked to a risk for heart arrhythmia. They also researched whether patients with genetic variants that affect their metabolism could change that association. Almost 400,000 people with a mean age of 56 years participated in the study. More than half of the participants were women.
The investigators analyzed the participants’ self-reported coffee consumption using a technique called Mendelian randomization to leverage genetic data with the participants’ relationship with caffeine, making it an even field and not relying on the participant consumption self-reporting for outcomes as in previous studies.
What they found, after the 4-year follow up, was nothing short of myth busting.
“We found no evidence that caffeine consumption leads to a greater risk of arrhythmias,” said senior and corresponding author Gregory Marcus, MD. “Our population-based study provides reassurance that common prohibitions against caffeine to reduce arrhythmia risk are likely unwarranted.”
There was no evidence of a heightened risk of arrhythmias in participants who were genetically predisposed to metabolize caffeine differently from those who were not. And, there was a 3% reduction of arrhythmias in patients who consumed higher amounts of coffee.
We are not lobbying for Big Caffeine, but this study adds to the reported health benefits linked to coffee, which already include reduced risk for cancer, diabetes, and Parkinson’s disease, with an added bonus of anti-inflammatory benefits. So, the next time you’re hesitant to pour that second cup of Joe, just go for it. Your heart can take it.
Bored? Feeling down? Don’t play Candy Crush
Now hang on, aren’t those the perfect times to play video games? If there’s nothing else to do, why not open Candy Crush and mindlessly power through the levels?
Because, according to a study by a group of Canadian researchers, it’s actually the worst thing you can do. Well, maybe not literally, but it’s not helpful. Researchers recruited 60 Candy Crush players who were at various levels in the game. They had the participants play early levels that were far too easy or levels balanced with their gameplay abilities.
Players in the easy-level group got bored and quit far earlier than did those in the advanced-level group. The group playing to their abilities were able to access a “flow” state and focus all their attention on the game. While this is all well and good for their gaming performance, according to the researchers, it confirms the theory that playing to escape boredom or negative emotions is more likely to lead to addiction. As with all addictions, the temporary high can give way to a self-repeating loop, causing patients to ignore real life and deepen depression.
The researchers hope their findings will encourage game developers to “consider implementing responsible video gaming tools directly within their games.” Comedy gold. Perhaps Canadians’ idea of capitalism is a little different from that of those south of the border.
Hiccups and vaccine refusal
Tonight, LOTME News dives into the fetid cesspool that is international politics and comes out with … hiccups?
But first, a word from our sponsor, Fearless Boxing Club of South Etobicoke, Ontario.
Are you looking to flout public health restrictions? Do you want to spend time in an enclosed space with other people who haven’t gotten the COVID-19 vaccine? Do you “feel safer waiting until more research is done on the side effects being discovered right now”? (We are not making this up.)
Then join the Fearless Boxing Club, because we “will not be accepting any vaccinated members.” Our founders, Mohammed Abedeen and Krystal Glazier-Roscoe, are working hard to exclude “those who received the experimental COVID vaccine.” (Still not making it up.)
And now, back to the news.
Brazilian president Jair Bolsonaro was hospitalized recently for a severe case of hiccups that may have been related to a stab wound he received in 2018. [Nope, didn’t make that up, either.]
Mr. Bolsonaro had been hiccuping for 10 days, and was experiencing abdominal pain and difficulty speaking, when he entered the hospital on July 14. Since being stabbed while on the campaign trail, he has undergone several operations, which may have led to the partial intestinal obstruction that caused his latest symptoms.
His medical team advised Mr. Bolsonaro to go on a diet to aid his recovery, but when he was released on July 18 he said, “I hope in 10 days I’ll be eating barbecued ribs.” (Maybe this is all just a lucid dream. Probably shouldn’t have had ribs right before bed.)
I dream of alien abductions
There he goes! It’s lunchtime and your colleague Tom is going on and on again about that time he was abducted by aliens. It sounds ridiculous, but he does make some convincing arguments. Tom thinks it was real, but could it have all just been in his head?
Lucid dreaming may help explain alleged alien abductions. During a lucid dream, people know that they’re dreaming, and can also have some control over how the dreams play out. During some dream states, a person can feel intense sensations, such as terror and paralysis, so it’s no wonder these dreams feel so real.
In a recent study, scientists encouraged 152 participants who had self-identified as lucid dreamers to dream about aliens. Many (75%) of the participants were able to dream about alien encounters, and 15% “achieved relatively realistic experiences,” the investigators reported.
So cut Tom some slack. He’s not crazy, he might just have lucid dreaming privileges. Tell him he should dream about something more fun, like a vacation in the Bahamas.
Follow your heart: Drink more coffee
It seems like the world is divided into coffee drinkers and non–coffee drinkers. Then there’s decaf and regular drinkers. Whichever camp you fall into, know this: The widespread belief that caffeine consumption has an effect on your heart is all beans.
In what is the largest investigation of its kind, researchers from the University of California, San Francisco, looked into whether drinking caffeinated coffee was linked to a risk for heart arrhythmia. They also researched whether patients with genetic variants that affect their metabolism could change that association. Almost 400,000 people with a mean age of 56 years participated in the study. More than half of the participants were women.
The investigators analyzed the participants’ self-reported coffee consumption using a technique called Mendelian randomization to leverage genetic data with the participants’ relationship with caffeine, making it an even field and not relying on the participant consumption self-reporting for outcomes as in previous studies.
What they found, after the 4-year follow up, was nothing short of myth busting.
“We found no evidence that caffeine consumption leads to a greater risk of arrhythmias,” said senior and corresponding author Gregory Marcus, MD. “Our population-based study provides reassurance that common prohibitions against caffeine to reduce arrhythmia risk are likely unwarranted.”
There was no evidence of a heightened risk of arrhythmias in participants who were genetically predisposed to metabolize caffeine differently from those who were not. And, there was a 3% reduction of arrhythmias in patients who consumed higher amounts of coffee.
We are not lobbying for Big Caffeine, but this study adds to the reported health benefits linked to coffee, which already include reduced risk for cancer, diabetes, and Parkinson’s disease, with an added bonus of anti-inflammatory benefits. So, the next time you’re hesitant to pour that second cup of Joe, just go for it. Your heart can take it.
Bored? Feeling down? Don’t play Candy Crush
Now hang on, aren’t those the perfect times to play video games? If there’s nothing else to do, why not open Candy Crush and mindlessly power through the levels?
Because, according to a study by a group of Canadian researchers, it’s actually the worst thing you can do. Well, maybe not literally, but it’s not helpful. Researchers recruited 60 Candy Crush players who were at various levels in the game. They had the participants play early levels that were far too easy or levels balanced with their gameplay abilities.
Players in the easy-level group got bored and quit far earlier than did those in the advanced-level group. The group playing to their abilities were able to access a “flow” state and focus all their attention on the game. While this is all well and good for their gaming performance, according to the researchers, it confirms the theory that playing to escape boredom or negative emotions is more likely to lead to addiction. As with all addictions, the temporary high can give way to a self-repeating loop, causing patients to ignore real life and deepen depression.
The researchers hope their findings will encourage game developers to “consider implementing responsible video gaming tools directly within their games.” Comedy gold. Perhaps Canadians’ idea of capitalism is a little different from that of those south of the border.
Hiccups and vaccine refusal
Tonight, LOTME News dives into the fetid cesspool that is international politics and comes out with … hiccups?
But first, a word from our sponsor, Fearless Boxing Club of South Etobicoke, Ontario.
Are you looking to flout public health restrictions? Do you want to spend time in an enclosed space with other people who haven’t gotten the COVID-19 vaccine? Do you “feel safer waiting until more research is done on the side effects being discovered right now”? (We are not making this up.)
Then join the Fearless Boxing Club, because we “will not be accepting any vaccinated members.” Our founders, Mohammed Abedeen and Krystal Glazier-Roscoe, are working hard to exclude “those who received the experimental COVID vaccine.” (Still not making it up.)
And now, back to the news.
Brazilian president Jair Bolsonaro was hospitalized recently for a severe case of hiccups that may have been related to a stab wound he received in 2018. [Nope, didn’t make that up, either.]
Mr. Bolsonaro had been hiccuping for 10 days, and was experiencing abdominal pain and difficulty speaking, when he entered the hospital on July 14. Since being stabbed while on the campaign trail, he has undergone several operations, which may have led to the partial intestinal obstruction that caused his latest symptoms.
His medical team advised Mr. Bolsonaro to go on a diet to aid his recovery, but when he was released on July 18 he said, “I hope in 10 days I’ll be eating barbecued ribs.” (Maybe this is all just a lucid dream. Probably shouldn’t have had ribs right before bed.)
I dream of alien abductions
There he goes! It’s lunchtime and your colleague Tom is going on and on again about that time he was abducted by aliens. It sounds ridiculous, but he does make some convincing arguments. Tom thinks it was real, but could it have all just been in his head?
Lucid dreaming may help explain alleged alien abductions. During a lucid dream, people know that they’re dreaming, and can also have some control over how the dreams play out. During some dream states, a person can feel intense sensations, such as terror and paralysis, so it’s no wonder these dreams feel so real.
In a recent study, scientists encouraged 152 participants who had self-identified as lucid dreamers to dream about aliens. Many (75%) of the participants were able to dream about alien encounters, and 15% “achieved relatively realistic experiences,” the investigators reported.
So cut Tom some slack. He’s not crazy, he might just have lucid dreaming privileges. Tell him he should dream about something more fun, like a vacation in the Bahamas.
Follow your heart: Drink more coffee
It seems like the world is divided into coffee drinkers and non–coffee drinkers. Then there’s decaf and regular drinkers. Whichever camp you fall into, know this: The widespread belief that caffeine consumption has an effect on your heart is all beans.
In what is the largest investigation of its kind, researchers from the University of California, San Francisco, looked into whether drinking caffeinated coffee was linked to a risk for heart arrhythmia. They also researched whether patients with genetic variants that affect their metabolism could change that association. Almost 400,000 people with a mean age of 56 years participated in the study. More than half of the participants were women.
The investigators analyzed the participants’ self-reported coffee consumption using a technique called Mendelian randomization to leverage genetic data with the participants’ relationship with caffeine, making it an even field and not relying on the participant consumption self-reporting for outcomes as in previous studies.
What they found, after the 4-year follow up, was nothing short of myth busting.
“We found no evidence that caffeine consumption leads to a greater risk of arrhythmias,” said senior and corresponding author Gregory Marcus, MD. “Our population-based study provides reassurance that common prohibitions against caffeine to reduce arrhythmia risk are likely unwarranted.”
There was no evidence of a heightened risk of arrhythmias in participants who were genetically predisposed to metabolize caffeine differently from those who were not. And, there was a 3% reduction of arrhythmias in patients who consumed higher amounts of coffee.
We are not lobbying for Big Caffeine, but this study adds to the reported health benefits linked to coffee, which already include reduced risk for cancer, diabetes, and Parkinson’s disease, with an added bonus of anti-inflammatory benefits. So, the next time you’re hesitant to pour that second cup of Joe, just go for it. Your heart can take it.
Bored? Feeling down? Don’t play Candy Crush
Now hang on, aren’t those the perfect times to play video games? If there’s nothing else to do, why not open Candy Crush and mindlessly power through the levels?
Because, according to a study by a group of Canadian researchers, it’s actually the worst thing you can do. Well, maybe not literally, but it’s not helpful. Researchers recruited 60 Candy Crush players who were at various levels in the game. They had the participants play early levels that were far too easy or levels balanced with their gameplay abilities.
Players in the easy-level group got bored and quit far earlier than did those in the advanced-level group. The group playing to their abilities were able to access a “flow” state and focus all their attention on the game. While this is all well and good for their gaming performance, according to the researchers, it confirms the theory that playing to escape boredom or negative emotions is more likely to lead to addiction. As with all addictions, the temporary high can give way to a self-repeating loop, causing patients to ignore real life and deepen depression.
The researchers hope their findings will encourage game developers to “consider implementing responsible video gaming tools directly within their games.” Comedy gold. Perhaps Canadians’ idea of capitalism is a little different from that of those south of the border.
Hiccups and vaccine refusal
Tonight, LOTME News dives into the fetid cesspool that is international politics and comes out with … hiccups?
But first, a word from our sponsor, Fearless Boxing Club of South Etobicoke, Ontario.
Are you looking to flout public health restrictions? Do you want to spend time in an enclosed space with other people who haven’t gotten the COVID-19 vaccine? Do you “feel safer waiting until more research is done on the side effects being discovered right now”? (We are not making this up.)
Then join the Fearless Boxing Club, because we “will not be accepting any vaccinated members.” Our founders, Mohammed Abedeen and Krystal Glazier-Roscoe, are working hard to exclude “those who received the experimental COVID vaccine.” (Still not making it up.)
And now, back to the news.
Brazilian president Jair Bolsonaro was hospitalized recently for a severe case of hiccups that may have been related to a stab wound he received in 2018. [Nope, didn’t make that up, either.]
Mr. Bolsonaro had been hiccuping for 10 days, and was experiencing abdominal pain and difficulty speaking, when he entered the hospital on July 14. Since being stabbed while on the campaign trail, he has undergone several operations, which may have led to the partial intestinal obstruction that caused his latest symptoms.
His medical team advised Mr. Bolsonaro to go on a diet to aid his recovery, but when he was released on July 18 he said, “I hope in 10 days I’ll be eating barbecued ribs.” (Maybe this is all just a lucid dream. Probably shouldn’t have had ribs right before bed.)









