User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
How nonadherence complicates cardiology, in two trials
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Each study adds new twist
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Gut metabolites may explain red meat–ASCVD link
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
FROM ATHEROSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Sexual dysfunction, hair loss linked with long COVID
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Should you sell your practice to a private equity firm?
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.
Obesity drug shortage triggers frustrations, workarounds
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
More evidence salt substitutes lower risk of CVD and death
Dietary salt substitutes not only lower blood pressure but also have a clear impact on hard clinical endpoints, lowering the risk of myocardial infarction (MI), stroke, and death from all causes and cardiovascular disease (CVD), a meta-analysis shows.
The blood pressure–mediated protective effects of salt substitutes on CVD and death are likely to apply to the roughly 1.28 billion people around the world who have high blood pressure, the researchers say.
“These findings are unlikely to reflect the play of chance and support the adoption of salt substitutes in clinical practice and public health policy as a strategy to reduce dietary sodium intake, increase dietary potassium intake, lower blood pressure, and prevent major cardiovascular events,” they write.
The study was published online in Heart.
Strong support for landmark study
In salt substitutes, a proportion of sodium chloride is replaced with potassium chloride. They are known to help lower blood pressure, but less is known about their impact on hard clinical endpoints, Maoyi Tian, PhD, with Harbin Medical University, China, and the George Institute for Global Health, Sydney, and colleagues note in their article.
In the landmark Salt Substitute and Stroke Study (SSaSS), salt substitutes cut the risk of MI, stroke, and early death, as reported previously by this news organization.
But SSaSS was conducted in China, and it was unclear whether these benefits would apply to people in other parts of the world.
To investigate, Dr. Tian and colleagues pooled data from 21 relevant parallel-group, step-wedge, or cluster randomized controlled trials published through August 2021, with 31,949 participants. The trials were conducted in Europe, the Western Pacific Region, the Americas, and South East Asia and reported the effect of a salt substitute on blood pressure or clinical outcomes.
A meta-analysis of blood pressure data from 19 trials that included 29,528 participants showed that salt substitutes lowered systolic blood pressure (SBP) by 4.61 mm Hg (95% confidence interval, −6.07 to −3.14) and diastolic blood pressure (DBP) by 1.61 mm Hg (95% CI, −2.42 to −0.79).
The proportion of sodium chloride in the salt substitutes varied from 33% to 75%; the proportion of potassium ranged from 25% to 65%.
Each 10% lower proportion of sodium chloride in the salt substitute was associated with a 1.53 mm Hg (95% CI, −3.02 to −0.03; P = .045) greater reduction in SBP and a 0.95 mm Hg (95% CI, −1.78 to −0.12; P = .025) greater reduction in DBP.
Reductions in blood pressure appeared consistent, irrespective of country, age, sex, history of high blood pressure, weight, baseline blood pressure, and baseline levels of urinary sodium and potassium.
Clear benefit on hard outcomes
Pooled data on clinical outcomes from five trials that included 24,306 participants, mostly from the SSaSS, showed clear protective effects of salt substitutes on total mortality (risk ratio, 0.89; 95% CI, 0.85-0.94), CV mortality (RR, 0.87; 95% CI, 0.81-0.94), and CV events (RR, 0.89; 95% CI, 0.85-0.94).
Dr. Tian and colleagues say that “broader population use of salt substitute is supported by the absence of any detectable adverse effect of salt substitutes on hyperkalemia in this review.”
They note, however, that all of the trials took “pragmatic steps to exclude participants at elevated risk of hyperkalemia, seeking to exclude those with chronic kidney disease or using medications that elevate serum potassium.”
Offering perspective on the study, Harlan Krumholz, MD, with Yale New Haven Hospital and Yale School of Medicine, both in New Haven, Conn., said it provides “useful information by bringing together the trial evidence on salt substitutes. The evidence is dominated by the SSaSS, but the others add context.”
Dr. Krumholz said that at this point, he thinks salt substitutes “could be included in recommendations to patients.”
“SSaSS was conducted in villages in China, so that is where the evidence is strongest and most relevant, but this is a low-cost and seemingly safe strategy that could be tried by anyone without contraindications, such as kidney disease or taking a potassium-sparing medication or potassium supplement,” Dr. Krumholz told this news organization.
Johanna Contreras, MD, heart failure and transplant cardiologist at the Mount Sinai Hospital, New York, agrees that in the absence of contraindications, salt substitutes should be recommended.
“Americans put salt on everything and don’t even think about it. The salt substitutes are very helpful,” Dr. Contreras said in an interview.
“People who don’t have high blood pressure should limit salt intake, because what we have seen is that if you have high blood pressure in your family – even if you don’t have high blood pressure in your 20s or 30s – you’re likely to develop high blood pressure,” Dr. Contreras said.
“Therefore, it’s wise early on to start protecting yourself and using low salt and salt substitutes,” she added.
The study had no specific funding. Dr. Tian, Dr. Krumholz, and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dietary salt substitutes not only lower blood pressure but also have a clear impact on hard clinical endpoints, lowering the risk of myocardial infarction (MI), stroke, and death from all causes and cardiovascular disease (CVD), a meta-analysis shows.
The blood pressure–mediated protective effects of salt substitutes on CVD and death are likely to apply to the roughly 1.28 billion people around the world who have high blood pressure, the researchers say.
“These findings are unlikely to reflect the play of chance and support the adoption of salt substitutes in clinical practice and public health policy as a strategy to reduce dietary sodium intake, increase dietary potassium intake, lower blood pressure, and prevent major cardiovascular events,” they write.
The study was published online in Heart.
Strong support for landmark study
In salt substitutes, a proportion of sodium chloride is replaced with potassium chloride. They are known to help lower blood pressure, but less is known about their impact on hard clinical endpoints, Maoyi Tian, PhD, with Harbin Medical University, China, and the George Institute for Global Health, Sydney, and colleagues note in their article.
In the landmark Salt Substitute and Stroke Study (SSaSS), salt substitutes cut the risk of MI, stroke, and early death, as reported previously by this news organization.
But SSaSS was conducted in China, and it was unclear whether these benefits would apply to people in other parts of the world.
To investigate, Dr. Tian and colleagues pooled data from 21 relevant parallel-group, step-wedge, or cluster randomized controlled trials published through August 2021, with 31,949 participants. The trials were conducted in Europe, the Western Pacific Region, the Americas, and South East Asia and reported the effect of a salt substitute on blood pressure or clinical outcomes.
A meta-analysis of blood pressure data from 19 trials that included 29,528 participants showed that salt substitutes lowered systolic blood pressure (SBP) by 4.61 mm Hg (95% confidence interval, −6.07 to −3.14) and diastolic blood pressure (DBP) by 1.61 mm Hg (95% CI, −2.42 to −0.79).
The proportion of sodium chloride in the salt substitutes varied from 33% to 75%; the proportion of potassium ranged from 25% to 65%.
Each 10% lower proportion of sodium chloride in the salt substitute was associated with a 1.53 mm Hg (95% CI, −3.02 to −0.03; P = .045) greater reduction in SBP and a 0.95 mm Hg (95% CI, −1.78 to −0.12; P = .025) greater reduction in DBP.
Reductions in blood pressure appeared consistent, irrespective of country, age, sex, history of high blood pressure, weight, baseline blood pressure, and baseline levels of urinary sodium and potassium.
Clear benefit on hard outcomes
Pooled data on clinical outcomes from five trials that included 24,306 participants, mostly from the SSaSS, showed clear protective effects of salt substitutes on total mortality (risk ratio, 0.89; 95% CI, 0.85-0.94), CV mortality (RR, 0.87; 95% CI, 0.81-0.94), and CV events (RR, 0.89; 95% CI, 0.85-0.94).
Dr. Tian and colleagues say that “broader population use of salt substitute is supported by the absence of any detectable adverse effect of salt substitutes on hyperkalemia in this review.”
They note, however, that all of the trials took “pragmatic steps to exclude participants at elevated risk of hyperkalemia, seeking to exclude those with chronic kidney disease or using medications that elevate serum potassium.”
Offering perspective on the study, Harlan Krumholz, MD, with Yale New Haven Hospital and Yale School of Medicine, both in New Haven, Conn., said it provides “useful information by bringing together the trial evidence on salt substitutes. The evidence is dominated by the SSaSS, but the others add context.”
Dr. Krumholz said that at this point, he thinks salt substitutes “could be included in recommendations to patients.”
“SSaSS was conducted in villages in China, so that is where the evidence is strongest and most relevant, but this is a low-cost and seemingly safe strategy that could be tried by anyone without contraindications, such as kidney disease or taking a potassium-sparing medication or potassium supplement,” Dr. Krumholz told this news organization.
Johanna Contreras, MD, heart failure and transplant cardiologist at the Mount Sinai Hospital, New York, agrees that in the absence of contraindications, salt substitutes should be recommended.
“Americans put salt on everything and don’t even think about it. The salt substitutes are very helpful,” Dr. Contreras said in an interview.
“People who don’t have high blood pressure should limit salt intake, because what we have seen is that if you have high blood pressure in your family – even if you don’t have high blood pressure in your 20s or 30s – you’re likely to develop high blood pressure,” Dr. Contreras said.
“Therefore, it’s wise early on to start protecting yourself and using low salt and salt substitutes,” she added.
The study had no specific funding. Dr. Tian, Dr. Krumholz, and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dietary salt substitutes not only lower blood pressure but also have a clear impact on hard clinical endpoints, lowering the risk of myocardial infarction (MI), stroke, and death from all causes and cardiovascular disease (CVD), a meta-analysis shows.
The blood pressure–mediated protective effects of salt substitutes on CVD and death are likely to apply to the roughly 1.28 billion people around the world who have high blood pressure, the researchers say.
“These findings are unlikely to reflect the play of chance and support the adoption of salt substitutes in clinical practice and public health policy as a strategy to reduce dietary sodium intake, increase dietary potassium intake, lower blood pressure, and prevent major cardiovascular events,” they write.
The study was published online in Heart.
Strong support for landmark study
In salt substitutes, a proportion of sodium chloride is replaced with potassium chloride. They are known to help lower blood pressure, but less is known about their impact on hard clinical endpoints, Maoyi Tian, PhD, with Harbin Medical University, China, and the George Institute for Global Health, Sydney, and colleagues note in their article.
In the landmark Salt Substitute and Stroke Study (SSaSS), salt substitutes cut the risk of MI, stroke, and early death, as reported previously by this news organization.
But SSaSS was conducted in China, and it was unclear whether these benefits would apply to people in other parts of the world.
To investigate, Dr. Tian and colleagues pooled data from 21 relevant parallel-group, step-wedge, or cluster randomized controlled trials published through August 2021, with 31,949 participants. The trials were conducted in Europe, the Western Pacific Region, the Americas, and South East Asia and reported the effect of a salt substitute on blood pressure or clinical outcomes.
A meta-analysis of blood pressure data from 19 trials that included 29,528 participants showed that salt substitutes lowered systolic blood pressure (SBP) by 4.61 mm Hg (95% confidence interval, −6.07 to −3.14) and diastolic blood pressure (DBP) by 1.61 mm Hg (95% CI, −2.42 to −0.79).
The proportion of sodium chloride in the salt substitutes varied from 33% to 75%; the proportion of potassium ranged from 25% to 65%.
Each 10% lower proportion of sodium chloride in the salt substitute was associated with a 1.53 mm Hg (95% CI, −3.02 to −0.03; P = .045) greater reduction in SBP and a 0.95 mm Hg (95% CI, −1.78 to −0.12; P = .025) greater reduction in DBP.
Reductions in blood pressure appeared consistent, irrespective of country, age, sex, history of high blood pressure, weight, baseline blood pressure, and baseline levels of urinary sodium and potassium.
Clear benefit on hard outcomes
Pooled data on clinical outcomes from five trials that included 24,306 participants, mostly from the SSaSS, showed clear protective effects of salt substitutes on total mortality (risk ratio, 0.89; 95% CI, 0.85-0.94), CV mortality (RR, 0.87; 95% CI, 0.81-0.94), and CV events (RR, 0.89; 95% CI, 0.85-0.94).
Dr. Tian and colleagues say that “broader population use of salt substitute is supported by the absence of any detectable adverse effect of salt substitutes on hyperkalemia in this review.”
They note, however, that all of the trials took “pragmatic steps to exclude participants at elevated risk of hyperkalemia, seeking to exclude those with chronic kidney disease or using medications that elevate serum potassium.”
Offering perspective on the study, Harlan Krumholz, MD, with Yale New Haven Hospital and Yale School of Medicine, both in New Haven, Conn., said it provides “useful information by bringing together the trial evidence on salt substitutes. The evidence is dominated by the SSaSS, but the others add context.”
Dr. Krumholz said that at this point, he thinks salt substitutes “could be included in recommendations to patients.”
“SSaSS was conducted in villages in China, so that is where the evidence is strongest and most relevant, but this is a low-cost and seemingly safe strategy that could be tried by anyone without contraindications, such as kidney disease or taking a potassium-sparing medication or potassium supplement,” Dr. Krumholz told this news organization.
Johanna Contreras, MD, heart failure and transplant cardiologist at the Mount Sinai Hospital, New York, agrees that in the absence of contraindications, salt substitutes should be recommended.
“Americans put salt on everything and don’t even think about it. The salt substitutes are very helpful,” Dr. Contreras said in an interview.
“People who don’t have high blood pressure should limit salt intake, because what we have seen is that if you have high blood pressure in your family – even if you don’t have high blood pressure in your 20s or 30s – you’re likely to develop high blood pressure,” Dr. Contreras said.
“Therefore, it’s wise early on to start protecting yourself and using low salt and salt substitutes,” she added.
The study had no specific funding. Dr. Tian, Dr. Krumholz, and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Meet a champion climber with type 1 diabetes
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Short walks after meals can cut diabetes risk
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Saddled with med school debt, yet left out of loan forgiveness plans
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.
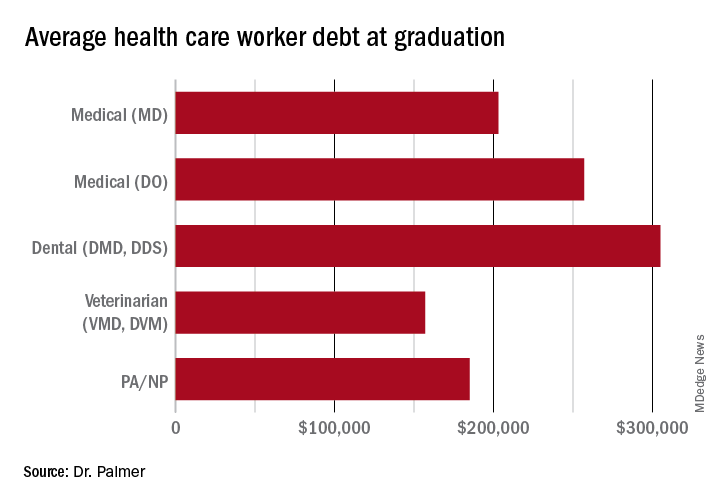
The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.

The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.

The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
Weight-loss surgery has a big effect on marriage
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
FROM ANNALS OF SURGERY