User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Both potatoes and beans reduced insulin resistance, weight in controlled study
Low energy–density diets that are based either on potatoes or beans similarly reduced insulin resistance in adults with poor blood glucose control, according to a controlled feeding study in 36 individuals.
Potatoes have gotten a bad rap for their high glycemic index, but they have little fat and a low energy density, wrote the study investigators. In fact, “cooling of gelatinized potatoes generates appreciable levels of slowly digested starch (resistant starch type 3) and substantially lowers the blood glucose response that potatoes elicit.”
“There is a view that potatoes are a less healthy plant food, but there is very little empirical data from randomized trials to support this view,” senior investigator John P. Kirwan, PhD, said in an interview.
Dry beans and peas (known as pulses) also contain resistant starch that improves insulin sensitivity and glucose tolerance, and multiple studies support pulses as part of a low-glycemic diet to improve glucose control in adults, the researchers explained, but because the density of food often guides how much people eat, they hypothesized that potatoes could substitute for beans and provide similar glucose control benefits.
In a study published in the Journal of Medicinal Food, the researchers randomized 36 adults aged 18-60 years with insulin resistance to 8 weeks of a low energy–density diet (1 kcal/g) high in either potatoes or beans. The baseline body mass index ranged from 25 to 40 kg/m2. Insulin resistance was defined using the homeostatic model assessment of insulin resistance (HOMA-IR) with a score greater than 2.
The controlled diet consisted of 50%-55% carbohydrates, 30%-35% fats, and 15%-20% protein. Each meal in the potato group included a side of potatoes, and each meal in the bean group included a side of beans.
The primary outcome was the mean change in blood glucose concentration; the researchers also assessed weight loss.
A total of 14 individuals in the potato group and 17 in the bean group completed the study; but data from the 18 individuals in each group were included in an intent-to-treat analysis.
Among study completers, HOMA-IR in the bean group showed an average decrease of 1.4 from baseline (P = .02 ); a similar decrease of 1.3 occurred in the potato group (P < .05) with no significant difference between the two diets.
Overall compliance with both diets was roughly 88%. Body weight reductions were similar in both groups and significantly reduced from baseline over the study period, with average reductions in intent-to-treat analysis of 5.82 kg in the potato group and 4.0 kg in the bean group. BMI also was significantly reduced from baseline in both potato and bean groups (2.04 kg/m2 and 1.35 kg/m2, respectively). Although baseline differences were not significant, “BMI at baseline was higher and the reduction in response to the treatment was significantly greater in the potato diet compared with the bean diet,” the researchers noted. The effect on blood glucose response was not significantly different between the two groups or from baseline, they said.
The findings were limited by several factors including the small size, relatively short study period, and controlled nature of the study diet, the researchers noted. “The addition of a typical Western diet would have enhanced our understanding of the effect of low energy–dense diets on metabolic outcomes,” they noted in their discussion.
However, both diets led to a reduction in body weight, and the low energy density of both potato and bean diets promoted weight loss without affecting appetite or requiring calorie restriction, the researchers explained. Therefore, “this weight loss if sustained over time could have a substantial impact on body weight,” they said.
“We hypothesized that there would be equivalence between the potato and bean diet and this hypothesis proved to be correct,” said Dr. Kirwan, of the Pennington Biomedical Research Center, Baton Rouge, La., in an interview.
The take-home message for clinicians is that, though small, the study was very well-controlled, Dr. Kirwan emphasized. “Clinicians ought to consider the health benefits of the potato when it is cooked and served appropriately.”
Looking ahead, larger randomized controlled trials with additional control arms, longer time of at least 12 weeks, and different patient populations are needed, Dr. Kirwan added.
Findings mitigate food myths
The debate continues about whether there are foods that are “good” or “evil;” or foods that one “should not eat” or “should eat,” said Amy Rothberg, MD, associate professor of internal medicine and of nutritional sciences at the University of Michigan, Ann Arbor, in an interview.
“This study dispels the myth that incorporating a small portion of potato into the diet (although these are not potatoes that are fried, or are topped with cheese, bacon, sour cream, etc.) results in deleterious metabolic outcomes when compared to a diet that is comprised of beans (pulses) as part of a low energy–dense diet,” she explained.
“The diet in both groups was of low energy density, which has been shown to result in fewer calories consumed, weight loss, and improvement in insulin resistance,” so the similarity in results was not so surprising, said Dr. Rothberg.
For the clinical takeaway, Dr. Rothberg agreed with the study authors: “Clinicians may counsel their patients that they can still consume a small potato (with the caveat above regarding cooking methods and toppings) as part of a balanced meal so long as they are keeping their overall calories low and not exceeding their metabolic requirements based on body weight/BMI,” she said.
As for additional research, studies with a longer time frame and a larger and more diverse study population are needed, including populations with common insulin resistance comorbidities such as type 2 diabetes, fatty liver disease, and cardiovascular disease, Dr. Rothberg noted.
Consumer considerations, with caveats
The key message for consumers is that, “based on this very small study of short duration, consuming a small portion of potato as part of an overall balanced, low-energy diet did not produce adverse effects on glucose or insulin when compared to a diet of pulses known to have favorable effects on glucose and insulin,” Dr. Rothberg told this news organization. However, “consumers should note that, although the results from this small study are encouraging, it would be premature to extrapolate the findings from this study to other populations,” she said. Also, keep in mind that the study was supported in part by the Alliance for Potato Research, although the authors stated that none of the funders (Alliance for Potato Research and Education and the National Institutes of Health) had any role in the design, analysis, or writing of the article, she added.
The study was supported in part by the Alliance for Potato Research and Education and the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The researchers and Dr. Rothberg had no financial conflicts to disclose.
Low energy–density diets that are based either on potatoes or beans similarly reduced insulin resistance in adults with poor blood glucose control, according to a controlled feeding study in 36 individuals.
Potatoes have gotten a bad rap for their high glycemic index, but they have little fat and a low energy density, wrote the study investigators. In fact, “cooling of gelatinized potatoes generates appreciable levels of slowly digested starch (resistant starch type 3) and substantially lowers the blood glucose response that potatoes elicit.”
“There is a view that potatoes are a less healthy plant food, but there is very little empirical data from randomized trials to support this view,” senior investigator John P. Kirwan, PhD, said in an interview.
Dry beans and peas (known as pulses) also contain resistant starch that improves insulin sensitivity and glucose tolerance, and multiple studies support pulses as part of a low-glycemic diet to improve glucose control in adults, the researchers explained, but because the density of food often guides how much people eat, they hypothesized that potatoes could substitute for beans and provide similar glucose control benefits.
In a study published in the Journal of Medicinal Food, the researchers randomized 36 adults aged 18-60 years with insulin resistance to 8 weeks of a low energy–density diet (1 kcal/g) high in either potatoes or beans. The baseline body mass index ranged from 25 to 40 kg/m2. Insulin resistance was defined using the homeostatic model assessment of insulin resistance (HOMA-IR) with a score greater than 2.
The controlled diet consisted of 50%-55% carbohydrates, 30%-35% fats, and 15%-20% protein. Each meal in the potato group included a side of potatoes, and each meal in the bean group included a side of beans.
The primary outcome was the mean change in blood glucose concentration; the researchers also assessed weight loss.
A total of 14 individuals in the potato group and 17 in the bean group completed the study; but data from the 18 individuals in each group were included in an intent-to-treat analysis.
Among study completers, HOMA-IR in the bean group showed an average decrease of 1.4 from baseline (P = .02 ); a similar decrease of 1.3 occurred in the potato group (P < .05) with no significant difference between the two diets.
Overall compliance with both diets was roughly 88%. Body weight reductions were similar in both groups and significantly reduced from baseline over the study period, with average reductions in intent-to-treat analysis of 5.82 kg in the potato group and 4.0 kg in the bean group. BMI also was significantly reduced from baseline in both potato and bean groups (2.04 kg/m2 and 1.35 kg/m2, respectively). Although baseline differences were not significant, “BMI at baseline was higher and the reduction in response to the treatment was significantly greater in the potato diet compared with the bean diet,” the researchers noted. The effect on blood glucose response was not significantly different between the two groups or from baseline, they said.
The findings were limited by several factors including the small size, relatively short study period, and controlled nature of the study diet, the researchers noted. “The addition of a typical Western diet would have enhanced our understanding of the effect of low energy–dense diets on metabolic outcomes,” they noted in their discussion.
However, both diets led to a reduction in body weight, and the low energy density of both potato and bean diets promoted weight loss without affecting appetite or requiring calorie restriction, the researchers explained. Therefore, “this weight loss if sustained over time could have a substantial impact on body weight,” they said.
“We hypothesized that there would be equivalence between the potato and bean diet and this hypothesis proved to be correct,” said Dr. Kirwan, of the Pennington Biomedical Research Center, Baton Rouge, La., in an interview.
The take-home message for clinicians is that, though small, the study was very well-controlled, Dr. Kirwan emphasized. “Clinicians ought to consider the health benefits of the potato when it is cooked and served appropriately.”
Looking ahead, larger randomized controlled trials with additional control arms, longer time of at least 12 weeks, and different patient populations are needed, Dr. Kirwan added.
Findings mitigate food myths
The debate continues about whether there are foods that are “good” or “evil;” or foods that one “should not eat” or “should eat,” said Amy Rothberg, MD, associate professor of internal medicine and of nutritional sciences at the University of Michigan, Ann Arbor, in an interview.
“This study dispels the myth that incorporating a small portion of potato into the diet (although these are not potatoes that are fried, or are topped with cheese, bacon, sour cream, etc.) results in deleterious metabolic outcomes when compared to a diet that is comprised of beans (pulses) as part of a low energy–dense diet,” she explained.
“The diet in both groups was of low energy density, which has been shown to result in fewer calories consumed, weight loss, and improvement in insulin resistance,” so the similarity in results was not so surprising, said Dr. Rothberg.
For the clinical takeaway, Dr. Rothberg agreed with the study authors: “Clinicians may counsel their patients that they can still consume a small potato (with the caveat above regarding cooking methods and toppings) as part of a balanced meal so long as they are keeping their overall calories low and not exceeding their metabolic requirements based on body weight/BMI,” she said.
As for additional research, studies with a longer time frame and a larger and more diverse study population are needed, including populations with common insulin resistance comorbidities such as type 2 diabetes, fatty liver disease, and cardiovascular disease, Dr. Rothberg noted.
Consumer considerations, with caveats
The key message for consumers is that, “based on this very small study of short duration, consuming a small portion of potato as part of an overall balanced, low-energy diet did not produce adverse effects on glucose or insulin when compared to a diet of pulses known to have favorable effects on glucose and insulin,” Dr. Rothberg told this news organization. However, “consumers should note that, although the results from this small study are encouraging, it would be premature to extrapolate the findings from this study to other populations,” she said. Also, keep in mind that the study was supported in part by the Alliance for Potato Research, although the authors stated that none of the funders (Alliance for Potato Research and Education and the National Institutes of Health) had any role in the design, analysis, or writing of the article, she added.
The study was supported in part by the Alliance for Potato Research and Education and the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The researchers and Dr. Rothberg had no financial conflicts to disclose.
Low energy–density diets that are based either on potatoes or beans similarly reduced insulin resistance in adults with poor blood glucose control, according to a controlled feeding study in 36 individuals.
Potatoes have gotten a bad rap for their high glycemic index, but they have little fat and a low energy density, wrote the study investigators. In fact, “cooling of gelatinized potatoes generates appreciable levels of slowly digested starch (resistant starch type 3) and substantially lowers the blood glucose response that potatoes elicit.”
“There is a view that potatoes are a less healthy plant food, but there is very little empirical data from randomized trials to support this view,” senior investigator John P. Kirwan, PhD, said in an interview.
Dry beans and peas (known as pulses) also contain resistant starch that improves insulin sensitivity and glucose tolerance, and multiple studies support pulses as part of a low-glycemic diet to improve glucose control in adults, the researchers explained, but because the density of food often guides how much people eat, they hypothesized that potatoes could substitute for beans and provide similar glucose control benefits.
In a study published in the Journal of Medicinal Food, the researchers randomized 36 adults aged 18-60 years with insulin resistance to 8 weeks of a low energy–density diet (1 kcal/g) high in either potatoes or beans. The baseline body mass index ranged from 25 to 40 kg/m2. Insulin resistance was defined using the homeostatic model assessment of insulin resistance (HOMA-IR) with a score greater than 2.
The controlled diet consisted of 50%-55% carbohydrates, 30%-35% fats, and 15%-20% protein. Each meal in the potato group included a side of potatoes, and each meal in the bean group included a side of beans.
The primary outcome was the mean change in blood glucose concentration; the researchers also assessed weight loss.
A total of 14 individuals in the potato group and 17 in the bean group completed the study; but data from the 18 individuals in each group were included in an intent-to-treat analysis.
Among study completers, HOMA-IR in the bean group showed an average decrease of 1.4 from baseline (P = .02 ); a similar decrease of 1.3 occurred in the potato group (P < .05) with no significant difference between the two diets.
Overall compliance with both diets was roughly 88%. Body weight reductions were similar in both groups and significantly reduced from baseline over the study period, with average reductions in intent-to-treat analysis of 5.82 kg in the potato group and 4.0 kg in the bean group. BMI also was significantly reduced from baseline in both potato and bean groups (2.04 kg/m2 and 1.35 kg/m2, respectively). Although baseline differences were not significant, “BMI at baseline was higher and the reduction in response to the treatment was significantly greater in the potato diet compared with the bean diet,” the researchers noted. The effect on blood glucose response was not significantly different between the two groups or from baseline, they said.
The findings were limited by several factors including the small size, relatively short study period, and controlled nature of the study diet, the researchers noted. “The addition of a typical Western diet would have enhanced our understanding of the effect of low energy–dense diets on metabolic outcomes,” they noted in their discussion.
However, both diets led to a reduction in body weight, and the low energy density of both potato and bean diets promoted weight loss without affecting appetite or requiring calorie restriction, the researchers explained. Therefore, “this weight loss if sustained over time could have a substantial impact on body weight,” they said.
“We hypothesized that there would be equivalence between the potato and bean diet and this hypothesis proved to be correct,” said Dr. Kirwan, of the Pennington Biomedical Research Center, Baton Rouge, La., in an interview.
The take-home message for clinicians is that, though small, the study was very well-controlled, Dr. Kirwan emphasized. “Clinicians ought to consider the health benefits of the potato when it is cooked and served appropriately.”
Looking ahead, larger randomized controlled trials with additional control arms, longer time of at least 12 weeks, and different patient populations are needed, Dr. Kirwan added.
Findings mitigate food myths
The debate continues about whether there are foods that are “good” or “evil;” or foods that one “should not eat” or “should eat,” said Amy Rothberg, MD, associate professor of internal medicine and of nutritional sciences at the University of Michigan, Ann Arbor, in an interview.
“This study dispels the myth that incorporating a small portion of potato into the diet (although these are not potatoes that are fried, or are topped with cheese, bacon, sour cream, etc.) results in deleterious metabolic outcomes when compared to a diet that is comprised of beans (pulses) as part of a low energy–dense diet,” she explained.
“The diet in both groups was of low energy density, which has been shown to result in fewer calories consumed, weight loss, and improvement in insulin resistance,” so the similarity in results was not so surprising, said Dr. Rothberg.
For the clinical takeaway, Dr. Rothberg agreed with the study authors: “Clinicians may counsel their patients that they can still consume a small potato (with the caveat above regarding cooking methods and toppings) as part of a balanced meal so long as they are keeping their overall calories low and not exceeding their metabolic requirements based on body weight/BMI,” she said.
As for additional research, studies with a longer time frame and a larger and more diverse study population are needed, including populations with common insulin resistance comorbidities such as type 2 diabetes, fatty liver disease, and cardiovascular disease, Dr. Rothberg noted.
Consumer considerations, with caveats
The key message for consumers is that, “based on this very small study of short duration, consuming a small portion of potato as part of an overall balanced, low-energy diet did not produce adverse effects on glucose or insulin when compared to a diet of pulses known to have favorable effects on glucose and insulin,” Dr. Rothberg told this news organization. However, “consumers should note that, although the results from this small study are encouraging, it would be premature to extrapolate the findings from this study to other populations,” she said. Also, keep in mind that the study was supported in part by the Alliance for Potato Research, although the authors stated that none of the funders (Alliance for Potato Research and Education and the National Institutes of Health) had any role in the design, analysis, or writing of the article, she added.
The study was supported in part by the Alliance for Potato Research and Education and the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The researchers and Dr. Rothberg had no financial conflicts to disclose.
FROM THE JOURNAL OF MEDICINAL FOOD
Looking for a healthy meat substitute? Consider the potato
Boil ‘em, mash ‘em, include ‘em in a balanced diet
It’s kind of funny that, even though potatoes are vegetables and vegetables are generally considered to be healthy foods, not many people think of potatoes as being particularly good for you. And that’s hardly surprising since we usually either consume them in the form of French fries or potato chips, neither of which are known for their healthiness.
In fact, some previous research shows that potatoes are a food to avoid, particularly for people with insulin resistance. However, a new study from England goes against the grain and asserts that the potato is perfectly fine for insulin-resistant individuals and filled with valuable nutrients and health benefits. Which is great news for the state of Idaho and the potato organization funding the research. Of course there’s a potato organization.
For the study, a group of obese, overweight, or insulin-resistant individuals received a diet of either beans, peas, and meat or fish or white potatoes with meat or fish for 8 weeks; both diets were heavy in fruits and vegetables and both diets replaced about 40% of typical meat consumption with either beans and peas or potatoes. By the end of the study, those on the potato diet experienced health benefits equivalent to those on the bean and pea diet, including losing roughly equivalent amounts of weight and similarly reducing the body’s insulin response.
The researchers noted that, because people tend to eat the same amount of food no matter what, replacing something like meat with dense, low-calorie potatoes meant study participants could eat normally yet consume much fewer calories. So you could make a delicious, healthy stew without the brace of conies and the nice fish, which would make Smeagol very happy.
You won’t have ‘monkeypox’ to kick around anymore
It’s true. No more monkeypox. It’s gone. It’s history. Adios. The World Health Organization said that the disease formerly known as monkeypox will now be called mpox. What? You didn’t think it had been cured, did you? You did? Really? Silly readers.
“Mpox will become a preferred term, replacing monkeypox, after a transition period of 1 year. This serves to mitigate the concerns raised by experts about confusion caused by a name change in the midst of a global outbreak,” WHO said in a statement announcing the change.
The stigma attached to the name was the main problem. New York City Health Commissioner Dr. Ashwin Vasan had sent a letter to WHO earlier this year, according to CNN, saying that there was “growing concern for the potentially devastating and stigmatizing effects that the messaging around the ‘monkeypox’ virus can have on … vulnerable communities.”
We here at LOTME applaud the fight against stigmas of any sort, but we sensed there was more to this name change business, so our dedicated team of investigative journalists went into action. Sure enough, while rooting through WHO Director-General Tedros Adhanom Ghebreyesus’s garbage, we found a list of the names that had been rejected in favor of mpox:
- K-pop (already taken)
- Keeping up with the Kardashi-pox
- Trumpox
- Pox the magic dragon
- Monkey plague (didn’t really solve the problem)
- Hockey pox
- Mission mpoxible
- Jurassic Pox
- The pox that refreshes
- Debbie
Feet catch what the ears miss
The spectrum of frequencies that can be heard by human ears varies from person to person. Then there’s the matter of personal taste in music and volume level. But what really gets people moving? A new study shows that it’s more about the frequency of the sound than the volume.
For the study, participants at a concert by electronic music duo Orphx at LIVELab – a research performance center on the McMaster University campus in Hamilton, Ont., that was specifically designed to study music and dance – filled out questionnaires before and after the show. They also wore motion-capture headbands to detect their movement throughout the concert. During the show the researchers turned very-low-frequency (VLF) sounds (8-37 Hz) on and off every 2.5 minutes. Movement speed was calculated during on and off periods.
Although the effects of subliminal messaging aren’t new, past studies have shown that participants were mostly aware of the messaging. In this study, the researchers found that the subjects’ movements increased by 11.8% when the VLF sounds were on, but without their awareness. The researchers and the participants attributed movement to the bass, as lower pitches tend to elicit stronger neural responses and thus movement, compared with higher pitches.
“Our whole sense of the beat is mediated by the vestibular system but nobody’s really, I think, effectively confirmed that,” Jonathan Cannon, an assistant professor of psychology, neuroscience, and behavior at McMaster who not involved in the study, told Live Science.
Not to say this study didn’t have its limitations, such as the effect of the surrounding crowd or vibrations of the floor influencing the need to dance. But it definitely makes you wonder about what’s actually playing in your favorite song.
Uncle Leonid wants you
Do you like to travel? Are you a bit of a thrill seeker? Do you have any extra socks? If you’re a physician who answered yes to those three questions, then we’ve got an opportunity for you.
Leonid Slutsky, leader of Russia’s populist Liberal Democratic Party and chairman of the foreign relations committee in the lower house of Russia’s parliament – yes, that Leonid Slutsky – recently made a bit of a recruiting pitch, although that’s not how ABC News described it.
Mr. Slutsky, a strong supporter of his country’s war against Ukraine, recently told the mothers of Russian soldiers “that the whole world is watching us. We are the largest state and when we do not have socks, shorts, doctors, intelligence, communications, or simply care for our children, questions arise that will be very difficult to answer.”
It’s probably not what he meant, but the lack of intelligence is pretty clear.
Boil ‘em, mash ‘em, include ‘em in a balanced diet
It’s kind of funny that, even though potatoes are vegetables and vegetables are generally considered to be healthy foods, not many people think of potatoes as being particularly good for you. And that’s hardly surprising since we usually either consume them in the form of French fries or potato chips, neither of which are known for their healthiness.
In fact, some previous research shows that potatoes are a food to avoid, particularly for people with insulin resistance. However, a new study from England goes against the grain and asserts that the potato is perfectly fine for insulin-resistant individuals and filled with valuable nutrients and health benefits. Which is great news for the state of Idaho and the potato organization funding the research. Of course there’s a potato organization.
For the study, a group of obese, overweight, or insulin-resistant individuals received a diet of either beans, peas, and meat or fish or white potatoes with meat or fish for 8 weeks; both diets were heavy in fruits and vegetables and both diets replaced about 40% of typical meat consumption with either beans and peas or potatoes. By the end of the study, those on the potato diet experienced health benefits equivalent to those on the bean and pea diet, including losing roughly equivalent amounts of weight and similarly reducing the body’s insulin response.
The researchers noted that, because people tend to eat the same amount of food no matter what, replacing something like meat with dense, low-calorie potatoes meant study participants could eat normally yet consume much fewer calories. So you could make a delicious, healthy stew without the brace of conies and the nice fish, which would make Smeagol very happy.
You won’t have ‘monkeypox’ to kick around anymore
It’s true. No more monkeypox. It’s gone. It’s history. Adios. The World Health Organization said that the disease formerly known as monkeypox will now be called mpox. What? You didn’t think it had been cured, did you? You did? Really? Silly readers.
“Mpox will become a preferred term, replacing monkeypox, after a transition period of 1 year. This serves to mitigate the concerns raised by experts about confusion caused by a name change in the midst of a global outbreak,” WHO said in a statement announcing the change.
The stigma attached to the name was the main problem. New York City Health Commissioner Dr. Ashwin Vasan had sent a letter to WHO earlier this year, according to CNN, saying that there was “growing concern for the potentially devastating and stigmatizing effects that the messaging around the ‘monkeypox’ virus can have on … vulnerable communities.”
We here at LOTME applaud the fight against stigmas of any sort, but we sensed there was more to this name change business, so our dedicated team of investigative journalists went into action. Sure enough, while rooting through WHO Director-General Tedros Adhanom Ghebreyesus’s garbage, we found a list of the names that had been rejected in favor of mpox:
- K-pop (already taken)
- Keeping up with the Kardashi-pox
- Trumpox
- Pox the magic dragon
- Monkey plague (didn’t really solve the problem)
- Hockey pox
- Mission mpoxible
- Jurassic Pox
- The pox that refreshes
- Debbie
Feet catch what the ears miss
The spectrum of frequencies that can be heard by human ears varies from person to person. Then there’s the matter of personal taste in music and volume level. But what really gets people moving? A new study shows that it’s more about the frequency of the sound than the volume.
For the study, participants at a concert by electronic music duo Orphx at LIVELab – a research performance center on the McMaster University campus in Hamilton, Ont., that was specifically designed to study music and dance – filled out questionnaires before and after the show. They also wore motion-capture headbands to detect their movement throughout the concert. During the show the researchers turned very-low-frequency (VLF) sounds (8-37 Hz) on and off every 2.5 minutes. Movement speed was calculated during on and off periods.
Although the effects of subliminal messaging aren’t new, past studies have shown that participants were mostly aware of the messaging. In this study, the researchers found that the subjects’ movements increased by 11.8% when the VLF sounds were on, but without their awareness. The researchers and the participants attributed movement to the bass, as lower pitches tend to elicit stronger neural responses and thus movement, compared with higher pitches.
“Our whole sense of the beat is mediated by the vestibular system but nobody’s really, I think, effectively confirmed that,” Jonathan Cannon, an assistant professor of psychology, neuroscience, and behavior at McMaster who not involved in the study, told Live Science.
Not to say this study didn’t have its limitations, such as the effect of the surrounding crowd or vibrations of the floor influencing the need to dance. But it definitely makes you wonder about what’s actually playing in your favorite song.
Uncle Leonid wants you
Do you like to travel? Are you a bit of a thrill seeker? Do you have any extra socks? If you’re a physician who answered yes to those three questions, then we’ve got an opportunity for you.
Leonid Slutsky, leader of Russia’s populist Liberal Democratic Party and chairman of the foreign relations committee in the lower house of Russia’s parliament – yes, that Leonid Slutsky – recently made a bit of a recruiting pitch, although that’s not how ABC News described it.
Mr. Slutsky, a strong supporter of his country’s war against Ukraine, recently told the mothers of Russian soldiers “that the whole world is watching us. We are the largest state and when we do not have socks, shorts, doctors, intelligence, communications, or simply care for our children, questions arise that will be very difficult to answer.”
It’s probably not what he meant, but the lack of intelligence is pretty clear.
Boil ‘em, mash ‘em, include ‘em in a balanced diet
It’s kind of funny that, even though potatoes are vegetables and vegetables are generally considered to be healthy foods, not many people think of potatoes as being particularly good for you. And that’s hardly surprising since we usually either consume them in the form of French fries or potato chips, neither of which are known for their healthiness.
In fact, some previous research shows that potatoes are a food to avoid, particularly for people with insulin resistance. However, a new study from England goes against the grain and asserts that the potato is perfectly fine for insulin-resistant individuals and filled with valuable nutrients and health benefits. Which is great news for the state of Idaho and the potato organization funding the research. Of course there’s a potato organization.
For the study, a group of obese, overweight, or insulin-resistant individuals received a diet of either beans, peas, and meat or fish or white potatoes with meat or fish for 8 weeks; both diets were heavy in fruits and vegetables and both diets replaced about 40% of typical meat consumption with either beans and peas or potatoes. By the end of the study, those on the potato diet experienced health benefits equivalent to those on the bean and pea diet, including losing roughly equivalent amounts of weight and similarly reducing the body’s insulin response.
The researchers noted that, because people tend to eat the same amount of food no matter what, replacing something like meat with dense, low-calorie potatoes meant study participants could eat normally yet consume much fewer calories. So you could make a delicious, healthy stew without the brace of conies and the nice fish, which would make Smeagol very happy.
You won’t have ‘monkeypox’ to kick around anymore
It’s true. No more monkeypox. It’s gone. It’s history. Adios. The World Health Organization said that the disease formerly known as monkeypox will now be called mpox. What? You didn’t think it had been cured, did you? You did? Really? Silly readers.
“Mpox will become a preferred term, replacing monkeypox, after a transition period of 1 year. This serves to mitigate the concerns raised by experts about confusion caused by a name change in the midst of a global outbreak,” WHO said in a statement announcing the change.
The stigma attached to the name was the main problem. New York City Health Commissioner Dr. Ashwin Vasan had sent a letter to WHO earlier this year, according to CNN, saying that there was “growing concern for the potentially devastating and stigmatizing effects that the messaging around the ‘monkeypox’ virus can have on … vulnerable communities.”
We here at LOTME applaud the fight against stigmas of any sort, but we sensed there was more to this name change business, so our dedicated team of investigative journalists went into action. Sure enough, while rooting through WHO Director-General Tedros Adhanom Ghebreyesus’s garbage, we found a list of the names that had been rejected in favor of mpox:
- K-pop (already taken)
- Keeping up with the Kardashi-pox
- Trumpox
- Pox the magic dragon
- Monkey plague (didn’t really solve the problem)
- Hockey pox
- Mission mpoxible
- Jurassic Pox
- The pox that refreshes
- Debbie
Feet catch what the ears miss
The spectrum of frequencies that can be heard by human ears varies from person to person. Then there’s the matter of personal taste in music and volume level. But what really gets people moving? A new study shows that it’s more about the frequency of the sound than the volume.
For the study, participants at a concert by electronic music duo Orphx at LIVELab – a research performance center on the McMaster University campus in Hamilton, Ont., that was specifically designed to study music and dance – filled out questionnaires before and after the show. They also wore motion-capture headbands to detect their movement throughout the concert. During the show the researchers turned very-low-frequency (VLF) sounds (8-37 Hz) on and off every 2.5 minutes. Movement speed was calculated during on and off periods.
Although the effects of subliminal messaging aren’t new, past studies have shown that participants were mostly aware of the messaging. In this study, the researchers found that the subjects’ movements increased by 11.8% when the VLF sounds were on, but without their awareness. The researchers and the participants attributed movement to the bass, as lower pitches tend to elicit stronger neural responses and thus movement, compared with higher pitches.
“Our whole sense of the beat is mediated by the vestibular system but nobody’s really, I think, effectively confirmed that,” Jonathan Cannon, an assistant professor of psychology, neuroscience, and behavior at McMaster who not involved in the study, told Live Science.
Not to say this study didn’t have its limitations, such as the effect of the surrounding crowd or vibrations of the floor influencing the need to dance. But it definitely makes you wonder about what’s actually playing in your favorite song.
Uncle Leonid wants you
Do you like to travel? Are you a bit of a thrill seeker? Do you have any extra socks? If you’re a physician who answered yes to those three questions, then we’ve got an opportunity for you.
Leonid Slutsky, leader of Russia’s populist Liberal Democratic Party and chairman of the foreign relations committee in the lower house of Russia’s parliament – yes, that Leonid Slutsky – recently made a bit of a recruiting pitch, although that’s not how ABC News described it.
Mr. Slutsky, a strong supporter of his country’s war against Ukraine, recently told the mothers of Russian soldiers “that the whole world is watching us. We are the largest state and when we do not have socks, shorts, doctors, intelligence, communications, or simply care for our children, questions arise that will be very difficult to answer.”
It’s probably not what he meant, but the lack of intelligence is pretty clear.
What is the genetic influence on the severity of COVID-19?
A striking characteristic of COVID-19 is that the severity of clinical outcomes is remarkably variable. Establishing a prognosis for individuals infected with COVID-19 remains a challenge.
Since the start of the COVID-19 pandemic, the heterogeneity of individuals who progress toward severe disease or death, along with the fact that individuals directly exposed to the virus do not necessarily become sick, supports the hypothesis that genetic risk or protective factors are at play.
In an interview with this news organization, Mayana Zatz, PhD, head professor of genetics and coordinator of the Human Genome and Stem Cell Study Center at the University of São Paulo, explained: “The first case that caught my eye was the case of my neighbors, a couple. He presented COVID-19 symptoms, but his wife, who took care of him, had absolutely no symptoms. I thought that it was strange, but we received 3,000 emails from people saying, ‘This happened to me, too.’”
Reports in the media about seven pairs of monozygotic (MZ) twins who died from COVID-19 within days of one another in Brazil also stood out, said the researcher.
, as well as their pathology. Dr. Zatz’s team analyzed the case of a 31-year-old Brazilian MZ twin brother pair who presented simultaneously with severe COVID-19 and the need for oxygen support, despite their age and good health conditions. Curiously, they were admitted and intubated on the same day, but neither of the twins knew about the other’s situation; they found out only when they were extubated.
The study was carried out at the USP with the collaboration of the State University of São Paulo. The authors mapped the genetic profile (by sequencing the genome responsible for coding proteins, or whole-exome sequencing) and the immune cell profile to evaluate innate and adaptive immunity.
The MZ twin brothers shared the same two rare genetic mutations, which may be associated with their increased risk of developing severe COVID-19. However, since these variants were not studied at the protein or functional level, their pathogenicity has yet to be determined. The twins also had [human leukocyte antigen (HLA)] alleles associated with severe COVID-19, which are important candidates for the mechanisms of innate and adaptive immunity and susceptibility to COVID-19 infection and manifestation.
But one particular oddity stood out to the researchers: One of the brothers required longer hospitalization, and only he reported symptoms of long COVID.
In the authors’ eyes, even though the patients shared genetic mutations potentially associated with the risk of developing severe COVID-19, the differences in clinical progression emphasize that, beyond genetic risk factors, continuous exposure to pathogens over a lifetime and other environmental factors mean that each individual’s immune response is unique, even in twins.
“There is no doubt that genetics contribute to the severity of COVID-19, and environmental factors sometimes give us the opportunity to study the disease, too. Such [is the case with] MZ twins who have genetic similarities, even with changes that take place over a lifetime,” José Eduardo Krieger, MD, PhD, professor of molecular medicine at the University of São Paulo Medical School (FMUSP), told this news organization. “Examining MZ twins is a strategy that may help, but, with n = 2, luck really needs to be on your side to get straight to the problem. You need to combine [these findings] with other studies to solve this conundrum,” said Dr. Krieger, who did not take part in the research.
Large cohorts
Genomic and computer resources allow for the study of large sets of data from thousands of individuals. In each of those sets of data, the signal offered by thousands of markers distributed throughout the genome can be studied. This is the possibility offered by various genomic studies of large cohorts of patients with different clinical manifestations.
“Researchers examine thousands of genetic variants throughout the genome from a large sample of individuals and have the chance, for example, to identify genetic variants that are more prevalent in patients who have presented with severe disease than in those who presented with milder disease,” said Dr. Krieger. “These associations highlight a chromosome region in which one or more genes explain, at least in part, the differences observed.”
Genomewide association studies have identified some genetic variants that indicate severity of COVID-19, with potential impact on the virus entering the cell, the immune response, or the development of cytokine storms.
One of these studies, COVID-19 Host Genetics Initiative (COVID-19 HGI), is an international, open-science collaboration for sharing scientific methods and resources with research groups across the world, with the goal of robustly mapping the host genetic determinants of SARS-CoV-2 infection and the severity of the resulting COVID-19 disease. At the start of 2021, the COVID-19 HGI combined genetic data from 49,562 cases and 2 million controls from 46 studies in 19 countries. A total of 853 samples from the BRACOVID study were included in the meta-analysis. The endeavor enabled the identification of 13 genomewide significant loci that are associated with SARS-CoV-2 infection or severe manifestations of COVID-19.
The BRACOVID study, in which Dr. Krieger participates, aims to identify host genetic factors that determine the severity of COVID-19. It is currently the largest project of its kind in Latin America. An article provides the analysis of the first 5,233 participants in the BRACOVID study, who were recruited in São Paulo. Of these participants, 3,533 had been infected with COVID-19 and hospitalized at either the Heart Institute or the Central Institute of the FMUSP General Hospital. The remaining 1,700 made up the control group, which included health care professionals and members of the general population. The controls were recruited through serology assays or PCR tests for SARS-CoV-2.
The researchers discovered a region of chromosome 1 that could play a role in modulating immune response and that could lead to an increase in the likelihood of hospitalization across a wide range of COVID-19 risk factors. This region of chromosome 1 was observed only in Brazilians with a strong European ancestry; however, this finding had not been mentioned in previous studies, suggesting that it could harbor a risk allele specific to the Brazilian population.
The study also confirmed most, but not all, of the regions recorded in the literature, which may be significant in identifying factors determining severity that are specific to a given population.
Including information from the BRACOVID study, other studies have enhanced the knowledge on affected organs. Combined data from 14,000 patients from nine countries evaluated a region of a single chromosome and found that carriers of a certain allele had a higher probability of experiencing various COVID-19 complications, such as severe respiratory failure, venous thromboembolism, and liver damage. The risk was even higher for individuals aged 60 years and over.
Discordant couples
Smaller sample sizes of underrepresented populations also provide relevant data for genomic studies. Dr. Zatz’s team carried out genomic studies on smaller groups, comparing serodiscordant couples (where one was infected and symptomatic while the partner remained asymptomatic and seronegative despite sharing the same bedroom during the infection). Their research found genetic variants related to immune response that were associated with susceptibility to infection and progression to severe COVID-19.
The team also went on to study a group of patients older than 90 years who recovered from COVID-19 with mild symptoms or who remained asymptomatic following a positive test for SARS-CoV-2. They compared these patients with a sample of elderly patients from the same city (São Paulo), sampled before the current pandemic. The researchers identified a genetic variant related to mucin production. “In individuals with mild COVID-19, the degradation of these mucins would be more efficient,” said Dr. Zatz. It is possible for this variant to interfere not only with the production of mucus, but also in its composition, as there is an exchange of amino acids in the protein.
“We continued the study by comparing the extremes, i.e., those in their 90s with mild COVID-19 and younger patients with severe COVID-19, including several who died,” said Dr. Zatz.
More personalized medicine
The specialists agreed that a genetic test to predict COVID-19 severity is still a long way away. The genetic component is too little understood to enable the evaluation of individual risk. It has been possible to identify several important areas but, as Dr. Krieger pointed out, a variant identified in a certain chromosome interval may not be just one gene. There may be various candidate genes, or there may be a regulatory sequence for a distant gene. Furthermore, there are regions with genes that make sense as moderators of COVID-19 severity, because they regulate an inflammatory or immunologic reaction, but evidence is still lacking.
Reaching the molecular mechanism would, in future, allow a medicine to be chosen for a given patient, as already happens with other diseases. It also could enable the discovery of new medicines following as-yet-unexplored lines of research. Dr. Zatz also considers the possibility of genetic therapy.
Even with the knowledge of human genetics, one part of the equation is missing: viral genetics. “Many of the individuals who were resistant to the Delta variant were later affected by Omicron,” she pointed out.
Significance of Brazil
“We have an infinite amount of genomic data worldwide, but the vast majority originates from White Americans of European origin,” said Dr. Krieger. Moreover, genomic associations of COVID-19 severity discovered in the Chinese population were not significant in the European population. Besides underscoring the importance of collaborating with international studies, this situation supports scientists’ interest in carrying out genetic studies within Brazil, he added.
“In the genomic study of the Brazilian population, we found 2 million variants that were not present in the European populations,” said Dr. Zatz.
Dr. Krieger mentioned a technical advantage that Brazil has. “Having been colonized by different ethnic groups and mixed many generations ago, Brazil has a population with a unique genetic structure; the recombinations are different. When preparing the samples, the regions break differently.” This factor could help to separate, in a candidate region, the gene that is significant from those that might not be.
In general, severe COVID-19 would be a complex phenomenon involving several genes and interactions with environmental factors. The Brazilian studies tried to find a factor that was unique to Brazil, but the significance of the differences remained unclear. “We found some signs that were specific to our population,” concluded Dr. Krieger. “But the reason that more people in Brazil died as a result of COVID-19 was not genetic,” he added.
Dr. Zatz and Dr. Krieger reported no conflicts of interest. This article was translated from the Medscape Portuguese edition.
A version of this article first appeared on Medscape.com.
A striking characteristic of COVID-19 is that the severity of clinical outcomes is remarkably variable. Establishing a prognosis for individuals infected with COVID-19 remains a challenge.
Since the start of the COVID-19 pandemic, the heterogeneity of individuals who progress toward severe disease or death, along with the fact that individuals directly exposed to the virus do not necessarily become sick, supports the hypothesis that genetic risk or protective factors are at play.
In an interview with this news organization, Mayana Zatz, PhD, head professor of genetics and coordinator of the Human Genome and Stem Cell Study Center at the University of São Paulo, explained: “The first case that caught my eye was the case of my neighbors, a couple. He presented COVID-19 symptoms, but his wife, who took care of him, had absolutely no symptoms. I thought that it was strange, but we received 3,000 emails from people saying, ‘This happened to me, too.’”
Reports in the media about seven pairs of monozygotic (MZ) twins who died from COVID-19 within days of one another in Brazil also stood out, said the researcher.
, as well as their pathology. Dr. Zatz’s team analyzed the case of a 31-year-old Brazilian MZ twin brother pair who presented simultaneously with severe COVID-19 and the need for oxygen support, despite their age and good health conditions. Curiously, they were admitted and intubated on the same day, but neither of the twins knew about the other’s situation; they found out only when they were extubated.
The study was carried out at the USP with the collaboration of the State University of São Paulo. The authors mapped the genetic profile (by sequencing the genome responsible for coding proteins, or whole-exome sequencing) and the immune cell profile to evaluate innate and adaptive immunity.
The MZ twin brothers shared the same two rare genetic mutations, which may be associated with their increased risk of developing severe COVID-19. However, since these variants were not studied at the protein or functional level, their pathogenicity has yet to be determined. The twins also had [human leukocyte antigen (HLA)] alleles associated with severe COVID-19, which are important candidates for the mechanisms of innate and adaptive immunity and susceptibility to COVID-19 infection and manifestation.
But one particular oddity stood out to the researchers: One of the brothers required longer hospitalization, and only he reported symptoms of long COVID.
In the authors’ eyes, even though the patients shared genetic mutations potentially associated with the risk of developing severe COVID-19, the differences in clinical progression emphasize that, beyond genetic risk factors, continuous exposure to pathogens over a lifetime and other environmental factors mean that each individual’s immune response is unique, even in twins.
“There is no doubt that genetics contribute to the severity of COVID-19, and environmental factors sometimes give us the opportunity to study the disease, too. Such [is the case with] MZ twins who have genetic similarities, even with changes that take place over a lifetime,” José Eduardo Krieger, MD, PhD, professor of molecular medicine at the University of São Paulo Medical School (FMUSP), told this news organization. “Examining MZ twins is a strategy that may help, but, with n = 2, luck really needs to be on your side to get straight to the problem. You need to combine [these findings] with other studies to solve this conundrum,” said Dr. Krieger, who did not take part in the research.
Large cohorts
Genomic and computer resources allow for the study of large sets of data from thousands of individuals. In each of those sets of data, the signal offered by thousands of markers distributed throughout the genome can be studied. This is the possibility offered by various genomic studies of large cohorts of patients with different clinical manifestations.
“Researchers examine thousands of genetic variants throughout the genome from a large sample of individuals and have the chance, for example, to identify genetic variants that are more prevalent in patients who have presented with severe disease than in those who presented with milder disease,” said Dr. Krieger. “These associations highlight a chromosome region in which one or more genes explain, at least in part, the differences observed.”
Genomewide association studies have identified some genetic variants that indicate severity of COVID-19, with potential impact on the virus entering the cell, the immune response, or the development of cytokine storms.
One of these studies, COVID-19 Host Genetics Initiative (COVID-19 HGI), is an international, open-science collaboration for sharing scientific methods and resources with research groups across the world, with the goal of robustly mapping the host genetic determinants of SARS-CoV-2 infection and the severity of the resulting COVID-19 disease. At the start of 2021, the COVID-19 HGI combined genetic data from 49,562 cases and 2 million controls from 46 studies in 19 countries. A total of 853 samples from the BRACOVID study were included in the meta-analysis. The endeavor enabled the identification of 13 genomewide significant loci that are associated with SARS-CoV-2 infection or severe manifestations of COVID-19.
The BRACOVID study, in which Dr. Krieger participates, aims to identify host genetic factors that determine the severity of COVID-19. It is currently the largest project of its kind in Latin America. An article provides the analysis of the first 5,233 participants in the BRACOVID study, who were recruited in São Paulo. Of these participants, 3,533 had been infected with COVID-19 and hospitalized at either the Heart Institute or the Central Institute of the FMUSP General Hospital. The remaining 1,700 made up the control group, which included health care professionals and members of the general population. The controls were recruited through serology assays or PCR tests for SARS-CoV-2.
The researchers discovered a region of chromosome 1 that could play a role in modulating immune response and that could lead to an increase in the likelihood of hospitalization across a wide range of COVID-19 risk factors. This region of chromosome 1 was observed only in Brazilians with a strong European ancestry; however, this finding had not been mentioned in previous studies, suggesting that it could harbor a risk allele specific to the Brazilian population.
The study also confirmed most, but not all, of the regions recorded in the literature, which may be significant in identifying factors determining severity that are specific to a given population.
Including information from the BRACOVID study, other studies have enhanced the knowledge on affected organs. Combined data from 14,000 patients from nine countries evaluated a region of a single chromosome and found that carriers of a certain allele had a higher probability of experiencing various COVID-19 complications, such as severe respiratory failure, venous thromboembolism, and liver damage. The risk was even higher for individuals aged 60 years and over.
Discordant couples
Smaller sample sizes of underrepresented populations also provide relevant data for genomic studies. Dr. Zatz’s team carried out genomic studies on smaller groups, comparing serodiscordant couples (where one was infected and symptomatic while the partner remained asymptomatic and seronegative despite sharing the same bedroom during the infection). Their research found genetic variants related to immune response that were associated with susceptibility to infection and progression to severe COVID-19.
The team also went on to study a group of patients older than 90 years who recovered from COVID-19 with mild symptoms or who remained asymptomatic following a positive test for SARS-CoV-2. They compared these patients with a sample of elderly patients from the same city (São Paulo), sampled before the current pandemic. The researchers identified a genetic variant related to mucin production. “In individuals with mild COVID-19, the degradation of these mucins would be more efficient,” said Dr. Zatz. It is possible for this variant to interfere not only with the production of mucus, but also in its composition, as there is an exchange of amino acids in the protein.
“We continued the study by comparing the extremes, i.e., those in their 90s with mild COVID-19 and younger patients with severe COVID-19, including several who died,” said Dr. Zatz.
More personalized medicine
The specialists agreed that a genetic test to predict COVID-19 severity is still a long way away. The genetic component is too little understood to enable the evaluation of individual risk. It has been possible to identify several important areas but, as Dr. Krieger pointed out, a variant identified in a certain chromosome interval may not be just one gene. There may be various candidate genes, or there may be a regulatory sequence for a distant gene. Furthermore, there are regions with genes that make sense as moderators of COVID-19 severity, because they regulate an inflammatory or immunologic reaction, but evidence is still lacking.
Reaching the molecular mechanism would, in future, allow a medicine to be chosen for a given patient, as already happens with other diseases. It also could enable the discovery of new medicines following as-yet-unexplored lines of research. Dr. Zatz also considers the possibility of genetic therapy.
Even with the knowledge of human genetics, one part of the equation is missing: viral genetics. “Many of the individuals who were resistant to the Delta variant were later affected by Omicron,” she pointed out.
Significance of Brazil
“We have an infinite amount of genomic data worldwide, but the vast majority originates from White Americans of European origin,” said Dr. Krieger. Moreover, genomic associations of COVID-19 severity discovered in the Chinese population were not significant in the European population. Besides underscoring the importance of collaborating with international studies, this situation supports scientists’ interest in carrying out genetic studies within Brazil, he added.
“In the genomic study of the Brazilian population, we found 2 million variants that were not present in the European populations,” said Dr. Zatz.
Dr. Krieger mentioned a technical advantage that Brazil has. “Having been colonized by different ethnic groups and mixed many generations ago, Brazil has a population with a unique genetic structure; the recombinations are different. When preparing the samples, the regions break differently.” This factor could help to separate, in a candidate region, the gene that is significant from those that might not be.
In general, severe COVID-19 would be a complex phenomenon involving several genes and interactions with environmental factors. The Brazilian studies tried to find a factor that was unique to Brazil, but the significance of the differences remained unclear. “We found some signs that were specific to our population,” concluded Dr. Krieger. “But the reason that more people in Brazil died as a result of COVID-19 was not genetic,” he added.
Dr. Zatz and Dr. Krieger reported no conflicts of interest. This article was translated from the Medscape Portuguese edition.
A version of this article first appeared on Medscape.com.
A striking characteristic of COVID-19 is that the severity of clinical outcomes is remarkably variable. Establishing a prognosis for individuals infected with COVID-19 remains a challenge.
Since the start of the COVID-19 pandemic, the heterogeneity of individuals who progress toward severe disease or death, along with the fact that individuals directly exposed to the virus do not necessarily become sick, supports the hypothesis that genetic risk or protective factors are at play.
In an interview with this news organization, Mayana Zatz, PhD, head professor of genetics and coordinator of the Human Genome and Stem Cell Study Center at the University of São Paulo, explained: “The first case that caught my eye was the case of my neighbors, a couple. He presented COVID-19 symptoms, but his wife, who took care of him, had absolutely no symptoms. I thought that it was strange, but we received 3,000 emails from people saying, ‘This happened to me, too.’”
Reports in the media about seven pairs of monozygotic (MZ) twins who died from COVID-19 within days of one another in Brazil also stood out, said the researcher.
, as well as their pathology. Dr. Zatz’s team analyzed the case of a 31-year-old Brazilian MZ twin brother pair who presented simultaneously with severe COVID-19 and the need for oxygen support, despite their age and good health conditions. Curiously, they were admitted and intubated on the same day, but neither of the twins knew about the other’s situation; they found out only when they were extubated.
The study was carried out at the USP with the collaboration of the State University of São Paulo. The authors mapped the genetic profile (by sequencing the genome responsible for coding proteins, or whole-exome sequencing) and the immune cell profile to evaluate innate and adaptive immunity.
The MZ twin brothers shared the same two rare genetic mutations, which may be associated with their increased risk of developing severe COVID-19. However, since these variants were not studied at the protein or functional level, their pathogenicity has yet to be determined. The twins also had [human leukocyte antigen (HLA)] alleles associated with severe COVID-19, which are important candidates for the mechanisms of innate and adaptive immunity and susceptibility to COVID-19 infection and manifestation.
But one particular oddity stood out to the researchers: One of the brothers required longer hospitalization, and only he reported symptoms of long COVID.
In the authors’ eyes, even though the patients shared genetic mutations potentially associated with the risk of developing severe COVID-19, the differences in clinical progression emphasize that, beyond genetic risk factors, continuous exposure to pathogens over a lifetime and other environmental factors mean that each individual’s immune response is unique, even in twins.
“There is no doubt that genetics contribute to the severity of COVID-19, and environmental factors sometimes give us the opportunity to study the disease, too. Such [is the case with] MZ twins who have genetic similarities, even with changes that take place over a lifetime,” José Eduardo Krieger, MD, PhD, professor of molecular medicine at the University of São Paulo Medical School (FMUSP), told this news organization. “Examining MZ twins is a strategy that may help, but, with n = 2, luck really needs to be on your side to get straight to the problem. You need to combine [these findings] with other studies to solve this conundrum,” said Dr. Krieger, who did not take part in the research.
Large cohorts
Genomic and computer resources allow for the study of large sets of data from thousands of individuals. In each of those sets of data, the signal offered by thousands of markers distributed throughout the genome can be studied. This is the possibility offered by various genomic studies of large cohorts of patients with different clinical manifestations.
“Researchers examine thousands of genetic variants throughout the genome from a large sample of individuals and have the chance, for example, to identify genetic variants that are more prevalent in patients who have presented with severe disease than in those who presented with milder disease,” said Dr. Krieger. “These associations highlight a chromosome region in which one or more genes explain, at least in part, the differences observed.”
Genomewide association studies have identified some genetic variants that indicate severity of COVID-19, with potential impact on the virus entering the cell, the immune response, or the development of cytokine storms.
One of these studies, COVID-19 Host Genetics Initiative (COVID-19 HGI), is an international, open-science collaboration for sharing scientific methods and resources with research groups across the world, with the goal of robustly mapping the host genetic determinants of SARS-CoV-2 infection and the severity of the resulting COVID-19 disease. At the start of 2021, the COVID-19 HGI combined genetic data from 49,562 cases and 2 million controls from 46 studies in 19 countries. A total of 853 samples from the BRACOVID study were included in the meta-analysis. The endeavor enabled the identification of 13 genomewide significant loci that are associated with SARS-CoV-2 infection or severe manifestations of COVID-19.
The BRACOVID study, in which Dr. Krieger participates, aims to identify host genetic factors that determine the severity of COVID-19. It is currently the largest project of its kind in Latin America. An article provides the analysis of the first 5,233 participants in the BRACOVID study, who were recruited in São Paulo. Of these participants, 3,533 had been infected with COVID-19 and hospitalized at either the Heart Institute or the Central Institute of the FMUSP General Hospital. The remaining 1,700 made up the control group, which included health care professionals and members of the general population. The controls were recruited through serology assays or PCR tests for SARS-CoV-2.
The researchers discovered a region of chromosome 1 that could play a role in modulating immune response and that could lead to an increase in the likelihood of hospitalization across a wide range of COVID-19 risk factors. This region of chromosome 1 was observed only in Brazilians with a strong European ancestry; however, this finding had not been mentioned in previous studies, suggesting that it could harbor a risk allele specific to the Brazilian population.
The study also confirmed most, but not all, of the regions recorded in the literature, which may be significant in identifying factors determining severity that are specific to a given population.
Including information from the BRACOVID study, other studies have enhanced the knowledge on affected organs. Combined data from 14,000 patients from nine countries evaluated a region of a single chromosome and found that carriers of a certain allele had a higher probability of experiencing various COVID-19 complications, such as severe respiratory failure, venous thromboembolism, and liver damage. The risk was even higher for individuals aged 60 years and over.
Discordant couples
Smaller sample sizes of underrepresented populations also provide relevant data for genomic studies. Dr. Zatz’s team carried out genomic studies on smaller groups, comparing serodiscordant couples (where one was infected and symptomatic while the partner remained asymptomatic and seronegative despite sharing the same bedroom during the infection). Their research found genetic variants related to immune response that were associated with susceptibility to infection and progression to severe COVID-19.
The team also went on to study a group of patients older than 90 years who recovered from COVID-19 with mild symptoms or who remained asymptomatic following a positive test for SARS-CoV-2. They compared these patients with a sample of elderly patients from the same city (São Paulo), sampled before the current pandemic. The researchers identified a genetic variant related to mucin production. “In individuals with mild COVID-19, the degradation of these mucins would be more efficient,” said Dr. Zatz. It is possible for this variant to interfere not only with the production of mucus, but also in its composition, as there is an exchange of amino acids in the protein.
“We continued the study by comparing the extremes, i.e., those in their 90s with mild COVID-19 and younger patients with severe COVID-19, including several who died,” said Dr. Zatz.
More personalized medicine
The specialists agreed that a genetic test to predict COVID-19 severity is still a long way away. The genetic component is too little understood to enable the evaluation of individual risk. It has been possible to identify several important areas but, as Dr. Krieger pointed out, a variant identified in a certain chromosome interval may not be just one gene. There may be various candidate genes, or there may be a regulatory sequence for a distant gene. Furthermore, there are regions with genes that make sense as moderators of COVID-19 severity, because they regulate an inflammatory or immunologic reaction, but evidence is still lacking.
Reaching the molecular mechanism would, in future, allow a medicine to be chosen for a given patient, as already happens with other diseases. It also could enable the discovery of new medicines following as-yet-unexplored lines of research. Dr. Zatz also considers the possibility of genetic therapy.
Even with the knowledge of human genetics, one part of the equation is missing: viral genetics. “Many of the individuals who were resistant to the Delta variant were later affected by Omicron,” she pointed out.
Significance of Brazil
“We have an infinite amount of genomic data worldwide, but the vast majority originates from White Americans of European origin,” said Dr. Krieger. Moreover, genomic associations of COVID-19 severity discovered in the Chinese population were not significant in the European population. Besides underscoring the importance of collaborating with international studies, this situation supports scientists’ interest in carrying out genetic studies within Brazil, he added.
“In the genomic study of the Brazilian population, we found 2 million variants that were not present in the European populations,” said Dr. Zatz.
Dr. Krieger mentioned a technical advantage that Brazil has. “Having been colonized by different ethnic groups and mixed many generations ago, Brazil has a population with a unique genetic structure; the recombinations are different. When preparing the samples, the regions break differently.” This factor could help to separate, in a candidate region, the gene that is significant from those that might not be.
In general, severe COVID-19 would be a complex phenomenon involving several genes and interactions with environmental factors. The Brazilian studies tried to find a factor that was unique to Brazil, but the significance of the differences remained unclear. “We found some signs that were specific to our population,” concluded Dr. Krieger. “But the reason that more people in Brazil died as a result of COVID-19 was not genetic,” he added.
Dr. Zatz and Dr. Krieger reported no conflicts of interest. This article was translated from the Medscape Portuguese edition.
A version of this article first appeared on Medscape.com.
Obesity tied to worse brain health in children
CHICAGO – Higher weight and body mass index (BMI) in preadolescents are linked with poor brain health, new research suggests.
Poor brain health has been linked with obesity in adults, but little has been known about the link in children.
Lead author Simone Kaltenhauser, a postgraduate research fellow in radiology and biomedical imaging at the Yale School of Medicine, New Haven, Conn., presented her findings at the annual meeting of the Radiological Society of North America.
To represent the national sociodemographic makeup, the researchers used baseline information from the Adolescent Brain Cognitive Development (ABCD) study, which included 11,878 children aged 9 and 10 years from 21 centers across the United States.
This study included 5,169 children (51.9% girls). Children who had had traumatic brain injury, eating disorders, neurodevelopmental problems, and psychiatric diseases were excluded from the final analysis.
The researchers analyzed information from structural MRI and resting-state functional MRI, which allowed them to measure brain activity by detecting changes in blood flow.
“We analyzed the average fractional anisotropy (FA), mean (MD), axial (AD) and radial diffusivity (RD), and neurite density (ND) of 35 white matter (WM) tracts; cortical thickness and surface of 68 regions; and functional connectivity of 91 predefined network correlations,” the authors explained.
They adjusted for age, sex, race/ethnicity, handedness, and socioeconomic status. They used linear models to determine associations between weight and BMI z-scores and the imaging metrics.
Loss of white matter integrity
Among children with obesity, there was pervasive loss of white matter integrity and neurite density, cortical gray matter thinning, and decreased connectivity within and between networks that have been associated with impulse control and reward-based decision-making.
“These changes persisted in a similar pattern also 2 years later,” she said.
A member of the audience asked whether a reverse relationship might be at work – that poor brain health might drive obesity rather than the other way around.
Ms. Kaltenhauser agreed that other factors could be driving the link and acknowledged as a limitation that they did not have access to genetic information on the children.
Information on the effects of overweight and obesity is critical, especially in the United States, where an estimated 1 in 5 children are obese.
Ms. Kaltenhauser said she hopes her study raises awareness of potential brain health consequences as well as the physical consequences of childhood obesity.
Senior author Sam Payabvash, MD, a neuroradiologist and assistant professor of radiology and biomedical imaging at the Yale School of Medicine, said in a press release that the study may help explain the findings from previous studies that show an association between higher BMI in children and poor cognitive functioning and academic performance.
“The longitudinal ABCD study gives us the opportunity to observe any changes that occur in children with higher weight and BMI z-scores,” Dr. Payabvash said. “We’ll need to watch over the next 6-10 years.”
Avenues for future research
Amit B. Desai, MD, a neuroradiologist with Mayo Clinic in Jacksonville, Fla., who was not part of the study, said that while the research demonstrates an association between brain structure and BMI and obesity, “there may be other lurking variables.”
“What’s happening at an earlier stage in life could be causing both,” he said.
He noted that he would like to see future studies involving children at even earlier ages, along with investigations of the role of genetics or socioeconomic factors.
Including older children would be interesting as well, he said.
“Myelination doesn’t complete until you’re in your late teens or early 20s, so there are structural changes happening in the brain much later on into adolescence and early adulthood,” Dr. Desai said.
It would be premature, he said, to conclude from these findings that if children have obesity, there must be something wrong with their brain.
He would like to see whether there are changes in this link if a child is obese early on and later has normal weight or if a child has normal weight early and then becomes obese.
“It’s definitely an eye-opening study, but [it] needs additional work to expand upon it,” he said.
Ms. Kaltenhauser and Dr. Desai report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Higher weight and body mass index (BMI) in preadolescents are linked with poor brain health, new research suggests.
Poor brain health has been linked with obesity in adults, but little has been known about the link in children.
Lead author Simone Kaltenhauser, a postgraduate research fellow in radiology and biomedical imaging at the Yale School of Medicine, New Haven, Conn., presented her findings at the annual meeting of the Radiological Society of North America.
To represent the national sociodemographic makeup, the researchers used baseline information from the Adolescent Brain Cognitive Development (ABCD) study, which included 11,878 children aged 9 and 10 years from 21 centers across the United States.
This study included 5,169 children (51.9% girls). Children who had had traumatic brain injury, eating disorders, neurodevelopmental problems, and psychiatric diseases were excluded from the final analysis.
The researchers analyzed information from structural MRI and resting-state functional MRI, which allowed them to measure brain activity by detecting changes in blood flow.
“We analyzed the average fractional anisotropy (FA), mean (MD), axial (AD) and radial diffusivity (RD), and neurite density (ND) of 35 white matter (WM) tracts; cortical thickness and surface of 68 regions; and functional connectivity of 91 predefined network correlations,” the authors explained.
They adjusted for age, sex, race/ethnicity, handedness, and socioeconomic status. They used linear models to determine associations between weight and BMI z-scores and the imaging metrics.
Loss of white matter integrity
Among children with obesity, there was pervasive loss of white matter integrity and neurite density, cortical gray matter thinning, and decreased connectivity within and between networks that have been associated with impulse control and reward-based decision-making.
“These changes persisted in a similar pattern also 2 years later,” she said.
A member of the audience asked whether a reverse relationship might be at work – that poor brain health might drive obesity rather than the other way around.
Ms. Kaltenhauser agreed that other factors could be driving the link and acknowledged as a limitation that they did not have access to genetic information on the children.
Information on the effects of overweight and obesity is critical, especially in the United States, where an estimated 1 in 5 children are obese.
Ms. Kaltenhauser said she hopes her study raises awareness of potential brain health consequences as well as the physical consequences of childhood obesity.
Senior author Sam Payabvash, MD, a neuroradiologist and assistant professor of radiology and biomedical imaging at the Yale School of Medicine, said in a press release that the study may help explain the findings from previous studies that show an association between higher BMI in children and poor cognitive functioning and academic performance.
“The longitudinal ABCD study gives us the opportunity to observe any changes that occur in children with higher weight and BMI z-scores,” Dr. Payabvash said. “We’ll need to watch over the next 6-10 years.”
Avenues for future research
Amit B. Desai, MD, a neuroradiologist with Mayo Clinic in Jacksonville, Fla., who was not part of the study, said that while the research demonstrates an association between brain structure and BMI and obesity, “there may be other lurking variables.”
“What’s happening at an earlier stage in life could be causing both,” he said.
He noted that he would like to see future studies involving children at even earlier ages, along with investigations of the role of genetics or socioeconomic factors.
Including older children would be interesting as well, he said.
“Myelination doesn’t complete until you’re in your late teens or early 20s, so there are structural changes happening in the brain much later on into adolescence and early adulthood,” Dr. Desai said.
It would be premature, he said, to conclude from these findings that if children have obesity, there must be something wrong with their brain.
He would like to see whether there are changes in this link if a child is obese early on and later has normal weight or if a child has normal weight early and then becomes obese.
“It’s definitely an eye-opening study, but [it] needs additional work to expand upon it,” he said.
Ms. Kaltenhauser and Dr. Desai report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Higher weight and body mass index (BMI) in preadolescents are linked with poor brain health, new research suggests.
Poor brain health has been linked with obesity in adults, but little has been known about the link in children.
Lead author Simone Kaltenhauser, a postgraduate research fellow in radiology and biomedical imaging at the Yale School of Medicine, New Haven, Conn., presented her findings at the annual meeting of the Radiological Society of North America.
To represent the national sociodemographic makeup, the researchers used baseline information from the Adolescent Brain Cognitive Development (ABCD) study, which included 11,878 children aged 9 and 10 years from 21 centers across the United States.
This study included 5,169 children (51.9% girls). Children who had had traumatic brain injury, eating disorders, neurodevelopmental problems, and psychiatric diseases were excluded from the final analysis.
The researchers analyzed information from structural MRI and resting-state functional MRI, which allowed them to measure brain activity by detecting changes in blood flow.
“We analyzed the average fractional anisotropy (FA), mean (MD), axial (AD) and radial diffusivity (RD), and neurite density (ND) of 35 white matter (WM) tracts; cortical thickness and surface of 68 regions; and functional connectivity of 91 predefined network correlations,” the authors explained.
They adjusted for age, sex, race/ethnicity, handedness, and socioeconomic status. They used linear models to determine associations between weight and BMI z-scores and the imaging metrics.
Loss of white matter integrity
Among children with obesity, there was pervasive loss of white matter integrity and neurite density, cortical gray matter thinning, and decreased connectivity within and between networks that have been associated with impulse control and reward-based decision-making.
“These changes persisted in a similar pattern also 2 years later,” she said.
A member of the audience asked whether a reverse relationship might be at work – that poor brain health might drive obesity rather than the other way around.
Ms. Kaltenhauser agreed that other factors could be driving the link and acknowledged as a limitation that they did not have access to genetic information on the children.
Information on the effects of overweight and obesity is critical, especially in the United States, where an estimated 1 in 5 children are obese.
Ms. Kaltenhauser said she hopes her study raises awareness of potential brain health consequences as well as the physical consequences of childhood obesity.
Senior author Sam Payabvash, MD, a neuroradiologist and assistant professor of radiology and biomedical imaging at the Yale School of Medicine, said in a press release that the study may help explain the findings from previous studies that show an association between higher BMI in children and poor cognitive functioning and academic performance.
“The longitudinal ABCD study gives us the opportunity to observe any changes that occur in children with higher weight and BMI z-scores,” Dr. Payabvash said. “We’ll need to watch over the next 6-10 years.”
Avenues for future research
Amit B. Desai, MD, a neuroradiologist with Mayo Clinic in Jacksonville, Fla., who was not part of the study, said that while the research demonstrates an association between brain structure and BMI and obesity, “there may be other lurking variables.”
“What’s happening at an earlier stage in life could be causing both,” he said.
He noted that he would like to see future studies involving children at even earlier ages, along with investigations of the role of genetics or socioeconomic factors.
Including older children would be interesting as well, he said.
“Myelination doesn’t complete until you’re in your late teens or early 20s, so there are structural changes happening in the brain much later on into adolescence and early adulthood,” Dr. Desai said.
It would be premature, he said, to conclude from these findings that if children have obesity, there must be something wrong with their brain.
He would like to see whether there are changes in this link if a child is obese early on and later has normal weight or if a child has normal weight early and then becomes obese.
“It’s definitely an eye-opening study, but [it] needs additional work to expand upon it,” he said.
Ms. Kaltenhauser and Dr. Desai report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT RSNA 2022
Lp(a) tied to more early CV events than familial hypercholesterolemia
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
New studies change beliefs about cardiovascular disease
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Rates of health care use after bariatric surgery in teens
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.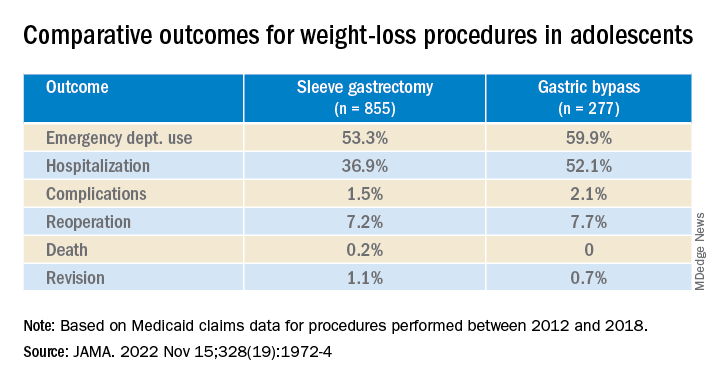
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Vitamin D fails to stave off statin-related muscle symptoms
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Transgender patients on hormone therapy require monitoring
PAU, France – Transgender patients on hormone therapy have an increased mortality risk and so must be closely monitored, especially in terms of cardiovascular health and oncology, reported Marie D’Assigny, MD, of the department of endocrinology, diabetes, and dietetics at Poitiers (France) University Hospital, at the Infogyn 2022 conference. Because transgender women (those assigned male at birth who have assumed a female gender identity) are at risk of breast cancer, they should also be recommended for breast cancer screening.
Transgender men and women, especially transgender women, “should be deemed high-risk cardiovascular patients, or even very high risk in some cases,” said Dr. D’Assigny. This means that they should be considered candidates for cholesterol-lowering medication earlier than their cisgender counterparts, and a target LDL cholesterol of less than 0.70 g/L (70 mg/dL) should be sought. Likewise, blood pressure must be strictly monitored, especially because it tends to rise when on hormone therapy.
Feminizing hormone therapy requires chemical castration with the use of anti-androgen drugs to achieve a blood testosterone level less than 0.5 ng/mL (1.73 nmol/L). Low-dose cyproterone acetate (< 25 to 50 mg/day) is usually used. Treatment is stopped if a patient undergoes an orchidectomy. For feminizing hormone therapy, administration of 17beta-estradiol transcutaneously (patch or gel) is recommended, because it is associated with a lower risk of thromboembolism than oral administration.
Masculinizing hormone therapy is based on administration of progestogens, then testosterone in the form of an injection (mostly testosterone enanthate via intramuscular injection every 10 days) or percutaneously (gel or patch). There are few contraindications, and treatment is generally well tolerated.
High mortality rate
A recent retrospective study highlighted the mortality and risk factors for death in transgender men and women receiving hormone therapy. More than 4,500 people, mostly male to female transgender women, were enrolled in this study, which was conducted over a 47-year period (1972-2018) at a specialist clinic at Amsterdam UMC.
Over the course of the study, the mortality rate in transgender men and women was twice that of the general population. The death rate was 10.8% in transgender women vs. 2.7% in transgender men, after a follow-up of 40,232 person-years and 17,285 person-years, respectively. In transgender women, mortality was nearly three times that of cisgender women in the general population.
Over the nearly 5 decades of study, there was no improvement in the mortality rate, even over the last 10 years when transgender issues started to be more recognized. The mortality trends are markedly distinct over the years from those observed in the cisgender population, and this is especially true for transgender women compared to transgender men. “Much is still to be done,” said Dr. D’Assigny.
According to the study, cause-specific mortality in transgender women was high for cardiovascular disease and lung cancer, possibly because of a higher smoking rate in this population. HIV-related disease and suicide remained very high in both transgender men and women.
People with gender dysphoria who do not receive treatment for gender reassignment have a suicide rate of 40%, reported François-Xavier Madec, MD, of Foch Hospital in Suresnes, France, at a previous presentation. For transgender men and women who receive care, this rate is lowered to 15%, which is still significantly higher than the rate of 1.6% observed in the general population.
“These causes of death don’t give any indication as to a specific effect of hormone treatment but show that monitoring and, if necessary, treatment of comorbidities and lifestyle-related factors are important in managing transgender patients,” said the study authors.
“Strengthening social acceptance and treating cardiovascular risk factors could also help to reduce mortality in transgender men and women,” they added.
Screening for osteoporosis
In addition to receiving cardiovascular risk factor assessment and monitoring, transgender men and women on hormone therapy should also undergo bone density testing “when risk factors for osteoporosis are present, especially in patients stopping hormone therapy after a gonadectomy,” said Dr. D’Assigny.
Calcium and vitamin D supplements are also recommended for all patients after a gonadectomy, especially in transgender men on testosterone. Osteoporosis screening is recommended for transgender men 10 years after starting treatment with testosterone, then every 10 years.
There is also the risk for breast cancer in transgender women, although the risk is lower than in cisgender women. This risk was highlighted in another study of more than 2,260 transgender women that was carried out by a team at Amsterdam UMC.
A total of 18 cases of breast cancer (15 invasive) were diagnosed after a median 18 years of hormone treatment. This represents an incidence of breast cancer that is 46 times higher than that expected in cisgender men of the same age but 3 times lower than in cisgender women.
The authors noted that “the risk of breast cancer in transgender women increases during a relatively short duration of hormone treatment,” going on to say that “these results suggest that breast cancer screening recommendations are relevant for transgender men and women on hormone therapy.”
Poorly attended screening
All of this means that transgender women older than age 50 years, as well as transgender men who have not had a mastectomy, should be offered a mammogram screening, taking into account the possible presence of implants in the former. Transgender women are also at risk for prostate cancer. Monitoring is personalized according to the individual risk of prostate disease, as it is for cisgender men.
There is no consensus on the monitoring of transgender men on hormone therapy for uterine cancer. Yet there is a risk. “Testosterone causes thinning of the endometrium, which may lead to dysplasia,” said Dr. D’Assigny. A physical examination once a year or a pelvic ultrasound scan every 2 years should form the basis of endometrial and ovarian appearance monitoring.
Transgender women are also at risk for prostate cancer. However, they are less likely to attend a prostate cancer screening test, said Dr. D’Assigny, which means “we need to raise awareness of their benefit in advance.” Vaginal swabs for transgender men and mammograms in transgender women “are resented, on both a physical and emotional level.” As a result, delays in diagnosis are common in transgender men and women.
Globally, access to care is still difficult for transgender patients because they don’t always receive appropriate gynecological monitoring, through fear of judgment or discrimination. Many transgender men and women are reluctant to see a gynecologist, even though they are at risk of gynecological cancers, as well as unwanted pregnancies in transgender men who have not undergone a hysterectomy.
In a demonstration of the collective desire to improve patient care for the transgender community, a literature review was recently published by a French team that analyzed gynecological monitoring methods in transgender patients. In September, the French National Authority for Health also issued a guidance memorandum on the transgender transition pathway, pending new recommendations scheduled for 2023.
A version of this article first appeared on Medscape.com.
This article was translated from the Medscape French edition.
PAU, France – Transgender patients on hormone therapy have an increased mortality risk and so must be closely monitored, especially in terms of cardiovascular health and oncology, reported Marie D’Assigny, MD, of the department of endocrinology, diabetes, and dietetics at Poitiers (France) University Hospital, at the Infogyn 2022 conference. Because transgender women (those assigned male at birth who have assumed a female gender identity) are at risk of breast cancer, they should also be recommended for breast cancer screening.
Transgender men and women, especially transgender women, “should be deemed high-risk cardiovascular patients, or even very high risk in some cases,” said Dr. D’Assigny. This means that they should be considered candidates for cholesterol-lowering medication earlier than their cisgender counterparts, and a target LDL cholesterol of less than 0.70 g/L (70 mg/dL) should be sought. Likewise, blood pressure must be strictly monitored, especially because it tends to rise when on hormone therapy.
Feminizing hormone therapy requires chemical castration with the use of anti-androgen drugs to achieve a blood testosterone level less than 0.5 ng/mL (1.73 nmol/L). Low-dose cyproterone acetate (< 25 to 50 mg/day) is usually used. Treatment is stopped if a patient undergoes an orchidectomy. For feminizing hormone therapy, administration of 17beta-estradiol transcutaneously (patch or gel) is recommended, because it is associated with a lower risk of thromboembolism than oral administration.
Masculinizing hormone therapy is based on administration of progestogens, then testosterone in the form of an injection (mostly testosterone enanthate via intramuscular injection every 10 days) or percutaneously (gel or patch). There are few contraindications, and treatment is generally well tolerated.
High mortality rate
A recent retrospective study highlighted the mortality and risk factors for death in transgender men and women receiving hormone therapy. More than 4,500 people, mostly male to female transgender women, were enrolled in this study, which was conducted over a 47-year period (1972-2018) at a specialist clinic at Amsterdam UMC.
Over the course of the study, the mortality rate in transgender men and women was twice that of the general population. The death rate was 10.8% in transgender women vs. 2.7% in transgender men, after a follow-up of 40,232 person-years and 17,285 person-years, respectively. In transgender women, mortality was nearly three times that of cisgender women in the general population.
Over the nearly 5 decades of study, there was no improvement in the mortality rate, even over the last 10 years when transgender issues started to be more recognized. The mortality trends are markedly distinct over the years from those observed in the cisgender population, and this is especially true for transgender women compared to transgender men. “Much is still to be done,” said Dr. D’Assigny.
According to the study, cause-specific mortality in transgender women was high for cardiovascular disease and lung cancer, possibly because of a higher smoking rate in this population. HIV-related disease and suicide remained very high in both transgender men and women.
People with gender dysphoria who do not receive treatment for gender reassignment have a suicide rate of 40%, reported François-Xavier Madec, MD, of Foch Hospital in Suresnes, France, at a previous presentation. For transgender men and women who receive care, this rate is lowered to 15%, which is still significantly higher than the rate of 1.6% observed in the general population.
“These causes of death don’t give any indication as to a specific effect of hormone treatment but show that monitoring and, if necessary, treatment of comorbidities and lifestyle-related factors are important in managing transgender patients,” said the study authors.
“Strengthening social acceptance and treating cardiovascular risk factors could also help to reduce mortality in transgender men and women,” they added.
Screening for osteoporosis
In addition to receiving cardiovascular risk factor assessment and monitoring, transgender men and women on hormone therapy should also undergo bone density testing “when risk factors for osteoporosis are present, especially in patients stopping hormone therapy after a gonadectomy,” said Dr. D’Assigny.
Calcium and vitamin D supplements are also recommended for all patients after a gonadectomy, especially in transgender men on testosterone. Osteoporosis screening is recommended for transgender men 10 years after starting treatment with testosterone, then every 10 years.
There is also the risk for breast cancer in transgender women, although the risk is lower than in cisgender women. This risk was highlighted in another study of more than 2,260 transgender women that was carried out by a team at Amsterdam UMC.
A total of 18 cases of breast cancer (15 invasive) were diagnosed after a median 18 years of hormone treatment. This represents an incidence of breast cancer that is 46 times higher than that expected in cisgender men of the same age but 3 times lower than in cisgender women.
The authors noted that “the risk of breast cancer in transgender women increases during a relatively short duration of hormone treatment,” going on to say that “these results suggest that breast cancer screening recommendations are relevant for transgender men and women on hormone therapy.”
Poorly attended screening
All of this means that transgender women older than age 50 years, as well as transgender men who have not had a mastectomy, should be offered a mammogram screening, taking into account the possible presence of implants in the former. Transgender women are also at risk for prostate cancer. Monitoring is personalized according to the individual risk of prostate disease, as it is for cisgender men.
There is no consensus on the monitoring of transgender men on hormone therapy for uterine cancer. Yet there is a risk. “Testosterone causes thinning of the endometrium, which may lead to dysplasia,” said Dr. D’Assigny. A physical examination once a year or a pelvic ultrasound scan every 2 years should form the basis of endometrial and ovarian appearance monitoring.
Transgender women are also at risk for prostate cancer. However, they are less likely to attend a prostate cancer screening test, said Dr. D’Assigny, which means “we need to raise awareness of their benefit in advance.” Vaginal swabs for transgender men and mammograms in transgender women “are resented, on both a physical and emotional level.” As a result, delays in diagnosis are common in transgender men and women.
Globally, access to care is still difficult for transgender patients because they don’t always receive appropriate gynecological monitoring, through fear of judgment or discrimination. Many transgender men and women are reluctant to see a gynecologist, even though they are at risk of gynecological cancers, as well as unwanted pregnancies in transgender men who have not undergone a hysterectomy.
In a demonstration of the collective desire to improve patient care for the transgender community, a literature review was recently published by a French team that analyzed gynecological monitoring methods in transgender patients. In September, the French National Authority for Health also issued a guidance memorandum on the transgender transition pathway, pending new recommendations scheduled for 2023.
A version of this article first appeared on Medscape.com.
This article was translated from the Medscape French edition.
PAU, France – Transgender patients on hormone therapy have an increased mortality risk and so must be closely monitored, especially in terms of cardiovascular health and oncology, reported Marie D’Assigny, MD, of the department of endocrinology, diabetes, and dietetics at Poitiers (France) University Hospital, at the Infogyn 2022 conference. Because transgender women (those assigned male at birth who have assumed a female gender identity) are at risk of breast cancer, they should also be recommended for breast cancer screening.
Transgender men and women, especially transgender women, “should be deemed high-risk cardiovascular patients, or even very high risk in some cases,” said Dr. D’Assigny. This means that they should be considered candidates for cholesterol-lowering medication earlier than their cisgender counterparts, and a target LDL cholesterol of less than 0.70 g/L (70 mg/dL) should be sought. Likewise, blood pressure must be strictly monitored, especially because it tends to rise when on hormone therapy.
Feminizing hormone therapy requires chemical castration with the use of anti-androgen drugs to achieve a blood testosterone level less than 0.5 ng/mL (1.73 nmol/L). Low-dose cyproterone acetate (< 25 to 50 mg/day) is usually used. Treatment is stopped if a patient undergoes an orchidectomy. For feminizing hormone therapy, administration of 17beta-estradiol transcutaneously (patch or gel) is recommended, because it is associated with a lower risk of thromboembolism than oral administration.
Masculinizing hormone therapy is based on administration of progestogens, then testosterone in the form of an injection (mostly testosterone enanthate via intramuscular injection every 10 days) or percutaneously (gel or patch). There are few contraindications, and treatment is generally well tolerated.
High mortality rate
A recent retrospective study highlighted the mortality and risk factors for death in transgender men and women receiving hormone therapy. More than 4,500 people, mostly male to female transgender women, were enrolled in this study, which was conducted over a 47-year period (1972-2018) at a specialist clinic at Amsterdam UMC.
Over the course of the study, the mortality rate in transgender men and women was twice that of the general population. The death rate was 10.8% in transgender women vs. 2.7% in transgender men, after a follow-up of 40,232 person-years and 17,285 person-years, respectively. In transgender women, mortality was nearly three times that of cisgender women in the general population.
Over the nearly 5 decades of study, there was no improvement in the mortality rate, even over the last 10 years when transgender issues started to be more recognized. The mortality trends are markedly distinct over the years from those observed in the cisgender population, and this is especially true for transgender women compared to transgender men. “Much is still to be done,” said Dr. D’Assigny.
According to the study, cause-specific mortality in transgender women was high for cardiovascular disease and lung cancer, possibly because of a higher smoking rate in this population. HIV-related disease and suicide remained very high in both transgender men and women.
People with gender dysphoria who do not receive treatment for gender reassignment have a suicide rate of 40%, reported François-Xavier Madec, MD, of Foch Hospital in Suresnes, France, at a previous presentation. For transgender men and women who receive care, this rate is lowered to 15%, which is still significantly higher than the rate of 1.6% observed in the general population.
“These causes of death don’t give any indication as to a specific effect of hormone treatment but show that monitoring and, if necessary, treatment of comorbidities and lifestyle-related factors are important in managing transgender patients,” said the study authors.
“Strengthening social acceptance and treating cardiovascular risk factors could also help to reduce mortality in transgender men and women,” they added.
Screening for osteoporosis
In addition to receiving cardiovascular risk factor assessment and monitoring, transgender men and women on hormone therapy should also undergo bone density testing “when risk factors for osteoporosis are present, especially in patients stopping hormone therapy after a gonadectomy,” said Dr. D’Assigny.
Calcium and vitamin D supplements are also recommended for all patients after a gonadectomy, especially in transgender men on testosterone. Osteoporosis screening is recommended for transgender men 10 years after starting treatment with testosterone, then every 10 years.
There is also the risk for breast cancer in transgender women, although the risk is lower than in cisgender women. This risk was highlighted in another study of more than 2,260 transgender women that was carried out by a team at Amsterdam UMC.
A total of 18 cases of breast cancer (15 invasive) were diagnosed after a median 18 years of hormone treatment. This represents an incidence of breast cancer that is 46 times higher than that expected in cisgender men of the same age but 3 times lower than in cisgender women.
The authors noted that “the risk of breast cancer in transgender women increases during a relatively short duration of hormone treatment,” going on to say that “these results suggest that breast cancer screening recommendations are relevant for transgender men and women on hormone therapy.”
Poorly attended screening
All of this means that transgender women older than age 50 years, as well as transgender men who have not had a mastectomy, should be offered a mammogram screening, taking into account the possible presence of implants in the former. Transgender women are also at risk for prostate cancer. Monitoring is personalized according to the individual risk of prostate disease, as it is for cisgender men.
There is no consensus on the monitoring of transgender men on hormone therapy for uterine cancer. Yet there is a risk. “Testosterone causes thinning of the endometrium, which may lead to dysplasia,” said Dr. D’Assigny. A physical examination once a year or a pelvic ultrasound scan every 2 years should form the basis of endometrial and ovarian appearance monitoring.
Transgender women are also at risk for prostate cancer. However, they are less likely to attend a prostate cancer screening test, said Dr. D’Assigny, which means “we need to raise awareness of their benefit in advance.” Vaginal swabs for transgender men and mammograms in transgender women “are resented, on both a physical and emotional level.” As a result, delays in diagnosis are common in transgender men and women.
Globally, access to care is still difficult for transgender patients because they don’t always receive appropriate gynecological monitoring, through fear of judgment or discrimination. Many transgender men and women are reluctant to see a gynecologist, even though they are at risk of gynecological cancers, as well as unwanted pregnancies in transgender men who have not undergone a hysterectomy.
In a demonstration of the collective desire to improve patient care for the transgender community, a literature review was recently published by a French team that analyzed gynecological monitoring methods in transgender patients. In September, the French National Authority for Health also issued a guidance memorandum on the transgender transition pathway, pending new recommendations scheduled for 2023.
A version of this article first appeared on Medscape.com.
This article was translated from the Medscape French edition.
New genetic variant linked to maturity-onset diabetes of the young
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
FROM THE LANCET REGIONAL HEALTH–EUROPE