User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
How cannabis-based therapeutics could help fight COVID inflammation
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
MADIT-CRT: Resynchronization linked to fewer heart failure hospitalizations
Patients with mild heart failure who received a cardiac resynchronization device had significantly reduced rates of hospitalizations for heart failure during follow-up of 1,820 patients for an average of 5.6 years, identifying in this post hoc analysis another benefit from this device that patients potentially receive in addition to an established survival advantage.
Extended follow-up of patients enrolled in the MADIT-CRT trial showed that patients with either New York Heart Association (NYHA) class I or II cardiomyopathy who received a cardiac resynchronization device with a defibrillator (CRT-D) had a significant reduction in all-cause hospitalization during follow-up, compared with control patients randomized to receive an implantable cardioverter defibrillator (ICD) device. This reduction in all hospitalizations was specifically driven by a significant reduction in cardiovascular hospitalizations, and the drop in cardiovascular hospitalizations was specifically driven by a cut in hospitalizations for heart failure (HHF), Sabu Thomas, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The data showed that during follow-up all-cause hospitalizations occurred in 73% of the CRT-D patients and 83% of those who received an ICD; cardiovascular hospitalizations happened in 29% of the CRT-D patients and in 43% of those with an ICD; and HHF occurred in 12% of the CRT-D patients and in 22% of those with an ICD, reported Dr. Thomas, a heart failure cardiologist at the University of Rochester (N.Y.) Medical Center. All three between-group differences were statistically significant for these post hoc endpoints.
These reduced hospitalizations also linked with better survival. Patients in the trial database with cardiovascular hospitalizations had a nearly fourfold higher rate of death, compared with nonhospitalized patients, Dr. Thomas said.
The findings “suggest that this device [CRT-D] has sustained benefit in these patients for up to 7 years,” said Dr. Thomas and his collaborator, Valentina Kutyifa, MD, in an interview. “However, this was only seen in patients with left bundle branch block [LBBB].” In patients with non-LBBB, CRT-D was not associated with a reduction in [cardiovascular] hospitalizations.
The LBBB connection
In a multivariate analysis, the 1,281 patients with LBBB (70% of the study cohort) who were more than 6 months out from device placement had a significant 43% relative cut in their incidence of cardiovascular hospitalizations, compared with that of control patients who received an ICD, while the 537 patients with non-LBBB showed no benefit from CRT-D treatment, compared with those who received an ICD, for reducing cardiovascular hospitalizations. (Data from two enrolled patients weren’t available for the analyses.) This finding that the HHF benefit focused in patients with LBBB was consistent with many prior observations that CRT-D was most effective in this patient subgroup.
The researchers also highlighted that their findings apply only to patients with NYHA functional class I or II heart failure with reduced ejection fraction (HFrEF), the only types of patients enrolled in the MADIT-CRT trial (15% had class I disease).
The results also showed that, during the first 6 months on CRT-D treatment, patients with a LBBB showed a significant 43% increase in their cardiovascular hospitalizations, compared with control patients, which may have been driven by device-related events. “We did not investigate this in detail, and it needs more study,” said Dr. Thomas and Dr. Kutyifa, a cardiac electrophysiologist at the University of Rochester.Their new findings extend the initial, prespecified results of the MADIT-CRT (Multicenter Automatic Defibrillator Implantation With Cardiac Resynchronization Therapy) trial, which was designed to examine a primary endpoint of death from any cause or a nonfatal heart failure event. During the initial average follow-up of 2.4 years, patients who received a CRT-D device had a significant relative reduction in this endpoint of 34%, compared with patients on ICD treatment, exclusively in patients with LBBB. Extended follow-up for as long as 7 years of the same cohort showed a continued significant reduction of all-cause death compared with controls, a 41% relative risk reduction, that again was only apparent in patients with LBBB.
The MADIT-CRT findings are generally consistent with prevailing CRT-D recommendations from the American College of Cardiology and American Heart Association from 2013 that give a class I indication (“is indicated”) for using the device in heart failure patients with LBBB, a QRS interval of at least 150 msec, NYHA class II-IV function, and a left ventricular ejection fraction no greater than 35%. A lesser, class IIa recommendation (“can be useful”) exists for patients with a narrower QRS of 120-149 msec with the other class I criteria, and for patients with non-LBBB the recommendation drops to class IIb (“may be considered”).
CRT-D ‘is mysterious,’ especially for non-LBBB patients
“Every time researchers have tried to move beyond the [existing] paradigm of who benefits from CRT-D, it’s never panned out,” commented Jeffrey J. Goldberger, MD, an electrophysiologist, professor, and chief of the cardiovascular division at the University of Miami. “The guidelines are pretty correct on who should get CRT-D. I wouldn’t say that no patients with non-LBBB should get it, but they are less likely to benefit,” although he conceded that responses to CRT-D are highly individualized and hard to predict.
“CRT is mysterious. I’ve had patients who did incredibly well on it,” but “once you start getting outside of where the benefits are proven, you start to run into issues,” Dr. Goldberger said in an interview. “The only solid predictor of a CRT-D response is in patients with LBBB.”
The hospitalizations for heart failure that the University of Rochester investigators assessed as an additional study outcome represent an “important endpoint, but one that is much more subjective than survival,” making its reliability “a bit of a gray area,” he said. The analyses are also limited by being post hoc and, hence, just hypothesis generating.
A recently published analysis of the same dataset by many of the same investigators hinted that CRT-D might reduce HHF in non-LBBB patients when the focus is on recurrent hospitalizations.
Despite the evidence of a survival benefit from CRT-D placement in selected patients, especially those with LBBB, “registry data have shown that use of CRT-D varies widely and has been as low as 27% of eligible patients,” noted Dr. Thomas and Dr. Kutyifa. “There is an opportunity here to understand the barriers to more widespread adoption of CRT-D in appropriate patients,” they said. It is also “possible that CRT-D is overused in non-LBBB patients” given that this subgroup receives about a third of CRT-D devices now. “Future studies should carefully investigate the role of CRT-D in non-LBBB patients.”
MADIT-CRT was funded by Boston Scientific, which markets several CRT-D devices. Dr. Thomas had no disclosures. Dr. Kutyifa has been a consultant to Biotronik and Zoll and has received research funding from Biotronik, Boston Scientific, Spire, and Zoll. Dr Goldberger is director of a not-for-profit think tank on risk stratification for sudden cardiac death that has received unrestricted educational grants from Abbott, Biotronik, Boston Scientific, and Medtronic.
SOURCE: Thomas S et al. HFSA 2020, Abstract 019.
Patients with mild heart failure who received a cardiac resynchronization device had significantly reduced rates of hospitalizations for heart failure during follow-up of 1,820 patients for an average of 5.6 years, identifying in this post hoc analysis another benefit from this device that patients potentially receive in addition to an established survival advantage.
Extended follow-up of patients enrolled in the MADIT-CRT trial showed that patients with either New York Heart Association (NYHA) class I or II cardiomyopathy who received a cardiac resynchronization device with a defibrillator (CRT-D) had a significant reduction in all-cause hospitalization during follow-up, compared with control patients randomized to receive an implantable cardioverter defibrillator (ICD) device. This reduction in all hospitalizations was specifically driven by a significant reduction in cardiovascular hospitalizations, and the drop in cardiovascular hospitalizations was specifically driven by a cut in hospitalizations for heart failure (HHF), Sabu Thomas, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The data showed that during follow-up all-cause hospitalizations occurred in 73% of the CRT-D patients and 83% of those who received an ICD; cardiovascular hospitalizations happened in 29% of the CRT-D patients and in 43% of those with an ICD; and HHF occurred in 12% of the CRT-D patients and in 22% of those with an ICD, reported Dr. Thomas, a heart failure cardiologist at the University of Rochester (N.Y.) Medical Center. All three between-group differences were statistically significant for these post hoc endpoints.
These reduced hospitalizations also linked with better survival. Patients in the trial database with cardiovascular hospitalizations had a nearly fourfold higher rate of death, compared with nonhospitalized patients, Dr. Thomas said.
The findings “suggest that this device [CRT-D] has sustained benefit in these patients for up to 7 years,” said Dr. Thomas and his collaborator, Valentina Kutyifa, MD, in an interview. “However, this was only seen in patients with left bundle branch block [LBBB].” In patients with non-LBBB, CRT-D was not associated with a reduction in [cardiovascular] hospitalizations.
The LBBB connection
In a multivariate analysis, the 1,281 patients with LBBB (70% of the study cohort) who were more than 6 months out from device placement had a significant 43% relative cut in their incidence of cardiovascular hospitalizations, compared with that of control patients who received an ICD, while the 537 patients with non-LBBB showed no benefit from CRT-D treatment, compared with those who received an ICD, for reducing cardiovascular hospitalizations. (Data from two enrolled patients weren’t available for the analyses.) This finding that the HHF benefit focused in patients with LBBB was consistent with many prior observations that CRT-D was most effective in this patient subgroup.
The researchers also highlighted that their findings apply only to patients with NYHA functional class I or II heart failure with reduced ejection fraction (HFrEF), the only types of patients enrolled in the MADIT-CRT trial (15% had class I disease).
The results also showed that, during the first 6 months on CRT-D treatment, patients with a LBBB showed a significant 43% increase in their cardiovascular hospitalizations, compared with control patients, which may have been driven by device-related events. “We did not investigate this in detail, and it needs more study,” said Dr. Thomas and Dr. Kutyifa, a cardiac electrophysiologist at the University of Rochester.Their new findings extend the initial, prespecified results of the MADIT-CRT (Multicenter Automatic Defibrillator Implantation With Cardiac Resynchronization Therapy) trial, which was designed to examine a primary endpoint of death from any cause or a nonfatal heart failure event. During the initial average follow-up of 2.4 years, patients who received a CRT-D device had a significant relative reduction in this endpoint of 34%, compared with patients on ICD treatment, exclusively in patients with LBBB. Extended follow-up for as long as 7 years of the same cohort showed a continued significant reduction of all-cause death compared with controls, a 41% relative risk reduction, that again was only apparent in patients with LBBB.
The MADIT-CRT findings are generally consistent with prevailing CRT-D recommendations from the American College of Cardiology and American Heart Association from 2013 that give a class I indication (“is indicated”) for using the device in heart failure patients with LBBB, a QRS interval of at least 150 msec, NYHA class II-IV function, and a left ventricular ejection fraction no greater than 35%. A lesser, class IIa recommendation (“can be useful”) exists for patients with a narrower QRS of 120-149 msec with the other class I criteria, and for patients with non-LBBB the recommendation drops to class IIb (“may be considered”).
CRT-D ‘is mysterious,’ especially for non-LBBB patients
“Every time researchers have tried to move beyond the [existing] paradigm of who benefits from CRT-D, it’s never panned out,” commented Jeffrey J. Goldberger, MD, an electrophysiologist, professor, and chief of the cardiovascular division at the University of Miami. “The guidelines are pretty correct on who should get CRT-D. I wouldn’t say that no patients with non-LBBB should get it, but they are less likely to benefit,” although he conceded that responses to CRT-D are highly individualized and hard to predict.
“CRT is mysterious. I’ve had patients who did incredibly well on it,” but “once you start getting outside of where the benefits are proven, you start to run into issues,” Dr. Goldberger said in an interview. “The only solid predictor of a CRT-D response is in patients with LBBB.”
The hospitalizations for heart failure that the University of Rochester investigators assessed as an additional study outcome represent an “important endpoint, but one that is much more subjective than survival,” making its reliability “a bit of a gray area,” he said. The analyses are also limited by being post hoc and, hence, just hypothesis generating.
A recently published analysis of the same dataset by many of the same investigators hinted that CRT-D might reduce HHF in non-LBBB patients when the focus is on recurrent hospitalizations.
Despite the evidence of a survival benefit from CRT-D placement in selected patients, especially those with LBBB, “registry data have shown that use of CRT-D varies widely and has been as low as 27% of eligible patients,” noted Dr. Thomas and Dr. Kutyifa. “There is an opportunity here to understand the barriers to more widespread adoption of CRT-D in appropriate patients,” they said. It is also “possible that CRT-D is overused in non-LBBB patients” given that this subgroup receives about a third of CRT-D devices now. “Future studies should carefully investigate the role of CRT-D in non-LBBB patients.”
MADIT-CRT was funded by Boston Scientific, which markets several CRT-D devices. Dr. Thomas had no disclosures. Dr. Kutyifa has been a consultant to Biotronik and Zoll and has received research funding from Biotronik, Boston Scientific, Spire, and Zoll. Dr Goldberger is director of a not-for-profit think tank on risk stratification for sudden cardiac death that has received unrestricted educational grants from Abbott, Biotronik, Boston Scientific, and Medtronic.
SOURCE: Thomas S et al. HFSA 2020, Abstract 019.
Patients with mild heart failure who received a cardiac resynchronization device had significantly reduced rates of hospitalizations for heart failure during follow-up of 1,820 patients for an average of 5.6 years, identifying in this post hoc analysis another benefit from this device that patients potentially receive in addition to an established survival advantage.
Extended follow-up of patients enrolled in the MADIT-CRT trial showed that patients with either New York Heart Association (NYHA) class I or II cardiomyopathy who received a cardiac resynchronization device with a defibrillator (CRT-D) had a significant reduction in all-cause hospitalization during follow-up, compared with control patients randomized to receive an implantable cardioverter defibrillator (ICD) device. This reduction in all hospitalizations was specifically driven by a significant reduction in cardiovascular hospitalizations, and the drop in cardiovascular hospitalizations was specifically driven by a cut in hospitalizations for heart failure (HHF), Sabu Thomas, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The data showed that during follow-up all-cause hospitalizations occurred in 73% of the CRT-D patients and 83% of those who received an ICD; cardiovascular hospitalizations happened in 29% of the CRT-D patients and in 43% of those with an ICD; and HHF occurred in 12% of the CRT-D patients and in 22% of those with an ICD, reported Dr. Thomas, a heart failure cardiologist at the University of Rochester (N.Y.) Medical Center. All three between-group differences were statistically significant for these post hoc endpoints.
These reduced hospitalizations also linked with better survival. Patients in the trial database with cardiovascular hospitalizations had a nearly fourfold higher rate of death, compared with nonhospitalized patients, Dr. Thomas said.
The findings “suggest that this device [CRT-D] has sustained benefit in these patients for up to 7 years,” said Dr. Thomas and his collaborator, Valentina Kutyifa, MD, in an interview. “However, this was only seen in patients with left bundle branch block [LBBB].” In patients with non-LBBB, CRT-D was not associated with a reduction in [cardiovascular] hospitalizations.
The LBBB connection
In a multivariate analysis, the 1,281 patients with LBBB (70% of the study cohort) who were more than 6 months out from device placement had a significant 43% relative cut in their incidence of cardiovascular hospitalizations, compared with that of control patients who received an ICD, while the 537 patients with non-LBBB showed no benefit from CRT-D treatment, compared with those who received an ICD, for reducing cardiovascular hospitalizations. (Data from two enrolled patients weren’t available for the analyses.) This finding that the HHF benefit focused in patients with LBBB was consistent with many prior observations that CRT-D was most effective in this patient subgroup.
The researchers also highlighted that their findings apply only to patients with NYHA functional class I or II heart failure with reduced ejection fraction (HFrEF), the only types of patients enrolled in the MADIT-CRT trial (15% had class I disease).
The results also showed that, during the first 6 months on CRT-D treatment, patients with a LBBB showed a significant 43% increase in their cardiovascular hospitalizations, compared with control patients, which may have been driven by device-related events. “We did not investigate this in detail, and it needs more study,” said Dr. Thomas and Dr. Kutyifa, a cardiac electrophysiologist at the University of Rochester.Their new findings extend the initial, prespecified results of the MADIT-CRT (Multicenter Automatic Defibrillator Implantation With Cardiac Resynchronization Therapy) trial, which was designed to examine a primary endpoint of death from any cause or a nonfatal heart failure event. During the initial average follow-up of 2.4 years, patients who received a CRT-D device had a significant relative reduction in this endpoint of 34%, compared with patients on ICD treatment, exclusively in patients with LBBB. Extended follow-up for as long as 7 years of the same cohort showed a continued significant reduction of all-cause death compared with controls, a 41% relative risk reduction, that again was only apparent in patients with LBBB.
The MADIT-CRT findings are generally consistent with prevailing CRT-D recommendations from the American College of Cardiology and American Heart Association from 2013 that give a class I indication (“is indicated”) for using the device in heart failure patients with LBBB, a QRS interval of at least 150 msec, NYHA class II-IV function, and a left ventricular ejection fraction no greater than 35%. A lesser, class IIa recommendation (“can be useful”) exists for patients with a narrower QRS of 120-149 msec with the other class I criteria, and for patients with non-LBBB the recommendation drops to class IIb (“may be considered”).
CRT-D ‘is mysterious,’ especially for non-LBBB patients
“Every time researchers have tried to move beyond the [existing] paradigm of who benefits from CRT-D, it’s never panned out,” commented Jeffrey J. Goldberger, MD, an electrophysiologist, professor, and chief of the cardiovascular division at the University of Miami. “The guidelines are pretty correct on who should get CRT-D. I wouldn’t say that no patients with non-LBBB should get it, but they are less likely to benefit,” although he conceded that responses to CRT-D are highly individualized and hard to predict.
“CRT is mysterious. I’ve had patients who did incredibly well on it,” but “once you start getting outside of where the benefits are proven, you start to run into issues,” Dr. Goldberger said in an interview. “The only solid predictor of a CRT-D response is in patients with LBBB.”
The hospitalizations for heart failure that the University of Rochester investigators assessed as an additional study outcome represent an “important endpoint, but one that is much more subjective than survival,” making its reliability “a bit of a gray area,” he said. The analyses are also limited by being post hoc and, hence, just hypothesis generating.
A recently published analysis of the same dataset by many of the same investigators hinted that CRT-D might reduce HHF in non-LBBB patients when the focus is on recurrent hospitalizations.
Despite the evidence of a survival benefit from CRT-D placement in selected patients, especially those with LBBB, “registry data have shown that use of CRT-D varies widely and has been as low as 27% of eligible patients,” noted Dr. Thomas and Dr. Kutyifa. “There is an opportunity here to understand the barriers to more widespread adoption of CRT-D in appropriate patients,” they said. It is also “possible that CRT-D is overused in non-LBBB patients” given that this subgroup receives about a third of CRT-D devices now. “Future studies should carefully investigate the role of CRT-D in non-LBBB patients.”
MADIT-CRT was funded by Boston Scientific, which markets several CRT-D devices. Dr. Thomas had no disclosures. Dr. Kutyifa has been a consultant to Biotronik and Zoll and has received research funding from Biotronik, Boston Scientific, Spire, and Zoll. Dr Goldberger is director of a not-for-profit think tank on risk stratification for sudden cardiac death that has received unrestricted educational grants from Abbott, Biotronik, Boston Scientific, and Medtronic.
SOURCE: Thomas S et al. HFSA 2020, Abstract 019.
FROM HFSA 2020
Survey finds European dermatologists unhappy with pandemic teledermatology experience
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
FROM THE EADV CONGRESS
COVID-19: U.S. sets new weekly high in children
the American Academy of Pediatrics announced Nov. 2.
For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
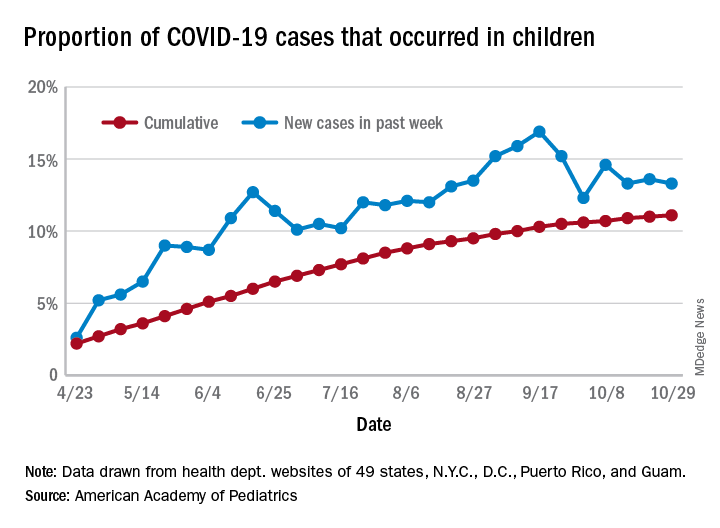
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
Biologics may protect psoriasis patients against severe COVID-19
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
FROM THE EADV CONGRESS
Updated heart failure measures add newer meds
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Health sector has spent $464 million on lobbying in 2020
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
COMPARE CRUSH: Crushed prehospital prasugrel misses mark in STEMI
Giving crushed prasugrel (Effient) to patients with ST-segment elevation myocardial infarction (STEMI) en route to a planned primary percutaneous coronary intervention (PCI) does not improve reperfusion rates, results of the COMPARE CRUSH trial show.
Patients assigned to prasugrel as crushed or integral tablets had similar rates of the study’s co-primary endpoints of thrombolysis in myocardial infarction (TIMI) 3 flow in the infarct-related artery at first angiography (31% vs. 32.7%; P = .64) and complete ST-segment resolution 1 hour post PCI (59.9% vs. 57.3%; P = .55).
“These findings hold in spite of the fact that crushed tablets of prasugrel led to more potent platelet inhibition compared with integral tablets,” said study author Georgios Vlachojannis, MD, PhD, University Medical Center Utrecht, the Netherlands.
“Whether faster and more potent antiplatelet therapy can improve coronary reperfusion in contemporary STEMI treatment regimen warrants further investigation.”
The results were reported in a late-breaking clinical science session at the Transcatheter Cardiovascular Therapeutics virtual annual meeting and published simultaneously in the journal Circulation. The meeting was sponsored by the Cardiovascular Research Foundation.
Fibrinolytics and glycoprotein IIb/IIIa inhibitors have demonstrated improved coronary reperfusion and outcomes when given pre hospital. Prior studies have also shown that early administration of a crushed P2Y12 inhibitor increases bioavailability and speeds platelet inhibition in STEMI patients, Dr. Vlachojannis noted.
However, the large randomized ATLANTIC trial, which compared prehospital to cath lab administration of crushed or integral ticagrelor (Brilinta), also found no difference in either TIMI flow in the infarct-related artery or ST-segment resolution.
Between November 2017 and March 2020, the investigator-initiated COMPARE CRUSH trial randomly allocated 727 STEMI patients (mean age, 62 years; 23% female) undergoing primary PCI to receive in the ambulance a 60-mg loading dose of prasugrel as either crushed or integral tablets.
The median time from onset of symptoms to first medical contact was 59 minutes, from first medical contact to study treatment 22 minutes, and from study treatment to primary PCI 57 minutes. These times did not differ between groups.
Platelet reactivity at the beginning of coronary angiography was significantly lower in the crushed group than in the integral group (P2Y12 reactivity units 192 vs. 227; P < .01). This resulted in significantly fewer patients in the crushed group with high platelet reactivity, defined as P2Y12 reactivity units >208, prior to the start of PCI (43.3% vs. 62.6%; P < .01).
There was no difference between the crushed and integral groups in the primary safety endpoint of TIMI major and BARC type 3 or higher bleeding within 48 hours after study treatment (0.4% vs 0.7%).
Death, MI, stroke, and urgent revascularization rates were also similar between groups during index hospitalization and at 30 days. Definite stent thrombosis occurred in one patient in the crushed group and two patients in the integral group.
In an exploratory analysis, the co-primary endpoint results were consistent across multiple subgroups, although there was a trend toward greater benefit on TIMI 3 flow in the crushed tablet group in patients older than age 75 years (P for interaction = .04), presenting with anterior infarction (P for interaction = .03), or with a history of prior PCI (P for interaction < .01).
“However, these results should be regarded as hypothesis-generating,” the authors wrote. “Opioids use in the ambulance was remarkably low in our study compared with the ATLANTIC trial, which might explain that we did not observe any significant interaction.”
Notably, morphine was used in half the ATLANTIC patients and was thought to have possibly delayed the absorption of ticagrelor.
During discussion following the presentation, Sunil V. Rao, MD, Duke University Medical Center, Durham, N.C., asked: “Based on what you found, which is really no clinical advantage but no safety issue either, are you having your patients with ST-segment MI administering crushed prasugrel now?”
Dr. Vlachojannis said they didn’t see any clinical impact but reiterated that high platelet reactivity was reduced by one-third. “If this now translates into a safer primary PCI procedure, we can’t say. The study wasn’t powered for this kind of endpoint. Is this enough to give you a recommendation, Sunil, I’m not sure.”
“What we know with COMPARE CRUSH, and this is important, is that we tried to give the medication as soon as possible and tried to give this medication in a formulation which has the most favorable pharmacodynamics profile, and we still see it’s not doing the job,” he added.
Fellow panelist Philippe Gabriel Steg, MD, Imperial College London, questioned whether treatment time may play a role in teasing out the relatively modest differences that platelet reactivity may have on clinical outcomes.
Dr. Vlachojannis said the time from symptom onset to first medical contact was very fast and similar to that in the ATLANTIC trial. “The short time intervals have certainly influenced the outcomes.”
Panelist Marco Valgimigli, MD, PhD, University Hospital Bern, Switzerland, followed up on the morphine issue, asking whether the investigators tested for an interaction between morphine or opioid use and platelet reactivity at the time of PCI.
“We haven’t looked into this but you probably have the ON-TIME 3 data in your mind when you’re asking this, where crushed ticagrelor given in the ambulance didn’t influence platelet reactivity at the time point of PCI,” Dr. Vlachojannis said. “We are going to look further into the data and certainly the platelet reactivity analysis is going to be very interesting in this data set.”
The study was an investigator-initiated trial sponsored by Maasstad Cardiovascular Research B.V. with unrestricted grants from Shanghai MicroPort Medical and Daiichi Sankyo. Dr. Vlachojannis declared receiving consulting fees from AstraZeneca, and research grants from Daiichi Sankyo and Shanghai MicroPort.
A version of this article originally appeared on Medscape.com.
Giving crushed prasugrel (Effient) to patients with ST-segment elevation myocardial infarction (STEMI) en route to a planned primary percutaneous coronary intervention (PCI) does not improve reperfusion rates, results of the COMPARE CRUSH trial show.
Patients assigned to prasugrel as crushed or integral tablets had similar rates of the study’s co-primary endpoints of thrombolysis in myocardial infarction (TIMI) 3 flow in the infarct-related artery at first angiography (31% vs. 32.7%; P = .64) and complete ST-segment resolution 1 hour post PCI (59.9% vs. 57.3%; P = .55).
“These findings hold in spite of the fact that crushed tablets of prasugrel led to more potent platelet inhibition compared with integral tablets,” said study author Georgios Vlachojannis, MD, PhD, University Medical Center Utrecht, the Netherlands.
“Whether faster and more potent antiplatelet therapy can improve coronary reperfusion in contemporary STEMI treatment regimen warrants further investigation.”
The results were reported in a late-breaking clinical science session at the Transcatheter Cardiovascular Therapeutics virtual annual meeting and published simultaneously in the journal Circulation. The meeting was sponsored by the Cardiovascular Research Foundation.
Fibrinolytics and glycoprotein IIb/IIIa inhibitors have demonstrated improved coronary reperfusion and outcomes when given pre hospital. Prior studies have also shown that early administration of a crushed P2Y12 inhibitor increases bioavailability and speeds platelet inhibition in STEMI patients, Dr. Vlachojannis noted.
However, the large randomized ATLANTIC trial, which compared prehospital to cath lab administration of crushed or integral ticagrelor (Brilinta), also found no difference in either TIMI flow in the infarct-related artery or ST-segment resolution.
Between November 2017 and March 2020, the investigator-initiated COMPARE CRUSH trial randomly allocated 727 STEMI patients (mean age, 62 years; 23% female) undergoing primary PCI to receive in the ambulance a 60-mg loading dose of prasugrel as either crushed or integral tablets.
The median time from onset of symptoms to first medical contact was 59 minutes, from first medical contact to study treatment 22 minutes, and from study treatment to primary PCI 57 minutes. These times did not differ between groups.
Platelet reactivity at the beginning of coronary angiography was significantly lower in the crushed group than in the integral group (P2Y12 reactivity units 192 vs. 227; P < .01). This resulted in significantly fewer patients in the crushed group with high platelet reactivity, defined as P2Y12 reactivity units >208, prior to the start of PCI (43.3% vs. 62.6%; P < .01).
There was no difference between the crushed and integral groups in the primary safety endpoint of TIMI major and BARC type 3 or higher bleeding within 48 hours after study treatment (0.4% vs 0.7%).
Death, MI, stroke, and urgent revascularization rates were also similar between groups during index hospitalization and at 30 days. Definite stent thrombosis occurred in one patient in the crushed group and two patients in the integral group.
In an exploratory analysis, the co-primary endpoint results were consistent across multiple subgroups, although there was a trend toward greater benefit on TIMI 3 flow in the crushed tablet group in patients older than age 75 years (P for interaction = .04), presenting with anterior infarction (P for interaction = .03), or with a history of prior PCI (P for interaction < .01).
“However, these results should be regarded as hypothesis-generating,” the authors wrote. “Opioids use in the ambulance was remarkably low in our study compared with the ATLANTIC trial, which might explain that we did not observe any significant interaction.”
Notably, morphine was used in half the ATLANTIC patients and was thought to have possibly delayed the absorption of ticagrelor.
During discussion following the presentation, Sunil V. Rao, MD, Duke University Medical Center, Durham, N.C., asked: “Based on what you found, which is really no clinical advantage but no safety issue either, are you having your patients with ST-segment MI administering crushed prasugrel now?”
Dr. Vlachojannis said they didn’t see any clinical impact but reiterated that high platelet reactivity was reduced by one-third. “If this now translates into a safer primary PCI procedure, we can’t say. The study wasn’t powered for this kind of endpoint. Is this enough to give you a recommendation, Sunil, I’m not sure.”
“What we know with COMPARE CRUSH, and this is important, is that we tried to give the medication as soon as possible and tried to give this medication in a formulation which has the most favorable pharmacodynamics profile, and we still see it’s not doing the job,” he added.
Fellow panelist Philippe Gabriel Steg, MD, Imperial College London, questioned whether treatment time may play a role in teasing out the relatively modest differences that platelet reactivity may have on clinical outcomes.
Dr. Vlachojannis said the time from symptom onset to first medical contact was very fast and similar to that in the ATLANTIC trial. “The short time intervals have certainly influenced the outcomes.”
Panelist Marco Valgimigli, MD, PhD, University Hospital Bern, Switzerland, followed up on the morphine issue, asking whether the investigators tested for an interaction between morphine or opioid use and platelet reactivity at the time of PCI.
“We haven’t looked into this but you probably have the ON-TIME 3 data in your mind when you’re asking this, where crushed ticagrelor given in the ambulance didn’t influence platelet reactivity at the time point of PCI,” Dr. Vlachojannis said. “We are going to look further into the data and certainly the platelet reactivity analysis is going to be very interesting in this data set.”
The study was an investigator-initiated trial sponsored by Maasstad Cardiovascular Research B.V. with unrestricted grants from Shanghai MicroPort Medical and Daiichi Sankyo. Dr. Vlachojannis declared receiving consulting fees from AstraZeneca, and research grants from Daiichi Sankyo and Shanghai MicroPort.
A version of this article originally appeared on Medscape.com.
Giving crushed prasugrel (Effient) to patients with ST-segment elevation myocardial infarction (STEMI) en route to a planned primary percutaneous coronary intervention (PCI) does not improve reperfusion rates, results of the COMPARE CRUSH trial show.
Patients assigned to prasugrel as crushed or integral tablets had similar rates of the study’s co-primary endpoints of thrombolysis in myocardial infarction (TIMI) 3 flow in the infarct-related artery at first angiography (31% vs. 32.7%; P = .64) and complete ST-segment resolution 1 hour post PCI (59.9% vs. 57.3%; P = .55).
“These findings hold in spite of the fact that crushed tablets of prasugrel led to more potent platelet inhibition compared with integral tablets,” said study author Georgios Vlachojannis, MD, PhD, University Medical Center Utrecht, the Netherlands.
“Whether faster and more potent antiplatelet therapy can improve coronary reperfusion in contemporary STEMI treatment regimen warrants further investigation.”
The results were reported in a late-breaking clinical science session at the Transcatheter Cardiovascular Therapeutics virtual annual meeting and published simultaneously in the journal Circulation. The meeting was sponsored by the Cardiovascular Research Foundation.
Fibrinolytics and glycoprotein IIb/IIIa inhibitors have demonstrated improved coronary reperfusion and outcomes when given pre hospital. Prior studies have also shown that early administration of a crushed P2Y12 inhibitor increases bioavailability and speeds platelet inhibition in STEMI patients, Dr. Vlachojannis noted.
However, the large randomized ATLANTIC trial, which compared prehospital to cath lab administration of crushed or integral ticagrelor (Brilinta), also found no difference in either TIMI flow in the infarct-related artery or ST-segment resolution.
Between November 2017 and March 2020, the investigator-initiated COMPARE CRUSH trial randomly allocated 727 STEMI patients (mean age, 62 years; 23% female) undergoing primary PCI to receive in the ambulance a 60-mg loading dose of prasugrel as either crushed or integral tablets.
The median time from onset of symptoms to first medical contact was 59 minutes, from first medical contact to study treatment 22 minutes, and from study treatment to primary PCI 57 minutes. These times did not differ between groups.
Platelet reactivity at the beginning of coronary angiography was significantly lower in the crushed group than in the integral group (P2Y12 reactivity units 192 vs. 227; P < .01). This resulted in significantly fewer patients in the crushed group with high platelet reactivity, defined as P2Y12 reactivity units >208, prior to the start of PCI (43.3% vs. 62.6%; P < .01).
There was no difference between the crushed and integral groups in the primary safety endpoint of TIMI major and BARC type 3 or higher bleeding within 48 hours after study treatment (0.4% vs 0.7%).
Death, MI, stroke, and urgent revascularization rates were also similar between groups during index hospitalization and at 30 days. Definite stent thrombosis occurred in one patient in the crushed group and two patients in the integral group.
In an exploratory analysis, the co-primary endpoint results were consistent across multiple subgroups, although there was a trend toward greater benefit on TIMI 3 flow in the crushed tablet group in patients older than age 75 years (P for interaction = .04), presenting with anterior infarction (P for interaction = .03), or with a history of prior PCI (P for interaction < .01).
“However, these results should be regarded as hypothesis-generating,” the authors wrote. “Opioids use in the ambulance was remarkably low in our study compared with the ATLANTIC trial, which might explain that we did not observe any significant interaction.”
Notably, morphine was used in half the ATLANTIC patients and was thought to have possibly delayed the absorption of ticagrelor.
During discussion following the presentation, Sunil V. Rao, MD, Duke University Medical Center, Durham, N.C., asked: “Based on what you found, which is really no clinical advantage but no safety issue either, are you having your patients with ST-segment MI administering crushed prasugrel now?”
Dr. Vlachojannis said they didn’t see any clinical impact but reiterated that high platelet reactivity was reduced by one-third. “If this now translates into a safer primary PCI procedure, we can’t say. The study wasn’t powered for this kind of endpoint. Is this enough to give you a recommendation, Sunil, I’m not sure.”
“What we know with COMPARE CRUSH, and this is important, is that we tried to give the medication as soon as possible and tried to give this medication in a formulation which has the most favorable pharmacodynamics profile, and we still see it’s not doing the job,” he added.
Fellow panelist Philippe Gabriel Steg, MD, Imperial College London, questioned whether treatment time may play a role in teasing out the relatively modest differences that platelet reactivity may have on clinical outcomes.
Dr. Vlachojannis said the time from symptom onset to first medical contact was very fast and similar to that in the ATLANTIC trial. “The short time intervals have certainly influenced the outcomes.”
Panelist Marco Valgimigli, MD, PhD, University Hospital Bern, Switzerland, followed up on the morphine issue, asking whether the investigators tested for an interaction between morphine or opioid use and platelet reactivity at the time of PCI.
“We haven’t looked into this but you probably have the ON-TIME 3 data in your mind when you’re asking this, where crushed ticagrelor given in the ambulance didn’t influence platelet reactivity at the time point of PCI,” Dr. Vlachojannis said. “We are going to look further into the data and certainly the platelet reactivity analysis is going to be very interesting in this data set.”
The study was an investigator-initiated trial sponsored by Maasstad Cardiovascular Research B.V. with unrestricted grants from Shanghai MicroPort Medical and Daiichi Sankyo. Dr. Vlachojannis declared receiving consulting fees from AstraZeneca, and research grants from Daiichi Sankyo and Shanghai MicroPort.
A version of this article originally appeared on Medscape.com.
Is the tide turning on the ‘grubby’ affair of EXCEL and the European guidelines?
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
Frivolous lawsuits: Still a big threat to doctors?
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.