User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Physician-Owned Hospitals: The Answer for Better Care?
This discussion was recorded on November 16, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Dr. Brian J. Miller, a hospitalist with Johns Hopkins University School of Medicine and a health policy expert, to discuss the current and renewed interest in physician-owned hospitals.
Welcome, Dr. Miller. It’s a pleasure to have you join me today.
Brian J. Miller, MD, MBA, MPH: Thank you for having me.
History and Controversies Surrounding Physician-Owned Hospitals
Dr. Glatter: I want to start off by having you describe the history associated with the moratorium on new physician-owned hospitals in 2010 that’s related ultimately to the Affordable Care Act, but also, the current and renewed media interest in physician-owned hospitals that’s linked to recent congressional hearings last month.
Dr. Miller: Thank you. I should note that my views are my own and don’t represent those of Hopkins or the American Enterprise Institute, where I’m a nonresident fellow nor the Medicare Payment Advisory Commission, of which I’m a Commissioner.
The story about physician-owned hospitals is an interesting one. Hospitals turned into health systems in the 1980s and 1990s, and physicians started to shift purely from an independent model into a more organized group practice or employed model. Physicians realized that they wanted an alternative operating arrangement. You want a choice of how you practice and what your employment is. And as community hospitals started to buy physicians and also establish their own physician groups de novo, physicians opened physician-owned hospitals.
Physician-owned hospitals fell into a couple of buckets. One is what we call community hospitals, or what the antitrust lawyers would call general acute care hospitals: those offering emergency room (ER) services, labor and delivery, primary care, general surgery — the whole regular gamut, except that some of the owners were physicians.
The other half of the marketplace ended up being specialty hospitals: those built around a specific medical specialty and series of procedures and chronic care. For example, cardiac hospitals often do CABG, TAVR, maybe abdominal aortic aneurysm (triple A) repairs, and they have cardiology clinics, cath labs, a cardiac intensive care unit (ICU), ER, etc. There were also orthopedic surgical specialty hospitals, which were sort of like an ambulatory surgery center (ASC) plus several beds. Then there were general surgical specialty hospitals. At one point, there were some women’s health–focused specialty hospitals.
The hospital industry, of course, as you can understand, didn’t exactly like this. They had a series of concerns about what we would historically call cherry-picking or lemon-dropping of patients. They were worried that physician-owned facilities didn’t want to serve public payer patients, and there was a whole series of reports and investigations.
Around the time the Affordable Care Act passed, the hospital industry had many concerns about physician-owned specialty hospitals, and there was a moratorium as part of the 2003 Medicare Modernization Act. As part of the bargaining over the hospital industry support for the Affordable Care Act, they traded their support for, among other things, their number one priority, which is a statutory prohibition on new or expanded physician-owned hospitals from participating in Medicare. That included both physician-owned community hospitals and physician-owned specialty hospitals.
Dr. Glatter: That was part of the impetus to prevent physicians from referring patients where they had an ownership stake. Certainly, hospitals can be owned by attorneys and nonprofit organizations, and certainly, ASCs can be owned by physicians. There is an ongoing issue in terms of physicians not being able to have an ownership stake. In terms of equity ownership, we know that certain other models allow this, but basically, it sounds like this is an issue with Medicare. That seems to be the crux of it, correct?
Dr. Miller: Yes. I would also add that it’s interesting when we look at other professions. When we look at lawyers, nonlawyers are actually not allowed to own an equity stake in a law practice. In many other professions, you either have corporate ownership or professional ownership, or the alternative is you have only professional ownership. I would say the hospital industry is one of the few areas where professional ownership not only is not allowed, but also is statutorily prohibited functionally through the Medicare program.
Unveiling the Dynamics of Hospital Ownership
Dr. Glatter: A recent study done by two PhDs looked at 2019 data on 20 of the most expensive diagnosis-related groups (DRGs). It examined the cost savings, and we’re talking over $1 billion in expenditures when you look at the data from general acute care hospitals vs physician-owned hospitals. This is what appears to me to be a key driver of the push to loosen restrictions on physician-owned hospitals. Isn’t that correct?
Dr. Miller: I would say that’s one of many components. There’s more history to this issue. I remember sitting at a think tank talking to someone several years ago about hospital consolidation as an issue. We went through the usual levers that us policy wonks go through. We talked about antitrust enforcement, certificate of need, rising hospital costs from consolidation, lower quality (or at least no quality gains, as shown by a New England Journal of Medicine study), and decrements in patient experience that result from the diseconomies of scale. They sort of pooh-poohed many of the policy ideas. They basically said that there was no hope for hospital consolidation as an issue.
Well, what about physician ownership? I started with my research team to comb through the literature and found a variety of studies — some of which were sort of entertaining, because they’d do things like study physician-owned specialty hospitals, nonprofit-owned specialty hospitals, and for-profit specialty hospitals and compare them with nonprofit or for-profit community hospitals, and then say physician-owned hospitals that were specialty were bad.
They mixed ownership and service markets right there in so many ways, I’m not sure where to start. My team did a systematic review of around 30 years of research, looking at the evidence base in this space. We found a couple of things.
We found that physician-owned community hospitals did not have a cost or quality difference, meaning that there was no definitive evidence that the physician-owned community hospitals were cheaper based on historical evidence, which was very old. That means there’s not specific harm from them. When you permit market entry for community hospitals, that promotes competition, which results in lower prices and higher quality.
Then we also looked at the specialty hospital markets — surgical specialty hospitals, orthopedic surgical specialty hospitals, and cardiac hospitals. We noted for cardiac hospitals, there wasn’t clear evidence about cost savings, but there was definitive evidence of higher quality, from things like 30-day mortality for significant procedures like treatment of acute MI, triple A repair, stuff like that.
For orthopedic surgical specialty hospitals, we noted lower costs and higher quality, which again fits with operationally what we would know. If you have a facility that’s doing 20 total hips a day, you’re creating a focused factory. Just like if you think about it for interventional cardiology, your boards have a minimum number of procedures that you have to do to stay certified because we know about the volume-quality relationship.
Then we looked at general surgical specialty hospitals. There wasn’t enough evidence to make a conclusive thought about costs, and there was a clear trend toward higher quality. I would say this recent study is important, but there is a whole bunch of other literature out there, too.
Exploring the Scope of Emergency Care in Physician-Owned Hospitals
Dr. Glatter: Certainly, your colleague Wang from Johns Hopkins has done important research in this sector. The paper, “Reconsidering the Ban on Physician-Owned Hospitals to Combat Consolidation,” by you and several colleagues, mentions and highlights the issues that you just described. I understand that it’s going to be published in the NYU Journal of Legislation and Public Policy.
One thing I want to bring up — and this is an important issue — is that the risk for patients has been talked about by the American Hospital Association and the Federation of American Hospitals, in terms of limited or no emergency services at such physician-owned hospitals and having to call 911 when patients need emergent care or stabilization. That’s been the rebuttal, along with an Office of Inspector General (OIG) report from 2008. Almost, I guess, three quarters of the patients that needed emergent care got this at publicly funded hospitals.
Dr. Miller: I’m familiar with the argument about emergency care. If you actually go and look at it, it differs by specialty market. Physician-owned community hospitals have ERs because that’s how they get their business. If you are running a hospital medicine floor, a general surgical specialty floor, you have a labor delivery unit, a primary care clinic, and a cardiology clinic. You have all the things that all the other hospitals have. The physician-owned community hospitals almost uniformly have an ER.
When you look at the physician-owned specialty hospitals, it’s a little more granular. If you look at the cardiac hospitals, they have ERs. They also have cardiac ICUs, operating rooms, etc. The area where the hospital industry had concerns — which I think is valid to point out — is that physician-owned orthopedic surgical specialty hospitals don’t have ERs. But this makes sense because of what that hospital functionally is: a factory for whatever the scope of procedures is, be it joint replacements or shoulder arthroscopy. The orthopedic surgical specialty hospital is like an ASC plus several hospital beds. Many of those did not have ERs because clinically it didn’t make sense.
What’s interesting, though, is that the hospital industry also operates specialty hospitals. If you go into many of the large systems, they have cardiac specialty hospitals and cancer specialty hospitals. I would say that some of them have ERs, as they appropriately should, and some of those specialty hospitals do not. They might have a community hospital down the street that’s part of that health system that has an ER, but some of the specialty hospitals don’t necessarily have a dedicated ER.
I agree, that’s a valid concern. I would say, though, the question is, what are the scope of services in that hospital? Is an ER required? Community hospitals should have ERs. It makes sense also for a cardiac hospital to have one. If you’re running a total joint replacement factory, it might not make clinical sense.
Dr. Glatter: The patients who are treated at that hospital, if they do have emergent conditions, need to have board-certified emergency physicians treating them, in my view because I’m an ER physician. Having surgeons that are not emergency physicians staff a department at a specialty orthopedic hospital or, say, a cancer hospital is not acceptable from my standpoint. That›s my opinion and recommendation, coming from emergency medicine.
Dr. Miller: I would say that anesthesiologists are actually highly qualified in critical care. The question is about clinical decompensation; if you’re doing a procedure, you have an anesthesiologist right there who is capable of critical care. The function of the ER is to either serve as a window into the hospital for patient volume or to serve as a referral for emergent complaints.
Dr. Glatter: An anesthesiologist — I’ll take issue with that — does not have the training of an emergency physician in terms of scope of practice.
Dr. Miller: My anesthesiology colleagues would probably disagree for managing an emergency during an operating room case.
Dr. Glatter: Fair enough, but I think in the general sense. The other issue is that, in terms of emergent responses to patients that decompensate, when you have to transfer a patient, that violates Medicare requirements. How is that even a valid issue or argument if you’re going to have to transfer a patient from your specialty hospital? That happens. Again, I know that you’re saying these hospitals are completely independent and can function, stabilize patients, and treat emergencies, but that’s not the reality across the country, in my opinion.
Dr. Miller: I don’t think that’s the case for the physician-owned specialty cardiac hospitals, for starters. Many of those have ICUs in addition to operating rooms as a matter of routine in addition to ERs. I don’t think that’s the case for physician-owned community hospitals, which have ERs, ICUs, medicine floors, and surgical floors. Physician-owned community hospitals are around half the market. Of that remaining market, a significant percentage are cardiac hospitals. If you’re taking an issue with orthopedic surgical specialty hospitals, that’s a clinical operational question that can and should be answered.
I’d also posit that the nonprofit and for-profit hospital industries also operate specialty hospitals. Any of these questions, we shouldn’t just be asking about physician-owned facilities; we should be asking about them across ownership types, because we’re talking about scope of service and quality and safety. The ownership in that case doesn’t matter. The broader question is, are orthopedic surgical specialty hospitals owned by physicians, tax-exempt hospitals, or tax-paying hospitals? Is that a valid clinical business model? Is it safe? Does it meet Medicare conditions of participation? I would say that’s what that question is, because other ownership models do operate those facilities.
Dr. Glatter: You make some valid points, and I do agree on some of them. I think that, ultimately, these models of care, and certainly cost and quality, are issues. Again, it goes back to being able, in my opinion, to provide emergent care, which seems to me a very important issue.
Dr. Miller: I agree that providing emergent care is an issue. It›s an issue in any site of care. The hospital industry posits that all hospital outpatient departments (HOPDs) have emergent care. I can tell you, having worked in HOPDs (I›ve trained in them during residency), the response if something emergent happens is to either call 911 or wheel the patient down to the ER in a wheelchair or stretcher. I think that these hospital claims about emergency care coverage — these are important questions, but we should be asking them across all clinical settings and say what is the appropriate scope of care provided? What is the appropriate level of acuity and ability to provide emergent or critical care? That›s an important question regardless of ownership model across the entire industry.
Deeper Dive Into Data on Physician-Owned Hospitals
Dr. Glatter: We need to really focus on that. I’ll agree with you on that.
There was a March 2023 report from Dobson | DaVanzo. It showed that physician-owned hospitals had lower Medicaid, dual-eligible, and uncompensated care and charity care discharges than full-service acute care hospitals. Physician-owned hospitals had less than half the proportion of Medicaid discharges compared with non–physician-owned hospitals. They were also less likely to care for dual-eligible patients overall compared with non–physician-owned hospitals.
In addition, when COVID hit, the physician-owned hospitals overall — and again, there may be exceptions — were not equipped to handle these patient surges in the acute setting of a public health emergency. There was a hospital in Texas that did pivot that I’m aware of — Renaissance Hospital, which ramped up a long-term care facility to become a COVID hospital — but I think that’s the exception. I think this report raises some valid concerns; I’ll let you rebut that.
Dr. Miller: A couple of things. One, I am not aware that there’s any clear market evidence or a systematic study that shows that physician-owned hospitals had trouble responding to COVID. I don’t think that assertion has been proven. The study was funded by the hospital industry. First of all, it was not a peer-reviewed study; it was funded by an industry that paid a consulting firm. It doesn’t mean that we still shouldn’t read it, but that brings bias into question. The joke in Washington is, pick your favorite statistician or economist, and they can say what you want and have a battle of economists and statisticians.
For example, in that study, they didn’t include the entire ownership universe of physician-owned hospitals. If we go to the peer-reviewed literature, there’s a great 2015 BMJ paper showing that the Medicaid payer mix is actually the same between physician-owned hospitals vs not. The mix of patients by ethnicity — for example, think about African American patients — was the same. I would be more inclined to believe the peer-reviewed literature in BMJ as opposed to an industry-funded study that was not peer-reviewed and not independent and has methodological questions.
Dr. Glatter: Those data are 8 years old, so I’d like to see more recent data. It would be interesting, just as a follow-up to that, to see where the needle has moved — if it has, for that matter — in terms of Medicaid patients that you’re referring to.
Dr. Miller: I tend to be skeptical of all industry research, regardless of who published it, because they have an economic incentive. If they’re selecting certain age groups or excluding certain hospitals, that makes you wonder about the validity of the study. Your job as an industry-funded researcher is that, essentially, you’re being paid to look for an answer. It’s not necessarily an honest evaluation of the data.
Dr. Glatter: I want to bring up another point about the Hospital Readmissions Reduction Program (HRRP) and the data on how physician-owned hospitals compared with acute care hospitals that are non–physician-owned and have you comment on that. The Dobson | DaVanzo study called into question that physician-owned hospitals treat fewer patients who are dual-eligible, which we know.
Dr. Miller: I don’t think we do know that.
Dr. Glatter: There are data that point to that, again, looking at the studies.
Dr. Miller: I’m saying that’s a single study funded by industry as opposed to an independent, academic, peer-reviewed literature paper. That would be like saying, during the debate of the Inflation Reduction Act (IRA), that you should read the pharmaceutical industries research but take any of it at pure face value as factual. Yes, we should read it. Yes, we should evaluate it on its own merits. I think, again, appropriately, you need to be concerned when people have an economic incentive.
The question about the HRRP I’m going to take a little broader, because I think that program is unfair to the industry overall. There are many factors that drive hospital readmission. Whether Mrs Smith went home and ate potato chips and then took her Lasix, that’s very much outside of the hospital industry’s control, and there’s some evidence that the HRRP increases mortality in some patient populations.
In terms of a quality metric, it’s unfair to the industry. I think we took an operating process, internal metric for the hospital industry, turned it into a quality metric, and attached it to a financial bonus, which is an inappropriate policy decision.
Rethinking Ownership Models and Empowering Clinicians
Dr. Glatter: I agree with you on that. One thing I do want to bring up is that whether the physician-owned hospitals are subject to many of the quality measures that full-service, acute care hospitals are. That really is, I think, a broader context.
Dr. Miller: Fifty-five percent of physician-owned hospitals are full-service community hospitals, so I would say at least half the market is 100% subject to that.
Dr. Glatter: If only 50% are, that’s already an issue.
Dr. Miller: Cardiac specialty hospitals — which, as I said, nonprofit and for-profit hospital chains also operate — are also subject to the appropriate quality measures, readmissions, etc. Just because we don’t necessarily have the best quality measurement in the system in the country, it doesn’t mean that we shouldn’t allow care specialization. As I’d point out, if we’re concerned about specialty hospitals, the concern shouldn’t just be about physician-owned specialty hospitals; it should be about specialty hospitals by and large. Many health systems run cardiac specialty hospitals, cancer specialty hospitals, and orthopedic specialty hospitals. If we’re going to have a discussion about concerns there, it should be about the entire industry of specialty hospitals.
I think specialty hospitals serve an important role in society, allowing for specialization and exploiting in a positive way the volume-quality relationship. Whether those are owned by a for-profit publicly traded company, a tax-exempt facility, or physicians, I think that is an important way to have innovation and care delivery because frankly, we haven’t had much innovation in care delivery. Much of what we do in terms of how we practice clinically hasn’t really changed in the 50 years since my late father graduated from medical school. We still have rounds, we’re still taking notes, we’re still operating in the same way. Many processes are manual. We don’t have the mass production and mass customization of care that we need.
When you have a focused factory, it allows you to design care in a way that drives up quality, not just for the average patient but also the patients at the tail ends, because you have time to focus on that specific service line and that specific patient population.
Physician-owned community hospitals offer an important opportunity for a different employment model. I remember going to the dermatologist and the dermatologist was depressed, shuffling around the room, sad, and I asked him why. He said he didn’t really like his employer, and I said, “Why don’t you pick another one?” He’s like, “There are only two large health systems I can work for. They all have the same clinical practice environment and functionally the same value.”
Physicians are increasingly burned out. They face monopsony power in who purchases their labor. They have little control. They don’t want to go through five committees, seven administrators, and attend 25 meetings just to change a single small process in clinical operations. If you’re an owner operator, you have a much better ability to do it.
Frankly, when many facilities do well now, when they do well clinically and do well financially, who benefits? The hospital administration and the hospital executives. The doctors aren’t benefiting. The nurses aren’t benefiting. The CNA is not benefiting. The secretary is not benefiting. The custodian is not benefiting. Shouldn’t the workers have a right to own and operate the business and do well when the business does well serving the community? That puts me in the weird space of agreeing with both conservatives and progressives.
Dr. Glatter: I agree with you. I think an ownership stake is always attractive. It helps with retention of employed persons. There›s no question that, when they have a stake, when they have skin in the game, they feel more empowered. I will not argue with you about that.
Dr. Miller: We don’t have business models where workers have that option in healthcare. Like the National Academy of Medicine said, one of the key drivers of burnout is the externalization of the locus of control over clinical practice, and the current business operating models guarantee an externalization of the locus of control over clinical practice.
If you actually look at the recent American Medical Association (AMA) meeting, there was a resolution to ban the corporate practice of medicine. They wanted to go more toward the legal professions model where only physicians can own and operate care delivery.
Dr. Glatter: Well, I think the shift is certainly something that the AMA would like and physicians collectively would agree with. Having a better lifestyle and being able to have control are factors in burnout.
Dr. Miller: It’s not just doctors. I think nurses want a better lifestyle. The nurses are treated as interchangeable lines on a spreadsheet. The nurses are an integral part of our clinical team. Why don’t we work together as a clinical unit to build a better delivery system? What better way to do that than to have clinicians in charge of it, right?
My favorite bakery that’s about 30 minutes away is owned by a baker. It is not owned by a large tax-exempt corporation. It’s owned by an owner operator who takes pride in their work. I think that is something that the profession would do well to return to. When I was a resident, one of my colleagues was already planning their retirement. That’s how depressed they were.
I went into medicine to actually care for patients. I think that we can make the world a better place for our patients. What that means is not only treating them with drugs and devices, but also creating a delivery system where they don’t have to wander from lobby to lobby in a 200,000 square-foot facility, wait in line for hours on end, get bills 6 months later, and fill out endless paper forms over and over again.
All of these basic processes in healthcare delivery that are broken could have and should have been fixed — and have been fixed in almost every other industry. I had to replace one of my car tires because I had a flat tire. The local tire shop has an app, and it sends me SMS text messages telling me when my appointment is and when my car is ready. We have solved all of these problems in many other businesses.
We have not solved them in healthcare delivery because, one, we have massive monopolies that are raising prices, have lower quality, and deliver a crappy patient experience, and we have also subjugated the clinical worker into a corporate automaton. We are functionally drones. We don’t have the agency and the authority to improve clinical operations anymore. It’s really depressing, and we should have that option again.
I trust my doctor. I trust the nurses that I work with, and I would like them to help make clinical decisions in a financially responsible and a sensible operational manner. We need to empower our workforce in order to do that so we can recapture the value of what it means to be a clinician again.
The current model of corporate employment: massive scale, more administrators, more processes, more emails, more meetings, more PowerPoint decks, more federal subsidies. The hospital industry has choices. It can improve clinical operations. It can show up in Washington and lobby for increased subsidies. It can invest in the market and not pay taxes for the tax-exempt facilities. Obviously, it makes the logical choices as an economic actor to show up, lobby for increased subsidies, and then also invest in the stock market.
Improving clinical operations is hard. It hasn’t happened. The Bureau of Labor Statistics shows that the private community hospital industry has had flat labor productivity growth, on average, for the past 25 years, and for some years it even declined. This is totally atypical across the economy.
We have failed our clinicians, and most importantly, we have failed our patients. I’ve been sick. My relatives have been sick, waiting hours, not able to get appointments, and redoing forms. It’s a total disaster. It’s time and reasonable to try an alternative ownership and operating model. There are obviously problems. The problems can and should be addressed, but it doesn’t mean that we should have a statutory prohibition on professionals owning and operating their own business.
Dr. Glatter: There was a report that $500 million was saved by limiting or banning or putting a moratorium on physician-owned hospitals by the Congressional Budget Office.
Dr. Miller: Yes, I’m very aware of those data. I’d say that the CBO also is off by 50% on the estimation of the implementation of the Part D program. They overestimated the Affordable Care Act market enrollment by over 10 million people — again, around 50%. They also estimated that the CMS Innovation Center initially would be a savings. Now they’ve re-estimated it as a 10-year expenditure and it has actually cost the taxpayers money.
The CBO is not transparent about what its assumptions are or its analysis and methods. As a researcher, we have to publish our information. It has to go through peer review. I want to know what goes into that $500 million figure — what the assumptions are and what the model is. It’s hard to comment without knowing how they came up with it.
Dr. Glatter: The points you make are very valid. Physicians and nurses want a better lifestyle.
Dr. Miller: It’s not even a better lifestyle. It’s about having a say in how clinical operations work and helping make them better. We want the delivery system to work better. This is an opportunity for us to do so.
Dr. Glatter: That translates into technology: obviously, generative artificial intelligence (AI) coming into the forefront, as we know, and changing care delivery models as you’re referring to, which is going to happen. It’s going to be a slow process. I think that the evolution is happening and will happen, as you accurately described.
Dr. Miller: The other thing that’s different now vs 20 years ago is that managed care is here, there, and everywhere, as Dr Seuss would say. You have utilization review and prior authorization, which I’ve experienced as a patient and a physician, and boy, is it not a fun process. There’s a large amount of friction that needs to be improved. If we’re worried about induced demand or inappropriate utilization, we have managed care right there to help police bad behavior.
Reforming Healthcare Systems and Restoring Patient-Centric Focus
Dr. Glatter: If you were to come up with, say, three bullet points of how we can work our way out of this current morass of where our healthcare systems exist, where do you see the solutions or how can we make and effect change?
Dr. Miller: I’d say there are a couple of things. One is, let business models compete fairly on an equal playing field. Let the physician-owned hospital compete with the tax-exempt hospital and the nonprofit hospital. Put them on an equal playing field. We have things like 340B, which favors tax-exempt hospitals. For-profit or tax-paying hospitals are not able to participate in that. That doesn’t make any sense just from a public policy perspective. Tax-paying hospitals and physician-owned hospitals pay taxes on investments, but tax-exempt hospitals don’t. I think, in public policy, we need to equalize the playing field between business models. Let the best business model win.
The other thing we need to do is to encourage the adoption of technology. The physician will eventually be an arbiter of tech-driven or AI-driven tools. In fact, at some point, the standard of care might be to use those tools. Not using those tools would be seen as negligence. If you think about placing a jugular or central venous catheter, to not use ultrasound would be considered insane. Thirty years ago, to use ultrasound would be considered novel. I think technology and AI will get us to that point of helping make care more efficient and more customized.
Those are the two biggest interventions, I would say. Third, every time we have a conversation in public policy, we need to remember what it is to be a patient. The decision should be driven not around any one industry’s profitability, but what it is to be a patient and how we can make that experience less burdensome, less expensive, or in plain English, suck less.
Dr. Glatter: Safety net hospitals and critical access hospitals are part of this discussion that, yes, we want everything to, in an ideal world, function more efficiently and effectively, with less cost and less red tape. The safety net of our nation is struggling.
Dr. Miller: I 100% agree. The Cook County hospitals of the world are deserving of our support and, frankly, our gratitude. Facilities like that have huge burdens of patients with Medicaid. We also still have millions of uninsured patients. The neighborhoods that they serve are also poorer. I think facilities like that are deserving of public support.
I also think we need to clearly define what those hospitals are. One of the challenges I’ve realized as I waded into this space is that market definitions of what a service market is for a hospital, its specialty type or what a safety net hospital is need to be more clearly defined because those facilities 100% are deserving of our support. We just need to be clear about what they are.
Regarding critical access hospitals, when you practice in a rural area, you have to think differently about care delivery. I’d say many of the rural systems are highly creative in how they structure clinical operations. Before the public health emergency, during the COVID pandemic, when we had a massive change in telehealth, rural hospitals were using — within the very narrow confines — as much telehealth as they could and should.
Rural hospitals also make greater use of nurse practitioners (NPs) and physician assistants (PAs). For many of the specialty services, I remember, your first call was an NP or a PA because the physician was downstairs doing procedures. They’d come up and assess the patient before the procedure, but most of your consult questions were answered by the NP or PA. I’m not saying that’s the model we should use nationwide, but that rural systems are highly innovative and creative; they’re deserving of our time, attention, and support, and frankly, we can learn from them.
Dr. Glatter: I want to thank you for your time and your expertise in this area. We’ll see how the congressional hearings affect the industry as a whole, how the needle moves, and whether the ban or moratorium on physician-owned hospitals continues to exist going forward.
Dr. Miller: I appreciate you having me. The hospital industry is one of the most important industries for health care. This is a time of inflection, right? We need to go back to the value of what it means to be a clinician and serve patients. Hospitals need to reorient themselves around that core concern. How do we help support clinicians — doctors, nurses, pharmacists, whomever it is — in serving patients? Hospitals have become too corporate, so I think that this is an expected pushback.
Dr. Glatter: Again, I want to thank you for your time. This was a very important discussion. Thank you for your expertise.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He disclosed no relevant financial relationships.Brian J. Miller, MD, MBA, MPH, is a hospitalist and an assistant professor of medicine at the Johns Hopkins University School of Medicine. He is also a nonresident fellow at the American Enterprise Institute. From 2014 to 2017, Dr. Miller worked at four federal regulatory agencies: Federal Trade Commission (FTC), Federal Communications Commission (FCC), Centers for Medicare & Medicaid Services (CMS), and the Food & Drug Administration (FDA). Dr. Miller disclosed ties with the Medicare Payment Advisory Commission.
A version of this article appeared on Medscape.com.
This discussion was recorded on November 16, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Dr. Brian J. Miller, a hospitalist with Johns Hopkins University School of Medicine and a health policy expert, to discuss the current and renewed interest in physician-owned hospitals.
Welcome, Dr. Miller. It’s a pleasure to have you join me today.
Brian J. Miller, MD, MBA, MPH: Thank you for having me.
History and Controversies Surrounding Physician-Owned Hospitals
Dr. Glatter: I want to start off by having you describe the history associated with the moratorium on new physician-owned hospitals in 2010 that’s related ultimately to the Affordable Care Act, but also, the current and renewed media interest in physician-owned hospitals that’s linked to recent congressional hearings last month.
Dr. Miller: Thank you. I should note that my views are my own and don’t represent those of Hopkins or the American Enterprise Institute, where I’m a nonresident fellow nor the Medicare Payment Advisory Commission, of which I’m a Commissioner.
The story about physician-owned hospitals is an interesting one. Hospitals turned into health systems in the 1980s and 1990s, and physicians started to shift purely from an independent model into a more organized group practice or employed model. Physicians realized that they wanted an alternative operating arrangement. You want a choice of how you practice and what your employment is. And as community hospitals started to buy physicians and also establish their own physician groups de novo, physicians opened physician-owned hospitals.
Physician-owned hospitals fell into a couple of buckets. One is what we call community hospitals, or what the antitrust lawyers would call general acute care hospitals: those offering emergency room (ER) services, labor and delivery, primary care, general surgery — the whole regular gamut, except that some of the owners were physicians.
The other half of the marketplace ended up being specialty hospitals: those built around a specific medical specialty and series of procedures and chronic care. For example, cardiac hospitals often do CABG, TAVR, maybe abdominal aortic aneurysm (triple A) repairs, and they have cardiology clinics, cath labs, a cardiac intensive care unit (ICU), ER, etc. There were also orthopedic surgical specialty hospitals, which were sort of like an ambulatory surgery center (ASC) plus several beds. Then there were general surgical specialty hospitals. At one point, there were some women’s health–focused specialty hospitals.
The hospital industry, of course, as you can understand, didn’t exactly like this. They had a series of concerns about what we would historically call cherry-picking or lemon-dropping of patients. They were worried that physician-owned facilities didn’t want to serve public payer patients, and there was a whole series of reports and investigations.
Around the time the Affordable Care Act passed, the hospital industry had many concerns about physician-owned specialty hospitals, and there was a moratorium as part of the 2003 Medicare Modernization Act. As part of the bargaining over the hospital industry support for the Affordable Care Act, they traded their support for, among other things, their number one priority, which is a statutory prohibition on new or expanded physician-owned hospitals from participating in Medicare. That included both physician-owned community hospitals and physician-owned specialty hospitals.
Dr. Glatter: That was part of the impetus to prevent physicians from referring patients where they had an ownership stake. Certainly, hospitals can be owned by attorneys and nonprofit organizations, and certainly, ASCs can be owned by physicians. There is an ongoing issue in terms of physicians not being able to have an ownership stake. In terms of equity ownership, we know that certain other models allow this, but basically, it sounds like this is an issue with Medicare. That seems to be the crux of it, correct?
Dr. Miller: Yes. I would also add that it’s interesting when we look at other professions. When we look at lawyers, nonlawyers are actually not allowed to own an equity stake in a law practice. In many other professions, you either have corporate ownership or professional ownership, or the alternative is you have only professional ownership. I would say the hospital industry is one of the few areas where professional ownership not only is not allowed, but also is statutorily prohibited functionally through the Medicare program.
Unveiling the Dynamics of Hospital Ownership
Dr. Glatter: A recent study done by two PhDs looked at 2019 data on 20 of the most expensive diagnosis-related groups (DRGs). It examined the cost savings, and we’re talking over $1 billion in expenditures when you look at the data from general acute care hospitals vs physician-owned hospitals. This is what appears to me to be a key driver of the push to loosen restrictions on physician-owned hospitals. Isn’t that correct?
Dr. Miller: I would say that’s one of many components. There’s more history to this issue. I remember sitting at a think tank talking to someone several years ago about hospital consolidation as an issue. We went through the usual levers that us policy wonks go through. We talked about antitrust enforcement, certificate of need, rising hospital costs from consolidation, lower quality (or at least no quality gains, as shown by a New England Journal of Medicine study), and decrements in patient experience that result from the diseconomies of scale. They sort of pooh-poohed many of the policy ideas. They basically said that there was no hope for hospital consolidation as an issue.
Well, what about physician ownership? I started with my research team to comb through the literature and found a variety of studies — some of which were sort of entertaining, because they’d do things like study physician-owned specialty hospitals, nonprofit-owned specialty hospitals, and for-profit specialty hospitals and compare them with nonprofit or for-profit community hospitals, and then say physician-owned hospitals that were specialty were bad.
They mixed ownership and service markets right there in so many ways, I’m not sure where to start. My team did a systematic review of around 30 years of research, looking at the evidence base in this space. We found a couple of things.
We found that physician-owned community hospitals did not have a cost or quality difference, meaning that there was no definitive evidence that the physician-owned community hospitals were cheaper based on historical evidence, which was very old. That means there’s not specific harm from them. When you permit market entry for community hospitals, that promotes competition, which results in lower prices and higher quality.
Then we also looked at the specialty hospital markets — surgical specialty hospitals, orthopedic surgical specialty hospitals, and cardiac hospitals. We noted for cardiac hospitals, there wasn’t clear evidence about cost savings, but there was definitive evidence of higher quality, from things like 30-day mortality for significant procedures like treatment of acute MI, triple A repair, stuff like that.
For orthopedic surgical specialty hospitals, we noted lower costs and higher quality, which again fits with operationally what we would know. If you have a facility that’s doing 20 total hips a day, you’re creating a focused factory. Just like if you think about it for interventional cardiology, your boards have a minimum number of procedures that you have to do to stay certified because we know about the volume-quality relationship.
Then we looked at general surgical specialty hospitals. There wasn’t enough evidence to make a conclusive thought about costs, and there was a clear trend toward higher quality. I would say this recent study is important, but there is a whole bunch of other literature out there, too.
Exploring the Scope of Emergency Care in Physician-Owned Hospitals
Dr. Glatter: Certainly, your colleague Wang from Johns Hopkins has done important research in this sector. The paper, “Reconsidering the Ban on Physician-Owned Hospitals to Combat Consolidation,” by you and several colleagues, mentions and highlights the issues that you just described. I understand that it’s going to be published in the NYU Journal of Legislation and Public Policy.
One thing I want to bring up — and this is an important issue — is that the risk for patients has been talked about by the American Hospital Association and the Federation of American Hospitals, in terms of limited or no emergency services at such physician-owned hospitals and having to call 911 when patients need emergent care or stabilization. That’s been the rebuttal, along with an Office of Inspector General (OIG) report from 2008. Almost, I guess, three quarters of the patients that needed emergent care got this at publicly funded hospitals.
Dr. Miller: I’m familiar with the argument about emergency care. If you actually go and look at it, it differs by specialty market. Physician-owned community hospitals have ERs because that’s how they get their business. If you are running a hospital medicine floor, a general surgical specialty floor, you have a labor delivery unit, a primary care clinic, and a cardiology clinic. You have all the things that all the other hospitals have. The physician-owned community hospitals almost uniformly have an ER.
When you look at the physician-owned specialty hospitals, it’s a little more granular. If you look at the cardiac hospitals, they have ERs. They also have cardiac ICUs, operating rooms, etc. The area where the hospital industry had concerns — which I think is valid to point out — is that physician-owned orthopedic surgical specialty hospitals don’t have ERs. But this makes sense because of what that hospital functionally is: a factory for whatever the scope of procedures is, be it joint replacements or shoulder arthroscopy. The orthopedic surgical specialty hospital is like an ASC plus several hospital beds. Many of those did not have ERs because clinically it didn’t make sense.
What’s interesting, though, is that the hospital industry also operates specialty hospitals. If you go into many of the large systems, they have cardiac specialty hospitals and cancer specialty hospitals. I would say that some of them have ERs, as they appropriately should, and some of those specialty hospitals do not. They might have a community hospital down the street that’s part of that health system that has an ER, but some of the specialty hospitals don’t necessarily have a dedicated ER.
I agree, that’s a valid concern. I would say, though, the question is, what are the scope of services in that hospital? Is an ER required? Community hospitals should have ERs. It makes sense also for a cardiac hospital to have one. If you’re running a total joint replacement factory, it might not make clinical sense.
Dr. Glatter: The patients who are treated at that hospital, if they do have emergent conditions, need to have board-certified emergency physicians treating them, in my view because I’m an ER physician. Having surgeons that are not emergency physicians staff a department at a specialty orthopedic hospital or, say, a cancer hospital is not acceptable from my standpoint. That›s my opinion and recommendation, coming from emergency medicine.
Dr. Miller: I would say that anesthesiologists are actually highly qualified in critical care. The question is about clinical decompensation; if you’re doing a procedure, you have an anesthesiologist right there who is capable of critical care. The function of the ER is to either serve as a window into the hospital for patient volume or to serve as a referral for emergent complaints.
Dr. Glatter: An anesthesiologist — I’ll take issue with that — does not have the training of an emergency physician in terms of scope of practice.
Dr. Miller: My anesthesiology colleagues would probably disagree for managing an emergency during an operating room case.
Dr. Glatter: Fair enough, but I think in the general sense. The other issue is that, in terms of emergent responses to patients that decompensate, when you have to transfer a patient, that violates Medicare requirements. How is that even a valid issue or argument if you’re going to have to transfer a patient from your specialty hospital? That happens. Again, I know that you’re saying these hospitals are completely independent and can function, stabilize patients, and treat emergencies, but that’s not the reality across the country, in my opinion.
Dr. Miller: I don’t think that’s the case for the physician-owned specialty cardiac hospitals, for starters. Many of those have ICUs in addition to operating rooms as a matter of routine in addition to ERs. I don’t think that’s the case for physician-owned community hospitals, which have ERs, ICUs, medicine floors, and surgical floors. Physician-owned community hospitals are around half the market. Of that remaining market, a significant percentage are cardiac hospitals. If you’re taking an issue with orthopedic surgical specialty hospitals, that’s a clinical operational question that can and should be answered.
I’d also posit that the nonprofit and for-profit hospital industries also operate specialty hospitals. Any of these questions, we shouldn’t just be asking about physician-owned facilities; we should be asking about them across ownership types, because we’re talking about scope of service and quality and safety. The ownership in that case doesn’t matter. The broader question is, are orthopedic surgical specialty hospitals owned by physicians, tax-exempt hospitals, or tax-paying hospitals? Is that a valid clinical business model? Is it safe? Does it meet Medicare conditions of participation? I would say that’s what that question is, because other ownership models do operate those facilities.
Dr. Glatter: You make some valid points, and I do agree on some of them. I think that, ultimately, these models of care, and certainly cost and quality, are issues. Again, it goes back to being able, in my opinion, to provide emergent care, which seems to me a very important issue.
Dr. Miller: I agree that providing emergent care is an issue. It›s an issue in any site of care. The hospital industry posits that all hospital outpatient departments (HOPDs) have emergent care. I can tell you, having worked in HOPDs (I›ve trained in them during residency), the response if something emergent happens is to either call 911 or wheel the patient down to the ER in a wheelchair or stretcher. I think that these hospital claims about emergency care coverage — these are important questions, but we should be asking them across all clinical settings and say what is the appropriate scope of care provided? What is the appropriate level of acuity and ability to provide emergent or critical care? That›s an important question regardless of ownership model across the entire industry.
Deeper Dive Into Data on Physician-Owned Hospitals
Dr. Glatter: We need to really focus on that. I’ll agree with you on that.
There was a March 2023 report from Dobson | DaVanzo. It showed that physician-owned hospitals had lower Medicaid, dual-eligible, and uncompensated care and charity care discharges than full-service acute care hospitals. Physician-owned hospitals had less than half the proportion of Medicaid discharges compared with non–physician-owned hospitals. They were also less likely to care for dual-eligible patients overall compared with non–physician-owned hospitals.
In addition, when COVID hit, the physician-owned hospitals overall — and again, there may be exceptions — were not equipped to handle these patient surges in the acute setting of a public health emergency. There was a hospital in Texas that did pivot that I’m aware of — Renaissance Hospital, which ramped up a long-term care facility to become a COVID hospital — but I think that’s the exception. I think this report raises some valid concerns; I’ll let you rebut that.
Dr. Miller: A couple of things. One, I am not aware that there’s any clear market evidence or a systematic study that shows that physician-owned hospitals had trouble responding to COVID. I don’t think that assertion has been proven. The study was funded by the hospital industry. First of all, it was not a peer-reviewed study; it was funded by an industry that paid a consulting firm. It doesn’t mean that we still shouldn’t read it, but that brings bias into question. The joke in Washington is, pick your favorite statistician or economist, and they can say what you want and have a battle of economists and statisticians.
For example, in that study, they didn’t include the entire ownership universe of physician-owned hospitals. If we go to the peer-reviewed literature, there’s a great 2015 BMJ paper showing that the Medicaid payer mix is actually the same between physician-owned hospitals vs not. The mix of patients by ethnicity — for example, think about African American patients — was the same. I would be more inclined to believe the peer-reviewed literature in BMJ as opposed to an industry-funded study that was not peer-reviewed and not independent and has methodological questions.
Dr. Glatter: Those data are 8 years old, so I’d like to see more recent data. It would be interesting, just as a follow-up to that, to see where the needle has moved — if it has, for that matter — in terms of Medicaid patients that you’re referring to.
Dr. Miller: I tend to be skeptical of all industry research, regardless of who published it, because they have an economic incentive. If they’re selecting certain age groups or excluding certain hospitals, that makes you wonder about the validity of the study. Your job as an industry-funded researcher is that, essentially, you’re being paid to look for an answer. It’s not necessarily an honest evaluation of the data.
Dr. Glatter: I want to bring up another point about the Hospital Readmissions Reduction Program (HRRP) and the data on how physician-owned hospitals compared with acute care hospitals that are non–physician-owned and have you comment on that. The Dobson | DaVanzo study called into question that physician-owned hospitals treat fewer patients who are dual-eligible, which we know.
Dr. Miller: I don’t think we do know that.
Dr. Glatter: There are data that point to that, again, looking at the studies.
Dr. Miller: I’m saying that’s a single study funded by industry as opposed to an independent, academic, peer-reviewed literature paper. That would be like saying, during the debate of the Inflation Reduction Act (IRA), that you should read the pharmaceutical industries research but take any of it at pure face value as factual. Yes, we should read it. Yes, we should evaluate it on its own merits. I think, again, appropriately, you need to be concerned when people have an economic incentive.
The question about the HRRP I’m going to take a little broader, because I think that program is unfair to the industry overall. There are many factors that drive hospital readmission. Whether Mrs Smith went home and ate potato chips and then took her Lasix, that’s very much outside of the hospital industry’s control, and there’s some evidence that the HRRP increases mortality in some patient populations.
In terms of a quality metric, it’s unfair to the industry. I think we took an operating process, internal metric for the hospital industry, turned it into a quality metric, and attached it to a financial bonus, which is an inappropriate policy decision.
Rethinking Ownership Models and Empowering Clinicians
Dr. Glatter: I agree with you on that. One thing I do want to bring up is that whether the physician-owned hospitals are subject to many of the quality measures that full-service, acute care hospitals are. That really is, I think, a broader context.
Dr. Miller: Fifty-five percent of physician-owned hospitals are full-service community hospitals, so I would say at least half the market is 100% subject to that.
Dr. Glatter: If only 50% are, that’s already an issue.
Dr. Miller: Cardiac specialty hospitals — which, as I said, nonprofit and for-profit hospital chains also operate — are also subject to the appropriate quality measures, readmissions, etc. Just because we don’t necessarily have the best quality measurement in the system in the country, it doesn’t mean that we shouldn’t allow care specialization. As I’d point out, if we’re concerned about specialty hospitals, the concern shouldn’t just be about physician-owned specialty hospitals; it should be about specialty hospitals by and large. Many health systems run cardiac specialty hospitals, cancer specialty hospitals, and orthopedic specialty hospitals. If we’re going to have a discussion about concerns there, it should be about the entire industry of specialty hospitals.
I think specialty hospitals serve an important role in society, allowing for specialization and exploiting in a positive way the volume-quality relationship. Whether those are owned by a for-profit publicly traded company, a tax-exempt facility, or physicians, I think that is an important way to have innovation and care delivery because frankly, we haven’t had much innovation in care delivery. Much of what we do in terms of how we practice clinically hasn’t really changed in the 50 years since my late father graduated from medical school. We still have rounds, we’re still taking notes, we’re still operating in the same way. Many processes are manual. We don’t have the mass production and mass customization of care that we need.
When you have a focused factory, it allows you to design care in a way that drives up quality, not just for the average patient but also the patients at the tail ends, because you have time to focus on that specific service line and that specific patient population.
Physician-owned community hospitals offer an important opportunity for a different employment model. I remember going to the dermatologist and the dermatologist was depressed, shuffling around the room, sad, and I asked him why. He said he didn’t really like his employer, and I said, “Why don’t you pick another one?” He’s like, “There are only two large health systems I can work for. They all have the same clinical practice environment and functionally the same value.”
Physicians are increasingly burned out. They face monopsony power in who purchases their labor. They have little control. They don’t want to go through five committees, seven administrators, and attend 25 meetings just to change a single small process in clinical operations. If you’re an owner operator, you have a much better ability to do it.
Frankly, when many facilities do well now, when they do well clinically and do well financially, who benefits? The hospital administration and the hospital executives. The doctors aren’t benefiting. The nurses aren’t benefiting. The CNA is not benefiting. The secretary is not benefiting. The custodian is not benefiting. Shouldn’t the workers have a right to own and operate the business and do well when the business does well serving the community? That puts me in the weird space of agreeing with both conservatives and progressives.
Dr. Glatter: I agree with you. I think an ownership stake is always attractive. It helps with retention of employed persons. There›s no question that, when they have a stake, when they have skin in the game, they feel more empowered. I will not argue with you about that.
Dr. Miller: We don’t have business models where workers have that option in healthcare. Like the National Academy of Medicine said, one of the key drivers of burnout is the externalization of the locus of control over clinical practice, and the current business operating models guarantee an externalization of the locus of control over clinical practice.
If you actually look at the recent American Medical Association (AMA) meeting, there was a resolution to ban the corporate practice of medicine. They wanted to go more toward the legal professions model where only physicians can own and operate care delivery.
Dr. Glatter: Well, I think the shift is certainly something that the AMA would like and physicians collectively would agree with. Having a better lifestyle and being able to have control are factors in burnout.
Dr. Miller: It’s not just doctors. I think nurses want a better lifestyle. The nurses are treated as interchangeable lines on a spreadsheet. The nurses are an integral part of our clinical team. Why don’t we work together as a clinical unit to build a better delivery system? What better way to do that than to have clinicians in charge of it, right?
My favorite bakery that’s about 30 minutes away is owned by a baker. It is not owned by a large tax-exempt corporation. It’s owned by an owner operator who takes pride in their work. I think that is something that the profession would do well to return to. When I was a resident, one of my colleagues was already planning their retirement. That’s how depressed they were.
I went into medicine to actually care for patients. I think that we can make the world a better place for our patients. What that means is not only treating them with drugs and devices, but also creating a delivery system where they don’t have to wander from lobby to lobby in a 200,000 square-foot facility, wait in line for hours on end, get bills 6 months later, and fill out endless paper forms over and over again.
All of these basic processes in healthcare delivery that are broken could have and should have been fixed — and have been fixed in almost every other industry. I had to replace one of my car tires because I had a flat tire. The local tire shop has an app, and it sends me SMS text messages telling me when my appointment is and when my car is ready. We have solved all of these problems in many other businesses.
We have not solved them in healthcare delivery because, one, we have massive monopolies that are raising prices, have lower quality, and deliver a crappy patient experience, and we have also subjugated the clinical worker into a corporate automaton. We are functionally drones. We don’t have the agency and the authority to improve clinical operations anymore. It’s really depressing, and we should have that option again.
I trust my doctor. I trust the nurses that I work with, and I would like them to help make clinical decisions in a financially responsible and a sensible operational manner. We need to empower our workforce in order to do that so we can recapture the value of what it means to be a clinician again.
The current model of corporate employment: massive scale, more administrators, more processes, more emails, more meetings, more PowerPoint decks, more federal subsidies. The hospital industry has choices. It can improve clinical operations. It can show up in Washington and lobby for increased subsidies. It can invest in the market and not pay taxes for the tax-exempt facilities. Obviously, it makes the logical choices as an economic actor to show up, lobby for increased subsidies, and then also invest in the stock market.
Improving clinical operations is hard. It hasn’t happened. The Bureau of Labor Statistics shows that the private community hospital industry has had flat labor productivity growth, on average, for the past 25 years, and for some years it even declined. This is totally atypical across the economy.
We have failed our clinicians, and most importantly, we have failed our patients. I’ve been sick. My relatives have been sick, waiting hours, not able to get appointments, and redoing forms. It’s a total disaster. It’s time and reasonable to try an alternative ownership and operating model. There are obviously problems. The problems can and should be addressed, but it doesn’t mean that we should have a statutory prohibition on professionals owning and operating their own business.
Dr. Glatter: There was a report that $500 million was saved by limiting or banning or putting a moratorium on physician-owned hospitals by the Congressional Budget Office.
Dr. Miller: Yes, I’m very aware of those data. I’d say that the CBO also is off by 50% on the estimation of the implementation of the Part D program. They overestimated the Affordable Care Act market enrollment by over 10 million people — again, around 50%. They also estimated that the CMS Innovation Center initially would be a savings. Now they’ve re-estimated it as a 10-year expenditure and it has actually cost the taxpayers money.
The CBO is not transparent about what its assumptions are or its analysis and methods. As a researcher, we have to publish our information. It has to go through peer review. I want to know what goes into that $500 million figure — what the assumptions are and what the model is. It’s hard to comment without knowing how they came up with it.
Dr. Glatter: The points you make are very valid. Physicians and nurses want a better lifestyle.
Dr. Miller: It’s not even a better lifestyle. It’s about having a say in how clinical operations work and helping make them better. We want the delivery system to work better. This is an opportunity for us to do so.
Dr. Glatter: That translates into technology: obviously, generative artificial intelligence (AI) coming into the forefront, as we know, and changing care delivery models as you’re referring to, which is going to happen. It’s going to be a slow process. I think that the evolution is happening and will happen, as you accurately described.
Dr. Miller: The other thing that’s different now vs 20 years ago is that managed care is here, there, and everywhere, as Dr Seuss would say. You have utilization review and prior authorization, which I’ve experienced as a patient and a physician, and boy, is it not a fun process. There’s a large amount of friction that needs to be improved. If we’re worried about induced demand or inappropriate utilization, we have managed care right there to help police bad behavior.
Reforming Healthcare Systems and Restoring Patient-Centric Focus
Dr. Glatter: If you were to come up with, say, three bullet points of how we can work our way out of this current morass of where our healthcare systems exist, where do you see the solutions or how can we make and effect change?
Dr. Miller: I’d say there are a couple of things. One is, let business models compete fairly on an equal playing field. Let the physician-owned hospital compete with the tax-exempt hospital and the nonprofit hospital. Put them on an equal playing field. We have things like 340B, which favors tax-exempt hospitals. For-profit or tax-paying hospitals are not able to participate in that. That doesn’t make any sense just from a public policy perspective. Tax-paying hospitals and physician-owned hospitals pay taxes on investments, but tax-exempt hospitals don’t. I think, in public policy, we need to equalize the playing field between business models. Let the best business model win.
The other thing we need to do is to encourage the adoption of technology. The physician will eventually be an arbiter of tech-driven or AI-driven tools. In fact, at some point, the standard of care might be to use those tools. Not using those tools would be seen as negligence. If you think about placing a jugular or central venous catheter, to not use ultrasound would be considered insane. Thirty years ago, to use ultrasound would be considered novel. I think technology and AI will get us to that point of helping make care more efficient and more customized.
Those are the two biggest interventions, I would say. Third, every time we have a conversation in public policy, we need to remember what it is to be a patient. The decision should be driven not around any one industry’s profitability, but what it is to be a patient and how we can make that experience less burdensome, less expensive, or in plain English, suck less.
Dr. Glatter: Safety net hospitals and critical access hospitals are part of this discussion that, yes, we want everything to, in an ideal world, function more efficiently and effectively, with less cost and less red tape. The safety net of our nation is struggling.
Dr. Miller: I 100% agree. The Cook County hospitals of the world are deserving of our support and, frankly, our gratitude. Facilities like that have huge burdens of patients with Medicaid. We also still have millions of uninsured patients. The neighborhoods that they serve are also poorer. I think facilities like that are deserving of public support.
I also think we need to clearly define what those hospitals are. One of the challenges I’ve realized as I waded into this space is that market definitions of what a service market is for a hospital, its specialty type or what a safety net hospital is need to be more clearly defined because those facilities 100% are deserving of our support. We just need to be clear about what they are.
Regarding critical access hospitals, when you practice in a rural area, you have to think differently about care delivery. I’d say many of the rural systems are highly creative in how they structure clinical operations. Before the public health emergency, during the COVID pandemic, when we had a massive change in telehealth, rural hospitals were using — within the very narrow confines — as much telehealth as they could and should.
Rural hospitals also make greater use of nurse practitioners (NPs) and physician assistants (PAs). For many of the specialty services, I remember, your first call was an NP or a PA because the physician was downstairs doing procedures. They’d come up and assess the patient before the procedure, but most of your consult questions were answered by the NP or PA. I’m not saying that’s the model we should use nationwide, but that rural systems are highly innovative and creative; they’re deserving of our time, attention, and support, and frankly, we can learn from them.
Dr. Glatter: I want to thank you for your time and your expertise in this area. We’ll see how the congressional hearings affect the industry as a whole, how the needle moves, and whether the ban or moratorium on physician-owned hospitals continues to exist going forward.
Dr. Miller: I appreciate you having me. The hospital industry is one of the most important industries for health care. This is a time of inflection, right? We need to go back to the value of what it means to be a clinician and serve patients. Hospitals need to reorient themselves around that core concern. How do we help support clinicians — doctors, nurses, pharmacists, whomever it is — in serving patients? Hospitals have become too corporate, so I think that this is an expected pushback.
Dr. Glatter: Again, I want to thank you for your time. This was a very important discussion. Thank you for your expertise.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He disclosed no relevant financial relationships.Brian J. Miller, MD, MBA, MPH, is a hospitalist and an assistant professor of medicine at the Johns Hopkins University School of Medicine. He is also a nonresident fellow at the American Enterprise Institute. From 2014 to 2017, Dr. Miller worked at four federal regulatory agencies: Federal Trade Commission (FTC), Federal Communications Commission (FCC), Centers for Medicare & Medicaid Services (CMS), and the Food & Drug Administration (FDA). Dr. Miller disclosed ties with the Medicare Payment Advisory Commission.
A version of this article appeared on Medscape.com.
This discussion was recorded on November 16, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Dr. Brian J. Miller, a hospitalist with Johns Hopkins University School of Medicine and a health policy expert, to discuss the current and renewed interest in physician-owned hospitals.
Welcome, Dr. Miller. It’s a pleasure to have you join me today.
Brian J. Miller, MD, MBA, MPH: Thank you for having me.
History and Controversies Surrounding Physician-Owned Hospitals
Dr. Glatter: I want to start off by having you describe the history associated with the moratorium on new physician-owned hospitals in 2010 that’s related ultimately to the Affordable Care Act, but also, the current and renewed media interest in physician-owned hospitals that’s linked to recent congressional hearings last month.
Dr. Miller: Thank you. I should note that my views are my own and don’t represent those of Hopkins or the American Enterprise Institute, where I’m a nonresident fellow nor the Medicare Payment Advisory Commission, of which I’m a Commissioner.
The story about physician-owned hospitals is an interesting one. Hospitals turned into health systems in the 1980s and 1990s, and physicians started to shift purely from an independent model into a more organized group practice or employed model. Physicians realized that they wanted an alternative operating arrangement. You want a choice of how you practice and what your employment is. And as community hospitals started to buy physicians and also establish their own physician groups de novo, physicians opened physician-owned hospitals.
Physician-owned hospitals fell into a couple of buckets. One is what we call community hospitals, or what the antitrust lawyers would call general acute care hospitals: those offering emergency room (ER) services, labor and delivery, primary care, general surgery — the whole regular gamut, except that some of the owners were physicians.
The other half of the marketplace ended up being specialty hospitals: those built around a specific medical specialty and series of procedures and chronic care. For example, cardiac hospitals often do CABG, TAVR, maybe abdominal aortic aneurysm (triple A) repairs, and they have cardiology clinics, cath labs, a cardiac intensive care unit (ICU), ER, etc. There were also orthopedic surgical specialty hospitals, which were sort of like an ambulatory surgery center (ASC) plus several beds. Then there were general surgical specialty hospitals. At one point, there were some women’s health–focused specialty hospitals.
The hospital industry, of course, as you can understand, didn’t exactly like this. They had a series of concerns about what we would historically call cherry-picking or lemon-dropping of patients. They were worried that physician-owned facilities didn’t want to serve public payer patients, and there was a whole series of reports and investigations.
Around the time the Affordable Care Act passed, the hospital industry had many concerns about physician-owned specialty hospitals, and there was a moratorium as part of the 2003 Medicare Modernization Act. As part of the bargaining over the hospital industry support for the Affordable Care Act, they traded their support for, among other things, their number one priority, which is a statutory prohibition on new or expanded physician-owned hospitals from participating in Medicare. That included both physician-owned community hospitals and physician-owned specialty hospitals.
Dr. Glatter: That was part of the impetus to prevent physicians from referring patients where they had an ownership stake. Certainly, hospitals can be owned by attorneys and nonprofit organizations, and certainly, ASCs can be owned by physicians. There is an ongoing issue in terms of physicians not being able to have an ownership stake. In terms of equity ownership, we know that certain other models allow this, but basically, it sounds like this is an issue with Medicare. That seems to be the crux of it, correct?
Dr. Miller: Yes. I would also add that it’s interesting when we look at other professions. When we look at lawyers, nonlawyers are actually not allowed to own an equity stake in a law practice. In many other professions, you either have corporate ownership or professional ownership, or the alternative is you have only professional ownership. I would say the hospital industry is one of the few areas where professional ownership not only is not allowed, but also is statutorily prohibited functionally through the Medicare program.
Unveiling the Dynamics of Hospital Ownership
Dr. Glatter: A recent study done by two PhDs looked at 2019 data on 20 of the most expensive diagnosis-related groups (DRGs). It examined the cost savings, and we’re talking over $1 billion in expenditures when you look at the data from general acute care hospitals vs physician-owned hospitals. This is what appears to me to be a key driver of the push to loosen restrictions on physician-owned hospitals. Isn’t that correct?
Dr. Miller: I would say that’s one of many components. There’s more history to this issue. I remember sitting at a think tank talking to someone several years ago about hospital consolidation as an issue. We went through the usual levers that us policy wonks go through. We talked about antitrust enforcement, certificate of need, rising hospital costs from consolidation, lower quality (or at least no quality gains, as shown by a New England Journal of Medicine study), and decrements in patient experience that result from the diseconomies of scale. They sort of pooh-poohed many of the policy ideas. They basically said that there was no hope for hospital consolidation as an issue.
Well, what about physician ownership? I started with my research team to comb through the literature and found a variety of studies — some of which were sort of entertaining, because they’d do things like study physician-owned specialty hospitals, nonprofit-owned specialty hospitals, and for-profit specialty hospitals and compare them with nonprofit or for-profit community hospitals, and then say physician-owned hospitals that were specialty were bad.
They mixed ownership and service markets right there in so many ways, I’m not sure where to start. My team did a systematic review of around 30 years of research, looking at the evidence base in this space. We found a couple of things.
We found that physician-owned community hospitals did not have a cost or quality difference, meaning that there was no definitive evidence that the physician-owned community hospitals were cheaper based on historical evidence, which was very old. That means there’s not specific harm from them. When you permit market entry for community hospitals, that promotes competition, which results in lower prices and higher quality.
Then we also looked at the specialty hospital markets — surgical specialty hospitals, orthopedic surgical specialty hospitals, and cardiac hospitals. We noted for cardiac hospitals, there wasn’t clear evidence about cost savings, but there was definitive evidence of higher quality, from things like 30-day mortality for significant procedures like treatment of acute MI, triple A repair, stuff like that.
For orthopedic surgical specialty hospitals, we noted lower costs and higher quality, which again fits with operationally what we would know. If you have a facility that’s doing 20 total hips a day, you’re creating a focused factory. Just like if you think about it for interventional cardiology, your boards have a minimum number of procedures that you have to do to stay certified because we know about the volume-quality relationship.
Then we looked at general surgical specialty hospitals. There wasn’t enough evidence to make a conclusive thought about costs, and there was a clear trend toward higher quality. I would say this recent study is important, but there is a whole bunch of other literature out there, too.
Exploring the Scope of Emergency Care in Physician-Owned Hospitals
Dr. Glatter: Certainly, your colleague Wang from Johns Hopkins has done important research in this sector. The paper, “Reconsidering the Ban on Physician-Owned Hospitals to Combat Consolidation,” by you and several colleagues, mentions and highlights the issues that you just described. I understand that it’s going to be published in the NYU Journal of Legislation and Public Policy.
One thing I want to bring up — and this is an important issue — is that the risk for patients has been talked about by the American Hospital Association and the Federation of American Hospitals, in terms of limited or no emergency services at such physician-owned hospitals and having to call 911 when patients need emergent care or stabilization. That’s been the rebuttal, along with an Office of Inspector General (OIG) report from 2008. Almost, I guess, three quarters of the patients that needed emergent care got this at publicly funded hospitals.
Dr. Miller: I’m familiar with the argument about emergency care. If you actually go and look at it, it differs by specialty market. Physician-owned community hospitals have ERs because that’s how they get their business. If you are running a hospital medicine floor, a general surgical specialty floor, you have a labor delivery unit, a primary care clinic, and a cardiology clinic. You have all the things that all the other hospitals have. The physician-owned community hospitals almost uniformly have an ER.
When you look at the physician-owned specialty hospitals, it’s a little more granular. If you look at the cardiac hospitals, they have ERs. They also have cardiac ICUs, operating rooms, etc. The area where the hospital industry had concerns — which I think is valid to point out — is that physician-owned orthopedic surgical specialty hospitals don’t have ERs. But this makes sense because of what that hospital functionally is: a factory for whatever the scope of procedures is, be it joint replacements or shoulder arthroscopy. The orthopedic surgical specialty hospital is like an ASC plus several hospital beds. Many of those did not have ERs because clinically it didn’t make sense.
What’s interesting, though, is that the hospital industry also operates specialty hospitals. If you go into many of the large systems, they have cardiac specialty hospitals and cancer specialty hospitals. I would say that some of them have ERs, as they appropriately should, and some of those specialty hospitals do not. They might have a community hospital down the street that’s part of that health system that has an ER, but some of the specialty hospitals don’t necessarily have a dedicated ER.
I agree, that’s a valid concern. I would say, though, the question is, what are the scope of services in that hospital? Is an ER required? Community hospitals should have ERs. It makes sense also for a cardiac hospital to have one. If you’re running a total joint replacement factory, it might not make clinical sense.
Dr. Glatter: The patients who are treated at that hospital, if they do have emergent conditions, need to have board-certified emergency physicians treating them, in my view because I’m an ER physician. Having surgeons that are not emergency physicians staff a department at a specialty orthopedic hospital or, say, a cancer hospital is not acceptable from my standpoint. That›s my opinion and recommendation, coming from emergency medicine.
Dr. Miller: I would say that anesthesiologists are actually highly qualified in critical care. The question is about clinical decompensation; if you’re doing a procedure, you have an anesthesiologist right there who is capable of critical care. The function of the ER is to either serve as a window into the hospital for patient volume or to serve as a referral for emergent complaints.
Dr. Glatter: An anesthesiologist — I’ll take issue with that — does not have the training of an emergency physician in terms of scope of practice.
Dr. Miller: My anesthesiology colleagues would probably disagree for managing an emergency during an operating room case.
Dr. Glatter: Fair enough, but I think in the general sense. The other issue is that, in terms of emergent responses to patients that decompensate, when you have to transfer a patient, that violates Medicare requirements. How is that even a valid issue or argument if you’re going to have to transfer a patient from your specialty hospital? That happens. Again, I know that you’re saying these hospitals are completely independent and can function, stabilize patients, and treat emergencies, but that’s not the reality across the country, in my opinion.
Dr. Miller: I don’t think that’s the case for the physician-owned specialty cardiac hospitals, for starters. Many of those have ICUs in addition to operating rooms as a matter of routine in addition to ERs. I don’t think that’s the case for physician-owned community hospitals, which have ERs, ICUs, medicine floors, and surgical floors. Physician-owned community hospitals are around half the market. Of that remaining market, a significant percentage are cardiac hospitals. If you’re taking an issue with orthopedic surgical specialty hospitals, that’s a clinical operational question that can and should be answered.
I’d also posit that the nonprofit and for-profit hospital industries also operate specialty hospitals. Any of these questions, we shouldn’t just be asking about physician-owned facilities; we should be asking about them across ownership types, because we’re talking about scope of service and quality and safety. The ownership in that case doesn’t matter. The broader question is, are orthopedic surgical specialty hospitals owned by physicians, tax-exempt hospitals, or tax-paying hospitals? Is that a valid clinical business model? Is it safe? Does it meet Medicare conditions of participation? I would say that’s what that question is, because other ownership models do operate those facilities.
Dr. Glatter: You make some valid points, and I do agree on some of them. I think that, ultimately, these models of care, and certainly cost and quality, are issues. Again, it goes back to being able, in my opinion, to provide emergent care, which seems to me a very important issue.
Dr. Miller: I agree that providing emergent care is an issue. It›s an issue in any site of care. The hospital industry posits that all hospital outpatient departments (HOPDs) have emergent care. I can tell you, having worked in HOPDs (I›ve trained in them during residency), the response if something emergent happens is to either call 911 or wheel the patient down to the ER in a wheelchair or stretcher. I think that these hospital claims about emergency care coverage — these are important questions, but we should be asking them across all clinical settings and say what is the appropriate scope of care provided? What is the appropriate level of acuity and ability to provide emergent or critical care? That›s an important question regardless of ownership model across the entire industry.
Deeper Dive Into Data on Physician-Owned Hospitals
Dr. Glatter: We need to really focus on that. I’ll agree with you on that.
There was a March 2023 report from Dobson | DaVanzo. It showed that physician-owned hospitals had lower Medicaid, dual-eligible, and uncompensated care and charity care discharges than full-service acute care hospitals. Physician-owned hospitals had less than half the proportion of Medicaid discharges compared with non–physician-owned hospitals. They were also less likely to care for dual-eligible patients overall compared with non–physician-owned hospitals.
In addition, when COVID hit, the physician-owned hospitals overall — and again, there may be exceptions — were not equipped to handle these patient surges in the acute setting of a public health emergency. There was a hospital in Texas that did pivot that I’m aware of — Renaissance Hospital, which ramped up a long-term care facility to become a COVID hospital — but I think that’s the exception. I think this report raises some valid concerns; I’ll let you rebut that.
Dr. Miller: A couple of things. One, I am not aware that there’s any clear market evidence or a systematic study that shows that physician-owned hospitals had trouble responding to COVID. I don’t think that assertion has been proven. The study was funded by the hospital industry. First of all, it was not a peer-reviewed study; it was funded by an industry that paid a consulting firm. It doesn’t mean that we still shouldn’t read it, but that brings bias into question. The joke in Washington is, pick your favorite statistician or economist, and they can say what you want and have a battle of economists and statisticians.
For example, in that study, they didn’t include the entire ownership universe of physician-owned hospitals. If we go to the peer-reviewed literature, there’s a great 2015 BMJ paper showing that the Medicaid payer mix is actually the same between physician-owned hospitals vs not. The mix of patients by ethnicity — for example, think about African American patients — was the same. I would be more inclined to believe the peer-reviewed literature in BMJ as opposed to an industry-funded study that was not peer-reviewed and not independent and has methodological questions.
Dr. Glatter: Those data are 8 years old, so I’d like to see more recent data. It would be interesting, just as a follow-up to that, to see where the needle has moved — if it has, for that matter — in terms of Medicaid patients that you’re referring to.
Dr. Miller: I tend to be skeptical of all industry research, regardless of who published it, because they have an economic incentive. If they’re selecting certain age groups or excluding certain hospitals, that makes you wonder about the validity of the study. Your job as an industry-funded researcher is that, essentially, you’re being paid to look for an answer. It’s not necessarily an honest evaluation of the data.
Dr. Glatter: I want to bring up another point about the Hospital Readmissions Reduction Program (HRRP) and the data on how physician-owned hospitals compared with acute care hospitals that are non–physician-owned and have you comment on that. The Dobson | DaVanzo study called into question that physician-owned hospitals treat fewer patients who are dual-eligible, which we know.
Dr. Miller: I don’t think we do know that.
Dr. Glatter: There are data that point to that, again, looking at the studies.
Dr. Miller: I’m saying that’s a single study funded by industry as opposed to an independent, academic, peer-reviewed literature paper. That would be like saying, during the debate of the Inflation Reduction Act (IRA), that you should read the pharmaceutical industries research but take any of it at pure face value as factual. Yes, we should read it. Yes, we should evaluate it on its own merits. I think, again, appropriately, you need to be concerned when people have an economic incentive.
The question about the HRRP I’m going to take a little broader, because I think that program is unfair to the industry overall. There are many factors that drive hospital readmission. Whether Mrs Smith went home and ate potato chips and then took her Lasix, that’s very much outside of the hospital industry’s control, and there’s some evidence that the HRRP increases mortality in some patient populations.
In terms of a quality metric, it’s unfair to the industry. I think we took an operating process, internal metric for the hospital industry, turned it into a quality metric, and attached it to a financial bonus, which is an inappropriate policy decision.
Rethinking Ownership Models and Empowering Clinicians
Dr. Glatter: I agree with you on that. One thing I do want to bring up is that whether the physician-owned hospitals are subject to many of the quality measures that full-service, acute care hospitals are. That really is, I think, a broader context.
Dr. Miller: Fifty-five percent of physician-owned hospitals are full-service community hospitals, so I would say at least half the market is 100% subject to that.
Dr. Glatter: If only 50% are, that’s already an issue.
Dr. Miller: Cardiac specialty hospitals — which, as I said, nonprofit and for-profit hospital chains also operate — are also subject to the appropriate quality measures, readmissions, etc. Just because we don’t necessarily have the best quality measurement in the system in the country, it doesn’t mean that we shouldn’t allow care specialization. As I’d point out, if we’re concerned about specialty hospitals, the concern shouldn’t just be about physician-owned specialty hospitals; it should be about specialty hospitals by and large. Many health systems run cardiac specialty hospitals, cancer specialty hospitals, and orthopedic specialty hospitals. If we’re going to have a discussion about concerns there, it should be about the entire industry of specialty hospitals.
I think specialty hospitals serve an important role in society, allowing for specialization and exploiting in a positive way the volume-quality relationship. Whether those are owned by a for-profit publicly traded company, a tax-exempt facility, or physicians, I think that is an important way to have innovation and care delivery because frankly, we haven’t had much innovation in care delivery. Much of what we do in terms of how we practice clinically hasn’t really changed in the 50 years since my late father graduated from medical school. We still have rounds, we’re still taking notes, we’re still operating in the same way. Many processes are manual. We don’t have the mass production and mass customization of care that we need.
When you have a focused factory, it allows you to design care in a way that drives up quality, not just for the average patient but also the patients at the tail ends, because you have time to focus on that specific service line and that specific patient population.
Physician-owned community hospitals offer an important opportunity for a different employment model. I remember going to the dermatologist and the dermatologist was depressed, shuffling around the room, sad, and I asked him why. He said he didn’t really like his employer, and I said, “Why don’t you pick another one?” He’s like, “There are only two large health systems I can work for. They all have the same clinical practice environment and functionally the same value.”
Physicians are increasingly burned out. They face monopsony power in who purchases their labor. They have little control. They don’t want to go through five committees, seven administrators, and attend 25 meetings just to change a single small process in clinical operations. If you’re an owner operator, you have a much better ability to do it.
Frankly, when many facilities do well now, when they do well clinically and do well financially, who benefits? The hospital administration and the hospital executives. The doctors aren’t benefiting. The nurses aren’t benefiting. The CNA is not benefiting. The secretary is not benefiting. The custodian is not benefiting. Shouldn’t the workers have a right to own and operate the business and do well when the business does well serving the community? That puts me in the weird space of agreeing with both conservatives and progressives.
Dr. Glatter: I agree with you. I think an ownership stake is always attractive. It helps with retention of employed persons. There›s no question that, when they have a stake, when they have skin in the game, they feel more empowered. I will not argue with you about that.
Dr. Miller: We don’t have business models where workers have that option in healthcare. Like the National Academy of Medicine said, one of the key drivers of burnout is the externalization of the locus of control over clinical practice, and the current business operating models guarantee an externalization of the locus of control over clinical practice.
If you actually look at the recent American Medical Association (AMA) meeting, there was a resolution to ban the corporate practice of medicine. They wanted to go more toward the legal professions model where only physicians can own and operate care delivery.
Dr. Glatter: Well, I think the shift is certainly something that the AMA would like and physicians collectively would agree with. Having a better lifestyle and being able to have control are factors in burnout.
Dr. Miller: It’s not just doctors. I think nurses want a better lifestyle. The nurses are treated as interchangeable lines on a spreadsheet. The nurses are an integral part of our clinical team. Why don’t we work together as a clinical unit to build a better delivery system? What better way to do that than to have clinicians in charge of it, right?
My favorite bakery that’s about 30 minutes away is owned by a baker. It is not owned by a large tax-exempt corporation. It’s owned by an owner operator who takes pride in their work. I think that is something that the profession would do well to return to. When I was a resident, one of my colleagues was already planning their retirement. That’s how depressed they were.
I went into medicine to actually care for patients. I think that we can make the world a better place for our patients. What that means is not only treating them with drugs and devices, but also creating a delivery system where they don’t have to wander from lobby to lobby in a 200,000 square-foot facility, wait in line for hours on end, get bills 6 months later, and fill out endless paper forms over and over again.
All of these basic processes in healthcare delivery that are broken could have and should have been fixed — and have been fixed in almost every other industry. I had to replace one of my car tires because I had a flat tire. The local tire shop has an app, and it sends me SMS text messages telling me when my appointment is and when my car is ready. We have solved all of these problems in many other businesses.
We have not solved them in healthcare delivery because, one, we have massive monopolies that are raising prices, have lower quality, and deliver a crappy patient experience, and we have also subjugated the clinical worker into a corporate automaton. We are functionally drones. We don’t have the agency and the authority to improve clinical operations anymore. It’s really depressing, and we should have that option again.
I trust my doctor. I trust the nurses that I work with, and I would like them to help make clinical decisions in a financially responsible and a sensible operational manner. We need to empower our workforce in order to do that so we can recapture the value of what it means to be a clinician again.
The current model of corporate employment: massive scale, more administrators, more processes, more emails, more meetings, more PowerPoint decks, more federal subsidies. The hospital industry has choices. It can improve clinical operations. It can show up in Washington and lobby for increased subsidies. It can invest in the market and not pay taxes for the tax-exempt facilities. Obviously, it makes the logical choices as an economic actor to show up, lobby for increased subsidies, and then also invest in the stock market.
Improving clinical operations is hard. It hasn’t happened. The Bureau of Labor Statistics shows that the private community hospital industry has had flat labor productivity growth, on average, for the past 25 years, and for some years it even declined. This is totally atypical across the economy.
We have failed our clinicians, and most importantly, we have failed our patients. I’ve been sick. My relatives have been sick, waiting hours, not able to get appointments, and redoing forms. It’s a total disaster. It’s time and reasonable to try an alternative ownership and operating model. There are obviously problems. The problems can and should be addressed, but it doesn’t mean that we should have a statutory prohibition on professionals owning and operating their own business.
Dr. Glatter: There was a report that $500 million was saved by limiting or banning or putting a moratorium on physician-owned hospitals by the Congressional Budget Office.
Dr. Miller: Yes, I’m very aware of those data. I’d say that the CBO also is off by 50% on the estimation of the implementation of the Part D program. They overestimated the Affordable Care Act market enrollment by over 10 million people — again, around 50%. They also estimated that the CMS Innovation Center initially would be a savings. Now they’ve re-estimated it as a 10-year expenditure and it has actually cost the taxpayers money.
The CBO is not transparent about what its assumptions are or its analysis and methods. As a researcher, we have to publish our information. It has to go through peer review. I want to know what goes into that $500 million figure — what the assumptions are and what the model is. It’s hard to comment without knowing how they came up with it.
Dr. Glatter: The points you make are very valid. Physicians and nurses want a better lifestyle.
Dr. Miller: It’s not even a better lifestyle. It’s about having a say in how clinical operations work and helping make them better. We want the delivery system to work better. This is an opportunity for us to do so.
Dr. Glatter: That translates into technology: obviously, generative artificial intelligence (AI) coming into the forefront, as we know, and changing care delivery models as you’re referring to, which is going to happen. It’s going to be a slow process. I think that the evolution is happening and will happen, as you accurately described.
Dr. Miller: The other thing that’s different now vs 20 years ago is that managed care is here, there, and everywhere, as Dr Seuss would say. You have utilization review and prior authorization, which I’ve experienced as a patient and a physician, and boy, is it not a fun process. There’s a large amount of friction that needs to be improved. If we’re worried about induced demand or inappropriate utilization, we have managed care right there to help police bad behavior.
Reforming Healthcare Systems and Restoring Patient-Centric Focus
Dr. Glatter: If you were to come up with, say, three bullet points of how we can work our way out of this current morass of where our healthcare systems exist, where do you see the solutions or how can we make and effect change?
Dr. Miller: I’d say there are a couple of things. One is, let business models compete fairly on an equal playing field. Let the physician-owned hospital compete with the tax-exempt hospital and the nonprofit hospital. Put them on an equal playing field. We have things like 340B, which favors tax-exempt hospitals. For-profit or tax-paying hospitals are not able to participate in that. That doesn’t make any sense just from a public policy perspective. Tax-paying hospitals and physician-owned hospitals pay taxes on investments, but tax-exempt hospitals don’t. I think, in public policy, we need to equalize the playing field between business models. Let the best business model win.
The other thing we need to do is to encourage the adoption of technology. The physician will eventually be an arbiter of tech-driven or AI-driven tools. In fact, at some point, the standard of care might be to use those tools. Not using those tools would be seen as negligence. If you think about placing a jugular or central venous catheter, to not use ultrasound would be considered insane. Thirty years ago, to use ultrasound would be considered novel. I think technology and AI will get us to that point of helping make care more efficient and more customized.
Those are the two biggest interventions, I would say. Third, every time we have a conversation in public policy, we need to remember what it is to be a patient. The decision should be driven not around any one industry’s profitability, but what it is to be a patient and how we can make that experience less burdensome, less expensive, or in plain English, suck less.
Dr. Glatter: Safety net hospitals and critical access hospitals are part of this discussion that, yes, we want everything to, in an ideal world, function more efficiently and effectively, with less cost and less red tape. The safety net of our nation is struggling.
Dr. Miller: I 100% agree. The Cook County hospitals of the world are deserving of our support and, frankly, our gratitude. Facilities like that have huge burdens of patients with Medicaid. We also still have millions of uninsured patients. The neighborhoods that they serve are also poorer. I think facilities like that are deserving of public support.
I also think we need to clearly define what those hospitals are. One of the challenges I’ve realized as I waded into this space is that market definitions of what a service market is for a hospital, its specialty type or what a safety net hospital is need to be more clearly defined because those facilities 100% are deserving of our support. We just need to be clear about what they are.
Regarding critical access hospitals, when you practice in a rural area, you have to think differently about care delivery. I’d say many of the rural systems are highly creative in how they structure clinical operations. Before the public health emergency, during the COVID pandemic, when we had a massive change in telehealth, rural hospitals were using — within the very narrow confines — as much telehealth as they could and should.
Rural hospitals also make greater use of nurse practitioners (NPs) and physician assistants (PAs). For many of the specialty services, I remember, your first call was an NP or a PA because the physician was downstairs doing procedures. They’d come up and assess the patient before the procedure, but most of your consult questions were answered by the NP or PA. I’m not saying that’s the model we should use nationwide, but that rural systems are highly innovative and creative; they’re deserving of our time, attention, and support, and frankly, we can learn from them.
Dr. Glatter: I want to thank you for your time and your expertise in this area. We’ll see how the congressional hearings affect the industry as a whole, how the needle moves, and whether the ban or moratorium on physician-owned hospitals continues to exist going forward.
Dr. Miller: I appreciate you having me. The hospital industry is one of the most important industries for health care. This is a time of inflection, right? We need to go back to the value of what it means to be a clinician and serve patients. Hospitals need to reorient themselves around that core concern. How do we help support clinicians — doctors, nurses, pharmacists, whomever it is — in serving patients? Hospitals have become too corporate, so I think that this is an expected pushback.
Dr. Glatter: Again, I want to thank you for your time. This was a very important discussion. Thank you for your expertise.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He disclosed no relevant financial relationships.Brian J. Miller, MD, MBA, MPH, is a hospitalist and an assistant professor of medicine at the Johns Hopkins University School of Medicine. He is also a nonresident fellow at the American Enterprise Institute. From 2014 to 2017, Dr. Miller worked at four federal regulatory agencies: Federal Trade Commission (FTC), Federal Communications Commission (FCC), Centers for Medicare & Medicaid Services (CMS), and the Food & Drug Administration (FDA). Dr. Miller disclosed ties with the Medicare Payment Advisory Commission.
A version of this article appeared on Medscape.com.
Doctors Win $7 Million Settlement in EEOC Forced Retirement Case
In a victory for clinicians who fought to keep working regardless of age,
In a statement, the US Equal Employment Opportunity Commission (EEOC) said the settlement will resolve an age and disability discrimination charge filed against Scripps Clinic Medical Group. The medical group is part of Scripps Health, a major provider of medical services in the San Diego region that operates five local hospitals.
The EECO said it found “reasonable cause” that the medical group violated the Age Discrimination in Employment Act and the Americans with Disabilities Act.
US health systems are facing lawsuits that claim they’ve engaged in age discrimination by requiring physicians to take cognitive tests when they reach specific ages.
The Scripps medical group’s mandatory retirement policy began in 2016 and was consistent with California law, which specifically allows for mandatory retirement of physicians in medical groups at age 70, Scripps said in a statement, adding that it rescinded the policy in 2018.
“This policy was put in place to enhance patient safety,” Scripps said. “The EEOC took the position while such a policy is expressly legal under California law; it is not allowed under federal law.”
The Federal Age Discrimination in Employment Act, passed in 1967, states that employers may not “fail or refuse to hire or to discharge any individual or otherwise discriminate against any individual with respect to his compensation, terms, conditions, or privileges of employment because of such individual’s age.” There are exceptions, however, in cases of public safety for professions such as air traffic controllers.
California law has a similar provision banning age discrimination, but it makes an exception for “any employee who has attained 70 years of age and is a physician employed by a professional medical corporation, the articles or bylaws of which provide for compulsory retirement.”
In 2020, an estimated 12% of US licensed physicians were at least 70 years old — more than 120,000 in total — up from 9% in a 2010, according to a Federation of State Medical Boards 2021 report.
Scripps Clinic Medical Group settled with the EEOC “without any admission of fault or wrongdoing to avoid the continued expense and distraction of litigation,” its statement said. It agreed to pay $6.875 million to the affected physicians.
When asked about how many physicians were affected by the policy, a Scripps human resources official said, “this was disputed but very few. The policy was only in effect for 2 years, 2016 and 2017. Additionally, by age 75, most doctors have retired. And those who have not almost always have voluntarily limited their practice.”
The Scripps official didn’t respond to questions about the number of patients served by the medical group and how many physicians it employs.
According to the EEOC, the medical group has agreed to tell employees that the policy has been scrapped and must “clarify that the company does not have any policy in which age is a factor in making employment decisions, including termination, retirement, and terms and conditions of employment.”
Scripps Clinic Medical Group also agreed to require division and department heads, executive leadership, and human resources employees to be trained regarding the Age Discrimination in Employment Act and the Americans with Disabilities Act.
A version of this article appeared on Medscape.com.
In a victory for clinicians who fought to keep working regardless of age,
In a statement, the US Equal Employment Opportunity Commission (EEOC) said the settlement will resolve an age and disability discrimination charge filed against Scripps Clinic Medical Group. The medical group is part of Scripps Health, a major provider of medical services in the San Diego region that operates five local hospitals.
The EECO said it found “reasonable cause” that the medical group violated the Age Discrimination in Employment Act and the Americans with Disabilities Act.
US health systems are facing lawsuits that claim they’ve engaged in age discrimination by requiring physicians to take cognitive tests when they reach specific ages.
The Scripps medical group’s mandatory retirement policy began in 2016 and was consistent with California law, which specifically allows for mandatory retirement of physicians in medical groups at age 70, Scripps said in a statement, adding that it rescinded the policy in 2018.
“This policy was put in place to enhance patient safety,” Scripps said. “The EEOC took the position while such a policy is expressly legal under California law; it is not allowed under federal law.”
The Federal Age Discrimination in Employment Act, passed in 1967, states that employers may not “fail or refuse to hire or to discharge any individual or otherwise discriminate against any individual with respect to his compensation, terms, conditions, or privileges of employment because of such individual’s age.” There are exceptions, however, in cases of public safety for professions such as air traffic controllers.
California law has a similar provision banning age discrimination, but it makes an exception for “any employee who has attained 70 years of age and is a physician employed by a professional medical corporation, the articles or bylaws of which provide for compulsory retirement.”
In 2020, an estimated 12% of US licensed physicians were at least 70 years old — more than 120,000 in total — up from 9% in a 2010, according to a Federation of State Medical Boards 2021 report.
Scripps Clinic Medical Group settled with the EEOC “without any admission of fault or wrongdoing to avoid the continued expense and distraction of litigation,” its statement said. It agreed to pay $6.875 million to the affected physicians.
When asked about how many physicians were affected by the policy, a Scripps human resources official said, “this was disputed but very few. The policy was only in effect for 2 years, 2016 and 2017. Additionally, by age 75, most doctors have retired. And those who have not almost always have voluntarily limited their practice.”
The Scripps official didn’t respond to questions about the number of patients served by the medical group and how many physicians it employs.
According to the EEOC, the medical group has agreed to tell employees that the policy has been scrapped and must “clarify that the company does not have any policy in which age is a factor in making employment decisions, including termination, retirement, and terms and conditions of employment.”
Scripps Clinic Medical Group also agreed to require division and department heads, executive leadership, and human resources employees to be trained regarding the Age Discrimination in Employment Act and the Americans with Disabilities Act.
A version of this article appeared on Medscape.com.
In a victory for clinicians who fought to keep working regardless of age,
In a statement, the US Equal Employment Opportunity Commission (EEOC) said the settlement will resolve an age and disability discrimination charge filed against Scripps Clinic Medical Group. The medical group is part of Scripps Health, a major provider of medical services in the San Diego region that operates five local hospitals.
The EECO said it found “reasonable cause” that the medical group violated the Age Discrimination in Employment Act and the Americans with Disabilities Act.
US health systems are facing lawsuits that claim they’ve engaged in age discrimination by requiring physicians to take cognitive tests when they reach specific ages.
The Scripps medical group’s mandatory retirement policy began in 2016 and was consistent with California law, which specifically allows for mandatory retirement of physicians in medical groups at age 70, Scripps said in a statement, adding that it rescinded the policy in 2018.
“This policy was put in place to enhance patient safety,” Scripps said. “The EEOC took the position while such a policy is expressly legal under California law; it is not allowed under federal law.”
The Federal Age Discrimination in Employment Act, passed in 1967, states that employers may not “fail or refuse to hire or to discharge any individual or otherwise discriminate against any individual with respect to his compensation, terms, conditions, or privileges of employment because of such individual’s age.” There are exceptions, however, in cases of public safety for professions such as air traffic controllers.
California law has a similar provision banning age discrimination, but it makes an exception for “any employee who has attained 70 years of age and is a physician employed by a professional medical corporation, the articles or bylaws of which provide for compulsory retirement.”
In 2020, an estimated 12% of US licensed physicians were at least 70 years old — more than 120,000 in total — up from 9% in a 2010, according to a Federation of State Medical Boards 2021 report.
Scripps Clinic Medical Group settled with the EEOC “without any admission of fault or wrongdoing to avoid the continued expense and distraction of litigation,” its statement said. It agreed to pay $6.875 million to the affected physicians.
When asked about how many physicians were affected by the policy, a Scripps human resources official said, “this was disputed but very few. The policy was only in effect for 2 years, 2016 and 2017. Additionally, by age 75, most doctors have retired. And those who have not almost always have voluntarily limited their practice.”
The Scripps official didn’t respond to questions about the number of patients served by the medical group and how many physicians it employs.
According to the EEOC, the medical group has agreed to tell employees that the policy has been scrapped and must “clarify that the company does not have any policy in which age is a factor in making employment decisions, including termination, retirement, and terms and conditions of employment.”
Scripps Clinic Medical Group also agreed to require division and department heads, executive leadership, and human resources employees to be trained regarding the Age Discrimination in Employment Act and the Americans with Disabilities Act.
A version of this article appeared on Medscape.com.
‘Real-World’ Registry Study of Upadacitinib Supports Clinical Trial Data
, results from an analysis of registry data showed.
“We know from clinical trials that upadacitinib is effective, but we have very little real-world experience on its effectiveness,” presenting study author Melinda Gooderham, MD, medical director of the Skin Center for Dermatology in Peterborough, Ontario, Canada, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference.
For the analysis, she and her coauthors evaluated the real-world clinical and patient-reported outcomes of 335 adults enrolled in the CorEvitas AD Registry from July 21, 2020, through Aug. 7, 2023. They included patients who were on upadacitinib for at least 4 weeks and persisted on the drug until the time of evaluation.
The CorEvitas AD Registry is a prospective, non-interventional registry of adults diagnosed with AD by a dermatologist or qualified dermatology practitioner. Outcomes measures included the proportion of patients who achieved skin clearance as defined by a Validated Investigators Global Assessment Scale for Atopic Dermatitis (vIGA) score of 0 or 1, an Eczema Area and Severity Index (EASI) score of 3 or less, a Peak Pruritus Numeric Rating Scale (PP-NRS) score of 0 or 1, Patient-Oriented Eczema Measure (POEM) scores of 0-2, Dermatology Life Quality Index (DLQI) scores of 0 or 1, or an Atopic Dermatitis Control Tool (ADCT) score of <7.
The researchers evaluated cross-sectional data from three different cohorts: data from the last registry visit (the overall cohort), data from a visit within 1 month to less than 5 months of upadacitinib initiation (the 1-5 months cohort), and data from a visit within 5-9 months following upadacitinib initiation (the 5-9 months cohort). They also conducted subgroup analyses of patients with prior use of biologics for AD (bio-experience) and those with no such history (bio-naive). Safety events were not assessed in this analysis.
The mean age of the 335 patients was 45.6 years, 51.6% were female, 64.2% were White, 64.2% were based in the United States and the rest were based in Canada. Most patients (70.8%) were treated with the 15-mg dose of upadacitinib. The median duration of treatment was 6.9 months. Slightly more than one-quarter of patients (28.1%) reported concomitant use of topical corticosteroids for AD, while 45.4% reported prior use of dupilumab and 6% reported prior use of tralokinumab.
Dr. Gooderham reported that 57.5% of patients in the total cohort total cohort achieved clear or almost clear skin (a vIGA-AD score of 0 or 1), with slight differences between the bio-naive (60.6%) and bio-experienced (54.1%) subgroups.
The other outcomes were similarly close between the 176 bio-naive and 159 bio-experienced patients. Specifically, 74.8% in the total cohort, 79.4% in the bio-naive subgroup, and 69.6% in the bio-experienced subgroup achieved an EASI score of 3 or less. In the measure of the worst itch in the past 24 hours, 45.3%, 47.7%, and 42.8% respectively achieved a PP-NRS of 0 or 1. In the patient-reported disease burden, 36.4%, 41%, and 31.4% achieved a POEM score of 0-2. In the quality of life measure, 39.8%, 42.8%, and 36.5% achieved a DLQI score of 0 or 1. In the measure of disease control, 69.3%, 70.5%, and 67.9% achieved an ADCT score of <7. In a combination of skin clearance and itch control, 40.9%, 43.2%, and 38.4% of the total cohort, bio-naive, and bio-experienced respectively achieved both an EASI score of 3 or less and a PP-NRS of 0 or 1.
The study outcomes were similar between the 1-5 months cohort and the 5-9 months cohort, but there was a trend toward more clearance the longer patients were on therapy.
“The findings suggest that low levels of disease severity are observed in patients on upadacitinib in a real-world setting,” Dr. Gooderham concluded. “This confirms what we see in the clinical trials.”
She disclosed that she is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including AbbVie.
, results from an analysis of registry data showed.
“We know from clinical trials that upadacitinib is effective, but we have very little real-world experience on its effectiveness,” presenting study author Melinda Gooderham, MD, medical director of the Skin Center for Dermatology in Peterborough, Ontario, Canada, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference.
For the analysis, she and her coauthors evaluated the real-world clinical and patient-reported outcomes of 335 adults enrolled in the CorEvitas AD Registry from July 21, 2020, through Aug. 7, 2023. They included patients who were on upadacitinib for at least 4 weeks and persisted on the drug until the time of evaluation.
The CorEvitas AD Registry is a prospective, non-interventional registry of adults diagnosed with AD by a dermatologist or qualified dermatology practitioner. Outcomes measures included the proportion of patients who achieved skin clearance as defined by a Validated Investigators Global Assessment Scale for Atopic Dermatitis (vIGA) score of 0 or 1, an Eczema Area and Severity Index (EASI) score of 3 or less, a Peak Pruritus Numeric Rating Scale (PP-NRS) score of 0 or 1, Patient-Oriented Eczema Measure (POEM) scores of 0-2, Dermatology Life Quality Index (DLQI) scores of 0 or 1, or an Atopic Dermatitis Control Tool (ADCT) score of <7.
The researchers evaluated cross-sectional data from three different cohorts: data from the last registry visit (the overall cohort), data from a visit within 1 month to less than 5 months of upadacitinib initiation (the 1-5 months cohort), and data from a visit within 5-9 months following upadacitinib initiation (the 5-9 months cohort). They also conducted subgroup analyses of patients with prior use of biologics for AD (bio-experience) and those with no such history (bio-naive). Safety events were not assessed in this analysis.
The mean age of the 335 patients was 45.6 years, 51.6% were female, 64.2% were White, 64.2% were based in the United States and the rest were based in Canada. Most patients (70.8%) were treated with the 15-mg dose of upadacitinib. The median duration of treatment was 6.9 months. Slightly more than one-quarter of patients (28.1%) reported concomitant use of topical corticosteroids for AD, while 45.4% reported prior use of dupilumab and 6% reported prior use of tralokinumab.
Dr. Gooderham reported that 57.5% of patients in the total cohort total cohort achieved clear or almost clear skin (a vIGA-AD score of 0 or 1), with slight differences between the bio-naive (60.6%) and bio-experienced (54.1%) subgroups.
The other outcomes were similarly close between the 176 bio-naive and 159 bio-experienced patients. Specifically, 74.8% in the total cohort, 79.4% in the bio-naive subgroup, and 69.6% in the bio-experienced subgroup achieved an EASI score of 3 or less. In the measure of the worst itch in the past 24 hours, 45.3%, 47.7%, and 42.8% respectively achieved a PP-NRS of 0 or 1. In the patient-reported disease burden, 36.4%, 41%, and 31.4% achieved a POEM score of 0-2. In the quality of life measure, 39.8%, 42.8%, and 36.5% achieved a DLQI score of 0 or 1. In the measure of disease control, 69.3%, 70.5%, and 67.9% achieved an ADCT score of <7. In a combination of skin clearance and itch control, 40.9%, 43.2%, and 38.4% of the total cohort, bio-naive, and bio-experienced respectively achieved both an EASI score of 3 or less and a PP-NRS of 0 or 1.
The study outcomes were similar between the 1-5 months cohort and the 5-9 months cohort, but there was a trend toward more clearance the longer patients were on therapy.
“The findings suggest that low levels of disease severity are observed in patients on upadacitinib in a real-world setting,” Dr. Gooderham concluded. “This confirms what we see in the clinical trials.”
She disclosed that she is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including AbbVie.
, results from an analysis of registry data showed.
“We know from clinical trials that upadacitinib is effective, but we have very little real-world experience on its effectiveness,” presenting study author Melinda Gooderham, MD, medical director of the Skin Center for Dermatology in Peterborough, Ontario, Canada, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference.
For the analysis, she and her coauthors evaluated the real-world clinical and patient-reported outcomes of 335 adults enrolled in the CorEvitas AD Registry from July 21, 2020, through Aug. 7, 2023. They included patients who were on upadacitinib for at least 4 weeks and persisted on the drug until the time of evaluation.
The CorEvitas AD Registry is a prospective, non-interventional registry of adults diagnosed with AD by a dermatologist or qualified dermatology practitioner. Outcomes measures included the proportion of patients who achieved skin clearance as defined by a Validated Investigators Global Assessment Scale for Atopic Dermatitis (vIGA) score of 0 or 1, an Eczema Area and Severity Index (EASI) score of 3 or less, a Peak Pruritus Numeric Rating Scale (PP-NRS) score of 0 or 1, Patient-Oriented Eczema Measure (POEM) scores of 0-2, Dermatology Life Quality Index (DLQI) scores of 0 or 1, or an Atopic Dermatitis Control Tool (ADCT) score of <7.
The researchers evaluated cross-sectional data from three different cohorts: data from the last registry visit (the overall cohort), data from a visit within 1 month to less than 5 months of upadacitinib initiation (the 1-5 months cohort), and data from a visit within 5-9 months following upadacitinib initiation (the 5-9 months cohort). They also conducted subgroup analyses of patients with prior use of biologics for AD (bio-experience) and those with no such history (bio-naive). Safety events were not assessed in this analysis.
The mean age of the 335 patients was 45.6 years, 51.6% were female, 64.2% were White, 64.2% were based in the United States and the rest were based in Canada. Most patients (70.8%) were treated with the 15-mg dose of upadacitinib. The median duration of treatment was 6.9 months. Slightly more than one-quarter of patients (28.1%) reported concomitant use of topical corticosteroids for AD, while 45.4% reported prior use of dupilumab and 6% reported prior use of tralokinumab.
Dr. Gooderham reported that 57.5% of patients in the total cohort total cohort achieved clear or almost clear skin (a vIGA-AD score of 0 or 1), with slight differences between the bio-naive (60.6%) and bio-experienced (54.1%) subgroups.
The other outcomes were similarly close between the 176 bio-naive and 159 bio-experienced patients. Specifically, 74.8% in the total cohort, 79.4% in the bio-naive subgroup, and 69.6% in the bio-experienced subgroup achieved an EASI score of 3 or less. In the measure of the worst itch in the past 24 hours, 45.3%, 47.7%, and 42.8% respectively achieved a PP-NRS of 0 or 1. In the patient-reported disease burden, 36.4%, 41%, and 31.4% achieved a POEM score of 0-2. In the quality of life measure, 39.8%, 42.8%, and 36.5% achieved a DLQI score of 0 or 1. In the measure of disease control, 69.3%, 70.5%, and 67.9% achieved an ADCT score of <7. In a combination of skin clearance and itch control, 40.9%, 43.2%, and 38.4% of the total cohort, bio-naive, and bio-experienced respectively achieved both an EASI score of 3 or less and a PP-NRS of 0 or 1.
The study outcomes were similar between the 1-5 months cohort and the 5-9 months cohort, but there was a trend toward more clearance the longer patients were on therapy.
“The findings suggest that low levels of disease severity are observed in patients on upadacitinib in a real-world setting,” Dr. Gooderham concluded. “This confirms what we see in the clinical trials.”
She disclosed that she is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including AbbVie.
FROM RAD 2023
What Makes Patients Vulnerable to Delusions of Parasitosis?
, reported researchers in a small retrospective case-control study.
Delusions of parasitosis (DOP) affects mostly middle-aged women and has associations with renal failure and some medications, wrote corresponding author Colleen Reisz, MD, a dermatologist with the department of internal medicine at the University of Missouri–Kansas City School of Medicine, and her coauthors. The study was published online December 15, 2023, in the Journal of the American Academy of Dermatology.
“We hypothesize that vulnerability to DOP emerges when multiple factors combine, such as age, sex, medications, and changes in [drug] clearance capacity,” Dr. Reisz and her coauthors wrote. “Changes in health care, such as the dramatic increase in stimulant prescriptions and alternatives to opioids in pain management, may be contributing to off target drug effects on the brain.”
To test their hypothesis, the researchers conducted a case-control study of biometric and pharmaceutical data from 34 patients with DOP which they compared to an age-matched control group of 53 women presenting with a dermatitis above the clavicle from a general dermatology practice between 2012 and 2020. They de-identified the data and performed statistical analysis on variables that included biometric data and intake of pharmaceuticals and nutraceuticals. Polypharmacy was defined as five or more drugs.
Of the 34 patients with DOP, 27 were women with a mean age of 58 years and 7 were men with a mean age of 60 years. Dr. Reisz and her colleagues observed statistical significance between cases and controls in terms of polypharmacy (P = .011), attention-deficit/hyperactivity disorder medications (P < .001), selective serotonin reuptake inhibitors (P = .005), opioids (P = .003), and gabapentin (P = .003).
In other findings, half of DOP cases presented with samples of perceived parasitic material, and four associated the perceived infestation with a single emotion-laden event. This prompted the researchers “to consider that DOP may share mechanisms with fear conditioning and extinction,” they wrote. “Fear conditioning refers to the process of memory acquisition and extinction. This process is essential for survival and has been studied in posttraumatic stress disorder.”
They acknowledged certain limitations of the study, including its retrospective single-center design and the lack of control for factors such as socioeconomic background and level of education.
“Patients with DOP should undergo detailed drug histories and examination of clearance profiles, especially renal function,” the researchers concluded.
Evan A. Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, said that delusional infestation is one of the most difficult medical conditions to treat and study.
“Though the numbers of cases in this research letter are small, they are instructive in demonstrating a high burden of polypharmacy including psychostimulants, opioids, and SSRIs in such patients,” he told this news organization. “Dermatologists should be performing detailed drug histories, obtaining comprehensive lab work, and considering the effects of medications — both illicit and prescribed — on clinical presentations. While in many cases, delusional patients refuse to consent to psychopharmacologic medications (or treatment in general), the elimination or decrease in dose of certain problematic medications may be helpful in and of themselves.”
The researchers reported having no financial disclosures. Dr. Rieder disclosed that he is a consultant for AbbVie, L’Oréal, Pierre Fabre, Procter & Gamble, and Unilever.
, reported researchers in a small retrospective case-control study.
Delusions of parasitosis (DOP) affects mostly middle-aged women and has associations with renal failure and some medications, wrote corresponding author Colleen Reisz, MD, a dermatologist with the department of internal medicine at the University of Missouri–Kansas City School of Medicine, and her coauthors. The study was published online December 15, 2023, in the Journal of the American Academy of Dermatology.
“We hypothesize that vulnerability to DOP emerges when multiple factors combine, such as age, sex, medications, and changes in [drug] clearance capacity,” Dr. Reisz and her coauthors wrote. “Changes in health care, such as the dramatic increase in stimulant prescriptions and alternatives to opioids in pain management, may be contributing to off target drug effects on the brain.”
To test their hypothesis, the researchers conducted a case-control study of biometric and pharmaceutical data from 34 patients with DOP which they compared to an age-matched control group of 53 women presenting with a dermatitis above the clavicle from a general dermatology practice between 2012 and 2020. They de-identified the data and performed statistical analysis on variables that included biometric data and intake of pharmaceuticals and nutraceuticals. Polypharmacy was defined as five or more drugs.
Of the 34 patients with DOP, 27 were women with a mean age of 58 years and 7 were men with a mean age of 60 years. Dr. Reisz and her colleagues observed statistical significance between cases and controls in terms of polypharmacy (P = .011), attention-deficit/hyperactivity disorder medications (P < .001), selective serotonin reuptake inhibitors (P = .005), opioids (P = .003), and gabapentin (P = .003).
In other findings, half of DOP cases presented with samples of perceived parasitic material, and four associated the perceived infestation with a single emotion-laden event. This prompted the researchers “to consider that DOP may share mechanisms with fear conditioning and extinction,” they wrote. “Fear conditioning refers to the process of memory acquisition and extinction. This process is essential for survival and has been studied in posttraumatic stress disorder.”
They acknowledged certain limitations of the study, including its retrospective single-center design and the lack of control for factors such as socioeconomic background and level of education.
“Patients with DOP should undergo detailed drug histories and examination of clearance profiles, especially renal function,” the researchers concluded.
Evan A. Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, said that delusional infestation is one of the most difficult medical conditions to treat and study.
“Though the numbers of cases in this research letter are small, they are instructive in demonstrating a high burden of polypharmacy including psychostimulants, opioids, and SSRIs in such patients,” he told this news organization. “Dermatologists should be performing detailed drug histories, obtaining comprehensive lab work, and considering the effects of medications — both illicit and prescribed — on clinical presentations. While in many cases, delusional patients refuse to consent to psychopharmacologic medications (or treatment in general), the elimination or decrease in dose of certain problematic medications may be helpful in and of themselves.”
The researchers reported having no financial disclosures. Dr. Rieder disclosed that he is a consultant for AbbVie, L’Oréal, Pierre Fabre, Procter & Gamble, and Unilever.
, reported researchers in a small retrospective case-control study.
Delusions of parasitosis (DOP) affects mostly middle-aged women and has associations with renal failure and some medications, wrote corresponding author Colleen Reisz, MD, a dermatologist with the department of internal medicine at the University of Missouri–Kansas City School of Medicine, and her coauthors. The study was published online December 15, 2023, in the Journal of the American Academy of Dermatology.
“We hypothesize that vulnerability to DOP emerges when multiple factors combine, such as age, sex, medications, and changes in [drug] clearance capacity,” Dr. Reisz and her coauthors wrote. “Changes in health care, such as the dramatic increase in stimulant prescriptions and alternatives to opioids in pain management, may be contributing to off target drug effects on the brain.”
To test their hypothesis, the researchers conducted a case-control study of biometric and pharmaceutical data from 34 patients with DOP which they compared to an age-matched control group of 53 women presenting with a dermatitis above the clavicle from a general dermatology practice between 2012 and 2020. They de-identified the data and performed statistical analysis on variables that included biometric data and intake of pharmaceuticals and nutraceuticals. Polypharmacy was defined as five or more drugs.
Of the 34 patients with DOP, 27 were women with a mean age of 58 years and 7 were men with a mean age of 60 years. Dr. Reisz and her colleagues observed statistical significance between cases and controls in terms of polypharmacy (P = .011), attention-deficit/hyperactivity disorder medications (P < .001), selective serotonin reuptake inhibitors (P = .005), opioids (P = .003), and gabapentin (P = .003).
In other findings, half of DOP cases presented with samples of perceived parasitic material, and four associated the perceived infestation with a single emotion-laden event. This prompted the researchers “to consider that DOP may share mechanisms with fear conditioning and extinction,” they wrote. “Fear conditioning refers to the process of memory acquisition and extinction. This process is essential for survival and has been studied in posttraumatic stress disorder.”
They acknowledged certain limitations of the study, including its retrospective single-center design and the lack of control for factors such as socioeconomic background and level of education.
“Patients with DOP should undergo detailed drug histories and examination of clearance profiles, especially renal function,” the researchers concluded.
Evan A. Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, said that delusional infestation is one of the most difficult medical conditions to treat and study.
“Though the numbers of cases in this research letter are small, they are instructive in demonstrating a high burden of polypharmacy including psychostimulants, opioids, and SSRIs in such patients,” he told this news organization. “Dermatologists should be performing detailed drug histories, obtaining comprehensive lab work, and considering the effects of medications — both illicit and prescribed — on clinical presentations. While in many cases, delusional patients refuse to consent to psychopharmacologic medications (or treatment in general), the elimination or decrease in dose of certain problematic medications may be helpful in and of themselves.”
The researchers reported having no financial disclosures. Dr. Rieder disclosed that he is a consultant for AbbVie, L’Oréal, Pierre Fabre, Procter & Gamble, and Unilever.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
FDA Approves Topical Gel For Wounds Associated With JEB and DEB
The FDA has approved a topical gel containing birch triterpenes for the treatment of partial thickness wounds in patients 6 months and older with junctional epidermolysis bullosa (JEB) and dystrophic epidermolysis bullosa (DEB).
The gel is marketed under the name Filsuvez. It is the first approved treatment for wounds associated with JEB and the second for patients with DEB, following the approval of Vyjuvek (Krystal Biotech), a topical gene therapy gel, in May 2023.
First developed by Amryt Pharma and intended for home use, Filsuvez is now marketed by Chiesi Global Rare Diseases, which acquired Amryt in January 2023. The gel is applied topically to the wound at each dressing change.
The approval of Filsuvez is based on results from the Efficacy and Safety Study of Oleogel-S10 in Epidermolysis Bullosa (EASE), a randomized, placebo-controlled study of 223 people, the largest-ever phase 3 clinical trial for the treatment of EB, according to the Chiesi news release. The gel was well tolerated and met the primary endpoint with statistical significance, with 41.3% of patients achieving first complete target wound closure within 45 days (compared with 28.9% on placebo).
“I am so excited to say that this is another hurdle cleared and milestone achieved for the EB Community,” Brett Kopelan, executive director at debra of America said in a blog post. “We are now on the road to being able to treat EB more effectively, and to make the worst disease you’ve never heard of chronic, but livable, by making use of multiple therapeutic options in conjunction with each other.”
The FDA has approved a topical gel containing birch triterpenes for the treatment of partial thickness wounds in patients 6 months and older with junctional epidermolysis bullosa (JEB) and dystrophic epidermolysis bullosa (DEB).
The gel is marketed under the name Filsuvez. It is the first approved treatment for wounds associated with JEB and the second for patients with DEB, following the approval of Vyjuvek (Krystal Biotech), a topical gene therapy gel, in May 2023.
First developed by Amryt Pharma and intended for home use, Filsuvez is now marketed by Chiesi Global Rare Diseases, which acquired Amryt in January 2023. The gel is applied topically to the wound at each dressing change.
The approval of Filsuvez is based on results from the Efficacy and Safety Study of Oleogel-S10 in Epidermolysis Bullosa (EASE), a randomized, placebo-controlled study of 223 people, the largest-ever phase 3 clinical trial for the treatment of EB, according to the Chiesi news release. The gel was well tolerated and met the primary endpoint with statistical significance, with 41.3% of patients achieving first complete target wound closure within 45 days (compared with 28.9% on placebo).
“I am so excited to say that this is another hurdle cleared and milestone achieved for the EB Community,” Brett Kopelan, executive director at debra of America said in a blog post. “We are now on the road to being able to treat EB more effectively, and to make the worst disease you’ve never heard of chronic, but livable, by making use of multiple therapeutic options in conjunction with each other.”
The FDA has approved a topical gel containing birch triterpenes for the treatment of partial thickness wounds in patients 6 months and older with junctional epidermolysis bullosa (JEB) and dystrophic epidermolysis bullosa (DEB).
The gel is marketed under the name Filsuvez. It is the first approved treatment for wounds associated with JEB and the second for patients with DEB, following the approval of Vyjuvek (Krystal Biotech), a topical gene therapy gel, in May 2023.
First developed by Amryt Pharma and intended for home use, Filsuvez is now marketed by Chiesi Global Rare Diseases, which acquired Amryt in January 2023. The gel is applied topically to the wound at each dressing change.
The approval of Filsuvez is based on results from the Efficacy and Safety Study of Oleogel-S10 in Epidermolysis Bullosa (EASE), a randomized, placebo-controlled study of 223 people, the largest-ever phase 3 clinical trial for the treatment of EB, according to the Chiesi news release. The gel was well tolerated and met the primary endpoint with statistical significance, with 41.3% of patients achieving first complete target wound closure within 45 days (compared with 28.9% on placebo).
“I am so excited to say that this is another hurdle cleared and milestone achieved for the EB Community,” Brett Kopelan, executive director at debra of America said in a blog post. “We are now on the road to being able to treat EB more effectively, and to make the worst disease you’ve never heard of chronic, but livable, by making use of multiple therapeutic options in conjunction with each other.”
Is It Time to Air Grievances?
‘Twas the night before Festivus and all through the house, everyone was griping.
In case you’ve only been watching Friends reruns lately, Festivus is a holiday that originated 25 years ago in the last season of Seinfeld. George’s father created it as an alternative to Christmas hype. In addition to an aluminum pole, the holiday features the annual airing of grievances, when one is encouraged to voice complaints. Aluminum poles haven’t replaced Christmas trees, but the spirit of Festivus is still with us in the widespread airing of grievances in 2023.
Complaining isn’t just a post-pandemic problem. Hector spends quite a bit of time complaining about Paris in the Iliad. That was a few pandemics ago. And repining is ubiquitous in literature — as human as walking on two limbs it seems. Ostensibly, we complain to effect change: Something is wrong and we expect it to be different. But that’s not the whole story. No one believes the weather will improve or the Patriots will play better because we complain about them. So why do we bother?
Even if nothing changes on the outside, it does seem to alter our internal state, serving a healthy psychological function. Putting to words what is aggravating can have the same benefit of deep breathing. We describe it as “getting something off our chest” because that’s what it feels like. We feel unburdened just by saying it out loud. Think about the last time you complained: Cranky staff, prior auths, Medicare, disrespectful patients, many of your colleagues will nod in agreement, validating your feelings and making you feel less isolated.
There are also maladaptive reasons for whining. It’s obviously an elementary way to get attention or to remove responsibility. It can also be a political weapon (office politics included). It’s such a potent way to connect that it’s used to build alliances and clout. “Washington is doing a great job,” said no candidate ever. No, if you want to get people on your side, find something irritating and complain to everyone how annoying it is. This solidifies “us” versus “them,” which can harm organizations and families alike.
Yet, eliminating all complaints is neither feasible, nor probably advisable. You could try to make your office a complaint-free zone, but the likely result would be to push any griping to the remote corners where you can no longer hear them. These criticisms might have uncovered missed opportunities, identify problems, and even improve cohesion if done in a safe and transparent setting. If they are left unaddressed or if the underlying culture isn’t sound, then they can propagate and lead to factions that harm productivity.
Griping is as much part of the holiday season as jingle bells and jelly donuts. I don’t believe complaining is up now because people were grumpier in 2023. Rather I think people just craved connection more than ever. So join in: Traffic after the time change, Tesla service, (super) late patients, prior auths, perioral dermatitis, post-COVID telogen effluvium.
I feel better.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X (formerly Twitter). Write to him at [email protected].
‘Twas the night before Festivus and all through the house, everyone was griping.
In case you’ve only been watching Friends reruns lately, Festivus is a holiday that originated 25 years ago in the last season of Seinfeld. George’s father created it as an alternative to Christmas hype. In addition to an aluminum pole, the holiday features the annual airing of grievances, when one is encouraged to voice complaints. Aluminum poles haven’t replaced Christmas trees, but the spirit of Festivus is still with us in the widespread airing of grievances in 2023.
Complaining isn’t just a post-pandemic problem. Hector spends quite a bit of time complaining about Paris in the Iliad. That was a few pandemics ago. And repining is ubiquitous in literature — as human as walking on two limbs it seems. Ostensibly, we complain to effect change: Something is wrong and we expect it to be different. But that’s not the whole story. No one believes the weather will improve or the Patriots will play better because we complain about them. So why do we bother?
Even if nothing changes on the outside, it does seem to alter our internal state, serving a healthy psychological function. Putting to words what is aggravating can have the same benefit of deep breathing. We describe it as “getting something off our chest” because that’s what it feels like. We feel unburdened just by saying it out loud. Think about the last time you complained: Cranky staff, prior auths, Medicare, disrespectful patients, many of your colleagues will nod in agreement, validating your feelings and making you feel less isolated.
There are also maladaptive reasons for whining. It’s obviously an elementary way to get attention or to remove responsibility. It can also be a political weapon (office politics included). It’s such a potent way to connect that it’s used to build alliances and clout. “Washington is doing a great job,” said no candidate ever. No, if you want to get people on your side, find something irritating and complain to everyone how annoying it is. This solidifies “us” versus “them,” which can harm organizations and families alike.
Yet, eliminating all complaints is neither feasible, nor probably advisable. You could try to make your office a complaint-free zone, but the likely result would be to push any griping to the remote corners where you can no longer hear them. These criticisms might have uncovered missed opportunities, identify problems, and even improve cohesion if done in a safe and transparent setting. If they are left unaddressed or if the underlying culture isn’t sound, then they can propagate and lead to factions that harm productivity.
Griping is as much part of the holiday season as jingle bells and jelly donuts. I don’t believe complaining is up now because people were grumpier in 2023. Rather I think people just craved connection more than ever. So join in: Traffic after the time change, Tesla service, (super) late patients, prior auths, perioral dermatitis, post-COVID telogen effluvium.
I feel better.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X (formerly Twitter). Write to him at [email protected].
‘Twas the night before Festivus and all through the house, everyone was griping.
In case you’ve only been watching Friends reruns lately, Festivus is a holiday that originated 25 years ago in the last season of Seinfeld. George’s father created it as an alternative to Christmas hype. In addition to an aluminum pole, the holiday features the annual airing of grievances, when one is encouraged to voice complaints. Aluminum poles haven’t replaced Christmas trees, but the spirit of Festivus is still with us in the widespread airing of grievances in 2023.
Complaining isn’t just a post-pandemic problem. Hector spends quite a bit of time complaining about Paris in the Iliad. That was a few pandemics ago. And repining is ubiquitous in literature — as human as walking on two limbs it seems. Ostensibly, we complain to effect change: Something is wrong and we expect it to be different. But that’s not the whole story. No one believes the weather will improve or the Patriots will play better because we complain about them. So why do we bother?
Even if nothing changes on the outside, it does seem to alter our internal state, serving a healthy psychological function. Putting to words what is aggravating can have the same benefit of deep breathing. We describe it as “getting something off our chest” because that’s what it feels like. We feel unburdened just by saying it out loud. Think about the last time you complained: Cranky staff, prior auths, Medicare, disrespectful patients, many of your colleagues will nod in agreement, validating your feelings and making you feel less isolated.
There are also maladaptive reasons for whining. It’s obviously an elementary way to get attention or to remove responsibility. It can also be a political weapon (office politics included). It’s such a potent way to connect that it’s used to build alliances and clout. “Washington is doing a great job,” said no candidate ever. No, if you want to get people on your side, find something irritating and complain to everyone how annoying it is. This solidifies “us” versus “them,” which can harm organizations and families alike.
Yet, eliminating all complaints is neither feasible, nor probably advisable. You could try to make your office a complaint-free zone, but the likely result would be to push any griping to the remote corners where you can no longer hear them. These criticisms might have uncovered missed opportunities, identify problems, and even improve cohesion if done in a safe and transparent setting. If they are left unaddressed or if the underlying culture isn’t sound, then they can propagate and lead to factions that harm productivity.
Griping is as much part of the holiday season as jingle bells and jelly donuts. I don’t believe complaining is up now because people were grumpier in 2023. Rather I think people just craved connection more than ever. So join in: Traffic after the time change, Tesla service, (super) late patients, prior auths, perioral dermatitis, post-COVID telogen effluvium.
I feel better.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X (formerly Twitter). Write to him at [email protected].
Paradoxical Eczema Risk Low With Biologic Psoriasis Treatments
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
FROM JAMA DERMATOLOGY
Study evaluates aesthetic concerns among Hispanic, Latinx women
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
AT ASDS 2023
Sodium deoxycholate and triamcinolone: A good mix?
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at [email protected]. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at [email protected]. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at [email protected]. She was an investigator in clinical trials of Kybella.
How to Reduce Cardiovascular Morbidity and Mortality in Psoriasis and PsA
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.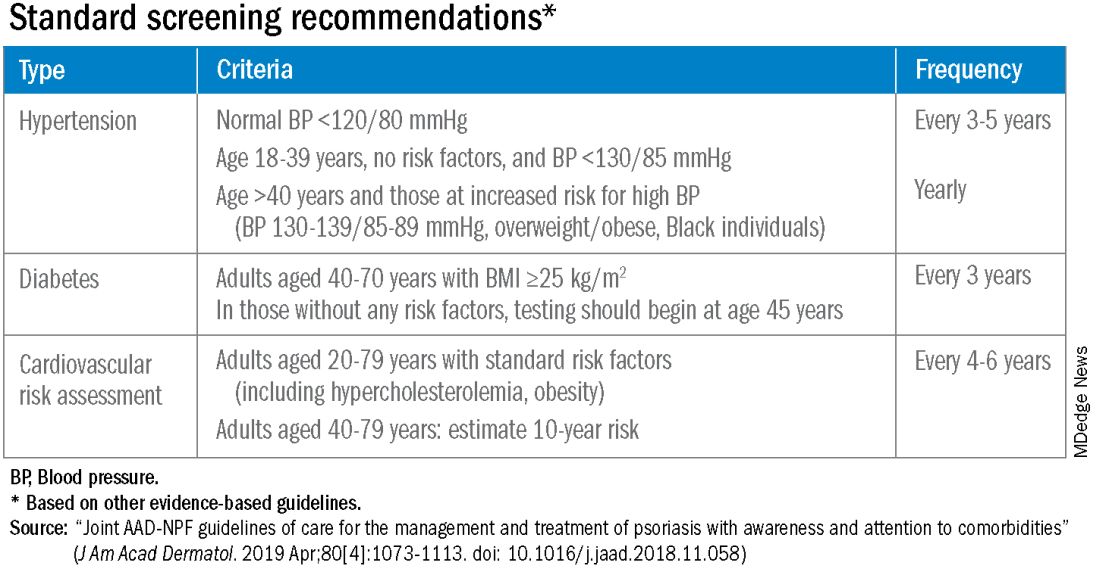
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.












