User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
ABIM grants MOC extension
Physicians will not lose their certification if they are unable to complete maintenance of certification requirements in 2020, the American Board of Internal Medicine announced.
ABIM President Richard Baron, MD, said in a letter sent to all diplomates.
Additionally, physicians “currently in their grace year will also be afforded an additional grace year in 2021,” the letter continued.
ABIM noted that many assessments were planned for the fall of 2020 and the organization will continue to offer them as planned for physicians who are able to take them. It added that more assessment dates for 2020 and 2021 will be sent out later this year.
“The next few weeks and months will challenge our health care system and country like never before,” Dr. Baron stated. “Our many internal medicine colleagues – and the clinical teams that support them – have been heroic in their response, often selflessly putting their own personal safety at risk while using their superb skills to provide care for others. They have inspired all of us.”
Physicians will not lose their certification if they are unable to complete maintenance of certification requirements in 2020, the American Board of Internal Medicine announced.
ABIM President Richard Baron, MD, said in a letter sent to all diplomates.
Additionally, physicians “currently in their grace year will also be afforded an additional grace year in 2021,” the letter continued.
ABIM noted that many assessments were planned for the fall of 2020 and the organization will continue to offer them as planned for physicians who are able to take them. It added that more assessment dates for 2020 and 2021 will be sent out later this year.
“The next few weeks and months will challenge our health care system and country like never before,” Dr. Baron stated. “Our many internal medicine colleagues – and the clinical teams that support them – have been heroic in their response, often selflessly putting their own personal safety at risk while using their superb skills to provide care for others. They have inspired all of us.”
Physicians will not lose their certification if they are unable to complete maintenance of certification requirements in 2020, the American Board of Internal Medicine announced.
ABIM President Richard Baron, MD, said in a letter sent to all diplomates.
Additionally, physicians “currently in their grace year will also be afforded an additional grace year in 2021,” the letter continued.
ABIM noted that many assessments were planned for the fall of 2020 and the organization will continue to offer them as planned for physicians who are able to take them. It added that more assessment dates for 2020 and 2021 will be sent out later this year.
“The next few weeks and months will challenge our health care system and country like never before,” Dr. Baron stated. “Our many internal medicine colleagues – and the clinical teams that support them – have been heroic in their response, often selflessly putting their own personal safety at risk while using their superb skills to provide care for others. They have inspired all of us.”
COVID 19: Confessions of an outpatient psychiatrist during the pandemic
It seems that some glitches would be inevitable. With a sudden shift to videoconferencing in private psychiatric practices, there were bound to be issues with both technology and privacy. One friend told me of such a glitch on the very first day she started telemental health: She was meeting with a patient who was sitting at her kitchen table. Unbeknownst to the patient, her husband walked into the kitchen behind her, fully naked, to get something from the refrigerator. “There was a full moon shot!” my friend said, initially quite shocked, and then eventually amused. As we all cope with a national tragedy and the total upheaval to our personal and professional lives, the stories just keep coming.
I left work on Friday, March 13, with plans to return on the following Monday to see patients. I had no idea that, by Sunday evening, I would be persuaded that for the safety of all I would need to shut down my real-life psychiatric practice and switch to a videoconferencing venue. I, along with many psychiatrists in Maryland, made this decision after Amy Huberman, MD, posted the following on the Maryland Psychiatric Society (MPS) listserv on Sunday, March 15:
“I want to make a case for starting video sessions with all your patients NOW. There is increasing evidence that the spread of coronavirus is driven primarily by asymptomatic or mildly ill people infected with the virus. Because of this, it’s not good enough to tell your patients not to come in if they have symptoms, or for you not to come into work if you have no symptoms. Even after I sent out a letter two weeks ago warning people not to come in if they had symptoms or had potentially come in contact with someone with COVID-19, several patients with coughs still came to my office, as well as several people who had just been on trips to New York City.
If we want to help slow the spread of this illness so that our health system has a better chance of being able to offer ventilators to the people who need them, we must limit all contacts as much as possible – even of asymptomatic people, given the emerging data.
I am planning to send out a message to all my patients today that they should do the same. Without the president or the media giving clear advice to people about what to do, it’s our job as physicians to do it.”
By that night, I had set up a home office with a blank wall behind me, windows in front of me, and books propping my computer at a height that would not have my patients looking up my nose. For the first time in over 20 years, I dusted my son’s Little League trophies, moved them and a 40,000 baseball card collection against the wall, carried a desk, chair, rug, houseplant, and a small Buddha into a room in which I would have some privacy, and my telepsychiatry practice found a home.
After some research, I registered for a free site called Doxy.me because it was HIPAA compliant and did not require patients to download an application; anyone with a camera on any Internet-enabled phone, computer, or tablet, could click on a link and enter my virtual waiting room. I soon discovered that images on the Doxy.me site are sometimes grainy and sometimes freeze up; in some sessions, we ended up switching to FaceTime, and as government mandates for HIPAA compliance relaxed, I offered to meet on any site that my patients might be comfortable with: if not Doxy.me (which remains my starting place for most sessions), Facetime, Skype, Zoom, or Whatsapp. I have not offered Bluejeans, Google Hangouts, or WebEx, and no one has requested those applications. I keep the phone next to the computer, and some sessions include a few minutes of tech support as I help patients (or they help me) navigate the various sites. In a few sessions, we could not get the audio to work and we used video on one venue while we talked on the phone. I haven’t figured out if the variations in the quality of the connection has to do with my Comcast connection, the fact that these websites are overloaded with users, or that my household now consists of three people, two large monitors, three laptops, two tablets, three cell phone lines (not to mention one dog and a transplanted cat), all going at the same time. The pets do not require any bandwidth, but all the people are talking to screens throughout the workday.
As my colleagues embarked on the same journey, the listserv questions and comments came quickly. What were the best platforms? Was it a good thing or a bad thing to suddenly be in people’s homes? Some felt the extraneous background to be helpful, others found it distracting and intrusive.
How do these sessions get coded for the purpose of billing? There was a tremendous amount of confusion over that, with the initial verdict being that Medicare wanted the place of service changed to “02” and that private insurers want one of two modifiers, and it was anyone’s guess which company wanted which modifier. Then there was the concern that Medicare was paying 25% less, until the MPS staff clarified that full fees would be paid, but the place of service should be filled in as “11” – not “02” – as with regular office visits, and the modifier “95” should be added on the Health Care Finance Administration claim form. We were left to wait and see what gets reimbursed and for what fees.
Could new patients be seen by videoconferencing? Could patients from other states be seen this way if the psychiatrist was not licensed in the state where the patient was calling from? One psychiatrist reported he had a patient in an adjacent state drive over the border into Maryland, but the patient brought her mother and the evaluation included unwanted input from the mom as the session consisted of the patient and her mother yelling at both each other in the car and at the psychiatrist on the screen!
Psychiatrists on the listserv began to comment that treatment sessions were intense and exhausting. I feel the literal face-to-face contact of another person’s head just inches from my own, with full eye contact, often gets to be a lot. No one asks why I’ve moved a trinket (ah, there are no trinkets) or gazes off around the room. I sometimes sit for long periods of time as I don’t even stand to see the patients to the door. Other patients move about or bounce their devices on their laps, and my stomach starts to feel queasy until I ask to have the device adjusted. In some sessions, I find I’m talking to partial heads, or that computer icons cover the patient’s mouth.
Being in people’s lives via screen has been interesting. Unlike my colleague, I have not had any streaking spouses, but I’ve greeted a few family members – often those serving as technical support – and I’ve toured part of a farm, met dogs, guinea pigs, and even a goat. I’ve made brief daily “visits” to a frightened patient in isolation on a COVID hospital unit and had the joy of celebrating the discharge to home. It’s odd to be in a bedroom with a patient, even virtually, and it is interesting to note where they choose to hold their sessions; I’ve had several patients hold sessions from their cars. Seeing my own image in the corner of the screen is also a bit distracting, and in one session, as I saw my own reaction, my patient said, “I knew you were going to make that face!”
The pandemic has usurped most of the activities of all of our lives, and without social interactions, travel, and work in the usual way, life does not hold its usual richness. In a few cases, I have ended the session after half the time as the patient insisted there was nothing to talk about. Many talk about the medical problems they can’t be seen for, what they are doing to keep safe (or not), how they are washing down their groceries, and who they are meeting with by Zoom. Of those who were terribly anxious before, some feel oddly calmer – the world has ramped up to meet their level of anxiety and they feel vindicated. No one thinks they are odd for worrying about germs on door knobs or elevator buttons. What were once neurotic fears are now our real-life reality. Others have been triggered by a paralyzing fear, often with panic attacks, and these sessions are certainly challenging as I figure out which medications will best help, while responding to requests for reassurance. And there is the troublesome aspect of trying to care for others who are fearful while living with the reality that these fears are not extraneous to our own lives: We, too, are scared for ourselves and our families.
For some people, stay-at-home mandates have been easier than for others. People who are naturally introverted, or those with social anxiety, have told me they find this time at home to be a relief. They no longer feel pressured to go out; there is permission to be alone, to read, or watch Netflix. No one is pressuring them to go to parties or look for a Tinder date. For others, the isolation and loneliness have been devastating, causing a range of emotions from being “stir crazy,” to triggering episodes of major depression and severe anxiety.
Health care workers in therapy talk about their fears of being contaminated with coronavirus, about the exposures they’ve had, their fears of bringing the virus home to family, and about the anger – sometimes rage – that their employers are not doing more to protect them.
Few people these past weeks are looking for insight into their patterns of behavior and emotion. Most of life has come to be about survival and not about personal striving. Students who are driven to excel are disappointed to have their scholastic worlds have switched to pass/fail. And for those struggling with milder forms of depression and anxiety, both the patients and I have all been a bit perplexed by losing the usual measures of what feelings are normal in a tragic world and we no longer use socializing as the hallmark that heralds a return to normalcy after a period of withdrawal.
In some aspects, it is not all been bad. I’ve enjoyed watching my neighbors walk by with their dogs through the window behind my computer screen and I’ve felt part of the daily evolution as the cherry tree outside that same window turns from dead brown wood to vibrant pink blossoms. I like the flexibility of my schedule and the sensation I always carry of being rushed has quelled. I take more walks and spend more time with the family members who are held captive with me. The dog, who no longer is left alone for hours each day, is certainly a winner.
Some of my colleagues tell me they are overwhelmed – patients they have not seen for years have returned, people are asking for more frequent sessions, and they are suddenly trying to work at home while homeschooling children. I have had only a few of those requests for crisis care, while new referrals are much quieter than normal. Some of my patients have even said that they simply aren’t comfortable meeting this way and they will see me at the other end of the pandemic. A few people I would have expected to hear from I have not, and I fear that those who have lost their jobs may avoiding the cost of treatment – this group I will reach out to in the coming weeks. A little extra time, however, has given me the opportunity to join the Johns Hopkins COVID-19 Mental Health team. And my first attempt at teaching a resident seminar by Zoom has gone well.
For some in the medical field, this has been a horrible and traumatic time; they are worked to exhaustion, and surrounded by distress, death, and personal fear with every shift. For others, life has come to a standstill as the elective procedures that fill their days have virtually stopped. For outpatient psychiatry, it’s been a bit of an in-between, we may feel an odd mix of relevant and useless all at the same time, as our services are appreciated by our patients, but as actual soldiers caring for the ill COVID patients, we are leaving that to our colleagues in the EDs, COVID units, and ICUs. As a physician who has not treated a patient in an ICU for decades, I wish I had something more concrete to contribute to the effort, and at the same time, I’m relieved that I don’t.
And what about the patients? How are they doing with remote psychiatry? Some are clearly flustered or frustrated by the technology issues. Other times sessions go smoothly, and the fact that we are talking through screens gets forgotten. Some like the convenience of not having to drive a far distance and no one misses my crowded parking lot.
Kristen, another doctor’s patient in Illinois, commented: “I appreciate the continuity in care, especially if the alternative is delaying appointments. I think that’s most important. The interaction helps manage added anxiety from isolating as well. I don’t think it diminishes the care I receive; it makes me feel that my doctor is still accessible. One other point, since I have had both telemedicine and in-person appointments with my current psychiatrist, is that during in-person meetings, he is usually on his computer and rarely looks at me or makes eye contact. In virtual meetings, I feel he is much more engaged with me.”
In normal times, I spend a good deal of time encouraging patients to work on building their relationships and community – these connections lead people to healthy and fulfilling lives – and now we talk about how to best be socially distant. We see each other as vectors of disease and to greet a friend with a handshake, much less a hug, would be unthinkable. Will our collective psyches ever recover? For those of us who will survive, that remains to be seen. In the meantime, perhaps we are all being forced to be more flexible and innovative.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
It seems that some glitches would be inevitable. With a sudden shift to videoconferencing in private psychiatric practices, there were bound to be issues with both technology and privacy. One friend told me of such a glitch on the very first day she started telemental health: She was meeting with a patient who was sitting at her kitchen table. Unbeknownst to the patient, her husband walked into the kitchen behind her, fully naked, to get something from the refrigerator. “There was a full moon shot!” my friend said, initially quite shocked, and then eventually amused. As we all cope with a national tragedy and the total upheaval to our personal and professional lives, the stories just keep coming.
I left work on Friday, March 13, with plans to return on the following Monday to see patients. I had no idea that, by Sunday evening, I would be persuaded that for the safety of all I would need to shut down my real-life psychiatric practice and switch to a videoconferencing venue. I, along with many psychiatrists in Maryland, made this decision after Amy Huberman, MD, posted the following on the Maryland Psychiatric Society (MPS) listserv on Sunday, March 15:
“I want to make a case for starting video sessions with all your patients NOW. There is increasing evidence that the spread of coronavirus is driven primarily by asymptomatic or mildly ill people infected with the virus. Because of this, it’s not good enough to tell your patients not to come in if they have symptoms, or for you not to come into work if you have no symptoms. Even after I sent out a letter two weeks ago warning people not to come in if they had symptoms or had potentially come in contact with someone with COVID-19, several patients with coughs still came to my office, as well as several people who had just been on trips to New York City.
If we want to help slow the spread of this illness so that our health system has a better chance of being able to offer ventilators to the people who need them, we must limit all contacts as much as possible – even of asymptomatic people, given the emerging data.
I am planning to send out a message to all my patients today that they should do the same. Without the president or the media giving clear advice to people about what to do, it’s our job as physicians to do it.”
By that night, I had set up a home office with a blank wall behind me, windows in front of me, and books propping my computer at a height that would not have my patients looking up my nose. For the first time in over 20 years, I dusted my son’s Little League trophies, moved them and a 40,000 baseball card collection against the wall, carried a desk, chair, rug, houseplant, and a small Buddha into a room in which I would have some privacy, and my telepsychiatry practice found a home.
After some research, I registered for a free site called Doxy.me because it was HIPAA compliant and did not require patients to download an application; anyone with a camera on any Internet-enabled phone, computer, or tablet, could click on a link and enter my virtual waiting room. I soon discovered that images on the Doxy.me site are sometimes grainy and sometimes freeze up; in some sessions, we ended up switching to FaceTime, and as government mandates for HIPAA compliance relaxed, I offered to meet on any site that my patients might be comfortable with: if not Doxy.me (which remains my starting place for most sessions), Facetime, Skype, Zoom, or Whatsapp. I have not offered Bluejeans, Google Hangouts, or WebEx, and no one has requested those applications. I keep the phone next to the computer, and some sessions include a few minutes of tech support as I help patients (or they help me) navigate the various sites. In a few sessions, we could not get the audio to work and we used video on one venue while we talked on the phone. I haven’t figured out if the variations in the quality of the connection has to do with my Comcast connection, the fact that these websites are overloaded with users, or that my household now consists of three people, two large monitors, three laptops, two tablets, three cell phone lines (not to mention one dog and a transplanted cat), all going at the same time. The pets do not require any bandwidth, but all the people are talking to screens throughout the workday.
As my colleagues embarked on the same journey, the listserv questions and comments came quickly. What were the best platforms? Was it a good thing or a bad thing to suddenly be in people’s homes? Some felt the extraneous background to be helpful, others found it distracting and intrusive.
How do these sessions get coded for the purpose of billing? There was a tremendous amount of confusion over that, with the initial verdict being that Medicare wanted the place of service changed to “02” and that private insurers want one of two modifiers, and it was anyone’s guess which company wanted which modifier. Then there was the concern that Medicare was paying 25% less, until the MPS staff clarified that full fees would be paid, but the place of service should be filled in as “11” – not “02” – as with regular office visits, and the modifier “95” should be added on the Health Care Finance Administration claim form. We were left to wait and see what gets reimbursed and for what fees.
Could new patients be seen by videoconferencing? Could patients from other states be seen this way if the psychiatrist was not licensed in the state where the patient was calling from? One psychiatrist reported he had a patient in an adjacent state drive over the border into Maryland, but the patient brought her mother and the evaluation included unwanted input from the mom as the session consisted of the patient and her mother yelling at both each other in the car and at the psychiatrist on the screen!
Psychiatrists on the listserv began to comment that treatment sessions were intense and exhausting. I feel the literal face-to-face contact of another person’s head just inches from my own, with full eye contact, often gets to be a lot. No one asks why I’ve moved a trinket (ah, there are no trinkets) or gazes off around the room. I sometimes sit for long periods of time as I don’t even stand to see the patients to the door. Other patients move about or bounce their devices on their laps, and my stomach starts to feel queasy until I ask to have the device adjusted. In some sessions, I find I’m talking to partial heads, or that computer icons cover the patient’s mouth.
Being in people’s lives via screen has been interesting. Unlike my colleague, I have not had any streaking spouses, but I’ve greeted a few family members – often those serving as technical support – and I’ve toured part of a farm, met dogs, guinea pigs, and even a goat. I’ve made brief daily “visits” to a frightened patient in isolation on a COVID hospital unit and had the joy of celebrating the discharge to home. It’s odd to be in a bedroom with a patient, even virtually, and it is interesting to note where they choose to hold their sessions; I’ve had several patients hold sessions from their cars. Seeing my own image in the corner of the screen is also a bit distracting, and in one session, as I saw my own reaction, my patient said, “I knew you were going to make that face!”
The pandemic has usurped most of the activities of all of our lives, and without social interactions, travel, and work in the usual way, life does not hold its usual richness. In a few cases, I have ended the session after half the time as the patient insisted there was nothing to talk about. Many talk about the medical problems they can’t be seen for, what they are doing to keep safe (or not), how they are washing down their groceries, and who they are meeting with by Zoom. Of those who were terribly anxious before, some feel oddly calmer – the world has ramped up to meet their level of anxiety and they feel vindicated. No one thinks they are odd for worrying about germs on door knobs or elevator buttons. What were once neurotic fears are now our real-life reality. Others have been triggered by a paralyzing fear, often with panic attacks, and these sessions are certainly challenging as I figure out which medications will best help, while responding to requests for reassurance. And there is the troublesome aspect of trying to care for others who are fearful while living with the reality that these fears are not extraneous to our own lives: We, too, are scared for ourselves and our families.
For some people, stay-at-home mandates have been easier than for others. People who are naturally introverted, or those with social anxiety, have told me they find this time at home to be a relief. They no longer feel pressured to go out; there is permission to be alone, to read, or watch Netflix. No one is pressuring them to go to parties or look for a Tinder date. For others, the isolation and loneliness have been devastating, causing a range of emotions from being “stir crazy,” to triggering episodes of major depression and severe anxiety.
Health care workers in therapy talk about their fears of being contaminated with coronavirus, about the exposures they’ve had, their fears of bringing the virus home to family, and about the anger – sometimes rage – that their employers are not doing more to protect them.
Few people these past weeks are looking for insight into their patterns of behavior and emotion. Most of life has come to be about survival and not about personal striving. Students who are driven to excel are disappointed to have their scholastic worlds have switched to pass/fail. And for those struggling with milder forms of depression and anxiety, both the patients and I have all been a bit perplexed by losing the usual measures of what feelings are normal in a tragic world and we no longer use socializing as the hallmark that heralds a return to normalcy after a period of withdrawal.
In some aspects, it is not all been bad. I’ve enjoyed watching my neighbors walk by with their dogs through the window behind my computer screen and I’ve felt part of the daily evolution as the cherry tree outside that same window turns from dead brown wood to vibrant pink blossoms. I like the flexibility of my schedule and the sensation I always carry of being rushed has quelled. I take more walks and spend more time with the family members who are held captive with me. The dog, who no longer is left alone for hours each day, is certainly a winner.
Some of my colleagues tell me they are overwhelmed – patients they have not seen for years have returned, people are asking for more frequent sessions, and they are suddenly trying to work at home while homeschooling children. I have had only a few of those requests for crisis care, while new referrals are much quieter than normal. Some of my patients have even said that they simply aren’t comfortable meeting this way and they will see me at the other end of the pandemic. A few people I would have expected to hear from I have not, and I fear that those who have lost their jobs may avoiding the cost of treatment – this group I will reach out to in the coming weeks. A little extra time, however, has given me the opportunity to join the Johns Hopkins COVID-19 Mental Health team. And my first attempt at teaching a resident seminar by Zoom has gone well.
For some in the medical field, this has been a horrible and traumatic time; they are worked to exhaustion, and surrounded by distress, death, and personal fear with every shift. For others, life has come to a standstill as the elective procedures that fill their days have virtually stopped. For outpatient psychiatry, it’s been a bit of an in-between, we may feel an odd mix of relevant and useless all at the same time, as our services are appreciated by our patients, but as actual soldiers caring for the ill COVID patients, we are leaving that to our colleagues in the EDs, COVID units, and ICUs. As a physician who has not treated a patient in an ICU for decades, I wish I had something more concrete to contribute to the effort, and at the same time, I’m relieved that I don’t.
And what about the patients? How are they doing with remote psychiatry? Some are clearly flustered or frustrated by the technology issues. Other times sessions go smoothly, and the fact that we are talking through screens gets forgotten. Some like the convenience of not having to drive a far distance and no one misses my crowded parking lot.
Kristen, another doctor’s patient in Illinois, commented: “I appreciate the continuity in care, especially if the alternative is delaying appointments. I think that’s most important. The interaction helps manage added anxiety from isolating as well. I don’t think it diminishes the care I receive; it makes me feel that my doctor is still accessible. One other point, since I have had both telemedicine and in-person appointments with my current psychiatrist, is that during in-person meetings, he is usually on his computer and rarely looks at me or makes eye contact. In virtual meetings, I feel he is much more engaged with me.”
In normal times, I spend a good deal of time encouraging patients to work on building their relationships and community – these connections lead people to healthy and fulfilling lives – and now we talk about how to best be socially distant. We see each other as vectors of disease and to greet a friend with a handshake, much less a hug, would be unthinkable. Will our collective psyches ever recover? For those of us who will survive, that remains to be seen. In the meantime, perhaps we are all being forced to be more flexible and innovative.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
It seems that some glitches would be inevitable. With a sudden shift to videoconferencing in private psychiatric practices, there were bound to be issues with both technology and privacy. One friend told me of such a glitch on the very first day she started telemental health: She was meeting with a patient who was sitting at her kitchen table. Unbeknownst to the patient, her husband walked into the kitchen behind her, fully naked, to get something from the refrigerator. “There was a full moon shot!” my friend said, initially quite shocked, and then eventually amused. As we all cope with a national tragedy and the total upheaval to our personal and professional lives, the stories just keep coming.
I left work on Friday, March 13, with plans to return on the following Monday to see patients. I had no idea that, by Sunday evening, I would be persuaded that for the safety of all I would need to shut down my real-life psychiatric practice and switch to a videoconferencing venue. I, along with many psychiatrists in Maryland, made this decision after Amy Huberman, MD, posted the following on the Maryland Psychiatric Society (MPS) listserv on Sunday, March 15:
“I want to make a case for starting video sessions with all your patients NOW. There is increasing evidence that the spread of coronavirus is driven primarily by asymptomatic or mildly ill people infected with the virus. Because of this, it’s not good enough to tell your patients not to come in if they have symptoms, or for you not to come into work if you have no symptoms. Even after I sent out a letter two weeks ago warning people not to come in if they had symptoms or had potentially come in contact with someone with COVID-19, several patients with coughs still came to my office, as well as several people who had just been on trips to New York City.
If we want to help slow the spread of this illness so that our health system has a better chance of being able to offer ventilators to the people who need them, we must limit all contacts as much as possible – even of asymptomatic people, given the emerging data.
I am planning to send out a message to all my patients today that they should do the same. Without the president or the media giving clear advice to people about what to do, it’s our job as physicians to do it.”
By that night, I had set up a home office with a blank wall behind me, windows in front of me, and books propping my computer at a height that would not have my patients looking up my nose. For the first time in over 20 years, I dusted my son’s Little League trophies, moved them and a 40,000 baseball card collection against the wall, carried a desk, chair, rug, houseplant, and a small Buddha into a room in which I would have some privacy, and my telepsychiatry practice found a home.
After some research, I registered for a free site called Doxy.me because it was HIPAA compliant and did not require patients to download an application; anyone with a camera on any Internet-enabled phone, computer, or tablet, could click on a link and enter my virtual waiting room. I soon discovered that images on the Doxy.me site are sometimes grainy and sometimes freeze up; in some sessions, we ended up switching to FaceTime, and as government mandates for HIPAA compliance relaxed, I offered to meet on any site that my patients might be comfortable with: if not Doxy.me (which remains my starting place for most sessions), Facetime, Skype, Zoom, or Whatsapp. I have not offered Bluejeans, Google Hangouts, or WebEx, and no one has requested those applications. I keep the phone next to the computer, and some sessions include a few minutes of tech support as I help patients (or they help me) navigate the various sites. In a few sessions, we could not get the audio to work and we used video on one venue while we talked on the phone. I haven’t figured out if the variations in the quality of the connection has to do with my Comcast connection, the fact that these websites are overloaded with users, or that my household now consists of three people, two large monitors, three laptops, two tablets, three cell phone lines (not to mention one dog and a transplanted cat), all going at the same time. The pets do not require any bandwidth, but all the people are talking to screens throughout the workday.
As my colleagues embarked on the same journey, the listserv questions and comments came quickly. What were the best platforms? Was it a good thing or a bad thing to suddenly be in people’s homes? Some felt the extraneous background to be helpful, others found it distracting and intrusive.
How do these sessions get coded for the purpose of billing? There was a tremendous amount of confusion over that, with the initial verdict being that Medicare wanted the place of service changed to “02” and that private insurers want one of two modifiers, and it was anyone’s guess which company wanted which modifier. Then there was the concern that Medicare was paying 25% less, until the MPS staff clarified that full fees would be paid, but the place of service should be filled in as “11” – not “02” – as with regular office visits, and the modifier “95” should be added on the Health Care Finance Administration claim form. We were left to wait and see what gets reimbursed and for what fees.
Could new patients be seen by videoconferencing? Could patients from other states be seen this way if the psychiatrist was not licensed in the state where the patient was calling from? One psychiatrist reported he had a patient in an adjacent state drive over the border into Maryland, but the patient brought her mother and the evaluation included unwanted input from the mom as the session consisted of the patient and her mother yelling at both each other in the car and at the psychiatrist on the screen!
Psychiatrists on the listserv began to comment that treatment sessions were intense and exhausting. I feel the literal face-to-face contact of another person’s head just inches from my own, with full eye contact, often gets to be a lot. No one asks why I’ve moved a trinket (ah, there are no trinkets) or gazes off around the room. I sometimes sit for long periods of time as I don’t even stand to see the patients to the door. Other patients move about or bounce their devices on their laps, and my stomach starts to feel queasy until I ask to have the device adjusted. In some sessions, I find I’m talking to partial heads, or that computer icons cover the patient’s mouth.
Being in people’s lives via screen has been interesting. Unlike my colleague, I have not had any streaking spouses, but I’ve greeted a few family members – often those serving as technical support – and I’ve toured part of a farm, met dogs, guinea pigs, and even a goat. I’ve made brief daily “visits” to a frightened patient in isolation on a COVID hospital unit and had the joy of celebrating the discharge to home. It’s odd to be in a bedroom with a patient, even virtually, and it is interesting to note where they choose to hold their sessions; I’ve had several patients hold sessions from their cars. Seeing my own image in the corner of the screen is also a bit distracting, and in one session, as I saw my own reaction, my patient said, “I knew you were going to make that face!”
The pandemic has usurped most of the activities of all of our lives, and without social interactions, travel, and work in the usual way, life does not hold its usual richness. In a few cases, I have ended the session after half the time as the patient insisted there was nothing to talk about. Many talk about the medical problems they can’t be seen for, what they are doing to keep safe (or not), how they are washing down their groceries, and who they are meeting with by Zoom. Of those who were terribly anxious before, some feel oddly calmer – the world has ramped up to meet their level of anxiety and they feel vindicated. No one thinks they are odd for worrying about germs on door knobs or elevator buttons. What were once neurotic fears are now our real-life reality. Others have been triggered by a paralyzing fear, often with panic attacks, and these sessions are certainly challenging as I figure out which medications will best help, while responding to requests for reassurance. And there is the troublesome aspect of trying to care for others who are fearful while living with the reality that these fears are not extraneous to our own lives: We, too, are scared for ourselves and our families.
For some people, stay-at-home mandates have been easier than for others. People who are naturally introverted, or those with social anxiety, have told me they find this time at home to be a relief. They no longer feel pressured to go out; there is permission to be alone, to read, or watch Netflix. No one is pressuring them to go to parties or look for a Tinder date. For others, the isolation and loneliness have been devastating, causing a range of emotions from being “stir crazy,” to triggering episodes of major depression and severe anxiety.
Health care workers in therapy talk about their fears of being contaminated with coronavirus, about the exposures they’ve had, their fears of bringing the virus home to family, and about the anger – sometimes rage – that their employers are not doing more to protect them.
Few people these past weeks are looking for insight into their patterns of behavior and emotion. Most of life has come to be about survival and not about personal striving. Students who are driven to excel are disappointed to have their scholastic worlds have switched to pass/fail. And for those struggling with milder forms of depression and anxiety, both the patients and I have all been a bit perplexed by losing the usual measures of what feelings are normal in a tragic world and we no longer use socializing as the hallmark that heralds a return to normalcy after a period of withdrawal.
In some aspects, it is not all been bad. I’ve enjoyed watching my neighbors walk by with their dogs through the window behind my computer screen and I’ve felt part of the daily evolution as the cherry tree outside that same window turns from dead brown wood to vibrant pink blossoms. I like the flexibility of my schedule and the sensation I always carry of being rushed has quelled. I take more walks and spend more time with the family members who are held captive with me. The dog, who no longer is left alone for hours each day, is certainly a winner.
Some of my colleagues tell me they are overwhelmed – patients they have not seen for years have returned, people are asking for more frequent sessions, and they are suddenly trying to work at home while homeschooling children. I have had only a few of those requests for crisis care, while new referrals are much quieter than normal. Some of my patients have even said that they simply aren’t comfortable meeting this way and they will see me at the other end of the pandemic. A few people I would have expected to hear from I have not, and I fear that those who have lost their jobs may avoiding the cost of treatment – this group I will reach out to in the coming weeks. A little extra time, however, has given me the opportunity to join the Johns Hopkins COVID-19 Mental Health team. And my first attempt at teaching a resident seminar by Zoom has gone well.
For some in the medical field, this has been a horrible and traumatic time; they are worked to exhaustion, and surrounded by distress, death, and personal fear with every shift. For others, life has come to a standstill as the elective procedures that fill their days have virtually stopped. For outpatient psychiatry, it’s been a bit of an in-between, we may feel an odd mix of relevant and useless all at the same time, as our services are appreciated by our patients, but as actual soldiers caring for the ill COVID patients, we are leaving that to our colleagues in the EDs, COVID units, and ICUs. As a physician who has not treated a patient in an ICU for decades, I wish I had something more concrete to contribute to the effort, and at the same time, I’m relieved that I don’t.
And what about the patients? How are they doing with remote psychiatry? Some are clearly flustered or frustrated by the technology issues. Other times sessions go smoothly, and the fact that we are talking through screens gets forgotten. Some like the convenience of not having to drive a far distance and no one misses my crowded parking lot.
Kristen, another doctor’s patient in Illinois, commented: “I appreciate the continuity in care, especially if the alternative is delaying appointments. I think that’s most important. The interaction helps manage added anxiety from isolating as well. I don’t think it diminishes the care I receive; it makes me feel that my doctor is still accessible. One other point, since I have had both telemedicine and in-person appointments with my current psychiatrist, is that during in-person meetings, he is usually on his computer and rarely looks at me or makes eye contact. In virtual meetings, I feel he is much more engaged with me.”
In normal times, I spend a good deal of time encouraging patients to work on building their relationships and community – these connections lead people to healthy and fulfilling lives – and now we talk about how to best be socially distant. We see each other as vectors of disease and to greet a friend with a handshake, much less a hug, would be unthinkable. Will our collective psyches ever recover? For those of us who will survive, that remains to be seen. In the meantime, perhaps we are all being forced to be more flexible and innovative.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
TWILIGHT-COMPLEX: Tap ticagrelor monotherapy early after complex PCI
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
FROM ACC 2020
COVID-19: What now?
“There are decades where nothing happens,” wrote Vladimir Lenin, “and there are weeks where decades happen.” Barely a dozen weeks ago, no one knew that the SARS-CoV-2 virus existed. Now, it has spread to almost every country on Earth, infecting over 1.8 million people whom we know about, and many more whom we do not. In so doing, it has crashed economies and health care systems, filled hospitals, emptied public spaces, and separated people from their workplaces and their friends on a scale that few of us have ever witnessed.
It has also triggered an avalanche of questions as to why our initial response was so thoroughly lethargic, rudderless, and uncoordinated; while there is plenty of blame to go around, that is for another time. The glaring question for many – including physicians trying to keep our private practices viable – is: What now?
The answer depends, of course, on how the pandemic plays out. No one yet knows exactly what will happen, but much depends on two properties of the virus, both of which are currently unknown. First: seasonality. Coronaviruses tend to thrive in winter and wane in the summer. That may also be true for SARS-CoV-2, but seasonal variations might not sufficiently slow the virus when it has so many immunologically naive hosts to infect. As I write this in mid-April, we wait anxiously to see what – if anything – summer temperatures do to its transmission in the Northern Hemisphere.
The second wild card is duration of immunity. Determining that will involve developing accurate serologic tests and administering them widely. Immune citizens, once identified, can return to work, care for the vulnerable, and anchor the economy during future outbreaks.
Even if we do get a summer hiatus, seasonal viruses typically return as winter approaches. We could conceivably still be mopping up from this outbreak when the virus – if it is seasonal – comes roaring back in October or November. Will we be ready? Or will it catch us with our pants amidships yet again?
I can envision two possibilities: Assuming we luck into a seasonal reprieve in the next few weeks, infection rates should drop, which could allow our private practices to return toward some semblance of normal – if health workers and patients alike can be convinced that our offices and clinics are safe. This might be accomplished as part of our overall preparation for a potential winter recurrence, by checking every patient’s temperature at the waiting room door. Similarly, all students should get a daily temperature check at school, as should all commuters, airline passengers, and individuals at any sizable gathering. Every fever should trigger a COVID-19 test, and every positive test should launch aggressive contact tracing and quarantines. Meanwhile, treatments and vaccines should get fast-tracked.
That’s what should happen. If it doesn’t, and COVID-19 recurs next winter, worse than before, it is anybody’s guess whether most private medical practices will be able to weather a second onslaught. Further government funding is not assured. We won’t have a vaccine by November. Chloroquine, hydroxychloroquine, and azithromycin might turn out to be helpful, but we can’t count on them.
Even if we do get lucky with seasonality, the question remains of how long it will take to restore public confidence and reboot the economy. Economies generally do not function like light switches that can be turned off for a while then simply turned back on, but act more like campfires. If you pour a bucket of water on one, it takes some time to get it cranked up again. After the “Great Recession” of 2008, it took nearly 10 years.
So now, with great reluctance, I must trot out a hoary old cliché: Hope for the best, but plan for the worst. Everyone’s situation will be different, of course, but I can make a few general suggestions. Perform a difficult mental exercise: What will you do if SARS-CoV-2 outlasts emergency funds from the Paycheck Protection and Economic Injury Disaster programs? Do the math – how long can you keep your practice afloat without floating further loans or dipping into personal savings? If you don’t know how many patients you need to see per day to break even, figure it out – now. On what day will you run out of money? When will you start putting your future at risk?
None of us thought we would ever have to face questions like these, of course – and how ironic is it that a medical emergency has forced them upon us? I sincerely hope that none of us will need to actually confront this Hobson’s choice in the coming months, but far better to address the hypothetical now than the reality later. As always, consult with your own attorney, accountant, and other business advisors before making any life-altering decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected]. He has no disclosures.
“There are decades where nothing happens,” wrote Vladimir Lenin, “and there are weeks where decades happen.” Barely a dozen weeks ago, no one knew that the SARS-CoV-2 virus existed. Now, it has spread to almost every country on Earth, infecting over 1.8 million people whom we know about, and many more whom we do not. In so doing, it has crashed economies and health care systems, filled hospitals, emptied public spaces, and separated people from their workplaces and their friends on a scale that few of us have ever witnessed.
It has also triggered an avalanche of questions as to why our initial response was so thoroughly lethargic, rudderless, and uncoordinated; while there is plenty of blame to go around, that is for another time. The glaring question for many – including physicians trying to keep our private practices viable – is: What now?
The answer depends, of course, on how the pandemic plays out. No one yet knows exactly what will happen, but much depends on two properties of the virus, both of which are currently unknown. First: seasonality. Coronaviruses tend to thrive in winter and wane in the summer. That may also be true for SARS-CoV-2, but seasonal variations might not sufficiently slow the virus when it has so many immunologically naive hosts to infect. As I write this in mid-April, we wait anxiously to see what – if anything – summer temperatures do to its transmission in the Northern Hemisphere.
The second wild card is duration of immunity. Determining that will involve developing accurate serologic tests and administering them widely. Immune citizens, once identified, can return to work, care for the vulnerable, and anchor the economy during future outbreaks.
Even if we do get a summer hiatus, seasonal viruses typically return as winter approaches. We could conceivably still be mopping up from this outbreak when the virus – if it is seasonal – comes roaring back in October or November. Will we be ready? Or will it catch us with our pants amidships yet again?
I can envision two possibilities: Assuming we luck into a seasonal reprieve in the next few weeks, infection rates should drop, which could allow our private practices to return toward some semblance of normal – if health workers and patients alike can be convinced that our offices and clinics are safe. This might be accomplished as part of our overall preparation for a potential winter recurrence, by checking every patient’s temperature at the waiting room door. Similarly, all students should get a daily temperature check at school, as should all commuters, airline passengers, and individuals at any sizable gathering. Every fever should trigger a COVID-19 test, and every positive test should launch aggressive contact tracing and quarantines. Meanwhile, treatments and vaccines should get fast-tracked.
That’s what should happen. If it doesn’t, and COVID-19 recurs next winter, worse than before, it is anybody’s guess whether most private medical practices will be able to weather a second onslaught. Further government funding is not assured. We won’t have a vaccine by November. Chloroquine, hydroxychloroquine, and azithromycin might turn out to be helpful, but we can’t count on them.
Even if we do get lucky with seasonality, the question remains of how long it will take to restore public confidence and reboot the economy. Economies generally do not function like light switches that can be turned off for a while then simply turned back on, but act more like campfires. If you pour a bucket of water on one, it takes some time to get it cranked up again. After the “Great Recession” of 2008, it took nearly 10 years.
So now, with great reluctance, I must trot out a hoary old cliché: Hope for the best, but plan for the worst. Everyone’s situation will be different, of course, but I can make a few general suggestions. Perform a difficult mental exercise: What will you do if SARS-CoV-2 outlasts emergency funds from the Paycheck Protection and Economic Injury Disaster programs? Do the math – how long can you keep your practice afloat without floating further loans or dipping into personal savings? If you don’t know how many patients you need to see per day to break even, figure it out – now. On what day will you run out of money? When will you start putting your future at risk?
None of us thought we would ever have to face questions like these, of course – and how ironic is it that a medical emergency has forced them upon us? I sincerely hope that none of us will need to actually confront this Hobson’s choice in the coming months, but far better to address the hypothetical now than the reality later. As always, consult with your own attorney, accountant, and other business advisors before making any life-altering decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected]. He has no disclosures.
“There are decades where nothing happens,” wrote Vladimir Lenin, “and there are weeks where decades happen.” Barely a dozen weeks ago, no one knew that the SARS-CoV-2 virus existed. Now, it has spread to almost every country on Earth, infecting over 1.8 million people whom we know about, and many more whom we do not. In so doing, it has crashed economies and health care systems, filled hospitals, emptied public spaces, and separated people from their workplaces and their friends on a scale that few of us have ever witnessed.
It has also triggered an avalanche of questions as to why our initial response was so thoroughly lethargic, rudderless, and uncoordinated; while there is plenty of blame to go around, that is for another time. The glaring question for many – including physicians trying to keep our private practices viable – is: What now?
The answer depends, of course, on how the pandemic plays out. No one yet knows exactly what will happen, but much depends on two properties of the virus, both of which are currently unknown. First: seasonality. Coronaviruses tend to thrive in winter and wane in the summer. That may also be true for SARS-CoV-2, but seasonal variations might not sufficiently slow the virus when it has so many immunologically naive hosts to infect. As I write this in mid-April, we wait anxiously to see what – if anything – summer temperatures do to its transmission in the Northern Hemisphere.
The second wild card is duration of immunity. Determining that will involve developing accurate serologic tests and administering them widely. Immune citizens, once identified, can return to work, care for the vulnerable, and anchor the economy during future outbreaks.
Even if we do get a summer hiatus, seasonal viruses typically return as winter approaches. We could conceivably still be mopping up from this outbreak when the virus – if it is seasonal – comes roaring back in October or November. Will we be ready? Or will it catch us with our pants amidships yet again?
I can envision two possibilities: Assuming we luck into a seasonal reprieve in the next few weeks, infection rates should drop, which could allow our private practices to return toward some semblance of normal – if health workers and patients alike can be convinced that our offices and clinics are safe. This might be accomplished as part of our overall preparation for a potential winter recurrence, by checking every patient’s temperature at the waiting room door. Similarly, all students should get a daily temperature check at school, as should all commuters, airline passengers, and individuals at any sizable gathering. Every fever should trigger a COVID-19 test, and every positive test should launch aggressive contact tracing and quarantines. Meanwhile, treatments and vaccines should get fast-tracked.
That’s what should happen. If it doesn’t, and COVID-19 recurs next winter, worse than before, it is anybody’s guess whether most private medical practices will be able to weather a second onslaught. Further government funding is not assured. We won’t have a vaccine by November. Chloroquine, hydroxychloroquine, and azithromycin might turn out to be helpful, but we can’t count on them.
Even if we do get lucky with seasonality, the question remains of how long it will take to restore public confidence and reboot the economy. Economies generally do not function like light switches that can be turned off for a while then simply turned back on, but act more like campfires. If you pour a bucket of water on one, it takes some time to get it cranked up again. After the “Great Recession” of 2008, it took nearly 10 years.
So now, with great reluctance, I must trot out a hoary old cliché: Hope for the best, but plan for the worst. Everyone’s situation will be different, of course, but I can make a few general suggestions. Perform a difficult mental exercise: What will you do if SARS-CoV-2 outlasts emergency funds from the Paycheck Protection and Economic Injury Disaster programs? Do the math – how long can you keep your practice afloat without floating further loans or dipping into personal savings? If you don’t know how many patients you need to see per day to break even, figure it out – now. On what day will you run out of money? When will you start putting your future at risk?
None of us thought we would ever have to face questions like these, of course – and how ironic is it that a medical emergency has forced them upon us? I sincerely hope that none of us will need to actually confront this Hobson’s choice in the coming months, but far better to address the hypothetical now than the reality later. As always, consult with your own attorney, accountant, and other business advisors before making any life-altering decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected]. He has no disclosures.
Presymptomatic or asymptomatic? ID experts on shifting terminology
They also addressed racial disparities surrounding COVID-19, and announced new IDSA guidelines for diagnosis and treatment of the illness.
Regarding the shifting thinking on symptoms and transmission of the novel coronavirus, when it comes to presymptomatic or asymptomatic, “pre” is really the right terminology, Carlos del Rio, MD, professor of medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, said during the briefing, because it’s not that people are asymptomatic but that they develop symptoms later and start transmitting the virus 24 to 48 hours before they develop symptoms.
“Clearly, this plays a role in transmission,” with some studies suggesting that 6% to 12% of transmissions occur during this presymptomatic stage, he explained.
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at University of Alabama at Birmingham, noted that early in the COVID-19 pandemic, the presymptomatic phase “could have been missed because we didn’t realize the wide ranging symptoms this disease has.”
This is turning out to be a “very interesting” virus with “fascinating” symptoms, she told reporters on the call.
The virus seems to have capacity to affect far more than just the respiratory tract. Initially, however, it was viewed “very much like a classic respiratory viral infection. As a result, a lot of people were refused testing because they were not showing the classic signs” of respiratory infection, Marrazzo noted.
It’s now clear that the range of symptoms is quite different, she said.
Notably, loss of smell seems to be “very characteristic and very specific to this infection. I can’t think of another common viral infection that causes loss of smell before you start to see other things,” Marrazzo said.
Data also suggest that gastrointestinal symptoms are common with COVID-19. Early data suggest that diarrhea probably occurs in about one third of patients. Some people have reported abdominal pain as the first sign, she said.
“Now that we know about the more wide range of symptoms associated [with COVID-19], we are being much more open to considering people perhaps having this infection. There is a lower index of suspicion and much lower threshold for diagnostic testing,” Marrazzo said, adding that there are still many barriers to testing and getting test results.
Stark Racial Disparities Need Greater Understanding
The second major topic of discussion at the briefing was the growing realization of racial disparities in COVID-19.
“Racial disparities in our country are not new but racial disparities in this disease are pretty stark,” del Rio said. “We live in a country where disparities have really colored a lot of what our diseases are, from HIV to diabetes to hypertension, and it’s not surprising that we are seeing this now with COVID-19.”
Marrazzo noted that, in Alabama, around 20% of the population is African American, yet almost 40% of COVID-19 deaths are occurring in this population. “The most stark statistics are coming out of Illinois and Michigan, where less than around 15% of the population is African American and yet 70% of the deaths are occurring in that group,” she said.
Both del Rio and Marrazzo agreed that understanding the racial differences in COVID-19 deaths is going to require a lot of analysis in the coming months.
Part of it likely reflects the challenge of social distancing in urban areas, Marrazzo said. “Social distancing is a luxury afforded by having a really big space, and space is money.”
The other long-standing challenge of unequal access to healthcare also likely plays a role, she said. This includes missing out on preventive health appointments and screenings, which can translate into more comorbidities, particularly hypertension.
The evolving evidence about the virus, and the stark conditions that frontline clinicians face, make this an especially challenging public health crisis, del Rio said.
“Taking care of these patients is incredibly taxing and my hat is off to physicians, residents, nurses, everybody working on this in the hospitals because they are really doing a yeoman’s work,” he said.
“These are not easy patients to take care of. Not only are [the frontline clinicians] providing care, they are caring for the patient and providing a comfort and someone to listen to when family can’t be present,” del Rio emphasized.
New Guidelines
The IDSA just released new guidelines for diagnosis and treatment of COVID-19.
“We are learning new things every day about this virus. Things are rapidly changing, and as we learn new things we have to adapt and make changes,” del Rio said.
del Rio noted that the guildelines “will evolve and change as more information comes out.”
This article first appeared on Medscape.com.
They also addressed racial disparities surrounding COVID-19, and announced new IDSA guidelines for diagnosis and treatment of the illness.
Regarding the shifting thinking on symptoms and transmission of the novel coronavirus, when it comes to presymptomatic or asymptomatic, “pre” is really the right terminology, Carlos del Rio, MD, professor of medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, said during the briefing, because it’s not that people are asymptomatic but that they develop symptoms later and start transmitting the virus 24 to 48 hours before they develop symptoms.
“Clearly, this plays a role in transmission,” with some studies suggesting that 6% to 12% of transmissions occur during this presymptomatic stage, he explained.
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at University of Alabama at Birmingham, noted that early in the COVID-19 pandemic, the presymptomatic phase “could have been missed because we didn’t realize the wide ranging symptoms this disease has.”
This is turning out to be a “very interesting” virus with “fascinating” symptoms, she told reporters on the call.
The virus seems to have capacity to affect far more than just the respiratory tract. Initially, however, it was viewed “very much like a classic respiratory viral infection. As a result, a lot of people were refused testing because they were not showing the classic signs” of respiratory infection, Marrazzo noted.
It’s now clear that the range of symptoms is quite different, she said.
Notably, loss of smell seems to be “very characteristic and very specific to this infection. I can’t think of another common viral infection that causes loss of smell before you start to see other things,” Marrazzo said.
Data also suggest that gastrointestinal symptoms are common with COVID-19. Early data suggest that diarrhea probably occurs in about one third of patients. Some people have reported abdominal pain as the first sign, she said.
“Now that we know about the more wide range of symptoms associated [with COVID-19], we are being much more open to considering people perhaps having this infection. There is a lower index of suspicion and much lower threshold for diagnostic testing,” Marrazzo said, adding that there are still many barriers to testing and getting test results.
Stark Racial Disparities Need Greater Understanding
The second major topic of discussion at the briefing was the growing realization of racial disparities in COVID-19.
“Racial disparities in our country are not new but racial disparities in this disease are pretty stark,” del Rio said. “We live in a country where disparities have really colored a lot of what our diseases are, from HIV to diabetes to hypertension, and it’s not surprising that we are seeing this now with COVID-19.”
Marrazzo noted that, in Alabama, around 20% of the population is African American, yet almost 40% of COVID-19 deaths are occurring in this population. “The most stark statistics are coming out of Illinois and Michigan, where less than around 15% of the population is African American and yet 70% of the deaths are occurring in that group,” she said.
Both del Rio and Marrazzo agreed that understanding the racial differences in COVID-19 deaths is going to require a lot of analysis in the coming months.
Part of it likely reflects the challenge of social distancing in urban areas, Marrazzo said. “Social distancing is a luxury afforded by having a really big space, and space is money.”
The other long-standing challenge of unequal access to healthcare also likely plays a role, she said. This includes missing out on preventive health appointments and screenings, which can translate into more comorbidities, particularly hypertension.
The evolving evidence about the virus, and the stark conditions that frontline clinicians face, make this an especially challenging public health crisis, del Rio said.
“Taking care of these patients is incredibly taxing and my hat is off to physicians, residents, nurses, everybody working on this in the hospitals because they are really doing a yeoman’s work,” he said.
“These are not easy patients to take care of. Not only are [the frontline clinicians] providing care, they are caring for the patient and providing a comfort and someone to listen to when family can’t be present,” del Rio emphasized.
New Guidelines
The IDSA just released new guidelines for diagnosis and treatment of COVID-19.
“We are learning new things every day about this virus. Things are rapidly changing, and as we learn new things we have to adapt and make changes,” del Rio said.
del Rio noted that the guildelines “will evolve and change as more information comes out.”
This article first appeared on Medscape.com.
They also addressed racial disparities surrounding COVID-19, and announced new IDSA guidelines for diagnosis and treatment of the illness.
Regarding the shifting thinking on symptoms and transmission of the novel coronavirus, when it comes to presymptomatic or asymptomatic, “pre” is really the right terminology, Carlos del Rio, MD, professor of medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, said during the briefing, because it’s not that people are asymptomatic but that they develop symptoms later and start transmitting the virus 24 to 48 hours before they develop symptoms.
“Clearly, this plays a role in transmission,” with some studies suggesting that 6% to 12% of transmissions occur during this presymptomatic stage, he explained.
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at University of Alabama at Birmingham, noted that early in the COVID-19 pandemic, the presymptomatic phase “could have been missed because we didn’t realize the wide ranging symptoms this disease has.”
This is turning out to be a “very interesting” virus with “fascinating” symptoms, she told reporters on the call.
The virus seems to have capacity to affect far more than just the respiratory tract. Initially, however, it was viewed “very much like a classic respiratory viral infection. As a result, a lot of people were refused testing because they were not showing the classic signs” of respiratory infection, Marrazzo noted.
It’s now clear that the range of symptoms is quite different, she said.
Notably, loss of smell seems to be “very characteristic and very specific to this infection. I can’t think of another common viral infection that causes loss of smell before you start to see other things,” Marrazzo said.
Data also suggest that gastrointestinal symptoms are common with COVID-19. Early data suggest that diarrhea probably occurs in about one third of patients. Some people have reported abdominal pain as the first sign, she said.
“Now that we know about the more wide range of symptoms associated [with COVID-19], we are being much more open to considering people perhaps having this infection. There is a lower index of suspicion and much lower threshold for diagnostic testing,” Marrazzo said, adding that there are still many barriers to testing and getting test results.
Stark Racial Disparities Need Greater Understanding
The second major topic of discussion at the briefing was the growing realization of racial disparities in COVID-19.
“Racial disparities in our country are not new but racial disparities in this disease are pretty stark,” del Rio said. “We live in a country where disparities have really colored a lot of what our diseases are, from HIV to diabetes to hypertension, and it’s not surprising that we are seeing this now with COVID-19.”
Marrazzo noted that, in Alabama, around 20% of the population is African American, yet almost 40% of COVID-19 deaths are occurring in this population. “The most stark statistics are coming out of Illinois and Michigan, where less than around 15% of the population is African American and yet 70% of the deaths are occurring in that group,” she said.
Both del Rio and Marrazzo agreed that understanding the racial differences in COVID-19 deaths is going to require a lot of analysis in the coming months.
Part of it likely reflects the challenge of social distancing in urban areas, Marrazzo said. “Social distancing is a luxury afforded by having a really big space, and space is money.”
The other long-standing challenge of unequal access to healthcare also likely plays a role, she said. This includes missing out on preventive health appointments and screenings, which can translate into more comorbidities, particularly hypertension.
The evolving evidence about the virus, and the stark conditions that frontline clinicians face, make this an especially challenging public health crisis, del Rio said.
“Taking care of these patients is incredibly taxing and my hat is off to physicians, residents, nurses, everybody working on this in the hospitals because they are really doing a yeoman’s work,” he said.
“These are not easy patients to take care of. Not only are [the frontline clinicians] providing care, they are caring for the patient and providing a comfort and someone to listen to when family can’t be present,” del Rio emphasized.
New Guidelines
The IDSA just released new guidelines for diagnosis and treatment of COVID-19.
“We are learning new things every day about this virus. Things are rapidly changing, and as we learn new things we have to adapt and make changes,” del Rio said.
del Rio noted that the guildelines “will evolve and change as more information comes out.”
This article first appeared on Medscape.com.
COVID-19 hits physician couple: Dramatically different responses
A physician couple who both had COVID-19 had very different responses — one ending up in intensive care, the other asymptomatic.
Their story, one of two people living together but with such different responses to the infection, illustrates how much is still to be learned about COVID-19, says Noopur Raje, MD, professor of medicine at Harvard Medical School and director of the Center for Multiple Myeloma at Massachusetts General Hospital (MGH) in Boston.
“After experiencing #Covid_19 from the patient/caregiver end despite both of us being physicians at a major academic medical center, this has been a challenge like no other I have experienced,” Raje (@NoopurRajeMD) wrote on Twitter.
She outlined their experiences in a Twitter thread and elaborated in an interview with Medscape Medical News.
Raje says that she wants clinicians to know how symptoms can evolve both quickly and suddenly.
She recalls how for 10 days, she cared for her COVID-19–positive husband at home, separated from him by a floor in their Boston townhouse and wearing a surgical mask and gloves to bring him food and fluids, as he was too weak to help himself.
Despite the high fevers, chills, extreme fatigue, and dramatic weight loss, Raje says she felt reasonably confident that her husband was getting better. His temperature had dropped from around 103 to 101, his heart rate was in the 80s, and his blood pressure was “OK,” she recalls.
But then Jag Singh, MD, an otherwise healthy 55-year-old Harvard professor and cardiologist, started to cough — and everything suddenly changed.
The cough sounded chesty, and he was weak and unwell. They decided that he needed medical help.
“I was planning on driving him to the hospital, but I ended up having to call 911, although we literally live across the street,” she said.
“We have stairs here and I wasn’t sure that he would be able to make it coming down with me trying to help him, so the safest thing was for me to call for help.”
Singh was admitted straight to the medical intensive care unit (MICU) while his wife waited at home.
“I was blown away when I saw Jag’s x-ray and CT scan and the bilateral pneumonia he had developed,” she commented. “I would not have believed it, the way he was clinically — and seeing that x-ray.
“Honestly, when I took him in to hospital, I thought he’d be there a couple of days — over the weekend — and I’d get him back Monday. But it didn’t turn out that way. He was there for about 9 days.”
That first night in the hospital, Singh consented to intubation — should he need it. “He called me then,” said Raje. “I said we’ve got to do what we’ve got to do, it’s OK — it is what it is, and we’ll do whatever it takes.”
He remained in the MICU overnight and through the next day, still breathing on his own, but with the looming prospect of mechanical ventilation.
“The good news is he maintained his oxygen saturations throughout,” said Raje. “I was able to see his vitals with EPIC [remote monitoring] ... It was crazy,” she recalls. “Seeing a respiratory rate of 26 was difficult. When you see that, you worry about somebody tiring with the breathing. His inflammatory markers kept climbing, his fevers persisted.”
Thankfully, he never needed the ventilator.
But by this time Raje had another worry: She, too, had tested positive and was now alone at home.
“I was unable to talk to my extended family as they all looked to us as physicians for support,” she tweeted. Both children came to Boston to see her, but she saw them only through a window.
Alone, she waited for the same symptoms that had slammed her husband; but they never came — something she wants caregivers to know.
“The fear and anxiety of taking care of somebody who’s COVID positive ... I am hoping that can be alleviated a little bit at least,” she said. “If you’ve been taking care of someone, chances are you’re probably positive already and if you’re not sick, the chances of you getting sick are really low, so don’t be afraid to take care of that person.”
Singh is recovering well at home now, almost a month into his illness. During the interview, conducted via Zoom, he could be heard coughing in the background.
While in the MICU, Singh was treated with azithromycin and hydroxychloroquine — standard at MGH for critically ill COVID-19 patients — and he was also enrolled into a double-blind, randomized, placebo-controlled trial of the investigational agent remdesivir (Gilead).
Raje is not sure what, if anything, helped him turn the corner.
“I saw his inflammatory markers get worse actually — I don’t think we can know if the drugs made a difference,” she says. “His first dose of hydroxychloroquine was Friday night when he was admitted, and the markers continued to climb until the next Thursday.”
In particular, his C-reactive protein (CRP) kept rising, reaching the 260 to 270 mg/dL range, “which to me was scary,” she said. “I do think he had a cytokine storm going, but I didn’t see those results.”
“Understanding the immune compartment is going to be so, so critically important and what it is that we can do to boost folks’ immune systems,” she said.
“If you have a very high viral load and your immune system is not 100% even though you’re otherwise healthy, you might be the person who ends up with that more serious response to this virus. Trying to study this in a focused way, looking at the immune compartment, looking at the antibody status, looking at the viral load — there’s so much more we need to look at. Until we get the vaccine, which is probably a year-and-a-half away, we need to look at how can we develop that herd immunity so we don’t have folks getting as critically ill as they do.”
Despite feeling perfectly healthy, Raje is still at home. Three weeks after her first test, she is still testing positive for COVID-19, waiting for two consecutive negative results 72 hours apart before she is allowed back to work at the hospital.
When she gets the green light, she plans to go work on the COVID-19 floor, if needed. “It’s people like us [who have had COVID-19] who have to get back in the trenches and do the work now,” she says.
“My biggest concern is that it’s a very isolating experience for the COVID-positive patient. We are doing complete-barrier nursing — they are completely alone. The only person who ever walks into the room is the nurse — and the physician goes in once a day. It’s so important that we don’t lose sight of compassion,” she says.
“That’s why, in terms of alleviating anxiety, it is so important we do antibody testing so that people can actually go in and take care of these folks.”
‘Look for red flag’
Raje wants physicians to warn their self-isolating patients and caregivers to look for red flags. “There are primary care physicians who reached out to me [after my tweets] and said ‘when someone calls me and says it’s been 5-7 days and I am still not feeling well, I am going to look at that more seriously.’
“Part of me wanting to share this experience was basically to dispel the notion that 2 weeks into this you’re going to be fine,” she said, because it is not widely appreciated, she feels that “in week 2, you could become pretty sick.”
This article first appeared on Medscape.com.
A physician couple who both had COVID-19 had very different responses — one ending up in intensive care, the other asymptomatic.
Their story, one of two people living together but with such different responses to the infection, illustrates how much is still to be learned about COVID-19, says Noopur Raje, MD, professor of medicine at Harvard Medical School and director of the Center for Multiple Myeloma at Massachusetts General Hospital (MGH) in Boston.
“After experiencing #Covid_19 from the patient/caregiver end despite both of us being physicians at a major academic medical center, this has been a challenge like no other I have experienced,” Raje (@NoopurRajeMD) wrote on Twitter.
She outlined their experiences in a Twitter thread and elaborated in an interview with Medscape Medical News.
Raje says that she wants clinicians to know how symptoms can evolve both quickly and suddenly.
She recalls how for 10 days, she cared for her COVID-19–positive husband at home, separated from him by a floor in their Boston townhouse and wearing a surgical mask and gloves to bring him food and fluids, as he was too weak to help himself.
Despite the high fevers, chills, extreme fatigue, and dramatic weight loss, Raje says she felt reasonably confident that her husband was getting better. His temperature had dropped from around 103 to 101, his heart rate was in the 80s, and his blood pressure was “OK,” she recalls.
But then Jag Singh, MD, an otherwise healthy 55-year-old Harvard professor and cardiologist, started to cough — and everything suddenly changed.
The cough sounded chesty, and he was weak and unwell. They decided that he needed medical help.
“I was planning on driving him to the hospital, but I ended up having to call 911, although we literally live across the street,” she said.
“We have stairs here and I wasn’t sure that he would be able to make it coming down with me trying to help him, so the safest thing was for me to call for help.”
Singh was admitted straight to the medical intensive care unit (MICU) while his wife waited at home.
“I was blown away when I saw Jag’s x-ray and CT scan and the bilateral pneumonia he had developed,” she commented. “I would not have believed it, the way he was clinically — and seeing that x-ray.
“Honestly, when I took him in to hospital, I thought he’d be there a couple of days — over the weekend — and I’d get him back Monday. But it didn’t turn out that way. He was there for about 9 days.”
That first night in the hospital, Singh consented to intubation — should he need it. “He called me then,” said Raje. “I said we’ve got to do what we’ve got to do, it’s OK — it is what it is, and we’ll do whatever it takes.”
He remained in the MICU overnight and through the next day, still breathing on his own, but with the looming prospect of mechanical ventilation.
“The good news is he maintained his oxygen saturations throughout,” said Raje. “I was able to see his vitals with EPIC [remote monitoring] ... It was crazy,” she recalls. “Seeing a respiratory rate of 26 was difficult. When you see that, you worry about somebody tiring with the breathing. His inflammatory markers kept climbing, his fevers persisted.”
Thankfully, he never needed the ventilator.
But by this time Raje had another worry: She, too, had tested positive and was now alone at home.
“I was unable to talk to my extended family as they all looked to us as physicians for support,” she tweeted. Both children came to Boston to see her, but she saw them only through a window.
Alone, she waited for the same symptoms that had slammed her husband; but they never came — something she wants caregivers to know.
“The fear and anxiety of taking care of somebody who’s COVID positive ... I am hoping that can be alleviated a little bit at least,” she said. “If you’ve been taking care of someone, chances are you’re probably positive already and if you’re not sick, the chances of you getting sick are really low, so don’t be afraid to take care of that person.”
Singh is recovering well at home now, almost a month into his illness. During the interview, conducted via Zoom, he could be heard coughing in the background.
While in the MICU, Singh was treated with azithromycin and hydroxychloroquine — standard at MGH for critically ill COVID-19 patients — and he was also enrolled into a double-blind, randomized, placebo-controlled trial of the investigational agent remdesivir (Gilead).
Raje is not sure what, if anything, helped him turn the corner.
“I saw his inflammatory markers get worse actually — I don’t think we can know if the drugs made a difference,” she says. “His first dose of hydroxychloroquine was Friday night when he was admitted, and the markers continued to climb until the next Thursday.”
In particular, his C-reactive protein (CRP) kept rising, reaching the 260 to 270 mg/dL range, “which to me was scary,” she said. “I do think he had a cytokine storm going, but I didn’t see those results.”
“Understanding the immune compartment is going to be so, so critically important and what it is that we can do to boost folks’ immune systems,” she said.
“If you have a very high viral load and your immune system is not 100% even though you’re otherwise healthy, you might be the person who ends up with that more serious response to this virus. Trying to study this in a focused way, looking at the immune compartment, looking at the antibody status, looking at the viral load — there’s so much more we need to look at. Until we get the vaccine, which is probably a year-and-a-half away, we need to look at how can we develop that herd immunity so we don’t have folks getting as critically ill as they do.”
Despite feeling perfectly healthy, Raje is still at home. Three weeks after her first test, she is still testing positive for COVID-19, waiting for two consecutive negative results 72 hours apart before she is allowed back to work at the hospital.
When she gets the green light, she plans to go work on the COVID-19 floor, if needed. “It’s people like us [who have had COVID-19] who have to get back in the trenches and do the work now,” she says.
“My biggest concern is that it’s a very isolating experience for the COVID-positive patient. We are doing complete-barrier nursing — they are completely alone. The only person who ever walks into the room is the nurse — and the physician goes in once a day. It’s so important that we don’t lose sight of compassion,” she says.
“That’s why, in terms of alleviating anxiety, it is so important we do antibody testing so that people can actually go in and take care of these folks.”
‘Look for red flag’
Raje wants physicians to warn their self-isolating patients and caregivers to look for red flags. “There are primary care physicians who reached out to me [after my tweets] and said ‘when someone calls me and says it’s been 5-7 days and I am still not feeling well, I am going to look at that more seriously.’
“Part of me wanting to share this experience was basically to dispel the notion that 2 weeks into this you’re going to be fine,” she said, because it is not widely appreciated, she feels that “in week 2, you could become pretty sick.”
This article first appeared on Medscape.com.
A physician couple who both had COVID-19 had very different responses — one ending up in intensive care, the other asymptomatic.
Their story, one of two people living together but with such different responses to the infection, illustrates how much is still to be learned about COVID-19, says Noopur Raje, MD, professor of medicine at Harvard Medical School and director of the Center for Multiple Myeloma at Massachusetts General Hospital (MGH) in Boston.
“After experiencing #Covid_19 from the patient/caregiver end despite both of us being physicians at a major academic medical center, this has been a challenge like no other I have experienced,” Raje (@NoopurRajeMD) wrote on Twitter.
She outlined their experiences in a Twitter thread and elaborated in an interview with Medscape Medical News.
Raje says that she wants clinicians to know how symptoms can evolve both quickly and suddenly.
She recalls how for 10 days, she cared for her COVID-19–positive husband at home, separated from him by a floor in their Boston townhouse and wearing a surgical mask and gloves to bring him food and fluids, as he was too weak to help himself.
Despite the high fevers, chills, extreme fatigue, and dramatic weight loss, Raje says she felt reasonably confident that her husband was getting better. His temperature had dropped from around 103 to 101, his heart rate was in the 80s, and his blood pressure was “OK,” she recalls.
But then Jag Singh, MD, an otherwise healthy 55-year-old Harvard professor and cardiologist, started to cough — and everything suddenly changed.
The cough sounded chesty, and he was weak and unwell. They decided that he needed medical help.
“I was planning on driving him to the hospital, but I ended up having to call 911, although we literally live across the street,” she said.
“We have stairs here and I wasn’t sure that he would be able to make it coming down with me trying to help him, so the safest thing was for me to call for help.”
Singh was admitted straight to the medical intensive care unit (MICU) while his wife waited at home.
“I was blown away when I saw Jag’s x-ray and CT scan and the bilateral pneumonia he had developed,” she commented. “I would not have believed it, the way he was clinically — and seeing that x-ray.
“Honestly, when I took him in to hospital, I thought he’d be there a couple of days — over the weekend — and I’d get him back Monday. But it didn’t turn out that way. He was there for about 9 days.”
That first night in the hospital, Singh consented to intubation — should he need it. “He called me then,” said Raje. “I said we’ve got to do what we’ve got to do, it’s OK — it is what it is, and we’ll do whatever it takes.”
He remained in the MICU overnight and through the next day, still breathing on his own, but with the looming prospect of mechanical ventilation.
“The good news is he maintained his oxygen saturations throughout,” said Raje. “I was able to see his vitals with EPIC [remote monitoring] ... It was crazy,” she recalls. “Seeing a respiratory rate of 26 was difficult. When you see that, you worry about somebody tiring with the breathing. His inflammatory markers kept climbing, his fevers persisted.”
Thankfully, he never needed the ventilator.
But by this time Raje had another worry: She, too, had tested positive and was now alone at home.
“I was unable to talk to my extended family as they all looked to us as physicians for support,” she tweeted. Both children came to Boston to see her, but she saw them only through a window.
Alone, she waited for the same symptoms that had slammed her husband; but they never came — something she wants caregivers to know.
“The fear and anxiety of taking care of somebody who’s COVID positive ... I am hoping that can be alleviated a little bit at least,” she said. “If you’ve been taking care of someone, chances are you’re probably positive already and if you’re not sick, the chances of you getting sick are really low, so don’t be afraid to take care of that person.”
Singh is recovering well at home now, almost a month into his illness. During the interview, conducted via Zoom, he could be heard coughing in the background.
While in the MICU, Singh was treated with azithromycin and hydroxychloroquine — standard at MGH for critically ill COVID-19 patients — and he was also enrolled into a double-blind, randomized, placebo-controlled trial of the investigational agent remdesivir (Gilead).
Raje is not sure what, if anything, helped him turn the corner.
“I saw his inflammatory markers get worse actually — I don’t think we can know if the drugs made a difference,” she says. “His first dose of hydroxychloroquine was Friday night when he was admitted, and the markers continued to climb until the next Thursday.”
In particular, his C-reactive protein (CRP) kept rising, reaching the 260 to 270 mg/dL range, “which to me was scary,” she said. “I do think he had a cytokine storm going, but I didn’t see those results.”
“Understanding the immune compartment is going to be so, so critically important and what it is that we can do to boost folks’ immune systems,” she said.
“If you have a very high viral load and your immune system is not 100% even though you’re otherwise healthy, you might be the person who ends up with that more serious response to this virus. Trying to study this in a focused way, looking at the immune compartment, looking at the antibody status, looking at the viral load — there’s so much more we need to look at. Until we get the vaccine, which is probably a year-and-a-half away, we need to look at how can we develop that herd immunity so we don’t have folks getting as critically ill as they do.”
Despite feeling perfectly healthy, Raje is still at home. Three weeks after her first test, she is still testing positive for COVID-19, waiting for two consecutive negative results 72 hours apart before she is allowed back to work at the hospital.
When she gets the green light, she plans to go work on the COVID-19 floor, if needed. “It’s people like us [who have had COVID-19] who have to get back in the trenches and do the work now,” she says.
“My biggest concern is that it’s a very isolating experience for the COVID-positive patient. We are doing complete-barrier nursing — they are completely alone. The only person who ever walks into the room is the nurse — and the physician goes in once a day. It’s so important that we don’t lose sight of compassion,” she says.
“That’s why, in terms of alleviating anxiety, it is so important we do antibody testing so that people can actually go in and take care of these folks.”
‘Look for red flag’
Raje wants physicians to warn their self-isolating patients and caregivers to look for red flags. “There are primary care physicians who reached out to me [after my tweets] and said ‘when someone calls me and says it’s been 5-7 days and I am still not feeling well, I am going to look at that more seriously.’
“Part of me wanting to share this experience was basically to dispel the notion that 2 weeks into this you’re going to be fine,” she said, because it is not widely appreciated, she feels that “in week 2, you could become pretty sick.”
This article first appeared on Medscape.com.
NYC hospitals require health care workers to report in person, even for phone and telehealth work
A social worker in New York City was home, caring for his sick son, when the hospital at which he works ordered him to report back to work. His son had COVID-19, yet his hospital told him he had to show up in person.
The social worker’s situation is just one of many NYC Health + Hospitals employees who could work remotely yet are required to report in person. His circumstances were described in a letter sent by Lichten & Bright, a law firm representing the New York City Health Services Employees Union, Local 768.
“Despite the fact that all or virtually all of the work social workers perform can be done remotely, only a handful…are being permitted to work from home,” said the letter, which was written on behalf of about 1000 social workers and 150 medical records specialists and addressed to NYC H+H CEO Mitchell Katz, MD.
Most social workers stopped seeing patients in person in early March. But many still face crowded conditions at several points during their work day. They take public transportation to work, come face-to-face with other health care workers and patients in elevators, and some attend daily meetings with up to 10 employees in conference rooms too small to stay six feet apart, the letter says.
“The social workers are scared to go to work,” said Daniel Bright, the letter’s author. “They’re baffled by the lack of any management response that would allow them to work from home. They are worried about getting exposed to the coronavirus while riding the subway or the bus to work or at work from a doctor or nurse or patient, and getting sick themselves or taking it home to their families.”
There is no good reason that the social workers should be compelled to be physically at work during the COVID-19 pandemic, Bright said. The handful of social workers at NYC H+H’s World Trade Center Environmental Health Center clinic at Bellevue who have been allowed to work from home on an ad hoc basis, he said, have done so successfully.
In response to Bright’s letter, the hospital system issued a statement that seemed to downplay workers’ assessment of the situation, and included the following: “NYC Health + Hospital social workers…play different roles in our system, from acting as front-line providers to navigating safe discharges and helping patients and families with important health care decisions. Depending on the facility, the department, and the role they play, decisions are made by our hospital leaders on whether their critical work could be done remotely.”
Recently, many medical associations have issued statements supporting the rights of health care workers to speak up without fear of repercussion. But NYC H+H social workers have been complying with the orders because they say they’re scared of retaliation: In daily video conference calls, an administrator at one of NYC H+H’s hospitals has shown exasperation when asked about working from home, multiple employees told Medscape Medical News. And other questions, they said, such as whether staff could receive hazard pay, were scoffed at. Instead, the administration mentioned disciplinary action for those who didn’t show up to work.
During Thursday’s call, a recording of which was obtained by Medscape, the CEO of one NYC H+H hospital chastised his employees for taking their concerns to the press.
“People are just taking things and you know, using things for their benefit to be able to create problems for us who are trying to do our jobs,” he said, adding that he refuses to be bullied or blackmailed and that he’ll continue to do what he needs to do as CEO — but he wanted people to know “some of the garbage I have to deal with.”
He also reminded employees of documentation people need to provide if they don’t come in to work for being sick or taking a personal leave so the hospital can verify that “you have a condition that warrants you being out.”
Christopher Miller, a spokesperson for the hospital system, said that “some employees in certain functions may be approved to telecommute.” But employees contacted by Medscape who see all of their clients remotely said their requests to telecommute have not been approved.
At this point, it’s no longer a theoretical problem. COVID-19 appears to have spread among a cluster of people reporting to work in one of the H+H hospitals, employees said. In some cases, employees’ family members also became ill.
This article first appeared on Medscape.com.
A social worker in New York City was home, caring for his sick son, when the hospital at which he works ordered him to report back to work. His son had COVID-19, yet his hospital told him he had to show up in person.
The social worker’s situation is just one of many NYC Health + Hospitals employees who could work remotely yet are required to report in person. His circumstances were described in a letter sent by Lichten & Bright, a law firm representing the New York City Health Services Employees Union, Local 768.
“Despite the fact that all or virtually all of the work social workers perform can be done remotely, only a handful…are being permitted to work from home,” said the letter, which was written on behalf of about 1000 social workers and 150 medical records specialists and addressed to NYC H+H CEO Mitchell Katz, MD.
Most social workers stopped seeing patients in person in early March. But many still face crowded conditions at several points during their work day. They take public transportation to work, come face-to-face with other health care workers and patients in elevators, and some attend daily meetings with up to 10 employees in conference rooms too small to stay six feet apart, the letter says.
“The social workers are scared to go to work,” said Daniel Bright, the letter’s author. “They’re baffled by the lack of any management response that would allow them to work from home. They are worried about getting exposed to the coronavirus while riding the subway or the bus to work or at work from a doctor or nurse or patient, and getting sick themselves or taking it home to their families.”
There is no good reason that the social workers should be compelled to be physically at work during the COVID-19 pandemic, Bright said. The handful of social workers at NYC H+H’s World Trade Center Environmental Health Center clinic at Bellevue who have been allowed to work from home on an ad hoc basis, he said, have done so successfully.
In response to Bright’s letter, the hospital system issued a statement that seemed to downplay workers’ assessment of the situation, and included the following: “NYC Health + Hospital social workers…play different roles in our system, from acting as front-line providers to navigating safe discharges and helping patients and families with important health care decisions. Depending on the facility, the department, and the role they play, decisions are made by our hospital leaders on whether their critical work could be done remotely.”
Recently, many medical associations have issued statements supporting the rights of health care workers to speak up without fear of repercussion. But NYC H+H social workers have been complying with the orders because they say they’re scared of retaliation: In daily video conference calls, an administrator at one of NYC H+H’s hospitals has shown exasperation when asked about working from home, multiple employees told Medscape Medical News. And other questions, they said, such as whether staff could receive hazard pay, were scoffed at. Instead, the administration mentioned disciplinary action for those who didn’t show up to work.
During Thursday’s call, a recording of which was obtained by Medscape, the CEO of one NYC H+H hospital chastised his employees for taking their concerns to the press.
“People are just taking things and you know, using things for their benefit to be able to create problems for us who are trying to do our jobs,” he said, adding that he refuses to be bullied or blackmailed and that he’ll continue to do what he needs to do as CEO — but he wanted people to know “some of the garbage I have to deal with.”
He also reminded employees of documentation people need to provide if they don’t come in to work for being sick or taking a personal leave so the hospital can verify that “you have a condition that warrants you being out.”
Christopher Miller, a spokesperson for the hospital system, said that “some employees in certain functions may be approved to telecommute.” But employees contacted by Medscape who see all of their clients remotely said their requests to telecommute have not been approved.
At this point, it’s no longer a theoretical problem. COVID-19 appears to have spread among a cluster of people reporting to work in one of the H+H hospitals, employees said. In some cases, employees’ family members also became ill.
This article first appeared on Medscape.com.
A social worker in New York City was home, caring for his sick son, when the hospital at which he works ordered him to report back to work. His son had COVID-19, yet his hospital told him he had to show up in person.
The social worker’s situation is just one of many NYC Health + Hospitals employees who could work remotely yet are required to report in person. His circumstances were described in a letter sent by Lichten & Bright, a law firm representing the New York City Health Services Employees Union, Local 768.
“Despite the fact that all or virtually all of the work social workers perform can be done remotely, only a handful…are being permitted to work from home,” said the letter, which was written on behalf of about 1000 social workers and 150 medical records specialists and addressed to NYC H+H CEO Mitchell Katz, MD.
Most social workers stopped seeing patients in person in early March. But many still face crowded conditions at several points during their work day. They take public transportation to work, come face-to-face with other health care workers and patients in elevators, and some attend daily meetings with up to 10 employees in conference rooms too small to stay six feet apart, the letter says.
“The social workers are scared to go to work,” said Daniel Bright, the letter’s author. “They’re baffled by the lack of any management response that would allow them to work from home. They are worried about getting exposed to the coronavirus while riding the subway or the bus to work or at work from a doctor or nurse or patient, and getting sick themselves or taking it home to their families.”
There is no good reason that the social workers should be compelled to be physically at work during the COVID-19 pandemic, Bright said. The handful of social workers at NYC H+H’s World Trade Center Environmental Health Center clinic at Bellevue who have been allowed to work from home on an ad hoc basis, he said, have done so successfully.
In response to Bright’s letter, the hospital system issued a statement that seemed to downplay workers’ assessment of the situation, and included the following: “NYC Health + Hospital social workers…play different roles in our system, from acting as front-line providers to navigating safe discharges and helping patients and families with important health care decisions. Depending on the facility, the department, and the role they play, decisions are made by our hospital leaders on whether their critical work could be done remotely.”
Recently, many medical associations have issued statements supporting the rights of health care workers to speak up without fear of repercussion. But NYC H+H social workers have been complying with the orders because they say they’re scared of retaliation: In daily video conference calls, an administrator at one of NYC H+H’s hospitals has shown exasperation when asked about working from home, multiple employees told Medscape Medical News. And other questions, they said, such as whether staff could receive hazard pay, were scoffed at. Instead, the administration mentioned disciplinary action for those who didn’t show up to work.
During Thursday’s call, a recording of which was obtained by Medscape, the CEO of one NYC H+H hospital chastised his employees for taking their concerns to the press.
“People are just taking things and you know, using things for their benefit to be able to create problems for us who are trying to do our jobs,” he said, adding that he refuses to be bullied or blackmailed and that he’ll continue to do what he needs to do as CEO — but he wanted people to know “some of the garbage I have to deal with.”
He also reminded employees of documentation people need to provide if they don’t come in to work for being sick or taking a personal leave so the hospital can verify that “you have a condition that warrants you being out.”
Christopher Miller, a spokesperson for the hospital system, said that “some employees in certain functions may be approved to telecommute.” But employees contacted by Medscape who see all of their clients remotely said their requests to telecommute have not been approved.
At this point, it’s no longer a theoretical problem. COVID-19 appears to have spread among a cluster of people reporting to work in one of the H+H hospitals, employees said. In some cases, employees’ family members also became ill.
This article first appeared on Medscape.com.
Doing things right vs. doing the right things
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)
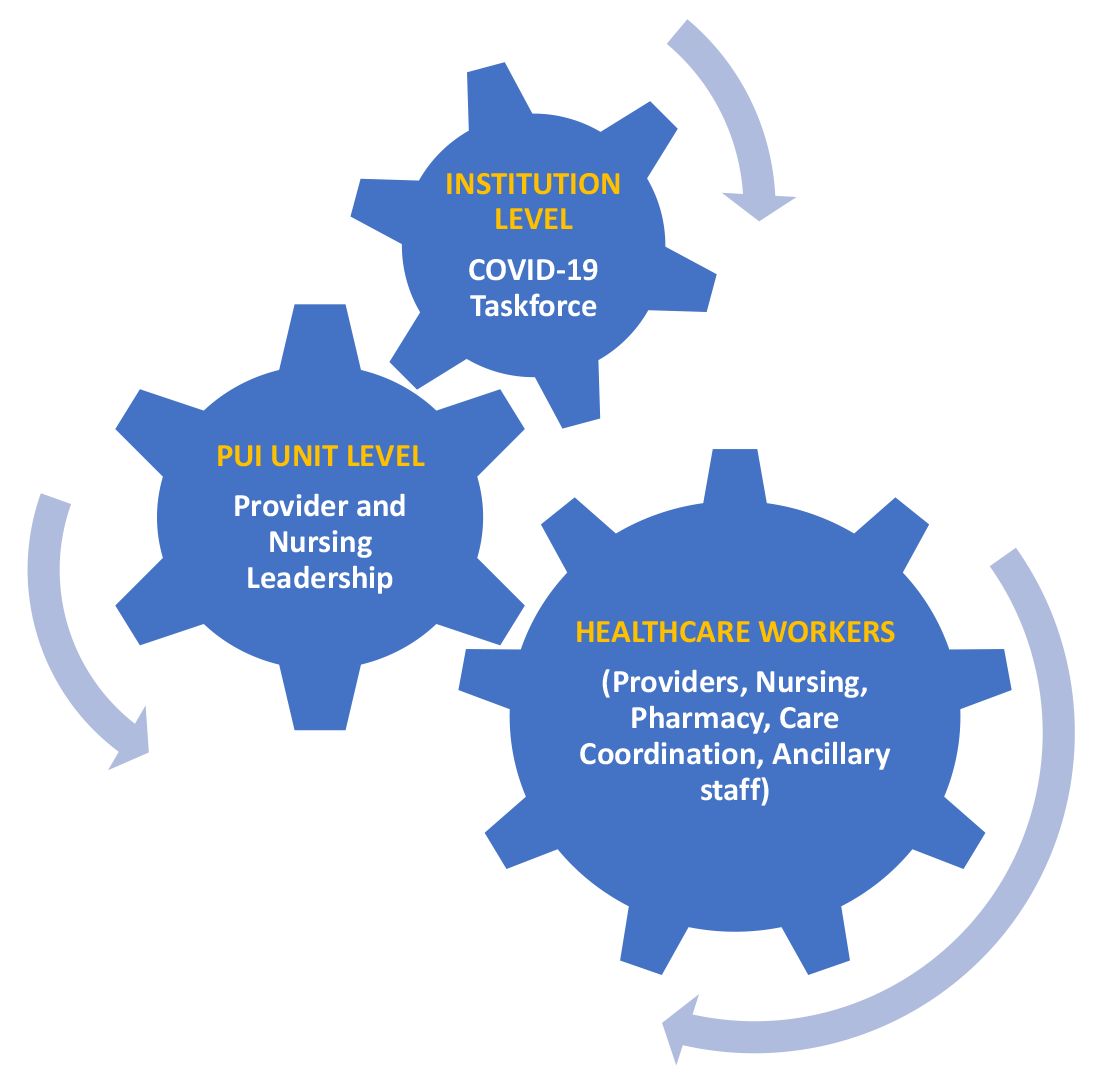
Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
A framework for a COVID-19 Person Under Investigation unit
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)

Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)

Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
Coronavirus tests are being fast-tracked by the FDA, but it’s unclear how accurate they are
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Hypertension goes unmedicated in 40% of adults
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.
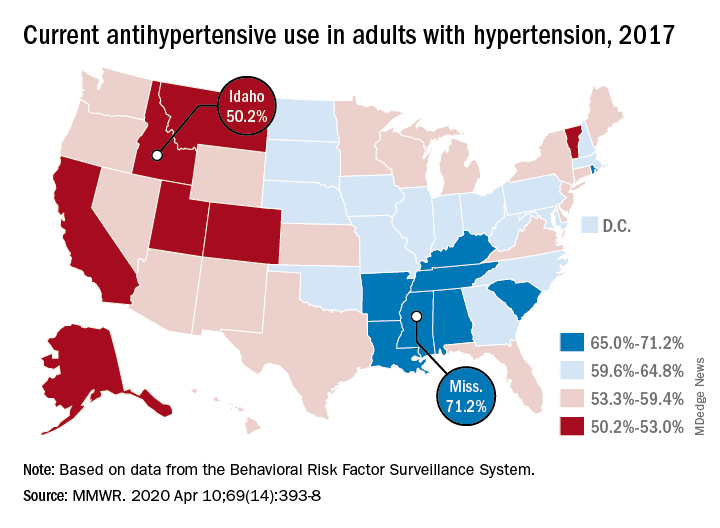
There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
FROM THE MMWR











