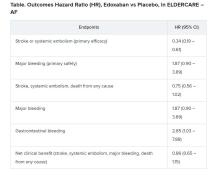
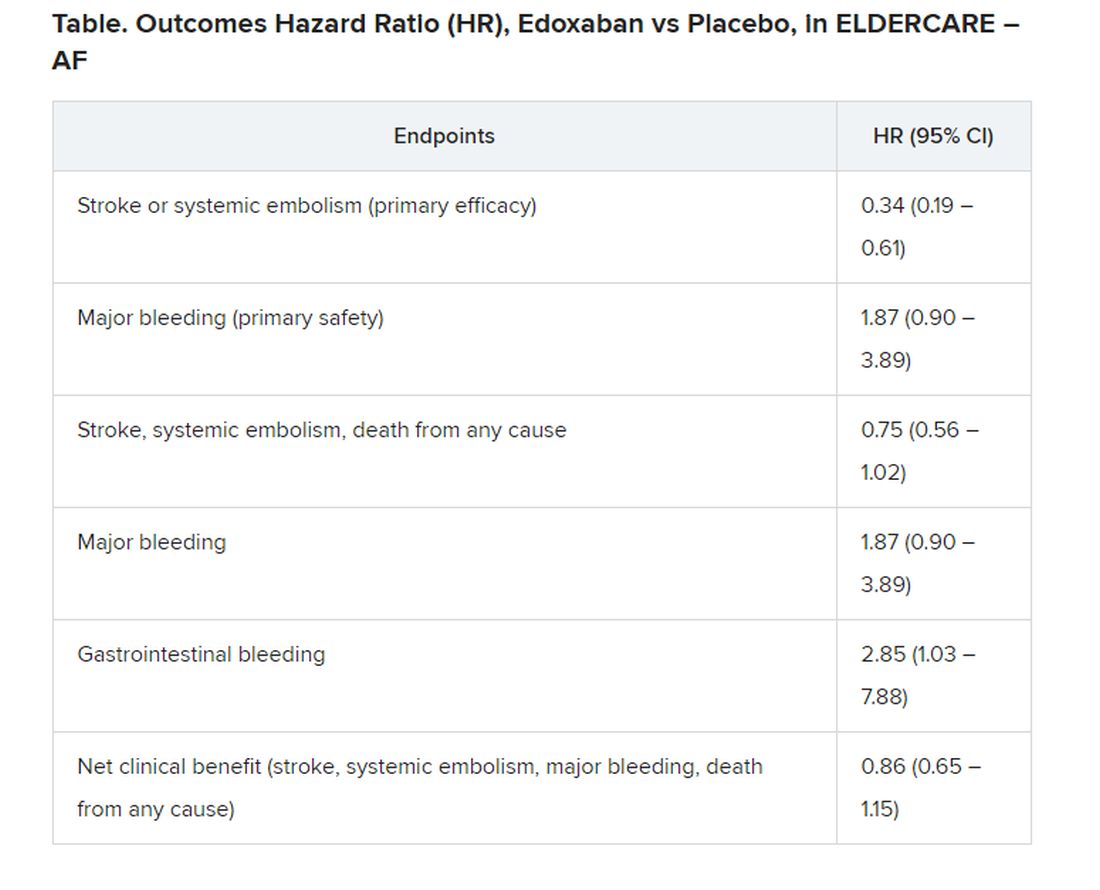
User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Early evolocumab quickly lowers LDL cholesterol after primary PCI
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
FROM ESC CONGRESS 2020
LoDoCo2: Added steam for colchicine as secondary prevention
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
Statins linked to reduced mortality in COVID-19
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
Who’s better off: Employed or self-employed physicians?
Self-employed physicians have the highest salaries, largest homes, and greatest wealth – yet they feel the least fairly compensated, according to an analysis of data from over 17,000 physicians.
A new examination of survey responses from the Medscape Physician Compensation Report 2020, which included information about income, job satisfaction, and more, compared responses from self-employed physicians, independent contractors, and employed physicians.
Income and wealth, benefits, and job satisfaction were compared. From the results of the questionnaire, self-employed physicians stand out among their peers across all categories: They enjoy greater income, wealth, and benefits and appear to be more satisfied by their choice of practice.
“The survey confirms that self-employed is the most satisfying, although the trend in health care is to take employed positions,” said Robert Scroggins, JD, CPA, certified health care business consultant with ScrogginsGreer, Cincinnati. “Doctors who become employees primarily do that to escape the management responsibilities for the practice. It seems to be more a decision to get away from something than to go toward something.”
The financial and work picture for self-employed physicians
Self-employed physicians reported the largest salaries for 2019 (average, $360,752), followed by independent contractors ($336,005). Employees reported the lowest average salary ($297,332).
The largest percentage of self-employed physicians (46%) work in an office-based group practice, followed by those in office-based solo practices (30%). Almost two-thirds of self-employed respondents are owners and 37% are partners.
Self-employed physicians are more likely to be older than 45 years; 79% fall into that age bracket, compared with 57% of employees and 70% of independent contractors.
Self-employed physicians reported the highest levels of wealth among their peers. About 44% of self-employed respondents declared a net wealth of over $2 million, compared with 25% of employees. Only 6% of contractors and employed physicians reported a net wealth of over $5 million, compared with 13% of self-employed physicians.
Self-employed physicians also managed their personal expenses slightly differently. They were more likely to pool their income with their spouse in a common account used for bills and expenses, regardless of how much they each earned (63% of self-employed respondents, compared with 58% of employees and 50% of independent contractors).
Perhaps unsurprisingly, self-employed physicians also reported having the largest homes, with an average square footage of 3,629 square feet, compared with 3,023 square feet for employees and 2,984 square feet for independent contractors. Self-employed physicians’ mortgages (average, $240,389) were similar to those of employed physicians’ mortgages but were higher than those of independent contractors’ mortgages (average, $213,740).
Self-employed physicians were also most likely to highly appraise their own performance: Half of all self-employed respondents felt “very satisfied” with their job performance, compared with 40% of employees and 44% of independent contractors.
When asked what they consider to be the most rewarding aspect of their job, self-employed physicians were more likely to choose gratitude and patient relationships than their peers (32%, compared with 26% of employees and 19% of independent contractors).
Despite their higher net wealth and larger salaries, self-employed physicians were least likely to feel fairly compensated; 49% of self-employed physicians said they did not feel fairly compensated for their work, compared with 40% of employees and 40% of independent contractors.
“Self-employed physicians may be better compensated than others of the same specialty who are employees, so some of that may be perception,” said Mr. Scroggins. “Or they feel they should be compensated to a far greater degree than those who are employed.”
Self-employed physicians were also more likely to respond that they would choose the same practice setting again, though across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
The financial and work picture for employed physicians
About a third (32%) of employed physician respondents work in hospitals; 28% work in private practices.
Employed physicians were most likely to report a salary increase from 2018 to 2019: 74%, compared with 45% of self-employed and 52% of independent contractors.
As for declines in income, self-employed physicians and independent contractors suffered a comparable loss, with 13% and 12% of them, respectively, reporting salary cuts greater than 10%. Decreases of up to 10% were felt mostly by the self-employed, with 17% experiencing such cuts, compared with 7% of employees and 10% of independent contractors.
In contrast, employees were the least likely of the three categories to have incurred large financial losses over the past year: 77% of employed respondents indicated that they had not experienced any significant financial losses in the past year, compared with 63% of self-employed physicians and 63% of independent contractors. They were also least likely to have made any investments at all over the past year – 21% of employees reported having made none at all in 2019, compared to 11% of self-employed physicians and 16% of independent contractors.
The financial and work picture for independent contractors
Just over half (52%) of all independent contractors who responded to our questionnaire work in hospitals, 15% work in group practices, 9% work in outpatient clinics, and just 2% work in solo practices.
Independent contractors were less likely than their peers to have received employment benefits such as health insurance, malpractice coverage, and paid time off. They were also less likely to be saving for retirement. Almost half (45%) of independent contractors said they received no employment benefits at all, compared to 20% of self-employed physicians and just 8% of employees.
What’s more, 27% of independent contractors do not currently put money into a 401(k) retirement account or tax-deferred college savings account on a regular basis, compared with 16% of self-employed physicians and 8% of employees. Similarly, they were less likely to put money into a taxable savings account (39% responded that they do not, compared with 32% of self-employed physicians and 27% of employees).
“Net worth and retirement funding findings do line up with what I’ve observed,” said Mr. Scroggins. “Those who have independent practices as opposed to working for a hospital do tend to more heavily fund retirement plan accounts, which is typically the biggest driver of building net worth.”
Despite the lack of retirement planning, independent contractors were more likely than their peers to derive satisfaction from making money at a job they like (18%, compared with 12% of employees and 11% of self-employed physicians). They’re also far more likely to be in emergency medicine (22% of independent contractors, compared with 3% of self-employed and 5% of employees) or psychiatry (11% of independent contractors, compared with 5% of self-employed and 6% of employees).
Among the three categories of physicians, independent contractors were least likely to say that they would choose the same practice setting again. Across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
Physicians who are considering leaving their own practice for a hospital setting should do so with caution and fully understand what they are getting into, said Mr. Scroggins. “If they’re just looking at compensation, they also should be looking very carefully at retirement plan benefits. If that’s their main method of saving and building net worth, then that’s a dramatic difference.”
And of course, there’s always the intangible value of feeling connected to a practice and its patients: “Physicians got into this line of work to treat patients and help people become healthier, and in hospitals they end up being more disconnected from their patients,” Mr. Scroggins said. “That’s a big factor as well.”
Editor’s note: Only differences that are statistically significant at a 95% confidence level between categories of employment have been included. Of the 13,893 responses included in this analysis, 3,860 physicians identified as self-employed, 9,262 as employees, and 772 as independent contractors.
A version of this article originally appeared on Medscape.com.
Self-employed physicians have the highest salaries, largest homes, and greatest wealth – yet they feel the least fairly compensated, according to an analysis of data from over 17,000 physicians.
A new examination of survey responses from the Medscape Physician Compensation Report 2020, which included information about income, job satisfaction, and more, compared responses from self-employed physicians, independent contractors, and employed physicians.
Income and wealth, benefits, and job satisfaction were compared. From the results of the questionnaire, self-employed physicians stand out among their peers across all categories: They enjoy greater income, wealth, and benefits and appear to be more satisfied by their choice of practice.
“The survey confirms that self-employed is the most satisfying, although the trend in health care is to take employed positions,” said Robert Scroggins, JD, CPA, certified health care business consultant with ScrogginsGreer, Cincinnati. “Doctors who become employees primarily do that to escape the management responsibilities for the practice. It seems to be more a decision to get away from something than to go toward something.”
The financial and work picture for self-employed physicians
Self-employed physicians reported the largest salaries for 2019 (average, $360,752), followed by independent contractors ($336,005). Employees reported the lowest average salary ($297,332).
The largest percentage of self-employed physicians (46%) work in an office-based group practice, followed by those in office-based solo practices (30%). Almost two-thirds of self-employed respondents are owners and 37% are partners.
Self-employed physicians are more likely to be older than 45 years; 79% fall into that age bracket, compared with 57% of employees and 70% of independent contractors.
Self-employed physicians reported the highest levels of wealth among their peers. About 44% of self-employed respondents declared a net wealth of over $2 million, compared with 25% of employees. Only 6% of contractors and employed physicians reported a net wealth of over $5 million, compared with 13% of self-employed physicians.
Self-employed physicians also managed their personal expenses slightly differently. They were more likely to pool their income with their spouse in a common account used for bills and expenses, regardless of how much they each earned (63% of self-employed respondents, compared with 58% of employees and 50% of independent contractors).
Perhaps unsurprisingly, self-employed physicians also reported having the largest homes, with an average square footage of 3,629 square feet, compared with 3,023 square feet for employees and 2,984 square feet for independent contractors. Self-employed physicians’ mortgages (average, $240,389) were similar to those of employed physicians’ mortgages but were higher than those of independent contractors’ mortgages (average, $213,740).
Self-employed physicians were also most likely to highly appraise their own performance: Half of all self-employed respondents felt “very satisfied” with their job performance, compared with 40% of employees and 44% of independent contractors.
When asked what they consider to be the most rewarding aspect of their job, self-employed physicians were more likely to choose gratitude and patient relationships than their peers (32%, compared with 26% of employees and 19% of independent contractors).
Despite their higher net wealth and larger salaries, self-employed physicians were least likely to feel fairly compensated; 49% of self-employed physicians said they did not feel fairly compensated for their work, compared with 40% of employees and 40% of independent contractors.
“Self-employed physicians may be better compensated than others of the same specialty who are employees, so some of that may be perception,” said Mr. Scroggins. “Or they feel they should be compensated to a far greater degree than those who are employed.”
Self-employed physicians were also more likely to respond that they would choose the same practice setting again, though across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
The financial and work picture for employed physicians
About a third (32%) of employed physician respondents work in hospitals; 28% work in private practices.
Employed physicians were most likely to report a salary increase from 2018 to 2019: 74%, compared with 45% of self-employed and 52% of independent contractors.
As for declines in income, self-employed physicians and independent contractors suffered a comparable loss, with 13% and 12% of them, respectively, reporting salary cuts greater than 10%. Decreases of up to 10% were felt mostly by the self-employed, with 17% experiencing such cuts, compared with 7% of employees and 10% of independent contractors.
In contrast, employees were the least likely of the three categories to have incurred large financial losses over the past year: 77% of employed respondents indicated that they had not experienced any significant financial losses in the past year, compared with 63% of self-employed physicians and 63% of independent contractors. They were also least likely to have made any investments at all over the past year – 21% of employees reported having made none at all in 2019, compared to 11% of self-employed physicians and 16% of independent contractors.
The financial and work picture for independent contractors
Just over half (52%) of all independent contractors who responded to our questionnaire work in hospitals, 15% work in group practices, 9% work in outpatient clinics, and just 2% work in solo practices.
Independent contractors were less likely than their peers to have received employment benefits such as health insurance, malpractice coverage, and paid time off. They were also less likely to be saving for retirement. Almost half (45%) of independent contractors said they received no employment benefits at all, compared to 20% of self-employed physicians and just 8% of employees.
What’s more, 27% of independent contractors do not currently put money into a 401(k) retirement account or tax-deferred college savings account on a regular basis, compared with 16% of self-employed physicians and 8% of employees. Similarly, they were less likely to put money into a taxable savings account (39% responded that they do not, compared with 32% of self-employed physicians and 27% of employees).
“Net worth and retirement funding findings do line up with what I’ve observed,” said Mr. Scroggins. “Those who have independent practices as opposed to working for a hospital do tend to more heavily fund retirement plan accounts, which is typically the biggest driver of building net worth.”
Despite the lack of retirement planning, independent contractors were more likely than their peers to derive satisfaction from making money at a job they like (18%, compared with 12% of employees and 11% of self-employed physicians). They’re also far more likely to be in emergency medicine (22% of independent contractors, compared with 3% of self-employed and 5% of employees) or psychiatry (11% of independent contractors, compared with 5% of self-employed and 6% of employees).
Among the three categories of physicians, independent contractors were least likely to say that they would choose the same practice setting again. Across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
Physicians who are considering leaving their own practice for a hospital setting should do so with caution and fully understand what they are getting into, said Mr. Scroggins. “If they’re just looking at compensation, they also should be looking very carefully at retirement plan benefits. If that’s their main method of saving and building net worth, then that’s a dramatic difference.”
And of course, there’s always the intangible value of feeling connected to a practice and its patients: “Physicians got into this line of work to treat patients and help people become healthier, and in hospitals they end up being more disconnected from their patients,” Mr. Scroggins said. “That’s a big factor as well.”
Editor’s note: Only differences that are statistically significant at a 95% confidence level between categories of employment have been included. Of the 13,893 responses included in this analysis, 3,860 physicians identified as self-employed, 9,262 as employees, and 772 as independent contractors.
A version of this article originally appeared on Medscape.com.
Self-employed physicians have the highest salaries, largest homes, and greatest wealth – yet they feel the least fairly compensated, according to an analysis of data from over 17,000 physicians.
A new examination of survey responses from the Medscape Physician Compensation Report 2020, which included information about income, job satisfaction, and more, compared responses from self-employed physicians, independent contractors, and employed physicians.
Income and wealth, benefits, and job satisfaction were compared. From the results of the questionnaire, self-employed physicians stand out among their peers across all categories: They enjoy greater income, wealth, and benefits and appear to be more satisfied by their choice of practice.
“The survey confirms that self-employed is the most satisfying, although the trend in health care is to take employed positions,” said Robert Scroggins, JD, CPA, certified health care business consultant with ScrogginsGreer, Cincinnati. “Doctors who become employees primarily do that to escape the management responsibilities for the practice. It seems to be more a decision to get away from something than to go toward something.”
The financial and work picture for self-employed physicians
Self-employed physicians reported the largest salaries for 2019 (average, $360,752), followed by independent contractors ($336,005). Employees reported the lowest average salary ($297,332).
The largest percentage of self-employed physicians (46%) work in an office-based group practice, followed by those in office-based solo practices (30%). Almost two-thirds of self-employed respondents are owners and 37% are partners.
Self-employed physicians are more likely to be older than 45 years; 79% fall into that age bracket, compared with 57% of employees and 70% of independent contractors.
Self-employed physicians reported the highest levels of wealth among their peers. About 44% of self-employed respondents declared a net wealth of over $2 million, compared with 25% of employees. Only 6% of contractors and employed physicians reported a net wealth of over $5 million, compared with 13% of self-employed physicians.
Self-employed physicians also managed their personal expenses slightly differently. They were more likely to pool their income with their spouse in a common account used for bills and expenses, regardless of how much they each earned (63% of self-employed respondents, compared with 58% of employees and 50% of independent contractors).
Perhaps unsurprisingly, self-employed physicians also reported having the largest homes, with an average square footage of 3,629 square feet, compared with 3,023 square feet for employees and 2,984 square feet for independent contractors. Self-employed physicians’ mortgages (average, $240,389) were similar to those of employed physicians’ mortgages but were higher than those of independent contractors’ mortgages (average, $213,740).
Self-employed physicians were also most likely to highly appraise their own performance: Half of all self-employed respondents felt “very satisfied” with their job performance, compared with 40% of employees and 44% of independent contractors.
When asked what they consider to be the most rewarding aspect of their job, self-employed physicians were more likely to choose gratitude and patient relationships than their peers (32%, compared with 26% of employees and 19% of independent contractors).
Despite their higher net wealth and larger salaries, self-employed physicians were least likely to feel fairly compensated; 49% of self-employed physicians said they did not feel fairly compensated for their work, compared with 40% of employees and 40% of independent contractors.
“Self-employed physicians may be better compensated than others of the same specialty who are employees, so some of that may be perception,” said Mr. Scroggins. “Or they feel they should be compensated to a far greater degree than those who are employed.”
Self-employed physicians were also more likely to respond that they would choose the same practice setting again, though across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
The financial and work picture for employed physicians
About a third (32%) of employed physician respondents work in hospitals; 28% work in private practices.
Employed physicians were most likely to report a salary increase from 2018 to 2019: 74%, compared with 45% of self-employed and 52% of independent contractors.
As for declines in income, self-employed physicians and independent contractors suffered a comparable loss, with 13% and 12% of them, respectively, reporting salary cuts greater than 10%. Decreases of up to 10% were felt mostly by the self-employed, with 17% experiencing such cuts, compared with 7% of employees and 10% of independent contractors.
In contrast, employees were the least likely of the three categories to have incurred large financial losses over the past year: 77% of employed respondents indicated that they had not experienced any significant financial losses in the past year, compared with 63% of self-employed physicians and 63% of independent contractors. They were also least likely to have made any investments at all over the past year – 21% of employees reported having made none at all in 2019, compared to 11% of self-employed physicians and 16% of independent contractors.
The financial and work picture for independent contractors
Just over half (52%) of all independent contractors who responded to our questionnaire work in hospitals, 15% work in group practices, 9% work in outpatient clinics, and just 2% work in solo practices.
Independent contractors were less likely than their peers to have received employment benefits such as health insurance, malpractice coverage, and paid time off. They were also less likely to be saving for retirement. Almost half (45%) of independent contractors said they received no employment benefits at all, compared to 20% of self-employed physicians and just 8% of employees.
What’s more, 27% of independent contractors do not currently put money into a 401(k) retirement account or tax-deferred college savings account on a regular basis, compared with 16% of self-employed physicians and 8% of employees. Similarly, they were less likely to put money into a taxable savings account (39% responded that they do not, compared with 32% of self-employed physicians and 27% of employees).
“Net worth and retirement funding findings do line up with what I’ve observed,” said Mr. Scroggins. “Those who have independent practices as opposed to working for a hospital do tend to more heavily fund retirement plan accounts, which is typically the biggest driver of building net worth.”
Despite the lack of retirement planning, independent contractors were more likely than their peers to derive satisfaction from making money at a job they like (18%, compared with 12% of employees and 11% of self-employed physicians). They’re also far more likely to be in emergency medicine (22% of independent contractors, compared with 3% of self-employed and 5% of employees) or psychiatry (11% of independent contractors, compared with 5% of self-employed and 6% of employees).
Among the three categories of physicians, independent contractors were least likely to say that they would choose the same practice setting again. Across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
Physicians who are considering leaving their own practice for a hospital setting should do so with caution and fully understand what they are getting into, said Mr. Scroggins. “If they’re just looking at compensation, they also should be looking very carefully at retirement plan benefits. If that’s their main method of saving and building net worth, then that’s a dramatic difference.”
And of course, there’s always the intangible value of feeling connected to a practice and its patients: “Physicians got into this line of work to treat patients and help people become healthier, and in hospitals they end up being more disconnected from their patients,” Mr. Scroggins said. “That’s a big factor as well.”
Editor’s note: Only differences that are statistically significant at a 95% confidence level between categories of employment have been included. Of the 13,893 responses included in this analysis, 3,860 physicians identified as self-employed, 9,262 as employees, and 772 as independent contractors.
A version of this article originally appeared on Medscape.com.
Minidose edoxaban may safely cut AFib stroke risk in the frail, very elderly
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
FROM ESC CONGRESS 2020
High mortality rates reported in large COVID-19 study
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Nightmares: An independent risk factor for heart disease?
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows. In what researchers describe as “surprising” findings, results from a large study of relatively young military veterans showed those who had nightmares two or more times per week had significantly increased risks for hypertension, myocardial infarction, or other heart problems.
“A diagnosis of PTSD incorporates sleep disturbance as a symptom. Thus, we were surprised to find that nightmares continued to be associated with CVD after controlling not only for PTSD and demographic factors, but also smoking and depression diagnosis,” said Christi Ulmer, PhD, of the department of psychiatry and behavioral sciences, Duke University Medical Center, Durham, N.C.
The findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Unclear mechanism
The study included 3,468 veterans (77% male) with a mean age of 38 years who had served one or two tours of duty since Sept. 11, 2001. Nearly one-third (31%) met criteria for PTSD, and 33% self-reported having at least one cardiovascular condition, such as heart problems, hypertension, stroke, and MI.
Nightmare frequency and severity was assessed using the Davidson Trauma Scale. Nightmares were considered frequent if they occurred two or more times per week and moderate to severe if they were at least moderately distressing. About 31% of veterans reported having frequent nightmares, and 35% reported moderately distressing nightmares over the past week.
After adjusting for age, race, and sex, frequent nightmares were associated with hypertension (odds ratio, 1.51; 95% confidence interval, 1.28-1.78), heart problems (OR, 1.50; 95% CI, 1.11-2.02), and MI (OR, 2.32; 95% CI, 1.18-4.54).
Associations between frequent nightmares and hypertension (OR, 1.43; 95% CI, 1.17-1.73) and heart problems (OR, 1.43; 95% CI, 1.00-2.05) remained significant after further adjusting for smoking, depression, and PTSD.
“Our cross-sectional findings set the stage for future research examining the possibility that nightmares may confer cardiovascular disease risks beyond those conferred by PTSD diagnosis alone,” Dr. Ulmer said in a news release.
Dr. Ulmer also said that, because the study was based on self-reported data, the findings are “very preliminary.” Before doctors adjust clinical practices, it’s important that our findings be replicated using longitudinal studies, clinically diagnosed medical conditions, and objectively assessed sleep,” she said.
She added that more research is needed to uncover mechanisms explaining these associations and determine if reducing the frequency and severity of nightmares can lead to improved cardiovascular health.
Timely research
Reached for comment, Rajkumar (Raj) Dasgupta, MD, of the University of Southern California, Los Angeles, noted “the correlation between nightmares and heart disease is a timely topic right now with COVID-19 as more people may be having nightmares.”
“If a patient mentions nightmares, I do think it’s important not to just glaze over it, but to talk more about it and document it in the patient record, especially in patients with cardiovascular disease, atrial fibrillation, diabetes, and hypertension,” said Dr. Dasgupta, who wasn’t involved in the study.
The research was supported by the Veterans Integrated Service Network 6 Mental Illness Research, Education and Clinical Center and the Department of Veterans Affairs HSR&D ADAPT Center at the Durham VA Health Care System. Dr. Ulmer and Dr. Dasgupta have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SLEEP 2020
IMPACT-AFib: Single mailing fails to budge oral anticoagulant uptake for AFib
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
FROM ESC CONGRESS 2020
REALITY trial supports restrictive transfusion in anemic MI
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
REPORTING FROM ESC CONGRESS 2020
First randomized trial reassures on ACEIs, ARBs in COVID-19
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.