User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
How Physician Mortgage Loans Work for Doctors With Debt
Tell someone you’re a doctor, and the reaction is often: “You must be rich.” But physicians who are just finishing medical school or are in their early careers might feel far from it. The average medical school debt is more than $200,000, with total debts including undergrad climbing well north of $250,000.
That leaves house-hunting physicians in a predicament. A key factor for lending institutions is the “debt to income” ratio, a calculation which indicates if you already have too much debt to pay your mortgage. That single equation could eliminate you from lenders’ mortgage requirements.
But young doctors are also in a unique situation. Yes, they carry above-average levels of debt, but they are on a path to substantial income in future years. That’s where the physician mortgage loan (PML) becomes a useful option.
What Is a Physician Mortgage Loan?
, according to Stephen Chang, MD, a radiologist, and a managing director at Acts Financial Advisors in McLean, Virginia.
The key features, according to James M. Dahle, MD, an emergency physician and founder of The White Coat Investor, include:
- No required down payment, which is typically 20% with a conventional loan.
- No private mortgage insurance (PMI). This is often a requirement of traditional loans, designed to protect the lender if the buyer misses payments. PMLs don’t involve PMI even if you don’t put down 20%.
- No pay stubs. With a conventional loan, pay stubs are often required to prove income level and reliability. PMLs will often allow an employment contract in place of those.
- Different consideration of the student loan burden.
Those are the upsides, of course, but there may be downsides. Dr. Dahle said a PML might involve slightly higher rates and fees than a conventional mortgage does but not always.
Who Is Best Suited for a Physician Mortgage Loan?
Financial advisers caution that everyone should first consider their full financial picture before applying for a mortgage, PML or otherwise. “If you don’t have the money saved for a down payment, one can ask if you are financially prepared to purchase a home,” says Cobin Soelberg, MD, an anesthesiologist and owner of Greeley Wealth Management, a financial planning firm serving physician families in Bend, Oregon.
If your savings are slim, you might need to build those accounts further before pursuing home ownership and the expenses that come along with it.
Your credit score can contribute to the equation. “With any loan product, we always recommend working to optimize your personal credit score as soon as possible before applying for a loan,” said Mark P. Eid, MD, a dermatologist and co–managing director (with Dr. Chang) at Act Financial Advisors. “Once you get into the high 700s, you’ve typically qualified for the best interest rates, so while that perfect 850 is nice to achieve, it’s by no means necessary.”
Also, assess your reasons for purchasing a home and whether it will fit your lifestyle in the coming years. “The main reason that [my wife and I] wanted to buy a home was for stability,” said Jordan Frey, MD, founder of The Prudent Plastic Surgeon. “After living in apartments for years, we wanted a place that was truly our own. We definitely felt disappointed and frustrated when worrying that our student debt may limit our ability to do this.”
Like many physicians, Dr. Frey had taken on a huge amount of debt, to the tune of half a million dollars in student loans and credit card debt when he finished training in 2020. The question Dr. Frey and his wife wrestled with was: “How much debt should we take on in addition to what we already have?”
What Are the Risks? What’s in the Fine Print?
The eased limitations of PMLs come with potential pitfalls, and physicians should not imagine that they have unlimited buying power.
“Many physicians buy more expensive or bigger houses than they need simply because banks are willing to lend physicians money,” Dr. Soelberg warns. “So, the doctor gets locked into a large mortgage and cannot build wealth, save for retirement, and repay their student loans.”
As you shop around, beware of omissions and scams. When meeting with lenders, Dr. Frey recalled that some didn’t even present PMLs as an option, and others presented them with unfavorable terms. He was careful to look for disadvantages hidden in the fine print, such as a potential “big hike in the rate a year later.”
But sometimes, a scam is not outright deception but is more like temptation. So it’s important to have your own best interests in mind without relying on lenders’ advice.
“When we were shopping around, some mortgage lenders would [offer] $1.5 million, and we thought ‘that makes no sense,’ ” said Dr. Frey. “[Physicians] have big future income, which makes us attractive to these lenders. No one in their right mind would give a mortgage like this to anyone else. They aren’t worried about whether it’s a smart decision for you or not.”
What Other Red Flags Should You Look Out for?
Dr. Frey recommends medical professionals beware of these red flags when shopping for PMLs:
- A request for any type of collateral, including your medical practice
- A rate that is much higher than others
- A lender is pushing you to borrow a higher amount than you’re comfortable with
- A lender attempts to influence your decision about the size of your down payment
Remember, if you are choosing an adjustable-rate mortgage (ARM), your rate will recalibrate on the basis of the market’s rates — for better or worse. This means that your payment might be higher or lower, taking current interest rates into account, based on the market.
Looking back, Dr. Frey said he might reconsider his decision to use a 10-year ARM. He and his wife chose it because the rate was low at the time, and they planned to pay off the mortgage quickly or move before it went up. But the uncertainty added an element of pressure.
How Can PMLs Contribute to Overall Financial Health?
Dr. Frey says his physician mortgage was “a huge advantage,” allowing him and his wife to put 0% down on their home without PMI. But most importantly, it fit within their overall financial plan, which included investing. “The money that we would have potentially used for a down payment, we used to buy a rental property, which then got us more income,” he says.
Of course, buying a rental property is not the only path to financial health and freedom. Many people approach a home as an investment that will eventually become fully their own. Others might put that down payment toward building a safety net of savings accounts.
Used strategically and intentionally, PMLs can put you on a more predictable financial path. And with less money stress, buying a home can be an exciting milestone as you plan your future and put down roots in a community.
A version of this article appeared on Medscape.com.
Tell someone you’re a doctor, and the reaction is often: “You must be rich.” But physicians who are just finishing medical school or are in their early careers might feel far from it. The average medical school debt is more than $200,000, with total debts including undergrad climbing well north of $250,000.
That leaves house-hunting physicians in a predicament. A key factor for lending institutions is the “debt to income” ratio, a calculation which indicates if you already have too much debt to pay your mortgage. That single equation could eliminate you from lenders’ mortgage requirements.
But young doctors are also in a unique situation. Yes, they carry above-average levels of debt, but they are on a path to substantial income in future years. That’s where the physician mortgage loan (PML) becomes a useful option.
What Is a Physician Mortgage Loan?
, according to Stephen Chang, MD, a radiologist, and a managing director at Acts Financial Advisors in McLean, Virginia.
The key features, according to James M. Dahle, MD, an emergency physician and founder of The White Coat Investor, include:
- No required down payment, which is typically 20% with a conventional loan.
- No private mortgage insurance (PMI). This is often a requirement of traditional loans, designed to protect the lender if the buyer misses payments. PMLs don’t involve PMI even if you don’t put down 20%.
- No pay stubs. With a conventional loan, pay stubs are often required to prove income level and reliability. PMLs will often allow an employment contract in place of those.
- Different consideration of the student loan burden.
Those are the upsides, of course, but there may be downsides. Dr. Dahle said a PML might involve slightly higher rates and fees than a conventional mortgage does but not always.
Who Is Best Suited for a Physician Mortgage Loan?
Financial advisers caution that everyone should first consider their full financial picture before applying for a mortgage, PML or otherwise. “If you don’t have the money saved for a down payment, one can ask if you are financially prepared to purchase a home,” says Cobin Soelberg, MD, an anesthesiologist and owner of Greeley Wealth Management, a financial planning firm serving physician families in Bend, Oregon.
If your savings are slim, you might need to build those accounts further before pursuing home ownership and the expenses that come along with it.
Your credit score can contribute to the equation. “With any loan product, we always recommend working to optimize your personal credit score as soon as possible before applying for a loan,” said Mark P. Eid, MD, a dermatologist and co–managing director (with Dr. Chang) at Act Financial Advisors. “Once you get into the high 700s, you’ve typically qualified for the best interest rates, so while that perfect 850 is nice to achieve, it’s by no means necessary.”
Also, assess your reasons for purchasing a home and whether it will fit your lifestyle in the coming years. “The main reason that [my wife and I] wanted to buy a home was for stability,” said Jordan Frey, MD, founder of The Prudent Plastic Surgeon. “After living in apartments for years, we wanted a place that was truly our own. We definitely felt disappointed and frustrated when worrying that our student debt may limit our ability to do this.”
Like many physicians, Dr. Frey had taken on a huge amount of debt, to the tune of half a million dollars in student loans and credit card debt when he finished training in 2020. The question Dr. Frey and his wife wrestled with was: “How much debt should we take on in addition to what we already have?”
What Are the Risks? What’s in the Fine Print?
The eased limitations of PMLs come with potential pitfalls, and physicians should not imagine that they have unlimited buying power.
“Many physicians buy more expensive or bigger houses than they need simply because banks are willing to lend physicians money,” Dr. Soelberg warns. “So, the doctor gets locked into a large mortgage and cannot build wealth, save for retirement, and repay their student loans.”
As you shop around, beware of omissions and scams. When meeting with lenders, Dr. Frey recalled that some didn’t even present PMLs as an option, and others presented them with unfavorable terms. He was careful to look for disadvantages hidden in the fine print, such as a potential “big hike in the rate a year later.”
But sometimes, a scam is not outright deception but is more like temptation. So it’s important to have your own best interests in mind without relying on lenders’ advice.
“When we were shopping around, some mortgage lenders would [offer] $1.5 million, and we thought ‘that makes no sense,’ ” said Dr. Frey. “[Physicians] have big future income, which makes us attractive to these lenders. No one in their right mind would give a mortgage like this to anyone else. They aren’t worried about whether it’s a smart decision for you or not.”
What Other Red Flags Should You Look Out for?
Dr. Frey recommends medical professionals beware of these red flags when shopping for PMLs:
- A request for any type of collateral, including your medical practice
- A rate that is much higher than others
- A lender is pushing you to borrow a higher amount than you’re comfortable with
- A lender attempts to influence your decision about the size of your down payment
Remember, if you are choosing an adjustable-rate mortgage (ARM), your rate will recalibrate on the basis of the market’s rates — for better or worse. This means that your payment might be higher or lower, taking current interest rates into account, based on the market.
Looking back, Dr. Frey said he might reconsider his decision to use a 10-year ARM. He and his wife chose it because the rate was low at the time, and they planned to pay off the mortgage quickly or move before it went up. But the uncertainty added an element of pressure.
How Can PMLs Contribute to Overall Financial Health?
Dr. Frey says his physician mortgage was “a huge advantage,” allowing him and his wife to put 0% down on their home without PMI. But most importantly, it fit within their overall financial plan, which included investing. “The money that we would have potentially used for a down payment, we used to buy a rental property, which then got us more income,” he says.
Of course, buying a rental property is not the only path to financial health and freedom. Many people approach a home as an investment that will eventually become fully their own. Others might put that down payment toward building a safety net of savings accounts.
Used strategically and intentionally, PMLs can put you on a more predictable financial path. And with less money stress, buying a home can be an exciting milestone as you plan your future and put down roots in a community.
A version of this article appeared on Medscape.com.
Tell someone you’re a doctor, and the reaction is often: “You must be rich.” But physicians who are just finishing medical school or are in their early careers might feel far from it. The average medical school debt is more than $200,000, with total debts including undergrad climbing well north of $250,000.
That leaves house-hunting physicians in a predicament. A key factor for lending institutions is the “debt to income” ratio, a calculation which indicates if you already have too much debt to pay your mortgage. That single equation could eliminate you from lenders’ mortgage requirements.
But young doctors are also in a unique situation. Yes, they carry above-average levels of debt, but they are on a path to substantial income in future years. That’s where the physician mortgage loan (PML) becomes a useful option.
What Is a Physician Mortgage Loan?
, according to Stephen Chang, MD, a radiologist, and a managing director at Acts Financial Advisors in McLean, Virginia.
The key features, according to James M. Dahle, MD, an emergency physician and founder of The White Coat Investor, include:
- No required down payment, which is typically 20% with a conventional loan.
- No private mortgage insurance (PMI). This is often a requirement of traditional loans, designed to protect the lender if the buyer misses payments. PMLs don’t involve PMI even if you don’t put down 20%.
- No pay stubs. With a conventional loan, pay stubs are often required to prove income level and reliability. PMLs will often allow an employment contract in place of those.
- Different consideration of the student loan burden.
Those are the upsides, of course, but there may be downsides. Dr. Dahle said a PML might involve slightly higher rates and fees than a conventional mortgage does but not always.
Who Is Best Suited for a Physician Mortgage Loan?
Financial advisers caution that everyone should first consider their full financial picture before applying for a mortgage, PML or otherwise. “If you don’t have the money saved for a down payment, one can ask if you are financially prepared to purchase a home,” says Cobin Soelberg, MD, an anesthesiologist and owner of Greeley Wealth Management, a financial planning firm serving physician families in Bend, Oregon.
If your savings are slim, you might need to build those accounts further before pursuing home ownership and the expenses that come along with it.
Your credit score can contribute to the equation. “With any loan product, we always recommend working to optimize your personal credit score as soon as possible before applying for a loan,” said Mark P. Eid, MD, a dermatologist and co–managing director (with Dr. Chang) at Act Financial Advisors. “Once you get into the high 700s, you’ve typically qualified for the best interest rates, so while that perfect 850 is nice to achieve, it’s by no means necessary.”
Also, assess your reasons for purchasing a home and whether it will fit your lifestyle in the coming years. “The main reason that [my wife and I] wanted to buy a home was for stability,” said Jordan Frey, MD, founder of The Prudent Plastic Surgeon. “After living in apartments for years, we wanted a place that was truly our own. We definitely felt disappointed and frustrated when worrying that our student debt may limit our ability to do this.”
Like many physicians, Dr. Frey had taken on a huge amount of debt, to the tune of half a million dollars in student loans and credit card debt when he finished training in 2020. The question Dr. Frey and his wife wrestled with was: “How much debt should we take on in addition to what we already have?”
What Are the Risks? What’s in the Fine Print?
The eased limitations of PMLs come with potential pitfalls, and physicians should not imagine that they have unlimited buying power.
“Many physicians buy more expensive or bigger houses than they need simply because banks are willing to lend physicians money,” Dr. Soelberg warns. “So, the doctor gets locked into a large mortgage and cannot build wealth, save for retirement, and repay their student loans.”
As you shop around, beware of omissions and scams. When meeting with lenders, Dr. Frey recalled that some didn’t even present PMLs as an option, and others presented them with unfavorable terms. He was careful to look for disadvantages hidden in the fine print, such as a potential “big hike in the rate a year later.”
But sometimes, a scam is not outright deception but is more like temptation. So it’s important to have your own best interests in mind without relying on lenders’ advice.
“When we were shopping around, some mortgage lenders would [offer] $1.5 million, and we thought ‘that makes no sense,’ ” said Dr. Frey. “[Physicians] have big future income, which makes us attractive to these lenders. No one in their right mind would give a mortgage like this to anyone else. They aren’t worried about whether it’s a smart decision for you or not.”
What Other Red Flags Should You Look Out for?
Dr. Frey recommends medical professionals beware of these red flags when shopping for PMLs:
- A request for any type of collateral, including your medical practice
- A rate that is much higher than others
- A lender is pushing you to borrow a higher amount than you’re comfortable with
- A lender attempts to influence your decision about the size of your down payment
Remember, if you are choosing an adjustable-rate mortgage (ARM), your rate will recalibrate on the basis of the market’s rates — for better or worse. This means that your payment might be higher or lower, taking current interest rates into account, based on the market.
Looking back, Dr. Frey said he might reconsider his decision to use a 10-year ARM. He and his wife chose it because the rate was low at the time, and they planned to pay off the mortgage quickly or move before it went up. But the uncertainty added an element of pressure.
How Can PMLs Contribute to Overall Financial Health?
Dr. Frey says his physician mortgage was “a huge advantage,” allowing him and his wife to put 0% down on their home without PMI. But most importantly, it fit within their overall financial plan, which included investing. “The money that we would have potentially used for a down payment, we used to buy a rental property, which then got us more income,” he says.
Of course, buying a rental property is not the only path to financial health and freedom. Many people approach a home as an investment that will eventually become fully their own. Others might put that down payment toward building a safety net of savings accounts.
Used strategically and intentionally, PMLs can put you on a more predictable financial path. And with less money stress, buying a home can be an exciting milestone as you plan your future and put down roots in a community.
A version of this article appeared on Medscape.com.
New Data to Change Practice on BP Control in Acute Stroke: INTERACT4
BASEL, SWITZERLAND — Early reduction of blood pressure has a beneficial effect in hemorrhagic stroke but a detrimental effect in ischemic stroke, new trial data show. The findings could shake up recommendations on control of blood pressure in acute stroke patients.
“This is the first time that we have randomized evidence of blood pressure control prior to reperfusion in ischemic stroke patients, and our data will challenge the current guidelines that recommend lowering blood pressure to below 180 mm Hg systolic in these patients,” said study coauthor Craig Anderson, MD, George Institute for Global Health, Sydney, Australia.
“And this study also clearly shows for the first time that getting blood pressure under control in hemorrhagic stroke patients in the first couple of hours has definitive benefits,” he added.
The findings were presented on May 16 at the European Stroke Organization Conference (ESOC) annual meeting and published online simultaneously in The New England Journal of Medicine.
A Test of Early BP Control
The trial was conducted to test the strategy of very early blood pressure control during patient transport in an ambulance after acute stroke, which investigators suspected could benefit patients with both types of stroke.
The hypothesis was that this would reduce bleeding in the brain for those with hemorrhagic stroke. For ischemic stroke patients, it was thought this strategy would speed up administration of thrombolysis, because guidelines recommend bringing blood pressure under control before thrombolysis.
For the INTERACT4 trial, which was conducted in China, 2404 patients with suspected acute stroke and elevated systolic blood pressure (≥ 150 mm Hg) who were assessed in the ambulance within 2 hours after symptom onset were randomized to receive immediate treatment with intravenous urapidil to lower the systolic blood pressure or usual blood pressure management (usual care group).
The median time between symptom onset and randomization was 61 minutes, and the mean blood pressure at randomization was 178/98 mm Hg.
Stroke was subsequently confirmed by imaging in 2240 patients, of whom 46% had a hemorrhagic stroke and 54% an ischemic stroke.
At the time of arrival at the hospital, the mean systolic blood pressure in the intervention group was 158 mm Hg, compared with 170 mm Hg in the usual care group.
The primary efficacy outcome was functional status as assessed by modified Rankin scale score at 90 days.
Overall, there was no difference between the two groups in terms of functional outcome scores (common odds ratio [OR], 1.00; 95% CI, 0.87-1.15), and the incidence of serious adverse events was similar.
But the study showed very different results in patients with hemorrhagic stroke vs those with ischemic stroke.
‘Slam-Dunk’ Effect
Anderson has led several previous trials of blood pressure control in stroke patients, some of which have suggested benefit of lowering blood pressure in those with hemorrhagic stroke, but he says the results of the current trial are more clear-cut.
“We have never seen such a slam-dunk effect as there was in INTERACT4,” Dr. Anderson said. “Not only did we show that early reduction of blood pressure in hemorrhagic stroke patients improved functional outcome, it also reduced bleeding in the brain, improved survival and quality of life, and reduced surgery and infection complications. That’s quite remarkable.”
The findings offer “clear evidence that for patients with hemorrhagic stroke, we must get the blood pressure under control as soon as possible and introduce systems of care to ensure this happens,” he added.
The reason for the clear findings in the current trial is probably the treatment time, Dr. Anderson said.
“This is the first trial in which blood pressure has been controlled in the ambulance and occurred much earlier than in the previous trials.”
Challenging Ischemic Stroke Guidelines
The INTERACT4 results in ischemic stroke patients are likely to be more controversial.
“Our results are clearly challenging longstanding beliefs around blood pressure control in ischemic stroke prior to thrombolysis,” Dr. Anderson said.
Current guidelines recommend a blood pressure < 185 mm Hg systolic before initiation of thrombolysis because of concerns about intracerebral hemorrhage, he noted. Often, blood pressure is lowered rapidly down to much lower levels in order give thrombolysis quickly.
“Our results suggest this may not be a good idea,” Dr. Anderson said. “I think these data will shake us up a bit and make us more cautious about reducing blood pressure in these patients. Personally, I wouldn’t touch the blood pressure at all in ischemic stroke patients after these results.”
He said the mechanisms behind the different stroke types would explain the results.
“If a patient is bleeding, it makes sense that higher blood pressure would make that worse,” Dr. Anderson said. “But when a patient has a blocked artery and ischemia in the brain, it seems likely that the extra pressure is needed to keep oxygen delivery to the ischemic tissue.”
Accurate Diagnosis Necessary
Because it is not possible to make an accurate diagnosis between ischemic and hemorrhagic stroke without a CT scan, Dr. Anderson stressed that at the present time, no action on blood pressure can be taken in the ambulance.
“There is a lot of interest in developing a lightweight brain scanner to be used in ambulances, but this won’t be routinely available for several years,” he said. “So for now, quick diagnosis of the type of stroke that is occurring on the patient’s arrival at the emergency department and, for hemorrhagic stroke patients, swift action to control blood pressure at this point is critical to preserving brain function.”
Commenting on the INTERACT4 results at the ESOC meeting, Simona Sacco, MD, professor of neurology at the University of L’Aquila, Italy, said this was a very important trial that would impact clinical practice.
“The data really reinforce that hemorrhagic stroke patients must have their blood pressure reduced as soon as possible,” she stated.
Dr. Sacco said the trial emphasizes the need to be able to distinguish between a hemorrhagic and ischemic stroke in a prehospital setting and supports the introduction of more mobile stroke units carrying CT scanners and calls for the development of biomarkers that can allow rapid differentiation between the two conditions.
In an accompanying editorial, Jonathan Edlow, MD, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, points out several aspects of the trial that may potentially limit the generalizability of the findings. These include use of urapidil as the antihypertensive agent, which is unavailable in the United States; all patients being of Han Chinese ethnicity; and an unusually high sensitivity of initial CT scans in detecting visible signs of ischemia or infarction in patients in acute ischemic stroke.
“These findings should be considered hypothesis-generating, and they make the case for validation of the trial results in other settings,” Dr. Edlow wrote.
The INTERACT4 trial was funded by the National Health and Medical Research Council of Australia, the George Institute for Global Health, several Chinese healthcare institutions, and Takeda Pharmaceuticals China. Disclosures for study and editorial authors are provided in the original articles.
A version of this article appeared on Medscape.com.
BASEL, SWITZERLAND — Early reduction of blood pressure has a beneficial effect in hemorrhagic stroke but a detrimental effect in ischemic stroke, new trial data show. The findings could shake up recommendations on control of blood pressure in acute stroke patients.
“This is the first time that we have randomized evidence of blood pressure control prior to reperfusion in ischemic stroke patients, and our data will challenge the current guidelines that recommend lowering blood pressure to below 180 mm Hg systolic in these patients,” said study coauthor Craig Anderson, MD, George Institute for Global Health, Sydney, Australia.
“And this study also clearly shows for the first time that getting blood pressure under control in hemorrhagic stroke patients in the first couple of hours has definitive benefits,” he added.
The findings were presented on May 16 at the European Stroke Organization Conference (ESOC) annual meeting and published online simultaneously in The New England Journal of Medicine.
A Test of Early BP Control
The trial was conducted to test the strategy of very early blood pressure control during patient transport in an ambulance after acute stroke, which investigators suspected could benefit patients with both types of stroke.
The hypothesis was that this would reduce bleeding in the brain for those with hemorrhagic stroke. For ischemic stroke patients, it was thought this strategy would speed up administration of thrombolysis, because guidelines recommend bringing blood pressure under control before thrombolysis.
For the INTERACT4 trial, which was conducted in China, 2404 patients with suspected acute stroke and elevated systolic blood pressure (≥ 150 mm Hg) who were assessed in the ambulance within 2 hours after symptom onset were randomized to receive immediate treatment with intravenous urapidil to lower the systolic blood pressure or usual blood pressure management (usual care group).
The median time between symptom onset and randomization was 61 minutes, and the mean blood pressure at randomization was 178/98 mm Hg.
Stroke was subsequently confirmed by imaging in 2240 patients, of whom 46% had a hemorrhagic stroke and 54% an ischemic stroke.
At the time of arrival at the hospital, the mean systolic blood pressure in the intervention group was 158 mm Hg, compared with 170 mm Hg in the usual care group.
The primary efficacy outcome was functional status as assessed by modified Rankin scale score at 90 days.
Overall, there was no difference between the two groups in terms of functional outcome scores (common odds ratio [OR], 1.00; 95% CI, 0.87-1.15), and the incidence of serious adverse events was similar.
But the study showed very different results in patients with hemorrhagic stroke vs those with ischemic stroke.
‘Slam-Dunk’ Effect
Anderson has led several previous trials of blood pressure control in stroke patients, some of which have suggested benefit of lowering blood pressure in those with hemorrhagic stroke, but he says the results of the current trial are more clear-cut.
“We have never seen such a slam-dunk effect as there was in INTERACT4,” Dr. Anderson said. “Not only did we show that early reduction of blood pressure in hemorrhagic stroke patients improved functional outcome, it also reduced bleeding in the brain, improved survival and quality of life, and reduced surgery and infection complications. That’s quite remarkable.”
The findings offer “clear evidence that for patients with hemorrhagic stroke, we must get the blood pressure under control as soon as possible and introduce systems of care to ensure this happens,” he added.
The reason for the clear findings in the current trial is probably the treatment time, Dr. Anderson said.
“This is the first trial in which blood pressure has been controlled in the ambulance and occurred much earlier than in the previous trials.”
Challenging Ischemic Stroke Guidelines
The INTERACT4 results in ischemic stroke patients are likely to be more controversial.
“Our results are clearly challenging longstanding beliefs around blood pressure control in ischemic stroke prior to thrombolysis,” Dr. Anderson said.
Current guidelines recommend a blood pressure < 185 mm Hg systolic before initiation of thrombolysis because of concerns about intracerebral hemorrhage, he noted. Often, blood pressure is lowered rapidly down to much lower levels in order give thrombolysis quickly.
“Our results suggest this may not be a good idea,” Dr. Anderson said. “I think these data will shake us up a bit and make us more cautious about reducing blood pressure in these patients. Personally, I wouldn’t touch the blood pressure at all in ischemic stroke patients after these results.”
He said the mechanisms behind the different stroke types would explain the results.
“If a patient is bleeding, it makes sense that higher blood pressure would make that worse,” Dr. Anderson said. “But when a patient has a blocked artery and ischemia in the brain, it seems likely that the extra pressure is needed to keep oxygen delivery to the ischemic tissue.”
Accurate Diagnosis Necessary
Because it is not possible to make an accurate diagnosis between ischemic and hemorrhagic stroke without a CT scan, Dr. Anderson stressed that at the present time, no action on blood pressure can be taken in the ambulance.
“There is a lot of interest in developing a lightweight brain scanner to be used in ambulances, but this won’t be routinely available for several years,” he said. “So for now, quick diagnosis of the type of stroke that is occurring on the patient’s arrival at the emergency department and, for hemorrhagic stroke patients, swift action to control blood pressure at this point is critical to preserving brain function.”
Commenting on the INTERACT4 results at the ESOC meeting, Simona Sacco, MD, professor of neurology at the University of L’Aquila, Italy, said this was a very important trial that would impact clinical practice.
“The data really reinforce that hemorrhagic stroke patients must have their blood pressure reduced as soon as possible,” she stated.
Dr. Sacco said the trial emphasizes the need to be able to distinguish between a hemorrhagic and ischemic stroke in a prehospital setting and supports the introduction of more mobile stroke units carrying CT scanners and calls for the development of biomarkers that can allow rapid differentiation between the two conditions.
In an accompanying editorial, Jonathan Edlow, MD, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, points out several aspects of the trial that may potentially limit the generalizability of the findings. These include use of urapidil as the antihypertensive agent, which is unavailable in the United States; all patients being of Han Chinese ethnicity; and an unusually high sensitivity of initial CT scans in detecting visible signs of ischemia or infarction in patients in acute ischemic stroke.
“These findings should be considered hypothesis-generating, and they make the case for validation of the trial results in other settings,” Dr. Edlow wrote.
The INTERACT4 trial was funded by the National Health and Medical Research Council of Australia, the George Institute for Global Health, several Chinese healthcare institutions, and Takeda Pharmaceuticals China. Disclosures for study and editorial authors are provided in the original articles.
A version of this article appeared on Medscape.com.
BASEL, SWITZERLAND — Early reduction of blood pressure has a beneficial effect in hemorrhagic stroke but a detrimental effect in ischemic stroke, new trial data show. The findings could shake up recommendations on control of blood pressure in acute stroke patients.
“This is the first time that we have randomized evidence of blood pressure control prior to reperfusion in ischemic stroke patients, and our data will challenge the current guidelines that recommend lowering blood pressure to below 180 mm Hg systolic in these patients,” said study coauthor Craig Anderson, MD, George Institute for Global Health, Sydney, Australia.
“And this study also clearly shows for the first time that getting blood pressure under control in hemorrhagic stroke patients in the first couple of hours has definitive benefits,” he added.
The findings were presented on May 16 at the European Stroke Organization Conference (ESOC) annual meeting and published online simultaneously in The New England Journal of Medicine.
A Test of Early BP Control
The trial was conducted to test the strategy of very early blood pressure control during patient transport in an ambulance after acute stroke, which investigators suspected could benefit patients with both types of stroke.
The hypothesis was that this would reduce bleeding in the brain for those with hemorrhagic stroke. For ischemic stroke patients, it was thought this strategy would speed up administration of thrombolysis, because guidelines recommend bringing blood pressure under control before thrombolysis.
For the INTERACT4 trial, which was conducted in China, 2404 patients with suspected acute stroke and elevated systolic blood pressure (≥ 150 mm Hg) who were assessed in the ambulance within 2 hours after symptom onset were randomized to receive immediate treatment with intravenous urapidil to lower the systolic blood pressure or usual blood pressure management (usual care group).
The median time between symptom onset and randomization was 61 minutes, and the mean blood pressure at randomization was 178/98 mm Hg.
Stroke was subsequently confirmed by imaging in 2240 patients, of whom 46% had a hemorrhagic stroke and 54% an ischemic stroke.
At the time of arrival at the hospital, the mean systolic blood pressure in the intervention group was 158 mm Hg, compared with 170 mm Hg in the usual care group.
The primary efficacy outcome was functional status as assessed by modified Rankin scale score at 90 days.
Overall, there was no difference between the two groups in terms of functional outcome scores (common odds ratio [OR], 1.00; 95% CI, 0.87-1.15), and the incidence of serious adverse events was similar.
But the study showed very different results in patients with hemorrhagic stroke vs those with ischemic stroke.
‘Slam-Dunk’ Effect
Anderson has led several previous trials of blood pressure control in stroke patients, some of which have suggested benefit of lowering blood pressure in those with hemorrhagic stroke, but he says the results of the current trial are more clear-cut.
“We have never seen such a slam-dunk effect as there was in INTERACT4,” Dr. Anderson said. “Not only did we show that early reduction of blood pressure in hemorrhagic stroke patients improved functional outcome, it also reduced bleeding in the brain, improved survival and quality of life, and reduced surgery and infection complications. That’s quite remarkable.”
The findings offer “clear evidence that for patients with hemorrhagic stroke, we must get the blood pressure under control as soon as possible and introduce systems of care to ensure this happens,” he added.
The reason for the clear findings in the current trial is probably the treatment time, Dr. Anderson said.
“This is the first trial in which blood pressure has been controlled in the ambulance and occurred much earlier than in the previous trials.”
Challenging Ischemic Stroke Guidelines
The INTERACT4 results in ischemic stroke patients are likely to be more controversial.
“Our results are clearly challenging longstanding beliefs around blood pressure control in ischemic stroke prior to thrombolysis,” Dr. Anderson said.
Current guidelines recommend a blood pressure < 185 mm Hg systolic before initiation of thrombolysis because of concerns about intracerebral hemorrhage, he noted. Often, blood pressure is lowered rapidly down to much lower levels in order give thrombolysis quickly.
“Our results suggest this may not be a good idea,” Dr. Anderson said. “I think these data will shake us up a bit and make us more cautious about reducing blood pressure in these patients. Personally, I wouldn’t touch the blood pressure at all in ischemic stroke patients after these results.”
He said the mechanisms behind the different stroke types would explain the results.
“If a patient is bleeding, it makes sense that higher blood pressure would make that worse,” Dr. Anderson said. “But when a patient has a blocked artery and ischemia in the brain, it seems likely that the extra pressure is needed to keep oxygen delivery to the ischemic tissue.”
Accurate Diagnosis Necessary
Because it is not possible to make an accurate diagnosis between ischemic and hemorrhagic stroke without a CT scan, Dr. Anderson stressed that at the present time, no action on blood pressure can be taken in the ambulance.
“There is a lot of interest in developing a lightweight brain scanner to be used in ambulances, but this won’t be routinely available for several years,” he said. “So for now, quick diagnosis of the type of stroke that is occurring on the patient’s arrival at the emergency department and, for hemorrhagic stroke patients, swift action to control blood pressure at this point is critical to preserving brain function.”
Commenting on the INTERACT4 results at the ESOC meeting, Simona Sacco, MD, professor of neurology at the University of L’Aquila, Italy, said this was a very important trial that would impact clinical practice.
“The data really reinforce that hemorrhagic stroke patients must have their blood pressure reduced as soon as possible,” she stated.
Dr. Sacco said the trial emphasizes the need to be able to distinguish between a hemorrhagic and ischemic stroke in a prehospital setting and supports the introduction of more mobile stroke units carrying CT scanners and calls for the development of biomarkers that can allow rapid differentiation between the two conditions.
In an accompanying editorial, Jonathan Edlow, MD, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, points out several aspects of the trial that may potentially limit the generalizability of the findings. These include use of urapidil as the antihypertensive agent, which is unavailable in the United States; all patients being of Han Chinese ethnicity; and an unusually high sensitivity of initial CT scans in detecting visible signs of ischemia or infarction in patients in acute ischemic stroke.
“These findings should be considered hypothesis-generating, and they make the case for validation of the trial results in other settings,” Dr. Edlow wrote.
The INTERACT4 trial was funded by the National Health and Medical Research Council of Australia, the George Institute for Global Health, several Chinese healthcare institutions, and Takeda Pharmaceuticals China. Disclosures for study and editorial authors are provided in the original articles.
A version of this article appeared on Medscape.com.
‘Big Breakthrough’: New Low-Field MRI Is Safer and Easier
For years, researchers and medical companies have explored low-field MRI systems (those with a magnetic field strength of less than 1 T) — searching for a feasible alternative to the loud, expensive machines requiring special rooms with shielding to block their powerful magnetic field.
Most low-field scanners in development are for brain scans only. In 2022, the US Food and Drug Administration (FDA) cleared the first portable MRI system — Hyperfine’s Swoop, designed for use at a patient’s bedside — for head and brain scans. But the technology has not been applied to whole-body MRI — until now.
In a new study published in Science, researchers from Hong Kong described a whole-body, ultra low–field MRI.
The device uses a 0.05 T magnet — one sixtieth the magnetic field strength of the standard 3 T MRI model common in hospitals today, said lead author Ed Wu, PhD, professor of biomedical engineering at The University of Hong Kong.
Because the field strength is so low, no protective shielding is needed. Patients and bystanders can safely use smart phones . And the scanner is safe for patients with implanted devices, like a cochlear implant or pacemaker, or any metal on their body or clothes. No hearing protection is required, either, because the machine is so quiet.
If all goes well, the technology could be commercially available in as little as a few years, Dr. Wu said.
But first, funding and FDA approval would be needed. “A company is going to have to come along and say, ‘This looks fantastic. We’re going to commercialize this, and we’re going to go through this certification process,’ ” said Andrew Webb, PhD, professor of radiology and the founding director of the C.J. Gorter MRI Center at the Leiden University Medical Center, Leiden, the Netherlands. (Dr. Webb was not involved in the study.)
Improving Access to MRI
One hope for this technology is to bring MRI to more people worldwide. Africa has less than one MRI scanner per million residents, whereas the United States has about 40.
While a new 3 T machine can cost about $1 million, the low-field version is much cheaper — only about $22,000 in materials cost per scanner, according to Dr. Wu.
A low magnetic field means less electricity, too — the machine can be plugged into a standard wall outlet. And because a fully shielded room isn’t needed, that could save another $100,000 in materials, Dr. Webb said.
Its ease of use could improve accessibility in countries with limited training, Dr. Webb pointed out.
“To be a technician is 2-3 years training for a regular MRI machine, a lot of it to do safety, a lot of it to do very subtle planning,” said Webb. “These [low-field] systems are much simpler.”
Challenges and the Future
The prototype weighs about 1.5 tons or 3000 lb. (A 3 T MRI can weigh between 6 and 13 tons or 12,000 and 26,000 lb.) That might sound like a lot, but it’s comparable to a mobile CT scanner, which is designed to be moved from room to room. Plus, “its weight can be substantially reduced if further optimized,” Dr. Wu said.
One challenge with low-field MRIs is image quality, which tends to be not as clear and detailed as those from high-power machines. To address this, the research team used deep learning (artificial intelligence) to enhance the image quality. “Computing power and large-scale data underpin our success, which tackles the physics and math problems that are traditionally considered intractable in existing MRI methodology,” Dr. Wu said.
Dr. Webb said he was impressed by the image quality shown in the study. They “look much higher quality than you would expect from such a low-field system,” he said. Still, only healthy volunteers were scanned. The true test will be using it to view subtle pathologies, Dr. Webb said.
That’s what Dr. Wu and his team are working on now — taking scans to diagnose various medical conditions. His group’s brain-only version of the low-field MRI has been used for diagnosis, he noted.
A version of this article appeared on Medscape.com.
For years, researchers and medical companies have explored low-field MRI systems (those with a magnetic field strength of less than 1 T) — searching for a feasible alternative to the loud, expensive machines requiring special rooms with shielding to block their powerful magnetic field.
Most low-field scanners in development are for brain scans only. In 2022, the US Food and Drug Administration (FDA) cleared the first portable MRI system — Hyperfine’s Swoop, designed for use at a patient’s bedside — for head and brain scans. But the technology has not been applied to whole-body MRI — until now.
In a new study published in Science, researchers from Hong Kong described a whole-body, ultra low–field MRI.
The device uses a 0.05 T magnet — one sixtieth the magnetic field strength of the standard 3 T MRI model common in hospitals today, said lead author Ed Wu, PhD, professor of biomedical engineering at The University of Hong Kong.
Because the field strength is so low, no protective shielding is needed. Patients and bystanders can safely use smart phones . And the scanner is safe for patients with implanted devices, like a cochlear implant or pacemaker, or any metal on their body or clothes. No hearing protection is required, either, because the machine is so quiet.
If all goes well, the technology could be commercially available in as little as a few years, Dr. Wu said.
But first, funding and FDA approval would be needed. “A company is going to have to come along and say, ‘This looks fantastic. We’re going to commercialize this, and we’re going to go through this certification process,’ ” said Andrew Webb, PhD, professor of radiology and the founding director of the C.J. Gorter MRI Center at the Leiden University Medical Center, Leiden, the Netherlands. (Dr. Webb was not involved in the study.)
Improving Access to MRI
One hope for this technology is to bring MRI to more people worldwide. Africa has less than one MRI scanner per million residents, whereas the United States has about 40.
While a new 3 T machine can cost about $1 million, the low-field version is much cheaper — only about $22,000 in materials cost per scanner, according to Dr. Wu.
A low magnetic field means less electricity, too — the machine can be plugged into a standard wall outlet. And because a fully shielded room isn’t needed, that could save another $100,000 in materials, Dr. Webb said.
Its ease of use could improve accessibility in countries with limited training, Dr. Webb pointed out.
“To be a technician is 2-3 years training for a regular MRI machine, a lot of it to do safety, a lot of it to do very subtle planning,” said Webb. “These [low-field] systems are much simpler.”
Challenges and the Future
The prototype weighs about 1.5 tons or 3000 lb. (A 3 T MRI can weigh between 6 and 13 tons or 12,000 and 26,000 lb.) That might sound like a lot, but it’s comparable to a mobile CT scanner, which is designed to be moved from room to room. Plus, “its weight can be substantially reduced if further optimized,” Dr. Wu said.
One challenge with low-field MRIs is image quality, which tends to be not as clear and detailed as those from high-power machines. To address this, the research team used deep learning (artificial intelligence) to enhance the image quality. “Computing power and large-scale data underpin our success, which tackles the physics and math problems that are traditionally considered intractable in existing MRI methodology,” Dr. Wu said.
Dr. Webb said he was impressed by the image quality shown in the study. They “look much higher quality than you would expect from such a low-field system,” he said. Still, only healthy volunteers were scanned. The true test will be using it to view subtle pathologies, Dr. Webb said.
That’s what Dr. Wu and his team are working on now — taking scans to diagnose various medical conditions. His group’s brain-only version of the low-field MRI has been used for diagnosis, he noted.
A version of this article appeared on Medscape.com.
For years, researchers and medical companies have explored low-field MRI systems (those with a magnetic field strength of less than 1 T) — searching for a feasible alternative to the loud, expensive machines requiring special rooms with shielding to block their powerful magnetic field.
Most low-field scanners in development are for brain scans only. In 2022, the US Food and Drug Administration (FDA) cleared the first portable MRI system — Hyperfine’s Swoop, designed for use at a patient’s bedside — for head and brain scans. But the technology has not been applied to whole-body MRI — until now.
In a new study published in Science, researchers from Hong Kong described a whole-body, ultra low–field MRI.
The device uses a 0.05 T magnet — one sixtieth the magnetic field strength of the standard 3 T MRI model common in hospitals today, said lead author Ed Wu, PhD, professor of biomedical engineering at The University of Hong Kong.
Because the field strength is so low, no protective shielding is needed. Patients and bystanders can safely use smart phones . And the scanner is safe for patients with implanted devices, like a cochlear implant or pacemaker, or any metal on their body or clothes. No hearing protection is required, either, because the machine is so quiet.
If all goes well, the technology could be commercially available in as little as a few years, Dr. Wu said.
But first, funding and FDA approval would be needed. “A company is going to have to come along and say, ‘This looks fantastic. We’re going to commercialize this, and we’re going to go through this certification process,’ ” said Andrew Webb, PhD, professor of radiology and the founding director of the C.J. Gorter MRI Center at the Leiden University Medical Center, Leiden, the Netherlands. (Dr. Webb was not involved in the study.)
Improving Access to MRI
One hope for this technology is to bring MRI to more people worldwide. Africa has less than one MRI scanner per million residents, whereas the United States has about 40.
While a new 3 T machine can cost about $1 million, the low-field version is much cheaper — only about $22,000 in materials cost per scanner, according to Dr. Wu.
A low magnetic field means less electricity, too — the machine can be plugged into a standard wall outlet. And because a fully shielded room isn’t needed, that could save another $100,000 in materials, Dr. Webb said.
Its ease of use could improve accessibility in countries with limited training, Dr. Webb pointed out.
“To be a technician is 2-3 years training for a regular MRI machine, a lot of it to do safety, a lot of it to do very subtle planning,” said Webb. “These [low-field] systems are much simpler.”
Challenges and the Future
The prototype weighs about 1.5 tons or 3000 lb. (A 3 T MRI can weigh between 6 and 13 tons or 12,000 and 26,000 lb.) That might sound like a lot, but it’s comparable to a mobile CT scanner, which is designed to be moved from room to room. Plus, “its weight can be substantially reduced if further optimized,” Dr. Wu said.
One challenge with low-field MRIs is image quality, which tends to be not as clear and detailed as those from high-power machines. To address this, the research team used deep learning (artificial intelligence) to enhance the image quality. “Computing power and large-scale data underpin our success, which tackles the physics and math problems that are traditionally considered intractable in existing MRI methodology,” Dr. Wu said.
Dr. Webb said he was impressed by the image quality shown in the study. They “look much higher quality than you would expect from such a low-field system,” he said. Still, only healthy volunteers were scanned. The true test will be using it to view subtle pathologies, Dr. Webb said.
That’s what Dr. Wu and his team are working on now — taking scans to diagnose various medical conditions. His group’s brain-only version of the low-field MRI has been used for diagnosis, he noted.
A version of this article appeared on Medscape.com.
Crossing State Lines: PA Licensure Compact Coming Soon
For decades, physicians and nurses who ventured across state lines to practice, particularly in locum tenens roles, have reaped the benefits of medical licensure compacts. Yet, the same courtesy has eluded physician assistants (PAs), until now.
In April, Virginia Governor Glenn Youngkin signed the bill enacting the PA Compact making Virginia the seventh state to join. The legislation opens a cross-state agreement with seven states and finally allows locum tenens PAs to practice across these state’s borders.
How the PA Compact Works
The interstate arrangement recognizes valid, unencumbered PA licenses issued by other states in the compact. PAs working within the seven states won’t need a separate license from any of those states to practice.
The states include Delaware, Nebraska, Utah, Washington, West Virginia, Wisconsin, and Virginia. While the compact has been approved, the American Academy of Physician Associates said it could take an additional 18-24 months for the states to execute it, giving PAs the access they need to work in the compact states.
How the PA Compact Helps
The PA Compact holds the promise of alleviating some of the travel barriers that PAs often encounter, especially when they work locum tenens or in telehealth and must traverse state lines to deliver essential healthcare. This agreement not only enhances healthcare access but also empowers facilities to recruit new PAs, thereby bridging gaps in their healthcare staffing and addressing public health emergencies more effectively.
PAs will also gain increased flexibility and additional opportunities to earn and benefit from the right to practice in more states without requiring a time-consuming and expensive licensure from each state.
One motivating factor behind developing an interstate compact for physician assistants is that the same types of compacts for physicians and nurses are highly successful. The Nurse Licensure Compact and the Interstate Medical Licensure Compact for physicians encompass 37 and 41 states, respectively. While the seven-state PA Compact is in its earliest stages, it will likely be equally beneficial for PAs.
A survey by Barton Associates found that 95% of PAs said they would be more likely to consider working in a different state if the PA Compact made it more accessible.
Other states have begun legislation to enact a PA Compact, including Colorado, New Hampshire, Maine, Michigan New York, Ohio, Oklahoma, Rhode Island, Tennessee, and Vermont.
If your state still needs to enact a compact or file for compact legislation, let your elected officials know that the PAs in your state want to join a compact.
A version of this article appeared on Medscape.com .
For decades, physicians and nurses who ventured across state lines to practice, particularly in locum tenens roles, have reaped the benefits of medical licensure compacts. Yet, the same courtesy has eluded physician assistants (PAs), until now.
In April, Virginia Governor Glenn Youngkin signed the bill enacting the PA Compact making Virginia the seventh state to join. The legislation opens a cross-state agreement with seven states and finally allows locum tenens PAs to practice across these state’s borders.
How the PA Compact Works
The interstate arrangement recognizes valid, unencumbered PA licenses issued by other states in the compact. PAs working within the seven states won’t need a separate license from any of those states to practice.
The states include Delaware, Nebraska, Utah, Washington, West Virginia, Wisconsin, and Virginia. While the compact has been approved, the American Academy of Physician Associates said it could take an additional 18-24 months for the states to execute it, giving PAs the access they need to work in the compact states.
How the PA Compact Helps
The PA Compact holds the promise of alleviating some of the travel barriers that PAs often encounter, especially when they work locum tenens or in telehealth and must traverse state lines to deliver essential healthcare. This agreement not only enhances healthcare access but also empowers facilities to recruit new PAs, thereby bridging gaps in their healthcare staffing and addressing public health emergencies more effectively.
PAs will also gain increased flexibility and additional opportunities to earn and benefit from the right to practice in more states without requiring a time-consuming and expensive licensure from each state.
One motivating factor behind developing an interstate compact for physician assistants is that the same types of compacts for physicians and nurses are highly successful. The Nurse Licensure Compact and the Interstate Medical Licensure Compact for physicians encompass 37 and 41 states, respectively. While the seven-state PA Compact is in its earliest stages, it will likely be equally beneficial for PAs.
A survey by Barton Associates found that 95% of PAs said they would be more likely to consider working in a different state if the PA Compact made it more accessible.
Other states have begun legislation to enact a PA Compact, including Colorado, New Hampshire, Maine, Michigan New York, Ohio, Oklahoma, Rhode Island, Tennessee, and Vermont.
If your state still needs to enact a compact or file for compact legislation, let your elected officials know that the PAs in your state want to join a compact.
A version of this article appeared on Medscape.com .
For decades, physicians and nurses who ventured across state lines to practice, particularly in locum tenens roles, have reaped the benefits of medical licensure compacts. Yet, the same courtesy has eluded physician assistants (PAs), until now.
In April, Virginia Governor Glenn Youngkin signed the bill enacting the PA Compact making Virginia the seventh state to join. The legislation opens a cross-state agreement with seven states and finally allows locum tenens PAs to practice across these state’s borders.
How the PA Compact Works
The interstate arrangement recognizes valid, unencumbered PA licenses issued by other states in the compact. PAs working within the seven states won’t need a separate license from any of those states to practice.
The states include Delaware, Nebraska, Utah, Washington, West Virginia, Wisconsin, and Virginia. While the compact has been approved, the American Academy of Physician Associates said it could take an additional 18-24 months for the states to execute it, giving PAs the access they need to work in the compact states.
How the PA Compact Helps
The PA Compact holds the promise of alleviating some of the travel barriers that PAs often encounter, especially when they work locum tenens or in telehealth and must traverse state lines to deliver essential healthcare. This agreement not only enhances healthcare access but also empowers facilities to recruit new PAs, thereby bridging gaps in their healthcare staffing and addressing public health emergencies more effectively.
PAs will also gain increased flexibility and additional opportunities to earn and benefit from the right to practice in more states without requiring a time-consuming and expensive licensure from each state.
One motivating factor behind developing an interstate compact for physician assistants is that the same types of compacts for physicians and nurses are highly successful. The Nurse Licensure Compact and the Interstate Medical Licensure Compact for physicians encompass 37 and 41 states, respectively. While the seven-state PA Compact is in its earliest stages, it will likely be equally beneficial for PAs.
A survey by Barton Associates found that 95% of PAs said they would be more likely to consider working in a different state if the PA Compact made it more accessible.
Other states have begun legislation to enact a PA Compact, including Colorado, New Hampshire, Maine, Michigan New York, Ohio, Oklahoma, Rhode Island, Tennessee, and Vermont.
If your state still needs to enact a compact or file for compact legislation, let your elected officials know that the PAs in your state want to join a compact.
A version of this article appeared on Medscape.com .
Follow-Up Outcomes Data Often Missing for FDA Drug Approvals Based on Surrogate Markers
Over the past few decades, the US Food and Drug Administration (FDA) has increasingly relied on surrogate measures such as blood tests instead of clinical outcomes for medication approvals. But critics say the agency lacks consistent standards to ensure the surrogate aligns with clinical outcomes that matter to patients — things like improvements in symptoms and gains in function.
Sometimes those decisions backfire. Consider: In July 2021, the FDA approved aducanumab for the treatment of Alzheimer’s disease, bucking the advice of an advisory panel for the agency that questioned the effectiveness of the medication. Regulators relied on data from the drugmaker, Biogen, showing the monoclonal antibody could reduce levels of amyloid beta plaques in blood — a surrogate marker officials hoped would translate to clinical benefit.
The FDA’s decision triggered significant controversy, and Biogen in January announced it is pulling it from the market this year, citing disappointing sales.
Although the case of aducanumab might seem extreme, given the stakes — Alzheimer’s remains a disease without an effective treatment — it’s far from unusual.
“When we prescribe a drug, there is an underlying assumption that the FDA has done its due diligence to confirm the drug is safe and of benefit,” said Reshma Ramachandran, MD, MPP, MHS, a researcher at Yale School of Medicine, New Haven, Connecticut, and a coauthor of a recent review of surrogate outcomes. “In fact, we found either no evidence or low-quality evidence.” Such markers are associated with clinical outcomes. “We just don’t know if they work meaningfully to treat the patient’s condition. The results were pretty shocking for us,” she said.
The FDA in 2018 released an Adult Surrogate Endpoint Table listing markers that can be used as substitutes for clinical outcomes to more quickly test, review, and approve new therapies. The analysis found the majority of these endpoints lacked subsequent confirmations, defined as published meta-analyses of clinical studies to validate the association between the marker and a clinical outcome important to patients.
In a paper published in JAMA, Dr. Ramachandran and her colleagues looked at 37 surrogate endpoints for nearly 3 dozen nononcologic diseases in the table.
Approval with surrogate markers implies responsibility for postapproval or validation studies — not just lab measures or imaging findings but mortality, morbidity, or improved quality of life, said Joshua D. Wallach, PhD, MS, assistant professor in the department of epidemiology at the Emory Rollins School of Public Health in Atlanta and lead author of the JAMA review.
Dr. Wallach said surrogate markers are easier to measure and do not require large and long trials. But the FDA has not provided clear rules for what makes a surrogate marker valid in clinical trials.
“They’ve said that at a minimum, it requires meta-analytical evidence from studies that have looked at the correlation or the association between the surrogate and the clinical outcome,” Dr. Wallach said. “Our understanding was that if that’s a minimum expectation, we should be able to find those studies in the literature. And the reality is that we were unable to find evidence from those types of studies supporting the association between the surrogate and the clinical outcome.”
Physicians generally do not receive training about the FDA approval process and the difference between biomarkers, surrogate markers, and clinical endpoints, Dr. Ramachandran said. “Our study shows that things are much more uncertain than we thought when it comes to the prescribing of new drugs,” she said.
Surrogate Markers on the Rise
Dr. Wallach’s group looked for published meta-analyses compiling randomized controlled trials reporting surrogate endpoints for more than 3 dozen chronic nononcologic conditions, including type 2 diabetes, Alzheimer’s, kidney disease, HIV, gout, and lupus. They found no meta-analyses at all for 59% of the surrogate markers, while for those that were studied, few reported high-strength evidence of an association with clinical outcomes.
The findings echo previous research. In a 2020 study in JAMA Network Open, researchers tallied primary endpoints for all FDA approvals of new drugs and therapies during three 3-year periods: 1995-1997, 2005-2007, and 2015-2017. The proportion of products whose approvals were based on the use of clinical endpoints decreased from 43.8% in 1995-1997 to 28.4% in 2005-2007 to 23.3% in 2015-2017. The share based on surrogate endpoints rose from 43.3% to roughly 60% over the same interval.
A 2017 study in the Journal of Health Economics found the use of “imperfect” surrogate endpoints helped support the approval of an average of 16 new drugs per year between 2010 and 2014 compared with six per year from 1998 to 2008.
Similar concerns about weak associations between surrogate markers and drugs used to treat cancer have been documented before, including in a 2020 study published in eClinicalMedicine. The researchers found the surrogate endpoints in the FDA table either were not tested or were tested but proven to be weak surrogates.
“And yet the FDA considered these as good enough not only for accelerated approval but also for regular approval,” said Bishal Gyawali, MD, PhD, associate professor in the department of oncology at Queen’s University, Kingston, Ontario, Canada, who led the group.
The use of surrogate endpoints is also increasing in Europe, said Huseyin Naci, MHS, PhD, associate professor of health policy at the London School of Economics and Political Science in England. He cited a cohort study of 298 randomized clinical trials (RCTs) in JAMA Oncology suggesting “contemporary oncology RCTs now largely measure putative surrogate endpoints.” Dr. Wallach called the FDA’s surrogate table “a great first step toward transparency. But a key column is missing from that table, telling us what is the basis for which the FDA allows drug companies to use the recognized surrogate markers. What is the evidence they are considering?”
If the agency allows companies the flexibility to validate surrogate endpoints, postmarketing studies designed to confirm the clinical utility of those endpoints should follow.
“We obviously want physicians to be guided by evidence when they’re selecting treatments, and they need to be able to interpret the clinical benefits of the drug that they’re prescribing,” he said. “This is really about having the research consumer, patients, and physicians, as well as industry, understand why certain markers are considered and not considered.”
Dr. Wallach reported receiving grants from the FDA (through the Yale University — Mayo Clinic Center of Excellence in Regulatory Science and Innovation), National Institute on Alcohol Abuse and Alcoholism (1K01AA028258), and Johnson & Johnson (through the Yale University Open Data Access Project); and consulting fees from Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC outside the submitted work. Dr. Ramachandran reported receiving grants from the Stavros Niarchos Foundation and FDA; receiving consulting fees from ReAct Action on Antibiotic Resistance strategy policy program outside the submitted work; and serving in an unpaid capacity as chair of the FDA task force for the nonprofit organization Doctors for America and in an unpaid capacity as board president for Universities Allied for Essential Medicines North America.
A version of this article appeared on Medscape.com.
Over the past few decades, the US Food and Drug Administration (FDA) has increasingly relied on surrogate measures such as blood tests instead of clinical outcomes for medication approvals. But critics say the agency lacks consistent standards to ensure the surrogate aligns with clinical outcomes that matter to patients — things like improvements in symptoms and gains in function.
Sometimes those decisions backfire. Consider: In July 2021, the FDA approved aducanumab for the treatment of Alzheimer’s disease, bucking the advice of an advisory panel for the agency that questioned the effectiveness of the medication. Regulators relied on data from the drugmaker, Biogen, showing the monoclonal antibody could reduce levels of amyloid beta plaques in blood — a surrogate marker officials hoped would translate to clinical benefit.
The FDA’s decision triggered significant controversy, and Biogen in January announced it is pulling it from the market this year, citing disappointing sales.
Although the case of aducanumab might seem extreme, given the stakes — Alzheimer’s remains a disease without an effective treatment — it’s far from unusual.
“When we prescribe a drug, there is an underlying assumption that the FDA has done its due diligence to confirm the drug is safe and of benefit,” said Reshma Ramachandran, MD, MPP, MHS, a researcher at Yale School of Medicine, New Haven, Connecticut, and a coauthor of a recent review of surrogate outcomes. “In fact, we found either no evidence or low-quality evidence.” Such markers are associated with clinical outcomes. “We just don’t know if they work meaningfully to treat the patient’s condition. The results were pretty shocking for us,” she said.
The FDA in 2018 released an Adult Surrogate Endpoint Table listing markers that can be used as substitutes for clinical outcomes to more quickly test, review, and approve new therapies. The analysis found the majority of these endpoints lacked subsequent confirmations, defined as published meta-analyses of clinical studies to validate the association between the marker and a clinical outcome important to patients.
In a paper published in JAMA, Dr. Ramachandran and her colleagues looked at 37 surrogate endpoints for nearly 3 dozen nononcologic diseases in the table.
Approval with surrogate markers implies responsibility for postapproval or validation studies — not just lab measures or imaging findings but mortality, morbidity, or improved quality of life, said Joshua D. Wallach, PhD, MS, assistant professor in the department of epidemiology at the Emory Rollins School of Public Health in Atlanta and lead author of the JAMA review.
Dr. Wallach said surrogate markers are easier to measure and do not require large and long trials. But the FDA has not provided clear rules for what makes a surrogate marker valid in clinical trials.
“They’ve said that at a minimum, it requires meta-analytical evidence from studies that have looked at the correlation or the association between the surrogate and the clinical outcome,” Dr. Wallach said. “Our understanding was that if that’s a minimum expectation, we should be able to find those studies in the literature. And the reality is that we were unable to find evidence from those types of studies supporting the association between the surrogate and the clinical outcome.”
Physicians generally do not receive training about the FDA approval process and the difference between biomarkers, surrogate markers, and clinical endpoints, Dr. Ramachandran said. “Our study shows that things are much more uncertain than we thought when it comes to the prescribing of new drugs,” she said.
Surrogate Markers on the Rise
Dr. Wallach’s group looked for published meta-analyses compiling randomized controlled trials reporting surrogate endpoints for more than 3 dozen chronic nononcologic conditions, including type 2 diabetes, Alzheimer’s, kidney disease, HIV, gout, and lupus. They found no meta-analyses at all for 59% of the surrogate markers, while for those that were studied, few reported high-strength evidence of an association with clinical outcomes.
The findings echo previous research. In a 2020 study in JAMA Network Open, researchers tallied primary endpoints for all FDA approvals of new drugs and therapies during three 3-year periods: 1995-1997, 2005-2007, and 2015-2017. The proportion of products whose approvals were based on the use of clinical endpoints decreased from 43.8% in 1995-1997 to 28.4% in 2005-2007 to 23.3% in 2015-2017. The share based on surrogate endpoints rose from 43.3% to roughly 60% over the same interval.
A 2017 study in the Journal of Health Economics found the use of “imperfect” surrogate endpoints helped support the approval of an average of 16 new drugs per year between 2010 and 2014 compared with six per year from 1998 to 2008.
Similar concerns about weak associations between surrogate markers and drugs used to treat cancer have been documented before, including in a 2020 study published in eClinicalMedicine. The researchers found the surrogate endpoints in the FDA table either were not tested or were tested but proven to be weak surrogates.
“And yet the FDA considered these as good enough not only for accelerated approval but also for regular approval,” said Bishal Gyawali, MD, PhD, associate professor in the department of oncology at Queen’s University, Kingston, Ontario, Canada, who led the group.
The use of surrogate endpoints is also increasing in Europe, said Huseyin Naci, MHS, PhD, associate professor of health policy at the London School of Economics and Political Science in England. He cited a cohort study of 298 randomized clinical trials (RCTs) in JAMA Oncology suggesting “contemporary oncology RCTs now largely measure putative surrogate endpoints.” Dr. Wallach called the FDA’s surrogate table “a great first step toward transparency. But a key column is missing from that table, telling us what is the basis for which the FDA allows drug companies to use the recognized surrogate markers. What is the evidence they are considering?”
If the agency allows companies the flexibility to validate surrogate endpoints, postmarketing studies designed to confirm the clinical utility of those endpoints should follow.
“We obviously want physicians to be guided by evidence when they’re selecting treatments, and they need to be able to interpret the clinical benefits of the drug that they’re prescribing,” he said. “This is really about having the research consumer, patients, and physicians, as well as industry, understand why certain markers are considered and not considered.”
Dr. Wallach reported receiving grants from the FDA (through the Yale University — Mayo Clinic Center of Excellence in Regulatory Science and Innovation), National Institute on Alcohol Abuse and Alcoholism (1K01AA028258), and Johnson & Johnson (through the Yale University Open Data Access Project); and consulting fees from Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC outside the submitted work. Dr. Ramachandran reported receiving grants from the Stavros Niarchos Foundation and FDA; receiving consulting fees from ReAct Action on Antibiotic Resistance strategy policy program outside the submitted work; and serving in an unpaid capacity as chair of the FDA task force for the nonprofit organization Doctors for America and in an unpaid capacity as board president for Universities Allied for Essential Medicines North America.
A version of this article appeared on Medscape.com.
Over the past few decades, the US Food and Drug Administration (FDA) has increasingly relied on surrogate measures such as blood tests instead of clinical outcomes for medication approvals. But critics say the agency lacks consistent standards to ensure the surrogate aligns with clinical outcomes that matter to patients — things like improvements in symptoms and gains in function.
Sometimes those decisions backfire. Consider: In July 2021, the FDA approved aducanumab for the treatment of Alzheimer’s disease, bucking the advice of an advisory panel for the agency that questioned the effectiveness of the medication. Regulators relied on data from the drugmaker, Biogen, showing the monoclonal antibody could reduce levels of amyloid beta plaques in blood — a surrogate marker officials hoped would translate to clinical benefit.
The FDA’s decision triggered significant controversy, and Biogen in January announced it is pulling it from the market this year, citing disappointing sales.
Although the case of aducanumab might seem extreme, given the stakes — Alzheimer’s remains a disease without an effective treatment — it’s far from unusual.
“When we prescribe a drug, there is an underlying assumption that the FDA has done its due diligence to confirm the drug is safe and of benefit,” said Reshma Ramachandran, MD, MPP, MHS, a researcher at Yale School of Medicine, New Haven, Connecticut, and a coauthor of a recent review of surrogate outcomes. “In fact, we found either no evidence or low-quality evidence.” Such markers are associated with clinical outcomes. “We just don’t know if they work meaningfully to treat the patient’s condition. The results were pretty shocking for us,” she said.
The FDA in 2018 released an Adult Surrogate Endpoint Table listing markers that can be used as substitutes for clinical outcomes to more quickly test, review, and approve new therapies. The analysis found the majority of these endpoints lacked subsequent confirmations, defined as published meta-analyses of clinical studies to validate the association between the marker and a clinical outcome important to patients.
In a paper published in JAMA, Dr. Ramachandran and her colleagues looked at 37 surrogate endpoints for nearly 3 dozen nononcologic diseases in the table.
Approval with surrogate markers implies responsibility for postapproval or validation studies — not just lab measures or imaging findings but mortality, morbidity, or improved quality of life, said Joshua D. Wallach, PhD, MS, assistant professor in the department of epidemiology at the Emory Rollins School of Public Health in Atlanta and lead author of the JAMA review.
Dr. Wallach said surrogate markers are easier to measure and do not require large and long trials. But the FDA has not provided clear rules for what makes a surrogate marker valid in clinical trials.
“They’ve said that at a minimum, it requires meta-analytical evidence from studies that have looked at the correlation or the association between the surrogate and the clinical outcome,” Dr. Wallach said. “Our understanding was that if that’s a minimum expectation, we should be able to find those studies in the literature. And the reality is that we were unable to find evidence from those types of studies supporting the association between the surrogate and the clinical outcome.”
Physicians generally do not receive training about the FDA approval process and the difference between biomarkers, surrogate markers, and clinical endpoints, Dr. Ramachandran said. “Our study shows that things are much more uncertain than we thought when it comes to the prescribing of new drugs,” she said.
Surrogate Markers on the Rise
Dr. Wallach’s group looked for published meta-analyses compiling randomized controlled trials reporting surrogate endpoints for more than 3 dozen chronic nononcologic conditions, including type 2 diabetes, Alzheimer’s, kidney disease, HIV, gout, and lupus. They found no meta-analyses at all for 59% of the surrogate markers, while for those that were studied, few reported high-strength evidence of an association with clinical outcomes.
The findings echo previous research. In a 2020 study in JAMA Network Open, researchers tallied primary endpoints for all FDA approvals of new drugs and therapies during three 3-year periods: 1995-1997, 2005-2007, and 2015-2017. The proportion of products whose approvals were based on the use of clinical endpoints decreased from 43.8% in 1995-1997 to 28.4% in 2005-2007 to 23.3% in 2015-2017. The share based on surrogate endpoints rose from 43.3% to roughly 60% over the same interval.
A 2017 study in the Journal of Health Economics found the use of “imperfect” surrogate endpoints helped support the approval of an average of 16 new drugs per year between 2010 and 2014 compared with six per year from 1998 to 2008.
Similar concerns about weak associations between surrogate markers and drugs used to treat cancer have been documented before, including in a 2020 study published in eClinicalMedicine. The researchers found the surrogate endpoints in the FDA table either were not tested or were tested but proven to be weak surrogates.
“And yet the FDA considered these as good enough not only for accelerated approval but also for regular approval,” said Bishal Gyawali, MD, PhD, associate professor in the department of oncology at Queen’s University, Kingston, Ontario, Canada, who led the group.
The use of surrogate endpoints is also increasing in Europe, said Huseyin Naci, MHS, PhD, associate professor of health policy at the London School of Economics and Political Science in England. He cited a cohort study of 298 randomized clinical trials (RCTs) in JAMA Oncology suggesting “contemporary oncology RCTs now largely measure putative surrogate endpoints.” Dr. Wallach called the FDA’s surrogate table “a great first step toward transparency. But a key column is missing from that table, telling us what is the basis for which the FDA allows drug companies to use the recognized surrogate markers. What is the evidence they are considering?”
If the agency allows companies the flexibility to validate surrogate endpoints, postmarketing studies designed to confirm the clinical utility of those endpoints should follow.
“We obviously want physicians to be guided by evidence when they’re selecting treatments, and they need to be able to interpret the clinical benefits of the drug that they’re prescribing,” he said. “This is really about having the research consumer, patients, and physicians, as well as industry, understand why certain markers are considered and not considered.”
Dr. Wallach reported receiving grants from the FDA (through the Yale University — Mayo Clinic Center of Excellence in Regulatory Science and Innovation), National Institute on Alcohol Abuse and Alcoholism (1K01AA028258), and Johnson & Johnson (through the Yale University Open Data Access Project); and consulting fees from Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC outside the submitted work. Dr. Ramachandran reported receiving grants from the Stavros Niarchos Foundation and FDA; receiving consulting fees from ReAct Action on Antibiotic Resistance strategy policy program outside the submitted work; and serving in an unpaid capacity as chair of the FDA task force for the nonprofit organization Doctors for America and in an unpaid capacity as board president for Universities Allied for Essential Medicines North America.
A version of this article appeared on Medscape.com.
FROM JAMA
What Health Risks Do Microplastics Pose?
The annual production of plastic worldwide has increased exponentially from about 2 million tons in 1950 to 460 million tons in 2019, and current levels are expected to triple by 2060.
Plastic contains more than 10,000 chemicals, including carcinogenic substances and endocrine disruptors. Plastic and associated chemicals are responsible for widespread pollution, contaminating aquatic (marine and freshwater), terrestrial, and atmospheric environments globally.
Atmospheric concentrations of plastic particles are on the rise, to the extent that in a remote station in the Eastern Alps in Austria, the contribution of micro- and nanoplastics (MNPs) to organic matter was comparable to data collected at an urban site.
The ocean is the ultimate destination for much of the plastic. All oceans, on the surface and in the depths, contain plastic, which is even found in polar sea ice. Many plastics seem to resist decomposition in the ocean and could persist in the environment for decades. Macro- and microplastic (MP) particles have been identified in hundreds of marine species, including species consumed by humans.
The quantity and fate of MP particles (> 10 µm) and smaller nanoplastics (< 10 µm) in aquatic environments are poorly understood, but what is most concerning is their ability to cross biologic barriers and the potential harm associated with their mobility in biologic systems.
MNP Exposure
MNPs can originate from a wide variety of sources, including food, beverages, and food product packaging. Water bottles represent a significant source of ingestible MNPs for people in their daily lives. Recent estimates, using stimulated Raman scattering imaging, documented a concentration of MNP of approximately 2.4 ± 1.3 × 105 particles per liter of bottled water. Around 90% are nanoplastics, which is two to three orders of magnitude higher than previously reported results for larger MPs.
MNPs enter the body primarily through ingestion or inhalation. For example, MNPs can be ingested by drinking liquids or eating food that has been stored or heated in plastic containers from which they have leaked or by using toothpaste that contains them. Infants are exposed to MPs from artificial milk preparation in polypropylene baby bottles, with higher levels than previously detected and ranging from 14,600 to 4,550,000 particles per capita per day.
MNP and Biologic Systems
The possible formation of hetero-aggregates between nanoplastics and natural organic matter has long been recognized as a potential challenge in the analysis of nanoplastics and can influence toxicologic results in biologic exposure. The direct visualization of such hetero-aggregates in real-world samples supports these concerns, but the analysis of MNPs with traditional techniques remains challenging. Unlike engineered nanoparticles (prepared in the laboratory as model systems), the nanoplastics in the environment are label-free and exhibit significant heterogeneity in chemical composition and morphology.
A systematic analysis of evidence on the toxic effects of MNPs on murine models, however, showed that 52.78% of biologic endpoints (related to glucose metabolism, reproduction, oxidative stress, and lipid metabolism) were significantly affected by MNP exposure.
Between Risk and Toxicity
MNP can enter the body in vivo through the digestive tract, respiratory tract, and skin contact. On average, humans could ingest from 0.1 to 5 g of MNP per week through various exposure routes.
MNPs are a potential risk factor for cardiovascular diseases, as suggested by a recent study on 257 patients with carotid atheromatous plaques. In 58.4% of cases, polyvinyl chloride was detected in the carotid artery plaque, with an average level of 5.2 ± 2.4 μg/mg of plaque. Patients with MNPs inside the atheroma had a higher risk (relative risk, 4.53) for a composite cardiovascular event of myocardial infarction, stroke, or death from any cause at 34 months of follow-up than participants where MNPs were not detectable inside the atheromatous plaque.
The potential link between inflammatory bowel disease (IBD) and MPs has been hypothesized by a study that reported a higher fecal MP concentration in patients with IBD than in healthy individuals. Fecal MP level was correlated with disease severity.
However, these studies have not demonstrated a causal relationship between MNPs and disease, and the way MNPs may influence cellular functions and induce stress responses is not yet well understood.
Future Scenarios
Current evidence confirms the fragmentation of plastic beyond the micrometer level and has unequivocally detected nanoplastics in real samples. As with many other particle distributions of the same size in the natural world, there are substantially more nanoplastics, despite their invisibility with conventional imaging techniques, than particles larger than the micron size.
The initial results of studies on MNPs in humans will stimulate future research on the amounts of MNPs that accumulate in tissue over a person’s lifetime. Researchers also will examine how the particles’ characteristics, including their chemical composition, size, and shape, can influence organs and tissues.
The way MNPs can cause harm, including through effects on the immune system and microbiome, will need to be clarified by investigating possible direct cytotoxic effects, consistent with the introductory statement of the Organization for Economic Cooperation and Development global policy forum on plastics, which states, “Plastic pollution is one of the great environmental challenges of the 21st century, causing wide-ranging damage to ecosystems and human health.”
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The annual production of plastic worldwide has increased exponentially from about 2 million tons in 1950 to 460 million tons in 2019, and current levels are expected to triple by 2060.
Plastic contains more than 10,000 chemicals, including carcinogenic substances and endocrine disruptors. Plastic and associated chemicals are responsible for widespread pollution, contaminating aquatic (marine and freshwater), terrestrial, and atmospheric environments globally.
Atmospheric concentrations of plastic particles are on the rise, to the extent that in a remote station in the Eastern Alps in Austria, the contribution of micro- and nanoplastics (MNPs) to organic matter was comparable to data collected at an urban site.
The ocean is the ultimate destination for much of the plastic. All oceans, on the surface and in the depths, contain plastic, which is even found in polar sea ice. Many plastics seem to resist decomposition in the ocean and could persist in the environment for decades. Macro- and microplastic (MP) particles have been identified in hundreds of marine species, including species consumed by humans.
The quantity and fate of MP particles (> 10 µm) and smaller nanoplastics (< 10 µm) in aquatic environments are poorly understood, but what is most concerning is their ability to cross biologic barriers and the potential harm associated with their mobility in biologic systems.
MNP Exposure
MNPs can originate from a wide variety of sources, including food, beverages, and food product packaging. Water bottles represent a significant source of ingestible MNPs for people in their daily lives. Recent estimates, using stimulated Raman scattering imaging, documented a concentration of MNP of approximately 2.4 ± 1.3 × 105 particles per liter of bottled water. Around 90% are nanoplastics, which is two to three orders of magnitude higher than previously reported results for larger MPs.
MNPs enter the body primarily through ingestion or inhalation. For example, MNPs can be ingested by drinking liquids or eating food that has been stored or heated in plastic containers from which they have leaked or by using toothpaste that contains them. Infants are exposed to MPs from artificial milk preparation in polypropylene baby bottles, with higher levels than previously detected and ranging from 14,600 to 4,550,000 particles per capita per day.
MNP and Biologic Systems
The possible formation of hetero-aggregates between nanoplastics and natural organic matter has long been recognized as a potential challenge in the analysis of nanoplastics and can influence toxicologic results in biologic exposure. The direct visualization of such hetero-aggregates in real-world samples supports these concerns, but the analysis of MNPs with traditional techniques remains challenging. Unlike engineered nanoparticles (prepared in the laboratory as model systems), the nanoplastics in the environment are label-free and exhibit significant heterogeneity in chemical composition and morphology.
A systematic analysis of evidence on the toxic effects of MNPs on murine models, however, showed that 52.78% of biologic endpoints (related to glucose metabolism, reproduction, oxidative stress, and lipid metabolism) were significantly affected by MNP exposure.
Between Risk and Toxicity
MNP can enter the body in vivo through the digestive tract, respiratory tract, and skin contact. On average, humans could ingest from 0.1 to 5 g of MNP per week through various exposure routes.
MNPs are a potential risk factor for cardiovascular diseases, as suggested by a recent study on 257 patients with carotid atheromatous plaques. In 58.4% of cases, polyvinyl chloride was detected in the carotid artery plaque, with an average level of 5.2 ± 2.4 μg/mg of plaque. Patients with MNPs inside the atheroma had a higher risk (relative risk, 4.53) for a composite cardiovascular event of myocardial infarction, stroke, or death from any cause at 34 months of follow-up than participants where MNPs were not detectable inside the atheromatous plaque.
The potential link between inflammatory bowel disease (IBD) and MPs has been hypothesized by a study that reported a higher fecal MP concentration in patients with IBD than in healthy individuals. Fecal MP level was correlated with disease severity.
However, these studies have not demonstrated a causal relationship between MNPs and disease, and the way MNPs may influence cellular functions and induce stress responses is not yet well understood.
Future Scenarios
Current evidence confirms the fragmentation of plastic beyond the micrometer level and has unequivocally detected nanoplastics in real samples. As with many other particle distributions of the same size in the natural world, there are substantially more nanoplastics, despite their invisibility with conventional imaging techniques, than particles larger than the micron size.
The initial results of studies on MNPs in humans will stimulate future research on the amounts of MNPs that accumulate in tissue over a person’s lifetime. Researchers also will examine how the particles’ characteristics, including their chemical composition, size, and shape, can influence organs and tissues.
The way MNPs can cause harm, including through effects on the immune system and microbiome, will need to be clarified by investigating possible direct cytotoxic effects, consistent with the introductory statement of the Organization for Economic Cooperation and Development global policy forum on plastics, which states, “Plastic pollution is one of the great environmental challenges of the 21st century, causing wide-ranging damage to ecosystems and human health.”
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The annual production of plastic worldwide has increased exponentially from about 2 million tons in 1950 to 460 million tons in 2019, and current levels are expected to triple by 2060.
Plastic contains more than 10,000 chemicals, including carcinogenic substances and endocrine disruptors. Plastic and associated chemicals are responsible for widespread pollution, contaminating aquatic (marine and freshwater), terrestrial, and atmospheric environments globally.
Atmospheric concentrations of plastic particles are on the rise, to the extent that in a remote station in the Eastern Alps in Austria, the contribution of micro- and nanoplastics (MNPs) to organic matter was comparable to data collected at an urban site.
The ocean is the ultimate destination for much of the plastic. All oceans, on the surface and in the depths, contain plastic, which is even found in polar sea ice. Many plastics seem to resist decomposition in the ocean and could persist in the environment for decades. Macro- and microplastic (MP) particles have been identified in hundreds of marine species, including species consumed by humans.
The quantity and fate of MP particles (> 10 µm) and smaller nanoplastics (< 10 µm) in aquatic environments are poorly understood, but what is most concerning is their ability to cross biologic barriers and the potential harm associated with their mobility in biologic systems.
MNP Exposure
MNPs can originate from a wide variety of sources, including food, beverages, and food product packaging. Water bottles represent a significant source of ingestible MNPs for people in their daily lives. Recent estimates, using stimulated Raman scattering imaging, documented a concentration of MNP of approximately 2.4 ± 1.3 × 105 particles per liter of bottled water. Around 90% are nanoplastics, which is two to three orders of magnitude higher than previously reported results for larger MPs.
MNPs enter the body primarily through ingestion or inhalation. For example, MNPs can be ingested by drinking liquids or eating food that has been stored or heated in plastic containers from which they have leaked or by using toothpaste that contains them. Infants are exposed to MPs from artificial milk preparation in polypropylene baby bottles, with higher levels than previously detected and ranging from 14,600 to 4,550,000 particles per capita per day.
MNP and Biologic Systems
The possible formation of hetero-aggregates between nanoplastics and natural organic matter has long been recognized as a potential challenge in the analysis of nanoplastics and can influence toxicologic results in biologic exposure. The direct visualization of such hetero-aggregates in real-world samples supports these concerns, but the analysis of MNPs with traditional techniques remains challenging. Unlike engineered nanoparticles (prepared in the laboratory as model systems), the nanoplastics in the environment are label-free and exhibit significant heterogeneity in chemical composition and morphology.
A systematic analysis of evidence on the toxic effects of MNPs on murine models, however, showed that 52.78% of biologic endpoints (related to glucose metabolism, reproduction, oxidative stress, and lipid metabolism) were significantly affected by MNP exposure.
Between Risk and Toxicity
MNP can enter the body in vivo through the digestive tract, respiratory tract, and skin contact. On average, humans could ingest from 0.1 to 5 g of MNP per week through various exposure routes.
MNPs are a potential risk factor for cardiovascular diseases, as suggested by a recent study on 257 patients with carotid atheromatous plaques. In 58.4% of cases, polyvinyl chloride was detected in the carotid artery plaque, with an average level of 5.2 ± 2.4 μg/mg of plaque. Patients with MNPs inside the atheroma had a higher risk (relative risk, 4.53) for a composite cardiovascular event of myocardial infarction, stroke, or death from any cause at 34 months of follow-up than participants where MNPs were not detectable inside the atheromatous plaque.
The potential link between inflammatory bowel disease (IBD) and MPs has been hypothesized by a study that reported a higher fecal MP concentration in patients with IBD than in healthy individuals. Fecal MP level was correlated with disease severity.
However, these studies have not demonstrated a causal relationship between MNPs and disease, and the way MNPs may influence cellular functions and induce stress responses is not yet well understood.
Future Scenarios
Current evidence confirms the fragmentation of plastic beyond the micrometer level and has unequivocally detected nanoplastics in real samples. As with many other particle distributions of the same size in the natural world, there are substantially more nanoplastics, despite their invisibility with conventional imaging techniques, than particles larger than the micron size.
The initial results of studies on MNPs in humans will stimulate future research on the amounts of MNPs that accumulate in tissue over a person’s lifetime. Researchers also will examine how the particles’ characteristics, including their chemical composition, size, and shape, can influence organs and tissues.
The way MNPs can cause harm, including through effects on the immune system and microbiome, will need to be clarified by investigating possible direct cytotoxic effects, consistent with the introductory statement of the Organization for Economic Cooperation and Development global policy forum on plastics, which states, “Plastic pollution is one of the great environmental challenges of the 21st century, causing wide-ranging damage to ecosystems and human health.”
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Serious Mental Illness Tied to Multiple Physical Illnesses
Serious mental illness (SMI), including bipolar disorder or schizophrenia spectrum disorders, is associated with a twofold increased risk for comorbid physical illness, results of a new meta-analysis showed.
“Although treatment of physical and mental health remains siloed in many health services globally, the high prevalence of physical multimorbidity attests to the urgent need for integrated care models that address both physical and mental health outcomes in people with severe mental illness,” the authors, led by Sean Halstead, MD, of The University of Queensland Medical School in Brisbane, Australia, wrote.
The findings were published online in The Lancet Psychiatry.
Shorter Lifespan?
SMI is associated with reduced life expectancy, and experts speculate that additional chronic illnesses — whether physical or psychiatric — may underlie this association.
While previous research has paired SMI with comorbid physical illnesses, the researchers noted that this study is the first to focus on both physical and psychiatric multimorbidity in individuals with SMI.
The investigators conducted a meta-analysis of 82 observational studies comprising 1.6 million individuals with SMI and 13.2 million control subjects to determine the risk for physical or psychiatric multimorbidity.
Studies were included if participants were diagnosed with either a schizophrenia spectrum disorder or bipolar disorder, and the study assessed either physical multimorbidity (at least two physical health conditions) or psychiatric multimorbidity (at least three psychiatric conditions), including the initial SMI.
Investigators found that individuals with SMI had more than a twofold increased risk for physical multimorbidity than those without SMI (odds ratio [OR], 2.40; 95% CI, 1.57-3.65; P = .0009).
Physical multimorbidity, which included cardiovascular, endocrine, neurological rental, gastrointestinal, musculoskeletal, and infectious disorders, was prevalent at similar rates in both schizophrenia spectrum disorder and bipolar disorder.
The ratio of physical multimorbidity was about four times higher in younger populations with SMI (mean age ≤ 40; OR, 3.99; 95% CI, 1.43-11.10) than in older populations (mean age > 40; OR, 1.55; 95% CI, 0.96-2.51; subgroup differences, P = .0013).
In terms of absolute prevalence, 25% of those with SMI had a physical multimorbidity, and 14% had a psychiatric multimorbidity, which were primarily anxiety and substance use disorders.
Investigators speculated that physical multimorbidity in SMI could stem from side effects of psychotropic medications, which are known to cause rapid cardiometabolic changes, including weight gain. In addition, lifestyle factors or nonmodifiable risk factors could also contribute to physical multimorbidity.
The study’s limitations included its small sample sizes for subgroup analyses and insufficient analysis for significant covariates, including smoking rates and symptom severity.
“While health services and treatment guidelines often operate on the assumption that individuals have a single principal diagnosis, these results attest to the clinical complexity many people with severe mental illness face in relation to burden of chronic disease,” the investigators wrote. They added that a greater understanding of the epidemiological manifestations of multimorbidity in SMI is “imperative.”
There was no source of funding for this study. Dr. Halstead is supported by the Australian Research Training Program scholarship. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com .
Serious mental illness (SMI), including bipolar disorder or schizophrenia spectrum disorders, is associated with a twofold increased risk for comorbid physical illness, results of a new meta-analysis showed.
“Although treatment of physical and mental health remains siloed in many health services globally, the high prevalence of physical multimorbidity attests to the urgent need for integrated care models that address both physical and mental health outcomes in people with severe mental illness,” the authors, led by Sean Halstead, MD, of The University of Queensland Medical School in Brisbane, Australia, wrote.
The findings were published online in The Lancet Psychiatry.
Shorter Lifespan?
SMI is associated with reduced life expectancy, and experts speculate that additional chronic illnesses — whether physical or psychiatric — may underlie this association.
While previous research has paired SMI with comorbid physical illnesses, the researchers noted that this study is the first to focus on both physical and psychiatric multimorbidity in individuals with SMI.
The investigators conducted a meta-analysis of 82 observational studies comprising 1.6 million individuals with SMI and 13.2 million control subjects to determine the risk for physical or psychiatric multimorbidity.
Studies were included if participants were diagnosed with either a schizophrenia spectrum disorder or bipolar disorder, and the study assessed either physical multimorbidity (at least two physical health conditions) or psychiatric multimorbidity (at least three psychiatric conditions), including the initial SMI.
Investigators found that individuals with SMI had more than a twofold increased risk for physical multimorbidity than those without SMI (odds ratio [OR], 2.40; 95% CI, 1.57-3.65; P = .0009).
Physical multimorbidity, which included cardiovascular, endocrine, neurological rental, gastrointestinal, musculoskeletal, and infectious disorders, was prevalent at similar rates in both schizophrenia spectrum disorder and bipolar disorder.
The ratio of physical multimorbidity was about four times higher in younger populations with SMI (mean age ≤ 40; OR, 3.99; 95% CI, 1.43-11.10) than in older populations (mean age > 40; OR, 1.55; 95% CI, 0.96-2.51; subgroup differences, P = .0013).
In terms of absolute prevalence, 25% of those with SMI had a physical multimorbidity, and 14% had a psychiatric multimorbidity, which were primarily anxiety and substance use disorders.
Investigators speculated that physical multimorbidity in SMI could stem from side effects of psychotropic medications, which are known to cause rapid cardiometabolic changes, including weight gain. In addition, lifestyle factors or nonmodifiable risk factors could also contribute to physical multimorbidity.
The study’s limitations included its small sample sizes for subgroup analyses and insufficient analysis for significant covariates, including smoking rates and symptom severity.
“While health services and treatment guidelines often operate on the assumption that individuals have a single principal diagnosis, these results attest to the clinical complexity many people with severe mental illness face in relation to burden of chronic disease,” the investigators wrote. They added that a greater understanding of the epidemiological manifestations of multimorbidity in SMI is “imperative.”
There was no source of funding for this study. Dr. Halstead is supported by the Australian Research Training Program scholarship. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com .
Serious mental illness (SMI), including bipolar disorder or schizophrenia spectrum disorders, is associated with a twofold increased risk for comorbid physical illness, results of a new meta-analysis showed.
“Although treatment of physical and mental health remains siloed in many health services globally, the high prevalence of physical multimorbidity attests to the urgent need for integrated care models that address both physical and mental health outcomes in people with severe mental illness,” the authors, led by Sean Halstead, MD, of The University of Queensland Medical School in Brisbane, Australia, wrote.
The findings were published online in The Lancet Psychiatry.
Shorter Lifespan?
SMI is associated with reduced life expectancy, and experts speculate that additional chronic illnesses — whether physical or psychiatric — may underlie this association.
While previous research has paired SMI with comorbid physical illnesses, the researchers noted that this study is the first to focus on both physical and psychiatric multimorbidity in individuals with SMI.
The investigators conducted a meta-analysis of 82 observational studies comprising 1.6 million individuals with SMI and 13.2 million control subjects to determine the risk for physical or psychiatric multimorbidity.
Studies were included if participants were diagnosed with either a schizophrenia spectrum disorder or bipolar disorder, and the study assessed either physical multimorbidity (at least two physical health conditions) or psychiatric multimorbidity (at least three psychiatric conditions), including the initial SMI.
Investigators found that individuals with SMI had more than a twofold increased risk for physical multimorbidity than those without SMI (odds ratio [OR], 2.40; 95% CI, 1.57-3.65; P = .0009).
Physical multimorbidity, which included cardiovascular, endocrine, neurological rental, gastrointestinal, musculoskeletal, and infectious disorders, was prevalent at similar rates in both schizophrenia spectrum disorder and bipolar disorder.
The ratio of physical multimorbidity was about four times higher in younger populations with SMI (mean age ≤ 40; OR, 3.99; 95% CI, 1.43-11.10) than in older populations (mean age > 40; OR, 1.55; 95% CI, 0.96-2.51; subgroup differences, P = .0013).
In terms of absolute prevalence, 25% of those with SMI had a physical multimorbidity, and 14% had a psychiatric multimorbidity, which were primarily anxiety and substance use disorders.
Investigators speculated that physical multimorbidity in SMI could stem from side effects of psychotropic medications, which are known to cause rapid cardiometabolic changes, including weight gain. In addition, lifestyle factors or nonmodifiable risk factors could also contribute to physical multimorbidity.
The study’s limitations included its small sample sizes for subgroup analyses and insufficient analysis for significant covariates, including smoking rates and symptom severity.
“While health services and treatment guidelines often operate on the assumption that individuals have a single principal diagnosis, these results attest to the clinical complexity many people with severe mental illness face in relation to burden of chronic disease,” the investigators wrote. They added that a greater understanding of the epidemiological manifestations of multimorbidity in SMI is “imperative.”
There was no source of funding for this study. Dr. Halstead is supported by the Australian Research Training Program scholarship. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com .
FROM THE LANCET PSYCHIATRY
CPAP Underperforms: The Sequel
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
Why Cardiac Biomarkers Don’t Help Predict Heart Disease
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.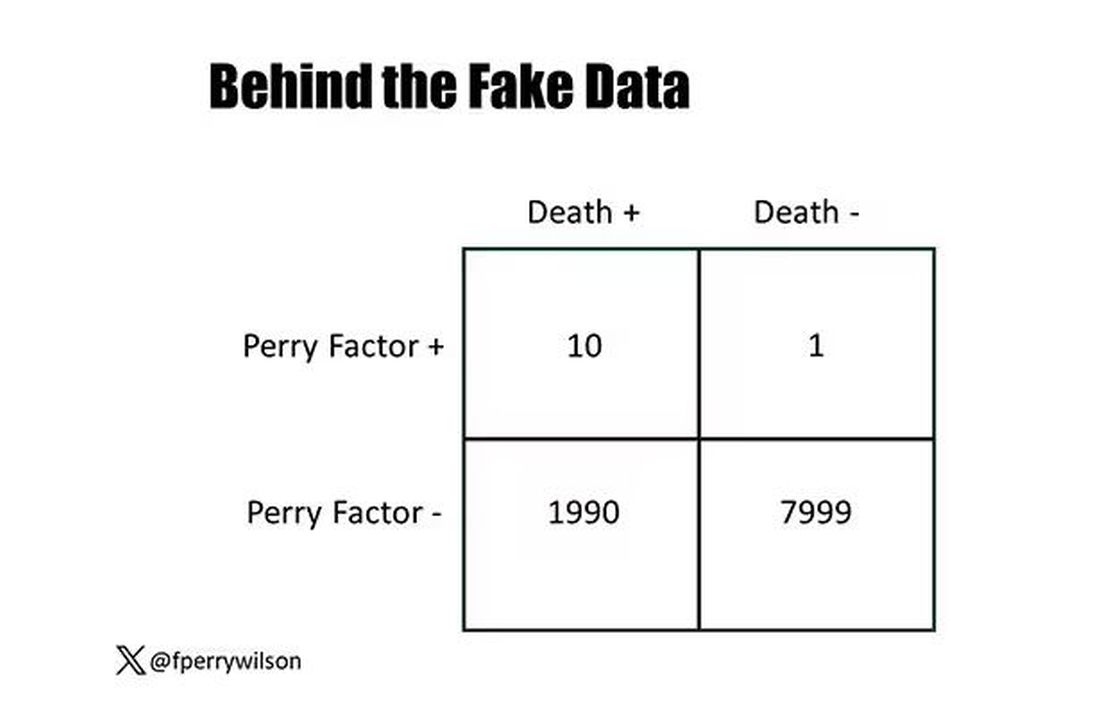
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.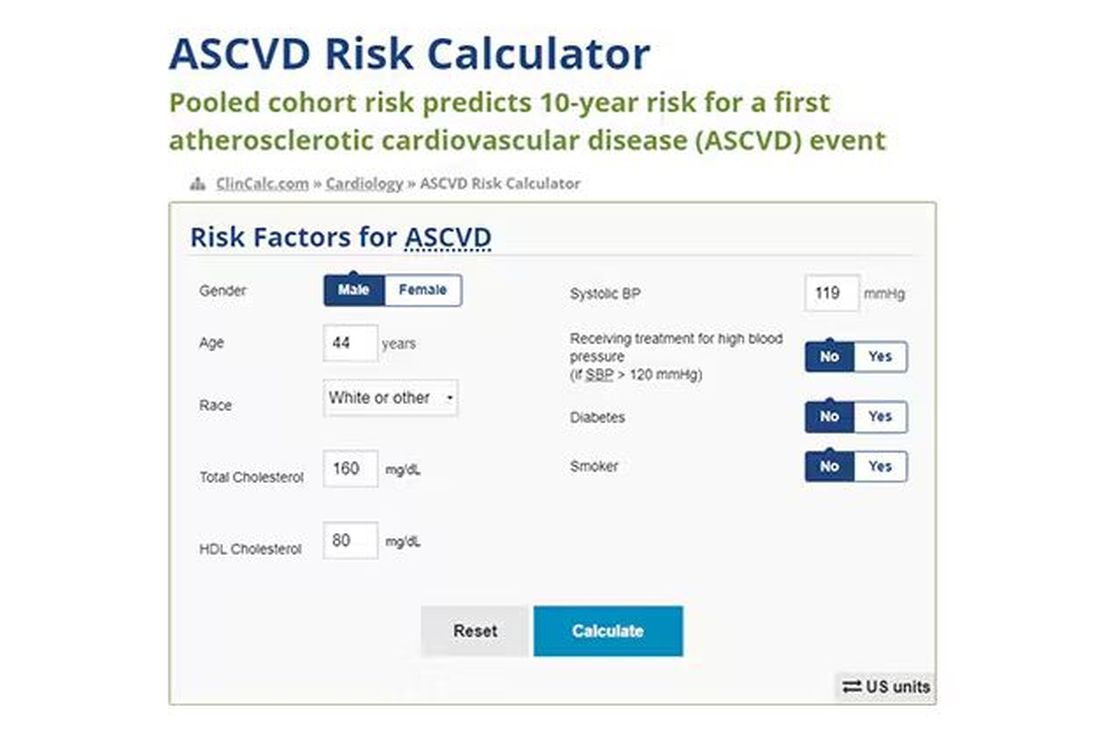
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.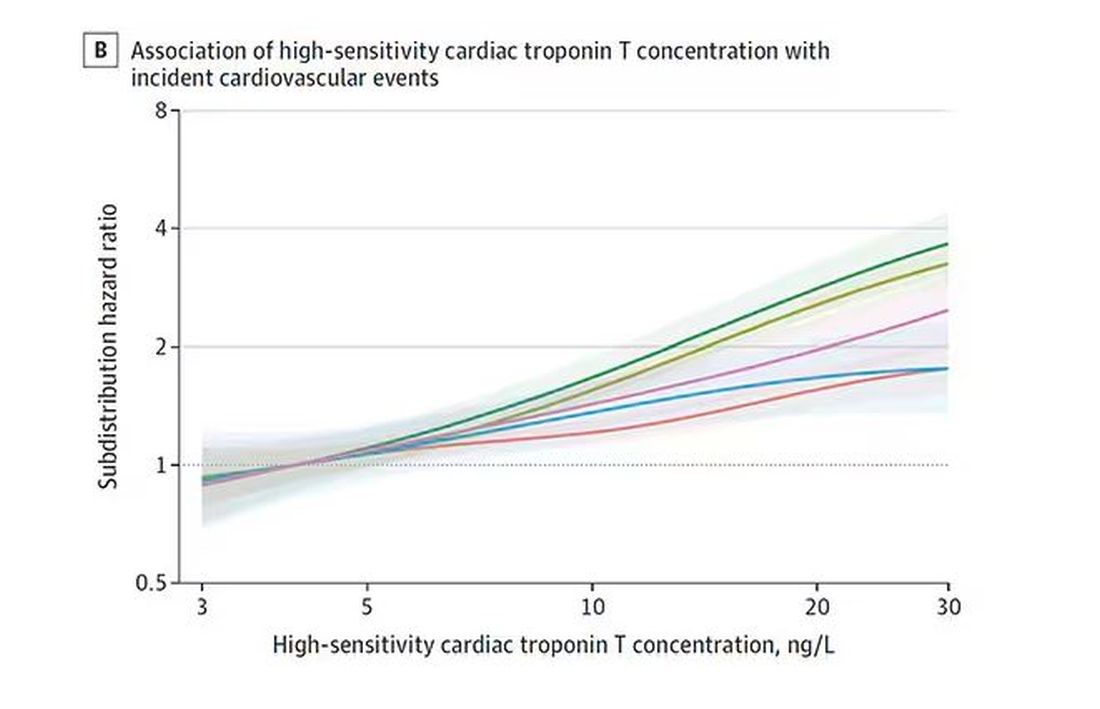
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.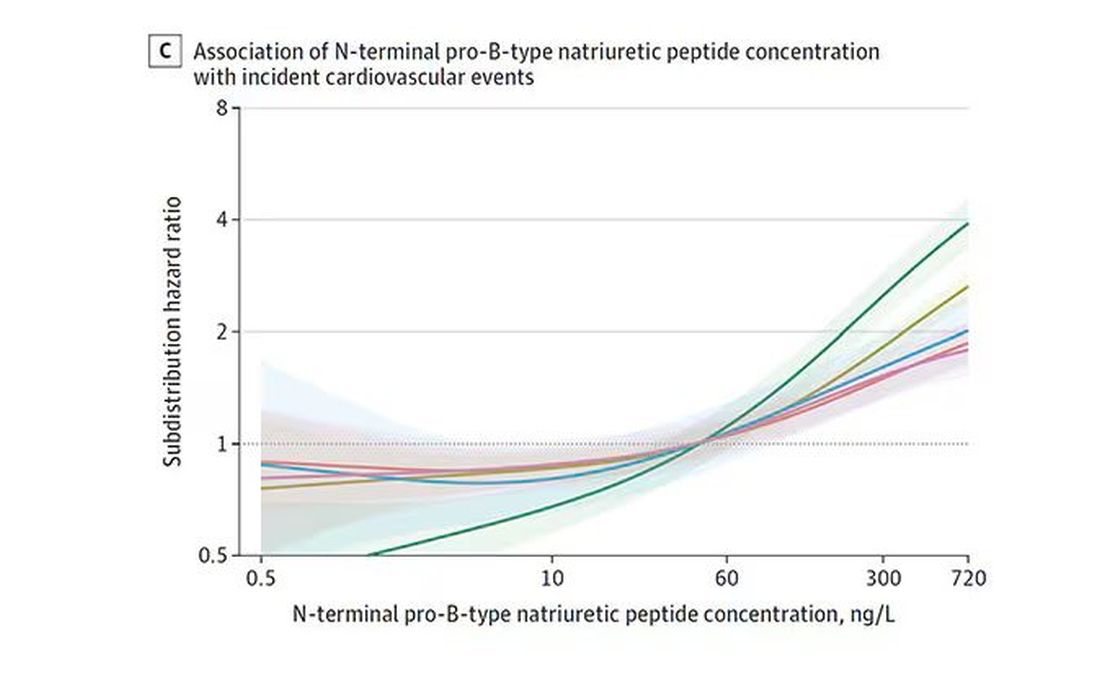
And CRP does a similar thing, with levels above 1.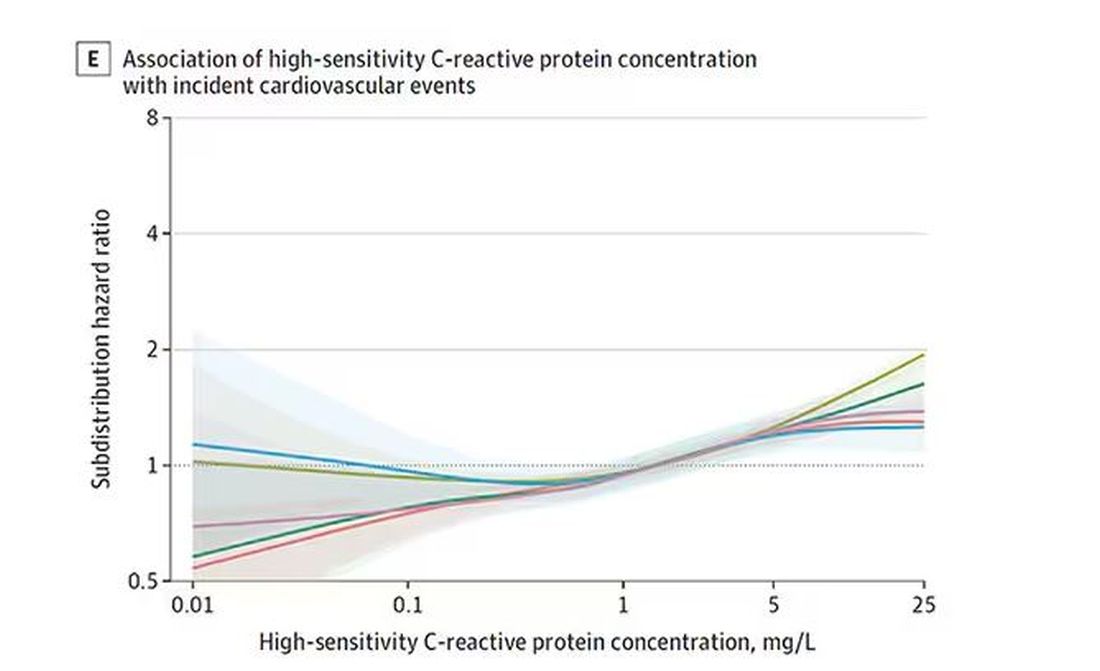
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.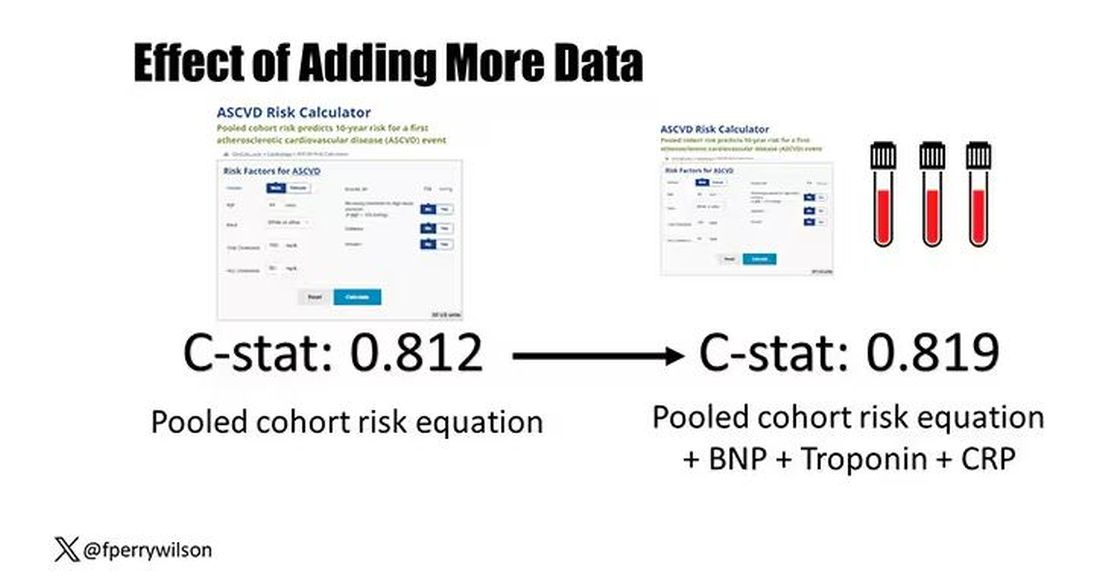
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Why Incorporating Obstetric History Matters for CVD Risk Management in Autoimmune Diseases
NEW YORK — Systemic autoimmune disease is well-recognized as a major risk factor for cardiovascular disease (CVD), but less recognized as a cardiovascular risk factor is a history of pregnancy complications, including preeclampsia, and cardiologists and rheumatologists need to include an obstetric history when managing patients with autoimmune diseases, a specialist in reproductive health in rheumatology told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
“Autoimmune diseases, lupus in particular, increase the risk for both cardiovascular disease and maternal placental syndromes,” Lisa R. Sammaritano, MD, a professor at Hospital for Special Surgery in New York City and a specialist in reproductive health issues in rheumatology patients, told attendees. “For those patients who have complications during pregnancy, it further increases their already increased risk for later cardiovascular disease.”
CVD Risk Double Whammy
A history of systemic lupus erythematosus (SLE) and problematic pregnancy can be a double whammy for CVD risk. Dr. Sammaritano cited a 2022 meta-analysis that showed patients with SLE had a 2.5 times greater risk for stroke and almost three times greater risk for myocardial infarction than people without SLE.
Maternal placental syndromes include pregnancy loss, restricted fetal growth, preeclampsia, premature membrane rupture, placental abruption, and intrauterine fetal demise, Dr. Sammaritano said. Hypertensive disorders of pregnancy, formerly called adverse pregnancy outcomes, she noted, include gestational hypertension, preeclampsia, and eclampsia.
Pregnancy complications can have an adverse effect on the mother’s postpartum cardiovascular health, Dr. Sammaritano noted, a fact borne out by the cardiovascular health after maternal placental syndromes population-based retrospective cohort study and a 2007 meta-analysis that found a history of preeclampsia doubles the risk for venous thromboembolism, stroke, and ischemic heart disease up to 15 years after pregnancy.
“It is always important to obtain a reproductive health history from patients with autoimmune diseases,” Dr. Sammaritano told this news organization in an interview. “This is an integral part of any medical history. In the usual setting, this includes not only pregnancy history but also use of contraception in reproductive-aged women. Unplanned pregnancy can lead to adverse outcomes in the setting of active or severe autoimmune disease or when teratogenic medications are used.”
Pregnancy history can be a factor in a woman’s cardiovascular health more than 15 years postpartum, even if a woman is no longer planning a pregnancy or is menopausal. “As such, this history is important in assessing every woman’s risk profile for CVD in addition to usual traditional risk factors,” Dr. Sammaritano said.
“It is even more important for women with autoimmune disorders, who have been shown to have an already increased risk for CVD independent of their pregnancy history, likely related to a chronic inflammatory state and other autoimmune-related factors such as presence of antiphospholipid antibodies [aPL] or use of corticosteroids.”
Timing of disease onset is also an issue, she said. “In patients with SLE, for example, onset of CVD is much earlier than in the general population,” Dr. Sammaritano said. “As a result, these patients should likely be assessed for risk — both traditional and other risk factors — earlier than the general population, especially if an adverse obstetric history is present.”
At the younger end of the age continuum, women with autoimmune disease, including SLE and antiphospholipid syndrome, who are pregnant should be put on guideline-directed low-dose aspirin preeclampsia prophylaxis, Dr. Sammaritano said. “Whether every patient with SLE needs this is still uncertain, but certainly, those with a history of renal disease, hypertension, or aPL antibody clearly do,” she added.
The evidence supporting hydroxychloroquine (HCQ) in these patients is controversial, but Dr. Sammaritano noted two meta-analyses, one in 2022 and the other in 2023, that showed that HCQ lowered the risk for preeclampsia in women.
“The clear benefit of HCQ in preventing maternal disease complications, including flare, means we recommend it regardless for all patients with SLE at baseline and during pregnancy [if tolerated],” Dr. Sammaritano said. “The benefit or optimal use of these medications in other autoimmune diseases is less studied and less certain.”
Dr. Sammaritano added in her presentation, “We really need better therapies and, hopefully, those will be on the way, but I think the takeaway message, particularly for practicing rheumatologists and cardiologists, is to ask the question about obstetric history. Many of us don’t. It doesn’t seem relevant in the moment, but it really is in terms of the patient’s long-term risk for cardiovascular disease.”
The Case for Treatment During Pregnancy
Prophylaxis against pregnancy complications in patients with autoimmune disease may be achievable, Taryn Youngstein, MBBS, consultant rheumatologist and codirector of the Centre of Excellence in Vasculitis Research, Imperial College London, London, England, told this news organization after Dr. Sammaritano’s presentation. At the 2023 American College of Rheumatology Annual Meeting, her group reported the safety and effectiveness of continuing tocilizumab in pregnant women with Takayasu arteritis, a large-vessel vasculitis predominantly affecting women of reproductive age.
“What traditionally happens is you would stop the biologic particularly before the third trimester because of safety and concerns that the monoclonal antibody is actively transported across the placenta, which means the baby gets much more concentration of the drug than the mum,” Dr. Youngstein said.
It’s a situation physicians must monitor closely, she said. “The mum is donating their immune system to the baby, but they’re also donating drug.”
“In high-risk patients, we would share decision-making with the patient,” Dr. Youngstein continued. “We have decided it’s too high of a risk for us to stop the drug, so we have been continuing the interleukin-6 [IL-6] inhibitor throughout the entire pregnancy.”
The data from Dr. Youngstein’s group showed that pregnant women with Takayasu arteritis who continued IL-6 inhibition therapy all carried to term with healthy births.
“We’ve shown that it’s relatively safe to do that, but you have to be very careful in monitoring the baby,” she said. This includes not giving the infant any live vaccines at birth because it will have the high levels of IL-6 inhibition, she said.
Dr. Sammaritano and Dr. Youngstein had no relevant financial relationships to disclose.
A version of this article appeared on Medscape.com.
NEW YORK — Systemic autoimmune disease is well-recognized as a major risk factor for cardiovascular disease (CVD), but less recognized as a cardiovascular risk factor is a history of pregnancy complications, including preeclampsia, and cardiologists and rheumatologists need to include an obstetric history when managing patients with autoimmune diseases, a specialist in reproductive health in rheumatology told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
“Autoimmune diseases, lupus in particular, increase the risk for both cardiovascular disease and maternal placental syndromes,” Lisa R. Sammaritano, MD, a professor at Hospital for Special Surgery in New York City and a specialist in reproductive health issues in rheumatology patients, told attendees. “For those patients who have complications during pregnancy, it further increases their already increased risk for later cardiovascular disease.”
CVD Risk Double Whammy
A history of systemic lupus erythematosus (SLE) and problematic pregnancy can be a double whammy for CVD risk. Dr. Sammaritano cited a 2022 meta-analysis that showed patients with SLE had a 2.5 times greater risk for stroke and almost three times greater risk for myocardial infarction than people without SLE.
Maternal placental syndromes include pregnancy loss, restricted fetal growth, preeclampsia, premature membrane rupture, placental abruption, and intrauterine fetal demise, Dr. Sammaritano said. Hypertensive disorders of pregnancy, formerly called adverse pregnancy outcomes, she noted, include gestational hypertension, preeclampsia, and eclampsia.
Pregnancy complications can have an adverse effect on the mother’s postpartum cardiovascular health, Dr. Sammaritano noted, a fact borne out by the cardiovascular health after maternal placental syndromes population-based retrospective cohort study and a 2007 meta-analysis that found a history of preeclampsia doubles the risk for venous thromboembolism, stroke, and ischemic heart disease up to 15 years after pregnancy.
“It is always important to obtain a reproductive health history from patients with autoimmune diseases,” Dr. Sammaritano told this news organization in an interview. “This is an integral part of any medical history. In the usual setting, this includes not only pregnancy history but also use of contraception in reproductive-aged women. Unplanned pregnancy can lead to adverse outcomes in the setting of active or severe autoimmune disease or when teratogenic medications are used.”
Pregnancy history can be a factor in a woman’s cardiovascular health more than 15 years postpartum, even if a woman is no longer planning a pregnancy or is menopausal. “As such, this history is important in assessing every woman’s risk profile for CVD in addition to usual traditional risk factors,” Dr. Sammaritano said.
“It is even more important for women with autoimmune disorders, who have been shown to have an already increased risk for CVD independent of their pregnancy history, likely related to a chronic inflammatory state and other autoimmune-related factors such as presence of antiphospholipid antibodies [aPL] or use of corticosteroids.”
Timing of disease onset is also an issue, she said. “In patients with SLE, for example, onset of CVD is much earlier than in the general population,” Dr. Sammaritano said. “As a result, these patients should likely be assessed for risk — both traditional and other risk factors — earlier than the general population, especially if an adverse obstetric history is present.”
At the younger end of the age continuum, women with autoimmune disease, including SLE and antiphospholipid syndrome, who are pregnant should be put on guideline-directed low-dose aspirin preeclampsia prophylaxis, Dr. Sammaritano said. “Whether every patient with SLE needs this is still uncertain, but certainly, those with a history of renal disease, hypertension, or aPL antibody clearly do,” she added.
The evidence supporting hydroxychloroquine (HCQ) in these patients is controversial, but Dr. Sammaritano noted two meta-analyses, one in 2022 and the other in 2023, that showed that HCQ lowered the risk for preeclampsia in women.
“The clear benefit of HCQ in preventing maternal disease complications, including flare, means we recommend it regardless for all patients with SLE at baseline and during pregnancy [if tolerated],” Dr. Sammaritano said. “The benefit or optimal use of these medications in other autoimmune diseases is less studied and less certain.”
Dr. Sammaritano added in her presentation, “We really need better therapies and, hopefully, those will be on the way, but I think the takeaway message, particularly for practicing rheumatologists and cardiologists, is to ask the question about obstetric history. Many of us don’t. It doesn’t seem relevant in the moment, but it really is in terms of the patient’s long-term risk for cardiovascular disease.”
The Case for Treatment During Pregnancy
Prophylaxis against pregnancy complications in patients with autoimmune disease may be achievable, Taryn Youngstein, MBBS, consultant rheumatologist and codirector of the Centre of Excellence in Vasculitis Research, Imperial College London, London, England, told this news organization after Dr. Sammaritano’s presentation. At the 2023 American College of Rheumatology Annual Meeting, her group reported the safety and effectiveness of continuing tocilizumab in pregnant women with Takayasu arteritis, a large-vessel vasculitis predominantly affecting women of reproductive age.
“What traditionally happens is you would stop the biologic particularly before the third trimester because of safety and concerns that the monoclonal antibody is actively transported across the placenta, which means the baby gets much more concentration of the drug than the mum,” Dr. Youngstein said.
It’s a situation physicians must monitor closely, she said. “The mum is donating their immune system to the baby, but they’re also donating drug.”
“In high-risk patients, we would share decision-making with the patient,” Dr. Youngstein continued. “We have decided it’s too high of a risk for us to stop the drug, so we have been continuing the interleukin-6 [IL-6] inhibitor throughout the entire pregnancy.”
The data from Dr. Youngstein’s group showed that pregnant women with Takayasu arteritis who continued IL-6 inhibition therapy all carried to term with healthy births.
“We’ve shown that it’s relatively safe to do that, but you have to be very careful in monitoring the baby,” she said. This includes not giving the infant any live vaccines at birth because it will have the high levels of IL-6 inhibition, she said.
Dr. Sammaritano and Dr. Youngstein had no relevant financial relationships to disclose.
A version of this article appeared on Medscape.com.
NEW YORK — Systemic autoimmune disease is well-recognized as a major risk factor for cardiovascular disease (CVD), but less recognized as a cardiovascular risk factor is a history of pregnancy complications, including preeclampsia, and cardiologists and rheumatologists need to include an obstetric history when managing patients with autoimmune diseases, a specialist in reproductive health in rheumatology told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
“Autoimmune diseases, lupus in particular, increase the risk for both cardiovascular disease and maternal placental syndromes,” Lisa R. Sammaritano, MD, a professor at Hospital for Special Surgery in New York City and a specialist in reproductive health issues in rheumatology patients, told attendees. “For those patients who have complications during pregnancy, it further increases their already increased risk for later cardiovascular disease.”
CVD Risk Double Whammy
A history of systemic lupus erythematosus (SLE) and problematic pregnancy can be a double whammy for CVD risk. Dr. Sammaritano cited a 2022 meta-analysis that showed patients with SLE had a 2.5 times greater risk for stroke and almost three times greater risk for myocardial infarction than people without SLE.
Maternal placental syndromes include pregnancy loss, restricted fetal growth, preeclampsia, premature membrane rupture, placental abruption, and intrauterine fetal demise, Dr. Sammaritano said. Hypertensive disorders of pregnancy, formerly called adverse pregnancy outcomes, she noted, include gestational hypertension, preeclampsia, and eclampsia.
Pregnancy complications can have an adverse effect on the mother’s postpartum cardiovascular health, Dr. Sammaritano noted, a fact borne out by the cardiovascular health after maternal placental syndromes population-based retrospective cohort study and a 2007 meta-analysis that found a history of preeclampsia doubles the risk for venous thromboembolism, stroke, and ischemic heart disease up to 15 years after pregnancy.
“It is always important to obtain a reproductive health history from patients with autoimmune diseases,” Dr. Sammaritano told this news organization in an interview. “This is an integral part of any medical history. In the usual setting, this includes not only pregnancy history but also use of contraception in reproductive-aged women. Unplanned pregnancy can lead to adverse outcomes in the setting of active or severe autoimmune disease or when teratogenic medications are used.”
Pregnancy history can be a factor in a woman’s cardiovascular health more than 15 years postpartum, even if a woman is no longer planning a pregnancy or is menopausal. “As such, this history is important in assessing every woman’s risk profile for CVD in addition to usual traditional risk factors,” Dr. Sammaritano said.
“It is even more important for women with autoimmune disorders, who have been shown to have an already increased risk for CVD independent of their pregnancy history, likely related to a chronic inflammatory state and other autoimmune-related factors such as presence of antiphospholipid antibodies [aPL] or use of corticosteroids.”
Timing of disease onset is also an issue, she said. “In patients with SLE, for example, onset of CVD is much earlier than in the general population,” Dr. Sammaritano said. “As a result, these patients should likely be assessed for risk — both traditional and other risk factors — earlier than the general population, especially if an adverse obstetric history is present.”
At the younger end of the age continuum, women with autoimmune disease, including SLE and antiphospholipid syndrome, who are pregnant should be put on guideline-directed low-dose aspirin preeclampsia prophylaxis, Dr. Sammaritano said. “Whether every patient with SLE needs this is still uncertain, but certainly, those with a history of renal disease, hypertension, or aPL antibody clearly do,” she added.
The evidence supporting hydroxychloroquine (HCQ) in these patients is controversial, but Dr. Sammaritano noted two meta-analyses, one in 2022 and the other in 2023, that showed that HCQ lowered the risk for preeclampsia in women.
“The clear benefit of HCQ in preventing maternal disease complications, including flare, means we recommend it regardless for all patients with SLE at baseline and during pregnancy [if tolerated],” Dr. Sammaritano said. “The benefit or optimal use of these medications in other autoimmune diseases is less studied and less certain.”
Dr. Sammaritano added in her presentation, “We really need better therapies and, hopefully, those will be on the way, but I think the takeaway message, particularly for practicing rheumatologists and cardiologists, is to ask the question about obstetric history. Many of us don’t. It doesn’t seem relevant in the moment, but it really is in terms of the patient’s long-term risk for cardiovascular disease.”
The Case for Treatment During Pregnancy
Prophylaxis against pregnancy complications in patients with autoimmune disease may be achievable, Taryn Youngstein, MBBS, consultant rheumatologist and codirector of the Centre of Excellence in Vasculitis Research, Imperial College London, London, England, told this news organization after Dr. Sammaritano’s presentation. At the 2023 American College of Rheumatology Annual Meeting, her group reported the safety and effectiveness of continuing tocilizumab in pregnant women with Takayasu arteritis, a large-vessel vasculitis predominantly affecting women of reproductive age.
“What traditionally happens is you would stop the biologic particularly before the third trimester because of safety and concerns that the monoclonal antibody is actively transported across the placenta, which means the baby gets much more concentration of the drug than the mum,” Dr. Youngstein said.
It’s a situation physicians must monitor closely, she said. “The mum is donating their immune system to the baby, but they’re also donating drug.”
“In high-risk patients, we would share decision-making with the patient,” Dr. Youngstein continued. “We have decided it’s too high of a risk for us to stop the drug, so we have been continuing the interleukin-6 [IL-6] inhibitor throughout the entire pregnancy.”
The data from Dr. Youngstein’s group showed that pregnant women with Takayasu arteritis who continued IL-6 inhibition therapy all carried to term with healthy births.
“We’ve shown that it’s relatively safe to do that, but you have to be very careful in monitoring the baby,” she said. This includes not giving the infant any live vaccines at birth because it will have the high levels of IL-6 inhibition, she said.
Dr. Sammaritano and Dr. Youngstein had no relevant financial relationships to disclose.
A version of this article appeared on Medscape.com.

