User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Fauci: Omicron ‘very different from other variants’
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
FDA panel backs first pill for COVID-19 by a small margin
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
Could an oral PCSK9 inhibitor be on the horizon?
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Merck’s COVID-19 pill may be less effective than first hoped
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
Did prior authorization refusals lead to this patient’s death?
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Does vitamin D benefit only those who are deficient?
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
U.S. obesity rates soar in early adulthood
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.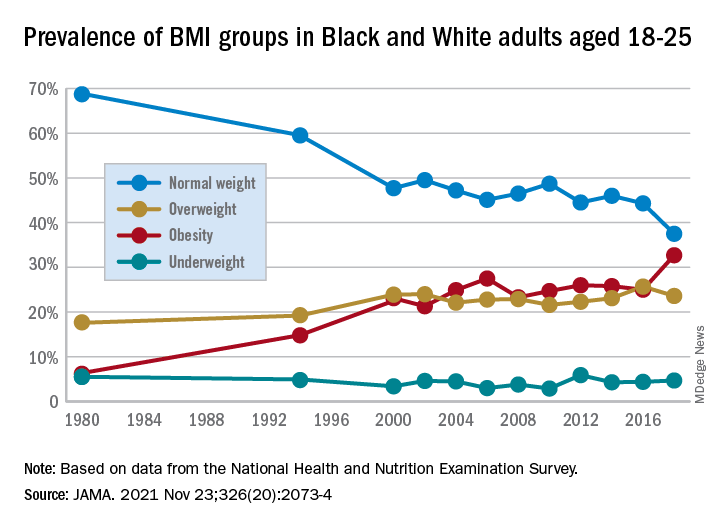
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hypertension may double the risk of late-onset epilepsy
new research suggests.
After excluding individuals with normal blood pressure who were taking antihypertensive medication, investigators found hypertension was linked to an almost 2.5-fold higher risk of epilepsy.
“Our findings further expand upon our knowledge of the negative effects hypertension has on brain health and, regarding epilepsy, that effect may be starting even in midlife,” said co–lead author Maria Stefanidou, MD, MSc, of Boston University.
“Practicing clinicians should be vigilant to diagnose hypertension, discuss with patients all potential long-term brain health outcomes, and need for treatment. Furthermore, in those presenting with new-onset epilepsy later in life, screening for potentially undiagnosed hypertension should be included in the initial workup,” she said.
The study was published online Nov. 17, 2021, in Epilepsia.
Unknown etiology
“New-onset epilepsy risk increases with increasing age over the age of 65 and can affect 15-20 per 1,000 older individuals. Although the most common causes for seizures in this age group are prior history of stroke and presence of dementia, for about 30%-40% of patients, the etiology of seizures remains unknown,” Dr. Stefanidou said.
“We wanted to study if modifiable vascular risk factors that are known to contribute both to vascular brain aging and to neurodegeneration may directly predict the development of epilepsy, even in the absence of clinical stroke or dementia,” she added.
To investigate, the researchers turned to data from participants in the Offspring Cohort of the Framingham Health Study (FHS). The original FHS was an ongoing longitudinal community-based study that first began in 1948. Offspring of the original cohort and their spouses (n = 5,124) were enrolled in the Offspring Cohort in 1971, with surveillance of these second-generation participants based on exam visits occurring every 4 years.
The study included participants who had attended exam 5 (1991-1995), were age 45 years or older, had available vascular risk factor (VRF) data, and available follow-up data on epilepsy status (n = 2,986; mean age, 58 years; 48% male).
The investigators conducted two statistical analyses. In the primary model, they adjusted for age and gender, while in a secondary model they also adjusted for prevalent and interim stroke. They also conducted an analysis that excluded participants treated with antihypertensive medication and had normal blood pressure.
Plausible mechanisms
During a mean follow-up of 19.2 years, 55 incident epilepsy cases were identified. The mean age of these patients was 73.8 years.
In the primary model, hypertension was associated with an almost twofold higher risk of developing epilepsy (hazard ratio, 1.97; 95% confidence interval, 1.13-3.45; P = .017).
Interestingly, the Framingham Stroke Risk Profile – a calculation based on an array of factors, including age/sex, systolic blood pressure, antihypertensive therapy, diabetes, history of cardiovascular disease, atrial fibrillation, and cigarette smoking – was not associated with incident epilepsy, and there was no other significant associated between any of the other VRFs when looked at independently.
When the researchers adjusted for prevalent and interim stroke, they continued to find an almost twofold higher risk of developing epilepsy (HR 1.93; 95% CI, 1.10-3.37; P = .022). An analysis that adjusted for competing risk of death obtained similar findings (HR, 1.98; 95% CI, 1.03-3.81; P = .042).
The model that excluded patients receiving antihypertensive treatment, whose blood pressure readings were normal (n = 2,162; 50 incident epilepsy cases) showed an even stronger association (HR, 2.44; 95% CI, 1.36-4.35; P = .003).
“Our results are based on an epidemiological, observational study, therefore our findings point to an association between hypertension and new-onset epilepsy later in life,” said Dr. Stefanidou.
She noted that because it was an observational study, “a cause-effect relationship cannot be established based on these results, but there is growing evidence from our, as well as other, similar cohorts that hypertension, a modifiable vascular risk factor, may indeed be an independent predictor of late-onset epilepsy.”
There are “plausible mechanisms” that support both a direct, and indirect, role of hypertension – for example, through accumulation of small vessel disease in the brain – but further research will be necessary to elucidate the exact mechanisms involved in the process,” she added.
‘Welcome addition’
In a joint comment, Hedley C.A. Emsley, PhD, professor of clinical neuroscience, Lancaster (England) University, and Jasmine Wall, MBBChir, academic clinical fellow in neurology, Lancaster University, described the study as a “welcome addition to this field,” noting that the Framingham Heart Study “lends itself well to an embedded observational study of this nature of late-onset epilepsy.”
Dr. Emsley and Dr. Wall, who were not involved in the research, said that the “apparent magnitude of increased late-onset epilepsy risk association with hypertension in the Stefanidou et al study is quite striking,” even allowing for the “relatively small sample size,” since their analysis and findings appear to “withstand exclusion of individuals who became normotensive on antihypertensive treatment.”
They noted that in recent years there has been a growing body of evidence highlighting the importance of hypertension in late-onset epilepsy epileptogenesis with subclinical cerebrovascular diseases, including “otherwise occult cerebral small vessel disease believed to be a frequent cause.”
The mechanisms “remain unclear,” but they could potentially include diffuse cerebral microangiopathy, structural and physiological changes, and/or blood-brain barrier dysfunction and leakage, they suggested.
“Although there is no current consensus over an age threshold that defines ‘late onset,’ we would argue that age thresholds used in such studies of late-onset epilepsy should be lower, to avoid missing younger adults at risk through vascular mechanisms,” Dr. Emsley and Dr. Wall added.
The study authors suggest that “potential pathophysiologic mechanisms can further be explored in future experimental studies and clinical trials.”
This study was funded by grants from the National Institutes of Health and Finding a Cure for Epilepsy/Seizures. Dr. Stefanidou disclosed relevant financial relationships. Dr. Emsley and Dr. Wall disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
After excluding individuals with normal blood pressure who were taking antihypertensive medication, investigators found hypertension was linked to an almost 2.5-fold higher risk of epilepsy.
“Our findings further expand upon our knowledge of the negative effects hypertension has on brain health and, regarding epilepsy, that effect may be starting even in midlife,” said co–lead author Maria Stefanidou, MD, MSc, of Boston University.
“Practicing clinicians should be vigilant to diagnose hypertension, discuss with patients all potential long-term brain health outcomes, and need for treatment. Furthermore, in those presenting with new-onset epilepsy later in life, screening for potentially undiagnosed hypertension should be included in the initial workup,” she said.
The study was published online Nov. 17, 2021, in Epilepsia.
Unknown etiology
“New-onset epilepsy risk increases with increasing age over the age of 65 and can affect 15-20 per 1,000 older individuals. Although the most common causes for seizures in this age group are prior history of stroke and presence of dementia, for about 30%-40% of patients, the etiology of seizures remains unknown,” Dr. Stefanidou said.
“We wanted to study if modifiable vascular risk factors that are known to contribute both to vascular brain aging and to neurodegeneration may directly predict the development of epilepsy, even in the absence of clinical stroke or dementia,” she added.
To investigate, the researchers turned to data from participants in the Offspring Cohort of the Framingham Health Study (FHS). The original FHS was an ongoing longitudinal community-based study that first began in 1948. Offspring of the original cohort and their spouses (n = 5,124) were enrolled in the Offspring Cohort in 1971, with surveillance of these second-generation participants based on exam visits occurring every 4 years.
The study included participants who had attended exam 5 (1991-1995), were age 45 years or older, had available vascular risk factor (VRF) data, and available follow-up data on epilepsy status (n = 2,986; mean age, 58 years; 48% male).
The investigators conducted two statistical analyses. In the primary model, they adjusted for age and gender, while in a secondary model they also adjusted for prevalent and interim stroke. They also conducted an analysis that excluded participants treated with antihypertensive medication and had normal blood pressure.
Plausible mechanisms
During a mean follow-up of 19.2 years, 55 incident epilepsy cases were identified. The mean age of these patients was 73.8 years.
In the primary model, hypertension was associated with an almost twofold higher risk of developing epilepsy (hazard ratio, 1.97; 95% confidence interval, 1.13-3.45; P = .017).
Interestingly, the Framingham Stroke Risk Profile – a calculation based on an array of factors, including age/sex, systolic blood pressure, antihypertensive therapy, diabetes, history of cardiovascular disease, atrial fibrillation, and cigarette smoking – was not associated with incident epilepsy, and there was no other significant associated between any of the other VRFs when looked at independently.
When the researchers adjusted for prevalent and interim stroke, they continued to find an almost twofold higher risk of developing epilepsy (HR 1.93; 95% CI, 1.10-3.37; P = .022). An analysis that adjusted for competing risk of death obtained similar findings (HR, 1.98; 95% CI, 1.03-3.81; P = .042).
The model that excluded patients receiving antihypertensive treatment, whose blood pressure readings were normal (n = 2,162; 50 incident epilepsy cases) showed an even stronger association (HR, 2.44; 95% CI, 1.36-4.35; P = .003).
“Our results are based on an epidemiological, observational study, therefore our findings point to an association between hypertension and new-onset epilepsy later in life,” said Dr. Stefanidou.
She noted that because it was an observational study, “a cause-effect relationship cannot be established based on these results, but there is growing evidence from our, as well as other, similar cohorts that hypertension, a modifiable vascular risk factor, may indeed be an independent predictor of late-onset epilepsy.”
There are “plausible mechanisms” that support both a direct, and indirect, role of hypertension – for example, through accumulation of small vessel disease in the brain – but further research will be necessary to elucidate the exact mechanisms involved in the process,” she added.
‘Welcome addition’
In a joint comment, Hedley C.A. Emsley, PhD, professor of clinical neuroscience, Lancaster (England) University, and Jasmine Wall, MBBChir, academic clinical fellow in neurology, Lancaster University, described the study as a “welcome addition to this field,” noting that the Framingham Heart Study “lends itself well to an embedded observational study of this nature of late-onset epilepsy.”
Dr. Emsley and Dr. Wall, who were not involved in the research, said that the “apparent magnitude of increased late-onset epilepsy risk association with hypertension in the Stefanidou et al study is quite striking,” even allowing for the “relatively small sample size,” since their analysis and findings appear to “withstand exclusion of individuals who became normotensive on antihypertensive treatment.”
They noted that in recent years there has been a growing body of evidence highlighting the importance of hypertension in late-onset epilepsy epileptogenesis with subclinical cerebrovascular diseases, including “otherwise occult cerebral small vessel disease believed to be a frequent cause.”
The mechanisms “remain unclear,” but they could potentially include diffuse cerebral microangiopathy, structural and physiological changes, and/or blood-brain barrier dysfunction and leakage, they suggested.
“Although there is no current consensus over an age threshold that defines ‘late onset,’ we would argue that age thresholds used in such studies of late-onset epilepsy should be lower, to avoid missing younger adults at risk through vascular mechanisms,” Dr. Emsley and Dr. Wall added.
The study authors suggest that “potential pathophysiologic mechanisms can further be explored in future experimental studies and clinical trials.”
This study was funded by grants from the National Institutes of Health and Finding a Cure for Epilepsy/Seizures. Dr. Stefanidou disclosed relevant financial relationships. Dr. Emsley and Dr. Wall disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
After excluding individuals with normal blood pressure who were taking antihypertensive medication, investigators found hypertension was linked to an almost 2.5-fold higher risk of epilepsy.
“Our findings further expand upon our knowledge of the negative effects hypertension has on brain health and, regarding epilepsy, that effect may be starting even in midlife,” said co–lead author Maria Stefanidou, MD, MSc, of Boston University.
“Practicing clinicians should be vigilant to diagnose hypertension, discuss with patients all potential long-term brain health outcomes, and need for treatment. Furthermore, in those presenting with new-onset epilepsy later in life, screening for potentially undiagnosed hypertension should be included in the initial workup,” she said.
The study was published online Nov. 17, 2021, in Epilepsia.
Unknown etiology
“New-onset epilepsy risk increases with increasing age over the age of 65 and can affect 15-20 per 1,000 older individuals. Although the most common causes for seizures in this age group are prior history of stroke and presence of dementia, for about 30%-40% of patients, the etiology of seizures remains unknown,” Dr. Stefanidou said.
“We wanted to study if modifiable vascular risk factors that are known to contribute both to vascular brain aging and to neurodegeneration may directly predict the development of epilepsy, even in the absence of clinical stroke or dementia,” she added.
To investigate, the researchers turned to data from participants in the Offspring Cohort of the Framingham Health Study (FHS). The original FHS was an ongoing longitudinal community-based study that first began in 1948. Offspring of the original cohort and their spouses (n = 5,124) were enrolled in the Offspring Cohort in 1971, with surveillance of these second-generation participants based on exam visits occurring every 4 years.
The study included participants who had attended exam 5 (1991-1995), were age 45 years or older, had available vascular risk factor (VRF) data, and available follow-up data on epilepsy status (n = 2,986; mean age, 58 years; 48% male).
The investigators conducted two statistical analyses. In the primary model, they adjusted for age and gender, while in a secondary model they also adjusted for prevalent and interim stroke. They also conducted an analysis that excluded participants treated with antihypertensive medication and had normal blood pressure.
Plausible mechanisms
During a mean follow-up of 19.2 years, 55 incident epilepsy cases were identified. The mean age of these patients was 73.8 years.
In the primary model, hypertension was associated with an almost twofold higher risk of developing epilepsy (hazard ratio, 1.97; 95% confidence interval, 1.13-3.45; P = .017).
Interestingly, the Framingham Stroke Risk Profile – a calculation based on an array of factors, including age/sex, systolic blood pressure, antihypertensive therapy, diabetes, history of cardiovascular disease, atrial fibrillation, and cigarette smoking – was not associated with incident epilepsy, and there was no other significant associated between any of the other VRFs when looked at independently.
When the researchers adjusted for prevalent and interim stroke, they continued to find an almost twofold higher risk of developing epilepsy (HR 1.93; 95% CI, 1.10-3.37; P = .022). An analysis that adjusted for competing risk of death obtained similar findings (HR, 1.98; 95% CI, 1.03-3.81; P = .042).
The model that excluded patients receiving antihypertensive treatment, whose blood pressure readings were normal (n = 2,162; 50 incident epilepsy cases) showed an even stronger association (HR, 2.44; 95% CI, 1.36-4.35; P = .003).
“Our results are based on an epidemiological, observational study, therefore our findings point to an association between hypertension and new-onset epilepsy later in life,” said Dr. Stefanidou.
She noted that because it was an observational study, “a cause-effect relationship cannot be established based on these results, but there is growing evidence from our, as well as other, similar cohorts that hypertension, a modifiable vascular risk factor, may indeed be an independent predictor of late-onset epilepsy.”
There are “plausible mechanisms” that support both a direct, and indirect, role of hypertension – for example, through accumulation of small vessel disease in the brain – but further research will be necessary to elucidate the exact mechanisms involved in the process,” she added.
‘Welcome addition’
In a joint comment, Hedley C.A. Emsley, PhD, professor of clinical neuroscience, Lancaster (England) University, and Jasmine Wall, MBBChir, academic clinical fellow in neurology, Lancaster University, described the study as a “welcome addition to this field,” noting that the Framingham Heart Study “lends itself well to an embedded observational study of this nature of late-onset epilepsy.”
Dr. Emsley and Dr. Wall, who were not involved in the research, said that the “apparent magnitude of increased late-onset epilepsy risk association with hypertension in the Stefanidou et al study is quite striking,” even allowing for the “relatively small sample size,” since their analysis and findings appear to “withstand exclusion of individuals who became normotensive on antihypertensive treatment.”
They noted that in recent years there has been a growing body of evidence highlighting the importance of hypertension in late-onset epilepsy epileptogenesis with subclinical cerebrovascular diseases, including “otherwise occult cerebral small vessel disease believed to be a frequent cause.”
The mechanisms “remain unclear,” but they could potentially include diffuse cerebral microangiopathy, structural and physiological changes, and/or blood-brain barrier dysfunction and leakage, they suggested.
“Although there is no current consensus over an age threshold that defines ‘late onset,’ we would argue that age thresholds used in such studies of late-onset epilepsy should be lower, to avoid missing younger adults at risk through vascular mechanisms,” Dr. Emsley and Dr. Wall added.
The study authors suggest that “potential pathophysiologic mechanisms can further be explored in future experimental studies and clinical trials.”
This study was funded by grants from the National Institutes of Health and Finding a Cure for Epilepsy/Seizures. Dr. Stefanidou disclosed relevant financial relationships. Dr. Emsley and Dr. Wall disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EPILEPSIA
Fueling an ‘already raging fire’: Fifth COVID surge approaches
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
Daily aspirin linked to increased risk of heart failure
Daily aspirin is associated with new onset heart failure independent of other risk factors, according to data derived from a database with follow-up from more than 30,000 patients who did not have HF when they were enrolled.
These data are not relevant to primary or secondary prevention of cardiovascular events but “refer only to starting aspirin for secondary prevention of HF in patients at high risk of HF or with symptomatic HF,” according to the senior investigator, Jan A. Staessen, MD, PhD, professor emeritus at the University of Leuven (Belgium).
In data from 30,827 patients at risk for HF enrolled in six observational studies, the hazard ratio (HR) for developing HF among those taking daily aspirin at baseline relative to those who were not was 1.26 (P ≤ .001) over 5.3 years of follow-up. In the 22,690 patients without a prior history cardiovascular disease (CVD), the HF risk increase for exposure to daily aspirin was about the same (HR 1.27; P = .001).
This study was launched because multiple conflicting studies have made the relationship between aspirin and HF risk unclear, according to the multinational team of authors, whose finding were published in ESC Heart Failure.
In principle, HF is recognized as a prothrombotic condition for which an antithrombotic therapy such as aspirin would be expected to have a protective role, but the investigators pointed out that the evidence is mixed. In a population-based Danish study of 12,277 patients with new-onset HF, for example, there was no relationship seen between aspirin use and a reduction in the composite outcome of all-cause mortality, myocardial infarction, or stroke.
Aspirin use linked to HF admissions
“Interestingly, this study reported that aspirin use was associated with an increased risk of readmissions for HF,” wrote the authors of the newly published data. “Uncertainty on aspirin use has been reflected in current guideline recommendations,” they added.
The population studied was drawn from the HOMAGE database, which has collated data on 46,437 participants in 21 studies. After the exclusion of studies with patients who already had HF as well as studies without information on HF incidence over time, six studies with 30,827 participants provided the basis for this analysis.
One study, ASCOT, which was randomized and blinded, served as the derivation data set. The remaining five studies, FLEMENGHO, HEALTH ABC, HULL LIFE LAB, PREDICTOR, and PROSPER, served as the validation data set.
In addition to identifying participants as aspirin users or nonusers at baseline, all of the studies had detailed baseline data on a wide variety of patient characteristics and risk factors, such as body mass index, blood cholesterol levels, blood glucose concentrations, blood pressure, and creatinine.
No patient in any trial was on an antithrombotic therapy other than aspirin at baseline.
Of the minority of patients with CVD at baseline, more than 80% had coronary heart disease. Only 2.8% of the total population had a prior myocardial infarction. In the study population overall, most (86%) had hypertension, and there was a sizeable proportion with diabetes (22%). The average age was 67 years, and 34% were women.
HF incidence on aspirin: 14.5/1000 person-years
Overall, the incidence rate of HF per 1,000 person-years for the entire population before adjustment was 14.5 in the group on daily aspirin versus 5.9 in the non-aspirin group. These absolute rates were lower in the discovery data set than in the validation set, but the relative differences in HF incidence rates for those who were versus those who were not on aspirin at baseline were similar.
Numerous sensitivity analyses supported the basic conclusions. This not only included one omitting patients with a history of CVD, but another that excluded patients who developed HF within the first 2 years. Stratified analyses looking for overall consistency across variables showed increased risk of new onset heart failure among those taking daily aspirin regardless of relative age, body weight, or blood pressure levels.
The most important limitation of this study was that it evaluated data taken from studies not originally designed to test the study hypothesis. In addition, only baseline data were available, so the drugs that patients took over the course of follow-up are unknown. However, the authors believe these data have a clinical message.
Given the consistency of these results, “our observations suggest that aspirin should be prescribed with caution in patients at risk of HF or having HF,” the investigators concluded.
“If such treatment is initiated in these patients, use low-dose aspirin,” Dr. Staessen told this news organization.
Aspirin for CVD versus HF risk
Many patients take low-dose aspirin to prevent the types of cardiovascular events, such as MI, that lead to heart failure. In attempting to address a controversy regarding aspirin and risk of new onset heart failure, it appears to create another regarding CVD risk reduction.
Deepak L. Bhatt, MD, executive director of Interventional Cardiovascular Programs at Brigham and Women’s Health, Boston, expressed some reluctance in applying these data to routine practice.
“It is important to emphasize that this pooled analysis draws upon six observational studies, not randomized trials of aspirin,” Dr. Bhatt said.
He called these findings “provocative,” but he said they “would need to be confirmed in databases of already completed randomized trials of aspirin versus a control before being actionable.” For Dr. Bhatt, one obstacle to a change in practice based on these data is that, “to my knowledge, no such signal [of a relationship between aspirin and incident heart failure] exists in the cumulative randomized data.”
Dr. Staessen reports no potential conflicts of interest for this study. Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PLx Pharma, for which he performs aspirin-related research.
Daily aspirin is associated with new onset heart failure independent of other risk factors, according to data derived from a database with follow-up from more than 30,000 patients who did not have HF when they were enrolled.
These data are not relevant to primary or secondary prevention of cardiovascular events but “refer only to starting aspirin for secondary prevention of HF in patients at high risk of HF or with symptomatic HF,” according to the senior investigator, Jan A. Staessen, MD, PhD, professor emeritus at the University of Leuven (Belgium).
In data from 30,827 patients at risk for HF enrolled in six observational studies, the hazard ratio (HR) for developing HF among those taking daily aspirin at baseline relative to those who were not was 1.26 (P ≤ .001) over 5.3 years of follow-up. In the 22,690 patients without a prior history cardiovascular disease (CVD), the HF risk increase for exposure to daily aspirin was about the same (HR 1.27; P = .001).
This study was launched because multiple conflicting studies have made the relationship between aspirin and HF risk unclear, according to the multinational team of authors, whose finding were published in ESC Heart Failure.
In principle, HF is recognized as a prothrombotic condition for which an antithrombotic therapy such as aspirin would be expected to have a protective role, but the investigators pointed out that the evidence is mixed. In a population-based Danish study of 12,277 patients with new-onset HF, for example, there was no relationship seen between aspirin use and a reduction in the composite outcome of all-cause mortality, myocardial infarction, or stroke.
Aspirin use linked to HF admissions
“Interestingly, this study reported that aspirin use was associated with an increased risk of readmissions for HF,” wrote the authors of the newly published data. “Uncertainty on aspirin use has been reflected in current guideline recommendations,” they added.
The population studied was drawn from the HOMAGE database, which has collated data on 46,437 participants in 21 studies. After the exclusion of studies with patients who already had HF as well as studies without information on HF incidence over time, six studies with 30,827 participants provided the basis for this analysis.
One study, ASCOT, which was randomized and blinded, served as the derivation data set. The remaining five studies, FLEMENGHO, HEALTH ABC, HULL LIFE LAB, PREDICTOR, and PROSPER, served as the validation data set.
In addition to identifying participants as aspirin users or nonusers at baseline, all of the studies had detailed baseline data on a wide variety of patient characteristics and risk factors, such as body mass index, blood cholesterol levels, blood glucose concentrations, blood pressure, and creatinine.
No patient in any trial was on an antithrombotic therapy other than aspirin at baseline.
Of the minority of patients with CVD at baseline, more than 80% had coronary heart disease. Only 2.8% of the total population had a prior myocardial infarction. In the study population overall, most (86%) had hypertension, and there was a sizeable proportion with diabetes (22%). The average age was 67 years, and 34% were women.
HF incidence on aspirin: 14.5/1000 person-years
Overall, the incidence rate of HF per 1,000 person-years for the entire population before adjustment was 14.5 in the group on daily aspirin versus 5.9 in the non-aspirin group. These absolute rates were lower in the discovery data set than in the validation set, but the relative differences in HF incidence rates for those who were versus those who were not on aspirin at baseline were similar.
Numerous sensitivity analyses supported the basic conclusions. This not only included one omitting patients with a history of CVD, but another that excluded patients who developed HF within the first 2 years. Stratified analyses looking for overall consistency across variables showed increased risk of new onset heart failure among those taking daily aspirin regardless of relative age, body weight, or blood pressure levels.
The most important limitation of this study was that it evaluated data taken from studies not originally designed to test the study hypothesis. In addition, only baseline data were available, so the drugs that patients took over the course of follow-up are unknown. However, the authors believe these data have a clinical message.
Given the consistency of these results, “our observations suggest that aspirin should be prescribed with caution in patients at risk of HF or having HF,” the investigators concluded.
“If such treatment is initiated in these patients, use low-dose aspirin,” Dr. Staessen told this news organization.
Aspirin for CVD versus HF risk
Many patients take low-dose aspirin to prevent the types of cardiovascular events, such as MI, that lead to heart failure. In attempting to address a controversy regarding aspirin and risk of new onset heart failure, it appears to create another regarding CVD risk reduction.
Deepak L. Bhatt, MD, executive director of Interventional Cardiovascular Programs at Brigham and Women’s Health, Boston, expressed some reluctance in applying these data to routine practice.
“It is important to emphasize that this pooled analysis draws upon six observational studies, not randomized trials of aspirin,” Dr. Bhatt said.
He called these findings “provocative,” but he said they “would need to be confirmed in databases of already completed randomized trials of aspirin versus a control before being actionable.” For Dr. Bhatt, one obstacle to a change in practice based on these data is that, “to my knowledge, no such signal [of a relationship between aspirin and incident heart failure] exists in the cumulative randomized data.”
Dr. Staessen reports no potential conflicts of interest for this study. Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PLx Pharma, for which he performs aspirin-related research.
Daily aspirin is associated with new onset heart failure independent of other risk factors, according to data derived from a database with follow-up from more than 30,000 patients who did not have HF when they were enrolled.
These data are not relevant to primary or secondary prevention of cardiovascular events but “refer only to starting aspirin for secondary prevention of HF in patients at high risk of HF or with symptomatic HF,” according to the senior investigator, Jan A. Staessen, MD, PhD, professor emeritus at the University of Leuven (Belgium).
In data from 30,827 patients at risk for HF enrolled in six observational studies, the hazard ratio (HR) for developing HF among those taking daily aspirin at baseline relative to those who were not was 1.26 (P ≤ .001) over 5.3 years of follow-up. In the 22,690 patients without a prior history cardiovascular disease (CVD), the HF risk increase for exposure to daily aspirin was about the same (HR 1.27; P = .001).
This study was launched because multiple conflicting studies have made the relationship between aspirin and HF risk unclear, according to the multinational team of authors, whose finding were published in ESC Heart Failure.
In principle, HF is recognized as a prothrombotic condition for which an antithrombotic therapy such as aspirin would be expected to have a protective role, but the investigators pointed out that the evidence is mixed. In a population-based Danish study of 12,277 patients with new-onset HF, for example, there was no relationship seen between aspirin use and a reduction in the composite outcome of all-cause mortality, myocardial infarction, or stroke.
Aspirin use linked to HF admissions
“Interestingly, this study reported that aspirin use was associated with an increased risk of readmissions for HF,” wrote the authors of the newly published data. “Uncertainty on aspirin use has been reflected in current guideline recommendations,” they added.
The population studied was drawn from the HOMAGE database, which has collated data on 46,437 participants in 21 studies. After the exclusion of studies with patients who already had HF as well as studies without information on HF incidence over time, six studies with 30,827 participants provided the basis for this analysis.
One study, ASCOT, which was randomized and blinded, served as the derivation data set. The remaining five studies, FLEMENGHO, HEALTH ABC, HULL LIFE LAB, PREDICTOR, and PROSPER, served as the validation data set.
In addition to identifying participants as aspirin users or nonusers at baseline, all of the studies had detailed baseline data on a wide variety of patient characteristics and risk factors, such as body mass index, blood cholesterol levels, blood glucose concentrations, blood pressure, and creatinine.
No patient in any trial was on an antithrombotic therapy other than aspirin at baseline.
Of the minority of patients with CVD at baseline, more than 80% had coronary heart disease. Only 2.8% of the total population had a prior myocardial infarction. In the study population overall, most (86%) had hypertension, and there was a sizeable proportion with diabetes (22%). The average age was 67 years, and 34% were women.
HF incidence on aspirin: 14.5/1000 person-years
Overall, the incidence rate of HF per 1,000 person-years for the entire population before adjustment was 14.5 in the group on daily aspirin versus 5.9 in the non-aspirin group. These absolute rates were lower in the discovery data set than in the validation set, but the relative differences in HF incidence rates for those who were versus those who were not on aspirin at baseline were similar.
Numerous sensitivity analyses supported the basic conclusions. This not only included one omitting patients with a history of CVD, but another that excluded patients who developed HF within the first 2 years. Stratified analyses looking for overall consistency across variables showed increased risk of new onset heart failure among those taking daily aspirin regardless of relative age, body weight, or blood pressure levels.
The most important limitation of this study was that it evaluated data taken from studies not originally designed to test the study hypothesis. In addition, only baseline data were available, so the drugs that patients took over the course of follow-up are unknown. However, the authors believe these data have a clinical message.
Given the consistency of these results, “our observations suggest that aspirin should be prescribed with caution in patients at risk of HF or having HF,” the investigators concluded.
“If such treatment is initiated in these patients, use low-dose aspirin,” Dr. Staessen told this news organization.
Aspirin for CVD versus HF risk
Many patients take low-dose aspirin to prevent the types of cardiovascular events, such as MI, that lead to heart failure. In attempting to address a controversy regarding aspirin and risk of new onset heart failure, it appears to create another regarding CVD risk reduction.
Deepak L. Bhatt, MD, executive director of Interventional Cardiovascular Programs at Brigham and Women’s Health, Boston, expressed some reluctance in applying these data to routine practice.
“It is important to emphasize that this pooled analysis draws upon six observational studies, not randomized trials of aspirin,” Dr. Bhatt said.
He called these findings “provocative,” but he said they “would need to be confirmed in databases of already completed randomized trials of aspirin versus a control before being actionable.” For Dr. Bhatt, one obstacle to a change in practice based on these data is that, “to my knowledge, no such signal [of a relationship between aspirin and incident heart failure] exists in the cumulative randomized data.”
Dr. Staessen reports no potential conflicts of interest for this study. Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PLx Pharma, for which he performs aspirin-related research.
FROM JACC HEART FAILURE