User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
No survival advantage for either torsemide or furosemide in HF: TRANSFORM-HF
CHICAGO – The choice of loop diuretic for decongestion in patients hospitalized with heart failure (HF) may make little difference to survival or readmission risk over the next year, at least when deciding between furosemide or torsemide, a randomized trial suggests.
Both drugs are old and widely used, but differences between the two loop diuretics in bioavailability, effects on potassium levels, and other features have led some clinicians to sometimes prefer torsemide. Until now, however, no randomized HF trials have compared the two drugs.
The new findings suggest clinicians can continue starting such patients with HF on either agent, at their discretion, without concern that the choice may compromise outcomes, say researchers from the TRANSFORM-HF trial, which compared furosemide-first and torsemide-first diuretic strategies in a diverse population of patients with HF.
Given that the two strategies were similarly effective for survival and rehospitalization, clinicians caring for patients with HF can focus more on “getting patients on the right dose for their loop diuretic, and prioritizing those therapies proven to improve clinical outcomes,” said Robert J. Mentz, MD, of Duke University Clinical Research Institute, Durham, N.C.
Dr. Mentz, a TRANSFORM-HF principal investigator, presented the primary results November 5 at the American Heart Association scientific sessions.
The trial had randomly assigned 2,859 patients hospitalized with HF and with a plan for oral loop diuretic therapy to initiate treatment with furosemide or torsemide. Clinicians were encouraged to maintain patients on the assigned diuretic, but crossovers to the other drug or other diuretic changes were allowed.
Rates of death from any cause, the primary endpoint, were about 26% in both groups over a median 17-month follow-up, regardless of ejection fraction (EF).
The composite rates of all-cause death or hospitalization at 12 months were also not significantly different, about 49% for those started on furosemide and about 47% for patients initially prescribed torsemide.
As a pragmatic comparative effectiveness trial, TRANSFORM-HF entered diverse patients with HF, broadly representative of actual clinical practice, who were managed according to routine practice and a streamlined study protocol at more than 60 U.S. centers, Dr. Mentz observed.
One of the pragmatic design’s advantages, he told this news organization, was “how efficient it was” as a randomized comparison of treatment strategies for clinical outcomes. It was “relatively low cost” and recruited patients quickly, compared with conventional randomized trials, “and we answered the question clearly.” The trial’s results, Dr. Mentz said, reflect “what happens in the real world.”
When might torsemide have the edge?
Although furosemide is the most commonly used loop diuretic in HF, and there are others besides it and torsemide, the latter has both known and theoretical advantages that set it apart. Torsemide is more than twice as potent as furosemide and more bioavailable, and its treatment effect lasts longer, the TRANSFORM-HF investigators have noted.
In addition, preclinical and small clinical studies suggest torsemide may have pleiotropic effects that might be theoretical advantages for patients with HF. For example, it appears to downregulate the renin-angiotensin-aldosterone system (RAAS) and reduce myocardial fibrosis and promote reverse ventricular remodeling, the group writes.
In practice, therefore, torsemide may be preferred in patients with furosemide resistance or “challenges with bioavailability, especially those with very advanced heart failure with congestion who may have gut edema, where oral furosemide and other loop diuretics are not effectively absorbed,” Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
In such patients, she said, torsemide “is considered to be a better choice for individuals who have diuretic resistance with advanced congestion.”
The drug’s apparent pleiotropic effects, such as RAAS inhibition, may have less relevance to the TRANSFORM-HF primary endpoint of all-cause mortality than to clinical outcomes more likely associated with successful decongestion, such as HF hospitalization, Dr. Bozkurt proposed.
The trial’s pragmatic design, however, made it more feasible to focus on all-cause mortality and less practical to use measures of successful decongestion, such as volume loss or reduction in natriuretic peptide levels, she observed. Those are endpoints of special interest when diuretics are compared, “especially for the subgroup of patients who are diuretic resistant.”
Over the last 20 years or so, “we’ve learned that hospitalized heart failure is a very different disease process with a different natural history,” observed Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, who was not part of the current study.
“So, the idea that something as nuanced as choice of one loop diuretic over the other, in that setting, would be sufficient to change the natural history, may be still a high bar for us,” he said in an interview.
“Based on these data, one would have to argue that whichever loop diuretic you select for the hospitalized patient – and a lot of that is driven by market exigencies right now – it turns out that the response is indistinguishable,” Dr. Yancy said. “That means if your hospital happens to have furosemide on the formulary, use it. If furosemide is not available but torsemide is available, use it.”
Dr. Yancy said he’d like to see a trial similar to TRANSFORM-HF but in outpatients receiving today’s guideline-directed medical therapy, which includes the sodium-glucose cotransporter 2 (SGLT2) inhibitors, drugs that increase the fractional excretion of sodium and have a “diureticlike” effect.
Such a trial, he said, would explore “the combination of not one, or two, but three agents with a diuretic effect – a loop diuretic, a mineralocorticoid antagonist, and an SGLT2 inhibitor – in ambulatory, optimized patients. It might make a difference.”
HF regardless of EF
The trial enrolled patients hospitalized with worsening or new-onset HF with a plan for long-term loop diuretic therapy who had either an EF of 40% or lower or, regardless of EF, elevated natriuretic peptide levels when hospitalized.
Of the 2,859 participants, whose mean age was about 65 years, about 36% were women and 34% African American. Overall, 1,428 were assigned to receive furosemide as their initial oral diuretic and 1,431 patients were assigned to the torsemide-first strategy.
The rate of death from any cause in both groups was 17 per 100 patient-years at a median of 17.4 months. The hazard ratio for torsemide vs. furosemide was 1.02 (95% confidence interval, 0.89-1.18; P = .77).
The corresponding HR at 12 months for all-cause death or hospitalization was 0.92 (95% CI, 0.83-1.02; P = .11). The relative risk for any hospitalization was 0.94 (95% CI, 0.84-1.07).
Pragmatic design: Other implications
Dosing was left to clinician discretion in the open-label study, as was whether patients maintained their assigned drug or switched over to the other agent. Indeed, 5.4% of patients crossed over to the other loop diuretic, and 2.8% went off loop diuretics entirely between in-hospital randomization and discharge, Dr. Mentz reported. By day 30, 6.7% had crossed over, and 7% had stopped taking loop diuretics.
The diuretic crossovers and discontinuations, Dr. Mentz said, likely biased the trial’s outcomes, such that the two strategies performed about equally well. Efforts were made, however, to at least partially overcome that limitation.
“We put measures in place to support adherence – sending letters to their primary doctors, giving them a wallet card so they would know which therapy they were on, having conversations about the importance of trying to stay on the randomized therapy,” Dr. Mentz said in an interview. Still, some clinicians saw differences between the two agents that prompted them, at some point, to switch patients from one loop diuretic to the other.
But interestingly, Dr. Mentz reported, the two strategies did not significantly differ in all-cause mortality or the composite of all-cause mortality or hospitalization in analysis by intention to treat.
Dr. Mentz discloses receiving honoraria from AstraZeneca, Bayer/Merck, Boehringer Ingelheim/Lilly, Cytokinetics, Pharmacosmos, Respicardia, Windtree Therapeutics, and Zoll; and research grants from American Regent and Novartis. Dr. Bozkurt discloses receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion. Dr. Yancy discloses a modest relationship with Abbott.
A version of this article first appeared on Medscape.com.
CHICAGO – The choice of loop diuretic for decongestion in patients hospitalized with heart failure (HF) may make little difference to survival or readmission risk over the next year, at least when deciding between furosemide or torsemide, a randomized trial suggests.
Both drugs are old and widely used, but differences between the two loop diuretics in bioavailability, effects on potassium levels, and other features have led some clinicians to sometimes prefer torsemide. Until now, however, no randomized HF trials have compared the two drugs.
The new findings suggest clinicians can continue starting such patients with HF on either agent, at their discretion, without concern that the choice may compromise outcomes, say researchers from the TRANSFORM-HF trial, which compared furosemide-first and torsemide-first diuretic strategies in a diverse population of patients with HF.
Given that the two strategies were similarly effective for survival and rehospitalization, clinicians caring for patients with HF can focus more on “getting patients on the right dose for their loop diuretic, and prioritizing those therapies proven to improve clinical outcomes,” said Robert J. Mentz, MD, of Duke University Clinical Research Institute, Durham, N.C.
Dr. Mentz, a TRANSFORM-HF principal investigator, presented the primary results November 5 at the American Heart Association scientific sessions.
The trial had randomly assigned 2,859 patients hospitalized with HF and with a plan for oral loop diuretic therapy to initiate treatment with furosemide or torsemide. Clinicians were encouraged to maintain patients on the assigned diuretic, but crossovers to the other drug or other diuretic changes were allowed.
Rates of death from any cause, the primary endpoint, were about 26% in both groups over a median 17-month follow-up, regardless of ejection fraction (EF).
The composite rates of all-cause death or hospitalization at 12 months were also not significantly different, about 49% for those started on furosemide and about 47% for patients initially prescribed torsemide.
As a pragmatic comparative effectiveness trial, TRANSFORM-HF entered diverse patients with HF, broadly representative of actual clinical practice, who were managed according to routine practice and a streamlined study protocol at more than 60 U.S. centers, Dr. Mentz observed.
One of the pragmatic design’s advantages, he told this news organization, was “how efficient it was” as a randomized comparison of treatment strategies for clinical outcomes. It was “relatively low cost” and recruited patients quickly, compared with conventional randomized trials, “and we answered the question clearly.” The trial’s results, Dr. Mentz said, reflect “what happens in the real world.”
When might torsemide have the edge?
Although furosemide is the most commonly used loop diuretic in HF, and there are others besides it and torsemide, the latter has both known and theoretical advantages that set it apart. Torsemide is more than twice as potent as furosemide and more bioavailable, and its treatment effect lasts longer, the TRANSFORM-HF investigators have noted.
In addition, preclinical and small clinical studies suggest torsemide may have pleiotropic effects that might be theoretical advantages for patients with HF. For example, it appears to downregulate the renin-angiotensin-aldosterone system (RAAS) and reduce myocardial fibrosis and promote reverse ventricular remodeling, the group writes.
In practice, therefore, torsemide may be preferred in patients with furosemide resistance or “challenges with bioavailability, especially those with very advanced heart failure with congestion who may have gut edema, where oral furosemide and other loop diuretics are not effectively absorbed,” Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
In such patients, she said, torsemide “is considered to be a better choice for individuals who have diuretic resistance with advanced congestion.”
The drug’s apparent pleiotropic effects, such as RAAS inhibition, may have less relevance to the TRANSFORM-HF primary endpoint of all-cause mortality than to clinical outcomes more likely associated with successful decongestion, such as HF hospitalization, Dr. Bozkurt proposed.
The trial’s pragmatic design, however, made it more feasible to focus on all-cause mortality and less practical to use measures of successful decongestion, such as volume loss or reduction in natriuretic peptide levels, she observed. Those are endpoints of special interest when diuretics are compared, “especially for the subgroup of patients who are diuretic resistant.”
Over the last 20 years or so, “we’ve learned that hospitalized heart failure is a very different disease process with a different natural history,” observed Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, who was not part of the current study.
“So, the idea that something as nuanced as choice of one loop diuretic over the other, in that setting, would be sufficient to change the natural history, may be still a high bar for us,” he said in an interview.
“Based on these data, one would have to argue that whichever loop diuretic you select for the hospitalized patient – and a lot of that is driven by market exigencies right now – it turns out that the response is indistinguishable,” Dr. Yancy said. “That means if your hospital happens to have furosemide on the formulary, use it. If furosemide is not available but torsemide is available, use it.”
Dr. Yancy said he’d like to see a trial similar to TRANSFORM-HF but in outpatients receiving today’s guideline-directed medical therapy, which includes the sodium-glucose cotransporter 2 (SGLT2) inhibitors, drugs that increase the fractional excretion of sodium and have a “diureticlike” effect.
Such a trial, he said, would explore “the combination of not one, or two, but three agents with a diuretic effect – a loop diuretic, a mineralocorticoid antagonist, and an SGLT2 inhibitor – in ambulatory, optimized patients. It might make a difference.”
HF regardless of EF
The trial enrolled patients hospitalized with worsening or new-onset HF with a plan for long-term loop diuretic therapy who had either an EF of 40% or lower or, regardless of EF, elevated natriuretic peptide levels when hospitalized.
Of the 2,859 participants, whose mean age was about 65 years, about 36% were women and 34% African American. Overall, 1,428 were assigned to receive furosemide as their initial oral diuretic and 1,431 patients were assigned to the torsemide-first strategy.
The rate of death from any cause in both groups was 17 per 100 patient-years at a median of 17.4 months. The hazard ratio for torsemide vs. furosemide was 1.02 (95% confidence interval, 0.89-1.18; P = .77).
The corresponding HR at 12 months for all-cause death or hospitalization was 0.92 (95% CI, 0.83-1.02; P = .11). The relative risk for any hospitalization was 0.94 (95% CI, 0.84-1.07).
Pragmatic design: Other implications
Dosing was left to clinician discretion in the open-label study, as was whether patients maintained their assigned drug or switched over to the other agent. Indeed, 5.4% of patients crossed over to the other loop diuretic, and 2.8% went off loop diuretics entirely between in-hospital randomization and discharge, Dr. Mentz reported. By day 30, 6.7% had crossed over, and 7% had stopped taking loop diuretics.
The diuretic crossovers and discontinuations, Dr. Mentz said, likely biased the trial’s outcomes, such that the two strategies performed about equally well. Efforts were made, however, to at least partially overcome that limitation.
“We put measures in place to support adherence – sending letters to their primary doctors, giving them a wallet card so they would know which therapy they were on, having conversations about the importance of trying to stay on the randomized therapy,” Dr. Mentz said in an interview. Still, some clinicians saw differences between the two agents that prompted them, at some point, to switch patients from one loop diuretic to the other.
But interestingly, Dr. Mentz reported, the two strategies did not significantly differ in all-cause mortality or the composite of all-cause mortality or hospitalization in analysis by intention to treat.
Dr. Mentz discloses receiving honoraria from AstraZeneca, Bayer/Merck, Boehringer Ingelheim/Lilly, Cytokinetics, Pharmacosmos, Respicardia, Windtree Therapeutics, and Zoll; and research grants from American Regent and Novartis. Dr. Bozkurt discloses receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion. Dr. Yancy discloses a modest relationship with Abbott.
A version of this article first appeared on Medscape.com.
CHICAGO – The choice of loop diuretic for decongestion in patients hospitalized with heart failure (HF) may make little difference to survival or readmission risk over the next year, at least when deciding between furosemide or torsemide, a randomized trial suggests.
Both drugs are old and widely used, but differences between the two loop diuretics in bioavailability, effects on potassium levels, and other features have led some clinicians to sometimes prefer torsemide. Until now, however, no randomized HF trials have compared the two drugs.
The new findings suggest clinicians can continue starting such patients with HF on either agent, at their discretion, without concern that the choice may compromise outcomes, say researchers from the TRANSFORM-HF trial, which compared furosemide-first and torsemide-first diuretic strategies in a diverse population of patients with HF.
Given that the two strategies were similarly effective for survival and rehospitalization, clinicians caring for patients with HF can focus more on “getting patients on the right dose for their loop diuretic, and prioritizing those therapies proven to improve clinical outcomes,” said Robert J. Mentz, MD, of Duke University Clinical Research Institute, Durham, N.C.
Dr. Mentz, a TRANSFORM-HF principal investigator, presented the primary results November 5 at the American Heart Association scientific sessions.
The trial had randomly assigned 2,859 patients hospitalized with HF and with a plan for oral loop diuretic therapy to initiate treatment with furosemide or torsemide. Clinicians were encouraged to maintain patients on the assigned diuretic, but crossovers to the other drug or other diuretic changes were allowed.
Rates of death from any cause, the primary endpoint, were about 26% in both groups over a median 17-month follow-up, regardless of ejection fraction (EF).
The composite rates of all-cause death or hospitalization at 12 months were also not significantly different, about 49% for those started on furosemide and about 47% for patients initially prescribed torsemide.
As a pragmatic comparative effectiveness trial, TRANSFORM-HF entered diverse patients with HF, broadly representative of actual clinical practice, who were managed according to routine practice and a streamlined study protocol at more than 60 U.S. centers, Dr. Mentz observed.
One of the pragmatic design’s advantages, he told this news organization, was “how efficient it was” as a randomized comparison of treatment strategies for clinical outcomes. It was “relatively low cost” and recruited patients quickly, compared with conventional randomized trials, “and we answered the question clearly.” The trial’s results, Dr. Mentz said, reflect “what happens in the real world.”
When might torsemide have the edge?
Although furosemide is the most commonly used loop diuretic in HF, and there are others besides it and torsemide, the latter has both known and theoretical advantages that set it apart. Torsemide is more than twice as potent as furosemide and more bioavailable, and its treatment effect lasts longer, the TRANSFORM-HF investigators have noted.
In addition, preclinical and small clinical studies suggest torsemide may have pleiotropic effects that might be theoretical advantages for patients with HF. For example, it appears to downregulate the renin-angiotensin-aldosterone system (RAAS) and reduce myocardial fibrosis and promote reverse ventricular remodeling, the group writes.
In practice, therefore, torsemide may be preferred in patients with furosemide resistance or “challenges with bioavailability, especially those with very advanced heart failure with congestion who may have gut edema, where oral furosemide and other loop diuretics are not effectively absorbed,” Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
In such patients, she said, torsemide “is considered to be a better choice for individuals who have diuretic resistance with advanced congestion.”
The drug’s apparent pleiotropic effects, such as RAAS inhibition, may have less relevance to the TRANSFORM-HF primary endpoint of all-cause mortality than to clinical outcomes more likely associated with successful decongestion, such as HF hospitalization, Dr. Bozkurt proposed.
The trial’s pragmatic design, however, made it more feasible to focus on all-cause mortality and less practical to use measures of successful decongestion, such as volume loss or reduction in natriuretic peptide levels, she observed. Those are endpoints of special interest when diuretics are compared, “especially for the subgroup of patients who are diuretic resistant.”
Over the last 20 years or so, “we’ve learned that hospitalized heart failure is a very different disease process with a different natural history,” observed Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, who was not part of the current study.
“So, the idea that something as nuanced as choice of one loop diuretic over the other, in that setting, would be sufficient to change the natural history, may be still a high bar for us,” he said in an interview.
“Based on these data, one would have to argue that whichever loop diuretic you select for the hospitalized patient – and a lot of that is driven by market exigencies right now – it turns out that the response is indistinguishable,” Dr. Yancy said. “That means if your hospital happens to have furosemide on the formulary, use it. If furosemide is not available but torsemide is available, use it.”
Dr. Yancy said he’d like to see a trial similar to TRANSFORM-HF but in outpatients receiving today’s guideline-directed medical therapy, which includes the sodium-glucose cotransporter 2 (SGLT2) inhibitors, drugs that increase the fractional excretion of sodium and have a “diureticlike” effect.
Such a trial, he said, would explore “the combination of not one, or two, but three agents with a diuretic effect – a loop diuretic, a mineralocorticoid antagonist, and an SGLT2 inhibitor – in ambulatory, optimized patients. It might make a difference.”
HF regardless of EF
The trial enrolled patients hospitalized with worsening or new-onset HF with a plan for long-term loop diuretic therapy who had either an EF of 40% or lower or, regardless of EF, elevated natriuretic peptide levels when hospitalized.
Of the 2,859 participants, whose mean age was about 65 years, about 36% were women and 34% African American. Overall, 1,428 were assigned to receive furosemide as their initial oral diuretic and 1,431 patients were assigned to the torsemide-first strategy.
The rate of death from any cause in both groups was 17 per 100 patient-years at a median of 17.4 months. The hazard ratio for torsemide vs. furosemide was 1.02 (95% confidence interval, 0.89-1.18; P = .77).
The corresponding HR at 12 months for all-cause death or hospitalization was 0.92 (95% CI, 0.83-1.02; P = .11). The relative risk for any hospitalization was 0.94 (95% CI, 0.84-1.07).
Pragmatic design: Other implications
Dosing was left to clinician discretion in the open-label study, as was whether patients maintained their assigned drug or switched over to the other agent. Indeed, 5.4% of patients crossed over to the other loop diuretic, and 2.8% went off loop diuretics entirely between in-hospital randomization and discharge, Dr. Mentz reported. By day 30, 6.7% had crossed over, and 7% had stopped taking loop diuretics.
The diuretic crossovers and discontinuations, Dr. Mentz said, likely biased the trial’s outcomes, such that the two strategies performed about equally well. Efforts were made, however, to at least partially overcome that limitation.
“We put measures in place to support adherence – sending letters to their primary doctors, giving them a wallet card so they would know which therapy they were on, having conversations about the importance of trying to stay on the randomized therapy,” Dr. Mentz said in an interview. Still, some clinicians saw differences between the two agents that prompted them, at some point, to switch patients from one loop diuretic to the other.
But interestingly, Dr. Mentz reported, the two strategies did not significantly differ in all-cause mortality or the composite of all-cause mortality or hospitalization in analysis by intention to treat.
Dr. Mentz discloses receiving honoraria from AstraZeneca, Bayer/Merck, Boehringer Ingelheim/Lilly, Cytokinetics, Pharmacosmos, Respicardia, Windtree Therapeutics, and Zoll; and research grants from American Regent and Novartis. Dr. Bozkurt discloses receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion. Dr. Yancy discloses a modest relationship with Abbott.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Acute heart failure risk assessment in ED improves outcomes: COACH
CHICAGO – Systematic mortality-risk assessment of patients who presented to hospital emergency departments for acute heart failure led to better patient outcomes in a controlled Canadian trial with more than 5,000 patients.
Thirty days after patients presented, the incidence of death from any cause or hospitalization for cardiovascular causes – one of two primary endpoints in the COACH study – was 12.1% among patients who underwent acute risk assessment and 14.5% in control patients who did not undergo this assessment, which translated into an adjusted, significant 12% relative risk reduction for the patients who underwent systematic assessment, Douglas S. Lee, MD, PhD, said at the American Heart Association scientific sessions.
The study’s second primary endpoint, the incidence of the same combined outcome 20 months after initial presentation, was 54.4% among the 2,480 patients assessed with the risk-assessment tool and 56.2% in the 2,972 controls, a significant, adjusted relative risk reduction of 5%.
This benefit was primarily driven by reductions in cardiovascular hospitalizations, which fell by an adjusted 16% in the intervention group compared with controls, and more specifically by hospitalizations for heart failure, which tallied a relative 20% less with the intervention. Both were significant between-group differences.
The other portion of the combined endpoint, all-cause mortality, was not significantly different between the patients who underwent the systematic emergency department assessment and the controls who were managed using usual emergency-department protocols.
Simultaneous with the report, the results also appeared online in the New England Journal of Medicine.
A pathway for early discharge and improved outcomes
“Implementation of this approach may lead to a pathway for early discharge from the hospital or emergency department, and improved patient outcomes,” said Dr. Lee, a professor at the University of Toronto, and a senior core scientist at the ICES Cardiovascular Research Program in Toronto.
“The treatment effect on the primary process outcome – patients admitted or discharged – will add useful insights into how intervention may improve,” commented Harriette Van Spall, MD, who was designated discussant for the report. The findings “fill an important knowledge gap,” added Dr. Van Spall, a cardiologist at McMaster University in Hamilton, Ont. The results “have important implications for health resource utilization,” she said.
The risk assessment tool used in the study is called the Emergency Heart failure Mortality Risk Grade for 30-day mortality (EHMRG30-ST), which was devised and validated by Dr. Lee and his associates. The assessment tool uses 11 clinical variables that include age, systolic blood pressure, heart rate, oxygen saturation, potassium and creatinine levels, and presence of ST depression on a 12-lead ECG.
The study design recommended that patients be discharged early and receive standardized transitional care as outpatients if they had a low risk of death within 7 days and within 30 days as estimated by the EHMRG30-ST. The protocol recommended that patients scored as high risk should be admitted to the hospital, and that clinicians use their clinical judgment for intermediate-risk patients but favor admission for intermediate to high risk and discharge for low to intermediate risk. The study ran at 10 hospitals in Ontario. Initially, all 10 hospitals assessed patients by usual care, and then, over time, each hospital began using the tool so that by the end of the study all 10 hospitals employed it. Among the 2,480 patients seen during the active phase, 2,442 actually underwent assessment, with 24% rated as low risk, 32% rated as intermediate risk, and 44% judged to have high risk.
The researchers also ran risk assessments retrospectively on the controls, who showed a roughly similar risk distribution, with 18% low risk, 28% intermediate risk, and 54% high risk.
The patients averaged 78 years of age, 55% were men, about 40% had diabetes, and about 64% had a prior heart failure diagnosis.
Heart failure admissions have become ‘a big deal’
Emergency department clinicians and heart failure cardiologists “have worked together for a long time” when making decisions about which patients with acute heart failure need hospital admission, commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis. These decisions “became a big deal” a decade ago when the U.S. Centers for Medicare & Medicaid Services launched the Hospital Readmissions Reduction Program that began to penalize hospitals for high rates of hospital readmissions for several conditions including heart failure, she said in an interview.
“If a heart failure patient is not admitted, they can’t be readmitted,” Dr. Walsh noted.
“Many risk-assessment tools exist for patients once they are hospitalized, but these tools have not been used in emergency departments. The take-home message is that we need to start risk assessment sooner, in the emergency department,” she said.
But the specific approach tested in the COACH trial needs more study and may need further tweaking to work in the United States, where it is not clear who would pay for a program like the one tested in the trial. Canada’s unified health care payment system makes the COACH approach more financially feasible, Dr. Walsh commented.
COACH received no commercial funding. Dr. Lee, Dr. Van Spall, and Dr. Walsh had no disclosures.
CHICAGO – Systematic mortality-risk assessment of patients who presented to hospital emergency departments for acute heart failure led to better patient outcomes in a controlled Canadian trial with more than 5,000 patients.
Thirty days after patients presented, the incidence of death from any cause or hospitalization for cardiovascular causes – one of two primary endpoints in the COACH study – was 12.1% among patients who underwent acute risk assessment and 14.5% in control patients who did not undergo this assessment, which translated into an adjusted, significant 12% relative risk reduction for the patients who underwent systematic assessment, Douglas S. Lee, MD, PhD, said at the American Heart Association scientific sessions.
The study’s second primary endpoint, the incidence of the same combined outcome 20 months after initial presentation, was 54.4% among the 2,480 patients assessed with the risk-assessment tool and 56.2% in the 2,972 controls, a significant, adjusted relative risk reduction of 5%.
This benefit was primarily driven by reductions in cardiovascular hospitalizations, which fell by an adjusted 16% in the intervention group compared with controls, and more specifically by hospitalizations for heart failure, which tallied a relative 20% less with the intervention. Both were significant between-group differences.
The other portion of the combined endpoint, all-cause mortality, was not significantly different between the patients who underwent the systematic emergency department assessment and the controls who were managed using usual emergency-department protocols.
Simultaneous with the report, the results also appeared online in the New England Journal of Medicine.
A pathway for early discharge and improved outcomes
“Implementation of this approach may lead to a pathway for early discharge from the hospital or emergency department, and improved patient outcomes,” said Dr. Lee, a professor at the University of Toronto, and a senior core scientist at the ICES Cardiovascular Research Program in Toronto.
“The treatment effect on the primary process outcome – patients admitted or discharged – will add useful insights into how intervention may improve,” commented Harriette Van Spall, MD, who was designated discussant for the report. The findings “fill an important knowledge gap,” added Dr. Van Spall, a cardiologist at McMaster University in Hamilton, Ont. The results “have important implications for health resource utilization,” she said.
The risk assessment tool used in the study is called the Emergency Heart failure Mortality Risk Grade for 30-day mortality (EHMRG30-ST), which was devised and validated by Dr. Lee and his associates. The assessment tool uses 11 clinical variables that include age, systolic blood pressure, heart rate, oxygen saturation, potassium and creatinine levels, and presence of ST depression on a 12-lead ECG.
The study design recommended that patients be discharged early and receive standardized transitional care as outpatients if they had a low risk of death within 7 days and within 30 days as estimated by the EHMRG30-ST. The protocol recommended that patients scored as high risk should be admitted to the hospital, and that clinicians use their clinical judgment for intermediate-risk patients but favor admission for intermediate to high risk and discharge for low to intermediate risk. The study ran at 10 hospitals in Ontario. Initially, all 10 hospitals assessed patients by usual care, and then, over time, each hospital began using the tool so that by the end of the study all 10 hospitals employed it. Among the 2,480 patients seen during the active phase, 2,442 actually underwent assessment, with 24% rated as low risk, 32% rated as intermediate risk, and 44% judged to have high risk.
The researchers also ran risk assessments retrospectively on the controls, who showed a roughly similar risk distribution, with 18% low risk, 28% intermediate risk, and 54% high risk.
The patients averaged 78 years of age, 55% were men, about 40% had diabetes, and about 64% had a prior heart failure diagnosis.
Heart failure admissions have become ‘a big deal’
Emergency department clinicians and heart failure cardiologists “have worked together for a long time” when making decisions about which patients with acute heart failure need hospital admission, commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis. These decisions “became a big deal” a decade ago when the U.S. Centers for Medicare & Medicaid Services launched the Hospital Readmissions Reduction Program that began to penalize hospitals for high rates of hospital readmissions for several conditions including heart failure, she said in an interview.
“If a heart failure patient is not admitted, they can’t be readmitted,” Dr. Walsh noted.
“Many risk-assessment tools exist for patients once they are hospitalized, but these tools have not been used in emergency departments. The take-home message is that we need to start risk assessment sooner, in the emergency department,” she said.
But the specific approach tested in the COACH trial needs more study and may need further tweaking to work in the United States, where it is not clear who would pay for a program like the one tested in the trial. Canada’s unified health care payment system makes the COACH approach more financially feasible, Dr. Walsh commented.
COACH received no commercial funding. Dr. Lee, Dr. Van Spall, and Dr. Walsh had no disclosures.
CHICAGO – Systematic mortality-risk assessment of patients who presented to hospital emergency departments for acute heart failure led to better patient outcomes in a controlled Canadian trial with more than 5,000 patients.
Thirty days after patients presented, the incidence of death from any cause or hospitalization for cardiovascular causes – one of two primary endpoints in the COACH study – was 12.1% among patients who underwent acute risk assessment and 14.5% in control patients who did not undergo this assessment, which translated into an adjusted, significant 12% relative risk reduction for the patients who underwent systematic assessment, Douglas S. Lee, MD, PhD, said at the American Heart Association scientific sessions.
The study’s second primary endpoint, the incidence of the same combined outcome 20 months after initial presentation, was 54.4% among the 2,480 patients assessed with the risk-assessment tool and 56.2% in the 2,972 controls, a significant, adjusted relative risk reduction of 5%.
This benefit was primarily driven by reductions in cardiovascular hospitalizations, which fell by an adjusted 16% in the intervention group compared with controls, and more specifically by hospitalizations for heart failure, which tallied a relative 20% less with the intervention. Both were significant between-group differences.
The other portion of the combined endpoint, all-cause mortality, was not significantly different between the patients who underwent the systematic emergency department assessment and the controls who were managed using usual emergency-department protocols.
Simultaneous with the report, the results also appeared online in the New England Journal of Medicine.
A pathway for early discharge and improved outcomes
“Implementation of this approach may lead to a pathway for early discharge from the hospital or emergency department, and improved patient outcomes,” said Dr. Lee, a professor at the University of Toronto, and a senior core scientist at the ICES Cardiovascular Research Program in Toronto.
“The treatment effect on the primary process outcome – patients admitted or discharged – will add useful insights into how intervention may improve,” commented Harriette Van Spall, MD, who was designated discussant for the report. The findings “fill an important knowledge gap,” added Dr. Van Spall, a cardiologist at McMaster University in Hamilton, Ont. The results “have important implications for health resource utilization,” she said.
The risk assessment tool used in the study is called the Emergency Heart failure Mortality Risk Grade for 30-day mortality (EHMRG30-ST), which was devised and validated by Dr. Lee and his associates. The assessment tool uses 11 clinical variables that include age, systolic blood pressure, heart rate, oxygen saturation, potassium and creatinine levels, and presence of ST depression on a 12-lead ECG.
The study design recommended that patients be discharged early and receive standardized transitional care as outpatients if they had a low risk of death within 7 days and within 30 days as estimated by the EHMRG30-ST. The protocol recommended that patients scored as high risk should be admitted to the hospital, and that clinicians use their clinical judgment for intermediate-risk patients but favor admission for intermediate to high risk and discharge for low to intermediate risk. The study ran at 10 hospitals in Ontario. Initially, all 10 hospitals assessed patients by usual care, and then, over time, each hospital began using the tool so that by the end of the study all 10 hospitals employed it. Among the 2,480 patients seen during the active phase, 2,442 actually underwent assessment, with 24% rated as low risk, 32% rated as intermediate risk, and 44% judged to have high risk.
The researchers also ran risk assessments retrospectively on the controls, who showed a roughly similar risk distribution, with 18% low risk, 28% intermediate risk, and 54% high risk.
The patients averaged 78 years of age, 55% were men, about 40% had diabetes, and about 64% had a prior heart failure diagnosis.
Heart failure admissions have become ‘a big deal’
Emergency department clinicians and heart failure cardiologists “have worked together for a long time” when making decisions about which patients with acute heart failure need hospital admission, commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis. These decisions “became a big deal” a decade ago when the U.S. Centers for Medicare & Medicaid Services launched the Hospital Readmissions Reduction Program that began to penalize hospitals for high rates of hospital readmissions for several conditions including heart failure, she said in an interview.
“If a heart failure patient is not admitted, they can’t be readmitted,” Dr. Walsh noted.
“Many risk-assessment tools exist for patients once they are hospitalized, but these tools have not been used in emergency departments. The take-home message is that we need to start risk assessment sooner, in the emergency department,” she said.
But the specific approach tested in the COACH trial needs more study and may need further tweaking to work in the United States, where it is not clear who would pay for a program like the one tested in the trial. Canada’s unified health care payment system makes the COACH approach more financially feasible, Dr. Walsh commented.
COACH received no commercial funding. Dr. Lee, Dr. Van Spall, and Dr. Walsh had no disclosures.
AT AHA 2022
In CABG, radial artery works best for second key graft: RAPCO at 15 years
Lower risk of MACE shown
CHICAGO – With more than 15 years of follow-up from two related trials, the best conduit for the second most important target vessel in coronary artery bypass grafting (CABG) appears to be resolved.
The radial artery (RA) graft is linked with a lower risk of major adverse cardiac events (MACE) relative to a saphenous vein (SV) or the free right internal thoracic artery (FRITA).
On the basis of these findings, “a radial artery graft should be considered in all isolated CABG operations unless there are contraindications,” reported David L. Hare, MBBS, director of research in the department of cardiology, University of Melbourne.
For the primary graft, there is general agreement that the left internal thoracic artery (LITA) is the first choice for the left anterior descending vessel, but the optimal graft for the second most important target has never been established, according to Dr. Hare.
Almost 25 years ago, two randomized controlled trials called RAPCO-RITA and RAPCO-SV were initiated to address the question. There is now 15 years of follow-up for both of the RAPCO (Radial Artery Patency and Clinical Outcomes) trials, which were presented together at the American Heart Association scientific sessions.
Two trials conducted simultaneously
The RAPCO-RITA trial randomized CABG patients less than 70 years of age (less than 60 years in those with diabetes) to grafting of the second target vessel with an RA or FRITA graft. The RAPCO-SV trial randomized those 70 years or older (60 years or older with diabetes) to an RA or SV graft.
The two primary endpoints were graft patency at 10 years and a composite MACE at 10 years. The assessment of the MACE endpoint, which consisted of cardiovascular mortality, acute myocardial infarction, and coronary revascularization, was later amended to include a comparison at 15 years.
Ten-year patency results, favoring the RA in both studies, were previously published in Circulation. In the new data presented at the meeting, the RA was associated with a significant reduction in MACE relative to the comparator graft in both studies.
“The main driver was a reduction in all-cause mortality,” Dr. Hare reported.
In RAPCO-RITA, 394 patients were randomized with follow-up data available for all but 1 patient at 15 years. Similarly, only 1 patient was lost to follow-up among the 225 randomized in RAPCO-SV. In both studies, baseline characteristics were well balanced.
MACE curves separate at 5 years
In RAPCO-RITA, the MACE survival curves began to separate at about 5 years and then gradually widened. By 15 years, the lower rate of MACE in the RA group (38% vs. 48%) translated into a 26% relative reduction (hazard ratio, 0.74; P = .04).
In RAPCO-SV, the pattern was similar, by 15 years, the rates of MACE were 60% and 73% for the RA and SV groups, respectively, translating into a 29% relative reduction (HR, 0.71; P = .04).
There was no heterogeneity in benefit across prespecified subgroups such as presence or absence of diabetes, gender, or age. In RAPCO-RITA, there was 8% absolute and 31% relative reduction in all-cause mortality. In RAPCO-SV, the absolute and relative reductions were 11% and 26%.
When the trial was initiated, Dr. Hare hypothesized that RITA would prove more durable than RA, so the outcome was not anticipated.
“This is the first randomized controlled trial program to address the question,” said Dr. Hare, who noted that there have been numerous retrospective and case control analyses that have produced mixed results in the past.
Discussant praises trial quality
The AHA-invited discussant, Marc Ruel, MD, chair of cardiac surgery, University of Ottawa (Ont.) Heart Institute, called these data “important,” and he congratulated Dr. Hare for conducting the first randomized trial to address the question about second graft durability.
However, he noted that, although the study was randomized, it was not blinded, and he questioned whether postoperative care, in particular, was similar. He also pointed out that the MACE rate seemed high, particularly among the older patients randomized in RAPCO-SV.
“All of the patients were referred to an independently run CABG rehab program that was quite separate from the trial but that provided identical mandated care,” Dr. Hare responded, indicating that there was no opportunity for differences in postprocedural management.
In the United States, the SV graft is often preferred on the basis of easy harvesting and handling characteristics, according to Dr. Hare, who estimated that fewer than 10% of the 200,000 CABG procedures performed in the United States employ the RA conduit for second target vessels. He believes the RAPCO trials data support a change.
“My personal view is [that, on the basis of] this data, given that it is from a controlled trial rather than from patient-level meta-analyses, all isolated CABG operations should be using a radial graft if it is suitable,” Dr. Hare said.
Dr. Hare reports financial relationships with Abbott, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, CSL-Biotherapies, Lundbeck, Menarini, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, and Vifor. Dr. Ruel reports financial relationships with Cryolife, Edwards, and Medtronic.
Lower risk of MACE shown
Lower risk of MACE shown
CHICAGO – With more than 15 years of follow-up from two related trials, the best conduit for the second most important target vessel in coronary artery bypass grafting (CABG) appears to be resolved.
The radial artery (RA) graft is linked with a lower risk of major adverse cardiac events (MACE) relative to a saphenous vein (SV) or the free right internal thoracic artery (FRITA).
On the basis of these findings, “a radial artery graft should be considered in all isolated CABG operations unless there are contraindications,” reported David L. Hare, MBBS, director of research in the department of cardiology, University of Melbourne.
For the primary graft, there is general agreement that the left internal thoracic artery (LITA) is the first choice for the left anterior descending vessel, but the optimal graft for the second most important target has never been established, according to Dr. Hare.
Almost 25 years ago, two randomized controlled trials called RAPCO-RITA and RAPCO-SV were initiated to address the question. There is now 15 years of follow-up for both of the RAPCO (Radial Artery Patency and Clinical Outcomes) trials, which were presented together at the American Heart Association scientific sessions.
Two trials conducted simultaneously
The RAPCO-RITA trial randomized CABG patients less than 70 years of age (less than 60 years in those with diabetes) to grafting of the second target vessel with an RA or FRITA graft. The RAPCO-SV trial randomized those 70 years or older (60 years or older with diabetes) to an RA or SV graft.
The two primary endpoints were graft patency at 10 years and a composite MACE at 10 years. The assessment of the MACE endpoint, which consisted of cardiovascular mortality, acute myocardial infarction, and coronary revascularization, was later amended to include a comparison at 15 years.
Ten-year patency results, favoring the RA in both studies, were previously published in Circulation. In the new data presented at the meeting, the RA was associated with a significant reduction in MACE relative to the comparator graft in both studies.
“The main driver was a reduction in all-cause mortality,” Dr. Hare reported.
In RAPCO-RITA, 394 patients were randomized with follow-up data available for all but 1 patient at 15 years. Similarly, only 1 patient was lost to follow-up among the 225 randomized in RAPCO-SV. In both studies, baseline characteristics were well balanced.
MACE curves separate at 5 years
In RAPCO-RITA, the MACE survival curves began to separate at about 5 years and then gradually widened. By 15 years, the lower rate of MACE in the RA group (38% vs. 48%) translated into a 26% relative reduction (hazard ratio, 0.74; P = .04).
In RAPCO-SV, the pattern was similar, by 15 years, the rates of MACE were 60% and 73% for the RA and SV groups, respectively, translating into a 29% relative reduction (HR, 0.71; P = .04).
There was no heterogeneity in benefit across prespecified subgroups such as presence or absence of diabetes, gender, or age. In RAPCO-RITA, there was 8% absolute and 31% relative reduction in all-cause mortality. In RAPCO-SV, the absolute and relative reductions were 11% and 26%.
When the trial was initiated, Dr. Hare hypothesized that RITA would prove more durable than RA, so the outcome was not anticipated.
“This is the first randomized controlled trial program to address the question,” said Dr. Hare, who noted that there have been numerous retrospective and case control analyses that have produced mixed results in the past.
Discussant praises trial quality
The AHA-invited discussant, Marc Ruel, MD, chair of cardiac surgery, University of Ottawa (Ont.) Heart Institute, called these data “important,” and he congratulated Dr. Hare for conducting the first randomized trial to address the question about second graft durability.
However, he noted that, although the study was randomized, it was not blinded, and he questioned whether postoperative care, in particular, was similar. He also pointed out that the MACE rate seemed high, particularly among the older patients randomized in RAPCO-SV.
“All of the patients were referred to an independently run CABG rehab program that was quite separate from the trial but that provided identical mandated care,” Dr. Hare responded, indicating that there was no opportunity for differences in postprocedural management.
In the United States, the SV graft is often preferred on the basis of easy harvesting and handling characteristics, according to Dr. Hare, who estimated that fewer than 10% of the 200,000 CABG procedures performed in the United States employ the RA conduit for second target vessels. He believes the RAPCO trials data support a change.
“My personal view is [that, on the basis of] this data, given that it is from a controlled trial rather than from patient-level meta-analyses, all isolated CABG operations should be using a radial graft if it is suitable,” Dr. Hare said.
Dr. Hare reports financial relationships with Abbott, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, CSL-Biotherapies, Lundbeck, Menarini, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, and Vifor. Dr. Ruel reports financial relationships with Cryolife, Edwards, and Medtronic.
CHICAGO – With more than 15 years of follow-up from two related trials, the best conduit for the second most important target vessel in coronary artery bypass grafting (CABG) appears to be resolved.
The radial artery (RA) graft is linked with a lower risk of major adverse cardiac events (MACE) relative to a saphenous vein (SV) or the free right internal thoracic artery (FRITA).
On the basis of these findings, “a radial artery graft should be considered in all isolated CABG operations unless there are contraindications,” reported David L. Hare, MBBS, director of research in the department of cardiology, University of Melbourne.
For the primary graft, there is general agreement that the left internal thoracic artery (LITA) is the first choice for the left anterior descending vessel, but the optimal graft for the second most important target has never been established, according to Dr. Hare.
Almost 25 years ago, two randomized controlled trials called RAPCO-RITA and RAPCO-SV were initiated to address the question. There is now 15 years of follow-up for both of the RAPCO (Radial Artery Patency and Clinical Outcomes) trials, which were presented together at the American Heart Association scientific sessions.
Two trials conducted simultaneously
The RAPCO-RITA trial randomized CABG patients less than 70 years of age (less than 60 years in those with diabetes) to grafting of the second target vessel with an RA or FRITA graft. The RAPCO-SV trial randomized those 70 years or older (60 years or older with diabetes) to an RA or SV graft.
The two primary endpoints were graft patency at 10 years and a composite MACE at 10 years. The assessment of the MACE endpoint, which consisted of cardiovascular mortality, acute myocardial infarction, and coronary revascularization, was later amended to include a comparison at 15 years.
Ten-year patency results, favoring the RA in both studies, were previously published in Circulation. In the new data presented at the meeting, the RA was associated with a significant reduction in MACE relative to the comparator graft in both studies.
“The main driver was a reduction in all-cause mortality,” Dr. Hare reported.
In RAPCO-RITA, 394 patients were randomized with follow-up data available for all but 1 patient at 15 years. Similarly, only 1 patient was lost to follow-up among the 225 randomized in RAPCO-SV. In both studies, baseline characteristics were well balanced.
MACE curves separate at 5 years
In RAPCO-RITA, the MACE survival curves began to separate at about 5 years and then gradually widened. By 15 years, the lower rate of MACE in the RA group (38% vs. 48%) translated into a 26% relative reduction (hazard ratio, 0.74; P = .04).
In RAPCO-SV, the pattern was similar, by 15 years, the rates of MACE were 60% and 73% for the RA and SV groups, respectively, translating into a 29% relative reduction (HR, 0.71; P = .04).
There was no heterogeneity in benefit across prespecified subgroups such as presence or absence of diabetes, gender, or age. In RAPCO-RITA, there was 8% absolute and 31% relative reduction in all-cause mortality. In RAPCO-SV, the absolute and relative reductions were 11% and 26%.
When the trial was initiated, Dr. Hare hypothesized that RITA would prove more durable than RA, so the outcome was not anticipated.
“This is the first randomized controlled trial program to address the question,” said Dr. Hare, who noted that there have been numerous retrospective and case control analyses that have produced mixed results in the past.
Discussant praises trial quality
The AHA-invited discussant, Marc Ruel, MD, chair of cardiac surgery, University of Ottawa (Ont.) Heart Institute, called these data “important,” and he congratulated Dr. Hare for conducting the first randomized trial to address the question about second graft durability.
However, he noted that, although the study was randomized, it was not blinded, and he questioned whether postoperative care, in particular, was similar. He also pointed out that the MACE rate seemed high, particularly among the older patients randomized in RAPCO-SV.
“All of the patients were referred to an independently run CABG rehab program that was quite separate from the trial but that provided identical mandated care,” Dr. Hare responded, indicating that there was no opportunity for differences in postprocedural management.
In the United States, the SV graft is often preferred on the basis of easy harvesting and handling characteristics, according to Dr. Hare, who estimated that fewer than 10% of the 200,000 CABG procedures performed in the United States employ the RA conduit for second target vessels. He believes the RAPCO trials data support a change.
“My personal view is [that, on the basis of] this data, given that it is from a controlled trial rather than from patient-level meta-analyses, all isolated CABG operations should be using a radial graft if it is suitable,” Dr. Hare said.
Dr. Hare reports financial relationships with Abbott, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, CSL-Biotherapies, Lundbeck, Menarini, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, and Vifor. Dr. Ruel reports financial relationships with Cryolife, Edwards, and Medtronic.
AT AHA 2022
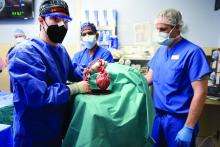
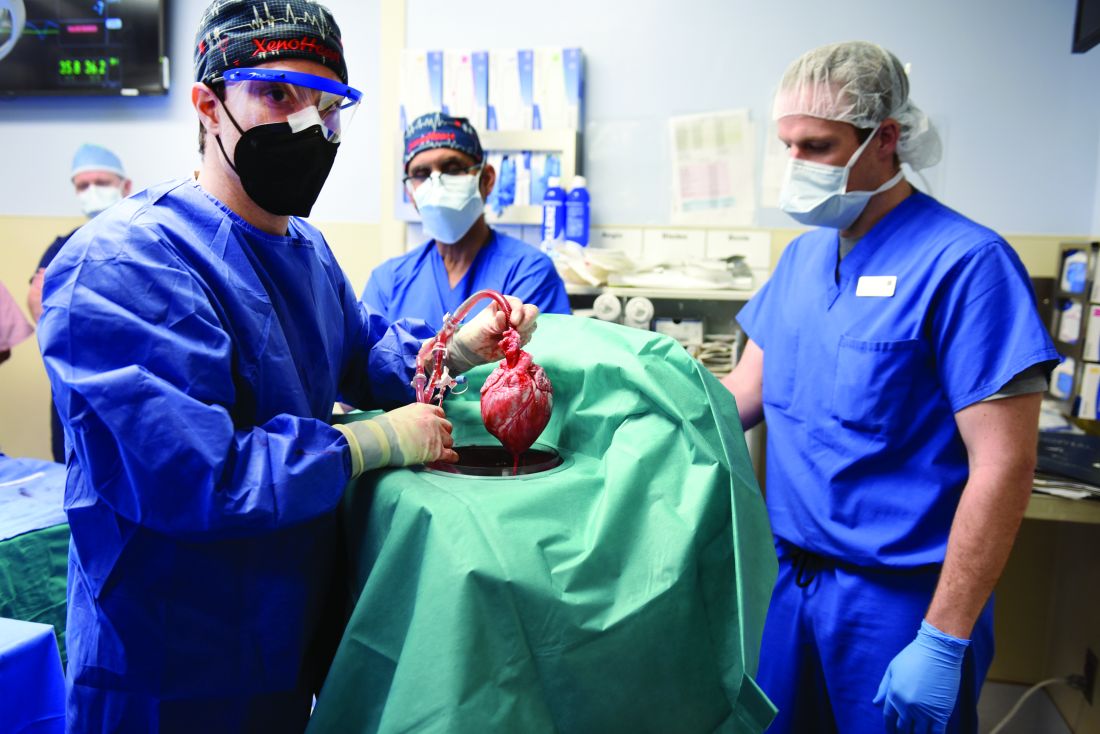
Puzzling, unique ECG from pig-to-human transplanted heart
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Diuretic agents equal to prevent CV events in hypertension: DCP
There was no difference in major cardiovascular outcomes with the use of two different diuretics – chlorthalidone or hydrochlorothiazide – in the treatment of hypertension in a new large randomized real-world study.
The Diuretic Comparison Project (DCP), which was conducted in more than 13,500 U.S. veterans age 65 years or over, showed almost identical rates of the primary composite endpoint, including myocardial infarction (MI), stroke, noncancer death, hospitalization for acute heart failure, or urgent revascularization, after a median of 2.4 years of follow-up.
There was no difference in any of the individual endpoints or other secondary cardiovascular outcomes.
However, in the subgroup of patients who had a history of MI or stroke (who made up about 10% of the study population), there was a significant reduction in the primary endpoint with chlorthalidone, whereas those without a history of MI or stroke appeared to have an increased risk for primary outcome events while receiving chlorthalidone compared with those receiving hydrochlorothiazide.
The DCP trial was presented at the American Heart Association scientific sessions by Areef Ishani, MD, director of the Minneapolis Primary Care and Specialty Care Integrated Care Community and director of the Veterans Affairs (VA) Midwest Health Care Network.
Asked how to interpret the result for clinical practice, Dr. Ishani said, “I think we can now say that either of these two drugs is appropriate to use for the treatment of hypertension.”
But he added that the decision on what to do with the subgroup of patients with previous MI or stroke was more “challenging.”
“We saw a highly significant benefit in this subgroup, but this was in the context of an overall negative trial,” he noted. “I think this is a discussion with the patients on how they want to hedge their bets. Because these two drugs are so similar, if they wanted to take one or the other because of this subgroup result I think that is a conversation to have, but I think we now need to conduct another trial specifically in this subgroup of patients to see if chlorthalidone really is of benefit in that group.”
Dr. Ishani explained that both chlorthalidone and hydrochlorothiazide have been around for more than 50 years and are considered first-line treatments for hypertension. Early studies suggested better cardiovascular outcomes and 24-hour blood pressure control with chlorthalidone, but recent observational studies have not shown more benefit with chlorthalidone. These studies have suggested that chlorthalidone may be associated with an increase in adverse events, such as hypokalemia, acute kidney injury, and chronic kidney disease.
Pragmatic study
The DCP trial was conducted to try to definitively answer this question of whether chlorthalidone is superior to hydrochlorothiazide. The pragmatic study had a “point-of-care” design that allowed participants and health care professionals to know which medication was being prescribed and to administer the medication in a real-world setting.
“Patients can continue with their normal care with their usual care team because we integrated this trial into primary care clinics,” Dr. Ishani said. “We followed participant results using their electronic health record. This study was nonintrusive, cost-effective, and inexpensive. Plus, we were able to recruit a large rural population, which is unusual for large, randomized trials, where we usually rely on big academic medical centers.”
Using VA electronic medical records, the investigators recruited primary care physicians who identified patients older than age 65 years who were receiving hydrochlorothiazide (25 mg or 50 mg) for hypertension. These patients (97% of whom were male) were then randomly assigned to continue receiving hydrochlorothiazide or to switch to an equivalent dose of chlorthalidone. Patients were followed through the electronic medical record as well as Medicare claims and the National Death Index.
Results after a median follow-up of 2.4 years showed no difference in blood pressure control between the two groups.
In terms of clinical events, the primary composite outcome of MI, stroke, noncancer death, hospitalization for acute heart failure, or urgent revascularization occurred in 10.4% of the chlorthalidone group and in 10.0% of the hydrochlorothiazide group (hazard ratio [HR], 1.04; 95% confidence interval [CI], 0.94-1.16; P = .4).
There was no difference in any individual components of the primary endpoint or the secondary outcomes of all-cause mortality, any revascularization, or erectile dysfunction.
In terms of adverse events, chlorthalidone was associated with an increase in hypokalemia (6% vs. 4.4%; HR, 1.38), but there was no difference in hospitalization for acute kidney injury.
Benefit in MI, stroke subgroup?
In the subgroup analysis, patients with a history of MI or stroke who were receiving chlorthalidone had a significant 27% reduction in the primary endpoint (HR, 0.73; 95% CI, 0.57-0.94). Conversely, patients without a history of MI or stroke appeared to do worse while taking chlorthalidone (HR, 1.12; 95% CI, 1.00-1.26).
“We were surprised by these results,” Dr. Ishani said. “We expected chlorthalidone to be more effective overall. However, learning about these differences in patients who have a history of cardiovascular disease may affect patient care. It’s best for people to talk with their health care clinicians about which of these medications is better for their individual needs.”
He added: “More research is needed to explore these results further because we don’t know how they may fit into treating the general population.”
Dr. Ishani noted that a limitations of this study was that most patients were receiving the low dose of chlorthalidone, and previous studies that suggested benefits with chlorthalidone used the higher dose.
“But the world has voted – we had 4,000 clinicians involved in this study, and the vast majority are using the low dose of hydrochlorothiazide. And this is a definitively negative study,” he said. “The world has also voted in that 10 times more patients were on hydrochlorothiazide than on chlorthalidone.”
Commenting on the study at an AHA press conference, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, pointed out that in all of the landmark National Institutes of Health hypertension trials, there was a signal for benefit with chlorthalidone compared with other antihypertensives.
“We’ve always had this concept that chlorthalidone is better,” she said. “But this study shows no difference in major cardiovascular endpoints. There was more hypokalemia with chlorthalidone, but that’s recognizable as chlorthalidone is a more potent diuretic.”
Other limitations of the DCP trial are its open-label design, which could interject some bias; the enduring effects of hydrochlorothiazide – most of these patients were receiving this agent as background therapy; and inability to look at the effectiveness of decongestion of the agents in such a pragmatic study, Dr. Bozkurt noted.
She said she would like to see more analysis in the subgroup of patients with previous MI or stroke. “Does this result mean that chlorthalidone is better for sicker patients or is this result just due to chance?” she asked.
“While this study demonstrates equal effectiveness of these two diuretics in the targeted population, the question of subgroups of patients for which we use a more potent diuretic I think remains unanswered,” she concluded.
Designated discussant of the DCP trial at the late-breaking trial session, Daniel Levy, MD, director of the Framingham Heart Study at the National Heart, Lung, and Blood Institute, reminded attendees that chlorthalidone had shown impressive results in previous important hypertension studies including SHEP and ALLHAT.
He said the current DCP was a pragmatic study addressing a knowledge gap that “would never have been performed by industry.”
Dr. Levy concluded that the results showing no difference in outcomes between the two diuretics were “compelling,” although a few questions remain.
These include a possible bias toward hydrochlorothiazide – patients were selected who were already taking that drug and so would have already had a favorable response to it. In addition, because the trial was conducted in an older male population, he questioned whether the results could be generalized to women and younger patients.
The DCP study was funded by the VA Cooperative Studies Program. Dr. Ishani reported no disclosures.
A version of this article first appeared on Medscape.com.
There was no difference in major cardiovascular outcomes with the use of two different diuretics – chlorthalidone or hydrochlorothiazide – in the treatment of hypertension in a new large randomized real-world study.
The Diuretic Comparison Project (DCP), which was conducted in more than 13,500 U.S. veterans age 65 years or over, showed almost identical rates of the primary composite endpoint, including myocardial infarction (MI), stroke, noncancer death, hospitalization for acute heart failure, or urgent revascularization, after a median of 2.4 years of follow-up.
There was no difference in any of the individual endpoints or other secondary cardiovascular outcomes.
However, in the subgroup of patients who had a history of MI or stroke (who made up about 10% of the study population), there was a significant reduction in the primary endpoint with chlorthalidone, whereas those without a history of MI or stroke appeared to have an increased risk for primary outcome events while receiving chlorthalidone compared with those receiving hydrochlorothiazide.
The DCP trial was presented at the American Heart Association scientific sessions by Areef Ishani, MD, director of the Minneapolis Primary Care and Specialty Care Integrated Care Community and director of the Veterans Affairs (VA) Midwest Health Care Network.
Asked how to interpret the result for clinical practice, Dr. Ishani said, “I think we can now say that either of these two drugs is appropriate to use for the treatment of hypertension.”
But he added that the decision on what to do with the subgroup of patients with previous MI or stroke was more “challenging.”
“We saw a highly significant benefit in this subgroup, but this was in the context of an overall negative trial,” he noted. “I think this is a discussion with the patients on how they want to hedge their bets. Because these two drugs are so similar, if they wanted to take one or the other because of this subgroup result I think that is a conversation to have, but I think we now need to conduct another trial specifically in this subgroup of patients to see if chlorthalidone really is of benefit in that group.”
Dr. Ishani explained that both chlorthalidone and hydrochlorothiazide have been around for more than 50 years and are considered first-line treatments for hypertension. Early studies suggested better cardiovascular outcomes and 24-hour blood pressure control with chlorthalidone, but recent observational studies have not shown more benefit with chlorthalidone. These studies have suggested that chlorthalidone may be associated with an increase in adverse events, such as hypokalemia, acute kidney injury, and chronic kidney disease.
Pragmatic study
The DCP trial was conducted to try to definitively answer this question of whether chlorthalidone is superior to hydrochlorothiazide. The pragmatic study had a “point-of-care” design that allowed participants and health care professionals to know which medication was being prescribed and to administer the medication in a real-world setting.
“Patients can continue with their normal care with their usual care team because we integrated this trial into primary care clinics,” Dr. Ishani said. “We followed participant results using their electronic health record. This study was nonintrusive, cost-effective, and inexpensive. Plus, we were able to recruit a large rural population, which is unusual for large, randomized trials, where we usually rely on big academic medical centers.”
Using VA electronic medical records, the investigators recruited primary care physicians who identified patients older than age 65 years who were receiving hydrochlorothiazide (25 mg or 50 mg) for hypertension. These patients (97% of whom were male) were then randomly assigned to continue receiving hydrochlorothiazide or to switch to an equivalent dose of chlorthalidone. Patients were followed through the electronic medical record as well as Medicare claims and the National Death Index.
Results after a median follow-up of 2.4 years showed no difference in blood pressure control between the two groups.
In terms of clinical events, the primary composite outcome of MI, stroke, noncancer death, hospitalization for acute heart failure, or urgent revascularization occurred in 10.4% of the chlorthalidone group and in 10.0% of the hydrochlorothiazide group (hazard ratio [HR], 1.04; 95% confidence interval [CI], 0.94-1.16; P = .4).
There was no difference in any individual components of the primary endpoint or the secondary outcomes of all-cause mortality, any revascularization, or erectile dysfunction.
In terms of adverse events, chlorthalidone was associated with an increase in hypokalemia (6% vs. 4.4%; HR, 1.38), but there was no difference in hospitalization for acute kidney injury.
Benefit in MI, stroke subgroup?
In the subgroup analysis, patients with a history of MI or stroke who were receiving chlorthalidone had a significant 27% reduction in the primary endpoint (HR, 0.73; 95% CI, 0.57-0.94). Conversely, patients without a history of MI or stroke appeared to do worse while taking chlorthalidone (HR, 1.12; 95% CI, 1.00-1.26).
“We were surprised by these results,” Dr. Ishani said. “We expected chlorthalidone to be more effective overall. However, learning about these differences in patients who have a history of cardiovascular disease may affect patient care. It’s best for people to talk with their health care clinicians about which of these medications is better for their individual needs.”
He added: “More research is needed to explore these results further because we don’t know how they may fit into treating the general population.”
Dr. Ishani noted that a limitations of this study was that most patients were receiving the low dose of chlorthalidone, and previous studies that suggested benefits with chlorthalidone used the higher dose.
“But the world has voted – we had 4,000 clinicians involved in this study, and the vast majority are using the low dose of hydrochlorothiazide. And this is a definitively negative study,” he said. “The world has also voted in that 10 times more patients were on hydrochlorothiazide than on chlorthalidone.”
Commenting on the study at an AHA press conference, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, pointed out that in all of the landmark National Institutes of Health hypertension trials, there was a signal for benefit with chlorthalidone compared with other antihypertensives.
“We’ve always had this concept that chlorthalidone is better,” she said. “But this study shows no difference in major cardiovascular endpoints. There was more hypokalemia with chlorthalidone, but that’s recognizable as chlorthalidone is a more potent diuretic.”
Other limitations of the DCP trial are its open-label design, which could interject some bias; the enduring effects of hydrochlorothiazide – most of these patients were receiving this agent as background therapy; and inability to look at the effectiveness of decongestion of the agents in such a pragmatic study, Dr. Bozkurt noted.
She said she would like to see more analysis in the subgroup of patients with previous MI or stroke. “Does this result mean that chlorthalidone is better for sicker patients or is this result just due to chance?” she asked.
“While this study demonstrates equal effectiveness of these two diuretics in the targeted population, the question of subgroups of patients for which we use a more potent diuretic I think remains unanswered,” she concluded.
Designated discussant of the DCP trial at the late-breaking trial session, Daniel Levy, MD, director of the Framingham Heart Study at the National Heart, Lung, and Blood Institute, reminded attendees that chlorthalidone had shown impressive results in previous important hypertension studies including SHEP and ALLHAT.
He said the current DCP was a pragmatic study addressing a knowledge gap that “would never have been performed by industry.”
Dr. Levy concluded that the results showing no difference in outcomes between the two diuretics were “compelling,” although a few questions remain.
These include a possible bias toward hydrochlorothiazide – patients were selected who were already taking that drug and so would have already had a favorable response to it. In addition, because the trial was conducted in an older male population, he questioned whether the results could be generalized to women and younger patients.
The DCP study was funded by the VA Cooperative Studies Program. Dr. Ishani reported no disclosures.
A version of this article first appeared on Medscape.com.
There was no difference in major cardiovascular outcomes with the use of two different diuretics – chlorthalidone or hydrochlorothiazide – in the treatment of hypertension in a new large randomized real-world study.
The Diuretic Comparison Project (DCP), which was conducted in more than 13,500 U.S. veterans age 65 years or over, showed almost identical rates of the primary composite endpoint, including myocardial infarction (MI), stroke, noncancer death, hospitalization for acute heart failure, or urgent revascularization, after a median of 2.4 years of follow-up.
There was no difference in any of the individual endpoints or other secondary cardiovascular outcomes.
However, in the subgroup of patients who had a history of MI or stroke (who made up about 10% of the study population), there was a significant reduction in the primary endpoint with chlorthalidone, whereas those without a history of MI or stroke appeared to have an increased risk for primary outcome events while receiving chlorthalidone compared with those receiving hydrochlorothiazide.
The DCP trial was presented at the American Heart Association scientific sessions by Areef Ishani, MD, director of the Minneapolis Primary Care and Specialty Care Integrated Care Community and director of the Veterans Affairs (VA) Midwest Health Care Network.
Asked how to interpret the result for clinical practice, Dr. Ishani said, “I think we can now say that either of these two drugs is appropriate to use for the treatment of hypertension.”
But he added that the decision on what to do with the subgroup of patients with previous MI or stroke was more “challenging.”
“We saw a highly significant benefit in this subgroup, but this was in the context of an overall negative trial,” he noted. “I think this is a discussion with the patients on how they want to hedge their bets. Because these two drugs are so similar, if they wanted to take one or the other because of this subgroup result I think that is a conversation to have, but I think we now need to conduct another trial specifically in this subgroup of patients to see if chlorthalidone really is of benefit in that group.”
Dr. Ishani explained that both chlorthalidone and hydrochlorothiazide have been around for more than 50 years and are considered first-line treatments for hypertension. Early studies suggested better cardiovascular outcomes and 24-hour blood pressure control with chlorthalidone, but recent observational studies have not shown more benefit with chlorthalidone. These studies have suggested that chlorthalidone may be associated with an increase in adverse events, such as hypokalemia, acute kidney injury, and chronic kidney disease.
Pragmatic study
The DCP trial was conducted to try to definitively answer this question of whether chlorthalidone is superior to hydrochlorothiazide. The pragmatic study had a “point-of-care” design that allowed participants and health care professionals to know which medication was being prescribed and to administer the medication in a real-world setting.
“Patients can continue with their normal care with their usual care team because we integrated this trial into primary care clinics,” Dr. Ishani said. “We followed participant results using their electronic health record. This study was nonintrusive, cost-effective, and inexpensive. Plus, we were able to recruit a large rural population, which is unusual for large, randomized trials, where we usually rely on big academic medical centers.”
Using VA electronic medical records, the investigators recruited primary care physicians who identified patients older than age 65 years who were receiving hydrochlorothiazide (25 mg or 50 mg) for hypertension. These patients (97% of whom were male) were then randomly assigned to continue receiving hydrochlorothiazide or to switch to an equivalent dose of chlorthalidone. Patients were followed through the electronic medical record as well as Medicare claims and the National Death Index.
Results after a median follow-up of 2.4 years showed no difference in blood pressure control between the two groups.
In terms of clinical events, the primary composite outcome of MI, stroke, noncancer death, hospitalization for acute heart failure, or urgent revascularization occurred in 10.4% of the chlorthalidone group and in 10.0% of the hydrochlorothiazide group (hazard ratio [HR], 1.04; 95% confidence interval [CI], 0.94-1.16; P = .4).
There was no difference in any individual components of the primary endpoint or the secondary outcomes of all-cause mortality, any revascularization, or erectile dysfunction.
In terms of adverse events, chlorthalidone was associated with an increase in hypokalemia (6% vs. 4.4%; HR, 1.38), but there was no difference in hospitalization for acute kidney injury.
Benefit in MI, stroke subgroup?
In the subgroup analysis, patients with a history of MI or stroke who were receiving chlorthalidone had a significant 27% reduction in the primary endpoint (HR, 0.73; 95% CI, 0.57-0.94). Conversely, patients without a history of MI or stroke appeared to do worse while taking chlorthalidone (HR, 1.12; 95% CI, 1.00-1.26).
“We were surprised by these results,” Dr. Ishani said. “We expected chlorthalidone to be more effective overall. However, learning about these differences in patients who have a history of cardiovascular disease may affect patient care. It’s best for people to talk with their health care clinicians about which of these medications is better for their individual needs.”
He added: “More research is needed to explore these results further because we don’t know how they may fit into treating the general population.”
Dr. Ishani noted that a limitations of this study was that most patients were receiving the low dose of chlorthalidone, and previous studies that suggested benefits with chlorthalidone used the higher dose.
“But the world has voted – we had 4,000 clinicians involved in this study, and the vast majority are using the low dose of hydrochlorothiazide. And this is a definitively negative study,” he said. “The world has also voted in that 10 times more patients were on hydrochlorothiazide than on chlorthalidone.”
Commenting on the study at an AHA press conference, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, pointed out that in all of the landmark National Institutes of Health hypertension trials, there was a signal for benefit with chlorthalidone compared with other antihypertensives.
“We’ve always had this concept that chlorthalidone is better,” she said. “But this study shows no difference in major cardiovascular endpoints. There was more hypokalemia with chlorthalidone, but that’s recognizable as chlorthalidone is a more potent diuretic.”
Other limitations of the DCP trial are its open-label design, which could interject some bias; the enduring effects of hydrochlorothiazide – most of these patients were receiving this agent as background therapy; and inability to look at the effectiveness of decongestion of the agents in such a pragmatic study, Dr. Bozkurt noted.
She said she would like to see more analysis in the subgroup of patients with previous MI or stroke. “Does this result mean that chlorthalidone is better for sicker patients or is this result just due to chance?” she asked.
“While this study demonstrates equal effectiveness of these two diuretics in the targeted population, the question of subgroups of patients for which we use a more potent diuretic I think remains unanswered,” she concluded.
Designated discussant of the DCP trial at the late-breaking trial session, Daniel Levy, MD, director of the Framingham Heart Study at the National Heart, Lung, and Blood Institute, reminded attendees that chlorthalidone had shown impressive results in previous important hypertension studies including SHEP and ALLHAT.
He said the current DCP was a pragmatic study addressing a knowledge gap that “would never have been performed by industry.”
Dr. Levy concluded that the results showing no difference in outcomes between the two diuretics were “compelling,” although a few questions remain.
These include a possible bias toward hydrochlorothiazide – patients were selected who were already taking that drug and so would have already had a favorable response to it. In addition, because the trial was conducted in an older male population, he questioned whether the results could be generalized to women and younger patients.
The DCP study was funded by the VA Cooperative Studies Program. Dr. Ishani reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Triglyceride-lowering fails to show CV benefit in large fibrate trial
Twenty-five percent reduction has no effect
CHICAGO – Despite a 25% reduction in triglycerides (TGs) along with similar reductions in very-low-density lipoprotein (VLDL), and remnant cholesterol, a novel agent failed to provide any protection in a multinational trial against a composite endpoint of major adverse cardiovascular events (MACE) in patients with type 2 diabetes.
“Our data further highlight the complexity of lipid mediators of residual risk among patients with insulin resistance who are receiving statin therapy,” reported Aruna Das Pradhan, MD, of Harvard Medical School, Boston, and Queen Mary University, London.
It is the most recent in a series of trials that have failed to associate a meaningful reduction in TGs with protection from a composite MACE endpoint. This is a pattern that dates back 20 years, even though earlier trials did suggest that hypertriglyceridemia was a targetable risk factor.
No benefit from fibrates seen in statin era
“We have not seen a significant cardiovascular event reduction with a fibrate in the statin era,” according to Karol Watson, MD, PhD, director of the UCLA Women’s Cardiovascular Health Center, Los Angeles.
In the statin era, which began soon after the Helsinki Heart Study was published in 1987, Dr. Watson counted at least five studies with fibrates that had a null result.
In the setting of good control of LDL cholesterol, “fibrates have not been shown to further lower CV risk,” said Dr. Watson, who was invited by the AHA to discuss the PROMINENT trial.
In PROMINENT, 10,497 patients with type 2 diabetes were randomized to pemafibrate, a peroxisome proliferator-activated receptor a (PPAR-a) agonist, or placebo. Pemafibrate is not currently available in North America or Europe, but it is licensed in Japan for the treatment of hypertriglyceridemia.
The primary efficacy endpoint of the double-blind trial was a composite endpoint of nonfatal myocardial infarction, ischemic stroke, coronary revascularization, or death.
The patients were eligible if they had TG levels from 200 to 400 mg/dL and HDL cholesterol levels of 40 mg/dL or below. Pemafibrate in a dose of 0.2 mg or placebo were taken twice daily. About two-thirds had a prior history of coronary heart disease. The goal was primary prevention in the remainder.
After a median follow-up of 3.4 years when the study was stopped for futility, the proportion of patients reaching a primary endpoint was slightly greater in the experimental arm (3.60 vs. 3.51 events per 100 patient-years). The hazard ratio, although not significant, was nominally in favor of placebo (hazard ratio, 1.03; P = .67).
When events within the composite endpoint were assessed individually, there was no signal of benefit for any outcome. The rates of death from any cause, although numerically higher in the pemafibrate group (2.44 vs. 2.34 per 100 patient years), were also comparable.
Lipid profile improved as predicted
Yet, in regard to an improvement in the lipid profile, pemafibrate performed as predicted. When compared to placebo 4 months into the trial, pemafibrate was associated with median reductions of 26.2% in TGs, 25.8% in VLDL, and 25.6% in remnant cholesterol, which is cholesterol transported in TG-rich lipoproteins after lipolysis and lipoprotein remodeling.
Furthermore, pemafibrate was associated with a median 27.6% reduction relative to placebo in apolipoprotein C-III and a median 4.8% reduction in apolipoprotein E, all of which would be expected to reduce CV risk.
The findings of PROMINENT were published online in the New England Journal of Medicine immediately after their presentation.
The findings of this study do not eliminate any hope for lowering residual CV risk with TG reductions, but they do suggest the relationship with other lipid subfractions is complex, according to Salim S. Virani, MD, PhD, a professor of cardiology at Baylor College of Medicine, Houston.
“I think that the lack of efficacy despite TG lowering may be largely due to a lack of an overall decrease in the apolipoprotein B level,” speculated Dr. Virani, who wrote an editorial that accompanied publication of the PROMINENT results.
He noted that pemafibrate is implicated in converting remnant cholesterol to LDL cholesterol, which might be one reason for a counterproductive effect on CV risk.
“In order for therapies that lower TG levels to be effective, they probably have to have mechanisms to increase clearance of TG-rich remnant lipoprotein cholesterol particles rather than just converting remnant lipoproteins to LDL,” Dr. Virani explained in an attempt to unravel the interplay of these variables.
Although this study enrolled patients “who would be predicted to have the most benefit from a TG-lowering strategy,” Dr. Watson agreed that these results do not necessarily extend to other means of lowering TG. However, it might draw into question the value of pemafibrate and perhaps other drugs in this class for treatment of hypertriglyceridemia. In addition to a lack of CV benefit, treatment was not without risks, including a higher rate of thromboembolism and adverse renal events.
Dr. Das Pradhan reported financial relationships with Denka, Medtelligence, Optum, Novo Nordisk, and Kowa, which provided funding for this trial. Dr. Watson reported financial relationships with Amarin, Amgen, Boehringer-Ingelheim, and Esperion.
Twenty-five percent reduction has no effect
Twenty-five percent reduction has no effect
CHICAGO – Despite a 25% reduction in triglycerides (TGs) along with similar reductions in very-low-density lipoprotein (VLDL), and remnant cholesterol, a novel agent failed to provide any protection in a multinational trial against a composite endpoint of major adverse cardiovascular events (MACE) in patients with type 2 diabetes.
“Our data further highlight the complexity of lipid mediators of residual risk among patients with insulin resistance who are receiving statin therapy,” reported Aruna Das Pradhan, MD, of Harvard Medical School, Boston, and Queen Mary University, London.
It is the most recent in a series of trials that have failed to associate a meaningful reduction in TGs with protection from a composite MACE endpoint. This is a pattern that dates back 20 years, even though earlier trials did suggest that hypertriglyceridemia was a targetable risk factor.
No benefit from fibrates seen in statin era
“We have not seen a significant cardiovascular event reduction with a fibrate in the statin era,” according to Karol Watson, MD, PhD, director of the UCLA Women’s Cardiovascular Health Center, Los Angeles.
In the statin era, which began soon after the Helsinki Heart Study was published in 1987, Dr. Watson counted at least five studies with fibrates that had a null result.
In the setting of good control of LDL cholesterol, “fibrates have not been shown to further lower CV risk,” said Dr. Watson, who was invited by the AHA to discuss the PROMINENT trial.
In PROMINENT, 10,497 patients with type 2 diabetes were randomized to pemafibrate, a peroxisome proliferator-activated receptor a (PPAR-a) agonist, or placebo. Pemafibrate is not currently available in North America or Europe, but it is licensed in Japan for the treatment of hypertriglyceridemia.
The primary efficacy endpoint of the double-blind trial was a composite endpoint of nonfatal myocardial infarction, ischemic stroke, coronary revascularization, or death.
The patients were eligible if they had TG levels from 200 to 400 mg/dL and HDL cholesterol levels of 40 mg/dL or below. Pemafibrate in a dose of 0.2 mg or placebo were taken twice daily. About two-thirds had a prior history of coronary heart disease. The goal was primary prevention in the remainder.
After a median follow-up of 3.4 years when the study was stopped for futility, the proportion of patients reaching a primary endpoint was slightly greater in the experimental arm (3.60 vs. 3.51 events per 100 patient-years). The hazard ratio, although not significant, was nominally in favor of placebo (hazard ratio, 1.03; P = .67).
When events within the composite endpoint were assessed individually, there was no signal of benefit for any outcome. The rates of death from any cause, although numerically higher in the pemafibrate group (2.44 vs. 2.34 per 100 patient years), were also comparable.
Lipid profile improved as predicted
Yet, in regard to an improvement in the lipid profile, pemafibrate performed as predicted. When compared to placebo 4 months into the trial, pemafibrate was associated with median reductions of 26.2% in TGs, 25.8% in VLDL, and 25.6% in remnant cholesterol, which is cholesterol transported in TG-rich lipoproteins after lipolysis and lipoprotein remodeling.
Furthermore, pemafibrate was associated with a median 27.6% reduction relative to placebo in apolipoprotein C-III and a median 4.8% reduction in apolipoprotein E, all of which would be expected to reduce CV risk.
The findings of PROMINENT were published online in the New England Journal of Medicine immediately after their presentation.
The findings of this study do not eliminate any hope for lowering residual CV risk with TG reductions, but they do suggest the relationship with other lipid subfractions is complex, according to Salim S. Virani, MD, PhD, a professor of cardiology at Baylor College of Medicine, Houston.
“I think that the lack of efficacy despite TG lowering may be largely due to a lack of an overall decrease in the apolipoprotein B level,” speculated Dr. Virani, who wrote an editorial that accompanied publication of the PROMINENT results.
He noted that pemafibrate is implicated in converting remnant cholesterol to LDL cholesterol, which might be one reason for a counterproductive effect on CV risk.
“In order for therapies that lower TG levels to be effective, they probably have to have mechanisms to increase clearance of TG-rich remnant lipoprotein cholesterol particles rather than just converting remnant lipoproteins to LDL,” Dr. Virani explained in an attempt to unravel the interplay of these variables.
Although this study enrolled patients “who would be predicted to have the most benefit from a TG-lowering strategy,” Dr. Watson agreed that these results do not necessarily extend to other means of lowering TG. However, it might draw into question the value of pemafibrate and perhaps other drugs in this class for treatment of hypertriglyceridemia. In addition to a lack of CV benefit, treatment was not without risks, including a higher rate of thromboembolism and adverse renal events.
Dr. Das Pradhan reported financial relationships with Denka, Medtelligence, Optum, Novo Nordisk, and Kowa, which provided funding for this trial. Dr. Watson reported financial relationships with Amarin, Amgen, Boehringer-Ingelheim, and Esperion.
CHICAGO – Despite a 25% reduction in triglycerides (TGs) along with similar reductions in very-low-density lipoprotein (VLDL), and remnant cholesterol, a novel agent failed to provide any protection in a multinational trial against a composite endpoint of major adverse cardiovascular events (MACE) in patients with type 2 diabetes.
“Our data further highlight the complexity of lipid mediators of residual risk among patients with insulin resistance who are receiving statin therapy,” reported Aruna Das Pradhan, MD, of Harvard Medical School, Boston, and Queen Mary University, London.
It is the most recent in a series of trials that have failed to associate a meaningful reduction in TGs with protection from a composite MACE endpoint. This is a pattern that dates back 20 years, even though earlier trials did suggest that hypertriglyceridemia was a targetable risk factor.
No benefit from fibrates seen in statin era
“We have not seen a significant cardiovascular event reduction with a fibrate in the statin era,” according to Karol Watson, MD, PhD, director of the UCLA Women’s Cardiovascular Health Center, Los Angeles.
In the statin era, which began soon after the Helsinki Heart Study was published in 1987, Dr. Watson counted at least five studies with fibrates that had a null result.
In the setting of good control of LDL cholesterol, “fibrates have not been shown to further lower CV risk,” said Dr. Watson, who was invited by the AHA to discuss the PROMINENT trial.
In PROMINENT, 10,497 patients with type 2 diabetes were randomized to pemafibrate, a peroxisome proliferator-activated receptor a (PPAR-a) agonist, or placebo. Pemafibrate is not currently available in North America or Europe, but it is licensed in Japan for the treatment of hypertriglyceridemia.
The primary efficacy endpoint of the double-blind trial was a composite endpoint of nonfatal myocardial infarction, ischemic stroke, coronary revascularization, or death.
The patients were eligible if they had TG levels from 200 to 400 mg/dL and HDL cholesterol levels of 40 mg/dL or below. Pemafibrate in a dose of 0.2 mg or placebo were taken twice daily. About two-thirds had a prior history of coronary heart disease. The goal was primary prevention in the remainder.
After a median follow-up of 3.4 years when the study was stopped for futility, the proportion of patients reaching a primary endpoint was slightly greater in the experimental arm (3.60 vs. 3.51 events per 100 patient-years). The hazard ratio, although not significant, was nominally in favor of placebo (hazard ratio, 1.03; P = .67).
When events within the composite endpoint were assessed individually, there was no signal of benefit for any outcome. The rates of death from any cause, although numerically higher in the pemafibrate group (2.44 vs. 2.34 per 100 patient years), were also comparable.
Lipid profile improved as predicted
Yet, in regard to an improvement in the lipid profile, pemafibrate performed as predicted. When compared to placebo 4 months into the trial, pemafibrate was associated with median reductions of 26.2% in TGs, 25.8% in VLDL, and 25.6% in remnant cholesterol, which is cholesterol transported in TG-rich lipoproteins after lipolysis and lipoprotein remodeling.
Furthermore, pemafibrate was associated with a median 27.6% reduction relative to placebo in apolipoprotein C-III and a median 4.8% reduction in apolipoprotein E, all of which would be expected to reduce CV risk.
The findings of PROMINENT were published online in the New England Journal of Medicine immediately after their presentation.
The findings of this study do not eliminate any hope for lowering residual CV risk with TG reductions, but they do suggest the relationship with other lipid subfractions is complex, according to Salim S. Virani, MD, PhD, a professor of cardiology at Baylor College of Medicine, Houston.
“I think that the lack of efficacy despite TG lowering may be largely due to a lack of an overall decrease in the apolipoprotein B level,” speculated Dr. Virani, who wrote an editorial that accompanied publication of the PROMINENT results.
He noted that pemafibrate is implicated in converting remnant cholesterol to LDL cholesterol, which might be one reason for a counterproductive effect on CV risk.
“In order for therapies that lower TG levels to be effective, they probably have to have mechanisms to increase clearance of TG-rich remnant lipoprotein cholesterol particles rather than just converting remnant lipoproteins to LDL,” Dr. Virani explained in an attempt to unravel the interplay of these variables.
Although this study enrolled patients “who would be predicted to have the most benefit from a TG-lowering strategy,” Dr. Watson agreed that these results do not necessarily extend to other means of lowering TG. However, it might draw into question the value of pemafibrate and perhaps other drugs in this class for treatment of hypertriglyceridemia. In addition to a lack of CV benefit, treatment was not without risks, including a higher rate of thromboembolism and adverse renal events.
Dr. Das Pradhan reported financial relationships with Denka, Medtelligence, Optum, Novo Nordisk, and Kowa, which provided funding for this trial. Dr. Watson reported financial relationships with Amarin, Amgen, Boehringer-Ingelheim, and Esperion.
AT AHA 2022
ACC/AHA issues updated guidance on aortic disease
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Working while sick: Why doctors don’t stay home when ill
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
Stroke risk rises with years of drinking in young adults
“The rate of stroke among young adults has been increasing over the last few decades, and stroke in young adults causes death and serious disability,” study coauthor Eue-Keun Choi, MD, PhD, of Seoul National University, Republic of Korea, said in a statement.
“If we could prevent stroke in young adults by reducing alcohol consumption, that could potentially have a substantial impact on the health of individuals and the overall burden of stroke on society,” Dr. Choi added.
The study was published online in Neurology.
Compounding effects
Using data from a Korean national health database, the researchers identified roughly 1.5 million adults aged 20-39 years (mean age 29.5 years, 72% male) who had four consecutive annual health examinations during which they were asked about their alcohol use.
During a median follow-up of roughly 6 years, 3,153 individuals suffered a stroke.
After multivariate adjustment accounting for other factors that could affect the risk for stroke, such as hypertension, smoking, and body mass index, the risk of stroke increased steadily with the number of years of moderate to heavy drinking, defined as 105 grams or more of alcohol per week.
Compared with light drinkers or teetotalers, stroke risk increased 19% with 2 years of moderate to heavy drinking and 22% and 23%, respectively, for 3 and 4 years of moderate or heaving drinking.
The positive dose-response relationship was chiefly driven by increased risk of hemorrhagic stroke; with 2, 3 and 4 years of moderate to heavy drinking, hemorrhagic stroke risk increased 30%, 42% and 36%, respectively, relative to light/no drinking.
“Since more than 90% of the burden of stroke overall can be attributed to potentially modifiable risk factors, including alcohol consumption, and since stroke in young adults severely impacts both the individual and society by limiting their activities during their most productive years, reducing alcohol consumption should be emphasized in young adults with heavy drinking habits as part of any strategy to prevent stroke,” Dr. Choi said.
A limitation of the study is that only Korean people were included, so the results may not apply to people of other races and ethnicities, they noted. In addition, people filled out questionnaires about their alcohol consumption, which may introduce recall bias.
Consistent evidence
“For decades, the evidence was suggestive that a moderate amount of alcohol daily is actually beneficial – one to two drinks in men and one drink in women – at reducing major vascular outcomes,” Pierre Fayad, MD, professor, department of neurological sciences, and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said in commenting on the research.
Yet, over the past few years, some research has found no evidence of benefit with moderate alcohol intake. There is, however, “consistent evidence” that shows a detrimental effect of excessive alcohol, particularly binge drinking, especially in young adults, Dr. Fayad said.
This study, he said, “adds to the known detrimental effects of excessive alcohol intake, in increasing the risk of stroke, particularly in young adults.
“The bottom line: Young adults who usually have a low risk of stroke increase their risk significantly by heavy alcohol drinking, and Koreans are equally at risk as other populations,” Dr. Fayad said.
The study was supported by the Korea Medical Device Development Fund and the Korea National Research Foundation. Dr. Choi and Dr. Fayad report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The rate of stroke among young adults has been increasing over the last few decades, and stroke in young adults causes death and serious disability,” study coauthor Eue-Keun Choi, MD, PhD, of Seoul National University, Republic of Korea, said in a statement.
“If we could prevent stroke in young adults by reducing alcohol consumption, that could potentially have a substantial impact on the health of individuals and the overall burden of stroke on society,” Dr. Choi added.
The study was published online in Neurology.
Compounding effects
Using data from a Korean national health database, the researchers identified roughly 1.5 million adults aged 20-39 years (mean age 29.5 years, 72% male) who had four consecutive annual health examinations during which they were asked about their alcohol use.
During a median follow-up of roughly 6 years, 3,153 individuals suffered a stroke.
After multivariate adjustment accounting for other factors that could affect the risk for stroke, such as hypertension, smoking, and body mass index, the risk of stroke increased steadily with the number of years of moderate to heavy drinking, defined as 105 grams or more of alcohol per week.
Compared with light drinkers or teetotalers, stroke risk increased 19% with 2 years of moderate to heavy drinking and 22% and 23%, respectively, for 3 and 4 years of moderate or heaving drinking.
The positive dose-response relationship was chiefly driven by increased risk of hemorrhagic stroke; with 2, 3 and 4 years of moderate to heavy drinking, hemorrhagic stroke risk increased 30%, 42% and 36%, respectively, relative to light/no drinking.
“Since more than 90% of the burden of stroke overall can be attributed to potentially modifiable risk factors, including alcohol consumption, and since stroke in young adults severely impacts both the individual and society by limiting their activities during their most productive years, reducing alcohol consumption should be emphasized in young adults with heavy drinking habits as part of any strategy to prevent stroke,” Dr. Choi said.
A limitation of the study is that only Korean people were included, so the results may not apply to people of other races and ethnicities, they noted. In addition, people filled out questionnaires about their alcohol consumption, which may introduce recall bias.
Consistent evidence
“For decades, the evidence was suggestive that a moderate amount of alcohol daily is actually beneficial – one to two drinks in men and one drink in women – at reducing major vascular outcomes,” Pierre Fayad, MD, professor, department of neurological sciences, and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said in commenting on the research.
Yet, over the past few years, some research has found no evidence of benefit with moderate alcohol intake. There is, however, “consistent evidence” that shows a detrimental effect of excessive alcohol, particularly binge drinking, especially in young adults, Dr. Fayad said.
This study, he said, “adds to the known detrimental effects of excessive alcohol intake, in increasing the risk of stroke, particularly in young adults.
“The bottom line: Young adults who usually have a low risk of stroke increase their risk significantly by heavy alcohol drinking, and Koreans are equally at risk as other populations,” Dr. Fayad said.
The study was supported by the Korea Medical Device Development Fund and the Korea National Research Foundation. Dr. Choi and Dr. Fayad report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The rate of stroke among young adults has been increasing over the last few decades, and stroke in young adults causes death and serious disability,” study coauthor Eue-Keun Choi, MD, PhD, of Seoul National University, Republic of Korea, said in a statement.
“If we could prevent stroke in young adults by reducing alcohol consumption, that could potentially have a substantial impact on the health of individuals and the overall burden of stroke on society,” Dr. Choi added.
The study was published online in Neurology.
Compounding effects
Using data from a Korean national health database, the researchers identified roughly 1.5 million adults aged 20-39 years (mean age 29.5 years, 72% male) who had four consecutive annual health examinations during which they were asked about their alcohol use.
During a median follow-up of roughly 6 years, 3,153 individuals suffered a stroke.
After multivariate adjustment accounting for other factors that could affect the risk for stroke, such as hypertension, smoking, and body mass index, the risk of stroke increased steadily with the number of years of moderate to heavy drinking, defined as 105 grams or more of alcohol per week.
Compared with light drinkers or teetotalers, stroke risk increased 19% with 2 years of moderate to heavy drinking and 22% and 23%, respectively, for 3 and 4 years of moderate or heaving drinking.
The positive dose-response relationship was chiefly driven by increased risk of hemorrhagic stroke; with 2, 3 and 4 years of moderate to heavy drinking, hemorrhagic stroke risk increased 30%, 42% and 36%, respectively, relative to light/no drinking.
“Since more than 90% of the burden of stroke overall can be attributed to potentially modifiable risk factors, including alcohol consumption, and since stroke in young adults severely impacts both the individual and society by limiting their activities during their most productive years, reducing alcohol consumption should be emphasized in young adults with heavy drinking habits as part of any strategy to prevent stroke,” Dr. Choi said.
A limitation of the study is that only Korean people were included, so the results may not apply to people of other races and ethnicities, they noted. In addition, people filled out questionnaires about their alcohol consumption, which may introduce recall bias.
Consistent evidence
“For decades, the evidence was suggestive that a moderate amount of alcohol daily is actually beneficial – one to two drinks in men and one drink in women – at reducing major vascular outcomes,” Pierre Fayad, MD, professor, department of neurological sciences, and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said in commenting on the research.
Yet, over the past few years, some research has found no evidence of benefit with moderate alcohol intake. There is, however, “consistent evidence” that shows a detrimental effect of excessive alcohol, particularly binge drinking, especially in young adults, Dr. Fayad said.
This study, he said, “adds to the known detrimental effects of excessive alcohol intake, in increasing the risk of stroke, particularly in young adults.
“The bottom line: Young adults who usually have a low risk of stroke increase their risk significantly by heavy alcohol drinking, and Koreans are equally at risk as other populations,” Dr. Fayad said.
The study was supported by the Korea Medical Device Development Fund and the Korea National Research Foundation. Dr. Choi and Dr. Fayad report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
A 95-year-old White male with hypertension presented with itchy patches and bullae on the trunk and extremities
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.