User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Clinical Pearl: Mohs Cantaloupe Analogy for the Dermatology Resident
Practice Gap
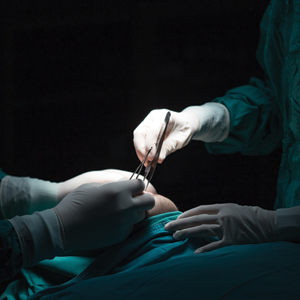
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
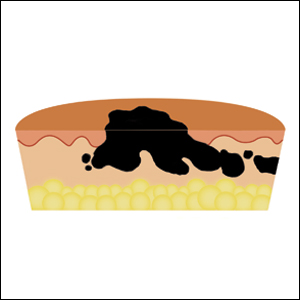
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).

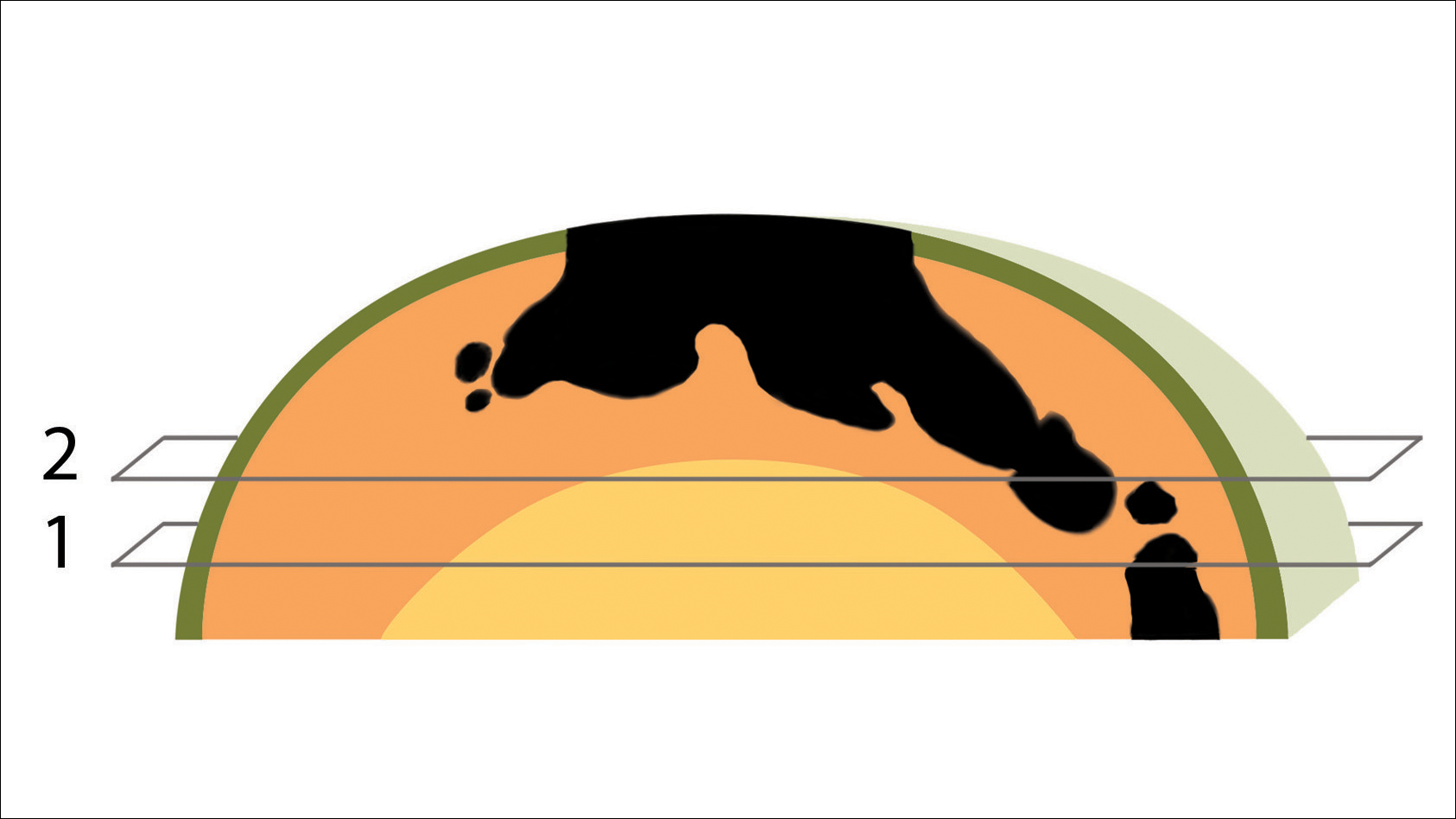
In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.
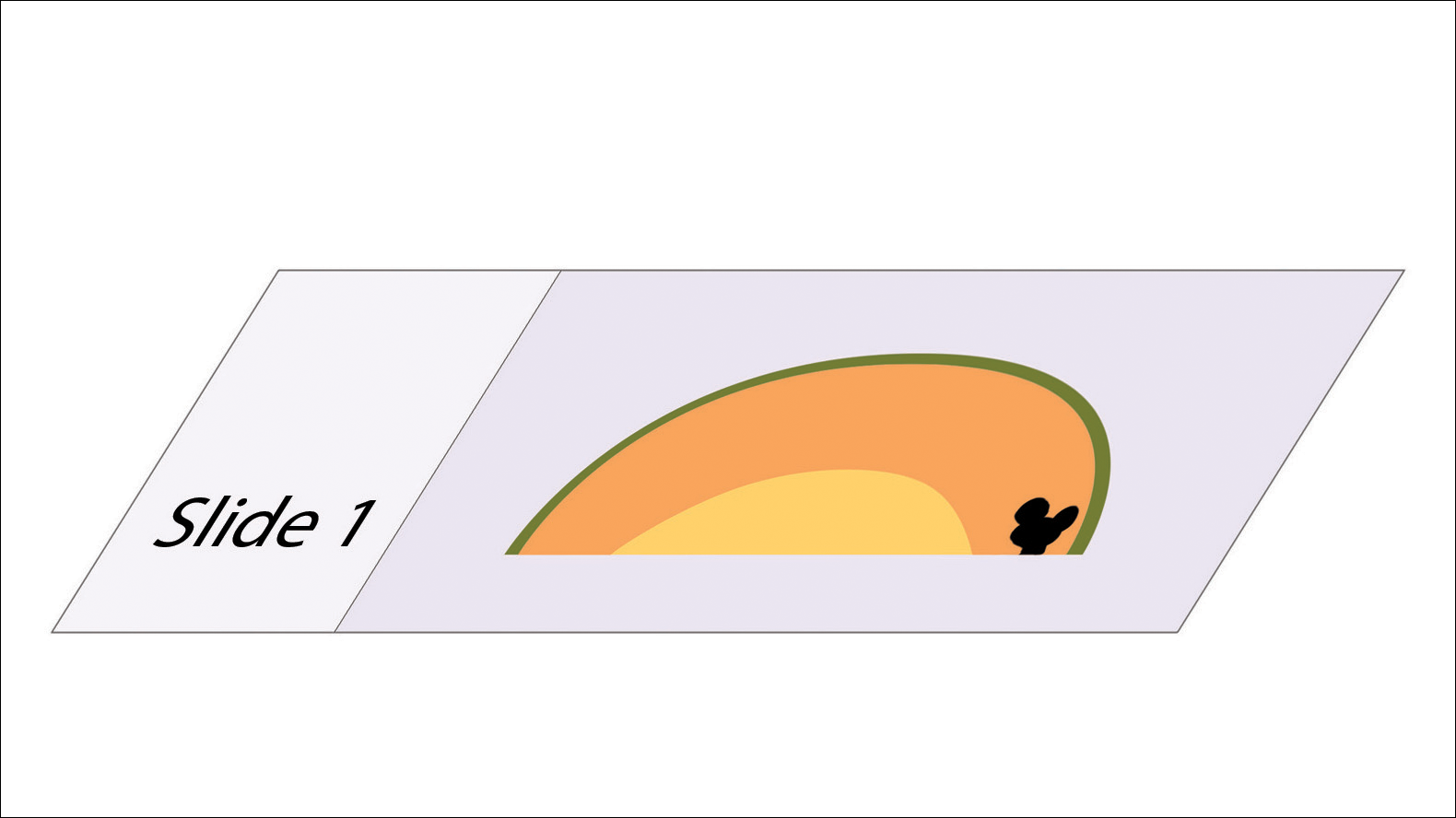
Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
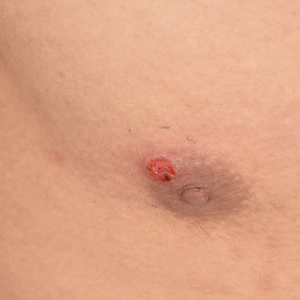
Painless Ulcer on the Areola
The Diagnosis: Primary Syphilitic Chancre of the Nipple
Because laboratory investigation was negative, a primary syphilitic chancre was suspected based on clinical findings, which was confirmed by a positive rapid plasma reagin with a titer of 1:32 and a positive Treponema pallidum particle agglutination assay. Results were negative for human immunodeficiency virus. On further inquiry, the patient acknowledged that the right areola had been traumatized during sexual activity with his regular male partner 1 month prior. In the last year he reported having had 5 different male partners. He was treated with a single dose of 2.4 million IU of intramuscular benzathine penicillin. Screening for other sexually transmitted infections revealed concomitant gonococcal infection of the pharynx and chlamydia proctitis, both of which were subsequently treated. On follow-up 2 weeks after presentation the ulcer had resolved, and he currently is undergoing serial rapid plasma reagin titer monitoring.
Primary syphilitic chancres can occur at any mucocutaneous site of inoculation, most frequently on the genitalia.1 Classically, after an incubation period of 9 to 90 days, a painless indurated ulcer forms2 and heals spontaneously after 3 to 6 weeks if left untreated.3 Chancres at extragenital sites are uncommon, occurring in approximately 2% of patients with primary syphilis.1 Of them, common sites include the lips and mouth (40%-70%),4 with areolar involvement rarely being reported. A PubMed search of articles indexed for MEDLINE using the terms nipple and chancre revealed 9 case reports in the English-language literature, with the first 2 cases being reported by Lee et al5 in 2006. The characteristics of these cases and our patient are summarized in the Table.5-12
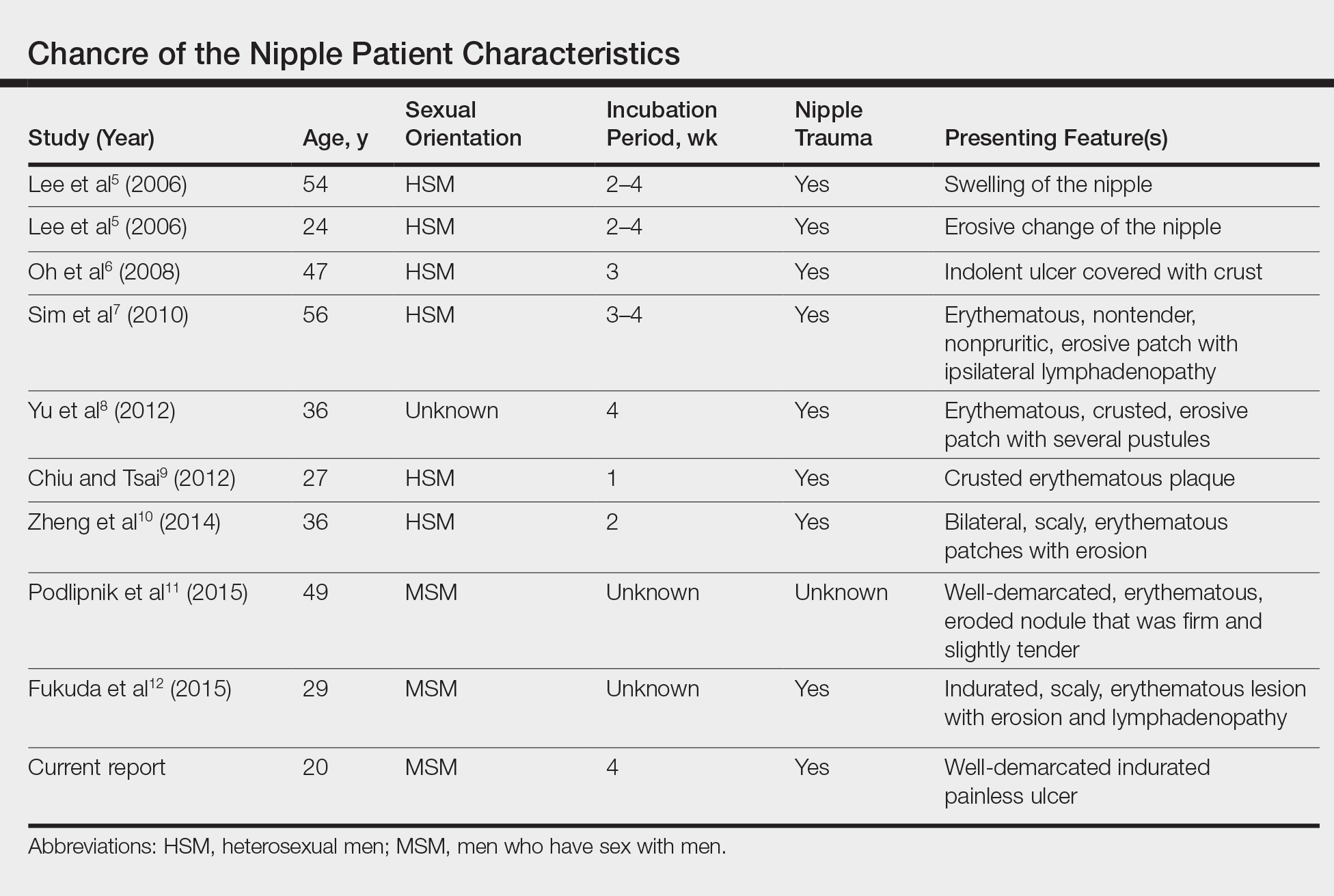
Oral contact or traumatization of the nipple by the patient's sexual partner was reported in all but one of these cases5-10,12; trauma was unknown in one case.11 Our patient reported a similar history of trauma to the nipple. It is known that transmission of syphilis can take place via kissing or oral contact, and it has been asserted that oral syphilitic lesions are highly infectious.13 Syphilis also can be transmitted by an already infected sexual partner sustaining minor trauma at the oral mucosa, allowing Treponema pallidum from the bloodstream to be inoculated onto the nipple. Another explanation for transmission could be the Koebner phenomenon, whereby trauma at the nipple of an already infected patient could lead to the formation of a chancre.6,8
The differential diagnosis includes erosive adenomatosis of the nipple, nipple eczema, Paget disease of the breast, and ulcerated basal cell carcinoma. Erosive adenomatosis of the nipple is a benign tumor of unilateral involvement that presents as an asymptomatic eroded/ulcerated papule. Clinically, it is similar to Paget disease of the breast. Eczema of the nipple usually is associated with pruritus and epidermal changes such as scaling.7,8 Paget disease of the breast arises from the extension of breast ductal carcinoma in situ onto the skin overlying the nipple. It can present as a unilateral nipple plaque with ulceration and bloody discharge. The diagnoses of erosive adenomatosis and Paget disease are confirmed with histologic examination. Basal cell carcinoma is the most common nonmelanoma skin cancer and can present as an ulcerated plaque, often with rolled borders, pearly edges, and overlying telangiectasia. It is known to be locally invasive. A punch biopsy and histopathologic examination would confirm the diagnosis of basal cell carcinoma.14
Extragenital chancres, especially those occurring at unusual sites, are uncommon. Therefore, a high index of suspicion is required to diagnose and initiate appropriate treatment for these patients.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Goh B. Syphilis in adults. Sex Transm Infect. 2005;81:448-452.
- Katz KA. Syphilis. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:2471-2492.
- Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187-209.
- Lee JY, Lin MH, Jung YC. Extragenital syphilitic chancre manifesting as a solitary nodule of the nipple. J Eur Acad Dermatol Venereol. 2006;20:886-887.
- Oh Y, Ahn S, Hong SP, et al. A case of extragenital chancre on a nipple from a human bite during sexual intercourse. Int J Dermatol. 2008;47:978-980.
- Sim JH, Lee MG, In SI, et al. Erythematous erosive patch on the left nipple--quiz case. diagnosis: extragenital syphilitic chancres. Arch Dermatol. 2010;146:81-86.
- Yu M, Lee HR, Han TY, et al. A solitary erosive patch on the left nipple. extragenital syphilitic chancres. Int J Dermatol. 2012;51:27-28.
- Chiu HY, Tsai TF. A crusted plaque on the right nipple. JAMA. 2012;308:403-404.
- Zheng S, Liu J, Xu XG, et al. Primary syphilis presenting as bilateral nipple-areola eczematoid lesions. Acta Derm Venereol. 2014;94:617-618.
- Podlipnik S, Giavedoni P, Alsina M, et al. An erythematous nodule on the nipple: an unusual presentation of primary syphilis. J Cutan Pathol. 2015;42:239-243.
- Fukuda H, Takahashi M, Kato K, et al. Multiple primary syphilis on the lip, nipple-areola and penis: an immunohistochemical examination of Treponema pallidum localization using an anti-T. pallidum antibody. J Dermatol. 2015;42:515-517.
- Yu X, Zheng H. Syphilitic chancre of the lips transmitted by kissing. Medicine (Baltimore). 2016;95:E3303.
- Carucci JA, Leffell DJ, Pettersen JS. Basal cell carcinoma. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:1294-1303.
The Diagnosis: Primary Syphilitic Chancre of the Nipple
Because laboratory investigation was negative, a primary syphilitic chancre was suspected based on clinical findings, which was confirmed by a positive rapid plasma reagin with a titer of 1:32 and a positive Treponema pallidum particle agglutination assay. Results were negative for human immunodeficiency virus. On further inquiry, the patient acknowledged that the right areola had been traumatized during sexual activity with his regular male partner 1 month prior. In the last year he reported having had 5 different male partners. He was treated with a single dose of 2.4 million IU of intramuscular benzathine penicillin. Screening for other sexually transmitted infections revealed concomitant gonococcal infection of the pharynx and chlamydia proctitis, both of which were subsequently treated. On follow-up 2 weeks after presentation the ulcer had resolved, and he currently is undergoing serial rapid plasma reagin titer monitoring.
Primary syphilitic chancres can occur at any mucocutaneous site of inoculation, most frequently on the genitalia.1 Classically, after an incubation period of 9 to 90 days, a painless indurated ulcer forms2 and heals spontaneously after 3 to 6 weeks if left untreated.3 Chancres at extragenital sites are uncommon, occurring in approximately 2% of patients with primary syphilis.1 Of them, common sites include the lips and mouth (40%-70%),4 with areolar involvement rarely being reported. A PubMed search of articles indexed for MEDLINE using the terms nipple and chancre revealed 9 case reports in the English-language literature, with the first 2 cases being reported by Lee et al5 in 2006. The characteristics of these cases and our patient are summarized in the Table.5-12

Oral contact or traumatization of the nipple by the patient's sexual partner was reported in all but one of these cases5-10,12; trauma was unknown in one case.11 Our patient reported a similar history of trauma to the nipple. It is known that transmission of syphilis can take place via kissing or oral contact, and it has been asserted that oral syphilitic lesions are highly infectious.13 Syphilis also can be transmitted by an already infected sexual partner sustaining minor trauma at the oral mucosa, allowing Treponema pallidum from the bloodstream to be inoculated onto the nipple. Another explanation for transmission could be the Koebner phenomenon, whereby trauma at the nipple of an already infected patient could lead to the formation of a chancre.6,8
The differential diagnosis includes erosive adenomatosis of the nipple, nipple eczema, Paget disease of the breast, and ulcerated basal cell carcinoma. Erosive adenomatosis of the nipple is a benign tumor of unilateral involvement that presents as an asymptomatic eroded/ulcerated papule. Clinically, it is similar to Paget disease of the breast. Eczema of the nipple usually is associated with pruritus and epidermal changes such as scaling.7,8 Paget disease of the breast arises from the extension of breast ductal carcinoma in situ onto the skin overlying the nipple. It can present as a unilateral nipple plaque with ulceration and bloody discharge. The diagnoses of erosive adenomatosis and Paget disease are confirmed with histologic examination. Basal cell carcinoma is the most common nonmelanoma skin cancer and can present as an ulcerated plaque, often with rolled borders, pearly edges, and overlying telangiectasia. It is known to be locally invasive. A punch biopsy and histopathologic examination would confirm the diagnosis of basal cell carcinoma.14
Extragenital chancres, especially those occurring at unusual sites, are uncommon. Therefore, a high index of suspicion is required to diagnose and initiate appropriate treatment for these patients.
The Diagnosis: Primary Syphilitic Chancre of the Nipple
Because laboratory investigation was negative, a primary syphilitic chancre was suspected based on clinical findings, which was confirmed by a positive rapid plasma reagin with a titer of 1:32 and a positive Treponema pallidum particle agglutination assay. Results were negative for human immunodeficiency virus. On further inquiry, the patient acknowledged that the right areola had been traumatized during sexual activity with his regular male partner 1 month prior. In the last year he reported having had 5 different male partners. He was treated with a single dose of 2.4 million IU of intramuscular benzathine penicillin. Screening for other sexually transmitted infections revealed concomitant gonococcal infection of the pharynx and chlamydia proctitis, both of which were subsequently treated. On follow-up 2 weeks after presentation the ulcer had resolved, and he currently is undergoing serial rapid plasma reagin titer monitoring.
Primary syphilitic chancres can occur at any mucocutaneous site of inoculation, most frequently on the genitalia.1 Classically, after an incubation period of 9 to 90 days, a painless indurated ulcer forms2 and heals spontaneously after 3 to 6 weeks if left untreated.3 Chancres at extragenital sites are uncommon, occurring in approximately 2% of patients with primary syphilis.1 Of them, common sites include the lips and mouth (40%-70%),4 with areolar involvement rarely being reported. A PubMed search of articles indexed for MEDLINE using the terms nipple and chancre revealed 9 case reports in the English-language literature, with the first 2 cases being reported by Lee et al5 in 2006. The characteristics of these cases and our patient are summarized in the Table.5-12

Oral contact or traumatization of the nipple by the patient's sexual partner was reported in all but one of these cases5-10,12; trauma was unknown in one case.11 Our patient reported a similar history of trauma to the nipple. It is known that transmission of syphilis can take place via kissing or oral contact, and it has been asserted that oral syphilitic lesions are highly infectious.13 Syphilis also can be transmitted by an already infected sexual partner sustaining minor trauma at the oral mucosa, allowing Treponema pallidum from the bloodstream to be inoculated onto the nipple. Another explanation for transmission could be the Koebner phenomenon, whereby trauma at the nipple of an already infected patient could lead to the formation of a chancre.6,8
The differential diagnosis includes erosive adenomatosis of the nipple, nipple eczema, Paget disease of the breast, and ulcerated basal cell carcinoma. Erosive adenomatosis of the nipple is a benign tumor of unilateral involvement that presents as an asymptomatic eroded/ulcerated papule. Clinically, it is similar to Paget disease of the breast. Eczema of the nipple usually is associated with pruritus and epidermal changes such as scaling.7,8 Paget disease of the breast arises from the extension of breast ductal carcinoma in situ onto the skin overlying the nipple. It can present as a unilateral nipple plaque with ulceration and bloody discharge. The diagnoses of erosive adenomatosis and Paget disease are confirmed with histologic examination. Basal cell carcinoma is the most common nonmelanoma skin cancer and can present as an ulcerated plaque, often with rolled borders, pearly edges, and overlying telangiectasia. It is known to be locally invasive. A punch biopsy and histopathologic examination would confirm the diagnosis of basal cell carcinoma.14
Extragenital chancres, especially those occurring at unusual sites, are uncommon. Therefore, a high index of suspicion is required to diagnose and initiate appropriate treatment for these patients.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Goh B. Syphilis in adults. Sex Transm Infect. 2005;81:448-452.
- Katz KA. Syphilis. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:2471-2492.
- Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187-209.
- Lee JY, Lin MH, Jung YC. Extragenital syphilitic chancre manifesting as a solitary nodule of the nipple. J Eur Acad Dermatol Venereol. 2006;20:886-887.
- Oh Y, Ahn S, Hong SP, et al. A case of extragenital chancre on a nipple from a human bite during sexual intercourse. Int J Dermatol. 2008;47:978-980.
- Sim JH, Lee MG, In SI, et al. Erythematous erosive patch on the left nipple--quiz case. diagnosis: extragenital syphilitic chancres. Arch Dermatol. 2010;146:81-86.
- Yu M, Lee HR, Han TY, et al. A solitary erosive patch on the left nipple. extragenital syphilitic chancres. Int J Dermatol. 2012;51:27-28.
- Chiu HY, Tsai TF. A crusted plaque on the right nipple. JAMA. 2012;308:403-404.
- Zheng S, Liu J, Xu XG, et al. Primary syphilis presenting as bilateral nipple-areola eczematoid lesions. Acta Derm Venereol. 2014;94:617-618.
- Podlipnik S, Giavedoni P, Alsina M, et al. An erythematous nodule on the nipple: an unusual presentation of primary syphilis. J Cutan Pathol. 2015;42:239-243.
- Fukuda H, Takahashi M, Kato K, et al. Multiple primary syphilis on the lip, nipple-areola and penis: an immunohistochemical examination of Treponema pallidum localization using an anti-T. pallidum antibody. J Dermatol. 2015;42:515-517.
- Yu X, Zheng H. Syphilitic chancre of the lips transmitted by kissing. Medicine (Baltimore). 2016;95:E3303.
- Carucci JA, Leffell DJ, Pettersen JS. Basal cell carcinoma. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:1294-1303.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Goh B. Syphilis in adults. Sex Transm Infect. 2005;81:448-452.
- Katz KA. Syphilis. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:2471-2492.
- Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187-209.
- Lee JY, Lin MH, Jung YC. Extragenital syphilitic chancre manifesting as a solitary nodule of the nipple. J Eur Acad Dermatol Venereol. 2006;20:886-887.
- Oh Y, Ahn S, Hong SP, et al. A case of extragenital chancre on a nipple from a human bite during sexual intercourse. Int J Dermatol. 2008;47:978-980.
- Sim JH, Lee MG, In SI, et al. Erythematous erosive patch on the left nipple--quiz case. diagnosis: extragenital syphilitic chancres. Arch Dermatol. 2010;146:81-86.
- Yu M, Lee HR, Han TY, et al. A solitary erosive patch on the left nipple. extragenital syphilitic chancres. Int J Dermatol. 2012;51:27-28.
- Chiu HY, Tsai TF. A crusted plaque on the right nipple. JAMA. 2012;308:403-404.
- Zheng S, Liu J, Xu XG, et al. Primary syphilis presenting as bilateral nipple-areola eczematoid lesions. Acta Derm Venereol. 2014;94:617-618.
- Podlipnik S, Giavedoni P, Alsina M, et al. An erythematous nodule on the nipple: an unusual presentation of primary syphilis. J Cutan Pathol. 2015;42:239-243.
- Fukuda H, Takahashi M, Kato K, et al. Multiple primary syphilis on the lip, nipple-areola and penis: an immunohistochemical examination of Treponema pallidum localization using an anti-T. pallidum antibody. J Dermatol. 2015;42:515-517.
- Yu X, Zheng H. Syphilitic chancre of the lips transmitted by kissing. Medicine (Baltimore). 2016;95:E3303.
- Carucci JA, Leffell DJ, Pettersen JS. Basal cell carcinoma. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:1294-1303.
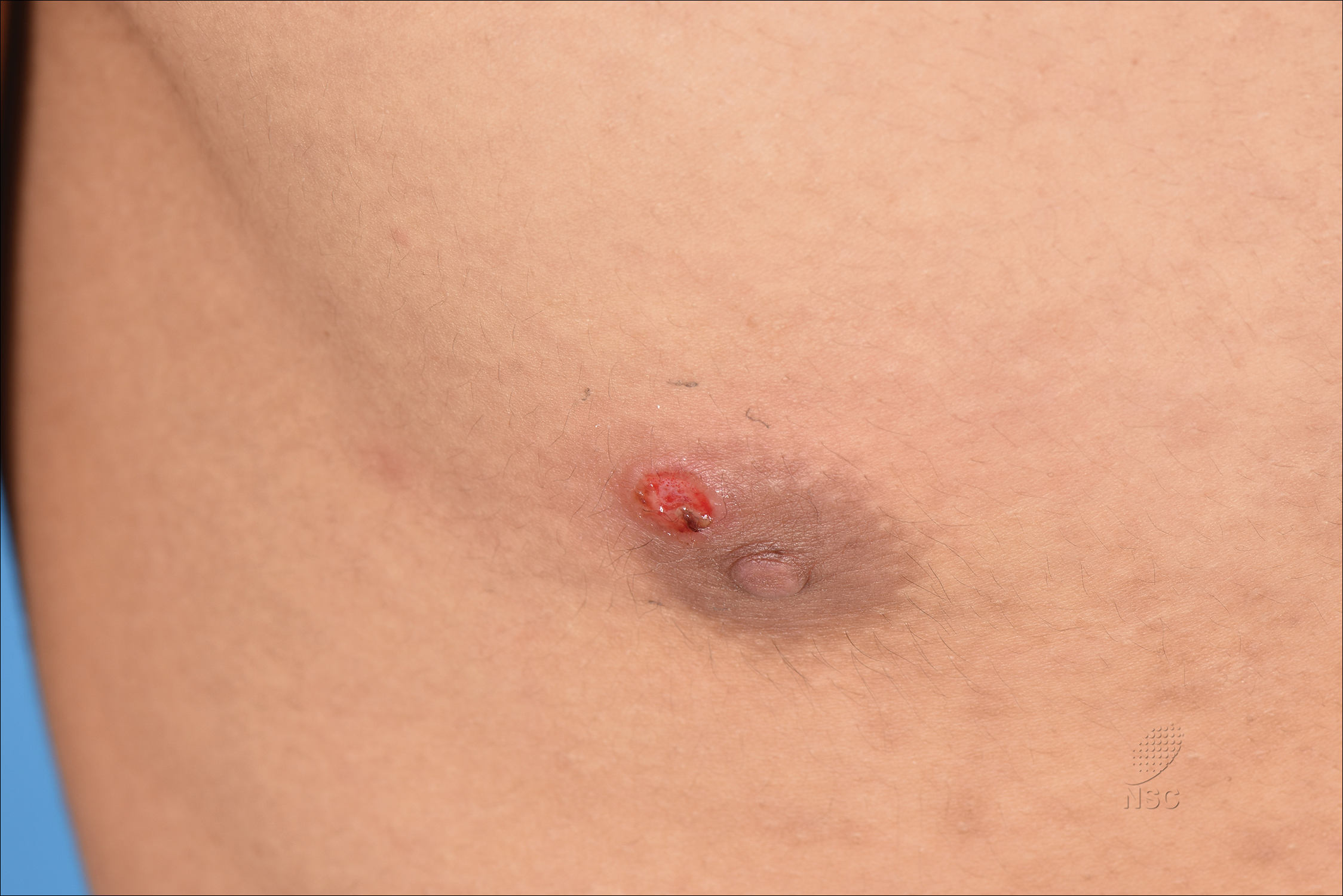
A previously healthy 20-year-old Chinese man presented to our dermatology outpatient clinic with a solitary painless ulcer on the right areola of 1 week's duration. Examination showed a small, slightly indurated ulcer with well-defined borders. No lesions were noted elsewhere. Swabs for pyogenic culture and herpes simplex virus polymerase chain reaction tests were sent, and he was treated empirically with oral cephalexin and tetracycline ointment 3%. At 1-week follow-up the ulcer had dried up and begun to heal, and results from the laboratory investigations were negative.
Deep Soft Tissue Mass of the Knee
The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
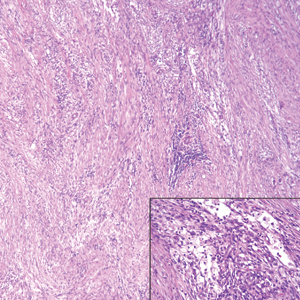
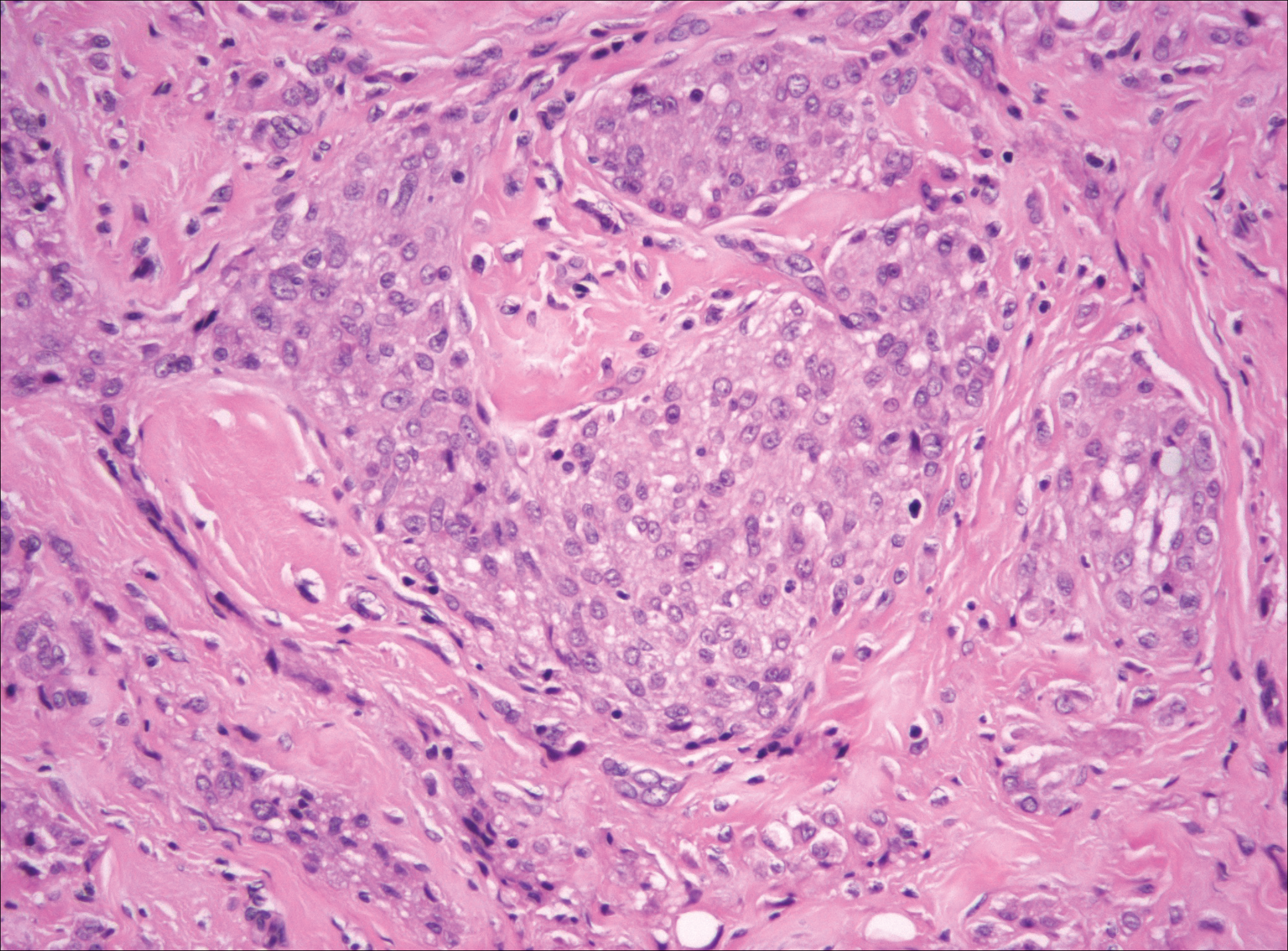
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
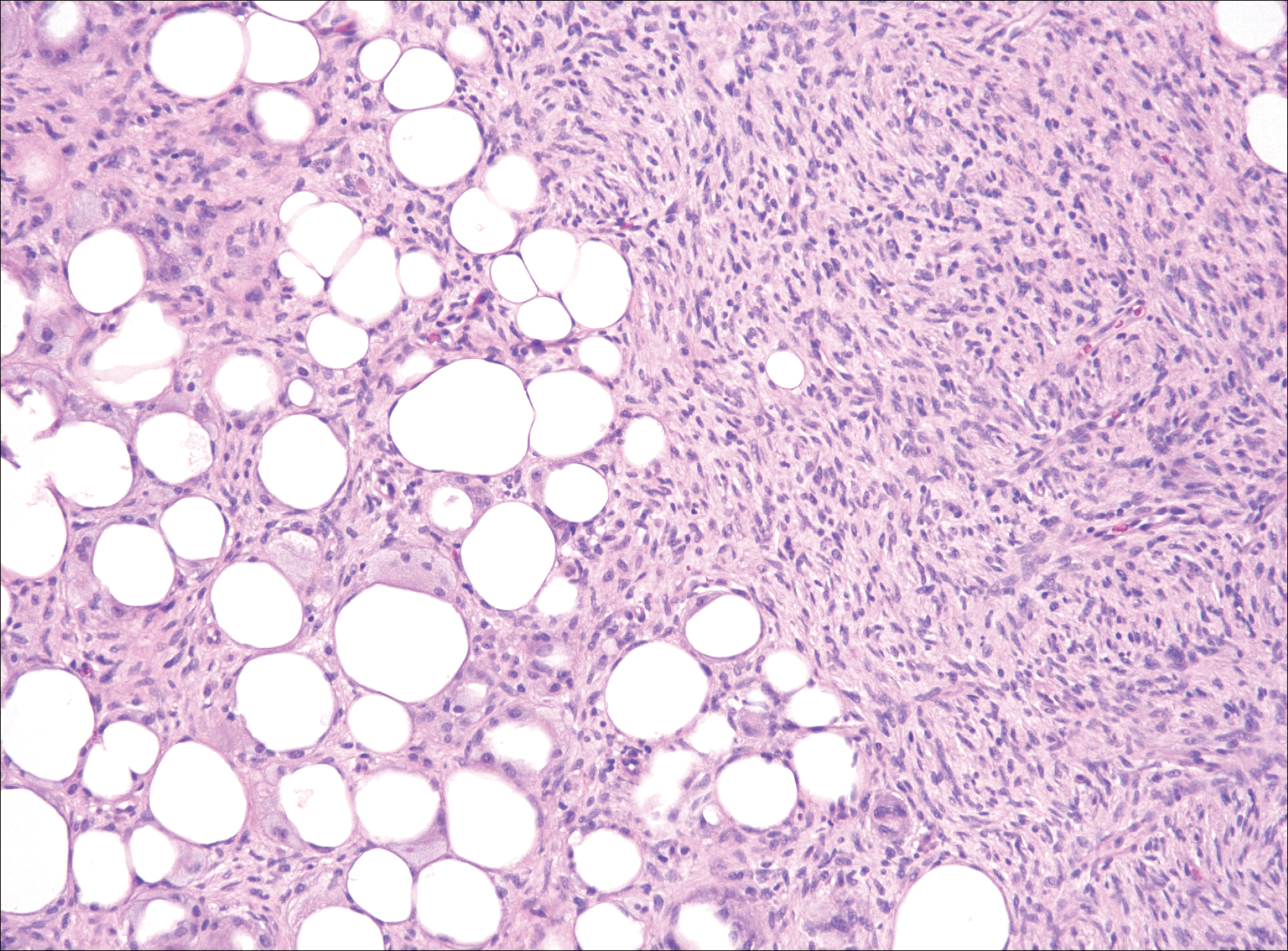
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.
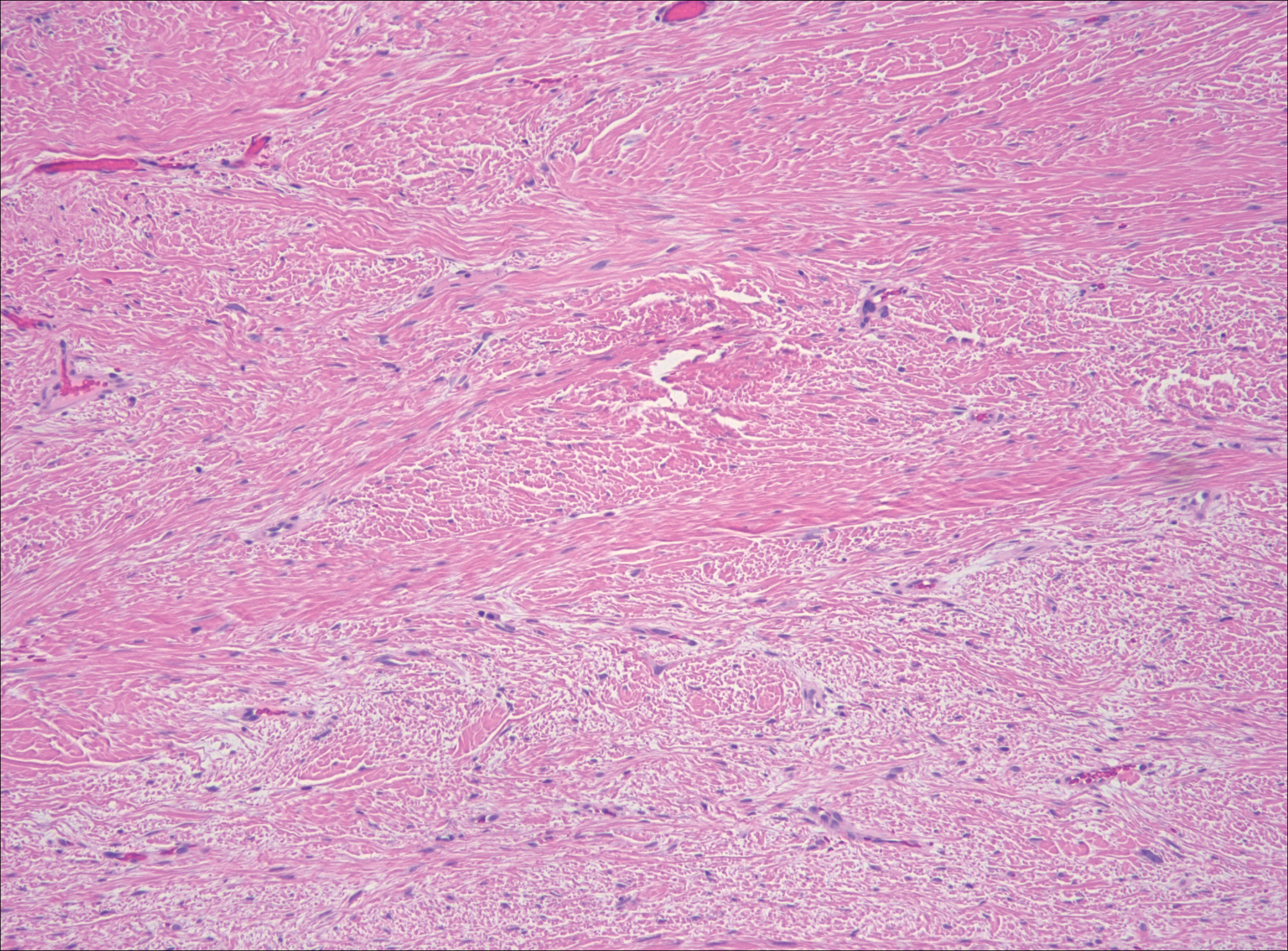
The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15
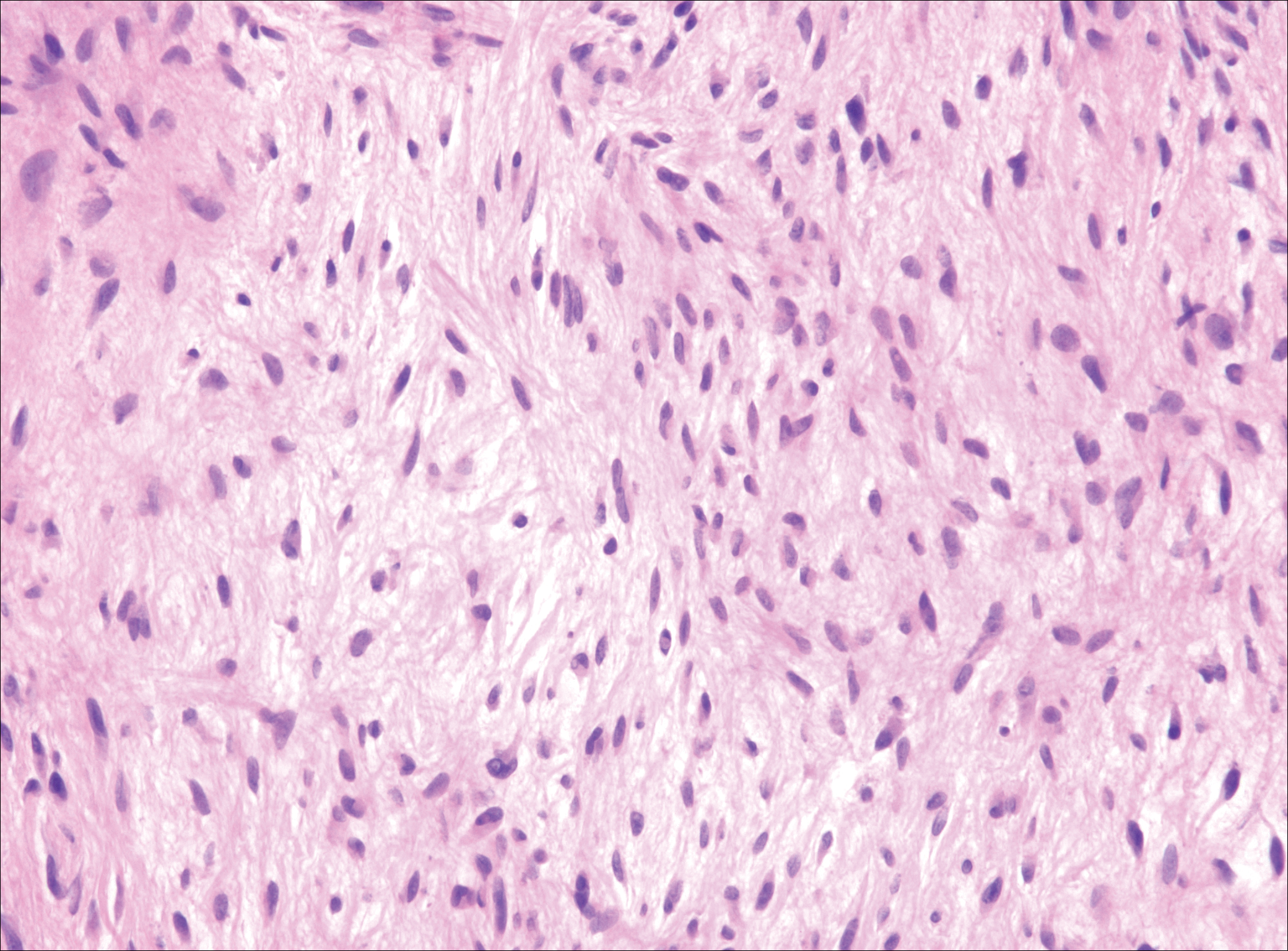
Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16
Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.
- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.

The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15

Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16

Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.

The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.

The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15

Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16

Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.

- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
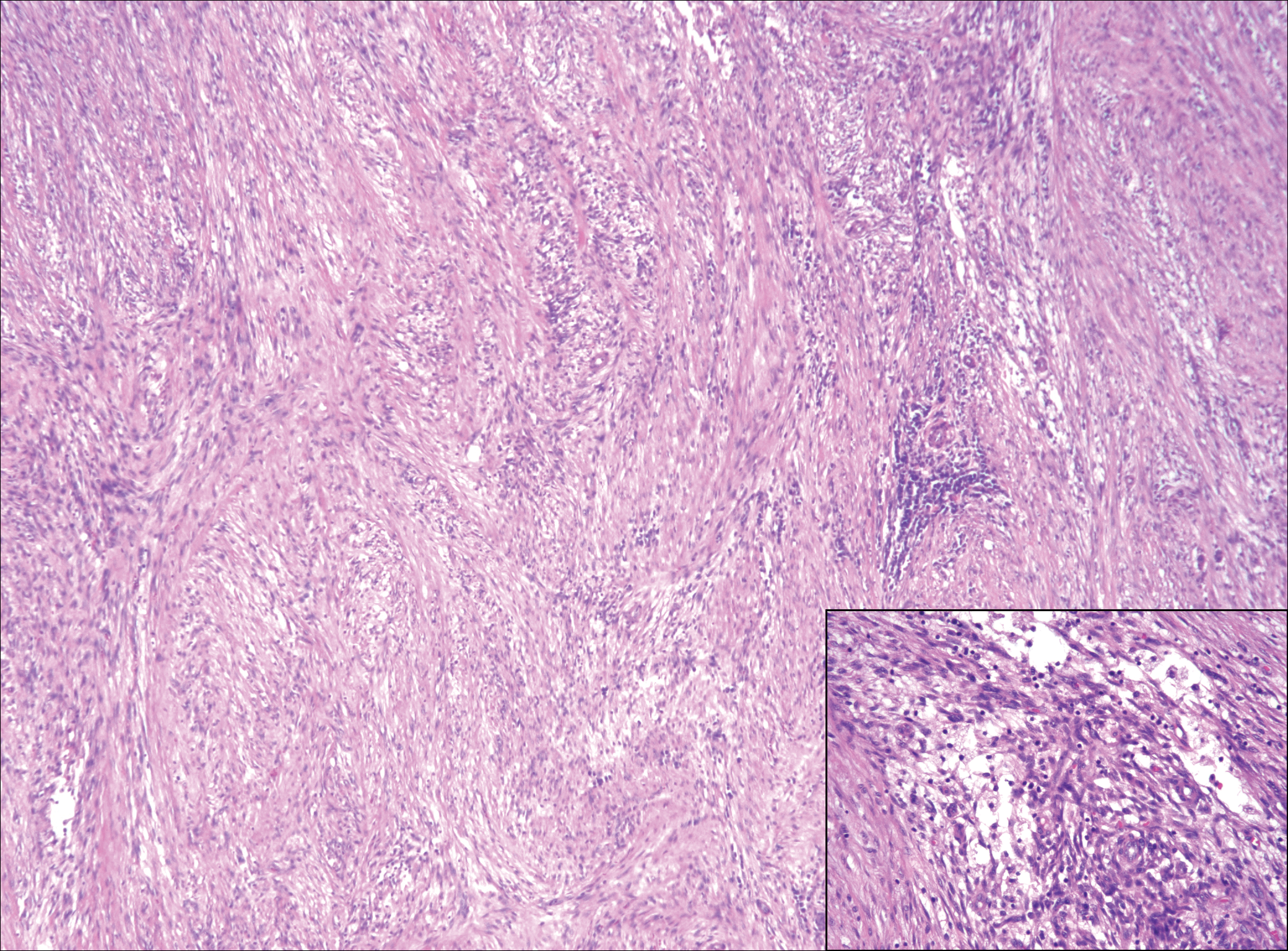
A 16-year-old adolescent girl presented with a bump over the left posterior knee of 1 month's duration. Her medical history was unremarkable. She denied recent trauma or injury to the area. On physical examination there was a visible and palpable tense nontender mass the size of an egg over the left posterior knee. Magnetic resonance imaging showed a lobulated mass-like focus of T2 hyperintensity centered at the subcutaneous tissues and superficial myofascial plane of the gastrocnemius on the posterior knee. Complete excision of the lesion was performed and demonstrated a 2.6.2 ×2.9.2 ×2.1-cm mass within subcutaneous adipose tissue. There was no microscopic involvement of skeletal muscle. Immunohistochemistry staining of the tumor was performed that was positive for smooth muscle actin and negative for desmin, S-100, CD34, pan-cytokeratin, and β-catenin. Fluorescent in situ hybridization testing demonstrated rearrangement of the ubiquitin-specific peptidase 6 gene, USP6, locus (17p13).
Patient Satisfaction and Quality of Care: A Prospective Study at Outpatient Dermatology Clinics
The Patient Protection and Affordable Care Act has increased the number of insured Americans by more than 20 million individuals.1 Approximately half of the newly insured have an income at or below 138% of the poverty level and are on average younger, sicker, and more likely to report poor to fair health compared to those individuals who already had health care coverage.2 Specialties such as dermatology are faced with the challenge of expanding access to these newly insured individuals while also improving quality of care.
Because of the complexity of defining quality in medicine, patient satisfaction is being used as a proxy for quality, with physicians evaluated and reimbursed based on patient satisfaction scores. Little research has been conducted to validate the relationship between patient satisfaction and quality; however, one study showed online reviews from patients on Yelp correlated with traditional markers of quality, such as mortality and readmission rates, lending credibility to the notion that patient satisfaction equates quality of care.3 Moreover, prospective studies have found positive correlations between patient satisfaction and compliance to therapy4,5; however, these studies may not give a complete picture of the relationship between patient satisfaction and quality of care, as other studies also have illustrated that, more often than not, factors extrinsic to actual medical care (eg, time spent in the waiting room) play a considerable role in patient satisfaction scores.6-9
When judging the quality of care that is provided, one study found that patients rate physicians based on interpersonal skills and not care delivered.8 Another important factor related to patient satisfaction is the anonymity of the surveys. Patients who have negative experiences are more likely to respond to online surveys than those who have positive experiences, skewing overall ratings.6 Additionally, because of Health Insurance Portability and Accountability Act regulations, physicians often are unable to respond directly to public patient reviews, resulting in an incomplete picture of the quality of care provided.
Ultimately, even if physicians do not agree that patient satisfaction correlates with quality of care, it is increasingly being used as a marker of such. Leading health care systems are embracing this new weight on patient satisfaction by increasing transparency and publishing patient satisfaction results online, allowing patients more access to physician reviews.
In dermatology, patient satisfaction serves an even more important role, as traditional markers of quality such as mortality and hospital readmission rates are not reasonable measures of patient care in this specialty, leaving patient satisfaction as one of the most accessible markers insurance companies and prospective patients can use to evaluate dermatologists. Furthermore, treatment modalities in dermatology often aim to improve quality of life, of which patient satisfaction arguably serves as an indicator. Ideally, patient satisfaction would allow physicians to identify areas where they may be better able to meet patients’ needs. However, patient satisfaction scores rarely are used as outcome measures in studies and are notoriously difficult to ascertain, as they tend to be inaccurate and may be unreliable in correlation with physician skill and training or may be skewed by patients’ desires to please their physicians.10 There also is a lack of standardized tools and scales to quantitatively judge outcomes in procedural surgeries.
Although patient satisfaction is being used as a measure of quality of care and is particularly necessary in a field such as dermatology that has outcome measures that are subjective in nature, there is a gap in the current literature regarding patient satisfaction and dermatology. To fill this gap, we conducted a prospective study of targeted interventions administered at outpatient dermatology clinics to determine if they resulted in statistically significant increases in patient satisfaction measures, particularly among Spanish-speaking patients.
Methods
We conducted a prospective study evaluating patient satisfaction in the outpatient dermatology clinics of LAC+USC Medical Center in Los Angeles, California, spanning over 1 year. During this time period, patients were randomly selected to participate and were asked to complete the Short-Form Patient Satisfaction Questionnaire (PSQ-18), which asked patients to rate their care experience on a 5-point Likert scale (1=strongly agree; 5=strongly disagree). The survey was separated into the following 7 subscales or categories looking at different aspects of care: general satisfaction, technical quality, interpersonal manner, communication, financial aspects, time spent with physician, and accessibility and convenience. Patients were given this survey both before and after targeted interventions to improve patient satisfaction were implemented. The targeted interventions were created based on literature review in the factors affecting patient satisfaction. The change in relative satisfaction was then determined using statistical analysis. The study was approved by the University of Southern California Health Science institutional review board.
Results
Of 470 patients surveyed, the average age was 49 years. Fifty percent of respondents were male, 70% self-identified as Hispanic, 45% spoke Spanish as their native language, and 69% reported a mean annual household income of less than $15,000. When scores were stratified, English-speaking patients were significantly more satisfied than Spanish-speaking patients in the categories of technical quality (P.0340), financial aspects (P.0301), interpersonal manner (P.0037), and time spent with physician (P.0059). Specifically, in the time spent with physician category, the lowest scores were found in females, patients aged 18 to 29 years, and patients with a mean annual household income less than $15,000. These demographics correlate well with many of the newly insured and intimate the need for improved patient satisfaction, particularly in this subset of patients.
After analyzing baseline patient satisfaction scores, we implemented targeted interventions such as creating a call tree, developing multilingual disease-specific patient handouts, instituting quarterly nursing in-services, which judged interpersonal and occupational nursing skills, and recruiting bilingual staff. These interventions were implemented simultaneously and were selected with the goal of reducing the impact of the language barrier between physicians and patients and increasing accessibility to clinics. Following approximately 3 months of these interventions, performance on many categories increased in our demographics that were lowest performing when we collected baseline data. In Spanish-speaking respondents, improvement in several categories approached statistical significance, including general satisfaction (P.110), interpersonal skills (P.080), and time spent with physician (P.096). When stratifying by income and age, patients with a mean annual household income less than $15,000 demonstrated an improved technical quality (P.066) subscale score, and participants aged 18 to 29 years showed improvement in both accessibility and convenience (P.053) and financial aspects (P.056) subscales.
Comment
The categories where improvements were found are noteworthy and suggest that certain aspects of care are more important than others. Although it seems intuitive that clinical acumen and training should be important contributors to patient satisfaction, one study that analyzed 1000 online comments regarding patient satisfaction with dermatologists on the website DrScore.com found that most comments concerned physician personality and interpersonal skills rather than medical judgment and acumen,4 suggesting that a patient’s perception of the character of the physician directly affects patient satisfaction scores. This notion was reiterated by other studies, including one that found that a patient’s perception of the physician’s kindness and empathy skills, is the most important measure of quality of care scores.8 Although this perception can be intimidating to some physicians, as certain interpersonal skills are difficult to change, it is reassuring to note that external environment and cues, such as the clinic building and staff, also seem to affect interpersonal ratings. As seen in our study, patient ratings of a physician’s interpersonal skills increased after educational materials for staff and patients were created and more bilingual staff was recruited. Other environmental changes, such as spending a few more minutes with patients and sitting down when talking to patients, are relatively easy to administer and can improve patient satisfaction scores.8
Although some of the scores in our study approached but did not reach statistical significance, likely because of a small sample size, they suggest that targeted interventions can improve patient satisfaction. They also suggested that targeted interventions are particularly useful in Spanish-speaking patients, younger patients, and patients from lower socioeconomic backgrounds, which are all characteristics of the newly insured under the Patient Protection and Affordable Health Care Act.
Our study also is unique in that dermatology as a specialty is lagging in quality improvement studies. In the few studies evaluating patient satisfaction in the literature, the care provided by dermatologists was painted in a positive light.6,11 One study evaluated 45 dermatology practices and reported average patient satisfaction scores of 3.46 and 4.72 of 5 on Yelp and ZocDoc, respectively.11 Another study looking at dermatologist ratings on DrScore.com found that the majority of patients were satisfied with the care they received.6
Although these studies seem encouraging, they have several limitations. First, their results were not stratified by patient demographics and therefore may not be generalizable to low-income populations that constitute much of the newly insured. Secondly, the observational nature and limited number of studies prohibit meaningful conclusions from being drawn and leave many questions unanswered. Additionally, although the raw patient satisfaction scores seem good, dermatology is lacking compared to the patient satisfaction scores within other specialties. A study of more than 28,000 Yelp reviews of 23 specialties found that dermatology ranked second to last, ahead of only psychiatry.7 Of course, given the observational nature of this study, it is impossible to generalize, as many confounders (eg, medical comorbidities, patient age) may have skewed the dermatology ranking. Regardless, there is always room for improvement, and luckily improving patient satisfaction is not an elusive goal.
Conclusion
As dermatologists, our interventions often improve quality of life; therefore, we are positioned to be leaders in the quality improvement field. Despite the numerous limitations of using patient satisfaction as a measure for quality of care, it is used by payers to determine reimbursement and patients to select providers. Encouraging initial data from our prospective study demonstrate that small interventions can increase patient satisfaction. Continued work to maximize patient satisfaction is needed to improve outcomes for our patients, help validate the quality of care being provided, and further solidify the importance of having insurers maintain sufficient dermatologists in their networks.
- Uberoi N, Finegold K, Gee E. Health insurance coverage and the Affordable Care Act, 2010-2016. US Department of Health & Human Services website. https://aspe.hhs.gov/system/files/pdf/187551/ACA2010-2016.pdf. Published March 3, 2016. Accessed May 18, 2018.
- Shatzer A, Long SK, Zuckerman S. Who are the newly insured as of early March 2014? Urban Institute Health Policy Center website. http://hrms.urban.org/briefs/Who-Are-the-Newly-Insured.html. Published May 22, 2014. Accessed May 18, 2018.
- Bardach NS, Asteria-Peñaloza R, Boscardin WJ, et al. The relationship between commercial website ratings and traditional hospital performance measures in the USA. BMJ Qual Saf. 2013;22:194-202.
- Kincey J, Bradshaw P, Ley P. Patient satisfaction and reported acceptance of medical advice in general practice. J R Coll Gen Pract. 1975;25:558-566.
- Francis V, Korsch BM, Morris MJ. Gaps in doctor-patient communication. patients’ response to medical advice. N Engl J Med. 1969;280:535-540.
- Ali ST, Feldman SR. Patient satisfaction in dermatology: a qualitative assessment. Dermatol Online J. 2014;20. pii:doj_21534.
- Internet study: highest educated & trained doctors get poorest online reviews. Vanguard Communications website. https://vanguard communications.net/best-online-doctor-reviews/. Published April 22, 2015. Accessed May 18, 2018.
- Uhas AA, Camacho FT, Feldman SR, et al. The relationship between physician friendliness and caring, and patient satisfaction: findings from an internet-based survey. Patient. 2008;1:91-96.
- Anderson RT, Camacho FT, Balkrishnan R. Willing to wait?: the influence of patient wait time on satisfaction with primary care. BMC Health Serv Res. 2007;7:31.
- Maibach HI, Gorouhi F. Evidence-Based Dermatology. 2nd ed. Shelton, CT: People’s Medical Publishing House-USA; 2011.
- Smith R, Lipoff J. Evaluation of dermatology practice online reviews. JAMA Dermatol. 2016;152:153-157.
The Patient Protection and Affordable Care Act has increased the number of insured Americans by more than 20 million individuals.1 Approximately half of the newly insured have an income at or below 138% of the poverty level and are on average younger, sicker, and more likely to report poor to fair health compared to those individuals who already had health care coverage.2 Specialties such as dermatology are faced with the challenge of expanding access to these newly insured individuals while also improving quality of care.
Because of the complexity of defining quality in medicine, patient satisfaction is being used as a proxy for quality, with physicians evaluated and reimbursed based on patient satisfaction scores. Little research has been conducted to validate the relationship between patient satisfaction and quality; however, one study showed online reviews from patients on Yelp correlated with traditional markers of quality, such as mortality and readmission rates, lending credibility to the notion that patient satisfaction equates quality of care.3 Moreover, prospective studies have found positive correlations between patient satisfaction and compliance to therapy4,5; however, these studies may not give a complete picture of the relationship between patient satisfaction and quality of care, as other studies also have illustrated that, more often than not, factors extrinsic to actual medical care (eg, time spent in the waiting room) play a considerable role in patient satisfaction scores.6-9
When judging the quality of care that is provided, one study found that patients rate physicians based on interpersonal skills and not care delivered.8 Another important factor related to patient satisfaction is the anonymity of the surveys. Patients who have negative experiences are more likely to respond to online surveys than those who have positive experiences, skewing overall ratings.6 Additionally, because of Health Insurance Portability and Accountability Act regulations, physicians often are unable to respond directly to public patient reviews, resulting in an incomplete picture of the quality of care provided.
Ultimately, even if physicians do not agree that patient satisfaction correlates with quality of care, it is increasingly being used as a marker of such. Leading health care systems are embracing this new weight on patient satisfaction by increasing transparency and publishing patient satisfaction results online, allowing patients more access to physician reviews.
In dermatology, patient satisfaction serves an even more important role, as traditional markers of quality such as mortality and hospital readmission rates are not reasonable measures of patient care in this specialty, leaving patient satisfaction as one of the most accessible markers insurance companies and prospective patients can use to evaluate dermatologists. Furthermore, treatment modalities in dermatology often aim to improve quality of life, of which patient satisfaction arguably serves as an indicator. Ideally, patient satisfaction would allow physicians to identify areas where they may be better able to meet patients’ needs. However, patient satisfaction scores rarely are used as outcome measures in studies and are notoriously difficult to ascertain, as they tend to be inaccurate and may be unreliable in correlation with physician skill and training or may be skewed by patients’ desires to please their physicians.10 There also is a lack of standardized tools and scales to quantitatively judge outcomes in procedural surgeries.
Although patient satisfaction is being used as a measure of quality of care and is particularly necessary in a field such as dermatology that has outcome measures that are subjective in nature, there is a gap in the current literature regarding patient satisfaction and dermatology. To fill this gap, we conducted a prospective study of targeted interventions administered at outpatient dermatology clinics to determine if they resulted in statistically significant increases in patient satisfaction measures, particularly among Spanish-speaking patients.
Methods
We conducted a prospective study evaluating patient satisfaction in the outpatient dermatology clinics of LAC+USC Medical Center in Los Angeles, California, spanning over 1 year. During this time period, patients were randomly selected to participate and were asked to complete the Short-Form Patient Satisfaction Questionnaire (PSQ-18), which asked patients to rate their care experience on a 5-point Likert scale (1=strongly agree; 5=strongly disagree). The survey was separated into the following 7 subscales or categories looking at different aspects of care: general satisfaction, technical quality, interpersonal manner, communication, financial aspects, time spent with physician, and accessibility and convenience. Patients were given this survey both before and after targeted interventions to improve patient satisfaction were implemented. The targeted interventions were created based on literature review in the factors affecting patient satisfaction. The change in relative satisfaction was then determined using statistical analysis. The study was approved by the University of Southern California Health Science institutional review board.
Results
Of 470 patients surveyed, the average age was 49 years. Fifty percent of respondents were male, 70% self-identified as Hispanic, 45% spoke Spanish as their native language, and 69% reported a mean annual household income of less than $15,000. When scores were stratified, English-speaking patients were significantly more satisfied than Spanish-speaking patients in the categories of technical quality (P.0340), financial aspects (P.0301), interpersonal manner (P.0037), and time spent with physician (P.0059). Specifically, in the time spent with physician category, the lowest scores were found in females, patients aged 18 to 29 years, and patients with a mean annual household income less than $15,000. These demographics correlate well with many of the newly insured and intimate the need for improved patient satisfaction, particularly in this subset of patients.
After analyzing baseline patient satisfaction scores, we implemented targeted interventions such as creating a call tree, developing multilingual disease-specific patient handouts, instituting quarterly nursing in-services, which judged interpersonal and occupational nursing skills, and recruiting bilingual staff. These interventions were implemented simultaneously and were selected with the goal of reducing the impact of the language barrier between physicians and patients and increasing accessibility to clinics. Following approximately 3 months of these interventions, performance on many categories increased in our demographics that were lowest performing when we collected baseline data. In Spanish-speaking respondents, improvement in several categories approached statistical significance, including general satisfaction (P.110), interpersonal skills (P.080), and time spent with physician (P.096). When stratifying by income and age, patients with a mean annual household income less than $15,000 demonstrated an improved technical quality (P.066) subscale score, and participants aged 18 to 29 years showed improvement in both accessibility and convenience (P.053) and financial aspects (P.056) subscales.
Comment
The categories where improvements were found are noteworthy and suggest that certain aspects of care are more important than others. Although it seems intuitive that clinical acumen and training should be important contributors to patient satisfaction, one study that analyzed 1000 online comments regarding patient satisfaction with dermatologists on the website DrScore.com found that most comments concerned physician personality and interpersonal skills rather than medical judgment and acumen,4 suggesting that a patient’s perception of the character of the physician directly affects patient satisfaction scores. This notion was reiterated by other studies, including one that found that a patient’s perception of the physician’s kindness and empathy skills, is the most important measure of quality of care scores.8 Although this perception can be intimidating to some physicians, as certain interpersonal skills are difficult to change, it is reassuring to note that external environment and cues, such as the clinic building and staff, also seem to affect interpersonal ratings. As seen in our study, patient ratings of a physician’s interpersonal skills increased after educational materials for staff and patients were created and more bilingual staff was recruited. Other environmental changes, such as spending a few more minutes with patients and sitting down when talking to patients, are relatively easy to administer and can improve patient satisfaction scores.8
Although some of the scores in our study approached but did not reach statistical significance, likely because of a small sample size, they suggest that targeted interventions can improve patient satisfaction. They also suggested that targeted interventions are particularly useful in Spanish-speaking patients, younger patients, and patients from lower socioeconomic backgrounds, which are all characteristics of the newly insured under the Patient Protection and Affordable Health Care Act.
Our study also is unique in that dermatology as a specialty is lagging in quality improvement studies. In the few studies evaluating patient satisfaction in the literature, the care provided by dermatologists was painted in a positive light.6,11 One study evaluated 45 dermatology practices and reported average patient satisfaction scores of 3.46 and 4.72 of 5 on Yelp and ZocDoc, respectively.11 Another study looking at dermatologist ratings on DrScore.com found that the majority of patients were satisfied with the care they received.6
Although these studies seem encouraging, they have several limitations. First, their results were not stratified by patient demographics and therefore may not be generalizable to low-income populations that constitute much of the newly insured. Secondly, the observational nature and limited number of studies prohibit meaningful conclusions from being drawn and leave many questions unanswered. Additionally, although the raw patient satisfaction scores seem good, dermatology is lacking compared to the patient satisfaction scores within other specialties. A study of more than 28,000 Yelp reviews of 23 specialties found that dermatology ranked second to last, ahead of only psychiatry.7 Of course, given the observational nature of this study, it is impossible to generalize, as many confounders (eg, medical comorbidities, patient age) may have skewed the dermatology ranking. Regardless, there is always room for improvement, and luckily improving patient satisfaction is not an elusive goal.
Conclusion
As dermatologists, our interventions often improve quality of life; therefore, we are positioned to be leaders in the quality improvement field. Despite the numerous limitations of using patient satisfaction as a measure for quality of care, it is used by payers to determine reimbursement and patients to select providers. Encouraging initial data from our prospective study demonstrate that small interventions can increase patient satisfaction. Continued work to maximize patient satisfaction is needed to improve outcomes for our patients, help validate the quality of care being provided, and further solidify the importance of having insurers maintain sufficient dermatologists in their networks.
The Patient Protection and Affordable Care Act has increased the number of insured Americans by more than 20 million individuals.1 Approximately half of the newly insured have an income at or below 138% of the poverty level and are on average younger, sicker, and more likely to report poor to fair health compared to those individuals who already had health care coverage.2 Specialties such as dermatology are faced with the challenge of expanding access to these newly insured individuals while also improving quality of care.
Because of the complexity of defining quality in medicine, patient satisfaction is being used as a proxy for quality, with physicians evaluated and reimbursed based on patient satisfaction scores. Little research has been conducted to validate the relationship between patient satisfaction and quality; however, one study showed online reviews from patients on Yelp correlated with traditional markers of quality, such as mortality and readmission rates, lending credibility to the notion that patient satisfaction equates quality of care.3 Moreover, prospective studies have found positive correlations between patient satisfaction and compliance to therapy4,5; however, these studies may not give a complete picture of the relationship between patient satisfaction and quality of care, as other studies also have illustrated that, more often than not, factors extrinsic to actual medical care (eg, time spent in the waiting room) play a considerable role in patient satisfaction scores.6-9
When judging the quality of care that is provided, one study found that patients rate physicians based on interpersonal skills and not care delivered.8 Another important factor related to patient satisfaction is the anonymity of the surveys. Patients who have negative experiences are more likely to respond to online surveys than those who have positive experiences, skewing overall ratings.6 Additionally, because of Health Insurance Portability and Accountability Act regulations, physicians often are unable to respond directly to public patient reviews, resulting in an incomplete picture of the quality of care provided.
Ultimately, even if physicians do not agree that patient satisfaction correlates with quality of care, it is increasingly being used as a marker of such. Leading health care systems are embracing this new weight on patient satisfaction by increasing transparency and publishing patient satisfaction results online, allowing patients more access to physician reviews.
In dermatology, patient satisfaction serves an even more important role, as traditional markers of quality such as mortality and hospital readmission rates are not reasonable measures of patient care in this specialty, leaving patient satisfaction as one of the most accessible markers insurance companies and prospective patients can use to evaluate dermatologists. Furthermore, treatment modalities in dermatology often aim to improve quality of life, of which patient satisfaction arguably serves as an indicator. Ideally, patient satisfaction would allow physicians to identify areas where they may be better able to meet patients’ needs. However, patient satisfaction scores rarely are used as outcome measures in studies and are notoriously difficult to ascertain, as they tend to be inaccurate and may be unreliable in correlation with physician skill and training or may be skewed by patients’ desires to please their physicians.10 There also is a lack of standardized tools and scales to quantitatively judge outcomes in procedural surgeries.
Although patient satisfaction is being used as a measure of quality of care and is particularly necessary in a field such as dermatology that has outcome measures that are subjective in nature, there is a gap in the current literature regarding patient satisfaction and dermatology. To fill this gap, we conducted a prospective study of targeted interventions administered at outpatient dermatology clinics to determine if they resulted in statistically significant increases in patient satisfaction measures, particularly among Spanish-speaking patients.
Methods
We conducted a prospective study evaluating patient satisfaction in the outpatient dermatology clinics of LAC+USC Medical Center in Los Angeles, California, spanning over 1 year. During this time period, patients were randomly selected to participate and were asked to complete the Short-Form Patient Satisfaction Questionnaire (PSQ-18), which asked patients to rate their care experience on a 5-point Likert scale (1=strongly agree; 5=strongly disagree). The survey was separated into the following 7 subscales or categories looking at different aspects of care: general satisfaction, technical quality, interpersonal manner, communication, financial aspects, time spent with physician, and accessibility and convenience. Patients were given this survey both before and after targeted interventions to improve patient satisfaction were implemented. The targeted interventions were created based on literature review in the factors affecting patient satisfaction. The change in relative satisfaction was then determined using statistical analysis. The study was approved by the University of Southern California Health Science institutional review board.
Results
Of 470 patients surveyed, the average age was 49 years. Fifty percent of respondents were male, 70% self-identified as Hispanic, 45% spoke Spanish as their native language, and 69% reported a mean annual household income of less than $15,000. When scores were stratified, English-speaking patients were significantly more satisfied than Spanish-speaking patients in the categories of technical quality (P.0340), financial aspects (P.0301), interpersonal manner (P.0037), and time spent with physician (P.0059). Specifically, in the time spent with physician category, the lowest scores were found in females, patients aged 18 to 29 years, and patients with a mean annual household income less than $15,000. These demographics correlate well with many of the newly insured and intimate the need for improved patient satisfaction, particularly in this subset of patients.
After analyzing baseline patient satisfaction scores, we implemented targeted interventions such as creating a call tree, developing multilingual disease-specific patient handouts, instituting quarterly nursing in-services, which judged interpersonal and occupational nursing skills, and recruiting bilingual staff. These interventions were implemented simultaneously and were selected with the goal of reducing the impact of the language barrier between physicians and patients and increasing accessibility to clinics. Following approximately 3 months of these interventions, performance on many categories increased in our demographics that were lowest performing when we collected baseline data. In Spanish-speaking respondents, improvement in several categories approached statistical significance, including general satisfaction (P.110), interpersonal skills (P.080), and time spent with physician (P.096). When stratifying by income and age, patients with a mean annual household income less than $15,000 demonstrated an improved technical quality (P.066) subscale score, and participants aged 18 to 29 years showed improvement in both accessibility and convenience (P.053) and financial aspects (P.056) subscales.
Comment
The categories where improvements were found are noteworthy and suggest that certain aspects of care are more important than others. Although it seems intuitive that clinical acumen and training should be important contributors to patient satisfaction, one study that analyzed 1000 online comments regarding patient satisfaction with dermatologists on the website DrScore.com found that most comments concerned physician personality and interpersonal skills rather than medical judgment and acumen,4 suggesting that a patient’s perception of the character of the physician directly affects patient satisfaction scores. This notion was reiterated by other studies, including one that found that a patient’s perception of the physician’s kindness and empathy skills, is the most important measure of quality of care scores.8 Although this perception can be intimidating to some physicians, as certain interpersonal skills are difficult to change, it is reassuring to note that external environment and cues, such as the clinic building and staff, also seem to affect interpersonal ratings. As seen in our study, patient ratings of a physician’s interpersonal skills increased after educational materials for staff and patients were created and more bilingual staff was recruited. Other environmental changes, such as spending a few more minutes with patients and sitting down when talking to patients, are relatively easy to administer and can improve patient satisfaction scores.8
Although some of the scores in our study approached but did not reach statistical significance, likely because of a small sample size, they suggest that targeted interventions can improve patient satisfaction. They also suggested that targeted interventions are particularly useful in Spanish-speaking patients, younger patients, and patients from lower socioeconomic backgrounds, which are all characteristics of the newly insured under the Patient Protection and Affordable Health Care Act.
Our study also is unique in that dermatology as a specialty is lagging in quality improvement studies. In the few studies evaluating patient satisfaction in the literature, the care provided by dermatologists was painted in a positive light.6,11 One study evaluated 45 dermatology practices and reported average patient satisfaction scores of 3.46 and 4.72 of 5 on Yelp and ZocDoc, respectively.11 Another study looking at dermatologist ratings on DrScore.com found that the majority of patients were satisfied with the care they received.6
Although these studies seem encouraging, they have several limitations. First, their results were not stratified by patient demographics and therefore may not be generalizable to low-income populations that constitute much of the newly insured. Secondly, the observational nature and limited number of studies prohibit meaningful conclusions from being drawn and leave many questions unanswered. Additionally, although the raw patient satisfaction scores seem good, dermatology is lacking compared to the patient satisfaction scores within other specialties. A study of more than 28,000 Yelp reviews of 23 specialties found that dermatology ranked second to last, ahead of only psychiatry.7 Of course, given the observational nature of this study, it is impossible to generalize, as many confounders (eg, medical comorbidities, patient age) may have skewed the dermatology ranking. Regardless, there is always room for improvement, and luckily improving patient satisfaction is not an elusive goal.
Conclusion
As dermatologists, our interventions often improve quality of life; therefore, we are positioned to be leaders in the quality improvement field. Despite the numerous limitations of using patient satisfaction as a measure for quality of care, it is used by payers to determine reimbursement and patients to select providers. Encouraging initial data from our prospective study demonstrate that small interventions can increase patient satisfaction. Continued work to maximize patient satisfaction is needed to improve outcomes for our patients, help validate the quality of care being provided, and further solidify the importance of having insurers maintain sufficient dermatologists in their networks.
- Uberoi N, Finegold K, Gee E. Health insurance coverage and the Affordable Care Act, 2010-2016. US Department of Health & Human Services website. https://aspe.hhs.gov/system/files/pdf/187551/ACA2010-2016.pdf. Published March 3, 2016. Accessed May 18, 2018.
- Shatzer A, Long SK, Zuckerman S. Who are the newly insured as of early March 2014? Urban Institute Health Policy Center website. http://hrms.urban.org/briefs/Who-Are-the-Newly-Insured.html. Published May 22, 2014. Accessed May 18, 2018.
- Bardach NS, Asteria-Peñaloza R, Boscardin WJ, et al. The relationship between commercial website ratings and traditional hospital performance measures in the USA. BMJ Qual Saf. 2013;22:194-202.
- Kincey J, Bradshaw P, Ley P. Patient satisfaction and reported acceptance of medical advice in general practice. J R Coll Gen Pract. 1975;25:558-566.
- Francis V, Korsch BM, Morris MJ. Gaps in doctor-patient communication. patients’ response to medical advice. N Engl J Med. 1969;280:535-540.
- Ali ST, Feldman SR. Patient satisfaction in dermatology: a qualitative assessment. Dermatol Online J. 2014;20. pii:doj_21534.
- Internet study: highest educated & trained doctors get poorest online reviews. Vanguard Communications website. https://vanguard communications.net/best-online-doctor-reviews/. Published April 22, 2015. Accessed May 18, 2018.
- Uhas AA, Camacho FT, Feldman SR, et al. The relationship between physician friendliness and caring, and patient satisfaction: findings from an internet-based survey. Patient. 2008;1:91-96.
- Anderson RT, Camacho FT, Balkrishnan R. Willing to wait?: the influence of patient wait time on satisfaction with primary care. BMC Health Serv Res. 2007;7:31.
- Maibach HI, Gorouhi F. Evidence-Based Dermatology. 2nd ed. Shelton, CT: People’s Medical Publishing House-USA; 2011.
- Smith R, Lipoff J. Evaluation of dermatology practice online reviews. JAMA Dermatol. 2016;152:153-157.
- Uberoi N, Finegold K, Gee E. Health insurance coverage and the Affordable Care Act, 2010-2016. US Department of Health & Human Services website. https://aspe.hhs.gov/system/files/pdf/187551/ACA2010-2016.pdf. Published March 3, 2016. Accessed May 18, 2018.
- Shatzer A, Long SK, Zuckerman S. Who are the newly insured as of early March 2014? Urban Institute Health Policy Center website. http://hrms.urban.org/briefs/Who-Are-the-Newly-Insured.html. Published May 22, 2014. Accessed May 18, 2018.
- Bardach NS, Asteria-Peñaloza R, Boscardin WJ, et al. The relationship between commercial website ratings and traditional hospital performance measures in the USA. BMJ Qual Saf. 2013;22:194-202.
- Kincey J, Bradshaw P, Ley P. Patient satisfaction and reported acceptance of medical advice in general practice. J R Coll Gen Pract. 1975;25:558-566.
- Francis V, Korsch BM, Morris MJ. Gaps in doctor-patient communication. patients’ response to medical advice. N Engl J Med. 1969;280:535-540.
- Ali ST, Feldman SR. Patient satisfaction in dermatology: a qualitative assessment. Dermatol Online J. 2014;20. pii:doj_21534.
- Internet study: highest educated & trained doctors get poorest online reviews. Vanguard Communications website. https://vanguard communications.net/best-online-doctor-reviews/. Published April 22, 2015. Accessed May 18, 2018.
- Uhas AA, Camacho FT, Feldman SR, et al. The relationship between physician friendliness and caring, and patient satisfaction: findings from an internet-based survey. Patient. 2008;1:91-96.
- Anderson RT, Camacho FT, Balkrishnan R. Willing to wait?: the influence of patient wait time on satisfaction with primary care. BMC Health Serv Res. 2007;7:31.
- Maibach HI, Gorouhi F. Evidence-Based Dermatology. 2nd ed. Shelton, CT: People’s Medical Publishing House-USA; 2011.
- Smith R, Lipoff J. Evaluation of dermatology practice online reviews. JAMA Dermatol. 2016;152:153-157.
Practice Points
- It is becoming increasingly important, particularly in the field of dermatology, to both measure and work to improve patient satisfaction scores.
- Preliminary research has found that simple interventions, such as providing disease-specific handouts and interpreter services, can improve satisfaction scores, making patient satisfaction an achievable goal.
Surgical Procedures for Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that has a social and psychosocial impact on patients with skin of color.1 It is characterized by recurrent abscesses, draining sinus tracts, and scarring in the intertriginous skin folds. The lesions are difficult to treat and present with considerable frustration for both patients and physicians. Although current treatment ladders can delay procedures and surgical intervention,1 some believe that surgery should be introduced earlier in HS management.2 In this article, we review current procedures for the management of HS, including cryoinsufflation, incision and drainage, deroofing, skin tissue–saving excision with electrosurgical peeling, and wide surgical excision, along with various closure techniques.
Cryoinsufflation
First described in 2014, cryoinsufflation is a novel method for treating sinus tracts.3 Lesions initially are identified on physical examination. Prior to the procedure, local anesthesia is administered to the lesion.3 A 21-gauge needle is mounted onto a cryosurgical unit and inserted into the opening of the sinus tract. Liquid nitrogen is sprayed into the tract for 5 seconds, followed by a 3-second pause; the process is repeated 3 times. Patients return for treatment sessions monthly until the tract is obliterated. This procedure was first performed on 2 patients with satisfactory results.3
Since the initial report, the investigators made 2 changes to refine the procedure.4 First, systemic antibiotics should be prescribed 2 months prior to the procedure to clear the sinus tracts of infection. Furthermore, a 21-gauge, olive-tipped cannula is recommended in lieu of a 21-gauge needle to mitigate the risk of adverse events such as air embolism.4
Incision and Drainage
Incision and drainage provides rapid pain relief for tense fluctuant abscesses, but recurrence is common and the procedure costs are high.5 For drainage, wide circumferential local anesthesia is administered followed by incision.6 Pus is eliminated using digital pressure or saline rinses.2 Following the elimination of pus, the wound may need gauze packing or placement of a wick for a few days.6 The general belief is that incision and drainage should be used, if necessary, to rapidly relieve the patient’s pain; however, other surgical options should be considered if the patient has had multiple incision and drainage procedures.7 Currently there are no randomized controlled trials (RCTs) on incision and drainage procedures in HS abscesses.
Deroofing
In 1959, Mullins et al8 first described the deroofing procedure, which was refined to preserve the floor of the sinus tract in the 1980s.9,10 Culp10 and Brown et al9 theorized that preservation of the exposed floor of the sinus tract allowed for the epithelial cells from sweat glands and hair follicle remnants to rapidly reepithelialize the wound. In 2010, van der Zee et al11 performed a prospective study of 88 deroofed lesions in which the investigators removed keratinous debris and epithelial remnants of the floor due to concern for recurrence in this area if the tissues remained. Only 17% (15/88) of the lesions recurred at a median follow-up of 34 months.11
In Hurley stage I or II HS, deroofing remains the primary procedure for persistent nodules and sinus tracts.2 The lesion is identified on physical examination and local anesthesia is administered, first to the area surrounding the lesion, then to the lesion itself.11 A blunt probe is used to identify openings and search for connecting fistulas. After defining the sinus tract, the roof and wings created by the incision are removed.11,12 The material on the floor of the tract is scraped away, and the wound is left to heal by secondary intention.11 In general, deroofed lesions heal with cosmetically acceptable scars. We have used this procedure in skin of color patients with good results and no difficulties with healing. Controlled trials with long-term follow-up are lacking in this population.
Skin Tissue–Saving Excision With Electrosurgical Peeling
Skin tissue–saving excision with electrosurgical peeling was first introduced in 2015.13 Blok et al14 described the procedure as a promising alternative to wide surgical excision for Hurley stage II or III HS. The procedure saves healthy tissue while completely removing lesional tissue, leading to rapid wound healing, excellent cosmesis, and a low risk of contractures2,14; however, recurrence rates are higher than those seen in wide surgical excision.15 There are no known RCTs with long-term follow-up for HS patients treated with skin tissue–saving excision with electrosurgical peeling.
The procedure typically is performed under general anesthesia.14 First, the sinus tract is palpated on physical examination and probed to delineate the extent of the tract. Next, the roof of the tract is incised electrosurgically with a wire loop tip coupled to an electrosurgical generator.14 Consecutive tangential excisions are made until the floor of the sinus tract is reached. The process of incising sinus tracts followed by tangential peeling off of tissue continues until the entire area is clear of lesional and fibrotic tissue. The wound margins are probed for the presence and subsequent removal of residual sinus tracts. Lastly, the electrosurgical generator is used to achieve hemostasis, steroids are injected to prevent the formation of hypergranulation tissue, and the wound is left to heal by secondary intention.14 Following intervention, recurrence rates appear to be similar to wide surgical excision.13,14
Wide Surgical Excision
Wide excision is a widely established technique consisting of surgical excision of a lesion plus an area of surrounding disease-free tissue such as subcutaneous fat or a lateral margin of intertriginous skin.15 Similar to other surgical techniques, wide excision is considered in cases of severe disease when pharmacologic management cannot remedy extensive fibrosis or architectural loss. It typically is performed in Hurley stage II and III HS, with pathology extending to involve deeper structures inaccessible to more superficial surgical methods.2 Prominent areas of use include gluteal, axillary, perineal, and perianal HS lesions on which conservative treatments have little effect and depend on wide excision to provide successful postoperative results.16 Although retrospective and prospective studies exist on wide excision in HS, there continues to be a dearth of RCTs. Based on the available literature, the primary motive for wide excision is lower recurrence rates (13% overall compared to 22% and 27% for local excision and deroofing, respectively) and longer asymptomatic periods compared to more local techniques.7,17 Wide excision combined with continued aggressive medical management and dietary modifications currently is an efficacious treatment in providing functional long-term results.6 These benefits, however, are not without their drawbacks, as the more extensive nature of wide surgical excision predisposes patients to larger wounds, surgery-induced infection, and prolonged recovery periods.6,15 If preoperative measurements are not wisely assessed, the excision also can extend to involve neurovascular bundles and other vital structures, contributing to greater postoperative morbidity.15 Ultrasonography provides useful anatomic information in HS, such as location and extent of fistulous tracts and fluid collections; these findings can assist in guiding the width and depth of the excision itself to ensure the entire area of HS involvement is removed.18 Published data revealed that 204 of 255 (80%) patients were markedly satisfied with postoperative outcomes of wide excision,19 which gives credence to the idea that although the complications of wide excision may not be as favorable, the long-lasting improvements in quality of life make wide surgical excision a suggested first-line treatment in all stages of HS.16,20
Closure Techniques
The best skin closure method following surgical excision is controversial and not well established in literature. Options include healing by secondary intention, primary (suture-based) closure, skin grafts, and skin flaps. Each of these methods has had moderate success in multiple observational studies, and the choice should be made based on individualized assessment of the patient’s HS lesion characteristics, ability to adhere to recovery protocols, and relevant demographics. A systematic review by Mehdizadeh et al17 provided the following recurrence rates for techniques utilized after wide excision: primary closure, 15%; flaps, 8%; and grafting, 6%. Despite conflicting evidence, allowing wounds to heal by secondary intention is best, based on the author’s experience (I.H.H.).
Secondary Intention
Healing by secondary intention refers to a wound that is intentionally left open to be filled in with granulation tissue and eventual epithelization over time rather than being approximated and closed via sutures or staples as in primary intention. It is a well-established option in wound management and results in a longer but more comfortable period of convalescence in postsurgical HS management.20 Patients can add regular moist wound dressings (eg, silastic foam dressing) to manage the wound at home and continue normal activities for most of the healing period; however, the recovery period can become excessively long and painful, and there is a high risk of formation of retractile scar bands at and around the healing site.12 Strict adherence to wound-healing protocols is paramount to minimizing unwanted complications.21 Secondary intention often is used after wide local excision and has been demonstrated to yield positive functional and aesthetic results in multiple studies, especially in the more severe Hurley stage II or III cases.21,22 It can be successfully employed after laser treatment and in surgical defects of all sizes with little to no contractures or reduced range of motion.6 Ultimately, the choice to heal via secondary intention should be made after thorough assessment of patient needs and with ample education to ensure compliance.
Primary Closure
Primary closure is the suture-mediated closing technique that is most often used in wound closure for lower-grade HS cases, especially smaller excisions. However, it is associated with potential complications. If HS lesions are not effectively excised, disease can then recur at the periphery of the excision and wound dehiscence can manifest more readily, especially as wound size increases.23 Consequently, primary closure is associated with the highest recurrence rates among closure techniques.17 Avoiding primary closure in active disease also is recommended due to the potential of burying residual foci of inflammation.6 Finally, primary closures lack skin coverage and thus often are not viable options in most perianal and genital lesions that require more extensive reconstruction. Retrospective case series and case reports exist on primary excision, but further study is needed.
Skin Grafts
Skin grafting is a technique of surgically transplanting a piece of healthy skin from one body site to another. Skin grafts typically are used when primary closure or skin flaps are not feasible (eg, in large wounds) and also when shorter time to wound closure is a greater concern in patient recovery.2,24 Additionally, skin grafts can be employed on large flat surfaces of the body, such as the buttocks or thighs, for timely wound closure when wound contraction is less effective or wound healing is slow via epithelization. Types of skin graft techniques include split-thickness skin graft (STSG), full-thickness skin graft, and recycled skin graft. All 3 types have demonstrated acceptable functional and aesthetic results in observational studies and case reports, and thus deciding which technique to use should include individualized assessment.2,25 The STSG has several advantages over the full-thickness skin graft, including hairlessness (ie, without hair follicles), ease of harvest, and a less complicated transfer to contaminated lesional areas such as those in HS.26 Additionally, STSGs allow for closure of even the largest wounds with minimal risk of serious infection. Split-thickness skin grafts are considered one of the most efficacious tools for axilla reconstruction; however, they require prolonged immobilization of the arm, result in sequelae in donor sites, and do not always prevent retractile scars.26 The recycled skin graft technique can be used to treat chronic gluteal HS, but reliability and outcomes have not been reported. Skin grafting after excision is associated with increased pain, immobilization, prolonged hospitalization, and longer healing times compared to skin flaps.19 In a systematic review of wound healing techniques following wide excision, grafting was shown to have the lowest recurrence rate (6%) compared to skin flaps (8%) and primary closure (15%).17 The absence of hair follicles and sweat glands in STSGs may be advantageous in HS because both hair follicles and sweat glands are thought to play more roles in the pathogenesis of HS.18,24 Most studies on skin grafts are limited to case reports.
Skin Flaps
Skin flaps are similar to skin grafts in that healthy skin is transplanted from one site to another; the difference is that flaps maintain an intact blood supply, whereas skin grafts depend on growth of new blood vessels.12,13 The primary advantage of skin flaps is that they provide the best quality of skin due to the thick tissue coverage, which is an important concern, especially in aesthetic scenarios. Additionally, they have been shown to provide shorter healing times than grafts, primary closure, and secondary healing, which can be especially important when functional disability is a concern in the postoperative period.26 However, their use should be limited due to several complications owing to their blood supply, as there is a high risk of ischemia to distant portions of flaps, which often can progress to necrosis and hemorrhage during the harvesting process.2 Thus, skin flaps are incredibly difficult to use in larger wounds and often require debulking due to their thickness. Additionally, skin flaps are definitive by nature, which can pose an issue if HS recurs locally. Skin flaps are recommended only when their use is mandatory, such as in the coverage of important anatomic structures (eg, exposed neurovascular bundles and large vessels).2 Advances have been made in flap construction, and now several types of flaps are employed in several body areas with differing indications and recommendations.2,21 As with skin grafts, most studies in the literature are case reports; therefore, further investigation is needed.
Combination Reconstructions
Combination reconstructions refer to the simultaneous use of multiple closure or healing techniques. By combining 2 or more methods, surgeons can utilize the advantages of each technique to provide an individualized approach that can substantially diminish wound surface area and accelerate wound healing.2 For example, with the starlike technique, 5 equilateral triangles bordering a foci of axillary disease are excised in addition to the central foci, and the edges of each triangle are then sutured together to create a final scar of considerably smaller size. The starlike technique allows the wound to be partially sutured while leaving the remaining area to heal by secondary intention.2 There are a small number of case series and prospective studies on combined reconstructions in HS but no RCTs.
Conclusion
- Smith MK, Nicholson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Janse I, Bieniek A, Horvath B, et al. Surgical procedures in hidradenitis suppurativa. Dermatol Clin. 2016;34:97-109.
- Pagliarello C, Fabrizi G, Feliciani C, et al. Cryoinsufflation for Hurley stage II hidradenitis suppurativa: a useful treatment option when systemic therapies should be avoided. JAMA Dermatol. 2014;150:765-766.
- Pagliarello C, Fabrizi G, di Nuzzo S. Cryoinsufflation for hidradenitis suppurativa: technical refinement to prevent complications. Dermatol Surg. 2016;42:130-132.
- Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168.
- Danby FW, Hazen PG, Boer J. New and traditional surgical approaches to hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5, suppl 1):S62-S65.
- Ellis LZ. Hidradenitis suppurativa: surgical and other management techniques. Dermatol Surg. 2012;38:517-536.
- Mullins JF, McCash WB, Boudreau RF. Treatment of chronic hidradenitis suppurativa: surgical modification. Postgrad Med. 1959;26:805-808.
- Brown SC, Kazzazi N, Lord PH. Surgical treatment of perineal hidradenitis suppurativa with special reference to recognition of the perianal form. Br J Surg. 1986;73:978-980.
- Culp CE. Chronic hidradenitis suppurativa of the anal canal. a surgical skin disease. Dis Colon Rectum. 1983;26:669-676.
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480.
- Lin CH, Chang KP, Huang SH. Deroofing: an effective method for treating chronic diffuse hidradenitis suppurativa. Dermatol Surg. 2016;42:273-275.
- Blok JL, Boersma M, Terra JB, et al. Surgery under general anaes-thesia in severe hidradenitis suppurativa: a study of 363 primary operations in 113 patients. J Eur Acad Dermatol Venereol. 2015;29:1590-1597.
- Blok JL, Spoo JR, Leeman FW, et al. Skin-Tissue-sparing Excision with Electrosurgical Peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Maghsoudi H, Almasi H, Miri Bonjar MR. Men, main victims of hidradenitis suppurativa (a prospective cohort study). Int J Surg. 2018;50:6-10.
- Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5, suppl 1):S70-S77.
- Wortsman X, Moreno C, Soto R, et al. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg. 2013;39:1835-1842.
- Kofler L, Schweinzer K, Heister M, et al. Surgical treatment of hidradenitis suppurativa: an analysis of postoperative outcome, cosmetic results and quality of life in 255 patients [published online February 17, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14892.
- Dini V, Oranges T, Rotella L, et al. Hidradenitis suppurativa and wound management. Int J Low Extrem Wounds. 2015;14:236-244.
- Humphries LS, Kueberuwa E, Beederman M, et al. Wide excision and healing by secondary intent for the surgical treatment of hidradenitis suppurativa: a single-center experience. J Plast Reconstr Aesthet Surg. 2016;69:554-566.
- Wollina U, Langner D, Heinig B, et al. Comorbidities, treatment, and outcome in severe anogenital inverse acne (hidradenitis suppurativa): a 15-year single center report. Int J Dermatol. 2017;56:109-115.
- Watson JD. Hidradenitis suppurativa—a clinical review. Br J Plast Surg. 1985;38:567-569.
- Sugio Y, Tomita K, Hosokawa K. Reconstruction after excision of hidradenitis suppurativa: are skin grafts better than flaps? Plast Reconstr Surg Glob Open. 2016;4:E1128.
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730.
- Nail-Barthelemy R, Stroumza N, Qassemyar Q, et al. Evaluation of the mobility of the shoulder and quality of life after perforator flaps for recalcitrant axillary hidradenitis [published online February 13, 2018]. Ann Chir Plast Esthet. pii:S0294-1260(18)30005-0. doi:10.1016/j.anplas.2018.01.003.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that has a social and psychosocial impact on patients with skin of color.1 It is characterized by recurrent abscesses, draining sinus tracts, and scarring in the intertriginous skin folds. The lesions are difficult to treat and present with considerable frustration for both patients and physicians. Although current treatment ladders can delay procedures and surgical intervention,1 some believe that surgery should be introduced earlier in HS management.2 In this article, we review current procedures for the management of HS, including cryoinsufflation, incision and drainage, deroofing, skin tissue–saving excision with electrosurgical peeling, and wide surgical excision, along with various closure techniques.
Cryoinsufflation
First described in 2014, cryoinsufflation is a novel method for treating sinus tracts.3 Lesions initially are identified on physical examination. Prior to the procedure, local anesthesia is administered to the lesion.3 A 21-gauge needle is mounted onto a cryosurgical unit and inserted into the opening of the sinus tract. Liquid nitrogen is sprayed into the tract for 5 seconds, followed by a 3-second pause; the process is repeated 3 times. Patients return for treatment sessions monthly until the tract is obliterated. This procedure was first performed on 2 patients with satisfactory results.3
Since the initial report, the investigators made 2 changes to refine the procedure.4 First, systemic antibiotics should be prescribed 2 months prior to the procedure to clear the sinus tracts of infection. Furthermore, a 21-gauge, olive-tipped cannula is recommended in lieu of a 21-gauge needle to mitigate the risk of adverse events such as air embolism.4
Incision and Drainage
Incision and drainage provides rapid pain relief for tense fluctuant abscesses, but recurrence is common and the procedure costs are high.5 For drainage, wide circumferential local anesthesia is administered followed by incision.6 Pus is eliminated using digital pressure or saline rinses.2 Following the elimination of pus, the wound may need gauze packing or placement of a wick for a few days.6 The general belief is that incision and drainage should be used, if necessary, to rapidly relieve the patient’s pain; however, other surgical options should be considered if the patient has had multiple incision and drainage procedures.7 Currently there are no randomized controlled trials (RCTs) on incision and drainage procedures in HS abscesses.
Deroofing
In 1959, Mullins et al8 first described the deroofing procedure, which was refined to preserve the floor of the sinus tract in the 1980s.9,10 Culp10 and Brown et al9 theorized that preservation of the exposed floor of the sinus tract allowed for the epithelial cells from sweat glands and hair follicle remnants to rapidly reepithelialize the wound. In 2010, van der Zee et al11 performed a prospective study of 88 deroofed lesions in which the investigators removed keratinous debris and epithelial remnants of the floor due to concern for recurrence in this area if the tissues remained. Only 17% (15/88) of the lesions recurred at a median follow-up of 34 months.11
In Hurley stage I or II HS, deroofing remains the primary procedure for persistent nodules and sinus tracts.2 The lesion is identified on physical examination and local anesthesia is administered, first to the area surrounding the lesion, then to the lesion itself.11 A blunt probe is used to identify openings and search for connecting fistulas. After defining the sinus tract, the roof and wings created by the incision are removed.11,12 The material on the floor of the tract is scraped away, and the wound is left to heal by secondary intention.11 In general, deroofed lesions heal with cosmetically acceptable scars. We have used this procedure in skin of color patients with good results and no difficulties with healing. Controlled trials with long-term follow-up are lacking in this population.
Skin Tissue–Saving Excision With Electrosurgical Peeling
Skin tissue–saving excision with electrosurgical peeling was first introduced in 2015.13 Blok et al14 described the procedure as a promising alternative to wide surgical excision for Hurley stage II or III HS. The procedure saves healthy tissue while completely removing lesional tissue, leading to rapid wound healing, excellent cosmesis, and a low risk of contractures2,14; however, recurrence rates are higher than those seen in wide surgical excision.15 There are no known RCTs with long-term follow-up for HS patients treated with skin tissue–saving excision with electrosurgical peeling.
The procedure typically is performed under general anesthesia.14 First, the sinus tract is palpated on physical examination and probed to delineate the extent of the tract. Next, the roof of the tract is incised electrosurgically with a wire loop tip coupled to an electrosurgical generator.14 Consecutive tangential excisions are made until the floor of the sinus tract is reached. The process of incising sinus tracts followed by tangential peeling off of tissue continues until the entire area is clear of lesional and fibrotic tissue. The wound margins are probed for the presence and subsequent removal of residual sinus tracts. Lastly, the electrosurgical generator is used to achieve hemostasis, steroids are injected to prevent the formation of hypergranulation tissue, and the wound is left to heal by secondary intention.14 Following intervention, recurrence rates appear to be similar to wide surgical excision.13,14
Wide Surgical Excision
Wide excision is a widely established technique consisting of surgical excision of a lesion plus an area of surrounding disease-free tissue such as subcutaneous fat or a lateral margin of intertriginous skin.15 Similar to other surgical techniques, wide excision is considered in cases of severe disease when pharmacologic management cannot remedy extensive fibrosis or architectural loss. It typically is performed in Hurley stage II and III HS, with pathology extending to involve deeper structures inaccessible to more superficial surgical methods.2 Prominent areas of use include gluteal, axillary, perineal, and perianal HS lesions on which conservative treatments have little effect and depend on wide excision to provide successful postoperative results.16 Although retrospective and prospective studies exist on wide excision in HS, there continues to be a dearth of RCTs. Based on the available literature, the primary motive for wide excision is lower recurrence rates (13% overall compared to 22% and 27% for local excision and deroofing, respectively) and longer asymptomatic periods compared to more local techniques.7,17 Wide excision combined with continued aggressive medical management and dietary modifications currently is an efficacious treatment in providing functional long-term results.6 These benefits, however, are not without their drawbacks, as the more extensive nature of wide surgical excision predisposes patients to larger wounds, surgery-induced infection, and prolonged recovery periods.6,15 If preoperative measurements are not wisely assessed, the excision also can extend to involve neurovascular bundles and other vital structures, contributing to greater postoperative morbidity.15 Ultrasonography provides useful anatomic information in HS, such as location and extent of fistulous tracts and fluid collections; these findings can assist in guiding the width and depth of the excision itself to ensure the entire area of HS involvement is removed.18 Published data revealed that 204 of 255 (80%) patients were markedly satisfied with postoperative outcomes of wide excision,19 which gives credence to the idea that although the complications of wide excision may not be as favorable, the long-lasting improvements in quality of life make wide surgical excision a suggested first-line treatment in all stages of HS.16,20
Closure Techniques
The best skin closure method following surgical excision is controversial and not well established in literature. Options include healing by secondary intention, primary (suture-based) closure, skin grafts, and skin flaps. Each of these methods has had moderate success in multiple observational studies, and the choice should be made based on individualized assessment of the patient’s HS lesion characteristics, ability to adhere to recovery protocols, and relevant demographics. A systematic review by Mehdizadeh et al17 provided the following recurrence rates for techniques utilized after wide excision: primary closure, 15%; flaps, 8%; and grafting, 6%. Despite conflicting evidence, allowing wounds to heal by secondary intention is best, based on the author’s experience (I.H.H.).
Secondary Intention
Healing by secondary intention refers to a wound that is intentionally left open to be filled in with granulation tissue and eventual epithelization over time rather than being approximated and closed via sutures or staples as in primary intention. It is a well-established option in wound management and results in a longer but more comfortable period of convalescence in postsurgical HS management.20 Patients can add regular moist wound dressings (eg, silastic foam dressing) to manage the wound at home and continue normal activities for most of the healing period; however, the recovery period can become excessively long and painful, and there is a high risk of formation of retractile scar bands at and around the healing site.12 Strict adherence to wound-healing protocols is paramount to minimizing unwanted complications.21 Secondary intention often is used after wide local excision and has been demonstrated to yield positive functional and aesthetic results in multiple studies, especially in the more severe Hurley stage II or III cases.21,22 It can be successfully employed after laser treatment and in surgical defects of all sizes with little to no contractures or reduced range of motion.6 Ultimately, the choice to heal via secondary intention should be made after thorough assessment of patient needs and with ample education to ensure compliance.
Primary Closure
Primary closure is the suture-mediated closing technique that is most often used in wound closure for lower-grade HS cases, especially smaller excisions. However, it is associated with potential complications. If HS lesions are not effectively excised, disease can then recur at the periphery of the excision and wound dehiscence can manifest more readily, especially as wound size increases.23 Consequently, primary closure is associated with the highest recurrence rates among closure techniques.17 Avoiding primary closure in active disease also is recommended due to the potential of burying residual foci of inflammation.6 Finally, primary closures lack skin coverage and thus often are not viable options in most perianal and genital lesions that require more extensive reconstruction. Retrospective case series and case reports exist on primary excision, but further study is needed.
Skin Grafts
Skin grafting is a technique of surgically transplanting a piece of healthy skin from one body site to another. Skin grafts typically are used when primary closure or skin flaps are not feasible (eg, in large wounds) and also when shorter time to wound closure is a greater concern in patient recovery.2,24 Additionally, skin grafts can be employed on large flat surfaces of the body, such as the buttocks or thighs, for timely wound closure when wound contraction is less effective or wound healing is slow via epithelization. Types of skin graft techniques include split-thickness skin graft (STSG), full-thickness skin graft, and recycled skin graft. All 3 types have demonstrated acceptable functional and aesthetic results in observational studies and case reports, and thus deciding which technique to use should include individualized assessment.2,25 The STSG has several advantages over the full-thickness skin graft, including hairlessness (ie, without hair follicles), ease of harvest, and a less complicated transfer to contaminated lesional areas such as those in HS.26 Additionally, STSGs allow for closure of even the largest wounds with minimal risk of serious infection. Split-thickness skin grafts are considered one of the most efficacious tools for axilla reconstruction; however, they require prolonged immobilization of the arm, result in sequelae in donor sites, and do not always prevent retractile scars.26 The recycled skin graft technique can be used to treat chronic gluteal HS, but reliability and outcomes have not been reported. Skin grafting after excision is associated with increased pain, immobilization, prolonged hospitalization, and longer healing times compared to skin flaps.19 In a systematic review of wound healing techniques following wide excision, grafting was shown to have the lowest recurrence rate (6%) compared to skin flaps (8%) and primary closure (15%).17 The absence of hair follicles and sweat glands in STSGs may be advantageous in HS because both hair follicles and sweat glands are thought to play more roles in the pathogenesis of HS.18,24 Most studies on skin grafts are limited to case reports.
Skin Flaps
Skin flaps are similar to skin grafts in that healthy skin is transplanted from one site to another; the difference is that flaps maintain an intact blood supply, whereas skin grafts depend on growth of new blood vessels.12,13 The primary advantage of skin flaps is that they provide the best quality of skin due to the thick tissue coverage, which is an important concern, especially in aesthetic scenarios. Additionally, they have been shown to provide shorter healing times than grafts, primary closure, and secondary healing, which can be especially important when functional disability is a concern in the postoperative period.26 However, their use should be limited due to several complications owing to their blood supply, as there is a high risk of ischemia to distant portions of flaps, which often can progress to necrosis and hemorrhage during the harvesting process.2 Thus, skin flaps are incredibly difficult to use in larger wounds and often require debulking due to their thickness. Additionally, skin flaps are definitive by nature, which can pose an issue if HS recurs locally. Skin flaps are recommended only when their use is mandatory, such as in the coverage of important anatomic structures (eg, exposed neurovascular bundles and large vessels).2 Advances have been made in flap construction, and now several types of flaps are employed in several body areas with differing indications and recommendations.2,21 As with skin grafts, most studies in the literature are case reports; therefore, further investigation is needed.
Combination Reconstructions
Combination reconstructions refer to the simultaneous use of multiple closure or healing techniques. By combining 2 or more methods, surgeons can utilize the advantages of each technique to provide an individualized approach that can substantially diminish wound surface area and accelerate wound healing.2 For example, with the starlike technique, 5 equilateral triangles bordering a foci of axillary disease are excised in addition to the central foci, and the edges of each triangle are then sutured together to create a final scar of considerably smaller size. The starlike technique allows the wound to be partially sutured while leaving the remaining area to heal by secondary intention.2 There are a small number of case series and prospective studies on combined reconstructions in HS but no RCTs.
Conclusion
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that has a social and psychosocial impact on patients with skin of color.1 It is characterized by recurrent abscesses, draining sinus tracts, and scarring in the intertriginous skin folds. The lesions are difficult to treat and present with considerable frustration for both patients and physicians. Although current treatment ladders can delay procedures and surgical intervention,1 some believe that surgery should be introduced earlier in HS management.2 In this article, we review current procedures for the management of HS, including cryoinsufflation, incision and drainage, deroofing, skin tissue–saving excision with electrosurgical peeling, and wide surgical excision, along with various closure techniques.
Cryoinsufflation
First described in 2014, cryoinsufflation is a novel method for treating sinus tracts.3 Lesions initially are identified on physical examination. Prior to the procedure, local anesthesia is administered to the lesion.3 A 21-gauge needle is mounted onto a cryosurgical unit and inserted into the opening of the sinus tract. Liquid nitrogen is sprayed into the tract for 5 seconds, followed by a 3-second pause; the process is repeated 3 times. Patients return for treatment sessions monthly until the tract is obliterated. This procedure was first performed on 2 patients with satisfactory results.3
Since the initial report, the investigators made 2 changes to refine the procedure.4 First, systemic antibiotics should be prescribed 2 months prior to the procedure to clear the sinus tracts of infection. Furthermore, a 21-gauge, olive-tipped cannula is recommended in lieu of a 21-gauge needle to mitigate the risk of adverse events such as air embolism.4
Incision and Drainage
Incision and drainage provides rapid pain relief for tense fluctuant abscesses, but recurrence is common and the procedure costs are high.5 For drainage, wide circumferential local anesthesia is administered followed by incision.6 Pus is eliminated using digital pressure or saline rinses.2 Following the elimination of pus, the wound may need gauze packing or placement of a wick for a few days.6 The general belief is that incision and drainage should be used, if necessary, to rapidly relieve the patient’s pain; however, other surgical options should be considered if the patient has had multiple incision and drainage procedures.7 Currently there are no randomized controlled trials (RCTs) on incision and drainage procedures in HS abscesses.
Deroofing
In 1959, Mullins et al8 first described the deroofing procedure, which was refined to preserve the floor of the sinus tract in the 1980s.9,10 Culp10 and Brown et al9 theorized that preservation of the exposed floor of the sinus tract allowed for the epithelial cells from sweat glands and hair follicle remnants to rapidly reepithelialize the wound. In 2010, van der Zee et al11 performed a prospective study of 88 deroofed lesions in which the investigators removed keratinous debris and epithelial remnants of the floor due to concern for recurrence in this area if the tissues remained. Only 17% (15/88) of the lesions recurred at a median follow-up of 34 months.11
In Hurley stage I or II HS, deroofing remains the primary procedure for persistent nodules and sinus tracts.2 The lesion is identified on physical examination and local anesthesia is administered, first to the area surrounding the lesion, then to the lesion itself.11 A blunt probe is used to identify openings and search for connecting fistulas. After defining the sinus tract, the roof and wings created by the incision are removed.11,12 The material on the floor of the tract is scraped away, and the wound is left to heal by secondary intention.11 In general, deroofed lesions heal with cosmetically acceptable scars. We have used this procedure in skin of color patients with good results and no difficulties with healing. Controlled trials with long-term follow-up are lacking in this population.
Skin Tissue–Saving Excision With Electrosurgical Peeling
Skin tissue–saving excision with electrosurgical peeling was first introduced in 2015.13 Blok et al14 described the procedure as a promising alternative to wide surgical excision for Hurley stage II or III HS. The procedure saves healthy tissue while completely removing lesional tissue, leading to rapid wound healing, excellent cosmesis, and a low risk of contractures2,14; however, recurrence rates are higher than those seen in wide surgical excision.15 There are no known RCTs with long-term follow-up for HS patients treated with skin tissue–saving excision with electrosurgical peeling.
The procedure typically is performed under general anesthesia.14 First, the sinus tract is palpated on physical examination and probed to delineate the extent of the tract. Next, the roof of the tract is incised electrosurgically with a wire loop tip coupled to an electrosurgical generator.14 Consecutive tangential excisions are made until the floor of the sinus tract is reached. The process of incising sinus tracts followed by tangential peeling off of tissue continues until the entire area is clear of lesional and fibrotic tissue. The wound margins are probed for the presence and subsequent removal of residual sinus tracts. Lastly, the electrosurgical generator is used to achieve hemostasis, steroids are injected to prevent the formation of hypergranulation tissue, and the wound is left to heal by secondary intention.14 Following intervention, recurrence rates appear to be similar to wide surgical excision.13,14
Wide Surgical Excision
Wide excision is a widely established technique consisting of surgical excision of a lesion plus an area of surrounding disease-free tissue such as subcutaneous fat or a lateral margin of intertriginous skin.15 Similar to other surgical techniques, wide excision is considered in cases of severe disease when pharmacologic management cannot remedy extensive fibrosis or architectural loss. It typically is performed in Hurley stage II and III HS, with pathology extending to involve deeper structures inaccessible to more superficial surgical methods.2 Prominent areas of use include gluteal, axillary, perineal, and perianal HS lesions on which conservative treatments have little effect and depend on wide excision to provide successful postoperative results.16 Although retrospective and prospective studies exist on wide excision in HS, there continues to be a dearth of RCTs. Based on the available literature, the primary motive for wide excision is lower recurrence rates (13% overall compared to 22% and 27% for local excision and deroofing, respectively) and longer asymptomatic periods compared to more local techniques.7,17 Wide excision combined with continued aggressive medical management and dietary modifications currently is an efficacious treatment in providing functional long-term results.6 These benefits, however, are not without their drawbacks, as the more extensive nature of wide surgical excision predisposes patients to larger wounds, surgery-induced infection, and prolonged recovery periods.6,15 If preoperative measurements are not wisely assessed, the excision also can extend to involve neurovascular bundles and other vital structures, contributing to greater postoperative morbidity.15 Ultrasonography provides useful anatomic information in HS, such as location and extent of fistulous tracts and fluid collections; these findings can assist in guiding the width and depth of the excision itself to ensure the entire area of HS involvement is removed.18 Published data revealed that 204 of 255 (80%) patients were markedly satisfied with postoperative outcomes of wide excision,19 which gives credence to the idea that although the complications of wide excision may not be as favorable, the long-lasting improvements in quality of life make wide surgical excision a suggested first-line treatment in all stages of HS.16,20
Closure Techniques
The best skin closure method following surgical excision is controversial and not well established in literature. Options include healing by secondary intention, primary (suture-based) closure, skin grafts, and skin flaps. Each of these methods has had moderate success in multiple observational studies, and the choice should be made based on individualized assessment of the patient’s HS lesion characteristics, ability to adhere to recovery protocols, and relevant demographics. A systematic review by Mehdizadeh et al17 provided the following recurrence rates for techniques utilized after wide excision: primary closure, 15%; flaps, 8%; and grafting, 6%. Despite conflicting evidence, allowing wounds to heal by secondary intention is best, based on the author’s experience (I.H.H.).
Secondary Intention
Healing by secondary intention refers to a wound that is intentionally left open to be filled in with granulation tissue and eventual epithelization over time rather than being approximated and closed via sutures or staples as in primary intention. It is a well-established option in wound management and results in a longer but more comfortable period of convalescence in postsurgical HS management.20 Patients can add regular moist wound dressings (eg, silastic foam dressing) to manage the wound at home and continue normal activities for most of the healing period; however, the recovery period can become excessively long and painful, and there is a high risk of formation of retractile scar bands at and around the healing site.12 Strict adherence to wound-healing protocols is paramount to minimizing unwanted complications.21 Secondary intention often is used after wide local excision and has been demonstrated to yield positive functional and aesthetic results in multiple studies, especially in the more severe Hurley stage II or III cases.21,22 It can be successfully employed after laser treatment and in surgical defects of all sizes with little to no contractures or reduced range of motion.6 Ultimately, the choice to heal via secondary intention should be made after thorough assessment of patient needs and with ample education to ensure compliance.
Primary Closure
Primary closure is the suture-mediated closing technique that is most often used in wound closure for lower-grade HS cases, especially smaller excisions. However, it is associated with potential complications. If HS lesions are not effectively excised, disease can then recur at the periphery of the excision and wound dehiscence can manifest more readily, especially as wound size increases.23 Consequently, primary closure is associated with the highest recurrence rates among closure techniques.17 Avoiding primary closure in active disease also is recommended due to the potential of burying residual foci of inflammation.6 Finally, primary closures lack skin coverage and thus often are not viable options in most perianal and genital lesions that require more extensive reconstruction. Retrospective case series and case reports exist on primary excision, but further study is needed.
Skin Grafts
Skin grafting is a technique of surgically transplanting a piece of healthy skin from one body site to another. Skin grafts typically are used when primary closure or skin flaps are not feasible (eg, in large wounds) and also when shorter time to wound closure is a greater concern in patient recovery.2,24 Additionally, skin grafts can be employed on large flat surfaces of the body, such as the buttocks or thighs, for timely wound closure when wound contraction is less effective or wound healing is slow via epithelization. Types of skin graft techniques include split-thickness skin graft (STSG), full-thickness skin graft, and recycled skin graft. All 3 types have demonstrated acceptable functional and aesthetic results in observational studies and case reports, and thus deciding which technique to use should include individualized assessment.2,25 The STSG has several advantages over the full-thickness skin graft, including hairlessness (ie, without hair follicles), ease of harvest, and a less complicated transfer to contaminated lesional areas such as those in HS.26 Additionally, STSGs allow for closure of even the largest wounds with minimal risk of serious infection. Split-thickness skin grafts are considered one of the most efficacious tools for axilla reconstruction; however, they require prolonged immobilization of the arm, result in sequelae in donor sites, and do not always prevent retractile scars.26 The recycled skin graft technique can be used to treat chronic gluteal HS, but reliability and outcomes have not been reported. Skin grafting after excision is associated with increased pain, immobilization, prolonged hospitalization, and longer healing times compared to skin flaps.19 In a systematic review of wound healing techniques following wide excision, grafting was shown to have the lowest recurrence rate (6%) compared to skin flaps (8%) and primary closure (15%).17 The absence of hair follicles and sweat glands in STSGs may be advantageous in HS because both hair follicles and sweat glands are thought to play more roles in the pathogenesis of HS.18,24 Most studies on skin grafts are limited to case reports.
Skin Flaps
Skin flaps are similar to skin grafts in that healthy skin is transplanted from one site to another; the difference is that flaps maintain an intact blood supply, whereas skin grafts depend on growth of new blood vessels.12,13 The primary advantage of skin flaps is that they provide the best quality of skin due to the thick tissue coverage, which is an important concern, especially in aesthetic scenarios. Additionally, they have been shown to provide shorter healing times than grafts, primary closure, and secondary healing, which can be especially important when functional disability is a concern in the postoperative period.26 However, their use should be limited due to several complications owing to their blood supply, as there is a high risk of ischemia to distant portions of flaps, which often can progress to necrosis and hemorrhage during the harvesting process.2 Thus, skin flaps are incredibly difficult to use in larger wounds and often require debulking due to their thickness. Additionally, skin flaps are definitive by nature, which can pose an issue if HS recurs locally. Skin flaps are recommended only when their use is mandatory, such as in the coverage of important anatomic structures (eg, exposed neurovascular bundles and large vessels).2 Advances have been made in flap construction, and now several types of flaps are employed in several body areas with differing indications and recommendations.2,21 As with skin grafts, most studies in the literature are case reports; therefore, further investigation is needed.
Combination Reconstructions
Combination reconstructions refer to the simultaneous use of multiple closure or healing techniques. By combining 2 or more methods, surgeons can utilize the advantages of each technique to provide an individualized approach that can substantially diminish wound surface area and accelerate wound healing.2 For example, with the starlike technique, 5 equilateral triangles bordering a foci of axillary disease are excised in addition to the central foci, and the edges of each triangle are then sutured together to create a final scar of considerably smaller size. The starlike technique allows the wound to be partially sutured while leaving the remaining area to heal by secondary intention.2 There are a small number of case series and prospective studies on combined reconstructions in HS but no RCTs.
Conclusion
- Smith MK, Nicholson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Janse I, Bieniek A, Horvath B, et al. Surgical procedures in hidradenitis suppurativa. Dermatol Clin. 2016;34:97-109.
- Pagliarello C, Fabrizi G, Feliciani C, et al. Cryoinsufflation for Hurley stage II hidradenitis suppurativa: a useful treatment option when systemic therapies should be avoided. JAMA Dermatol. 2014;150:765-766.
- Pagliarello C, Fabrizi G, di Nuzzo S. Cryoinsufflation for hidradenitis suppurativa: technical refinement to prevent complications. Dermatol Surg. 2016;42:130-132.
- Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168.
- Danby FW, Hazen PG, Boer J. New and traditional surgical approaches to hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5, suppl 1):S62-S65.
- Ellis LZ. Hidradenitis suppurativa: surgical and other management techniques. Dermatol Surg. 2012;38:517-536.
- Mullins JF, McCash WB, Boudreau RF. Treatment of chronic hidradenitis suppurativa: surgical modification. Postgrad Med. 1959;26:805-808.
- Brown SC, Kazzazi N, Lord PH. Surgical treatment of perineal hidradenitis suppurativa with special reference to recognition of the perianal form. Br J Surg. 1986;73:978-980.
- Culp CE. Chronic hidradenitis suppurativa of the anal canal. a surgical skin disease. Dis Colon Rectum. 1983;26:669-676.
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480.
- Lin CH, Chang KP, Huang SH. Deroofing: an effective method for treating chronic diffuse hidradenitis suppurativa. Dermatol Surg. 2016;42:273-275.
- Blok JL, Boersma M, Terra JB, et al. Surgery under general anaes-thesia in severe hidradenitis suppurativa: a study of 363 primary operations in 113 patients. J Eur Acad Dermatol Venereol. 2015;29:1590-1597.
- Blok JL, Spoo JR, Leeman FW, et al. Skin-Tissue-sparing Excision with Electrosurgical Peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Maghsoudi H, Almasi H, Miri Bonjar MR. Men, main victims of hidradenitis suppurativa (a prospective cohort study). Int J Surg. 2018;50:6-10.
- Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5, suppl 1):S70-S77.
- Wortsman X, Moreno C, Soto R, et al. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg. 2013;39:1835-1842.
- Kofler L, Schweinzer K, Heister M, et al. Surgical treatment of hidradenitis suppurativa: an analysis of postoperative outcome, cosmetic results and quality of life in 255 patients [published online February 17, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14892.
- Dini V, Oranges T, Rotella L, et al. Hidradenitis suppurativa and wound management. Int J Low Extrem Wounds. 2015;14:236-244.
- Humphries LS, Kueberuwa E, Beederman M, et al. Wide excision and healing by secondary intent for the surgical treatment of hidradenitis suppurativa: a single-center experience. J Plast Reconstr Aesthet Surg. 2016;69:554-566.
- Wollina U, Langner D, Heinig B, et al. Comorbidities, treatment, and outcome in severe anogenital inverse acne (hidradenitis suppurativa): a 15-year single center report. Int J Dermatol. 2017;56:109-115.
- Watson JD. Hidradenitis suppurativa—a clinical review. Br J Plast Surg. 1985;38:567-569.
- Sugio Y, Tomita K, Hosokawa K. Reconstruction after excision of hidradenitis suppurativa: are skin grafts better than flaps? Plast Reconstr Surg Glob Open. 2016;4:E1128.
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730.
- Nail-Barthelemy R, Stroumza N, Qassemyar Q, et al. Evaluation of the mobility of the shoulder and quality of life after perforator flaps for recalcitrant axillary hidradenitis [published online February 13, 2018]. Ann Chir Plast Esthet. pii:S0294-1260(18)30005-0. doi:10.1016/j.anplas.2018.01.003.
- Smith MK, Nicholson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Janse I, Bieniek A, Horvath B, et al. Surgical procedures in hidradenitis suppurativa. Dermatol Clin. 2016;34:97-109.
- Pagliarello C, Fabrizi G, Feliciani C, et al. Cryoinsufflation for Hurley stage II hidradenitis suppurativa: a useful treatment option when systemic therapies should be avoided. JAMA Dermatol. 2014;150:765-766.
- Pagliarello C, Fabrizi G, di Nuzzo S. Cryoinsufflation for hidradenitis suppurativa: technical refinement to prevent complications. Dermatol Surg. 2016;42:130-132.
- Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168.
- Danby FW, Hazen PG, Boer J. New and traditional surgical approaches to hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5, suppl 1):S62-S65.
- Ellis LZ. Hidradenitis suppurativa: surgical and other management techniques. Dermatol Surg. 2012;38:517-536.
- Mullins JF, McCash WB, Boudreau RF. Treatment of chronic hidradenitis suppurativa: surgical modification. Postgrad Med. 1959;26:805-808.
- Brown SC, Kazzazi N, Lord PH. Surgical treatment of perineal hidradenitis suppurativa with special reference to recognition of the perianal form. Br J Surg. 1986;73:978-980.
- Culp CE. Chronic hidradenitis suppurativa of the anal canal. a surgical skin disease. Dis Colon Rectum. 1983;26:669-676.
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480.
- Lin CH, Chang KP, Huang SH. Deroofing: an effective method for treating chronic diffuse hidradenitis suppurativa. Dermatol Surg. 2016;42:273-275.
- Blok JL, Boersma M, Terra JB, et al. Surgery under general anaes-thesia in severe hidradenitis suppurativa: a study of 363 primary operations in 113 patients. J Eur Acad Dermatol Venereol. 2015;29:1590-1597.
- Blok JL, Spoo JR, Leeman FW, et al. Skin-Tissue-sparing Excision with Electrosurgical Peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Maghsoudi H, Almasi H, Miri Bonjar MR. Men, main victims of hidradenitis suppurativa (a prospective cohort study). Int J Surg. 2018;50:6-10.
- Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5, suppl 1):S70-S77.
- Wortsman X, Moreno C, Soto R, et al. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg. 2013;39:1835-1842.
- Kofler L, Schweinzer K, Heister M, et al. Surgical treatment of hidradenitis suppurativa: an analysis of postoperative outcome, cosmetic results and quality of life in 255 patients [published online February 17, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14892.
- Dini V, Oranges T, Rotella L, et al. Hidradenitis suppurativa and wound management. Int J Low Extrem Wounds. 2015;14:236-244.
- Humphries LS, Kueberuwa E, Beederman M, et al. Wide excision and healing by secondary intent for the surgical treatment of hidradenitis suppurativa: a single-center experience. J Plast Reconstr Aesthet Surg. 2016;69:554-566.
- Wollina U, Langner D, Heinig B, et al. Comorbidities, treatment, and outcome in severe anogenital inverse acne (hidradenitis suppurativa): a 15-year single center report. Int J Dermatol. 2017;56:109-115.
- Watson JD. Hidradenitis suppurativa—a clinical review. Br J Plast Surg. 1985;38:567-569.
- Sugio Y, Tomita K, Hosokawa K. Reconstruction after excision of hidradenitis suppurativa: are skin grafts better than flaps? Plast Reconstr Surg Glob Open. 2016;4:E1128.
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730.
- Nail-Barthelemy R, Stroumza N, Qassemyar Q, et al. Evaluation of the mobility of the shoulder and quality of life after perforator flaps for recalcitrant axillary hidradenitis [published online February 13, 2018]. Ann Chir Plast Esthet. pii:S0294-1260(18)30005-0. doi:10.1016/j.anplas.2018.01.003.
Practice Points
- Surgical intervention currently is the only definitive treatment for hidradenitis suppurativa (HS).
- There is no consensus on the best surgical intervention for long-term outcomes in HS; rather, approach is based on clinical judgment dependent upon the location and severity of lesions.
- After wide excision, allow wounds to heal by secondary intention.
Economic Stewardship in Acne Management
We are fortunate to have plentiful acne treatment options available that cater to each patient’s clinical examination, predispositions, and triggers, but the choices are daunting amidst the vast prescription and over-the-counter (OTC) topicals available along with disparate insurance and cost nuances. In addition, when prescribing generic oral therapies, it is complicated to parse out regional differences in price, supply, and insurance coverage to advocate best for each patient and land upon the delicate balance between efficacy, safety, and financial stewardship, both at an individual and community level. I will outline some challenges and solutions to the management of acne amidst these complicated factors.
Oral Therapies
For isotretinoin, generic choices, cost, and tiering within insurance plans are perpetual moving targets despite the drug being the only member of its class.1 Prescriber resources include tandem searches of electronic medical record price estimates within each insurance formulary, individual pharmacy search engines, and compilation mobile applications such as GoodRx to select the most affordable version for each patient. As an example of a regional trend, isotretinoin generic coverage by one provider in Pennsylvania shifted earlier this year so that every patient, whether new to isotretinoin or midcourse, required a new prior authorization with more stringent coverage requirements including failure of 2 oral antibiotics. Swiftly thereafter, efforts across the state driven by the Pennsylvania Academy of Dermatology and Dermatologic Surgery and its members and fueled by poignant patient vignettes about fragmented and substandard patient care helped to reverse this policy and remove the prior authorization mandate.2
Tetracyclines have experienced broad cost swings, mostly based on disruption of manufacturing at the limited number of distribution sites in the United States. In 2011, a tetracycline shortage arose due to a major manufacturer’s recall3 and persisted with subsequent material shortages, as doxycycline became the preferred and cheaper member of the class. Doxycycline price tag hikes then occurred following Hurricane Sandy when an East Coast manufacturing site was damaged.3 Spironolactone backorder also has been frequent due to recent raw material shortages and delays in production,3 forcing pharmacies to refill small amounts of medication in various dosage forms despite patients owing the same copayment per prescription.
Topical Therapies
Topical retinoid prescription affordability has always been fraught with difficulty owing to age cutoffs because it is often restricted to patients younger than 25 or 40 years, depending on the plan,4,5 but the availability of adapalene gel 0.1% as an OTC preparation in 2016 has broadened retinoid access and use.6 Prescription benzoyl peroxide (BPO) products alone or in combination with retinoids or topical antibiotics (or other combination topical therapies) comprise a large number of branded prescriptions often not covered by insurance or are only affordable using proprietary and intermittently available coupon cards (eg, BPO-clindamycin, clindamycin-tretinoin, BPO-adapalene); therefore, prescribers tend to dispense the individual ingredients and instruct the patient to compound them at home. Furthermore, BPO products can be purchased in effective concentrations as OTC products, and patients looking to procure more affordable, albeit less effective, topical retinoids that also have less irritation potential reach for OTC nightly retinol creams nestled in the antiaging section of the pharmacy.7
Opportunities to be involved in the larger scaffold of patient advocacy also are plentiful at the state and national levels. For example, with the support of dermatology state societies and the American Academy of Dermatology Association, Pennsylvania House Bill 22118 and similar bills in other states call for reversal of the gag clause that prevents pharmacists from disclosing the best medication price to patients. Also guided by the American Academy of Dermatology Association, prior authorization model legislation to promote transparency across insurers in this haphazard process is emerging across the country.9,10
Final Thoughts
These examples of acne medication access and cost quandaries serve to embody the daily problem-solving that dermatologists execute as part of their growing administrative and economic duties. They also represent worthy efforts to consider on behalf of patients, their pocketbooks, and the prudent use of their dermatologists’ time.
- Borgonjen RJ, de Lange JA, van de Kerkhof PCM. Guideline-based clinical decision support in acne patients receiving isotretinoin: improving adherence and cost-effectiveness. J Eur Acad Dermatol Venereol. 2017;31:e440-e442.
- Oral isotretinoin therapy update. Highmark website. https://content.highmarkprc.com/Files/NewsletterNotices/HotTopics/ht-all-isotretinoin-therapy-update-051718.pdf. Published May 17, 2018. Accessed June 29, 2018.
- Drug shortages. US Food & Drug Administration website. https://www.fda.gov/drugs/drugsafety/drugshortages/default.htm. Updated June 19, 2018. Accessed June 19, 2018.
- Retinoids prior authorization criteria. Blue Cross and Blue Shield of Illinois website. https://www.bcbsil.com/provider/pdf/retinoids.pdf. Published March 2008. Accessed June 18, 2018.
- Davis SA, Huang KE, Feldman SR, et al. Trends in ambulatory health care usage for adult acne. J Cutan Med Surg. 2015;19:377-379.
- FDA approves Differin gel 0.1% for over-the-counter use to treat acne [press release]. Silver Spring, MD: US Food & Drug Administration; July 8, 2016.
- Rosamilia LL. Over-the-counter treatments for acne and rosacea. Semin Cutan Med Surg. 2016;35:87-95.
- An Act Providing for Consumer Prescription Drug Pricing Disclosure, HB 2211, Regular Sess (Pa 2018).
- An Act Providing for Preauthorizations Conducted by Utilization Review Entities Relating to Health Care Services, HB 1293, Regular Sess (Pa 2017).
- Step therapy legislation. American Academy of Dermatology website. https://www.aad.org/advocacy/state-policy/step-therapy-legislation. Accessed June 19, 2018.
We are fortunate to have plentiful acne treatment options available that cater to each patient’s clinical examination, predispositions, and triggers, but the choices are daunting amidst the vast prescription and over-the-counter (OTC) topicals available along with disparate insurance and cost nuances. In addition, when prescribing generic oral therapies, it is complicated to parse out regional differences in price, supply, and insurance coverage to advocate best for each patient and land upon the delicate balance between efficacy, safety, and financial stewardship, both at an individual and community level. I will outline some challenges and solutions to the management of acne amidst these complicated factors.
Oral Therapies
For isotretinoin, generic choices, cost, and tiering within insurance plans are perpetual moving targets despite the drug being the only member of its class.1 Prescriber resources include tandem searches of electronic medical record price estimates within each insurance formulary, individual pharmacy search engines, and compilation mobile applications such as GoodRx to select the most affordable version for each patient. As an example of a regional trend, isotretinoin generic coverage by one provider in Pennsylvania shifted earlier this year so that every patient, whether new to isotretinoin or midcourse, required a new prior authorization with more stringent coverage requirements including failure of 2 oral antibiotics. Swiftly thereafter, efforts across the state driven by the Pennsylvania Academy of Dermatology and Dermatologic Surgery and its members and fueled by poignant patient vignettes about fragmented and substandard patient care helped to reverse this policy and remove the prior authorization mandate.2
Tetracyclines have experienced broad cost swings, mostly based on disruption of manufacturing at the limited number of distribution sites in the United States. In 2011, a tetracycline shortage arose due to a major manufacturer’s recall3 and persisted with subsequent material shortages, as doxycycline became the preferred and cheaper member of the class. Doxycycline price tag hikes then occurred following Hurricane Sandy when an East Coast manufacturing site was damaged.3 Spironolactone backorder also has been frequent due to recent raw material shortages and delays in production,3 forcing pharmacies to refill small amounts of medication in various dosage forms despite patients owing the same copayment per prescription.
Topical Therapies
Topical retinoid prescription affordability has always been fraught with difficulty owing to age cutoffs because it is often restricted to patients younger than 25 or 40 years, depending on the plan,4,5 but the availability of adapalene gel 0.1% as an OTC preparation in 2016 has broadened retinoid access and use.6 Prescription benzoyl peroxide (BPO) products alone or in combination with retinoids or topical antibiotics (or other combination topical therapies) comprise a large number of branded prescriptions often not covered by insurance or are only affordable using proprietary and intermittently available coupon cards (eg, BPO-clindamycin, clindamycin-tretinoin, BPO-adapalene); therefore, prescribers tend to dispense the individual ingredients and instruct the patient to compound them at home. Furthermore, BPO products can be purchased in effective concentrations as OTC products, and patients looking to procure more affordable, albeit less effective, topical retinoids that also have less irritation potential reach for OTC nightly retinol creams nestled in the antiaging section of the pharmacy.7
Opportunities to be involved in the larger scaffold of patient advocacy also are plentiful at the state and national levels. For example, with the support of dermatology state societies and the American Academy of Dermatology Association, Pennsylvania House Bill 22118 and similar bills in other states call for reversal of the gag clause that prevents pharmacists from disclosing the best medication price to patients. Also guided by the American Academy of Dermatology Association, prior authorization model legislation to promote transparency across insurers in this haphazard process is emerging across the country.9,10
Final Thoughts
These examples of acne medication access and cost quandaries serve to embody the daily problem-solving that dermatologists execute as part of their growing administrative and economic duties. They also represent worthy efforts to consider on behalf of patients, their pocketbooks, and the prudent use of their dermatologists’ time.
We are fortunate to have plentiful acne treatment options available that cater to each patient’s clinical examination, predispositions, and triggers, but the choices are daunting amidst the vast prescription and over-the-counter (OTC) topicals available along with disparate insurance and cost nuances. In addition, when prescribing generic oral therapies, it is complicated to parse out regional differences in price, supply, and insurance coverage to advocate best for each patient and land upon the delicate balance between efficacy, safety, and financial stewardship, both at an individual and community level. I will outline some challenges and solutions to the management of acne amidst these complicated factors.
Oral Therapies
For isotretinoin, generic choices, cost, and tiering within insurance plans are perpetual moving targets despite the drug being the only member of its class.1 Prescriber resources include tandem searches of electronic medical record price estimates within each insurance formulary, individual pharmacy search engines, and compilation mobile applications such as GoodRx to select the most affordable version for each patient. As an example of a regional trend, isotretinoin generic coverage by one provider in Pennsylvania shifted earlier this year so that every patient, whether new to isotretinoin or midcourse, required a new prior authorization with more stringent coverage requirements including failure of 2 oral antibiotics. Swiftly thereafter, efforts across the state driven by the Pennsylvania Academy of Dermatology and Dermatologic Surgery and its members and fueled by poignant patient vignettes about fragmented and substandard patient care helped to reverse this policy and remove the prior authorization mandate.2
Tetracyclines have experienced broad cost swings, mostly based on disruption of manufacturing at the limited number of distribution sites in the United States. In 2011, a tetracycline shortage arose due to a major manufacturer’s recall3 and persisted with subsequent material shortages, as doxycycline became the preferred and cheaper member of the class. Doxycycline price tag hikes then occurred following Hurricane Sandy when an East Coast manufacturing site was damaged.3 Spironolactone backorder also has been frequent due to recent raw material shortages and delays in production,3 forcing pharmacies to refill small amounts of medication in various dosage forms despite patients owing the same copayment per prescription.
Topical Therapies
Topical retinoid prescription affordability has always been fraught with difficulty owing to age cutoffs because it is often restricted to patients younger than 25 or 40 years, depending on the plan,4,5 but the availability of adapalene gel 0.1% as an OTC preparation in 2016 has broadened retinoid access and use.6 Prescription benzoyl peroxide (BPO) products alone or in combination with retinoids or topical antibiotics (or other combination topical therapies) comprise a large number of branded prescriptions often not covered by insurance or are only affordable using proprietary and intermittently available coupon cards (eg, BPO-clindamycin, clindamycin-tretinoin, BPO-adapalene); therefore, prescribers tend to dispense the individual ingredients and instruct the patient to compound them at home. Furthermore, BPO products can be purchased in effective concentrations as OTC products, and patients looking to procure more affordable, albeit less effective, topical retinoids that also have less irritation potential reach for OTC nightly retinol creams nestled in the antiaging section of the pharmacy.7
Opportunities to be involved in the larger scaffold of patient advocacy also are plentiful at the state and national levels. For example, with the support of dermatology state societies and the American Academy of Dermatology Association, Pennsylvania House Bill 22118 and similar bills in other states call for reversal of the gag clause that prevents pharmacists from disclosing the best medication price to patients. Also guided by the American Academy of Dermatology Association, prior authorization model legislation to promote transparency across insurers in this haphazard process is emerging across the country.9,10
Final Thoughts
These examples of acne medication access and cost quandaries serve to embody the daily problem-solving that dermatologists execute as part of their growing administrative and economic duties. They also represent worthy efforts to consider on behalf of patients, their pocketbooks, and the prudent use of their dermatologists’ time.
- Borgonjen RJ, de Lange JA, van de Kerkhof PCM. Guideline-based clinical decision support in acne patients receiving isotretinoin: improving adherence and cost-effectiveness. J Eur Acad Dermatol Venereol. 2017;31:e440-e442.
- Oral isotretinoin therapy update. Highmark website. https://content.highmarkprc.com/Files/NewsletterNotices/HotTopics/ht-all-isotretinoin-therapy-update-051718.pdf. Published May 17, 2018. Accessed June 29, 2018.
- Drug shortages. US Food & Drug Administration website. https://www.fda.gov/drugs/drugsafety/drugshortages/default.htm. Updated June 19, 2018. Accessed June 19, 2018.
- Retinoids prior authorization criteria. Blue Cross and Blue Shield of Illinois website. https://www.bcbsil.com/provider/pdf/retinoids.pdf. Published March 2008. Accessed June 18, 2018.
- Davis SA, Huang KE, Feldman SR, et al. Trends in ambulatory health care usage for adult acne. J Cutan Med Surg. 2015;19:377-379.
- FDA approves Differin gel 0.1% for over-the-counter use to treat acne [press release]. Silver Spring, MD: US Food & Drug Administration; July 8, 2016.
- Rosamilia LL. Over-the-counter treatments for acne and rosacea. Semin Cutan Med Surg. 2016;35:87-95.
- An Act Providing for Consumer Prescription Drug Pricing Disclosure, HB 2211, Regular Sess (Pa 2018).
- An Act Providing for Preauthorizations Conducted by Utilization Review Entities Relating to Health Care Services, HB 1293, Regular Sess (Pa 2017).
- Step therapy legislation. American Academy of Dermatology website. https://www.aad.org/advocacy/state-policy/step-therapy-legislation. Accessed June 19, 2018.
- Borgonjen RJ, de Lange JA, van de Kerkhof PCM. Guideline-based clinical decision support in acne patients receiving isotretinoin: improving adherence and cost-effectiveness. J Eur Acad Dermatol Venereol. 2017;31:e440-e442.
- Oral isotretinoin therapy update. Highmark website. https://content.highmarkprc.com/Files/NewsletterNotices/HotTopics/ht-all-isotretinoin-therapy-update-051718.pdf. Published May 17, 2018. Accessed June 29, 2018.
- Drug shortages. US Food & Drug Administration website. https://www.fda.gov/drugs/drugsafety/drugshortages/default.htm. Updated June 19, 2018. Accessed June 19, 2018.
- Retinoids prior authorization criteria. Blue Cross and Blue Shield of Illinois website. https://www.bcbsil.com/provider/pdf/retinoids.pdf. Published March 2008. Accessed June 18, 2018.
- Davis SA, Huang KE, Feldman SR, et al. Trends in ambulatory health care usage for adult acne. J Cutan Med Surg. 2015;19:377-379.
- FDA approves Differin gel 0.1% for over-the-counter use to treat acne [press release]. Silver Spring, MD: US Food & Drug Administration; July 8, 2016.
- Rosamilia LL. Over-the-counter treatments for acne and rosacea. Semin Cutan Med Surg. 2016;35:87-95.
- An Act Providing for Consumer Prescription Drug Pricing Disclosure, HB 2211, Regular Sess (Pa 2018).
- An Act Providing for Preauthorizations Conducted by Utilization Review Entities Relating to Health Care Services, HB 1293, Regular Sess (Pa 2017).
- Step therapy legislation. American Academy of Dermatology website. https://www.aad.org/advocacy/state-policy/step-therapy-legislation. Accessed June 19, 2018.
Diet and Dermatology: Google Search Results for Acne, Psoriasis, and Eczema
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.

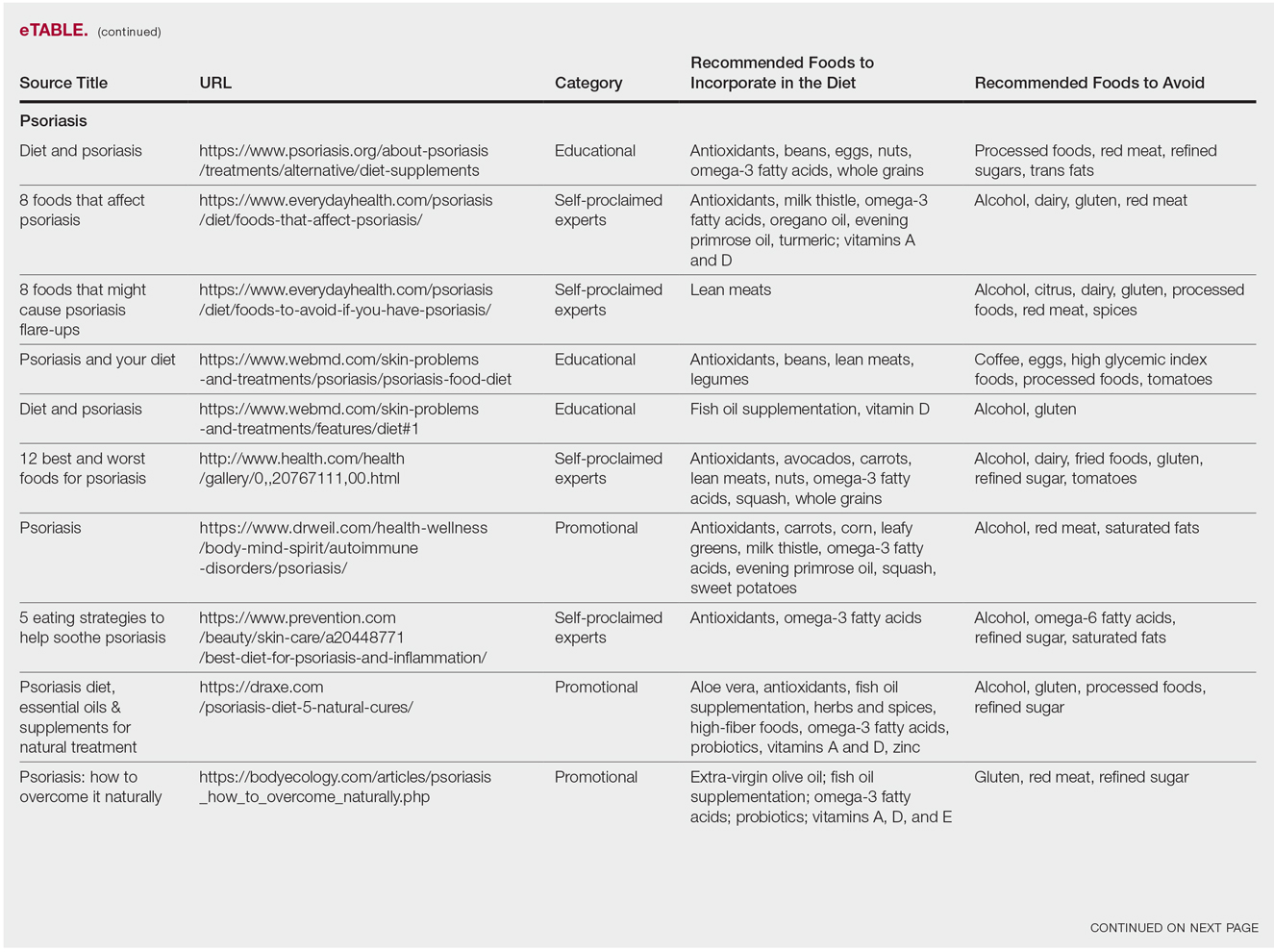
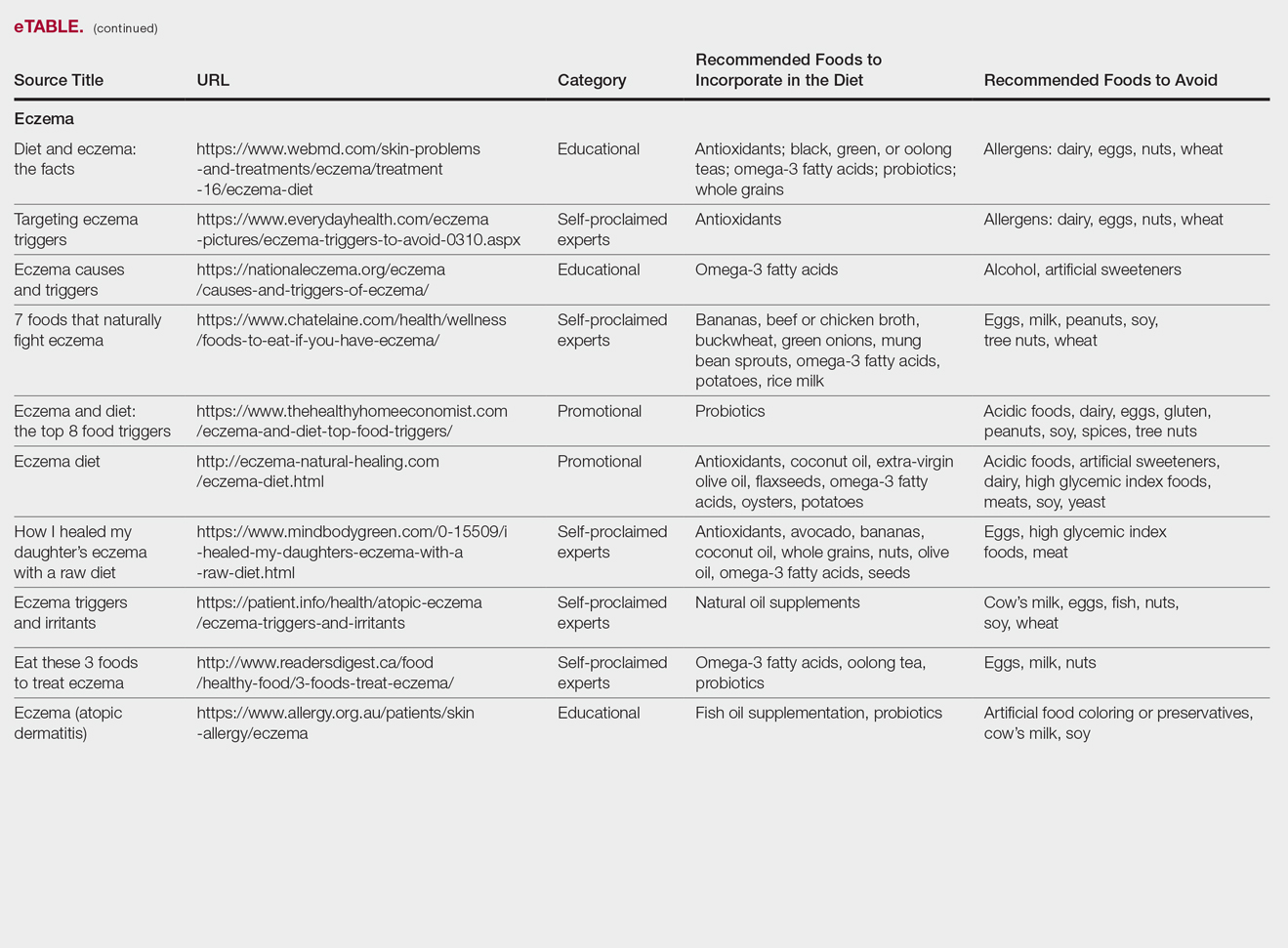
Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.



Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.



Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
Practice Points
- It is important physicians be well-informed regarding Internet discourse to discredit unfounded recommendations.
- It is likely that patients seeking medical advice regarding their dermatologic condition and treatment will have done prior research on the Internet.
- Oftentimes, the information on educational health websites can be confusing to patients.
- Because of widespread Internet access to health-related information, patients may opt to self-diagnose and therefore delay seeking professional care.
Intravascular Involvement of Cutaneous Squamous Cell Carcinoma
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.
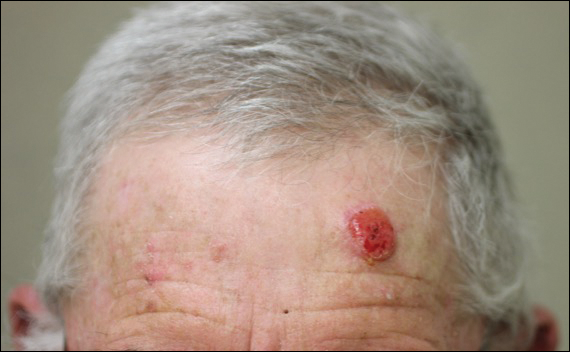
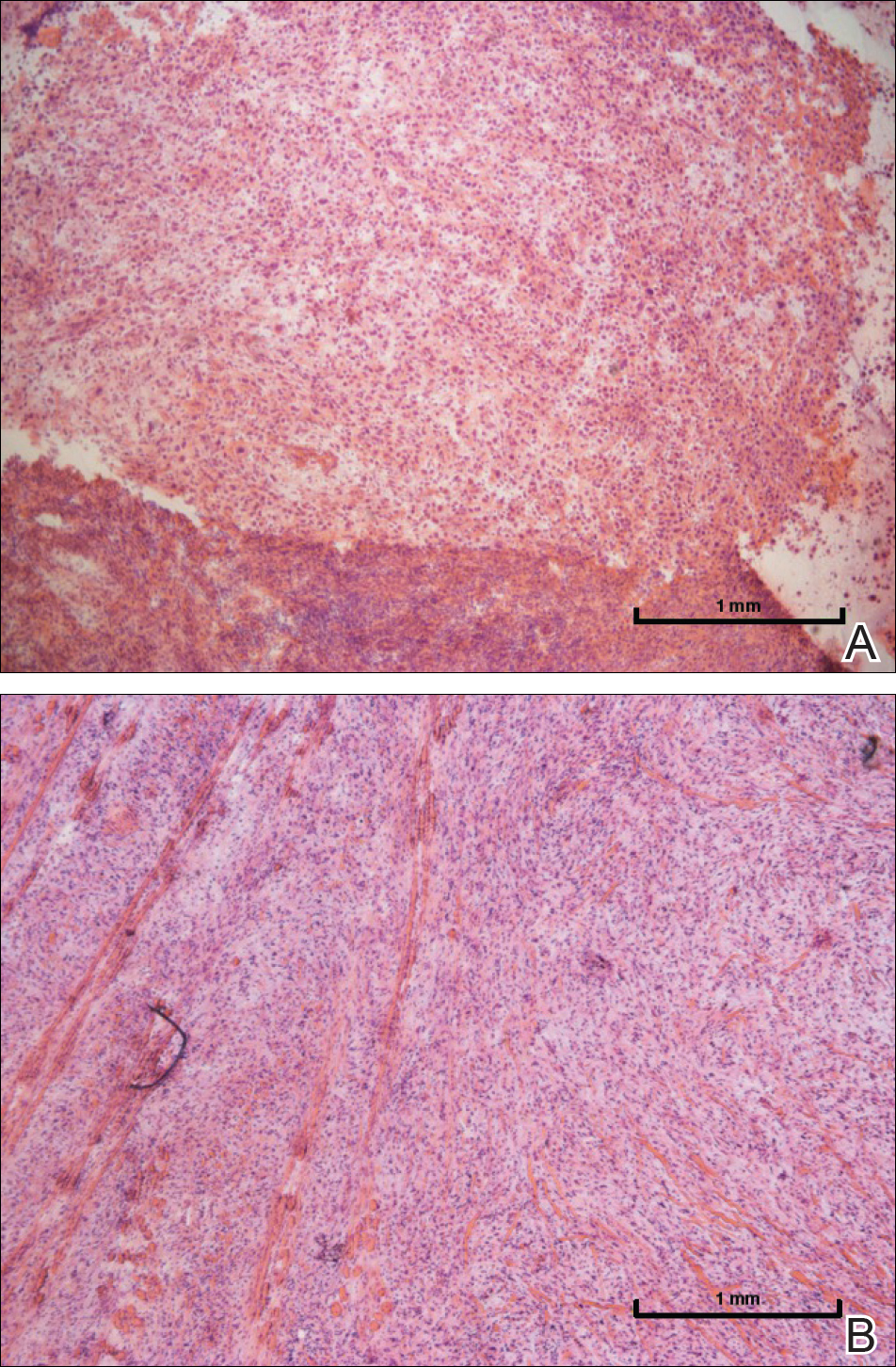
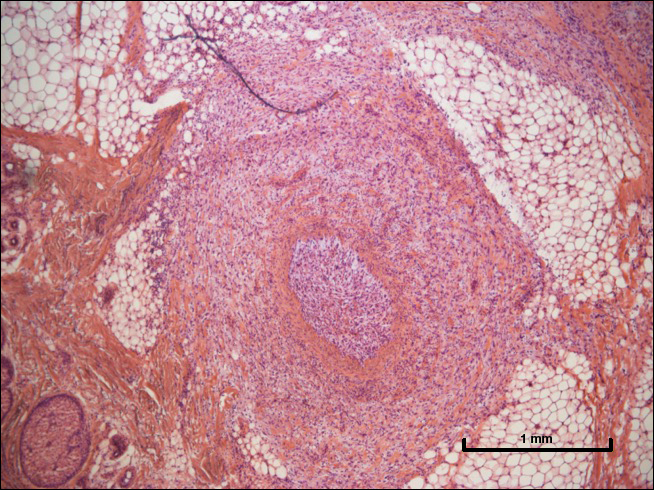
One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.



One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.



One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
Resident Pearl
- Intravascular (also referred to as lymphovascular when involving vessels and/or lymphatics) invasion of cutaneous squamous cell carcinoma can be considered a high-risk factor that may warrant adjuvant therapy.