User login
ISTANBUL, TURKEY – The investigational interleukin-17A inhibitor secukinumab was the talk of Europe’s premier dermatology conference as a result of a series of world-premiere presentations of not one, but three, phase III clinical trials.
The entire secukinumab pivotal phase III program results were presented together at the annual congress of the European Academy of Dermatology and Venereology. Based on these promising results, Novartis plans to file for Food and Drug Administration and European regulatory approval before the end of 2013.
The three randomized, double-blind, multicenter pivotal trials of secukinumab in psoriasis collectively included 3,367 patients with moderate to severe chronic plaque psoriasis. All three studies were strongly positive. Efficacy rates were higher than with current biologics, clinical improvement was remarkably rapid, and the safety profile was reassuring.
"Those of us working in this area of research are really excited by this new data and what it says for the treatment of psoriasis patients," commented Dr. Richard Langley of Dalhousie University, Halifax, N.S.
He presented the centerpiece of this trio of phase III trials, known as the FIXTURE trial. FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using 2 Dosing Regimens to Determine Efficacy in Psoriasis) was a 1,306-patient, double-blind, double-dummy trial conducted by 153 investigators in 37 countries. The trial featured a 1-year head-to-head comparison of secukinumab and the widely prescribed tumor necrosis factor (TNF) inhibitor etanercept (Enbrel). The IL-17A inhibitor outperformed etanercept in all of the prespecified primary and secondary outcome measures. Moreover, the two biologics proved equally safe.
"I think it’s interesting in comparing these two molecules that when we look at the data to date, we see comparable safety results. Etanercept is one of the most widely used biologics and has an excellent safety record. That’s reassuring for those of us who are investigating IL-17 signaling," observed Dr. Langley, also the current president of the Canadian Dermatology Association.
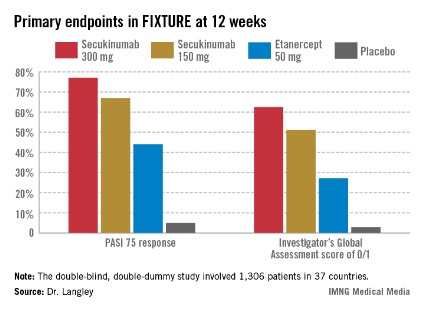
FIXTURE participants had a mean baseline Psoriasis Area and Severity Index (PASI) score of 24, an average body mass index of 34 kg/m2, and a mean age in the mid-40s, and were previous inadequate responders to conventional systemic agents and/or biologics. The two co-primary endpoints were 12-week rates of at least a 75% reduction in PASI score, or PASI 75 response, and an Investigator’s Global Assessment (IGA) rating of 0/1, corresponding to clear or nearly clear, on a modified 5-point scale that provides more clinically meaningful information than the older 6-point IGA scale. Patients randomized to the secukinumab 300-mg or 150-mg groups did significantly better on both outcome measures than those assigned to etanercept.
The onset of benefit with secukinumab was swift; a statistically significant improvement compared with etanercept was seen within 2 weeks in terms of IGA scores and within 3 weeks for PASI 75. Secukinumab at 300 mg per subcutaneous injection – the most effective dose across all three pivotal trials – achieved roughly a 50% reduction in PASI scores after 3 weeks, compared with 8 weeks for etanercept, the dermatologist explained.
The PASI 75 response rate at 12 weeks was 77.1% in patients receiving secukinumab at 300 mg, 67% for those receiving secukinumab at 150 mg, 44% for etanercept 50 mg, and 4.9% for placebo.
The highest response rates with secukinumab were seen at week 16 rather than week 12. In the secukinumab 300-mg group, the PASI 75 rate at week 16 was 86.7%, with that high level of response being retained throughout the remainder of the 52-week study. In contrast, the PASI 75 rate at week 16 for etanercept was less than 60%. The IGA 0/1 rate at week 16 was 75.5% in the secukinumab 300-mg group and 60% with secukinumab 150 mg. The peak IGA 0/1 rate in the etanercept arm was only about 45%, and it came much later, at week 26.
Looking at more stringent secondary endpoints, the investigators found that a PASI 90 response was achieved by week 16 in 72.4% of the high-dose secukinumab group, compared with about 30% with etanercept. Twenty-four percent of patients on high-dose secukinumab had a PASI 100 response at week 12, compared with 4% on etanercept. By week 16, 36.8% of the high-dose secukinumab group had a PASI 100 response. At week 52, 65% of the secukinumab 300-mg group maintained a PASI 90 response, compared with 33% of the etanercept group.
The most common adverse event documented in FIXTURE was nasopharyngitis, which occurred in roughly one-third of patients in all four study arms. The serious infection rate was 1%-1.2% in all four study arms. No signals of an increase in malignancies or major adverse cardiovascular events were noted in any of the active treatment groups. Overall, adverse events were similar in both secukinumab arms and comparable to etanercept. Injection-site reactions occurred in 6.1% of the etanercept group and in none of the patients assigned to secukinumab or placebo.
The incidence of treatment-emergent antidrug antibodies in the secukinumab arms was 0.4%, and when it occurred, it had no impact on treatment efficacy or safety. Patients on etanercept weren’t tested for the emergence of antidrug antibodies.
Candida infections occurred in 4.7% of patients on secukinumab 300 mg, 2.3% of those on 150 mg, and 1.2% of the etanercept group. All cases in patients on secukinumab were mild or moderate and easily treated. Candida infections are a side effect of special interest because patients with a genetic defect in the IL-17 pathway can develop chronic mucocutaneous candidiasis. A theoretical concern that pharmacologic inhibition of IL-17 might create a big problem in this regard has not materialized.
The other two phase III secukinumab clinical trials presented in Istanbul – ERASURE and SCULPTURE – showed efficacy and safety results similar to those of FIXTURE. Unlike FIXTURE, neither of those trials featured a head-to-head comparison with another biologic.
These were the first completed phase III studies for any of the three biologics in this novel, highly promising class targeting IL-17 in the treatment of psoriasis and other immune-mediated inflammatory diseases. The other two agents in the pipeline targeting IL-17, brodalumab and ixekizumab, remain in ongoing phase III trials.
Because of the pivotal role IL-17 plays in generating inflammatory cytokines downstream, all three drugs are being studied for additional indications beyond psoriasis. For example, secukinumab, an IgG1 human monoclonal antibody, is in phase III clinical trials for psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis, and in phase II for muscular sclerosis and asthma.
One audience member asked Dr. Langley for his thoughts regarding the absence of injection-site reactions in the secukinumab-treated patients. He replied that one obvious factor is that secukinumab injections are given much less frequently: once monthly during maintenance therapy as compared to once weekly with etanercept. In any event, he doesn’t consider injection-site reactions to be clinically relevant.
"Injection-site reactions don’t seem to be a major issue with any of the subcutaneous therapies. It has never in 15 years of using different biologics been an issue that’s made me stop treatment for a patient," he added.
Secukinumab was dosed at baseline, again weekly through week 4, then once monthly from week 8 through the study’s end. Etanercept was dosed at 50 mg twice weekly for 12 weeks, then once weekly in accord with the labeling instructions in most countries. Nonresponders at 12 weeks in the placebo arm were at that point randomized to secukinumab at 300 mg or 150 mg. The statistical analysis in FIXTURE was performed by nonresponder imputation.
"Those who are interested in statistics will know that that’s the most rigorous way that you can look at the data, because anybody who drops out of the study, whether they withdraw consent, have a protocol violation, or have an adverse event, even if they were responding at that time, are considered a failure or nonresponder," Dr. Langley explained.
Asked how he sees secukinumab fitting into patient management, Dr. Langley replied, "Most of us are forced to use conventional therapies and phototherapy to get approval for biologics, but after that I think the safety and efficacy of this drug put it right at the top. I could definitely use this drug as a first-line biologic for patients with plain psoriasis without psoriatic arthritis."
Coinvestigator Dr. Kristian Reich, professor of dermatology at Georg-August University in Göttingen, Germany, said he will probably continue to use TNF inhibitors in psoriasis patients with prominent arthritis, where he believes the TNF inhibitors are particularly beneficial, but he anticipates using secukinumab widely in others.
Dr. Bruce E. Strober, another active clinical trial researcher in psoriasis, said he likes what he sees in the IL-17 inhibitor data.
"I predict as a specialty we’ll become very confident in these drugs 5 years from now. A lot of us will be using them as first line in many patients. That’s my prediction. For now, though, I’m a big fan of TNF inhibition," added Dr. Strober, vice chair of dermatology at the University of Connecticut Medical Center, Farmington.
The secukinumab development program is sponsored by Novartis. Dr. Langley, Dr. Reich, and Dr. Strober disclosed having received research grants from and serving as consultants to Novartis and numerous other companies developing biologic agents for psoriasis.
ISTANBUL, TURKEY – The investigational interleukin-17A inhibitor secukinumab was the talk of Europe’s premier dermatology conference as a result of a series of world-premiere presentations of not one, but three, phase III clinical trials.
The entire secukinumab pivotal phase III program results were presented together at the annual congress of the European Academy of Dermatology and Venereology. Based on these promising results, Novartis plans to file for Food and Drug Administration and European regulatory approval before the end of 2013.

The three randomized, double-blind, multicenter pivotal trials of secukinumab in psoriasis collectively included 3,367 patients with moderate to severe chronic plaque psoriasis. All three studies were strongly positive. Efficacy rates were higher than with current biologics, clinical improvement was remarkably rapid, and the safety profile was reassuring.
"Those of us working in this area of research are really excited by this new data and what it says for the treatment of psoriasis patients," commented Dr. Richard Langley of Dalhousie University, Halifax, N.S.
He presented the centerpiece of this trio of phase III trials, known as the FIXTURE trial. FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using 2 Dosing Regimens to Determine Efficacy in Psoriasis) was a 1,306-patient, double-blind, double-dummy trial conducted by 153 investigators in 37 countries. The trial featured a 1-year head-to-head comparison of secukinumab and the widely prescribed tumor necrosis factor (TNF) inhibitor etanercept (Enbrel). The IL-17A inhibitor outperformed etanercept in all of the prespecified primary and secondary outcome measures. Moreover, the two biologics proved equally safe.
"I think it’s interesting in comparing these two molecules that when we look at the data to date, we see comparable safety results. Etanercept is one of the most widely used biologics and has an excellent safety record. That’s reassuring for those of us who are investigating IL-17 signaling," observed Dr. Langley, also the current president of the Canadian Dermatology Association.
FIXTURE participants had a mean baseline Psoriasis Area and Severity Index (PASI) score of 24, an average body mass index of 34 kg/m2, and a mean age in the mid-40s, and were previous inadequate responders to conventional systemic agents and/or biologics. The two co-primary endpoints were 12-week rates of at least a 75% reduction in PASI score, or PASI 75 response, and an Investigator’s Global Assessment (IGA) rating of 0/1, corresponding to clear or nearly clear, on a modified 5-point scale that provides more clinically meaningful information than the older 6-point IGA scale. Patients randomized to the secukinumab 300-mg or 150-mg groups did significantly better on both outcome measures than those assigned to etanercept.
The onset of benefit with secukinumab was swift; a statistically significant improvement compared with etanercept was seen within 2 weeks in terms of IGA scores and within 3 weeks for PASI 75. Secukinumab at 300 mg per subcutaneous injection – the most effective dose across all three pivotal trials – achieved roughly a 50% reduction in PASI scores after 3 weeks, compared with 8 weeks for etanercept, the dermatologist explained.
The PASI 75 response rate at 12 weeks was 77.1% in patients receiving secukinumab at 300 mg, 67% for those receiving secukinumab at 150 mg, 44% for etanercept 50 mg, and 4.9% for placebo.
The highest response rates with secukinumab were seen at week 16 rather than week 12. In the secukinumab 300-mg group, the PASI 75 rate at week 16 was 86.7%, with that high level of response being retained throughout the remainder of the 52-week study. In contrast, the PASI 75 rate at week 16 for etanercept was less than 60%. The IGA 0/1 rate at week 16 was 75.5% in the secukinumab 300-mg group and 60% with secukinumab 150 mg. The peak IGA 0/1 rate in the etanercept arm was only about 45%, and it came much later, at week 26.
Looking at more stringent secondary endpoints, the investigators found that a PASI 90 response was achieved by week 16 in 72.4% of the high-dose secukinumab group, compared with about 30% with etanercept. Twenty-four percent of patients on high-dose secukinumab had a PASI 100 response at week 12, compared with 4% on etanercept. By week 16, 36.8% of the high-dose secukinumab group had a PASI 100 response. At week 52, 65% of the secukinumab 300-mg group maintained a PASI 90 response, compared with 33% of the etanercept group.
The most common adverse event documented in FIXTURE was nasopharyngitis, which occurred in roughly one-third of patients in all four study arms. The serious infection rate was 1%-1.2% in all four study arms. No signals of an increase in malignancies or major adverse cardiovascular events were noted in any of the active treatment groups. Overall, adverse events were similar in both secukinumab arms and comparable to etanercept. Injection-site reactions occurred in 6.1% of the etanercept group and in none of the patients assigned to secukinumab or placebo.
The incidence of treatment-emergent antidrug antibodies in the secukinumab arms was 0.4%, and when it occurred, it had no impact on treatment efficacy or safety. Patients on etanercept weren’t tested for the emergence of antidrug antibodies.
Candida infections occurred in 4.7% of patients on secukinumab 300 mg, 2.3% of those on 150 mg, and 1.2% of the etanercept group. All cases in patients on secukinumab were mild or moderate and easily treated. Candida infections are a side effect of special interest because patients with a genetic defect in the IL-17 pathway can develop chronic mucocutaneous candidiasis. A theoretical concern that pharmacologic inhibition of IL-17 might create a big problem in this regard has not materialized.
The other two phase III secukinumab clinical trials presented in Istanbul – ERASURE and SCULPTURE – showed efficacy and safety results similar to those of FIXTURE. Unlike FIXTURE, neither of those trials featured a head-to-head comparison with another biologic.
These were the first completed phase III studies for any of the three biologics in this novel, highly promising class targeting IL-17 in the treatment of psoriasis and other immune-mediated inflammatory diseases. The other two agents in the pipeline targeting IL-17, brodalumab and ixekizumab, remain in ongoing phase III trials.
Because of the pivotal role IL-17 plays in generating inflammatory cytokines downstream, all three drugs are being studied for additional indications beyond psoriasis. For example, secukinumab, an IgG1 human monoclonal antibody, is in phase III clinical trials for psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis, and in phase II for muscular sclerosis and asthma.
One audience member asked Dr. Langley for his thoughts regarding the absence of injection-site reactions in the secukinumab-treated patients. He replied that one obvious factor is that secukinumab injections are given much less frequently: once monthly during maintenance therapy as compared to once weekly with etanercept. In any event, he doesn’t consider injection-site reactions to be clinically relevant.
"Injection-site reactions don’t seem to be a major issue with any of the subcutaneous therapies. It has never in 15 years of using different biologics been an issue that’s made me stop treatment for a patient," he added.
Secukinumab was dosed at baseline, again weekly through week 4, then once monthly from week 8 through the study’s end. Etanercept was dosed at 50 mg twice weekly for 12 weeks, then once weekly in accord with the labeling instructions in most countries. Nonresponders at 12 weeks in the placebo arm were at that point randomized to secukinumab at 300 mg or 150 mg. The statistical analysis in FIXTURE was performed by nonresponder imputation.
"Those who are interested in statistics will know that that’s the most rigorous way that you can look at the data, because anybody who drops out of the study, whether they withdraw consent, have a protocol violation, or have an adverse event, even if they were responding at that time, are considered a failure or nonresponder," Dr. Langley explained.
Asked how he sees secukinumab fitting into patient management, Dr. Langley replied, "Most of us are forced to use conventional therapies and phototherapy to get approval for biologics, but after that I think the safety and efficacy of this drug put it right at the top. I could definitely use this drug as a first-line biologic for patients with plain psoriasis without psoriatic arthritis."
Coinvestigator Dr. Kristian Reich, professor of dermatology at Georg-August University in Göttingen, Germany, said he will probably continue to use TNF inhibitors in psoriasis patients with prominent arthritis, where he believes the TNF inhibitors are particularly beneficial, but he anticipates using secukinumab widely in others.
Dr. Bruce E. Strober, another active clinical trial researcher in psoriasis, said he likes what he sees in the IL-17 inhibitor data.
"I predict as a specialty we’ll become very confident in these drugs 5 years from now. A lot of us will be using them as first line in many patients. That’s my prediction. For now, though, I’m a big fan of TNF inhibition," added Dr. Strober, vice chair of dermatology at the University of Connecticut Medical Center, Farmington.
The secukinumab development program is sponsored by Novartis. Dr. Langley, Dr. Reich, and Dr. Strober disclosed having received research grants from and serving as consultants to Novartis and numerous other companies developing biologic agents for psoriasis.
ISTANBUL, TURKEY – The investigational interleukin-17A inhibitor secukinumab was the talk of Europe’s premier dermatology conference as a result of a series of world-premiere presentations of not one, but three, phase III clinical trials.
The entire secukinumab pivotal phase III program results were presented together at the annual congress of the European Academy of Dermatology and Venereology. Based on these promising results, Novartis plans to file for Food and Drug Administration and European regulatory approval before the end of 2013.

The three randomized, double-blind, multicenter pivotal trials of secukinumab in psoriasis collectively included 3,367 patients with moderate to severe chronic plaque psoriasis. All three studies were strongly positive. Efficacy rates were higher than with current biologics, clinical improvement was remarkably rapid, and the safety profile was reassuring.
"Those of us working in this area of research are really excited by this new data and what it says for the treatment of psoriasis patients," commented Dr. Richard Langley of Dalhousie University, Halifax, N.S.
He presented the centerpiece of this trio of phase III trials, known as the FIXTURE trial. FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using 2 Dosing Regimens to Determine Efficacy in Psoriasis) was a 1,306-patient, double-blind, double-dummy trial conducted by 153 investigators in 37 countries. The trial featured a 1-year head-to-head comparison of secukinumab and the widely prescribed tumor necrosis factor (TNF) inhibitor etanercept (Enbrel). The IL-17A inhibitor outperformed etanercept in all of the prespecified primary and secondary outcome measures. Moreover, the two biologics proved equally safe.
"I think it’s interesting in comparing these two molecules that when we look at the data to date, we see comparable safety results. Etanercept is one of the most widely used biologics and has an excellent safety record. That’s reassuring for those of us who are investigating IL-17 signaling," observed Dr. Langley, also the current president of the Canadian Dermatology Association.
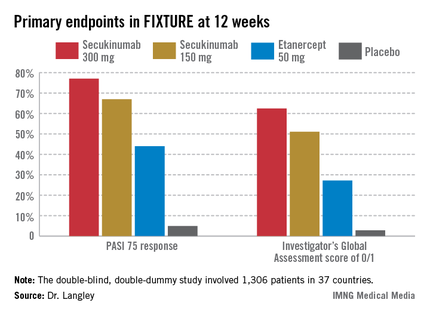
FIXTURE participants had a mean baseline Psoriasis Area and Severity Index (PASI) score of 24, an average body mass index of 34 kg/m2, and a mean age in the mid-40s, and were previous inadequate responders to conventional systemic agents and/or biologics. The two co-primary endpoints were 12-week rates of at least a 75% reduction in PASI score, or PASI 75 response, and an Investigator’s Global Assessment (IGA) rating of 0/1, corresponding to clear or nearly clear, on a modified 5-point scale that provides more clinically meaningful information than the older 6-point IGA scale. Patients randomized to the secukinumab 300-mg or 150-mg groups did significantly better on both outcome measures than those assigned to etanercept.
The onset of benefit with secukinumab was swift; a statistically significant improvement compared with etanercept was seen within 2 weeks in terms of IGA scores and within 3 weeks for PASI 75. Secukinumab at 300 mg per subcutaneous injection – the most effective dose across all three pivotal trials – achieved roughly a 50% reduction in PASI scores after 3 weeks, compared with 8 weeks for etanercept, the dermatologist explained.
The PASI 75 response rate at 12 weeks was 77.1% in patients receiving secukinumab at 300 mg, 67% for those receiving secukinumab at 150 mg, 44% for etanercept 50 mg, and 4.9% for placebo.
The highest response rates with secukinumab were seen at week 16 rather than week 12. In the secukinumab 300-mg group, the PASI 75 rate at week 16 was 86.7%, with that high level of response being retained throughout the remainder of the 52-week study. In contrast, the PASI 75 rate at week 16 for etanercept was less than 60%. The IGA 0/1 rate at week 16 was 75.5% in the secukinumab 300-mg group and 60% with secukinumab 150 mg. The peak IGA 0/1 rate in the etanercept arm was only about 45%, and it came much later, at week 26.
Looking at more stringent secondary endpoints, the investigators found that a PASI 90 response was achieved by week 16 in 72.4% of the high-dose secukinumab group, compared with about 30% with etanercept. Twenty-four percent of patients on high-dose secukinumab had a PASI 100 response at week 12, compared with 4% on etanercept. By week 16, 36.8% of the high-dose secukinumab group had a PASI 100 response. At week 52, 65% of the secukinumab 300-mg group maintained a PASI 90 response, compared with 33% of the etanercept group.
The most common adverse event documented in FIXTURE was nasopharyngitis, which occurred in roughly one-third of patients in all four study arms. The serious infection rate was 1%-1.2% in all four study arms. No signals of an increase in malignancies or major adverse cardiovascular events were noted in any of the active treatment groups. Overall, adverse events were similar in both secukinumab arms and comparable to etanercept. Injection-site reactions occurred in 6.1% of the etanercept group and in none of the patients assigned to secukinumab or placebo.
The incidence of treatment-emergent antidrug antibodies in the secukinumab arms was 0.4%, and when it occurred, it had no impact on treatment efficacy or safety. Patients on etanercept weren’t tested for the emergence of antidrug antibodies.
Candida infections occurred in 4.7% of patients on secukinumab 300 mg, 2.3% of those on 150 mg, and 1.2% of the etanercept group. All cases in patients on secukinumab were mild or moderate and easily treated. Candida infections are a side effect of special interest because patients with a genetic defect in the IL-17 pathway can develop chronic mucocutaneous candidiasis. A theoretical concern that pharmacologic inhibition of IL-17 might create a big problem in this regard has not materialized.
The other two phase III secukinumab clinical trials presented in Istanbul – ERASURE and SCULPTURE – showed efficacy and safety results similar to those of FIXTURE. Unlike FIXTURE, neither of those trials featured a head-to-head comparison with another biologic.
These were the first completed phase III studies for any of the three biologics in this novel, highly promising class targeting IL-17 in the treatment of psoriasis and other immune-mediated inflammatory diseases. The other two agents in the pipeline targeting IL-17, brodalumab and ixekizumab, remain in ongoing phase III trials.
Because of the pivotal role IL-17 plays in generating inflammatory cytokines downstream, all three drugs are being studied for additional indications beyond psoriasis. For example, secukinumab, an IgG1 human monoclonal antibody, is in phase III clinical trials for psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis, and in phase II for muscular sclerosis and asthma.
One audience member asked Dr. Langley for his thoughts regarding the absence of injection-site reactions in the secukinumab-treated patients. He replied that one obvious factor is that secukinumab injections are given much less frequently: once monthly during maintenance therapy as compared to once weekly with etanercept. In any event, he doesn’t consider injection-site reactions to be clinically relevant.
"Injection-site reactions don’t seem to be a major issue with any of the subcutaneous therapies. It has never in 15 years of using different biologics been an issue that’s made me stop treatment for a patient," he added.
Secukinumab was dosed at baseline, again weekly through week 4, then once monthly from week 8 through the study’s end. Etanercept was dosed at 50 mg twice weekly for 12 weeks, then once weekly in accord with the labeling instructions in most countries. Nonresponders at 12 weeks in the placebo arm were at that point randomized to secukinumab at 300 mg or 150 mg. The statistical analysis in FIXTURE was performed by nonresponder imputation.
"Those who are interested in statistics will know that that’s the most rigorous way that you can look at the data, because anybody who drops out of the study, whether they withdraw consent, have a protocol violation, or have an adverse event, even if they were responding at that time, are considered a failure or nonresponder," Dr. Langley explained.
Asked how he sees secukinumab fitting into patient management, Dr. Langley replied, "Most of us are forced to use conventional therapies and phototherapy to get approval for biologics, but after that I think the safety and efficacy of this drug put it right at the top. I could definitely use this drug as a first-line biologic for patients with plain psoriasis without psoriatic arthritis."
Coinvestigator Dr. Kristian Reich, professor of dermatology at Georg-August University in Göttingen, Germany, said he will probably continue to use TNF inhibitors in psoriasis patients with prominent arthritis, where he believes the TNF inhibitors are particularly beneficial, but he anticipates using secukinumab widely in others.
Dr. Bruce E. Strober, another active clinical trial researcher in psoriasis, said he likes what he sees in the IL-17 inhibitor data.
"I predict as a specialty we’ll become very confident in these drugs 5 years from now. A lot of us will be using them as first line in many patients. That’s my prediction. For now, though, I’m a big fan of TNF inhibition," added Dr. Strober, vice chair of dermatology at the University of Connecticut Medical Center, Farmington.
The secukinumab development program is sponsored by Novartis. Dr. Langley, Dr. Reich, and Dr. Strober disclosed having received research grants from and serving as consultants to Novartis and numerous other companies developing biologic agents for psoriasis.
AT THE EADV CONGRESS
Major finding: The PASI 75 response rate at 12 weeks in patients with moderate to severe chronic plaque psoriasis was 77.1% in those randomized to the investigational interleukin-17A inhibitor secukinumab at 300 mg, 67% for secukinumab at 150 mg, 44% for etanercept 50 mg, and 4.9% for placebo.
Data source: The FIXTURE trial was a randomized, double-blind, double-dummy, year-long, phase III trial involving 1,306 patients with chronic plaque psoriasis not adequately responsive to prior systemic therapies.
Disclosures: The secukinumab development program is sponsored by Novartis. Dr. Langley, Dr. Reich, and Dr. Strober disclosed having received research grants from and serving as consultants to Novartis and numerous other companies developing biologic agents for psoriasis.