User login
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
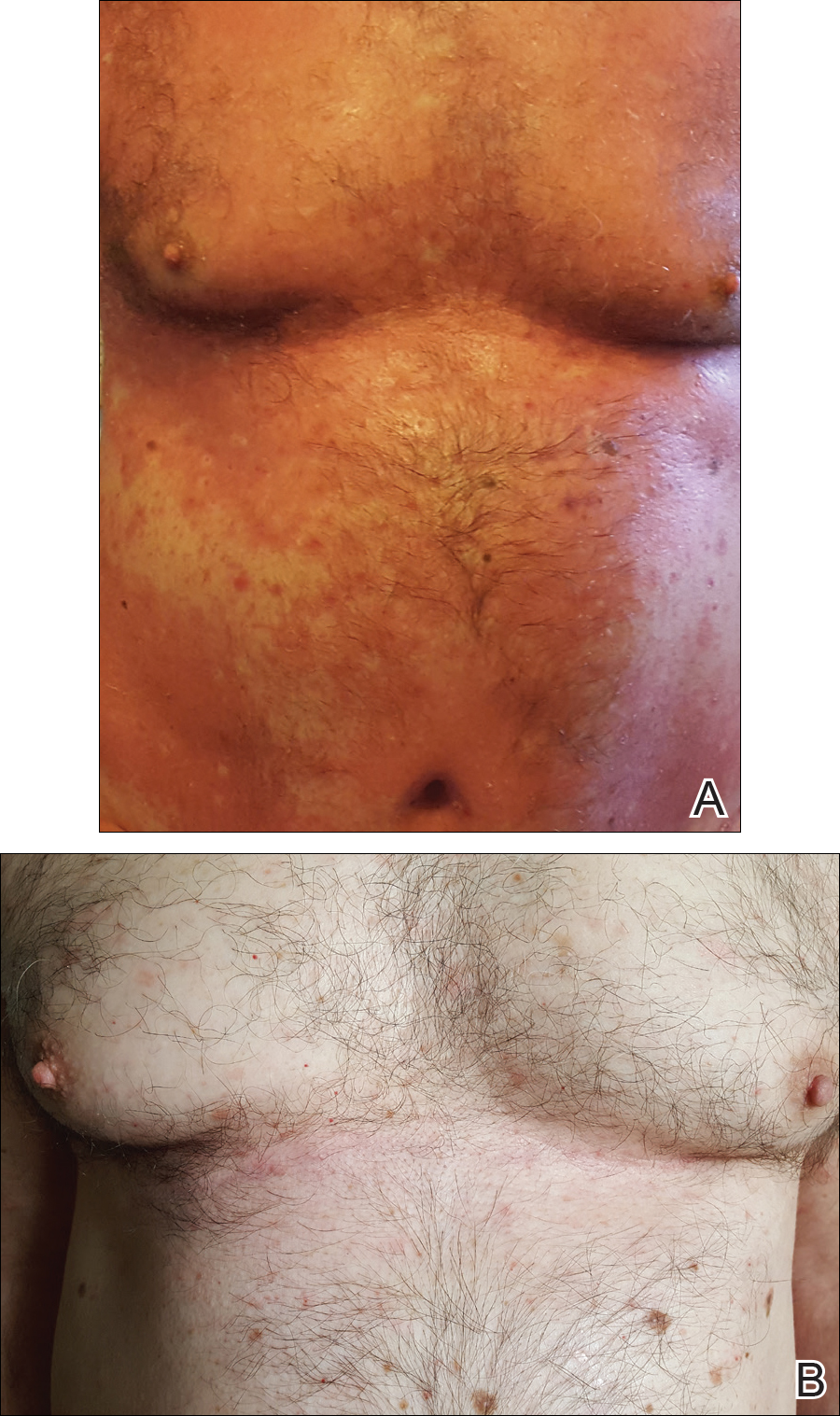
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.
Patient 2
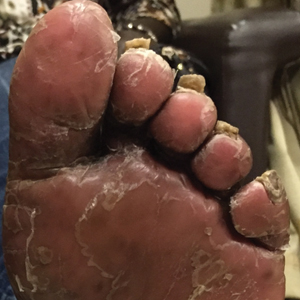
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.
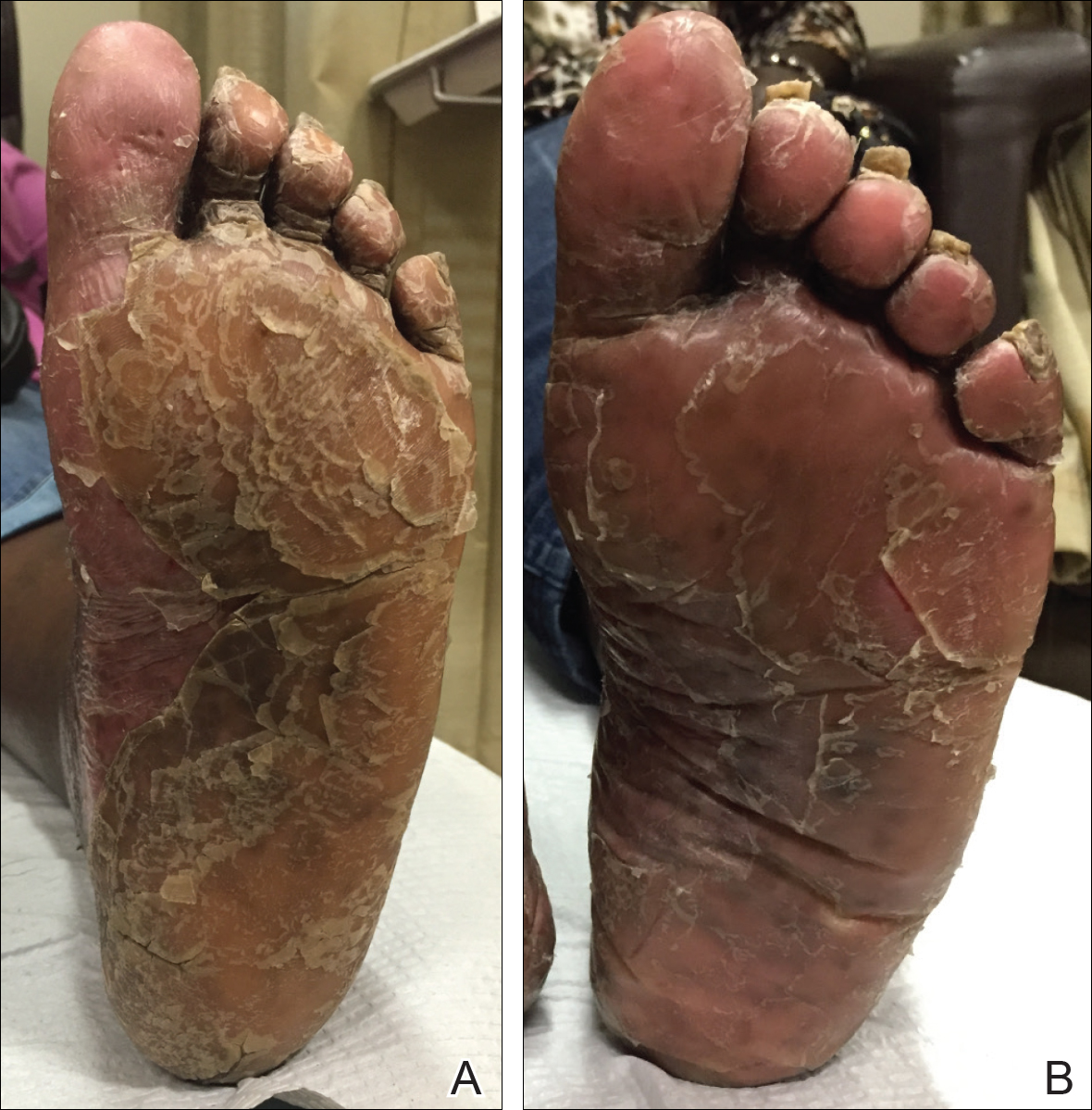
By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Practice Points
- In patients with pityriasis rubra pilaris (PRP) who have not responded to topical treatments, off-label treatment with systemic therapies approved for plaque psoriasis can be considered.
- Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP.