User login
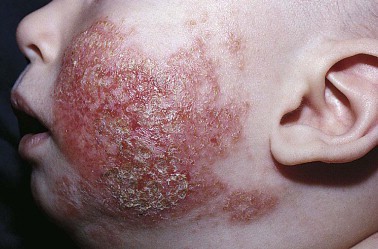
PRAGUE – Long-term therapy utilizing a pimecrolimus-based strategy for mild to moderate atopic dermatitis beginning in infancy proved as effective and safe as conventional therapy with topical corticosteroids in a 5-year randomized study.
"Given that pimecrolimus cream 1% is not associated with topical corticosteroid-specific side effects, such as hypothalamic-pituitary-adrenal axis suppression or skin atrophy, which can limit their long-term use, pimecrolimus cream may represent the drug of choice for long-term atopic dermatitis management," said Dr. Thomas A. Luger, professor and chairman of the department of dermatology at the University of Müenster (Germany).
Currently, pimecrolimus is not approved for use in patients under 2 years of age, he noted.
Dr. Luger, who is an investigator on the study’s steering committee, reported on 2,418 infants with mild to moderate atopic dermatitis randomized to daily use of an emollient for their dry skin plus either pimecrolimus cream 1% (Elidel) or low-potency to midpotency topical steroids on an as-needed basis. The participants’ mean age at enrollment was 7 months. Fifty-three percent of infants had moderate atopic dermatitis based on the Investigator’s Global Assessment; the rest had mild disease.
This was a 5-year, international, open-label randomized study designed to reflect real world, as-needed use of topical medications. Patients in the pimecrolimus group applied the topical calcineurin inhibitor at the first sign of an atopic dermatitis flare. If pimecrolimus didn’t control the itching, redness, and/or lesion elevation to the parents’ satisfaction, the child was switched to a low-potency topical steroid. If that didn’t quell the episode, a midpotency topical steroid was used. And if the child’s skin disease worsened despite the midpotency topical steroid, the patient went to the clinic. If the physician determined systemic therapy was needed, the child was removed from the study.
Treatment success was defined as a score of 0 or 1, meaning clear or almost clear, on the Investigator’s Global Assessment. By week 3 of the study, more than 50% of patients in both treatment arms met that standard. The success rate gradually increased over a 5-year period to about 90% overall in both groups and greater than 95% for facial atopic dermatitis.
At baseline, the participants’ mean total body surface area affected by atopic dermatitis was 17%, and 22% of infants had a total body surface area of 30% or greater. By week 3, however, the mean total body surface area had dropped below 5% in both groups. The total body surface area progressively improved, reaching a mean of 0% at week 78 and staying that way for the remainder of the 5-year study, Dr. Luger reported at the annual congress of the European Academy of Dermatology and Venereology.
Of children in the pimecrolimus cohort, 70% completed the 5-year study, as did 72% in the topical steroid arm. During the course of the study, 38% of completers in the pimecrolimus arm used a low-potency topical steroid, and 41% used a midpotency topical steroid.
The two treatment strategies proved similarly well tolerated. The rate of discontinuation because of adverse events was 0.6% in the pimecrolimus cohort and 1.1% in the topical steroids group. The incidence and time to onset of all adverse events were similar in the two study arms. Serious adverse events, the majority of which were infections, occurred in 20% of the pimecrolimus group and 17% of the topical steroids group.
The study was funded by Novartis, which markets Elidel. Dr. Luger is a consultant to and a paid speaker for the pharmaceutical company.
PRAGUE – Long-term therapy utilizing a pimecrolimus-based strategy for mild to moderate atopic dermatitis beginning in infancy proved as effective and safe as conventional therapy with topical corticosteroids in a 5-year randomized study.
"Given that pimecrolimus cream 1% is not associated with topical corticosteroid-specific side effects, such as hypothalamic-pituitary-adrenal axis suppression or skin atrophy, which can limit their long-term use, pimecrolimus cream may represent the drug of choice for long-term atopic dermatitis management," said Dr. Thomas A. Luger, professor and chairman of the department of dermatology at the University of Müenster (Germany).
Currently, pimecrolimus is not approved for use in patients under 2 years of age, he noted.
Dr. Luger, who is an investigator on the study’s steering committee, reported on 2,418 infants with mild to moderate atopic dermatitis randomized to daily use of an emollient for their dry skin plus either pimecrolimus cream 1% (Elidel) or low-potency to midpotency topical steroids on an as-needed basis. The participants’ mean age at enrollment was 7 months. Fifty-three percent of infants had moderate atopic dermatitis based on the Investigator’s Global Assessment; the rest had mild disease.
This was a 5-year, international, open-label randomized study designed to reflect real world, as-needed use of topical medications. Patients in the pimecrolimus group applied the topical calcineurin inhibitor at the first sign of an atopic dermatitis flare. If pimecrolimus didn’t control the itching, redness, and/or lesion elevation to the parents’ satisfaction, the child was switched to a low-potency topical steroid. If that didn’t quell the episode, a midpotency topical steroid was used. And if the child’s skin disease worsened despite the midpotency topical steroid, the patient went to the clinic. If the physician determined systemic therapy was needed, the child was removed from the study.
Treatment success was defined as a score of 0 or 1, meaning clear or almost clear, on the Investigator’s Global Assessment. By week 3 of the study, more than 50% of patients in both treatment arms met that standard. The success rate gradually increased over a 5-year period to about 90% overall in both groups and greater than 95% for facial atopic dermatitis.
At baseline, the participants’ mean total body surface area affected by atopic dermatitis was 17%, and 22% of infants had a total body surface area of 30% or greater. By week 3, however, the mean total body surface area had dropped below 5% in both groups. The total body surface area progressively improved, reaching a mean of 0% at week 78 and staying that way for the remainder of the 5-year study, Dr. Luger reported at the annual congress of the European Academy of Dermatology and Venereology.
Of children in the pimecrolimus cohort, 70% completed the 5-year study, as did 72% in the topical steroid arm. During the course of the study, 38% of completers in the pimecrolimus arm used a low-potency topical steroid, and 41% used a midpotency topical steroid.
The two treatment strategies proved similarly well tolerated. The rate of discontinuation because of adverse events was 0.6% in the pimecrolimus cohort and 1.1% in the topical steroids group. The incidence and time to onset of all adverse events were similar in the two study arms. Serious adverse events, the majority of which were infections, occurred in 20% of the pimecrolimus group and 17% of the topical steroids group.
The study was funded by Novartis, which markets Elidel. Dr. Luger is a consultant to and a paid speaker for the pharmaceutical company.
PRAGUE – Long-term therapy utilizing a pimecrolimus-based strategy for mild to moderate atopic dermatitis beginning in infancy proved as effective and safe as conventional therapy with topical corticosteroids in a 5-year randomized study.
"Given that pimecrolimus cream 1% is not associated with topical corticosteroid-specific side effects, such as hypothalamic-pituitary-adrenal axis suppression or skin atrophy, which can limit their long-term use, pimecrolimus cream may represent the drug of choice for long-term atopic dermatitis management," said Dr. Thomas A. Luger, professor and chairman of the department of dermatology at the University of Müenster (Germany).
Currently, pimecrolimus is not approved for use in patients under 2 years of age, he noted.
Dr. Luger, who is an investigator on the study’s steering committee, reported on 2,418 infants with mild to moderate atopic dermatitis randomized to daily use of an emollient for their dry skin plus either pimecrolimus cream 1% (Elidel) or low-potency to midpotency topical steroids on an as-needed basis. The participants’ mean age at enrollment was 7 months. Fifty-three percent of infants had moderate atopic dermatitis based on the Investigator’s Global Assessment; the rest had mild disease.
This was a 5-year, international, open-label randomized study designed to reflect real world, as-needed use of topical medications. Patients in the pimecrolimus group applied the topical calcineurin inhibitor at the first sign of an atopic dermatitis flare. If pimecrolimus didn’t control the itching, redness, and/or lesion elevation to the parents’ satisfaction, the child was switched to a low-potency topical steroid. If that didn’t quell the episode, a midpotency topical steroid was used. And if the child’s skin disease worsened despite the midpotency topical steroid, the patient went to the clinic. If the physician determined systemic therapy was needed, the child was removed from the study.
Treatment success was defined as a score of 0 or 1, meaning clear or almost clear, on the Investigator’s Global Assessment. By week 3 of the study, more than 50% of patients in both treatment arms met that standard. The success rate gradually increased over a 5-year period to about 90% overall in both groups and greater than 95% for facial atopic dermatitis.
At baseline, the participants’ mean total body surface area affected by atopic dermatitis was 17%, and 22% of infants had a total body surface area of 30% or greater. By week 3, however, the mean total body surface area had dropped below 5% in both groups. The total body surface area progressively improved, reaching a mean of 0% at week 78 and staying that way for the remainder of the 5-year study, Dr. Luger reported at the annual congress of the European Academy of Dermatology and Venereology.
Of children in the pimecrolimus cohort, 70% completed the 5-year study, as did 72% in the topical steroid arm. During the course of the study, 38% of completers in the pimecrolimus arm used a low-potency topical steroid, and 41% used a midpotency topical steroid.
The two treatment strategies proved similarly well tolerated. The rate of discontinuation because of adverse events was 0.6% in the pimecrolimus cohort and 1.1% in the topical steroids group. The incidence and time to onset of all adverse events were similar in the two study arms. Serious adverse events, the majority of which were infections, occurred in 20% of the pimecrolimus group and 17% of the topical steroids group.
The study was funded by Novartis, which markets Elidel. Dr. Luger is a consultant to and a paid speaker for the pharmaceutical company.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: After 5 years of participation in a study of pimecrolimus- versus topical corticosteroid-based treatment strategies for infants with atopic dermatitis, about 90% of patients in both study arms were rated clear or almost clear.
Data Source: This was a randomized, international, prospective open-label study involving 2,418 infants with mild to moderate atopic dermatitis.
Disclosures: The study was funded by Novartis, which markets Elidel. Dr. Luger is a consultant to and a paid speaker for the pharmaceutical company.