User login
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
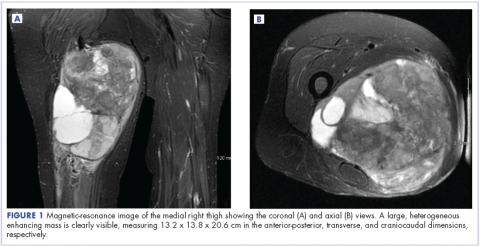
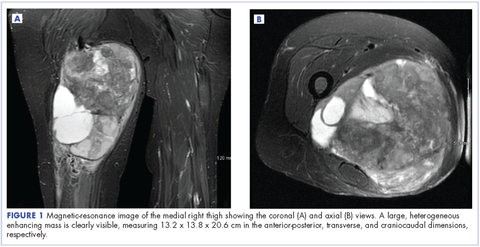
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
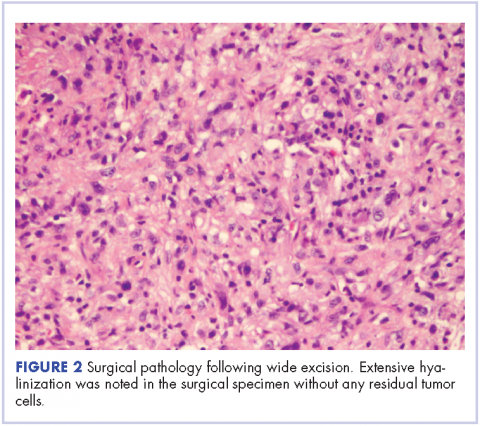
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.