User login
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
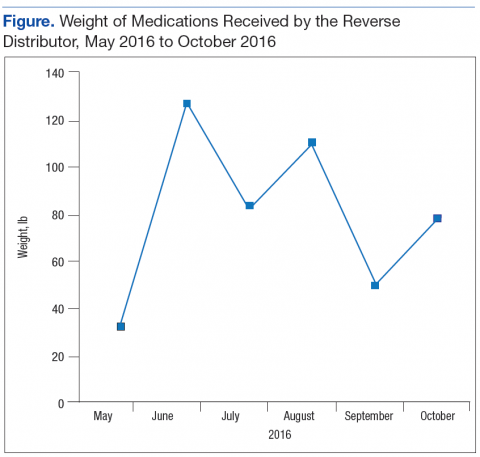
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.