User login
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
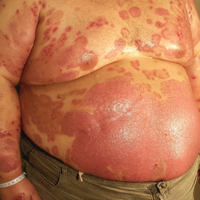
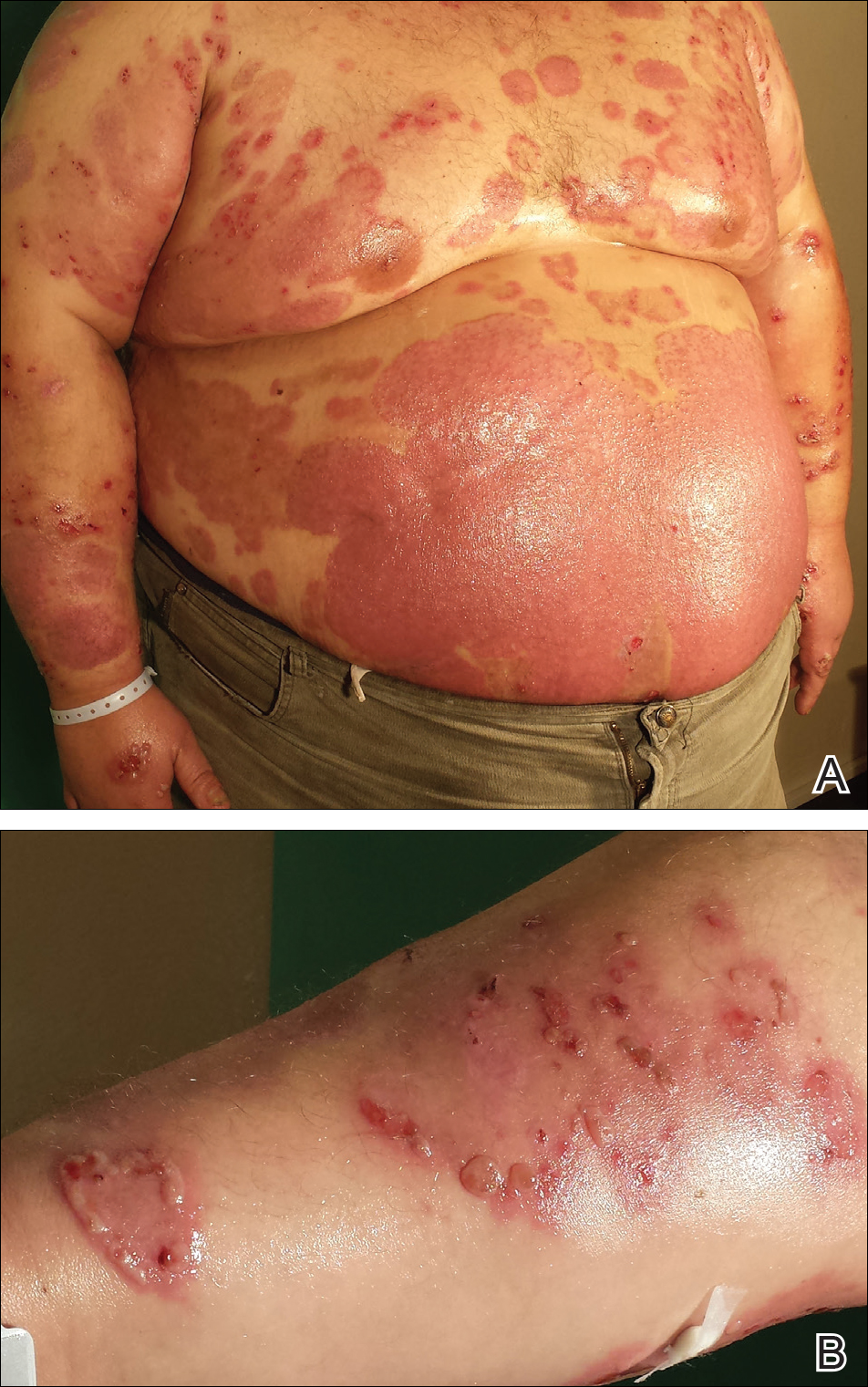
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.
The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
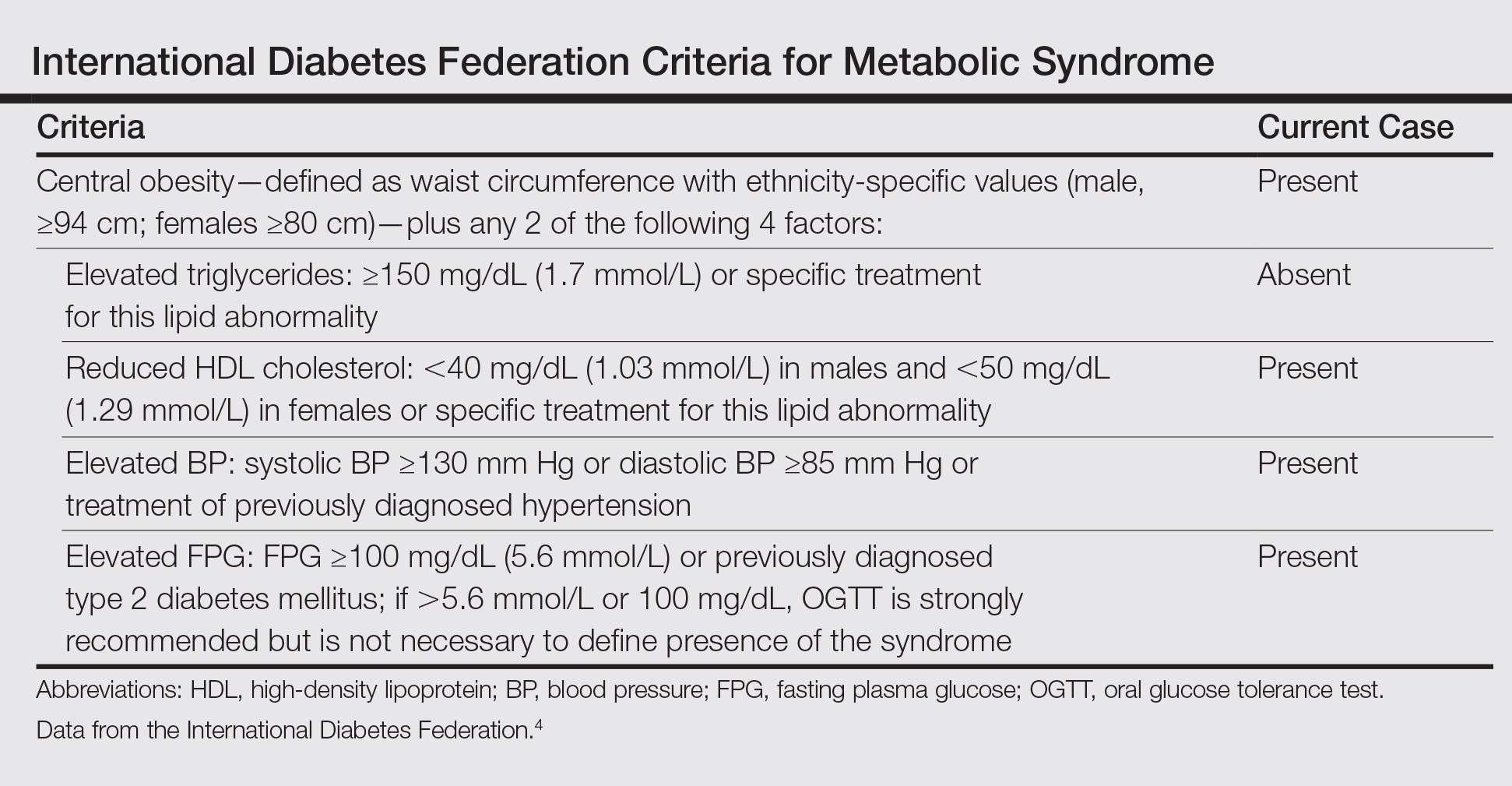
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).

Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.

The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).


Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.

The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).


Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
Practice Points
- Metabolic syndrome and psoriasis vulgaris (PV) may promote development of bullous pemphigoid (BP) in patients younger than 60 years.
- Methotrexate may be a therapeutic solution for BP coexisting with PV and metabolic syndrome.