User login
IN THIS ARTICLE
- Progress and treatment timeline with long- and rapid-acting insulin
- Progress and treatment timeline with continuous subcutaneous insulin infusion
- American Diabetes Association criteria for diagnosis of diabetes
- Blood glucose and A1C goals for type 1 diabetes by age-group
A 5-year-old Caucasian girl presents to the primary care practitioner’s office with chief complaints of polydipsia, polyuria with nocturia, polyphagia, and weight loss over the past three weeks. Her medical history includes a four-year history of keratosis pilaris (KP). The child experienced a KP flare-up two weeks ago; application of triamcinolone acetonide cream yielded no improvement. She also has xerosis, which is treated daily with OTC moisturizing lotion. She was born vaginally and breast-fed and is up to date on her immunizations. There is no family history of diabetes or autoimmune diseases.
Physical examination reveals a weight of 54 lb (95th percentile); height, 47 in (97th percentile); and BMI, 17.2. Vital signs include a blood pressure of 105/55 mm Hg; pulse, 85 beats/min and regular; temperature, 98.2°F; and respiratory rate, 22 breaths/min. KP is noted on the patient’s eyebrows, bilateral upper arms, and bilateral cheeks; the affected skin is erythemic and rough to the touch. Her physical examination findings are otherwise unremarkable.
The child’s urine is tested in the office for glucose and ketones, with results of 4+ glucose and 3+ ketones. These results and the child’s history prompt her admission to the pediatric ICU at a nearby hospital for further treatment with a diagnosis of new-onset type 1 diabetes (T1D) and diabetic ketoacidosis (DKA).
The diagnosis is confirmed at the hospital with laboratory results that include venous glucose, 418 mg/dL (normal range, 70 to 100 mg/dL) and A1C, 10.5% (range, 4.0% to 5.6%). Venous blood gas results include pH, 7.278 (7.32 to 7.42); PCO2, 29.6 mm Hg (39 to 54 mm Hg); HCO3, 13.8 mEq/L (19 to 25 mEq/L); base excess, –12 mmol/L (–4 to +2 mmol/L); beta hydroxybutyrate, 6.0 mmol/L (0.4 to 0.5 mmol/L); insulin antibody, 0.9 U/mL (< 0.4 U/mL); glutamic acid decarboxylase, 166 U/mL (< 0.5 U/mL); and venous lactate, 1.79 mmol/L (0.69 to 2.75 mmol/L).
The child is treated initially with an IV insulin infusion for 24 hours, then transitioned to subcutaneous insulin therapy once the DKA resolves and glucose levels are within normal limits. The child remains hospitalized for four days. Discharge medications include insulin glargine, 8 U/d, and insulin lispro before each meal, at bedtime, and at 0200 hours, with dosing based on sliding scales. Dietary orders include 45 to 60 g carbohydrates per meal, along with two snacks of 15 g carbohydrates.
The child is instructed to exercise at least 30 min/d (unless hypoglycemic events occur more than once per week or ketones are found in the blood or urine), drink plenty of water, and avoid concentrated sweets. Education is provided via the Diabetes Educator; the family takes home the beginner T1D booklet and is instructed to log the child’s blood glucose levels and return with this information in two weeks.
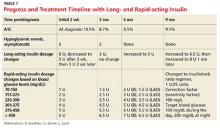
In the first three months, the patient experiences eight asymptomatic hypoglycemic events; for the next seven months, after dosing changes, she remains hyperglycemic most of the time (see Table 1). Insulin doses are adjusted, ranging from weekly to every three months, but glycemic goals are not achieved with the subcutaneous insulin injections. Use of continuous subcutaneous insulin infusion, the “insulin pump,” is then considered. Ten months postdiagnosis, the child begins a five-day-long saline (placebo) pump trial to determine whether the pump is appropriate for her and her lifestyle. After the trial, the decision is made to move forward with the insulin pump, initiated 11 months postdiagnosis.
The practitioner remains in frequent communication with the child’s mother in an effort to maintain glycemic control. After three months on the insulin pump, the child’s A1C is reduced to 7.9%, which is within the target range for her age-group (see Table 2). The child is now maintaining glycemic goals with the use of the insulin pump and close monitoring by the practitioner.
Continue for the discussion >>
DISCUSSION
According to the Juvenile Diabetes Research Foundation, as many as 1.25 million Americans are currently living with T1D; from 2001 to 2009, the prevalence of T1D in people younger than 20 increased by 23%.1 The overall prevalence of diabetes (both types 1 and 2) is predicted to be one in every three people by 2050 if current trends continue.2 According to the American Diabetes Association (ADA), 18,436 US youths are diagnosed with T1D every year, and T1D accounts for about 5% of diabetes cases in the US population.2
Diagnosis
Diabetes is diagnosed based on blood test results that fall within the parameters set by the ADA diagnostic criteria (see Table 3).3 In addition to diagnostic testing for diabetes recommended by the ADA guidelines, blood tests are ordered for autoantibodies that are associated with T1D, to distinguish between type 1 and type 2 diabetes. (T1D results from cellular-mediated autoimmune destruction of the insulin-producing beta cells in the pancreas.4) Upon initial diagnosis, about 85% to 90% of T1D patients have one or more autoantibodies present in blood work, such as autoantibodies to islet cells or to insulin, glutamic acid decarboxylase (GAD65), or tyrosine phosphatases IA-2 and IA-2B.4
In this case study, the child had an elevated GAD65 value and a positive screening for an insulin autoantibody, which explained the destruction of her beta cells. The patient also had KP and xerosis, which are clinical manifestations commonly seen in T1D. In one study of children with T1D, 22% had xerosis, compared with 3% of healthy, age-matched controls, and KP was also significantly more common in T1D patients than in controls (12% vs 1.5%).5
The presence of ketones in the case patient’s urine also suggests T1D, rather than type 2.4 The differential diagnosis for T1D includes type 2 diabetes mellitus, monogenic diabetes mellitus (formerly known as maturity-onset diabetes of the young), secondary hyperglycemia, and other endocrine disorders.6
Acute complications associated with T1D include hypoglycemia, hyperglycemia, and DKA. Long-term complications may include diabetic retinopathy, cataracts, gastroparesis, hypertension, renal failure, coronary artery disease, peripheral vascular disease, diabetic neuropathy, and increased risk for infection.7 These complications can likely be prevented by good glycemic control, proper diet, exercise, and avoidance of nicotine.7
Unfortunately, T1D cannot currently be prevented, although research studies are under way. TrialNet is currently conducting a “Pathway to Prevention” trial; the researchers are testing ways to delay and prevent T1D, as well as slow its progression after diagnosis.8 Potential participants (family members of a T1D patient) are screened for T1D autoantibodies. If test results are positive, these participants are included in the prevention pathway study.
Continue for management >>
Management
Most cases of T1D are diagnosed in patients younger than 18.9 Management of the child with T1D involves many challenges. The patient will experience an initial honeymoon period, that is, a brief remission during which the pancreas begins to secrete some insulin again and exogenous insulin demands are lower. However, this period is temporary, lasting only a few weeks, months, or years. Once pancreatic insulin secretion stops (as a result of complete beta-cell destruction), the exogenous insulin demands increase. In the case study, the child’s insulin demands were initially low, and she experienced hypoglycemia. Once she transitioned out of the honeymoon period, however, her blood glucose levels rose because her pancreas was producing little to no insulin.
As the patient ages, physical growth and hormone changes also alter the demand for insulin. A key factor to keep in mind is lifestyle changes: The child may need age-appropriate supervision and adjustments in exercise, diet, and diabetes education regimens when school routines and self-care capacities change. The child with T1D can only be educated as far as his or her cognitive ability will allow, but autonomy should increase with age.
Helping the patient reach glycemic goals requires special consideration, based on the child’s age. Whereas the target A1C for an adult with diabetes is below 7%, that for a young child is either < 7.5% or < 8.5%, depending on age (see Table 4).9 According to Danne et al, approximately 60% of children younger than 6 years have an imperfect awareness of hypoglycemia. 10 Because the risk for a hypoglycemic event is increased in this age-group, their target A1C is higher.10
This is also an important age for brain development: The metabolism of glucose in the brain of a young child occurs at double the rate of that in an adult brain.11 Between ages 1 and 6 years, the brain increases in size dramatically, reaching 90% of its adult volume by age 6.11 In retrospective studies reviewed by Arbelaez et al,data show that frequent, severe hypoglycemic and hyperglycemic events are associated with poor cognitive function, particularly memory and attention.11 Due to the timing of brain development and the risk for glycemic extremes in young children, practitioners are advised to follow the ADA recommendations shown in Table 4.9
Continue for the conclusion >>
CONCLUSION
T1D is the most common chronic, serious, potentially life-threatening disease among children and adolescents. This lifelong illness is challenging to control, especially when managing the honeymoon period and addressing the increasing insulin demands in a growing child. Once a diagnosis is confirmed, the challenges persist, as each patient needs an individualized treatment regimen with ongoing adjustments. Knowledge of the ADA guidelines for age-appropriate A1C goals is essential for the practitioner who manages a growing child with T1D in order to achieve glycemic control while avoiding hypoglycemia. Preventing hypoglycemia is of the utmost importance, especially in a child too young to recognize symptoms.
Considering all the changes that a child with T1D is likely to experience, it is also important to remember that the foremost goal is for this child to live a healthy life. Thus, practitioners must educate both patients and parents regarding the complications that can arise with poor glycemic control and encourage adherence to the insulin therapy.
T1D requires vigilant monitoring and ongoing adjusted insulin therapy. Understanding age-appropriate treatment and maintaining good communications with patients and their parents are key to successful management of this disease.
REFERENCES
1. Juvenile Diabetes Research Foundation. Type 1 diabetes facts (2014). http://jdrf.org/about-jdrf/fact-sheets/type-1-diabetes-facts/. Accessed February 8, 2016.
2. American Diabetes Association. Fast facts: data and statistics about diabetes (2015). http://professional2.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/Fast_Facts_12-2015a.pdf. Accessed February 8, 2016.
3. American Diabetes Association. Executive summary: standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S5-S13.
4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):81-90.
5. Pavlovic MD, Milenkovic T, Dinic M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30(8):1964-1967.
6. Khardori R. Type 1 diabetes mellitus differential diagnosis (updated 2015). http://emedicine.medscape.com/article/117739-differential. Accessed February 8, 2016.
7. Lamb WH. Pediatric type 1 diabetes mellitus (updated 2015). http://emedicine.medscape.com/article/919999-overview. Accessed February 8, 2016.
8. Type 1 Diabetes TrialNet. TrialNet Pathway to Prevention (2014). www.pathway2prevention.org/study/. Accessed February 8, 2016.
9. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66. VIII. Diabetes care in specific populations. http://care.diabetesjournals.org/content/36/Supplement_1/S11.full#sec-128. Accessed February 8, 2016.
10. Danne T, Philotheou A, Goldman D, et al. A randomized trial comparing the rate of hypoglycemia—assessed using continuous glucose monitoring—in 125 preschool children with type 1 diabetes treated with insulin glargine or NPH insulin (the PRESCHOOL study). Pediatr Diabetes. 2013;14(8):593-601.
11. Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes. 2013;14(8):541-553.
IN THIS ARTICLE
- Progress and treatment timeline with long- and rapid-acting insulin
- Progress and treatment timeline with continuous subcutaneous insulin infusion
- American Diabetes Association criteria for diagnosis of diabetes
- Blood glucose and A1C goals for type 1 diabetes by age-group
A 5-year-old Caucasian girl presents to the primary care practitioner’s office with chief complaints of polydipsia, polyuria with nocturia, polyphagia, and weight loss over the past three weeks. Her medical history includes a four-year history of keratosis pilaris (KP). The child experienced a KP flare-up two weeks ago; application of triamcinolone acetonide cream yielded no improvement. She also has xerosis, which is treated daily with OTC moisturizing lotion. She was born vaginally and breast-fed and is up to date on her immunizations. There is no family history of diabetes or autoimmune diseases.
Physical examination reveals a weight of 54 lb (95th percentile); height, 47 in (97th percentile); and BMI, 17.2. Vital signs include a blood pressure of 105/55 mm Hg; pulse, 85 beats/min and regular; temperature, 98.2°F; and respiratory rate, 22 breaths/min. KP is noted on the patient’s eyebrows, bilateral upper arms, and bilateral cheeks; the affected skin is erythemic and rough to the touch. Her physical examination findings are otherwise unremarkable.
The child’s urine is tested in the office for glucose and ketones, with results of 4+ glucose and 3+ ketones. These results and the child’s history prompt her admission to the pediatric ICU at a nearby hospital for further treatment with a diagnosis of new-onset type 1 diabetes (T1D) and diabetic ketoacidosis (DKA).
The diagnosis is confirmed at the hospital with laboratory results that include venous glucose, 418 mg/dL (normal range, 70 to 100 mg/dL) and A1C, 10.5% (range, 4.0% to 5.6%). Venous blood gas results include pH, 7.278 (7.32 to 7.42); PCO2, 29.6 mm Hg (39 to 54 mm Hg); HCO3, 13.8 mEq/L (19 to 25 mEq/L); base excess, –12 mmol/L (–4 to +2 mmol/L); beta hydroxybutyrate, 6.0 mmol/L (0.4 to 0.5 mmol/L); insulin antibody, 0.9 U/mL (< 0.4 U/mL); glutamic acid decarboxylase, 166 U/mL (< 0.5 U/mL); and venous lactate, 1.79 mmol/L (0.69 to 2.75 mmol/L).
The child is treated initially with an IV insulin infusion for 24 hours, then transitioned to subcutaneous insulin therapy once the DKA resolves and glucose levels are within normal limits. The child remains hospitalized for four days. Discharge medications include insulin glargine, 8 U/d, and insulin lispro before each meal, at bedtime, and at 0200 hours, with dosing based on sliding scales. Dietary orders include 45 to 60 g carbohydrates per meal, along with two snacks of 15 g carbohydrates.
The child is instructed to exercise at least 30 min/d (unless hypoglycemic events occur more than once per week or ketones are found in the blood or urine), drink plenty of water, and avoid concentrated sweets. Education is provided via the Diabetes Educator; the family takes home the beginner T1D booklet and is instructed to log the child’s blood glucose levels and return with this information in two weeks.
In the first three months, the patient experiences eight asymptomatic hypoglycemic events; for the next seven months, after dosing changes, she remains hyperglycemic most of the time (see Table 1). Insulin doses are adjusted, ranging from weekly to every three months, but glycemic goals are not achieved with the subcutaneous insulin injections. Use of continuous subcutaneous insulin infusion, the “insulin pump,” is then considered. Ten months postdiagnosis, the child begins a five-day-long saline (placebo) pump trial to determine whether the pump is appropriate for her and her lifestyle. After the trial, the decision is made to move forward with the insulin pump, initiated 11 months postdiagnosis.
The practitioner remains in frequent communication with the child’s mother in an effort to maintain glycemic control. After three months on the insulin pump, the child’s A1C is reduced to 7.9%, which is within the target range for her age-group (see Table 2). The child is now maintaining glycemic goals with the use of the insulin pump and close monitoring by the practitioner.
Continue for the discussion >>
DISCUSSION
According to the Juvenile Diabetes Research Foundation, as many as 1.25 million Americans are currently living with T1D; from 2001 to 2009, the prevalence of T1D in people younger than 20 increased by 23%.1 The overall prevalence of diabetes (both types 1 and 2) is predicted to be one in every three people by 2050 if current trends continue.2 According to the American Diabetes Association (ADA), 18,436 US youths are diagnosed with T1D every year, and T1D accounts for about 5% of diabetes cases in the US population.2
Diagnosis
Diabetes is diagnosed based on blood test results that fall within the parameters set by the ADA diagnostic criteria (see Table 3).3 In addition to diagnostic testing for diabetes recommended by the ADA guidelines, blood tests are ordered for autoantibodies that are associated with T1D, to distinguish between type 1 and type 2 diabetes. (T1D results from cellular-mediated autoimmune destruction of the insulin-producing beta cells in the pancreas.4) Upon initial diagnosis, about 85% to 90% of T1D patients have one or more autoantibodies present in blood work, such as autoantibodies to islet cells or to insulin, glutamic acid decarboxylase (GAD65), or tyrosine phosphatases IA-2 and IA-2B.4
In this case study, the child had an elevated GAD65 value and a positive screening for an insulin autoantibody, which explained the destruction of her beta cells. The patient also had KP and xerosis, which are clinical manifestations commonly seen in T1D. In one study of children with T1D, 22% had xerosis, compared with 3% of healthy, age-matched controls, and KP was also significantly more common in T1D patients than in controls (12% vs 1.5%).5
The presence of ketones in the case patient’s urine also suggests T1D, rather than type 2.4 The differential diagnosis for T1D includes type 2 diabetes mellitus, monogenic diabetes mellitus (formerly known as maturity-onset diabetes of the young), secondary hyperglycemia, and other endocrine disorders.6
Acute complications associated with T1D include hypoglycemia, hyperglycemia, and DKA. Long-term complications may include diabetic retinopathy, cataracts, gastroparesis, hypertension, renal failure, coronary artery disease, peripheral vascular disease, diabetic neuropathy, and increased risk for infection.7 These complications can likely be prevented by good glycemic control, proper diet, exercise, and avoidance of nicotine.7
Unfortunately, T1D cannot currently be prevented, although research studies are under way. TrialNet is currently conducting a “Pathway to Prevention” trial; the researchers are testing ways to delay and prevent T1D, as well as slow its progression after diagnosis.8 Potential participants (family members of a T1D patient) are screened for T1D autoantibodies. If test results are positive, these participants are included in the prevention pathway study.
Continue for management >>
Management
Most cases of T1D are diagnosed in patients younger than 18.9 Management of the child with T1D involves many challenges. The patient will experience an initial honeymoon period, that is, a brief remission during which the pancreas begins to secrete some insulin again and exogenous insulin demands are lower. However, this period is temporary, lasting only a few weeks, months, or years. Once pancreatic insulin secretion stops (as a result of complete beta-cell destruction), the exogenous insulin demands increase. In the case study, the child’s insulin demands were initially low, and she experienced hypoglycemia. Once she transitioned out of the honeymoon period, however, her blood glucose levels rose because her pancreas was producing little to no insulin.
As the patient ages, physical growth and hormone changes also alter the demand for insulin. A key factor to keep in mind is lifestyle changes: The child may need age-appropriate supervision and adjustments in exercise, diet, and diabetes education regimens when school routines and self-care capacities change. The child with T1D can only be educated as far as his or her cognitive ability will allow, but autonomy should increase with age.
Helping the patient reach glycemic goals requires special consideration, based on the child’s age. Whereas the target A1C for an adult with diabetes is below 7%, that for a young child is either < 7.5% or < 8.5%, depending on age (see Table 4).9 According to Danne et al, approximately 60% of children younger than 6 years have an imperfect awareness of hypoglycemia. 10 Because the risk for a hypoglycemic event is increased in this age-group, their target A1C is higher.10
This is also an important age for brain development: The metabolism of glucose in the brain of a young child occurs at double the rate of that in an adult brain.11 Between ages 1 and 6 years, the brain increases in size dramatically, reaching 90% of its adult volume by age 6.11 In retrospective studies reviewed by Arbelaez et al,data show that frequent, severe hypoglycemic and hyperglycemic events are associated with poor cognitive function, particularly memory and attention.11 Due to the timing of brain development and the risk for glycemic extremes in young children, practitioners are advised to follow the ADA recommendations shown in Table 4.9
Continue for the conclusion >>
CONCLUSION
T1D is the most common chronic, serious, potentially life-threatening disease among children and adolescents. This lifelong illness is challenging to control, especially when managing the honeymoon period and addressing the increasing insulin demands in a growing child. Once a diagnosis is confirmed, the challenges persist, as each patient needs an individualized treatment regimen with ongoing adjustments. Knowledge of the ADA guidelines for age-appropriate A1C goals is essential for the practitioner who manages a growing child with T1D in order to achieve glycemic control while avoiding hypoglycemia. Preventing hypoglycemia is of the utmost importance, especially in a child too young to recognize symptoms.
Considering all the changes that a child with T1D is likely to experience, it is also important to remember that the foremost goal is for this child to live a healthy life. Thus, practitioners must educate both patients and parents regarding the complications that can arise with poor glycemic control and encourage adherence to the insulin therapy.
T1D requires vigilant monitoring and ongoing adjusted insulin therapy. Understanding age-appropriate treatment and maintaining good communications with patients and their parents are key to successful management of this disease.
REFERENCES
1. Juvenile Diabetes Research Foundation. Type 1 diabetes facts (2014). http://jdrf.org/about-jdrf/fact-sheets/type-1-diabetes-facts/. Accessed February 8, 2016.
2. American Diabetes Association. Fast facts: data and statistics about diabetes (2015). http://professional2.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/Fast_Facts_12-2015a.pdf. Accessed February 8, 2016.
3. American Diabetes Association. Executive summary: standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S5-S13.
4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):81-90.
5. Pavlovic MD, Milenkovic T, Dinic M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30(8):1964-1967.
6. Khardori R. Type 1 diabetes mellitus differential diagnosis (updated 2015). http://emedicine.medscape.com/article/117739-differential. Accessed February 8, 2016.
7. Lamb WH. Pediatric type 1 diabetes mellitus (updated 2015). http://emedicine.medscape.com/article/919999-overview. Accessed February 8, 2016.
8. Type 1 Diabetes TrialNet. TrialNet Pathway to Prevention (2014). www.pathway2prevention.org/study/. Accessed February 8, 2016.
9. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66. VIII. Diabetes care in specific populations. http://care.diabetesjournals.org/content/36/Supplement_1/S11.full#sec-128. Accessed February 8, 2016.
10. Danne T, Philotheou A, Goldman D, et al. A randomized trial comparing the rate of hypoglycemia—assessed using continuous glucose monitoring—in 125 preschool children with type 1 diabetes treated with insulin glargine or NPH insulin (the PRESCHOOL study). Pediatr Diabetes. 2013;14(8):593-601.
11. Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes. 2013;14(8):541-553.
IN THIS ARTICLE
- Progress and treatment timeline with long- and rapid-acting insulin
- Progress and treatment timeline with continuous subcutaneous insulin infusion
- American Diabetes Association criteria for diagnosis of diabetes
- Blood glucose and A1C goals for type 1 diabetes by age-group
A 5-year-old Caucasian girl presents to the primary care practitioner’s office with chief complaints of polydipsia, polyuria with nocturia, polyphagia, and weight loss over the past three weeks. Her medical history includes a four-year history of keratosis pilaris (KP). The child experienced a KP flare-up two weeks ago; application of triamcinolone acetonide cream yielded no improvement. She also has xerosis, which is treated daily with OTC moisturizing lotion. She was born vaginally and breast-fed and is up to date on her immunizations. There is no family history of diabetes or autoimmune diseases.
Physical examination reveals a weight of 54 lb (95th percentile); height, 47 in (97th percentile); and BMI, 17.2. Vital signs include a blood pressure of 105/55 mm Hg; pulse, 85 beats/min and regular; temperature, 98.2°F; and respiratory rate, 22 breaths/min. KP is noted on the patient’s eyebrows, bilateral upper arms, and bilateral cheeks; the affected skin is erythemic and rough to the touch. Her physical examination findings are otherwise unremarkable.
The child’s urine is tested in the office for glucose and ketones, with results of 4+ glucose and 3+ ketones. These results and the child’s history prompt her admission to the pediatric ICU at a nearby hospital for further treatment with a diagnosis of new-onset type 1 diabetes (T1D) and diabetic ketoacidosis (DKA).
The diagnosis is confirmed at the hospital with laboratory results that include venous glucose, 418 mg/dL (normal range, 70 to 100 mg/dL) and A1C, 10.5% (range, 4.0% to 5.6%). Venous blood gas results include pH, 7.278 (7.32 to 7.42); PCO2, 29.6 mm Hg (39 to 54 mm Hg); HCO3, 13.8 mEq/L (19 to 25 mEq/L); base excess, –12 mmol/L (–4 to +2 mmol/L); beta hydroxybutyrate, 6.0 mmol/L (0.4 to 0.5 mmol/L); insulin antibody, 0.9 U/mL (< 0.4 U/mL); glutamic acid decarboxylase, 166 U/mL (< 0.5 U/mL); and venous lactate, 1.79 mmol/L (0.69 to 2.75 mmol/L).
The child is treated initially with an IV insulin infusion for 24 hours, then transitioned to subcutaneous insulin therapy once the DKA resolves and glucose levels are within normal limits. The child remains hospitalized for four days. Discharge medications include insulin glargine, 8 U/d, and insulin lispro before each meal, at bedtime, and at 0200 hours, with dosing based on sliding scales. Dietary orders include 45 to 60 g carbohydrates per meal, along with two snacks of 15 g carbohydrates.
The child is instructed to exercise at least 30 min/d (unless hypoglycemic events occur more than once per week or ketones are found in the blood or urine), drink plenty of water, and avoid concentrated sweets. Education is provided via the Diabetes Educator; the family takes home the beginner T1D booklet and is instructed to log the child’s blood glucose levels and return with this information in two weeks.
In the first three months, the patient experiences eight asymptomatic hypoglycemic events; for the next seven months, after dosing changes, she remains hyperglycemic most of the time (see Table 1). Insulin doses are adjusted, ranging from weekly to every three months, but glycemic goals are not achieved with the subcutaneous insulin injections. Use of continuous subcutaneous insulin infusion, the “insulin pump,” is then considered. Ten months postdiagnosis, the child begins a five-day-long saline (placebo) pump trial to determine whether the pump is appropriate for her and her lifestyle. After the trial, the decision is made to move forward with the insulin pump, initiated 11 months postdiagnosis.
The practitioner remains in frequent communication with the child’s mother in an effort to maintain glycemic control. After three months on the insulin pump, the child’s A1C is reduced to 7.9%, which is within the target range for her age-group (see Table 2). The child is now maintaining glycemic goals with the use of the insulin pump and close monitoring by the practitioner.
Continue for the discussion >>
DISCUSSION
According to the Juvenile Diabetes Research Foundation, as many as 1.25 million Americans are currently living with T1D; from 2001 to 2009, the prevalence of T1D in people younger than 20 increased by 23%.1 The overall prevalence of diabetes (both types 1 and 2) is predicted to be one in every three people by 2050 if current trends continue.2 According to the American Diabetes Association (ADA), 18,436 US youths are diagnosed with T1D every year, and T1D accounts for about 5% of diabetes cases in the US population.2
Diagnosis
Diabetes is diagnosed based on blood test results that fall within the parameters set by the ADA diagnostic criteria (see Table 3).3 In addition to diagnostic testing for diabetes recommended by the ADA guidelines, blood tests are ordered for autoantibodies that are associated with T1D, to distinguish between type 1 and type 2 diabetes. (T1D results from cellular-mediated autoimmune destruction of the insulin-producing beta cells in the pancreas.4) Upon initial diagnosis, about 85% to 90% of T1D patients have one or more autoantibodies present in blood work, such as autoantibodies to islet cells or to insulin, glutamic acid decarboxylase (GAD65), or tyrosine phosphatases IA-2 and IA-2B.4
In this case study, the child had an elevated GAD65 value and a positive screening for an insulin autoantibody, which explained the destruction of her beta cells. The patient also had KP and xerosis, which are clinical manifestations commonly seen in T1D. In one study of children with T1D, 22% had xerosis, compared with 3% of healthy, age-matched controls, and KP was also significantly more common in T1D patients than in controls (12% vs 1.5%).5
The presence of ketones in the case patient’s urine also suggests T1D, rather than type 2.4 The differential diagnosis for T1D includes type 2 diabetes mellitus, monogenic diabetes mellitus (formerly known as maturity-onset diabetes of the young), secondary hyperglycemia, and other endocrine disorders.6
Acute complications associated with T1D include hypoglycemia, hyperglycemia, and DKA. Long-term complications may include diabetic retinopathy, cataracts, gastroparesis, hypertension, renal failure, coronary artery disease, peripheral vascular disease, diabetic neuropathy, and increased risk for infection.7 These complications can likely be prevented by good glycemic control, proper diet, exercise, and avoidance of nicotine.7
Unfortunately, T1D cannot currently be prevented, although research studies are under way. TrialNet is currently conducting a “Pathway to Prevention” trial; the researchers are testing ways to delay and prevent T1D, as well as slow its progression after diagnosis.8 Potential participants (family members of a T1D patient) are screened for T1D autoantibodies. If test results are positive, these participants are included in the prevention pathway study.
Continue for management >>
Management
Most cases of T1D are diagnosed in patients younger than 18.9 Management of the child with T1D involves many challenges. The patient will experience an initial honeymoon period, that is, a brief remission during which the pancreas begins to secrete some insulin again and exogenous insulin demands are lower. However, this period is temporary, lasting only a few weeks, months, or years. Once pancreatic insulin secretion stops (as a result of complete beta-cell destruction), the exogenous insulin demands increase. In the case study, the child’s insulin demands were initially low, and she experienced hypoglycemia. Once she transitioned out of the honeymoon period, however, her blood glucose levels rose because her pancreas was producing little to no insulin.
As the patient ages, physical growth and hormone changes also alter the demand for insulin. A key factor to keep in mind is lifestyle changes: The child may need age-appropriate supervision and adjustments in exercise, diet, and diabetes education regimens when school routines and self-care capacities change. The child with T1D can only be educated as far as his or her cognitive ability will allow, but autonomy should increase with age.
Helping the patient reach glycemic goals requires special consideration, based on the child’s age. Whereas the target A1C for an adult with diabetes is below 7%, that for a young child is either < 7.5% or < 8.5%, depending on age (see Table 4).9 According to Danne et al, approximately 60% of children younger than 6 years have an imperfect awareness of hypoglycemia. 10 Because the risk for a hypoglycemic event is increased in this age-group, their target A1C is higher.10
This is also an important age for brain development: The metabolism of glucose in the brain of a young child occurs at double the rate of that in an adult brain.11 Between ages 1 and 6 years, the brain increases in size dramatically, reaching 90% of its adult volume by age 6.11 In retrospective studies reviewed by Arbelaez et al,data show that frequent, severe hypoglycemic and hyperglycemic events are associated with poor cognitive function, particularly memory and attention.11 Due to the timing of brain development and the risk for glycemic extremes in young children, practitioners are advised to follow the ADA recommendations shown in Table 4.9
Continue for the conclusion >>
CONCLUSION
T1D is the most common chronic, serious, potentially life-threatening disease among children and adolescents. This lifelong illness is challenging to control, especially when managing the honeymoon period and addressing the increasing insulin demands in a growing child. Once a diagnosis is confirmed, the challenges persist, as each patient needs an individualized treatment regimen with ongoing adjustments. Knowledge of the ADA guidelines for age-appropriate A1C goals is essential for the practitioner who manages a growing child with T1D in order to achieve glycemic control while avoiding hypoglycemia. Preventing hypoglycemia is of the utmost importance, especially in a child too young to recognize symptoms.
Considering all the changes that a child with T1D is likely to experience, it is also important to remember that the foremost goal is for this child to live a healthy life. Thus, practitioners must educate both patients and parents regarding the complications that can arise with poor glycemic control and encourage adherence to the insulin therapy.
T1D requires vigilant monitoring and ongoing adjusted insulin therapy. Understanding age-appropriate treatment and maintaining good communications with patients and their parents are key to successful management of this disease.
REFERENCES
1. Juvenile Diabetes Research Foundation. Type 1 diabetes facts (2014). http://jdrf.org/about-jdrf/fact-sheets/type-1-diabetes-facts/. Accessed February 8, 2016.
2. American Diabetes Association. Fast facts: data and statistics about diabetes (2015). http://professional2.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/Fast_Facts_12-2015a.pdf. Accessed February 8, 2016.
3. American Diabetes Association. Executive summary: standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S5-S13.
4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):81-90.
5. Pavlovic MD, Milenkovic T, Dinic M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30(8):1964-1967.
6. Khardori R. Type 1 diabetes mellitus differential diagnosis (updated 2015). http://emedicine.medscape.com/article/117739-differential. Accessed February 8, 2016.
7. Lamb WH. Pediatric type 1 diabetes mellitus (updated 2015). http://emedicine.medscape.com/article/919999-overview. Accessed February 8, 2016.
8. Type 1 Diabetes TrialNet. TrialNet Pathway to Prevention (2014). www.pathway2prevention.org/study/. Accessed February 8, 2016.
9. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66. VIII. Diabetes care in specific populations. http://care.diabetesjournals.org/content/36/Supplement_1/S11.full#sec-128. Accessed February 8, 2016.
10. Danne T, Philotheou A, Goldman D, et al. A randomized trial comparing the rate of hypoglycemia—assessed using continuous glucose monitoring—in 125 preschool children with type 1 diabetes treated with insulin glargine or NPH insulin (the PRESCHOOL study). Pediatr Diabetes. 2013;14(8):593-601.
11. Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes. 2013;14(8):541-553.