User login
Psoriasis is a T cell–mediated inflammatory disease that manifests as erythematous scaling plaques of the skin. In recent decades, our understanding of psoriasis has transformed from a disease isolated to the skin to a systemic disease impacting the overall health of those affected.
With recent elucidation of the pathways driving psoriasis, development of targeted therapies has resulted in an influx of options to the market. Navigating the options can seem overwhelming even to the seasoned clinician. Becoming familiar with a sound treatment approach during residency will create a foundation for biologic use in psoriasis patients throughout your career. Here we offer an approach to choosing biologic treatments based on individual patient characteristics, including disease severity, comorbidities, and ultimate treatment goals.
Immune Pathogenesis
Although the pathogenesis of psoriasis is complex and outside the scope of this article, we do recommend clinicians keep in mind the current understanding of pathways involved and ways our therapies alter them. Briefly, psoriasis is a T cell–mediated disease in which IL-12 and IL-23 released by activated dendritic cells activate T helper cells including TH1, TH17, and TH22. These cells produce additional cytokines, including IFN-γ, tumor necrosis factor (TNF) α, IL-17, and IL-22, which propagate the immune response and lead to keratinocyte hyperproliferation. In general, psoriasis medications work by altering T-cell activation, effector cytokines, or cytokine receptors.
Comorbidities
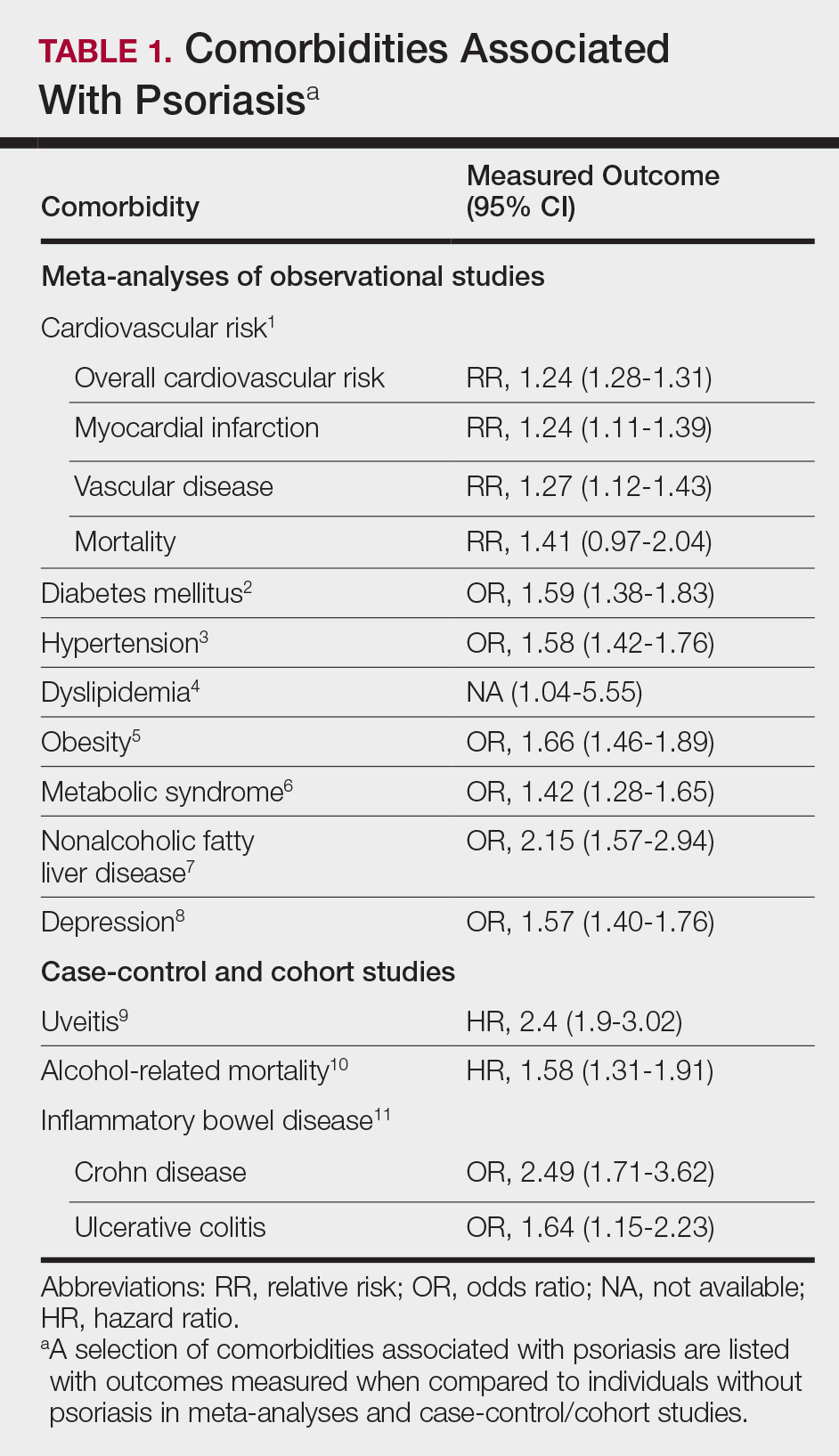
A targeted approach should take into consideration the immune dysregulation shared by psoriasis and associated comorbidities (Table 1). One goal of biologic treatments is to improve comorbidities when possible. At minimum, selected treatments should not exacerbate these conditions.
Treatment Goals
Establishing treatment goals can help shape patient expectations and provide a plan for clinicians. In 2017, the National Psoriasis Foundation published a treat-to-target approach using body surface area (BSA) measurements at baseline, 3 months, and then every 6 months after starting a new treatment.12 The target response is a decrease in psoriasis to 1% or less BSA at 3 months and to maintain this response when evaluated at 6-month intervals. Alternatively, a target of 3% BSA after 3 months is satisfactory if the patient improves by 75% BSA overall. If these targets are not met after 6 months, therapeutic alternatives can be considered.12
Biologic Treatment of Psoriasis
Treatment options for patients with psoriasis depend first on disease severity. Topicals and phototherapy are first line for mild to moderate disease. For moderate to severe disease, addition of systemic agents such as methotrexate, cyclosporine, or acitretin; small-molecular-weight immunomodulators such as apremilast; or biologic medications should be considered. Current biologics available for moderate to severe plaque psoriasis target TNF-α, IL-12/IL-23, IL-23, IL-17A, or IL-17A receptor.
TNF-α Inhibitors
Tumor necrosis factor α inhibitors have been available for treatment of autoimmune disease for nearly 20 years. These medications block either soluble cytokine or membrane-bound cytokine. All are given as subcutaneous injections, except for infliximab, which is a weight-based infusion.
Efficacy
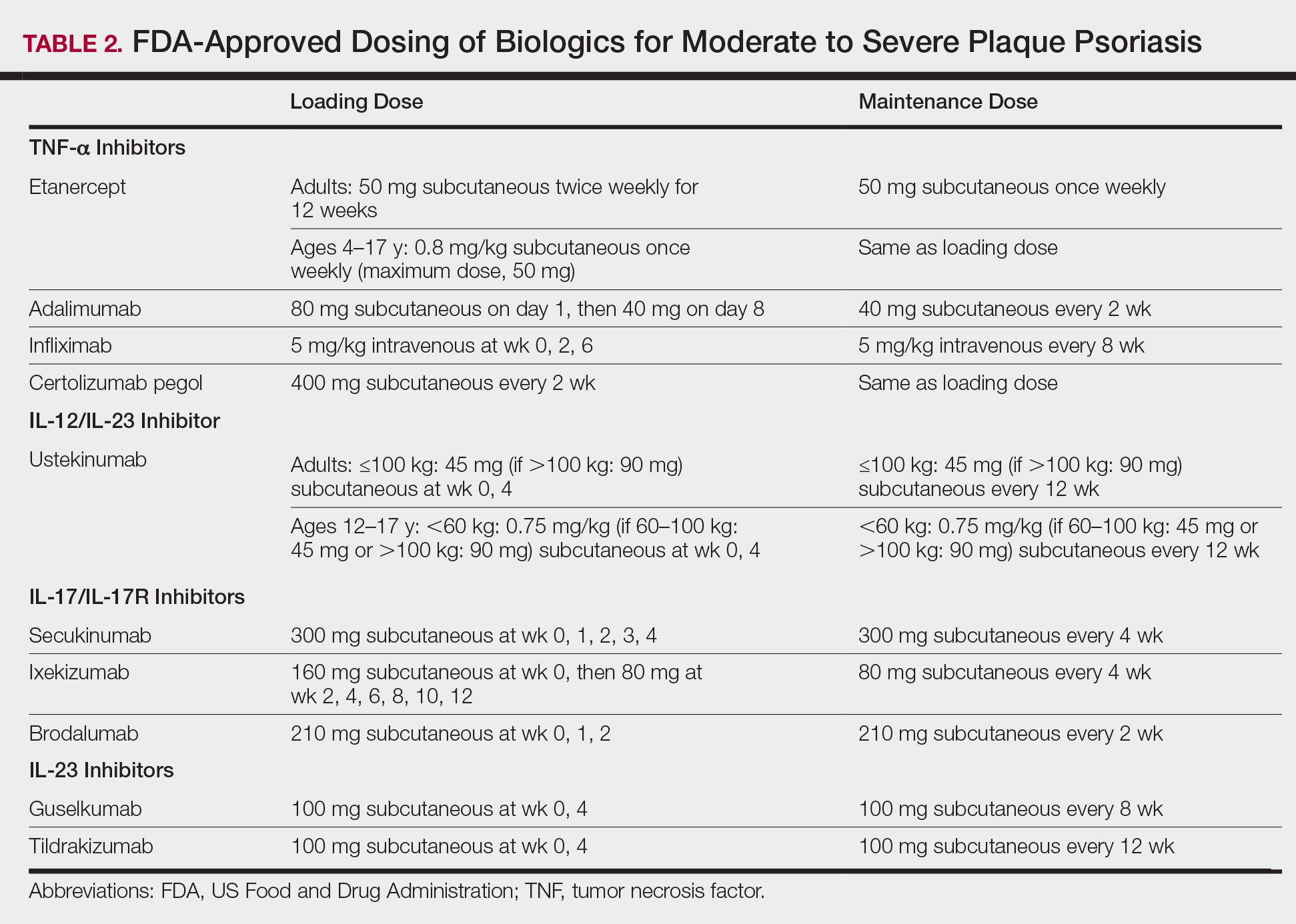
Tumor necrosis factor α inhibitors are the first class to demonstrate long-term efficacy and safety in both psoriasis and psoriatic arthritis (PsA). Etanercept was approved for adults with PsA in 2002 and psoriasis in 2004, and later for pediatric psoriasis (≥4 years of age) in 2016 (Table 2). Although etanercept has a sustained safety profile, the response rates are not as high as other anti–TNF-α inhibitors. Adalimumab is one of the most prescribed biologics, with a total of 10 indications at present, including PsA. Infliximab is an intravenous infusion that demonstrates a rapid and sustained response in most patients. The dose and dosing interval can be adjusted according to response. Certolizumab pegol was approved for PsA in 2013 and for psoriasis in 2018.
Tumor necrosis factor α inhibitors maintain efficacy well and work best when dosed continuously. Both neutralizing and nonneutralizing antibodies form with these agents. Neutralizing antibodies may contribute to decreased efficacy, particularly for the chimeric antibody infliximab. One approach to mitigate loss of efficacy is the short-term addition of low-dose methotrexate (eg, 7.5–15 mg weekly) for 3 to 6 months until response is recaptured.
Safety
To evaluate long-term safety, a multicenter prospective registry study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]) was initiated in 2007 to follow clinical outcomes. Data through 2013 showed no significant increase in rates of infection, malignancy, or major adverse cardiovascular events in more than 12,000 patients.13
Conflicting information exists in the literature regarding risk for malignancy with TNF-α inhibitors. One recent retrospective cohort study suggested a slightly increased risk for malignancies other than nonmelanoma skin cancers in patients on TNF-α inhibitors for more than 12 months (relative risk, 1.54).14 Reports of increased risk for cutaneous squamous cell carcinomas necessitate regular skin checks.15 A potential risk for lymphoma has been noted, though having psoriasis itself imparts an increased risk for Hodgkin and cutaneous T-cell lymphoma.16
Reactivation of tuberculosis and hepatitis have been reported with TNF-α inhibition. Data suggest that infliximab may be associated with more serious infections.13
Demyelinating conditions such as multiple sclerosis have occurred de novo or worsened in patients on TNF-α inhibitors.17 Tumor necrosis factor α blockers should be avoided in patients with decompensated heart failure. Rare cases of liver enzyme elevation and cytopenia have been noted. Additionally, lupuslike syndromes, which are generally reversible upon discontinuation, have occurred in some patients.
Patient Selection
Tumor necrosis factor α inhibitors are the treatment of choice for patients with comorbid PsA. This class halts progression of joint destruction over time.18Select TNF-α inhibitors are indicated for inflammatory bowel disease (IBD) and are a preferred treatment in this patient population. Specifically, adalimumab and infliximab are approved for both Crohn disease (CD) and ulcerative colitis. Certolizumab pegol is approved for CD.
Tumor necrosis factor α is upregulated in obesity, cardiovascular disease, and atherosclerotic plaques. Evidence suggests that TNF-α blockers may lower cardiovascular risk over time.19 For patients with obesity, infliximab is a good option, as it is the only TNF-α inhibitor with weight-based dosing.
In patients with frequent infections or history of hepatitis C, etanercept has been the biologic most commonly used when no alternatives exist, in part due to its shorter half-life.
IL-12/IL-23 Inhibitor
Ustekinumab is a monoclonal antibody that binds the p40 subunit shared by IL-12 and IL-23, blocking their ability to bind receptors. IL-12 and IL-23 play a role in activating naïve T cells to become TH1 or TH17 cells, respectively.
Efficacy and Safety
Clinical trials demonstrate long-term efficacy of ustekinumab, which was approved for psoriasis in 2009, PsA in 2013, and later pediatric psoriasis (≥12 years of age) in 2017. Dosing is listed in Table 2.
Laboratory abnormalities did not arise in trials. Periodic tuberculosis screening is required. Prospective data over 5 years showed very low rates of adverse events (AEs), serious infections, malignancies, and major adverse cardiovascular events.20 Ustekinumab did not worsen or improve demyelinating disease and appears safe in this population.
Patient Selection
Ustekinumab is approved for PsA and is a good option for those who are not candidates for TNF-α and IL-17 inhibitors. Ustekinumab also is approved for CD. The dosing interval of 12 weeks makes ustekinumab convenient for patients. Two dosages exist based on the patient’s weight, offering an advantage to obese patients.
IL-17/IL-17R Inhibitors
Activated TH17 cells produce the IL-17 cytokine family, which stimulates keratinocyte proliferation and dermal inflammation. Secukinumab is a fully human monoclonal antibody, and ixekizumab is a humanized monoclonal antibody; both target IL-17A. Brodalumab targets the IL-17A receptor.
Efficacy and Safety
IL-17 inhibitors showed impressive and rapid responses in trials.21-23 The subsets of patients who responded well and continued treatment in extension trials demonstrated that these treatments maintain efficacy over time.24-26
In addition to tuberculosis reactivation, there is a small increased risk for cutaneous candidiasis with IL-17 inhibitors, which can be managed without stopping treatment. Laboratory abnormalities were limited to mild neutropenia, which was not associated with increased risk for infection.21-23 With ixekizumab, neutropenia was seen more commonly in the first 12 weeks.22
IL-17 is highly expressed in the gut mucosa, and its inhibition is thought to weaken the barrier function of the gut mucosa, promoting inflammation. As a consequence, this class is contraindicated in patients with IBD due to exacerbations of existing IBD and cases of new-onset IBD.21-23 Symptoms of diarrhea, abdominal pain, blood in stool, or nighttime stooling on review of gastrointestinal tract symptoms should prompt further evaluation.
Brodalumab has a unique warning for risk for suicidal ideation and behavior.23 Depression is more common in the psoriasis population in general; therefore, physicians should be aware of this potential comorbidity regardless of the treatment plan. Because the response rates are so impressive with brodalumab, the Risk Evaluation and Mitigation Strategy (REMS) program was established to ensure understanding of this risk so that patients can be appropriately counseled and managed.
Patient Selection
The improvement in psoriasis is rapid and may occur as early as week 2 to 3 of treatment after initiation of IL-17 inhibitors. Ixekizumab and secukinumab also are approved for PsA. Although improvement in joint disease is not as fast as with the anti-TNF inhibitors, notable improvement occurs by week 20 to 24.27
IL-23 Inhibitors
Guselkumab and tildrakizumab are the newest biologics for psoriasis, approved in 2017 and 2018, respectively. Both are monoclonal antibodies against the p19 subunit of IL-23, which blocks activation of TH17 cells.
Efficacy and Safety
Guselkumab and tildrakizumab demonstrated efficacy with minimal AEs or precautions noted thus far.28,29 Infections are again a risk, making tuberculosis testing the only recommended monitoring.
Patient Selection
Both medications offer another effective and safe option for patients with psoriasis. Similar to ustekinumab, the dosing interval of 12 weeks for tildrakizumab is ideal for patients who have needle phobia or are unable to administer their own injections.
Special Populations
Pregnancy
Antibodies cross the placenta as pregnancy progresses, with the highest rate in the third trimester. Certolizumab pegol has shown the lowest concentrations in infant serum, possibly due to its unique structure lacking the fragment crystallizable region required for passage through the placenta.30 For this reason, certolizumab pegol is a treatment of choice if biologic therapy is warranted during pregnancy.
Much of the pregnancy data for the remaining TNF-α inhibitors come from patients with rheumatoid arthritis or CD. In these populations, rates of major birth defects and miscarriages do not differ greatly from untreated women with these conditions.31 One retrospective study of unintentional pregnancies in women receiving ustekinumab showed rates of AEs similar to the general population.32
Pregnancy data for IL-17 or IL-23 inhibitors are largely limited to animal studies. One retrospective study of women exposed to secukinumab early in gestation showed no increased risk for pregnancy-related AEs.33 Discontinuation is still recommended for patients who become pregnant.
Pediatric Patients
Etanercept is approved for pediatric psoriatic patients 4 years and older. Children with juvenile idiopathic arthritis who are 2 years and older can receive etanercept. Ustekinumab is safe and effective for pediatric psoriatic patients 12 years and older, offering a second biologic option in children.
Although not approved for pediatric psoriasis, adalimumab is approved in pediatric CD (≥6 years of age) and for juvenile idiopathic arthritis (≥2 years of age). Infliximab is approved for children 6 years and older with CD or ulcerative colitis.
Monitoring
Periodic tuberculosis screening is recommended for all biologics. For patients with latent tuberculosis, biologics may be restarted after 1 month of treatment of tuberculosis.
Prior to initiation of biologics, patients should be screened for hepatitis with hepatitis B surface antigen and antibody, hepatitis B core antibody, and hepatitis C antibody. Patients at risk for human immunodeficiency virus also should be screened.
Generally, complete blood cell count and comprehensive metabolic profile are advisable prior to starting a biologic. Opinions differ on frequency of repeating laboratory work. Complete blood cell count and comprehensive metabolic profile should be monitored at least every 3 to 6 months in patients on TNF-α inhibitors, and neutrophil count should be monitored during the induction phase of IL-17 inhibitors.
All patients with psoriasis should maintain age-appropriate cancer screenings, especially those on biologics. If malignancy is discovered, biologic medication should be discontinued. Debate exists as to when therapy can be safely restarted following treatment of malignancy. Patients who are considered at low risk for recurrence may opt to restart a biologic after 5 years, or sooner if symptoms warrant.34 This decision should involve the patient’s cancer specialist.
Conclusion
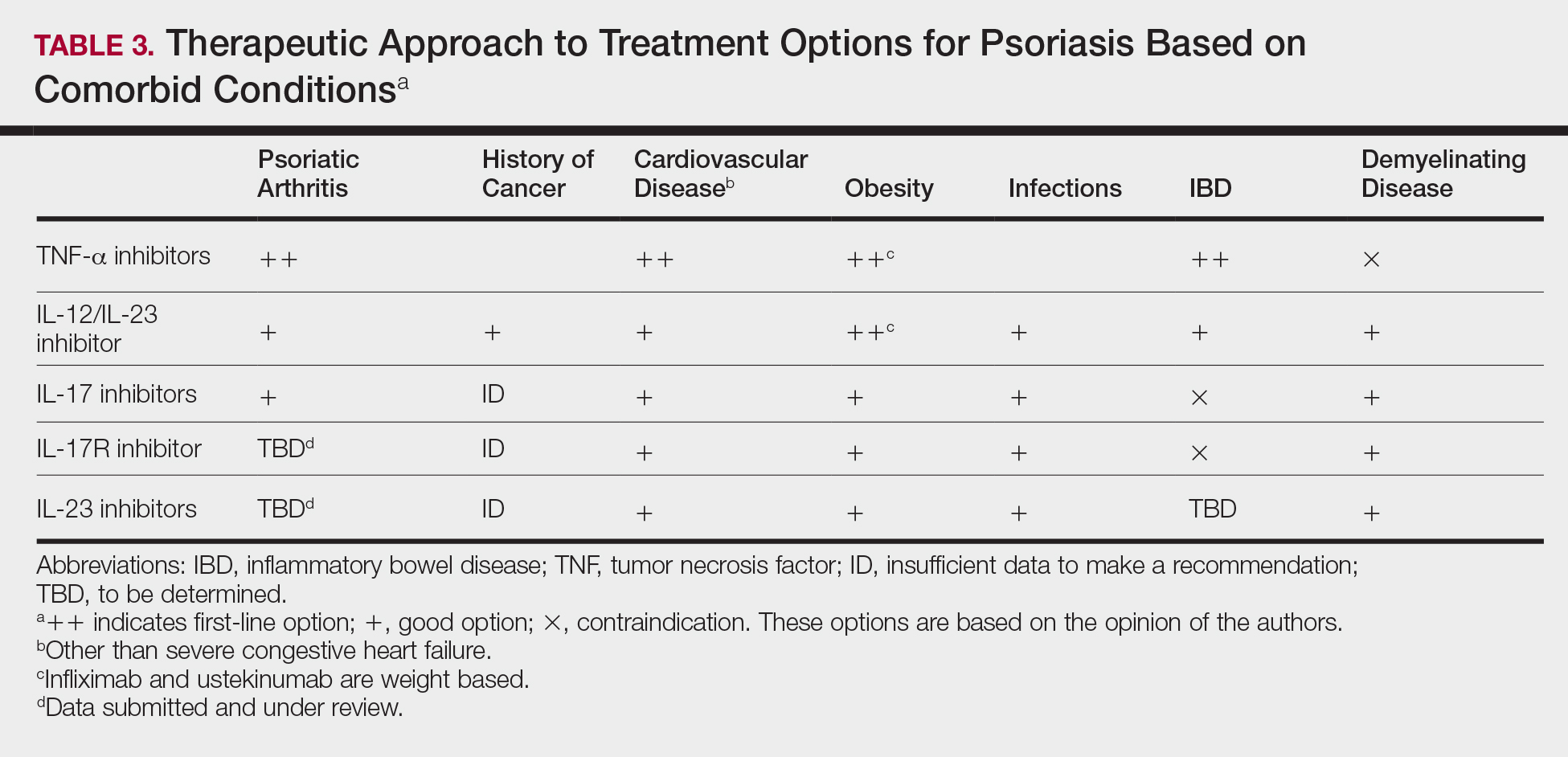
Treatment choices are based on psoriasis type and severity, comorbidities, patient preferences, and drug accessibility. One approach is detailed in Table 3. As research advances the understanding of psoriasis, this field will continue to rapidly change. Knowledge of the immunopathogenesis of psoriasis and its relation to comorbidities can direct your decision-making for individual patients.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84-91.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-433.
- Candia R, Ruiz A, Torres-Robles R, et al. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662.
- Chi CC, Tung TH, Wang J, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135:415-422.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Gaeta M, Castelvecchio S, Ricci C, et al. Role of psoriasis as independent predictor of cardiovascular disease: a meta-regression analysis. Int J Cardiol. 2013;168:2282-2288.
- Ma C, Harskamp CT, Armstrong EJ, et al. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168:486-495.
- Parisi R, Webb RT, Carr MJ, et al. Alcohol-related mortality in patients with psoriasis: a population-based cohort study. JAMA Dermatol. 2017;153:1256-1262.
- Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77:657-666.e8.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- van Lümig PP, Menting SP, van den Reek JM, et al. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J Eur Acad Dermatol Venereol. 2015;29:752-760.
- Gelfand JM, Berlin J, Van Voorhees A, et al. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139:1425-1429.
- Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885-1888.
- Finckh A, Simard JF, Duryea J, et al. The effectiveness of anti-tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2006;54:54-59.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79:824-830.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.e1183.
- Gottlieb AB, Strand V, Kishimoto M, et al. Ixekizumab improves patient-reported outcomes up to 52 weeks in bDMARD-naïve patients with active psoriatic arthritis (SPIRIT-P1). Rheumatology (Oxford). 2018;57:1777-1788.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Komaki F, Komaki Y, Micic D, et al. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J Autoimmun. 2017;76:38-52.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database [published online ahead of print June 21, 2018]. Br J Dermatol. doi:10.1111/bjd.16901.
- Elandt K, Aletaha D. Treating rheumatic patients with a malignancy. Arthritis Res Ther. 2011;13:223.
Psoriasis is a T cell–mediated inflammatory disease that manifests as erythematous scaling plaques of the skin. In recent decades, our understanding of psoriasis has transformed from a disease isolated to the skin to a systemic disease impacting the overall health of those affected.
With recent elucidation of the pathways driving psoriasis, development of targeted therapies has resulted in an influx of options to the market. Navigating the options can seem overwhelming even to the seasoned clinician. Becoming familiar with a sound treatment approach during residency will create a foundation for biologic use in psoriasis patients throughout your career. Here we offer an approach to choosing biologic treatments based on individual patient characteristics, including disease severity, comorbidities, and ultimate treatment goals.
Immune Pathogenesis
Although the pathogenesis of psoriasis is complex and outside the scope of this article, we do recommend clinicians keep in mind the current understanding of pathways involved and ways our therapies alter them. Briefly, psoriasis is a T cell–mediated disease in which IL-12 and IL-23 released by activated dendritic cells activate T helper cells including TH1, TH17, and TH22. These cells produce additional cytokines, including IFN-γ, tumor necrosis factor (TNF) α, IL-17, and IL-22, which propagate the immune response and lead to keratinocyte hyperproliferation. In general, psoriasis medications work by altering T-cell activation, effector cytokines, or cytokine receptors.
Comorbidities
A targeted approach should take into consideration the immune dysregulation shared by psoriasis and associated comorbidities (Table 1). One goal of biologic treatments is to improve comorbidities when possible. At minimum, selected treatments should not exacerbate these conditions.

Treatment Goals
Establishing treatment goals can help shape patient expectations and provide a plan for clinicians. In 2017, the National Psoriasis Foundation published a treat-to-target approach using body surface area (BSA) measurements at baseline, 3 months, and then every 6 months after starting a new treatment.12 The target response is a decrease in psoriasis to 1% or less BSA at 3 months and to maintain this response when evaluated at 6-month intervals. Alternatively, a target of 3% BSA after 3 months is satisfactory if the patient improves by 75% BSA overall. If these targets are not met after 6 months, therapeutic alternatives can be considered.12
Biologic Treatment of Psoriasis
Treatment options for patients with psoriasis depend first on disease severity. Topicals and phototherapy are first line for mild to moderate disease. For moderate to severe disease, addition of systemic agents such as methotrexate, cyclosporine, or acitretin; small-molecular-weight immunomodulators such as apremilast; or biologic medications should be considered. Current biologics available for moderate to severe plaque psoriasis target TNF-α, IL-12/IL-23, IL-23, IL-17A, or IL-17A receptor.
TNF-α Inhibitors
Tumor necrosis factor α inhibitors have been available for treatment of autoimmune disease for nearly 20 years. These medications block either soluble cytokine or membrane-bound cytokine. All are given as subcutaneous injections, except for infliximab, which is a weight-based infusion.
Efficacy
Tumor necrosis factor α inhibitors are the first class to demonstrate long-term efficacy and safety in both psoriasis and psoriatic arthritis (PsA). Etanercept was approved for adults with PsA in 2002 and psoriasis in 2004, and later for pediatric psoriasis (≥4 years of age) in 2016 (Table 2). Although etanercept has a sustained safety profile, the response rates are not as high as other anti–TNF-α inhibitors. Adalimumab is one of the most prescribed biologics, with a total of 10 indications at present, including PsA. Infliximab is an intravenous infusion that demonstrates a rapid and sustained response in most patients. The dose and dosing interval can be adjusted according to response. Certolizumab pegol was approved for PsA in 2013 and for psoriasis in 2018.

Tumor necrosis factor α inhibitors maintain efficacy well and work best when dosed continuously. Both neutralizing and nonneutralizing antibodies form with these agents. Neutralizing antibodies may contribute to decreased efficacy, particularly for the chimeric antibody infliximab. One approach to mitigate loss of efficacy is the short-term addition of low-dose methotrexate (eg, 7.5–15 mg weekly) for 3 to 6 months until response is recaptured.
Safety
To evaluate long-term safety, a multicenter prospective registry study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]) was initiated in 2007 to follow clinical outcomes. Data through 2013 showed no significant increase in rates of infection, malignancy, or major adverse cardiovascular events in more than 12,000 patients.13
Conflicting information exists in the literature regarding risk for malignancy with TNF-α inhibitors. One recent retrospective cohort study suggested a slightly increased risk for malignancies other than nonmelanoma skin cancers in patients on TNF-α inhibitors for more than 12 months (relative risk, 1.54).14 Reports of increased risk for cutaneous squamous cell carcinomas necessitate regular skin checks.15 A potential risk for lymphoma has been noted, though having psoriasis itself imparts an increased risk for Hodgkin and cutaneous T-cell lymphoma.16
Reactivation of tuberculosis and hepatitis have been reported with TNF-α inhibition. Data suggest that infliximab may be associated with more serious infections.13
Demyelinating conditions such as multiple sclerosis have occurred de novo or worsened in patients on TNF-α inhibitors.17 Tumor necrosis factor α blockers should be avoided in patients with decompensated heart failure. Rare cases of liver enzyme elevation and cytopenia have been noted. Additionally, lupuslike syndromes, which are generally reversible upon discontinuation, have occurred in some patients.
Patient Selection
Tumor necrosis factor α inhibitors are the treatment of choice for patients with comorbid PsA. This class halts progression of joint destruction over time.18Select TNF-α inhibitors are indicated for inflammatory bowel disease (IBD) and are a preferred treatment in this patient population. Specifically, adalimumab and infliximab are approved for both Crohn disease (CD) and ulcerative colitis. Certolizumab pegol is approved for CD.
Tumor necrosis factor α is upregulated in obesity, cardiovascular disease, and atherosclerotic plaques. Evidence suggests that TNF-α blockers may lower cardiovascular risk over time.19 For patients with obesity, infliximab is a good option, as it is the only TNF-α inhibitor with weight-based dosing.
In patients with frequent infections or history of hepatitis C, etanercept has been the biologic most commonly used when no alternatives exist, in part due to its shorter half-life.
IL-12/IL-23 Inhibitor
Ustekinumab is a monoclonal antibody that binds the p40 subunit shared by IL-12 and IL-23, blocking their ability to bind receptors. IL-12 and IL-23 play a role in activating naïve T cells to become TH1 or TH17 cells, respectively.
Efficacy and Safety
Clinical trials demonstrate long-term efficacy of ustekinumab, which was approved for psoriasis in 2009, PsA in 2013, and later pediatric psoriasis (≥12 years of age) in 2017. Dosing is listed in Table 2.
Laboratory abnormalities did not arise in trials. Periodic tuberculosis screening is required. Prospective data over 5 years showed very low rates of adverse events (AEs), serious infections, malignancies, and major adverse cardiovascular events.20 Ustekinumab did not worsen or improve demyelinating disease and appears safe in this population.
Patient Selection
Ustekinumab is approved for PsA and is a good option for those who are not candidates for TNF-α and IL-17 inhibitors. Ustekinumab also is approved for CD. The dosing interval of 12 weeks makes ustekinumab convenient for patients. Two dosages exist based on the patient’s weight, offering an advantage to obese patients.
IL-17/IL-17R Inhibitors
Activated TH17 cells produce the IL-17 cytokine family, which stimulates keratinocyte proliferation and dermal inflammation. Secukinumab is a fully human monoclonal antibody, and ixekizumab is a humanized monoclonal antibody; both target IL-17A. Brodalumab targets the IL-17A receptor.
Efficacy and Safety
IL-17 inhibitors showed impressive and rapid responses in trials.21-23 The subsets of patients who responded well and continued treatment in extension trials demonstrated that these treatments maintain efficacy over time.24-26
In addition to tuberculosis reactivation, there is a small increased risk for cutaneous candidiasis with IL-17 inhibitors, which can be managed without stopping treatment. Laboratory abnormalities were limited to mild neutropenia, which was not associated with increased risk for infection.21-23 With ixekizumab, neutropenia was seen more commonly in the first 12 weeks.22
IL-17 is highly expressed in the gut mucosa, and its inhibition is thought to weaken the barrier function of the gut mucosa, promoting inflammation. As a consequence, this class is contraindicated in patients with IBD due to exacerbations of existing IBD and cases of new-onset IBD.21-23 Symptoms of diarrhea, abdominal pain, blood in stool, or nighttime stooling on review of gastrointestinal tract symptoms should prompt further evaluation.
Brodalumab has a unique warning for risk for suicidal ideation and behavior.23 Depression is more common in the psoriasis population in general; therefore, physicians should be aware of this potential comorbidity regardless of the treatment plan. Because the response rates are so impressive with brodalumab, the Risk Evaluation and Mitigation Strategy (REMS) program was established to ensure understanding of this risk so that patients can be appropriately counseled and managed.
Patient Selection
The improvement in psoriasis is rapid and may occur as early as week 2 to 3 of treatment after initiation of IL-17 inhibitors. Ixekizumab and secukinumab also are approved for PsA. Although improvement in joint disease is not as fast as with the anti-TNF inhibitors, notable improvement occurs by week 20 to 24.27
IL-23 Inhibitors
Guselkumab and tildrakizumab are the newest biologics for psoriasis, approved in 2017 and 2018, respectively. Both are monoclonal antibodies against the p19 subunit of IL-23, which blocks activation of TH17 cells.
Efficacy and Safety
Guselkumab and tildrakizumab demonstrated efficacy with minimal AEs or precautions noted thus far.28,29 Infections are again a risk, making tuberculosis testing the only recommended monitoring.
Patient Selection
Both medications offer another effective and safe option for patients with psoriasis. Similar to ustekinumab, the dosing interval of 12 weeks for tildrakizumab is ideal for patients who have needle phobia or are unable to administer their own injections.
Special Populations
Pregnancy
Antibodies cross the placenta as pregnancy progresses, with the highest rate in the third trimester. Certolizumab pegol has shown the lowest concentrations in infant serum, possibly due to its unique structure lacking the fragment crystallizable region required for passage through the placenta.30 For this reason, certolizumab pegol is a treatment of choice if biologic therapy is warranted during pregnancy.
Much of the pregnancy data for the remaining TNF-α inhibitors come from patients with rheumatoid arthritis or CD. In these populations, rates of major birth defects and miscarriages do not differ greatly from untreated women with these conditions.31 One retrospective study of unintentional pregnancies in women receiving ustekinumab showed rates of AEs similar to the general population.32
Pregnancy data for IL-17 or IL-23 inhibitors are largely limited to animal studies. One retrospective study of women exposed to secukinumab early in gestation showed no increased risk for pregnancy-related AEs.33 Discontinuation is still recommended for patients who become pregnant.
Pediatric Patients
Etanercept is approved for pediatric psoriatic patients 4 years and older. Children with juvenile idiopathic arthritis who are 2 years and older can receive etanercept. Ustekinumab is safe and effective for pediatric psoriatic patients 12 years and older, offering a second biologic option in children.
Although not approved for pediatric psoriasis, adalimumab is approved in pediatric CD (≥6 years of age) and for juvenile idiopathic arthritis (≥2 years of age). Infliximab is approved for children 6 years and older with CD or ulcerative colitis.
Monitoring
Periodic tuberculosis screening is recommended for all biologics. For patients with latent tuberculosis, biologics may be restarted after 1 month of treatment of tuberculosis.
Prior to initiation of biologics, patients should be screened for hepatitis with hepatitis B surface antigen and antibody, hepatitis B core antibody, and hepatitis C antibody. Patients at risk for human immunodeficiency virus also should be screened.
Generally, complete blood cell count and comprehensive metabolic profile are advisable prior to starting a biologic. Opinions differ on frequency of repeating laboratory work. Complete blood cell count and comprehensive metabolic profile should be monitored at least every 3 to 6 months in patients on TNF-α inhibitors, and neutrophil count should be monitored during the induction phase of IL-17 inhibitors.
All patients with psoriasis should maintain age-appropriate cancer screenings, especially those on biologics. If malignancy is discovered, biologic medication should be discontinued. Debate exists as to when therapy can be safely restarted following treatment of malignancy. Patients who are considered at low risk for recurrence may opt to restart a biologic after 5 years, or sooner if symptoms warrant.34 This decision should involve the patient’s cancer specialist.

Conclusion
Treatment choices are based on psoriasis type and severity, comorbidities, patient preferences, and drug accessibility. One approach is detailed in Table 3. As research advances the understanding of psoriasis, this field will continue to rapidly change. Knowledge of the immunopathogenesis of psoriasis and its relation to comorbidities can direct your decision-making for individual patients.
Psoriasis is a T cell–mediated inflammatory disease that manifests as erythematous scaling plaques of the skin. In recent decades, our understanding of psoriasis has transformed from a disease isolated to the skin to a systemic disease impacting the overall health of those affected.
With recent elucidation of the pathways driving psoriasis, development of targeted therapies has resulted in an influx of options to the market. Navigating the options can seem overwhelming even to the seasoned clinician. Becoming familiar with a sound treatment approach during residency will create a foundation for biologic use in psoriasis patients throughout your career. Here we offer an approach to choosing biologic treatments based on individual patient characteristics, including disease severity, comorbidities, and ultimate treatment goals.
Immune Pathogenesis
Although the pathogenesis of psoriasis is complex and outside the scope of this article, we do recommend clinicians keep in mind the current understanding of pathways involved and ways our therapies alter them. Briefly, psoriasis is a T cell–mediated disease in which IL-12 and IL-23 released by activated dendritic cells activate T helper cells including TH1, TH17, and TH22. These cells produce additional cytokines, including IFN-γ, tumor necrosis factor (TNF) α, IL-17, and IL-22, which propagate the immune response and lead to keratinocyte hyperproliferation. In general, psoriasis medications work by altering T-cell activation, effector cytokines, or cytokine receptors.
Comorbidities
A targeted approach should take into consideration the immune dysregulation shared by psoriasis and associated comorbidities (Table 1). One goal of biologic treatments is to improve comorbidities when possible. At minimum, selected treatments should not exacerbate these conditions.

Treatment Goals
Establishing treatment goals can help shape patient expectations and provide a plan for clinicians. In 2017, the National Psoriasis Foundation published a treat-to-target approach using body surface area (BSA) measurements at baseline, 3 months, and then every 6 months after starting a new treatment.12 The target response is a decrease in psoriasis to 1% or less BSA at 3 months and to maintain this response when evaluated at 6-month intervals. Alternatively, a target of 3% BSA after 3 months is satisfactory if the patient improves by 75% BSA overall. If these targets are not met after 6 months, therapeutic alternatives can be considered.12
Biologic Treatment of Psoriasis
Treatment options for patients with psoriasis depend first on disease severity. Topicals and phototherapy are first line for mild to moderate disease. For moderate to severe disease, addition of systemic agents such as methotrexate, cyclosporine, or acitretin; small-molecular-weight immunomodulators such as apremilast; or biologic medications should be considered. Current biologics available for moderate to severe plaque psoriasis target TNF-α, IL-12/IL-23, IL-23, IL-17A, or IL-17A receptor.
TNF-α Inhibitors
Tumor necrosis factor α inhibitors have been available for treatment of autoimmune disease for nearly 20 years. These medications block either soluble cytokine or membrane-bound cytokine. All are given as subcutaneous injections, except for infliximab, which is a weight-based infusion.
Efficacy
Tumor necrosis factor α inhibitors are the first class to demonstrate long-term efficacy and safety in both psoriasis and psoriatic arthritis (PsA). Etanercept was approved for adults with PsA in 2002 and psoriasis in 2004, and later for pediatric psoriasis (≥4 years of age) in 2016 (Table 2). Although etanercept has a sustained safety profile, the response rates are not as high as other anti–TNF-α inhibitors. Adalimumab is one of the most prescribed biologics, with a total of 10 indications at present, including PsA. Infliximab is an intravenous infusion that demonstrates a rapid and sustained response in most patients. The dose and dosing interval can be adjusted according to response. Certolizumab pegol was approved for PsA in 2013 and for psoriasis in 2018.

Tumor necrosis factor α inhibitors maintain efficacy well and work best when dosed continuously. Both neutralizing and nonneutralizing antibodies form with these agents. Neutralizing antibodies may contribute to decreased efficacy, particularly for the chimeric antibody infliximab. One approach to mitigate loss of efficacy is the short-term addition of low-dose methotrexate (eg, 7.5–15 mg weekly) for 3 to 6 months until response is recaptured.
Safety
To evaluate long-term safety, a multicenter prospective registry study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]) was initiated in 2007 to follow clinical outcomes. Data through 2013 showed no significant increase in rates of infection, malignancy, or major adverse cardiovascular events in more than 12,000 patients.13
Conflicting information exists in the literature regarding risk for malignancy with TNF-α inhibitors. One recent retrospective cohort study suggested a slightly increased risk for malignancies other than nonmelanoma skin cancers in patients on TNF-α inhibitors for more than 12 months (relative risk, 1.54).14 Reports of increased risk for cutaneous squamous cell carcinomas necessitate regular skin checks.15 A potential risk for lymphoma has been noted, though having psoriasis itself imparts an increased risk for Hodgkin and cutaneous T-cell lymphoma.16
Reactivation of tuberculosis and hepatitis have been reported with TNF-α inhibition. Data suggest that infliximab may be associated with more serious infections.13
Demyelinating conditions such as multiple sclerosis have occurred de novo or worsened in patients on TNF-α inhibitors.17 Tumor necrosis factor α blockers should be avoided in patients with decompensated heart failure. Rare cases of liver enzyme elevation and cytopenia have been noted. Additionally, lupuslike syndromes, which are generally reversible upon discontinuation, have occurred in some patients.
Patient Selection
Tumor necrosis factor α inhibitors are the treatment of choice for patients with comorbid PsA. This class halts progression of joint destruction over time.18Select TNF-α inhibitors are indicated for inflammatory bowel disease (IBD) and are a preferred treatment in this patient population. Specifically, adalimumab and infliximab are approved for both Crohn disease (CD) and ulcerative colitis. Certolizumab pegol is approved for CD.
Tumor necrosis factor α is upregulated in obesity, cardiovascular disease, and atherosclerotic plaques. Evidence suggests that TNF-α blockers may lower cardiovascular risk over time.19 For patients with obesity, infliximab is a good option, as it is the only TNF-α inhibitor with weight-based dosing.
In patients with frequent infections or history of hepatitis C, etanercept has been the biologic most commonly used when no alternatives exist, in part due to its shorter half-life.
IL-12/IL-23 Inhibitor
Ustekinumab is a monoclonal antibody that binds the p40 subunit shared by IL-12 and IL-23, blocking their ability to bind receptors. IL-12 and IL-23 play a role in activating naïve T cells to become TH1 or TH17 cells, respectively.
Efficacy and Safety
Clinical trials demonstrate long-term efficacy of ustekinumab, which was approved for psoriasis in 2009, PsA in 2013, and later pediatric psoriasis (≥12 years of age) in 2017. Dosing is listed in Table 2.
Laboratory abnormalities did not arise in trials. Periodic tuberculosis screening is required. Prospective data over 5 years showed very low rates of adverse events (AEs), serious infections, malignancies, and major adverse cardiovascular events.20 Ustekinumab did not worsen or improve demyelinating disease and appears safe in this population.
Patient Selection
Ustekinumab is approved for PsA and is a good option for those who are not candidates for TNF-α and IL-17 inhibitors. Ustekinumab also is approved for CD. The dosing interval of 12 weeks makes ustekinumab convenient for patients. Two dosages exist based on the patient’s weight, offering an advantage to obese patients.
IL-17/IL-17R Inhibitors
Activated TH17 cells produce the IL-17 cytokine family, which stimulates keratinocyte proliferation and dermal inflammation. Secukinumab is a fully human monoclonal antibody, and ixekizumab is a humanized monoclonal antibody; both target IL-17A. Brodalumab targets the IL-17A receptor.
Efficacy and Safety
IL-17 inhibitors showed impressive and rapid responses in trials.21-23 The subsets of patients who responded well and continued treatment in extension trials demonstrated that these treatments maintain efficacy over time.24-26
In addition to tuberculosis reactivation, there is a small increased risk for cutaneous candidiasis with IL-17 inhibitors, which can be managed without stopping treatment. Laboratory abnormalities were limited to mild neutropenia, which was not associated with increased risk for infection.21-23 With ixekizumab, neutropenia was seen more commonly in the first 12 weeks.22
IL-17 is highly expressed in the gut mucosa, and its inhibition is thought to weaken the barrier function of the gut mucosa, promoting inflammation. As a consequence, this class is contraindicated in patients with IBD due to exacerbations of existing IBD and cases of new-onset IBD.21-23 Symptoms of diarrhea, abdominal pain, blood in stool, or nighttime stooling on review of gastrointestinal tract symptoms should prompt further evaluation.
Brodalumab has a unique warning for risk for suicidal ideation and behavior.23 Depression is more common in the psoriasis population in general; therefore, physicians should be aware of this potential comorbidity regardless of the treatment plan. Because the response rates are so impressive with brodalumab, the Risk Evaluation and Mitigation Strategy (REMS) program was established to ensure understanding of this risk so that patients can be appropriately counseled and managed.
Patient Selection
The improvement in psoriasis is rapid and may occur as early as week 2 to 3 of treatment after initiation of IL-17 inhibitors. Ixekizumab and secukinumab also are approved for PsA. Although improvement in joint disease is not as fast as with the anti-TNF inhibitors, notable improvement occurs by week 20 to 24.27
IL-23 Inhibitors
Guselkumab and tildrakizumab are the newest biologics for psoriasis, approved in 2017 and 2018, respectively. Both are monoclonal antibodies against the p19 subunit of IL-23, which blocks activation of TH17 cells.
Efficacy and Safety
Guselkumab and tildrakizumab demonstrated efficacy with minimal AEs or precautions noted thus far.28,29 Infections are again a risk, making tuberculosis testing the only recommended monitoring.
Patient Selection
Both medications offer another effective and safe option for patients with psoriasis. Similar to ustekinumab, the dosing interval of 12 weeks for tildrakizumab is ideal for patients who have needle phobia or are unable to administer their own injections.
Special Populations
Pregnancy
Antibodies cross the placenta as pregnancy progresses, with the highest rate in the third trimester. Certolizumab pegol has shown the lowest concentrations in infant serum, possibly due to its unique structure lacking the fragment crystallizable region required for passage through the placenta.30 For this reason, certolizumab pegol is a treatment of choice if biologic therapy is warranted during pregnancy.
Much of the pregnancy data for the remaining TNF-α inhibitors come from patients with rheumatoid arthritis or CD. In these populations, rates of major birth defects and miscarriages do not differ greatly from untreated women with these conditions.31 One retrospective study of unintentional pregnancies in women receiving ustekinumab showed rates of AEs similar to the general population.32
Pregnancy data for IL-17 or IL-23 inhibitors are largely limited to animal studies. One retrospective study of women exposed to secukinumab early in gestation showed no increased risk for pregnancy-related AEs.33 Discontinuation is still recommended for patients who become pregnant.
Pediatric Patients
Etanercept is approved for pediatric psoriatic patients 4 years and older. Children with juvenile idiopathic arthritis who are 2 years and older can receive etanercept. Ustekinumab is safe and effective for pediatric psoriatic patients 12 years and older, offering a second biologic option in children.
Although not approved for pediatric psoriasis, adalimumab is approved in pediatric CD (≥6 years of age) and for juvenile idiopathic arthritis (≥2 years of age). Infliximab is approved for children 6 years and older with CD or ulcerative colitis.
Monitoring
Periodic tuberculosis screening is recommended for all biologics. For patients with latent tuberculosis, biologics may be restarted after 1 month of treatment of tuberculosis.
Prior to initiation of biologics, patients should be screened for hepatitis with hepatitis B surface antigen and antibody, hepatitis B core antibody, and hepatitis C antibody. Patients at risk for human immunodeficiency virus also should be screened.
Generally, complete blood cell count and comprehensive metabolic profile are advisable prior to starting a biologic. Opinions differ on frequency of repeating laboratory work. Complete blood cell count and comprehensive metabolic profile should be monitored at least every 3 to 6 months in patients on TNF-α inhibitors, and neutrophil count should be monitored during the induction phase of IL-17 inhibitors.
All patients with psoriasis should maintain age-appropriate cancer screenings, especially those on biologics. If malignancy is discovered, biologic medication should be discontinued. Debate exists as to when therapy can be safely restarted following treatment of malignancy. Patients who are considered at low risk for recurrence may opt to restart a biologic after 5 years, or sooner if symptoms warrant.34 This decision should involve the patient’s cancer specialist.

Conclusion
Treatment choices are based on psoriasis type and severity, comorbidities, patient preferences, and drug accessibility. One approach is detailed in Table 3. As research advances the understanding of psoriasis, this field will continue to rapidly change. Knowledge of the immunopathogenesis of psoriasis and its relation to comorbidities can direct your decision-making for individual patients.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84-91.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-433.
- Candia R, Ruiz A, Torres-Robles R, et al. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662.
- Chi CC, Tung TH, Wang J, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135:415-422.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Gaeta M, Castelvecchio S, Ricci C, et al. Role of psoriasis as independent predictor of cardiovascular disease: a meta-regression analysis. Int J Cardiol. 2013;168:2282-2288.
- Ma C, Harskamp CT, Armstrong EJ, et al. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168:486-495.
- Parisi R, Webb RT, Carr MJ, et al. Alcohol-related mortality in patients with psoriasis: a population-based cohort study. JAMA Dermatol. 2017;153:1256-1262.
- Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77:657-666.e8.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- van Lümig PP, Menting SP, van den Reek JM, et al. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J Eur Acad Dermatol Venereol. 2015;29:752-760.
- Gelfand JM, Berlin J, Van Voorhees A, et al. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139:1425-1429.
- Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885-1888.
- Finckh A, Simard JF, Duryea J, et al. The effectiveness of anti-tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2006;54:54-59.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79:824-830.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.e1183.
- Gottlieb AB, Strand V, Kishimoto M, et al. Ixekizumab improves patient-reported outcomes up to 52 weeks in bDMARD-naïve patients with active psoriatic arthritis (SPIRIT-P1). Rheumatology (Oxford). 2018;57:1777-1788.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Komaki F, Komaki Y, Micic D, et al. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J Autoimmun. 2017;76:38-52.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database [published online ahead of print June 21, 2018]. Br J Dermatol. doi:10.1111/bjd.16901.
- Elandt K, Aletaha D. Treating rheumatic patients with a malignancy. Arthritis Res Ther. 2011;13:223.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84-91.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-433.
- Candia R, Ruiz A, Torres-Robles R, et al. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662.
- Chi CC, Tung TH, Wang J, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135:415-422.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Gaeta M, Castelvecchio S, Ricci C, et al. Role of psoriasis as independent predictor of cardiovascular disease: a meta-regression analysis. Int J Cardiol. 2013;168:2282-2288.
- Ma C, Harskamp CT, Armstrong EJ, et al. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168:486-495.
- Parisi R, Webb RT, Carr MJ, et al. Alcohol-related mortality in patients with psoriasis: a population-based cohort study. JAMA Dermatol. 2017;153:1256-1262.
- Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77:657-666.e8.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- van Lümig PP, Menting SP, van den Reek JM, et al. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J Eur Acad Dermatol Venereol. 2015;29:752-760.
- Gelfand JM, Berlin J, Van Voorhees A, et al. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139:1425-1429.
- Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885-1888.
- Finckh A, Simard JF, Duryea J, et al. The effectiveness of anti-tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2006;54:54-59.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79:824-830.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.e1183.
- Gottlieb AB, Strand V, Kishimoto M, et al. Ixekizumab improves patient-reported outcomes up to 52 weeks in bDMARD-naïve patients with active psoriatic arthritis (SPIRIT-P1). Rheumatology (Oxford). 2018;57:1777-1788.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Komaki F, Komaki Y, Micic D, et al. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J Autoimmun. 2017;76:38-52.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database [published online ahead of print June 21, 2018]. Br J Dermatol. doi:10.1111/bjd.16901.
- Elandt K, Aletaha D. Treating rheumatic patients with a malignancy. Arthritis Res Ther. 2011;13:223.
Practice Points
- Psoriasis affects millions of Americans and is associated with a growing list of comorbidities.
- With the increasing number of biologic treatment options available, the clinician must keep in mind the immune pathways involved in psoriasis and the ways our therapies alter them.
- Consider disease severity, comorbidities, patient preferences, and drug accessibility when choosing psoriasis treatments.