User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Janssen/J&J COVID-19 vaccine cuts transmission, new data show
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Consider connections between depression, chronic medical comorbidities
For many adults, depression and chronic medical conditions are inextricably linked.
In fact, the prevalence of depression is 2-10 times higher among people with chronic medical conditions, particularly in people with chronic pain, where the prevalence reaches 40%-60%, according to Jonathan E. Alpert, MD, PhD.
“About 60% of adults over 65 have two or more chronic conditions, of which depression is the single most common comorbidity,” Dr. Alpert, chair of the department of psychiatry and behavioral sciences at the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Premorbid depression is a risk factor for a number of medical conditions, such as heart disease. We also know that medical illness is a risk factor for depression. Comorbid depression predicts poorer health outcomes, including disability, hospital readmission, and mortality. It is also associated with up to severalfold higher general medical costs.”
Despite the pervasive nature of depression on other medical conditions, a limited evidence base exists to guide clinicians on treatment approaches.
“Most major depressive disorder randomized clinical trials exclude individuals with active medical illness, but we do know that medical comorbidity is associated with poorer depression outcomes,” Dr. Alpert said. For example, the STAR*D trial found that people with major depressive disorder plus medical comorbidity had lower remission rates, compared with those who had MDD alone (P < .001), while a large analysis from University of Pittsburgh researchers found that people with medical comorbidities had higher depression recurrence rates.
An assessment of the relationship between medical conditions and depression should include thinking about the association between the medical illness itself and medications with depressive symptoms.
“Are the medications contributing to depressive symptoms?” he asked. “We also want to be thinking of the impact of medical illness and medications on antidepressant pharmacokinetics and pharmacodynamics. We also want to know about the evidence for antidepressant safety, tolerability, efficacy, and anticipated drug-drug interactions among individuals with the medical illness. You also want to enhance focus on treatment adherence and coordination of care.”
Nontraditional routes of antidepressant administration exist for patients who have difficulty swallowing pills. Food and Drug Administration–approved options include transdermal selegiline; intranasal esketamine; liquid forms of fluoxetine, escitalopram, paroxetine, nortriptyline, doxepin, imipramine, and lithium; and oral disintegrating tablet forms of mirtazapine and selegiline. As for non–FDA-approved forms of antidepressant administration, small studies or case reports have appeared in the medical literature regarding intravenous ketamine, citalopram, amitriptyline, mirtazapine, maprotiline, and lithium; intramuscular ketamine and amitriptyline; and rectal forms of antidepressants such as trazodone, amitriptyline, doxepin, fluoxetine, and lamotrigine.
“It’s good to keep in mind that, when you’re not able to use by mouth antidepressants or typical tablet forms of antidepressants, there are other options available,” said Dr. Alpert, who is also chair of the American Psychiatric Association’s Council on Research.
Metabolism of medications occurs primarily in the liver, he continued, but some metabolic enzymes also line the intestinal tract. The metabolism of a substrate may be inhibited or induced by other drugs.
“If someone is on drug A and we give drug B, and drug B is inhibiting the metabolism of drug A, there will be a very rapid impact – hours to just a few days,” Dr. Alpert said. “The substrate levels rise very quickly, so within hours or days of taking drug B, drug A levels can rise steeply.” On the other hand, if someone is on drug A and you give a drug B – which induces the enzymes that usually metabolize drug A – the impact will be gradual. “That’s because induction requires increased synthesis of the metabolic enzyme responsible for metabolizing drug A,” he said. “That happens over days to weeks.”
Medications that are potential inducers of metabolism include carbamazepine, phenobarbital, phenytoin, primidone, prednisone, ritonavir, rifampin, chronic alcohol use, chronic smoking, St. John’s wort, and consumption of large quantities of cruciferous vegetables and charbroiled meats.
On the other hand, potential inhibitors of metabolism include antifungals, macrolide antibiotics, fluoroquinolones, antiretrovirals, isoniazid, antimalarials, disulfiram, SSRIs, phenothiazines, valproic acid, nefazodone, duloxetine, bupropion, beta-blockers, acute alcohol use, cimetidine, quinidine, calcium channel blockers, grapefruit juice, propafenone, and amiodarone.
“When treating people with significant medical comorbidity, start low and go slow, but persevere,” Dr. Alpert advised. “We want to always think about the risk of treating versus the risk of not treating, or not treating actively enough. Often, people with comorbid medical illness require the same or even more assertive treatment with pharmacotherapy for their depression as people without medical illness. So, we don’t want to make the mistake of undertreating depression. We also want to anticipate and address challenges with adherence.”
He also recommended being mindful of the most salient side effects for a given condition, such as lowered seizure threshold or QT prolongation in populations with brain injury or with cardiovascular disease, and to leverage dual benefits when they might exist, such as using [selective norepinephrine reuptake inhibitors] for depression and pain or hot flashes, or bupropion for depression and smoking cessation, or mirtazapine, which is effective for nausea, cachexia, or insomnia, as well as depression itself.
“We want to collaborate closely and regularly with other treaters, sharing our notes and diagnostic impressions,” Dr. Alpert said. “We want to use all the tools in the box in addition to pharmacotherapy, thinking about psychotherapy, neuromodulation, and peer navigators. We want to strive for measurement-based care using rating scales when we can, to augment our treatment. And
Dr. Alpert reports having received speaker’s honoraria, consulting fees, and research support from numerous pharmaceutical companies.
For many adults, depression and chronic medical conditions are inextricably linked.
In fact, the prevalence of depression is 2-10 times higher among people with chronic medical conditions, particularly in people with chronic pain, where the prevalence reaches 40%-60%, according to Jonathan E. Alpert, MD, PhD.
“About 60% of adults over 65 have two or more chronic conditions, of which depression is the single most common comorbidity,” Dr. Alpert, chair of the department of psychiatry and behavioral sciences at the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Premorbid depression is a risk factor for a number of medical conditions, such as heart disease. We also know that medical illness is a risk factor for depression. Comorbid depression predicts poorer health outcomes, including disability, hospital readmission, and mortality. It is also associated with up to severalfold higher general medical costs.”
Despite the pervasive nature of depression on other medical conditions, a limited evidence base exists to guide clinicians on treatment approaches.
“Most major depressive disorder randomized clinical trials exclude individuals with active medical illness, but we do know that medical comorbidity is associated with poorer depression outcomes,” Dr. Alpert said. For example, the STAR*D trial found that people with major depressive disorder plus medical comorbidity had lower remission rates, compared with those who had MDD alone (P < .001), while a large analysis from University of Pittsburgh researchers found that people with medical comorbidities had higher depression recurrence rates.
An assessment of the relationship between medical conditions and depression should include thinking about the association between the medical illness itself and medications with depressive symptoms.
“Are the medications contributing to depressive symptoms?” he asked. “We also want to be thinking of the impact of medical illness and medications on antidepressant pharmacokinetics and pharmacodynamics. We also want to know about the evidence for antidepressant safety, tolerability, efficacy, and anticipated drug-drug interactions among individuals with the medical illness. You also want to enhance focus on treatment adherence and coordination of care.”
Nontraditional routes of antidepressant administration exist for patients who have difficulty swallowing pills. Food and Drug Administration–approved options include transdermal selegiline; intranasal esketamine; liquid forms of fluoxetine, escitalopram, paroxetine, nortriptyline, doxepin, imipramine, and lithium; and oral disintegrating tablet forms of mirtazapine and selegiline. As for non–FDA-approved forms of antidepressant administration, small studies or case reports have appeared in the medical literature regarding intravenous ketamine, citalopram, amitriptyline, mirtazapine, maprotiline, and lithium; intramuscular ketamine and amitriptyline; and rectal forms of antidepressants such as trazodone, amitriptyline, doxepin, fluoxetine, and lamotrigine.
“It’s good to keep in mind that, when you’re not able to use by mouth antidepressants or typical tablet forms of antidepressants, there are other options available,” said Dr. Alpert, who is also chair of the American Psychiatric Association’s Council on Research.
Metabolism of medications occurs primarily in the liver, he continued, but some metabolic enzymes also line the intestinal tract. The metabolism of a substrate may be inhibited or induced by other drugs.
“If someone is on drug A and we give drug B, and drug B is inhibiting the metabolism of drug A, there will be a very rapid impact – hours to just a few days,” Dr. Alpert said. “The substrate levels rise very quickly, so within hours or days of taking drug B, drug A levels can rise steeply.” On the other hand, if someone is on drug A and you give a drug B – which induces the enzymes that usually metabolize drug A – the impact will be gradual. “That’s because induction requires increased synthesis of the metabolic enzyme responsible for metabolizing drug A,” he said. “That happens over days to weeks.”
Medications that are potential inducers of metabolism include carbamazepine, phenobarbital, phenytoin, primidone, prednisone, ritonavir, rifampin, chronic alcohol use, chronic smoking, St. John’s wort, and consumption of large quantities of cruciferous vegetables and charbroiled meats.
On the other hand, potential inhibitors of metabolism include antifungals, macrolide antibiotics, fluoroquinolones, antiretrovirals, isoniazid, antimalarials, disulfiram, SSRIs, phenothiazines, valproic acid, nefazodone, duloxetine, bupropion, beta-blockers, acute alcohol use, cimetidine, quinidine, calcium channel blockers, grapefruit juice, propafenone, and amiodarone.
“When treating people with significant medical comorbidity, start low and go slow, but persevere,” Dr. Alpert advised. “We want to always think about the risk of treating versus the risk of not treating, or not treating actively enough. Often, people with comorbid medical illness require the same or even more assertive treatment with pharmacotherapy for their depression as people without medical illness. So, we don’t want to make the mistake of undertreating depression. We also want to anticipate and address challenges with adherence.”
He also recommended being mindful of the most salient side effects for a given condition, such as lowered seizure threshold or QT prolongation in populations with brain injury or with cardiovascular disease, and to leverage dual benefits when they might exist, such as using [selective norepinephrine reuptake inhibitors] for depression and pain or hot flashes, or bupropion for depression and smoking cessation, or mirtazapine, which is effective for nausea, cachexia, or insomnia, as well as depression itself.
“We want to collaborate closely and regularly with other treaters, sharing our notes and diagnostic impressions,” Dr. Alpert said. “We want to use all the tools in the box in addition to pharmacotherapy, thinking about psychotherapy, neuromodulation, and peer navigators. We want to strive for measurement-based care using rating scales when we can, to augment our treatment. And
Dr. Alpert reports having received speaker’s honoraria, consulting fees, and research support from numerous pharmaceutical companies.
For many adults, depression and chronic medical conditions are inextricably linked.
In fact, the prevalence of depression is 2-10 times higher among people with chronic medical conditions, particularly in people with chronic pain, where the prevalence reaches 40%-60%, according to Jonathan E. Alpert, MD, PhD.
“About 60% of adults over 65 have two or more chronic conditions, of which depression is the single most common comorbidity,” Dr. Alpert, chair of the department of psychiatry and behavioral sciences at the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Premorbid depression is a risk factor for a number of medical conditions, such as heart disease. We also know that medical illness is a risk factor for depression. Comorbid depression predicts poorer health outcomes, including disability, hospital readmission, and mortality. It is also associated with up to severalfold higher general medical costs.”
Despite the pervasive nature of depression on other medical conditions, a limited evidence base exists to guide clinicians on treatment approaches.
“Most major depressive disorder randomized clinical trials exclude individuals with active medical illness, but we do know that medical comorbidity is associated with poorer depression outcomes,” Dr. Alpert said. For example, the STAR*D trial found that people with major depressive disorder plus medical comorbidity had lower remission rates, compared with those who had MDD alone (P < .001), while a large analysis from University of Pittsburgh researchers found that people with medical comorbidities had higher depression recurrence rates.
An assessment of the relationship between medical conditions and depression should include thinking about the association between the medical illness itself and medications with depressive symptoms.
“Are the medications contributing to depressive symptoms?” he asked. “We also want to be thinking of the impact of medical illness and medications on antidepressant pharmacokinetics and pharmacodynamics. We also want to know about the evidence for antidepressant safety, tolerability, efficacy, and anticipated drug-drug interactions among individuals with the medical illness. You also want to enhance focus on treatment adherence and coordination of care.”
Nontraditional routes of antidepressant administration exist for patients who have difficulty swallowing pills. Food and Drug Administration–approved options include transdermal selegiline; intranasal esketamine; liquid forms of fluoxetine, escitalopram, paroxetine, nortriptyline, doxepin, imipramine, and lithium; and oral disintegrating tablet forms of mirtazapine and selegiline. As for non–FDA-approved forms of antidepressant administration, small studies or case reports have appeared in the medical literature regarding intravenous ketamine, citalopram, amitriptyline, mirtazapine, maprotiline, and lithium; intramuscular ketamine and amitriptyline; and rectal forms of antidepressants such as trazodone, amitriptyline, doxepin, fluoxetine, and lamotrigine.
“It’s good to keep in mind that, when you’re not able to use by mouth antidepressants or typical tablet forms of antidepressants, there are other options available,” said Dr. Alpert, who is also chair of the American Psychiatric Association’s Council on Research.
Metabolism of medications occurs primarily in the liver, he continued, but some metabolic enzymes also line the intestinal tract. The metabolism of a substrate may be inhibited or induced by other drugs.
“If someone is on drug A and we give drug B, and drug B is inhibiting the metabolism of drug A, there will be a very rapid impact – hours to just a few days,” Dr. Alpert said. “The substrate levels rise very quickly, so within hours or days of taking drug B, drug A levels can rise steeply.” On the other hand, if someone is on drug A and you give a drug B – which induces the enzymes that usually metabolize drug A – the impact will be gradual. “That’s because induction requires increased synthesis of the metabolic enzyme responsible for metabolizing drug A,” he said. “That happens over days to weeks.”
Medications that are potential inducers of metabolism include carbamazepine, phenobarbital, phenytoin, primidone, prednisone, ritonavir, rifampin, chronic alcohol use, chronic smoking, St. John’s wort, and consumption of large quantities of cruciferous vegetables and charbroiled meats.
On the other hand, potential inhibitors of metabolism include antifungals, macrolide antibiotics, fluoroquinolones, antiretrovirals, isoniazid, antimalarials, disulfiram, SSRIs, phenothiazines, valproic acid, nefazodone, duloxetine, bupropion, beta-blockers, acute alcohol use, cimetidine, quinidine, calcium channel blockers, grapefruit juice, propafenone, and amiodarone.
“When treating people with significant medical comorbidity, start low and go slow, but persevere,” Dr. Alpert advised. “We want to always think about the risk of treating versus the risk of not treating, or not treating actively enough. Often, people with comorbid medical illness require the same or even more assertive treatment with pharmacotherapy for their depression as people without medical illness. So, we don’t want to make the mistake of undertreating depression. We also want to anticipate and address challenges with adherence.”
He also recommended being mindful of the most salient side effects for a given condition, such as lowered seizure threshold or QT prolongation in populations with brain injury or with cardiovascular disease, and to leverage dual benefits when they might exist, such as using [selective norepinephrine reuptake inhibitors] for depression and pain or hot flashes, or bupropion for depression and smoking cessation, or mirtazapine, which is effective for nausea, cachexia, or insomnia, as well as depression itself.
“We want to collaborate closely and regularly with other treaters, sharing our notes and diagnostic impressions,” Dr. Alpert said. “We want to use all the tools in the box in addition to pharmacotherapy, thinking about psychotherapy, neuromodulation, and peer navigators. We want to strive for measurement-based care using rating scales when we can, to augment our treatment. And
Dr. Alpert reports having received speaker’s honoraria, consulting fees, and research support from numerous pharmaceutical companies.
FROM NPA 2021
Screening tool may help better predict suicide attempts in adolescents
Researchers have developed a proprietary computer adaptive screening tool that may help emergency departments more accurately predict suicide attempts in adolescents, according to a recent study in JAMA Psychiatry.
The computerized adaptive screen for suicidal youth (CASSY) had an area under the curve (AUC) of 0.87 in an independent validation cohort that predicted an adolescent suicide attempt within 3 months, according to Cheryl A. King, PhD, of the department of psychiatry at the University of Michigan in Ann Arbor, and colleagues. CASSY’s adaptive design, which presents different questions based on a respondent’s answers, means “an individual’s initial item responses are used to determine a provisional estimate of their standing on the measured trait,” the researchers said.
Dr. King and colleagues evaluated the CASSY algorithm in a first study that consisted of 2,845 adolescents who were mean 15.1 years old, mostly girls (63%) enrolled from 13 different emergency departments across the United States within the Pediatric Emergency Care Applied Research Network (PECARN) between June 2015 and July 2016. To develop the CASSY algorithm, the participants received a 92-item self-report survey at baseline with three “anchor” questions from the Ask Suicide-Screening Questions (ASQ) and Columbia–Suicide Severity Rating Scale (C-SSRS). Based on the answers to the baseline survey, the researchers categorized participants as being at low, medium, or high risk for a suicide attempt, and followed participants for 3 months to record suicide attempts reported by a patient or parent.
Retention of participants at 3 months was 72.9%, leaving data available for 2,075 adolescents for review. The researchers found that the AUC was 0.89 (95% confidence interval, 0.85-0.91) in the first study, with a sensitivity of 82.4% and a specificity of 80%. Participants answered a mean number of 11 items during an assessment (range, 5-21 items) administered in a median time of 1 minute, 24 seconds.
In a second study consisting of a validation cohort, 4,050 adolescents from 14 PECARN emergency departments and 1 Indian Health Service hospital were followed, with a retention of 2,754 participants (69.5%) at the end of 3 months. Of the adolescents available at the end of 3 months, 62.1% were girls with a mean age of 15.0 years. The AUC for this validation group was 0.87 (95% CI, 0.85-0.89). Of these participants, 71.5% reported no previous suicide attempts, 9% reported one prior attempt, 18.2% reported multiple attempts, and 1.2% had an unknown number of suicide attempts. During the 3-month window of the second study, 6.0% of participants had at least one suicide attempt.
The researchers said that while the CASSY instrument may be advantageous for some emergency departments, “a standard screen such as the ASQ, which consists of fewer items, may be preferred in some settings, particularly those in which the cost and technical setup of a computerized adaptive screen poses too high a barrier.”
Dr. King and colleagues concluded.
Climbing adolescent suicide rate
In an interview, Igor Galynker, MD, PhD, professor in the department of psychiatry, and director of the suicide lab and the Zirinsky Center for Bipolar Disorder at the Icahn School of Medicine at Mount Sinai, New York, said the study by Dr. King and colleagues is important during a time when the suicide rate for adolescents is substantially increasing.
According to data from the CDC’s Web-based Injury Statistics Query and Reporting System, 1,750 adolescents died of suicide in 2018, and the rate of deaths by suicide has increased by 62% since the year 2000. “The issue really needs to be addressed,” said Dr. Galynker, who was not involved with the study.
Some methods of screening suicidal ideation that open with a direct question can miss suicide attempts in individuals who do not express these suicidal ideations, he explained, and the problem can be magnified in adolescent patients. “This is particularly difficult with adolescents because they’re notoriously poor historians. They cannot describe their feelings as well. It’s even more important to have methods that work for suicide prevention for adolescents and to support those predictors which do not rely on self-report,” he said.
Dr. Galynker said that CASSY is innovative because asking whether the patient is suicidal is not the “gateway question” and does not categorize people into groups determined to be at low, medium, or high risk for a suicide attempt.
“When you categorize people – adolescents in this particular case – you remove clinical judgment from [the] clinician. You deprive [the] clinician of exercising their clinical judgment in terms of somebody is or is not likely to die by suicide. That’s a serious problem,” he said, noting it may be one reason why these screening tools have difficulty identifying patients at risk of suicide.
Regarding limitations, the 3-month follow-up window for patients in the study may be too long to be clinically meaningful.
“If somebody is in treatment, 3 months is a long time. You want to know whether somebody is going to attempt suicide before the next time you see them, which is usually a month or a week,” he said.
But a strength of the CASSY instrument, Dr. Galynker said, is its ability to capture the patient’s mental state in the moment, as opposed to relying only a patient’s electronic medical record. The study also demonstrates “it should be possible to introduce detailed suicide risk assessment in the emergency rooms, and [it] should be done,” he said.
This study was funded with support from the Health Resources and Services Administration, the Maternal and Child Health Bureau, and the Emergency Medical Services for Children Network Development Demonstration Program, and a grant by the National Institute of Mental Health for the Emergency Department Screen for Teens at Risk for Suicide. Twelve authors reported personal and institutional relationships in the form of fees, grants, consultancies, royalties, copyrighted work, founding of technologies, and scientific council memberships for a variety of agencies, societies, foundations, and other organizations inside and outside of the study. Dr. Galynker reported his work unrelated to the study is supported by the National Institute of Mental Health and the American Foundation for Suicide Prevention. But he has no proprietary interests.
Researchers have developed a proprietary computer adaptive screening tool that may help emergency departments more accurately predict suicide attempts in adolescents, according to a recent study in JAMA Psychiatry.
The computerized adaptive screen for suicidal youth (CASSY) had an area under the curve (AUC) of 0.87 in an independent validation cohort that predicted an adolescent suicide attempt within 3 months, according to Cheryl A. King, PhD, of the department of psychiatry at the University of Michigan in Ann Arbor, and colleagues. CASSY’s adaptive design, which presents different questions based on a respondent’s answers, means “an individual’s initial item responses are used to determine a provisional estimate of their standing on the measured trait,” the researchers said.
Dr. King and colleagues evaluated the CASSY algorithm in a first study that consisted of 2,845 adolescents who were mean 15.1 years old, mostly girls (63%) enrolled from 13 different emergency departments across the United States within the Pediatric Emergency Care Applied Research Network (PECARN) between June 2015 and July 2016. To develop the CASSY algorithm, the participants received a 92-item self-report survey at baseline with three “anchor” questions from the Ask Suicide-Screening Questions (ASQ) and Columbia–Suicide Severity Rating Scale (C-SSRS). Based on the answers to the baseline survey, the researchers categorized participants as being at low, medium, or high risk for a suicide attempt, and followed participants for 3 months to record suicide attempts reported by a patient or parent.
Retention of participants at 3 months was 72.9%, leaving data available for 2,075 adolescents for review. The researchers found that the AUC was 0.89 (95% confidence interval, 0.85-0.91) in the first study, with a sensitivity of 82.4% and a specificity of 80%. Participants answered a mean number of 11 items during an assessment (range, 5-21 items) administered in a median time of 1 minute, 24 seconds.
In a second study consisting of a validation cohort, 4,050 adolescents from 14 PECARN emergency departments and 1 Indian Health Service hospital were followed, with a retention of 2,754 participants (69.5%) at the end of 3 months. Of the adolescents available at the end of 3 months, 62.1% were girls with a mean age of 15.0 years. The AUC for this validation group was 0.87 (95% CI, 0.85-0.89). Of these participants, 71.5% reported no previous suicide attempts, 9% reported one prior attempt, 18.2% reported multiple attempts, and 1.2% had an unknown number of suicide attempts. During the 3-month window of the second study, 6.0% of participants had at least one suicide attempt.
The researchers said that while the CASSY instrument may be advantageous for some emergency departments, “a standard screen such as the ASQ, which consists of fewer items, may be preferred in some settings, particularly those in which the cost and technical setup of a computerized adaptive screen poses too high a barrier.”
Dr. King and colleagues concluded.
Climbing adolescent suicide rate
In an interview, Igor Galynker, MD, PhD, professor in the department of psychiatry, and director of the suicide lab and the Zirinsky Center for Bipolar Disorder at the Icahn School of Medicine at Mount Sinai, New York, said the study by Dr. King and colleagues is important during a time when the suicide rate for adolescents is substantially increasing.
According to data from the CDC’s Web-based Injury Statistics Query and Reporting System, 1,750 adolescents died of suicide in 2018, and the rate of deaths by suicide has increased by 62% since the year 2000. “The issue really needs to be addressed,” said Dr. Galynker, who was not involved with the study.
Some methods of screening suicidal ideation that open with a direct question can miss suicide attempts in individuals who do not express these suicidal ideations, he explained, and the problem can be magnified in adolescent patients. “This is particularly difficult with adolescents because they’re notoriously poor historians. They cannot describe their feelings as well. It’s even more important to have methods that work for suicide prevention for adolescents and to support those predictors which do not rely on self-report,” he said.
Dr. Galynker said that CASSY is innovative because asking whether the patient is suicidal is not the “gateway question” and does not categorize people into groups determined to be at low, medium, or high risk for a suicide attempt.
“When you categorize people – adolescents in this particular case – you remove clinical judgment from [the] clinician. You deprive [the] clinician of exercising their clinical judgment in terms of somebody is or is not likely to die by suicide. That’s a serious problem,” he said, noting it may be one reason why these screening tools have difficulty identifying patients at risk of suicide.
Regarding limitations, the 3-month follow-up window for patients in the study may be too long to be clinically meaningful.
“If somebody is in treatment, 3 months is a long time. You want to know whether somebody is going to attempt suicide before the next time you see them, which is usually a month or a week,” he said.
But a strength of the CASSY instrument, Dr. Galynker said, is its ability to capture the patient’s mental state in the moment, as opposed to relying only a patient’s electronic medical record. The study also demonstrates “it should be possible to introduce detailed suicide risk assessment in the emergency rooms, and [it] should be done,” he said.
This study was funded with support from the Health Resources and Services Administration, the Maternal and Child Health Bureau, and the Emergency Medical Services for Children Network Development Demonstration Program, and a grant by the National Institute of Mental Health for the Emergency Department Screen for Teens at Risk for Suicide. Twelve authors reported personal and institutional relationships in the form of fees, grants, consultancies, royalties, copyrighted work, founding of technologies, and scientific council memberships for a variety of agencies, societies, foundations, and other organizations inside and outside of the study. Dr. Galynker reported his work unrelated to the study is supported by the National Institute of Mental Health and the American Foundation for Suicide Prevention. But he has no proprietary interests.
Researchers have developed a proprietary computer adaptive screening tool that may help emergency departments more accurately predict suicide attempts in adolescents, according to a recent study in JAMA Psychiatry.
The computerized adaptive screen for suicidal youth (CASSY) had an area under the curve (AUC) of 0.87 in an independent validation cohort that predicted an adolescent suicide attempt within 3 months, according to Cheryl A. King, PhD, of the department of psychiatry at the University of Michigan in Ann Arbor, and colleagues. CASSY’s adaptive design, which presents different questions based on a respondent’s answers, means “an individual’s initial item responses are used to determine a provisional estimate of their standing on the measured trait,” the researchers said.
Dr. King and colleagues evaluated the CASSY algorithm in a first study that consisted of 2,845 adolescents who were mean 15.1 years old, mostly girls (63%) enrolled from 13 different emergency departments across the United States within the Pediatric Emergency Care Applied Research Network (PECARN) between June 2015 and July 2016. To develop the CASSY algorithm, the participants received a 92-item self-report survey at baseline with three “anchor” questions from the Ask Suicide-Screening Questions (ASQ) and Columbia–Suicide Severity Rating Scale (C-SSRS). Based on the answers to the baseline survey, the researchers categorized participants as being at low, medium, or high risk for a suicide attempt, and followed participants for 3 months to record suicide attempts reported by a patient or parent.
Retention of participants at 3 months was 72.9%, leaving data available for 2,075 adolescents for review. The researchers found that the AUC was 0.89 (95% confidence interval, 0.85-0.91) in the first study, with a sensitivity of 82.4% and a specificity of 80%. Participants answered a mean number of 11 items during an assessment (range, 5-21 items) administered in a median time of 1 minute, 24 seconds.
In a second study consisting of a validation cohort, 4,050 adolescents from 14 PECARN emergency departments and 1 Indian Health Service hospital were followed, with a retention of 2,754 participants (69.5%) at the end of 3 months. Of the adolescents available at the end of 3 months, 62.1% were girls with a mean age of 15.0 years. The AUC for this validation group was 0.87 (95% CI, 0.85-0.89). Of these participants, 71.5% reported no previous suicide attempts, 9% reported one prior attempt, 18.2% reported multiple attempts, and 1.2% had an unknown number of suicide attempts. During the 3-month window of the second study, 6.0% of participants had at least one suicide attempt.
The researchers said that while the CASSY instrument may be advantageous for some emergency departments, “a standard screen such as the ASQ, which consists of fewer items, may be preferred in some settings, particularly those in which the cost and technical setup of a computerized adaptive screen poses too high a barrier.”
Dr. King and colleagues concluded.
Climbing adolescent suicide rate
In an interview, Igor Galynker, MD, PhD, professor in the department of psychiatry, and director of the suicide lab and the Zirinsky Center for Bipolar Disorder at the Icahn School of Medicine at Mount Sinai, New York, said the study by Dr. King and colleagues is important during a time when the suicide rate for adolescents is substantially increasing.
According to data from the CDC’s Web-based Injury Statistics Query and Reporting System, 1,750 adolescents died of suicide in 2018, and the rate of deaths by suicide has increased by 62% since the year 2000. “The issue really needs to be addressed,” said Dr. Galynker, who was not involved with the study.
Some methods of screening suicidal ideation that open with a direct question can miss suicide attempts in individuals who do not express these suicidal ideations, he explained, and the problem can be magnified in adolescent patients. “This is particularly difficult with adolescents because they’re notoriously poor historians. They cannot describe their feelings as well. It’s even more important to have methods that work for suicide prevention for adolescents and to support those predictors which do not rely on self-report,” he said.
Dr. Galynker said that CASSY is innovative because asking whether the patient is suicidal is not the “gateway question” and does not categorize people into groups determined to be at low, medium, or high risk for a suicide attempt.
“When you categorize people – adolescents in this particular case – you remove clinical judgment from [the] clinician. You deprive [the] clinician of exercising their clinical judgment in terms of somebody is or is not likely to die by suicide. That’s a serious problem,” he said, noting it may be one reason why these screening tools have difficulty identifying patients at risk of suicide.
Regarding limitations, the 3-month follow-up window for patients in the study may be too long to be clinically meaningful.
“If somebody is in treatment, 3 months is a long time. You want to know whether somebody is going to attempt suicide before the next time you see them, which is usually a month or a week,” he said.
But a strength of the CASSY instrument, Dr. Galynker said, is its ability to capture the patient’s mental state in the moment, as opposed to relying only a patient’s electronic medical record. The study also demonstrates “it should be possible to introduce detailed suicide risk assessment in the emergency rooms, and [it] should be done,” he said.
This study was funded with support from the Health Resources and Services Administration, the Maternal and Child Health Bureau, and the Emergency Medical Services for Children Network Development Demonstration Program, and a grant by the National Institute of Mental Health for the Emergency Department Screen for Teens at Risk for Suicide. Twelve authors reported personal and institutional relationships in the form of fees, grants, consultancies, royalties, copyrighted work, founding of technologies, and scientific council memberships for a variety of agencies, societies, foundations, and other organizations inside and outside of the study. Dr. Galynker reported his work unrelated to the study is supported by the National Institute of Mental Health and the American Foundation for Suicide Prevention. But he has no proprietary interests.
FROM JAMA PSYCHIATRY
Being in the now
Mindfulness as an intervention in challenging, changing, and uncertain times
The COVID-19 pandemic, multiple national displays of racial and social injustice, and recent political strife have left many feeling uncertain, anxious, sad, angry, grief-stricken, and struggling to cope. Coping may be especially difficult for our clients already grappling with mental health concerns, and many are looking to mental health professionals to restore a sense of well-being.
As professionals, we may be unsure about the best approach; after all, we haven’t experienced anything like this before! We’re facing many unknowns and unanswered questions, but one thing we do know is that we’re dealing with constant change. And, in fact, the only certainty is continued change and uncertainty. The truth of uncertainty can be challenging to contend with, especially when so much, including our country’s future, is in question. In times like this, there is likely no perfect treatment, but mindfulness can serve as a powerful intervention for coping with uncertainty and change, and for managing a range of difficult reactions.
The ‘what’ of mindfulness: Awareness, being in the now, and nonattachment
It’s crucial that we understand what mindfulness really is. It’s become something of a buzzword in American society, complete with misconceptions. Mindfulness has roots in many faith traditions, but as it’s practiced in the Western world, it usually has roots in Hinduism and Buddhism.
Mindfulness roughly means “awareness”; this is an approximate translation of the Pali (an ancient Indian language) word “Sati.” Mindfulness is moment-to-moment awareness and acceptance of our present experiences, thoughts, and feelings, without judgment or attachment. Attachment relates to the continually changing nature of all thoughts, feelings, and situations. Because everything is continuously changing, we needn’t become attached; attachment can keep us from being in the now. Acceptance means facing the now, which is essential when we feel tempted to avoid or deny painful feelings or situations. Acceptance doesn’t mean that we’ve resigned to being in pain forever; it merely means that we’re willing to see things as they actually are right now. This honest assessment of the present can prepare us for next steps.
Being in touch with the now helps us reconnect with ourselves, promote clarity about our situation and choices, and increases our awareness of our thoughts and feelings, moment to moment. It can also help us realize when we’ve fallen into unhelpful or catastrophic thinking, the risk of which is high during intense stress and uncertainty like what we’re facing now. Mindfulness helps us catch ourselves so we have the opportunity to make different choices, and feel better.
The how of mindfulness: Symptom management and changes in the brain
Research on mindfulness suggests that it can improve coping with anxiety,1 regulate mood,2 improve depression,3 reduce rumination,4 and mitigate trauma symptom severity.
Because mindfulness can effectively address psychiatric concerns, mindfulness-based clinical interventions such as mindfulness-based stress reduction and mindfulness-based cognitive therapy have been developed. These may reduce anxiety,5 depression, and posttraumatic stress disorder.6 Mindfulness can have a powerful impact on the brain; it’s been shown to improve the functioning of the regions associated with emotional regulation7 and change the regions related to awareness and fear.8 So, whether mindfulness is practiced in our clients’ everyday lives or used as the basis of therapeutic programs, it can promote well-being.
The how of mindfulness: In everyday life and treatment
How can we help our clients enjoy mindfulness’ benefits? I suggest that we start with ourselves. We’ll be more effective at guiding our clients in using mindfulness if we have our own experience.
And, mindfulness may help us to be more attentive to and effective in treatment. There is research demonstrating that treatment providers can benefit from mindfulness practices,9 and that clinicians who practice mindfulness report higher levels of empathy toward their clients.10 Because mindfulness is about attention and nonjudgmental and nonattached observation, it can be incorporated into many aspects of everyday life. Many options are available; we might encourage our clients to begin their day with a mindful pause, simply breathing and observing thoughts, feelings, sensations, or anything else that comes up. If they find themselves fixated on negative thinking or feelings, nonjudgmentally recognizing these experiences as temporary can help to prevent immersion and overwhelm. , perhaps during tasks such as housekeeping, working, talking with others, exercising, and even eating.
It can be beneficial to practice mindfulness before, during, and after situations that our clients know may bring on increased stress, anxiety, negative mood, and other undesirable experiences, such as watching the news or using some forms of social media. For clients who want more structure or guidance, several mobile apps are available, such as InsightTimer, Ten Percent Happier, or for Black clients, Liberate, which may be especially helpful for the impacts of racial injustice. Apps may also help clients who want to establish a formal mindfulness meditation practice, which may decrease anxiety and depression in some clinical populations.11 And, of course, with training, we can incorporate mindfulness into treatment. We may encourage clients to start our treatment or therapy sessions with a mindful pause to help them attain calm and focus, and depending on their concerns and needs, during times at which they feel particularly strong emotions. Clients may consider taking a Mindfulness-Based Stress Reduction course if something more intensive is needed, or clinicians may consider becoming trained in mindfulness-based cognitive therapy. Because recognition is increasing that mindfulness can address many clinical concerns, and because we’re contending with unprecedented challenges, mindfulness training for clinicians has become widely available.
Calm, clarity, and choices
None of us as individuals can eliminate the strife our country is living through, and none of us as clinicians can completely prevent or alleviate our clients’ pain. But by employing mindfulness, we can help clients cope with change and uncertainty, gain greater awareness of themselves and their experiences, feel calmer, attain more clarity to make better choices, and ultimately, feel better.
References
1. Bernstein A et al. J Cogn Psychother. 2011;25(2):99-113.
2. Remmers C et al. Mindfulness. 2016;7(4):829-37.
3. Rodrigues MF et al. Trends Psychiatry Psychother. Jul-Sep 2017;39(3):207-15.
4. Chambers R et al. Cogn Ther Res. 2008;32(3):303-22.
5. Montero-Marin et al. Psychol Med. 2019 Oct;49(13)2118-33.
6. Khusid MA, Vythilingam M. Mil Med. 2016 Sep;181(9):961-8.
7. Kral TRA et al. Neuroimage. 2018 Nov 1;181:301-13.
8. Desbordes G et al. Front Hum Neurosci. 2012 Nov 1. doi: 10.33891/fnhum.2012.00292.
9. Escuriex BF, Labbé EE. Mindfulness. 2011;2(4):242-53.
10. Aiken GA. Dissertation Abstracts Int Sec B: Sci Eng. 2006;67(4-B),2212.
11. Goyal M et al. JAMA Intern Med. 2014 Mar;174:357-68.
Dr. Collins is a Brooklyn-based licensed counseling psychologist, educator, and speaker. She is experienced in addressing a wide range of mental health concerns within youth, adult, and family populations. Her work has a strong social justice emphasis, and she is particularly skilled at working with clients of color. She has been a mindfulness practitioner for 10 years and is passionate about sharing the practice with others. Dr. Collins has no conflicts of interest.
Mindfulness as an intervention in challenging, changing, and uncertain times
Mindfulness as an intervention in challenging, changing, and uncertain times
The COVID-19 pandemic, multiple national displays of racial and social injustice, and recent political strife have left many feeling uncertain, anxious, sad, angry, grief-stricken, and struggling to cope. Coping may be especially difficult for our clients already grappling with mental health concerns, and many are looking to mental health professionals to restore a sense of well-being.
As professionals, we may be unsure about the best approach; after all, we haven’t experienced anything like this before! We’re facing many unknowns and unanswered questions, but one thing we do know is that we’re dealing with constant change. And, in fact, the only certainty is continued change and uncertainty. The truth of uncertainty can be challenging to contend with, especially when so much, including our country’s future, is in question. In times like this, there is likely no perfect treatment, but mindfulness can serve as a powerful intervention for coping with uncertainty and change, and for managing a range of difficult reactions.
The ‘what’ of mindfulness: Awareness, being in the now, and nonattachment
It’s crucial that we understand what mindfulness really is. It’s become something of a buzzword in American society, complete with misconceptions. Mindfulness has roots in many faith traditions, but as it’s practiced in the Western world, it usually has roots in Hinduism and Buddhism.
Mindfulness roughly means “awareness”; this is an approximate translation of the Pali (an ancient Indian language) word “Sati.” Mindfulness is moment-to-moment awareness and acceptance of our present experiences, thoughts, and feelings, without judgment or attachment. Attachment relates to the continually changing nature of all thoughts, feelings, and situations. Because everything is continuously changing, we needn’t become attached; attachment can keep us from being in the now. Acceptance means facing the now, which is essential when we feel tempted to avoid or deny painful feelings or situations. Acceptance doesn’t mean that we’ve resigned to being in pain forever; it merely means that we’re willing to see things as they actually are right now. This honest assessment of the present can prepare us for next steps.
Being in touch with the now helps us reconnect with ourselves, promote clarity about our situation and choices, and increases our awareness of our thoughts and feelings, moment to moment. It can also help us realize when we’ve fallen into unhelpful or catastrophic thinking, the risk of which is high during intense stress and uncertainty like what we’re facing now. Mindfulness helps us catch ourselves so we have the opportunity to make different choices, and feel better.
The how of mindfulness: Symptom management and changes in the brain
Research on mindfulness suggests that it can improve coping with anxiety,1 regulate mood,2 improve depression,3 reduce rumination,4 and mitigate trauma symptom severity.
Because mindfulness can effectively address psychiatric concerns, mindfulness-based clinical interventions such as mindfulness-based stress reduction and mindfulness-based cognitive therapy have been developed. These may reduce anxiety,5 depression, and posttraumatic stress disorder.6 Mindfulness can have a powerful impact on the brain; it’s been shown to improve the functioning of the regions associated with emotional regulation7 and change the regions related to awareness and fear.8 So, whether mindfulness is practiced in our clients’ everyday lives or used as the basis of therapeutic programs, it can promote well-being.
The how of mindfulness: In everyday life and treatment
How can we help our clients enjoy mindfulness’ benefits? I suggest that we start with ourselves. We’ll be more effective at guiding our clients in using mindfulness if we have our own experience.
And, mindfulness may help us to be more attentive to and effective in treatment. There is research demonstrating that treatment providers can benefit from mindfulness practices,9 and that clinicians who practice mindfulness report higher levels of empathy toward their clients.10 Because mindfulness is about attention and nonjudgmental and nonattached observation, it can be incorporated into many aspects of everyday life. Many options are available; we might encourage our clients to begin their day with a mindful pause, simply breathing and observing thoughts, feelings, sensations, or anything else that comes up. If they find themselves fixated on negative thinking or feelings, nonjudgmentally recognizing these experiences as temporary can help to prevent immersion and overwhelm. , perhaps during tasks such as housekeeping, working, talking with others, exercising, and even eating.
It can be beneficial to practice mindfulness before, during, and after situations that our clients know may bring on increased stress, anxiety, negative mood, and other undesirable experiences, such as watching the news or using some forms of social media. For clients who want more structure or guidance, several mobile apps are available, such as InsightTimer, Ten Percent Happier, or for Black clients, Liberate, which may be especially helpful for the impacts of racial injustice. Apps may also help clients who want to establish a formal mindfulness meditation practice, which may decrease anxiety and depression in some clinical populations.11 And, of course, with training, we can incorporate mindfulness into treatment. We may encourage clients to start our treatment or therapy sessions with a mindful pause to help them attain calm and focus, and depending on their concerns and needs, during times at which they feel particularly strong emotions. Clients may consider taking a Mindfulness-Based Stress Reduction course if something more intensive is needed, or clinicians may consider becoming trained in mindfulness-based cognitive therapy. Because recognition is increasing that mindfulness can address many clinical concerns, and because we’re contending with unprecedented challenges, mindfulness training for clinicians has become widely available.
Calm, clarity, and choices
None of us as individuals can eliminate the strife our country is living through, and none of us as clinicians can completely prevent or alleviate our clients’ pain. But by employing mindfulness, we can help clients cope with change and uncertainty, gain greater awareness of themselves and their experiences, feel calmer, attain more clarity to make better choices, and ultimately, feel better.
References
1. Bernstein A et al. J Cogn Psychother. 2011;25(2):99-113.
2. Remmers C et al. Mindfulness. 2016;7(4):829-37.
3. Rodrigues MF et al. Trends Psychiatry Psychother. Jul-Sep 2017;39(3):207-15.
4. Chambers R et al. Cogn Ther Res. 2008;32(3):303-22.
5. Montero-Marin et al. Psychol Med. 2019 Oct;49(13)2118-33.
6. Khusid MA, Vythilingam M. Mil Med. 2016 Sep;181(9):961-8.
7. Kral TRA et al. Neuroimage. 2018 Nov 1;181:301-13.
8. Desbordes G et al. Front Hum Neurosci. 2012 Nov 1. doi: 10.33891/fnhum.2012.00292.
9. Escuriex BF, Labbé EE. Mindfulness. 2011;2(4):242-53.
10. Aiken GA. Dissertation Abstracts Int Sec B: Sci Eng. 2006;67(4-B),2212.
11. Goyal M et al. JAMA Intern Med. 2014 Mar;174:357-68.
Dr. Collins is a Brooklyn-based licensed counseling psychologist, educator, and speaker. She is experienced in addressing a wide range of mental health concerns within youth, adult, and family populations. Her work has a strong social justice emphasis, and she is particularly skilled at working with clients of color. She has been a mindfulness practitioner for 10 years and is passionate about sharing the practice with others. Dr. Collins has no conflicts of interest.
The COVID-19 pandemic, multiple national displays of racial and social injustice, and recent political strife have left many feeling uncertain, anxious, sad, angry, grief-stricken, and struggling to cope. Coping may be especially difficult for our clients already grappling with mental health concerns, and many are looking to mental health professionals to restore a sense of well-being.
As professionals, we may be unsure about the best approach; after all, we haven’t experienced anything like this before! We’re facing many unknowns and unanswered questions, but one thing we do know is that we’re dealing with constant change. And, in fact, the only certainty is continued change and uncertainty. The truth of uncertainty can be challenging to contend with, especially when so much, including our country’s future, is in question. In times like this, there is likely no perfect treatment, but mindfulness can serve as a powerful intervention for coping with uncertainty and change, and for managing a range of difficult reactions.
The ‘what’ of mindfulness: Awareness, being in the now, and nonattachment
It’s crucial that we understand what mindfulness really is. It’s become something of a buzzword in American society, complete with misconceptions. Mindfulness has roots in many faith traditions, but as it’s practiced in the Western world, it usually has roots in Hinduism and Buddhism.
Mindfulness roughly means “awareness”; this is an approximate translation of the Pali (an ancient Indian language) word “Sati.” Mindfulness is moment-to-moment awareness and acceptance of our present experiences, thoughts, and feelings, without judgment or attachment. Attachment relates to the continually changing nature of all thoughts, feelings, and situations. Because everything is continuously changing, we needn’t become attached; attachment can keep us from being in the now. Acceptance means facing the now, which is essential when we feel tempted to avoid or deny painful feelings or situations. Acceptance doesn’t mean that we’ve resigned to being in pain forever; it merely means that we’re willing to see things as they actually are right now. This honest assessment of the present can prepare us for next steps.
Being in touch with the now helps us reconnect with ourselves, promote clarity about our situation and choices, and increases our awareness of our thoughts and feelings, moment to moment. It can also help us realize when we’ve fallen into unhelpful or catastrophic thinking, the risk of which is high during intense stress and uncertainty like what we’re facing now. Mindfulness helps us catch ourselves so we have the opportunity to make different choices, and feel better.
The how of mindfulness: Symptom management and changes in the brain
Research on mindfulness suggests that it can improve coping with anxiety,1 regulate mood,2 improve depression,3 reduce rumination,4 and mitigate trauma symptom severity.
Because mindfulness can effectively address psychiatric concerns, mindfulness-based clinical interventions such as mindfulness-based stress reduction and mindfulness-based cognitive therapy have been developed. These may reduce anxiety,5 depression, and posttraumatic stress disorder.6 Mindfulness can have a powerful impact on the brain; it’s been shown to improve the functioning of the regions associated with emotional regulation7 and change the regions related to awareness and fear.8 So, whether mindfulness is practiced in our clients’ everyday lives or used as the basis of therapeutic programs, it can promote well-being.
The how of mindfulness: In everyday life and treatment
How can we help our clients enjoy mindfulness’ benefits? I suggest that we start with ourselves. We’ll be more effective at guiding our clients in using mindfulness if we have our own experience.
And, mindfulness may help us to be more attentive to and effective in treatment. There is research demonstrating that treatment providers can benefit from mindfulness practices,9 and that clinicians who practice mindfulness report higher levels of empathy toward their clients.10 Because mindfulness is about attention and nonjudgmental and nonattached observation, it can be incorporated into many aspects of everyday life. Many options are available; we might encourage our clients to begin their day with a mindful pause, simply breathing and observing thoughts, feelings, sensations, or anything else that comes up. If they find themselves fixated on negative thinking or feelings, nonjudgmentally recognizing these experiences as temporary can help to prevent immersion and overwhelm. , perhaps during tasks such as housekeeping, working, talking with others, exercising, and even eating.
It can be beneficial to practice mindfulness before, during, and after situations that our clients know may bring on increased stress, anxiety, negative mood, and other undesirable experiences, such as watching the news or using some forms of social media. For clients who want more structure or guidance, several mobile apps are available, such as InsightTimer, Ten Percent Happier, or for Black clients, Liberate, which may be especially helpful for the impacts of racial injustice. Apps may also help clients who want to establish a formal mindfulness meditation practice, which may decrease anxiety and depression in some clinical populations.11 And, of course, with training, we can incorporate mindfulness into treatment. We may encourage clients to start our treatment or therapy sessions with a mindful pause to help them attain calm and focus, and depending on their concerns and needs, during times at which they feel particularly strong emotions. Clients may consider taking a Mindfulness-Based Stress Reduction course if something more intensive is needed, or clinicians may consider becoming trained in mindfulness-based cognitive therapy. Because recognition is increasing that mindfulness can address many clinical concerns, and because we’re contending with unprecedented challenges, mindfulness training for clinicians has become widely available.
Calm, clarity, and choices
None of us as individuals can eliminate the strife our country is living through, and none of us as clinicians can completely prevent or alleviate our clients’ pain. But by employing mindfulness, we can help clients cope with change and uncertainty, gain greater awareness of themselves and their experiences, feel calmer, attain more clarity to make better choices, and ultimately, feel better.
References
1. Bernstein A et al. J Cogn Psychother. 2011;25(2):99-113.
2. Remmers C et al. Mindfulness. 2016;7(4):829-37.
3. Rodrigues MF et al. Trends Psychiatry Psychother. Jul-Sep 2017;39(3):207-15.
4. Chambers R et al. Cogn Ther Res. 2008;32(3):303-22.
5. Montero-Marin et al. Psychol Med. 2019 Oct;49(13)2118-33.
6. Khusid MA, Vythilingam M. Mil Med. 2016 Sep;181(9):961-8.
7. Kral TRA et al. Neuroimage. 2018 Nov 1;181:301-13.
8. Desbordes G et al. Front Hum Neurosci. 2012 Nov 1. doi: 10.33891/fnhum.2012.00292.
9. Escuriex BF, Labbé EE. Mindfulness. 2011;2(4):242-53.
10. Aiken GA. Dissertation Abstracts Int Sec B: Sci Eng. 2006;67(4-B),2212.
11. Goyal M et al. JAMA Intern Med. 2014 Mar;174:357-68.
Dr. Collins is a Brooklyn-based licensed counseling psychologist, educator, and speaker. She is experienced in addressing a wide range of mental health concerns within youth, adult, and family populations. Her work has a strong social justice emphasis, and she is particularly skilled at working with clients of color. She has been a mindfulness practitioner for 10 years and is passionate about sharing the practice with others. Dr. Collins has no conflicts of interest.
Psychiatrists’ happiness, well-being hit hard by COVID-19
Events of the past year have taken a huge toll on the happiness, wellness, and lifestyles of many, but especially those in the health care field, including psychiatrists.
The newly released Medscape Psychiatrist Lifestyle, Happiness & Burnout Report 2021 reveals how psychiatrists are coping with burnout and trying to maintain personal wellness, and how they view their workplaces and their futures amid the ongoing COVID-19 pandemic.
Before the pandemic hit in March 2020, 84% of psychiatrists who responded to the survey reported being happy outside of work, similar to the percentage (82%) of physicians overall.
But as the pandemic has worn on, feelings have shifted, and there are clear signs of strain on those in the health care field. Now, just over half (55%) of psychiatrists say they are happy outside of work, similar to the percentage (58%) of physicians overall.
Perhaps not surprising given the specific challenges of COVID-19, infectious disease physicians, pulmonologists, rheumatologists, and intensivists currently rank lowest in happiness outside of work.
Anxiety, depression, burnout
With the ongoing COVID-19 pandemic, more than three quarters (77%) of psychiatrists surveyed report experiencing some degree of anxiety about their future, the same percentage as for physicians overall.
This year, more psychiatrists reported being either burned out or burned out and depressed (41% vs. 35% last year). About two-thirds of psychiatrists said burnout has had at least a moderate impact on their lives; 5% consider the impact so severe that they are thinking of leaving medicine altogether.
The majority of burned-out psychiatrists (63%) said they felt that way even before the pandemic began; for about one-third (37%), burnout set in after the pandemic began.
in the workplace (39%) and spending too many hours at work (37%).
Psychiatrists’ top tactic to cope with burnout is talking with family or friends (53%), followed by isolating themselves from others (51%), sleeping (45%), and exercising (43%); 42% said they eat junk food to cope; 35% play music; and 25% drink alcohol.
Most psychiatrists (63%) suffering burnout and/or depression don’t plan on seeking professional help. About one-third are currently seeking help or plan to do so, the highest proportion among all specialties.
Considering their symptoms not severe enough (57%) and feeling that they could deal with the problem on their own (41%) are the top reasons for not seeking professional help; 36% said they were too busy to get help, and 17% said they didn’t want to risk disclosing a problem.
Fifteen percent of psychiatrists who are burned out, depressed, or both have contemplated suicide, and 2% have attempted suicide.
Striving for work-life balance
Work-life balance is the most pressing workplace issue for 45% of psychiatrists, and 44% would sacrifice some of their salary for better work-life balance. These figures are about the same for physicians overall.
Forty-seven percent of psychiatrists take 3-4 weeks of vacation each year; 16% take 5 or more weeks. In this there was no change from last year’s report.
About one-third (35%) of psychiatrists generally make time to focus on their own well-being, the same proportion as physicians overall.
About two-thirds (68%) of psychiatrists exercise two or more times per week. Half of psychiatrists said they are currently trying to lose weight; about one-quarter are trying to maintain their current weight.
About one-quarter (26%) of psychiatrists said they do not drink alcohol at all; 17% have five or more drinks per week.
Most psychiatrists are currently in a committed relationship, with 81% either married or living with a partner. Among psychiatrists who are married or living with a partner, 43% are with someone who also works in medicine. About 81% of psychiatrists say their marriages are very good or good. These percentages are similar to those of physicians overall (85%).
Most psychiatrists (58%) spend up to 10 hours per week online for personal reasons; 82% spend this amount of time online each week for work.
It’s likely that the amount of time spent online for work will increase, given the pandemic-fueled surge in telemedicine. Yet even when their personal and professional Internet use are combined, psychiatrists, on average, spend far less time online than the nearly 7 hours per day of the average Internet user, according to recent data.
Findings from the latest happiness, wellness, and lifestyle survey are based on 12,339 Medscape member physicians practicing in the United States who completed an online survey conducted between Aug. 30 and Nov. 5, 2020.
A version of this article first appeared on Medscape.com.
Events of the past year have taken a huge toll on the happiness, wellness, and lifestyles of many, but especially those in the health care field, including psychiatrists.
The newly released Medscape Psychiatrist Lifestyle, Happiness & Burnout Report 2021 reveals how psychiatrists are coping with burnout and trying to maintain personal wellness, and how they view their workplaces and their futures amid the ongoing COVID-19 pandemic.
Before the pandemic hit in March 2020, 84% of psychiatrists who responded to the survey reported being happy outside of work, similar to the percentage (82%) of physicians overall.
But as the pandemic has worn on, feelings have shifted, and there are clear signs of strain on those in the health care field. Now, just over half (55%) of psychiatrists say they are happy outside of work, similar to the percentage (58%) of physicians overall.
Perhaps not surprising given the specific challenges of COVID-19, infectious disease physicians, pulmonologists, rheumatologists, and intensivists currently rank lowest in happiness outside of work.
Anxiety, depression, burnout
With the ongoing COVID-19 pandemic, more than three quarters (77%) of psychiatrists surveyed report experiencing some degree of anxiety about their future, the same percentage as for physicians overall.
This year, more psychiatrists reported being either burned out or burned out and depressed (41% vs. 35% last year). About two-thirds of psychiatrists said burnout has had at least a moderate impact on their lives; 5% consider the impact so severe that they are thinking of leaving medicine altogether.
The majority of burned-out psychiatrists (63%) said they felt that way even before the pandemic began; for about one-third (37%), burnout set in after the pandemic began.
in the workplace (39%) and spending too many hours at work (37%).
Psychiatrists’ top tactic to cope with burnout is talking with family or friends (53%), followed by isolating themselves from others (51%), sleeping (45%), and exercising (43%); 42% said they eat junk food to cope; 35% play music; and 25% drink alcohol.
Most psychiatrists (63%) suffering burnout and/or depression don’t plan on seeking professional help. About one-third are currently seeking help or plan to do so, the highest proportion among all specialties.
Considering their symptoms not severe enough (57%) and feeling that they could deal with the problem on their own (41%) are the top reasons for not seeking professional help; 36% said they were too busy to get help, and 17% said they didn’t want to risk disclosing a problem.
Fifteen percent of psychiatrists who are burned out, depressed, or both have contemplated suicide, and 2% have attempted suicide.
Striving for work-life balance
Work-life balance is the most pressing workplace issue for 45% of psychiatrists, and 44% would sacrifice some of their salary for better work-life balance. These figures are about the same for physicians overall.
Forty-seven percent of psychiatrists take 3-4 weeks of vacation each year; 16% take 5 or more weeks. In this there was no change from last year’s report.
About one-third (35%) of psychiatrists generally make time to focus on their own well-being, the same proportion as physicians overall.
About two-thirds (68%) of psychiatrists exercise two or more times per week. Half of psychiatrists said they are currently trying to lose weight; about one-quarter are trying to maintain their current weight.
About one-quarter (26%) of psychiatrists said they do not drink alcohol at all; 17% have five or more drinks per week.
Most psychiatrists are currently in a committed relationship, with 81% either married or living with a partner. Among psychiatrists who are married or living with a partner, 43% are with someone who also works in medicine. About 81% of psychiatrists say their marriages are very good or good. These percentages are similar to those of physicians overall (85%).
Most psychiatrists (58%) spend up to 10 hours per week online for personal reasons; 82% spend this amount of time online each week for work.
It’s likely that the amount of time spent online for work will increase, given the pandemic-fueled surge in telemedicine. Yet even when their personal and professional Internet use are combined, psychiatrists, on average, spend far less time online than the nearly 7 hours per day of the average Internet user, according to recent data.
Findings from the latest happiness, wellness, and lifestyle survey are based on 12,339 Medscape member physicians practicing in the United States who completed an online survey conducted between Aug. 30 and Nov. 5, 2020.
A version of this article first appeared on Medscape.com.
Events of the past year have taken a huge toll on the happiness, wellness, and lifestyles of many, but especially those in the health care field, including psychiatrists.
The newly released Medscape Psychiatrist Lifestyle, Happiness & Burnout Report 2021 reveals how psychiatrists are coping with burnout and trying to maintain personal wellness, and how they view their workplaces and their futures amid the ongoing COVID-19 pandemic.
Before the pandemic hit in March 2020, 84% of psychiatrists who responded to the survey reported being happy outside of work, similar to the percentage (82%) of physicians overall.
But as the pandemic has worn on, feelings have shifted, and there are clear signs of strain on those in the health care field. Now, just over half (55%) of psychiatrists say they are happy outside of work, similar to the percentage (58%) of physicians overall.
Perhaps not surprising given the specific challenges of COVID-19, infectious disease physicians, pulmonologists, rheumatologists, and intensivists currently rank lowest in happiness outside of work.
Anxiety, depression, burnout
With the ongoing COVID-19 pandemic, more than three quarters (77%) of psychiatrists surveyed report experiencing some degree of anxiety about their future, the same percentage as for physicians overall.
This year, more psychiatrists reported being either burned out or burned out and depressed (41% vs. 35% last year). About two-thirds of psychiatrists said burnout has had at least a moderate impact on their lives; 5% consider the impact so severe that they are thinking of leaving medicine altogether.
The majority of burned-out psychiatrists (63%) said they felt that way even before the pandemic began; for about one-third (37%), burnout set in after the pandemic began.
in the workplace (39%) and spending too many hours at work (37%).
Psychiatrists’ top tactic to cope with burnout is talking with family or friends (53%), followed by isolating themselves from others (51%), sleeping (45%), and exercising (43%); 42% said they eat junk food to cope; 35% play music; and 25% drink alcohol.
Most psychiatrists (63%) suffering burnout and/or depression don’t plan on seeking professional help. About one-third are currently seeking help or plan to do so, the highest proportion among all specialties.
Considering their symptoms not severe enough (57%) and feeling that they could deal with the problem on their own (41%) are the top reasons for not seeking professional help; 36% said they were too busy to get help, and 17% said they didn’t want to risk disclosing a problem.
Fifteen percent of psychiatrists who are burned out, depressed, or both have contemplated suicide, and 2% have attempted suicide.
Striving for work-life balance
Work-life balance is the most pressing workplace issue for 45% of psychiatrists, and 44% would sacrifice some of their salary for better work-life balance. These figures are about the same for physicians overall.
Forty-seven percent of psychiatrists take 3-4 weeks of vacation each year; 16% take 5 or more weeks. In this there was no change from last year’s report.
About one-third (35%) of psychiatrists generally make time to focus on their own well-being, the same proportion as physicians overall.
About two-thirds (68%) of psychiatrists exercise two or more times per week. Half of psychiatrists said they are currently trying to lose weight; about one-quarter are trying to maintain their current weight.
About one-quarter (26%) of psychiatrists said they do not drink alcohol at all; 17% have five or more drinks per week.
Most psychiatrists are currently in a committed relationship, with 81% either married or living with a partner. Among psychiatrists who are married or living with a partner, 43% are with someone who also works in medicine. About 81% of psychiatrists say their marriages are very good or good. These percentages are similar to those of physicians overall (85%).
Most psychiatrists (58%) spend up to 10 hours per week online for personal reasons; 82% spend this amount of time online each week for work.
It’s likely that the amount of time spent online for work will increase, given the pandemic-fueled surge in telemedicine. Yet even when their personal and professional Internet use are combined, psychiatrists, on average, spend far less time online than the nearly 7 hours per day of the average Internet user, according to recent data.
Findings from the latest happiness, wellness, and lifestyle survey are based on 12,339 Medscape member physicians practicing in the United States who completed an online survey conducted between Aug. 30 and Nov. 5, 2020.
A version of this article first appeared on Medscape.com.
Variants spur new FDA guidance on COVID vaccines, tests, drugs
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
Tips offered for treating co-occurring ADHD and SUDs
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
FROM NPA 2021
Emerging research shows link between suicidality, ‘high-potency’ cannabis products
Number of suicides positive for marijuana on rise soared among Colorado youth
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
Number of suicides positive for marijuana on rise soared among Colorado youth
Number of suicides positive for marijuana on rise soared among Colorado youth
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
FROM NPA 2021
How does an emotionally drained workforce move on post pandemic?
Psychiatric community is facing ‘triple challenges’ tied to COVID
When cases of COVID-19 began to surge in New York City in March 2020, Carol A. Bernstein, MD, did her best to practice psychiatry and carry out administrative tasks from a home office, but by mid-May, she became stir-crazy.
“I just couldn’t stand it, anymore,” Dr. Bernstein said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “I came back to work at least just to see my colleagues, because I felt so disconnected. Normally, in a disaster, people come together – whether it’s responding to an earthquake or a fire or whatever. People come together to provide themselves with support. They hug each other and hold each other’s hands. We could not and cannot do that in this pandemic.”
According to Dr. Bernstein, stress, fear, and uncertainty triggered by the COVID-19 pandemic require special attention to the needs of health care personnel.
“Taking care of yourself and encouraging others to do the same sustains the ability to care for those in need,” said Dr. Bernstein, who is vice chair for faculty development and well-being in the departments of psychiatry and behavioral science and obstetrics and gynecology at Montefiore Medical Center/Albert Einstein College of Medicine, New York. “This includes both meeting practical needs as well as physical and emotional self-care. Everyone is impacted by this, so emotional support needs to be available to everyone. In the psychiatric community, we have triple challenges. We have to take care of our patients, our colleagues, and ourselves. It’s a lot.”
Specific challenges for health care workers include the potential for a surge in care demand and uncertainty about future outbreaks.
“Although we don’t have [personal protective] and respirator shortages at the moment, we’re worried about the vaccine shortages,” she said. Then there’s the fact that patients with comorbid conditions have the highest risk of death and the task of providing supportive care as well as medical care. “Of course, we still have a risk of becoming infected or infecting our families. There is additional psychological stress: fear, grief, frustration, guilt, insomnia, and exhaustion.”
Now, more than a year removed from the start of the pandemic, health care personnel are experiencing compassion fatigue, which she described as the inability to feel compassion for our patients because of our inability to feel compassion for ourselves. “We’re certainly experiencing burnout, although the primary aspect of burnout that we are experiencing is emotional exhaustion,” said Dr. Bernstein, who also is a past president of the American Psychiatric Association.
General risk factors for burnout and distress include sleep deprivation, high levels of work/life conflict, work interrupted by personal concerns, high levels of anger, loneliness, or anxiety, the stress of work relationships/work outcomes, anxiety about competency, difficulty “unplugging” after work, and regular use of alcohol and other drugs. At the same time, she continued, signs of burnout and secondary traumatic stress include sadness, depression, or apathy; feeling easily frustrated; feeling isolated and disconnected from others; excessive worry or fear about something bad happening; feeling like a failure, and feeling tired, exhausted, or overwhelmed.
“Why is this crisis so hard for us docs?” she asked. “Because This can lead to medical errors and unprofessional behavior. There are significant feelings of guilt that ‘I’m not doing enough.’
“This was true for a lot of us in psychiatry who were working virtually early during the pandemic while our medicine colleagues were on the front lines exposing themselves to COVID. Even the people working on the COVID units at the height on the initial surge felt guilty because treatment algorithms were changing almost every day. Fortunately, protocols are more established now, but the sense of not doing enough is pervasive and makes it difficult for us to ask for help.”
Fear of the unknown also posed a challenge to the workforce. “We didn’t know what we were dealing with at first,” she said. “The loss of control and autonomy, which is a major driver of burnout in the best of circumstances, was particularly true here in New York. People were told what to do. They were deployed into new circumstances. We experienced a significant loss of control, both of the virus and of what we were doing, and a widespread sense of isolation and loneliness.”
To cultivate resilience going forward, Dr. Bernstein advocates for the concept of psychological flexibility, which she defined as the ability to stay in contact with the present moment regardless of unpleasant thoughts, feelings, and bodily sensations, while choosing one’s behaviors based on the situation and personal values. “It is understanding that you can feel demoralized and bad one minute and better the next day,” she said. “This is a key concept for being able to continuously adapt under stressful circumstances and to tolerate uncertainty.”
She advises clinicians to identify safe areas and behaviors, and to maximize their ability to care for themselves and their families – including keeping in touch with colleagues and people you care about. “You also want to take advantage of calming skills and the maintenance of natural body rhythms,” she said. “This includes sensible nutrition and getting adequate rest and exercise.”
Dr. Bernstein also emphasized the importance of trying to maintain hope and optimism while not denying risk. “We also have to think about ethics, to provide the best possible care given the circumstances,” she said. “The crisis standards of care are necessarily different. We are not ethically required to offer futile care, but we must tell the truth.”
She pointed out that resilience is sometimes thought of as returning to the way you were before a stressful or life-altering event. “But here we refer to it as using your coping resources, connecting to others, and cultivating your values and purpose in life as you ride through this time of stress,” Dr. Bernstein said. “You are aware of the time it takes to develop and test for treatment and vaccine efficacy, and to then roll out these interventions, so you do know there will be an end to this, hopefully by the summer. While you won’t forget this time, focus on what you can control, your positive relationships, remind yourself of your purpose, and practice gratitude for what you are thankful for in your life. We need to cultivate what is positive and promote the message that emotional health should have the same priority level as physical health. The goal is to flourish.”
Dr. Bernstein reported having no financial disclosures.
Psychiatric community is facing ‘triple challenges’ tied to COVID
Psychiatric community is facing ‘triple challenges’ tied to COVID
When cases of COVID-19 began to surge in New York City in March 2020, Carol A. Bernstein, MD, did her best to practice psychiatry and carry out administrative tasks from a home office, but by mid-May, she became stir-crazy.
“I just couldn’t stand it, anymore,” Dr. Bernstein said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “I came back to work at least just to see my colleagues, because I felt so disconnected. Normally, in a disaster, people come together – whether it’s responding to an earthquake or a fire or whatever. People come together to provide themselves with support. They hug each other and hold each other’s hands. We could not and cannot do that in this pandemic.”
According to Dr. Bernstein, stress, fear, and uncertainty triggered by the COVID-19 pandemic require special attention to the needs of health care personnel.
“Taking care of yourself and encouraging others to do the same sustains the ability to care for those in need,” said Dr. Bernstein, who is vice chair for faculty development and well-being in the departments of psychiatry and behavioral science and obstetrics and gynecology at Montefiore Medical Center/Albert Einstein College of Medicine, New York. “This includes both meeting practical needs as well as physical and emotional self-care. Everyone is impacted by this, so emotional support needs to be available to everyone. In the psychiatric community, we have triple challenges. We have to take care of our patients, our colleagues, and ourselves. It’s a lot.”
Specific challenges for health care workers include the potential for a surge in care demand and uncertainty about future outbreaks.
“Although we don’t have [personal protective] and respirator shortages at the moment, we’re worried about the vaccine shortages,” she said. Then there’s the fact that patients with comorbid conditions have the highest risk of death and the task of providing supportive care as well as medical care. “Of course, we still have a risk of becoming infected or infecting our families. There is additional psychological stress: fear, grief, frustration, guilt, insomnia, and exhaustion.”
Now, more than a year removed from the start of the pandemic, health care personnel are experiencing compassion fatigue, which she described as the inability to feel compassion for our patients because of our inability to feel compassion for ourselves. “We’re certainly experiencing burnout, although the primary aspect of burnout that we are experiencing is emotional exhaustion,” said Dr. Bernstein, who also is a past president of the American Psychiatric Association.
General risk factors for burnout and distress include sleep deprivation, high levels of work/life conflict, work interrupted by personal concerns, high levels of anger, loneliness, or anxiety, the stress of work relationships/work outcomes, anxiety about competency, difficulty “unplugging” after work, and regular use of alcohol and other drugs. At the same time, she continued, signs of burnout and secondary traumatic stress include sadness, depression, or apathy; feeling easily frustrated; feeling isolated and disconnected from others; excessive worry or fear about something bad happening; feeling like a failure, and feeling tired, exhausted, or overwhelmed.
“Why is this crisis so hard for us docs?” she asked. “Because This can lead to medical errors and unprofessional behavior. There are significant feelings of guilt that ‘I’m not doing enough.’
“This was true for a lot of us in psychiatry who were working virtually early during the pandemic while our medicine colleagues were on the front lines exposing themselves to COVID. Even the people working on the COVID units at the height on the initial surge felt guilty because treatment algorithms were changing almost every day. Fortunately, protocols are more established now, but the sense of not doing enough is pervasive and makes it difficult for us to ask for help.”
Fear of the unknown also posed a challenge to the workforce. “We didn’t know what we were dealing with at first,” she said. “The loss of control and autonomy, which is a major driver of burnout in the best of circumstances, was particularly true here in New York. People were told what to do. They were deployed into new circumstances. We experienced a significant loss of control, both of the virus and of what we were doing, and a widespread sense of isolation and loneliness.”
To cultivate resilience going forward, Dr. Bernstein advocates for the concept of psychological flexibility, which she defined as the ability to stay in contact with the present moment regardless of unpleasant thoughts, feelings, and bodily sensations, while choosing one’s behaviors based on the situation and personal values. “It is understanding that you can feel demoralized and bad one minute and better the next day,” she said. “This is a key concept for being able to continuously adapt under stressful circumstances and to tolerate uncertainty.”
She advises clinicians to identify safe areas and behaviors, and to maximize their ability to care for themselves and their families – including keeping in touch with colleagues and people you care about. “You also want to take advantage of calming skills and the maintenance of natural body rhythms,” she said. “This includes sensible nutrition and getting adequate rest and exercise.”
Dr. Bernstein also emphasized the importance of trying to maintain hope and optimism while not denying risk. “We also have to think about ethics, to provide the best possible care given the circumstances,” she said. “The crisis standards of care are necessarily different. We are not ethically required to offer futile care, but we must tell the truth.”
She pointed out that resilience is sometimes thought of as returning to the way you were before a stressful or life-altering event. “But here we refer to it as using your coping resources, connecting to others, and cultivating your values and purpose in life as you ride through this time of stress,” Dr. Bernstein said. “You are aware of the time it takes to develop and test for treatment and vaccine efficacy, and to then roll out these interventions, so you do know there will be an end to this, hopefully by the summer. While you won’t forget this time, focus on what you can control, your positive relationships, remind yourself of your purpose, and practice gratitude for what you are thankful for in your life. We need to cultivate what is positive and promote the message that emotional health should have the same priority level as physical health. The goal is to flourish.”
Dr. Bernstein reported having no financial disclosures.
When cases of COVID-19 began to surge in New York City in March 2020, Carol A. Bernstein, MD, did her best to practice psychiatry and carry out administrative tasks from a home office, but by mid-May, she became stir-crazy.
“I just couldn’t stand it, anymore,” Dr. Bernstein said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “I came back to work at least just to see my colleagues, because I felt so disconnected. Normally, in a disaster, people come together – whether it’s responding to an earthquake or a fire or whatever. People come together to provide themselves with support. They hug each other and hold each other’s hands. We could not and cannot do that in this pandemic.”
According to Dr. Bernstein, stress, fear, and uncertainty triggered by the COVID-19 pandemic require special attention to the needs of health care personnel.
“Taking care of yourself and encouraging others to do the same sustains the ability to care for those in need,” said Dr. Bernstein, who is vice chair for faculty development and well-being in the departments of psychiatry and behavioral science and obstetrics and gynecology at Montefiore Medical Center/Albert Einstein College of Medicine, New York. “This includes both meeting practical needs as well as physical and emotional self-care. Everyone is impacted by this, so emotional support needs to be available to everyone. In the psychiatric community, we have triple challenges. We have to take care of our patients, our colleagues, and ourselves. It’s a lot.”
Specific challenges for health care workers include the potential for a surge in care demand and uncertainty about future outbreaks.
“Although we don’t have [personal protective] and respirator shortages at the moment, we’re worried about the vaccine shortages,” she said. Then there’s the fact that patients with comorbid conditions have the highest risk of death and the task of providing supportive care as well as medical care. “Of course, we still have a risk of becoming infected or infecting our families. There is additional psychological stress: fear, grief, frustration, guilt, insomnia, and exhaustion.”
Now, more than a year removed from the start of the pandemic, health care personnel are experiencing compassion fatigue, which she described as the inability to feel compassion for our patients because of our inability to feel compassion for ourselves. “We’re certainly experiencing burnout, although the primary aspect of burnout that we are experiencing is emotional exhaustion,” said Dr. Bernstein, who also is a past president of the American Psychiatric Association.
General risk factors for burnout and distress include sleep deprivation, high levels of work/life conflict, work interrupted by personal concerns, high levels of anger, loneliness, or anxiety, the stress of work relationships/work outcomes, anxiety about competency, difficulty “unplugging” after work, and regular use of alcohol and other drugs. At the same time, she continued, signs of burnout and secondary traumatic stress include sadness, depression, or apathy; feeling easily frustrated; feeling isolated and disconnected from others; excessive worry or fear about something bad happening; feeling like a failure, and feeling tired, exhausted, or overwhelmed.
“Why is this crisis so hard for us docs?” she asked. “Because This can lead to medical errors and unprofessional behavior. There are significant feelings of guilt that ‘I’m not doing enough.’
“This was true for a lot of us in psychiatry who were working virtually early during the pandemic while our medicine colleagues were on the front lines exposing themselves to COVID. Even the people working on the COVID units at the height on the initial surge felt guilty because treatment algorithms were changing almost every day. Fortunately, protocols are more established now, but the sense of not doing enough is pervasive and makes it difficult for us to ask for help.”
Fear of the unknown also posed a challenge to the workforce. “We didn’t know what we were dealing with at first,” she said. “The loss of control and autonomy, which is a major driver of burnout in the best of circumstances, was particularly true here in New York. People were told what to do. They were deployed into new circumstances. We experienced a significant loss of control, both of the virus and of what we were doing, and a widespread sense of isolation and loneliness.”
To cultivate resilience going forward, Dr. Bernstein advocates for the concept of psychological flexibility, which she defined as the ability to stay in contact with the present moment regardless of unpleasant thoughts, feelings, and bodily sensations, while choosing one’s behaviors based on the situation and personal values. “It is understanding that you can feel demoralized and bad one minute and better the next day,” she said. “This is a key concept for being able to continuously adapt under stressful circumstances and to tolerate uncertainty.”
She advises clinicians to identify safe areas and behaviors, and to maximize their ability to care for themselves and their families – including keeping in touch with colleagues and people you care about. “You also want to take advantage of calming skills and the maintenance of natural body rhythms,” she said. “This includes sensible nutrition and getting adequate rest and exercise.”
Dr. Bernstein also emphasized the importance of trying to maintain hope and optimism while not denying risk. “We also have to think about ethics, to provide the best possible care given the circumstances,” she said. “The crisis standards of care are necessarily different. We are not ethically required to offer futile care, but we must tell the truth.”
She pointed out that resilience is sometimes thought of as returning to the way you were before a stressful or life-altering event. “But here we refer to it as using your coping resources, connecting to others, and cultivating your values and purpose in life as you ride through this time of stress,” Dr. Bernstein said. “You are aware of the time it takes to develop and test for treatment and vaccine efficacy, and to then roll out these interventions, so you do know there will be an end to this, hopefully by the summer. While you won’t forget this time, focus on what you can control, your positive relationships, remind yourself of your purpose, and practice gratitude for what you are thankful for in your life. We need to cultivate what is positive and promote the message that emotional health should have the same priority level as physical health. The goal is to flourish.”
Dr. Bernstein reported having no financial disclosures.
FROM NPA 2021
Short sleep predicts incident dementia and all-cause mortality
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).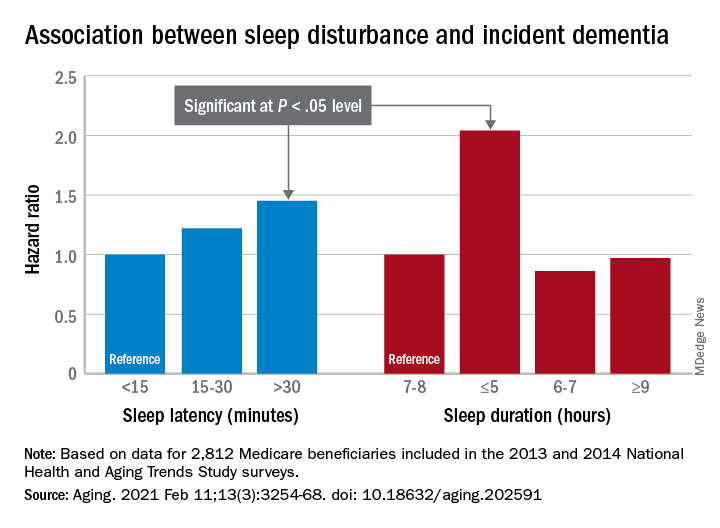
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
FROM AGING
















