User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
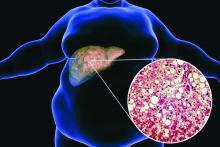
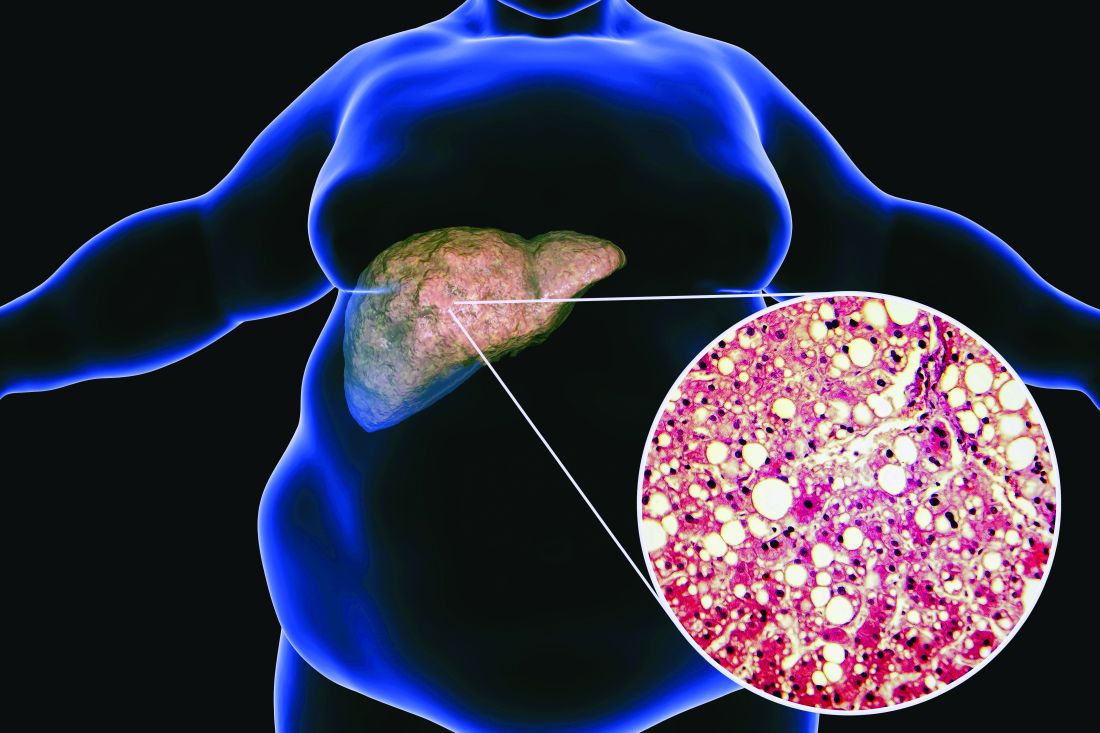
Bariatric surgery may up risk for epilepsy
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Balanced fat intake links with less type 2 diabetes
Researchers published the study covered in this summary on Preprints with The Lancet as a preprint that has not yet been peer reviewed.
Key takeaways
- Adults in China who consumed a “balanced,” moderate ratio (middle three quintiles) of animal-to-vegetable cooking oil had a lower rate of developing type 2 diabetes during a median follow-up of 8.6 years, compared with those who consumed the lowest ratio (first quintile), after multivariable adjustment using prospectively collected data.
- The results also indicate that increasing animal cooking oil (such as lard, tallow, or butter) and vegetable cooking oil (such as peanut or soybean oil) consumption were each positively associated with a higher rate of developing type 2 diabetes.
- Those who consumed the highest ratio (fifth quintile) of animal-to-vegetable cooking oil had a nonsignificant difference in their rate of developing type 2 diabetes, compared with those in the first quintile.
Why this matters
- The findings suggest that consuming a diet with a “balanced” moderate intake of animal and vegetable oil might lower the risk of type 2 diabetes, which would reduce disease burden and health care expenditures.
- The results imply that using a single source of cooking oil, either animal or vegetable, contributes to the incidence of type 2 diabetes.
- This is the first large epidemiological study showing a relationship between the ratio of animal- and vegetable-derived fats in people’s diets and their risk for incident type 2 diabetes.
Study design
- The researchers used data collected prospectively starting in 2010-2012 from 7,274 adult residents of Guizhou province, China, with follow-up assessment in 2020 after a median of 8.6 years.
- At baseline, participants underwent an oral glucose tolerance test and provided information on demographics, family medical history, and personal medical history, including whether they had been diagnosed with type 2 diabetes or were taking antihyperglycemic medications. The study did not include anyone with a history of diabetes.
- Data on intake of animal and vegetable cooking oil came from a dietary questionnaire.
- The authors calculated hazard ratios for development of type 2 diabetes after adjusting for multiple potential confounders.
Key results
- The study cohort averaged 44 years old, and 53% were women.
- During a median follow-up of 8.6 years, 747 people developed type 2 diabetes.
- Compared with those who had the lowest intake of animal cooking oil (first quintile), those with the highest intake (fifth quintile) had a significant 28% increased relative rate for developing type 2 diabetes after adjustment for several potential confounders.
- Compared with those with the lowest intake of vegetable cooking oil, those with the highest intake had a significant 56% increased rate of developing type 2 diabetes after adjustment.
- Compared with adults with the lowest animal-to-vegetable cooking oil ratio (first quintile), those in the second, third, and fourth quintiles for this ratio had significantly lower adjusted relative rates of developing type 2 diabetes, with adjusted hazard ratios of 0.79, 0.65, and 0.68, respectively. Those in the highest quintile (fifth quintile) did not have a significantly different risk, compared with the first quintile.
- The protective effect of a balanced ratio of animal-to-vegetable cooking oils was stronger in people who lived in rural districts and in those who had obesity.
Limitations
- The dietary information came from participants’ self-reports, which may have produced biased data.
- The study only included information about animal and vegetable cooking oil consumed at home.
- There may have been residual confounding from variables not included in the study.
- The time of diagnosis of type 2 diabetes may have been inaccurate because follow-up occurred only once.
- The study may have underestimated the incidence of type 2 diabetes because of a lack of information about hemoglobin A1c levels at follow-up.
Disclosures
- The study did not receive commercial funding.
- The authors reported no financial disclosures.
This is a summary of a preprint article “The consumption ratio of animal cooking oil to vegetable cooking oil and reduced risk of type 2 diabetes mellitus: A prospective cohort study in Southwest China” written by researchers primarily from Zunyi Medical University, China, on Preprints with The Lancet. This study has not yet been peer reviewed. The full text of the study can be found on papers.ssrn.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on Preprints with The Lancet as a preprint that has not yet been peer reviewed.
Key takeaways
- Adults in China who consumed a “balanced,” moderate ratio (middle three quintiles) of animal-to-vegetable cooking oil had a lower rate of developing type 2 diabetes during a median follow-up of 8.6 years, compared with those who consumed the lowest ratio (first quintile), after multivariable adjustment using prospectively collected data.
- The results also indicate that increasing animal cooking oil (such as lard, tallow, or butter) and vegetable cooking oil (such as peanut or soybean oil) consumption were each positively associated with a higher rate of developing type 2 diabetes.
- Those who consumed the highest ratio (fifth quintile) of animal-to-vegetable cooking oil had a nonsignificant difference in their rate of developing type 2 diabetes, compared with those in the first quintile.
Why this matters
- The findings suggest that consuming a diet with a “balanced” moderate intake of animal and vegetable oil might lower the risk of type 2 diabetes, which would reduce disease burden and health care expenditures.
- The results imply that using a single source of cooking oil, either animal or vegetable, contributes to the incidence of type 2 diabetes.
- This is the first large epidemiological study showing a relationship between the ratio of animal- and vegetable-derived fats in people’s diets and their risk for incident type 2 diabetes.
Study design
- The researchers used data collected prospectively starting in 2010-2012 from 7,274 adult residents of Guizhou province, China, with follow-up assessment in 2020 after a median of 8.6 years.
- At baseline, participants underwent an oral glucose tolerance test and provided information on demographics, family medical history, and personal medical history, including whether they had been diagnosed with type 2 diabetes or were taking antihyperglycemic medications. The study did not include anyone with a history of diabetes.
- Data on intake of animal and vegetable cooking oil came from a dietary questionnaire.
- The authors calculated hazard ratios for development of type 2 diabetes after adjusting for multiple potential confounders.
Key results
- The study cohort averaged 44 years old, and 53% were women.
- During a median follow-up of 8.6 years, 747 people developed type 2 diabetes.
- Compared with those who had the lowest intake of animal cooking oil (first quintile), those with the highest intake (fifth quintile) had a significant 28% increased relative rate for developing type 2 diabetes after adjustment for several potential confounders.
- Compared with those with the lowest intake of vegetable cooking oil, those with the highest intake had a significant 56% increased rate of developing type 2 diabetes after adjustment.
- Compared with adults with the lowest animal-to-vegetable cooking oil ratio (first quintile), those in the second, third, and fourth quintiles for this ratio had significantly lower adjusted relative rates of developing type 2 diabetes, with adjusted hazard ratios of 0.79, 0.65, and 0.68, respectively. Those in the highest quintile (fifth quintile) did not have a significantly different risk, compared with the first quintile.
- The protective effect of a balanced ratio of animal-to-vegetable cooking oils was stronger in people who lived in rural districts and in those who had obesity.
Limitations
- The dietary information came from participants’ self-reports, which may have produced biased data.
- The study only included information about animal and vegetable cooking oil consumed at home.
- There may have been residual confounding from variables not included in the study.
- The time of diagnosis of type 2 diabetes may have been inaccurate because follow-up occurred only once.
- The study may have underestimated the incidence of type 2 diabetes because of a lack of information about hemoglobin A1c levels at follow-up.
Disclosures
- The study did not receive commercial funding.
- The authors reported no financial disclosures.
This is a summary of a preprint article “The consumption ratio of animal cooking oil to vegetable cooking oil and reduced risk of type 2 diabetes mellitus: A prospective cohort study in Southwest China” written by researchers primarily from Zunyi Medical University, China, on Preprints with The Lancet. This study has not yet been peer reviewed. The full text of the study can be found on papers.ssrn.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on Preprints with The Lancet as a preprint that has not yet been peer reviewed.
Key takeaways
- Adults in China who consumed a “balanced,” moderate ratio (middle three quintiles) of animal-to-vegetable cooking oil had a lower rate of developing type 2 diabetes during a median follow-up of 8.6 years, compared with those who consumed the lowest ratio (first quintile), after multivariable adjustment using prospectively collected data.
- The results also indicate that increasing animal cooking oil (such as lard, tallow, or butter) and vegetable cooking oil (such as peanut or soybean oil) consumption were each positively associated with a higher rate of developing type 2 diabetes.
- Those who consumed the highest ratio (fifth quintile) of animal-to-vegetable cooking oil had a nonsignificant difference in their rate of developing type 2 diabetes, compared with those in the first quintile.
Why this matters
- The findings suggest that consuming a diet with a “balanced” moderate intake of animal and vegetable oil might lower the risk of type 2 diabetes, which would reduce disease burden and health care expenditures.
- The results imply that using a single source of cooking oil, either animal or vegetable, contributes to the incidence of type 2 diabetes.
- This is the first large epidemiological study showing a relationship between the ratio of animal- and vegetable-derived fats in people’s diets and their risk for incident type 2 diabetes.
Study design
- The researchers used data collected prospectively starting in 2010-2012 from 7,274 adult residents of Guizhou province, China, with follow-up assessment in 2020 after a median of 8.6 years.
- At baseline, participants underwent an oral glucose tolerance test and provided information on demographics, family medical history, and personal medical history, including whether they had been diagnosed with type 2 diabetes or were taking antihyperglycemic medications. The study did not include anyone with a history of diabetes.
- Data on intake of animal and vegetable cooking oil came from a dietary questionnaire.
- The authors calculated hazard ratios for development of type 2 diabetes after adjusting for multiple potential confounders.
Key results
- The study cohort averaged 44 years old, and 53% were women.
- During a median follow-up of 8.6 years, 747 people developed type 2 diabetes.
- Compared with those who had the lowest intake of animal cooking oil (first quintile), those with the highest intake (fifth quintile) had a significant 28% increased relative rate for developing type 2 diabetes after adjustment for several potential confounders.
- Compared with those with the lowest intake of vegetable cooking oil, those with the highest intake had a significant 56% increased rate of developing type 2 diabetes after adjustment.
- Compared with adults with the lowest animal-to-vegetable cooking oil ratio (first quintile), those in the second, third, and fourth quintiles for this ratio had significantly lower adjusted relative rates of developing type 2 diabetes, with adjusted hazard ratios of 0.79, 0.65, and 0.68, respectively. Those in the highest quintile (fifth quintile) did not have a significantly different risk, compared with the first quintile.
- The protective effect of a balanced ratio of animal-to-vegetable cooking oils was stronger in people who lived in rural districts and in those who had obesity.
Limitations
- The dietary information came from participants’ self-reports, which may have produced biased data.
- The study only included information about animal and vegetable cooking oil consumed at home.
- There may have been residual confounding from variables not included in the study.
- The time of diagnosis of type 2 diabetes may have been inaccurate because follow-up occurred only once.
- The study may have underestimated the incidence of type 2 diabetes because of a lack of information about hemoglobin A1c levels at follow-up.
Disclosures
- The study did not receive commercial funding.
- The authors reported no financial disclosures.
This is a summary of a preprint article “The consumption ratio of animal cooking oil to vegetable cooking oil and reduced risk of type 2 diabetes mellitus: A prospective cohort study in Southwest China” written by researchers primarily from Zunyi Medical University, China, on Preprints with The Lancet. This study has not yet been peer reviewed. The full text of the study can be found on papers.ssrn.com.
A version of this article first appeared on Medscape.com.
Physician bias may prevent quality care for patients with disabilities
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.
Strong link found between enterovirus and type 1 diabetes
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
AT EASD 2022
How to improve diagnosis of HFpEF, common in diabetes
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Once-weekly insulin promising in phase 3 trial in type 2 diabetes
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Hormones’ impact described in transgender rheumatology patients
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
Ezetimibe-statin combo lowers liver fat in open-label trial
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
FROM EASD 2022
Does COVID-19 cause type 1 diabetes in children? Time will tell
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Aspirin primary prevention benefit in those with raised Lp(a)?
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.