User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Does PREDICT accurately estimate breast cancer survival?
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
FROM NPJ BREAST CANCER
‘Obesity paradox’ in AFib challenged as mortality climbs with BMI
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.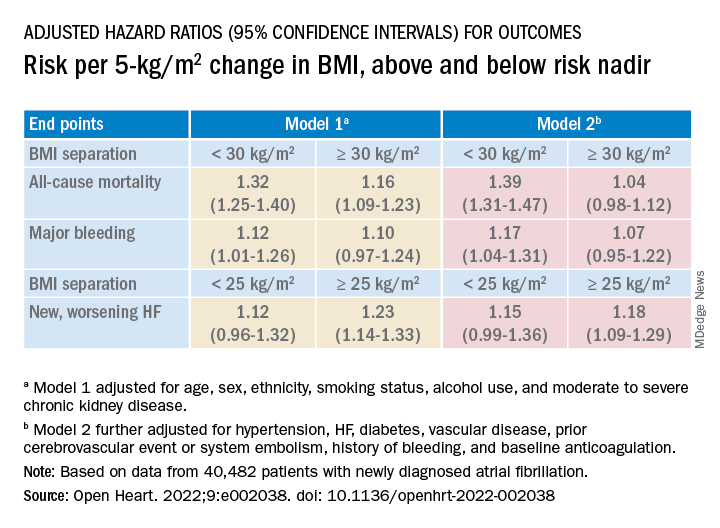
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Strength training overcomes bone effects of vegan diet
People who maintain a vegan diet show significant deficits in bone microarchitecture, compared with omnivores; however, resistance training not only appears to improve those deficits but may have a stronger effect in vegans, suggesting an important strategy in maintaining bone health with a vegan diet.
“We expected better bone structure in both vegans and omnivores who reported resistance training,” first author Robert Wakolbinger-Habel, MD, PhD, of St. Vincent Hospital Vienna and the Medical University of Vienna, said in an interview.
“However, we expected [there would still be] differences in structure between vegans and omnivores [who practiced resistance training], as previous literature reported higher fracture rates in vegans,” he said. “Still, the positive message is that ‘pumping iron’ could counterbalance these differences between vegans and omnivores.”
The research was published online in The Endocrine Society’s Journal of Clinical Endocrinology & Metabolism.
Exercise significantly impacts bone health in vegans
The potential effects of the plant-based vegan diet on bone health have been reported in studies linking the diet to an increased risk of fractures and lower bone mineral density (BMD), with common theories including lower bone- and muscle-building protein in vegan diets.
However, most previous studies have not considered other key factors, such as the effects of exercise, the authors noted.
“While previous studies on bone health in vegans only took BMD, biochemical and nutritional parameters into account, they did not consider the significant effects of physical activity,” they wrote.
“By ignoring these effects, important factors influencing bone health are neglected.”
For the study, 88 participants were enrolled in Vienna, with vegan participants recruited with the help of the Austrian Vegan Society.
Importantly, the study documented participants’ bone microarchitecture, a key measure of bone strength that has also not been previously investigated in vegans, using high-resolution peripheral quantitative CT.
Inclusion criteria included maintaining an omnivore diet of meat and plant-based foods or a vegan diet for at least 5 years, not being underweight or obese (body mass index [BMI], 18.5-30 kg/m2), being age 30-50 years, and being premenopausal.
Of the participants, 43 were vegan and 45 were omnivores, with generally equal ratios of men and women.
Vegan bone deficits disappear with strength training
Overall, compared with omnivores, the vegan group showed significant deficits in 7 of 14 measures of BMI-adjusted trabecular and cortical structure (all P < .05).
Among participants who reported no resistance training, vegans still showed significant decreases in bone microarchitecture, compared with omnivores, including radius trabecular BMD, radius trabecular bone volume fraction, and other tibial and cortical bone microarchitecture measures.
However, among those who did report progressive resistant training (20 vegans and 25 omnivores), defined as using machines, free weights, or bodyweight resistance exercises at least once a week, those differences disappeared and there were no significant differences in BMI-adjusted bone microarchitecture between vegans and omnivores after the 5 years.
Of note, no significant differences in bone microarchitecture were observed between those who performed exclusively aerobic activities and those who reported no sports activities in the vegan or omnivore group.
Based on the findings, “other types of exercise such as aerobics, cycling, etc, would not be sufficient for a similar positive effect on bone [as resistance training],” Dr. Wakolbinger-Habel said.
Although the findings suggest that resistance training seemed to allow vegans to “catch up” with omnivores in terms of bone microarchitecture, Dr. Wakolbinger-Habel cautioned that a study limitation is the relatively low number of participants.
“The absolute numbers suggest that in vegans the differences, and the relative effect, respectively of resistance training might be larger,” he said. “However, the number of participants in the subgroups is small and it is still an observational study, so we need to be careful in drawing causal conclusions.”
Serum bone markers were within normal ranges across all subgroups. And although there were some correlations between nutrient intake and bone microarchitecture among vegans who did and did not practice resistance training, no conclusions could be drawn from that data, the authors noted.
“Based on our data, the structural [differences between vegans and omnivores] cannot solely be explained by deficits in certain nutrients according to lifestyle,” the authors concluded.
Mechanisms
The mechanisms by which progressive resistance training could result in the benefits include that mechanical loads trigger stimulation of key pathways involved in bone formation, or mechanotransduction, the authors explained.
The unique effects have been observed in other studies, including one study showing that, among young adult runners, the addition of resistance training once a week was associated with significantly greater BMD.
“Veganism is a global trend with strongly increasing numbers of people worldwide adhering to a purely plant-based diet,” first author Christian Muschitz, MD, also of St. Vincent Hospital Vienna and the Medical University of Vienna, said in a press statement.
“Our study showed resistance training offsets diminished bone structure in vegan people when compared to omnivores,” he said.
Dr. Wakolbinger-Habel recommended that, based on the findings, “exercise, including resistance training, should be strongly advocated [for vegans], I would say, at least two times per week.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People who maintain a vegan diet show significant deficits in bone microarchitecture, compared with omnivores; however, resistance training not only appears to improve those deficits but may have a stronger effect in vegans, suggesting an important strategy in maintaining bone health with a vegan diet.
“We expected better bone structure in both vegans and omnivores who reported resistance training,” first author Robert Wakolbinger-Habel, MD, PhD, of St. Vincent Hospital Vienna and the Medical University of Vienna, said in an interview.
“However, we expected [there would still be] differences in structure between vegans and omnivores [who practiced resistance training], as previous literature reported higher fracture rates in vegans,” he said. “Still, the positive message is that ‘pumping iron’ could counterbalance these differences between vegans and omnivores.”
The research was published online in The Endocrine Society’s Journal of Clinical Endocrinology & Metabolism.
Exercise significantly impacts bone health in vegans
The potential effects of the plant-based vegan diet on bone health have been reported in studies linking the diet to an increased risk of fractures and lower bone mineral density (BMD), with common theories including lower bone- and muscle-building protein in vegan diets.
However, most previous studies have not considered other key factors, such as the effects of exercise, the authors noted.
“While previous studies on bone health in vegans only took BMD, biochemical and nutritional parameters into account, they did not consider the significant effects of physical activity,” they wrote.
“By ignoring these effects, important factors influencing bone health are neglected.”
For the study, 88 participants were enrolled in Vienna, with vegan participants recruited with the help of the Austrian Vegan Society.
Importantly, the study documented participants’ bone microarchitecture, a key measure of bone strength that has also not been previously investigated in vegans, using high-resolution peripheral quantitative CT.
Inclusion criteria included maintaining an omnivore diet of meat and plant-based foods or a vegan diet for at least 5 years, not being underweight or obese (body mass index [BMI], 18.5-30 kg/m2), being age 30-50 years, and being premenopausal.
Of the participants, 43 were vegan and 45 were omnivores, with generally equal ratios of men and women.
Vegan bone deficits disappear with strength training
Overall, compared with omnivores, the vegan group showed significant deficits in 7 of 14 measures of BMI-adjusted trabecular and cortical structure (all P < .05).
Among participants who reported no resistance training, vegans still showed significant decreases in bone microarchitecture, compared with omnivores, including radius trabecular BMD, radius trabecular bone volume fraction, and other tibial and cortical bone microarchitecture measures.
However, among those who did report progressive resistant training (20 vegans and 25 omnivores), defined as using machines, free weights, or bodyweight resistance exercises at least once a week, those differences disappeared and there were no significant differences in BMI-adjusted bone microarchitecture between vegans and omnivores after the 5 years.
Of note, no significant differences in bone microarchitecture were observed between those who performed exclusively aerobic activities and those who reported no sports activities in the vegan or omnivore group.
Based on the findings, “other types of exercise such as aerobics, cycling, etc, would not be sufficient for a similar positive effect on bone [as resistance training],” Dr. Wakolbinger-Habel said.
Although the findings suggest that resistance training seemed to allow vegans to “catch up” with omnivores in terms of bone microarchitecture, Dr. Wakolbinger-Habel cautioned that a study limitation is the relatively low number of participants.
“The absolute numbers suggest that in vegans the differences, and the relative effect, respectively of resistance training might be larger,” he said. “However, the number of participants in the subgroups is small and it is still an observational study, so we need to be careful in drawing causal conclusions.”
Serum bone markers were within normal ranges across all subgroups. And although there were some correlations between nutrient intake and bone microarchitecture among vegans who did and did not practice resistance training, no conclusions could be drawn from that data, the authors noted.
“Based on our data, the structural [differences between vegans and omnivores] cannot solely be explained by deficits in certain nutrients according to lifestyle,” the authors concluded.
Mechanisms
The mechanisms by which progressive resistance training could result in the benefits include that mechanical loads trigger stimulation of key pathways involved in bone formation, or mechanotransduction, the authors explained.
The unique effects have been observed in other studies, including one study showing that, among young adult runners, the addition of resistance training once a week was associated with significantly greater BMD.
“Veganism is a global trend with strongly increasing numbers of people worldwide adhering to a purely plant-based diet,” first author Christian Muschitz, MD, also of St. Vincent Hospital Vienna and the Medical University of Vienna, said in a press statement.
“Our study showed resistance training offsets diminished bone structure in vegan people when compared to omnivores,” he said.
Dr. Wakolbinger-Habel recommended that, based on the findings, “exercise, including resistance training, should be strongly advocated [for vegans], I would say, at least two times per week.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People who maintain a vegan diet show significant deficits in bone microarchitecture, compared with omnivores; however, resistance training not only appears to improve those deficits but may have a stronger effect in vegans, suggesting an important strategy in maintaining bone health with a vegan diet.
“We expected better bone structure in both vegans and omnivores who reported resistance training,” first author Robert Wakolbinger-Habel, MD, PhD, of St. Vincent Hospital Vienna and the Medical University of Vienna, said in an interview.
“However, we expected [there would still be] differences in structure between vegans and omnivores [who practiced resistance training], as previous literature reported higher fracture rates in vegans,” he said. “Still, the positive message is that ‘pumping iron’ could counterbalance these differences between vegans and omnivores.”
The research was published online in The Endocrine Society’s Journal of Clinical Endocrinology & Metabolism.
Exercise significantly impacts bone health in vegans
The potential effects of the plant-based vegan diet on bone health have been reported in studies linking the diet to an increased risk of fractures and lower bone mineral density (BMD), with common theories including lower bone- and muscle-building protein in vegan diets.
However, most previous studies have not considered other key factors, such as the effects of exercise, the authors noted.
“While previous studies on bone health in vegans only took BMD, biochemical and nutritional parameters into account, they did not consider the significant effects of physical activity,” they wrote.
“By ignoring these effects, important factors influencing bone health are neglected.”
For the study, 88 participants were enrolled in Vienna, with vegan participants recruited with the help of the Austrian Vegan Society.
Importantly, the study documented participants’ bone microarchitecture, a key measure of bone strength that has also not been previously investigated in vegans, using high-resolution peripheral quantitative CT.
Inclusion criteria included maintaining an omnivore diet of meat and plant-based foods or a vegan diet for at least 5 years, not being underweight or obese (body mass index [BMI], 18.5-30 kg/m2), being age 30-50 years, and being premenopausal.
Of the participants, 43 were vegan and 45 were omnivores, with generally equal ratios of men and women.
Vegan bone deficits disappear with strength training
Overall, compared with omnivores, the vegan group showed significant deficits in 7 of 14 measures of BMI-adjusted trabecular and cortical structure (all P < .05).
Among participants who reported no resistance training, vegans still showed significant decreases in bone microarchitecture, compared with omnivores, including radius trabecular BMD, radius trabecular bone volume fraction, and other tibial and cortical bone microarchitecture measures.
However, among those who did report progressive resistant training (20 vegans and 25 omnivores), defined as using machines, free weights, or bodyweight resistance exercises at least once a week, those differences disappeared and there were no significant differences in BMI-adjusted bone microarchitecture between vegans and omnivores after the 5 years.
Of note, no significant differences in bone microarchitecture were observed between those who performed exclusively aerobic activities and those who reported no sports activities in the vegan or omnivore group.
Based on the findings, “other types of exercise such as aerobics, cycling, etc, would not be sufficient for a similar positive effect on bone [as resistance training],” Dr. Wakolbinger-Habel said.
Although the findings suggest that resistance training seemed to allow vegans to “catch up” with omnivores in terms of bone microarchitecture, Dr. Wakolbinger-Habel cautioned that a study limitation is the relatively low number of participants.
“The absolute numbers suggest that in vegans the differences, and the relative effect, respectively of resistance training might be larger,” he said. “However, the number of participants in the subgroups is small and it is still an observational study, so we need to be careful in drawing causal conclusions.”
Serum bone markers were within normal ranges across all subgroups. And although there were some correlations between nutrient intake and bone microarchitecture among vegans who did and did not practice resistance training, no conclusions could be drawn from that data, the authors noted.
“Based on our data, the structural [differences between vegans and omnivores] cannot solely be explained by deficits in certain nutrients according to lifestyle,” the authors concluded.
Mechanisms
The mechanisms by which progressive resistance training could result in the benefits include that mechanical loads trigger stimulation of key pathways involved in bone formation, or mechanotransduction, the authors explained.
The unique effects have been observed in other studies, including one study showing that, among young adult runners, the addition of resistance training once a week was associated with significantly greater BMD.
“Veganism is a global trend with strongly increasing numbers of people worldwide adhering to a purely plant-based diet,” first author Christian Muschitz, MD, also of St. Vincent Hospital Vienna and the Medical University of Vienna, said in a press statement.
“Our study showed resistance training offsets diminished bone structure in vegan people when compared to omnivores,” he said.
Dr. Wakolbinger-Habel recommended that, based on the findings, “exercise, including resistance training, should be strongly advocated [for vegans], I would say, at least two times per week.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Patients who won’t pay: What’s your recourse?
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Blood pressure smartphone app fails to beat standard self-monitoring
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Poor sleep raises risk for fatty liver disease
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Risk factors in children linked to stroke as soon as 30s, 40s
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
How nonadherence complicates cardiology, in two trials
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Each study adds new twist
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Gut metabolites may explain red meat–ASCVD link
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
FROM ATHEROSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Should you sell your practice to a private equity firm?
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.









