User login
Psoriasis Treatment in Patients With Human Immunodeficiency Virus

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8
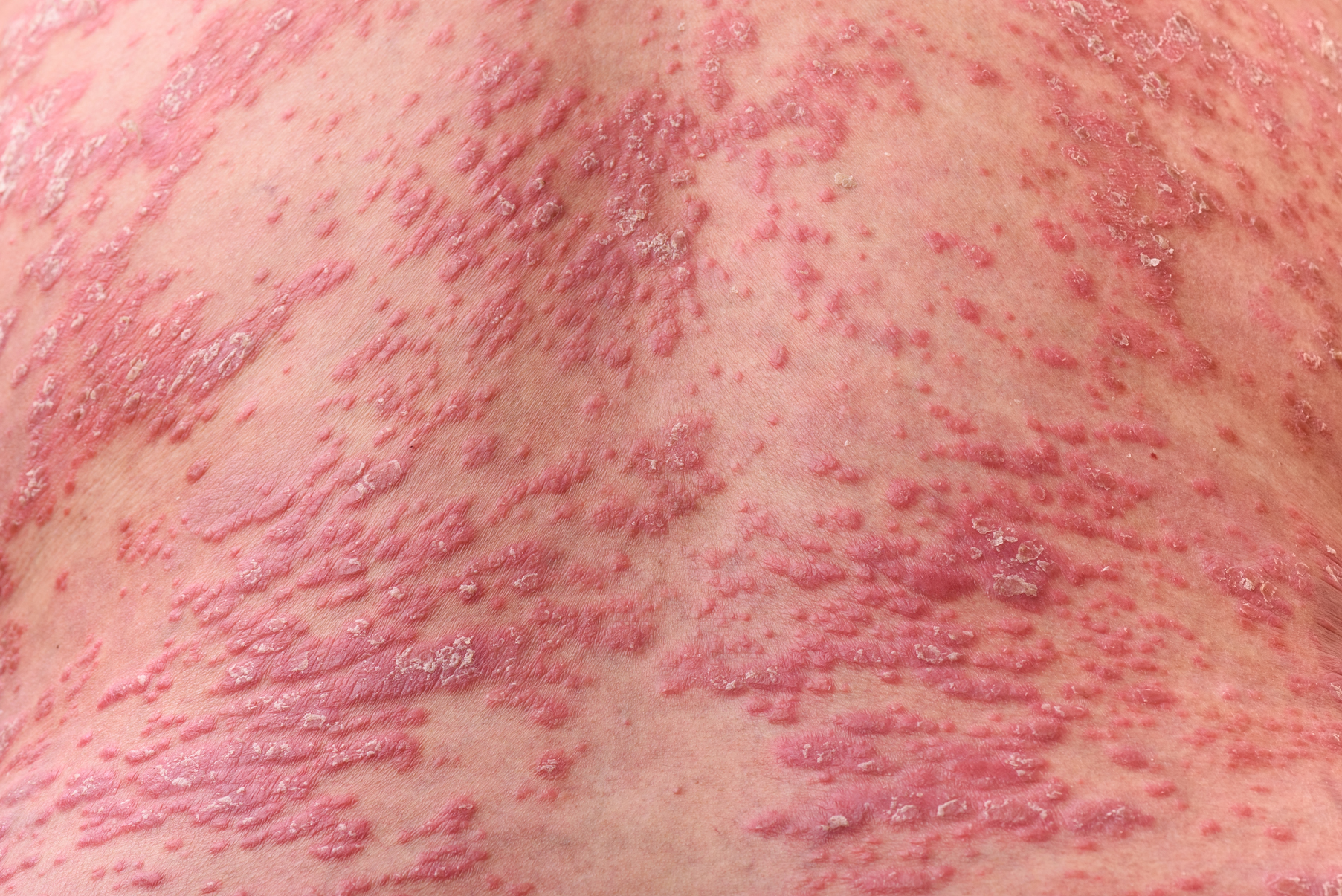
Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
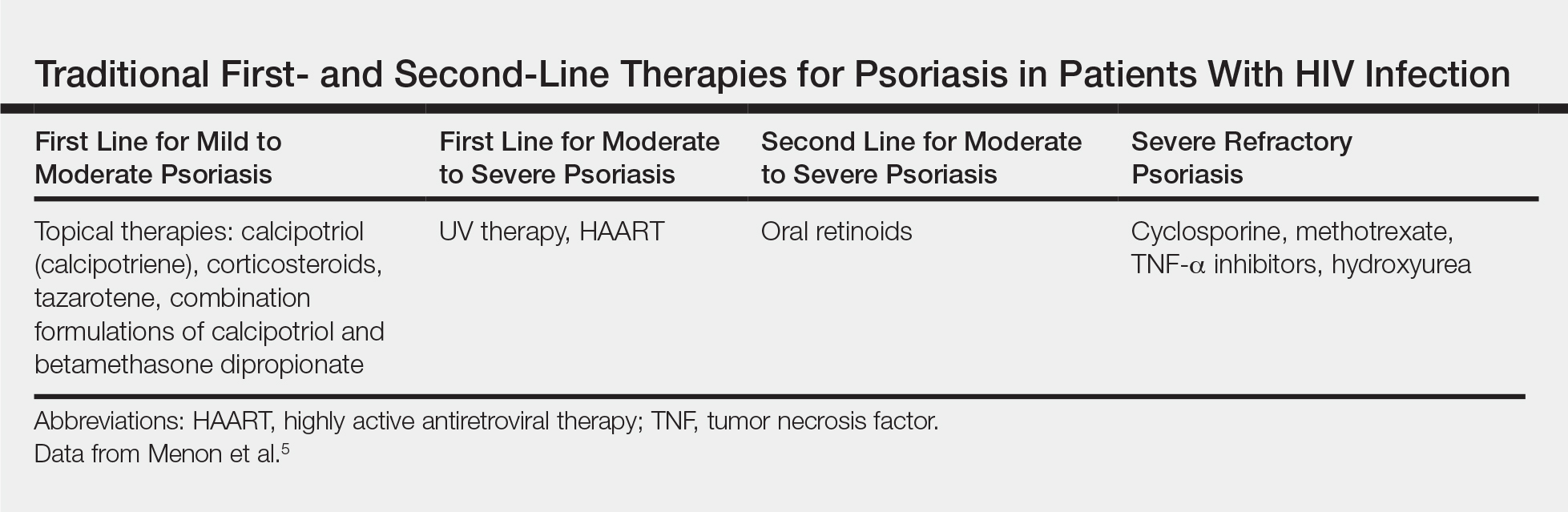
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1
Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16
Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8

Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1

Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16

Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8

Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1

Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16

Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
A 50-year-old man with Fitzpatrick skin type IV presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. The patient’s medical history was positive for human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected), for which clobetasol spray and calcitriol ointment had been prescribed. The patient’s CD4 count was 460 at presentation, and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to presentation. For the last 5 months, the patient had been undergoing phototherapy 3 times weekly for treatment of psoriasis.
An apremilast starter pack was initiated with the dosage titrated from 10 mg to 30 mg over the course of 1 week. The patient was maintained on a dose of 30 mg twice daily after 1 week, while continuing clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the patient’s affected BSA was 0%. Apremilast was continued, and phototherapy was reduced to once weekly. After 7 months of concomitant treatment with apremilast, phototherapy was discontinued after clearance was maintained. Phototherapy was reinitiated twice weekly after a mild flare (3% BSA affected).
The patient continued apremilast for a total of 20 months until it became cost prohibitive. After discontinuing apremilast for 4 months, he presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with intention to apply for an apremilast financial assistance program.
This case was adapted from Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7
Novel chip system could improve preclinical drug studies
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of anticancer therapies.
Major finding: Overall, results support the utility of the system to assess both off-target toxicity and on-target efficacy for various anticancer drugs.
Study details: A study exploring the utility of a multi-organ-on-a-chip system to assess safety and effectiveness of anticancer therapies in the preclinical setting.
Disclosures: The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
Source: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
Hippocampal cerebral blood flow upped with antihypertensive use in Alzheimer’s
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
FROM HYPERTENSION
PALOMA-3 biomarker analysis: Liquid biopsy could ID progression risk
CHICAGO – Tumor protein 53 (TP53) mutation, fibroblast growth factor receptor 1 (FGFR1) amplification, and tumor purity in plasma each predict early progression on palbociclib and/or fulvestrant in patients with advanced estrogen receptor–positive (ER+) breast cancer, according to genomic analyses of PALOMA-3 trial data.
Overall, the presence of one or more of these genomic changes identified 131 out of 310 patients from the phase 3 trial who had baseline samples available, Ben O’Leary, MBBS, said at the annual meeting of the American Society of Clinical Oncology.
“So, a significant minority of patients – 42.3% – potentially who fall into a more poor-prognosis group,” said Dr. O’Leary of the Institute of Cancer Research at the Royal Marsden Hospital in London.
The findings suggest that a “liquid biopsy” at the start of treatment could identify patients at risk for progression.
The PALOMA-3 trial randomized 521 patients with ER+, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer who had previously progressed on endocrine therapy 2:1 to CDK4/CDK6 inhibition with palbociclib plus fulvestrant (P+F) or placebo plus fulvestrant (F), and it showed that adding palbociclib significantly improved progression-free survival (PFS) (N Engl J Med. Jul 16 2015;373:209-19).
For the current analysis, the investigators assessed circulating tumor DNA (ctDNA) in baseline plasma samples from 459 study participants in an effort to identify genomic biomarkers for progression, to examine the association between baseline tumor fraction and clinical outcome, and to explore differences in predictive markers by treatment arm. A custom amplicon-sequencing analysis was performed to look for mutations in 17 different relevant genes, and another was used to estimate tumor fraction by looking at about 800 common germline single-nucleotide polymorphisms and to assess copy-number gain in the amplification status in 11 different genes, Dr. O’Leary said.
Results for mutations and circulating nucleic acids were available in 203 and 107 patients from the P+F and F groups, respectively, and on multivariable analysis of all 310 patients (including palbociclib as a variable in the model and with ctDNA fraction as a continuous variable), higher baseline tumor purity in plasma was associated with highly significantly worse PFS (HR 1.2 per 10% increase in purity), and baseline TP53 mutation and FGFR1 amplification each were associated with significantly shorter PFS (HRs, 1.8 and 2.9, respectively).
“[It is] very important to note ... that we did look specifically for interaction between our genomic changes and treatment, and we didn’t find any evidence of a significant interaction, so these genomic markers [are] prognostic rather than predictive in terms of the two treatment arms of the trial,” he said.
A survival analysis showed a median PFS of 3.7 vs. 12.7 months in patients with vs. without TP53 mutation in the P+F arm, and 1.8 vs. 5.4 months, respectively, in the F arm, with similar HRs of 2.0 and 2.3 in the arms, respectively.
“Even in the [P+F] arm, you see almost half of the patients with a TP53 mutation ... have relapsed by 2 months, the earliest clinical assessment in the trial,” he noted.
For FGFR1, the PFS was 3.9 vs. 12 months with vs. without amplification in the P+F arms, and 1.8 vs. 5.8 months, respectively in th F arm, with HRs of 3.4 and 3.6, respectively.
These findings are notable because markers of early progression on endocrine therapy in combination with CDK4/6 inhibitors remain limited – despite the key role of these combinations in treating ER+ advanced breast cancer, Dr. O’Leary explained.
“Although many patients derive a great deal of benefit from these combinations, there are a subset of patients who will relapse relatively early, and ... we don’t have an established means of identifying those patients at the present,” he said. “From the technical perspective, liquid biopsies have emerged in recent years as a promising means of genotyping patients’ cancers from circulating tumor DNA, and in addition, the overall level of circulating tumor DNA – the fractional purity – has been associated with poor prognosis, specifically in the triple-negative breast cancer setting.”
The results, which require independent validation, could potentially inform future clinical trials of CDK4/6 inhibitor combinations in advanced ER+ breast cancer to identify a high-risk group of patients who require escalation of therapy, he concluded.
Dr. O’Leary reported receiving research funding from Pfizer to his institution.
SOURCE: O’Leary B et al. ASCO 2019, Abstract 1010.
CHICAGO – Tumor protein 53 (TP53) mutation, fibroblast growth factor receptor 1 (FGFR1) amplification, and tumor purity in plasma each predict early progression on palbociclib and/or fulvestrant in patients with advanced estrogen receptor–positive (ER+) breast cancer, according to genomic analyses of PALOMA-3 trial data.
Overall, the presence of one or more of these genomic changes identified 131 out of 310 patients from the phase 3 trial who had baseline samples available, Ben O’Leary, MBBS, said at the annual meeting of the American Society of Clinical Oncology.
“So, a significant minority of patients – 42.3% – potentially who fall into a more poor-prognosis group,” said Dr. O’Leary of the Institute of Cancer Research at the Royal Marsden Hospital in London.
The findings suggest that a “liquid biopsy” at the start of treatment could identify patients at risk for progression.
The PALOMA-3 trial randomized 521 patients with ER+, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer who had previously progressed on endocrine therapy 2:1 to CDK4/CDK6 inhibition with palbociclib plus fulvestrant (P+F) or placebo plus fulvestrant (F), and it showed that adding palbociclib significantly improved progression-free survival (PFS) (N Engl J Med. Jul 16 2015;373:209-19).
For the current analysis, the investigators assessed circulating tumor DNA (ctDNA) in baseline plasma samples from 459 study participants in an effort to identify genomic biomarkers for progression, to examine the association between baseline tumor fraction and clinical outcome, and to explore differences in predictive markers by treatment arm. A custom amplicon-sequencing analysis was performed to look for mutations in 17 different relevant genes, and another was used to estimate tumor fraction by looking at about 800 common germline single-nucleotide polymorphisms and to assess copy-number gain in the amplification status in 11 different genes, Dr. O’Leary said.
Results for mutations and circulating nucleic acids were available in 203 and 107 patients from the P+F and F groups, respectively, and on multivariable analysis of all 310 patients (including palbociclib as a variable in the model and with ctDNA fraction as a continuous variable), higher baseline tumor purity in plasma was associated with highly significantly worse PFS (HR 1.2 per 10% increase in purity), and baseline TP53 mutation and FGFR1 amplification each were associated with significantly shorter PFS (HRs, 1.8 and 2.9, respectively).
“[It is] very important to note ... that we did look specifically for interaction between our genomic changes and treatment, and we didn’t find any evidence of a significant interaction, so these genomic markers [are] prognostic rather than predictive in terms of the two treatment arms of the trial,” he said.
A survival analysis showed a median PFS of 3.7 vs. 12.7 months in patients with vs. without TP53 mutation in the P+F arm, and 1.8 vs. 5.4 months, respectively, in the F arm, with similar HRs of 2.0 and 2.3 in the arms, respectively.
“Even in the [P+F] arm, you see almost half of the patients with a TP53 mutation ... have relapsed by 2 months, the earliest clinical assessment in the trial,” he noted.
For FGFR1, the PFS was 3.9 vs. 12 months with vs. without amplification in the P+F arms, and 1.8 vs. 5.8 months, respectively in th F arm, with HRs of 3.4 and 3.6, respectively.
These findings are notable because markers of early progression on endocrine therapy in combination with CDK4/6 inhibitors remain limited – despite the key role of these combinations in treating ER+ advanced breast cancer, Dr. O’Leary explained.
“Although many patients derive a great deal of benefit from these combinations, there are a subset of patients who will relapse relatively early, and ... we don’t have an established means of identifying those patients at the present,” he said. “From the technical perspective, liquid biopsies have emerged in recent years as a promising means of genotyping patients’ cancers from circulating tumor DNA, and in addition, the overall level of circulating tumor DNA – the fractional purity – has been associated with poor prognosis, specifically in the triple-negative breast cancer setting.”
The results, which require independent validation, could potentially inform future clinical trials of CDK4/6 inhibitor combinations in advanced ER+ breast cancer to identify a high-risk group of patients who require escalation of therapy, he concluded.
Dr. O’Leary reported receiving research funding from Pfizer to his institution.
SOURCE: O’Leary B et al. ASCO 2019, Abstract 1010.
CHICAGO – Tumor protein 53 (TP53) mutation, fibroblast growth factor receptor 1 (FGFR1) amplification, and tumor purity in plasma each predict early progression on palbociclib and/or fulvestrant in patients with advanced estrogen receptor–positive (ER+) breast cancer, according to genomic analyses of PALOMA-3 trial data.
Overall, the presence of one or more of these genomic changes identified 131 out of 310 patients from the phase 3 trial who had baseline samples available, Ben O’Leary, MBBS, said at the annual meeting of the American Society of Clinical Oncology.
“So, a significant minority of patients – 42.3% – potentially who fall into a more poor-prognosis group,” said Dr. O’Leary of the Institute of Cancer Research at the Royal Marsden Hospital in London.
The findings suggest that a “liquid biopsy” at the start of treatment could identify patients at risk for progression.
The PALOMA-3 trial randomized 521 patients with ER+, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer who had previously progressed on endocrine therapy 2:1 to CDK4/CDK6 inhibition with palbociclib plus fulvestrant (P+F) or placebo plus fulvestrant (F), and it showed that adding palbociclib significantly improved progression-free survival (PFS) (N Engl J Med. Jul 16 2015;373:209-19).
For the current analysis, the investigators assessed circulating tumor DNA (ctDNA) in baseline plasma samples from 459 study participants in an effort to identify genomic biomarkers for progression, to examine the association between baseline tumor fraction and clinical outcome, and to explore differences in predictive markers by treatment arm. A custom amplicon-sequencing analysis was performed to look for mutations in 17 different relevant genes, and another was used to estimate tumor fraction by looking at about 800 common germline single-nucleotide polymorphisms and to assess copy-number gain in the amplification status in 11 different genes, Dr. O’Leary said.
Results for mutations and circulating nucleic acids were available in 203 and 107 patients from the P+F and F groups, respectively, and on multivariable analysis of all 310 patients (including palbociclib as a variable in the model and with ctDNA fraction as a continuous variable), higher baseline tumor purity in plasma was associated with highly significantly worse PFS (HR 1.2 per 10% increase in purity), and baseline TP53 mutation and FGFR1 amplification each were associated with significantly shorter PFS (HRs, 1.8 and 2.9, respectively).
“[It is] very important to note ... that we did look specifically for interaction between our genomic changes and treatment, and we didn’t find any evidence of a significant interaction, so these genomic markers [are] prognostic rather than predictive in terms of the two treatment arms of the trial,” he said.
A survival analysis showed a median PFS of 3.7 vs. 12.7 months in patients with vs. without TP53 mutation in the P+F arm, and 1.8 vs. 5.4 months, respectively, in the F arm, with similar HRs of 2.0 and 2.3 in the arms, respectively.
“Even in the [P+F] arm, you see almost half of the patients with a TP53 mutation ... have relapsed by 2 months, the earliest clinical assessment in the trial,” he noted.
For FGFR1, the PFS was 3.9 vs. 12 months with vs. without amplification in the P+F arms, and 1.8 vs. 5.8 months, respectively in th F arm, with HRs of 3.4 and 3.6, respectively.
These findings are notable because markers of early progression on endocrine therapy in combination with CDK4/6 inhibitors remain limited – despite the key role of these combinations in treating ER+ advanced breast cancer, Dr. O’Leary explained.
“Although many patients derive a great deal of benefit from these combinations, there are a subset of patients who will relapse relatively early, and ... we don’t have an established means of identifying those patients at the present,” he said. “From the technical perspective, liquid biopsies have emerged in recent years as a promising means of genotyping patients’ cancers from circulating tumor DNA, and in addition, the overall level of circulating tumor DNA – the fractional purity – has been associated with poor prognosis, specifically in the triple-negative breast cancer setting.”
The results, which require independent validation, could potentially inform future clinical trials of CDK4/6 inhibitor combinations in advanced ER+ breast cancer to identify a high-risk group of patients who require escalation of therapy, he concluded.
Dr. O’Leary reported receiving research funding from Pfizer to his institution.
SOURCE: O’Leary B et al. ASCO 2019, Abstract 1010.
REPORTING FROM ASCO 2019
CSF neurofilament light level could aid in diagnosis
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
FROM JAMA NEUROLOGY
Gepant Safety & Lack of Liver Toxicity: Highlights from AAN 2019
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
Niraparib-pembrolizumab combo finds niche in breast, ovarian cancers
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
FROM JAMA ONCOLOGY
TAILORx: Clinical data add value to recurrence score
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
REPORTING FROM ASCO 2019
FDA approves trastuzumab-anns for HER2-positive breast, gastric cancer
The Food and Drug Administration has approved Amgen’s trastuzumab-anns as a trastuzumab biosimilar for the treatment of HER2-positive breast cancer and gastric cancer.
This biosimilar, to be marketed as Kanjinti, is the fifth trastuzumab biosimilar to be approved by the agency, according to the FDA.
Approval was based in part on the LILAC study, which demonstrated that the biosimilar, previously called ABP-980, had similar efficacy and comparable cardiac safety with trastuzumab.
In the phase 3 study, 725 patients with HER2-positive early breast cancer were randomized to neoadjuvant treatment with trastuzumab-anns or trastuzumab, plus paclitaxel, for four cycles following four cycles of chemotherapy. The primary pathological complete response endpoint was achieved in 48% of those in the biosimilar arm, compared with 40.5% in the trastuzumab arm. Patients then went on to receive adjuvant treatment with ABP 980 or trastuzumab every 3 weeks for up to 1 year following surgery.
Grade 3 or worse adverse events during the neoadjuvant phase occurred in 15% of patients in the ABP 980 group and 14% in the trastuzumab group. The most frequent grade 3 event in both study arms was neutropenia. In the adjuvant phase, grade 3 or worse adverse events occurred in 9% of those continuing ABP 980 and in 6% of those continuing trastuzumab. The most frequent events in both arms were infections, infestations, and neutropenia.
Trastuzumab-anns is indicated for adjuvant treatment of HER2-overexpressing node positive or node negative breast cancer, first-line treatment of HER2-overexpressing metastatic breast cancer, and first-line treatment of patients with HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. The FDA indicates patients should be selected based on an FDA-approved companion diagnostic for a trastuzumab product.
The biosimilar includes a boxed warning for cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity.
The Food and Drug Administration has approved Amgen’s trastuzumab-anns as a trastuzumab biosimilar for the treatment of HER2-positive breast cancer and gastric cancer.
This biosimilar, to be marketed as Kanjinti, is the fifth trastuzumab biosimilar to be approved by the agency, according to the FDA.
Approval was based in part on the LILAC study, which demonstrated that the biosimilar, previously called ABP-980, had similar efficacy and comparable cardiac safety with trastuzumab.
In the phase 3 study, 725 patients with HER2-positive early breast cancer were randomized to neoadjuvant treatment with trastuzumab-anns or trastuzumab, plus paclitaxel, for four cycles following four cycles of chemotherapy. The primary pathological complete response endpoint was achieved in 48% of those in the biosimilar arm, compared with 40.5% in the trastuzumab arm. Patients then went on to receive adjuvant treatment with ABP 980 or trastuzumab every 3 weeks for up to 1 year following surgery.
Grade 3 or worse adverse events during the neoadjuvant phase occurred in 15% of patients in the ABP 980 group and 14% in the trastuzumab group. The most frequent grade 3 event in both study arms was neutropenia. In the adjuvant phase, grade 3 or worse adverse events occurred in 9% of those continuing ABP 980 and in 6% of those continuing trastuzumab. The most frequent events in both arms were infections, infestations, and neutropenia.
Trastuzumab-anns is indicated for adjuvant treatment of HER2-overexpressing node positive or node negative breast cancer, first-line treatment of HER2-overexpressing metastatic breast cancer, and first-line treatment of patients with HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. The FDA indicates patients should be selected based on an FDA-approved companion diagnostic for a trastuzumab product.
The biosimilar includes a boxed warning for cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity.
The Food and Drug Administration has approved Amgen’s trastuzumab-anns as a trastuzumab biosimilar for the treatment of HER2-positive breast cancer and gastric cancer.
This biosimilar, to be marketed as Kanjinti, is the fifth trastuzumab biosimilar to be approved by the agency, according to the FDA.
Approval was based in part on the LILAC study, which demonstrated that the biosimilar, previously called ABP-980, had similar efficacy and comparable cardiac safety with trastuzumab.
In the phase 3 study, 725 patients with HER2-positive early breast cancer were randomized to neoadjuvant treatment with trastuzumab-anns or trastuzumab, plus paclitaxel, for four cycles following four cycles of chemotherapy. The primary pathological complete response endpoint was achieved in 48% of those in the biosimilar arm, compared with 40.5% in the trastuzumab arm. Patients then went on to receive adjuvant treatment with ABP 980 or trastuzumab every 3 weeks for up to 1 year following surgery.
Grade 3 or worse adverse events during the neoadjuvant phase occurred in 15% of patients in the ABP 980 group and 14% in the trastuzumab group. The most frequent grade 3 event in both study arms was neutropenia. In the adjuvant phase, grade 3 or worse adverse events occurred in 9% of those continuing ABP 980 and in 6% of those continuing trastuzumab. The most frequent events in both arms were infections, infestations, and neutropenia.
Trastuzumab-anns is indicated for adjuvant treatment of HER2-overexpressing node positive or node negative breast cancer, first-line treatment of HER2-overexpressing metastatic breast cancer, and first-line treatment of patients with HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. The FDA indicates patients should be selected based on an FDA-approved companion diagnostic for a trastuzumab product.
The biosimilar includes a boxed warning for cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity.
Breast cancer linked to 23% higher risk for new diabetes
SAN FRANCISCO – a new Danish study finds.
The findings are “quite a clear signal of increased diabetes following breast cancer,” said epidemiologist and study coauthor Reimar W. Thomsen, MD, PhD, of Aarhus (Denmark) University Hospital, in an interview. “It’s very important to tell [patients with breast cancer] what they may expect in the long term.”
He spoke at the annual scientific sessions of the American Diabetes Association, where he presented the study findings.
Much of the research into links between breast cancer and diabetes has focused on whether diabetes is a risk factor for breast cancer, and not the other way around. A 2018 meta-analysis of 18 studies found a slightly higher risk of breast cancer in women with diabetes (summary relative risk, 1.13; 95% confidence interval, 1.04-1.24). However, the researchers found evidence that the risk factor might be adiposity, and not diabetes itself (Diabetes. 2018 Jul;67[Supplement 1]. doi: 10.2337/db18-180-OR).
For the new study, researchers used health registries to track women in Denmark for up to 12 years, during 2005-2016. They compared 33,909 women who were older than 50 years and who had new-onset breast cancer with 313,998 women without breast cancer in a matched comparison cohort. The average age in both groups was 66 years; obesity was rare (4% vs. 3%, respectively), but statin therapy (21% in both groups) and hormone replacement therapy (36% vs. 32%) were more prevalent.
In the first year after a breast cancer diagnosis, the women in the breast cancer group were 15% more likely to develop diabetes (per use of diabetes medication or hospital-diagnosed diabetes) than those in the comparison group (adjusted hazard ratio, 1.15; 95% CI, 1.01-1.30) with adjustments for factors such as age, marital status, residence, medical history, medications, and comorbidity.
Over a median follow-up period of 5.2 years, the risk of diabetes was 23% higher in the breast cancer group, at 8.4 new cases per 1,000 women, compared with 6.8 new cases per 1,000 women in the comparison group (aHR, 1.23; 95% CI, 1.16-1.30). Unadjusted hazard ratios were similar.
Women in the breast cancer group who developed diabetes were more likely to use insulin-based therapy, suggesting they had more severe diabetes, compared with those in the control group (5% vs. 2%, respectively; P less than .00001). They were also more likely to be treated with insulin only (4% vs. 1%, P less than .00001).
It is not clear why patients with breast cancer face a higher risk of diabetes. Dr. Thomsen speculated that cancer drugs might play a role and he noted that cancer itself can cause inflammation and “lead to consequences.”
A 2018 study linked usage of hormone therapies, including tamoxifen (HR, 2.25; 95% CI, 1.19-4.26; P = .013) and aromatase inhibitors (HR, 4.27;95% CI, 1.42-12.84), in patients with breast cancer to higher levels of diabetes, compared with patients who did not use hormone therapy (J Clin Oncol. 2018;36[20]:2061-9).
Dr. Thomsen emphasized that physicians should monitor patients with breast cancer for diabetes. “It develops over time, and the risk is increasing, so you need to be aware of that.”
No study funding was reported. One of the researchers reported numerous ties to a range of drug companies. Dr. Thomsen and the other researchers reported no relevant disclosures.
SAN FRANCISCO – a new Danish study finds.
The findings are “quite a clear signal of increased diabetes following breast cancer,” said epidemiologist and study coauthor Reimar W. Thomsen, MD, PhD, of Aarhus (Denmark) University Hospital, in an interview. “It’s very important to tell [patients with breast cancer] what they may expect in the long term.”
He spoke at the annual scientific sessions of the American Diabetes Association, where he presented the study findings.
Much of the research into links between breast cancer and diabetes has focused on whether diabetes is a risk factor for breast cancer, and not the other way around. A 2018 meta-analysis of 18 studies found a slightly higher risk of breast cancer in women with diabetes (summary relative risk, 1.13; 95% confidence interval, 1.04-1.24). However, the researchers found evidence that the risk factor might be adiposity, and not diabetes itself (Diabetes. 2018 Jul;67[Supplement 1]. doi: 10.2337/db18-180-OR).
For the new study, researchers used health registries to track women in Denmark for up to 12 years, during 2005-2016. They compared 33,909 women who were older than 50 years and who had new-onset breast cancer with 313,998 women without breast cancer in a matched comparison cohort. The average age in both groups was 66 years; obesity was rare (4% vs. 3%, respectively), but statin therapy (21% in both groups) and hormone replacement therapy (36% vs. 32%) were more prevalent.
In the first year after a breast cancer diagnosis, the women in the breast cancer group were 15% more likely to develop diabetes (per use of diabetes medication or hospital-diagnosed diabetes) than those in the comparison group (adjusted hazard ratio, 1.15; 95% CI, 1.01-1.30) with adjustments for factors such as age, marital status, residence, medical history, medications, and comorbidity.
Over a median follow-up period of 5.2 years, the risk of diabetes was 23% higher in the breast cancer group, at 8.4 new cases per 1,000 women, compared with 6.8 new cases per 1,000 women in the comparison group (aHR, 1.23; 95% CI, 1.16-1.30). Unadjusted hazard ratios were similar.
Women in the breast cancer group who developed diabetes were more likely to use insulin-based therapy, suggesting they had more severe diabetes, compared with those in the control group (5% vs. 2%, respectively; P less than .00001). They were also more likely to be treated with insulin only (4% vs. 1%, P less than .00001).
It is not clear why patients with breast cancer face a higher risk of diabetes. Dr. Thomsen speculated that cancer drugs might play a role and he noted that cancer itself can cause inflammation and “lead to consequences.”
A 2018 study linked usage of hormone therapies, including tamoxifen (HR, 2.25; 95% CI, 1.19-4.26; P = .013) and aromatase inhibitors (HR, 4.27;95% CI, 1.42-12.84), in patients with breast cancer to higher levels of diabetes, compared with patients who did not use hormone therapy (J Clin Oncol. 2018;36[20]:2061-9).
Dr. Thomsen emphasized that physicians should monitor patients with breast cancer for diabetes. “It develops over time, and the risk is increasing, so you need to be aware of that.”
No study funding was reported. One of the researchers reported numerous ties to a range of drug companies. Dr. Thomsen and the other researchers reported no relevant disclosures.
SAN FRANCISCO – a new Danish study finds.
The findings are “quite a clear signal of increased diabetes following breast cancer,” said epidemiologist and study coauthor Reimar W. Thomsen, MD, PhD, of Aarhus (Denmark) University Hospital, in an interview. “It’s very important to tell [patients with breast cancer] what they may expect in the long term.”
He spoke at the annual scientific sessions of the American Diabetes Association, where he presented the study findings.
Much of the research into links between breast cancer and diabetes has focused on whether diabetes is a risk factor for breast cancer, and not the other way around. A 2018 meta-analysis of 18 studies found a slightly higher risk of breast cancer in women with diabetes (summary relative risk, 1.13; 95% confidence interval, 1.04-1.24). However, the researchers found evidence that the risk factor might be adiposity, and not diabetes itself (Diabetes. 2018 Jul;67[Supplement 1]. doi: 10.2337/db18-180-OR).
For the new study, researchers used health registries to track women in Denmark for up to 12 years, during 2005-2016. They compared 33,909 women who were older than 50 years and who had new-onset breast cancer with 313,998 women without breast cancer in a matched comparison cohort. The average age in both groups was 66 years; obesity was rare (4% vs. 3%, respectively), but statin therapy (21% in both groups) and hormone replacement therapy (36% vs. 32%) were more prevalent.
In the first year after a breast cancer diagnosis, the women in the breast cancer group were 15% more likely to develop diabetes (per use of diabetes medication or hospital-diagnosed diabetes) than those in the comparison group (adjusted hazard ratio, 1.15; 95% CI, 1.01-1.30) with adjustments for factors such as age, marital status, residence, medical history, medications, and comorbidity.
Over a median follow-up period of 5.2 years, the risk of diabetes was 23% higher in the breast cancer group, at 8.4 new cases per 1,000 women, compared with 6.8 new cases per 1,000 women in the comparison group (aHR, 1.23; 95% CI, 1.16-1.30). Unadjusted hazard ratios were similar.
Women in the breast cancer group who developed diabetes were more likely to use insulin-based therapy, suggesting they had more severe diabetes, compared with those in the control group (5% vs. 2%, respectively; P less than .00001). They were also more likely to be treated with insulin only (4% vs. 1%, P less than .00001).
It is not clear why patients with breast cancer face a higher risk of diabetes. Dr. Thomsen speculated that cancer drugs might play a role and he noted that cancer itself can cause inflammation and “lead to consequences.”
A 2018 study linked usage of hormone therapies, including tamoxifen (HR, 2.25; 95% CI, 1.19-4.26; P = .013) and aromatase inhibitors (HR, 4.27;95% CI, 1.42-12.84), in patients with breast cancer to higher levels of diabetes, compared with patients who did not use hormone therapy (J Clin Oncol. 2018;36[20]:2061-9).
Dr. Thomsen emphasized that physicians should monitor patients with breast cancer for diabetes. “It develops over time, and the risk is increasing, so you need to be aware of that.”
No study funding was reported. One of the researchers reported numerous ties to a range of drug companies. Dr. Thomsen and the other researchers reported no relevant disclosures.
REPORTING FROM ADA 2019