User login
Failed ATEMPT: T-DM1 no safer in early HER2+ breast cancer
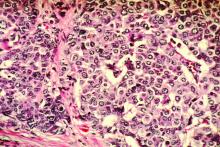
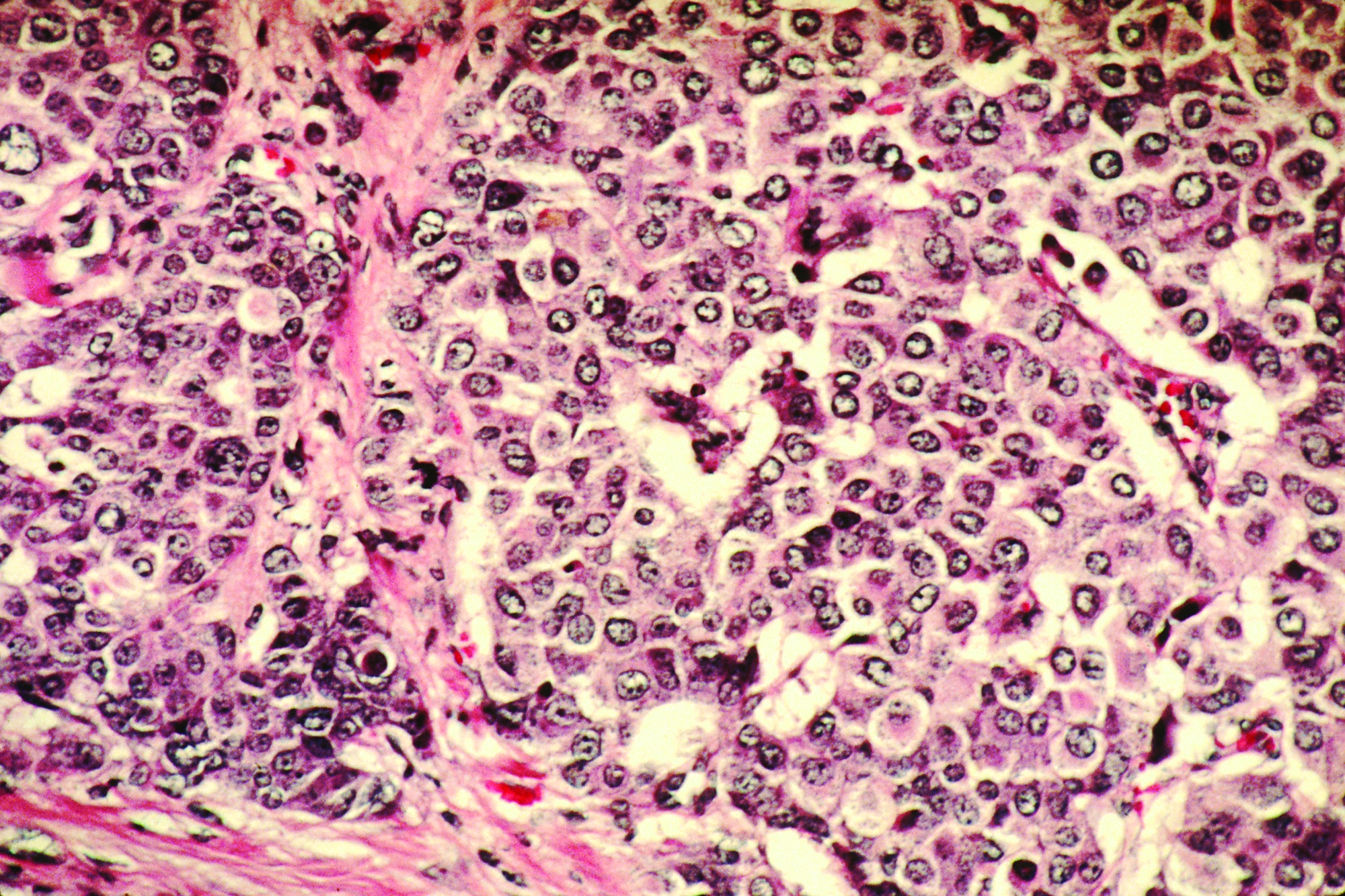
SAN ANTONIO – Nice try, ATEMPT investigators, but trastuzumab emtansine (T-DM1) does not have a disease-free survival or safety advantage over paclitaxel plus trastuzumab in the adjuvant setting for patients with stage 1 HER2-positive breast cancer.
For 497 patients randomized on a 3:1 basis to receive either T-DM1 every 3 weeks for 17 cycles or paclitaxel plus trastuzumab (TH) weekly for 12 cycles followed by trastuzumab every 3 weeks for an additional 13 cycles, there were no significant between-regimen differences in the co-primary endpoints of 3-year disease-free survival (DFS) or clinically relevant toxicities, reported Sara M. Tolaney, MD, MPH of Dana-Farber Cancer Institute, Boston.
“While there was no difference in the overall incidence of clinically relevant toxicities between the two arms, there were differences in the toxicity profiles that were seen between T-DM1 and TH. It’s also important to know that not all toxicities that are significant for our patients are captured in this clinically relevant toxicity endpoint, such as alopecia, and patient-reported outcomes should be considered when assessing the tolerability of therapy,” she said at the San Antonio Breast Cancer Symposium.
For patients with stage 1, HER2-positive breast cancer at high risk of recurrence, paclitaxel and trastuzumab is associated with a 93% disease survival rate.
T-DM1, an drug antibody conjugate of trastuzumab and DM1, a cytotoxic agent, is active against metastatic HER2-positive breast cancer and in patients with residual disease after neoadjuvant HER2-directed therapy.
“Importantly, T-DM1 has been associated with less toxicity when compared to chemotherapy with trastuzumab,” said Dr. Tolaney.
To see whether T-DM1 could be a less toxic treatment option for patients with stage 1 HER2-positive breast cancer at risk for recurrence, the investigators enrolled 512 patients who were within 90 days of surgery, had N0 or microscopic N1 disease, left ventricular ejection fraction (LVEF) of 50% or greater, and no prior invasive breast cancer.
The patients were stratified by age (younger than 55 or 55 and older), planned radiation (yes or no), and planned hormonal therapy (yes or no), and then randomly assigned on a 3:1 basis to receive either T-DM1 3.6 mg/kg intravenously every 3 weeks for 17 cycles, or TH, consisting of paclitaxel 80 mg/m2 plus trastuzumab 2 mg/kg intravenously weekly for 12 cycles, followed by trastuzumab 6 mg/kg every 3 weeks for an additional 13 cycles.
A total of 383 patients assigned to T-DM1 and 114 assigned to TH were included in the intention-to-treat analysis.
The study arms were well balanced by tumor size, histologic grade, hormone receptor status, and HER2 status by fluorescent in situ hybridization (1+, 2+, 3+ or not performed).
Three-year disease-free survival with was 97.7% with T-DM1 and 93.2% with TH, but the study was not powered to detect efficacy differences between the two adjuvant regimens, Dr. Tolaney noted.
In all, 46% of patients in each arm had clinically relevant toxicities. Grade 3 or greater nonhematologic toxicities were seen in 10% of those on T-DM1, vs. 11% of those on TH. Grade 2 or greater neurotoxicity was seen in 11% of patients and 23%, respectively.
Four patients on T-DM1 had grade 4 hematologic toxicity vs. none on TH. Febrile neutropenia was not seen among patients on T-DM1, but occurred in two patients on TH.
The incidence of toxicity requiring a dose delay was 28% and 26%, respectively, while nearly 3 times as many patients on T-DM1 had toxicities requiring early discontinuation (17% vs. 6%).
T-DM1 was also associated with higher incidences of grade 2 or greater thrombocytopenia (11% vs. 1%), alanine aminotransferase elevation (9% vs. 4%), and bilirubin increase (5% vs. 1%).
Three patients on T-DM1 and one on TH had symptomatic heart failure. Asymptomatic declines in LVEF were seen in five and seven patients, respectively.
“Given the low event rate seen in this trial, T-DM1 may be considered an alternative treatment approach to TH for select patients with stage 1 HER2-positive disease who are concerned about specific TH-related side effects and who understand the potential for T-DM1 toxicities. There may be some patients and physicians, however, who will want longer follow-up before adopting such an approach,” Dr. Tolaney said.
In the question and response session, an audience member said, “I would like to add one more toxicity that has not been considered, which is financial toxicity. There’s a huge difference in the price of both regimens, and the total cost of care.”
Dr. Tolaney replied that “certainly we did consider this and we had our pharmacist do some calculations looking at this financial toxicity, and it is true that a year of T-DM1 does cost a little more than two times as much as TH.”
She agreed that financial toxicity is a very important consideration when making treatment decisions, “but I think there are differences in toxicity profiles that do need to be considered when making individual decisions for our patients.”
Invited discussant A. Jo Chien, MD of the University of California, San Francisco noted that 75% of all patients enrolled in ATEMPT had hormone receptor positive disease “and therefore 3 years median follow-up is relatively short for this cohort.
“Due to the high rates of discontinuation in the T-DM1 arm, it is important to remember that duration of toxicity is a contributor to overall tolerability, which often is not well characterized by standard toxicity assessments, which often just report highest-grade toxicity at one point in time. High-grade toxicities that are short-lived may be acceptable, but low-grade toxicities for longer duration may not,” she said.
The ATEMPT trial was funded by Genentech. Dr. Tolaney has disclosed advisory board participation, institutional research funds, honoraria, and travel expense reimbursement from the company. Dr. Chien disclosed institutional research funding from Merck, Puma, Seattle Genetics, Astellas, and Amgen.
SOURCE: Tolaney SM et al. SABCS 2019, Abstract GS1-05.
SAN ANTONIO – Nice try, ATEMPT investigators, but trastuzumab emtansine (T-DM1) does not have a disease-free survival or safety advantage over paclitaxel plus trastuzumab in the adjuvant setting for patients with stage 1 HER2-positive breast cancer.
For 497 patients randomized on a 3:1 basis to receive either T-DM1 every 3 weeks for 17 cycles or paclitaxel plus trastuzumab (TH) weekly for 12 cycles followed by trastuzumab every 3 weeks for an additional 13 cycles, there were no significant between-regimen differences in the co-primary endpoints of 3-year disease-free survival (DFS) or clinically relevant toxicities, reported Sara M. Tolaney, MD, MPH of Dana-Farber Cancer Institute, Boston.
“While there was no difference in the overall incidence of clinically relevant toxicities between the two arms, there were differences in the toxicity profiles that were seen between T-DM1 and TH. It’s also important to know that not all toxicities that are significant for our patients are captured in this clinically relevant toxicity endpoint, such as alopecia, and patient-reported outcomes should be considered when assessing the tolerability of therapy,” she said at the San Antonio Breast Cancer Symposium.
For patients with stage 1, HER2-positive breast cancer at high risk of recurrence, paclitaxel and trastuzumab is associated with a 93% disease survival rate.
T-DM1, an drug antibody conjugate of trastuzumab and DM1, a cytotoxic agent, is active against metastatic HER2-positive breast cancer and in patients with residual disease after neoadjuvant HER2-directed therapy.
“Importantly, T-DM1 has been associated with less toxicity when compared to chemotherapy with trastuzumab,” said Dr. Tolaney.
To see whether T-DM1 could be a less toxic treatment option for patients with stage 1 HER2-positive breast cancer at risk for recurrence, the investigators enrolled 512 patients who were within 90 days of surgery, had N0 or microscopic N1 disease, left ventricular ejection fraction (LVEF) of 50% or greater, and no prior invasive breast cancer.
The patients were stratified by age (younger than 55 or 55 and older), planned radiation (yes or no), and planned hormonal therapy (yes or no), and then randomly assigned on a 3:1 basis to receive either T-DM1 3.6 mg/kg intravenously every 3 weeks for 17 cycles, or TH, consisting of paclitaxel 80 mg/m2 plus trastuzumab 2 mg/kg intravenously weekly for 12 cycles, followed by trastuzumab 6 mg/kg every 3 weeks for an additional 13 cycles.
A total of 383 patients assigned to T-DM1 and 114 assigned to TH were included in the intention-to-treat analysis.
The study arms were well balanced by tumor size, histologic grade, hormone receptor status, and HER2 status by fluorescent in situ hybridization (1+, 2+, 3+ or not performed).
Three-year disease-free survival with was 97.7% with T-DM1 and 93.2% with TH, but the study was not powered to detect efficacy differences between the two adjuvant regimens, Dr. Tolaney noted.
In all, 46% of patients in each arm had clinically relevant toxicities. Grade 3 or greater nonhematologic toxicities were seen in 10% of those on T-DM1, vs. 11% of those on TH. Grade 2 or greater neurotoxicity was seen in 11% of patients and 23%, respectively.
Four patients on T-DM1 had grade 4 hematologic toxicity vs. none on TH. Febrile neutropenia was not seen among patients on T-DM1, but occurred in two patients on TH.
The incidence of toxicity requiring a dose delay was 28% and 26%, respectively, while nearly 3 times as many patients on T-DM1 had toxicities requiring early discontinuation (17% vs. 6%).
T-DM1 was also associated with higher incidences of grade 2 or greater thrombocytopenia (11% vs. 1%), alanine aminotransferase elevation (9% vs. 4%), and bilirubin increase (5% vs. 1%).
Three patients on T-DM1 and one on TH had symptomatic heart failure. Asymptomatic declines in LVEF were seen in five and seven patients, respectively.
“Given the low event rate seen in this trial, T-DM1 may be considered an alternative treatment approach to TH for select patients with stage 1 HER2-positive disease who are concerned about specific TH-related side effects and who understand the potential for T-DM1 toxicities. There may be some patients and physicians, however, who will want longer follow-up before adopting such an approach,” Dr. Tolaney said.
In the question and response session, an audience member said, “I would like to add one more toxicity that has not been considered, which is financial toxicity. There’s a huge difference in the price of both regimens, and the total cost of care.”
Dr. Tolaney replied that “certainly we did consider this and we had our pharmacist do some calculations looking at this financial toxicity, and it is true that a year of T-DM1 does cost a little more than two times as much as TH.”
She agreed that financial toxicity is a very important consideration when making treatment decisions, “but I think there are differences in toxicity profiles that do need to be considered when making individual decisions for our patients.”
Invited discussant A. Jo Chien, MD of the University of California, San Francisco noted that 75% of all patients enrolled in ATEMPT had hormone receptor positive disease “and therefore 3 years median follow-up is relatively short for this cohort.
“Due to the high rates of discontinuation in the T-DM1 arm, it is important to remember that duration of toxicity is a contributor to overall tolerability, which often is not well characterized by standard toxicity assessments, which often just report highest-grade toxicity at one point in time. High-grade toxicities that are short-lived may be acceptable, but low-grade toxicities for longer duration may not,” she said.
The ATEMPT trial was funded by Genentech. Dr. Tolaney has disclosed advisory board participation, institutional research funds, honoraria, and travel expense reimbursement from the company. Dr. Chien disclosed institutional research funding from Merck, Puma, Seattle Genetics, Astellas, and Amgen.
SOURCE: Tolaney SM et al. SABCS 2019, Abstract GS1-05.
SAN ANTONIO – Nice try, ATEMPT investigators, but trastuzumab emtansine (T-DM1) does not have a disease-free survival or safety advantage over paclitaxel plus trastuzumab in the adjuvant setting for patients with stage 1 HER2-positive breast cancer.
For 497 patients randomized on a 3:1 basis to receive either T-DM1 every 3 weeks for 17 cycles or paclitaxel plus trastuzumab (TH) weekly for 12 cycles followed by trastuzumab every 3 weeks for an additional 13 cycles, there were no significant between-regimen differences in the co-primary endpoints of 3-year disease-free survival (DFS) or clinically relevant toxicities, reported Sara M. Tolaney, MD, MPH of Dana-Farber Cancer Institute, Boston.
“While there was no difference in the overall incidence of clinically relevant toxicities between the two arms, there were differences in the toxicity profiles that were seen between T-DM1 and TH. It’s also important to know that not all toxicities that are significant for our patients are captured in this clinically relevant toxicity endpoint, such as alopecia, and patient-reported outcomes should be considered when assessing the tolerability of therapy,” she said at the San Antonio Breast Cancer Symposium.
For patients with stage 1, HER2-positive breast cancer at high risk of recurrence, paclitaxel and trastuzumab is associated with a 93% disease survival rate.
T-DM1, an drug antibody conjugate of trastuzumab and DM1, a cytotoxic agent, is active against metastatic HER2-positive breast cancer and in patients with residual disease after neoadjuvant HER2-directed therapy.
“Importantly, T-DM1 has been associated with less toxicity when compared to chemotherapy with trastuzumab,” said Dr. Tolaney.
To see whether T-DM1 could be a less toxic treatment option for patients with stage 1 HER2-positive breast cancer at risk for recurrence, the investigators enrolled 512 patients who were within 90 days of surgery, had N0 or microscopic N1 disease, left ventricular ejection fraction (LVEF) of 50% or greater, and no prior invasive breast cancer.
The patients were stratified by age (younger than 55 or 55 and older), planned radiation (yes or no), and planned hormonal therapy (yes or no), and then randomly assigned on a 3:1 basis to receive either T-DM1 3.6 mg/kg intravenously every 3 weeks for 17 cycles, or TH, consisting of paclitaxel 80 mg/m2 plus trastuzumab 2 mg/kg intravenously weekly for 12 cycles, followed by trastuzumab 6 mg/kg every 3 weeks for an additional 13 cycles.
A total of 383 patients assigned to T-DM1 and 114 assigned to TH were included in the intention-to-treat analysis.
The study arms were well balanced by tumor size, histologic grade, hormone receptor status, and HER2 status by fluorescent in situ hybridization (1+, 2+, 3+ or not performed).
Three-year disease-free survival with was 97.7% with T-DM1 and 93.2% with TH, but the study was not powered to detect efficacy differences between the two adjuvant regimens, Dr. Tolaney noted.
In all, 46% of patients in each arm had clinically relevant toxicities. Grade 3 or greater nonhematologic toxicities were seen in 10% of those on T-DM1, vs. 11% of those on TH. Grade 2 or greater neurotoxicity was seen in 11% of patients and 23%, respectively.
Four patients on T-DM1 had grade 4 hematologic toxicity vs. none on TH. Febrile neutropenia was not seen among patients on T-DM1, but occurred in two patients on TH.
The incidence of toxicity requiring a dose delay was 28% and 26%, respectively, while nearly 3 times as many patients on T-DM1 had toxicities requiring early discontinuation (17% vs. 6%).
T-DM1 was also associated with higher incidences of grade 2 or greater thrombocytopenia (11% vs. 1%), alanine aminotransferase elevation (9% vs. 4%), and bilirubin increase (5% vs. 1%).
Three patients on T-DM1 and one on TH had symptomatic heart failure. Asymptomatic declines in LVEF were seen in five and seven patients, respectively.
“Given the low event rate seen in this trial, T-DM1 may be considered an alternative treatment approach to TH for select patients with stage 1 HER2-positive disease who are concerned about specific TH-related side effects and who understand the potential for T-DM1 toxicities. There may be some patients and physicians, however, who will want longer follow-up before adopting such an approach,” Dr. Tolaney said.
In the question and response session, an audience member said, “I would like to add one more toxicity that has not been considered, which is financial toxicity. There’s a huge difference in the price of both regimens, and the total cost of care.”
Dr. Tolaney replied that “certainly we did consider this and we had our pharmacist do some calculations looking at this financial toxicity, and it is true that a year of T-DM1 does cost a little more than two times as much as TH.”
She agreed that financial toxicity is a very important consideration when making treatment decisions, “but I think there are differences in toxicity profiles that do need to be considered when making individual decisions for our patients.”
Invited discussant A. Jo Chien, MD of the University of California, San Francisco noted that 75% of all patients enrolled in ATEMPT had hormone receptor positive disease “and therefore 3 years median follow-up is relatively short for this cohort.
“Due to the high rates of discontinuation in the T-DM1 arm, it is important to remember that duration of toxicity is a contributor to overall tolerability, which often is not well characterized by standard toxicity assessments, which often just report highest-grade toxicity at one point in time. High-grade toxicities that are short-lived may be acceptable, but low-grade toxicities for longer duration may not,” she said.
The ATEMPT trial was funded by Genentech. Dr. Tolaney has disclosed advisory board participation, institutional research funds, honoraria, and travel expense reimbursement from the company. Dr. Chien disclosed institutional research funding from Merck, Puma, Seattle Genetics, Astellas, and Amgen.
SOURCE: Tolaney SM et al. SABCS 2019, Abstract GS1-05.
REPORTING FROM SABCS 2019
Key clinical point: Trastuzumab emtansine did not have a lower incidence of toxicities compared with trastuzumab/paclitaxel.
Major finding: In each trial arm, 46% of patients had clinically relevant toxicities.
Study details: Randomized phase 2 trial in 497 patients with stage 1 HER2-positive breast cancer.
Disclosures: The ATEMPT trial was funded by Genentech. Dr. Tolaney has disclosed advisory board participation, institutional research funds, honoraria, and travel expense reimbursement from the company. Dr. Chien disclosed institutional research funding from Merck, Puma, Seattle Genetics, Astellas, and Amgen.
Source: Tolaney SM et al. SABCS 2019. Abstract GS1-05.
Adjuvant denosumab falls short in early-stage breast cancer
Adjuvant denosumab did not improve bone metastasis–free survival and related outcomes in women with early-stage breast cancer, according to a phase 3 trial.
“We hypothesised that denosumab would modify the clinical course of early breast cancer, delaying the development of clinical bone metastases with or without disease recurrence at other sites,” reported Robert Coleman, MBBS, MD, of the University of Sheffield, England, and colleagues. Their report is in The Lancet Oncology.
The randomized, placebo-controlled, phase 3 D-CARE study included 4,509 women with early-stage, high-risk disease. The effects of adding denosumab to standard-of-care adjuvant or neoadjuvant chemotherapy was studied in 389 institutions around the globe. In the initial treatment phase, study patients received denosumab or placebo every 3-4 weeks in combination with adjuvant or neoadjuvant chemotherapy for approximately 6 months.
After completion of chemotherapy, the dosing interval was extended to every 12 weeks (range, 14 days) for a total of 5 years. The median age of women who received denosumab was 50 years (range, 44-59 years), 65% of whom were hormone receptor positive, HER2-negative. In the study, patients were stratified by various factors, including type of therapy, age, lymph node status, geographical region, and others. The primary outcome was a composite endpoint of bone metastasis–free survival.
At 5-year follow-up, the researchers found no significant difference in bone metastasis–free survival between the denosumab and placebo treatment arms (median survival not reached in either arm; P = .70). With respect to safety, the most frequently seen grade 3 or higher treatment-emergent adverse events were neutropenia (15% vs. 15%), febrile neutropenia (5% vs. 6%), and leukopenia (3% vs. 3%). Positively adjudicated osteonecrosis of the jaw occurred in 122 (5%) of 2,241 patients treated with denosumab versus 4 (less than 1%) of 2,218 patients treated with placebo, Dr. Coleman and colleagues wrote.
The researchers acknowledged that a key limitation of the study was the smaller than anticipated number of events for efficacy outcomes. As a result, the study protocol was modified, which could have limited the statistical power of the study. “The results of this study do not support a role for denosumab as an antitumour agent in this setting,” they concluded.
Amgen funded the study. The authors reported financial affiliations with AbbVie, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Covance, Lilly, Medivation, Merck Serono, Merck Sharp and Dohme, Novartis, Pfizer, and several others.
SOURCE: Coleman R et al. Lancet Oncol. 2019 Dec 2. doi: 10.1016/S1470-2045(19)30687-4.
Adjuvant denosumab did not improve bone metastasis–free survival and related outcomes in women with early-stage breast cancer, according to a phase 3 trial.
“We hypothesised that denosumab would modify the clinical course of early breast cancer, delaying the development of clinical bone metastases with or without disease recurrence at other sites,” reported Robert Coleman, MBBS, MD, of the University of Sheffield, England, and colleagues. Their report is in The Lancet Oncology.
The randomized, placebo-controlled, phase 3 D-CARE study included 4,509 women with early-stage, high-risk disease. The effects of adding denosumab to standard-of-care adjuvant or neoadjuvant chemotherapy was studied in 389 institutions around the globe. In the initial treatment phase, study patients received denosumab or placebo every 3-4 weeks in combination with adjuvant or neoadjuvant chemotherapy for approximately 6 months.
After completion of chemotherapy, the dosing interval was extended to every 12 weeks (range, 14 days) for a total of 5 years. The median age of women who received denosumab was 50 years (range, 44-59 years), 65% of whom were hormone receptor positive, HER2-negative. In the study, patients were stratified by various factors, including type of therapy, age, lymph node status, geographical region, and others. The primary outcome was a composite endpoint of bone metastasis–free survival.
At 5-year follow-up, the researchers found no significant difference in bone metastasis–free survival between the denosumab and placebo treatment arms (median survival not reached in either arm; P = .70). With respect to safety, the most frequently seen grade 3 or higher treatment-emergent adverse events were neutropenia (15% vs. 15%), febrile neutropenia (5% vs. 6%), and leukopenia (3% vs. 3%). Positively adjudicated osteonecrosis of the jaw occurred in 122 (5%) of 2,241 patients treated with denosumab versus 4 (less than 1%) of 2,218 patients treated with placebo, Dr. Coleman and colleagues wrote.
The researchers acknowledged that a key limitation of the study was the smaller than anticipated number of events for efficacy outcomes. As a result, the study protocol was modified, which could have limited the statistical power of the study. “The results of this study do not support a role for denosumab as an antitumour agent in this setting,” they concluded.
Amgen funded the study. The authors reported financial affiliations with AbbVie, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Covance, Lilly, Medivation, Merck Serono, Merck Sharp and Dohme, Novartis, Pfizer, and several others.
SOURCE: Coleman R et al. Lancet Oncol. 2019 Dec 2. doi: 10.1016/S1470-2045(19)30687-4.
Adjuvant denosumab did not improve bone metastasis–free survival and related outcomes in women with early-stage breast cancer, according to a phase 3 trial.
“We hypothesised that denosumab would modify the clinical course of early breast cancer, delaying the development of clinical bone metastases with or without disease recurrence at other sites,” reported Robert Coleman, MBBS, MD, of the University of Sheffield, England, and colleagues. Their report is in The Lancet Oncology.
The randomized, placebo-controlled, phase 3 D-CARE study included 4,509 women with early-stage, high-risk disease. The effects of adding denosumab to standard-of-care adjuvant or neoadjuvant chemotherapy was studied in 389 institutions around the globe. In the initial treatment phase, study patients received denosumab or placebo every 3-4 weeks in combination with adjuvant or neoadjuvant chemotherapy for approximately 6 months.
After completion of chemotherapy, the dosing interval was extended to every 12 weeks (range, 14 days) for a total of 5 years. The median age of women who received denosumab was 50 years (range, 44-59 years), 65% of whom were hormone receptor positive, HER2-negative. In the study, patients were stratified by various factors, including type of therapy, age, lymph node status, geographical region, and others. The primary outcome was a composite endpoint of bone metastasis–free survival.
At 5-year follow-up, the researchers found no significant difference in bone metastasis–free survival between the denosumab and placebo treatment arms (median survival not reached in either arm; P = .70). With respect to safety, the most frequently seen grade 3 or higher treatment-emergent adverse events were neutropenia (15% vs. 15%), febrile neutropenia (5% vs. 6%), and leukopenia (3% vs. 3%). Positively adjudicated osteonecrosis of the jaw occurred in 122 (5%) of 2,241 patients treated with denosumab versus 4 (less than 1%) of 2,218 patients treated with placebo, Dr. Coleman and colleagues wrote.
The researchers acknowledged that a key limitation of the study was the smaller than anticipated number of events for efficacy outcomes. As a result, the study protocol was modified, which could have limited the statistical power of the study. “The results of this study do not support a role for denosumab as an antitumour agent in this setting,” they concluded.
Amgen funded the study. The authors reported financial affiliations with AbbVie, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Covance, Lilly, Medivation, Merck Serono, Merck Sharp and Dohme, Novartis, Pfizer, and several others.
SOURCE: Coleman R et al. Lancet Oncol. 2019 Dec 2. doi: 10.1016/S1470-2045(19)30687-4.
FROM LANCET ONCOLOGY
First generics for Gilenya approved by FDA
The Food and Drug Administration has approved the first generics of fingolimod (Gilenya) for the treatment of relapsing forms of multiple sclerosis.
The three generic fingolimod applications came from HEC Pharm, Biocon, and Sun Pharmaceutical Industries.
Fingolimod is a widely used, orally administered treatment option for relapsing forms of multiple sclerosis in adults. The most common adverse events associated with fingolimod in clinical trials include headache, elevation of liver enzymes, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in the extremities.
The drug must be dispensed with a medication guide that contains important information on its usage and risk, the FDA noted. Serious risks associated with fingolimod include slowing of the heart rate, vision problems, posterior reversible encephalopathy syndrome, respiratory problems, liver injury, increased blood pressure, skin cancer, and risk of serious infection including a rare and often deadly brain infection called progressive multifocal leukoencephalopathy. Fingolimod can also cause harm to a developing fetus.
Find the full press release on the FDA website.
The Food and Drug Administration has approved the first generics of fingolimod (Gilenya) for the treatment of relapsing forms of multiple sclerosis.
The three generic fingolimod applications came from HEC Pharm, Biocon, and Sun Pharmaceutical Industries.
Fingolimod is a widely used, orally administered treatment option for relapsing forms of multiple sclerosis in adults. The most common adverse events associated with fingolimod in clinical trials include headache, elevation of liver enzymes, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in the extremities.
The drug must be dispensed with a medication guide that contains important information on its usage and risk, the FDA noted. Serious risks associated with fingolimod include slowing of the heart rate, vision problems, posterior reversible encephalopathy syndrome, respiratory problems, liver injury, increased blood pressure, skin cancer, and risk of serious infection including a rare and often deadly brain infection called progressive multifocal leukoencephalopathy. Fingolimod can also cause harm to a developing fetus.
Find the full press release on the FDA website.
The Food and Drug Administration has approved the first generics of fingolimod (Gilenya) for the treatment of relapsing forms of multiple sclerosis.
The three generic fingolimod applications came from HEC Pharm, Biocon, and Sun Pharmaceutical Industries.
Fingolimod is a widely used, orally administered treatment option for relapsing forms of multiple sclerosis in adults. The most common adverse events associated with fingolimod in clinical trials include headache, elevation of liver enzymes, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in the extremities.
The drug must be dispensed with a medication guide that contains important information on its usage and risk, the FDA noted. Serious risks associated with fingolimod include slowing of the heart rate, vision problems, posterior reversible encephalopathy syndrome, respiratory problems, liver injury, increased blood pressure, skin cancer, and risk of serious infection including a rare and often deadly brain infection called progressive multifocal leukoencephalopathy. Fingolimod can also cause harm to a developing fetus.
Find the full press release on the FDA website.
Bilateral mastectomy reduces second breast cancer risk, but not deaths
Bilateral mastectomy significantly decreases the risk for a second contralateral breast cancer, but does not decrease the risk of death, compared with breast-conserving therapy, results of a large retrospective study indicate.
Among 245,418 patients followed for a median of 6.7 years, the risk of death from breast cancer was similar for those who had undergone either breast-conserving therapy or bilateral mastectomy (BLM) but was 20% higher among women who had undergone unilateral mastectomy (ULM) when compared with breast-conserving therapy, reported Allison W. Kurian, MD, MSc, from Stanford (Calif.) University, and colleagues.
“Second breast cancers are rare, and their reduction should be weighed against the harms associated with BLM,” they wrote in a study published online in Cancer.
The investigators extracted data from the Surveillance, Epidemiology, and End Results program on all women diagnosed with American Joint Committee on Cancer stage 0 to stage III unilateral breast cancer in California from 1998 to 2015 who were treated with either BLM versus breast-conserving therapy, including surgery and radiation or unilateral mastectomy.
They calculated the absolute excess risk of contralateral breast cancer as the observed minus expected number of breast cancers in the general population divided by 10,000 person-years at risk.
Of 421,643 women with a first diagnosis of primary breast cancer during the study period, 245,418 met the study criteria. Of this cohort, 7,784 (3.2%) developed a contralateral second breast cancer more than 6 months after diagnosis of the first, after a median 6.7 years of follow-up.
Slightly more than half of the cohort (52.1%) had undergone breast-conserving therapy, 37.5% underwent unilateral mastectomy, and 7.6% had bilateral mastectomy. An additional 2.9% of patients were women aged 70 years and older with stage I hormone receptor–positive, HER2-negative disease who underwent breast-conserving surgery without radiation (percentages exceed 100% because of rounding).
A multivariate-adjusted model showed that, as might be expected, patients who underwent bilateral mastectomy had a 90% reduction in risk of contralateral cancer (hazard ratio, 0.10; P less than .001), compared with breast-conserving therapy. In contrast, patients who underwent unilateral mastectomy had a slight but significant increase in risk for a second contralateral breast cancer (HR, 1.07; P = .008).
The absolute excess risk for second contralateral breast cancer was 5 per 10,000 person-years with breast-conserving therapy, 13.6 per 10,000 person-years with unilateral mastectomy, and –28.6 per 10,000 person-years with bilateral mastectomy.
When they looked at risk for death, however they found that, compared with breast-conserving therapy, breast-conserving surgery alone (HR, 1.36; P = .0001) and unilateral mastectomy (HR, 1.21; P less than .001), but not bilateral mastectomy (HR, 1.03; P = .35) were significantly associated with increased risk for breast cancer death.
The authors noted that their estimates of absolute risk of second contralateral breast cancer jibe with those of earlier studies, and can help clinicians frame the discussion of the benefits versus risks for individual patients.
“What one patient might consider to be a negligible benefit of BLM, weighed against its potential harms of greater pain, recovery time, and impact on body image and employment, might appear worthwhile to another,” they wrote.
The study was funded by the National Cancer Institute, National Institutes of Health, Department of Health & Human Services, Suzanne Pride Bryan Fund for Breast Cancer Research, Jan Weimer Faculty Chair for Breast Oncology, and the BRCA Foundation. Dr. Kurian disclosed institutional research funding from Myriad Genetics.
SOURCE: Kurin AW et al. Cancer. 2019 Nov 21. doi: 10.1002/cncr.32618.
Bilateral mastectomy significantly decreases the risk for a second contralateral breast cancer, but does not decrease the risk of death, compared with breast-conserving therapy, results of a large retrospective study indicate.
Among 245,418 patients followed for a median of 6.7 years, the risk of death from breast cancer was similar for those who had undergone either breast-conserving therapy or bilateral mastectomy (BLM) but was 20% higher among women who had undergone unilateral mastectomy (ULM) when compared with breast-conserving therapy, reported Allison W. Kurian, MD, MSc, from Stanford (Calif.) University, and colleagues.
“Second breast cancers are rare, and their reduction should be weighed against the harms associated with BLM,” they wrote in a study published online in Cancer.
The investigators extracted data from the Surveillance, Epidemiology, and End Results program on all women diagnosed with American Joint Committee on Cancer stage 0 to stage III unilateral breast cancer in California from 1998 to 2015 who were treated with either BLM versus breast-conserving therapy, including surgery and radiation or unilateral mastectomy.
They calculated the absolute excess risk of contralateral breast cancer as the observed minus expected number of breast cancers in the general population divided by 10,000 person-years at risk.
Of 421,643 women with a first diagnosis of primary breast cancer during the study period, 245,418 met the study criteria. Of this cohort, 7,784 (3.2%) developed a contralateral second breast cancer more than 6 months after diagnosis of the first, after a median 6.7 years of follow-up.
Slightly more than half of the cohort (52.1%) had undergone breast-conserving therapy, 37.5% underwent unilateral mastectomy, and 7.6% had bilateral mastectomy. An additional 2.9% of patients were women aged 70 years and older with stage I hormone receptor–positive, HER2-negative disease who underwent breast-conserving surgery without radiation (percentages exceed 100% because of rounding).
A multivariate-adjusted model showed that, as might be expected, patients who underwent bilateral mastectomy had a 90% reduction in risk of contralateral cancer (hazard ratio, 0.10; P less than .001), compared with breast-conserving therapy. In contrast, patients who underwent unilateral mastectomy had a slight but significant increase in risk for a second contralateral breast cancer (HR, 1.07; P = .008).
The absolute excess risk for second contralateral breast cancer was 5 per 10,000 person-years with breast-conserving therapy, 13.6 per 10,000 person-years with unilateral mastectomy, and –28.6 per 10,000 person-years with bilateral mastectomy.
When they looked at risk for death, however they found that, compared with breast-conserving therapy, breast-conserving surgery alone (HR, 1.36; P = .0001) and unilateral mastectomy (HR, 1.21; P less than .001), but not bilateral mastectomy (HR, 1.03; P = .35) were significantly associated with increased risk for breast cancer death.
The authors noted that their estimates of absolute risk of second contralateral breast cancer jibe with those of earlier studies, and can help clinicians frame the discussion of the benefits versus risks for individual patients.
“What one patient might consider to be a negligible benefit of BLM, weighed against its potential harms of greater pain, recovery time, and impact on body image and employment, might appear worthwhile to another,” they wrote.
The study was funded by the National Cancer Institute, National Institutes of Health, Department of Health & Human Services, Suzanne Pride Bryan Fund for Breast Cancer Research, Jan Weimer Faculty Chair for Breast Oncology, and the BRCA Foundation. Dr. Kurian disclosed institutional research funding from Myriad Genetics.
SOURCE: Kurin AW et al. Cancer. 2019 Nov 21. doi: 10.1002/cncr.32618.
Bilateral mastectomy significantly decreases the risk for a second contralateral breast cancer, but does not decrease the risk of death, compared with breast-conserving therapy, results of a large retrospective study indicate.
Among 245,418 patients followed for a median of 6.7 years, the risk of death from breast cancer was similar for those who had undergone either breast-conserving therapy or bilateral mastectomy (BLM) but was 20% higher among women who had undergone unilateral mastectomy (ULM) when compared with breast-conserving therapy, reported Allison W. Kurian, MD, MSc, from Stanford (Calif.) University, and colleagues.
“Second breast cancers are rare, and their reduction should be weighed against the harms associated with BLM,” they wrote in a study published online in Cancer.
The investigators extracted data from the Surveillance, Epidemiology, and End Results program on all women diagnosed with American Joint Committee on Cancer stage 0 to stage III unilateral breast cancer in California from 1998 to 2015 who were treated with either BLM versus breast-conserving therapy, including surgery and radiation or unilateral mastectomy.
They calculated the absolute excess risk of contralateral breast cancer as the observed minus expected number of breast cancers in the general population divided by 10,000 person-years at risk.
Of 421,643 women with a first diagnosis of primary breast cancer during the study period, 245,418 met the study criteria. Of this cohort, 7,784 (3.2%) developed a contralateral second breast cancer more than 6 months after diagnosis of the first, after a median 6.7 years of follow-up.
Slightly more than half of the cohort (52.1%) had undergone breast-conserving therapy, 37.5% underwent unilateral mastectomy, and 7.6% had bilateral mastectomy. An additional 2.9% of patients were women aged 70 years and older with stage I hormone receptor–positive, HER2-negative disease who underwent breast-conserving surgery without radiation (percentages exceed 100% because of rounding).
A multivariate-adjusted model showed that, as might be expected, patients who underwent bilateral mastectomy had a 90% reduction in risk of contralateral cancer (hazard ratio, 0.10; P less than .001), compared with breast-conserving therapy. In contrast, patients who underwent unilateral mastectomy had a slight but significant increase in risk for a second contralateral breast cancer (HR, 1.07; P = .008).
The absolute excess risk for second contralateral breast cancer was 5 per 10,000 person-years with breast-conserving therapy, 13.6 per 10,000 person-years with unilateral mastectomy, and –28.6 per 10,000 person-years with bilateral mastectomy.
When they looked at risk for death, however they found that, compared with breast-conserving therapy, breast-conserving surgery alone (HR, 1.36; P = .0001) and unilateral mastectomy (HR, 1.21; P less than .001), but not bilateral mastectomy (HR, 1.03; P = .35) were significantly associated with increased risk for breast cancer death.
The authors noted that their estimates of absolute risk of second contralateral breast cancer jibe with those of earlier studies, and can help clinicians frame the discussion of the benefits versus risks for individual patients.
“What one patient might consider to be a negligible benefit of BLM, weighed against its potential harms of greater pain, recovery time, and impact on body image and employment, might appear worthwhile to another,” they wrote.
The study was funded by the National Cancer Institute, National Institutes of Health, Department of Health & Human Services, Suzanne Pride Bryan Fund for Breast Cancer Research, Jan Weimer Faculty Chair for Breast Oncology, and the BRCA Foundation. Dr. Kurian disclosed institutional research funding from Myriad Genetics.
SOURCE: Kurin AW et al. Cancer. 2019 Nov 21. doi: 10.1002/cncr.32618.
FROM CANCER
More phase 3 ubrogepant data published as FDA decision nears
published Dec. 4 in the New England Journal of Medicine. In addition, about 38% of patients who receive ubrogepant no longer have their most bothersome migraine-associated symptom, such as photophobia, phonophobia, or nausea, at 2 hours, compared with 28% of patients who receive placebo, said David W. Dodick, MD, and colleagues.
Dr. Dodick, professor of neurology at the Mayo Clinic in Phoenix, and his coauthors described efficacy and safety results from the ACHIEVE I trial. Another phase 3 study of ubrogepant, ACHIEVE II, was published in JAMA in November. That trial evaluated 25- and 50-mg doses of ubrogepant versus placebo and found rates of pain freedom and absence of the most bothersome symptom in the placebo and active treatment arms that were similar to those in ACHIEVE I.
Assessing a gepant for acute migraine treatment
Ubrogepant is an oral calcitonin gene–related peptide (CGRP) receptor antagonist. Allergan, the company developing the drug, has said it expects the Food and Drug Administration to decide in December whether to approve the drug.
To compare ubrogepant 50 mg, ubrogepant 100 mg, and placebo for the acute treatment of migraine, investigators conducted the randomized ACHIEVE I trial. Researchers enrolled 1,672 adults with migraine with or without aura. They excluded patients with clinically significant cardiovascular or cerebrovascular disease. During the trial, patients treated a single migraine attack, and they had the option to take a second dose. In all, 1,436 participants took an initial dose. Patients had an average age of 40.5 years, about 88% were women, and 82% were white.
In ACHIEVE I, the most common adverse events within 48 hours of treatment were nausea, somnolence, and dry mouth, and these events occurred more frequently in the 100-mg–dose group, Dr. Dodick and colleagues reported. Among patients who received ubrogepant, serious adverse events more than 48 hours after treatment but within 30 days of treatment included appendicitis, spontaneous abortion, pericardial effusion, and seizure. No serious adverse events occurred in the placebo group.
The authors noted that, “there was no active comparator and no evaluation of consistency of effect across multiple migraine attacks; therefore it is not possible to determine whether the drug is more or less effective than standard therapies or consistently effective with repeated use.” In addition, “safety and side-effect data from this trial were based on evaluation of a single attack, and therefore safety after repeated use cannot be inferred; an extension trial has assessed the long-term safety of ubrogepant,” they said.
The present trial was performed well, commented Alan M. Rapoport, MD. “The coprimary endpoints of pain freedom and most bothersome symptom freedom, both at 2 hours after dosing, were statistically superior for both doses of ubrogepant versus placebo,” he said. “Some of the secondary endpoints, such as pain relief at 2 hours post dose and sustained pain relief from 2 to 24 hours, were statistically better than placebo.”
“Based on this data, I suspect that the FDA would approve this gepant after appropriate safety data,” said Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles and editor-in-chief of Neurology Reviews. “Many more patients need to take this drug before we can be sure it is safe and effective.”
The CGRP therapeutic landscape
“Other gepants have been shown to be effective, although some have caused a degree of liver toxicity,” said Dr. Rapoport. “Blocking the effect of CGRP on the migraine peripheral nervous system, in this case by preventing the ligand from docking at its receptor by administering an oral CGRP receptor blocker, appears to be effective.” Researchers are studying another oral gepant for similar approval, he added.
Ubrogepant stands to join other treatments targeting CGRP.
“There are currently three, and soon to be four, injectable monoclonal antibodies against CGRP functionality, which are preventive, not acute-care drugs,” Dr. Rapoport said. “The first released was a subcutaneous injection of a CGRP receptor blocker, and the other two are subcutaneous injections of CGRP ligand blockers. The last drug will be an intravenous infusion of a ligand blocker. These recently approved migraine treatments have greatly improved the lives of many of our patients, even when other preventives have failed. I expect ubrogepant and other gepants will do the same for the acute care of migraine.”
Allergan funded the trials of ubrogepant, and some of the authors are Allergan employees and stockholders. Dr. Dodick reported consulting fees and advisory board fees from Allergan and various pharmaceutical companies.
SOURCE: Dodick DW et al. N Engl J Med. 2019;381(23):2230-41. doi: 10.1056/NEJMoa1813049.
published Dec. 4 in the New England Journal of Medicine. In addition, about 38% of patients who receive ubrogepant no longer have their most bothersome migraine-associated symptom, such as photophobia, phonophobia, or nausea, at 2 hours, compared with 28% of patients who receive placebo, said David W. Dodick, MD, and colleagues.
Dr. Dodick, professor of neurology at the Mayo Clinic in Phoenix, and his coauthors described efficacy and safety results from the ACHIEVE I trial. Another phase 3 study of ubrogepant, ACHIEVE II, was published in JAMA in November. That trial evaluated 25- and 50-mg doses of ubrogepant versus placebo and found rates of pain freedom and absence of the most bothersome symptom in the placebo and active treatment arms that were similar to those in ACHIEVE I.
Assessing a gepant for acute migraine treatment
Ubrogepant is an oral calcitonin gene–related peptide (CGRP) receptor antagonist. Allergan, the company developing the drug, has said it expects the Food and Drug Administration to decide in December whether to approve the drug.
To compare ubrogepant 50 mg, ubrogepant 100 mg, and placebo for the acute treatment of migraine, investigators conducted the randomized ACHIEVE I trial. Researchers enrolled 1,672 adults with migraine with or without aura. They excluded patients with clinically significant cardiovascular or cerebrovascular disease. During the trial, patients treated a single migraine attack, and they had the option to take a second dose. In all, 1,436 participants took an initial dose. Patients had an average age of 40.5 years, about 88% were women, and 82% were white.
In ACHIEVE I, the most common adverse events within 48 hours of treatment were nausea, somnolence, and dry mouth, and these events occurred more frequently in the 100-mg–dose group, Dr. Dodick and colleagues reported. Among patients who received ubrogepant, serious adverse events more than 48 hours after treatment but within 30 days of treatment included appendicitis, spontaneous abortion, pericardial effusion, and seizure. No serious adverse events occurred in the placebo group.
The authors noted that, “there was no active comparator and no evaluation of consistency of effect across multiple migraine attacks; therefore it is not possible to determine whether the drug is more or less effective than standard therapies or consistently effective with repeated use.” In addition, “safety and side-effect data from this trial were based on evaluation of a single attack, and therefore safety after repeated use cannot be inferred; an extension trial has assessed the long-term safety of ubrogepant,” they said.
The present trial was performed well, commented Alan M. Rapoport, MD. “The coprimary endpoints of pain freedom and most bothersome symptom freedom, both at 2 hours after dosing, were statistically superior for both doses of ubrogepant versus placebo,” he said. “Some of the secondary endpoints, such as pain relief at 2 hours post dose and sustained pain relief from 2 to 24 hours, were statistically better than placebo.”
“Based on this data, I suspect that the FDA would approve this gepant after appropriate safety data,” said Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles and editor-in-chief of Neurology Reviews. “Many more patients need to take this drug before we can be sure it is safe and effective.”
The CGRP therapeutic landscape
“Other gepants have been shown to be effective, although some have caused a degree of liver toxicity,” said Dr. Rapoport. “Blocking the effect of CGRP on the migraine peripheral nervous system, in this case by preventing the ligand from docking at its receptor by administering an oral CGRP receptor blocker, appears to be effective.” Researchers are studying another oral gepant for similar approval, he added.
Ubrogepant stands to join other treatments targeting CGRP.
“There are currently three, and soon to be four, injectable monoclonal antibodies against CGRP functionality, which are preventive, not acute-care drugs,” Dr. Rapoport said. “The first released was a subcutaneous injection of a CGRP receptor blocker, and the other two are subcutaneous injections of CGRP ligand blockers. The last drug will be an intravenous infusion of a ligand blocker. These recently approved migraine treatments have greatly improved the lives of many of our patients, even when other preventives have failed. I expect ubrogepant and other gepants will do the same for the acute care of migraine.”
Allergan funded the trials of ubrogepant, and some of the authors are Allergan employees and stockholders. Dr. Dodick reported consulting fees and advisory board fees from Allergan and various pharmaceutical companies.
SOURCE: Dodick DW et al. N Engl J Med. 2019;381(23):2230-41. doi: 10.1056/NEJMoa1813049.
published Dec. 4 in the New England Journal of Medicine. In addition, about 38% of patients who receive ubrogepant no longer have their most bothersome migraine-associated symptom, such as photophobia, phonophobia, or nausea, at 2 hours, compared with 28% of patients who receive placebo, said David W. Dodick, MD, and colleagues.
Dr. Dodick, professor of neurology at the Mayo Clinic in Phoenix, and his coauthors described efficacy and safety results from the ACHIEVE I trial. Another phase 3 study of ubrogepant, ACHIEVE II, was published in JAMA in November. That trial evaluated 25- and 50-mg doses of ubrogepant versus placebo and found rates of pain freedom and absence of the most bothersome symptom in the placebo and active treatment arms that were similar to those in ACHIEVE I.
Assessing a gepant for acute migraine treatment
Ubrogepant is an oral calcitonin gene–related peptide (CGRP) receptor antagonist. Allergan, the company developing the drug, has said it expects the Food and Drug Administration to decide in December whether to approve the drug.
To compare ubrogepant 50 mg, ubrogepant 100 mg, and placebo for the acute treatment of migraine, investigators conducted the randomized ACHIEVE I trial. Researchers enrolled 1,672 adults with migraine with or without aura. They excluded patients with clinically significant cardiovascular or cerebrovascular disease. During the trial, patients treated a single migraine attack, and they had the option to take a second dose. In all, 1,436 participants took an initial dose. Patients had an average age of 40.5 years, about 88% were women, and 82% were white.
In ACHIEVE I, the most common adverse events within 48 hours of treatment were nausea, somnolence, and dry mouth, and these events occurred more frequently in the 100-mg–dose group, Dr. Dodick and colleagues reported. Among patients who received ubrogepant, serious adverse events more than 48 hours after treatment but within 30 days of treatment included appendicitis, spontaneous abortion, pericardial effusion, and seizure. No serious adverse events occurred in the placebo group.
The authors noted that, “there was no active comparator and no evaluation of consistency of effect across multiple migraine attacks; therefore it is not possible to determine whether the drug is more or less effective than standard therapies or consistently effective with repeated use.” In addition, “safety and side-effect data from this trial were based on evaluation of a single attack, and therefore safety after repeated use cannot be inferred; an extension trial has assessed the long-term safety of ubrogepant,” they said.
The present trial was performed well, commented Alan M. Rapoport, MD. “The coprimary endpoints of pain freedom and most bothersome symptom freedom, both at 2 hours after dosing, were statistically superior for both doses of ubrogepant versus placebo,” he said. “Some of the secondary endpoints, such as pain relief at 2 hours post dose and sustained pain relief from 2 to 24 hours, were statistically better than placebo.”
“Based on this data, I suspect that the FDA would approve this gepant after appropriate safety data,” said Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles and editor-in-chief of Neurology Reviews. “Many more patients need to take this drug before we can be sure it is safe and effective.”
The CGRP therapeutic landscape
“Other gepants have been shown to be effective, although some have caused a degree of liver toxicity,” said Dr. Rapoport. “Blocking the effect of CGRP on the migraine peripheral nervous system, in this case by preventing the ligand from docking at its receptor by administering an oral CGRP receptor blocker, appears to be effective.” Researchers are studying another oral gepant for similar approval, he added.
Ubrogepant stands to join other treatments targeting CGRP.
“There are currently three, and soon to be four, injectable monoclonal antibodies against CGRP functionality, which are preventive, not acute-care drugs,” Dr. Rapoport said. “The first released was a subcutaneous injection of a CGRP receptor blocker, and the other two are subcutaneous injections of CGRP ligand blockers. The last drug will be an intravenous infusion of a ligand blocker. These recently approved migraine treatments have greatly improved the lives of many of our patients, even when other preventives have failed. I expect ubrogepant and other gepants will do the same for the acute care of migraine.”
Allergan funded the trials of ubrogepant, and some of the authors are Allergan employees and stockholders. Dr. Dodick reported consulting fees and advisory board fees from Allergan and various pharmaceutical companies.
SOURCE: Dodick DW et al. N Engl J Med. 2019;381(23):2230-41. doi: 10.1056/NEJMoa1813049.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Compared with placebo, ubrogepant tablets result in higher rates of pain freedom and freedom from the most bothersome migraine-associated symptom at 2 hours following treatment.
Major finding: About 20% of patients who receive tablets containing 50 mg or 100 mg of ubrogepant for the acute treatment of migraine are pain free 2 hours later, compared with 12% of patients who receive placebo. In addition, about 38% of patients who receive ubrogepant no longer have their most bothersome migraine-associated symptom, such as photophobia, phonophobia, or nausea, at 2 hours, compared with 28% of patients who receive placebo.
Study details: A randomized trial that enrolled 1,672 adults with migraine with or without aura. Participants treated a single migraine attack.
Disclosures: Allergan funded the trial, and some of the authors are Allergan employees and stockholders. Dr. Dodick reported consulting fees and advisory board fees from Allergan and various pharmaceutical companies.
Source: Dodick DW et al. N Engl J Med. 2019;381(23):2230-41. doi: 10.1056/NEJMoa1813049.
Survival gains in HR+/HER2– MBC trials yet to be seen in real world
The introduction over the last decade of new systemic therapies for the treatment of hormone receptor positive, HER2-negative metastatic breast cancer has not translated into improved survival in a real-world setting, results of a retrospective study suggest.
Among 2,197 patients who received at least one line of systemic therapy for hormone receptor positive, HER2-negative metastatic breast cancer (HR+/HER2– MBC) from 2003 to 2013, there were no significant differences in median duration of hormonal therapy or median overall survival (OS) for patients treated in any of three time spans during that 10-year period, reported Dan Le, MD, MHA, of BC Cancer, Surrey, B.C., and colleagues.
“Despite the introduction of 9 new adjuvant therapies and 2 new metastatic treatments, survival in the metastatic setting for HR-positive, HER2-negative breast cancer did not improve between 2003 and 2013,” they wrote in a report published in Cancer.
Improvements in adjuvant therapy such as the introduction of cyclin-dependent kinase inhibitors (CDKI) may result in fewer relapses but may also affect the response of relapsed cancers to additional lines of therapy, the authors contended.
“Improved adjuvant therapy means that the cancers that do relapse may have more adverse biology, either intrinsically or because of selective pressure and clonal evolution from exposure to more and better drugs in the adjuvant setting. These factors could, in part, explain the lack of improved survival over time observed in this study,” they wrote.
To see whether significant increases in progression-free survival (PFS) in a clinical trial translated into improved outcomes – including OS – in population-based settings, the investigators identified 2,432 patients with HR+/HER2– MBC from data in the prospective Breast Cancer Outcomes Unit Database of BC Cancer. Of this group, 2,197 received at least one line of systemic therapy after an MBC diagnosis, and 1,752 received first and/or second-line hormonal therapy as well.
The patients were treated in one of three time cohorts: from 2003 through 2005, 2007 through 2009, or 2011 through 2013.
Nine new adjuvant systemic therapies with or without neoadjuvant therapy were approved by BC Cancer during the study period. For the entire decade of the study, the mean survival time was 3.1 years, and the median OS was 2.0 years.
The longest survival for patients diagnosed from 2003 through 2005 was 14.6 years, with 18.1% of these patients living at least 5 years after diagnosis. For patients diagnosed from 2007 through 2009, the longest survival was 10.6 years, with 17.7% of these patients living 5 years or longer post diagnosis. For patients in the most recent cohort (with patients diagnosed after August 2012 excluded), the longest survival was 6.6 years, with 17.3% living at least 5 years after diagnosis.
Overall, patients had a median of 9 months of first-line hormonal treatment, and 6.1 months of second-line hormonal therapy, with nearly identical duration across all three time cohorts.
“Ultimately, it seems likely that the greater the proportion of patients we cure with modern adjuvant therapy, the more challenging it will be to improve outcomes for patients with relapsed disease. This underscores the importance of 1) making continued progress in the adjuvant management of potentially curable breast cancer by first studying new therapeutic agents in the metastatic setting and 2) developing a better understanding of how selective pressure and clonal evolution may lead to more resistant biologic phenotypes in MBC,” the investigators wrote.
No specific study funding was disclosed. No authors disclosed potential conflicts of interest.
SOURCE: Le D et al. Cancer 2019 Nov 21. doi: 10.1002/cncr.32631.
The introduction over the last decade of new systemic therapies for the treatment of hormone receptor positive, HER2-negative metastatic breast cancer has not translated into improved survival in a real-world setting, results of a retrospective study suggest.
Among 2,197 patients who received at least one line of systemic therapy for hormone receptor positive, HER2-negative metastatic breast cancer (HR+/HER2– MBC) from 2003 to 2013, there were no significant differences in median duration of hormonal therapy or median overall survival (OS) for patients treated in any of three time spans during that 10-year period, reported Dan Le, MD, MHA, of BC Cancer, Surrey, B.C., and colleagues.
“Despite the introduction of 9 new adjuvant therapies and 2 new metastatic treatments, survival in the metastatic setting for HR-positive, HER2-negative breast cancer did not improve between 2003 and 2013,” they wrote in a report published in Cancer.
Improvements in adjuvant therapy such as the introduction of cyclin-dependent kinase inhibitors (CDKI) may result in fewer relapses but may also affect the response of relapsed cancers to additional lines of therapy, the authors contended.
“Improved adjuvant therapy means that the cancers that do relapse may have more adverse biology, either intrinsically or because of selective pressure and clonal evolution from exposure to more and better drugs in the adjuvant setting. These factors could, in part, explain the lack of improved survival over time observed in this study,” they wrote.
To see whether significant increases in progression-free survival (PFS) in a clinical trial translated into improved outcomes – including OS – in population-based settings, the investigators identified 2,432 patients with HR+/HER2– MBC from data in the prospective Breast Cancer Outcomes Unit Database of BC Cancer. Of this group, 2,197 received at least one line of systemic therapy after an MBC diagnosis, and 1,752 received first and/or second-line hormonal therapy as well.
The patients were treated in one of three time cohorts: from 2003 through 2005, 2007 through 2009, or 2011 through 2013.
Nine new adjuvant systemic therapies with or without neoadjuvant therapy were approved by BC Cancer during the study period. For the entire decade of the study, the mean survival time was 3.1 years, and the median OS was 2.0 years.
The longest survival for patients diagnosed from 2003 through 2005 was 14.6 years, with 18.1% of these patients living at least 5 years after diagnosis. For patients diagnosed from 2007 through 2009, the longest survival was 10.6 years, with 17.7% of these patients living 5 years or longer post diagnosis. For patients in the most recent cohort (with patients diagnosed after August 2012 excluded), the longest survival was 6.6 years, with 17.3% living at least 5 years after diagnosis.
Overall, patients had a median of 9 months of first-line hormonal treatment, and 6.1 months of second-line hormonal therapy, with nearly identical duration across all three time cohorts.
“Ultimately, it seems likely that the greater the proportion of patients we cure with modern adjuvant therapy, the more challenging it will be to improve outcomes for patients with relapsed disease. This underscores the importance of 1) making continued progress in the adjuvant management of potentially curable breast cancer by first studying new therapeutic agents in the metastatic setting and 2) developing a better understanding of how selective pressure and clonal evolution may lead to more resistant biologic phenotypes in MBC,” the investigators wrote.
No specific study funding was disclosed. No authors disclosed potential conflicts of interest.
SOURCE: Le D et al. Cancer 2019 Nov 21. doi: 10.1002/cncr.32631.
The introduction over the last decade of new systemic therapies for the treatment of hormone receptor positive, HER2-negative metastatic breast cancer has not translated into improved survival in a real-world setting, results of a retrospective study suggest.
Among 2,197 patients who received at least one line of systemic therapy for hormone receptor positive, HER2-negative metastatic breast cancer (HR+/HER2– MBC) from 2003 to 2013, there were no significant differences in median duration of hormonal therapy or median overall survival (OS) for patients treated in any of three time spans during that 10-year period, reported Dan Le, MD, MHA, of BC Cancer, Surrey, B.C., and colleagues.
“Despite the introduction of 9 new adjuvant therapies and 2 new metastatic treatments, survival in the metastatic setting for HR-positive, HER2-negative breast cancer did not improve between 2003 and 2013,” they wrote in a report published in Cancer.
Improvements in adjuvant therapy such as the introduction of cyclin-dependent kinase inhibitors (CDKI) may result in fewer relapses but may also affect the response of relapsed cancers to additional lines of therapy, the authors contended.
“Improved adjuvant therapy means that the cancers that do relapse may have more adverse biology, either intrinsically or because of selective pressure and clonal evolution from exposure to more and better drugs in the adjuvant setting. These factors could, in part, explain the lack of improved survival over time observed in this study,” they wrote.
To see whether significant increases in progression-free survival (PFS) in a clinical trial translated into improved outcomes – including OS – in population-based settings, the investigators identified 2,432 patients with HR+/HER2– MBC from data in the prospective Breast Cancer Outcomes Unit Database of BC Cancer. Of this group, 2,197 received at least one line of systemic therapy after an MBC diagnosis, and 1,752 received first and/or second-line hormonal therapy as well.
The patients were treated in one of three time cohorts: from 2003 through 2005, 2007 through 2009, or 2011 through 2013.
Nine new adjuvant systemic therapies with or without neoadjuvant therapy were approved by BC Cancer during the study period. For the entire decade of the study, the mean survival time was 3.1 years, and the median OS was 2.0 years.
The longest survival for patients diagnosed from 2003 through 2005 was 14.6 years, with 18.1% of these patients living at least 5 years after diagnosis. For patients diagnosed from 2007 through 2009, the longest survival was 10.6 years, with 17.7% of these patients living 5 years or longer post diagnosis. For patients in the most recent cohort (with patients diagnosed after August 2012 excluded), the longest survival was 6.6 years, with 17.3% living at least 5 years after diagnosis.
Overall, patients had a median of 9 months of first-line hormonal treatment, and 6.1 months of second-line hormonal therapy, with nearly identical duration across all three time cohorts.
“Ultimately, it seems likely that the greater the proportion of patients we cure with modern adjuvant therapy, the more challenging it will be to improve outcomes for patients with relapsed disease. This underscores the importance of 1) making continued progress in the adjuvant management of potentially curable breast cancer by first studying new therapeutic agents in the metastatic setting and 2) developing a better understanding of how selective pressure and clonal evolution may lead to more resistant biologic phenotypes in MBC,” the investigators wrote.
No specific study funding was disclosed. No authors disclosed potential conflicts of interest.
SOURCE: Le D et al. Cancer 2019 Nov 21. doi: 10.1002/cncr.32631.
FROM CANCER
Single-fraction radiation just misses mark for spinal compression relief
Single-fraction radiation could not be shown to be noninferior to multi-fraction radiation at improving walking function in patients with spinal compression from metastatic cancer, but the small differences seen in a noninferiority trial may not matter to patients, investigators suggest.
Among 686 patients with spinal compression from metastatic cancer randomly assigned in a clinical trial to receive either 8 Gy of radiation in a single fraction or 20 Gy delivered in 5 fractions over 5 consecutive days, 69.3% of patients in the single-fraction arm had good ambulatory status at 8 weeks, compared with 72.7% of patients in the multi-fraction arm (P for noninferiority = .06), reported Peter J Hoskin, BSc, MBBS, MD, of Mount Vernon Cancer Centre in Northwood, England, and colleagues.
The trial did not meet the endpoint of noninferiority of single-fraction radiation for improving ambulation at 8 weeks because the lower limit of the 95% confidence interval (CI) was –11.5%, overlapping the noninferiority margin of –11%.
“However, for all other time points, the CI limits were within the noninferiority margin, and the observed risk differences between single-fraction and multi-fraction radiotherapy groups in ambulatory status were small and unlikely to be of clinical importance,” the investigators wrote in JAMA.
The authors note that although radiotherapy is widely used as a palliative measure for patients with spinal canal compression caused my metastatic disease, there is no agreement on the optimum schedule, with some guidelines recommending higher doses in multiple fractions, and others recommending a single 8 Gy does for patients with painful spinal sites.
To see whether single-fraction radiation could be noninferior to multi-fraction, the investigators enrolled patients in 42 sites in the United Kingdom and 5 in Australia into the SCORAD trial, and randomly assigned them to either single-fraction (345 patients) or multi-fraction (341 patients) radiation. The median age of those enrolled was 70 years, and 44% had prostate cancer, 19% had lung cancer, and 12% had breast cancer.
As noted, the primary endpoint of noninferiority of single-fraction radiation at improving ambulatory status at week 8 was not met. Ambulatory status was based on a 4-point scale and was classified as either grade 1: ambulatory without the use of aids and grade 5 of 5 of muscle power, or grade 2: ambulatory with aids or grade 4 of 5 of muscle power.
An analysis of secondary endpoints showed that the difference in ambulatory status grade 1 or 2 in the single- vs. multi-fraction group at week 1 was −0.4% (P value for noninferiority = .004), at week 4 it was −0.7% (P value for noninferiority = .01), and at week 12 it was 4.1% (P value for noninferiority = .002).
Overall survival rates at 12 weeks were 50% in the single-fraction group vs. 55% in the multi-fraction group; this difference was not statistically significant.
Of 11 other secondary endpoints analyzed, including ambulatory and safety endpoints, the between-group differences were not statistically significant or did not meet noninferiority criteria, the authors noted.
They concluded that although the trial did not meet the primary endpoint, ”the extent to which the lower bound of the CI overlapped with the noninferiority margin should be taken into account when interpreting the clinical importance of these findings.”
Cancer Research UK and Cancer Council Queensland funded the trial. Dr. Hoskin reported being supported by the National Institute for Health Research Manchester Biomedical Research Centre.
SOURCE: Hoskin PJ et al. JAMA 2019 Dec 3. doi: 10.1001/jama.2019.17913.
Single-fraction radiation could not be shown to be noninferior to multi-fraction radiation at improving walking function in patients with spinal compression from metastatic cancer, but the small differences seen in a noninferiority trial may not matter to patients, investigators suggest.
Among 686 patients with spinal compression from metastatic cancer randomly assigned in a clinical trial to receive either 8 Gy of radiation in a single fraction or 20 Gy delivered in 5 fractions over 5 consecutive days, 69.3% of patients in the single-fraction arm had good ambulatory status at 8 weeks, compared with 72.7% of patients in the multi-fraction arm (P for noninferiority = .06), reported Peter J Hoskin, BSc, MBBS, MD, of Mount Vernon Cancer Centre in Northwood, England, and colleagues.
The trial did not meet the endpoint of noninferiority of single-fraction radiation for improving ambulation at 8 weeks because the lower limit of the 95% confidence interval (CI) was –11.5%, overlapping the noninferiority margin of –11%.
“However, for all other time points, the CI limits were within the noninferiority margin, and the observed risk differences between single-fraction and multi-fraction radiotherapy groups in ambulatory status were small and unlikely to be of clinical importance,” the investigators wrote in JAMA.
The authors note that although radiotherapy is widely used as a palliative measure for patients with spinal canal compression caused my metastatic disease, there is no agreement on the optimum schedule, with some guidelines recommending higher doses in multiple fractions, and others recommending a single 8 Gy does for patients with painful spinal sites.
To see whether single-fraction radiation could be noninferior to multi-fraction, the investigators enrolled patients in 42 sites in the United Kingdom and 5 in Australia into the SCORAD trial, and randomly assigned them to either single-fraction (345 patients) or multi-fraction (341 patients) radiation. The median age of those enrolled was 70 years, and 44% had prostate cancer, 19% had lung cancer, and 12% had breast cancer.
As noted, the primary endpoint of noninferiority of single-fraction radiation at improving ambulatory status at week 8 was not met. Ambulatory status was based on a 4-point scale and was classified as either grade 1: ambulatory without the use of aids and grade 5 of 5 of muscle power, or grade 2: ambulatory with aids or grade 4 of 5 of muscle power.
An analysis of secondary endpoints showed that the difference in ambulatory status grade 1 or 2 in the single- vs. multi-fraction group at week 1 was −0.4% (P value for noninferiority = .004), at week 4 it was −0.7% (P value for noninferiority = .01), and at week 12 it was 4.1% (P value for noninferiority = .002).
Overall survival rates at 12 weeks were 50% in the single-fraction group vs. 55% in the multi-fraction group; this difference was not statistically significant.
Of 11 other secondary endpoints analyzed, including ambulatory and safety endpoints, the between-group differences were not statistically significant or did not meet noninferiority criteria, the authors noted.
They concluded that although the trial did not meet the primary endpoint, ”the extent to which the lower bound of the CI overlapped with the noninferiority margin should be taken into account when interpreting the clinical importance of these findings.”
Cancer Research UK and Cancer Council Queensland funded the trial. Dr. Hoskin reported being supported by the National Institute for Health Research Manchester Biomedical Research Centre.
SOURCE: Hoskin PJ et al. JAMA 2019 Dec 3. doi: 10.1001/jama.2019.17913.
Single-fraction radiation could not be shown to be noninferior to multi-fraction radiation at improving walking function in patients with spinal compression from metastatic cancer, but the small differences seen in a noninferiority trial may not matter to patients, investigators suggest.
Among 686 patients with spinal compression from metastatic cancer randomly assigned in a clinical trial to receive either 8 Gy of radiation in a single fraction or 20 Gy delivered in 5 fractions over 5 consecutive days, 69.3% of patients in the single-fraction arm had good ambulatory status at 8 weeks, compared with 72.7% of patients in the multi-fraction arm (P for noninferiority = .06), reported Peter J Hoskin, BSc, MBBS, MD, of Mount Vernon Cancer Centre in Northwood, England, and colleagues.
The trial did not meet the endpoint of noninferiority of single-fraction radiation for improving ambulation at 8 weeks because the lower limit of the 95% confidence interval (CI) was –11.5%, overlapping the noninferiority margin of –11%.
“However, for all other time points, the CI limits were within the noninferiority margin, and the observed risk differences between single-fraction and multi-fraction radiotherapy groups in ambulatory status were small and unlikely to be of clinical importance,” the investigators wrote in JAMA.
The authors note that although radiotherapy is widely used as a palliative measure for patients with spinal canal compression caused my metastatic disease, there is no agreement on the optimum schedule, with some guidelines recommending higher doses in multiple fractions, and others recommending a single 8 Gy does for patients with painful spinal sites.
To see whether single-fraction radiation could be noninferior to multi-fraction, the investigators enrolled patients in 42 sites in the United Kingdom and 5 in Australia into the SCORAD trial, and randomly assigned them to either single-fraction (345 patients) or multi-fraction (341 patients) radiation. The median age of those enrolled was 70 years, and 44% had prostate cancer, 19% had lung cancer, and 12% had breast cancer.
As noted, the primary endpoint of noninferiority of single-fraction radiation at improving ambulatory status at week 8 was not met. Ambulatory status was based on a 4-point scale and was classified as either grade 1: ambulatory without the use of aids and grade 5 of 5 of muscle power, or grade 2: ambulatory with aids or grade 4 of 5 of muscle power.
An analysis of secondary endpoints showed that the difference in ambulatory status grade 1 or 2 in the single- vs. multi-fraction group at week 1 was −0.4% (P value for noninferiority = .004), at week 4 it was −0.7% (P value for noninferiority = .01), and at week 12 it was 4.1% (P value for noninferiority = .002).
Overall survival rates at 12 weeks were 50% in the single-fraction group vs. 55% in the multi-fraction group; this difference was not statistically significant.
Of 11 other secondary endpoints analyzed, including ambulatory and safety endpoints, the between-group differences were not statistically significant or did not meet noninferiority criteria, the authors noted.
They concluded that although the trial did not meet the primary endpoint, ”the extent to which the lower bound of the CI overlapped with the noninferiority margin should be taken into account when interpreting the clinical importance of these findings.”
Cancer Research UK and Cancer Council Queensland funded the trial. Dr. Hoskin reported being supported by the National Institute for Health Research Manchester Biomedical Research Centre.
SOURCE: Hoskin PJ et al. JAMA 2019 Dec 3. doi: 10.1001/jama.2019.17913.
FROM JAMA
Antibiotic use may increase the risk of Parkinson’s disease
according to a report published in Movement Disorders. Associations were found for broad-spectrum antibiotics and those that act against anaerobic bacteria and fungi. The timing of antibiotic exposure also seemed to matter.
In a nationwide case-control study, Finnish researchers compared data on antibiotic use in 13,976 individuals diagnosed with Parkinson’s disease between 1998 and 2014 with antibiotic-use data from 40,697 controls. The strongest connection with Parkinson’s disease risk was found for oral exposure to macrolides and lincosamides (adjusted odds ratio up to 1.416). After correction for multiple comparisons, exposure to antianaerobics and tetracyclines 10-15 years before the index date, and antifungal medications 1-5 years before the index date were positively associated with Parkinson’s disease risk. In post hoc analyses, further positive associations were found for broad-spectrum antibiotics.
Tuomas H. Mertsalmi, MD, from the Helsinki University Hospital and coauthors reported that this was the first study to explore a possible connection between antimicrobial use and Parkinson’s disease.
“In Parkinson’s disease, several studies have described alterations of gut microbiota composition, and changes in fecal microbiota abundance have been found to be associated with gastrointestinal and motor symptoms,” they wrote.
Commenting on the delay between the exposure and diagnosis for the most strongly associated antimicrobials, the authors noted that this 10-15 year lag was comparable with what has been found between the peripheral initiation of Parkinson’s disease and its motor manifestation.
“This would also explain the lack of association between antibiotic exposure 1-5 years before index date – if antibiotic exposure could induce or contribute to the pathogenesis of Parkinson’s disease in the gastrointestinal tract, it would probably take several years before the clinical manifestation of Parkinson’s disease,” they wrote.
With regards to the association seen for sulfonamides and trimethoprim – which was 1-5 years before the index date – they speculated this could reflect treatment for urinary tract infections, which individuals with Parkinson’s disease might be more susceptible to in the prodromal phase of the disease.
The authors noted that infectious disease has also been associated with Parkinson’s disease, and that their analysis did not include information about why the antimicrobial agents were prescribed. However, they pointed out that the associations were only for certain antibiotic classes, which makes it unlikely that the association was related to greater burden of infectious disease among individuals with Parkinson’s disease.
The pattern of associations supports the hypothesis that effects on gut microbiota could link antibiotics to Parkinson’s disease. “The link between antibiotic exposure and Parkinson’s disease fits the current view that in a significant proportion of patients the pathology of Parkinson’s disease may originate in the gut, possibly related to microbial changes, years before the onset of typical Parkinson’s disease motor symptoms such as slowness, muscle stiffness, and shaking of the extremities. It was known that bacterial composition of the intestine in patients with Parkinson’s disease is abnormal, but the cause is unclear. Our results suggest that some commonly used antibiotics, which are known to strongly influence the gut microbiota, could be a predisposing factor,” said lead investigator Filip Scheperjans, MD, PhD, from the department of neurology at Helsinki University Hospital.
The findings may have implications for antibiotic prescribing practices in the future, said Dr. Scheperjans. “In addition to the problem of antibiotic resistance, antimicrobial prescribing should also take into account their potentially long-lasting effects on the gut microbiome and the development of certain diseases.”
The study was funded by the Finnish Parkinson Foundation, the Finnish Medical Foundation, the Maire Taponen Foundation, and the Academy of Finland. One author declared relevant patents and his position as founder and chief executive of a private company. No other conflicts of interest were declared.
SOURCE: Mertsalmi TH et al. Mov Disord. 2019 Nov 18. doi: 10.1002/mds.27924.
according to a report published in Movement Disorders. Associations were found for broad-spectrum antibiotics and those that act against anaerobic bacteria and fungi. The timing of antibiotic exposure also seemed to matter.
In a nationwide case-control study, Finnish researchers compared data on antibiotic use in 13,976 individuals diagnosed with Parkinson’s disease between 1998 and 2014 with antibiotic-use data from 40,697 controls. The strongest connection with Parkinson’s disease risk was found for oral exposure to macrolides and lincosamides (adjusted odds ratio up to 1.416). After correction for multiple comparisons, exposure to antianaerobics and tetracyclines 10-15 years before the index date, and antifungal medications 1-5 years before the index date were positively associated with Parkinson’s disease risk. In post hoc analyses, further positive associations were found for broad-spectrum antibiotics.
Tuomas H. Mertsalmi, MD, from the Helsinki University Hospital and coauthors reported that this was the first study to explore a possible connection between antimicrobial use and Parkinson’s disease.
“In Parkinson’s disease, several studies have described alterations of gut microbiota composition, and changes in fecal microbiota abundance have been found to be associated with gastrointestinal and motor symptoms,” they wrote.
Commenting on the delay between the exposure and diagnosis for the most strongly associated antimicrobials, the authors noted that this 10-15 year lag was comparable with what has been found between the peripheral initiation of Parkinson’s disease and its motor manifestation.
“This would also explain the lack of association between antibiotic exposure 1-5 years before index date – if antibiotic exposure could induce or contribute to the pathogenesis of Parkinson’s disease in the gastrointestinal tract, it would probably take several years before the clinical manifestation of Parkinson’s disease,” they wrote.
With regards to the association seen for sulfonamides and trimethoprim – which was 1-5 years before the index date – they speculated this could reflect treatment for urinary tract infections, which individuals with Parkinson’s disease might be more susceptible to in the prodromal phase of the disease.
The authors noted that infectious disease has also been associated with Parkinson’s disease, and that their analysis did not include information about why the antimicrobial agents were prescribed. However, they pointed out that the associations were only for certain antibiotic classes, which makes it unlikely that the association was related to greater burden of infectious disease among individuals with Parkinson’s disease.
The pattern of associations supports the hypothesis that effects on gut microbiota could link antibiotics to Parkinson’s disease. “The link between antibiotic exposure and Parkinson’s disease fits the current view that in a significant proportion of patients the pathology of Parkinson’s disease may originate in the gut, possibly related to microbial changes, years before the onset of typical Parkinson’s disease motor symptoms such as slowness, muscle stiffness, and shaking of the extremities. It was known that bacterial composition of the intestine in patients with Parkinson’s disease is abnormal, but the cause is unclear. Our results suggest that some commonly used antibiotics, which are known to strongly influence the gut microbiota, could be a predisposing factor,” said lead investigator Filip Scheperjans, MD, PhD, from the department of neurology at Helsinki University Hospital.
The findings may have implications for antibiotic prescribing practices in the future, said Dr. Scheperjans. “In addition to the problem of antibiotic resistance, antimicrobial prescribing should also take into account their potentially long-lasting effects on the gut microbiome and the development of certain diseases.”
The study was funded by the Finnish Parkinson Foundation, the Finnish Medical Foundation, the Maire Taponen Foundation, and the Academy of Finland. One author declared relevant patents and his position as founder and chief executive of a private company. No other conflicts of interest were declared.
SOURCE: Mertsalmi TH et al. Mov Disord. 2019 Nov 18. doi: 10.1002/mds.27924.
according to a report published in Movement Disorders. Associations were found for broad-spectrum antibiotics and those that act against anaerobic bacteria and fungi. The timing of antibiotic exposure also seemed to matter.
In a nationwide case-control study, Finnish researchers compared data on antibiotic use in 13,976 individuals diagnosed with Parkinson’s disease between 1998 and 2014 with antibiotic-use data from 40,697 controls. The strongest connection with Parkinson’s disease risk was found for oral exposure to macrolides and lincosamides (adjusted odds ratio up to 1.416). After correction for multiple comparisons, exposure to antianaerobics and tetracyclines 10-15 years before the index date, and antifungal medications 1-5 years before the index date were positively associated with Parkinson’s disease risk. In post hoc analyses, further positive associations were found for broad-spectrum antibiotics.
Tuomas H. Mertsalmi, MD, from the Helsinki University Hospital and coauthors reported that this was the first study to explore a possible connection between antimicrobial use and Parkinson’s disease.
“In Parkinson’s disease, several studies have described alterations of gut microbiota composition, and changes in fecal microbiota abundance have been found to be associated with gastrointestinal and motor symptoms,” they wrote.
Commenting on the delay between the exposure and diagnosis for the most strongly associated antimicrobials, the authors noted that this 10-15 year lag was comparable with what has been found between the peripheral initiation of Parkinson’s disease and its motor manifestation.
“This would also explain the lack of association between antibiotic exposure 1-5 years before index date – if antibiotic exposure could induce or contribute to the pathogenesis of Parkinson’s disease in the gastrointestinal tract, it would probably take several years before the clinical manifestation of Parkinson’s disease,” they wrote.
With regards to the association seen for sulfonamides and trimethoprim – which was 1-5 years before the index date – they speculated this could reflect treatment for urinary tract infections, which individuals with Parkinson’s disease might be more susceptible to in the prodromal phase of the disease.
The authors noted that infectious disease has also been associated with Parkinson’s disease, and that their analysis did not include information about why the antimicrobial agents were prescribed. However, they pointed out that the associations were only for certain antibiotic classes, which makes it unlikely that the association was related to greater burden of infectious disease among individuals with Parkinson’s disease.
The pattern of associations supports the hypothesis that effects on gut microbiota could link antibiotics to Parkinson’s disease. “The link between antibiotic exposure and Parkinson’s disease fits the current view that in a significant proportion of patients the pathology of Parkinson’s disease may originate in the gut, possibly related to microbial changes, years before the onset of typical Parkinson’s disease motor symptoms such as slowness, muscle stiffness, and shaking of the extremities. It was known that bacterial composition of the intestine in patients with Parkinson’s disease is abnormal, but the cause is unclear. Our results suggest that some commonly used antibiotics, which are known to strongly influence the gut microbiota, could be a predisposing factor,” said lead investigator Filip Scheperjans, MD, PhD, from the department of neurology at Helsinki University Hospital.
The findings may have implications for antibiotic prescribing practices in the future, said Dr. Scheperjans. “In addition to the problem of antibiotic resistance, antimicrobial prescribing should also take into account their potentially long-lasting effects on the gut microbiome and the development of certain diseases.”
The study was funded by the Finnish Parkinson Foundation, the Finnish Medical Foundation, the Maire Taponen Foundation, and the Academy of Finland. One author declared relevant patents and his position as founder and chief executive of a private company. No other conflicts of interest were declared.
SOURCE: Mertsalmi TH et al. Mov Disord. 2019 Nov 18. doi: 10.1002/mds.27924.
FROM MOVEMENT DISORDERS
Trial finds three drugs equally effective for established status epilepticus
according to a study published Nov. 27 in the New England Journal of Medicine. The effectiveness and safety of the intravenous medications do not differ significantly, the researchers wrote.
“Having three equally effective second-line intravenous medications means that the clinician may choose a drug that takes into account individual situations,” wrote Phil E.M. Smith, MD, in an accompanying editorial (doi: 10.1056/NEJMe1913775). Clinicians may consider “factors such as the presumed underlying cause of status epilepticus; coexisting conditions, including allergy, liver and renal disease, hypotension, propensity to cardiac arrhythmia, and alcohol and drug dependence; the currently prescribed antiepileptic treatment; the cost of the medication; and governmental agency drug approval,” said Dr. Smith, who is affiliated with University Hospital of Wales in Cardiff.
A gap in guidance
Evidence supports benzodiazepines as the initial treatment for status epilepticus, but these drugs do not work in up to a third of patients, said first study author Jaideep Kapur, MBBS, PhD, and colleagues. “Clinical guidelines emphasize the need for rapid control of benzodiazepine-refractory status epilepticus but do not provide guidance regarding the choice of medication on the basis of either efficacy or safety,” they wrote. Dr. Kapur is a professor of neurology and the director of UVA Brain Institute at University of Virginia in Charlottesville.
Levetiracetam, fosphenytoin, and valproate are the three most commonly used medications for benzodiazepine-refractory status epilepticus. The Food and Drug Administration has labeled fosphenytoin for this indication in adults, and none of the drugs is approved for children. To determine the superiority or inferiority of these medications, the researchers conducted the Established Status Epilepticus Treatment Trial (ESETT). The blinded, comparative-effectiveness trial enrolled 384 patients at 57 hospital EDs in the United States. Patients were aged 2 years or older, had received a generally accepted cumulative dose of benzodiazepines for generalized convulsive seizures lasting more than 5 minutes and continued to have persistent or recurrent convulsions between 5-30 minutes after the last dose of benzodiazepine.
Patients randomly received one of the three trial drugs, which “were identical in appearance, formulation, packaging, and administration,” the authors said. The primary outcome was absence of clinically apparent seizures and improving responsiveness at 60 minutes after the start of the infusion without administration of additional anticonvulsant medication. ED physicians determined the presence of seizure and improvement in responsiveness.
Trial was stopped for futility
The trial included 400 enrollments of 384 unique patients during 2015-2017. Sixteen patients were enrolled twice, and their second enrollments were not included in the intention-to-treat analysis. A planned interim analysis after 400 enrollments to assess the likelihood of success or futility found that the trial had met the futility criterion. “There was a 1% chance of showing a most effective or least effective treatment if the trial were to continue to the maximum sample size” of 795 patients, Dr. Kapur and coauthors wrote. The researchers continued enrollment in a pediatric subcohort for a planned subgroup analysis by age.
In all, 55% of the patients were male, 43% were black, and 16% were Hispanic. The population was 39% children and adolescents, 48% adults aged 18-65 years, and 13% older than 65 years. Most patients had a final diagnosis of status epilepticus (87%). Other final diagnoses included psychogenic nonepileptic seizures (10%).
At 60 minutes after treatment administration, absence of seizures and improved responsiveness occurred in 47% of patients who received levetiracetam, 45% who received fosphenytoin, and 46% who received valproate.
In 39 patients for whom the researchers had reliable information about time to seizure cessation, median time to seizure cessation numerically favored valproate (7 minutes for valproate vs. 10.5 minutes for levetiracetam vs. 11.7 minutes for fosphenytoin), but the number of patients was limited, the authors noted.
“Hypotension and endotracheal intubation were more frequent with fosphenytoin than with the other two drugs, and deaths were more frequent with levetiracetam, but these differences were not significant,” wrote Dr. Kapur and colleagues. Seven patients who received levetiracetam died, compared with three who received fosphenytoin and two who received valproate. Life-threatening hypotension occurred in 3.2% of patients who received fosphenytoin, compared with 1.6% who received valproate and 0.7% who received levetiracetam. Endotracheal intubation occurred in 26.4% or patients who received fosphenytoin, compared with 20% of patients in the levetiracetam group and 16.8% in the valproate group.
The trial’s limitations include the enrollment of patients with psychogenic nonepileptic seizures and the use of clinical instead of electroencephalographic criteria for the primary outcome measure, the investigators wrote.
Dr. Smith noted that third- and fourth-line management of status epilepticus is not supported by high-quality evidence, and further studies are needed. Given the evidence from ESETT, “the practical challenge for the management of status epilepticus remains the same as in the past: ensuring that clinicians are familiar with, and follow, a treatment protocol,” he said.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Kapur had no financial disclosures. A coauthor holds a patent on intravenous carbamazepine and intellectual property on intravenous topiramate. Other coauthors have ties to pharmaceutical and medical device companies.
Dr. Smith is coeditor of Practical Neurology and a member of the U.K. National Institute for Health and Clinical Excellence (NICE) guidelines committee for epilepsy.
SOURCE: Kapur J et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1905795.
according to a study published Nov. 27 in the New England Journal of Medicine. The effectiveness and safety of the intravenous medications do not differ significantly, the researchers wrote.
“Having three equally effective second-line intravenous medications means that the clinician may choose a drug that takes into account individual situations,” wrote Phil E.M. Smith, MD, in an accompanying editorial (doi: 10.1056/NEJMe1913775). Clinicians may consider “factors such as the presumed underlying cause of status epilepticus; coexisting conditions, including allergy, liver and renal disease, hypotension, propensity to cardiac arrhythmia, and alcohol and drug dependence; the currently prescribed antiepileptic treatment; the cost of the medication; and governmental agency drug approval,” said Dr. Smith, who is affiliated with University Hospital of Wales in Cardiff.
A gap in guidance
Evidence supports benzodiazepines as the initial treatment for status epilepticus, but these drugs do not work in up to a third of patients, said first study author Jaideep Kapur, MBBS, PhD, and colleagues. “Clinical guidelines emphasize the need for rapid control of benzodiazepine-refractory status epilepticus but do not provide guidance regarding the choice of medication on the basis of either efficacy or safety,” they wrote. Dr. Kapur is a professor of neurology and the director of UVA Brain Institute at University of Virginia in Charlottesville.
Levetiracetam, fosphenytoin, and valproate are the three most commonly used medications for benzodiazepine-refractory status epilepticus. The Food and Drug Administration has labeled fosphenytoin for this indication in adults, and none of the drugs is approved for children. To determine the superiority or inferiority of these medications, the researchers conducted the Established Status Epilepticus Treatment Trial (ESETT). The blinded, comparative-effectiveness trial enrolled 384 patients at 57 hospital EDs in the United States. Patients were aged 2 years or older, had received a generally accepted cumulative dose of benzodiazepines for generalized convulsive seizures lasting more than 5 minutes and continued to have persistent or recurrent convulsions between 5-30 minutes after the last dose of benzodiazepine.
Patients randomly received one of the three trial drugs, which “were identical in appearance, formulation, packaging, and administration,” the authors said. The primary outcome was absence of clinically apparent seizures and improving responsiveness at 60 minutes after the start of the infusion without administration of additional anticonvulsant medication. ED physicians determined the presence of seizure and improvement in responsiveness.
Trial was stopped for futility
The trial included 400 enrollments of 384 unique patients during 2015-2017. Sixteen patients were enrolled twice, and their second enrollments were not included in the intention-to-treat analysis. A planned interim analysis after 400 enrollments to assess the likelihood of success or futility found that the trial had met the futility criterion. “There was a 1% chance of showing a most effective or least effective treatment if the trial were to continue to the maximum sample size” of 795 patients, Dr. Kapur and coauthors wrote. The researchers continued enrollment in a pediatric subcohort for a planned subgroup analysis by age.
In all, 55% of the patients were male, 43% were black, and 16% were Hispanic. The population was 39% children and adolescents, 48% adults aged 18-65 years, and 13% older than 65 years. Most patients had a final diagnosis of status epilepticus (87%). Other final diagnoses included psychogenic nonepileptic seizures (10%).
At 60 minutes after treatment administration, absence of seizures and improved responsiveness occurred in 47% of patients who received levetiracetam, 45% who received fosphenytoin, and 46% who received valproate.
In 39 patients for whom the researchers had reliable information about time to seizure cessation, median time to seizure cessation numerically favored valproate (7 minutes for valproate vs. 10.5 minutes for levetiracetam vs. 11.7 minutes for fosphenytoin), but the number of patients was limited, the authors noted.
“Hypotension and endotracheal intubation were more frequent with fosphenytoin than with the other two drugs, and deaths were more frequent with levetiracetam, but these differences were not significant,” wrote Dr. Kapur and colleagues. Seven patients who received levetiracetam died, compared with three who received fosphenytoin and two who received valproate. Life-threatening hypotension occurred in 3.2% of patients who received fosphenytoin, compared with 1.6% who received valproate and 0.7% who received levetiracetam. Endotracheal intubation occurred in 26.4% or patients who received fosphenytoin, compared with 20% of patients in the levetiracetam group and 16.8% in the valproate group.
The trial’s limitations include the enrollment of patients with psychogenic nonepileptic seizures and the use of clinical instead of electroencephalographic criteria for the primary outcome measure, the investigators wrote.
Dr. Smith noted that third- and fourth-line management of status epilepticus is not supported by high-quality evidence, and further studies are needed. Given the evidence from ESETT, “the practical challenge for the management of status epilepticus remains the same as in the past: ensuring that clinicians are familiar with, and follow, a treatment protocol,” he said.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Kapur had no financial disclosures. A coauthor holds a patent on intravenous carbamazepine and intellectual property on intravenous topiramate. Other coauthors have ties to pharmaceutical and medical device companies.
Dr. Smith is coeditor of Practical Neurology and a member of the U.K. National Institute for Health and Clinical Excellence (NICE) guidelines committee for epilepsy.
SOURCE: Kapur J et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1905795.
according to a study published Nov. 27 in the New England Journal of Medicine. The effectiveness and safety of the intravenous medications do not differ significantly, the researchers wrote.
“Having three equally effective second-line intravenous medications means that the clinician may choose a drug that takes into account individual situations,” wrote Phil E.M. Smith, MD, in an accompanying editorial (doi: 10.1056/NEJMe1913775). Clinicians may consider “factors such as the presumed underlying cause of status epilepticus; coexisting conditions, including allergy, liver and renal disease, hypotension, propensity to cardiac arrhythmia, and alcohol and drug dependence; the currently prescribed antiepileptic treatment; the cost of the medication; and governmental agency drug approval,” said Dr. Smith, who is affiliated with University Hospital of Wales in Cardiff.
A gap in guidance
Evidence supports benzodiazepines as the initial treatment for status epilepticus, but these drugs do not work in up to a third of patients, said first study author Jaideep Kapur, MBBS, PhD, and colleagues. “Clinical guidelines emphasize the need for rapid control of benzodiazepine-refractory status epilepticus but do not provide guidance regarding the choice of medication on the basis of either efficacy or safety,” they wrote. Dr. Kapur is a professor of neurology and the director of UVA Brain Institute at University of Virginia in Charlottesville.
Levetiracetam, fosphenytoin, and valproate are the three most commonly used medications for benzodiazepine-refractory status epilepticus. The Food and Drug Administration has labeled fosphenytoin for this indication in adults, and none of the drugs is approved for children. To determine the superiority or inferiority of these medications, the researchers conducted the Established Status Epilepticus Treatment Trial (ESETT). The blinded, comparative-effectiveness trial enrolled 384 patients at 57 hospital EDs in the United States. Patients were aged 2 years or older, had received a generally accepted cumulative dose of benzodiazepines for generalized convulsive seizures lasting more than 5 minutes and continued to have persistent or recurrent convulsions between 5-30 minutes after the last dose of benzodiazepine.
Patients randomly received one of the three trial drugs, which “were identical in appearance, formulation, packaging, and administration,” the authors said. The primary outcome was absence of clinically apparent seizures and improving responsiveness at 60 minutes after the start of the infusion without administration of additional anticonvulsant medication. ED physicians determined the presence of seizure and improvement in responsiveness.
Trial was stopped for futility
The trial included 400 enrollments of 384 unique patients during 2015-2017. Sixteen patients were enrolled twice, and their second enrollments were not included in the intention-to-treat analysis. A planned interim analysis after 400 enrollments to assess the likelihood of success or futility found that the trial had met the futility criterion. “There was a 1% chance of showing a most effective or least effective treatment if the trial were to continue to the maximum sample size” of 795 patients, Dr. Kapur and coauthors wrote. The researchers continued enrollment in a pediatric subcohort for a planned subgroup analysis by age.
In all, 55% of the patients were male, 43% were black, and 16% were Hispanic. The population was 39% children and adolescents, 48% adults aged 18-65 years, and 13% older than 65 years. Most patients had a final diagnosis of status epilepticus (87%). Other final diagnoses included psychogenic nonepileptic seizures (10%).
At 60 minutes after treatment administration, absence of seizures and improved responsiveness occurred in 47% of patients who received levetiracetam, 45% who received fosphenytoin, and 46% who received valproate.
In 39 patients for whom the researchers had reliable information about time to seizure cessation, median time to seizure cessation numerically favored valproate (7 minutes for valproate vs. 10.5 minutes for levetiracetam vs. 11.7 minutes for fosphenytoin), but the number of patients was limited, the authors noted.
“Hypotension and endotracheal intubation were more frequent with fosphenytoin than with the other two drugs, and deaths were more frequent with levetiracetam, but these differences were not significant,” wrote Dr. Kapur and colleagues. Seven patients who received levetiracetam died, compared with three who received fosphenytoin and two who received valproate. Life-threatening hypotension occurred in 3.2% of patients who received fosphenytoin, compared with 1.6% who received valproate and 0.7% who received levetiracetam. Endotracheal intubation occurred in 26.4% or patients who received fosphenytoin, compared with 20% of patients in the levetiracetam group and 16.8% in the valproate group.
The trial’s limitations include the enrollment of patients with psychogenic nonepileptic seizures and the use of clinical instead of electroencephalographic criteria for the primary outcome measure, the investigators wrote.
Dr. Smith noted that third- and fourth-line management of status epilepticus is not supported by high-quality evidence, and further studies are needed. Given the evidence from ESETT, “the practical challenge for the management of status epilepticus remains the same as in the past: ensuring that clinicians are familiar with, and follow, a treatment protocol,” he said.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Kapur had no financial disclosures. A coauthor holds a patent on intravenous carbamazepine and intellectual property on intravenous topiramate. Other coauthors have ties to pharmaceutical and medical device companies.
Dr. Smith is coeditor of Practical Neurology and a member of the U.K. National Institute for Health and Clinical Excellence (NICE) guidelines committee for epilepsy.
SOURCE: Kapur J et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1905795.
FROM NEJM
Key clinical point: Among children and adults with benzodiazepine-refractory status epilepticus, fosphenytoin, valproate, and levetiracetam each stop seizures by 60 minutes in approximately half of patients.
Major finding: Absence of seizures and improved responsiveness occurred in 47% of patients who received levetiracetam, 45% who received fosphenytoin, and 46% who received valproate.
Study details: The Established Status Epilepticus Treatment Trial (ESETT) was a blinded, comparative-effectiveness trial that enrolled 384 patients at 57 hospital EDs in the United States.
Disclosures: The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Kapur had no financial disclosures. A coauthor holds a patent on intravenous carbamazepine and intellectual property on intravenous topiramate. Other coauthors have ties to pharmaceutical and medical device companies.
Source: Kapur J et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1905795.
Supplemental MRI found to benefit women with dense breast tissue
The use of supplemental MRI screening in women with extremely dense breast tissue and normal results on mammography led to the diagnosis of significantly fewer interval cancers, compared with mammography alone during a 2-year screening period, results from a randomized trial show.
“Women with extremely dense breast tissue have an increased risk of breast cancer, and their cancers are also less likely to be detected on mammography,” Dutch researchers led by Marije F. Bakker, PhD, of Utrecht (The Netherlands) University and colleagues wrote for the Dense Tissue and Early Breast Neoplasm Screening (DENSE) Trial Study Group in an article published in the New England Journal of Medicine.
“Such patients may benefit from a tailored breast-screening strategy, supplemented with more sensitive imaging methods. The benefit of supplemental imaging is the subject of a worldwide debate. In the United States, a federal law directs breast-density reporting, but supplemental screening is not recommended in American guidelines. Although supplemental imaging increases the rate of cancer detection in women with dense breasts, the question remains whether it improves health outcomes,” they said.
In the DENSE trial, researchers assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to a group that was invited to undergo supplemental MRI or to a group that received mammography screening only. The women were between the ages of 50 and 75 years and were enrolled between December 2011 and November 2015 as part of the Dutch population-based digital mammography screening program. The primary outcome was the between-group difference in the incidence of interval cancers during a 2-year screening period.
Dr. Bakker and associates found that the interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the MRI invitation group, compared with 5 per 1,000 among the 32,312 women in the mammography-only group, a difference of 2.5 per 1,000 screenings (P less than 0.001). Among the women who were invited to undergo MRI, 59% actually underwent the procedure. Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the women who had undergone MRI, which translated to 0.8 per 1,000 screenings. The remaining 16 were diagnosed in those who had not undergone MRI, which translated into 4.9 per 1,000 screenings.
“Undergoing supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings and resulted in a false positive rate of 8.0% (79.8 per 1,000 screenings),” the researchers wrote. “Of the women who underwent a breast biopsy on the basis of an MRI indication, 26.3% had breast cancer and 73.7% did not.”
Dr. Bakker and coauthors acknowledged certain limitations of the trial, including the fact that it was not large enough to examine the effect of MRI screening on breast cancer–specific or overall mortality. “This outcome would require a much larger sample size and longer follow-up,” they wrote. “The lower rate of interval cancers that we found among participants who underwent MRI is indicative of and prerequisite for an effect on mortality. After that, a reduction in the number of advanced cancers would also be required to show a mortality benefit, which would require several years of follow-up.”
In an accompanying editorial, Dan L. Longo, MD, noted that the study provides high-quality data from a randomized trial where none existed (N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMe1912943). “It appears to show that among women with dense breasts, the risk of interval cancers is halved by following a negative mammogram with MRI screening,” wrote Dr. Longo, who is deputy editor of the New England Journal of Medicine, as well as professor of medicine at Harvard Medical School, Boston. “But is a reduction in interval cancers an appropriate surrogate for improved overall survival? It appears that most of the cancers that were detected on supplemental MRI screening were found at an early stage. Ductal carcinoma in situ was 10 times more frequent among patients undergoing MRI, and these diagnoses were likely to lead to treatments. What remains unclear is whether the tumors would never otherwise have been detected or threatened the patient’s survival.”
The trial was supported by the University Medical Center Utrecht (the Netherlands), the Netherlands Organization for Health Research and Development, the Dutch Cancer Society, the Dutch Pink Ribbon–A Sister’s Hope organization, Stichting Kankerpreventie Midden-West, and Bayer Pharmaceuticals, with an in-kind contribution from Volpara Health Technologies.
The researchers reported having no relevant financial disclosures other than the trial funding. Dr. Longo is employed by the New England Journal of Medicine as deputy editor.
SOURCE: Bakker MF et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1903986.
The use of supplemental MRI screening in women with extremely dense breast tissue and normal results on mammography led to the diagnosis of significantly fewer interval cancers, compared with mammography alone during a 2-year screening period, results from a randomized trial show.
“Women with extremely dense breast tissue have an increased risk of breast cancer, and their cancers are also less likely to be detected on mammography,” Dutch researchers led by Marije F. Bakker, PhD, of Utrecht (The Netherlands) University and colleagues wrote for the Dense Tissue and Early Breast Neoplasm Screening (DENSE) Trial Study Group in an article published in the New England Journal of Medicine.
“Such patients may benefit from a tailored breast-screening strategy, supplemented with more sensitive imaging methods. The benefit of supplemental imaging is the subject of a worldwide debate. In the United States, a federal law directs breast-density reporting, but supplemental screening is not recommended in American guidelines. Although supplemental imaging increases the rate of cancer detection in women with dense breasts, the question remains whether it improves health outcomes,” they said.
In the DENSE trial, researchers assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to a group that was invited to undergo supplemental MRI or to a group that received mammography screening only. The women were between the ages of 50 and 75 years and were enrolled between December 2011 and November 2015 as part of the Dutch population-based digital mammography screening program. The primary outcome was the between-group difference in the incidence of interval cancers during a 2-year screening period.
Dr. Bakker and associates found that the interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the MRI invitation group, compared with 5 per 1,000 among the 32,312 women in the mammography-only group, a difference of 2.5 per 1,000 screenings (P less than 0.001). Among the women who were invited to undergo MRI, 59% actually underwent the procedure. Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the women who had undergone MRI, which translated to 0.8 per 1,000 screenings. The remaining 16 were diagnosed in those who had not undergone MRI, which translated into 4.9 per 1,000 screenings.
“Undergoing supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings and resulted in a false positive rate of 8.0% (79.8 per 1,000 screenings),” the researchers wrote. “Of the women who underwent a breast biopsy on the basis of an MRI indication, 26.3% had breast cancer and 73.7% did not.”
Dr. Bakker and coauthors acknowledged certain limitations of the trial, including the fact that it was not large enough to examine the effect of MRI screening on breast cancer–specific or overall mortality. “This outcome would require a much larger sample size and longer follow-up,” they wrote. “The lower rate of interval cancers that we found among participants who underwent MRI is indicative of and prerequisite for an effect on mortality. After that, a reduction in the number of advanced cancers would also be required to show a mortality benefit, which would require several years of follow-up.”
In an accompanying editorial, Dan L. Longo, MD, noted that the study provides high-quality data from a randomized trial where none existed (N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMe1912943). “It appears to show that among women with dense breasts, the risk of interval cancers is halved by following a negative mammogram with MRI screening,” wrote Dr. Longo, who is deputy editor of the New England Journal of Medicine, as well as professor of medicine at Harvard Medical School, Boston. “But is a reduction in interval cancers an appropriate surrogate for improved overall survival? It appears that most of the cancers that were detected on supplemental MRI screening were found at an early stage. Ductal carcinoma in situ was 10 times more frequent among patients undergoing MRI, and these diagnoses were likely to lead to treatments. What remains unclear is whether the tumors would never otherwise have been detected or threatened the patient’s survival.”
The trial was supported by the University Medical Center Utrecht (the Netherlands), the Netherlands Organization for Health Research and Development, the Dutch Cancer Society, the Dutch Pink Ribbon–A Sister’s Hope organization, Stichting Kankerpreventie Midden-West, and Bayer Pharmaceuticals, with an in-kind contribution from Volpara Health Technologies.
The researchers reported having no relevant financial disclosures other than the trial funding. Dr. Longo is employed by the New England Journal of Medicine as deputy editor.
SOURCE: Bakker MF et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1903986.
The use of supplemental MRI screening in women with extremely dense breast tissue and normal results on mammography led to the diagnosis of significantly fewer interval cancers, compared with mammography alone during a 2-year screening period, results from a randomized trial show.
“Women with extremely dense breast tissue have an increased risk of breast cancer, and their cancers are also less likely to be detected on mammography,” Dutch researchers led by Marije F. Bakker, PhD, of Utrecht (The Netherlands) University and colleagues wrote for the Dense Tissue and Early Breast Neoplasm Screening (DENSE) Trial Study Group in an article published in the New England Journal of Medicine.
“Such patients may benefit from a tailored breast-screening strategy, supplemented with more sensitive imaging methods. The benefit of supplemental imaging is the subject of a worldwide debate. In the United States, a federal law directs breast-density reporting, but supplemental screening is not recommended in American guidelines. Although supplemental imaging increases the rate of cancer detection in women with dense breasts, the question remains whether it improves health outcomes,” they said.
In the DENSE trial, researchers assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to a group that was invited to undergo supplemental MRI or to a group that received mammography screening only. The women were between the ages of 50 and 75 years and were enrolled between December 2011 and November 2015 as part of the Dutch population-based digital mammography screening program. The primary outcome was the between-group difference in the incidence of interval cancers during a 2-year screening period.
Dr. Bakker and associates found that the interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the MRI invitation group, compared with 5 per 1,000 among the 32,312 women in the mammography-only group, a difference of 2.5 per 1,000 screenings (P less than 0.001). Among the women who were invited to undergo MRI, 59% actually underwent the procedure. Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the women who had undergone MRI, which translated to 0.8 per 1,000 screenings. The remaining 16 were diagnosed in those who had not undergone MRI, which translated into 4.9 per 1,000 screenings.
“Undergoing supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings and resulted in a false positive rate of 8.0% (79.8 per 1,000 screenings),” the researchers wrote. “Of the women who underwent a breast biopsy on the basis of an MRI indication, 26.3% had breast cancer and 73.7% did not.”
Dr. Bakker and coauthors acknowledged certain limitations of the trial, including the fact that it was not large enough to examine the effect of MRI screening on breast cancer–specific or overall mortality. “This outcome would require a much larger sample size and longer follow-up,” they wrote. “The lower rate of interval cancers that we found among participants who underwent MRI is indicative of and prerequisite for an effect on mortality. After that, a reduction in the number of advanced cancers would also be required to show a mortality benefit, which would require several years of follow-up.”
In an accompanying editorial, Dan L. Longo, MD, noted that the study provides high-quality data from a randomized trial where none existed (N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMe1912943). “It appears to show that among women with dense breasts, the risk of interval cancers is halved by following a negative mammogram with MRI screening,” wrote Dr. Longo, who is deputy editor of the New England Journal of Medicine, as well as professor of medicine at Harvard Medical School, Boston. “But is a reduction in interval cancers an appropriate surrogate for improved overall survival? It appears that most of the cancers that were detected on supplemental MRI screening were found at an early stage. Ductal carcinoma in situ was 10 times more frequent among patients undergoing MRI, and these diagnoses were likely to lead to treatments. What remains unclear is whether the tumors would never otherwise have been detected or threatened the patient’s survival.”
The trial was supported by the University Medical Center Utrecht (the Netherlands), the Netherlands Organization for Health Research and Development, the Dutch Cancer Society, the Dutch Pink Ribbon–A Sister’s Hope organization, Stichting Kankerpreventie Midden-West, and Bayer Pharmaceuticals, with an in-kind contribution from Volpara Health Technologies.
The researchers reported having no relevant financial disclosures other than the trial funding. Dr. Longo is employed by the New England Journal of Medicine as deputy editor.
SOURCE: Bakker MF et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1903986.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The interval cancer rate was 2.5 per 1,000 screenings among women in the MRI invitation group, compared with 5 per 1,000 among women in the mammography-only group, a difference of 2.5 per 1,000 screenings (P less than 0.001).
Study details: A multicenter, randomized study of 40,373 women between the ages of 50 and 75 years. One-quarter were offered supplemental MRI to the mammography all received.
Disclosures: The trial was supported by the University Medical Center Utrecht, the Netherlands Organization for Health Research and Development, the Dutch Cancer Society, the Dutch Pink Ribbon–A Sister’s Hope organization, Stichting Kankerpreventie Midden-West, and Bayer Pharmaceuticals, with an in-kind contribution from Volpara Health Technologies.
Source: Bakker MF et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1903986.