User login
APOE genotype directly regulates alpha-synuclein accumulation
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
FROM SCIENCE TRANSLATIONAL MEDICINE
Abbreviated MRI equals standard protocol for high-risk breast cancer screens
CHICAGO – An abbreviated magnetic resonance imaging (MRI) protocol for screening individuals at high risk for breast cancer performed as well as a standard protocol, in about half the time and with greater patient satisfaction.
The abbreviated protocol also resulted in fewer false positive findings, with 5% fewer patients receiving biopsies for benign lesions than with a standard protocol (8.4% versus 13.7%, P less than .001).
Findings from the prospective 10-month trial conducted in the province of Ontario were presented by Jean Seely, MD, professor of radiology at the University of Ottawa, at the annual meeting of the Radiological Society of North America.
“The abbreviated protocol was shown to be as effective as the standard protocol in high-risk screening breast MRI, supporting previous studies,” said Dr. Seely. The shorter protocol took 16.3 minutes on average, compared with 27 minutes for the standard MRI protocol. This difference resulted in a 50% increase in institutional capacity, or a jump from two to three patients screened per hour.
In the province of Ontario, said Dr. Seely, women assessed at being at a 25% or greater lifetime risk of breast cancer receive MRIs as part of the Ontario Breast Screening Program (OBSP), which calculates risk by using the International Breast Cancer Intervention Study model.
For high-risk patients, the OBSP model provides annual mammography and an MRI for women between the ages of 30 and 60 years. Not only is the half-hour duration of the standard protocol resource-intensive, especially in regions with limited scanner availability, but patients may either be reluctant to undergo a half-hour scan, or not tolerate a lengthy scan very well.
Dr. Seely cited previous work (J Clin Oncol. 2014 Aug 1;32[22]:2304-10) showing that an abbreviated MRI protocol has similar accuracy as the full standard protocol. With the foundation of evidence from this study, Dr. Seely and her collaborators compared outcomes for high-risk patients who were screened with an abbreviated versus a standard protocol.
The abbreviated protocol, approved by the American College of Radiology, omits a final round of image acquisition sequences at the 9-minute mark after gadolinium administration, instead performing acquisition at 1, 2, 3, and 4 minutes after contrast delivery. Total time required for this protocol is just over 13 minutes, said Dr. Seely, and additional diagnostic MRIs were not required.
The trial was constructed so that the abbreviated protocol was used for the entire OBSP cohort for 10 months in 2018. Results were compared with those from the 12 previous months, when OBSP patients’ MRIs were performed using the standard protocol.
A total of 881 patients received standard-protocol MRIs; about three quarters (651) of those patients had previous MRI screening, while the remaining 230 patients had a baseline screen via the standard protocol.
Of the 658 patients in the abbreviated protocol group, 135, or about 20%, received the briefer scans as a baseline screen; the remast of the patients in this arm had received earlier MRI screening.
In addition to tracking scanning times, Dr. Seely and her collaborators also compared cancer detection rates, Breast Imaging Reporting and Data System (BI-RADS) assessment categories, positive predictive values, and the abnormal interpretation rate – that is, how many scans fell into BI-RADS categories 0, 4, and 5.
No significant difference was found between the rates of BI-RADs 0, 3, or 5 studies between the groups. Significantly fewer abbreviated scans fell into the BI-RADS 4 category, however (9.3% vs. 14.9%; P less than .001).
Similarly, the abnormal interpretation rate was 12.5% for the abbreviated protocol, compared with 17.5% for the standard protocol (P less than .007), with a correspondingly lower biopsy rate of 8.4% for the abbreviated protocol, compared with 13.7% for the standard protocol (P less than .001). The overall cancer detection rate did not differ between groups.
She and her colleagues will continue to track outcomes for those receiving abbreviated screening within the province of Ontario to track performance over time.
Dr. Seely reported that she had no relevant conflicts of interest. She reported no funding source beyond the province of Ontario.
SOURCE: Seely J et al. RSNA 2019, Session RC-215-04.
CHICAGO – An abbreviated magnetic resonance imaging (MRI) protocol for screening individuals at high risk for breast cancer performed as well as a standard protocol, in about half the time and with greater patient satisfaction.
The abbreviated protocol also resulted in fewer false positive findings, with 5% fewer patients receiving biopsies for benign lesions than with a standard protocol (8.4% versus 13.7%, P less than .001).
Findings from the prospective 10-month trial conducted in the province of Ontario were presented by Jean Seely, MD, professor of radiology at the University of Ottawa, at the annual meeting of the Radiological Society of North America.
“The abbreviated protocol was shown to be as effective as the standard protocol in high-risk screening breast MRI, supporting previous studies,” said Dr. Seely. The shorter protocol took 16.3 minutes on average, compared with 27 minutes for the standard MRI protocol. This difference resulted in a 50% increase in institutional capacity, or a jump from two to three patients screened per hour.
In the province of Ontario, said Dr. Seely, women assessed at being at a 25% or greater lifetime risk of breast cancer receive MRIs as part of the Ontario Breast Screening Program (OBSP), which calculates risk by using the International Breast Cancer Intervention Study model.
For high-risk patients, the OBSP model provides annual mammography and an MRI for women between the ages of 30 and 60 years. Not only is the half-hour duration of the standard protocol resource-intensive, especially in regions with limited scanner availability, but patients may either be reluctant to undergo a half-hour scan, or not tolerate a lengthy scan very well.
Dr. Seely cited previous work (J Clin Oncol. 2014 Aug 1;32[22]:2304-10) showing that an abbreviated MRI protocol has similar accuracy as the full standard protocol. With the foundation of evidence from this study, Dr. Seely and her collaborators compared outcomes for high-risk patients who were screened with an abbreviated versus a standard protocol.
The abbreviated protocol, approved by the American College of Radiology, omits a final round of image acquisition sequences at the 9-minute mark after gadolinium administration, instead performing acquisition at 1, 2, 3, and 4 minutes after contrast delivery. Total time required for this protocol is just over 13 minutes, said Dr. Seely, and additional diagnostic MRIs were not required.
The trial was constructed so that the abbreviated protocol was used for the entire OBSP cohort for 10 months in 2018. Results were compared with those from the 12 previous months, when OBSP patients’ MRIs were performed using the standard protocol.
A total of 881 patients received standard-protocol MRIs; about three quarters (651) of those patients had previous MRI screening, while the remaining 230 patients had a baseline screen via the standard protocol.
Of the 658 patients in the abbreviated protocol group, 135, or about 20%, received the briefer scans as a baseline screen; the remast of the patients in this arm had received earlier MRI screening.
In addition to tracking scanning times, Dr. Seely and her collaborators also compared cancer detection rates, Breast Imaging Reporting and Data System (BI-RADS) assessment categories, positive predictive values, and the abnormal interpretation rate – that is, how many scans fell into BI-RADS categories 0, 4, and 5.
No significant difference was found between the rates of BI-RADs 0, 3, or 5 studies between the groups. Significantly fewer abbreviated scans fell into the BI-RADS 4 category, however (9.3% vs. 14.9%; P less than .001).
Similarly, the abnormal interpretation rate was 12.5% for the abbreviated protocol, compared with 17.5% for the standard protocol (P less than .007), with a correspondingly lower biopsy rate of 8.4% for the abbreviated protocol, compared with 13.7% for the standard protocol (P less than .001). The overall cancer detection rate did not differ between groups.
She and her colleagues will continue to track outcomes for those receiving abbreviated screening within the province of Ontario to track performance over time.
Dr. Seely reported that she had no relevant conflicts of interest. She reported no funding source beyond the province of Ontario.
SOURCE: Seely J et al. RSNA 2019, Session RC-215-04.
CHICAGO – An abbreviated magnetic resonance imaging (MRI) protocol for screening individuals at high risk for breast cancer performed as well as a standard protocol, in about half the time and with greater patient satisfaction.
The abbreviated protocol also resulted in fewer false positive findings, with 5% fewer patients receiving biopsies for benign lesions than with a standard protocol (8.4% versus 13.7%, P less than .001).
Findings from the prospective 10-month trial conducted in the province of Ontario were presented by Jean Seely, MD, professor of radiology at the University of Ottawa, at the annual meeting of the Radiological Society of North America.
“The abbreviated protocol was shown to be as effective as the standard protocol in high-risk screening breast MRI, supporting previous studies,” said Dr. Seely. The shorter protocol took 16.3 minutes on average, compared with 27 minutes for the standard MRI protocol. This difference resulted in a 50% increase in institutional capacity, or a jump from two to three patients screened per hour.
In the province of Ontario, said Dr. Seely, women assessed at being at a 25% or greater lifetime risk of breast cancer receive MRIs as part of the Ontario Breast Screening Program (OBSP), which calculates risk by using the International Breast Cancer Intervention Study model.
For high-risk patients, the OBSP model provides annual mammography and an MRI for women between the ages of 30 and 60 years. Not only is the half-hour duration of the standard protocol resource-intensive, especially in regions with limited scanner availability, but patients may either be reluctant to undergo a half-hour scan, or not tolerate a lengthy scan very well.
Dr. Seely cited previous work (J Clin Oncol. 2014 Aug 1;32[22]:2304-10) showing that an abbreviated MRI protocol has similar accuracy as the full standard protocol. With the foundation of evidence from this study, Dr. Seely and her collaborators compared outcomes for high-risk patients who were screened with an abbreviated versus a standard protocol.
The abbreviated protocol, approved by the American College of Radiology, omits a final round of image acquisition sequences at the 9-minute mark after gadolinium administration, instead performing acquisition at 1, 2, 3, and 4 minutes after contrast delivery. Total time required for this protocol is just over 13 minutes, said Dr. Seely, and additional diagnostic MRIs were not required.
The trial was constructed so that the abbreviated protocol was used for the entire OBSP cohort for 10 months in 2018. Results were compared with those from the 12 previous months, when OBSP patients’ MRIs were performed using the standard protocol.
A total of 881 patients received standard-protocol MRIs; about three quarters (651) of those patients had previous MRI screening, while the remaining 230 patients had a baseline screen via the standard protocol.
Of the 658 patients in the abbreviated protocol group, 135, or about 20%, received the briefer scans as a baseline screen; the remast of the patients in this arm had received earlier MRI screening.
In addition to tracking scanning times, Dr. Seely and her collaborators also compared cancer detection rates, Breast Imaging Reporting and Data System (BI-RADS) assessment categories, positive predictive values, and the abnormal interpretation rate – that is, how many scans fell into BI-RADS categories 0, 4, and 5.
No significant difference was found between the rates of BI-RADs 0, 3, or 5 studies between the groups. Significantly fewer abbreviated scans fell into the BI-RADS 4 category, however (9.3% vs. 14.9%; P less than .001).
Similarly, the abnormal interpretation rate was 12.5% for the abbreviated protocol, compared with 17.5% for the standard protocol (P less than .007), with a correspondingly lower biopsy rate of 8.4% for the abbreviated protocol, compared with 13.7% for the standard protocol (P less than .001). The overall cancer detection rate did not differ between groups.
She and her colleagues will continue to track outcomes for those receiving abbreviated screening within the province of Ontario to track performance over time.
Dr. Seely reported that she had no relevant conflicts of interest. She reported no funding source beyond the province of Ontario.
SOURCE: Seely J et al. RSNA 2019, Session RC-215-04.
REPORTING FROM RSNA 2019
Data emerging to support personalized nutrition in oncology
SAN DIEGO – When Dawn Lemanne, MD, MPH, meets with cancer patients and their families, the question invariably comes up: “What should I eat?”
“The answer always is, ‘It depends,’” Dr. Lemanne, an oncologist who founded Oregon Integrative Oncology in Ashland, said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine. “The answers are not the same for each of these patients.”
According to Dr. Lemanne, targeted nutrition is evolving as a key component of cancer care. One of the goals of this approach is to decrease mTOR signaling. Normally, mTOR signaling promotes cell proliferation and metabolism; aberrant mTOR signaling can contribute to cancer initiation and progression.
“When mTOR speaks it says, ‘grow,’” said Dr. Lemanne, who is also an assistant professor of clinical medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona in Tucson. This message is meant to be heard by normal tissues, to stimulate normal tissue proliferation, such as in growing children or when a wound needs to be healed.
“However, cancer cells can hear and respond to mTOR’s message,” she said. “Normal cells may listen to mTOR’s ‘grow’ message or not, depending on the task they perform. Once we reach adulthood, we all likely have some precancerous or cancerous cells around, but they’re usually dormant. That’s why once you’re an adult, however, you don’t want too much mTOR signaling, because that might stimulate growth of things you definitely don’t want to grow.”
Having excessive levels of the growth hormone insulin-like growth factor-1 (IGF-1) also appears to play a role in cancer risk. Researchers studying members of a South American clan with Laron dwarfism – an inherited IGF-1 deficiency – found that besides being very short, affected members of this family rarely develop cancer (Cells. 2019;8[6]:596). “They also don’t get diabetes,” Dr. Lemanne said. “What we see in those with Laron dwarfism is that mTOR signaling is missing.”
She went on to note that studying type 2 diabetes gives physicians “a clue as to what dietary measures we might offer our patients in terms of decreasing their risk of dying from cancer or getting cancer.” The most common types of cancer are indeed more common in patients with type 2 diabetes. In addition, once someone with type 2 diabetes is diagnosed with cancer, their prognosis is poorer, compared with a cancer patient without diabetes.
“Metformin is often prescribed to patients with type 2 diabetes because it helps keep blood sugar low,” she said. “What’s fascinating is that diabetics on metformin develop cancer less frequently than diabetics not taking this drug. And also interesting, those diabetics who do develop cancer seem to do better if they’re on metformin before and after diagnosis.”
On the other hand, exogenous insulin therapy given to people with type 2 diabetes doubles the risk of cancer. Consistent with this is the two-decades-old finding that an elevated fasting insulin level also is associated with a poor breast cancer prognosis (J Clin Oncol. 2002 Jan 1;20[1]:42-51). “It’s really important to understand that, in a person destined to become a type 2 diabetic, the level of fasting insulin rises long before fasting glucose becomes abnormally high,” Dr. Lemanne explained. “A normal fasting glucose doesn’t let you off the hook in terms of checking your patient for insulin resistance.
“We will miss diagnosing many patients with dangerous insulin resistance and prediabetes if we don’t check the fasting glucose and the fasting insulin levels together. If the fasting insulin level is high, it’s important to limit carbohydrate intake enough to bring it down permanently, even when the fasting glucose is normal, or the patient is likely at increased risk for developing cancer.”
Two large, prospective randomized trials have examined breast cancer and diet: the Women’s Intervention Study (WINS) and the Women’s Health Eating and Living Study (WHEL). Patients in both trials had early stage breast cancer and were put on low-fat diets. In the end, there was a weak to negligible connection between breast cancer survival and dietary fat restriction. “That kind of shook up the oncology world,” Dr. Lemanne said, “because before these two studies, everyone ‘knew’ that dietary fat was related to breast cancer risk. These studies showed that wasn’t the case at all.”
According to Dr. Lemanne, unexpectedly, moderate carbohydrate restriction has been associated with lower risk of breast cancer recurrence in patients with postmenopausal hormone-receptor expressing breast cancer. Researchers at the University of California, San Diego, conducted a subanalysis of 265 postmenopausal patients with estrogen receptor positive breast cancer from the WHEL cohort (Cancer Epidemiol Biomarkers Prev. 2014 23[7]:1273-9). The recurrence risk was halved in those who cut their carbohydrate intake after diagnosis. The amount of decrease was modest, only 27 grams per day – the equivalent of one banana. “That is on par with a lot of our drugs, and maybe a little bit better,” she said. The effect was strongest if the breast tumor expressed IGF-1 receptor. Dr. Lemanne pointed out that decreasing dietary carbohydrate load was not the only treatment. These patients also had appropriate conventional cancer treatments, including surgery, radiation, and chemotherapy. “If we cut just some of the daily carb load in these patients, they might have a better cancer prognosis,” she said.
Overweight or obese patients with colon cancer also may benefit from moderate carbohydrate restriction. The CALGB 89803 study assessed 1,011 subjects with stage III colon cancer. It found that the subjects in the highest quintile of daily glycemic load and total carbohydrate intake had an increased risk of cancer recurrence and mortality (hazard ratio, 2.26; J Nat Cancer Inst. 2012;104[22]:1702-11). “This is pretty strong evidence that glycemic load and total carbohydrate intake play a role in colon cancer recurrence, but there’s a caveat here,” she said. “The effect was seen only in patients who were overweight or obese.” There was no association between carbohydrate intake and colon cancer recurrence in the absence of overweight or obesity.
Based on existing evidence, she said,
“That’s pretty modest; that’s 400 calories of carbohydrates per day,” Dr. Lemanne said. “I tell patients that they can have fruit, starchy vegetables, and even very small amounts of healthy whole grains, although I’m not a fan of grains due to the heavy carbohydrate load. All those things are OK. We’re not talking about jelly beans and white sugar.
“I also have them measure their fasting glucose each day, because different people have different blood glucose responses to the same food.” The goals she aims for with many of her patients are a fasting morning glucose between 79 and 83 mg/dL consistently, an HbA1c of 5.4 or less, and a BMI of 24.9 kg/m2 or less. “This set of goals, however, has to be individualized,” she said.
The ketogenic diet is another form of carb restriction, “but it’s much more drastic,” Dr. Lemanne said. “Most people require a carbohydrate load below 30 grams a day to enter a state of ketosis. But ketosis lowers the blood sugar and dampens the mTOR signaling.”
Evidence is emerging to support the use of a ketogenic diet as an adjunct to radiation therapy and as part of a complete course of treatment for glioblastoma multiforme and cancer cachexia. As an adjunct to radiation, a ketogenic diet decreases insulin and IGF-1 signaling. “This causes normal cells to enter dormancy, decreasing oxidative damage in normal cells,” Dr. Lemanne said. “There is also suppression of tumor angiogenesis, and thus poor DNA repair of radiation damage in tumor cells (Cancer Metastasis Rev. 2014;33[1]:217-29). Being in ketosis widens the therapeutic window. There are many animal studies which show that the ketogenic diet is helpful in cancer, mainly when combined with other anticancer treatments, such as radiation. Unfortunately, the evidence in humans is very anecdotal.”
One study found that if you feed mice with cancer ketogenic chow versus standard chow, they have a modestly improved survival (a mean of 43 days vs. 33 days; PLoS ONE. 2012;7[5]:e36197). However, when radiation was added to the keto diet, there was a dramatic improvement in survival (P less than 0.001). In fact, 75% survived to 250 days. “That’s pretty spectacular,” Dr. Lemanne said.
A ketogenic diet is standard therapy for several nonmalignant conditions, including glucose transporter 1 deficiency syndrome, pyruvate dehydrogenase deficiency syndrome, and refractory infantile epilepsy. The three major ketone bodies involved in human nutrition are acetoacetate, beta hydroxybutyrate, and acetone. Dr. Lemanne said beta hydroxybutyrate decreases inflammation and inhibits hexadecynoic acids (which induces apoptosis in cancer cells). Beta hydroxybutyrate also increases sirtuins, innate immunity, and seizure threshold; modulates circadian rhythm; and decreases insulin levels, she said.
In one case report from the scientific literature, a 38-year-old male with glioblastoma multiforme was placed on a hypocaloric ketogenic diet (Front Nutr. 2018 Mar 29;5:20). The patient had surgery, radiation, chemotherapy, and hyperbaric oxygen, and was given high doses of green tea extract in an attempt to antagonize glutamine metabolism. Two years after the beginning of his treatment, he was alive and had maintained a good level of tumor regression.
“We’ll see how he does,” said Dr. Lemanne, who was not involved in the report. “In my experience, I have a patient right now with a diagnosis of glioblastoma multiforme. She’s getting a keto diet in combo with intensive chemo, radiation, and surgery. She’s also had some hyperbaric oxygen and IV ozone therapy and is taking repurposed drugs. She has exceeded her expected survival, but she continues to have disease and symptoms. We are by no means out of the woods with this patient. But the keto diet has been quite feasible for her, because she has a lot of family and outside support.”
A ketogenic diet also may benefit patients with cancer cachexia, which is a loss of lean tissue. “Cancer cachexia is not completely understood,” Dr. Lemanne said. “What we know is that it is caused by inflammation created by the tumor itself, and this, in turn results in severe insulin resistance. Therefore, giving more calories as carbohydrate makes the cancer cachexia situation worse. Animal models of cancer cachexia have shown that the ketogenic diet normalizes metabolism and prevents lean tissue loss. Human studies are underway; we’ll see how they turn out.”
She closed her presentation by noting that in copious amounts of animal studies, fasting has been linked to improvements in chemotherapy efficacy and decreased side effects. In one study carried out at the University of Southern California in Los Angeles, volunteers fasted up to 140 hours before chemotherapy and an additional 156 hours afterward (Aging. 2009;1[12]:988-1007). The researchers found that the fasting was well-tolerated.
“The patients had some mild light-headedness, but there were no adverse effects on tumor volume or serum tumor markers,” Dr. Lemanne said. A more recent study of patients on cisplatin found that acaloric fasting led to decreased DNA damage in white blood cells, decreased IFG-1, and better white blood cell counts (BMC Cancer. 2016 Jun 10;16:360). “The benefits are immediate, and the optimal fasting time appears to be 48 hours,” Dr. Lemanne said.
One of her patients is a 64-year-old man on adjuvant cisplatin-based chemotherapy for cholangiocarcinoma. He fasts 24 hours before and 24 hours after each infusion, and has experienced no emesis or nausea. “His immune suppression and anemia are much milder than we expected, and he has not required any treatment for chemotherapy-related side effects,” Dr. Lemanne said. “That’s a big monetary value.”
Fasting 13 hours overnight has been associated with fewer breast cancer-related problems in patients already diagnosed with the disease. Chronic caloric restriction, just cutting calories by 25%-40% daily, has been shown to delay all diseases of aging, including cancer, and is associated with increased longevity in many species. “Chronic caloric restriction is difficult, however, because it results in chronic hunger and weight loss,” she said. “Occasional fasting is superior to chronic caloric restriction because it maintains normal weight, preserves lean muscle mass, enhances tumor sensitivity to chemotherapy and radiotherapy, and diminishes the side effects of chemotherapy.”
Dr. Lemanne reported having no financial disclosures.
SAN DIEGO – When Dawn Lemanne, MD, MPH, meets with cancer patients and their families, the question invariably comes up: “What should I eat?”
“The answer always is, ‘It depends,’” Dr. Lemanne, an oncologist who founded Oregon Integrative Oncology in Ashland, said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine. “The answers are not the same for each of these patients.”
According to Dr. Lemanne, targeted nutrition is evolving as a key component of cancer care. One of the goals of this approach is to decrease mTOR signaling. Normally, mTOR signaling promotes cell proliferation and metabolism; aberrant mTOR signaling can contribute to cancer initiation and progression.
“When mTOR speaks it says, ‘grow,’” said Dr. Lemanne, who is also an assistant professor of clinical medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona in Tucson. This message is meant to be heard by normal tissues, to stimulate normal tissue proliferation, such as in growing children or when a wound needs to be healed.
“However, cancer cells can hear and respond to mTOR’s message,” she said. “Normal cells may listen to mTOR’s ‘grow’ message or not, depending on the task they perform. Once we reach adulthood, we all likely have some precancerous or cancerous cells around, but they’re usually dormant. That’s why once you’re an adult, however, you don’t want too much mTOR signaling, because that might stimulate growth of things you definitely don’t want to grow.”
Having excessive levels of the growth hormone insulin-like growth factor-1 (IGF-1) also appears to play a role in cancer risk. Researchers studying members of a South American clan with Laron dwarfism – an inherited IGF-1 deficiency – found that besides being very short, affected members of this family rarely develop cancer (Cells. 2019;8[6]:596). “They also don’t get diabetes,” Dr. Lemanne said. “What we see in those with Laron dwarfism is that mTOR signaling is missing.”
She went on to note that studying type 2 diabetes gives physicians “a clue as to what dietary measures we might offer our patients in terms of decreasing their risk of dying from cancer or getting cancer.” The most common types of cancer are indeed more common in patients with type 2 diabetes. In addition, once someone with type 2 diabetes is diagnosed with cancer, their prognosis is poorer, compared with a cancer patient without diabetes.
“Metformin is often prescribed to patients with type 2 diabetes because it helps keep blood sugar low,” she said. “What’s fascinating is that diabetics on metformin develop cancer less frequently than diabetics not taking this drug. And also interesting, those diabetics who do develop cancer seem to do better if they’re on metformin before and after diagnosis.”
On the other hand, exogenous insulin therapy given to people with type 2 diabetes doubles the risk of cancer. Consistent with this is the two-decades-old finding that an elevated fasting insulin level also is associated with a poor breast cancer prognosis (J Clin Oncol. 2002 Jan 1;20[1]:42-51). “It’s really important to understand that, in a person destined to become a type 2 diabetic, the level of fasting insulin rises long before fasting glucose becomes abnormally high,” Dr. Lemanne explained. “A normal fasting glucose doesn’t let you off the hook in terms of checking your patient for insulin resistance.
“We will miss diagnosing many patients with dangerous insulin resistance and prediabetes if we don’t check the fasting glucose and the fasting insulin levels together. If the fasting insulin level is high, it’s important to limit carbohydrate intake enough to bring it down permanently, even when the fasting glucose is normal, or the patient is likely at increased risk for developing cancer.”
Two large, prospective randomized trials have examined breast cancer and diet: the Women’s Intervention Study (WINS) and the Women’s Health Eating and Living Study (WHEL). Patients in both trials had early stage breast cancer and were put on low-fat diets. In the end, there was a weak to negligible connection between breast cancer survival and dietary fat restriction. “That kind of shook up the oncology world,” Dr. Lemanne said, “because before these two studies, everyone ‘knew’ that dietary fat was related to breast cancer risk. These studies showed that wasn’t the case at all.”
According to Dr. Lemanne, unexpectedly, moderate carbohydrate restriction has been associated with lower risk of breast cancer recurrence in patients with postmenopausal hormone-receptor expressing breast cancer. Researchers at the University of California, San Diego, conducted a subanalysis of 265 postmenopausal patients with estrogen receptor positive breast cancer from the WHEL cohort (Cancer Epidemiol Biomarkers Prev. 2014 23[7]:1273-9). The recurrence risk was halved in those who cut their carbohydrate intake after diagnosis. The amount of decrease was modest, only 27 grams per day – the equivalent of one banana. “That is on par with a lot of our drugs, and maybe a little bit better,” she said. The effect was strongest if the breast tumor expressed IGF-1 receptor. Dr. Lemanne pointed out that decreasing dietary carbohydrate load was not the only treatment. These patients also had appropriate conventional cancer treatments, including surgery, radiation, and chemotherapy. “If we cut just some of the daily carb load in these patients, they might have a better cancer prognosis,” she said.
Overweight or obese patients with colon cancer also may benefit from moderate carbohydrate restriction. The CALGB 89803 study assessed 1,011 subjects with stage III colon cancer. It found that the subjects in the highest quintile of daily glycemic load and total carbohydrate intake had an increased risk of cancer recurrence and mortality (hazard ratio, 2.26; J Nat Cancer Inst. 2012;104[22]:1702-11). “This is pretty strong evidence that glycemic load and total carbohydrate intake play a role in colon cancer recurrence, but there’s a caveat here,” she said. “The effect was seen only in patients who were overweight or obese.” There was no association between carbohydrate intake and colon cancer recurrence in the absence of overweight or obesity.
Based on existing evidence, she said,
“That’s pretty modest; that’s 400 calories of carbohydrates per day,” Dr. Lemanne said. “I tell patients that they can have fruit, starchy vegetables, and even very small amounts of healthy whole grains, although I’m not a fan of grains due to the heavy carbohydrate load. All those things are OK. We’re not talking about jelly beans and white sugar.
“I also have them measure their fasting glucose each day, because different people have different blood glucose responses to the same food.” The goals she aims for with many of her patients are a fasting morning glucose between 79 and 83 mg/dL consistently, an HbA1c of 5.4 or less, and a BMI of 24.9 kg/m2 or less. “This set of goals, however, has to be individualized,” she said.
The ketogenic diet is another form of carb restriction, “but it’s much more drastic,” Dr. Lemanne said. “Most people require a carbohydrate load below 30 grams a day to enter a state of ketosis. But ketosis lowers the blood sugar and dampens the mTOR signaling.”
Evidence is emerging to support the use of a ketogenic diet as an adjunct to radiation therapy and as part of a complete course of treatment for glioblastoma multiforme and cancer cachexia. As an adjunct to radiation, a ketogenic diet decreases insulin and IGF-1 signaling. “This causes normal cells to enter dormancy, decreasing oxidative damage in normal cells,” Dr. Lemanne said. “There is also suppression of tumor angiogenesis, and thus poor DNA repair of radiation damage in tumor cells (Cancer Metastasis Rev. 2014;33[1]:217-29). Being in ketosis widens the therapeutic window. There are many animal studies which show that the ketogenic diet is helpful in cancer, mainly when combined with other anticancer treatments, such as radiation. Unfortunately, the evidence in humans is very anecdotal.”
One study found that if you feed mice with cancer ketogenic chow versus standard chow, they have a modestly improved survival (a mean of 43 days vs. 33 days; PLoS ONE. 2012;7[5]:e36197). However, when radiation was added to the keto diet, there was a dramatic improvement in survival (P less than 0.001). In fact, 75% survived to 250 days. “That’s pretty spectacular,” Dr. Lemanne said.
A ketogenic diet is standard therapy for several nonmalignant conditions, including glucose transporter 1 deficiency syndrome, pyruvate dehydrogenase deficiency syndrome, and refractory infantile epilepsy. The three major ketone bodies involved in human nutrition are acetoacetate, beta hydroxybutyrate, and acetone. Dr. Lemanne said beta hydroxybutyrate decreases inflammation and inhibits hexadecynoic acids (which induces apoptosis in cancer cells). Beta hydroxybutyrate also increases sirtuins, innate immunity, and seizure threshold; modulates circadian rhythm; and decreases insulin levels, she said.
In one case report from the scientific literature, a 38-year-old male with glioblastoma multiforme was placed on a hypocaloric ketogenic diet (Front Nutr. 2018 Mar 29;5:20). The patient had surgery, radiation, chemotherapy, and hyperbaric oxygen, and was given high doses of green tea extract in an attempt to antagonize glutamine metabolism. Two years after the beginning of his treatment, he was alive and had maintained a good level of tumor regression.
“We’ll see how he does,” said Dr. Lemanne, who was not involved in the report. “In my experience, I have a patient right now with a diagnosis of glioblastoma multiforme. She’s getting a keto diet in combo with intensive chemo, radiation, and surgery. She’s also had some hyperbaric oxygen and IV ozone therapy and is taking repurposed drugs. She has exceeded her expected survival, but she continues to have disease and symptoms. We are by no means out of the woods with this patient. But the keto diet has been quite feasible for her, because she has a lot of family and outside support.”
A ketogenic diet also may benefit patients with cancer cachexia, which is a loss of lean tissue. “Cancer cachexia is not completely understood,” Dr. Lemanne said. “What we know is that it is caused by inflammation created by the tumor itself, and this, in turn results in severe insulin resistance. Therefore, giving more calories as carbohydrate makes the cancer cachexia situation worse. Animal models of cancer cachexia have shown that the ketogenic diet normalizes metabolism and prevents lean tissue loss. Human studies are underway; we’ll see how they turn out.”
She closed her presentation by noting that in copious amounts of animal studies, fasting has been linked to improvements in chemotherapy efficacy and decreased side effects. In one study carried out at the University of Southern California in Los Angeles, volunteers fasted up to 140 hours before chemotherapy and an additional 156 hours afterward (Aging. 2009;1[12]:988-1007). The researchers found that the fasting was well-tolerated.
“The patients had some mild light-headedness, but there were no adverse effects on tumor volume or serum tumor markers,” Dr. Lemanne said. A more recent study of patients on cisplatin found that acaloric fasting led to decreased DNA damage in white blood cells, decreased IFG-1, and better white blood cell counts (BMC Cancer. 2016 Jun 10;16:360). “The benefits are immediate, and the optimal fasting time appears to be 48 hours,” Dr. Lemanne said.
One of her patients is a 64-year-old man on adjuvant cisplatin-based chemotherapy for cholangiocarcinoma. He fasts 24 hours before and 24 hours after each infusion, and has experienced no emesis or nausea. “His immune suppression and anemia are much milder than we expected, and he has not required any treatment for chemotherapy-related side effects,” Dr. Lemanne said. “That’s a big monetary value.”
Fasting 13 hours overnight has been associated with fewer breast cancer-related problems in patients already diagnosed with the disease. Chronic caloric restriction, just cutting calories by 25%-40% daily, has been shown to delay all diseases of aging, including cancer, and is associated with increased longevity in many species. “Chronic caloric restriction is difficult, however, because it results in chronic hunger and weight loss,” she said. “Occasional fasting is superior to chronic caloric restriction because it maintains normal weight, preserves lean muscle mass, enhances tumor sensitivity to chemotherapy and radiotherapy, and diminishes the side effects of chemotherapy.”
Dr. Lemanne reported having no financial disclosures.
SAN DIEGO – When Dawn Lemanne, MD, MPH, meets with cancer patients and their families, the question invariably comes up: “What should I eat?”
“The answer always is, ‘It depends,’” Dr. Lemanne, an oncologist who founded Oregon Integrative Oncology in Ashland, said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine. “The answers are not the same for each of these patients.”
According to Dr. Lemanne, targeted nutrition is evolving as a key component of cancer care. One of the goals of this approach is to decrease mTOR signaling. Normally, mTOR signaling promotes cell proliferation and metabolism; aberrant mTOR signaling can contribute to cancer initiation and progression.
“When mTOR speaks it says, ‘grow,’” said Dr. Lemanne, who is also an assistant professor of clinical medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona in Tucson. This message is meant to be heard by normal tissues, to stimulate normal tissue proliferation, such as in growing children or when a wound needs to be healed.
“However, cancer cells can hear and respond to mTOR’s message,” she said. “Normal cells may listen to mTOR’s ‘grow’ message or not, depending on the task they perform. Once we reach adulthood, we all likely have some precancerous or cancerous cells around, but they’re usually dormant. That’s why once you’re an adult, however, you don’t want too much mTOR signaling, because that might stimulate growth of things you definitely don’t want to grow.”
Having excessive levels of the growth hormone insulin-like growth factor-1 (IGF-1) also appears to play a role in cancer risk. Researchers studying members of a South American clan with Laron dwarfism – an inherited IGF-1 deficiency – found that besides being very short, affected members of this family rarely develop cancer (Cells. 2019;8[6]:596). “They also don’t get diabetes,” Dr. Lemanne said. “What we see in those with Laron dwarfism is that mTOR signaling is missing.”
She went on to note that studying type 2 diabetes gives physicians “a clue as to what dietary measures we might offer our patients in terms of decreasing their risk of dying from cancer or getting cancer.” The most common types of cancer are indeed more common in patients with type 2 diabetes. In addition, once someone with type 2 diabetes is diagnosed with cancer, their prognosis is poorer, compared with a cancer patient without diabetes.
“Metformin is often prescribed to patients with type 2 diabetes because it helps keep blood sugar low,” she said. “What’s fascinating is that diabetics on metformin develop cancer less frequently than diabetics not taking this drug. And also interesting, those diabetics who do develop cancer seem to do better if they’re on metformin before and after diagnosis.”
On the other hand, exogenous insulin therapy given to people with type 2 diabetes doubles the risk of cancer. Consistent with this is the two-decades-old finding that an elevated fasting insulin level also is associated with a poor breast cancer prognosis (J Clin Oncol. 2002 Jan 1;20[1]:42-51). “It’s really important to understand that, in a person destined to become a type 2 diabetic, the level of fasting insulin rises long before fasting glucose becomes abnormally high,” Dr. Lemanne explained. “A normal fasting glucose doesn’t let you off the hook in terms of checking your patient for insulin resistance.
“We will miss diagnosing many patients with dangerous insulin resistance and prediabetes if we don’t check the fasting glucose and the fasting insulin levels together. If the fasting insulin level is high, it’s important to limit carbohydrate intake enough to bring it down permanently, even when the fasting glucose is normal, or the patient is likely at increased risk for developing cancer.”
Two large, prospective randomized trials have examined breast cancer and diet: the Women’s Intervention Study (WINS) and the Women’s Health Eating and Living Study (WHEL). Patients in both trials had early stage breast cancer and were put on low-fat diets. In the end, there was a weak to negligible connection between breast cancer survival and dietary fat restriction. “That kind of shook up the oncology world,” Dr. Lemanne said, “because before these two studies, everyone ‘knew’ that dietary fat was related to breast cancer risk. These studies showed that wasn’t the case at all.”
According to Dr. Lemanne, unexpectedly, moderate carbohydrate restriction has been associated with lower risk of breast cancer recurrence in patients with postmenopausal hormone-receptor expressing breast cancer. Researchers at the University of California, San Diego, conducted a subanalysis of 265 postmenopausal patients with estrogen receptor positive breast cancer from the WHEL cohort (Cancer Epidemiol Biomarkers Prev. 2014 23[7]:1273-9). The recurrence risk was halved in those who cut their carbohydrate intake after diagnosis. The amount of decrease was modest, only 27 grams per day – the equivalent of one banana. “That is on par with a lot of our drugs, and maybe a little bit better,” she said. The effect was strongest if the breast tumor expressed IGF-1 receptor. Dr. Lemanne pointed out that decreasing dietary carbohydrate load was not the only treatment. These patients also had appropriate conventional cancer treatments, including surgery, radiation, and chemotherapy. “If we cut just some of the daily carb load in these patients, they might have a better cancer prognosis,” she said.
Overweight or obese patients with colon cancer also may benefit from moderate carbohydrate restriction. The CALGB 89803 study assessed 1,011 subjects with stage III colon cancer. It found that the subjects in the highest quintile of daily glycemic load and total carbohydrate intake had an increased risk of cancer recurrence and mortality (hazard ratio, 2.26; J Nat Cancer Inst. 2012;104[22]:1702-11). “This is pretty strong evidence that glycemic load and total carbohydrate intake play a role in colon cancer recurrence, but there’s a caveat here,” she said. “The effect was seen only in patients who were overweight or obese.” There was no association between carbohydrate intake and colon cancer recurrence in the absence of overweight or obesity.
Based on existing evidence, she said,
“That’s pretty modest; that’s 400 calories of carbohydrates per day,” Dr. Lemanne said. “I tell patients that they can have fruit, starchy vegetables, and even very small amounts of healthy whole grains, although I’m not a fan of grains due to the heavy carbohydrate load. All those things are OK. We’re not talking about jelly beans and white sugar.
“I also have them measure their fasting glucose each day, because different people have different blood glucose responses to the same food.” The goals she aims for with many of her patients are a fasting morning glucose between 79 and 83 mg/dL consistently, an HbA1c of 5.4 or less, and a BMI of 24.9 kg/m2 or less. “This set of goals, however, has to be individualized,” she said.
The ketogenic diet is another form of carb restriction, “but it’s much more drastic,” Dr. Lemanne said. “Most people require a carbohydrate load below 30 grams a day to enter a state of ketosis. But ketosis lowers the blood sugar and dampens the mTOR signaling.”
Evidence is emerging to support the use of a ketogenic diet as an adjunct to radiation therapy and as part of a complete course of treatment for glioblastoma multiforme and cancer cachexia. As an adjunct to radiation, a ketogenic diet decreases insulin and IGF-1 signaling. “This causes normal cells to enter dormancy, decreasing oxidative damage in normal cells,” Dr. Lemanne said. “There is also suppression of tumor angiogenesis, and thus poor DNA repair of radiation damage in tumor cells (Cancer Metastasis Rev. 2014;33[1]:217-29). Being in ketosis widens the therapeutic window. There are many animal studies which show that the ketogenic diet is helpful in cancer, mainly when combined with other anticancer treatments, such as radiation. Unfortunately, the evidence in humans is very anecdotal.”
One study found that if you feed mice with cancer ketogenic chow versus standard chow, they have a modestly improved survival (a mean of 43 days vs. 33 days; PLoS ONE. 2012;7[5]:e36197). However, when radiation was added to the keto diet, there was a dramatic improvement in survival (P less than 0.001). In fact, 75% survived to 250 days. “That’s pretty spectacular,” Dr. Lemanne said.
A ketogenic diet is standard therapy for several nonmalignant conditions, including glucose transporter 1 deficiency syndrome, pyruvate dehydrogenase deficiency syndrome, and refractory infantile epilepsy. The three major ketone bodies involved in human nutrition are acetoacetate, beta hydroxybutyrate, and acetone. Dr. Lemanne said beta hydroxybutyrate decreases inflammation and inhibits hexadecynoic acids (which induces apoptosis in cancer cells). Beta hydroxybutyrate also increases sirtuins, innate immunity, and seizure threshold; modulates circadian rhythm; and decreases insulin levels, she said.
In one case report from the scientific literature, a 38-year-old male with glioblastoma multiforme was placed on a hypocaloric ketogenic diet (Front Nutr. 2018 Mar 29;5:20). The patient had surgery, radiation, chemotherapy, and hyperbaric oxygen, and was given high doses of green tea extract in an attempt to antagonize glutamine metabolism. Two years after the beginning of his treatment, he was alive and had maintained a good level of tumor regression.
“We’ll see how he does,” said Dr. Lemanne, who was not involved in the report. “In my experience, I have a patient right now with a diagnosis of glioblastoma multiforme. She’s getting a keto diet in combo with intensive chemo, radiation, and surgery. She’s also had some hyperbaric oxygen and IV ozone therapy and is taking repurposed drugs. She has exceeded her expected survival, but she continues to have disease and symptoms. We are by no means out of the woods with this patient. But the keto diet has been quite feasible for her, because she has a lot of family and outside support.”
A ketogenic diet also may benefit patients with cancer cachexia, which is a loss of lean tissue. “Cancer cachexia is not completely understood,” Dr. Lemanne said. “What we know is that it is caused by inflammation created by the tumor itself, and this, in turn results in severe insulin resistance. Therefore, giving more calories as carbohydrate makes the cancer cachexia situation worse. Animal models of cancer cachexia have shown that the ketogenic diet normalizes metabolism and prevents lean tissue loss. Human studies are underway; we’ll see how they turn out.”
She closed her presentation by noting that in copious amounts of animal studies, fasting has been linked to improvements in chemotherapy efficacy and decreased side effects. In one study carried out at the University of Southern California in Los Angeles, volunteers fasted up to 140 hours before chemotherapy and an additional 156 hours afterward (Aging. 2009;1[12]:988-1007). The researchers found that the fasting was well-tolerated.
“The patients had some mild light-headedness, but there were no adverse effects on tumor volume or serum tumor markers,” Dr. Lemanne said. A more recent study of patients on cisplatin found that acaloric fasting led to decreased DNA damage in white blood cells, decreased IFG-1, and better white blood cell counts (BMC Cancer. 2016 Jun 10;16:360). “The benefits are immediate, and the optimal fasting time appears to be 48 hours,” Dr. Lemanne said.
One of her patients is a 64-year-old man on adjuvant cisplatin-based chemotherapy for cholangiocarcinoma. He fasts 24 hours before and 24 hours after each infusion, and has experienced no emesis or nausea. “His immune suppression and anemia are much milder than we expected, and he has not required any treatment for chemotherapy-related side effects,” Dr. Lemanne said. “That’s a big monetary value.”
Fasting 13 hours overnight has been associated with fewer breast cancer-related problems in patients already diagnosed with the disease. Chronic caloric restriction, just cutting calories by 25%-40% daily, has been shown to delay all diseases of aging, including cancer, and is associated with increased longevity in many species. “Chronic caloric restriction is difficult, however, because it results in chronic hunger and weight loss,” she said. “Occasional fasting is superior to chronic caloric restriction because it maintains normal weight, preserves lean muscle mass, enhances tumor sensitivity to chemotherapy and radiotherapy, and diminishes the side effects of chemotherapy.”
Dr. Lemanne reported having no financial disclosures.
REPORTING FROM A NATURAL SUPPLEMENTS UPDATE
Breast cancer chemoprophylaxis in high-risk women: How persistent is the impact of an aromatase inhibitor after 5 years of use?
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
Serum levels of neurofilament light are increased before clinical onset of MS
(MS), according to research published in the January issue of JAMA Neurology. These results lend weight to the idea that MS has a prodromal phase, and this phase appears to be associated with neurodegeneration, according to the authors.
Patients often have CNS lesions of various stages of development at the time of their first demyelinating event, and this finding was one basis for neurologists’ hypothesis of a prodromal phase of MS. The finding that one-third of patients with radiologically isolated syndrome develop MS within 5 years also lends credence to this idea. Diagnosing MS early would enable early treatment that could prevent demyelination and the progression of neurodegeneration.
Researchers compared presymptomatic and symptomatic samples
With this idea in mind, Kjetil Bjornevik, MD, PhD, a member of the neuroepidemiology research group at Harvard TH Chan School of Public Health in Boston, and colleagues evaluated whether serum levels of NfL, a marker of ongoing neuroaxonal degeneration, were increased in the years before and around the time of clinical onset of MS. For their study population, the investigators chose active-duty U.S. military personnel who have at least one serum sample stored in the U.S. Department of Defense Serum Repository. Samples are collected after routine HIV type 1 antibody testing.
Within this population, Dr. Bjornevik and colleagues identified patients with MS who had at least one presymptomatic serum sample. The date of clinical MS onset was defined as the date of the first neurologic symptoms attributable to MS documented in the medical record. The investigators randomly selected two control individuals from the population and matched them to each case by age, sex, race or ethnicity, and dates of sample collection. Eligible controls were on active duty on the date of onset of the matched case.
Dr. Bjornevik and colleagues identified 245 patients with MS. Among this sample, the researchers selected two groups that each included 30 cases and 30 controls. The first group included patients who had provided at least one serum sample before MS onset and one sample within 2 years after MS onset. The second group included cases with at least two presymptomatic serum samples, one of which was collected more than 5 years before MS diagnosis, and the other of which was collected between 2 and 5 years before diagnosis. The investigators handled pairs of serum samples in the same way and assayed them in the same batch. The order of the samples in each pair was arranged at random.
Levels were higher in cases than in controls
About 77% of the population was male. Sixty percent of participants were white, 28% were black, and 6.7% were Hispanic. The population’s mean age at first sample collection was approximately 27 years. Mean age at MS onset was approximately 31 years.
For patients who provided samples before and after the clinical onset of MS, serum NfL levels were higher than in matched controls at both points. Most patients who passed from the presymptomatic stage to the symptomatic stage had a significant increase in serum NfL level (i.e., from a median of 25.0 pg/mL to a median of 45.1 pg/mL). Serum NfL levels at the two time points in controls did not differ significantly. For any given patient, an increase in serum NfL level from the presymptomatic measurement to the symptomatic measurement was associated with an increased risk of MS.
In patients with two presymptomatic samples, serum NfL levels were significantly higher in both samples than in the corresponding samples from matched controls. In cases, the earlier sample was collected at a median of 6 years before clinical onset of MS, and the later sample was collected at a median of 1 year before clinical onset. The serum NfL levels increased significantly between the two points for cases (i.e., a median increase of 1.3 pg/mL per year), but there was no significant difference in serum NfL level between the two samples in controls. A within-patient increase in presymptomatic serum NfL level was associated with an increased risk of MS.
Population included few women
“Our study differs from previous studies on the prodromal phase of MS because these have used indirect markers of this phase, which included unspecific symptoms or disturbances occurring before the clinical onset, compared with a marker of neurodegeneration,” wrote Dr. Bjornevik and colleagues. Initiation of treatment with disease-modifying therapy is associated with reductions in serum NfL levels, and this association could explain why some patients in the current study had higher NfL levels before MS onset than afterward. Furthermore, serum NfL levels are highly associated with levels of NfL in cerebrospinal fluid. “Thus, our findings of a presymptomatic increase in serum NfL not only suggest the presence of a prodromal phase in MS, but also that this phase is associated with neurodegeneration,” wrote the investigators.
The study’s well-defined population helped to minimize selection bias, and the blinded, randomized method of analyzing the serum samples eliminated artifactual differences in serum NfL concentrations. But the small sample size precluded analyses that could have influenced clinical practice, wrote Dr. Bjornevik and colleagues. For example, the researchers could not evaluate distinct cutoffs in serum NfL level that could mark the beginning of the prodromal phase of MS. Nor could they determine whether presymptomatic serum NfL levels varied with age at clinical onset, sex, or race. The small number of women in the sample was another limitation of the study.
The Swiss National Research Foundation and the National Institute of Neurologic Disorders and Stroke funded the study. Several of the investigators received fees from various drug companies that were unrelated to the study, and one researcher received grants from the National Institutes of Health during the study.
SOURCE: Bjornevik K et al. JAMA Neurol. 2020;77(1):58-64.
(MS), according to research published in the January issue of JAMA Neurology. These results lend weight to the idea that MS has a prodromal phase, and this phase appears to be associated with neurodegeneration, according to the authors.
Patients often have CNS lesions of various stages of development at the time of their first demyelinating event, and this finding was one basis for neurologists’ hypothesis of a prodromal phase of MS. The finding that one-third of patients with radiologically isolated syndrome develop MS within 5 years also lends credence to this idea. Diagnosing MS early would enable early treatment that could prevent demyelination and the progression of neurodegeneration.
Researchers compared presymptomatic and symptomatic samples
With this idea in mind, Kjetil Bjornevik, MD, PhD, a member of the neuroepidemiology research group at Harvard TH Chan School of Public Health in Boston, and colleagues evaluated whether serum levels of NfL, a marker of ongoing neuroaxonal degeneration, were increased in the years before and around the time of clinical onset of MS. For their study population, the investigators chose active-duty U.S. military personnel who have at least one serum sample stored in the U.S. Department of Defense Serum Repository. Samples are collected after routine HIV type 1 antibody testing.
Within this population, Dr. Bjornevik and colleagues identified patients with MS who had at least one presymptomatic serum sample. The date of clinical MS onset was defined as the date of the first neurologic symptoms attributable to MS documented in the medical record. The investigators randomly selected two control individuals from the population and matched them to each case by age, sex, race or ethnicity, and dates of sample collection. Eligible controls were on active duty on the date of onset of the matched case.
Dr. Bjornevik and colleagues identified 245 patients with MS. Among this sample, the researchers selected two groups that each included 30 cases and 30 controls. The first group included patients who had provided at least one serum sample before MS onset and one sample within 2 years after MS onset. The second group included cases with at least two presymptomatic serum samples, one of which was collected more than 5 years before MS diagnosis, and the other of which was collected between 2 and 5 years before diagnosis. The investigators handled pairs of serum samples in the same way and assayed them in the same batch. The order of the samples in each pair was arranged at random.
Levels were higher in cases than in controls
About 77% of the population was male. Sixty percent of participants were white, 28% were black, and 6.7% were Hispanic. The population’s mean age at first sample collection was approximately 27 years. Mean age at MS onset was approximately 31 years.
For patients who provided samples before and after the clinical onset of MS, serum NfL levels were higher than in matched controls at both points. Most patients who passed from the presymptomatic stage to the symptomatic stage had a significant increase in serum NfL level (i.e., from a median of 25.0 pg/mL to a median of 45.1 pg/mL). Serum NfL levels at the two time points in controls did not differ significantly. For any given patient, an increase in serum NfL level from the presymptomatic measurement to the symptomatic measurement was associated with an increased risk of MS.
In patients with two presymptomatic samples, serum NfL levels were significantly higher in both samples than in the corresponding samples from matched controls. In cases, the earlier sample was collected at a median of 6 years before clinical onset of MS, and the later sample was collected at a median of 1 year before clinical onset. The serum NfL levels increased significantly between the two points for cases (i.e., a median increase of 1.3 pg/mL per year), but there was no significant difference in serum NfL level between the two samples in controls. A within-patient increase in presymptomatic serum NfL level was associated with an increased risk of MS.
Population included few women
“Our study differs from previous studies on the prodromal phase of MS because these have used indirect markers of this phase, which included unspecific symptoms or disturbances occurring before the clinical onset, compared with a marker of neurodegeneration,” wrote Dr. Bjornevik and colleagues. Initiation of treatment with disease-modifying therapy is associated with reductions in serum NfL levels, and this association could explain why some patients in the current study had higher NfL levels before MS onset than afterward. Furthermore, serum NfL levels are highly associated with levels of NfL in cerebrospinal fluid. “Thus, our findings of a presymptomatic increase in serum NfL not only suggest the presence of a prodromal phase in MS, but also that this phase is associated with neurodegeneration,” wrote the investigators.
The study’s well-defined population helped to minimize selection bias, and the blinded, randomized method of analyzing the serum samples eliminated artifactual differences in serum NfL concentrations. But the small sample size precluded analyses that could have influenced clinical practice, wrote Dr. Bjornevik and colleagues. For example, the researchers could not evaluate distinct cutoffs in serum NfL level that could mark the beginning of the prodromal phase of MS. Nor could they determine whether presymptomatic serum NfL levels varied with age at clinical onset, sex, or race. The small number of women in the sample was another limitation of the study.
The Swiss National Research Foundation and the National Institute of Neurologic Disorders and Stroke funded the study. Several of the investigators received fees from various drug companies that were unrelated to the study, and one researcher received grants from the National Institutes of Health during the study.
SOURCE: Bjornevik K et al. JAMA Neurol. 2020;77(1):58-64.
(MS), according to research published in the January issue of JAMA Neurology. These results lend weight to the idea that MS has a prodromal phase, and this phase appears to be associated with neurodegeneration, according to the authors.
Patients often have CNS lesions of various stages of development at the time of their first demyelinating event, and this finding was one basis for neurologists’ hypothesis of a prodromal phase of MS. The finding that one-third of patients with radiologically isolated syndrome develop MS within 5 years also lends credence to this idea. Diagnosing MS early would enable early treatment that could prevent demyelination and the progression of neurodegeneration.
Researchers compared presymptomatic and symptomatic samples
With this idea in mind, Kjetil Bjornevik, MD, PhD, a member of the neuroepidemiology research group at Harvard TH Chan School of Public Health in Boston, and colleagues evaluated whether serum levels of NfL, a marker of ongoing neuroaxonal degeneration, were increased in the years before and around the time of clinical onset of MS. For their study population, the investigators chose active-duty U.S. military personnel who have at least one serum sample stored in the U.S. Department of Defense Serum Repository. Samples are collected after routine HIV type 1 antibody testing.
Within this population, Dr. Bjornevik and colleagues identified patients with MS who had at least one presymptomatic serum sample. The date of clinical MS onset was defined as the date of the first neurologic symptoms attributable to MS documented in the medical record. The investigators randomly selected two control individuals from the population and matched them to each case by age, sex, race or ethnicity, and dates of sample collection. Eligible controls were on active duty on the date of onset of the matched case.
Dr. Bjornevik and colleagues identified 245 patients with MS. Among this sample, the researchers selected two groups that each included 30 cases and 30 controls. The first group included patients who had provided at least one serum sample before MS onset and one sample within 2 years after MS onset. The second group included cases with at least two presymptomatic serum samples, one of which was collected more than 5 years before MS diagnosis, and the other of which was collected between 2 and 5 years before diagnosis. The investigators handled pairs of serum samples in the same way and assayed them in the same batch. The order of the samples in each pair was arranged at random.
Levels were higher in cases than in controls
About 77% of the population was male. Sixty percent of participants were white, 28% were black, and 6.7% were Hispanic. The population’s mean age at first sample collection was approximately 27 years. Mean age at MS onset was approximately 31 years.
For patients who provided samples before and after the clinical onset of MS, serum NfL levels were higher than in matched controls at both points. Most patients who passed from the presymptomatic stage to the symptomatic stage had a significant increase in serum NfL level (i.e., from a median of 25.0 pg/mL to a median of 45.1 pg/mL). Serum NfL levels at the two time points in controls did not differ significantly. For any given patient, an increase in serum NfL level from the presymptomatic measurement to the symptomatic measurement was associated with an increased risk of MS.
In patients with two presymptomatic samples, serum NfL levels were significantly higher in both samples than in the corresponding samples from matched controls. In cases, the earlier sample was collected at a median of 6 years before clinical onset of MS, and the later sample was collected at a median of 1 year before clinical onset. The serum NfL levels increased significantly between the two points for cases (i.e., a median increase of 1.3 pg/mL per year), but there was no significant difference in serum NfL level between the two samples in controls. A within-patient increase in presymptomatic serum NfL level was associated with an increased risk of MS.
Population included few women
“Our study differs from previous studies on the prodromal phase of MS because these have used indirect markers of this phase, which included unspecific symptoms or disturbances occurring before the clinical onset, compared with a marker of neurodegeneration,” wrote Dr. Bjornevik and colleagues. Initiation of treatment with disease-modifying therapy is associated with reductions in serum NfL levels, and this association could explain why some patients in the current study had higher NfL levels before MS onset than afterward. Furthermore, serum NfL levels are highly associated with levels of NfL in cerebrospinal fluid. “Thus, our findings of a presymptomatic increase in serum NfL not only suggest the presence of a prodromal phase in MS, but also that this phase is associated with neurodegeneration,” wrote the investigators.
The study’s well-defined population helped to minimize selection bias, and the blinded, randomized method of analyzing the serum samples eliminated artifactual differences in serum NfL concentrations. But the small sample size precluded analyses that could have influenced clinical practice, wrote Dr. Bjornevik and colleagues. For example, the researchers could not evaluate distinct cutoffs in serum NfL level that could mark the beginning of the prodromal phase of MS. Nor could they determine whether presymptomatic serum NfL levels varied with age at clinical onset, sex, or race. The small number of women in the sample was another limitation of the study.
The Swiss National Research Foundation and the National Institute of Neurologic Disorders and Stroke funded the study. Several of the investigators received fees from various drug companies that were unrelated to the study, and one researcher received grants from the National Institutes of Health during the study.
SOURCE: Bjornevik K et al. JAMA Neurol. 2020;77(1):58-64.
FROM JAMA NEUROLOGY
New tools could help predict complication risks in lung and breast cancer
In this edition of “How I Will Treat My Next Patient,” I highlight the potential role of new models for predicting risks of common, clinically important situations in general oncology practice: severe neutropenia in lung cancer patients and locoregional recurrence of breast cancer.
Predicting neutropenia
Accurate, lung cancer–specific prediction models would be useful to estimate risk of chemotherapy-induced neutropenia (CIN), especially febrile neutropenia (FN), since that particular toxicity is linked to infection, dose delays and dose reductions that can compromise treatment efficacy, and poor health-related quality of life. Lung cancer patients are often older adults, with advanced disease and comorbid conditions, so they are a particularly vulnerable population for CIN.
Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators published a model for predicting risk of severe CIN in advanced lung cancer patients, based on 10 pretreatment variables (Lung Cancer. 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004). They developed their model to overcome limitations of the previously published work of Gary H. Lyman, MD, and colleagues that is not specific to lung cancer and incorporated relative dose intensity as a predictor (Cancer. 2011;117:1917-27). Relative dose intensity is not determined until after a treatment course is completed.
The new prediction model was based on a lung cancer data set encompassing 11,352 patients from 67 phase 2-3 cooperative group studies conducted between 1991 and 2010. In this data set, the Lyman model had an area under the curve of 0.8772 in patients with small cell lung cancer, but an area under the curve of just 0.6787 in non–small cell lung cancer.
The derivation model was derived from about two-thirds of the patients, randomly selected. The validation set was conducted using the remaining third. The variables included were readily clinically available: age, gender, weight, body mass index, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status. Their model had an area under the curve of 0.8348 in the training set and 0.8234 in the testing set.
How these results influence practice
The risk of an initial episode of FN is highest during a patient’s initial cycle of chemotherapy, when most patients are receiving full-dose treatment, often without prophylactic measures. Guidelines from the National Comprehensive Cancer Network suggest the use of prophylactic growth factors in patients with more than a 20% risk of FN, and considering using prophylaxis in patients with 10%-20% risk of FN. Underestimating those risks and failure to take adequate precautions may be particularly important for patients with lung cancer who are generally older adults, with comorbid conditions.
The comprehensive risk model for neutropenic complications that was developed by Dr. Lyman and colleagues was based on a large, prospective cohort including nearly 3,800 patients. The model had a 90% sensitivity and 96% predictive value, but was not lung cancer specific and, in this latest study, did not perform as well in the 85% of lung cancer patients with non–small cell lung cancer. The Lyman data, however, was obtained in cancer patients treated with investigator-choice chemotherapy in community practices. It remains the National Comprehensive Cancer Network standard for evaluating FN risk in patients embarking on chemotherapy for advanced malignancies. That should remain the case, pending the additional validation testing of the new lung cancer–specific model at independent institutions, treating heterogeneous patients in real-world settings.
Locoregional recurrence
A retrospective cohort analysis of SWOG 8814, a phase 3 study of tamoxifen alone versus chemotherapy plus by tamoxifen in postmenopausal, node-positive, hormone receptor–positive breast cancer patients suggests that the 21-gene assay recurrence score (RS) can aid decisions about radiotherapy (RT).
Wendy A. Woodward, MD, PhD, and colleagues, analyzed patients who underwent mastectomy or breast-conserving surgery as their local therapy (JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559). They found that patients with an intermediate or high RS – according to the 21-gene assay OncotypeDX – had more locoregional recurrences (LRR; breast, chest wall, axilla, internal mammary, supraclavicular or infraclavicular nodes).
There were 367 patients in SWOG 8814 who received tamoxifen alone or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen. LRR was observed in 5.8% of patients with a low RS (less than 18) and in 13.8% of patients with an intermediate or high RS (more than 18). The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
In the subset of patients with one to three positive nodes who had mastectomy without radiotherapy, the LRR was 1.5% for those with low RS and 11.1% for those with intermediate or high RS (P = .051). No difference by RS was found in the 10-year rates of LRR among patients with four or more involved nodes who received a mastectomy without RT (25.9% vs. 27.0%; P = .27).
In multivariate analysis, incorporating RS, type of surgery, and number of involved nodes, intermediate or high RS was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). The investigators suggested that RS, when available, should be one of the factors considered in selecting patients for postmastectomy RT.
How these results influence practice
Selecting the node-positive, hormone receptor–positive, breast cancer patients who should receive postmastectomy RT is difficult and controversial. This is particularly true for those postmenopausal patients with fewer than four involved nodes, no lymphatic or vascular invasion, and no extracapsular spread of disease into the axillary fat. Limited information exists on the ability of genomic assays to identify LRR risk.
Eleftherios P. Mamounas, MD, and colleagues examined the results of NSABP B-28, a trial of chemotherapy plus tamoxifen (J Natl Cancer Inst. 2017;109[4]. doi:10.1093/jnci/djw259). Postmastectomy RT was not permitted. They found high RS correlated with greater LRR and low RS with decreased LRR among patients with one to three positive nodes. At first blush, the prospectively treated cohort of SWOG 8814 represents a uniformly treated cohort with long-term follow-up (median, 8.5 years) and extends in an independent analysis the findings of NSABP B-28.
However, as Dr. Woodward and colleagues point out, the current study has limitations. The use of RT was extracted retrospectively and may be underreported. More modern chemotherapy and RT may lower LRR from the risks observed in SWOG 8814. Finally, the modest numbers of LRR events precluded secondary analysis of RS as a continuous variable. This is important because the risk group cutoffs suggested by the authors are not aligned with those in the recently published TailorRx study or the ongoing RxPonder trial.
The TailorRT (Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer) study examines the safety of omitting RT among patients with low RS and one to three positive nodes. Until the TailorRT results are reported, the controversy regarding the role of postmastectomy RT in this group will continue for patients with low nodal tumor burden and less aggressive tumor features, including low RS.
An observed LRR risk of 11.1% in SWOG 8814 among patients with N1 disease and an RS above 18 suggest that genomic risk could be one of the factors that may justify postmastectomy RT in postmenopausal patients with node-positive, hormone receptor–positive breast cancer until additional data emerge from the contemporary trials.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight the potential role of new models for predicting risks of common, clinically important situations in general oncology practice: severe neutropenia in lung cancer patients and locoregional recurrence of breast cancer.
Predicting neutropenia
Accurate, lung cancer–specific prediction models would be useful to estimate risk of chemotherapy-induced neutropenia (CIN), especially febrile neutropenia (FN), since that particular toxicity is linked to infection, dose delays and dose reductions that can compromise treatment efficacy, and poor health-related quality of life. Lung cancer patients are often older adults, with advanced disease and comorbid conditions, so they are a particularly vulnerable population for CIN.
Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators published a model for predicting risk of severe CIN in advanced lung cancer patients, based on 10 pretreatment variables (Lung Cancer. 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004). They developed their model to overcome limitations of the previously published work of Gary H. Lyman, MD, and colleagues that is not specific to lung cancer and incorporated relative dose intensity as a predictor (Cancer. 2011;117:1917-27). Relative dose intensity is not determined until after a treatment course is completed.
The new prediction model was based on a lung cancer data set encompassing 11,352 patients from 67 phase 2-3 cooperative group studies conducted between 1991 and 2010. In this data set, the Lyman model had an area under the curve of 0.8772 in patients with small cell lung cancer, but an area under the curve of just 0.6787 in non–small cell lung cancer.
The derivation model was derived from about two-thirds of the patients, randomly selected. The validation set was conducted using the remaining third. The variables included were readily clinically available: age, gender, weight, body mass index, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status. Their model had an area under the curve of 0.8348 in the training set and 0.8234 in the testing set.
How these results influence practice
The risk of an initial episode of FN is highest during a patient’s initial cycle of chemotherapy, when most patients are receiving full-dose treatment, often without prophylactic measures. Guidelines from the National Comprehensive Cancer Network suggest the use of prophylactic growth factors in patients with more than a 20% risk of FN, and considering using prophylaxis in patients with 10%-20% risk of FN. Underestimating those risks and failure to take adequate precautions may be particularly important for patients with lung cancer who are generally older adults, with comorbid conditions.
The comprehensive risk model for neutropenic complications that was developed by Dr. Lyman and colleagues was based on a large, prospective cohort including nearly 3,800 patients. The model had a 90% sensitivity and 96% predictive value, but was not lung cancer specific and, in this latest study, did not perform as well in the 85% of lung cancer patients with non–small cell lung cancer. The Lyman data, however, was obtained in cancer patients treated with investigator-choice chemotherapy in community practices. It remains the National Comprehensive Cancer Network standard for evaluating FN risk in patients embarking on chemotherapy for advanced malignancies. That should remain the case, pending the additional validation testing of the new lung cancer–specific model at independent institutions, treating heterogeneous patients in real-world settings.
Locoregional recurrence
A retrospective cohort analysis of SWOG 8814, a phase 3 study of tamoxifen alone versus chemotherapy plus by tamoxifen in postmenopausal, node-positive, hormone receptor–positive breast cancer patients suggests that the 21-gene assay recurrence score (RS) can aid decisions about radiotherapy (RT).
Wendy A. Woodward, MD, PhD, and colleagues, analyzed patients who underwent mastectomy or breast-conserving surgery as their local therapy (JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559). They found that patients with an intermediate or high RS – according to the 21-gene assay OncotypeDX – had more locoregional recurrences (LRR; breast, chest wall, axilla, internal mammary, supraclavicular or infraclavicular nodes).
There were 367 patients in SWOG 8814 who received tamoxifen alone or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen. LRR was observed in 5.8% of patients with a low RS (less than 18) and in 13.8% of patients with an intermediate or high RS (more than 18). The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
In the subset of patients with one to three positive nodes who had mastectomy without radiotherapy, the LRR was 1.5% for those with low RS and 11.1% for those with intermediate or high RS (P = .051). No difference by RS was found in the 10-year rates of LRR among patients with four or more involved nodes who received a mastectomy without RT (25.9% vs. 27.0%; P = .27).
In multivariate analysis, incorporating RS, type of surgery, and number of involved nodes, intermediate or high RS was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). The investigators suggested that RS, when available, should be one of the factors considered in selecting patients for postmastectomy RT.
How these results influence practice
Selecting the node-positive, hormone receptor–positive, breast cancer patients who should receive postmastectomy RT is difficult and controversial. This is particularly true for those postmenopausal patients with fewer than four involved nodes, no lymphatic or vascular invasion, and no extracapsular spread of disease into the axillary fat. Limited information exists on the ability of genomic assays to identify LRR risk.
Eleftherios P. Mamounas, MD, and colleagues examined the results of NSABP B-28, a trial of chemotherapy plus tamoxifen (J Natl Cancer Inst. 2017;109[4]. doi:10.1093/jnci/djw259). Postmastectomy RT was not permitted. They found high RS correlated with greater LRR and low RS with decreased LRR among patients with one to three positive nodes. At first blush, the prospectively treated cohort of SWOG 8814 represents a uniformly treated cohort with long-term follow-up (median, 8.5 years) and extends in an independent analysis the findings of NSABP B-28.
However, as Dr. Woodward and colleagues point out, the current study has limitations. The use of RT was extracted retrospectively and may be underreported. More modern chemotherapy and RT may lower LRR from the risks observed in SWOG 8814. Finally, the modest numbers of LRR events precluded secondary analysis of RS as a continuous variable. This is important because the risk group cutoffs suggested by the authors are not aligned with those in the recently published TailorRx study or the ongoing RxPonder trial.
The TailorRT (Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer) study examines the safety of omitting RT among patients with low RS and one to three positive nodes. Until the TailorRT results are reported, the controversy regarding the role of postmastectomy RT in this group will continue for patients with low nodal tumor burden and less aggressive tumor features, including low RS.
An observed LRR risk of 11.1% in SWOG 8814 among patients with N1 disease and an RS above 18 suggest that genomic risk could be one of the factors that may justify postmastectomy RT in postmenopausal patients with node-positive, hormone receptor–positive breast cancer until additional data emerge from the contemporary trials.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight the potential role of new models for predicting risks of common, clinically important situations in general oncology practice: severe neutropenia in lung cancer patients and locoregional recurrence of breast cancer.
Predicting neutropenia
Accurate, lung cancer–specific prediction models would be useful to estimate risk of chemotherapy-induced neutropenia (CIN), especially febrile neutropenia (FN), since that particular toxicity is linked to infection, dose delays and dose reductions that can compromise treatment efficacy, and poor health-related quality of life. Lung cancer patients are often older adults, with advanced disease and comorbid conditions, so they are a particularly vulnerable population for CIN.
Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators published a model for predicting risk of severe CIN in advanced lung cancer patients, based on 10 pretreatment variables (Lung Cancer. 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004). They developed their model to overcome limitations of the previously published work of Gary H. Lyman, MD, and colleagues that is not specific to lung cancer and incorporated relative dose intensity as a predictor (Cancer. 2011;117:1917-27). Relative dose intensity is not determined until after a treatment course is completed.
The new prediction model was based on a lung cancer data set encompassing 11,352 patients from 67 phase 2-3 cooperative group studies conducted between 1991 and 2010. In this data set, the Lyman model had an area under the curve of 0.8772 in patients with small cell lung cancer, but an area under the curve of just 0.6787 in non–small cell lung cancer.
The derivation model was derived from about two-thirds of the patients, randomly selected. The validation set was conducted using the remaining third. The variables included were readily clinically available: age, gender, weight, body mass index, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status. Their model had an area under the curve of 0.8348 in the training set and 0.8234 in the testing set.
How these results influence practice
The risk of an initial episode of FN is highest during a patient’s initial cycle of chemotherapy, when most patients are receiving full-dose treatment, often without prophylactic measures. Guidelines from the National Comprehensive Cancer Network suggest the use of prophylactic growth factors in patients with more than a 20% risk of FN, and considering using prophylaxis in patients with 10%-20% risk of FN. Underestimating those risks and failure to take adequate precautions may be particularly important for patients with lung cancer who are generally older adults, with comorbid conditions.
The comprehensive risk model for neutropenic complications that was developed by Dr. Lyman and colleagues was based on a large, prospective cohort including nearly 3,800 patients. The model had a 90% sensitivity and 96% predictive value, but was not lung cancer specific and, in this latest study, did not perform as well in the 85% of lung cancer patients with non–small cell lung cancer. The Lyman data, however, was obtained in cancer patients treated with investigator-choice chemotherapy in community practices. It remains the National Comprehensive Cancer Network standard for evaluating FN risk in patients embarking on chemotherapy for advanced malignancies. That should remain the case, pending the additional validation testing of the new lung cancer–specific model at independent institutions, treating heterogeneous patients in real-world settings.
Locoregional recurrence
A retrospective cohort analysis of SWOG 8814, a phase 3 study of tamoxifen alone versus chemotherapy plus by tamoxifen in postmenopausal, node-positive, hormone receptor–positive breast cancer patients suggests that the 21-gene assay recurrence score (RS) can aid decisions about radiotherapy (RT).
Wendy A. Woodward, MD, PhD, and colleagues, analyzed patients who underwent mastectomy or breast-conserving surgery as their local therapy (JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559). They found that patients with an intermediate or high RS – according to the 21-gene assay OncotypeDX – had more locoregional recurrences (LRR; breast, chest wall, axilla, internal mammary, supraclavicular or infraclavicular nodes).
There were 367 patients in SWOG 8814 who received tamoxifen alone or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen. LRR was observed in 5.8% of patients with a low RS (less than 18) and in 13.8% of patients with an intermediate or high RS (more than 18). The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
In the subset of patients with one to three positive nodes who had mastectomy without radiotherapy, the LRR was 1.5% for those with low RS and 11.1% for those with intermediate or high RS (P = .051). No difference by RS was found in the 10-year rates of LRR among patients with four or more involved nodes who received a mastectomy without RT (25.9% vs. 27.0%; P = .27).
In multivariate analysis, incorporating RS, type of surgery, and number of involved nodes, intermediate or high RS was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). The investigators suggested that RS, when available, should be one of the factors considered in selecting patients for postmastectomy RT.
How these results influence practice
Selecting the node-positive, hormone receptor–positive, breast cancer patients who should receive postmastectomy RT is difficult and controversial. This is particularly true for those postmenopausal patients with fewer than four involved nodes, no lymphatic or vascular invasion, and no extracapsular spread of disease into the axillary fat. Limited information exists on the ability of genomic assays to identify LRR risk.
Eleftherios P. Mamounas, MD, and colleagues examined the results of NSABP B-28, a trial of chemotherapy plus tamoxifen (J Natl Cancer Inst. 2017;109[4]. doi:10.1093/jnci/djw259). Postmastectomy RT was not permitted. They found high RS correlated with greater LRR and low RS with decreased LRR among patients with one to three positive nodes. At first blush, the prospectively treated cohort of SWOG 8814 represents a uniformly treated cohort with long-term follow-up (median, 8.5 years) and extends in an independent analysis the findings of NSABP B-28.
However, as Dr. Woodward and colleagues point out, the current study has limitations. The use of RT was extracted retrospectively and may be underreported. More modern chemotherapy and RT may lower LRR from the risks observed in SWOG 8814. Finally, the modest numbers of LRR events precluded secondary analysis of RS as a continuous variable. This is important because the risk group cutoffs suggested by the authors are not aligned with those in the recently published TailorRx study or the ongoing RxPonder trial.
The TailorRT (Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer) study examines the safety of omitting RT among patients with low RS and one to three positive nodes. Until the TailorRT results are reported, the controversy regarding the role of postmastectomy RT in this group will continue for patients with low nodal tumor burden and less aggressive tumor features, including low RS.
An observed LRR risk of 11.1% in SWOG 8814 among patients with N1 disease and an RS above 18 suggest that genomic risk could be one of the factors that may justify postmastectomy RT in postmenopausal patients with node-positive, hormone receptor–positive breast cancer until additional data emerge from the contemporary trials.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Should supplemental MRI be used in otherwise average-risk women with extremely dense breasts?
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3
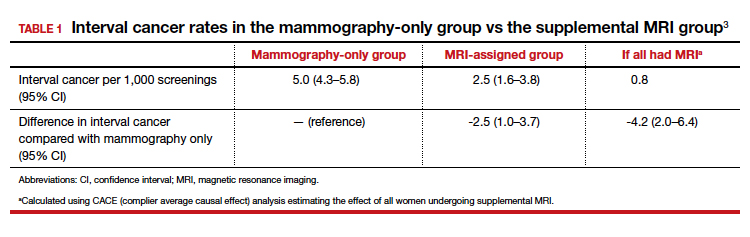
Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.
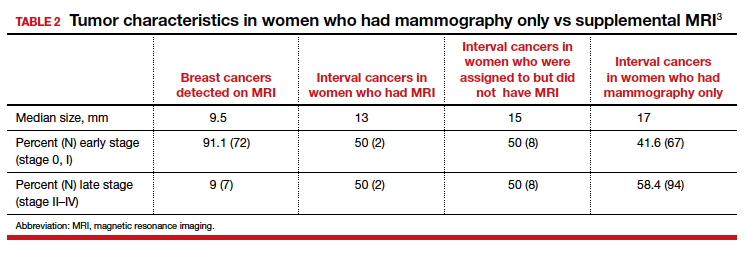
Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3

Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.

Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3

Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.

Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
Global project reveals cancer’s genomic playbook
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE
Rate of suicide is higher in people with neurologic disorders
The absolute risk difference is small, but statistically significant. “These findings do not necessarily warrant changing the management of treatment for individual patients,” wrote Annette Erlangsen, PhD, a researcher at the Danish Research Institute for Suicide Prevention in Hellerup, and colleagues. “As with all patients, physicians should be aware of the potential for depression, demoralization, and suicide.”
In addition, dementia, Alzheimer’s disease, and intellectual disabilities may be associated with lower suicide rates, according to the study, which was published in JAMA.
“Plausible mechanisms” could underlie the association between neurologic disease and suicide, the authors wrote. A neurologic diagnosis “may constitute a distressing life event,” and the diseases may have psychological, physical, and psychiatric effects. Patients may see themselves as a burden or have less financial security. In addition, the diseases may entail “communication difficulties, poor sleep, and pain.” Neurologic diseases may alter brain circuitry and functioning and influence aggression and impulsivity. “People with neurologic disorders may also have easier access to toxic medication,” they added.
More than a dozen conditions examined
Prior studies have found associations between neurologic conditions and rates of suicide, but data have been inconclusive or inconsistent for some of the disorders. To examine whether people with neurologic disorders have higher suicide rates, relative to people without these disorders, the researchers conducted a retrospective study. They analyzed data from more than 7.3 million people aged 15 years or older who lived in Denmark between 1980 and 2016. The cohort included more than 1.2 million people with neurologic disorders. The investigators identified neurologic disorders using ICD codes for head injury, stroke, epilepsy, polyneuropathy, diseases of the myoneural junction, Parkinson’s disease, multiple sclerosis, CNS infections, meningitis, encephalitis, amyotrophic lateral sclerosis, Huntington’s disease, dementia, intellectual disability, and other brain disorders. They compared incidence rates using a Poisson regression model and adjusted for time period, sex, age, region, socioeconomic status, comorbidity, self-harm or psychiatric hospitalization prior to a neurologic diagnosis, and whether a person lived alone.
In all, 35,483 people in the cohort died by suicide at an average age of about 52 years; 77.4% were male. About 15% of those who died by suicide had a neurologic disorder. The suicide incidence rate among people with a neurologic disorder was 44.0 per 100,000 person-years, whereas the rate among people without a neurologic disorder was 20.1 per 100,000 person-years.
The adjusted incidence rate ratio for people with a neurologic disorder was 1.8. The rate ratio was highest during the 3 months after diagnosis, at 3.1. Huntington’s disease and amyotrophic lateral sclerosis were associated with “the largest excess adjusted [incidence rate ratios] of suicide mortality,” with a rate ratio of 4.9 for each condition, the researchers reported. The adjusted incidence rate ratio was 1.7 for head injury, 1.3 for stroke, 1.7 for epilepsy, 1.4 for intracerebral hemorrhage, 1.3 for cerebral infarction, 1.3 for subarachnoid hemorrhage, 1.7 for polyneuropathy and peripheral neuropathy, 2.2 for Guillain-Barré syndrome, 1.9 for diseases of myoneural junction and muscle, 1.8 for other brain disorders, 1.7 for Parkinson’s disease, 2.2 for multiple sclerosis, and 1.6 for CNS infection.
Compared with people without a neurologic condition, people with dementia, Alzheimer’s disease, and intellectual disabilities had lower suicide rates, with adjusted incidence rate ratios of 0.8, 0.2, and 0.6, respectively. “However, the adjusted [incidence rate ratio] for people with dementia during the first month after diagnosis was 3.0,” the researchers wrote.
In addition, the suicide rate increased with an increasing cumulative number of hospital contacts for neurologic conditions.
Overall incidence rates declined
“Over the study period, the suicide incidence rate for people with neurological disorders decreased from 78.6 per 100,000 person-years during the 1980-1999 years to 27.3 per 100,000 person-years during the 2000-2016 years,” wrote Dr. Erlangsen and colleagues. “The suicide incidence rate for those without a disorder decreased from 26.3 to 12.7 during the same time spans. ... The decline in the overall suicide rate over time did not affect the relative risk pattern.”
The decline in the general suicide rate in Denmark “has largely been attributed to means restriction, such as efforts to limit availability of firearms and particularly toxic medication,” the authors added.
In those time spans, the adjusted incidence rate ratio for suicide among those with dementia decreased from 2.4 to 1.0, and among those with multiple sclerosis from 2.0 to 1.0. “It is possible that the improvements observed for dementia and multiple sclerosis may be related to improvements in treatment and intensified community-based support,” Dr. Erlangsen and coauthors wrote.
When the researchers used people with rheumatoid arthritis as a reference group, those with a neurologic disorder had a higher suicide rate per 100,000 person-years, 30.2 versus 18.4. The adjusted incidence rate ratio for that comparison was 1.4.
In patients with Huntington’s disease, depression mediated by hyperactivity in the hypothalamic-pituitary-adrenal axis may contribute to the risk of suicide. “Witnessing the course of the disease in one’s parent” also may contribute the risk, the researchers wrote.
The analysis may have missed people with neurologic disorders diagnosed before 1977 if they did not have subsequent contact with a hospital, the investigators noted. In addition, diagnoses given in primary care were not included, suicide deaths may be underrecorded, and “adjusting for preexisting mental disorders could be viewed as overadjusting,” they wrote.
The study was supported by a grant from the Psychiatric Research Foundation in Denmark. The authors reported that they had no disclosures.
SOURCE: Erlangsen A et al. JAMA. 2020 Feb 4. doi: 10.1001/jama.2019.21834.
The absolute risk difference is small, but statistically significant. “These findings do not necessarily warrant changing the management of treatment for individual patients,” wrote Annette Erlangsen, PhD, a researcher at the Danish Research Institute for Suicide Prevention in Hellerup, and colleagues. “As with all patients, physicians should be aware of the potential for depression, demoralization, and suicide.”
In addition, dementia, Alzheimer’s disease, and intellectual disabilities may be associated with lower suicide rates, according to the study, which was published in JAMA.
“Plausible mechanisms” could underlie the association between neurologic disease and suicide, the authors wrote. A neurologic diagnosis “may constitute a distressing life event,” and the diseases may have psychological, physical, and psychiatric effects. Patients may see themselves as a burden or have less financial security. In addition, the diseases may entail “communication difficulties, poor sleep, and pain.” Neurologic diseases may alter brain circuitry and functioning and influence aggression and impulsivity. “People with neurologic disorders may also have easier access to toxic medication,” they added.
More than a dozen conditions examined
Prior studies have found associations between neurologic conditions and rates of suicide, but data have been inconclusive or inconsistent for some of the disorders. To examine whether people with neurologic disorders have higher suicide rates, relative to people without these disorders, the researchers conducted a retrospective study. They analyzed data from more than 7.3 million people aged 15 years or older who lived in Denmark between 1980 and 2016. The cohort included more than 1.2 million people with neurologic disorders. The investigators identified neurologic disorders using ICD codes for head injury, stroke, epilepsy, polyneuropathy, diseases of the myoneural junction, Parkinson’s disease, multiple sclerosis, CNS infections, meningitis, encephalitis, amyotrophic lateral sclerosis, Huntington’s disease, dementia, intellectual disability, and other brain disorders. They compared incidence rates using a Poisson regression model and adjusted for time period, sex, age, region, socioeconomic status, comorbidity, self-harm or psychiatric hospitalization prior to a neurologic diagnosis, and whether a person lived alone.
In all, 35,483 people in the cohort died by suicide at an average age of about 52 years; 77.4% were male. About 15% of those who died by suicide had a neurologic disorder. The suicide incidence rate among people with a neurologic disorder was 44.0 per 100,000 person-years, whereas the rate among people without a neurologic disorder was 20.1 per 100,000 person-years.
The adjusted incidence rate ratio for people with a neurologic disorder was 1.8. The rate ratio was highest during the 3 months after diagnosis, at 3.1. Huntington’s disease and amyotrophic lateral sclerosis were associated with “the largest excess adjusted [incidence rate ratios] of suicide mortality,” with a rate ratio of 4.9 for each condition, the researchers reported. The adjusted incidence rate ratio was 1.7 for head injury, 1.3 for stroke, 1.7 for epilepsy, 1.4 for intracerebral hemorrhage, 1.3 for cerebral infarction, 1.3 for subarachnoid hemorrhage, 1.7 for polyneuropathy and peripheral neuropathy, 2.2 for Guillain-Barré syndrome, 1.9 for diseases of myoneural junction and muscle, 1.8 for other brain disorders, 1.7 for Parkinson’s disease, 2.2 for multiple sclerosis, and 1.6 for CNS infection.
Compared with people without a neurologic condition, people with dementia, Alzheimer’s disease, and intellectual disabilities had lower suicide rates, with adjusted incidence rate ratios of 0.8, 0.2, and 0.6, respectively. “However, the adjusted [incidence rate ratio] for people with dementia during the first month after diagnosis was 3.0,” the researchers wrote.
In addition, the suicide rate increased with an increasing cumulative number of hospital contacts for neurologic conditions.
Overall incidence rates declined
“Over the study period, the suicide incidence rate for people with neurological disorders decreased from 78.6 per 100,000 person-years during the 1980-1999 years to 27.3 per 100,000 person-years during the 2000-2016 years,” wrote Dr. Erlangsen and colleagues. “The suicide incidence rate for those without a disorder decreased from 26.3 to 12.7 during the same time spans. ... The decline in the overall suicide rate over time did not affect the relative risk pattern.”
The decline in the general suicide rate in Denmark “has largely been attributed to means restriction, such as efforts to limit availability of firearms and particularly toxic medication,” the authors added.
In those time spans, the adjusted incidence rate ratio for suicide among those with dementia decreased from 2.4 to 1.0, and among those with multiple sclerosis from 2.0 to 1.0. “It is possible that the improvements observed for dementia and multiple sclerosis may be related to improvements in treatment and intensified community-based support,” Dr. Erlangsen and coauthors wrote.
When the researchers used people with rheumatoid arthritis as a reference group, those with a neurologic disorder had a higher suicide rate per 100,000 person-years, 30.2 versus 18.4. The adjusted incidence rate ratio for that comparison was 1.4.
In patients with Huntington’s disease, depression mediated by hyperactivity in the hypothalamic-pituitary-adrenal axis may contribute to the risk of suicide. “Witnessing the course of the disease in one’s parent” also may contribute the risk, the researchers wrote.
The analysis may have missed people with neurologic disorders diagnosed before 1977 if they did not have subsequent contact with a hospital, the investigators noted. In addition, diagnoses given in primary care were not included, suicide deaths may be underrecorded, and “adjusting for preexisting mental disorders could be viewed as overadjusting,” they wrote.
The study was supported by a grant from the Psychiatric Research Foundation in Denmark. The authors reported that they had no disclosures.
SOURCE: Erlangsen A et al. JAMA. 2020 Feb 4. doi: 10.1001/jama.2019.21834.
The absolute risk difference is small, but statistically significant. “These findings do not necessarily warrant changing the management of treatment for individual patients,” wrote Annette Erlangsen, PhD, a researcher at the Danish Research Institute for Suicide Prevention in Hellerup, and colleagues. “As with all patients, physicians should be aware of the potential for depression, demoralization, and suicide.”
In addition, dementia, Alzheimer’s disease, and intellectual disabilities may be associated with lower suicide rates, according to the study, which was published in JAMA.
“Plausible mechanisms” could underlie the association between neurologic disease and suicide, the authors wrote. A neurologic diagnosis “may constitute a distressing life event,” and the diseases may have psychological, physical, and psychiatric effects. Patients may see themselves as a burden or have less financial security. In addition, the diseases may entail “communication difficulties, poor sleep, and pain.” Neurologic diseases may alter brain circuitry and functioning and influence aggression and impulsivity. “People with neurologic disorders may also have easier access to toxic medication,” they added.
More than a dozen conditions examined
Prior studies have found associations between neurologic conditions and rates of suicide, but data have been inconclusive or inconsistent for some of the disorders. To examine whether people with neurologic disorders have higher suicide rates, relative to people without these disorders, the researchers conducted a retrospective study. They analyzed data from more than 7.3 million people aged 15 years or older who lived in Denmark between 1980 and 2016. The cohort included more than 1.2 million people with neurologic disorders. The investigators identified neurologic disorders using ICD codes for head injury, stroke, epilepsy, polyneuropathy, diseases of the myoneural junction, Parkinson’s disease, multiple sclerosis, CNS infections, meningitis, encephalitis, amyotrophic lateral sclerosis, Huntington’s disease, dementia, intellectual disability, and other brain disorders. They compared incidence rates using a Poisson regression model and adjusted for time period, sex, age, region, socioeconomic status, comorbidity, self-harm or psychiatric hospitalization prior to a neurologic diagnosis, and whether a person lived alone.
In all, 35,483 people in the cohort died by suicide at an average age of about 52 years; 77.4% were male. About 15% of those who died by suicide had a neurologic disorder. The suicide incidence rate among people with a neurologic disorder was 44.0 per 100,000 person-years, whereas the rate among people without a neurologic disorder was 20.1 per 100,000 person-years.
The adjusted incidence rate ratio for people with a neurologic disorder was 1.8. The rate ratio was highest during the 3 months after diagnosis, at 3.1. Huntington’s disease and amyotrophic lateral sclerosis were associated with “the largest excess adjusted [incidence rate ratios] of suicide mortality,” with a rate ratio of 4.9 for each condition, the researchers reported. The adjusted incidence rate ratio was 1.7 for head injury, 1.3 for stroke, 1.7 for epilepsy, 1.4 for intracerebral hemorrhage, 1.3 for cerebral infarction, 1.3 for subarachnoid hemorrhage, 1.7 for polyneuropathy and peripheral neuropathy, 2.2 for Guillain-Barré syndrome, 1.9 for diseases of myoneural junction and muscle, 1.8 for other brain disorders, 1.7 for Parkinson’s disease, 2.2 for multiple sclerosis, and 1.6 for CNS infection.
Compared with people without a neurologic condition, people with dementia, Alzheimer’s disease, and intellectual disabilities had lower suicide rates, with adjusted incidence rate ratios of 0.8, 0.2, and 0.6, respectively. “However, the adjusted [incidence rate ratio] for people with dementia during the first month after diagnosis was 3.0,” the researchers wrote.
In addition, the suicide rate increased with an increasing cumulative number of hospital contacts for neurologic conditions.
Overall incidence rates declined
“Over the study period, the suicide incidence rate for people with neurological disorders decreased from 78.6 per 100,000 person-years during the 1980-1999 years to 27.3 per 100,000 person-years during the 2000-2016 years,” wrote Dr. Erlangsen and colleagues. “The suicide incidence rate for those without a disorder decreased from 26.3 to 12.7 during the same time spans. ... The decline in the overall suicide rate over time did not affect the relative risk pattern.”
The decline in the general suicide rate in Denmark “has largely been attributed to means restriction, such as efforts to limit availability of firearms and particularly toxic medication,” the authors added.
In those time spans, the adjusted incidence rate ratio for suicide among those with dementia decreased from 2.4 to 1.0, and among those with multiple sclerosis from 2.0 to 1.0. “It is possible that the improvements observed for dementia and multiple sclerosis may be related to improvements in treatment and intensified community-based support,” Dr. Erlangsen and coauthors wrote.
When the researchers used people with rheumatoid arthritis as a reference group, those with a neurologic disorder had a higher suicide rate per 100,000 person-years, 30.2 versus 18.4. The adjusted incidence rate ratio for that comparison was 1.4.
In patients with Huntington’s disease, depression mediated by hyperactivity in the hypothalamic-pituitary-adrenal axis may contribute to the risk of suicide. “Witnessing the course of the disease in one’s parent” also may contribute the risk, the researchers wrote.
The analysis may have missed people with neurologic disorders diagnosed before 1977 if they did not have subsequent contact with a hospital, the investigators noted. In addition, diagnoses given in primary care were not included, suicide deaths may be underrecorded, and “adjusting for preexisting mental disorders could be viewed as overadjusting,” they wrote.
The study was supported by a grant from the Psychiatric Research Foundation in Denmark. The authors reported that they had no disclosures.
SOURCE: Erlangsen A et al. JAMA. 2020 Feb 4. doi: 10.1001/jama.2019.21834.
FROM JAMA
Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate (FULL)
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29