User login
Atrial Fibrillation and Bleeding in Patients With Chronic Lymphocytic Leukemia Treated with Ibrutinib in the Veterans Health Administration (FULL)
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
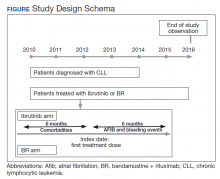
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
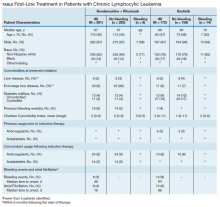
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
Radiotherapeutic Care of Patients With Stage IV Lung Cancer with Thoracic Symptoms in the Veterans Health Administration (FULL)
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Basal ganglia microcircuits offer clues to Parkinson’s symptoms
, according to a new study using a mouse model of disease.
Parkinson’s disease is characterized by a range of cognitive and motor symptoms, which appear at different disease stages. While recent research has pointed to specific neuronal subpopulations, or microcircuits, operating in the basal ganglia, researchers lacked a clear understanding of how they might correspond with specific symptom domains.
In a study published online March 15 in Nature Neuroscience, lead author Varoth Lilascharoen, PhD, of the University of California, San Diego, and colleagues reported that two different neuronal subpopulations within the external globus pallidus, an important nucleus within the basal ganglia, are associated, respectively, with movement and with reversal learning (having to adapt to a reward pattern that is the reverse of a previous pattern). This is the first time, the investigators said, that the contributions of specific microcircuits in the basal ganglia have been linked to different behaviors.
Using electrophysiology, viral tracing, and other approaches, Dr. Lilascharoen and colleagues demonstrated that two microcircuits or populations of parvalbumin-expressing neurons could be manipulated to exacerbate or alleviate the motor or cognitive deficits in the dopamine-depleted mice.
One of these microcircuits, made up of substantia nigra pars reticulata-projecting GPe-PV neurons, could be manipulated in ways that promoted or inhibited the mice’s movement. The other, which comprises parafascicular thalamus-projecting GPe-PV neurons, could be manipulated to affect reversal learning, the researchers found. Activation or inhibition of either circuit was not seen affecting function in the other.
The results shed light on the functional organization of the different basal ganglia nuclei at the circuit level, and suggest, the authors argued, that differences in how different neuronal subpopulations adapt to dopamine loss could explain some of the patterns of progression seen in Parkinson’s disease.
The findings “establish the differential contributions from two distinct GPe-PV microcircuits in specific Parkinsonian-like behaviors linked to early and late stages of the disease,” Dr. Lilascharoen and colleagues wrote in their analysis. “[F]urther elucidation of the detailed connectivity of GPe subpopulations to their downstream targets … is needed to fully define the function of each microcircuit and design better therapeutic strategies for the various behavioral impairments of Parkinson’s disease.”
Commenting on the research, Stefan Lang, MD, PhD, of the University of Calgary in Alberta said, “While Parkinson’s disease is often referred to as a movement disorder, it is well known that nonmotor symptoms, including cognitive and behavioral impairment, are common and debilitating. Impairment of basal ganglia function is known to contribute to these different symptom domains, though the specific circuits have never been elucidated. [Dr.] Lilascharoen et al. tease apart specific basal ganglia circuits associated with motor and behavioral symptoms, thereby providing evidence that distinct microcircuits might contribute to unique behaviours. As technological advances in neuromodulatory therapies continue to improve the spatial and temporal resolution of stimulation, future treatments may allow for specific targeting of behavioral impairment symptoms in Parkinson’s disease.”
Dr. Lilascharoen and Dr. Lang did not report outside funding or conflicts of interest.
, according to a new study using a mouse model of disease.
Parkinson’s disease is characterized by a range of cognitive and motor symptoms, which appear at different disease stages. While recent research has pointed to specific neuronal subpopulations, or microcircuits, operating in the basal ganglia, researchers lacked a clear understanding of how they might correspond with specific symptom domains.
In a study published online March 15 in Nature Neuroscience, lead author Varoth Lilascharoen, PhD, of the University of California, San Diego, and colleagues reported that two different neuronal subpopulations within the external globus pallidus, an important nucleus within the basal ganglia, are associated, respectively, with movement and with reversal learning (having to adapt to a reward pattern that is the reverse of a previous pattern). This is the first time, the investigators said, that the contributions of specific microcircuits in the basal ganglia have been linked to different behaviors.
Using electrophysiology, viral tracing, and other approaches, Dr. Lilascharoen and colleagues demonstrated that two microcircuits or populations of parvalbumin-expressing neurons could be manipulated to exacerbate or alleviate the motor or cognitive deficits in the dopamine-depleted mice.
One of these microcircuits, made up of substantia nigra pars reticulata-projecting GPe-PV neurons, could be manipulated in ways that promoted or inhibited the mice’s movement. The other, which comprises parafascicular thalamus-projecting GPe-PV neurons, could be manipulated to affect reversal learning, the researchers found. Activation or inhibition of either circuit was not seen affecting function in the other.
The results shed light on the functional organization of the different basal ganglia nuclei at the circuit level, and suggest, the authors argued, that differences in how different neuronal subpopulations adapt to dopamine loss could explain some of the patterns of progression seen in Parkinson’s disease.
The findings “establish the differential contributions from two distinct GPe-PV microcircuits in specific Parkinsonian-like behaviors linked to early and late stages of the disease,” Dr. Lilascharoen and colleagues wrote in their analysis. “[F]urther elucidation of the detailed connectivity of GPe subpopulations to their downstream targets … is needed to fully define the function of each microcircuit and design better therapeutic strategies for the various behavioral impairments of Parkinson’s disease.”
Commenting on the research, Stefan Lang, MD, PhD, of the University of Calgary in Alberta said, “While Parkinson’s disease is often referred to as a movement disorder, it is well known that nonmotor symptoms, including cognitive and behavioral impairment, are common and debilitating. Impairment of basal ganglia function is known to contribute to these different symptom domains, though the specific circuits have never been elucidated. [Dr.] Lilascharoen et al. tease apart specific basal ganglia circuits associated with motor and behavioral symptoms, thereby providing evidence that distinct microcircuits might contribute to unique behaviours. As technological advances in neuromodulatory therapies continue to improve the spatial and temporal resolution of stimulation, future treatments may allow for specific targeting of behavioral impairment symptoms in Parkinson’s disease.”
Dr. Lilascharoen and Dr. Lang did not report outside funding or conflicts of interest.
, according to a new study using a mouse model of disease.
Parkinson’s disease is characterized by a range of cognitive and motor symptoms, which appear at different disease stages. While recent research has pointed to specific neuronal subpopulations, or microcircuits, operating in the basal ganglia, researchers lacked a clear understanding of how they might correspond with specific symptom domains.
In a study published online March 15 in Nature Neuroscience, lead author Varoth Lilascharoen, PhD, of the University of California, San Diego, and colleagues reported that two different neuronal subpopulations within the external globus pallidus, an important nucleus within the basal ganglia, are associated, respectively, with movement and with reversal learning (having to adapt to a reward pattern that is the reverse of a previous pattern). This is the first time, the investigators said, that the contributions of specific microcircuits in the basal ganglia have been linked to different behaviors.
Using electrophysiology, viral tracing, and other approaches, Dr. Lilascharoen and colleagues demonstrated that two microcircuits or populations of parvalbumin-expressing neurons could be manipulated to exacerbate or alleviate the motor or cognitive deficits in the dopamine-depleted mice.
One of these microcircuits, made up of substantia nigra pars reticulata-projecting GPe-PV neurons, could be manipulated in ways that promoted or inhibited the mice’s movement. The other, which comprises parafascicular thalamus-projecting GPe-PV neurons, could be manipulated to affect reversal learning, the researchers found. Activation or inhibition of either circuit was not seen affecting function in the other.
The results shed light on the functional organization of the different basal ganglia nuclei at the circuit level, and suggest, the authors argued, that differences in how different neuronal subpopulations adapt to dopamine loss could explain some of the patterns of progression seen in Parkinson’s disease.
The findings “establish the differential contributions from two distinct GPe-PV microcircuits in specific Parkinsonian-like behaviors linked to early and late stages of the disease,” Dr. Lilascharoen and colleagues wrote in their analysis. “[F]urther elucidation of the detailed connectivity of GPe subpopulations to their downstream targets … is needed to fully define the function of each microcircuit and design better therapeutic strategies for the various behavioral impairments of Parkinson’s disease.”
Commenting on the research, Stefan Lang, MD, PhD, of the University of Calgary in Alberta said, “While Parkinson’s disease is often referred to as a movement disorder, it is well known that nonmotor symptoms, including cognitive and behavioral impairment, are common and debilitating. Impairment of basal ganglia function is known to contribute to these different symptom domains, though the specific circuits have never been elucidated. [Dr.] Lilascharoen et al. tease apart specific basal ganglia circuits associated with motor and behavioral symptoms, thereby providing evidence that distinct microcircuits might contribute to unique behaviours. As technological advances in neuromodulatory therapies continue to improve the spatial and temporal resolution of stimulation, future treatments may allow for specific targeting of behavioral impairment symptoms in Parkinson’s disease.”
Dr. Lilascharoen and Dr. Lang did not report outside funding or conflicts of interest.
FROM NATURE NEUROSCIENCE
FDA approves loncastuximab for diffuse large B-cell lymphomas
The Food and Drug Administration granted an accelerated approval April 24, 2021, for a new drug for use in patients with relapsed/refractory diffuse large B-cell lymphomas (DLBCL) who have tried at least two prior systemic therapies.
The new product, loncastuximab tesirine-lpyl (Zynlonta, ADC Therapeutics), is the first and only CD19-targeted antibody-drug conjugate approved for this disease.
DLBCL is the most common type of non-Hodgkin lymphoma in the United States, but the indication also includes DLBCL not otherwise specified, DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma.
“There is a significant unmet need for treatment options for patients with [relapsed or refractory] DLBCL, including those who have been heavily pretreated and have difficult-to-treat disease,” Paolo F. Caimi, MD, University Hospitals Cleveland Medical Center and Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, said in a company press release.
The company also cited data from previous clinical trials showing that more than 40% of first-line DLBCL treatments fail, and that these patients have a poor prognosis, worsening with each line of therapy that is tried.
Accelerated approval based on ORR
The accelerated approval was based on overall response rate data from the single-arm LOTIS-2 trial. All patients received the new drug, administered as a 30-minute infusion once every 3 weeks for 1 year.
The trial was conducted in 145 patients with relapsed/refractory DLBCL who had already tried at least two lines of systemic therapy. Dr. Caimi noted that this included patients who had been heavily pretreated, as the population included patients who previously received stem cell transplant or chimeric antigen receptor T-cell therapy.
The ORR was 48.3% (70/145 patients), which included a complete response rate of 24.1% (35/145 patients) and a partial response rate of 24.1% (35/145 patients).
Patients had a median time to response of 1.3 months and the median duration of response for the 70 responders was 10.3 months.
“Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial,” the company noted.
A phase 3 confirmatory is underway: the LOTIS 5 trial (NCT04384484) compares the combination of loncastuximab tesirine and rituximab versus chemoimmunotherapy in patients with relapsed/refractory DLBCL.
The company also noted that in a pooled safety population the most common adverse reactions (≥20%) were thrombocytopenia, an increase in levels of the liver enzyme gamma-glutamyltransferase, neutropenia, anemia, hyperglycemia, transaminase elevation, fatigue, hypoalbuminemia, rash, edema, nausea, and musculoskeletal pain.
In the LOTIS-2 trial, the most common (≥10%) grade 3 or higher treatment-emergent adverse events were neutropenia (26.2%), thrombocytopenia (17.9%), GGT increase (17.2%) and anemia (10.3%).
Permanent treatment discontinuation as the result of an adverse reaction occurred in 19% of patients, and these included a GGT increase, edema, and effusion.
Dose reductions because of an adverse reaction occurred in 8% of patients, and most were the result of a GGT increase. Dosage interruptions because of an adverse reaction occurred in 49% of patients, and these included a GGT increase, neutropenia, thrombocytopenia, and edema.
Warnings on effusions, infections, and skin reactions
The product carries a warning that serious effusion and edema has been reported. Grade 3 edema occurred in 3% (primarily peripheral edema or ascites), grade 3 pleural effusion occurred in 3%, and grade 3 or 4 pericardial effusion occurred in 1%.
Prescribers are recommended to monitor patients for new or worsening edema or effusions, and to consider diagnostic imaging in patients who develop symptoms of pleural effusion or pericardial effusion, such as new or worsened dyspnea, chest pain, and/or ascites such as swelling in the abdomen and bloating.
The product also carries a warning about fatal and serious infections, including opportunistic infections, and serious cutaneous reactions, including photosensitivity reaction, rash (including exfoliative and maculopapular), and erythema.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration granted an accelerated approval April 24, 2021, for a new drug for use in patients with relapsed/refractory diffuse large B-cell lymphomas (DLBCL) who have tried at least two prior systemic therapies.
The new product, loncastuximab tesirine-lpyl (Zynlonta, ADC Therapeutics), is the first and only CD19-targeted antibody-drug conjugate approved for this disease.
DLBCL is the most common type of non-Hodgkin lymphoma in the United States, but the indication also includes DLBCL not otherwise specified, DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma.
“There is a significant unmet need for treatment options for patients with [relapsed or refractory] DLBCL, including those who have been heavily pretreated and have difficult-to-treat disease,” Paolo F. Caimi, MD, University Hospitals Cleveland Medical Center and Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, said in a company press release.
The company also cited data from previous clinical trials showing that more than 40% of first-line DLBCL treatments fail, and that these patients have a poor prognosis, worsening with each line of therapy that is tried.
Accelerated approval based on ORR
The accelerated approval was based on overall response rate data from the single-arm LOTIS-2 trial. All patients received the new drug, administered as a 30-minute infusion once every 3 weeks for 1 year.
The trial was conducted in 145 patients with relapsed/refractory DLBCL who had already tried at least two lines of systemic therapy. Dr. Caimi noted that this included patients who had been heavily pretreated, as the population included patients who previously received stem cell transplant or chimeric antigen receptor T-cell therapy.
The ORR was 48.3% (70/145 patients), which included a complete response rate of 24.1% (35/145 patients) and a partial response rate of 24.1% (35/145 patients).
Patients had a median time to response of 1.3 months and the median duration of response for the 70 responders was 10.3 months.
“Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial,” the company noted.
A phase 3 confirmatory is underway: the LOTIS 5 trial (NCT04384484) compares the combination of loncastuximab tesirine and rituximab versus chemoimmunotherapy in patients with relapsed/refractory DLBCL.
The company also noted that in a pooled safety population the most common adverse reactions (≥20%) were thrombocytopenia, an increase in levels of the liver enzyme gamma-glutamyltransferase, neutropenia, anemia, hyperglycemia, transaminase elevation, fatigue, hypoalbuminemia, rash, edema, nausea, and musculoskeletal pain.
In the LOTIS-2 trial, the most common (≥10%) grade 3 or higher treatment-emergent adverse events were neutropenia (26.2%), thrombocytopenia (17.9%), GGT increase (17.2%) and anemia (10.3%).
Permanent treatment discontinuation as the result of an adverse reaction occurred in 19% of patients, and these included a GGT increase, edema, and effusion.
Dose reductions because of an adverse reaction occurred in 8% of patients, and most were the result of a GGT increase. Dosage interruptions because of an adverse reaction occurred in 49% of patients, and these included a GGT increase, neutropenia, thrombocytopenia, and edema.
Warnings on effusions, infections, and skin reactions
The product carries a warning that serious effusion and edema has been reported. Grade 3 edema occurred in 3% (primarily peripheral edema or ascites), grade 3 pleural effusion occurred in 3%, and grade 3 or 4 pericardial effusion occurred in 1%.
Prescribers are recommended to monitor patients for new or worsening edema or effusions, and to consider diagnostic imaging in patients who develop symptoms of pleural effusion or pericardial effusion, such as new or worsened dyspnea, chest pain, and/or ascites such as swelling in the abdomen and bloating.
The product also carries a warning about fatal and serious infections, including opportunistic infections, and serious cutaneous reactions, including photosensitivity reaction, rash (including exfoliative and maculopapular), and erythema.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration granted an accelerated approval April 24, 2021, for a new drug for use in patients with relapsed/refractory diffuse large B-cell lymphomas (DLBCL) who have tried at least two prior systemic therapies.
The new product, loncastuximab tesirine-lpyl (Zynlonta, ADC Therapeutics), is the first and only CD19-targeted antibody-drug conjugate approved for this disease.
DLBCL is the most common type of non-Hodgkin lymphoma in the United States, but the indication also includes DLBCL not otherwise specified, DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma.
“There is a significant unmet need for treatment options for patients with [relapsed or refractory] DLBCL, including those who have been heavily pretreated and have difficult-to-treat disease,” Paolo F. Caimi, MD, University Hospitals Cleveland Medical Center and Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, said in a company press release.
The company also cited data from previous clinical trials showing that more than 40% of first-line DLBCL treatments fail, and that these patients have a poor prognosis, worsening with each line of therapy that is tried.
Accelerated approval based on ORR
The accelerated approval was based on overall response rate data from the single-arm LOTIS-2 trial. All patients received the new drug, administered as a 30-minute infusion once every 3 weeks for 1 year.
The trial was conducted in 145 patients with relapsed/refractory DLBCL who had already tried at least two lines of systemic therapy. Dr. Caimi noted that this included patients who had been heavily pretreated, as the population included patients who previously received stem cell transplant or chimeric antigen receptor T-cell therapy.
The ORR was 48.3% (70/145 patients), which included a complete response rate of 24.1% (35/145 patients) and a partial response rate of 24.1% (35/145 patients).
Patients had a median time to response of 1.3 months and the median duration of response for the 70 responders was 10.3 months.
“Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial,” the company noted.
A phase 3 confirmatory is underway: the LOTIS 5 trial (NCT04384484) compares the combination of loncastuximab tesirine and rituximab versus chemoimmunotherapy in patients with relapsed/refractory DLBCL.
The company also noted that in a pooled safety population the most common adverse reactions (≥20%) were thrombocytopenia, an increase in levels of the liver enzyme gamma-glutamyltransferase, neutropenia, anemia, hyperglycemia, transaminase elevation, fatigue, hypoalbuminemia, rash, edema, nausea, and musculoskeletal pain.
In the LOTIS-2 trial, the most common (≥10%) grade 3 or higher treatment-emergent adverse events were neutropenia (26.2%), thrombocytopenia (17.9%), GGT increase (17.2%) and anemia (10.3%).
Permanent treatment discontinuation as the result of an adverse reaction occurred in 19% of patients, and these included a GGT increase, edema, and effusion.
Dose reductions because of an adverse reaction occurred in 8% of patients, and most were the result of a GGT increase. Dosage interruptions because of an adverse reaction occurred in 49% of patients, and these included a GGT increase, neutropenia, thrombocytopenia, and edema.
Warnings on effusions, infections, and skin reactions
The product carries a warning that serious effusion and edema has been reported. Grade 3 edema occurred in 3% (primarily peripheral edema or ascites), grade 3 pleural effusion occurred in 3%, and grade 3 or 4 pericardial effusion occurred in 1%.
Prescribers are recommended to monitor patients for new or worsening edema or effusions, and to consider diagnostic imaging in patients who develop symptoms of pleural effusion or pericardial effusion, such as new or worsened dyspnea, chest pain, and/or ascites such as swelling in the abdomen and bloating.
The product also carries a warning about fatal and serious infections, including opportunistic infections, and serious cutaneous reactions, including photosensitivity reaction, rash (including exfoliative and maculopapular), and erythema.
A version of this article first appeared on Medscape.com.
GENUINE improvements: Ublituximab plus ibrutinib for CLL
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
CLL patients: Diagnostic difficulties, treatment confusion with COVID-19
Chronic lymphocytic leukemia (CLL) patients present significant problems with regard to COVID-19 disease, according to a literature review by Yousef Roosta, MD, of Urmia (Iran) University of Medical Sciences, and colleagues.
Diagnostic interaction between CLL and COVID-19 provides a major challenge. CLL patients have a lower rate of anti–SARS-CoV-2 IgG development, and evidence shows worse therapeutic outcomes in these patients, according to study published in Leukemia Research Reports.
The researchers assessed 20 retrieved articles, 11 of which examined patients with CLL and with concomitant COVID-19; and 9 articles were designed as prospective or retrospective case series of such patients. The studies were assessed qualitatively by the QUADAS-2 tool.
Troubling results
Although the overall prevalence of CLL and COVID-19 concurrence was low, at 0.6% (95% confidence interval 0.5%-0.7%) according to the meta-analysis, the results showed some special challenges in the diagnosis and care of these patients.
Diagnostic difficulties are a unique problem. Lymphopenia is common in patients with COVID-19, while lymphocytosis may be considered a transient or even rare finding. The interplay between the two diseases is sometimes very misleading for specialists, and in patients with lymphocytosis, the diagnosis of CLL may be completely ignored, according to the researchers. They added that when performing a diagnostic approach for concurrent COVID-19 and CLL, due to differences in the amount and type of immune response, “relying on serological testing, and especially the evaluation of the anti–SARS-CoV-2 IgG levels may not be beneficial,” they indicated.
In addition, studies showed unacceptable therapeutic outcome in patients with concurrent CLL and COVID-19, with mortality ranging from 33% to 41.7%, showing a need to revise current treatment protocols, according to the authors. In one study, 85.7% of surviving patients showed a considerable decrease in functional class and significant fatigue, with such a poor prognosis occurring more commonly in the elderly.
With regard to treatment, “it is quite obvious that despite the use of current standard protocols, the prognosis of these patients will be much worse than the prognosis of CLL patients with no evidence of COVID-19. Even in the first-line treatment protocol for these patients, there is no agreement in combination therapy with selected CLL drugs along with management protocols of COVID-19 patients,” the researchers stated.
“[The] different hematological behaviors of two diseases might mimic the detection of COVID-19 in the CLL state and vise versa. Also, due to the low level of immune response against SARS-CoV-2 in CLL patients, both scheduled immunological-based diagnosis and treatment may fail,” the researchers added.
The authors reported that they had no disclosures.
Chronic lymphocytic leukemia (CLL) patients present significant problems with regard to COVID-19 disease, according to a literature review by Yousef Roosta, MD, of Urmia (Iran) University of Medical Sciences, and colleagues.
Diagnostic interaction between CLL and COVID-19 provides a major challenge. CLL patients have a lower rate of anti–SARS-CoV-2 IgG development, and evidence shows worse therapeutic outcomes in these patients, according to study published in Leukemia Research Reports.
The researchers assessed 20 retrieved articles, 11 of which examined patients with CLL and with concomitant COVID-19; and 9 articles were designed as prospective or retrospective case series of such patients. The studies were assessed qualitatively by the QUADAS-2 tool.
Troubling results
Although the overall prevalence of CLL and COVID-19 concurrence was low, at 0.6% (95% confidence interval 0.5%-0.7%) according to the meta-analysis, the results showed some special challenges in the diagnosis and care of these patients.
Diagnostic difficulties are a unique problem. Lymphopenia is common in patients with COVID-19, while lymphocytosis may be considered a transient or even rare finding. The interplay between the two diseases is sometimes very misleading for specialists, and in patients with lymphocytosis, the diagnosis of CLL may be completely ignored, according to the researchers. They added that when performing a diagnostic approach for concurrent COVID-19 and CLL, due to differences in the amount and type of immune response, “relying on serological testing, and especially the evaluation of the anti–SARS-CoV-2 IgG levels may not be beneficial,” they indicated.
In addition, studies showed unacceptable therapeutic outcome in patients with concurrent CLL and COVID-19, with mortality ranging from 33% to 41.7%, showing a need to revise current treatment protocols, according to the authors. In one study, 85.7% of surviving patients showed a considerable decrease in functional class and significant fatigue, with such a poor prognosis occurring more commonly in the elderly.
With regard to treatment, “it is quite obvious that despite the use of current standard protocols, the prognosis of these patients will be much worse than the prognosis of CLL patients with no evidence of COVID-19. Even in the first-line treatment protocol for these patients, there is no agreement in combination therapy with selected CLL drugs along with management protocols of COVID-19 patients,” the researchers stated.
“[The] different hematological behaviors of two diseases might mimic the detection of COVID-19 in the CLL state and vise versa. Also, due to the low level of immune response against SARS-CoV-2 in CLL patients, both scheduled immunological-based diagnosis and treatment may fail,” the researchers added.
The authors reported that they had no disclosures.
Chronic lymphocytic leukemia (CLL) patients present significant problems with regard to COVID-19 disease, according to a literature review by Yousef Roosta, MD, of Urmia (Iran) University of Medical Sciences, and colleagues.
Diagnostic interaction between CLL and COVID-19 provides a major challenge. CLL patients have a lower rate of anti–SARS-CoV-2 IgG development, and evidence shows worse therapeutic outcomes in these patients, according to study published in Leukemia Research Reports.
The researchers assessed 20 retrieved articles, 11 of which examined patients with CLL and with concomitant COVID-19; and 9 articles were designed as prospective or retrospective case series of such patients. The studies were assessed qualitatively by the QUADAS-2 tool.
Troubling results
Although the overall prevalence of CLL and COVID-19 concurrence was low, at 0.6% (95% confidence interval 0.5%-0.7%) according to the meta-analysis, the results showed some special challenges in the diagnosis and care of these patients.
Diagnostic difficulties are a unique problem. Lymphopenia is common in patients with COVID-19, while lymphocytosis may be considered a transient or even rare finding. The interplay between the two diseases is sometimes very misleading for specialists, and in patients with lymphocytosis, the diagnosis of CLL may be completely ignored, according to the researchers. They added that when performing a diagnostic approach for concurrent COVID-19 and CLL, due to differences in the amount and type of immune response, “relying on serological testing, and especially the evaluation of the anti–SARS-CoV-2 IgG levels may not be beneficial,” they indicated.
In addition, studies showed unacceptable therapeutic outcome in patients with concurrent CLL and COVID-19, with mortality ranging from 33% to 41.7%, showing a need to revise current treatment protocols, according to the authors. In one study, 85.7% of surviving patients showed a considerable decrease in functional class and significant fatigue, with such a poor prognosis occurring more commonly in the elderly.
With regard to treatment, “it is quite obvious that despite the use of current standard protocols, the prognosis of these patients will be much worse than the prognosis of CLL patients with no evidence of COVID-19. Even in the first-line treatment protocol for these patients, there is no agreement in combination therapy with selected CLL drugs along with management protocols of COVID-19 patients,” the researchers stated.
“[The] different hematological behaviors of two diseases might mimic the detection of COVID-19 in the CLL state and vise versa. Also, due to the low level of immune response against SARS-CoV-2 in CLL patients, both scheduled immunological-based diagnosis and treatment may fail,” the researchers added.
The authors reported that they had no disclosures.
FROM LEUKEMIA RESEARCH REPORTS
ZUMA-2, TRANSCEND data pique interest in earlier CAR T for R/R MCL
The “remarkable” efficacy of chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory mantle cell lymphoma as observed in recent trials supports its evaluation earlier in the course of treatment, according to Roch Houot, MD, PhD.
Patients with relapsed or refractory mantle cell lymphoma (MCL) who progress after treatment with a Bruton’s tyrosine kinase inhibitor (BTKi) have poor clinical outcomes, Dr. Houot, professor of hematology at Rennes (France) University Hospital, explained at the 3rd European CAR T-cell meeting.
Objective response rates in patients who relapse after BTKi therapy range from 25% to 42%, and median overall survival (OS) is less than 10 months with standard therapies, he said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
The recent ZUMA-2 and TRANSCEND NHL 001 trials evaluating the CD19 CAR T-cell products brexucabtagene autoleucel (brexu-cel; Tecartus) and lisocabtagene maraleucel (liso-cel; Breyanzi), respectively, in patients with relapsed or refractory MCL after BTKi therapy, showed dramatically improved outcomes, compared with outcomes seen previously with standard salvage therapies.
The ORR in 68 patients treated with brexu-cel in ZUMA-2 was 92%, including complete response (CR) in 40 patients (67%) and partial response (PR) in 15 patients (25%) with the rare, aggressive subtype of B-cell lymphoma.
“Interestingly, among patients who achieved a CR, 70% remained in remission after a median follow-up of 17.5 months,” he said.
Median duration of response, progression-free survival, and overall survival were not reached at that time, and ongoing responses were consistent across prognostic subgroups, he added.
The ZUMA-2 findings led to accelerated approval of brexu-cel by the Food and Drug Administration in July 2020, as well as priority medicine designation by the European Medicines Agency in December 2020, for the treatment of MCL after two or more prior lines of systemic therapy including a BTKi.
The TRANSCEND study also included patients with MCL who were relapsed or refractory after two or more lines of therapy.
The ORR was 84% in 32 patients who completed treatment – including CRs in 66% and PRs in 19%. An additional 3% had stable disease and 9% of patients progressed, Dr. Houot said.
“The follow-up of the TRANSCEND study is still very short – the median is 5.9 months – so we don’t have survival data yet for these patients,” he noted.
Still, the efficacy in these studies is excellent, particularly considering the challenges of treating MCL patients who relapse or are refractory after BTKi treatment, he said, noting that most patients in both studies had poor prognostic factors.
Toxicities in both studies were similar to those seen in studies of patients with aggressive B-cell lymphomas who were treated with these drugs, he added.
“Longer follow-up is needed to better evaluate long-term efficacy,” he said, concluding that the results nonetheless “support evaluation of CAR T-cell therapy earlier in the therapeutic strategy of mantle cell lymphoma.”
Dr. Houot reported having no disclosures.
The “remarkable” efficacy of chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory mantle cell lymphoma as observed in recent trials supports its evaluation earlier in the course of treatment, according to Roch Houot, MD, PhD.
Patients with relapsed or refractory mantle cell lymphoma (MCL) who progress after treatment with a Bruton’s tyrosine kinase inhibitor (BTKi) have poor clinical outcomes, Dr. Houot, professor of hematology at Rennes (France) University Hospital, explained at the 3rd European CAR T-cell meeting.
Objective response rates in patients who relapse after BTKi therapy range from 25% to 42%, and median overall survival (OS) is less than 10 months with standard therapies, he said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
The recent ZUMA-2 and TRANSCEND NHL 001 trials evaluating the CD19 CAR T-cell products brexucabtagene autoleucel (brexu-cel; Tecartus) and lisocabtagene maraleucel (liso-cel; Breyanzi), respectively, in patients with relapsed or refractory MCL after BTKi therapy, showed dramatically improved outcomes, compared with outcomes seen previously with standard salvage therapies.
The ORR in 68 patients treated with brexu-cel in ZUMA-2 was 92%, including complete response (CR) in 40 patients (67%) and partial response (PR) in 15 patients (25%) with the rare, aggressive subtype of B-cell lymphoma.
“Interestingly, among patients who achieved a CR, 70% remained in remission after a median follow-up of 17.5 months,” he said.
Median duration of response, progression-free survival, and overall survival were not reached at that time, and ongoing responses were consistent across prognostic subgroups, he added.
The ZUMA-2 findings led to accelerated approval of brexu-cel by the Food and Drug Administration in July 2020, as well as priority medicine designation by the European Medicines Agency in December 2020, for the treatment of MCL after two or more prior lines of systemic therapy including a BTKi.
The TRANSCEND study also included patients with MCL who were relapsed or refractory after two or more lines of therapy.
The ORR was 84% in 32 patients who completed treatment – including CRs in 66% and PRs in 19%. An additional 3% had stable disease and 9% of patients progressed, Dr. Houot said.
“The follow-up of the TRANSCEND study is still very short – the median is 5.9 months – so we don’t have survival data yet for these patients,” he noted.
Still, the efficacy in these studies is excellent, particularly considering the challenges of treating MCL patients who relapse or are refractory after BTKi treatment, he said, noting that most patients in both studies had poor prognostic factors.
Toxicities in both studies were similar to those seen in studies of patients with aggressive B-cell lymphomas who were treated with these drugs, he added.
“Longer follow-up is needed to better evaluate long-term efficacy,” he said, concluding that the results nonetheless “support evaluation of CAR T-cell therapy earlier in the therapeutic strategy of mantle cell lymphoma.”
Dr. Houot reported having no disclosures.
The “remarkable” efficacy of chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory mantle cell lymphoma as observed in recent trials supports its evaluation earlier in the course of treatment, according to Roch Houot, MD, PhD.
Patients with relapsed or refractory mantle cell lymphoma (MCL) who progress after treatment with a Bruton’s tyrosine kinase inhibitor (BTKi) have poor clinical outcomes, Dr. Houot, professor of hematology at Rennes (France) University Hospital, explained at the 3rd European CAR T-cell meeting.
Objective response rates in patients who relapse after BTKi therapy range from 25% to 42%, and median overall survival (OS) is less than 10 months with standard therapies, he said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
The recent ZUMA-2 and TRANSCEND NHL 001 trials evaluating the CD19 CAR T-cell products brexucabtagene autoleucel (brexu-cel; Tecartus) and lisocabtagene maraleucel (liso-cel; Breyanzi), respectively, in patients with relapsed or refractory MCL after BTKi therapy, showed dramatically improved outcomes, compared with outcomes seen previously with standard salvage therapies.
The ORR in 68 patients treated with brexu-cel in ZUMA-2 was 92%, including complete response (CR) in 40 patients (67%) and partial response (PR) in 15 patients (25%) with the rare, aggressive subtype of B-cell lymphoma.
“Interestingly, among patients who achieved a CR, 70% remained in remission after a median follow-up of 17.5 months,” he said.
Median duration of response, progression-free survival, and overall survival were not reached at that time, and ongoing responses were consistent across prognostic subgroups, he added.
The ZUMA-2 findings led to accelerated approval of brexu-cel by the Food and Drug Administration in July 2020, as well as priority medicine designation by the European Medicines Agency in December 2020, for the treatment of MCL after two or more prior lines of systemic therapy including a BTKi.
The TRANSCEND study also included patients with MCL who were relapsed or refractory after two or more lines of therapy.
The ORR was 84% in 32 patients who completed treatment – including CRs in 66% and PRs in 19%. An additional 3% had stable disease and 9% of patients progressed, Dr. Houot said.
“The follow-up of the TRANSCEND study is still very short – the median is 5.9 months – so we don’t have survival data yet for these patients,” he noted.
Still, the efficacy in these studies is excellent, particularly considering the challenges of treating MCL patients who relapse or are refractory after BTKi treatment, he said, noting that most patients in both studies had poor prognostic factors.
Toxicities in both studies were similar to those seen in studies of patients with aggressive B-cell lymphomas who were treated with these drugs, he added.
“Longer follow-up is needed to better evaluate long-term efficacy,” he said, concluding that the results nonetheless “support evaluation of CAR T-cell therapy earlier in the therapeutic strategy of mantle cell lymphoma.”
Dr. Houot reported having no disclosures.
FROM CART21
Ubrogepant safety and efficacy not affected by triptan therapy
according to a study published in Headache.
“The goal is to get migraine attacks under control as quickly as you can with as few adverse events as possible,” said lead author Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad, California. “Ubrogepant is very efficacious and well tolerated because it has few adverse events.”
Migraine disorder is the third most prevalent disease, and at least one person living in 25% of all U.S. households has the condition.
Clinicians have a wide range of medications at their disposal to treat migraines. These drug classes include triptans, ditans, NSAIDs, dihydroergotamine, and combination analgesics. Although numerous pharmacologic options are available to manage this patient population, an estimated 95% of patients who take oral medications to alleviate their migraine symptoms still fail to achieve relief with at least one acute episode.
Triptans remain a common option and first-line choice for acute migraine relief, but poor tolerability, among other factors, continue to limit their effectiveness. Moreover, their vasoconstrictive properties preclude their use in specific patient populations, such as those who have hypertension, peripheral vascular disease, and cerebral vascular accident. These circumstances, combined with other unmet clinical needs in migraine, have prompted researchers to explore new options, including a newer class of drugs – CGRP receptor antagonists. An endogenous protein, CGRP, has inflammatory and pronociceptive properties that play an active role in contributing to migraine pathogenesis.
Efficacy analyzed by triptan response
To investigate the effects of anti-CGRP treatment in patients with migraine who have a previous history of triptan use, researchers conducted two phase 3, randomized, double-blind, multicenter, single-attack trials, known as ACHIEVE I and ACHIEVE II.
Trial participants ranged in age from 18 to 75 years with a documented history of migraines with or without aura. In ACHIEVE I, investigators randomized 1,327 participants 1:1:1 to receive placebo, ubrogepant 50 mg, or ubrogepant 100 mg and placebo. Randomized patients in ACHIEVE II (n = 1,355) received placebo, ubrogepant 25 mg, or ubrogepant 50 mg to treat a single episode. During the screening process, researchers further placed patients in one of three groups based on their previous triptan use – triptan responder, triptan-insufficient responder, and triptan naive. Patients were further randomized based on their previous experience with triptans and whether they currently used them for migraine prevention. Patients participating in the study had up to 60 days to treat one qualifying migraine of moderate or severe nature at home.
The studies had two primary endpoints. The first was freedom from pain at the 2-hour mark following the initial dose, defined as decreased headache severity from moderate or severe at baseline to no pain. The other primary endpoint was the absence of most bothersome migraine-associated symptom (MBS) – photophobia, phonophobia, or nausea – 2 hours after the initial ubrogepant dose.
The pooled analysis collated data from 1,799 patients in both studies (placebo, n = 912; ubrogepant 50 mg, n = 887). Patients fell into the following categories: 682 triptan responders (placebo, n = 350; ubrogepant, n = 332); 451 triptan-insufficient responders (placebo, n = 223; ubrogepant, n = 228), and 666 triptan naive (placebo, n = 339; ubrogepant, n = 337).
Based on the data, approximately 25% of the patients enrolled in the study fell into the triptan-insufficient category. Of this subpopulation, about 80% of the patients in each treatment group experienced insufficient efficacy when using triptans. In each treatment group of insufficient responders, approximately 17% of patients cited tolerability issues, and 3% had contraindications that precluded them from triptan therapy.
The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs did not differ appreciably across historical triptan experience subgroups. The highest percentage of participants experiencing a treatment-related TEAE in the pooled ubrogepant 50-mg treatment group was found in the triptan-insufficient responders (10.4%), whereas the highest percentage in the placebo group was found in the triptan-naive subgroup (9.7%). No serious AEs (SAEs) were reported in any subgroup.
The researchers concluded that “ubrogepant efficacy and tolerability did not differ for the acute treatment of migraine in participants classified as triptan responders, triptan-insufficient responders, and triptan naive based on their historical experience with triptans.”
Payers limit use
Despite the promising data, payer hurdles limit ubrogepant’s use, said Stewart Tepper, MD, who was asked to comment on the study. Dr. Tepper, a professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., was not involved in the study. “The study shows that ubrogepant will work as well as those who have not responded well to triptans, which is key,” Dr. Tepper said. “However, payers have set up step edits in which patients aren’t allowed to get ubrogepant unless they fail therapy with at least two triptans.”
Dr. Blumenfeld discounts the rationale behind requiring patients to try a second triptan after failing initial triptan therapy. “There are plenty of studies showing that if you fail one triptan, you’re likely to fail another,” he said. “Why would you put the patient on a different triptan when you could switch them to another drug with a different mechanism of action?”
The results showed that more patients in the ubrogepant 50-mg arm achieved pain freedom than those in the placebo arm within 2 hours after the initial dose in each group.
“Migraine is a very disabling condition, so you want to get the attack under control as quickly as possible while limiting the risk for potential side effects,” said Dr. Blumenfeld. “Ubrogepant is very efficacious and with very few adverse effects, but its use is limited because insurance companies require the failure of several triptans.”
One limitation of the study is that it is a subanalysis.
Dr. Blumenfeld has disclosed advisory board service, consulting, speaking, and authorship for AbbVie, Alder, Amgen, Biohaven, Lilly, Novartis, Teva, Theranica, and Zoscano.
according to a study published in Headache.
“The goal is to get migraine attacks under control as quickly as you can with as few adverse events as possible,” said lead author Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad, California. “Ubrogepant is very efficacious and well tolerated because it has few adverse events.”
Migraine disorder is the third most prevalent disease, and at least one person living in 25% of all U.S. households has the condition.
Clinicians have a wide range of medications at their disposal to treat migraines. These drug classes include triptans, ditans, NSAIDs, dihydroergotamine, and combination analgesics. Although numerous pharmacologic options are available to manage this patient population, an estimated 95% of patients who take oral medications to alleviate their migraine symptoms still fail to achieve relief with at least one acute episode.
Triptans remain a common option and first-line choice for acute migraine relief, but poor tolerability, among other factors, continue to limit their effectiveness. Moreover, their vasoconstrictive properties preclude their use in specific patient populations, such as those who have hypertension, peripheral vascular disease, and cerebral vascular accident. These circumstances, combined with other unmet clinical needs in migraine, have prompted researchers to explore new options, including a newer class of drugs – CGRP receptor antagonists. An endogenous protein, CGRP, has inflammatory and pronociceptive properties that play an active role in contributing to migraine pathogenesis.
Efficacy analyzed by triptan response
To investigate the effects of anti-CGRP treatment in patients with migraine who have a previous history of triptan use, researchers conducted two phase 3, randomized, double-blind, multicenter, single-attack trials, known as ACHIEVE I and ACHIEVE II.
Trial participants ranged in age from 18 to 75 years with a documented history of migraines with or without aura. In ACHIEVE I, investigators randomized 1,327 participants 1:1:1 to receive placebo, ubrogepant 50 mg, or ubrogepant 100 mg and placebo. Randomized patients in ACHIEVE II (n = 1,355) received placebo, ubrogepant 25 mg, or ubrogepant 50 mg to treat a single episode. During the screening process, researchers further placed patients in one of three groups based on their previous triptan use – triptan responder, triptan-insufficient responder, and triptan naive. Patients were further randomized based on their previous experience with triptans and whether they currently used them for migraine prevention. Patients participating in the study had up to 60 days to treat one qualifying migraine of moderate or severe nature at home.
The studies had two primary endpoints. The first was freedom from pain at the 2-hour mark following the initial dose, defined as decreased headache severity from moderate or severe at baseline to no pain. The other primary endpoint was the absence of most bothersome migraine-associated symptom (MBS) – photophobia, phonophobia, or nausea – 2 hours after the initial ubrogepant dose.
The pooled analysis collated data from 1,799 patients in both studies (placebo, n = 912; ubrogepant 50 mg, n = 887). Patients fell into the following categories: 682 triptan responders (placebo, n = 350; ubrogepant, n = 332); 451 triptan-insufficient responders (placebo, n = 223; ubrogepant, n = 228), and 666 triptan naive (placebo, n = 339; ubrogepant, n = 337).
Based on the data, approximately 25% of the patients enrolled in the study fell into the triptan-insufficient category. Of this subpopulation, about 80% of the patients in each treatment group experienced insufficient efficacy when using triptans. In each treatment group of insufficient responders, approximately 17% of patients cited tolerability issues, and 3% had contraindications that precluded them from triptan therapy.
The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs did not differ appreciably across historical triptan experience subgroups. The highest percentage of participants experiencing a treatment-related TEAE in the pooled ubrogepant 50-mg treatment group was found in the triptan-insufficient responders (10.4%), whereas the highest percentage in the placebo group was found in the triptan-naive subgroup (9.7%). No serious AEs (SAEs) were reported in any subgroup.
The researchers concluded that “ubrogepant efficacy and tolerability did not differ for the acute treatment of migraine in participants classified as triptan responders, triptan-insufficient responders, and triptan naive based on their historical experience with triptans.”
Payers limit use
Despite the promising data, payer hurdles limit ubrogepant’s use, said Stewart Tepper, MD, who was asked to comment on the study. Dr. Tepper, a professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., was not involved in the study. “The study shows that ubrogepant will work as well as those who have not responded well to triptans, which is key,” Dr. Tepper said. “However, payers have set up step edits in which patients aren’t allowed to get ubrogepant unless they fail therapy with at least two triptans.”
Dr. Blumenfeld discounts the rationale behind requiring patients to try a second triptan after failing initial triptan therapy. “There are plenty of studies showing that if you fail one triptan, you’re likely to fail another,” he said. “Why would you put the patient on a different triptan when you could switch them to another drug with a different mechanism of action?”
The results showed that more patients in the ubrogepant 50-mg arm achieved pain freedom than those in the placebo arm within 2 hours after the initial dose in each group.
“Migraine is a very disabling condition, so you want to get the attack under control as quickly as possible while limiting the risk for potential side effects,” said Dr. Blumenfeld. “Ubrogepant is very efficacious and with very few adverse effects, but its use is limited because insurance companies require the failure of several triptans.”
One limitation of the study is that it is a subanalysis.
Dr. Blumenfeld has disclosed advisory board service, consulting, speaking, and authorship for AbbVie, Alder, Amgen, Biohaven, Lilly, Novartis, Teva, Theranica, and Zoscano.
according to a study published in Headache.
“The goal is to get migraine attacks under control as quickly as you can with as few adverse events as possible,” said lead author Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad, California. “Ubrogepant is very efficacious and well tolerated because it has few adverse events.”
Migraine disorder is the third most prevalent disease, and at least one person living in 25% of all U.S. households has the condition.
Clinicians have a wide range of medications at their disposal to treat migraines. These drug classes include triptans, ditans, NSAIDs, dihydroergotamine, and combination analgesics. Although numerous pharmacologic options are available to manage this patient population, an estimated 95% of patients who take oral medications to alleviate their migraine symptoms still fail to achieve relief with at least one acute episode.
Triptans remain a common option and first-line choice for acute migraine relief, but poor tolerability, among other factors, continue to limit their effectiveness. Moreover, their vasoconstrictive properties preclude their use in specific patient populations, such as those who have hypertension, peripheral vascular disease, and cerebral vascular accident. These circumstances, combined with other unmet clinical needs in migraine, have prompted researchers to explore new options, including a newer class of drugs – CGRP receptor antagonists. An endogenous protein, CGRP, has inflammatory and pronociceptive properties that play an active role in contributing to migraine pathogenesis.
Efficacy analyzed by triptan response
To investigate the effects of anti-CGRP treatment in patients with migraine who have a previous history of triptan use, researchers conducted two phase 3, randomized, double-blind, multicenter, single-attack trials, known as ACHIEVE I and ACHIEVE II.
Trial participants ranged in age from 18 to 75 years with a documented history of migraines with or without aura. In ACHIEVE I, investigators randomized 1,327 participants 1:1:1 to receive placebo, ubrogepant 50 mg, or ubrogepant 100 mg and placebo. Randomized patients in ACHIEVE II (n = 1,355) received placebo, ubrogepant 25 mg, or ubrogepant 50 mg to treat a single episode. During the screening process, researchers further placed patients in one of three groups based on their previous triptan use – triptan responder, triptan-insufficient responder, and triptan naive. Patients were further randomized based on their previous experience with triptans and whether they currently used them for migraine prevention. Patients participating in the study had up to 60 days to treat one qualifying migraine of moderate or severe nature at home.
The studies had two primary endpoints. The first was freedom from pain at the 2-hour mark following the initial dose, defined as decreased headache severity from moderate or severe at baseline to no pain. The other primary endpoint was the absence of most bothersome migraine-associated symptom (MBS) – photophobia, phonophobia, or nausea – 2 hours after the initial ubrogepant dose.
The pooled analysis collated data from 1,799 patients in both studies (placebo, n = 912; ubrogepant 50 mg, n = 887). Patients fell into the following categories: 682 triptan responders (placebo, n = 350; ubrogepant, n = 332); 451 triptan-insufficient responders (placebo, n = 223; ubrogepant, n = 228), and 666 triptan naive (placebo, n = 339; ubrogepant, n = 337).
Based on the data, approximately 25% of the patients enrolled in the study fell into the triptan-insufficient category. Of this subpopulation, about 80% of the patients in each treatment group experienced insufficient efficacy when using triptans. In each treatment group of insufficient responders, approximately 17% of patients cited tolerability issues, and 3% had contraindications that precluded them from triptan therapy.
The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs did not differ appreciably across historical triptan experience subgroups. The highest percentage of participants experiencing a treatment-related TEAE in the pooled ubrogepant 50-mg treatment group was found in the triptan-insufficient responders (10.4%), whereas the highest percentage in the placebo group was found in the triptan-naive subgroup (9.7%). No serious AEs (SAEs) were reported in any subgroup.
The researchers concluded that “ubrogepant efficacy and tolerability did not differ for the acute treatment of migraine in participants classified as triptan responders, triptan-insufficient responders, and triptan naive based on their historical experience with triptans.”
Payers limit use
Despite the promising data, payer hurdles limit ubrogepant’s use, said Stewart Tepper, MD, who was asked to comment on the study. Dr. Tepper, a professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., was not involved in the study. “The study shows that ubrogepant will work as well as those who have not responded well to triptans, which is key,” Dr. Tepper said. “However, payers have set up step edits in which patients aren’t allowed to get ubrogepant unless they fail therapy with at least two triptans.”
Dr. Blumenfeld discounts the rationale behind requiring patients to try a second triptan after failing initial triptan therapy. “There are plenty of studies showing that if you fail one triptan, you’re likely to fail another,” he said. “Why would you put the patient on a different triptan when you could switch them to another drug with a different mechanism of action?”
The results showed that more patients in the ubrogepant 50-mg arm achieved pain freedom than those in the placebo arm within 2 hours after the initial dose in each group.
“Migraine is a very disabling condition, so you want to get the attack under control as quickly as possible while limiting the risk for potential side effects,” said Dr. Blumenfeld. “Ubrogepant is very efficacious and with very few adverse effects, but its use is limited because insurance companies require the failure of several triptans.”
One limitation of the study is that it is a subanalysis.
Dr. Blumenfeld has disclosed advisory board service, consulting, speaking, and authorship for AbbVie, Alder, Amgen, Biohaven, Lilly, Novartis, Teva, Theranica, and Zoscano.
FROM HEADACHE
Evidence favors lower-dose R-CHOP for fit, very elderly DLBCL patients
A dose-adjusted R-CHOP may be the best treatment for elderly patients with diffuse large beta-cell lymphoma (DLBCL), according to a review of 38 studies that examined this aged population.
In addition, treatment choices based on new tools such as the Comprehensive Geriatric Assessment appeared to provide useful guidance based on the comorbidities and frailty index of this group of patients, according to Alda Tavares, MD, of Hospital Pedro Hispano, Matosinhos (Portugal) Local Health Unit, and Ilídia Moreira, MD, of the Portuguese Institute of Oncology of Porto.
Study characteristics
Of the 38 studies assessed, 13 were retrospective and 25 were phase II/III clinical trials. Most of these studies investigated the efficacy of dose-adjusted R-CHOP regimen, according to the review published online in Critical Reviews in Oncology/Hematology.
Alternative therapeutic drugs as well as the use of geriatric assessment were also investigated.
In terms of the elderly populations assessed, 11 out of 38 studies included at least 30 patients over age 80 years, although 11 other studies did not specify the number of patients older than 80 years. Eight of the studies included exclusively patients aged 80 years and over. Three of these studies were phase II trials.
Only six of the clinical trials required a geriatric assessment tool for inclusion criteria or therapeutic regime choice, using the Cumulative Illness Rating Scale–Geriatric (CIRS-G), the performance in activities of daily living (ADL) and/or instrumental activities of daily living (IADL) tools.
Most of the studies investigated the efficacy of R-CHOP regimen at different doses and variations, with 11 studies using alternative anthracycline in place of doxorubicin.
MiniCHOP mattered
Elderly patients over 80 years achieved complete response (CR) rates from 37.2% to 66.7% and 2-year overall survival (OS) from 31.9% to 64.7% across the studies reviewed. Overall, for fit patients aged 80 and over, the strongest evidence favored the use of an R-miniCHOP regimen, according to the authors.
In the 25 studies with treatment based on R-CHOP/modified R-CHOP or immunochemotherapy with an alternative anthracycline, the CR rate was below 50% in three studies and over 60% in the majority. Higher CR rates of 71%-88.9% were achieved in eight studies.
For patients over 80 years, the strongest evidence favored rituximab/ofatumumab-miniCHOP, based on two studies. In both studies, patients over 80 years old, without significant comorbidities, received CHOP regime with a dose reduction of about 50% (miniCHOP: cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, and 1 mg vincristine on day 1 of each cycle, and prednisone 40 mg/m2 on days 1-5) plus an anti–CD-20 antibody (rituximab 375 mg/m2 or ofatumumab 1,000 mg). The first of these studies obtained CR rate of 62% and 2-year OS of 59% with low toxicities. The second study achieved slightly better results, according to the reviewers, who suggested the difference was possibly because of a prephase treatment and/or the use of ofatumumab.
One study group developed a simple prognostic model based on multivariate analysis of 108 patients aged 80 years and older treated in their study with R-CHOP at full (48%) or reduced dose (51%). Patients with at least two out of three risk factors (age > 85 years, revised International Prognostic Index score 3-5 and CIRS > 5) had worse survival than did those with 0-1 risk factors, with a median OS of 12 months vs. 45 months, P = .001, respectively).
“All these studies results favor the tailored treatment approach,” the reviewers stated. “More prospective studies are still needed to demonstrate and validate the adequate tools for the selection of patients and their optimal treatment. They would provide the grounds for clinical therapeutic decision, aiming for tailored treatment and fulfilling best individual expectations and outcome,” they concluded.
The authors reported that they received no research funds for the study and that they had no disclosures.
A dose-adjusted R-CHOP may be the best treatment for elderly patients with diffuse large beta-cell lymphoma (DLBCL), according to a review of 38 studies that examined this aged population.
In addition, treatment choices based on new tools such as the Comprehensive Geriatric Assessment appeared to provide useful guidance based on the comorbidities and frailty index of this group of patients, according to Alda Tavares, MD, of Hospital Pedro Hispano, Matosinhos (Portugal) Local Health Unit, and Ilídia Moreira, MD, of the Portuguese Institute of Oncology of Porto.
Study characteristics
Of the 38 studies assessed, 13 were retrospective and 25 were phase II/III clinical trials. Most of these studies investigated the efficacy of dose-adjusted R-CHOP regimen, according to the review published online in Critical Reviews in Oncology/Hematology.
Alternative therapeutic drugs as well as the use of geriatric assessment were also investigated.
In terms of the elderly populations assessed, 11 out of 38 studies included at least 30 patients over age 80 years, although 11 other studies did not specify the number of patients older than 80 years. Eight of the studies included exclusively patients aged 80 years and over. Three of these studies were phase II trials.
Only six of the clinical trials required a geriatric assessment tool for inclusion criteria or therapeutic regime choice, using the Cumulative Illness Rating Scale–Geriatric (CIRS-G), the performance in activities of daily living (ADL) and/or instrumental activities of daily living (IADL) tools.
Most of the studies investigated the efficacy of R-CHOP regimen at different doses and variations, with 11 studies using alternative anthracycline in place of doxorubicin.
MiniCHOP mattered
Elderly patients over 80 years achieved complete response (CR) rates from 37.2% to 66.7% and 2-year overall survival (OS) from 31.9% to 64.7% across the studies reviewed. Overall, for fit patients aged 80 and over, the strongest evidence favored the use of an R-miniCHOP regimen, according to the authors.
In the 25 studies with treatment based on R-CHOP/modified R-CHOP or immunochemotherapy with an alternative anthracycline, the CR rate was below 50% in three studies and over 60% in the majority. Higher CR rates of 71%-88.9% were achieved in eight studies.
For patients over 80 years, the strongest evidence favored rituximab/ofatumumab-miniCHOP, based on two studies. In both studies, patients over 80 years old, without significant comorbidities, received CHOP regime with a dose reduction of about 50% (miniCHOP: cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, and 1 mg vincristine on day 1 of each cycle, and prednisone 40 mg/m2 on days 1-5) plus an anti–CD-20 antibody (rituximab 375 mg/m2 or ofatumumab 1,000 mg). The first of these studies obtained CR rate of 62% and 2-year OS of 59% with low toxicities. The second study achieved slightly better results, according to the reviewers, who suggested the difference was possibly because of a prephase treatment and/or the use of ofatumumab.
One study group developed a simple prognostic model based on multivariate analysis of 108 patients aged 80 years and older treated in their study with R-CHOP at full (48%) or reduced dose (51%). Patients with at least two out of three risk factors (age > 85 years, revised International Prognostic Index score 3-5 and CIRS > 5) had worse survival than did those with 0-1 risk factors, with a median OS of 12 months vs. 45 months, P = .001, respectively).
“All these studies results favor the tailored treatment approach,” the reviewers stated. “More prospective studies are still needed to demonstrate and validate the adequate tools for the selection of patients and their optimal treatment. They would provide the grounds for clinical therapeutic decision, aiming for tailored treatment and fulfilling best individual expectations and outcome,” they concluded.
The authors reported that they received no research funds for the study and that they had no disclosures.
A dose-adjusted R-CHOP may be the best treatment for elderly patients with diffuse large beta-cell lymphoma (DLBCL), according to a review of 38 studies that examined this aged population.
In addition, treatment choices based on new tools such as the Comprehensive Geriatric Assessment appeared to provide useful guidance based on the comorbidities and frailty index of this group of patients, according to Alda Tavares, MD, of Hospital Pedro Hispano, Matosinhos (Portugal) Local Health Unit, and Ilídia Moreira, MD, of the Portuguese Institute of Oncology of Porto.
Study characteristics
Of the 38 studies assessed, 13 were retrospective and 25 were phase II/III clinical trials. Most of these studies investigated the efficacy of dose-adjusted R-CHOP regimen, according to the review published online in Critical Reviews in Oncology/Hematology.
Alternative therapeutic drugs as well as the use of geriatric assessment were also investigated.
In terms of the elderly populations assessed, 11 out of 38 studies included at least 30 patients over age 80 years, although 11 other studies did not specify the number of patients older than 80 years. Eight of the studies included exclusively patients aged 80 years and over. Three of these studies were phase II trials.
Only six of the clinical trials required a geriatric assessment tool for inclusion criteria or therapeutic regime choice, using the Cumulative Illness Rating Scale–Geriatric (CIRS-G), the performance in activities of daily living (ADL) and/or instrumental activities of daily living (IADL) tools.
Most of the studies investigated the efficacy of R-CHOP regimen at different doses and variations, with 11 studies using alternative anthracycline in place of doxorubicin.
MiniCHOP mattered
Elderly patients over 80 years achieved complete response (CR) rates from 37.2% to 66.7% and 2-year overall survival (OS) from 31.9% to 64.7% across the studies reviewed. Overall, for fit patients aged 80 and over, the strongest evidence favored the use of an R-miniCHOP regimen, according to the authors.
In the 25 studies with treatment based on R-CHOP/modified R-CHOP or immunochemotherapy with an alternative anthracycline, the CR rate was below 50% in three studies and over 60% in the majority. Higher CR rates of 71%-88.9% were achieved in eight studies.
For patients over 80 years, the strongest evidence favored rituximab/ofatumumab-miniCHOP, based on two studies. In both studies, patients over 80 years old, without significant comorbidities, received CHOP regime with a dose reduction of about 50% (miniCHOP: cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, and 1 mg vincristine on day 1 of each cycle, and prednisone 40 mg/m2 on days 1-5) plus an anti–CD-20 antibody (rituximab 375 mg/m2 or ofatumumab 1,000 mg). The first of these studies obtained CR rate of 62% and 2-year OS of 59% with low toxicities. The second study achieved slightly better results, according to the reviewers, who suggested the difference was possibly because of a prephase treatment and/or the use of ofatumumab.
One study group developed a simple prognostic model based on multivariate analysis of 108 patients aged 80 years and older treated in their study with R-CHOP at full (48%) or reduced dose (51%). Patients with at least two out of three risk factors (age > 85 years, revised International Prognostic Index score 3-5 and CIRS > 5) had worse survival than did those with 0-1 risk factors, with a median OS of 12 months vs. 45 months, P = .001, respectively).
“All these studies results favor the tailored treatment approach,” the reviewers stated. “More prospective studies are still needed to demonstrate and validate the adequate tools for the selection of patients and their optimal treatment. They would provide the grounds for clinical therapeutic decision, aiming for tailored treatment and fulfilling best individual expectations and outcome,” they concluded.
The authors reported that they received no research funds for the study and that they had no disclosures.
FROM CRITICAL REVIEWS IN ONCOLOGY/HEMATOLOGY
Can exercise prevent cognitive decline in patients with early Parkinson’s disease?
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY