User login
American Headache Society updates guideline on neuroimaging for migraine
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
FROM HEADACHE
HIV free 30 months after stem cell transplant, is the London patient cured?
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
FROM CROI 2020
TBI deaths from falls on the rise
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.
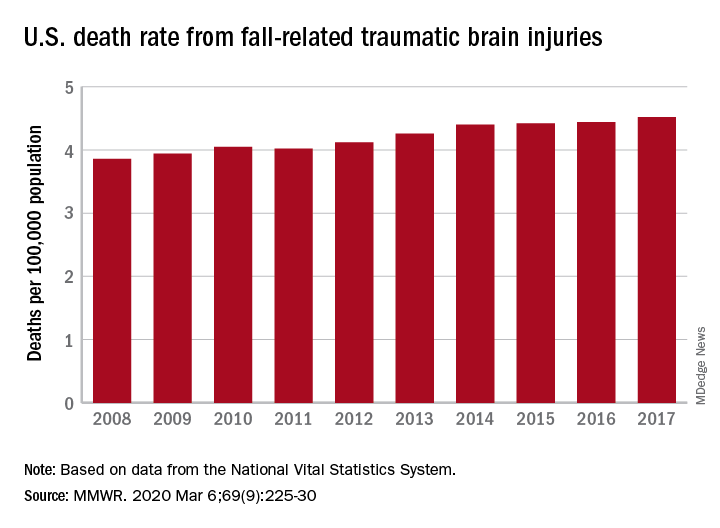
Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
FROM MMWR
Stress-related disorders linked to later neurodegenerative diseases
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
FROM JAMA NEUROLOGY
USPSTF again deems evidence insufficient to recommend cognitive impairment screening in older adults
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
FROM JAMA
As costs for neurologic drugs rise, adherence to therapy drops
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
FROM NEUROLOGY
Zilucoplan improved efficacy outcomes in myasthenia gravis
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
FROM JAMA NEUROLOGY
Safer CAR uses modified NK cells for advanced CLL, NHL
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Antiepileptic drugs may not independently impair cognition
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
FROM NEUROLOGY
Thrombectomy access lags for U.S. stroke patients
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
FROM STROKE



