User login
Cirrhosis, Child-Pugh score predict ERCP complications
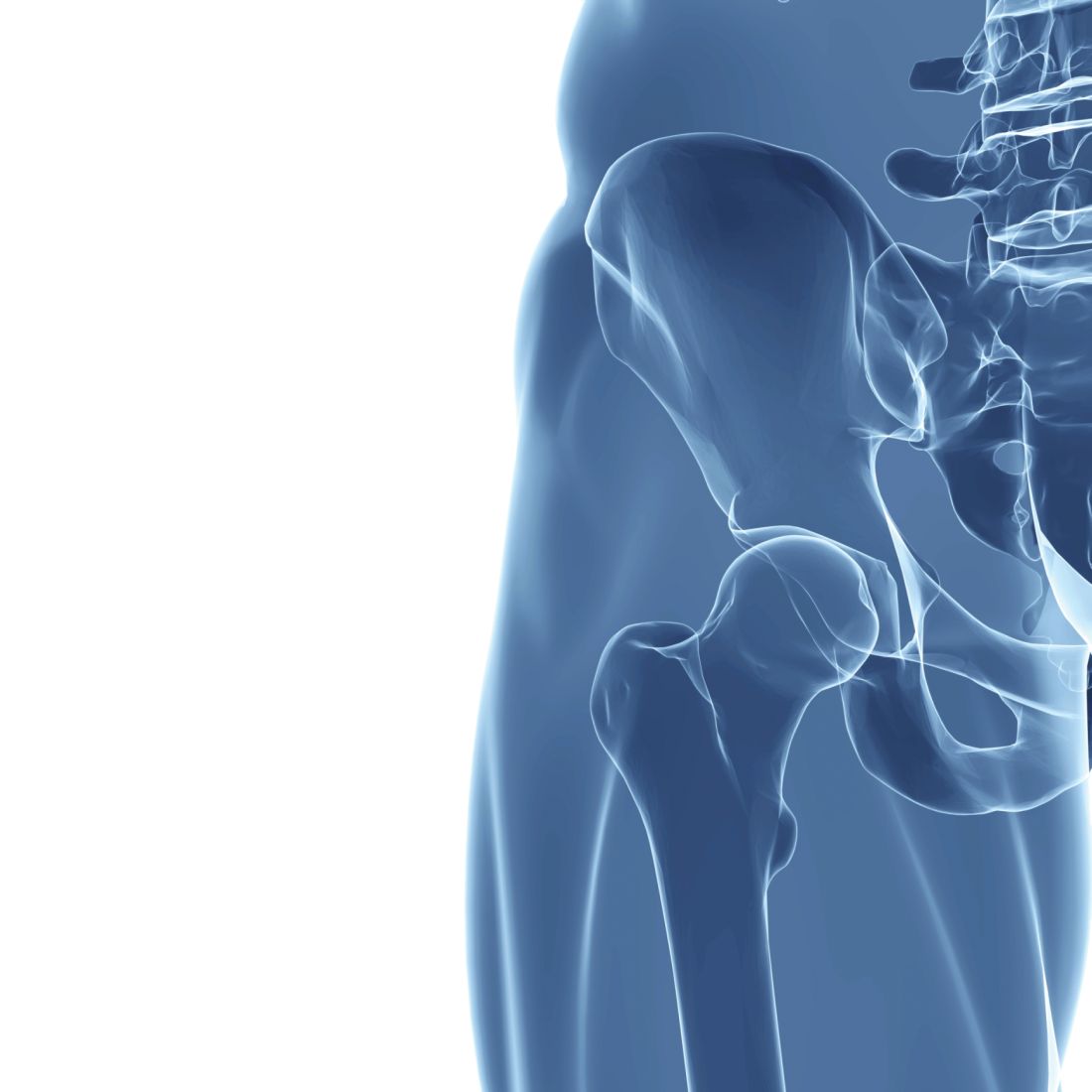
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
FROM ACG 2020
Antibiotics fail to improve colon ischemia outcomes
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
FROM ACG 2020
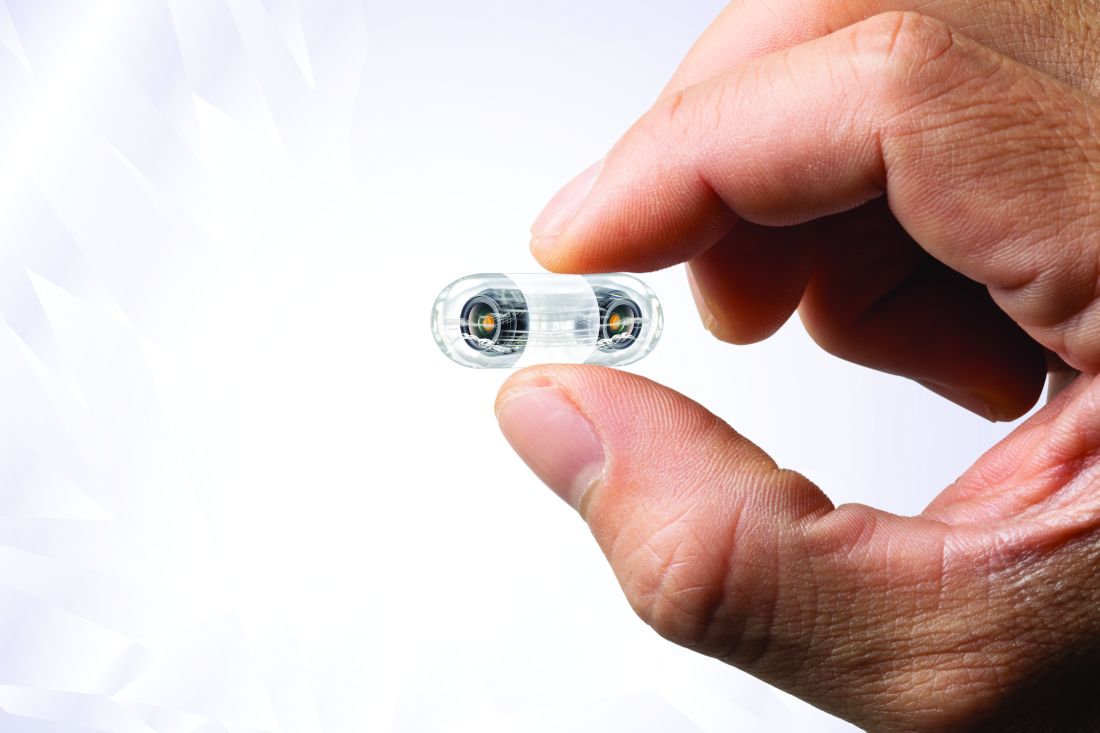
Video capsule endoscopy shows superiority, may reduce coronavirus exposure
Video capsule endoscopy (VCE) offers an alternative triage tool for acute GI bleeding that may reduce personnel exposure to SARS-CoV-2, based on a cohort study with historical controls.
VCE should be considered even when rapid coronavirus testing is available, as active bleeding is more likely to be detected when evaluated sooner, potentially sparing patients from invasive procedures, reported lead author Shahrad Hakimian, MD, of the University of Massachusetts Medical Center, Worchester, and colleagues.
“Endoscopists and staff are at high risk of exposure to coronavirus through aerosols, as well as unintended, unrecognized splashes that are well known to occur frequently during routine endoscopy,” Dr. Hakimian said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
Although pretesting and delaying procedures as needed may mitigate risks of viral exposure, “many urgent procedures, such as endoscopic evaluation of gastrointestinal bleeding, can’t really wait,” Dr. Hakimian said.
Current guidelines recommend early upper endoscopy and/or colonoscopy for evaluation of GI bleeding, but Dr. Hakimian noted that two out of three initial tests are nondiagnostic, so multiple procedures are often needed to find an answer.
In 2018, a randomized, controlled trial coauthored by Dr. Hakimian’s colleagues demonstrated how VCE may be a better approach, as it more frequently detected active bleeding than standard of care (adjusted hazard ratio, 2.77; 95% confidence interval, 1.36-5.64).
The present study built on these findings in the context of the COVID-19 pandemic.
Dr. Hakimian and colleagues analyzed data from 50 consecutive, hemodynamically stable patients with severe anemia or suspected GI bleeding who underwent VCE as a first-line diagnostic modality from mid-March to mid-May 2020 (COVID arm). These patients were compared with 57 consecutive patients who were evaluated for acute GI bleeding or severe anemia with standard of care prior to the COVID-19 pandemic (pre-COVID arm).
Characteristics of the two cohorts were generally similar, although the COVID arm included a slightly older population, with a median age of 68 years, compared with a median age of 61.8 years for the pre-COVID arm (P = .03). Among presenting symptoms, hematochezia was less common in the COVID group (4% vs. 18%; P = .03). Comorbidities were not significantly different between cohorts.
Per the study design, 100% of patients in the COVID arm underwent VCE as their first diagnostic modality. In the pre-COVID arm, 82% of patients first underwent upper endoscopy, followed by colonoscopy (12%) and VCE (5%).
The main outcome, bleeding localization, did not differ between groups, whether this was confined to the first test, or in terms of final localization. But VCE was significantly better at detecting active bleeding or stigmata of bleeding, at a rate of 66%, compared with 28% in the pre-COVID group (P < .001). Patients in the COVID arm were also significantly less likely to need any invasive procedures (44% vs. 96%; P < .001).
No intergroup differences were observed in rates of blood transfusion, in-hospital or GI-bleed mortality, rebleeding, or readmission for bleeding.
“VCE appears to be a safe alternative to traditional diagnostic evaluation of GI bleeding in the era of COVID,” Dr. Hakimian concluded, noting that “the VCE-first strategy reduces the risk of staff exposure to endoscopic aerosols, conserves personal protective equipment, and reduces staff utilization.”
According to Neil Sengupta, MD, of the University of Chicago, “a VCE-first strategy in GI bleeding may be a useful triage tool in the COVID-19 era to determine which patients truly benefit from invasive endoscopy,” although he also noted that “further data are needed to determine the efficacy and safety of this approach.”
Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., had a similar opinion.
“VCE appears to be a reasonable alternative in this patient group, at least as a first step,” Dr. Hookey said. “However, whether it truly makes a difference in the decision making process would have to be assessed prospectively via a randomized controlled trial or a decision analysis done in real time at various steps of the patient’s care path.”
Erik A. Holzwanger, MD, a gastroenterology fellow at Tufts Medical Center in Boston, suggested that these findings may “serve as a foundation” for similar studies, “as it appears COVID-19 will be an ongoing obstacle in endoscopy for the foreseeable future.”
“It would be interesting to have further discussion of timing of VCE, any COVID-19 transmission to staff during the VCE placement, and discussion of what constituted proceeding with endoscopic intervention [high-risk lesion, active bleeding] in both groups,” he added.
David Cave, MD, PhD, coauthor of the present study and the 2015 ACG clinical guideline for small bowel bleeding, said that VCE is gaining ground as the diagnostic of choice for GI bleeding, and patients prefer it, since it does not require anesthesia.
“This abstract is another clear pointer to the way in which, we should in the future, investigate gastrointestinal bleeding, both acute and chronic,” Dr. Cave said. “We are at an inflection point of transition to a new technology.”
Dr. Cave disclosed relationships with Medtronic and Olympus. The other investigators and interviewees reported no conflicts of interest.
Video capsule endoscopy (VCE) offers an alternative triage tool for acute GI bleeding that may reduce personnel exposure to SARS-CoV-2, based on a cohort study with historical controls.
VCE should be considered even when rapid coronavirus testing is available, as active bleeding is more likely to be detected when evaluated sooner, potentially sparing patients from invasive procedures, reported lead author Shahrad Hakimian, MD, of the University of Massachusetts Medical Center, Worchester, and colleagues.
“Endoscopists and staff are at high risk of exposure to coronavirus through aerosols, as well as unintended, unrecognized splashes that are well known to occur frequently during routine endoscopy,” Dr. Hakimian said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
Although pretesting and delaying procedures as needed may mitigate risks of viral exposure, “many urgent procedures, such as endoscopic evaluation of gastrointestinal bleeding, can’t really wait,” Dr. Hakimian said.
Current guidelines recommend early upper endoscopy and/or colonoscopy for evaluation of GI bleeding, but Dr. Hakimian noted that two out of three initial tests are nondiagnostic, so multiple procedures are often needed to find an answer.
In 2018, a randomized, controlled trial coauthored by Dr. Hakimian’s colleagues demonstrated how VCE may be a better approach, as it more frequently detected active bleeding than standard of care (adjusted hazard ratio, 2.77; 95% confidence interval, 1.36-5.64).
The present study built on these findings in the context of the COVID-19 pandemic.
Dr. Hakimian and colleagues analyzed data from 50 consecutive, hemodynamically stable patients with severe anemia or suspected GI bleeding who underwent VCE as a first-line diagnostic modality from mid-March to mid-May 2020 (COVID arm). These patients were compared with 57 consecutive patients who were evaluated for acute GI bleeding or severe anemia with standard of care prior to the COVID-19 pandemic (pre-COVID arm).
Characteristics of the two cohorts were generally similar, although the COVID arm included a slightly older population, with a median age of 68 years, compared with a median age of 61.8 years for the pre-COVID arm (P = .03). Among presenting symptoms, hematochezia was less common in the COVID group (4% vs. 18%; P = .03). Comorbidities were not significantly different between cohorts.
Per the study design, 100% of patients in the COVID arm underwent VCE as their first diagnostic modality. In the pre-COVID arm, 82% of patients first underwent upper endoscopy, followed by colonoscopy (12%) and VCE (5%).
The main outcome, bleeding localization, did not differ between groups, whether this was confined to the first test, or in terms of final localization. But VCE was significantly better at detecting active bleeding or stigmata of bleeding, at a rate of 66%, compared with 28% in the pre-COVID group (P < .001). Patients in the COVID arm were also significantly less likely to need any invasive procedures (44% vs. 96%; P < .001).
No intergroup differences were observed in rates of blood transfusion, in-hospital or GI-bleed mortality, rebleeding, or readmission for bleeding.
“VCE appears to be a safe alternative to traditional diagnostic evaluation of GI bleeding in the era of COVID,” Dr. Hakimian concluded, noting that “the VCE-first strategy reduces the risk of staff exposure to endoscopic aerosols, conserves personal protective equipment, and reduces staff utilization.”
According to Neil Sengupta, MD, of the University of Chicago, “a VCE-first strategy in GI bleeding may be a useful triage tool in the COVID-19 era to determine which patients truly benefit from invasive endoscopy,” although he also noted that “further data are needed to determine the efficacy and safety of this approach.”
Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., had a similar opinion.
“VCE appears to be a reasonable alternative in this patient group, at least as a first step,” Dr. Hookey said. “However, whether it truly makes a difference in the decision making process would have to be assessed prospectively via a randomized controlled trial or a decision analysis done in real time at various steps of the patient’s care path.”
Erik A. Holzwanger, MD, a gastroenterology fellow at Tufts Medical Center in Boston, suggested that these findings may “serve as a foundation” for similar studies, “as it appears COVID-19 will be an ongoing obstacle in endoscopy for the foreseeable future.”
“It would be interesting to have further discussion of timing of VCE, any COVID-19 transmission to staff during the VCE placement, and discussion of what constituted proceeding with endoscopic intervention [high-risk lesion, active bleeding] in both groups,” he added.
David Cave, MD, PhD, coauthor of the present study and the 2015 ACG clinical guideline for small bowel bleeding, said that VCE is gaining ground as the diagnostic of choice for GI bleeding, and patients prefer it, since it does not require anesthesia.
“This abstract is another clear pointer to the way in which, we should in the future, investigate gastrointestinal bleeding, both acute and chronic,” Dr. Cave said. “We are at an inflection point of transition to a new technology.”
Dr. Cave disclosed relationships with Medtronic and Olympus. The other investigators and interviewees reported no conflicts of interest.
Video capsule endoscopy (VCE) offers an alternative triage tool for acute GI bleeding that may reduce personnel exposure to SARS-CoV-2, based on a cohort study with historical controls.
VCE should be considered even when rapid coronavirus testing is available, as active bleeding is more likely to be detected when evaluated sooner, potentially sparing patients from invasive procedures, reported lead author Shahrad Hakimian, MD, of the University of Massachusetts Medical Center, Worchester, and colleagues.
“Endoscopists and staff are at high risk of exposure to coronavirus through aerosols, as well as unintended, unrecognized splashes that are well known to occur frequently during routine endoscopy,” Dr. Hakimian said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
Although pretesting and delaying procedures as needed may mitigate risks of viral exposure, “many urgent procedures, such as endoscopic evaluation of gastrointestinal bleeding, can’t really wait,” Dr. Hakimian said.
Current guidelines recommend early upper endoscopy and/or colonoscopy for evaluation of GI bleeding, but Dr. Hakimian noted that two out of three initial tests are nondiagnostic, so multiple procedures are often needed to find an answer.
In 2018, a randomized, controlled trial coauthored by Dr. Hakimian’s colleagues demonstrated how VCE may be a better approach, as it more frequently detected active bleeding than standard of care (adjusted hazard ratio, 2.77; 95% confidence interval, 1.36-5.64).
The present study built on these findings in the context of the COVID-19 pandemic.
Dr. Hakimian and colleagues analyzed data from 50 consecutive, hemodynamically stable patients with severe anemia or suspected GI bleeding who underwent VCE as a first-line diagnostic modality from mid-March to mid-May 2020 (COVID arm). These patients were compared with 57 consecutive patients who were evaluated for acute GI bleeding or severe anemia with standard of care prior to the COVID-19 pandemic (pre-COVID arm).
Characteristics of the two cohorts were generally similar, although the COVID arm included a slightly older population, with a median age of 68 years, compared with a median age of 61.8 years for the pre-COVID arm (P = .03). Among presenting symptoms, hematochezia was less common in the COVID group (4% vs. 18%; P = .03). Comorbidities were not significantly different between cohorts.
Per the study design, 100% of patients in the COVID arm underwent VCE as their first diagnostic modality. In the pre-COVID arm, 82% of patients first underwent upper endoscopy, followed by colonoscopy (12%) and VCE (5%).
The main outcome, bleeding localization, did not differ between groups, whether this was confined to the first test, or in terms of final localization. But VCE was significantly better at detecting active bleeding or stigmata of bleeding, at a rate of 66%, compared with 28% in the pre-COVID group (P < .001). Patients in the COVID arm were also significantly less likely to need any invasive procedures (44% vs. 96%; P < .001).
No intergroup differences were observed in rates of blood transfusion, in-hospital or GI-bleed mortality, rebleeding, or readmission for bleeding.
“VCE appears to be a safe alternative to traditional diagnostic evaluation of GI bleeding in the era of COVID,” Dr. Hakimian concluded, noting that “the VCE-first strategy reduces the risk of staff exposure to endoscopic aerosols, conserves personal protective equipment, and reduces staff utilization.”
According to Neil Sengupta, MD, of the University of Chicago, “a VCE-first strategy in GI bleeding may be a useful triage tool in the COVID-19 era to determine which patients truly benefit from invasive endoscopy,” although he also noted that “further data are needed to determine the efficacy and safety of this approach.”
Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., had a similar opinion.
“VCE appears to be a reasonable alternative in this patient group, at least as a first step,” Dr. Hookey said. “However, whether it truly makes a difference in the decision making process would have to be assessed prospectively via a randomized controlled trial or a decision analysis done in real time at various steps of the patient’s care path.”
Erik A. Holzwanger, MD, a gastroenterology fellow at Tufts Medical Center in Boston, suggested that these findings may “serve as a foundation” for similar studies, “as it appears COVID-19 will be an ongoing obstacle in endoscopy for the foreseeable future.”
“It would be interesting to have further discussion of timing of VCE, any COVID-19 transmission to staff during the VCE placement, and discussion of what constituted proceeding with endoscopic intervention [high-risk lesion, active bleeding] in both groups,” he added.
David Cave, MD, PhD, coauthor of the present study and the 2015 ACG clinical guideline for small bowel bleeding, said that VCE is gaining ground as the diagnostic of choice for GI bleeding, and patients prefer it, since it does not require anesthesia.
“This abstract is another clear pointer to the way in which, we should in the future, investigate gastrointestinal bleeding, both acute and chronic,” Dr. Cave said. “We are at an inflection point of transition to a new technology.”
Dr. Cave disclosed relationships with Medtronic and Olympus. The other investigators and interviewees reported no conflicts of interest.
FROM ACG 2020
Statins may lower risk of colorectal cancer
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
FROM ACG 2020
AHA adds recovery, emotional support to CPR guidelines
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
FROM CIRCULATION
COVID-19: Convalescent plasma falls short in phase 2 trial
Convalescent plasma may not prevent progression to severe disease or reduce mortality risk in hospitalized patients with moderate COVID-19, based on a phase 2 trial involving more than 400 patients in India.
The PLACID trial offers real-world data with “high generalizability,” according to lead author Anup Agarwal, MD, of the Indian Council of Medical Research, New Delhi, and colleagues.
“Evidence suggests that convalescent plasma collected from survivors of COVID-19 contains receptor binding domain specific antibodies with potent antiviral activity,” the investigators wrote in the BMJ. “However, effective titers of antiviral neutralizing antibodies, optimal timing for convalescent plasma treatment, optimal timing for plasma donation, and the severity class of patients who are likely to benefit from convalescent plasma remain unclear.”
According to Dr. Agarwal and colleagues, case series and observational studies have suggested that convalescent plasma may reduce viral load, hospital stay, and mortality, but randomized controlled trials to date have ended prematurely because of issues with enrollment and design, making PLACID the first randomized controlled trial of its kind to reach completion.
The open-label, multicenter study involved 464 hospitalized adults who tested positive for SARS-CoV-2 via reverse transcription polymerase chain reaction (RT-PCR). Enrollment also required a respiratory rate of more than 24 breaths/min with an oxygen saturation (SpO2) of 93% or less on room air, or a partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2 /FiO2 ) ratio between 200 and 300 mm Hg.
Patients were randomly assigned in a 1:1 ratio to receive either best standard of care (control), or best standard of care plus convalescent plasma, which was given in two doses of 200 mL, 24 hours apart. Patients were assessed via clinical examination, chest imaging, and serial laboratory testing, the latter of which included neutralizing antibody titers on days 0, 3, and 7.
The primary outcome was a 28-day composite of progression to severe disease (PaO2/FiO2 ratio < 100 mm Hg) and all-cause mortality. An array of secondary outcomes were also reported, including symptom resolution, total duration of respiratory support, change in oxygen requirement, and others.
In the convalescent plasma group, 19% of patients progressed to severe disease or died within 28 days, compared with 18% of those in the control group (risk ratio, 1.04; 95% confidence interval, 0.71-1.54), suggesting no statistically significant benefit from the intervention. This lack of benefit was also found in a subgroup analysis of patients with detectable titers of antibodies to SARS-CoV-2, and when progression to severe disease and all-cause mortality were analyzed independently across all patients.
Still, at day 7, patients treated with convalescent plasma were significantly more likely to have resolution of fatigue (RR, 1.21; 95% CI, 1.02-1.42) and shortness of breath (RR, 1.16; 95% CI, 1.02-1.32). And at the same time point, patients treated with convalescent plasma were 20% more likely to test negative for SARS-CoV-2 RNA (RR, 1.2; 95% CI, 1.04-1.5).
In an accompanying editorial, Elizabeth B. Pathak, PhD, of the Women’s Institute for Independent Social Enquiry, Olney, Md., suggested that the reported symptom improvements need to be viewed with skepticism.
“These results should be interpreted with caution, because the trial was not blinded, so knowledge of treatment status could have influenced the reporting of subjective symptoms by patients who survived to day 7,” Dr. Pathak wrote.
Dr. Pathak noted that convalescent plasma did appear to have an antiviral effect, based on the higher rate of negative RNA test results at day 7. She hypothesized that the lack of major corresponding clinical benefit could be explained by detrimental thrombotic processes.
“The net effect of plasma is prothrombotic,” Dr. Pathak wrote, which should raise safety concerns, since “COVID-19 is a life-threatening thrombotic disorder.”
According to Dr. Pathak, large-scale datasets may be giving a false sense of security. She cited a recent safety analysis of 20,000 U.S. patients who received convalescent plasma, in which the investigators excluded 88.2% of cardiac events and 66.3% of thrombotic events, as these were deemed unrelated to transfusion; but this decision was made by the treating physician, without independent review or a defined protocol.
Michael J. Joyner, MD, of the Mayo Clinic in Rochester, Minn., was the lead author of the above safety study, and is leading the Food and Drug Administration expanded access program for convalescent plasma in patients with COVID-19. He suggested that the study by Dr. Agarwal and colleagues was admirable, but flaws in the treatment protocol cast doubt upon the efficacy findings.
“It is very impressive that these investigators performed a large trial of convalescent plasma in the midst of a pandemic,” Dr. Joyner said. “Unfortunately it is unclear how generalizable the findings are because many of the units of plasma had either very low or no antibody titers and because the plasma was given late in the course of the disease. It has been known since at least the 1930s that antibody therapy works best when enough product is given either prophylactically or early in the course of disease.”
Dr. Joyner had a more positive interpretation of the reported symptom improvements.
“It is also interesting to note that while there was no mortality benefit, that – even with the limitations of the study – there was some evidence of improved patient physiology at 7 days,” he said. “So, at one level, [this is] a negative study, but at least [there are] some hints of efficacy given the suboptimal use case in the patients studied.”
The study was funded by the Indian Council of Medical Research, which employs several of the authors and PLACID Trial Collaborators. Dr. Pathak and Dr. Joyner reported no conflicts of interest.
SOURCE: Agarwal A et al. BMJ. 2020 Oct 23. doi: 10.1136/bmj.m3939 .
Convalescent plasma may not prevent progression to severe disease or reduce mortality risk in hospitalized patients with moderate COVID-19, based on a phase 2 trial involving more than 400 patients in India.
The PLACID trial offers real-world data with “high generalizability,” according to lead author Anup Agarwal, MD, of the Indian Council of Medical Research, New Delhi, and colleagues.
“Evidence suggests that convalescent plasma collected from survivors of COVID-19 contains receptor binding domain specific antibodies with potent antiviral activity,” the investigators wrote in the BMJ. “However, effective titers of antiviral neutralizing antibodies, optimal timing for convalescent plasma treatment, optimal timing for plasma donation, and the severity class of patients who are likely to benefit from convalescent plasma remain unclear.”
According to Dr. Agarwal and colleagues, case series and observational studies have suggested that convalescent plasma may reduce viral load, hospital stay, and mortality, but randomized controlled trials to date have ended prematurely because of issues with enrollment and design, making PLACID the first randomized controlled trial of its kind to reach completion.
The open-label, multicenter study involved 464 hospitalized adults who tested positive for SARS-CoV-2 via reverse transcription polymerase chain reaction (RT-PCR). Enrollment also required a respiratory rate of more than 24 breaths/min with an oxygen saturation (SpO2) of 93% or less on room air, or a partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2 /FiO2 ) ratio between 200 and 300 mm Hg.
Patients were randomly assigned in a 1:1 ratio to receive either best standard of care (control), or best standard of care plus convalescent plasma, which was given in two doses of 200 mL, 24 hours apart. Patients were assessed via clinical examination, chest imaging, and serial laboratory testing, the latter of which included neutralizing antibody titers on days 0, 3, and 7.
The primary outcome was a 28-day composite of progression to severe disease (PaO2/FiO2 ratio < 100 mm Hg) and all-cause mortality. An array of secondary outcomes were also reported, including symptom resolution, total duration of respiratory support, change in oxygen requirement, and others.
In the convalescent plasma group, 19% of patients progressed to severe disease or died within 28 days, compared with 18% of those in the control group (risk ratio, 1.04; 95% confidence interval, 0.71-1.54), suggesting no statistically significant benefit from the intervention. This lack of benefit was also found in a subgroup analysis of patients with detectable titers of antibodies to SARS-CoV-2, and when progression to severe disease and all-cause mortality were analyzed independently across all patients.
Still, at day 7, patients treated with convalescent plasma were significantly more likely to have resolution of fatigue (RR, 1.21; 95% CI, 1.02-1.42) and shortness of breath (RR, 1.16; 95% CI, 1.02-1.32). And at the same time point, patients treated with convalescent plasma were 20% more likely to test negative for SARS-CoV-2 RNA (RR, 1.2; 95% CI, 1.04-1.5).
In an accompanying editorial, Elizabeth B. Pathak, PhD, of the Women’s Institute for Independent Social Enquiry, Olney, Md., suggested that the reported symptom improvements need to be viewed with skepticism.
“These results should be interpreted with caution, because the trial was not blinded, so knowledge of treatment status could have influenced the reporting of subjective symptoms by patients who survived to day 7,” Dr. Pathak wrote.
Dr. Pathak noted that convalescent plasma did appear to have an antiviral effect, based on the higher rate of negative RNA test results at day 7. She hypothesized that the lack of major corresponding clinical benefit could be explained by detrimental thrombotic processes.
“The net effect of plasma is prothrombotic,” Dr. Pathak wrote, which should raise safety concerns, since “COVID-19 is a life-threatening thrombotic disorder.”
According to Dr. Pathak, large-scale datasets may be giving a false sense of security. She cited a recent safety analysis of 20,000 U.S. patients who received convalescent plasma, in which the investigators excluded 88.2% of cardiac events and 66.3% of thrombotic events, as these were deemed unrelated to transfusion; but this decision was made by the treating physician, without independent review or a defined protocol.
Michael J. Joyner, MD, of the Mayo Clinic in Rochester, Minn., was the lead author of the above safety study, and is leading the Food and Drug Administration expanded access program for convalescent plasma in patients with COVID-19. He suggested that the study by Dr. Agarwal and colleagues was admirable, but flaws in the treatment protocol cast doubt upon the efficacy findings.
“It is very impressive that these investigators performed a large trial of convalescent plasma in the midst of a pandemic,” Dr. Joyner said. “Unfortunately it is unclear how generalizable the findings are because many of the units of plasma had either very low or no antibody titers and because the plasma was given late in the course of the disease. It has been known since at least the 1930s that antibody therapy works best when enough product is given either prophylactically or early in the course of disease.”
Dr. Joyner had a more positive interpretation of the reported symptom improvements.
“It is also interesting to note that while there was no mortality benefit, that – even with the limitations of the study – there was some evidence of improved patient physiology at 7 days,” he said. “So, at one level, [this is] a negative study, but at least [there are] some hints of efficacy given the suboptimal use case in the patients studied.”
The study was funded by the Indian Council of Medical Research, which employs several of the authors and PLACID Trial Collaborators. Dr. Pathak and Dr. Joyner reported no conflicts of interest.
SOURCE: Agarwal A et al. BMJ. 2020 Oct 23. doi: 10.1136/bmj.m3939 .
Convalescent plasma may not prevent progression to severe disease or reduce mortality risk in hospitalized patients with moderate COVID-19, based on a phase 2 trial involving more than 400 patients in India.
The PLACID trial offers real-world data with “high generalizability,” according to lead author Anup Agarwal, MD, of the Indian Council of Medical Research, New Delhi, and colleagues.
“Evidence suggests that convalescent plasma collected from survivors of COVID-19 contains receptor binding domain specific antibodies with potent antiviral activity,” the investigators wrote in the BMJ. “However, effective titers of antiviral neutralizing antibodies, optimal timing for convalescent plasma treatment, optimal timing for plasma donation, and the severity class of patients who are likely to benefit from convalescent plasma remain unclear.”
According to Dr. Agarwal and colleagues, case series and observational studies have suggested that convalescent plasma may reduce viral load, hospital stay, and mortality, but randomized controlled trials to date have ended prematurely because of issues with enrollment and design, making PLACID the first randomized controlled trial of its kind to reach completion.
The open-label, multicenter study involved 464 hospitalized adults who tested positive for SARS-CoV-2 via reverse transcription polymerase chain reaction (RT-PCR). Enrollment also required a respiratory rate of more than 24 breaths/min with an oxygen saturation (SpO2) of 93% or less on room air, or a partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2 /FiO2 ) ratio between 200 and 300 mm Hg.
Patients were randomly assigned in a 1:1 ratio to receive either best standard of care (control), or best standard of care plus convalescent plasma, which was given in two doses of 200 mL, 24 hours apart. Patients were assessed via clinical examination, chest imaging, and serial laboratory testing, the latter of which included neutralizing antibody titers on days 0, 3, and 7.
The primary outcome was a 28-day composite of progression to severe disease (PaO2/FiO2 ratio < 100 mm Hg) and all-cause mortality. An array of secondary outcomes were also reported, including symptom resolution, total duration of respiratory support, change in oxygen requirement, and others.
In the convalescent plasma group, 19% of patients progressed to severe disease or died within 28 days, compared with 18% of those in the control group (risk ratio, 1.04; 95% confidence interval, 0.71-1.54), suggesting no statistically significant benefit from the intervention. This lack of benefit was also found in a subgroup analysis of patients with detectable titers of antibodies to SARS-CoV-2, and when progression to severe disease and all-cause mortality were analyzed independently across all patients.
Still, at day 7, patients treated with convalescent plasma were significantly more likely to have resolution of fatigue (RR, 1.21; 95% CI, 1.02-1.42) and shortness of breath (RR, 1.16; 95% CI, 1.02-1.32). And at the same time point, patients treated with convalescent plasma were 20% more likely to test negative for SARS-CoV-2 RNA (RR, 1.2; 95% CI, 1.04-1.5).
In an accompanying editorial, Elizabeth B. Pathak, PhD, of the Women’s Institute for Independent Social Enquiry, Olney, Md., suggested that the reported symptom improvements need to be viewed with skepticism.
“These results should be interpreted with caution, because the trial was not blinded, so knowledge of treatment status could have influenced the reporting of subjective symptoms by patients who survived to day 7,” Dr. Pathak wrote.
Dr. Pathak noted that convalescent plasma did appear to have an antiviral effect, based on the higher rate of negative RNA test results at day 7. She hypothesized that the lack of major corresponding clinical benefit could be explained by detrimental thrombotic processes.
“The net effect of plasma is prothrombotic,” Dr. Pathak wrote, which should raise safety concerns, since “COVID-19 is a life-threatening thrombotic disorder.”
According to Dr. Pathak, large-scale datasets may be giving a false sense of security. She cited a recent safety analysis of 20,000 U.S. patients who received convalescent plasma, in which the investigators excluded 88.2% of cardiac events and 66.3% of thrombotic events, as these were deemed unrelated to transfusion; but this decision was made by the treating physician, without independent review or a defined protocol.
Michael J. Joyner, MD, of the Mayo Clinic in Rochester, Minn., was the lead author of the above safety study, and is leading the Food and Drug Administration expanded access program for convalescent plasma in patients with COVID-19. He suggested that the study by Dr. Agarwal and colleagues was admirable, but flaws in the treatment protocol cast doubt upon the efficacy findings.
“It is very impressive that these investigators performed a large trial of convalescent plasma in the midst of a pandemic,” Dr. Joyner said. “Unfortunately it is unclear how generalizable the findings are because many of the units of plasma had either very low or no antibody titers and because the plasma was given late in the course of the disease. It has been known since at least the 1930s that antibody therapy works best when enough product is given either prophylactically or early in the course of disease.”
Dr. Joyner had a more positive interpretation of the reported symptom improvements.
“It is also interesting to note that while there was no mortality benefit, that – even with the limitations of the study – there was some evidence of improved patient physiology at 7 days,” he said. “So, at one level, [this is] a negative study, but at least [there are] some hints of efficacy given the suboptimal use case in the patients studied.”
The study was funded by the Indian Council of Medical Research, which employs several of the authors and PLACID Trial Collaborators. Dr. Pathak and Dr. Joyner reported no conflicts of interest.
SOURCE: Agarwal A et al. BMJ. 2020 Oct 23. doi: 10.1136/bmj.m3939 .
FROM BMJ
Low DHT linked to hip fracture in men
In older men, circulating levels of dihydrotestosterone (DHT) and sex hormone–binding globulin (SHBG) independently predict risk of hip fracture, but testosterone does not, according to a study involving more than 1,000 men.
These findings could influence clinical measurement of male hormone levels and possibly intervention for low DHT, reported lead author Emily A. Rosenberg, MD, of Brigham and Women’s Hospital in Boston and colleagues.
“Male aging is associated with a decrease in serum sex hormones, and this decline has been shown to influence bone health, although the links between androgen levels in men and bone mineral density and fracture risk remain an ongoing source of debate,” the investigators wrote in Metabolism.
According to Dr. Rosenberg and colleagues, most previous studies in this area have focused on total or bioavailable testosterone; however, DHT demonstrates greater affinity with and slower dissociation from the androgen receptor, which could translate to a more significant role in bone metabolism. With the advent of mass spectrometry–based DHT assays, it is now possible to accurately measure small concentrations of DHT in blood, they added.
Their prospective, multicenter, cohort study involved 1,128 men who were 65 years or older and without history of cardiovascular disease. Beginning in 1989-1990, participants underwent a baseline examination that included standardized medical history questionnaires, physical exam, and laboratory testing. Additional participants joined the study in 1992-1993, and in 1994-1995, a subset of participants (n = 439) underwent dual-energy x-ray absorptiometry (DXA) scanning.
Hormone assays were conducted in 2010 using frozen serum samples from 1994-1995. Testosterone and DHT were measured by liquid chromatography–tandem mass spectrometry assay, while SHBG was measured by fluoroimmunoassay.
The primary outcome, incident hip fracture, was identified from medical records through 2013. Secondary outcomes included lean body mass and bone mineral density of the hip. A variety of covariates were also recorded, including age, sex, weight, alcohol consumption, smoking status, and others.
After a median follow-up of 10.2 years (interquartile range, 5.9-15.5 years), 106 cases of hip fracture occurred, which translated to an incidence rate of 0.89 per 100 person-years. Cox regression models mutually adjusted for covariates, and the other analyses showed that each standard deviation increase in DHT correlated with a 26% decreased risk of hip fracture (adjusted hazard ratio, 0.74; 95% confidence interval, 0.55-1.00; P = .049). Conversely, each standard deviation increase in SBHG was associated with a 26% increased risk of hip fracture (aHR, 1.26; 95% CI, 1.01-1.58; P = .045). In contrast with both DHT and SBHG, testosterone was not significantly associated with the primary outcome (aHR, 1.16; 95% CI, 0.86-1.56; P = .324).
Further analysis showed that testosterone, DHT, and SBHG were not significantly associated with bone mineral density of the hip. In adjusted models, testosterone and DHT were independently associated with higher lean body mass; however, in mutually adjusted models, these associations were not statistically significant, although they remained similar and positive.
“More research is needed to determine the mechanism(s) by which DHT may affect bone health and whether interventions that regulate DHT might be used to reduce risk of hip fracture,” the investigators concluded. “While our results require confirmation, there may be a role for measurement of DHT along with testosterone when the clinical scenario requires measurement of male hormone levels.”
The study was funded by the National Heart, Lung and Blood Institute and the National Institute on Aging. The investigators reported no conflicts of interest.
SOURCE: Rosenberg EA et al. Metabolism. 2020 Oct 12. doi: 10.1016/j.metabol.2020.154399.
In older men, circulating levels of dihydrotestosterone (DHT) and sex hormone–binding globulin (SHBG) independently predict risk of hip fracture, but testosterone does not, according to a study involving more than 1,000 men.
These findings could influence clinical measurement of male hormone levels and possibly intervention for low DHT, reported lead author Emily A. Rosenberg, MD, of Brigham and Women’s Hospital in Boston and colleagues.
“Male aging is associated with a decrease in serum sex hormones, and this decline has been shown to influence bone health, although the links between androgen levels in men and bone mineral density and fracture risk remain an ongoing source of debate,” the investigators wrote in Metabolism.
According to Dr. Rosenberg and colleagues, most previous studies in this area have focused on total or bioavailable testosterone; however, DHT demonstrates greater affinity with and slower dissociation from the androgen receptor, which could translate to a more significant role in bone metabolism. With the advent of mass spectrometry–based DHT assays, it is now possible to accurately measure small concentrations of DHT in blood, they added.
Their prospective, multicenter, cohort study involved 1,128 men who were 65 years or older and without history of cardiovascular disease. Beginning in 1989-1990, participants underwent a baseline examination that included standardized medical history questionnaires, physical exam, and laboratory testing. Additional participants joined the study in 1992-1993, and in 1994-1995, a subset of participants (n = 439) underwent dual-energy x-ray absorptiometry (DXA) scanning.
Hormone assays were conducted in 2010 using frozen serum samples from 1994-1995. Testosterone and DHT were measured by liquid chromatography–tandem mass spectrometry assay, while SHBG was measured by fluoroimmunoassay.
The primary outcome, incident hip fracture, was identified from medical records through 2013. Secondary outcomes included lean body mass and bone mineral density of the hip. A variety of covariates were also recorded, including age, sex, weight, alcohol consumption, smoking status, and others.
After a median follow-up of 10.2 years (interquartile range, 5.9-15.5 years), 106 cases of hip fracture occurred, which translated to an incidence rate of 0.89 per 100 person-years. Cox regression models mutually adjusted for covariates, and the other analyses showed that each standard deviation increase in DHT correlated with a 26% decreased risk of hip fracture (adjusted hazard ratio, 0.74; 95% confidence interval, 0.55-1.00; P = .049). Conversely, each standard deviation increase in SBHG was associated with a 26% increased risk of hip fracture (aHR, 1.26; 95% CI, 1.01-1.58; P = .045). In contrast with both DHT and SBHG, testosterone was not significantly associated with the primary outcome (aHR, 1.16; 95% CI, 0.86-1.56; P = .324).
Further analysis showed that testosterone, DHT, and SBHG were not significantly associated with bone mineral density of the hip. In adjusted models, testosterone and DHT were independently associated with higher lean body mass; however, in mutually adjusted models, these associations were not statistically significant, although they remained similar and positive.
“More research is needed to determine the mechanism(s) by which DHT may affect bone health and whether interventions that regulate DHT might be used to reduce risk of hip fracture,” the investigators concluded. “While our results require confirmation, there may be a role for measurement of DHT along with testosterone when the clinical scenario requires measurement of male hormone levels.”
The study was funded by the National Heart, Lung and Blood Institute and the National Institute on Aging. The investigators reported no conflicts of interest.
SOURCE: Rosenberg EA et al. Metabolism. 2020 Oct 12. doi: 10.1016/j.metabol.2020.154399.
In older men, circulating levels of dihydrotestosterone (DHT) and sex hormone–binding globulin (SHBG) independently predict risk of hip fracture, but testosterone does not, according to a study involving more than 1,000 men.
These findings could influence clinical measurement of male hormone levels and possibly intervention for low DHT, reported lead author Emily A. Rosenberg, MD, of Brigham and Women’s Hospital in Boston and colleagues.
“Male aging is associated with a decrease in serum sex hormones, and this decline has been shown to influence bone health, although the links between androgen levels in men and bone mineral density and fracture risk remain an ongoing source of debate,” the investigators wrote in Metabolism.
According to Dr. Rosenberg and colleagues, most previous studies in this area have focused on total or bioavailable testosterone; however, DHT demonstrates greater affinity with and slower dissociation from the androgen receptor, which could translate to a more significant role in bone metabolism. With the advent of mass spectrometry–based DHT assays, it is now possible to accurately measure small concentrations of DHT in blood, they added.
Their prospective, multicenter, cohort study involved 1,128 men who were 65 years or older and without history of cardiovascular disease. Beginning in 1989-1990, participants underwent a baseline examination that included standardized medical history questionnaires, physical exam, and laboratory testing. Additional participants joined the study in 1992-1993, and in 1994-1995, a subset of participants (n = 439) underwent dual-energy x-ray absorptiometry (DXA) scanning.
Hormone assays were conducted in 2010 using frozen serum samples from 1994-1995. Testosterone and DHT were measured by liquid chromatography–tandem mass spectrometry assay, while SHBG was measured by fluoroimmunoassay.
The primary outcome, incident hip fracture, was identified from medical records through 2013. Secondary outcomes included lean body mass and bone mineral density of the hip. A variety of covariates were also recorded, including age, sex, weight, alcohol consumption, smoking status, and others.
After a median follow-up of 10.2 years (interquartile range, 5.9-15.5 years), 106 cases of hip fracture occurred, which translated to an incidence rate of 0.89 per 100 person-years. Cox regression models mutually adjusted for covariates, and the other analyses showed that each standard deviation increase in DHT correlated with a 26% decreased risk of hip fracture (adjusted hazard ratio, 0.74; 95% confidence interval, 0.55-1.00; P = .049). Conversely, each standard deviation increase in SBHG was associated with a 26% increased risk of hip fracture (aHR, 1.26; 95% CI, 1.01-1.58; P = .045). In contrast with both DHT and SBHG, testosterone was not significantly associated with the primary outcome (aHR, 1.16; 95% CI, 0.86-1.56; P = .324).
Further analysis showed that testosterone, DHT, and SBHG were not significantly associated with bone mineral density of the hip. In adjusted models, testosterone and DHT were independently associated with higher lean body mass; however, in mutually adjusted models, these associations were not statistically significant, although they remained similar and positive.
“More research is needed to determine the mechanism(s) by which DHT may affect bone health and whether interventions that regulate DHT might be used to reduce risk of hip fracture,” the investigators concluded. “While our results require confirmation, there may be a role for measurement of DHT along with testosterone when the clinical scenario requires measurement of male hormone levels.”
The study was funded by the National Heart, Lung and Blood Institute and the National Institute on Aging. The investigators reported no conflicts of interest.
SOURCE: Rosenberg EA et al. Metabolism. 2020 Oct 12. doi: 10.1016/j.metabol.2020.154399.
FROM METABOLISM
More data on impact of corticosteroids on COVID-19 mortality in patients with COPD
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
FROM LANCET RESPIRATORY MEDICINE
Creeping fat in Crohn’s linked with microbial translocation
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
FROM CELL
Vedolizumab shows long-term safety, efficacy
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at http://ow.ly/szHz30rdKyx.
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at http://ow.ly/szHz30rdKyx.
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at http://ow.ly/szHz30rdKyx.
FROM ALIMENTARY PHARMACOLOGY & THERAPEUTICS