User login
Brain/body connection: Treating depression in patients with cardiovascular disease
Depression can exacerbate cardiovascular disease (CVD), and CVD can exacerbate depression (Figure). Thus, effectively treating depression enhances heart disease treatment, particularly if psychiatrists and medical physicians collaborate in providing patient care.
This article describes a patient with risk factors for heart disease, illustrates the physiologic pathways that link depression and CVD, and offers clinical tips to help you improve outcomes for patients with both disorders.
Case report: Trying to ‘get going’
Mr. D, age 51, presents with vegetative symptoms and a personal and family history of CVD, depression, and substance abuse disorders. He was born in a small town in Kentucky and raised in Louisville’s poorest neighborhood. After his mother died at age 42 of “hardening of the arteries,” his father started drinking more, working less, and “never really got going again.”
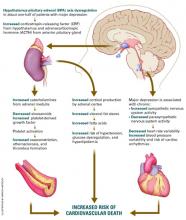
Figure Neuroendocrine pathways by which depression may cause or promote CVD
Among patients with a recent myocardial infarction (MI), as many as two-thirds report depressive symptoms.1 Major depression has been reported in:
- 16% to 22% of patients hospitalized post-MI,2,3 compared with 5% in the general population and 10% in the primary care population4
- 15% of patients with unstable angina5 and 20% of patients undergoing coronary artery bypass (CABG) surgery.6
Among the annual 1.5 million Americans who have an acute MI or unstable angina, 40% develop depression immediately thereafter. These 600,000 depressed patients are three times more likely to die within 6 months, compared with post-MI patients who are not depressed.7
Mr. D worked 20 years as a construction contractor, often running several work crews at once. At age 41, he slid into a depressive episode after his second divorce. He struggled with low energy, disturbed sleep, hopelessness, and increased smoking and drinking for 1 year, but he did not seek help.
Two years later, he suffered an inferior wall transmural myocardial infarction. His CVD risk factors included family history of early heart disease, smoking for 32 years, and elevated low-density lipoprotein (LDL) cholesterol. After subsequent episodes of unstable angina, stents were placed in two coronary arteries. Though his cardiologist cleared him to return to work, he felt able to work only part-time and erratically.
During a visit to their family doctor several years later, Mr. D’s wife suggested that her husband might be depressed. Reluctantly, Mr. D consulted a psychiatrist.
The psychiatrist diagnosed major depressive disorder and prescribed sertraline, 50 mg/d. Within 2 months, Mr. D’s symptoms had dropped by 50% on a symptom severity measure. He did not refill his prescription, however, because of concerns about sexual side effects. Two months later he was hospitalized for another episode of unstable angina. His depression had returned within 1 month of stopping sertraline.
The psychiatrist switched him to citalopram, 20 mg/d, and carefully monitored depressive symptoms, side effects, and medication adherence. Aside from talking with the psychiatrist for a half-hour in his family doctor’s office every few weeks, Mr. D refused to undergo psychotherapy. He eventually achieved depression remission with a combination of citalopram, 20 mg/d, and nefazodone, 200 mg/d.
Depression-CVD connection
As in Mr. D’s case, depression and CVD commonly occur together, often with serious consequences (Box). 1-7 The association between depression and CVD is not limited to depression’s effect on existing disease, however. Depression often precedes coronary disease by about 30 years—suggesting possible cause and effect. Two systematic reviews8,9 found that depression increased CVD risk by 64%.
Seven well-controlled studies5-7,10-13 compared the relative effect of depression on the cardiovascular system with that of established CVD predictors. All seven found depression’s independent effect to be significant and comparable to or greater than that of ejection fraction, previous MI history, or number of vessels with >50% narrowing.
Comorbid depression and CVD usually persists months or years,14 and most studies indicate a dose-response relationship; the more severe the depression, the greater the risk for CVD to develop or progress.8,15
The link between depression treatment and CVD risk has not been well-studied. The only randomized, controlled trial found that cognitive therapy for depression did not significantly reduce cardiac events among patients with known CVD.16
Possible mechanisms
Depression’s effect on CVD. How does depression affect CVD development and progression? Both behavioral and biological pathways may be involved.17 The behavioral pathway proposes that depression triggers behaviors—such as smoking, overeating, and sedentary lifestyles—that increase the risk of developing or worsening CVD. The biological pathway proposes that neuroendocrine changes during depression accelerate CVD development.
About one-half of persons with major depression exhibit hypothalamic-pituitary-adrenal (HPA) axis dysregulation, with excessive secretion of corticotropin releasing factor (CRF) and chronically elevated cortisol.18 This HPA dysregulation is related to defective negative feedback at the paraventricular nucleus of the hypothalamus. Chronic HPA axis dysregulation promotes vascular inflammation, and several studies have reported C-reactive protein elevation and cytokine changes in patients with major depression.19,20
Major depression is also associated with excessive sympathetic and diminished parasym-pathetic nervous system activity, potentially contributing to hypertension, increased resting heart rate, decreased heart rate variability, and altered endothelial function.2,21,22 Each of these factors facilitates arterial plaque formation.
Depression may also exacerbate chronic anxiety and other forms of distress. The combined effects of an overtaxed central nervous system, neuroendocrine dysregulation, and unhealthy behaviors may eventually overwhelm the cardiovascular system.
CVD’s effect on depression. How does CVD contribute to depression? The vascular depression hypothesis23 proposes that diffuse heart and brain atherosclerosis restricts perfusion of limbic and cortical structures that regulate mood. A first depressive episode after acute MI or CABG probably represents exacerbation of cerebrovascular insufficiency that preceded the coronary event.
Table
Four keys to effectively treat depression in patients with heart disease
|
In practical terms, this means that pathways linking depression and heart disease include not only biological factors but also:
- psychological factors such as depression, anxiety, and chronic stress
- behavioral factors such as smoking, physical inactivity, and high-fat diet.
How to improve outcomes
Patients with CVD commonly do not receive effective depression treatment:
- Internists and family physicians give preferential attention to physical illness.
- Patients may have insufficient access to mental health specialists.
- Physicians do not adequately monitor depression treatment.
- Patients are reluctant to accept the stigma of mental illness.
By collaborating with primary care physicians, you can improve the likelihood that depression treatment will achieve remission and prevent relapse (Table).
Risk factors for CVD. Depression contributes to heart disease by exacerbating four major CVD risk factors—smoking, diabetes, obesity, and physical inactivity. By effectively treating depression, you may help patients avoid common depressive symptoms—such as overeating and sedentary behaviors—that are related to low energy or fatigue.
Educate middle-aged patients with depression about CVD’s associated risk. Prochaska’s “stages of change” (see Related resources) can help them stop smoking, lose weight, and exercise.
Access to cardiac care. Depressed patients may be less motivated than nondepressed patients to pursue cardiac care.24 Therefore, you may need to:
- encourage your patients to take advantage of indicated state-of-the-art care, including stents, bypass surgery, and medications
- understand patients’ complex cardiac regimens and help them adhere when depression interferes with their motivation.
Effective depression treatment
Patient history. For depressed patients older than 40, take a careful inventory of CVD risk factors:
- family history of heart disease before age 60 for men and age 70 for women
- personal history of smoking, blood pressure >140/90 mm Hg, LDL cholesterol >100 mg/dL, type 2 diabetes, body mass index >30, or physical inactivity (<30 minutes of walking 3 days a week).
In general, the more risk factors, the greater the risk of CVD.
Antidepressant selection. Selective serotonin reuptake inhibitors (SSRIs) are safe and effective for treating major depression in CVD and congestive heart failure.25 Venlafaxine at doses >300 mg/d may increase blood pressure, so use this drug with caution in depressed patients with hypertension.
No controlled clinical trials have gauged the safety and efficacy of bupropion or mirtazapine in patients with CVD.
Tricyclic antidepressants are contraindicated for 6 months post-MI because they may contribute to arrhythmias. Avoid using them in depressed patients with CVD or conduction defects because of their quinidine-like effects on conduction.
Cardiac medications. Contrary to folk wisdom, beta blockers do not cause depression.26 Whether or not a patient is depressed, our primary care and cardiology colleagues can use beta blockers to help regulate the peripheral autonomic nervous system, reducing high blood pressure and the risk of arrhythmias.
SSRIs may increase blood levels of beta blockers, warfarin, and other cardiac medications via cytochrome P-450 isoenzyme inhibition. Make sure warfarin levels and other cardiac drug effects are well monitored when you adjust psychotropic dosages.
Divalproex and SSRIs also may reduce platelet aggregation. Patients who are receiving concomitant aspirin or warfarin may bruise or bleed easily and require dosage reductions or medication changes.
Psychotherapy. All patients with major or minor depression and CVD are considered high-risk and are candidates for a trial of brief psychotherapy. Therapeutic goals are to achieve full remission of depressive symptoms as rapidly as possible, prevent relapse, and maximize adherence to cardiac and depression drug regimens.
Collaborate closely with the cardiologist or primary care physician during the patient’s depressive episode and occasionally during maintenance treatment. Discuss or share notes on the patient’s depressive and cardiac disorders, medication management, symptom monitoring, and behavior changes needed to reduce cardiac risk.
With your added support, patients with depression and CVD are more likely to adhere to antidepressant medications and achieve symptom remission.
- National Institute of Mental Health. Depression and heart disease. www.nimh.nih.gov/publicat/depheart.cfm.
- Dewan NA, Suresh DP, Blomkalns A. Selecting safe psychotropics for post-MI patients. Current Psychiatry. 2003;2(3):15-21.
- Prochaska JO, Norcross JC, DiClemente CC. Changing for good. New York: Avon, 1994.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Wulsin is a consultant to Pfizer Inc. and Janssen Pharmaceutica.
Dr. Vieweg is a speaker for Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Cassem N, Hackett T. Psychiatric condition in a coronary care unit. Ann Intern Med 1971;75:9-14.
2. Glassman A, Shapiro P. Depression and the course of coronary artery disease. Am J Psychiatry 1998;155:4-11.
3. Carney R, Freedland K, Sheline Y, Weiss E. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol 1997;20:196-200.
4. Katon W, Schulbert H. Epidemiology of depression in primary care. Gen Hosp Psychiatry 1992;14:237-47.
5. Lesperance F, Frasure-Smith N, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000;160:1354-60.
6. Connerney I, Shapiro P, McLaughlin J, et al. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001;358:1766-71.
7. Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995;91:999-1005.
8. Rugulies R. Depression as a predictor for coronary heart disease. Am J Prev Med 2002;23:51-61.
9. Wulsin L, Singal B. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003;65:201-10.
10. Carney R, Rich M, Freedland K, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988;50:627-33.
11. Ladwig K, Roll G, Breithardt G, Borggrefe M. Extracardiac contributions to chest pain perception in patients 6 months after acute myocardial infarction. Am Heart J 1999;137:528-34.
12. Levine J, Covino N, Slack W, et al. Psychological predictors of subsequent medical care among patients hospitalized with cardiac disease. J Cardiopulm Rehabil 1996;16:109-16.
13. Lesperance F, Frasure-Smith N, Talajic M, Bourassa M. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002;105:1049-53.
14. Dwight M, Stoudemire A. Effects of depressive disorders on coronary artery disease: a review. Harv Rev Psychiatry 1997;5:115-122.
15. Penninx B, Beekman A, Honig A, et al. Depression and cardiac mortality. Arch Gen Psychiatry 2001;58:221-7.
16. Writing committee of the ENRICHD investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction. JAMA 2003;289:3106-16.
17. Carney RM, Freedland K, Miller G, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
18. Musselman D, Evans D, Nemeroff C. The relationship of depression to cardiovascular disease. Arch Gen Psychiatry 1998;55:580-92.
19. Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med 1999;61:476-86.
20. Miller G, Cohen S, Herbert T. Pathways linking major depression and immunity in ambulatory female patients. Psychosom Med 1999;61:850-60.
21. Carney R, Freedland K, Stein P. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med 2000;62:639-47.
22. Carney R, Freedland K, Miller G, Jaffe A. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
23. Alexopoulos G, Meyers B, Young R, et al. Vascular depression hypothesis. Psychosom Med 1997;58:113-121.
24. Ziegelstein R, Fauerbach J, Stevens S, et al. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000;160:1818-23.
25. Glassman AH, O’Connor C, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002;288:701-9.
26. Ko D, Hebert P, Coffey C, et al. B-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002;288:351-7.
Depression can exacerbate cardiovascular disease (CVD), and CVD can exacerbate depression (Figure). Thus, effectively treating depression enhances heart disease treatment, particularly if psychiatrists and medical physicians collaborate in providing patient care.
This article describes a patient with risk factors for heart disease, illustrates the physiologic pathways that link depression and CVD, and offers clinical tips to help you improve outcomes for patients with both disorders.
Case report: Trying to ‘get going’
Mr. D, age 51, presents with vegetative symptoms and a personal and family history of CVD, depression, and substance abuse disorders. He was born in a small town in Kentucky and raised in Louisville’s poorest neighborhood. After his mother died at age 42 of “hardening of the arteries,” his father started drinking more, working less, and “never really got going again.”
Figure Neuroendocrine pathways by which depression may cause or promote CVD
Among patients with a recent myocardial infarction (MI), as many as two-thirds report depressive symptoms.1 Major depression has been reported in:
- 16% to 22% of patients hospitalized post-MI,2,3 compared with 5% in the general population and 10% in the primary care population4
- 15% of patients with unstable angina5 and 20% of patients undergoing coronary artery bypass (CABG) surgery.6
Among the annual 1.5 million Americans who have an acute MI or unstable angina, 40% develop depression immediately thereafter. These 600,000 depressed patients are three times more likely to die within 6 months, compared with post-MI patients who are not depressed.7
Mr. D worked 20 years as a construction contractor, often running several work crews at once. At age 41, he slid into a depressive episode after his second divorce. He struggled with low energy, disturbed sleep, hopelessness, and increased smoking and drinking for 1 year, but he did not seek help.
Two years later, he suffered an inferior wall transmural myocardial infarction. His CVD risk factors included family history of early heart disease, smoking for 32 years, and elevated low-density lipoprotein (LDL) cholesterol. After subsequent episodes of unstable angina, stents were placed in two coronary arteries. Though his cardiologist cleared him to return to work, he felt able to work only part-time and erratically.
During a visit to their family doctor several years later, Mr. D’s wife suggested that her husband might be depressed. Reluctantly, Mr. D consulted a psychiatrist.
The psychiatrist diagnosed major depressive disorder and prescribed sertraline, 50 mg/d. Within 2 months, Mr. D’s symptoms had dropped by 50% on a symptom severity measure. He did not refill his prescription, however, because of concerns about sexual side effects. Two months later he was hospitalized for another episode of unstable angina. His depression had returned within 1 month of stopping sertraline.
The psychiatrist switched him to citalopram, 20 mg/d, and carefully monitored depressive symptoms, side effects, and medication adherence. Aside from talking with the psychiatrist for a half-hour in his family doctor’s office every few weeks, Mr. D refused to undergo psychotherapy. He eventually achieved depression remission with a combination of citalopram, 20 mg/d, and nefazodone, 200 mg/d.
Depression-CVD connection
As in Mr. D’s case, depression and CVD commonly occur together, often with serious consequences (Box). 1-7 The association between depression and CVD is not limited to depression’s effect on existing disease, however. Depression often precedes coronary disease by about 30 years—suggesting possible cause and effect. Two systematic reviews8,9 found that depression increased CVD risk by 64%.
Seven well-controlled studies5-7,10-13 compared the relative effect of depression on the cardiovascular system with that of established CVD predictors. All seven found depression’s independent effect to be significant and comparable to or greater than that of ejection fraction, previous MI history, or number of vessels with >50% narrowing.
Comorbid depression and CVD usually persists months or years,14 and most studies indicate a dose-response relationship; the more severe the depression, the greater the risk for CVD to develop or progress.8,15
The link between depression treatment and CVD risk has not been well-studied. The only randomized, controlled trial found that cognitive therapy for depression did not significantly reduce cardiac events among patients with known CVD.16
Possible mechanisms
Depression’s effect on CVD. How does depression affect CVD development and progression? Both behavioral and biological pathways may be involved.17 The behavioral pathway proposes that depression triggers behaviors—such as smoking, overeating, and sedentary lifestyles—that increase the risk of developing or worsening CVD. The biological pathway proposes that neuroendocrine changes during depression accelerate CVD development.
About one-half of persons with major depression exhibit hypothalamic-pituitary-adrenal (HPA) axis dysregulation, with excessive secretion of corticotropin releasing factor (CRF) and chronically elevated cortisol.18 This HPA dysregulation is related to defective negative feedback at the paraventricular nucleus of the hypothalamus. Chronic HPA axis dysregulation promotes vascular inflammation, and several studies have reported C-reactive protein elevation and cytokine changes in patients with major depression.19,20
Major depression is also associated with excessive sympathetic and diminished parasym-pathetic nervous system activity, potentially contributing to hypertension, increased resting heart rate, decreased heart rate variability, and altered endothelial function.2,21,22 Each of these factors facilitates arterial plaque formation.
Depression may also exacerbate chronic anxiety and other forms of distress. The combined effects of an overtaxed central nervous system, neuroendocrine dysregulation, and unhealthy behaviors may eventually overwhelm the cardiovascular system.
CVD’s effect on depression. How does CVD contribute to depression? The vascular depression hypothesis23 proposes that diffuse heart and brain atherosclerosis restricts perfusion of limbic and cortical structures that regulate mood. A first depressive episode after acute MI or CABG probably represents exacerbation of cerebrovascular insufficiency that preceded the coronary event.
Table
Four keys to effectively treat depression in patients with heart disease
|
In practical terms, this means that pathways linking depression and heart disease include not only biological factors but also:
- psychological factors such as depression, anxiety, and chronic stress
- behavioral factors such as smoking, physical inactivity, and high-fat diet.
How to improve outcomes
Patients with CVD commonly do not receive effective depression treatment:
- Internists and family physicians give preferential attention to physical illness.
- Patients may have insufficient access to mental health specialists.
- Physicians do not adequately monitor depression treatment.
- Patients are reluctant to accept the stigma of mental illness.
By collaborating with primary care physicians, you can improve the likelihood that depression treatment will achieve remission and prevent relapse (Table).
Risk factors for CVD. Depression contributes to heart disease by exacerbating four major CVD risk factors—smoking, diabetes, obesity, and physical inactivity. By effectively treating depression, you may help patients avoid common depressive symptoms—such as overeating and sedentary behaviors—that are related to low energy or fatigue.
Educate middle-aged patients with depression about CVD’s associated risk. Prochaska’s “stages of change” (see Related resources) can help them stop smoking, lose weight, and exercise.
Access to cardiac care. Depressed patients may be less motivated than nondepressed patients to pursue cardiac care.24 Therefore, you may need to:
- encourage your patients to take advantage of indicated state-of-the-art care, including stents, bypass surgery, and medications
- understand patients’ complex cardiac regimens and help them adhere when depression interferes with their motivation.
Effective depression treatment
Patient history. For depressed patients older than 40, take a careful inventory of CVD risk factors:
- family history of heart disease before age 60 for men and age 70 for women
- personal history of smoking, blood pressure >140/90 mm Hg, LDL cholesterol >100 mg/dL, type 2 diabetes, body mass index >30, or physical inactivity (<30 minutes of walking 3 days a week).
In general, the more risk factors, the greater the risk of CVD.
Antidepressant selection. Selective serotonin reuptake inhibitors (SSRIs) are safe and effective for treating major depression in CVD and congestive heart failure.25 Venlafaxine at doses >300 mg/d may increase blood pressure, so use this drug with caution in depressed patients with hypertension.
No controlled clinical trials have gauged the safety and efficacy of bupropion or mirtazapine in patients with CVD.
Tricyclic antidepressants are contraindicated for 6 months post-MI because they may contribute to arrhythmias. Avoid using them in depressed patients with CVD or conduction defects because of their quinidine-like effects on conduction.
Cardiac medications. Contrary to folk wisdom, beta blockers do not cause depression.26 Whether or not a patient is depressed, our primary care and cardiology colleagues can use beta blockers to help regulate the peripheral autonomic nervous system, reducing high blood pressure and the risk of arrhythmias.
SSRIs may increase blood levels of beta blockers, warfarin, and other cardiac medications via cytochrome P-450 isoenzyme inhibition. Make sure warfarin levels and other cardiac drug effects are well monitored when you adjust psychotropic dosages.
Divalproex and SSRIs also may reduce platelet aggregation. Patients who are receiving concomitant aspirin or warfarin may bruise or bleed easily and require dosage reductions or medication changes.
Psychotherapy. All patients with major or minor depression and CVD are considered high-risk and are candidates for a trial of brief psychotherapy. Therapeutic goals are to achieve full remission of depressive symptoms as rapidly as possible, prevent relapse, and maximize adherence to cardiac and depression drug regimens.
Collaborate closely with the cardiologist or primary care physician during the patient’s depressive episode and occasionally during maintenance treatment. Discuss or share notes on the patient’s depressive and cardiac disorders, medication management, symptom monitoring, and behavior changes needed to reduce cardiac risk.
With your added support, patients with depression and CVD are more likely to adhere to antidepressant medications and achieve symptom remission.
- National Institute of Mental Health. Depression and heart disease. www.nimh.nih.gov/publicat/depheart.cfm.
- Dewan NA, Suresh DP, Blomkalns A. Selecting safe psychotropics for post-MI patients. Current Psychiatry. 2003;2(3):15-21.
- Prochaska JO, Norcross JC, DiClemente CC. Changing for good. New York: Avon, 1994.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Wulsin is a consultant to Pfizer Inc. and Janssen Pharmaceutica.
Dr. Vieweg is a speaker for Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Depression can exacerbate cardiovascular disease (CVD), and CVD can exacerbate depression (Figure). Thus, effectively treating depression enhances heart disease treatment, particularly if psychiatrists and medical physicians collaborate in providing patient care.
This article describes a patient with risk factors for heart disease, illustrates the physiologic pathways that link depression and CVD, and offers clinical tips to help you improve outcomes for patients with both disorders.
Case report: Trying to ‘get going’
Mr. D, age 51, presents with vegetative symptoms and a personal and family history of CVD, depression, and substance abuse disorders. He was born in a small town in Kentucky and raised in Louisville’s poorest neighborhood. After his mother died at age 42 of “hardening of the arteries,” his father started drinking more, working less, and “never really got going again.”
Figure Neuroendocrine pathways by which depression may cause or promote CVD
Among patients with a recent myocardial infarction (MI), as many as two-thirds report depressive symptoms.1 Major depression has been reported in:
- 16% to 22% of patients hospitalized post-MI,2,3 compared with 5% in the general population and 10% in the primary care population4
- 15% of patients with unstable angina5 and 20% of patients undergoing coronary artery bypass (CABG) surgery.6
Among the annual 1.5 million Americans who have an acute MI or unstable angina, 40% develop depression immediately thereafter. These 600,000 depressed patients are three times more likely to die within 6 months, compared with post-MI patients who are not depressed.7
Mr. D worked 20 years as a construction contractor, often running several work crews at once. At age 41, he slid into a depressive episode after his second divorce. He struggled with low energy, disturbed sleep, hopelessness, and increased smoking and drinking for 1 year, but he did not seek help.
Two years later, he suffered an inferior wall transmural myocardial infarction. His CVD risk factors included family history of early heart disease, smoking for 32 years, and elevated low-density lipoprotein (LDL) cholesterol. After subsequent episodes of unstable angina, stents were placed in two coronary arteries. Though his cardiologist cleared him to return to work, he felt able to work only part-time and erratically.
During a visit to their family doctor several years later, Mr. D’s wife suggested that her husband might be depressed. Reluctantly, Mr. D consulted a psychiatrist.
The psychiatrist diagnosed major depressive disorder and prescribed sertraline, 50 mg/d. Within 2 months, Mr. D’s symptoms had dropped by 50% on a symptom severity measure. He did not refill his prescription, however, because of concerns about sexual side effects. Two months later he was hospitalized for another episode of unstable angina. His depression had returned within 1 month of stopping sertraline.
The psychiatrist switched him to citalopram, 20 mg/d, and carefully monitored depressive symptoms, side effects, and medication adherence. Aside from talking with the psychiatrist for a half-hour in his family doctor’s office every few weeks, Mr. D refused to undergo psychotherapy. He eventually achieved depression remission with a combination of citalopram, 20 mg/d, and nefazodone, 200 mg/d.
Depression-CVD connection
As in Mr. D’s case, depression and CVD commonly occur together, often with serious consequences (Box). 1-7 The association between depression and CVD is not limited to depression’s effect on existing disease, however. Depression often precedes coronary disease by about 30 years—suggesting possible cause and effect. Two systematic reviews8,9 found that depression increased CVD risk by 64%.
Seven well-controlled studies5-7,10-13 compared the relative effect of depression on the cardiovascular system with that of established CVD predictors. All seven found depression’s independent effect to be significant and comparable to or greater than that of ejection fraction, previous MI history, or number of vessels with >50% narrowing.
Comorbid depression and CVD usually persists months or years,14 and most studies indicate a dose-response relationship; the more severe the depression, the greater the risk for CVD to develop or progress.8,15
The link between depression treatment and CVD risk has not been well-studied. The only randomized, controlled trial found that cognitive therapy for depression did not significantly reduce cardiac events among patients with known CVD.16
Possible mechanisms
Depression’s effect on CVD. How does depression affect CVD development and progression? Both behavioral and biological pathways may be involved.17 The behavioral pathway proposes that depression triggers behaviors—such as smoking, overeating, and sedentary lifestyles—that increase the risk of developing or worsening CVD. The biological pathway proposes that neuroendocrine changes during depression accelerate CVD development.
About one-half of persons with major depression exhibit hypothalamic-pituitary-adrenal (HPA) axis dysregulation, with excessive secretion of corticotropin releasing factor (CRF) and chronically elevated cortisol.18 This HPA dysregulation is related to defective negative feedback at the paraventricular nucleus of the hypothalamus. Chronic HPA axis dysregulation promotes vascular inflammation, and several studies have reported C-reactive protein elevation and cytokine changes in patients with major depression.19,20
Major depression is also associated with excessive sympathetic and diminished parasym-pathetic nervous system activity, potentially contributing to hypertension, increased resting heart rate, decreased heart rate variability, and altered endothelial function.2,21,22 Each of these factors facilitates arterial plaque formation.
Depression may also exacerbate chronic anxiety and other forms of distress. The combined effects of an overtaxed central nervous system, neuroendocrine dysregulation, and unhealthy behaviors may eventually overwhelm the cardiovascular system.
CVD’s effect on depression. How does CVD contribute to depression? The vascular depression hypothesis23 proposes that diffuse heart and brain atherosclerosis restricts perfusion of limbic and cortical structures that regulate mood. A first depressive episode after acute MI or CABG probably represents exacerbation of cerebrovascular insufficiency that preceded the coronary event.
Table
Four keys to effectively treat depression in patients with heart disease
|
In practical terms, this means that pathways linking depression and heart disease include not only biological factors but also:
- psychological factors such as depression, anxiety, and chronic stress
- behavioral factors such as smoking, physical inactivity, and high-fat diet.
How to improve outcomes
Patients with CVD commonly do not receive effective depression treatment:
- Internists and family physicians give preferential attention to physical illness.
- Patients may have insufficient access to mental health specialists.
- Physicians do not adequately monitor depression treatment.
- Patients are reluctant to accept the stigma of mental illness.
By collaborating with primary care physicians, you can improve the likelihood that depression treatment will achieve remission and prevent relapse (Table).
Risk factors for CVD. Depression contributes to heart disease by exacerbating four major CVD risk factors—smoking, diabetes, obesity, and physical inactivity. By effectively treating depression, you may help patients avoid common depressive symptoms—such as overeating and sedentary behaviors—that are related to low energy or fatigue.
Educate middle-aged patients with depression about CVD’s associated risk. Prochaska’s “stages of change” (see Related resources) can help them stop smoking, lose weight, and exercise.
Access to cardiac care. Depressed patients may be less motivated than nondepressed patients to pursue cardiac care.24 Therefore, you may need to:
- encourage your patients to take advantage of indicated state-of-the-art care, including stents, bypass surgery, and medications
- understand patients’ complex cardiac regimens and help them adhere when depression interferes with their motivation.
Effective depression treatment
Patient history. For depressed patients older than 40, take a careful inventory of CVD risk factors:
- family history of heart disease before age 60 for men and age 70 for women
- personal history of smoking, blood pressure >140/90 mm Hg, LDL cholesterol >100 mg/dL, type 2 diabetes, body mass index >30, or physical inactivity (<30 minutes of walking 3 days a week).
In general, the more risk factors, the greater the risk of CVD.
Antidepressant selection. Selective serotonin reuptake inhibitors (SSRIs) are safe and effective for treating major depression in CVD and congestive heart failure.25 Venlafaxine at doses >300 mg/d may increase blood pressure, so use this drug with caution in depressed patients with hypertension.
No controlled clinical trials have gauged the safety and efficacy of bupropion or mirtazapine in patients with CVD.
Tricyclic antidepressants are contraindicated for 6 months post-MI because they may contribute to arrhythmias. Avoid using them in depressed patients with CVD or conduction defects because of their quinidine-like effects on conduction.
Cardiac medications. Contrary to folk wisdom, beta blockers do not cause depression.26 Whether or not a patient is depressed, our primary care and cardiology colleagues can use beta blockers to help regulate the peripheral autonomic nervous system, reducing high blood pressure and the risk of arrhythmias.
SSRIs may increase blood levels of beta blockers, warfarin, and other cardiac medications via cytochrome P-450 isoenzyme inhibition. Make sure warfarin levels and other cardiac drug effects are well monitored when you adjust psychotropic dosages.
Divalproex and SSRIs also may reduce platelet aggregation. Patients who are receiving concomitant aspirin or warfarin may bruise or bleed easily and require dosage reductions or medication changes.
Psychotherapy. All patients with major or minor depression and CVD are considered high-risk and are candidates for a trial of brief psychotherapy. Therapeutic goals are to achieve full remission of depressive symptoms as rapidly as possible, prevent relapse, and maximize adherence to cardiac and depression drug regimens.
Collaborate closely with the cardiologist or primary care physician during the patient’s depressive episode and occasionally during maintenance treatment. Discuss or share notes on the patient’s depressive and cardiac disorders, medication management, symptom monitoring, and behavior changes needed to reduce cardiac risk.
With your added support, patients with depression and CVD are more likely to adhere to antidepressant medications and achieve symptom remission.
- National Institute of Mental Health. Depression and heart disease. www.nimh.nih.gov/publicat/depheart.cfm.
- Dewan NA, Suresh DP, Blomkalns A. Selecting safe psychotropics for post-MI patients. Current Psychiatry. 2003;2(3):15-21.
- Prochaska JO, Norcross JC, DiClemente CC. Changing for good. New York: Avon, 1994.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Wulsin is a consultant to Pfizer Inc. and Janssen Pharmaceutica.
Dr. Vieweg is a speaker for Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Cassem N, Hackett T. Psychiatric condition in a coronary care unit. Ann Intern Med 1971;75:9-14.
2. Glassman A, Shapiro P. Depression and the course of coronary artery disease. Am J Psychiatry 1998;155:4-11.
3. Carney R, Freedland K, Sheline Y, Weiss E. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol 1997;20:196-200.
4. Katon W, Schulbert H. Epidemiology of depression in primary care. Gen Hosp Psychiatry 1992;14:237-47.
5. Lesperance F, Frasure-Smith N, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000;160:1354-60.
6. Connerney I, Shapiro P, McLaughlin J, et al. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001;358:1766-71.
7. Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995;91:999-1005.
8. Rugulies R. Depression as a predictor for coronary heart disease. Am J Prev Med 2002;23:51-61.
9. Wulsin L, Singal B. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003;65:201-10.
10. Carney R, Rich M, Freedland K, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988;50:627-33.
11. Ladwig K, Roll G, Breithardt G, Borggrefe M. Extracardiac contributions to chest pain perception in patients 6 months after acute myocardial infarction. Am Heart J 1999;137:528-34.
12. Levine J, Covino N, Slack W, et al. Psychological predictors of subsequent medical care among patients hospitalized with cardiac disease. J Cardiopulm Rehabil 1996;16:109-16.
13. Lesperance F, Frasure-Smith N, Talajic M, Bourassa M. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002;105:1049-53.
14. Dwight M, Stoudemire A. Effects of depressive disorders on coronary artery disease: a review. Harv Rev Psychiatry 1997;5:115-122.
15. Penninx B, Beekman A, Honig A, et al. Depression and cardiac mortality. Arch Gen Psychiatry 2001;58:221-7.
16. Writing committee of the ENRICHD investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction. JAMA 2003;289:3106-16.
17. Carney RM, Freedland K, Miller G, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
18. Musselman D, Evans D, Nemeroff C. The relationship of depression to cardiovascular disease. Arch Gen Psychiatry 1998;55:580-92.
19. Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med 1999;61:476-86.
20. Miller G, Cohen S, Herbert T. Pathways linking major depression and immunity in ambulatory female patients. Psychosom Med 1999;61:850-60.
21. Carney R, Freedland K, Stein P. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med 2000;62:639-47.
22. Carney R, Freedland K, Miller G, Jaffe A. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
23. Alexopoulos G, Meyers B, Young R, et al. Vascular depression hypothesis. Psychosom Med 1997;58:113-121.
24. Ziegelstein R, Fauerbach J, Stevens S, et al. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000;160:1818-23.
25. Glassman AH, O’Connor C, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002;288:701-9.
26. Ko D, Hebert P, Coffey C, et al. B-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002;288:351-7.
1. Cassem N, Hackett T. Psychiatric condition in a coronary care unit. Ann Intern Med 1971;75:9-14.
2. Glassman A, Shapiro P. Depression and the course of coronary artery disease. Am J Psychiatry 1998;155:4-11.
3. Carney R, Freedland K, Sheline Y, Weiss E. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol 1997;20:196-200.
4. Katon W, Schulbert H. Epidemiology of depression in primary care. Gen Hosp Psychiatry 1992;14:237-47.
5. Lesperance F, Frasure-Smith N, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000;160:1354-60.
6. Connerney I, Shapiro P, McLaughlin J, et al. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001;358:1766-71.
7. Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995;91:999-1005.
8. Rugulies R. Depression as a predictor for coronary heart disease. Am J Prev Med 2002;23:51-61.
9. Wulsin L, Singal B. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003;65:201-10.
10. Carney R, Rich M, Freedland K, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988;50:627-33.
11. Ladwig K, Roll G, Breithardt G, Borggrefe M. Extracardiac contributions to chest pain perception in patients 6 months after acute myocardial infarction. Am Heart J 1999;137:528-34.
12. Levine J, Covino N, Slack W, et al. Psychological predictors of subsequent medical care among patients hospitalized with cardiac disease. J Cardiopulm Rehabil 1996;16:109-16.
13. Lesperance F, Frasure-Smith N, Talajic M, Bourassa M. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002;105:1049-53.
14. Dwight M, Stoudemire A. Effects of depressive disorders on coronary artery disease: a review. Harv Rev Psychiatry 1997;5:115-122.
15. Penninx B, Beekman A, Honig A, et al. Depression and cardiac mortality. Arch Gen Psychiatry 2001;58:221-7.
16. Writing committee of the ENRICHD investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction. JAMA 2003;289:3106-16.
17. Carney RM, Freedland K, Miller G, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
18. Musselman D, Evans D, Nemeroff C. The relationship of depression to cardiovascular disease. Arch Gen Psychiatry 1998;55:580-92.
19. Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med 1999;61:476-86.
20. Miller G, Cohen S, Herbert T. Pathways linking major depression and immunity in ambulatory female patients. Psychosom Med 1999;61:850-60.
21. Carney R, Freedland K, Stein P. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med 2000;62:639-47.
22. Carney R, Freedland K, Miller G, Jaffe A. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
23. Alexopoulos G, Meyers B, Young R, et al. Vascular depression hypothesis. Psychosom Med 1997;58:113-121.
24. Ziegelstein R, Fauerbach J, Stevens S, et al. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000;160:1818-23.
25. Glassman AH, O’Connor C, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002;288:701-9.
26. Ko D, Hebert P, Coffey C, et al. B-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002;288:351-7.
How to prevent hyperprolactinemia in patients taking antipsychotics
Antipsychotics have long been linked with hyperprolactinemia.1 This phenomenon was first considered a drug class effect, but the arrival of clozapine, better deliniation of dopamine receptor subtypes, and identification of the four principal CNS dopamine pathways revealed that hyperprolactinemia was not a universal consequence of antipsychotic use.
We now know that most atypical antipsychotics are less likely to induce hyperprolactinemia than older antipsychotics, but we don’t know why. The most likely explanation is that most of the newer agents block dopamine D2 minimally in the hypothalamic tuberoinfundibular pathway.2 Evidence is emerging that atypical agents elevate serum prolactin levels at least transiently—but usually less than typical antipsychotics—and this effect varies, depending on each compound’s dopamine-binding properties.
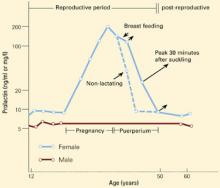
Figure 1 CHANGES IN PROLACTIN LEVELS OVER TIME
Mean serum prolactin concentrations from puberty until menopause. For nursing women, the length of the arrows depicts the increase in serum prolactin concentration associated with each episode of suckling. The Y-axis expresses serum prolactin concentration in both ng/ml and mg/l.
Source: Adapted and reprinted with permission from Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
Prolactin physiology
Prolactin—a large peptide containing 198 amino acids—was the first anterior pituitary gland hormone to be isolated in pure form.3 Despite its molecular weight of approximately 23,000, the hormone easily crosses the blood-brain barrier.4
Similar to other anterior pituitary hormones, prolactin is secreted episodically. Its secretion is inhibited by dopamine release from the hypothalamus and enhanced by different prolactin-releasing factors. Prolactin is the only anterior pituitary hormone that is produced by tuberoinfundibular neurons governed by dopamine.5 Dopamine stimulates lactotrope D2 receptors and inhibits adenylate cyclase, resulting in reduced prolactin synthesis and release.
Serum prolactin concentrations change during various life stages (Figure 1).6 Estrogen’s effects on prolactin gene expression regulate prolactin synthesis, resulting in higher prolactin levels in premenopausal women than in men.
Prolactin secretion
Normally, prolactin is secreted in pulses—approximately 14 in a 24-hour period, with an interpulse interval of about 80 minutes.5 A bimodal daily pattern of secretion is superimposed upon this pattern, with peak levels at night and trough levels at noon. Stress—including surgery and general anesthesia, exercise, and hypoglycemia—may transiently increase prolactin levels.
Endocrine regulation. Estrogen modulates the response of hypothalamic factors that control prolactin production. It stimulates decreased prolactin response to dopamine and increased response to thyrotropic-releasing hormone.
Insulin also stimulates prolactin secretion—probably by inducing hypoglycemia. Serum insulin level changes within physiologic ranges appear to affect prolactin regulation.
Neuroendocrine regulation. The hypothalamus blunts prolactin secretion primarily via dopamine release. This modulation occurs principally within the tuberoinfundibular dopamine pathway. The D2 subtype is the only dopamine receptor in the anterior pituitary gland:
- a decrease in dopamine levels reaching the anterior pituitary gland increases the number of D2 receptors
- to a lesser extent, estrogen decreases the number of D2 receptors.
Dopamine-modulated reductions in action potential discharge from lactotrophs and in calcium flux leads to decreased intracellular calcium and decreased prolactin secretion.5
Most hormones are target-organ agents and are regulated via a feedback loop that includes the peripheral circulation. Prolactin, however, is not considered to have a specific target organ. It is its own inhibiting factor, using an autoregulatory, pituitary-to-hypothalamus short-loop feedback circuit.
For example, prolactin-secreting tumors or drugs that elevate hormone levels lead to an increase in dopamine. In contrast, hypophysectomy decreases dopamine. In this setting, prolactin injections will restore normal dopamine levels. Prolactin-releasing factors include thyrotropic-releasing hormone, vasoactive intestinal peptide, and serotonin.
Prolactin’s actions
Many tissues—including breast, liver, ovary, testis, and prostate—have prolactin receptors. These receptors are stimulated with equal potency by prolactin and growth hormone.
The principal site of prolactin action is the mammary gland, where the hormone initiates and maintains lactation after childbirth. Major stimuli for breast development are estrogen, progesterone, prolactin, and placental mammotropic hormones. Other stimuli include insulin, cortisol, and thyroid hormone.7
Gonadotropin secretion is influenced by prolactin via the hypothalamus. Prolactin-mediated inhibition of luteinizing hormone-releasing hormone secretion impairs gonadotropin release and inhibits gonadal function.
Table 1
COMMON CLINICAL EFFECTS IN PATIENTS WITH HYPERPROLACTINEMIA
| Organ or syndrome | Clinical effects |
|---|---|
| Behavior | Direct effects Secondary effects due to hypogonadism Possible cognitive impairment |
| Bones | Decreased bone mineral density due to testosterone or estrogen deficits |
| Breast | Engorgement Lactation unrelated to breast feeding |
| Cardiovascular system | Possible adverse effects due to low levels of testosterone or estrogen |
| Menstrual function | Absence of ovulation Amenorrhea |
| Sexual function | Reduced libido Reduced arousal Orgasmic dysfunction |
| Source: Adapted and reprinted with permission from Dickson RA, Glazer WM. Neurolepticinduced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86. | |
Diagnosis of hyperprolactinemia
Pathologic hyperprolactinemia is defined as consistently elevated serum prolactin concentration (>20 ng/ml) in the absence of pregnancy or postpartum lactation. Because of the pulsatile nature of prolactin secretion, a definitive diagnosis of hyperprolactinemia requires three serum prolactin levels taken on different mornings.
Clinical presentation. Hyperprolactinemia—the most common hypothalamic-pituitary disturbance—usually presents with clinical features of gonadal dysfunction (Table 1).8 Symptoms and signs related to a brain mass—headache, visual field disturbances, ophthalmoplegia, and reduced visual acuity—may predominate with a large pituitary tumor. The patient may first present to a primary care physician or to a clinical specialist, such as a gynecologist, neurologist, ophthalmologist, pediatrician, psychiatrist, or urologist.
Thirty to 80% of women with hyperprolactinemia develop galactorrhea,9 although some women with galactorrhea have normal prolactin levels. Men with hyperprolactinemia usually have gonadal dysfunction, which unfortunately is often attributed to “psychogenic” causes. Particularly in men, prolactin is implicated in the control of libido.
Causes. Hyperprolactinemia may be caused by any process that inhibits dopamine synthesis, the neurotransmitter’s transport to the anterior pituitary gland, or its action at the lactotrope dopamine receptors ( Table 2).9 In this article, we will limit our discussion to antipsychotic drugs. have long-term effects on bone density. Trabecular bone mass
Estrogen and prolactin. During pregnancy, the rise in estrogen levels probably stimulates an increase in prolactin. Increased prolactin levels are also found in women taking estrogen-containing oral contraceptives, although this effect is very small with low-estrogen formulations.
Table 2
CAUSES OF PATHOLOGIC HYPERPROLACTINEMIA
| Hypothalamic disease | Tumor, infiltrative disease, pseudotumor cerebri, cranial radiation |
| Pituitary disease | Prolactinoma, acromegaly, Cushing’s disease, glycoprotein-producing tumor, other tumors, pituitary stalk section, empty sella, infiltrative disease |
| Medications | Antipsychotics, dopamine receptor blockers, antidepressants, antihypertensives, estrogens, opiates, cimetidine |
| Others | Primary hypothyroidism, chronic renal failure, cirrhosis, neurogenic and idiopathic causes |
| Source: Adapted and reprinted from Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders, 1995:394-405. Copyright 1995, with permission from Elsevier Science. | |
Functions of the pituitary lactotrophs regulated by estrogen include prolactin gene expression, release, storage, and cellular expression.2 Estradiol inhibition of dopamine synthesis in the tuberoinfundibular dopaminergic neurons may contribute to some gender differences in neurocognitive function and to psychiatric conditions’ clinical features.
Hyperprolactinemia and bone density. Besides causing galactorrhea and sexual dysfunction, hyperprolactinemia may has been found to be reduced in young women with amenorrhea secondary to hyperprolactinemia. This trabecular osteopenia is reversible—spinal bone density decreases progressively without treatment and improves when hyperprolactinemia is treated. Menstrual function appears to best predict risk of progressive spinal osteopenia in women with hyperprolactinemia. Estradiol level is a stronger predictor of clinical course than is the prolactin level.10
Antipsychotic drugs and hyperprolactinemia
Among the four principal dopamine pathways in the brain, the tuberoinfundibular pathway is a system of short axons at the base of the hypothalamus that releases dopamine into the portal veins of the pituitary gland. Terminals in the median eminence of the hypothalamus release dopamine that travels down the pituitary stalk in the portal veins.
Typical antipsychotics block dopamine receptors both in the striatum and in the hypothalamus.11 This finding suggests that the older drugs lack specificity of dopamine blockade. Prolactin elevations in patients treated with older antipsychotics may be associated with sexual dysfunction—a common cause of drug noncompliance, particularly in men.12
Antipsychotics and sexual side effects. Patients taking antipsy-chotics often complain—spontaneously or after focused questioning—of sexual side effects caused by drug-induced hyperprolactinemia. Assessing antipsychotic-induced sexual dysfunction may be confounded by the psychoses being treated, patient compliance, and sexuality’s complexities. Antipsychotics are generally believed to reduce desire, cause orgasmic dysfunction, and lead to difficulties during sexual performance.8
Atypical antipsychotics
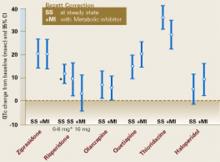
A recent study designed to assess the effect of three atypical antipsychotics on serum prolactin levels enrolled 18 men with schizophrenia (mean age 32) taking clozapine, 300 to 400 mg/d; risperidone, 1 to 3 mg/d; or olanzapine, 10 to 20 mg/d, for at least 8 weeks.13 The study participants were instructed not to take their antipsychotics the night before the study. Baseline prolactin levels were measured in the morning, the men took the full daily dose of their medications, and prolactin levels were measured every 60 minutes over the next 8 hours and again at 24 hours.
Mean baseline prolactin values of clozapine (9 ng/ml, SD=5) and olanzapine (9 ng/ml, SD=5) were in the normal range (<20 ng/ml), compared with those of risperidone (27 ng/ml, SD=14). Three of the six patients taking risperidone had hyperprolactinemia at baseline. Prolactin values doubled within 6 hours of administration of all three medications. There was no comparable increase in prolactin levels in five control subjects not taking antipsychotics.
The authors concluded that these atypical antipsychotics raise prolactin levels but more transiently than typical antipsychotics. They suggested that the differences among the three drugs may be attributed to each drug’s binding properties to pituitary dopamine D2 receptors. A similar study in four patients with first-episode schizophrenia found serum prolactin levels increased from <10 ng/ml at baseline to peak levels of 80 to 120 ng/ml within 60 to 90 minutes after patients took a full daily dose of quetiapine, 700 to 800 mg/d.14
Risperidone. A study sponsored by Janssen Pharmaceutica15 reviewed the manufacturer’s experience with prolactin and its potential to induce side effects, using data from premarketing studies comparing risperidone with haloperidol. Amenorrhea and galactorrhea were assessed in women; ejaculatory dysfunction, erectile dysfunction, and gynecomastia were assessed in men.
Table 3
HYPERPROLACTINEMIA-RELATED SIDE EFFECTS REPORTED BY PATIENTS TAKING RISPERIDONE AND OLANZAPINE
| Gender and complaint | Taking risperidone (%) | Taking olanzapine (%) | Difference (P-value) |
|---|---|---|---|
| Women | |||
| Galactorrhea | 11 of 47 (23.4%) | 11 of 49 (22.4%) | 1.00 |
| Amenorrhea | 11 of 46 (23.9%) | 9 of 45 (20.0%) | 0.80 |
| Men | |||
| Gynecomastia | 9 of 115 (7.8%) | 4 of 115 (3.5%) | 0.25 |
| Sexual dysfunction | 36 of 115 (31.3%) | 34 of 114 (29.8%) | 0.89 |
| Source: Adapted and reprinted with permission from Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry2001;158:765-74. | |||
Both risperidone and haloperidol were associated with dose-related increases in plasma prolactin concentration in men and women. In women, neither risperidone dosage nor end-point prolactin concentrations were correlated with adverse events. In men:
- adverse events did not correlate with plasma prolactin concentrations
- the incidence of adverse events was dose-related
- the incidence of adverse events associated with risperidone, 4 to 10 mg/d, was not significantly greater than in patients taking placebo.
Another Janssen-sponsored study compared potential hyperprolactinemia-related side effects of risperidone and olanzapine but did not report prolactin concentrations. The authors found no significant differences between the drugs, based on breast features/menstrual changes in women and chest features/sexual dysfunction in men (Table 3).16
Olanzapine. A study sponsored by Eli Lilly and Co.17 assessed the effects of olanzapine on prolactin concentration in women previously treated with risperidone. The authors enrolled 20 Korean women with schizophrenia treated with risperidone (mean dosage 3.5 mg/d) and complaining of menstrual disturbances, galactorrhea, and/or sexual dysfunction. The mean serum prolactin concentration with risperidone was 132.2 ng/ml.
Over 2 weeks, patients were switched from risperidone to olanzapine (mean dosage 9.1 mg/d). After 8 weeks, the mean serum prolactin concentration was measured at 23.4 ng/ml. The authors noted improved menstrual function and reduced sexual side effects with olanzapine.
Conclusion
The package inserts of all atypical antipsychotics list hyperprolactinemia as a potential risk in patients taking these medications. The clinical significance of hyperprolactinemia associated with antipsychotic use is being explored but requires further elucidation.
Based on our understanding of the long-term course of untreated hyperprolactinemia—derived largely from patients not taking antipsychotics—it seems reasonable to ask patients taking atypical antipsychotics at least once a year about chest/breast complaints and sexual dysfunction. This recommendation would seem particularly relevant in patients taking risperidone at dosages >6 mg/d for sustained periods. In the absence of specific complaints, hyperprolactinemia associated with risperidone should be evaluated case by case, including perhaps endocrinology consultation.
Related resources
- Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J Clin Psychiatry 2002;63(suppl 4):56-62.
- Smith S, Wheeler MJ, Murray R, O’Keane V. The effects of antipsychotic-induced hyperprolactinemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002;22(2):109-14. Available at: http://www.psychiatry.wustl.edu/Resources/LiteratureList/2002/May/Smith.PDF.
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
Dr. Vieweg reports that he is on the speakers bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Clemens JA, Smalstig EB, Sawyer BD. Antipsychotic drugs stimulate prolactin release. Psychopharmacol. 1974;40:123-7.
2. Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res 1999;35(suppl):S67-S73.
3. West ES, Todd WR. The hormones. In: West ES, Todd WR (eds). Textbook of biochemistry. New York: The Macmillan Co., 1961;1315-54.
4. Belchetz PE, Ridley RM, Baker HF. Studies on the accessibility of prolactin and growth hormone to brain: effect of opiate agonists on hormone levels in serial, simultaneous plasma and cerebrospinal fluid samples in the rhesus monkey. Brain Res 1982;239:310-4.
5. Cooke NE. Prolactin: basic physiology. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Company, 1995;368-93.
6. Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
7. Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K. The anterior pituitary. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds). Williams textbook of endocrinology. Philadelphia: W.B. Saunders Co., 1998;249-340.
8. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86.
9. Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Co., 1995;394-405.
10. Biller BM, Baum HB, Rosenthal DI, Saxe VC, Charpie PM, Kilibanski A. Progressive trabecular osteopenia in women with hyperprolactinemic amenorrhea. J Clin Endocrinol Metab 1992;75:692-7.
11. Baron JC, Martinot JL, Cambon H, et al. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacol 1989;99:463-72.
12. Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis 1982;170:463-7.
13. Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 2002;159:133-5.
14. Alexiadis M, Whitehorn D, Woodley H, Kopala L. Prolactin elevation with quetiapine (letter). Am J Psychiatry 2002;159(Sept):1608-9.
15. Kleinberg DL, Davis JM, De Coster R, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999;19:57-61.
16. Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001;158:765-74.
17. Kim K, Pae C, Chae J, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry 2002;63:408-13.
Antipsychotics have long been linked with hyperprolactinemia.1 This phenomenon was first considered a drug class effect, but the arrival of clozapine, better deliniation of dopamine receptor subtypes, and identification of the four principal CNS dopamine pathways revealed that hyperprolactinemia was not a universal consequence of antipsychotic use.
We now know that most atypical antipsychotics are less likely to induce hyperprolactinemia than older antipsychotics, but we don’t know why. The most likely explanation is that most of the newer agents block dopamine D2 minimally in the hypothalamic tuberoinfundibular pathway.2 Evidence is emerging that atypical agents elevate serum prolactin levels at least transiently—but usually less than typical antipsychotics—and this effect varies, depending on each compound’s dopamine-binding properties.
Figure 1 CHANGES IN PROLACTIN LEVELS OVER TIME
Mean serum prolactin concentrations from puberty until menopause. For nursing women, the length of the arrows depicts the increase in serum prolactin concentration associated with each episode of suckling. The Y-axis expresses serum prolactin concentration in both ng/ml and mg/l.
Source: Adapted and reprinted with permission from Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
Prolactin physiology
Prolactin—a large peptide containing 198 amino acids—was the first anterior pituitary gland hormone to be isolated in pure form.3 Despite its molecular weight of approximately 23,000, the hormone easily crosses the blood-brain barrier.4
Similar to other anterior pituitary hormones, prolactin is secreted episodically. Its secretion is inhibited by dopamine release from the hypothalamus and enhanced by different prolactin-releasing factors. Prolactin is the only anterior pituitary hormone that is produced by tuberoinfundibular neurons governed by dopamine.5 Dopamine stimulates lactotrope D2 receptors and inhibits adenylate cyclase, resulting in reduced prolactin synthesis and release.
Serum prolactin concentrations change during various life stages (Figure 1).6 Estrogen’s effects on prolactin gene expression regulate prolactin synthesis, resulting in higher prolactin levels in premenopausal women than in men.
Prolactin secretion
Normally, prolactin is secreted in pulses—approximately 14 in a 24-hour period, with an interpulse interval of about 80 minutes.5 A bimodal daily pattern of secretion is superimposed upon this pattern, with peak levels at night and trough levels at noon. Stress—including surgery and general anesthesia, exercise, and hypoglycemia—may transiently increase prolactin levels.
Endocrine regulation. Estrogen modulates the response of hypothalamic factors that control prolactin production. It stimulates decreased prolactin response to dopamine and increased response to thyrotropic-releasing hormone.
Insulin also stimulates prolactin secretion—probably by inducing hypoglycemia. Serum insulin level changes within physiologic ranges appear to affect prolactin regulation.
Neuroendocrine regulation. The hypothalamus blunts prolactin secretion primarily via dopamine release. This modulation occurs principally within the tuberoinfundibular dopamine pathway. The D2 subtype is the only dopamine receptor in the anterior pituitary gland:
- a decrease in dopamine levels reaching the anterior pituitary gland increases the number of D2 receptors
- to a lesser extent, estrogen decreases the number of D2 receptors.
Dopamine-modulated reductions in action potential discharge from lactotrophs and in calcium flux leads to decreased intracellular calcium and decreased prolactin secretion.5
Most hormones are target-organ agents and are regulated via a feedback loop that includes the peripheral circulation. Prolactin, however, is not considered to have a specific target organ. It is its own inhibiting factor, using an autoregulatory, pituitary-to-hypothalamus short-loop feedback circuit.
For example, prolactin-secreting tumors or drugs that elevate hormone levels lead to an increase in dopamine. In contrast, hypophysectomy decreases dopamine. In this setting, prolactin injections will restore normal dopamine levels. Prolactin-releasing factors include thyrotropic-releasing hormone, vasoactive intestinal peptide, and serotonin.
Prolactin’s actions
Many tissues—including breast, liver, ovary, testis, and prostate—have prolactin receptors. These receptors are stimulated with equal potency by prolactin and growth hormone.
The principal site of prolactin action is the mammary gland, where the hormone initiates and maintains lactation after childbirth. Major stimuli for breast development are estrogen, progesterone, prolactin, and placental mammotropic hormones. Other stimuli include insulin, cortisol, and thyroid hormone.7
Gonadotropin secretion is influenced by prolactin via the hypothalamus. Prolactin-mediated inhibition of luteinizing hormone-releasing hormone secretion impairs gonadotropin release and inhibits gonadal function.
Table 1
COMMON CLINICAL EFFECTS IN PATIENTS WITH HYPERPROLACTINEMIA
| Organ or syndrome | Clinical effects |
|---|---|
| Behavior | Direct effects Secondary effects due to hypogonadism Possible cognitive impairment |
| Bones | Decreased bone mineral density due to testosterone or estrogen deficits |
| Breast | Engorgement Lactation unrelated to breast feeding |
| Cardiovascular system | Possible adverse effects due to low levels of testosterone or estrogen |
| Menstrual function | Absence of ovulation Amenorrhea |
| Sexual function | Reduced libido Reduced arousal Orgasmic dysfunction |
| Source: Adapted and reprinted with permission from Dickson RA, Glazer WM. Neurolepticinduced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86. | |
Diagnosis of hyperprolactinemia
Pathologic hyperprolactinemia is defined as consistently elevated serum prolactin concentration (>20 ng/ml) in the absence of pregnancy or postpartum lactation. Because of the pulsatile nature of prolactin secretion, a definitive diagnosis of hyperprolactinemia requires three serum prolactin levels taken on different mornings.
Clinical presentation. Hyperprolactinemia—the most common hypothalamic-pituitary disturbance—usually presents with clinical features of gonadal dysfunction (Table 1).8 Symptoms and signs related to a brain mass—headache, visual field disturbances, ophthalmoplegia, and reduced visual acuity—may predominate with a large pituitary tumor. The patient may first present to a primary care physician or to a clinical specialist, such as a gynecologist, neurologist, ophthalmologist, pediatrician, psychiatrist, or urologist.
Thirty to 80% of women with hyperprolactinemia develop galactorrhea,9 although some women with galactorrhea have normal prolactin levels. Men with hyperprolactinemia usually have gonadal dysfunction, which unfortunately is often attributed to “psychogenic” causes. Particularly in men, prolactin is implicated in the control of libido.
Causes. Hyperprolactinemia may be caused by any process that inhibits dopamine synthesis, the neurotransmitter’s transport to the anterior pituitary gland, or its action at the lactotrope dopamine receptors ( Table 2).9 In this article, we will limit our discussion to antipsychotic drugs. have long-term effects on bone density. Trabecular bone mass
Estrogen and prolactin. During pregnancy, the rise in estrogen levels probably stimulates an increase in prolactin. Increased prolactin levels are also found in women taking estrogen-containing oral contraceptives, although this effect is very small with low-estrogen formulations.
Table 2
CAUSES OF PATHOLOGIC HYPERPROLACTINEMIA
| Hypothalamic disease | Tumor, infiltrative disease, pseudotumor cerebri, cranial radiation |
| Pituitary disease | Prolactinoma, acromegaly, Cushing’s disease, glycoprotein-producing tumor, other tumors, pituitary stalk section, empty sella, infiltrative disease |
| Medications | Antipsychotics, dopamine receptor blockers, antidepressants, antihypertensives, estrogens, opiates, cimetidine |
| Others | Primary hypothyroidism, chronic renal failure, cirrhosis, neurogenic and idiopathic causes |
| Source: Adapted and reprinted from Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders, 1995:394-405. Copyright 1995, with permission from Elsevier Science. | |
Functions of the pituitary lactotrophs regulated by estrogen include prolactin gene expression, release, storage, and cellular expression.2 Estradiol inhibition of dopamine synthesis in the tuberoinfundibular dopaminergic neurons may contribute to some gender differences in neurocognitive function and to psychiatric conditions’ clinical features.
Hyperprolactinemia and bone density. Besides causing galactorrhea and sexual dysfunction, hyperprolactinemia may has been found to be reduced in young women with amenorrhea secondary to hyperprolactinemia. This trabecular osteopenia is reversible—spinal bone density decreases progressively without treatment and improves when hyperprolactinemia is treated. Menstrual function appears to best predict risk of progressive spinal osteopenia in women with hyperprolactinemia. Estradiol level is a stronger predictor of clinical course than is the prolactin level.10
Antipsychotic drugs and hyperprolactinemia
Among the four principal dopamine pathways in the brain, the tuberoinfundibular pathway is a system of short axons at the base of the hypothalamus that releases dopamine into the portal veins of the pituitary gland. Terminals in the median eminence of the hypothalamus release dopamine that travels down the pituitary stalk in the portal veins.
Typical antipsychotics block dopamine receptors both in the striatum and in the hypothalamus.11 This finding suggests that the older drugs lack specificity of dopamine blockade. Prolactin elevations in patients treated with older antipsychotics may be associated with sexual dysfunction—a common cause of drug noncompliance, particularly in men.12
Antipsychotics and sexual side effects. Patients taking antipsy-chotics often complain—spontaneously or after focused questioning—of sexual side effects caused by drug-induced hyperprolactinemia. Assessing antipsychotic-induced sexual dysfunction may be confounded by the psychoses being treated, patient compliance, and sexuality’s complexities. Antipsychotics are generally believed to reduce desire, cause orgasmic dysfunction, and lead to difficulties during sexual performance.8
Atypical antipsychotics
A recent study designed to assess the effect of three atypical antipsychotics on serum prolactin levels enrolled 18 men with schizophrenia (mean age 32) taking clozapine, 300 to 400 mg/d; risperidone, 1 to 3 mg/d; or olanzapine, 10 to 20 mg/d, for at least 8 weeks.13 The study participants were instructed not to take their antipsychotics the night before the study. Baseline prolactin levels were measured in the morning, the men took the full daily dose of their medications, and prolactin levels were measured every 60 minutes over the next 8 hours and again at 24 hours.
Mean baseline prolactin values of clozapine (9 ng/ml, SD=5) and olanzapine (9 ng/ml, SD=5) were in the normal range (<20 ng/ml), compared with those of risperidone (27 ng/ml, SD=14). Three of the six patients taking risperidone had hyperprolactinemia at baseline. Prolactin values doubled within 6 hours of administration of all three medications. There was no comparable increase in prolactin levels in five control subjects not taking antipsychotics.
The authors concluded that these atypical antipsychotics raise prolactin levels but more transiently than typical antipsychotics. They suggested that the differences among the three drugs may be attributed to each drug’s binding properties to pituitary dopamine D2 receptors. A similar study in four patients with first-episode schizophrenia found serum prolactin levels increased from <10 ng/ml at baseline to peak levels of 80 to 120 ng/ml within 60 to 90 minutes after patients took a full daily dose of quetiapine, 700 to 800 mg/d.14
Risperidone. A study sponsored by Janssen Pharmaceutica15 reviewed the manufacturer’s experience with prolactin and its potential to induce side effects, using data from premarketing studies comparing risperidone with haloperidol. Amenorrhea and galactorrhea were assessed in women; ejaculatory dysfunction, erectile dysfunction, and gynecomastia were assessed in men.
Table 3
HYPERPROLACTINEMIA-RELATED SIDE EFFECTS REPORTED BY PATIENTS TAKING RISPERIDONE AND OLANZAPINE
| Gender and complaint | Taking risperidone (%) | Taking olanzapine (%) | Difference (P-value) |
|---|---|---|---|
| Women | |||
| Galactorrhea | 11 of 47 (23.4%) | 11 of 49 (22.4%) | 1.00 |
| Amenorrhea | 11 of 46 (23.9%) | 9 of 45 (20.0%) | 0.80 |
| Men | |||
| Gynecomastia | 9 of 115 (7.8%) | 4 of 115 (3.5%) | 0.25 |
| Sexual dysfunction | 36 of 115 (31.3%) | 34 of 114 (29.8%) | 0.89 |
| Source: Adapted and reprinted with permission from Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry2001;158:765-74. | |||
Both risperidone and haloperidol were associated with dose-related increases in plasma prolactin concentration in men and women. In women, neither risperidone dosage nor end-point prolactin concentrations were correlated with adverse events. In men:
- adverse events did not correlate with plasma prolactin concentrations
- the incidence of adverse events was dose-related
- the incidence of adverse events associated with risperidone, 4 to 10 mg/d, was not significantly greater than in patients taking placebo.
Another Janssen-sponsored study compared potential hyperprolactinemia-related side effects of risperidone and olanzapine but did not report prolactin concentrations. The authors found no significant differences between the drugs, based on breast features/menstrual changes in women and chest features/sexual dysfunction in men (Table 3).16
Olanzapine. A study sponsored by Eli Lilly and Co.17 assessed the effects of olanzapine on prolactin concentration in women previously treated with risperidone. The authors enrolled 20 Korean women with schizophrenia treated with risperidone (mean dosage 3.5 mg/d) and complaining of menstrual disturbances, galactorrhea, and/or sexual dysfunction. The mean serum prolactin concentration with risperidone was 132.2 ng/ml.
Over 2 weeks, patients were switched from risperidone to olanzapine (mean dosage 9.1 mg/d). After 8 weeks, the mean serum prolactin concentration was measured at 23.4 ng/ml. The authors noted improved menstrual function and reduced sexual side effects with olanzapine.
Conclusion
The package inserts of all atypical antipsychotics list hyperprolactinemia as a potential risk in patients taking these medications. The clinical significance of hyperprolactinemia associated with antipsychotic use is being explored but requires further elucidation.
Based on our understanding of the long-term course of untreated hyperprolactinemia—derived largely from patients not taking antipsychotics—it seems reasonable to ask patients taking atypical antipsychotics at least once a year about chest/breast complaints and sexual dysfunction. This recommendation would seem particularly relevant in patients taking risperidone at dosages >6 mg/d for sustained periods. In the absence of specific complaints, hyperprolactinemia associated with risperidone should be evaluated case by case, including perhaps endocrinology consultation.
Related resources
- Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J Clin Psychiatry 2002;63(suppl 4):56-62.
- Smith S, Wheeler MJ, Murray R, O’Keane V. The effects of antipsychotic-induced hyperprolactinemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002;22(2):109-14. Available at: http://www.psychiatry.wustl.edu/Resources/LiteratureList/2002/May/Smith.PDF.
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
Dr. Vieweg reports that he is on the speakers bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Antipsychotics have long been linked with hyperprolactinemia.1 This phenomenon was first considered a drug class effect, but the arrival of clozapine, better deliniation of dopamine receptor subtypes, and identification of the four principal CNS dopamine pathways revealed that hyperprolactinemia was not a universal consequence of antipsychotic use.
We now know that most atypical antipsychotics are less likely to induce hyperprolactinemia than older antipsychotics, but we don’t know why. The most likely explanation is that most of the newer agents block dopamine D2 minimally in the hypothalamic tuberoinfundibular pathway.2 Evidence is emerging that atypical agents elevate serum prolactin levels at least transiently—but usually less than typical antipsychotics—and this effect varies, depending on each compound’s dopamine-binding properties.
Figure 1 CHANGES IN PROLACTIN LEVELS OVER TIME
Mean serum prolactin concentrations from puberty until menopause. For nursing women, the length of the arrows depicts the increase in serum prolactin concentration associated with each episode of suckling. The Y-axis expresses serum prolactin concentration in both ng/ml and mg/l.
Source: Adapted and reprinted with permission from Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
Prolactin physiology
Prolactin—a large peptide containing 198 amino acids—was the first anterior pituitary gland hormone to be isolated in pure form.3 Despite its molecular weight of approximately 23,000, the hormone easily crosses the blood-brain barrier.4
Similar to other anterior pituitary hormones, prolactin is secreted episodically. Its secretion is inhibited by dopamine release from the hypothalamus and enhanced by different prolactin-releasing factors. Prolactin is the only anterior pituitary hormone that is produced by tuberoinfundibular neurons governed by dopamine.5 Dopamine stimulates lactotrope D2 receptors and inhibits adenylate cyclase, resulting in reduced prolactin synthesis and release.
Serum prolactin concentrations change during various life stages (Figure 1).6 Estrogen’s effects on prolactin gene expression regulate prolactin synthesis, resulting in higher prolactin levels in premenopausal women than in men.
Prolactin secretion
Normally, prolactin is secreted in pulses—approximately 14 in a 24-hour period, with an interpulse interval of about 80 minutes.5 A bimodal daily pattern of secretion is superimposed upon this pattern, with peak levels at night and trough levels at noon. Stress—including surgery and general anesthesia, exercise, and hypoglycemia—may transiently increase prolactin levels.
Endocrine regulation. Estrogen modulates the response of hypothalamic factors that control prolactin production. It stimulates decreased prolactin response to dopamine and increased response to thyrotropic-releasing hormone.
Insulin also stimulates prolactin secretion—probably by inducing hypoglycemia. Serum insulin level changes within physiologic ranges appear to affect prolactin regulation.
Neuroendocrine regulation. The hypothalamus blunts prolactin secretion primarily via dopamine release. This modulation occurs principally within the tuberoinfundibular dopamine pathway. The D2 subtype is the only dopamine receptor in the anterior pituitary gland:
- a decrease in dopamine levels reaching the anterior pituitary gland increases the number of D2 receptors
- to a lesser extent, estrogen decreases the number of D2 receptors.
Dopamine-modulated reductions in action potential discharge from lactotrophs and in calcium flux leads to decreased intracellular calcium and decreased prolactin secretion.5
Most hormones are target-organ agents and are regulated via a feedback loop that includes the peripheral circulation. Prolactin, however, is not considered to have a specific target organ. It is its own inhibiting factor, using an autoregulatory, pituitary-to-hypothalamus short-loop feedback circuit.
For example, prolactin-secreting tumors or drugs that elevate hormone levels lead to an increase in dopamine. In contrast, hypophysectomy decreases dopamine. In this setting, prolactin injections will restore normal dopamine levels. Prolactin-releasing factors include thyrotropic-releasing hormone, vasoactive intestinal peptide, and serotonin.
Prolactin’s actions
Many tissues—including breast, liver, ovary, testis, and prostate—have prolactin receptors. These receptors are stimulated with equal potency by prolactin and growth hormone.
The principal site of prolactin action is the mammary gland, where the hormone initiates and maintains lactation after childbirth. Major stimuli for breast development are estrogen, progesterone, prolactin, and placental mammotropic hormones. Other stimuli include insulin, cortisol, and thyroid hormone.7
Gonadotropin secretion is influenced by prolactin via the hypothalamus. Prolactin-mediated inhibition of luteinizing hormone-releasing hormone secretion impairs gonadotropin release and inhibits gonadal function.
Table 1
COMMON CLINICAL EFFECTS IN PATIENTS WITH HYPERPROLACTINEMIA
| Organ or syndrome | Clinical effects |
|---|---|
| Behavior | Direct effects Secondary effects due to hypogonadism Possible cognitive impairment |
| Bones | Decreased bone mineral density due to testosterone or estrogen deficits |
| Breast | Engorgement Lactation unrelated to breast feeding |
| Cardiovascular system | Possible adverse effects due to low levels of testosterone or estrogen |
| Menstrual function | Absence of ovulation Amenorrhea |
| Sexual function | Reduced libido Reduced arousal Orgasmic dysfunction |
| Source: Adapted and reprinted with permission from Dickson RA, Glazer WM. Neurolepticinduced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86. | |
Diagnosis of hyperprolactinemia
Pathologic hyperprolactinemia is defined as consistently elevated serum prolactin concentration (>20 ng/ml) in the absence of pregnancy or postpartum lactation. Because of the pulsatile nature of prolactin secretion, a definitive diagnosis of hyperprolactinemia requires three serum prolactin levels taken on different mornings.
Clinical presentation. Hyperprolactinemia—the most common hypothalamic-pituitary disturbance—usually presents with clinical features of gonadal dysfunction (Table 1).8 Symptoms and signs related to a brain mass—headache, visual field disturbances, ophthalmoplegia, and reduced visual acuity—may predominate with a large pituitary tumor. The patient may first present to a primary care physician or to a clinical specialist, such as a gynecologist, neurologist, ophthalmologist, pediatrician, psychiatrist, or urologist.
Thirty to 80% of women with hyperprolactinemia develop galactorrhea,9 although some women with galactorrhea have normal prolactin levels. Men with hyperprolactinemia usually have gonadal dysfunction, which unfortunately is often attributed to “psychogenic” causes. Particularly in men, prolactin is implicated in the control of libido.
Causes. Hyperprolactinemia may be caused by any process that inhibits dopamine synthesis, the neurotransmitter’s transport to the anterior pituitary gland, or its action at the lactotrope dopamine receptors ( Table 2).9 In this article, we will limit our discussion to antipsychotic drugs. have long-term effects on bone density. Trabecular bone mass
Estrogen and prolactin. During pregnancy, the rise in estrogen levels probably stimulates an increase in prolactin. Increased prolactin levels are also found in women taking estrogen-containing oral contraceptives, although this effect is very small with low-estrogen formulations.
Table 2
CAUSES OF PATHOLOGIC HYPERPROLACTINEMIA
| Hypothalamic disease | Tumor, infiltrative disease, pseudotumor cerebri, cranial radiation |
| Pituitary disease | Prolactinoma, acromegaly, Cushing’s disease, glycoprotein-producing tumor, other tumors, pituitary stalk section, empty sella, infiltrative disease |
| Medications | Antipsychotics, dopamine receptor blockers, antidepressants, antihypertensives, estrogens, opiates, cimetidine |
| Others | Primary hypothyroidism, chronic renal failure, cirrhosis, neurogenic and idiopathic causes |
| Source: Adapted and reprinted from Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders, 1995:394-405. Copyright 1995, with permission from Elsevier Science. | |
Functions of the pituitary lactotrophs regulated by estrogen include prolactin gene expression, release, storage, and cellular expression.2 Estradiol inhibition of dopamine synthesis in the tuberoinfundibular dopaminergic neurons may contribute to some gender differences in neurocognitive function and to psychiatric conditions’ clinical features.
Hyperprolactinemia and bone density. Besides causing galactorrhea and sexual dysfunction, hyperprolactinemia may has been found to be reduced in young women with amenorrhea secondary to hyperprolactinemia. This trabecular osteopenia is reversible—spinal bone density decreases progressively without treatment and improves when hyperprolactinemia is treated. Menstrual function appears to best predict risk of progressive spinal osteopenia in women with hyperprolactinemia. Estradiol level is a stronger predictor of clinical course than is the prolactin level.10
Antipsychotic drugs and hyperprolactinemia
Among the four principal dopamine pathways in the brain, the tuberoinfundibular pathway is a system of short axons at the base of the hypothalamus that releases dopamine into the portal veins of the pituitary gland. Terminals in the median eminence of the hypothalamus release dopamine that travels down the pituitary stalk in the portal veins.
Typical antipsychotics block dopamine receptors both in the striatum and in the hypothalamus.11 This finding suggests that the older drugs lack specificity of dopamine blockade. Prolactin elevations in patients treated with older antipsychotics may be associated with sexual dysfunction—a common cause of drug noncompliance, particularly in men.12
Antipsychotics and sexual side effects. Patients taking antipsy-chotics often complain—spontaneously or after focused questioning—of sexual side effects caused by drug-induced hyperprolactinemia. Assessing antipsychotic-induced sexual dysfunction may be confounded by the psychoses being treated, patient compliance, and sexuality’s complexities. Antipsychotics are generally believed to reduce desire, cause orgasmic dysfunction, and lead to difficulties during sexual performance.8
Atypical antipsychotics
A recent study designed to assess the effect of three atypical antipsychotics on serum prolactin levels enrolled 18 men with schizophrenia (mean age 32) taking clozapine, 300 to 400 mg/d; risperidone, 1 to 3 mg/d; or olanzapine, 10 to 20 mg/d, for at least 8 weeks.13 The study participants were instructed not to take their antipsychotics the night before the study. Baseline prolactin levels were measured in the morning, the men took the full daily dose of their medications, and prolactin levels were measured every 60 minutes over the next 8 hours and again at 24 hours.
Mean baseline prolactin values of clozapine (9 ng/ml, SD=5) and olanzapine (9 ng/ml, SD=5) were in the normal range (<20 ng/ml), compared with those of risperidone (27 ng/ml, SD=14). Three of the six patients taking risperidone had hyperprolactinemia at baseline. Prolactin values doubled within 6 hours of administration of all three medications. There was no comparable increase in prolactin levels in five control subjects not taking antipsychotics.
The authors concluded that these atypical antipsychotics raise prolactin levels but more transiently than typical antipsychotics. They suggested that the differences among the three drugs may be attributed to each drug’s binding properties to pituitary dopamine D2 receptors. A similar study in four patients with first-episode schizophrenia found serum prolactin levels increased from <10 ng/ml at baseline to peak levels of 80 to 120 ng/ml within 60 to 90 minutes after patients took a full daily dose of quetiapine, 700 to 800 mg/d.14
Risperidone. A study sponsored by Janssen Pharmaceutica15 reviewed the manufacturer’s experience with prolactin and its potential to induce side effects, using data from premarketing studies comparing risperidone with haloperidol. Amenorrhea and galactorrhea were assessed in women; ejaculatory dysfunction, erectile dysfunction, and gynecomastia were assessed in men.
Table 3
HYPERPROLACTINEMIA-RELATED SIDE EFFECTS REPORTED BY PATIENTS TAKING RISPERIDONE AND OLANZAPINE
| Gender and complaint | Taking risperidone (%) | Taking olanzapine (%) | Difference (P-value) |
|---|---|---|---|
| Women | |||
| Galactorrhea | 11 of 47 (23.4%) | 11 of 49 (22.4%) | 1.00 |
| Amenorrhea | 11 of 46 (23.9%) | 9 of 45 (20.0%) | 0.80 |
| Men | |||
| Gynecomastia | 9 of 115 (7.8%) | 4 of 115 (3.5%) | 0.25 |
| Sexual dysfunction | 36 of 115 (31.3%) | 34 of 114 (29.8%) | 0.89 |
| Source: Adapted and reprinted with permission from Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry2001;158:765-74. | |||
Both risperidone and haloperidol were associated with dose-related increases in plasma prolactin concentration in men and women. In women, neither risperidone dosage nor end-point prolactin concentrations were correlated with adverse events. In men:
- adverse events did not correlate with plasma prolactin concentrations
- the incidence of adverse events was dose-related
- the incidence of adverse events associated with risperidone, 4 to 10 mg/d, was not significantly greater than in patients taking placebo.
Another Janssen-sponsored study compared potential hyperprolactinemia-related side effects of risperidone and olanzapine but did not report prolactin concentrations. The authors found no significant differences between the drugs, based on breast features/menstrual changes in women and chest features/sexual dysfunction in men (Table 3).16
Olanzapine. A study sponsored by Eli Lilly and Co.17 assessed the effects of olanzapine on prolactin concentration in women previously treated with risperidone. The authors enrolled 20 Korean women with schizophrenia treated with risperidone (mean dosage 3.5 mg/d) and complaining of menstrual disturbances, galactorrhea, and/or sexual dysfunction. The mean serum prolactin concentration with risperidone was 132.2 ng/ml.
Over 2 weeks, patients were switched from risperidone to olanzapine (mean dosage 9.1 mg/d). After 8 weeks, the mean serum prolactin concentration was measured at 23.4 ng/ml. The authors noted improved menstrual function and reduced sexual side effects with olanzapine.
Conclusion
The package inserts of all atypical antipsychotics list hyperprolactinemia as a potential risk in patients taking these medications. The clinical significance of hyperprolactinemia associated with antipsychotic use is being explored but requires further elucidation.
Based on our understanding of the long-term course of untreated hyperprolactinemia—derived largely from patients not taking antipsychotics—it seems reasonable to ask patients taking atypical antipsychotics at least once a year about chest/breast complaints and sexual dysfunction. This recommendation would seem particularly relevant in patients taking risperidone at dosages >6 mg/d for sustained periods. In the absence of specific complaints, hyperprolactinemia associated with risperidone should be evaluated case by case, including perhaps endocrinology consultation.
Related resources
- Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J Clin Psychiatry 2002;63(suppl 4):56-62.
- Smith S, Wheeler MJ, Murray R, O’Keane V. The effects of antipsychotic-induced hyperprolactinemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002;22(2):109-14. Available at: http://www.psychiatry.wustl.edu/Resources/LiteratureList/2002/May/Smith.PDF.
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
Dr. Vieweg reports that he is on the speakers bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Clemens JA, Smalstig EB, Sawyer BD. Antipsychotic drugs stimulate prolactin release. Psychopharmacol. 1974;40:123-7.
2. Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res 1999;35(suppl):S67-S73.
3. West ES, Todd WR. The hormones. In: West ES, Todd WR (eds). Textbook of biochemistry. New York: The Macmillan Co., 1961;1315-54.
4. Belchetz PE, Ridley RM, Baker HF. Studies on the accessibility of prolactin and growth hormone to brain: effect of opiate agonists on hormone levels in serial, simultaneous plasma and cerebrospinal fluid samples in the rhesus monkey. Brain Res 1982;239:310-4.
5. Cooke NE. Prolactin: basic physiology. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Company, 1995;368-93.
6. Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
7. Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K. The anterior pituitary. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds). Williams textbook of endocrinology. Philadelphia: W.B. Saunders Co., 1998;249-340.
8. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86.
9. Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Co., 1995;394-405.
10. Biller BM, Baum HB, Rosenthal DI, Saxe VC, Charpie PM, Kilibanski A. Progressive trabecular osteopenia in women with hyperprolactinemic amenorrhea. J Clin Endocrinol Metab 1992;75:692-7.
11. Baron JC, Martinot JL, Cambon H, et al. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacol 1989;99:463-72.
12. Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis 1982;170:463-7.
13. Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 2002;159:133-5.
14. Alexiadis M, Whitehorn D, Woodley H, Kopala L. Prolactin elevation with quetiapine (letter). Am J Psychiatry 2002;159(Sept):1608-9.
15. Kleinberg DL, Davis JM, De Coster R, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999;19:57-61.
16. Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001;158:765-74.
17. Kim K, Pae C, Chae J, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry 2002;63:408-13.
1. Clemens JA, Smalstig EB, Sawyer BD. Antipsychotic drugs stimulate prolactin release. Psychopharmacol. 1974;40:123-7.
2. Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res 1999;35(suppl):S67-S73.
3. West ES, Todd WR. The hormones. In: West ES, Todd WR (eds). Textbook of biochemistry. New York: The Macmillan Co., 1961;1315-54.
4. Belchetz PE, Ridley RM, Baker HF. Studies on the accessibility of prolactin and growth hormone to brain: effect of opiate agonists on hormone levels in serial, simultaneous plasma and cerebrospinal fluid samples in the rhesus monkey. Brain Res 1982;239:310-4.
5. Cooke NE. Prolactin: basic physiology. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Company, 1995;368-93.
6. Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
7. Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K. The anterior pituitary. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds). Williams textbook of endocrinology. Philadelphia: W.B. Saunders Co., 1998;249-340.
8. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86.
9. Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Co., 1995;394-405.
10. Biller BM, Baum HB, Rosenthal DI, Saxe VC, Charpie PM, Kilibanski A. Progressive trabecular osteopenia in women with hyperprolactinemic amenorrhea. J Clin Endocrinol Metab 1992;75:692-7.
11. Baron JC, Martinot JL, Cambon H, et al. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacol 1989;99:463-72.
12. Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis 1982;170:463-7.
13. Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 2002;159:133-5.
14. Alexiadis M, Whitehorn D, Woodley H, Kopala L. Prolactin elevation with quetiapine (letter). Am J Psychiatry 2002;159(Sept):1608-9.
15. Kleinberg DL, Davis JM, De Coster R, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999;19:57-61.
16. Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001;158:765-74.
17. Kim K, Pae C, Chae J, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry 2002;63:408-13.
How to prevent hyperprolactinemia in patients taking antipsychotics
Antipsychotics have long been linked with hyperprolactinemia.1 This phenomenon was first considered a drug class effect, but the arrival of clozapine, better deliniation of dopamine receptor subtypes, and identification of the four principal CNS dopamine pathways revealed that hyperprolactinemia was not a universal consequence of antipsychotic use.
We now know that most atypical antipsychotics are less likely to induce hyperprolactinemia than older antipsychotics, but we don’t know why. The most likely explanation is that most of the newer agents block dopamine D2 minimally in the hypothalamic tuberoinfundibular pathway.2 Evidence is emerging that atypical agents elevate serum prolactin levels at least transiently—but usually less than typical antipsychotics—and this effect varies, depending on each compound’s dopamine-binding properties.
Figure 1 CHANGES IN PROLACTIN LEVELS OVER TIME
Mean serum prolactin concentrations from puberty until menopause. For nursing women, the length of the arrows depicts the increase in serum prolactin concentration associated with each episode of suckling. The Y-axis expresses serum prolactin concentration in both ng/ml and mg/l.
Source: Adapted and reprinted with permission from Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
Prolactin physiology
Prolactin—a large peptide containing 198 amino acids—was the first anterior pituitary gland hormone to be isolated in pure form.3 Despite its molecular weight of approximately 23,000, the hormone easily crosses the blood-brain barrier.4
Similar to other anterior pituitary hormones, prolactin is secreted episodically. Its secretion is inhibited by dopamine release from the hypothalamus and enhanced by different prolactin-releasing factors. Prolactin is the only anterior pituitary hormone that is produced by tuberoinfundibular neurons governed by dopamine.5 Dopamine stimulates lactotrope D2 receptors and inhibits adenylate cyclase, resulting in reduced prolactin synthesis and release.
Serum prolactin concentrations change during various life stages (Figure 1).6 Estrogen’s effects on prolactin gene expression regulate prolactin synthesis, resulting in higher prolactin levels in premenopausal women than in men.
Prolactin secretion
Normally, prolactin is secreted in pulses—approximately 14 in a 24-hour period, with an interpulse interval of about 80 minutes.5 A bimodal daily pattern of secretion is superimposed upon this pattern, with peak levels at night and trough levels at noon. Stress—including surgery and general anesthesia, exercise, and hypoglycemia—may transiently increase prolactin levels.
Endocrine regulation. Estrogen modulates the response of hypothalamic factors that control prolactin production. It stimulates decreased prolactin response to dopamine and increased response to thyrotropic-releasing hormone.
Insulin also stimulates prolactin secretion—probably by inducing hypoglycemia. Serum insulin level changes within physiologic ranges appear to affect prolactin regulation.
Neuroendocrine regulation. The hypothalamus blunts prolactin secretion primarily via dopamine release. This modulation occurs principally within the tuberoinfundibular dopamine pathway. The D2 subtype is the only dopamine receptor in the anterior pituitary gland:
- a decrease in dopamine levels reaching the anterior pituitary gland increases the number of D2 receptors
- to a lesser extent, estrogen decreases the number of D2 receptors.
Dopamine-modulated reductions in action potential discharge from lactotrophs and in calcium flux leads to decreased intracellular calcium and decreased prolactin secretion.5
Most hormones are target-organ agents and are regulated via a feedback loop that includes the peripheral circulation. Prolactin, however, is not considered to have a specific target organ. It is its own inhibiting factor, using an autoregulatory, pituitary-to-hypothalamus short-loop feedback circuit.
For example, prolactin-secreting tumors or drugs that elevate hormone levels lead to an increase in dopamine. In contrast, hypophysectomy decreases dopamine. In this setting, prolactin injections will restore normal dopamine levels. Prolactin-releasing factors include thyrotropic-releasing hormone, vasoactive intestinal peptide, and serotonin.
Prolactin’s actions
Many tissues—including breast, liver, ovary, testis, and prostate—have prolactin receptors. These receptors are stimulated with equal potency by prolactin and growth hormone.
The principal site of prolactin action is the mammary gland, where the hormone initiates and maintains lactation after childbirth. Major stimuli for breast development are estrogen, progesterone, prolactin, and placental mammotropic hormones. Other stimuli include insulin, cortisol, and thyroid hormone.7
Gonadotropin secretion is influenced by prolactin via the hypothalamus. Prolactin-mediated inhibition of luteinizing hormone-releasing hormone secretion impairs gonadotropin release and inhibits gonadal function.
Table 1
COMMON CLINICAL EFFECTS IN PATIENTS WITH HYPERPROLACTINEMIA
| Organ or syndrome | Clinical effects |
|---|---|
| Behavior | Direct effects Secondary effects due to hypogonadism Possible cognitive impairment |
| Bones | Decreased bone mineral density due to testosterone or estrogen deficits |
| Breast | Engorgement Lactation unrelated to breast feeding |
| Cardiovascular system | Possible adverse effects due to low levels of testosterone or estrogen |
| Menstrual function | Absence of ovulation Amenorrhea |
| Sexual function | Reduced libido Reduced arousal Orgasmic dysfunction |
| Source: Adapted and reprinted with permission from Dickson RA, Glazer WM. Neurolepticinduced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86. | |
Diagnosis of hyperprolactinemia
Pathologic hyperprolactinemia is defined as consistently elevated serum prolactin concentration (>20 ng/ml) in the absence of pregnancy or postpartum lactation. Because of the pulsatile nature of prolactin secretion, a definitive diagnosis of hyperprolactinemia requires three serum prolactin levels taken on different mornings.
Clinical presentation. Hyperprolactinemia—the most common hypothalamic-pituitary disturbance—usually presents with clinical features of gonadal dysfunction (Table 1).8 Symptoms and signs related to a brain mass—headache, visual field disturbances, ophthalmoplegia, and reduced visual acuity—may predominate with a large pituitary tumor. The patient may first present to a primary care physician or to a clinical specialist, such as a gynecologist, neurologist, ophthalmologist, pediatrician, psychiatrist, or urologist.
Thirty to 80% of women with hyperprolactinemia develop galactorrhea,9 although some women with galactorrhea have normal prolactin levels. Men with hyperprolactinemia usually have gonadal dysfunction, which unfortunately is often attributed to “psychogenic” causes. Particularly in men, prolactin is implicated in the control of libido.
Causes. Hyperprolactinemia may be caused by any process that inhibits dopamine synthesis, the neurotransmitter’s transport to the anterior pituitary gland, or its action at the lactotrope dopamine receptors ( Table 2).9 In this article, we will limit our discussion to antipsychotic drugs. have long-term effects on bone density. Trabecular bone mass
Estrogen and prolactin. During pregnancy, the rise in estrogen levels probably stimulates an increase in prolactin. Increased prolactin levels are also found in women taking estrogen-containing oral contraceptives, although this effect is very small with low-estrogen formulations.
Table 2
CAUSES OF PATHOLOGIC HYPERPROLACTINEMIA
| Hypothalamic disease | Tumor, infiltrative disease, pseudotumor cerebri, cranial radiation |
| Pituitary disease | Prolactinoma, acromegaly, Cushing’s disease, glycoprotein-producing tumor, other tumors, pituitary stalk section, empty sella, infiltrative disease |
| Medications | Antipsychotics, dopamine receptor blockers, antidepressants, antihypertensives, estrogens, opiates, cimetidine |
| Others | Primary hypothyroidism, chronic renal failure, cirrhosis, neurogenic and idiopathic causes |
| Source: Adapted and reprinted from Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders, 1995:394-405. Copyright 1995, with permission from Elsevier Science. | |
Functions of the pituitary lactotrophs regulated by estrogen include prolactin gene expression, release, storage, and cellular expression.2 Estradiol inhibition of dopamine synthesis in the tuberoinfundibular dopaminergic neurons may contribute to some gender differences in neurocognitive function and to psychiatric conditions’ clinical features.
Hyperprolactinemia and bone density. Besides causing galactorrhea and sexual dysfunction, hyperprolactinemia may has been found to be reduced in young women with amenorrhea secondary to hyperprolactinemia. This trabecular osteopenia is reversible—spinal bone density decreases progressively without treatment and improves when hyperprolactinemia is treated. Menstrual function appears to best predict risk of progressive spinal osteopenia in women with hyperprolactinemia. Estradiol level is a stronger predictor of clinical course than is the prolactin level.10
Antipsychotic drugs and hyperprolactinemia
Among the four principal dopamine pathways in the brain, the tuberoinfundibular pathway is a system of short axons at the base of the hypothalamus that releases dopamine into the portal veins of the pituitary gland. Terminals in the median eminence of the hypothalamus release dopamine that travels down the pituitary stalk in the portal veins.
Typical antipsychotics block dopamine receptors both in the striatum and in the hypothalamus.11 This finding suggests that the older drugs lack specificity of dopamine blockade. Prolactin elevations in patients treated with older antipsychotics may be associated with sexual dysfunction—a common cause of drug noncompliance, particularly in men.12
Antipsychotics and sexual side effects. Patients taking antipsy-chotics often complain—spontaneously or after focused questioning—of sexual side effects caused by drug-induced hyperprolactinemia. Assessing antipsychotic-induced sexual dysfunction may be confounded by the psychoses being treated, patient compliance, and sexuality’s complexities. Antipsychotics are generally believed to reduce desire, cause orgasmic dysfunction, and lead to difficulties during sexual performance.8
Atypical antipsychotics
A recent study designed to assess the effect of three atypical antipsychotics on serum prolactin levels enrolled 18 men with schizophrenia (mean age 32) taking clozapine, 300 to 400 mg/d; risperidone, 1 to 3 mg/d; or olanzapine, 10 to 20 mg/d, for at least 8 weeks.13 The study participants were instructed not to take their antipsychotics the night before the study. Baseline prolactin levels were measured in the morning, the men took the full daily dose of their medications, and prolactin levels were measured every 60 minutes over the next 8 hours and again at 24 hours.
Mean baseline prolactin values of clozapine (9 ng/ml, SD=5) and olanzapine (9 ng/ml, SD=5) were in the normal range (<20 ng/ml), compared with those of risperidone (27 ng/ml, SD=14). Three of the six patients taking risperidone had hyperprolactinemia at baseline. Prolactin values doubled within 6 hours of administration of all three medications. There was no comparable increase in prolactin levels in five control subjects not taking antipsychotics.
The authors concluded that these atypical antipsychotics raise prolactin levels but more transiently than typical antipsychotics. They suggested that the differences among the three drugs may be attributed to each drug’s binding properties to pituitary dopamine D2 receptors. A similar study in four patients with first-episode schizophrenia found serum prolactin levels increased from <10 ng/ml at baseline to peak levels of 80 to 120 ng/ml within 60 to 90 minutes after patients took a full daily dose of quetiapine, 700 to 800 mg/d.14
Risperidone. A study sponsored by Janssen Pharmaceutica15 reviewed the manufacturer’s experience with prolactin and its potential to induce side effects, using data from premarketing studies comparing risperidone with haloperidol. Amenorrhea and galactorrhea were assessed in women; ejaculatory dysfunction, erectile dysfunction, and gynecomastia were assessed in men.
Table 3
HYPERPROLACTINEMIA-RELATED SIDE EFFECTS REPORTED BY PATIENTS TAKING RISPERIDONE AND OLANZAPINE
| Gender and complaint | Taking risperidone (%) | Taking olanzapine (%) | Difference (P-value) |
|---|---|---|---|
| Women | |||
| Galactorrhea | 11 of 47 (23.4%) | 11 of 49 (22.4%) | 1.00 |
| Amenorrhea | 11 of 46 (23.9%) | 9 of 45 (20.0%) | 0.80 |
| Men | |||
| Gynecomastia | 9 of 115 (7.8%) | 4 of 115 (3.5%) | 0.25 |
| Sexual dysfunction | 36 of 115 (31.3%) | 34 of 114 (29.8%) | 0.89 |
| Source: Adapted and reprinted with permission from Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry2001;158:765-74. | |||
Both risperidone and haloperidol were associated with dose-related increases in plasma prolactin concentration in men and women. In women, neither risperidone dosage nor end-point prolactin concentrations were correlated with adverse events. In men:
- adverse events did not correlate with plasma prolactin concentrations
- the incidence of adverse events was dose-related
- the incidence of adverse events associated with risperidone, 4 to 10 mg/d, was not significantly greater than in patients taking placebo.
Another Janssen-sponsored study compared potential hyperprolactinemia-related side effects of risperidone and olanzapine but did not report prolactin concentrations. The authors found no significant differences between the drugs, based on breast features/menstrual changes in women and chest features/sexual dysfunction in men (Table 3).16
Olanzapine. A study sponsored by Eli Lilly and Co.17 assessed the effects of olanzapine on prolactin concentration in women previously treated with risperidone. The authors enrolled 20 Korean women with schizophrenia treated with risperidone (mean dosage 3.5 mg/d) and complaining of menstrual disturbances, galactorrhea, and/or sexual dysfunction. The mean serum prolactin concentration with risperidone was 132.2 ng/ml.
Over 2 weeks, patients were switched from risperidone to olanzapine (mean dosage 9.1 mg/d). After 8 weeks, the mean serum prolactin concentration was measured at 23.4 ng/ml. The authors noted improved menstrual function and reduced sexual side effects with olanzapine.
Conclusion
The package inserts of all atypical antipsychotics list hyperprolactinemia as a potential risk in patients taking these medications. The clinical significance of hyperprolactinemia associated with antipsychotic use is being explored but requires further elucidation.
Based on our understanding of the long-term course of untreated hyperprolactinemia—derived largely from patients not taking antipsychotics—it seems reasonable to ask patients taking atypical antipsychotics at least once a year about chest/breast complaints and sexual dysfunction. This recommendation would seem particularly relevant in patients taking risperidone at dosages >6 mg/d for sustained periods. In the absence of specific complaints, hyperprolactinemia associated with risperidone should be evaluated case by case, including perhaps endocrinology consultation.
Related resources
- Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J Clin Psychiatry 2002;63(suppl 4):56-62.
- Smith S, Wheeler MJ, Murray R, O’Keane V. The effects of antipsychotic-induced hyperprolactinemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002;22(2):109-14. Available at: http://www.psychiatry.wustl.edu/Resources/LiteratureList/2002/May/Smith.PDF.
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
Dr. Vieweg reports that he is on the speakers bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Clemens JA, Smalstig EB, Sawyer BD. Antipsychotic drugs stimulate prolactin release. Psychopharmacol. 1974;40:123-7.
2. Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res 1999;35(suppl):S67-S73.
3. West ES, Todd WR. The hormones. In: West ES, Todd WR (eds). Textbook of biochemistry. New York: The Macmillan Co., 1961;1315-54.
4. Belchetz PE, Ridley RM, Baker HF. Studies on the accessibility of prolactin and growth hormone to brain: effect of opiate agonists on hormone levels in serial, simultaneous plasma and cerebrospinal fluid samples in the rhesus monkey. Brain Res 1982;239:310-4.
5. Cooke NE. Prolactin: basic physiology. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Company, 1995;368-93.
6. Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
7. Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K. The anterior pituitary. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds). Williams textbook of endocrinology. Philadelphia: W.B. Saunders Co., 1998;249-340.
8. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86.
9. Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Co., 1995;394-405.
10. Biller BM, Baum HB, Rosenthal DI, Saxe VC, Charpie PM, Kilibanski A. Progressive trabecular osteopenia in women with hyperprolactinemic amenorrhea. J Clin Endocrinol Metab 1992;75:692-7.
11. Baron JC, Martinot JL, Cambon H, et al. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacol 1989;99:463-72.
12. Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis 1982;170:463-7.
13. Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 2002;159:133-5.
14. Alexiadis M, Whitehorn D, Woodley H, Kopala L. Prolactin elevation with quetiapine (letter). Am J Psychiatry 2002;159(Sept):1608-9.
15. Kleinberg DL, Davis JM, De Coster R, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999;19:57-61.
16. Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001;158:765-74.
17. Kim K, Pae C, Chae J, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry 2002;63:408-13.
Antipsychotics have long been linked with hyperprolactinemia.1 This phenomenon was first considered a drug class effect, but the arrival of clozapine, better deliniation of dopamine receptor subtypes, and identification of the four principal CNS dopamine pathways revealed that hyperprolactinemia was not a universal consequence of antipsychotic use.
We now know that most atypical antipsychotics are less likely to induce hyperprolactinemia than older antipsychotics, but we don’t know why. The most likely explanation is that most of the newer agents block dopamine D2 minimally in the hypothalamic tuberoinfundibular pathway.2 Evidence is emerging that atypical agents elevate serum prolactin levels at least transiently—but usually less than typical antipsychotics—and this effect varies, depending on each compound’s dopamine-binding properties.
Figure 1 CHANGES IN PROLACTIN LEVELS OVER TIME
Mean serum prolactin concentrations from puberty until menopause. For nursing women, the length of the arrows depicts the increase in serum prolactin concentration associated with each episode of suckling. The Y-axis expresses serum prolactin concentration in both ng/ml and mg/l.
Source: Adapted and reprinted with permission from Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
Prolactin physiology
Prolactin—a large peptide containing 198 amino acids—was the first anterior pituitary gland hormone to be isolated in pure form.3 Despite its molecular weight of approximately 23,000, the hormone easily crosses the blood-brain barrier.4
Similar to other anterior pituitary hormones, prolactin is secreted episodically. Its secretion is inhibited by dopamine release from the hypothalamus and enhanced by different prolactin-releasing factors. Prolactin is the only anterior pituitary hormone that is produced by tuberoinfundibular neurons governed by dopamine.5 Dopamine stimulates lactotrope D2 receptors and inhibits adenylate cyclase, resulting in reduced prolactin synthesis and release.
Serum prolactin concentrations change during various life stages (Figure 1).6 Estrogen’s effects on prolactin gene expression regulate prolactin synthesis, resulting in higher prolactin levels in premenopausal women than in men.
Prolactin secretion
Normally, prolactin is secreted in pulses—approximately 14 in a 24-hour period, with an interpulse interval of about 80 minutes.5 A bimodal daily pattern of secretion is superimposed upon this pattern, with peak levels at night and trough levels at noon. Stress—including surgery and general anesthesia, exercise, and hypoglycemia—may transiently increase prolactin levels.
Endocrine regulation. Estrogen modulates the response of hypothalamic factors that control prolactin production. It stimulates decreased prolactin response to dopamine and increased response to thyrotropic-releasing hormone.
Insulin also stimulates prolactin secretion—probably by inducing hypoglycemia. Serum insulin level changes within physiologic ranges appear to affect prolactin regulation.
Neuroendocrine regulation. The hypothalamus blunts prolactin secretion primarily via dopamine release. This modulation occurs principally within the tuberoinfundibular dopamine pathway. The D2 subtype is the only dopamine receptor in the anterior pituitary gland:
- a decrease in dopamine levels reaching the anterior pituitary gland increases the number of D2 receptors
- to a lesser extent, estrogen decreases the number of D2 receptors.
Dopamine-modulated reductions in action potential discharge from lactotrophs and in calcium flux leads to decreased intracellular calcium and decreased prolactin secretion.5
Most hormones are target-organ agents and are regulated via a feedback loop that includes the peripheral circulation. Prolactin, however, is not considered to have a specific target organ. It is its own inhibiting factor, using an autoregulatory, pituitary-to-hypothalamus short-loop feedback circuit.
For example, prolactin-secreting tumors or drugs that elevate hormone levels lead to an increase in dopamine. In contrast, hypophysectomy decreases dopamine. In this setting, prolactin injections will restore normal dopamine levels. Prolactin-releasing factors include thyrotropic-releasing hormone, vasoactive intestinal peptide, and serotonin.
Prolactin’s actions
Many tissues—including breast, liver, ovary, testis, and prostate—have prolactin receptors. These receptors are stimulated with equal potency by prolactin and growth hormone.
The principal site of prolactin action is the mammary gland, where the hormone initiates and maintains lactation after childbirth. Major stimuli for breast development are estrogen, progesterone, prolactin, and placental mammotropic hormones. Other stimuli include insulin, cortisol, and thyroid hormone.7
Gonadotropin secretion is influenced by prolactin via the hypothalamus. Prolactin-mediated inhibition of luteinizing hormone-releasing hormone secretion impairs gonadotropin release and inhibits gonadal function.
Table 1
COMMON CLINICAL EFFECTS IN PATIENTS WITH HYPERPROLACTINEMIA
| Organ or syndrome | Clinical effects |
|---|---|
| Behavior | Direct effects Secondary effects due to hypogonadism Possible cognitive impairment |
| Bones | Decreased bone mineral density due to testosterone or estrogen deficits |
| Breast | Engorgement Lactation unrelated to breast feeding |
| Cardiovascular system | Possible adverse effects due to low levels of testosterone or estrogen |
| Menstrual function | Absence of ovulation Amenorrhea |
| Sexual function | Reduced libido Reduced arousal Orgasmic dysfunction |
| Source: Adapted and reprinted with permission from Dickson RA, Glazer WM. Neurolepticinduced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86. | |
Diagnosis of hyperprolactinemia
Pathologic hyperprolactinemia is defined as consistently elevated serum prolactin concentration (>20 ng/ml) in the absence of pregnancy or postpartum lactation. Because of the pulsatile nature of prolactin secretion, a definitive diagnosis of hyperprolactinemia requires three serum prolactin levels taken on different mornings.
Clinical presentation. Hyperprolactinemia—the most common hypothalamic-pituitary disturbance—usually presents with clinical features of gonadal dysfunction (Table 1).8 Symptoms and signs related to a brain mass—headache, visual field disturbances, ophthalmoplegia, and reduced visual acuity—may predominate with a large pituitary tumor. The patient may first present to a primary care physician or to a clinical specialist, such as a gynecologist, neurologist, ophthalmologist, pediatrician, psychiatrist, or urologist.
Thirty to 80% of women with hyperprolactinemia develop galactorrhea,9 although some women with galactorrhea have normal prolactin levels. Men with hyperprolactinemia usually have gonadal dysfunction, which unfortunately is often attributed to “psychogenic” causes. Particularly in men, prolactin is implicated in the control of libido.
Causes. Hyperprolactinemia may be caused by any process that inhibits dopamine synthesis, the neurotransmitter’s transport to the anterior pituitary gland, or its action at the lactotrope dopamine receptors ( Table 2).9 In this article, we will limit our discussion to antipsychotic drugs. have long-term effects on bone density. Trabecular bone mass
Estrogen and prolactin. During pregnancy, the rise in estrogen levels probably stimulates an increase in prolactin. Increased prolactin levels are also found in women taking estrogen-containing oral contraceptives, although this effect is very small with low-estrogen formulations.
Table 2
CAUSES OF PATHOLOGIC HYPERPROLACTINEMIA
| Hypothalamic disease | Tumor, infiltrative disease, pseudotumor cerebri, cranial radiation |
| Pituitary disease | Prolactinoma, acromegaly, Cushing’s disease, glycoprotein-producing tumor, other tumors, pituitary stalk section, empty sella, infiltrative disease |
| Medications | Antipsychotics, dopamine receptor blockers, antidepressants, antihypertensives, estrogens, opiates, cimetidine |
| Others | Primary hypothyroidism, chronic renal failure, cirrhosis, neurogenic and idiopathic causes |
| Source: Adapted and reprinted from Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders, 1995:394-405. Copyright 1995, with permission from Elsevier Science. | |
Functions of the pituitary lactotrophs regulated by estrogen include prolactin gene expression, release, storage, and cellular expression.2 Estradiol inhibition of dopamine synthesis in the tuberoinfundibular dopaminergic neurons may contribute to some gender differences in neurocognitive function and to psychiatric conditions’ clinical features.
Hyperprolactinemia and bone density. Besides causing galactorrhea and sexual dysfunction, hyperprolactinemia may has been found to be reduced in young women with amenorrhea secondary to hyperprolactinemia. This trabecular osteopenia is reversible—spinal bone density decreases progressively without treatment and improves when hyperprolactinemia is treated. Menstrual function appears to best predict risk of progressive spinal osteopenia in women with hyperprolactinemia. Estradiol level is a stronger predictor of clinical course than is the prolactin level.10
Antipsychotic drugs and hyperprolactinemia
Among the four principal dopamine pathways in the brain, the tuberoinfundibular pathway is a system of short axons at the base of the hypothalamus that releases dopamine into the portal veins of the pituitary gland. Terminals in the median eminence of the hypothalamus release dopamine that travels down the pituitary stalk in the portal veins.
Typical antipsychotics block dopamine receptors both in the striatum and in the hypothalamus.11 This finding suggests that the older drugs lack specificity of dopamine blockade. Prolactin elevations in patients treated with older antipsychotics may be associated with sexual dysfunction—a common cause of drug noncompliance, particularly in men.12
Antipsychotics and sexual side effects. Patients taking antipsy-chotics often complain—spontaneously or after focused questioning—of sexual side effects caused by drug-induced hyperprolactinemia. Assessing antipsychotic-induced sexual dysfunction may be confounded by the psychoses being treated, patient compliance, and sexuality’s complexities. Antipsychotics are generally believed to reduce desire, cause orgasmic dysfunction, and lead to difficulties during sexual performance.8
Atypical antipsychotics
A recent study designed to assess the effect of three atypical antipsychotics on serum prolactin levels enrolled 18 men with schizophrenia (mean age 32) taking clozapine, 300 to 400 mg/d; risperidone, 1 to 3 mg/d; or olanzapine, 10 to 20 mg/d, for at least 8 weeks.13 The study participants were instructed not to take their antipsychotics the night before the study. Baseline prolactin levels were measured in the morning, the men took the full daily dose of their medications, and prolactin levels were measured every 60 minutes over the next 8 hours and again at 24 hours.
Mean baseline prolactin values of clozapine (9 ng/ml, SD=5) and olanzapine (9 ng/ml, SD=5) were in the normal range (<20 ng/ml), compared with those of risperidone (27 ng/ml, SD=14). Three of the six patients taking risperidone had hyperprolactinemia at baseline. Prolactin values doubled within 6 hours of administration of all three medications. There was no comparable increase in prolactin levels in five control subjects not taking antipsychotics.
The authors concluded that these atypical antipsychotics raise prolactin levels but more transiently than typical antipsychotics. They suggested that the differences among the three drugs may be attributed to each drug’s binding properties to pituitary dopamine D2 receptors. A similar study in four patients with first-episode schizophrenia found serum prolactin levels increased from <10 ng/ml at baseline to peak levels of 80 to 120 ng/ml within 60 to 90 minutes after patients took a full daily dose of quetiapine, 700 to 800 mg/d.14
Risperidone. A study sponsored by Janssen Pharmaceutica15 reviewed the manufacturer’s experience with prolactin and its potential to induce side effects, using data from premarketing studies comparing risperidone with haloperidol. Amenorrhea and galactorrhea were assessed in women; ejaculatory dysfunction, erectile dysfunction, and gynecomastia were assessed in men.
Table 3
HYPERPROLACTINEMIA-RELATED SIDE EFFECTS REPORTED BY PATIENTS TAKING RISPERIDONE AND OLANZAPINE
| Gender and complaint | Taking risperidone (%) | Taking olanzapine (%) | Difference (P-value) |
|---|---|---|---|
| Women | |||
| Galactorrhea | 11 of 47 (23.4%) | 11 of 49 (22.4%) | 1.00 |
| Amenorrhea | 11 of 46 (23.9%) | 9 of 45 (20.0%) | 0.80 |
| Men | |||
| Gynecomastia | 9 of 115 (7.8%) | 4 of 115 (3.5%) | 0.25 |
| Sexual dysfunction | 36 of 115 (31.3%) | 34 of 114 (29.8%) | 0.89 |
| Source: Adapted and reprinted with permission from Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry2001;158:765-74. | |||
Both risperidone and haloperidol were associated with dose-related increases in plasma prolactin concentration in men and women. In women, neither risperidone dosage nor end-point prolactin concentrations were correlated with adverse events. In men:
- adverse events did not correlate with plasma prolactin concentrations
- the incidence of adverse events was dose-related
- the incidence of adverse events associated with risperidone, 4 to 10 mg/d, was not significantly greater than in patients taking placebo.
Another Janssen-sponsored study compared potential hyperprolactinemia-related side effects of risperidone and olanzapine but did not report prolactin concentrations. The authors found no significant differences between the drugs, based on breast features/menstrual changes in women and chest features/sexual dysfunction in men (Table 3).16
Olanzapine. A study sponsored by Eli Lilly and Co.17 assessed the effects of olanzapine on prolactin concentration in women previously treated with risperidone. The authors enrolled 20 Korean women with schizophrenia treated with risperidone (mean dosage 3.5 mg/d) and complaining of menstrual disturbances, galactorrhea, and/or sexual dysfunction. The mean serum prolactin concentration with risperidone was 132.2 ng/ml.
Over 2 weeks, patients were switched from risperidone to olanzapine (mean dosage 9.1 mg/d). After 8 weeks, the mean serum prolactin concentration was measured at 23.4 ng/ml. The authors noted improved menstrual function and reduced sexual side effects with olanzapine.
Conclusion
The package inserts of all atypical antipsychotics list hyperprolactinemia as a potential risk in patients taking these medications. The clinical significance of hyperprolactinemia associated with antipsychotic use is being explored but requires further elucidation.
Based on our understanding of the long-term course of untreated hyperprolactinemia—derived largely from patients not taking antipsychotics—it seems reasonable to ask patients taking atypical antipsychotics at least once a year about chest/breast complaints and sexual dysfunction. This recommendation would seem particularly relevant in patients taking risperidone at dosages >6 mg/d for sustained periods. In the absence of specific complaints, hyperprolactinemia associated with risperidone should be evaluated case by case, including perhaps endocrinology consultation.
Related resources
- Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J Clin Psychiatry 2002;63(suppl 4):56-62.
- Smith S, Wheeler MJ, Murray R, O’Keane V. The effects of antipsychotic-induced hyperprolactinemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002;22(2):109-14. Available at: http://www.psychiatry.wustl.edu/Resources/LiteratureList/2002/May/Smith.PDF.
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
Dr. Vieweg reports that he is on the speakers bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Antipsychotics have long been linked with hyperprolactinemia.1 This phenomenon was first considered a drug class effect, but the arrival of clozapine, better deliniation of dopamine receptor subtypes, and identification of the four principal CNS dopamine pathways revealed that hyperprolactinemia was not a universal consequence of antipsychotic use.
We now know that most atypical antipsychotics are less likely to induce hyperprolactinemia than older antipsychotics, but we don’t know why. The most likely explanation is that most of the newer agents block dopamine D2 minimally in the hypothalamic tuberoinfundibular pathway.2 Evidence is emerging that atypical agents elevate serum prolactin levels at least transiently—but usually less than typical antipsychotics—and this effect varies, depending on each compound’s dopamine-binding properties.
Figure 1 CHANGES IN PROLACTIN LEVELS OVER TIME
Mean serum prolactin concentrations from puberty until menopause. For nursing women, the length of the arrows depicts the increase in serum prolactin concentration associated with each episode of suckling. The Y-axis expresses serum prolactin concentration in both ng/ml and mg/l.
Source: Adapted and reprinted with permission from Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
Prolactin physiology
Prolactin—a large peptide containing 198 amino acids—was the first anterior pituitary gland hormone to be isolated in pure form.3 Despite its molecular weight of approximately 23,000, the hormone easily crosses the blood-brain barrier.4
Similar to other anterior pituitary hormones, prolactin is secreted episodically. Its secretion is inhibited by dopamine release from the hypothalamus and enhanced by different prolactin-releasing factors. Prolactin is the only anterior pituitary hormone that is produced by tuberoinfundibular neurons governed by dopamine.5 Dopamine stimulates lactotrope D2 receptors and inhibits adenylate cyclase, resulting in reduced prolactin synthesis and release.
Serum prolactin concentrations change during various life stages (Figure 1).6 Estrogen’s effects on prolactin gene expression regulate prolactin synthesis, resulting in higher prolactin levels in premenopausal women than in men.
Prolactin secretion
Normally, prolactin is secreted in pulses—approximately 14 in a 24-hour period, with an interpulse interval of about 80 minutes.5 A bimodal daily pattern of secretion is superimposed upon this pattern, with peak levels at night and trough levels at noon. Stress—including surgery and general anesthesia, exercise, and hypoglycemia—may transiently increase prolactin levels.
Endocrine regulation. Estrogen modulates the response of hypothalamic factors that control prolactin production. It stimulates decreased prolactin response to dopamine and increased response to thyrotropic-releasing hormone.
Insulin also stimulates prolactin secretion—probably by inducing hypoglycemia. Serum insulin level changes within physiologic ranges appear to affect prolactin regulation.
Neuroendocrine regulation. The hypothalamus blunts prolactin secretion primarily via dopamine release. This modulation occurs principally within the tuberoinfundibular dopamine pathway. The D2 subtype is the only dopamine receptor in the anterior pituitary gland:
- a decrease in dopamine levels reaching the anterior pituitary gland increases the number of D2 receptors
- to a lesser extent, estrogen decreases the number of D2 receptors.
Dopamine-modulated reductions in action potential discharge from lactotrophs and in calcium flux leads to decreased intracellular calcium and decreased prolactin secretion.5
Most hormones are target-organ agents and are regulated via a feedback loop that includes the peripheral circulation. Prolactin, however, is not considered to have a specific target organ. It is its own inhibiting factor, using an autoregulatory, pituitary-to-hypothalamus short-loop feedback circuit.
For example, prolactin-secreting tumors or drugs that elevate hormone levels lead to an increase in dopamine. In contrast, hypophysectomy decreases dopamine. In this setting, prolactin injections will restore normal dopamine levels. Prolactin-releasing factors include thyrotropic-releasing hormone, vasoactive intestinal peptide, and serotonin.
Prolactin’s actions
Many tissues—including breast, liver, ovary, testis, and prostate—have prolactin receptors. These receptors are stimulated with equal potency by prolactin and growth hormone.
The principal site of prolactin action is the mammary gland, where the hormone initiates and maintains lactation after childbirth. Major stimuli for breast development are estrogen, progesterone, prolactin, and placental mammotropic hormones. Other stimuli include insulin, cortisol, and thyroid hormone.7
Gonadotropin secretion is influenced by prolactin via the hypothalamus. Prolactin-mediated inhibition of luteinizing hormone-releasing hormone secretion impairs gonadotropin release and inhibits gonadal function.
Table 1
COMMON CLINICAL EFFECTS IN PATIENTS WITH HYPERPROLACTINEMIA
| Organ or syndrome | Clinical effects |
|---|---|
| Behavior | Direct effects Secondary effects due to hypogonadism Possible cognitive impairment |
| Bones | Decreased bone mineral density due to testosterone or estrogen deficits |
| Breast | Engorgement Lactation unrelated to breast feeding |
| Cardiovascular system | Possible adverse effects due to low levels of testosterone or estrogen |
| Menstrual function | Absence of ovulation Amenorrhea |
| Sexual function | Reduced libido Reduced arousal Orgasmic dysfunction |
| Source: Adapted and reprinted with permission from Dickson RA, Glazer WM. Neurolepticinduced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86. | |
Diagnosis of hyperprolactinemia
Pathologic hyperprolactinemia is defined as consistently elevated serum prolactin concentration (>20 ng/ml) in the absence of pregnancy or postpartum lactation. Because of the pulsatile nature of prolactin secretion, a definitive diagnosis of hyperprolactinemia requires three serum prolactin levels taken on different mornings.
Clinical presentation. Hyperprolactinemia—the most common hypothalamic-pituitary disturbance—usually presents with clinical features of gonadal dysfunction (Table 1).8 Symptoms and signs related to a brain mass—headache, visual field disturbances, ophthalmoplegia, and reduced visual acuity—may predominate with a large pituitary tumor. The patient may first present to a primary care physician or to a clinical specialist, such as a gynecologist, neurologist, ophthalmologist, pediatrician, psychiatrist, or urologist.
Thirty to 80% of women with hyperprolactinemia develop galactorrhea,9 although some women with galactorrhea have normal prolactin levels. Men with hyperprolactinemia usually have gonadal dysfunction, which unfortunately is often attributed to “psychogenic” causes. Particularly in men, prolactin is implicated in the control of libido.
Causes. Hyperprolactinemia may be caused by any process that inhibits dopamine synthesis, the neurotransmitter’s transport to the anterior pituitary gland, or its action at the lactotrope dopamine receptors ( Table 2).9 In this article, we will limit our discussion to antipsychotic drugs. have long-term effects on bone density. Trabecular bone mass
Estrogen and prolactin. During pregnancy, the rise in estrogen levels probably stimulates an increase in prolactin. Increased prolactin levels are also found in women taking estrogen-containing oral contraceptives, although this effect is very small with low-estrogen formulations.
Table 2
CAUSES OF PATHOLOGIC HYPERPROLACTINEMIA
| Hypothalamic disease | Tumor, infiltrative disease, pseudotumor cerebri, cranial radiation |
| Pituitary disease | Prolactinoma, acromegaly, Cushing’s disease, glycoprotein-producing tumor, other tumors, pituitary stalk section, empty sella, infiltrative disease |
| Medications | Antipsychotics, dopamine receptor blockers, antidepressants, antihypertensives, estrogens, opiates, cimetidine |
| Others | Primary hypothyroidism, chronic renal failure, cirrhosis, neurogenic and idiopathic causes |
| Source: Adapted and reprinted from Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders, 1995:394-405. Copyright 1995, with permission from Elsevier Science. | |
Functions of the pituitary lactotrophs regulated by estrogen include prolactin gene expression, release, storage, and cellular expression.2 Estradiol inhibition of dopamine synthesis in the tuberoinfundibular dopaminergic neurons may contribute to some gender differences in neurocognitive function and to psychiatric conditions’ clinical features.
Hyperprolactinemia and bone density. Besides causing galactorrhea and sexual dysfunction, hyperprolactinemia may has been found to be reduced in young women with amenorrhea secondary to hyperprolactinemia. This trabecular osteopenia is reversible—spinal bone density decreases progressively without treatment and improves when hyperprolactinemia is treated. Menstrual function appears to best predict risk of progressive spinal osteopenia in women with hyperprolactinemia. Estradiol level is a stronger predictor of clinical course than is the prolactin level.10
Antipsychotic drugs and hyperprolactinemia
Among the four principal dopamine pathways in the brain, the tuberoinfundibular pathway is a system of short axons at the base of the hypothalamus that releases dopamine into the portal veins of the pituitary gland. Terminals in the median eminence of the hypothalamus release dopamine that travels down the pituitary stalk in the portal veins.
Typical antipsychotics block dopamine receptors both in the striatum and in the hypothalamus.11 This finding suggests that the older drugs lack specificity of dopamine blockade. Prolactin elevations in patients treated with older antipsychotics may be associated with sexual dysfunction—a common cause of drug noncompliance, particularly in men.12
Antipsychotics and sexual side effects. Patients taking antipsy-chotics often complain—spontaneously or after focused questioning—of sexual side effects caused by drug-induced hyperprolactinemia. Assessing antipsychotic-induced sexual dysfunction may be confounded by the psychoses being treated, patient compliance, and sexuality’s complexities. Antipsychotics are generally believed to reduce desire, cause orgasmic dysfunction, and lead to difficulties during sexual performance.8
Atypical antipsychotics
A recent study designed to assess the effect of three atypical antipsychotics on serum prolactin levels enrolled 18 men with schizophrenia (mean age 32) taking clozapine, 300 to 400 mg/d; risperidone, 1 to 3 mg/d; or olanzapine, 10 to 20 mg/d, for at least 8 weeks.13 The study participants were instructed not to take their antipsychotics the night before the study. Baseline prolactin levels were measured in the morning, the men took the full daily dose of their medications, and prolactin levels were measured every 60 minutes over the next 8 hours and again at 24 hours.
Mean baseline prolactin values of clozapine (9 ng/ml, SD=5) and olanzapine (9 ng/ml, SD=5) were in the normal range (<20 ng/ml), compared with those of risperidone (27 ng/ml, SD=14). Three of the six patients taking risperidone had hyperprolactinemia at baseline. Prolactin values doubled within 6 hours of administration of all three medications. There was no comparable increase in prolactin levels in five control subjects not taking antipsychotics.
The authors concluded that these atypical antipsychotics raise prolactin levels but more transiently than typical antipsychotics. They suggested that the differences among the three drugs may be attributed to each drug’s binding properties to pituitary dopamine D2 receptors. A similar study in four patients with first-episode schizophrenia found serum prolactin levels increased from <10 ng/ml at baseline to peak levels of 80 to 120 ng/ml within 60 to 90 minutes after patients took a full daily dose of quetiapine, 700 to 800 mg/d.14
Risperidone. A study sponsored by Janssen Pharmaceutica15 reviewed the manufacturer’s experience with prolactin and its potential to induce side effects, using data from premarketing studies comparing risperidone with haloperidol. Amenorrhea and galactorrhea were assessed in women; ejaculatory dysfunction, erectile dysfunction, and gynecomastia were assessed in men.
Table 3
HYPERPROLACTINEMIA-RELATED SIDE EFFECTS REPORTED BY PATIENTS TAKING RISPERIDONE AND OLANZAPINE
| Gender and complaint | Taking risperidone (%) | Taking olanzapine (%) | Difference (P-value) |
|---|---|---|---|
| Women | |||
| Galactorrhea | 11 of 47 (23.4%) | 11 of 49 (22.4%) | 1.00 |
| Amenorrhea | 11 of 46 (23.9%) | 9 of 45 (20.0%) | 0.80 |
| Men | |||
| Gynecomastia | 9 of 115 (7.8%) | 4 of 115 (3.5%) | 0.25 |
| Sexual dysfunction | 36 of 115 (31.3%) | 34 of 114 (29.8%) | 0.89 |
| Source: Adapted and reprinted with permission from Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry2001;158:765-74. | |||
Both risperidone and haloperidol were associated with dose-related increases in plasma prolactin concentration in men and women. In women, neither risperidone dosage nor end-point prolactin concentrations were correlated with adverse events. In men:
- adverse events did not correlate with plasma prolactin concentrations
- the incidence of adverse events was dose-related
- the incidence of adverse events associated with risperidone, 4 to 10 mg/d, was not significantly greater than in patients taking placebo.
Another Janssen-sponsored study compared potential hyperprolactinemia-related side effects of risperidone and olanzapine but did not report prolactin concentrations. The authors found no significant differences between the drugs, based on breast features/menstrual changes in women and chest features/sexual dysfunction in men (Table 3).16
Olanzapine. A study sponsored by Eli Lilly and Co.17 assessed the effects of olanzapine on prolactin concentration in women previously treated with risperidone. The authors enrolled 20 Korean women with schizophrenia treated with risperidone (mean dosage 3.5 mg/d) and complaining of menstrual disturbances, galactorrhea, and/or sexual dysfunction. The mean serum prolactin concentration with risperidone was 132.2 ng/ml.
Over 2 weeks, patients were switched from risperidone to olanzapine (mean dosage 9.1 mg/d). After 8 weeks, the mean serum prolactin concentration was measured at 23.4 ng/ml. The authors noted improved menstrual function and reduced sexual side effects with olanzapine.
Conclusion
The package inserts of all atypical antipsychotics list hyperprolactinemia as a potential risk in patients taking these medications. The clinical significance of hyperprolactinemia associated with antipsychotic use is being explored but requires further elucidation.
Based on our understanding of the long-term course of untreated hyperprolactinemia—derived largely from patients not taking antipsychotics—it seems reasonable to ask patients taking atypical antipsychotics at least once a year about chest/breast complaints and sexual dysfunction. This recommendation would seem particularly relevant in patients taking risperidone at dosages >6 mg/d for sustained periods. In the absence of specific complaints, hyperprolactinemia associated with risperidone should be evaluated case by case, including perhaps endocrinology consultation.
Related resources
- Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J Clin Psychiatry 2002;63(suppl 4):56-62.
- Smith S, Wheeler MJ, Murray R, O’Keane V. The effects of antipsychotic-induced hyperprolactinemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002;22(2):109-14. Available at: http://www.psychiatry.wustl.edu/Resources/LiteratureList/2002/May/Smith.PDF.
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
Dr. Vieweg reports that he is on the speakers bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
Dr. Fernandez reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Clemens JA, Smalstig EB, Sawyer BD. Antipsychotic drugs stimulate prolactin release. Psychopharmacol. 1974;40:123-7.
2. Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res 1999;35(suppl):S67-S73.
3. West ES, Todd WR. The hormones. In: West ES, Todd WR (eds). Textbook of biochemistry. New York: The Macmillan Co., 1961;1315-54.
4. Belchetz PE, Ridley RM, Baker HF. Studies on the accessibility of prolactin and growth hormone to brain: effect of opiate agonists on hormone levels in serial, simultaneous plasma and cerebrospinal fluid samples in the rhesus monkey. Brain Res 1982;239:310-4.
5. Cooke NE. Prolactin: basic physiology. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Company, 1995;368-93.
6. Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
7. Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K. The anterior pituitary. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds). Williams textbook of endocrinology. Philadelphia: W.B. Saunders Co., 1998;249-340.
8. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86.
9. Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Co., 1995;394-405.
10. Biller BM, Baum HB, Rosenthal DI, Saxe VC, Charpie PM, Kilibanski A. Progressive trabecular osteopenia in women with hyperprolactinemic amenorrhea. J Clin Endocrinol Metab 1992;75:692-7.
11. Baron JC, Martinot JL, Cambon H, et al. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacol 1989;99:463-72.
12. Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis 1982;170:463-7.
13. Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 2002;159:133-5.
14. Alexiadis M, Whitehorn D, Woodley H, Kopala L. Prolactin elevation with quetiapine (letter). Am J Psychiatry 2002;159(Sept):1608-9.
15. Kleinberg DL, Davis JM, De Coster R, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999;19:57-61.
16. Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001;158:765-74.
17. Kim K, Pae C, Chae J, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry 2002;63:408-13.
1. Clemens JA, Smalstig EB, Sawyer BD. Antipsychotic drugs stimulate prolactin release. Psychopharmacol. 1974;40:123-7.
2. Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res 1999;35(suppl):S67-S73.
3. West ES, Todd WR. The hormones. In: West ES, Todd WR (eds). Textbook of biochemistry. New York: The Macmillan Co., 1961;1315-54.
4. Belchetz PE, Ridley RM, Baker HF. Studies on the accessibility of prolactin and growth hormone to brain: effect of opiate agonists on hormone levels in serial, simultaneous plasma and cerebrospinal fluid samples in the rhesus monkey. Brain Res 1982;239:310-4.
5. Cooke NE. Prolactin: basic physiology. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Company, 1995;368-93.
6. Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
7. Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K. The anterior pituitary. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds). Williams textbook of endocrinology. Philadelphia: W.B. Saunders Co., 1998;249-340.
8. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86.
9. Vance ML, Thorner MO. Prolactin: hyperprolactinemic syndromes and management. In: DeGroot LJ, Besser M, Burger HG, et al (eds). Endocrinology. Philadelphia: W.B. Saunders Co., 1995;394-405.
10. Biller BM, Baum HB, Rosenthal DI, Saxe VC, Charpie PM, Kilibanski A. Progressive trabecular osteopenia in women with hyperprolactinemic amenorrhea. J Clin Endocrinol Metab 1992;75:692-7.
11. Baron JC, Martinot JL, Cambon H, et al. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacol 1989;99:463-72.
12. Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis 1982;170:463-7.
13. Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 2002;159:133-5.
14. Alexiadis M, Whitehorn D, Woodley H, Kopala L. Prolactin elevation with quetiapine (letter). Am J Psychiatry 2002;159(Sept):1608-9.
15. Kleinberg DL, Davis JM, De Coster R, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999;19:57-61.
16. Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001;158:765-74.
17. Kim K, Pae C, Chae J, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry 2002;63:408-13.
Weight control and antipsychotics: How to tip the scales away from diabetes and heart disease
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight control and antipsychotics: How to tip the scales away from diabetes and heart disease
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Strategies to prevent fatal arrhythmias in patients taking antipsychotics
Before approving the antipsychotic agent ziprasidone last year, the Food and Drug Administration required specific safety data on whether the drug might cause the life-threatening arrhythmia known as torsade de pointes.
The FDA’s action, which delayed the drug’s approval for 3 years, underscores growing concern about the risk of cardiovascular effects with the use of antipsychotic and other agents known to prolong the cardiac QT interval. This concern has led to withdrawal of some drugs before reaching the market (e.g., the atypical neuroleptic sertindole), the addition of “black box” warnings in the labeling of some antipsychotics, and withdrawal from the market of antihistamines terfenadine and astemizole and the GI stimulant cisapride.
Torsade de pointes is a polymorphic ventricular tachycardia (VT), a rare arrhythmia that can cause sudden death. Because torsade can occur with the use of some antipsychotics, the psychiatrist needs to consider cardiovascular safety when selecting among available agents. To help with these decisions, here is information about the documented and potential electrocardiographic features of commonly prescribed antipsychotic drugs, as well as background on QT interval prolongation and torsade de pointes.
Torsade de pointes
Named for a ballet movement, torsade de pointes describes bursts of “twisting of the points,” a variation of the morphology of the QRS vector about the isoelectric axis from positive to net negative and back again. As seen on an ECG (Figure 1), the first beat of torsade de pointes is a normal ventricular complex preceded by a P wave. This is followed by a premature ventricular contraction (PVC) with a short coupling interval. After a compensatory pause, a second normal beat is followed by a second PVC, which is the first beat of a polymorphic VT. We know tachycardia is present because the ventricular beats appear close together. We know the arrhythmia is ventricular in origin because the ventricular complexes are wide. Finally, we note the ventricular complexes vary in configuration—that is, the shape (morphology) varies from beat to beat.
Figure 1 Typical ECG features of torsade de pointes
Sinus beat with normal ventricular complex (1) followed by premature ventricular contraction (PVC) (2) with short coupling interval. After a long pause (long refractory period), another sinus beat (3) is followed by another PVC (4) with a short coupling interval. The second PVC (4) is the first beat of polymorphic ventricular tachycardiaIn torsade, the stimulus for the VT moves within the ventricle, changing its shape from beat to beat. This multifocal VT differs from the more common unifocal VT, in which all the QRS complexes appear the same.
Drug-induced torsade de pointes
Although the term torsade de pointes was first described in 1966,1 the drug-induced form of this arrhythmia has been recognized for nearly a century.
Quinidine Around 1920, cardiologists first used quinidine to help restore normal sinus rhythm in patients with atrial fibrillation, most commonly due to rheumatic heart disease.2
In 1964, Selzer and Wray3 studied the use of quinidine to convert atrial fibrillation to normal sinus rhythm in more than 200 patients seen during 4 years in a cardiopulmonary clinic. In a subgroup of eight patients, these researchers documented 10 reactions (including five documented episodes of ventricular fibrillation/ventricular flutter) among 36 syncopal episodes that developed within 1 to 6.5 hours of quinidine administration. Symptoms were nonspecific and included nausea, faintness, and feeling ill. It is now recognized that torsade de pointes was the principal rhythm disturbance in those eight patients. Syncope usually occurs early in treatment and may be found in 5% to 10% of patients taking quinidine.
TCAs and antipsychotics Tricyclic antidepressants (TCAs) and antipsychotics that have quinidine-like properties (e.g., thioridazine) also may be associated with QT interval prolongation and torsade de pointes.4-9 In high doses (particularly in overdose), TCAs may induce widening of the QRS complex. Fowler et al reported episodes of VT in five patients taking thioridazine—one of whom died.10
Mehtonen et al reported sudden unexpected deaths associated with antipsychotic or antidepressant drugs among 31 women and 18 men in a survey of autopsies performed from 1985 to 1988 in Finland. The authors documented therapeutic use of phenothiazines in all but 3 of the 49 cases. Thioridazine was involved in more than half the deaths. In 15 of the deaths, thioridazine was the only antipsychotic drug taken. Drugs other than thioridazine were documented in only 5 of the 49 sudden cardiac deaths.11
Figure 2 Normal ECG in sinus rhythm
In this typical lead II of a surface ECG, the P wave (atrial depolarization) leads to right and left atrial contraction and the QRS complex (ventricular depolarization) leads to left and right ventricular contraction. The ST segment represents isoelectric ventricular repolarization, and the T wave represents directional repolarization. The QT interval includes both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval, or ST segment plus T wave).
QT interval as a marker for torsade
The incidence of torsade is unknown, but it is an uncommon cardiac abnormality. In the United States, torsade probably accounts for less than 5% of the 300,000 sudden cardiac deaths that occur each year. Because torsade de pointes is rare, regulatory agencies and clinicians use the QT interval as a surrogate ECG marker for risk of torsade de pointes. Heart rate can affect the QT interval, so various formulae are used to correct the QT interval for heart rate (QTc).
What is the QT interval? In a normal ECG (Figure 2), the P wave derives from right and left atrial electrical depolarization. The pacemaker of the heart is located in the sino-atrial node (SAN) in the superior portion of the right atrium. From the SAN, electrical signals travel down three intra-atrial pathways, activating the right atrium, then travel to the atrioventricular node (AVN). Bachmann’s bundle—a fourth atrial pathway—passes from the SAN to depolarize the left atrium. From the AVN, the electrical signal travels through the left and right bundle branches to activate their respective ventricles.
Electrical depolarization of the left and right ventricles produces the QRS complex. Most of the electrical forces making up this complex arise in the left ventricle, which is much larger than the right ventricle.
The electrical circuitry of the heart activates the left and right atria in such a fashion that these chambers eject blood into their respective ventricles just before these chambers contract. Optimal ventricular filling maximizes ventricular ejection of blood (Starling’s law). Ventricular repolarization (JT interval—electrical recovery) follows ventricular depolarization. On the surface ECG, the JT interval consists of an isoelectric event—the ST segment running from the end of the QRS complex to the beginning of the T wave—and the T wave itself (directional electrical recovery).
The QT interval, then, consists of both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval). Ventricular repolarization makes up by far the greater portion of the QT interval.
Correcting the QT interval (QTc) In 1920, Bazett noted that as the heart rate slowed, the QT interval lengthened.12 From personal and reported observations, he derived an equation called the Bazett formula that corrects (or normalizes) the QT interval to a heart rate of 60 beats/min (QTc). In the Bazett formula, the QTc interval is the measured QT interval divided by the square root of the RR interval (time between sequential QRS complexes—the determinant of heart rate) measured in seconds (QTc = QT/RR).
The Bazett formula is most widely used to estimate the QTc interval, although at least 20 other formulae have been developed in response to the original’s perceived inadequacies.13-15 Bazett’s formula is used in most automated interpretations of the ECG.
Up to age 55, the normal QTc interval ranges from 350 to 430 msec for men and 350 to 450 msec for women, and it tends to increase with age. Most cases of torsade occur when the QT or QTc interval is greater than 500 msec.14 A QTc interval between 450 and 500 msec is cause for concern; a QTc interval that exceeds 500 msec is cause for alarm.
Factors that cause variations in QTc
Factors that can affect the QTc interval and increase the risk of torsade de pointes include electrolyte imbalances, medication use and overdose, cardiac disease, liver disease, endocrine disorders such as diabetes and hypothyroidism, and CNS injury (Table 1).
Table 1
Risk factors contributing to QTc interval prolongation
| Risk factor | Causes/implications |
|---|---|
| Sex (female) | QT intervals longer in women than in men QT interval longer during first half of menstrual cycle |
| Age (elderly) | Increased risk for CAD Multiple medications Pharmacokinetic/pharmacodynamic changes |
| Electrolyte imbalance Hypokalemia, hypomagnesemia Hypocalcemia | Diuretic use Excessive vomiting or diarrhea Postprandial hypokalemia |
| Congenital long QT syndrome | Associated with torsade and sudden death |
| Cardiac disease, with history of acute or chronic myocardial ischemia, CHF, cardiac arrhythmias, bradycardia | Increased risk of cardiac arrhythmias |
| Drugs known to prolong QTc interval | May potentiate QTc prolongation |
| Medication overdose with drugs that prolong the QTc interval | QTc prolongation generally dose-dependent |
| Concomitant medications, liver disease | Adverse events with cytochrome P-450 enzyme system inhibition, leading to increased drug levels that can increase QT interval |
| Endocrine/metabolic disorders Diabetes, obesity Hypothyroidism, pituitary insufficiency | Via electrolytes or cardiovascular disease |
| CNS injury Stroke, infection, trauma | Via autonomic nervous system dysfunction |
Circadian patterns The QTc interval varies throughout the 24-hour day, with nocturnal values about 20 msec greater than daytime measurements. These differences are driven by changes in autonomic (sympathetic and parasympathetic) tone.16,17 In 20 normal subjects, circadian variability was 76 ± 19 msec (range 35 to 108 msec) from day to night.17 This circadian variation may be accentuated in patients with cardiovascular disease.
Sex. At birth, QTc interval measurements do no vary by sex.18 At puberty, however, the male QTc interval shortens and remains shorter than its female counterpart by about 20 msec until age 50 to 55, coincident with a decline in male testosterone levels. This sex difference appears to be androgen driven. About 70% of torsade de pointes cases occur in women.18
Menstrual cycle QTc interval measurements are stable throughout the menstrual cycle if quinidine-like drugs are not given.
Variations were seen, however, when Rodriguez et al studied the effect of IV low-dose ibutilide (an antiarrhythmic agent known to prolong the QT interval) on the QTc intervals of 58 healthy subjects (38 men and 20 women, ages 21 to 40). During 1 month, men were studied once and women studied three times, coincident with the three phases of the menstrual cycle. The greatest increase in QTc intervals measurements occurred in women during the first half of their menstrual cycles.19
Age and cardiovascular disease Two congenital long QT syndromes may be associated with sudden death, mostly in children and young adults:
- The Jervell and Lange-Nielsen syndrome is marked by severe congenital deafness and autosomal recessive inheritance.
- The Romano-Ward syndrome has normal hearing and autosomal dominant inheritance.20
Congenital long QT syndrome (LQTS) occurs in about one in 5,000 births and accounts for about 3,000 to 4,000 deaths per year in the United States. Nine percent of pediatric LQTS subjects present with sudden cardiac death. More than 71% of patients will die before age 15 if not treated.
Elderly persons tend to have longer QTc intervals than do younger subjects, even when both groups are free of cardiovascular disease.21 Also, age-matched subjects with cardiovascular disease tend to have longer QTc intervals than do those free of cardiovascular disease.
Electrolytes Electrolyte disturbances, particularly hypokalemia and hypomagnesemia, may contribute to or even cause QT interval prolongation.22
Hypokalemia prolongs the cardiac action potential and may cause early afterdepolarization, leading to torsade.23 Low potassium levels reduce the net outward potassium current during phase 3 of the cardiac action potential. Hypomagnesemia may contribute to gross U wave alternans, lengthening the cardiac action potential and setting the stage for torsade.24 Various factors may contribute to electrolyte disturbances, including use of diuretics and excessive vomiting and diarrhea. Even postprandial states may induce hypokalemia.
Intensive exercise and agitation may be associated with hypokalemia.25 Serum potassium may be lower in severely agitated patients (3.59 mmol/L) than in mildly agitated patients (3.79 mmol/L). The mean QTc interval of psychiatric emergency patients may be prolonged (453±40 msec),5 with QTc intervals of psychiatric inpatients longer than those of psychiatric outpatients. Altered potassium states probably explain these observations. Mechanisms that link exercise and agitation with hypokalemia remain to be elucidated.
Metabolic factors Drugs may alter phase 3 potassium flow, thereby disrupting the synchrony of action of individual cardiac cells during repolarization. This change may induce early afterdepolarizations and torsade.23
Five percent to 10% of Americans of European descent have genetic profiles that make them poor metabolizers of drugs that are metabolized by the cytochrome P-450 isoenzyme 2D6. The Pfizer Inc. 054 study assessed the potential for metabolic inhibitors such as paroxetine to raise antipsychotic drug levels in these patients and induce QTc interval prolongation.26
In response to FDA concerns about QTc interval prolongation associated with the use of ziprasidone, Pfizer studied the potential for QTc interval prolongation when antipsychotics are given with and without metabolic inhibitors of cytochrome P-450 isoenzymes 2D6 (paroxetine), 3A4 (ketoconazole), and 1A2 (fluvoxamine). The study population of 183 subjects (mean age:men, 37.1 years, women 38.8 years) was three-quarters young men with schizophrenia, in good health otherwise and possessing normal ECGs—i.e., patients with a low risk of developing cardiac arrhythmias.
Figure 3 Antipsychotic drugs and QTc interval changes
Six antipsychotic drugs and QTc interval changes from baseline when given with and without metabolic inhibitors. QTc interval changes (in msec) when given without a metabolic inhibitor were ziprasidone, 20.3; risperidone, 11.6; olanzapine, 6.8; quetiapine, 14.5; thioridazine, 35.6; and haloperidol 4.7.
Reprinted from: “FDA Psychopharmacological Drugs Advisory Committee. 19 July 2000. Briefing Documents for Zeldox Capsules (Ziprasidone HCL). Pfizer.” Available from Central Research Division, Pfizer, Inc., Eastern Point Road, Groton, CT 06340, (860) 441-4100.Over the course of about 1 week, daily doses were escalated to ziprasidone, 160 mg; risperidone, 8 mg and 16 mg; olanzapine, 20 mg; quetiapine, 750 mg; thioridazine, 300 mg; and haloperidol, 15 mg. Thioridazine (35.6 msec) and ziprasidone (20.3 msec) showed the greatest QTc interval increase following drug administration (Figure 3). Co-administration of a metabolic inhibitor did not further prolong the QTc interval for these two drugs.
Of the six drugs studied, only thioridazine and ziprasidone showed QTc interval increases 5% compared with baseline measurements.
Co-administration of a metabolic inhibitor caused the greatest increase in QTc intervals for quetiapine (from 14.5 to 19.7 msec). This value closely approached the steady-state ziprasidone measurement (20.3 msec). Because quetiapine is more likely than the other antipsychotic drugs studied to increase heart rate, it may be argued that the Bazett formula’s limitations in estimating the QTc interval at higher heart rates contributed to the quetiapine study findings.
Table 2
Relative risk of QTc interval prolongation with common antipsychotic agents
| Risk level | Agent |
|---|---|
| ECG required or strongly recommended before prescribing (most commonly associated with QTc interval prolongation and torsade de pointes) | Thioridazine Mesoridazine Droperidol Pimozide Haloperidol in large doses IV (commonly ≥ 100 mg/d) |
| Mild to moderate risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Quetiapine Ziprasidone Chlorpromazine |
| Little or no risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Haloperidol (oral) Olanzapine Risperidone Clozapine |
Recommendations
Taking a careful history is key to cardiovascular assessment before prescribing an antipsychotic. An ECG is indicated for patients with:
- Personal or family history of syncope or sudden death;
- Personal history of angina pectoris, myocardial infarction, congestive heart failure, cardiac arrhythmias, hypokalemia, hypomagnesemia, or significant cardiac risk factors.
The relative cardiovascular risks associated with antipsychotic agents are shown in Table 2.
An ECG also is required or strongly recommended before prescribing the antipsychotic drugs most commonly associated with QT prolongation and torsade de pointes—droperidol, haloperidol in large doses IV (commonly 100 mg/d), mesoridazine, pimozide, and thioridazine.
The FDA has strengthened the warning labels required for these agents, adding “black box” warnings about the risks of prolonged QTc intervals, torsade de pointes, and sudden death for droperidol, mesoridazine, and thioridazine. Thioridazine, for example, is indicated only for patients with schizophrenia who fail to show an acceptable response to other antipsychotic drugs. Its use is contraindicated in patients who take:
- fluvoxamine, propranolol, and pindolol;
- any drug that inhibits the cytochrome P-450 2D6 isoenzyme (e.g., fluoxetine, paroxetine);
- agents known to prolong the QTc interval.
Use of thioridazine also is contraindicated in patients known to have reduced levels of the cytochrome P450 2D6 isozyme, as well as in patients with congenital LQTS or a history of cardiac arrhythmias. Psychiatrists are advised to read the warnings and prescribing information in the labeling of all antipsychotics for potential cardiovascular side effects.
When the psychiatrist receives a report of suspected QTc interval prolongation on a patient’s ECG, the following steps are recommended:
- Obtain another ECG.
- Assess serum potassium, magnesium, calcium, and thyroid hormone levels.
In patients with confirmed QTc interval prolongation, any complaint of palpitations, presyncope, or syncope are grounds for urgent referral to a cardiologist.
Related resources
- European Society of Cardiology guidelines: www.escardio.org/scinfo/Guidelines/Haverkamp.pdf
- Sudden Arrhythmia Death Syndromes Foundation (SADSF): www.sads.org (800) 786-7723.
- Drugs that prolong the QT interval and/or induce torsade de pointes. Georgetown Center for Education and Research Therapeutics: www.torsades.org
1. Dessertenne F. Tachycardie ventriculaire a deux foyers opposes variables. Arch Mal Coeur Vaiss 1966;59(2):263-72.
2. Clark-Kennedy AE. Quinidine in the treatment of auricular fibrillation. Quart J Med 1922;16:204-35.
3. Selzer A, Wray W. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation 1964;30:17-26.
4. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 2000;355:1048-52.
5. Hatta K, Takahashi T, Nakamura H, Yamashiro H, Yonezawa Y. Prolonged QT interval in acute psychotic patients. Psychiatry Res 2000;94(3):279-85.
6. Welch R. Antipsychotic agents and QT changes. J Psychiatry Neurosci 2000;25(2):154-60.
7. Fayek M, Kingsbury SJ, Zada J, Simpson GM. Cardiac effects of antipsychotic medications. Psychiatr Serv 2001;52(5):607-9.
8. Kelly HG, Fay JE, Laverty SG. Thioridazine hydrochloride (Mellaril): its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J 1963;89:546-54.
9. Donatini B, LeBlaye I, Krupp P. Transient cardiac pacing is insufficiently used to treat arrhythmia associated with thioridazine. Cardiology 1992;81(6):340-1.
10. Fowler NO, McCall D, et al. Electrocardiographic changes and cardiac arrhythmias in patients receiving psychotropic drugs. Am J Cardiol 1976;37:223-30.
11. Mehtonen OP, Aranko K, Malkonen L, Vapaatalo H. A survey of sudden death associated with the use of antipsychotic or antidepressant drugs: 49 cases in Finland. Acta Psychiatr Scand 1991;84:58-64.
12 Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920;7:353-70.
13. Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol 1993;72(suppl):17B-22B.
14. Bednar MM, et al. The QT Interval. Prog Cardiovas Dis 2001;43(5, pt 2):1-45.
15. Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 2001;12(4):411-20.
16. Browne K, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol 1983;52(1):55-9.
17. Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol 1991;67(8):774-6.
18. Woosley R, Sketch MH. Gender and drug-induced torsade de pointes. Bethesda, Md: American College of Cardiology, 1998; ACCEL 30, No. 2.
19. Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 2001;285(10):1322-6.
20. Vincent GM. Long QT syndrome. Cardiology Clinics 1999;18:309-25.
21. Khan SP, Dahlvani S, Vieweg WVR, Bernardo NL, Lewis RE. Electrocardiographic QT interval in a geropsychiatric inpatient population: a preliminary study. Med Psychiatr 1998;1:71-4.
22. Compton SJ, Lux RL, Ramsey MR, et al. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation 1996;94(5):1018-22.
23. Tan HL, Hou CJY, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann Intern Med 1995;122(9):701-14.
24. Jackman WM, Friday KJ, Anderson JL, et al. The long QT syndromes: a critical review, new clinical observations, and a unifying hypothesis. Prog Cardiovas Dis 1988;31(2):115-72.
25. Hatta K, Takahashi T, Nakamura H, et al. Hypokalemia and agitation in acute psychotic patients. Psychiatry Res 1999;86(1):85-8.
26. Food and Drug Administration Advisory Committee: Zeldox capsules (ziprasidone): summary of efficacy and safety and overall benefit risk relationship. Bethesda, Md: Food and Drug Administration, July 19, 2000.
Before approving the antipsychotic agent ziprasidone last year, the Food and Drug Administration required specific safety data on whether the drug might cause the life-threatening arrhythmia known as torsade de pointes.
The FDA’s action, which delayed the drug’s approval for 3 years, underscores growing concern about the risk of cardiovascular effects with the use of antipsychotic and other agents known to prolong the cardiac QT interval. This concern has led to withdrawal of some drugs before reaching the market (e.g., the atypical neuroleptic sertindole), the addition of “black box” warnings in the labeling of some antipsychotics, and withdrawal from the market of antihistamines terfenadine and astemizole and the GI stimulant cisapride.
Torsade de pointes is a polymorphic ventricular tachycardia (VT), a rare arrhythmia that can cause sudden death. Because torsade can occur with the use of some antipsychotics, the psychiatrist needs to consider cardiovascular safety when selecting among available agents. To help with these decisions, here is information about the documented and potential electrocardiographic features of commonly prescribed antipsychotic drugs, as well as background on QT interval prolongation and torsade de pointes.
Torsade de pointes
Named for a ballet movement, torsade de pointes describes bursts of “twisting of the points,” a variation of the morphology of the QRS vector about the isoelectric axis from positive to net negative and back again. As seen on an ECG (Figure 1), the first beat of torsade de pointes is a normal ventricular complex preceded by a P wave. This is followed by a premature ventricular contraction (PVC) with a short coupling interval. After a compensatory pause, a second normal beat is followed by a second PVC, which is the first beat of a polymorphic VT. We know tachycardia is present because the ventricular beats appear close together. We know the arrhythmia is ventricular in origin because the ventricular complexes are wide. Finally, we note the ventricular complexes vary in configuration—that is, the shape (morphology) varies from beat to beat.
Figure 1 Typical ECG features of torsade de pointes
Sinus beat with normal ventricular complex (1) followed by premature ventricular contraction (PVC) (2) with short coupling interval. After a long pause (long refractory period), another sinus beat (3) is followed by another PVC (4) with a short coupling interval. The second PVC (4) is the first beat of polymorphic ventricular tachycardiaIn torsade, the stimulus for the VT moves within the ventricle, changing its shape from beat to beat. This multifocal VT differs from the more common unifocal VT, in which all the QRS complexes appear the same.
Drug-induced torsade de pointes
Although the term torsade de pointes was first described in 1966,1 the drug-induced form of this arrhythmia has been recognized for nearly a century.
Quinidine Around 1920, cardiologists first used quinidine to help restore normal sinus rhythm in patients with atrial fibrillation, most commonly due to rheumatic heart disease.2
In 1964, Selzer and Wray3 studied the use of quinidine to convert atrial fibrillation to normal sinus rhythm in more than 200 patients seen during 4 years in a cardiopulmonary clinic. In a subgroup of eight patients, these researchers documented 10 reactions (including five documented episodes of ventricular fibrillation/ventricular flutter) among 36 syncopal episodes that developed within 1 to 6.5 hours of quinidine administration. Symptoms were nonspecific and included nausea, faintness, and feeling ill. It is now recognized that torsade de pointes was the principal rhythm disturbance in those eight patients. Syncope usually occurs early in treatment and may be found in 5% to 10% of patients taking quinidine.
TCAs and antipsychotics Tricyclic antidepressants (TCAs) and antipsychotics that have quinidine-like properties (e.g., thioridazine) also may be associated with QT interval prolongation and torsade de pointes.4-9 In high doses (particularly in overdose), TCAs may induce widening of the QRS complex. Fowler et al reported episodes of VT in five patients taking thioridazine—one of whom died.10
Mehtonen et al reported sudden unexpected deaths associated with antipsychotic or antidepressant drugs among 31 women and 18 men in a survey of autopsies performed from 1985 to 1988 in Finland. The authors documented therapeutic use of phenothiazines in all but 3 of the 49 cases. Thioridazine was involved in more than half the deaths. In 15 of the deaths, thioridazine was the only antipsychotic drug taken. Drugs other than thioridazine were documented in only 5 of the 49 sudden cardiac deaths.11
Figure 2 Normal ECG in sinus rhythm
In this typical lead II of a surface ECG, the P wave (atrial depolarization) leads to right and left atrial contraction and the QRS complex (ventricular depolarization) leads to left and right ventricular contraction. The ST segment represents isoelectric ventricular repolarization, and the T wave represents directional repolarization. The QT interval includes both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval, or ST segment plus T wave).
QT interval as a marker for torsade
The incidence of torsade is unknown, but it is an uncommon cardiac abnormality. In the United States, torsade probably accounts for less than 5% of the 300,000 sudden cardiac deaths that occur each year. Because torsade de pointes is rare, regulatory agencies and clinicians use the QT interval as a surrogate ECG marker for risk of torsade de pointes. Heart rate can affect the QT interval, so various formulae are used to correct the QT interval for heart rate (QTc).
What is the QT interval? In a normal ECG (Figure 2), the P wave derives from right and left atrial electrical depolarization. The pacemaker of the heart is located in the sino-atrial node (SAN) in the superior portion of the right atrium. From the SAN, electrical signals travel down three intra-atrial pathways, activating the right atrium, then travel to the atrioventricular node (AVN). Bachmann’s bundle—a fourth atrial pathway—passes from the SAN to depolarize the left atrium. From the AVN, the electrical signal travels through the left and right bundle branches to activate their respective ventricles.
Electrical depolarization of the left and right ventricles produces the QRS complex. Most of the electrical forces making up this complex arise in the left ventricle, which is much larger than the right ventricle.
The electrical circuitry of the heart activates the left and right atria in such a fashion that these chambers eject blood into their respective ventricles just before these chambers contract. Optimal ventricular filling maximizes ventricular ejection of blood (Starling’s law). Ventricular repolarization (JT interval—electrical recovery) follows ventricular depolarization. On the surface ECG, the JT interval consists of an isoelectric event—the ST segment running from the end of the QRS complex to the beginning of the T wave—and the T wave itself (directional electrical recovery).
The QT interval, then, consists of both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval). Ventricular repolarization makes up by far the greater portion of the QT interval.
Correcting the QT interval (QTc) In 1920, Bazett noted that as the heart rate slowed, the QT interval lengthened.12 From personal and reported observations, he derived an equation called the Bazett formula that corrects (or normalizes) the QT interval to a heart rate of 60 beats/min (QTc). In the Bazett formula, the QTc interval is the measured QT interval divided by the square root of the RR interval (time between sequential QRS complexes—the determinant of heart rate) measured in seconds (QTc = QT/RR).
The Bazett formula is most widely used to estimate the QTc interval, although at least 20 other formulae have been developed in response to the original’s perceived inadequacies.13-15 Bazett’s formula is used in most automated interpretations of the ECG.
Up to age 55, the normal QTc interval ranges from 350 to 430 msec for men and 350 to 450 msec for women, and it tends to increase with age. Most cases of torsade occur when the QT or QTc interval is greater than 500 msec.14 A QTc interval between 450 and 500 msec is cause for concern; a QTc interval that exceeds 500 msec is cause for alarm.
Factors that cause variations in QTc
Factors that can affect the QTc interval and increase the risk of torsade de pointes include electrolyte imbalances, medication use and overdose, cardiac disease, liver disease, endocrine disorders such as diabetes and hypothyroidism, and CNS injury (Table 1).
Table 1
Risk factors contributing to QTc interval prolongation
| Risk factor | Causes/implications |
|---|---|
| Sex (female) | QT intervals longer in women than in men QT interval longer during first half of menstrual cycle |
| Age (elderly) | Increased risk for CAD Multiple medications Pharmacokinetic/pharmacodynamic changes |
| Electrolyte imbalance Hypokalemia, hypomagnesemia Hypocalcemia | Diuretic use Excessive vomiting or diarrhea Postprandial hypokalemia |
| Congenital long QT syndrome | Associated with torsade and sudden death |
| Cardiac disease, with history of acute or chronic myocardial ischemia, CHF, cardiac arrhythmias, bradycardia | Increased risk of cardiac arrhythmias |
| Drugs known to prolong QTc interval | May potentiate QTc prolongation |
| Medication overdose with drugs that prolong the QTc interval | QTc prolongation generally dose-dependent |
| Concomitant medications, liver disease | Adverse events with cytochrome P-450 enzyme system inhibition, leading to increased drug levels that can increase QT interval |
| Endocrine/metabolic disorders Diabetes, obesity Hypothyroidism, pituitary insufficiency | Via electrolytes or cardiovascular disease |
| CNS injury Stroke, infection, trauma | Via autonomic nervous system dysfunction |
Circadian patterns The QTc interval varies throughout the 24-hour day, with nocturnal values about 20 msec greater than daytime measurements. These differences are driven by changes in autonomic (sympathetic and parasympathetic) tone.16,17 In 20 normal subjects, circadian variability was 76 ± 19 msec (range 35 to 108 msec) from day to night.17 This circadian variation may be accentuated in patients with cardiovascular disease.
Sex. At birth, QTc interval measurements do no vary by sex.18 At puberty, however, the male QTc interval shortens and remains shorter than its female counterpart by about 20 msec until age 50 to 55, coincident with a decline in male testosterone levels. This sex difference appears to be androgen driven. About 70% of torsade de pointes cases occur in women.18
Menstrual cycle QTc interval measurements are stable throughout the menstrual cycle if quinidine-like drugs are not given.
Variations were seen, however, when Rodriguez et al studied the effect of IV low-dose ibutilide (an antiarrhythmic agent known to prolong the QT interval) on the QTc intervals of 58 healthy subjects (38 men and 20 women, ages 21 to 40). During 1 month, men were studied once and women studied three times, coincident with the three phases of the menstrual cycle. The greatest increase in QTc intervals measurements occurred in women during the first half of their menstrual cycles.19
Age and cardiovascular disease Two congenital long QT syndromes may be associated with sudden death, mostly in children and young adults:
- The Jervell and Lange-Nielsen syndrome is marked by severe congenital deafness and autosomal recessive inheritance.
- The Romano-Ward syndrome has normal hearing and autosomal dominant inheritance.20
Congenital long QT syndrome (LQTS) occurs in about one in 5,000 births and accounts for about 3,000 to 4,000 deaths per year in the United States. Nine percent of pediatric LQTS subjects present with sudden cardiac death. More than 71% of patients will die before age 15 if not treated.
Elderly persons tend to have longer QTc intervals than do younger subjects, even when both groups are free of cardiovascular disease.21 Also, age-matched subjects with cardiovascular disease tend to have longer QTc intervals than do those free of cardiovascular disease.
Electrolytes Electrolyte disturbances, particularly hypokalemia and hypomagnesemia, may contribute to or even cause QT interval prolongation.22
Hypokalemia prolongs the cardiac action potential and may cause early afterdepolarization, leading to torsade.23 Low potassium levels reduce the net outward potassium current during phase 3 of the cardiac action potential. Hypomagnesemia may contribute to gross U wave alternans, lengthening the cardiac action potential and setting the stage for torsade.24 Various factors may contribute to electrolyte disturbances, including use of diuretics and excessive vomiting and diarrhea. Even postprandial states may induce hypokalemia.
Intensive exercise and agitation may be associated with hypokalemia.25 Serum potassium may be lower in severely agitated patients (3.59 mmol/L) than in mildly agitated patients (3.79 mmol/L). The mean QTc interval of psychiatric emergency patients may be prolonged (453±40 msec),5 with QTc intervals of psychiatric inpatients longer than those of psychiatric outpatients. Altered potassium states probably explain these observations. Mechanisms that link exercise and agitation with hypokalemia remain to be elucidated.
Metabolic factors Drugs may alter phase 3 potassium flow, thereby disrupting the synchrony of action of individual cardiac cells during repolarization. This change may induce early afterdepolarizations and torsade.23
Five percent to 10% of Americans of European descent have genetic profiles that make them poor metabolizers of drugs that are metabolized by the cytochrome P-450 isoenzyme 2D6. The Pfizer Inc. 054 study assessed the potential for metabolic inhibitors such as paroxetine to raise antipsychotic drug levels in these patients and induce QTc interval prolongation.26
In response to FDA concerns about QTc interval prolongation associated with the use of ziprasidone, Pfizer studied the potential for QTc interval prolongation when antipsychotics are given with and without metabolic inhibitors of cytochrome P-450 isoenzymes 2D6 (paroxetine), 3A4 (ketoconazole), and 1A2 (fluvoxamine). The study population of 183 subjects (mean age:men, 37.1 years, women 38.8 years) was three-quarters young men with schizophrenia, in good health otherwise and possessing normal ECGs—i.e., patients with a low risk of developing cardiac arrhythmias.
Figure 3 Antipsychotic drugs and QTc interval changes
Six antipsychotic drugs and QTc interval changes from baseline when given with and without metabolic inhibitors. QTc interval changes (in msec) when given without a metabolic inhibitor were ziprasidone, 20.3; risperidone, 11.6; olanzapine, 6.8; quetiapine, 14.5; thioridazine, 35.6; and haloperidol 4.7.
Reprinted from: “FDA Psychopharmacological Drugs Advisory Committee. 19 July 2000. Briefing Documents for Zeldox Capsules (Ziprasidone HCL). Pfizer.” Available from Central Research Division, Pfizer, Inc., Eastern Point Road, Groton, CT 06340, (860) 441-4100.Over the course of about 1 week, daily doses were escalated to ziprasidone, 160 mg; risperidone, 8 mg and 16 mg; olanzapine, 20 mg; quetiapine, 750 mg; thioridazine, 300 mg; and haloperidol, 15 mg. Thioridazine (35.6 msec) and ziprasidone (20.3 msec) showed the greatest QTc interval increase following drug administration (Figure 3). Co-administration of a metabolic inhibitor did not further prolong the QTc interval for these two drugs.
Of the six drugs studied, only thioridazine and ziprasidone showed QTc interval increases 5% compared with baseline measurements.
Co-administration of a metabolic inhibitor caused the greatest increase in QTc intervals for quetiapine (from 14.5 to 19.7 msec). This value closely approached the steady-state ziprasidone measurement (20.3 msec). Because quetiapine is more likely than the other antipsychotic drugs studied to increase heart rate, it may be argued that the Bazett formula’s limitations in estimating the QTc interval at higher heart rates contributed to the quetiapine study findings.
Table 2
Relative risk of QTc interval prolongation with common antipsychotic agents
| Risk level | Agent |
|---|---|
| ECG required or strongly recommended before prescribing (most commonly associated with QTc interval prolongation and torsade de pointes) | Thioridazine Mesoridazine Droperidol Pimozide Haloperidol in large doses IV (commonly ≥ 100 mg/d) |
| Mild to moderate risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Quetiapine Ziprasidone Chlorpromazine |
| Little or no risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Haloperidol (oral) Olanzapine Risperidone Clozapine |
Recommendations
Taking a careful history is key to cardiovascular assessment before prescribing an antipsychotic. An ECG is indicated for patients with:
- Personal or family history of syncope or sudden death;
- Personal history of angina pectoris, myocardial infarction, congestive heart failure, cardiac arrhythmias, hypokalemia, hypomagnesemia, or significant cardiac risk factors.
The relative cardiovascular risks associated with antipsychotic agents are shown in Table 2.
An ECG also is required or strongly recommended before prescribing the antipsychotic drugs most commonly associated with QT prolongation and torsade de pointes—droperidol, haloperidol in large doses IV (commonly 100 mg/d), mesoridazine, pimozide, and thioridazine.
The FDA has strengthened the warning labels required for these agents, adding “black box” warnings about the risks of prolonged QTc intervals, torsade de pointes, and sudden death for droperidol, mesoridazine, and thioridazine. Thioridazine, for example, is indicated only for patients with schizophrenia who fail to show an acceptable response to other antipsychotic drugs. Its use is contraindicated in patients who take:
- fluvoxamine, propranolol, and pindolol;
- any drug that inhibits the cytochrome P-450 2D6 isoenzyme (e.g., fluoxetine, paroxetine);
- agents known to prolong the QTc interval.
Use of thioridazine also is contraindicated in patients known to have reduced levels of the cytochrome P450 2D6 isozyme, as well as in patients with congenital LQTS or a history of cardiac arrhythmias. Psychiatrists are advised to read the warnings and prescribing information in the labeling of all antipsychotics for potential cardiovascular side effects.
When the psychiatrist receives a report of suspected QTc interval prolongation on a patient’s ECG, the following steps are recommended:
- Obtain another ECG.
- Assess serum potassium, magnesium, calcium, and thyroid hormone levels.
In patients with confirmed QTc interval prolongation, any complaint of palpitations, presyncope, or syncope are grounds for urgent referral to a cardiologist.
Related resources
- European Society of Cardiology guidelines: www.escardio.org/scinfo/Guidelines/Haverkamp.pdf
- Sudden Arrhythmia Death Syndromes Foundation (SADSF): www.sads.org (800) 786-7723.
- Drugs that prolong the QT interval and/or induce torsade de pointes. Georgetown Center for Education and Research Therapeutics: www.torsades.org
Before approving the antipsychotic agent ziprasidone last year, the Food and Drug Administration required specific safety data on whether the drug might cause the life-threatening arrhythmia known as torsade de pointes.
The FDA’s action, which delayed the drug’s approval for 3 years, underscores growing concern about the risk of cardiovascular effects with the use of antipsychotic and other agents known to prolong the cardiac QT interval. This concern has led to withdrawal of some drugs before reaching the market (e.g., the atypical neuroleptic sertindole), the addition of “black box” warnings in the labeling of some antipsychotics, and withdrawal from the market of antihistamines terfenadine and astemizole and the GI stimulant cisapride.
Torsade de pointes is a polymorphic ventricular tachycardia (VT), a rare arrhythmia that can cause sudden death. Because torsade can occur with the use of some antipsychotics, the psychiatrist needs to consider cardiovascular safety when selecting among available agents. To help with these decisions, here is information about the documented and potential electrocardiographic features of commonly prescribed antipsychotic drugs, as well as background on QT interval prolongation and torsade de pointes.
Torsade de pointes
Named for a ballet movement, torsade de pointes describes bursts of “twisting of the points,” a variation of the morphology of the QRS vector about the isoelectric axis from positive to net negative and back again. As seen on an ECG (Figure 1), the first beat of torsade de pointes is a normal ventricular complex preceded by a P wave. This is followed by a premature ventricular contraction (PVC) with a short coupling interval. After a compensatory pause, a second normal beat is followed by a second PVC, which is the first beat of a polymorphic VT. We know tachycardia is present because the ventricular beats appear close together. We know the arrhythmia is ventricular in origin because the ventricular complexes are wide. Finally, we note the ventricular complexes vary in configuration—that is, the shape (morphology) varies from beat to beat.
Figure 1 Typical ECG features of torsade de pointes
Sinus beat with normal ventricular complex (1) followed by premature ventricular contraction (PVC) (2) with short coupling interval. After a long pause (long refractory period), another sinus beat (3) is followed by another PVC (4) with a short coupling interval. The second PVC (4) is the first beat of polymorphic ventricular tachycardiaIn torsade, the stimulus for the VT moves within the ventricle, changing its shape from beat to beat. This multifocal VT differs from the more common unifocal VT, in which all the QRS complexes appear the same.
Drug-induced torsade de pointes
Although the term torsade de pointes was first described in 1966,1 the drug-induced form of this arrhythmia has been recognized for nearly a century.
Quinidine Around 1920, cardiologists first used quinidine to help restore normal sinus rhythm in patients with atrial fibrillation, most commonly due to rheumatic heart disease.2
In 1964, Selzer and Wray3 studied the use of quinidine to convert atrial fibrillation to normal sinus rhythm in more than 200 patients seen during 4 years in a cardiopulmonary clinic. In a subgroup of eight patients, these researchers documented 10 reactions (including five documented episodes of ventricular fibrillation/ventricular flutter) among 36 syncopal episodes that developed within 1 to 6.5 hours of quinidine administration. Symptoms were nonspecific and included nausea, faintness, and feeling ill. It is now recognized that torsade de pointes was the principal rhythm disturbance in those eight patients. Syncope usually occurs early in treatment and may be found in 5% to 10% of patients taking quinidine.
TCAs and antipsychotics Tricyclic antidepressants (TCAs) and antipsychotics that have quinidine-like properties (e.g., thioridazine) also may be associated with QT interval prolongation and torsade de pointes.4-9 In high doses (particularly in overdose), TCAs may induce widening of the QRS complex. Fowler et al reported episodes of VT in five patients taking thioridazine—one of whom died.10
Mehtonen et al reported sudden unexpected deaths associated with antipsychotic or antidepressant drugs among 31 women and 18 men in a survey of autopsies performed from 1985 to 1988 in Finland. The authors documented therapeutic use of phenothiazines in all but 3 of the 49 cases. Thioridazine was involved in more than half the deaths. In 15 of the deaths, thioridazine was the only antipsychotic drug taken. Drugs other than thioridazine were documented in only 5 of the 49 sudden cardiac deaths.11
Figure 2 Normal ECG in sinus rhythm
In this typical lead II of a surface ECG, the P wave (atrial depolarization) leads to right and left atrial contraction and the QRS complex (ventricular depolarization) leads to left and right ventricular contraction. The ST segment represents isoelectric ventricular repolarization, and the T wave represents directional repolarization. The QT interval includes both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval, or ST segment plus T wave).
QT interval as a marker for torsade
The incidence of torsade is unknown, but it is an uncommon cardiac abnormality. In the United States, torsade probably accounts for less than 5% of the 300,000 sudden cardiac deaths that occur each year. Because torsade de pointes is rare, regulatory agencies and clinicians use the QT interval as a surrogate ECG marker for risk of torsade de pointes. Heart rate can affect the QT interval, so various formulae are used to correct the QT interval for heart rate (QTc).
What is the QT interval? In a normal ECG (Figure 2), the P wave derives from right and left atrial electrical depolarization. The pacemaker of the heart is located in the sino-atrial node (SAN) in the superior portion of the right atrium. From the SAN, electrical signals travel down three intra-atrial pathways, activating the right atrium, then travel to the atrioventricular node (AVN). Bachmann’s bundle—a fourth atrial pathway—passes from the SAN to depolarize the left atrium. From the AVN, the electrical signal travels through the left and right bundle branches to activate their respective ventricles.
Electrical depolarization of the left and right ventricles produces the QRS complex. Most of the electrical forces making up this complex arise in the left ventricle, which is much larger than the right ventricle.
The electrical circuitry of the heart activates the left and right atria in such a fashion that these chambers eject blood into their respective ventricles just before these chambers contract. Optimal ventricular filling maximizes ventricular ejection of blood (Starling’s law). Ventricular repolarization (JT interval—electrical recovery) follows ventricular depolarization. On the surface ECG, the JT interval consists of an isoelectric event—the ST segment running from the end of the QRS complex to the beginning of the T wave—and the T wave itself (directional electrical recovery).
The QT interval, then, consists of both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval). Ventricular repolarization makes up by far the greater portion of the QT interval.
Correcting the QT interval (QTc) In 1920, Bazett noted that as the heart rate slowed, the QT interval lengthened.12 From personal and reported observations, he derived an equation called the Bazett formula that corrects (or normalizes) the QT interval to a heart rate of 60 beats/min (QTc). In the Bazett formula, the QTc interval is the measured QT interval divided by the square root of the RR interval (time between sequential QRS complexes—the determinant of heart rate) measured in seconds (QTc = QT/RR).
The Bazett formula is most widely used to estimate the QTc interval, although at least 20 other formulae have been developed in response to the original’s perceived inadequacies.13-15 Bazett’s formula is used in most automated interpretations of the ECG.
Up to age 55, the normal QTc interval ranges from 350 to 430 msec for men and 350 to 450 msec for women, and it tends to increase with age. Most cases of torsade occur when the QT or QTc interval is greater than 500 msec.14 A QTc interval between 450 and 500 msec is cause for concern; a QTc interval that exceeds 500 msec is cause for alarm.
Factors that cause variations in QTc
Factors that can affect the QTc interval and increase the risk of torsade de pointes include electrolyte imbalances, medication use and overdose, cardiac disease, liver disease, endocrine disorders such as diabetes and hypothyroidism, and CNS injury (Table 1).
Table 1
Risk factors contributing to QTc interval prolongation
| Risk factor | Causes/implications |
|---|---|
| Sex (female) | QT intervals longer in women than in men QT interval longer during first half of menstrual cycle |
| Age (elderly) | Increased risk for CAD Multiple medications Pharmacokinetic/pharmacodynamic changes |
| Electrolyte imbalance Hypokalemia, hypomagnesemia Hypocalcemia | Diuretic use Excessive vomiting or diarrhea Postprandial hypokalemia |
| Congenital long QT syndrome | Associated with torsade and sudden death |
| Cardiac disease, with history of acute or chronic myocardial ischemia, CHF, cardiac arrhythmias, bradycardia | Increased risk of cardiac arrhythmias |
| Drugs known to prolong QTc interval | May potentiate QTc prolongation |
| Medication overdose with drugs that prolong the QTc interval | QTc prolongation generally dose-dependent |
| Concomitant medications, liver disease | Adverse events with cytochrome P-450 enzyme system inhibition, leading to increased drug levels that can increase QT interval |
| Endocrine/metabolic disorders Diabetes, obesity Hypothyroidism, pituitary insufficiency | Via electrolytes or cardiovascular disease |
| CNS injury Stroke, infection, trauma | Via autonomic nervous system dysfunction |
Circadian patterns The QTc interval varies throughout the 24-hour day, with nocturnal values about 20 msec greater than daytime measurements. These differences are driven by changes in autonomic (sympathetic and parasympathetic) tone.16,17 In 20 normal subjects, circadian variability was 76 ± 19 msec (range 35 to 108 msec) from day to night.17 This circadian variation may be accentuated in patients with cardiovascular disease.
Sex. At birth, QTc interval measurements do no vary by sex.18 At puberty, however, the male QTc interval shortens and remains shorter than its female counterpart by about 20 msec until age 50 to 55, coincident with a decline in male testosterone levels. This sex difference appears to be androgen driven. About 70% of torsade de pointes cases occur in women.18
Menstrual cycle QTc interval measurements are stable throughout the menstrual cycle if quinidine-like drugs are not given.
Variations were seen, however, when Rodriguez et al studied the effect of IV low-dose ibutilide (an antiarrhythmic agent known to prolong the QT interval) on the QTc intervals of 58 healthy subjects (38 men and 20 women, ages 21 to 40). During 1 month, men were studied once and women studied three times, coincident with the three phases of the menstrual cycle. The greatest increase in QTc intervals measurements occurred in women during the first half of their menstrual cycles.19
Age and cardiovascular disease Two congenital long QT syndromes may be associated with sudden death, mostly in children and young adults:
- The Jervell and Lange-Nielsen syndrome is marked by severe congenital deafness and autosomal recessive inheritance.
- The Romano-Ward syndrome has normal hearing and autosomal dominant inheritance.20
Congenital long QT syndrome (LQTS) occurs in about one in 5,000 births and accounts for about 3,000 to 4,000 deaths per year in the United States. Nine percent of pediatric LQTS subjects present with sudden cardiac death. More than 71% of patients will die before age 15 if not treated.
Elderly persons tend to have longer QTc intervals than do younger subjects, even when both groups are free of cardiovascular disease.21 Also, age-matched subjects with cardiovascular disease tend to have longer QTc intervals than do those free of cardiovascular disease.
Electrolytes Electrolyte disturbances, particularly hypokalemia and hypomagnesemia, may contribute to or even cause QT interval prolongation.22
Hypokalemia prolongs the cardiac action potential and may cause early afterdepolarization, leading to torsade.23 Low potassium levels reduce the net outward potassium current during phase 3 of the cardiac action potential. Hypomagnesemia may contribute to gross U wave alternans, lengthening the cardiac action potential and setting the stage for torsade.24 Various factors may contribute to electrolyte disturbances, including use of diuretics and excessive vomiting and diarrhea. Even postprandial states may induce hypokalemia.
Intensive exercise and agitation may be associated with hypokalemia.25 Serum potassium may be lower in severely agitated patients (3.59 mmol/L) than in mildly agitated patients (3.79 mmol/L). The mean QTc interval of psychiatric emergency patients may be prolonged (453±40 msec),5 with QTc intervals of psychiatric inpatients longer than those of psychiatric outpatients. Altered potassium states probably explain these observations. Mechanisms that link exercise and agitation with hypokalemia remain to be elucidated.
Metabolic factors Drugs may alter phase 3 potassium flow, thereby disrupting the synchrony of action of individual cardiac cells during repolarization. This change may induce early afterdepolarizations and torsade.23
Five percent to 10% of Americans of European descent have genetic profiles that make them poor metabolizers of drugs that are metabolized by the cytochrome P-450 isoenzyme 2D6. The Pfizer Inc. 054 study assessed the potential for metabolic inhibitors such as paroxetine to raise antipsychotic drug levels in these patients and induce QTc interval prolongation.26
In response to FDA concerns about QTc interval prolongation associated with the use of ziprasidone, Pfizer studied the potential for QTc interval prolongation when antipsychotics are given with and without metabolic inhibitors of cytochrome P-450 isoenzymes 2D6 (paroxetine), 3A4 (ketoconazole), and 1A2 (fluvoxamine). The study population of 183 subjects (mean age:men, 37.1 years, women 38.8 years) was three-quarters young men with schizophrenia, in good health otherwise and possessing normal ECGs—i.e., patients with a low risk of developing cardiac arrhythmias.
Figure 3 Antipsychotic drugs and QTc interval changes
Six antipsychotic drugs and QTc interval changes from baseline when given with and without metabolic inhibitors. QTc interval changes (in msec) when given without a metabolic inhibitor were ziprasidone, 20.3; risperidone, 11.6; olanzapine, 6.8; quetiapine, 14.5; thioridazine, 35.6; and haloperidol 4.7.
Reprinted from: “FDA Psychopharmacological Drugs Advisory Committee. 19 July 2000. Briefing Documents for Zeldox Capsules (Ziprasidone HCL). Pfizer.” Available from Central Research Division, Pfizer, Inc., Eastern Point Road, Groton, CT 06340, (860) 441-4100.Over the course of about 1 week, daily doses were escalated to ziprasidone, 160 mg; risperidone, 8 mg and 16 mg; olanzapine, 20 mg; quetiapine, 750 mg; thioridazine, 300 mg; and haloperidol, 15 mg. Thioridazine (35.6 msec) and ziprasidone (20.3 msec) showed the greatest QTc interval increase following drug administration (Figure 3). Co-administration of a metabolic inhibitor did not further prolong the QTc interval for these two drugs.
Of the six drugs studied, only thioridazine and ziprasidone showed QTc interval increases 5% compared with baseline measurements.
Co-administration of a metabolic inhibitor caused the greatest increase in QTc intervals for quetiapine (from 14.5 to 19.7 msec). This value closely approached the steady-state ziprasidone measurement (20.3 msec). Because quetiapine is more likely than the other antipsychotic drugs studied to increase heart rate, it may be argued that the Bazett formula’s limitations in estimating the QTc interval at higher heart rates contributed to the quetiapine study findings.
Table 2
Relative risk of QTc interval prolongation with common antipsychotic agents
| Risk level | Agent |
|---|---|
| ECG required or strongly recommended before prescribing (most commonly associated with QTc interval prolongation and torsade de pointes) | Thioridazine Mesoridazine Droperidol Pimozide Haloperidol in large doses IV (commonly ≥ 100 mg/d) |
| Mild to moderate risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Quetiapine Ziprasidone Chlorpromazine |
| Little or no risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Haloperidol (oral) Olanzapine Risperidone Clozapine |
Recommendations
Taking a careful history is key to cardiovascular assessment before prescribing an antipsychotic. An ECG is indicated for patients with:
- Personal or family history of syncope or sudden death;
- Personal history of angina pectoris, myocardial infarction, congestive heart failure, cardiac arrhythmias, hypokalemia, hypomagnesemia, or significant cardiac risk factors.
The relative cardiovascular risks associated with antipsychotic agents are shown in Table 2.
An ECG also is required or strongly recommended before prescribing the antipsychotic drugs most commonly associated with QT prolongation and torsade de pointes—droperidol, haloperidol in large doses IV (commonly 100 mg/d), mesoridazine, pimozide, and thioridazine.
The FDA has strengthened the warning labels required for these agents, adding “black box” warnings about the risks of prolonged QTc intervals, torsade de pointes, and sudden death for droperidol, mesoridazine, and thioridazine. Thioridazine, for example, is indicated only for patients with schizophrenia who fail to show an acceptable response to other antipsychotic drugs. Its use is contraindicated in patients who take:
- fluvoxamine, propranolol, and pindolol;
- any drug that inhibits the cytochrome P-450 2D6 isoenzyme (e.g., fluoxetine, paroxetine);
- agents known to prolong the QTc interval.
Use of thioridazine also is contraindicated in patients known to have reduced levels of the cytochrome P450 2D6 isozyme, as well as in patients with congenital LQTS or a history of cardiac arrhythmias. Psychiatrists are advised to read the warnings and prescribing information in the labeling of all antipsychotics for potential cardiovascular side effects.
When the psychiatrist receives a report of suspected QTc interval prolongation on a patient’s ECG, the following steps are recommended:
- Obtain another ECG.
- Assess serum potassium, magnesium, calcium, and thyroid hormone levels.
In patients with confirmed QTc interval prolongation, any complaint of palpitations, presyncope, or syncope are grounds for urgent referral to a cardiologist.
Related resources
- European Society of Cardiology guidelines: www.escardio.org/scinfo/Guidelines/Haverkamp.pdf
- Sudden Arrhythmia Death Syndromes Foundation (SADSF): www.sads.org (800) 786-7723.
- Drugs that prolong the QT interval and/or induce torsade de pointes. Georgetown Center for Education and Research Therapeutics: www.torsades.org
1. Dessertenne F. Tachycardie ventriculaire a deux foyers opposes variables. Arch Mal Coeur Vaiss 1966;59(2):263-72.
2. Clark-Kennedy AE. Quinidine in the treatment of auricular fibrillation. Quart J Med 1922;16:204-35.
3. Selzer A, Wray W. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation 1964;30:17-26.
4. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 2000;355:1048-52.
5. Hatta K, Takahashi T, Nakamura H, Yamashiro H, Yonezawa Y. Prolonged QT interval in acute psychotic patients. Psychiatry Res 2000;94(3):279-85.
6. Welch R. Antipsychotic agents and QT changes. J Psychiatry Neurosci 2000;25(2):154-60.
7. Fayek M, Kingsbury SJ, Zada J, Simpson GM. Cardiac effects of antipsychotic medications. Psychiatr Serv 2001;52(5):607-9.
8. Kelly HG, Fay JE, Laverty SG. Thioridazine hydrochloride (Mellaril): its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J 1963;89:546-54.
9. Donatini B, LeBlaye I, Krupp P. Transient cardiac pacing is insufficiently used to treat arrhythmia associated with thioridazine. Cardiology 1992;81(6):340-1.
10. Fowler NO, McCall D, et al. Electrocardiographic changes and cardiac arrhythmias in patients receiving psychotropic drugs. Am J Cardiol 1976;37:223-30.
11. Mehtonen OP, Aranko K, Malkonen L, Vapaatalo H. A survey of sudden death associated with the use of antipsychotic or antidepressant drugs: 49 cases in Finland. Acta Psychiatr Scand 1991;84:58-64.
12 Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920;7:353-70.
13. Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol 1993;72(suppl):17B-22B.
14. Bednar MM, et al. The QT Interval. Prog Cardiovas Dis 2001;43(5, pt 2):1-45.
15. Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 2001;12(4):411-20.
16. Browne K, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol 1983;52(1):55-9.
17. Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol 1991;67(8):774-6.
18. Woosley R, Sketch MH. Gender and drug-induced torsade de pointes. Bethesda, Md: American College of Cardiology, 1998; ACCEL 30, No. 2.
19. Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 2001;285(10):1322-6.
20. Vincent GM. Long QT syndrome. Cardiology Clinics 1999;18:309-25.
21. Khan SP, Dahlvani S, Vieweg WVR, Bernardo NL, Lewis RE. Electrocardiographic QT interval in a geropsychiatric inpatient population: a preliminary study. Med Psychiatr 1998;1:71-4.
22. Compton SJ, Lux RL, Ramsey MR, et al. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation 1996;94(5):1018-22.
23. Tan HL, Hou CJY, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann Intern Med 1995;122(9):701-14.
24. Jackman WM, Friday KJ, Anderson JL, et al. The long QT syndromes: a critical review, new clinical observations, and a unifying hypothesis. Prog Cardiovas Dis 1988;31(2):115-72.
25. Hatta K, Takahashi T, Nakamura H, et al. Hypokalemia and agitation in acute psychotic patients. Psychiatry Res 1999;86(1):85-8.
26. Food and Drug Administration Advisory Committee: Zeldox capsules (ziprasidone): summary of efficacy and safety and overall benefit risk relationship. Bethesda, Md: Food and Drug Administration, July 19, 2000.
1. Dessertenne F. Tachycardie ventriculaire a deux foyers opposes variables. Arch Mal Coeur Vaiss 1966;59(2):263-72.
2. Clark-Kennedy AE. Quinidine in the treatment of auricular fibrillation. Quart J Med 1922;16:204-35.
3. Selzer A, Wray W. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation 1964;30:17-26.
4. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 2000;355:1048-52.
5. Hatta K, Takahashi T, Nakamura H, Yamashiro H, Yonezawa Y. Prolonged QT interval in acute psychotic patients. Psychiatry Res 2000;94(3):279-85.
6. Welch R. Antipsychotic agents and QT changes. J Psychiatry Neurosci 2000;25(2):154-60.
7. Fayek M, Kingsbury SJ, Zada J, Simpson GM. Cardiac effects of antipsychotic medications. Psychiatr Serv 2001;52(5):607-9.
8. Kelly HG, Fay JE, Laverty SG. Thioridazine hydrochloride (Mellaril): its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J 1963;89:546-54.
9. Donatini B, LeBlaye I, Krupp P. Transient cardiac pacing is insufficiently used to treat arrhythmia associated with thioridazine. Cardiology 1992;81(6):340-1.
10. Fowler NO, McCall D, et al. Electrocardiographic changes and cardiac arrhythmias in patients receiving psychotropic drugs. Am J Cardiol 1976;37:223-30.
11. Mehtonen OP, Aranko K, Malkonen L, Vapaatalo H. A survey of sudden death associated with the use of antipsychotic or antidepressant drugs: 49 cases in Finland. Acta Psychiatr Scand 1991;84:58-64.
12 Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920;7:353-70.
13. Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol 1993;72(suppl):17B-22B.
14. Bednar MM, et al. The QT Interval. Prog Cardiovas Dis 2001;43(5, pt 2):1-45.
15. Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 2001;12(4):411-20.
16. Browne K, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol 1983;52(1):55-9.
17. Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol 1991;67(8):774-6.
18. Woosley R, Sketch MH. Gender and drug-induced torsade de pointes. Bethesda, Md: American College of Cardiology, 1998; ACCEL 30, No. 2.
19. Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 2001;285(10):1322-6.
20. Vincent GM. Long QT syndrome. Cardiology Clinics 1999;18:309-25.
21. Khan SP, Dahlvani S, Vieweg WVR, Bernardo NL, Lewis RE. Electrocardiographic QT interval in a geropsychiatric inpatient population: a preliminary study. Med Psychiatr 1998;1:71-4.
22. Compton SJ, Lux RL, Ramsey MR, et al. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation 1996;94(5):1018-22.
23. Tan HL, Hou CJY, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann Intern Med 1995;122(9):701-14.
24. Jackman WM, Friday KJ, Anderson JL, et al. The long QT syndromes: a critical review, new clinical observations, and a unifying hypothesis. Prog Cardiovas Dis 1988;31(2):115-72.
25. Hatta K, Takahashi T, Nakamura H, et al. Hypokalemia and agitation in acute psychotic patients. Psychiatry Res 1999;86(1):85-8.
26. Food and Drug Administration Advisory Committee: Zeldox capsules (ziprasidone): summary of efficacy and safety and overall benefit risk relationship. Bethesda, Md: Food and Drug Administration, July 19, 2000.
A diagnosis that’s yours to make: Accidental hypothermia in the elderly
You may well be the first specialist to evaluate an elderly patient with accidental hypothermia, a severe medical illness, because patients with this condition may present initially with cognitive impairment and disruptive behavior. This problem is particularly evident when evaluating elderly patients. Accidental hypothermia commonly mimics major mental illness, may be induced or exacerbated by psychotropic medications, is commonly fatal, and may remain unrecognized without a high index of suspicion.
Hypothermia is defined as a fall in body temperature below 95°F or 35°C (Box 1). Clinical mercury thermometers commonly range between 96°F and 106°F. Thus, the family member or clinician may not suspect hypothermia after the initial temperature measurement.
The diagnosis of accidental hypothermia is straightforward if there is a history of environmental exposure, but such evidence is often lacking in urban settings and among the elderly. Also, particularly in the elderly, hypothermia may occur at room temperature, secondary to diseases that strike the hypothalamic thermoregulatory center.
Subjects with core body temperatures dropping from 95°F to 90°F develop amnesia, dysarthria, confusion, and disruptive behavior.1 Further cooling as the body temperature falls to 82.4°F yields stupor, paradoxical undressing, and hallucinations. These characteristics are illustrated in the accompanying vignette of Ms. B.
![]()
The body’s thermoregulatory center located in the hypothalamus normally maintains core body temperature between 97.5°F (36.5°C) and 99.5°F (37.5°C). When body temperature declines, heat production increases by shivering, and heat loss is reduced by decreasing cutaneous blood flow.
Accidental hypothermia is defined as an unintentional fall in body temperature below 95°F (35°C). The coordinated systems responsible for thermoregulation start to fail. Heat loss through radiation, conduction, convection, respiration, and evaporation occurs because compensatory physiologic mechanisms are both limited and impaired.
Schizophrenia and the hypothalamus
Over the course of 6 months before her death, Ms. B. showed evidence of both thermoregulatory dysfunction and autonomic nervous system instability. We do not know if these hypothalamic problems were separate from, or intrinsic parts of, her schizophrenia.
The hypothalamus regulates autonomic, endocrine, and visceral function. Hypothalamic dysfunction may be an intrinsic part of schizophrenia. Such dysfunction occurs most commonly in the periventricular and supraoptic nuclei of the hypothalamus.2 These areas are adjacent to hypothalamic areas regulating body temperature.3
Lesions in anterior parts of the hypothalamus (temperaturesensitive neurons in the preoptic nuclei—located close to nuclei controlling thirst and osmotic regulation) may induce hyperthermia, impairing heat-dissipating mechanisms including vasodilatation and sweating. Lesions in posterior parts of the hypothalamus may impair heat conservation and heat production mechanisms and induce hypothermia.4
Associated medical problems
Independent of drug treatment, metabolic and cardiovascular problems occur more frequently in patients suffering from schizophrenia than they do in the general population.5 Ms. B. developed hypertension, diabetes mellitus, dyslipidemia, and coronary artery disease.
Diabetes mellitus in particular is a risk factor for hypothermia and may be found in more than 10 percent of elderly patients who suffered thermoregulatory failure before dying.6 Diabetes may impair autonomic system vasomotor stability and the body’s ability to vasoconstrict to preserve body heat.
Dementia and hypothermia
Cognitive impairment is a core feature of schizophrenia,7 and dementia is a common outcome among elderly patients suffering with the disorder.8 We don’t know whether Ms. B.’s progressive cognitive deterioration derived from dementia associated with schizophrenia or from a separate process such as Alzheimer’s disease.
Alzheimer’s disease may limit behavioral responses to cooling or even recognition that the body temperature is dropping.9 This disease is associated with weight loss (and attendant loss of body fat that acts, in part, as insulation), hypothalamic pathologic changes, and decreased serotonin activity in the hypothalamus. The processes leading to Ms. B.’s progressive cognitive impairment most likely contributed to hypothalamic dysregulation and subsequent accidental hypothermia.
Ms. B.’s repeated disrobing during her stay at the adult care facility was ascribed to dementia. Serial body temperature measurements were not available, so we do not know the extent to which the disrobing may have been paradoxical—that is, undressing when cold rather than dressing more warmly. Paradoxical undressing is found during moderate (82.4°F to 90°F) hypothermia.1
Medications and hypothermia
Normally, mild hypothermia induces vasoconstriction and initial increases in heart rate and cardiac output. (The latter increase is principally driven by the accelerated heart rate rather than increased stroke volume.) These changes tend to protect the patient from further lowering of body temperature. But Ms. B.’s medications included the vasodilator, isosorbide dinitrate; the beta-blocker, metoprolol; and the angiotensin-converting enzyme (ACE) inhibitor, lisinopril. All these agents impaired her capacities to vasoconstrict and to increase cardiac output, thereby reducing her ability to conserve body heat.
Over the course of several months, Ms. B., a woman in her mid-70s, manifested features of accidental hypothermia, which went undiagnosed amid a backdrop of a long history of schizophrenia and a more recent history of dementia.
In 1996, almost 5 years before developing accidental hypothermia, Ms. B. sought care for paranoia, nervousness, and dysphoria. The records showed a history of cigarette abuse, diet-controlled type 2 diabetes mellitus of more than 20 years duration, and kidney surgery. She was cognitively intact and had received doses of up to 3 mg/bid of risperidone and desipramine. A few months later, temazepam was added for insomnia. Still later, following the death of her husband, lorazepam was added.
Until late 1999, Ms. B. remained psychiatrically stable. Then she became more anxious and her lorazepam dosage was increased. But in June 2000, she was admitted to a local hospital following a month of confusion, weakness, and slurred speech. The precipitating event was a fall. A head CT scan showed brain atrophy and white-matter disease. Extensive condylomata led to a partial vulvectomy. Her lowest recorded oral temperature was 95.6°F.
Ms. B. returned to a residential home briefly but was readmitted when she was found unresponsive; hypotension and bradycardia were detected. Cardiac catheterization showed normal left ventricular function and severe 3-vessel coronary artery disease with a 50% obstruction of the left main coronary artery. This procedure was complicated by severe agitation, confusion, and a large post-catheterization hematoma requiring blood transfusions.
Following discussions with the cardiac surgeons, the family considered Ms. B. too ill to undergo coronary artery bypass surgery. The lowest recorded oral temperature was 94°F.
Ms. B. returned to the residential home—but not for long. In August 2000, she was again taken to the hospital. She was confused, threatening to harm herself with a knife, and eating “hair grease.” Her medications now included temazepam, lorazepam, risperidone, paroxetine, and desipramine—plus aspirin, verapamil, lisinopril, metoprolol, amlodipine, and isosorbide dinitrate for coronary heart disease and hypertension. The admission database included a temperature of 96.2°F. She received a Global Assessment of Functioning score of 20 contrasted with a high score of 70 the preceding year.
Ms. B.’s hospital stay lasted 2 months. Confusion and disorientation persisted one month after admission while still undergoing psychiatric care. Midway during her hospitalization, she underwent a cholecystectomy.
When she was discharged to an assisted living facility, Ms. B. required assistance with self-care and restraint with a posey vest. Dementia was considered the major psychiatric problem. Medications now included amlodipine, aspirin, famotidine, isosorbide dinitrate, lisinopril, metoprolol, oxybutynin, metoclopramide, lorazepam 0.5 mg 3 times a day, and risperidone 1 mg twice daily.
Two weeks later, Ms. B. was still confused and disoriented. Risperidone was increased to 1 mg 3 times daily and lorazepam was increased to 0.5 mg 4 times daily. A week later, the nursing staff noted further deterioration. She would wander, on occasion even into the street. Subsequently, she began disrobing for no apparent reason, 3 to 4 times a week.
In early December 2000, nurses called an ambulance because Ms. B. was “lethargic, unresponsive to name call.” The ambulance crew noted she was “foaming at the mouth,” lying "naked" in bed, and very “cold” to the touch. At the hospital, hypothermia was documented with a body temperature of 84°F rectally. (Of note, the patient’s roommate manifested a normal body temperature, was cognitively intact, and did not complain that their room was cold.) Medications at the time of admission included lisinopril 10 mg/d, aspirin 325 mg/d, amlodipine 10 mg/d, oxybutynin 5 mg twice daily, lorazepam 0.5 mg 3 times daily, metoprolol 50 mg twice daily, famotidine 20 mg twice daily, isosorbide dinitrate 10 mg 3 times daily, metoclopramide 10 mg 4 times daily, and risperidone 1 mg twice daily.
Initially, Ms. B. manifested bradycardia requiring temporary pacing, and hemoconcentration without explanation for the low body temperature. Despite return to normal body temperature within 24 hours, vasomotor instability, body temperatures ranging between 95.9°F and 100.1°F, encephalopathy, and general organ failure persisted. Ms. B. was pronounced dead on the 18th hospital day. An autopsy was not performed.
Amlodipine, a calcium channel blocker, enhances vasodilatation and may also have limited Ms. B.’s capacity to vasoconstrict. Calcium channel blockers may have variable effects on intraoperative core body temperature in humans.10
Phenothiazines, particularly the low-potency agents in this class, are the antipsychotic drugs most commonly associated with drug-induced hypothermia.6,9,11 Phenothiazines seem to have a direct effect on hypothalamic thermoregulation. About a month before developing moderate hypothermia, Ms. B. received an increase in her risperidone dosage from 1 mg twice daily to 1 mg 3 times daily because of agitation. The package insert for risperidone states:
- Medical conditions
- Hypoglycemia
- Hypothyroidism
- Adrenal insufficiency
- Hypopituitarism
- Stroke
- Malnutrition
- Shock
- Sepsis
- Hepatic or renal failure
- Burns
- Exfoliative dermatitis
- Immobility or debilitation
- Hypothalamic disorders
- Parkinson’s disease
- Spinal cord injury
- Diabetic ketoacidosis
- Psychiatric conditions
- Alzheimer’s disease
- Schizophrenia
- Medications
- Ethanol
- Phenothiazines
- Barbiturates
- Anesthetics
- Neuromuscular blockers
*Adapted from Danzl DF. Hypothermia. Harrison’s 15th Ed., Principles of Internal Medicine, New York: McGraw-Hill, 2001, p. 107.
“Disruption of body temperature regulation has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with Risperdal use. Caution is advised when prescribing for patients who will be exposed to temperature extremes.”12
Lorazepam very rarely may be associated with hypothermia. In animal studies, zolpidem, diazepam, and lorazepam produced comparable dose-dependent hypothermia.13 Ms. B. had her dosage of lorazepam increased from 0.5 mg 3 times daily to 0.5 mg 4 times daily because of increasing agitation and wandering. About 10 days before developing moderate hypothermia, she became more lethargic and the nursing staff was directed to withhold lorazepam if she appeared unduly sedated. At this point, Ms. B. may have had a drug-induced delirium superimposed upon dementia or a toxic-metabolic encephalopathy superimposed upon dementia. In her case, we do not know if druginduced or metabolic-induced changes (or a combination of the two) best explained her change in mental status.
Once accidental hypothermia sets in
During the days before Ms. B. developed moderate hypothermia, the temperature outside the assisted living facility ranged from 25°F to 40°F. When she was found by the nursing staff to be unusually unresponsive, she was wearing her nightgown under bed sheets. Even if her room temperature had been at 70°F, an almost 30°F gradient would exist between that and normal body temperature (98.6°F). In complete thermodysregulation, her body temperature of 84°F could have been reached within 5 to 8 hours. The colder the room, the faster her body would cool in the presence of thermodysregulation.
Although sepsis and adverse environmental exposure are the most common conditions leading to hypothermia, up to onethird of cases of accidental hypothermia in the elderly occur during the warmer months, with one-half of these cases found in the hospital.6 In cases of accidental hypothermia occurring during the winter, one-half occur in a normal room temperature setting.9
In a United Kingdom study, about 25% of elderly patients with hypothermia died.9 Still, the severity of underlying disease is more predictive of mortality than is the degree of hypothermia.14 Ms. B.’s fatal clinical course was that of multiple organ failure complicated by hypothermia. No mention was made in the hospital records of her vulnerability to hypothermia. This vulnerability placed significant burden on the assisted living facility staff.
Hypothermia should be considered in the differential diagnosis of confusion and disruptive behavior in the elderly patient. In Ms. B.’s case, an early diagnosis of accidental hypothermia by a psychiatrist could have made a difference.
Related resources Oriented to mental health issues
- Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
- Murphy PJ. Hypothermia. In Oxford Textbook of Geriatric Medicine. Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. New York: Oxford University Press, 2000:857-863.
- Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992; 10:311-327.
- Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981; 10:384-387.
Drug brand names
- Amlodipine • Norvasc
- Famotidine • Pepcid
- Isosorbide dinitrate • Isordil
- Lisinopril • Prinivil
- Metoclopramide • Reglan
- Metoprolol • Lopressor
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The author reports that he is on the speakers’ bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
1. Danzl DF, Pozos RS. Accidental hypothermia. N Engl J Med. 1994;331:1756-1760.
2. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs. 1997;7:121-138.
3. Grossman SP. Physiology of thirst. In: Schnur DB, Kirch DG, eds. Water balance in schizophrenia. Washington, DC: American Psychiatric Press, Inc., 1996;53-87.
4. Guyton AC, Hall JE. Behavioral and Motivational Mechanisms of the Brain—The Limbic System and the Hypothalamus. Textbook of Medical Physiology. Philadelphia: W.B. Saunders, 1996;749-760.
5. Fontaine KR, Heo M, Harrigan EP, Shear CL, et al. Estimating the consequences of antipsychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277-288.
6. Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
7. Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749-754.
8. Vieweg V, Tucker R, Talbot PC, Blair CE, Lewis R. Mini-Mental State Examination scores of subjects with nondementing diagnoses on admission to a geropsychiatric hospital. Med Psychiatry. 2001;4:19-22.
9. Murphy PJ. Hypothermia. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. Oxford Textbook of Geriatric Medicine. New York: Oxford University Press, 2000;857-863.
10. Vassilieff N, Rosencher N, Sessler DL, Conseiller C, Lienhart A. Nifedipine and intraoperative core body temperature in humans. Anesthesiology. 1994;80:123-128.
11. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
12. Physicians’ Desk Reference. 54th ed. Montvale, NJ: Medical Economics Company, Inc., 2000.
13. Elliott EE, White JM. The acute effects of zolpidem compared to diazepam and lorazepam using radiotelemetry. Neuropharmacology. 2001;40:717-721.
14. Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981;10:384-387.
You may well be the first specialist to evaluate an elderly patient with accidental hypothermia, a severe medical illness, because patients with this condition may present initially with cognitive impairment and disruptive behavior. This problem is particularly evident when evaluating elderly patients. Accidental hypothermia commonly mimics major mental illness, may be induced or exacerbated by psychotropic medications, is commonly fatal, and may remain unrecognized without a high index of suspicion.
Hypothermia is defined as a fall in body temperature below 95°F or 35°C (Box 1). Clinical mercury thermometers commonly range between 96°F and 106°F. Thus, the family member or clinician may not suspect hypothermia after the initial temperature measurement.
The diagnosis of accidental hypothermia is straightforward if there is a history of environmental exposure, but such evidence is often lacking in urban settings and among the elderly. Also, particularly in the elderly, hypothermia may occur at room temperature, secondary to diseases that strike the hypothalamic thermoregulatory center.
Subjects with core body temperatures dropping from 95°F to 90°F develop amnesia, dysarthria, confusion, and disruptive behavior.1 Further cooling as the body temperature falls to 82.4°F yields stupor, paradoxical undressing, and hallucinations. These characteristics are illustrated in the accompanying vignette of Ms. B.
![]()
The body’s thermoregulatory center located in the hypothalamus normally maintains core body temperature between 97.5°F (36.5°C) and 99.5°F (37.5°C). When body temperature declines, heat production increases by shivering, and heat loss is reduced by decreasing cutaneous blood flow.
Accidental hypothermia is defined as an unintentional fall in body temperature below 95°F (35°C). The coordinated systems responsible for thermoregulation start to fail. Heat loss through radiation, conduction, convection, respiration, and evaporation occurs because compensatory physiologic mechanisms are both limited and impaired.
Schizophrenia and the hypothalamus
Over the course of 6 months before her death, Ms. B. showed evidence of both thermoregulatory dysfunction and autonomic nervous system instability. We do not know if these hypothalamic problems were separate from, or intrinsic parts of, her schizophrenia.
The hypothalamus regulates autonomic, endocrine, and visceral function. Hypothalamic dysfunction may be an intrinsic part of schizophrenia. Such dysfunction occurs most commonly in the periventricular and supraoptic nuclei of the hypothalamus.2 These areas are adjacent to hypothalamic areas regulating body temperature.3
Lesions in anterior parts of the hypothalamus (temperaturesensitive neurons in the preoptic nuclei—located close to nuclei controlling thirst and osmotic regulation) may induce hyperthermia, impairing heat-dissipating mechanisms including vasodilatation and sweating. Lesions in posterior parts of the hypothalamus may impair heat conservation and heat production mechanisms and induce hypothermia.4
Associated medical problems
Independent of drug treatment, metabolic and cardiovascular problems occur more frequently in patients suffering from schizophrenia than they do in the general population.5 Ms. B. developed hypertension, diabetes mellitus, dyslipidemia, and coronary artery disease.
Diabetes mellitus in particular is a risk factor for hypothermia and may be found in more than 10 percent of elderly patients who suffered thermoregulatory failure before dying.6 Diabetes may impair autonomic system vasomotor stability and the body’s ability to vasoconstrict to preserve body heat.
Dementia and hypothermia
Cognitive impairment is a core feature of schizophrenia,7 and dementia is a common outcome among elderly patients suffering with the disorder.8 We don’t know whether Ms. B.’s progressive cognitive deterioration derived from dementia associated with schizophrenia or from a separate process such as Alzheimer’s disease.
Alzheimer’s disease may limit behavioral responses to cooling or even recognition that the body temperature is dropping.9 This disease is associated with weight loss (and attendant loss of body fat that acts, in part, as insulation), hypothalamic pathologic changes, and decreased serotonin activity in the hypothalamus. The processes leading to Ms. B.’s progressive cognitive impairment most likely contributed to hypothalamic dysregulation and subsequent accidental hypothermia.
Ms. B.’s repeated disrobing during her stay at the adult care facility was ascribed to dementia. Serial body temperature measurements were not available, so we do not know the extent to which the disrobing may have been paradoxical—that is, undressing when cold rather than dressing more warmly. Paradoxical undressing is found during moderate (82.4°F to 90°F) hypothermia.1
Medications and hypothermia
Normally, mild hypothermia induces vasoconstriction and initial increases in heart rate and cardiac output. (The latter increase is principally driven by the accelerated heart rate rather than increased stroke volume.) These changes tend to protect the patient from further lowering of body temperature. But Ms. B.’s medications included the vasodilator, isosorbide dinitrate; the beta-blocker, metoprolol; and the angiotensin-converting enzyme (ACE) inhibitor, lisinopril. All these agents impaired her capacities to vasoconstrict and to increase cardiac output, thereby reducing her ability to conserve body heat.
Over the course of several months, Ms. B., a woman in her mid-70s, manifested features of accidental hypothermia, which went undiagnosed amid a backdrop of a long history of schizophrenia and a more recent history of dementia.
In 1996, almost 5 years before developing accidental hypothermia, Ms. B. sought care for paranoia, nervousness, and dysphoria. The records showed a history of cigarette abuse, diet-controlled type 2 diabetes mellitus of more than 20 years duration, and kidney surgery. She was cognitively intact and had received doses of up to 3 mg/bid of risperidone and desipramine. A few months later, temazepam was added for insomnia. Still later, following the death of her husband, lorazepam was added.
Until late 1999, Ms. B. remained psychiatrically stable. Then she became more anxious and her lorazepam dosage was increased. But in June 2000, she was admitted to a local hospital following a month of confusion, weakness, and slurred speech. The precipitating event was a fall. A head CT scan showed brain atrophy and white-matter disease. Extensive condylomata led to a partial vulvectomy. Her lowest recorded oral temperature was 95.6°F.
Ms. B. returned to a residential home briefly but was readmitted when she was found unresponsive; hypotension and bradycardia were detected. Cardiac catheterization showed normal left ventricular function and severe 3-vessel coronary artery disease with a 50% obstruction of the left main coronary artery. This procedure was complicated by severe agitation, confusion, and a large post-catheterization hematoma requiring blood transfusions.
Following discussions with the cardiac surgeons, the family considered Ms. B. too ill to undergo coronary artery bypass surgery. The lowest recorded oral temperature was 94°F.
Ms. B. returned to the residential home—but not for long. In August 2000, she was again taken to the hospital. She was confused, threatening to harm herself with a knife, and eating “hair grease.” Her medications now included temazepam, lorazepam, risperidone, paroxetine, and desipramine—plus aspirin, verapamil, lisinopril, metoprolol, amlodipine, and isosorbide dinitrate for coronary heart disease and hypertension. The admission database included a temperature of 96.2°F. She received a Global Assessment of Functioning score of 20 contrasted with a high score of 70 the preceding year.
Ms. B.’s hospital stay lasted 2 months. Confusion and disorientation persisted one month after admission while still undergoing psychiatric care. Midway during her hospitalization, she underwent a cholecystectomy.
When she was discharged to an assisted living facility, Ms. B. required assistance with self-care and restraint with a posey vest. Dementia was considered the major psychiatric problem. Medications now included amlodipine, aspirin, famotidine, isosorbide dinitrate, lisinopril, metoprolol, oxybutynin, metoclopramide, lorazepam 0.5 mg 3 times a day, and risperidone 1 mg twice daily.
Two weeks later, Ms. B. was still confused and disoriented. Risperidone was increased to 1 mg 3 times daily and lorazepam was increased to 0.5 mg 4 times daily. A week later, the nursing staff noted further deterioration. She would wander, on occasion even into the street. Subsequently, she began disrobing for no apparent reason, 3 to 4 times a week.
In early December 2000, nurses called an ambulance because Ms. B. was “lethargic, unresponsive to name call.” The ambulance crew noted she was “foaming at the mouth,” lying "naked" in bed, and very “cold” to the touch. At the hospital, hypothermia was documented with a body temperature of 84°F rectally. (Of note, the patient’s roommate manifested a normal body temperature, was cognitively intact, and did not complain that their room was cold.) Medications at the time of admission included lisinopril 10 mg/d, aspirin 325 mg/d, amlodipine 10 mg/d, oxybutynin 5 mg twice daily, lorazepam 0.5 mg 3 times daily, metoprolol 50 mg twice daily, famotidine 20 mg twice daily, isosorbide dinitrate 10 mg 3 times daily, metoclopramide 10 mg 4 times daily, and risperidone 1 mg twice daily.
Initially, Ms. B. manifested bradycardia requiring temporary pacing, and hemoconcentration without explanation for the low body temperature. Despite return to normal body temperature within 24 hours, vasomotor instability, body temperatures ranging between 95.9°F and 100.1°F, encephalopathy, and general organ failure persisted. Ms. B. was pronounced dead on the 18th hospital day. An autopsy was not performed.
Amlodipine, a calcium channel blocker, enhances vasodilatation and may also have limited Ms. B.’s capacity to vasoconstrict. Calcium channel blockers may have variable effects on intraoperative core body temperature in humans.10
Phenothiazines, particularly the low-potency agents in this class, are the antipsychotic drugs most commonly associated with drug-induced hypothermia.6,9,11 Phenothiazines seem to have a direct effect on hypothalamic thermoregulation. About a month before developing moderate hypothermia, Ms. B. received an increase in her risperidone dosage from 1 mg twice daily to 1 mg 3 times daily because of agitation. The package insert for risperidone states:
- Medical conditions
- Hypoglycemia
- Hypothyroidism
- Adrenal insufficiency
- Hypopituitarism
- Stroke
- Malnutrition
- Shock
- Sepsis
- Hepatic or renal failure
- Burns
- Exfoliative dermatitis
- Immobility or debilitation
- Hypothalamic disorders
- Parkinson’s disease
- Spinal cord injury
- Diabetic ketoacidosis
- Psychiatric conditions
- Alzheimer’s disease
- Schizophrenia
- Medications
- Ethanol
- Phenothiazines
- Barbiturates
- Anesthetics
- Neuromuscular blockers
*Adapted from Danzl DF. Hypothermia. Harrison’s 15th Ed., Principles of Internal Medicine, New York: McGraw-Hill, 2001, p. 107.
“Disruption of body temperature regulation has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with Risperdal use. Caution is advised when prescribing for patients who will be exposed to temperature extremes.”12
Lorazepam very rarely may be associated with hypothermia. In animal studies, zolpidem, diazepam, and lorazepam produced comparable dose-dependent hypothermia.13 Ms. B. had her dosage of lorazepam increased from 0.5 mg 3 times daily to 0.5 mg 4 times daily because of increasing agitation and wandering. About 10 days before developing moderate hypothermia, she became more lethargic and the nursing staff was directed to withhold lorazepam if she appeared unduly sedated. At this point, Ms. B. may have had a drug-induced delirium superimposed upon dementia or a toxic-metabolic encephalopathy superimposed upon dementia. In her case, we do not know if druginduced or metabolic-induced changes (or a combination of the two) best explained her change in mental status.
Once accidental hypothermia sets in
During the days before Ms. B. developed moderate hypothermia, the temperature outside the assisted living facility ranged from 25°F to 40°F. When she was found by the nursing staff to be unusually unresponsive, she was wearing her nightgown under bed sheets. Even if her room temperature had been at 70°F, an almost 30°F gradient would exist between that and normal body temperature (98.6°F). In complete thermodysregulation, her body temperature of 84°F could have been reached within 5 to 8 hours. The colder the room, the faster her body would cool in the presence of thermodysregulation.
Although sepsis and adverse environmental exposure are the most common conditions leading to hypothermia, up to onethird of cases of accidental hypothermia in the elderly occur during the warmer months, with one-half of these cases found in the hospital.6 In cases of accidental hypothermia occurring during the winter, one-half occur in a normal room temperature setting.9
In a United Kingdom study, about 25% of elderly patients with hypothermia died.9 Still, the severity of underlying disease is more predictive of mortality than is the degree of hypothermia.14 Ms. B.’s fatal clinical course was that of multiple organ failure complicated by hypothermia. No mention was made in the hospital records of her vulnerability to hypothermia. This vulnerability placed significant burden on the assisted living facility staff.
Hypothermia should be considered in the differential diagnosis of confusion and disruptive behavior in the elderly patient. In Ms. B.’s case, an early diagnosis of accidental hypothermia by a psychiatrist could have made a difference.
Related resources Oriented to mental health issues
- Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
- Murphy PJ. Hypothermia. In Oxford Textbook of Geriatric Medicine. Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. New York: Oxford University Press, 2000:857-863.
- Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992; 10:311-327.
- Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981; 10:384-387.
Drug brand names
- Amlodipine • Norvasc
- Famotidine • Pepcid
- Isosorbide dinitrate • Isordil
- Lisinopril • Prinivil
- Metoclopramide • Reglan
- Metoprolol • Lopressor
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The author reports that he is on the speakers’ bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
You may well be the first specialist to evaluate an elderly patient with accidental hypothermia, a severe medical illness, because patients with this condition may present initially with cognitive impairment and disruptive behavior. This problem is particularly evident when evaluating elderly patients. Accidental hypothermia commonly mimics major mental illness, may be induced or exacerbated by psychotropic medications, is commonly fatal, and may remain unrecognized without a high index of suspicion.
Hypothermia is defined as a fall in body temperature below 95°F or 35°C (Box 1). Clinical mercury thermometers commonly range between 96°F and 106°F. Thus, the family member or clinician may not suspect hypothermia after the initial temperature measurement.
The diagnosis of accidental hypothermia is straightforward if there is a history of environmental exposure, but such evidence is often lacking in urban settings and among the elderly. Also, particularly in the elderly, hypothermia may occur at room temperature, secondary to diseases that strike the hypothalamic thermoregulatory center.
Subjects with core body temperatures dropping from 95°F to 90°F develop amnesia, dysarthria, confusion, and disruptive behavior.1 Further cooling as the body temperature falls to 82.4°F yields stupor, paradoxical undressing, and hallucinations. These characteristics are illustrated in the accompanying vignette of Ms. B.
![]()
The body’s thermoregulatory center located in the hypothalamus normally maintains core body temperature between 97.5°F (36.5°C) and 99.5°F (37.5°C). When body temperature declines, heat production increases by shivering, and heat loss is reduced by decreasing cutaneous blood flow.
Accidental hypothermia is defined as an unintentional fall in body temperature below 95°F (35°C). The coordinated systems responsible for thermoregulation start to fail. Heat loss through radiation, conduction, convection, respiration, and evaporation occurs because compensatory physiologic mechanisms are both limited and impaired.
Schizophrenia and the hypothalamus
Over the course of 6 months before her death, Ms. B. showed evidence of both thermoregulatory dysfunction and autonomic nervous system instability. We do not know if these hypothalamic problems were separate from, or intrinsic parts of, her schizophrenia.
The hypothalamus regulates autonomic, endocrine, and visceral function. Hypothalamic dysfunction may be an intrinsic part of schizophrenia. Such dysfunction occurs most commonly in the periventricular and supraoptic nuclei of the hypothalamus.2 These areas are adjacent to hypothalamic areas regulating body temperature.3
Lesions in anterior parts of the hypothalamus (temperaturesensitive neurons in the preoptic nuclei—located close to nuclei controlling thirst and osmotic regulation) may induce hyperthermia, impairing heat-dissipating mechanisms including vasodilatation and sweating. Lesions in posterior parts of the hypothalamus may impair heat conservation and heat production mechanisms and induce hypothermia.4
Associated medical problems
Independent of drug treatment, metabolic and cardiovascular problems occur more frequently in patients suffering from schizophrenia than they do in the general population.5 Ms. B. developed hypertension, diabetes mellitus, dyslipidemia, and coronary artery disease.
Diabetes mellitus in particular is a risk factor for hypothermia and may be found in more than 10 percent of elderly patients who suffered thermoregulatory failure before dying.6 Diabetes may impair autonomic system vasomotor stability and the body’s ability to vasoconstrict to preserve body heat.
Dementia and hypothermia
Cognitive impairment is a core feature of schizophrenia,7 and dementia is a common outcome among elderly patients suffering with the disorder.8 We don’t know whether Ms. B.’s progressive cognitive deterioration derived from dementia associated with schizophrenia or from a separate process such as Alzheimer’s disease.
Alzheimer’s disease may limit behavioral responses to cooling or even recognition that the body temperature is dropping.9 This disease is associated with weight loss (and attendant loss of body fat that acts, in part, as insulation), hypothalamic pathologic changes, and decreased serotonin activity in the hypothalamus. The processes leading to Ms. B.’s progressive cognitive impairment most likely contributed to hypothalamic dysregulation and subsequent accidental hypothermia.
Ms. B.’s repeated disrobing during her stay at the adult care facility was ascribed to dementia. Serial body temperature measurements were not available, so we do not know the extent to which the disrobing may have been paradoxical—that is, undressing when cold rather than dressing more warmly. Paradoxical undressing is found during moderate (82.4°F to 90°F) hypothermia.1
Medications and hypothermia
Normally, mild hypothermia induces vasoconstriction and initial increases in heart rate and cardiac output. (The latter increase is principally driven by the accelerated heart rate rather than increased stroke volume.) These changes tend to protect the patient from further lowering of body temperature. But Ms. B.’s medications included the vasodilator, isosorbide dinitrate; the beta-blocker, metoprolol; and the angiotensin-converting enzyme (ACE) inhibitor, lisinopril. All these agents impaired her capacities to vasoconstrict and to increase cardiac output, thereby reducing her ability to conserve body heat.
Over the course of several months, Ms. B., a woman in her mid-70s, manifested features of accidental hypothermia, which went undiagnosed amid a backdrop of a long history of schizophrenia and a more recent history of dementia.
In 1996, almost 5 years before developing accidental hypothermia, Ms. B. sought care for paranoia, nervousness, and dysphoria. The records showed a history of cigarette abuse, diet-controlled type 2 diabetes mellitus of more than 20 years duration, and kidney surgery. She was cognitively intact and had received doses of up to 3 mg/bid of risperidone and desipramine. A few months later, temazepam was added for insomnia. Still later, following the death of her husband, lorazepam was added.
Until late 1999, Ms. B. remained psychiatrically stable. Then she became more anxious and her lorazepam dosage was increased. But in June 2000, she was admitted to a local hospital following a month of confusion, weakness, and slurred speech. The precipitating event was a fall. A head CT scan showed brain atrophy and white-matter disease. Extensive condylomata led to a partial vulvectomy. Her lowest recorded oral temperature was 95.6°F.
Ms. B. returned to a residential home briefly but was readmitted when she was found unresponsive; hypotension and bradycardia were detected. Cardiac catheterization showed normal left ventricular function and severe 3-vessel coronary artery disease with a 50% obstruction of the left main coronary artery. This procedure was complicated by severe agitation, confusion, and a large post-catheterization hematoma requiring blood transfusions.
Following discussions with the cardiac surgeons, the family considered Ms. B. too ill to undergo coronary artery bypass surgery. The lowest recorded oral temperature was 94°F.
Ms. B. returned to the residential home—but not for long. In August 2000, she was again taken to the hospital. She was confused, threatening to harm herself with a knife, and eating “hair grease.” Her medications now included temazepam, lorazepam, risperidone, paroxetine, and desipramine—plus aspirin, verapamil, lisinopril, metoprolol, amlodipine, and isosorbide dinitrate for coronary heart disease and hypertension. The admission database included a temperature of 96.2°F. She received a Global Assessment of Functioning score of 20 contrasted with a high score of 70 the preceding year.
Ms. B.’s hospital stay lasted 2 months. Confusion and disorientation persisted one month after admission while still undergoing psychiatric care. Midway during her hospitalization, she underwent a cholecystectomy.
When she was discharged to an assisted living facility, Ms. B. required assistance with self-care and restraint with a posey vest. Dementia was considered the major psychiatric problem. Medications now included amlodipine, aspirin, famotidine, isosorbide dinitrate, lisinopril, metoprolol, oxybutynin, metoclopramide, lorazepam 0.5 mg 3 times a day, and risperidone 1 mg twice daily.
Two weeks later, Ms. B. was still confused and disoriented. Risperidone was increased to 1 mg 3 times daily and lorazepam was increased to 0.5 mg 4 times daily. A week later, the nursing staff noted further deterioration. She would wander, on occasion even into the street. Subsequently, she began disrobing for no apparent reason, 3 to 4 times a week.
In early December 2000, nurses called an ambulance because Ms. B. was “lethargic, unresponsive to name call.” The ambulance crew noted she was “foaming at the mouth,” lying "naked" in bed, and very “cold” to the touch. At the hospital, hypothermia was documented with a body temperature of 84°F rectally. (Of note, the patient’s roommate manifested a normal body temperature, was cognitively intact, and did not complain that their room was cold.) Medications at the time of admission included lisinopril 10 mg/d, aspirin 325 mg/d, amlodipine 10 mg/d, oxybutynin 5 mg twice daily, lorazepam 0.5 mg 3 times daily, metoprolol 50 mg twice daily, famotidine 20 mg twice daily, isosorbide dinitrate 10 mg 3 times daily, metoclopramide 10 mg 4 times daily, and risperidone 1 mg twice daily.
Initially, Ms. B. manifested bradycardia requiring temporary pacing, and hemoconcentration without explanation for the low body temperature. Despite return to normal body temperature within 24 hours, vasomotor instability, body temperatures ranging between 95.9°F and 100.1°F, encephalopathy, and general organ failure persisted. Ms. B. was pronounced dead on the 18th hospital day. An autopsy was not performed.
Amlodipine, a calcium channel blocker, enhances vasodilatation and may also have limited Ms. B.’s capacity to vasoconstrict. Calcium channel blockers may have variable effects on intraoperative core body temperature in humans.10
Phenothiazines, particularly the low-potency agents in this class, are the antipsychotic drugs most commonly associated with drug-induced hypothermia.6,9,11 Phenothiazines seem to have a direct effect on hypothalamic thermoregulation. About a month before developing moderate hypothermia, Ms. B. received an increase in her risperidone dosage from 1 mg twice daily to 1 mg 3 times daily because of agitation. The package insert for risperidone states:
- Medical conditions
- Hypoglycemia
- Hypothyroidism
- Adrenal insufficiency
- Hypopituitarism
- Stroke
- Malnutrition
- Shock
- Sepsis
- Hepatic or renal failure
- Burns
- Exfoliative dermatitis
- Immobility or debilitation
- Hypothalamic disorders
- Parkinson’s disease
- Spinal cord injury
- Diabetic ketoacidosis
- Psychiatric conditions
- Alzheimer’s disease
- Schizophrenia
- Medications
- Ethanol
- Phenothiazines
- Barbiturates
- Anesthetics
- Neuromuscular blockers
*Adapted from Danzl DF. Hypothermia. Harrison’s 15th Ed., Principles of Internal Medicine, New York: McGraw-Hill, 2001, p. 107.
“Disruption of body temperature regulation has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with Risperdal use. Caution is advised when prescribing for patients who will be exposed to temperature extremes.”12
Lorazepam very rarely may be associated with hypothermia. In animal studies, zolpidem, diazepam, and lorazepam produced comparable dose-dependent hypothermia.13 Ms. B. had her dosage of lorazepam increased from 0.5 mg 3 times daily to 0.5 mg 4 times daily because of increasing agitation and wandering. About 10 days before developing moderate hypothermia, she became more lethargic and the nursing staff was directed to withhold lorazepam if she appeared unduly sedated. At this point, Ms. B. may have had a drug-induced delirium superimposed upon dementia or a toxic-metabolic encephalopathy superimposed upon dementia. In her case, we do not know if druginduced or metabolic-induced changes (or a combination of the two) best explained her change in mental status.
Once accidental hypothermia sets in
During the days before Ms. B. developed moderate hypothermia, the temperature outside the assisted living facility ranged from 25°F to 40°F. When she was found by the nursing staff to be unusually unresponsive, she was wearing her nightgown under bed sheets. Even if her room temperature had been at 70°F, an almost 30°F gradient would exist between that and normal body temperature (98.6°F). In complete thermodysregulation, her body temperature of 84°F could have been reached within 5 to 8 hours. The colder the room, the faster her body would cool in the presence of thermodysregulation.
Although sepsis and adverse environmental exposure are the most common conditions leading to hypothermia, up to onethird of cases of accidental hypothermia in the elderly occur during the warmer months, with one-half of these cases found in the hospital.6 In cases of accidental hypothermia occurring during the winter, one-half occur in a normal room temperature setting.9
In a United Kingdom study, about 25% of elderly patients with hypothermia died.9 Still, the severity of underlying disease is more predictive of mortality than is the degree of hypothermia.14 Ms. B.’s fatal clinical course was that of multiple organ failure complicated by hypothermia. No mention was made in the hospital records of her vulnerability to hypothermia. This vulnerability placed significant burden on the assisted living facility staff.
Hypothermia should be considered in the differential diagnosis of confusion and disruptive behavior in the elderly patient. In Ms. B.’s case, an early diagnosis of accidental hypothermia by a psychiatrist could have made a difference.
Related resources Oriented to mental health issues
- Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
- Murphy PJ. Hypothermia. In Oxford Textbook of Geriatric Medicine. Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. New York: Oxford University Press, 2000:857-863.
- Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992; 10:311-327.
- Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981; 10:384-387.
Drug brand names
- Amlodipine • Norvasc
- Famotidine • Pepcid
- Isosorbide dinitrate • Isordil
- Lisinopril • Prinivil
- Metoclopramide • Reglan
- Metoprolol • Lopressor
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The author reports that he is on the speakers’ bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
1. Danzl DF, Pozos RS. Accidental hypothermia. N Engl J Med. 1994;331:1756-1760.
2. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs. 1997;7:121-138.
3. Grossman SP. Physiology of thirst. In: Schnur DB, Kirch DG, eds. Water balance in schizophrenia. Washington, DC: American Psychiatric Press, Inc., 1996;53-87.
4. Guyton AC, Hall JE. Behavioral and Motivational Mechanisms of the Brain—The Limbic System and the Hypothalamus. Textbook of Medical Physiology. Philadelphia: W.B. Saunders, 1996;749-760.
5. Fontaine KR, Heo M, Harrigan EP, Shear CL, et al. Estimating the consequences of antipsychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277-288.
6. Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
7. Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749-754.
8. Vieweg V, Tucker R, Talbot PC, Blair CE, Lewis R. Mini-Mental State Examination scores of subjects with nondementing diagnoses on admission to a geropsychiatric hospital. Med Psychiatry. 2001;4:19-22.
9. Murphy PJ. Hypothermia. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. Oxford Textbook of Geriatric Medicine. New York: Oxford University Press, 2000;857-863.
10. Vassilieff N, Rosencher N, Sessler DL, Conseiller C, Lienhart A. Nifedipine and intraoperative core body temperature in humans. Anesthesiology. 1994;80:123-128.
11. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
12. Physicians’ Desk Reference. 54th ed. Montvale, NJ: Medical Economics Company, Inc., 2000.
13. Elliott EE, White JM. The acute effects of zolpidem compared to diazepam and lorazepam using radiotelemetry. Neuropharmacology. 2001;40:717-721.
14. Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981;10:384-387.
1. Danzl DF, Pozos RS. Accidental hypothermia. N Engl J Med. 1994;331:1756-1760.
2. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs. 1997;7:121-138.
3. Grossman SP. Physiology of thirst. In: Schnur DB, Kirch DG, eds. Water balance in schizophrenia. Washington, DC: American Psychiatric Press, Inc., 1996;53-87.
4. Guyton AC, Hall JE. Behavioral and Motivational Mechanisms of the Brain—The Limbic System and the Hypothalamus. Textbook of Medical Physiology. Philadelphia: W.B. Saunders, 1996;749-760.
5. Fontaine KR, Heo M, Harrigan EP, Shear CL, et al. Estimating the consequences of antipsychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277-288.
6. Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
7. Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749-754.
8. Vieweg V, Tucker R, Talbot PC, Blair CE, Lewis R. Mini-Mental State Examination scores of subjects with nondementing diagnoses on admission to a geropsychiatric hospital. Med Psychiatry. 2001;4:19-22.
9. Murphy PJ. Hypothermia. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. Oxford Textbook of Geriatric Medicine. New York: Oxford University Press, 2000;857-863.
10. Vassilieff N, Rosencher N, Sessler DL, Conseiller C, Lienhart A. Nifedipine and intraoperative core body temperature in humans. Anesthesiology. 1994;80:123-128.
11. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
12. Physicians’ Desk Reference. 54th ed. Montvale, NJ: Medical Economics Company, Inc., 2000.
13. Elliott EE, White JM. The acute effects of zolpidem compared to diazepam and lorazepam using radiotelemetry. Neuropharmacology. 2001;40:717-721.
14. Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981;10:384-387.