User login
PICC Use in Adults With Pneumonia
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | <0.001 |
| Gender | <0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | <0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | <0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | <0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | <0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | <0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | <0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | <0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | <0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | <0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | <0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | <0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | <0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | <0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | <0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | <0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | <0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | <0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | <0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | <0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | <0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | <0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | <0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | <0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | <0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | <0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | <0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | <0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | <0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | <0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | <0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | <0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | <0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | <0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | <0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | <0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | <0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | <0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
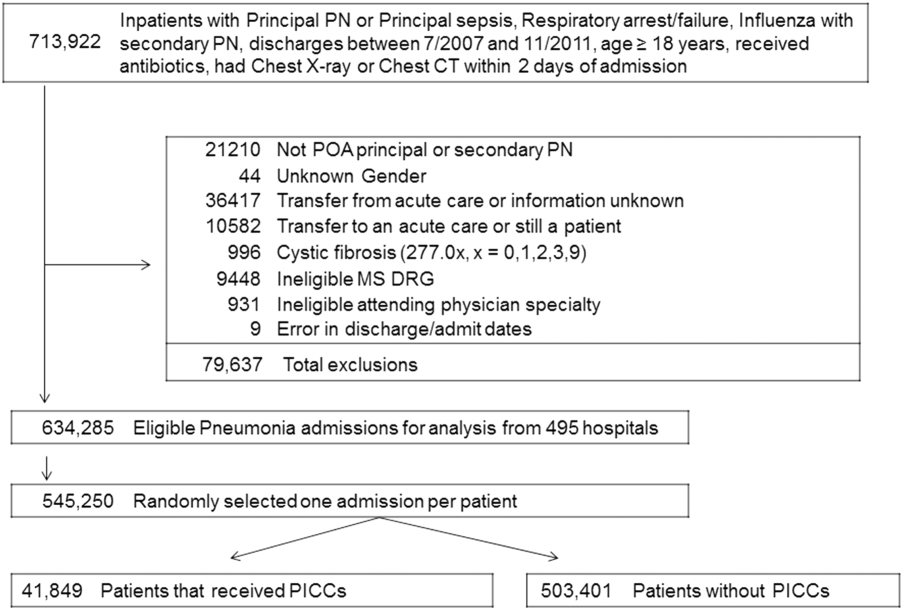
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).
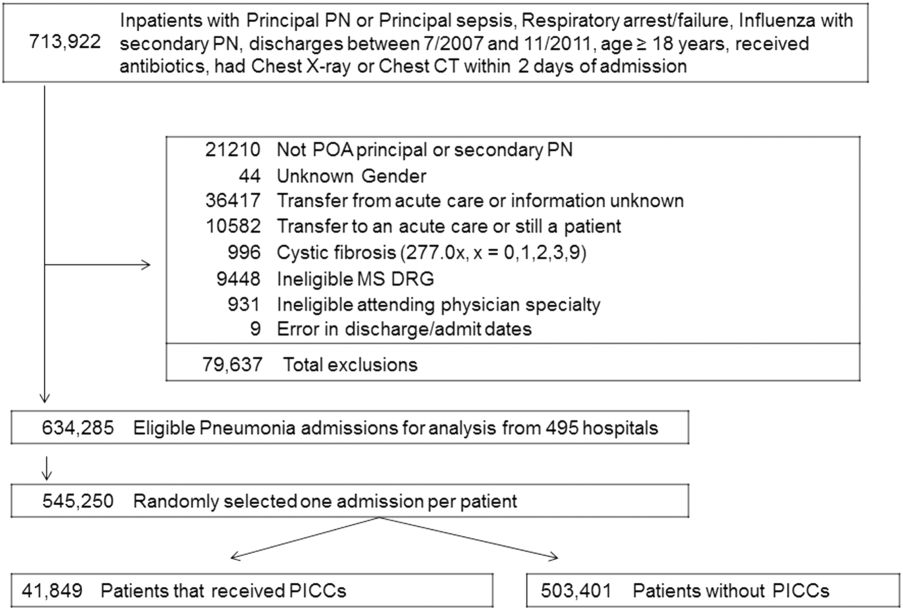
Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P < .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P < 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P < 0.001) than CAP (56.9% vs 70.1%, P < 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P < 0.001) and both noninvasive (17.5% vs 8.1%, P < 0.001) and invasive ventilation (28.6% vs 8.8%, P < 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P < 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had <5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |
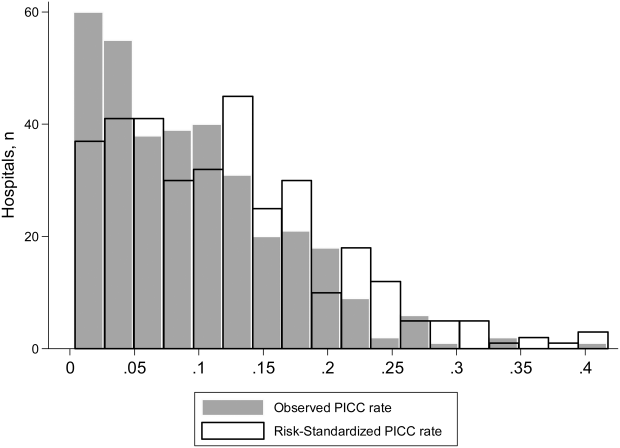
A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P < 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | <0.001 |
| Gender | <0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | <0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | <0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | <0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | <0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | <0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | <0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | <0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | <0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | <0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | <0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | <0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | <0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | <0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | <0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | <0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | <0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | <0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | <0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | <0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | <0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | <0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | <0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | <0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | <0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | <0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | <0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | <0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | <0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | <0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | <0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | <0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | <0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | <0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | <0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | <0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | <0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | <0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).

Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P < .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P < 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P < 0.001) than CAP (56.9% vs 70.1%, P < 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P < 0.001) and both noninvasive (17.5% vs 8.1%, P < 0.001) and invasive ventilation (28.6% vs 8.8%, P < 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P < 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had <5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |

A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P < 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | <0.001 |
| Gender | <0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | <0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | <0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | <0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | <0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | <0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | <0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | <0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | <0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | <0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | <0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | <0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | <0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | <0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | <0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | <0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | <0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | <0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | <0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | <0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | <0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | <0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | <0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | <0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | <0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | <0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | <0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | <0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | <0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | <0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | <0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | <0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | <0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | <0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | <0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | <0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | <0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | <0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).

Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P < .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P < 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P < 0.001) than CAP (56.9% vs 70.1%, P < 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P < 0.001) and both noninvasive (17.5% vs 8.1%, P < 0.001) and invasive ventilation (28.6% vs 8.8%, P < 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P < 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had <5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |

A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P < 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
Assessing Vascular Nursing Experience
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
© 2015 Society of Hospital Medicine
Navigating Venous Access
Reliable venous access is fundamental for the safe and effective care of hospitalized patients. Venous access devices (VADs) are conduits for this purpose, providing delivery of intravenous medications, accurate measurement of central venous pressure, or administration of life‐saving blood products. Despite this important role, VADs are also often the source of hospital‐acquired complications. Although inpatient providers must balance the relative risks of VADs against their benefits, the evidence supporting such decisions is often limited. Advances in technology, scattered research, and growing availability of novel devices has only further fragmented provider knowledge in the field of vascular access.[1]
It is not surprising, then, that survey‐based studies of hospitalists reveal important knowledge gaps with regard to practices associated with VADs.[2] In this narrative review, we seek to bridge this gap by providing a concise and pragmatic overview of the fundamentals of venous access. We focus specifically on parameters that influence decisions regarding VAD placement in hospitalized patients, providing key takeaways for practicing hospitalists.
METHODS
To compile this review, we systematically searched Medline (via Ovid) for several keywords, including: peripheral intravenous catheters, ultrasound‐guided peripheral catheter, intraosseous, midline, peripherally inserted central catheter, central venous catheters, and vascular access device complications. We concentrated on full‐length articles in English only; no date restrictions were placed on the search. We reviewed guidelines and consensus statements (eg, from the Center for Disease Control [CDC] or Choosing Wisely criteria) as appropriate. Additional studies of interest were identified through content experts (M.P., C.M.R.) and bibliographies of included studies.
SCIENTIFIC PRINCIPLES UNDERPINNING VENOUS ACCESS
It is useful to begin by reviewing VAD‐related nomenclature and physiology. In the simplest sense, a VAD consists of a hub (providing access to various connectors), a hollow tube divided into 1 or many sections (lumens), and a tip that may terminate within a central or peripheral blood vessel. VADs are classified as central venous catheters (eg, centrally inserted central catheters [CICCs] or peripherally inserted central catheters [PICCs]) or peripheral intravenous catheters (eg, midlines or peripheral intravenous catheters) based on site of entry and location of the catheter tip. Therefore, VADs entering via proximal or distal veins of the arm are often referred to as peripheral lines, as their site of entry and tip both reside within peripheral veins. Conversely, the term central line is often used when VADs enter or terminate in a central vein (eg, subclavian vein insertion with the catheter tip in the lower superior vena cava).
Attention to a host of clinical and theoretical parameters is important when choosing a device for venous access. Some such parameters are summarized in Table 1.
| Parameter | Major Considerations |
|---|---|
| |
| Desired flow rate | Smaller diameter veins susceptible to damage with high flow rates. |
| Short, large‐bore catheters facilitate rapid infusion. | |
| Nature of infusion | pH, viscosity, and temperature may damage vessels. |
| Vesicants and irritants should always be administered into larger, central veins. | |
| Desired duration of vascular access, or dwell time | Vessel thrombosis or phlebitis increase over time with catheter in place. |
| Intermittent infusions increase complications in central catheters; often tunneled catheters are recommended. | |
| Urgency of placement | Access to large caliber vessels is often needed in emergencies. |
| Critically ill or hemodynamically unstable patients may require urgent access for invasive monitoring or rapid infusions. | |
| Patients with trauma often require large volumes of blood products and reliable access to central veins. | |
| Number of device lumens | VADs may have single or multiple lumens. |
| Multilumen allows for multiple functions (eg, infusion of multiple agents, measurement of central venous pressures, blood draws). | |
| Device gauge | In general, use of a smaller‐gauge catheter is preferred to prevent complications. |
| However, larger catheter diameter may be needed for specific clinical needs (eg, blood transfusion). | |
| Device coating | VADs may have antithrombotic or anti‐infective coatings. |
| These devices may be of value in patients at high risk of complications. | |
| Such devices, however, may be more costly than their counterparts. | |
| Self‐care compatibility | VADs that can be cared for by patients are ideal for outpatient care. |
| Conversely, VADs such as peripheral catheters, are highly prone to dislodgement and should be reserved for supervised settings only. | |
VENOUS ACCESS DEVICES
We will organize our discussion of VADs based on whether they terminate in peripheral or central vessels. These anatomical considerations are relevant as they determine physical characteristics, compatibility with particular infusates, dwell time, and risk of complications associated with each VAD discussed in Table 2.
| Complications | Major Considerations |
|---|---|
| |
| Infection | VADs breach the integrity of skin and permit skin pathogens to enter the blood stream (extraluminal infection). |
| Inadequate antisepsis of the VAD hub, including poor hand hygiene, failure to "scrub the hub," and multiple manipulations may also increase the risk of VAD‐related infection (endoluminal infection). | |
| Infections may be local (eg, exit‐site infections) or may spread hematogenously (eg, CLABSI). | |
| Type of VAD, duration of therapy, and host characteristics interact to influence infection risk. | |
| VADs with antiseptic coatings (eg, chlorhexidine) or antibiotic coatings (eg, minocycline) may reduce risk of infection in high‐risk patients. | |
| Antiseptic‐impregnated dressings may reduce risk of extraluminal infection. | |
| Venous thrombosis | VADs predispose to venous stasis and thrombosis. |
| Duration of VAD use, type and care of the VAD, and patient characteristics affect risk of thromboembolism. | |
| VAD tip position is a key determinant of venous thrombosis; central VADs that do not terminate at the cavo‐atrial junction should be repositioned to reduce the risk of thrombosis. | |
| Antithrombotic coated or eluting devices may reduce risk of thrombosis, though definitive data are lacking. | |
| Phlebitis | Inflammation caused by damage to tunica media.[18] |
| 3 types of phlebitis: | |
| Chemical: due to irritation of media from the infusate. | |
| Mechanical: VAD physically damages the vessel. | |
| Infective: bacteria invade vein and inflame vessel wall. | |
| Phlebitis may be limited by close attention to infusate compatibility with peripheral veins, appropriate dilution, and prompt removal of catheters that show signs of inflammation. | |
| Phlebitis may be prevented in PICCs by ensuring at least a 2:1 vein:catheter ratio. | |
| Extravasation | Extravasation (also called infiltration) is defined as leakage of infusate from intravascular to extravascular space. |
| Extravasation of vesicants/emrritants is particularly worrisome. | |
| May result in severe tissue injury, blistering, and tissue necrosis.[11] | |
| VADs should be checked frequently for adequate flushing and position prior to each infusion to minimize risk. | |
| Any VAD with redness, swelling, and tenderness at the entry site or problems with flushing should not be used without further examination and review of position. | |
Peripheral Venous Access
Short Peripheral Intravenous Catheter
Approximately 200 million peripheral intravenous catheters (PIVs) are placed annually in the United States, making them the most common intravenous catheter.[3] PIVs are short devices, 3 to 6 cm in length, that enter and terminate in peripheral veins (Figure 1A). Placement is recommended in forearm veins rather than those of the hand, wrist, or upper arm, as forearm sites are less prone to occlusion, accidental removal, and phlebitis.[4] Additionally, placement in hand veins impedes activities of daily living (eg, hand washing) and is not preferred by patients.[5] PIV size ranges from 24 gauge (smallest) to 14 gauge (largest); larger catheters are often reserved for fluid resuscitation or blood transfusion as they accommodate greater flow and limit hemolysis. To decrease risk of phlebitis and thrombosis, the shortest catheter and smallest diameter should be used. However, unless adequately secured, smaller diameter catheters are also associated with greater rates of accidental removal.[4, 5]
By definition, PIVs are short‐term devices. The CDC currently recommends removal and replacement of these devices no more frequently than every 72 to 96 hours in adults. However, a recent randomized controlled trial found that replacing PIVs when clinically indicated (eg, device failure, phlebitis) rather than on a routine schedule added 30 hours to their lifespan without an increase in complications.[6] A systematic review by the Cochrane Collaboration echoes these findings.[3] These data have thus been incorporated into recommendations from the Infusion Nurses Society (INS) and the National Health Service in the United Kingdom.[5, 7] In hospitalized patients, this approach is relevant, as it preserves venous access sites, maximizes device dwell, and limits additional PIV insertions. In turn, these differences may reduce the need for invasive VADs such as PICCs. Furthermore, the projected 5‐year savings from implementation of clinically indicated PIV removal policies is US$300 million and 1 million health‐worker hours in the United States alone.[4]
PIVs offer many advantages. First, they are minimally invasive and require little training to insert. Second, they can be used for diverse indications in patients requiring short‐term (1 week) venous access. Third, PIVs do not require imaging to ensure correct placement; palpation of superficial veins is sufficient. Fourth, PIVs exhibit a risk of bloodstream infection that is about 40‐fold lower than more invasive, longer‐dwelling VADs[8] (0.06 bacteremia per 1000 catheter‐days).
Despite these advantages, PIVs also have important drawbacks. First, a quarter of all PIVs fail through occlusion or accidental dislodgement.[4] Infiltration, extravasation, and hematoma formation are important adverse events that may occur in such cases. Second, thrombophlebitis (pain and redness at the insertion site) is frequent, and may require device removal, especially in patients with catheters 20 guage.[9] Third, despite their relative safety, PIVs can cause localized or hematogenous infection. Septic thrombophlebitis (superficial thrombosis and bloodstream infection) and catheter‐related bloodstream infection, though rare, have been reported with PIVs and may lead to serious complications.[8, 10] In fact, some suggest that the overall burden of bloodstream infection risk posed by PIVs may be similar to that of CICCs given the substantially greater number of devices used and greater number of device days.[8]
PIVs and other peripheral VADs are not suitable for infusion of vesicants or irritants, which require larger, central veins for delivery. Vesicants (drugs that cause blistering on infusion) include chemotherapeutic agents (eg, dactinomycin, paclitaxel) and commonly used nonchemotherapeutical agents (eg, diazepam, piperacillin, vancomycin, esmolol, or total parenteral nutrition [TPN]).[11] Irritants (phlebitogenic drugs) cause short‐term inflammation and pain, and thus should not be peripherally infused for prolonged durations. Common irritants in the hospital setting include acyclovir, dobutamine, penicillin, and potassium chloride.
Of note, about one‐quarter of PIV insertions fail owing to difficult intravenous access.[12] Ultrasound‐guided peripheral intravenous (USGPIV) catheter placement is emerging as a technique to provide peripheral access for such patients to avoid placement of central venous access devices. Novel, longer devices (>8 cm) with built‐in guide wires have been developed to increase placement success of USGPIVs. These new designs provide easier access into deeper arm veins (brachial or basilic) not otherwise accessible by short PIVs. Although studies comparing the efficacy of USGPIV devices to other VADs are limited, a recent systematic review showed that time to successful cannulation was shorter, and fewer attempts were required to place USGPIVs compared to PIVs.[13] A recent study in France found that USGPIVs met the infusion needs of patients with difficult veins with minimal increase in complications.[14] Despite these encouraging data, future studies are needed to better evaluate this technology.
Midline Catheter
A midline is a VAD that is between 7.5 to 25 cm in length and is typically inserted into veins above the antecubital fossa. The catheter tip resides in a peripheral upper arm vein, often the basilic or cephalic vein, terminating just short of the subclavian vein (Figure 1B). Midline‐like devices were first developed in the 1950s and were initially used as an alternative to PIVs because they were thought to allow longer dwell times.[15] However, because they were originally constructed with a fairly rigid material, infiltration, mechanical phlebitis, and inflammation were common and tempered enthusiasm for their use.[15, 16] Newer midline devices obviate many of these problems and are inserted by ultrasound guidance and modified Seldinger technique.[17] Despite these advances, data regarding comparative efficacy are limited.
Midlines offer longer dwell times than standard PIVs owing to termination in the larger diameter basilic and brachial veins of the arm. Additionally, owing to their length, midlines are less prone to dislodgement. As they are inserted with greater antisepsis than PIVs and better secured to the skin, they are more durable than PIVs.[5, 9, 18] Current INS standards recommend use of midlines for 1 to 4 weeks.[5] Because they terminate in a peripheral vein, medications and infusions compatible with midlines are identical to those that are infused through a PIV. Thus, TPN, vesicants or irritants, or drugs that feature a pH <5 or pH >9, or >500 mOsm should not be infused through a midline.[15] New evidence suggests that diluted solutions of vancomycin (usually pH <5) may be safe to infuse for short durations (<6 days) through a midline, and that concentration rather than pH may be more important in this regard.[19] Although it is possible that the use of midlines may extend to agents typically not deemed peripheral access compatible, limited evidence exists to support such a strategy at this time.
Midlines offer several advantages. First, because blood flow is greater in the more proximal veins of the arm, midlines can accommodate infusions at rates of 100 to 150 mL/min compared to 20 to 40 mL/min in smaller peripheral veins. Higher flow rates offer greater hemodilution (dilution of the infusion with blood), decreasing the likelihood of phlebitis and infiltration.[20] Second, midlines do not require x‐ray verification of tip placement; thus, their use is often favored in resource‐depleted settings such as skilled nursing facilities. Third, midlines offer longer dwell times than peripheral intravenous catheters and can thus serve as bridge devices for short‐term intravenous antibiotics or peripheral‐compatible infusions in an outpatient setting. Available evidence suggests that midlines are associated with low rates of bloodstream infection (0.30.8 per 1000 catheter‐days).[17] The most frequent complications include phlebitis (4.2%) and occlusion (3.3%).[20] Given these favorable statistics, midlines may offer a good alternative to PIVs in select patients who require peripheral infusions of intermediate duration.
Intraosseous Vascular Access
Intraosseous (IO) devices access the vascular system by piercing cortical bone. These devices provide access to the intramedullary cavity and venous plexi of long bones such as the tibia, femur, or humerus. Several insertion devices are now commercially available and have enhanced the ease and safety of IO placement. Using these newer devices, IO access may be obtained in 1 to 2 minutes with minimal training. By comparison, a central venous catheter often requires 10 to 15 minutes to insert with substantial training efforts for providers.[21, 22, 23]
IO devices thus offer several advantages. First, given the rapidity with which they can be inserted, they are often preferred in emergency settings (eg, trauma). Second, these devices are versatile and can accommodate both central and peripheral infusates.[24] Third, a recent meta‐analysis found that IOs have a low complication rate of 0.8%, with extravasation of infusate through the cortical entry site being the most common adverse event.[21] Of note, this study also reported zero local or distal infectious complications, a finding that may relate to the shorter dwell of these devices.[21] Some animal studies suggest that fat embolism from bone may occur at high rates with IO VADs.[25] However, death or significant morbidity from fat emboli in humans following IO access has not been described. Whether such emboli occur or are clinically significant in the context of IO devices remains unclear at this time.[21]
Central Venous Access Devices
Central venous access devices (CVADs) share in common tip termination in the cavo‐atrial junction, either in the lower portion of the superior vena cava or in the upper portion of the right atrium. CVADs can be safely used for irritant or vesicant medications as well as for blood withdrawal, blood exchange procedures (eg, dialysis), and hemodynamic monitoring. Traditionally, these devices are 15 to 25 cm in length and are directly inserted in the deep veins of the supra‐ or infraclavicular area, including the internal jugular, brachiocephalic, subclavian, or axillary veins. PICCs are unique CVADs in that they enter through peripheral veins but terminate in the proximity of the cavoatrial junction. Regarding nomenclature, CICC will be used to denote devices that enter directly into veins of the neck or chest, whereas PICC will be used for devices that are inserted peripherally but terminate centrally.
Peripherally Inserted Central Catheter
PICCs are inserted into peripheral veins of the upper arm (eg, brachial, basilica, or cephalic vein) and advanced such that the tip resides at the cavoatrial junction (Figure 1C). PICCs offer prolonged dwell times and are thus indicated when patients require venous access for weeks or months.[26] Additionally, they can accommodate a variety of infusates and are safer to insert than CICCs, given placement in peripheral veins of the arm rather than central veins of the chest/neck. Thus, insertion complications such as pneumothorax, hemothorax, or significant bleeding are rare with PICCs. In fact, a recent study reported that PICC insertion by hospitalists was associated with low rates of insertion or infectious complications.[27]
However, like CICCs, PICCs are associated with central lineassociated bloodstream infection (CLABSI), a serious complication known to prolong length of hospital stay, increase costs, and carry a 12% to 25% associated mortality.[28, 29] In the United States alone, over 250,000 CLASBI cases occur per year drawing considerable attention from the CDC and Joint Commission, who now mandate reporting and nonpayment for hospital‐acquired CLABSI.[30, 31, 32] A recent systematic review and meta‐analysis found that PICCs are associated with a substantial risk of CLABSI in hospitalized patients.[33] Importantly, no difference in CLABSI rates between PICCs and CICCs in hospitalized patients was evident in this meta‐analysis. Therefore, current guidelines specifically recommend against use of PICCs over CICCs as a strategy to reduce CLABSI.[34] Additionally, PICCs are associated with 2.5‐fold greater risk of deep vein thrombosis (DVT) compared to CICCs; thus, they should be used with caution in patients with cancer or those with underlying hypercoagulable states.
Of particular import to hospitalists is the fact that PICC placement is contraindicated in patients with stage IIIB or greater chronic kidney disease (CKD). In such patients, sequelae of PICC use, such as phlebitis or central vein stenosis, can be devastating in patients with CKD.[35] In a recent study, prior PICC placement was the strongest predictor of subsequent arteriovenous graft failure.[36] For this reason, Choosing Wisely recommendations call for avoidance of PICCs in such patients.[37]
Centrally Inserted Central Catheter
CICCs are CVADs placed by puncture and cannulation of the internal jugular, subclavian, brachiocephalic, or femoral veins (Figure 1D) and compose the vast majority of VADs placed in ICU settings.[38, 39] Central termination of CICCs allows for a variety of infusions, including irritants, vesicants, and vasopressors, as well as blood withdrawal and hemodynamic monitoring. CICCs are typically used for 7 to 14 days, but may remain for longer durations if they remain complication free and clinically necessary.[40] A key advantage of CICCs is that they can be placed in emergent settings to facilitate quick access for rapid infusion or hemodynamic monitoring. In particular, CICCs are inserted in the femoral vein and may be useful in emergency settings. However, owing to risk of infection and inability to monitor central pressures, these femoral devices should be replaced with a proper CICC or PICC when possible. Importantly, although CICCs are almost exclusively used in intensive or emergency care, PICCs may also be considered in such settings.[41, 42] CICCs usually have multiple lumens and often serve several simultaneous functions such as both infusions and hemodynamic monitoring.
Despite their benefits, CICCs have several disadvantages. First, insertion requires an experienced clinician and has historically been a task limited to physicians. However, this is changing rapidly (especially in Europe and Australia) where specially trained nurses are assuming responsibility for CICC placement.[43] Second, these devices are historically more likely to be associated with CLABSI, with estimates of infection rates varying between 2 and 5 infections per 1000 catheter‐days.[44] Third, CICCs pose a significant DVT risk, with rates around 22 DVTs per 1000 catheter‐days.[45] However, compared to PICCs, the DVT risk appears lower, and CICC use may be preferable in patients at high risk of DVT, such as critically ill or cancer populations.[46] An important note to prevent CICC insertion complications relates to use of ultrasound, a practice that has been associated with decreased accidental arterial puncture and hematoma formation. The role of ultrasound guidance with PICCs as well as implications for thrombotic and infectious events remains less characterized at this point.[47]
Tunneled Central Venous Access Devices
Tunneled devices (either CICCs or PICCs) are characterized by the fact that the insertion site on the skin and site of ultimate venipuncture are physically separated (Figure 1E). Tunneling limits bacterial entry from the extraluminal aspect of the CVAD to the bloodstream. For example, internal jugular veins are often ideal sites of puncture but inappropriate sites for catheter placement, as providing care to this area is challenging and may increase risk of infection.[34] Tunneling to the infraclavicular area provides a better option, as it provides an exit site that can be adequately cared for. Importantly, any CVAD (PICCs or CICCs) can be tunneled. Additionally, tunneled CICCs may be used in patients with chronic or impending renal failure where PICCs are contraindicated because entry into dialysis‐relevant vessels is to be avoided.[48] Such devices also allow regular blood sampling in patients who require frequent testing but have limited peripheral access, such as those with hematological malignancies. Additionally, tunneled catheters are more comfortable for patients and viewed as being more socially acceptable than nontunneled devices. However, the more invasive and permanent nature of these devices often requires deliberation prior to insertion.
Of note, tunneled devices and ports may be used as long‐term (>3 months to years) VADs. As our focus in this review is short‐term devices, we will not expand the discussion of these devices as they are almost always used for prolonged durations.[7]
OPERATIONALIZING THE DATA: AN ALGORITHMIC APPROACH TO VENOUS ACCESS
Hospitalists should consider approaching venous access using an algorithm based on a number of parameters. For example, a critically ill patient who requires vasopressor support and hemodynamic monitoring will need a CICC or a PICC. Given the potential greater risk of thromboses from PICCs, a CICC is preferable for critically ill patients provided an experienced inserter is available. Conversely, patients who require short‐term (<710 days) venous access for infusion of nonirritant or nonvesicant therapy often only require a PIV. In patients with poor or difficult venous access, USGPIVs or midlines may be ideal and preferred over short PIVs. Finally, patients who require longer‐term or home‐based treatment may benefit from early placement of a midline or a PICC, depending again on the nature of the infusion, duration of treatment, and available venous access sites.
An algorithmic approach considering these parameters is suggested in Figure 2, and a brief overview of the devices and their considerations is shown in Table 3.
| Vascular Access Device | Central/Peripheral | Anatomical Location of Placement | Desired Duration of Placement | Common Uses | BSI Risk (Per 1,000 Catheter‐Days) | Thrombosis Risk | Important Considerations |
|---|---|---|---|---|---|---|---|
| |||||||
| Small peripheral IV | Peripheral | Peripheral veins, usually forearm | 710 days | Fluid resuscitation, most medications, blood products | 0.06[8] | Virtually no risk | Consider necessity of PIV daily and remove unnecessary devices |
| Midline | Peripheral | Inserted around antecubital fossa, reside within basilic or cephalic vein of the arm | 24 weeks | Long‐term medications excluding TPN, vesicants, corrosives | 0.30.8[17] | Insufficient data | Can be used as bridge devices for patients to complete short‐term antibiotics/emnfusions as an outpatient |
| Peripherally inserted central catheter | Central | Inserted into peripheral arm vein and advanced to larger veins (eg, internal jugular or subclavian) to the CAJ | >1 week, <3 months | Large variety of infusates, including TPN, vesicants, corrosives | 2.4[44] | 6.30% | Contraindicated in patients with CKD stage IIIb or higher |
| Centrally inserted central catheters | Central | Inserted above (internal jugular vein, brachiocephalic vein, subclavian vein), or below the clavicle (axillary vein) | >1 week, <3 months | Same infusate variety as PICC, measurement of central venous pressures, common in trauma/emergent settings | 2.3[44] | 1.30% | Given lower rates of DVT than PICC, preferred in ICU and hypercoagulable environments |
| Tunneled CICCs | Central | Placed percutaneously in any large vein in the arm, chest, neck or groin | >3 months to years | Central infusates, as in any CVAD; used for patients with CKD stage IIIb or greater when a PICC is indicated | Insufficient data | Insufficient data | May be superior when insertion site and puncture site are not congruent and may increase risk of infection |
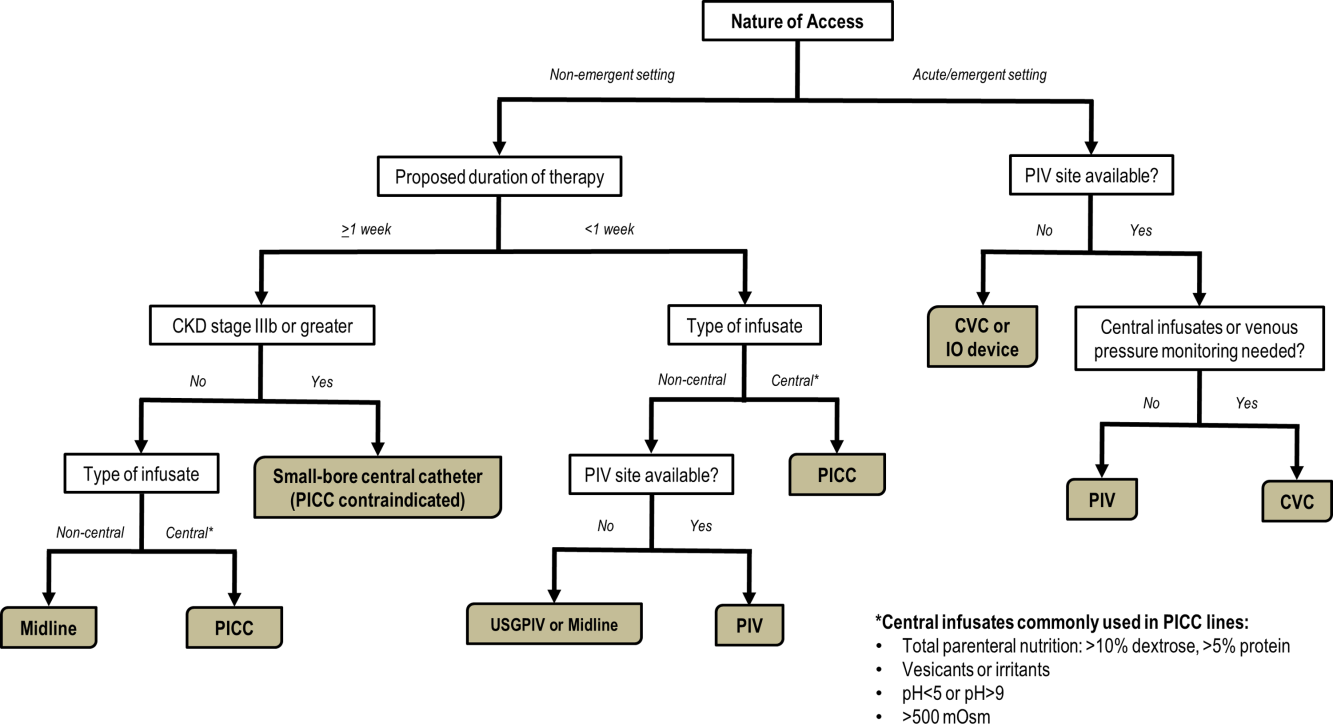
CONCLUSIONS
With strides in technology and progress in medicine, hospitalists have access to an array of options for venous access. However, every VAD has limitations that can be easily overlooked in a perfunctory decision‐making process. The data presented in this review thus provide a first step to improving safety in this evolving science. Studies that further determine appropriateness of VADs in hospitalized settings are necessary. Only through such progressive scientific enquiry will complication‐free venous access be realized.
Disclosure
Nothing to report.
- , . The need for comparative data in vascular access: the rationale and design of the PICC registry. J Vasc Access. 2013; 18(4): 219–224.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013; 8(6): 309–314.
- , , , . Clinically‐indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2013; 4: CD007798.
- , , , et al. Cost‐effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014; 12(1): 51–58.
- Infusion Nurses Society. Infusion Nursing Standards of Practice. Norwood, MA; Infusion Nurses Society; 2011.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012; 380(9847): 1066–1074.
- , , , et al. epic3: national evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. J Hosp Infect. 2014; 86(suppl 1): S1–S70.
- . Short peripheral intravenous catheters and infections. J Infus Nurs. 2012; 35(4): 230–240.
- , , , et al. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: a large multi‐centre prospective study. J Adv Nurs. 2014; 70(11): 2539–2549.
- , . Intravenous catheter complications in the hand and forearm. J Trauma. 2004; 56(1): 123–127.
- , , , . Vesicant extravasation part I: Mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006; 33(6): 1134–1141.
- , . Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005; 34(5): 345–359.
- , , . Ultrasound‐guided peripheral venous access: a systematic review of randomized‐controlled trials. Eur J Emerg Med. 2014; 21(1): 18–23.
- , , , et al. Difficult peripheral venous access: clinical evaluation of a catheter inserted with the Seldinger method under ultrasound guidance. J Crit Care. 2014; 29(5): 823–827.
- . Choosing the right intravenous catheter. Home Healthc Nurse. 2007; 25(8): 523–531; quiz 532–523.
- Adverse reactions associated with midline catheters—United States, 1992–1995. MMWR Morb Mortal Wkly Rep. 1996; 45(5): 101–103.
- , , . The risk of midline catheterization in hospitalized patients. A prospective study. Ann Intern Med. 1995; 123(11): 841–844.
- . Midline catheters: indications, complications and maintenance. Nurs Stand. 2007; 22(11): 48–57; quiz 58.
- , . Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access. 2014; 15(4): 251–256.
- . Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004; 27(5): 313–321.
- . Vascular access in resuscitation: is there a role for the intraosseous route? Anesthesiology. 2014; 120(4): 1015–1031.
- , , , et al. A new system for sternal intraosseous infusion in adults. Prehosp Emerg Care. 2000; 4(2): 173–177.
- , , , , , . Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department: a prospective, randomized study. Resuscitation. 2010; 81(8): 994–999.
- , , , , . Comparison study of intraosseous, central intravenous, and peripheral intravenous infusions of emergency drugs. Am J Dis Child. 1990; 144(1): 112–117.
- , , , , . The safety of intraosseous infusions: risks of fat and bone marrow emboli to the lungs. Ann Emerg Med. 1989; 18(10): 1062–1067.
- , , , , . ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr. 2009; 28(4): 365–377.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009; 4(6): E1–E4.
- Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011; 60(8): 243–248.
- , , , . Hospital costs of central line‐associated bloodstream infections and cost‐effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Eff Resour Alloc. 2010; 8: 8.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006; 81(9): 1159–1171.
- The Joint Commission. Preventing Central Line‐Associated Bloodstream Infections: A Global Challenge, a Global Perspective. Oak Brook, IL: Joint Commission Resources; 2012.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011; 39(4 suppl 1): S1–S34.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013; 34(9): 908–918.
- Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, American Hospital Association, Association for Professionals in Infection Control and Epidemiology, The Joint Commission. Compendium of Strategies to Prevent Healthcare‐Associated Infections in Acute Care Hospitals: 2014 Updates. Available at: http://www.shea‐online.org. Accessed August 1, 2014.
- , , , , . Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008; 21(2): 186–191.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012; 60(4): 601–608.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012; 7(10): 1664–1672.
- , , , et al. Do physicians know which of their patients have central venous catheters? A multi‐center observational study. Ann Intern Med. 2014; 161(8): 562–567.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011; 77(4): 304–308.
- , , , , , . Peripherally inserted central catheter‐related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014; 12(6): 847–854.
- , , , . An in vitro study comparing a peripherally inserted central catheter to a conventional central venous catheter: no difference in static and dynamic pressure transmission. BMC Anesthesiol. 2010; 10: 18.
- , , , et al. Clinical experience with power‐injectable PICCs in intensive care patients. Crit Care. 2012; 16(1): R21.
- , , , et al. Nurse‐led central venous catheter insertion‐procedural characteristics and outcomes of three intensive care based catheter placement services. Int J Nurs Stud. 2012; 49(2): 162–168.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010; 38(2): 149–153.
- , , , et al. Which central venous catheters have the highest rate of catheter‐associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg. 2013; 74(2): 454–460; discussion 461–452.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013; 382(9889): 311–325.
- , , , , . Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015; 1: CD011447.
- , , , et al. Tunneled jugular small‐bore central catheters as an alternative to peripherally inserted central catheters for intermediate‐term venous access in patients with hemodialysis and chronic renal insufficiency. Radiology. 1999; 213(1): 303–306.
Reliable venous access is fundamental for the safe and effective care of hospitalized patients. Venous access devices (VADs) are conduits for this purpose, providing delivery of intravenous medications, accurate measurement of central venous pressure, or administration of life‐saving blood products. Despite this important role, VADs are also often the source of hospital‐acquired complications. Although inpatient providers must balance the relative risks of VADs against their benefits, the evidence supporting such decisions is often limited. Advances in technology, scattered research, and growing availability of novel devices has only further fragmented provider knowledge in the field of vascular access.[1]
It is not surprising, then, that survey‐based studies of hospitalists reveal important knowledge gaps with regard to practices associated with VADs.[2] In this narrative review, we seek to bridge this gap by providing a concise and pragmatic overview of the fundamentals of venous access. We focus specifically on parameters that influence decisions regarding VAD placement in hospitalized patients, providing key takeaways for practicing hospitalists.
METHODS
To compile this review, we systematically searched Medline (via Ovid) for several keywords, including: peripheral intravenous catheters, ultrasound‐guided peripheral catheter, intraosseous, midline, peripherally inserted central catheter, central venous catheters, and vascular access device complications. We concentrated on full‐length articles in English only; no date restrictions were placed on the search. We reviewed guidelines and consensus statements (eg, from the Center for Disease Control [CDC] or Choosing Wisely criteria) as appropriate. Additional studies of interest were identified through content experts (M.P., C.M.R.) and bibliographies of included studies.
SCIENTIFIC PRINCIPLES UNDERPINNING VENOUS ACCESS
It is useful to begin by reviewing VAD‐related nomenclature and physiology. In the simplest sense, a VAD consists of a hub (providing access to various connectors), a hollow tube divided into 1 or many sections (lumens), and a tip that may terminate within a central or peripheral blood vessel. VADs are classified as central venous catheters (eg, centrally inserted central catheters [CICCs] or peripherally inserted central catheters [PICCs]) or peripheral intravenous catheters (eg, midlines or peripheral intravenous catheters) based on site of entry and location of the catheter tip. Therefore, VADs entering via proximal or distal veins of the arm are often referred to as peripheral lines, as their site of entry and tip both reside within peripheral veins. Conversely, the term central line is often used when VADs enter or terminate in a central vein (eg, subclavian vein insertion with the catheter tip in the lower superior vena cava).
Attention to a host of clinical and theoretical parameters is important when choosing a device for venous access. Some such parameters are summarized in Table 1.
| Parameter | Major Considerations |
|---|---|
| |
| Desired flow rate | Smaller diameter veins susceptible to damage with high flow rates. |
| Short, large‐bore catheters facilitate rapid infusion. | |
| Nature of infusion | pH, viscosity, and temperature may damage vessels. |
| Vesicants and irritants should always be administered into larger, central veins. | |
| Desired duration of vascular access, or dwell time | Vessel thrombosis or phlebitis increase over time with catheter in place. |
| Intermittent infusions increase complications in central catheters; often tunneled catheters are recommended. | |
| Urgency of placement | Access to large caliber vessels is often needed in emergencies. |
| Critically ill or hemodynamically unstable patients may require urgent access for invasive monitoring or rapid infusions. | |
| Patients with trauma often require large volumes of blood products and reliable access to central veins. | |
| Number of device lumens | VADs may have single or multiple lumens. |
| Multilumen allows for multiple functions (eg, infusion of multiple agents, measurement of central venous pressures, blood draws). | |
| Device gauge | In general, use of a smaller‐gauge catheter is preferred to prevent complications. |
| However, larger catheter diameter may be needed for specific clinical needs (eg, blood transfusion). | |
| Device coating | VADs may have antithrombotic or anti‐infective coatings. |
| These devices may be of value in patients at high risk of complications. | |
| Such devices, however, may be more costly than their counterparts. | |
| Self‐care compatibility | VADs that can be cared for by patients are ideal for outpatient care. |
| Conversely, VADs such as peripheral catheters, are highly prone to dislodgement and should be reserved for supervised settings only. | |
VENOUS ACCESS DEVICES
We will organize our discussion of VADs based on whether they terminate in peripheral or central vessels. These anatomical considerations are relevant as they determine physical characteristics, compatibility with particular infusates, dwell time, and risk of complications associated with each VAD discussed in Table 2.
| Complications | Major Considerations |
|---|---|
| |
| Infection | VADs breach the integrity of skin and permit skin pathogens to enter the blood stream (extraluminal infection). |
| Inadequate antisepsis of the VAD hub, including poor hand hygiene, failure to "scrub the hub," and multiple manipulations may also increase the risk of VAD‐related infection (endoluminal infection). | |
| Infections may be local (eg, exit‐site infections) or may spread hematogenously (eg, CLABSI). | |
| Type of VAD, duration of therapy, and host characteristics interact to influence infection risk. | |
| VADs with antiseptic coatings (eg, chlorhexidine) or antibiotic coatings (eg, minocycline) may reduce risk of infection in high‐risk patients. | |
| Antiseptic‐impregnated dressings may reduce risk of extraluminal infection. | |
| Venous thrombosis | VADs predispose to venous stasis and thrombosis. |
| Duration of VAD use, type and care of the VAD, and patient characteristics affect risk of thromboembolism. | |
| VAD tip position is a key determinant of venous thrombosis; central VADs that do not terminate at the cavo‐atrial junction should be repositioned to reduce the risk of thrombosis. | |
| Antithrombotic coated or eluting devices may reduce risk of thrombosis, though definitive data are lacking. | |
| Phlebitis | Inflammation caused by damage to tunica media.[18] |
| 3 types of phlebitis: | |
| Chemical: due to irritation of media from the infusate. | |
| Mechanical: VAD physically damages the vessel. | |
| Infective: bacteria invade vein and inflame vessel wall. | |
| Phlebitis may be limited by close attention to infusate compatibility with peripheral veins, appropriate dilution, and prompt removal of catheters that show signs of inflammation. | |
| Phlebitis may be prevented in PICCs by ensuring at least a 2:1 vein:catheter ratio. | |
| Extravasation | Extravasation (also called infiltration) is defined as leakage of infusate from intravascular to extravascular space. |
| Extravasation of vesicants/emrritants is particularly worrisome. | |
| May result in severe tissue injury, blistering, and tissue necrosis.[11] | |
| VADs should be checked frequently for adequate flushing and position prior to each infusion to minimize risk. | |
| Any VAD with redness, swelling, and tenderness at the entry site or problems with flushing should not be used without further examination and review of position. | |
Peripheral Venous Access
Short Peripheral Intravenous Catheter
Approximately 200 million peripheral intravenous catheters (PIVs) are placed annually in the United States, making them the most common intravenous catheter.[3] PIVs are short devices, 3 to 6 cm in length, that enter and terminate in peripheral veins (Figure 1A). Placement is recommended in forearm veins rather than those of the hand, wrist, or upper arm, as forearm sites are less prone to occlusion, accidental removal, and phlebitis.[4] Additionally, placement in hand veins impedes activities of daily living (eg, hand washing) and is not preferred by patients.[5] PIV size ranges from 24 gauge (smallest) to 14 gauge (largest); larger catheters are often reserved for fluid resuscitation or blood transfusion as they accommodate greater flow and limit hemolysis. To decrease risk of phlebitis and thrombosis, the shortest catheter and smallest diameter should be used. However, unless adequately secured, smaller diameter catheters are also associated with greater rates of accidental removal.[4, 5]

By definition, PIVs are short‐term devices. The CDC currently recommends removal and replacement of these devices no more frequently than every 72 to 96 hours in adults. However, a recent randomized controlled trial found that replacing PIVs when clinically indicated (eg, device failure, phlebitis) rather than on a routine schedule added 30 hours to their lifespan without an increase in complications.[6] A systematic review by the Cochrane Collaboration echoes these findings.[3] These data have thus been incorporated into recommendations from the Infusion Nurses Society (INS) and the National Health Service in the United Kingdom.[5, 7] In hospitalized patients, this approach is relevant, as it preserves venous access sites, maximizes device dwell, and limits additional PIV insertions. In turn, these differences may reduce the need for invasive VADs such as PICCs. Furthermore, the projected 5‐year savings from implementation of clinically indicated PIV removal policies is US$300 million and 1 million health‐worker hours in the United States alone.[4]
PIVs offer many advantages. First, they are minimally invasive and require little training to insert. Second, they can be used for diverse indications in patients requiring short‐term (1 week) venous access. Third, PIVs do not require imaging to ensure correct placement; palpation of superficial veins is sufficient. Fourth, PIVs exhibit a risk of bloodstream infection that is about 40‐fold lower than more invasive, longer‐dwelling VADs[8] (0.06 bacteremia per 1000 catheter‐days).
Despite these advantages, PIVs also have important drawbacks. First, a quarter of all PIVs fail through occlusion or accidental dislodgement.[4] Infiltration, extravasation, and hematoma formation are important adverse events that may occur in such cases. Second, thrombophlebitis (pain and redness at the insertion site) is frequent, and may require device removal, especially in patients with catheters 20 guage.[9] Third, despite their relative safety, PIVs can cause localized or hematogenous infection. Septic thrombophlebitis (superficial thrombosis and bloodstream infection) and catheter‐related bloodstream infection, though rare, have been reported with PIVs and may lead to serious complications.[8, 10] In fact, some suggest that the overall burden of bloodstream infection risk posed by PIVs may be similar to that of CICCs given the substantially greater number of devices used and greater number of device days.[8]
PIVs and other peripheral VADs are not suitable for infusion of vesicants or irritants, which require larger, central veins for delivery. Vesicants (drugs that cause blistering on infusion) include chemotherapeutic agents (eg, dactinomycin, paclitaxel) and commonly used nonchemotherapeutical agents (eg, diazepam, piperacillin, vancomycin, esmolol, or total parenteral nutrition [TPN]).[11] Irritants (phlebitogenic drugs) cause short‐term inflammation and pain, and thus should not be peripherally infused for prolonged durations. Common irritants in the hospital setting include acyclovir, dobutamine, penicillin, and potassium chloride.
Of note, about one‐quarter of PIV insertions fail owing to difficult intravenous access.[12] Ultrasound‐guided peripheral intravenous (USGPIV) catheter placement is emerging as a technique to provide peripheral access for such patients to avoid placement of central venous access devices. Novel, longer devices (>8 cm) with built‐in guide wires have been developed to increase placement success of USGPIVs. These new designs provide easier access into deeper arm veins (brachial or basilic) not otherwise accessible by short PIVs. Although studies comparing the efficacy of USGPIV devices to other VADs are limited, a recent systematic review showed that time to successful cannulation was shorter, and fewer attempts were required to place USGPIVs compared to PIVs.[13] A recent study in France found that USGPIVs met the infusion needs of patients with difficult veins with minimal increase in complications.[14] Despite these encouraging data, future studies are needed to better evaluate this technology.
Midline Catheter
A midline is a VAD that is between 7.5 to 25 cm in length and is typically inserted into veins above the antecubital fossa. The catheter tip resides in a peripheral upper arm vein, often the basilic or cephalic vein, terminating just short of the subclavian vein (Figure 1B). Midline‐like devices were first developed in the 1950s and were initially used as an alternative to PIVs because they were thought to allow longer dwell times.[15] However, because they were originally constructed with a fairly rigid material, infiltration, mechanical phlebitis, and inflammation were common and tempered enthusiasm for their use.[15, 16] Newer midline devices obviate many of these problems and are inserted by ultrasound guidance and modified Seldinger technique.[17] Despite these advances, data regarding comparative efficacy are limited.
Midlines offer longer dwell times than standard PIVs owing to termination in the larger diameter basilic and brachial veins of the arm. Additionally, owing to their length, midlines are less prone to dislodgement. As they are inserted with greater antisepsis than PIVs and better secured to the skin, they are more durable than PIVs.[5, 9, 18] Current INS standards recommend use of midlines for 1 to 4 weeks.[5] Because they terminate in a peripheral vein, medications and infusions compatible with midlines are identical to those that are infused through a PIV. Thus, TPN, vesicants or irritants, or drugs that feature a pH <5 or pH >9, or >500 mOsm should not be infused through a midline.[15] New evidence suggests that diluted solutions of vancomycin (usually pH <5) may be safe to infuse for short durations (<6 days) through a midline, and that concentration rather than pH may be more important in this regard.[19] Although it is possible that the use of midlines may extend to agents typically not deemed peripheral access compatible, limited evidence exists to support such a strategy at this time.
Midlines offer several advantages. First, because blood flow is greater in the more proximal veins of the arm, midlines can accommodate infusions at rates of 100 to 150 mL/min compared to 20 to 40 mL/min in smaller peripheral veins. Higher flow rates offer greater hemodilution (dilution of the infusion with blood), decreasing the likelihood of phlebitis and infiltration.[20] Second, midlines do not require x‐ray verification of tip placement; thus, their use is often favored in resource‐depleted settings such as skilled nursing facilities. Third, midlines offer longer dwell times than peripheral intravenous catheters and can thus serve as bridge devices for short‐term intravenous antibiotics or peripheral‐compatible infusions in an outpatient setting. Available evidence suggests that midlines are associated with low rates of bloodstream infection (0.30.8 per 1000 catheter‐days).[17] The most frequent complications include phlebitis (4.2%) and occlusion (3.3%).[20] Given these favorable statistics, midlines may offer a good alternative to PIVs in select patients who require peripheral infusions of intermediate duration.
Intraosseous Vascular Access
Intraosseous (IO) devices access the vascular system by piercing cortical bone. These devices provide access to the intramedullary cavity and venous plexi of long bones such as the tibia, femur, or humerus. Several insertion devices are now commercially available and have enhanced the ease and safety of IO placement. Using these newer devices, IO access may be obtained in 1 to 2 minutes with minimal training. By comparison, a central venous catheter often requires 10 to 15 minutes to insert with substantial training efforts for providers.[21, 22, 23]
IO devices thus offer several advantages. First, given the rapidity with which they can be inserted, they are often preferred in emergency settings (eg, trauma). Second, these devices are versatile and can accommodate both central and peripheral infusates.[24] Third, a recent meta‐analysis found that IOs have a low complication rate of 0.8%, with extravasation of infusate through the cortical entry site being the most common adverse event.[21] Of note, this study also reported zero local or distal infectious complications, a finding that may relate to the shorter dwell of these devices.[21] Some animal studies suggest that fat embolism from bone may occur at high rates with IO VADs.[25] However, death or significant morbidity from fat emboli in humans following IO access has not been described. Whether such emboli occur or are clinically significant in the context of IO devices remains unclear at this time.[21]
Central Venous Access Devices
Central venous access devices (CVADs) share in common tip termination in the cavo‐atrial junction, either in the lower portion of the superior vena cava or in the upper portion of the right atrium. CVADs can be safely used for irritant or vesicant medications as well as for blood withdrawal, blood exchange procedures (eg, dialysis), and hemodynamic monitoring. Traditionally, these devices are 15 to 25 cm in length and are directly inserted in the deep veins of the supra‐ or infraclavicular area, including the internal jugular, brachiocephalic, subclavian, or axillary veins. PICCs are unique CVADs in that they enter through peripheral veins but terminate in the proximity of the cavoatrial junction. Regarding nomenclature, CICC will be used to denote devices that enter directly into veins of the neck or chest, whereas PICC will be used for devices that are inserted peripherally but terminate centrally.
Peripherally Inserted Central Catheter
PICCs are inserted into peripheral veins of the upper arm (eg, brachial, basilica, or cephalic vein) and advanced such that the tip resides at the cavoatrial junction (Figure 1C). PICCs offer prolonged dwell times and are thus indicated when patients require venous access for weeks or months.[26] Additionally, they can accommodate a variety of infusates and are safer to insert than CICCs, given placement in peripheral veins of the arm rather than central veins of the chest/neck. Thus, insertion complications such as pneumothorax, hemothorax, or significant bleeding are rare with PICCs. In fact, a recent study reported that PICC insertion by hospitalists was associated with low rates of insertion or infectious complications.[27]
However, like CICCs, PICCs are associated with central lineassociated bloodstream infection (CLABSI), a serious complication known to prolong length of hospital stay, increase costs, and carry a 12% to 25% associated mortality.[28, 29] In the United States alone, over 250,000 CLASBI cases occur per year drawing considerable attention from the CDC and Joint Commission, who now mandate reporting and nonpayment for hospital‐acquired CLABSI.[30, 31, 32] A recent systematic review and meta‐analysis found that PICCs are associated with a substantial risk of CLABSI in hospitalized patients.[33] Importantly, no difference in CLABSI rates between PICCs and CICCs in hospitalized patients was evident in this meta‐analysis. Therefore, current guidelines specifically recommend against use of PICCs over CICCs as a strategy to reduce CLABSI.[34] Additionally, PICCs are associated with 2.5‐fold greater risk of deep vein thrombosis (DVT) compared to CICCs; thus, they should be used with caution in patients with cancer or those with underlying hypercoagulable states.
Of particular import to hospitalists is the fact that PICC placement is contraindicated in patients with stage IIIB or greater chronic kidney disease (CKD). In such patients, sequelae of PICC use, such as phlebitis or central vein stenosis, can be devastating in patients with CKD.[35] In a recent study, prior PICC placement was the strongest predictor of subsequent arteriovenous graft failure.[36] For this reason, Choosing Wisely recommendations call for avoidance of PICCs in such patients.[37]
Centrally Inserted Central Catheter
CICCs are CVADs placed by puncture and cannulation of the internal jugular, subclavian, brachiocephalic, or femoral veins (Figure 1D) and compose the vast majority of VADs placed in ICU settings.[38, 39] Central termination of CICCs allows for a variety of infusions, including irritants, vesicants, and vasopressors, as well as blood withdrawal and hemodynamic monitoring. CICCs are typically used for 7 to 14 days, but may remain for longer durations if they remain complication free and clinically necessary.[40] A key advantage of CICCs is that they can be placed in emergent settings to facilitate quick access for rapid infusion or hemodynamic monitoring. In particular, CICCs are inserted in the femoral vein and may be useful in emergency settings. However, owing to risk of infection and inability to monitor central pressures, these femoral devices should be replaced with a proper CICC or PICC when possible. Importantly, although CICCs are almost exclusively used in intensive or emergency care, PICCs may also be considered in such settings.[41, 42] CICCs usually have multiple lumens and often serve several simultaneous functions such as both infusions and hemodynamic monitoring.
Despite their benefits, CICCs have several disadvantages. First, insertion requires an experienced clinician and has historically been a task limited to physicians. However, this is changing rapidly (especially in Europe and Australia) where specially trained nurses are assuming responsibility for CICC placement.[43] Second, these devices are historically more likely to be associated with CLABSI, with estimates of infection rates varying between 2 and 5 infections per 1000 catheter‐days.[44] Third, CICCs pose a significant DVT risk, with rates around 22 DVTs per 1000 catheter‐days.[45] However, compared to PICCs, the DVT risk appears lower, and CICC use may be preferable in patients at high risk of DVT, such as critically ill or cancer populations.[46] An important note to prevent CICC insertion complications relates to use of ultrasound, a practice that has been associated with decreased accidental arterial puncture and hematoma formation. The role of ultrasound guidance with PICCs as well as implications for thrombotic and infectious events remains less characterized at this point.[47]
Tunneled Central Venous Access Devices
Tunneled devices (either CICCs or PICCs) are characterized by the fact that the insertion site on the skin and site of ultimate venipuncture are physically separated (Figure 1E). Tunneling limits bacterial entry from the extraluminal aspect of the CVAD to the bloodstream. For example, internal jugular veins are often ideal sites of puncture but inappropriate sites for catheter placement, as providing care to this area is challenging and may increase risk of infection.[34] Tunneling to the infraclavicular area provides a better option, as it provides an exit site that can be adequately cared for. Importantly, any CVAD (PICCs or CICCs) can be tunneled. Additionally, tunneled CICCs may be used in patients with chronic or impending renal failure where PICCs are contraindicated because entry into dialysis‐relevant vessels is to be avoided.[48] Such devices also allow regular blood sampling in patients who require frequent testing but have limited peripheral access, such as those with hematological malignancies. Additionally, tunneled catheters are more comfortable for patients and viewed as being more socially acceptable than nontunneled devices. However, the more invasive and permanent nature of these devices often requires deliberation prior to insertion.
Of note, tunneled devices and ports may be used as long‐term (>3 months to years) VADs. As our focus in this review is short‐term devices, we will not expand the discussion of these devices as they are almost always used for prolonged durations.[7]
OPERATIONALIZING THE DATA: AN ALGORITHMIC APPROACH TO VENOUS ACCESS
Hospitalists should consider approaching venous access using an algorithm based on a number of parameters. For example, a critically ill patient who requires vasopressor support and hemodynamic monitoring will need a CICC or a PICC. Given the potential greater risk of thromboses from PICCs, a CICC is preferable for critically ill patients provided an experienced inserter is available. Conversely, patients who require short‐term (<710 days) venous access for infusion of nonirritant or nonvesicant therapy often only require a PIV. In patients with poor or difficult venous access, USGPIVs or midlines may be ideal and preferred over short PIVs. Finally, patients who require longer‐term or home‐based treatment may benefit from early placement of a midline or a PICC, depending again on the nature of the infusion, duration of treatment, and available venous access sites.
An algorithmic approach considering these parameters is suggested in Figure 2, and a brief overview of the devices and their considerations is shown in Table 3.
| Vascular Access Device | Central/Peripheral | Anatomical Location of Placement | Desired Duration of Placement | Common Uses | BSI Risk (Per 1,000 Catheter‐Days) | Thrombosis Risk | Important Considerations |
|---|---|---|---|---|---|---|---|
| |||||||
| Small peripheral IV | Peripheral | Peripheral veins, usually forearm | 710 days | Fluid resuscitation, most medications, blood products | 0.06[8] | Virtually no risk | Consider necessity of PIV daily and remove unnecessary devices |
| Midline | Peripheral | Inserted around antecubital fossa, reside within basilic or cephalic vein of the arm | 24 weeks | Long‐term medications excluding TPN, vesicants, corrosives | 0.30.8[17] | Insufficient data | Can be used as bridge devices for patients to complete short‐term antibiotics/emnfusions as an outpatient |
| Peripherally inserted central catheter | Central | Inserted into peripheral arm vein and advanced to larger veins (eg, internal jugular or subclavian) to the CAJ | >1 week, <3 months | Large variety of infusates, including TPN, vesicants, corrosives | 2.4[44] | 6.30% | Contraindicated in patients with CKD stage IIIb or higher |
| Centrally inserted central catheters | Central | Inserted above (internal jugular vein, brachiocephalic vein, subclavian vein), or below the clavicle (axillary vein) | >1 week, <3 months | Same infusate variety as PICC, measurement of central venous pressures, common in trauma/emergent settings | 2.3[44] | 1.30% | Given lower rates of DVT than PICC, preferred in ICU and hypercoagulable environments |
| Tunneled CICCs | Central | Placed percutaneously in any large vein in the arm, chest, neck or groin | >3 months to years | Central infusates, as in any CVAD; used for patients with CKD stage IIIb or greater when a PICC is indicated | Insufficient data | Insufficient data | May be superior when insertion site and puncture site are not congruent and may increase risk of infection |

CONCLUSIONS
With strides in technology and progress in medicine, hospitalists have access to an array of options for venous access. However, every VAD has limitations that can be easily overlooked in a perfunctory decision‐making process. The data presented in this review thus provide a first step to improving safety in this evolving science. Studies that further determine appropriateness of VADs in hospitalized settings are necessary. Only through such progressive scientific enquiry will complication‐free venous access be realized.
Disclosure
Nothing to report.
Reliable venous access is fundamental for the safe and effective care of hospitalized patients. Venous access devices (VADs) are conduits for this purpose, providing delivery of intravenous medications, accurate measurement of central venous pressure, or administration of life‐saving blood products. Despite this important role, VADs are also often the source of hospital‐acquired complications. Although inpatient providers must balance the relative risks of VADs against their benefits, the evidence supporting such decisions is often limited. Advances in technology, scattered research, and growing availability of novel devices has only further fragmented provider knowledge in the field of vascular access.[1]
It is not surprising, then, that survey‐based studies of hospitalists reveal important knowledge gaps with regard to practices associated with VADs.[2] In this narrative review, we seek to bridge this gap by providing a concise and pragmatic overview of the fundamentals of venous access. We focus specifically on parameters that influence decisions regarding VAD placement in hospitalized patients, providing key takeaways for practicing hospitalists.
METHODS
To compile this review, we systematically searched Medline (via Ovid) for several keywords, including: peripheral intravenous catheters, ultrasound‐guided peripheral catheter, intraosseous, midline, peripherally inserted central catheter, central venous catheters, and vascular access device complications. We concentrated on full‐length articles in English only; no date restrictions were placed on the search. We reviewed guidelines and consensus statements (eg, from the Center for Disease Control [CDC] or Choosing Wisely criteria) as appropriate. Additional studies of interest were identified through content experts (M.P., C.M.R.) and bibliographies of included studies.
SCIENTIFIC PRINCIPLES UNDERPINNING VENOUS ACCESS
It is useful to begin by reviewing VAD‐related nomenclature and physiology. In the simplest sense, a VAD consists of a hub (providing access to various connectors), a hollow tube divided into 1 or many sections (lumens), and a tip that may terminate within a central or peripheral blood vessel. VADs are classified as central venous catheters (eg, centrally inserted central catheters [CICCs] or peripherally inserted central catheters [PICCs]) or peripheral intravenous catheters (eg, midlines or peripheral intravenous catheters) based on site of entry and location of the catheter tip. Therefore, VADs entering via proximal or distal veins of the arm are often referred to as peripheral lines, as their site of entry and tip both reside within peripheral veins. Conversely, the term central line is often used when VADs enter or terminate in a central vein (eg, subclavian vein insertion with the catheter tip in the lower superior vena cava).
Attention to a host of clinical and theoretical parameters is important when choosing a device for venous access. Some such parameters are summarized in Table 1.
| Parameter | Major Considerations |
|---|---|
| |
| Desired flow rate | Smaller diameter veins susceptible to damage with high flow rates. |
| Short, large‐bore catheters facilitate rapid infusion. | |
| Nature of infusion | pH, viscosity, and temperature may damage vessels. |
| Vesicants and irritants should always be administered into larger, central veins. | |
| Desired duration of vascular access, or dwell time | Vessel thrombosis or phlebitis increase over time with catheter in place. |
| Intermittent infusions increase complications in central catheters; often tunneled catheters are recommended. | |
| Urgency of placement | Access to large caliber vessels is often needed in emergencies. |
| Critically ill or hemodynamically unstable patients may require urgent access for invasive monitoring or rapid infusions. | |
| Patients with trauma often require large volumes of blood products and reliable access to central veins. | |
| Number of device lumens | VADs may have single or multiple lumens. |
| Multilumen allows for multiple functions (eg, infusion of multiple agents, measurement of central venous pressures, blood draws). | |
| Device gauge | In general, use of a smaller‐gauge catheter is preferred to prevent complications. |
| However, larger catheter diameter may be needed for specific clinical needs (eg, blood transfusion). | |
| Device coating | VADs may have antithrombotic or anti‐infective coatings. |
| These devices may be of value in patients at high risk of complications. | |
| Such devices, however, may be more costly than their counterparts. | |
| Self‐care compatibility | VADs that can be cared for by patients are ideal for outpatient care. |
| Conversely, VADs such as peripheral catheters, are highly prone to dislodgement and should be reserved for supervised settings only. | |
VENOUS ACCESS DEVICES
We will organize our discussion of VADs based on whether they terminate in peripheral or central vessels. These anatomical considerations are relevant as they determine physical characteristics, compatibility with particular infusates, dwell time, and risk of complications associated with each VAD discussed in Table 2.
| Complications | Major Considerations |
|---|---|
| |
| Infection | VADs breach the integrity of skin and permit skin pathogens to enter the blood stream (extraluminal infection). |
| Inadequate antisepsis of the VAD hub, including poor hand hygiene, failure to "scrub the hub," and multiple manipulations may also increase the risk of VAD‐related infection (endoluminal infection). | |
| Infections may be local (eg, exit‐site infections) or may spread hematogenously (eg, CLABSI). | |
| Type of VAD, duration of therapy, and host characteristics interact to influence infection risk. | |
| VADs with antiseptic coatings (eg, chlorhexidine) or antibiotic coatings (eg, minocycline) may reduce risk of infection in high‐risk patients. | |
| Antiseptic‐impregnated dressings may reduce risk of extraluminal infection. | |
| Venous thrombosis | VADs predispose to venous stasis and thrombosis. |
| Duration of VAD use, type and care of the VAD, and patient characteristics affect risk of thromboembolism. | |
| VAD tip position is a key determinant of venous thrombosis; central VADs that do not terminate at the cavo‐atrial junction should be repositioned to reduce the risk of thrombosis. | |
| Antithrombotic coated or eluting devices may reduce risk of thrombosis, though definitive data are lacking. | |
| Phlebitis | Inflammation caused by damage to tunica media.[18] |
| 3 types of phlebitis: | |
| Chemical: due to irritation of media from the infusate. | |
| Mechanical: VAD physically damages the vessel. | |
| Infective: bacteria invade vein and inflame vessel wall. | |
| Phlebitis may be limited by close attention to infusate compatibility with peripheral veins, appropriate dilution, and prompt removal of catheters that show signs of inflammation. | |
| Phlebitis may be prevented in PICCs by ensuring at least a 2:1 vein:catheter ratio. | |
| Extravasation | Extravasation (also called infiltration) is defined as leakage of infusate from intravascular to extravascular space. |
| Extravasation of vesicants/emrritants is particularly worrisome. | |
| May result in severe tissue injury, blistering, and tissue necrosis.[11] | |
| VADs should be checked frequently for adequate flushing and position prior to each infusion to minimize risk. | |
| Any VAD with redness, swelling, and tenderness at the entry site or problems with flushing should not be used without further examination and review of position. | |
Peripheral Venous Access
Short Peripheral Intravenous Catheter
Approximately 200 million peripheral intravenous catheters (PIVs) are placed annually in the United States, making them the most common intravenous catheter.[3] PIVs are short devices, 3 to 6 cm in length, that enter and terminate in peripheral veins (Figure 1A). Placement is recommended in forearm veins rather than those of the hand, wrist, or upper arm, as forearm sites are less prone to occlusion, accidental removal, and phlebitis.[4] Additionally, placement in hand veins impedes activities of daily living (eg, hand washing) and is not preferred by patients.[5] PIV size ranges from 24 gauge (smallest) to 14 gauge (largest); larger catheters are often reserved for fluid resuscitation or blood transfusion as they accommodate greater flow and limit hemolysis. To decrease risk of phlebitis and thrombosis, the shortest catheter and smallest diameter should be used. However, unless adequately secured, smaller diameter catheters are also associated with greater rates of accidental removal.[4, 5]

By definition, PIVs are short‐term devices. The CDC currently recommends removal and replacement of these devices no more frequently than every 72 to 96 hours in adults. However, a recent randomized controlled trial found that replacing PIVs when clinically indicated (eg, device failure, phlebitis) rather than on a routine schedule added 30 hours to their lifespan without an increase in complications.[6] A systematic review by the Cochrane Collaboration echoes these findings.[3] These data have thus been incorporated into recommendations from the Infusion Nurses Society (INS) and the National Health Service in the United Kingdom.[5, 7] In hospitalized patients, this approach is relevant, as it preserves venous access sites, maximizes device dwell, and limits additional PIV insertions. In turn, these differences may reduce the need for invasive VADs such as PICCs. Furthermore, the projected 5‐year savings from implementation of clinically indicated PIV removal policies is US$300 million and 1 million health‐worker hours in the United States alone.[4]
PIVs offer many advantages. First, they are minimally invasive and require little training to insert. Second, they can be used for diverse indications in patients requiring short‐term (1 week) venous access. Third, PIVs do not require imaging to ensure correct placement; palpation of superficial veins is sufficient. Fourth, PIVs exhibit a risk of bloodstream infection that is about 40‐fold lower than more invasive, longer‐dwelling VADs[8] (0.06 bacteremia per 1000 catheter‐days).
Despite these advantages, PIVs also have important drawbacks. First, a quarter of all PIVs fail through occlusion or accidental dislodgement.[4] Infiltration, extravasation, and hematoma formation are important adverse events that may occur in such cases. Second, thrombophlebitis (pain and redness at the insertion site) is frequent, and may require device removal, especially in patients with catheters 20 guage.[9] Third, despite their relative safety, PIVs can cause localized or hematogenous infection. Septic thrombophlebitis (superficial thrombosis and bloodstream infection) and catheter‐related bloodstream infection, though rare, have been reported with PIVs and may lead to serious complications.[8, 10] In fact, some suggest that the overall burden of bloodstream infection risk posed by PIVs may be similar to that of CICCs given the substantially greater number of devices used and greater number of device days.[8]
PIVs and other peripheral VADs are not suitable for infusion of vesicants or irritants, which require larger, central veins for delivery. Vesicants (drugs that cause blistering on infusion) include chemotherapeutic agents (eg, dactinomycin, paclitaxel) and commonly used nonchemotherapeutical agents (eg, diazepam, piperacillin, vancomycin, esmolol, or total parenteral nutrition [TPN]).[11] Irritants (phlebitogenic drugs) cause short‐term inflammation and pain, and thus should not be peripherally infused for prolonged durations. Common irritants in the hospital setting include acyclovir, dobutamine, penicillin, and potassium chloride.
Of note, about one‐quarter of PIV insertions fail owing to difficult intravenous access.[12] Ultrasound‐guided peripheral intravenous (USGPIV) catheter placement is emerging as a technique to provide peripheral access for such patients to avoid placement of central venous access devices. Novel, longer devices (>8 cm) with built‐in guide wires have been developed to increase placement success of USGPIVs. These new designs provide easier access into deeper arm veins (brachial or basilic) not otherwise accessible by short PIVs. Although studies comparing the efficacy of USGPIV devices to other VADs are limited, a recent systematic review showed that time to successful cannulation was shorter, and fewer attempts were required to place USGPIVs compared to PIVs.[13] A recent study in France found that USGPIVs met the infusion needs of patients with difficult veins with minimal increase in complications.[14] Despite these encouraging data, future studies are needed to better evaluate this technology.
Midline Catheter
A midline is a VAD that is between 7.5 to 25 cm in length and is typically inserted into veins above the antecubital fossa. The catheter tip resides in a peripheral upper arm vein, often the basilic or cephalic vein, terminating just short of the subclavian vein (Figure 1B). Midline‐like devices were first developed in the 1950s and were initially used as an alternative to PIVs because they were thought to allow longer dwell times.[15] However, because they were originally constructed with a fairly rigid material, infiltration, mechanical phlebitis, and inflammation were common and tempered enthusiasm for their use.[15, 16] Newer midline devices obviate many of these problems and are inserted by ultrasound guidance and modified Seldinger technique.[17] Despite these advances, data regarding comparative efficacy are limited.
Midlines offer longer dwell times than standard PIVs owing to termination in the larger diameter basilic and brachial veins of the arm. Additionally, owing to their length, midlines are less prone to dislodgement. As they are inserted with greater antisepsis than PIVs and better secured to the skin, they are more durable than PIVs.[5, 9, 18] Current INS standards recommend use of midlines for 1 to 4 weeks.[5] Because they terminate in a peripheral vein, medications and infusions compatible with midlines are identical to those that are infused through a PIV. Thus, TPN, vesicants or irritants, or drugs that feature a pH <5 or pH >9, or >500 mOsm should not be infused through a midline.[15] New evidence suggests that diluted solutions of vancomycin (usually pH <5) may be safe to infuse for short durations (<6 days) through a midline, and that concentration rather than pH may be more important in this regard.[19] Although it is possible that the use of midlines may extend to agents typically not deemed peripheral access compatible, limited evidence exists to support such a strategy at this time.
Midlines offer several advantages. First, because blood flow is greater in the more proximal veins of the arm, midlines can accommodate infusions at rates of 100 to 150 mL/min compared to 20 to 40 mL/min in smaller peripheral veins. Higher flow rates offer greater hemodilution (dilution of the infusion with blood), decreasing the likelihood of phlebitis and infiltration.[20] Second, midlines do not require x‐ray verification of tip placement; thus, their use is often favored in resource‐depleted settings such as skilled nursing facilities. Third, midlines offer longer dwell times than peripheral intravenous catheters and can thus serve as bridge devices for short‐term intravenous antibiotics or peripheral‐compatible infusions in an outpatient setting. Available evidence suggests that midlines are associated with low rates of bloodstream infection (0.30.8 per 1000 catheter‐days).[17] The most frequent complications include phlebitis (4.2%) and occlusion (3.3%).[20] Given these favorable statistics, midlines may offer a good alternative to PIVs in select patients who require peripheral infusions of intermediate duration.
Intraosseous Vascular Access
Intraosseous (IO) devices access the vascular system by piercing cortical bone. These devices provide access to the intramedullary cavity and venous plexi of long bones such as the tibia, femur, or humerus. Several insertion devices are now commercially available and have enhanced the ease and safety of IO placement. Using these newer devices, IO access may be obtained in 1 to 2 minutes with minimal training. By comparison, a central venous catheter often requires 10 to 15 minutes to insert with substantial training efforts for providers.[21, 22, 23]
IO devices thus offer several advantages. First, given the rapidity with which they can be inserted, they are often preferred in emergency settings (eg, trauma). Second, these devices are versatile and can accommodate both central and peripheral infusates.[24] Third, a recent meta‐analysis found that IOs have a low complication rate of 0.8%, with extravasation of infusate through the cortical entry site being the most common adverse event.[21] Of note, this study also reported zero local or distal infectious complications, a finding that may relate to the shorter dwell of these devices.[21] Some animal studies suggest that fat embolism from bone may occur at high rates with IO VADs.[25] However, death or significant morbidity from fat emboli in humans following IO access has not been described. Whether such emboli occur or are clinically significant in the context of IO devices remains unclear at this time.[21]
Central Venous Access Devices
Central venous access devices (CVADs) share in common tip termination in the cavo‐atrial junction, either in the lower portion of the superior vena cava or in the upper portion of the right atrium. CVADs can be safely used for irritant or vesicant medications as well as for blood withdrawal, blood exchange procedures (eg, dialysis), and hemodynamic monitoring. Traditionally, these devices are 15 to 25 cm in length and are directly inserted in the deep veins of the supra‐ or infraclavicular area, including the internal jugular, brachiocephalic, subclavian, or axillary veins. PICCs are unique CVADs in that they enter through peripheral veins but terminate in the proximity of the cavoatrial junction. Regarding nomenclature, CICC will be used to denote devices that enter directly into veins of the neck or chest, whereas PICC will be used for devices that are inserted peripherally but terminate centrally.
Peripherally Inserted Central Catheter
PICCs are inserted into peripheral veins of the upper arm (eg, brachial, basilica, or cephalic vein) and advanced such that the tip resides at the cavoatrial junction (Figure 1C). PICCs offer prolonged dwell times and are thus indicated when patients require venous access for weeks or months.[26] Additionally, they can accommodate a variety of infusates and are safer to insert than CICCs, given placement in peripheral veins of the arm rather than central veins of the chest/neck. Thus, insertion complications such as pneumothorax, hemothorax, or significant bleeding are rare with PICCs. In fact, a recent study reported that PICC insertion by hospitalists was associated with low rates of insertion or infectious complications.[27]
However, like CICCs, PICCs are associated with central lineassociated bloodstream infection (CLABSI), a serious complication known to prolong length of hospital stay, increase costs, and carry a 12% to 25% associated mortality.[28, 29] In the United States alone, over 250,000 CLASBI cases occur per year drawing considerable attention from the CDC and Joint Commission, who now mandate reporting and nonpayment for hospital‐acquired CLABSI.[30, 31, 32] A recent systematic review and meta‐analysis found that PICCs are associated with a substantial risk of CLABSI in hospitalized patients.[33] Importantly, no difference in CLABSI rates between PICCs and CICCs in hospitalized patients was evident in this meta‐analysis. Therefore, current guidelines specifically recommend against use of PICCs over CICCs as a strategy to reduce CLABSI.[34] Additionally, PICCs are associated with 2.5‐fold greater risk of deep vein thrombosis (DVT) compared to CICCs; thus, they should be used with caution in patients with cancer or those with underlying hypercoagulable states.
Of particular import to hospitalists is the fact that PICC placement is contraindicated in patients with stage IIIB or greater chronic kidney disease (CKD). In such patients, sequelae of PICC use, such as phlebitis or central vein stenosis, can be devastating in patients with CKD.[35] In a recent study, prior PICC placement was the strongest predictor of subsequent arteriovenous graft failure.[36] For this reason, Choosing Wisely recommendations call for avoidance of PICCs in such patients.[37]
Centrally Inserted Central Catheter
CICCs are CVADs placed by puncture and cannulation of the internal jugular, subclavian, brachiocephalic, or femoral veins (Figure 1D) and compose the vast majority of VADs placed in ICU settings.[38, 39] Central termination of CICCs allows for a variety of infusions, including irritants, vesicants, and vasopressors, as well as blood withdrawal and hemodynamic monitoring. CICCs are typically used for 7 to 14 days, but may remain for longer durations if they remain complication free and clinically necessary.[40] A key advantage of CICCs is that they can be placed in emergent settings to facilitate quick access for rapid infusion or hemodynamic monitoring. In particular, CICCs are inserted in the femoral vein and may be useful in emergency settings. However, owing to risk of infection and inability to monitor central pressures, these femoral devices should be replaced with a proper CICC or PICC when possible. Importantly, although CICCs are almost exclusively used in intensive or emergency care, PICCs may also be considered in such settings.[41, 42] CICCs usually have multiple lumens and often serve several simultaneous functions such as both infusions and hemodynamic monitoring.
Despite their benefits, CICCs have several disadvantages. First, insertion requires an experienced clinician and has historically been a task limited to physicians. However, this is changing rapidly (especially in Europe and Australia) where specially trained nurses are assuming responsibility for CICC placement.[43] Second, these devices are historically more likely to be associated with CLABSI, with estimates of infection rates varying between 2 and 5 infections per 1000 catheter‐days.[44] Third, CICCs pose a significant DVT risk, with rates around 22 DVTs per 1000 catheter‐days.[45] However, compared to PICCs, the DVT risk appears lower, and CICC use may be preferable in patients at high risk of DVT, such as critically ill or cancer populations.[46] An important note to prevent CICC insertion complications relates to use of ultrasound, a practice that has been associated with decreased accidental arterial puncture and hematoma formation. The role of ultrasound guidance with PICCs as well as implications for thrombotic and infectious events remains less characterized at this point.[47]
Tunneled Central Venous Access Devices
Tunneled devices (either CICCs or PICCs) are characterized by the fact that the insertion site on the skin and site of ultimate venipuncture are physically separated (Figure 1E). Tunneling limits bacterial entry from the extraluminal aspect of the CVAD to the bloodstream. For example, internal jugular veins are often ideal sites of puncture but inappropriate sites for catheter placement, as providing care to this area is challenging and may increase risk of infection.[34] Tunneling to the infraclavicular area provides a better option, as it provides an exit site that can be adequately cared for. Importantly, any CVAD (PICCs or CICCs) can be tunneled. Additionally, tunneled CICCs may be used in patients with chronic or impending renal failure where PICCs are contraindicated because entry into dialysis‐relevant vessels is to be avoided.[48] Such devices also allow regular blood sampling in patients who require frequent testing but have limited peripheral access, such as those with hematological malignancies. Additionally, tunneled catheters are more comfortable for patients and viewed as being more socially acceptable than nontunneled devices. However, the more invasive and permanent nature of these devices often requires deliberation prior to insertion.
Of note, tunneled devices and ports may be used as long‐term (>3 months to years) VADs. As our focus in this review is short‐term devices, we will not expand the discussion of these devices as they are almost always used for prolonged durations.[7]
OPERATIONALIZING THE DATA: AN ALGORITHMIC APPROACH TO VENOUS ACCESS
Hospitalists should consider approaching venous access using an algorithm based on a number of parameters. For example, a critically ill patient who requires vasopressor support and hemodynamic monitoring will need a CICC or a PICC. Given the potential greater risk of thromboses from PICCs, a CICC is preferable for critically ill patients provided an experienced inserter is available. Conversely, patients who require short‐term (<710 days) venous access for infusion of nonirritant or nonvesicant therapy often only require a PIV. In patients with poor or difficult venous access, USGPIVs or midlines may be ideal and preferred over short PIVs. Finally, patients who require longer‐term or home‐based treatment may benefit from early placement of a midline or a PICC, depending again on the nature of the infusion, duration of treatment, and available venous access sites.
An algorithmic approach considering these parameters is suggested in Figure 2, and a brief overview of the devices and their considerations is shown in Table 3.
| Vascular Access Device | Central/Peripheral | Anatomical Location of Placement | Desired Duration of Placement | Common Uses | BSI Risk (Per 1,000 Catheter‐Days) | Thrombosis Risk | Important Considerations |
|---|---|---|---|---|---|---|---|
| |||||||
| Small peripheral IV | Peripheral | Peripheral veins, usually forearm | 710 days | Fluid resuscitation, most medications, blood products | 0.06[8] | Virtually no risk | Consider necessity of PIV daily and remove unnecessary devices |
| Midline | Peripheral | Inserted around antecubital fossa, reside within basilic or cephalic vein of the arm | 24 weeks | Long‐term medications excluding TPN, vesicants, corrosives | 0.30.8[17] | Insufficient data | Can be used as bridge devices for patients to complete short‐term antibiotics/emnfusions as an outpatient |
| Peripherally inserted central catheter | Central | Inserted into peripheral arm vein and advanced to larger veins (eg, internal jugular or subclavian) to the CAJ | >1 week, <3 months | Large variety of infusates, including TPN, vesicants, corrosives | 2.4[44] | 6.30% | Contraindicated in patients with CKD stage IIIb or higher |
| Centrally inserted central catheters | Central | Inserted above (internal jugular vein, brachiocephalic vein, subclavian vein), or below the clavicle (axillary vein) | >1 week, <3 months | Same infusate variety as PICC, measurement of central venous pressures, common in trauma/emergent settings | 2.3[44] | 1.30% | Given lower rates of DVT than PICC, preferred in ICU and hypercoagulable environments |
| Tunneled CICCs | Central | Placed percutaneously in any large vein in the arm, chest, neck or groin | >3 months to years | Central infusates, as in any CVAD; used for patients with CKD stage IIIb or greater when a PICC is indicated | Insufficient data | Insufficient data | May be superior when insertion site and puncture site are not congruent and may increase risk of infection |

CONCLUSIONS
With strides in technology and progress in medicine, hospitalists have access to an array of options for venous access. However, every VAD has limitations that can be easily overlooked in a perfunctory decision‐making process. The data presented in this review thus provide a first step to improving safety in this evolving science. Studies that further determine appropriateness of VADs in hospitalized settings are necessary. Only through such progressive scientific enquiry will complication‐free venous access be realized.
Disclosure
Nothing to report.
- , . The need for comparative data in vascular access: the rationale and design of the PICC registry. J Vasc Access. 2013; 18(4): 219–224.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013; 8(6): 309–314.
- , , , . Clinically‐indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2013; 4: CD007798.
- , , , et al. Cost‐effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014; 12(1): 51–58.
- Infusion Nurses Society. Infusion Nursing Standards of Practice. Norwood, MA; Infusion Nurses Society; 2011.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012; 380(9847): 1066–1074.
- , , , et al. epic3: national evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. J Hosp Infect. 2014; 86(suppl 1): S1–S70.
- . Short peripheral intravenous catheters and infections. J Infus Nurs. 2012; 35(4): 230–240.
- , , , et al. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: a large multi‐centre prospective study. J Adv Nurs. 2014; 70(11): 2539–2549.
- , . Intravenous catheter complications in the hand and forearm. J Trauma. 2004; 56(1): 123–127.
- , , , . Vesicant extravasation part I: Mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006; 33(6): 1134–1141.
- , . Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005; 34(5): 345–359.
- , , . Ultrasound‐guided peripheral venous access: a systematic review of randomized‐controlled trials. Eur J Emerg Med. 2014; 21(1): 18–23.
- , , , et al. Difficult peripheral venous access: clinical evaluation of a catheter inserted with the Seldinger method under ultrasound guidance. J Crit Care. 2014; 29(5): 823–827.
- . Choosing the right intravenous catheter. Home Healthc Nurse. 2007; 25(8): 523–531; quiz 532–523.
- Adverse reactions associated with midline catheters—United States, 1992–1995. MMWR Morb Mortal Wkly Rep. 1996; 45(5): 101–103.
- , , . The risk of midline catheterization in hospitalized patients. A prospective study. Ann Intern Med. 1995; 123(11): 841–844.
- . Midline catheters: indications, complications and maintenance. Nurs Stand. 2007; 22(11): 48–57; quiz 58.
- , . Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access. 2014; 15(4): 251–256.
- . Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004; 27(5): 313–321.
- . Vascular access in resuscitation: is there a role for the intraosseous route? Anesthesiology. 2014; 120(4): 1015–1031.
- , , , et al. A new system for sternal intraosseous infusion in adults. Prehosp Emerg Care. 2000; 4(2): 173–177.
- , , , , , . Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department: a prospective, randomized study. Resuscitation. 2010; 81(8): 994–999.
- , , , , . Comparison study of intraosseous, central intravenous, and peripheral intravenous infusions of emergency drugs. Am J Dis Child. 1990; 144(1): 112–117.
- , , , , . The safety of intraosseous infusions: risks of fat and bone marrow emboli to the lungs. Ann Emerg Med. 1989; 18(10): 1062–1067.
- , , , , . ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr. 2009; 28(4): 365–377.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009; 4(6): E1–E4.
- Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011; 60(8): 243–248.
- , , , . Hospital costs of central line‐associated bloodstream infections and cost‐effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Eff Resour Alloc. 2010; 8: 8.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006; 81(9): 1159–1171.
- The Joint Commission. Preventing Central Line‐Associated Bloodstream Infections: A Global Challenge, a Global Perspective. Oak Brook, IL: Joint Commission Resources; 2012.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011; 39(4 suppl 1): S1–S34.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013; 34(9): 908–918.
- Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, American Hospital Association, Association for Professionals in Infection Control and Epidemiology, The Joint Commission. Compendium of Strategies to Prevent Healthcare‐Associated Infections in Acute Care Hospitals: 2014 Updates. Available at: http://www.shea‐online.org. Accessed August 1, 2014.
- , , , , . Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008; 21(2): 186–191.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012; 60(4): 601–608.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012; 7(10): 1664–1672.
- , , , et al. Do physicians know which of their patients have central venous catheters? A multi‐center observational study. Ann Intern Med. 2014; 161(8): 562–567.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011; 77(4): 304–308.
- , , , , , . Peripherally inserted central catheter‐related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014; 12(6): 847–854.
- , , , . An in vitro study comparing a peripherally inserted central catheter to a conventional central venous catheter: no difference in static and dynamic pressure transmission. BMC Anesthesiol. 2010; 10: 18.
- , , , et al. Clinical experience with power‐injectable PICCs in intensive care patients. Crit Care. 2012; 16(1): R21.
- , , , et al. Nurse‐led central venous catheter insertion‐procedural characteristics and outcomes of three intensive care based catheter placement services. Int J Nurs Stud. 2012; 49(2): 162–168.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010; 38(2): 149–153.
- , , , et al. Which central venous catheters have the highest rate of catheter‐associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg. 2013; 74(2): 454–460; discussion 461–452.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013; 382(9889): 311–325.
- , , , , . Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015; 1: CD011447.
- , , , et al. Tunneled jugular small‐bore central catheters as an alternative to peripherally inserted central catheters for intermediate‐term venous access in patients with hemodialysis and chronic renal insufficiency. Radiology. 1999; 213(1): 303–306.
- , . The need for comparative data in vascular access: the rationale and design of the PICC registry. J Vasc Access. 2013; 18(4): 219–224.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013; 8(6): 309–314.
- , , , . Clinically‐indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2013; 4: CD007798.
- , , , et al. Cost‐effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014; 12(1): 51–58.
- Infusion Nurses Society. Infusion Nursing Standards of Practice. Norwood, MA; Infusion Nurses Society; 2011.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012; 380(9847): 1066–1074.
- , , , et al. epic3: national evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. J Hosp Infect. 2014; 86(suppl 1): S1–S70.
- . Short peripheral intravenous catheters and infections. J Infus Nurs. 2012; 35(4): 230–240.
- , , , et al. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: a large multi‐centre prospective study. J Adv Nurs. 2014; 70(11): 2539–2549.
- , . Intravenous catheter complications in the hand and forearm. J Trauma. 2004; 56(1): 123–127.
- , , , . Vesicant extravasation part I: Mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006; 33(6): 1134–1141.
- , . Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005; 34(5): 345–359.
- , , . Ultrasound‐guided peripheral venous access: a systematic review of randomized‐controlled trials. Eur J Emerg Med. 2014; 21(1): 18–23.
- , , , et al. Difficult peripheral venous access: clinical evaluation of a catheter inserted with the Seldinger method under ultrasound guidance. J Crit Care. 2014; 29(5): 823–827.
- . Choosing the right intravenous catheter. Home Healthc Nurse. 2007; 25(8): 523–531; quiz 532–523.
- Adverse reactions associated with midline catheters—United States, 1992–1995. MMWR Morb Mortal Wkly Rep. 1996; 45(5): 101–103.
- , , . The risk of midline catheterization in hospitalized patients. A prospective study. Ann Intern Med. 1995; 123(11): 841–844.
- . Midline catheters: indications, complications and maintenance. Nurs Stand. 2007; 22(11): 48–57; quiz 58.
- , . Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access. 2014; 15(4): 251–256.
- . Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004; 27(5): 313–321.
- . Vascular access in resuscitation: is there a role for the intraosseous route? Anesthesiology. 2014; 120(4): 1015–1031.
- , , , et al. A new system for sternal intraosseous infusion in adults. Prehosp Emerg Care. 2000; 4(2): 173–177.
- , , , , , . Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department: a prospective, randomized study. Resuscitation. 2010; 81(8): 994–999.
- , , , , . Comparison study of intraosseous, central intravenous, and peripheral intravenous infusions of emergency drugs. Am J Dis Child. 1990; 144(1): 112–117.
- , , , , . The safety of intraosseous infusions: risks of fat and bone marrow emboli to the lungs. Ann Emerg Med. 1989; 18(10): 1062–1067.
- , , , , . ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr. 2009; 28(4): 365–377.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009; 4(6): E1–E4.
- Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011; 60(8): 243–248.
- , , , . Hospital costs of central line‐associated bloodstream infections and cost‐effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Eff Resour Alloc. 2010; 8: 8.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006; 81(9): 1159–1171.
- The Joint Commission. Preventing Central Line‐Associated Bloodstream Infections: A Global Challenge, a Global Perspective. Oak Brook, IL: Joint Commission Resources; 2012.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011; 39(4 suppl 1): S1–S34.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013; 34(9): 908–918.
- Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, American Hospital Association, Association for Professionals in Infection Control and Epidemiology, The Joint Commission. Compendium of Strategies to Prevent Healthcare‐Associated Infections in Acute Care Hospitals: 2014 Updates. Available at: http://www.shea‐online.org. Accessed August 1, 2014.
- , , , , . Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008; 21(2): 186–191.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012; 60(4): 601–608.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012; 7(10): 1664–1672.
- , , , et al. Do physicians know which of their patients have central venous catheters? A multi‐center observational study. Ann Intern Med. 2014; 161(8): 562–567.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011; 77(4): 304–308.
- , , , , , . Peripherally inserted central catheter‐related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014; 12(6): 847–854.
- , , , . An in vitro study comparing a peripherally inserted central catheter to a conventional central venous catheter: no difference in static and dynamic pressure transmission. BMC Anesthesiol. 2010; 10: 18.
- , , , et al. Clinical experience with power‐injectable PICCs in intensive care patients. Crit Care. 2012; 16(1): R21.
- , , , et al. Nurse‐led central venous catheter insertion‐procedural characteristics and outcomes of three intensive care based catheter placement services. Int J Nurs Stud. 2012; 49(2): 162–168.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010; 38(2): 149–153.
- , , , et al. Which central venous catheters have the highest rate of catheter‐associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg. 2013; 74(2): 454–460; discussion 461–452.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013; 382(9889): 311–325.
- , , , , . Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015; 1: CD011447.
- , , , et al. Tunneled jugular small‐bore central catheters as an alternative to peripherally inserted central catheters for intermediate‐term venous access in patients with hemodialysis and chronic renal insufficiency. Radiology. 1999; 213(1): 303–306.
Mobilization in Severe Sepsis
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]
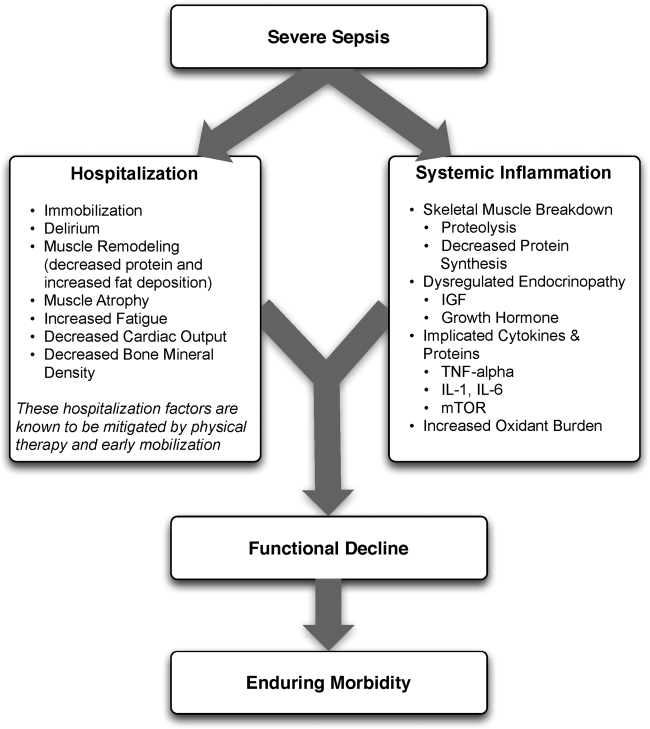
Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]
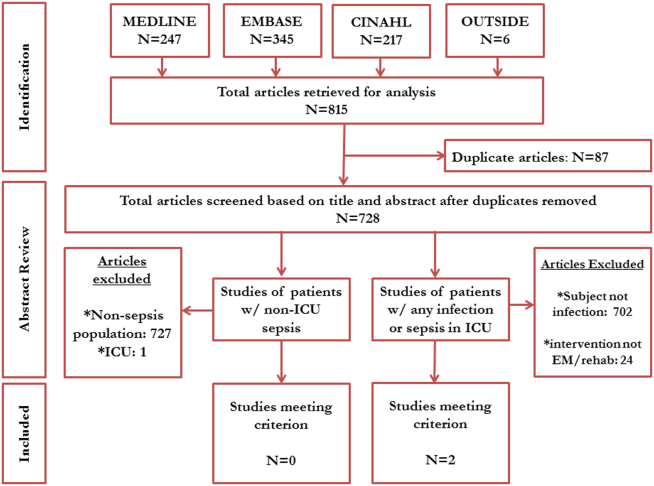
EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]

Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]

EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]

Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]

EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
Perioperative ACE‐Inhibitor Management
The management of perioperative medications is a tale of progressive scientific enquiry. Although long‐term use of agents such as aspirin or statins improves clinical outcomes, use during surgery ranges from problematic to protective. The delicate balance between proven long‐term benefits in the nonoperative setting versus short‐term uncertainty in the perioperative setting must be assessed using a lens that incorporates patient risk, surgical process, and pharmacodynamic principles. Advances in our understanding of perioperative physiology, coupled with robust clinical and outcome data, have led to new knowledge and insights regarding how best to manage these medications. As well illustrated by the near 180 change in the use of perioperative ‐blockers to prevent adverse cardiovascular outcomes, these new data have led to substantial progress in surgical safety and patient outcomes.
In this issue of the Journal of Hospital Medicine, 2 retrospective cohort studies add to this growing body of evidence by examining risks associated with use of angiotensin‐converting enzyme (ACE) inhibitors in the perioperative setting. In the first study, conducted at a single academic medical center, Nielson and colleagues evaluate the association of preoperative ACE‐inhibitor use with hypotension and acute kidney injury in patients undergoing major elective orthopedic surgery.[1] The authors report that patients receiving ACE‐inhibitors were not only more likely to experience hypotension after induction of anesthesia (12.2% vs 6.7%, P = 0.005), but also were more likely to develop postoperative acute kidney injury (odds ratio [OR]: 2.68, 95% confidence interval [CI]: 1.25‐1.99). In the second study which focused solely on patients who were receiving preoperative ACE‐inhibitor therapy, Mudumbai and colleagues used a national Veterans Affairs database to examine the association between failure to resume ACE‐inhibitor treatment after surgery and outcomes at 30 days.[2] The authors found that failure to resume treatment 14 days after surgery was not only common (affecting 1 in 4 patients), but was also associated with increased 30‐day mortality (hazard ratio: 3.44, 95% CI: 3.30‐3.60).[2] Taken together, these 2 studies shed new light on clinical practice and policy implications for the use of these agents in the surgical period. Both sets of authors should be congratulated on moving the needle forward in this enquiry.
ACE‐inhibitors physiologically mediate their effects by preventing the formation of the potent vasoconstrictor angiotensin‐II from its precursor angiotensin‐I. In doing so, they decrease arterial resistance, increase venous capacitance, decrease glomerular filtration pressure, and promote natriuresis. These vascular effects have key benefits for the management of a number of chronic diseases including hypertension, congestive heart failure, and diabetic nephropathy. However, these clinical alterations are often problematic in the perioperative setting. For example, ACE‐inhibitors may cause vasoplegia during anesthetic administration, commonly manifested as hypotension during induction.[3] This hemodynamic alteration has been viewed as being so precarious, that some authors recommend withholding ACE‐inhibitors prior to major cardiovascular procedures such as coronary artery bypass grafting.[4] Although hypotension leads to management challenges (often increasing vasoconstrictor requirements), several studies report that ACE‐inhibitor use during surgery may also be associated with increased risks of acute kidney injury and mortality.[5, 6] However, despite these data, existing literature has not shown a consistent association between ACE‐inhibitor use and adverse postoperative outcomes. For example, a propensity‐matched cohort study of 79,228 patients at the Cleveland Clinic found no difference in hemodynamic characteristics, vasopressor requirements, or cardiorespiratory complications among patients who were or were not using ACE‐inhibitors during noncardiac surgery.[7] Furthermore, some research has also found that withdrawal of ACE‐inhibitors may itself lead to harm. For instance, a contemporary study reported that withdrawal of ACE‐inhibitor therapy in patients undergoing coronary artery bypass was associated with increased in‐hospital cardiovascular events.[8] Given this uncertainty, it is not surprising that management of ACE‐inhibitors in the perioperative period remains a subject of ongoing controversy with clinical reviews often recommending consideration of the risks and benefits associated with use of these agents.[9]
It is important to note that driver of this ambiguity is the very design of relevant studies. For instance, studies that focus on patients undergoing coronary artery bypass grafting surgery have limited external validity, as these patients are very different from those who undergo elective hip or knee replacement (such as those included by Nielsen and colleagues). Additionally, many studies suffer from methodological constraints or biases. For instance, retrospective observational studies often suffer from selection bias and residual confounding; that is, the individuals chosen for inclusion in the study and the variables available for analysis are often limited by available data, curtailing the conclusions that can be generated. Although the use of multivariable regression or propensity score techniques helps address these limitations, residual confounding by unmeasured variables always remains a threat to statistical inference. The potential influence for this bias is particularly relevant when examining the results of the study by Nielsen and colleagues. Another important limitation is survivor bias, a problem inherent in the study by Mudumbai and colleagues. Put simply, for a patient to resume ACE‐inhibitors after surgery, this same patient, must also survive for at least 15 to 30 days after surgery. Thus, for some patients, failure to resume this treatment may simply be a marker of early mortality rather than failure to resume the ACE itself. The potential influence of this bias is supported by an included sensitivity analysis, where a large change in the adjusted OR was observed when patients suffering early mortality were excluded. This swing in effect size suggests that biases related to comparing patients who survived to those who did not with respect to ACE use may, in part, account for the results of the study.
These limitations aside, the studies brought forth by both authors help inform practice with respect to the use of these agents during surgery. In this context, 3 paradigms are relevant for practicing hospitalists.
First, if a patient is maintained on an ACE‐inhibitor before surgery, should the medication be temporarily held before surgery to minimize hypotension during anesthesia? The study by Nielsen and colleagues (comparing those on ACE‐inhibitor treatment to those without), in addition to the evidence generated from other studies in this area[10, 11, 12] suggest that this is a rational decision. Although the existence of a withdrawal state from abrupt cessation of ACE‐inhibitor use is theoretically plausible, this has yet to be reliably reported in the literature. Given the short half‐life of most ACE‐inhibitors, cessation 24 hours before surgery appears to be the most pragmatic clinical approach.
Second, if a patient is on an ACE‐inhibitor before surgery, when should the medication be resumed after surgery? The findings from the study by Mudumbai and colleagues, in addition to contemporary evidence,[7, 8, 13] support the resumption of these agents as soon as possible following operative intervention. Once hemodynamic stability and volume status have been assured, risks associated with postoperative ACE‐inhibitor use appear to be outweighed by benefits, though specific care is likely necessary in those with preexisting renal dysfunction.[14] A program that ensures reconciliation of medications in the postoperative setting may be valuable in ensuring that such treatment is restarted.
Third, if a patient is not on ACE‐inhibitor therapy, should this be started before surgery for perioperative or long‐term benefit? Although neither study examines this issue, the potential for significant risk make this an unattractive option. Future interventional studies with thoughtfully weighed safety parameters may be necessary to assess whether such a paradigm may be valuable.
The studies included in this issue of the Journal of Hospital Medicine suggest that the use of ACE‐inhibitors during the perioperative period may be considered a function of time and place. Resuming ACE‐inhibitors and cessation of treatment at specific intervals in relation to surgery can help ensure positive outcomes. Hospitalists have an important role in this regard, as they are ideally situated to manage these agents in the many patients undergoing surgery across the United States.
Disclosures: Dr. Wijeysundera is supported by a Clinician‐Scientist Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto. Dr. Chopra is supported by a Career‐Development Award (1K08HS022835‐01) from the Agency of Healthcare Research and Quality.
- , , , . Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283–288.
- , , , , , . Thirty‐day mortality risk associated with postoperative nonresumption of angiotensin‐converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289–296.
- , , , , , . Clinical consequences of withholding versus administering renin‐angiotensin‐aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319–325.
- , , , , . Angiotensin‐converting enzyme inhibitors increase vasoconstrictor requirements after cardiopulmonary bypass. Anesth Analg. 1995;80(3):473–479.
- , , , et al. Effects of angiotensin‐converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2009;54(19):1778–1784.
- , , , . Renin‐angiotensin blockade is associated with increased mortality after vascular surgery. Can J Anaesth. 2010;57(8):736–744.
- , , , , , . Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114(3):552–560.
- , , , et al. Patterns of use of perioperative angiotensin‐converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in‐hospital morbidity and mortality. Circulation. 2012;126(3):261–269.
- , , , . Renin‐angiotensin system antagonists in the perioperative setting: clinical consequences and recommendations for practice. Postgrad Med J. 2011;87(1029):472–481.
- , , , et al; TRIBE‐AKI Consortium. Preoperative angiotensin‐converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery. Nephrol Dial Transplant. 2013;28(11):2787–2799.
- , , , , , . Effects of renin‐angiotensin system inhibitors on the occurrence of acute kidney injury following off‐pump coronary artery bypass grafting. Circulation J. 2010;74(9):1852–1858.
- , , , , , . Prophylactic vasopressin in patients receiving the angiotensin‐converting enzyme inhibitor ramipril undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2010;24(2):230–238.
- , , , , . Neither diabetes nor glucose‐lowering drugs are associated with mortality after noncardiac surgery in patients with coronary artery disease or heart failure. Can J Cardiol. 2013;29(4):423–428.
- , , , , , . Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev. 2008;(4):CD003590.
The management of perioperative medications is a tale of progressive scientific enquiry. Although long‐term use of agents such as aspirin or statins improves clinical outcomes, use during surgery ranges from problematic to protective. The delicate balance between proven long‐term benefits in the nonoperative setting versus short‐term uncertainty in the perioperative setting must be assessed using a lens that incorporates patient risk, surgical process, and pharmacodynamic principles. Advances in our understanding of perioperative physiology, coupled with robust clinical and outcome data, have led to new knowledge and insights regarding how best to manage these medications. As well illustrated by the near 180 change in the use of perioperative ‐blockers to prevent adverse cardiovascular outcomes, these new data have led to substantial progress in surgical safety and patient outcomes.
In this issue of the Journal of Hospital Medicine, 2 retrospective cohort studies add to this growing body of evidence by examining risks associated with use of angiotensin‐converting enzyme (ACE) inhibitors in the perioperative setting. In the first study, conducted at a single academic medical center, Nielson and colleagues evaluate the association of preoperative ACE‐inhibitor use with hypotension and acute kidney injury in patients undergoing major elective orthopedic surgery.[1] The authors report that patients receiving ACE‐inhibitors were not only more likely to experience hypotension after induction of anesthesia (12.2% vs 6.7%, P = 0.005), but also were more likely to develop postoperative acute kidney injury (odds ratio [OR]: 2.68, 95% confidence interval [CI]: 1.25‐1.99). In the second study which focused solely on patients who were receiving preoperative ACE‐inhibitor therapy, Mudumbai and colleagues used a national Veterans Affairs database to examine the association between failure to resume ACE‐inhibitor treatment after surgery and outcomes at 30 days.[2] The authors found that failure to resume treatment 14 days after surgery was not only common (affecting 1 in 4 patients), but was also associated with increased 30‐day mortality (hazard ratio: 3.44, 95% CI: 3.30‐3.60).[2] Taken together, these 2 studies shed new light on clinical practice and policy implications for the use of these agents in the surgical period. Both sets of authors should be congratulated on moving the needle forward in this enquiry.
ACE‐inhibitors physiologically mediate their effects by preventing the formation of the potent vasoconstrictor angiotensin‐II from its precursor angiotensin‐I. In doing so, they decrease arterial resistance, increase venous capacitance, decrease glomerular filtration pressure, and promote natriuresis. These vascular effects have key benefits for the management of a number of chronic diseases including hypertension, congestive heart failure, and diabetic nephropathy. However, these clinical alterations are often problematic in the perioperative setting. For example, ACE‐inhibitors may cause vasoplegia during anesthetic administration, commonly manifested as hypotension during induction.[3] This hemodynamic alteration has been viewed as being so precarious, that some authors recommend withholding ACE‐inhibitors prior to major cardiovascular procedures such as coronary artery bypass grafting.[4] Although hypotension leads to management challenges (often increasing vasoconstrictor requirements), several studies report that ACE‐inhibitor use during surgery may also be associated with increased risks of acute kidney injury and mortality.[5, 6] However, despite these data, existing literature has not shown a consistent association between ACE‐inhibitor use and adverse postoperative outcomes. For example, a propensity‐matched cohort study of 79,228 patients at the Cleveland Clinic found no difference in hemodynamic characteristics, vasopressor requirements, or cardiorespiratory complications among patients who were or were not using ACE‐inhibitors during noncardiac surgery.[7] Furthermore, some research has also found that withdrawal of ACE‐inhibitors may itself lead to harm. For instance, a contemporary study reported that withdrawal of ACE‐inhibitor therapy in patients undergoing coronary artery bypass was associated with increased in‐hospital cardiovascular events.[8] Given this uncertainty, it is not surprising that management of ACE‐inhibitors in the perioperative period remains a subject of ongoing controversy with clinical reviews often recommending consideration of the risks and benefits associated with use of these agents.[9]
It is important to note that driver of this ambiguity is the very design of relevant studies. For instance, studies that focus on patients undergoing coronary artery bypass grafting surgery have limited external validity, as these patients are very different from those who undergo elective hip or knee replacement (such as those included by Nielsen and colleagues). Additionally, many studies suffer from methodological constraints or biases. For instance, retrospective observational studies often suffer from selection bias and residual confounding; that is, the individuals chosen for inclusion in the study and the variables available for analysis are often limited by available data, curtailing the conclusions that can be generated. Although the use of multivariable regression or propensity score techniques helps address these limitations, residual confounding by unmeasured variables always remains a threat to statistical inference. The potential influence for this bias is particularly relevant when examining the results of the study by Nielsen and colleagues. Another important limitation is survivor bias, a problem inherent in the study by Mudumbai and colleagues. Put simply, for a patient to resume ACE‐inhibitors after surgery, this same patient, must also survive for at least 15 to 30 days after surgery. Thus, for some patients, failure to resume this treatment may simply be a marker of early mortality rather than failure to resume the ACE itself. The potential influence of this bias is supported by an included sensitivity analysis, where a large change in the adjusted OR was observed when patients suffering early mortality were excluded. This swing in effect size suggests that biases related to comparing patients who survived to those who did not with respect to ACE use may, in part, account for the results of the study.
These limitations aside, the studies brought forth by both authors help inform practice with respect to the use of these agents during surgery. In this context, 3 paradigms are relevant for practicing hospitalists.
First, if a patient is maintained on an ACE‐inhibitor before surgery, should the medication be temporarily held before surgery to minimize hypotension during anesthesia? The study by Nielsen and colleagues (comparing those on ACE‐inhibitor treatment to those without), in addition to the evidence generated from other studies in this area[10, 11, 12] suggest that this is a rational decision. Although the existence of a withdrawal state from abrupt cessation of ACE‐inhibitor use is theoretically plausible, this has yet to be reliably reported in the literature. Given the short half‐life of most ACE‐inhibitors, cessation 24 hours before surgery appears to be the most pragmatic clinical approach.
Second, if a patient is on an ACE‐inhibitor before surgery, when should the medication be resumed after surgery? The findings from the study by Mudumbai and colleagues, in addition to contemporary evidence,[7, 8, 13] support the resumption of these agents as soon as possible following operative intervention. Once hemodynamic stability and volume status have been assured, risks associated with postoperative ACE‐inhibitor use appear to be outweighed by benefits, though specific care is likely necessary in those with preexisting renal dysfunction.[14] A program that ensures reconciliation of medications in the postoperative setting may be valuable in ensuring that such treatment is restarted.
Third, if a patient is not on ACE‐inhibitor therapy, should this be started before surgery for perioperative or long‐term benefit? Although neither study examines this issue, the potential for significant risk make this an unattractive option. Future interventional studies with thoughtfully weighed safety parameters may be necessary to assess whether such a paradigm may be valuable.
The studies included in this issue of the Journal of Hospital Medicine suggest that the use of ACE‐inhibitors during the perioperative period may be considered a function of time and place. Resuming ACE‐inhibitors and cessation of treatment at specific intervals in relation to surgery can help ensure positive outcomes. Hospitalists have an important role in this regard, as they are ideally situated to manage these agents in the many patients undergoing surgery across the United States.
Disclosures: Dr. Wijeysundera is supported by a Clinician‐Scientist Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto. Dr. Chopra is supported by a Career‐Development Award (1K08HS022835‐01) from the Agency of Healthcare Research and Quality.
The management of perioperative medications is a tale of progressive scientific enquiry. Although long‐term use of agents such as aspirin or statins improves clinical outcomes, use during surgery ranges from problematic to protective. The delicate balance between proven long‐term benefits in the nonoperative setting versus short‐term uncertainty in the perioperative setting must be assessed using a lens that incorporates patient risk, surgical process, and pharmacodynamic principles. Advances in our understanding of perioperative physiology, coupled with robust clinical and outcome data, have led to new knowledge and insights regarding how best to manage these medications. As well illustrated by the near 180 change in the use of perioperative ‐blockers to prevent adverse cardiovascular outcomes, these new data have led to substantial progress in surgical safety and patient outcomes.
In this issue of the Journal of Hospital Medicine, 2 retrospective cohort studies add to this growing body of evidence by examining risks associated with use of angiotensin‐converting enzyme (ACE) inhibitors in the perioperative setting. In the first study, conducted at a single academic medical center, Nielson and colleagues evaluate the association of preoperative ACE‐inhibitor use with hypotension and acute kidney injury in patients undergoing major elective orthopedic surgery.[1] The authors report that patients receiving ACE‐inhibitors were not only more likely to experience hypotension after induction of anesthesia (12.2% vs 6.7%, P = 0.005), but also were more likely to develop postoperative acute kidney injury (odds ratio [OR]: 2.68, 95% confidence interval [CI]: 1.25‐1.99). In the second study which focused solely on patients who were receiving preoperative ACE‐inhibitor therapy, Mudumbai and colleagues used a national Veterans Affairs database to examine the association between failure to resume ACE‐inhibitor treatment after surgery and outcomes at 30 days.[2] The authors found that failure to resume treatment 14 days after surgery was not only common (affecting 1 in 4 patients), but was also associated with increased 30‐day mortality (hazard ratio: 3.44, 95% CI: 3.30‐3.60).[2] Taken together, these 2 studies shed new light on clinical practice and policy implications for the use of these agents in the surgical period. Both sets of authors should be congratulated on moving the needle forward in this enquiry.
ACE‐inhibitors physiologically mediate their effects by preventing the formation of the potent vasoconstrictor angiotensin‐II from its precursor angiotensin‐I. In doing so, they decrease arterial resistance, increase venous capacitance, decrease glomerular filtration pressure, and promote natriuresis. These vascular effects have key benefits for the management of a number of chronic diseases including hypertension, congestive heart failure, and diabetic nephropathy. However, these clinical alterations are often problematic in the perioperative setting. For example, ACE‐inhibitors may cause vasoplegia during anesthetic administration, commonly manifested as hypotension during induction.[3] This hemodynamic alteration has been viewed as being so precarious, that some authors recommend withholding ACE‐inhibitors prior to major cardiovascular procedures such as coronary artery bypass grafting.[4] Although hypotension leads to management challenges (often increasing vasoconstrictor requirements), several studies report that ACE‐inhibitor use during surgery may also be associated with increased risks of acute kidney injury and mortality.[5, 6] However, despite these data, existing literature has not shown a consistent association between ACE‐inhibitor use and adverse postoperative outcomes. For example, a propensity‐matched cohort study of 79,228 patients at the Cleveland Clinic found no difference in hemodynamic characteristics, vasopressor requirements, or cardiorespiratory complications among patients who were or were not using ACE‐inhibitors during noncardiac surgery.[7] Furthermore, some research has also found that withdrawal of ACE‐inhibitors may itself lead to harm. For instance, a contemporary study reported that withdrawal of ACE‐inhibitor therapy in patients undergoing coronary artery bypass was associated with increased in‐hospital cardiovascular events.[8] Given this uncertainty, it is not surprising that management of ACE‐inhibitors in the perioperative period remains a subject of ongoing controversy with clinical reviews often recommending consideration of the risks and benefits associated with use of these agents.[9]
It is important to note that driver of this ambiguity is the very design of relevant studies. For instance, studies that focus on patients undergoing coronary artery bypass grafting surgery have limited external validity, as these patients are very different from those who undergo elective hip or knee replacement (such as those included by Nielsen and colleagues). Additionally, many studies suffer from methodological constraints or biases. For instance, retrospective observational studies often suffer from selection bias and residual confounding; that is, the individuals chosen for inclusion in the study and the variables available for analysis are often limited by available data, curtailing the conclusions that can be generated. Although the use of multivariable regression or propensity score techniques helps address these limitations, residual confounding by unmeasured variables always remains a threat to statistical inference. The potential influence for this bias is particularly relevant when examining the results of the study by Nielsen and colleagues. Another important limitation is survivor bias, a problem inherent in the study by Mudumbai and colleagues. Put simply, for a patient to resume ACE‐inhibitors after surgery, this same patient, must also survive for at least 15 to 30 days after surgery. Thus, for some patients, failure to resume this treatment may simply be a marker of early mortality rather than failure to resume the ACE itself. The potential influence of this bias is supported by an included sensitivity analysis, where a large change in the adjusted OR was observed when patients suffering early mortality were excluded. This swing in effect size suggests that biases related to comparing patients who survived to those who did not with respect to ACE use may, in part, account for the results of the study.
These limitations aside, the studies brought forth by both authors help inform practice with respect to the use of these agents during surgery. In this context, 3 paradigms are relevant for practicing hospitalists.
First, if a patient is maintained on an ACE‐inhibitor before surgery, should the medication be temporarily held before surgery to minimize hypotension during anesthesia? The study by Nielsen and colleagues (comparing those on ACE‐inhibitor treatment to those without), in addition to the evidence generated from other studies in this area[10, 11, 12] suggest that this is a rational decision. Although the existence of a withdrawal state from abrupt cessation of ACE‐inhibitor use is theoretically plausible, this has yet to be reliably reported in the literature. Given the short half‐life of most ACE‐inhibitors, cessation 24 hours before surgery appears to be the most pragmatic clinical approach.
Second, if a patient is on an ACE‐inhibitor before surgery, when should the medication be resumed after surgery? The findings from the study by Mudumbai and colleagues, in addition to contemporary evidence,[7, 8, 13] support the resumption of these agents as soon as possible following operative intervention. Once hemodynamic stability and volume status have been assured, risks associated with postoperative ACE‐inhibitor use appear to be outweighed by benefits, though specific care is likely necessary in those with preexisting renal dysfunction.[14] A program that ensures reconciliation of medications in the postoperative setting may be valuable in ensuring that such treatment is restarted.
Third, if a patient is not on ACE‐inhibitor therapy, should this be started before surgery for perioperative or long‐term benefit? Although neither study examines this issue, the potential for significant risk make this an unattractive option. Future interventional studies with thoughtfully weighed safety parameters may be necessary to assess whether such a paradigm may be valuable.
The studies included in this issue of the Journal of Hospital Medicine suggest that the use of ACE‐inhibitors during the perioperative period may be considered a function of time and place. Resuming ACE‐inhibitors and cessation of treatment at specific intervals in relation to surgery can help ensure positive outcomes. Hospitalists have an important role in this regard, as they are ideally situated to manage these agents in the many patients undergoing surgery across the United States.
Disclosures: Dr. Wijeysundera is supported by a Clinician‐Scientist Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto. Dr. Chopra is supported by a Career‐Development Award (1K08HS022835‐01) from the Agency of Healthcare Research and Quality.
- , , , . Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283–288.
- , , , , , . Thirty‐day mortality risk associated with postoperative nonresumption of angiotensin‐converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289–296.
- , , , , , . Clinical consequences of withholding versus administering renin‐angiotensin‐aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319–325.
- , , , , . Angiotensin‐converting enzyme inhibitors increase vasoconstrictor requirements after cardiopulmonary bypass. Anesth Analg. 1995;80(3):473–479.
- , , , et al. Effects of angiotensin‐converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2009;54(19):1778–1784.
- , , , . Renin‐angiotensin blockade is associated with increased mortality after vascular surgery. Can J Anaesth. 2010;57(8):736–744.
- , , , , , . Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114(3):552–560.
- , , , et al. Patterns of use of perioperative angiotensin‐converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in‐hospital morbidity and mortality. Circulation. 2012;126(3):261–269.
- , , , . Renin‐angiotensin system antagonists in the perioperative setting: clinical consequences and recommendations for practice. Postgrad Med J. 2011;87(1029):472–481.
- , , , et al; TRIBE‐AKI Consortium. Preoperative angiotensin‐converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery. Nephrol Dial Transplant. 2013;28(11):2787–2799.
- , , , , , . Effects of renin‐angiotensin system inhibitors on the occurrence of acute kidney injury following off‐pump coronary artery bypass grafting. Circulation J. 2010;74(9):1852–1858.
- , , , , , . Prophylactic vasopressin in patients receiving the angiotensin‐converting enzyme inhibitor ramipril undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2010;24(2):230–238.
- , , , , . Neither diabetes nor glucose‐lowering drugs are associated with mortality after noncardiac surgery in patients with coronary artery disease or heart failure. Can J Cardiol. 2013;29(4):423–428.
- , , , , , . Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev. 2008;(4):CD003590.
- , , , . Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283–288.
- , , , , , . Thirty‐day mortality risk associated with postoperative nonresumption of angiotensin‐converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289–296.
- , , , , , . Clinical consequences of withholding versus administering renin‐angiotensin‐aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319–325.
- , , , , . Angiotensin‐converting enzyme inhibitors increase vasoconstrictor requirements after cardiopulmonary bypass. Anesth Analg. 1995;80(3):473–479.
- , , , et al. Effects of angiotensin‐converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2009;54(19):1778–1784.
- , , , . Renin‐angiotensin blockade is associated with increased mortality after vascular surgery. Can J Anaesth. 2010;57(8):736–744.
- , , , , , . Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114(3):552–560.
- , , , et al. Patterns of use of perioperative angiotensin‐converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in‐hospital morbidity and mortality. Circulation. 2012;126(3):261–269.
- , , , . Renin‐angiotensin system antagonists in the perioperative setting: clinical consequences and recommendations for practice. Postgrad Med J. 2011;87(1029):472–481.
- , , , et al; TRIBE‐AKI Consortium. Preoperative angiotensin‐converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery. Nephrol Dial Transplant. 2013;28(11):2787–2799.
- , , , , , . Effects of renin‐angiotensin system inhibitors on the occurrence of acute kidney injury following off‐pump coronary artery bypass grafting. Circulation J. 2010;74(9):1852–1858.
- , , , , , . Prophylactic vasopressin in patients receiving the angiotensin‐converting enzyme inhibitor ramipril undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2010;24(2):230–238.
- , , , , . Neither diabetes nor glucose‐lowering drugs are associated with mortality after noncardiac surgery in patients with coronary artery disease or heart failure. Can J Cardiol. 2013;29(4):423–428.
- , , , , , . Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev. 2008;(4):CD003590.
Hospitalist Experiences With PICCs
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
Hospitalist Experiences Regarding PICCs
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
Copyright © 2013 Society of Hospital Medicine
Rethinking Cardiac Risk Reduction
Noncardiac surgery is frequently associated with major adverse cardiac events. Prevention of these events has traditionally focused on risk estimation and targeted interventions prior to surgical intervention.1 Cardiac risk is assessed through clinical prediction rules, such as the revised cardiac risk index (RCRI) or the American Society of Anesthesiology (ASA) classification. In patients deemed to be at high risk of adverse cardiac events, discretionary preoperative testing, medical treatments, and interventions are implemented.2 However, even when executed optimally, this approach fails to protect all patients. Thus, many patients undergoing noncardiac surgery continue to experience perioperative myocardial infarction (MI) and death.3
Recently, a prospective international study involving 15,133 patients reported that cardiac troponin levels measured within 3 days of noncardiac surgery were strongly associated with 30‐day mortality.4 This study is but the latest in a series of investigations that have concluded that postoperative measurement of cardiac troponin is a better predictor of cardiac outcomes than preoperative‐risk algorithms.48 Intuitively, this link is not surprising. Surgery represents the ultimate physiologic stress test. In the setting of hemodynamic changes and sustained oxygen supply:demand mismatch, it is hardly shocking that those with significant epicardial coronary stenosis or major subendocardial ischemia suffer poorer outcomes. What is perhaps most important about the association between troponin release and clinical outcomes, however, is the foundation it provides for a novel framework to improve perioperative care: using troponin measurement to guide treatment and interventions in the postoperative setting.
MODEL TO IMPROVE POSTOPERATIVE CARDIAC OUTCOMES
We hypothesize that the postoperative period can be seized as a golden moment to ameliorate cardiac risk and improve clinical outcomes for 3 reasons. First, those who release cardiac troponin during surgery declare themselves as harboring myocardial territories in jeopardy. In effect, the peripheral presence of troponin amounts to a cry for help in a population where many cardiac events are silent and thus remain clinically unnoticed.9, 10 Routine postoperative monitoring for this biomarker can thus help identify a population that would ordinarily escape detection until the precipitation of a major clinical event. Second, as the pathophysiology of cardiac events centers around rupture of vulnerable plaque (type‐1 MI), supply:demand mismatch (type‐2 MI), or both of these phenomena, escalation or institution of important medical risk‐reducing treatments (such as aspirin, beta‐blockers, or statins), can be established to improve outcomes. The ability to initiate these treatments in a monitored setting may offset and address many of the risks feared with these therapies, including hypotension, stroke, or rhabdomyolysis. Third, as patients convalesce from surgery, timely and discretionary cardiac testing or interventions can be undertaken in those identified as being at elevated cardiovascular risk prior to discharge. In sum, the trifecta of clearly recognizing those at risk, instituting interventions in controlled settings, and performing well‐timed interventions could translate into greater odds of survival in this cohort.
Owing to their intimate involvement with patients in the perioperative setting, hospitalists are uniquely suited for implementing a troponin surveillance paradigm. Yet, how may such a schema be realized? In Figure 1, we outline a pathway with which to implement this approach. This model replicates perioperative management workflow, calling for 3 discrete interventionspreoperative assessment, perioperative surveillance, and postoperative risk stratification:
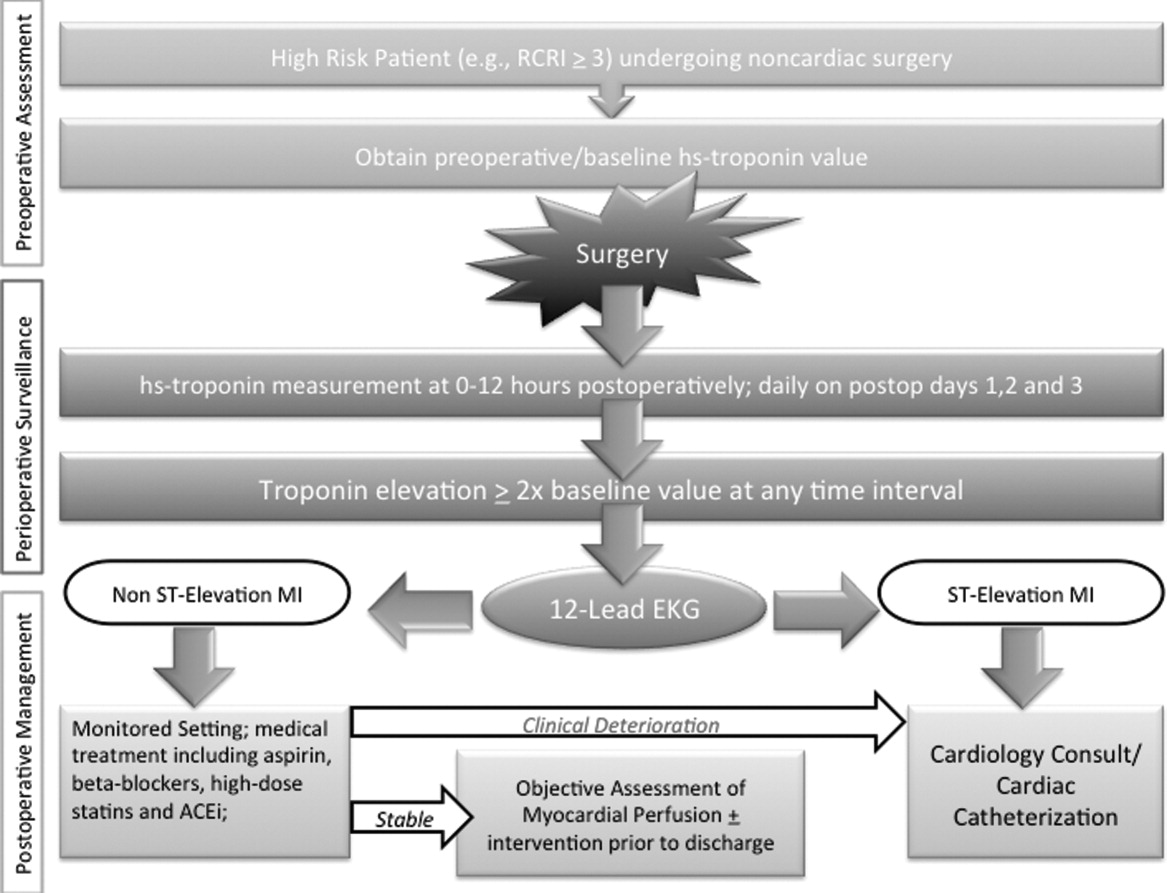
-
Preoperative assessment: Patients estimated to be at high risk of perioperative cardiac events (eg, RCRI 3) should undergo baseline, preoperative high‐sensitivity troponin measurement, as the frequency of major adverse cardiac outcomes is significant this group (11%).11 The importance of a baseline measurement of troponin cannot be overstated, as biologic variation of this marker can impact subsequent management.12, 13 Hospitalists can obtain these assays in preoperative clinics at the time of initial review and risk‐estimation.
-
Perioperative surveillance: Following the model implemented by a recent study, serial troponin surveillance should commence 612 hours after surgery, then daily on the first, second, and third day.4 As the optimal cardiac troponin threshold for the diagnosis of perioperative ischemia remains uncertain, doubling of baseline troponin levels may serve as a logical signal for further evaluation.14 The electrocardiographic pattern may be used as a branch point for immediate management at this stage: ST‐segment elevation MI may be treated aggressively with an early invasive strategy and cardiac catheterization when apropos (much as in the nonoperative context). However, as most perioperative cardiac events are non‐ST elevation MIs, treatment of this subset should center on initiation or escalation of medical therapy. Thus, achieving heart‐rate control in order to preserve coronary perfusion by careful titration of beta‐blockers, and initiation/up‐titration of statins to temper unstable atherosclerotic plaques represent cornerstones of this approach. Other potential therapeutic entities such as initiation of angiotensin‐converting‐enzyme (ACE)‐inhibitors in the context of left‐ventricular dysfunction, and/or antiplatelet agents such as aspirin, may also be relevant risk‐reducing tools.
-
Postoperative risk stratification: Prior to hospital discharge, objective assessment of myocardial perfusion and viability should occur in selected individuals with troponin elevations. Modalities that may be utilized in this context include either noninvasive imaging or occasional coronary angiography in patients with unstable coronary syndromes. Defining an optimal approach must be contextual, as interventions that may subsequently mandate uninterrupted antiplatelet treatment may be logistically challenging in this setting.
LIMITATIONS
Although this implementation model is plausible, it generates many questions that must be tested through the lens of a randomized controlled study. For instance, what is the optimal strategy for intervention in the postoperative setting? Though medical treatment with statins and beta‐blockade may represent the mainstay of treatment, are these therapies safe during potential clinical instability? Can net benefit be realized through judicious use of coronary angiography and revascularization? Can the artifact of heightened awareness and reporting of postoperative cardiac events mar the reported quality of a hospital? Finally, though cardiac troponin has been shown to be a strong and independent predictor of mortality, which troponin assay, reference range, and troponometric standard to use remain unclear.15 Whether or not these interventions translate into lesser mortality and improved clinical outcomes represents the raison d'etre for this experimental approach. As no alternative approach to define and target patients at high risk of adverse outcome after seemingly uneventful surgery currently exists, we believe that this hypothetical paradigm is worthy of further investigation.
CONCLUSION
For decades, perioperative practitioners have searched for a divining rod to reduce risk during surgery. While our efforts have been focused on the preoperative setting, the postoperative setting represents an as yet untapped and potentially profound period in the quest to improving surgical outcomes. Through our proximity to patients in the perioperative setting, hospitalists are ideal agents to test, deliver, and bring about this change. It is time for a postoperative carpe diem.
- , , , et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy. JAMA. 2001;285(14):1865–1873.
- , , , et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110(11):859–866.
- , , , et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144.
- VISION Study Investigators. Association between postoperative troponin levels and 30‐day mortlality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–2304.
- , , , et al. Postoperative levels of cardiac troponin versus CK‐MB and high‐sensitivity C‐reactive protein for the prediction of 1‐year cardiovascular outcome in patients undergoing vascular surgery. Coron Artery Dis. 2011;22(6):428–434.
- , , , et al. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long‐term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27(1):66–72.
- , , , et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology. 2011;114(4):796–806.
- , , . Outcomes in vascular surgical patients with isolated postoperative troponin leak: a meta‐analysis. Anaesthesia. 2011;66(7):604–610.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523–528.
- , , , , . Perioperative myocardial necrosis in patients at high cardiovascular risk undergoing elective non‐cardiac surgery. Heart. 2012;98(10):792–798.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049.
- , , , , . Biologic variation of a novel cardiac troponin I assay. Clin Chem. 2011;57(7):1080–1081.
- , , , , . Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52–58.
- , , , et al. Relationship between perioperative troponin elevation and other indicators of myocardial injury in vascular surgery patients. Br J Anaesth. 2006;96(3):303–309.
- , , , et al. Which troponometric best predicts midterm outcome after coronary artery bypass graft surgery? Ann Thorac Surg. 2011;91(6):1860–1867.
Noncardiac surgery is frequently associated with major adverse cardiac events. Prevention of these events has traditionally focused on risk estimation and targeted interventions prior to surgical intervention.1 Cardiac risk is assessed through clinical prediction rules, such as the revised cardiac risk index (RCRI) or the American Society of Anesthesiology (ASA) classification. In patients deemed to be at high risk of adverse cardiac events, discretionary preoperative testing, medical treatments, and interventions are implemented.2 However, even when executed optimally, this approach fails to protect all patients. Thus, many patients undergoing noncardiac surgery continue to experience perioperative myocardial infarction (MI) and death.3
Recently, a prospective international study involving 15,133 patients reported that cardiac troponin levels measured within 3 days of noncardiac surgery were strongly associated with 30‐day mortality.4 This study is but the latest in a series of investigations that have concluded that postoperative measurement of cardiac troponin is a better predictor of cardiac outcomes than preoperative‐risk algorithms.48 Intuitively, this link is not surprising. Surgery represents the ultimate physiologic stress test. In the setting of hemodynamic changes and sustained oxygen supply:demand mismatch, it is hardly shocking that those with significant epicardial coronary stenosis or major subendocardial ischemia suffer poorer outcomes. What is perhaps most important about the association between troponin release and clinical outcomes, however, is the foundation it provides for a novel framework to improve perioperative care: using troponin measurement to guide treatment and interventions in the postoperative setting.
MODEL TO IMPROVE POSTOPERATIVE CARDIAC OUTCOMES
We hypothesize that the postoperative period can be seized as a golden moment to ameliorate cardiac risk and improve clinical outcomes for 3 reasons. First, those who release cardiac troponin during surgery declare themselves as harboring myocardial territories in jeopardy. In effect, the peripheral presence of troponin amounts to a cry for help in a population where many cardiac events are silent and thus remain clinically unnoticed.9, 10 Routine postoperative monitoring for this biomarker can thus help identify a population that would ordinarily escape detection until the precipitation of a major clinical event. Second, as the pathophysiology of cardiac events centers around rupture of vulnerable plaque (type‐1 MI), supply:demand mismatch (type‐2 MI), or both of these phenomena, escalation or institution of important medical risk‐reducing treatments (such as aspirin, beta‐blockers, or statins), can be established to improve outcomes. The ability to initiate these treatments in a monitored setting may offset and address many of the risks feared with these therapies, including hypotension, stroke, or rhabdomyolysis. Third, as patients convalesce from surgery, timely and discretionary cardiac testing or interventions can be undertaken in those identified as being at elevated cardiovascular risk prior to discharge. In sum, the trifecta of clearly recognizing those at risk, instituting interventions in controlled settings, and performing well‐timed interventions could translate into greater odds of survival in this cohort.
Owing to their intimate involvement with patients in the perioperative setting, hospitalists are uniquely suited for implementing a troponin surveillance paradigm. Yet, how may such a schema be realized? In Figure 1, we outline a pathway with which to implement this approach. This model replicates perioperative management workflow, calling for 3 discrete interventionspreoperative assessment, perioperative surveillance, and postoperative risk stratification:

-
Preoperative assessment: Patients estimated to be at high risk of perioperative cardiac events (eg, RCRI 3) should undergo baseline, preoperative high‐sensitivity troponin measurement, as the frequency of major adverse cardiac outcomes is significant this group (11%).11 The importance of a baseline measurement of troponin cannot be overstated, as biologic variation of this marker can impact subsequent management.12, 13 Hospitalists can obtain these assays in preoperative clinics at the time of initial review and risk‐estimation.
-
Perioperative surveillance: Following the model implemented by a recent study, serial troponin surveillance should commence 612 hours after surgery, then daily on the first, second, and third day.4 As the optimal cardiac troponin threshold for the diagnosis of perioperative ischemia remains uncertain, doubling of baseline troponin levels may serve as a logical signal for further evaluation.14 The electrocardiographic pattern may be used as a branch point for immediate management at this stage: ST‐segment elevation MI may be treated aggressively with an early invasive strategy and cardiac catheterization when apropos (much as in the nonoperative context). However, as most perioperative cardiac events are non‐ST elevation MIs, treatment of this subset should center on initiation or escalation of medical therapy. Thus, achieving heart‐rate control in order to preserve coronary perfusion by careful titration of beta‐blockers, and initiation/up‐titration of statins to temper unstable atherosclerotic plaques represent cornerstones of this approach. Other potential therapeutic entities such as initiation of angiotensin‐converting‐enzyme (ACE)‐inhibitors in the context of left‐ventricular dysfunction, and/or antiplatelet agents such as aspirin, may also be relevant risk‐reducing tools.
-
Postoperative risk stratification: Prior to hospital discharge, objective assessment of myocardial perfusion and viability should occur in selected individuals with troponin elevations. Modalities that may be utilized in this context include either noninvasive imaging or occasional coronary angiography in patients with unstable coronary syndromes. Defining an optimal approach must be contextual, as interventions that may subsequently mandate uninterrupted antiplatelet treatment may be logistically challenging in this setting.
LIMITATIONS
Although this implementation model is plausible, it generates many questions that must be tested through the lens of a randomized controlled study. For instance, what is the optimal strategy for intervention in the postoperative setting? Though medical treatment with statins and beta‐blockade may represent the mainstay of treatment, are these therapies safe during potential clinical instability? Can net benefit be realized through judicious use of coronary angiography and revascularization? Can the artifact of heightened awareness and reporting of postoperative cardiac events mar the reported quality of a hospital? Finally, though cardiac troponin has been shown to be a strong and independent predictor of mortality, which troponin assay, reference range, and troponometric standard to use remain unclear.15 Whether or not these interventions translate into lesser mortality and improved clinical outcomes represents the raison d'etre for this experimental approach. As no alternative approach to define and target patients at high risk of adverse outcome after seemingly uneventful surgery currently exists, we believe that this hypothetical paradigm is worthy of further investigation.
CONCLUSION
For decades, perioperative practitioners have searched for a divining rod to reduce risk during surgery. While our efforts have been focused on the preoperative setting, the postoperative setting represents an as yet untapped and potentially profound period in the quest to improving surgical outcomes. Through our proximity to patients in the perioperative setting, hospitalists are ideal agents to test, deliver, and bring about this change. It is time for a postoperative carpe diem.
Noncardiac surgery is frequently associated with major adverse cardiac events. Prevention of these events has traditionally focused on risk estimation and targeted interventions prior to surgical intervention.1 Cardiac risk is assessed through clinical prediction rules, such as the revised cardiac risk index (RCRI) or the American Society of Anesthesiology (ASA) classification. In patients deemed to be at high risk of adverse cardiac events, discretionary preoperative testing, medical treatments, and interventions are implemented.2 However, even when executed optimally, this approach fails to protect all patients. Thus, many patients undergoing noncardiac surgery continue to experience perioperative myocardial infarction (MI) and death.3
Recently, a prospective international study involving 15,133 patients reported that cardiac troponin levels measured within 3 days of noncardiac surgery were strongly associated with 30‐day mortality.4 This study is but the latest in a series of investigations that have concluded that postoperative measurement of cardiac troponin is a better predictor of cardiac outcomes than preoperative‐risk algorithms.48 Intuitively, this link is not surprising. Surgery represents the ultimate physiologic stress test. In the setting of hemodynamic changes and sustained oxygen supply:demand mismatch, it is hardly shocking that those with significant epicardial coronary stenosis or major subendocardial ischemia suffer poorer outcomes. What is perhaps most important about the association between troponin release and clinical outcomes, however, is the foundation it provides for a novel framework to improve perioperative care: using troponin measurement to guide treatment and interventions in the postoperative setting.
MODEL TO IMPROVE POSTOPERATIVE CARDIAC OUTCOMES
We hypothesize that the postoperative period can be seized as a golden moment to ameliorate cardiac risk and improve clinical outcomes for 3 reasons. First, those who release cardiac troponin during surgery declare themselves as harboring myocardial territories in jeopardy. In effect, the peripheral presence of troponin amounts to a cry for help in a population where many cardiac events are silent and thus remain clinically unnoticed.9, 10 Routine postoperative monitoring for this biomarker can thus help identify a population that would ordinarily escape detection until the precipitation of a major clinical event. Second, as the pathophysiology of cardiac events centers around rupture of vulnerable plaque (type‐1 MI), supply:demand mismatch (type‐2 MI), or both of these phenomena, escalation or institution of important medical risk‐reducing treatments (such as aspirin, beta‐blockers, or statins), can be established to improve outcomes. The ability to initiate these treatments in a monitored setting may offset and address many of the risks feared with these therapies, including hypotension, stroke, or rhabdomyolysis. Third, as patients convalesce from surgery, timely and discretionary cardiac testing or interventions can be undertaken in those identified as being at elevated cardiovascular risk prior to discharge. In sum, the trifecta of clearly recognizing those at risk, instituting interventions in controlled settings, and performing well‐timed interventions could translate into greater odds of survival in this cohort.
Owing to their intimate involvement with patients in the perioperative setting, hospitalists are uniquely suited for implementing a troponin surveillance paradigm. Yet, how may such a schema be realized? In Figure 1, we outline a pathway with which to implement this approach. This model replicates perioperative management workflow, calling for 3 discrete interventionspreoperative assessment, perioperative surveillance, and postoperative risk stratification:

-
Preoperative assessment: Patients estimated to be at high risk of perioperative cardiac events (eg, RCRI 3) should undergo baseline, preoperative high‐sensitivity troponin measurement, as the frequency of major adverse cardiac outcomes is significant this group (11%).11 The importance of a baseline measurement of troponin cannot be overstated, as biologic variation of this marker can impact subsequent management.12, 13 Hospitalists can obtain these assays in preoperative clinics at the time of initial review and risk‐estimation.
-
Perioperative surveillance: Following the model implemented by a recent study, serial troponin surveillance should commence 612 hours after surgery, then daily on the first, second, and third day.4 As the optimal cardiac troponin threshold for the diagnosis of perioperative ischemia remains uncertain, doubling of baseline troponin levels may serve as a logical signal for further evaluation.14 The electrocardiographic pattern may be used as a branch point for immediate management at this stage: ST‐segment elevation MI may be treated aggressively with an early invasive strategy and cardiac catheterization when apropos (much as in the nonoperative context). However, as most perioperative cardiac events are non‐ST elevation MIs, treatment of this subset should center on initiation or escalation of medical therapy. Thus, achieving heart‐rate control in order to preserve coronary perfusion by careful titration of beta‐blockers, and initiation/up‐titration of statins to temper unstable atherosclerotic plaques represent cornerstones of this approach. Other potential therapeutic entities such as initiation of angiotensin‐converting‐enzyme (ACE)‐inhibitors in the context of left‐ventricular dysfunction, and/or antiplatelet agents such as aspirin, may also be relevant risk‐reducing tools.
-
Postoperative risk stratification: Prior to hospital discharge, objective assessment of myocardial perfusion and viability should occur in selected individuals with troponin elevations. Modalities that may be utilized in this context include either noninvasive imaging or occasional coronary angiography in patients with unstable coronary syndromes. Defining an optimal approach must be contextual, as interventions that may subsequently mandate uninterrupted antiplatelet treatment may be logistically challenging in this setting.
LIMITATIONS
Although this implementation model is plausible, it generates many questions that must be tested through the lens of a randomized controlled study. For instance, what is the optimal strategy for intervention in the postoperative setting? Though medical treatment with statins and beta‐blockade may represent the mainstay of treatment, are these therapies safe during potential clinical instability? Can net benefit be realized through judicious use of coronary angiography and revascularization? Can the artifact of heightened awareness and reporting of postoperative cardiac events mar the reported quality of a hospital? Finally, though cardiac troponin has been shown to be a strong and independent predictor of mortality, which troponin assay, reference range, and troponometric standard to use remain unclear.15 Whether or not these interventions translate into lesser mortality and improved clinical outcomes represents the raison d'etre for this experimental approach. As no alternative approach to define and target patients at high risk of adverse outcome after seemingly uneventful surgery currently exists, we believe that this hypothetical paradigm is worthy of further investigation.
CONCLUSION
For decades, perioperative practitioners have searched for a divining rod to reduce risk during surgery. While our efforts have been focused on the preoperative setting, the postoperative setting represents an as yet untapped and potentially profound period in the quest to improving surgical outcomes. Through our proximity to patients in the perioperative setting, hospitalists are ideal agents to test, deliver, and bring about this change. It is time for a postoperative carpe diem.
- , , , et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy. JAMA. 2001;285(14):1865–1873.
- , , , et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110(11):859–866.
- , , , et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144.
- VISION Study Investigators. Association between postoperative troponin levels and 30‐day mortlality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–2304.
- , , , et al. Postoperative levels of cardiac troponin versus CK‐MB and high‐sensitivity C‐reactive protein for the prediction of 1‐year cardiovascular outcome in patients undergoing vascular surgery. Coron Artery Dis. 2011;22(6):428–434.
- , , , et al. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long‐term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27(1):66–72.
- , , , et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology. 2011;114(4):796–806.
- , , . Outcomes in vascular surgical patients with isolated postoperative troponin leak: a meta‐analysis. Anaesthesia. 2011;66(7):604–610.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523–528.
- , , , , . Perioperative myocardial necrosis in patients at high cardiovascular risk undergoing elective non‐cardiac surgery. Heart. 2012;98(10):792–798.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049.
- , , , , . Biologic variation of a novel cardiac troponin I assay. Clin Chem. 2011;57(7):1080–1081.
- , , , , . Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52–58.
- , , , et al. Relationship between perioperative troponin elevation and other indicators of myocardial injury in vascular surgery patients. Br J Anaesth. 2006;96(3):303–309.
- , , , et al. Which troponometric best predicts midterm outcome after coronary artery bypass graft surgery? Ann Thorac Surg. 2011;91(6):1860–1867.
- , , , et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy. JAMA. 2001;285(14):1865–1873.
- , , , et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110(11):859–866.
- , , , et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144.
- VISION Study Investigators. Association between postoperative troponin levels and 30‐day mortlality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–2304.
- , , , et al. Postoperative levels of cardiac troponin versus CK‐MB and high‐sensitivity C‐reactive protein for the prediction of 1‐year cardiovascular outcome in patients undergoing vascular surgery. Coron Artery Dis. 2011;22(6):428–434.
- , , , et al. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long‐term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27(1):66–72.
- , , , et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology. 2011;114(4):796–806.
- , , . Outcomes in vascular surgical patients with isolated postoperative troponin leak: a meta‐analysis. Anaesthesia. 2011;66(7):604–610.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523–528.
- , , , , . Perioperative myocardial necrosis in patients at high cardiovascular risk undergoing elective non‐cardiac surgery. Heart. 2012;98(10):792–798.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049.
- , , , , . Biologic variation of a novel cardiac troponin I assay. Clin Chem. 2011;57(7):1080–1081.
- , , , , . Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52–58.
- , , , et al. Relationship between perioperative troponin elevation and other indicators of myocardial injury in vascular surgery patients. Br J Anaesth. 2006;96(3):303–309.
- , , , et al. Which troponometric best predicts midterm outcome after coronary artery bypass graft surgery? Ann Thorac Surg. 2011;91(6):1860–1867.
Statin Withdrawal After Major Surgery
Accumulating evidence suggests that perioperative treatment with 3‐hydroxy‐3‐methylglutaryl coenzyme‐A (HMG‐CoA) reductase inhibitors (or, statins) reduces the incidence of cardiovascular events during noncardiac surgery.16 This evidence has lead the European Society of Cardiology (ESC) and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) to endorse the use of perioperative statins in patients already on this treatment or those at high‐risk of cardiovascular events.7, 8
However, statins are available only in oral formulation. Consequently, prolonged bowel recovery or clinical instability may interfere with use during surgery. Furthermore, many clinicians may not recognize the imperative of postoperative statin resumption, viewing them principally as lipid‐lowering entities and not as agents of perioperative benefit. Failure to resume statins postoperatively can be catastrophic, as the ensuing inflammation and thrombosis frequently culminates in myocardial infarction (MI) or death.9, 10
In this article, we review the potent anti‐inflammatory properties of statins and their role in preventing perioperative cardiac events. We outline the biochemical basis for perioperative statin benefit, summarizing the basic, clinical, and experimental evidence regarding statin withdrawal. We conclude by presenting strategies to avert postoperative statin cessation and outline a research agenda dedicated to informing this practice.
METHODS
We performed a literature search using MEDLINE via Ovid (1946present), EMBASE (1946present), Biosis (1926present), and Cochrane CENTRAL (1960present). We used Boolean logic to search for key terms including statins, 3‐hydroxy‐3‐methylglutaryl CoA reductase inhibitors, death, MI, stroke, acute coronary syndrome (ACS), and statin withdrawal or cessation. All studies published in full‐text or abstract form were included. A total of 489 articles were retrieved by this search (last updated March 15, 2012). For this narrative review, we focused on studies that examined adverse outcomes associated with statin withdrawal.
BIOCHEMICAL BASIS OF STATIN PLEIOTROPICITY
The nonlipid‐lowering or pleiotropic properties of statins are especially valuable in the perioperative setting.16, 11 Perioperative cardiac complications occur due to oxygen supply:demand mismatch, vascular inflammation, or a combination of these states. A significant perisurgical catecholamine surge produces unopposed sympathetic effects,12 increasing the risk of rupture of vulnerable coronary plaques, thrombus formation, and adverse cardiac events.13, 14 Similarly, augmented inflammatory responses and increased circulating coagulation factors further predispose to a hazardous perioperative milieu.15 Statins attenuate this vascular inflammatory response by suppressing the synthesis of mevalonate by inhibiting HMG‐CoA reductase. Suppression of mevalonate synthesis reduces the bioavailability of 2 important isoprenoid molecules: farnesyl‐pyrophosphate and geranylgeranyl‐pyrophosphate.16 Diminution of these isoprenoid intermediaries leads to reductions in the active intracellular signaling molecules Ras, Rho, and Rac, which play critical roles in vascular reactivity, endothelial function, and coagulation and inflammatory pathways.1723 The cumulative effect of these cellular changes is diminished inflammation during periods of surgical stress (Figure 1).
While the perioperative pleiotropicity of statins is of inherent clinical value, several studies have shown that these effects are lost and even reversed when statins are withdrawn.2428 During statin treatment, absence of isoprenoid intermediaries induces cytosolic accumulation of nonactivated Rho and Rac proteins. Abrupt cessation of statins activates Rho/Rac‐kinase pathways, leading to unregulated inflammation, platelet hyper‐activation, and endothelial dysfunction.24, 25, 28, 29 For instance, statin withdrawal in mice‐models leads to an overshoot activation of Rho, resulting in down‐regulation of endothelial nitric oxide production,25 activation of nicotinamide adenine dinucleotide phosphate (NAD[P]H)‐oxidase, and increased superoxide production.29 In another mouse‐model, statin withdrawal was associated with up‐regulation of key pro‐thrombotic molecules including platelet factor 4 and beta‐thromboglobulin.24 In human studies, a platelet hyper‐activation state (manifested by increased platelet P‐selectin expression and enhanced platelet aggregation) occurs after statin discontinuation.27 Furthermore, withdrawal of statins in patients with hyperlipidemia increases inflammatory markers such as C‐reactive protein and interleukin‐6.26 In the perioperative context, absence of these important anti‐inflammatory properties increases the risk of cardiac events.9, 10
EVIDENCE SUGGESTING BENEFIT FROM PERIOPERATIVE STATIN TREATMENT
Retrospective studies first suggested clinical benefit from perioperative statin treatment. In a case‐control study involving 2816 patients undergoing vascular surgery at Erasmus Medical Center, statin use was associated with substantially decreased postoperative mortality (adjusted odd ratio [OR] 0.22, 95% confidence interval [CI] 0.100.47).5 In a subsequent retrospective cohort study of 780,591 patients who underwent major noncardiac surgery, the risk of postoperative mortality was considerably lower among statin users (unadjusted OR 0.68, 95% CI 0.640.72) compared to patients who did not receive, or received delayed treatment with statins.3 A third retrospective study of 1163 vascular surgery patients found that statins prevented perioperative cardiac complications including death, MI, congestive heart failure, and ventricular tachyarrhythmias (OR 0.52, 95% CI 0.350.76).4
The benefit from statin treatment found in retrospective studies prompted the first double‐blinded, randomized controlled trial (RCT) of perioperative statin use. In 2004, Durazzo and colleagues1 randomized 100 statin‐naive patients scheduled to undergo elective aortic, femoro‐popliteal, or carotid surgery to receive either 20 mg of atorvastatin or placebo for 45 days. Vascular surgery was performed, on average, 31 days after randomization. Atorvastatin therapy reduced the incidence of death from cardiac causes, nonfatal acute MI, ischemic stroke, and unstable angina (26% in the placebo group vs 8% in the atorvastatin group; P = 0.031).1 Although the small size of the trial rendered it underpowered to show a mortality benefit, this remains the first RCT to demonstrate a protective perioperative effect of statins.
In the 2009 Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE)‐III trial, Schouten and colleagues6 randomized 497 high‐risk, statin‐naive patients undergoing vascular surgery to receive, in addition to a beta‐blocker, either fluvastatin or placebo before surgery (median of 37 days). Postoperative myocardial ischemia (hazard ratio [HR] 0.55, 95% CI 0.340.88), and combined death from cardiovascular causes or nonfatal MI (HR 0.47, 95% CI 0.240.94), occurred less frequently in the treatment group.6 In 2009, the same group published DECREASE‐IV, a multicenter, prospective, open‐label, 2 2 factorial design trial of 1066 intermediate‐risk patients, scheduled to undergo elective, noncardiac surgery. Patients were assigned to bisoprolol, fluvastatin, combination treatment, or control therapy before surgery (median of 34 days). Although those randomized to fluvastatin demonstrated lower incidence of 30‐day cardiac death and MI than control (HR 0.65, 95% CI 0.351.10), these outcomes failed to reach statistical significance as the trial was principally powered to examine the effects of perioperative beta‐blockade.30
Using this pool of data, a meta‐analysis of 15 studies (223,010 patients) found a substantial 38% reduction in the risk of mortality after cardiac surgery (1.9% vs 3.1%; P = 0.0001) and an even greater 59% reduction in the risk of mortality following vascular surgery (1.7% vs 6.1%; P = 0.0001) with perioperative statin therapy. When including noncardiac surgery, a 44% reduction in mortality was observed (2.2% vs 3.2%; P < 0.01).2 We performed a similar meta‐analysis of 15 RCTs involving 2292 patients to determine whether perioperative statin treatment in statin‐naive patients, undergoing either cardiac or noncardiac surgery, improved clinical outcomes. Our analysis also found statistically significant reductions in the risk of MI associated with perioperative statin use in both cardiac and noncardiac surgery (risk reduction [RR] 0.53, 95% CI 0.380.74) and atrial fibrillation in statin‐naive patients undergoing cardiac surgery (RR 0.56, 95% CI 0.450.69).31 Taken together, a large volume of evidence supports the use of statins in surgical settings.
In view of this evidence, the ACCF/AHA perioperative guidelines for noncardiac surgery endorsed statins as an important risk‐reducing intervention in those undergoing noncardiac surgery, and recommended continued use in patients on chronic statin treatment scheduled for noncardiac surgery (Level of Evidence B, Class I; Benefits >>> Risk). Initiating statins in patients undergoing vascular surgery, with or without risk factors, was considered reasonable (Level of Evidence B, Class IIa; Benefits >> Risk).7 Current ESC perioperative guidelines in noncardiac surgery offer similar recommendations to those of ACCF/AHA, but differ by categorizing the recommendation to initiate statins in patients at high cardiovascular risk as a Class I recommendation.8
CLINICAL CONSEQUENCES OF STATIN WITHDRAWAL
Although statins provide important cardiac benefits, an important limitation to their perioperative use remains their oral‐only formulation. Thus, patients who are unable to resume oral intake may fail to resume treatment. Perioperative statin cessation has been hypothesized to lead to a statin withdrawal phenomenon. The evidence that supports the existence of this phenomenon comes from 3 distinct populations: ACS, ischemic stroke, and perioperative patients (Table 1).
| Author (ref) Year | Design | Study Population | Sample Size (N) (Total/ Continuation/Discontinuation) | Clinical Setting and Context | Timing of Statin Discontinuation | Study Outcome | Results (Withdrawal vs Continuation) |
|---|---|---|---|---|---|---|---|
| |||||||
| Heeschen et al33 2002 | Retrospective cohort | Mostly men (68%) in early 60s | 1616/379/86 | Chest pain in ACS | During or after admission | Incidence of death and nonfatal MI | OR (95% CI) |
| 2.93 (1.646.27) | |||||||
| Spencer et al32 2004 | Retrospective cohort | Mostly men (62%) in late 60s | 68,506/9001/487 | NonST‐segment elevation MI | During the first 24 hr of hospitalization | In‐hospital death | HR (95% CI) |
| 1.83 (1.582.13) | |||||||
| Daskalopoulou et al34 2008 | Retrospective cohort | 60% men in late 60s | 9939/2026/137 Non‐users 2124 (reference group) | Acute MI | Within 1 yr of the coronary event | 1‐yr all‐cause mortality | HR (95% CI) |
| 1.88 (1.133.07)* | |||||||
| Colivicchi et al36 2007 | Prospective cohort | 51% men in early 70s | 631/385/246 | Ischemic stroke | Mean 48.6 days | 1‐yr all‐cause mortality | HR (95% CI) |
| 2.78 (1.963.72) | |||||||
| Blanco et al37 2007 | RCT | 51% men in mid‐60s | 215/46/43 | Ischemic stroke | N/A | Risk of death or dependency at 3 mo | OR (95% CI) |
| 4.66 (1.4614.91) | |||||||
| Early neurologic deterioration | OR (95% CI) | ||||||
| 7.08 (2.7318.37) | |||||||
| Le Manach et al9 2007 | Quasi‐experimental (prepost) | Mostly men (89%) in late‐60s | 669/178/491 | Infra‐renal aortic surgery | Median of 4 days off statins | Postoperative troponin release, MI | OR (95% CI) |
| 2.9 (1.65.5) | |||||||
| Schouten et al10 2007 | Prospective cohort | Mostly men (75%) in mid‐60s | 298/228/70 | Aortic and lower extremity vascular surgery | Median of 3 days off statins | Postoperative troponin release | HR (95% CI) |
| 4.6 (2.29.6) | |||||||
| Combination of postoperative MI and CV death | HR (95% CI) | ||||||
| 7.5 (2.820.1) | |||||||
| Schouten et al6 2009 | RCT | Mostly men (75%) in mid‐60s | 250/189/61 | Vascular surgery (carotid, abdominal aortic, endovascular, and lower extremity arterial) | Median of 2 days off statins | Postoperative myocardial ischemia and combined death from cardiovascular causes or nonfatal MI | OR (95% CI) |
| 1.1 (0.482.52) | |||||||
Statin Withdrawal in Acute Coronary Syndromes
Several studies have demonstrated an association between statin withdrawal and heightened risk of cardiovascular events in ACS.3234 In a retrospective analysis of 1616 patients presenting with ACS, withdrawal of statins during or after admission was associated with more frequent death and nonfatal MI compared to those who continued therapy (OR 2.93, 95% CI 1.646.27).33 In another retrospective observational study of 68,606 nonST‐segment elevation MI patients, statin cessation during the first 24 hours of hospitalization was independently associated with adverse outcomes including in‐hospital death (adjusted HR 1.83; 95% CI 1.582.13), cardiac arrest, and cardiogenic shock.32 In a population‐based, cohort study in the United Kingdom, statin cessation following an acute MI was independently associated with greater all‐cause mortality at 1‐year (adjusted HR 1.88, 95% CI 1.133.07).34
The significantly increased risk of adverse outcomes associated with the interruption of statins in ACS may be moderated by vascular inflammation related to the inciting coronary event, as statin discontinuation in patients with stable cardiac conditions was not associated with increased risk of cardiovascular events in a large‐scale, double‐blind, parallel‐group study.35
Statin Withdrawal in Ischemic Stroke
Adverse events associated with statin withdrawal have also been reported in patients with cerebrovascular disease. In a prospective observational study of 631 consecutive stroke survivors, those who discontinued statins (owing to mild adverse effects or unclear reasons) experienced increased mortality during the first year after the event (adjusted HR 2.78, 95% CI 1.963.72).36 Using a controversial study design aimed at evaluating the effects of stopping oral intake (including chronic medications) during the first days of acute stroke, Blanco and colleagues37 randomized 89 stroke victims on chronic statins to either continue medications or experience statin withdrawal following admission. Statin withdrawal was independently associated with increased risk of mortality and dependency at 3 months (OR 4.66, 95% CI 1.4614.91).37
Perioperative Statin Withdrawal
In the perioperative setting, statin withdrawal has also been associated with adverse outcomes. Using a quasi‐experimental design, Le Manach et al.9 evaluated the risk of cardiac complications after infra‐renal aortic surgery when immediate, postoperative resumption of statins was adopted at their institution. The investigators compared the risk of developing MI, cardiac death, or abnormal troponin release in 491 patients who did not get early postoperative statin resumption (pre‐intervention group) to 178 patients who did. Statin withdrawal for 4 days was demonstrated to be an independent predictor of postoperative troponin leak and MI (OR 2.9, 95% CI 1.65.5). Similarly, Schouten et al.10 investigated the risk of adverse events related to interruption of long‐term statins by examining cardiac outcomes in 298 statin users undergoing major vascular surgery. Among the 70 patients who experienced statin withdrawal, an increased risk of postoperative troponin release (HR 4.6, 95% CI 2.29.6), and the composite endpoint of MI and cardiovascular death (HR 7.5, 95% CI 2.820.1), was observed compared to those who resumed treatment. Not unexpectedly, the most common reason for statin cessation was inability to take oral medications after surgery. However, even in patients who discontinued statins, the use of extended‐release fluvastatin was associated with fewer perioperative cardiac events than other statins. Furthermore, extended‐release fluvastatin was also held for 2 days following surgery (owing to inability to take the drug orally), in 25% of patients in the DECREASE‐III study. However, no impact in the rate of adverse outcomes was noted despite this interruption (OR 1.1, 95% CI 0.482.52).6 Although the authors surmised that the extended formulation of fluvastatin had provided sustained levels of statin activity despite lack of timely oral intake, it is important to note that this theory may not be generalizable to chronic statin users, as they were not enrolled in this study. Conversely, some patients may have experienced postoperative ileus for longer than 2 days, perhaps resulting in confounding or attenuation of the effect noted in the study.
CLINICAL INSIGHTS INTO FAILURE OF POSTOPERATIVE STATIN RESUMPTION
We hypothesize that failure to resume perioperative statins may occur for 4 cardinal reasons. First, resumption of an oral agent frequently proves clinically challenging when complications such as postoperative ileus, nausea, and vomiting peak. To date, no intravenous statin formulations are available, although phase‐I studies are currently underway.38 Second, it is not inconceivable that perioperative clinical instability may overshadow the resumption of statin treatment. Third, clinicians may also remain concerned regarding adverse effects of statins, a thought compounded by US Food and Drug Administration statin package inserts that specifically advocate for statins to be withheld during surgery. However, although the occurrence of elevated liver function tests and myopathy are theoretically important, the overwhelming majority of perioperative statin studies in noncardiac surgery have not found this to be a major occurrence.39 Nonetheless, a lack of uniform definitions and appropriate surveillance for adverse events are important limitations to this finding. In our recent systematic review, we were unable to provide refined estimates of these important side effects owing to differences in definition, variations in screening, and absence of standardized cutoffs used in studies.31 Finally, an important reason for failing to resume postoperative statins is that many physicians simply fail to recognize the perioperative importance of these agents.
STRATEGIES TO IMPROVE PERIOPERATIVE STATIN RESUMPTION
Using the existing evidence, we propose the following 4 clinical strategies to assist in avoiding a statin withdrawal state.
Nasogastric Administration
Utilizing a post‐pyloric nasogastric tube is a straightforward solution to provide statins in those who cannot otherwise tolerate oral intake due to nausea or emesis. Although this solution is hardly innovative, it is relevant as it forces consideration of the need to resume postoperative statins by available means. While the development of a high nasogastric output or a prolonged ileus may limit the applicability of this intervention, it is important that this option be considered as opposed to expectant watching for the clinical return of bowel function. Simvastatin, atorvastatin, rosuvastatin, and pravastatin can be crushed and delivered through this route.40
Development of Reminder Systems
Computerized reminder systems have proved important in ensuring the resumption of deep venous thrombosis prophylaxis and other preventative care compliance in hospitalized patients.41, 42 Using this process, pharmacist‐ or electronic health record‐based reminder systems could be implemented to ensure that statins are restarted when clinically feasible. Further studies are needed to test whether this approach can lead to improved outcomes.
Medication Reconciliation Prior to Hospital Discharge
Statin withdrawal highlights the pertinence of a robust, medical reconciliation process prior to the patient's departure from the hospital. In this context, the development of policies using single‐ or multi‐faceted interventions that promote cooperation between inpatient physicians, surgeons, and pharmacists with outpatient primary care providers are necessary.43
Preoperative Transition to Extended Release Statin Formulations
An innovative approach to minimizing statin withdrawal involves preoperative transition to an extended‐release statin formulation. This strategy may be of particular value in patients where prolonged bowel nonavailability is likely, such as those undergoing gastrointestinal surgery, or when prolonged postoperative dietary restriction (eg, NPO [nil per os]: nothing by mouth) status is expected (Figure 2).
CONCLUSIONS AND FUTURE DIRECTIONS
Sudden withdrawal of perioperative statins results in adverse clinical outcomes. Individuals engaged in the care of patients during surgery such as hospitalists, anesthesiologists, and surgeons must become more cognizant of a statin withdrawal state.
An important limitation associated with the study of perioperative statin withdrawal remains the ambiguity regarding the extent of the problem in the United States. Therefore, a logical first step could be the use of infrastructure within the National Surgical Quality Improvement Program (NSQIP) to understand the epidemiology of perioperative statin use and consequences associated with statin discontinuation.44 Mandating such quality reporting could easily be built into current NSQIP performance metrics. These data would help inform a research agenda targeting patients that experience statin withdrawal and strategies most likely to prevent it.
Note Added in Proof
Disclosure: Nothing to report.
- , , , et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39(5):967–975.
- , , , , , . Improved postoperative outcomes associated with preoperative statin therapy. Anesthesiology. 2006;105(6):1260–1272; quiz 1289–1290.
- , , , , . Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092–2099.
- , , , et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol. 2005;45(3):336–342.
- , , , et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation. 2003;107(14):1848–1851.
- , , , et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980–989.
- , , , et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):e418–e499.
- , , , et al. Guidelines for pre‐operative cardiac risk assessment and perioperative cardiac management in non‐cardiac surgery. Eur Heart J. 2009;30(22):2769–2812.
- , , , et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg. 2007;104(6):1326–1333.
- , , , et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316–320.
- . Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 suppl 1):III39–III43.
- , , , , , . Persistent endocrine stress response in patients undergoing cardiac surgery. J Endocrinol Invest. 1998;21(1):12–19.
- , , , , , . Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol. 1996;57(1):37–44.
- , , , , , . Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J. 2005;173(6):627–634.
- . Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153–184.
- , . Isoprenoid metabolism and the pleiotropic effects of statins. Curr Atheroscler Rep. 2003;5(5):372–378.
- , , , . Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97(12):1129–1135.
- , , , et al. Roles of rho‐associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49(6):698–705.
- , , , et al. Inhibition of rho‐associated kinase results in suppression of neointimal formation of balloon‐injured arteries. Circulation. 2000;101(17):2030–2033.
- , , , . Reduced expression of endothelial cell markers after 1 year treatment with simvastatin and atorvastatin in patients with coronary heart disease. Atherosclerosis. 2002;162(1):179–185.
- , , , et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108(10):1429–1437.
- , , . Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37–44.
- , , , et al. Inhibition of geranylgeranylation reduces angiotensin II‐mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59(3):646–654.
- , , , et al. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke. 2003;34(2):551–557.
- , , , et al. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102(25):3104–3110.
- , , , et al. Changes of plasma inflammatory markers after withdrawal of statin therapy in patients with hyperlipidemia. Clin Chim Acta. 2006;366(1–2):269–273.
- , , , et al. Platelet hyperactivity after statin treatment discontinuation. Thromb Haemost. 2003;90(3):476–482.
- , , , et al. Rebound inflammatory response during the acute phase of myocardial infarction after simvastatin withdrawal. Atherosclerosis. 2009;207(1):191–194.
- , . Effects of statins on endothelium and signaling mechanisms. Stroke. 2004;35(11 suppl 1):2708–2711.
- , , , et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- , , , et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta‐analysis. Arch Surg. 2012;147(2):181–189.
- , , , et al. Early withdrawal of statin therapy in patients with non‐ST‐segment elevation myocardial infarction: National Registry of Myocardial Infarction. Arch Intern Med. 2004;164(19):2162–2168.
- , , , , , . Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105(12):1446–1452.
- , , , , , . Discontinuation of statin therapy following an acute myocardial infarction: a population‐based study. Eur Heart J. 2008;29(17):2083–2091.
- . There is no evidence for an increase in acute coronary syndromes after short‐term abrupt discontinuation of statins in stable cardiac patients. Circulation. 2004;110(16):2333–2335.
- , , , . Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke. 2007;38(10):2652–2657.
- , , , et al. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69(9):904–910.
- , , , et al. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39(2):433–438.
- , , , et al. Safety of perioperative statin use in high‐risk patients undergoing major vascular surgery. Am J Cardiol. 2005;95(5):658–660.
- Lexi‐Comp Online™, Lexi‐Drugs™, Hudson, OH: Lexi‐Comp, Inc; December 7, 2011.
- , , , , , . A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965–970.
- , , , et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–977.
- , , , . Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: a systematic review. Br J Clin Pharmacol. January 13, 2012. doi: 10.1111/j.1365–2125.2012.04178.x.
- American College of Surgeons National Surgical Quality Improvement Program. Available at: http://www.acsnsqip.org. Accessed December 15, 2012.
Accumulating evidence suggests that perioperative treatment with 3‐hydroxy‐3‐methylglutaryl coenzyme‐A (HMG‐CoA) reductase inhibitors (or, statins) reduces the incidence of cardiovascular events during noncardiac surgery.16 This evidence has lead the European Society of Cardiology (ESC) and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) to endorse the use of perioperative statins in patients already on this treatment or those at high‐risk of cardiovascular events.7, 8
However, statins are available only in oral formulation. Consequently, prolonged bowel recovery or clinical instability may interfere with use during surgery. Furthermore, many clinicians may not recognize the imperative of postoperative statin resumption, viewing them principally as lipid‐lowering entities and not as agents of perioperative benefit. Failure to resume statins postoperatively can be catastrophic, as the ensuing inflammation and thrombosis frequently culminates in myocardial infarction (MI) or death.9, 10
In this article, we review the potent anti‐inflammatory properties of statins and their role in preventing perioperative cardiac events. We outline the biochemical basis for perioperative statin benefit, summarizing the basic, clinical, and experimental evidence regarding statin withdrawal. We conclude by presenting strategies to avert postoperative statin cessation and outline a research agenda dedicated to informing this practice.
METHODS
We performed a literature search using MEDLINE via Ovid (1946present), EMBASE (1946present), Biosis (1926present), and Cochrane CENTRAL (1960present). We used Boolean logic to search for key terms including statins, 3‐hydroxy‐3‐methylglutaryl CoA reductase inhibitors, death, MI, stroke, acute coronary syndrome (ACS), and statin withdrawal or cessation. All studies published in full‐text or abstract form were included. A total of 489 articles were retrieved by this search (last updated March 15, 2012). For this narrative review, we focused on studies that examined adverse outcomes associated with statin withdrawal.
BIOCHEMICAL BASIS OF STATIN PLEIOTROPICITY
The nonlipid‐lowering or pleiotropic properties of statins are especially valuable in the perioperative setting.16, 11 Perioperative cardiac complications occur due to oxygen supply:demand mismatch, vascular inflammation, or a combination of these states. A significant perisurgical catecholamine surge produces unopposed sympathetic effects,12 increasing the risk of rupture of vulnerable coronary plaques, thrombus formation, and adverse cardiac events.13, 14 Similarly, augmented inflammatory responses and increased circulating coagulation factors further predispose to a hazardous perioperative milieu.15 Statins attenuate this vascular inflammatory response by suppressing the synthesis of mevalonate by inhibiting HMG‐CoA reductase. Suppression of mevalonate synthesis reduces the bioavailability of 2 important isoprenoid molecules: farnesyl‐pyrophosphate and geranylgeranyl‐pyrophosphate.16 Diminution of these isoprenoid intermediaries leads to reductions in the active intracellular signaling molecules Ras, Rho, and Rac, which play critical roles in vascular reactivity, endothelial function, and coagulation and inflammatory pathways.1723 The cumulative effect of these cellular changes is diminished inflammation during periods of surgical stress (Figure 1).

While the perioperative pleiotropicity of statins is of inherent clinical value, several studies have shown that these effects are lost and even reversed when statins are withdrawn.2428 During statin treatment, absence of isoprenoid intermediaries induces cytosolic accumulation of nonactivated Rho and Rac proteins. Abrupt cessation of statins activates Rho/Rac‐kinase pathways, leading to unregulated inflammation, platelet hyper‐activation, and endothelial dysfunction.24, 25, 28, 29 For instance, statin withdrawal in mice‐models leads to an overshoot activation of Rho, resulting in down‐regulation of endothelial nitric oxide production,25 activation of nicotinamide adenine dinucleotide phosphate (NAD[P]H)‐oxidase, and increased superoxide production.29 In another mouse‐model, statin withdrawal was associated with up‐regulation of key pro‐thrombotic molecules including platelet factor 4 and beta‐thromboglobulin.24 In human studies, a platelet hyper‐activation state (manifested by increased platelet P‐selectin expression and enhanced platelet aggregation) occurs after statin discontinuation.27 Furthermore, withdrawal of statins in patients with hyperlipidemia increases inflammatory markers such as C‐reactive protein and interleukin‐6.26 In the perioperative context, absence of these important anti‐inflammatory properties increases the risk of cardiac events.9, 10
EVIDENCE SUGGESTING BENEFIT FROM PERIOPERATIVE STATIN TREATMENT
Retrospective studies first suggested clinical benefit from perioperative statin treatment. In a case‐control study involving 2816 patients undergoing vascular surgery at Erasmus Medical Center, statin use was associated with substantially decreased postoperative mortality (adjusted odd ratio [OR] 0.22, 95% confidence interval [CI] 0.100.47).5 In a subsequent retrospective cohort study of 780,591 patients who underwent major noncardiac surgery, the risk of postoperative mortality was considerably lower among statin users (unadjusted OR 0.68, 95% CI 0.640.72) compared to patients who did not receive, or received delayed treatment with statins.3 A third retrospective study of 1163 vascular surgery patients found that statins prevented perioperative cardiac complications including death, MI, congestive heart failure, and ventricular tachyarrhythmias (OR 0.52, 95% CI 0.350.76).4
The benefit from statin treatment found in retrospective studies prompted the first double‐blinded, randomized controlled trial (RCT) of perioperative statin use. In 2004, Durazzo and colleagues1 randomized 100 statin‐naive patients scheduled to undergo elective aortic, femoro‐popliteal, or carotid surgery to receive either 20 mg of atorvastatin or placebo for 45 days. Vascular surgery was performed, on average, 31 days after randomization. Atorvastatin therapy reduced the incidence of death from cardiac causes, nonfatal acute MI, ischemic stroke, and unstable angina (26% in the placebo group vs 8% in the atorvastatin group; P = 0.031).1 Although the small size of the trial rendered it underpowered to show a mortality benefit, this remains the first RCT to demonstrate a protective perioperative effect of statins.
In the 2009 Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE)‐III trial, Schouten and colleagues6 randomized 497 high‐risk, statin‐naive patients undergoing vascular surgery to receive, in addition to a beta‐blocker, either fluvastatin or placebo before surgery (median of 37 days). Postoperative myocardial ischemia (hazard ratio [HR] 0.55, 95% CI 0.340.88), and combined death from cardiovascular causes or nonfatal MI (HR 0.47, 95% CI 0.240.94), occurred less frequently in the treatment group.6 In 2009, the same group published DECREASE‐IV, a multicenter, prospective, open‐label, 2 2 factorial design trial of 1066 intermediate‐risk patients, scheduled to undergo elective, noncardiac surgery. Patients were assigned to bisoprolol, fluvastatin, combination treatment, or control therapy before surgery (median of 34 days). Although those randomized to fluvastatin demonstrated lower incidence of 30‐day cardiac death and MI than control (HR 0.65, 95% CI 0.351.10), these outcomes failed to reach statistical significance as the trial was principally powered to examine the effects of perioperative beta‐blockade.30
Using this pool of data, a meta‐analysis of 15 studies (223,010 patients) found a substantial 38% reduction in the risk of mortality after cardiac surgery (1.9% vs 3.1%; P = 0.0001) and an even greater 59% reduction in the risk of mortality following vascular surgery (1.7% vs 6.1%; P = 0.0001) with perioperative statin therapy. When including noncardiac surgery, a 44% reduction in mortality was observed (2.2% vs 3.2%; P < 0.01).2 We performed a similar meta‐analysis of 15 RCTs involving 2292 patients to determine whether perioperative statin treatment in statin‐naive patients, undergoing either cardiac or noncardiac surgery, improved clinical outcomes. Our analysis also found statistically significant reductions in the risk of MI associated with perioperative statin use in both cardiac and noncardiac surgery (risk reduction [RR] 0.53, 95% CI 0.380.74) and atrial fibrillation in statin‐naive patients undergoing cardiac surgery (RR 0.56, 95% CI 0.450.69).31 Taken together, a large volume of evidence supports the use of statins in surgical settings.
In view of this evidence, the ACCF/AHA perioperative guidelines for noncardiac surgery endorsed statins as an important risk‐reducing intervention in those undergoing noncardiac surgery, and recommended continued use in patients on chronic statin treatment scheduled for noncardiac surgery (Level of Evidence B, Class I; Benefits >>> Risk). Initiating statins in patients undergoing vascular surgery, with or without risk factors, was considered reasonable (Level of Evidence B, Class IIa; Benefits >> Risk).7 Current ESC perioperative guidelines in noncardiac surgery offer similar recommendations to those of ACCF/AHA, but differ by categorizing the recommendation to initiate statins in patients at high cardiovascular risk as a Class I recommendation.8
CLINICAL CONSEQUENCES OF STATIN WITHDRAWAL
Although statins provide important cardiac benefits, an important limitation to their perioperative use remains their oral‐only formulation. Thus, patients who are unable to resume oral intake may fail to resume treatment. Perioperative statin cessation has been hypothesized to lead to a statin withdrawal phenomenon. The evidence that supports the existence of this phenomenon comes from 3 distinct populations: ACS, ischemic stroke, and perioperative patients (Table 1).
| Author (ref) Year | Design | Study Population | Sample Size (N) (Total/ Continuation/Discontinuation) | Clinical Setting and Context | Timing of Statin Discontinuation | Study Outcome | Results (Withdrawal vs Continuation) |
|---|---|---|---|---|---|---|---|
| |||||||
| Heeschen et al33 2002 | Retrospective cohort | Mostly men (68%) in early 60s | 1616/379/86 | Chest pain in ACS | During or after admission | Incidence of death and nonfatal MI | OR (95% CI) |
| 2.93 (1.646.27) | |||||||
| Spencer et al32 2004 | Retrospective cohort | Mostly men (62%) in late 60s | 68,506/9001/487 | NonST‐segment elevation MI | During the first 24 hr of hospitalization | In‐hospital death | HR (95% CI) |
| 1.83 (1.582.13) | |||||||
| Daskalopoulou et al34 2008 | Retrospective cohort | 60% men in late 60s | 9939/2026/137 Non‐users 2124 (reference group) | Acute MI | Within 1 yr of the coronary event | 1‐yr all‐cause mortality | HR (95% CI) |
| 1.88 (1.133.07)* | |||||||
| Colivicchi et al36 2007 | Prospective cohort | 51% men in early 70s | 631/385/246 | Ischemic stroke | Mean 48.6 days | 1‐yr all‐cause mortality | HR (95% CI) |
| 2.78 (1.963.72) | |||||||
| Blanco et al37 2007 | RCT | 51% men in mid‐60s | 215/46/43 | Ischemic stroke | N/A | Risk of death or dependency at 3 mo | OR (95% CI) |
| 4.66 (1.4614.91) | |||||||
| Early neurologic deterioration | OR (95% CI) | ||||||
| 7.08 (2.7318.37) | |||||||
| Le Manach et al9 2007 | Quasi‐experimental (prepost) | Mostly men (89%) in late‐60s | 669/178/491 | Infra‐renal aortic surgery | Median of 4 days off statins | Postoperative troponin release, MI | OR (95% CI) |
| 2.9 (1.65.5) | |||||||
| Schouten et al10 2007 | Prospective cohort | Mostly men (75%) in mid‐60s | 298/228/70 | Aortic and lower extremity vascular surgery | Median of 3 days off statins | Postoperative troponin release | HR (95% CI) |
| 4.6 (2.29.6) | |||||||
| Combination of postoperative MI and CV death | HR (95% CI) | ||||||
| 7.5 (2.820.1) | |||||||
| Schouten et al6 2009 | RCT | Mostly men (75%) in mid‐60s | 250/189/61 | Vascular surgery (carotid, abdominal aortic, endovascular, and lower extremity arterial) | Median of 2 days off statins | Postoperative myocardial ischemia and combined death from cardiovascular causes or nonfatal MI | OR (95% CI) |
| 1.1 (0.482.52) | |||||||
Statin Withdrawal in Acute Coronary Syndromes
Several studies have demonstrated an association between statin withdrawal and heightened risk of cardiovascular events in ACS.3234 In a retrospective analysis of 1616 patients presenting with ACS, withdrawal of statins during or after admission was associated with more frequent death and nonfatal MI compared to those who continued therapy (OR 2.93, 95% CI 1.646.27).33 In another retrospective observational study of 68,606 nonST‐segment elevation MI patients, statin cessation during the first 24 hours of hospitalization was independently associated with adverse outcomes including in‐hospital death (adjusted HR 1.83; 95% CI 1.582.13), cardiac arrest, and cardiogenic shock.32 In a population‐based, cohort study in the United Kingdom, statin cessation following an acute MI was independently associated with greater all‐cause mortality at 1‐year (adjusted HR 1.88, 95% CI 1.133.07).34
The significantly increased risk of adverse outcomes associated with the interruption of statins in ACS may be moderated by vascular inflammation related to the inciting coronary event, as statin discontinuation in patients with stable cardiac conditions was not associated with increased risk of cardiovascular events in a large‐scale, double‐blind, parallel‐group study.35
Statin Withdrawal in Ischemic Stroke
Adverse events associated with statin withdrawal have also been reported in patients with cerebrovascular disease. In a prospective observational study of 631 consecutive stroke survivors, those who discontinued statins (owing to mild adverse effects or unclear reasons) experienced increased mortality during the first year after the event (adjusted HR 2.78, 95% CI 1.963.72).36 Using a controversial study design aimed at evaluating the effects of stopping oral intake (including chronic medications) during the first days of acute stroke, Blanco and colleagues37 randomized 89 stroke victims on chronic statins to either continue medications or experience statin withdrawal following admission. Statin withdrawal was independently associated with increased risk of mortality and dependency at 3 months (OR 4.66, 95% CI 1.4614.91).37
Perioperative Statin Withdrawal
In the perioperative setting, statin withdrawal has also been associated with adverse outcomes. Using a quasi‐experimental design, Le Manach et al.9 evaluated the risk of cardiac complications after infra‐renal aortic surgery when immediate, postoperative resumption of statins was adopted at their institution. The investigators compared the risk of developing MI, cardiac death, or abnormal troponin release in 491 patients who did not get early postoperative statin resumption (pre‐intervention group) to 178 patients who did. Statin withdrawal for 4 days was demonstrated to be an independent predictor of postoperative troponin leak and MI (OR 2.9, 95% CI 1.65.5). Similarly, Schouten et al.10 investigated the risk of adverse events related to interruption of long‐term statins by examining cardiac outcomes in 298 statin users undergoing major vascular surgery. Among the 70 patients who experienced statin withdrawal, an increased risk of postoperative troponin release (HR 4.6, 95% CI 2.29.6), and the composite endpoint of MI and cardiovascular death (HR 7.5, 95% CI 2.820.1), was observed compared to those who resumed treatment. Not unexpectedly, the most common reason for statin cessation was inability to take oral medications after surgery. However, even in patients who discontinued statins, the use of extended‐release fluvastatin was associated with fewer perioperative cardiac events than other statins. Furthermore, extended‐release fluvastatin was also held for 2 days following surgery (owing to inability to take the drug orally), in 25% of patients in the DECREASE‐III study. However, no impact in the rate of adverse outcomes was noted despite this interruption (OR 1.1, 95% CI 0.482.52).6 Although the authors surmised that the extended formulation of fluvastatin had provided sustained levels of statin activity despite lack of timely oral intake, it is important to note that this theory may not be generalizable to chronic statin users, as they were not enrolled in this study. Conversely, some patients may have experienced postoperative ileus for longer than 2 days, perhaps resulting in confounding or attenuation of the effect noted in the study.
CLINICAL INSIGHTS INTO FAILURE OF POSTOPERATIVE STATIN RESUMPTION
We hypothesize that failure to resume perioperative statins may occur for 4 cardinal reasons. First, resumption of an oral agent frequently proves clinically challenging when complications such as postoperative ileus, nausea, and vomiting peak. To date, no intravenous statin formulations are available, although phase‐I studies are currently underway.38 Second, it is not inconceivable that perioperative clinical instability may overshadow the resumption of statin treatment. Third, clinicians may also remain concerned regarding adverse effects of statins, a thought compounded by US Food and Drug Administration statin package inserts that specifically advocate for statins to be withheld during surgery. However, although the occurrence of elevated liver function tests and myopathy are theoretically important, the overwhelming majority of perioperative statin studies in noncardiac surgery have not found this to be a major occurrence.39 Nonetheless, a lack of uniform definitions and appropriate surveillance for adverse events are important limitations to this finding. In our recent systematic review, we were unable to provide refined estimates of these important side effects owing to differences in definition, variations in screening, and absence of standardized cutoffs used in studies.31 Finally, an important reason for failing to resume postoperative statins is that many physicians simply fail to recognize the perioperative importance of these agents.
STRATEGIES TO IMPROVE PERIOPERATIVE STATIN RESUMPTION
Using the existing evidence, we propose the following 4 clinical strategies to assist in avoiding a statin withdrawal state.
Nasogastric Administration
Utilizing a post‐pyloric nasogastric tube is a straightforward solution to provide statins in those who cannot otherwise tolerate oral intake due to nausea or emesis. Although this solution is hardly innovative, it is relevant as it forces consideration of the need to resume postoperative statins by available means. While the development of a high nasogastric output or a prolonged ileus may limit the applicability of this intervention, it is important that this option be considered as opposed to expectant watching for the clinical return of bowel function. Simvastatin, atorvastatin, rosuvastatin, and pravastatin can be crushed and delivered through this route.40
Development of Reminder Systems
Computerized reminder systems have proved important in ensuring the resumption of deep venous thrombosis prophylaxis and other preventative care compliance in hospitalized patients.41, 42 Using this process, pharmacist‐ or electronic health record‐based reminder systems could be implemented to ensure that statins are restarted when clinically feasible. Further studies are needed to test whether this approach can lead to improved outcomes.
Medication Reconciliation Prior to Hospital Discharge
Statin withdrawal highlights the pertinence of a robust, medical reconciliation process prior to the patient's departure from the hospital. In this context, the development of policies using single‐ or multi‐faceted interventions that promote cooperation between inpatient physicians, surgeons, and pharmacists with outpatient primary care providers are necessary.43
Preoperative Transition to Extended Release Statin Formulations
An innovative approach to minimizing statin withdrawal involves preoperative transition to an extended‐release statin formulation. This strategy may be of particular value in patients where prolonged bowel nonavailability is likely, such as those undergoing gastrointestinal surgery, or when prolonged postoperative dietary restriction (eg, NPO [nil per os]: nothing by mouth) status is expected (Figure 2).

CONCLUSIONS AND FUTURE DIRECTIONS
Sudden withdrawal of perioperative statins results in adverse clinical outcomes. Individuals engaged in the care of patients during surgery such as hospitalists, anesthesiologists, and surgeons must become more cognizant of a statin withdrawal state.
An important limitation associated with the study of perioperative statin withdrawal remains the ambiguity regarding the extent of the problem in the United States. Therefore, a logical first step could be the use of infrastructure within the National Surgical Quality Improvement Program (NSQIP) to understand the epidemiology of perioperative statin use and consequences associated with statin discontinuation.44 Mandating such quality reporting could easily be built into current NSQIP performance metrics. These data would help inform a research agenda targeting patients that experience statin withdrawal and strategies most likely to prevent it.
Note Added in Proof
Disclosure: Nothing to report.
Accumulating evidence suggests that perioperative treatment with 3‐hydroxy‐3‐methylglutaryl coenzyme‐A (HMG‐CoA) reductase inhibitors (or, statins) reduces the incidence of cardiovascular events during noncardiac surgery.16 This evidence has lead the European Society of Cardiology (ESC) and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) to endorse the use of perioperative statins in patients already on this treatment or those at high‐risk of cardiovascular events.7, 8
However, statins are available only in oral formulation. Consequently, prolonged bowel recovery or clinical instability may interfere with use during surgery. Furthermore, many clinicians may not recognize the imperative of postoperative statin resumption, viewing them principally as lipid‐lowering entities and not as agents of perioperative benefit. Failure to resume statins postoperatively can be catastrophic, as the ensuing inflammation and thrombosis frequently culminates in myocardial infarction (MI) or death.9, 10
In this article, we review the potent anti‐inflammatory properties of statins and their role in preventing perioperative cardiac events. We outline the biochemical basis for perioperative statin benefit, summarizing the basic, clinical, and experimental evidence regarding statin withdrawal. We conclude by presenting strategies to avert postoperative statin cessation and outline a research agenda dedicated to informing this practice.
METHODS
We performed a literature search using MEDLINE via Ovid (1946present), EMBASE (1946present), Biosis (1926present), and Cochrane CENTRAL (1960present). We used Boolean logic to search for key terms including statins, 3‐hydroxy‐3‐methylglutaryl CoA reductase inhibitors, death, MI, stroke, acute coronary syndrome (ACS), and statin withdrawal or cessation. All studies published in full‐text or abstract form were included. A total of 489 articles were retrieved by this search (last updated March 15, 2012). For this narrative review, we focused on studies that examined adverse outcomes associated with statin withdrawal.
BIOCHEMICAL BASIS OF STATIN PLEIOTROPICITY
The nonlipid‐lowering or pleiotropic properties of statins are especially valuable in the perioperative setting.16, 11 Perioperative cardiac complications occur due to oxygen supply:demand mismatch, vascular inflammation, or a combination of these states. A significant perisurgical catecholamine surge produces unopposed sympathetic effects,12 increasing the risk of rupture of vulnerable coronary plaques, thrombus formation, and adverse cardiac events.13, 14 Similarly, augmented inflammatory responses and increased circulating coagulation factors further predispose to a hazardous perioperative milieu.15 Statins attenuate this vascular inflammatory response by suppressing the synthesis of mevalonate by inhibiting HMG‐CoA reductase. Suppression of mevalonate synthesis reduces the bioavailability of 2 important isoprenoid molecules: farnesyl‐pyrophosphate and geranylgeranyl‐pyrophosphate.16 Diminution of these isoprenoid intermediaries leads to reductions in the active intracellular signaling molecules Ras, Rho, and Rac, which play critical roles in vascular reactivity, endothelial function, and coagulation and inflammatory pathways.1723 The cumulative effect of these cellular changes is diminished inflammation during periods of surgical stress (Figure 1).

While the perioperative pleiotropicity of statins is of inherent clinical value, several studies have shown that these effects are lost and even reversed when statins are withdrawn.2428 During statin treatment, absence of isoprenoid intermediaries induces cytosolic accumulation of nonactivated Rho and Rac proteins. Abrupt cessation of statins activates Rho/Rac‐kinase pathways, leading to unregulated inflammation, platelet hyper‐activation, and endothelial dysfunction.24, 25, 28, 29 For instance, statin withdrawal in mice‐models leads to an overshoot activation of Rho, resulting in down‐regulation of endothelial nitric oxide production,25 activation of nicotinamide adenine dinucleotide phosphate (NAD[P]H)‐oxidase, and increased superoxide production.29 In another mouse‐model, statin withdrawal was associated with up‐regulation of key pro‐thrombotic molecules including platelet factor 4 and beta‐thromboglobulin.24 In human studies, a platelet hyper‐activation state (manifested by increased platelet P‐selectin expression and enhanced platelet aggregation) occurs after statin discontinuation.27 Furthermore, withdrawal of statins in patients with hyperlipidemia increases inflammatory markers such as C‐reactive protein and interleukin‐6.26 In the perioperative context, absence of these important anti‐inflammatory properties increases the risk of cardiac events.9, 10
EVIDENCE SUGGESTING BENEFIT FROM PERIOPERATIVE STATIN TREATMENT
Retrospective studies first suggested clinical benefit from perioperative statin treatment. In a case‐control study involving 2816 patients undergoing vascular surgery at Erasmus Medical Center, statin use was associated with substantially decreased postoperative mortality (adjusted odd ratio [OR] 0.22, 95% confidence interval [CI] 0.100.47).5 In a subsequent retrospective cohort study of 780,591 patients who underwent major noncardiac surgery, the risk of postoperative mortality was considerably lower among statin users (unadjusted OR 0.68, 95% CI 0.640.72) compared to patients who did not receive, or received delayed treatment with statins.3 A third retrospective study of 1163 vascular surgery patients found that statins prevented perioperative cardiac complications including death, MI, congestive heart failure, and ventricular tachyarrhythmias (OR 0.52, 95% CI 0.350.76).4
The benefit from statin treatment found in retrospective studies prompted the first double‐blinded, randomized controlled trial (RCT) of perioperative statin use. In 2004, Durazzo and colleagues1 randomized 100 statin‐naive patients scheduled to undergo elective aortic, femoro‐popliteal, or carotid surgery to receive either 20 mg of atorvastatin or placebo for 45 days. Vascular surgery was performed, on average, 31 days after randomization. Atorvastatin therapy reduced the incidence of death from cardiac causes, nonfatal acute MI, ischemic stroke, and unstable angina (26% in the placebo group vs 8% in the atorvastatin group; P = 0.031).1 Although the small size of the trial rendered it underpowered to show a mortality benefit, this remains the first RCT to demonstrate a protective perioperative effect of statins.
In the 2009 Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE)‐III trial, Schouten and colleagues6 randomized 497 high‐risk, statin‐naive patients undergoing vascular surgery to receive, in addition to a beta‐blocker, either fluvastatin or placebo before surgery (median of 37 days). Postoperative myocardial ischemia (hazard ratio [HR] 0.55, 95% CI 0.340.88), and combined death from cardiovascular causes or nonfatal MI (HR 0.47, 95% CI 0.240.94), occurred less frequently in the treatment group.6 In 2009, the same group published DECREASE‐IV, a multicenter, prospective, open‐label, 2 2 factorial design trial of 1066 intermediate‐risk patients, scheduled to undergo elective, noncardiac surgery. Patients were assigned to bisoprolol, fluvastatin, combination treatment, or control therapy before surgery (median of 34 days). Although those randomized to fluvastatin demonstrated lower incidence of 30‐day cardiac death and MI than control (HR 0.65, 95% CI 0.351.10), these outcomes failed to reach statistical significance as the trial was principally powered to examine the effects of perioperative beta‐blockade.30
Using this pool of data, a meta‐analysis of 15 studies (223,010 patients) found a substantial 38% reduction in the risk of mortality after cardiac surgery (1.9% vs 3.1%; P = 0.0001) and an even greater 59% reduction in the risk of mortality following vascular surgery (1.7% vs 6.1%; P = 0.0001) with perioperative statin therapy. When including noncardiac surgery, a 44% reduction in mortality was observed (2.2% vs 3.2%; P < 0.01).2 We performed a similar meta‐analysis of 15 RCTs involving 2292 patients to determine whether perioperative statin treatment in statin‐naive patients, undergoing either cardiac or noncardiac surgery, improved clinical outcomes. Our analysis also found statistically significant reductions in the risk of MI associated with perioperative statin use in both cardiac and noncardiac surgery (risk reduction [RR] 0.53, 95% CI 0.380.74) and atrial fibrillation in statin‐naive patients undergoing cardiac surgery (RR 0.56, 95% CI 0.450.69).31 Taken together, a large volume of evidence supports the use of statins in surgical settings.
In view of this evidence, the ACCF/AHA perioperative guidelines for noncardiac surgery endorsed statins as an important risk‐reducing intervention in those undergoing noncardiac surgery, and recommended continued use in patients on chronic statin treatment scheduled for noncardiac surgery (Level of Evidence B, Class I; Benefits >>> Risk). Initiating statins in patients undergoing vascular surgery, with or without risk factors, was considered reasonable (Level of Evidence B, Class IIa; Benefits >> Risk).7 Current ESC perioperative guidelines in noncardiac surgery offer similar recommendations to those of ACCF/AHA, but differ by categorizing the recommendation to initiate statins in patients at high cardiovascular risk as a Class I recommendation.8
CLINICAL CONSEQUENCES OF STATIN WITHDRAWAL
Although statins provide important cardiac benefits, an important limitation to their perioperative use remains their oral‐only formulation. Thus, patients who are unable to resume oral intake may fail to resume treatment. Perioperative statin cessation has been hypothesized to lead to a statin withdrawal phenomenon. The evidence that supports the existence of this phenomenon comes from 3 distinct populations: ACS, ischemic stroke, and perioperative patients (Table 1).
| Author (ref) Year | Design | Study Population | Sample Size (N) (Total/ Continuation/Discontinuation) | Clinical Setting and Context | Timing of Statin Discontinuation | Study Outcome | Results (Withdrawal vs Continuation) |
|---|---|---|---|---|---|---|---|
| |||||||
| Heeschen et al33 2002 | Retrospective cohort | Mostly men (68%) in early 60s | 1616/379/86 | Chest pain in ACS | During or after admission | Incidence of death and nonfatal MI | OR (95% CI) |
| 2.93 (1.646.27) | |||||||
| Spencer et al32 2004 | Retrospective cohort | Mostly men (62%) in late 60s | 68,506/9001/487 | NonST‐segment elevation MI | During the first 24 hr of hospitalization | In‐hospital death | HR (95% CI) |
| 1.83 (1.582.13) | |||||||
| Daskalopoulou et al34 2008 | Retrospective cohort | 60% men in late 60s | 9939/2026/137 Non‐users 2124 (reference group) | Acute MI | Within 1 yr of the coronary event | 1‐yr all‐cause mortality | HR (95% CI) |
| 1.88 (1.133.07)* | |||||||
| Colivicchi et al36 2007 | Prospective cohort | 51% men in early 70s | 631/385/246 | Ischemic stroke | Mean 48.6 days | 1‐yr all‐cause mortality | HR (95% CI) |
| 2.78 (1.963.72) | |||||||
| Blanco et al37 2007 | RCT | 51% men in mid‐60s | 215/46/43 | Ischemic stroke | N/A | Risk of death or dependency at 3 mo | OR (95% CI) |
| 4.66 (1.4614.91) | |||||||
| Early neurologic deterioration | OR (95% CI) | ||||||
| 7.08 (2.7318.37) | |||||||
| Le Manach et al9 2007 | Quasi‐experimental (prepost) | Mostly men (89%) in late‐60s | 669/178/491 | Infra‐renal aortic surgery | Median of 4 days off statins | Postoperative troponin release, MI | OR (95% CI) |
| 2.9 (1.65.5) | |||||||
| Schouten et al10 2007 | Prospective cohort | Mostly men (75%) in mid‐60s | 298/228/70 | Aortic and lower extremity vascular surgery | Median of 3 days off statins | Postoperative troponin release | HR (95% CI) |
| 4.6 (2.29.6) | |||||||
| Combination of postoperative MI and CV death | HR (95% CI) | ||||||
| 7.5 (2.820.1) | |||||||
| Schouten et al6 2009 | RCT | Mostly men (75%) in mid‐60s | 250/189/61 | Vascular surgery (carotid, abdominal aortic, endovascular, and lower extremity arterial) | Median of 2 days off statins | Postoperative myocardial ischemia and combined death from cardiovascular causes or nonfatal MI | OR (95% CI) |
| 1.1 (0.482.52) | |||||||
Statin Withdrawal in Acute Coronary Syndromes
Several studies have demonstrated an association between statin withdrawal and heightened risk of cardiovascular events in ACS.3234 In a retrospective analysis of 1616 patients presenting with ACS, withdrawal of statins during or after admission was associated with more frequent death and nonfatal MI compared to those who continued therapy (OR 2.93, 95% CI 1.646.27).33 In another retrospective observational study of 68,606 nonST‐segment elevation MI patients, statin cessation during the first 24 hours of hospitalization was independently associated with adverse outcomes including in‐hospital death (adjusted HR 1.83; 95% CI 1.582.13), cardiac arrest, and cardiogenic shock.32 In a population‐based, cohort study in the United Kingdom, statin cessation following an acute MI was independently associated with greater all‐cause mortality at 1‐year (adjusted HR 1.88, 95% CI 1.133.07).34
The significantly increased risk of adverse outcomes associated with the interruption of statins in ACS may be moderated by vascular inflammation related to the inciting coronary event, as statin discontinuation in patients with stable cardiac conditions was not associated with increased risk of cardiovascular events in a large‐scale, double‐blind, parallel‐group study.35
Statin Withdrawal in Ischemic Stroke
Adverse events associated with statin withdrawal have also been reported in patients with cerebrovascular disease. In a prospective observational study of 631 consecutive stroke survivors, those who discontinued statins (owing to mild adverse effects or unclear reasons) experienced increased mortality during the first year after the event (adjusted HR 2.78, 95% CI 1.963.72).36 Using a controversial study design aimed at evaluating the effects of stopping oral intake (including chronic medications) during the first days of acute stroke, Blanco and colleagues37 randomized 89 stroke victims on chronic statins to either continue medications or experience statin withdrawal following admission. Statin withdrawal was independently associated with increased risk of mortality and dependency at 3 months (OR 4.66, 95% CI 1.4614.91).37
Perioperative Statin Withdrawal
In the perioperative setting, statin withdrawal has also been associated with adverse outcomes. Using a quasi‐experimental design, Le Manach et al.9 evaluated the risk of cardiac complications after infra‐renal aortic surgery when immediate, postoperative resumption of statins was adopted at their institution. The investigators compared the risk of developing MI, cardiac death, or abnormal troponin release in 491 patients who did not get early postoperative statin resumption (pre‐intervention group) to 178 patients who did. Statin withdrawal for 4 days was demonstrated to be an independent predictor of postoperative troponin leak and MI (OR 2.9, 95% CI 1.65.5). Similarly, Schouten et al.10 investigated the risk of adverse events related to interruption of long‐term statins by examining cardiac outcomes in 298 statin users undergoing major vascular surgery. Among the 70 patients who experienced statin withdrawal, an increased risk of postoperative troponin release (HR 4.6, 95% CI 2.29.6), and the composite endpoint of MI and cardiovascular death (HR 7.5, 95% CI 2.820.1), was observed compared to those who resumed treatment. Not unexpectedly, the most common reason for statin cessation was inability to take oral medications after surgery. However, even in patients who discontinued statins, the use of extended‐release fluvastatin was associated with fewer perioperative cardiac events than other statins. Furthermore, extended‐release fluvastatin was also held for 2 days following surgery (owing to inability to take the drug orally), in 25% of patients in the DECREASE‐III study. However, no impact in the rate of adverse outcomes was noted despite this interruption (OR 1.1, 95% CI 0.482.52).6 Although the authors surmised that the extended formulation of fluvastatin had provided sustained levels of statin activity despite lack of timely oral intake, it is important to note that this theory may not be generalizable to chronic statin users, as they were not enrolled in this study. Conversely, some patients may have experienced postoperative ileus for longer than 2 days, perhaps resulting in confounding or attenuation of the effect noted in the study.
CLINICAL INSIGHTS INTO FAILURE OF POSTOPERATIVE STATIN RESUMPTION
We hypothesize that failure to resume perioperative statins may occur for 4 cardinal reasons. First, resumption of an oral agent frequently proves clinically challenging when complications such as postoperative ileus, nausea, and vomiting peak. To date, no intravenous statin formulations are available, although phase‐I studies are currently underway.38 Second, it is not inconceivable that perioperative clinical instability may overshadow the resumption of statin treatment. Third, clinicians may also remain concerned regarding adverse effects of statins, a thought compounded by US Food and Drug Administration statin package inserts that specifically advocate for statins to be withheld during surgery. However, although the occurrence of elevated liver function tests and myopathy are theoretically important, the overwhelming majority of perioperative statin studies in noncardiac surgery have not found this to be a major occurrence.39 Nonetheless, a lack of uniform definitions and appropriate surveillance for adverse events are important limitations to this finding. In our recent systematic review, we were unable to provide refined estimates of these important side effects owing to differences in definition, variations in screening, and absence of standardized cutoffs used in studies.31 Finally, an important reason for failing to resume postoperative statins is that many physicians simply fail to recognize the perioperative importance of these agents.
STRATEGIES TO IMPROVE PERIOPERATIVE STATIN RESUMPTION
Using the existing evidence, we propose the following 4 clinical strategies to assist in avoiding a statin withdrawal state.
Nasogastric Administration
Utilizing a post‐pyloric nasogastric tube is a straightforward solution to provide statins in those who cannot otherwise tolerate oral intake due to nausea or emesis. Although this solution is hardly innovative, it is relevant as it forces consideration of the need to resume postoperative statins by available means. While the development of a high nasogastric output or a prolonged ileus may limit the applicability of this intervention, it is important that this option be considered as opposed to expectant watching for the clinical return of bowel function. Simvastatin, atorvastatin, rosuvastatin, and pravastatin can be crushed and delivered through this route.40
Development of Reminder Systems
Computerized reminder systems have proved important in ensuring the resumption of deep venous thrombosis prophylaxis and other preventative care compliance in hospitalized patients.41, 42 Using this process, pharmacist‐ or electronic health record‐based reminder systems could be implemented to ensure that statins are restarted when clinically feasible. Further studies are needed to test whether this approach can lead to improved outcomes.
Medication Reconciliation Prior to Hospital Discharge
Statin withdrawal highlights the pertinence of a robust, medical reconciliation process prior to the patient's departure from the hospital. In this context, the development of policies using single‐ or multi‐faceted interventions that promote cooperation between inpatient physicians, surgeons, and pharmacists with outpatient primary care providers are necessary.43
Preoperative Transition to Extended Release Statin Formulations
An innovative approach to minimizing statin withdrawal involves preoperative transition to an extended‐release statin formulation. This strategy may be of particular value in patients where prolonged bowel nonavailability is likely, such as those undergoing gastrointestinal surgery, or when prolonged postoperative dietary restriction (eg, NPO [nil per os]: nothing by mouth) status is expected (Figure 2).

CONCLUSIONS AND FUTURE DIRECTIONS
Sudden withdrawal of perioperative statins results in adverse clinical outcomes. Individuals engaged in the care of patients during surgery such as hospitalists, anesthesiologists, and surgeons must become more cognizant of a statin withdrawal state.
An important limitation associated with the study of perioperative statin withdrawal remains the ambiguity regarding the extent of the problem in the United States. Therefore, a logical first step could be the use of infrastructure within the National Surgical Quality Improvement Program (NSQIP) to understand the epidemiology of perioperative statin use and consequences associated with statin discontinuation.44 Mandating such quality reporting could easily be built into current NSQIP performance metrics. These data would help inform a research agenda targeting patients that experience statin withdrawal and strategies most likely to prevent it.
Note Added in Proof
Disclosure: Nothing to report.
- , , , et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39(5):967–975.
- , , , , , . Improved postoperative outcomes associated with preoperative statin therapy. Anesthesiology. 2006;105(6):1260–1272; quiz 1289–1290.
- , , , , . Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092–2099.
- , , , et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol. 2005;45(3):336–342.
- , , , et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation. 2003;107(14):1848–1851.
- , , , et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980–989.
- , , , et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):e418–e499.
- , , , et al. Guidelines for pre‐operative cardiac risk assessment and perioperative cardiac management in non‐cardiac surgery. Eur Heart J. 2009;30(22):2769–2812.
- , , , et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg. 2007;104(6):1326–1333.
- , , , et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316–320.
- . Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 suppl 1):III39–III43.
- , , , , , . Persistent endocrine stress response in patients undergoing cardiac surgery. J Endocrinol Invest. 1998;21(1):12–19.
- , , , , , . Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol. 1996;57(1):37–44.
- , , , , , . Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J. 2005;173(6):627–634.
- . Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153–184.
- , . Isoprenoid metabolism and the pleiotropic effects of statins. Curr Atheroscler Rep. 2003;5(5):372–378.
- , , , . Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97(12):1129–1135.
- , , , et al. Roles of rho‐associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49(6):698–705.
- , , , et al. Inhibition of rho‐associated kinase results in suppression of neointimal formation of balloon‐injured arteries. Circulation. 2000;101(17):2030–2033.
- , , , . Reduced expression of endothelial cell markers after 1 year treatment with simvastatin and atorvastatin in patients with coronary heart disease. Atherosclerosis. 2002;162(1):179–185.
- , , , et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108(10):1429–1437.
- , , . Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37–44.
- , , , et al. Inhibition of geranylgeranylation reduces angiotensin II‐mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59(3):646–654.
- , , , et al. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke. 2003;34(2):551–557.
- , , , et al. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102(25):3104–3110.
- , , , et al. Changes of plasma inflammatory markers after withdrawal of statin therapy in patients with hyperlipidemia. Clin Chim Acta. 2006;366(1–2):269–273.
- , , , et al. Platelet hyperactivity after statin treatment discontinuation. Thromb Haemost. 2003;90(3):476–482.
- , , , et al. Rebound inflammatory response during the acute phase of myocardial infarction after simvastatin withdrawal. Atherosclerosis. 2009;207(1):191–194.
- , . Effects of statins on endothelium and signaling mechanisms. Stroke. 2004;35(11 suppl 1):2708–2711.
- , , , et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- , , , et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta‐analysis. Arch Surg. 2012;147(2):181–189.
- , , , et al. Early withdrawal of statin therapy in patients with non‐ST‐segment elevation myocardial infarction: National Registry of Myocardial Infarction. Arch Intern Med. 2004;164(19):2162–2168.
- , , , , , . Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105(12):1446–1452.
- , , , , , . Discontinuation of statin therapy following an acute myocardial infarction: a population‐based study. Eur Heart J. 2008;29(17):2083–2091.
- . There is no evidence for an increase in acute coronary syndromes after short‐term abrupt discontinuation of statins in stable cardiac patients. Circulation. 2004;110(16):2333–2335.
- , , , . Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke. 2007;38(10):2652–2657.
- , , , et al. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69(9):904–910.
- , , , et al. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39(2):433–438.
- , , , et al. Safety of perioperative statin use in high‐risk patients undergoing major vascular surgery. Am J Cardiol. 2005;95(5):658–660.
- Lexi‐Comp Online™, Lexi‐Drugs™, Hudson, OH: Lexi‐Comp, Inc; December 7, 2011.
- , , , , , . A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965–970.
- , , , et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–977.
- , , , . Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: a systematic review. Br J Clin Pharmacol. January 13, 2012. doi: 10.1111/j.1365–2125.2012.04178.x.
- American College of Surgeons National Surgical Quality Improvement Program. Available at: http://www.acsnsqip.org. Accessed December 15, 2012.
- , , , et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39(5):967–975.
- , , , , , . Improved postoperative outcomes associated with preoperative statin therapy. Anesthesiology. 2006;105(6):1260–1272; quiz 1289–1290.
- , , , , . Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092–2099.
- , , , et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol. 2005;45(3):336–342.
- , , , et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation. 2003;107(14):1848–1851.
- , , , et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980–989.
- , , , et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):e418–e499.
- , , , et al. Guidelines for pre‐operative cardiac risk assessment and perioperative cardiac management in non‐cardiac surgery. Eur Heart J. 2009;30(22):2769–2812.
- , , , et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg. 2007;104(6):1326–1333.
- , , , et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316–320.
- . Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 suppl 1):III39–III43.
- , , , , , . Persistent endocrine stress response in patients undergoing cardiac surgery. J Endocrinol Invest. 1998;21(1):12–19.
- , , , , , . Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol. 1996;57(1):37–44.
- , , , , , . Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J. 2005;173(6):627–634.
- . Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153–184.
- , . Isoprenoid metabolism and the pleiotropic effects of statins. Curr Atheroscler Rep. 2003;5(5):372–378.
- , , , . Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97(12):1129–1135.
- , , , et al. Roles of rho‐associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49(6):698–705.
- , , , et al. Inhibition of rho‐associated kinase results in suppression of neointimal formation of balloon‐injured arteries. Circulation. 2000;101(17):2030–2033.
- , , , . Reduced expression of endothelial cell markers after 1 year treatment with simvastatin and atorvastatin in patients with coronary heart disease. Atherosclerosis. 2002;162(1):179–185.
- , , , et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108(10):1429–1437.
- , , . Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37–44.
- , , , et al. Inhibition of geranylgeranylation reduces angiotensin II‐mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59(3):646–654.
- , , , et al. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke. 2003;34(2):551–557.
- , , , et al. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102(25):3104–3110.
- , , , et al. Changes of plasma inflammatory markers after withdrawal of statin therapy in patients with hyperlipidemia. Clin Chim Acta. 2006;366(1–2):269–273.
- , , , et al. Platelet hyperactivity after statin treatment discontinuation. Thromb Haemost. 2003;90(3):476–482.
- , , , et al. Rebound inflammatory response during the acute phase of myocardial infarction after simvastatin withdrawal. Atherosclerosis. 2009;207(1):191–194.
- , . Effects of statins on endothelium and signaling mechanisms. Stroke. 2004;35(11 suppl 1):2708–2711.
- , , , et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- , , , et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta‐analysis. Arch Surg. 2012;147(2):181–189.
- , , , et al. Early withdrawal of statin therapy in patients with non‐ST‐segment elevation myocardial infarction: National Registry of Myocardial Infarction. Arch Intern Med. 2004;164(19):2162–2168.
- , , , , , . Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105(12):1446–1452.
- , , , , , . Discontinuation of statin therapy following an acute myocardial infarction: a population‐based study. Eur Heart J. 2008;29(17):2083–2091.
- . There is no evidence for an increase in acute coronary syndromes after short‐term abrupt discontinuation of statins in stable cardiac patients. Circulation. 2004;110(16):2333–2335.
- , , , . Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke. 2007;38(10):2652–2657.
- , , , et al. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69(9):904–910.
- , , , et al. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39(2):433–438.
- , , , et al. Safety of perioperative statin use in high‐risk patients undergoing major vascular surgery. Am J Cardiol. 2005;95(5):658–660.
- Lexi‐Comp Online™, Lexi‐Drugs™, Hudson, OH: Lexi‐Comp, Inc; December 7, 2011.
- , , , , , . A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965–970.
- , , , et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–977.
- , , , . Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: a systematic review. Br J Clin Pharmacol. January 13, 2012. doi: 10.1111/j.1365–2125.2012.04178.x.
- American College of Surgeons National Surgical Quality Improvement Program. Available at: http://www.acsnsqip.org. Accessed December 15, 2012.