User login
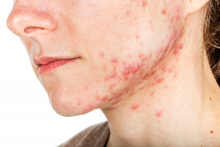
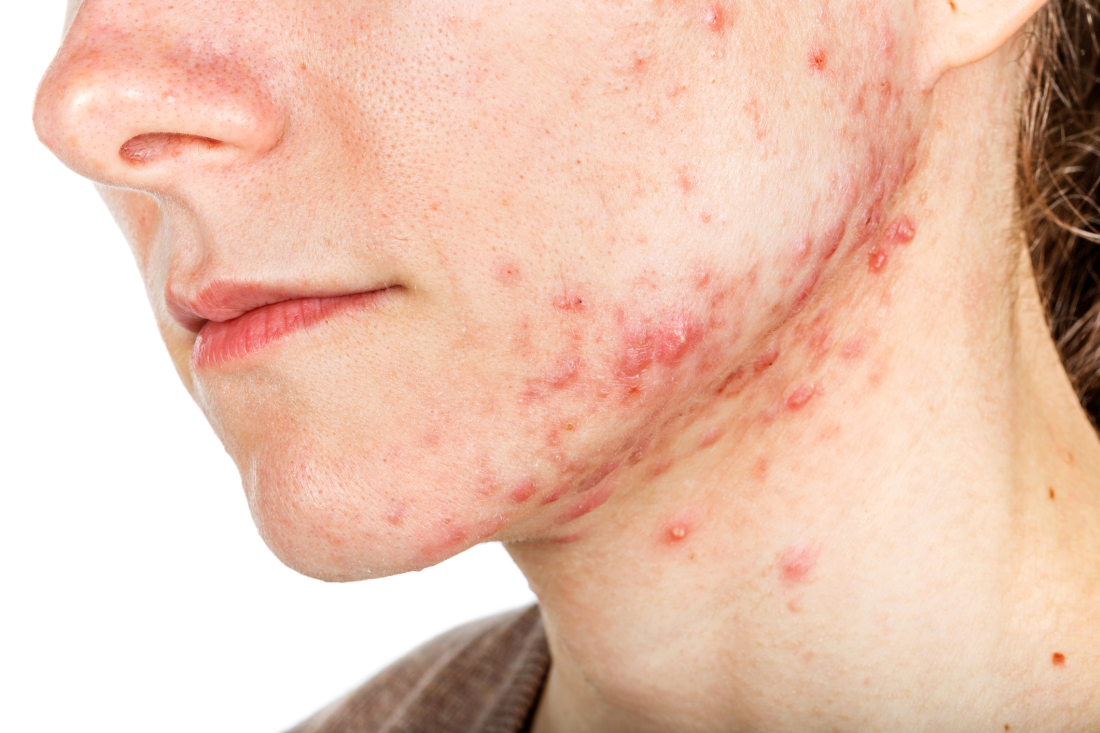
New rosacea clinical management guidelines focus on symptomatology
, as was previously practiced, according to an update on options for managing rosacea published in the Journal of the American Academy of Dermatology.
The update, by the National Rosacea Society Expert Committee, is based on a review of the evidence, and is a follow-up to the classification system for rosacea that was updated in 2017, which recommended classification of rosacea based on phenotype (Am Acad Dermatol. 2018;78:148-155).
The key take-away is “that patients shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” lead author of the management update, Diane Thiboutot, MD, professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey, said in an interview.
“There is an opportunity for physicians to recognize that the symptom complex of rosacea differs widely and treatments should be selected to address the symptoms experienced by the patient, particularly with regard to ocular rosacea,” she said.
Until there were updated guidelines on rosacea classification, published in 2018, relying primarily on diagnostic subtypes “tended to limit consideration of the full range of potential signs and symptoms as well as the frequent simultaneous occurrence of more than one subtype or the potential progression from one subtype to another,” Dr. Thiboutot and coauthors wrote in the management update (J Am Acad Dermatol 2020;82:1501-10).
“The more we learn, the more complex rosacea becomes,” she said in the interview. “The clinical manifestations of rosacea are so varied, ranging from skin erythema, eye findings, papules and pustules to rhinophyma, [that] it calls into question, if all of these are actually one disease (rosacea) or if they represent localized reaction patterns to a multitude of stimuli that vary among individuals.”
Etiology and impact
Dr. Thiboutot and colleagues summarized the management options and recommendations from a committee of 27 experts who assessed the data on rosacea therapies using the updated standard classification system. They also highlighted the suspected systemic nature of rosacea etiology and its psychosocial impact on those with the condition.
“Recent studies have found an association between rosacea and increased risk of a growing number of systemic disorders, including cardiovascular, gastrointestinal, neurologic, and autoimmune diseases as well as certain types of cancer,” the authors wrote. “These findings further elevate the clinical significance of rosacea as growing evidence of its potential link with systemic inflammation is increasingly understood.”
Dr. Thiboutot said that research has implicated both the innate and adaptive immune systems and the neuromuscular system in rosacea’s underpinnings.
“Many of the triggers associated with clinical exacerbation of rosacea are known to activate the immune system and/or the neurovasculature, such as demodex, sunlight, alcohol, and changes in temperature,” she said, adding that therapies targeting the neurovascular effects of rosacea are particularly needed.
More than 50% of patients with rosacea have ocular manifestations, with symptoms such as “dryness, burning and stinging, light sensitivity, blurred vision, and foreign body sensation,” the authors reported.
Diagnosis and management
Without definitive laboratory tests, it’s essential that the clinical diagnosis takes into account not only the physical examination findings, but also patient history, the authors stressed, since “some features may not be visually evident or present at the time of the patient’s visit.”
The authors also recommend taking into account patients’ perception and response to their appearance and its effects on their emotional, social, and professional lives when selecting interventions.
“Rosacea’s unsightly and conspicuous appearance often has significant emotional ramifications, potentially resulting in depression or anxiety, and frequently interferes with social and occupational interactions,” the authors wrote. “It may also be advisable to remind patients that normalization of skin tone and color is the goal rather than complete eradication of facial coloration, which can leave the face with a sallow appearance.”
Therapy will often require multiple agents, such as topical and oral agents for inflammatory papules/pustules of rosacea. If insufficient, adjunctive therapy with oral antibiotics or retinoids may be options, though the latter requires prevention of pregnancy during treatment. The authors also discussed pharmacological treatments for facial erythema and flushing, with all these drugs organized in tables according to symptoms and their levels of evidence and effectiveness.
Despite limited clinical evidence, the authors noted success with two types of laser therapy – pulsed-dye and potassium titanyl phosphate – for telangiectasia and erythema. They also noted the option of intense pulsed light for flushing, ocular symptoms, and meibomian gland dysfunction, and of ablative lasers for rhinophymatous nose. But they highlighted the importance of being cautious when using these therapies on darker skin.
In their discussion of ocular rosacea, the authors described the various manifestations and the two “mainstays” of treatment: “eyelash hygiene and oral [omega-3] supplementation, followed by topical azithromycin or calcineurin inhibitors.” In addition to pharmacological and light therapy options, attention to environmental contributors and conscientious skin care regimens can benefit patients with rosacea as well.
“Clinicians may advise patients to keep a daily diary of lifestyle and environmental factors that appear to affect their rosacea to help identify and avoid their personal triggers,” the authors wrote. “The most common factors are sun exposure, emotional stress, hot weather, wind, heavy exercise, alcohol consumption, hot baths, cold weather, spicy foods, humidity, indoor heat, certain skin-care products, heated beverages, certain medications, medical conditions, certain fruits, marinated meats, certain vegetables, and dairy products.”
The paper also emphasizes the importance of gentle skin care given the highly sensitive and easily irritated skin of patients with rosacea. Sunscreen use, particularly with mineral sunscreens that provide physical barriers and reflect ultraviolet light, should be a key aspect of patients’ skin care, and clinicians should advise patients to seek out gentle, nonirritating cleansers.
Funding was provided by the National Rosacea Society, which receives funding from patients and corporations that include Aclaris Therapeutics, Allergan, Bayer, Cutanea Life Sciences, and Galderma Laboratories. Dr. Thiboutot consults for Galderma. Six of the other nine coauthors have financial links to industry through advisory boards, consulting, or research funding.
SOURCE: Thiboutot D et al. J Am Acad Dermatol. 2020;82:1501-10.
, as was previously practiced, according to an update on options for managing rosacea published in the Journal of the American Academy of Dermatology.
The update, by the National Rosacea Society Expert Committee, is based on a review of the evidence, and is a follow-up to the classification system for rosacea that was updated in 2017, which recommended classification of rosacea based on phenotype (Am Acad Dermatol. 2018;78:148-155).
The key take-away is “that patients shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” lead author of the management update, Diane Thiboutot, MD, professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey, said in an interview.
“There is an opportunity for physicians to recognize that the symptom complex of rosacea differs widely and treatments should be selected to address the symptoms experienced by the patient, particularly with regard to ocular rosacea,” she said.
Until there were updated guidelines on rosacea classification, published in 2018, relying primarily on diagnostic subtypes “tended to limit consideration of the full range of potential signs and symptoms as well as the frequent simultaneous occurrence of more than one subtype or the potential progression from one subtype to another,” Dr. Thiboutot and coauthors wrote in the management update (J Am Acad Dermatol 2020;82:1501-10).
“The more we learn, the more complex rosacea becomes,” she said in the interview. “The clinical manifestations of rosacea are so varied, ranging from skin erythema, eye findings, papules and pustules to rhinophyma, [that] it calls into question, if all of these are actually one disease (rosacea) or if they represent localized reaction patterns to a multitude of stimuli that vary among individuals.”
Etiology and impact
Dr. Thiboutot and colleagues summarized the management options and recommendations from a committee of 27 experts who assessed the data on rosacea therapies using the updated standard classification system. They also highlighted the suspected systemic nature of rosacea etiology and its psychosocial impact on those with the condition.
“Recent studies have found an association between rosacea and increased risk of a growing number of systemic disorders, including cardiovascular, gastrointestinal, neurologic, and autoimmune diseases as well as certain types of cancer,” the authors wrote. “These findings further elevate the clinical significance of rosacea as growing evidence of its potential link with systemic inflammation is increasingly understood.”
Dr. Thiboutot said that research has implicated both the innate and adaptive immune systems and the neuromuscular system in rosacea’s underpinnings.
“Many of the triggers associated with clinical exacerbation of rosacea are known to activate the immune system and/or the neurovasculature, such as demodex, sunlight, alcohol, and changes in temperature,” she said, adding that therapies targeting the neurovascular effects of rosacea are particularly needed.
More than 50% of patients with rosacea have ocular manifestations, with symptoms such as “dryness, burning and stinging, light sensitivity, blurred vision, and foreign body sensation,” the authors reported.
Diagnosis and management
Without definitive laboratory tests, it’s essential that the clinical diagnosis takes into account not only the physical examination findings, but also patient history, the authors stressed, since “some features may not be visually evident or present at the time of the patient’s visit.”
The authors also recommend taking into account patients’ perception and response to their appearance and its effects on their emotional, social, and professional lives when selecting interventions.
“Rosacea’s unsightly and conspicuous appearance often has significant emotional ramifications, potentially resulting in depression or anxiety, and frequently interferes with social and occupational interactions,” the authors wrote. “It may also be advisable to remind patients that normalization of skin tone and color is the goal rather than complete eradication of facial coloration, which can leave the face with a sallow appearance.”
Therapy will often require multiple agents, such as topical and oral agents for inflammatory papules/pustules of rosacea. If insufficient, adjunctive therapy with oral antibiotics or retinoids may be options, though the latter requires prevention of pregnancy during treatment. The authors also discussed pharmacological treatments for facial erythema and flushing, with all these drugs organized in tables according to symptoms and their levels of evidence and effectiveness.
Despite limited clinical evidence, the authors noted success with two types of laser therapy – pulsed-dye and potassium titanyl phosphate – for telangiectasia and erythema. They also noted the option of intense pulsed light for flushing, ocular symptoms, and meibomian gland dysfunction, and of ablative lasers for rhinophymatous nose. But they highlighted the importance of being cautious when using these therapies on darker skin.
In their discussion of ocular rosacea, the authors described the various manifestations and the two “mainstays” of treatment: “eyelash hygiene and oral [omega-3] supplementation, followed by topical azithromycin or calcineurin inhibitors.” In addition to pharmacological and light therapy options, attention to environmental contributors and conscientious skin care regimens can benefit patients with rosacea as well.
“Clinicians may advise patients to keep a daily diary of lifestyle and environmental factors that appear to affect their rosacea to help identify and avoid their personal triggers,” the authors wrote. “The most common factors are sun exposure, emotional stress, hot weather, wind, heavy exercise, alcohol consumption, hot baths, cold weather, spicy foods, humidity, indoor heat, certain skin-care products, heated beverages, certain medications, medical conditions, certain fruits, marinated meats, certain vegetables, and dairy products.”
The paper also emphasizes the importance of gentle skin care given the highly sensitive and easily irritated skin of patients with rosacea. Sunscreen use, particularly with mineral sunscreens that provide physical barriers and reflect ultraviolet light, should be a key aspect of patients’ skin care, and clinicians should advise patients to seek out gentle, nonirritating cleansers.
Funding was provided by the National Rosacea Society, which receives funding from patients and corporations that include Aclaris Therapeutics, Allergan, Bayer, Cutanea Life Sciences, and Galderma Laboratories. Dr. Thiboutot consults for Galderma. Six of the other nine coauthors have financial links to industry through advisory boards, consulting, or research funding.
SOURCE: Thiboutot D et al. J Am Acad Dermatol. 2020;82:1501-10.
, as was previously practiced, according to an update on options for managing rosacea published in the Journal of the American Academy of Dermatology.
The update, by the National Rosacea Society Expert Committee, is based on a review of the evidence, and is a follow-up to the classification system for rosacea that was updated in 2017, which recommended classification of rosacea based on phenotype (Am Acad Dermatol. 2018;78:148-155).
The key take-away is “that patients shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” lead author of the management update, Diane Thiboutot, MD, professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey, said in an interview.
“There is an opportunity for physicians to recognize that the symptom complex of rosacea differs widely and treatments should be selected to address the symptoms experienced by the patient, particularly with regard to ocular rosacea,” she said.
Until there were updated guidelines on rosacea classification, published in 2018, relying primarily on diagnostic subtypes “tended to limit consideration of the full range of potential signs and symptoms as well as the frequent simultaneous occurrence of more than one subtype or the potential progression from one subtype to another,” Dr. Thiboutot and coauthors wrote in the management update (J Am Acad Dermatol 2020;82:1501-10).
“The more we learn, the more complex rosacea becomes,” she said in the interview. “The clinical manifestations of rosacea are so varied, ranging from skin erythema, eye findings, papules and pustules to rhinophyma, [that] it calls into question, if all of these are actually one disease (rosacea) or if they represent localized reaction patterns to a multitude of stimuli that vary among individuals.”
Etiology and impact
Dr. Thiboutot and colleagues summarized the management options and recommendations from a committee of 27 experts who assessed the data on rosacea therapies using the updated standard classification system. They also highlighted the suspected systemic nature of rosacea etiology and its psychosocial impact on those with the condition.
“Recent studies have found an association between rosacea and increased risk of a growing number of systemic disorders, including cardiovascular, gastrointestinal, neurologic, and autoimmune diseases as well as certain types of cancer,” the authors wrote. “These findings further elevate the clinical significance of rosacea as growing evidence of its potential link with systemic inflammation is increasingly understood.”
Dr. Thiboutot said that research has implicated both the innate and adaptive immune systems and the neuromuscular system in rosacea’s underpinnings.
“Many of the triggers associated with clinical exacerbation of rosacea are known to activate the immune system and/or the neurovasculature, such as demodex, sunlight, alcohol, and changes in temperature,” she said, adding that therapies targeting the neurovascular effects of rosacea are particularly needed.
More than 50% of patients with rosacea have ocular manifestations, with symptoms such as “dryness, burning and stinging, light sensitivity, blurred vision, and foreign body sensation,” the authors reported.
Diagnosis and management
Without definitive laboratory tests, it’s essential that the clinical diagnosis takes into account not only the physical examination findings, but also patient history, the authors stressed, since “some features may not be visually evident or present at the time of the patient’s visit.”
The authors also recommend taking into account patients’ perception and response to their appearance and its effects on their emotional, social, and professional lives when selecting interventions.
“Rosacea’s unsightly and conspicuous appearance often has significant emotional ramifications, potentially resulting in depression or anxiety, and frequently interferes with social and occupational interactions,” the authors wrote. “It may also be advisable to remind patients that normalization of skin tone and color is the goal rather than complete eradication of facial coloration, which can leave the face with a sallow appearance.”
Therapy will often require multiple agents, such as topical and oral agents for inflammatory papules/pustules of rosacea. If insufficient, adjunctive therapy with oral antibiotics or retinoids may be options, though the latter requires prevention of pregnancy during treatment. The authors also discussed pharmacological treatments for facial erythema and flushing, with all these drugs organized in tables according to symptoms and their levels of evidence and effectiveness.
Despite limited clinical evidence, the authors noted success with two types of laser therapy – pulsed-dye and potassium titanyl phosphate – for telangiectasia and erythema. They also noted the option of intense pulsed light for flushing, ocular symptoms, and meibomian gland dysfunction, and of ablative lasers for rhinophymatous nose. But they highlighted the importance of being cautious when using these therapies on darker skin.
In their discussion of ocular rosacea, the authors described the various manifestations and the two “mainstays” of treatment: “eyelash hygiene and oral [omega-3] supplementation, followed by topical azithromycin or calcineurin inhibitors.” In addition to pharmacological and light therapy options, attention to environmental contributors and conscientious skin care regimens can benefit patients with rosacea as well.
“Clinicians may advise patients to keep a daily diary of lifestyle and environmental factors that appear to affect their rosacea to help identify and avoid their personal triggers,” the authors wrote. “The most common factors are sun exposure, emotional stress, hot weather, wind, heavy exercise, alcohol consumption, hot baths, cold weather, spicy foods, humidity, indoor heat, certain skin-care products, heated beverages, certain medications, medical conditions, certain fruits, marinated meats, certain vegetables, and dairy products.”
The paper also emphasizes the importance of gentle skin care given the highly sensitive and easily irritated skin of patients with rosacea. Sunscreen use, particularly with mineral sunscreens that provide physical barriers and reflect ultraviolet light, should be a key aspect of patients’ skin care, and clinicians should advise patients to seek out gentle, nonirritating cleansers.
Funding was provided by the National Rosacea Society, which receives funding from patients and corporations that include Aclaris Therapeutics, Allergan, Bayer, Cutanea Life Sciences, and Galderma Laboratories. Dr. Thiboutot consults for Galderma. Six of the other nine coauthors have financial links to industry through advisory boards, consulting, or research funding.
SOURCE: Thiboutot D et al. J Am Acad Dermatol. 2020;82:1501-10.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Extremely preterm infants fare better with corticosteroid and magnesium combo
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
FROM OBSTETRICS & GYNECOLOGY
Novel inflammatory syndrome in children possibly linked to COVID-19
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
Standing orders for vaccines may improve pediatric vaccination rates
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
FROM PEDIATRICS
Sleep in the time of COVID-19
Mass social distancing and social isolation to prevent the spread of a deadly disease, along with technological tools that allow social communication and continued work and school, is an unprecedented situation.
The current reality of most people’s lives during the COVID-19 pandemic has the potential to induce or exacerbate sleep problems, though it may also present some with an opportunity to improve sleep, wrote Ellemarije Altena, PhD, of the University of Bordeaux (France), and her colleagues in a recent research review in the Journal of Sleep Research.
The review was conducted by a task force of the European Academy for Cognitive Behavioural Therapy for Insomnia. The European CBT-I Academy is an initiative of the European Insomnia Network to promote implementation and dissemination of treatment.
After discussing the known effects of stress, confinement, and altered schedules on sleep, the authors present recommendations on ways to manage sleep problems such as insomnia in the general public and potentially encourage people to take advantage of the opportunity to align their schedules with their natural circadian rhythms. Physicians may find the recommendations helpful in advising patients with sleep problems related to the COVID-19 emergency.
“Being forced to stay at home, work from home, do homeschooling with children, drastically minimize outings, reduce social interaction or work many more hours under stressful circumstances, and in parallel manage the attendant health risks, can have a major impact on daily functioning and nighttime sleep,” Dr. Altena and colleagues wrote.
There may also be a lag time in physicians hearing about changes in sleep or sleeping problems from patients, said Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah in Salt Lake City. “There may actually be some improvement in sleep durations given that most folks are working from home with more time with family and less work-related stress,” he said in an interview. “In terms of sleep or other effects on worsening of psychiatric problems, it is still not clear what the overall effects are going to be.”
Although daylight has the biggest impact on regulating circadian rhythms, artificial light, meal times, diet, and amount of physical activity can also have an influence. Negative effects on sleep can result from both excessively high activity levels, such as stress and work overload, or excessively low levels, such as from depression or confinement, the authors note.
The current situation also opens the door to interactions between stress, sleep, anxiety, and risk of PTSD. “Those sensitive to stress-related sleep disruption are more likely to develop chronic insomnia,” which, in combination with a major stressor, is a risk factor for PTSD, the authors write. They note that 7% of Wuhan residents, the city in China where the virus appears to have originated, particularly women, reported PTSD symptoms after the COVID-19 outbreak, and anxiety was highest in those under age 35 years and those who followed news about the disease for more than 3 hours a day.
Better sleep quality and fewer early morning awakenings, however, appeared to be protective against PTSD symptoms. The authors note the value of physical exercise, cognitive interventions, and relaxation techniques, including meditation, for reducing stress and milder symptoms of PTSD.
“Some patients are sleeping a bit better because of the pace of things has slowed down a bit,” said Anne C. Trainor, a nurse practitioner and instructor in the neurology department’s sleep disorders program at Oregon Health & Science University in Portland, who was not involved in the study. “Keeping a regular schedule for sleeping and eating, getting exercise daily – preferably in sunlight and not just before bedtime – and using relaxation or mindfulness practice and cognitive interventions to help manage anxiety” were the key takeaways from this review, Ms. Trainor said in an interview.
Home confinement, stressors and sleep
A wide range of stressors could affect sleep during COVID-19 social distancing interventions, including “major changes in routines, living with uncertainty,” and anxiety about health, the economic situation, and how long this situation will last, the authors write.
Parents must juggle work, homeschooling, and ordinary household errands and management. Meanwhile, entrepreneurs, small business owners ,and workers in entertainment, hospitality and food service must contend with anxiety about job uncertainty and financial security. For anyone working from home, disruptions to work and home routines can make it difficult to associate being home with relaxation – and sleep.
“The more regular our sleep schedule is the better quality our sleep tends to be, but it is a struggle when we don’t have separate spaces to work and parent in,” Ms. Trainor said.
At the same time, “confinement-related stress may be caused by an inability to engage in rewarding activities, such as visiting friends and family, shopping, attending cultural and sports events, and visiting bars or restaurants,” the authors write. “Spending more time with family in a limited space can also induce stress, particularly in situations where there are preexisting family difficulties.”
Being stuck at home may lead to less daylight exposure than usual, reduced physical activity, and increased eating, which can contribute to weight gain and other health risks. However, “the effect of stress from confinement, loss of work, and health concerns needs to be individualized and may be difficult to generalize,” Dr. Sundar said.
The authors of the review note the established associations between too little social interaction, increased stress, and poor sleep quality, though loneliness mediates this relationship. Loneliness is also a risk during this time, with or without online social interaction.
Children and teens may also have difficulty sleeping, which can affect their behavioral and emotional regulation, and primary caregivers experience more stress while juggling childcare, household duties, and work.
“While many parents share childcare and household responsibilities, in most families these tasks are still predominantly managed by mothers,” the authors added.
“Sharing responsibilities between parents and not overworking just one parent is key,” said Brandon M. Seay MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. He also recommended trying to incorporate work into the day while kids are doing online learning.
Ms. Trainor agreed that trading off responsibilities between parents is ideal, though the challenge is greater for single parents. It may be possible for some to take family leave, but not all families have that option, she said.
The study authors also point out a Catch-22 for many people: The blurred boundary between home life and work life can undermine work productivity and efficiency, thereby increasing stress. “Healthy sleep may be a key protective factor to cope positively with these challenges, although adequate opportunity to sleep may be affected by increased time pressure of work, childcare, and household requirements.”
Dr. Seay advises adults to try to get at least 6-8 hours of sleep each night, even taking advantage of a later waking time – if the kids also sleep in – to help. “If anything, the ability to sleep later and wake up later is of benefit for a lot of my teenage patients,” he said in an interview.
In fact, the study authors also address possible positive effects on sleep for some people during the current situation. Since social support can improve sleep quality, social media interaction might provide some social support, though it’s not the same as meeting people in person and “screen exposure may hamper sleep quality when used close to bedtime.”
Some people may actually have an opportunity to get more daylight exposure or exercise, which can improve sleep, and some, especially night owls and teenagers, may be able to align their daily schedules more closely to their natural circadian rhythms.
“Given that we are not bound by usual work or social schedules, there may be a tendency to drift to our sleep chronotypes,” especially for teenagers, Dr. Sundar said.
For some, this may be their first opportunity to learn what their chronotype is, Dr. Seay said.
“It is always advantageous to ‘obey’ your natural sleep timing, [although] it simply isn’t always the most efficient outside of our current situation,” he said. “Use this as a time to figure out your natural sleep timing if you constantly have issues being able to wake up in the morning. Now that you don’t have to be up for work or school, you can figure out what time works for you.”
At the same time, if you have an extreme circadian rhythm disorder, especially an irregular one, it may still be best to try to keep a regular sleep schedule to avoid feeling isolated if others are socializing while you’re asleep, Ms. Trainor said.
The authors similarly note the limits of potential benefits during this time, noting that they “may not be enough to counteract the negative effects of the increased work and family requirements, as well as the overwhelming levels of stress and anxiety about the well-being of oneself and others, and the negative effects of confinement for family social reactions.”
Treating stress, anxiety, and insomnia
The first-line treatment for chronic insomnia is cognitive-behavioral therapy for insomnia, but “recent evidence shows that cognitive-behavioral therapy can also serve to treat sudden-onset (acute) insomnia due to rapid stress-causing situation changes,” the authors noted. They also reviewed the key elements of CBT-I: stimulus control, sleep hygiene, relaxation interventions, cognitive reappraisal, paradoxical intention, and sleep restriction.
CBT-I lends very naturally to telemedicine, Dr. Seay, Dr. Sundar, and Ms. Trainor all agreed.
“I actually see this current situation as an opportunity for health care practices and providers to expand the reach of telemedicine – due to necessity – which will hopefully continue after confinement has been lifted worldwide,” Dr. Seay said.
Dr. Sundar pointed to research supporting CBT-I online and several apps that can be used for it, such as SHUTi and Sleepio. Ms. Trainor noted that the Cleveland Clinic offers a basic CBT-I online class for $40.
The authors note that prescribing medication is generally discouraged because it lacks evidence for long-term effectiveness of chronic insomnia, but it might be worth considering as a second-line therapy for acute insomnia from outside stressors, such as home confinement, if CBT-I doesn’t work or isn’t possible. Pharmacologic treatment can include benzodiazepines, hypnotic benzodiazepine receptor agonists, or sedating antidepressants, particularly if used for a comorbid mood disorder.
The authors then offer general recommendations for improving sleep that doctors can pass on to their patients:
- Get up and go to bed at approximately the same times daily.
- Schedule 15-minute breaks during the day to manage stress and reflect on worries and the situation.
- Reserve the bed for sleep and sex only; not for working, watching TV, using the computer, or doing other activities.
- Try to follow your natural sleep rhythm as much as possible.
- Use social media as stress relief, an opportunity to communicate with friends and family, and distraction, especially with uplifting stories or humor.
- Leave devices out of the bedroom.
- Limit your exposure to news about the COVID-19 pandemic.
- Exercise regularly, ideally in daylight.
- Look for ways to stay busy and distracted, including making your home or bedroom more comfortable if possible.
- Get as much daylight during the day as possible, and keep lights dim or dark at night.
- Engage in familiar, comfortable, relaxing activities before bedtime.
- If your daily activity level is lower, eat less as well, ideally at least 2 hours before going to bed.
The authors also offered recommendations specifically for families:
- Divide child care, home maintenance, and chores between adults, being sure not to let the lion’s share fall on women.
- Maintain regular sleep times for children and spend the 30 minutes before their bedtime doing a calming, familiar activity that both the children and parents enjoy.
- “While using computer, smartphones, and watching TV more than usual may be inevitable in confinement, avoid technological devices after dinner or too close to bedtime.”
- Ensure your child has daily physical activity, keep a relatively consistent schedule or routine, expose them to as much daylight or bright light as possible during the day, and try to limit their bed use only to sleeping if possible. “Parents need to be involved in setting schedules for sleep and meal times so that kids do not get into sleep patterns that are difficult to change when school starts back,” Dr. Sundar said. “Limiting screen time is also important especially during nighttime.”
- Reassure children if they wake up anxious at night.
SOURCE: Altena E et al. J Sleep Res. 2020 Apr 4. doi: 10.1111/jsr.13052.
Mass social distancing and social isolation to prevent the spread of a deadly disease, along with technological tools that allow social communication and continued work and school, is an unprecedented situation.
The current reality of most people’s lives during the COVID-19 pandemic has the potential to induce or exacerbate sleep problems, though it may also present some with an opportunity to improve sleep, wrote Ellemarije Altena, PhD, of the University of Bordeaux (France), and her colleagues in a recent research review in the Journal of Sleep Research.
The review was conducted by a task force of the European Academy for Cognitive Behavioural Therapy for Insomnia. The European CBT-I Academy is an initiative of the European Insomnia Network to promote implementation and dissemination of treatment.
After discussing the known effects of stress, confinement, and altered schedules on sleep, the authors present recommendations on ways to manage sleep problems such as insomnia in the general public and potentially encourage people to take advantage of the opportunity to align their schedules with their natural circadian rhythms. Physicians may find the recommendations helpful in advising patients with sleep problems related to the COVID-19 emergency.
“Being forced to stay at home, work from home, do homeschooling with children, drastically minimize outings, reduce social interaction or work many more hours under stressful circumstances, and in parallel manage the attendant health risks, can have a major impact on daily functioning and nighttime sleep,” Dr. Altena and colleagues wrote.
There may also be a lag time in physicians hearing about changes in sleep or sleeping problems from patients, said Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah in Salt Lake City. “There may actually be some improvement in sleep durations given that most folks are working from home with more time with family and less work-related stress,” he said in an interview. “In terms of sleep or other effects on worsening of psychiatric problems, it is still not clear what the overall effects are going to be.”
Although daylight has the biggest impact on regulating circadian rhythms, artificial light, meal times, diet, and amount of physical activity can also have an influence. Negative effects on sleep can result from both excessively high activity levels, such as stress and work overload, or excessively low levels, such as from depression or confinement, the authors note.
The current situation also opens the door to interactions between stress, sleep, anxiety, and risk of PTSD. “Those sensitive to stress-related sleep disruption are more likely to develop chronic insomnia,” which, in combination with a major stressor, is a risk factor for PTSD, the authors write. They note that 7% of Wuhan residents, the city in China where the virus appears to have originated, particularly women, reported PTSD symptoms after the COVID-19 outbreak, and anxiety was highest in those under age 35 years and those who followed news about the disease for more than 3 hours a day.
Better sleep quality and fewer early morning awakenings, however, appeared to be protective against PTSD symptoms. The authors note the value of physical exercise, cognitive interventions, and relaxation techniques, including meditation, for reducing stress and milder symptoms of PTSD.
“Some patients are sleeping a bit better because of the pace of things has slowed down a bit,” said Anne C. Trainor, a nurse practitioner and instructor in the neurology department’s sleep disorders program at Oregon Health & Science University in Portland, who was not involved in the study. “Keeping a regular schedule for sleeping and eating, getting exercise daily – preferably in sunlight and not just before bedtime – and using relaxation or mindfulness practice and cognitive interventions to help manage anxiety” were the key takeaways from this review, Ms. Trainor said in an interview.
Home confinement, stressors and sleep
A wide range of stressors could affect sleep during COVID-19 social distancing interventions, including “major changes in routines, living with uncertainty,” and anxiety about health, the economic situation, and how long this situation will last, the authors write.
Parents must juggle work, homeschooling, and ordinary household errands and management. Meanwhile, entrepreneurs, small business owners ,and workers in entertainment, hospitality and food service must contend with anxiety about job uncertainty and financial security. For anyone working from home, disruptions to work and home routines can make it difficult to associate being home with relaxation – and sleep.
“The more regular our sleep schedule is the better quality our sleep tends to be, but it is a struggle when we don’t have separate spaces to work and parent in,” Ms. Trainor said.
At the same time, “confinement-related stress may be caused by an inability to engage in rewarding activities, such as visiting friends and family, shopping, attending cultural and sports events, and visiting bars or restaurants,” the authors write. “Spending more time with family in a limited space can also induce stress, particularly in situations where there are preexisting family difficulties.”
Being stuck at home may lead to less daylight exposure than usual, reduced physical activity, and increased eating, which can contribute to weight gain and other health risks. However, “the effect of stress from confinement, loss of work, and health concerns needs to be individualized and may be difficult to generalize,” Dr. Sundar said.
The authors of the review note the established associations between too little social interaction, increased stress, and poor sleep quality, though loneliness mediates this relationship. Loneliness is also a risk during this time, with or without online social interaction.
Children and teens may also have difficulty sleeping, which can affect their behavioral and emotional regulation, and primary caregivers experience more stress while juggling childcare, household duties, and work.
“While many parents share childcare and household responsibilities, in most families these tasks are still predominantly managed by mothers,” the authors added.
“Sharing responsibilities between parents and not overworking just one parent is key,” said Brandon M. Seay MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. He also recommended trying to incorporate work into the day while kids are doing online learning.
Ms. Trainor agreed that trading off responsibilities between parents is ideal, though the challenge is greater for single parents. It may be possible for some to take family leave, but not all families have that option, she said.
The study authors also point out a Catch-22 for many people: The blurred boundary between home life and work life can undermine work productivity and efficiency, thereby increasing stress. “Healthy sleep may be a key protective factor to cope positively with these challenges, although adequate opportunity to sleep may be affected by increased time pressure of work, childcare, and household requirements.”
Dr. Seay advises adults to try to get at least 6-8 hours of sleep each night, even taking advantage of a later waking time – if the kids also sleep in – to help. “If anything, the ability to sleep later and wake up later is of benefit for a lot of my teenage patients,” he said in an interview.
In fact, the study authors also address possible positive effects on sleep for some people during the current situation. Since social support can improve sleep quality, social media interaction might provide some social support, though it’s not the same as meeting people in person and “screen exposure may hamper sleep quality when used close to bedtime.”
Some people may actually have an opportunity to get more daylight exposure or exercise, which can improve sleep, and some, especially night owls and teenagers, may be able to align their daily schedules more closely to their natural circadian rhythms.
“Given that we are not bound by usual work or social schedules, there may be a tendency to drift to our sleep chronotypes,” especially for teenagers, Dr. Sundar said.
For some, this may be their first opportunity to learn what their chronotype is, Dr. Seay said.
“It is always advantageous to ‘obey’ your natural sleep timing, [although] it simply isn’t always the most efficient outside of our current situation,” he said. “Use this as a time to figure out your natural sleep timing if you constantly have issues being able to wake up in the morning. Now that you don’t have to be up for work or school, you can figure out what time works for you.”
At the same time, if you have an extreme circadian rhythm disorder, especially an irregular one, it may still be best to try to keep a regular sleep schedule to avoid feeling isolated if others are socializing while you’re asleep, Ms. Trainor said.
The authors similarly note the limits of potential benefits during this time, noting that they “may not be enough to counteract the negative effects of the increased work and family requirements, as well as the overwhelming levels of stress and anxiety about the well-being of oneself and others, and the negative effects of confinement for family social reactions.”
Treating stress, anxiety, and insomnia
The first-line treatment for chronic insomnia is cognitive-behavioral therapy for insomnia, but “recent evidence shows that cognitive-behavioral therapy can also serve to treat sudden-onset (acute) insomnia due to rapid stress-causing situation changes,” the authors noted. They also reviewed the key elements of CBT-I: stimulus control, sleep hygiene, relaxation interventions, cognitive reappraisal, paradoxical intention, and sleep restriction.
CBT-I lends very naturally to telemedicine, Dr. Seay, Dr. Sundar, and Ms. Trainor all agreed.
“I actually see this current situation as an opportunity for health care practices and providers to expand the reach of telemedicine – due to necessity – which will hopefully continue after confinement has been lifted worldwide,” Dr. Seay said.
Dr. Sundar pointed to research supporting CBT-I online and several apps that can be used for it, such as SHUTi and Sleepio. Ms. Trainor noted that the Cleveland Clinic offers a basic CBT-I online class for $40.
The authors note that prescribing medication is generally discouraged because it lacks evidence for long-term effectiveness of chronic insomnia, but it might be worth considering as a second-line therapy for acute insomnia from outside stressors, such as home confinement, if CBT-I doesn’t work or isn’t possible. Pharmacologic treatment can include benzodiazepines, hypnotic benzodiazepine receptor agonists, or sedating antidepressants, particularly if used for a comorbid mood disorder.
The authors then offer general recommendations for improving sleep that doctors can pass on to their patients:
- Get up and go to bed at approximately the same times daily.
- Schedule 15-minute breaks during the day to manage stress and reflect on worries and the situation.
- Reserve the bed for sleep and sex only; not for working, watching TV, using the computer, or doing other activities.
- Try to follow your natural sleep rhythm as much as possible.
- Use social media as stress relief, an opportunity to communicate with friends and family, and distraction, especially with uplifting stories or humor.
- Leave devices out of the bedroom.
- Limit your exposure to news about the COVID-19 pandemic.
- Exercise regularly, ideally in daylight.
- Look for ways to stay busy and distracted, including making your home or bedroom more comfortable if possible.
- Get as much daylight during the day as possible, and keep lights dim or dark at night.
- Engage in familiar, comfortable, relaxing activities before bedtime.
- If your daily activity level is lower, eat less as well, ideally at least 2 hours before going to bed.
The authors also offered recommendations specifically for families:
- Divide child care, home maintenance, and chores between adults, being sure not to let the lion’s share fall on women.
- Maintain regular sleep times for children and spend the 30 minutes before their bedtime doing a calming, familiar activity that both the children and parents enjoy.
- “While using computer, smartphones, and watching TV more than usual may be inevitable in confinement, avoid technological devices after dinner or too close to bedtime.”
- Ensure your child has daily physical activity, keep a relatively consistent schedule or routine, expose them to as much daylight or bright light as possible during the day, and try to limit their bed use only to sleeping if possible. “Parents need to be involved in setting schedules for sleep and meal times so that kids do not get into sleep patterns that are difficult to change when school starts back,” Dr. Sundar said. “Limiting screen time is also important especially during nighttime.”
- Reassure children if they wake up anxious at night.
SOURCE: Altena E et al. J Sleep Res. 2020 Apr 4. doi: 10.1111/jsr.13052.
Mass social distancing and social isolation to prevent the spread of a deadly disease, along with technological tools that allow social communication and continued work and school, is an unprecedented situation.
The current reality of most people’s lives during the COVID-19 pandemic has the potential to induce or exacerbate sleep problems, though it may also present some with an opportunity to improve sleep, wrote Ellemarije Altena, PhD, of the University of Bordeaux (France), and her colleagues in a recent research review in the Journal of Sleep Research.
The review was conducted by a task force of the European Academy for Cognitive Behavioural Therapy for Insomnia. The European CBT-I Academy is an initiative of the European Insomnia Network to promote implementation and dissemination of treatment.
After discussing the known effects of stress, confinement, and altered schedules on sleep, the authors present recommendations on ways to manage sleep problems such as insomnia in the general public and potentially encourage people to take advantage of the opportunity to align their schedules with their natural circadian rhythms. Physicians may find the recommendations helpful in advising patients with sleep problems related to the COVID-19 emergency.
“Being forced to stay at home, work from home, do homeschooling with children, drastically minimize outings, reduce social interaction or work many more hours under stressful circumstances, and in parallel manage the attendant health risks, can have a major impact on daily functioning and nighttime sleep,” Dr. Altena and colleagues wrote.
There may also be a lag time in physicians hearing about changes in sleep or sleeping problems from patients, said Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah in Salt Lake City. “There may actually be some improvement in sleep durations given that most folks are working from home with more time with family and less work-related stress,” he said in an interview. “In terms of sleep or other effects on worsening of psychiatric problems, it is still not clear what the overall effects are going to be.”
Although daylight has the biggest impact on regulating circadian rhythms, artificial light, meal times, diet, and amount of physical activity can also have an influence. Negative effects on sleep can result from both excessively high activity levels, such as stress and work overload, or excessively low levels, such as from depression or confinement, the authors note.
The current situation also opens the door to interactions between stress, sleep, anxiety, and risk of PTSD. “Those sensitive to stress-related sleep disruption are more likely to develop chronic insomnia,” which, in combination with a major stressor, is a risk factor for PTSD, the authors write. They note that 7% of Wuhan residents, the city in China where the virus appears to have originated, particularly women, reported PTSD symptoms after the COVID-19 outbreak, and anxiety was highest in those under age 35 years and those who followed news about the disease for more than 3 hours a day.
Better sleep quality and fewer early morning awakenings, however, appeared to be protective against PTSD symptoms. The authors note the value of physical exercise, cognitive interventions, and relaxation techniques, including meditation, for reducing stress and milder symptoms of PTSD.
“Some patients are sleeping a bit better because of the pace of things has slowed down a bit,” said Anne C. Trainor, a nurse practitioner and instructor in the neurology department’s sleep disorders program at Oregon Health & Science University in Portland, who was not involved in the study. “Keeping a regular schedule for sleeping and eating, getting exercise daily – preferably in sunlight and not just before bedtime – and using relaxation or mindfulness practice and cognitive interventions to help manage anxiety” were the key takeaways from this review, Ms. Trainor said in an interview.
Home confinement, stressors and sleep
A wide range of stressors could affect sleep during COVID-19 social distancing interventions, including “major changes in routines, living with uncertainty,” and anxiety about health, the economic situation, and how long this situation will last, the authors write.
Parents must juggle work, homeschooling, and ordinary household errands and management. Meanwhile, entrepreneurs, small business owners ,and workers in entertainment, hospitality and food service must contend with anxiety about job uncertainty and financial security. For anyone working from home, disruptions to work and home routines can make it difficult to associate being home with relaxation – and sleep.
“The more regular our sleep schedule is the better quality our sleep tends to be, but it is a struggle when we don’t have separate spaces to work and parent in,” Ms. Trainor said.
At the same time, “confinement-related stress may be caused by an inability to engage in rewarding activities, such as visiting friends and family, shopping, attending cultural and sports events, and visiting bars or restaurants,” the authors write. “Spending more time with family in a limited space can also induce stress, particularly in situations where there are preexisting family difficulties.”
Being stuck at home may lead to less daylight exposure than usual, reduced physical activity, and increased eating, which can contribute to weight gain and other health risks. However, “the effect of stress from confinement, loss of work, and health concerns needs to be individualized and may be difficult to generalize,” Dr. Sundar said.
The authors of the review note the established associations between too little social interaction, increased stress, and poor sleep quality, though loneliness mediates this relationship. Loneliness is also a risk during this time, with or without online social interaction.
Children and teens may also have difficulty sleeping, which can affect their behavioral and emotional regulation, and primary caregivers experience more stress while juggling childcare, household duties, and work.
“While many parents share childcare and household responsibilities, in most families these tasks are still predominantly managed by mothers,” the authors added.
“Sharing responsibilities between parents and not overworking just one parent is key,” said Brandon M. Seay MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. He also recommended trying to incorporate work into the day while kids are doing online learning.
Ms. Trainor agreed that trading off responsibilities between parents is ideal, though the challenge is greater for single parents. It may be possible for some to take family leave, but not all families have that option, she said.
The study authors also point out a Catch-22 for many people: The blurred boundary between home life and work life can undermine work productivity and efficiency, thereby increasing stress. “Healthy sleep may be a key protective factor to cope positively with these challenges, although adequate opportunity to sleep may be affected by increased time pressure of work, childcare, and household requirements.”
Dr. Seay advises adults to try to get at least 6-8 hours of sleep each night, even taking advantage of a later waking time – if the kids also sleep in – to help. “If anything, the ability to sleep later and wake up later is of benefit for a lot of my teenage patients,” he said in an interview.
In fact, the study authors also address possible positive effects on sleep for some people during the current situation. Since social support can improve sleep quality, social media interaction might provide some social support, though it’s not the same as meeting people in person and “screen exposure may hamper sleep quality when used close to bedtime.”
Some people may actually have an opportunity to get more daylight exposure or exercise, which can improve sleep, and some, especially night owls and teenagers, may be able to align their daily schedules more closely to their natural circadian rhythms.
“Given that we are not bound by usual work or social schedules, there may be a tendency to drift to our sleep chronotypes,” especially for teenagers, Dr. Sundar said.
For some, this may be their first opportunity to learn what their chronotype is, Dr. Seay said.
“It is always advantageous to ‘obey’ your natural sleep timing, [although] it simply isn’t always the most efficient outside of our current situation,” he said. “Use this as a time to figure out your natural sleep timing if you constantly have issues being able to wake up in the morning. Now that you don’t have to be up for work or school, you can figure out what time works for you.”
At the same time, if you have an extreme circadian rhythm disorder, especially an irregular one, it may still be best to try to keep a regular sleep schedule to avoid feeling isolated if others are socializing while you’re asleep, Ms. Trainor said.
The authors similarly note the limits of potential benefits during this time, noting that they “may not be enough to counteract the negative effects of the increased work and family requirements, as well as the overwhelming levels of stress and anxiety about the well-being of oneself and others, and the negative effects of confinement for family social reactions.”
Treating stress, anxiety, and insomnia
The first-line treatment for chronic insomnia is cognitive-behavioral therapy for insomnia, but “recent evidence shows that cognitive-behavioral therapy can also serve to treat sudden-onset (acute) insomnia due to rapid stress-causing situation changes,” the authors noted. They also reviewed the key elements of CBT-I: stimulus control, sleep hygiene, relaxation interventions, cognitive reappraisal, paradoxical intention, and sleep restriction.
CBT-I lends very naturally to telemedicine, Dr. Seay, Dr. Sundar, and Ms. Trainor all agreed.
“I actually see this current situation as an opportunity for health care practices and providers to expand the reach of telemedicine – due to necessity – which will hopefully continue after confinement has been lifted worldwide,” Dr. Seay said.
Dr. Sundar pointed to research supporting CBT-I online and several apps that can be used for it, such as SHUTi and Sleepio. Ms. Trainor noted that the Cleveland Clinic offers a basic CBT-I online class for $40.
The authors note that prescribing medication is generally discouraged because it lacks evidence for long-term effectiveness of chronic insomnia, but it might be worth considering as a second-line therapy for acute insomnia from outside stressors, such as home confinement, if CBT-I doesn’t work or isn’t possible. Pharmacologic treatment can include benzodiazepines, hypnotic benzodiazepine receptor agonists, or sedating antidepressants, particularly if used for a comorbid mood disorder.
The authors then offer general recommendations for improving sleep that doctors can pass on to their patients:
- Get up and go to bed at approximately the same times daily.
- Schedule 15-minute breaks during the day to manage stress and reflect on worries and the situation.
- Reserve the bed for sleep and sex only; not for working, watching TV, using the computer, or doing other activities.
- Try to follow your natural sleep rhythm as much as possible.
- Use social media as stress relief, an opportunity to communicate with friends and family, and distraction, especially with uplifting stories or humor.
- Leave devices out of the bedroom.
- Limit your exposure to news about the COVID-19 pandemic.
- Exercise regularly, ideally in daylight.
- Look for ways to stay busy and distracted, including making your home or bedroom more comfortable if possible.
- Get as much daylight during the day as possible, and keep lights dim or dark at night.
- Engage in familiar, comfortable, relaxing activities before bedtime.
- If your daily activity level is lower, eat less as well, ideally at least 2 hours before going to bed.
The authors also offered recommendations specifically for families:
- Divide child care, home maintenance, and chores between adults, being sure not to let the lion’s share fall on women.
- Maintain regular sleep times for children and spend the 30 minutes before their bedtime doing a calming, familiar activity that both the children and parents enjoy.
- “While using computer, smartphones, and watching TV more than usual may be inevitable in confinement, avoid technological devices after dinner or too close to bedtime.”
- Ensure your child has daily physical activity, keep a relatively consistent schedule or routine, expose them to as much daylight or bright light as possible during the day, and try to limit their bed use only to sleeping if possible. “Parents need to be involved in setting schedules for sleep and meal times so that kids do not get into sleep patterns that are difficult to change when school starts back,” Dr. Sundar said. “Limiting screen time is also important especially during nighttime.”
- Reassure children if they wake up anxious at night.
SOURCE: Altena E et al. J Sleep Res. 2020 Apr 4. doi: 10.1111/jsr.13052.
FROM JOURNAL OF SLEEP RESEARCH
iPLEDGE allows at-home pregnancy tests during pandemic
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
Liraglutide gives adolescents with obesity an edge in managing weight loss
Prescribing liraglutide plus lifestyle therapy for adolescents with obesity resulted in greater weight loss and greater reduction in body mass index, compared with those prescribed lifestyle therapy alone, according to findings from a new study published in The New England Journal of Medicine.
Liraglutide with lifestyle therapy also “compared favorably in terms of [body mass index] reduction,” compared with other pediatric weight-management programs in the United States and with use of orlistat, wrote Aaron S. Kelly, PhD, of the University of Minnesota, Minneapolis, and colleagues. The study abstract was presented during a virtual news conference held by The Endocrine Society. It had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
The study included adolescents aged 12-17 years, who had obesity (BMI, ≥30 kg/m2) and had responded poorly to recommendations involving lifestyle therapy only, as judged by the site investigator and documented in the participant’s medical records. The adolescents participated at one of five sites in Belgium, Mexico, Russia, Sweden, and the United States.
In the randomized, controlled, double-blind trial, 125 participants received 3 mg liraglutide, and 126 received placebo for 56 weeks, during which both groups received lifestyle therapy, “defined as counseling about healthy nutrition and physical activity for weight loss,” the authors wrote.
After 12-weeks of run-in, the treatment period lasted 56 weeks, with a follow-up 26 weeks after treatment ended. The liraglutide group retained 80.8% of its participants, and the placebo group, 79.4%.
At week 56, there were no significant differences between the groups in blood pressure, fasting lipids, fasting plasma glucose, or hemoglobin A1c, the authors noted.
However, in the liraglutide group, 43.3% of participants lost at least 5% of their BMI, compared with 18.7% in the control group. Similarly, 26.1% of those in the liraglutide group had a BMI reduction of at least 10%, compared with 8.1% in the control group.
Participants in the liraglutide group also saw a greater reduction in BMI, compared with those in the placebo group (estimated difference, 4.64 percentage points), and those taking liraglutide lost 9.9 pounds (4.5 kg) more than those receiving placebo – a relative reduction of 5%. The authors noted that a weight loss of 3%-5% “significantly improves some health-related outcomes in adults.”
In addition, the liraglutide group had a BMI standard-deviation score that was 0.22 lower than that in the placebo group (P = .002), but after the participants discontinued with the trial, “a greater increase in the BMI standard-deviation score was observed with liraglutide than with placebo (0.15),” the authors reported.
“Although evidence in children is limited, a change in BMI standard-deviation score of at least 0.20 has been suggested to be clinically meaningful,” they wrote. “Some studies indicate that even temporary weight loss may have long-term benefits, but the extent to which this applies in adolescents and the extent to which long-term adherence to pharmacotherapy can be expected are unknown.”
The researchers added that the reduction in standard-deviation score seen in this study, of 0.22, was a bigger reduction than that seen in lifestyle therapy trials from the U.S. Preventive Services Task Force and from an overview of six Cochrane reviews. Their trial also, however, had a fairly high adherence rate, over 80%.
No notable differences in cardiometabolic markers or in quality of life showed up between the liraglutide and placebo groups. The heterogeneous treatment response in this and past studies suggests the need for future trials to “characterize predictors of treatment response to identify patients who would benefit the most from treatment,” the authors wrote.
About twice as many participants taking liraglutide experienced gastrointestinal adverse events compared with those receiving placebo (64.8% vs. 36.5%, respectively). Those symptoms, a known side effect of this drug type, included nausea, vomiting, and diarrhea and occurred primarily during escalation of the drug dose before then dropping in frequency. Still, the authors note that the high rate of gastrointestinal effects “suggests that this treatment may not be suitable for all patients.”
None of the adolescents receiving the placebo stopped treatment, but 10.4% of those taking liraglutide discontinued. One participant in the liraglutide group died by suicide, but the death was determined to be unrelated to the therapy.
Although the 0.22 reduction in the BMI standard-deviation score was for the intent-to-treat population, the authors calculated that the difference would have been 0.26 “if all participants had adhered to the treatment throughout the trial.”
Novo Nordisk funded the research. Several of the authors reported that they are employees of the company.
The abstract will also be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will ost ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Source: Kelly AS et al. NEJM. 2020 Mar 31. doi: 10.1056/NEJMoa1916038.
Prescribing liraglutide plus lifestyle therapy for adolescents with obesity resulted in greater weight loss and greater reduction in body mass index, compared with those prescribed lifestyle therapy alone, according to findings from a new study published in The New England Journal of Medicine.
Liraglutide with lifestyle therapy also “compared favorably in terms of [body mass index] reduction,” compared with other pediatric weight-management programs in the United States and with use of orlistat, wrote Aaron S. Kelly, PhD, of the University of Minnesota, Minneapolis, and colleagues. The study abstract was presented during a virtual news conference held by The Endocrine Society. It had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
The study included adolescents aged 12-17 years, who had obesity (BMI, ≥30 kg/m2) and had responded poorly to recommendations involving lifestyle therapy only, as judged by the site investigator and documented in the participant’s medical records. The adolescents participated at one of five sites in Belgium, Mexico, Russia, Sweden, and the United States.
In the randomized, controlled, double-blind trial, 125 participants received 3 mg liraglutide, and 126 received placebo for 56 weeks, during which both groups received lifestyle therapy, “defined as counseling about healthy nutrition and physical activity for weight loss,” the authors wrote.
After 12-weeks of run-in, the treatment period lasted 56 weeks, with a follow-up 26 weeks after treatment ended. The liraglutide group retained 80.8% of its participants, and the placebo group, 79.4%.
At week 56, there were no significant differences between the groups in blood pressure, fasting lipids, fasting plasma glucose, or hemoglobin A1c, the authors noted.
However, in the liraglutide group, 43.3% of participants lost at least 5% of their BMI, compared with 18.7% in the control group. Similarly, 26.1% of those in the liraglutide group had a BMI reduction of at least 10%, compared with 8.1% in the control group.
Participants in the liraglutide group also saw a greater reduction in BMI, compared with those in the placebo group (estimated difference, 4.64 percentage points), and those taking liraglutide lost 9.9 pounds (4.5 kg) more than those receiving placebo – a relative reduction of 5%. The authors noted that a weight loss of 3%-5% “significantly improves some health-related outcomes in adults.”
In addition, the liraglutide group had a BMI standard-deviation score that was 0.22 lower than that in the placebo group (P = .002), but after the participants discontinued with the trial, “a greater increase in the BMI standard-deviation score was observed with liraglutide than with placebo (0.15),” the authors reported.
“Although evidence in children is limited, a change in BMI standard-deviation score of at least 0.20 has been suggested to be clinically meaningful,” they wrote. “Some studies indicate that even temporary weight loss may have long-term benefits, but the extent to which this applies in adolescents and the extent to which long-term adherence to pharmacotherapy can be expected are unknown.”
The researchers added that the reduction in standard-deviation score seen in this study, of 0.22, was a bigger reduction than that seen in lifestyle therapy trials from the U.S. Preventive Services Task Force and from an overview of six Cochrane reviews. Their trial also, however, had a fairly high adherence rate, over 80%.
No notable differences in cardiometabolic markers or in quality of life showed up between the liraglutide and placebo groups. The heterogeneous treatment response in this and past studies suggests the need for future trials to “characterize predictors of treatment response to identify patients who would benefit the most from treatment,” the authors wrote.
About twice as many participants taking liraglutide experienced gastrointestinal adverse events compared with those receiving placebo (64.8% vs. 36.5%, respectively). Those symptoms, a known side effect of this drug type, included nausea, vomiting, and diarrhea and occurred primarily during escalation of the drug dose before then dropping in frequency. Still, the authors note that the high rate of gastrointestinal effects “suggests that this treatment may not be suitable for all patients.”
None of the adolescents receiving the placebo stopped treatment, but 10.4% of those taking liraglutide discontinued. One participant in the liraglutide group died by suicide, but the death was determined to be unrelated to the therapy.
Although the 0.22 reduction in the BMI standard-deviation score was for the intent-to-treat population, the authors calculated that the difference would have been 0.26 “if all participants had adhered to the treatment throughout the trial.”
Novo Nordisk funded the research. Several of the authors reported that they are employees of the company.
The abstract will also be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will ost ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Source: Kelly AS et al. NEJM. 2020 Mar 31. doi: 10.1056/NEJMoa1916038.
Prescribing liraglutide plus lifestyle therapy for adolescents with obesity resulted in greater weight loss and greater reduction in body mass index, compared with those prescribed lifestyle therapy alone, according to findings from a new study published in The New England Journal of Medicine.
Liraglutide with lifestyle therapy also “compared favorably in terms of [body mass index] reduction,” compared with other pediatric weight-management programs in the United States and with use of orlistat, wrote Aaron S. Kelly, PhD, of the University of Minnesota, Minneapolis, and colleagues. The study abstract was presented during a virtual news conference held by The Endocrine Society. It had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
The study included adolescents aged 12-17 years, who had obesity (BMI, ≥30 kg/m2) and had responded poorly to recommendations involving lifestyle therapy only, as judged by the site investigator and documented in the participant’s medical records. The adolescents participated at one of five sites in Belgium, Mexico, Russia, Sweden, and the United States.
In the randomized, controlled, double-blind trial, 125 participants received 3 mg liraglutide, and 126 received placebo for 56 weeks, during which both groups received lifestyle therapy, “defined as counseling about healthy nutrition and physical activity for weight loss,” the authors wrote.
After 12-weeks of run-in, the treatment period lasted 56 weeks, with a follow-up 26 weeks after treatment ended. The liraglutide group retained 80.8% of its participants, and the placebo group, 79.4%.
At week 56, there were no significant differences between the groups in blood pressure, fasting lipids, fasting plasma glucose, or hemoglobin A1c, the authors noted.
However, in the liraglutide group, 43.3% of participants lost at least 5% of their BMI, compared with 18.7% in the control group. Similarly, 26.1% of those in the liraglutide group had a BMI reduction of at least 10%, compared with 8.1% in the control group.
Participants in the liraglutide group also saw a greater reduction in BMI, compared with those in the placebo group (estimated difference, 4.64 percentage points), and those taking liraglutide lost 9.9 pounds (4.5 kg) more than those receiving placebo – a relative reduction of 5%. The authors noted that a weight loss of 3%-5% “significantly improves some health-related outcomes in adults.”
In addition, the liraglutide group had a BMI standard-deviation score that was 0.22 lower than that in the placebo group (P = .002), but after the participants discontinued with the trial, “a greater increase in the BMI standard-deviation score was observed with liraglutide than with placebo (0.15),” the authors reported.
“Although evidence in children is limited, a change in BMI standard-deviation score of at least 0.20 has been suggested to be clinically meaningful,” they wrote. “Some studies indicate that even temporary weight loss may have long-term benefits, but the extent to which this applies in adolescents and the extent to which long-term adherence to pharmacotherapy can be expected are unknown.”
The researchers added that the reduction in standard-deviation score seen in this study, of 0.22, was a bigger reduction than that seen in lifestyle therapy trials from the U.S. Preventive Services Task Force and from an overview of six Cochrane reviews. Their trial also, however, had a fairly high adherence rate, over 80%.
No notable differences in cardiometabolic markers or in quality of life showed up between the liraglutide and placebo groups. The heterogeneous treatment response in this and past studies suggests the need for future trials to “characterize predictors of treatment response to identify patients who would benefit the most from treatment,” the authors wrote.
About twice as many participants taking liraglutide experienced gastrointestinal adverse events compared with those receiving placebo (64.8% vs. 36.5%, respectively). Those symptoms, a known side effect of this drug type, included nausea, vomiting, and diarrhea and occurred primarily during escalation of the drug dose before then dropping in frequency. Still, the authors note that the high rate of gastrointestinal effects “suggests that this treatment may not be suitable for all patients.”
None of the adolescents receiving the placebo stopped treatment, but 10.4% of those taking liraglutide discontinued. One participant in the liraglutide group died by suicide, but the death was determined to be unrelated to the therapy.
Although the 0.22 reduction in the BMI standard-deviation score was for the intent-to-treat population, the authors calculated that the difference would have been 0.26 “if all participants had adhered to the treatment throughout the trial.”
Novo Nordisk funded the research. Several of the authors reported that they are employees of the company.
The abstract will also be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will ost ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Source: Kelly AS et al. NEJM. 2020 Mar 31. doi: 10.1056/NEJMoa1916038.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
PARP inhibitors not cost effective for platinum-resistant ovarian cancer
For patients with platinum-resistant ovarian cancer with BRCA1/2 mutations, third- or fourth-line therapy with poly (ADP-ribose) polymerase (PARP) inhibitors is less cost effective than non–platinum-based chemotherapy or bevacizumab-containing regimens, according to a study published in Gynecologic Oncology.
Compared with PARP inhibitors, intravenous chemotherapy regimens tend to produce lower response rates and shorter median progression-free survival (PFS) in this difficult-to-treat population, according to study author Juliet E. Wolford, MD, of the University of California, Irvine, and colleagues.
PARP inhibitors also have the advantages of oral administration and being well tolerated, the researchers noted. However, they found the expense of PARP inhibitors remains substantially greater per month of PFS, even after accounting for the costs of infusion and toxicity management related to chemotherapy.
“We initially wanted to do this study because we suspected that, when including the costs of infusions and costs of managing toxicities, even though the PARP [inhibitors] were more expensive, they would ultimately be more cost effective because they were well tolerated, oral, and more effective,” Dr. Wolford said in an interview. “Surprisingly, the high costs of the PARP [inhibitors] outweighs any other factors so much so that the costs of receiving infusions or managing the adverse events becomes negligible.”
Dr. Wolford and colleagues developed a model using median PFS and toxicity data from regulatory trials to show patient response, complications (hematologic and nonhematologic), progression, and death.
The researchers compared olaparib, rucaparib, and niraparib individually to non–platinum-based chemotherapy regimens and to regimens containing bevacizumab. The team then estimated the costs of intravenous drugs, infusions, toxicity management, and supportive care, based on 2017 Medicare data.
The cost of non–platinum-based intravenous chemotherapy was $6,412 per quality-adjusted month of PFS, a little more than half the cost of bevacizumab-containing regimens, which was $12,187 per month of PFS.
The cost of PARP inhibitors was much higher: $18,970 per month of PFS for niraparib, $16,637 per month of PFS for rucaparib, and $16,327 per month of PFS for olaparib.
“An interesting, albeit not unexpected, phenomenon we observed in our analyses was that, with the relatively higher response rates and/or duration of response associated with PARP [inhibitor] treatment, higher drug costs are incurred,” Dr. Wolford and colleagues wrote.
“The longer patients remain progression free, the longer they remain on treatment and accumulate treatment-related cost,” the authors wrote, noting that complete responses are rare during recurrence treatment, so patients tend to receive salvage therapy until their disease progresses.
However, Dr. Wolford pointed out that using a model requires making assumptions and that “clinical decisions are not derived from a simulation.
“This type of simulation can facilitate the recognition of the financial burden the use of these novel treatments can place on our patients but, more importantly, can highlight the importance of identifying predictive biomarkers,” she said. “We need to be able to distinguish those patients who will benefit the most from the treatment in order to circumvent patients from experiencing financial toxicity from a therapy they will not derive benefit from.”
In their paper, Dr. Wolford and colleagues also pointed out that the new drugs’ cost-effectiveness could substantially improve with minimal reductions in cost, according to many models.
“Such reductions to improve the affordability of many novel molecules can be achieved through mechanisms which result in more widespread use and increased awareness and accessibility of the targeted agent in clinical practice,” the authors wrote.
Further, this study focused on platinum-resistant patients, who are particularly difficult to treat. Expanding the use of PARP inhibitors or identifying the most clinically meaningful uses of them could improve their cost-effectiveness, including possibly using them earlier in the disease course, the authors noted.
“We know from SOLO-1, PRIMA, and PAOLA-1 studies that using the PARP [inhibitors] as frontline maintenance therapy can have a significant benefit, so likely the trend will be to use the PARP [inhibitors] earlier in the disease course and utilizing the antiangiogenic therapy for recurrences when patients begin to develop platinum resistance,” Dr. Wolford said. “It is important to note, however, that, for the frontline trials, we only have PFS data, as the overall survival data is not yet mature.”
The high current costs of PARP inhibitors also follow a common trend with new oncologic agents, Dr. Wolford noted. “When they are first introduced, the high costs are reflective of the high developmental costs. As use of the novel therapies becomes more pervasive, with the approval of additional indications, the costs will eventually decrease over time.”
Dr. Wolford and colleagues did not report any external funding for this study. Some authors disclosed relationships with a range of pharmaceutical, device, and cancer-related businesses.
SOURCE: Wolford JE et al. Gynecol Oncol. 2020 Mar 13. doi: 10.1016/j.ygyno.2020.02.030.
For patients with platinum-resistant ovarian cancer with BRCA1/2 mutations, third- or fourth-line therapy with poly (ADP-ribose) polymerase (PARP) inhibitors is less cost effective than non–platinum-based chemotherapy or bevacizumab-containing regimens, according to a study published in Gynecologic Oncology.
Compared with PARP inhibitors, intravenous chemotherapy regimens tend to produce lower response rates and shorter median progression-free survival (PFS) in this difficult-to-treat population, according to study author Juliet E. Wolford, MD, of the University of California, Irvine, and colleagues.
PARP inhibitors also have the advantages of oral administration and being well tolerated, the researchers noted. However, they found the expense of PARP inhibitors remains substantially greater per month of PFS, even after accounting for the costs of infusion and toxicity management related to chemotherapy.
“We initially wanted to do this study because we suspected that, when including the costs of infusions and costs of managing toxicities, even though the PARP [inhibitors] were more expensive, they would ultimately be more cost effective because they were well tolerated, oral, and more effective,” Dr. Wolford said in an interview. “Surprisingly, the high costs of the PARP [inhibitors] outweighs any other factors so much so that the costs of receiving infusions or managing the adverse events becomes negligible.”
Dr. Wolford and colleagues developed a model using median PFS and toxicity data from regulatory trials to show patient response, complications (hematologic and nonhematologic), progression, and death.
The researchers compared olaparib, rucaparib, and niraparib individually to non–platinum-based chemotherapy regimens and to regimens containing bevacizumab. The team then estimated the costs of intravenous drugs, infusions, toxicity management, and supportive care, based on 2017 Medicare data.
The cost of non–platinum-based intravenous chemotherapy was $6,412 per quality-adjusted month of PFS, a little more than half the cost of bevacizumab-containing regimens, which was $12,187 per month of PFS.
The cost of PARP inhibitors was much higher: $18,970 per month of PFS for niraparib, $16,637 per month of PFS for rucaparib, and $16,327 per month of PFS for olaparib.
“An interesting, albeit not unexpected, phenomenon we observed in our analyses was that, with the relatively higher response rates and/or duration of response associated with PARP [inhibitor] treatment, higher drug costs are incurred,” Dr. Wolford and colleagues wrote.
“The longer patients remain progression free, the longer they remain on treatment and accumulate treatment-related cost,” the authors wrote, noting that complete responses are rare during recurrence treatment, so patients tend to receive salvage therapy until their disease progresses.
However, Dr. Wolford pointed out that using a model requires making assumptions and that “clinical decisions are not derived from a simulation.
“This type of simulation can facilitate the recognition of the financial burden the use of these novel treatments can place on our patients but, more importantly, can highlight the importance of identifying predictive biomarkers,” she said. “We need to be able to distinguish those patients who will benefit the most from the treatment in order to circumvent patients from experiencing financial toxicity from a therapy they will not derive benefit from.”
In their paper, Dr. Wolford and colleagues also pointed out that the new drugs’ cost-effectiveness could substantially improve with minimal reductions in cost, according to many models.
“Such reductions to improve the affordability of many novel molecules can be achieved through mechanisms which result in more widespread use and increased awareness and accessibility of the targeted agent in clinical practice,” the authors wrote.
Further, this study focused on platinum-resistant patients, who are particularly difficult to treat. Expanding the use of PARP inhibitors or identifying the most clinically meaningful uses of them could improve their cost-effectiveness, including possibly using them earlier in the disease course, the authors noted.
“We know from SOLO-1, PRIMA, and PAOLA-1 studies that using the PARP [inhibitors] as frontline maintenance therapy can have a significant benefit, so likely the trend will be to use the PARP [inhibitors] earlier in the disease course and utilizing the antiangiogenic therapy for recurrences when patients begin to develop platinum resistance,” Dr. Wolford said. “It is important to note, however, that, for the frontline trials, we only have PFS data, as the overall survival data is not yet mature.”
The high current costs of PARP inhibitors also follow a common trend with new oncologic agents, Dr. Wolford noted. “When they are first introduced, the high costs are reflective of the high developmental costs. As use of the novel therapies becomes more pervasive, with the approval of additional indications, the costs will eventually decrease over time.”
Dr. Wolford and colleagues did not report any external funding for this study. Some authors disclosed relationships with a range of pharmaceutical, device, and cancer-related businesses.
SOURCE: Wolford JE et al. Gynecol Oncol. 2020 Mar 13. doi: 10.1016/j.ygyno.2020.02.030.
For patients with platinum-resistant ovarian cancer with BRCA1/2 mutations, third- or fourth-line therapy with poly (ADP-ribose) polymerase (PARP) inhibitors is less cost effective than non–platinum-based chemotherapy or bevacizumab-containing regimens, according to a study published in Gynecologic Oncology.
Compared with PARP inhibitors, intravenous chemotherapy regimens tend to produce lower response rates and shorter median progression-free survival (PFS) in this difficult-to-treat population, according to study author Juliet E. Wolford, MD, of the University of California, Irvine, and colleagues.
PARP inhibitors also have the advantages of oral administration and being well tolerated, the researchers noted. However, they found the expense of PARP inhibitors remains substantially greater per month of PFS, even after accounting for the costs of infusion and toxicity management related to chemotherapy.
“We initially wanted to do this study because we suspected that, when including the costs of infusions and costs of managing toxicities, even though the PARP [inhibitors] were more expensive, they would ultimately be more cost effective because they were well tolerated, oral, and more effective,” Dr. Wolford said in an interview. “Surprisingly, the high costs of the PARP [inhibitors] outweighs any other factors so much so that the costs of receiving infusions or managing the adverse events becomes negligible.”
Dr. Wolford and colleagues developed a model using median PFS and toxicity data from regulatory trials to show patient response, complications (hematologic and nonhematologic), progression, and death.
The researchers compared olaparib, rucaparib, and niraparib individually to non–platinum-based chemotherapy regimens and to regimens containing bevacizumab. The team then estimated the costs of intravenous drugs, infusions, toxicity management, and supportive care, based on 2017 Medicare data.
The cost of non–platinum-based intravenous chemotherapy was $6,412 per quality-adjusted month of PFS, a little more than half the cost of bevacizumab-containing regimens, which was $12,187 per month of PFS.
The cost of PARP inhibitors was much higher: $18,970 per month of PFS for niraparib, $16,637 per month of PFS for rucaparib, and $16,327 per month of PFS for olaparib.
“An interesting, albeit not unexpected, phenomenon we observed in our analyses was that, with the relatively higher response rates and/or duration of response associated with PARP [inhibitor] treatment, higher drug costs are incurred,” Dr. Wolford and colleagues wrote.
“The longer patients remain progression free, the longer they remain on treatment and accumulate treatment-related cost,” the authors wrote, noting that complete responses are rare during recurrence treatment, so patients tend to receive salvage therapy until their disease progresses.
However, Dr. Wolford pointed out that using a model requires making assumptions and that “clinical decisions are not derived from a simulation.
“This type of simulation can facilitate the recognition of the financial burden the use of these novel treatments can place on our patients but, more importantly, can highlight the importance of identifying predictive biomarkers,” she said. “We need to be able to distinguish those patients who will benefit the most from the treatment in order to circumvent patients from experiencing financial toxicity from a therapy they will not derive benefit from.”
In their paper, Dr. Wolford and colleagues also pointed out that the new drugs’ cost-effectiveness could substantially improve with minimal reductions in cost, according to many models.
“Such reductions to improve the affordability of many novel molecules can be achieved through mechanisms which result in more widespread use and increased awareness and accessibility of the targeted agent in clinical practice,” the authors wrote.
Further, this study focused on platinum-resistant patients, who are particularly difficult to treat. Expanding the use of PARP inhibitors or identifying the most clinically meaningful uses of them could improve their cost-effectiveness, including possibly using them earlier in the disease course, the authors noted.
“We know from SOLO-1, PRIMA, and PAOLA-1 studies that using the PARP [inhibitors] as frontline maintenance therapy can have a significant benefit, so likely the trend will be to use the PARP [inhibitors] earlier in the disease course and utilizing the antiangiogenic therapy for recurrences when patients begin to develop platinum resistance,” Dr. Wolford said. “It is important to note, however, that, for the frontline trials, we only have PFS data, as the overall survival data is not yet mature.”
The high current costs of PARP inhibitors also follow a common trend with new oncologic agents, Dr. Wolford noted. “When they are first introduced, the high costs are reflective of the high developmental costs. As use of the novel therapies becomes more pervasive, with the approval of additional indications, the costs will eventually decrease over time.”
Dr. Wolford and colleagues did not report any external funding for this study. Some authors disclosed relationships with a range of pharmaceutical, device, and cancer-related businesses.
SOURCE: Wolford JE et al. Gynecol Oncol. 2020 Mar 13. doi: 10.1016/j.ygyno.2020.02.030.
FROM GYNECOLOGIC ONCOLOGY
Sexual-minority youth at greater risk for physical, sexual violence
U.S. high school students who identify as gay, lesbian, bisexual, or questioning – “sexual minorities” – faced twice the risk of physical or sexual assault in the past year compared with their heterosexual peers, according to findings reported in a research letter.
Sexual-minority females were particularly more likely to experience physical violence while sexual-minority boys had a fourfold increased risk of sexual violence.
“The results of our study suggest the existence of a crisis of violence against sexual minority adolescents,” Theodore L. Caputi, MPH, of Harvard Medical School, Boston, and colleagues reported in JAMA Pediatrics. “Given the substantial physical and emotional consequences of violence for those subjected to it and the large existing health disparities among sexual minority adolescents, addressing both physical and sexual violence against sexual minority adolescents should become a public health priority.”
Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery in the Mount Sinai Health System, New York, said he was not surprised by the findings because adolescents who may feel more vulnerable relative to their peers are likely to be more of a target. They may not have the supports they need, he said, which will affect their resiliency and their ability to push back.
“These patients are at ages where their parents might be among their supporters,” Dr. Safer said in an interview. “People in their circle may not be aware of their circumstances.”
He emphasized the need for physicians to ensure their offices are safe places for sexual-minority youth to talk to adolescent patients about their gender and sexual identity as well as any history of victimization, and to involve parents in being an ally of their child.
The researchers analyzed data from the nationally representative 2015 and 2017 National Youth Risk Behavior Surveys administered to public and private high school students in grades 9-12 by the Centers for Disease Control and Prevention. The 28,811 total respondents represented a 60% response rate both years.
After indicating their sex as male or female and their sexual orientation, respondents reported whether, in the past year, they had experienced a physical fight at school, a physical fight anywhere, or physical violence from a romantic partner. They also reported whether they had been sexually assaulted in the past year by a romantic partner or ever been forced to have intercourse. The 2017 survey included an additional question about sexual assault by anyone in the past year.
Most youth (87%) identified themselves as heterosexual while 2% were gay/lesbian, 7% were bisexual, and 4% were unsure. Sexual minorities reported a higher prevalence of all forms of violence and assault, compared with their heterosexual counterparts. Although risk of a physical fight in the past year differed by a small amount (28% of sexual-minority youth vs. 22% of heterosexual youth), the gap was considerably greater for risk of physical violence by a romantic partner (12% of sexual-minority youth vs. 5% of heterosexual youth).
More than three times as many sexual-minority adolescents (18%) as heterosexual adolescents (5%) said they had ever been forced to have intercourse, and a similarly high proportion of sexual-minority students (21%) had been sexually assaulted in the past year, compared with heterosexual students (8%). After accounting for survey year, sex, age, race/ethnicity, English language proficiency, and grade level, youth who identified as anything other than heterosexual were about twice as likely as their heterosexual counterparts to have experienced physical or sexual violence, including physical violence by a romantic partner (adjusted risk ratio, 1.97) or sexual assault by anyone (aRR, 2.10), in the past year. The risk of physical violence by a romantic partner or sexual assault by anyone was even greater for bisexual youth (aRR, 2.22 and aRR, 2.36, respectively).
The increased likelihood of physical violence and sexual violence differed by sex. Girls who identified as lesbian, bisexual, or questioning were more likely than heterosexual girls to have been in a fight at school or anywhere else (aRR, 1.91 and aRR, 1.74, respectively). Boys who were gay, bisexual, or questioning, meanwhile, were over four times more likely than heterosexual boys to have had forced intercourse or any kind of sexual assault (aRR, 4.70 and aRR, 4.64, respectively).
These findings point to the need for physicians to be “specifically talking to youth about gender identity and sexual orientation. Validating what kids are feeling is important,” Dr. Safer said in an interview.
Key to that process is making sure the physician’s office feels like a safe place for LGBTQ youth to have these kinds of conversations. “Most primary care and pediatric and adolescent care practices are not feeling well equipped to take care of these kids and are not necessarily serving as a good resource for these kids,” Dr. Safer said.
It’s also important for physicians to ask youth about potential violence or abuse they have experienced, including depression and sequelae from lack of support, for which gender- and sexual-minority youth are at greater risk, he said. Finally, doctors need to engage parents in the conversation.
“As a medical professional, you need to be asking the questions and really be out there as an ally, especially for pediatric and adolescent patients, and you need to be helping the parents of your patients be allies too,” Dr. Safer said.
The study was limited by having a binary question only about respondent’s sex and no data collection about transgender youth. The study’s cross-sectional design also precludes the ability to claim causation about any of the associations.
The research was funded by the Marshall Aid Commemoration Commission, Stanford (Calif.) University, and the National Institutes of Health. The authors had no disclosures.
SOURCE: Caputi TL et al. JAMA Pediatr. 2019 Mar 9. doi: 10.1001/jamapediatrics.2019.6291.
U.S. high school students who identify as gay, lesbian, bisexual, or questioning – “sexual minorities” – faced twice the risk of physical or sexual assault in the past year compared with their heterosexual peers, according to findings reported in a research letter.
Sexual-minority females were particularly more likely to experience physical violence while sexual-minority boys had a fourfold increased risk of sexual violence.
“The results of our study suggest the existence of a crisis of violence against sexual minority adolescents,” Theodore L. Caputi, MPH, of Harvard Medical School, Boston, and colleagues reported in JAMA Pediatrics. “Given the substantial physical and emotional consequences of violence for those subjected to it and the large existing health disparities among sexual minority adolescents, addressing both physical and sexual violence against sexual minority adolescents should become a public health priority.”
Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery in the Mount Sinai Health System, New York, said he was not surprised by the findings because adolescents who may feel more vulnerable relative to their peers are likely to be more of a target. They may not have the supports they need, he said, which will affect their resiliency and their ability to push back.
“These patients are at ages where their parents might be among their supporters,” Dr. Safer said in an interview. “People in their circle may not be aware of their circumstances.”
He emphasized the need for physicians to ensure their offices are safe places for sexual-minority youth to talk to adolescent patients about their gender and sexual identity as well as any history of victimization, and to involve parents in being an ally of their child.
The researchers analyzed data from the nationally representative 2015 and 2017 National Youth Risk Behavior Surveys administered to public and private high school students in grades 9-12 by the Centers for Disease Control and Prevention. The 28,811 total respondents represented a 60% response rate both years.
After indicating their sex as male or female and their sexual orientation, respondents reported whether, in the past year, they had experienced a physical fight at school, a physical fight anywhere, or physical violence from a romantic partner. They also reported whether they had been sexually assaulted in the past year by a romantic partner or ever been forced to have intercourse. The 2017 survey included an additional question about sexual assault by anyone in the past year.
Most youth (87%) identified themselves as heterosexual while 2% were gay/lesbian, 7% were bisexual, and 4% were unsure. Sexual minorities reported a higher prevalence of all forms of violence and assault, compared with their heterosexual counterparts. Although risk of a physical fight in the past year differed by a small amount (28% of sexual-minority youth vs. 22% of heterosexual youth), the gap was considerably greater for risk of physical violence by a romantic partner (12% of sexual-minority youth vs. 5% of heterosexual youth).
More than three times as many sexual-minority adolescents (18%) as heterosexual adolescents (5%) said they had ever been forced to have intercourse, and a similarly high proportion of sexual-minority students (21%) had been sexually assaulted in the past year, compared with heterosexual students (8%). After accounting for survey year, sex, age, race/ethnicity, English language proficiency, and grade level, youth who identified as anything other than heterosexual were about twice as likely as their heterosexual counterparts to have experienced physical or sexual violence, including physical violence by a romantic partner (adjusted risk ratio, 1.97) or sexual assault by anyone (aRR, 2.10), in the past year. The risk of physical violence by a romantic partner or sexual assault by anyone was even greater for bisexual youth (aRR, 2.22 and aRR, 2.36, respectively).
The increased likelihood of physical violence and sexual violence differed by sex. Girls who identified as lesbian, bisexual, or questioning were more likely than heterosexual girls to have been in a fight at school or anywhere else (aRR, 1.91 and aRR, 1.74, respectively). Boys who were gay, bisexual, or questioning, meanwhile, were over four times more likely than heterosexual boys to have had forced intercourse or any kind of sexual assault (aRR, 4.70 and aRR, 4.64, respectively).
These findings point to the need for physicians to be “specifically talking to youth about gender identity and sexual orientation. Validating what kids are feeling is important,” Dr. Safer said in an interview.
Key to that process is making sure the physician’s office feels like a safe place for LGBTQ youth to have these kinds of conversations. “Most primary care and pediatric and adolescent care practices are not feeling well equipped to take care of these kids and are not necessarily serving as a good resource for these kids,” Dr. Safer said.
It’s also important for physicians to ask youth about potential violence or abuse they have experienced, including depression and sequelae from lack of support, for which gender- and sexual-minority youth are at greater risk, he said. Finally, doctors need to engage parents in the conversation.
“As a medical professional, you need to be asking the questions and really be out there as an ally, especially for pediatric and adolescent patients, and you need to be helping the parents of your patients be allies too,” Dr. Safer said.
The study was limited by having a binary question only about respondent’s sex and no data collection about transgender youth. The study’s cross-sectional design also precludes the ability to claim causation about any of the associations.
The research was funded by the Marshall Aid Commemoration Commission, Stanford (Calif.) University, and the National Institutes of Health. The authors had no disclosures.
SOURCE: Caputi TL et al. JAMA Pediatr. 2019 Mar 9. doi: 10.1001/jamapediatrics.2019.6291.
U.S. high school students who identify as gay, lesbian, bisexual, or questioning – “sexual minorities” – faced twice the risk of physical or sexual assault in the past year compared with their heterosexual peers, according to findings reported in a research letter.
Sexual-minority females were particularly more likely to experience physical violence while sexual-minority boys had a fourfold increased risk of sexual violence.
“The results of our study suggest the existence of a crisis of violence against sexual minority adolescents,” Theodore L. Caputi, MPH, of Harvard Medical School, Boston, and colleagues reported in JAMA Pediatrics. “Given the substantial physical and emotional consequences of violence for those subjected to it and the large existing health disparities among sexual minority adolescents, addressing both physical and sexual violence against sexual minority adolescents should become a public health priority.”
Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery in the Mount Sinai Health System, New York, said he was not surprised by the findings because adolescents who may feel more vulnerable relative to their peers are likely to be more of a target. They may not have the supports they need, he said, which will affect their resiliency and their ability to push back.
“These patients are at ages where their parents might be among their supporters,” Dr. Safer said in an interview. “People in their circle may not be aware of their circumstances.”
He emphasized the need for physicians to ensure their offices are safe places for sexual-minority youth to talk to adolescent patients about their gender and sexual identity as well as any history of victimization, and to involve parents in being an ally of their child.
The researchers analyzed data from the nationally representative 2015 and 2017 National Youth Risk Behavior Surveys administered to public and private high school students in grades 9-12 by the Centers for Disease Control and Prevention. The 28,811 total respondents represented a 60% response rate both years.
After indicating their sex as male or female and their sexual orientation, respondents reported whether, in the past year, they had experienced a physical fight at school, a physical fight anywhere, or physical violence from a romantic partner. They also reported whether they had been sexually assaulted in the past year by a romantic partner or ever been forced to have intercourse. The 2017 survey included an additional question about sexual assault by anyone in the past year.
Most youth (87%) identified themselves as heterosexual while 2% were gay/lesbian, 7% were bisexual, and 4% were unsure. Sexual minorities reported a higher prevalence of all forms of violence and assault, compared with their heterosexual counterparts. Although risk of a physical fight in the past year differed by a small amount (28% of sexual-minority youth vs. 22% of heterosexual youth), the gap was considerably greater for risk of physical violence by a romantic partner (12% of sexual-minority youth vs. 5% of heterosexual youth).
More than three times as many sexual-minority adolescents (18%) as heterosexual adolescents (5%) said they had ever been forced to have intercourse, and a similarly high proportion of sexual-minority students (21%) had been sexually assaulted in the past year, compared with heterosexual students (8%). After accounting for survey year, sex, age, race/ethnicity, English language proficiency, and grade level, youth who identified as anything other than heterosexual were about twice as likely as their heterosexual counterparts to have experienced physical or sexual violence, including physical violence by a romantic partner (adjusted risk ratio, 1.97) or sexual assault by anyone (aRR, 2.10), in the past year. The risk of physical violence by a romantic partner or sexual assault by anyone was even greater for bisexual youth (aRR, 2.22 and aRR, 2.36, respectively).
The increased likelihood of physical violence and sexual violence differed by sex. Girls who identified as lesbian, bisexual, or questioning were more likely than heterosexual girls to have been in a fight at school or anywhere else (aRR, 1.91 and aRR, 1.74, respectively). Boys who were gay, bisexual, or questioning, meanwhile, were over four times more likely than heterosexual boys to have had forced intercourse or any kind of sexual assault (aRR, 4.70 and aRR, 4.64, respectively).
These findings point to the need for physicians to be “specifically talking to youth about gender identity and sexual orientation. Validating what kids are feeling is important,” Dr. Safer said in an interview.
Key to that process is making sure the physician’s office feels like a safe place for LGBTQ youth to have these kinds of conversations. “Most primary care and pediatric and adolescent care practices are not feeling well equipped to take care of these kids and are not necessarily serving as a good resource for these kids,” Dr. Safer said.
It’s also important for physicians to ask youth about potential violence or abuse they have experienced, including depression and sequelae from lack of support, for which gender- and sexual-minority youth are at greater risk, he said. Finally, doctors need to engage parents in the conversation.
“As a medical professional, you need to be asking the questions and really be out there as an ally, especially for pediatric and adolescent patients, and you need to be helping the parents of your patients be allies too,” Dr. Safer said.
The study was limited by having a binary question only about respondent’s sex and no data collection about transgender youth. The study’s cross-sectional design also precludes the ability to claim causation about any of the associations.
The research was funded by the Marshall Aid Commemoration Commission, Stanford (Calif.) University, and the National Institutes of Health. The authors had no disclosures.
SOURCE: Caputi TL et al. JAMA Pediatr. 2019 Mar 9. doi: 10.1001/jamapediatrics.2019.6291.
FROM JAMA PEDIATRICS
Gender pronouns in EMR preferred by many gender nonconforming teens
Most transgender and gender nonconforming youth would like their preferred name and pronouns be recorded throughout their EMRs, but very few are ever asked for that identity information outside of gender specialty clinic settings, according to a recent research letter in JAMA Pediatrics.
The findings are not surprising, said Cora Breuner, MD, a professor of pediatrics in adolescent medicine at Seattle Children’s Hospital in Washington, because “we know that use of gender-affirming language when accessing health care is extremely important to transgender youth.”
“Use of gender-affirming language in the health care system is associated with improved mental health outcomes in this population,” Dr Breuner said in an interview.
But the authors of the study noted that EMRs often lack the functions needed to provide gender-affirming care.
“To better support this vulnerable group of youths, health systems and EMRs should allow for EMR-wide name and pronoun documentation, even when a patient has not legally changed their name,” Gina M. Sequeira, MD, of UPMC Children’s Hospital of Pittsburgh and associates wrote.
Although many providers have begun routinely asking patients for both their gender identity and their sex assigned at birth, these questions leave out a patient’s preferred name and pronouns – crucial components of respectful and affirming care, the authors explained.
At a specialty gender clinic, the authors surveyed 204 transgender youths, aged 12-26 years, regarding how their name and pronouns are recorded in their EMR files. Just over half the respondents were under age 18 years (56%), and most were white (86%). Most were transmasculine (59%), with 21% transfeminine and 20% nonbinary.
Most respondents (69%) went by a name other than their legal one, yet only 9% said they were frequently or always asked in clinical settings outside specialty gender centers whether they wanted their preferred name and pronouns noted in the EMR.
A majority (79%), however, said they wanted their name and pronouns noted throughout their EMR. The youths’ preferences varied according to their gender identity and how many people were aware of their gender identity, but not by age, race/ethnicity, or perceived amount of parental support.
Only two-thirds (67%) of 42 transfeminine patients wished their EMR to include their preferred name, compared with most (85%) of 121 transmasculine patients and nearly all (92%) of 37 nonbinary respondents (P = .007). Pronouns preferences were similar: All but one nonbinary respondent wanted their pronouns in the EMR, compared with 84% of transmasculine and 64% of transfeminine respondents (P=.0003).
“It may be that transfeminine patients have more pressure to ‘stay’ their assigned gender,” Dr Breuner said regarding these findings. “ ‘Outness’ may be challenging, and thus they remain in their traditional gender norms, but further research on this theory is warranted.”
Among those who were “out to everyone,” most (88%) wanted their preferred name and pronouns recorded in the EMR, and the proportion was similar for those “out to most.” But only 65% of those “out to few or no one” preferred their name and pronouns be noted in the EMR, a similar proportion for those “out to some.”
Of 7 youths who did not wish to include their name and pronouns throughout their EMR, all but one said they didn’t think it was necessary because they believed they already “passed” well enough as their gender. Just one person said they did not want name and pronouns recorded for confidentiality reasons.
However, confidentiality is still an important consideration particularly for minors, the authors and Dr. Breuner pointed out.
“It is essential to discuss confidentiality with the youth as parents may have access to the medical records younger than 18 years of age,” Dr. Breuner said.
The authors noted the study’s limitation in using a convenience sample but they and Dr. Breuner said that the findings still demonstrate transgender youths’ desire for EMRs to include their name and pronouns.
The research was funded by grants from the National Institutes of Health. The authors had no industry disclosures.
SOURCE: Sequeira GM et al. JAMA Pediatrics. 2020 Feb 23. doi: 10.1001/jamapediatrics.2019.6071.
Most transgender and gender nonconforming youth would like their preferred name and pronouns be recorded throughout their EMRs, but very few are ever asked for that identity information outside of gender specialty clinic settings, according to a recent research letter in JAMA Pediatrics.
The findings are not surprising, said Cora Breuner, MD, a professor of pediatrics in adolescent medicine at Seattle Children’s Hospital in Washington, because “we know that use of gender-affirming language when accessing health care is extremely important to transgender youth.”
“Use of gender-affirming language in the health care system is associated with improved mental health outcomes in this population,” Dr Breuner said in an interview.
But the authors of the study noted that EMRs often lack the functions needed to provide gender-affirming care.
“To better support this vulnerable group of youths, health systems and EMRs should allow for EMR-wide name and pronoun documentation, even when a patient has not legally changed their name,” Gina M. Sequeira, MD, of UPMC Children’s Hospital of Pittsburgh and associates wrote.
Although many providers have begun routinely asking patients for both their gender identity and their sex assigned at birth, these questions leave out a patient’s preferred name and pronouns – crucial components of respectful and affirming care, the authors explained.
At a specialty gender clinic, the authors surveyed 204 transgender youths, aged 12-26 years, regarding how their name and pronouns are recorded in their EMR files. Just over half the respondents were under age 18 years (56%), and most were white (86%). Most were transmasculine (59%), with 21% transfeminine and 20% nonbinary.
Most respondents (69%) went by a name other than their legal one, yet only 9% said they were frequently or always asked in clinical settings outside specialty gender centers whether they wanted their preferred name and pronouns noted in the EMR.
A majority (79%), however, said they wanted their name and pronouns noted throughout their EMR. The youths’ preferences varied according to their gender identity and how many people were aware of their gender identity, but not by age, race/ethnicity, or perceived amount of parental support.
Only two-thirds (67%) of 42 transfeminine patients wished their EMR to include their preferred name, compared with most (85%) of 121 transmasculine patients and nearly all (92%) of 37 nonbinary respondents (P = .007). Pronouns preferences were similar: All but one nonbinary respondent wanted their pronouns in the EMR, compared with 84% of transmasculine and 64% of transfeminine respondents (P=.0003).
“It may be that transfeminine patients have more pressure to ‘stay’ their assigned gender,” Dr Breuner said regarding these findings. “ ‘Outness’ may be challenging, and thus they remain in their traditional gender norms, but further research on this theory is warranted.”
Among those who were “out to everyone,” most (88%) wanted their preferred name and pronouns recorded in the EMR, and the proportion was similar for those “out to most.” But only 65% of those “out to few or no one” preferred their name and pronouns be noted in the EMR, a similar proportion for those “out to some.”
Of 7 youths who did not wish to include their name and pronouns throughout their EMR, all but one said they didn’t think it was necessary because they believed they already “passed” well enough as their gender. Just one person said they did not want name and pronouns recorded for confidentiality reasons.
However, confidentiality is still an important consideration particularly for minors, the authors and Dr. Breuner pointed out.
“It is essential to discuss confidentiality with the youth as parents may have access to the medical records younger than 18 years of age,” Dr. Breuner said.
The authors noted the study’s limitation in using a convenience sample but they and Dr. Breuner said that the findings still demonstrate transgender youths’ desire for EMRs to include their name and pronouns.
The research was funded by grants from the National Institutes of Health. The authors had no industry disclosures.
SOURCE: Sequeira GM et al. JAMA Pediatrics. 2020 Feb 23. doi: 10.1001/jamapediatrics.2019.6071.
Most transgender and gender nonconforming youth would like their preferred name and pronouns be recorded throughout their EMRs, but very few are ever asked for that identity information outside of gender specialty clinic settings, according to a recent research letter in JAMA Pediatrics.
The findings are not surprising, said Cora Breuner, MD, a professor of pediatrics in adolescent medicine at Seattle Children’s Hospital in Washington, because “we know that use of gender-affirming language when accessing health care is extremely important to transgender youth.”
“Use of gender-affirming language in the health care system is associated with improved mental health outcomes in this population,” Dr Breuner said in an interview.
But the authors of the study noted that EMRs often lack the functions needed to provide gender-affirming care.
“To better support this vulnerable group of youths, health systems and EMRs should allow for EMR-wide name and pronoun documentation, even when a patient has not legally changed their name,” Gina M. Sequeira, MD, of UPMC Children’s Hospital of Pittsburgh and associates wrote.
Although many providers have begun routinely asking patients for both their gender identity and their sex assigned at birth, these questions leave out a patient’s preferred name and pronouns – crucial components of respectful and affirming care, the authors explained.
At a specialty gender clinic, the authors surveyed 204 transgender youths, aged 12-26 years, regarding how their name and pronouns are recorded in their EMR files. Just over half the respondents were under age 18 years (56%), and most were white (86%). Most were transmasculine (59%), with 21% transfeminine and 20% nonbinary.
Most respondents (69%) went by a name other than their legal one, yet only 9% said they were frequently or always asked in clinical settings outside specialty gender centers whether they wanted their preferred name and pronouns noted in the EMR.
A majority (79%), however, said they wanted their name and pronouns noted throughout their EMR. The youths’ preferences varied according to their gender identity and how many people were aware of their gender identity, but not by age, race/ethnicity, or perceived amount of parental support.
Only two-thirds (67%) of 42 transfeminine patients wished their EMR to include their preferred name, compared with most (85%) of 121 transmasculine patients and nearly all (92%) of 37 nonbinary respondents (P = .007). Pronouns preferences were similar: All but one nonbinary respondent wanted their pronouns in the EMR, compared with 84% of transmasculine and 64% of transfeminine respondents (P=.0003).
“It may be that transfeminine patients have more pressure to ‘stay’ their assigned gender,” Dr Breuner said regarding these findings. “ ‘Outness’ may be challenging, and thus they remain in their traditional gender norms, but further research on this theory is warranted.”
Among those who were “out to everyone,” most (88%) wanted their preferred name and pronouns recorded in the EMR, and the proportion was similar for those “out to most.” But only 65% of those “out to few or no one” preferred their name and pronouns be noted in the EMR, a similar proportion for those “out to some.”
Of 7 youths who did not wish to include their name and pronouns throughout their EMR, all but one said they didn’t think it was necessary because they believed they already “passed” well enough as their gender. Just one person said they did not want name and pronouns recorded for confidentiality reasons.
However, confidentiality is still an important consideration particularly for minors, the authors and Dr. Breuner pointed out.
“It is essential to discuss confidentiality with the youth as parents may have access to the medical records younger than 18 years of age,” Dr. Breuner said.
The authors noted the study’s limitation in using a convenience sample but they and Dr. Breuner said that the findings still demonstrate transgender youths’ desire for EMRs to include their name and pronouns.
The research was funded by grants from the National Institutes of Health. The authors had no industry disclosures.
SOURCE: Sequeira GM et al. JAMA Pediatrics. 2020 Feb 23. doi: 10.1001/jamapediatrics.2019.6071.
FROM JAMA PEDIATRICS